WO2014141960A1 - 二次電池用活物質、二次電池用電極、二次電池、電池パック、電動車両、電力貯蔵システム、電動工具および電子機器 - Google Patents
二次電池用活物質、二次電池用電極、二次電池、電池パック、電動車両、電力貯蔵システム、電動工具および電子機器 Download PDFInfo
- Publication number
- WO2014141960A1 WO2014141960A1 PCT/JP2014/055573 JP2014055573W WO2014141960A1 WO 2014141960 A1 WO2014141960 A1 WO 2014141960A1 JP 2014055573 W JP2014055573 W JP 2014055573W WO 2014141960 A1 WO2014141960 A1 WO 2014141960A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- active material
- carbon
- secondary battery
- particles
- measured
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/58—Selection of substances as active materials, active masses, active liquids of inorganic compounds other than oxides or hydroxides, e.g. sulfides, selenides, tellurides, halogenides or LiCoFy; of polyanionic structures, e.g. phosphates, silicates or borates
- H01M4/5825—Oxygenated metallic salts or polyanionic structures, e.g. borates, phosphates, silicates, olivines
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B60—VEHICLES IN GENERAL
- B60L—PROPULSION OF ELECTRICALLY-PROPELLED VEHICLES; SUPPLYING ELECTRIC POWER FOR AUXILIARY EQUIPMENT OF ELECTRICALLY-PROPELLED VEHICLES; ELECTRODYNAMIC BRAKE SYSTEMS FOR VEHICLES IN GENERAL; MAGNETIC SUSPENSION OR LEVITATION FOR VEHICLES; MONITORING OPERATING VARIABLES OF ELECTRICALLY-PROPELLED VEHICLES; ELECTRIC SAFETY DEVICES FOR ELECTRICALLY-PROPELLED VEHICLES
- B60L50/00—Electric propulsion with power supplied within the vehicle
- B60L50/50—Electric propulsion with power supplied within the vehicle using propulsion power supplied by batteries or fuel cells
- B60L50/60—Electric propulsion with power supplied within the vehicle using propulsion power supplied by batteries or fuel cells using power supplied by batteries
- B60L50/64—Constructional details of batteries specially adapted for electric vehicles
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B60—VEHICLES IN GENERAL
- B60L—PROPULSION OF ELECTRICALLY-PROPELLED VEHICLES; SUPPLYING ELECTRIC POWER FOR AUXILIARY EQUIPMENT OF ELECTRICALLY-PROPELLED VEHICLES; ELECTRODYNAMIC BRAKE SYSTEMS FOR VEHICLES IN GENERAL; MAGNETIC SUSPENSION OR LEVITATION FOR VEHICLES; MONITORING OPERATING VARIABLES OF ELECTRICALLY-PROPELLED VEHICLES; ELECTRIC SAFETY DEVICES FOR ELECTRICALLY-PROPELLED VEHICLES
- B60L58/00—Methods or circuit arrangements for monitoring or controlling batteries or fuel cells, specially adapted for electric vehicles
- B60L58/10—Methods or circuit arrangements for monitoring or controlling batteries or fuel cells, specially adapted for electric vehicles for monitoring or controlling batteries
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/04—Construction or manufacture in general
- H01M10/0431—Cells with wound or folded electrodes
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/052—Li-accumulators
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/056—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes
- H01M10/0564—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes the electrolyte being constituted of organic materials only
- H01M10/0566—Liquid materials
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/42—Methods or arrangements for servicing or maintenance of secondary cells or secondary half-cells
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/362—Composites
- H01M4/366—Composites as layered products
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/58—Selection of substances as active materials, active masses, active liquids of inorganic compounds other than oxides or hydroxides, e.g. sulfides, selenides, tellurides, halogenides or LiCoFy; of polyanionic structures, e.g. phosphates, silicates or borates
- H01M4/583—Carbonaceous material, e.g. graphite-intercalation compounds or CFx
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/58—Selection of substances as active materials, active masses, active liquids of inorganic compounds other than oxides or hydroxides, e.g. sulfides, selenides, tellurides, halogenides or LiCoFy; of polyanionic structures, e.g. phosphates, silicates or borates
- H01M4/583—Carbonaceous material, e.g. graphite-intercalation compounds or CFx
- H01M4/587—Carbonaceous material, e.g. graphite-intercalation compounds or CFx for inserting or intercalating light metals
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/60—Selection of substances as active materials, active masses, active liquids of organic compounds
- H01M4/602—Polymers
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M2004/021—Physical characteristics, e.g. porosity, surface area
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M2004/026—Electrodes composed of, or comprising, active material characterised by the polarity
- H01M2004/028—Positive electrodes
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/10—Energy storage using batteries
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P70/00—Climate change mitigation technologies in the production process for final industrial or consumer products
- Y02P70/50—Manufacturing or production processes characterised by the final manufactured product
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02T—CLIMATE CHANGE MITIGATION TECHNOLOGIES RELATED TO TRANSPORTATION
- Y02T10/00—Road transport of goods or passengers
- Y02T10/60—Other road transportation technologies with climate change mitigation effect
- Y02T10/70—Energy storage systems for electromobility, e.g. batteries
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S903/00—Hybrid electric vehicles, HEVS
- Y10S903/902—Prime movers comprising electrical and internal combustion motors
- Y10S903/903—Prime movers comprising electrical and internal combustion motors having energy storing means, e.g. battery, capacitor
Definitions
- a variety of electronic devices such as mobile phones and personal digital assistants (PDAs) are widely used, and there is a demand for further downsizing, weight reduction, and longer life of the electronic devices. Accordingly, as a power source, development of a battery, in particular, a secondary battery that is small and lightweight and capable of obtaining a high energy density is in progress.
- the secondary battery includes an electrolyte solution together with a positive electrode and a negative electrode, and the positive electrode includes an active material (positive electrode active material) related to an electrode reaction (charge / discharge reaction).
- an active material positive electrode active material
- a lithium compound such as LiCoO 2
- a layered rock salt type crystal structure or a lithium compound (such as LiMn 2 O 4 ) having a spinel type crystal structure is widely used.
- polyanionic compounds more specifically lithium compounds having an olivine type crystal structure (such as LiFePO 4 ) are also used (see, for example, Patent Document 1).
- particles including a core and a coating are synthesized in the presence of a carbon source (conductive carbon) (see, for example, Patent Document 9).
- This core is composed of Li x M 1-y M ′ y (XO 4 ) n (M is a transition metal element, M ′ is Mg 2+ , X is P, etc., 0 ⁇ x ⁇ 2, 0 ⁇ y ⁇ 0.6 1 ⁇ n ⁇ 1.5).
- Y may or may not be included as a constituent element in the polyanionic compound, but is preferably included. This is because the structural stability of the active material 10 and the operating potential of the secondary battery are improved.
- the type of Y is not particularly limited as long as it is a halogen element, or more specifically, any one or more of group 17 elements in the long-period periodic table.
- the halogen element is, for example, one or more of fluorine (F), chlorine (Cl), bromine (Br), and iodine (I), and among these, F is preferable. This is because F has a particularly high electronegativity, and thus a higher effect can be obtained.
- the type of Z is not particularly limited as long as it is any one or more of Fe, Co, and Ni, but among them, Fe is more preferable. This is because the distortion of Mn crystals due to the electronic structure change during the oxidation-reduction reaction (divalent / trivalent) is alleviated. Moreover, since the redox reaction (bivalent / trivalent) of Fe itself also occurs, a higher battery capacity can be obtained.
- f is not particularly limited as long as 0 ⁇ f ⁇ 1 is satisfied, but it is preferable that 0.5 ⁇ f ⁇ 0.9 is satisfied. This is because a high energy density can be obtained and the oxidation-reduction reaction is stabilized, so that both advantages can be achieved.
- coated carbon material 4 is provided on the surface of the active material particles 1 is to prevent the surface of the highly reactive active material particles 1 from being exposed. Thereby, decomposition reactions, such as electrolyte solution, on the surface of the active material particles 1 are suppressed.
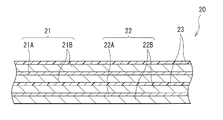
- This secondary battery includes, for example, a pair of insulating plates 12 and 13 and a wound electrode body 20 in a hollow cylindrical battery can 11.
- the wound electrode body 20 is wound, for example, after the positive electrode 21 and the negative electrode 22 are laminated via the separator 23.
- the battery can 11 has, for example, a hollow structure in which one end is closed and the other end is opened.
- the battery can 11 is formed of one or more of iron, aluminum, and alloys thereof. Has been. Nickel or the like may be plated on the surface of the battery can 11.
- the pair of insulating plates 12 and 13 are disposed so as to sandwich the wound electrode body 20, and extend perpendicular to the winding peripheral surface of the wound electrode body 20.
- LiNi 1-z M z O 2 (4) (M is Co, Mn, Fe, Al, V, Sn, Mg, Ti, Sr, Ca, Zr, Mo, Tc, Ru, Ta, W, Re, Yb, Cu, Zn, Ba, B, Cr, Si , Ga, P, Sb, and Nb, z satisfies 0.005 ⁇ z ⁇ 0.5.)
- Si alloys and Si compounds include SiB 4 , SiB 6 , Mg 2 Si, Ni 2 Si, TiSi 2 , MoSi 2 , CoSi 2 , NiSi 2 , CaSi 2 , CrSi 2 , Cu 5 Si, FeSi 2. , MnSi 2 , NbSi 2 , TaSi 2 , VSi 2 , WSi 2 , ZnSi 2 , SiC, Si 3 N 4 , Si 2 N 2 O, SiO v (0 ⁇ v ⁇ 2), and LiSiO. It should be noted, v in SiO v may be 0.2 ⁇ v ⁇ 1.4.
- a vacuum deposition method for example, a vacuum deposition method, a sputtering method, an ion plating method, a laser ablation method, a thermal chemical vapor deposition, a chemical vapor deposition (CVD) method, and a plasma chemical vapor deposition method.
- the liquid phase method include an electrolytic plating method and an electroless plating method.
- the thermal spraying method is a method of spraying a molten or semi-molten negative electrode active material onto the negative electrode current collector 22A.
- the firing method is, for example, a method in which a mixture dispersed in a solvent is applied to the negative electrode current collector 22A using a coating method and then heat-treated at a temperature higher than the melting point of the negative electrode binder or the like.
- an atmosphere firing method, a reaction firing method, a hot press firing method, or the like can be used.
- This secondary battery is manufactured by the following procedure, for example.
- the negative electrode 22 is produced by the same procedure as that of the positive electrode 21 described above.
- a negative electrode mixture in which a negative electrode active material, a negative electrode binder, a negative electrode conductive agent, and the like are mixed is dispersed in an organic solvent or the like to obtain a paste-like negative electrode mixture slurry.
- the negative electrode mixture slurry is applied to both surfaces of the negative electrode current collector 22A and then dried to form the negative electrode active material layer 22B, and then the negative electrode active material layer 22B is compression molded.
- the positive electrode 33 and the negative electrode 34 are manufactured by the same manufacturing procedure as that of the positive electrode 21 and the negative electrode 22.
- the positive electrode active material layer 33B is formed on both surfaces of the positive electrode current collector 33A to produce the positive electrode 33
- the negative electrode active material layer 34B is formed on both surfaces of the negative electrode current collector 34A to produce the negative electrode 34.
- the precursor solution is applied to the positive electrode 33 and the negative electrode 34 to form a gel electrolyte layer 36.
- a secondary battery which is a power storage source
- An electric power tool is a tool in which a movable part (for example, a drill etc.) moves, using a secondary battery as a driving power source.
- An electronic device is a device that exhibits various functions using a secondary battery as a driving power source (power supply source).
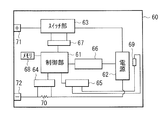
- the control unit 61 controls the operation of the entire battery pack (including the usage state of the power supply 62), and includes, for example, a central processing unit (CPU).
- the power source 62 includes one or more secondary batteries (not shown).
- the power source 62 is, for example, an assembled battery including two or more secondary batteries, and the connection form of these secondary batteries may be in series, in parallel, or a mixture of both.
- the power source 62 includes six secondary batteries connected in two parallel three series.
- the switch unit 63 switches the usage state of the power source 62 (whether or not the power source 62 can be connected to an external device) according to an instruction from the control unit 61.
- the switch unit 63 includes, for example, a charge control switch, a discharge control switch, a charging diode, a discharging diode (all not shown), and the like.
- the charge control switch and the discharge control switch are semiconductor switches such as a field effect transistor (MOSFET) using a metal oxide semiconductor, for example.
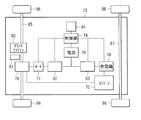
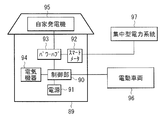
- the power source 91 is connected to, for example, an electric device 94 installed inside the house 89 and can be connected to an electric vehicle 96 stopped outside the house 89.
- the power source 91 is connected to, for example, a private generator 95 installed in a house 89 via a power hub 93 and can be connected to an external centralized power system 97 via the smart meter 92 and the power hub 93. It has become.
- the control unit 90 controls the operation of the entire power storage system (including the usage state of the power supply 91), and includes, for example, a CPU.
- the power source 91 includes one or more secondary batteries (not shown).
- the smart meter 92 is, for example, a network-compatible power meter installed in a house 89 on the power demand side, and can communicate with the power supply side. Accordingly, for example, the smart meter 92 enables efficient and stable energy supply by controlling the balance between supply and demand in the house 89 while communicating with the outside.
- the carbon source material was added to the slurry and stirred.
- the types and mixing amounts (carbon source amount: g) of the carbon source material are as shown in Table 1.
- the slurry was put into a vessel of a bead mill and wet pulverized.
- the appropriate ranges derived from the results of the examples are explained for the respective ranges of the ratio B / A and the ratio D / C.
- the explanation does not completely deny the possibility that the ratio B / A and the ratio D / C are out of the above-mentioned range. That is, the appropriate range described above is a particularly preferable range for obtaining the effect of the present technology. Therefore, if the effect of the present technology can be obtained, the ratio B / A and the ratio D / C are slightly higher than the above range. You may come off.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Inorganic Chemistry (AREA)
- Manufacturing & Machinery (AREA)
- Composite Materials (AREA)
- Power Engineering (AREA)
- Sustainable Energy (AREA)
- Sustainable Development (AREA)
- Transportation (AREA)
- Mechanical Engineering (AREA)
- Life Sciences & Earth Sciences (AREA)
- Crystallography & Structural Chemistry (AREA)
- Physics & Mathematics (AREA)
- Condensed Matter Physics & Semiconductors (AREA)
- General Physics & Mathematics (AREA)
- Battery Electrode And Active Subsutance (AREA)
- Battery Mounting, Suspending (AREA)
- Secondary Cells (AREA)
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US14/773,661 US9825296B2 (en) | 2013-03-12 | 2014-03-05 | Secondary battery-use active material, secondary battery-use electrode, secondary battery, battery pack, electric vehicle, electric power storage system, electric power tool, and electronic apparatus |
| CN201480012248.8A CN105027337B (zh) | 2013-03-12 | 2014-03-05 | 二次电池用活性物质、二次电池用电极、二次电池、电池组、电动车辆、电力储存系统、电动工具以及电子设备 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2013-048881 | 2013-03-12 | ||
| JP2013048881A JP6028630B2 (ja) | 2013-03-12 | 2013-03-12 | 二次電池用活物質、二次電池用電極、二次電池、電池パック、電動車両、電力貯蔵システム、電動工具および電子機器 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2014141960A1 true WO2014141960A1 (ja) | 2014-09-18 |
Family
ID=51536626
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2014/055573 Ceased WO2014141960A1 (ja) | 2013-03-12 | 2014-03-05 | 二次電池用活物質、二次電池用電極、二次電池、電池パック、電動車両、電力貯蔵システム、電動工具および電子機器 |
Country Status (4)
| Country | Link |
|---|---|
| US (1) | US9825296B2 (enExample) |
| JP (1) | JP6028630B2 (enExample) |
| CN (1) | CN105027337B (enExample) |
| WO (1) | WO2014141960A1 (enExample) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN105914346A (zh) * | 2015-02-20 | 2016-08-31 | 丰田自动车株式会社 | 非水电解质二次电池及其制造方法 |
| EP3145001A1 (en) * | 2015-09-16 | 2017-03-22 | Samsung Electronics Co., Ltd. | Electrode active material, electrode and secondary battery including the same, and method of preparing the electrode active material |
Families Citing this family (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016105366A (ja) * | 2014-12-01 | 2016-06-09 | ソニー株式会社 | 二次電池用活物質、二次電池用電極、二次電池、電池パック、電動車両、電力貯蔵システム、電動工具および電子機器 |
| CN104577115A (zh) * | 2014-12-26 | 2015-04-29 | 青海时代新能源科技有限公司 | 一种锂离子电池正极材料、其制备方法及应用 |
| JP6853616B2 (ja) * | 2015-03-24 | 2021-03-31 | 株式会社東芝 | 非水電解質二次電池用電極、非水電解質二次電池、及び電池パック |
| JP6665551B2 (ja) * | 2016-01-22 | 2020-03-13 | 株式会社Gsユアサ | バッテリ装置および二次電池の不正使用判断方法 |
| HUE068831T2 (hu) * | 2019-05-30 | 2025-01-28 | Acondicionamiento Tarrasense | Funkcionalizált lítium anód akkumulátorokhoz |
| CN115380403A (zh) | 2021-03-19 | 2022-11-22 | 积水化学工业株式会社 | 非水电解质二次电池用正极、以及使用了该正极的非水电解质二次电池、电池模块和电池系统 |
| CN115377358A (zh) * | 2022-09-29 | 2022-11-22 | 欣旺达电动汽车电池有限公司 | 二次电池和用电设备 |
| CN115528296B (zh) * | 2022-09-29 | 2023-12-29 | 欣旺达动力科技股份有限公司 | 一种二次电池 |
| CN118367139A (zh) * | 2023-01-18 | 2024-07-19 | 宁德时代新能源科技股份有限公司 | 正极活性材料、及其制备方法、正极极片、二次电池、电池模块、电池包和用电装置 |
| CN118117082B (zh) * | 2024-04-30 | 2024-09-13 | 浙江锂宸新材料科技有限公司 | 提高氧化亚硅包碳利用率的方法及其产品与应用 |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2009075289A1 (ja) * | 2007-12-10 | 2009-06-18 | Sumitomo Osaka Cement Co., Ltd. | 電極材料およびその製造方法、並びに、電極および電池 |
| JP2010218884A (ja) * | 2009-03-17 | 2010-09-30 | Nippon Chem Ind Co Ltd | リチウムリン系複合酸化物炭素複合体、その製造方法、リチウム二次電池用正極活物質及びリチウム二次電池 |
| JP2012104290A (ja) * | 2010-11-08 | 2012-05-31 | Sony Corp | 非水電解質電池用正極活物質、非水電解質電池用正極および非水電解質電池 |
| WO2013005705A1 (ja) * | 2011-07-04 | 2013-01-10 | 昭栄化学工業株式会社 | リチウムイオン二次電池用の正極材料、正極部材、リチウムイオン二次電池及び前記正極材料の製造方法 |
| JP2013048053A (ja) * | 2011-08-29 | 2013-03-07 | Sony Corp | 活物質、電極、二次電池、電池パック、電動車両、電力貯蔵システム、電動工具および電子機器 |
Family Cites Families (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5910382A (en) | 1996-04-23 | 1999-06-08 | Board Of Regents, University Of Texas Systems | Cathode materials for secondary (rechargeable) lithium batteries |
| JP4768901B2 (ja) | 1999-06-03 | 2011-09-07 | チタン工業株式会社 | リチウムチタン複合酸化物及びその製造方法、並びにその用途 |
| JP2001110414A (ja) | 1999-10-04 | 2001-04-20 | Nippon Telegr & Teleph Corp <Ntt> | リチウム二次電池正極活物質およびリチウム二次電池 |
| CA2320661A1 (fr) | 2000-09-26 | 2002-03-26 | Hydro-Quebec | Nouveau procede de synthese de materiaux limpo4 a structure olivine |
| JP3921931B2 (ja) | 2000-09-29 | 2007-05-30 | ソニー株式会社 | 正極活物質及び非水電解質電池 |
| JP2002313320A (ja) | 2001-04-09 | 2002-10-25 | Asahi Kasei Corp | 非水系二次電池 |
| JP4075451B2 (ja) | 2001-05-15 | 2008-04-16 | 株式会社豊田中央研究所 | リチウム二次電池 |
| JP4953525B2 (ja) | 2001-07-23 | 2012-06-13 | パナソニック株式会社 | 非水電解質二次電池およびその製造法 |
| JP4310942B2 (ja) | 2001-07-24 | 2009-08-12 | 新神戸電機株式会社 | 非水電解液リチウム二次電池 |
| JP2003123764A (ja) | 2001-10-09 | 2003-04-25 | Hitachi Maxell Ltd | 非水二次電池 |
| JP4190912B2 (ja) | 2003-02-24 | 2008-12-03 | 住友大阪セメント株式会社 | リチウムイオン電池用正極活物質及びそれを有するリチウムイオン電池 |
| JP4527423B2 (ja) | 2004-03-18 | 2010-08-18 | 大日本印刷株式会社 | 活物質層用塗工組成物、非水電解液二次電池用電極板、及び非水電解液二次電池 |
| JP5235307B2 (ja) | 2007-01-23 | 2013-07-10 | 三洋電機株式会社 | 非水電解質二次電池 |
| JP5293936B2 (ja) | 2007-05-21 | 2013-09-18 | 戸田工業株式会社 | 非水電解質二次電池用オリビン型複合酸化物及びその製造方法、並びに二次電池 |
| CN101980956A (zh) | 2008-03-31 | 2011-02-23 | 户田工业株式会社 | 磷酸铁锂颗粒粉末的制造方法、橄榄石型结构的磷酸铁锂颗粒粉末、使用该磷酸铁锂颗粒粉末的正极材料片和非水溶剂类二次电池 |
| JP5334506B2 (ja) | 2008-09-08 | 2013-11-06 | 花王株式会社 | 非水電解質二次電池の正極用組成物の製造方法 |
| CN102067362B (zh) | 2008-12-05 | 2013-12-25 | Jx日矿日石金属株式会社 | 锂离子二次电池用正极活性物质、使用其的二次电池用正极及使用该正极的锂离子二次电池 |
| JP5784292B2 (ja) | 2010-09-13 | 2015-09-24 | 三井金属鉱業株式会社 | マグネタイト粒子 |
| JP5772197B2 (ja) | 2011-05-09 | 2015-09-02 | ソニー株式会社 | リチウムイオン二次電池用活物質、リチウムイオン二次電池用電極、リチウムイオン二次電池、電子機器、電動工具、電動車両および電力貯蔵システム |
| JP6011888B2 (ja) * | 2012-08-01 | 2016-10-25 | トヨタ自動車株式会社 | 非水電解液二次電池 |
-
2013
- 2013-03-12 JP JP2013048881A patent/JP6028630B2/ja active Active
-
2014
- 2014-03-05 WO PCT/JP2014/055573 patent/WO2014141960A1/ja not_active Ceased
- 2014-03-05 CN CN201480012248.8A patent/CN105027337B/zh active Active
- 2014-03-05 US US14/773,661 patent/US9825296B2/en active Active
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2009075289A1 (ja) * | 2007-12-10 | 2009-06-18 | Sumitomo Osaka Cement Co., Ltd. | 電極材料およびその製造方法、並びに、電極および電池 |
| JP2010218884A (ja) * | 2009-03-17 | 2010-09-30 | Nippon Chem Ind Co Ltd | リチウムリン系複合酸化物炭素複合体、その製造方法、リチウム二次電池用正極活物質及びリチウム二次電池 |
| JP2012104290A (ja) * | 2010-11-08 | 2012-05-31 | Sony Corp | 非水電解質電池用正極活物質、非水電解質電池用正極および非水電解質電池 |
| WO2013005705A1 (ja) * | 2011-07-04 | 2013-01-10 | 昭栄化学工業株式会社 | リチウムイオン二次電池用の正極材料、正極部材、リチウムイオン二次電池及び前記正極材料の製造方法 |
| JP2013048053A (ja) * | 2011-08-29 | 2013-03-07 | Sony Corp | 活物質、電極、二次電池、電池パック、電動車両、電力貯蔵システム、電動工具および電子機器 |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN105914346A (zh) * | 2015-02-20 | 2016-08-31 | 丰田自动车株式会社 | 非水电解质二次电池及其制造方法 |
| CN105914346B (zh) * | 2015-02-20 | 2019-05-03 | 丰田自动车株式会社 | 非水电解质二次电池及其制造方法 |
| US10431814B2 (en) | 2015-02-20 | 2019-10-01 | Toyota Jidosha Kabushiki Kaisha | Non-aqueous electrolyte secondary battery and method for manufacturing the same |
| EP3145001A1 (en) * | 2015-09-16 | 2017-03-22 | Samsung Electronics Co., Ltd. | Electrode active material, electrode and secondary battery including the same, and method of preparing the electrode active material |
| US10516157B2 (en) | 2015-09-16 | 2019-12-24 | Samsung Electronics Co., Ltd. | Electrode active material, electrode and secondary battery including the same, and method of preparing the electrode active material |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2014175238A (ja) | 2014-09-22 |
| CN105027337B (zh) | 2018-05-04 |
| US20160043398A1 (en) | 2016-02-11 |
| US9825296B2 (en) | 2017-11-21 |
| CN105027337A (zh) | 2015-11-04 |
| JP6028630B2 (ja) | 2016-11-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6028630B2 (ja) | 二次電池用活物質、二次電池用電極、二次電池、電池パック、電動車両、電力貯蔵システム、電動工具および電子機器 | |
| JP6135253B2 (ja) | 電極、リチウム二次電池、電池パック、電動車両、電力貯蔵システム、電動工具および電子機器 | |
| KR101988071B1 (ko) | 정극 활물질, 정극, 이차 전지, 전지 팩, 전동 차량, 전력 저장 시스템, 전동 공구 및 전자 기기 | |
| JP6136612B2 (ja) | リチウムイオン二次電池用電極、リチウムイオン二次電池、電池パック、電動車両、電力貯蔵システム、電動工具および電子機器 | |
| JP5924237B2 (ja) | リチウムイオン二次電池用活物質、リチウムイオン二次電池用電極、リチウムイオン二次電池、電池パック、電動車両、電力貯蔵システム、電動工具および電子機器 | |
| JP6809487B2 (ja) | リチウムイオン二次電池用正極活物質、リチウムイオン二次電池用正極、リチウムイオン二次電池、電池パック、電動車両、電力貯蔵システム、電動工具および電子機器 | |
| JP6249388B2 (ja) | リチウムイオン二次電池用正極、リチウムイオン二次電池、電池パック、電動車両、電力貯蔵システム、電動工具ならびに電子機器 | |
| JP2013048053A (ja) | 活物質、電極、二次電池、電池パック、電動車両、電力貯蔵システム、電動工具および電子機器 | |
| JP2013218875A (ja) | 正極活物質、正極、二次電池、電池パック、電動車両、電力貯蔵システム、電動工具および電子機器 | |
| JP6179372B2 (ja) | リチウムイオン二次電池用活物質、リチウムイオン二次電池用電極、リチウムイオン二次電池、電池パック、電動車両、電力貯蔵システム、電動工具および電子機器 | |
| WO2016009794A1 (ja) | 二次電池用負極活物質、二次電池用負極、二次電池、電池パック、電動車両、電力貯蔵システム、電動工具および電子機器 | |
| JP2013048061A (ja) | 二次電池、電池パック、電動車両、電力貯蔵システム、電動工具および電子機器 | |
| JP2014112476A (ja) | 二次電池用活物質、二次電池用電極、二次電池、電池パック、電動車両、電力貯蔵システム、電動工具および電子機器 | |
| JP2014103032A (ja) | 二次電池用活物質、二次電池用電極、二次電池、電池パック、電動車両、電力貯蔵システム、電動工具および電子機器 | |
| JP6596815B2 (ja) | 二次電池用活物質、二次電池用電極、二次電池、電動車両および電子機器 | |
| JP2017191662A (ja) | 二次電池、電池パック、電動車両、電力貯蔵システム、電動工具及び電子機器 | |
| WO2015037353A1 (ja) | 二次電池用負極、二次電池、電池パック、電動車両、電力貯蔵システム、電動工具および電子機器 | |
| JP2017130476A (ja) | リチウムイオン二次電池用電極、リチウムイオン二次電池、電池パック、電動車両、電力貯蔵システム、電動工具および電子機器 | |
| JPWO2017159073A1 (ja) | 二次電池用負極、二次電池、電池パック、電動車両、電力貯蔵システム、電動工具および電子機器 | |
| WO2016088471A1 (ja) | 二次電池用活物質、二次電池用電極、二次電池、電池パック、電動車両、電力貯蔵システム、電動工具および電子機器 | |
| JP2013218787A (ja) | 正極活物質、正極、二次電池、電池パック、電動車両、電力貯蔵システム、電動工具および電子機器 | |
| WO2016059861A1 (ja) | 二次電池、電池パック、電動車両、電力貯蔵システム、電動工具および電子機器 | |
| JP2015156280A (ja) | 二次電池用活物質、二次電池用電極、二次電池、電池パック、電動車両、電力貯蔵システム、電動工具および電子機器 | |
| JP2014017071A (ja) | 二次電池、電池パック、電動車両、電力貯蔵システム、電動工具および電子機器 | |
| WO2015194314A1 (ja) | 二次電池用活物質、二次電池用電極、二次電池、電池パック、電動車両、電力貯蔵システム、電動工具および電子機器 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 201480012248.8 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 14765230 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 14773661 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 14765230 Country of ref document: EP Kind code of ref document: A1 |