CN1271715A - Method for obtaining biphenol A - Google Patents
Method for obtaining biphenol A Download PDFInfo
- Publication number
- CN1271715A CN1271715A CN 99118071 CN99118071A CN1271715A CN 1271715 A CN1271715 A CN 1271715A CN 99118071 CN99118071 CN 99118071 CN 99118071 A CN99118071 A CN 99118071A CN 1271715 A CN1271715 A CN 1271715A
- Authority
- CN
- China
- Prior art keywords
- phenol
- reaction
- acetone
- mother liquor
- adducts
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Landscapes
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Abstract
The subject of the invention is a method for obtaining biphenol A by multistep operational crystallizations and distilling distributes, as well as recovery from heat catalytic decomposition of by products in the process of condensation reaction between phenol and acetone taking strongly acidic cation exchanger as catalyzer. According to the invention, the condensation reaction between phenol and acetone and the reaction of isomerizing by products into biphenol A are simultaneously carried out in presence of a gelatinous sulfonated-styrene-divinylbenzene copolymer in step 1, by products are introduced into step 1 in the form of anhydrous mother liquor from step 7, the reaction temperature is 50-85 DEG C and the contacting time is at least 5 hours.
Description
Theme of the present invention is in the phenol condensation with acetone reaction process that exists as catalyzer with strongly acidic cation exchanger, distributes and obtain from the approach that by product thermocatalysis decomposition course reclaims the method for dihydroxyphenyl propane by the crystallization and the distillation of multistep operation.
Known have some methods of obtaining high purity 2,2 a pair ofs (4 '-hydroxy phenyl) propane (claiming dihydroxyphenyl propane again).Dihydroxyphenyl propane is a kind of raw material of producing resin, mainly is Resins, epoxy and polycarbonate, comprises that raw material must satisfy the optical polycarbonates of special high purity and color requirement.
In the patent description of EP0313165A1, proposed a kind of integrated process of obtaining dihydroxyphenyl propane: acetone and phenol are introduced condensation reactor with all round-robin isomerization products.Can adopt any effective condensation catalyst in the dihydroxyphenyl propane process that obtains.Preferred reactor contains modification or unmodified Zeo-karb, the acid large pores cation exchange resin of sulfonated polystyrene-Vinylstyrene form for example, and temperature is 50 ℃ to 90 ℃.Reaction product is delivered in the thickener, reclaims unreacted acetone and phenol with excessive water, is recycled to condensation reactor.The product of crude bisphenol form is delivered to crystallizer, obtains solid bisphenol a/phenol adducts.Suspension is delivered in the separator, adducts is separated with the mother liquor that contains by product, and send to refining.The by product mother liquor that contains that obtains in the separator is delivered to the isomerization work area.In the isomerization work area, temperature remains on 60~90 ℃ of scopes.This work area contains the acidic cation-exchange resin that uses after the mercaptan modification, be preferably acid micropore or large pores cation exchange resin, for example through the sulfonated polystyrene-Vinylstyrene of 10% couple-2-(mercapto ethyl) amine modification.The concentration of required dihydroxyphenyl propane increases to some extent in the isomerization product, is recycled to the concentration response device.
In the patent specification of EP0332203A1, a kind of method of obtaining high-purity dihydroxyphenyl propane is proposed, comprise main procedure and so-called process.In main procedure, phenol and acetone reaction, reaction mixture is treated, obtain the solution of regulation bisphenol A concentration, this is so-called first concentration control stage, next carry out bisphenol a/phenol adducts first crystallisation stage, adducts and mother liquor first separation phase are removed phenol to the stage of obtaining high-purity dihydroxyphenyl propane from adducts.Inferior process comprises second step of reaction that australol and phenol react, dihydroxyphenyl propane second concentration control stage, bisphenol a/phenol adducts second crystallisation stage, the secondary crystal thing of adducts and second mother liquor, second separation phase, second mother liquor was handled to the stage that obtains australol and phenol, and precondition is that first mother liquor from main procedure enters time process.Second adducts crystal from inferior process is delivered to main procedure.
By in the solution of patent of invention EP0332203A1, also required another to obtain the different methods of high-purity dihydroxyphenyl propane.Main procedure comprises: phenol and acetone react and shift out first step of reaction of catalyzer, the crystallisation process that bisphenol a/phenol and adducts are separated out, isolating stage of adducts crystal and mother liquor, the stage of removing phenol from adducts.Inferior process comprises: australol and phenol react in the presence of acidic catalyst and shift out catalyzer acquisition second and contains phenol solution, contain from second and to shift out phenol the phenol solution and obtain crude bisphenol, from crude bisphenol, isolate lower boiling and high boiling substance by distillation and obtain the rectifying dihydroxyphenyl propane, isolated lower boiling and high boiling substance are handled obtaining australol and phenol.Deliver to time process from the mother liquor of main procedure, and deliver to main procedure from the rectifying dihydroxyphenyl propane of inferior process.
By the main drawback of the solution of EP0332203 patent is too complicated by introducing the method that so-called process obtain high-purity dihydroxyphenyl propane, inferior process comprises that some unit operations and complicacy and main procedure are similar, and this can make the cost of investment and the process cost of the dihydroxyphenyl propane production equipment that adopts this method improve greatly.The dihydroxyphenyl propane purity of according to said method obtaining is also lower, and product contains from 0.3 to 2% impurity.
International Patent Application PCT WO 94/19302 has described the method for producing dihydroxyphenyl propane in a kind of novel acetone and phenol condensation reactor.This method comprises: acetone and phenol reactant, generate mixture after the reaction contain dihydroxyphenyl propane and water, and will react the back mixture and be cooled to acquisition bisphenol a/phenol adducts crystal.Adducts is separated with mother liquor, from adducts crystal, remove phenol and obtain dihydroxyphenyl propane.The method is characterized in that being reflected in the column reactor that some orifice plates are arranged of acetone and phenol carry out, keep the solid granular catalyst flooded operation, and in the reaction process, neutral air communication is crossed reactor to cat head, with this reach catalyst mix and after the reaction of the purpose of reacting back mixture drainage water-come out from reactor the water content of mixture be minimized.When this invention scheme was characterised in that cooling stages, reaction back mixture had the crystallizer internal cooling of two filtering elements an inside, and grain is pumped out by first filtering element through the part less than predetermined grain-size in the reaction mixture after the crystallization.Be incorporated into then in the equipment, all crystals is transferred to solution, be recycled in the crystallizer by second filtering element again.After the scheduled time, mixture pumped out through second filtering element after the varying cyclically direction of the filtering element of flowing through, Recycle design became the reaction of partial crystallization, and sent to dissolution of crystals, got back to crystallizer by first filtering element then.This characteristic feature of an invention is to be used in the steam stripped vertical unit of suspension reaction at one, and is all higher to the selectivity and its efficient of dihydroxyphenyl propane.
Poland Patent specification sheets 153396 and 164289 proposes a kind of method of obtaining dihydroxyphenyl propane, and this method is mixed with mixture behind the partial reaction of taking from reaction system and obtained reacting feeding mixture by circulation being contained phenol liquid.According to Poland Patent 153396, the initial concentration of dihydroxyphenyl propane is the 12-20% (weight) of dihydroxyphenyl propane in the incoming mixture of Huo Deing in this way, simultaneously, its irregular part content is less than 1/4 in total by product, and the mol ratio of phenol and acetone reaches (5-30): 1 scope level.React under the 60-90 ℃ of temperature, hand over the agent weight ratio to should be (0.05-0.5) mutually at macropore and micropore positively charged ion: 1 mixture is to carry out in the presence of the catalyzer.Reaction is carried out through this mode, makes that to obtain in the mixture ultimate density of dihydroxyphenyl propane higher, equals 21-35% (weight), and by-products content is 12-24% (weight) simultaneously.Because containing phenol liquid is recycled in the reaction system, the impurity level in the system can accumulate.For avoiding accumulating the material of not expecting in the production process, it is carried out respective handling removes impurity, with the dihydroxyphenyl propane that base is purified and recovery is managed mutually by from reaction system, taking out at least a portion liquid stream.
The objective of the invention is further to improve the method for after the reaction that contains high density production process by product, obtaining high-purity dihydroxyphenyl propane the mixture.
According to the present invention, in the 1st step, in the presence of the sulfonated phenylethylene and divinyl benzene copolymer of gel structure, carry out the condensation reaction of phenol and acetone and the reaction that by product is isomerizated into dihydroxyphenyl propane simultaneously, by product is to introduce step 1 with the anhydrous mother liquor form of step 7, this step is to operate under 50-85 ℃ of temperature, is at least duration of contact 5 hours.The 2nd step was with the mixture cooling of reaction back, obtained the suspension of bisphenol a/phenol adducts in containing phenol solution.The 3rd step was that the suspension of bisphenol a/phenol adducts in containing phenol solution that will obtain in the step 2 is distributed into the bisphenol a/phenol adducts and contains phenol mother liquor I, then with containing phenol liquid washing adducts crystal.The 4th step was that the bisphenol a/phenol adducts that will be in the step 3 obtains is dissolved in and contains phenol solution.In ensuing the 5th step, the suspension that obtains in the step 4 is distributed into bisphenol a/phenol adducts and mother liquor II, mother liquor II loops back step 3 and 4, and the bisphenol a/phenol adducts crystal is with containing the phenol solution washing and adding colour stabilizer.In the 6th step, isolate dihydroxyphenyl propane by the phenol of removing in the bisphenol a/phenol adducts that obtains in the step 5.In ensuing the 7th step, contain phenol mother liquor I distillation with what obtain in the step 3, shift out wherein acetone, water and part phenol, the part that the dehydration of step 7 contains the phenol mother liquor is circulated to the step 1 of process.In the 8th and the 9th step subsequently, the rest part dehydration mother liquor that obtains in the step 7 is carried out the thermocatalysis decomposition reaction, 5% to 70% of basic catalyst total amount is introduced step 8, and the basic catalyst of its surplus is incorporated into step 9.The overhead product that step 9 obtains contains phenol, australol, its dimer and oligopolymer and production process by product, and it was directly delivered to for the 10th step, in the presence of the macroporous type cationite, these products is rearranged into dihydroxyphenyl propane.Overhead product is recycled to the step 1 and 2 of production process after the rearrangement of step 10.
The mother liquor II that obtains in the step 5 was delivered to for the 11st step with phenol that reclaims in the step 7 and acetone from the mother liquor I of step 3, under 50-85 ℃ temperature, they are contacted with divinyl benzene copolymer with the sulfonated phenylethylene of gel structure, and then be recycled to step 3 and/or 4.
The present invention also comprises another kind of method, wherein the 1st step was in the presence of the sulfonated phenylethylene and divinyl benzene copolymer of gel structure, under 50-85 ℃ of temperature, carry out the condensation reaction of phenol and acetone, in step, after the reaction of step 1, distillate unreacted acetone, water and part phenol at 1a the mixture with respect to feed steam total amount 5-50% (weight).In the 7th step, the overhead product that obtains among the step 1a is divided into water, acetone and phenol through distillation.The mother liquor of part step 3 is delivered to the 10a step of process, carries out the reaction that by product is isomerizated into dihydroxyphenyl propane in the presence of sulfonated phenylethylene and divinyl benzene copolymer.The overhead product of step 10a is incorporated into the overhead product of step 10, is circulated to the step 1 of process.
Mixture through concentration, distillates phenol, acetone and the aqueous distillate of charging 5-50% (weight) after the reaction that step 11 is come out in 11a goes on foot, and then this cut is delivered to step 7, and enriched material is delivered to step 3 and/or 4 simultaneously.
Used phenol condensation with acetone catalysts is sulfonated phenylethylene and 2~6% (weight) divinyl benzene copolymer of gel structure in the step 1 and 11, and wherein the sulfo group of 5-45% is neutralized by amineothiot promotor molecule.
Used amidosulphuric acid is Cisteamine and 2,2-dimethylthiazole alkane.
Used isomerization catalyst is the sulfonated phenylethylene and the divinyl benzene copolymer of macroporous structure among the step 10a.
Distillate part phenol by underpressure distillation in the step 6, isolate dihydroxyphenyl propane by the method for removing remaining phenol with water vapor discharge method from bis-phenol/A phenol adducts then.
Used basic catalyst is NaHCO in the step 8 and 9
3, NaOH, NaH
2PO
2Or the mixture of these compounds.
Colour stabilizer used in the step 5 is for containing the mixture of 1: 1 to 1: 20 acid sodium carbonate of weight ratio or potassium and lactic acid or oxysuccinic acid or citric acid or R-Glyceric acid or their sodium, potassium or calcium salt.
Mixture contacts with anionite under 40-85 ℃ of temperature after step 1 and 11 the reaction.Fresh phenol materials flow is introduced before the process earlier with the sulfo group of 10-90% wherein and is contacted with mercapto-amine neutral acidic ion exchange resin, it is in the presence of the 5-1000ppm compounds containing thiol groups, be heated to 40-100 ℃ of 0.5~5 hour time, behind the phenol and condensation of acetone by this approach acquisition, reaction back mixture contacts with anionite under 40-85 ℃ temperature.
Have now found that the phenol solution recrystallization of bisphenol a/phenol adducts can have the essence influence to refining effect, even, also do not reach above-mentioned effect to bisphenol a/phenol adducts washing several times.But, to isolated bisphenol a/phenol adducts in re-crystallization step with in addition washing or effectively of phenol solution.Concerning washing for the second time, can use the back from adducts, to remove the phenol that reclaims in the phenol operation.The purity of this phenol is identical with the commercial goods.If increase the amount of washing with phenol by the fresh phenol that adds with raw material mode introducing process, then the effect of washing meeting for the second time is better.Fresh raw material phenol is introduced production process in this step, and in a manner described, before the step 1 of delivering to condensation reaction, use and to render a service the other favourable influence of generation to the purification operations of bisphenol a/phenol adducts through the circulation of materials flow after the corresponding crystallization and washing soln.The characteristics of the dihydroxyphenyl propane that obtains by the present invention are high-purity grade bis phenol As that waits.
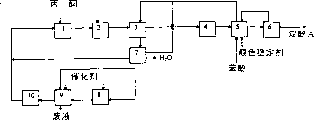
Embodiment 1
Illustrate by the illustrated skeleton diagram of Fig. 1 by originating party case of the present invention.This block diagram illustrates a kind of basic skills, and the 1st step was that consumption is 45m in the base
3Gel structure sulfonated phenylethylene and 4% (weight) divinyl benzene copolymer-Amberlyst 31 exist down, introduce the reaction mixture of 3400kg/h amount.Incoming mixture composed as follows:
Dihydroxyphenyl propane 272kg/h
By product 612kg/h
Phenol 2,272kg/h
Acetone 230kg/h
Water 14kg/h
In the step 1, carry out the condensation reaction of phenol and acetone and the reaction that by product is isomerizated into dihydroxyphenyl propane simultaneously, by product wherein is the dehydration mother liquor form with step 7, introduces step 1 with the amount of 623.6kg/h, and temperature of reaction is 72 ℃, and be 5 hours duration of contact.The 2nd step was that reaction back mixture is cooled to 39 ℃, obtained the suspension of bisphenol a/phenol in containing phenol solution.The 3rd step was that the suspension with the bisphenol a/phenol adducts is distributed into adducts crystal and mother liquor I, and adducts crystal washs with the phenol solution that contains that step 5 obtains.The 4th step was the bisphenol a/phenol adducts that step 3 obtains to be dissolved in contain phenol solution, and ensuing the 5th step is that the suspension that will obtain in the step 4 is distributed into bisphenol a/phenol adducts crystal and mother liquor II, and mother liquor II is recycled to step 3 and 4.The dihydroxyphenyl propane crystal is 1: 4 NaHCO with containing phenol solution washing and adding the 20ppm ratio
3Colour stabilizer with the lactic mixt form.The 6th step was to isolate dihydroxyphenyl propane by removing phenol the adducts that obtains from step 5.Obtain the 1150kg dihydroxyphenyl propane.The characteristics of final product: colourity 10 (APHA colour code) molten state colourity 25 (APHA colour code) selectivity 95% of content of bisphenol A 99.91% ortho para content of isomer 58ppm trisphenol 49ppm50% methanol solution
The 7th step was the mother liquor I distillation that step 3 is obtained, and shifted out acetone, water and part phenol.The part that the dehydration of step 7 contains phenol liquid is recycled to the step 1 of process, and rest part then delivered to for the 8th and the 9th step, carried out the reaction that thermocatalysis resolves into australol and phenol.In step 8, introduce 50kg NaHPO
3, in step 9, introduce 9-30kgNaHPO
3The overhead product that step 9 obtains contains phenol, australol and linear dimer thereof and oligopolymer and by product, this overhead product was delivered to for the 10th step, in the presence of macroporous type cationite AMberlyst15, these products are rearranged into dihydroxyphenyl propane, and overhead product is recycled to the step 1 and 2 of process after the rearrangement of step 10.Embodiment 2
Skeleton diagram similar embodiment 1 shown in Fig. 2, just the materials flow working cycle has part to change.
The mother liquor II that step 5 obtains delivers to the 11st step of process together with phenol that reclaims in the step 7 and acetone from the mother liquor I of step 3, under 61 ℃ temperature, with them and 10m
3Cationite Purolite CT-124 contact, 18% sulfo group neutralizes with cisteamine in the cationite wherein, and then is recycled to step 3 and 4.Incoming mixture by the 3700kg/h amount, obtain the 1350kg dihydroxyphenyl propane, its characteristics are as follows: colourity 8 (APHA colour code) molten state colourity 20 (APHA colour code) selectivity 96% embodiment 3 of content of bisphenol A 99.90% ortho para content of isomer 64ppm trisphenol content 57ppm50% methanol solution
Fig. 3 illustrates the expanding system of a method illustrated in figures 1 and 2.
The first step is at 20m
317% sulfo group used 2,2-dimethylthiazole alkane neutral Purolite C-124 exists down, carry out the condensation reaction of phenol and acetone, temperature of reaction is 62 ℃, in 1a goes on foot, to distillate unreacted acetone, water and the part phenol of relative feed steam amount 25% (weight) from the reaction mixture distillation of step 1.In the 7th step, the overhead product that obtains among the step 1a is distributed into water, acetone and phenol through distillation.The mother liquor I of part step 3 delivered to for the 8th and 9 steps, carry out the thermocatalysis decomposition reaction, the overhead product that obtains in the step 9 entered for the 10th step with the partial mother liquid I of step 3, in the presence of catalyst A mberlyst15, carried out the reaction that n-propyl phenol and dipolymer thereof and oligopolymer are rearranged into dihydroxyphenyl propane.Next, in the step, also be at 10a in the presence of Amberlyst15, carry out the reaction that by product is isomerizated into dihydroxyphenyl propane, the catabolite of step 10a is recycled to step 1.The characteristics of the dihydroxyphenyl propane that obtains are as follows: colourity 3 (APHA colour code) molten state colourity 10 (APHA colour code) selectivity 96% embodiment 4 of content of bisphenol A 99.96% ortho para content of isomer 69ppm trisphenol content 32ppm50% methanol solution
Fig. 4 illustrates the another program by the inventive method.
Mixture through concentration, distillates phenol, acetone and the aqueous distillate of inlet amount 30% 11a goes on foot after the reaction that step 11 is come out, and then this cut is delivered to step 7, and enriched material is sent to step 3 and/or 4.The characteristics of the dihydroxyphenyl propane that obtains are as follows: colourity 4 (APHA colour code) molten state colourity 12 (APHA colour code) selectivity 96% embodiment 4 of content of bisphenol A 99.92% ortho para content of isomer 73ppm trisphenol content 35ppm50% methanol solution
Scheme shown in Figure 5 is that the scheme of EXAMPLE III is improved.
Mixture was concentrated by phenol, acetone and the aqueous distillate that distillates with respect to inlet amount 20% in the 11a step after the reaction of step 11, then this cut was sent into step 7.The characteristics of the dihydroxyphenyl propane that obtains are as follows: colourity 5 (APHA colour code) fusion colourity 14 (APHA colour code) selectivity 97% of content of bisphenol A 99.91% ortho para content of isomer 62ppm trisphenol content 31ppm50% methanol solution
Claims (15)
1. in the acetone and phenol condensation reaction that one kind exists as catalyzer with strongly acidic cation exchanger, the method of distributing and obtain dihydroxyphenyl propane by crystallization and distillation from the approach that production process by product thermocatalysis decomposition course reclaims, it is characterized in that in the 1st step, in the presence of the sulfonated phenylethylene and divinyl benzene copolymer of gel structure, under 50-85 ℃ of temperature, carry out the condensation reaction of phenol and acetone and the reaction that by product is isomerizated into phenol A simultaneously, by product is to introduce step 1 with the anhydrous mother liquor form of step 7, the 2nd step was with the mixture cooling of reaction back, obtain the suspension of bisphenol a/phenol adducts in containing phenol solution, the 3rd step was that the suspension of bisphenol a/phenol adducts in containing phenol solution that will obtain in the step 2 is distributed into the bisphenol a/phenol adducts and contains phenol mother liquor I, then with containing phenol solution washing adducts crystal, the 4th step was that the bisphenol a/phenol adducts that will be in the step 3 obtains is dissolved in and contains phenol solution, in ensuing the 5th step, the suspension that obtains in the step 4 is distributed into bisphenol a/phenol adducts and mother liquor II, mother liquor II loops back step 3 and 4, the bisphenol a/phenol adducts crystal is with containing the phenol solution washing and adding colour stabilizer, in the 6th step, isolate dihydroxyphenyl propane by the phenol of removing in the bisphenol a/phenol adducts that obtains in the step 5, in ensuing the 7th step, the phenol mother liquor I that contains that obtains in the step 3 is distilled, remove acetone wherein, water and part phenol, the part that the dehydration of step 7 contains phenol liquid is circulated to the step 1 of process, in the 8th and the 9th step subsequently, the rest part dehydration mother liquor that obtains in the step 7 is carried out the thermocatalysis decomposition reaction, 5% to 70% of basic catalyst total amount is introduced step 8, and the basic catalyst of its surplus is incorporated into step 9, the overhead product that step 9 obtains contains phenol, australol, its dimer and oligopolymer and production process by product, it was directly delivered to for the 10th step, in the presence of the macroporous type cationite, these products are rearranged into dihydroxyphenyl propane, and overhead product is recycled to the step 1 and 2 of production process after the rearrangement of step 10.
2. press the method for claim 1, it is characterized in that the mother liquor II that will obtain in the step 5 delivered to for the 11st step with phenol that reclaims in the step 7 and acetone from the mother liquor I of step 3, under 50-85 ℃ temperature, they are contacted with divinyl benzene copolymer with the sulfonated phenylethylene of gel structure, and then be recycled to step 3 and/or 4.
3. press the method for claim 2, it is characterized in that wherein the 1st step was in the presence of the sulfonated phenylethylene and divinyl benzene copolymer of gel structure, under 50-85 ℃ of temperature, carry out the condensation reaction of phenol and acetone, in 1a goes on foot, after the reaction of step 1, distillate unreacted acetone the mixture with respect to feed steam total amount 5-50% (weight), water and part phenol, in the 7th step, the overhead product that obtains among the step 1a is divided into water through distillation, acetone and phenol, simultaneously, the 10a that the mother liquor I of part step 3 delivers to production process with the product of step 10 goes on foot, carry out the reaction that by product is isomerizated into dihydroxyphenyl propane in the presence of benzene iodide ethene and divinyl benzene copolymer, the isomerization product of step 10a is recycled to the step 1 of production process.
4. press the method for claim 2 or 3, it is characterized in that after the reaction that step 11 is come out mixture 11a the step through concentration, distillate phenol, acetone and the aqueous distillate of charging 5-50% (weight), then this cut is delivered to step 7, enriched material is delivered to step 3 and/or 4 simultaneously.
5. by the method for claim 1, it is characterized in that be at least 5 hours the duration of contact of reaction mixture and condensation catalyst in the step 1.
6. press the method for claim 4, it is characterized in that phenol condensation with acetone catalysts used in step 1 and 11 is sulfonated phenylethylene and 2.6% (weight) divinyl benzene copolymer of gel structure, wherein the sulfo group of 5-45% is neutralized by amineothiot promotor molecule.
7. by the method for claim 6, it is characterized in that used promotor is amineothiot.
8. by the method for claim 7, it is characterized in that used amineothiot is Cisteamine and 2,2-dimethylthiazole alkane.
9. by the method for claim 3 or 6, it is characterized in that isomerization catalyst used among the step 10a is the sulfonated phenylethylene and the divinyl benzene copolymer of macroporous structure.
10. by the method for claim 1 or 2 or 3, it is characterized in that distillating part phenol by underpressure distillation in the step 6, isolate dihydroxyphenyl propane by the method for removing remaining phenol with water vapor discharge method from bis-phenol/A phenol adducts then.
11. the method by claim 1 or 2 or 3 is characterized in that basic catalyst used in step 8 and 9 is NaHCO
3, NaOH, NaH
2PO
2Or the mixture of these compounds.
12., it is characterized in that colour stabilizer used in the step 5 is for containing the mixture of 1: 1 to 1: 20 acid sodium carbonate of weight ratio or potassium and lactic acid or oxysuccinic acid or citric acid or R-Glyceric acid or their sodium, potassium or calcium salt by the method for claim 1 or 3.
13. by claim 1 or 2 or 3 method, it is characterized in that step 1 and 11 reaction after mixture under 40-85 ℃ of temperature, contact with anionite.
14., it is characterized in that fresh phenol materials flow is introduced before the process earlier with 10~90% sulfo group wherein to contact with mercapto-amine neutral acidic ion exchange resin by claim 1 or 2 or 3 method.
15. method by claim 1 or 2 or 3, it is characterized in that containing the phenol materials flow introduces before the step 1 and 11, in the presence of the 5-1000ppm compounds containing thiol groups, be heated to 40-100 ℃ of 0.5~5 hour time, behind the phenol and condensation of acetone by this approach acquisition, reaction back mixture contacts with anionite under 40-85 ℃ temperature.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PL332879A PL194718B1 (en) | 1999-04-27 | 1999-04-27 | Method of obtaining biphenol a |
| PLP332879 | 1999-04-27 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN1271715A true CN1271715A (en) | 2000-11-01 |
Family
ID=20074265
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN 99118071 Pending CN1271715A (en) | 1999-04-27 | 1999-08-25 | Method for obtaining biphenol A |
Country Status (3)
| Country | Link |
|---|---|
| CN (1) | CN1271715A (en) |
| IN (1) | IN187893B (en) |
| PL (1) | PL194718B1 (en) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN108611189A (en) * | 2016-12-09 | 2018-10-02 | 丰益(上海)生物技术研发中心有限公司 | The refinery practice of bisphenol-A and alkyl phenol in a kind of control grease |
| CN109415284A (en) * | 2016-07-12 | 2019-03-01 | 沙特基础工业全球技术有限公司 | The preparation of bisphenol-A |
| CN112409573A (en) * | 2019-08-23 | 2021-02-26 | 南通星辰合成材料有限公司 | Byproduct polyphenol epoxy resin and preparation method and application thereof |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN114835559B (en) * | 2022-07-04 | 2022-09-09 | 山东亿科化学有限责任公司 | Catalytic method for synthesizing bisphenol F |
-
1999
- 1999-04-27 PL PL332879A patent/PL194718B1/en not_active IP Right Cessation
- 1999-07-12 IN IN622CA1999 patent/IN187893B/en unknown
- 1999-08-25 CN CN 99118071 patent/CN1271715A/en active Pending
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN109415284A (en) * | 2016-07-12 | 2019-03-01 | 沙特基础工业全球技术有限公司 | The preparation of bisphenol-A |
| CN109415284B (en) * | 2016-07-12 | 2022-06-17 | 沙特基础工业全球技术有限公司 | Preparation of bisphenol A |
| CN108611189A (en) * | 2016-12-09 | 2018-10-02 | 丰益(上海)生物技术研发中心有限公司 | The refinery practice of bisphenol-A and alkyl phenol in a kind of control grease |
| CN108611189B (en) * | 2016-12-09 | 2023-02-21 | 丰益(上海)生物技术研发中心有限公司 | Refining process for controlling bisphenol A and alkylphenol in grease |
| CN112409573A (en) * | 2019-08-23 | 2021-02-26 | 南通星辰合成材料有限公司 | Byproduct polyphenol epoxy resin and preparation method and application thereof |
| CN112409573B (en) * | 2019-08-23 | 2023-06-20 | 南通星辰合成材料有限公司 | Byproduct polyphenol epoxy resin and preparation method and application thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| PL332879A1 (en) | 2000-11-06 |
| IN187893B (en) | 2002-07-20 |
| PL194718B1 (en) | 2007-06-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN101636371B (en) | Process for producing bisphenol a | |
| EP0558552B1 (en) | A process to obtain high-purity bisphenol a | |
| KR100788091B1 (en) | Process for producing bisphenol a | |
| KR101322711B1 (en) | Process for producing high-purity bisphenol a and production apparatus | |
| CN1914142A (en) | Dehydration of recycle streams in bisphenol A production | |
| CN1189438C (en) | Method for the production of bisphenol-A | |
| CN1271715A (en) | Method for obtaining biphenol A | |
| RU2419600C2 (en) | Method of producing bisphenol a | |
| EP1809589B1 (en) | A method to obtain visually pure bisphenol a | |
| CN100537504C (en) | Economical Purification of bisphenol A | |
| CN100582069C (en) | Method for producing bisphenol A | |
| CN1331831C (en) | Process for producing bisphenol A | |
| CN1250504C (en) | Bisphenol production | |
| US6294702B1 (en) | Method for continuous production of dihydroxydiphenylalkanes | |
| JP2004010566A (en) | Method for producing bisphenol a | |
| KR101090193B1 (en) | Process For Production Of Bisphenol A | |
| KR102349519B1 (en) | Method for preparing bisphenol-a | |
| US20240308944A1 (en) | Method for the manufacture of bisphenol a | |
| CN1300729A (en) | Preparation of high purity bisphenol A | |
| EP3024809A1 (en) | A method to obtain bisphenol a | |
| JPH09323989A (en) | Process for producing trioxane |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C06 | Publication | ||
| PB01 | Publication | ||
| C02 | Deemed withdrawal of patent application after publication (patent law 2001) | ||
| WD01 | Invention patent application deemed withdrawn after publication |