WO2015166755A1 - Copper nanoparticle dispersion and method for manufacturing electroconductive substrate - Google Patents
Copper nanoparticle dispersion and method for manufacturing electroconductive substrate Download PDFInfo
- Publication number
- WO2015166755A1 WO2015166755A1 PCT/JP2015/060007 JP2015060007W WO2015166755A1 WO 2015166755 A1 WO2015166755 A1 WO 2015166755A1 JP 2015060007 W JP2015060007 W JP 2015060007W WO 2015166755 A1 WO2015166755 A1 WO 2015166755A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- copper
- nanoparticle dispersion
- acid
- conductive substrate
- copper nanoparticle
- Prior art date
Links
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F1/00—Metallic powder; Treatment of metallic powder, e.g. to facilitate working or to improve properties
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F9/00—Making metallic powder or suspensions thereof
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01B—CABLES; CONDUCTORS; INSULATORS; SELECTION OF MATERIALS FOR THEIR CONDUCTIVE, INSULATING OR DIELECTRIC PROPERTIES
- H01B1/00—Conductors or conductive bodies characterised by the conductive materials; Selection of materials as conductors
- H01B1/02—Conductors or conductive bodies characterised by the conductive materials; Selection of materials as conductors mainly consisting of metals or alloys
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01B—CABLES; CONDUCTORS; INSULATORS; SELECTION OF MATERIALS FOR THEIR CONDUCTIVE, INSULATING OR DIELECTRIC PROPERTIES
- H01B1/00—Conductors or conductive bodies characterised by the conductive materials; Selection of materials as conductors
- H01B1/20—Conductive material dispersed in non-conductive organic material
- H01B1/22—Conductive material dispersed in non-conductive organic material the conductive material comprising metals or alloys
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01B—CABLES; CONDUCTORS; INSULATORS; SELECTION OF MATERIALS FOR THEIR CONDUCTIVE, INSULATING OR DIELECTRIC PROPERTIES
- H01B13/00—Apparatus or processes specially adapted for manufacturing conductors or cables
-
- H—ELECTRICITY
- H05—ELECTRIC TECHNIQUES NOT OTHERWISE PROVIDED FOR
- H05K—PRINTED CIRCUITS; CASINGS OR CONSTRUCTIONAL DETAILS OF ELECTRIC APPARATUS; MANUFACTURE OF ASSEMBLAGES OF ELECTRICAL COMPONENTS
- H05K3/00—Apparatus or processes for manufacturing printed circuits
Definitions
- the present invention relates to a copper nanoparticle dispersion and a method for producing a conductive substrate using the dispersion.
- metal particle dispersions using silver particles are difficult to oxidize and are excellent in conductivity, but silver itself is expensive or has problems of ion migration. Therefore, development of a metal particle dispersion using copper as an inexpensive metal with excellent migration resistance is required. However, since copper particles are generally more easily oxidized than silver particles, there is a problem that conductivity is difficult to be expressed.
- Patent Document 1 a compound containing copper and a reducing compound are mixed, and a step of producing a composite compound that can be thermally decomposed in an alkylamine to form copper, and the composite compound is heated in an alkylamine. And a process for producing copper fine particles coated with an alkylamine.
- the coated copper fine particles have a narrow and fine particle size distribution, excellent storage stability and can be sintered at a low temperature.
- the dispersibility of copper fine particles is poor, the coating suitability is poor, and unevenness occurs when coating on a resin substrate such as a PET film, as in the comparative example described later. was there.
- Patent Document 2 describes a specific metal fine particle, a polymer dispersant having a specific polyester skeleton, a metal fine particle dispersion containing a dispersion medium, and a method for producing a conductive substrate using the dispersion.
- Patent Document 2 it is described that the specific polymer dispersant exhibits a high effect on the dispersibility of the metal fine particles and is easily volatilized in a subsequent sintering step.
- the technique of Patent Document 2 retains dispersibility only with a dispersant, organic substances are likely to remain during sintering, and firing of a metal fine particle coating film requires long firing with high microwave output. It has become.
- Patent Document 3 describes a method for producing a conductive metal thin film using metal nanoparticles synthesized from a solution containing a metal precursor, an acid, an amine, and a reducing agent. It is also stated that it is good.
- Patent Document 3 is a technique that focuses on improving the conductivity by firing at a high temperature of 200 ° C. or higher using a reducing atmosphere.
- the technique specifically disclosed in Patent Document 3 has poor dispersibility of metal nanoparticles as in the comparative example described later, a certain degree of conductivity can be obtained by high-temperature firing. . Therefore, in order to coat and sinter on a PET film or the like, it is necessary to study further lowering the sintering temperature.
- JP 2012-72418 A International Publication No. 2011/040189 Pamphlet International Publication No. 2013/147535 Pamphlet
- the dispersant having a low molecular weight has insufficient dispersibility and applicability, and attempts to improve dispersibility and applicability have resulted in insufficient sinterability at low temperatures or in a short time.
- the substrate that can be used is limited because high temperature firing is required, In the firing, the volume resistivity of the substrate obtained by leaving the polymer dispersant in the metal film is increased, and there are cases where sufficient performance as a conductive substrate cannot be obtained.
- the present invention has been made under such circumstances, and a copper nanoparticle dispersion excellent in oxidation resistance, dispersibility, applicability, and sinterability at low temperature or in a short time, and low temperature or short It aims at providing the manufacturing method of an electroconductive board
- the present inventors have combined copper nanoparticles with a carboxylic acid, an alkylamine, and a polymer dispersant having a specific amine value or acid value.
- a copper nanoparticle dispersion having a volume average particle size of 500 nm or less, it is excellent in oxidation resistance, dispersibility, and coating suitability, and is organic from the film even when baked at a low temperature or in a short time. Since the components are easily decomposed or removed, and the nanoparticles are uniformly and densely arranged in the coating film, it has been found that a film having high conductivity can be formed.
- the present invention has been completed based on such knowledge.
- the method for producing a conductive substrate according to the first aspect of the present invention includes copper nanoparticles, a carboxylic acid, an alkylamine, a polymer dispersant, and a solvent, and the polymer dispersant is an amine.
- substrate of the 2nd aspect which concerns on this invention is a compound containing copper, a reducing compound, carboxylic acid, and the mixture containing an alkylamine, or copper carboxylate, a reducing compound, and alkyl.
- Preparing copper nanoparticles by heating any of the amine-containing mixtures; By dispersing the copper nanoparticles in a solvent with a polymer dispersant having one of an amine value and an acid value of 30 to 160 mgKOH / g and the other of an amine value and an acid value of 0 to 160 mgKOH / g.
- a step of preparing a copper nanoparticle dispersion having a volume average particle size of 500 nm or less by a dynamic light scattering method Applying the copper nanoparticle dispersion on a substrate to form a coating film; And a step of firing the coating film.
- the copper nanoparticle dispersion according to the present invention contains copper nanoparticles, a carboxylic acid, an alkylamine, a polymer dispersant, and a solvent.
- the polymer dispersant has an amine value and an acid value. One of these is 30 to 160 mgKOH / g, the other one of the amine value and the acid value is 0 to 160 mgKOH / g, and the volume average particle diameter by dynamic light scattering method is 500 nm or less.
- the polymer dispersant has a low 90% thermal weight loss temperature of 420 ° C. or less, low temperature sinterability and after sintering. It is preferable from the point which the electroconductivity of a coating film is excellent.
- the carboxylic acid has 10 or less carbon atoms, and the low-temperature firing property and the conductivity of the coated film after sintering are excellent. It is preferable from the point.
- the baking step is a step of baking by plasma or a step of baking by irradiation with flash light, which can be performed at a low temperature or for a short time. It is preferable because a conductive substrate having excellent conductivity can be obtained even on a low heat-resistant substrate.
- a method having a step of chemically etching the obtained sintered film after the firing step is also preferably used. Since the sintered film formed in the present invention is imparted with smoothness and adhesion, a chemical etching method can be used to form a fine conductive pattern.
- the copper nanoparticle dispersion excellent in oxidation resistance, dispersibility, applicability, and sinterability at a low temperature or in a short time, and excellent conductivity by firing at a low temperature or in a short time. It is possible to provide a method for manufacturing a conductive substrate capable of obtaining a conductive substrate having a property.
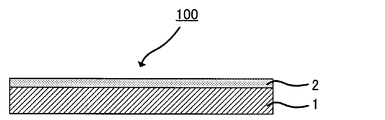
- FIG. 1 is a schematic view showing an example of a conductive substrate obtained by the production method of the present invention.
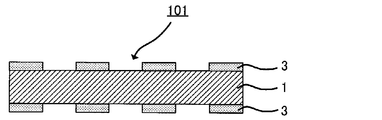
- FIG. 2 is a schematic view showing another example of a conductive substrate obtained by the production method of the present invention.
- (meth) acryl represents each of acryl and methacryl
- (meth) acrylate represents each of acrylate and methacrylate.
- the copper nanoparticle dispersion according to the present invention contains copper nanoparticles, a carboxylic acid, an alkylamine, a polymer dispersant, and a solvent, and the polymer dispersant has one of an amine value and an acid value. 30 to 160 mgKOH / g, the other of the amine value and the acid value is 0 to 160 mgKOH / g, and the volume average particle size by dynamic light scattering is 500 nm or less.
- the copper nanoparticles have a relatively low molecular weight carboxylic acid and an alkylamine, one of an amine value and an acid value of 30 to 160 mgKOH / g, and the other of the amine value and the acid value of 0 to 160 mgKOH / g.
- the volume average particle size is dispersed so as to be 500 nm or less. Therefore, it is estimated that the following specific effects are exhibited by their synergistic action.
- the polymer dispersant having a specific amine value and acid value has at least one kind of basic and acidic functional groups, and acid-base interaction with carboxylic acid or alkylamine on the surface of the copper nanoparticles. It can be considered that there is something attached due to the above.
- the carboxylic acid that can be strongly adsorbed on the surface of the copper nanoparticles is present stably surrounding the copper nanoparticles in the solvent due to the strong adsorption.
- the alkylamine has a charge different from that of the carboxylic acid at the adsorption site and further adheres to the copper nanoparticles
- the low molecular weight dispersants of carboxylic acid and alkylamine can adhere more closely to the copper nanoparticles. It is estimated to be.
- the polymer dispersant is selected so as to have a specific amine value and acid value, the polymer dispersant can be stably attached to the copper nanoparticles to which both the carboxylic acid and the alkylamine are attached. It is presumed that due to the steric hindrance of the polymer chain, aggregation of copper nanoparticles is less likely to occur, and excellent dispersibility of the copper nanoparticles can be achieved.
- the amine value and acid value of the polymer dispersant are too low or too high. In either case, the polymer dispersant cannot be stably attached, but rather the dispersibility deteriorates.
- the copper nanoparticles are surrounded by a polymer dispersant having a specific amine value and acid value and are stably dispersed uniformly in a fine particle size, film formation of a polymer chain Depending on the properties, it is estimated that the coating suitability of the copper nanoparticle dispersion is excellent and the smoothness of the coating film is increased.
- the copper nanoparticles are uniformly arranged in the coating film, and a high-density film can be formed. Since the relatively low molecular weight alkylamine is weakly adsorbed on the surface of the copper nanoparticles, it is easily detached during drying when forming a coating film. On the other hand, it is considered that the polymer dispersing agent remains to prevent non-uniform aggregation of the copper nanoparticles, and the copper nanoparticles are arranged at a higher density during drying. It is presumed that carboxylic acid is easily desorbed even during drying, low temperature or short time baking because it is mixed with easily desorbed alkylamine.
- the polymer dispersant since the polymer dispersant has the specific amine value and acid value, the adsorption is not too strong, and the polymer dispersant is uniformly disposed around the copper nanoparticles, so that the low temperature is low in the subsequent firing step. It is presumed that a metal thin film having excellent electrical conductivity can be obtained by fusion of copper nanoparticles arranged at high density, which is easily desorbed or decomposed even after baking for a short time. When the volume average particle size of the dispersion exceeds 500 nm in the system as in the present application, the copper nanoparticles cannot be arranged at high density, so that they are non-uniformly baked and can adhere stably to the copper nanoparticles.
- the alkylamine has a function of scavenging protons in the amino group, and therefore adheres to the surface of the copper nanoparticles during production, thereby suppressing oxidation of copper atoms during production and in the dispersion.
- the present invention has an effect of suppressing the oxidation of copper nanoparticles in the present invention because the copper nanoparticles are closely surrounded with a carboxylic acid that can be strongly adsorbed on the surface of the copper nanoparticles, and further a polymer dispersant is attached. Is estimated to be high. From these, it is presumed that sintering inhibition due to oxidation during firing hardly occurs, and a film having high conductivity can be formed after firing.
- the copper nanoparticle dispersion of the present invention may contain other components in addition to the above essential components as long as the effects of the present invention are not impaired.
- each configuration of the copper nanoparticle dispersion will be described in detail in order.
- the copper nanoparticles are typically metallic copper particles.
- copper is a metal that is very easily oxidized
- the surface of the metallic copper nanoparticles is partially oxidized into an oxide. It may be included.
- the copper nanoparticles mean particles having a diameter of the order of nm (nanometer), that is, less than 1 ⁇ m. In the present invention, by using such copper nanoparticles, sintering at a low temperature is easy to proceed, and the printability of fine wiring is improved.
- the copper nanoparticles used in the present invention are preferably particles having an average primary particle size of 1 nm to 100 nm, and more preferably 10 nm to 100 nm, from the viewpoint of achieving both dispersibility, applicability, low temperature firing, and conductivity. Of these particles, it is preferable.
- the average primary particle diameter of the said copper nanoparticle can be calculated
- TEM transmission electron micrograph
- the method for preparing the copper nanoparticles may be appropriately selected from conventionally known methods. For example, a physical method of pulverizing metal powder by mechanochemical method, etc .; chemical dry method such as chemical vapor deposition method (CVD method), vapor deposition method, sputtering method, thermal plasma method, laser method; thermal decomposition method
- CVD method chemical vapor deposition method
- vapor deposition method vapor deposition method
- sputtering method thermal plasma method
- laser method thermal decomposition method
- the copper nanoparticles can be obtained using a chemical wet method such as a chemical reduction method, an electrolysis method, an ultrasonic method, a laser ablation method, a supercritical fluid method, or a microwave synthesis method.
- fine particles are produced by bringing a vapor of a metal heated and brought into contact with a low vapor pressure liquid containing a dispersant under a high vacuum.
- a chemical reduction method there is a method in which a copper-containing compound and a reducing agent are mixed in a solvent in the presence of a complexing agent and an organic protective agent.
- commercially available copper nanoparticles can be used as appropriate.
- copper nanoparticles are coated with carboxylic acid and alkylamine, among them, as will be described in detail later, using carboxylic acid and alkylamine as an organic protective agent, a copper-containing compound and a reducing agent, A method of mixing copper in a solvent to produce copper nanoparticles is preferably used.
- the content of the copper nanoparticles may be appropriately selected according to the use, but from the viewpoint of dispersibility, 0.01% with respect to the total amount of the copper nanoparticle dispersion.
- the content is preferably from 90 to 90% by mass, and more preferably from 0.1 to 85% by mass.
- the carboxylic acid used in the present invention is a compound that can be bonded to copper by an oxygen atom as a ligand. Therefore, in the copper nanoparticle dispersion, the carboxylic acid contributing to the dispersion usually exists in a state of being bonded to copper by at least one oxygen atom.
- carboxylic acid examples include saturated aliphatic monocarboxylic acids such as acetic acid, propanoic acid, butanoic acid, pentanoic acid, hexanoic acid, heptanoic acid, octanoic acid, nonanoic acid, decanoic acid, dodecanoic acid, and hexadecanoic acid; oleic acid, Unsaturated aliphatic monocarboxylic acids such as linoleic acid; Aliphatic polycarboxylic acids such as oxalic acid, malonic acid, and succinic acid; Aliphatic unsaturated polycarboxylic acids such as maleic acid; Aromatic monocarboxylic acids such as benzoic acid Examples thereof include, but are not limited to, acids; aromatic polycarboxylic acids such as phthalic acid; and hydroxycarboxylic acids such as citric acid.
- saturated aliphatic monocarboxylic acids such as acetic acid, propanoic acid,
- a carboxylic acid having 10 or less carbon atoms is preferable from the viewpoint that the low-temperature baking property is improved and the conductivity is improved.
- carboxylic acids having 2 or more carbon atoms are used from the viewpoint of dispersibility.
- an aliphatic carboxylic acid having 10 or less carbon atoms is preferable from the viewpoint of improving dispersibility and low-temperature calcination properties and improving conductivity.
- the aliphatic carboxylic acid may be either saturated or unsaturated.
- carboxylic acid used in the present invention one carboxylic acid may be used, or two or more carboxylic acids may be mixed and used.
- the carboxylic acid used in the present invention is preferably a carboxylic acid having one or two carboxyl groups in the molecule, since the polarity is relatively weak and easily desorbed at the time of firing. It is preferable to use a carboxylic acid having a group.
- the carboxylic acid used in the present invention is preferably not too high in molecular weight, and preferably has a molecular weight of 300 or less, more preferably 200 or less, from the viewpoint that it easily desorbs during firing. Moreover, it is preferable that a boiling point is 400 degrees C or less, Furthermore, it is preferable that it is 300 degrees C or less.
- the molecular weight of the carboxylic acid is preferably 50 or more from the viewpoint of desorption during storage of nanoparticles and storage and prevention of volatilization. Moreover, it is preferable that a boiling point is 50 degreeC or more.
- the content of the carboxylic acid may be appropriately selected according to the use, but from the viewpoint of low-temperature calcinability, 0.1 to 30 masses relative to 100 mass parts of copper. Part, more preferably in the range of 0.1 to 20 parts by weight.
- the content of the carboxylic acid may be appropriately selected according to the use, but from the viewpoint of low-temperature calcinability, 0.05% with respect to the total amount of the copper nanoparticle dispersion. It is preferably ⁇ 15% by mass, and more preferably in the range of 0.05 to 10% by mass.
- the alkylamine used in the present invention can be appropriately selected from known alkylamines according to properties expected for the produced copper nanoparticle dispersion. It is presumed that the alkylamine has a function of capturing protons, thereby preventing the copper atom from being oxidized.
- Alkylamine has a structure in which an amino group is bonded to a part of an alkyl group.
- RNH 2 R is a hydrocarbon chain
- R 1 is a secondary amino group
- R 2 NH R 1 and R 2 may be the same or different in the hydrocarbon chain
- the alkyl chain may be linear, branched or cyclic.
- the hydrocarbon chain may contain atoms other than carbon such as oxygen, silicon, nitrogen, sulfur, and phosphorus.
- it may be an alkylamine having a substituent such as an alkoxy group, an alkoxysilyl group, or an alkylthio group, or may have two amino groups in the molecule.
- alkylamine (monoamine) having one amino group in the molecule examples include 2-ethoxyethylamine, dipropylamine, dibutylamine, hexylamine, cyclohexylamine, heptylamine, 3-methoxypropylamine, and 3-ethoxypropyl.
- Alkylamines such as amine, 3-butoxypropylamine, octylamine, nonylamine, decylamine, 3-aminopropyltriethoxysilane, dodecylamine, hexadecylamine, octadecylamine and oleylamine are industrially produced and easily available. It is practical.
- examples of the alkyldiamine having two amino groups in the molecule include ethylenediamine, N, N-dimethylethylenediamine, N, N′-dimethylethylenediamine, N, N-diethylethylenediamine, N, N′-diethylethylenediamine, 1 , 3-propanediamine, 2,2-dimethyl-1,3-propanediamine, N, N-dimethyl-1,3-diaminopropane, N, N′-dimethyl-1,3-diaminopropane, N, N— Diethyl-1,3-diaminopropane, 1,4-diaminobutane, 1,5-diamino-2-methylpentane, 1,6-diaminohexane, N, N′-dimethyl-1,6-diaminohexane, 1, 7-diaminoheptane, 1,8-diaminooctane,
- alkylamine used in the present invention one type of alkylamine may be used, or two or more types of alkylamine may be mixed and used.
- the alkylamine used in the present invention is preferably an alkylamine having one or two amino groups in the molecule from the viewpoint that the polarity is relatively weak and that it is easily eliminated during firing.
- the alkylamine used in the present invention is preferably not too high in molecular weight, and preferably has a molecular weight of 300 or less, more preferably 200 or less, from the viewpoint of easy desorption during firing.
- the alkylamine used in the present invention preferably has 8 or less carbon atoms, and more preferably 6 or less, from the viewpoint that it is easily detached during firing.
- the alkylamine used in the present invention preferably has a boiling point of 300 ° C. or lower, more preferably 200 ° C. or lower, from the viewpoint of easy desorption during firing.
- the molecular weight of the alkylamine is preferably 50 or more from the viewpoints of desorption during storage and storage and prevention of volatilization.
- the boiling point is preferably 23 ° C. or higher, and more preferably 50 ° C. or higher.
- the content of the alkylamine may be appropriately selected depending on the use, but from the viewpoint of low-temperature calcinability, 0.1 to 30 masses with respect to 100 mass parts of copper. Part, more preferably in the range of 0.1 to 20 parts by weight.
- the content of the alkylamine may be appropriately selected according to the use, but from the viewpoint of oxidation resistance and low-temperature sinterability, it is based on the total amount of the copper nanoparticle dispersion. 0.05 to 15% by mass, more preferably 0.05 to 10% by mass.
- the polymer dispersant used in the present invention is a polymer dispersant in which one of the amine value and the acid value is 30 to 160 mgKOH / g, and the other one of the amine value and the acid value is 0 to 160 mgKOH / g, Having at least one of a functional functional group and an acidic functional group.
- a functional functional group and an acidic functional group include primary, secondary, or tertiary amino groups, nitrogen-containing heterocycles such as pyridine, pyrimidine, and pyrazine.
- acidic functional groups include carboxylic acid groups, phosphoric acid groups, and sulfonic acid groups.
- the amine value indicates the total amount of free base and base, and is expressed in mg of potassium hydroxide equivalent to the hydrochloric acid required to neutralize 1 g of the sample.
- the acid value represents the total amount of free acid and acid, and is expressed in mg of potassium hydroxide required to neutralize 1 g of the sample.
- the amine value can be measured by a method according to JIS-K7237, and the acid value can be measured by a method according to JIS-K0070.
- the polymer dispersant used in the present invention has the above-mentioned specific amine value and acid value with respect to copper nanoparticles to which both carboxylic acid and alkylamine are attached, and an appropriate amount of basic functional group or acidity. Since it has a functional group, it is presumed that it adsorbs stably to the copper nanoparticles, while causing a steric hindrance by the polymer chain portion and stably preventing aggregation of the copper nanoparticles. As shown in the examples and comparative examples described later, for the copper nanoparticles to which both carboxylic acid and alkylamine are attached, the amine value and acid value of the polymer dispersant are too low or too high.
- the polymer dispersant cannot be stably attached, but rather the dispersibility deteriorates.
- the polymer dispersant when combined with a carboxylic acid having a low dispersibility but excellent low-temperature calcinability and having a small number of carbon atoms, it is clear that the polymer dispersant cannot be dispersed if the amine value and acid value are too low or too high. It was made.
- the polymer dispersant having the specific amine value and acid value is stably adsorbed to the copper nanoparticles, whereby dispersibility and dispersion stability are improved.
- the diameter can be reduced. Therefore, when the above polymer dispersant is used, the smoothness and uniformity of the coating film of the copper nanoparticle dispersion are excellent, and the copper nanoparticles in the coating film are arranged at high density. Therefore, sintering is easy to proceed uniformly, and the copper nanoparticles are easily fused.
- the polymer dispersant is easily decomposed or volatilized by baking due to a synergistic effect with the alkylamine, and the resulting conductive substrate suppresses the remaining organic components. As a result, the obtained metal film is presumed to be excellent in conductivity.
- the polymer dispersant used in the present invention has an amine value and an acid value of 30 to 160 mgKOH / g and the other one of the amine value and the acid value is 0 to 160 mgKOH / g for the reasons described above.
- One of the amine value and the acid value is preferably 40 to 140 mgKOH / g, and the other one of the amine value and the acid value is preferably 0 to 140 mgKOH / g.
- the polymer dispersant used in the present invention preferably has a weight average molecular weight of 800 or more, more preferably 900 or more, particularly 1000 or more, from the viewpoint of excellent dispersibility and coating suitability. Is preferred. On the other hand, from the viewpoint of excellent low-temperature calcinability, it is preferably 30000 or less, more preferably 20000 or less, and particularly preferably 10,000 or less.
- the weight average molecular weight in this invention can be measured by the gel permeation chromatography (GPC) method (polystyrene conversion).
- the polymer dispersant used in the present invention has a 90% thermogravimetric decrease temperature of 450 ° C. or lower, and further 420 ° C. or lower, so that the low-temperature firing property and the conductivity of the coated film after sintering are reduced. It is preferable from an excellent point.
- the 90% thermogravimetric decrease temperature is a value measured by thermogravimetry (TG) as follows. Using a thermogravimetry apparatus (for example, DTG-60A manufactured by Shimadzu Corporation), about 5 mg of a sample is measured in a nitrogen atmosphere. The temperature rising rate is 10 ° C./min, and the temperature is measured from room temperature (23 ° C.) to 600 ° C. In the present invention, the temperature at which 90% is reduced based on the sample weight at room temperature is defined as the 90% thermogravimetric decrease temperature.
- the polymer dispersant having one of an amine value and an acid value of 30 to 160 mg KOH / g and the other one of an amine value and an acid value used in the present invention is usually from 0 to 160 mg KOH / g. It can be appropriately selected from polymer dispersants used for dispersing colorants.
- polymer dispersant examples include (co) polymers of unsaturated carboxylic acid esters such as polyacrylic acid esters; (partial) amine salts of (co) polymers of unsaturated carboxylic acid such as polyacrylic acid , (Partial) ammonium salts and (partial) alkylamine salts; hydroxyl group-containing unsaturated carboxylic acid ester (co) polymers such as hydroxyl group-containing polyacrylates and their modified products; polyurethanes; unsaturated polyamides; Long chain polyaminoamide phosphates; Polyethyleneimine derivatives (amides and their bases obtained by reaction of poly (lower alkyleneimines) with free carboxyl group-containing polyesters); Polyallylamine derivatives (polyallylamine and free radicals) Polyester, polyamide or ester and amide having carboxyl group Compound reaction product obtained by the reaction of one or more compounds selected from the three compounds of (polyester amide)), and the like.
- the polymer dispersant used in the present invention preferably has a polyester skeleton or a polyether skeleton in at least one of the main chain and the side chain.
- a polymer dispersant is excellent in conductivity of the fired film because it is easily decomposed by baking at a low temperature due to its skeletal structure, and organic matter hardly remains.
- one type of polymer dispersant may be used, or two or more types may be used in combination, and the content depends on the type of copper nanoparticles used.
- the copper nanoparticles 100 parts by weight it is usually in the range of 0.1 to 100 parts by weight, preferably 1 to 50 parts by weight, and 2 to 30 parts by weight. It is more preferable.
- the content of the polymer dispersant may be appropriately selected according to the use, but from the viewpoint of dispersibility, coating suitability, and low-temperature firing properties, the copper nanoparticle dispersion
- the amount is preferably from 0.05 to 25% by mass, more preferably from 0.5 to 15% by mass, based on the total amount. If content of the said polymer dispersing agent is more than the said lower limit, the dispersibility and dispersion stability of a copper nanoparticle dispersion can be made excellent. Moreover, if it is below the said upper limit, it is excellent in the electroconductivity of the film
- the solvent is not particularly limited as long as it is an organic solvent that does not react with each component in the copper nanoparticle dispersion and can dissolve or disperse them.
- An organic solvent conventionally used for the copper nanoparticle dispersion may be appropriately selected and used.
- the solvent used in the present invention MBA (3-methoxybutyl acetate), PGMEA (propylene glycol monomethyl ether acetate), DMDG (diethylene glycol dimethyl ether), diethylene glycol methyl ethyl ether, PGME (propylene glycol monomethyl ether) or these are used. What mixed is preferable from the point of the solubility of the said polymer dispersing agent, and the applicability
- the content of the solvent in the copper nanoparticle dispersion of the present invention is not particularly limited as long as it can uniformly dissolve or disperse each component of the copper nanoparticle dispersion.
- the solid content in the copper nanoparticle dispersion is preferably in the range of 5 to 95% by mass, more preferably in the range of 10 to 90% by mass. By being the said range, it can be set as the viscosity suitable for application
- the copper nanoparticle dispersion of the present invention may appropriately contain other known components conventionally used in copper nanoparticle dispersions as needed, as long as the effects of the present invention are not impaired.
- Other components include, for example, complexing agents, organic protective agents, reducing agents, surfactants for improving wettability, silane coupling agents for improving adhesion, antifoaming agents, repellency inhibitors, and antioxidants. Agents, anti-aggregation agents, viscosity modifiers, and the like.
- the other dispersing agent may be contained.
- a resin binder such as an acrylic resin, a polyester resin, a cellulose resin, and an olefin resin may be added from the viewpoints of film forming property, printability, and dispersibility within a range that does not impair the effects of the present invention.
- the copper nanoparticle dispersion of the present invention has a volume average particle size of 500 nm or less by dynamic light scattering method, and preferably 450 nm or less. Since the copper nanoparticle dispersion of the present invention has such a small dispersed particle size, excellent smoothness and uniformity of the coating film and high density arrangement of the copper nanoparticles in the coating film are realized. To do.
- the volume average particle diameter by the dynamic light scattering method in the copper nanoparticle dispersion is a dispersion average particle diameter of copper nanoparticles dispersed in a dispersion medium containing at least a solvent, and is a laser light scattering particle size distribution analyzer. It is measured by.
- the copper nano particle dispersion is appropriately diluted to a concentration that can be measured with a laser light scattering particle size distribution meter with a solvent used in the copper nano particle dispersion (for example, And can be measured at 23 ° C. by a dynamic light scattering method using a laser light scattering particle size distribution analyzer (for example, Nikkiso Nanotrac particle size distribution analyzer UPA-EX150).
- a laser light scattering particle size distribution analyzer for example, Nikkiso Nanotrac particle size distribution analyzer UPA-EX150.
- the manufacturing method of a copper nanoparticle dispersion should just be a method in which a copper nanoparticle can disperse
- a copper nanoparticle can disperse distribute favorably
- the copper nanoparticles are dispersed in the solvent with the above-described polymer dispersant by a conventionally known method.
- copper nanoparticles produced using carboxylic acid and alkylamine as a protective agent during production may be used, or other protective agents may be used.
- the copper nanoparticle protective agent produced in this manner may be substituted with a carboxylic acid or an alkylamine by a known method.
- the method for producing a copper nanoparticle dispersion includes a compound containing copper, a reducing compound, a mixture containing a carboxylic acid and an alkylamine, or a copper carboxylate, a reducing compound and an alkylamine.
- the compound containing copper, a reducing compound, a carboxylic acid, and a mixture containing an alkylamine or a mixture containing copper carboxylate, a reducing compound, and an alkylamine is the mixture at the time of heating. good.
- a compound containing copper (hereinafter sometimes referred to as a copper-containing compound) is a copper-containing compound capable of forming a complex compound such as a complex with a reducing compound. Used as a metal source for particles.
- the copper-containing compound include copper hydroxide, copper oxalate, copper acetate, copper propionate, copper butyrate, copper isobutyrate, copper valerate, copper isovalerate, copper caproate, copper enanthate, and caprylic acid.
- Examples include organic acid salts and inorganic acid salts of copper such as copper phosphate, and complex compounds represented by acetylacetonato copper coordinated with acetylacetone.
- the copper-containing compound other than copper carboxylate and carboxylic acid be used as a carboxylate copper such as fatty acid copper. This is because copper carboxylate serves as a carboxylic acid source to be coated on the copper nanoparticles and easily forms a complex compound such as a complex with the reducing compound.
- a reducing compound having a reducing action is mixed with the copper carboxylate to produce a composite compound such as a complex of copper and the reducing compound.
- the composite compound may be generated using a mixture of the copper-containing compound other than copper carboxylate, the reducing compound, and the carboxylic acid.
- the reducing compound used in this case for example, the reducing compound described in JP2012-72418A can be appropriately selected and used.
- reducing compounds having an amino group such as hydrazine, hydrazine hydrate, hydroxylamine, and derivatives thereof are preferably used.
- a reduction reaction occurs directly when copper carboxylate and a reducing compound are mixed, it is desirable to suppress the reduction reaction by mixing in a cooled environment.
- a compound containing copper such as fatty acid copper and a reducing compound are preferably mixed by cooling to 30 ° C. or lower, more preferably 25 ° C. or lower, and most preferably 20 ° C. or lower.
- a reducing compound having a reducing action with copper carboxylate to form a complex compound such as a complex of copper and a reducing compound.
- the mixture of the copper carboxylate and the reducing compound produced above is mixed with a sufficient amount of alkylamine and heated to spontaneously decompose the copper carboxylate. It is preferable to obtain copper nanoparticles by forming and aggregating copper atoms. At this time, since the surface of the copper nanoparticles is coated with a carboxylic acid and an alkylamine, stable coated copper nanoparticles that are hardly oxidized by oxidation in the air can be obtained. By adjusting the molecular weight of carboxylic acid or alkylamine, it is possible to adjust the primary particle size of the produced copper nanoparticles to a desired size.
- the mixing ratio of the alkylamine to the mixture of the copper carboxylate and the reducing compound may be appropriately selected according to the use, but from the viewpoint of oxidation resistance and low-temperature calcination, it is based on 1 mol of the copper carboxylate.
- the amount is preferably 1 to 10 moles, more preferably 2 to 6 moles.
- the second step of heating a complex compound such as alkylamine in the presence of an alkylamine to produce copper nanoparticles can be performed simultaneously or sequentially in one container.
- copper nanoparticles can be produced by heating.
- the heating temperature in the presence of alkylamine is preferably 60 ° C to 150 ° C.
- the first step is performed by cooling to about 30 ° C. or less, and the second step It is preferable that the process is performed by heating to 60 ° C. to 150 ° C.
- a copper nanoparticle dispersion having a volume average particle size of 500 nm or less by dynamic light scattering method is prepared by dispersing the copper nanoparticles obtained in the preparation step with the specific polymer dispersant in a solvent. To do. For example, after the specific polymer dispersant is mixed and stirred in the solvent to prepare a polymer dispersant solution, the polymer dispersant solution is mixed with the copper nanoparticles obtained in the preparation step, as necessary. Accordingly, a copper nanoparticle dispersion can be prepared by mixing other components and dispersing them using a known stirrer or disperser.
- the copper nanoparticle dispersion obtained in the present invention is preferably used for a conductive substrate described later, and particularly preferably for conductive pattern printing. Since the copper nanoparticle dispersion of the present invention can be fired at a low temperature and in a short time, it is suitably used for low-temperature or short-time firing applications such as plasma firing and flash light firing described later.
- the copper nanoparticle dispersion obtained in the present invention can be further applied to a seed layer for plating and various metal films, and can be used, for example, for mirror surfaces for optical devices and various decoration applications.
- the method for producing a conductive substrate according to the first aspect of the present invention comprises a copper nanoparticle, a carboxylic acid, an alkylamine, and one of an amine value and an acid value of 30 to 160 mgKOH / g, an amine value and an acid value.
- a copper nanoparticle dispersion containing a polymer dispersant of 0 to 160 mgKOH / g and a solvent, and having a volume average particle diameter of 500 nm or less by dynamic light scattering method is applied onto a substrate. And a step of forming a coating film and a step of baking the coating film.
- substrate of the 2nd aspect which concerns on this invention is a compound containing copper, a reducing compound, carboxylic acid, and the mixture containing an alkylamine, or copper carboxylate, a reducing compound, and alkyl.
- a conductive substrate of the present invention According to the method for producing a conductive substrate of the present invention, as described above, copper nanoparticles having a small dispersed particle diameter, excellent in oxidation resistance, dispersibility, applicability, and sintering property at low temperature or in a short time. Since the dispersion is used, it is possible to form a smooth and highly uniform coating film in which copper nanoparticles whose oxidation is suppressed are present uniformly and at a high density. As a result, a conductive substrate having good pattern accuracy and excellent conductivity after sintering can be obtained.
- FIG. 1 is a schematic view showing an example of a conductive substrate obtained by the production method of the present invention.
- a conductive substrate 100 shown in FIG. 1 includes a metal film 2 formed on one surface of a base material 1 by baking a coating film of a copper nanoparticle dispersion.
- FIG. 2 is a schematic view showing another example of a conductive substrate obtained by the production method of the present invention.
- a conductive substrate 101 shown in FIG. 2 is provided with a patterned metal film 3 on both surfaces of a base material 1 by baking a coating film of a copper nanoparticle dispersion.
- the conductive substrate obtained by the production method of the present invention may be provided with a metal film obtained by firing a coating film of a copper nanoparticle dispersion only on one surface of a base material.
- a metal film obtained by firing a coating film of a copper nanoparticle dispersion on both surfaces of a base material may be used.
- the metal film provided on only one surface of the base material and the metal film provided on both surfaces of the base material are each a solid metal film having no pattern even if it is a patterned metal film. There may be.
- the metal film provided on both surfaces of the base material may be a metal film having one surface that is a patterned metal film and the other surface that is a solid film.
- the metal film provided on both surfaces of the substrate is a patterned metal film on both surfaces
- the patterns on both surfaces may be the same or different.
- the patterned metal films provided on both surfaces of the substrate have the same pattern, the patterns on both surfaces may be at the same position or at different positions on both surfaces.
- a step of preparing a copper nanoparticle dispersion, and a compound containing copper in the method for producing a conductive substrate according to the second aspect of the present invention A step of preparing copper nanoparticles by heating either a mixture containing a reducing compound, a carboxylic acid and an alkylamine, or a mixture containing copper carboxylate, a reducing compound and an alkylamine; A step of preparing a copper nanoparticle dispersion having a volume average particle diameter of 500 nm or less by a dynamic light scattering method by dispersing particles in a solvent with a polymer dispersant having the specific amine value and acid value.
- distribution on a base material, and forming a coating film> (Base material)
- the base material used for this invention suitably from the base materials used for an electroconductive board
- inorganic materials such as glass, alumina, silica, and SUS foil can be used, and polymer materials and paper can also be used. Since the metal fine particle dispersion for conductive substrate according to the present invention can obtain a metal film having excellent conductivity even when fired at a lower temperature than before, soda lime glass, which has been difficult to apply conventionally, Even molecular materials can be suitably used, and are particularly useful in that a resin film can be used.
- the resin film examples include polyimide, polyamide, polyamideimide, polyethylene terephthalate (PET), polyethylene naphthalate (PEN), polyphenylene sulfide, polyether ether ketone, polyether sulfone, polycarbonate, polyether imide, epoxy resin, and phenol resin. Glass-epoxy resins, polyphenylene ethers, acrylic resins, polyolefins such as polyethylene and polypropylene, polycycloolefins such as polynorbornene, and liquid crystalline polymer compounds.
- a resin film having a glass transition temperature of 100 ° C. or less, such as PET can be used.
- the glass transition temperature is determined by differential scanning calorimetry (DSC) measurement measured according to JIS-K7121.
- the treatment method for the substrate surface can be appropriately selected from conventionally known methods. Specifically, for example, corona treatment, UV treatment, vacuum ultraviolet lamp treatment, dry treatment such as plasma treatment, amine silane coupling agent, imidazole silane coupling agent, titanium coupling agent, aluminum coupling agent treatment, etc. Chemical layer treatment, porous silica, porous membrane formation treatment such as cellulose-based receiving layer, active energy ray curable resin layer, thermosetting resin layer, thermoplastic resin layer and other resin layer formation treatment .
- the shape of the substrate may be appropriately selected depending on the application, and may be flat or curved, but is usually flat.
- the thickness of the substrate may be appropriately set according to the application, and may be, for example, about 10 ⁇ m to 1 mm.
- the method for applying the copper nanoparticle dispersion onto the substrate may be appropriately selected from conventionally known application or printing methods. Among these, gravure printing, gravure offset printing, reverse offset printing, flexographic printing, screen printing, and ink jet printing are preferable because fine patterning can be performed when printing a conductive pattern.
- the coating method includes a case where the entire surface is coated. In the case of whole surface application, a pattern can be formed by a chemical etching method as described later on a copper nanoparticle sintered film obtained by baking the coating film.
- the copper nanoparticle dispersion on the substrate may be dried by a usual method after coating.
- the film thickness of the coated film after drying can be controlled by appropriately changing the coating amount, the average primary particle diameter of the copper nanoparticles, etc., and may be appropriately adjusted according to the application, but is usually 0.
- the range is from 0.01 to 50 ⁇ m, and preferably from 0.1 to 20 ⁇ m.
- This step is a step of forming a metal film by baking the coating film obtained in the above step to form a sintered film.
- the firing method can be appropriately selected from conventionally known firing methods. Specific examples of the firing method include, for example, methods such as heating by a firing furnace (oven), infrared heating, various laser annealing, ultraviolet light, visible light, light irradiation firing with flash light, microwave heating, and the like. In addition, it is preferably performed in an inert gas atmosphere or a reducing gas atmosphere. In the case of an air atmosphere, it is preferable that heating is performed instantaneously in order to prevent oxidation during firing. Since the copper nanoparticle dispersion of the present invention can be fired at a low temperature or in a short time, it can be fired at a lower temperature than the conventional method.
- the firing step is plasma firing, in particular, firing by surface wave plasma generated by application of microwave energy, or firing by flash light irradiation (hereinafter referred to as flash light firing). It is preferable that any one of the above.
- flash light firing firing by flash light irradiation
- thermal damage to the substrate can be reduced, and oxidation of the metal during firing can be suppressed.
- it is baking for a short time there also exists a merit that productivity is high.
- microwave firing Firing using microwave surface wave plasma is preferably performed in an inert gas atmosphere or a reducing gas atmosphere from the viewpoint of the conductivity of the obtained sintered film.
- the microwave surface wave plasma is preferably generated in a reducing gas atmosphere, and more preferably generated in a hydrogen gas atmosphere.
- the baking may be performed at a temperature of about 50 to 200 ° C. for about 1 minute to 2 hours. This treatment may be performed under reduced pressure. By this firing, organic substances are oxidatively decomposed and removed, and the sintering of the copper nanoparticles is promoted in the microwave surface wave plasma treatment.
- the method for generating the microwave surface wave plasma may be appropriately selected from conventionally known methods.
- the method described in International Publication No. 2011/040189 pamphlet can be used.
- Flash light firing is a method of firing in an extremely short time by irradiation with flash light.
- flash light refers to light having a relatively short lighting time, and the lighting time is referred to as a pulse width.
- the light source of the flash light is not particularly limited, and examples thereof include a flash lamp and a laser in which a rare gas such as xenon is sealed. Among them, it is preferable to irradiate light having a continuous wavelength spectrum from ultraviolet to infrared, and specifically, it is preferable to use a xenon flash lamp. When such a light source is used, the same effect as when UV irradiation is performed simultaneously with heating can be obtained, and baking can be performed in an extremely short time.
- the polymer dispersant used in the present invention is uniformly present around the copper nanoparticles due to a synergistic effect with the alkylamine and carboxylic acid, and therefore easily decomposes or volatilizes by irradiation with flash light. Since it is easy and hardly remains on the metal film, it can be easily sintered even by irradiation with flash light for a very short time. Therefore, flash light baking is preferably used in the present invention.
- the pulse width of the flash light may be appropriately adjusted, but is preferably set between 1 ⁇ s and 10,000 ⁇ s, and more preferably within the range of 10 ⁇ s to 5000 ⁇ s.
- the irradiation energy per one flash light is preferably 0.1J / cm 2 ⁇ 100J / cm 2, 0.5J / cm 2 ⁇ 50J / cm 2 is more preferable.
- the number of times of flash light irradiation may be appropriately adjusted according to the composition, film thickness, area, etc. of the coating film, and the number of times of irradiation may be only once or may be repeated two or more times. Good.
- the number of irradiation is preferably 1 to 100 times, and more preferably 1 to 50 times.
- the irradiation interval of the flash light may be adjusted as appropriate.
- the irradiation interval is preferably set within a range of 10 ⁇ sec to 2 seconds, and more preferably set within a range of 100 ⁇ sec to 1 second.
- the flash light As described above, it is possible to suppress the influence on the base material and to suppress the oxidation of the copper nanoparticles, and the alkylamine and carboxylic acid contained in the copper nanoparticle dispersion
- the above-described polymer dispersant can be easily detached or decomposed to obtain a conductive substrate having excellent conductivity.
- Such flash light baking can heat only the coating film of the copper nanoparticle dispersion and its vicinity, and can burn the coating film at a low temperature and in a short time.
- a nanoparticle sintered film can be formed.
- the heating temperature and the processing depth can be controlled by appropriately adjusting the pulse width and irradiation energy of the flash light. As a result, a non-uniform film is rarely formed and grain growth can be prevented, so that a very dense and smooth film can be obtained.
- baking is possible in a very short time, the oxidation of copper nanoparticles can be suppressed, and a sintered film having excellent conductivity can be obtained.
- the flash light baking can be performed in the atmosphere at atmospheric pressure, but may be performed in an inert gas atmosphere, a reducing gas atmosphere, or a reduced pressure. Moreover, you may perform flash light baking, heating a coating film.
- the thickness of the metal film of the conductive substrate thus obtained may be adjusted as appropriate according to the application, but the thickness is usually about 0.01 to 50 ⁇ m, and 0.05 nm to 30 ⁇ m. Preferably, the thickness is 0.1 to 20 ⁇ m.
- the volume resistivity of the metal film is preferably 1.0 ⁇ 10 ⁇ 4 ⁇ ⁇ cm or less.
- the sintered film obtained using the copper nanoparticle dispersion of the present invention has a smooth surface and low resistance, and is less likely to generate voids at the interface with the substrate, and has good adhesion. And it has a moderate space
- the sintered film obtained by using the copper nanoparticle dispersion of the present invention can also form fine wiring by further chemically etching after forming the sintered film by the entire surface coating as described above. Is suitable.
- Pattern formation by chemical etching is performed by applying a photoresist or laminating a dry film resist to a sintered film formed on the entire surface to form a photoresist layer, and then exposing and developing by a photolithography method using a photomask. After the pattern is formed, etching with ferric chloride, cupric chloride, phosphorous nitric acid or the like is performed, and the remaining resist is peeled off to form a patterned metal film.
- the manufacturing method of the present invention is a pattern in which a copper nanoparticle dispersion is applied in a pattern on a substrate to form a coating film, and the coating film is baked to form a patterned metal film. It may be a method for manufacturing a conductive substrate.
- the conductive substrate obtained by the method for producing a conductive substrate of the present invention has good pattern accuracy and excellent conductivity.
- An electronic member using such a conductive substrate can be effectively used for an electromagnetic wave shielding film having a low surface resistance, a conductive film, a flexible printed wiring board, and the like.
- Example 1 (1) Synthesis of copper nanoparticles In a 200 ml three-necked flask, 10.0 g of copper hydroxide (0.1 mol, manufactured by Wako Pure Chemical Industries), 31.5 g of nonanoic acid (0.2 mol, manufactured by Tokyo Chemical Industry, boiling point) 254 ° C.) and 18.5 g (20 ml, manufactured by Kanto Chemical) of propylene glycol monomethyl ether (PGME). The mixture was heated to 100 ° C. with stirring and the temperature was maintained for 20 minutes.
- copper hydroxide 0.1 mol, manufactured by Wako Pure Chemical Industries
- nonanoic acid 0.2 mol, manufactured by Tokyo Chemical Industry, boiling point 254 ° C.
- PGME propylene glycol monomethyl ether
- the average primary particle size was 39 nm.
- the obtained copper nanoparticles were dispersed in toluene, and this was dropped onto a TEM substrate (manufactured by Hitachi High-Tech Fielding Co., Ltd., Cu grid with elastic carbon support film) and dried to prepare a sample for observation.
- Particle images were measured with TEM (Hitachi High-Technology H-7650), and the average value of the length of the longest portion of 100 randomly selected primary particles was defined as the average primary particle size.
- the main structure of the copper nanoparticle was Cu (Cubic), and a part of Cu 2 O (Cubic) structure was observed, and Cu (111)
- the peak intensity of Cu 2 O (111) relative to was about 3%.
- the copper nanoparticle dispersion 1 is applied to a PET film (Cosmo Shine A4100) having a thickness of 100 ⁇ m with a wire bar and dried to form a coating film having a thickness of 0.5 ⁇ m. It was. Thereafter, while introducing hydrogen gas at an introduction pressure of 20 Pa, using a microwave surface wave plasma processing apparatus (MSP-1500, manufactured by Microelectronics), firing was performed at a microwave output of 450 W for 300 seconds to obtain a conductive substrate.
- MSP-1500 microwave surface wave plasma processing apparatus
- the copper nanoparticle dispersion 1 is applied to a PET film (Cosmo Shine A4100) having a thickness of 100 ⁇ m with a wire bar and dried to obtain a coating having a thickness of 0.5 ⁇ m. A membrane was obtained. Thereafter, using a pulsed xenon lamp device (SINTERON 2000 (manufactured by Xenon Corporation)), irradiation was performed once with a pulse width of 500 ⁇ sec and an applied voltage of 3.7 kV to obtain a conductive substrate.
- a pulsed xenon lamp device SINTERON 2000 (manufactured by Xenon Corporation)
- Example 2 In Example 1, (2), instead of Solsperse 41000, Solsperse 53095 (manufactured by Nippon Lubrizol, acid value 47 mgKOH / g, amine value 0 mgKOH / g, weight average molecular weight 3900, 90% thermogravimetric decrease temperature is 360.7. C.) was used in the same manner as (2) of Example 1 except that the copper nanoparticle dispersion 2 was obtained. Using the obtained copper nanoparticle dispersion 2, a conductive substrate was produced by plasma firing in the same manner as (3) of Example 1. Using the obtained copper nanoparticle dispersion 2, a conductive substrate was produced by flash light baking in the same manner as in (4) of Example 1.
- Example 3 In Example 1 (2), instead of Solsperse 41000, Solsperse 71000 (manufactured by Nippon Lubrizol, acid value 0 mgKOH / g, amine value 77.4 mgKOH / g, weight average molecular weight 4700, 90% thermogravimetric reduction temperature is 405. .9 ° C) was used in the same manner as (2) of Example 1 to obtain a copper nanoparticle dispersion 3. Using the obtained copper nanoparticle dispersion 3, a conductive substrate was produced by plasma firing in the same manner as (3) of Example 1. Using the obtained copper nanoparticle dispersion 3, a conductive substrate was produced by flash light baking in the same manner as in (4) of Example 1.
- Example 4 In Example 1, (2), instead of Solsperse 41000, Disper byk-111 (manufactured by Big Chemie Japan, acid value 129 mgKOH / g, amine value 0 mgKOH / g, weight average molecular weight 1700, 90% thermogravimetric decrease temperature is 320. .4 ° C.) was used in the same manner as (2) of Example 1 to obtain a copper nanoparticle dispersion 4. Using the obtained copper nanoparticle dispersion 4, a conductive substrate was produced by plasma firing in the same manner as (3) of Example 1. Using the obtained copper nanoparticle dispersion 4, a conductive substrate was produced by flash light baking in the same manner as in Example 1 (4).
- Disper byk-111 manufactured by Big Chemie Japan, acid value 129 mgKOH / g, amine value 0 mgKOH / g, weight average molecular weight 1700, 90% thermogravimetric decrease temperature is 320. .4 ° C.
- Example 5 In Example 1 (2), instead of Solsperse 41000, Disper byk-145 (manufactured by Big Chemie Japan, acid value 76 mgKOH / g, amine value 71 mgKOH / g, weight average molecular weight 1800, 90% thermogravimetric decrease temperature is 378 .2 ° C.) was used in the same manner as (2) of Example 1 to obtain a copper nanoparticle dispersion 5. Using the obtained copper nanoparticle dispersion 5, a conductive substrate was produced by plasma firing in the same manner as in (1) of Example 1. Using the obtained copper nanoparticle dispersion 5, a conductive substrate was produced by flash light baking in the same manner as in Example 1 (4).
- Disper byk-145 manufactured by Big Chemie Japan, acid value 76 mgKOH / g, amine value 71 mgKOH / g, weight average molecular weight 1800, 90% thermogravimetric decrease temperature is 378 .2 ° C.
- Example 6 In Example 1 (2), instead of Solsperse 41000, Disper byk-180 (manufactured by Big Chemie Japan, acid value 94 mgKOH / g, amine value 94 mgKOH / g, THF-soluble component weight average molecular weight 1000, 90% heat A copper nanoparticle dispersion 6 was obtained in the same manner as (2) of Example 1 except that the weight reduction temperature was 350.2 ° C. Using the obtained copper nanoparticle dispersion 6, a conductive substrate was produced by plasma firing in the same manner as in (1) of Example 1. Using the obtained copper nanoparticle dispersion 6, a conductive substrate was produced by flash light baking in the same manner as in Example 1 (4).
- Example 7 (1) Synthesis of copper nanoparticles In Example 1, instead of 40.5 g (0.4 mol) of hexylamine, 35.7 g of 3-methoxypropylamine (0.4 mol, manufactured by Guangei Chemical Industry Co., Ltd., boiling point 116 ° C.) was used. Copper nanoparticles coated with nonanoic acid and 3-methoxypropylamine were obtained in the same manner as Example 1 except for using.
- the average primary particle size was 65 nm.
- the main structure of the copper nanoparticle was Cu (Cubic), and a part of Cu 2 O (Cubic) structure was observed, and Cu (111)
- the peak intensity of Cu 2 O (111) relative to was about 1%.
- a copper nanoparticle dispersion was prepared in the same manner as (2) of Example 1 except that the copper nanoparticle coated with nonanoic acid and 3-methoxypropylamine obtained above was used. A particle dispersion 7 was obtained.
- Example 8 (1) Synthesis of copper nanoparticles In Example 1, instead of 40.5 g (0.4 mol) of hexylamine, 41.3 g of 3-ethoxypropylamine (0.4 mol, manufactured by Guangei Chemical Industry Co., Ltd., boiling point 135 ° C.) was used. In the same manner as in Example 1 except that 63.0 g (100 ml) of hexane was added instead of 33.0 g (50 ml) of hexane, copper nano-particles coated with nonanoic acid and 3-ethoxypropylamine were used. Particles were obtained.
- the average primary particle size was 65 nm.
- the main structure of the copper nanoparticle was Cu (Cubic), and a part of Cu 2 O (Cubic) structure was observed, and Cu (111)
- the peak intensity of Cu 2 O (111) relative to was about 5%.
- Example 2 Preparation of copper nanoparticle dispersion
- the copper nanoparticles coated with nonanoic acid and 3-ethoxypropylamine obtained above were used as the copper nanoparticles, and instead of Solsperse 41000
- Solsperse 71000 (manufactured by Nippon Lubrizol, acid value 0 mgKOH / g, amine value 77.4 mgKOH / g, weight average molecular weight 4700, 90% thermogravimetric decrease temperature is 405.9 ° C.) is used.
- a copper nanoparticle dispersion 8 was obtained.
- Example 9 (1) Synthesis of copper nanoparticles In Example 8, instead of 31.5 g (0.2 mol) of nonanoic acid, 34.5 g of decanoic acid (0.2 mol, Kao Lunak 10-98, boiling point 268 ° C.) was used. Except for the above, copper nanoparticles coated with decanoic acid and 3-ethoxypropylamine were obtained in the same manner as in Example 8.
- the average primary particle size was 48 nm.
- the main structure of the copper nanoparticle was Cu (Cubic), and a part of Cu 2 O (Cubic) structure was observed, and Cu (111)
- the peak intensity of Cu 2 O (111) relative to was about 7%.
- a copper nanoparticle dispersion was prepared in the same manner as (2) of Example 1 except that the copper nanoparticle coated with decanoic acid and 3-ethoxypropylamine obtained above was used. A particle dispersion 9 was obtained.
- Example 10 In Example 1 (2), copper nanoparticles coated with decanoic acid and 3-ethoxypropylamine obtained in the same manner as in Example 9 (1) were used as copper nanoparticles, and instead of Solsperse 41000, In addition, Solsperse 71000 (manufactured by Nippon Lubrizol, acid value 0 mgKOH / g, amine value 77.4 mgKOH / g, weight average molecular weight 4700, 90% thermogravimetric decrease temperature is 405.9 ° C.) is used.
- a copper nanoparticle dispersion 10 was obtained. Using the obtained copper nanoparticle dispersion 10, a conductive substrate was produced by plasma firing in the same manner as (3) of Example 1. Using the obtained copper nanoparticle dispersion 10, a conductive substrate was produced by flash light baking in the same manner as in (4) of Example 1.
- Example 11 In Example 1 (2), copper nanoparticles coated with decanoic acid and 3-ethoxypropylamine obtained in the same manner as in Example 9 (1) were used as copper nanoparticles, and instead of Solsperse 41000, In addition, Disper byk-102 (manufactured by Big Chemie Japan, acid value 101 mgKOH / g, amine value 0 mgKOH / g, weight average molecular weight 1400, 90% thermogravimetric decrease temperature is 327.5 ° C.) is used. In the same manner as (2), a copper nanoparticle dispersion 11 was obtained. Using the obtained copper nanoparticle dispersion 11, a conductive substrate was produced by plasma firing in the same manner as in (1) of Example 1. Using the obtained copper nanoparticle dispersion 11, a conductive substrate was produced by flash light baking in the same manner as in Example 1 (4).
- Disper byk-102 manufactured by Big Chemie Japan, acid value 101 mgKOH / g, amine value 0 mgKOH / g,
- Example 12 In Example 1 (2), copper nanoparticles coated with decanoic acid and 3-ethoxypropylamine obtained in the same manner as in Example 9 (1) were used as copper nanoparticles, and instead of Solsperse 41000, In addition, Disper byk-106 (manufactured by Big Chemie Japan, acid value 132 mgKOH / g, amine value 74 mgKOH / g, weight average molecular weight 1400, 90% thermogravimetric decrease temperature is 405.3 ° C.) is used. In the same manner as (2), a copper nanoparticle dispersion 12 was obtained. Using the obtained copper nanoparticle dispersion 12, a conductive substrate was produced by plasma firing in the same manner as in (1) of Example 1. Using the obtained copper nanoparticle dispersion 12, a conductive substrate was produced by flash light baking in the same manner as in (4) of Example 1.
- Example 13 In Example 1 (2), copper nanoparticles coated with decanoic acid and 3-ethoxypropylamine obtained in the same manner as in Example 9 (1) were used as copper nanoparticles, and instead of Solsperse 41000, In addition, Disper byk-111 (manufactured by Big Chemie Japan, acid value 129 mgKOH / g, amine value 0 mgKOH / g, weight average molecular weight 1700, 90% thermogravimetric decrease temperature is 320.4 ° C.) In the same manner as (2), a copper nanoparticle dispersion 13 was obtained. Using the obtained copper nanoparticle dispersion 13, a conductive substrate was produced by plasma firing in the same manner as (3) of Example 1. Using the obtained copper nanoparticle dispersion 13, a conductive substrate was produced by flash light baking in the same manner as in Example 1 (4).
- Example 14 (1) Synthesis of copper nanoparticles In Example 1, instead of hexylamine 40.5 g (0.4 mol), 40.9 g of dimethylaminopropylamine (0.4 mol, manufactured by Guangei Chemical Industry Co., Ltd., boiling point 135 ° C.) was used. Except for the above, copper nanoparticles coated with nonanoic acid and dimethylaminopropylamine were obtained in the same manner as in Example 1.
- the average primary particle size was 64 nm.
- the main structure of the copper nanoparticle was Cu (Cubic), and a part of Cu 2 O (Cubic) structure was observed, and Cu (111)
- the peak intensity of Cu 2 O (111) relative to was about 1%.
- Example 15 (1) Synthesis of copper nanoparticles In Example 1, instead of 31.5 g (0.2 mol) of nonanoic acid, 28.2 g of oleic acid (0.1 mol, manufactured by Kanto Chemical Co., Ltd., boiling point: 360 ° C.) was used, and hexylamine was used. Instead of 40.5 g (0.4 mol), oleic acid and octylamine were obtained in the same manner as in Example 1 except that 51.7 g (0.4 mol, manufactured by Tokyo Chemical Industry Co., Ltd., boiling point: 176 ° C.) was used. Copper nanoparticles coated with were obtained.
- the average primary particle size was 28 nm.
- the main structure of the copper nanoparticle was Cu (Cubic), and a part of Cu 2 O (Cubic) structure was observed, and Cu (111)
- the peak intensity of Cu 2 O (111) relative to was about 12%.
- Example 1 The nonanoic acid obtained in the same manner as in Example 1 (1) and copper nanoparticles coated with hexylamine (3.0 parts by mass) and PGME (4.5 parts by mass) were mixed, and a paint shaker (manufactured by Asada Tekko) was mixed. Then, 2 mm zirconia beads were preliminarily dispersed for 1 hour, and 0.1 mm zirconia beads were further dispersed for 2 hours as the main dispersion. However, the copper nanoparticles were not aggregated and dispersed, and a copper nanoparticle dispersion could not be obtained.
- Example 2 In the same manner as in Example 1 (1), copper nanoparticles coated with nonanoic acid and hexylamine were obtained.
- the copper nanoparticles in Example 2 (2) instead of Solsperse 41000, Solsperse 16000 (manufactured by Nippon Lubrizol, acid value 20 mgKOH / g, amine value 0 mgKOH / g, weight average molecular weight 4200, 90% thermogravimetric weight) Dispersion was carried out in the same manner as in (2) of Example 1 except that the reduction temperature was 405.5 ° C. However, the copper nanoparticles were not aggregated and dispersed, and a copper nanoparticle dispersion could not be obtained.
- Example 3 In the same manner as in Example 1 (1), copper nanoparticles coated with nonanoic acid and hexylamine were obtained.
- Example 1 (2) instead of Solsperse 41000, the copper nanoparticles were Solsperse 76500 (manufactured by Nippon Lubrizol, acid value 0 mgKOH / g, amine value 15.2 mgKOH / g, 90% thermogravimetric decrease temperature) Dispersion was carried out in the same manner as (2) of Example 1 except that over 600 ° C. (32% remaining even at 600 ° C.) was used. However, the copper nanoparticles were not aggregated and dispersed, and a copper nanoparticle dispersion could not be obtained.
- Example 4 In the same manner as in Example 9 (1), copper nanoparticles coated with decanoic acid and 3-ethoxypropylamine were obtained.
- Example 1 (2) the copper nanoparticles were replaced with Dispers byk-130 (manufactured by Big Chemie Japan, acid value of less than 3 mgKOH / g, amine value of 190 mgKOH / g, 90% thermogravimetric reduction temperature). Was 474.8 ° C.) in the same manner as in Example 1 (2). However, the copper nanoparticles were not aggregated and dispersed, and a copper nanoparticle dispersion could not be obtained.
- Example 5 In the same manner as in Example 15 (1), copper nanoparticles coated with oleic acid and octylamine were obtained.
- Example 1 (2) the copper nanoparticles were replaced with Dispers byk-130 (manufactured by Big Chemie Japan, acid value of less than 3 mgKOH / g, amine value of 190 mgKOH / g, 90% thermogravimetric reduction temperature).
- Dispers byk-130 manufactured by Big Chemie Japan, acid value of less than 3 mgKOH / g, amine value of 190 mgKOH / g, 90% thermogravimetric reduction temperature.
- 474.8 ° C. was used to obtain Comparative Copper Nanoparticle Dispersion 5.
- a conductive substrate was produced by plasma firing in the same manner as (3) of Example 1.
- a conductive substrate was produced by flash light baking in the same manner as in (4) of Example 1.
- Example 6 (Comparative Example 6) (1) Synthesis of copper nanoparticles In Example 1, instead of 31.5 g (0.2 mol) of nonanoic acid, 28.2 g of oleic acid (0.1 mol, manufactured by Kanto Chemical Co., Ltd., boiling point: 360 ° C.) was used. In the same manner as in Example 1, copper nanoparticles coated with oleic acid and hexylamine were obtained.
- the average primary particle size was 24 nm.
- the main structure of the copper nanoparticle was Cu (Cubic), and a part of Cu 2 O (Cubic) structure was observed, and Cu (111)
- the peak intensity of Cu 2 O (111) relative to was about 17%.
- Example 7 In the same manner as in Comparative Example 6 (1), copper nanoparticles coated with oleic acid and hexylamine were obtained.
- Example 1 (2) instead of Solsperse 41000, the copper nanoparticles were Solsperse 76500 (manufactured by Nippon Lubrizol, acid value 0 mgKOH / g, amine value 15.2 mgKOH / g, 90% thermogravimetric decrease temperature)
- a comparative copper nanoparticle dispersion 7 was obtained by dispersing in the same manner as in (2) of Example 1 except that over 600 ° C (32% remaining even at 600 ° C) was used.
- Example 7 Using the obtained comparative copper nanoparticle dispersion 7, a conductive substrate was produced by plasma firing in the same manner as in Example 1 (3). Using the obtained comparative copper nanoparticle dispersion 7, a conductive substrate was produced by flash light baking in the same manner as in Example 1 (4).
- Conductivity evaluation was performed on the conductive substrate. Using a surface resistance meter ("Loresta GP" manufactured by Dia Instruments, PSP probe type), 4 probes are brought into contact with the metal film of the conductive substrate of each example and comparative example, and the sheet resistance value is determined by the 4 probe method. It was measured. The evaluation results are shown in Table 1. The lower the sheet resistance value, the better the conductivity. The upper limit of sheet resistance measured by this measurement method was 10 8 ⁇ / ⁇ . In the table, O.D. L. Represents Over Load.
- a copper nanoparticle dispersion containing a carboxylic acid, an alkylamine, a polymer dispersant having a specific amine value and an acid value, and a solvent according to the present invention can be dispersed and applied. It has been clarified that it has excellent sinterability at a low temperature or in a short time, and a high conductivity with a surface resistance of 1 ⁇ / ⁇ or less can be obtained. In particular, it was found that the use of a carboxylic acid having 10 or less carbon atoms improves the conductivity.
- the copper nanoparticle dispersion which concerns on this invention since it is excellent in application
- the copper nanoparticles obtained in Examples 1 to 15 have good oxidation resistance at the time of production.
- the oxidation resistance at the time of production of the copper nanoparticles It was also found to be excellent.
- the copper nanoparticle dispersion of Comparative Example 1 containing a carboxylic acid and an alkylamine but not containing a polymer dispersant has poor dispersibility, and even if a coating film is formed, it has repelling and is not uniform.
- Example 16 In the same manner as in Example 3, a copper nanoparticle dispersion 3 was obtained.
- the obtained copper nanoparticle dispersion 3 was applied to a PET film (Cosmo Shine A4100) having a thickness of 100 ⁇ m with a wire bar and dried to form a coating film having a thickness of 0.5 ⁇ m.
- a microwave surface wave plasma processing apparatus MSP-1500, manufactured by Microelectronics
- firing was performed for 300 seconds at a microwave output of 450 W, and the copper nanoparticle dispersion was fired.
- the resulting sintered film (copper film) was used as a PET film with a copper thin film.
- a UV curable ink was applied to a cleaned glass substrate with a spin coater, and the PET film with a copper thin film obtained above was affixed to prevent bubbles from entering, and the glass and the film were fixed by UV irradiation from the back side. .
- a commercially available naphthoquinone-based positive resist was spin-coated on the surface of the PET film with a copper thin film and hot-plate dried at 100 ° C. for 3 minutes to form a dry film thickness of about 1 ⁇ m.
- a positive resist film surface was contacted with a photomask, exposed at 40 mJ / cm 2 , then paddle developed with NMD-3 (manufactured by Tokyo Ohka Kogyo Co., Ltd.) for 20 seconds, and rinsed with pure water to obtain a resist pattern. Further, etching was performed for 80 seconds while being rocked with a phosphoric acid acetate etching solution at 23 ° C., and then rinsed with pure water and etched.
- NMD-3 manufactured by Tokyo Ohka Kogyo Co., Ltd.
- ⁇ Smoothness evaluation> The cross section of the obtained copper wiring pattern was measured with a scanning electron microscope (SEM, scanning electron microscope “S-4800” manufactured by Hitachi High-Technology), and a cross-sectional observation image of 100,000 times was obtained. An uneven line (length 2 ⁇ m) at the interface between the substrate and the sintered film is extracted, and from the uneven line, the maximum height (Rz, the difference between the most peak and the most valley, according to the definition of JIS B0601 (2001)) Asked. Uneven lines were extracted from any 10 locations in the surface, and the average value of the maximum heights was defined as the average roughness.
- corrugated line converted the image obtained by SEM observation into black-and-white, and extracted the line along the metal sintered film side by making a white part into the metal sintered film side. Parts with obvious irregularities such as foreign matter and filler contained in the film were not used. As a result, the unevenness of the interface between the base material and the metal sintered film was 12.4 nm, and it was confirmed that the above-mentioned interface was smooth.
- Adhesion evaluation> The obtained copper wiring pattern was subjected to a peel test using an adhesive tape (trade name: Scotch Mending Tape, manufactured by Sumitomo 3M). As a result, it was confirmed that the wiring did not peel off, and it was confirmed that the adhesion was good even after etching.
Landscapes
- Engineering & Computer Science (AREA)
- Manufacturing & Machinery (AREA)
- Physics & Mathematics (AREA)
- Chemical & Material Sciences (AREA)
- Dispersion Chemistry (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Microelectronics & Electronic Packaging (AREA)
- Manufacture Of Metal Powder And Suspensions Thereof (AREA)
- Conductive Materials (AREA)
- Powder Metallurgy (AREA)
- Manufacturing Of Electric Cables (AREA)
Abstract
Provided is a method for manufacturing an electroconductive substrate with which it is possible to obtain an electroconductive substrate that has exceptional electroconductivity due to being sintered at a low temperature or for a short period of time. The present invention is a method for manufacturing an electroconductive substrate, which has: a step for forming a coating film by coating a base material with a copper nanoparticle dispersion that contains copper nanoparticles, a carboxylic acid, an alkylamine, a polymer dispersing agent, and a solvent; and a step for sintering the coating film. The polymer dispersing agent has one of either an amine value or an acid value of 30-160 mgKOH/g and the other of either the amine value or the acid value at 0-160 mgKOH/g, and the volume-average particle diameter is 500 nm or less as obtained by dynamic light scattering.
Description
本発明は、銅ナノ粒子分散体、及び当該分散体を用いた導電性基板の製造方法に関するものである。
The present invention relates to a copper nanoparticle dispersion and a method for producing a conductive substrate using the dispersion.
金属粒子を分散させた金属粒子分散体を用いて、スクリーン印刷やインクジェット印刷などの印刷プロセスにより、基材に直接パターンを印刷し、金属粒子を焼結させることにより、回路パターンを形成する手法が注目されている。基材に直接パターンを印刷する手法によれば、従来のフォトレジスト法等と比較して、生産性が飛躍的に向上する。
A method of forming a circuit pattern by printing a pattern directly on a base material by a printing process such as screen printing or ink jet printing using a metal particle dispersion in which metal particles are dispersed, and sintering the metal particles. Attention has been paid. According to the technique of printing a pattern directly on a substrate, productivity is dramatically improved as compared with a conventional photoresist method or the like.
金属粒子は、微細化することにより、劇的に融点が低下することが知られている。これは、金属粒子の粒径が小さくなるのに伴って、粒子の比表面積が増加し、表面エネルギーが増大することによるものである。この効果を利用すれば、金属粒子同士の焼結を従来よりも低温で進行させることができ、従来用いることが困難であった、耐熱性の低い樹脂基材に対しても、印刷による回路形成が可能となると期待される。しかしながら、金属粒子の粒径を小さくするにつれて凝集しやすくなり、分散性や分散安定性を確保できなくなり、塗布適性に問題が生じる。塗布適性に問題が生じて均一性の高い塗膜が形成できないと、回路パターンの精度が悪くなったり、金属粒子同士が均一且つ密に存在できず、焼結後の導電性が悪くなる。このように、金属粒子分散体の分散安定性や塗布適性と低温焼成性を両立することは、従来、困難であった。
It is known that the melting point of metal particles dramatically decreases as they become finer. This is because the specific surface area of the particles increases and the surface energy increases as the particle size of the metal particles decreases. If this effect is utilized, the sintering of metal particles can proceed at a lower temperature than in the past, and circuit formation by printing is possible even for resin substrates with low heat resistance that have been difficult to use conventionally. Is expected to be possible. However, as the particle size of the metal particles is reduced, aggregation tends to occur, dispersibility and dispersion stability cannot be ensured, and a problem arises in coating suitability. If there is a problem in coating suitability and a highly uniform coating film cannot be formed, the accuracy of the circuit pattern will deteriorate, the metal particles will not be present uniformly and densely, and the conductivity after sintering will deteriorate. Thus, it has been difficult in the past to achieve both the dispersion stability and coating suitability of the metal particle dispersion and the low-temperature firing property.
また、銀粒子を用いた金属粒子分散体は、酸化し難く、導電性にも優れているが、銀自体が高価であったり、イオンマイグレーションの問題などがある。そこで、安価で耐マイグレーションに優れた金属として、銅を用いた金属粒子分散体の開発が求められている。しかしながら、銅粒子は一般的に銀粒子と比較して酸化されやすいため、導電性が発現しにくい課題がある。
Also, metal particle dispersions using silver particles are difficult to oxidize and are excellent in conductivity, but silver itself is expensive or has problems of ion migration. Therefore, development of a metal particle dispersion using copper as an inexpensive metal with excellent migration resistance is required. However, since copper particles are generally more easily oxidized than silver particles, there is a problem that conductivity is difficult to be expressed.
特許文献1には、銅を含む化合物と還元性化合物を混合して、アルキルアミン中で熱分解して銅を生成可能な複合化合物を生成する工程と、当該複合化合物をアルキルアミン中で加熱してアルキルアミンで被覆された銅微粒子を生成する工程とを有することを特徴とする被覆銅微粒子の製造方法が記載されている。特許文献1によれば、当該被覆銅微粒子は、粒径分布が狭く微細であり、保存性に優れると共に低温での焼結が可能であると記載されている。
しかしながら、特許文献1の技術によれば、後述の比較例のように、銅微粒子の分散性が悪く、塗布適性が悪く、PETフィルム等の樹脂基材上に塗布する際にムラが生じるという課題があった。更に、分散性を高めようとするとアルキルアミンを大量に使用する必要があり、焼結性が悪化するという問題があった。 In Patent Document 1, a compound containing copper and a reducing compound are mixed, and a step of producing a composite compound that can be thermally decomposed in an alkylamine to form copper, and the composite compound is heated in an alkylamine. And a process for producing copper fine particles coated with an alkylamine. According to Patent Document 1, it is described that the coated copper fine particles have a narrow and fine particle size distribution, excellent storage stability and can be sintered at a low temperature.
However, according to the technique of Patent Document 1, the dispersibility of copper fine particles is poor, the coating suitability is poor, and unevenness occurs when coating on a resin substrate such as a PET film, as in the comparative example described later. was there. Furthermore, when trying to improve dispersibility, it is necessary to use a large amount of alkylamine, and there is a problem that the sinterability deteriorates.
しかしながら、特許文献1の技術によれば、後述の比較例のように、銅微粒子の分散性が悪く、塗布適性が悪く、PETフィルム等の樹脂基材上に塗布する際にムラが生じるという課題があった。更に、分散性を高めようとするとアルキルアミンを大量に使用する必要があり、焼結性が悪化するという問題があった。 In Patent Document 1, a compound containing copper and a reducing compound are mixed, and a step of producing a composite compound that can be thermally decomposed in an alkylamine to form copper, and the composite compound is heated in an alkylamine. And a process for producing copper fine particles coated with an alkylamine. According to Patent Document 1, it is described that the coated copper fine particles have a narrow and fine particle size distribution, excellent storage stability and can be sintered at a low temperature.
However, according to the technique of Patent Document 1, the dispersibility of copper fine particles is poor, the coating suitability is poor, and unevenness occurs when coating on a resin substrate such as a PET film, as in the comparative example described later. was there. Furthermore, when trying to improve dispersibility, it is necessary to use a large amount of alkylamine, and there is a problem that the sinterability deteriorates.
また、特許文献2には、特定の金属微粒子と、特定のポリエステル骨格を有する高分子分散剤と、分散媒を含有する金属微粒子分散体と当該分散体を用いた導電性基板の製造方法が記載されている。特許文献2によれば、上記特定の高分子分散剤が金属微粒子の分散性に高い効果を示し、しかも後の焼結工程で容易に揮散されると記載されている。
しかしながら、特許文献2の技術は、分散剤のみで分散性を保持しているため、焼結時に有機物が残存しやすく、金属微粒子塗工膜の焼成には高いマイクロ波出力による長時間焼成が必要となっている。実際には当該文献の実施例に記載の通りポリエチレンナフタレート(PEN)フィルム上での金属微粒子塗工膜の焼成が限界であり、より安価で汎用的な低耐熱基材であるPETフィルム等に塗工して特許文献2と同条件で焼成しようすると導電性が発現する前に基材が変形し焼成できないという課題があった。PETフィルム等に塗工して焼成するには更なる焼結温度の低温化が必要であった。また、特許文献2の技術では、焼結時に有機物が残存しやすいことから、塗膜表面と内部で焼結度に傾斜が生じやすいため、焼結後に化学エッチングすることが困難であった。Patent Document 2 describes a specific metal fine particle, a polymer dispersant having a specific polyester skeleton, a metal fine particle dispersion containing a dispersion medium, and a method for producing a conductive substrate using the dispersion. Has been. According to Patent Document 2, it is described that the specific polymer dispersant exhibits a high effect on the dispersibility of the metal fine particles and is easily volatilized in a subsequent sintering step.
However, since the technique ofPatent Document 2 retains dispersibility only with a dispersant, organic substances are likely to remain during sintering, and firing of a metal fine particle coating film requires long firing with high microwave output. It has become. Actually, as described in the examples of the document, firing of metal fine particle coating film on polyethylene naphthalate (PEN) film is the limit, and PET film, which is a cheaper and versatile low heat resistant substrate, etc. When it was applied and fired under the same conditions as in Patent Document 2, there was a problem that the base material was deformed and could not be fired before conductivity was developed. In order to coat and fire a PET film or the like, it was necessary to further lower the sintering temperature. Further, in the technique of Patent Document 2, since organic substances are likely to remain at the time of sintering, it is difficult to perform chemical etching after sintering because the degree of sintering tends to be inclined between the coating film surface and inside.
しかしながら、特許文献2の技術は、分散剤のみで分散性を保持しているため、焼結時に有機物が残存しやすく、金属微粒子塗工膜の焼成には高いマイクロ波出力による長時間焼成が必要となっている。実際には当該文献の実施例に記載の通りポリエチレンナフタレート(PEN)フィルム上での金属微粒子塗工膜の焼成が限界であり、より安価で汎用的な低耐熱基材であるPETフィルム等に塗工して特許文献2と同条件で焼成しようすると導電性が発現する前に基材が変形し焼成できないという課題があった。PETフィルム等に塗工して焼成するには更なる焼結温度の低温化が必要であった。また、特許文献2の技術では、焼結時に有機物が残存しやすいことから、塗膜表面と内部で焼結度に傾斜が生じやすいため、焼結後に化学エッチングすることが困難であった。
However, since the technique of
また、特許文献3には、金属プレカーサー、酸、アミン、および還元剤を含む溶液から合成された金属ナノ粒子を用いる、導電性金属薄膜の製造方法が記載されており、分散剤で分散しても良い旨が記載されている。しかしながら、特許文献3は、還元性雰囲気を用いて200℃以上の高温での焼成により導電性を向上することに着目した技術である。特許文献3の具体的に開示されている技術は、後述の比較例のように、金属ナノ粒子の分散性が悪いものであるが、高温焼成によれば、ある程度の導電性を得ることができる。したがって、PETフィルム等に塗工して焼成するには更なる焼結温度の低温化の検討が必要であった。
Patent Document 3 describes a method for producing a conductive metal thin film using metal nanoparticles synthesized from a solution containing a metal precursor, an acid, an amine, and a reducing agent. It is also stated that it is good. However, Patent Document 3 is a technique that focuses on improving the conductivity by firing at a high temperature of 200 ° C. or higher using a reducing atmosphere. Although the technique specifically disclosed in Patent Document 3 has poor dispersibility of metal nanoparticles as in the comparative example described later, a certain degree of conductivity can be obtained by high-temperature firing. . Therefore, in order to coat and sinter on a PET film or the like, it is necessary to study further lowering the sintering temperature.
上述のように、従来、銅粒子分散体は、焼成時に有機成分が残存するのを抑制するために、比較的低分子量の分散剤が用いられてきた。しかしながら、低分子量の分散剤では、分散性や塗布適性が不十分であり、分散性や塗布適性を向上しようとすると低温又は短時間での焼結性も不十分となった。或いは、銅粒子の分散性や分散安定性を向上するために、分散剤として高分子分散剤を用いて銅粒子を分散させると、高温焼成が必要なため使用可能な基板が限定されたり、低温焼成では金属膜に当該高分子分散剤が残存して得られた基板の体積抵抗率が高くなり、導電性基板として十分な性能が得られない場合があった。一方で、銅粒子は酸化されやすいという問題もあった。そのため、銅の酸化を抑制しながら、分散性、及び塗布適性に優れ、且つ、低温又は短時間での焼成後に高い導電性を有する膜が形成可能な分散体を得ることは非常に難しい課題であった。
本発明は、このような状況下になされたものであり、耐酸化性、分散性、塗布適性、及び低温又は短時間での焼結性に優れた銅ナノ粒子分散体、並びに、低温又は短時間での焼成により、優れた導電性を有する導電性基板を得ることができる、導電性基板の製造方法を提供することを目的とする。 As described above, conventionally, in the copper particle dispersion, a relatively low molecular weight dispersant has been used in order to prevent the organic component from remaining during firing. However, the dispersant having a low molecular weight has insufficient dispersibility and applicability, and attempts to improve dispersibility and applicability have resulted in insufficient sinterability at low temperatures or in a short time. Alternatively, in order to improve the dispersibility and dispersion stability of the copper particles, if the copper particles are dispersed using a polymer dispersant as a dispersant, the substrate that can be used is limited because high temperature firing is required, In the firing, the volume resistivity of the substrate obtained by leaving the polymer dispersant in the metal film is increased, and there are cases where sufficient performance as a conductive substrate cannot be obtained. On the other hand, there is a problem that copper particles are easily oxidized. Therefore, it is a very difficult problem to obtain a dispersion that is excellent in dispersibility and coating suitability while suppressing copper oxidation, and that can form a film having high conductivity after firing at a low temperature or in a short time. there were.
The present invention has been made under such circumstances, and a copper nanoparticle dispersion excellent in oxidation resistance, dispersibility, applicability, and sinterability at low temperature or in a short time, and low temperature or short It aims at providing the manufacturing method of an electroconductive board | substrate which can obtain the electroconductive board | substrate which has the outstanding electroconductivity by baking by time.
本発明は、このような状況下になされたものであり、耐酸化性、分散性、塗布適性、及び低温又は短時間での焼結性に優れた銅ナノ粒子分散体、並びに、低温又は短時間での焼成により、優れた導電性を有する導電性基板を得ることができる、導電性基板の製造方法を提供することを目的とする。 As described above, conventionally, in the copper particle dispersion, a relatively low molecular weight dispersant has been used in order to prevent the organic component from remaining during firing. However, the dispersant having a low molecular weight has insufficient dispersibility and applicability, and attempts to improve dispersibility and applicability have resulted in insufficient sinterability at low temperatures or in a short time. Alternatively, in order to improve the dispersibility and dispersion stability of the copper particles, if the copper particles are dispersed using a polymer dispersant as a dispersant, the substrate that can be used is limited because high temperature firing is required, In the firing, the volume resistivity of the substrate obtained by leaving the polymer dispersant in the metal film is increased, and there are cases where sufficient performance as a conductive substrate cannot be obtained. On the other hand, there is a problem that copper particles are easily oxidized. Therefore, it is a very difficult problem to obtain a dispersion that is excellent in dispersibility and coating suitability while suppressing copper oxidation, and that can form a film having high conductivity after firing at a low temperature or in a short time. there were.
The present invention has been made under such circumstances, and a copper nanoparticle dispersion excellent in oxidation resistance, dispersibility, applicability, and sinterability at low temperature or in a short time, and low temperature or short It aims at providing the manufacturing method of an electroconductive board | substrate which can obtain the electroconductive board | substrate which has the outstanding electroconductivity by baking by time.
本発明者らは、前記目的を達成するために鋭意研究を重ねた結果、銅ナノ粒子に、カルボン酸と、アルキルアミンと、特定のアミン価又は酸価を有する高分子分散剤とを組み合わせて体積平均粒径が500nm以下である銅ナノ粒子分散体とすることにより、耐酸化性、分散性、及び塗布適性に優れるとともに、低温又は短時間で焼成した場合であっても、膜中から有機成分が分解乃至除去されやすく、且つ塗膜中でナノ粒子が均一で高密度に配置されることから、高い導電性を有する膜が形成できるとの知見を得た。
本発明は、係る知見に基づいて完成したものである。 As a result of intensive studies to achieve the above object, the present inventors have combined copper nanoparticles with a carboxylic acid, an alkylamine, and a polymer dispersant having a specific amine value or acid value. By making a copper nanoparticle dispersion having a volume average particle size of 500 nm or less, it is excellent in oxidation resistance, dispersibility, and coating suitability, and is organic from the film even when baked at a low temperature or in a short time. Since the components are easily decomposed or removed, and the nanoparticles are uniformly and densely arranged in the coating film, it has been found that a film having high conductivity can be formed.
The present invention has been completed based on such knowledge.
本発明は、係る知見に基づいて完成したものである。 As a result of intensive studies to achieve the above object, the present inventors have combined copper nanoparticles with a carboxylic acid, an alkylamine, and a polymer dispersant having a specific amine value or acid value. By making a copper nanoparticle dispersion having a volume average particle size of 500 nm or less, it is excellent in oxidation resistance, dispersibility, and coating suitability, and is organic from the film even when baked at a low temperature or in a short time. Since the components are easily decomposed or removed, and the nanoparticles are uniformly and densely arranged in the coating film, it has been found that a film having high conductivity can be formed.
The present invention has been completed based on such knowledge.
本発明に係る第一の態様の導電性基板の製造方法は、銅ナノ粒子と、カルボン酸と、アルキルアミンと、高分子分散剤と、溶剤とを含有し、前記高分子分散剤は、アミン価及び酸価の一方が30~160mgKOH/g、アミン価及び酸価の他の一方が0~160mgKOH/gであり、動的光散乱法による体積平均粒径が500nm以下である銅ナノ粒子分散体を、基材上に塗布して塗膜を形成する工程と、当該塗膜を焼成する工程とを有することを特徴とする。
The method for producing a conductive substrate according to the first aspect of the present invention includes copper nanoparticles, a carboxylic acid, an alkylamine, a polymer dispersant, and a solvent, and the polymer dispersant is an amine. Dispersion of copper nanoparticles in which one of the valence and acid value is 30 to 160 mgKOH / g, the other one of the amine value and acid value is 0 to 160 mgKOH / g, and the volume average particle size by dynamic light scattering is 500 nm or less It has the process of apply | coating a body on a base material, and forming the coating film, and the process of baking the said coating film.
また、本発明に係る第二の態様の導電性基板の製造方法は、銅を含む化合物、還元性化合物、カルボン酸、及びアルキルアミンを含む混合物、又は、カルボン酸銅、還元性化合物、及びアルキルアミンを含む混合物のいずれかを加熱することにより銅ナノ粒子を調製する工程と、
前記銅ナノ粒子を、溶剤中で、アミン価及び酸価の一方が30~160mgKOH/g、アミン価及び酸価の他の一方が0~160mgKOH/gである高分子分散剤により分散することにより、動的光散乱法による体積平均粒径が500nm以下である銅ナノ粒子分散体を調製する工程と、
前記銅ナノ粒子分散体を、基材上に塗布して塗膜を形成する工程と、
当該塗膜を焼成する工程とを有することを特徴とする。 Moreover, the manufacturing method of the electroconductive board | substrate of the 2nd aspect which concerns on this invention is a compound containing copper, a reducing compound, carboxylic acid, and the mixture containing an alkylamine, or copper carboxylate, a reducing compound, and alkyl. Preparing copper nanoparticles by heating any of the amine-containing mixtures;
By dispersing the copper nanoparticles in a solvent with a polymer dispersant having one of an amine value and an acid value of 30 to 160 mgKOH / g and the other of an amine value and an acid value of 0 to 160 mgKOH / g. A step of preparing a copper nanoparticle dispersion having a volume average particle size of 500 nm or less by a dynamic light scattering method;
Applying the copper nanoparticle dispersion on a substrate to form a coating film;
And a step of firing the coating film.
前記銅ナノ粒子を、溶剤中で、アミン価及び酸価の一方が30~160mgKOH/g、アミン価及び酸価の他の一方が0~160mgKOH/gである高分子分散剤により分散することにより、動的光散乱法による体積平均粒径が500nm以下である銅ナノ粒子分散体を調製する工程と、
前記銅ナノ粒子分散体を、基材上に塗布して塗膜を形成する工程と、
当該塗膜を焼成する工程とを有することを特徴とする。 Moreover, the manufacturing method of the electroconductive board | substrate of the 2nd aspect which concerns on this invention is a compound containing copper, a reducing compound, carboxylic acid, and the mixture containing an alkylamine, or copper carboxylate, a reducing compound, and alkyl. Preparing copper nanoparticles by heating any of the amine-containing mixtures;
By dispersing the copper nanoparticles in a solvent with a polymer dispersant having one of an amine value and an acid value of 30 to 160 mgKOH / g and the other of an amine value and an acid value of 0 to 160 mgKOH / g. A step of preparing a copper nanoparticle dispersion having a volume average particle size of 500 nm or less by a dynamic light scattering method;
Applying the copper nanoparticle dispersion on a substrate to form a coating film;
And a step of firing the coating film.
また、本発明に係る銅ナノ粒子分散体は、銅ナノ粒子と、カルボン酸と、アルキルアミンと、高分子分散剤と、溶剤とを含有し、前記高分子分散剤は、アミン価及び酸価の一方が30~160mgKOH/g、アミン価及び酸価の他の一方が0~160mgKOH/gであり、動的光散乱法による体積平均粒径が500nm以下であることを特徴とする。
Further, the copper nanoparticle dispersion according to the present invention contains copper nanoparticles, a carboxylic acid, an alkylamine, a polymer dispersant, and a solvent. The polymer dispersant has an amine value and an acid value. One of these is 30 to 160 mgKOH / g, the other one of the amine value and the acid value is 0 to 160 mgKOH / g, and the volume average particle diameter by dynamic light scattering method is 500 nm or less.
本発明に係る導電性基板の製造方法、及び銅ナノ粒子分散体においては、前記高分子分散剤は、90%熱重量減少温度が420℃以下であることが、低温焼成性及び焼結後の塗膜の導電性が優れる点から好ましい。
In the method for producing a conductive substrate and the copper nanoparticle dispersion according to the present invention, the polymer dispersant has a low 90% thermal weight loss temperature of 420 ° C. or less, low temperature sinterability and after sintering. It is preferable from the point which the electroconductivity of a coating film is excellent.
本発明に係る導電性基板の製造方法、及び銅ナノ粒子分散体においては、前記カルボン酸は、炭素数が10以下であることが、低温焼成性及び焼結後の塗膜の導電性が優れる点から好ましい。
In the method for producing a conductive substrate and the copper nanoparticle dispersion according to the present invention, the carboxylic acid has 10 or less carbon atoms, and the low-temperature firing property and the conductivity of the coated film after sintering are excellent. It is preferable from the point.
また、本発明に係る導電性基板の製造方法においては、前記焼成する工程が、プラズマにより焼成する工程であるか、フラッシュ光の照射により焼成する工程であることが、低温乃至短時間焼成が可能であり、低耐熱性の基材上にも優れた導電性を有する導電性基板を得ることができる点から好ましい。
Further, in the method for manufacturing a conductive substrate according to the present invention, the baking step is a step of baking by plasma or a step of baking by irradiation with flash light, which can be performed at a low temperature or for a short time. It is preferable because a conductive substrate having excellent conductivity can be obtained even on a low heat-resistant substrate.
また、本発明に係る導電性基板の製造方法においては、前記焼成する工程後に、更に、得られた焼結膜を化学エッチングする工程を有する方法も好適に用いられる。本発明で形成される前記焼結膜は平滑性及び密着性が付与されるため、微細な導電性パターンを形成するのに化学エッチング法を用いることが可能である。
In the method for producing a conductive substrate according to the present invention, a method having a step of chemically etching the obtained sintered film after the firing step is also preferably used. Since the sintered film formed in the present invention is imparted with smoothness and adhesion, a chemical etching method can be used to form a fine conductive pattern.
本発明によれば、耐酸化性、分散性、塗布適性、及び低温又は短時間での焼結性に優れた、銅ナノ粒子分散体、並びに、低温又は短時間での焼成により、優れた導電性を有する導電性基板を得ることができる導電性基板の製造方法を提供することができる。
According to the present invention, the copper nanoparticle dispersion excellent in oxidation resistance, dispersibility, applicability, and sinterability at a low temperature or in a short time, and excellent conductivity by firing at a low temperature or in a short time. It is possible to provide a method for manufacturing a conductive substrate capable of obtaining a conductive substrate having a property.
以下、本発明に係る銅ナノ粒子分散体、及び導電性基板の製造方法について説明する。
なお、本発明において(メタ)アクリルとは、アクリル及びメタクリルの各々を表し、(メタ)アクリレートとは、アクリレート及びメタクリレートの各々を表す。 Hereinafter, the copper nanoparticle dispersion according to the present invention and the method for producing a conductive substrate will be described.
In the present invention, (meth) acryl represents each of acryl and methacryl, and (meth) acrylate represents each of acrylate and methacrylate.
なお、本発明において(メタ)アクリルとは、アクリル及びメタクリルの各々を表し、(メタ)アクリレートとは、アクリレート及びメタクリレートの各々を表す。 Hereinafter, the copper nanoparticle dispersion according to the present invention and the method for producing a conductive substrate will be described.
In the present invention, (meth) acryl represents each of acryl and methacryl, and (meth) acrylate represents each of acrylate and methacrylate.
[銅ナノ粒子分散体]
本発明に係る銅ナノ粒子分散体は、銅ナノ粒子と、カルボン酸と、アルキルアミンと、高分子分散剤と、溶剤とを含有し、前記高分子分散剤は、アミン価及び酸価の一方が30~160mgKOH/g、アミン価及び酸価の他の一方が0~160mgKOH/gであり、動的光散乱法による体積平均粒径が500nm以下であることを特徴とする。 [Copper nanoparticle dispersion]
The copper nanoparticle dispersion according to the present invention contains copper nanoparticles, a carboxylic acid, an alkylamine, a polymer dispersant, and a solvent, and the polymer dispersant has one of an amine value and an acid value. 30 to 160 mgKOH / g, the other of the amine value and the acid value is 0 to 160 mgKOH / g, and the volume average particle size by dynamic light scattering is 500 nm or less.
本発明に係る銅ナノ粒子分散体は、銅ナノ粒子と、カルボン酸と、アルキルアミンと、高分子分散剤と、溶剤とを含有し、前記高分子分散剤は、アミン価及び酸価の一方が30~160mgKOH/g、アミン価及び酸価の他の一方が0~160mgKOH/gであり、動的光散乱法による体積平均粒径が500nm以下であることを特徴とする。 [Copper nanoparticle dispersion]
The copper nanoparticle dispersion according to the present invention contains copper nanoparticles, a carboxylic acid, an alkylamine, a polymer dispersant, and a solvent, and the polymer dispersant has one of an amine value and an acid value. 30 to 160 mgKOH / g, the other of the amine value and the acid value is 0 to 160 mgKOH / g, and the volume average particle size by dynamic light scattering is 500 nm or less.
本発明においては、銅ナノ粒子に、比較的低分子量のカルボン酸及びアルキルアミンと、アミン価及び酸価の一方が30~160mgKOH/g、アミン価及び酸価の他の一方が0~160mgKOH/gである高分子分散剤とを組み合わせて、体積平均粒径が500nm以下となるように分散することから、以下のようなそれらの相乗作用により、上述のような特定の効果を発揮すると推定される。
銅ナノ粒子の分散性を付与するには、銅ナノ粒子表面に強く吸着する官能基を有する化合物を用いることが望ましいが、一方で、化合物の吸着が強すぎると焼成工程で当該化合物が銅ナノ粒子から脱離し難くなり、結果として導電性悪化の要因となると考えられる。その点、本発明においては、銅ナノ粒子表面に強く吸着し得る比較的低分子量のカルボン酸と、銅ナノ粒子表面に弱く吸着する比較的低分子量のアルキルアミンとの両方と、更に、塩基性及び酸性官能基の少なくとも1種を有する特定のアミン価及び酸価を有する高分子分散剤が、銅ナノ粒子に付着して、溶剤中に当該銅ナノ粒子が分散されてなる。本発明においては、特定のアミン価及び酸価を有する高分子分散剤は、塩基性及び酸性官能基の少なくとも1種を有し、銅ナノ粒子表面のカルボン酸やアルキルアミンとの酸塩基相互作用により付着しているものが存在していることが考えられる。
本発明においては、銅ナノ粒子表面に強く吸着し得るカルボン酸が、強い吸着により溶剤中でも銅ナノ粒子を取り囲んで安定して存在する。そして、アルキルアミンがカルボン酸とは異なる電荷を吸着部位に持って、更に銅ナノ粒子に付着することにより、カルボン酸とアルキルアミンという低分子量分散剤が、銅ナノ粒子に、より密に付着できると推定される。更に、高分子分散剤が特定のアミン価及び酸価を有するように選択したことから、カルボン酸とアルキルアミンの両方が付着している銅ナノ粒子に安定的に付着でき、当該高分子分散剤のポリマー鎖の立体障害により、銅ナノ粒子同士の凝集がより生じ難くなり、銅ナノ粒子の優れた分散性を達成できると推定される。後述の比較例で示したように、カルボン酸とアルキルアミンの両方が付着している銅ナノ粒子に対しては、高分子分散剤のアミン価及び酸価が低すぎても、或いは高すぎても、いずれも高分子分散剤が安定的に付着できず、むしろ分散性は悪化する。
また、本発明においては、銅ナノ粒子が特定のアミン価及び酸価を有する高分子分散剤に取り囲まれて、安定して細かい粒径で均一に分散されていることから、ポリマー鎖の成膜性によって、銅ナノ粒子分散体の塗布適性が優れ、且つ塗膜の平滑性が高くなると推定される。
また、分散体の体積平均粒径を500nm以下とすることで、塗膜中で銅ナノ粒子が均一に配置され、高密度な膜を形成できる。比較的低分子量のアルキルアミンは、銅ナノ粒子表面に弱く吸着しているため、塗膜を形成する際の乾燥時に脱離し易い。その一方で、前記高分子分散剤が残留することで銅ナノ粒子の不均一な凝集を防ぎ、乾燥時に銅ナノ粒子は、より高密度に配列すると考えられる。脱離し易いアルキルアミンと混在していることから、カルボン酸も、乾燥時や低温や短時間の焼成でも脱離し易くなっていると推定される。また、上記高分子分散剤は、上記特定のアミン価及び酸価を有することから吸着が強すぎず、また、均一に銅ナノ粒子の周囲に配置されていることから、続く焼成工程で、低温や短時間の焼成でも脱離乃至分解しやすく、高密度に配列した銅ナノ粒子が融着することにより、優れた導電性を有する金属薄膜が得られると推定される。本願のような系において分散体の体積平均粒径が500nmを超えるような場合には、銅ナノ粒子が高密度に配列できないため不均一に焼成され、また、銅ナノ粒子に安定的に付着できていない高分子分散剤が塗膜中に存在することにより低温や短時間の焼成での焼結を阻害し、優れた導電性が得られないと推定される。
また、銅ナノ粒子は、大気中で合成すると特に酸化されやすい。そのため、ナノ粒子製造時に酸化を抑制して、直ちに溶剤中に分散させて酸素に触れ難くすることにより、酸化を抑制できる。本発明において、アルキルアミンは、アミノ基がプロトンを捕捉する機能を有するため、製造時に銅ナノ粒子表面に付着することにより、製造時及び分散体中での銅原子が酸化されることを抑制していると推定される。更に、銅ナノ粒子表面に強く吸着し得るカルボン酸と共に、銅ナノ粒子を密に取り囲み、更に高分子分散剤が付着していることから、本発明においては、銅ナノ粒子の酸化を抑制する効果が高くなっていることが推定される。これらのことから、焼成時の酸化による焼結阻害も生じ難くなり、焼成後に高い導電性を有する膜を形成可能になると推定される。 In the present invention, the copper nanoparticles have a relatively low molecular weight carboxylic acid and an alkylamine, one of an amine value and an acid value of 30 to 160 mgKOH / g, and the other of the amine value and the acid value of 0 to 160 mgKOH / g. In combination with the polymer dispersant that is g, the volume average particle size is dispersed so as to be 500 nm or less. Therefore, it is estimated that the following specific effects are exhibited by their synergistic action. The
In order to impart dispersibility of the copper nanoparticles, it is desirable to use a compound having a functional group that strongly adsorbs to the surface of the copper nanoparticles. It is considered that it becomes difficult to detach from the particles, and as a result, it becomes a factor of deterioration of conductivity. In that respect, in the present invention, both a relatively low molecular weight carboxylic acid that can be strongly adsorbed on the surface of the copper nanoparticles and a relatively low molecular weight alkylamine that is weakly adsorbed on the surface of the copper nanoparticles, And a polymer dispersant having a specific amine value and acid value having at least one kind of acidic functional group is attached to the copper nanoparticles, and the copper nanoparticles are dispersed in the solvent. In the present invention, the polymer dispersant having a specific amine value and acid value has at least one kind of basic and acidic functional groups, and acid-base interaction with carboxylic acid or alkylamine on the surface of the copper nanoparticles. It can be considered that there is something attached due to the above.
In the present invention, the carboxylic acid that can be strongly adsorbed on the surface of the copper nanoparticles is present stably surrounding the copper nanoparticles in the solvent due to the strong adsorption. And, since the alkylamine has a charge different from that of the carboxylic acid at the adsorption site and further adheres to the copper nanoparticles, the low molecular weight dispersants of carboxylic acid and alkylamine can adhere more closely to the copper nanoparticles. It is estimated to be. Furthermore, since the polymer dispersant is selected so as to have a specific amine value and acid value, the polymer dispersant can be stably attached to the copper nanoparticles to which both the carboxylic acid and the alkylamine are attached. It is presumed that due to the steric hindrance of the polymer chain, aggregation of copper nanoparticles is less likely to occur, and excellent dispersibility of the copper nanoparticles can be achieved. As shown in the comparative example described later, for the copper nanoparticles to which both carboxylic acid and alkylamine are attached, the amine value and acid value of the polymer dispersant are too low or too high. In either case, the polymer dispersant cannot be stably attached, but rather the dispersibility deteriorates.
In the present invention, since the copper nanoparticles are surrounded by a polymer dispersant having a specific amine value and acid value and are stably dispersed uniformly in a fine particle size, film formation of a polymer chain Depending on the properties, it is estimated that the coating suitability of the copper nanoparticle dispersion is excellent and the smoothness of the coating film is increased.
Moreover, by setting the volume average particle diameter of the dispersion to 500 nm or less, the copper nanoparticles are uniformly arranged in the coating film, and a high-density film can be formed. Since the relatively low molecular weight alkylamine is weakly adsorbed on the surface of the copper nanoparticles, it is easily detached during drying when forming a coating film. On the other hand, it is considered that the polymer dispersing agent remains to prevent non-uniform aggregation of the copper nanoparticles, and the copper nanoparticles are arranged at a higher density during drying. It is presumed that carboxylic acid is easily desorbed even during drying, low temperature or short time baking because it is mixed with easily desorbed alkylamine. In addition, since the polymer dispersant has the specific amine value and acid value, the adsorption is not too strong, and the polymer dispersant is uniformly disposed around the copper nanoparticles, so that the low temperature is low in the subsequent firing step. It is presumed that a metal thin film having excellent electrical conductivity can be obtained by fusion of copper nanoparticles arranged at high density, which is easily desorbed or decomposed even after baking for a short time. When the volume average particle size of the dispersion exceeds 500 nm in the system as in the present application, the copper nanoparticles cannot be arranged at high density, so that they are non-uniformly baked and can adhere stably to the copper nanoparticles. It is presumed that an unsatisfactory polymer dispersant is present in the coating film, which inhibits sintering at a low temperature or a short time and prevents excellent conductivity.
Copper nanoparticles are particularly easily oxidized when synthesized in the atmosphere. Therefore, oxidation can be suppressed by suppressing oxidation at the time of nanoparticle production and immediately dispersing in a solvent to make it difficult to touch oxygen. In the present invention, the alkylamine has a function of scavenging protons in the amino group, and therefore adheres to the surface of the copper nanoparticles during production, thereby suppressing oxidation of copper atoms during production and in the dispersion. It is estimated that In addition, the present invention has an effect of suppressing the oxidation of copper nanoparticles in the present invention because the copper nanoparticles are closely surrounded with a carboxylic acid that can be strongly adsorbed on the surface of the copper nanoparticles, and further a polymer dispersant is attached. Is estimated to be high. From these, it is presumed that sintering inhibition due to oxidation during firing hardly occurs, and a film having high conductivity can be formed after firing.
銅ナノ粒子の分散性を付与するには、銅ナノ粒子表面に強く吸着する官能基を有する化合物を用いることが望ましいが、一方で、化合物の吸着が強すぎると焼成工程で当該化合物が銅ナノ粒子から脱離し難くなり、結果として導電性悪化の要因となると考えられる。その点、本発明においては、銅ナノ粒子表面に強く吸着し得る比較的低分子量のカルボン酸と、銅ナノ粒子表面に弱く吸着する比較的低分子量のアルキルアミンとの両方と、更に、塩基性及び酸性官能基の少なくとも1種を有する特定のアミン価及び酸価を有する高分子分散剤が、銅ナノ粒子に付着して、溶剤中に当該銅ナノ粒子が分散されてなる。本発明においては、特定のアミン価及び酸価を有する高分子分散剤は、塩基性及び酸性官能基の少なくとも1種を有し、銅ナノ粒子表面のカルボン酸やアルキルアミンとの酸塩基相互作用により付着しているものが存在していることが考えられる。
本発明においては、銅ナノ粒子表面に強く吸着し得るカルボン酸が、強い吸着により溶剤中でも銅ナノ粒子を取り囲んで安定して存在する。そして、アルキルアミンがカルボン酸とは異なる電荷を吸着部位に持って、更に銅ナノ粒子に付着することにより、カルボン酸とアルキルアミンという低分子量分散剤が、銅ナノ粒子に、より密に付着できると推定される。更に、高分子分散剤が特定のアミン価及び酸価を有するように選択したことから、カルボン酸とアルキルアミンの両方が付着している銅ナノ粒子に安定的に付着でき、当該高分子分散剤のポリマー鎖の立体障害により、銅ナノ粒子同士の凝集がより生じ難くなり、銅ナノ粒子の優れた分散性を達成できると推定される。後述の比較例で示したように、カルボン酸とアルキルアミンの両方が付着している銅ナノ粒子に対しては、高分子分散剤のアミン価及び酸価が低すぎても、或いは高すぎても、いずれも高分子分散剤が安定的に付着できず、むしろ分散性は悪化する。
また、本発明においては、銅ナノ粒子が特定のアミン価及び酸価を有する高分子分散剤に取り囲まれて、安定して細かい粒径で均一に分散されていることから、ポリマー鎖の成膜性によって、銅ナノ粒子分散体の塗布適性が優れ、且つ塗膜の平滑性が高くなると推定される。
また、分散体の体積平均粒径を500nm以下とすることで、塗膜中で銅ナノ粒子が均一に配置され、高密度な膜を形成できる。比較的低分子量のアルキルアミンは、銅ナノ粒子表面に弱く吸着しているため、塗膜を形成する際の乾燥時に脱離し易い。その一方で、前記高分子分散剤が残留することで銅ナノ粒子の不均一な凝集を防ぎ、乾燥時に銅ナノ粒子は、より高密度に配列すると考えられる。脱離し易いアルキルアミンと混在していることから、カルボン酸も、乾燥時や低温や短時間の焼成でも脱離し易くなっていると推定される。また、上記高分子分散剤は、上記特定のアミン価及び酸価を有することから吸着が強すぎず、また、均一に銅ナノ粒子の周囲に配置されていることから、続く焼成工程で、低温や短時間の焼成でも脱離乃至分解しやすく、高密度に配列した銅ナノ粒子が融着することにより、優れた導電性を有する金属薄膜が得られると推定される。本願のような系において分散体の体積平均粒径が500nmを超えるような場合には、銅ナノ粒子が高密度に配列できないため不均一に焼成され、また、銅ナノ粒子に安定的に付着できていない高分子分散剤が塗膜中に存在することにより低温や短時間の焼成での焼結を阻害し、優れた導電性が得られないと推定される。
また、銅ナノ粒子は、大気中で合成すると特に酸化されやすい。そのため、ナノ粒子製造時に酸化を抑制して、直ちに溶剤中に分散させて酸素に触れ難くすることにより、酸化を抑制できる。本発明において、アルキルアミンは、アミノ基がプロトンを捕捉する機能を有するため、製造時に銅ナノ粒子表面に付着することにより、製造時及び分散体中での銅原子が酸化されることを抑制していると推定される。更に、銅ナノ粒子表面に強く吸着し得るカルボン酸と共に、銅ナノ粒子を密に取り囲み、更に高分子分散剤が付着していることから、本発明においては、銅ナノ粒子の酸化を抑制する効果が高くなっていることが推定される。これらのことから、焼成時の酸化による焼結阻害も生じ難くなり、焼成後に高い導電性を有する膜を形成可能になると推定される。 In the present invention, the copper nanoparticles have a relatively low molecular weight carboxylic acid and an alkylamine, one of an amine value and an acid value of 30 to 160 mgKOH / g, and the other of the amine value and the acid value of 0 to 160 mgKOH / g. In combination with the polymer dispersant that is g, the volume average particle size is dispersed so as to be 500 nm or less. Therefore, it is estimated that the following specific effects are exhibited by their synergistic action. The
In order to impart dispersibility of the copper nanoparticles, it is desirable to use a compound having a functional group that strongly adsorbs to the surface of the copper nanoparticles. It is considered that it becomes difficult to detach from the particles, and as a result, it becomes a factor of deterioration of conductivity. In that respect, in the present invention, both a relatively low molecular weight carboxylic acid that can be strongly adsorbed on the surface of the copper nanoparticles and a relatively low molecular weight alkylamine that is weakly adsorbed on the surface of the copper nanoparticles, And a polymer dispersant having a specific amine value and acid value having at least one kind of acidic functional group is attached to the copper nanoparticles, and the copper nanoparticles are dispersed in the solvent. In the present invention, the polymer dispersant having a specific amine value and acid value has at least one kind of basic and acidic functional groups, and acid-base interaction with carboxylic acid or alkylamine on the surface of the copper nanoparticles. It can be considered that there is something attached due to the above.
In the present invention, the carboxylic acid that can be strongly adsorbed on the surface of the copper nanoparticles is present stably surrounding the copper nanoparticles in the solvent due to the strong adsorption. And, since the alkylamine has a charge different from that of the carboxylic acid at the adsorption site and further adheres to the copper nanoparticles, the low molecular weight dispersants of carboxylic acid and alkylamine can adhere more closely to the copper nanoparticles. It is estimated to be. Furthermore, since the polymer dispersant is selected so as to have a specific amine value and acid value, the polymer dispersant can be stably attached to the copper nanoparticles to which both the carboxylic acid and the alkylamine are attached. It is presumed that due to the steric hindrance of the polymer chain, aggregation of copper nanoparticles is less likely to occur, and excellent dispersibility of the copper nanoparticles can be achieved. As shown in the comparative example described later, for the copper nanoparticles to which both carboxylic acid and alkylamine are attached, the amine value and acid value of the polymer dispersant are too low or too high. In either case, the polymer dispersant cannot be stably attached, but rather the dispersibility deteriorates.
In the present invention, since the copper nanoparticles are surrounded by a polymer dispersant having a specific amine value and acid value and are stably dispersed uniformly in a fine particle size, film formation of a polymer chain Depending on the properties, it is estimated that the coating suitability of the copper nanoparticle dispersion is excellent and the smoothness of the coating film is increased.
Moreover, by setting the volume average particle diameter of the dispersion to 500 nm or less, the copper nanoparticles are uniformly arranged in the coating film, and a high-density film can be formed. Since the relatively low molecular weight alkylamine is weakly adsorbed on the surface of the copper nanoparticles, it is easily detached during drying when forming a coating film. On the other hand, it is considered that the polymer dispersing agent remains to prevent non-uniform aggregation of the copper nanoparticles, and the copper nanoparticles are arranged at a higher density during drying. It is presumed that carboxylic acid is easily desorbed even during drying, low temperature or short time baking because it is mixed with easily desorbed alkylamine. In addition, since the polymer dispersant has the specific amine value and acid value, the adsorption is not too strong, and the polymer dispersant is uniformly disposed around the copper nanoparticles, so that the low temperature is low in the subsequent firing step. It is presumed that a metal thin film having excellent electrical conductivity can be obtained by fusion of copper nanoparticles arranged at high density, which is easily desorbed or decomposed even after baking for a short time. When the volume average particle size of the dispersion exceeds 500 nm in the system as in the present application, the copper nanoparticles cannot be arranged at high density, so that they are non-uniformly baked and can adhere stably to the copper nanoparticles. It is presumed that an unsatisfactory polymer dispersant is present in the coating film, which inhibits sintering at a low temperature or a short time and prevents excellent conductivity.
Copper nanoparticles are particularly easily oxidized when synthesized in the atmosphere. Therefore, oxidation can be suppressed by suppressing oxidation at the time of nanoparticle production and immediately dispersing in a solvent to make it difficult to touch oxygen. In the present invention, the alkylamine has a function of scavenging protons in the amino group, and therefore adheres to the surface of the copper nanoparticles during production, thereby suppressing oxidation of copper atoms during production and in the dispersion. It is estimated that In addition, the present invention has an effect of suppressing the oxidation of copper nanoparticles in the present invention because the copper nanoparticles are closely surrounded with a carboxylic acid that can be strongly adsorbed on the surface of the copper nanoparticles, and further a polymer dispersant is attached. Is estimated to be high. From these, it is presumed that sintering inhibition due to oxidation during firing hardly occurs, and a film having high conductivity can be formed after firing.
本発明の銅ナノ粒子分散体は、上記必須成分の他、本発明の効果が損なわれない限り、他の成分を含有してもよいものである。
以下、銅ナノ粒子分散体の各構成について順に詳細に説明する。 The copper nanoparticle dispersion of the present invention may contain other components in addition to the above essential components as long as the effects of the present invention are not impaired.
Hereinafter, each configuration of the copper nanoparticle dispersion will be described in detail in order.
以下、銅ナノ粒子分散体の各構成について順に詳細に説明する。 The copper nanoparticle dispersion of the present invention may contain other components in addition to the above essential components as long as the effects of the present invention are not impaired.
Hereinafter, each configuration of the copper nanoparticle dispersion will be described in detail in order.
<銅ナノ粒子>
本発明において銅ナノ粒子は、典型的には金属状態の銅粒子であるが、銅は非常に酸化され易い金属のため、金属状態の銅ナノ粒子の表面が一部酸化されて酸化物となっている場合が含まれていてもよいものである。 <Copper nanoparticles>
In the present invention, the copper nanoparticles are typically metallic copper particles. However, since copper is a metal that is very easily oxidized, the surface of the metallic copper nanoparticles is partially oxidized into an oxide. It may be included.
本発明において銅ナノ粒子は、典型的には金属状態の銅粒子であるが、銅は非常に酸化され易い金属のため、金属状態の銅ナノ粒子の表面が一部酸化されて酸化物となっている場合が含まれていてもよいものである。 <Copper nanoparticles>
In the present invention, the copper nanoparticles are typically metallic copper particles. However, since copper is a metal that is very easily oxidized, the surface of the metallic copper nanoparticles is partially oxidized into an oxide. It may be included.
また、銅ナノ粒子とは、直径がnm(ナノメートル)オーダー、すなわち1μm未満の粒子をいう。本発明では、このような銅ナノ粒子を用いることにより、低温での焼結が進行し易く、また微細配線の印刷性が良好になる。本発明で用いられる銅ナノ粒子としては、中でも、分散性と塗布適性、低温焼成、導電性を両立させる点から、平均一次粒径が1nm~100nmの粒子であることが好ましく、更に10nm~100nmの粒子であることが好ましい。下限値未満だと、分散安定性や塗布適性を付与するために、高分子分散剤が多量に必要となり、結果として導電性の悪化につながる恐れがあるからである。上限値を超えると、分散粒径が大きくなり、結果として導電性の悪化につながる恐れがあるからである。
なお、上記銅ナノ粒子の平均一次粒径は、電子顕微鏡写真から一次粒子の大きさを直接計測する方法で求めることができる。具体的には、透過型電子顕微鏡写真(TEM)(例えば、日立ハイテク製 H-7650)にて粒子像を測定し、ランダムに選択した100個の一次粒子の最長部の長さの平均値を平均一次粒径とすることができる。 The copper nanoparticles mean particles having a diameter of the order of nm (nanometer), that is, less than 1 μm. In the present invention, by using such copper nanoparticles, sintering at a low temperature is easy to proceed, and the printability of fine wiring is improved. The copper nanoparticles used in the present invention are preferably particles having an average primary particle size of 1 nm to 100 nm, and more preferably 10 nm to 100 nm, from the viewpoint of achieving both dispersibility, applicability, low temperature firing, and conductivity. Of these particles, it is preferable. This is because if it is less than the lower limit, a large amount of a polymeric dispersant is required to impart dispersion stability and coating suitability, and as a result, there is a risk of deteriorating conductivity. If the upper limit is exceeded, the dispersed particle size becomes large, and as a result, there is a possibility that the conductivity is deteriorated.
In addition, the average primary particle diameter of the said copper nanoparticle can be calculated | required by the method of measuring the magnitude | size of a primary particle directly from an electron micrograph. Specifically, a particle image was measured with a transmission electron micrograph (TEM) (for example, H-7650 manufactured by Hitachi High-Tech), and the average value of the length of the longest part of 100 randomly selected primary particles was calculated. The average primary particle size can be obtained.
なお、上記銅ナノ粒子の平均一次粒径は、電子顕微鏡写真から一次粒子の大きさを直接計測する方法で求めることができる。具体的には、透過型電子顕微鏡写真(TEM)(例えば、日立ハイテク製 H-7650)にて粒子像を測定し、ランダムに選択した100個の一次粒子の最長部の長さの平均値を平均一次粒径とすることができる。 The copper nanoparticles mean particles having a diameter of the order of nm (nanometer), that is, less than 1 μm. In the present invention, by using such copper nanoparticles, sintering at a low temperature is easy to proceed, and the printability of fine wiring is improved. The copper nanoparticles used in the present invention are preferably particles having an average primary particle size of 1 nm to 100 nm, and more preferably 10 nm to 100 nm, from the viewpoint of achieving both dispersibility, applicability, low temperature firing, and conductivity. Of these particles, it is preferable. This is because if it is less than the lower limit, a large amount of a polymeric dispersant is required to impart dispersion stability and coating suitability, and as a result, there is a risk of deteriorating conductivity. If the upper limit is exceeded, the dispersed particle size becomes large, and as a result, there is a possibility that the conductivity is deteriorated.
In addition, the average primary particle diameter of the said copper nanoparticle can be calculated | required by the method of measuring the magnitude | size of a primary particle directly from an electron micrograph. Specifically, a particle image was measured with a transmission electron micrograph (TEM) (for example, H-7650 manufactured by Hitachi High-Tech), and the average value of the length of the longest part of 100 randomly selected primary particles was calculated. The average primary particle size can be obtained.
上記銅ナノ粒子の調製方法は、従来公知の方法から適宜選択すればよい。例えば、メカノケミカル法などにより金属粉を粉砕する物理的な方法;化学気相法(CVD法)や蒸着法、スパッタ法、熱プラズマ法、レーザー法のような化学的な乾式法;熱分解法、化学還元法、電気分解法、超音波法、レーザーアブレーション法、超臨界流体法、マイクロ波合成法等による化学的な湿式法等を用いて銅ナノ粒子を得ることができる。
The method for preparing the copper nanoparticles may be appropriately selected from conventionally known methods. For example, a physical method of pulverizing metal powder by mechanochemical method, etc .; chemical dry method such as chemical vapor deposition method (CVD method), vapor deposition method, sputtering method, thermal plasma method, laser method; thermal decomposition method The copper nanoparticles can be obtained using a chemical wet method such as a chemical reduction method, an electrolysis method, an ultrasonic method, a laser ablation method, a supercritical fluid method, or a microwave synthesis method.
例えば、蒸着法では、高真空下で分散剤を含む低蒸気圧液体中に加熱蒸発した金属の蒸気を接触させて微粒子を製造する。
また、化学還元法の1種としては、錯化剤及び有機保護剤の存在下で、含銅化合物と還元剤とを溶剤中で混合して生成する方法が挙げられる。
なお、上記の方法の他、市販の銅ナノ粒子を適宜用いることができる。
本発明においては、銅ナノ粒子にカルボン酸とアルキルアミンを被覆させることから、中でも、後に詳述するように、有機保護剤としてカルボン酸とアルキルアミンとを用いて、含銅化合物と還元剤とを溶剤中で混合して銅ナノ粒子を生成する方法が好適に用いられる。 For example, in the vapor deposition method, fine particles are produced by bringing a vapor of a metal heated and brought into contact with a low vapor pressure liquid containing a dispersant under a high vacuum.
Further, as one type of chemical reduction method, there is a method in which a copper-containing compound and a reducing agent are mixed in a solvent in the presence of a complexing agent and an organic protective agent.
In addition to the above method, commercially available copper nanoparticles can be used as appropriate.
In the present invention, since copper nanoparticles are coated with carboxylic acid and alkylamine, among them, as will be described in detail later, using carboxylic acid and alkylamine as an organic protective agent, a copper-containing compound and a reducing agent, A method of mixing copper in a solvent to produce copper nanoparticles is preferably used.
また、化学還元法の1種としては、錯化剤及び有機保護剤の存在下で、含銅化合物と還元剤とを溶剤中で混合して生成する方法が挙げられる。
なお、上記の方法の他、市販の銅ナノ粒子を適宜用いることができる。
本発明においては、銅ナノ粒子にカルボン酸とアルキルアミンを被覆させることから、中でも、後に詳述するように、有機保護剤としてカルボン酸とアルキルアミンとを用いて、含銅化合物と還元剤とを溶剤中で混合して銅ナノ粒子を生成する方法が好適に用いられる。 For example, in the vapor deposition method, fine particles are produced by bringing a vapor of a metal heated and brought into contact with a low vapor pressure liquid containing a dispersant under a high vacuum.
Further, as one type of chemical reduction method, there is a method in which a copper-containing compound and a reducing agent are mixed in a solvent in the presence of a complexing agent and an organic protective agent.
In addition to the above method, commercially available copper nanoparticles can be used as appropriate.
In the present invention, since copper nanoparticles are coated with carboxylic acid and alkylamine, among them, as will be described in detail later, using carboxylic acid and alkylamine as an organic protective agent, a copper-containing compound and a reducing agent, A method of mixing copper in a solvent to produce copper nanoparticles is preferably used.
本発明の銅ナノ粒子分散体において、銅ナノ粒子の含有量は、用途に応じて適宜選択されれば良いが、分散性の点から、銅ナノ粒子分散体の全量に対して、0.01~90質量%であることが好ましく、更に、0.1~85質量%の範囲内であることがより好ましい。
In the copper nanoparticle dispersion of the present invention, the content of the copper nanoparticles may be appropriately selected according to the use, but from the viewpoint of dispersibility, 0.01% with respect to the total amount of the copper nanoparticle dispersion. The content is preferably from 90 to 90% by mass, and more preferably from 0.1 to 85% by mass.
<カルボン酸>
本発明において使用されるカルボン酸は、配位子として、酸素原子により銅に結合し得る化合物である。従って、銅ナノ粒子分散体において、分散に寄与している当該カルボン酸は、通常、少なくとも1つの酸素原子により銅に結合した状態で存在する。
カルボン酸としては、例えば、酢酸、プロパン酸、ブタン酸、ペンタン酸、ヘキサン酸、ヘプタン酸、オクタン酸、ノナン酸、デカン酸、ドデカン酸、ヘキサデカン酸等の飽和脂肪族モノカルボン酸;オレイン酸、リノール酸等の等の不飽和脂肪族モノカルボン酸;シュウ酸、マロン酸、コハク酸等の脂肪族ポリカルボン酸;マレイン酸等の脂肪族不飽和ポリカルボン酸;安息香酸等の芳香族モノカルボン酸;フタル酸等の芳香族ポリカルボン酸;クエン酸等のヒドロキシカルボン酸などが挙げられるが、これらに限定されるものではない。
中でも、炭素数が10以下のカルボン酸であることが、低温焼成性が良好になり、導電性が向上する点から好ましい。また、分散性の点から炭素数が2以上のカルボン酸が用いられる。特に、炭素数が10以下の脂肪族カルボン酸であることが、分散性、低温焼成性が良好になり、導電性が向上する点から好ましい。なお、当該脂肪族カルボン酸は、飽和、不飽和のどちらでもよい。 <Carboxylic acid>
The carboxylic acid used in the present invention is a compound that can be bonded to copper by an oxygen atom as a ligand. Therefore, in the copper nanoparticle dispersion, the carboxylic acid contributing to the dispersion usually exists in a state of being bonded to copper by at least one oxygen atom.
Examples of the carboxylic acid include saturated aliphatic monocarboxylic acids such as acetic acid, propanoic acid, butanoic acid, pentanoic acid, hexanoic acid, heptanoic acid, octanoic acid, nonanoic acid, decanoic acid, dodecanoic acid, and hexadecanoic acid; oleic acid, Unsaturated aliphatic monocarboxylic acids such as linoleic acid; Aliphatic polycarboxylic acids such as oxalic acid, malonic acid, and succinic acid; Aliphatic unsaturated polycarboxylic acids such as maleic acid; Aromatic monocarboxylic acids such as benzoic acid Examples thereof include, but are not limited to, acids; aromatic polycarboxylic acids such as phthalic acid; and hydroxycarboxylic acids such as citric acid.
Among these, a carboxylic acid having 10 or less carbon atoms is preferable from the viewpoint that the low-temperature baking property is improved and the conductivity is improved. Also, carboxylic acids having 2 or more carbon atoms are used from the viewpoint of dispersibility. In particular, an aliphatic carboxylic acid having 10 or less carbon atoms is preferable from the viewpoint of improving dispersibility and low-temperature calcination properties and improving conductivity. The aliphatic carboxylic acid may be either saturated or unsaturated.
本発明において使用されるカルボン酸は、配位子として、酸素原子により銅に結合し得る化合物である。従って、銅ナノ粒子分散体において、分散に寄与している当該カルボン酸は、通常、少なくとも1つの酸素原子により銅に結合した状態で存在する。
カルボン酸としては、例えば、酢酸、プロパン酸、ブタン酸、ペンタン酸、ヘキサン酸、ヘプタン酸、オクタン酸、ノナン酸、デカン酸、ドデカン酸、ヘキサデカン酸等の飽和脂肪族モノカルボン酸;オレイン酸、リノール酸等の等の不飽和脂肪族モノカルボン酸;シュウ酸、マロン酸、コハク酸等の脂肪族ポリカルボン酸;マレイン酸等の脂肪族不飽和ポリカルボン酸;安息香酸等の芳香族モノカルボン酸;フタル酸等の芳香族ポリカルボン酸;クエン酸等のヒドロキシカルボン酸などが挙げられるが、これらに限定されるものではない。
中でも、炭素数が10以下のカルボン酸であることが、低温焼成性が良好になり、導電性が向上する点から好ましい。また、分散性の点から炭素数が2以上のカルボン酸が用いられる。特に、炭素数が10以下の脂肪族カルボン酸であることが、分散性、低温焼成性が良好になり、導電性が向上する点から好ましい。なお、当該脂肪族カルボン酸は、飽和、不飽和のどちらでもよい。 <Carboxylic acid>
The carboxylic acid used in the present invention is a compound that can be bonded to copper by an oxygen atom as a ligand. Therefore, in the copper nanoparticle dispersion, the carboxylic acid contributing to the dispersion usually exists in a state of being bonded to copper by at least one oxygen atom.
Examples of the carboxylic acid include saturated aliphatic monocarboxylic acids such as acetic acid, propanoic acid, butanoic acid, pentanoic acid, hexanoic acid, heptanoic acid, octanoic acid, nonanoic acid, decanoic acid, dodecanoic acid, and hexadecanoic acid; oleic acid, Unsaturated aliphatic monocarboxylic acids such as linoleic acid; Aliphatic polycarboxylic acids such as oxalic acid, malonic acid, and succinic acid; Aliphatic unsaturated polycarboxylic acids such as maleic acid; Aromatic monocarboxylic acids such as benzoic acid Examples thereof include, but are not limited to, acids; aromatic polycarboxylic acids such as phthalic acid; and hydroxycarboxylic acids such as citric acid.
Among these, a carboxylic acid having 10 or less carbon atoms is preferable from the viewpoint that the low-temperature baking property is improved and the conductivity is improved. Also, carboxylic acids having 2 or more carbon atoms are used from the viewpoint of dispersibility. In particular, an aliphatic carboxylic acid having 10 or less carbon atoms is preferable from the viewpoint of improving dispersibility and low-temperature calcination properties and improving conductivity. The aliphatic carboxylic acid may be either saturated or unsaturated.
本発明で用いられるカルボン酸は、1種のカルボン酸を使用しても良いが、2種以上のカルボン酸を混合して使用してもよい。
本発明で用いられるカルボン酸は、極性が比較的弱く、焼成時に脱離しやすい点から、分子内に一つもしくは二つのカルボキシル基を有するカルボン酸を用いることが好ましく、更に分子内に一つのカルボキシル基を有するカルボン酸を用いることが好ましい。 As the carboxylic acid used in the present invention, one carboxylic acid may be used, or two or more carboxylic acids may be mixed and used.
The carboxylic acid used in the present invention is preferably a carboxylic acid having one or two carboxyl groups in the molecule, since the polarity is relatively weak and easily desorbed at the time of firing. It is preferable to use a carboxylic acid having a group.
本発明で用いられるカルボン酸は、極性が比較的弱く、焼成時に脱離しやすい点から、分子内に一つもしくは二つのカルボキシル基を有するカルボン酸を用いることが好ましく、更に分子内に一つのカルボキシル基を有するカルボン酸を用いることが好ましい。 As the carboxylic acid used in the present invention, one carboxylic acid may be used, or two or more carboxylic acids may be mixed and used.
The carboxylic acid used in the present invention is preferably a carboxylic acid having one or two carboxyl groups in the molecule, since the polarity is relatively weak and easily desorbed at the time of firing. It is preferable to use a carboxylic acid having a group.
また、本発明で用いられるカルボン酸は、焼成時に脱離しやすい点から、分子量が高過ぎないことが好ましく、分子量が300以下であることが好ましく、更に200以下であることが好ましい。また、沸点が400℃以下であることが好ましく、更に300℃以下であることが好ましい。一方で、ナノ粒子作製時、保管時の脱離、揮発防止の点から、カルボン酸の分子量は50以上であることが好ましい。また、沸点が50℃以上であることが好ましい。
In addition, the carboxylic acid used in the present invention is preferably not too high in molecular weight, and preferably has a molecular weight of 300 or less, more preferably 200 or less, from the viewpoint that it easily desorbs during firing. Moreover, it is preferable that a boiling point is 400 degrees C or less, Furthermore, it is preferable that it is 300 degrees C or less. On the other hand, the molecular weight of the carboxylic acid is preferably 50 or more from the viewpoint of desorption during storage of nanoparticles and storage and prevention of volatilization. Moreover, it is preferable that a boiling point is 50 degreeC or more.
本発明の銅ナノ粒子分散体において、カルボン酸の含有量は、用途に応じて適宜選択されれば良いが、低温焼成性の点から、銅100質量部に対して、0.1~30質量部であることが好ましく、更に、0.1~20質量部の範囲内であることがより好ましい。
本発明の銅ナノ粒子分散体において、カルボン酸の含有量は、用途に応じて適宜選択されれば良いが、低温焼成性の点から、銅ナノ粒子分散体の全量に対して、0.05~15質量%であることが好ましく、更に、0.05~10質量%の範囲内であることがより好ましい。 In the copper nanoparticle dispersion of the present invention, the content of the carboxylic acid may be appropriately selected according to the use, but from the viewpoint of low-temperature calcinability, 0.1 to 30 masses relative to 100 mass parts of copper. Part, more preferably in the range of 0.1 to 20 parts by weight.
In the copper nanoparticle dispersion of the present invention, the content of the carboxylic acid may be appropriately selected according to the use, but from the viewpoint of low-temperature calcinability, 0.05% with respect to the total amount of the copper nanoparticle dispersion. It is preferably ˜15% by mass, and more preferably in the range of 0.05 to 10% by mass.
本発明の銅ナノ粒子分散体において、カルボン酸の含有量は、用途に応じて適宜選択されれば良いが、低温焼成性の点から、銅ナノ粒子分散体の全量に対して、0.05~15質量%であることが好ましく、更に、0.05~10質量%の範囲内であることがより好ましい。 In the copper nanoparticle dispersion of the present invention, the content of the carboxylic acid may be appropriately selected according to the use, but from the viewpoint of low-temperature calcinability, 0.1 to 30 masses relative to 100 mass parts of copper. Part, more preferably in the range of 0.1 to 20 parts by weight.
In the copper nanoparticle dispersion of the present invention, the content of the carboxylic acid may be appropriately selected according to the use, but from the viewpoint of low-temperature calcinability, 0.05% with respect to the total amount of the copper nanoparticle dispersion. It is preferably ˜15% by mass, and more preferably in the range of 0.05 to 10% by mass.
<アルキルアミン>
本発明において使用されるアルキルアミンは、製造される銅ナノ粒子分散体に期待される特性等に応じて、公知のアルキルアミンから適宜選択して用いることができる。
アルキルアミンは、プロトンを捕捉する機能を有することにより、銅原子が酸化されることを防止していると推定される。
アルキルアミンはアルキル基の一部にアミノ基の結合した構造を有している。銅原子に対して配位結合を形成するために、使用するアルキルアミンに含まれるアミノ基の少なくとも1つが一級アミノ基であるRNH2(Rは炭化水素鎖)または二級アミノ基であるR1R2NH(R1、R2は炭化水素鎖で同じであっても異なっていてもよい)であることが望ましい。アルキル鎖は直鎖、分岐鎖、又は環状のいずれであっても良い。また、前記炭化水素鎖には酸素、珪素、窒素、硫黄、リンなどの炭素以外の原子が含有されていても良い。例えば具体的には、アルコキシ基、アルコキシシリル基、アルキルチオ基等の置換基を有するアルキルアミンであっても良く、分子内に二つのアミノ基を有していても良いものである。 <Alkylamine>
The alkylamine used in the present invention can be appropriately selected from known alkylamines according to properties expected for the produced copper nanoparticle dispersion.
It is presumed that the alkylamine has a function of capturing protons, thereby preventing the copper atom from being oxidized.
Alkylamine has a structure in which an amino group is bonded to a part of an alkyl group. In order to form a coordination bond to a copper atom, RNH 2 (R is a hydrocarbon chain) in which at least one of the amino groups contained in the alkylamine used is a primary amino group or R 1 is a secondary amino group R 2 NH (R 1 and R 2 may be the same or different in the hydrocarbon chain) is desirable. The alkyl chain may be linear, branched or cyclic. The hydrocarbon chain may contain atoms other than carbon such as oxygen, silicon, nitrogen, sulfur, and phosphorus. For example, specifically, it may be an alkylamine having a substituent such as an alkoxy group, an alkoxysilyl group, or an alkylthio group, or may have two amino groups in the molecule.
本発明において使用されるアルキルアミンは、製造される銅ナノ粒子分散体に期待される特性等に応じて、公知のアルキルアミンから適宜選択して用いることができる。
アルキルアミンは、プロトンを捕捉する機能を有することにより、銅原子が酸化されることを防止していると推定される。
アルキルアミンはアルキル基の一部にアミノ基の結合した構造を有している。銅原子に対して配位結合を形成するために、使用するアルキルアミンに含まれるアミノ基の少なくとも1つが一級アミノ基であるRNH2(Rは炭化水素鎖)または二級アミノ基であるR1R2NH(R1、R2は炭化水素鎖で同じであっても異なっていてもよい)であることが望ましい。アルキル鎖は直鎖、分岐鎖、又は環状のいずれであっても良い。また、前記炭化水素鎖には酸素、珪素、窒素、硫黄、リンなどの炭素以外の原子が含有されていても良い。例えば具体的には、アルコキシ基、アルコキシシリル基、アルキルチオ基等の置換基を有するアルキルアミンであっても良く、分子内に二つのアミノ基を有していても良いものである。 <Alkylamine>
The alkylamine used in the present invention can be appropriately selected from known alkylamines according to properties expected for the produced copper nanoparticle dispersion.
It is presumed that the alkylamine has a function of capturing protons, thereby preventing the copper atom from being oxidized.
Alkylamine has a structure in which an amino group is bonded to a part of an alkyl group. In order to form a coordination bond to a copper atom, RNH 2 (R is a hydrocarbon chain) in which at least one of the amino groups contained in the alkylamine used is a primary amino group or R 1 is a secondary amino group R 2 NH (R 1 and R 2 may be the same or different in the hydrocarbon chain) is desirable. The alkyl chain may be linear, branched or cyclic. The hydrocarbon chain may contain atoms other than carbon such as oxygen, silicon, nitrogen, sulfur, and phosphorus. For example, specifically, it may be an alkylamine having a substituent such as an alkoxy group, an alkoxysilyl group, or an alkylthio group, or may have two amino groups in the molecule.
分子内に一つのアミノ基を有するアルキルアミン(モノアミン)としては、例えば、2-エトキシエチルアミン、ジプロピルアミン、ジブチルアミン、ヘキシルアミン、シクロヘキシルアミン、ヘプチルアミン、3-メトキシプロピルアミン、3-エトキシプロピルアミン、3-ブトキシプロピルアミン、オクチルアミン、ノニルアミン、デシルアミン、3-アミノプロピルトリエトキシシラン、ドデシルアミン、ヘキサデシルアミン、オクタデシルアミン、オレイルアミン等のアルキルアミンは工業的に生産され入手が容易な点で実用的である。
Examples of the alkylamine (monoamine) having one amino group in the molecule include 2-ethoxyethylamine, dipropylamine, dibutylamine, hexylamine, cyclohexylamine, heptylamine, 3-methoxypropylamine, and 3-ethoxypropyl. Alkylamines such as amine, 3-butoxypropylamine, octylamine, nonylamine, decylamine, 3-aminopropyltriethoxysilane, dodecylamine, hexadecylamine, octadecylamine and oleylamine are industrially produced and easily available. It is practical.
一方、分子内に二つのアミノ基を有するアルキルジアミンとして、例えば、エチレンジアミン、N,N-ジメチルエチレンジアミン、N,N’-ジメチルエチレンジアミン、N,N-ジエチルエチレンジアミン、N,N’-ジエチルエチレンジアミン、1,3-プロパンジアミン、2,2-ジメチル-1,3-プロパンジアミン、N,N-ジメチル-1,3-ジアミノプロパン、N,N’-ジメチル-1,3-ジアミノプロパン、N,N-ジエチル-1,3-ジアミノプロパン、1,4-ジアミノブタン、1,5-ジアミノ-2-メチルペンタン、1,6-ジアミノヘキサン、N,N’-ジメチル-1,6-ジアミノヘキサン、1,7-ジアミノヘプタン、1,8-ジアミノオクタン、3-ジメチルアミノプロピルアミン、3-ジエチルアミノプロピルアミン、3-ジブチルアミノプロピルアミン、3-メチルアミノプロピルアミン等が挙げられるが、これらに限定されるものではない。
On the other hand, examples of the alkyldiamine having two amino groups in the molecule include ethylenediamine, N, N-dimethylethylenediamine, N, N′-dimethylethylenediamine, N, N-diethylethylenediamine, N, N′-diethylethylenediamine, 1 , 3-propanediamine, 2,2-dimethyl-1,3-propanediamine, N, N-dimethyl-1,3-diaminopropane, N, N′-dimethyl-1,3-diaminopropane, N, N— Diethyl-1,3-diaminopropane, 1,4-diaminobutane, 1,5-diamino-2-methylpentane, 1,6-diaminohexane, N, N′-dimethyl-1,6-diaminohexane, 1, 7-diaminoheptane, 1,8-diaminooctane, 3-dimethylaminopropylamine, 3-diethylamino Propylamine, 3-dibutylaminopropylamine, 3 but methylaminopropylamine and the like, but is not limited thereto.
本発明で用いられるアルキルアミンは、1種のアルキルアミンを使用しても良いが、2種以上のアルキルアミンを混合して使用してもよい。
本発明で用いられるアルキルアミンは、極性が比較的弱く、焼成時に脱離しやすい点から、分子内に一つもしくは二つのアミノ基を有するアルキルアミンを用いることが好ましい。 As the alkylamine used in the present invention, one type of alkylamine may be used, or two or more types of alkylamine may be mixed and used.
The alkylamine used in the present invention is preferably an alkylamine having one or two amino groups in the molecule from the viewpoint that the polarity is relatively weak and that it is easily eliminated during firing.
本発明で用いられるアルキルアミンは、極性が比較的弱く、焼成時に脱離しやすい点から、分子内に一つもしくは二つのアミノ基を有するアルキルアミンを用いることが好ましい。 As the alkylamine used in the present invention, one type of alkylamine may be used, or two or more types of alkylamine may be mixed and used.
The alkylamine used in the present invention is preferably an alkylamine having one or two amino groups in the molecule from the viewpoint that the polarity is relatively weak and that it is easily eliminated during firing.
また、本発明で用いられるアルキルアミンは、焼成時に脱離しやすい点から、分子量が高すぎないことが好ましく、分子量が300以下であることが好ましく、更に200以下であることが好ましい。また、本発明で用いられるアルキルアミンは、焼成時に脱離しやすい点から、炭素数が8以下であることが好ましく、更に6以下であることが好ましい。
また、本発明で用いられるアルキルアミンは、焼成時に脱離しやすい点から、沸点が300℃以下であることが好ましく、更に200℃以下であることが好ましい。一方で、ナノ粒子作製時、保管時の脱離、揮発防止の点から、アルキルアミンの分子量は50以上であることが好ましい。また、沸点は23℃以上であることが好ましく、更に50℃以上であることが好ましい。 In addition, the alkylamine used in the present invention is preferably not too high in molecular weight, and preferably has a molecular weight of 300 or less, more preferably 200 or less, from the viewpoint of easy desorption during firing. In addition, the alkylamine used in the present invention preferably has 8 or less carbon atoms, and more preferably 6 or less, from the viewpoint that it is easily detached during firing.
In addition, the alkylamine used in the present invention preferably has a boiling point of 300 ° C. or lower, more preferably 200 ° C. or lower, from the viewpoint of easy desorption during firing. On the other hand, the molecular weight of the alkylamine is preferably 50 or more from the viewpoints of desorption during storage and storage and prevention of volatilization. The boiling point is preferably 23 ° C. or higher, and more preferably 50 ° C. or higher.
また、本発明で用いられるアルキルアミンは、焼成時に脱離しやすい点から、沸点が300℃以下であることが好ましく、更に200℃以下であることが好ましい。一方で、ナノ粒子作製時、保管時の脱離、揮発防止の点から、アルキルアミンの分子量は50以上であることが好ましい。また、沸点は23℃以上であることが好ましく、更に50℃以上であることが好ましい。 In addition, the alkylamine used in the present invention is preferably not too high in molecular weight, and preferably has a molecular weight of 300 or less, more preferably 200 or less, from the viewpoint of easy desorption during firing. In addition, the alkylamine used in the present invention preferably has 8 or less carbon atoms, and more preferably 6 or less, from the viewpoint that it is easily detached during firing.
In addition, the alkylamine used in the present invention preferably has a boiling point of 300 ° C. or lower, more preferably 200 ° C. or lower, from the viewpoint of easy desorption during firing. On the other hand, the molecular weight of the alkylamine is preferably 50 or more from the viewpoints of desorption during storage and storage and prevention of volatilization. The boiling point is preferably 23 ° C. or higher, and more preferably 50 ° C. or higher.
本発明の銅ナノ粒子分散体において、アルキルアミンの含有量は、用途に応じて適宜選択されれば良いが、低温焼成性の点から、銅100質量部に対して、0.1~30質量部であることが好ましく、更に、0.1~20質量部の範囲内であることがより好ましい。
本発明の銅ナノ粒子分散体において、アルキルアミンの含有量は、用途に応じて適宜選択されれば良いが、耐酸化性、低温焼成性の点から、銅ナノ粒子分散体の全量に対して、0.05~15質量%であることが好ましく、更に、0.05~10質量%の範囲内であることがより好ましい。 In the copper nanoparticle dispersion of the present invention, the content of the alkylamine may be appropriately selected depending on the use, but from the viewpoint of low-temperature calcinability, 0.1 to 30 masses with respect to 100 mass parts of copper. Part, more preferably in the range of 0.1 to 20 parts by weight.
In the copper nanoparticle dispersion of the present invention, the content of the alkylamine may be appropriately selected according to the use, but from the viewpoint of oxidation resistance and low-temperature sinterability, it is based on the total amount of the copper nanoparticle dispersion. 0.05 to 15% by mass, more preferably 0.05 to 10% by mass.
本発明の銅ナノ粒子分散体において、アルキルアミンの含有量は、用途に応じて適宜選択されれば良いが、耐酸化性、低温焼成性の点から、銅ナノ粒子分散体の全量に対して、0.05~15質量%であることが好ましく、更に、0.05~10質量%の範囲内であることがより好ましい。 In the copper nanoparticle dispersion of the present invention, the content of the alkylamine may be appropriately selected depending on the use, but from the viewpoint of low-temperature calcinability, 0.1 to 30 masses with respect to 100 mass parts of copper. Part, more preferably in the range of 0.1 to 20 parts by weight.
In the copper nanoparticle dispersion of the present invention, the content of the alkylamine may be appropriately selected according to the use, but from the viewpoint of oxidation resistance and low-temperature sinterability, it is based on the total amount of the copper nanoparticle dispersion. 0.05 to 15% by mass, more preferably 0.05 to 10% by mass.
<高分子分散剤>
本発明において用いられる高分子分散剤は、アミン価及び酸価の一方が30~160mgKOH/g、アミン価及び酸価の他の一方が0~160mgKOH/gである高分子分散剤であり、塩基性官能基及び酸性官能基の少なくとも1種を有するものである。
塩基性官能基としては一級、二級、又は三級アミノ基、ピリジン、ピリミジン、ピラジン等の含窒素ヘテロ環等をあげることができる。また、酸性官能基としては、カルボン酸基、リン酸基、スルホン酸基等が挙げられる。
なお、アミン価とは、遊離塩基、塩基の総量を示すもので、試料1gを中和するのに要する塩酸に対して当量の水酸化カリウムのmg数で表したものである。また、酸価とは、遊離酸、酸の総量を示すもので、試料1gを中和するのに要する水酸化カリウムのmg数で表したものである。アミン価はJIS-K7237に準拠した方法で、酸価はJIS-K0070に準拠した方法で測定することができる。 <Polymer dispersant>
The polymer dispersant used in the present invention is a polymer dispersant in which one of the amine value and the acid value is 30 to 160 mgKOH / g, and the other one of the amine value and the acid value is 0 to 160 mgKOH / g, Having at least one of a functional functional group and an acidic functional group.
Examples of basic functional groups include primary, secondary, or tertiary amino groups, nitrogen-containing heterocycles such as pyridine, pyrimidine, and pyrazine. Examples of acidic functional groups include carboxylic acid groups, phosphoric acid groups, and sulfonic acid groups.
The amine value indicates the total amount of free base and base, and is expressed in mg of potassium hydroxide equivalent to the hydrochloric acid required to neutralize 1 g of the sample. The acid value represents the total amount of free acid and acid, and is expressed in mg of potassium hydroxide required to neutralize 1 g of the sample. The amine value can be measured by a method according to JIS-K7237, and the acid value can be measured by a method according to JIS-K0070.
本発明において用いられる高分子分散剤は、アミン価及び酸価の一方が30~160mgKOH/g、アミン価及び酸価の他の一方が0~160mgKOH/gである高分子分散剤であり、塩基性官能基及び酸性官能基の少なくとも1種を有するものである。
塩基性官能基としては一級、二級、又は三級アミノ基、ピリジン、ピリミジン、ピラジン等の含窒素ヘテロ環等をあげることができる。また、酸性官能基としては、カルボン酸基、リン酸基、スルホン酸基等が挙げられる。
なお、アミン価とは、遊離塩基、塩基の総量を示すもので、試料1gを中和するのに要する塩酸に対して当量の水酸化カリウムのmg数で表したものである。また、酸価とは、遊離酸、酸の総量を示すもので、試料1gを中和するのに要する水酸化カリウムのmg数で表したものである。アミン価はJIS-K7237に準拠した方法で、酸価はJIS-K0070に準拠した方法で測定することができる。 <Polymer dispersant>
The polymer dispersant used in the present invention is a polymer dispersant in which one of the amine value and the acid value is 30 to 160 mgKOH / g, and the other one of the amine value and the acid value is 0 to 160 mgKOH / g, Having at least one of a functional functional group and an acidic functional group.
Examples of basic functional groups include primary, secondary, or tertiary amino groups, nitrogen-containing heterocycles such as pyridine, pyrimidine, and pyrazine. Examples of acidic functional groups include carboxylic acid groups, phosphoric acid groups, and sulfonic acid groups.
The amine value indicates the total amount of free base and base, and is expressed in mg of potassium hydroxide equivalent to the hydrochloric acid required to neutralize 1 g of the sample. The acid value represents the total amount of free acid and acid, and is expressed in mg of potassium hydroxide required to neutralize 1 g of the sample. The amine value can be measured by a method according to JIS-K7237, and the acid value can be measured by a method according to JIS-K0070.
本発明において用いられる高分子分散剤は、カルボン酸及びアルキルアミンの両方が付着した銅ナノ粒子に対して、上記特定のアミン価及び酸価を有し、適切な量の塩基性官能基や酸性官能基を有することから、前記銅ナノ粒子に安定して吸着し、一方でポリマー鎖部分により立体障害をもたらし、銅ナノ粒子同士の凝集を安定して防止していると推定される。
後述の実施例及び比較例に示すように、カルボン酸とアルキルアミンの両方が付着した銅ナノ粒子に対しては、高分子分散剤のアミン価及び酸価が低すぎても、或いは高すぎても、いずれも高分子分散剤が安定的に付着できず、むしろ分散性は悪化する。特に、分散性は低いが低温焼成性に優れる炭素数が小さいカルボン酸と組み合わせる場合には、高分子分散剤のアミン価及び酸価が低すぎても、高すぎても、分散できないことが明らかにされた。 The polymer dispersant used in the present invention has the above-mentioned specific amine value and acid value with respect to copper nanoparticles to which both carboxylic acid and alkylamine are attached, and an appropriate amount of basic functional group or acidity. Since it has a functional group, it is presumed that it adsorbs stably to the copper nanoparticles, while causing a steric hindrance by the polymer chain portion and stably preventing aggregation of the copper nanoparticles.
As shown in the examples and comparative examples described later, for the copper nanoparticles to which both carboxylic acid and alkylamine are attached, the amine value and acid value of the polymer dispersant are too low or too high. In either case, the polymer dispersant cannot be stably attached, but rather the dispersibility deteriorates. In particular, when combined with a carboxylic acid having a low dispersibility but excellent low-temperature calcinability and having a small number of carbon atoms, it is clear that the polymer dispersant cannot be dispersed if the amine value and acid value are too low or too high. It was made.
後述の実施例及び比較例に示すように、カルボン酸とアルキルアミンの両方が付着した銅ナノ粒子に対しては、高分子分散剤のアミン価及び酸価が低すぎても、或いは高すぎても、いずれも高分子分散剤が安定的に付着できず、むしろ分散性は悪化する。特に、分散性は低いが低温焼成性に優れる炭素数が小さいカルボン酸と組み合わせる場合には、高分子分散剤のアミン価及び酸価が低すぎても、高すぎても、分散できないことが明らかにされた。 The polymer dispersant used in the present invention has the above-mentioned specific amine value and acid value with respect to copper nanoparticles to which both carboxylic acid and alkylamine are attached, and an appropriate amount of basic functional group or acidity. Since it has a functional group, it is presumed that it adsorbs stably to the copper nanoparticles, while causing a steric hindrance by the polymer chain portion and stably preventing aggregation of the copper nanoparticles.
As shown in the examples and comparative examples described later, for the copper nanoparticles to which both carboxylic acid and alkylamine are attached, the amine value and acid value of the polymer dispersant are too low or too high. In either case, the polymer dispersant cannot be stably attached, but rather the dispersibility deteriorates. In particular, when combined with a carboxylic acid having a low dispersibility but excellent low-temperature calcinability and having a small number of carbon atoms, it is clear that the polymer dispersant cannot be dispersed if the amine value and acid value are too low or too high. It was made.
本発明においては、上記特定のアミン価及び酸価を有する高分子分散剤が、銅ナノ粒子と安定して吸着することにより、分散性及び分散安定性が向上するため、銅ナノ粒子の分散粒径を小さくすることができる。そのため、上記高分子分散剤を用いると銅ナノ粒子分散体の塗膜の平滑性、均一性が優れたものとなり、また塗膜中の銅ナノ粒子は高密度に配列する。従って、焼結が均一に進行し易く、銅ナノ粒子同士が融着し易い。また当該高分子分散剤は、上記アルキルアミンとの相乗効果によって焼成により分解乃至揮散されやすく、得られた導電性基板は、有機成分の残存が抑制される。これらの結果、得られた金属膜は導電性に優れると推定される。
In the present invention, the polymer dispersant having the specific amine value and acid value is stably adsorbed to the copper nanoparticles, whereby dispersibility and dispersion stability are improved. The diameter can be reduced. Therefore, when the above polymer dispersant is used, the smoothness and uniformity of the coating film of the copper nanoparticle dispersion are excellent, and the copper nanoparticles in the coating film are arranged at high density. Therefore, sintering is easy to proceed uniformly, and the copper nanoparticles are easily fused. In addition, the polymer dispersant is easily decomposed or volatilized by baking due to a synergistic effect with the alkylamine, and the resulting conductive substrate suppresses the remaining organic components. As a result, the obtained metal film is presumed to be excellent in conductivity.
本発明において用いられる高分子分散剤は、上述の理由から、アミン価及び酸価の一方が30~160mgKOH/g、アミン価及び酸価の他の一方が0~160mgKOH/gであるが、中でも、アミン価及び酸価の一方が40~140mgKOH/gであり、アミン価及び酸価の他の一方が0~140mgKOH/gであることが、好ましい。
The polymer dispersant used in the present invention has an amine value and an acid value of 30 to 160 mgKOH / g and the other one of the amine value and the acid value is 0 to 160 mgKOH / g for the reasons described above. One of the amine value and the acid value is preferably 40 to 140 mgKOH / g, and the other one of the amine value and the acid value is preferably 0 to 140 mgKOH / g.
本発明に用いられる高分子分散剤としては、分散性と塗布適性が優れる点から、重量平均分子量が、800以上であることが好ましく、更に900以上であることが好ましく、特に1000以上であることが好ましい。一方で、低温焼成性が優れる点から、30000以下であることが好ましく、更に20000以下であることが好ましく、特に10000以下であることが好ましい。なお、本発明における重量平均分子量は、ゲルパーミエーションクロマトグラフィー(GPC)法(ポリスチレンン換算)で測定することができる。
The polymer dispersant used in the present invention preferably has a weight average molecular weight of 800 or more, more preferably 900 or more, particularly 1000 or more, from the viewpoint of excellent dispersibility and coating suitability. Is preferred. On the other hand, from the viewpoint of excellent low-temperature calcinability, it is preferably 30000 or less, more preferably 20000 or less, and particularly preferably 10,000 or less. In addition, the weight average molecular weight in this invention can be measured by the gel permeation chromatography (GPC) method (polystyrene conversion).
また、本発明に用いられる高分子分散剤は、90%熱重量減少温度が450℃以下であること、更に420℃以下であることが、低温焼成性及び焼結後の塗膜の導電性が優れる点から好ましい。なお、ここでの90%熱重量減少温度は、熱重量測定(TG)により次のようにして測定した値とする。熱重量測定装置(例えば、島津製作所製DTG-60A)を用い、試料約5mgについて窒素雰囲気下で測定する。昇温速度は10℃/分とし、室温(23℃)~600℃まで測定する。本発明においては、室温時点の試料重量を基準に90%が減量した時点の温度を90%熱重量減少温度と定義する。
Further, the polymer dispersant used in the present invention has a 90% thermogravimetric decrease temperature of 450 ° C. or lower, and further 420 ° C. or lower, so that the low-temperature firing property and the conductivity of the coated film after sintering are reduced. It is preferable from an excellent point. Here, the 90% thermogravimetric decrease temperature is a value measured by thermogravimetry (TG) as follows. Using a thermogravimetry apparatus (for example, DTG-60A manufactured by Shimadzu Corporation), about 5 mg of a sample is measured in a nitrogen atmosphere. The temperature rising rate is 10 ° C./min, and the temperature is measured from room temperature (23 ° C.) to 600 ° C. In the present invention, the temperature at which 90% is reduced based on the sample weight at room temperature is defined as the 90% thermogravimetric decrease temperature.
本発明において用いられるアミン価及び酸価の一方が30~160mgKOH/g、アミン価及び酸価の他の一方が0~160mgKOH/gである高分子分散剤は、通常、塗料、インキ分野などで着色剤の分散に用いられている高分子分散剤から適宜選択して用いることができる。
上記高分子分散剤としては、例えば、ポリアクリル酸エステル等の不飽和カルボン酸エステルの(共)重合体類;ポリアクリル酸等の不飽和カルボン酸の(共)重合体の(部分)アミン塩、(部分)アンモニウム塩や(部分)アルキルアミン塩類;水酸基含有ポリアクリル酸エステル等の水酸基含有不飽和カルボン酸エステルの(共)重合体やそれらの変性物;ポリウレタン類;不飽和ポリアミド類;ポリシロキサン類;長鎖ポリアミノアミドリン酸塩類;ポリエチレンイミン誘導体(ポリ(低級アルキレンイミン)と遊離カルボキシル基含有ポリエステルとの反応により得られるアミドやそれらの塩基);ポリアリルアミン誘導体(ポリアリルアミンと、遊離のカルボキシル基を有するポリエステル、ポリアミド又はエステルとアミドの共縮合物(ポリエステルアミド)の3種の化合物の中から選ばれる1種以上の化合物とを反応させて得られる反応生成物)等が挙げられる。 The polymer dispersant having one of an amine value and an acid value of 30 to 160 mg KOH / g and the other one of an amine value and an acid value used in the present invention is usually from 0 to 160 mg KOH / g. It can be appropriately selected from polymer dispersants used for dispersing colorants.
Examples of the polymer dispersant include (co) polymers of unsaturated carboxylic acid esters such as polyacrylic acid esters; (partial) amine salts of (co) polymers of unsaturated carboxylic acid such as polyacrylic acid , (Partial) ammonium salts and (partial) alkylamine salts; hydroxyl group-containing unsaturated carboxylic acid ester (co) polymers such as hydroxyl group-containing polyacrylates and their modified products; polyurethanes; unsaturated polyamides; Long chain polyaminoamide phosphates; Polyethyleneimine derivatives (amides and their bases obtained by reaction of poly (lower alkyleneimines) with free carboxyl group-containing polyesters); Polyallylamine derivatives (polyallylamine and free radicals) Polyester, polyamide or ester and amide having carboxyl group Compound reaction product obtained by the reaction of one or more compounds selected from the three compounds of (polyester amide)), and the like.
上記高分子分散剤としては、例えば、ポリアクリル酸エステル等の不飽和カルボン酸エステルの(共)重合体類;ポリアクリル酸等の不飽和カルボン酸の(共)重合体の(部分)アミン塩、(部分)アンモニウム塩や(部分)アルキルアミン塩類;水酸基含有ポリアクリル酸エステル等の水酸基含有不飽和カルボン酸エステルの(共)重合体やそれらの変性物;ポリウレタン類;不飽和ポリアミド類;ポリシロキサン類;長鎖ポリアミノアミドリン酸塩類;ポリエチレンイミン誘導体(ポリ(低級アルキレンイミン)と遊離カルボキシル基含有ポリエステルとの反応により得られるアミドやそれらの塩基);ポリアリルアミン誘導体(ポリアリルアミンと、遊離のカルボキシル基を有するポリエステル、ポリアミド又はエステルとアミドの共縮合物(ポリエステルアミド)の3種の化合物の中から選ばれる1種以上の化合物とを反応させて得られる反応生成物)等が挙げられる。 The polymer dispersant having one of an amine value and an acid value of 30 to 160 mg KOH / g and the other one of an amine value and an acid value used in the present invention is usually from 0 to 160 mg KOH / g. It can be appropriately selected from polymer dispersants used for dispersing colorants.
Examples of the polymer dispersant include (co) polymers of unsaturated carboxylic acid esters such as polyacrylic acid esters; (partial) amine salts of (co) polymers of unsaturated carboxylic acid such as polyacrylic acid , (Partial) ammonium salts and (partial) alkylamine salts; hydroxyl group-containing unsaturated carboxylic acid ester (co) polymers such as hydroxyl group-containing polyacrylates and their modified products; polyurethanes; unsaturated polyamides; Long chain polyaminoamide phosphates; Polyethyleneimine derivatives (amides and their bases obtained by reaction of poly (lower alkyleneimines) with free carboxyl group-containing polyesters); Polyallylamine derivatives (polyallylamine and free radicals) Polyester, polyamide or ester and amide having carboxyl group Compound reaction product obtained by the reaction of one or more compounds selected from the three compounds of (polyester amide)), and the like.
本発明において用いられる高分子分散剤としては、主鎖及び側鎖の少なくとも一方に、ポリエステル骨格又はポリエーテル骨格を有することが好ましい。このような高分子分散剤は、その骨格構造に起因して、低温での焼成により分解されやすく、有機物が残存しにくいため、焼成後の膜の導電性に優れている。
The polymer dispersant used in the present invention preferably has a polyester skeleton or a polyether skeleton in at least one of the main chain and the side chain. Such a polymer dispersant is excellent in conductivity of the fired film because it is easily decomposed by baking at a low temperature due to its skeletal structure, and organic matter hardly remains.
本発明の銅ナノ粒子分散体において、上記高分子分散剤としては、1種用いてもよいし、2種以上を組み合わせて用いてもよく、その含有量は、用いる銅ナノ粒子の種類等に応じて適宜設定されるが、銅ナノ粒子100質量部に対して、通常、0.1~100質量部の範囲であり、1~50質量部であることが好ましく、2~30質量部であることがより好ましい。
本発明の銅ナノ粒子分散体において、上記高分子分散剤の含有量は、用途に応じて適宜選択されれば良いが、分散性、塗布適性、低温焼成性の点から、銅ナノ粒子分散体の全量に対して、0.05~25質量%であることが好ましく、更に、0.5~15質量%の範囲内であることがより好ましい。
上記高分子分散剤の含有量が上記下限値以上であれば、銅ナノ粒子分散体の分散性及び分散安定性を優れたものとすることができる。また上記上限値以下であれば、焼成後の膜の導電性に優れている。 In the copper nanoparticle dispersion of the present invention, one type of polymer dispersant may be used, or two or more types may be used in combination, and the content depends on the type of copper nanoparticles used. Depending on thecopper nanoparticles 100 parts by weight, it is usually in the range of 0.1 to 100 parts by weight, preferably 1 to 50 parts by weight, and 2 to 30 parts by weight. It is more preferable.
In the copper nanoparticle dispersion of the present invention, the content of the polymer dispersant may be appropriately selected according to the use, but from the viewpoint of dispersibility, coating suitability, and low-temperature firing properties, the copper nanoparticle dispersion The amount is preferably from 0.05 to 25% by mass, more preferably from 0.5 to 15% by mass, based on the total amount.
If content of the said polymer dispersing agent is more than the said lower limit, the dispersibility and dispersion stability of a copper nanoparticle dispersion can be made excellent. Moreover, if it is below the said upper limit, it is excellent in the electroconductivity of the film | membrane after baking.
本発明の銅ナノ粒子分散体において、上記高分子分散剤の含有量は、用途に応じて適宜選択されれば良いが、分散性、塗布適性、低温焼成性の点から、銅ナノ粒子分散体の全量に対して、0.05~25質量%であることが好ましく、更に、0.5~15質量%の範囲内であることがより好ましい。
上記高分子分散剤の含有量が上記下限値以上であれば、銅ナノ粒子分散体の分散性及び分散安定性を優れたものとすることができる。また上記上限値以下であれば、焼成後の膜の導電性に優れている。 In the copper nanoparticle dispersion of the present invention, one type of polymer dispersant may be used, or two or more types may be used in combination, and the content depends on the type of copper nanoparticles used. Depending on the
In the copper nanoparticle dispersion of the present invention, the content of the polymer dispersant may be appropriately selected according to the use, but from the viewpoint of dispersibility, coating suitability, and low-temperature firing properties, the copper nanoparticle dispersion The amount is preferably from 0.05 to 25% by mass, more preferably from 0.5 to 15% by mass, based on the total amount.
If content of the said polymer dispersing agent is more than the said lower limit, the dispersibility and dispersion stability of a copper nanoparticle dispersion can be made excellent. Moreover, if it is below the said upper limit, it is excellent in the electroconductivity of the film | membrane after baking.
<溶剤>
本発明の銅ナノ粒子分散体において、溶剤は、銅ナノ粒子分散体中の各成分とは反応せず、これらを溶解もしくは分散可能な有機溶剤であればよく、特に限定されない。銅ナノ粒子分散体に従来用いられている有機溶剤を適宜選択して用いれば良い。中でも、本発明に用いられる溶剤としては、MBA(酢酸3-メトキシブチル)、PGMEA(プロピレングリコールモノメチルエーテルアセテート)、DMDG(ジエチレングリコールジメチルエーテル)、ジエチレングリコールメチルエチルエーテル、PGME(プロピレングリコールモノメチルエーテル)又はこれらを混合したものが、上記高分子分散剤の溶解性や塗布適性の点から好ましい。 <Solvent>
In the copper nanoparticle dispersion of the present invention, the solvent is not particularly limited as long as it is an organic solvent that does not react with each component in the copper nanoparticle dispersion and can dissolve or disperse them. An organic solvent conventionally used for the copper nanoparticle dispersion may be appropriately selected and used. Among them, as the solvent used in the present invention, MBA (3-methoxybutyl acetate), PGMEA (propylene glycol monomethyl ether acetate), DMDG (diethylene glycol dimethyl ether), diethylene glycol methyl ethyl ether, PGME (propylene glycol monomethyl ether) or these are used. What mixed is preferable from the point of the solubility of the said polymer dispersing agent, and the applicability | paintability.
本発明の銅ナノ粒子分散体において、溶剤は、銅ナノ粒子分散体中の各成分とは反応せず、これらを溶解もしくは分散可能な有機溶剤であればよく、特に限定されない。銅ナノ粒子分散体に従来用いられている有機溶剤を適宜選択して用いれば良い。中でも、本発明に用いられる溶剤としては、MBA(酢酸3-メトキシブチル)、PGMEA(プロピレングリコールモノメチルエーテルアセテート)、DMDG(ジエチレングリコールジメチルエーテル)、ジエチレングリコールメチルエチルエーテル、PGME(プロピレングリコールモノメチルエーテル)又はこれらを混合したものが、上記高分子分散剤の溶解性や塗布適性の点から好ましい。 <Solvent>
In the copper nanoparticle dispersion of the present invention, the solvent is not particularly limited as long as it is an organic solvent that does not react with each component in the copper nanoparticle dispersion and can dissolve or disperse them. An organic solvent conventionally used for the copper nanoparticle dispersion may be appropriately selected and used. Among them, as the solvent used in the present invention, MBA (3-methoxybutyl acetate), PGMEA (propylene glycol monomethyl ether acetate), DMDG (diethylene glycol dimethyl ether), diethylene glycol methyl ethyl ether, PGME (propylene glycol monomethyl ether) or these are used. What mixed is preferable from the point of the solubility of the said polymer dispersing agent, and the applicability | paintability.
本発明の銅ナノ粒子分散体における溶剤の含有量は、該銅ナノ粒子分散体の各構成を均一に溶解又は分散することができるものであればよく、特に限定されない。本発明においては、該銅ナノ粒子分散体中の固形分含有量が、5~95質量%の範囲が好ましく、10~90質量%の範囲がより好ましい。上記範囲であることにより、塗布に適した粘度とすることができる。
The content of the solvent in the copper nanoparticle dispersion of the present invention is not particularly limited as long as it can uniformly dissolve or disperse each component of the copper nanoparticle dispersion. In the present invention, the solid content in the copper nanoparticle dispersion is preferably in the range of 5 to 95% by mass, more preferably in the range of 10 to 90% by mass. By being the said range, it can be set as the viscosity suitable for application | coating.
<その他の成分>
本発明の銅ナノ粒子分散体には、本発明の効果を損なわない範囲で、必要に応じて、従来銅ナノ粒子分散体に用いられている公知のその他の成分を適宜含有してもよい。
その他の成分としては、例えば、錯化剤、有機保護剤、還元剤、濡れ性向上のための界面活性剤、密着性向上のためのシランカップリング剤、消泡剤、ハジキ防止剤、酸化防止剤、凝集防止剤、粘度調製剤、等が挙げられる。また、本発明の効果が損なわれない限り、他の分散剤が含まれていてもよい。更に、本発明の効果が損なわれない範囲で、造膜性、印刷適性や分散性の点から、アクリル樹脂、ポリエステル樹脂、セルロース樹脂、オレフィン樹脂等の樹脂バインダーを添加してもよい。 <Other ingredients>
The copper nanoparticle dispersion of the present invention may appropriately contain other known components conventionally used in copper nanoparticle dispersions as needed, as long as the effects of the present invention are not impaired.
Other components include, for example, complexing agents, organic protective agents, reducing agents, surfactants for improving wettability, silane coupling agents for improving adhesion, antifoaming agents, repellency inhibitors, and antioxidants. Agents, anti-aggregation agents, viscosity modifiers, and the like. Moreover, as long as the effect of this invention is not impaired, the other dispersing agent may be contained. Furthermore, a resin binder such as an acrylic resin, a polyester resin, a cellulose resin, and an olefin resin may be added from the viewpoints of film forming property, printability, and dispersibility within a range that does not impair the effects of the present invention.
本発明の銅ナノ粒子分散体には、本発明の効果を損なわない範囲で、必要に応じて、従来銅ナノ粒子分散体に用いられている公知のその他の成分を適宜含有してもよい。
その他の成分としては、例えば、錯化剤、有機保護剤、還元剤、濡れ性向上のための界面活性剤、密着性向上のためのシランカップリング剤、消泡剤、ハジキ防止剤、酸化防止剤、凝集防止剤、粘度調製剤、等が挙げられる。また、本発明の効果が損なわれない限り、他の分散剤が含まれていてもよい。更に、本発明の効果が損なわれない範囲で、造膜性、印刷適性や分散性の点から、アクリル樹脂、ポリエステル樹脂、セルロース樹脂、オレフィン樹脂等の樹脂バインダーを添加してもよい。 <Other ingredients>
The copper nanoparticle dispersion of the present invention may appropriately contain other known components conventionally used in copper nanoparticle dispersions as needed, as long as the effects of the present invention are not impaired.
Other components include, for example, complexing agents, organic protective agents, reducing agents, surfactants for improving wettability, silane coupling agents for improving adhesion, antifoaming agents, repellency inhibitors, and antioxidants. Agents, anti-aggregation agents, viscosity modifiers, and the like. Moreover, as long as the effect of this invention is not impaired, the other dispersing agent may be contained. Furthermore, a resin binder such as an acrylic resin, a polyester resin, a cellulose resin, and an olefin resin may be added from the viewpoints of film forming property, printability, and dispersibility within a range that does not impair the effects of the present invention.
<銅ナノ粒子分散体の体積平均粒径>
本発明の銅ナノ粒子分散体は、動的光散乱法による体積平均粒径が500nm以下であるが、中でも450nm以下であることが好ましい。本発明の銅ナノ粒子分散体はこのように銅ナノ粒子の分散粒径が小さいことから、塗膜の優れた平滑性、均一性、及び、塗膜中の銅ナノ粒子の高密度配列を実現する。
銅ナノ粒子分散体中の動的光散乱法による体積平均粒径は、少なくとも溶剤を含有する分散媒体中に分散している銅ナノ粒子の分散平均粒径であって、レーザー光散乱粒度分布計により測定されるものである。レーザー光散乱粒度分布計による粒径の測定としては、銅ナノ粒子分散体に用いられている溶剤で、銅ナノ粒子分散体をレーザー光散乱粒度分布計で測定可能な濃度に適宜希釈(例えば、1000倍など)し、レーザー光散乱粒度分布計(例えば、日機装製ナノトラック粒度分布測定装置UPA-EX150)を用いて動的光散乱法により23℃にて測定することができる。 <Volume average particle diameter of copper nanoparticle dispersion>
The copper nanoparticle dispersion of the present invention has a volume average particle size of 500 nm or less by dynamic light scattering method, and preferably 450 nm or less. Since the copper nanoparticle dispersion of the present invention has such a small dispersed particle size, excellent smoothness and uniformity of the coating film and high density arrangement of the copper nanoparticles in the coating film are realized. To do.
The volume average particle diameter by the dynamic light scattering method in the copper nanoparticle dispersion is a dispersion average particle diameter of copper nanoparticles dispersed in a dispersion medium containing at least a solvent, and is a laser light scattering particle size distribution analyzer. It is measured by. As the measurement of the particle size with a laser light scattering particle size distribution meter, the copper nano particle dispersion is appropriately diluted to a concentration that can be measured with a laser light scattering particle size distribution meter with a solvent used in the copper nano particle dispersion (for example, And can be measured at 23 ° C. by a dynamic light scattering method using a laser light scattering particle size distribution analyzer (for example, Nikkiso Nanotrac particle size distribution analyzer UPA-EX150).
本発明の銅ナノ粒子分散体は、動的光散乱法による体積平均粒径が500nm以下であるが、中でも450nm以下であることが好ましい。本発明の銅ナノ粒子分散体はこのように銅ナノ粒子の分散粒径が小さいことから、塗膜の優れた平滑性、均一性、及び、塗膜中の銅ナノ粒子の高密度配列を実現する。
銅ナノ粒子分散体中の動的光散乱法による体積平均粒径は、少なくとも溶剤を含有する分散媒体中に分散している銅ナノ粒子の分散平均粒径であって、レーザー光散乱粒度分布計により測定されるものである。レーザー光散乱粒度分布計による粒径の測定としては、銅ナノ粒子分散体に用いられている溶剤で、銅ナノ粒子分散体をレーザー光散乱粒度分布計で測定可能な濃度に適宜希釈(例えば、1000倍など)し、レーザー光散乱粒度分布計(例えば、日機装製ナノトラック粒度分布測定装置UPA-EX150)を用いて動的光散乱法により23℃にて測定することができる。 <Volume average particle diameter of copper nanoparticle dispersion>
The copper nanoparticle dispersion of the present invention has a volume average particle size of 500 nm or less by dynamic light scattering method, and preferably 450 nm or less. Since the copper nanoparticle dispersion of the present invention has such a small dispersed particle size, excellent smoothness and uniformity of the coating film and high density arrangement of the copper nanoparticles in the coating film are realized. To do.
The volume average particle diameter by the dynamic light scattering method in the copper nanoparticle dispersion is a dispersion average particle diameter of copper nanoparticles dispersed in a dispersion medium containing at least a solvent, and is a laser light scattering particle size distribution analyzer. It is measured by. As the measurement of the particle size with a laser light scattering particle size distribution meter, the copper nano particle dispersion is appropriately diluted to a concentration that can be measured with a laser light scattering particle size distribution meter with a solvent used in the copper nano particle dispersion (for example, And can be measured at 23 ° C. by a dynamic light scattering method using a laser light scattering particle size distribution analyzer (for example, Nikkiso Nanotrac particle size distribution analyzer UPA-EX150).
<銅ナノ粒子分散体の製造方法>
本発明において、銅ナノ粒子分散体の製造方法は、銅ナノ粒子が良好に分散できる方法であればよく、従来公知の方法から適宜選択して用いることができる。例えば、まずカルボン酸及びアルキルアミンが付着した銅ナノ粒子を準備し、当該銅ナノ粒子を、従来公知の方法により、溶剤中で上記高分子分散剤により分散する方法が挙げられる。
カルボン酸及びアルキルアミンが付着した銅ナノ粒子を準備する方法としては、製造時に保護剤としてカルボン酸及びアルキルアミンを用いて製造された銅ナノ粒子を用いても良いし、他の保護剤を用いて製造された銅ナノ粒子の保護剤を公知の方法でカルボン酸やアルキルアミンに置換しても良い。 <Method for producing copper nanoparticle dispersion>
In this invention, the manufacturing method of a copper nanoparticle dispersion should just be a method in which a copper nanoparticle can disperse | distribute favorably, and can be suitably selected and used from a conventionally well-known method. For example, first, copper nanoparticles to which carboxylic acid and alkylamine are attached are prepared, and the copper nanoparticles are dispersed in the solvent with the above-described polymer dispersant by a conventionally known method.
As a method for preparing copper nanoparticles having carboxylic acid and alkylamine attached thereto, copper nanoparticles produced using carboxylic acid and alkylamine as a protective agent during production may be used, or other protective agents may be used. The copper nanoparticle protective agent produced in this manner may be substituted with a carboxylic acid or an alkylamine by a known method.
本発明において、銅ナノ粒子分散体の製造方法は、銅ナノ粒子が良好に分散できる方法であればよく、従来公知の方法から適宜選択して用いることができる。例えば、まずカルボン酸及びアルキルアミンが付着した銅ナノ粒子を準備し、当該銅ナノ粒子を、従来公知の方法により、溶剤中で上記高分子分散剤により分散する方法が挙げられる。
カルボン酸及びアルキルアミンが付着した銅ナノ粒子を準備する方法としては、製造時に保護剤としてカルボン酸及びアルキルアミンを用いて製造された銅ナノ粒子を用いても良いし、他の保護剤を用いて製造された銅ナノ粒子の保護剤を公知の方法でカルボン酸やアルキルアミンに置換しても良い。 <Method for producing copper nanoparticle dispersion>
In this invention, the manufacturing method of a copper nanoparticle dispersion should just be a method in which a copper nanoparticle can disperse | distribute favorably, and can be suitably selected and used from a conventionally well-known method. For example, first, copper nanoparticles to which carboxylic acid and alkylamine are attached are prepared, and the copper nanoparticles are dispersed in the solvent with the above-described polymer dispersant by a conventionally known method.
As a method for preparing copper nanoparticles having carboxylic acid and alkylamine attached thereto, copper nanoparticles produced using carboxylic acid and alkylamine as a protective agent during production may be used, or other protective agents may be used. The copper nanoparticle protective agent produced in this manner may be substituted with a carboxylic acid or an alkylamine by a known method.
中でも、本発明において、銅ナノ粒子分散体の製造方法は、銅を含む化合物、還元性化合物、カルボン酸、及びアルキルアミンを含む混合物、又は、カルボン酸銅、還元性化合物、及びアルキルアミンを含む混合物のいずれかを加熱することにより銅ナノ粒子を調製する工程と、前記銅ナノ粒子を、アミン価及び酸価の一方が30~160mgKOH/g、アミン価及び酸価の他の一方が0~160mgKOH/gである高分子分散剤により溶剤中で分散することにより、動的光散乱法による体積平均粒径が500nm以下である銅ナノ粒子分散体を調製する工程とを有することが好ましい。
なお、銅を含む化合物、還元性化合物、カルボン酸、及びアルキルアミンを含む混合物、又は、カルボン酸銅、還元性化合物、及びアルキルアミンを含む混合物は、加熱する時点で当該混合物となっていれば良い。 Among them, in the present invention, the method for producing a copper nanoparticle dispersion includes a compound containing copper, a reducing compound, a mixture containing a carboxylic acid and an alkylamine, or a copper carboxylate, a reducing compound and an alkylamine. A step of preparing copper nanoparticles by heating any of the mixture, and the copper nanoparticles having an amine value and an acid value of 30 to 160 mg KOH / g, and the other of the amine value and the acid value of 0 to It is preferable to have the process of preparing the copper nanoparticle dispersion whose volume average particle diameter by a dynamic light-scattering method is 500 nm or less by disperse | distributing in a solvent by the polymer dispersing agent which is 160 mgKOH / g.
In addition, if the compound containing copper, a reducing compound, a carboxylic acid, and a mixture containing an alkylamine or a mixture containing copper carboxylate, a reducing compound, and an alkylamine is the mixture at the time of heating. good.
なお、銅を含む化合物、還元性化合物、カルボン酸、及びアルキルアミンを含む混合物、又は、カルボン酸銅、還元性化合物、及びアルキルアミンを含む混合物は、加熱する時点で当該混合物となっていれば良い。 Among them, in the present invention, the method for producing a copper nanoparticle dispersion includes a compound containing copper, a reducing compound, a mixture containing a carboxylic acid and an alkylamine, or a copper carboxylate, a reducing compound and an alkylamine. A step of preparing copper nanoparticles by heating any of the mixture, and the copper nanoparticles having an amine value and an acid value of 30 to 160 mg KOH / g, and the other of the amine value and the acid value of 0 to It is preferable to have the process of preparing the copper nanoparticle dispersion whose volume average particle diameter by a dynamic light-scattering method is 500 nm or less by disperse | distributing in a solvent by the polymer dispersing agent which is 160 mgKOH / g.
In addition, if the compound containing copper, a reducing compound, a carboxylic acid, and a mixture containing an alkylamine or a mixture containing copper carboxylate, a reducing compound, and an alkylamine is the mixture at the time of heating. good.
(銅ナノ粒子を調製する工程)
上記銅ナノ粒子を調製する工程において、銅を含む化合物(以下、含銅化合物ということがある)は、還元性化合物との間で錯体等の複合化合物を生成可能な含銅化合物が、銅ナノ粒子の金属源として用いられる。当該含銅化合物としては、例えば、水酸化銅、シュウ酸銅、酢酸銅、プロピオン酸銅、酪酸銅、イソ酪酸銅、吉草酸銅、イソ吉草酸銅、カプロン酸銅、エナント酸銅、カプリル酸銅、ノナン酸銅、カプリン酸銅、ピバリン酸銅、マロン酸銅、コハク酸銅、マレイン酸銅、安息香酸銅、クエン酸銅、酒石酸銅、硝酸銅、亜硝酸銅、亜硫酸銅、硫酸銅、リン酸銅のような銅の有機酸塩や無機酸塩等が例示される他、アセチルアセトンが配位結合したアセチルアセトナト銅に代表される錯化合物等が挙げられる。 (Step of preparing copper nanoparticles)
In the step of preparing the copper nanoparticles, a compound containing copper (hereinafter sometimes referred to as a copper-containing compound) is a copper-containing compound capable of forming a complex compound such as a complex with a reducing compound. Used as a metal source for particles. Examples of the copper-containing compound include copper hydroxide, copper oxalate, copper acetate, copper propionate, copper butyrate, copper isobutyrate, copper valerate, copper isovalerate, copper caproate, copper enanthate, and caprylic acid. Copper, copper nonanoate, copper caprate, copper pivalate, copper malonate, copper succinate, copper maleate, copper benzoate, copper citrate, copper tartrate, copper nitrate, copper nitrite, copper sulfite, copper sulfate, Examples include organic acid salts and inorganic acid salts of copper such as copper phosphate, and complex compounds represented by acetylacetonato copper coordinated with acetylacetone.
上記銅ナノ粒子を調製する工程において、銅を含む化合物(以下、含銅化合物ということがある)は、還元性化合物との間で錯体等の複合化合物を生成可能な含銅化合物が、銅ナノ粒子の金属源として用いられる。当該含銅化合物としては、例えば、水酸化銅、シュウ酸銅、酢酸銅、プロピオン酸銅、酪酸銅、イソ酪酸銅、吉草酸銅、イソ吉草酸銅、カプロン酸銅、エナント酸銅、カプリル酸銅、ノナン酸銅、カプリン酸銅、ピバリン酸銅、マロン酸銅、コハク酸銅、マレイン酸銅、安息香酸銅、クエン酸銅、酒石酸銅、硝酸銅、亜硝酸銅、亜硫酸銅、硫酸銅、リン酸銅のような銅の有機酸塩や無機酸塩等が例示される他、アセチルアセトンが配位結合したアセチルアセトナト銅に代表される錯化合物等が挙げられる。 (Step of preparing copper nanoparticles)
In the step of preparing the copper nanoparticles, a compound containing copper (hereinafter sometimes referred to as a copper-containing compound) is a copper-containing compound capable of forming a complex compound such as a complex with a reducing compound. Used as a metal source for particles. Examples of the copper-containing compound include copper hydroxide, copper oxalate, copper acetate, copper propionate, copper butyrate, copper isobutyrate, copper valerate, copper isovalerate, copper caproate, copper enanthate, and caprylic acid. Copper, copper nonanoate, copper caprate, copper pivalate, copper malonate, copper succinate, copper maleate, copper benzoate, copper citrate, copper tartrate, copper nitrate, copper nitrite, copper sulfite, copper sulfate, Examples include organic acid salts and inorganic acid salts of copper such as copper phosphate, and complex compounds represented by acetylacetonato copper coordinated with acetylacetone.
金属源として用いられる含銅化合物として、カルボン酸銅を用いない場合には、カルボン酸銅以外の上記含銅化合物とカルボン酸により、脂肪酸銅のようなカルボン酸銅とすることが好ましい。カルボン酸銅は、銅ナノ粒子に被覆するカルボン酸源となると共に、還元性化合物との間で錯体等の複合化合物を生成しやすいからである。
In the case where copper carboxylate is not used as the copper-containing compound used as the metal source, it is preferable that the copper-containing compound other than copper carboxylate and carboxylic acid be used as a carboxylate copper such as fatty acid copper. This is because copper carboxylate serves as a carboxylic acid source to be coated on the copper nanoparticles and easily forms a complex compound such as a complex with the reducing compound.
次に、上記カルボン酸銅に対して、還元作用を有する還元性化合物を混合して、銅と還元性化合物との錯体等の複合化合物を生成させることが好ましい。金属源として用いられる含銅化合物としてカルボン酸銅を用いない場合に、カルボン酸銅以外の上記含銅化合物、還元性化合物、及びカルボン酸の混合物を用いて、上記複合化合物を生成しても良い。
この際に使用される還元性化合物としては、例えば、特開2012-72418号公報に記載の還元性化合物を適宜選択して用いることができる。
中でも、ヒドラジン、ヒドラジンの水和物、ヒドロキシルアミン及びこれらの誘導体等のアミノ基を有する還元性化合物が好適に用いられる。 Next, it is preferable that a reducing compound having a reducing action is mixed with the copper carboxylate to produce a composite compound such as a complex of copper and the reducing compound. When copper carboxylate is not used as the copper-containing compound used as the metal source, the composite compound may be generated using a mixture of the copper-containing compound other than copper carboxylate, the reducing compound, and the carboxylic acid. .
As the reducing compound used in this case, for example, the reducing compound described in JP2012-72418A can be appropriately selected and used.
Among these, reducing compounds having an amino group such as hydrazine, hydrazine hydrate, hydroxylamine, and derivatives thereof are preferably used.
この際に使用される還元性化合物としては、例えば、特開2012-72418号公報に記載の還元性化合物を適宜選択して用いることができる。
中でも、ヒドラジン、ヒドラジンの水和物、ヒドロキシルアミン及びこれらの誘導体等のアミノ基を有する還元性化合物が好適に用いられる。 Next, it is preferable that a reducing compound having a reducing action is mixed with the copper carboxylate to produce a composite compound such as a complex of copper and the reducing compound. When copper carboxylate is not used as the copper-containing compound used as the metal source, the composite compound may be generated using a mixture of the copper-containing compound other than copper carboxylate, the reducing compound, and the carboxylic acid. .
As the reducing compound used in this case, for example, the reducing compound described in JP2012-72418A can be appropriately selected and used.
Among these, reducing compounds having an amino group such as hydrazine, hydrazine hydrate, hydroxylamine, and derivatives thereof are preferably used.
また、カルボン酸銅と還元性化合物とを混合した際に、直接的に還元反応を生じる場合には、冷却した環境で混合することで還元反応を抑制することが望ましい。例えば、脂肪酸銅等の銅を含む化合物と還元性化合物とを、30℃以下に冷却して混合を行うことが好ましく、更に好ましくは25℃以下、最も好ましくは20℃以下である。
その他、カルボン酸銅に対して、還元作用を有する還元性化合物を混合して、銅と還元性化合物との錯体等の複合化合物を生成させる条件は、特開2012-72418号公報を参照して適宜選択することができる。 In addition, when a reduction reaction occurs directly when copper carboxylate and a reducing compound are mixed, it is desirable to suppress the reduction reaction by mixing in a cooled environment. For example, a compound containing copper such as fatty acid copper and a reducing compound are preferably mixed by cooling to 30 ° C. or lower, more preferably 25 ° C. or lower, and most preferably 20 ° C. or lower.
In addition, for conditions for mixing a reducing compound having a reducing action with copper carboxylate to form a complex compound such as a complex of copper and a reducing compound, refer to JP 2012-72418 A It can be selected appropriately.
その他、カルボン酸銅に対して、還元作用を有する還元性化合物を混合して、銅と還元性化合物との錯体等の複合化合物を生成させる条件は、特開2012-72418号公報を参照して適宜選択することができる。 In addition, when a reduction reaction occurs directly when copper carboxylate and a reducing compound are mixed, it is desirable to suppress the reduction reaction by mixing in a cooled environment. For example, a compound containing copper such as fatty acid copper and a reducing compound are preferably mixed by cooling to 30 ° C. or lower, more preferably 25 ° C. or lower, and most preferably 20 ° C. or lower.
In addition, for conditions for mixing a reducing compound having a reducing action with copper carboxylate to form a complex compound such as a complex of copper and a reducing compound, refer to JP 2012-72418 A It can be selected appropriately.
本発明における銅ナノ粒子を調製する工程においては、上記で生成したカルボン酸銅と還元性化合物との混合物を、十分な量のアルキルアミンと混合して加熱し、カルボン酸銅の自発的分解反応により銅原子が生成して凝集することで銅ナノ粒子を得ることが好ましい。この際に、銅ナノ粒子表面が、カルボン酸とアルキルアミンにより被覆されることから、空気中の酸化で酸化され難い、安定な被覆銅ナノ粒子を得ることができる。カルボン酸やアルキルアミンの分子量を調整することで、生成する銅ナノ粒子の1次粒径を所望の大きさに調節することが可能である。
上記カルボン酸銅と還元性化合物との混合物に対する、アルキルアミンの混合比は、用途に応じて適宜選択されれば良いが、耐酸化性、低温焼成性の点から、カルボン酸銅1モルに対して、1~10モルであることが好ましく、更に、2~6モルの範囲内であることがより好ましい。 In the step of preparing the copper nanoparticles in the present invention, the mixture of the copper carboxylate and the reducing compound produced above is mixed with a sufficient amount of alkylamine and heated to spontaneously decompose the copper carboxylate. It is preferable to obtain copper nanoparticles by forming and aggregating copper atoms. At this time, since the surface of the copper nanoparticles is coated with a carboxylic acid and an alkylamine, stable coated copper nanoparticles that are hardly oxidized by oxidation in the air can be obtained. By adjusting the molecular weight of carboxylic acid or alkylamine, it is possible to adjust the primary particle size of the produced copper nanoparticles to a desired size.
The mixing ratio of the alkylamine to the mixture of the copper carboxylate and the reducing compound may be appropriately selected according to the use, but from the viewpoint of oxidation resistance and low-temperature calcination, it is based on 1 mol of the copper carboxylate. The amount is preferably 1 to 10 moles, more preferably 2 to 6 moles.
上記カルボン酸銅と還元性化合物との混合物に対する、アルキルアミンの混合比は、用途に応じて適宜選択されれば良いが、耐酸化性、低温焼成性の点から、カルボン酸銅1モルに対して、1~10モルであることが好ましく、更に、2~6モルの範囲内であることがより好ましい。 In the step of preparing the copper nanoparticles in the present invention, the mixture of the copper carboxylate and the reducing compound produced above is mixed with a sufficient amount of alkylamine and heated to spontaneously decompose the copper carboxylate. It is preferable to obtain copper nanoparticles by forming and aggregating copper atoms. At this time, since the surface of the copper nanoparticles is coated with a carboxylic acid and an alkylamine, stable coated copper nanoparticles that are hardly oxidized by oxidation in the air can be obtained. By adjusting the molecular weight of carboxylic acid or alkylamine, it is possible to adjust the primary particle size of the produced copper nanoparticles to a desired size.
The mixing ratio of the alkylamine to the mixture of the copper carboxylate and the reducing compound may be appropriately selected according to the use, but from the viewpoint of oxidation resistance and low-temperature calcination, it is based on 1 mol of the copper carboxylate. The amount is preferably 1 to 10 moles, more preferably 2 to 6 moles.
銅を含む化合物、還元性化合物、カルボン酸、及びアルキルアミンを含む混合物、又は、カルボン酸銅、還元性化合物、及びアルキルアミンを含む混合物のいずれかを加熱することにより銅ナノ粒子を調製する工程の実施形態において、銅を含む化合物、還元性化合物及びカルボン酸を混合して、又は、カルボン酸銅及び還元性化合物を混合して、錯体等の複合化合物を調製する第1工程と、当該錯体等の複合化合物をアルキルアミンの存在下に加熱して銅ナノ粒子を生成させる第2工程とを、1つの容器内で同時に又は逐次に行うことができる。好ましくはこれに極性溶媒を添加して可溶化した後に、加熱することにより銅ナノ粒子を生成することができる。アルキルアミンの存在下に加熱する温度としては、60℃~150℃であることが好ましい。
銅ナノ粒子を調製する際には、前記第1工程と第2工程とを、逐次的に行うことが好ましく、前記第1の工程が約30℃以下に冷却して行われ、前記第2の工程が60℃~150℃に加熱して行われることが好ましい。 A step of preparing copper nanoparticles by heating either a compound containing copper, a reducing compound, a carboxylic acid, and a mixture containing an alkylamine, or a mixture containing copper carboxylate, a reducing compound, and an alkylamine. In the embodiment, a first step of preparing a complex compound such as a complex by mixing a compound containing copper, a reducing compound and a carboxylic acid, or mixing a copper carboxylate and a reducing compound, and the complex The second step of heating a complex compound such as alkylamine in the presence of an alkylamine to produce copper nanoparticles can be performed simultaneously or sequentially in one container. Preferably, after adding a polar solvent thereto and solubilizing it, copper nanoparticles can be produced by heating. The heating temperature in the presence of alkylamine is preferably 60 ° C to 150 ° C.
When preparing copper nanoparticles, it is preferable to sequentially perform the first step and the second step, the first step is performed by cooling to about 30 ° C. or less, and the second step It is preferable that the process is performed by heating to 60 ° C. to 150 ° C.
銅ナノ粒子を調製する際には、前記第1工程と第2工程とを、逐次的に行うことが好ましく、前記第1の工程が約30℃以下に冷却して行われ、前記第2の工程が60℃~150℃に加熱して行われることが好ましい。 A step of preparing copper nanoparticles by heating either a compound containing copper, a reducing compound, a carboxylic acid, and a mixture containing an alkylamine, or a mixture containing copper carboxylate, a reducing compound, and an alkylamine. In the embodiment, a first step of preparing a complex compound such as a complex by mixing a compound containing copper, a reducing compound and a carboxylic acid, or mixing a copper carboxylate and a reducing compound, and the complex The second step of heating a complex compound such as alkylamine in the presence of an alkylamine to produce copper nanoparticles can be performed simultaneously or sequentially in one container. Preferably, after adding a polar solvent thereto and solubilizing it, copper nanoparticles can be produced by heating. The heating temperature in the presence of alkylamine is preferably 60 ° C to 150 ° C.
When preparing copper nanoparticles, it is preferable to sequentially perform the first step and the second step, the first step is performed by cooling to about 30 ° C. or less, and the second step It is preferable that the process is performed by heating to 60 ° C. to 150 ° C.
(分散体を調製する工程)
前記調製工程で得られた銅ナノ粒子を、溶剤中で前記特定の高分子分散剤により分散することにより、動的光散乱法による体積平均粒径が500nm以下である銅ナノ粒子分散体を調製する。
例えば、前記特定の高分子分散剤を前記溶剤に混合、攪拌し、高分子分散剤溶液を調製した後、当該高分子分散剤溶液に、前記調製工程で得られた銅ナノ粒子と、必要に応じて他の成分を混合し、公知の攪拌機、又は分散機等を用いて分散させることよって、銅ナノ粒子分散体を調製することができる。 (Process for preparing a dispersion)
A copper nanoparticle dispersion having a volume average particle size of 500 nm or less by dynamic light scattering method is prepared by dispersing the copper nanoparticles obtained in the preparation step with the specific polymer dispersant in a solvent. To do.
For example, after the specific polymer dispersant is mixed and stirred in the solvent to prepare a polymer dispersant solution, the polymer dispersant solution is mixed with the copper nanoparticles obtained in the preparation step, as necessary. Accordingly, a copper nanoparticle dispersion can be prepared by mixing other components and dispersing them using a known stirrer or disperser.
前記調製工程で得られた銅ナノ粒子を、溶剤中で前記特定の高分子分散剤により分散することにより、動的光散乱法による体積平均粒径が500nm以下である銅ナノ粒子分散体を調製する。
例えば、前記特定の高分子分散剤を前記溶剤に混合、攪拌し、高分子分散剤溶液を調製した後、当該高分子分散剤溶液に、前記調製工程で得られた銅ナノ粒子と、必要に応じて他の成分を混合し、公知の攪拌機、又は分散機等を用いて分散させることよって、銅ナノ粒子分散体を調製することができる。 (Process for preparing a dispersion)
A copper nanoparticle dispersion having a volume average particle size of 500 nm or less by dynamic light scattering method is prepared by dispersing the copper nanoparticles obtained in the preparation step with the specific polymer dispersant in a solvent. To do.
For example, after the specific polymer dispersant is mixed and stirred in the solvent to prepare a polymer dispersant solution, the polymer dispersant solution is mixed with the copper nanoparticles obtained in the preparation step, as necessary. Accordingly, a copper nanoparticle dispersion can be prepared by mixing other components and dispersing them using a known stirrer or disperser.
本発明で得られる銅ナノ粒子分散体は、後述する導電性基板に好適に用いられ、特に導電性パターン印刷用に好適に用いられる。本発明の銅ナノ粒子分散体は、低温や短時間で焼成可能なため、後述するプラズマ焼成やフラッシュ光焼成等の低温又は短時間焼成用途に好適に用いられる。
本発明で得られる銅ナノ粒子分散体は、更に、めっき用のシード層や各種金属膜に応用することができ、例えば、光学装置用の鏡面や、各種装飾用途等に用いることができる。 The copper nanoparticle dispersion obtained in the present invention is preferably used for a conductive substrate described later, and particularly preferably for conductive pattern printing. Since the copper nanoparticle dispersion of the present invention can be fired at a low temperature and in a short time, it is suitably used for low-temperature or short-time firing applications such as plasma firing and flash light firing described later.
The copper nanoparticle dispersion obtained in the present invention can be further applied to a seed layer for plating and various metal films, and can be used, for example, for mirror surfaces for optical devices and various decoration applications.
本発明で得られる銅ナノ粒子分散体は、更に、めっき用のシード層や各種金属膜に応用することができ、例えば、光学装置用の鏡面や、各種装飾用途等に用いることができる。 The copper nanoparticle dispersion obtained in the present invention is preferably used for a conductive substrate described later, and particularly preferably for conductive pattern printing. Since the copper nanoparticle dispersion of the present invention can be fired at a low temperature and in a short time, it is suitably used for low-temperature or short-time firing applications such as plasma firing and flash light firing described later.
The copper nanoparticle dispersion obtained in the present invention can be further applied to a seed layer for plating and various metal films, and can be used, for example, for mirror surfaces for optical devices and various decoration applications.
[導電性基板の製造方法]
本発明に係る第一の態様の導電性基板の製造方法は、銅ナノ粒子と、カルボン酸と、アルキルアミンと、アミン価及び酸価の一方が30~160mgKOH/g、アミン価及び酸価の他の一方が0~160mgKOH/gである高分子分散剤と、溶剤とを含有し、動的光散乱法による体積平均粒径が500nm以下である銅ナノ粒子分散体を、基材上に塗布して塗膜を形成する工程と、当該塗膜を焼成する工程とを有することを特徴とする。
また、本発明に係る第二の態様の導電性基板の製造方法は、銅を含む化合物、還元性化合物、カルボン酸、及びアルキルアミンを含む混合物、又は、カルボン酸銅、還元性化合物、及びアルキルアミンを含む混合物のいずれかを加熱することにより銅ナノ粒子を調製する工程と、前記銅ナノ粒子を、溶剤中でアミン価及び酸価の一方が30~160mgKOH/g、アミン価及び酸価の他の一方が0~160mgKOH/gである高分子分散剤により分散することにより、動的光散乱法による体積平均粒径が500nm以下である銅ナノ粒子分散体を調製する工程と、前記銅ナノ粒子分散体を、基材上に塗布して塗膜を形成する工程と、当該塗膜を焼成する工程とを有することを特徴とする。 [Method of manufacturing conductive substrate]
The method for producing a conductive substrate according to the first aspect of the present invention comprises a copper nanoparticle, a carboxylic acid, an alkylamine, and one of an amine value and an acid value of 30 to 160 mgKOH / g, an amine value and an acid value. A copper nanoparticle dispersion containing a polymer dispersant of 0 to 160 mgKOH / g and a solvent, and having a volume average particle diameter of 500 nm or less by dynamic light scattering method is applied onto a substrate. And a step of forming a coating film and a step of baking the coating film.
Moreover, the manufacturing method of the electroconductive board | substrate of the 2nd aspect which concerns on this invention is a compound containing copper, a reducing compound, carboxylic acid, and the mixture containing an alkylamine, or copper carboxylate, a reducing compound, and alkyl. A step of preparing copper nanoparticles by heating any one of a mixture containing an amine, and the copper nanoparticles having an amine value and an acid value of 30 to 160 mgKOH / g, an amine value and an acid value in a solvent; A step of preparing a copper nanoparticle dispersion having a volume average particle diameter of 500 nm or less by a dynamic light scattering method by dispersing with a polymer dispersant having the other one of 0 to 160 mgKOH / g; It has the process of apply | coating a particle dispersion on a base material, forming a coating film, and the process of baking the said coating film.
本発明に係る第一の態様の導電性基板の製造方法は、銅ナノ粒子と、カルボン酸と、アルキルアミンと、アミン価及び酸価の一方が30~160mgKOH/g、アミン価及び酸価の他の一方が0~160mgKOH/gである高分子分散剤と、溶剤とを含有し、動的光散乱法による体積平均粒径が500nm以下である銅ナノ粒子分散体を、基材上に塗布して塗膜を形成する工程と、当該塗膜を焼成する工程とを有することを特徴とする。
また、本発明に係る第二の態様の導電性基板の製造方法は、銅を含む化合物、還元性化合物、カルボン酸、及びアルキルアミンを含む混合物、又は、カルボン酸銅、還元性化合物、及びアルキルアミンを含む混合物のいずれかを加熱することにより銅ナノ粒子を調製する工程と、前記銅ナノ粒子を、溶剤中でアミン価及び酸価の一方が30~160mgKOH/g、アミン価及び酸価の他の一方が0~160mgKOH/gである高分子分散剤により分散することにより、動的光散乱法による体積平均粒径が500nm以下である銅ナノ粒子分散体を調製する工程と、前記銅ナノ粒子分散体を、基材上に塗布して塗膜を形成する工程と、当該塗膜を焼成する工程とを有することを特徴とする。 [Method of manufacturing conductive substrate]
The method for producing a conductive substrate according to the first aspect of the present invention comprises a copper nanoparticle, a carboxylic acid, an alkylamine, and one of an amine value and an acid value of 30 to 160 mgKOH / g, an amine value and an acid value. A copper nanoparticle dispersion containing a polymer dispersant of 0 to 160 mgKOH / g and a solvent, and having a volume average particle diameter of 500 nm or less by dynamic light scattering method is applied onto a substrate. And a step of forming a coating film and a step of baking the coating film.
Moreover, the manufacturing method of the electroconductive board | substrate of the 2nd aspect which concerns on this invention is a compound containing copper, a reducing compound, carboxylic acid, and the mixture containing an alkylamine, or copper carboxylate, a reducing compound, and alkyl. A step of preparing copper nanoparticles by heating any one of a mixture containing an amine, and the copper nanoparticles having an amine value and an acid value of 30 to 160 mgKOH / g, an amine value and an acid value in a solvent; A step of preparing a copper nanoparticle dispersion having a volume average particle diameter of 500 nm or less by a dynamic light scattering method by dispersing with a polymer dispersant having the other one of 0 to 160 mgKOH / g; It has the process of apply | coating a particle dispersion on a base material, forming a coating film, and the process of baking the said coating film.
本発明の導電性基板の製造方法によれば、上述のように、耐酸化性、分散性、塗布適性、及び低温又は短時間での焼結性に優れた、分散粒径の小さい銅ナノ粒子分散体を用いることから、酸化が抑制された銅ナノ粒子が均一且つ高密度に存在している、平滑で均一性の高い塗膜を形成できる。その結果、パターン精度が良好で、焼結後に優れた導電性を有する導電性基板を得ることができる。
According to the method for producing a conductive substrate of the present invention, as described above, copper nanoparticles having a small dispersed particle diameter, excellent in oxidation resistance, dispersibility, applicability, and sintering property at low temperature or in a short time. Since the dispersion is used, it is possible to form a smooth and highly uniform coating film in which copper nanoparticles whose oxidation is suppressed are present uniformly and at a high density. As a result, a conductive substrate having good pattern accuracy and excellent conductivity after sintering can be obtained.
図1は、本発明の製造方法により得られる導電性基板の一例を示す概略図である。図1に示される導電性基板100は、基材1の一方の面に、銅ナノ粒子分散体の塗膜が焼成されてなる金属膜2を備えたものである。
また、図2は、本発明の製造方法により得られる導電性基板の他の一例を示す概略図である。図2に示される導電性基板101は、基材1の両面に、銅ナノ粒子分散体の塗膜が焼成されてなり、パターン状金属膜3を備えたものである。
このように、本発明の製造方法により得られる導電性基板は、基材の一方の面のみに銅ナノ粒子分散体の塗膜が焼成されてなる金属膜を備えたものであっても良いし、基材の両面に銅ナノ粒子分散体の塗膜が焼成されてなる金属膜を備えたものであっても良い。また、基材の一方の面のみに備えた金属膜、及び、基材の両面に備えた金属膜はそれぞれ、パターン状金属膜であっても、パターンを有していないベタ膜の金属膜であっても良い。更に、基材の両面に備えた金属膜は、一方の面がパターン状金属膜で、他方の面がベタ膜の金属膜であっても良い。また、基材の両面に備えた金属膜が、両面ともパターン状金属膜である場合に、当該両面のパターンは、同じであっても異なっていても良い。更に、基材の両面に備えたパターン状金属膜が、同じパターンを有する場合に、当該両面のパターンは、両面で同じ位置にあっても異なる位置にあっても良い。 FIG. 1 is a schematic view showing an example of a conductive substrate obtained by the production method of the present invention. Aconductive substrate 100 shown in FIG. 1 includes a metal film 2 formed on one surface of a base material 1 by baking a coating film of a copper nanoparticle dispersion.
FIG. 2 is a schematic view showing another example of a conductive substrate obtained by the production method of the present invention. Aconductive substrate 101 shown in FIG. 2 is provided with a patterned metal film 3 on both surfaces of a base material 1 by baking a coating film of a copper nanoparticle dispersion.
As described above, the conductive substrate obtained by the production method of the present invention may be provided with a metal film obtained by firing a coating film of a copper nanoparticle dispersion only on one surface of a base material. Further, a metal film obtained by firing a coating film of a copper nanoparticle dispersion on both surfaces of a base material may be used. In addition, the metal film provided on only one surface of the base material and the metal film provided on both surfaces of the base material are each a solid metal film having no pattern even if it is a patterned metal film. There may be. Furthermore, the metal film provided on both surfaces of the base material may be a metal film having one surface that is a patterned metal film and the other surface that is a solid film. Moreover, when the metal film provided on both surfaces of the substrate is a patterned metal film on both surfaces, the patterns on both surfaces may be the same or different. Further, when the patterned metal films provided on both surfaces of the substrate have the same pattern, the patterns on both surfaces may be at the same position or at different positions on both surfaces.
また、図2は、本発明の製造方法により得られる導電性基板の他の一例を示す概略図である。図2に示される導電性基板101は、基材1の両面に、銅ナノ粒子分散体の塗膜が焼成されてなり、パターン状金属膜3を備えたものである。
このように、本発明の製造方法により得られる導電性基板は、基材の一方の面のみに銅ナノ粒子分散体の塗膜が焼成されてなる金属膜を備えたものであっても良いし、基材の両面に銅ナノ粒子分散体の塗膜が焼成されてなる金属膜を備えたものであっても良い。また、基材の一方の面のみに備えた金属膜、及び、基材の両面に備えた金属膜はそれぞれ、パターン状金属膜であっても、パターンを有していないベタ膜の金属膜であっても良い。更に、基材の両面に備えた金属膜は、一方の面がパターン状金属膜で、他方の面がベタ膜の金属膜であっても良い。また、基材の両面に備えた金属膜が、両面ともパターン状金属膜である場合に、当該両面のパターンは、同じであっても異なっていても良い。更に、基材の両面に備えたパターン状金属膜が、同じパターンを有する場合に、当該両面のパターンは、両面で同じ位置にあっても異なる位置にあっても良い。 FIG. 1 is a schematic view showing an example of a conductive substrate obtained by the production method of the present invention. A
FIG. 2 is a schematic view showing another example of a conductive substrate obtained by the production method of the present invention. A
As described above, the conductive substrate obtained by the production method of the present invention may be provided with a metal film obtained by firing a coating film of a copper nanoparticle dispersion only on one surface of a base material. Further, a metal film obtained by firing a coating film of a copper nanoparticle dispersion on both surfaces of a base material may be used. In addition, the metal film provided on only one surface of the base material and the metal film provided on both surfaces of the base material are each a solid metal film having no pattern even if it is a patterned metal film. There may be. Furthermore, the metal film provided on both surfaces of the base material may be a metal film having one surface that is a patterned metal film and the other surface that is a solid film. Moreover, when the metal film provided on both surfaces of the substrate is a patterned metal film on both surfaces, the patterns on both surfaces may be the same or different. Further, when the patterned metal films provided on both surfaces of the substrate have the same pattern, the patterns on both surfaces may be at the same position or at different positions on both surfaces.
本発明に係る第一の態様の導電性基板の製造方法における、銅ナノ粒子分散体を準備する工程や、本発明に係る第二の態様の導電性基板の製造方法における、銅を含む化合物、還元性化合物、カルボン酸、及びアルキルアミンを含む混合物、又は、カルボン酸銅、還元性化合物、及びアルキルアミンを含む混合物のいずれかを加熱することにより銅ナノ粒子を調製する工程と、前記銅ナノ粒子を、溶剤中で上記特定のアミン価及び酸価を有する高分子分散剤により分散することにより、動的光散乱法による体積平均粒径が500nm以下である銅ナノ粒子分散体を調製する工程については、上述した銅ナノ粒子を調製する工程と銅ナノ粒子分散体を調製する工程と同様であってよいので、ここでの説明を省略する。
以下、第一の態様と第二の態様に共通する、塗膜を形成する工程と、当該塗膜を焼成する工程の各工程について、順に説明する。
なお、本発明に係る上記導電性基板の製造方法は、本発明の効果が損なわれない限り、必要に応じて他の工程を有していてもよいものである。 In the method for producing a conductive substrate according to the first aspect of the present invention, a step of preparing a copper nanoparticle dispersion, and a compound containing copper in the method for producing a conductive substrate according to the second aspect of the present invention, A step of preparing copper nanoparticles by heating either a mixture containing a reducing compound, a carboxylic acid and an alkylamine, or a mixture containing copper carboxylate, a reducing compound and an alkylamine; A step of preparing a copper nanoparticle dispersion having a volume average particle diameter of 500 nm or less by a dynamic light scattering method by dispersing particles in a solvent with a polymer dispersant having the specific amine value and acid value. Since it may be the same as the step of preparing the copper nanoparticles and the step of preparing the copper nanoparticle dispersion described above, the description thereof is omitted here.
Hereafter, each process of the process of forming a coating film and the process of baking the said coating film which are common to a 1st aspect and a 2nd aspect is demonstrated in order.
In addition, the manufacturing method of the said electroconductive board | substrate which concerns on this invention may have another process as needed, unless the effect of this invention is impaired.
以下、第一の態様と第二の態様に共通する、塗膜を形成する工程と、当該塗膜を焼成する工程の各工程について、順に説明する。
なお、本発明に係る上記導電性基板の製造方法は、本発明の効果が損なわれない限り、必要に応じて他の工程を有していてもよいものである。 In the method for producing a conductive substrate according to the first aspect of the present invention, a step of preparing a copper nanoparticle dispersion, and a compound containing copper in the method for producing a conductive substrate according to the second aspect of the present invention, A step of preparing copper nanoparticles by heating either a mixture containing a reducing compound, a carboxylic acid and an alkylamine, or a mixture containing copper carboxylate, a reducing compound and an alkylamine; A step of preparing a copper nanoparticle dispersion having a volume average particle diameter of 500 nm or less by a dynamic light scattering method by dispersing particles in a solvent with a polymer dispersant having the specific amine value and acid value. Since it may be the same as the step of preparing the copper nanoparticles and the step of preparing the copper nanoparticle dispersion described above, the description thereof is omitted here.
Hereafter, each process of the process of forming a coating film and the process of baking the said coating film which are common to a 1st aspect and a 2nd aspect is demonstrated in order.
In addition, the manufacturing method of the said electroconductive board | substrate which concerns on this invention may have another process as needed, unless the effect of this invention is impaired.
<銅ナノ粒子分散体を、基材上に塗布して塗膜を形成する工程>
(基材)
本発明に用いられる基材は、導電性基板に用いられる基材の中から、用途に応じて適宜選択すればよい。例えば、ガラス、アルミナ、シリカ、SUS箔などの無機材料を用いることができ、さらに高分子材料や、紙などを用いることもできる。前記本発明に係る導電性基板用金属微粒子分散体は、従来よりも低温で焼成しても導電性に優れた金属膜が得られることから、従来適用が困難であったソーダライムガラスや、高分子材料であっても好適に用いることができ、特に樹脂フィルムを用いることができる点で非常に有用である。 <The process of apply | coating a copper nanoparticle dispersion | distribution on a base material, and forming a coating film>
(Base material)
What is necessary is just to select the base material used for this invention suitably from the base materials used for an electroconductive board | substrate according to a use. For example, inorganic materials such as glass, alumina, silica, and SUS foil can be used, and polymer materials and paper can also be used. Since the metal fine particle dispersion for conductive substrate according to the present invention can obtain a metal film having excellent conductivity even when fired at a lower temperature than before, soda lime glass, which has been difficult to apply conventionally, Even molecular materials can be suitably used, and are particularly useful in that a resin film can be used.
(基材)
本発明に用いられる基材は、導電性基板に用いられる基材の中から、用途に応じて適宜選択すればよい。例えば、ガラス、アルミナ、シリカ、SUS箔などの無機材料を用いることができ、さらに高分子材料や、紙などを用いることもできる。前記本発明に係る導電性基板用金属微粒子分散体は、従来よりも低温で焼成しても導電性に優れた金属膜が得られることから、従来適用が困難であったソーダライムガラスや、高分子材料であっても好適に用いることができ、特に樹脂フィルムを用いることができる点で非常に有用である。 <The process of apply | coating a copper nanoparticle dispersion | distribution on a base material, and forming a coating film>
(Base material)
What is necessary is just to select the base material used for this invention suitably from the base materials used for an electroconductive board | substrate according to a use. For example, inorganic materials such as glass, alumina, silica, and SUS foil can be used, and polymer materials and paper can also be used. Since the metal fine particle dispersion for conductive substrate according to the present invention can obtain a metal film having excellent conductivity even when fired at a lower temperature than before, soda lime glass, which has been difficult to apply conventionally, Even molecular materials can be suitably used, and are particularly useful in that a resin film can be used.
樹脂フィルムとしては、例えば、ポリイミド、ポリアミド、ポリアミドイミド、ポリエチレンテレフタレート(PET)、ポリエチレンナフタレート(PEN)、ポリフェニレンスルフィド、ポリエーテルエーテルケトン、ポリエーテルスルホン、ポリカーボネート、ポリエーテルイミド、エポキシ樹脂、フェノール樹脂、ガラス-エポキシ樹脂、ポリフェニレンエーテル、アクリル樹脂、ポリエチレン、ポリプロピレン等のポリオレフィン、ポリノルボルネン等のポリシクロオレフィン、液晶性高分子化合物等が挙げられる。特に本発明においては、PETのようなガラス転移温度が100℃以下の樹脂フィルムを用いることも可能である。なお、ここでのガラス転移温度は、JIS-K7121に準じて測定した示差走査熱量分析(DSC)測定によるものである。
Examples of the resin film include polyimide, polyamide, polyamideimide, polyethylene terephthalate (PET), polyethylene naphthalate (PEN), polyphenylene sulfide, polyether ether ketone, polyether sulfone, polycarbonate, polyether imide, epoxy resin, and phenol resin. Glass-epoxy resins, polyphenylene ethers, acrylic resins, polyolefins such as polyethylene and polypropylene, polycycloolefins such as polynorbornene, and liquid crystalline polymer compounds. In particular, in the present invention, a resin film having a glass transition temperature of 100 ° C. or less, such as PET, can be used. The glass transition temperature here is determined by differential scanning calorimetry (DSC) measurement measured according to JIS-K7121.
また、基材表面には、前記銅ナノ粒子分散体の塗膜をパターン状に形成した場合におけるパターンの形状を制御したり、前記銅ナノ粒子分散体の塗膜との間の密着性を付与するための処理を行ってもよい。基材表面の処理方法としては、従来公知の方法の中から適宜選択することができる。具体的には、例えば、コロナ処理、UV処理、真空紫外ランプ処理、プラズマ処理などのドライ処理、アミン系シランカップリング剤、イミダゾール系シランカップリング剤、チタンカップリング剤、アルミニウムカップリング剤処理などの薬液処理、多孔質シリカや、セルロース系受容層などの多孔質膜形成処理、活性エネルギー線硬化型樹脂層、熱硬化型樹脂層、熱可塑性樹脂層などの樹脂層形成処理を行うことができる。当該処理により、基材表面に撥液性を持たせることにより、基材に銅ナノ粒子分散体の塗膜をパターン状に形成した際、塗布液の濡れ広がりを抑え、高精細なパターンを形成することが可能である。また、基材表面に多孔質膜などのインク受容層を形成することにより、溶媒成分が浸透し、高精細なパターンを形成することが可能である。逆に、基材表面に親液性を持たせることで、基材に対する塗布性を向上させることができる。これらの基材表面の処理は、用途や目的に応じて使い分けることができる。
In addition, on the surface of the base material, when the coating film of the copper nanoparticle dispersion is formed in a pattern, the shape of the pattern is controlled, or adhesion between the coating film of the copper nanoparticle dispersion is given. You may perform the process for doing. The treatment method for the substrate surface can be appropriately selected from conventionally known methods. Specifically, for example, corona treatment, UV treatment, vacuum ultraviolet lamp treatment, dry treatment such as plasma treatment, amine silane coupling agent, imidazole silane coupling agent, titanium coupling agent, aluminum coupling agent treatment, etc. Chemical layer treatment, porous silica, porous membrane formation treatment such as cellulose-based receiving layer, active energy ray curable resin layer, thermosetting resin layer, thermoplastic resin layer and other resin layer formation treatment . By providing the substrate surface with liquid repellency by this treatment, when the coating film of copper nanoparticle dispersion is formed in a pattern on the substrate, wetting spread of the coating solution is suppressed and a high-definition pattern is formed Is possible. In addition, by forming an ink receiving layer such as a porous film on the surface of the substrate, it is possible to penetrate the solvent component and form a high-definition pattern. On the contrary, the applicability | paintability with respect to a base material can be improved by giving lyophilic property to the base-material surface. These treatments on the surface of the substrate can be used properly according to the purpose and purpose.
当該基材の形状は、用途に応じて適宜選択すればよく、平板状であっても、曲面を有するものであってもよいが、通常は平板状である。平板状の基材を用いる場合、当該基材の厚みは、用途に応じて適宜設定すればよく、例えば10μm~1mm程度のものとすることができる。
The shape of the substrate may be appropriately selected depending on the application, and may be flat or curved, but is usually flat. In the case of using a flat substrate, the thickness of the substrate may be appropriately set according to the application, and may be, for example, about 10 μm to 1 mm.
(塗布方法)
上記銅ナノ粒子分散体を上記基材上に塗布する方法は、従来公知の塗布乃至印刷方法の中から適宜選択すればよい。中でも、導電性パターンを印刷するに当たり、微細なパターニングを行うことができる点から、グラビア印刷、グラビアオフセット印刷、反転オフセット印刷、フレキソ印刷、スクリーン印刷、及びインクジェット印刷が好ましい。或いは、塗布方法には全面を塗布する場合も包含される。全面塗布の場合には、当該塗膜を焼成処理して得られる銅ナノ粒子焼結膜に対して、後述するような化学エッチング法によりパターンを形成することが可能である。 (Application method)
The method for applying the copper nanoparticle dispersion onto the substrate may be appropriately selected from conventionally known application or printing methods. Among these, gravure printing, gravure offset printing, reverse offset printing, flexographic printing, screen printing, and ink jet printing are preferable because fine patterning can be performed when printing a conductive pattern. Alternatively, the coating method includes a case where the entire surface is coated. In the case of whole surface application, a pattern can be formed by a chemical etching method as described later on a copper nanoparticle sintered film obtained by baking the coating film.
上記銅ナノ粒子分散体を上記基材上に塗布する方法は、従来公知の塗布乃至印刷方法の中から適宜選択すればよい。中でも、導電性パターンを印刷するに当たり、微細なパターニングを行うことができる点から、グラビア印刷、グラビアオフセット印刷、反転オフセット印刷、フレキソ印刷、スクリーン印刷、及びインクジェット印刷が好ましい。或いは、塗布方法には全面を塗布する場合も包含される。全面塗布の場合には、当該塗膜を焼成処理して得られる銅ナノ粒子焼結膜に対して、後述するような化学エッチング法によりパターンを形成することが可能である。 (Application method)
The method for applying the copper nanoparticle dispersion onto the substrate may be appropriately selected from conventionally known application or printing methods. Among these, gravure printing, gravure offset printing, reverse offset printing, flexographic printing, screen printing, and ink jet printing are preferable because fine patterning can be performed when printing a conductive pattern. Alternatively, the coating method includes a case where the entire surface is coated. In the case of whole surface application, a pattern can be formed by a chemical etching method as described later on a copper nanoparticle sintered film obtained by baking the coating film.
基材上の銅ナノ粒子分散体は、塗布後、通常の方法で乾燥してもよい。乾燥後の塗膜の膜厚は、適宜塗布量や銅ナノ粒子の平均一次粒子径等を変化させて制御することができ、用途に応じて適宜調整すればよいものであるが、通常、0.01~50μmの範囲であり、好ましくは、0.1~20μmである。
The copper nanoparticle dispersion on the substrate may be dried by a usual method after coating. The film thickness of the coated film after drying can be controlled by appropriately changing the coating amount, the average primary particle diameter of the copper nanoparticles, etc., and may be appropriately adjusted according to the application, but is usually 0. The range is from 0.01 to 50 μm, and preferably from 0.1 to 20 μm.
<塗膜を焼成する工程>
本工程は、上記工程で得られた塗膜を焼成することにより、焼結膜とし、金属膜を形成する工程である。
焼成方法は、従来公知の焼成方法の中から適宜選択して用いることができる。焼成方法の具体例としては、例えば、焼成炉(オーブン)により加熱する方法の他、赤外線加熱、各種レーザーアニール、紫外線、可視光、フラッシュ光による光照射焼成、マイクロ波加熱などの方法が挙げられ、不活性ガス雰囲気下又は還元性ガス雰囲気下で行われることが好ましく、また大気雰囲気の場合には、焼成時の酸化を防ぐため、瞬間的に加熱が行われることが好ましい。
本発明の銅ナノ粒子分散体は、低温や、短時間で焼成可能なため、従来の方法よりも低温で焼成することができる。 <Step of baking the coating film>
This step is a step of forming a metal film by baking the coating film obtained in the above step to form a sintered film.
The firing method can be appropriately selected from conventionally known firing methods. Specific examples of the firing method include, for example, methods such as heating by a firing furnace (oven), infrared heating, various laser annealing, ultraviolet light, visible light, light irradiation firing with flash light, microwave heating, and the like. In addition, it is preferably performed in an inert gas atmosphere or a reducing gas atmosphere. In the case of an air atmosphere, it is preferable that heating is performed instantaneously in order to prevent oxidation during firing.
Since the copper nanoparticle dispersion of the present invention can be fired at a low temperature or in a short time, it can be fired at a lower temperature than the conventional method.
本工程は、上記工程で得られた塗膜を焼成することにより、焼結膜とし、金属膜を形成する工程である。
焼成方法は、従来公知の焼成方法の中から適宜選択して用いることができる。焼成方法の具体例としては、例えば、焼成炉(オーブン)により加熱する方法の他、赤外線加熱、各種レーザーアニール、紫外線、可視光、フラッシュ光による光照射焼成、マイクロ波加熱などの方法が挙げられ、不活性ガス雰囲気下又は還元性ガス雰囲気下で行われることが好ましく、また大気雰囲気の場合には、焼成時の酸化を防ぐため、瞬間的に加熱が行われることが好ましい。
本発明の銅ナノ粒子分散体は、低温や、短時間で焼成可能なため、従来の方法よりも低温で焼成することができる。 <Step of baking the coating film>
This step is a step of forming a metal film by baking the coating film obtained in the above step to form a sintered film.
The firing method can be appropriately selected from conventionally known firing methods. Specific examples of the firing method include, for example, methods such as heating by a firing furnace (oven), infrared heating, various laser annealing, ultraviolet light, visible light, light irradiation firing with flash light, microwave heating, and the like. In addition, it is preferably performed in an inert gas atmosphere or a reducing gas atmosphere. In the case of an air atmosphere, it is preferable that heating is performed instantaneously in order to prevent oxidation during firing.
Since the copper nanoparticle dispersion of the present invention can be fired at a low temperature or in a short time, it can be fired at a lower temperature than the conventional method.
本発明においては、中でも、焼成する工程が、プラズマ焼成、中でもマイクロ波エネルギーの印加により発生する表面波プラズマにより焼成する工程、又は、フラッシュ光の照射により焼成する工程(以下、フラッシュ光焼成と称することがある。)のいずれかであることが好ましい。
これらの方法を用いると、基材への熱ダメージを少なくすることができると共に、焼成時の金属の酸化も抑制できる。また、短時間焼成であるため、生産性が高いというメリットもある。 In the present invention, in particular, the firing step is plasma firing, in particular, firing by surface wave plasma generated by application of microwave energy, or firing by flash light irradiation (hereinafter referred to as flash light firing). It is preferable that any one of the above.
When these methods are used, thermal damage to the substrate can be reduced, and oxidation of the metal during firing can be suppressed. Moreover, since it is baking for a short time, there also exists a merit that productivity is high.
これらの方法を用いると、基材への熱ダメージを少なくすることができると共に、焼成時の金属の酸化も抑制できる。また、短時間焼成であるため、生産性が高いというメリットもある。 In the present invention, in particular, the firing step is plasma firing, in particular, firing by surface wave plasma generated by application of microwave energy, or firing by flash light irradiation (hereinafter referred to as flash light firing). It is preferable that any one of the above.
When these methods are used, thermal damage to the substrate can be reduced, and oxidation of the metal during firing can be suppressed. Moreover, since it is baking for a short time, there also exists a merit that productivity is high.
(プラズマ焼成)
マイクロ波表面波プラズマを用いた焼成は、不活性ガス雰囲気下又は還元性ガス雰囲気下で行うのが、得られる焼結膜の導電性の観点から好ましい。
特に、本発明においては、マイクロ波表面波プラズマを、還元性ガス雰囲気下で発生させることが好ましく、中でも、水素ガス雰囲気下で発生させることがより好ましい。これにより、銅ナノ粒子表面に存在する絶縁性の酸化物が還元除去され、導電性能の良好な導電パターンが形成される。 (Plasma firing)
Firing using microwave surface wave plasma is preferably performed in an inert gas atmosphere or a reducing gas atmosphere from the viewpoint of the conductivity of the obtained sintered film.
In particular, in the present invention, the microwave surface wave plasma is preferably generated in a reducing gas atmosphere, and more preferably generated in a hydrogen gas atmosphere. Thereby, the insulating oxide which exists in the copper nanoparticle surface is reduced and removed, and the conductive pattern with favorable conductive performance is formed.
マイクロ波表面波プラズマを用いた焼成は、不活性ガス雰囲気下又は還元性ガス雰囲気下で行うのが、得られる焼結膜の導電性の観点から好ましい。
特に、本発明においては、マイクロ波表面波プラズマを、還元性ガス雰囲気下で発生させることが好ましく、中でも、水素ガス雰囲気下で発生させることがより好ましい。これにより、銅ナノ粒子表面に存在する絶縁性の酸化物が還元除去され、導電性能の良好な導電パターンが形成される。 (Plasma firing)
Firing using microwave surface wave plasma is preferably performed in an inert gas atmosphere or a reducing gas atmosphere from the viewpoint of the conductivity of the obtained sintered film.
In particular, in the present invention, the microwave surface wave plasma is preferably generated in a reducing gas atmosphere, and more preferably generated in a hydrogen gas atmosphere. Thereby, the insulating oxide which exists in the copper nanoparticle surface is reduced and removed, and the conductive pattern with favorable conductive performance is formed.
マイクロ波表面波プラズマ処理の前に、銅ナノ粒子分散体を塗布した塗膜に含まれるアルキルアミン、カルボン酸、高分子分散剤等の有機物を除去するために、大気下又は酸素を含む雰囲気下、50~200℃程度の温度で1分から2時間程度焼成してもよい。なお、この処理は減圧下で行ってもよい。この焼成により、有機物が酸化分解除去され、マイクロ波表面波プラズマ処理において、銅ナノ粒子の焼結が促進される。
Prior to microwave surface wave plasma treatment, in order to remove organic substances such as alkylamines, carboxylic acids, polymer dispersants, etc. contained in the coating film coated with copper nanoparticle dispersion, in the atmosphere or in an atmosphere containing oxygen The baking may be performed at a temperature of about 50 to 200 ° C. for about 1 minute to 2 hours. This treatment may be performed under reduced pressure. By this firing, organic substances are oxidatively decomposed and removed, and the sintering of the copper nanoparticles is promoted in the microwave surface wave plasma treatment.
前記マイクロ波表面波プラズマの発生方法は、従来公知の方法の中から適宜選択すればよい。例えば、国際公開第2011/040189号パンフレットに記載の方法を用いることができる。
The method for generating the microwave surface wave plasma may be appropriately selected from conventionally known methods. For example, the method described in International Publication No. 2011/040189 pamphlet can be used.
(フラッシュ光焼成)
フラッシュ光焼成とは、フラッシュ光の照射により極めて短時間で焼成する方法である。ここで、本発明においてフラッシュ光とは、点灯時間が比較的短時間の光のことをいい、当該点灯時間をパルス幅という。フラッシュ光の光源は特に限定されないが、キセノン等の希ガスが封入されたフラッシュランプやレーザー等が挙げられる。中でも、紫外線から赤外線までの連続的な波長スペクトルをもつ光を照射することが好ましく、具体的には、キセノンフラッシュランプを用いることが好ましい。このような光源を用いた場合には、加熱と同時にUV照射を行ったのと同様の効果を得ることができ、極めて短時間で焼成が可能となる。また、このような光源を用いた場合には、パルス幅と照射エネルギーを制御することにより、銅ナノ粒子分散体の塗膜、及びその近傍のみを加熱することができ、基材に対する熱の影響を抑えることができる。特に、本発明で用いられる上記高分子分散剤は、上記アルキルアミン及びカルボン酸との相乗効果により、銅ナノ粒子の周囲に均一に存在することから、フラッシュ光の照射により容易に分解乃至揮発しやすく、金属膜に残存しにくいため、極短時間のフラッシュ光の照射であっても容易に焼結させることができる。そのため、本発明においてフラッシュ光焼成は好適に用いられる。 (Flash light firing)
Flash light firing is a method of firing in an extremely short time by irradiation with flash light. Here, in the present invention, flash light refers to light having a relatively short lighting time, and the lighting time is referred to as a pulse width. The light source of the flash light is not particularly limited, and examples thereof include a flash lamp and a laser in which a rare gas such as xenon is sealed. Among them, it is preferable to irradiate light having a continuous wavelength spectrum from ultraviolet to infrared, and specifically, it is preferable to use a xenon flash lamp. When such a light source is used, the same effect as when UV irradiation is performed simultaneously with heating can be obtained, and baking can be performed in an extremely short time. In addition, when such a light source is used, by controlling the pulse width and irradiation energy, only the coating film of the copper nanoparticle dispersion and its vicinity can be heated, and the influence of heat on the substrate Can be suppressed. In particular, the polymer dispersant used in the present invention is uniformly present around the copper nanoparticles due to a synergistic effect with the alkylamine and carboxylic acid, and therefore easily decomposes or volatilizes by irradiation with flash light. Since it is easy and hardly remains on the metal film, it can be easily sintered even by irradiation with flash light for a very short time. Therefore, flash light baking is preferably used in the present invention.
フラッシュ光焼成とは、フラッシュ光の照射により極めて短時間で焼成する方法である。ここで、本発明においてフラッシュ光とは、点灯時間が比較的短時間の光のことをいい、当該点灯時間をパルス幅という。フラッシュ光の光源は特に限定されないが、キセノン等の希ガスが封入されたフラッシュランプやレーザー等が挙げられる。中でも、紫外線から赤外線までの連続的な波長スペクトルをもつ光を照射することが好ましく、具体的には、キセノンフラッシュランプを用いることが好ましい。このような光源を用いた場合には、加熱と同時にUV照射を行ったのと同様の効果を得ることができ、極めて短時間で焼成が可能となる。また、このような光源を用いた場合には、パルス幅と照射エネルギーを制御することにより、銅ナノ粒子分散体の塗膜、及びその近傍のみを加熱することができ、基材に対する熱の影響を抑えることができる。特に、本発明で用いられる上記高分子分散剤は、上記アルキルアミン及びカルボン酸との相乗効果により、銅ナノ粒子の周囲に均一に存在することから、フラッシュ光の照射により容易に分解乃至揮発しやすく、金属膜に残存しにくいため、極短時間のフラッシュ光の照射であっても容易に焼結させることができる。そのため、本発明においてフラッシュ光焼成は好適に用いられる。 (Flash light firing)
Flash light firing is a method of firing in an extremely short time by irradiation with flash light. Here, in the present invention, flash light refers to light having a relatively short lighting time, and the lighting time is referred to as a pulse width. The light source of the flash light is not particularly limited, and examples thereof include a flash lamp and a laser in which a rare gas such as xenon is sealed. Among them, it is preferable to irradiate light having a continuous wavelength spectrum from ultraviolet to infrared, and specifically, it is preferable to use a xenon flash lamp. When such a light source is used, the same effect as when UV irradiation is performed simultaneously with heating can be obtained, and baking can be performed in an extremely short time. In addition, when such a light source is used, by controlling the pulse width and irradiation energy, only the coating film of the copper nanoparticle dispersion and its vicinity can be heated, and the influence of heat on the substrate Can be suppressed. In particular, the polymer dispersant used in the present invention is uniformly present around the copper nanoparticles due to a synergistic effect with the alkylamine and carboxylic acid, and therefore easily decomposes or volatilizes by irradiation with flash light. Since it is easy and hardly remains on the metal film, it can be easily sintered even by irradiation with flash light for a very short time. Therefore, flash light baking is preferably used in the present invention.
本発明において、フラッシュ光のパルス幅は、適宜調整すればよいものであるが、1μs~10000μsの間で設定されることが好ましく、10μs~5000μsの範囲内とすることがより好ましい。また、フラッシュ光の1回あたりの照射エネルギーは、0.1J/cm2~100J/cm2が好ましく、0.5J/cm2~50J/cm2がより好ましい。
フラッシュ光焼成においてフラッシュ光の照射回数は、塗膜の組成や、膜厚、面積などに応じて適宜調整すればよく、照射回数は1回のみであってもよく、2回以上繰り返し行ってもよい。中でも、照射回数を1~100回とすることが好ましく、1~50回とすることが好ましい。フラッシュ光を複数回照射する場合には、フラッシュ光の照射間隔は適宜調整すればよい。中でも照射間隔を10μ秒~2秒の範囲内で設定することが好ましく、100μ秒~1秒の範囲内に設定することがより好ましい。
フラッシュ光を上記のように設定することにより、基材への影響を抑えるとともに、銅ナノ粒子の酸化を抑制することが可能であり、且つ、銅ナノ粒子分散体に含まれるアルキルアミン、カルボン酸、上記高分子分散剤も脱離乃至分解しやすく導電性に優れた導電性基板を得ることができる。 In the present invention, the pulse width of the flash light may be appropriately adjusted, but is preferably set between 1 μs and 10,000 μs, and more preferably within the range of 10 μs to 5000 μs. The irradiation energy per one flash light is preferably 0.1J /cm 2 ~ 100J / cm 2, 0.5J / cm 2 ~ 50J / cm 2 is more preferable.
In flash light firing, the number of times of flash light irradiation may be appropriately adjusted according to the composition, film thickness, area, etc. of the coating film, and the number of times of irradiation may be only once or may be repeated two or more times. Good. Among these, the number of irradiation is preferably 1 to 100 times, and more preferably 1 to 50 times. When the flash light is irradiated a plurality of times, the irradiation interval of the flash light may be adjusted as appropriate. In particular, the irradiation interval is preferably set within a range of 10 μsec to 2 seconds, and more preferably set within a range of 100 μsec to 1 second.
By setting the flash light as described above, it is possible to suppress the influence on the base material and to suppress the oxidation of the copper nanoparticles, and the alkylamine and carboxylic acid contained in the copper nanoparticle dispersion The above-described polymer dispersant can be easily detached or decomposed to obtain a conductive substrate having excellent conductivity.
フラッシュ光焼成においてフラッシュ光の照射回数は、塗膜の組成や、膜厚、面積などに応じて適宜調整すればよく、照射回数は1回のみであってもよく、2回以上繰り返し行ってもよい。中でも、照射回数を1~100回とすることが好ましく、1~50回とすることが好ましい。フラッシュ光を複数回照射する場合には、フラッシュ光の照射間隔は適宜調整すればよい。中でも照射間隔を10μ秒~2秒の範囲内で設定することが好ましく、100μ秒~1秒の範囲内に設定することがより好ましい。
フラッシュ光を上記のように設定することにより、基材への影響を抑えるとともに、銅ナノ粒子の酸化を抑制することが可能であり、且つ、銅ナノ粒子分散体に含まれるアルキルアミン、カルボン酸、上記高分子分散剤も脱離乃至分解しやすく導電性に優れた導電性基板を得ることができる。 In the present invention, the pulse width of the flash light may be appropriately adjusted, but is preferably set between 1 μs and 10,000 μs, and more preferably within the range of 10 μs to 5000 μs. The irradiation energy per one flash light is preferably 0.1J /
In flash light firing, the number of times of flash light irradiation may be appropriately adjusted according to the composition, film thickness, area, etc. of the coating film, and the number of times of irradiation may be only once or may be repeated two or more times. Good. Among these, the number of irradiation is preferably 1 to 100 times, and more preferably 1 to 50 times. When the flash light is irradiated a plurality of times, the irradiation interval of the flash light may be adjusted as appropriate. In particular, the irradiation interval is preferably set within a range of 10 μsec to 2 seconds, and more preferably set within a range of 100 μsec to 1 second.
By setting the flash light as described above, it is possible to suppress the influence on the base material and to suppress the oxidation of the copper nanoparticles, and the alkylamine and carboxylic acid contained in the copper nanoparticle dispersion The above-described polymer dispersant can be easily detached or decomposed to obtain a conductive substrate having excellent conductivity.
このようなフラッシュ光焼成は、銅ナノ粒子分散体の塗膜、及びその近傍のみを加熱することができ、前記塗膜を低温かつ短時間で焼成することが可能であり、緻密かつ平滑な銅ナノ粒子焼結膜を形成することができる。フラッシュ光焼成は、フラッシュ光のパルス幅と照射エネルギーを適宜調整することで、加熱温度と処理深さを制御することができる。その結果、不均一な膜が形成されることが少なく、また粒成長を防ぐことができるため、非常に緻密で、平滑な膜が得られる。また、極めて短時間で焼成が可能であるので、銅ナノ粒子の酸化を抑えることができ、導電性に優れた焼結膜を得ることができる。
上記フラッシュ光焼成は、大気中、大気圧下で行うことが可能であるが、不活性ガス雰囲気下、還元性ガス雰囲気下、減圧下で行ってもよい。また、塗膜を加熱しながら、フラッシュ光焼成を行ってもよい。 Such flash light baking can heat only the coating film of the copper nanoparticle dispersion and its vicinity, and can burn the coating film at a low temperature and in a short time. A nanoparticle sintered film can be formed. In the flash light firing, the heating temperature and the processing depth can be controlled by appropriately adjusting the pulse width and irradiation energy of the flash light. As a result, a non-uniform film is rarely formed and grain growth can be prevented, so that a very dense and smooth film can be obtained. Moreover, since baking is possible in a very short time, the oxidation of copper nanoparticles can be suppressed, and a sintered film having excellent conductivity can be obtained.
The flash light baking can be performed in the atmosphere at atmospheric pressure, but may be performed in an inert gas atmosphere, a reducing gas atmosphere, or a reduced pressure. Moreover, you may perform flash light baking, heating a coating film.
上記フラッシュ光焼成は、大気中、大気圧下で行うことが可能であるが、不活性ガス雰囲気下、還元性ガス雰囲気下、減圧下で行ってもよい。また、塗膜を加熱しながら、フラッシュ光焼成を行ってもよい。 Such flash light baking can heat only the coating film of the copper nanoparticle dispersion and its vicinity, and can burn the coating film at a low temperature and in a short time. A nanoparticle sintered film can be formed. In the flash light firing, the heating temperature and the processing depth can be controlled by appropriately adjusting the pulse width and irradiation energy of the flash light. As a result, a non-uniform film is rarely formed and grain growth can be prevented, so that a very dense and smooth film can be obtained. Moreover, since baking is possible in a very short time, the oxidation of copper nanoparticles can be suppressed, and a sintered film having excellent conductivity can be obtained.
The flash light baking can be performed in the atmosphere at atmospheric pressure, but may be performed in an inert gas atmosphere, a reducing gas atmosphere, or a reduced pressure. Moreover, you may perform flash light baking, heating a coating film.
このようにして得られた導電性基板の金属膜の厚みは、用途に応じて適宜調整すればよいものであるが、通常、厚みが0.01~50μm程度であり、0.05nm~30μmであることが好ましく、0.1~20μmであることがより好ましい。
また、上記金属膜の体積抵抗率は、1.0×10-4Ω・cm以下であることが好ましい。 The thickness of the metal film of the conductive substrate thus obtained may be adjusted as appropriate according to the application, but the thickness is usually about 0.01 to 50 μm, and 0.05 nm to 30 μm. Preferably, the thickness is 0.1 to 20 μm.
The volume resistivity of the metal film is preferably 1.0 × 10 −4 Ω · cm or less.
また、上記金属膜の体積抵抗率は、1.0×10-4Ω・cm以下であることが好ましい。 The thickness of the metal film of the conductive substrate thus obtained may be adjusted as appropriate according to the application, but the thickness is usually about 0.01 to 50 μm, and 0.05 nm to 30 μm. Preferably, the thickness is 0.1 to 20 μm.
The volume resistivity of the metal film is preferably 1.0 × 10 −4 Ω · cm or less.
また、本発明の銅ナノ粒子分散体を用いて得られた焼結膜は、表面が平滑で低抵抗であって、また、基材との界面に空隙が発生しにくく、密着性が良好で、且つ適度な空隙を有している。従来は、表面が平滑で低抵抗で且つ適度な空隙を有している焼結膜を得ることが困難であり、化学的エッチングによりパターンを形成することは困難であった。しかし、本発明の銅ナノ粒子分散体を用いて得られた焼結膜は、前述のように全面塗布による焼結膜を形成後に、更に化学的エッチングすることにより、微細な配線を形成することにも適している。
In addition, the sintered film obtained using the copper nanoparticle dispersion of the present invention has a smooth surface and low resistance, and is less likely to generate voids at the interface with the substrate, and has good adhesion. And it has a moderate space | gap. Conventionally, it has been difficult to obtain a sintered film having a smooth surface, low resistance, and appropriate voids, and it has been difficult to form a pattern by chemical etching. However, the sintered film obtained by using the copper nanoparticle dispersion of the present invention can also form fine wiring by further chemically etching after forming the sintered film by the entire surface coating as described above. Is suitable.
化学的エッチングによるパターン形成は、全面塗布による焼結膜に対し、フォトレジストを塗布するか又はドライフィルムレジストをラミネートしてフォトレジスト層を形成し、フォトマスクを用いたフォトリソグラフィ法により露光、現像してパターンを形成した後、塩化第二鉄、塩化第二銅、リン硝酢酸などによるエッチングを行い、残ったレジストを剥離して、パターン状の金属膜を形成することができる。
Pattern formation by chemical etching is performed by applying a photoresist or laminating a dry film resist to a sintered film formed on the entire surface to form a photoresist layer, and then exposing and developing by a photolithography method using a photomask. After the pattern is formed, etching with ferric chloride, cupric chloride, phosphorous nitric acid or the like is performed, and the remaining resist is peeled off to form a patterned metal film.
また、本発明の製造方法は、基材上に、銅ナノ粒子分散体をパターン状に塗布して、塗膜を形成し、該塗膜を焼成して、パターン状の金属膜を形成するパターン状導電性基板の製造方法であってもよい。
本発明の導電性基板の製造方法により得られた導電性基板は、パターン精度が良好で、優れた導電性を有する。このような導電性基板を用いた電子部材としては、表面抵抗の低い電磁波シールド用フィルム、導電膜、フレキシブルプリント配線板などに有効に利用することができる。 Moreover, the manufacturing method of the present invention is a pattern in which a copper nanoparticle dispersion is applied in a pattern on a substrate to form a coating film, and the coating film is baked to form a patterned metal film. It may be a method for manufacturing a conductive substrate.
The conductive substrate obtained by the method for producing a conductive substrate of the present invention has good pattern accuracy and excellent conductivity. An electronic member using such a conductive substrate can be effectively used for an electromagnetic wave shielding film having a low surface resistance, a conductive film, a flexible printed wiring board, and the like.
本発明の導電性基板の製造方法により得られた導電性基板は、パターン精度が良好で、優れた導電性を有する。このような導電性基板を用いた電子部材としては、表面抵抗の低い電磁波シールド用フィルム、導電膜、フレキシブルプリント配線板などに有効に利用することができる。 Moreover, the manufacturing method of the present invention is a pattern in which a copper nanoparticle dispersion is applied in a pattern on a substrate to form a coating film, and the coating film is baked to form a patterned metal film. It may be a method for manufacturing a conductive substrate.
The conductive substrate obtained by the method for producing a conductive substrate of the present invention has good pattern accuracy and excellent conductivity. An electronic member using such a conductive substrate can be effectively used for an electromagnetic wave shielding film having a low surface resistance, a conductive film, a flexible printed wiring board, and the like.
以下、本発明について実施例を示して具体的に説明する。これらの記載により本発明を制限するものではない。
(実施例1)
(1)銅ナノ粒子の合成
200ml三ッ口フラスコ中に、水酸化銅 10.0g(0.1mol、和光純薬工業製)、ノナン酸 31.5g(0.2mol、東京化成工業製、沸点254℃)、プロピレングリコールモノメチルエーテル(PGME) 18.5g(20ml、関東化学製)を量り取った。この混合液を撹拌しながら100℃まで加熱し、その温度を20分維持した。その後、ヘキシルアミン 40.5g(0.4mol、東京化成工業製、沸点130℃)を添加し、100℃で10分加熱、撹拌した。この混合液を、氷浴を用いて10℃まで冷却した後、氷浴中でヒドラジン一水和物 10.0g(0.2mol、関東化学製)をPGME 18.5g(20ml、関東化学製)に溶解させた溶液を添加し、10分撹拌した。その後、反応溶液を100℃まで加熱し、その温度を10分維持した。30℃まで冷却後、ヘキサン 33g(50ml、関東化学製)を添加した。遠心分離後、上澄み液を除去した。沈殿物をヘキサンで洗浄し、ノナン酸とヘキシルアミンで被覆された銅ナノ粒子を得た。 Hereinafter, the present invention will be specifically described with reference to examples. These descriptions do not limit the present invention.
Example 1
(1) Synthesis of copper nanoparticles In a 200 ml three-necked flask, 10.0 g of copper hydroxide (0.1 mol, manufactured by Wako Pure Chemical Industries), 31.5 g of nonanoic acid (0.2 mol, manufactured by Tokyo Chemical Industry, boiling point) 254 ° C.) and 18.5 g (20 ml, manufactured by Kanto Chemical) of propylene glycol monomethyl ether (PGME). The mixture was heated to 100 ° C. with stirring and the temperature was maintained for 20 minutes. Thereafter, 40.5 g of hexylamine (0.4 mol, manufactured by Tokyo Chemical Industry Co., Ltd., boiling point: 130 ° C.) was added, and the mixture was heated and stirred at 100 ° C. for 10 minutes. After cooling this mixed solution to 10 ° C. using an ice bath, 10.0 g (0.2 mol, manufactured by Kanto Chemical Co., Ltd.) of hydrazine monohydrate and 18.5 g of PGME (20 ml, manufactured by Kanto Chemical Co., Ltd.) were used in the ice bath. The solution dissolved in was added and stirred for 10 minutes. Thereafter, the reaction solution was heated to 100 ° C., and the temperature was maintained for 10 minutes. After cooling to 30 ° C., 33 g of hexane (50 ml, manufactured by Kanto Chemical Co., Inc.) was added. After centrifugation, the supernatant was removed. The precipitate was washed with hexane to obtain copper nanoparticles coated with nonanoic acid and hexylamine.
(実施例1)
(1)銅ナノ粒子の合成
200ml三ッ口フラスコ中に、水酸化銅 10.0g(0.1mol、和光純薬工業製)、ノナン酸 31.5g(0.2mol、東京化成工業製、沸点254℃)、プロピレングリコールモノメチルエーテル(PGME) 18.5g(20ml、関東化学製)を量り取った。この混合液を撹拌しながら100℃まで加熱し、その温度を20分維持した。その後、ヘキシルアミン 40.5g(0.4mol、東京化成工業製、沸点130℃)を添加し、100℃で10分加熱、撹拌した。この混合液を、氷浴を用いて10℃まで冷却した後、氷浴中でヒドラジン一水和物 10.0g(0.2mol、関東化学製)をPGME 18.5g(20ml、関東化学製)に溶解させた溶液を添加し、10分撹拌した。その後、反応溶液を100℃まで加熱し、その温度を10分維持した。30℃まで冷却後、ヘキサン 33g(50ml、関東化学製)を添加した。遠心分離後、上澄み液を除去した。沈殿物をヘキサンで洗浄し、ノナン酸とヘキシルアミンで被覆された銅ナノ粒子を得た。 Hereinafter, the present invention will be specifically described with reference to examples. These descriptions do not limit the present invention.
Example 1
(1) Synthesis of copper nanoparticles In a 200 ml three-necked flask, 10.0 g of copper hydroxide (0.1 mol, manufactured by Wako Pure Chemical Industries), 31.5 g of nonanoic acid (0.2 mol, manufactured by Tokyo Chemical Industry, boiling point) 254 ° C.) and 18.5 g (20 ml, manufactured by Kanto Chemical) of propylene glycol monomethyl ether (PGME). The mixture was heated to 100 ° C. with stirring and the temperature was maintained for 20 minutes. Thereafter, 40.5 g of hexylamine (0.4 mol, manufactured by Tokyo Chemical Industry Co., Ltd., boiling point: 130 ° C.) was added, and the mixture was heated and stirred at 100 ° C. for 10 minutes. After cooling this mixed solution to 10 ° C. using an ice bath, 10.0 g (0.2 mol, manufactured by Kanto Chemical Co., Ltd.) of hydrazine monohydrate and 18.5 g of PGME (20 ml, manufactured by Kanto Chemical Co., Ltd.) were used in the ice bath. The solution dissolved in was added and stirred for 10 minutes. Thereafter, the reaction solution was heated to 100 ° C., and the temperature was maintained for 10 minutes. After cooling to 30 ° C., 33 g of hexane (50 ml, manufactured by Kanto Chemical Co., Inc.) was added. After centrifugation, the supernatant was removed. The precipitate was washed with hexane to obtain copper nanoparticles coated with nonanoic acid and hexylamine.
得られた銅ナノ粒子を透過型電子顕微鏡(TEM)で観察したところ、平均一次粒径は39nmであった。具体的には、得られた銅ナノ粒子をトルエンに分散させ、これをTEM基板(日立ハイテクフィールディング製、エラスチックカーボン支持膜付Cuグリッド)へ滴下し、乾燥させることで観察用サンプルを作製した。TEM(日立ハイテク製 H-7650)にて粒子像を測定し、ランダムに選択した100個の一次粒子の最長部の長さの平均値を平均一次粒径とした。
またX線回折装置にて得られた銅ナノ粒子の結晶構造を測定したところ、銅ナノ粒子の主構造はCu(Cubic)で一部Cu2O(Cubic)構造が見られ、Cu(111)に対するCu2O(111)のピーク強度は約3%であった。 When the obtained copper nanoparticles were observed with a transmission electron microscope (TEM), the average primary particle size was 39 nm. Specifically, the obtained copper nanoparticles were dispersed in toluene, and this was dropped onto a TEM substrate (manufactured by Hitachi High-Tech Fielding Co., Ltd., Cu grid with elastic carbon support film) and dried to prepare a sample for observation. Particle images were measured with TEM (Hitachi High-Technology H-7650), and the average value of the length of the longest portion of 100 randomly selected primary particles was defined as the average primary particle size.
Moreover, when the crystal structure of the copper nanoparticle obtained by the X-ray diffractometer was measured, the main structure of the copper nanoparticle was Cu (Cubic), and a part of Cu 2 O (Cubic) structure was observed, and Cu (111) The peak intensity of Cu 2 O (111) relative to was about 3%.
またX線回折装置にて得られた銅ナノ粒子の結晶構造を測定したところ、銅ナノ粒子の主構造はCu(Cubic)で一部Cu2O(Cubic)構造が見られ、Cu(111)に対するCu2O(111)のピーク強度は約3%であった。 When the obtained copper nanoparticles were observed with a transmission electron microscope (TEM), the average primary particle size was 39 nm. Specifically, the obtained copper nanoparticles were dispersed in toluene, and this was dropped onto a TEM substrate (manufactured by Hitachi High-Tech Fielding Co., Ltd., Cu grid with elastic carbon support film) and dried to prepare a sample for observation. Particle images were measured with TEM (Hitachi High-Technology H-7650), and the average value of the length of the longest portion of 100 randomly selected primary particles was defined as the average primary particle size.
Moreover, when the crystal structure of the copper nanoparticle obtained by the X-ray diffractometer was measured, the main structure of the copper nanoparticle was Cu (Cubic), and a part of Cu 2 O (Cubic) structure was observed, and Cu (111) The peak intensity of Cu 2 O (111) relative to was about 3%.
(2)銅ナノ粒子分散体の調製
上記で得られたノナン酸及びヘキシルアミンで被覆された銅ナノ粒子 3.0質量部、ソルスパース41000(日本ルーブリゾール製、酸価50mgKOH/g、アミン価0mgKOH/g、重量平均分子量3500、90%熱重量減少温度が370.4℃) 0.3質量部、PGME 4.2質量部を混合し、ペイントシェーカー(浅田鉄工製)にて予備分散として2mmジルコニアビーズで1時間、さらに本分散として0.1mmジルコニアビーズで2時間分散し、銅ナノ粒子分散体1を得た。 (2) Preparation of copper nanoparticle dispersion 3.0 parts by mass of copper nanoparticles coated with nonanoic acid and hexylamine obtained above, Solsperse 41000 (manufactured by Nippon Lubrizol, acid value 50 mgKOH / g, amine value 0 mgKOH / G, weight average molecular weight 3500, 90% thermogravimetric reduction temperature is 370.4 ° C.) 0.3 parts by mass and 4.2 parts by mass of PGME are mixed, and 2 mm zirconia is preliminarily dispersed in a paint shaker (manufactured by Asada Tekko). Dispersion was carried out for 1 hour with beads and further for 2 hours with 0.1 mm zirconia beads as the main dispersion to obtain a copper nanoparticle dispersion 1.
上記で得られたノナン酸及びヘキシルアミンで被覆された銅ナノ粒子 3.0質量部、ソルスパース41000(日本ルーブリゾール製、酸価50mgKOH/g、アミン価0mgKOH/g、重量平均分子量3500、90%熱重量減少温度が370.4℃) 0.3質量部、PGME 4.2質量部を混合し、ペイントシェーカー(浅田鉄工製)にて予備分散として2mmジルコニアビーズで1時間、さらに本分散として0.1mmジルコニアビーズで2時間分散し、銅ナノ粒子分散体1を得た。 (2) Preparation of copper nanoparticle dispersion 3.0 parts by mass of copper nanoparticles coated with nonanoic acid and hexylamine obtained above, Solsperse 41000 (manufactured by Nippon Lubrizol, acid value 50 mgKOH / g, amine value 0 mgKOH / G, weight average molecular weight 3500, 90% thermogravimetric reduction temperature is 370.4 ° C.) 0.3 parts by mass and 4.2 parts by mass of PGME are mixed, and 2 mm zirconia is preliminarily dispersed in a paint shaker (manufactured by Asada Tekko). Dispersion was carried out for 1 hour with beads and further for 2 hours with 0.1 mm zirconia beads as the main dispersion to obtain a copper nanoparticle dispersion 1.
(3)プラズマ焼成による導電性基板の製造
上記銅ナノ粒子分散体1を、厚さ100μmのPETフィルム(コスモシャイン A4100)にワイヤーバーで塗布、乾燥して、膜厚が0.5μmの塗膜とした。
その後、水素ガスを導入圧力20Paで導入しながら、マイクロ波表面波プラズマ処理装置(MSP-1500、ミクロ電子製)を用いて、マイクロ波出力450Wで300秒間焼成し、導電性基板を得た。 (3) Production of conductive substrate by plasma firing The copper nanoparticle dispersion 1 is applied to a PET film (Cosmo Shine A4100) having a thickness of 100 μm with a wire bar and dried to form a coating film having a thickness of 0.5 μm. It was.
Thereafter, while introducing hydrogen gas at an introduction pressure of 20 Pa, using a microwave surface wave plasma processing apparatus (MSP-1500, manufactured by Microelectronics), firing was performed at a microwave output of 450 W for 300 seconds to obtain a conductive substrate.
上記銅ナノ粒子分散体1を、厚さ100μmのPETフィルム(コスモシャイン A4100)にワイヤーバーで塗布、乾燥して、膜厚が0.5μmの塗膜とした。
その後、水素ガスを導入圧力20Paで導入しながら、マイクロ波表面波プラズマ処理装置(MSP-1500、ミクロ電子製)を用いて、マイクロ波出力450Wで300秒間焼成し、導電性基板を得た。 (3) Production of conductive substrate by plasma firing The copper nanoparticle dispersion 1 is applied to a PET film (Cosmo Shine A4100) having a thickness of 100 μm with a wire bar and dried to form a coating film having a thickness of 0.5 μm. It was.
Thereafter, while introducing hydrogen gas at an introduction pressure of 20 Pa, using a microwave surface wave plasma processing apparatus (MSP-1500, manufactured by Microelectronics), firing was performed at a microwave output of 450 W for 300 seconds to obtain a conductive substrate.
(4)フラッシュ光焼成による導電性基板の製造
上記銅ナノ粒子分散体1を、厚さ100μmのPETフィルム(コスモシャイン A4100)にワイヤーバーで塗布、乾燥して、膜厚が0.5μmの塗膜とした。
その後、パルスドキセノンランプ装置(SINTERON 2000(Xenon Corporation製))を用いて、パルス幅500μ秒、印加電圧3.7kVで1回照射して、導電性基板を得た。 (4) Production of conductive substrate by flash light baking The copper nanoparticle dispersion 1 is applied to a PET film (Cosmo Shine A4100) having a thickness of 100 μm with a wire bar and dried to obtain a coating having a thickness of 0.5 μm. A membrane was obtained.
Thereafter, using a pulsed xenon lamp device (SINTERON 2000 (manufactured by Xenon Corporation)), irradiation was performed once with a pulse width of 500 μsec and an applied voltage of 3.7 kV to obtain a conductive substrate.
上記銅ナノ粒子分散体1を、厚さ100μmのPETフィルム(コスモシャイン A4100)にワイヤーバーで塗布、乾燥して、膜厚が0.5μmの塗膜とした。
その後、パルスドキセノンランプ装置(SINTERON 2000(Xenon Corporation製))を用いて、パルス幅500μ秒、印加電圧3.7kVで1回照射して、導電性基板を得た。 (4) Production of conductive substrate by flash light baking The copper nanoparticle dispersion 1 is applied to a PET film (Cosmo Shine A4100) having a thickness of 100 μm with a wire bar and dried to obtain a coating having a thickness of 0.5 μm. A membrane was obtained.
Thereafter, using a pulsed xenon lamp device (SINTERON 2000 (manufactured by Xenon Corporation)), irradiation was performed once with a pulse width of 500 μsec and an applied voltage of 3.7 kV to obtain a conductive substrate.
(実施例2)
実施例1の(2)において、ソルスパース41000の代わりに、ソルスパース53095(日本ルーブリゾール製、酸価47mgKOH/g、アミン価0mgKOH/g、重量平均分子量3900、90%熱重量減少温度が360.7℃)を用いた以外は実施例1の(2)と同様にして、銅ナノ粒子分散体2を得た。
得られた銅ナノ粒子分散体2を用いて、実施例1の(3)と同様にして、プラズマ焼成により導電性基板を製造した。
得られた銅ナノ粒子分散体2を用いて、実施例1の(4)と同様にして、フラッシュ光焼成により導電性基板を製造した。 (Example 2)
In Example 1, (2), instead of Solsperse 41000, Solsperse 53095 (manufactured by Nippon Lubrizol, acid value 47 mgKOH / g, amine value 0 mgKOH / g, weight average molecular weight 3900, 90% thermogravimetric decrease temperature is 360.7. C.) was used in the same manner as (2) of Example 1 except that thecopper nanoparticle dispersion 2 was obtained.
Using the obtainedcopper nanoparticle dispersion 2, a conductive substrate was produced by plasma firing in the same manner as (3) of Example 1.
Using the obtainedcopper nanoparticle dispersion 2, a conductive substrate was produced by flash light baking in the same manner as in (4) of Example 1.
実施例1の(2)において、ソルスパース41000の代わりに、ソルスパース53095(日本ルーブリゾール製、酸価47mgKOH/g、アミン価0mgKOH/g、重量平均分子量3900、90%熱重量減少温度が360.7℃)を用いた以外は実施例1の(2)と同様にして、銅ナノ粒子分散体2を得た。
得られた銅ナノ粒子分散体2を用いて、実施例1の(3)と同様にして、プラズマ焼成により導電性基板を製造した。
得られた銅ナノ粒子分散体2を用いて、実施例1の(4)と同様にして、フラッシュ光焼成により導電性基板を製造した。 (Example 2)
In Example 1, (2), instead of Solsperse 41000, Solsperse 53095 (manufactured by Nippon Lubrizol, acid value 47 mgKOH / g, amine value 0 mgKOH / g, weight average molecular weight 3900, 90% thermogravimetric decrease temperature is 360.7. C.) was used in the same manner as (2) of Example 1 except that the
Using the obtained
Using the obtained
(実施例3)
実施例1の(2)において、ソルスパース41000の代わりに、ソルスパース71000(日本ルーブリゾール製、酸価0mgKOH/g、アミン価77.4mgKOH/g、重量平均分子量4700、90%熱重量減少温度が405.9℃)を用いた以外は実施例1の(2)と同様にして、銅ナノ粒子分散体3を得た。
得られた銅ナノ粒子分散体3を用いて、実施例1の(3)と同様にして、プラズマ焼成により導電性基板を製造した。
得られた銅ナノ粒子分散体3を用いて、実施例1の(4)と同様にして、フラッシュ光焼成により導電性基板を製造した。 Example 3
In Example 1 (2), instead of Solsperse 41000, Solsperse 71000 (manufactured by Nippon Lubrizol, acid value 0 mgKOH / g, amine value 77.4 mgKOH / g, weight average molecular weight 4700, 90% thermogravimetric reduction temperature is 405. .9 ° C) was used in the same manner as (2) of Example 1 to obtain acopper nanoparticle dispersion 3.
Using the obtainedcopper nanoparticle dispersion 3, a conductive substrate was produced by plasma firing in the same manner as (3) of Example 1.
Using the obtainedcopper nanoparticle dispersion 3, a conductive substrate was produced by flash light baking in the same manner as in (4) of Example 1.
実施例1の(2)において、ソルスパース41000の代わりに、ソルスパース71000(日本ルーブリゾール製、酸価0mgKOH/g、アミン価77.4mgKOH/g、重量平均分子量4700、90%熱重量減少温度が405.9℃)を用いた以外は実施例1の(2)と同様にして、銅ナノ粒子分散体3を得た。
得られた銅ナノ粒子分散体3を用いて、実施例1の(3)と同様にして、プラズマ焼成により導電性基板を製造した。
得られた銅ナノ粒子分散体3を用いて、実施例1の(4)と同様にして、フラッシュ光焼成により導電性基板を製造した。 Example 3
In Example 1 (2), instead of Solsperse 41000, Solsperse 71000 (manufactured by Nippon Lubrizol, acid value 0 mgKOH / g, amine value 77.4 mgKOH / g, weight average molecular weight 4700, 90% thermogravimetric reduction temperature is 405. .9 ° C) was used in the same manner as (2) of Example 1 to obtain a
Using the obtained
Using the obtained
(実施例4)
実施例1の(2)において、ソルスパース41000の代わりに、ディスパーbyk-111(ビックケミー・ジャパン製、酸価129mgKOH/g、アミン価0mgKOH/g、重量平均分子量1700、90%熱重量減少温度が320.4℃)を用いた以外は実施例1の(2)と同様にして、銅ナノ粒子分散体4を得た。
得られた銅ナノ粒子分散体4を用いて、実施例1の(3)と同様にして、プラズマ焼成により導電性基板を製造した。
得られた銅ナノ粒子分散体4を用いて、実施例1の(4)と同様にして、フラッシュ光焼成により導電性基板を製造した。 Example 4
In Example 1, (2), instead of Solsperse 41000, Disper byk-111 (manufactured by Big Chemie Japan, acid value 129 mgKOH / g, amine value 0 mgKOH / g, weight average molecular weight 1700, 90% thermogravimetric decrease temperature is 320. .4 ° C.) was used in the same manner as (2) of Example 1 to obtain a copper nanoparticle dispersion 4.
Using the obtained copper nanoparticle dispersion 4, a conductive substrate was produced by plasma firing in the same manner as (3) of Example 1.
Using the obtained copper nanoparticle dispersion 4, a conductive substrate was produced by flash light baking in the same manner as in Example 1 (4).
実施例1の(2)において、ソルスパース41000の代わりに、ディスパーbyk-111(ビックケミー・ジャパン製、酸価129mgKOH/g、アミン価0mgKOH/g、重量平均分子量1700、90%熱重量減少温度が320.4℃)を用いた以外は実施例1の(2)と同様にして、銅ナノ粒子分散体4を得た。
得られた銅ナノ粒子分散体4を用いて、実施例1の(3)と同様にして、プラズマ焼成により導電性基板を製造した。
得られた銅ナノ粒子分散体4を用いて、実施例1の(4)と同様にして、フラッシュ光焼成により導電性基板を製造した。 Example 4
In Example 1, (2), instead of Solsperse 41000, Disper byk-111 (manufactured by Big Chemie Japan, acid value 129 mgKOH / g, amine value 0 mgKOH / g, weight average molecular weight 1700, 90% thermogravimetric decrease temperature is 320. .4 ° C.) was used in the same manner as (2) of Example 1 to obtain a copper nanoparticle dispersion 4.
Using the obtained copper nanoparticle dispersion 4, a conductive substrate was produced by plasma firing in the same manner as (3) of Example 1.
Using the obtained copper nanoparticle dispersion 4, a conductive substrate was produced by flash light baking in the same manner as in Example 1 (4).
(実施例5)
実施例1の(2)において、ソルスパース41000の代わりに、ディスパーbyk-145(ビックケミー・ジャパン製、酸価76mgKOH/g、アミン価71mgKOH/g、重量平均分子量1800、90%熱重量減少温度が378.2℃)を用いた以外は実施例1の(2)と同様にして、銅ナノ粒子分散体5を得た。
得られた銅ナノ粒子分散体5を用いて、実施例1の(3)と同様にして、プラズマ焼成により導電性基板を製造した。
得られた銅ナノ粒子分散体5を用いて、実施例1の(4)と同様にして、フラッシュ光焼成により導電性基板を製造した。 (Example 5)
In Example 1 (2), instead of Solsperse 41000, Disper byk-145 (manufactured by Big Chemie Japan, acid value 76 mgKOH / g, amine value 71 mgKOH / g, weight average molecular weight 1800, 90% thermogravimetric decrease temperature is 378 .2 ° C.) was used in the same manner as (2) of Example 1 to obtain a copper nanoparticle dispersion 5.
Using the obtained copper nanoparticle dispersion 5, a conductive substrate was produced by plasma firing in the same manner as in (1) of Example 1.
Using the obtained copper nanoparticle dispersion 5, a conductive substrate was produced by flash light baking in the same manner as in Example 1 (4).
実施例1の(2)において、ソルスパース41000の代わりに、ディスパーbyk-145(ビックケミー・ジャパン製、酸価76mgKOH/g、アミン価71mgKOH/g、重量平均分子量1800、90%熱重量減少温度が378.2℃)を用いた以外は実施例1の(2)と同様にして、銅ナノ粒子分散体5を得た。
得られた銅ナノ粒子分散体5を用いて、実施例1の(3)と同様にして、プラズマ焼成により導電性基板を製造した。
得られた銅ナノ粒子分散体5を用いて、実施例1の(4)と同様にして、フラッシュ光焼成により導電性基板を製造した。 (Example 5)
In Example 1 (2), instead of Solsperse 41000, Disper byk-145 (manufactured by Big Chemie Japan, acid value 76 mgKOH / g, amine value 71 mgKOH / g, weight average molecular weight 1800, 90% thermogravimetric decrease temperature is 378 .2 ° C.) was used in the same manner as (2) of Example 1 to obtain a copper nanoparticle dispersion 5.
Using the obtained copper nanoparticle dispersion 5, a conductive substrate was produced by plasma firing in the same manner as in (1) of Example 1.
Using the obtained copper nanoparticle dispersion 5, a conductive substrate was produced by flash light baking in the same manner as in Example 1 (4).
(実施例6)
実施例1の(2)において、ソルスパース41000の代わりに、ディスパーbyk-180(ビックケミー・ジャパン製、酸価94mgKOH/g、アミン価94mgKOH/g、THF可溶分の重量平均分子量1000、90%熱重量減少温度が350.2℃)を用いた以外は実施例1の(2)と同様にして、銅ナノ粒子分散体6を得た。
得られた銅ナノ粒子分散体6を用いて、実施例1の(3)と同様にして、プラズマ焼成により導電性基板を製造した。
得られた銅ナノ粒子分散体6を用いて、実施例1の(4)と同様にして、フラッシュ光焼成により導電性基板を製造した。 (Example 6)
In Example 1 (2), instead of Solsperse 41000, Disper byk-180 (manufactured by Big Chemie Japan, acid value 94 mgKOH / g, amine value 94 mgKOH / g, THF-soluble component weight average molecular weight 1000, 90% heat A copper nanoparticle dispersion 6 was obtained in the same manner as (2) of Example 1 except that the weight reduction temperature was 350.2 ° C.
Using the obtained copper nanoparticle dispersion 6, a conductive substrate was produced by plasma firing in the same manner as in (1) of Example 1.
Using the obtained copper nanoparticle dispersion 6, a conductive substrate was produced by flash light baking in the same manner as in Example 1 (4).
実施例1の(2)において、ソルスパース41000の代わりに、ディスパーbyk-180(ビックケミー・ジャパン製、酸価94mgKOH/g、アミン価94mgKOH/g、THF可溶分の重量平均分子量1000、90%熱重量減少温度が350.2℃)を用いた以外は実施例1の(2)と同様にして、銅ナノ粒子分散体6を得た。
得られた銅ナノ粒子分散体6を用いて、実施例1の(3)と同様にして、プラズマ焼成により導電性基板を製造した。
得られた銅ナノ粒子分散体6を用いて、実施例1の(4)と同様にして、フラッシュ光焼成により導電性基板を製造した。 (Example 6)
In Example 1 (2), instead of Solsperse 41000, Disper byk-180 (manufactured by Big Chemie Japan, acid value 94 mgKOH / g, amine value 94 mgKOH / g, THF-soluble component weight average molecular weight 1000, 90% heat A copper nanoparticle dispersion 6 was obtained in the same manner as (2) of Example 1 except that the weight reduction temperature was 350.2 ° C.
Using the obtained copper nanoparticle dispersion 6, a conductive substrate was produced by plasma firing in the same manner as in (1) of Example 1.
Using the obtained copper nanoparticle dispersion 6, a conductive substrate was produced by flash light baking in the same manner as in Example 1 (4).
(実施例7)
(1)銅ナノ粒子の合成
実施例1において、ヘキシルアミン 40.5g(0.4mol)の代わりに、3-メトキシプロピルアミン 35.7g(0.4mol、広栄化学工業製、沸点116℃)を用いた以外は、実施例1と同様にして、ノナン酸と3-メトキシプロピルアミンで被覆された銅ナノ粒子を得た。 (Example 7)
(1) Synthesis of copper nanoparticles In Example 1, instead of 40.5 g (0.4 mol) of hexylamine, 35.7 g of 3-methoxypropylamine (0.4 mol, manufactured by Guangei Chemical Industry Co., Ltd., boiling point 116 ° C.) was used. Copper nanoparticles coated with nonanoic acid and 3-methoxypropylamine were obtained in the same manner as Example 1 except for using.
(1)銅ナノ粒子の合成
実施例1において、ヘキシルアミン 40.5g(0.4mol)の代わりに、3-メトキシプロピルアミン 35.7g(0.4mol、広栄化学工業製、沸点116℃)を用いた以外は、実施例1と同様にして、ノナン酸と3-メトキシプロピルアミンで被覆された銅ナノ粒子を得た。 (Example 7)
(1) Synthesis of copper nanoparticles In Example 1, instead of 40.5 g (0.4 mol) of hexylamine, 35.7 g of 3-methoxypropylamine (0.4 mol, manufactured by Guangei Chemical Industry Co., Ltd., boiling point 116 ° C.) was used. Copper nanoparticles coated with nonanoic acid and 3-methoxypropylamine were obtained in the same manner as Example 1 except for using.
得られた銅ナノ粒子を、実施例1と同様にして透過型電子顕微鏡(TEM)で観察したところ、平均一次粒径は65nmであった。
またX線回折装置にて得られた銅ナノ粒子の結晶構造を測定したところ、銅ナノ粒子の主構造はCu(Cubic)で一部Cu2O(Cubic)構造が見られ、Cu(111)に対するCu2O(111)のピーク強度は約1%であった。 When the obtained copper nanoparticles were observed with a transmission electron microscope (TEM) in the same manner as in Example 1, the average primary particle size was 65 nm.
Moreover, when the crystal structure of the copper nanoparticle obtained by the X-ray diffractometer was measured, the main structure of the copper nanoparticle was Cu (Cubic), and a part of Cu 2 O (Cubic) structure was observed, and Cu (111) The peak intensity of Cu 2 O (111) relative to was about 1%.
またX線回折装置にて得られた銅ナノ粒子の結晶構造を測定したところ、銅ナノ粒子の主構造はCu(Cubic)で一部Cu2O(Cubic)構造が見られ、Cu(111)に対するCu2O(111)のピーク強度は約1%であった。 When the obtained copper nanoparticles were observed with a transmission electron microscope (TEM) in the same manner as in Example 1, the average primary particle size was 65 nm.
Moreover, when the crystal structure of the copper nanoparticle obtained by the X-ray diffractometer was measured, the main structure of the copper nanoparticle was Cu (Cubic), and a part of Cu 2 O (Cubic) structure was observed, and Cu (111) The peak intensity of Cu 2 O (111) relative to was about 1%.
(2)銅ナノ粒子分散体の調製
上記で得られたノナン酸と3-メトキシプロピルアミンで被覆された銅ナノ粒子を用いた以外は、実施例1の(2)と同様にして、銅ナノ粒子分散体7を得た。 (2) Preparation of copper nanoparticle dispersion A copper nanoparticle dispersion was prepared in the same manner as (2) of Example 1 except that the copper nanoparticle coated with nonanoic acid and 3-methoxypropylamine obtained above was used. A particle dispersion 7 was obtained.
上記で得られたノナン酸と3-メトキシプロピルアミンで被覆された銅ナノ粒子を用いた以外は、実施例1の(2)と同様にして、銅ナノ粒子分散体7を得た。 (2) Preparation of copper nanoparticle dispersion A copper nanoparticle dispersion was prepared in the same manner as (2) of Example 1 except that the copper nanoparticle coated with nonanoic acid and 3-methoxypropylamine obtained above was used. A particle dispersion 7 was obtained.
(3)プラズマ焼成による導電性基板の製造
得られた銅ナノ粒子分散体7を用いて、実施例1の(3)と同様にして、プラズマ焼成により導電性基板を製造した。
(4)フラッシュ光焼成による導電性基板の製造
得られた銅ナノ粒子分散体7を用いて、実施例1の(4)と同様にして、フラッシュ光焼成により導電性基板を製造した。 (3) Production of conductive substrate by plasma firing Using the obtained copper nanoparticle dispersion 7, a conductive substrate was produced by plasma firing in the same manner as in (1) of Example 1.
(4) Production of conductive substrate by flash light firing Using the obtained copper nanoparticle dispersion 7, a conductive substrate was produced by flash light firing in the same manner as in (4) of Example 1.
得られた銅ナノ粒子分散体7を用いて、実施例1の(3)と同様にして、プラズマ焼成により導電性基板を製造した。
(4)フラッシュ光焼成による導電性基板の製造
得られた銅ナノ粒子分散体7を用いて、実施例1の(4)と同様にして、フラッシュ光焼成により導電性基板を製造した。 (3) Production of conductive substrate by plasma firing Using the obtained copper nanoparticle dispersion 7, a conductive substrate was produced by plasma firing in the same manner as in (1) of Example 1.
(4) Production of conductive substrate by flash light firing Using the obtained copper nanoparticle dispersion 7, a conductive substrate was produced by flash light firing in the same manner as in (4) of Example 1.
(実施例8)
(1)銅ナノ粒子の合成
実施例1において、ヘキシルアミン 40.5g(0.4mol)の代わりに、3-エトキシプロピルアミン 41.3g(0.4mol、広栄化学工業製、沸点135℃)を用い、ヘキサン33.0g(50ml)を添加する代わりに、ヘキサン66.0g(100ml)を添加した以外は、実施例1と同様にして、ノナン酸と3-エトキシプロピルアミンで被覆された銅ナノ粒子を得た。 (Example 8)
(1) Synthesis of copper nanoparticles In Example 1, instead of 40.5 g (0.4 mol) of hexylamine, 41.3 g of 3-ethoxypropylamine (0.4 mol, manufactured by Guangei Chemical Industry Co., Ltd., boiling point 135 ° C.) was used. In the same manner as in Example 1 except that 63.0 g (100 ml) of hexane was added instead of 33.0 g (50 ml) of hexane, copper nano-particles coated with nonanoic acid and 3-ethoxypropylamine were used. Particles were obtained.
(1)銅ナノ粒子の合成
実施例1において、ヘキシルアミン 40.5g(0.4mol)の代わりに、3-エトキシプロピルアミン 41.3g(0.4mol、広栄化学工業製、沸点135℃)を用い、ヘキサン33.0g(50ml)を添加する代わりに、ヘキサン66.0g(100ml)を添加した以外は、実施例1と同様にして、ノナン酸と3-エトキシプロピルアミンで被覆された銅ナノ粒子を得た。 (Example 8)
(1) Synthesis of copper nanoparticles In Example 1, instead of 40.5 g (0.4 mol) of hexylamine, 41.3 g of 3-ethoxypropylamine (0.4 mol, manufactured by Guangei Chemical Industry Co., Ltd., boiling point 135 ° C.) was used. In the same manner as in Example 1 except that 63.0 g (100 ml) of hexane was added instead of 33.0 g (50 ml) of hexane, copper nano-particles coated with nonanoic acid and 3-ethoxypropylamine were used. Particles were obtained.
得られた銅ナノ粒子を、実施例1と同様にして透過型電子顕微鏡(TEM)で観察したところ、平均一次粒径は65nmであった。
またX線回折装置にて得られた銅ナノ粒子の結晶構造を測定したところ、銅ナノ粒子の主構造はCu(Cubic)で一部Cu2O(Cubic)構造が見られ、Cu(111)に対するCu2O(111)のピーク強度は約5%であった。 When the obtained copper nanoparticles were observed with a transmission electron microscope (TEM) in the same manner as in Example 1, the average primary particle size was 65 nm.
Moreover, when the crystal structure of the copper nanoparticle obtained by the X-ray diffractometer was measured, the main structure of the copper nanoparticle was Cu (Cubic), and a part of Cu 2 O (Cubic) structure was observed, and Cu (111) The peak intensity of Cu 2 O (111) relative to was about 5%.
またX線回折装置にて得られた銅ナノ粒子の結晶構造を測定したところ、銅ナノ粒子の主構造はCu(Cubic)で一部Cu2O(Cubic)構造が見られ、Cu(111)に対するCu2O(111)のピーク強度は約5%であった。 When the obtained copper nanoparticles were observed with a transmission electron microscope (TEM) in the same manner as in Example 1, the average primary particle size was 65 nm.
Moreover, when the crystal structure of the copper nanoparticle obtained by the X-ray diffractometer was measured, the main structure of the copper nanoparticle was Cu (Cubic), and a part of Cu 2 O (Cubic) structure was observed, and Cu (111) The peak intensity of Cu 2 O (111) relative to was about 5%.
(2)銅ナノ粒子分散体の調製
実施例1の(2)において、銅ナノ粒子として上記で得られたノナン酸と3-エトキシプロピルアミンで被覆された銅ナノ粒子を用い、ソルスパース41000の代わりに、ソルスパース71000(日本ルーブリゾール製、酸価0mgKOH/g、アミン価77.4mgKOH/g、重量平均分子量4700、90%熱重量減少温度が405.9℃)を用いた以外は実施例1の(2)と同様にして、銅ナノ粒子分散体8を得た。 (2) Preparation of copper nanoparticle dispersion In Example 1 (2), the copper nanoparticles coated with nonanoic acid and 3-ethoxypropylamine obtained above were used as the copper nanoparticles, and instead of Solsperse 41000 In addition, Solsperse 71000 (manufactured by Nippon Lubrizol, acid value 0 mgKOH / g, amine value 77.4 mgKOH / g, weight average molecular weight 4700, 90% thermogravimetric decrease temperature is 405.9 ° C.) is used. In the same manner as (2), a copper nanoparticle dispersion 8 was obtained.
実施例1の(2)において、銅ナノ粒子として上記で得られたノナン酸と3-エトキシプロピルアミンで被覆された銅ナノ粒子を用い、ソルスパース41000の代わりに、ソルスパース71000(日本ルーブリゾール製、酸価0mgKOH/g、アミン価77.4mgKOH/g、重量平均分子量4700、90%熱重量減少温度が405.9℃)を用いた以外は実施例1の(2)と同様にして、銅ナノ粒子分散体8を得た。 (2) Preparation of copper nanoparticle dispersion In Example 1 (2), the copper nanoparticles coated with nonanoic acid and 3-ethoxypropylamine obtained above were used as the copper nanoparticles, and instead of Solsperse 41000 In addition, Solsperse 71000 (manufactured by Nippon Lubrizol, acid value 0 mgKOH / g, amine value 77.4 mgKOH / g, weight average molecular weight 4700, 90% thermogravimetric decrease temperature is 405.9 ° C.) is used. In the same manner as (2), a copper nanoparticle dispersion 8 was obtained.
(3)プラズマ焼成による導電性基板の製造
得られた銅ナノ粒子分散体8を用いて、実施例1の(3)と同様にして、プラズマ焼成により導電性基板を製造した。
(4)フラッシュ光焼成による導電性基板の製造
得られた銅ナノ粒子分散体8を用いて、実施例1の(4)と同様にして、フラッシュ光焼成により導電性基板を製造した。 (3) Production of conductive substrate by plasma firing Using the obtained copper nanoparticle dispersion 8, a conductive substrate was produced by plasma firing in the same manner as (3) of Example 1.
(4) Production of conductive substrate by flash light firing Using the obtained copper nanoparticle dispersion 8, a conductive substrate was produced by flash light firing in the same manner as in (4) of Example 1.
得られた銅ナノ粒子分散体8を用いて、実施例1の(3)と同様にして、プラズマ焼成により導電性基板を製造した。
(4)フラッシュ光焼成による導電性基板の製造
得られた銅ナノ粒子分散体8を用いて、実施例1の(4)と同様にして、フラッシュ光焼成により導電性基板を製造した。 (3) Production of conductive substrate by plasma firing Using the obtained copper nanoparticle dispersion 8, a conductive substrate was produced by plasma firing in the same manner as (3) of Example 1.
(4) Production of conductive substrate by flash light firing Using the obtained copper nanoparticle dispersion 8, a conductive substrate was produced by flash light firing in the same manner as in (4) of Example 1.
(実施例9)
(1)銅ナノ粒子の合成
実施例8において、ノナン酸 31.5g(0.2mol)の代わりに、デカン酸 34.5g(0.2mol、花王製ルナック10-98、沸点268℃)を用いた以外は、実施例8と同様にして、デカン酸と3-エトキシプロピルアミンで被覆された銅ナノ粒子を得た。 Example 9
(1) Synthesis of copper nanoparticles In Example 8, instead of 31.5 g (0.2 mol) of nonanoic acid, 34.5 g of decanoic acid (0.2 mol, Kao Lunak 10-98, boiling point 268 ° C.) was used. Except for the above, copper nanoparticles coated with decanoic acid and 3-ethoxypropylamine were obtained in the same manner as in Example 8.
(1)銅ナノ粒子の合成
実施例8において、ノナン酸 31.5g(0.2mol)の代わりに、デカン酸 34.5g(0.2mol、花王製ルナック10-98、沸点268℃)を用いた以外は、実施例8と同様にして、デカン酸と3-エトキシプロピルアミンで被覆された銅ナノ粒子を得た。 Example 9
(1) Synthesis of copper nanoparticles In Example 8, instead of 31.5 g (0.2 mol) of nonanoic acid, 34.5 g of decanoic acid (0.2 mol, Kao Lunak 10-98, boiling point 268 ° C.) was used. Except for the above, copper nanoparticles coated with decanoic acid and 3-ethoxypropylamine were obtained in the same manner as in Example 8.
得られた銅ナノ粒子を、実施例1と同様にして透過型電子顕微鏡(TEM)で観察したところ、平均一次粒径は48nmであった。
またX線回折装置にて得られた銅ナノ粒子の結晶構造を測定したところ、銅ナノ粒子の主構造はCu(Cubic)で一部Cu2O(Cubic)構造が見られ、Cu(111)に対するCu2O(111)のピーク強度は約7%であった。 When the obtained copper nanoparticles were observed with a transmission electron microscope (TEM) in the same manner as in Example 1, the average primary particle size was 48 nm.
Moreover, when the crystal structure of the copper nanoparticle obtained by the X-ray diffractometer was measured, the main structure of the copper nanoparticle was Cu (Cubic), and a part of Cu 2 O (Cubic) structure was observed, and Cu (111) The peak intensity of Cu 2 O (111) relative to was about 7%.
またX線回折装置にて得られた銅ナノ粒子の結晶構造を測定したところ、銅ナノ粒子の主構造はCu(Cubic)で一部Cu2O(Cubic)構造が見られ、Cu(111)に対するCu2O(111)のピーク強度は約7%であった。 When the obtained copper nanoparticles were observed with a transmission electron microscope (TEM) in the same manner as in Example 1, the average primary particle size was 48 nm.
Moreover, when the crystal structure of the copper nanoparticle obtained by the X-ray diffractometer was measured, the main structure of the copper nanoparticle was Cu (Cubic), and a part of Cu 2 O (Cubic) structure was observed, and Cu (111) The peak intensity of Cu 2 O (111) relative to was about 7%.
(2)銅ナノ粒子分散体の調製
上記で得られたデカン酸と3-エトキシプロピルアミンで被覆された銅ナノ粒子を用いた以外は、実施例1の(2)と同様にして、銅ナノ粒子分散体9を得た。 (2) Preparation of copper nanoparticle dispersion A copper nanoparticle dispersion was prepared in the same manner as (2) of Example 1 except that the copper nanoparticle coated with decanoic acid and 3-ethoxypropylamine obtained above was used. A particle dispersion 9 was obtained.
上記で得られたデカン酸と3-エトキシプロピルアミンで被覆された銅ナノ粒子を用いた以外は、実施例1の(2)と同様にして、銅ナノ粒子分散体9を得た。 (2) Preparation of copper nanoparticle dispersion A copper nanoparticle dispersion was prepared in the same manner as (2) of Example 1 except that the copper nanoparticle coated with decanoic acid and 3-ethoxypropylamine obtained above was used. A particle dispersion 9 was obtained.
(3)プラズマ焼成による導電性基板の製造
得られた銅ナノ粒子分散体9を用いて、実施例1の(3)と同様にして、プラズマ焼成により導電性基板を製造した。
(4)フラッシュ光焼成による導電性基板の製造
得られた銅ナノ粒子分散体9を用いて、実施例1の(4)と同様にして、フラッシュ光焼成により導電性基板を製造した。 (3) Production of conductive substrate by plasma firing Using the obtained copper nanoparticle dispersion 9, a conductive substrate was produced by plasma firing in the same manner as in (1) of Example 1.
(4) Production of conductive substrate by flash light firing Using the obtained copper nanoparticle dispersion 9, a conductive substrate was produced by flash light firing in the same manner as in (4) of Example 1.
得られた銅ナノ粒子分散体9を用いて、実施例1の(3)と同様にして、プラズマ焼成により導電性基板を製造した。
(4)フラッシュ光焼成による導電性基板の製造
得られた銅ナノ粒子分散体9を用いて、実施例1の(4)と同様にして、フラッシュ光焼成により導電性基板を製造した。 (3) Production of conductive substrate by plasma firing Using the obtained copper nanoparticle dispersion 9, a conductive substrate was produced by plasma firing in the same manner as in (1) of Example 1.
(4) Production of conductive substrate by flash light firing Using the obtained copper nanoparticle dispersion 9, a conductive substrate was produced by flash light firing in the same manner as in (4) of Example 1.
(実施例10)
実施例1の(2)において、銅ナノ粒子として、実施例9の(1)と同様にして得られたデカン酸と3-エトキシプロピルアミンで被覆された銅ナノ粒子を用い、ソルスパース41000の代わりに、ソルスパース71000(日本ルーブリゾール製、酸価0mgKOH/g、アミン価77.4mgKOH/g、重量平均分子量4700、90%熱重量減少温度が405.9℃)を用いた以外は実施例1の(2)と同様にして、銅ナノ粒子分散体10を得た。
得られた銅ナノ粒子分散体10を用いて、実施例1の(3)と同様にして、プラズマ焼成により導電性基板を製造した。
得られた銅ナノ粒子分散体10を用いて、実施例1の(4)と同様にして、フラッシュ光焼成により導電性基板を製造した。 (Example 10)
In Example 1 (2), copper nanoparticles coated with decanoic acid and 3-ethoxypropylamine obtained in the same manner as in Example 9 (1) were used as copper nanoparticles, and instead of Solsperse 41000, In addition, Solsperse 71000 (manufactured by Nippon Lubrizol, acid value 0 mgKOH / g, amine value 77.4 mgKOH / g, weight average molecular weight 4700, 90% thermogravimetric decrease temperature is 405.9 ° C.) is used. In the same manner as (2), a copper nanoparticle dispersion 10 was obtained.
Using the obtained copper nanoparticle dispersion 10, a conductive substrate was produced by plasma firing in the same manner as (3) of Example 1.
Using the obtained copper nanoparticle dispersion 10, a conductive substrate was produced by flash light baking in the same manner as in (4) of Example 1.
実施例1の(2)において、銅ナノ粒子として、実施例9の(1)と同様にして得られたデカン酸と3-エトキシプロピルアミンで被覆された銅ナノ粒子を用い、ソルスパース41000の代わりに、ソルスパース71000(日本ルーブリゾール製、酸価0mgKOH/g、アミン価77.4mgKOH/g、重量平均分子量4700、90%熱重量減少温度が405.9℃)を用いた以外は実施例1の(2)と同様にして、銅ナノ粒子分散体10を得た。
得られた銅ナノ粒子分散体10を用いて、実施例1の(3)と同様にして、プラズマ焼成により導電性基板を製造した。
得られた銅ナノ粒子分散体10を用いて、実施例1の(4)と同様にして、フラッシュ光焼成により導電性基板を製造した。 (Example 10)
In Example 1 (2), copper nanoparticles coated with decanoic acid and 3-ethoxypropylamine obtained in the same manner as in Example 9 (1) were used as copper nanoparticles, and instead of Solsperse 41000, In addition, Solsperse 71000 (manufactured by Nippon Lubrizol, acid value 0 mgKOH / g, amine value 77.4 mgKOH / g, weight average molecular weight 4700, 90% thermogravimetric decrease temperature is 405.9 ° C.) is used. In the same manner as (2), a copper nanoparticle dispersion 10 was obtained.
Using the obtained copper nanoparticle dispersion 10, a conductive substrate was produced by plasma firing in the same manner as (3) of Example 1.
Using the obtained copper nanoparticle dispersion 10, a conductive substrate was produced by flash light baking in the same manner as in (4) of Example 1.
(実施例11)
実施例1の(2)において、銅ナノ粒子として、実施例9の(1)と同様にして得られたデカン酸と3-エトキシプロピルアミンで被覆された銅ナノ粒子を用い、ソルスパース41000の代わりに、ディスパーbyk-102(ビックケミー・ジャパン製、酸価101mgKOH/g、アミン価0mgKOH/g、重量平均分子量1400、90%熱重量減少温度が327.5℃)を用いた以外は実施例1の(2)と同様にして、銅ナノ粒子分散体11を得た。
得られた銅ナノ粒子分散体11を用いて、実施例1の(3)と同様にして、プラズマ焼成により導電性基板を製造した。
得られた銅ナノ粒子分散体11を用いて、実施例1の(4)と同様にして、フラッシュ光焼成により導電性基板を製造した。 (Example 11)
In Example 1 (2), copper nanoparticles coated with decanoic acid and 3-ethoxypropylamine obtained in the same manner as in Example 9 (1) were used as copper nanoparticles, and instead of Solsperse 41000, In addition, Disper byk-102 (manufactured by Big Chemie Japan,acid value 101 mgKOH / g, amine value 0 mgKOH / g, weight average molecular weight 1400, 90% thermogravimetric decrease temperature is 327.5 ° C.) is used. In the same manner as (2), a copper nanoparticle dispersion 11 was obtained.
Using the obtained copper nanoparticle dispersion 11, a conductive substrate was produced by plasma firing in the same manner as in (1) of Example 1.
Using the obtained copper nanoparticle dispersion 11, a conductive substrate was produced by flash light baking in the same manner as in Example 1 (4).
実施例1の(2)において、銅ナノ粒子として、実施例9の(1)と同様にして得られたデカン酸と3-エトキシプロピルアミンで被覆された銅ナノ粒子を用い、ソルスパース41000の代わりに、ディスパーbyk-102(ビックケミー・ジャパン製、酸価101mgKOH/g、アミン価0mgKOH/g、重量平均分子量1400、90%熱重量減少温度が327.5℃)を用いた以外は実施例1の(2)と同様にして、銅ナノ粒子分散体11を得た。
得られた銅ナノ粒子分散体11を用いて、実施例1の(3)と同様にして、プラズマ焼成により導電性基板を製造した。
得られた銅ナノ粒子分散体11を用いて、実施例1の(4)と同様にして、フラッシュ光焼成により導電性基板を製造した。 (Example 11)
In Example 1 (2), copper nanoparticles coated with decanoic acid and 3-ethoxypropylamine obtained in the same manner as in Example 9 (1) were used as copper nanoparticles, and instead of Solsperse 41000, In addition, Disper byk-102 (manufactured by Big Chemie Japan,
Using the obtained copper nanoparticle dispersion 11, a conductive substrate was produced by plasma firing in the same manner as in (1) of Example 1.
Using the obtained copper nanoparticle dispersion 11, a conductive substrate was produced by flash light baking in the same manner as in Example 1 (4).
(実施例12)
実施例1の(2)において、銅ナノ粒子として、実施例9の(1)と同様にして得られたデカン酸と3-エトキシプロピルアミンで被覆された銅ナノ粒子を用い、ソルスパース41000の代わりに、ディスパーbyk-106(ビックケミー・ジャパン製、酸価132mgKOH/g、アミン価74mgKOH/g、重量平均分子量1400、90%熱重量減少温度が405.3℃)を用いた以外は実施例1の(2)と同様にして、銅ナノ粒子分散体12を得た。
得られた銅ナノ粒子分散体12を用いて、実施例1の(3)と同様にして、プラズマ焼成により導電性基板を製造した。
得られた銅ナノ粒子分散体12を用いて、実施例1の(4)と同様にして、フラッシュ光焼成により導電性基板を製造した。 Example 12
In Example 1 (2), copper nanoparticles coated with decanoic acid and 3-ethoxypropylamine obtained in the same manner as in Example 9 (1) were used as copper nanoparticles, and instead of Solsperse 41000, In addition, Disper byk-106 (manufactured by Big Chemie Japan, acid value 132 mgKOH / g, amine value 74 mgKOH / g, weight average molecular weight 1400, 90% thermogravimetric decrease temperature is 405.3 ° C.) is used. In the same manner as (2), a copper nanoparticle dispersion 12 was obtained.
Using the obtained copper nanoparticle dispersion 12, a conductive substrate was produced by plasma firing in the same manner as in (1) of Example 1.
Using the obtained copper nanoparticle dispersion 12, a conductive substrate was produced by flash light baking in the same manner as in (4) of Example 1.
実施例1の(2)において、銅ナノ粒子として、実施例9の(1)と同様にして得られたデカン酸と3-エトキシプロピルアミンで被覆された銅ナノ粒子を用い、ソルスパース41000の代わりに、ディスパーbyk-106(ビックケミー・ジャパン製、酸価132mgKOH/g、アミン価74mgKOH/g、重量平均分子量1400、90%熱重量減少温度が405.3℃)を用いた以外は実施例1の(2)と同様にして、銅ナノ粒子分散体12を得た。
得られた銅ナノ粒子分散体12を用いて、実施例1の(3)と同様にして、プラズマ焼成により導電性基板を製造した。
得られた銅ナノ粒子分散体12を用いて、実施例1の(4)と同様にして、フラッシュ光焼成により導電性基板を製造した。 Example 12
In Example 1 (2), copper nanoparticles coated with decanoic acid and 3-ethoxypropylamine obtained in the same manner as in Example 9 (1) were used as copper nanoparticles, and instead of Solsperse 41000, In addition, Disper byk-106 (manufactured by Big Chemie Japan, acid value 132 mgKOH / g, amine value 74 mgKOH / g, weight average molecular weight 1400, 90% thermogravimetric decrease temperature is 405.3 ° C.) is used. In the same manner as (2), a copper nanoparticle dispersion 12 was obtained.
Using the obtained copper nanoparticle dispersion 12, a conductive substrate was produced by plasma firing in the same manner as in (1) of Example 1.
Using the obtained copper nanoparticle dispersion 12, a conductive substrate was produced by flash light baking in the same manner as in (4) of Example 1.
(実施例13)
実施例1の(2)において、銅ナノ粒子として、実施例9の(1)と同様にして得られたデカン酸と3-エトキシプロピルアミンで被覆された銅ナノ粒子を用い、ソルスパース41000の代わりに、ディスパーbyk-111(ビックケミー・ジャパン製、酸価129mgKOH/g、アミン価0mgKOH/g、重量平均分子量1700、90%熱重量減少温度が320.4℃)を用いた以外は実施例1の(2)と同様にして、銅ナノ粒子分散体13を得た。
得られた銅ナノ粒子分散体13を用いて、実施例1の(3)と同様にして、プラズマ焼成により導電性基板を製造した。
得られた銅ナノ粒子分散体13を用いて、実施例1の(4)と同様にして、フラッシュ光焼成により導電性基板を製造した。 (Example 13)
In Example 1 (2), copper nanoparticles coated with decanoic acid and 3-ethoxypropylamine obtained in the same manner as in Example 9 (1) were used as copper nanoparticles, and instead of Solsperse 41000, In addition, Disper byk-111 (manufactured by Big Chemie Japan, acid value 129 mgKOH / g, amine value 0 mgKOH / g, weight average molecular weight 1700, 90% thermogravimetric decrease temperature is 320.4 ° C.) In the same manner as (2), a copper nanoparticle dispersion 13 was obtained.
Using the obtained copper nanoparticle dispersion 13, a conductive substrate was produced by plasma firing in the same manner as (3) of Example 1.
Using the obtained copper nanoparticle dispersion 13, a conductive substrate was produced by flash light baking in the same manner as in Example 1 (4).
実施例1の(2)において、銅ナノ粒子として、実施例9の(1)と同様にして得られたデカン酸と3-エトキシプロピルアミンで被覆された銅ナノ粒子を用い、ソルスパース41000の代わりに、ディスパーbyk-111(ビックケミー・ジャパン製、酸価129mgKOH/g、アミン価0mgKOH/g、重量平均分子量1700、90%熱重量減少温度が320.4℃)を用いた以外は実施例1の(2)と同様にして、銅ナノ粒子分散体13を得た。
得られた銅ナノ粒子分散体13を用いて、実施例1の(3)と同様にして、プラズマ焼成により導電性基板を製造した。
得られた銅ナノ粒子分散体13を用いて、実施例1の(4)と同様にして、フラッシュ光焼成により導電性基板を製造した。 (Example 13)
In Example 1 (2), copper nanoparticles coated with decanoic acid and 3-ethoxypropylamine obtained in the same manner as in Example 9 (1) were used as copper nanoparticles, and instead of Solsperse 41000, In addition, Disper byk-111 (manufactured by Big Chemie Japan, acid value 129 mgKOH / g, amine value 0 mgKOH / g, weight average molecular weight 1700, 90% thermogravimetric decrease temperature is 320.4 ° C.) In the same manner as (2), a copper nanoparticle dispersion 13 was obtained.
Using the obtained copper nanoparticle dispersion 13, a conductive substrate was produced by plasma firing in the same manner as (3) of Example 1.
Using the obtained copper nanoparticle dispersion 13, a conductive substrate was produced by flash light baking in the same manner as in Example 1 (4).
(実施例14)
(1)銅ナノ粒子の合成
実施例1において、ヘキシルアミン 40.5g(0.4mol)の代わりに、ジメチルアミノプロピルアミン 40.9g(0.4mol、広栄化学工業製、沸点135℃)を用いた以外は、実施例1と同様にして、ノナン酸とジメチルアミノプロピルアミンで被覆された銅ナノ粒子を得た。 (Example 14)
(1) Synthesis of copper nanoparticles In Example 1, instead of hexylamine 40.5 g (0.4 mol), 40.9 g of dimethylaminopropylamine (0.4 mol, manufactured by Guangei Chemical Industry Co., Ltd., boiling point 135 ° C.) was used. Except for the above, copper nanoparticles coated with nonanoic acid and dimethylaminopropylamine were obtained in the same manner as in Example 1.
(1)銅ナノ粒子の合成
実施例1において、ヘキシルアミン 40.5g(0.4mol)の代わりに、ジメチルアミノプロピルアミン 40.9g(0.4mol、広栄化学工業製、沸点135℃)を用いた以外は、実施例1と同様にして、ノナン酸とジメチルアミノプロピルアミンで被覆された銅ナノ粒子を得た。 (Example 14)
(1) Synthesis of copper nanoparticles In Example 1, instead of hexylamine 40.5 g (0.4 mol), 40.9 g of dimethylaminopropylamine (0.4 mol, manufactured by Guangei Chemical Industry Co., Ltd., boiling point 135 ° C.) was used. Except for the above, copper nanoparticles coated with nonanoic acid and dimethylaminopropylamine were obtained in the same manner as in Example 1.
得られた銅ナノ粒子を、実施例1と同様にして透過型電子顕微鏡(TEM)で観察したところ、平均一次粒径は64nmであった。
またX線回折装置にて得られた銅ナノ粒子の結晶構造を測定したところ、銅ナノ粒子の主構造はCu(Cubic)で一部Cu2O(Cubic)構造が見られ、Cu(111)に対するCu2O(111)のピーク強度は約1%であった。 When the obtained copper nanoparticles were observed with a transmission electron microscope (TEM) in the same manner as in Example 1, the average primary particle size was 64 nm.
Moreover, when the crystal structure of the copper nanoparticle obtained by the X-ray diffractometer was measured, the main structure of the copper nanoparticle was Cu (Cubic), and a part of Cu 2 O (Cubic) structure was observed, and Cu (111) The peak intensity of Cu 2 O (111) relative to was about 1%.
またX線回折装置にて得られた銅ナノ粒子の結晶構造を測定したところ、銅ナノ粒子の主構造はCu(Cubic)で一部Cu2O(Cubic)構造が見られ、Cu(111)に対するCu2O(111)のピーク強度は約1%であった。 When the obtained copper nanoparticles were observed with a transmission electron microscope (TEM) in the same manner as in Example 1, the average primary particle size was 64 nm.
Moreover, when the crystal structure of the copper nanoparticle obtained by the X-ray diffractometer was measured, the main structure of the copper nanoparticle was Cu (Cubic), and a part of Cu 2 O (Cubic) structure was observed, and Cu (111) The peak intensity of Cu 2 O (111) relative to was about 1%.
(2)銅ナノ粒子分散体の調製
上記で得られたノナン酸とジメチルアミノプロピルアミンで被覆された銅ナノ粒子を用い、ソルスパース41000の代わりに、ソルスパース71000(日本ルーブリゾール製、酸価0mgKOH/g、アミン価77.4mgKOH/g、重量平均分子量 4700、90%熱重量減少温度が405.9℃)を用いた以外は、実施例1の(2)と同様にして、銅ナノ粒子分散体14を得た。 (2) Preparation of Copper Nanoparticle Dispersion Using the copper nanoparticle coated with nonanoic acid and dimethylaminopropylamine obtained above, Solsperse 71000 (manufactured by Nippon Lubrizol, acid value 0 mgKOH / g, amine value 77.4 mgKOH / g, weight average molecular weight 4700, 90% thermogravimetric reduction temperature is 405.9 ° C.) 14 was obtained.
上記で得られたノナン酸とジメチルアミノプロピルアミンで被覆された銅ナノ粒子を用い、ソルスパース41000の代わりに、ソルスパース71000(日本ルーブリゾール製、酸価0mgKOH/g、アミン価77.4mgKOH/g、重量平均分子量 4700、90%熱重量減少温度が405.9℃)を用いた以外は、実施例1の(2)と同様にして、銅ナノ粒子分散体14を得た。 (2) Preparation of Copper Nanoparticle Dispersion Using the copper nanoparticle coated with nonanoic acid and dimethylaminopropylamine obtained above, Solsperse 71000 (manufactured by Nippon Lubrizol, acid value 0 mgKOH / g, amine value 77.4 mgKOH / g, weight average molecular weight 4700, 90% thermogravimetric reduction temperature is 405.9 ° C.) 14 was obtained.
(3)プラズマ焼成による導電性基板の製造
得られた銅ナノ粒子分散体14を用いて、実施例1の(3)と同様にして、プラズマ焼成により導電性基板を製造した。
(4)フラッシュ光焼成による導電性基板の製造
得られた銅ナノ粒子分散体14を用いて、実施例1の(4)と同様にして、フラッシュ光焼成により導電性基板を製造した。 (3) Production of conductive substrate by plasma firing Using the obtained copper nanoparticle dispersion 14, a conductive substrate was produced by plasma firing in the same manner as (3) of Example 1.
(4) Production of conductive substrate by flash light firing Using the obtained copper nanoparticle dispersion 14, a conductive substrate was produced by flash light firing in the same manner as in (4) of Example 1.
得られた銅ナノ粒子分散体14を用いて、実施例1の(3)と同様にして、プラズマ焼成により導電性基板を製造した。
(4)フラッシュ光焼成による導電性基板の製造
得られた銅ナノ粒子分散体14を用いて、実施例1の(4)と同様にして、フラッシュ光焼成により導電性基板を製造した。 (3) Production of conductive substrate by plasma firing Using the obtained copper nanoparticle dispersion 14, a conductive substrate was produced by plasma firing in the same manner as (3) of Example 1.
(4) Production of conductive substrate by flash light firing Using the obtained copper nanoparticle dispersion 14, a conductive substrate was produced by flash light firing in the same manner as in (4) of Example 1.
(実施例15)
(1)銅ナノ粒子の合成
実施例1において、ノナン酸 31.5g(0.2mol)の代わりに、オレイン酸 28.2g(0.1mol、関東化学製、沸点360℃)を用い、ヘキシルアミン 40.5g(0.4mol)の代わりに、オクチルアミン51.7g(0.4mol、東京化成工業製、沸点176℃)を用いた以外は、実施例1と同様にして、オレイン酸とオクチルアミンで被覆された銅ナノ粒子を得た。 (Example 15)
(1) Synthesis of copper nanoparticles In Example 1, instead of 31.5 g (0.2 mol) of nonanoic acid, 28.2 g of oleic acid (0.1 mol, manufactured by Kanto Chemical Co., Ltd., boiling point: 360 ° C.) was used, and hexylamine was used. Instead of 40.5 g (0.4 mol), oleic acid and octylamine were obtained in the same manner as in Example 1 except that 51.7 g (0.4 mol, manufactured by Tokyo Chemical Industry Co., Ltd., boiling point: 176 ° C.) was used. Copper nanoparticles coated with were obtained.
(1)銅ナノ粒子の合成
実施例1において、ノナン酸 31.5g(0.2mol)の代わりに、オレイン酸 28.2g(0.1mol、関東化学製、沸点360℃)を用い、ヘキシルアミン 40.5g(0.4mol)の代わりに、オクチルアミン51.7g(0.4mol、東京化成工業製、沸点176℃)を用いた以外は、実施例1と同様にして、オレイン酸とオクチルアミンで被覆された銅ナノ粒子を得た。 (Example 15)
(1) Synthesis of copper nanoparticles In Example 1, instead of 31.5 g (0.2 mol) of nonanoic acid, 28.2 g of oleic acid (0.1 mol, manufactured by Kanto Chemical Co., Ltd., boiling point: 360 ° C.) was used, and hexylamine was used. Instead of 40.5 g (0.4 mol), oleic acid and octylamine were obtained in the same manner as in Example 1 except that 51.7 g (0.4 mol, manufactured by Tokyo Chemical Industry Co., Ltd., boiling point: 176 ° C.) was used. Copper nanoparticles coated with were obtained.
得られた銅ナノ粒子を、実施例1と同様にして透過型電子顕微鏡(TEM)で観察したところ、平均一次粒径は28nmであった。
またX線回折装置にて得られた銅ナノ粒子の結晶構造を測定したところ、銅ナノ粒子の主構造はCu(Cubic)で一部Cu2O(Cubic)構造が見られ、Cu(111)に対するCu2O(111)のピーク強度は約12%であった。 When the obtained copper nanoparticles were observed with a transmission electron microscope (TEM) in the same manner as in Example 1, the average primary particle size was 28 nm.
Moreover, when the crystal structure of the copper nanoparticle obtained by the X-ray diffractometer was measured, the main structure of the copper nanoparticle was Cu (Cubic), and a part of Cu 2 O (Cubic) structure was observed, and Cu (111) The peak intensity of Cu 2 O (111) relative to was about 12%.
またX線回折装置にて得られた銅ナノ粒子の結晶構造を測定したところ、銅ナノ粒子の主構造はCu(Cubic)で一部Cu2O(Cubic)構造が見られ、Cu(111)に対するCu2O(111)のピーク強度は約12%であった。 When the obtained copper nanoparticles were observed with a transmission electron microscope (TEM) in the same manner as in Example 1, the average primary particle size was 28 nm.
Moreover, when the crystal structure of the copper nanoparticle obtained by the X-ray diffractometer was measured, the main structure of the copper nanoparticle was Cu (Cubic), and a part of Cu 2 O (Cubic) structure was observed, and Cu (111) The peak intensity of Cu 2 O (111) relative to was about 12%.
(2)銅ナノ粒子分散体の調製
上記で得られたオレイン酸とオクチルアミンで被覆された銅ナノ粒子を用い、ソルスパース41000の代わりに、ソルスパース71000(日本ルーブリゾール製、酸価0mgKOH/g、アミン価77.4mgKOH/g、重量平均分子量4700、90%熱重量減少温度が405.9℃)を用いた以外は、実施例1の(2)と同様にして、銅ナノ粒子分散体15を得た。 (2) Preparation of Copper Nanoparticle Dispersion Using the copper nanoparticles coated with oleic acid and octylamine obtained above, instead of Solsperse 41000, Solsperse 71000 (manufactured by Nippon Lubrizol, acid value 0 mgKOH / g, The copper nanoparticle dispersion 15 was prepared in the same manner as (2) of Example 1 except that an amine value of 77.4 mgKOH / g, a weight average molecular weight of 4700, and a 90% thermogravimetric decrease temperature of 405.9 ° C. were used. Obtained.
上記で得られたオレイン酸とオクチルアミンで被覆された銅ナノ粒子を用い、ソルスパース41000の代わりに、ソルスパース71000(日本ルーブリゾール製、酸価0mgKOH/g、アミン価77.4mgKOH/g、重量平均分子量4700、90%熱重量減少温度が405.9℃)を用いた以外は、実施例1の(2)と同様にして、銅ナノ粒子分散体15を得た。 (2) Preparation of Copper Nanoparticle Dispersion Using the copper nanoparticles coated with oleic acid and octylamine obtained above, instead of Solsperse 41000, Solsperse 71000 (manufactured by Nippon Lubrizol, acid value 0 mgKOH / g, The copper nanoparticle dispersion 15 was prepared in the same manner as (2) of Example 1 except that an amine value of 77.4 mgKOH / g, a weight average molecular weight of 4700, and a 90% thermogravimetric decrease temperature of 405.9 ° C. were used. Obtained.
(3)プラズマ焼成による導電性基板の製造
得られた銅ナノ粒子分散体15を用いて、実施例1の(3)と同様にして、プラズマ焼成により導電性基板を製造した。
(4)フラッシュ光焼成による導電性基板の製造
得られた銅ナノ粒子分散体15を用いて、実施例1の(4)と同様にして、フラッシュ光焼成により導電性基板を製造した。 (3) Production of conductive substrate by plasma firing Using the obtained copper nanoparticle dispersion 15, a conductive substrate was produced by plasma firing in the same manner as (3) of Example 1.
(4) Production of conductive substrate by flash light firing Using the obtained copper nanoparticle dispersion 15, a conductive substrate was produced by flash light firing in the same manner as in (4) of Example 1.
得られた銅ナノ粒子分散体15を用いて、実施例1の(3)と同様にして、プラズマ焼成により導電性基板を製造した。
(4)フラッシュ光焼成による導電性基板の製造
得られた銅ナノ粒子分散体15を用いて、実施例1の(4)と同様にして、フラッシュ光焼成により導電性基板を製造した。 (3) Production of conductive substrate by plasma firing Using the obtained copper nanoparticle dispersion 15, a conductive substrate was produced by plasma firing in the same manner as (3) of Example 1.
(4) Production of conductive substrate by flash light firing Using the obtained copper nanoparticle dispersion 15, a conductive substrate was produced by flash light firing in the same manner as in (4) of Example 1.
(比較例1)
実施例1の(1)と同様にして得られたノナン酸とヘキシルアミンで被覆された銅ナノ粒子 3.0質量部、PGME 4.5質量部を混合し、ペイントシェーカー(浅田鉄工製)にて予備分散として2mmジルコニアビーズで1時間、さらに本分散として0.1mmジルコニアビーズで2時間分散した。しかしながら、銅ナノ粒子が凝集して分散されず、銅ナノ粒子分散体を得ることはできなかった。 (Comparative Example 1)
The nonanoic acid obtained in the same manner as in Example 1 (1) and copper nanoparticles coated with hexylamine (3.0 parts by mass) and PGME (4.5 parts by mass) were mixed, and a paint shaker (manufactured by Asada Tekko) was mixed. Then, 2 mm zirconia beads were preliminarily dispersed for 1 hour, and 0.1 mm zirconia beads were further dispersed for 2 hours as the main dispersion. However, the copper nanoparticles were not aggregated and dispersed, and a copper nanoparticle dispersion could not be obtained.
実施例1の(1)と同様にして得られたノナン酸とヘキシルアミンで被覆された銅ナノ粒子 3.0質量部、PGME 4.5質量部を混合し、ペイントシェーカー(浅田鉄工製)にて予備分散として2mmジルコニアビーズで1時間、さらに本分散として0.1mmジルコニアビーズで2時間分散した。しかしながら、銅ナノ粒子が凝集して分散されず、銅ナノ粒子分散体を得ることはできなかった。 (Comparative Example 1)
The nonanoic acid obtained in the same manner as in Example 1 (1) and copper nanoparticles coated with hexylamine (3.0 parts by mass) and PGME (4.5 parts by mass) were mixed, and a paint shaker (manufactured by Asada Tekko) was mixed. Then, 2 mm zirconia beads were preliminarily dispersed for 1 hour, and 0.1 mm zirconia beads were further dispersed for 2 hours as the main dispersion. However, the copper nanoparticles were not aggregated and dispersed, and a copper nanoparticle dispersion could not be obtained.
(比較例2)
実施例1の(1)と同様にして、ノナン酸とヘキシルアミンで被覆された銅ナノ粒子を得た。
当該銅ナノ粒子を、実施例1の(2)において、ソルスパース41000の代わりに、ソルスパース16000(日本ルーブリゾール製、酸価20mgKOH/g、アミン価0mgKOH/g、重量平均分子量4200、90%熱重量減少温度が405.5℃)を用いた以外は実施例1の(2)と同様にして分散した。しかし、銅ナノ粒子が凝集して分散されず、銅ナノ粒子分散体を得ることはできなかった。 (Comparative Example 2)
In the same manner as in Example 1 (1), copper nanoparticles coated with nonanoic acid and hexylamine were obtained.
The copper nanoparticles in Example 2 (2), instead of Solsperse 41000, Solsperse 16000 (manufactured by Nippon Lubrizol, acid value 20 mgKOH / g, amine value 0 mgKOH / g, weight average molecular weight 4200, 90% thermogravimetric weight) Dispersion was carried out in the same manner as in (2) of Example 1 except that the reduction temperature was 405.5 ° C. However, the copper nanoparticles were not aggregated and dispersed, and a copper nanoparticle dispersion could not be obtained.
実施例1の(1)と同様にして、ノナン酸とヘキシルアミンで被覆された銅ナノ粒子を得た。
当該銅ナノ粒子を、実施例1の(2)において、ソルスパース41000の代わりに、ソルスパース16000(日本ルーブリゾール製、酸価20mgKOH/g、アミン価0mgKOH/g、重量平均分子量4200、90%熱重量減少温度が405.5℃)を用いた以外は実施例1の(2)と同様にして分散した。しかし、銅ナノ粒子が凝集して分散されず、銅ナノ粒子分散体を得ることはできなかった。 (Comparative Example 2)
In the same manner as in Example 1 (1), copper nanoparticles coated with nonanoic acid and hexylamine were obtained.
The copper nanoparticles in Example 2 (2), instead of Solsperse 41000, Solsperse 16000 (manufactured by Nippon Lubrizol, acid value 20 mgKOH / g, amine value 0 mgKOH / g, weight average molecular weight 4200, 90% thermogravimetric weight) Dispersion was carried out in the same manner as in (2) of Example 1 except that the reduction temperature was 405.5 ° C. However, the copper nanoparticles were not aggregated and dispersed, and a copper nanoparticle dispersion could not be obtained.
(比較例3)
実施例1の(1)と同様にして、ノナン酸とヘキシルアミンで被覆された銅ナノ粒子を得た。
当該銅ナノ粒子を、実施例1の(2)において、ソルスパース41000の代わりに、ソルスパース76500(日本ルーブリゾール製、酸価0mgKOH/g、アミン価15.2mgKOH/g、90%熱重量減少温度が600℃超過(600℃でも32%残存))を用いた以外は実施例1の(2)と同様にして分散した。しかし、銅ナノ粒子が凝集して分散されず、銅ナノ粒子分散体を得ることはできなかった。 (Comparative Example 3)
In the same manner as in Example 1 (1), copper nanoparticles coated with nonanoic acid and hexylamine were obtained.
In Example 1 (2), instead of Solsperse 41000, the copper nanoparticles were Solsperse 76500 (manufactured by Nippon Lubrizol, acid value 0 mgKOH / g, amine value 15.2 mgKOH / g, 90% thermogravimetric decrease temperature) Dispersion was carried out in the same manner as (2) of Example 1 except that over 600 ° C. (32% remaining even at 600 ° C.) was used. However, the copper nanoparticles were not aggregated and dispersed, and a copper nanoparticle dispersion could not be obtained.
実施例1の(1)と同様にして、ノナン酸とヘキシルアミンで被覆された銅ナノ粒子を得た。
当該銅ナノ粒子を、実施例1の(2)において、ソルスパース41000の代わりに、ソルスパース76500(日本ルーブリゾール製、酸価0mgKOH/g、アミン価15.2mgKOH/g、90%熱重量減少温度が600℃超過(600℃でも32%残存))を用いた以外は実施例1の(2)と同様にして分散した。しかし、銅ナノ粒子が凝集して分散されず、銅ナノ粒子分散体を得ることはできなかった。 (Comparative Example 3)
In the same manner as in Example 1 (1), copper nanoparticles coated with nonanoic acid and hexylamine were obtained.
In Example 1 (2), instead of Solsperse 41000, the copper nanoparticles were Solsperse 76500 (manufactured by Nippon Lubrizol, acid value 0 mgKOH / g, amine value 15.2 mgKOH / g, 90% thermogravimetric decrease temperature) Dispersion was carried out in the same manner as (2) of Example 1 except that over 600 ° C. (32% remaining even at 600 ° C.) was used. However, the copper nanoparticles were not aggregated and dispersed, and a copper nanoparticle dispersion could not be obtained.
(比較例4)
実施例9の(1)と同様にして、デカン酸と3-エトキシプロピルアミンで被覆された銅ナノ粒子を得た。
当該銅ナノ粒子を、実施例1の(2)において、ソルスパース41000の代わりに、ディスパーbyk-130(ビックケミー・ジャパン製、酸価3mgKOH/g未満、アミン価190mgKOH/g、90%熱重量減少温度が474.8℃)を用いた以外は実施例1の(2)と同様にして分散した。しかし、銅ナノ粒子が凝集して分散されず、銅ナノ粒子分散体を得ることはできなかった。 (Comparative Example 4)
In the same manner as in Example 9 (1), copper nanoparticles coated with decanoic acid and 3-ethoxypropylamine were obtained.
In Example 1 (2), the copper nanoparticles were replaced with Dispers byk-130 (manufactured by Big Chemie Japan, acid value of less than 3 mgKOH / g, amine value of 190 mgKOH / g, 90% thermogravimetric reduction temperature). Was 474.8 ° C.) in the same manner as in Example 1 (2). However, the copper nanoparticles were not aggregated and dispersed, and a copper nanoparticle dispersion could not be obtained.
実施例9の(1)と同様にして、デカン酸と3-エトキシプロピルアミンで被覆された銅ナノ粒子を得た。
当該銅ナノ粒子を、実施例1の(2)において、ソルスパース41000の代わりに、ディスパーbyk-130(ビックケミー・ジャパン製、酸価3mgKOH/g未満、アミン価190mgKOH/g、90%熱重量減少温度が474.8℃)を用いた以外は実施例1の(2)と同様にして分散した。しかし、銅ナノ粒子が凝集して分散されず、銅ナノ粒子分散体を得ることはできなかった。 (Comparative Example 4)
In the same manner as in Example 9 (1), copper nanoparticles coated with decanoic acid and 3-ethoxypropylamine were obtained.
In Example 1 (2), the copper nanoparticles were replaced with Dispers byk-130 (manufactured by Big Chemie Japan, acid value of less than 3 mgKOH / g, amine value of 190 mgKOH / g, 90% thermogravimetric reduction temperature). Was 474.8 ° C.) in the same manner as in Example 1 (2). However, the copper nanoparticles were not aggregated and dispersed, and a copper nanoparticle dispersion could not be obtained.
(比較例5)
実施例15の(1)と同様にして、オレイン酸とオクチルアミンで被覆された銅ナノ粒子を得た。
当該銅ナノ粒子を、実施例1の(2)において、ソルスパース41000の代わりに、ディスパーbyk-130(ビックケミー・ジャパン製、酸価3mgKOH/g未満、アミン価190mgKOH/g、90%熱重量減少温度が474.8℃)を用いた以外は実施例1の(2)と同様にして分散して、比較銅ナノ粒子分散体5を得た。
得られた比較銅ナノ粒子分散体5を用いて、実施例1の(3)と同様にして、プラズマ焼成により導電性基板を製造した。
得られた比較銅ナノ粒子分散体5を用いて、実施例1の(4)と同様にして、フラッシュ光焼成により導電性基板を製造した。 (Comparative Example 5)
In the same manner as in Example 15 (1), copper nanoparticles coated with oleic acid and octylamine were obtained.
In Example 1 (2), the copper nanoparticles were replaced with Dispers byk-130 (manufactured by Big Chemie Japan, acid value of less than 3 mgKOH / g, amine value of 190 mgKOH / g, 90% thermogravimetric reduction temperature). Was used in the same manner as in (2) of Example 1 except that 474.8 ° C. was used to obtain Comparative Copper Nanoparticle Dispersion 5.
Using the obtained comparative copper nanoparticle dispersion 5, a conductive substrate was produced by plasma firing in the same manner as (3) of Example 1.
Using the obtained comparative copper nanoparticle dispersion 5, a conductive substrate was produced by flash light baking in the same manner as in (4) of Example 1.
実施例15の(1)と同様にして、オレイン酸とオクチルアミンで被覆された銅ナノ粒子を得た。
当該銅ナノ粒子を、実施例1の(2)において、ソルスパース41000の代わりに、ディスパーbyk-130(ビックケミー・ジャパン製、酸価3mgKOH/g未満、アミン価190mgKOH/g、90%熱重量減少温度が474.8℃)を用いた以外は実施例1の(2)と同様にして分散して、比較銅ナノ粒子分散体5を得た。
得られた比較銅ナノ粒子分散体5を用いて、実施例1の(3)と同様にして、プラズマ焼成により導電性基板を製造した。
得られた比較銅ナノ粒子分散体5を用いて、実施例1の(4)と同様にして、フラッシュ光焼成により導電性基板を製造した。 (Comparative Example 5)
In the same manner as in Example 15 (1), copper nanoparticles coated with oleic acid and octylamine were obtained.
In Example 1 (2), the copper nanoparticles were replaced with Dispers byk-130 (manufactured by Big Chemie Japan, acid value of less than 3 mgKOH / g, amine value of 190 mgKOH / g, 90% thermogravimetric reduction temperature). Was used in the same manner as in (2) of Example 1 except that 474.8 ° C. was used to obtain Comparative Copper Nanoparticle Dispersion 5.
Using the obtained comparative copper nanoparticle dispersion 5, a conductive substrate was produced by plasma firing in the same manner as (3) of Example 1.
Using the obtained comparative copper nanoparticle dispersion 5, a conductive substrate was produced by flash light baking in the same manner as in (4) of Example 1.
(比較例6)
(1)銅ナノ粒子の合成
実施例1において、ノナン酸 31.5g(0.2mol)の代わりに、オレイン酸
28.2g(0.1mol、関東化学製、沸点360℃)を用いた以外は、実施例1と同様にして、オレイン酸とヘキシルアミンで被覆された銅ナノ粒子を得た。 (Comparative Example 6)
(1) Synthesis of copper nanoparticles In Example 1, instead of 31.5 g (0.2 mol) of nonanoic acid, 28.2 g of oleic acid (0.1 mol, manufactured by Kanto Chemical Co., Ltd., boiling point: 360 ° C.) was used. In the same manner as in Example 1, copper nanoparticles coated with oleic acid and hexylamine were obtained.
(1)銅ナノ粒子の合成
実施例1において、ノナン酸 31.5g(0.2mol)の代わりに、オレイン酸
28.2g(0.1mol、関東化学製、沸点360℃)を用いた以外は、実施例1と同様にして、オレイン酸とヘキシルアミンで被覆された銅ナノ粒子を得た。 (Comparative Example 6)
(1) Synthesis of copper nanoparticles In Example 1, instead of 31.5 g (0.2 mol) of nonanoic acid, 28.2 g of oleic acid (0.1 mol, manufactured by Kanto Chemical Co., Ltd., boiling point: 360 ° C.) was used. In the same manner as in Example 1, copper nanoparticles coated with oleic acid and hexylamine were obtained.
得られた銅ナノ粒子を、実施例1と同様にして透過型電子顕微鏡(TEM)で観察したところ、平均一次粒径は24nmであった。
またX線回折装置にて得られた銅ナノ粒子の結晶構造を測定したところ、銅ナノ粒子の主構造はCu(Cubic)で一部Cu2O(Cubic)構造が見られ、Cu(111)に対するCu2O(111)のピーク強度は約17%であった。 When the obtained copper nanoparticles were observed with a transmission electron microscope (TEM) in the same manner as in Example 1, the average primary particle size was 24 nm.
Moreover, when the crystal structure of the copper nanoparticle obtained by the X-ray diffractometer was measured, the main structure of the copper nanoparticle was Cu (Cubic), and a part of Cu 2 O (Cubic) structure was observed, and Cu (111) The peak intensity of Cu 2 O (111) relative to was about 17%.
またX線回折装置にて得られた銅ナノ粒子の結晶構造を測定したところ、銅ナノ粒子の主構造はCu(Cubic)で一部Cu2O(Cubic)構造が見られ、Cu(111)に対するCu2O(111)のピーク強度は約17%であった。 When the obtained copper nanoparticles were observed with a transmission electron microscope (TEM) in the same manner as in Example 1, the average primary particle size was 24 nm.
Moreover, when the crystal structure of the copper nanoparticle obtained by the X-ray diffractometer was measured, the main structure of the copper nanoparticle was Cu (Cubic), and a part of Cu 2 O (Cubic) structure was observed, and Cu (111) The peak intensity of Cu 2 O (111) relative to was about 17%.
(2)銅ナノ粒子分散体の調製
上記で得られたオレイン酸とヘキシルアミンで被覆された銅ナノ粒子を用い、ソルスパース41000の代わりに、ソルスパース16000(日本ルーブリゾール製、酸価20mgKOH/g、アミン価0mgKOH/g、重量平均分子量4200、90%熱重量減少温度が405.5℃)を用いた以外は、実施例1の(2)と同様にして、比較銅ナノ粒子分散体6を得た。 (2) Preparation of copper nanoparticle dispersion Using the copper nanoparticles coated with oleic acid and hexylamine obtained above, Solsperse 16000 (manufactured by Nippon Lubrizol, acid value 20 mgKOH / g, instead of Solsperse 41000, Comparative Copper Nanoparticle Dispersion 6 was obtained in the same manner as (2) of Example 1 except that the amine value was 0 mg KOH / g, the weight average molecular weight was 4200, and the 90% thermogravimetric decrease temperature was 405.5 ° C. It was.
上記で得られたオレイン酸とヘキシルアミンで被覆された銅ナノ粒子を用い、ソルスパース41000の代わりに、ソルスパース16000(日本ルーブリゾール製、酸価20mgKOH/g、アミン価0mgKOH/g、重量平均分子量4200、90%熱重量減少温度が405.5℃)を用いた以外は、実施例1の(2)と同様にして、比較銅ナノ粒子分散体6を得た。 (2) Preparation of copper nanoparticle dispersion Using the copper nanoparticles coated with oleic acid and hexylamine obtained above, Solsperse 16000 (manufactured by Nippon Lubrizol, acid value 20 mgKOH / g, instead of Solsperse 41000, Comparative Copper Nanoparticle Dispersion 6 was obtained in the same manner as (2) of Example 1 except that the amine value was 0 mg KOH / g, the weight average molecular weight was 4200, and the 90% thermogravimetric decrease temperature was 405.5 ° C. It was.
(3)プラズマ焼成による導電性基板の製造
得られた比較銅ナノ粒子分散体6を用いて、実施例1の(3)と同様にして、プラズマ焼成により導電性基板を製造した。
(4)フラッシュ光焼成による導電性基板の製造
得られた比較銅ナノ粒子分散体6を用いて、実施例1の(4)と同様にして、フラッシュ光焼成により導電性基板を製造した。 (3) Production of Conductive Substrate by Plasma Firing Using the obtained comparative copper nanoparticle dispersion 6, a conductive substrate was produced by plasma firing in the same manner as (3) of Example 1.
(4) Production of conductive substrate by flash light firing Using the obtained comparative copper nanoparticle dispersion 6, a conductive substrate was produced by flash light firing in the same manner as in (4) of Example 1.
得られた比較銅ナノ粒子分散体6を用いて、実施例1の(3)と同様にして、プラズマ焼成により導電性基板を製造した。
(4)フラッシュ光焼成による導電性基板の製造
得られた比較銅ナノ粒子分散体6を用いて、実施例1の(4)と同様にして、フラッシュ光焼成により導電性基板を製造した。 (3) Production of Conductive Substrate by Plasma Firing Using the obtained comparative copper nanoparticle dispersion 6, a conductive substrate was produced by plasma firing in the same manner as (3) of Example 1.
(4) Production of conductive substrate by flash light firing Using the obtained comparative copper nanoparticle dispersion 6, a conductive substrate was produced by flash light firing in the same manner as in (4) of Example 1.
(比較例7)
比較例6の(1)と同様にして、オレイン酸とヘキシルアミンで被覆された銅ナノ粒子を得た。
当該銅ナノ粒子を、実施例1の(2)において、ソルスパース41000の代わりに、ソルスパース76500(日本ルーブリゾール製、酸価0mgKOH/g、アミン価15.2mgKOH/g、90%熱重量減少温度が600℃超過(600℃でも32%残存))を用いた以外は実施例1の(2)と同様にして分散して、比較銅ナノ粒子分散体7を得た。
得られた比較銅ナノ粒子分散体7を用いて、実施例1の(3)と同様にして、プラズマ焼成により導電性基板を製造した。
得られた比較銅ナノ粒子分散体7を用いて、実施例1の(4)と同様にして、フラッシュ光焼成により導電性基板を製造した。 (Comparative Example 7)
In the same manner as in Comparative Example 6 (1), copper nanoparticles coated with oleic acid and hexylamine were obtained.
In Example 1 (2), instead of Solsperse 41000, the copper nanoparticles were Solsperse 76500 (manufactured by Nippon Lubrizol, acid value 0 mgKOH / g, amine value 15.2 mgKOH / g, 90% thermogravimetric decrease temperature) A comparative copper nanoparticle dispersion 7 was obtained by dispersing in the same manner as in (2) of Example 1 except that over 600 ° C (32% remaining even at 600 ° C) was used.
Using the obtained comparative copper nanoparticle dispersion 7, a conductive substrate was produced by plasma firing in the same manner as in Example 1 (3).
Using the obtained comparative copper nanoparticle dispersion 7, a conductive substrate was produced by flash light baking in the same manner as in Example 1 (4).
比較例6の(1)と同様にして、オレイン酸とヘキシルアミンで被覆された銅ナノ粒子を得た。
当該銅ナノ粒子を、実施例1の(2)において、ソルスパース41000の代わりに、ソルスパース76500(日本ルーブリゾール製、酸価0mgKOH/g、アミン価15.2mgKOH/g、90%熱重量減少温度が600℃超過(600℃でも32%残存))を用いた以外は実施例1の(2)と同様にして分散して、比較銅ナノ粒子分散体7を得た。
得られた比較銅ナノ粒子分散体7を用いて、実施例1の(3)と同様にして、プラズマ焼成により導電性基板を製造した。
得られた比較銅ナノ粒子分散体7を用いて、実施例1の(4)と同様にして、フラッシュ光焼成により導電性基板を製造した。 (Comparative Example 7)
In the same manner as in Comparative Example 6 (1), copper nanoparticles coated with oleic acid and hexylamine were obtained.
In Example 1 (2), instead of Solsperse 41000, the copper nanoparticles were Solsperse 76500 (manufactured by Nippon Lubrizol, acid value 0 mgKOH / g, amine value 15.2 mgKOH / g, 90% thermogravimetric decrease temperature) A comparative copper nanoparticle dispersion 7 was obtained by dispersing in the same manner as in (2) of Example 1 except that over 600 ° C (32% remaining even at 600 ° C) was used.
Using the obtained comparative copper nanoparticle dispersion 7, a conductive substrate was produced by plasma firing in the same manner as in Example 1 (3).
Using the obtained comparative copper nanoparticle dispersion 7, a conductive substrate was produced by flash light baking in the same manner as in Example 1 (4).
[評価]
<分散性評価>
銅ナノ粒子の分散性の評価として、各実施例及び比較例で得られた銅ナノ粒子分散体中の銅ナノ粒子の分散平均粒径の測定を行った。分散平均粒径の測定には、日機装製「マイクロトラック粒度分布計UPA-EX150」を用いた。分散平均粒径の値は体積平均粒径の値を用いることとし、また12時間放置後に沈降してしまう分散体は分散不可とした。各実施例及び比較例の分散体の分散平均粒径の結果を、表1に示す。 [Evaluation]
<Dispersibility evaluation>
As an evaluation of the dispersibility of the copper nanoparticles, the dispersion average particle diameter of the copper nanoparticles in the copper nanoparticle dispersion obtained in each of the examples and the comparative examples was measured. For the measurement of the dispersion average particle size, Nikkiso “Microtrac particle size distribution meter UPA-EX150” was used. The value of the volume average particle diameter was used as the value of the dispersion average particle diameter, and the dispersion that settled after standing for 12 hours was not dispersible. Table 1 shows the results of dispersion average particle diameters of the dispersions of Examples and Comparative Examples.
<分散性評価>
銅ナノ粒子の分散性の評価として、各実施例及び比較例で得られた銅ナノ粒子分散体中の銅ナノ粒子の分散平均粒径の測定を行った。分散平均粒径の測定には、日機装製「マイクロトラック粒度分布計UPA-EX150」を用いた。分散平均粒径の値は体積平均粒径の値を用いることとし、また12時間放置後に沈降してしまう分散体は分散不可とした。各実施例及び比較例の分散体の分散平均粒径の結果を、表1に示す。 [Evaluation]
<Dispersibility evaluation>
As an evaluation of the dispersibility of the copper nanoparticles, the dispersion average particle diameter of the copper nanoparticles in the copper nanoparticle dispersion obtained in each of the examples and the comparative examples was measured. For the measurement of the dispersion average particle size, Nikkiso “Microtrac particle size distribution meter UPA-EX150” was used. The value of the volume average particle diameter was used as the value of the dispersion average particle diameter, and the dispersion that settled after standing for 12 hours was not dispersible. Table 1 shows the results of dispersion average particle diameters of the dispersions of Examples and Comparative Examples.
<塗布適性評価>
各実施例及び比較例で得られた銅ナノ粒子分散体の塗膜を形成した後、焼成前に金属微粒子分散体の塗膜の膜質を目視で観察することにより塗布適性評価を行った。各実施例及び比較例の分散体の塗布適性評価の結果を、表1に示す。
[塗布適性評価基準]
A:はじきがなく、塗膜が均一である。
B:はじきがあり、塗膜が不均一である。 <Applicability evaluation>
After forming the coating film of the copper nanoparticle dispersion obtained in each Example and Comparative Example, the coating suitability was evaluated by visually observing the film quality of the coating film of the metal fine particle dispersion before firing. Table 1 shows the results of the applicability evaluation of the dispersions of Examples and Comparative Examples.
[Applicability evaluation criteria]
A: There is no repellency and the coating film is uniform.
B: There is repellency and the coating film is non-uniform.
各実施例及び比較例で得られた銅ナノ粒子分散体の塗膜を形成した後、焼成前に金属微粒子分散体の塗膜の膜質を目視で観察することにより塗布適性評価を行った。各実施例及び比較例の分散体の塗布適性評価の結果を、表1に示す。
[塗布適性評価基準]
A:はじきがなく、塗膜が均一である。
B:はじきがあり、塗膜が不均一である。 <Applicability evaluation>
After forming the coating film of the copper nanoparticle dispersion obtained in each Example and Comparative Example, the coating suitability was evaluated by visually observing the film quality of the coating film of the metal fine particle dispersion before firing. Table 1 shows the results of the applicability evaluation of the dispersions of Examples and Comparative Examples.
[Applicability evaluation criteria]
A: There is no repellency and the coating film is uniform.
B: There is repellency and the coating film is non-uniform.
<導電性評価>
導電性基板について、導電性評価を行った。表面抵抗計(ダイアインスツルメンツ製「ロレスタGP」、PSPプローブタイプ)を用いて、各実施例及び比較例の導電性基板の金属膜に4探針を接触させ、4探針法によりシート抵抗値を測定した。評価の結果を、表1に示す。シート抵抗値が低いほど導電性に優れている。なお、本測定法によるシート抵抗値の測定上限は108Ω/□であった。表中、O.L.とは、Over Loadを表す。 <Electrical conductivity evaluation>
Conductivity evaluation was performed on the conductive substrate. Using a surface resistance meter ("Loresta GP" manufactured by Dia Instruments, PSP probe type), 4 probes are brought into contact with the metal film of the conductive substrate of each example and comparative example, and the sheet resistance value is determined by the 4 probe method. It was measured. The evaluation results are shown in Table 1. The lower the sheet resistance value, the better the conductivity. The upper limit of sheet resistance measured by this measurement method was 10 8 Ω / □. In the table, O.D. L. Represents Over Load.
導電性基板について、導電性評価を行った。表面抵抗計(ダイアインスツルメンツ製「ロレスタGP」、PSPプローブタイプ)を用いて、各実施例及び比較例の導電性基板の金属膜に4探針を接触させ、4探針法によりシート抵抗値を測定した。評価の結果を、表1に示す。シート抵抗値が低いほど導電性に優れている。なお、本測定法によるシート抵抗値の測定上限は108Ω/□であった。表中、O.L.とは、Over Loadを表す。 <Electrical conductivity evaluation>
Conductivity evaluation was performed on the conductive substrate. Using a surface resistance meter ("Loresta GP" manufactured by Dia Instruments, PSP probe type), 4 probes are brought into contact with the metal film of the conductive substrate of each example and comparative example, and the sheet resistance value is determined by the 4 probe method. It was measured. The evaluation results are shown in Table 1. The lower the sheet resistance value, the better the conductivity. The upper limit of sheet resistance measured by this measurement method was 10 8 Ω / □. In the table, O.D. L. Represents Over Load.
<結果のまとめ>
実施例1~15により、本発明に係るカルボン酸とアルキルアミンと特定のアミン価及び酸価を有する高分子分散剤と溶剤とを含有する銅ナノ粒子分散体は、分散性、及び塗布適性に優れること、更に、低温又は短時間での焼結性に優れ、表面抵抗1Ω/□以下の高い導電性が得られることが明らかにされた。中でも、炭素数10以下のカルボン酸を用いると、導電性が向上することがわかった。また、本発明に係る銅ナノ粒子分散体を用いると、このように塗布適性に優れることから、回路パターンを精度よく形成することが可能である。また、実施例1~15で得られた銅ナノ粒子は、製造時の耐酸化性が良好であり、中でも、炭素数10以下のカルボン酸を用いると、銅ナノ粒子の製造時の耐酸化性にも優れることが分かった。
一方、カルボン酸とアルキルアミンとを含むが、高分子分散剤を含まない、比較例1の銅ナノ粒子分散体は、分散性が悪く、塗膜を形成しても、はじきがあり、不均一な塗膜しか形成できなかった。また、比較例2、3及び4の結果から、デカン酸やノナン酸など炭素数が小さいカルボン酸とアルキルアミンとを含む場合、本願で特定した値よりも小さい又は大きいアミン価又は酸価を有する高分子分散剤では、分散できないことが明らかにされた。
特許文献3の実施例に対応する比較例5は、本願で特定した値よりも大きいアミン価を有する高分子分散剤を用いても、カルボン酸として炭素数が大きいオレイン酸を被覆しているため、分散可能ではあるが、分散性が悪く、均一な塗膜を形成できなかった。また、低温又は短時間の焼成では、優れた導電性が得られないことが明らかにされた。
同様に、カルボン酸として炭素数が大きいオレイン酸を用いた場合には、本願で特定した値よりも小さいアミン価又は酸価を有する高分子分散剤でも、分散可能ではあるが、分散性が悪く、低温又は短時間の焼成では、優れた導電性が得られないことが明らかにされた。
一方、カルボン酸として炭素数が大きいオレイン酸を用いた場合でも、本願で特定したアミン価又は酸価を有する高分子分散剤では、優れた分散性が得られるため、低温又は短時間の焼成でも、表面抵抗1Ω/□以下の高い導電性が得られる(実施例15)。 <Summary of results>
According to Examples 1 to 15, a copper nanoparticle dispersion containing a carboxylic acid, an alkylamine, a polymer dispersant having a specific amine value and an acid value, and a solvent according to the present invention can be dispersed and applied. It has been clarified that it has excellent sinterability at a low temperature or in a short time, and a high conductivity with a surface resistance of 1 Ω / □ or less can be obtained. In particular, it was found that the use of a carboxylic acid having 10 or less carbon atoms improves the conductivity. Moreover, when the copper nanoparticle dispersion which concerns on this invention is used, since it is excellent in application | coating suitability in this way, it is possible to form a circuit pattern accurately. In addition, the copper nanoparticles obtained in Examples 1 to 15 have good oxidation resistance at the time of production. In particular, when a carboxylic acid having 10 or less carbon atoms is used, the oxidation resistance at the time of production of the copper nanoparticles. It was also found to be excellent.
On the other hand, the copper nanoparticle dispersion of Comparative Example 1 containing a carboxylic acid and an alkylamine but not containing a polymer dispersant has poor dispersibility, and even if a coating film is formed, it has repelling and is not uniform. Only a thin film could be formed. Moreover, from the results of Comparative Examples 2, 3 and 4, when a carboxylic acid having a small carbon number such as decanoic acid or nonanoic acid and an alkylamine are included, the amine value or acid value is smaller or larger than the value specified in this application. It was revealed that the polymer dispersant cannot be dispersed.
Since the comparative example 5 corresponding to the Example ofpatent document 3 coat | covers oleic acid with a large carbon number as carboxylic acid, even if it uses the polymer dispersing agent which has an amine value larger than the value specified by this application. Although dispersible, the dispersibility was poor and a uniform coating film could not be formed. Further, it has been clarified that excellent conductivity cannot be obtained by low-temperature or short-time firing.
Similarly, when oleic acid having a large number of carbon atoms is used as the carboxylic acid, even a polymer dispersant having an amine value or acid value smaller than the value specified in the present application can be dispersed, but the dispersibility is poor. It has been clarified that excellent conductivity cannot be obtained by low-temperature or short-time firing.
On the other hand, even when oleic acid having a large number of carbon atoms is used as the carboxylic acid, the polymer dispersant having the amine value or acid value specified in the present application can provide excellent dispersibility, so that it can be fired at low temperature or in a short time. A high conductivity with a surface resistance of 1 Ω / □ or less is obtained (Example 15).
実施例1~15により、本発明に係るカルボン酸とアルキルアミンと特定のアミン価及び酸価を有する高分子分散剤と溶剤とを含有する銅ナノ粒子分散体は、分散性、及び塗布適性に優れること、更に、低温又は短時間での焼結性に優れ、表面抵抗1Ω/□以下の高い導電性が得られることが明らかにされた。中でも、炭素数10以下のカルボン酸を用いると、導電性が向上することがわかった。また、本発明に係る銅ナノ粒子分散体を用いると、このように塗布適性に優れることから、回路パターンを精度よく形成することが可能である。また、実施例1~15で得られた銅ナノ粒子は、製造時の耐酸化性が良好であり、中でも、炭素数10以下のカルボン酸を用いると、銅ナノ粒子の製造時の耐酸化性にも優れることが分かった。
一方、カルボン酸とアルキルアミンとを含むが、高分子分散剤を含まない、比較例1の銅ナノ粒子分散体は、分散性が悪く、塗膜を形成しても、はじきがあり、不均一な塗膜しか形成できなかった。また、比較例2、3及び4の結果から、デカン酸やノナン酸など炭素数が小さいカルボン酸とアルキルアミンとを含む場合、本願で特定した値よりも小さい又は大きいアミン価又は酸価を有する高分子分散剤では、分散できないことが明らかにされた。
特許文献3の実施例に対応する比較例5は、本願で特定した値よりも大きいアミン価を有する高分子分散剤を用いても、カルボン酸として炭素数が大きいオレイン酸を被覆しているため、分散可能ではあるが、分散性が悪く、均一な塗膜を形成できなかった。また、低温又は短時間の焼成では、優れた導電性が得られないことが明らかにされた。
同様に、カルボン酸として炭素数が大きいオレイン酸を用いた場合には、本願で特定した値よりも小さいアミン価又は酸価を有する高分子分散剤でも、分散可能ではあるが、分散性が悪く、低温又は短時間の焼成では、優れた導電性が得られないことが明らかにされた。
一方、カルボン酸として炭素数が大きいオレイン酸を用いた場合でも、本願で特定したアミン価又は酸価を有する高分子分散剤では、優れた分散性が得られるため、低温又は短時間の焼成でも、表面抵抗1Ω/□以下の高い導電性が得られる(実施例15)。 <Summary of results>
According to Examples 1 to 15, a copper nanoparticle dispersion containing a carboxylic acid, an alkylamine, a polymer dispersant having a specific amine value and an acid value, and a solvent according to the present invention can be dispersed and applied. It has been clarified that it has excellent sinterability at a low temperature or in a short time, and a high conductivity with a surface resistance of 1 Ω / □ or less can be obtained. In particular, it was found that the use of a carboxylic acid having 10 or less carbon atoms improves the conductivity. Moreover, when the copper nanoparticle dispersion which concerns on this invention is used, since it is excellent in application | coating suitability in this way, it is possible to form a circuit pattern accurately. In addition, the copper nanoparticles obtained in Examples 1 to 15 have good oxidation resistance at the time of production. In particular, when a carboxylic acid having 10 or less carbon atoms is used, the oxidation resistance at the time of production of the copper nanoparticles. It was also found to be excellent.
On the other hand, the copper nanoparticle dispersion of Comparative Example 1 containing a carboxylic acid and an alkylamine but not containing a polymer dispersant has poor dispersibility, and even if a coating film is formed, it has repelling and is not uniform. Only a thin film could be formed. Moreover, from the results of Comparative Examples 2, 3 and 4, when a carboxylic acid having a small carbon number such as decanoic acid or nonanoic acid and an alkylamine are included, the amine value or acid value is smaller or larger than the value specified in this application. It was revealed that the polymer dispersant cannot be dispersed.
Since the comparative example 5 corresponding to the Example of
Similarly, when oleic acid having a large number of carbon atoms is used as the carboxylic acid, even a polymer dispersant having an amine value or acid value smaller than the value specified in the present application can be dispersed, but the dispersibility is poor. It has been clarified that excellent conductivity cannot be obtained by low-temperature or short-time firing.
On the other hand, even when oleic acid having a large number of carbon atoms is used as the carboxylic acid, the polymer dispersant having the amine value or acid value specified in the present application can provide excellent dispersibility, so that it can be fired at low temperature or in a short time. A high conductivity with a surface resistance of 1 Ω / □ or less is obtained (Example 15).
(実施例16)
実施例3と同様にして、銅ナノ粒子分散体3を得た。
得られた銅ナノ粒子分散体3を、厚さ100μmのPETフィルム(コスモシャイン A4100)にワイヤーバーで塗布、乾燥して、膜厚が0.5μmの塗膜とした。
その後、水素ガスを導入圧力20Paで導入しながら、マイクロ波表面波プラズマ処理装置(MSP-1500、ミクロ電子製)を用いて、マイクロ波出力450Wで300秒間焼成し、銅ナノ粒子分散体が焼成されてなる焼結膜(銅膜)とし、銅薄膜付PETフィルムとした。
洗浄済みガラス基板にUV硬化系インクをスピンコーターにより塗布し、気泡が入らないように、上記で得られた銅薄膜付PETフィルムを貼り付け、背面からUV照射することでガラスとフィルムを固定した。次に市販のナフトキノン系ポジ型レジストを銅薄膜付PETフィルム面上にスピンコート塗布し、100℃で3分間ホットプレート乾燥させ、乾燥膜厚を約1μmに製膜した。さらにポジ型レジスト膜面にフォトマスクをコンタクトさせ、40mJ/cm2で露光、次いでNMD-3(東京応化工業製)により20秒間パドル現像し、純水によってリンスし、レジストパターンを得た。さらに23℃のリン硝酢酸系エッチング液により、揺動させながらエッチング80秒間、その後純水によってリンスし、エッチング処理した。その後、全面を100mJ/cm2で露光、次いでNMD-3(東京応化工業製)により60秒間パドル現像し、純水によってリンスすることで線幅3.8μmの銅配線パターンをPETフィルム上に形成することができた。 (Example 16)
In the same manner as in Example 3, acopper nanoparticle dispersion 3 was obtained.
The obtainedcopper nanoparticle dispersion 3 was applied to a PET film (Cosmo Shine A4100) having a thickness of 100 μm with a wire bar and dried to form a coating film having a thickness of 0.5 μm.
Then, while introducing hydrogen gas at an introduction pressure of 20 Pa, using a microwave surface wave plasma processing apparatus (MSP-1500, manufactured by Microelectronics), firing was performed for 300 seconds at a microwave output of 450 W, and the copper nanoparticle dispersion was fired. The resulting sintered film (copper film) was used as a PET film with a copper thin film.
A UV curable ink was applied to a cleaned glass substrate with a spin coater, and the PET film with a copper thin film obtained above was affixed to prevent bubbles from entering, and the glass and the film were fixed by UV irradiation from the back side. . Next, a commercially available naphthoquinone-based positive resist was spin-coated on the surface of the PET film with a copper thin film and hot-plate dried at 100 ° C. for 3 minutes to form a dry film thickness of about 1 μm. Further, a positive resist film surface was contacted with a photomask, exposed at 40 mJ / cm 2 , then paddle developed with NMD-3 (manufactured by Tokyo Ohka Kogyo Co., Ltd.) for 20 seconds, and rinsed with pure water to obtain a resist pattern. Further, etching was performed for 80 seconds while being rocked with a phosphoric acid acetate etching solution at 23 ° C., and then rinsed with pure water and etched. Thereafter, the entire surface is exposed at 100 mJ / cm 2 , then paddle developed for 60 seconds with NMD-3 (manufactured by Tokyo Ohka Kogyo Co., Ltd.), and rinsed with pure water to form a copper wiring pattern with a line width of 3.8 μm on the PET film. We were able to.
実施例3と同様にして、銅ナノ粒子分散体3を得た。
得られた銅ナノ粒子分散体3を、厚さ100μmのPETフィルム(コスモシャイン A4100)にワイヤーバーで塗布、乾燥して、膜厚が0.5μmの塗膜とした。
その後、水素ガスを導入圧力20Paで導入しながら、マイクロ波表面波プラズマ処理装置(MSP-1500、ミクロ電子製)を用いて、マイクロ波出力450Wで300秒間焼成し、銅ナノ粒子分散体が焼成されてなる焼結膜(銅膜)とし、銅薄膜付PETフィルムとした。
洗浄済みガラス基板にUV硬化系インクをスピンコーターにより塗布し、気泡が入らないように、上記で得られた銅薄膜付PETフィルムを貼り付け、背面からUV照射することでガラスとフィルムを固定した。次に市販のナフトキノン系ポジ型レジストを銅薄膜付PETフィルム面上にスピンコート塗布し、100℃で3分間ホットプレート乾燥させ、乾燥膜厚を約1μmに製膜した。さらにポジ型レジスト膜面にフォトマスクをコンタクトさせ、40mJ/cm2で露光、次いでNMD-3(東京応化工業製)により20秒間パドル現像し、純水によってリンスし、レジストパターンを得た。さらに23℃のリン硝酢酸系エッチング液により、揺動させながらエッチング80秒間、その後純水によってリンスし、エッチング処理した。その後、全面を100mJ/cm2で露光、次いでNMD-3(東京応化工業製)により60秒間パドル現像し、純水によってリンスすることで線幅3.8μmの銅配線パターンをPETフィルム上に形成することができた。 (Example 16)
In the same manner as in Example 3, a
The obtained
Then, while introducing hydrogen gas at an introduction pressure of 20 Pa, using a microwave surface wave plasma processing apparatus (MSP-1500, manufactured by Microelectronics), firing was performed for 300 seconds at a microwave output of 450 W, and the copper nanoparticle dispersion was fired. The resulting sintered film (copper film) was used as a PET film with a copper thin film.
A UV curable ink was applied to a cleaned glass substrate with a spin coater, and the PET film with a copper thin film obtained above was affixed to prevent bubbles from entering, and the glass and the film were fixed by UV irradiation from the back side. . Next, a commercially available naphthoquinone-based positive resist was spin-coated on the surface of the PET film with a copper thin film and hot-plate dried at 100 ° C. for 3 minutes to form a dry film thickness of about 1 μm. Further, a positive resist film surface was contacted with a photomask, exposed at 40 mJ / cm 2 , then paddle developed with NMD-3 (manufactured by Tokyo Ohka Kogyo Co., Ltd.) for 20 seconds, and rinsed with pure water to obtain a resist pattern. Further, etching was performed for 80 seconds while being rocked with a phosphoric acid acetate etching solution at 23 ° C., and then rinsed with pure water and etched. Thereafter, the entire surface is exposed at 100 mJ / cm 2 , then paddle developed for 60 seconds with NMD-3 (manufactured by Tokyo Ohka Kogyo Co., Ltd.), and rinsed with pure water to form a copper wiring pattern with a line width of 3.8 μm on the PET film. We were able to.
<平滑性評価>
得られた銅配線パターンの断面を走査型電子顕微鏡(SEM、日立ハイテクノロジー製走査型電子顕微鏡「S-4800」)で測定し、10万倍の断面観察像を得た。基板と焼結膜の界面の凹凸ライン(長さ2μm)を抽出し、該凹凸ラインから、最大高さ(Rz、最も山の部分と最も谷の部分の差、JIS B0601(2001)の定義に従う)を求めた。凹凸ラインは面内の任意の10箇所から抽出し、それぞれの最大高さの平均値を、平均粗さとした。
なお、凹凸ラインは、SEM観察で得られた画像を白黒に変換し、白色の部分を金属焼結膜側として、金属焼結膜側に沿ったラインを抽出した。異物や、フィルム中に含有されるフィラーなど、明らかな凹凸異常がある部分は使用しなかった。
その結果、基材と金属焼結膜との界面の凹凸は12.4nmであり、前述の界面は平滑であることが確認された。
<密着性評価>
得られた銅配線パターンを粘着テープ(商品名:スコッチメンディングテープ、住友スリーエム製)で剥離試験を行った。その結果、配線に剥がれがないことが確認され、エッチング後であっても密着性が良好であることが確認された。 <Smoothness evaluation>
The cross section of the obtained copper wiring pattern was measured with a scanning electron microscope (SEM, scanning electron microscope “S-4800” manufactured by Hitachi High-Technology), and a cross-sectional observation image of 100,000 times was obtained. An uneven line (length 2 μm) at the interface between the substrate and the sintered film is extracted, and from the uneven line, the maximum height (Rz, the difference between the most peak and the most valley, according to the definition of JIS B0601 (2001)) Asked. Uneven lines were extracted from any 10 locations in the surface, and the average value of the maximum heights was defined as the average roughness.
In addition, the uneven | corrugated line converted the image obtained by SEM observation into black-and-white, and extracted the line along the metal sintered film side by making a white part into the metal sintered film side. Parts with obvious irregularities such as foreign matter and filler contained in the film were not used.
As a result, the unevenness of the interface between the base material and the metal sintered film was 12.4 nm, and it was confirmed that the above-mentioned interface was smooth.
<Adhesion evaluation>
The obtained copper wiring pattern was subjected to a peel test using an adhesive tape (trade name: Scotch Mending Tape, manufactured by Sumitomo 3M). As a result, it was confirmed that the wiring did not peel off, and it was confirmed that the adhesion was good even after etching.
得られた銅配線パターンの断面を走査型電子顕微鏡(SEM、日立ハイテクノロジー製走査型電子顕微鏡「S-4800」)で測定し、10万倍の断面観察像を得た。基板と焼結膜の界面の凹凸ライン(長さ2μm)を抽出し、該凹凸ラインから、最大高さ(Rz、最も山の部分と最も谷の部分の差、JIS B0601(2001)の定義に従う)を求めた。凹凸ラインは面内の任意の10箇所から抽出し、それぞれの最大高さの平均値を、平均粗さとした。
なお、凹凸ラインは、SEM観察で得られた画像を白黒に変換し、白色の部分を金属焼結膜側として、金属焼結膜側に沿ったラインを抽出した。異物や、フィルム中に含有されるフィラーなど、明らかな凹凸異常がある部分は使用しなかった。
その結果、基材と金属焼結膜との界面の凹凸は12.4nmであり、前述の界面は平滑であることが確認された。
<密着性評価>
得られた銅配線パターンを粘着テープ(商品名:スコッチメンディングテープ、住友スリーエム製)で剥離試験を行った。その結果、配線に剥がれがないことが確認され、エッチング後であっても密着性が良好であることが確認された。 <Smoothness evaluation>
The cross section of the obtained copper wiring pattern was measured with a scanning electron microscope (SEM, scanning electron microscope “S-4800” manufactured by Hitachi High-Technology), and a cross-sectional observation image of 100,000 times was obtained. An uneven line (
In addition, the uneven | corrugated line converted the image obtained by SEM observation into black-and-white, and extracted the line along the metal sintered film side by making a white part into the metal sintered film side. Parts with obvious irregularities such as foreign matter and filler contained in the film were not used.
As a result, the unevenness of the interface between the base material and the metal sintered film was 12.4 nm, and it was confirmed that the above-mentioned interface was smooth.
<Adhesion evaluation>
The obtained copper wiring pattern was subjected to a peel test using an adhesive tape (trade name: Scotch Mending Tape, manufactured by Sumitomo 3M). As a result, it was confirmed that the wiring did not peel off, and it was confirmed that the adhesion was good even after etching.
1 基材
2 金属膜
3 パターン状金属膜
100 導電性基板
101 導電性基板 DESCRIPTION OF SYMBOLS 1Base material 2 Metal film 3 Patterned metal film 100 Conductive substrate 101 Conductive substrate
2 金属膜
3 パターン状金属膜
100 導電性基板
101 導電性基板 DESCRIPTION OF SYMBOLS 1
Claims (13)
- 銅ナノ粒子と、カルボン酸と、アルキルアミンと、高分子分散剤と、溶剤とを含有し、前記高分子分散剤は、アミン価及び酸価の一方が30~160mgKOH/g、アミン価及び酸価の他の一方が0~160mgKOH/gであり、動的光散乱法による体積平均粒径が500nm以下である銅ナノ粒子分散体を、基材上に塗布して塗膜を形成する工程と、
当該塗膜を焼成する工程とを有する、導電性基板の製造方法。 Copper nanoparticles, a carboxylic acid, an alkylamine, a polymer dispersant, and a solvent, wherein the polymer dispersant has an amine value and an acid value of 30 to 160 mgKOH / g, an amine value and an acid. The other of the valence is 0 to 160 mgKOH / g, and a copper nanoparticle dispersion having a volume average particle diameter of 500 nm or less by a dynamic light scattering method is applied on a substrate to form a coating film; ,
A method for producing a conductive substrate, comprising a step of firing the coating film. - 前記高分子分散剤は、90%熱重量減少温度が420℃以下である、請求項1に記載の導電性基板の製造方法。 The method for producing a conductive substrate according to claim 1, wherein the polymer dispersant has a 90% thermal weight loss temperature of 420 ° C or lower.
- 前記カルボン酸は、炭素数が10以下である、請求項1又は2に記載の導電性基板の製造方法。 The method for producing a conductive substrate according to claim 1 or 2, wherein the carboxylic acid has 10 or less carbon atoms.
- 前記焼成する工程が、プラズマにより焼成する工程であるか、フラッシュ光の照射により焼成する工程である、請求項1乃至3のいずれか一項に記載の導電性基板の製造方法。 The method for producing a conductive substrate according to any one of claims 1 to 3, wherein the baking step is a step of baking by plasma or a step of baking by irradiation with flash light.
- 前記焼成する工程後に、更に、得られた焼結膜を化学エッチングする工程を有する、請求項1乃至4のいずれか一項に記載の導電性基板の製造方法。 The method for producing a conductive substrate according to any one of claims 1 to 4, further comprising a step of chemically etching the obtained sintered film after the baking step.
- 銅を含む化合物、還元性化合物、カルボン酸、及びアルキルアミンを含む混合物、又は、カルボン酸銅、還元性化合物、及びアルキルアミンを含む混合物のいずれかを加熱することにより銅ナノ粒子を調製する工程と、
前記銅ナノ粒子を、溶剤中で、アミン価及び酸価の一方が30~160mgKOH/g、アミン価及び酸価の他の一方が0~160mgKOH/gである高分子分散剤により分散することにより、動的光散乱法による体積平均粒径が500nm以下である銅ナノ粒子分散体を調製する工程と、
前記銅ナノ粒子分散体を、基材上に塗布して塗膜を形成する工程と、
当該塗膜を焼成する工程とを有する、導電性基板の製造方法。 A step of preparing copper nanoparticles by heating either a compound containing copper, a reducing compound, a carboxylic acid, and a mixture containing an alkylamine, or a mixture containing copper carboxylate, a reducing compound, and an alkylamine. When,
By dispersing the copper nanoparticles in a solvent with a polymer dispersant having one of an amine value and an acid value of 30 to 160 mgKOH / g and the other of an amine value and an acid value of 0 to 160 mgKOH / g. A step of preparing a copper nanoparticle dispersion having a volume average particle size of 500 nm or less by a dynamic light scattering method;
Applying the copper nanoparticle dispersion on a substrate to form a coating film;
A method for producing a conductive substrate, comprising a step of firing the coating film. - 前記高分子分散剤は、90%熱重量減少温度が420℃以下である、請求項6に記載の導電性基板の製造方法。 The method for producing a conductive substrate according to claim 6, wherein the polymer dispersant has a 90% thermal weight loss temperature of 420 ° C or lower.
- 前記カルボン酸は、炭素数が10以下である、請求項6又は7に記載の導電性基板の製造方法。 The method for producing a conductive substrate according to claim 6 or 7, wherein the carboxylic acid has 10 or less carbon atoms.
- 前記焼成する工程が、プラズマにより焼成する工程であるか、フラッシュ光の照射により焼成する工程である、請求項6乃至8のいずれか一項に記載の導電性基板の製造方法。 The method for manufacturing a conductive substrate according to any one of claims 6 to 8, wherein the baking step is a step of baking by plasma or a step of baking by irradiation with flash light.
- 前記焼成する工程後に、更に、得られた焼結膜を化学エッチングする工程を有する、請求項6乃至9のいずれか一項に記載の導電性基板の製造方法。 The method for manufacturing a conductive substrate according to any one of claims 6 to 9, further comprising a step of chemically etching the obtained sintered film after the baking step.
- 銅ナノ粒子と、カルボン酸と、アルキルアミンと、高分子分散剤と、溶剤とを含有し、前記高分子分散剤は、アミン価及び酸価の一方が30~160mgKOH/g、アミン価及び酸価の他の一方が0~160mgKOH/gであり、動的光散乱法による体積平均粒径が500nm以下である、銅ナノ粒子分散体。 Copper nanoparticles, a carboxylic acid, an alkylamine, a polymer dispersant, and a solvent, wherein the polymer dispersant has an amine value and an acid value of 30 to 160 mgKOH / g, an amine value and an acid. One of the other values is 0 to 160 mgKOH / g, and the copper nanoparticle dispersion has a volume average particle diameter of 500 nm or less by a dynamic light scattering method.
- 前記高分子分散剤は、90%熱重量減少温度が420℃以下である、請求項11に記載の銅ナノ粒子分散体。 The copper nanoparticle dispersion according to claim 11, wherein the polymer dispersant has a 90% thermal weight loss temperature of 420 ° C. or less.
- 前記カルボン酸は、炭素数が10以下である、請求項11又は12に記載の銅ナノ粒子分散体。 13. The copper nanoparticle dispersion according to claim 11 or 12, wherein the carboxylic acid has 10 or less carbon atoms.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2014-092473 | 2014-04-28 | ||
| JP2014092473A JP5994811B2 (en) | 2014-04-28 | 2014-04-28 | Copper nanoparticle dispersion and method for producing conductive substrate |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2015166755A1 true WO2015166755A1 (en) | 2015-11-05 |
Family
ID=54358492
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2015/060007 WO2015166755A1 (en) | 2014-04-28 | 2015-03-30 | Copper nanoparticle dispersion and method for manufacturing electroconductive substrate |
Country Status (2)
| Country | Link |
|---|---|
| JP (1) | JP5994811B2 (en) |
| WO (1) | WO2015166755A1 (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20210155818A1 (en) * | 2017-07-27 | 2021-05-27 | Asahi Kasei Kabushiki Kaisha | Copper Oxide Ink and Method for Producing Conductive Substrate Using Same, Product Containing Coating Film and Method for Producing Product Using Same, Method for Producing Product with Conductive Pattern, and Product with Conductive Pattern |
| WO2023017747A1 (en) * | 2021-08-10 | 2023-02-16 | 株式会社ダイセル | Electroconductive ink |
Families Citing this family (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11104813B2 (en) * | 2015-06-02 | 2021-08-31 | Asahi Kasei Kabushiki Kaisha | Dispersion |
| JP6642218B2 (en) * | 2016-04-05 | 2020-02-05 | 日油株式会社 | Copper paste composition for laser etching |
| JPWO2018003399A1 (en) * | 2016-06-30 | 2018-07-05 | 株式会社コイネックス | Copper wiring and electronic equipment, touchpad, touch panel using the same |
| US11328835B2 (en) | 2017-03-16 | 2022-05-10 | Asahi Kasei Kabushiki Kaisha | Dispersing element, method for manufacturing structure with conductive pattern using the same, and structure with conductive pattern |
| KR102225197B1 (en) | 2017-03-16 | 2021-03-09 | 아사히 가세이 가부시키가이샤 | Dispersion, and a method of manufacturing a structure with a conductive pattern using the same, and a structure with a conductive pattern |
| JP2018170228A (en) * | 2017-03-30 | 2018-11-01 | 日立化成株式会社 | Conductor-forming composition, and joint body, and method for producing the same |
| JP2018168439A (en) * | 2017-03-30 | 2018-11-01 | 日立化成株式会社 | Composition used for producing electromagnetic wave shield body, and method for producing electromagnetic wave shield body |
| WO2019017363A1 (en) | 2017-07-18 | 2019-01-24 | 旭化成株式会社 | Structure including electroconductive pattern regions, method for producing same, laminate, method for producing same, and copper wiring |
| JP7430483B2 (en) * | 2017-11-10 | 2024-02-13 | 旭化成株式会社 | Structure with conductive pattern area and manufacturing method thereof |
| JP2019211243A (en) * | 2018-05-31 | 2019-12-12 | 旭化成株式会社 | RFID tag |
| JP2020084242A (en) * | 2018-11-20 | 2020-06-04 | 京セラ株式会社 | Manufacturing method of copper particle for conjugation, conjugation paste and semiconductor device, and electronic and electric component |
| JP2020100888A (en) * | 2018-12-25 | 2020-07-02 | 京セラ株式会社 | Copper particle, producing method for copper particle, copper paste, and semiconductor device and electric/electronic component |
| JP7269565B2 (en) * | 2019-03-29 | 2023-05-09 | 学校法人 関西大学 | Conductive ink composition and conductive laminate |
| JP7434786B2 (en) * | 2019-09-27 | 2024-02-21 | Dic株式会社 | Copper/copper oxide fine particle paste |
| WO2022196620A1 (en) * | 2021-03-17 | 2022-09-22 | 京セラ株式会社 | Paste composition, semiconductor device, electrical component and electronic component |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2009074171A (en) * | 2007-08-30 | 2009-04-09 | Mitsuboshi Belting Ltd | Metal colloidal particle and fluid dispersion thereof |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5661273B2 (en) * | 2008-11-26 | 2015-01-28 | 三ツ星ベルト株式会社 | Colloidal metal particles, paste thereof and method for producing the same |
| KR20110027487A (en) * | 2009-09-10 | 2011-03-16 | 삼성전자주식회사 | Composition for metal pattern and method of forming metal pattern using the same |
| JP5623861B2 (en) * | 2010-10-14 | 2014-11-12 | 株式会社東芝 | Metal nanoparticle dispersion composition |
| JP5715851B2 (en) * | 2011-02-24 | 2015-05-13 | 東芝テック株式会社 | Method for producing printed matter using nanoparticle ink composition |
| JP6127433B2 (en) * | 2012-10-03 | 2017-05-17 | 大日本印刷株式会社 | Metal particle dispersion and article |
-
2014
- 2014-04-28 JP JP2014092473A patent/JP5994811B2/en active Active
-
2015
- 2015-03-30 WO PCT/JP2015/060007 patent/WO2015166755A1/en active Application Filing
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2009074171A (en) * | 2007-08-30 | 2009-04-09 | Mitsuboshi Belting Ltd | Metal colloidal particle and fluid dispersion thereof |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20210155818A1 (en) * | 2017-07-27 | 2021-05-27 | Asahi Kasei Kabushiki Kaisha | Copper Oxide Ink and Method for Producing Conductive Substrate Using Same, Product Containing Coating Film and Method for Producing Product Using Same, Method for Producing Product with Conductive Pattern, and Product with Conductive Pattern |
| US11760895B2 (en) * | 2017-07-27 | 2023-09-19 | Asahi Kasei Kabushiki Kaisha | Copper oxide ink and method for producing conductive substrate using same, product containing coating film and method for producing product using same, method for producing product with conductive pattern, and product with conductive pattern |
| WO2023017747A1 (en) * | 2021-08-10 | 2023-02-16 | 株式会社ダイセル | Electroconductive ink |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2015210973A (en) | 2015-11-24 |
| JP5994811B2 (en) | 2016-09-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5994811B2 (en) | Copper nanoparticle dispersion and method for producing conductive substrate | |
| JP5700864B2 (en) | Copper fine particle dispersion, conductive film forming method, and circuit board | |
| EP2952546B1 (en) | Composition for forming electrically conductive film, and method for producing electrically conductive film | |
| JP5715851B2 (en) | Method for producing printed matter using nanoparticle ink composition | |
| US20090053400A1 (en) | Ink jet printable compositions for preparing electronic devices and patterns | |
| TW201437299A (en) | Composition for electroconductive film formation and method of forming electroconductive film by using the same | |
| JP7403512B2 (en) | Copper oxide ink and a method for manufacturing a conductive substrate using the same, a product including a coating film and a method for manufacturing a product using the same, a method for manufacturing a product with a conductive pattern, and a product with a conductive pattern | |
| JP2008176951A (en) | Silver-based particulate ink paste | |
| JP6404614B2 (en) | Manufacturing method of core-shell type metal fine particles, core-shell type metal fine particles, conductive ink and substrate | |
| WO2014061556A1 (en) | Metal particle dispersion for conductive substrate, production method therefor, and production method for conductive substrate | |
| JP6331386B2 (en) | Coated copper nanoparticles, copper nanoparticle dispersion, and method for producing conductive substrate | |
| JP2015149121A (en) | Copper particle dispersion paste and manufacturing method of conductive substrate | |
| JP6331385B2 (en) | Coated copper nanoparticles, copper nanoparticle dispersion, and method for producing conductive substrate | |
| JP6562196B2 (en) | Copper fine particle sintered body and method for producing conductive substrate | |
| JP2017022125A (en) | Copper nanoparticle dispersion and method of manufacturing conductive substrate | |
| JP6036185B2 (en) | High purity metal nanoparticle dispersion and method for producing the same | |
| WO2016125355A1 (en) | Electroconductive microparticles | |
| JP2016110690A (en) | Conductive substrate | |
| JP2012218020A (en) | Bonding method | |
| JP2006269557A (en) | Method of forming circuit pattern, circuit pattern formed by using same, and laminate | |
| JP6237098B2 (en) | Dispersant, metal particle dispersion for conductive substrate, and method for producing conductive substrate | |
| JP2020119737A (en) | Conductive paste, base material with conductive film, production method of base material with conductive film | |
| JP2019160689A (en) | Dispersion, product containing coating film, method for producing structure with conductive pattern, and structure with conductive pattern | |
| JP5821485B2 (en) | Copper fine particle dispersion, pattern forming method, and copper pattern film manufacturing method | |
| JP6111587B2 (en) | Method for manufacturing conductive substrate |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 15785825 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 15785825 Country of ref document: EP Kind code of ref document: A1 |