WO2013111332A1 - テレフタル酸カリウム塩からの有用化学品の製造法 - Google Patents
テレフタル酸カリウム塩からの有用化学品の製造法 Download PDFInfo
- Publication number
- WO2013111332A1 WO2013111332A1 PCT/JP2012/051854 JP2012051854W WO2013111332A1 WO 2013111332 A1 WO2013111332 A1 WO 2013111332A1 JP 2012051854 W JP2012051854 W JP 2012051854W WO 2013111332 A1 WO2013111332 A1 WO 2013111332A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- terephthalate
- terephthalic acid
- acid
- seq
- solid
- Prior art date
Links
- 238000004519 manufacturing process Methods 0.000 title claims abstract description 90
- LRUDDHYVRFQYCN-UHFFFAOYSA-L dipotassium;terephthalate Chemical compound [K+].[K+].[O-]C(=O)C1=CC=C(C([O-])=O)C=C1 LRUDDHYVRFQYCN-UHFFFAOYSA-L 0.000 title claims abstract description 64
- 239000000126 substance Substances 0.000 title claims abstract description 28
- KKEYFWRCBNTPAC-UHFFFAOYSA-N terephthalic acid group Chemical group C(C1=CC=C(C(=O)O)C=C1)(=O)O KKEYFWRCBNTPAC-UHFFFAOYSA-N 0.000 claims abstract description 691
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 claims abstract description 313
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 claims abstract description 255
- YQUVCSBJEUQKSH-UHFFFAOYSA-N 3,4-dihydroxybenzoic acid Chemical compound OC(=O)C1=CC=C(O)C(O)=C1 YQUVCSBJEUQKSH-UHFFFAOYSA-N 0.000 claims abstract description 202
- LRHPLDYGYMQRHN-UHFFFAOYSA-N N-Butanol Chemical compound CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 claims abstract description 197
- LNTHITQWFMADLM-UHFFFAOYSA-N gallic acid Chemical compound OC(=O)C1=CC(O)=C(O)C(O)=C1 LNTHITQWFMADLM-UHFFFAOYSA-N 0.000 claims abstract description 143
- 244000005700 microbiome Species 0.000 claims abstract description 117
- 229910052700 potassium Inorganic materials 0.000 claims abstract description 74
- 239000011591 potassium Substances 0.000 claims abstract description 74
- 235000004515 gallic acid Nutrition 0.000 claims abstract description 72
- 229940074391 gallic acid Drugs 0.000 claims abstract description 72
- 239000002904 solvent Substances 0.000 claims abstract description 55
- 229920000728 polyester Polymers 0.000 claims abstract description 51
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 claims abstract description 34
- 238000010438 heat treatment Methods 0.000 claims abstract description 26
- 150000003503 terephthalic acid derivatives Chemical class 0.000 claims abstract description 15
- KKEYFWRCBNTPAC-UHFFFAOYSA-L terephthalate(2-) Chemical compound [O-]C(=O)C1=CC=C(C([O-])=O)C=C1 KKEYFWRCBNTPAC-UHFFFAOYSA-L 0.000 claims description 203
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 claims description 183
- 108090000623 proteins and genes Proteins 0.000 claims description 176
- 102000004169 proteins and genes Human genes 0.000 claims description 136
- 238000000034 method Methods 0.000 claims description 89
- 239000000243 solution Substances 0.000 claims description 81
- 239000007787 solid Substances 0.000 claims description 80
- 150000001413 amino acids Chemical class 0.000 claims description 75
- -1 Polyethylene Polymers 0.000 claims description 71
- 238000006243 chemical reaction Methods 0.000 claims description 71
- 239000002699 waste material Substances 0.000 claims description 69
- 239000007810 chemical reaction solvent Substances 0.000 claims description 63
- 229920000139 polyethylene terephthalate Polymers 0.000 claims description 51
- 239000005020 polyethylene terephthalate Substances 0.000 claims description 51
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 42
- 241000588724 Escherichia coli Species 0.000 claims description 40
- 239000007788 liquid Substances 0.000 claims description 34
- 229920002215 polytrimethylene terephthalate Polymers 0.000 claims description 34
- 229920001707 polybutylene terephthalate Polymers 0.000 claims description 33
- 238000000926 separation method Methods 0.000 claims description 33
- 238000012691 depolymerization reaction Methods 0.000 claims description 32
- 239000007864 aqueous solution Substances 0.000 claims description 30
- 230000000694 effects Effects 0.000 claims description 28
- 239000002253 acid Substances 0.000 claims description 27
- 230000008569 process Effects 0.000 claims description 22
- 150000003839 salts Chemical class 0.000 claims description 21
- 238000007667 floating Methods 0.000 claims description 18
- 108091022889 lactaldehyde reductase Proteins 0.000 claims description 17
- 108010057617 Lactaldehyde dehydrogenase Proteins 0.000 claims description 16
- 239000012736 aqueous medium Substances 0.000 claims description 14
- 230000000295 complement effect Effects 0.000 claims description 13
- 238000001035 drying Methods 0.000 claims description 10
- WGCNASOHLSPBMP-UHFFFAOYSA-N hydroxyacetaldehyde Natural products OCC=O WGCNASOHLSPBMP-UHFFFAOYSA-N 0.000 claims description 10
- 150000002009 diols Chemical class 0.000 claims description 9
- 238000001291 vacuum drying Methods 0.000 claims description 9
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 claims description 8
- 239000004698 Polyethylene Substances 0.000 claims description 8
- 150000002334 glycols Chemical class 0.000 claims description 8
- 229920000573 polyethylene Polymers 0.000 claims description 8
- 239000000843 powder Substances 0.000 claims description 8
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 claims description 7
- 239000005977 Ethylene Substances 0.000 claims description 7
- 238000011426 transformation method Methods 0.000 claims description 7
- 230000002708 enhancing effect Effects 0.000 claims description 6
- 102000040811 transporter activity Human genes 0.000 claims description 4
- 108091092194 transporter activity Proteins 0.000 claims description 4
- 239000000725 suspension Substances 0.000 claims description 3
- 230000010189 intracellular transport Effects 0.000 claims description 2
- 125000003275 alpha amino acid group Chemical group 0.000 claims 14
- 238000006116 polymerization reaction Methods 0.000 claims 2
- 238000002360 preparation method Methods 0.000 claims 1
- 239000002994 raw material Substances 0.000 abstract description 34
- 101710088194 Dehydrogenase Proteins 0.000 abstract description 19
- 108010082309 4-hydroxybenzoate 3-monooxygenase Proteins 0.000 abstract description 11
- 108010088582 Terephthalate 1,2-dioxygenase Proteins 0.000 abstract description 3
- 108020004414 DNA Proteins 0.000 description 167
- 235000018102 proteins Nutrition 0.000 description 114
- 210000004027 cell Anatomy 0.000 description 38
- 239000002585 base Substances 0.000 description 37
- 102000004020 Oxygenases Human genes 0.000 description 34
- 108090000417 Oxygenases Proteins 0.000 description 34
- 239000013612 plasmid Substances 0.000 description 31
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 29
- 239000008103 glucose Substances 0.000 description 29
- 108010078791 Carrier Proteins Proteins 0.000 description 25
- 239000002609 medium Substances 0.000 description 25
- 102000004316 Oxidoreductases Human genes 0.000 description 23
- 108090000854 Oxidoreductases Proteins 0.000 description 23
- 239000000047 product Substances 0.000 description 23
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 21
- 239000013615 primer Substances 0.000 description 21
- 239000000203 mixture Substances 0.000 description 20
- 238000010276 construction Methods 0.000 description 19
- 230000007423 decrease Effects 0.000 description 19
- FJKROLUGYXJWQN-UHFFFAOYSA-N 4-hydroxybenzoic acid Chemical compound OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 18
- 241000589518 Comamonas testosteroni Species 0.000 description 18
- 241001646716 Escherichia coli K-12 Species 0.000 description 18
- 238000010367 cloning Methods 0.000 description 18
- 238000012258 culturing Methods 0.000 description 18
- PWKNBLFSJAVFAB-UHFFFAOYSA-N 1-fluoro-2-nitrobenzene Chemical compound [O-][N+](=O)C1=CC=CC=C1F PWKNBLFSJAVFAB-UHFFFAOYSA-N 0.000 description 16
- 235000001014 amino acid Nutrition 0.000 description 16
- 239000012634 fragment Substances 0.000 description 16
- VIQSRHWJEKERKR-UHFFFAOYSA-L disodium;terephthalate Chemical compound [Na+].[Na+].[O-]C(=O)C1=CC=C(C([O-])=O)C=C1 VIQSRHWJEKERKR-UHFFFAOYSA-L 0.000 description 15
- 239000000463 material Substances 0.000 description 15
- 238000003756 stirring Methods 0.000 description 15
- 241000894006 Bacteria Species 0.000 description 14
- BSABBBMNWQWLLU-UHFFFAOYSA-N lactaldehyde Chemical compound CC(O)C=O BSABBBMNWQWLLU-UHFFFAOYSA-N 0.000 description 14
- GPSDUZXPYCFOSQ-UHFFFAOYSA-N m-toluic acid Chemical compound CC1=CC=CC(C(O)=O)=C1 GPSDUZXPYCFOSQ-UHFFFAOYSA-N 0.000 description 14
- 108091008146 restriction endonucleases Proteins 0.000 description 14
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 12
- 238000004064 recycling Methods 0.000 description 12
- 229920006395 saturated elastomer Polymers 0.000 description 12
- 102000004190 Enzymes Human genes 0.000 description 11
- 108090000790 Enzymes Proteins 0.000 description 11
- 108020004511 Recombinant DNA Proteins 0.000 description 11
- 150000001875 compounds Chemical class 0.000 description 11
- FFPQSNUAVYJZDH-UHFFFAOYSA-N diazanium;terephthalate Chemical compound [NH4+].[NH4+].[O-]C(=O)C1=CC=C(C([O-])=O)C=C1 FFPQSNUAVYJZDH-UHFFFAOYSA-N 0.000 description 11
- 238000005516 engineering process Methods 0.000 description 11
- 230000004060 metabolic process Effects 0.000 description 11
- 239000000523 sample Substances 0.000 description 11
- 239000011734 sodium Substances 0.000 description 11
- 229910052708 sodium Inorganic materials 0.000 description 11
- 239000013604 expression vector Substances 0.000 description 10
- 230000035772 mutation Effects 0.000 description 10
- 229940090248 4-hydroxybenzoic acid Drugs 0.000 description 9
- VHUUQVKOLVNVRT-UHFFFAOYSA-N Ammonium hydroxide Chemical compound [NH4+].[OH-] VHUUQVKOLVNVRT-UHFFFAOYSA-N 0.000 description 9
- 108020001019 DNA Primers Proteins 0.000 description 9
- 239000003155 DNA primer Substances 0.000 description 9
- 108010074633 Mixed Function Oxygenases Proteins 0.000 description 9
- 235000011114 ammonium hydroxide Nutrition 0.000 description 9
- 238000004458 analytical method Methods 0.000 description 9
- 239000001963 growth medium Substances 0.000 description 9
- 238000004128 high performance liquid chromatography Methods 0.000 description 9
- 230000014759 maintenance of location Effects 0.000 description 9
- 239000013598 vector Substances 0.000 description 9
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical group NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 8
- 102000008109 Mixed Function Oxygenases Human genes 0.000 description 8
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 8
- 239000000706 filtrate Substances 0.000 description 8
- 239000002773 nucleotide Substances 0.000 description 8
- 125000003729 nucleotide group Chemical group 0.000 description 8
- 229920003023 plastic Polymers 0.000 description 8
- 239000004033 plastic Substances 0.000 description 8
- 229910001414 potassium ion Inorganic materials 0.000 description 8
- 238000005070 sampling Methods 0.000 description 8
- 239000004474 valine Substances 0.000 description 8
- 239000003643 water by type Substances 0.000 description 8
- NPYPAHLBTDXSSS-UHFFFAOYSA-N Potassium ion Chemical compound [K+] NPYPAHLBTDXSSS-UHFFFAOYSA-N 0.000 description 7
- 238000002835 absorbance Methods 0.000 description 7
- 238000007792 addition Methods 0.000 description 7
- 229910021529 ammonia Inorganic materials 0.000 description 7
- 230000001976 improved effect Effects 0.000 description 7
- 239000000411 inducer Substances 0.000 description 7
- 229910001220 stainless steel Inorganic materials 0.000 description 7
- 239000010935 stainless steel Substances 0.000 description 7
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 6
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 6
- 108700039691 Genetic Promoter Regions Proteins 0.000 description 6
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 6
- 241000316848 Rhodococcus <scale insect> Species 0.000 description 6
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 6
- 101150081631 aldA gene Proteins 0.000 description 6
- 229910052783 alkali metal Inorganic materials 0.000 description 6
- 238000001914 filtration Methods 0.000 description 6
- 101150030625 fucO gene Proteins 0.000 description 6
- 230000001965 increasing effect Effects 0.000 description 6
- 238000000746 purification Methods 0.000 description 6
- 239000000758 substrate Substances 0.000 description 6
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonium chloride Substances [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 5
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical group CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 5
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 5
- 239000003513 alkali Substances 0.000 description 5
- 150000008044 alkali metal hydroxides Chemical class 0.000 description 5
- BFNBIHQBYMNNAN-UHFFFAOYSA-N ammonium sulfate Chemical compound N.N.OS(O)(=O)=O BFNBIHQBYMNNAN-UHFFFAOYSA-N 0.000 description 5
- 229910052921 ammonium sulfate Inorganic materials 0.000 description 5
- 235000011130 ammonium sulphate Nutrition 0.000 description 5
- 239000013611 chromosomal DNA Substances 0.000 description 5
- KTVIXTQDYHMGHF-UHFFFAOYSA-L cobalt(2+) sulfate Chemical compound [Co+2].[O-]S([O-])(=O)=O KTVIXTQDYHMGHF-UHFFFAOYSA-L 0.000 description 5
- 210000004748 cultured cell Anatomy 0.000 description 5
- 239000000284 extract Substances 0.000 description 5
- 125000001909 leucine group Chemical group [H]N(*)C(C(*)=O)C([H])([H])C(C([H])([H])[H])C([H])([H])[H] 0.000 description 5
- 230000000813 microbial effect Effects 0.000 description 5
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 5
- COLNVLDHVKWLRT-QMMMGPOBSA-N phenylalanine group Chemical group N[C@@H](CC1=CC=CC=C1)C(=O)O COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 5
- 230000032258 transport Effects 0.000 description 5
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 5
- 125000001493 tyrosinyl group Chemical group [H]OC1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 description 5
- 101710111736 1,2-dihydroxy-3,5-cyclohexadiene-1,4-dicarboxylate dehydrogenase Proteins 0.000 description 4
- 101100070555 Arabidopsis thaliana HSFA4C gene Proteins 0.000 description 4
- 101100523550 Arabidopsis thaliana RABF2A gene Proteins 0.000 description 4
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 4
- 241000589519 Comamonas Species 0.000 description 4
- 102000016680 Dioxygenases Human genes 0.000 description 4
- 108010028143 Dioxygenases Proteins 0.000 description 4
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 4
- 239000004471 Glycine Chemical group 0.000 description 4
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Chemical compound OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 description 4
- 239000004743 Polypropylene Substances 0.000 description 4
- 241000589516 Pseudomonas Species 0.000 description 4
- 101100198283 Scheffersomyces stipitis (strain ATCC 58785 / CBS 6054 / NBRC 10063 / NRRL Y-11545) DHG2 gene Proteins 0.000 description 4
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 4
- 235000004279 alanine Nutrition 0.000 description 4
- 238000011088 calibration curve Methods 0.000 description 4
- 229910052799 carbon Inorganic materials 0.000 description 4
- 238000004817 gas chromatography Methods 0.000 description 4
- 238000009396 hybridization Methods 0.000 description 4
- 238000005259 measurement Methods 0.000 description 4
- 229910052751 metal Inorganic materials 0.000 description 4
- 239000002184 metal Substances 0.000 description 4
- 238000010369 molecular cloning Methods 0.000 description 4
- 229910000402 monopotassium phosphate Inorganic materials 0.000 description 4
- 235000019796 monopotassium phosphate Nutrition 0.000 description 4
- 108020004707 nucleic acids Proteins 0.000 description 4
- 150000007523 nucleic acids Chemical class 0.000 description 4
- 102000039446 nucleic acids Human genes 0.000 description 4
- PJNZPQUBCPKICU-UHFFFAOYSA-N phosphoric acid;potassium Chemical compound [K].OP(O)(O)=O PJNZPQUBCPKICU-UHFFFAOYSA-N 0.000 description 4
- 229920001155 polypropylene Polymers 0.000 description 4
- 101150076874 rha-1 gene Proteins 0.000 description 4
- 239000012047 saturated solution Substances 0.000 description 4
- 229910001415 sodium ion Inorganic materials 0.000 description 4
- LPXPTNMVRIOKMN-UHFFFAOYSA-M sodium nitrite Chemical compound [Na+].[O-]N=O LPXPTNMVRIOKMN-UHFFFAOYSA-M 0.000 description 4
- 230000005030 transcription termination Effects 0.000 description 4
- TVKBBTQJNQDZRU-UHFFFAOYSA-N 2,4-dioxopentanedioic acid Chemical compound OC(=O)C(=O)CC(=O)C(O)=O TVKBBTQJNQDZRU-UHFFFAOYSA-N 0.000 description 3
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 3
- 241001485655 Corynebacterium glutamicum ATCC 13032 Species 0.000 description 3
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical group C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 3
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 3
- 239000003963 antioxidant agent Substances 0.000 description 3
- 235000006708 antioxidants Nutrition 0.000 description 3
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 3
- 229910000019 calcium carbonate Inorganic materials 0.000 description 3
- 238000005119 centrifugation Methods 0.000 description 3
- 239000003638 chemical reducing agent Substances 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- JZCCFEFSEZPSOG-UHFFFAOYSA-L copper(II) sulfate pentahydrate Chemical compound O.O.O.O.O.[Cu+2].[O-]S([O-])(=O)=O JZCCFEFSEZPSOG-UHFFFAOYSA-L 0.000 description 3
- 238000012217 deletion Methods 0.000 description 3
- 230000037430 deletion Effects 0.000 description 3
- 239000011521 glass Substances 0.000 description 3
- 150000004688 heptahydrates Chemical class 0.000 description 3
- 230000003100 immobilizing effect Effects 0.000 description 3
- 239000000395 magnesium oxide Substances 0.000 description 3
- CPLXHLVBOLITMK-UHFFFAOYSA-N magnesium oxide Inorganic materials [Mg]=O CPLXHLVBOLITMK-UHFFFAOYSA-N 0.000 description 3
- WRUGWIBCXHJTDG-UHFFFAOYSA-L magnesium sulfate heptahydrate Chemical compound O.O.O.O.O.O.O.[Mg+2].[O-]S([O-])(=O)=O WRUGWIBCXHJTDG-UHFFFAOYSA-L 0.000 description 3
- 229940061634 magnesium sulfate heptahydrate Drugs 0.000 description 3
- AXZKOIWUVFPNLO-UHFFFAOYSA-N magnesium;oxygen(2-) Chemical compound [O-2].[Mg+2] AXZKOIWUVFPNLO-UHFFFAOYSA-N 0.000 description 3
- CDUFCUKTJFSWPL-UHFFFAOYSA-L manganese(II) sulfate tetrahydrate Chemical compound O.O.O.O.[Mn+2].[O-]S([O-])(=O)=O CDUFCUKTJFSWPL-UHFFFAOYSA-L 0.000 description 3
- 238000002156 mixing Methods 0.000 description 3
- 239000007800 oxidant agent Substances 0.000 description 3
- 239000001301 oxygen Substances 0.000 description 3
- 229910052760 oxygen Inorganic materials 0.000 description 3
- 238000011084 recovery Methods 0.000 description 3
- 230000010076 replication Effects 0.000 description 3
- 238000011160 research Methods 0.000 description 3
- 230000035897 transcription Effects 0.000 description 3
- 238000013518 transcription Methods 0.000 description 3
- 230000009466 transformation Effects 0.000 description 3
- 125000002987 valine group Chemical group [H]N([H])C([H])(C(*)=O)C([H])(C([H])([H])[H])C([H])([H])[H] 0.000 description 3
- 238000005406 washing Methods 0.000 description 3
- RZLVQBNCHSJZPX-UHFFFAOYSA-L zinc sulfate heptahydrate Chemical compound O.O.O.O.O.O.O.[Zn+2].[O-]S([O-])(=O)=O RZLVQBNCHSJZPX-UHFFFAOYSA-L 0.000 description 3
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 2
- CDOWNLMZVKJRSC-UHFFFAOYSA-N 2-hydroxyterephthalic acid Chemical compound OC(=O)C1=CC=C(C(O)=O)C(O)=C1 CDOWNLMZVKJRSC-UHFFFAOYSA-N 0.000 description 2
- VRMXCPVFSJVVCA-UHFFFAOYSA-N 2-oxo-2H-pyran-4,6-dicarboxylic acid Chemical compound OC(=O)C=1C=C(C(O)=O)OC(=O)C=1 VRMXCPVFSJVVCA-UHFFFAOYSA-N 0.000 description 2
- 229920001817 Agar Polymers 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- 241001061264 Astragalus Species 0.000 description 2
- 241001453380 Burkholderia Species 0.000 description 2
- BHPQYMZQTOCNFJ-UHFFFAOYSA-N Calcium cation Chemical compound [Ca+2] BHPQYMZQTOCNFJ-UHFFFAOYSA-N 0.000 description 2
- YASYEJJMZJALEJ-UHFFFAOYSA-N Citric acid monohydrate Chemical compound O.OC(=O)CC(O)(C(O)=O)CC(O)=O YASYEJJMZJALEJ-UHFFFAOYSA-N 0.000 description 2
- 241000186216 Corynebacterium Species 0.000 description 2
- QXNVGIXVLWOKEQ-UHFFFAOYSA-N Disodium Chemical class [Na][Na] QXNVGIXVLWOKEQ-UHFFFAOYSA-N 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 2
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 2
- 229910021578 Iron(III) chloride Inorganic materials 0.000 description 2
- CSNNHWWHGAXBCP-UHFFFAOYSA-L Magnesium sulfate Chemical compound [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 description 2
- 108091028043 Nucleic acid sequence Proteins 0.000 description 2
- 241000589776 Pseudomonas putida Species 0.000 description 2
- 241000232299 Ralstonia Species 0.000 description 2
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 2
- 238000005273 aeration Methods 0.000 description 2
- 239000008272 agar Substances 0.000 description 2
- 229910052782 aluminium Inorganic materials 0.000 description 2
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 2
- AVKUERGKIZMTKX-NJBDSQKTSA-N ampicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=CC=C1 AVKUERGKIZMTKX-NJBDSQKTSA-N 0.000 description 2
- 229960000723 ampicillin Drugs 0.000 description 2
- 230000003078 antioxidant effect Effects 0.000 description 2
- 235000006533 astragalus Nutrition 0.000 description 2
- 230000001580 bacterial effect Effects 0.000 description 2
- KGBXLFKZBHKPEV-UHFFFAOYSA-N boric acid Chemical compound OB(O)O KGBXLFKZBHKPEV-UHFFFAOYSA-N 0.000 description 2
- 239000004327 boric acid Substances 0.000 description 2
- 239000000872 buffer Substances 0.000 description 2
- WERYXYBDKMZEQL-UHFFFAOYSA-N butane-1,4-diol Chemical compound OCCCCO WERYXYBDKMZEQL-UHFFFAOYSA-N 0.000 description 2
- LLSDKQJKOVVTOJ-UHFFFAOYSA-L calcium chloride dihydrate Chemical compound O.O.[Cl-].[Cl-].[Ca+2] LLSDKQJKOVVTOJ-UHFFFAOYSA-L 0.000 description 2
- 229940052299 calcium chloride dihydrate Drugs 0.000 description 2
- 229910001424 calcium ion Inorganic materials 0.000 description 2
- 229940041514 candida albicans extract Drugs 0.000 description 2
- 210000000349 chromosome Anatomy 0.000 description 2
- 229960002303 citric acid monohydrate Drugs 0.000 description 2
- 229910000361 cobalt sulfate Inorganic materials 0.000 description 2
- 229940044175 cobalt sulfate Drugs 0.000 description 2
- 238000002425 crystallisation Methods 0.000 description 2
- 238000005520 cutting process Methods 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- KCIDZIIHRGYJAE-YGFYJFDDSA-L dipotassium;[(2r,3r,4s,5r,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl] phosphate Chemical compound [K+].[K+].OC[C@H]1O[C@H](OP([O-])([O-])=O)[C@H](O)[C@@H](O)[C@H]1O KCIDZIIHRGYJAE-YGFYJFDDSA-L 0.000 description 2
- 238000004090 dissolution Methods 0.000 description 2
- 239000012153 distilled water Substances 0.000 description 2
- 239000013613 expression plasmid Substances 0.000 description 2
- 239000011888 foil Substances 0.000 description 2
- 239000012535 impurity Substances 0.000 description 2
- 238000003780 insertion Methods 0.000 description 2
- 230000037431 insertion Effects 0.000 description 2
- 229910000358 iron sulfate Inorganic materials 0.000 description 2
- RBTARNINKXHZNM-UHFFFAOYSA-K iron trichloride Chemical compound Cl[Fe](Cl)Cl RBTARNINKXHZNM-UHFFFAOYSA-K 0.000 description 2
- BAUYGSIQEAFULO-UHFFFAOYSA-L iron(2+) sulfate (anhydrous) Chemical compound [Fe+2].[O-]S([O-])(=O)=O BAUYGSIQEAFULO-UHFFFAOYSA-L 0.000 description 2
- RUTXIHLAWFEWGM-UHFFFAOYSA-H iron(3+) sulfate Chemical compound [Fe+3].[Fe+3].[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O RUTXIHLAWFEWGM-UHFFFAOYSA-H 0.000 description 2
- 229910000360 iron(III) sulfate Inorganic materials 0.000 description 2
- 238000002955 isolation Methods 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 229910052757 nitrogen Inorganic materials 0.000 description 2
- 235000015097 nutrients Nutrition 0.000 description 2
- 239000008363 phosphate buffer Substances 0.000 description 2
- XAEFZNCEHLXOMS-UHFFFAOYSA-M potassium benzoate Chemical compound [K+].[O-]C(=O)C1=CC=CC=C1 XAEFZNCEHLXOMS-UHFFFAOYSA-M 0.000 description 2
- 239000011347 resin Substances 0.000 description 2
- 229920005989 resin Polymers 0.000 description 2
- 238000005185 salting out Methods 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- 235000010288 sodium nitrite Nutrition 0.000 description 2
- GEHJYWRUCIMESM-UHFFFAOYSA-L sodium sulfite Chemical compound [Na+].[Na+].[O-]S([O-])=O GEHJYWRUCIMESM-UHFFFAOYSA-L 0.000 description 2
- 235000019345 sodium thiosulphate Nutrition 0.000 description 2
- 239000002689 soil Substances 0.000 description 2
- 238000006467 substitution reaction Methods 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- 210000004233 talus Anatomy 0.000 description 2
- 238000012546 transfer Methods 0.000 description 2
- 238000013519 translation Methods 0.000 description 2
- 229940088594 vitamin Drugs 0.000 description 2
- 239000011782 vitamin Substances 0.000 description 2
- 235000013343 vitamin Nutrition 0.000 description 2
- 229930003231 vitamin Natural products 0.000 description 2
- 239000012138 yeast extract Substances 0.000 description 2
- DNIAPMSPPWPWGF-VKHMYHEASA-N (+)-propylene glycol Chemical compound C[C@H](O)CO DNIAPMSPPWPWGF-VKHMYHEASA-N 0.000 description 1
- BJEPYKJPYRNKOW-REOHCLBHSA-N (S)-malic acid Chemical compound OC(=O)[C@@H](O)CC(O)=O BJEPYKJPYRNKOW-REOHCLBHSA-N 0.000 description 1
- YPFDHNVEDLHUCE-UHFFFAOYSA-N 1,3-propanediol Substances OCCCO YPFDHNVEDLHUCE-UHFFFAOYSA-N 0.000 description 1
- PAWQVTBBRAZDMG-UHFFFAOYSA-N 2-(3-bromo-2-fluorophenyl)acetic acid Chemical compound OC(=O)CC1=CC=CC(Br)=C1F PAWQVTBBRAZDMG-UHFFFAOYSA-N 0.000 description 1
- BKFXSOCDAQACQM-UHFFFAOYSA-N 3-chlorophthalic acid Chemical compound OC(=O)C1=CC=CC(Cl)=C1C(O)=O BKFXSOCDAQACQM-UHFFFAOYSA-N 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 1
- BTBUEUYNUDRHOZ-UHFFFAOYSA-N Borate Chemical compound [O-]B([O-])[O-] BTBUEUYNUDRHOZ-UHFFFAOYSA-N 0.000 description 1
- 108020004705 Codon Proteins 0.000 description 1
- 229920000742 Cotton Polymers 0.000 description 1
- 230000004544 DNA amplification Effects 0.000 description 1
- 108010014303 DNA-directed DNA polymerase Proteins 0.000 description 1
- 102000016928 DNA-directed DNA polymerase Human genes 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- 241000588722 Escherichia Species 0.000 description 1
- 241000672609 Escherichia coli BL21 Species 0.000 description 1
- 101900203280 Escherichia coli Lactaldehyde dehydrogenase Proteins 0.000 description 1
- 229930091371 Fructose Natural products 0.000 description 1
- 239000005715 Fructose Substances 0.000 description 1
- RFSUNEUAIZKAJO-ARQDHWQXSA-N Fructose Chemical compound OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O RFSUNEUAIZKAJO-ARQDHWQXSA-N 0.000 description 1
- 108700007698 Genetic Terminator Regions Proteins 0.000 description 1
- 241000238631 Hexapoda Species 0.000 description 1
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical compound SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 1
- HBBGRARXTFLTSG-UHFFFAOYSA-N Lithium ion Chemical compound [Li+] HBBGRARXTFLTSG-UHFFFAOYSA-N 0.000 description 1
- 240000002853 Nelumbo nucifera Species 0.000 description 1
- 235000006508 Nelumbo nucifera Nutrition 0.000 description 1
- 235000006510 Nelumbo pentapetala Nutrition 0.000 description 1
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 1
- 239000001888 Peptone Substances 0.000 description 1
- 108010080698 Peptones Proteins 0.000 description 1
- 241000512220 Polaromonas Species 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- 108010001267 Protein Subunits Proteins 0.000 description 1
- 102000002067 Protein Subunits Human genes 0.000 description 1
- 241000589517 Pseudomonas aeruginosa Species 0.000 description 1
- 241000320117 Pseudomonas putida KT2440 Species 0.000 description 1
- 108020005091 Replication Origin Proteins 0.000 description 1
- 241001459443 Rhodococcus jostii RHA1 Species 0.000 description 1
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 1
- 238000012300 Sequence Analysis Methods 0.000 description 1
- DWAQJAXMDSEUJJ-UHFFFAOYSA-M Sodium bisulfite Chemical compound [Na+].OS([O-])=O DWAQJAXMDSEUJJ-UHFFFAOYSA-M 0.000 description 1
- FKNQFGJONOIPTF-UHFFFAOYSA-N Sodium cation Chemical compound [Na+] FKNQFGJONOIPTF-UHFFFAOYSA-N 0.000 description 1
- 108091081024 Start codon Proteins 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- 108010006785 Taq Polymerase Proteins 0.000 description 1
- BZHJMEDXRYGGRV-UHFFFAOYSA-N Vinyl chloride Chemical compound ClC=C BZHJMEDXRYGGRV-UHFFFAOYSA-N 0.000 description 1
- 244000131415 Zanthoxylum piperitum Species 0.000 description 1
- 235000008853 Zanthoxylum piperitum Nutrition 0.000 description 1
- 240000008042 Zea mays Species 0.000 description 1
- 235000005824 Zea mays ssp. parviglumis Nutrition 0.000 description 1
- 235000002017 Zea mays subsp mays Nutrition 0.000 description 1
- AZFNGPAYDKGCRB-XCPIVNJJSA-M [(1s,2s)-2-amino-1,2-diphenylethyl]-(4-methylphenyl)sulfonylazanide;chlororuthenium(1+);1-methyl-4-propan-2-ylbenzene Chemical compound [Ru+]Cl.CC(C)C1=CC=C(C)C=C1.C1=CC(C)=CC=C1S(=O)(=O)[N-][C@@H](C=1C=CC=CC=1)[C@@H](N)C1=CC=CC=C1 AZFNGPAYDKGCRB-XCPIVNJJSA-M 0.000 description 1
- 238000010306 acid treatment Methods 0.000 description 1
- 125000003295 alanine group Chemical group N[C@@H](C)C(=O)* 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 150000001299 aldehydes Chemical class 0.000 description 1
- 239000000783 alginic acid Substances 0.000 description 1
- 235000010443 alginic acid Nutrition 0.000 description 1
- 229920000615 alginic acid Polymers 0.000 description 1
- 229960001126 alginic acid Drugs 0.000 description 1
- 150000001340 alkali metals Chemical class 0.000 description 1
- 239000012670 alkaline solution Substances 0.000 description 1
- BJEPYKJPYRNKOW-UHFFFAOYSA-N alpha-hydroxysuccinic acid Natural products OC(=O)C(O)CC(O)=O BJEPYKJPYRNKOW-UHFFFAOYSA-N 0.000 description 1
- 235000019270 ammonium chloride Nutrition 0.000 description 1
- 230000003321 amplification Effects 0.000 description 1
- 210000004102 animal cell Anatomy 0.000 description 1
- 239000003125 aqueous solvent Substances 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 229960005070 ascorbic acid Drugs 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- LFYJSSARVMHQJB-QIXNEVBVSA-N bakuchiol Chemical compound CC(C)=CCC[C@@](C)(C=C)\C=C\C1=CC=C(O)C=C1 LFYJSSARVMHQJB-QIXNEVBVSA-N 0.000 description 1
- 230000037429 base substitution Effects 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 239000011616 biotin Substances 0.000 description 1
- 229960002685 biotin Drugs 0.000 description 1
- 235000020958 biotin Nutrition 0.000 description 1
- 239000007853 buffer solution Substances 0.000 description 1
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 1
- 159000000007 calcium salts Chemical class 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 239000004202 carbamide Substances 0.000 description 1
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 1
- 239000000679 carrageenan Substances 0.000 description 1
- 235000010418 carrageenan Nutrition 0.000 description 1
- 229920001525 carrageenan Polymers 0.000 description 1
- 229940113118 carrageenan Drugs 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 238000003163 cell fusion method Methods 0.000 description 1
- 210000000170 cell membrane Anatomy 0.000 description 1
- 238000012824 chemical production Methods 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 229960004106 citric acid Drugs 0.000 description 1
- MEYVLGVRTYSQHI-UHFFFAOYSA-L cobalt(2+) sulfate heptahydrate Chemical compound O.O.O.O.O.O.O.[Co+2].[O-]S([O-])(=O)=O MEYVLGVRTYSQHI-UHFFFAOYSA-L 0.000 description 1
- 239000000571 coke Substances 0.000 description 1
- 238000004440 column chromatography Methods 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 235000005822 corn Nutrition 0.000 description 1
- 230000008025 crystallization Effects 0.000 description 1
- 235000018417 cysteine Nutrition 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 238000006297 dehydration reaction Methods 0.000 description 1
- RCRBCNZJGBTYDI-UHFFFAOYSA-L dilithium;terephthalate Chemical compound [Li+].[Li+].[O-]C(=O)C1=CC=C(C([O-])=O)C=C1 RCRBCNZJGBTYDI-UHFFFAOYSA-L 0.000 description 1
- ZPWVASYFFYYZEW-UHFFFAOYSA-L dipotassium hydrogen phosphate Chemical compound [K+].[K+].OP([O-])([O-])=O ZPWVASYFFYYZEW-UHFFFAOYSA-L 0.000 description 1
- 229910000396 dipotassium phosphate Inorganic materials 0.000 description 1
- 235000019797 dipotassium phosphate Nutrition 0.000 description 1
- BNIILDVGGAEEIG-UHFFFAOYSA-L disodium hydrogen phosphate Chemical compound [Na+].[Na+].OP([O-])([O-])=O BNIILDVGGAEEIG-UHFFFAOYSA-L 0.000 description 1
- 238000004821 distillation Methods 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 238000004520 electroporation Methods 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 238000006911 enzymatic reaction Methods 0.000 description 1
- FYIBGDKNYYMMAG-UHFFFAOYSA-N ethane-1,2-diol;terephthalic acid Chemical compound OCCO.OC(=O)C1=CC=C(C(O)=O)C=C1 FYIBGDKNYYMMAG-UHFFFAOYSA-N 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 235000013305 food Nutrition 0.000 description 1
- 108020001507 fusion proteins Proteins 0.000 description 1
- 102000037865 fusion proteins Human genes 0.000 description 1
- 238000001502 gel electrophoresis Methods 0.000 description 1
- 230000002068 genetic effect Effects 0.000 description 1
- 230000005484 gravity Effects 0.000 description 1
- 230000012010 growth Effects 0.000 description 1
- 229940093915 gynecological organic acid Drugs 0.000 description 1
- 229910052736 halogen Inorganic materials 0.000 description 1
- 150000002367 halogens Chemical class 0.000 description 1
- 230000006801 homologous recombination Effects 0.000 description 1
- 238000002744 homologous recombination Methods 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 238000009776 industrial production Methods 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 229910017053 inorganic salt Inorganic materials 0.000 description 1
- 239000003456 ion exchange resin Substances 0.000 description 1
- 229920003303 ion-exchange polymer Polymers 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- SURQXAFEQWPFPV-UHFFFAOYSA-L iron(2+) sulfate heptahydrate Chemical compound O.O.O.O.O.O.O.[Fe+2].[O-]S([O-])(=O)=O SURQXAFEQWPFPV-UHFFFAOYSA-L 0.000 description 1
- 229910001416 lithium ion Inorganic materials 0.000 description 1
- 229910052943 magnesium sulfate Inorganic materials 0.000 description 1
- 235000019341 magnesium sulphate Nutrition 0.000 description 1
- 239000001630 malic acid Substances 0.000 description 1
- 235000011090 malic acid Nutrition 0.000 description 1
- 235000013372 meat Nutrition 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- 229910021645 metal ion Inorganic materials 0.000 description 1
- 239000011259 mixed solution Substances 0.000 description 1
- 235000013379 molasses Nutrition 0.000 description 1
- 150000002823 nitrates Chemical class 0.000 description 1
- 239000002736 nonionic surfactant Substances 0.000 description 1
- 238000003199 nucleic acid amplification method Methods 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 238000010979 pH adjustment Methods 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 235000019319 peptone Nutrition 0.000 description 1
- 230000035699 permeability Effects 0.000 description 1
- 150000007965 phenolic acids Chemical class 0.000 description 1
- 235000009048 phenolic acids Nutrition 0.000 description 1
- 239000013600 plasmid vector Substances 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 229920000166 polytrimethylene carbonate Polymers 0.000 description 1
- 239000004304 potassium nitrite Substances 0.000 description 1
- 235000010289 potassium nitrite Nutrition 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 238000010188 recombinant method Methods 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 238000012827 research and development Methods 0.000 description 1
- 239000013605 shuttle vector Substances 0.000 description 1
- 239000002002 slurry Substances 0.000 description 1
- 239000001509 sodium citrate Substances 0.000 description 1
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 1
- 235000010267 sodium hydrogen sulphite Nutrition 0.000 description 1
- KKCBUQHMOMHUOY-UHFFFAOYSA-N sodium oxide Chemical compound [O-2].[Na+].[Na+] KKCBUQHMOMHUOY-UHFFFAOYSA-N 0.000 description 1
- 229910001948 sodium oxide Inorganic materials 0.000 description 1
- 159000000000 sodium salts Chemical class 0.000 description 1
- 235000010265 sodium sulphite Nutrition 0.000 description 1
- AKHNMLFCWUSKQB-UHFFFAOYSA-L sodium thiosulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=S AKHNMLFCWUSKQB-UHFFFAOYSA-L 0.000 description 1
- 239000006104 solid solution Substances 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 239000004575 stone Substances 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-L sulfite Chemical class [O-]S([O-])=O LSNNMFCWUKXFEE-UHFFFAOYSA-L 0.000 description 1
- RVUXIPACAZKWHU-UHFFFAOYSA-N sulfuric acid;heptahydrate Chemical compound O.O.O.O.O.O.O.OS(O)(=O)=O RVUXIPACAZKWHU-UHFFFAOYSA-N 0.000 description 1
- 239000006228 supernatant Substances 0.000 description 1
- 150000003504 terephthalic acids Chemical class 0.000 description 1
- 229940101691 thiamine 10 mg Drugs 0.000 description 1
- DPJRMOMPQZCRJU-UHFFFAOYSA-M thiamine hydrochloride Chemical compound Cl.[Cl-].CC1=C(CCO)SC=[N+]1CC1=CN=C(C)N=C1N DPJRMOMPQZCRJU-UHFFFAOYSA-M 0.000 description 1
- 229960000344 thiamine hydrochloride Drugs 0.000 description 1
- 235000019190 thiamine hydrochloride Nutrition 0.000 description 1
- 239000011747 thiamine hydrochloride Substances 0.000 description 1
- 150000004764 thiosulfuric acid derivatives Chemical class 0.000 description 1
- 101150102505 tphB gene Proteins 0.000 description 1
- 230000005758 transcription activity Effects 0.000 description 1
- 239000012137 tryptone Substances 0.000 description 1
- 238000009281 ultraviolet germicidal irradiation Methods 0.000 description 1
- 230000004580 weight loss Effects 0.000 description 1
- 239000008096 xylene Substances 0.000 description 1
- UHVMMEOXYDMDKI-JKYCWFKZSA-L zinc;1-(5-cyanopyridin-2-yl)-3-[(1s,2s)-2-(6-fluoro-2-hydroxy-3-propanoylphenyl)cyclopropyl]urea;diacetate Chemical compound [Zn+2].CC([O-])=O.CC([O-])=O.CCC(=O)C1=CC=C(F)C([C@H]2[C@H](C2)NC(=O)NC=2N=CC(=CC=2)C#N)=C1O UHVMMEOXYDMDKI-JKYCWFKZSA-L 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12P—FERMENTATION OR ENZYME-USING PROCESSES TO SYNTHESISE A DESIRED CHEMICAL COMPOUND OR COMPOSITION OR TO SEPARATE OPTICAL ISOMERS FROM A RACEMIC MIXTURE
- C12P7/00—Preparation of oxygen-containing organic compounds
- C12P7/40—Preparation of oxygen-containing organic compounds containing a carboxyl group including Peroxycarboxylic acids
- C12P7/44—Polycarboxylic acids
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12P—FERMENTATION OR ENZYME-USING PROCESSES TO SYNTHESISE A DESIRED CHEMICAL COMPOUND OR COMPOSITION OR TO SEPARATE OPTICAL ISOMERS FROM A RACEMIC MIXTURE
- C12P7/00—Preparation of oxygen-containing organic compounds
- C12P7/40—Preparation of oxygen-containing organic compounds containing a carboxyl group including Peroxycarboxylic acids
- C12P7/42—Hydroxy-carboxylic acids
Definitions
- the present invention uses a terephthalic acid potassium salt as a raw material and a microorganism expressing terephthalic acid 1,2-dioxygenase to produce terephthalic acid-1,2-cis-dihydrodiol (hereinafter referred to as TPA-DHD as necessary). And a method of converting TPA-DHD into protocatechuic acid or gallic acid, and a method of obtaining a raw material potassium terephthalate by depolymerization of waste polyester.
- terephthalic acid potassium salt means at least one terephthalic acid such as terephthalic acid dipotassium salt, terephthalic acid 1-potassium 4-sodium salt and terephthalic acid 1-potassium 4-ammonium salt.
- a carboxyl group residue forms a salt with potassium ion.
- Terephthalic acid is mainly produced in large quantities as a raw material for terephthalic acid-based polyesters such as polyethylene terephthalate (hereinafter abbreviated as PET), polytrimethylene terephthalate (hereinafter abbreviated as PTT), and polybutylene terephthalate (hereinafter abbreviated as PBT). It is an inexpensive chemical product. Since terephthalic acid is inexpensive, technologies for producing useful chemicals such as TPA-DHD, 2-pyrone-4,6-dicarboxylic acid, protocatechuic acid, and gallic acid using terephthalic acid as a raw material using microorganisms have also been developed. (References 1 to 5). It is known that 2-hydroxyterephthalic acid, which is a raw material for pharmaceuticals and resin materials, can be produced by dehydration reaction of TPA-DHD (Patent Documents 1 and 2).
- terephthalate having excellent water solubility to the medium instead of terephthalic acid itself. Since sodium hydroxide is cheaper than potassium hydroxide, sodium salt of terephthalic acid has been used as a raw material so far, but useful chemicals were produced using microorganisms using potassium salt of terephthalic acid as a raw material. There are no reports and reports that the use of terephthalic acid potassium salt is superior to terephthalic acid sodium salt in terms of productivity of the target compound.
- terephthalic acid-based polyester Regarding the recycling of terephthalic acid-based polyester, a number of recycling technologies have been developed and commercialized, especially focusing on the recycling of discarded PET bottles. However, the recycling cost is high, and more profitable recycling technology is required. Thus, in the manufacture of chemicals using terephthalic acid as a raw material, using terephthalic acid derived from waste polyester as a raw material is an important research and development issue because it leads to the solution of environmental problems and the reduction of manufacturing costs.
- Patent Literature 6 to 11 As a recycling method for waste polyester, in addition to the material recycling method for obtaining the original polyester, the chemical recycling method for chemically depolymerizing polyester to obtain terephthalic acid, bis-2-hydroxyethyl terephthalate, etc. (Patent Literature) 6 to 11) are known. It is known that depolymerization can be achieved by heating a polyester such as PET in an ethylene glycol reaction solvent or an alcohol reaction solvent containing an alkali metal hydroxide such as sodium hydroxide. In this case, sodium hydroxide is generally used as the alkali metal hydroxide because sodium hydroxide is inexpensive.
- Non-Patent Document 1 Although it is known that PET can be depolymerized in an ethylene glycol solvent containing potassium hydroxide (Non-Patent Document 1), the result of comparing the differences in PET depolymerization between potassium hydroxide and sodium hydroxide in an ethylene glycol solvent Has not been reported. Furthermore, after depolymerizing PET, PTT or PBT in an ethylene glycol reaction solvent containing potassium hydroxide to obtain potassium terephthalate, the microorganism is used to convert the potassium terephthalate into another useful chemical. There is no report on the recycling technology of waste polyester to be converted.
- Patent Document 12 An experimental example in which waste PET is depolymerized in a 1-butanol reaction solvent containing sodium hydroxide and terephthalic acid is obtained by addition of sulfuric acid has been reported (Patent Document 12). A 1-butanol reaction containing potassium hydroxide is reported. There have been no reports of cases in which waste PET is depolymerized in a solvent, and cases in which the difference between potassium hydroxide and sodium hydroxide in a depolymerization reaction of polyester in a 1-butanol reaction solvent containing an alkali is compared.
- E Escherichia coli
- K-12 strain which is often used in the basic research field and industrial production field, is obtained by using ethylene glycol with lactaldehyde reductase and lactaldehyde dehydrogenase. It is known that it can be converted to glycolic acid, and it has also been reported that the introduction of mutations into lactaldehyde reductase improves the metabolic ability of ethylene glycol (Non-Patent Documents 2 to 4).
- An object of the present invention is to produce TPA-DHD using an alkali metal terephthalate as a raw material and to improve the productivity of these compounds when converting the produced TPA-DHD into protocatechuic acid using a microorganism. And a method for obtaining the alkali metal terephthalate by depolymerization of waste polyester.
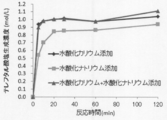
- the inventors of the present invention have studied the productivity of protocatechuic acid by recombinant Escherichia coli in the process of depolymerizing PET, a terephthalic acid polyester, in an alkaline solution to obtain terephthalate.
- terephthalic acid potassium salt terephthalic acid dipotassium salt, 1-potassium 4-ammonium salt or terephthalic acid 1-potassium 4-sodium salt
- protocatechuic acid was used instead of terephthalic acid disodium salt.
- the solubility of the terephthalate salt as a raw material is low, the amount of liquid introduced from the raw material tank increases, and the production amount of the target compound per culture tank decreases. Therefore, it is preferable to use terephthalate having high water solubility as a raw material. Therefore, when the solubility of terephthalic acid disodium salt, terephthalic acid 1-potassium 4-sodium salt and terephthalic acid dipotassium salt in water at 30 ° C. was examined, the solubility of terephthalic acid dipotassium salt was about 1.0 ⁇ M.
- terephthalic acid potassium salts such as terephthalic acid dipotassium salt and terephthalic acid 1-potassium 4-sodium salt were found to be superior to terephthalic acid disodium salt in the production of target compounds by microorganisms such as Escherichia coli. .
- the solubility of ammonium terephthalate in water at 30 ° C was investigated.
- the solubility of acid 1-potassium 4-ammonium salt is about 0.85 M
- the solubility of terephthalic acid 1-sodium 4-ammonium salt is about 0.61 M
- the solubility of terephthalic acid diammonium salt is about 0.51 M. It has been found that potassium 4-ammonium salt has high water solubility.
- terephthalic acid 1-potassium 4-ammonium salt it has been found that the productivity of the target compound by microorganisms such as Escherichia coli is excellent, and the problem of the present invention has been solved.
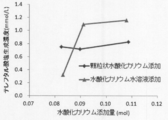
- the present inventors have found that by changing the depolymerization reaction solvent from ethylene glycol to 1-butanol, the depolymerization reaction temperature can be lowered and the terephthalate recovery efficiency is unexpectedly excellent. Furthermore, in the depolymerization reaction in 1-butanol, it has been unexpectedly found that the use of potassium hydroxide as the alkali metal hydroxide is superior to sodium hydroxide in that the depolymerization efficiency of terephthalate is superior. It was.
- the present invention relates to the following (1) to (24).
- a process for producing terephthalic acid 1,2-cis-dihydrodiol by reacting with terephthalic acid in an aqueous medium containing terephthalic acid salt to produce terephthalic acid 1,2-cis-dihydrodiol, wherein the terephthalic acid salt comprises The method further comprises 0.5 times or more and 2 times or less of potassium in terms of mole relative to all terephthalic acid contained in terephthalate.
- A DNA encoding a protein comprising the amino acid sequence shown in SEQ ID NO: 2.
- B conversion from terephthalic acid to terephthalic acid 1,2-cis-dihydrodiol, including a sequence in which one or several amino acids are deleted, substituted and / or added in the amino acid sequence shown in SEQ ID NO: 2 DNA encoding a protein having a function related to.
- C DNA consisting of the base sequence shown in SEQ ID NO: 1.
- (D) hybridizes under stringent conditions with DNA comprising a sequence complementary to all or part of the base sequence shown in SEQ ID NO: 1, and from terephthalic acid to terephthalic acid 1,2-cis-dihydro DNA encoding a protein having a function related to conversion to a diol.
- (E) DNA encoding a protein comprising the amino acid sequence shown in SEQ ID NO: 4.
- terephthalic acid selected from the group consisting of dipotassium terephthalate, 1-potassium 4-sodium terephthalate, and 1-potassium 4-ammonium terephthalate as the aqueous medium containing the terephthalate
- the microorganism described in (1) is a microorganism in which the intracellular transport ability of terephthalic acid is enhanced by introducing the DNA shown in (m), (n), (o) or (p) below.
- N DNA encoding a protein comprising a sequence in which one or several amino acids are deleted, substituted and / or added in the amino acid sequence shown in SEQ ID NO: 8 and having terephthalic acid transporter activity.
- O DNA consisting of the base sequence represented by SEQ ID NO: 7.
- P DNA that hybridizes under stringent conditions with DNA consisting of a sequence complementary to all or part of the base sequence shown in SEQ ID NO: 7 and encodes a protein having terephthalic acid transporter activity .
- the microorganism further has a DNA shown in (q), (r), (s) or (t) below, and has the ability to produce protocatechuic acid from terephthalic acid, from (1) (4) characterized in that terephthalic acid 1,2-cis-dihydrodiol is produced from terephthalate by the method described in any one of (4), and terephthalic acid 1,2-cis-dihydrodiol is further converted into protocatechuic acid.
- a production method of protocatechuic acid. (Q) DNA encoding a protein comprising the amino acid sequence represented by SEQ ID NO: 10.
- (R) includes a sequence in which one or several amino acids are deleted, substituted and / or added in the amino acid sequence shown in SEQ ID NO: 10, and converts 1,2-cis-dihydrodiol terephthalate to protocatechuic acid DNA encoding a protein having activity.
- the microorganism further has the DNA shown in (q), (r), (s) or (t) and the DNA shown in (u), (v), (w) or (x),
- a process for producing gallic acid characterized in that the acid 1,2-cis-dihydrodiol is converted into gallic acid.
- U DNA encoding a protein comprising the amino acid sequence represented by SEQ ID NO: 12.
- (V) a DNA encoding a protein comprising a sequence in which one or several amino acids are deleted, substituted and / or added in the amino acid sequence shown in SEQ ID NO: 12 and having an activity of converting protocatechuic acid to gallic acid .
- (X) a protein that hybridizes under stringent conditions with DNA consisting of a sequence complementary to all or part of the base sequence shown in SEQ ID NO: 11 and has an activity of converting protocatechuic acid to gallic acid DNA encoding (8)
- the above (q), (r), (s ) Or (t) in addition to the DNA shown in (u), (v), (w) or (x) above, treatment of the microorganism or the culture obtained by the transformation method
- a method for producing gallic acid comprising converting terephthalic acid 1,2-cis-dihydrodiol into gallic acid using a product.
- the terephthalic acid 1,2-cis-dihydrodiol according to any one of (1) to (4), further comprising the step of obtaining the terephthalate salt by the following steps (A) to (D): Manufacturing method.
- step (B) Of the solid foreign substances contained in the depolymerization reaction solution of the waste obtained in step (A), a process of removing solid foreign substances floating in the solution by a floating sorting method, (C) A step of recovering solids in the solution other than the suspended solid foreign matter by a solid-liquid separation method from the solution subjected to the treatment in the step (B), (D) The solid collected in step (C) is subjected to a heat drying treatment, a vacuum drying treatment or a centrifugal separation treatment to reduce the content of glycols in the solid, and the remaining solid is treated with terephthalate.
- Step (10) of obtaining an acid salt The step of recovering the ethylene glycol reaction solvent after recovering the solid by the solid-liquid separation method in the step (C), and using the solvent as the ethylene glycol reaction solvent in the step (A)
- (12) The ethylene glycol reaction solvent after recovering the solid matter by the solid-liquid separation method in the step (C) is recovered, and the solvent is used as the ethylene glycol reaction solvent in the step (A).
- the method for producing protocatechuic acid according to (11), wherein a terephthalate obtained by repeatedly using the solvent without discarding is used.
- the ethylene glycol reaction solvent after recovering the solid by the solid-liquid separation method in the step (C) is recovered, and the solvent is used as the ethylene glycol reaction solvent in the step (A).
- the method for producing gallic acid according to (13), wherein terephthalate obtained by repeatedly using the solvent without discarding is used.
- a butanol reaction solvent containing potassium hydroxide or 1-butanol containing both potassium hydroxide and sodium hydroxide
- step (F) Of the solid foreign matters contained in the waste depolymerization reaction solution obtained in step (E), a step of removing solid foreign matters floating in the solution by a floating sorting method, (G) A step of recovering solids in the solution other than the suspended solid foreign matters from the solution subjected to the treatment in the step (F) by a solid-liquid separation method, (H) The solid matter recovered in step (G) is subjected to heat drying treatment, vacuum drying treatment or centrifugal separation treatment to reduce the contents of 1-butanol and glycols in the solid matter and remain.
- Step (19) of obtaining a solid as a terephthalate salt The 1-butanol reaction solvent after recovering the solid by the solid-liquid separation method in the step (G) is recovered, and the solvent is recovered as 1-butanol in the step (E).
- a process for producing diols (20) The method for producing protocatechuic acid according to (5) or (6), further comprising the step of obtaining the terephthalate by the steps (E) to (H).
- the 1-butanol reaction solvent after recovering the solid by the solid-liquid separation method in the step (G) is recovered, and the solvent is used as the 1-butanol reaction solvent in the step (E).
- the method for producing protocatechuic acid according to (20), wherein a terephthalate obtained by repeatedly using a glycol reaction solvent without being discarded is used.
- the 1-butanol reaction solvent after the solid is recovered by the solid-liquid separation method in the step (G) is recovered, and the solvent is used as the 1-butanol reaction solvent in the step (E).
- TPA- DHD when terephthalate containing 0.5 to 2 times the amount of potassium is used as a raw material with respect to the total terephthalic acid contained in the terephthalate in terms of mole, TPA- DHD can be manufactured efficiently. Moreover, according to the present invention, the obtained TPA-DHD can be efficiently converted into protocatechuic acid or gallic acid. Furthermore, terephthalic acid potassium salt suitable as a raw material for terephthalic acid derivatives by microorganisms can be efficiently prepared by depolymerization of waste polyester.
- terephthalic acid salt As a form of terephthalic acid used as a raw material for compound production by microorganisms, terephthalic acid salt is generally preferable because terephthalic acid salt has higher water solubility than terephthalic acid itself.
- the terephthalate used for such purposes include terephthalic acid dipotassium salt, terephthalic acid 1-potassium 4-sodium salt, terephthalic acid 1-potassium 4-ammonium salt, terephthalic acid disodium salt, terephthalic acid 1-sodium 4 -Ammonium salts and diammonium terephthalic acid salts.
- the terephthalic acid dipotassium salt is a salt in which the carboxyl groups at the 1-position and 4-position of terephthalic acid are ion-bonded to potassium ions.
- the terephthalic acid 1-potassium 4-sodium salt is a salt in which the carboxyl group residue at the 1-position of terephthalic acid is ion-bonded with potassium ion, and the carboxyl group residue at the 4-position of terephthalic acid is ion-bonded with sodium ion. is there.
- the terephthalic acid 1-potassium 4-ammonium salt is a salt in which the carboxyl group residue at the 1-position of terephthalic acid is ion-bonded with potassium ion and the carboxyl group residue at the 4-position of terephthalic acid is ion-bonded with ammonium ion. is there.
- the terephthalic acid disodium salt is a salt in which the carboxyl group residues at positions 1 and 4 of terephthalic acid are ion-bonded to sodium ions.
- the terephthalic acid 1-sodium 4-ammonium salt is a salt in which the carboxyl group residue at the 1-position of terephthalic acid is ion-bonded with sodium ions and the carboxyl group residue at the 4-position of terephthalic acid is ion-bonded with ammonium ions. is there.
- the diammonium terephthalate is a salt in which the carboxyl groups at the 1st and 4th positions of terephthalic acid are ion-bonded to ammonium ions.
- Each terephthalic acid powder has a molar ratio of twice as much potassium hydroxide, a molar ratio of 1 time of potassium hydroxide and 1 time of sodium hydroxide, a molar ratio of 1 time of potassium hydroxide and 1 Aqueous ammonia solution containing twice the amount of ammonia, 2 times the molar amount of sodium hydroxide, an aqueous ammonia solution containing the molar amount of 1 time sodium hydroxide and 1 time amount of ammonia, and a molar amount of 2 times the amount of ammonia After adding an aqueous ammonia solution containing water to an appropriate amount of water, the solution of each terephthalate is stir
- the terephthalic acid salt used in the present invention is a terephthalic acid salt containing 0.5 times or more and 2 times or less of potassium in terms of moles based on the total terephthalic acid contained in the terephthalate. More preferably, it is a terephthalate salt containing 0.6 times or more and 2 times or less of potassium in terms of moles relative to the total terephthalic acid contained in the terephthalate.
- potassium defined as 0.5 to 2 times the amount of all terephthalic acid contained in terephthalate is potassium derived from potassium terephthalate, that is, carboxyl of terephthalic acid. This is the amount of potassium that forms an ionic bond with the base residue.
- potassium (ion) derived from potassium terephthalate may be contained in the aqueous solvent in an amount of 0.5 to 2 times the amount of all terephthalic acid in terms of mole.
- the terephthalate any form of terephthalate powder, an aqueous solution in which terephthalate is dissolved, or a suspension of terephthalate in which terephthalate powder is mixed in a slurry state in the aqueous solution of terephthalate Even so, it can be used in the present invention.
- terephthalate includes any form of terephthalate.
- terephthalic acid containing at least one potassium terephthalate selected from the group consisting of dipotassium terephthalate, 1-potassium 4-sodium terephthalate, and 1-potassium 4-ammonium terephthalate is used.
- a terephthalic acid salt which is a salt and contains 0.5 times or more and 2 times or less of potassium in terms of mole relative to the total terephthalic acid contained in the terephthalic acid salt can be used.
- a terephthalate having a single component of terephthalate selected from dipotassium terephthalate, 1-potassium 4-sodium terephthalate and 1-potassium 4-ammonium terephthalate may be used.
- the terephthalate used in the present invention satisfies the above potassium content, in addition to the potassium terephthalate, disodium terephthalate, 1-sodium 4-ammonium terephthalate, diammonium terephthalate, etc. May be included.
- terephthalic acid 1-potassium 4-sodium salt and terephthalic acid disodium salt when using a mixture of two types of terephthalic acid salt, terephthalic acid 1-potassium 4-sodium salt and terephthalic acid disodium salt, the molar ratio of terephthalic acid 1-potassium 4-sodium salt to terephthalic acid disodium salt is It is possible to use terephthalate containing at least one amount.
- terephthalic acid dipotassium salt contains at least 1/3 times the molar ratio of terephthalic acid disodium salt. Terephthalate can be used.
- a mixture of three or more types of terephthalate can also be used.
- the mixture is 0.5 times or more and 2 times in terms of moles based on the total terephthalic acid contained in the terephthalate.
- the following potassium must be included.
- one or more types of potassium terephthalate selected from the group consisting of dipotassium terephthalate, 1-potassium 4-sodium terephthalate, 1-potassium 4-ammonium terephthalate, and disodium terephthalate
- a terephthalate containing two or more terephthalates selected from the group consisting of 1-sodium 4-ammonium terephthalate and diammonium terephthalate can be used.
- aqueous solution prepared by mixing terephthalic acid dipotassium salt, terephthalic acid disodium salt and terephthalic acid diammonium salt in a molar ratio of 1: 2: 1.
- the aqueous solution contains 0.5 times the amount of potassium relative to the total terephthalic acid, and 0.5 mol of potassium hydroxide, 1.0 mol of sodium hydroxide and 0.5 mol of ammonia are added to water and dissolved with respect to 1 mol of terephthalic acid. Can also be obtained.
- terephthalic acid dipotassium salt or 1-terephthalic acid salt obtained by depolymerizing waste polyester in ethylene glycol solvent or 1-butanol solvent containing potassium hydroxide or a mixture of potassium hydroxide and sodium hydroxide.
- a terephthalate salt containing potassium 4-sodium salt can be used as a raw material for producing a compound by a microorganism.
- terephthalate obtained by depolymerization of waste polyester since the price per mole is lower than sodium hydroxide or aqueous sodium hydroxide than potassium hydroxide, high-purity terephthalic acid and sodium hydroxide or ammonia are used for the terephthalate obtained by depolymerization of waste polyester.
- terephthalate produced by adding water may lower the manufacturing cost.
- Specific examples thereof include, for example, terephthalic acid salt obtained by mixing terephthalic acid dipotassium salt obtained by depolymerization of waste polyester, high-purity terephthalic acid, and aqueous ammonia in a molar ratio of 6: 4: 8, respectively.
- aqueous solution of terephthalate thus obtained is equivalent to an aqueous solution prepared by mixing terephthalic acid dipotassium salt and terephthalic acid diammonium salt in a molar ratio of 6: 4.
- potassium terephthalate When potassium terephthalate is prepared from waste polyester using the method disclosed in the present invention, in addition to potassium ions, sodium ions, ammonium ions, other metal ions such as calcium ions and lithium ions may be mixed as foreign substances. is there.
- terephthalic acid is used as long as it is a terephthalate containing 0.5 to 2 times the amount of potassium in terms of moles relative to the total terephthalic acid contained in the terephthalate.
- a terephthalate such as calcium salt or lithium terephthalate may be mixed if its content is low.
- TPA-DHD a process for producing TPA-DHD, protocatechuic acid and gallic acid from terephthalic acid
- terephthalic acid is oxidized by terephthalic acid 1,2-dioxygenase and converted to TPA-DHD.
- TPA-DHD can be converted to protocatechuic acid by TPA-DHD dehydrogenase.
- parahydroxybenzoic acid hydroxylase or improved parahydroxybenzoic acid hydroxylase for example, leucine at position 199 or leucine at position 200 of parahydroxybenzoic acid hydroxylase is replaced with valine or glycine, and positions 385 or 386)
- Protocatechuic acid can be converted to gallic acid by a mutant in which the tyrosine is substituted with phenylalanine, valine or alanine.
- the terephthalic acid 1,2-dioxygenase used in the present invention comprises an oxygenase component and a reductase component.
- the oxygenase component consists of two large and small subunits.
- Examples of the oxygenase large subunit protein include a protein having an amino acid sequence derived from the Comamonas testeroni 72W2 strain represented by SEQ ID NO: 2. This strain was received on January 24, 2012 by an independent administrative agency, National Institute for Product Evaluation and Patent Microorganisms Depositary Center (NPMD) (2-5-8 Kazusa-Kamashita, Kisarazu City, Chiba Prefecture 292-0818, Japan) NITE ABP-1209 is deposited internationally and can be sold.
- NPMD National Institute for Product Evaluation and Patent Microorganisms Depositary Center
- the oxygenase large subunit protein used in the present invention includes an amino acid sequence in which one or several amino acids are deleted, substituted or added in the amino acid sequence of SEQ ID NO: 2, and terephthalic acid to TPA-DHD. Proteins having a function related to the conversion to can be mentioned.
- the protein comprises an amino acid sequence having 75% or more identity, preferably 90% or more, particularly preferably 95% or more identity with the amino acid sequence of SEQ ID NO: 2, and terephthalic acid to TPA-DHD. Proteins having a function related to the conversion of can be mentioned.
- function related to the conversion from terephthalic acid to TPA-DHD means terephthalic acid together with terephthalic acid 1,2-dioxygenase / oxygenase small subunit protein and terephthalic acid 1,2-dioxygenase / reductase protein. Means a function capable of producing TPA-DHD when reacted with.
- terephthalic acid 1,2-dioxygenase / oxygenase small subunit protein examples include a protein having the amino acid sequence derived from the above-mentioned Comamonas testosteroni 72W2 strain represented by SEQ ID NO: 4. Further, the terephthalic acid 1,2-dioxygenase / oxygenase small subunit protein used in the present invention is an amino acid sequence of SEQ ID NO: 4 from an amino acid sequence in which one or several amino acids are deleted, substituted or added. And a protein having a function related to the conversion from terephthalic acid to TPA-DHD.
- the protein comprises an amino acid sequence having 75% or more identity, preferably 90% or more, particularly preferably 95% or more identity with the amino acid sequence of SEQ ID NO: 4, and terephthalic acid to TPA-DHD.
- proteins having a function related to the conversion of can be mentioned.
- “function related to the conversion from terephthalic acid to TPA-DHD” means terephthalic acid together with terephthalic acid 1,2-dioxygenase / oxygenase large subunit protein and terephthalic acid 1,2-dioxygenase / reductase protein.
- Examples of the terephthalic acid 1,2-dioxygenase reductase protein used in the present invention include a protein having the amino acid sequence derived from the above-mentioned Comamonas testosteroni 72W2 strain represented by SEQ ID NO: 6.
- the terephthalic acid 1,2-dioxygenase / reductase protein used in the present invention comprises an amino acid sequence in which one or several amino acids are deleted, substituted or added in the amino acid sequence of SEQ ID NO: 6, and Examples thereof include proteins having a function related to the conversion of terephthalic acid to TPA-DHD.
- the protein comprises an amino acid sequence having 75% or more identity, preferably 90% or more, particularly preferably 95% or more identity with the amino acid sequence of SEQ ID NO: 6, and terephthalic acid to TPA-DHD Proteins having a function related to the conversion of can be mentioned.
- “functions related to the conversion from terephthalic acid to TPA-DHD” means terephthalic acid 1,2-dioxygenase / oxygenase large subunit protein and terephthalic acid 1,2-dioxygenase / oxygenase small subunit protein In addition, it means a function capable of producing TPA-DHD when reacted with terephthalic acid.
- the terephthalic acid transporter protein used in the present invention examples include a protein having an amino acid sequence derived from Rhodococcus jostii RHA1 strain represented by SEQ ID NO: 8.
- the terephthalic acid transporter protein used in the present invention is composed of an amino acid sequence in which one or several amino acids are deleted, substituted or added in the amino acid sequence of SEQ ID NO: 8, and transports terephthalic acid into cells.
- a protein having an ability can be mentioned.
- the protein comprises an amino acid sequence having 75% or more identity, preferably 90% or more, particularly preferably 95% or more identity with the amino acid sequence of SEQ ID NO: 8, and transports terephthalic acid into cells.
- TPA-DHD dehydrogenase protein used in the present invention examples include a protein having an amino acid sequence derived from the above-mentioned Comamonas testosteroni 72W2 strain represented by SEQ ID NO: 10.
- the TPA-DHD dehydrogenase protein used in the present invention includes an amino acid sequence in which one or several amino acids are deleted, substituted or added in the amino acid sequence of SEQ ID NO: 10, and TPA-DHD is converted to protocatechuic acid.
- proteins having the activity of converting to The protein comprises an amino acid sequence having 75% or more identity, preferably 90% or more, particularly preferably 95% or more identity with the amino acid sequence of SEQ ID NO: 10, and TPA-DHD is converted to protocatechuic acid.
- a protein having an activity to convert can be mentioned.
- Examples of the parahydroxybenzoic acid hydroxylase used in the present invention include, for example, a Pseudomonas aeruginosa PAO strain GenBank (hereinafter referred to as GenBank GB) protein of GB accession number AAG03636, or Pseudomonas putida ( Pseudomonas putida ) a protein of GB accession number AAN69138 of the KT2440 strain, or a protein having an amino acid sequence derived from the Corynebacterium glutamicum ATCC13032 strain represented by SEQ ID NO: 12.
- the parahydroxybenzoic acid hydroxylase protein used in the present invention is an amino acid sequence in which one or several amino acids are deleted, substituted or added in the amino acid sequence of SEQ ID NO: 12, and protocatechuic acid is gallic. Examples thereof include proteins having an activity of converting to an acid.
- the protein comprises an amino acid sequence having 75% or more identity, preferably 90% or more, particularly preferably 95% or more identity with the amino acid sequence of SEQ ID NO: 12, and converts protocatechuic acid to gallic acid.
- lactaldehyde reductase used in the present invention examples include the protein of GB accession number AAB40449 of Escherichia coli K-12 strain.
- the lactaldehyde reductase protein used in the present invention includes an amino acid sequence in which one or several amino acids are deleted, substituted or added in the amino acid sequence of GB accession number AAB40449, and ethylene glycol is glycosylated.
- a protein having an activity of converting to an aldehyde can be mentioned.
- the protein comprises an amino acid sequence having 75% or more identity, preferably 90% or more, particularly preferably 95% or more identity with the amino acid sequence of GB accession number AAB40449, and ethylene glycol is glycoaldehyde. Examples thereof include proteins having the activity of converting to.
- lactaldehyde dehydrogenase used in the present invention examples include the protein of GB accession number AAC74497 of Escherichia coli K-12 strain.
- the lactaldehyde dehydrogenase protein used in the present invention includes an amino acid sequence in which one or several amino acids are deleted, substituted or added in the amino acid sequence of GB accession number AAC74497.
- proteins having an activity of converting to an acid The protein comprises an amino acid sequence having 75% or more identity, preferably 90% or more, particularly preferably 95% or more identity with the amino acid sequence of GB accession number AAC74497, and glycoaldehyde is glycolic acid. Examples thereof include proteins having the activity of converting to.
- NBRC National Institute of Advanced Industrial Science and Technology, Biogenetic Resources Division
- NBRP Biogenetic Resources Division
- CGSC Coli Genetic Stock Center
- Terephthalic acid 1,2-dioxygenase / oxygenase large subunit protein, terephthalic acid 1,2-dioxygenase / oxygenase small subunit protein, terephthalic acid 1,2-dioxygenase / reductase protein, TPA-DHD Dehydrogenase protein, terephthalic acid transporter protein, lactaldehyde reductase protein, lactaldehyde dehydrogenase protein, consisting of amino acid sequences in which one or several amino acids are deleted, substituted or added, and each target enzyme Proteins with activity include Molecular Cloning, A Laboratory Manual, Second Edition, Cold Spring Harbor Laboratory Press (1989) (hereinafter abbreviated as Molecular Cloning 2nd Edition), Current Protocols in Molecular Biology, John Wiley & Sons (1987-1997) (hereinafter abbreviated as Current Protocols in Molecular Biology), Nucleic Acids Res., 10, 6487 (1982), Proc.
- the number of one or several amino acids to be deleted, substituted or added is not particularly limited as long as the individual enzyme activity is maintained, but is preferably within the number of differences from the original amino acid sequence. 20 is preferable, 1 to 10 is more preferable, and 1 to 5 is particularly preferable.
- Examples of the DNA encoding terephthalic acid 1,2-dioxygenase / oxygenase large subunit / protein used in the present invention include a DNA having the base sequence represented by SEQ ID NO: 1.
- DNA encoding terephthalic acid 1,2-dioxygenase / oxygenase small subunit / protein used in the present invention include DNA having the base sequence represented by SEQ ID NO: 3.
- Examples of the terephthalic acid 1,2-dioxygenase / reductase protein used in the present invention include DNA having the base sequence represented by SEQ ID NO: 5.
- Examples of the DNA encoding the terephthalic acid transporter protein used in the present invention include a DNA having the base sequence represented by SEQ ID NO: 7.
- TPA-DHD dehydrogenase protein used in the present invention examples include DNA having the base sequence represented by SEQ ID NO: 9.
- Examples of the parahydroxybenzoic acid hydroxylase protein used in the present invention include DNA having the base sequence represented by SEQ ID NO: 11.
- lactaldehyde reductase protein used in the present invention examples include DNA having a base sequence from 13420th to 4571th of GB accession number U2958.
- lactaldehyde dehydrogenase protein used in the present invention examples include DNA having a base sequence from 1486256 to 1487695 in GB accession number NC_000913.
- the DNA used in the present invention includes DNA into which mutations such as substitution mutations, deletion mutations and insertion mutations have been introduced within a range in which the protein encoded by each DNA does not lose the target enzyme activity, such as SEQ ID NO: 1, DNA that hybridizes under stringent conditions by a hybridization method using all or part of the DNA represented by 3, 5, 7, 9, or 11 as a probe is also included. Specifically, DNA that hybridizes under stringent conditions is 0.1 times after hybridization at 65 ° C. in the presence of 0.7 to 1.0 ⁇ M NaCl using a filter on which DNA is immobilized.
- microorganism Used in the present invention used to produce TPA-DHD from terephthalate containing 0.5 to 2 times the amount of potassium in terms of moles relative to the total terephthalic acid contained in terephthalate
- the microorganism include DNA encoding the terephthalic acid 1,2-dioxygenase / oxygenase large subunit, DNA encoding the terephthalic acid 1,2-dioxygenase / oxygenase small subunit, and the terephthalic acid 1,2-dioxy Any microorganism can be used as long as it has a DNA encoding oxygenase / oxygenase / reductase and has the ability to produce TPA-DHD from terephthalic acid.
- a microorganism having the DNA described in (1) and having the ability to produce TPA-DHD from terephthalic acid can be used.
- the microorganism having such properties may be a transformant obtained by introducing one or more of the DNAs described in (1) above into a host cell using a recombinant technique.
- microorganisms having such properties can be obtained by using one or more DNAs described in (4) above using recombinant technology. It may be a transformant obtained by introduction into a host cell.
- the microorganism used in the present invention for producing protocatechuic acid has a DNA encoding a protein having TPA-DHD dehydrogenase activity in addition to the above-mentioned ability to produce TPA-DHD from terephthalic acid, and TPA.
- Any microorganism can be used as long as it has the ability to produce protocatechuic acid from DHD. That is, the microorganism described in (1) or (4) having the DNA described in (5) and having the ability to produce protocatechuic acid from TPA-DHD can be used.
- microorganisms having such properties can be used to recombine one or more of the DNAs described in (5) above using recombinant technology.
- a microorganism having a DNA encoding a terephthalic acid transporter and having enhanced terephthalic acid transport ability is used. Is preferred.
- microorganisms having such properties can be used to transfer one or more of the DNAs described in (5) above to host cells using recombinant technology.
- a transformant obtained by introduction can be used.
- the microorganism used in the present invention for producing gallic acid includes DNA encoding a protein having TPA-DHD dehydrogenase activity in addition to the above-mentioned ability to produce TPA-DHD from terephthalic acid, and parahydroxybenzoic acid
- Any microorganism can be used as long as it has a DNA encoding a protein having a hydroxylase and has the ability to produce gallic acid from TPA-DHD. That is, in addition to the DNA described in (5) above, a microorganism having the DNA described in (7) above and capable of producing gallic acid from terephthalic acid can be used.
- microorganisms having such properties can recombine one or more DNAs described in (7) above. It may be a transformant obtained by introducing it into a host cell using a technique.
- a microorganism having DNA encoding a terephthalic acid transporter and having enhanced terephthalic acid transport ability should be used. Is preferred.
- microorganisms having such properties can be obtained by using one or more of the DNAs described in (7) above by recombinant technology. Or a transformant obtained by introduction into a host cell.
- the microorganism used in the present invention which decomposes ethylene glycol mixed in terephthalate, produces TPA-DHD, protocatechuic acid or gallic acid from the above-mentioned terephthalic acid.
- Microorganisms with enhanced ethylene glycol resolution can be used by introducing mutation into lactaldehyde reductase and lactaldehyde dehydrogenase and / or enhancing the expression level of lactaldehyde reductase and lactaldehyde dehydrogenase. .
- Bacteria such as Escherichia coli K-12 strain, Comamonas testosteroni 72W2 strain, Rhodococcus josti RHA1 strain, Corynebacterium glutamicum ATCC13032 strain described above are the culture conditions recommended by the above-mentioned microorganism distribution agencies or commonly used known conditions. Culture by method. After culturing, the chromosomal DNA of the microorganism is isolated and purified by a known method (for example, the method described in Current Protocols in Molecular Biology). From this chromosomal DNA, a fragment containing a DNA encoding the target protein can be obtained by a hybridization method or a PCR method using a synthetic DNA.
- DNA encoding the protein of interest can also be obtained by chemical synthesis.
- the synthetic DNA is, for example, a nucleotide sequence represented by SEQ ID NO: 1 of DNA encoding terephthalic acid 1,2-dioxygenase / oxygenase large subunit protein derived from Comamonas testosteroni 72W2 strain, and Comamonas testosteroni 72W2 strain Terephthalic acid 1,2-dioxygenase-oxygenase small subunit protein-derived DNA sequence represented by SEQ ID NO: 3, and terephthalic acid 1,2-dioxygenase reductase derived from Comamonas testosteroni 72W2 -It can design based on the base sequence represented by sequence number 5 of DNA which codes protein.
- the synthetic DNA encoding the terephthalic acid transporter protein can be designed based on the base sequence represented by SEQ ID NO: 7 of the DNA encoding the terephthalic acid transporter protein of Rhodococcus josti RHA1 strain.
- the synthetic DNA encoding TPA-DHD dehydrogenase can be designed based on the nucleotide sequence represented by SEQ ID NO: 9 of the DNA encoding TPA-DHD dehydrogenase protein of Comamonas testosteroni 72W2.
- SEQ ID NO: 11 of DNA encoding parahydroxybenzoate hydroxylase protein protein in which 385th tyrosine is substituted with phenylalanine
- SEQ ID NO: 11 of DNA encoding parahydroxybenzoate hydroxylase protein protein in which 385th tyrosine is substituted with phenylalanine
- Corynebacterium glutamicum ATCC13032 strain It can design based on the base sequence represented by these.
- Synthetic DNA encoding lactaldehyde reductase is designed based on the nucleotide sequence from 13420th to 4571th of GB accession number U2958 of DNA encoding lactaldehyde reductase protein of Escherichia coli K-12 strain can do.
- Synthetic DNA encoding lactaldehyde dehydrogenase is designed based on the nucleotide sequence from 1486256 to 1487695 in GB accession number NC_000913 of DNA encoding lactaldehyde dehydrogenase protein of Escherichia coli K-12 strain can do.
- any plasmid vector, phage vector, etc. can be used as long as they are vectors capable of autonomous replication in Escherichia coli K12 strain.
- pUC19 Gene, 33, 103 (1985)
- pUC18 pBR322
- pHelix1 manufactured by Roche Diagnostics
- ZAP® Express Stratagene, Strategies, 5, 58 (1992)
- pBluescript II SK (+) (Stratagene) Nucleic Acids Res., 17, 17, 9494 (1989)
- pUC118 manufactured by Takara Bio Inc.
- Escherichia coli used as a host for the recombinant DNA obtained by ligating the above-obtained DNA to the vector can be used as long as it belongs to Escherichia coli.
- Escherichia coli C600 Genetics, 39, 440 (1954)
- Escherichia coli Y1088 Science, 222,778 ( 1983), Escherichia coli Y1090 (Science, 222, 778 (1983)]
- Escherichia coli NM522 J. Mol.
- Rhodococcus genus Comamonas (Comamonas) genus Corynebacterium (Corynebacterium) genus Pseudomonas (Pseudomonas) genus, Poraromonasu (Polaromonas) genus, in a microorganism belonging to Ralstonia (Ralstonia) genus or Burkholderia (Burkholderia spp.)
- a vector capable of autonomous replication among these microorganisms is used.
- the recombinant DNA can be introduced into the host microorganism using a shuttle vector capable of autonomous replication in both microorganisms of both the microorganism and the Escherichia coli K12 strain.
- any method can be used as long as it is a method for introducing DNA into the above host cell.
- a method using calcium ions [Proc.cNatl. Acad. Sci., USA, 69, 2110]. (1972)), electroporation method (Nucleic Acids Res., 16, 6127 (1988)), junction transfer method (J. G. C. Ottow, Ann. Rev. Microbiol., Vol. 29, p. 80 ( 1975)], cell fusion methods [MH Gabor, J. Bacteriol., Vol. 137, p. 1346 (1979)], and the like.
- Recombinant DNA can be extracted from the transformant obtained as described above, and the base sequence of the DNA used in the present invention contained in the recombinant DNA can be determined.
- a commonly used base sequence analysis method for example, dideoxy method [Proc.tlNatl. Acad. Sci. USA, 74, 54631977 (1977)] or 3730xl type DNA analyzer (manufactured by Applied Biosystems), etc. Can be used.
- the target DNA can also be prepared by chemical synthesis using the 8905 type DNA synthesizer manufactured by Perceptive Biosystems based on the DNA base sequence determined above.
- a DNA fragment having an appropriate length containing a portion encoding the protein used in the present invention can be prepared as necessary. Further, the production rate of the protein can be improved by substituting the base in the base sequence of the protein-encoding portion so as to be an optimal codon for host expression.
- the transformant that expresses the DNA used in the present invention is prepared by inserting the above DNA fragment downstream of the promoter of an appropriate expression vector to produce a recombinant DNA and adapting the recombinant DNA to the expression vector. Can be obtained by introduction into a host cell.
- any bacteria, yeast, animal cells, insect cells, plant cells, etc. can be used so long as they can express the target gene.
- a microorganism that does not have the ability to metabolize TPA-DHD can be used. More preferably, mention may be made of bacteria or Pseudomonas (Pseudomonas) bacteria of the genus Escherichia having no metabolism of TPA-DHD. More preferable examples include Escherichia coli K-12 strain and Pseudomonas putida KT2440 strain.
- the expression vector one that can replicate autonomously in the host cell or can be integrated into a chromosome and contains a promoter at a position where the DNA used in the present invention can be transcribed is used.
- the recombinant DNA containing the DNA used in the present invention can be autonomously replicated in a prokaryotic organism, and at the same time, a promoter, a ribosome binding sequence, and the present invention are used. It is preferably a recombinant DNA composed of DNA to be transcribed and a transcription termination sequence. A gene that controls the promoter may also be included.
- a plasmid having a replication origin derived from ColE1 for example, a pUC series plasmid, a pBR322 series plasmid, or a derivative thereof can be mentioned.
- the “derivative” means one obtained by modifying a plasmid by base substitution, deletion, insertion, addition and / or inversion.
- the modification here includes modification by mutation treatment with a mutation agent, UV irradiation, or natural mutation.
- examples of the vector include pUC19 (Gene, 33, 103 (1985)), pUC18, pBR322, pHelix1 (manufactured by Roche Diagnostics), pKK233-2 (manufactured by Amersham Pharmacia Biotech). ), PSE280 (Invitrogen), pGEMEX-1 (Promega), pQE-8 (Qiagen), pET-3 (Novagen) pBluescriptII SK (+), pBluescript II KS (+) (Stratagene) PSTV28 (manufactured by Takara Bio Inc.), pUC118 (manufactured by Takara Bio Inc.) and the like can be used.
- any promoter can be used so long as it can be expressed in a host cell such as Escherichia coli.
- artificially designed such as trp promoter (Ptrp), lac promoter (Plac), PL promoter, PR promoter, etc., promoters derived from Escherichia coli such as T7 promoter, phages, etc., and tac promoter, lacT7 promoter A modified promoter or the like, and a Pm promoter controlled by the XylS protein of the P. topidae TOL plasmid can be used.
- a transcription termination sequence is not necessarily required for the expression of the DNA used in the present invention, but it is preferable to arrange the transcription termination sequence immediately below the structural gene.
- a promoter having a transcription activity stronger than that of a natural promoter is used as a promoter for expressing a gene encoding any of the above proteins, or as a terminator for terminating transcription of a gene encoding the protein. Examples include using a terminator having a transcription termination activity stronger than that of a natural terminator, using a high copy number vector as an expression vector, or incorporating it into a chromosome by homologous recombination.
- Terephthalic acid 1,2-dioxygenase / oxygenase large subunit / protein, terephthalic acid 1,2-dioxygenase / oxygenase small subunit / protein, and terephthalic acid 1,2-dioxygenase / reductase obtained as described above -TPA-DHD can be produced from terephthalate by using a microorganism expressing a protein. For example, after culturing the microorganism in a liquid medium, terephthalate containing 0.5 times or more and 2 times or less potassium in terms of molar relative to the total terephthalic acid contained in the terephthalate is added to the culture solution of the microorganism.
- TPA-DHD By adding to a concentration of 0.1 ⁇ M to 1 ⁇ M, TPA-DHD can be generated and accumulated, and TPA-DHD can be collected from the culture solution.
- the method of culturing the microorganism used in the present invention in a medium can be performed according to a usual method used for culturing microorganisms.
- TPA-DHD can also be produced by adding and culturing TPA-DHD by adding the cultured cells or treated product of the cultured cells and collecting TPA-DHD from the medium.
- Terephthalic acid 1,2-dioxygenase / oxygenase large subunit protein, terephthalic acid 1,2-dioxygenase / oxygenase small subunit protein, terephthalic acid 1,2-dioxygenase / reductase protein, and TPA-DHD dehydrogenase Protocatechuic acid can be produced from terephthalate by using a microorganism that expresses. For example, after culturing the microorganism in a liquid medium, terephthalate containing 0.5 times or more and 2 times or less potassium in terms of molar relative to the total terephthalic acid contained in the terephthalate is added to the culture solution of the microorganism.
- protocatechuic acid By adding to a concentration of 0.1 to 1 ⁇ M, protocatechuic acid can be produced and accumulated, and protocatechuic acid can be collected from the culture solution.
- the method of culturing the microorganism used in the present invention in a medium can be performed according to a usual method used for culturing microorganisms.
- the microorganism is contained in an aqueous medium containing terephthalate containing 0.5 to 2 times the amount of potassium in terms of moles relative to the total terephthalic acid contained in the terephthalate.
- Protocatechuic acid can also be produced by adding cultivated microbial cells or treated products of the cultured microbial cells to produce and accumulate protocatechuic acid and collecting protocatechuic acid from the medium.
- TPA-DHD is produced from terephthalate by the method according to any one of (1) to (4), it is shown in (q), (r), (s) or (t).
- TPA-DHD is converted to protocatechuic acid using a culture of another microorganism obtained by introducing DNA by a transformation method or a treated product of the culture, and protocatechuic acid is collected from the culture solution or the medium Protocatechuic acid can also be produced.
- Terephthalic acid 1,2-dioxygenase / oxygenase large subunit / protein, terephthalic acid 1,2-dioxygenase / oxygenase small subunit / protein, terephthalic acid 1,2-dioxygenase / reductase / protein, TPA-DHD dehydrogenase, And gallic acid can be produced from terephthalate by using a microorganism expressing parahydroxybenzoate hydroxylase.
- terephthalate containing 0.5 times or more and 2 times or less potassium in terms of molar relative to the total terephthalic acid contained in the terephthalate is added to the culture solution of the microorganism.
- gallic acid can be generated and accumulated, and gallic acid can be collected from the culture solution.
- the method of culturing the microorganism used in the present invention in a medium can be performed according to a usual method used for culturing microorganisms.
- the microorganism is contained in an aqueous medium containing terephthalate containing 0.5 to 2 times the amount of potassium in terms of moles relative to the total terephthalic acid contained in the terephthalate.
- the gallic acid can also be produced by adding and cultivating gallic acid by adding the cultured microbial cell or the treated product of the cultured microbial cell, and collecting gallic acid from the medium.
- TPA-DHD is produced from terephthalate by the method according to any one of (1) to (4), it is shown in (q), (r), (s) or (t).
- TPA- Gallic acid can also be produced by converting DHD to gallic acid and collecting gallic acid from the culture medium or the medium.
- the terephthalate containing 0.5 to 2 times the amount of potassium in terms of moles relative to the total terephthalic acid contained in terephthalate is dipotassium terephthalate, 1-potassium 4-sodium terephthalate and terephthalate.
- High-purity terephthalate containing potassium terephthalate such as acid 1-potassium 4-ammonium salt may be used, but it may be obtained from waste polyester.
- the following describes a method of obtaining terephthalate containing 0.5 to 2 times the amount of potassium in terms of moles relative to the total terephthalic acid contained in the terephthalate used as a raw material for compound production by microorganisms from waste polyester. .
- any shape such as a container, a film, a sheet, a part, a fiber, a granular pulverized material, and a powdered pulverized material can be used.
- These polyesters are the main components (the main component means, for example, that 80% or more of PET and / or PTT and / or PBT are included) and foreign components (the main components include, for example, polyethylene and polypropylene).
- the polyester crushed material thus obtained is weighed and the terephthalic acid content (number of moles) is measured, and then placed in a metallic reaction kettle. Furthermore, after adding potassium hydroxide or a mixture of potassium hydroxide and sodium hydroxide and a reaction solvent of either ethylene glycol or 1-butanol to the reaction kettle, the polyester crushed material is heated by heating for an appropriate time. A depolymerization reaction is performed. Inclusion of an appropriate amount of water in the solution for the depolymerization reaction allows the reaction to proceed more quickly and improves the yield of recoverable terephthalate.
- the potassium hydroxide or sodium hydroxide to be added can be added in the form of solid particles or an aqueous solution, but it is about twice as much as the number of moles of terephthalic acid in the crushed polyester put in the reaction vessel.
- the number of moles is preferably added so that the total number of moles is preferably 2 to 2.2 times. More preferably, the total amount of alkali added is 2.10 times the total number of terephthalic acids in the crushed polyester.
- the amount of ethylene glycol or 1-butanol to be added is preferably 3 to 10 times the volume of the polyester crushed material. More preferably, the total amount of solvent added is 5 times. Although it is desirable to add an appropriate amount of water to the depolymerization reaction solution, the total amount of water contained in the depolymerization reaction solution is 1 to 5 times the number of moles of terephthalic acid in the crushed polyester. The amount is desirable.
- the pressure in the reaction vessel is preferably a pressure in the vicinity of atmospheric pressure, for example, 0.9 to 1.1 atm (91.193 to 111.458 kPa).
- the reaction temperature when ethylene glycol is used as the reaction solvent is a temperature at which water evaporates and ethylene glycol does not evaporate.
- the pressure in the reaction vessel is 1 atm, 100 to 196 ° C. is preferable.
- the reaction is performed at a pressure lower than 1 atm, the reaction is performed within a range of 100 to 196 ° C. at a temperature at which water evaporates and ethylene glycol does not evaporate.
- the reaction is performed at a pressure higher than 1 atm, the reaction is performed within a range of 100 to 196 ° C.
- the reaction temperature when 1-butanol is used as the reaction solvent is a temperature at which water evaporates and 1-butanol does not evaporate.
- the pressure in the reaction vessel is 1 atm, 100 to 116 ° C. is preferable.
- the reaction is performed at a pressure lower than 1 atm, the reaction is performed within a range of 100 to 116 ° C. at a temperature at which water evaporates and 1-butanol does not evaporate.
- the reaction is performed at a pressure higher than 1 atm, the reaction is performed within a range of 100 to 116 ° C. at a temperature at which water evaporates and 1-butanol does not evaporate.
- the heating time is preferably the time for the polyester crushed material to be completely decomposed, usually 10 to 240 minutes, but the dissolution rate decreases in the order of PET, PTT, PBT, so when depolymerizing PTT and PBT, the heat decomposition time It is desirable to lengthen. Volatile substances such as moisture that evaporate during the heating reaction are recovered by a condenser.
- the solvent as a depolymerization reaction solvent for the PET, PTT, or PBT.
- the solubility of terephthalate in 1-butanol solvent is low, it is possible to reduce the production cost by reusing the 1-butanol solvent used in the depolymerization reaction as the depolymerization reaction solvent for PET, PTT, or PBT. Therefore, it is preferable.
- the ethylene glycol solvent or 1-butanol solvent used for the depolymerization reaction of PET, PTT, or PBT can be repeatedly used for the depolymerization reaction of the polyester by separating the produced terephthalate.
- the obtained terephthalate solid may be washed with an appropriate amount of ethylene glycol or 1-butanol.
- polyester waste containing PTT or PBT is depolymerized, 1,3-propanediol or 1,4-butanediol is mixed in the solid, so that it is washed with ethylene glycol or 1-butanol. It is preferable to apply.
- ethylene glycol is mixed into the solid, and the ethylene glycol can be removed by drying under reduced pressure as described below. The ethylene glycol may be removed by washing with butanol.
- the solid matter of terephthalate thus obtained contains a significant amount of ethylene glycol or 1-butanol. If necessary, ethylene in the solid matter can be obtained using a vacuum dryer or the like. A liquid substance is separated using a treatment for reducing glycol or 1-butanol, or a centrifuge.
- TPA-DHD protocatechuic acid or gallic acid using terephthalate containing 0.5 to 2 times the amount of potassium in terms of moles relative to the total terephthalic acid contained in the terephthalate thus obtained
- terephthalate containing 0.5 to 2 times the amount of potassium in terms of moles relative to the total terephthalic acid contained in the terephthalate thus obtained
- the terephthalate obtained by dissolving the terephthalate in an appropriate amount of water and then removing the foreign matter by a filtration method.
- An aqueous solution may be used for the production of compounds by microorganisms.
- the present invention In order to decompose ethylene glycol mixed in terephthalate obtained by depolymerization of waste polyester in an ethylene glycol solvent, the present invention with enhanced expression levels of lactaldehyde / reductase and lactaldehyde / dehydrogenase is used.
- terephthalate containing ethylene glycol and containing 0.5 to 2 times the amount of potassium in terms of moles relative to the total terephthalic acid contained in the terephthalate TPA-DHD protocatechuic acid or gallic acid can also be produced while decomposing ethylene glycol by adding it to the culture broth to a concentration of 0.1 to 1 ⁇ M.
- TPA-DHD, protocatechuic acid or gallic acid can be generated and accumulated by adding cultured cells of microorganisms or processed cells of the cultured cells, and TPA-DHD, protocatechuic acid or gallic acid can be collected from the medium. .
- the microorganism used in the present invention can be cultured in a normal nutrient medium containing a carbon source, a nitrogen source, an inorganic salt, various vitamins, etc.
- a carbon source include sugars such as glucose, sucrose, and fructose, Alcohols such as ethanol and methanol, organic acids such as citric acid, malic acid and succinic acid, waste molasses and the like are used.
- the nitrogen source for example, ammonia, ammonium sulfate, ammonium chloride, ammonium nitrate, urea or the like is used alone or in combination.
- inorganic salts that can be used include potassium monohydrogen phosphate, potassium dihydrogen phosphate, and magnesium sulfate.
- the raw material for producing TPA-DHD, protocatechuic acid or gallic acid is 0.5 times or more and 2 times or less in terms of mole relative to the total terephthalic acid contained in terephthalate prepared as described above.
- potassium-containing terephthalate is 0.5 times or more and 2 times or less in terms of mole relative to the total terephthalic acid contained in terephthalate prepared as described above.
- the culture is usually carried out under aerobic conditions such as aeration stirring and shaking.
- the culture temperature is not particularly limited as long as the microorganism used in the present invention can grow, and the pH during the culture is not particularly limited as long as the microorganism used in the present invention can grow.
- the pH adjustment during the culture can be performed by adding an acid or an alkali.
- a processed product of the cultured cells one obtained by immobilizing a microorganism used in the present invention on a carrier may be used.
- cells recovered from the culture or washed with an appropriate buffer for example, a phosphate buffer (pH 6 to 10) of about 0.02 to 0.2 ⁇ M can be used.
- the extract containing the protein used in the present invention obtained by crushing the microbial cells recovered from the culture by means of ultrasonic waves, squeezing or the like, and extracting the crushed material with water or the like
- a product obtained by further immobilizing a partially purified component of the protein used in the present invention obtained by subjecting the extract to further treatment with ammonium sulfate salting out, column chromatography, etc. on a carrier is also used for the TPA-DHD, protocatech of the present invention. It can be used for the production of acid or gallic acid.
- Immobilization of these cells, disrupted cells, extracts, or purified enzymes is carried out by immobilizing the cells on an appropriate carrier such as acrylamide monomer, alginic acid, or carrageenan according to a commonly used method known per se. It can be performed by the method of making it.
- an appropriate carrier such as acrylamide monomer, alginic acid, or carrageenan according to a commonly used method known per se. It can be performed by the method of making it.
- the aqueous medium used for the reaction can be an aqueous solution containing terephthalate or an appropriate buffer, for example, a phosphate buffer (pH 6 to 10) of about 0.02 to 0.2 ⁇ M.
- a phosphate buffer pH 6 to 10
- 0.05-2.0% (w / v) of toluene, xylene, nonionic surfactant, etc. can be added when it is necessary to further increase the substance permeability of the cell membrane of the cells.
- the concentration of terephthalate used as a reaction raw material in the aqueous medium is suitably about 0.1 to 1 ⁇ M.
- the enzyme reaction temperature and pH in the above aqueous medium are not particularly limited, but are usually 10 to 60 ° C., preferably 15 to 50 ° C., and the pH in the reaction solution is 5 to 10, preferably about 6 to 9. can do.
- the pH can be adjusted by adding acid or alkali.
- the enzyme used in the invention can be obtained by collecting the bacterial cell extract as it is or by collecting it by centrifugation, filtration or the like and suspending it in water or a buffer solution.
- the enzyme thus obtained is reacted in the presence of terephthalate, and it is advantageous that the concentration of terephthalate in the reaction solution is as high as possible within a range not inhibiting the activity of the enzyme.
- the reaction may be performed by any method of standing, stirring and shaking.
- a method in which an enzyme is immobilized on a suitable support, packed in a column, and a solution containing terephthalate is allowed to flow can be used.
- the reaction is usually carried out at 10 to 60 ° C., preferably 15 to 50 ° C., pH 5 to 9, preferably pH 6 to 9.
- the production yield of TPA-DHD, protocatechuic acid or gallic acid may be further improved.
- Antioxidants / reducing agents include ascorbic acid, isoalcorbic acid, cysteine, sulfites such as sodium sulfite and sodium bisulfite, and thiosulfates such as sodium thiosulfate.
- the concentration to be added varies depending on the kind of the antioxidant / reducing agent, but it is desirable to add it at a concentration that does not inhibit the production of TPA-DHD, protocatechuic acid or gallic acid, usually 0.001 to 5% (w / v), preferably 0.005 to 1%.
- the production yield of TPA-DHD, protocatechuic acid or gallic acid may be further improved.
- the oxidizing agent include nitrates such as sodium nitrite and potassium nitrite, metal salts such as ferric chloride and ferric sulfate, halogen, peroxo acid, etc., preferably sodium nitrite, ferric chloride, A ferric sulfate is mentioned.
- the addition concentration varies depending on the type of oxidizing agent, but it is desirable to add it at a concentration that does not inhibit the production of TPA-DHD, protocatechuic acid or gallic acid, and is usually 0.001 to 0.05% (w / v), preferably 0.005 to 0.02%. It is.
- TPA-DHD protocatechuic acid or gallic acid in the culture solution or reaction solution after completion of the culture is freed from insoluble components such as bacterial cells from the culture solution by centrifugation or the like, if necessary. Extraction methods using organic solvents, methods using activated carbon, methods using ion exchange resins, crystallization methods, precipitation methods such as salting out, distillation methods, etc., alone or in combination, TPA-DHD, protocatechuic acid or gallic acid Acid can be collected.
- a salt is formed by lowering the pH by adding sulfuric acid or hydrochloric acid in the purification step.
- the carboxylic acid present can be in the free acid state.
- Example 1 Measurement of solubility of various terephthalate salts Terephthalic acid (powder, Sigma-Aldrich Japan; unless otherwise specified, the reagent was Sigma-Aldrich Japan) 2.99 g (0.018 mol) and potassium hydroxide (granular) 0.036 mol was put into a 50 mL glass beaker and made up to about 15 mL with distilled water. The mixture was covered with aluminum foil, heated with a hot stirrer (manufactured by ASONE) for 60 minutes at 50 ° C., allowed to cool, and cooled to 30 ° C.
- a hot stirrer manufactured by ASONE
- the concentration of terephthalic acid in the supernatant was measured and used as the solubility of dipotassium terephthalate.
- the sample was diluted 16000 times with 2.5% acetonitrile aqueous solution, analyzed using high performance liquid chromatography (Water, LCT Premier XE) under the separation conditions shown in Table 1, and the concentration was known. Calculation was based on the ratio of the area of the terephthalic acid peak relative to the aqueous terephthalic acid solution.
- terephthalic acid dipotassium salt Similar to the above method for preparing an aqueous solution of terephthalic acid dipotassium salt, 0.036 mol of sodium hydroxide (granular) or 0.036 mol of 28% ammonia water for 2.99 g (0.018 mol) of terephthalic acid (powder), Or disodium terephthalate by adding 0.018 mol of potassium hydroxide and 0.018 mol of sodium hydroxide, or 0.018 mol of potassium hydroxide and 0.018 mol of 28% aqueous ammonia, or 0.018 mol of sodium hydroxide and 0.018 mol of 28% aqueous ammonia After preparing an aqueous solution of terephthalic acid diammonium salt, terephthalic acid 1-potassium 4-sodium salt, terephthalic acid 1-potassium 4-ammonium salt, or terephthalic acid 1-sodium 4-ammonium salt, Solubility was measured.
- the solubility of each terephthalate obtained is shown in Table 2. From the results in Table 2, dipotassium terephthalate, 1-potassium 4-sodium terephthalate, 1-potassium 4-ammonium terephthalate, disodium terephthalate, 1-sodium 4-ammonium terephthalate, and diammonium terephthalate in this order. It has been found that the solubility in water is high and the solubility of a salt in which at least one carboxyl group residue of terephthalic acid forms a salt with potassium is excellent.
- Example 2 Difference in productivity of TPA-DHD due to difference in terephthalate salt Based on the results of Example 1, 0.6 M terephthalic acid based on terephthalic acid, potassium hydroxide, sodium hydroxide, 28% ammonia water and distilled water 500 mL each of dipotassium aqueous solution, 0.6 M 1-potassium 4-sodium terephthalate aqueous solution, 0.6 M 1-potassium 4-ammonium terephthalate aqueous solution, and 0.6 M disodium terephthalate aqueous solution were prepared. 30 ml of these terephthalate solutions and 7 g of glucose were mixed to obtain a glucose / substrate mixture.
- the TPA-DHD production plasmid pUXPEaLT_tphA2A3A1_tpaK constructed in Reference Example 3 was introduced into E. coli M7032 strain (obtained from CGSC of Yale University) by a transformation method to obtain a recombinant E. coli strain M7032 (pUXPEaLT_tphA2A3A1_tpaK).
- This transformant was added to 1 ⁇ ml of LB liquid medium (10 g / l tryptone (Difco), 5 g / l dried yeast extract (Difco), 10 g / l salt) containing ampicillin final concentration of 100 mg / l. Sodium] overnight.
- LB liquid medium 10 g / l tryptone (Difco), 5 g / l dried yeast extract (Difco), 10 g / l salt
- ampicillin final concentration of 100 mg / l. Sodium overnight.
- 10% of F6.6W / P100 / G2 medium containing ampicillin final concentration of 100 mg / l was inoculated with 1% of this overnight culture and cultured at 30 ° C. for 24 hours.
- F6.6W / P100 / G2 medium means potassium dihydrogen phosphate ⁇ ⁇ 13.6 g / L, citric acid monohydrate 2.1 g / L, ammonium sulfate 9.9 g / L, iron (II) sulfate heptahydrate ⁇ ⁇ 1.703 g / L, glucose 20 mg / L, magnesium sulfate heptahydrate 246 mg / ml, calcium chloride dihydrate 12.9 mg / L, thiamine hydrochloride 10 mg / L, magnesium oxide 10.75 mg / L, calcium carbonate 2 mg / L, zinc sulfate heptahydrate 4.5 mg / ml, manganese (II) sulfate tetrahydrate 1.12 mg / L, copper (II) sulfate pentahydrate 0.25 mg / L, cobalt sulfate (II) heptahydrate Japanese aqueous solution 0.28 g /
- the F6.6W / P10 / G1 medium is 1.36 ⁇ ⁇ ⁇ ⁇ g / L potassium dihydrogen phosphate, 2.1 g / L citric acid monohydrate, 9.9 g / L ammonium sulfate, 1.70303 iron sulfate (II) sulfate heptahydrate 1.703 g / L, glucose 20 mg / L, magnesium sulfate heptahydrate 246 mg / ml, calcium chloride dihydrate 12.9 mg / L, thiamine 10 mg / L, magnesium oxide 10.75 mg / L, calcium carbonate 2 mg / L L, zinc sulfate heptahydrate 4.5 mg / ml, manganese sulfate tetrahydrate 1.12 mg / L, copper sulfate pentahydrate 0.2 5mg / L, cobalt sulfate heptahydrate 0.28 g / L, boric acid 0.06 mg An a
- the aeration condition of the jar fermenter culture was adjusted to 1 vvm, and the pH of the culture was adjusted using aqueous ammonia and sulfuric acid.
- the dissolved oxygen in the culture solution was adjusted by adjusting the saturation oxygen concentration to 100%, and controlled by the rotation speed of the stirring blade so as to maintain the dissolved oxygen that decreases with the growth of the cells at 15%.
- the main culture medium was inoculated with 0.1% of the preculture and cultured under the above conditions.
- an inducer m-toluic acid is added to a final concentration of 1 mM, and terephthalic acid 1,2-dioxygenase protein, terephthalic acid 1, 2-dioxygenase reductase protein and terephthalate transporter protein were expressed.
- the glucose concentration was measured over time, and the glucose / substrate mixture was administered when the glucose concentration reached zero.
- TPA-DHD was identified based on the expected accurate mass value of TPA-DHD among substances appearing with a decrease in terephthalic acid. TPA-DHD was quantified based on a calibration curve prepared from the decrease in terephthalic acid and the increase in the TPA-DHD peak area.
- Table 3 shows the ratio of TPA-DHD production from each terephthalate salt after 48 hours of culture as 100%. From the results shown in Table 3, it was revealed that the productivity of TPA-DHD was superior to that of the disodium terephthalic acid salt in all of the three types of potassium terephthalic acid salt.
- Example 3 Difference in productivity of TPA-DHD depending on the concentration of potassium ion in terephthalate From the results of Example 2, it was shown that the productivity of TPA-DHD is excellent when terephthalic acid potassium salt is used as a raw material. Therefore, the influence of the potassium ion concentration in terephthalate on TPA-DHD was examined as follows.
- Example 4 Difference in protocatechuic acid productivity due to difference in terephthalate
- the protocatechuic acid production plasmid pUXPEaLT_tphA2A3BA1_tpaK constructed in Reference Example 2 was introduced into Escherichia coli M7032 by transformation to obtain protocatechuic acid-producing Escherichia coli M7032 (pUXPEaLT_tphA2A3BA1_tpaK).
- Example 2 After preparing a pre-culture solution of the present protocatechuic acid-producing bacterium, 2% of the pre-culture solution was inoculated into the main culture medium to perform main culture.
- the inducer m-toluic acid is added to a final concentration of 1 mM, and terephthalic acid 1,2-dioxygenase protein, terephthalic acid 1,2 Dioxygenase reductase protein, terephthalate dihydrodiol dehydrogenase protein, and terephthalate transporter protein were expressed.
- the glucose concentration was measured over time, and the glucose / substrate mixture was administered when the glucose concentration reached zero.
- Table 5 shows the ratio of protocatechuic acid production from each terephthalate after 48 hours of culture as 100%. From the results shown in Table 5, it was clarified that all of the three types of potassium terephthalate had better protocatechuic acid productivity than disodium terephthalate.
- Example 5 Difference in gallic acid productivity due to difference in terephthalate
- the gallic acid plasmid pUXPEaLT_HFM145_tphA2A3BA1_tpaK constructed in Reference Example 4 was introduced into E. coli M7032 strain by transformation to obtain a gallic acid producing E. coli strain M7032 (pUXPEaLT_HFM145_tphA2A3BA1_tpaK). Subsequently, after preparing a preculture solution of this gallic acid-producing bacterium in the same manner as in Example 2, 2% of this preculture solution was inoculated into the main culture medium, and main culture was performed under the above conditions.
- an inducer m-toluic acid is added to a final concentration of 1 mM, and terephthalic acid 1,2-dioxygenase protein, terephthalic acid 1,2- Dioxygenase reductase protein, terephthalate dihydrodiol dehydrogenase protein, terephthalate transporter protein, and improved parahydroxybenzoate hydroxylase were expressed.
- the glucose concentration was measured over time, and the glucose / substrate mixture was administered when the glucose concentration reached zero.
- terephthalic acid and protocatechuic acid were carried out by combining the retention time of high performance liquid chromatography and the accurate mass value from the mass spectrometer as compared with terephthalic acid and gallic acid as standard products.
- Table 5 shows the ratio of gallic acid production from each terephthalate salt after 48 hours of culture as 100%. From the results shown in Table 6, it was revealed that all of the three types of potassium terephthalate had better gallic acid productivity than disodium terephthalate.
- Example 6 Depolymerization of waste PET in ethylene glycol solvent (1) Difference in depolymerization rate and depolymerization efficiency of waste PET depending on the type of alkali metal hydroxide After collecting 22 kg of waste PET bottles and separating the label and cap , Washed and air dried. This washed waste PET bottle was commissioned by Nippon Coke Kogyo Co., Ltd. and pulverized into grains having a size passing through a 5 mm square screen. This pulverized product was used as a waste plastic bottle crushed product in the following steps.
- the concentration of terephthalate contained in each of the solvents was 0.23 M for the dipotassium terephthalate saturated solution, 0.11 M for the disodium terephthalate saturated solution, and 0.20 M for the 1-potassium 4-sodium terephthalate saturated solution. .
- the contents were separated by a filter paper and separated into a filtrate and a residue, and the weight of each residue and the total amount of terephthalic acid in the residue were measured. From the results shown in Table 8, it was found that the total amount of terephthalic acid in the residue after filtration was large in the order of potassium hydroxide, a mixture of potassium hydroxide and sodium hydroxide, and sodium hydroxide.
- Example 7 Depolymerization of PTT in ethylene glycol solvent 13 g of PTT cotton (Nishikawa Sangyo Co., Ltd., Fine Smooth Solotex Watt Refill Pack RC7954) and ethylene saturated with dipotassium terephthalate prepared in Example 6 (3) 130 g glycol solvent and 0.13 mol potassium hydroxide or 130 g solution saturated with disodium terephthalate and 0.13 mol sodium hydroxide or 130 g 1-potassium 4-sodium terephthalate saturated solution and 0.066 mol potassium hydroxide and water 0.066 mol of sodium oxide was put into a 200 mL stainless steel beaker and hydrolyzed by heating at 160 ° C. for 60 minutes while stirring vigorously with a hot stirrer.
- the contents were dispensed after the temperature was allowed to cool and the internal temperature was below 40 ° C.
- the contents were separated by filter paper (manufactured by Whatman, grade 540) and separated into filtrate and residue, and the weight of each residue and the total amount of terephthalic acid in the residue were measured. From the results shown in Table 9, it was found that the total amount of terephthalic acid in the residue after filtration was large in the order of potassium hydroxide, a mixture of potassium hydroxide and sodium hydroxide, and sodium hydroxide.
- Example 8 Depolymerization of PBT in ethylene glycol solvent After adding 15 g of crushed PBT astragalus (PBT Astragalus 103TW white (RLVH001) purchased from Amazon.com) to a 200 mL stainless steel beaker, terephthalate prepared in Example 6 (3) was added.
- PBT Astragalus 103TW white RLVH001
- the mixture was hydrolyzed by heating at 160 ° C. for 120 minutes while stirring vigorously with a hot stirrer.
- the contents were dispensed after the temperature was allowed to cool and the internal temperature was below 40 ° C.
- the contents were separated by a filter paper and separated into a filtrate and a residue, and the weight of each residue and the total amount of terephthalic acid in the residue were measured. From the results shown in Table 10, it was found that the total amount of terephthalic acid in the residue after filtration was large in the order of potassium hydroxide, a mixture of potassium hydroxide and sodium hydroxide, and sodium hydroxide.
- Example 9.1 Difference between potassium hydroxide and sodium hydroxide in depolymerization of waste PET in 1-butanol
- the solubility of 1-butanol in dipotassium terephthalate and disodium terephthalate was the same as in Example 1. As a result, it was 0.2 mM and 1.9 mM, respectively. Thus, it was found that, unlike the dissolution in ethylene glycol, it is extremely difficult to dissolve in 1-butanol.
- Example 10 Difference between potassium hydroxide and sodium hydroxide in depolymerization of PTT in 0.1-butanol After dropping 13 g of PTT wadding (Nishikawa Sangyo Co., Ltd., Fine Smooth Solotex Wata Refill Pack RC7954) into a 200 mL stainless steel beaker and 130 g of 1-butanol, 0.13 mol of potassium hydroxide (granular), or Sodium hydroxide (granular) 0.13 mol was added. While stirring vigorously with a hot stirrer, heat treatment was performed at 100 ° C. for 90 minutes in the case of potassium hydroxide, and heat treatment at 120 ° C. for 120 minutes in the case of sodium hydroxide.
- PTT wadding Nehikawa Sangyo Co., Ltd., Fine Smooth Solotex Wata Refill Pack RC7954
- the contents were naturally cooled, and the contents were discharged after the internal temperature became 40 ° C. or lower.
- the contents were filtered with a filter paper (Whatman, grade 540) and separated into filtrate and residue, and the weight of each residue and the total amount of terephthalic acid in the residue were measured.
- Example 11.1 Difference between potassium hydroxide and sodium hydroxide in depolymerization of PBT in butanol After adding 15 g of the waste PBT lotus prepared in Example 7 and 75 g of 1-butanol to a 200 mL stainless steel beaker, 0.15 mol of potassium hydroxide (granular) or sodium hydroxide (granular) ) 0.15 mol was added, and after heat treatment at 100 ° C. for 240 minutes with vigorous stirring with a hot stirrer, the mixture was naturally cooled, and the contents were discharged after the internal temperature reached 40 ° C. or less.
- the contents were separated by filter paper and separated into filtrate and residue, and the weight of each residue and the total amount of terephthalic acid in the residue were measured.
- Example 12 Degradation of ethylene glycol when TPA-DHD is produced from terephthalate containing ethylene glycol TeTPA-DHD producing bacteria having ethylene glycol resolving ability are transformed into E. coli K-12 M7032 by pUXPEaLT_tpaA_tpaK_Pm_fucO_I7L_aldA constructed in Reference Example 3 E. coli M7032 (pUXPEaLT_tpaA_tpaK_Pm_fucO_I7L_aldA) strain was constructed by introducing by the method.
- Escherichia coli M7032 (pUXPEaLT_tpaA_tpaK_Pm_fucO_I7L_aldA) strain and E. coli K-12 M7032 strain as a control were cultured in a minijar fermenter. Simultaneously with administration of dipotassium terephthalate as the raw material, 0.278 ml (final concentration 72 mM) of ethylene glycol was added.
- TPA-DHD was identified based on the expected accurate mass value of TPA-DHD among substances appearing with a decrease in terephthalic acid. TPA-DHD was quantified based on a calibration curve prepared from the decrease in terephthalic acid and the increase in the TPA-DHD peak area.
- TPA-DHD As a result, an increase in TPA-DHD was observed with a decrease in terephthalic acid.
- the amount of TPA-DHD produced after 48 hours of culture was 10.5 g / L and 11.1 g / L after 48 hours of culturing of E. coli M7032 (pUXPEaLT_tpaA_tpaK_Pm_fucO_I7L_aldA) and E. coli K-12 M7032, respectively. It was. Along with this, an increase in TPA-DHD was observed.
- the amount of ethylene glycol in the culture solution after 48 hours of culture was measured under the conditions shown in Table 14 using a gas chromatography (GC) mass spectrometer (GC: Agilent 7890A, mass spectrometer: Agilent 5975C). analyzed.
- the ethylene glycol was identified by combining the measurement of the retention time of gas chromatography and the measurement using m / z62 from the mass spectrometer as the target ion, compared with the standard ethylene glycol.
- the E. coli M7032 ⁇ (pUXPEaLT_tpaA_tpaK_Pm_fucO_I7L_aldA) strain and the E. coli M7032 strain after 48 hours of culture were found to have an ethylene glycol concentration of 37.1 mM and 0.0 mM, respectively. It was found that ethylene glycol contained in terephthalate can be decomposed by enhancement.
- Example 13 Production of protocatechuic acid from terephthalate obtained by depolymerization of PET Using protocatechuic acid-producing Escherichia coli strain M7032 (pUXPEaLT_tphA2A3BA1_tpaK) constructed in Reference Example 2, this protocatechuic acid-producing bacterium was produced in the same manner as in Example 2. After preparing the preculture, 2% of the preculture was inoculated into the main culture medium, and main culture was performed.
- turbidity of the cells reached about 7 at an absorbance of 600 nm
- an inducer m-toluic acid was added to a final concentration of 1 mM, and terephthalic acid 1,2-dioxygenase protein, terephthalic acid 1,2 Dioxygenase reductase protein, terephthalate dihydrodiol dehydrogenase protein, and terephthalate transporter protein were expressed.
- the glucose concentration was measured over time, and when the glucose concentration reached 0, the PET-derived terephthalate prepared in Example 6 was dissolved in 0.6 M, mixed with glucose, and administered to the culture apparatus.
- Table 15 shows the ratio of protocatechuic acid production from each terephthalate salt after 52 hours of culture as 100%. From the results shown in Table 15, it was revealed that the productivity of protocatechuic acid was superior to that of the disodium terephthalic acid salt in both of the two types of terephthalic acid potassium salt.
- Example 14 Production of TPA-DHD from terephthalate obtained by depolymerization of PTT
- This TPA-DHD-producing recombinant Escherichia coli strain M7032 (pUXPEaLT_tphA2A3A1_tpaK) constructed in Reference Example 2 was used in the same manner as in Example 2 to produce this TPA.
- pUXPEaLT_tphA2A3A1_tpaK constructed in Reference Example 2 was used in the same manner as in Example 2 to produce this TPA.
- 2% of the preculture was inoculated into the main culture medium to perform main culture.
- turbidity of the cells reached about 7 at an absorbance of 600 nm
- an inducer m-toluic acid was added to a final concentration of 1 mM, and terephthalic acid 1,2-dioxygenase protein, terephthalic acid 1,2 -Dioxygenase reductase protein and terephthalate transporter protein were expressed.
- the glucose concentration was measured over time.
- the PTT-derived terephthalate prepared in Example 7 was dissolved in 0.6 M, mixed with glucose, and administered to the culture apparatus.
- TPA-DHD was identified based on the expected accurate mass value of TPA-DHD among substances appearing with a decrease in terephthalic acid. TPA-DHD was quantified based on a calibration curve prepared from the decrease in terephthalic acid and the increase in the TPA-DHD peak area.
- Table 16 shows the ratio of TPA-DHD production from each terephthalate salt after 48 hours of culture as 100%. From the results shown in Table 16, it was revealed that both of the two types of potassium terephthalate had better productivity of TPA-DHD than disodium terephthalate.
- Example 15 Production of protocatechuic acid from terephthalate obtained by depolymerization of PTT Using protocatechuic acid-producing Escherichia coli strain M7032 (pUXPEaLT_tphA2A3BA1_tpaK) constructed in Reference Example 2, this protocatechuic acid producing bacterium was produced in the same manner as in Example 2. After preparing the preculture, 2% of the preculture was inoculated into the main culture medium, and main culture was performed.
- turbidity of the cells reached about 7 at an absorbance of 600 nm
- an inducer m-toluic acid was added to a final concentration of 1 mM, and terephthalic acid 1,2-dioxygenase protein, terephthalic acid 1,2 Dioxygenase reductase protein, terephthalate dihydrodiol dehydrogenase protein, and terephthalate transporter protein were expressed.
- the glucose concentration was measured over time, and when the glucose concentration reached 0, the PBT-derived terephthalate prepared in Example 8 was dissolved in 0.6 M, mixed with glucose, and administered to the culture apparatus.
- Table 17 shows the ratio of protocatechuic acid production from each terephthalate after 48 hours of culture as 100%. From the results shown in Table 17, it was revealed that the productivity of protocatechuic acid was superior to that of the two types of potassium terephthalate salts than the disodium salt of terephthalic acid.
- Example 16 Production of TPA-DHD from terephthalate obtained by depolymerization of PET in 1-butanol solvent Using TPA-DHD-producing recombinant Escherichia coli strain M7032 (pUXPEaLT_tphA2A3A1_tpaK) constructed in Reference Example 2, In the same manner as in Example 2, after preparing a preculture solution of the present TPA-DHD-producing bacterium, 2% of the preculture solution was inoculated into the main culture medium, and main culture was performed.
- TPA-DHD was identified based on the expected accurate mass value of TPA-DHD among substances appearing with a decrease in terephthalic acid. TPA-DHD was quantified based on a calibration curve prepared from the decrease in terephthalic acid and the increase in the TPA-DHD peak area.
- Table 18 shows the ratio of TPA-DHD production from each terephthalate salt after 35 hours of culture as 100%. From the results shown in Table 18, it was clarified that both of the two types of potassium terephthalate had better productivity of TPA-DHD than disodium terephthalate.
- Reference Example 1 Construction of the expression vector pUXPEaLT19
- transcription of the target gene depends on the pseudogene, and the target gene is also maintained while maintaining the translation efficiency of the pseudogene.
- An expression system to be translated was constructed. More specifically, in order to insert a terminator sequence into pUC19 (manufactured by Takara Bio Inc.), six synthetic DNAs represented by SEQ ID NOs: 13 to 18 were synthesized.
- sequence number between the BglII site and the PacI site of pUTPELT19 was determined by PCR using PrimeSTAR DNA polymerase (manufactured by Takara Bio; unless otherwise specified, this enzyme was used as the DNA amplification enzyme for the PCR reaction).
- PrimeSTAR DNA polymerase manufactured by Takara Bio; unless otherwise specified, this enzyme was used as the DNA amplification enzyme for the PCR reaction.
- An expression plasmid pUTPEaLT19 substituted with the sequence represented by 25 was constructed.
- the XylS-Pm promoter region was obtained by amplification by a PCR reaction using two types of DNA primers (SEQ ID NOs: 26 and 27) using the plasmid pJB866 obtained from the National Institute of Genetics as a template.
- the amplified DNA fragment was purified by gel electrophoresis and incorporated into a pT7Blue-T vector (Novagen), to obtain XylS-Pm A plasmid pT7-xylS_Pm that retains the entire promoter region was constructed.
- the expression vector pUXPEaLT19 was constructed by excising the XylS-Pm promoter region from the plasmid pT7-xylS_Pm with the restriction enzyme SbfI and the restriction enzyme BamHI and inserting it between the SbfI and BglII sites of pUTPEaLT19.
- the collected soil sample was added to 5 ⁇ ml of W liquid medium containing 10 ⁇ m terephthalic acid in a 15 ⁇ m falcon tube, and then cultured at 30 ° C. with shaking.
- the composition of the W liquid medium was as follows: potassium dihydrogen phosphate 0.85 g / L, disodium hydrogen phosphate 4.9 g / L, ammonium sulfate 0.5 g / L, magnesium sulfate heptahydrate 9.5 mg / L, iron sulfate (II ) Heptahydrate 9.5 mg / L, Magnesium oxide 10.75 mg / L, Calcium carbonate 2 mg / L, Zinc sulfate heptahydrate 4.5 mg / ml, Manganese (II) sulfate tetrahydrate 1.12mg / L, Sulfuric acid Copper (II) pentahydrate 0.25 mg / L, cobalt sulfate (II) hept
- the culture broth was applied to an agar plate of W liquid medium containing 10 mM mM terephthalic acid and cultured at 30 ° C. to select a strain that grows using terephthalic acid as the sole carbon source. Colonies on an agar plate were cultured for 3 days in 5 ml of W liquid medium containing 10 mM terephthalic acid. By determining the 16S DNA sequence of this strain, this strain was identified as Comamonas testosteroni and named 72W2 strain.
- the base sequence of the operon encoding the terephthalic acid metabolism gene group of Comamonas testosteroni strain YZW-D is GB accession from the National Center for Biotechnology Information (hereinafter abbreviated as NCBI) GB database. Obtained as number AY923836.
- NCBI National Center for Biotechnology Information
- the base sequences of two operons encoding terephthalic acid metabolism genes possessed by the Comonamonus genus E6 strain were obtained from the NCBI GB database.
- Comomanas testosteroni strain YZW-D used base numbers 8242 to 11908 in GB accession number AY923836 as the base sequence of the operon of the terephthalic acid metabolism gene group.
- base numbers 3260-6926 in GB accession number AB238678.1 and base numbers 4911-8777 in GB accession number AB238679.1 are terephthalate, respectively. It was used as the base sequence of the operon of the acid metabolism gene group.
- degenerate primers were designed to clone DNA encoding the terephthalic acid metabolism gene group of Comamonas testosteroni 72W2.
- a PacI recognition site was added to the 5 'primer (SEQ ID NO: 28), and an SgfI recognition site and a NotI recognition site were added to the 3' primer (SEQ ID NO: 29).
- tphA2 gene-tphA3 gene-tphB gene-tphA1 gene tphA2 gene-tphA3 gene-tphB gene-tphA1 gene
- tphA2A3BA1 gene the operon of the terephthalic acid metabolism gene group (tphA2 gene-tphA3 gene-tphB gene-tphA1 gene; hereinafter abbreviated as tphA2A3BA1 gene) using the genomic DNA of Comamonas testosteroni 72W2 as a template
- the region was amplified by a PCR reaction.
- the amplified DNA fragment was recovered using a QIAquick PCR purification kit (manufactured by Qiagen; hereinafter, this kit was used unless otherwise specified).
- tphA2 gene (SEQ ID NO: 1) encoding terephthalic acid 1,2-dioxygenase / oxygenase / large subunit / protein, terephthalic acid 1,2-di- TphA3 gene (SEQ ID NO: 3) encoding oxygenase, oxygenase, small subunit, protein, tphB gene (SEQ ID NO: 9) encoding TPA-DHD dehydrogenase protein, terephthalate 1,2-dioxygenase, reductase protein It was confirmed that it encodes the encoding tphA1 gene (SEQ ID NO: 5).
- tphA2A3BA1 An operon of the tphA2A3BA1 gene (SEQ ID NO: 30) was excised from the PCR product with the restriction enzymes PacI and NotI and incorporated between the PacI and NotI sites of the expression vector pUXPEaLT19 described in Reference Example 1 to convert protophthalic acid from terephthalic acid. Plasmid pUXPEaLT_tphA2A3BA1 expressing the enzyme group involved in the conversion to was constructed.
- the nucleotide sequence of the tpaK gene was obtained from the NCBI GB database as the sequence of nucleotide numbers 175046 to 176425 (SEQ ID NO: 7) in the accession number CP000432.
- the amplified DNA fragment was recovered using a PCR purification kit.
- the tpaK gene was excised from the PCR product with the restriction enzymes PacI and NotI, and SgfI of the plasmid pUXPEaLT_tphA2A3BA1 described above was used.
- the protocatechuic acid production plasmid pUXPEaLT_tphA2A3BA1_tpaK was constructed by incorporating it between the site and the NotI site.
- Reference Example 3 Construction of TPA-DHD production plasmid Using the pUXPEaLT_tphA2A3BA1_tpaK (100 ng) constructed in Reference Example 2 (4) as a template, PCR was performed using the two types of DNA primers (SEQ ID NO: 28 and SEQ ID NO: 33). Amplified by reaction. In addition, the tphA1 gene region was amplified by PCR reaction using two types of DNA primers (SEQ ID NO: 29 and SEQ ID NO: 34). These amplified DNA fragments were recovered using a PCR purification kit.
- pUXPEaLT_tphA2A3A1_tpaK was constructed by incorporating it between the PacI and HindIII sites of the expression vector pUXPEaLT_tphA2A3BA1 constructed in Reference Example 2 (3).
- Reference Example 4 Construction of gallic acid production plasmid
- a plasmid for expressing the parahydroxybenzoic acid hydroxylase gene together with the protocatechuic acid production gene group was constructed as follows. Specifically, two types of DNAs (SEQ ID NO: 11) encoding parahydroxybenzoate hydroxylase (SEQ ID NO: 12) disclosed in a published patent publication (publication number: JP-A-2009-213392) are used as templates. DNA encoding the parahydroxybenzoate hydroxylase protein was amplified by PCR reaction using DNA primers (SEQ ID NO: 35 and SEQ ID NO: 36). The amplified DNA fragment was recovered using a PCR purification kit.
- the gallic acid production plasmid pUXPEaLT_HFM145_tphA2A3BA1_tpaK was constructed by incorporating the DNA into the PacI site of pUXPEaLT_tphA2A3BA1_tpaK constructed above.
- coli K-12 MG1655 strain obtained from NBRP as a template was amplified by PCR reaction. This amplified DNA fragment was excised from the PCR product with the restriction enzyme PacI site and the restriction enzyme NotI and incorporated into the expression vector pUXPEaLT19 constructed in Reference Example 1 to construct the plasmid pUXPEaLT_fucO_I7L.
- Plasmid pUXPEaLTEk_fucO_I7L_aldA was constructed by cutting out from the synthetic DNA having the sequence shown in SEQ ID NO: 41 with restriction enzyme NotI and restriction enzyme AscI and incorporating it between NotI and AscI sites of plasmid pUEPEaLT_fucO_I7L_aldA. Subsequently, an expression unit containing the fucO gene and the aldA gene (hereinafter abbreviated as fucO-aldA expression unit) was amplified from the plasmid pUXPEaLTEX_fucO_I7L_aldA by PCR using two types of DNA primers (SEQ ID NO: 42 and SEQ ID NO: 43).
- the plasmid pUXPEaLT_tpaA_tpaK_PmfucO_I7L_aldA was constructed by cutting out from the PCR product using the restriction enzyme AscI site and incorporating it into the expression vector pUXPEaLT_tphA2A3A1_tpaK constructed in the above (Reference Example 3).
- a method for producing terephthalic acid-1,2-cis-dihydrodiol using a terephthalic acid potassium salt as a raw material and a microorganism expressing terephthalic acid 1,2-dioxygenase, and further TPA-DHD can be provided in a method for converting chlorophthalic acid to phenolic acids such as protocatechuic acid and gallic acid, and a method for obtaining potassium terephthalate as a raw material by depolymerization of waste polyester.
- SEQ ID NO: 1 DNA encoding terephthalic acid 1,2-dioxygenase / oxygenase large subunit protein
- SEQ ID NO: 2 Terephthalic acid 1,2-dioxygenase / oxygenase large subunit / protein
- SEQ ID NO: 3 DNA encoding terephthalic acid 1,2-dioxygenase / oxygenase small subunit / protein
- SEQ ID NO: 4 Terephthalic acid 1,2-dioxygenase / oxygenase small subunit / protein
- SEQ ID NO: 6 Terephthalate 1,2-dioxygenase / reductase / protein
- SEQ ID NO: 7 DNA encoding terephthalate transporter / protein
- SEQ ID NO: 8 Terephthalic acid transporter protein
- coli lactaldehyde, reductase No. 38 PCR primer for cloning of E. coli lactaldehyde / reductase SEQ ID NO: 39: PCR primer for cloning of E. coli lactaldehyde / dehydrogenase SEQ ID NO: 40: PCR primer for cloning of Escherichia coli lactaldehyde dehydrogenase SEQ ID NO: 41: Sequence between NotI site and AscI site of plasmid pUXPEaLTEk_fucO_I7L_aldA SEQ ID NO: 42: PCR primer for cloning of expression unit containing fucO gene and aldA gene 43: PCR primers for expression unit cloning containing fucO gene and aldA gene
Landscapes
- Organic Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Zoology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Wood Science & Technology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Microbiology (AREA)
- General Chemical & Material Sciences (AREA)
- Biotechnology (AREA)
- Health & Medical Sciences (AREA)
- Biochemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Genetics & Genomics (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Separation, Recovery Or Treatment Of Waste Materials Containing Plastics (AREA)
Priority Applications (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201280034139.7A CN103703137B (zh) | 2012-01-27 | 2012-01-27 | 由对苯二甲酸钾盐制造有用化学品的方法 |
| IN978MUN2014 IN2014MN00978A (enEXAMPLES) | 2012-01-27 | 2012-01-27 | |
| KR1020147001278A KR101645105B1 (ko) | 2012-01-27 | 2012-01-27 | 테레프탈산칼륨염으로부터의 유용화학품의 제조법 |
| JP2013555086A JP6042827B2 (ja) | 2012-01-27 | 2012-01-27 | テレフタル酸カリウム塩からの有用化学品の製造法 |
| PCT/JP2012/051854 WO2013111332A1 (ja) | 2012-01-27 | 2012-01-27 | テレフタル酸カリウム塩からの有用化学品の製造法 |
| EP12866812.6A EP2722393B1 (en) | 2012-01-27 | 2012-01-27 | Method for producing useful chemical substance from terephthalic acid potassium salt |
| US14/143,912 US9394549B2 (en) | 2012-01-27 | 2013-12-30 | Method for producing useful chemical substance from terephthalic acid potassium salt |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PCT/JP2012/051854 WO2013111332A1 (ja) | 2012-01-27 | 2012-01-27 | テレフタル酸カリウム塩からの有用化学品の製造法 |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US14/143,912 Continuation-In-Part US9394549B2 (en) | 2012-01-27 | 2013-12-30 | Method for producing useful chemical substance from terephthalic acid potassium salt |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2013111332A1 true WO2013111332A1 (ja) | 2013-08-01 |
Family
ID=48873093
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2012/051854 WO2013111332A1 (ja) | 2012-01-27 | 2012-01-27 | テレフタル酸カリウム塩からの有用化学品の製造法 |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US9394549B2 (enEXAMPLES) |
| EP (1) | EP2722393B1 (enEXAMPLES) |
| JP (1) | JP6042827B2 (enEXAMPLES) |
| KR (1) | KR101645105B1 (enEXAMPLES) |
| CN (1) | CN103703137B (enEXAMPLES) |
| IN (1) | IN2014MN00978A (enEXAMPLES) |
| WO (1) | WO2013111332A1 (enEXAMPLES) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016003291A (ja) * | 2014-06-18 | 2016-01-12 | 住友ベークライト株式会社 | ポリエステル樹脂用単量体およびポリエステル樹脂 |
| WO2021002396A1 (ja) * | 2019-07-02 | 2021-01-07 | 花王株式会社 | 没食子酸含有組成物の製造方法 |
| WO2021182639A1 (ja) * | 2020-03-13 | 2021-09-16 | 国立研究開発法人森林研究・整備機構 | 2-ピロン-4,6-ジカルボン酸の製造方法 |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR3028529B1 (fr) | 2014-11-19 | 2016-12-30 | Inst Nat De La Rech Agronomique Inra | Procede de production d'au moins un metabolite d'interet par transformation d'un pentose dans un microorganisme |
| EP3909947A4 (en) * | 2019-02-12 | 2023-03-15 | Korea Research Institute of Chemical Technology | Method of producing high-purity 2-pyrone-4,6-dicarboxylic acid and method of producing intermediate for same |
| KR102596398B1 (ko) * | 2019-04-08 | 2023-11-02 | 고려대학교 산학협력단 | 폴리에틸렌 테레프탈레이트로부터 고부가가치 화합물 생산방법 |
| CN110607313B (zh) * | 2019-09-27 | 2021-06-22 | 内蒙古伊品生物科技有限公司 | 一种高产l-赖氨酸的重组菌株及其构建方法与应用 |
| CN115724713B (zh) * | 2022-05-11 | 2024-04-12 | 沈阳工业大学 | 一种制取对苯二甲酸二钾的方法 |
Citations (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3544622A (en) | 1965-03-10 | 1970-12-01 | Du Pont | Alkaline saponification of polyethylene terephthalate at high temperatures using controlled amount of sodium hydroxide |
| US4542239A (en) | 1981-11-18 | 1985-09-17 | Board Of Control Of Michigan Technological University | Process for recovering terephthalic acid from waste polyethylene terephthalate |
| US5068414A (en) | 1990-01-26 | 1991-11-26 | Celgene Corporation | Hydroxyterephthalic acid |
| US5124479A (en) | 1990-01-26 | 1992-06-23 | Celgene Corporation | Process for the preparation of hydroxyterephthalic acid |
| JPH1121374A (ja) | 1997-07-07 | 1999-01-26 | Unitika Ltd | Pet樹脂廃棄物の化学的処理方法 |
| JP2000169623A (ja) | 1998-12-10 | 2000-06-20 | Is:Kk | ポリエチレンテレフタレ―ト廃棄物のケミカルリサイクル方法 |
| JP2002060542A (ja) | 2000-08-17 | 2002-02-26 | Teijin Ltd | ポリエステル廃棄物からの有効成分回収方法 |
| WO2005082826A1 (es) | 2004-01-27 | 2005-09-09 | Fregoso-Infante Arturo Guadalu | Procedimiento quimico de reciclado de polietilentereftalato (pet) de desecho |
| JP2007104942A (ja) | 2005-10-12 | 2007-04-26 | Tokyo Univ Of Agriculture & Technology | テレフタル酸の代謝に関与する新規遺伝子 |
| JP2008278823A (ja) * | 2007-05-11 | 2008-11-20 | Toyota Industries Corp | 遺伝子破壊株、組換えプラスミド、形質転換体、及び3−カルボキシムコノラクトンの製造方法 |
| JP2009065839A (ja) | 2007-09-10 | 2009-04-02 | Genaris Inc | 没食子酸の製造法 |
| JP2009213392A (ja) | 2008-03-10 | 2009-09-24 | Genaris Inc | 改良型没食子酸合成酵素および没食子酸の製造法 |
| JP2010207094A (ja) * | 2009-03-06 | 2010-09-24 | Genaris Inc | プロトカテク酸の製造法 |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3629328A (en) * | 1969-05-20 | 1971-12-21 | Celanese Corp | Purification of organic acids |
-
2012
- 2012-01-27 JP JP2013555086A patent/JP6042827B2/ja active Active
- 2012-01-27 WO PCT/JP2012/051854 patent/WO2013111332A1/ja active Application Filing
- 2012-01-27 CN CN201280034139.7A patent/CN103703137B/zh not_active Expired - Fee Related
- 2012-01-27 KR KR1020147001278A patent/KR101645105B1/ko not_active Expired - Fee Related
- 2012-01-27 IN IN978MUN2014 patent/IN2014MN00978A/en unknown
- 2012-01-27 EP EP12866812.6A patent/EP2722393B1/en not_active Not-in-force
-
2013
- 2013-12-30 US US14/143,912 patent/US9394549B2/en not_active Expired - Fee Related
Patent Citations (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3544622A (en) | 1965-03-10 | 1970-12-01 | Du Pont | Alkaline saponification of polyethylene terephthalate at high temperatures using controlled amount of sodium hydroxide |
| US4542239A (en) | 1981-11-18 | 1985-09-17 | Board Of Control Of Michigan Technological University | Process for recovering terephthalic acid from waste polyethylene terephthalate |
| US5068414A (en) | 1990-01-26 | 1991-11-26 | Celgene Corporation | Hydroxyterephthalic acid |
| US5124479A (en) | 1990-01-26 | 1992-06-23 | Celgene Corporation | Process for the preparation of hydroxyterephthalic acid |
| JPH1121374A (ja) | 1997-07-07 | 1999-01-26 | Unitika Ltd | Pet樹脂廃棄物の化学的処理方法 |
| JP3715812B2 (ja) | 1998-12-10 | 2005-11-16 | 株式会社アイエス | ポリエチレンテレフタレート廃棄物のケミカルリサイクル方法 |
| JP2000169623A (ja) | 1998-12-10 | 2000-06-20 | Is:Kk | ポリエチレンテレフタレ―ト廃棄物のケミカルリサイクル方法 |
| JP2002060542A (ja) | 2000-08-17 | 2002-02-26 | Teijin Ltd | ポリエステル廃棄物からの有効成分回収方法 |
| WO2005082826A1 (es) | 2004-01-27 | 2005-09-09 | Fregoso-Infante Arturo Guadalu | Procedimiento quimico de reciclado de polietilentereftalato (pet) de desecho |
| JP2007104942A (ja) | 2005-10-12 | 2007-04-26 | Tokyo Univ Of Agriculture & Technology | テレフタル酸の代謝に関与する新規遺伝子 |
| JP2008278823A (ja) * | 2007-05-11 | 2008-11-20 | Toyota Industries Corp | 遺伝子破壊株、組換えプラスミド、形質転換体、及び3−カルボキシムコノラクトンの製造方法 |
| JP2009065839A (ja) | 2007-09-10 | 2009-04-02 | Genaris Inc | 没食子酸の製造法 |
| JP2009213392A (ja) | 2008-03-10 | 2009-09-24 | Genaris Inc | 改良型没食子酸合成酵素および没食子酸の製造法 |
| JP2010207094A (ja) * | 2009-03-06 | 2010-09-24 | Genaris Inc | プロトカテク酸の製造法 |
Non-Patent Citations (30)
| Title |
|---|
| "Current Protocols in Molecular Biology", 1987, JOHN WILEY & SONS |
| "Molecular Cloning, A Laboratory Manual", 1989, COLD SPRING HARBOR LABORATORY PRESS |
| "Molecular Cloning: A laboratory manual", 1989, COLD SPRING HARBOR LABORATORY PRESS |
| BORONAT A. ET AL.: "Experimental evolution of a metabolic pathway for ethylene glycol utilization by Escherichia coli", J. BACTERIOL., vol. 153, 1983, pages 134 - 139, XP009058663 * |
| GENE, vol. 33, 1985, pages 103 |
| GENE, vol. 34, 1985, pages 315 |
| GENE, vol. 38, 1985, pages 275 |
| GENETICS, vol. 39, 1954, pages 440 |
| GOJE A. S. ET AL.: "Chemical Recycling, Kinetics And Thermodynamics of Hydrolysis of Poly (Ethylene Terephthalate) Waste With Nonaqueous Potassium Hydroxide Solution", POLYMER-PLASTICS TECHNOLOGY AND ENGINEERING, vol. 43, no. 2, 2004, pages 369 - 388, XP008172693 * |
| J. BACTERIOL., vol. 153, 1983, pages 134 |
| J. BACTERIOL., vol. 171, 1989, pages 6097 |
| J. BIOL. CHEM., vol. 273, 1998, pages 8308 |
| J. G. C. OTTOW, ANN. REV.MICROBIOL., vol. 29, 1975, pages 80 |
| J. MOL. BIOL., vol. 16, 1966, pages 118 |
| J. MOL. BIOL., vol. 166, 1983, pages 1 |
| M.H. GABOR, J. BACTERIOL., vol. 137, 1979, pages 1346 |
| MCLEOD M. P. ET AL.: "The complete genome of Rhodococcus sp. RHA1 provides insights into a catabolic powerhouse", PROC. NATL. ACAD. SCI. USA., vol. 103, 2006, pages 15582 - 15587, XP002601293 * |
| NUCLEIC ACIDS RES., vol. 10, 1982, pages 6487 |
| NUCLEIC ACIDS RES., vol. 13, 1985, pages 4431 |
| NUCLEIC ACIDS RES., vol. 16, 1988, pages 6127 |
| NUCLEIC ACIDS RES., vol. 17, 1989, pages 9494 |
| POLYM.-PLASTICS TECH. ENG., vol. 43, 2004, pages 369 |
| PROC. NATL. ACAD. SCI. USA, vol. 74, 1977, pages 5463 |
| PROC. NATL. ACAD. SCI., USA, vol. 69, 1972, pages 2110 |
| PROC. NATL. ACAD. SCI., USA, vol. 79, 1982, pages 6409 |
| PROC. NATL. ACAD. SCI., USA, vol. 82, 1985, pages 488 |
| SCIENCE, vol. 222, 1983, pages 778 |
| SHUKLA S. R. ET AL.: "Glycolysis of polyethylene terephthalate waste fibers", JOURNAL OF APPLIED POLYMER SCIENCE, vol. 97, 2005, pages 513 - 517, XP055081732 * |
| STRATEGIES, vol. 5, 1992, pages 81 |
| TATSUYA NISHI: "Development of phenolic- compounds producing technologies using microorganisms", BIO INDUSTRY, vol. 28, 2011, pages 44 - 49, XP008172694 * |
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016003291A (ja) * | 2014-06-18 | 2016-01-12 | 住友ベークライト株式会社 | ポリエステル樹脂用単量体およびポリエステル樹脂 |
| WO2021002396A1 (ja) * | 2019-07-02 | 2021-01-07 | 花王株式会社 | 没食子酸含有組成物の製造方法 |
| US12103911B2 (en) | 2019-07-02 | 2024-10-01 | Kao Corporation | Method for producing composition containing gallic acid |
| JP7579071B2 (ja) | 2019-07-02 | 2024-11-07 | 花王株式会社 | 没食子酸含有組成物の製造方法 |
| WO2021182639A1 (ja) * | 2020-03-13 | 2021-09-16 | 国立研究開発法人森林研究・整備機構 | 2-ピロン-4,6-ジカルボン酸の製造方法 |
| JP2021141874A (ja) * | 2020-03-13 | 2021-09-24 | 国立研究開発法人森林研究・整備機構 | 2−ピロン−4,6−ジカルボン酸の製造方法 |
| JP7392928B2 (ja) | 2020-03-13 | 2023-12-06 | 国立研究開発法人森林研究・整備機構 | 2-ピロン-4,6-ジカルボン酸の製造方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| EP2722393A1 (en) | 2014-04-23 |
| US20140120593A1 (en) | 2014-05-01 |
| EP2722393B1 (en) | 2019-01-09 |
| KR20140033495A (ko) | 2014-03-18 |
| JP6042827B2 (ja) | 2016-12-14 |
| KR101645105B1 (ko) | 2016-08-02 |
| IN2014MN00978A (enEXAMPLES) | 2015-04-24 |
| EP2722393A4 (en) | 2015-03-04 |
| JPWO2013111332A1 (ja) | 2015-05-11 |
| CN103703137A (zh) | 2014-04-02 |
| US9394549B2 (en) | 2016-07-19 |
| CN103703137B (zh) | 2016-11-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6042827B2 (ja) | テレフタル酸カリウム塩からの有用化学品の製造法 | |
| JP4886775B2 (ja) | 補酵素再生によるグリコール酸の生産方法 | |
| JP7380768B2 (ja) | アルデヒドの製造方法 | |
| JP4954985B2 (ja) | 補酵素合成強化によるグリコール酸の生産方法 | |
| JP2022530467A (ja) | 再生可能資源からの化学物質の生成 | |
| JP7194950B2 (ja) | ヒドロキシチロソールの製造 | |
| JP2008029329A (ja) | 酵母及びl−乳酸の製造方法 | |
| CN108949652A (zh) | 一种工程菌及其生产咖啡酸的应用 | |
| WO2015118051A1 (en) | Modified microorganism for improved production of fine chemicals on sucrose | |
| CN106884001B (zh) | 一种重组嗜碱芽孢杆菌及其制备方法和应用以及制备d-乳酸的方法 | |
| JP5651989B2 (ja) | コハク酸の製造方法 | |
| JP6097907B2 (ja) | イソフタル酸輸送タンパク質を保有する微生物及びその利用 | |
| JP6527708B2 (ja) | 新規微生物、ならびにそれを用いる2,3−ジヒドロキシナフタレンの製造法 | |
| JP5678356B2 (ja) | フェノール酸の製造法 | |
| US11667923B2 (en) | Recombinant Candida cell and preparation process and use thereof | |
| KR102797614B1 (ko) | 에탄올 및 글리세롤을 폴리하이드록시알카노에이트로 전환하기 위한 재조합 슈도모나스 푸티다 바이오촉매의 개발 | |
| CN114276970B (zh) | 一种产1,3-丙二醇的基因工程菌 | |
| KR101326583B1 (ko) | 고광학순도의 젖산 생산용 형질전환체 및 이를 이용한 젖산 생산 방법 | |
| EP2679668B1 (en) | Transformant for producing lactic acid having high optical purity, and method for producing lactic acid using same | |
| JP5866604B2 (ja) | 新規微生物、ならびにそれを用いる2,3−ジヒドロキシ安息香酸およびサリチル酸の製造法 | |
| JP2017121203A (ja) | ジヒドロキシナフタレンの製造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 12866812 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2013555086 Country of ref document: JP Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 20147001278 Country of ref document: KR Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2012866812 Country of ref document: EP |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |