WO2015166755A1 - Dispersion de nanoparticules de cuivre et procédé de fabrication de substrat électroconducteur - Google Patents
Dispersion de nanoparticules de cuivre et procédé de fabrication de substrat électroconducteur Download PDFInfo
- Publication number
- WO2015166755A1 WO2015166755A1 PCT/JP2015/060007 JP2015060007W WO2015166755A1 WO 2015166755 A1 WO2015166755 A1 WO 2015166755A1 JP 2015060007 W JP2015060007 W JP 2015060007W WO 2015166755 A1 WO2015166755 A1 WO 2015166755A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- copper
- nanoparticle dispersion
- acid
- conductive substrate
- copper nanoparticle
- Prior art date
Links
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F1/00—Metallic powder; Treatment of metallic powder, e.g. to facilitate working or to improve properties
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F9/00—Making metallic powder or suspensions thereof
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01B—CABLES; CONDUCTORS; INSULATORS; SELECTION OF MATERIALS FOR THEIR CONDUCTIVE, INSULATING OR DIELECTRIC PROPERTIES
- H01B1/00—Conductors or conductive bodies characterised by the conductive materials; Selection of materials as conductors
- H01B1/02—Conductors or conductive bodies characterised by the conductive materials; Selection of materials as conductors mainly consisting of metals or alloys
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01B—CABLES; CONDUCTORS; INSULATORS; SELECTION OF MATERIALS FOR THEIR CONDUCTIVE, INSULATING OR DIELECTRIC PROPERTIES
- H01B1/00—Conductors or conductive bodies characterised by the conductive materials; Selection of materials as conductors
- H01B1/20—Conductive material dispersed in non-conductive organic material
- H01B1/22—Conductive material dispersed in non-conductive organic material the conductive material comprising metals or alloys
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01B—CABLES; CONDUCTORS; INSULATORS; SELECTION OF MATERIALS FOR THEIR CONDUCTIVE, INSULATING OR DIELECTRIC PROPERTIES
- H01B13/00—Apparatus or processes specially adapted for manufacturing conductors or cables
-
- H—ELECTRICITY
- H05—ELECTRIC TECHNIQUES NOT OTHERWISE PROVIDED FOR
- H05K—PRINTED CIRCUITS; CASINGS OR CONSTRUCTIONAL DETAILS OF ELECTRIC APPARATUS; MANUFACTURE OF ASSEMBLAGES OF ELECTRICAL COMPONENTS
- H05K3/00—Apparatus or processes for manufacturing printed circuits
Definitions
- the present invention relates to a copper nanoparticle dispersion and a method for producing a conductive substrate using the dispersion.
- metal particle dispersions using silver particles are difficult to oxidize and are excellent in conductivity, but silver itself is expensive or has problems of ion migration. Therefore, development of a metal particle dispersion using copper as an inexpensive metal with excellent migration resistance is required. However, since copper particles are generally more easily oxidized than silver particles, there is a problem that conductivity is difficult to be expressed.
- Patent Document 1 a compound containing copper and a reducing compound are mixed, and a step of producing a composite compound that can be thermally decomposed in an alkylamine to form copper, and the composite compound is heated in an alkylamine. And a process for producing copper fine particles coated with an alkylamine.
- the coated copper fine particles have a narrow and fine particle size distribution, excellent storage stability and can be sintered at a low temperature.
- the dispersibility of copper fine particles is poor, the coating suitability is poor, and unevenness occurs when coating on a resin substrate such as a PET film, as in the comparative example described later. was there.
- Patent Document 2 describes a specific metal fine particle, a polymer dispersant having a specific polyester skeleton, a metal fine particle dispersion containing a dispersion medium, and a method for producing a conductive substrate using the dispersion.
- Patent Document 2 it is described that the specific polymer dispersant exhibits a high effect on the dispersibility of the metal fine particles and is easily volatilized in a subsequent sintering step.
- the technique of Patent Document 2 retains dispersibility only with a dispersant, organic substances are likely to remain during sintering, and firing of a metal fine particle coating film requires long firing with high microwave output. It has become.
- Patent Document 3 describes a method for producing a conductive metal thin film using metal nanoparticles synthesized from a solution containing a metal precursor, an acid, an amine, and a reducing agent. It is also stated that it is good.
- Patent Document 3 is a technique that focuses on improving the conductivity by firing at a high temperature of 200 ° C. or higher using a reducing atmosphere.
- the technique specifically disclosed in Patent Document 3 has poor dispersibility of metal nanoparticles as in the comparative example described later, a certain degree of conductivity can be obtained by high-temperature firing. . Therefore, in order to coat and sinter on a PET film or the like, it is necessary to study further lowering the sintering temperature.
- JP 2012-72418 A International Publication No. 2011/040189 Pamphlet International Publication No. 2013/147535 Pamphlet
- the dispersant having a low molecular weight has insufficient dispersibility and applicability, and attempts to improve dispersibility and applicability have resulted in insufficient sinterability at low temperatures or in a short time.
- the substrate that can be used is limited because high temperature firing is required, In the firing, the volume resistivity of the substrate obtained by leaving the polymer dispersant in the metal film is increased, and there are cases where sufficient performance as a conductive substrate cannot be obtained.
- the present invention has been made under such circumstances, and a copper nanoparticle dispersion excellent in oxidation resistance, dispersibility, applicability, and sinterability at low temperature or in a short time, and low temperature or short It aims at providing the manufacturing method of an electroconductive board
- the present inventors have combined copper nanoparticles with a carboxylic acid, an alkylamine, and a polymer dispersant having a specific amine value or acid value.
- a copper nanoparticle dispersion having a volume average particle size of 500 nm or less, it is excellent in oxidation resistance, dispersibility, and coating suitability, and is organic from the film even when baked at a low temperature or in a short time. Since the components are easily decomposed or removed, and the nanoparticles are uniformly and densely arranged in the coating film, it has been found that a film having high conductivity can be formed.
- the present invention has been completed based on such knowledge.
- the method for producing a conductive substrate according to the first aspect of the present invention includes copper nanoparticles, a carboxylic acid, an alkylamine, a polymer dispersant, and a solvent, and the polymer dispersant is an amine.
- substrate of the 2nd aspect which concerns on this invention is a compound containing copper, a reducing compound, carboxylic acid, and the mixture containing an alkylamine, or copper carboxylate, a reducing compound, and alkyl.
- Preparing copper nanoparticles by heating any of the amine-containing mixtures; By dispersing the copper nanoparticles in a solvent with a polymer dispersant having one of an amine value and an acid value of 30 to 160 mgKOH / g and the other of an amine value and an acid value of 0 to 160 mgKOH / g.
- a step of preparing a copper nanoparticle dispersion having a volume average particle size of 500 nm or less by a dynamic light scattering method Applying the copper nanoparticle dispersion on a substrate to form a coating film; And a step of firing the coating film.
- the copper nanoparticle dispersion according to the present invention contains copper nanoparticles, a carboxylic acid, an alkylamine, a polymer dispersant, and a solvent.
- the polymer dispersant has an amine value and an acid value. One of these is 30 to 160 mgKOH / g, the other one of the amine value and the acid value is 0 to 160 mgKOH / g, and the volume average particle diameter by dynamic light scattering method is 500 nm or less.
- the polymer dispersant has a low 90% thermal weight loss temperature of 420 ° C. or less, low temperature sinterability and after sintering. It is preferable from the point which the electroconductivity of a coating film is excellent.
- the carboxylic acid has 10 or less carbon atoms, and the low-temperature firing property and the conductivity of the coated film after sintering are excellent. It is preferable from the point.
- the baking step is a step of baking by plasma or a step of baking by irradiation with flash light, which can be performed at a low temperature or for a short time. It is preferable because a conductive substrate having excellent conductivity can be obtained even on a low heat-resistant substrate.
- a method having a step of chemically etching the obtained sintered film after the firing step is also preferably used. Since the sintered film formed in the present invention is imparted with smoothness and adhesion, a chemical etching method can be used to form a fine conductive pattern.
- the copper nanoparticle dispersion excellent in oxidation resistance, dispersibility, applicability, and sinterability at a low temperature or in a short time, and excellent conductivity by firing at a low temperature or in a short time. It is possible to provide a method for manufacturing a conductive substrate capable of obtaining a conductive substrate having a property.
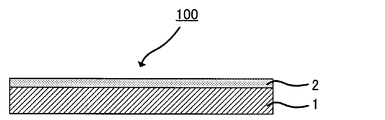
- FIG. 1 is a schematic view showing an example of a conductive substrate obtained by the production method of the present invention.
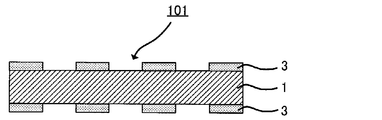
- FIG. 2 is a schematic view showing another example of a conductive substrate obtained by the production method of the present invention.
- (meth) acryl represents each of acryl and methacryl
- (meth) acrylate represents each of acrylate and methacrylate.
- the copper nanoparticle dispersion according to the present invention contains copper nanoparticles, a carboxylic acid, an alkylamine, a polymer dispersant, and a solvent, and the polymer dispersant has one of an amine value and an acid value. 30 to 160 mgKOH / g, the other of the amine value and the acid value is 0 to 160 mgKOH / g, and the volume average particle size by dynamic light scattering is 500 nm or less.
- the copper nanoparticles have a relatively low molecular weight carboxylic acid and an alkylamine, one of an amine value and an acid value of 30 to 160 mgKOH / g, and the other of the amine value and the acid value of 0 to 160 mgKOH / g.
- the volume average particle size is dispersed so as to be 500 nm or less. Therefore, it is estimated that the following specific effects are exhibited by their synergistic action.
- the polymer dispersant having a specific amine value and acid value has at least one kind of basic and acidic functional groups, and acid-base interaction with carboxylic acid or alkylamine on the surface of the copper nanoparticles. It can be considered that there is something attached due to the above.
- the carboxylic acid that can be strongly adsorbed on the surface of the copper nanoparticles is present stably surrounding the copper nanoparticles in the solvent due to the strong adsorption.
- the alkylamine has a charge different from that of the carboxylic acid at the adsorption site and further adheres to the copper nanoparticles
- the low molecular weight dispersants of carboxylic acid and alkylamine can adhere more closely to the copper nanoparticles. It is estimated to be.
- the polymer dispersant is selected so as to have a specific amine value and acid value, the polymer dispersant can be stably attached to the copper nanoparticles to which both the carboxylic acid and the alkylamine are attached. It is presumed that due to the steric hindrance of the polymer chain, aggregation of copper nanoparticles is less likely to occur, and excellent dispersibility of the copper nanoparticles can be achieved.
- the amine value and acid value of the polymer dispersant are too low or too high. In either case, the polymer dispersant cannot be stably attached, but rather the dispersibility deteriorates.
- the copper nanoparticles are surrounded by a polymer dispersant having a specific amine value and acid value and are stably dispersed uniformly in a fine particle size, film formation of a polymer chain Depending on the properties, it is estimated that the coating suitability of the copper nanoparticle dispersion is excellent and the smoothness of the coating film is increased.
- the copper nanoparticles are uniformly arranged in the coating film, and a high-density film can be formed. Since the relatively low molecular weight alkylamine is weakly adsorbed on the surface of the copper nanoparticles, it is easily detached during drying when forming a coating film. On the other hand, it is considered that the polymer dispersing agent remains to prevent non-uniform aggregation of the copper nanoparticles, and the copper nanoparticles are arranged at a higher density during drying. It is presumed that carboxylic acid is easily desorbed even during drying, low temperature or short time baking because it is mixed with easily desorbed alkylamine.
- the polymer dispersant since the polymer dispersant has the specific amine value and acid value, the adsorption is not too strong, and the polymer dispersant is uniformly disposed around the copper nanoparticles, so that the low temperature is low in the subsequent firing step. It is presumed that a metal thin film having excellent electrical conductivity can be obtained by fusion of copper nanoparticles arranged at high density, which is easily desorbed or decomposed even after baking for a short time. When the volume average particle size of the dispersion exceeds 500 nm in the system as in the present application, the copper nanoparticles cannot be arranged at high density, so that they are non-uniformly baked and can adhere stably to the copper nanoparticles.
- the alkylamine has a function of scavenging protons in the amino group, and therefore adheres to the surface of the copper nanoparticles during production, thereby suppressing oxidation of copper atoms during production and in the dispersion.
- the present invention has an effect of suppressing the oxidation of copper nanoparticles in the present invention because the copper nanoparticles are closely surrounded with a carboxylic acid that can be strongly adsorbed on the surface of the copper nanoparticles, and further a polymer dispersant is attached. Is estimated to be high. From these, it is presumed that sintering inhibition due to oxidation during firing hardly occurs, and a film having high conductivity can be formed after firing.
- the copper nanoparticle dispersion of the present invention may contain other components in addition to the above essential components as long as the effects of the present invention are not impaired.
- each configuration of the copper nanoparticle dispersion will be described in detail in order.
- the copper nanoparticles are typically metallic copper particles.
- copper is a metal that is very easily oxidized
- the surface of the metallic copper nanoparticles is partially oxidized into an oxide. It may be included.
- the copper nanoparticles mean particles having a diameter of the order of nm (nanometer), that is, less than 1 ⁇ m. In the present invention, by using such copper nanoparticles, sintering at a low temperature is easy to proceed, and the printability of fine wiring is improved.
- the copper nanoparticles used in the present invention are preferably particles having an average primary particle size of 1 nm to 100 nm, and more preferably 10 nm to 100 nm, from the viewpoint of achieving both dispersibility, applicability, low temperature firing, and conductivity. Of these particles, it is preferable.
- the average primary particle diameter of the said copper nanoparticle can be calculated
- TEM transmission electron micrograph
- the method for preparing the copper nanoparticles may be appropriately selected from conventionally known methods. For example, a physical method of pulverizing metal powder by mechanochemical method, etc .; chemical dry method such as chemical vapor deposition method (CVD method), vapor deposition method, sputtering method, thermal plasma method, laser method; thermal decomposition method
- CVD method chemical vapor deposition method
- vapor deposition method vapor deposition method
- sputtering method thermal plasma method
- laser method thermal decomposition method
- the copper nanoparticles can be obtained using a chemical wet method such as a chemical reduction method, an electrolysis method, an ultrasonic method, a laser ablation method, a supercritical fluid method, or a microwave synthesis method.
- fine particles are produced by bringing a vapor of a metal heated and brought into contact with a low vapor pressure liquid containing a dispersant under a high vacuum.
- a chemical reduction method there is a method in which a copper-containing compound and a reducing agent are mixed in a solvent in the presence of a complexing agent and an organic protective agent.
- commercially available copper nanoparticles can be used as appropriate.
- copper nanoparticles are coated with carboxylic acid and alkylamine, among them, as will be described in detail later, using carboxylic acid and alkylamine as an organic protective agent, a copper-containing compound and a reducing agent, A method of mixing copper in a solvent to produce copper nanoparticles is preferably used.
- the content of the copper nanoparticles may be appropriately selected according to the use, but from the viewpoint of dispersibility, 0.01% with respect to the total amount of the copper nanoparticle dispersion.
- the content is preferably from 90 to 90% by mass, and more preferably from 0.1 to 85% by mass.
- the carboxylic acid used in the present invention is a compound that can be bonded to copper by an oxygen atom as a ligand. Therefore, in the copper nanoparticle dispersion, the carboxylic acid contributing to the dispersion usually exists in a state of being bonded to copper by at least one oxygen atom.
- carboxylic acid examples include saturated aliphatic monocarboxylic acids such as acetic acid, propanoic acid, butanoic acid, pentanoic acid, hexanoic acid, heptanoic acid, octanoic acid, nonanoic acid, decanoic acid, dodecanoic acid, and hexadecanoic acid; oleic acid, Unsaturated aliphatic monocarboxylic acids such as linoleic acid; Aliphatic polycarboxylic acids such as oxalic acid, malonic acid, and succinic acid; Aliphatic unsaturated polycarboxylic acids such as maleic acid; Aromatic monocarboxylic acids such as benzoic acid Examples thereof include, but are not limited to, acids; aromatic polycarboxylic acids such as phthalic acid; and hydroxycarboxylic acids such as citric acid.
- saturated aliphatic monocarboxylic acids such as acetic acid, propanoic acid,
- a carboxylic acid having 10 or less carbon atoms is preferable from the viewpoint that the low-temperature baking property is improved and the conductivity is improved.
- carboxylic acids having 2 or more carbon atoms are used from the viewpoint of dispersibility.
- an aliphatic carboxylic acid having 10 or less carbon atoms is preferable from the viewpoint of improving dispersibility and low-temperature calcination properties and improving conductivity.
- the aliphatic carboxylic acid may be either saturated or unsaturated.
- carboxylic acid used in the present invention one carboxylic acid may be used, or two or more carboxylic acids may be mixed and used.
- the carboxylic acid used in the present invention is preferably a carboxylic acid having one or two carboxyl groups in the molecule, since the polarity is relatively weak and easily desorbed at the time of firing. It is preferable to use a carboxylic acid having a group.
- the carboxylic acid used in the present invention is preferably not too high in molecular weight, and preferably has a molecular weight of 300 or less, more preferably 200 or less, from the viewpoint that it easily desorbs during firing. Moreover, it is preferable that a boiling point is 400 degrees C or less, Furthermore, it is preferable that it is 300 degrees C or less.
- the molecular weight of the carboxylic acid is preferably 50 or more from the viewpoint of desorption during storage of nanoparticles and storage and prevention of volatilization. Moreover, it is preferable that a boiling point is 50 degreeC or more.
- the content of the carboxylic acid may be appropriately selected according to the use, but from the viewpoint of low-temperature calcinability, 0.1 to 30 masses relative to 100 mass parts of copper. Part, more preferably in the range of 0.1 to 20 parts by weight.
- the content of the carboxylic acid may be appropriately selected according to the use, but from the viewpoint of low-temperature calcinability, 0.05% with respect to the total amount of the copper nanoparticle dispersion. It is preferably ⁇ 15% by mass, and more preferably in the range of 0.05 to 10% by mass.
- the alkylamine used in the present invention can be appropriately selected from known alkylamines according to properties expected for the produced copper nanoparticle dispersion. It is presumed that the alkylamine has a function of capturing protons, thereby preventing the copper atom from being oxidized.
- Alkylamine has a structure in which an amino group is bonded to a part of an alkyl group.
- RNH 2 R is a hydrocarbon chain
- R 1 is a secondary amino group
- R 2 NH R 1 and R 2 may be the same or different in the hydrocarbon chain
- the alkyl chain may be linear, branched or cyclic.
- the hydrocarbon chain may contain atoms other than carbon such as oxygen, silicon, nitrogen, sulfur, and phosphorus.
- it may be an alkylamine having a substituent such as an alkoxy group, an alkoxysilyl group, or an alkylthio group, or may have two amino groups in the molecule.
- alkylamine (monoamine) having one amino group in the molecule examples include 2-ethoxyethylamine, dipropylamine, dibutylamine, hexylamine, cyclohexylamine, heptylamine, 3-methoxypropylamine, and 3-ethoxypropyl.
- Alkylamines such as amine, 3-butoxypropylamine, octylamine, nonylamine, decylamine, 3-aminopropyltriethoxysilane, dodecylamine, hexadecylamine, octadecylamine and oleylamine are industrially produced and easily available. It is practical.
- examples of the alkyldiamine having two amino groups in the molecule include ethylenediamine, N, N-dimethylethylenediamine, N, N′-dimethylethylenediamine, N, N-diethylethylenediamine, N, N′-diethylethylenediamine, 1 , 3-propanediamine, 2,2-dimethyl-1,3-propanediamine, N, N-dimethyl-1,3-diaminopropane, N, N′-dimethyl-1,3-diaminopropane, N, N— Diethyl-1,3-diaminopropane, 1,4-diaminobutane, 1,5-diamino-2-methylpentane, 1,6-diaminohexane, N, N′-dimethyl-1,6-diaminohexane, 1, 7-diaminoheptane, 1,8-diaminooctane,
- alkylamine used in the present invention one type of alkylamine may be used, or two or more types of alkylamine may be mixed and used.
- the alkylamine used in the present invention is preferably an alkylamine having one or two amino groups in the molecule from the viewpoint that the polarity is relatively weak and that it is easily eliminated during firing.
- the alkylamine used in the present invention is preferably not too high in molecular weight, and preferably has a molecular weight of 300 or less, more preferably 200 or less, from the viewpoint of easy desorption during firing.
- the alkylamine used in the present invention preferably has 8 or less carbon atoms, and more preferably 6 or less, from the viewpoint that it is easily detached during firing.
- the alkylamine used in the present invention preferably has a boiling point of 300 ° C. or lower, more preferably 200 ° C. or lower, from the viewpoint of easy desorption during firing.
- the molecular weight of the alkylamine is preferably 50 or more from the viewpoints of desorption during storage and storage and prevention of volatilization.
- the boiling point is preferably 23 ° C. or higher, and more preferably 50 ° C. or higher.
- the content of the alkylamine may be appropriately selected depending on the use, but from the viewpoint of low-temperature calcinability, 0.1 to 30 masses with respect to 100 mass parts of copper. Part, more preferably in the range of 0.1 to 20 parts by weight.
- the content of the alkylamine may be appropriately selected according to the use, but from the viewpoint of oxidation resistance and low-temperature sinterability, it is based on the total amount of the copper nanoparticle dispersion. 0.05 to 15% by mass, more preferably 0.05 to 10% by mass.
- the polymer dispersant used in the present invention is a polymer dispersant in which one of the amine value and the acid value is 30 to 160 mgKOH / g, and the other one of the amine value and the acid value is 0 to 160 mgKOH / g, Having at least one of a functional functional group and an acidic functional group.
- a functional functional group and an acidic functional group include primary, secondary, or tertiary amino groups, nitrogen-containing heterocycles such as pyridine, pyrimidine, and pyrazine.
- acidic functional groups include carboxylic acid groups, phosphoric acid groups, and sulfonic acid groups.
- the amine value indicates the total amount of free base and base, and is expressed in mg of potassium hydroxide equivalent to the hydrochloric acid required to neutralize 1 g of the sample.
- the acid value represents the total amount of free acid and acid, and is expressed in mg of potassium hydroxide required to neutralize 1 g of the sample.
- the amine value can be measured by a method according to JIS-K7237, and the acid value can be measured by a method according to JIS-K0070.
- the polymer dispersant used in the present invention has the above-mentioned specific amine value and acid value with respect to copper nanoparticles to which both carboxylic acid and alkylamine are attached, and an appropriate amount of basic functional group or acidity. Since it has a functional group, it is presumed that it adsorbs stably to the copper nanoparticles, while causing a steric hindrance by the polymer chain portion and stably preventing aggregation of the copper nanoparticles. As shown in the examples and comparative examples described later, for the copper nanoparticles to which both carboxylic acid and alkylamine are attached, the amine value and acid value of the polymer dispersant are too low or too high.
- the polymer dispersant cannot be stably attached, but rather the dispersibility deteriorates.
- the polymer dispersant when combined with a carboxylic acid having a low dispersibility but excellent low-temperature calcinability and having a small number of carbon atoms, it is clear that the polymer dispersant cannot be dispersed if the amine value and acid value are too low or too high. It was made.
- the polymer dispersant having the specific amine value and acid value is stably adsorbed to the copper nanoparticles, whereby dispersibility and dispersion stability are improved.
- the diameter can be reduced. Therefore, when the above polymer dispersant is used, the smoothness and uniformity of the coating film of the copper nanoparticle dispersion are excellent, and the copper nanoparticles in the coating film are arranged at high density. Therefore, sintering is easy to proceed uniformly, and the copper nanoparticles are easily fused.
- the polymer dispersant is easily decomposed or volatilized by baking due to a synergistic effect with the alkylamine, and the resulting conductive substrate suppresses the remaining organic components. As a result, the obtained metal film is presumed to be excellent in conductivity.
- the polymer dispersant used in the present invention has an amine value and an acid value of 30 to 160 mgKOH / g and the other one of the amine value and the acid value is 0 to 160 mgKOH / g for the reasons described above.
- One of the amine value and the acid value is preferably 40 to 140 mgKOH / g, and the other one of the amine value and the acid value is preferably 0 to 140 mgKOH / g.
- the polymer dispersant used in the present invention preferably has a weight average molecular weight of 800 or more, more preferably 900 or more, particularly 1000 or more, from the viewpoint of excellent dispersibility and coating suitability. Is preferred. On the other hand, from the viewpoint of excellent low-temperature calcinability, it is preferably 30000 or less, more preferably 20000 or less, and particularly preferably 10,000 or less.
- the weight average molecular weight in this invention can be measured by the gel permeation chromatography (GPC) method (polystyrene conversion).
- the polymer dispersant used in the present invention has a 90% thermogravimetric decrease temperature of 450 ° C. or lower, and further 420 ° C. or lower, so that the low-temperature firing property and the conductivity of the coated film after sintering are reduced. It is preferable from an excellent point.
- the 90% thermogravimetric decrease temperature is a value measured by thermogravimetry (TG) as follows. Using a thermogravimetry apparatus (for example, DTG-60A manufactured by Shimadzu Corporation), about 5 mg of a sample is measured in a nitrogen atmosphere. The temperature rising rate is 10 ° C./min, and the temperature is measured from room temperature (23 ° C.) to 600 ° C. In the present invention, the temperature at which 90% is reduced based on the sample weight at room temperature is defined as the 90% thermogravimetric decrease temperature.
- the polymer dispersant having one of an amine value and an acid value of 30 to 160 mg KOH / g and the other one of an amine value and an acid value used in the present invention is usually from 0 to 160 mg KOH / g. It can be appropriately selected from polymer dispersants used for dispersing colorants.
- polymer dispersant examples include (co) polymers of unsaturated carboxylic acid esters such as polyacrylic acid esters; (partial) amine salts of (co) polymers of unsaturated carboxylic acid such as polyacrylic acid , (Partial) ammonium salts and (partial) alkylamine salts; hydroxyl group-containing unsaturated carboxylic acid ester (co) polymers such as hydroxyl group-containing polyacrylates and their modified products; polyurethanes; unsaturated polyamides; Long chain polyaminoamide phosphates; Polyethyleneimine derivatives (amides and their bases obtained by reaction of poly (lower alkyleneimines) with free carboxyl group-containing polyesters); Polyallylamine derivatives (polyallylamine and free radicals) Polyester, polyamide or ester and amide having carboxyl group Compound reaction product obtained by the reaction of one or more compounds selected from the three compounds of (polyester amide)), and the like.
- the polymer dispersant used in the present invention preferably has a polyester skeleton or a polyether skeleton in at least one of the main chain and the side chain.
- a polymer dispersant is excellent in conductivity of the fired film because it is easily decomposed by baking at a low temperature due to its skeletal structure, and organic matter hardly remains.
- one type of polymer dispersant may be used, or two or more types may be used in combination, and the content depends on the type of copper nanoparticles used.
- the copper nanoparticles 100 parts by weight it is usually in the range of 0.1 to 100 parts by weight, preferably 1 to 50 parts by weight, and 2 to 30 parts by weight. It is more preferable.
- the content of the polymer dispersant may be appropriately selected according to the use, but from the viewpoint of dispersibility, coating suitability, and low-temperature firing properties, the copper nanoparticle dispersion
- the amount is preferably from 0.05 to 25% by mass, more preferably from 0.5 to 15% by mass, based on the total amount. If content of the said polymer dispersing agent is more than the said lower limit, the dispersibility and dispersion stability of a copper nanoparticle dispersion can be made excellent. Moreover, if it is below the said upper limit, it is excellent in the electroconductivity of the film
- the solvent is not particularly limited as long as it is an organic solvent that does not react with each component in the copper nanoparticle dispersion and can dissolve or disperse them.
- An organic solvent conventionally used for the copper nanoparticle dispersion may be appropriately selected and used.
- the solvent used in the present invention MBA (3-methoxybutyl acetate), PGMEA (propylene glycol monomethyl ether acetate), DMDG (diethylene glycol dimethyl ether), diethylene glycol methyl ethyl ether, PGME (propylene glycol monomethyl ether) or these are used. What mixed is preferable from the point of the solubility of the said polymer dispersing agent, and the applicability
- the content of the solvent in the copper nanoparticle dispersion of the present invention is not particularly limited as long as it can uniformly dissolve or disperse each component of the copper nanoparticle dispersion.
- the solid content in the copper nanoparticle dispersion is preferably in the range of 5 to 95% by mass, more preferably in the range of 10 to 90% by mass. By being the said range, it can be set as the viscosity suitable for application
- the copper nanoparticle dispersion of the present invention may appropriately contain other known components conventionally used in copper nanoparticle dispersions as needed, as long as the effects of the present invention are not impaired.
- Other components include, for example, complexing agents, organic protective agents, reducing agents, surfactants for improving wettability, silane coupling agents for improving adhesion, antifoaming agents, repellency inhibitors, and antioxidants. Agents, anti-aggregation agents, viscosity modifiers, and the like.
- the other dispersing agent may be contained.
- a resin binder such as an acrylic resin, a polyester resin, a cellulose resin, and an olefin resin may be added from the viewpoints of film forming property, printability, and dispersibility within a range that does not impair the effects of the present invention.
- the copper nanoparticle dispersion of the present invention has a volume average particle size of 500 nm or less by dynamic light scattering method, and preferably 450 nm or less. Since the copper nanoparticle dispersion of the present invention has such a small dispersed particle size, excellent smoothness and uniformity of the coating film and high density arrangement of the copper nanoparticles in the coating film are realized. To do.
- the volume average particle diameter by the dynamic light scattering method in the copper nanoparticle dispersion is a dispersion average particle diameter of copper nanoparticles dispersed in a dispersion medium containing at least a solvent, and is a laser light scattering particle size distribution analyzer. It is measured by.
- the copper nano particle dispersion is appropriately diluted to a concentration that can be measured with a laser light scattering particle size distribution meter with a solvent used in the copper nano particle dispersion (for example, And can be measured at 23 ° C. by a dynamic light scattering method using a laser light scattering particle size distribution analyzer (for example, Nikkiso Nanotrac particle size distribution analyzer UPA-EX150).
- a laser light scattering particle size distribution analyzer for example, Nikkiso Nanotrac particle size distribution analyzer UPA-EX150.
- the manufacturing method of a copper nanoparticle dispersion should just be a method in which a copper nanoparticle can disperse
- a copper nanoparticle can disperse distribute favorably
- the copper nanoparticles are dispersed in the solvent with the above-described polymer dispersant by a conventionally known method.
- copper nanoparticles produced using carboxylic acid and alkylamine as a protective agent during production may be used, or other protective agents may be used.
- the copper nanoparticle protective agent produced in this manner may be substituted with a carboxylic acid or an alkylamine by a known method.
- the method for producing a copper nanoparticle dispersion includes a compound containing copper, a reducing compound, a mixture containing a carboxylic acid and an alkylamine, or a copper carboxylate, a reducing compound and an alkylamine.
- the compound containing copper, a reducing compound, a carboxylic acid, and a mixture containing an alkylamine or a mixture containing copper carboxylate, a reducing compound, and an alkylamine is the mixture at the time of heating. good.
- a compound containing copper (hereinafter sometimes referred to as a copper-containing compound) is a copper-containing compound capable of forming a complex compound such as a complex with a reducing compound. Used as a metal source for particles.
- the copper-containing compound include copper hydroxide, copper oxalate, copper acetate, copper propionate, copper butyrate, copper isobutyrate, copper valerate, copper isovalerate, copper caproate, copper enanthate, and caprylic acid.
- Examples include organic acid salts and inorganic acid salts of copper such as copper phosphate, and complex compounds represented by acetylacetonato copper coordinated with acetylacetone.
- the copper-containing compound other than copper carboxylate and carboxylic acid be used as a carboxylate copper such as fatty acid copper. This is because copper carboxylate serves as a carboxylic acid source to be coated on the copper nanoparticles and easily forms a complex compound such as a complex with the reducing compound.
- a reducing compound having a reducing action is mixed with the copper carboxylate to produce a composite compound such as a complex of copper and the reducing compound.
- the composite compound may be generated using a mixture of the copper-containing compound other than copper carboxylate, the reducing compound, and the carboxylic acid.
- the reducing compound used in this case for example, the reducing compound described in JP2012-72418A can be appropriately selected and used.
- reducing compounds having an amino group such as hydrazine, hydrazine hydrate, hydroxylamine, and derivatives thereof are preferably used.
- a reduction reaction occurs directly when copper carboxylate and a reducing compound are mixed, it is desirable to suppress the reduction reaction by mixing in a cooled environment.
- a compound containing copper such as fatty acid copper and a reducing compound are preferably mixed by cooling to 30 ° C. or lower, more preferably 25 ° C. or lower, and most preferably 20 ° C. or lower.
- a reducing compound having a reducing action with copper carboxylate to form a complex compound such as a complex of copper and a reducing compound.
- the mixture of the copper carboxylate and the reducing compound produced above is mixed with a sufficient amount of alkylamine and heated to spontaneously decompose the copper carboxylate. It is preferable to obtain copper nanoparticles by forming and aggregating copper atoms. At this time, since the surface of the copper nanoparticles is coated with a carboxylic acid and an alkylamine, stable coated copper nanoparticles that are hardly oxidized by oxidation in the air can be obtained. By adjusting the molecular weight of carboxylic acid or alkylamine, it is possible to adjust the primary particle size of the produced copper nanoparticles to a desired size.
- the mixing ratio of the alkylamine to the mixture of the copper carboxylate and the reducing compound may be appropriately selected according to the use, but from the viewpoint of oxidation resistance and low-temperature calcination, it is based on 1 mol of the copper carboxylate.
- the amount is preferably 1 to 10 moles, more preferably 2 to 6 moles.
- the second step of heating a complex compound such as alkylamine in the presence of an alkylamine to produce copper nanoparticles can be performed simultaneously or sequentially in one container.
- copper nanoparticles can be produced by heating.
- the heating temperature in the presence of alkylamine is preferably 60 ° C to 150 ° C.
- the first step is performed by cooling to about 30 ° C. or less, and the second step It is preferable that the process is performed by heating to 60 ° C. to 150 ° C.
- a copper nanoparticle dispersion having a volume average particle size of 500 nm or less by dynamic light scattering method is prepared by dispersing the copper nanoparticles obtained in the preparation step with the specific polymer dispersant in a solvent. To do. For example, after the specific polymer dispersant is mixed and stirred in the solvent to prepare a polymer dispersant solution, the polymer dispersant solution is mixed with the copper nanoparticles obtained in the preparation step, as necessary. Accordingly, a copper nanoparticle dispersion can be prepared by mixing other components and dispersing them using a known stirrer or disperser.
- the copper nanoparticle dispersion obtained in the present invention is preferably used for a conductive substrate described later, and particularly preferably for conductive pattern printing. Since the copper nanoparticle dispersion of the present invention can be fired at a low temperature and in a short time, it is suitably used for low-temperature or short-time firing applications such as plasma firing and flash light firing described later.
- the copper nanoparticle dispersion obtained in the present invention can be further applied to a seed layer for plating and various metal films, and can be used, for example, for mirror surfaces for optical devices and various decoration applications.
- the method for producing a conductive substrate according to the first aspect of the present invention comprises a copper nanoparticle, a carboxylic acid, an alkylamine, and one of an amine value and an acid value of 30 to 160 mgKOH / g, an amine value and an acid value.
- a copper nanoparticle dispersion containing a polymer dispersant of 0 to 160 mgKOH / g and a solvent, and having a volume average particle diameter of 500 nm or less by dynamic light scattering method is applied onto a substrate. And a step of forming a coating film and a step of baking the coating film.
- substrate of the 2nd aspect which concerns on this invention is a compound containing copper, a reducing compound, carboxylic acid, and the mixture containing an alkylamine, or copper carboxylate, a reducing compound, and alkyl.
- a conductive substrate of the present invention According to the method for producing a conductive substrate of the present invention, as described above, copper nanoparticles having a small dispersed particle diameter, excellent in oxidation resistance, dispersibility, applicability, and sintering property at low temperature or in a short time. Since the dispersion is used, it is possible to form a smooth and highly uniform coating film in which copper nanoparticles whose oxidation is suppressed are present uniformly and at a high density. As a result, a conductive substrate having good pattern accuracy and excellent conductivity after sintering can be obtained.
- FIG. 1 is a schematic view showing an example of a conductive substrate obtained by the production method of the present invention.
- a conductive substrate 100 shown in FIG. 1 includes a metal film 2 formed on one surface of a base material 1 by baking a coating film of a copper nanoparticle dispersion.
- FIG. 2 is a schematic view showing another example of a conductive substrate obtained by the production method of the present invention.
- a conductive substrate 101 shown in FIG. 2 is provided with a patterned metal film 3 on both surfaces of a base material 1 by baking a coating film of a copper nanoparticle dispersion.
- the conductive substrate obtained by the production method of the present invention may be provided with a metal film obtained by firing a coating film of a copper nanoparticle dispersion only on one surface of a base material.
- a metal film obtained by firing a coating film of a copper nanoparticle dispersion on both surfaces of a base material may be used.
- the metal film provided on only one surface of the base material and the metal film provided on both surfaces of the base material are each a solid metal film having no pattern even if it is a patterned metal film. There may be.
- the metal film provided on both surfaces of the base material may be a metal film having one surface that is a patterned metal film and the other surface that is a solid film.
- the metal film provided on both surfaces of the substrate is a patterned metal film on both surfaces
- the patterns on both surfaces may be the same or different.
- the patterned metal films provided on both surfaces of the substrate have the same pattern, the patterns on both surfaces may be at the same position or at different positions on both surfaces.
- a step of preparing a copper nanoparticle dispersion, and a compound containing copper in the method for producing a conductive substrate according to the second aspect of the present invention A step of preparing copper nanoparticles by heating either a mixture containing a reducing compound, a carboxylic acid and an alkylamine, or a mixture containing copper carboxylate, a reducing compound and an alkylamine; A step of preparing a copper nanoparticle dispersion having a volume average particle diameter of 500 nm or less by a dynamic light scattering method by dispersing particles in a solvent with a polymer dispersant having the specific amine value and acid value.
- distribution on a base material, and forming a coating film> (Base material)
- the base material used for this invention suitably from the base materials used for an electroconductive board
- inorganic materials such as glass, alumina, silica, and SUS foil can be used, and polymer materials and paper can also be used. Since the metal fine particle dispersion for conductive substrate according to the present invention can obtain a metal film having excellent conductivity even when fired at a lower temperature than before, soda lime glass, which has been difficult to apply conventionally, Even molecular materials can be suitably used, and are particularly useful in that a resin film can be used.
- the resin film examples include polyimide, polyamide, polyamideimide, polyethylene terephthalate (PET), polyethylene naphthalate (PEN), polyphenylene sulfide, polyether ether ketone, polyether sulfone, polycarbonate, polyether imide, epoxy resin, and phenol resin. Glass-epoxy resins, polyphenylene ethers, acrylic resins, polyolefins such as polyethylene and polypropylene, polycycloolefins such as polynorbornene, and liquid crystalline polymer compounds.
- a resin film having a glass transition temperature of 100 ° C. or less, such as PET can be used.
- the glass transition temperature is determined by differential scanning calorimetry (DSC) measurement measured according to JIS-K7121.
- the treatment method for the substrate surface can be appropriately selected from conventionally known methods. Specifically, for example, corona treatment, UV treatment, vacuum ultraviolet lamp treatment, dry treatment such as plasma treatment, amine silane coupling agent, imidazole silane coupling agent, titanium coupling agent, aluminum coupling agent treatment, etc. Chemical layer treatment, porous silica, porous membrane formation treatment such as cellulose-based receiving layer, active energy ray curable resin layer, thermosetting resin layer, thermoplastic resin layer and other resin layer formation treatment .
- the shape of the substrate may be appropriately selected depending on the application, and may be flat or curved, but is usually flat.
- the thickness of the substrate may be appropriately set according to the application, and may be, for example, about 10 ⁇ m to 1 mm.
- the method for applying the copper nanoparticle dispersion onto the substrate may be appropriately selected from conventionally known application or printing methods. Among these, gravure printing, gravure offset printing, reverse offset printing, flexographic printing, screen printing, and ink jet printing are preferable because fine patterning can be performed when printing a conductive pattern.
- the coating method includes a case where the entire surface is coated. In the case of whole surface application, a pattern can be formed by a chemical etching method as described later on a copper nanoparticle sintered film obtained by baking the coating film.
- the copper nanoparticle dispersion on the substrate may be dried by a usual method after coating.
- the film thickness of the coated film after drying can be controlled by appropriately changing the coating amount, the average primary particle diameter of the copper nanoparticles, etc., and may be appropriately adjusted according to the application, but is usually 0.
- the range is from 0.01 to 50 ⁇ m, and preferably from 0.1 to 20 ⁇ m.
- This step is a step of forming a metal film by baking the coating film obtained in the above step to form a sintered film.
- the firing method can be appropriately selected from conventionally known firing methods. Specific examples of the firing method include, for example, methods such as heating by a firing furnace (oven), infrared heating, various laser annealing, ultraviolet light, visible light, light irradiation firing with flash light, microwave heating, and the like. In addition, it is preferably performed in an inert gas atmosphere or a reducing gas atmosphere. In the case of an air atmosphere, it is preferable that heating is performed instantaneously in order to prevent oxidation during firing. Since the copper nanoparticle dispersion of the present invention can be fired at a low temperature or in a short time, it can be fired at a lower temperature than the conventional method.
- the firing step is plasma firing, in particular, firing by surface wave plasma generated by application of microwave energy, or firing by flash light irradiation (hereinafter referred to as flash light firing). It is preferable that any one of the above.
- flash light firing firing by flash light irradiation
- thermal damage to the substrate can be reduced, and oxidation of the metal during firing can be suppressed.
- it is baking for a short time there also exists a merit that productivity is high.
- microwave firing Firing using microwave surface wave plasma is preferably performed in an inert gas atmosphere or a reducing gas atmosphere from the viewpoint of the conductivity of the obtained sintered film.
- the microwave surface wave plasma is preferably generated in a reducing gas atmosphere, and more preferably generated in a hydrogen gas atmosphere.
- the baking may be performed at a temperature of about 50 to 200 ° C. for about 1 minute to 2 hours. This treatment may be performed under reduced pressure. By this firing, organic substances are oxidatively decomposed and removed, and the sintering of the copper nanoparticles is promoted in the microwave surface wave plasma treatment.
- the method for generating the microwave surface wave plasma may be appropriately selected from conventionally known methods.
- the method described in International Publication No. 2011/040189 pamphlet can be used.
- Flash light firing is a method of firing in an extremely short time by irradiation with flash light.
- flash light refers to light having a relatively short lighting time, and the lighting time is referred to as a pulse width.
- the light source of the flash light is not particularly limited, and examples thereof include a flash lamp and a laser in which a rare gas such as xenon is sealed. Among them, it is preferable to irradiate light having a continuous wavelength spectrum from ultraviolet to infrared, and specifically, it is preferable to use a xenon flash lamp. When such a light source is used, the same effect as when UV irradiation is performed simultaneously with heating can be obtained, and baking can be performed in an extremely short time.
- the polymer dispersant used in the present invention is uniformly present around the copper nanoparticles due to a synergistic effect with the alkylamine and carboxylic acid, and therefore easily decomposes or volatilizes by irradiation with flash light. Since it is easy and hardly remains on the metal film, it can be easily sintered even by irradiation with flash light for a very short time. Therefore, flash light baking is preferably used in the present invention.
- the pulse width of the flash light may be appropriately adjusted, but is preferably set between 1 ⁇ s and 10,000 ⁇ s, and more preferably within the range of 10 ⁇ s to 5000 ⁇ s.
- the irradiation energy per one flash light is preferably 0.1J / cm 2 ⁇ 100J / cm 2, 0.5J / cm 2 ⁇ 50J / cm 2 is more preferable.
- the number of times of flash light irradiation may be appropriately adjusted according to the composition, film thickness, area, etc. of the coating film, and the number of times of irradiation may be only once or may be repeated two or more times. Good.
- the number of irradiation is preferably 1 to 100 times, and more preferably 1 to 50 times.
- the irradiation interval of the flash light may be adjusted as appropriate.
- the irradiation interval is preferably set within a range of 10 ⁇ sec to 2 seconds, and more preferably set within a range of 100 ⁇ sec to 1 second.
- the flash light As described above, it is possible to suppress the influence on the base material and to suppress the oxidation of the copper nanoparticles, and the alkylamine and carboxylic acid contained in the copper nanoparticle dispersion
- the above-described polymer dispersant can be easily detached or decomposed to obtain a conductive substrate having excellent conductivity.
- Such flash light baking can heat only the coating film of the copper nanoparticle dispersion and its vicinity, and can burn the coating film at a low temperature and in a short time.
- a nanoparticle sintered film can be formed.
- the heating temperature and the processing depth can be controlled by appropriately adjusting the pulse width and irradiation energy of the flash light. As a result, a non-uniform film is rarely formed and grain growth can be prevented, so that a very dense and smooth film can be obtained.
- baking is possible in a very short time, the oxidation of copper nanoparticles can be suppressed, and a sintered film having excellent conductivity can be obtained.
- the flash light baking can be performed in the atmosphere at atmospheric pressure, but may be performed in an inert gas atmosphere, a reducing gas atmosphere, or a reduced pressure. Moreover, you may perform flash light baking, heating a coating film.
- the thickness of the metal film of the conductive substrate thus obtained may be adjusted as appropriate according to the application, but the thickness is usually about 0.01 to 50 ⁇ m, and 0.05 nm to 30 ⁇ m. Preferably, the thickness is 0.1 to 20 ⁇ m.
- the volume resistivity of the metal film is preferably 1.0 ⁇ 10 ⁇ 4 ⁇ ⁇ cm or less.
- the sintered film obtained using the copper nanoparticle dispersion of the present invention has a smooth surface and low resistance, and is less likely to generate voids at the interface with the substrate, and has good adhesion. And it has a moderate space
- the sintered film obtained by using the copper nanoparticle dispersion of the present invention can also form fine wiring by further chemically etching after forming the sintered film by the entire surface coating as described above. Is suitable.
- Pattern formation by chemical etching is performed by applying a photoresist or laminating a dry film resist to a sintered film formed on the entire surface to form a photoresist layer, and then exposing and developing by a photolithography method using a photomask. After the pattern is formed, etching with ferric chloride, cupric chloride, phosphorous nitric acid or the like is performed, and the remaining resist is peeled off to form a patterned metal film.
- the manufacturing method of the present invention is a pattern in which a copper nanoparticle dispersion is applied in a pattern on a substrate to form a coating film, and the coating film is baked to form a patterned metal film. It may be a method for manufacturing a conductive substrate.
- the conductive substrate obtained by the method for producing a conductive substrate of the present invention has good pattern accuracy and excellent conductivity.
- An electronic member using such a conductive substrate can be effectively used for an electromagnetic wave shielding film having a low surface resistance, a conductive film, a flexible printed wiring board, and the like.
- Example 1 (1) Synthesis of copper nanoparticles In a 200 ml three-necked flask, 10.0 g of copper hydroxide (0.1 mol, manufactured by Wako Pure Chemical Industries), 31.5 g of nonanoic acid (0.2 mol, manufactured by Tokyo Chemical Industry, boiling point) 254 ° C.) and 18.5 g (20 ml, manufactured by Kanto Chemical) of propylene glycol monomethyl ether (PGME). The mixture was heated to 100 ° C. with stirring and the temperature was maintained for 20 minutes.
- copper hydroxide 0.1 mol, manufactured by Wako Pure Chemical Industries
- nonanoic acid 0.2 mol, manufactured by Tokyo Chemical Industry, boiling point 254 ° C.
- PGME propylene glycol monomethyl ether
- the average primary particle size was 39 nm.
- the obtained copper nanoparticles were dispersed in toluene, and this was dropped onto a TEM substrate (manufactured by Hitachi High-Tech Fielding Co., Ltd., Cu grid with elastic carbon support film) and dried to prepare a sample for observation.
- Particle images were measured with TEM (Hitachi High-Technology H-7650), and the average value of the length of the longest portion of 100 randomly selected primary particles was defined as the average primary particle size.
- the main structure of the copper nanoparticle was Cu (Cubic), and a part of Cu 2 O (Cubic) structure was observed, and Cu (111)
- the peak intensity of Cu 2 O (111) relative to was about 3%.
- the copper nanoparticle dispersion 1 is applied to a PET film (Cosmo Shine A4100) having a thickness of 100 ⁇ m with a wire bar and dried to form a coating film having a thickness of 0.5 ⁇ m. It was. Thereafter, while introducing hydrogen gas at an introduction pressure of 20 Pa, using a microwave surface wave plasma processing apparatus (MSP-1500, manufactured by Microelectronics), firing was performed at a microwave output of 450 W for 300 seconds to obtain a conductive substrate.
- MSP-1500 microwave surface wave plasma processing apparatus
- the copper nanoparticle dispersion 1 is applied to a PET film (Cosmo Shine A4100) having a thickness of 100 ⁇ m with a wire bar and dried to obtain a coating having a thickness of 0.5 ⁇ m. A membrane was obtained. Thereafter, using a pulsed xenon lamp device (SINTERON 2000 (manufactured by Xenon Corporation)), irradiation was performed once with a pulse width of 500 ⁇ sec and an applied voltage of 3.7 kV to obtain a conductive substrate.
- a pulsed xenon lamp device SINTERON 2000 (manufactured by Xenon Corporation)
- Example 2 In Example 1, (2), instead of Solsperse 41000, Solsperse 53095 (manufactured by Nippon Lubrizol, acid value 47 mgKOH / g, amine value 0 mgKOH / g, weight average molecular weight 3900, 90% thermogravimetric decrease temperature is 360.7. C.) was used in the same manner as (2) of Example 1 except that the copper nanoparticle dispersion 2 was obtained. Using the obtained copper nanoparticle dispersion 2, a conductive substrate was produced by plasma firing in the same manner as (3) of Example 1. Using the obtained copper nanoparticle dispersion 2, a conductive substrate was produced by flash light baking in the same manner as in (4) of Example 1.
- Example 3 In Example 1 (2), instead of Solsperse 41000, Solsperse 71000 (manufactured by Nippon Lubrizol, acid value 0 mgKOH / g, amine value 77.4 mgKOH / g, weight average molecular weight 4700, 90% thermogravimetric reduction temperature is 405. .9 ° C) was used in the same manner as (2) of Example 1 to obtain a copper nanoparticle dispersion 3. Using the obtained copper nanoparticle dispersion 3, a conductive substrate was produced by plasma firing in the same manner as (3) of Example 1. Using the obtained copper nanoparticle dispersion 3, a conductive substrate was produced by flash light baking in the same manner as in (4) of Example 1.
- Example 4 In Example 1, (2), instead of Solsperse 41000, Disper byk-111 (manufactured by Big Chemie Japan, acid value 129 mgKOH / g, amine value 0 mgKOH / g, weight average molecular weight 1700, 90% thermogravimetric decrease temperature is 320. .4 ° C.) was used in the same manner as (2) of Example 1 to obtain a copper nanoparticle dispersion 4. Using the obtained copper nanoparticle dispersion 4, a conductive substrate was produced by plasma firing in the same manner as (3) of Example 1. Using the obtained copper nanoparticle dispersion 4, a conductive substrate was produced by flash light baking in the same manner as in Example 1 (4).
- Disper byk-111 manufactured by Big Chemie Japan, acid value 129 mgKOH / g, amine value 0 mgKOH / g, weight average molecular weight 1700, 90% thermogravimetric decrease temperature is 320. .4 ° C.
- Example 5 In Example 1 (2), instead of Solsperse 41000, Disper byk-145 (manufactured by Big Chemie Japan, acid value 76 mgKOH / g, amine value 71 mgKOH / g, weight average molecular weight 1800, 90% thermogravimetric decrease temperature is 378 .2 ° C.) was used in the same manner as (2) of Example 1 to obtain a copper nanoparticle dispersion 5. Using the obtained copper nanoparticle dispersion 5, a conductive substrate was produced by plasma firing in the same manner as in (1) of Example 1. Using the obtained copper nanoparticle dispersion 5, a conductive substrate was produced by flash light baking in the same manner as in Example 1 (4).
- Disper byk-145 manufactured by Big Chemie Japan, acid value 76 mgKOH / g, amine value 71 mgKOH / g, weight average molecular weight 1800, 90% thermogravimetric decrease temperature is 378 .2 ° C.
- Example 6 In Example 1 (2), instead of Solsperse 41000, Disper byk-180 (manufactured by Big Chemie Japan, acid value 94 mgKOH / g, amine value 94 mgKOH / g, THF-soluble component weight average molecular weight 1000, 90% heat A copper nanoparticle dispersion 6 was obtained in the same manner as (2) of Example 1 except that the weight reduction temperature was 350.2 ° C. Using the obtained copper nanoparticle dispersion 6, a conductive substrate was produced by plasma firing in the same manner as in (1) of Example 1. Using the obtained copper nanoparticle dispersion 6, a conductive substrate was produced by flash light baking in the same manner as in Example 1 (4).
- Example 7 (1) Synthesis of copper nanoparticles In Example 1, instead of 40.5 g (0.4 mol) of hexylamine, 35.7 g of 3-methoxypropylamine (0.4 mol, manufactured by Guangei Chemical Industry Co., Ltd., boiling point 116 ° C.) was used. Copper nanoparticles coated with nonanoic acid and 3-methoxypropylamine were obtained in the same manner as Example 1 except for using.
- the average primary particle size was 65 nm.
- the main structure of the copper nanoparticle was Cu (Cubic), and a part of Cu 2 O (Cubic) structure was observed, and Cu (111)
- the peak intensity of Cu 2 O (111) relative to was about 1%.
- a copper nanoparticle dispersion was prepared in the same manner as (2) of Example 1 except that the copper nanoparticle coated with nonanoic acid and 3-methoxypropylamine obtained above was used. A particle dispersion 7 was obtained.
- Example 8 (1) Synthesis of copper nanoparticles In Example 1, instead of 40.5 g (0.4 mol) of hexylamine, 41.3 g of 3-ethoxypropylamine (0.4 mol, manufactured by Guangei Chemical Industry Co., Ltd., boiling point 135 ° C.) was used. In the same manner as in Example 1 except that 63.0 g (100 ml) of hexane was added instead of 33.0 g (50 ml) of hexane, copper nano-particles coated with nonanoic acid and 3-ethoxypropylamine were used. Particles were obtained.
- the average primary particle size was 65 nm.
- the main structure of the copper nanoparticle was Cu (Cubic), and a part of Cu 2 O (Cubic) structure was observed, and Cu (111)
- the peak intensity of Cu 2 O (111) relative to was about 5%.
- Example 2 Preparation of copper nanoparticle dispersion
- the copper nanoparticles coated with nonanoic acid and 3-ethoxypropylamine obtained above were used as the copper nanoparticles, and instead of Solsperse 41000
- Solsperse 71000 (manufactured by Nippon Lubrizol, acid value 0 mgKOH / g, amine value 77.4 mgKOH / g, weight average molecular weight 4700, 90% thermogravimetric decrease temperature is 405.9 ° C.) is used.
- a copper nanoparticle dispersion 8 was obtained.
- Example 9 (1) Synthesis of copper nanoparticles In Example 8, instead of 31.5 g (0.2 mol) of nonanoic acid, 34.5 g of decanoic acid (0.2 mol, Kao Lunak 10-98, boiling point 268 ° C.) was used. Except for the above, copper nanoparticles coated with decanoic acid and 3-ethoxypropylamine were obtained in the same manner as in Example 8.
- the average primary particle size was 48 nm.
- the main structure of the copper nanoparticle was Cu (Cubic), and a part of Cu 2 O (Cubic) structure was observed, and Cu (111)
- the peak intensity of Cu 2 O (111) relative to was about 7%.
- a copper nanoparticle dispersion was prepared in the same manner as (2) of Example 1 except that the copper nanoparticle coated with decanoic acid and 3-ethoxypropylamine obtained above was used. A particle dispersion 9 was obtained.
- Example 10 In Example 1 (2), copper nanoparticles coated with decanoic acid and 3-ethoxypropylamine obtained in the same manner as in Example 9 (1) were used as copper nanoparticles, and instead of Solsperse 41000, In addition, Solsperse 71000 (manufactured by Nippon Lubrizol, acid value 0 mgKOH / g, amine value 77.4 mgKOH / g, weight average molecular weight 4700, 90% thermogravimetric decrease temperature is 405.9 ° C.) is used.
- a copper nanoparticle dispersion 10 was obtained. Using the obtained copper nanoparticle dispersion 10, a conductive substrate was produced by plasma firing in the same manner as (3) of Example 1. Using the obtained copper nanoparticle dispersion 10, a conductive substrate was produced by flash light baking in the same manner as in (4) of Example 1.
- Example 11 In Example 1 (2), copper nanoparticles coated with decanoic acid and 3-ethoxypropylamine obtained in the same manner as in Example 9 (1) were used as copper nanoparticles, and instead of Solsperse 41000, In addition, Disper byk-102 (manufactured by Big Chemie Japan, acid value 101 mgKOH / g, amine value 0 mgKOH / g, weight average molecular weight 1400, 90% thermogravimetric decrease temperature is 327.5 ° C.) is used. In the same manner as (2), a copper nanoparticle dispersion 11 was obtained. Using the obtained copper nanoparticle dispersion 11, a conductive substrate was produced by plasma firing in the same manner as in (1) of Example 1. Using the obtained copper nanoparticle dispersion 11, a conductive substrate was produced by flash light baking in the same manner as in Example 1 (4).
- Disper byk-102 manufactured by Big Chemie Japan, acid value 101 mgKOH / g, amine value 0 mgKOH / g,
- Example 12 In Example 1 (2), copper nanoparticles coated with decanoic acid and 3-ethoxypropylamine obtained in the same manner as in Example 9 (1) were used as copper nanoparticles, and instead of Solsperse 41000, In addition, Disper byk-106 (manufactured by Big Chemie Japan, acid value 132 mgKOH / g, amine value 74 mgKOH / g, weight average molecular weight 1400, 90% thermogravimetric decrease temperature is 405.3 ° C.) is used. In the same manner as (2), a copper nanoparticle dispersion 12 was obtained. Using the obtained copper nanoparticle dispersion 12, a conductive substrate was produced by plasma firing in the same manner as in (1) of Example 1. Using the obtained copper nanoparticle dispersion 12, a conductive substrate was produced by flash light baking in the same manner as in (4) of Example 1.
- Example 13 In Example 1 (2), copper nanoparticles coated with decanoic acid and 3-ethoxypropylamine obtained in the same manner as in Example 9 (1) were used as copper nanoparticles, and instead of Solsperse 41000, In addition, Disper byk-111 (manufactured by Big Chemie Japan, acid value 129 mgKOH / g, amine value 0 mgKOH / g, weight average molecular weight 1700, 90% thermogravimetric decrease temperature is 320.4 ° C.) In the same manner as (2), a copper nanoparticle dispersion 13 was obtained. Using the obtained copper nanoparticle dispersion 13, a conductive substrate was produced by plasma firing in the same manner as (3) of Example 1. Using the obtained copper nanoparticle dispersion 13, a conductive substrate was produced by flash light baking in the same manner as in Example 1 (4).
- Example 14 (1) Synthesis of copper nanoparticles In Example 1, instead of hexylamine 40.5 g (0.4 mol), 40.9 g of dimethylaminopropylamine (0.4 mol, manufactured by Guangei Chemical Industry Co., Ltd., boiling point 135 ° C.) was used. Except for the above, copper nanoparticles coated with nonanoic acid and dimethylaminopropylamine were obtained in the same manner as in Example 1.
- the average primary particle size was 64 nm.
- the main structure of the copper nanoparticle was Cu (Cubic), and a part of Cu 2 O (Cubic) structure was observed, and Cu (111)
- the peak intensity of Cu 2 O (111) relative to was about 1%.
- Example 15 (1) Synthesis of copper nanoparticles In Example 1, instead of 31.5 g (0.2 mol) of nonanoic acid, 28.2 g of oleic acid (0.1 mol, manufactured by Kanto Chemical Co., Ltd., boiling point: 360 ° C.) was used, and hexylamine was used. Instead of 40.5 g (0.4 mol), oleic acid and octylamine were obtained in the same manner as in Example 1 except that 51.7 g (0.4 mol, manufactured by Tokyo Chemical Industry Co., Ltd., boiling point: 176 ° C.) was used. Copper nanoparticles coated with were obtained.
- the average primary particle size was 28 nm.
- the main structure of the copper nanoparticle was Cu (Cubic), and a part of Cu 2 O (Cubic) structure was observed, and Cu (111)
- the peak intensity of Cu 2 O (111) relative to was about 12%.
- Example 1 The nonanoic acid obtained in the same manner as in Example 1 (1) and copper nanoparticles coated with hexylamine (3.0 parts by mass) and PGME (4.5 parts by mass) were mixed, and a paint shaker (manufactured by Asada Tekko) was mixed. Then, 2 mm zirconia beads were preliminarily dispersed for 1 hour, and 0.1 mm zirconia beads were further dispersed for 2 hours as the main dispersion. However, the copper nanoparticles were not aggregated and dispersed, and a copper nanoparticle dispersion could not be obtained.
- Example 2 In the same manner as in Example 1 (1), copper nanoparticles coated with nonanoic acid and hexylamine were obtained.
- the copper nanoparticles in Example 2 (2) instead of Solsperse 41000, Solsperse 16000 (manufactured by Nippon Lubrizol, acid value 20 mgKOH / g, amine value 0 mgKOH / g, weight average molecular weight 4200, 90% thermogravimetric weight) Dispersion was carried out in the same manner as in (2) of Example 1 except that the reduction temperature was 405.5 ° C. However, the copper nanoparticles were not aggregated and dispersed, and a copper nanoparticle dispersion could not be obtained.
- Example 3 In the same manner as in Example 1 (1), copper nanoparticles coated with nonanoic acid and hexylamine were obtained.
- Example 1 (2) instead of Solsperse 41000, the copper nanoparticles were Solsperse 76500 (manufactured by Nippon Lubrizol, acid value 0 mgKOH / g, amine value 15.2 mgKOH / g, 90% thermogravimetric decrease temperature) Dispersion was carried out in the same manner as (2) of Example 1 except that over 600 ° C. (32% remaining even at 600 ° C.) was used. However, the copper nanoparticles were not aggregated and dispersed, and a copper nanoparticle dispersion could not be obtained.
- Example 4 In the same manner as in Example 9 (1), copper nanoparticles coated with decanoic acid and 3-ethoxypropylamine were obtained.
- Example 1 (2) the copper nanoparticles were replaced with Dispers byk-130 (manufactured by Big Chemie Japan, acid value of less than 3 mgKOH / g, amine value of 190 mgKOH / g, 90% thermogravimetric reduction temperature). Was 474.8 ° C.) in the same manner as in Example 1 (2). However, the copper nanoparticles were not aggregated and dispersed, and a copper nanoparticle dispersion could not be obtained.
- Example 5 In the same manner as in Example 15 (1), copper nanoparticles coated with oleic acid and octylamine were obtained.
- Example 1 (2) the copper nanoparticles were replaced with Dispers byk-130 (manufactured by Big Chemie Japan, acid value of less than 3 mgKOH / g, amine value of 190 mgKOH / g, 90% thermogravimetric reduction temperature).
- Dispers byk-130 manufactured by Big Chemie Japan, acid value of less than 3 mgKOH / g, amine value of 190 mgKOH / g, 90% thermogravimetric reduction temperature.
- 474.8 ° C. was used to obtain Comparative Copper Nanoparticle Dispersion 5.
- a conductive substrate was produced by plasma firing in the same manner as (3) of Example 1.
- a conductive substrate was produced by flash light baking in the same manner as in (4) of Example 1.
- Example 6 (Comparative Example 6) (1) Synthesis of copper nanoparticles In Example 1, instead of 31.5 g (0.2 mol) of nonanoic acid, 28.2 g of oleic acid (0.1 mol, manufactured by Kanto Chemical Co., Ltd., boiling point: 360 ° C.) was used. In the same manner as in Example 1, copper nanoparticles coated with oleic acid and hexylamine were obtained.
- the average primary particle size was 24 nm.
- the main structure of the copper nanoparticle was Cu (Cubic), and a part of Cu 2 O (Cubic) structure was observed, and Cu (111)
- the peak intensity of Cu 2 O (111) relative to was about 17%.
- Example 7 In the same manner as in Comparative Example 6 (1), copper nanoparticles coated with oleic acid and hexylamine were obtained.
- Example 1 (2) instead of Solsperse 41000, the copper nanoparticles were Solsperse 76500 (manufactured by Nippon Lubrizol, acid value 0 mgKOH / g, amine value 15.2 mgKOH / g, 90% thermogravimetric decrease temperature)
- a comparative copper nanoparticle dispersion 7 was obtained by dispersing in the same manner as in (2) of Example 1 except that over 600 ° C (32% remaining even at 600 ° C) was used.
- Example 7 Using the obtained comparative copper nanoparticle dispersion 7, a conductive substrate was produced by plasma firing in the same manner as in Example 1 (3). Using the obtained comparative copper nanoparticle dispersion 7, a conductive substrate was produced by flash light baking in the same manner as in Example 1 (4).
- Conductivity evaluation was performed on the conductive substrate. Using a surface resistance meter ("Loresta GP" manufactured by Dia Instruments, PSP probe type), 4 probes are brought into contact with the metal film of the conductive substrate of each example and comparative example, and the sheet resistance value is determined by the 4 probe method. It was measured. The evaluation results are shown in Table 1. The lower the sheet resistance value, the better the conductivity. The upper limit of sheet resistance measured by this measurement method was 10 8 ⁇ / ⁇ . In the table, O.D. L. Represents Over Load.
- a copper nanoparticle dispersion containing a carboxylic acid, an alkylamine, a polymer dispersant having a specific amine value and an acid value, and a solvent according to the present invention can be dispersed and applied. It has been clarified that it has excellent sinterability at a low temperature or in a short time, and a high conductivity with a surface resistance of 1 ⁇ / ⁇ or less can be obtained. In particular, it was found that the use of a carboxylic acid having 10 or less carbon atoms improves the conductivity.
- the copper nanoparticle dispersion which concerns on this invention since it is excellent in application
- the copper nanoparticles obtained in Examples 1 to 15 have good oxidation resistance at the time of production.
- the oxidation resistance at the time of production of the copper nanoparticles It was also found to be excellent.
- the copper nanoparticle dispersion of Comparative Example 1 containing a carboxylic acid and an alkylamine but not containing a polymer dispersant has poor dispersibility, and even if a coating film is formed, it has repelling and is not uniform.
- Example 16 In the same manner as in Example 3, a copper nanoparticle dispersion 3 was obtained.
- the obtained copper nanoparticle dispersion 3 was applied to a PET film (Cosmo Shine A4100) having a thickness of 100 ⁇ m with a wire bar and dried to form a coating film having a thickness of 0.5 ⁇ m.
- a microwave surface wave plasma processing apparatus MSP-1500, manufactured by Microelectronics
- firing was performed for 300 seconds at a microwave output of 450 W, and the copper nanoparticle dispersion was fired.
- the resulting sintered film (copper film) was used as a PET film with a copper thin film.
- a UV curable ink was applied to a cleaned glass substrate with a spin coater, and the PET film with a copper thin film obtained above was affixed to prevent bubbles from entering, and the glass and the film were fixed by UV irradiation from the back side. .
- a commercially available naphthoquinone-based positive resist was spin-coated on the surface of the PET film with a copper thin film and hot-plate dried at 100 ° C. for 3 minutes to form a dry film thickness of about 1 ⁇ m.
- a positive resist film surface was contacted with a photomask, exposed at 40 mJ / cm 2 , then paddle developed with NMD-3 (manufactured by Tokyo Ohka Kogyo Co., Ltd.) for 20 seconds, and rinsed with pure water to obtain a resist pattern. Further, etching was performed for 80 seconds while being rocked with a phosphoric acid acetate etching solution at 23 ° C., and then rinsed with pure water and etched.
- NMD-3 manufactured by Tokyo Ohka Kogyo Co., Ltd.
- ⁇ Smoothness evaluation> The cross section of the obtained copper wiring pattern was measured with a scanning electron microscope (SEM, scanning electron microscope “S-4800” manufactured by Hitachi High-Technology), and a cross-sectional observation image of 100,000 times was obtained. An uneven line (length 2 ⁇ m) at the interface between the substrate and the sintered film is extracted, and from the uneven line, the maximum height (Rz, the difference between the most peak and the most valley, according to the definition of JIS B0601 (2001)) Asked. Uneven lines were extracted from any 10 locations in the surface, and the average value of the maximum heights was defined as the average roughness.
- corrugated line converted the image obtained by SEM observation into black-and-white, and extracted the line along the metal sintered film side by making a white part into the metal sintered film side. Parts with obvious irregularities such as foreign matter and filler contained in the film were not used. As a result, the unevenness of the interface between the base material and the metal sintered film was 12.4 nm, and it was confirmed that the above-mentioned interface was smooth.
- Adhesion evaluation> The obtained copper wiring pattern was subjected to a peel test using an adhesive tape (trade name: Scotch Mending Tape, manufactured by Sumitomo 3M). As a result, it was confirmed that the wiring did not peel off, and it was confirmed that the adhesion was good even after etching.
Landscapes
- Engineering & Computer Science (AREA)
- Manufacturing & Machinery (AREA)
- Microelectronics & Electronic Packaging (AREA)
- Physics & Mathematics (AREA)
- Chemical & Material Sciences (AREA)
- Dispersion Chemistry (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Manufacture Of Metal Powder And Suspensions Thereof (AREA)
- Conductive Materials (AREA)
- Powder Metallurgy (AREA)
- Manufacturing Of Electric Cables (AREA)
Abstract
La présente invention porte sur un procédé de fabrication de substrat électroconducteur avec lequel il est possible d'obtenir un substrat électroconducteur qui présente une électroconductivité exceptionnelle du fait qu'il est fritté à une basse température ou pendant une courte période de temps. La présente invention est un procédé de fabrication de substrat électroconducteur, qui a : une étape de formation d'un film de revêtement par application en revêtement d'une matière de base avec une dispersion de nanoparticules de cuivre qui contient des nanoparticules de cuivre, un acide carboxylique, une alkylamine, un agent dispersant polymère, et un solvant ; et une étape de frittage du film de revêtement. L'agent dispersant polymère a l'un de soit une valeur d'amine soit une valeur d'acide de 30 à 160 mg de KOH/g et l'autre de soit la valeur d'amine soit la valeur d'acide à 0 à 160 mg de KOH/g, et le diamètre de particule moyen en volume est de 500 nm au maximum tel qu'obtenu par la diffusion dynamique de la lumière.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2014-092473 | 2014-04-28 | ||
| JP2014092473A JP5994811B2 (ja) | 2014-04-28 | 2014-04-28 | 銅ナノ粒子分散体、及び導電性基板の製造方法 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2015166755A1 true WO2015166755A1 (fr) | 2015-11-05 |
Family
ID=54358492
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2015/060007 WO2015166755A1 (fr) | 2014-04-28 | 2015-03-30 | Dispersion de nanoparticules de cuivre et procédé de fabrication de substrat électroconducteur |
Country Status (2)
| Country | Link |
|---|---|
| JP (1) | JP5994811B2 (fr) |
| WO (1) | WO2015166755A1 (fr) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20210155818A1 (en) * | 2017-07-27 | 2021-05-27 | Asahi Kasei Kabushiki Kaisha | Copper Oxide Ink and Method for Producing Conductive Substrate Using Same, Product Containing Coating Film and Method for Producing Product Using Same, Method for Producing Product with Conductive Pattern, and Product with Conductive Pattern |
| WO2023017747A1 (fr) * | 2021-08-10 | 2023-02-16 | 株式会社ダイセル | Encre électroconductrice |
Families Citing this family (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN107614614B (zh) * | 2015-06-02 | 2020-03-31 | 旭化成株式会社 | 分散体 |
| JP6642218B2 (ja) * | 2016-04-05 | 2020-02-05 | 日油株式会社 | レーザーエッチング用銅ペースト組成物 |
| WO2018003399A1 (fr) * | 2016-06-30 | 2018-01-04 | 株式会社コイネックス | Câblage en cuivre et son procédé de fabrication, et appareil électronique, pavé tactile et panneau tactile l'utilisant |
| US11328835B2 (en) | 2017-03-16 | 2022-05-10 | Asahi Kasei Kabushiki Kaisha | Dispersing element, method for manufacturing structure with conductive pattern using the same, and structure with conductive pattern |
| CN110366761B (zh) | 2017-03-16 | 2022-04-29 | 旭化成株式会社 | 分散体以及使用其的带导电性图案的结构体的制造方法和带导电性图案的结构体 |
| JP2018170228A (ja) * | 2017-03-30 | 2018-11-01 | 日立化成株式会社 | 導体形成用組成物、並びに接合体及びその製造方法 |
| JP2018168439A (ja) * | 2017-03-30 | 2018-11-01 | 日立化成株式会社 | 電磁波遮蔽体の製造に用いられる組成物、及び電磁波遮蔽体の製造方法 |
| JP7005625B2 (ja) | 2017-07-18 | 2022-01-21 | 旭化成株式会社 | 導電性パターン領域を有する構造体及びその製造方法、積層体及びその製造方法、並びに、銅配線 |
| JP7430483B2 (ja) * | 2017-11-10 | 2024-02-13 | 旭化成株式会社 | 導電性パターン領域付構造体及びその製造方法 |
| JP2019211243A (ja) * | 2018-05-31 | 2019-12-12 | 旭化成株式会社 | Rfidタグ |
| JP2020084242A (ja) * | 2018-11-20 | 2020-06-04 | 京セラ株式会社 | 接合用銅粒子の製造方法、接合用ペースト及び半導体装置並びに電気・電子部品 |
| JP2020100888A (ja) * | 2018-12-25 | 2020-07-02 | 京セラ株式会社 | 銅粒子、銅粒子の製造方法、銅ペースト及び半導体装置並びに電気・電子部品 |
| JP7269565B2 (ja) * | 2019-03-29 | 2023-05-09 | 学校法人 関西大学 | 導電性インキ組成物及び導電性積層体 |
| JP7434786B2 (ja) * | 2019-09-27 | 2024-02-21 | Dic株式会社 | 銅/酸化銅微粒子ペースト |
| US20240157483A1 (en) * | 2021-03-17 | 2024-05-16 | Kyocera Corporation | Paste composition, semiconductor device, electrical component and electronic component |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2009074171A (ja) * | 2007-08-30 | 2009-04-09 | Mitsuboshi Belting Ltd | 金属コロイド粒子およびその分散液 |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5661273B2 (ja) * | 2008-11-26 | 2015-01-28 | 三ツ星ベルト株式会社 | 金属コロイド粒子及びそのペースト並びにその製造方法 |
| KR20110027487A (ko) * | 2009-09-10 | 2011-03-16 | 삼성전자주식회사 | 금속 패턴 형성용 조성물 및 이를 이용한 금속 패턴 형성방법 |
| JP5623861B2 (ja) * | 2010-10-14 | 2014-11-12 | 株式会社東芝 | 金属ナノ粒子分散組成物 |
| JP5715851B2 (ja) * | 2011-02-24 | 2015-05-13 | 東芝テック株式会社 | ナノ粒子インク組成物を用いた印刷物の製造方法 |
| JP6127433B2 (ja) * | 2012-10-03 | 2017-05-17 | 大日本印刷株式会社 | 金属粒子分散体、及び物品 |
-
2014
- 2014-04-28 JP JP2014092473A patent/JP5994811B2/ja active Active
-
2015
- 2015-03-30 WO PCT/JP2015/060007 patent/WO2015166755A1/fr active Application Filing
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2009074171A (ja) * | 2007-08-30 | 2009-04-09 | Mitsuboshi Belting Ltd | 金属コロイド粒子およびその分散液 |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20210155818A1 (en) * | 2017-07-27 | 2021-05-27 | Asahi Kasei Kabushiki Kaisha | Copper Oxide Ink and Method for Producing Conductive Substrate Using Same, Product Containing Coating Film and Method for Producing Product Using Same, Method for Producing Product with Conductive Pattern, and Product with Conductive Pattern |
| US11760895B2 (en) * | 2017-07-27 | 2023-09-19 | Asahi Kasei Kabushiki Kaisha | Copper oxide ink and method for producing conductive substrate using same, product containing coating film and method for producing product using same, method for producing product with conductive pattern, and product with conductive pattern |
| WO2023017747A1 (fr) * | 2021-08-10 | 2023-02-16 | 株式会社ダイセル | Encre électroconductrice |
Also Published As
| Publication number | Publication date |
|---|---|
| JP5994811B2 (ja) | 2016-09-21 |
| JP2015210973A (ja) | 2015-11-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5994811B2 (ja) | 銅ナノ粒子分散体、及び導電性基板の製造方法 | |
| JP5700864B2 (ja) | 銅微粒子分散液、導電膜形成方法及び回路基板 | |
| EP2952546B1 (fr) | Composition permettant de former un film conducteur électriquement et procédé de production d'un film conducteur électriquement | |
| JP5715851B2 (ja) | ナノ粒子インク組成物を用いた印刷物の製造方法 | |
| US20090053400A1 (en) | Ink jet printable compositions for preparing electronic devices and patterns | |
| TW201437299A (zh) | 導電膜形成用組成物及使用其的導電膜的製造方法 | |
| JP2008176951A (ja) | 銀系微粒子インクペースト | |
| WO2018169012A1 (fr) | Dispersion, procédé de production d'une structure équipée d'un motif conducteur en utilisant une dispersion, et structure équipée d'un motif conducteur | |
| JP6404614B2 (ja) | コアシェル型金属微粒子の製造方法、コアシェル型金属微粒子、導電性インクおよび基板の製造方法 | |
| WO2014061556A1 (fr) | Dispersion de particules métalliques destinée à un substrat conducteur, son procédé de production et procédé de production d'un substrat conducteur | |
| JP6331386B2 (ja) | 被覆銅ナノ粒子、銅ナノ粒子分散体、及び導電性基板の製造方法 | |
| JP2015149121A (ja) | 銅粒子分散ペースト、及び導電性基板の製造方法 | |
| JP6331385B2 (ja) | 被覆銅ナノ粒子、銅ナノ粒子分散体、及び導電性基板の製造方法 | |
| JP6562196B2 (ja) | 銅微粒子焼結体と導電性基板の製造方法 | |
| JP2017022125A (ja) | 銅ナノ粒子分散体、及び導電性基板の製造方法 | |
| JP6036185B2 (ja) | 高純度の金属ナノ粒子分散体ならびにその製造方法 | |
| JPWO2016125355A1 (ja) | 導電性の微小粒子 | |
| JP2016110690A (ja) | 導電性基板 | |
| JP2012218020A (ja) | 接合方法 | |
| JP6237098B2 (ja) | 分散剤、導電性基板用金属粒子分散体、及び導電性基板の製造方法 | |
| JP2020119737A (ja) | 導電性ペースト、導電膜付き基材、導電膜付き基材の製造方法 | |
| JP2019160689A (ja) | 分散体、塗膜を含む製品、導電性パターン付き構造体の製造方法、及び、導電性パターン付き構造体 | |
| JP5821485B2 (ja) | 銅微粒子分散体、パターン形成方法、及び銅パターン膜の製造方法 | |
| JP2006278936A (ja) | 金属層を備えた基板の製造方法。 | |
| JP6111587B2 (ja) | 導電性基板の製造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 15785825 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 15785825 Country of ref document: EP Kind code of ref document: A1 |