WO2013146817A1 - 吸収性物品 - Google Patents
吸収性物品 Download PDFInfo
- Publication number
- WO2013146817A1 WO2013146817A1 PCT/JP2013/058859 JP2013058859W WO2013146817A1 WO 2013146817 A1 WO2013146817 A1 WO 2013146817A1 JP 2013058859 W JP2013058859 W JP 2013058859W WO 2013146817 A1 WO2013146817 A1 WO 2013146817A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- absorbent article
- chain hydrocarbon
- acid
- blood
- hydrocarbon moiety
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/42—Use of materials characterised by their function or physical properties
- A61L15/46—Deodorants or malodour counteractants, e.g. to inhibit the formation of ammonia or bacteria
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/15—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators
- A61F13/45—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators characterised by the shape
- A61F13/47—Sanitary towels, incontinence pads or napkins
- A61F13/4704—Sanitary towels, incontinence pads or napkins having preferential bending zones, e.g. fold lines or grooves
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/15—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators
- A61F13/45—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators characterised by the shape
- A61F13/47—Sanitary towels, incontinence pads or napkins
- A61F13/472—Sanitary towels, incontinence pads or napkins specially adapted for female use
- A61F13/47218—Sanitary towels, incontinence pads or napkins specially adapted for female use with a raised crotch region, e.g. hump
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/15—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators
- A61F13/45—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators characterised by the shape
- A61F13/47—Sanitary towels, incontinence pads or napkins
- A61F13/472—Sanitary towels, incontinence pads or napkins specially adapted for female use
- A61F13/47218—Sanitary towels, incontinence pads or napkins specially adapted for female use with a raised crotch region, e.g. hump
- A61F13/47227—Sanitary towels, incontinence pads or napkins specially adapted for female use with a raised crotch region, e.g. hump for interlabial use
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/15—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators
- A61F13/51—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators characterised by the outer layers of the pads
- A61F13/511—Topsheet, i.e. the permeable cover or layer facing the skin
- A61F13/51104—Topsheet, i.e. the permeable cover or layer facing the skin the top sheet having a three-dimensional cross-section, e.g. corrugations, embossments, recesses or projections
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/15—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators
- A61F13/51—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators characterised by the outer layers of the pads
- A61F13/515—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators characterised by the outer layers of the pads characterised by the interconnection of the topsheet and the backsheet
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/15—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators
- A61F13/53—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators characterised by the absorbing medium
- A61F13/534—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators characterised by the absorbing medium having an inhomogeneous composition through the thickness of the pad
- A61F13/535—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators characterised by the absorbing medium having an inhomogeneous composition through the thickness of the pad inhomogeneous in the plane of the pad, e.g. core absorbent layers being of different sizes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/22—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons containing macromolecular materials
- A61L15/28—Polysaccharides or their derivatives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L15/00—Chemical aspects of, or use of materials for, bandages, dressings or absorbent pads
- A61L15/16—Bandages, dressings or absorbent pads for physiological fluids such as urine or blood, e.g. sanitary towels, tampons
- A61L15/42—Use of materials characterised by their function or physical properties
- A61L15/60—Liquid-swellable gel-forming materials, e.g. super-absorbents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/15—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators
- A61F13/15203—Properties of the article, e.g. stiffness or absorbency
- A61F2013/15284—Properties of the article, e.g. stiffness or absorbency characterized by quantifiable properties
- A61F2013/15292—Resistance, i.e. modulus or strength
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/15—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators
- A61F13/15203—Properties of the article, e.g. stiffness or absorbency
- A61F2013/15284—Properties of the article, e.g. stiffness or absorbency characterized by quantifiable properties
- A61F2013/15406—Basis weight
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/15—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators
- A61F13/51—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators characterised by the outer layers of the pads
- A61F2013/51078—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators characterised by the outer layers of the pads being embossed
- A61F2013/5108—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators characterised by the outer layers of the pads being embossed in grids
Definitions
- the present invention relates to an absorbent article.
- a top sheet has a protrusion having cushioning properties on the skin contact surface for the purpose of fitting to the wearer's body.
- the top sheet has a protrusion on the skin contact surface, so that even when the wearer moves the body, the protrusion is expected to fit the wearer's body and hardly leak. ing.
- Patent Document 1 describes an absorbent article that is intended to provide an absorbent article that is excellent in leakage prevention performance and has a good wearing feeling.
- the absorbent article described in Patent Document 1 has the following configuration: a liquid impermeable leak-proof layer and a substance that includes a liquid-retaining absorbent layer disposed on the skin contact surface side of the leak-proof layer.
- the absorbent article in the vertically long absorbent article, the absorbent article is concavely curved toward the skin contact surface side in the longitudinal direction, and the side part of the excretory part contact position becomes narrower in the central direction in plan view.
- the three-dimensional shape is preferably a central three-dimensional absorption portion 7 made of an absorbent member, and specifically, formed from pulp or the like.
- the convex curved three-dimensional part includes pulp
- the bulk is reduced, and the compressive force is applied from the width direction.
- the article is deformed into a shape that protrudes excessively toward the wearer's skin, and the absorbent article is distorted.
- leakage of menstrual blood, uncomfortable feeling at the time of wearing, and the like are likely to occur. Therefore, the present disclosure continues to fit the excretion opening of the wearer having a complicated shape in both the dry state before absorbing menstrual blood and the wet state after absorbing menstrual blood,
- An object of the present invention is to provide an absorbent article that can be reduced in size because it is difficult to leak.
- a liquid-permeable top sheet, a liquid-impermeable back sheet, the liquid-permeable top sheet, and a liquid-impermeable back sheet An absorbent article including the absorbent article, wherein the absorbent article has a middle-high part protruding in the thickness direction of the absorbent article in the excretory opening contact area, and the middle-high part is the above-mentioned Including a part of the top sheet and a cushion part disposed between the top sheet and the absorber, and having a central part and an outer peripheral part surrounding the central part.
- the middle-high portion has a compression work WC of 10 to 50 (N ⁇ m / m 2 ) and a compression resilience RC of 35 to 100% in the central portion;
- the absorber is 80-230 mm,
- the absorbent article of the present disclosure fits the excretion opening of a wearer having a complex shape in both the dry state before absorbing menstrual blood and the wet state after absorbing menstrual blood. Since it is difficult to leak, it can be reduced in size.
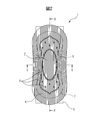
- FIG. 1 is a perspective view of an absorbent article according to one of the embodiments of the present disclosure.
- FIG. 2 is a front view of the absorbent article 1 shown in FIG.
- FIG. 3 is a rear view of the absorbent article 1 shown in FIG.
- FIG. 4 is an end view taken along the line XX of FIG.
- FIG. 5 is an end view of the YY end surface of FIG.
- FIG. 6 is a schematic view showing an example of an apparatus for measuring the compressive force.
- FIG. 7 is a diagram illustrating an example of the shape of the cushion portion before being incorporated into the absorbent article.
- FIG. 8 is an electron micrograph of the skin contact surface of the top sheet in a sanitary napkin in which the top sheet contains tri-C2L oil fatty acid glycerides.
- FIG. 9 is a photomicrograph of menstrual blood with or without a blood modifying agent.
- FIG. 10 is a diagram for explaining a method of measuring the surface tension.
- FIG. 1 is a perspective view of an absorbent article according to one of the embodiments of the present disclosure.
- the absorbent article 1 shown in FIG. 1 is a sanitary napkin.
- the right back corresponds to the front of the wearer
- the left front corresponds to the rear of the wearer, but the absorbent article 1 shown in FIG.
- it has a symmetrical shape in the front-rear direction and the left-right symmetry.
- the absorbent article 1 shown in FIG. 1 includes a liquid-permeable top sheet 4, a liquid-impermeable back sheet (not shown), a liquid-permeable top sheet 4 and a liquid-impermeable back sheet. And an absorbent body 7 therebetween.
- FIG. 1 also shows the pressing part 3 and the peeling part 5 fixed to the back sheet.
- the absorbent article 1 shown in FIG. 1 has a middle-high part 2 that protrudes in the thickness direction of the absorbent article 1 in the excretory opening contact area.
- the top sheet 4 has a plurality of ridge groove structures extending in the longitudinal direction on the skin contact surface.
- the boundary is indicated by a solid line.
- a wide region surrounded by two solid lines means a buttock, and a narrow region surrounded by two solid lines means a groove
- a plurality of flanges and a plurality of grooves are alternately arranged in the width direction of the absorbent article 1.
- the direction orthogonal to the longitudinal direction of the absorbent article may be referred to as the “width direction”.
- the “middle / high portion” means a portion including a part of the top sheet and the cushion portion, and in the middle / high portion, the thickness is generally reduced from the top portion toward the outer edge.
- the “central portion” regarding the middle-high portion is preferably 0 to about 50% of the distance from the top of the middle-high portion to the outer edge of the middle-high portion in the front view of the absorbent article as shown in FIG. More preferably, it means 0 to about 40%, and even more preferably 0 to about 30% of the area, and the remaining area means the “periphery” of the middle and high parts.
- FIG. 2 is a front view of the absorbent article 1 shown in FIG. 1 and is a view observed from the skin contact surface side of the top sheet 4.
- the upper side corresponds to the front of the wearer and the lower side corresponds to the rear of the wearer.
- the absorbent article 1 shown in FIG. 2 includes a liquid-permeable top sheet 4, a liquid-impermeable back sheet (not shown), a liquid-permeable top sheet 4 and a liquid-impermeable back sheet. And an absorbent body 7 therebetween.
- the absorbent article 1 shown in FIG. 2 also has a middle-high part 2 projecting in the thickness direction of the absorbent article 1 in the excretory opening contact area, particularly in the labia contact area.
- the middle-high part 2 includes a part of the top sheet 4 and a cushion part 6 disposed between the top sheet 4 and the absorber 7.
- FIG. 2 also shows the pressing part 3 and the peeling part 5 fixed to the back sheet.
- FIG. 3 is a rear view of the absorbent article 1 shown in FIG. 1 and is a view observed from the clothing contact surface side of the back sheet 8.
- the upper side corresponds to the front of the wearer and the lower side corresponds to the rear of the wearer.
- the fixing portion 9 is coated on the clothing contact surface of the liquid-impermeable back sheet 8, and the peeling portion 5 is temporarily fixed to the fixing portion 9. .
- a wearer can peel the peeling part 5 from the fixing
- FIG. 4 is an end view taken along the line XX in FIG.
- a liquid-impermeable back sheet 8 an absorbent body 7, and a liquid-permeable top sheet 4 are laminated in order from the bottom, and in the excretory opening contact area, the cushion portion 6 is disposed between the absorber 7 and the liquid-permeable top sheet 4.
- the middle-high part 2 includes a part of the top sheet 4 and the cushion part 6, and the absorbent article 1 is more specifically near the outer edge of the cushion part 6.
- the cushion part 6 is formed from an air-through nonwoven fabric and has a maximum thickness of about 15 mm.
- the density of the cushion portion 6 in the outer peripheral portion 12 is higher than the density of the cushion portion 6 in the central portion 11.
- FIG. 5 is an end view of the YY end face of FIG.
- the absorbent article 1 shown in FIG. 5 has the pressing part 3 formed by pressing the top sheet 4 and the absorber 7 in the vicinity of the outer edge of the cushion part 6, more specifically, outside the cushion part 6.
- the top sheet 4 has a plurality of groove structures on its skin contact surface.
- the density of the cushion portion 6 in the outer peripheral portion 12 is higher than the density of the cushion portion 6 in the central portion 11.
- FIG. 5 shows the elastic member 13.
- the density of the cushion portion 6 in the outer peripheral portion 12 is higher than the density of the cushion portion 6 in the central portion 11.
- the density of the cushion part in the outer peripheral part is the same as the density of the cushion part in the central part, and in the absorbent article of still another embodiment of the present disclosure, the density of the cushion part in the outer peripheral part is The density is lower than the density of the cushion part in the central part.
- the cushion part is not particularly limited, and preferably includes a nonwoven fabric in which the intersections of the fibers are heat-sealed.
- a nonwoven fabric in which the intersection of each fiber is heat-sealed the thing containing a natural fiber, a chemical fiber, or both is mentioned, for example. Since the intersection of each fiber is heat-sealed, the cushion part, and thus the mid-high part, is excellent in shape stability both when dry before absorbing menstrual blood and when wet after absorbing menstrual blood. .
- the non-woven fabric in which the intersections of the fibers are heat-sealed preferably contains about 50 to about 100% by weight of thermoplastic chemical fiber and more preferably about 70 to about 100% for heat-sealing each fiber. Including mass%.
- the raw material of the thermoplastic chemical fiber include polyethylene (PE), polypropylene (PP), polyethylene terephthalate (PET), PE and PP graft polymers, and examples of the fiber type of the thermoplastic chemical fiber. , Single fibers, composite fibers, heat-shrinkable fibers, heat-extensible fibers, atypical fibers, three-dimensional crimped fibers, split fibers, and the like.
- the thermoplastic chemical fiber may be a fiber containing a hydrophilic agent, a water repellent or the like, or a fiber coated with them. it can.
- the thermoplastic chemical fiber may be a fiber that has been hydrophilized by corona treatment, plasma treatment, or the like.
- the said fiber can contain inorganic fillers, such as a titanium oxide, barium sulfate, a calcium carbonate.
- inorganic fillers such as a titanium oxide, barium sulfate, a calcium carbonate.
- a core and / or a sheath can contain the said inorganic filler.
- the fiber constituting the cushion part is preferably a composite fiber rather than a single fiber, and more preferably a core-sheath type composite fiber including polyethylene in the sheath part in consideration of compression recovery at the middle and high parts.
- the composite fiber examples include a core-sheath type composite fiber in which the melting point of the core component is higher than the melting point of the sheath component, for example, an eccentric type core-sheath type composite fiber, and a side-by-side type composite fiber having two different melting points.
- examples of the atypical fiber include atypical fibers such as a hollow type, a flat type, a Y type, and a C type
- examples of the three-dimensional crimped fibers include three-dimensional crimped fibers such as latent crimps and actual crimps
- the split fibers include split fibers that are split by a physical load such as water flow, heat, and embossing.
- the above fibers preferably have a fineness of about 1.1 to about 8.8 dtex in consideration of ease of menstrual blood and the like, and touch.
- Examples of the natural fiber include cellulose such as pulverized pulp, cotton, etc., regenerated cellulose such as rayon and fibril rayon, semi-synthetic cellulose such as acetate and triacetate, and any combination thereof. In view of cost and formability, pulverized pulp is preferable.
- the cushion part contains the above-mentioned cellulose, regenerated cellulose and / or semi-synthetic cellulose, menstrual blood is drawn in a state where it is difficult to apply body pressure to a mid-high part such as a sleeping posture, and leakage of menstrual blood is suppressed.
- the cushion part is a nonwoven fabric made of a chemical fiber, for example, 100% thermoplastic chemical fiber, menstrual blood may flow on the outermost surface of the top sheet, causing leakage.
- the cushion portion can be formed by web forming, for example, a dry method (card method, spunbond method, melt blown method, airlaid method, TOW, etc.), a wet method, or a combination thereof.
- a dry method card method, spunbond method, melt blown method, airlaid method, TOW, etc.
- a wet method or a combination thereof.
- the method for bonding the cushion portion include thermal bonding, needle punching, chemical bonding, and hydroentanglement method.
- the cushion part is not particularly limited.
- an air-through nonwoven fabric for example, an air-through nonwoven fabric, a point bond nonwoven fabric, a spunbond nonwoven fabric, a spunbond / meltblown / spunbond nonwoven fabric (SMS nonwoven fabric), and a thermal fusion where each fiber intersection is thermally fused.
- SMS nonwoven fabric spunbond / meltblown / spunbond nonwoven fabric
- thermal fusion where each fiber intersection is thermally fused.
- a spunlace nonwoven fabric having intersections and more preferably an air-through nonwoven fabric. This is because the air-through nonwoven fabric is bulky, has a low density, and is excellent in the touch.
- the cushion portion has a maximum thickness of about 3 to about 30 mm, preferably has a maximum thickness of about 4 to about 20 mm, and more preferably about 5 to about 10 mm. Has maximum thickness.
- the maximum thickness of the cushion part is within the above range, it can fit into the excretion opening of the wearer, in particular, the labia, at the time of wearing, and can hardly cause leakage.
- the maximum thickness of the said cushion part means not the cushion part taken out from the absorbent article but the maximum thickness of the cushion part in the state of an absorbent article.
- the middle-high portion has a compression work WC of about 10 to about 50 (N ⁇ m / m 2 ) in the central portion, preferably about 15 to about 40 (N ⁇ m / m). m 2 ) and more preferably about 20 to about 35 (N ⁇ m / m 2 ).
- the compression work WC When the compression work WC is less than about 10 (N ⁇ m / m 2 ), the middle and high parts are difficult to be compressed. It becomes difficult to continue. When the compression work WC exceeded 50 (N ⁇ m / m 2 ), the mid-high part was compressed only by the self-weight of menstrual blood, a gap was formed between the labia and the mid-high part, and menstrual blood was repeatedly excreted In some cases, menstrual blood tends to spread to the topsheet.
- the mid-high section has a compression resilience RC of about 35 to about 100%, preferably about 40 to about 100%, and more preferably in the middle. It has a compression resilience RC of about 50 to about 100%.
- the compression resilience RC is less than about 35%, after the body pressure from the wearer is added, menstrual blood is absorbed, etc., the bulk of the middle and high parts does not recover quickly, and the middle and high parts continue to contact the labia It becomes difficult, and a gap is formed between the small labia and the middle and high parts, which easily leads to leakage.
- the compression work WC is a value indicating compressibility, that is, ease of compression, and a larger value means that compression is easier.
- the compression resilience RC is a value indicating the recoverability of the bulk after compression, and the closer the value is to 100%, the higher the recoverability is. In this specification, “compressibility” and “recoverability” may be collectively referred to as “compression recoverability”.
- the middle and high parts have the compression work WC in the above-described range at the center, so that the middle part of the middle and high parts can continue to bury the wearer's small labia.
- the middle and high parts are crushed temporarily by having the compression resilience RC in the above-mentioned range at the center part (when body pressure from the wearer is applied, menstrual blood is discharged) Etc.)
- the bulk quickly recovers, the middle part of the middle and high part can be buried in the wearer's small labia and the middle part of the middle and high part can be kept in contact with the labia.
- the compression resilience RC and the compression work WC are measured using a compression tester, for example, KES (handy compression tester, model: KES-G5) manufactured by Kato Tech Co., Ltd.
- KES hand compression tester, model: KES-G5 manufactured by Kato Tech Co., Ltd.
- the test conditions are set as follows. ⁇ Compression terminal area: 2.0 cm 2 ⁇ Speed: 0.1 cm / sec ⁇ Maximum compressive force: 4900 N / m 2
- the middle-high portion has the compression work WC and the compression resilience RC in the above-described range at the center portion thereof, so that the middle-high portion, more specifically, the cushion portion is compressed and compressed.
- the recovery can be repeated quickly and it can have a pump function to carry absorbed menstrual blood. That is, the menstrual blood absorbed by the cushion portion can be quickly transferred to the absorber.
- the cushion portion is preferably about 50 to about 1,000 g / m 2 , more preferably about 100 to 500 g / m 2 , and still more preferably about 150 to about 300 g / m 2 .
- the cushion portion preferably has an average basis weight within the above range at the center portion.
- the “average basis weight” means the basis weight of the entire sample obtained by dividing the mass of the sample (for example, a cushion part, an absorber, etc.) by the area.
- the cushion portion is preferably about 0.1 to about 3.6 N, more preferably about 0.2 to about 3.0 N, and even more preferably about 0.3 to about 2. It has a compression force of 0N.
- the absorbent article is compressed by the wearer's leg or the like when worn, the absorbent article is bent so that the skin contact surface of the top sheet protrudes in the width direction of the absorbent article in accordance with the reduction of the gap (hereinafter, It may be referred to as “developing”.
- the compressive force is less than about 0.1 N, after the absorbent article is deformed in a convex shape, the compression from the wearer's leg is lost and the cushion part is repelled to quickly return the absorbent article to its original shape. It may not be possible. Moreover, when the said compressive force exceeds about 3.6 N, the repulsive force of a cushion part will be too large, and there exists a tendency for an absorbent article to become difficult to carry out convex deformation to begin with.
- FIG. 6 is a schematic view showing an example of an apparatus for measuring the compressive force.
- FIG. 6A is a view of the compressive force measuring device 21 as viewed from above
- FIG. 6B is a view of the compressive force measuring device 21 as viewed from the side.
- a compressive force measuring device 21 shown in FIG. 6 includes an operating table 23 that can reciprocate left and right on a stand 22 and a digital force gauge that is installed on the operating table 23 and measures compressive force. 24 and a sample stage 25 on which a sample to be measured is placed.
- the sample stage 25 has a sample 26 to be measured, two cylindrical compression parts 27 that are arranged so as to sandwich the sample 26, and a stopper for fixing one of the compression parts. 28 is arranged.
- Examples of the stand 22 include a digital force gauge stand manufactured by Nidec Sympo Corporation, such as FGS-50X-H.
- Examples of the digital force gauge 24 include a digital force gauge manufactured by Nidec-Simpo Corporation, for example, a FGP-0.5 worn with a push adapter (square, side length: 15 mm).
- Examples of the compression part 27 include an AS-one plastic petri dish (mass: 38.5 g, diameter: 100 mm, height: 30 mm).
- the sample 26, specifically, the cushion part taken out from the absorbent article is placed between the two compression parts 27.
- the cushion part is set such that the two compression parts 27 compress the center position in the longitudinal direction of the cushion part in the width direction of the cushion part.
- the longitudinal direction and width direction regarding a cushion part mean the longitudinal direction and width direction of the absorbent article which the said cushion part comprises.
- the digital force gauge 24 is arranged so that the adapter compresses around a position 6 mm from the bottom of the compression part 27.
- the digital force gauge 24 is moved 20 mm so as to push the sample 26 at a speed of 100 mm / min, the sample 26 is compressed, the maximum value at that time is read, and the value of the compression force is adopted.
- the density of the cushion portion constituting the middle-high portion is different between the outer peripheral portion and the central portion of the middle-high portion, and the density of the cushion portion in the outer peripheral portion is the cushion portion in the central portion.
- the absorbent article may be referred to as “absorbent article including a cushion portion having a density difference”).
- the shape of the labia of adult women is not constant and varies from person to person, but the labia of adult women is less likely to deform when the body is moved than the labia.
- the large labia differs greatly in the degree of deformation when the body is moved depending on the body shape of the woman. When the body is moved, the large labia is very easily deformed when the body is moved.
- the relatively low-density cushion part in the middle part of the middle and high parts has low rigidity, and when in contact with the small labia, it deforms along the shape of the small labia, Small labia can be buried.
- the relatively high-density cushion part in the outer periphery of the middle-high part has high rigidity, and keeps contacting the boundary between the small labia and the large labia or the large labia when dry before absorbing menstrual blood. Can do.
- the relatively high-density cushion part in the outer peripheral part of the middle-high part has a fast recovery property after compression, so that it comes into contact with the excretory opening having a complicated shape even when wet after absorbing menstrual blood. You can continue.
- the “excretion opening contact area” means an area in contact with the wearer's excretion opening, and the above “excretion opening” mainly means the labia minora, but the outer peripheral part of the middle and high parts However, it does not prevent you from touching the labia.
- the middle part of the middle and high part is easily compressed, so that it deforms into a concave shape along the shape of the wearer's small labia while wearing, and the small labia is buried.
- the top sheet is brought into close contact with the vaginal opening to prevent menstrual blood from spreading excessively on the top sheet.
- the top sheet is in close contact with the vaginal opening, so that the distance between the vaginal opening and the absorber is close during wearing, and the cushion part is mainly heated.
- the menstrual blood passes through the top sheet and the cushion portion without being diffused in the plane direction, and is quickly transferred to the absorbent body. Once the passage of menstrual blood is made, the area is hydrophilized. Therefore, menstrual blood passes through the top sheet and the cushion portion and absorbs quickly after absorption of menstrual blood for the second and subsequent times. Transition.
- the absorbent article including the cushion part having the above density difference
- body pressure is applied, the volume of the cushion part is temporarily reduced, and a large amount of menstrual blood stays in the cushion part where the volume is reduced. Even so, when the body pressure becomes weak, the bulk of the cushion part is quickly recovered, centering on the outer peripheral part of the middle and high parts, so that menstrual blood is quickly transferred to the absorbent body.
- the absorbent article containing the cushion part which has the said density difference can be formed by squeezing a top sheet and an absorber at least in the outer edge vicinity of a cushion part, for example.
- a top sheet and an absorber at least in the outer edge vicinity of a cushion part, for example.
- the cushion part in the outer peripheral part of the middle and high parts can be made higher than the density of the cushion portion in the middle portion of the middle-high portion.
- the degree of compression of the cushion portion is low and the thickness thereof is not as thin as that of the outer peripheral portion.
- An absorbent article including a cushion part having the above density difference, more specifically, an absorbent article having a squeezed part formed by squeezing at least a top sheet and an absorber near the outer edge of the cushion part, for example, , (I) squeezing the top sheet and the absorbent body on the outside of the cushion part, and (ii) squeezing the top sheet, the cushion part and the absorbent body on the outer edge of the cushion part. It can be manufactured by forming a part.
- the absorbent article containing the cushion part which has the said density difference is, for example, squeezing the outer edge of a cushion part, and then a liquid-impermeable back sheet, an absorber, a cushion part, and a top sheet in order.
- the top sheet By having at least a compressed portion formed by pressing the top sheet and the absorbent body in the vicinity of the outer edge of the cushion section, the top sheet is difficult to peel from the absorbent body when wet after absorbing menstrual blood, for example. Moreover, the high density of the cushion part in the outer peripheral part of a middle-high part can be hold
- the vicinity of the outer edge” regarding the cushion part is a concept including not only the outer edge of the cushion part but also the inner side of the outer edge of the cushion part and the outer side of the outer edge of the cushion part.
- “near” is preferably a range of ⁇ 15%, more preferably ⁇ 10% of the distance from the center of the cushion portion to the outer edge of the cushion portion in the planar direction of the absorbent article, and more preferably ⁇ 10%. Means a range of ⁇ 5%.
- center regarding a cushion part means the center of the longitudinal direction and width direction of an absorbent article.
- FIGS. 1 to 5 there is a squeezed portion formed by continuously squeezing the top sheet and the absorbent body on the outside of the cushion portion, but the absorbency in another embodiment of the present disclosure
- a pressing part formed by intermittently pressing the top sheet and the absorbent body outside the cushion part.
- the absorbent article in still another embodiment of the present invention there is a pressing portion formed by continuously or intermittently pressing the top sheet, the cushion portion, and the absorbent body at the edge of the cushion portion, and the present invention.
- the pressing part can be formed by a method known in the art, for example, a pair of upper and lower embossing rolls, in which a pressing pattern is formed on one side, at a temperature below the melting point of the fiber,
- the squeezed portion can be formed by squeezing at a pressure of about 100 to about 1000 N / cm.
- the shape of the cushion part before being incorporated into the absorbent article is not particularly limited.
- the projection in the thickness direction of the absorbent article has a shape similar to the labia, for example, a substantially circular shape, a substantially elliptical shape, It can be a substantially rounded rectangle, a figure surrounded by two arcs, or the like.
- the cushion portion may have a constant thickness in the thickness direction of the absorbent article, the thickness may increase from the center toward the outer edge, or the thickness may increase from the center toward the outer edge. Can be thinned.
- the cushion part when the thickness of the cushion part becomes thinner from the center toward the outer edge, when the absorber and the top sheet are squeezed, the density of the cushion part in the outer peripheral part of the middle and high parts is Since it may be difficult to increase the density of the cushion part, in the absorbent article including the cushion part having the above density difference, the cushion part has a constant thickness or the thickness from the center to the outer edge. What becomes thick toward it is preferable. Further, the basis weight of the cushion portion may vary depending on the location.
- FIG. 7 is a diagram showing an example of the shape of the cushion portion before being incorporated into the absorbent article.
- the projection in the thickness direction of the absorbent article is substantially elliptical and has a constant thickness.
- the cushion portion 6 shown in FIG. 7B has a shape in which the projection in the thickness direction of the absorbent article is formed of two arcs and has a constant thickness.
- the projection in the thickness direction of the absorbent article is a substantially rounded rectangle and the thickness is constant.
- the projection in the thickness direction of the absorbent article is substantially elliptical, and the thickness decreases from the center toward the outer edge.
- the density of the cushion portion at the middle portion of the mid-high portion is preferably about 0.001 to 0.1 g / cm 3 , more preferably about 0.005 to about 0.08 g / cm 3 , and still more preferably about 0.01. To about 0.05 g / cm 3 .
- the density is less than about 0.001 g / cm 3 , the compression recovery property tends to be insufficient when wet after absorbing menstrual blood. If the density is greater than about 0.1 g / cm 3 , it tends to deform along the wearer's small labia and make it difficult to fit the small labia.
- the density of the cushion part in the center part and outer peripheral part of a middle-high part is measured as follows.
- a two-dimensional laser displacement meter measure the thickness t (cm) of the cushion portion at the position to be measured.
- Examples of the two-dimensional laser displacement meter include a high-precision two-dimensional laser displacement meter LJ-G series (model: LJ-G030) manufactured by Keyence Corporation.
- the thickness of the cushion part at the position to be measured is calculated by subtracting the thickness of the region where the cushion part does not exist from the thickness of the absorbent article at the position to be measured.
- the ratio of the density at different positions of the cushion portion can be simply compared by their thickness. That is, when the basis weight of the cushion portion is constant, it means that the thinner the cushion portion, the higher the density of the cushion portion.
- the density of the cushion part in the outer peripheral part of the middle-high part is higher than the density of the cushion part in the middle part of the middle-high part, preferably about 1.1 to about 5. 0 times higher, more preferably about 1.2 to about 4.0 times higher, and more preferably about 1.5 to about 3.0 times higher.
- the ratio is less than about 1.1 times, when wet, the cushion part at the outer periphery of the middle and high parts tends to have insufficient recovery after compression, and the ratio is about 5.0 times.
- the rigidity of the cushion part in the outer peripheral part of a middle-high part is high, and there exists a tendency for a wearer to remember a foreign material feeling easily.
- the cushion portion preferably has a longitudinal length of the absorbent article, preferably about 10 to about 100 mm, of about 30 to about 200 mm, more preferably about 40 to about 170 mm, and more preferably about 50 to about 100 mm. More preferably from about 20 to about 70 mm, and even more preferably from about 25 to about 50 mm, in the width direction of the absorbent article.
- the middle and high parts fit into the excretion opening of the wearer, particularly the labia, and leakage can be suppressed. If the size of the cushion part is smaller than the above range, the middle and high part will not fit into the wearer's excretion opening, especially the labia, and leakage will easily occur, and if the size of the cushion part is larger than the above range, it will be worn Sometimes it is easy to feel a sense of incongruity, and a gap with the labia tends to occur, and leakage tends to occur.
- longitudinal direction means the longitudinal direction of the absorbent article
- width direction means the width direction of the absorbent article
- thickness direction means the thickness direction of the absorbent article
- the cushion part preferably has a maximum thickness of about 50% or more after absorbing 2 g of equine EDTA blood and before absorbing horse EDTA blood, and has a maximum thickness of about 60% or more. More preferably, and even more preferably a maximum thickness of about 70% or more.
- 2 g of equine EDTA blood is dripped is that the amount of menstrual blood excreted at one time by humans is generally 2 g.
- 2 g of equine EDTA blood is dripped on the entire cushion part using a pipette.
- the cushion part contains a water-repellent material, the equine EDTA blood is difficult to enter the cushion part. In such a case, equine EDTA blood may be absorbed by the cushion portion while pressurizing the cushion portion.
- the maximum thickness of the cushion part after absorbing 2 g of equine EDTA blood is measured after 1 minute has elapsed since all of the equine EDTA blood has been absorbed. Further, the maximum thickness of the cushion portion is measured using the above-described two-dimensional laser displacement meter. The EDTA blood will be described later.
- the absorbent article has a curved structure that curves with the middle and high portions facing inward.
- the absorbent article fits to the curve of the wearer's body, and absorbed menstrual blood is less likely to leak.
- the curved structure can be formed, for example, by passing an elastic member, for example, a rubber thread, a stretchable film, etc., on both sides in the longitudinal direction of the absorbent article, and applying tension to both sides in the longitudinal direction of the absorbent article. .
- the absorbent article has a flat structure that is not curved with the middle-high portion facing inward.
- the liquid-permeable top sheet has a plurality of ridges and a plurality of grooves extending in the longitudinal direction of the absorbent article on the skin contact surface (
- a top sheet having a plurality of ridges and a plurality of grooves extending in the longitudinal direction of the absorbent article may be simply referred to as a top sheet having a ridge groove structure).
- the top sheet can be produced according to the methods described in JP-A-2008-025078, JP-A-2008-025079 and the like.
- the top sheet is manufactured according to the method described in Japanese Patent Application Laid-Open Nos. 2011-222010, 2011-2226011, and the like, and has a ridge groove structure. It is.
- the top sheet having the ridge groove structure is a pair of gear rolls having a rotation axis perpendicular to the conveying direction, and a gap between the plurality of teeth arranged on the outer peripheral surfaces of the gear rolls while meshing with each other. Can be formed by fluid treatment through the topsheet to be treated.
- the draw ratio of the gear roll is preferably about 105% or more, more preferably about 105 to about 500%, further preferably about 120 to 300%, and further preferably about 130%. About 200%.
- the stretch ratio is less than about 105%, the stretchability of the top sheet is insufficient, the cushion part is easily crushed during the manufacture of absorbent articles, and when the stretch ratio is greater than about 500%, There is a tendency that the top sheet is easily torn during the manufacture of the property article.
- the said draw ratio is the following formula when the gear pitch is P and the gear biting depth is D: Means the value calculated by.
- the liquid-permeable top sheet has a plurality of slits.
- the slit can be widened and the cushion portion can be prevented from being excessively crushed during the manufacture of the absorbent article.
- the top sheet having the plurality of slits can be formed by passing the top sheet through a slit roll in which vertically long slits are arranged in a staggered manner.
- the top sheet having the plurality of slits can be manufactured, for example, as described in JP-T-2002-528174.
- the liquid-permeable top sheet has a plurality of pin openings.
- a top sheet having a ridge groove structure, slits, pin openings, etc. has an excessive cushion part due to a change in the shape of the groove structure of the top sheet, opening / closing of the slit and pin openings, etc. during manufacture of the absorbent article. Can be prevented from being crushed. From this point of view, it is preferable that the ridge groove structure, slit, pin opening portion, etc. of the top sheet are present at least in the portion in contact with the cushion portion, i.e., the portion constituting the middle-high portion. May be present.
- the shape of the absorbent body is not particularly limited as long as it matches the shape of a female body and shorts, and examples thereof include a rectangular shape, an elliptical shape, and a saddle shape.
- the middle part of the middle and high parts can continue to bury the wearer's labia, and when the middle and high parts are temporarily crushed (when body pressure from the wearer is applied, Even when menstrual blood is drained, the bulk quickly recovers, the middle part of the middle and high part is buried in the wearer's small labia, and the middle part of the middle and high part is kept in contact with the labia be able to. Therefore, in the absorbent article of the present disclosure, the absorber can be made smaller than before.
- the absorbent body preferably has a longitudinal length of about 80 to about 230 mm, more preferably about 100 to 200 mm, and even more preferably about 120 to 170 mm.
- the absorbent body preferably has a length in the width direction of about 30 to about 90 mm, more preferably about 35 to about 80 mm, and more preferably about 40 to about 70 mm.
- the absorbent body comes into contact with the wearer's buttocks, legs, inner thighs, etc., and the wearer feels uncomfortable. And may cause skin problems for wearers with sensitive skin.
- the absorbent body is likely to be twisted, and as a result, the absorbent article and the skin There is a case where a gap is formed and a mole is induced.
- the absorbent capacity of the absorbent body tends to be insufficient, and the psychological state before wearing. May give a feeling of anxiety.
- the highly rigid absorbent body in the absorbent article has an excessive size, it becomes difficult for the middle and high portions to come into contact with the excretory opening, particularly the labia.
- the absorbent article of the present disclosure can be in an arbitrary shape such as a rectangle, an ellipse, and a saddle, and may have a so-called flap that prevents displacement from clothes, for example, shorts.
- the absorbent article of this indication preferably has a longitudinal length of about 100 to about 260 mm, more preferably about 120 to about 220 mm, and even more preferably about 150 to about 190 mm, and preferably about 40 to about 100 mm, more Preferably, it has a width in the width direction (excluding the flap portion) of about 45 to about 90 mm, and more preferably about 50 to 80 mm.
- the mid-high section has an IOB of about 0.00 to about 0.60, a melting point of about 45 ° C. or less, and about 0.00 to about 100 g of water at 25 ° C.
- IOB Inorganic Organic Balance
- IOB is an index indicating a balance between hydrophilicity and lipophilicity.
- Oda et al. IOB value calculated by inorganic value / organic value.
- the inorganic value and the organic value are represented by Fujita Minoru, “Prediction of organic compounds and conceptual diagram of organic compounds”, chemistry area Vol. 11, no. 10 (1957) p. 719-725).
- Table 1 summarizes the organic and inorganic values of the major groups by Mr. Fujita.
- the IOB is about 0.00 to about 0.60, preferably about 0.00 to about 0.50, and preferably about 0.00 to about 0.40. More preferred is about 0.00 to about 0.30. This is because the lower the IOB, the higher the organicity and the higher the affinity with blood cells.
- melting point means a peak top temperature of an endothermic peak when changing from a solid state to a liquid state when measured with a differential scanning calorimeter at a heating rate of 10 ° C./min.
- the melting point can be measured using, for example, a DSC-60 type DSC measuring apparatus manufactured by Shimadzu Corporation.
- the blood modifying agent may be liquid at room temperature or solid as long as it has a melting point of about 45 ° C. or lower, that is, whether the melting point is about 25 ° C. or higher, or less than about 25 ° C. Well, and can have a melting point of, for example, about ⁇ 5 ° C., about ⁇ 20 ° C., and the like. The reason why the melting point of the blood modifying agent is about 45 ° C. or less will be described later.
- the blood modifying agent has a lower melting point, but preferably has a low vapor pressure.
- the blood pressure of the blood modifying agent is preferably about 0 to about 200 Pa at 25 ° C. (1 atm), more preferably about 0 to about 100 Pa, and further about 0 to about 10 Pa. More preferably, it is about 0 to about 1 Pa, and even more preferably about 0.0 to about 0.1 Pa. Yes.
- the vapor pressure is preferably about 0 to about 700 Pa at 40 ° C. (1 atm), and is about 0 to about 100 Pa.
- the melting point of the blood modifying agent can be properly used according to the climate, the length of wearing time, and the like. For example, in an area where the average temperature is about 10 ° C. or less, even when the menstrual blood is excreted and then cooled by the ambient temperature by adopting a blood modifying agent having a melting point of about 10 ° C. or less.
- the blood modifying agent stably reforms the blood.
- the melting point of the blood modifying agent is preferably higher in the range of 45 ° C. or lower. This is because the blood modifying agent is difficult to move even when it is worn for a long time, and is hardly affected by sweat, friction at the time of wearing, and the like.
- the water solubility of 0.00-0.05 g was obtained by adding 0.05 g of sample to 100 g of deionized water at 25 ° C., leaving it to stand for 24 hours, and after 24 hours, gently stirring as necessary. Next, it can be measured by visually evaluating whether or not the sample is dissolved.
- dissolution includes a case where the sample is completely dissolved in deionized water to form a uniform mixture and a case where the sample is completely emulsified.
- “Complete” means that there is no lump of sample in deionized water.
- top sheet is coated with a surfactant for the purpose of changing blood surface tension and the like to absorb blood rapidly.
- surfactants generally have high water solubility
- top sheets coated with surfactants are well-suited to hydrophilic components (blood plasma, etc.) in blood, but rather leave blood on the top sheet.
- the blood modifying agent has low water solubility, unlike the conventionally known surfactants, blood is not allowed to remain on the top sheet, but is quickly transferred to the absorber.
- solubility in 100 g of water at 25 ° C. may be simply referred to as “water solubility”.
- a weight average molecular weight means the value of polystyrene conversion calculated
- GPC measurement conditions include the following. Model: Hitachi High-Technologies Corporation High-Performance Liquid Chromatogram Lachrom Elite Column: SHODEX KF-801, KF-803 and KF-804 manufactured by Showa Denko K.K. Eluent: THF Flow rate: 1.0 mL / min Driving amount: 100 ⁇ L Detection: RI (differential refractometer)
- the weight average molecular weight described in the Example of this specification is measured on the said conditions.
- the blood modifying agent is preferably the following (i) to (iii), (I) hydrocarbons, (Ii) from (ii-1) a hydrocarbon moiety and (ii-2) a carbonyl group (—CO—) and an oxy group (—O—) inserted between the CC single bonds of the hydrocarbon moiety.
- the hydrocarbon moiety A compound having one or a plurality of the same or different groups selected from the group consisting of a carboxyl group (—COOH) and a hydroxyl group (—OH), which replaces a hydrogen atom; As well as any combination thereof.
- hydrocarbon means a compound composed of carbon and hydrogen, and a chain hydrocarbon, for example, a paraffinic hydrocarbon (also referred to as an alkane, which does not include a double bond and a triple bond).
- a paraffinic hydrocarbon also referred to as an alkane, which does not include a double bond and a triple bond.
- Olefinic hydrocarbons including one double bond, also referred to as alkene
- acetylenic hydrocarbons including one triple bond, also referred to as alkyne
- hydrocarbons containing two or more bonds selected from the above and cyclic hydrocarbons such as aromatic hydrocarbons and alicyclic hydrocarbons.
- the hydrocarbon is preferably a chain hydrocarbon and an alicyclic hydrocarbon, more preferably a chain hydrocarbon, a paraffinic hydrocarbon, an olefinic hydrocarbon, and two double bonds. More preferred are hydrocarbons (not including triple bonds), and more preferred are paraffinic hydrocarbons.
- the chain hydrocarbon includes a straight chain hydrocarbon and a branched chain hydrocarbon.
- each oxy group (—O—) is not adjacent. Therefore, the compounds (ii) and (iii) do not include compounds having a continuous oxy group (so-called peroxides).
- At least one hydrogen atom in the hydrocarbon moiety is more than a hydroxyl group (-) than in a compound in which at least one hydrogen atom in the hydrocarbon moiety is substituted with a carboxyl group (-COOH).
- Compounds substituted with OH) are preferred.
- Table 1 since the carboxyl group binds to menstrual metals and the like, and the inorganic value increases significantly from 150 to 400 or more, the blood modifying agent having a carboxyl group is used at the time of use. This is because the value of IOB exceeds about 0.60, and the affinity with blood cells may be reduced.
- the blood modifying agent is more preferably the following (i ′) to (iii ′), (I ′) hydrocarbon, (Ii ′) (ii′-1) a hydrocarbon moiety and (ii′-2) a carbonyl bond (—CO—), an ester bond (—COO) inserted between the C—C single bonds of the hydrocarbon moiety.
- the blood modifying agent preferably has about 1.8 or less carbonyl bonds (—CO—) and 2 or less ester bonds (—COO—) per 10 carbon atoms in the hydrocarbon moiety. About 1.5 or less carbonate bonds (—OCOO—), about 6 or less ether bonds (—O—), about 0.8 or less carboxyl groups (—COOH), and / or hydroxyl groups (—OH) ) In a compound having about 1.2 or less.
- the blood modifying agent is more preferably the following (A) to (F), (A) (A1) a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing hydrogen atoms in the chain hydrocarbon moiety, (A2) a chain hydrocarbon moiety, and the chain An ester with a compound having one carboxyl group for substituting a hydrogen atom in the hydrocarbon moiety, (B) (B1) a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing hydrogen atoms in the chain hydrocarbon moiety, (B2) a chain hydrocarbon moiety, and the chain An ether with a compound having one hydroxyl group replacing a hydrogen atom of the hydrocarbon moiety, (C) (C1) a carboxylic acid, a hydroxy acid, an alkoxy acid or an oxo acid containing a chain hydrocarbon moiety and 2 to 4 carboxyl groups replacing a hydrogen atom in the chain hydrocarbon moiety; C2) an ester of a compound having a chain hydrocarbon mo
- (A1) A compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing hydrogen atoms in the chain hydrocarbon moiety (hereinafter sometimes referred to as “compound (A1)”)
- a chain hydrocarbon tetraol such as an alkanetetraol, such as pentaerythritol
- a chain hydrocarbon triol such as an alkanetriol, such as glycerine
- a chain hydrocarbon diol such as an alkanediol, such as, for example, Glycol.
- Examples of the compound (A2) having a chain hydrocarbon moiety and one carboxyl group that replaces the hydrogen atom of the chain hydrocarbon moiety include: , Compounds in which one hydrogen atom on a hydrocarbon is substituted with one carboxyl group (—COOH), for example, fatty acids.
- Examples of the compound (A) include (a 1 ) an ester of a chain hydrocarbon tetraol and at least one fatty acid, (a 2 ) an ester of a chain hydrocarbon triol and at least one fatty acid, and (a 3 ) Esters of chain hydrocarbon diols with at least one fatty acid.
- ester of (a 1 ) chain hydrocarbon tetraol and at least one fatty acid examples include the following formula (1): Tetraesters of pentaerythritol and fatty acids of the following formula (2): Triesters of pentaerythritol and fatty acids of the following formula (3): Diester of pentaerythritol and fatty acid of the following formula (4): And monoesters of pentaerythritol and fatty acids. (Wherein R 1 to R 4 are each a chain hydrocarbon)
- the ester of pentaerythritol and fatty acid has the above-mentioned IOB, melting point and water solubility.
- a saturated fatty acid for example, a C 2 to C 30 saturated fatty acid, for example, acetic acid (C 2 ) (C 2 represents the number of carbon atoms, R 1 C, R 2 C, R 3 C or R 4 C, the same applies hereinafter), propanoic acid (C 3 ), butanoic acid (C 4 ) and isomers thereof such as 2-methylpropanoic acid (C 4 ), Pentanoic acid (C 5 ) and isomers thereof such as 2-methylbutanoic acid (C 5 ), 2,2-dimethylpropanoic acid (C 5 ), hexanoic acid (C 6 ), heptanoic acid (C 7 ), octanoic acid (C 8) Beauty isomers thereof, e.g., 2-ethylhexanoic acid (C 8), nonanoic acid (C 9), decanoic acid (C 10), dode
- the fatty acid can also be an unsaturated fatty acid.
- unsaturated fatty acid include C 3 to C 20 unsaturated fatty acids such as monounsaturated fatty acids such as crotonic acid (C 4 ), myristoleic acid (C 14 ), palmitoleic acid (C 16 ), Oleic acid (C 18 ), elaidic acid (C 18 ), vaccenic acid (C 18 ), gadoleic acid (C 20 ), eicosenoic acid (C 20 ), etc., diunsaturated fatty acids such as linoleic acid (C 18 ), Triunsaturated fatty acids such as eicosadienoic acid (C 20 ), such as linolenic acid, such as ⁇ -linolenic acid (C 18 ) and ⁇ -linolenic acid (C 18 ), pinolenic acid (C 18 ), eleostearic acid, For example, ⁇ -eleostearic acid (C
- the ester of pentaerythritol and fatty acid is an ester of pentaerythritol and a fatty acid derived from a saturated fatty acid, that is, an ester of pentaerythritol and a saturated fatty acid, considering the possibility of modification by oxidation or the like. preferable.
- the ester of pentaerythritol and fatty acid is preferably a diester, triester or tetraester, more preferably a triester or tetraester, in order to reduce IOB and make it more hydrophobic. And tetraesters are more preferred.
- the total number of carbon atoms of the fatty acid constituting the tetraester of pentaerythritol and fatty acid that is, in the above formula (1), R 1 C, R 2 C, R 3 C and When the total number of carbon atoms in the R 4 C portion is 15, IOB is 0.60. Therefore, in the tetraester of pentaerythritol and fatty acid, the IOB satisfies the requirement of about 0.00 to about 0.60 when the total number of carbons is about 15 or more.
- tetraester of pentaerythritol and fatty acid for example, pentaerythritol, hexanoic acid (C 6 ), heptanoic acid (C 7 ), octanoic acid (C 8 ), for example, 2-ethylhexanoic acid (C 8 ), And tetraesters with nonanoic acid (C 9 ), decanoic acid (C 10 ) and / or dodecanoic acid (C 12 ).
- the total number of carbon atoms of the fatty acid constituting the triester of pentaerythritol and fatty acid that is, in the above formula (2), R 1 C, R 2 C and R 3 C moieties
- the IOB satisfies the requirement of about 0.00 to about 0.60 when the total number of carbon atoms of the fatty acid is about 19 or more.
- the total number of carbon atoms of the fatty acid constituting the diester of pentaerythritol and fatty acid that is, the total number of carbon atoms of the R 1 C and R 2 C moieties in the above formula (3) is In the case of 22, the IOB is 0.59. Therefore, in the diester of pentaerythritol and fatty acid, the IOB satisfies the requirement of about 0.00 to about 0.60 when the total number of carbon atoms of the fatty acid is about 22 or more.
- the number of carbons of the fatty acid constituting the monoester of pentaerythritol and fatty acid that is, in the above formula (4), the IOB is 25 when the carbon number of the R 1 C portion is 25. 0.60. Therefore, in the monoester of pentaerythritol and fatty acid, the IOB satisfies the requirement of about 0.00 to about 0.60 when the number of carbon atoms of the fatty acid is about 25 or more. In the above calculation, the influence of double bond, triple bond, iso branch, and tert branch is not taken into consideration.
- esters of pentaerythritol and fatty acids examples include Unistar H-408BRS, H-2408BRS-22 (mixed product) and the like (manufactured by NOF Corporation).
- ester of (a 2 ) chain hydrocarbon triol and at least one fatty acid examples include the following formula (5): Triester of glycerin and fatty acid of the following formula (6): Diesters of glycerin and fatty acids and the following formula (7): (Wherein R 5 to R 7 are each a chain hydrocarbon) Monoester of glycerin and fatty acid.
- the ester of the glycerin and the fatty acid constituting the ester of the glycerin and the fatty acid, as long as the ester of the glycerin and the fatty acid satisfies the requirements for the IOB, melting point and water solubility,
- the fatty acids listed in “Ester of (a 1 ) chain hydrocarbon tetraol and at least one fatty acid”, that is, saturated fatty acids and unsaturated fatty acids, and the like are modified by oxidation or the like.
- it is preferable to use an ester of glycerin and a fatty acid derived from a saturated fatty acid that is, an ester of glycerin and a saturated fatty acid.
- ester of glycerin and fatty acid is preferably a diester or triester, and more preferably a triester, in order to reduce IOB and make it more hydrophobic.
- the triester of glycerin and a fatty acid is also referred to as a triglyceride.
- triester of glycerin and octanoic acid C 8
- triester of glycerin and decanoic acid C 10
- glycerin and dodecanoic acid C 12
- triesters of glycerin and 2 or 3 fatty acids and mixtures thereof.
- Examples of the triesters of glycerin and two or more fatty acids include triesters of glycerin and octanoic acid (C 8 ) and decanoic acid (C 10 ), glycerin, octanoic acid (C 8 ), and decane.
- Examples thereof include triesters with (C 16 ) and octadecanoic acid (C 18 ).
- the total number of carbon atoms of the fatty acid constituting the triester of glycerin and fatty acid that is, R 5 C in formula (5) in order to make the melting point about 45 ° C. or less.
- R 6 C and R 7 C moieties preferably have a total carbon number of about 40 or less.
- the total number of carbon atoms of the fatty acid constituting the triester of glycerin and fatty acid that is, in the formula (5), R 5 C, R 6 C and R 7 C moieties.
- the IOB is 0.60. Therefore, in the triester of glycerin and fatty acid, the IOB satisfies the requirement of about 0.00 to about 0.60 when the total number of carbon atoms of the fatty acid is about 12 or more.
- the triester of glycerin and a fatty acid is a so-called fat and is a component that can constitute a human body, and thus is preferable from the viewpoint of safety.
- triesters of glycerin and fatty acids include tricoconut oil fatty acid glyceride, NA36, panacet 800, panacet 800B and panacet 810S, and tri-C2L oil fatty acid glyceride and tri-CL oil fatty acid glyceride (above, manufactured by NOF Corporation). ) And the like.
- the diester of glycerin and fatty acid is also called diglyceride.
- diester of glycerin and decanoic acid (C 10 ) diester of glycerin and dodecanoic acid (C 12 ), glycerin and hexadecanoic acid (C 16 ) Examples include diesters, diesters of glycerin and two fatty acids, and mixtures thereof.
- the total number of carbon atoms of the fatty acid constituting the diester of glycerin and fatty acid that is, the total number of carbon atoms of the R 5 C and R 6 C moieties in the formula (6) is 16.
- the IOB is 0.58. Accordingly, in the diester of glycerin and fatty acid, the IOB satisfies the requirement of about 0.00 to about 0.60 when the total number of carbon atoms of the fatty acid is about 16 or more.
- the monoester of the glycerin and the fatty acid is also referred to as a monoglyceride, and examples thereof include glycerin icosanoic acid (C 20 ) monoester, glycerin docosanoic acid (C 22 ) monoester, and the like.
- the monoester of glycerin and fatty acid the number of carbon atoms of the fatty acid constituting the monoester of glycerin and fatty acid, that is, when the carbon number of the R 5 C moiety in the formula (7) is 19, the IOB is 0.59. It becomes. Therefore, in the monoester of glycerin and fatty acid, when the carbon number of the fatty acid is about 19 or more, the IOB satisfies the requirement of about 0.00 to about 0.60.
- ester of (a 3 ) chain hydrocarbon diol and at least one fatty acid examples include C 2 to C 6 chain hydrocarbon diols such as C 2 to C 6 glycols such as ethylene glycol, propylene glycol, butylene. Examples thereof include monoesters or diesters of glycol, pentylene glycol or hexylene glycol and a fatty acid.
- ester of the chain hydrocarbon diol and at least one fatty acid for example, the following formula (8): R 8 COOC k H 2k OCOR 9 (8) (Wherein k is an integer from 2 to 6 and R 8 and R 9 are each a chain hydrocarbon) A diester of a C 2 -C 6 glycol with a fatty acid and the following formula (9): R 8 COOC k H 2k OH (9) (Wherein k is an integer from 2 to 6 and R 8 is a chain hydrocarbon) And monoesters of C 2 -C 6 glycols and fatty acids.
- the fatty acid to be esterified (corresponding to R 8 COOH and R 9 COOH in formula (8) and formula (9)) is C 2 -C 6 glycol.
- an ester of a fatty acid satisfying the above requirements of IOB, melting point and water solubility for example, “(a 1 ) ester of chain hydrocarbon tetraol and at least one fatty acid”
- the fatty acids listed in the above that is, saturated fatty acids and unsaturated fatty acids are mentioned, and saturated fatty acids are preferred in consideration of the possibility of modification by oxidation or the like.
- the ester of a C 2 ⁇ C 6 glycols and fatty acid in view of the potential for degradation by oxidation and the like, derived from saturated fatty acids, esters of C 2 ⁇ C 6 glycols and fatty acid, Nachi Suwa, C 2 An ester of ⁇ C 6 glycol and saturated fatty acid is preferred.
- the ester of C 2 -C 6 glycol and fatty acid is an ester of glycol and fatty acid derived from glycol having a large carbon number, for example, butylene glycol, in order to reduce IOB and make it more hydrophobic.
- An ester of glycol and fatty acid derived from pentylene glycol or hexylene glycol is preferable.
- ester of C 2 -C 6 glycol and fatty acid is preferably a diester in order to reduce IOB and make it more hydrophobic.
- examples of commercial products of the ester of C 2 -C 6 glycol and fatty acid include Compol BL and Compol BS (manufactured by NOF Corporation).
- Compound (B1) Compounds having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing hydrogen atoms in the chain hydrocarbon moiety are listed as “Compound (A)” as Compound (A1). For example, pentaerythritol, glycerin, and glycol.
- Examples of the compound (B2) having a chain hydrocarbon moiety and one hydroxyl group replacing the hydrogen atom of the chain hydrocarbon moiety include: , Compounds in which one hydrogen atom of a hydrocarbon is replaced by one hydroxyl group (—OH), for example, aliphatic monohydric alcohols, for example, saturated aliphatic monohydric alcohols and unsaturated aliphatic monohydric alcohols Is mentioned.
- saturated aliphatic monohydric alcohol examples include C 1 to C 20 saturated aliphatic monohydric alcohols such as methyl alcohol (C 1 ) (C 1 represents the number of carbon atoms, the same shall apply hereinafter), ethyl alcohol ( C 2 ), propyl alcohol (C 3 ) and isomers thereof such as isopropyl alcohol (C 3 ), butyl alcohol (C 4 ) and isomers thereof such as sec-butyl alcohol (C 4 ) and tert-butyl alcohol (C 4 ), pentyl alcohol (C 5 ), hexyl alcohol (C 6 ), heptyl alcohol (C 7 ), octyl alcohol (C 8 ) and isomers thereof such as 2-ethylhexyl alcohol (C 8 ), nonyl alcohol (C 9), decyl alcohol (C 10), dodecyl alcohol (C 12), tetradecyl alcohol (C 14), Hexadecyl alcohol (C 16), to
- Examples of the unsaturated aliphatic monohydric alcohol include those obtained by substituting one of the C—C single bonds of the saturated aliphatic monohydric alcohol with a C ⁇ C double bond, such as oleyl alcohol. It is commercially available from Shin Nippon Rika Co., Ltd. under the names of the Jamaica Coal series and the Angelo All series.
- Examples of the compound (B) include (b 1 ) ethers of chain hydrocarbon tetraol and at least one aliphatic monohydric alcohol, such as monoether, diether, triether and tetraether, preferably diether, triether.
- Ethers and tetraethers more preferably triethers and tetraethers, and even more preferably tetraethers, ethers of (b 2 ) chain hydrocarbon triols and at least one aliphatic monohydric alcohol, such as monoethers, diethers and Triethers, preferably diethers and triethers, and more preferably triethers, and (b 3 ) ethers of chain hydrocarbon diols with at least one aliphatic monohydric alcohol, such as monoethers and diethers, and preferably Diether It is below.
- Examples of the ether of the chain hydrocarbon tetraol and at least one aliphatic monohydric alcohol include, for example, the following formulas (10) to (13): (In the formula, R 10 to R 13 are each a chain hydrocarbon.) And tetraethers, triethers, diethers and monoethers of pentaerythritol and aliphatic monohydric alcohols.
- Examples of the ether of the chain hydrocarbon triol and at least one aliphatic monohydric alcohol include, for example, the following formulas (14) to (16): (Wherein R 14 to R 16 are each a chain hydrocarbon.) And triether, diether and monoether of glycerin and aliphatic monohydric alcohol.
- Examples of the ether of the chain hydrocarbon diol and at least one aliphatic monohydric alcohol include the following formula (17): R 17 OC n H 2n OR 18 (17) (Wherein n is an integer from 2 to 6 and R 17 and R 18 are each a chain hydrocarbon) A diether of a C 2 -C 6 glycol and an aliphatic monohydric alcohol, and the following formula (18): R 17 OC n H 2n OH (18) (Wherein n is an integer from 2 to 6 and R 17 is a chain hydrocarbon) And monoethers of C 2 -C 6 glycols and aliphatic monohydric alcohols.
- the total number of carbon atoms of the aliphatic monohydric alcohol constituting the tetraether of pentaerythritol and aliphatic monohydric alcohol that is, in the above formula (10)
- the IOB is 0.44. Therefore, in the tetraether of pentaerythritol and aliphatic monohydric alcohol, when the total number of carbon atoms of the aliphatic monohydric alcohol is about 4 or more, the IOB is about 0.00 to about 0.60. Fulfill.
- the triether of pentaerythritol and aliphatic monohydric alcohol the total number of carbon atoms of the aliphatic monohydric alcohol constituting the triether of pentaerythritol and aliphatic monohydric alcohol, that is, in the above formula (11),
- IOB is 0.57. Therefore, the triether of pentaerythritol and aliphatic monohydric alcohol satisfies the requirement that the IOB is about 0.00 to about 0.60 when the total number of carbon atoms of the aliphatic monohydric alcohol is about 9 or more. Fulfill.
- the total number of carbon atoms of the aliphatic monohydric alcohol constituting the diether of pentaerythritol and aliphatic monohydric alcohol that is, in the formula (12), R 10
- the IOB is 0.60. Therefore, in the diether of pentaerythritol and aliphatic monohydric alcohol, when the total number of carbon atoms of the aliphatic monohydric alcohol is about 15 or more, the IOB satisfies the requirement of about 0.00 to about 0.60. .
- the carbon number of the aliphatic monohydric alcohol constituting the monoether of pentaerythritol and aliphatic monohydric alcohol that is, in the above formula (13), R 10
- the IOB is 0.59. Therefore, in the monoether of pentaerythritol and aliphatic monohydric alcohol, the IOB satisfies the requirement of about 0.00 to about 0.60 when the aliphatic monohydric alcohol has about 22 or more carbon atoms.
- the total number of carbon atoms of the aliphatic monohydric alcohol constituting the triether of glycerin and aliphatic monohydric alcohol that is, in the formula (14), R
- the IOB is 0.50. Therefore, in the above triether of glycerin and aliphatic monohydric alcohol, when the total number of carbon atoms of the aliphatic monohydric alcohol is about 3 or more, IOB satisfies the requirement of about 0.00 to about 0.60. .
- the total number of carbon atoms of the aliphatic monohydric alcohol constituting the diether of glycerin and aliphatic monohydric alcohol that is, in the formula (15), R 14 and R 15
- the IOB is 0.58. Therefore, in the diether of glycerin and aliphatic monohydric alcohol, the IOB satisfies the requirement of about 0.00 to about 0.60 when the total number of carbon atoms of the aliphatic monohydric alcohol is about 9 or more.
- the carbon number of the aliphatic monohydric alcohol constituting the monoether of glycerin and aliphatic monohydric alcohol that is, the carbon of R 14 moiety in the formula (16).
- the IOB is 0.58. Therefore, in the monoether of glycerin and aliphatic monohydric alcohol, the IOB satisfies the requirement of about 0.00 to about 0.60 when the aliphatic monohydric alcohol has about 16 or more carbon atoms.
- the IOB is about 0. Satisfy requirements of 00 to about 0.60.
- Compound (B) can be produced by dehydrating condensation of compound (B1) and compound (B2) in the presence of an acid catalyst.
- C1 Carboxylic acids, hydroxy acids, alkoxy acids or oxo acids (hereinafter referred to as “compounds”) containing a chain hydrocarbon moiety and 2 to 4 carboxyl groups that replace the hydrogen atoms of the chain hydrocarbon moiety.
- C1) may be referred to as, for example, a chain hydrocarbon carboxylic acid having 2 to 4 carboxyl groups, such as a chain hydrocarbon dicarboxylic acid such as an alkanedicarboxylic acid such as ethanedioic acid.
- the compound (C1) includes a chain hydrocarbon hydroxy acid having 2 to 4 carboxyl groups, for example, a chain chain having 2 to 4 carboxyl groups such as malic acid, tartaric acid, citric acid, isocitric acid and the like. Hydrocarbon alkoxy acids such as O-acetylcitric acid and chain hydrocarbon oxoacids having 2 to 4 carboxyl groups are included.
- C2 Examples of the compound having a chain hydrocarbon moiety and one hydroxyl group substituting a hydrogen atom of the chain hydrocarbon moiety include those listed in the section of “Compound (B)”, for example, An aliphatic monohydric alcohol is mentioned.
- compound (D) a compound having any one bond selected from the group consisting of a carbonate bond (—OCOO—)
- compound (D) a compound having any one bond selected from the group consisting of a carbonate bond (—OCOO—)
- compound (D) aliphatic
- examples include ethers of monohydric alcohols and aliphatic monohydric alcohols, (d 2 ) dialkyl ketones, esters of (d 3 ) fatty acids and aliphatic monohydric alcohols, and (d 4 ) dialkyl carbonates.
- the ether As the aliphatic monohydric alcohol constituting the ether (corresponding to R 19 OH and R 20 OH in the formula (19)), the ether satisfies the requirements for the IOB, melting point and water solubility.
- the aliphatic monohydric alcohol listed in the section of “Compound (B)” is not particularly limited.
- the total number of carbon atoms of the aliphatic monohydric alcohol constituting the ether that is, the number of carbon atoms of R 19 and R 20 in the above formula (19). Since the IOB is 0.50 when the sum of the two is 2, the IOB requirement is satisfied if the total number of carbon atoms is about 2 or more. However, when the total number of carbon atoms is about 6, the water solubility is as high as about 2 g, which is problematic from the viewpoint of vapor pressure. In order to satisfy the requirement of water solubility of about 0.00 to about 0.05 g, the total number of carbon atoms is preferably about 8 or more.
- dialkylketone As the dialkyl ketone, the following formula (20): R 21 COR 22 (20) (Wherein R 21 and R 22 are each an alkyl group) The compound which has is mentioned.
- the IOB requirement is satisfied if the total number of carbon atoms is about 5 or more.
- the water solubility is as high as about 2 g. Therefore, in order to satisfy the requirement of water solubility of about 0.00 to about 0.05 g, the total number of carbon atoms is preferably about 8 or more.
- the carbon number is preferably about 10 or more, and more preferably about 12 or more.
- the melting point is about ⁇ 50 ° C.
- the vapor pressure is about 230 Pa at 20 ° C.
- the dialkyl ketone can be obtained by a known method, for example, by oxidizing a secondary alcohol with chromic acid or the like.
- ester of the fatty acid and the aliphatic monohydric alcohol include the following formula (21): R 23 COOR 24 (21) (Wherein R 23 and R 24 are each a chain hydrocarbon) The compound which has is mentioned.
- Examples of the fatty acid constituting the ester are listed in “(a 1 ) ester of chain hydrocarbon tetraol and at least one fatty acid”.
- Fatty acids that is, saturated fatty acids or unsaturated fatty acids can be mentioned, and saturated fatty acids are preferable in consideration of the possibility of modification by oxidation or the like.
- Examples of the aliphatic monohydric alcohol constituting the ester include the aliphatic monohydric alcohols listed in the section “Compound (B)”.
- the total number of carbon atoms of the fatty acid and the aliphatic monohydric alcohol is 5
- the above IOB requirement is satisfied when the total number of carbon atoms in the R 23 C and R 24 portions is about 5 or more.
- the total number of carbon atoms is preferably about 12 or more. If the total number of carbon atoms is about 11 or more, the water solubility can satisfy the requirement of about 0.00 to about 0.05 g.
- ester of the fatty acid and the aliphatic monohydric alcohol examples include, for example, an ester of dodecanoic acid (C 12 ) and dodecyl alcohol (C 12 ), tetradecanoic acid (C 14 ), and dodecyl alcohol (C 12 ).
- ester of the above fatty acids and aliphatic monohydric alcohols examples include Electol WE20 and Electol WE40 (manufactured by NOF Corporation).
- the dialkyl carbonate since the IOB is 0.57 when the total number of carbon atoms of R 25 and R 26 is 6, if the total number of carbon atoms of R 25 and R 26 is about 6 or more, Satisfy requirements. In consideration of water solubility, the total number of carbon atoms of R 25 and R 26 is preferably about 7 or more, and more preferably about 9 or more.
- the dialkyl carbonate can be synthesized by a reaction between phosgene and an alcohol, a reaction between a chlorinated formate and an alcohol or an alcoholate, and a reaction between silver carbonate and an alkyl iodide.
- Examples of the polyoxy C 2 -C 6 alkylene glycol, or an ester or ether thereof include (e 1 ) polyoxy C 2 -C 6 alkylene glycol, (e 2 ) polyoxy C An ester of 2 to C 6 alkylene glycol and at least one fatty acid, (e 3 ) an ether of polyoxy C 2 to C 6 alkylene glycol and at least one aliphatic monohydric alcohol, (e 4 ) polyoxy C 2 to C 6 Esters of alkylene glycol with chain hydrocarbon tetracarboxylic acid, chain hydrocarbon tricarboxylic acid, or chain hydrocarbon dicarboxylic acid, and (e 5 ) polyoxy C 2 -C 6 alkylene glycol and chain hydrocarbon tetra Examples include ethers with all, chain hydrocarbon triols, or chain hydrocarbon diols.
- the oxy C 2 -C 6 alkylene skeleton is preferably an oxypropylene skeleton, an oxybutylene skeleton, an oxypentylene skeleton, or an oxyhexylene skeleton from the viewpoint of lowering the IOB of the polyoxy C 2 -C 6 alkylene glycol. More preferred is an oxybutylene skeleton, an oxypentylene skeleton or an oxyhexylene skeleton.
- the polyoxy C 2 -C 6 alkylene glycol is represented by the following formula (23): HO- (C m H 2m O) n -H (23) (Where m is an integer from 2 to 6) Can be represented by:
- the homopolymer of formula (23) may include a homopolymer of propylene glycol, butylene glycol, pentylene glycol or hexylene glycol. From the above, in formula (23), m is about 3 to about 6, more preferably about 4 to about 6, and n is 2 or more.
- the value of n is such that the polyoxy C 2 -C 6 alkylene glycol has an IOB of about 0.00 to about 0.60, a melting point of about 45 ° C. or less, and 100 g of water at 25 ° C. A value having a water solubility of about 0.00 to about 0.05 g.
- the weight average molecular weight of the polyoxy C 4 -C 6 alkylene glycol is preferably about 200 to about 10,000, more preferably about 250 to about 8,000, and even more preferably, It is in the range of about 250 to about 5,000.
- the weight average molecular weight of polyoxy C 3 alkylene glycol, ie, polypropylene glycol is preferably about 1,000 to about 10,000, more preferably about 3,000 to about 8 , And more preferably in the range of about 4,000 to about 5,000. This is because, when the weight average molecular weight is less than about 1,000, the water solubility does not satisfy the requirement, and the higher the weight average molecular weight, the more the absorber transfer speed and the whiteness of the top sheet tend to improve.
- ester of polyoxy C 2 -C 6 alkylene glycol and at least one fatty acid examples include the OH terminal of the polyoxy C 2 -C 6 alkylene glycol described in the section “(e 1 ) polyoxy C 2 -C 6 alkylene glycol”. In which one or both of them are esterified with a fatty acid, that is, monoesters and diesters.
- Examples of the fatty acid to be esterified in the ester of polyoxy C 2 -C 6 alkylene glycol and at least one fatty acid are listed in “Ester of (a 1 ) chain hydrocarbon tetraol and at least one fatty acid”.
- Fatty acid that is, saturated fatty acid or unsaturated fatty acid, and saturated fatty acid is preferable in consideration of possibility of modification by oxidation or the like.
- Examples of commercially available esters of the above polyoxy C 2 -C 6 alkylene glycol and fatty acid include Wilbright cp9 (manufactured by NOF Corporation).
- Examples of the aliphatic monohydric alcohol to be etherified in the ether of polyoxy C 2 -C 6 alkylene glycol and at least one aliphatic monohydric alcohol include, for example, the aliphatic enumerated in the section of “Compound (B)”. A monohydric alcohol is mentioned.
- Esters of the above polyoxy C 2 -C 6 alkylene glycol and chain hydrocarbon tetracarboxylic acid, chain hydrocarbon tricarboxylic acid, or chain hydrocarbon dicarboxylic acid are commercially available, and chain hydrocarbon tetracarboxylic acid. It can be produced by polycondensing an oxy C 2 -C 6 alkylene glycol to an acid, a chain hydrocarbon tricarboxylic acid, or a chain hydrocarbon dicarboxylic acid under known conditions.
- chain hydrocarbon tetraol, chain hydrocarbon triol, and chain hydrocarbon diol to be etherified include those described in the section of “Compound (A)”, such as pentaerythritol, glycerin, and glycol. Is mentioned.
- Examples of commercially available ethers of the above polyoxy C 2 -C 6 alkylene glycol and chain hydrocarbon tetraol, chain hydrocarbon triol, or chain hydrocarbon diol include, for example, Unilube (trademark) 5TP-300KB, and And Uniol (trademark) TG-3000 and TG-4000 (manufactured by NOF Corporation).
- Unilube (trademark) 5TP-300KB is a compound obtained by polycondensation of 65 mol of propylene glycol and 5 mol of ethylene glycol to 1 mol of pentaerythritol, its IOB is 0.39, and its melting point is less than 45 ° C. Yes, and the water solubility was less than 0.05 g.
- Uniol TM TG-3000 is a compound obtained by polycondensation of 50 mol of propylene glycol with 1 mol of glycerin, its IOB is 0.42, its melting point is less than 45 ° C., and its water solubility is 0.05 g. And the weight average molecular weight was about 3,000.
- Uniol TM TG-4000 is a compound obtained by polycondensation of 70 mol of propylene glycol with 1 mol of glycerin, its IOB is 0.40, its melting point is less than 45 ° C., and its water solubility is 0.05 g. And the weight average molecular weight was about 4,000.
- the ether of the polyoxy C 2 -C 6 alkylene glycol and the chain hydrocarbon tetraol, the chain hydrocarbon triol, or the chain hydrocarbon diol is also a chain hydrocarbon tetraol, a chain hydrocarbon triol, or It can be produced by adding C 2 -C 6 alkylene oxide to a chain hydrocarbon diol under known conditions.
- the chain hydrocarbon Since the chain hydrocarbon has an inorganic value of 0, the IOB is 0.00 and the water solubility is approximately 0 g. Therefore, if the melting point is about 45 ° C. or less, the blood It can be included in the modifier.
- the chain hydrocarbon include (f 1 ) chain alkanes such as straight chain alkanes and branched chain alkanes. For example, in the case of straight chain alkanes, the melting point is about 45 ° C. or less. In general, those having 22 or less carbon atoms are included. Moreover, when the vapor pressure is taken into consideration, those having 13 or more carbon atoms are generally included.
- the melting point may be lower at the same number of carbons than that of a straight chain alkane. Therefore, those having 22 or more carbon atoms may be included.
- a commercial item of the above-mentioned hydrocarbon for example, Pearl Ream 6 (NOF Corporation) can be mentioned.
- the above-mentioned blood modifying agent is considered in detail together with the examples, but it has been found that it has at least an action of lowering blood viscosity and surface tension.
- menstrual blood to be absorbed by the absorbent article contains proteins such as the endometrial wall. Cheap. Therefore, menstrual blood to be absorbed by the absorbent article tends to have a high viscosity, and when the top sheet is a nonwoven fabric or a woven fabric, the menstrual blood tends to clog between the fibers, and the wearer feels sticky. Almost, and menstrual blood diffuses on the surface of the topsheet, making it easier to leak.
- the mid-high portion includes a blood modifying agent that has been found to have at least the effect of lowering blood viscosity and surface tension
- the top sheet is a nonwoven or woven fabric, between the fibers of the top sheet
- menstrual blood is not easily clogged in the cushion portion, and menstrual blood can be quickly transferred from the top sheet to the absorbent body via the cushion portion.
- the absorbent article of the present disclosure has a cushion portion between the top sheet and the absorbent body in the middle and high portions, and therefore the interval between the top sheet and the absorbent body tends to be larger than that of conventional absorbent articles. There is. Therefore, when the middle and high parts contain a blood modifying agent, blood that has reached the top sheet can be quickly transferred to the absorbent body via the cushion part, and therefore it is more difficult to leak.
- a blood modifying agent having an IOB of about 0.00 to about 0.60 is highly organic and easily enters between blood cells, so that it stabilizes the blood cells and makes it difficult to form a monetary structure in the blood cells. .
- the above-mentioned modifying agent stabilizes blood cells and makes it difficult to form a monetary structure on the blood cells, so that the absorbent body can easily absorb menstrual blood.
- SAP an acrylic superabsorbent polymer
- the collected blood cells cover the SAP surface and the SAP does not easily exhibit the absorption performance.
- the blood modifying agent having high affinity for red blood cells protects the red blood cell membrane, the red blood cells are hardly destroyed.
- liquid-permeable top sheet those normally used in the art can be employed without particular limitation, for example, a sheet-like material having a structure that allows blood to pass through, for example, an apertured film, A woven fabric, a nonwoven fabric, etc. are mentioned.
- the fibers constituting the woven fabric and the nonwoven fabric include natural fibers and chemical fibers.
- natural fibers include cellulose such as pulverized pulp and cotton.
- chemical fibers include rayon and fibrillar rayon. And regenerated cellulose, semi-synthetic cellulose such as acetate and triacetate, thermoplastic hydrophobic chemical fibers, and thermoplastic hydrophobic chemical fibers subjected to hydrophilic treatment.
- thermoplastic hydrophobic chemical fiber examples include single fibers such as polyethylene (PE), polypropylene (PP), and polyethylene terephthalate (PET), and fibers made of a graft polymer of PE and PP.
- nonwoven fabric examples include air-through nonwoven fabric, spunbond nonwoven fabric, point bond nonwoven fabric, spunlace nonwoven fabric, needle punch nonwoven fabric, melt blown nonwoven fabric, and combinations thereof (for example, SMS).
- liquid-impermeable back sheet examples include films containing PE, PP, etc., resin films having air permeability, those obtained by bonding a resin film having air permeability to a nonwoven fabric such as spunbond or spunlace, and composite materials such as SMS.
- a layer nonwoven fabric etc. are mentioned.
- a low density polyethylene (LDPE) film having a basis weight of about 15 to about 30 g / m 2 is preferred.
- Constituent elements of the absorbent core include, for example, hydrophilic fibers such as cellulose such as pulverized pulp and cotton, regenerated cellulose such as rayon and fibril rayon, semi-synthetic cellulose such as acetate and triacetate, particulate polymer, and fibrous polymer. , Thermoplastic hydrophobic chemical fibers, hydrophobized thermoplastic hydrophobic chemical fibers, and combinations thereof.
- a superabsorbent polymer for example, a granular material such as sodium acrylate copolymer may be mentioned.
- the core wrap is not particularly limited as long as it is liquid permeable and has a barrier property that does not allow the polymer absorber to permeate, and examples thereof include woven fabric and nonwoven fabric.
- examples of the woven fabric and non-woven fabric include natural fibers, chemical fibers, and tissues.
- the absorbent body As a second example of the absorbent body, one formed from an absorbent sheet or a polymer sheet can be cited, and the thickness is preferably about 0.3 to about 5.0 mm.
- the absorbent sheet and polymer sheet can be used without particular limitation as long as they are usually used in absorbent articles such as sanitary napkins.
- the blood modifying agent can be present at any location, such as the entire surface of the top sheet or the central region near the vaginal opening, with respect to the planar direction of the top sheet.
- the middle and high portions include the blood modifying agent
- the blood modifying agent when the liquid-permeable top sheet is formed from a nonwoven fabric or a woven fabric, the blood modifying agent is provided between the fibers of the nonwoven fabric or the woven fabric.
- the blood modifying agent is preferably adhered to the surface of the non-woven fiber in the form of droplets or particles, or the surface of the fiber can be covered.
- the middle and high portions contain the blood modifying agent
- the blood modifying agent closes the aperture of the apertured film.
- the blood modifying agent can be attached in the form of droplets or particles on the surface of the apertured film, for example. This is because when the blood modifying agent closes the gap between the fibers of the nonwoven fabric or the woven fabric or the aperture of the apertured film, the absorbed liquid may be inhibited from transferring to the absorber.
- the blood modifying agent preferably has a large surface area in order to quickly migrate to absorbed blood, and is present in the form of droplets or particles.
- the blood modifying agent preferably has a small particle size.
- the middle and high portions contain the blood modifying agent
- these are hydrophilic agents. It is preferable that the surface is hydrophilized by coating or mixing. If the original material is hydrophilic, then it has an IOB of about 0.00 to about 0.60 and is coated with a highly organic and lipophilic modifier, so that the lipophilic region and hydrophilic The area will coexist sparsely. Thereby, a certain absorption performance is exhibited for menstrual blood composed of a hydrophilic component (plasma and the like) and a lipophilic component (blood cell and the like).
- the method for applying the blood modifying agent is not particularly limited, and is heated as necessary, for example, a non-contact coater, for example, a spiral. It can apply
- a non-contact type coater is preferable from the viewpoint that the droplet-like or particulate modifier is uniformly dispersed throughout and the point of not damaging the material.
- the blood modifying agent is applied from the control seam HMA gun as it is when it is liquid at room temperature, or heated to lower the viscosity, and heated so as to be liquefied when it is solid at room temperature. be able to. By increasing the air pressure of the control seam HMA gun, the particulate blood modifying agent can be applied.
- the blood modifying agent can be applied when producing a topsheet material, for example, a nonwoven fabric, or a production line for producing an absorbent article. Can also be applied.
- the middle and high portions contain the blood modifying agent, from the viewpoint of suppressing the capital investment, it is preferable to apply the blood modifying agent in the production line of the absorbent article, and the blood modifying agent falls off. In order to suppress contamination of the line, it is preferable to apply the blood modifying agent downstream of the production line, specifically, immediately before the product is enclosed in the individual package.
- the blood modifying agent may also have an action as a lubricant.
- the top sheet is a nonwoven fabric, the friction between the fibers can be reduced, so that the flexibility of the entire nonwoven fabric is improved.
- a top sheet is a resin film, friction with a top sheet and skin can be reduced.
- the blood modifying agent preferably has a weight average molecular weight of about 2,000 or less, and more preferably has a weight average molecular weight of 1,000 or less. preferable. This is because when the weight average molecular weight increases, it becomes difficult to lower the viscosity of the blood modifying agent to a viscosity suitable for coating, and there is a case where the blood modifying agent should be diluted with a solvent. Moreover, when the weight average molecular weight is increased, the blood modifying agent is tacky, which may cause discomfort to the wearer.
- the absorbent article is suitable for absorbent articles intended to absorb blood, such as sanitary napkins and panty liners.
- an air-through non-woven fabric having a core-sheath structure formed from a polyethylene (PE) sheath and a polyethylene terephthalate (PET) core and made of a hydrophilic treated composite fiber (fineness: 2.2 dtex) Amount: 30 g / m 2 ) was prepared.
- the cushion portion before being incorporated into the absorbent article has a shape in which the projection in the thickness direction of the absorbent article is substantially elliptical and has a constant thickness, as shown in FIG.
- the length in the longitudinal direction was 70 mm, and the length in the width direction was 30 mm.
- the absorbent body was formed by winding a mixture (basis weight: 250 g / m 2 ) of pulp and SAP blended at 90:10 (mass ratio) with a tissue (basis weight: 14 g / m 2 ).
- a back sheet a film containing PE (basis weight: 23 g / m 2 ) coated with an adhesive was prepared.
- the absorbent body, the cushion part, and the top sheet were laminated in order, and the absorbent body and the top sheet were squeezed to form a middle and high part as shown in FIGS.
- the absorbent article and the back sheet are bonded and cut as shown in FIGS. 1-1 was produced.
- Absorbent article no. In 1-1 the length of the middle-high portion in the longitudinal direction was about 70 mm, and the length of the middle-high portion in the width direction was about 35 mm. Also, the absorbent article No. The longitudinal length of 1-1 was about 176 mm, and the length in the width direction was about 80 mm.
- the absorbent article No. 1 was changed except that the types of materials constituting the cushion portion, the average basis weight, and the like were changed as described in Table 2 below. 1-2 to No. 1-6 was produced.
- the absorbent article No. 1-2 to No. 1-4 is an absorbent article no. 1-1 and the basis weight of the air-through nonwoven fabric constituting the cushion portion are different.
- 1-2, no. 1-3 and no. 1-4 cushion portions were formed by laminating 3, 10 and 25 air-through nonwoven fabrics (basis weight: 30 g / m 2 ) with an adhesive, respectively.
- the cushion part 1-4 was made to have a smaller size as the air-through nonwoven fabric of the upper layer and to have a shape as shown in FIG. 7D as a whole.
- Absorbent article no. 1-5 and No. 1 The pulp and web in 1-6 were molded into the shape shown in FIG. 7 (d).
- the compression resilience RC and the compression work WC in the middle portion of 1-6 middle and high portions are also shown in Table 2 below.
- Absorbent article no. 1-1-No. A plurality of sheets 1-6 were prepared and worn by a plurality of subjects, and evaluated for compressibility and recoverability (leakage) according to the following criteria. In addition, since it is difficult to experience the recoverability directly, it was determined that the recoverability was sufficient when there was no leakage.
- the absorbent article No. 1-3 and No. 1 1-4 the thickness of the cushion portion at the middle portion of the middle-high portion is substantially the same as the thickness of the original cushion portion, but the thickness of the cushion portion at the outer peripheral portion of the middle-high portion is equal to the thickness of the original cushion portion. It was even thinner. Therefore, the absorbent article No. 1-3 and No. 1 In 1-4, it is clear that the density of the cushion part in the outer peripheral part of the middle-high part is higher than the density of the cushion part in the middle part of the middle-high part.
- Example 2 Absorbent article no. 2-1. Prepare 2-8 each, and have multiple subjects wear them, and evaluated the bendability and recoverability from the folded absorbent article according to the following criteria: . ⁇ : No problem in bending and recovery. X: There was a problem in bendability and / or recoverability. The results are shown in Table 3 below.
- Panacet 810s manufactured by NOF Corporation, triester of glycerin and fatty acid
- the absorbent article No By applying panacet 810s with a basis weight of 5.0 g / m 2 from a control seam HMA gun at room temperature, centering on the middle and high portions of 2-4, absorbent article No. 3-1.
- panacet 810s When confirmed with an electron microscope, panacet 810s was in the form of fine particles and adhered to the surface of the fibers of the top and middle portions of the top sheet.
- Example 3 Absorbent article no. When 10 subjects wore 2-4, in a total of 20 tests, they answered that the surface contamination area was small and leakage was small. In addition, both dry and wet, it deformed following the movement of the leg, and it was answered that it was excellent in the fit between the mid-high and the labia. Next, the absorbent article No. When 10 subjects wore 3-1, the absorbent article no. Compared with 2-4, the answer was that the area of surface contamination was small and the leakage was small.
- Example 4 [Other blood modifying agent data] A commercially available sanitary napkin was prepared.
- the sanitary napkin includes a top sheet formed from an air-through nonwoven fabric (composite fiber made of polyester and polyethylene terephthalate, basis weight: 35 g / m 2 ) treated with a hydrophilic agent, and an air-through nonwoven fabric (composite made of polyester and polyethylene terephthalate).
- Second sheet formed from fibers, basis weight: 30 g / m 2 ), pulp (basis weight: 150 to 450 g / m 2 , more in the center), acrylic superabsorbent polymer (basis weight: 15 g / m 2 ) And an absorbent body containing a tissue as a core wrap, a side sheet treated with a water repellent, and a back sheet made of a polyethylene film.
- fatty acids manufactured by NOF Corporation C 8 fatty to C 10 are contained in approximately 85:15 weight ratio of triesters of glycerol with fatty acids, the weight average molecular weight: about 480 ⁇ Panasate 800, manufactured by NOF Corporation All fatty acids are octanoic acid (C 8 ), triester of glycerin and fatty acid, weight average molecular weight: about 470
- Panaceate 800B manufactured by NOF Corporation All fatty acids are 2-ethylhexanoic acid (C 8 ), triester of glycerin and fatty acid, weight average molecular weight: about 470 ⁇ NA36, manufactured by NOF Corporation C 16 fatty acid: fatty acid C 18: (including both saturated and unsaturated fatty acids) C 20 fatty acid approximately 5: contained at a weight ratio of 3: 92 Triester of glycerin and fatty acid, weight average molecular weight: about 880
- C 8 fatty C 10: fatty acid
- C 12 fatty acid
- C 14 (including both saturated and unsaturated fatty acids)
- C 16 is approximately 4 : Triester of glycerin and fatty acid, contained in a mass ratio of 8: 60: 25: 3, weight average molecular weight: 670 -Caprylic acid diglyceride, manufactured by NOF Corporation
- Fatty acid is octanoic acid, diester of glycerin and fatty acid, weight average molecular weight: 340
- Uniol PB1000R polybutylene glycol manufactured by NOF Corporation, weight average molecular weight: about 1000 [(E 2 ) ester of polyoxy C 2 -C 6 alkylene glycol and at least one fatty acid] -Wilbright cp9, manufactured by NOF Co., Ltd.
- Uniol TG-3000 manufactured by NOF Corporation Polyglycol glycol glyceryl ether, about 16 repeating units, weight average molecular weight: about 3,000 ⁇ Uniol TG-4000, glyceryl ether of polypropylene glycol manufactured by NOF Corporation, about 16 repeating units, weight average molecular weight: about 4,000
- [Other materials] -NA50 manufactured by NOF Corporation Hydrogen added to NA36 to reduce the ratio of double bonds derived from the unsaturated fatty acid as a raw material Triester of glycerin and fatty acid, weight average molecular weight: about 880 -(Caprylic acid / capric acid) monoglyceride, NOF Corporation octanoic acid (C 8 ) and decanoic acid (C 10 ) in a mass ratio of approximately 85:15, monoester of glycerin and fatty acid, Weight average molecular weight: about 220 -Monomuls 90-L2 lauric acid monoglyceride, manufactured by Cognis Japan
- PEG 1500 manufactured by NOF Corporation, polyethylene glycol, weight average molecular weight: about 1,500 to about 1,600 Nonionic S-6, polyoxyethylene monostearate manufactured by NOF Corporation, about 7 repeating units, weight average molecular weight: about 880 -Wilbright s753, NOF Corporation polyoxyethylene polyoxypropylene polyoxybutylene glycerin, weight average molecular weight: about 960
- the IOB, melting point and water solubility of the above sample are shown in Table 4 below.
- the water solubility was measured according to the method described above, but 20.0 g was added to 100 g of demineralized water, and the sample dissolved after 24 hours was evaluated as “20 g ⁇ ”. Samples that dissolved 0.05 g but did not dissolve 1.00 g were evaluated as 0.05 to 1.00 g.
- “ ⁇ 45” means that the melting point is less than 45 ° C.
- the skin contact surface of the top sheet of the sanitary napkin was coated with the blood modifying agent described above.
- Each blood modifying agent is heated as it is if the blood modifying agent is liquid at room temperature, and if the blood modifying agent is solid at room temperature, it is heated to melting point + 20 ° C., and then control seam HMA gun
- Each blood modifying agent was atomized and applied to the entire skin contact surface of the top sheet so that the basis weight was approximately 5 g / m 2 .
- FIG. 8 is an electron micrograph of the skin contact surface of the top sheet in a sanitary napkin (No. 3-5) in which the top sheet contains tri-C2L oil fatty acid glycerides. As is clear from FIG. 8, the tri-C2L oil fatty acid glyceride is in the form of fine particles and adheres to the fiber surface.
- Rewetting rate (%) 100 ⁇ (filter paper mass after test ⁇ initial filter paper mass) / 6
- absorption body transfer rate which is the time for blood to transfer from the top sheet to the absorber, was measured after the second drop of blood.
- absorption body transfer speed means the time from when blood is introduced into the top sheet until the redness of blood is not seen on the surface and inside of the top sheet.
- the whiteness of the skin contact surface of the top sheet after the absorber transfer speed test was visually evaluated according to the following criteria.
- the rewetting rate was 22.7% and the absorber transfer rate was more than 60 seconds, but all triesters of glycerin and fatty acids had a rewetting rate. Since it is 7.0% or less and the absorber transfer speed is 8 seconds or less, it can be seen that the absorption performance is greatly improved. However, among the triesters of glycerin and fatty acid, NA50 having a melting point exceeding 45 ° C. did not significantly improve the absorption performance.
- a blood modifying agent having an IOB of about 0.00 to about 0.60, a melting point of about 45 ° C. or less, and a water solubility of about 0.00 to about 0.05 g for 100 g of water at 25 ° C. It was found that the absorption performance was greatly improved.
- Example 4 is an example in which the sanitary napkin does not have a predetermined middle and high part, no. 4-1.
- the 4-32 sanitary napkin is No. 4 containing panacet 810s. No. 4-7 is obtained because the same result is obtained.
- the blood modifying agent used in the 4-32 sanitary napkin is an absorbent article no.
- the absorbent article No. 2 coated with the blood modifying agent Panasate 810s It is considered that a result equivalent to 3-1 can be obtained.
- Example 5 The rewetting rate was evaluated according to the procedure described above for various animal blood.
- the blood used in the experiment is as follows. [Animal species] (1) Human (2) Horse (3) Sheep
- Defibrinated blood After collecting blood, stirred for about 5 minutes in an Erlenmeyer flask with glass beads
- ⁇ EDTA blood 65 mL of venous blood plus 0.5 mL of 12% EDTA / 2K physiological saline
- Serum or plasma supernatant after centrifuging defibrinated blood or EDTA blood, respectively, at room temperature at about 1900 G for 10 minutes.
- Blood cell serum is removed from the blood, and the residue is phosphate buffered saline (PBS ) Washed twice and then added with phosphate buffered saline for the removed serum
- An absorbent article was produced in the same manner as in Example 4 except that the tri-C2L oil fatty acid glyceride was applied so that the basis weight was approximately 5 g / m 2, and the rewetting rate was evaluated for the various blood types described above. . The measurement was performed 3 times for each blood, and the average value was adopted. The results are shown in Table 5 below.
- Example 4 The same tendency as the horse EDTA blood obtained in Example 4 was also obtained in human and sheep blood. Similar trends were also observed in defibrinated blood and EDTA blood.
- Tri C2L oil fatty acid glyceride is applied to the skin contact surface of the top sheet formed from an air-through nonwoven fabric (a composite fiber composed of polyester and polyethylene terephthalate, basis weight: 35 g / m 2 ) using a control seam HMA gun. It is atomized and applied so that the basis weight is approximately 5 g / m 2 . Moreover, the thing which has not apply
- the mass (c) of the top sheet containing the cell strainer + horse EDTA blood is measured.
- the post-test absorption amount (g) per 1 g of the top sheet is calculated according to the following formula.
- Absorption after test [mass (c) ⁇ mass (a)] / 0.2 (9)
- the top sheet containing a blood modifying agent has low blood retention and can be quickly transferred to an absorber after absorbing blood.
- Example 7 [Viscosity of blood containing blood modifying agents] The viscosity of blood containing a blood modifying agent was measured using a Rheometric Expansion System ARES (Rheometric Scientific, Inc). 2% by mass of panacet 810s was added to equine defibrinated blood, lightly stirred to form a sample, the sample was placed on a parallel plate with a diameter of 50 mm, the gap was 100 ⁇ m, and the viscosity was measured at 37 ⁇ 0.5 ° C. . Because of the parallel plate, the sample did not have a uniform shear rate, but the average shear rate displayed on the instrument was 10 s ⁇ 1 .
- the viscosity of equine defibrinated blood containing 2% by mass of panacet 810s was 5.9 mPa ⁇ s, while the viscosity of equine defibrinated blood containing no blood modifying agent was 50.4 mPa ⁇ s. Therefore, it can be seen that equine defibrinated blood containing 2% by mass of panacet 810s decreases the viscosity by about 90% compared to the case where no blood modifying agent is contained.
- blood contains components such as blood cells and is known to have thixotropic properties
- the blood modifying agent of the present disclosure lowers the viscosity of blood in a low viscosity region. By reducing the viscosity of the blood, the absorbed menstrual blood is quickly transferred from the top sheet to the absorber.
- Example 8 [Micrograph of blood containing blood modifying agent] A healthy volunteer's menstrual blood is collected on a food protective wrap film, and a portion of the panacet 810s dispersed in 10 times the mass of phosphate buffered saline is used, and the concentration of panacet 810s is 1% by mass.
- Menstrual blood was appropriately applied to a slide glass, covered with a cover glass, and the state of red blood cells was observed with an optical microscope.
- a photomicrograph of menstrual blood containing no blood modifying agent is shown in FIG. 9 (a)
- a photomicrograph of menstrual blood containing panacet 810s is shown in FIG. 9 (b).
- red blood cells form a collective mass such as remuneration, but in menstrual blood that includes panacet 810s, It can be seen that it is stably dispersed. Therefore, it is suggested that the blood modifying agent functions to stabilize red blood cells in the blood.
- Example 9 [Surface tension of blood containing blood modifying agents]
- the surface tension of blood containing a blood modifying agent was measured by a pendant drop method using a contact angle meter Drop Master 500 manufactured by Kyowa Interface Science Co., Ltd.
- the surface tension was measured after adding a predetermined amount of blood modifying agent to sheep defibrinated blood and shaking sufficiently. Although the measurement is automatically performed by the instrument, the surface tension ⁇ is obtained by the following equation (see FIG. 10).
- the density ⁇ is defined in 5. of “Density Test Method and Density / Mass / Capacity Conversion Table” of JIS K 2249-1995. The measurement was performed at the temperature shown in Table 7 below in accordance with the vibration density test method. For measurement, DA-505 of Kyoto Electronics Industry Co., Ltd. was used. The results are shown in Table 7.
- the blood modifying agent has a water solubility of about 0.00 to about 0.05 g in 100 g of water at 25 ° C., but the solubility in water is very low. It can be seen that the surface tension of blood can be lowered. By reducing the surface tension of the blood, the absorbed blood is not transferred between the fibers of the top sheet, but is quickly transferred to the absorber.
- the present invention relates to the following J1 to J15.
- An absorbent article comprising a liquid-permeable top sheet, a liquid-impermeable back sheet, and an absorbent body between the liquid-permeable top sheet and the liquid-impermeable back sheet,
- the absorbent article has a middle-high portion protruding in the thickness direction of the absorbent article in the excretory opening contact area,
- the middle-high part includes a part of the top sheet, a cushion part disposed between the top sheet and the absorber, and has a central part and an outer peripheral part surrounding the central part,
- the cushion part has a maximum thickness of 3 to 30 mm
- the middle-high part has a compression work WC of 10 to 50 (N ⁇ m / m 2 ) and a compression resilience RC of 35 to 100% in the central part, and the absorber is 80 to 230 mm. Having a longitudinal length of the absorbent article and a widthwise length of the absorbent article of 30 to 90 mm.
- the middle-high portion further includes a blood modifying agent having an IOB of 0.00 to 0.60, a melting point of 45 ° C. or lower, and a water solubility of 0.00 to 0.05 g with respect to 100 g of water at 25 ° C.
- the blood modifying agent comprises the following (i) to (iii): (I) hydrocarbons, (Ii) from (ii-1) a hydrocarbon moiety and (ii-2) a carbonyl group (—CO—) and an oxy group (—O—) inserted between the CC single bonds of the hydrocarbon moiety.
- —COOH carboxyl group
- —OH hydroxyl group
- the blood modifying agent comprises the following (i ′) to (iii ′), (I ′) hydrocarbon, (Ii ′) (ii′-1) a hydrocarbon moiety and (ii′-2) a carbonyl bond (—CO—), an ester bond (—COO) inserted between the C—C single bonds of the hydrocarbon moiety.
- the blood modifying agent comprises the following (A) to (F), (A) (A1) a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing hydrogen atoms in the chain hydrocarbon moiety, (A2) a chain hydrocarbon moiety, and the chain An ester with a compound having one carboxyl group for substituting a hydrogen atom in the hydrocarbon moiety, (B) (B1) a compound having a chain hydrocarbon moiety and 2 to 4 hydroxyl groups replacing hydrogen atoms in the chain hydrocarbon moiety, (B2) a chain hydrocarbon moiety, and the chain An ether with a compound having one hydroxyl group replacing a hydrogen atom of the hydrocarbon moiety, (C) (C1) a carboxylic acid, a hydroxy acid, an alkoxy acid or an oxo acid containing a chain hydrocarbon moiety and 2 to 4 carboxyl groups replacing a hydrogen atom in the chain hydrocarbon moiety; C2) an ester of a compound having a chain hydrocarbon mo
- the blood modifying agent comprises (a 1 ) an ester of a chain hydrocarbon tetraol and at least one fatty acid, (a 2 ) an ester of a chain hydrocarbon triol and at least one fatty acid, (a 3 ) a chain An ester of a hydrocarbon diol and at least one fatty acid, (b 1 ) an ether of a chain hydrocarbon tetraol and at least one aliphatic monohydric alcohol, (b 2 ) a chain hydrocarbon triol and at least one aliphatic Ethers with monohydric alcohols, (b 3 ) ethers of chain hydrocarbon diols with at least one aliphatic monohydric alcohol, (c 1 ) chain hydrocarbon tetracarboxylic acids having 4 carboxyl groups, hydroxy acids , alkoxy acid or an oxo acid, at least one ester of an aliphatic monohydric alcohol, (c 2) a chain hydrocarbon tricaprate with 3 carboxyl groups
Landscapes
- Health & Medical Sciences (AREA)
- Epidemiology (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Heart & Thoracic Surgery (AREA)
- Vascular Medicine (AREA)
- Biomedical Technology (AREA)
- Chemical & Material Sciences (AREA)
- Hematology (AREA)
- Materials Engineering (AREA)
- Dispersion Chemistry (AREA)
- Absorbent Articles And Supports Therefor (AREA)
- Orthopedics, Nursing, And Contraception (AREA)
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201380028070.1A CN104334133A (zh) | 2012-03-30 | 2013-03-26 | 吸收性物品 |
| EP13767763.9A EP2832326B1 (en) | 2012-03-30 | 2013-03-26 | Absorbent article |
| AU2013241415A AU2013241415B2 (en) | 2012-03-30 | 2013-03-26 | Absorbent article |
| SG11201405410VA SG11201405410VA (en) | 2012-03-30 | 2013-03-26 | Absorbent article |
| US14/389,256 US9233185B2 (en) | 2012-03-30 | 2013-03-26 | Absorbent article |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2012080539A JP5726120B2 (ja) | 2012-03-30 | 2012-03-30 | 吸収性物品 |
| JP2012-080539 | 2012-03-30 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2013146817A1 true WO2013146817A1 (ja) | 2013-10-03 |
Family
ID=49260068
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2013/058859 Ceased WO2013146817A1 (ja) | 2012-03-30 | 2013-03-26 | 吸収性物品 |
Country Status (9)
| Country | Link |
|---|---|
| US (1) | US9233185B2 (enExample) |
| EP (1) | EP2832326B1 (enExample) |
| JP (1) | JP5726120B2 (enExample) |
| CN (1) | CN104334133A (enExample) |
| AR (1) | AR090528A1 (enExample) |
| AU (1) | AU2013241415B2 (enExample) |
| SG (1) | SG11201405410VA (enExample) |
| TW (1) | TWI577351B (enExample) |
| WO (1) | WO2013146817A1 (enExample) |
Families Citing this family (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2015127289A1 (en) | 2014-02-21 | 2015-08-27 | Attends Healthcare Products, Inc. | Absorbent article with fluid control features |
| CN107205852B (zh) * | 2014-07-22 | 2018-10-09 | 花王株式会社 | 短裤型一次性尿布 |
| JP6501294B2 (ja) * | 2014-12-22 | 2019-04-17 | 日本製紙クレシア株式会社 | 吸収性物品 |
| WO2017209006A1 (ja) * | 2016-05-31 | 2017-12-07 | 花王株式会社 | 長繊維不織布 |
| USD843565S1 (en) * | 2017-03-27 | 2019-03-19 | Amber Adams | Combination tampon and menstrual pad |
| US12016756B2 (en) | 2017-12-19 | 2024-06-25 | Lyv Life, Inc. | Ultrathin absorbent hygienic pads |
| USD926974S1 (en) * | 2019-03-01 | 2021-08-03 | Lyv Life, Inc. | Ultrathin absorbent bladder liner with absorbent pod |
| USD917044S1 (en) | 2019-03-01 | 2021-04-20 | Lyv Life, Inc. | Ultrathin absorbent asymmetrical bladder liner with channels |
| USD917043S1 (en) | 2019-03-01 | 2021-04-20 | Lyv Life, Inc. | Ultrathin absorbent asymmetrical bladder liner |
| WO2025061888A1 (en) * | 2023-09-19 | 2025-03-27 | Melo & Co Bv | Moisture absorbing undergarment |
Citations (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2002528174A (ja) | 1998-10-23 | 2002-09-03 | ザ、プロクター、エンド、ギャンブル、カンパニー | 選択的開閉自在開口を含むトップシートを有する吸収体 |
| JP2003024372A (ja) * | 2001-07-12 | 2003-01-28 | Uni Charm Corp | 吸収性物品 |
| JP2003052750A (ja) * | 2001-08-10 | 2003-02-25 | Uni Charm Corp | 吸収性物品 |
| JP2004049529A (ja) * | 2002-07-19 | 2004-02-19 | Kao Corp | 吸収性物品 |
| JP2006115996A (ja) | 2004-10-20 | 2006-05-11 | Kao Corp | 吸収性物品 |
| JP2008025078A (ja) | 2006-06-23 | 2008-02-07 | Uni Charm Corp | 不織布 |
| JP2008023365A (ja) * | 2007-10-03 | 2008-02-07 | Oji Paper Co Ltd | 折り返し部を有する吸収体製品 |
| JP2008025079A (ja) | 2006-06-23 | 2008-02-07 | Uni Charm Corp | 不織布 |
| JP2009297048A (ja) * | 2008-06-10 | 2009-12-24 | Kao Corp | 吸収性物品 |
| JP2011067484A (ja) * | 2009-09-28 | 2011-04-07 | Kao Corp | 吸収性物品 |
| JP2011104059A (ja) * | 2009-11-16 | 2011-06-02 | Kao Corp | 吸収性物品 |
| JP2011120696A (ja) * | 2009-12-09 | 2011-06-23 | Kao Corp | 吸収性物品 |
| JP2011226010A (ja) | 2010-04-16 | 2011-11-10 | Uni Charm Corp | 凹凸を有する不織布を簡易に製造する方法、及び不織布を簡易に加工する方法 |
| JP2011226011A (ja) | 2010-04-16 | 2011-11-10 | Uni Charm Corp | 伸長性繊維と伸縮性繊維とを含む、凹凸を有する不織布、及び当該不織布を製造する方法 |
Family Cites Families (101)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3929135A (en) | 1974-12-20 | 1975-12-30 | Procter & Gamble | Absorptive structure having tapered capillaries |
| JPS5717081A (en) | 1981-05-25 | 1982-01-28 | Noriko Ikegami | Electronic interpreter |
| US4588630A (en) | 1984-06-13 | 1986-05-13 | Chicopee | Apertured fusible fabrics |
| JPH0775616B2 (ja) | 1987-07-30 | 1995-08-16 | 花王株式会社 | 衛生用品の表面材 |
| ES2065900T3 (es) | 1987-07-30 | 1995-03-01 | Kao Corp | Articulo sanitario. |
| US4759754A (en) | 1987-08-26 | 1988-07-26 | Personal Products Company | Sanitary napkin |
| JPH082364B2 (ja) | 1987-12-16 | 1996-01-17 | 花王株式会社 | 衛生用品の表面材 |
| JPH0720856B2 (ja) | 1988-12-01 | 1995-03-08 | 株式会社大塚製薬工場 | 皮膚の殺菌・清拭用組成物 |
| JP2728921B2 (ja) | 1989-03-01 | 1998-03-18 | 花王株式会社 | 凹凸を有する不織布の製造方法 |
| US5334176A (en) | 1991-07-23 | 1994-08-02 | The Procter & Gamble Company | Absorbent core for use in catamenial products |
| DK0549784T4 (da) | 1991-07-23 | 2000-08-28 | Procter & Gamble | Buet, formet absorberende artikel |
| US5591150A (en) | 1991-07-23 | 1997-01-07 | The Procter And Gamble Company | Sanitary napkin having a resilient body-conforming portion |
| MA22660A1 (fr) | 1991-10-01 | 1993-04-01 | Procter & Gamble | Article absorbant comportant des rabats et des zones d'extensibilite differentielle . |
| JP3091283B2 (ja) | 1991-12-09 | 2000-09-25 | 花王株式会社 | 生理用ナプキン |
| JPH065614A (ja) | 1992-06-17 | 1994-01-14 | Murata Mfg Co Ltd | 熱処理装置 |
| AU6776394A (en) | 1993-05-21 | 1994-12-20 | Procter & Gamble Company, The | Absorbent article having anti-wicking crimp seal |
| JPH0784697A (ja) | 1993-09-10 | 1995-03-31 | Canon Inc | 情報処理装置 |
| US5614283A (en) | 1994-12-22 | 1997-03-25 | Tredegar Industries | Absorbent composite with three-dimensional film surface for use in absorbent disposable products |
| JP2974934B2 (ja) | 1995-06-02 | 1999-11-10 | ザ、プロクター、エンド、ギャンブル、カンパニー | 生理用ナプキン |
| US5650214A (en) | 1996-05-31 | 1997-07-22 | The Procter & Gamble Company | Web materials exhibiting elastic-like behavior and soft, cloth-like texture |
| US5891126A (en) | 1996-08-30 | 1999-04-06 | The Procter & Gamble Company | Absorbent interlabial device treated with a polysiloxane emollient |
| JPH1095810A (ja) | 1996-09-24 | 1998-04-14 | Oji Paper Co Ltd | 高吸水性樹脂及びその製造方法 |
| TW538745U (en) * | 1997-05-29 | 2003-06-21 | Kao Corp | Absorbent article |
| ZA985679B (en) | 1997-06-30 | 1999-01-26 | Procter & Gamble | Absorbent article with multi-layered extensible wings |
| DE69724667T2 (de) | 1997-12-05 | 2004-06-24 | The Procter & Gamble Company, Cincinnati | Zusammengesetzte damenbinde mit flügeln und ausdehnungszonen |
| JPH11299827A (ja) * | 1998-04-20 | 1999-11-02 | Uni Charm Corp | 吸収性物品 |
| US6730819B1 (en) | 1999-03-05 | 2004-05-04 | The Procter & Gamble Company | Articles comprising oxidizing and hemolytic agents |
| EP1034804A1 (en) | 1999-03-05 | 2000-09-13 | The Procter & Gamble Company | Articles comprising an oxidising agent and a hemolytic agent |
| KR20020003562A (ko) * | 1999-04-16 | 2002-01-12 | 로날드 디. 맥크레이 | 흡수 용품 및 그의 제조 방법 |
| US6153209A (en) | 1999-09-28 | 2000-11-28 | The Procter & Gamble Company | Article having a transferable breathable skin care composition thereon |
| JP3748743B2 (ja) | 1999-10-04 | 2006-02-22 | ユニ・チャーム株式会社 | 吸収性物品およびその製造方法 |
| JP3611761B2 (ja) | 1999-11-02 | 2005-01-19 | 花王株式会社 | 吸収性物品 |
| US20010044614A1 (en) | 1999-12-23 | 2001-11-22 | Damay Emmanuelle Cecile | Reducing agents for feminine care products |
| JP3701208B2 (ja) | 2000-03-13 | 2005-09-28 | ユニ・チャーム株式会社 | 開孔シートとこの開孔シートを使用した吸収性物品および前記開孔シートの製造方法。 |
| ES2338297T3 (es) | 2001-04-17 | 2010-05-06 | THE PROCTER & GAMBLE COMPANY | Un articulo absorbente que comprende un agente capaz de proporcionar una percepcion al usuario. |
| JP3922895B2 (ja) * | 2001-06-29 | 2007-05-30 | ユニ・チャーム株式会社 | 生理用ナプキン |
| SE519783C2 (sv) | 2001-08-31 | 2003-04-08 | Sca Hygiene Prod Ab | Absorberande alster innefattande öppningar i absorptionskroppen |
| US20030082219A1 (en) | 2001-10-01 | 2003-05-01 | The Procter & Gamble Company | Skin care compositions comprising low concentrations of skin treatment agents |
| SG103382A1 (en) | 2002-01-11 | 2004-04-29 | Uni Charm Corp | Colored absorbent article |
| JP4278963B2 (ja) | 2002-03-26 | 2009-06-17 | ユニ・チャーム株式会社 | 吸収性物品 |
| JP4693847B2 (ja) | 2002-03-26 | 2011-06-01 | ユニ・チャーム株式会社 | 吸収性物品およびその製造方法 |
| DE60231704D1 (de) | 2002-06-12 | 2009-05-07 | Sca Hygiene Prod Ab | Absorbierender Artikel mit einer Hautpflegezusammensetzung |
| US9035123B2 (en) | 2002-10-01 | 2015-05-19 | The Procter & Gamble Company | Absorbent article having a lotioned topsheet |
| JP4390445B2 (ja) * | 2002-12-05 | 2009-12-24 | ユニ・チャーム株式会社 | 縦長の吸収性物品 |
| US8030535B2 (en) | 2002-12-18 | 2011-10-04 | The Procter & Gamble Company | Sanitary napkin for clean body benefit |
| JP2005095759A (ja) | 2003-09-24 | 2005-04-14 | San-Dia Polymer Ltd | 吸収剤とこれを用いてなる吸収性物品 |
| US20050096614A1 (en) | 2003-10-29 | 2005-05-05 | Perez Roberto C. | Cover layer for an absorbent article |
| JP4476102B2 (ja) | 2003-12-11 | 2010-06-09 | 花王株式会社 | 吸収性物品の表面シート |
| JP4964768B2 (ja) | 2004-06-21 | 2012-07-04 | ザ プロクター アンド ギャンブル カンパニー | ローション含有トップシートを伴う吸収性物品 |
| US8211078B2 (en) | 2005-02-17 | 2012-07-03 | The Procter And Gamble Company | Sanitary napkins capable of taking complex three-dimensional shape in use |
| JP4870365B2 (ja) * | 2005-02-23 | 2012-02-08 | ユニ・チャーム株式会社 | 生理用ナプキン |
| JP4514630B2 (ja) | 2005-03-16 | 2010-07-28 | 花王株式会社 | 吸収性物品の表面シート |
| JP4523866B2 (ja) | 2005-03-31 | 2010-08-11 | ユニ・チャーム株式会社 | 吸収性物品 |
| WO2006130646A1 (en) | 2005-06-02 | 2006-12-07 | The Procter & Gamble Company | Absorbent article having traverse reinforcing element |
| JP4792249B2 (ja) | 2005-07-11 | 2011-10-12 | ユニ・チャーム株式会社 | 体液吸収性物品 |
| CA2644815C (en) | 2006-03-14 | 2011-10-04 | The Procter & Gamble Company | Absorbent articles with lotions |
| JP5154048B2 (ja) | 2006-06-23 | 2013-02-27 | ユニ・チャーム株式会社 | 不織布 |
| JP5123512B2 (ja) | 2006-06-23 | 2013-01-23 | ユニ・チャーム株式会社 | 不織布 |
| US8304600B2 (en) | 2006-06-23 | 2012-11-06 | Uni-Charm Corporation | Absorbent article |
| JP5123513B2 (ja) | 2006-06-23 | 2013-01-23 | ユニ・チャーム株式会社 | 吸収体 |
| JP5123497B2 (ja) | 2006-06-23 | 2013-01-23 | ユニ・チャーム株式会社 | 不織布、不織布製造方法及び不織布製造装置 |
| JP5069891B2 (ja) | 2006-06-23 | 2012-11-07 | ユニ・チャーム株式会社 | 不織布 |
| JP5328089B2 (ja) | 2006-06-23 | 2013-10-30 | ユニ・チャーム株式会社 | 多層不織布及び多層不織布の製造方法 |
| JP5328088B2 (ja) | 2006-06-23 | 2013-10-30 | ユニ・チャーム株式会社 | 不織布 |
| JP5069890B2 (ja) | 2006-06-23 | 2012-11-07 | ユニ・チャーム株式会社 | 不織布 |
| CN101484109B (zh) * | 2006-07-05 | 2013-01-23 | 尤妮佳股份有限公司 | 吸收性物品 |
| JP4986673B2 (ja) | 2007-03-27 | 2012-07-25 | 花王株式会社 | 吸収性物品 |
| JP5021380B2 (ja) | 2006-07-05 | 2012-09-05 | 花王株式会社 | 吸収性物品 |
| EP2036521A4 (en) | 2006-07-05 | 2012-06-20 | Kao Corp | ABSORBENT ARTICLE |
| MY145921A (en) | 2006-12-05 | 2012-05-15 | Uni Charm Corp | Non-woven fabric, non-woven fabric manufacturing method and absorbent articles |
| JP4939196B2 (ja) | 2006-12-12 | 2012-05-23 | ユニ・チャーム株式会社 | 不織布、不織布の製造方法及び吸収性物品 |
| JP4939192B2 (ja) | 2006-12-05 | 2012-05-23 | ユニ・チャーム株式会社 | 不織布、不織布の製造方法及び吸収性物品 |
| US8343123B2 (en) | 2006-12-13 | 2013-01-01 | Uni-Charm Corporation | Absorbent article |
| AR065378A1 (es) | 2007-02-16 | 2009-06-03 | Procter & Gamble | Articulo absorbente con locion que comprende un material de polipropilenglicol |
| JP4979424B2 (ja) | 2007-03-20 | 2012-07-18 | 花王株式会社 | 吸収性物品 |
| JP4979423B2 (ja) | 2007-03-20 | 2012-07-18 | 花王株式会社 | 吸収性物品 |
| JP5114087B2 (ja) | 2007-04-17 | 2013-01-09 | ユニ・チャーム株式会社 | 不織布、及び、吸収性物品 |
| JP4879074B2 (ja) | 2007-04-17 | 2012-02-15 | ユニ・チャーム株式会社 | 不織布製造方法 |
| US8273941B2 (en) | 2007-04-17 | 2012-09-25 | Uni-Charm Corporation | Nonwoven fabric, method for producing nonwoven fabric, and absorbent article |
| EP1992366B1 (en) | 2007-05-15 | 2011-07-06 | The Procter & Gamble Company | Use of a lotion composition on an absorbent article for reducing adherence of feces or menses to the skin |
| JP5161491B2 (ja) | 2007-05-30 | 2013-03-13 | ユニ・チャーム株式会社 | 吸収性物品 |
| JP5568210B2 (ja) | 2007-06-13 | 2014-08-06 | ユニ・チャーム株式会社 | 不織布シートの製造方法 |
| JP5497987B2 (ja) | 2007-06-22 | 2014-05-21 | ユニ・チャーム株式会社 | 不織布およびその製造方法 |
| JP5254363B2 (ja) | 2008-02-15 | 2013-08-07 | ザ プロクター アンド ギャンブル カンパニー | ポリプロピレングリコール材料を含むローションを備える吸収性物品 |
| JP2009201878A (ja) | 2008-02-29 | 2009-09-10 | Uni Charm Corp | 吸収性物品 |
| JP5258380B2 (ja) * | 2008-05-15 | 2013-08-07 | ユニ・チャーム株式会社 | 吸収性物品 |
| JP5371361B2 (ja) | 2008-10-10 | 2013-12-18 | ユニ・チャーム株式会社 | 吸収性物品及び吸収性物品の製造方法 |
| JP5455363B2 (ja) | 2008-12-25 | 2014-03-26 | ユニ・チャーム株式会社 | 薄型吸収性物品 |
| EA201101219A1 (ru) * | 2009-03-19 | 2012-03-30 | Юничарм Корпорейшн | Абсорбирующее изделие |
| JP5520124B2 (ja) | 2009-05-14 | 2014-06-11 | ユニ・チャーム株式会社 | 透液性繊維不織布 |
| CN102427790B (zh) | 2009-05-20 | 2016-01-06 | 花王株式会社 | 吸收体和吸收性物品 |
| JP5401177B2 (ja) | 2009-06-04 | 2014-01-29 | 花王株式会社 | 吸収性物品 |
| JP5590834B2 (ja) | 2009-08-11 | 2014-09-17 | ユニ・チャーム株式会社 | 不織布およびその製造方法 |
| JP5623052B2 (ja) | 2009-09-29 | 2014-11-12 | ユニ・チャーム株式会社 | 不織布の製造方法 |
| JP5530693B2 (ja) | 2009-09-30 | 2014-06-25 | 大王製紙株式会社 | 吸収性物品 |
| JP5421720B2 (ja) | 2009-10-09 | 2014-02-19 | ユニ・チャーム株式会社 | 不織布 |
| JP5395630B2 (ja) | 2009-11-13 | 2014-01-22 | 花王株式会社 | 吸収性物品 |
| JP5749907B2 (ja) | 2010-08-31 | 2015-07-15 | ユニ・チャーム株式会社 | 吸収性物品及び生理用ナプキン |
| US8426671B2 (en) * | 2011-02-11 | 2013-04-23 | Polymer Group, Inc. | Liquid management layer for personal care absorbent articles |
| TWI448277B (zh) | 2011-03-31 | 2014-08-11 | Uni Charm Corp | Absorbent items |
| US9498384B2 (en) * | 2011-12-01 | 2016-11-22 | Leigh E. Wood | Assembled intermediate comprising staple fiber nonwoven web and articles |
-
2012
- 2012-03-30 JP JP2012080539A patent/JP5726120B2/ja active Active
-
2013
- 2013-03-26 AU AU2013241415A patent/AU2013241415B2/en not_active Ceased
- 2013-03-26 EP EP13767763.9A patent/EP2832326B1/en not_active Not-in-force
- 2013-03-26 CN CN201380028070.1A patent/CN104334133A/zh active Pending
- 2013-03-26 SG SG11201405410VA patent/SG11201405410VA/en unknown
- 2013-03-26 US US14/389,256 patent/US9233185B2/en not_active Expired - Fee Related
- 2013-03-26 WO PCT/JP2013/058859 patent/WO2013146817A1/ja not_active Ceased
- 2013-03-27 AR ARP130101010A patent/AR090528A1/es unknown
- 2013-03-29 TW TW102111517A patent/TWI577351B/zh not_active IP Right Cessation
Patent Citations (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2002528174A (ja) | 1998-10-23 | 2002-09-03 | ザ、プロクター、エンド、ギャンブル、カンパニー | 選択的開閉自在開口を含むトップシートを有する吸収体 |
| JP2003024372A (ja) * | 2001-07-12 | 2003-01-28 | Uni Charm Corp | 吸収性物品 |
| JP2003052750A (ja) * | 2001-08-10 | 2003-02-25 | Uni Charm Corp | 吸収性物品 |
| JP2004049529A (ja) * | 2002-07-19 | 2004-02-19 | Kao Corp | 吸収性物品 |
| JP2006115996A (ja) | 2004-10-20 | 2006-05-11 | Kao Corp | 吸収性物品 |
| JP2008025079A (ja) | 2006-06-23 | 2008-02-07 | Uni Charm Corp | 不織布 |
| JP2008025078A (ja) | 2006-06-23 | 2008-02-07 | Uni Charm Corp | 不織布 |
| JP2008023365A (ja) * | 2007-10-03 | 2008-02-07 | Oji Paper Co Ltd | 折り返し部を有する吸収体製品 |
| JP2009297048A (ja) * | 2008-06-10 | 2009-12-24 | Kao Corp | 吸収性物品 |
| JP2011067484A (ja) * | 2009-09-28 | 2011-04-07 | Kao Corp | 吸収性物品 |
| JP2011104059A (ja) * | 2009-11-16 | 2011-06-02 | Kao Corp | 吸収性物品 |
| JP2011120696A (ja) * | 2009-12-09 | 2011-06-23 | Kao Corp | 吸収性物品 |
| JP2011226010A (ja) | 2010-04-16 | 2011-11-10 | Uni Charm Corp | 凹凸を有する不織布を簡易に製造する方法、及び不織布を簡易に加工する方法 |
| JP2011226011A (ja) | 2010-04-16 | 2011-11-10 | Uni Charm Corp | 伸長性繊維と伸縮性繊維とを含む、凹凸を有する不織布、及び当該不織布を製造する方法 |
Non-Patent Citations (2)
| Title |
|---|
| FUJITA A.: "Organic compound predictions and organic paradigms", KAGAKU NO RYOIKI, vol. 11, no. 10, 1957, pages 719 - 725 |
| See also references of EP2832326A4 |
Also Published As
| Publication number | Publication date |
|---|---|
| AU2013241415A1 (en) | 2014-10-09 |
| SG11201405410VA (en) | 2014-11-27 |
| AR090528A1 (es) | 2014-11-19 |
| US20150051566A1 (en) | 2015-02-19 |
| EP2832326A4 (en) | 2015-07-29 |
| JP5726120B2 (ja) | 2015-05-27 |
| AU2013241415B2 (en) | 2017-05-18 |
| TW201402088A (zh) | 2014-01-16 |
| US9233185B2 (en) | 2016-01-12 |
| EP2832326B1 (en) | 2017-01-18 |
| JP2013208280A (ja) | 2013-10-10 |
| EP2832326A1 (en) | 2015-02-04 |
| CN104334133A (zh) | 2015-02-04 |
| TWI577351B (zh) | 2017-04-11 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6057664B2 (ja) | 吸収性物品、及びその製造方法 | |
| JP5122007B1 (ja) | 吸収性物品 | |
| JP5726120B2 (ja) | 吸収性物品 | |
| JP5925015B2 (ja) | 吸収性物品 | |
| JP5726121B2 (ja) | 吸収性物品 | |
| JP5717686B2 (ja) | 吸収性物品 | |
| JP5745487B2 (ja) | 吸収性物品 | |
| JP5847055B2 (ja) | 吸収性物品 | |
| JP6092508B2 (ja) | 吸収性物品 | |
| JP5963639B2 (ja) | 吸収性物品 | |
| JP6037606B2 (ja) | 吸収性物品、吸収性物品のトップシートおよびそのトップシートの製造方法 | |
| JP2013208419A (ja) | 吸収性物品 | |
| JP5709788B2 (ja) | 吸収性物品 | |
| JP5769655B2 (ja) | 吸収性物品 | |
| JP5762346B2 (ja) | 吸収性物品 | |
| JP2017109125A (ja) | 吸収性物品 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 13767763 Country of ref document: EP Kind code of ref document: A1 |
|
| REEP | Request for entry into the european phase |
Ref document number: 2013767763 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2013767763 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: IDP00201405554 Country of ref document: ID |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 14389256 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2013241415 Country of ref document: AU Date of ref document: 20130326 Kind code of ref document: A |