BR112013020086B1 - Anticorpo isolado ou seu fragmento de ligação a antígeno, composição compreendendo o anticorpo ou fragmento de ligação a antígeno, kit, uso de uma quantidade eficaz de um anticorpo ou seu fragmento de ligação a antígeno e método para inibir a formação da oligômeros de alfatoxina - Google Patents
Anticorpo isolado ou seu fragmento de ligação a antígeno, composição compreendendo o anticorpo ou fragmento de ligação a antígeno, kit, uso de uma quantidade eficaz de um anticorpo ou seu fragmento de ligação a antígeno e método para inibir a formação da oligômeros de alfatoxina Download PDFInfo
- Publication number
- BR112013020086B1 BR112013020086B1 BR112013020086-3A BR112013020086A BR112013020086B1 BR 112013020086 B1 BR112013020086 B1 BR 112013020086B1 BR 112013020086 A BR112013020086 A BR 112013020086A BR 112013020086 B1 BR112013020086 B1 BR 112013020086B1
- Authority
- BR
- Brazil
- Prior art keywords
- antibody
- seq
- toxin
- alpha
- nos
- Prior art date
Links
- 239000012634 fragment Substances 0.000 title claims abstract description 346
- 230000027455 binding Effects 0.000 title claims abstract description 265
- 102000036639 antigens Human genes 0.000 title claims abstract description 207
- 108091007433 antigens Proteins 0.000 title claims abstract description 206
- 239000000427 antigen Substances 0.000 title claims abstract description 204
- 238000000034 method Methods 0.000 title claims abstract description 126
- 239000000203 mixture Substances 0.000 title claims abstract description 67
- 239000002776 alpha toxin Substances 0.000 claims abstract description 466
- 101710092462 Alpha-hemolysin Proteins 0.000 claims abstract description 349
- 101710124951 Phospholipase C Proteins 0.000 claims abstract description 346
- 101710197219 Alpha-toxin Proteins 0.000 claims abstract description 345
- 230000015572 biosynthetic process Effects 0.000 claims abstract description 47
- 230000002401 inhibitory effect Effects 0.000 claims abstract description 24
- 125000003275 alpha amino acid group Chemical group 0.000 claims description 179
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 91
- 102000004196 processed proteins & peptides Human genes 0.000 claims description 81
- 229920001184 polypeptide Polymers 0.000 claims description 76
- 101100112922 Candida albicans CDR3 gene Proteins 0.000 claims description 67
- 241000191967 Staphylococcus aureus Species 0.000 claims description 39
- 241001465754 Metazoa Species 0.000 claims description 33
- 206010035664 Pneumonia Diseases 0.000 claims description 33
- 108010092231 staphylococcal alpha-toxin Proteins 0.000 claims description 33
- 238000003556 assay Methods 0.000 claims description 28
- 239000000178 monomer Substances 0.000 claims description 27
- 230000006037 cell lysis Effects 0.000 claims description 23
- 102000004127 Cytokines Human genes 0.000 claims description 19
- 108090000695 Cytokines Proteins 0.000 claims description 19
- 206010040872 skin infection Diseases 0.000 claims description 17
- 230000000770 proinflammatory effect Effects 0.000 claims description 11
- 206010018910 Haemolysis Diseases 0.000 claims description 8
- 230000001461 cytolytic effect Effects 0.000 claims description 8
- 230000008588 hemolysis Effects 0.000 claims description 8
- 230000008595 infiltration Effects 0.000 claims description 8
- 238000001764 infiltration Methods 0.000 claims description 8
- 239000003242 anti bacterial agent Substances 0.000 claims description 7
- 239000003795 chemical substances by application Substances 0.000 claims description 7
- 239000008194 pharmaceutical composition Substances 0.000 claims description 7
- 230000003115 biocidal effect Effects 0.000 claims description 4
- MYPYJXKWCTUITO-KIIOPKALSA-N chembl3301825 Chemical compound O([C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1OC1=C2C=C3C=C1OC1=CC=C(C=C1Cl)[C@@H](O)[C@H](C(N[C@@H](CC(N)=O)C(=O)N[C@H]3C(=O)N[C@H]1C(=O)N[C@H](C(N[C@H](C3=CC(O)=CC(O)=C3C=3C(O)=CC=C1C=3)C(O)=O)=O)[C@H](O)C1=CC=C(C(=C1)Cl)O2)=O)NC(=O)[C@@H](CC(C)C)NC)[C@H]1C[C@](C)(N)C(O)[C@H](C)O1 MYPYJXKWCTUITO-KIIOPKALSA-N 0.000 claims description 2
- 102100035361 Cerebellar degeneration-related protein 2 Human genes 0.000 claims 5
- 101000737796 Homo sapiens Cerebellar degeneration-related protein 2 Proteins 0.000 claims 5
- 238000004519 manufacturing process Methods 0.000 abstract description 24
- 210000004027 cell Anatomy 0.000 description 123
- 235000001014 amino acid Nutrition 0.000 description 122
- 229940024606 amino acid Drugs 0.000 description 110
- 150000001413 amino acids Chemical class 0.000 description 110
- 208000015181 infectious disease Diseases 0.000 description 98
- 238000006467 substitution reaction Methods 0.000 description 78
- 241000282414 Homo sapiens Species 0.000 description 75
- 108090000623 proteins and genes Proteins 0.000 description 69
- 125000000539 amino acid group Chemical group 0.000 description 65
- 241000699670 Mus sp. Species 0.000 description 57
- 102000004169 proteins and genes Human genes 0.000 description 49
- 235000018102 proteins Nutrition 0.000 description 46
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 42
- 230000005764 inhibitory process Effects 0.000 description 40
- 210000003743 erythrocyte Anatomy 0.000 description 39
- 125000003729 nucleotide group Chemical group 0.000 description 38
- 102000005962 receptors Human genes 0.000 description 37
- 108020003175 receptors Proteins 0.000 description 37
- 239000002773 nucleotide Substances 0.000 description 36
- 230000001147 anti-toxic effect Effects 0.000 description 35
- 230000000694 effects Effects 0.000 description 35
- 150000007523 nucleic acids Chemical class 0.000 description 34
- 201000010099 disease Diseases 0.000 description 33
- 230000014509 gene expression Effects 0.000 description 32
- 102000039446 nucleic acids Human genes 0.000 description 32
- 108020004707 nucleic acids Proteins 0.000 description 32
- 230000006870 function Effects 0.000 description 31
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 29
- 239000002953 phosphate buffered saline Substances 0.000 description 29
- 238000009396 hybridization Methods 0.000 description 28
- 238000005516 engineering process Methods 0.000 description 27
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 26
- 238000012360 testing method Methods 0.000 description 26
- 230000001580 bacterial effect Effects 0.000 description 25
- 238000006384 oligomerization reaction Methods 0.000 description 25
- 239000012636 effector Substances 0.000 description 24
- 210000004408 hybridoma Anatomy 0.000 description 24
- 239000002609 medium Substances 0.000 description 23
- 239000003053 toxin Substances 0.000 description 23
- 239000000872 buffer Substances 0.000 description 22
- 239000003550 marker Substances 0.000 description 22
- 108700012359 toxins Proteins 0.000 description 22
- 108020004414 DNA Proteins 0.000 description 21
- 230000009089 cytolysis Effects 0.000 description 21
- 108091033319 polynucleotide Proteins 0.000 description 21
- 102000040430 polynucleotide Human genes 0.000 description 21
- 239000002157 polynucleotide Substances 0.000 description 21
- 230000004083 survival effect Effects 0.000 description 21
- 230000001225 therapeutic effect Effects 0.000 description 21
- 231100000765 toxin Toxicity 0.000 description 21
- 241000283973 Oryctolagus cuniculus Species 0.000 description 20
- 230000003053 immunization Effects 0.000 description 20
- 238000002649 immunization Methods 0.000 description 20
- 230000001404 mediated effect Effects 0.000 description 20
- 239000011148 porous material Substances 0.000 description 20
- 238000010494 dissociation reaction Methods 0.000 description 19
- 230000005593 dissociations Effects 0.000 description 19
- 238000000338 in vitro Methods 0.000 description 19
- 239000000523 sample Substances 0.000 description 19
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 18
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 18
- 210000003491 skin Anatomy 0.000 description 18
- 241000894006 Bacteria Species 0.000 description 17
- 230000013595 glycosylation Effects 0.000 description 17
- 238000006206 glycosylation reaction Methods 0.000 description 17
- 230000001965 increasing effect Effects 0.000 description 17
- 241001529936 Murinae Species 0.000 description 16
- 108010059993 Vancomycin Proteins 0.000 description 16
- 230000004071 biological effect Effects 0.000 description 16
- 210000004602 germ cell Anatomy 0.000 description 16
- 210000002966 serum Anatomy 0.000 description 16
- 239000000126 substance Substances 0.000 description 16
- MYPYJXKWCTUITO-LYRMYLQWSA-N vancomycin Chemical compound O([C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1OC1=C2C=C3C=C1OC1=CC=C(C=C1Cl)[C@@H](O)[C@H](C(N[C@@H](CC(N)=O)C(=O)N[C@H]3C(=O)N[C@H]1C(=O)N[C@H](C(N[C@@H](C3=CC(O)=CC(O)=C3C=3C(O)=CC=C1C=3)C(O)=O)=O)[C@H](O)C1=CC=C(C(=C1)Cl)O2)=O)NC(=O)[C@@H](CC(C)C)NC)[C@H]1C[C@](C)(N)[C@H](O)[C@H](C)O1 MYPYJXKWCTUITO-LYRMYLQWSA-N 0.000 description 16
- 229960003165 vancomycin Drugs 0.000 description 16
- MYPYJXKWCTUITO-UHFFFAOYSA-N vancomycin Natural products O1C(C(=C2)Cl)=CC=C2C(O)C(C(NC(C2=CC(O)=CC(O)=C2C=2C(O)=CC=C3C=2)C(O)=O)=O)NC(=O)C3NC(=O)C2NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(CC(C)C)NC)C(O)C(C=C3Cl)=CC=C3OC3=CC2=CC1=C3OC1OC(CO)C(O)C(O)C1OC1CC(C)(N)C(O)C(C)O1 MYPYJXKWCTUITO-UHFFFAOYSA-N 0.000 description 16
- 108060003951 Immunoglobulin Proteins 0.000 description 15
- 102000018358 immunoglobulin Human genes 0.000 description 15
- 230000003902 lesion Effects 0.000 description 15
- 210000001519 tissue Anatomy 0.000 description 15
- 238000011282 treatment Methods 0.000 description 15
- 238000012217 deletion Methods 0.000 description 14
- 230000037430 deletion Effects 0.000 description 14
- 230000002829 reductive effect Effects 0.000 description 14
- 241000894007 species Species 0.000 description 14
- 239000006228 supernatant Substances 0.000 description 14
- 230000000295 complement effect Effects 0.000 description 13
- 238000003780 insertion Methods 0.000 description 13
- 230000037431 insertion Effects 0.000 description 13
- 210000004072 lung Anatomy 0.000 description 13
- 230000003389 potentiating effect Effects 0.000 description 13
- 239000011780 sodium chloride Substances 0.000 description 13
- 208000024891 symptom Diseases 0.000 description 13
- NFGXHKASABOEEW-UHFFFAOYSA-N 1-methylethyl 11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate Chemical compound COC(C)(C)CCCC(C)CC=CC(C)=CC(=O)OC(C)C NFGXHKASABOEEW-UHFFFAOYSA-N 0.000 description 12
- 241000124008 Mammalia Species 0.000 description 12
- 238000007792 addition Methods 0.000 description 12
- 230000010056 antibody-dependent cellular cytotoxicity Effects 0.000 description 12
- 239000002552 dosage form Substances 0.000 description 12
- 239000003814 drug Substances 0.000 description 12
- 230000002949 hemolytic effect Effects 0.000 description 12
- 239000003112 inhibitor Substances 0.000 description 12
- 230000003993 interaction Effects 0.000 description 12
- 238000000746 purification Methods 0.000 description 12
- 102000003855 L-lactate dehydrogenase Human genes 0.000 description 11
- 108700023483 L-lactate dehydrogenases Proteins 0.000 description 11
- 102100029193 Low affinity immunoglobulin gamma Fc region receptor III-A Human genes 0.000 description 11
- 108091028043 Nucleic acid sequence Proteins 0.000 description 11
- 230000000890 antigenic effect Effects 0.000 description 11
- 238000005119 centrifugation Methods 0.000 description 11
- 230000004048 modification Effects 0.000 description 11
- 238000012986 modification Methods 0.000 description 11
- 230000035772 mutation Effects 0.000 description 11
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 10
- 238000002965 ELISA Methods 0.000 description 10
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 10
- 101710099301 Low affinity immunoglobulin gamma Fc region receptor III-A Proteins 0.000 description 10
- 241000699666 Mus <mouse, genus> Species 0.000 description 10
- 206010041925 Staphylococcal infections Diseases 0.000 description 10
- 238000004113 cell culture Methods 0.000 description 10
- 239000006143 cell culture medium Substances 0.000 description 10
- 238000009472 formulation Methods 0.000 description 10
- 230000004044 response Effects 0.000 description 10
- 239000013598 vector Substances 0.000 description 10
- 206010040070 Septic Shock Diseases 0.000 description 9
- 206010044248 Toxic shock syndrome Diseases 0.000 description 9
- 231100000650 Toxic shock syndrome Toxicity 0.000 description 9
- 239000007640 basal medium Substances 0.000 description 9
- 238000001514 detection method Methods 0.000 description 9
- 208000035475 disorder Diseases 0.000 description 9
- 229940079593 drug Drugs 0.000 description 9
- 230000004927 fusion Effects 0.000 description 9
- 108020001507 fusion proteins Proteins 0.000 description 9
- 102000037865 fusion proteins Human genes 0.000 description 9
- 238000001727 in vivo Methods 0.000 description 9
- 239000007924 injection Substances 0.000 description 9
- 238000002347 injection Methods 0.000 description 9
- 208000015688 methicillin-resistant staphylococcus aureus infectious disease Diseases 0.000 description 9
- 230000001338 necrotic effect Effects 0.000 description 9
- 238000002823 phage display Methods 0.000 description 9
- 239000013612 plasmid Substances 0.000 description 9
- 230000008569 process Effects 0.000 description 9
- 230000009467 reduction Effects 0.000 description 9
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 8
- 238000011746 C57BL/6J (JAX™ mouse strain) Methods 0.000 description 8
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 8
- 206010035226 Plasma cell myeloma Diseases 0.000 description 8
- 208000021326 Ritter disease Diseases 0.000 description 8
- 206010041929 Staphylococcal scalded skin syndrome Diseases 0.000 description 8
- -1 aminoglycosides Substances 0.000 description 8
- 230000004540 complement-dependent cytotoxicity Effects 0.000 description 8
- 239000012530 fluid Substances 0.000 description 8
- 239000001963 growth medium Substances 0.000 description 8
- 239000000463 material Substances 0.000 description 8
- 238000005259 measurement Methods 0.000 description 8
- 239000012528 membrane Substances 0.000 description 8
- 238000002703 mutagenesis Methods 0.000 description 8
- 231100000350 mutagenesis Toxicity 0.000 description 8
- 201000000050 myeloid neoplasm Diseases 0.000 description 8
- 238000006386 neutralization reaction Methods 0.000 description 8
- 235000015097 nutrients Nutrition 0.000 description 8
- 230000001717 pathogenic effect Effects 0.000 description 8
- 239000000047 product Substances 0.000 description 8
- 239000007787 solid Substances 0.000 description 8
- 239000000243 solution Substances 0.000 description 8
- 238000001262 western blot Methods 0.000 description 8
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 7
- 208000035484 Cellulite Diseases 0.000 description 7
- 206010016228 Fasciitis Diseases 0.000 description 7
- 108010087819 Fc receptors Proteins 0.000 description 7
- 102000009109 Fc receptors Human genes 0.000 description 7
- 108091034117 Oligonucleotide Proteins 0.000 description 7
- 206010049752 Peau d'orange Diseases 0.000 description 7
- 229920002684 Sepharose Polymers 0.000 description 7
- 241000191940 Staphylococcus Species 0.000 description 7
- 239000002671 adjuvant Substances 0.000 description 7
- 238000013459 approach Methods 0.000 description 7
- 210000001772 blood platelet Anatomy 0.000 description 7
- 235000011089 carbon dioxide Nutrition 0.000 description 7
- 230000036232 cellulite Effects 0.000 description 7
- 238000010367 cloning Methods 0.000 description 7
- 230000008030 elimination Effects 0.000 description 7
- 238000003379 elimination reaction Methods 0.000 description 7
- 150000002632 lipids Chemical class 0.000 description 7
- 210000004698 lymphocyte Anatomy 0.000 description 7
- 230000000069 prophylactic effect Effects 0.000 description 7
- 238000012216 screening Methods 0.000 description 7
- 239000002023 wood Substances 0.000 description 7
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 6
- 239000004471 Glycine Substances 0.000 description 6
- 102100026120 IgG receptor FcRn large subunit p51 Human genes 0.000 description 6
- 101710177940 IgG receptor FcRn large subunit p51 Proteins 0.000 description 6
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 6
- RJQXTJLFIWVMTO-TYNCELHUSA-N Methicillin Chemical compound COC1=CC=CC(OC)=C1C(=O)N[C@@H]1C(=O)N2[C@@H](C(O)=O)C(C)(C)S[C@@H]21 RJQXTJLFIWVMTO-TYNCELHUSA-N 0.000 description 6
- 108010029485 Protein Isoforms Proteins 0.000 description 6
- 102000001708 Protein Isoforms Human genes 0.000 description 6
- 239000006146 Roswell Park Memorial Institute medium Substances 0.000 description 6
- 208000027418 Wounds and injury Diseases 0.000 description 6
- 210000004369 blood Anatomy 0.000 description 6
- 239000008280 blood Substances 0.000 description 6
- 238000010790 dilution Methods 0.000 description 6
- 239000012895 dilution Substances 0.000 description 6
- 238000002474 experimental method Methods 0.000 description 6
- 239000012091 fetal bovine serum Substances 0.000 description 6
- 230000006872 improvement Effects 0.000 description 6
- 230000000670 limiting effect Effects 0.000 description 6
- 229960003085 meticillin Drugs 0.000 description 6
- 210000001616 monocyte Anatomy 0.000 description 6
- 238000011321 prophylaxis Methods 0.000 description 6
- 239000001488 sodium phosphate Substances 0.000 description 6
- 229910000162 sodium phosphate Inorganic materials 0.000 description 6
- 239000000758 substrate Substances 0.000 description 6
- 238000002198 surface plasmon resonance spectroscopy Methods 0.000 description 6
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical compound [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 description 6
- 102000004190 Enzymes Human genes 0.000 description 5
- 108090000790 Enzymes Proteins 0.000 description 5
- 108010067060 Immunoglobulin Variable Region Proteins 0.000 description 5
- 102000017727 Immunoglobulin Variable Region Human genes 0.000 description 5
- 229920001213 Polysorbate 20 Polymers 0.000 description 5
- 108020004511 Recombinant DNA Proteins 0.000 description 5
- 206010062255 Soft tissue infection Diseases 0.000 description 5
- 206010052428 Wound Diseases 0.000 description 5
- 238000013019 agitation Methods 0.000 description 5
- 229940088710 antibiotic agent Drugs 0.000 description 5
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 5
- 210000000270 basal cell Anatomy 0.000 description 5
- 210000000170 cell membrane Anatomy 0.000 description 5
- 230000008859 change Effects 0.000 description 5
- 238000012512 characterization method Methods 0.000 description 5
- 238000006243 chemical reaction Methods 0.000 description 5
- 239000003153 chemical reaction reagent Substances 0.000 description 5
- 239000002299 complementary DNA Substances 0.000 description 5
- 235000018417 cysteine Nutrition 0.000 description 5
- 238000011161 development Methods 0.000 description 5
- 230000018109 developmental process Effects 0.000 description 5
- 239000000032 diagnostic agent Substances 0.000 description 5
- 229940039227 diagnostic agent Drugs 0.000 description 5
- 238000000502 dialysis Methods 0.000 description 5
- 230000001747 exhibiting effect Effects 0.000 description 5
- 239000013604 expression vector Substances 0.000 description 5
- 230000002068 genetic effect Effects 0.000 description 5
- NFJRQODDTXZEBV-MXIFXDQUSA-M gleptoferron Chemical compound [Fe].[O-]O.OC[C@H]1O[C@H](OCC(O)C(O)C(O)C(O)C(O)C(O)=O)[C@H](O)[C@@H](O)[C@@H]1O NFJRQODDTXZEBV-MXIFXDQUSA-M 0.000 description 5
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 5
- 235000004554 glutamine Nutrition 0.000 description 5
- 210000005260 human cell Anatomy 0.000 description 5
- 239000006166 lysate Substances 0.000 description 5
- 210000002540 macrophage Anatomy 0.000 description 5
- 230000017074 necrotic cell death Effects 0.000 description 5
- 229910052760 oxygen Inorganic materials 0.000 description 5
- 239000001301 oxygen Substances 0.000 description 5
- 239000002245 particle Substances 0.000 description 5
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 5
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 5
- 238000002360 preparation method Methods 0.000 description 5
- 230000002285 radioactive effect Effects 0.000 description 5
- 239000001509 sodium citrate Substances 0.000 description 5
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 5
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 5
- 239000007790 solid phase Substances 0.000 description 5
- 208000004048 staphylococcal pneumonia Diseases 0.000 description 5
- 238000003786 synthesis reaction Methods 0.000 description 5
- 238000002560 therapeutic procedure Methods 0.000 description 5
- 239000000304 virulence factor Substances 0.000 description 5
- 230000007923 virulence factor Effects 0.000 description 5
- FUOOLUPWFVMBKG-UHFFFAOYSA-N 2-Aminoisobutyric acid Chemical compound CC(C)(N)C(O)=O FUOOLUPWFVMBKG-UHFFFAOYSA-N 0.000 description 4
- WZRJTRPJURQBRM-UHFFFAOYSA-N 4-amino-n-(5-methyl-1,2-oxazol-3-yl)benzenesulfonamide;5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-diamine Chemical compound O1C(C)=CC(NS(=O)(=O)C=2C=CC(N)=CC=2)=N1.COC1=C(OC)C(OC)=CC(CC=2C(=NC(N)=NC=2)N)=C1 WZRJTRPJURQBRM-UHFFFAOYSA-N 0.000 description 4
- 241000588724 Escherichia coli Species 0.000 description 4
- ZHNUHDYFZUAESO-UHFFFAOYSA-N Formamide Chemical compound NC=O ZHNUHDYFZUAESO-UHFFFAOYSA-N 0.000 description 4
- 102000005720 Glutathione transferase Human genes 0.000 description 4
- 108010070675 Glutathione transferase Proteins 0.000 description 4
- 241000282412 Homo Species 0.000 description 4
- 108010054477 Immunoglobulin Fab Fragments Proteins 0.000 description 4
- 102000001706 Immunoglobulin Fab Fragments Human genes 0.000 description 4
- 206010061218 Inflammation Diseases 0.000 description 4
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 4
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 4
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 4
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 4
- 206010028851 Necrosis Diseases 0.000 description 4
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 4
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 4
- 206010040047 Sepsis Diseases 0.000 description 4
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 4
- 208000002847 Surgical Wound Diseases 0.000 description 4
- 238000012452 Xenomouse strains Methods 0.000 description 4
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 4
- 230000009471 action Effects 0.000 description 4
- 230000004913 activation Effects 0.000 description 4
- 239000004480 active ingredient Substances 0.000 description 4
- 238000005273 aeration Methods 0.000 description 4
- 235000004279 alanine Nutrition 0.000 description 4
- 210000003719 b-lymphocyte Anatomy 0.000 description 4
- UCMIRNVEIXFBKS-UHFFFAOYSA-N beta-alanine Chemical compound NCCC(O)=O UCMIRNVEIXFBKS-UHFFFAOYSA-N 0.000 description 4
- 230000001413 cellular effect Effects 0.000 description 4
- 229940047766 co-trimoxazole Drugs 0.000 description 4
- 239000013078 crystal Substances 0.000 description 4
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 4
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 description 4
- 230000016396 cytokine production Effects 0.000 description 4
- 230000034994 death Effects 0.000 description 4
- 230000002733 dermonecrotic effect Effects 0.000 description 4
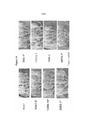
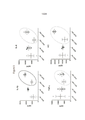
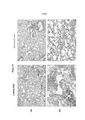
- 238000010586 diagram Methods 0.000 description 4
- 239000003937 drug carrier Substances 0.000 description 4
- 239000002095 exotoxin Substances 0.000 description 4
- 231100000776 exotoxin Toxicity 0.000 description 4
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 4
- 230000002163 immunogen Effects 0.000 description 4
- 230000005847 immunogenicity Effects 0.000 description 4
- 238000011534 incubation Methods 0.000 description 4
- 230000004054 inflammatory process Effects 0.000 description 4
- 239000007928 intraperitoneal injection Substances 0.000 description 4
- 238000002955 isolation Methods 0.000 description 4
- 210000003734 kidney Anatomy 0.000 description 4
- 210000000265 leukocyte Anatomy 0.000 description 4
- 239000003446 ligand Substances 0.000 description 4
- 239000002502 liposome Substances 0.000 description 4
- 239000007788 liquid Substances 0.000 description 4
- 210000000822 natural killer cell Anatomy 0.000 description 4
- 239000013642 negative control Substances 0.000 description 4
- 239000005022 packaging material Substances 0.000 description 4
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 4
- 229940071643 prefilled syringe Drugs 0.000 description 4
- 238000012545 processing Methods 0.000 description 4
- 238000011084 recovery Methods 0.000 description 4
- 238000011160 research Methods 0.000 description 4
- 208000013223 septicemia Diseases 0.000 description 4
- 238000009097 single-agent therapy Methods 0.000 description 4
- 238000001356 surgical procedure Methods 0.000 description 4
- 239000000725 suspension Substances 0.000 description 4
- 208000011580 syndromic disease Diseases 0.000 description 4
- 230000002195 synergetic effect Effects 0.000 description 4
- 230000009885 systemic effect Effects 0.000 description 4
- 235000002374 tyrosine Nutrition 0.000 description 4
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 4
- NHBKXEKEPDILRR-UHFFFAOYSA-N 2,3-bis(butanoylsulfanyl)propyl butanoate Chemical compound CCCC(=O)OCC(SC(=O)CCC)CSC(=O)CCC NHBKXEKEPDILRR-UHFFFAOYSA-N 0.000 description 3
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 3
- 241000283707 Capra Species 0.000 description 3
- 241000282693 Cercopithecidae Species 0.000 description 3
- 238000001712 DNA sequencing Methods 0.000 description 3
- 206010015988 Eyelid infection Diseases 0.000 description 3
- 206010016952 Food poisoning Diseases 0.000 description 3
- 208000019331 Foodborne disease Diseases 0.000 description 3
- 206010017711 Gangrene Diseases 0.000 description 3
- 108700005091 Immunoglobulin Genes Proteins 0.000 description 3
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 3
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 3
- 206010031252 Osteomyelitis Diseases 0.000 description 3
- 206010035734 Pneumonia staphylococcal Diseases 0.000 description 3
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 3
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 3
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 3
- 208000031650 Surgical Wound Infection Diseases 0.000 description 3
- 239000004098 Tetracycline Substances 0.000 description 3
- 101710120037 Toxin CcdB Proteins 0.000 description 3
- 206010000269 abscess Diseases 0.000 description 3
- 239000002253 acid Substances 0.000 description 3
- 230000001154 acute effect Effects 0.000 description 3
- QWCKQJZIFLGMSD-UHFFFAOYSA-N alpha-aminobutyric acid Chemical compound CCC(N)C(O)=O QWCKQJZIFLGMSD-UHFFFAOYSA-N 0.000 description 3
- 238000004458 analytical method Methods 0.000 description 3
- 235000009582 asparagine Nutrition 0.000 description 3
- 229960001230 asparagine Drugs 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 239000011230 binding agent Substances 0.000 description 3
- 229960002685 biotin Drugs 0.000 description 3
- 235000020958 biotin Nutrition 0.000 description 3
- 239000011616 biotin Substances 0.000 description 3
- 230000000903 blocking effect Effects 0.000 description 3
- 230000003915 cell function Effects 0.000 description 3
- 210000000349 chromosome Anatomy 0.000 description 3
- 238000002648 combination therapy Methods 0.000 description 3
- 230000009918 complex formation Effects 0.000 description 3
- 150000001875 compounds Chemical class 0.000 description 3
- 239000013068 control sample Substances 0.000 description 3
- 230000001086 cytosolic effect Effects 0.000 description 3
- 238000003745 diagnosis Methods 0.000 description 3
- 231100000676 disease causative agent Toxicity 0.000 description 3
- 206010014665 endocarditis Diseases 0.000 description 3
- 239000000499 gel Substances 0.000 description 3
- 229960004198 guanidine Drugs 0.000 description 3
- 230000002209 hydrophobic effect Effects 0.000 description 3
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 3
- 230000028993 immune response Effects 0.000 description 3
- 229940127121 immunoconjugate Drugs 0.000 description 3
- 229940072221 immunoglobulins Drugs 0.000 description 3
- 230000001939 inductive effect Effects 0.000 description 3
- 208000014674 injury Diseases 0.000 description 3
- 238000007912 intraperitoneal administration Methods 0.000 description 3
- 210000003292 kidney cell Anatomy 0.000 description 3
- 239000012669 liquid formulation Substances 0.000 description 3
- 210000004962 mammalian cell Anatomy 0.000 description 3
- 108020004999 messenger RNA Proteins 0.000 description 3
- 229930182817 methionine Natural products 0.000 description 3
- 239000007758 minimum essential medium Substances 0.000 description 3
- 239000003607 modifier Substances 0.000 description 3
- 238000012544 monitoring process Methods 0.000 description 3
- 206010033675 panniculitis Diseases 0.000 description 3
- 244000052769 pathogen Species 0.000 description 3
- 239000008188 pellet Substances 0.000 description 3
- 230000004481 post-translational protein modification Effects 0.000 description 3
- 238000000159 protein binding assay Methods 0.000 description 3
- 238000011002 quantification Methods 0.000 description 3
- 230000003248 secreting effect Effects 0.000 description 3
- 235000004400 serine Nutrition 0.000 description 3
- 239000001632 sodium acetate Substances 0.000 description 3
- 235000017281 sodium acetate Nutrition 0.000 description 3
- 238000010561 standard procedure Methods 0.000 description 3
- 238000003756 stirring Methods 0.000 description 3
- 238000007920 subcutaneous administration Methods 0.000 description 3
- 210000004304 subcutaneous tissue Anatomy 0.000 description 3
- 239000004094 surface-active agent Substances 0.000 description 3
- 230000008685 targeting Effects 0.000 description 3
- 235000019364 tetracycline Nutrition 0.000 description 3
- 150000003522 tetracyclines Chemical class 0.000 description 3
- 230000000451 tissue damage Effects 0.000 description 3
- 231100000827 tissue damage Toxicity 0.000 description 3
- 238000012546 transfer Methods 0.000 description 3
- SGKRLCUYIXIAHR-AKNGSSGZSA-N (4s,4ar,5s,5ar,6r,12ar)-4-(dimethylamino)-1,5,10,11,12a-pentahydroxy-6-methyl-3,12-dioxo-4a,5,5a,6-tetrahydro-4h-tetracene-2-carboxamide Chemical compound C1=CC=C2[C@H](C)[C@@H]([C@H](O)[C@@H]3[C@](C(O)=C(C(N)=O)C(=O)[C@H]3N(C)C)(O)C3=O)C3=C(O)C2=C1O SGKRLCUYIXIAHR-AKNGSSGZSA-N 0.000 description 2
- FFTVPQUHLQBXQZ-KVUCHLLUSA-N (4s,4as,5ar,12ar)-4,7-bis(dimethylamino)-1,10,11,12a-tetrahydroxy-3,12-dioxo-4a,5,5a,6-tetrahydro-4h-tetracene-2-carboxamide Chemical compound C1C2=C(N(C)C)C=CC(O)=C2C(O)=C2[C@@H]1C[C@H]1[C@H](N(C)C)C(=O)C(C(N)=O)=C(O)[C@@]1(O)C2=O FFTVPQUHLQBXQZ-KVUCHLLUSA-N 0.000 description 2
- LMDZBCPBFSXMTL-UHFFFAOYSA-N 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide Chemical compound CCN=C=NCCCN(C)C LMDZBCPBFSXMTL-UHFFFAOYSA-N 0.000 description 2
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 2
- QRXMUCSWCMTJGU-UHFFFAOYSA-N 5-bromo-4-chloro-3-indolyl phosphate Chemical compound C1=C(Br)C(Cl)=C2C(OP(O)(=O)O)=CNC2=C1 QRXMUCSWCMTJGU-UHFFFAOYSA-N 0.000 description 2
- SLXKOJJOQWFEFD-UHFFFAOYSA-N 6-aminohexanoic acid Chemical compound NCCCCCC(O)=O SLXKOJJOQWFEFD-UHFFFAOYSA-N 0.000 description 2
- 108010066676 Abrin Proteins 0.000 description 2
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 2
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 2
- 206010001889 Alveolitis Diseases 0.000 description 2
- 239000004475 Arginine Substances 0.000 description 2
- 206010060968 Arthritis infective Diseases 0.000 description 2
- 206010003445 Ascites Diseases 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- 208000031729 Bacteremia Diseases 0.000 description 2
- 201000001178 Bacterial Pneumonia Diseases 0.000 description 2
- 208000035143 Bacterial infection Diseases 0.000 description 2
- 101100454807 Caenorhabditis elegans lgg-1 gene Proteins 0.000 description 2
- 108010076119 Caseins Proteins 0.000 description 2
- 102000000844 Cell Surface Receptors Human genes 0.000 description 2
- 108010001857 Cell Surface Receptors Proteins 0.000 description 2
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 2
- 208000003322 Coinfection Diseases 0.000 description 2
- 108020004635 Complementary DNA Proteins 0.000 description 2
- 241000699800 Cricetinae Species 0.000 description 2
- 241000699802 Cricetulus griseus Species 0.000 description 2
- 102100025698 Cytosolic carboxypeptidase 4 Human genes 0.000 description 2
- 108010013198 Daptomycin Proteins 0.000 description 2
- BWGNESOTFCXPMA-UHFFFAOYSA-N Dihydrogen disulfide Chemical compound SS BWGNESOTFCXPMA-UHFFFAOYSA-N 0.000 description 2
- 206010061818 Disease progression Diseases 0.000 description 2
- 108010000912 Egg Proteins Proteins 0.000 description 2
- 102000002322 Egg Proteins Human genes 0.000 description 2
- 206010051814 Eschar Diseases 0.000 description 2
- 241001198387 Escherichia coli BL21(DE3) Species 0.000 description 2
- 208000010201 Exanthema Diseases 0.000 description 2
- XZWYTXMRWQJBGX-VXBMVYAYSA-N FLAG peptide Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@@H](N)CC(O)=O)CC1=CC=C(O)C=C1 XZWYTXMRWQJBGX-VXBMVYAYSA-N 0.000 description 2
- 108010020195 FLAG peptide Proteins 0.000 description 2
- 241000287828 Gallus gallus Species 0.000 description 2
- 108700004714 Gelonium multiflorum GEL Proteins 0.000 description 2
- 239000006147 Glasgow's Minimal Essential Medium Substances 0.000 description 2
- 235000010469 Glycine max Nutrition 0.000 description 2
- ZRALSGWEFCBTJO-UHFFFAOYSA-N Guanidine Chemical compound NC(N)=N ZRALSGWEFCBTJO-UHFFFAOYSA-N 0.000 description 2
- WZUVPPKBWHMQCE-UHFFFAOYSA-N Haematoxylin Chemical compound C12=CC(O)=C(O)C=C2CC2(O)C1C1=CC=C(O)C(O)=C1OC2 WZUVPPKBWHMQCE-UHFFFAOYSA-N 0.000 description 2
- 102100026122 High affinity immunoglobulin gamma Fc receptor I Human genes 0.000 description 2
- 101000932590 Homo sapiens Cytosolic carboxypeptidase 4 Proteins 0.000 description 2
- 101000913074 Homo sapiens High affinity immunoglobulin gamma Fc receptor I Proteins 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 2
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical compound SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 2
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 2
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 2
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 2
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 2
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 2
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 2
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 2
- 108010014603 Leukocidins Proteins 0.000 description 2
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 2
- 239000004472 Lysine Substances 0.000 description 2
- 201000009906 Meningitis Diseases 0.000 description 2
- 101001033003 Mus musculus Granzyme F Proteins 0.000 description 2
- 241001467552 Mycobacterium bovis BCG Species 0.000 description 2
- NQTADLQHYWFPDB-UHFFFAOYSA-N N-Hydroxysuccinimide Chemical compound ON1C(=O)CCC1=O NQTADLQHYWFPDB-UHFFFAOYSA-N 0.000 description 2
- 206010028980 Neoplasm Diseases 0.000 description 2
- 239000000020 Nitrocellulose Substances 0.000 description 2
- 108020005187 Oligonucleotide Probes Proteins 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- 206010036410 Postoperative wound infection Diseases 0.000 description 2
- 241000589516 Pseudomonas Species 0.000 description 2
- 206010037660 Pyrexia Diseases 0.000 description 2
- 239000012980 RPMI-1640 medium Substances 0.000 description 2
- 108010039491 Ricin Proteins 0.000 description 2
- 241000283984 Rodentia Species 0.000 description 2
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 2
- 108010090804 Streptavidin Proteins 0.000 description 2
- 241000193996 Streptococcus pyogenes Species 0.000 description 2
- 241001505901 Streptococcus sp. 'group A' Species 0.000 description 2
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 2
- 239000004473 Threonine Substances 0.000 description 2
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 2
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 2
- 206010000496 acne Diseases 0.000 description 2
- DZBUGLKDJFMEHC-UHFFFAOYSA-N acridine Chemical compound C1=CC=CC2=CC3=CC=CC=C3N=C21 DZBUGLKDJFMEHC-UHFFFAOYSA-N 0.000 description 2
- 230000009824 affinity maturation Effects 0.000 description 2
- 229960000723 ampicillin Drugs 0.000 description 2
- AVKUERGKIZMTKX-NJBDSQKTSA-N ampicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=CC=C1 AVKUERGKIZMTKX-NJBDSQKTSA-N 0.000 description 2
- 238000000540 analysis of variance Methods 0.000 description 2
- 230000000941 anti-staphylcoccal effect Effects 0.000 description 2
- 238000011091 antibody purification Methods 0.000 description 2
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 2
- 238000003149 assay kit Methods 0.000 description 2
- 229960000190 bacillus calmette–guérin vaccine Drugs 0.000 description 2
- 208000022362 bacterial infectious disease Diseases 0.000 description 2
- 230000004888 barrier function Effects 0.000 description 2
- 239000002585 base Substances 0.000 description 2
- 239000012472 biological sample Substances 0.000 description 2
- 210000000601 blood cell Anatomy 0.000 description 2
- 229940098773 bovine serum albumin Drugs 0.000 description 2
- 150000001720 carbohydrates Chemical class 0.000 description 2
- 235000014633 carbohydrates Nutrition 0.000 description 2
- 239000012876 carrier material Substances 0.000 description 2
- 239000005018 casein Substances 0.000 description 2
- BECPQYXYKAMYBN-UHFFFAOYSA-N casein, tech. Chemical compound NCCCCC(C(O)=O)N=C(O)C(CC(O)=O)N=C(O)C(CCC(O)=N)N=C(O)C(CC(C)C)N=C(O)C(CCC(O)=O)N=C(O)C(CC(O)=O)N=C(O)C(CCC(O)=O)N=C(O)C(C(C)O)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=O)N=C(O)C(CCC(O)=O)N=C(O)C(COP(O)(O)=O)N=C(O)C(CCC(O)=N)N=C(O)C(N)CC1=CC=CC=C1 BECPQYXYKAMYBN-UHFFFAOYSA-N 0.000 description 2
- 235000021240 caseins Nutrition 0.000 description 2
- 230000011712 cell development Effects 0.000 description 2
- 230000004663 cell proliferation Effects 0.000 description 2
- 230000008614 cellular interaction Effects 0.000 description 2
- 229940106164 cephalexin Drugs 0.000 description 2
- ZAIPMKNFIOOWCQ-UEKVPHQBSA-N cephalexin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@@H]3N(C2=O)C(=C(CS3)C)C(O)=O)=CC=CC=C1 ZAIPMKNFIOOWCQ-UEKVPHQBSA-N 0.000 description 2
- 235000013330 chicken meat Nutrition 0.000 description 2
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 2
- 238000004587 chromatography analysis Methods 0.000 description 2
- 229960002227 clindamycin Drugs 0.000 description 2
- KDLRVYVGXIQJDK-AWPVFWJPSA-N clindamycin Chemical compound CN1C[C@H](CCC)C[C@H]1C(=O)N[C@H]([C@H](C)Cl)[C@@H]1[C@H](O)[C@H](O)[C@@H](O)[C@@H](SC)O1 KDLRVYVGXIQJDK-AWPVFWJPSA-N 0.000 description 2
- 108010062119 complement 1q receptor Proteins 0.000 description 2
- 230000024203 complement activation Effects 0.000 description 2
- 230000001010 compromised effect Effects 0.000 description 2
- 230000021615 conjugation Effects 0.000 description 2
- 238000007796 conventional method Methods 0.000 description 2
- 230000008878 coupling Effects 0.000 description 2
- 238000010168 coupling process Methods 0.000 description 2
- 238000005859 coupling reaction Methods 0.000 description 2
- 231100000433 cytotoxic Toxicity 0.000 description 2
- 231100000599 cytotoxic agent Toxicity 0.000 description 2
- 230000001472 cytotoxic effect Effects 0.000 description 2
- 230000006378 damage Effects 0.000 description 2
- DOAKLVKFURWEDJ-QCMAZARJSA-N daptomycin Chemical compound C([C@H]1C(=O)O[C@H](C)[C@@H](C(NCC(=O)N[C@@H](CCCN)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@H](CO)C(=O)N[C@H](C(=O)N1)[C@H](C)CC(O)=O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)CCCCCCCCC)C(=O)C1=CC=CC=C1N DOAKLVKFURWEDJ-QCMAZARJSA-N 0.000 description 2
- 229960005484 daptomycin Drugs 0.000 description 2
- 239000003085 diluting agent Substances 0.000 description 2
- 206010013023 diphtheria Diseases 0.000 description 2
- 230000005750 disease progression Effects 0.000 description 2
- 229960003722 doxycycline Drugs 0.000 description 2
- 230000009977 dual effect Effects 0.000 description 2
- 239000000975 dye Substances 0.000 description 2
- 230000004064 dysfunction Effects 0.000 description 2
- 210000002969 egg yolk Anatomy 0.000 description 2
- 235000013345 egg yolk Nutrition 0.000 description 2
- 238000002330 electrospray ionisation mass spectrometry Methods 0.000 description 2
- 210000002889 endothelial cell Anatomy 0.000 description 2
- 230000002255 enzymatic effect Effects 0.000 description 2
- 210000002615 epidermis Anatomy 0.000 description 2
- 231100000333 eschar Toxicity 0.000 description 2
- 201000005884 exanthem Diseases 0.000 description 2
- 210000002950 fibroblast Anatomy 0.000 description 2
- BTCSSZJGUNDROE-UHFFFAOYSA-N gamma-aminobutyric acid Chemical compound NCCCC(O)=O BTCSSZJGUNDROE-UHFFFAOYSA-N 0.000 description 2
- 150000004676 glycans Chemical class 0.000 description 2
- 150000002337 glycosamines Chemical group 0.000 description 2
- PJJJBBJSCAKJQF-UHFFFAOYSA-N guanidinium chloride Chemical compound [Cl-].NC(N)=[NH2+] PJJJBBJSCAKJQF-UHFFFAOYSA-N 0.000 description 2
- 210000003709 heart valve Anatomy 0.000 description 2
- 239000003228 hemolysin Substances 0.000 description 2
- 125000000487 histidyl group Chemical group [H]N([H])C(C(=O)O*)C([H])([H])C1=C([H])N([H])C([H])=N1 0.000 description 2
- 238000007489 histopathology method Methods 0.000 description 2
- 229910052739 hydrogen Inorganic materials 0.000 description 2
- 210000000987 immune system Anatomy 0.000 description 2
- 230000016784 immunoglobulin production Effects 0.000 description 2
- 238000001114 immunoprecipitation Methods 0.000 description 2
- 230000001771 impaired effect Effects 0.000 description 2
- 238000000099 in vitro assay Methods 0.000 description 2
- 230000006698 induction Effects 0.000 description 2
- 239000004615 ingredient Substances 0.000 description 2
- 239000003999 initiator Substances 0.000 description 2
- 239000002198 insoluble material Substances 0.000 description 2
- 238000001990 intravenous administration Methods 0.000 description 2
- 230000009545 invasion Effects 0.000 description 2
- 229960000310 isoleucine Drugs 0.000 description 2
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 2
- BPHPUYQFMNQIOC-NXRLNHOXSA-N isopropyl beta-D-thiogalactopyranoside Chemical compound CC(C)S[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O BPHPUYQFMNQIOC-NXRLNHOXSA-N 0.000 description 2
- 210000002510 keratinocyte Anatomy 0.000 description 2
- 108010045069 keyhole-limpet hemocyanin Proteins 0.000 description 2
- TYZROVQLWOKYKF-ZDUSSCGKSA-N linezolid Chemical compound O=C1O[C@@H](CNC(=O)C)CN1C(C=C1F)=CC=C1N1CCOCC1 TYZROVQLWOKYKF-ZDUSSCGKSA-N 0.000 description 2
- 229960003907 linezolid Drugs 0.000 description 2
- 239000008176 lyophilized powder Substances 0.000 description 2
- 239000012139 lysis buffer Substances 0.000 description 2
- 239000011159 matrix material Substances 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 229960004023 minocycline Drugs 0.000 description 2
- 238000010172 mouse model Methods 0.000 description 2
- 230000003472 neutralizing effect Effects 0.000 description 2
- 210000000440 neutrophil Anatomy 0.000 description 2
- 229920001220 nitrocellulos Polymers 0.000 description 2
- 230000009871 nonspecific binding Effects 0.000 description 2
- 230000037311 normal skin Effects 0.000 description 2
- 230000000050 nutritive effect Effects 0.000 description 2
- 239000002751 oligonucleotide probe Substances 0.000 description 2
- 239000003960 organic solvent Substances 0.000 description 2
- 210000001672 ovary Anatomy 0.000 description 2
- 230000002018 overexpression Effects 0.000 description 2
- 238000004806 packaging method and process Methods 0.000 description 2
- 230000007918 pathogenicity Effects 0.000 description 2
- 102000013415 peroxidase activity proteins Human genes 0.000 description 2
- 108040007629 peroxidase activity proteins Proteins 0.000 description 2
- 239000008177 pharmaceutical agent Substances 0.000 description 2
- 239000000825 pharmaceutical preparation Substances 0.000 description 2
- 229940127557 pharmaceutical product Drugs 0.000 description 2
- 239000012071 phase Substances 0.000 description 2
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 2
- 229920001223 polyethylene glycol Polymers 0.000 description 2
- 229920001282 polysaccharide Polymers 0.000 description 2
- 239000005017 polysaccharide Substances 0.000 description 2
- 230000002685 pulmonary effect Effects 0.000 description 2
- 206010037844 rash Diseases 0.000 description 2
- 238000003259 recombinant expression Methods 0.000 description 2
- 238000005215 recombination Methods 0.000 description 2
- 230000006798 recombination Effects 0.000 description 2
- 230000029058 respiratory gaseous exchange Effects 0.000 description 2
- 150000003839 salts Chemical class 0.000 description 2
- 239000012723 sample buffer Substances 0.000 description 2
- FSYKKLYZXJSNPZ-UHFFFAOYSA-N sarcosine Chemical compound C[NH2+]CC([O-])=O FSYKKLYZXJSNPZ-UHFFFAOYSA-N 0.000 description 2
- 239000006152 selective media Substances 0.000 description 2
- 230000035945 sensitivity Effects 0.000 description 2
- 238000013207 serial dilution Methods 0.000 description 2
- 229910000029 sodium carbonate Inorganic materials 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- ATHGHQPFGPMSJY-UHFFFAOYSA-N spermidine Chemical compound NCCCCNCCCN ATHGHQPFGPMSJY-UHFFFAOYSA-N 0.000 description 2
- 239000007921 spray Substances 0.000 description 2
- 238000012916 structural analysis Methods 0.000 description 2
- 230000008961 swelling Effects 0.000 description 2
- 229940040944 tetracyclines Drugs 0.000 description 2
- 229940124597 therapeutic agent Drugs 0.000 description 2
- FYSNRJHAOHDILO-UHFFFAOYSA-N thionyl chloride Chemical compound ClS(Cl)=O FYSNRJHAOHDILO-UHFFFAOYSA-N 0.000 description 2
- 235000008521 threonine Nutrition 0.000 description 2
- 238000004448 titration Methods 0.000 description 2
- 230000000699 topical effect Effects 0.000 description 2
- 231100000331 toxic Toxicity 0.000 description 2
- 230000002588 toxic effect Effects 0.000 description 2
- 238000013518 transcription Methods 0.000 description 2
- 230000035897 transcription Effects 0.000 description 2
- 230000008733 trauma Effects 0.000 description 2
- 239000001974 tryptic soy broth Substances 0.000 description 2
- 108010050327 trypticase-soy broth Proteins 0.000 description 2
- 239000004474 valine Substances 0.000 description 2
- 230000001018 virulence Effects 0.000 description 2
- 239000011782 vitamin Substances 0.000 description 2
- 235000013343 vitamin Nutrition 0.000 description 2
- 229940088594 vitamin Drugs 0.000 description 2
- 229930003231 vitamin Natural products 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 1
- BVAUMRCGVHUWOZ-ZETCQYMHSA-N (2s)-2-(cyclohexylazaniumyl)propanoate Chemical compound OC(=O)[C@H](C)NC1CCCCC1 BVAUMRCGVHUWOZ-ZETCQYMHSA-N 0.000 description 1
- MRTPISKDZDHEQI-YFKPBYRVSA-N (2s)-2-(tert-butylamino)propanoic acid Chemical compound OC(=O)[C@H](C)NC(C)(C)C MRTPISKDZDHEQI-YFKPBYRVSA-N 0.000 description 1
- NPDBDJFLKKQMCM-SCSAIBSYSA-N (2s)-2-amino-3,3-dimethylbutanoic acid Chemical compound CC(C)(C)[C@H](N)C(O)=O NPDBDJFLKKQMCM-SCSAIBSYSA-N 0.000 description 1
- ASWBNKHCZGQVJV-UHFFFAOYSA-N (3-hexadecanoyloxy-2-hydroxypropyl) 2-(trimethylazaniumyl)ethyl phosphate Chemical compound CCCCCCCCCCCCCCCC(=O)OCC(O)COP([O-])(=O)OCC[N+](C)(C)C ASWBNKHCZGQVJV-UHFFFAOYSA-N 0.000 description 1
- MZOFCQQQCNRIBI-VMXHOPILSA-N (3s)-4-[[(2s)-1-[[(2s)-1-[[(1s)-1-carboxy-2-hydroxyethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-3-[[2-[[(2s)-2,6-diaminohexanoyl]amino]acetyl]amino]-4-oxobutanoic acid Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN MZOFCQQQCNRIBI-VMXHOPILSA-N 0.000 description 1
- SOVUOXKZCCAWOJ-HJYUBDRYSA-N (4s,4as,5ar,12ar)-9-[[2-(tert-butylamino)acetyl]amino]-4,7-bis(dimethylamino)-1,10,11,12a-tetrahydroxy-3,12-dioxo-4a,5,5a,6-tetrahydro-4h-tetracene-2-carboxamide Chemical compound C1C2=C(N(C)C)C=C(NC(=O)CNC(C)(C)C)C(O)=C2C(O)=C2[C@@H]1C[C@H]1[C@H](N(C)C)C(=O)C(C(N)=O)=C(O)[C@@]1(O)C2=O SOVUOXKZCCAWOJ-HJYUBDRYSA-N 0.000 description 1
- QGKMIGUHVLGJBR-UHFFFAOYSA-M (4z)-1-(3-methylbutyl)-4-[[1-(3-methylbutyl)quinolin-1-ium-4-yl]methylidene]quinoline;iodide Chemical compound [I-].C12=CC=CC=C2N(CCC(C)C)C=CC1=CC1=CC=[N+](CCC(C)C)C2=CC=CC=C12 QGKMIGUHVLGJBR-UHFFFAOYSA-M 0.000 description 1
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 1
- UFBJCMHMOXMLKC-UHFFFAOYSA-N 2,4-dinitrophenol Chemical compound OC1=CC=C([N+]([O-])=O)C=C1[N+]([O-])=O UFBJCMHMOXMLKC-UHFFFAOYSA-N 0.000 description 1
- FALRKNHUBBKYCC-UHFFFAOYSA-N 2-(chloromethyl)pyridine-3-carbonitrile Chemical compound ClCC1=NC=CC=C1C#N FALRKNHUBBKYCC-UHFFFAOYSA-N 0.000 description 1
- IZXIZTKNFFYFOF-UHFFFAOYSA-N 2-Oxazolidone Chemical class O=C1NCCO1 IZXIZTKNFFYFOF-UHFFFAOYSA-N 0.000 description 1
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 1
- OTLLEIBWKHEHGU-UHFFFAOYSA-N 2-[5-[[5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy]-3,4-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-3,5-dihydroxy-4-phosphonooxyhexanedioic acid Chemical compound C1=NC=2C(N)=NC=NC=2N1C(C(C1O)O)OC1COC1C(CO)OC(OC(C(O)C(OP(O)(O)=O)C(O)C(O)=O)C(O)=O)C(O)C1O OTLLEIBWKHEHGU-UHFFFAOYSA-N 0.000 description 1
- XMTQQYYKAHVGBJ-UHFFFAOYSA-N 3-(3,4-DICHLOROPHENYL)-1,1-DIMETHYLUREA Chemical compound CN(C)C(=O)NC1=CC=C(Cl)C(Cl)=C1 XMTQQYYKAHVGBJ-UHFFFAOYSA-N 0.000 description 1
- 102000036664 ADAM10 Human genes 0.000 description 1
- 108091007504 ADAM10 Proteins 0.000 description 1
- 208000035657 Abasia Diseases 0.000 description 1
- 206010069754 Acquired gene mutation Diseases 0.000 description 1
- 235000001674 Agaricus brunnescens Nutrition 0.000 description 1
- 229920000936 Agarose Polymers 0.000 description 1
- 108010088751 Albumins Proteins 0.000 description 1
- 102000009027 Albumins Human genes 0.000 description 1
- 108700028369 Alleles Proteins 0.000 description 1
- 108010032595 Antibody Binding Sites Proteins 0.000 description 1
- 101001084702 Arabidopsis thaliana Histone H2B.10 Proteins 0.000 description 1
- 101000669426 Aspergillus restrictus Ribonuclease mitogillin Proteins 0.000 description 1
- 102100022717 Atypical chemokine receptor 1 Human genes 0.000 description 1
- 241000271566 Aves Species 0.000 description 1
- 108090001008 Avidin Proteins 0.000 description 1
- 206010004053 Bacterial toxaemia Diseases 0.000 description 1
- 241000606124 Bacteroides fragilis Species 0.000 description 1
- 238000012492 Biacore method Methods 0.000 description 1
- 208000031462 Bovine Mastitis Diseases 0.000 description 1
- 206010006563 Bullous impetigo Diseases 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 241000282472 Canis lupus familiaris Species 0.000 description 1
- 101710158575 Cap-specific mRNA (nucleoside-2'-O-)-methyltransferase Proteins 0.000 description 1
- 101710132601 Capsid protein Proteins 0.000 description 1
- 206010007882 Cellulitis Diseases 0.000 description 1
- 206010008479 Chest Pain Diseases 0.000 description 1
- 208000017667 Chronic Disease Diseases 0.000 description 1
- 101000597253 Clostridium novyi Phospholipase C Proteins 0.000 description 1
- 241000193468 Clostridium perfringens Species 0.000 description 1
- 108010065152 Coagulase Proteins 0.000 description 1
- 101710094648 Coat protein Proteins 0.000 description 1
- 108020004705 Codon Proteins 0.000 description 1
- 206010010071 Coma Diseases 0.000 description 1
- 108091035707 Consensus sequence Proteins 0.000 description 1
- 241000186216 Corynebacterium Species 0.000 description 1
- 206010011224 Cough Diseases 0.000 description 1
- 244000168525 Croton tiglium Species 0.000 description 1
- 102000053602 DNA Human genes 0.000 description 1
- 101710121036 Delta-hemolysin Proteins 0.000 description 1
- 108010008532 Deoxyribonuclease I Proteins 0.000 description 1
- 102000007260 Deoxyribonuclease I Human genes 0.000 description 1
- 108010045579 Desmoglein 1 Proteins 0.000 description 1
- 102100034579 Desmoglein-1 Human genes 0.000 description 1
- 206010012735 Diarrhoea Diseases 0.000 description 1
- BVTJGGGYKAMDBN-UHFFFAOYSA-N Dioxetane Chemical class C1COO1 BVTJGGGYKAMDBN-UHFFFAOYSA-N 0.000 description 1
- 108010053187 Diphtheria Toxin Proteins 0.000 description 1
- 102000016607 Diphtheria Toxin Human genes 0.000 description 1
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 1
- 238000012286 ELISA Assay Methods 0.000 description 1
- 208000030814 Eating disease Diseases 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- 206010059284 Epidermal necrosis Diseases 0.000 description 1
- 241000283086 Equidae Species 0.000 description 1
- 241000672609 Escherichia coli BL21 Species 0.000 description 1
- 206010063560 Excessive granulation tissue Diseases 0.000 description 1
- 101710082714 Exotoxin A Proteins 0.000 description 1
- 108010021468 Fc gamma receptor IIA Proteins 0.000 description 1
- 108010008177 Fd immunoglobulins Proteins 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 102000008946 Fibrinogen Human genes 0.000 description 1
- 108010049003 Fibrinogen Proteins 0.000 description 1
- 241000724791 Filamentous phage Species 0.000 description 1
- 239000012739 FreeStyle 293 Expression medium Substances 0.000 description 1
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 1
- SXRSQZLOMIGNAQ-UHFFFAOYSA-N Glutaraldehyde Chemical compound O=CCCCC=O SXRSQZLOMIGNAQ-UHFFFAOYSA-N 0.000 description 1
- 244000068988 Glycine max Species 0.000 description 1
- 108090000288 Glycoproteins Proteins 0.000 description 1
- 102000003886 Glycoproteins Human genes 0.000 description 1
- 239000007995 HEPES buffer Substances 0.000 description 1
- 102000001554 Hemoglobins Human genes 0.000 description 1
- 108010054147 Hemoglobins Proteins 0.000 description 1
- 108010006464 Hemolysin Proteins Proteins 0.000 description 1
- LYCVKHSJGDMDLM-LURJTMIESA-N His-Gly Chemical compound OC(=O)CNC(=O)[C@@H](N)CC1=CN=CN1 LYCVKHSJGDMDLM-LURJTMIESA-N 0.000 description 1
- 101000678879 Homo sapiens Atypical chemokine receptor 1 Proteins 0.000 description 1
- 101000917826 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor II-a Proteins 0.000 description 1
- 101000917824 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor II-b Proteins 0.000 description 1
- 101000917858 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor III-A Proteins 0.000 description 1
- 101000917839 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor III-B Proteins 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 1
- PMMYEEVYMWASQN-DMTCNVIQSA-N Hydroxyproline Chemical compound O[C@H]1CN[C@H](C(O)=O)C1 PMMYEEVYMWASQN-DMTCNVIQSA-N 0.000 description 1
- 208000001953 Hypotension Diseases 0.000 description 1
- 108010073807 IgG Receptors Proteins 0.000 description 1
- 102000009490 IgG Receptors Human genes 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 102000012745 Immunoglobulin Subunits Human genes 0.000 description 1
- 108010079585 Immunoglobulin Subunits Proteins 0.000 description 1
- 102100034343 Integrase Human genes 0.000 description 1
- 102000004889 Interleukin-6 Human genes 0.000 description 1
- 108090001005 Interleukin-6 Proteins 0.000 description 1
- PIWKPBJCKXDKJR-UHFFFAOYSA-N Isoflurane Chemical compound FC(F)OC(Cl)C(F)(F)F PIWKPBJCKXDKJR-UHFFFAOYSA-N 0.000 description 1
- SNDPXSYFESPGGJ-BYPYZUCNSA-N L-2-aminopentanoic acid Chemical compound CCC[C@H](N)C(O)=O SNDPXSYFESPGGJ-BYPYZUCNSA-N 0.000 description 1
- AHLPHDHHMVZTML-BYPYZUCNSA-N L-Ornithine Chemical compound NCCC[C@H](N)C(O)=O AHLPHDHHMVZTML-BYPYZUCNSA-N 0.000 description 1
- ZGUNAGUHMKGQNY-ZETCQYMHSA-N L-alpha-phenylglycine zwitterion Chemical compound OC(=O)[C@@H](N)C1=CC=CC=C1 ZGUNAGUHMKGQNY-ZETCQYMHSA-N 0.000 description 1
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 1
- RHGKLRLOHDJJDR-BYPYZUCNSA-N L-citrulline Chemical compound NC(=O)NCCC[C@H]([NH3+])C([O-])=O RHGKLRLOHDJJDR-BYPYZUCNSA-N 0.000 description 1
- 229930182816 L-glutamine Natural products 0.000 description 1
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 1
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 1
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 1
- SNDPXSYFESPGGJ-UHFFFAOYSA-N L-norVal-OH Natural products CCCC(N)C(O)=O SNDPXSYFESPGGJ-UHFFFAOYSA-N 0.000 description 1
- LRQKBLKVPFOOQJ-YFKPBYRVSA-N L-norleucine Chemical compound CCCC[C@H]([NH3+])C([O-])=O LRQKBLKVPFOOQJ-YFKPBYRVSA-N 0.000 description 1
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical compound C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 description 1
- 206010024264 Lethargy Diseases 0.000 description 1
- 239000002841 Lewis acid Substances 0.000 description 1
- 239000002879 Lewis base Substances 0.000 description 1
- 239000000232 Lipid Bilayer Substances 0.000 description 1
- 102100029204 Low affinity immunoglobulin gamma Fc region receptor II-a Human genes 0.000 description 1
- 108060001084 Luciferase Proteins 0.000 description 1
- 239000005089 Luciferase Substances 0.000 description 1
- 241000282560 Macaca mulatta Species 0.000 description 1
- 101710125418 Major capsid protein Proteins 0.000 description 1
- 244000302512 Momordica charantia Species 0.000 description 1
- 235000009811 Momordica charantia Nutrition 0.000 description 1
- 208000034486 Multi-organ failure Diseases 0.000 description 1
- 208000010718 Multiple Organ Failure Diseases 0.000 description 1
- 101710135898 Myc proto-oncogene protein Proteins 0.000 description 1
- 102100038895 Myc proto-oncogene protein Human genes 0.000 description 1
- 230000004988 N-glycosylation Effects 0.000 description 1
- CHJJGSNFBQVOTG-UHFFFAOYSA-N N-methyl-guanidine Natural products CNC(N)=N CHJJGSNFBQVOTG-UHFFFAOYSA-N 0.000 description 1
- RHGKLRLOHDJJDR-UHFFFAOYSA-N Ndelta-carbamoyl-DL-ornithine Natural products OC(=O)C(N)CCCNC(N)=O RHGKLRLOHDJJDR-UHFFFAOYSA-N 0.000 description 1
- 101710141454 Nucleoprotein Proteins 0.000 description 1
- AHLPHDHHMVZTML-UHFFFAOYSA-N Orn-delta-NH2 Natural products NCCCC(N)C(O)=O AHLPHDHHMVZTML-UHFFFAOYSA-N 0.000 description 1
- UTJLXEIPEHZYQJ-UHFFFAOYSA-N Ornithine Natural products OC(=O)C(C)CCCN UTJLXEIPEHZYQJ-UHFFFAOYSA-N 0.000 description 1
- 238000012408 PCR amplification Methods 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 208000002193 Pain Diseases 0.000 description 1
- 241000609499 Palicourea Species 0.000 description 1
- 241000282520 Papio Species 0.000 description 1
- 241000237988 Patellidae Species 0.000 description 1
- 108091005804 Peptidases Proteins 0.000 description 1
- 102000035195 Peptidases Human genes 0.000 description 1
- 102000007079 Peptide Fragments Human genes 0.000 description 1
- 108010033276 Peptide Fragments Proteins 0.000 description 1
- 102000004160 Phosphoric Monoester Hydrolases Human genes 0.000 description 1
- 108090000608 Phosphoric Monoester Hydrolases Proteins 0.000 description 1
- 241000219506 Phytolacca Species 0.000 description 1
- 101100413173 Phytolacca americana PAP2 gene Proteins 0.000 description 1
- 241000235648 Pichia Species 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 101000606032 Pomacea maculata Perivitellin-2 31 kDa subunit Proteins 0.000 description 1
- 101000606027 Pomacea maculata Perivitellin-2 67 kDa subunit Proteins 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- 101710083689 Probable capsid protein Proteins 0.000 description 1
- 239000004365 Protease Substances 0.000 description 1
- 108010001267 Protein Subunits Proteins 0.000 description 1
- 102000002067 Protein Subunits Human genes 0.000 description 1
- 241000589517 Pseudomonas aeruginosa Species 0.000 description 1
- 206010058824 Pulmonary necrosis Diseases 0.000 description 1
- 108010092799 RNA-directed DNA polymerase Proteins 0.000 description 1
- 208000035415 Reinfection Diseases 0.000 description 1
- 108010083644 Ribonucleases Proteins 0.000 description 1
- 102000006382 Ribonucleases Human genes 0.000 description 1
- 229910006124 SOCl2 Inorganic materials 0.000 description 1
- 240000003946 Saponaria officinalis Species 0.000 description 1
- 108010077895 Sarcosine Proteins 0.000 description 1
- 239000012506 Sephacryl® Substances 0.000 description 1
- 229920005654 Sephadex Polymers 0.000 description 1
- 239000012507 Sephadex™ Substances 0.000 description 1
- 241000700584 Simplexvirus Species 0.000 description 1
- 108010003723 Single-Domain Antibodies Proteins 0.000 description 1
- 108020004682 Single-Stranded DNA Proteins 0.000 description 1
- 102220497176 Small vasohibin-binding protein_T47D_mutation Human genes 0.000 description 1
- 101000815632 Streptococcus suis (strain 05ZYH33) Rqc2 homolog RqcH Proteins 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- 206010042496 Sunburn Diseases 0.000 description 1
- 208000033809 Suppuration Diseases 0.000 description 1
- 108010053950 Teicoplanin Proteins 0.000 description 1
- 108010034949 Thyroglobulin Proteins 0.000 description 1
- 102000009843 Thyroglobulin Human genes 0.000 description 1
- 208000013222 Toxemia Diseases 0.000 description 1
- 206010044223 Toxic epidermal necrolysis Diseases 0.000 description 1
- 231100000087 Toxic epidermal necrolysis Toxicity 0.000 description 1
- 101710150448 Transcriptional regulator Myc Proteins 0.000 description 1
- 108700019146 Transgenes Proteins 0.000 description 1
- 241000255993 Trichoplusia ni Species 0.000 description 1
- 101710162629 Trypsin inhibitor Proteins 0.000 description 1
- 229940122618 Trypsin inhibitor Drugs 0.000 description 1
- 102000004243 Tubulin Human genes 0.000 description 1
- 108090000704 Tubulin Proteins 0.000 description 1
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 1
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 1
- 206010053613 Type IV hypersensitivity reaction Diseases 0.000 description 1
- 101150117115 V gene Proteins 0.000 description 1
- 240000001866 Vernicia fordii Species 0.000 description 1
- 241000607265 Vibrio vulnificus Species 0.000 description 1
- 241000700605 Viruses Species 0.000 description 1
- 206010047700 Vomiting Diseases 0.000 description 1
- 206010048038 Wound infection Diseases 0.000 description 1
- ATBOMIWRCZXYSZ-XZBBILGWSA-N [1-[2,3-dihydroxypropoxy(hydroxy)phosphoryl]oxy-3-hexadecanoyloxypropan-2-yl] (9e,12e)-octadeca-9,12-dienoate Chemical compound CCCCCCCCCCCCCCCC(=O)OCC(COP(O)(=O)OCC(O)CO)OC(=O)CCCCCCC\C=C\C\C=C\CCCCC ATBOMIWRCZXYSZ-XZBBILGWSA-N 0.000 description 1
- 230000001594 aberrant effect Effects 0.000 description 1
- 239000002250 absorbent Substances 0.000 description 1
- 230000002745 absorbent Effects 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 230000003213 activating effect Effects 0.000 description 1
- 206010069351 acute lung injury Diseases 0.000 description 1
- 230000000996 additive effect Effects 0.000 description 1
- 230000001464 adherent effect Effects 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 238000005276 aerator Methods 0.000 description 1
- 238000001042 affinity chromatography Methods 0.000 description 1
- 230000002776 aggregation Effects 0.000 description 1
- 238000004220 aggregation Methods 0.000 description 1
- 125000000217 alkyl group Chemical group 0.000 description 1
- AWUCVROLDVIAJX-UHFFFAOYSA-N alpha-glycerophosphate Natural products OCC(O)COP(O)(O)=O AWUCVROLDVIAJX-UHFFFAOYSA-N 0.000 description 1
- WNROFYMDJYEPJX-UHFFFAOYSA-K aluminium hydroxide Chemical compound [OH-].[OH-].[OH-].[Al+3] WNROFYMDJYEPJX-UHFFFAOYSA-K 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 230000006229 amino acid addition Effects 0.000 description 1
- 229960002684 aminocaproic acid Drugs 0.000 description 1
- 229940126575 aminoglycoside Drugs 0.000 description 1
- BFNBIHQBYMNNAN-UHFFFAOYSA-N ammonium sulfate Chemical compound N.N.OS(O)(=O)=O BFNBIHQBYMNNAN-UHFFFAOYSA-N 0.000 description 1
- 229910052921 ammonium sulfate Inorganic materials 0.000 description 1
- 235000011130 ammonium sulphate Nutrition 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 235000021120 animal protein Nutrition 0.000 description 1
- 230000008485 antagonism Effects 0.000 description 1
- 230000000845 anti-microbial effect Effects 0.000 description 1
- 230000000840 anti-viral effect Effects 0.000 description 1
- 230000009830 antibody antigen interaction Effects 0.000 description 1
- 210000000628 antibody-producing cell Anatomy 0.000 description 1
- 230000006907 apoptotic process Effects 0.000 description 1
- 238000000149 argon plasma sintering Methods 0.000 description 1
- 229940009098 aspartate Drugs 0.000 description 1
- 235000003704 aspartic acid Nutrition 0.000 description 1
- 238000002820 assay format Methods 0.000 description 1
- 230000002238 attenuated effect Effects 0.000 description 1
- 210000003578 bacterial chromosome Anatomy 0.000 description 1
- 230000037365 barrier function of the epidermis Effects 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 229940000635 beta-alanine Drugs 0.000 description 1
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 1
- 230000001588 bifunctional effect Effects 0.000 description 1
- 102000023732 binding proteins Human genes 0.000 description 1
- 108091008324 binding proteins Proteins 0.000 description 1
- 238000010170 biological method Methods 0.000 description 1
- 238000004061 bleaching Methods 0.000 description 1
- 230000017531 blood circulation Effects 0.000 description 1
- 210000000988 bone and bone Anatomy 0.000 description 1
- 239000006172 buffering agent Substances 0.000 description 1
- 230000003139 buffering effect Effects 0.000 description 1
- 210000004899 c-terminal region Anatomy 0.000 description 1
- PPKJUHVNTMYXOD-PZGPJMECSA-N c49ws9n75l Chemical compound O=C([C@@H]1N(C2=O)CC[C@H]1S(=O)(=O)CCN(CC)CC)O[C@H](C(C)C)[C@H](C)\C=C\C(=O)NC\C=C\C(\C)=C\[C@@H](O)CC(=O)CC1=NC2=CO1.N([C@@H]1C(=O)N[C@@H](C(N2CCC[C@H]2C(=O)N(C)[C@@H](CC=2C=CC(=CC=2)N(C)C)C(=O)N2C[C@@H](CS[C@H]3C4CCN(CC4)C3)C(=O)C[C@H]2C(=O)N[C@H](C(=O)O[C@@H]1C)C=1C=CC=CC=1)=O)CC)C(=O)C1=NC=CC=C1O PPKJUHVNTMYXOD-PZGPJMECSA-N 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 244000309466 calf Species 0.000 description 1
- 201000011510 cancer Diseases 0.000 description 1
- 229960003669 carbenicillin Drugs 0.000 description 1
- FPPNZSSZRUTDAP-UWFZAAFLSA-N carbenicillin Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)C(C(O)=O)C1=CC=CC=C1 FPPNZSSZRUTDAP-UWFZAAFLSA-N 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 238000010523 cascade reaction Methods 0.000 description 1
- 230000010261 cell growth Effects 0.000 description 1
- DDTDNCYHLGRFBM-YZEKDTGTSA-N chembl2367892 Chemical compound CC(=O)N[C@H]1[C@@H](O)[C@H](O)[C@H](CO)O[C@H]1O[C@@H]([C@H]1C(N[C@@H](C2=CC(O)=CC(O[C@@H]3[C@H]([C@H](O)[C@H](O)[C@@H](CO)O3)O)=C2C=2C(O)=CC=C(C=2)[C@@H](NC(=O)[C@@H]2NC(=O)[C@@H]3C=4C=C(O)C=C(C=4)OC=4C(O)=CC=C(C=4)[C@@H](N)C(=O)N[C@H](CC=4C=C(Cl)C(O5)=CC=4)C(=O)N3)C(=O)N1)C(O)=O)=O)C(C=C1Cl)=CC=C1OC1=C(O[C@H]3[C@H]([C@@H](O)[C@H](O)[C@H](CO)O3)NC(C)=O)C5=CC2=C1 DDTDNCYHLGRFBM-YZEKDTGTSA-N 0.000 description 1
- 210000004978 chinese hamster ovary cell Anatomy 0.000 description 1
- 229960005091 chloramphenicol Drugs 0.000 description 1
- WIIZWVCIJKGZOK-RKDXNWHRSA-N chloramphenicol Chemical compound ClC(Cl)C(=O)N[C@H](CO)[C@H](O)C1=CC=C([N+]([O-])=O)C=C1 WIIZWVCIJKGZOK-RKDXNWHRSA-N 0.000 description 1
- 235000012000 cholesterol Nutrition 0.000 description 1
- 239000013611 chromosomal DNA Substances 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 208000020832 chronic kidney disease Diseases 0.000 description 1
- 230000004087 circulation Effects 0.000 description 1
- 229960002173 citrulline Drugs 0.000 description 1
- 235000013477 citrulline Nutrition 0.000 description 1
- 238000003776 cleavage reaction Methods 0.000 description 1
- 238000011260 co-administration Methods 0.000 description 1
- 238000004440 column chromatography Methods 0.000 description 1
- 238000012875 competitive assay Methods 0.000 description 1
- 238000004590 computer program Methods 0.000 description 1
- 230000001268 conjugating effect Effects 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 230000009091 contractile dysfunction Effects 0.000 description 1
- 238000005336 cracking Methods 0.000 description 1
- 238000004132 cross linking Methods 0.000 description 1
- 238000009295 crossflow filtration Methods 0.000 description 1
- 238000002425 crystallisation Methods 0.000 description 1
- 230000008025 crystallization Effects 0.000 description 1
- 238000012926 crystallographic analysis Methods 0.000 description 1
- 238000012866 crystallographic experiment Methods 0.000 description 1
- 238000012136 culture method Methods 0.000 description 1
- 239000012228 culture supernatant Substances 0.000 description 1
- 210000001151 cytotoxic T lymphocyte Anatomy 0.000 description 1
- 229940127089 cytotoxic agent Drugs 0.000 description 1
- 239000002254 cytotoxic agent Substances 0.000 description 1
- 239000002619 cytotoxin Substances 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 230000000994 depressogenic effect Effects 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 238000003795 desorption Methods 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- 238000002405 diagnostic procedure Methods 0.000 description 1
- 239000000385 dialysis solution Substances 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- SWSQBOPZIKWTGO-UHFFFAOYSA-N dimethylaminoamidine Natural products CN(C)C(N)=N SWSQBOPZIKWTGO-UHFFFAOYSA-N 0.000 description 1
- 230000006806 disease prevention Effects 0.000 description 1
- KAKKHKRHCKCAGH-UHFFFAOYSA-L disodium;(4-nitrophenyl) phosphate;hexahydrate Chemical compound O.O.O.O.O.O.[Na+].[Na+].[O-][N+](=O)C1=CC=C(OP([O-])([O-])=O)C=C1 KAKKHKRHCKCAGH-UHFFFAOYSA-L 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- PMMYEEVYMWASQN-UHFFFAOYSA-N dl-hydroxyproline Natural products OC1C[NH2+]C(C([O-])=O)C1 PMMYEEVYMWASQN-UHFFFAOYSA-N 0.000 description 1
- 150000002066 eicosanoids Chemical class 0.000 description 1
- 239000003792 electrolyte Substances 0.000 description 1
- 239000012149 elution buffer Substances 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- 208000028208 end stage renal disease Diseases 0.000 description 1
- 201000000523 end stage renal failure Diseases 0.000 description 1
- 239000002158 endotoxin Substances 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- 108010028531 enomycin Proteins 0.000 description 1
- 239000000147 enterotoxin Substances 0.000 description 1
- 231100000655 enterotoxin Toxicity 0.000 description 1
- YQGOJNYOYNNSMM-UHFFFAOYSA-N eosin Chemical compound [Na+].OC(=O)C1=CC=CC=C1C1=C2C=C(Br)C(=O)C(Br)=C2OC2=C(Br)C(O)=C(Br)C=C21 YQGOJNYOYNNSMM-UHFFFAOYSA-N 0.000 description 1
- 210000003617 erythrocyte membrane Anatomy 0.000 description 1
- 239000003797 essential amino acid Substances 0.000 description 1
- 235000020776 essential amino acid Nutrition 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 210000003527 eukaryotic cell Anatomy 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 230000005284 excitation Effects 0.000 description 1
- 201000001155 extrinsic allergic alveolitis Diseases 0.000 description 1
- 206010016256 fatigue Diseases 0.000 description 1
- 238000013230 female C57BL/6J mice Methods 0.000 description 1
- 210000003754 fetus Anatomy 0.000 description 1
- 229940012952 fibrinogen Drugs 0.000 description 1
- 102000036072 fibronectin binding proteins Human genes 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 238000000684 flow cytometry Methods 0.000 description 1
- GNBHRKFJIUUOQI-UHFFFAOYSA-N fluorescein Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 GNBHRKFJIUUOQI-UHFFFAOYSA-N 0.000 description 1
- 238000002866 fluorescence resonance energy transfer Methods 0.000 description 1
- 238000009432 framing Methods 0.000 description 1
- 230000033581 fucosylation Effects 0.000 description 1
- 229960003692 gamma aminobutyric acid Drugs 0.000 description 1
- 238000001502 gel electrophoresis Methods 0.000 description 1
- 238000001641 gel filtration chromatography Methods 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 229930195712 glutamate Natural products 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- 125000003630 glycyl group Chemical group [H]N([H])C([H])([H])C(*)=O 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 210000001126 granulation tissue Anatomy 0.000 description 1
- 210000004013 groin Anatomy 0.000 description 1
- 238000003306 harvesting Methods 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 210000003958 hematopoietic stem cell Anatomy 0.000 description 1
- 108060003552 hemocyanin Proteins 0.000 description 1
- 206010073071 hepatocellular carcinoma Diseases 0.000 description 1
- 231100000844 hepatocellular carcinoma Toxicity 0.000 description 1
- 208000021760 high fever Diseases 0.000 description 1
- 239000012510 hollow fiber Substances 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- 238000012872 hydroxylapatite chromatography Methods 0.000 description 1
- 229960002591 hydroxyproline Drugs 0.000 description 1
- 208000022098 hypersensitivity pneumonitis Diseases 0.000 description 1
- 230000036543 hypotension Effects 0.000 description 1
- 239000012216 imaging agent Substances 0.000 description 1
- 210000002865 immune cell Anatomy 0.000 description 1
- 230000001900 immune effect Effects 0.000 description 1
- 239000007943 implant Substances 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 230000028709 inflammatory response Effects 0.000 description 1
- 230000004941 influx Effects 0.000 description 1
- 238000011081 inoculation Methods 0.000 description 1
- 229910052500 inorganic mineral Inorganic materials 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000005342 ion exchange Methods 0.000 description 1
- 238000004255 ion exchange chromatography Methods 0.000 description 1
- 229920000831 ionic polymer Polymers 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 229960002725 isoflurane Drugs 0.000 description 1
- 238000006317 isomerization reaction Methods 0.000 description 1
- 238000012933 kinetic analysis Methods 0.000 description 1
- 150000002605 large molecules Chemical class 0.000 description 1
- 230000001665 lethal effect Effects 0.000 description 1
- 150000007517 lewis acids Chemical class 0.000 description 1
- 150000007527 lewis bases Chemical class 0.000 description 1
- 229940041028 lincosamides Drugs 0.000 description 1
- 239000012160 loading buffer Substances 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 210000005265 lung cell Anatomy 0.000 description 1
- 210000001165 lymph node Anatomy 0.000 description 1
- 230000001926 lymphatic effect Effects 0.000 description 1
- 210000004324 lymphatic system Anatomy 0.000 description 1
- 239000012931 lyophilized formulation Substances 0.000 description 1
- 125000003588 lysine group Chemical group [H]N([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 description 1
- 239000003120 macrolide antibiotic agent Substances 0.000 description 1
- 229940041033 macrolides Drugs 0.000 description 1
- 229920002521 macromolecule Polymers 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 206010025482 malaise Diseases 0.000 description 1
- 238000013507 mapping Methods 0.000 description 1
- 238000004949 mass spectrometry Methods 0.000 description 1
- 230000008774 maternal effect Effects 0.000 description 1
- 230000035800 maturation Effects 0.000 description 1
- 238000005399 mechanical ventilation Methods 0.000 description 1
- 230000010534 mechanism of action Effects 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 229910021645 metal ion Inorganic materials 0.000 description 1
- 229960000485 methotrexate Drugs 0.000 description 1
- 239000011859 microparticle Substances 0.000 description 1
- 239000011707 mineral Substances 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 108010010621 modeccin Proteins 0.000 description 1
- 238000000302 molecular modelling Methods 0.000 description 1
- 210000003097 mucus Anatomy 0.000 description 1
- 208000029744 multiple organ dysfunction syndrome Diseases 0.000 description 1
- 231100000219 mutagenic Toxicity 0.000 description 1
- 230000003505 mutagenic effect Effects 0.000 description 1
- 210000000066 myeloid cell Anatomy 0.000 description 1
- 230000017095 negative regulation of cell growth Effects 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 239000002547 new drug Substances 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 238000001668 nucleic acid synthesis Methods 0.000 description 1
- 239000002777 nucleoside Substances 0.000 description 1
- 150000003833 nucleoside derivatives Chemical class 0.000 description 1
- 230000003606 oligomerizing effect Effects 0.000 description 1
- 206010030861 ophthalmia neonatorum Diseases 0.000 description 1
- 244000039328 opportunistic pathogen Species 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 229960003104 ornithine Drugs 0.000 description 1
- 238000012261 overproduction Methods 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 230000001590 oxidative effect Effects 0.000 description 1
- 238000007911 parenteral administration Methods 0.000 description 1
- 230000008506 pathogenesis Effects 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 230000010412 perfusion Effects 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- 210000005259 peripheral blood Anatomy 0.000 description 1
- 239000011886 peripheral blood Substances 0.000 description 1
- 108010076042 phenomycin Proteins 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- WTJKGGKOPKCXLL-RRHRGVEJSA-N phosphatidylcholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCC=CCCCCCCCC WTJKGGKOPKCXLL-RRHRGVEJSA-N 0.000 description 1
- 230000026731 phosphorylation Effects 0.000 description 1
- 238000006366 phosphorylation reaction Methods 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 230000007084 physiological dysfunction Effects 0.000 description 1
- 229920001983 poloxamer Polymers 0.000 description 1
- 229920002401 polyacrylamide Polymers 0.000 description 1
- 230000008488 polyadenylation Effects 0.000 description 1
- 108010054442 polyalanine Chemical group 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 230000001323 posttranslational effect Effects 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 1
- 239000000651 prodrug Substances 0.000 description 1
- 229940002612 prodrug Drugs 0.000 description 1
- 239000013587 production medium Substances 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 230000009145 protein modification Effects 0.000 description 1
- 238000001742 protein purification Methods 0.000 description 1
- 231100000654 protein toxin Toxicity 0.000 description 1
- 230000004850 protein–protein interaction Effects 0.000 description 1
- 230000002797 proteolythic effect Effects 0.000 description 1
- 230000005180 public health Effects 0.000 description 1
- 239000002510 pyrogen Substances 0.000 description 1
- 229940052337 quinupristin/dalfopristin Drugs 0.000 description 1
- 238000001525 receptor binding assay Methods 0.000 description 1
- 238000010188 recombinant method Methods 0.000 description 1
- PYWVYCXTNDRMGF-UHFFFAOYSA-N rhodamine B Chemical compound [Cl-].C=12C=CC(=[N+](CC)CC)C=C2OC2=CC(N(CC)CC)=CC=C2C=1C1=CC=CC=C1C(O)=O PYWVYCXTNDRMGF-UHFFFAOYSA-N 0.000 description 1
- 238000002702 ribosome display Methods 0.000 description 1
- 229940043230 sarcosine Drugs 0.000 description 1
- 230000007017 scission Effects 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 238000002864 sequence alignment Methods 0.000 description 1
- 125000003607 serino group Chemical group [H]N([H])[C@]([H])(C(=O)[*])C(O[H])([H])[H] 0.000 description 1
- 239000012679 serum free medium Substances 0.000 description 1
- 208000026425 severe pneumonia Diseases 0.000 description 1
- 230000035939 shock Effects 0.000 description 1
- 230000037432 silent mutation Effects 0.000 description 1
- 238000002741 site-directed mutagenesis Methods 0.000 description 1
- 231100000046 skin rash Toxicity 0.000 description 1
- 150000003384 small molecules Chemical class 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 230000000392 somatic effect Effects 0.000 description 1
- 230000037439 somatic mutation Effects 0.000 description 1
- 125000006850 spacer group Chemical group 0.000 description 1
- 230000009870 specific binding Effects 0.000 description 1
- 238000001228 spectrum Methods 0.000 description 1
- 229940063673 spermidine Drugs 0.000 description 1
- 210000000952 spleen Anatomy 0.000 description 1
- 210000004988 splenocyte Anatomy 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 230000010473 stable expression Effects 0.000 description 1
- 238000010186 staining Methods 0.000 description 1
- 208000015339 staphylococcus aureus infection Diseases 0.000 description 1
- 239000008174 sterile solution Substances 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 239000007929 subcutaneous injection Substances 0.000 description 1
- 238000010254 subcutaneous injection Methods 0.000 description 1
- 229940014800 succinic anhydride Drugs 0.000 description 1
- 229910052717 sulfur Inorganic materials 0.000 description 1
- 230000001629 suppression Effects 0.000 description 1
- 229920001059 synthetic polymer Polymers 0.000 description 1
- 229960001608 teicoplanin Drugs 0.000 description 1
- 238000005496 tempering Methods 0.000 description 1
- 229960002180 tetracycline Drugs 0.000 description 1
- 229930101283 tetracycline Natural products 0.000 description 1
- 230000004797 therapeutic response Effects 0.000 description 1
- 125000003396 thiol group Chemical group [H]S* 0.000 description 1
- 229960002175 thyroglobulin Drugs 0.000 description 1
- 229960004089 tigecycline Drugs 0.000 description 1
- 238000011200 topical administration Methods 0.000 description 1
- FGMPLJWBKKVCDB-UHFFFAOYSA-N trans-L-hydroxy-proline Natural products ON1CCCC1C(O)=O FGMPLJWBKKVCDB-UHFFFAOYSA-N 0.000 description 1
- 238000001890 transfection Methods 0.000 description 1
- 230000009261 transgenic effect Effects 0.000 description 1
- 238000011830 transgenic mouse model Methods 0.000 description 1
- 230000010474 transient expression Effects 0.000 description 1
- 238000003146 transient transfection Methods 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 229930013292 trichothecene Natural products 0.000 description 1
- 150000003327 trichothecene derivatives Chemical class 0.000 description 1
- LZMSXDHGHZKXJD-VJANTYMQSA-N trypanothione disulfide Chemical compound OC(=O)[C@@H](N)CCC(=O)N[C@H]1CSSC[C@H](NC(=O)CC[C@H](N)C(O)=O)C(=O)NCC(=O)NCCCNCCCCNC(=O)CNC1=O LZMSXDHGHZKXJD-VJANTYMQSA-N 0.000 description 1
- 239000002753 trypsin inhibitor Substances 0.000 description 1
- 229960005486 vaccine Drugs 0.000 description 1
- 230000017260 vegetative to reproductive phase transition of meristem Effects 0.000 description 1
- 108700026220 vif Genes Proteins 0.000 description 1
- 210000001835 viscera Anatomy 0.000 description 1
- 231100000009 visible necrosis Toxicity 0.000 description 1
- 230000008673 vomiting Effects 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 238000002424 x-ray crystallography Methods 0.000 description 1
- 210000005253 yeast cell Anatomy 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/12—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from bacteria
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/12—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from bacteria
- C07K16/1267—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from bacteria from Gram-positive bacteria
- C07K16/1271—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from bacteria from Gram-positive bacteria from Micrococcaceae (F), e.g. Staphylococcus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/04—Peptides having up to 20 amino acids in a fully defined sequence; Derivatives thereof
- A61K38/14—Peptides containing saccharide radicals; Derivatives thereof, e.g. bleomycin, phleomycin, muramylpeptides or vancomycin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/40—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum bacterial
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
- A61P15/14—Drugs for genital or sexual disorders; Contraceptives for lactation disorders, e.g. galactorrhoea
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/02—Drugs for dermatological disorders for treating wounds, ulcers, burns, scars, keloids, or the like
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P39/00—General protective or antinoxious agents
- A61P39/02—Antidotes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/569—Immunoassay; Biospecific binding assay; Materials therefor for microorganisms, e.g. protozoa, bacteria, viruses
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/569—Immunoassay; Biospecific binding assay; Materials therefor for microorganisms, e.g. protozoa, bacteria, viruses
- G01N33/56911—Bacteria
- G01N33/56938—Staphylococcus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2299/00—Coordinates from 3D structures of peptides, e.g. proteins or enzymes
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/21—Immunoglobulins specific features characterized by taxonomic origin from primates, e.g. man
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/24—Immunoglobulins specific features characterized by taxonomic origin containing regions, domains or residues from different species, e.g. chimeric, humanized or veneered
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/34—Identification of a linear epitope shorter than 20 amino acid residues or of a conformational epitope defined by amino acid residues
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/76—Antagonist effect on antigen, e.g. neutralization or inhibition of binding
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/92—Affinity (KD), association rate (Ka), dissociation rate (Kd) or EC50 value
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/195—Assays involving biological materials from specific organisms or of a specific nature from bacteria
- G01N2333/305—Assays involving biological materials from specific organisms or of a specific nature from bacteria from Micrococcaceae (F)
- G01N2333/31—Assays involving biological materials from specific organisms or of a specific nature from bacteria from Micrococcaceae (F) from Staphylococcus (G)
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2800/00—Detection or diagnosis of diseases
- G01N2800/20—Dermatological disorders
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2800/00—Detection or diagnosis of diseases
- G01N2800/44—Multiple drug resistance
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2800/00—Detection or diagnosis of diseases
- G01N2800/70—Mechanisms involved in disease identification
- G01N2800/709—Toxin induced
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Immunology (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Molecular Biology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Epidemiology (AREA)
- Biochemistry (AREA)
- Urology & Nephrology (AREA)
- Biomedical Technology (AREA)
- Hematology (AREA)
- Microbiology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Biophysics (AREA)
- Genetics & Genomics (AREA)
- Cell Biology (AREA)
- Biotechnology (AREA)
- Physics & Mathematics (AREA)
- Virology (AREA)
- Tropical Medicine & Parasitology (AREA)
- Food Science & Technology (AREA)
- General Physics & Mathematics (AREA)
- Analytical Chemistry (AREA)
- Pathology (AREA)
- Mycology (AREA)
- Gastroenterology & Hepatology (AREA)
- Dermatology (AREA)
- Endocrinology (AREA)
- Reproductive Health (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201161440581P | 2011-02-08 | 2011-02-08 | |
| US61/440,581 | 2011-02-08 | ||
| PCT/US2012/024201 WO2012109285A2 (en) | 2011-02-08 | 2012-02-07 | Antibodies that specifically bind staphylococcus aureus alpha toxin and methods of use |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| BR112013020086A2 BR112013020086A2 (pt) | 2016-11-22 |
| BR112013020086B1 true BR112013020086B1 (pt) | 2020-12-08 |
Family
ID=46639171
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| BR112013020086-3A BR112013020086B1 (pt) | 2011-02-08 | 2012-02-07 | Anticorpo isolado ou seu fragmento de ligação a antígeno, composição compreendendo o anticorpo ou fragmento de ligação a antígeno, kit, uso de uma quantidade eficaz de um anticorpo ou seu fragmento de ligação a antígeno e método para inibir a formação da oligômeros de alfatoxina |
Country Status (24)
| Country | Link |
|---|---|
| US (1) | US9527905B2 (enExample) |
| EP (2) | EP2673373B3 (enExample) |
| JP (4) | JP2014508748A (enExample) |
| KR (1) | KR101993839B1 (enExample) |
| CN (2) | CN103443285B9 (enExample) |
| AU (1) | AU2012214531C1 (enExample) |
| BR (1) | BR112013020086B1 (enExample) |
| CA (1) | CA2826566C (enExample) |
| CY (1) | CY1121389T1 (enExample) |
| DK (1) | DK2673373T6 (enExample) |
| ES (1) | ES2709065T7 (enExample) |
| HR (1) | HRP20182017T4 (enExample) |
| HU (1) | HUE040734T2 (enExample) |
| LT (1) | LT2673373T (enExample) |
| MX (3) | MX345285B (enExample) |
| PL (1) | PL2673373T6 (enExample) |
| PT (1) | PT2673373T (enExample) |
| RS (1) | RS58190B2 (enExample) |
| RU (1) | RU2620065C2 (enExample) |
| SG (3) | SG10201600899PA (enExample) |
| SI (1) | SI2673373T1 (enExample) |
| SM (1) | SMT201800635T1 (enExample) |
| TR (1) | TR201817932T4 (enExample) |
| WO (1) | WO2012109285A2 (enExample) |
Families Citing this family (35)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| HRP20180458T1 (hr) | 2011-06-10 | 2018-06-29 | Medimmune Limited | Molekule koje vežu anti-pseudomonas psl i njihova uporaba |
| AU2012336028A1 (en) * | 2011-11-07 | 2014-06-26 | Medimmune, Llc | Combination therapies using anti- Pseudomonas Psl and PcrV binding molecules |
| US9988439B2 (en) | 2011-12-23 | 2018-06-05 | Nicholas B. Lydon | Immunoglobulins and variants directed against pathogenic microbes |
| WO2013096948A1 (en) | 2011-12-23 | 2013-06-27 | Lydon Nicholas B | Immunoglobulins and variants directed against pathogenic microbes |
| JOP20200236A1 (ar) | 2012-09-21 | 2017-06-16 | Regeneron Pharma | الأجسام المضادة لمضاد cd3 وجزيئات ربط الأنتيجين ثنائية التحديد التي تربط cd3 وcd20 واستخداماتها |
| ES2776179T3 (es) | 2012-11-06 | 2020-07-29 | Medimmune Llc | Anticuerpos dirigidos contra determinantes de la superficie de S. aureus |
| RU2661406C2 (ru) * | 2012-11-06 | 2018-07-16 | МЕДИММЬЮН, ЭлЭлСи | Способы лечения заболеваний, ассоциированных с s. aureus |
| BR112015010240A2 (pt) * | 2012-11-06 | 2017-08-22 | Medimmune Ltd | Terapias de combinação através do uso de mo-léculas de ligação de psl e de pcrv anti-pseudomonas |
| CN105873946A (zh) * | 2013-10-17 | 2016-08-17 | 阿尔萨尼斯生物科学有限责任公司 | 交叉反应性金黄色葡萄球菌抗体序列 |
| EP3094348B1 (en) * | 2014-01-14 | 2025-03-05 | AbVacc, Inc. | Targeting immunological functions to the site of bacterial infections using cell wall targeting domains of bacteriolysins |
| TWI701042B (zh) | 2014-03-19 | 2020-08-11 | 美商再生元醫藥公司 | 用於腫瘤治療之方法及抗體組成物 |
| TWI719938B (zh) * | 2014-06-19 | 2021-03-01 | 美商麥迪紐有限責任公司 | 多重細菌感染之治療 |
| AU2015303147A1 (en) * | 2014-08-12 | 2017-02-23 | Arsanis Biosciences Gmbh | Predicting S. aureus disease |
| PT3221359T (pt) | 2014-11-17 | 2020-06-23 | Regeneron Pharma | Métodos para o tratamento de tumores usando anticorpos biespecíficos cd3xcd20 |
| EP3766987B1 (en) * | 2015-09-24 | 2023-08-02 | F. Hoffmann-La Roche AG | Alpha-hemolysin variants |
| WO2017075188A2 (en) * | 2015-10-30 | 2017-05-04 | Medimmune, Llc | Methods of using anti-alpha toxin antibody |
| TW202311284A (zh) | 2017-01-03 | 2023-03-16 | 美商再生元醫藥公司 | 抗金黃色葡萄球菌溶血素a毒素之人類抗體 |
| WO2018148494A1 (en) * | 2017-02-09 | 2018-08-16 | Bluefin Biomedicine, Inc. | Anti-ilt3 antibodies and antibody drug conjugates |
| CN108456250B (zh) * | 2017-02-17 | 2025-11-28 | 恺兴生命科技(上海)有限公司 | 靶向il-13ra2的抗体及其应用 |
| CN109384844B (zh) * | 2017-08-04 | 2020-12-18 | 中国人民解放军军事医学科学院基础医学研究所 | 一种抗金黄色葡萄球菌α溶血素单克隆抗体及应用 |
| WO2020023644A2 (en) | 2018-07-24 | 2020-01-30 | Medimmune, Llc | Antibody directed against s. aureus clumping factor a (clfa) |
| US11396542B2 (en) | 2018-08-21 | 2022-07-26 | Synkrino Biotherapeutics, Inc. | Astrotactin1-based compositions and pharmaceutical formulations |
| IL280875B2 (en) | 2018-08-31 | 2024-12-01 | Regeneron Pharma | Dosing strategy that mitigates cytokine release syndrome for cd3/c20 bispecific antibodies |
| AU2019359207A1 (en) | 2018-10-09 | 2021-05-20 | Humabs Biomed Sa | Antibodies directed against Staphylococcus aureus leukotoxins |
| CN113164602A (zh) | 2018-10-09 | 2021-07-23 | 免疫医疗有限责任公司 | 抗金黄色葡萄球菌抗体的组合 |
| WO2020185986A1 (en) | 2019-03-13 | 2020-09-17 | Medimmune, Llc | Decreasing staphylococcus aureus infections in colonized patients |
| WO2020210650A1 (en) | 2019-04-12 | 2020-10-15 | Medimmune, Llc | Neutralization of tgf-beta or alpha-v-beta-8 integrin for s. aureus infections |
| CN112538112B (zh) | 2019-09-20 | 2023-10-27 | 迈威(上海)生物科技股份有限公司 | 抗α-溶血素的抗体及其应用 |
| CN113444171A (zh) * | 2020-03-25 | 2021-09-28 | 兴盟生物医药(苏州)有限公司 | 金黄色葡萄球菌α-毒素特异性抗体及其应用 |
| WO2022148480A1 (zh) * | 2021-01-11 | 2022-07-14 | 星济生物(苏州)有限公司 | 靶向金黄色葡萄球菌α-毒素的抗原结合蛋白及其应用 |
| CN112941136B (zh) * | 2021-03-24 | 2023-04-28 | 成都欧林生物科技股份有限公司 | 一种重组金黄色葡萄球菌疫苗hi抗原蛋白的纯化方法 |
| CN114455048B (zh) * | 2022-03-02 | 2023-03-14 | 江苏微导纳米科技股份有限公司 | 一种桨杆 |
| CN117106077B (zh) * | 2022-04-28 | 2024-07-16 | 珠海泰诺麦博制药股份有限公司 | 特异性结合金黄色葡萄球菌Hla毒素的全人源单克隆抗体 |
| CN116589566A (zh) * | 2022-11-18 | 2023-08-15 | 昆明医科大学第一附属医院 | 一种HIV-1中和抗体的改造重组单克隆IgM抗体及其应用 |
| CN116023486B (zh) * | 2023-01-10 | 2024-10-08 | 西北农林科技大学 | 金黄色葡萄球菌α-溶血素纳米抗体、制备方法及其应用 |
Family Cites Families (23)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| IL85035A0 (en) | 1987-01-08 | 1988-06-30 | Int Genetic Eng | Polynucleotide molecule,a chimeric antibody with specificity for human b cell surface antigen,a process for the preparation and methods utilizing the same |
| CA2103059C (en) | 1991-06-14 | 2005-03-22 | Paul J. Carter | Method for making humanized antibodies |
| CA2253710A1 (en) | 1996-04-25 | 1997-10-30 | Spectrametrix Inc. | Analyte assay using particulate labels |
| ATE309385T1 (de) | 2000-06-28 | 2005-11-15 | Glycofi Inc | Verfahren für die herstellung modifizierter glykoproteine |
| US6596541B2 (en) | 2000-10-31 | 2003-07-22 | Regeneron Pharmaceuticals, Inc. | Methods of modifying eukaryotic cells |
| ES2649037T3 (es) | 2000-12-12 | 2018-01-09 | Medimmune, Llc | Moléculas con semividas prolongadas, composiciones y usos de las mismas |
| US20030226155A1 (en) | 2001-08-30 | 2003-12-04 | Biorexis Pharmaceutical Corporation | Modified transferrin-antibody fusion proteins |
| ES2338105T3 (es) | 2003-05-14 | 2010-05-04 | Kenta Biotech Ag | Anticuerpo monoclonal humano especifico para lipopolisacaridos (lps) de serotipo iats 06 pseudomonas aeruginosa. |
| CN1324049C (zh) | 2003-08-04 | 2007-07-04 | 中国疾病预防控制中心病毒病预防控制所 | 人源抗sars冠状病毒基因工程抗体 |
| CA2885854C (en) * | 2004-04-13 | 2017-02-21 | F. Hoffmann-La Roche Ag | Anti-p-selectin antibodies |
| JP2008513406A (ja) * | 2004-09-22 | 2008-05-01 | グラクソスミスクライン バイオロジカルズ ソシエテ アノニム | 免疫原性組成物 |
| NZ598048A (en) | 2006-01-17 | 2013-05-31 | Synthon Biopharmaceuticals Bv | Compositions and methods for humanization and optimization of n-glycans in plants |
| TW200813091A (en) * | 2006-04-10 | 2008-03-16 | Amgen Fremont Inc | Targeted binding agents directed to uPAR and uses thereof |
| EP2719397A1 (en) * | 2006-06-12 | 2014-04-16 | GlaxoSmithKline Biologicals SA | Use of alpha-toxin for treating and preventing staphylococcus infections |
| EP2164869A2 (en) * | 2007-05-31 | 2010-03-24 | Merck Sharp & Dohme Corp. | Antigen-binding proteins targeting s. aureus orf0657n |
| CN101802008B (zh) * | 2007-08-21 | 2015-04-01 | 安美基公司 | 人类c-fms抗原结合蛋白 |
| KR20100072228A (ko) * | 2007-08-31 | 2010-06-30 | 유니버시티 오브 시카고 | 스타필로코커스 폐 질환 및 상태에 대한 면역화와 관련된 방법 및 조성물 |
| US8172115B1 (en) | 2008-04-10 | 2012-05-08 | Spencer Forrest, Inc. | Hair building solids dispenser for one handed operation |
| KR20110044991A (ko) * | 2008-07-02 | 2011-05-03 | 이머전트 프로덕트 디벨롭먼트 시애틀, 엘엘씨 | TNF-α 길항제 다-표적 결합 단백질 |
| EP2208787A1 (en) * | 2009-01-19 | 2010-07-21 | Université de Liège | A recombinant alpha-hemolysin polypeptide of Staphylococcus aureus, having a deletion in the stem domain and heterologous sequences inserted |
| GB2467939A (en) * | 2009-02-20 | 2010-08-25 | Univ Sheffield | Removal of bacteria from media |
| JP5395264B2 (ja) * | 2009-06-22 | 2014-01-22 | ワイス・エルエルシー | 黄色ブドウ球菌(staphylococcusaureus)抗原の免疫原性組成物 |
| ES2776179T3 (es) | 2012-11-06 | 2020-07-29 | Medimmune Llc | Anticuerpos dirigidos contra determinantes de la superficie de S. aureus |
-
2012
- 2012-02-07 US US13/983,804 patent/US9527905B2/en active Active
- 2012-02-07 SG SG10201600899PA patent/SG10201600899PA/en unknown
- 2012-02-07 CN CN201280007986.4A patent/CN103443285B9/zh active Active
- 2012-02-07 BR BR112013020086-3A patent/BR112013020086B1/pt active IP Right Grant
- 2012-02-07 MX MX2013009127A patent/MX345285B/es active IP Right Grant
- 2012-02-07 AU AU2012214531A patent/AU2012214531C1/en active Active
- 2012-02-07 RS RS20181450A patent/RS58190B2/sr unknown
- 2012-02-07 SG SG10201913690SA patent/SG10201913690SA/en unknown
- 2012-02-07 SM SM20180635T patent/SMT201800635T1/it unknown
- 2012-02-07 KR KR1020137023512A patent/KR101993839B1/ko active Active
- 2012-02-07 LT LTEP12745207.6T patent/LT2673373T/lt unknown
- 2012-02-07 PL PL12745207T patent/PL2673373T6/pl unknown
- 2012-02-07 MX MX2017001101A patent/MX375113B/es unknown
- 2012-02-07 ES ES12745207T patent/ES2709065T7/es active Active
- 2012-02-07 CN CN201710480750.7A patent/CN107474133B/zh active Active
- 2012-02-07 EP EP12745207.6A patent/EP2673373B3/en active Active
- 2012-02-07 TR TR2018/17932T patent/TR201817932T4/en unknown
- 2012-02-07 JP JP2013553501A patent/JP2014508748A/ja active Pending
- 2012-02-07 SG SG2013057831A patent/SG192212A1/en unknown
- 2012-02-07 DK DK12745207.6T patent/DK2673373T6/da active
- 2012-02-07 WO PCT/US2012/024201 patent/WO2012109285A2/en not_active Ceased
- 2012-02-07 RU RU2013141134A patent/RU2620065C2/ru active
- 2012-02-07 EP EP18190790.8A patent/EP3470526A3/en active Pending
- 2012-02-07 CA CA2826566A patent/CA2826566C/en active Active
- 2012-02-07 SI SI201231470T patent/SI2673373T1/sl unknown
- 2012-02-07 PT PT12745207T patent/PT2673373T/pt unknown
- 2012-02-07 HU HUE12745207A patent/HUE040734T2/hu unknown
- 2012-02-07 HR HRP20182017TT patent/HRP20182017T4/hr unknown
-
2013
- 2013-08-07 MX MX2020009700A patent/MX2020009700A/es unknown
-
2017
- 2017-02-13 JP JP2017023905A patent/JP6367393B2/ja active Active
-
2018
- 2018-07-04 JP JP2018127340A patent/JP6723293B2/ja active Active
- 2018-11-28 CY CY20181101266T patent/CY1121389T1/el unknown
-
2020
- 2020-06-23 JP JP2020108083A patent/JP7047017B2/ja active Active
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7047017B2 (ja) | 黄色ブドウ球菌(Staphylococcus aureus)α毒素に特異的に結合する抗体及び使用方法 | |
| AU2012214531A1 (en) | Antibodies that specifically bind Staphylococcus aureus alpha toxin and methods of use | |
| AU2020233635B2 (en) | Neutralizing anti-influenza b antibodies and uses thereof | |
| CN113667013A (zh) | 中和抗甲型流感抗体及其用途 | |
| KR20160138220A (ko) | 당뇨병성 황반 부종의 치료를 위한 조성물 및 방법 | |
| KR20180101436A (ko) | 인플루엔자 a의 치료 방법 | |
| HK40007158A (en) | Antibodies that specifically bind staphylococcus aureus alpha toxin and methods of use | |
| HK1191978A (en) | Antibodies that specifically bind staphylococcus aureus alpha toxin and methods of use | |
| HK1191978B (en) | Antibodies that specifically bind staphylococcus aureus alpha toxin and methods of use | |
| HK1248257B (zh) | 特异性结合金黄色葡萄球菌α毒素的抗体以及使用方法 | |
| HK1191977B (en) | Antibodies that specifically bind staphylococcus aureus alpha toxin and methods of use | |
| HK1191977A (en) | Antibodies that specifically bind staphylococcus aureus alpha toxin and methods of use | |
| HK40064533A (en) | Neutralizing anti-influenza a antibodies and uses thereof | |
| HK40064536B (zh) | 中和抗甲型流感抗体及其用途 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| B07D | Technical examination (opinion) related to article 229 of industrial property law [chapter 7.4 patent gazette] | ||
| B06F | Objections, documents and/or translations needed after an examination request according [chapter 6.6 patent gazette] | ||
| B07E | Notification of approval relating to section 229 industrial property law [chapter 7.5 patent gazette] |
Free format text: NOTIFICACAO DE ANUENCIA RELACIONADA COM O ART 229 DA LPI |
|
| B06U | Preliminary requirement: requests with searches performed by other patent offices: procedure suspended [chapter 6.21 patent gazette] | ||
| B06A | Patent application procedure suspended [chapter 6.1 patent gazette] | ||
| B09A | Decision: intention to grant [chapter 9.1 patent gazette] | ||
| B16A | Patent or certificate of addition of invention granted [chapter 16.1 patent gazette] |
Free format text: PRAZO DE VALIDADE: 20 (VINTE) ANOS CONTADOS A PARTIR DE 07/02/2012, OBSERVADAS AS CONDICOES LEGAIS. |