CN1271715A - 获得双酚a的方法 - Google Patents
获得双酚a的方法 Download PDFInfo
- Publication number
- CN1271715A CN1271715A CN 99118071 CN99118071A CN1271715A CN 1271715 A CN1271715 A CN 1271715A CN 99118071 CN99118071 CN 99118071 CN 99118071 A CN99118071 A CN 99118071A CN 1271715 A CN1271715 A CN 1271715A
- Authority
- CN
- China
- Prior art keywords
- phenol
- bisphenol
- acetone
- mother liquor
- adduct
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- IISBACLAFKSPIT-UHFFFAOYSA-N bisphenol A Chemical compound C=1C=C(O)C=CC=1C(C)(C)C1=CC=C(O)C=C1 IISBACLAFKSPIT-UHFFFAOYSA-N 0.000 title claims abstract description 173
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 claims abstract description 122
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 claims abstract description 78
- 238000000034 method Methods 0.000 claims abstract description 70
- 239000012452 mother liquor Substances 0.000 claims abstract description 37
- 239000006227 byproduct Substances 0.000 claims abstract description 23
- 238000006243 chemical reaction Methods 0.000 claims abstract description 19
- 239000003054 catalyst Substances 0.000 claims abstract description 16
- MYRTYDVEIRVNKP-UHFFFAOYSA-N divinylbenzene Substances C=CC1=CC=CC=C1C=C MYRTYDVEIRVNKP-UHFFFAOYSA-N 0.000 claims abstract description 16
- 238000006482 condensation reaction Methods 0.000 claims abstract description 14
- 238000006317 isomerization reaction Methods 0.000 claims abstract description 14
- 229920001577 copolymer Polymers 0.000 claims abstract description 12
- 150000003440 styrenes Chemical class 0.000 claims abstract description 10
- 230000002378 acidificating effect Effects 0.000 claims abstract description 9
- 150000001768 cations Chemical class 0.000 claims abstract description 8
- 238000002425 crystallisation Methods 0.000 claims abstract description 8
- 230000008025 crystallization Effects 0.000 claims abstract description 7
- 238000004821 distillation Methods 0.000 claims abstract description 7
- 238000000354 decomposition reaction Methods 0.000 claims abstract description 4
- 238000009826 distribution Methods 0.000 claims abstract description 3
- 238000011084 recovery Methods 0.000 claims abstract description 3
- 229940106691 bisphenol a Drugs 0.000 claims description 78
- 239000000203 mixture Substances 0.000 claims description 21
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 16
- YQUQWHNMBPIWGK-UHFFFAOYSA-N 4-isopropylphenol Chemical compound CC(C)C1=CC=C(O)C=C1 YQUQWHNMBPIWGK-UHFFFAOYSA-N 0.000 claims description 14
- 239000013078 crystal Substances 0.000 claims description 14
- 238000004519 manufacturing process Methods 0.000 claims description 12
- 239000011541 reaction mixture Substances 0.000 claims description 12
- 239000000725 suspension Substances 0.000 claims description 11
- 239000000047 product Substances 0.000 claims description 10
- NWUYHJFMYQTDRP-UHFFFAOYSA-N 1,2-bis(ethenyl)benzene;1-ethenyl-2-ethylbenzene;styrene Chemical compound C=CC1=CC=CC=C1.CCC1=CC=CC=C1C=C.C=CC1=CC=CC=C1C=C NWUYHJFMYQTDRP-UHFFFAOYSA-N 0.000 claims description 6
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 claims description 6
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 claims description 6
- 150000001875 compounds Chemical class 0.000 claims description 6
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 claims description 6
- 125000000020 sulfo group Chemical group O=S(=O)([*])O[H] 0.000 claims description 6
- 230000018044 dehydration Effects 0.000 claims description 5
- 238000006297 dehydration reaction Methods 0.000 claims description 5
- 239000007788 liquid Substances 0.000 claims description 5
- 239000003381 stabilizer Substances 0.000 claims description 5
- RSPCKAHMRANGJZ-UHFFFAOYSA-N thiohydroxylamine Chemical compound SN RSPCKAHMRANGJZ-UHFFFAOYSA-N 0.000 claims description 5
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 claims description 4
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 claims description 4
- 150000001450 anions Chemical class 0.000 claims description 4
- 239000000539 dimer Substances 0.000 claims description 4
- 239000011591 potassium Substances 0.000 claims description 4
- 229910052700 potassium Inorganic materials 0.000 claims description 4
- SNPQRYOQWLOTFA-UHFFFAOYSA-N 2,2-dimethyl-1,3-thiazolidine Chemical compound CC1(C)NCCS1 SNPQRYOQWLOTFA-UHFFFAOYSA-N 0.000 claims description 3
- 229930185605 Bisphenol Natural products 0.000 claims description 3
- 239000012223 aqueous fraction Substances 0.000 claims description 3
- 239000007809 chemical reaction catalyst Substances 0.000 claims description 3
- 239000012141 concentrate Substances 0.000 claims description 3
- 239000004310 lactic acid Substances 0.000 claims description 3
- 235000014655 lactic acid Nutrition 0.000 claims description 3
- BJEPYKJPYRNKOW-REOHCLBHSA-N (S)-malic acid Chemical compound OC(=O)[C@@H](O)CC(O)=O BJEPYKJPYRNKOW-REOHCLBHSA-N 0.000 claims description 2
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 claims description 2
- BJEPYKJPYRNKOW-UHFFFAOYSA-N alpha-hydroxysuccinic acid Natural products OC(=O)C(O)CC(O)=O BJEPYKJPYRNKOW-UHFFFAOYSA-N 0.000 claims description 2
- 159000000007 calcium salts Chemical class 0.000 claims description 2
- 235000015165 citric acid Nutrition 0.000 claims description 2
- 239000003456 ion exchange resin Substances 0.000 claims description 2
- 229920003303 ion-exchange polymer Polymers 0.000 claims description 2
- 239000001630 malic acid Substances 0.000 claims description 2
- 235000011090 malic acid Nutrition 0.000 claims description 2
- 235000007686 potassium Nutrition 0.000 claims description 2
- 239000011734 sodium Substances 0.000 claims description 2
- 229910052708 sodium Inorganic materials 0.000 claims description 2
- 229910000029 sodium carbonate Inorganic materials 0.000 claims description 2
- 235000017550 sodium carbonate Nutrition 0.000 claims description 2
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 claims 2
- 239000002253 acid Substances 0.000 claims 2
- ZYUVGYBAPZYKSA-UHFFFAOYSA-N 5-(3-hydroxybutan-2-yl)-4-methylbenzene-1,3-diol Chemical compound CC(O)C(C)C1=CC(O)=CC(O)=C1C ZYUVGYBAPZYKSA-UHFFFAOYSA-N 0.000 claims 1
- 150000007513 acids Chemical class 0.000 claims 1
- 125000005365 aminothiol group Chemical group 0.000 claims 1
- NHADDZMCASKINP-HTRCEHHLSA-N decarboxydihydrocitrinin Natural products C1=C(O)C(C)=C2[C@H](C)[C@@H](C)OCC2=C1O NHADDZMCASKINP-HTRCEHHLSA-N 0.000 claims 1
- 235000011187 glycerol Nutrition 0.000 claims 1
- 125000003396 thiol group Chemical class [H]S* 0.000 claims 1
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 12
- 241001550224 Apha Species 0.000 description 8
- 239000012451 post-reaction mixture Substances 0.000 description 6
- 239000000126 substance Substances 0.000 description 6
- 238000009835 boiling Methods 0.000 description 4
- 238000009833 condensation Methods 0.000 description 4
- 230000005494 condensation Effects 0.000 description 4
- YZUPZGFPHUVJKC-UHFFFAOYSA-N 1-bromo-2-methoxyethane Chemical compound COCCBr YZUPZGFPHUVJKC-UHFFFAOYSA-N 0.000 description 3
- CQOZJDNCADWEKH-UHFFFAOYSA-N 2-[3,3-bis(2-hydroxyphenyl)propyl]phenol Chemical compound OC1=CC=CC=C1CCC(C=1C(=CC=CC=1)O)C1=CC=CC=C1O CQOZJDNCADWEKH-UHFFFAOYSA-N 0.000 description 3
- 238000003421 catalytic decomposition reaction Methods 0.000 description 3
- 239000003729 cation exchange resin Substances 0.000 description 3
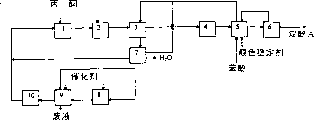
- 238000010586 diagram Methods 0.000 description 3
- 239000012535 impurity Substances 0.000 description 3
- 239000002994 raw material Substances 0.000 description 3
- 238000000926 separation method Methods 0.000 description 3
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 2
- 238000001816 cooling Methods 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 238000002156 mixing Methods 0.000 description 2
- 150000002989 phenols Chemical class 0.000 description 2
- 229920000515 polycarbonate Polymers 0.000 description 2
- 239000004417 polycarbonate Substances 0.000 description 2
- 238000001953 recrystallisation Methods 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- RBNPOMFGQQGHHO-UHFFFAOYSA-N -2,3-Dihydroxypropanoic acid Natural products OCC(O)C(O)=O RBNPOMFGQQGHHO-UHFFFAOYSA-N 0.000 description 1
- CRBJBYGJVIBWIY-UHFFFAOYSA-N 2-isopropylphenol Chemical compound CC(C)C1=CC=CC=C1O CRBJBYGJVIBWIY-UHFFFAOYSA-N 0.000 description 1
- RBNPOMFGQQGHHO-UWTATZPHSA-N D-glyceric acid Chemical compound OC[C@@H](O)C(O)=O RBNPOMFGQQGHHO-UWTATZPHSA-N 0.000 description 1
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 description 1
- 239000005977 Ethylene Substances 0.000 description 1
- LSDPWZHWYPCBBB-UHFFFAOYSA-N Methanethiol Chemical compound SC LSDPWZHWYPCBBB-UHFFFAOYSA-N 0.000 description 1
- 229920000265 Polyparaphenylene Polymers 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- 239000003377 acid catalyst Substances 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 150000001555 benzenes Chemical class 0.000 description 1
- 125000002091 cationic group Chemical group 0.000 description 1
- 229920001429 chelating resin Polymers 0.000 description 1
- 239000007795 chemical reaction product Substances 0.000 description 1
- 239000003431 cross linking reagent Substances 0.000 description 1
- 239000003822 epoxy resin Substances 0.000 description 1
- 239000012467 final product Substances 0.000 description 1
- LNEPOXFFQSENCJ-UHFFFAOYSA-N haloperidol Chemical compound C1CC(O)(C=2C=CC(Cl)=CC=2)CCN1CCCC(=O)C1=CC=C(F)C=C1 LNEPOXFFQSENCJ-UHFFFAOYSA-N 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 238000005192 partition Methods 0.000 description 1
- 229920000647 polyepoxide Polymers 0.000 description 1
- -1 polyphenylene Polymers 0.000 description 1
- 229920003053 polystyrene-divinylbenzene Polymers 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 238000011403 purification operation Methods 0.000 description 1
- 230000008707 rearrangement Effects 0.000 description 1
- 238000004064 recycling Methods 0.000 description 1
- 238000007670 refining Methods 0.000 description 1
- 239000011347 resin Substances 0.000 description 1
- 229920005989 resin Polymers 0.000 description 1
- 235000017557 sodium bicarbonate Nutrition 0.000 description 1
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 1
- IIACRCGMVDHOTQ-UHFFFAOYSA-N sulfamic acid Chemical compound NS(O)(=O)=O IIACRCGMVDHOTQ-UHFFFAOYSA-N 0.000 description 1
- PXQLVRUNWNTZOS-UHFFFAOYSA-N sulfanyl Chemical class [SH] PXQLVRUNWNTZOS-UHFFFAOYSA-N 0.000 description 1
- 238000010977 unit operation Methods 0.000 description 1
- 238000005292 vacuum distillation Methods 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
Images
Landscapes
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PL332879A PL194718B1 (pl) | 1999-04-27 | 1999-04-27 | Sposób otrzymywania bisfenolu A |
| PLP332879 | 1999-04-27 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN1271715A true CN1271715A (zh) | 2000-11-01 |
Family
ID=20074265
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN 99118071 Pending CN1271715A (zh) | 1999-04-27 | 1999-08-25 | 获得双酚a的方法 |
Country Status (3)
| Country | Link |
|---|---|
| CN (1) | CN1271715A (enExample) |
| IN (1) | IN187893B (enExample) |
| PL (1) | PL194718B1 (enExample) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN108611189A (zh) * | 2016-12-09 | 2018-10-02 | 丰益(上海)生物技术研发中心有限公司 | 一种控制油脂中双酚a和烷基酚的精炼工艺 |
| CN109415284A (zh) * | 2016-07-12 | 2019-03-01 | 沙特基础工业全球技术有限公司 | 双酚a的制备 |
| CN112409573A (zh) * | 2019-08-23 | 2021-02-26 | 南通星辰合成材料有限公司 | 一种副产多元酚环氧树脂及其制造方法与应用 |
| CN117597324A (zh) * | 2021-07-05 | 2024-02-23 | Sabic环球技术有限责任公司 | 制造双酚a的方法 |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN114835559B (zh) * | 2022-07-04 | 2022-09-09 | 山东亿科化学有限责任公司 | 一种合成双酚f的催化方法 |
-
1999
- 1999-04-27 PL PL332879A patent/PL194718B1/pl not_active IP Right Cessation
- 1999-07-12 IN IN622CA1999 patent/IN187893B/en unknown
- 1999-08-25 CN CN 99118071 patent/CN1271715A/zh active Pending
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN109415284A (zh) * | 2016-07-12 | 2019-03-01 | 沙特基础工业全球技术有限公司 | 双酚a的制备 |
| CN109415284B (zh) * | 2016-07-12 | 2022-06-17 | 沙特基础工业全球技术有限公司 | 双酚a的制备 |
| CN108611189A (zh) * | 2016-12-09 | 2018-10-02 | 丰益(上海)生物技术研发中心有限公司 | 一种控制油脂中双酚a和烷基酚的精炼工艺 |
| CN108611189B (zh) * | 2016-12-09 | 2023-02-21 | 丰益(上海)生物技术研发中心有限公司 | 一种控制油脂中双酚a和烷基酚的精炼工艺 |
| CN112409573A (zh) * | 2019-08-23 | 2021-02-26 | 南通星辰合成材料有限公司 | 一种副产多元酚环氧树脂及其制造方法与应用 |
| CN112409573B (zh) * | 2019-08-23 | 2023-06-20 | 南通星辰合成材料有限公司 | 一种副产多元酚环氧树脂及其制造方法与应用 |
| CN117597324A (zh) * | 2021-07-05 | 2024-02-23 | Sabic环球技术有限责任公司 | 制造双酚a的方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| PL194718B1 (pl) | 2007-06-29 |
| IN187893B (enExample) | 2002-07-20 |
| PL332879A1 (en) | 2000-11-06 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN1169765C (zh) | 生产双酚a的方法 | |
| EP0558552B1 (en) | A process to obtain high-purity bisphenol a | |
| CN1914142B (zh) | 双酚a生产中循环流的脱水 | |
| RU2422429C2 (ru) | Способ получения бисфенола а высокой чистоты и производственная установка | |
| CN1034361A (zh) | 制备高纯度2,2-二(4-羟苯基)丙烷的方法 | |
| TWI593665B (zh) | Method for producing bisphenol A. | |
| CN1271715A (zh) | 获得双酚a的方法 | |
| CN1250504C (zh) | 双酚的制备 | |
| JP2007520502A (ja) | 異性体形成の低減されたビスフェノールaの製造 | |
| JP4012436B2 (ja) | ビスフェノールaの製造方法 | |
| JP2002255881A (ja) | ビスフェノールaの製造方法 | |
| JP4904064B2 (ja) | ビスフェノールaの製造方法 | |
| CN1795155A (zh) | 双酚a的制造方法 | |
| CN100424060C (zh) | 双酚-a的提纯方法 | |
| JPH0558611B2 (enExample) | ||
| JP4333276B2 (ja) | 芳香族ポリカーボネートの製造方法 | |
| KR102349519B1 (ko) | 비스페놀a의 제조방법 | |
| JP4615831B2 (ja) | ビスフェノールaの製造におけるフェノールの回収方法 | |
| JP2000229899A (ja) | ビスフェノールaの製造方法 | |
| CN1300729A (zh) | 制备高纯度双酚a的方法 | |
| JP2003160524A (ja) | ビスフェノールaの製造方法及びその装置 | |
| RO119296B1 (ro) | Procedeu de obţinere a 2,2-bis-(4-hidroxi fenil) propanului prin cristalizare ca aduct | |
| PL199344B1 (pl) | Sposób otrzymywania bisfenolu A |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C06 | Publication | ||
| PB01 | Publication | ||
| C02 | Deemed withdrawal of patent application after publication (patent law 2001) | ||
| WD01 | Invention patent application deemed withdrawn after publication |