RU2239455C2 - Фармацевтические композиции, содержащие агонист или антагонист аденозинового рецептора - Google Patents
Фармацевтические композиции, содержащие агонист или антагонист аденозинового рецептора Download PDFInfo
- Publication number
- RU2239455C2 RU2239455C2 RU2002109228/15A RU2002109228A RU2239455C2 RU 2239455 C2 RU2239455 C2 RU 2239455C2 RU 2002109228/15 A RU2002109228/15 A RU 2002109228/15A RU 2002109228 A RU2002109228 A RU 2002109228A RU 2239455 C2 RU2239455 C2 RU 2239455C2
- Authority
- RU
- Russia
- Prior art keywords
- group
- adenosine
- active ingredient
- iodobenzyl
- alkyl
- Prior art date
Links
- 239000008194 pharmaceutical composition Substances 0.000 title claims abstract description 28
- 239000000556 agonist Substances 0.000 title abstract description 14
- 108050000203 Adenosine receptors Proteins 0.000 title abstract description 10
- 102000009346 Adenosine receptors Human genes 0.000 title abstract description 10
- 239000005557 antagonist Substances 0.000 title description 9
- 230000000694 effects Effects 0.000 claims abstract description 65
- 230000035755 proliferation Effects 0.000 claims abstract description 54
- 108010017080 Granulocyte Colony-Stimulating Factor Proteins 0.000 claims abstract description 44
- 102000004269 Granulocyte Colony-Stimulating Factor Human genes 0.000 claims abstract description 44
- 239000003814 drug Substances 0.000 claims abstract description 37
- 229940079593 drug Drugs 0.000 claims abstract description 34
- 201000002364 leukopenia Diseases 0.000 claims abstract description 32
- 231100001022 leukopenia Toxicity 0.000 claims abstract description 32
- 238000011282 treatment Methods 0.000 claims abstract description 31
- 239000000203 mixture Substances 0.000 claims abstract description 20
- 231100000331 toxic Toxicity 0.000 claims abstract description 18
- 230000002588 toxic effect Effects 0.000 claims abstract description 18
- 230000028327 secretion Effects 0.000 claims abstract description 13
- 230000012010 growth Effects 0.000 claims abstract description 8
- OIRDTQYFTABQOQ-KQYNXXCUSA-N adenosine Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O OIRDTQYFTABQOQ-KQYNXXCUSA-N 0.000 claims description 102
- 239000004480 active ingredient Substances 0.000 claims description 93
- 210000004027 cell Anatomy 0.000 claims description 61
- IPSYPUKKXMNCNQ-PFHKOEEOSA-N (2s,3s,4r,5r)-5-[2-chloro-6-[(3-iodophenyl)methylamino]purin-9-yl]-3,4-dihydroxy-n-methyloxolane-2-carboxamide Chemical compound O[C@@H]1[C@H](O)[C@@H](C(=O)NC)O[C@H]1N1C2=NC(Cl)=NC(NCC=3C=C(I)C=CC=3)=C2N=C1 IPSYPUKKXMNCNQ-PFHKOEEOSA-N 0.000 claims description 52
- 229960005305 adenosine Drugs 0.000 claims description 51
- 239000002126 C01EB10 - Adenosine Substances 0.000 claims description 49
- HUJXGQILHAUCCV-MOROJQBDSA-N 3-iodobenzyl-5'-N-methylcarboxamidoadenosine Chemical compound O[C@@H]1[C@H](O)[C@@H](C(=O)NC)O[C@H]1N1C2=NC=NC(NCC=3C=C(I)C=CC=3)=C2N=C1 HUJXGQILHAUCCV-MOROJQBDSA-N 0.000 claims description 47
- -1 nitro, hydroxyl Chemical group 0.000 claims description 42
- 210000002798 bone marrow cell Anatomy 0.000 claims description 41
- 210000000265 leukocyte Anatomy 0.000 claims description 39
- 229910052739 hydrogen Inorganic materials 0.000 claims description 33
- 239000001257 hydrogen Substances 0.000 claims description 33
- 125000000217 alkyl group Chemical group 0.000 claims description 31
- 206010028980 Neoplasm Diseases 0.000 claims description 30
- 125000000008 (C1-C10) alkyl group Chemical group 0.000 claims description 29
- 239000002246 antineoplastic agent Substances 0.000 claims description 28
- 230000037396 body weight Effects 0.000 claims description 26
- 229940044683 chemotherapy drug Drugs 0.000 claims description 23
- 229910052736 halogen Inorganic materials 0.000 claims description 23
- 150000001875 compounds Chemical class 0.000 claims description 22
- 150000002367 halogens Chemical class 0.000 claims description 22
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 claims description 22
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 claims description 20
- 238000004519 manufacturing process Methods 0.000 claims description 20
- 150000002431 hydrogen Chemical class 0.000 claims description 18
- 230000002401 inhibitory effect Effects 0.000 claims description 18
- 102000005962 receptors Human genes 0.000 claims description 17
- 108020003175 receptors Proteins 0.000 claims description 17
- 210000004881 tumor cell Anatomy 0.000 claims description 16
- 230000001939 inductive effect Effects 0.000 claims description 15
- 125000006376 (C3-C10) cycloalkyl group Chemical group 0.000 claims description 14
- 230000002265 prevention Effects 0.000 claims description 14
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims description 13
- 230000010261 cell growth Effects 0.000 claims description 13
- 125000000027 (C1-C10) alkoxy group Chemical group 0.000 claims description 11
- 230000002159 abnormal effect Effects 0.000 claims description 11
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims description 11
- 150000003839 salts Chemical class 0.000 claims description 11
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims description 10
- 125000003545 alkoxy group Chemical group 0.000 claims description 9
- 201000011510 cancer Diseases 0.000 claims description 9
- 150000003833 nucleoside derivatives Chemical group 0.000 claims description 9
- 229910052760 oxygen Inorganic materials 0.000 claims description 9
- 125000001424 substituent group Chemical group 0.000 claims description 9
- 125000001188 haloalkyl group Chemical group 0.000 claims description 8
- 230000004069 differentiation Effects 0.000 claims description 7
- 229910052717 sulfur Inorganic materials 0.000 claims description 7
- 239000002593 adenosine A3 receptor agonist Substances 0.000 claims description 6
- 150000003931 anilides Chemical group 0.000 claims description 6
- MWEQTWJABOLLOS-UHFFFAOYSA-L disodium;[[[5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-oxidophosphoryl] hydrogen phosphate;trihydrate Chemical compound O.O.O.[Na+].[Na+].C1=NC=2C(N)=NC=NC=2N1C1OC(COP(O)(=O)OP([O-])(=O)OP(O)([O-])=O)C(O)C1O MWEQTWJABOLLOS-UHFFFAOYSA-L 0.000 claims description 6
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical class CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 claims description 5
- 125000000738 acetamido group Chemical group [H]C([H])([H])C(=O)N([H])[*] 0.000 claims description 5
- 230000009471 action Effects 0.000 claims description 5
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims description 5
- 125000004432 carbon atom Chemical group C* 0.000 claims description 5
- 239000001301 oxygen Substances 0.000 claims description 5
- 125000000951 phenoxy group Chemical group [H]C1=C([H])C([H])=C(O*)C([H])=C1[H] 0.000 claims description 5
- 229940122216 Adenosine A3 receptor agonist Drugs 0.000 claims description 4
- LSNNMFCWUKXFEE-UHFFFAOYSA-M Bisulfite Chemical compound OS([O-])=O LSNNMFCWUKXFEE-UHFFFAOYSA-M 0.000 claims description 4
- 125000006374 C2-C10 alkenyl group Chemical group 0.000 claims description 4
- 239000013543 active substance Substances 0.000 claims description 4
- 125000000623 heterocyclic group Chemical group 0.000 claims description 4
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 claims description 4
- 125000000547 substituted alkyl group Chemical group 0.000 claims description 4
- 125000003107 substituted aryl group Chemical group 0.000 claims description 4
- 125000000446 sulfanediyl group Chemical group *S* 0.000 claims description 4
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 claims description 3
- 125000004414 alkyl thio group Chemical group 0.000 claims description 3
- 125000000304 alkynyl group Chemical group 0.000 claims description 3
- 125000004103 aminoalkyl group Chemical group 0.000 claims description 3
- 125000003118 aryl group Chemical group 0.000 claims description 3
- 239000011593 sulfur Substances 0.000 claims description 3
- 125000000101 thioether group Chemical group 0.000 claims description 3
- XTPOZVLRZZIEBW-SCFUHWHPSA-N (2r,3r,4s,5r)-2-[6-[2-(4-aminophenyl)ethylamino]purin-9-yl]-5-(hydroxymethyl)oxolane-3,4-diol Chemical compound C1=CC(N)=CC=C1CCNC1=NC=NC2=C1N=CN2[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 XTPOZVLRZZIEBW-SCFUHWHPSA-N 0.000 claims description 2
- 125000006832 (C1-C10) alkylene group Chemical group 0.000 claims description 2
- 125000003853 4-amino-3-iodobenzyl group Chemical group [H]N([H])C1=C(I)C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 claims description 2
- 125000003282 alkyl amino group Chemical group 0.000 claims description 2
- 125000002877 alkyl aryl group Chemical group 0.000 claims description 2
- 125000003368 amide group Chemical group 0.000 claims description 2
- 230000000259 anti-tumor effect Effects 0.000 claims description 2
- 208000008784 apnea Diseases 0.000 claims description 2
- 125000002648 azanetriyl group Chemical group *N(*)* 0.000 claims description 2
- 125000000852 azido group Chemical group *N=[N+]=[N-] 0.000 claims description 2
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 claims description 2
- USIUVYZYUHIAEV-UHFFFAOYSA-N diphenyl ether Chemical compound C=1C=CC=CC=1OC1=CC=CC=C1 USIUVYZYUHIAEV-UHFFFAOYSA-N 0.000 claims description 2
- RTZKZFJDLAIYFH-UHFFFAOYSA-N ether Substances CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 claims description 2
- 125000000286 phenylethyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])([H])* 0.000 claims description 2
- 125000005030 pyridylthio group Chemical group N1=C(C=CC=C1)S* 0.000 claims description 2
- 125000005309 thioalkoxy group Chemical group 0.000 claims description 2
- FDEACFAXFCKCHZ-MOROJQBDSA-N (2s,3s,4r,5r)-5-(6-amino-2-hex-1-ynylpurin-9-yl)-n-ethyl-3,4-dihydroxyoxolane-2-carboxamide Chemical compound C12=NC(C#CCCCC)=NC(N)=C2N=CN1[C@@H]1O[C@H](C(=O)NCC)[C@@H](O)[C@H]1O FDEACFAXFCKCHZ-MOROJQBDSA-N 0.000 claims 15
- 238000009472 formulation Methods 0.000 claims 5
- 125000000020 sulfo group Chemical group O=S(=O)([*])O[H] 0.000 claims 4
- 229930024421 Adenine Natural products 0.000 claims 2
- GFFGJBXGBJISGV-UHFFFAOYSA-N Adenine Chemical compound NC1=NC=NC2=C1N=CN2 GFFGJBXGBJISGV-UHFFFAOYSA-N 0.000 claims 2
- 229960000643 adenine Drugs 0.000 claims 2
- WHYVKEGFJPLEJZ-UHFFFAOYSA-N n-[(3-iodophenyl)methyl]-7h-purin-6-amine Chemical compound IC1=CC=CC(CNC=2C=3NC=NC=3N=CN=2)=C1 WHYVKEGFJPLEJZ-UHFFFAOYSA-N 0.000 claims 2
- UMMMZHIKPLYIHG-LPWJYYESSA-N (1s,2r,3s,4r)-4-(6-amino-2-chloropurin-9-yl)cyclopentane-1,2,3-triol Chemical compound C1=NC=2C(N)=NC(Cl)=NC=2N1[C@@H]1C[C@H](O)[C@@H](O)[C@H]1O UMMMZHIKPLYIHG-LPWJYYESSA-N 0.000 claims 1
- OZCCPYHYSIPRDM-PAPYEOQZSA-N (1s,2r,3s,4r)-4-[6-amino-2-(2-phenylethylamino)purin-9-yl]cyclopentane-1,2,3-triol Chemical compound N=1C=2N([C@H]3[C@@H]([C@H](O)[C@@H](O)C3)O)C=NC=2C(N)=NC=1NCCC1=CC=CC=C1 OZCCPYHYSIPRDM-PAPYEOQZSA-N 0.000 claims 1
- 125000004209 (C1-C8) alkyl group Chemical group 0.000 claims 1
- 125000006681 (C2-C10) alkylene group Chemical group 0.000 claims 1
- NFHVNDANLVCJHE-UHFFFAOYSA-N 2-chloro-n-[(3-iodophenyl)methyl]-9-(oxolan-2-yl)purin-6-amine Chemical compound C=12N=CN(C3OCCC3)C2=NC(Cl)=NC=1NCC1=CC=CC(I)=C1 NFHVNDANLVCJHE-UHFFFAOYSA-N 0.000 claims 1
- ATOXAERIKXFYQV-UHFFFAOYSA-N 2-chloro-n-[(3-iodophenyl)methyl]-9-methylpurin-6-amine Chemical compound N1=C(Cl)N=C2N(C)C=NC2=C1NCC1=CC=CC(I)=C1 ATOXAERIKXFYQV-UHFFFAOYSA-N 0.000 claims 1
- OJUZWXZBQQALOH-UHFFFAOYSA-N 2-n-hexyl-6-n-[(3-iodophenyl)methyl]-9-methylpurine-2,6-diamine Chemical compound C=12N=CN(C)C2=NC(NCCCCCC)=NC=1NCC1=CC=CC(I)=C1 OJUZWXZBQQALOH-UHFFFAOYSA-N 0.000 claims 1
- APXSDGLADFOEDD-UHFFFAOYSA-N 4-[6-[(3-iodophenyl)methylamino]purin-9-yl]butanenitrile Chemical compound IC1=CC=CC(CNC=2C=3N=CN(CCCC#N)C=3N=CN=2)=C1 APXSDGLADFOEDD-UHFFFAOYSA-N 0.000 claims 1
- XBNTWQABLLKTFL-UHFFFAOYSA-N 6-n-[(3-iodophenyl)methyl]-2-n,2-n,9-trimethylpurine-2,6-diamine Chemical compound C=12N=CN(C)C2=NC(N(C)C)=NC=1NCC1=CC=CC(I)=C1 XBNTWQABLLKTFL-UHFFFAOYSA-N 0.000 claims 1
- IKLSILSIWVEUSQ-UHFFFAOYSA-N 6-n-[(3-iodophenyl)methyl]-2-n,9-dimethylpurine-2,6-diamine Chemical compound C=12N=CN(C)C2=NC(NC)=NC=1NCC1=CC=CC(I)=C1 IKLSILSIWVEUSQ-UHFFFAOYSA-N 0.000 claims 1
- AZPDSYNWGWXMJJ-UHFFFAOYSA-N 6-n-[(3-iodophenyl)methyl]-9-methyl-2-n-propylpurine-2,6-diamine Chemical compound C=12N=CN(C)C2=NC(NCCC)=NC=1NCC1=CC=CC(I)=C1 AZPDSYNWGWXMJJ-UHFFFAOYSA-N 0.000 claims 1
- CRCODYWJYJOVSH-UHFFFAOYSA-N 6-n-[(3-iodophenyl)methyl]-9-methylpurine-2,6-diamine Chemical compound N1=C(N)N=C2N(C)C=NC2=C1NCC1=CC=CC(I)=C1 CRCODYWJYJOVSH-UHFFFAOYSA-N 0.000 claims 1
- 125000006294 amino alkylene group Chemical group 0.000 claims 1
- 238000011394 anticancer treatment Methods 0.000 claims 1
- ISRXYCXDEAVHOY-UHFFFAOYSA-N n-[(3-iodophenyl)methyl]-2-methoxy-9-methylpurin-6-amine Chemical compound C=12N=CN(C)C2=NC(OC)=NC=1NCC1=CC=CC(I)=C1 ISRXYCXDEAVHOY-UHFFFAOYSA-N 0.000 claims 1
- NZJYPBRWWQVBBD-UHFFFAOYSA-N n-[(3-iodophenyl)methyl]-9-methyl-2-methylsulfanylpurin-6-amine Chemical compound C=12N=CN(C)C2=NC(SC)=NC=1NCC1=CC=CC(I)=C1 NZJYPBRWWQVBBD-UHFFFAOYSA-N 0.000 claims 1
- IQIXQKYLZKCEFF-UHFFFAOYSA-N n-[(3-iodophenyl)methyl]-9-methyl-2-pyridin-4-ylsulfanylpurin-6-amine Chemical compound N1=C(SC=2C=CN=CC=2)N=C2N(C)C=NC2=C1NCC1=CC=CC(I)=C1 IQIXQKYLZKCEFF-UHFFFAOYSA-N 0.000 claims 1
- 125000003729 nucleotide group Chemical group 0.000 claims 1
- 230000005764 inhibitory process Effects 0.000 abstract description 12
- 239000000126 substance Substances 0.000 abstract description 6
- 230000006698 induction Effects 0.000 abstract description 4
- 239000003795 chemical substances by application Substances 0.000 abstract description 3
- 230000002411 adverse Effects 0.000 abstract 1
- 230000002547 anomalous effect Effects 0.000 abstract 1
- 230000007721 medicinal effect Effects 0.000 abstract 1
- 238000011321 prophylaxis Methods 0.000 abstract 1
- 241000699670 Mus sp. Species 0.000 description 59
- 239000003379 purinergic P1 receptor agonist Substances 0.000 description 39
- 238000002474 experimental method Methods 0.000 description 24
- 239000000670 adrenergic alpha-2 receptor antagonist Substances 0.000 description 19
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 19
- 238000000034 method Methods 0.000 description 19
- 239000002953 phosphate buffered saline Substances 0.000 description 19
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 description 18
- 229960002949 fluorouracil Drugs 0.000 description 18
- 210000000440 neutrophil Anatomy 0.000 description 18
- 238000004458 analytical method Methods 0.000 description 15
- IQFYYKKMVGJFEH-XLPZGREQSA-N Thymidine Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 IQFYYKKMVGJFEH-XLPZGREQSA-N 0.000 description 14
- 229940122614 Adenosine receptor agonist Drugs 0.000 description 13
- 206010025323 Lymphomas Diseases 0.000 description 13
- IQFYYKKMVGJFEH-UHFFFAOYSA-N beta-L-thymidine Natural products O=C1NC(=O)C(C)=CN1C1OC(CO)C(O)C1 IQFYYKKMVGJFEH-UHFFFAOYSA-N 0.000 description 13
- 230000001225 therapeutic effect Effects 0.000 description 12
- FFBDFADSZUINTG-UHFFFAOYSA-N DPCPX Chemical compound N1C=2C(=O)N(CCC)C(=O)N(CCC)C=2N=C1C1CCCC1 FFBDFADSZUINTG-UHFFFAOYSA-N 0.000 description 11
- 238000002512 chemotherapy Methods 0.000 description 11
- 229940090044 injection Drugs 0.000 description 11
- 239000007924 injection Substances 0.000 description 11
- 238000002347 injection Methods 0.000 description 11
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 description 10
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 10
- 229960004397 cyclophosphamide Drugs 0.000 description 10
- 229940127089 cytotoxic agent Drugs 0.000 description 10
- 238000007912 intraperitoneal administration Methods 0.000 description 10
- TWWFAXQOKNBUCR-UHFFFAOYSA-N N-[9-chloro-2-(2-furanyl)-[1,2,4]triazolo[1,5-c]quinazolin-5-yl]-2-phenylacetamide Chemical compound N12N=C(C=3OC=CC=3)N=C2C2=CC(Cl)=CC=C2N=C1NC(=O)CC1=CC=CC=C1 TWWFAXQOKNBUCR-UHFFFAOYSA-N 0.000 description 9
- 230000004614 tumor growth Effects 0.000 description 9
- 206010009944 Colon cancer Diseases 0.000 description 8
- 238000001727 in vivo Methods 0.000 description 8
- 125000003342 alkenyl group Chemical group 0.000 description 7
- 230000004663 cell proliferation Effects 0.000 description 7
- 239000002254 cytotoxic agent Substances 0.000 description 7
- 230000009977 dual effect Effects 0.000 description 7
- IORPOFJLSIHJOG-UHFFFAOYSA-N 3,7-dimethyl-1-prop-2-ynylpurine-2,6-dione Chemical compound CN1C(=O)N(CC#C)C(=O)C2=C1N=CN2C IORPOFJLSIHJOG-UHFFFAOYSA-N 0.000 description 6
- 241000699666 Mus <mouse, genus> Species 0.000 description 6
- 239000012979 RPMI medium Substances 0.000 description 6
- 210000004369 blood Anatomy 0.000 description 6
- 239000008280 blood Substances 0.000 description 6
- 239000003937 drug carrier Substances 0.000 description 6
- 238000011081 inoculation Methods 0.000 description 6
- 201000001441 melanoma Diseases 0.000 description 6
- 208000004235 neutropenia Diseases 0.000 description 6
- UUSHFEVEROROSP-UHFFFAOYSA-N propyl 6-ethyl-5-ethylsulfanylcarbonyl-2-phenyl-4-propylpyridine-3-carboxylate Chemical compound CCCOC(=O)C1=C(CCC)C(C(=O)SCC)=C(CC)N=C1C1=CC=CC=C1 UUSHFEVEROROSP-UHFFFAOYSA-N 0.000 description 6
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 5
- 208000001382 Experimental Melanoma Diseases 0.000 description 5
- 125000001589 carboacyl group Chemical group 0.000 description 5
- 230000000973 chemotherapeutic effect Effects 0.000 description 5
- 229960004679 doxorubicin Drugs 0.000 description 5
- 239000012091 fetal bovine serum Substances 0.000 description 5
- 239000000296 purinergic P1 receptor antagonist Substances 0.000 description 5
- 239000000243 solution Substances 0.000 description 5
- 230000001988 toxicity Effects 0.000 description 5
- 231100000419 toxicity Toxicity 0.000 description 5
- 230000004580 weight loss Effects 0.000 description 5
- 101150046889 ADORA3 gene Proteins 0.000 description 4
- KDCGOANMDULRCW-UHFFFAOYSA-N Purine Natural products N1=CNC2=NC=NC2=C1 KDCGOANMDULRCW-UHFFFAOYSA-N 0.000 description 4
- 239000000654 additive Substances 0.000 description 4
- 150000003835 adenosine derivatives Chemical class 0.000 description 4
- 102000030621 adenylate cyclase Human genes 0.000 description 4
- 108060000200 adenylate cyclase Proteins 0.000 description 4
- 230000001093 anti-cancer Effects 0.000 description 4
- 238000004113 cell culture Methods 0.000 description 4
- 125000000753 cycloalkyl group Chemical group 0.000 description 4
- 239000003085 diluting agent Substances 0.000 description 4
- 230000006870 function Effects 0.000 description 4
- 210000000987 immune system Anatomy 0.000 description 4
- 230000001965 increasing effect Effects 0.000 description 4
- 230000001404 mediated effect Effects 0.000 description 4
- 239000002777 nucleoside Substances 0.000 description 4
- 230000001681 protective effect Effects 0.000 description 4
- 230000009467 reduction Effects 0.000 description 4
- 230000004936 stimulating effect Effects 0.000 description 4
- 230000000638 stimulation Effects 0.000 description 4
- NHBKXEKEPDILRR-UHFFFAOYSA-N 2,3-bis(butanoylsulfanyl)propyl butanoate Chemical compound CCCC(=O)OCC(SC(=O)CCC)CSC(=O)CCC NHBKXEKEPDILRR-UHFFFAOYSA-N 0.000 description 3
- GVNVAWHJIKLAGL-UHFFFAOYSA-N 2-(cyclohexen-1-yl)cyclohexan-1-one Chemical compound O=C1CCCCC1C1=CCCCC1 GVNVAWHJIKLAGL-UHFFFAOYSA-N 0.000 description 3
- 101150007969 ADORA1 gene Proteins 0.000 description 3
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 3
- 101150065749 Churc1 gene Proteins 0.000 description 3
- 208000030453 Drug-Related Side Effects and Adverse reaction Diseases 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- 238000012404 In vitro experiment Methods 0.000 description 3
- 229910019142 PO4 Inorganic materials 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- 102100038239 Protein Churchill Human genes 0.000 description 3
- 241000700159 Rattus Species 0.000 description 3
- 239000006146 Roswell Park Memorial Institute medium Substances 0.000 description 3
- 230000000996 additive effect Effects 0.000 description 3
- 229940121359 adenosine receptor antagonist Drugs 0.000 description 3
- 125000003236 benzoyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C(*)=O 0.000 description 3
- 230000004071 biological effect Effects 0.000 description 3
- 230000001767 chemoprotection Effects 0.000 description 3
- 229940124444 chemoprotective agent Drugs 0.000 description 3
- 201000010099 disease Diseases 0.000 description 3
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 3
- 239000003102 growth factor Substances 0.000 description 3
- 125000005843 halogen group Chemical group 0.000 description 3
- 150000002430 hydrocarbons Chemical group 0.000 description 3
- 238000011534 incubation Methods 0.000 description 3
- 230000003993 interaction Effects 0.000 description 3
- 230000002147 killing effect Effects 0.000 description 3
- 230000003211 malignant effect Effects 0.000 description 3
- PSGAAPLEWMOORI-PEINSRQWSA-N medroxyprogesterone acetate Chemical compound C([C@@]12C)CC(=O)C=C1[C@@H](C)C[C@@H]1[C@@H]2CC[C@]2(C)[C@@](OC(C)=O)(C(C)=O)CC[C@H]21 PSGAAPLEWMOORI-PEINSRQWSA-N 0.000 description 3
- SQMWSBKSHWARHU-SDBHATRESA-N n6-cyclopentyladenosine Chemical class O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C2=NC=NC(NC3CCCC3)=C2N=C1 SQMWSBKSHWARHU-SDBHATRESA-N 0.000 description 3
- 210000005259 peripheral blood Anatomy 0.000 description 3
- 239000011886 peripheral blood Substances 0.000 description 3
- 239000000546 pharmaceutical excipient Substances 0.000 description 3
- 239000010452 phosphate Substances 0.000 description 3
- 239000002243 precursor Substances 0.000 description 3
- 238000002360 preparation method Methods 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- 229940002612 prodrug Drugs 0.000 description 3
- 239000000651 prodrug Substances 0.000 description 3
- 238000011084 recovery Methods 0.000 description 3
- 239000000725 suspension Substances 0.000 description 3
- 238000002560 therapeutic procedure Methods 0.000 description 3
- 125000002023 trifluoromethyl group Chemical group FC(F)(F)* 0.000 description 3
- 230000004565 tumor cell growth Effects 0.000 description 3
- BUHVIAUBTBOHAG-FOYDDCNASA-N (2r,3r,4s,5r)-2-[6-[[2-(3,5-dimethoxyphenyl)-2-(2-methylphenyl)ethyl]amino]purin-9-yl]-5-(hydroxymethyl)oxolane-3,4-diol Chemical compound COC1=CC(OC)=CC(C(CNC=2C=3N=CN(C=3N=CN=2)[C@H]2[C@@H]([C@H](O)[C@@H](CO)O2)O)C=2C(=CC=CC=2)C)=C1 BUHVIAUBTBOHAG-FOYDDCNASA-N 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical group N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- 101000695686 Bacteroides fragilis Metallo-beta-lactamase type 2 Proteins 0.000 description 2
- 241000283690 Bos taurus Species 0.000 description 2
- 238000011746 C57BL/6J (JAX™ mouse strain) Methods 0.000 description 2
- 239000004215 Carbon black (E152) Substances 0.000 description 2
- PIICEJLVQHRZGT-UHFFFAOYSA-N Ethylenediamine Chemical compound NCCN PIICEJLVQHRZGT-UHFFFAOYSA-N 0.000 description 2
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 2
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 2
- 241001529936 Murinae Species 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- CZPWVGJYEJSRLH-UHFFFAOYSA-N Pyrimidine Chemical compound C1=CN=CN=C1 CZPWVGJYEJSRLH-UHFFFAOYSA-N 0.000 description 2
- 150000003838 adenosines Chemical class 0.000 description 2
- 239000002671 adjuvant Substances 0.000 description 2
- 230000000845 anti-microbial effect Effects 0.000 description 2
- 229940041181 antineoplastic drug Drugs 0.000 description 2
- 210000001185 bone marrow Anatomy 0.000 description 2
- 150000001768 cations Chemical class 0.000 description 2
- 210000003169 central nervous system Anatomy 0.000 description 2
- OSASVXMJTNOKOY-UHFFFAOYSA-N chlorobutanol Chemical compound CC(C)(O)C(Cl)(Cl)Cl OSASVXMJTNOKOY-UHFFFAOYSA-N 0.000 description 2
- 238000002648 combination therapy Methods 0.000 description 2
- 125000004093 cyano group Chemical group *C#N 0.000 description 2
- 229940108605 cyclophosphamide injection Drugs 0.000 description 2
- 231100000433 cytotoxic Toxicity 0.000 description 2
- 230000001472 cytotoxic effect Effects 0.000 description 2
- 231100000673 dose–response relationship Toxicity 0.000 description 2
- 239000012636 effector Substances 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- 230000001605 fetal effect Effects 0.000 description 2
- 230000005714 functional activity Effects 0.000 description 2
- 230000009036 growth inhibition Effects 0.000 description 2
- 230000003394 haemopoietic effect Effects 0.000 description 2
- 125000005842 heteroatom Chemical group 0.000 description 2
- 229930195733 hydrocarbon Natural products 0.000 description 2
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- 238000000338 in vitro Methods 0.000 description 2
- 238000000099 in vitro assay Methods 0.000 description 2
- 238000010348 incorporation Methods 0.000 description 2
- 208000015181 infectious disease Diseases 0.000 description 2
- 238000003780 insertion Methods 0.000 description 2
- 230000037431 insertion Effects 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 229910052744 lithium Inorganic materials 0.000 description 2
- 210000004698 lymphocyte Anatomy 0.000 description 2
- 210000002540 macrophage Anatomy 0.000 description 2
- 102000006240 membrane receptors Human genes 0.000 description 2
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 2
- 230000002556 myelotoxic effect Effects 0.000 description 2
- 230000017095 negative regulation of cell growth Effects 0.000 description 2
- 229910052757 nitrogen Inorganic materials 0.000 description 2
- 230000002093 peripheral effect Effects 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 2
- 239000002504 physiological saline solution Substances 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- IGFXRKMLLMBKSA-UHFFFAOYSA-N purine Chemical compound N1=C[N]C2=NC=NC2=C1 IGFXRKMLLMBKSA-UHFFFAOYSA-N 0.000 description 2
- 239000002464 receptor antagonist Substances 0.000 description 2
- 229940044551 receptor antagonist Drugs 0.000 description 2
- 230000002829 reductive effect Effects 0.000 description 2
- 230000001105 regulatory effect Effects 0.000 description 2
- 210000002966 serum Anatomy 0.000 description 2
- 239000012453 solvate Substances 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- 210000000689 upper leg Anatomy 0.000 description 2
- 239000003981 vehicle Substances 0.000 description 2
- 230000003313 weakening effect Effects 0.000 description 2
- PEJBIQLCISLFIP-CJMZEECMSA-N (2R,3R,4S,5R)-2-[6-amino-6-(3-oxatricyclo[3.2.1.02,4]octan-6-yl)-1-oxido-8H-purin-1-ium-9-yl]-5-(hydroxymethyl)oxolane-3,4-diol Chemical compound C12C(CC(C3C1O3)C2)C1(C2=NCN([C@H]3[C@H](O)[C@H](O)[C@@H](CO)O3)C2=NC=[N+]1[O-])N PEJBIQLCISLFIP-CJMZEECMSA-N 0.000 description 1
- XSMYYYQVWPZWIZ-IDTAVKCVSA-N (2r,3r,4s,5r)-2-[2-chloro-6-(cyclopentylamino)purin-9-yl]-5-(hydroxymethyl)oxolane-3,4-diol Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C2=NC(Cl)=NC(NC3CCCC3)=C2N=C1 XSMYYYQVWPZWIZ-IDTAVKCVSA-N 0.000 description 1
- UHJRJLPSYJUCPF-SDBHATRESA-N (2r,3r,4s,5r)-2-[6-amino-2-(cyclohexylamino)purin-9-yl]-5-(hydroxymethyl)oxolane-3,4-diol Chemical compound N=1C=2N([C@H]3[C@@H]([C@H](O)[C@@H](CO)O3)O)C=NC=2C(N)=NC=1NC1CCCCC1 UHJRJLPSYJUCPF-SDBHATRESA-N 0.000 description 1
- LDYMCRRFCMRFKB-MOROJQBDSA-N (2s,3s,4r,5r)-5-[6-[(4-aminophenyl)methylamino]purin-9-yl]-3,4-dihydroxy-n-methyloxolane-2-carboxamide Chemical compound O[C@@H]1[C@H](O)[C@@H](C(=O)NC)O[C@H]1N1C2=NC=NC(NCC=3C=CC(N)=CC=3)=C2N=C1 LDYMCRRFCMRFKB-MOROJQBDSA-N 0.000 description 1
- 125000004178 (C1-C4) alkyl group Chemical group 0.000 description 1
- 125000004169 (C1-C6) alkyl group Chemical group 0.000 description 1
- 125000006586 (C3-C10) cycloalkylene group Chemical group 0.000 description 1
- 125000006552 (C3-C8) cycloalkyl group Chemical group 0.000 description 1
- ASJSAQIRZKANQN-CRCLSJGQSA-N 2-deoxy-D-ribose Chemical compound OC[C@@H](O)[C@@H](O)CC=O ASJSAQIRZKANQN-CRCLSJGQSA-N 0.000 description 1
- 125000002941 2-furyl group Chemical group O1C([*])=C([H])C([H])=C1[H] 0.000 description 1
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 description 1
- 125000001494 2-propynyl group Chemical group [H]C#CC([H])([H])* 0.000 description 1
- QFVHZQCOUORWEI-UHFFFAOYSA-N 4-[(4-anilino-5-sulfonaphthalen-1-yl)diazenyl]-5-hydroxynaphthalene-2,7-disulfonic acid Chemical compound C=12C(O)=CC(S(O)(=O)=O)=CC2=CC(S(O)(=O)=O)=CC=1N=NC(C1=CC=CC(=C11)S(O)(=O)=O)=CC=C1NC1=CC=CC=C1 QFVHZQCOUORWEI-UHFFFAOYSA-N 0.000 description 1
- HIQIXEFWDLTDED-UHFFFAOYSA-N 4-hydroxy-1-piperidin-4-ylpyrrolidin-2-one Chemical compound O=C1CC(O)CN1C1CCNCC1 HIQIXEFWDLTDED-UHFFFAOYSA-N 0.000 description 1
- 125000002373 5 membered heterocyclic group Chemical group 0.000 description 1
- 125000004070 6 membered heterocyclic group Chemical group 0.000 description 1
- ORAQXGJNNYRIAI-UHFFFAOYSA-N 6-ethyl-5-ethylsulfanylcarbonyl-2-phenyl-3,4-dipropyl-4H-pyridine-3-carboxylic acid Chemical compound CCCC1C(=C(N=C(C1(CCC)C(=O)O)C2=CC=CC=C2)CC)C(=O)SCC ORAQXGJNNYRIAI-UHFFFAOYSA-N 0.000 description 1
- LRFVTYWOQMYALW-UHFFFAOYSA-N 9H-xanthine Chemical class O=C1NC(=O)NC2=C1NC=N2 LRFVTYWOQMYALW-UHFFFAOYSA-N 0.000 description 1
- 229940124049 Adenosine A2 receptor antagonist Drugs 0.000 description 1
- 108010060261 Adenosine A3 Receptor Proteins 0.000 description 1
- 102000008161 Adenosine A3 Receptor Human genes 0.000 description 1
- 229940123786 Adenosine A3 receptor antagonist Drugs 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 1
- 208000023275 Autoimmune disease Diseases 0.000 description 1
- DWRXFEITVBNRMK-UHFFFAOYSA-N Beta-D-1-Arabinofuranosylthymine Natural products O=C1NC(=O)C(C)=CN1C1C(O)C(O)C(CO)O1 DWRXFEITVBNRMK-UHFFFAOYSA-N 0.000 description 1
- 206010065553 Bone marrow failure Diseases 0.000 description 1
- COVZYZSDYWQREU-UHFFFAOYSA-N Busulfan Chemical compound CS(=O)(=O)OCCCCOS(C)(=O)=O COVZYZSDYWQREU-UHFFFAOYSA-N 0.000 description 1
- 125000000882 C2-C6 alkenyl group Chemical group 0.000 description 1
- 0 CCN[C@@](C)CCC*C Chemical compound CCN[C@@](C)CCC*C 0.000 description 1
- UGTJLJZQQFGTJD-UHFFFAOYSA-N Carbonylcyanide-3-chlorophenylhydrazone Chemical compound ClC1=CC=CC(NN=C(C#N)C#N)=C1 UGTJLJZQQFGTJD-UHFFFAOYSA-N 0.000 description 1
- 108010001857 Cell Surface Receptors Proteins 0.000 description 1
- 244000205754 Colocasia esculenta Species 0.000 description 1
- 235000006481 Colocasia esculenta Nutrition 0.000 description 1
- 208000027205 Congenital disease Diseases 0.000 description 1
- 102000004127 Cytokines Human genes 0.000 description 1
- 108090000695 Cytokines Proteins 0.000 description 1
- HMFHBZSHGGEWLO-SOOFDHNKSA-N D-ribofuranose Chemical compound OC[C@H]1OC(O)[C@H](O)[C@@H]1O HMFHBZSHGGEWLO-SOOFDHNKSA-N 0.000 description 1
- 101100545204 Danio rerio zdhhc18a gene Proteins 0.000 description 1
- 239000004593 Epoxy Substances 0.000 description 1
- 102000003951 Erythropoietin Human genes 0.000 description 1
- 108090000394 Erythropoietin Proteins 0.000 description 1
- 108091006027 G proteins Proteins 0.000 description 1
- 102000030782 GTP binding Human genes 0.000 description 1
- 108091000058 GTP-Binding Proteins 0.000 description 1
- 108091006101 Gi proteins Proteins 0.000 description 1
- 102000034354 Gi proteins Human genes 0.000 description 1
- 108091006065 Gs proteins Proteins 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 208000026350 Inborn Genetic disease Diseases 0.000 description 1
- 102000014150 Interferons Human genes 0.000 description 1
- 108010050904 Interferons Proteins 0.000 description 1
- 102000010445 Lactoferrin Human genes 0.000 description 1
- 108010063045 Lactoferrin Proteins 0.000 description 1
- 102000007651 Macrophage Colony-Stimulating Factor Human genes 0.000 description 1
- 108010046938 Macrophage Colony-Stimulating Factor Proteins 0.000 description 1
- 208000024556 Mendelian disease Diseases 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 241000699660 Mus musculus Species 0.000 description 1
- 101000746384 Mus musculus Granulocyte colony-stimulating factor Proteins 0.000 description 1
- PWTBZOIUWZOPFT-UHFFFAOYSA-N Nc1nc(NCCc(cc2)ccc2O)nc2nc(-c3ccc[o]3)n[n]12 Chemical compound Nc1nc(NCCc(cc2)ccc2O)nc2nc(-c3ccc[o]3)n[n]12 PWTBZOIUWZOPFT-UHFFFAOYSA-N 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 235000019483 Peanut oil Nutrition 0.000 description 1
- 229930182555 Penicillin Natural products 0.000 description 1
- 101150018368 Pigv gene Proteins 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 208000004880 Polyuria Diseases 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- 201000004681 Psoriasis Diseases 0.000 description 1
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 1
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 1
- PYMYPHUHKUWMLA-LMVFSUKVSA-N Ribose Natural products OC[C@@H](O)[C@@H](O)[C@@H](O)C=O PYMYPHUHKUWMLA-LMVFSUKVSA-N 0.000 description 1
- 235000019486 Sunflower oil Nutrition 0.000 description 1
- 230000005856 abnormality Effects 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 239000003449 adenosine A2 receptor antagonist Substances 0.000 description 1
- 239000002580 adenosine A3 receptor antagonist Substances 0.000 description 1
- 230000001270 agonistic effect Effects 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- 229910052783 alkali metal Inorganic materials 0.000 description 1
- 150000001340 alkali metals Chemical class 0.000 description 1
- 125000004466 alkoxycarbonylamino group Chemical group 0.000 description 1
- 125000005037 alkyl phenyl group Chemical group 0.000 description 1
- 125000002947 alkylene group Chemical group 0.000 description 1
- HMFHBZSHGGEWLO-UHFFFAOYSA-N alpha-D-Furanose-Ribose Natural products OCC1OC(O)C(O)C1O HMFHBZSHGGEWLO-UHFFFAOYSA-N 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 125000002431 aminoalkoxy group Chemical group 0.000 description 1
- 230000000202 analgesic effect Effects 0.000 description 1
- 229940035676 analgesics Drugs 0.000 description 1
- 230000003042 antagnostic effect Effects 0.000 description 1
- 239000000730 antalgic agent Substances 0.000 description 1
- 230000003110 anti-inflammatory effect Effects 0.000 description 1
- 238000011319 anticancer therapy Methods 0.000 description 1
- 229940125681 anticonvulsant agent Drugs 0.000 description 1
- 239000001961 anticonvulsive agent Substances 0.000 description 1
- 229940121375 antifungal agent Drugs 0.000 description 1
- 239000003429 antifungal agent Substances 0.000 description 1
- 239000004599 antimicrobial Substances 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 239000003200 antithyroid agent Substances 0.000 description 1
- 229940043671 antithyroid preparations Drugs 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 210000003651 basophil Anatomy 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 150000008107 benzenesulfonic acids Chemical class 0.000 description 1
- 125000000051 benzyloxy group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])O* 0.000 description 1
- HMFHBZSHGGEWLO-TXICZTDVSA-N beta-D-ribose Chemical group OC[C@H]1O[C@@H](O)[C@H](O)[C@@H]1O HMFHBZSHGGEWLO-TXICZTDVSA-N 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 201000000053 blastoma Diseases 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 238000004820 blood count Methods 0.000 description 1
- 238000010322 bone marrow transplantation Methods 0.000 description 1
- 239000006172 buffering agent Substances 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 230000001914 calming effect Effects 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 230000022131 cell cycle Effects 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 239000002738 chelating agent Substances 0.000 description 1
- WIIZWVCIJKGZOK-RKDXNWHRSA-N chloramphenicol Chemical compound ClC(Cl)C(=O)N[C@H](CO)[C@H](O)C1=CC=C([N+]([O-])=O)C=C1 WIIZWVCIJKGZOK-RKDXNWHRSA-N 0.000 description 1
- 229960005091 chloramphenicol Drugs 0.000 description 1
- 229960004926 chlorobutanol Drugs 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 238000011284 combination treatment Methods 0.000 description 1
- 230000002301 combined effect Effects 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 230000009073 conformational modification Effects 0.000 description 1
- 235000005687 corn oil Nutrition 0.000 description 1
- 239000002285 corn oil Substances 0.000 description 1
- 235000012343 cottonseed oil Nutrition 0.000 description 1
- 239000002385 cottonseed oil Substances 0.000 description 1
- 230000001186 cumulative effect Effects 0.000 description 1
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 1
- 125000001511 cyclopentyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 239000000824 cytostatic agent Substances 0.000 description 1
- 230000001085 cytostatic effect Effects 0.000 description 1
- 231100000599 cytotoxic agent Toxicity 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 238000012217 deletion Methods 0.000 description 1
- 230000037430 deletion Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 125000004663 dialkyl amino group Chemical group 0.000 description 1
- 239000012954 diazonium Chemical group 0.000 description 1
- IJGRMHOSHXDMSA-UHFFFAOYSA-O diazynium Chemical group [NH+]#N IJGRMHOSHXDMSA-UHFFFAOYSA-O 0.000 description 1
- 235000005911 diet Nutrition 0.000 description 1
- 230000037213 diet Effects 0.000 description 1
- XEYBRNLFEZDVAW-ARSRFYASSA-N dinoprostone Chemical compound CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O XEYBRNLFEZDVAW-ARSRFYASSA-N 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 239000002612 dispersion medium Substances 0.000 description 1
- 238000010494 dissociation reaction Methods 0.000 description 1
- 230000005593 dissociations Effects 0.000 description 1
- 230000035619 diuresis Effects 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 230000004821 effect on bone Effects 0.000 description 1
- 230000008030 elimination Effects 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- 201000008184 embryoma Diseases 0.000 description 1
- 210000002889 endothelial cell Anatomy 0.000 description 1
- 210000003979 eosinophil Anatomy 0.000 description 1
- 125000003700 epoxy group Chemical group 0.000 description 1
- 229940105423 erythropoietin Drugs 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- BEFDCLMNVWHSGT-UHFFFAOYSA-N ethenylcyclopentane Chemical compound C=CC1CCCC1 BEFDCLMNVWHSGT-UHFFFAOYSA-N 0.000 description 1
- NTNZTEQNFHNYBC-UHFFFAOYSA-N ethyl 2-aminoacetate Chemical compound CCOC(=O)CN NTNZTEQNFHNYBC-UHFFFAOYSA-N 0.000 description 1
- 210000003722 extracellular fluid Anatomy 0.000 description 1
- 210000002950 fibroblast Anatomy 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 102000034356 gene-regulatory proteins Human genes 0.000 description 1
- 108091006104 gene-regulatory proteins Proteins 0.000 description 1
- 235000011187 glycerol Nutrition 0.000 description 1
- 210000003714 granulocyte Anatomy 0.000 description 1
- 230000007085 granulocyte colony-stimulating factor production Effects 0.000 description 1
- 125000000262 haloalkenyl group Chemical group 0.000 description 1
- 125000002768 hydroxyalkyl group Chemical group 0.000 description 1
- 201000004108 hypersplenism Diseases 0.000 description 1
- 238000005462 in vivo assay Methods 0.000 description 1
- 230000002458 infectious effect Effects 0.000 description 1
- 230000036512 infertility Effects 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 229940079322 interferon Drugs 0.000 description 1
- 230000003834 intracellular effect Effects 0.000 description 1
- 238000007913 intrathecal administration Methods 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 150000002540 isothiocyanates Chemical group 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- CSSYQJWUGATIHM-IKGCZBKSSA-N l-phenylalanyl-l-lysyl-l-cysteinyl-l-arginyl-l-arginyl-l-tryptophyl-l-glutaminyl-l-tryptophyl-l-arginyl-l-methionyl-l-lysyl-l-lysyl-l-leucylglycyl-l-alanyl-l-prolyl-l-seryl-l-isoleucyl-l-threonyl-l-cysteinyl-l-valyl-l-arginyl-l-arginyl-l-alanyl-l-phenylal Chemical compound C([C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)NCC(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CS)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CC=CC=C1 CSSYQJWUGATIHM-IKGCZBKSSA-N 0.000 description 1
- 229940078795 lactoferrin Drugs 0.000 description 1
- 235000021242 lactoferrin Nutrition 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- 230000001050 lubricating effect Effects 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 210000004962 mammalian cell Anatomy 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 230000035800 maturation Effects 0.000 description 1
- 229940126601 medicinal product Drugs 0.000 description 1
- 108020004084 membrane receptors Proteins 0.000 description 1
- 125000004492 methyl ester group Chemical group 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 210000001616 monocyte Anatomy 0.000 description 1
- 230000002410 myeloprotective effect Effects 0.000 description 1
- 125000001624 naphthyl group Chemical group 0.000 description 1
- MRWXACSTFXYYMV-FDDDBJFASA-N nebularine Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C2=NC=NC=C2N=C1 MRWXACSTFXYYMV-FDDDBJFASA-N 0.000 description 1
- 239000002858 neurotransmitter agent Substances 0.000 description 1
- 230000003472 neutralizing effect Effects 0.000 description 1
- 125000004433 nitrogen atom Chemical group N* 0.000 description 1
- 239000002687 nonaqueous vehicle Substances 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 238000011580 nude mouse model Methods 0.000 description 1
- 239000004006 olive oil Substances 0.000 description 1
- 235000008390 olive oil Nutrition 0.000 description 1
- 210000004789 organ system Anatomy 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 125000004430 oxygen atom Chemical group O* 0.000 description 1
- 238000007911 parenteral administration Methods 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 239000000312 peanut oil Substances 0.000 description 1
- 150000002960 penicillins Chemical class 0.000 description 1
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 1
- 229960003742 phenol Drugs 0.000 description 1
- 125000001484 phenothiazinyl group Chemical class C1(=CC=CC=2SC3=CC=CC=C3NC12)* 0.000 description 1
- 125000002467 phosphate group Chemical group [H]OP(=O)(O[H])O[*] 0.000 description 1
- 230000004962 physiological condition Effects 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- OXCMYAYHXIHQOA-UHFFFAOYSA-N potassium;[2-butyl-5-chloro-3-[[4-[2-(1,2,4-triaza-3-azanidacyclopenta-1,4-dien-5-yl)phenyl]phenyl]methyl]imidazol-4-yl]methanol Chemical compound [K+].CCCCC1=NC(Cl)=C(CO)N1CC1=CC=C(C=2C(=CC=CC=2)C2=N[N-]N=N2)C=C1 OXCMYAYHXIHQOA-UHFFFAOYSA-N 0.000 description 1
- 230000003389 potentiating effect Effects 0.000 description 1
- 239000013630 prepared media Substances 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 235000018102 proteins Nutrition 0.000 description 1
- 102000004169 proteins and genes Human genes 0.000 description 1
- 108090000623 proteins and genes Proteins 0.000 description 1
- 239000002212 purine nucleoside Substances 0.000 description 1
- JWVCLYRUEFBMGU-UHFFFAOYSA-N quinazoline Chemical compound N1=CN=CC2=CC=CC=C21 JWVCLYRUEFBMGU-UHFFFAOYSA-N 0.000 description 1
- 229940044601 receptor agonist Drugs 0.000 description 1
- 239000000018 receptor agonist Substances 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- 239000008159 sesame oil Substances 0.000 description 1
- 235000011803 sesame oil Nutrition 0.000 description 1
- 230000019491 signal transduction Effects 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 229940075582 sorbic acid Drugs 0.000 description 1
- 235000010199 sorbic acid Nutrition 0.000 description 1
- 239000004334 sorbic acid Substances 0.000 description 1
- 239000003549 soybean oil Substances 0.000 description 1
- 235000012424 soybean oil Nutrition 0.000 description 1
- 201000009225 splenic sequestration Diseases 0.000 description 1
- 208000010110 spontaneous platelet aggregation Diseases 0.000 description 1
- 238000010186 staining Methods 0.000 description 1
- 238000010254 subcutaneous injection Methods 0.000 description 1
- 239000007929 subcutaneous injection Substances 0.000 description 1
- 238000006467 substitution reaction Methods 0.000 description 1
- 229940124530 sulfonamide Drugs 0.000 description 1
- 150000003456 sulfonamides Chemical class 0.000 description 1
- 239000002600 sunflower oil Substances 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 239000006188 syrup Substances 0.000 description 1
- 235000020357 syrup Nutrition 0.000 description 1
- 229940065721 systemic for obstructive airway disease xanthines Drugs 0.000 description 1
- 239000003826 tablet Substances 0.000 description 1
- 235000020679 tap water Nutrition 0.000 description 1
- 239000008399 tap water Substances 0.000 description 1
- 102000055501 telomere Human genes 0.000 description 1
- 108091035539 telomere Proteins 0.000 description 1
- 210000003411 telomere Anatomy 0.000 description 1
- 210000001694 thigh bone Anatomy 0.000 description 1
- 150000007970 thio esters Chemical group 0.000 description 1
- 229940104230 thymidine Drugs 0.000 description 1
- 238000011269 treatment regimen Methods 0.000 description 1
- 125000004950 trifluoroalkyl group Chemical group 0.000 description 1
- 230000006442 vascular tone Effects 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- 210000003462 vein Anatomy 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
- 230000029663 wound healing Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7042—Compounds having saccharide radicals and heterocyclic rings
- A61K31/7052—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides
- A61K31/706—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/519—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim ortho- or peri-condensed with heterocyclic rings
- A61K31/52—Purines, e.g. adenine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7042—Compounds having saccharide radicals and heterocyclic rings
- A61K31/7052—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides
- A61K31/706—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom
- A61K31/7064—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines
- A61K31/7076—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines containing purines, e.g. adenosine, adenylic acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7042—Compounds having saccharide radicals and heterocyclic rings
- A61K31/7052—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides
- A61K31/706—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom
- A61K31/7064—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines
- A61K31/7076—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines containing purines, e.g. adenosine, adenylic acid
- A61K31/708—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines containing purines, e.g. adenosine, adenylic acid having oxo groups directly attached to the purine ring system, e.g. guanosine, guanylic acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P39/00—General protective or antinoxious agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical & Material Sciences (AREA)
- Veterinary Medicine (AREA)
- Medicinal Chemistry (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Molecular Biology (AREA)
- General Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Toxicology (AREA)
- Immunology (AREA)
- Oncology (AREA)
- Hematology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| IL13186499A IL131864A0 (en) | 1999-09-10 | 1999-09-10 | Pharmaceutical compositions comprising an adenosine receptor agonist or antagonist |
| IL131864 | 1999-09-10 | ||
| IL133680 | 1999-12-23 | ||
| IL13368099A IL133680A0 (en) | 1999-09-10 | 1999-12-23 | Pharmaceutical compositions comprising an adenosine receptor agonist or antagonist |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2002109228A RU2002109228A (ru) | 2004-01-10 |
| RU2239455C2 true RU2239455C2 (ru) | 2004-11-10 |
Family
ID=26323880
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2002109228/15A RU2239455C2 (ru) | 1999-09-10 | 2000-09-08 | Фармацевтические композиции, содержащие агонист или антагонист аденозинового рецептора |
Country Status (19)
| Country | Link |
|---|---|
| US (2) | US7064112B1 (enExample) |
| EP (1) | EP1261322B1 (enExample) |
| JP (2) | JP4980530B2 (enExample) |
| KR (2) | KR100584797B1 (enExample) |
| CN (1) | CN100358512C (enExample) |
| AU (1) | AU782826B2 (enExample) |
| BR (1) | BR0013905A (enExample) |
| CA (1) | CA2384111C (enExample) |
| DE (1) | DE60037277T2 (enExample) |
| DK (1) | DK1261322T3 (enExample) |
| ES (1) | ES2296637T3 (enExample) |
| HK (1) | HK1052653B (enExample) |
| HU (1) | HU226913B1 (enExample) |
| IL (1) | IL133680A0 (enExample) |
| MX (1) | MXPA02002546A (enExample) |
| PL (1) | PL199852B1 (enExample) |
| PT (1) | PT1261322E (enExample) |
| RU (1) | RU2239455C2 (enExample) |
| WO (1) | WO2001019360A2 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2391103C2 (ru) * | 2004-10-15 | 2010-06-10 | Си Ви Терапьютикс, Инк. | Способ предупреждения и лечения ремоделирования дыхательных путей и воспаления легких с применением антагонистов аденозиновых рецепторов a2b |
Families Citing this family (51)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
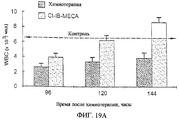
| IL121272A (en) * | 1997-07-10 | 2000-06-01 | Can Fite Technologies Ltd | Pharmaceutical compositions comprising adenosine and their use for treating or preventing leukopenia |
| CN1234358C (zh) | 2000-06-21 | 2006-01-04 | 弗·哈夫曼-拉罗切有限公司 | 苯并噻唑衍生物 |
| EP1365776B1 (en) * | 2001-01-16 | 2005-04-13 | Can-Fite Biopharma Ltd. | Use of an adenosine a3 receptor agonist for inhibition of viral replication |
| US20020115635A1 (en) * | 2001-02-21 | 2002-08-22 | Pnina Fishman | Modulation of GSK-3beta activity and its different uses |
| US20040204481A1 (en) * | 2001-04-12 | 2004-10-14 | Pnina Fishman | Activation of natural killer cells by adenosine A3 receptor agonists |
| US7049303B2 (en) * | 2001-11-07 | 2006-05-23 | Medical Research Council | Inhibition of viruses |
| US7414036B2 (en) | 2002-01-25 | 2008-08-19 | Muscagen Limited | Compounds useful as A3 adenosine receptor agonists |
| ATE371191T1 (de) * | 2002-10-22 | 2007-09-15 | Can Fite Biopharma Ltd | Die verwendung von dem a3 adenosin rezeptor als marker eines krankheitszustandes |
| US7087761B2 (en) | 2003-01-07 | 2006-08-08 | Hoffmann-La Roche Inc. | Cyclization process for substituted benzothiazole derivatives |
| EP1753760B1 (en) | 2004-05-24 | 2008-01-02 | F.Hoffmann-La Roche Ag | 4-hydroxy-4-methyl-piperidine-1-carboxylic acid (4-methoxy-7-morpholin-4-yl-benzothiazol-2-yl)-amide |
| DE602005020286D1 (de) | 2004-05-26 | 2010-05-12 | Inotek Pharmaceuticals Corp | Purinderivate als adenosin a 1 rezeptoragonisten und anwendungsverfahren dafür |
| US7825102B2 (en) | 2004-07-28 | 2010-11-02 | Can-Fite Biopharma Ltd. | Treatment of dry eye conditions |
| BRPI0515506A (pt) | 2004-09-20 | 2008-07-29 | Inotek Pharmaceuticals Corp | derivados de purina e métodos de uso dos mesmos |
| CN101056857B (zh) | 2004-11-05 | 2010-12-01 | 弗·哈夫曼-拉罗切有限公司 | 异烟酸衍生物的制备方法 |
| MX2007005525A (es) * | 2004-11-08 | 2007-07-05 | Can Fite Biopharma Ltd | Tratamiento terapeutico de resorcion ose acelerada. |
| CA2586420A1 (en) * | 2004-11-22 | 2007-04-12 | King Pharmaceuticals Research & Development, Inc. | Enhancing treatment of cancer and hif-1 mediated disoders with adenosine a3 receptor antagonists |
| KR100973609B1 (ko) | 2005-03-23 | 2010-08-03 | 에프. 호프만-라 로슈 아게 | mGluR2 길항제로서 아세틸렌일-피라졸로-피리미딘유도체 |
| CA2623721C (en) | 2005-09-27 | 2014-05-13 | F. Hoffmann-La Roche Ag | Oxadiazolyl pyrazolo-pyrimidines as mglur2 antagonists |
| AU2006320578B2 (en) | 2005-11-30 | 2013-01-31 | Inotek Pharmaceuticals Corporation | Purine derivatives and methods of use thereof |
| KR101037095B1 (ko) * | 2006-01-27 | 2011-05-26 | 캔-파이트 바이오파마 리미티드 | 건성안 장애의 치료를 위한 아데노신 a3 수용체 아고니스트 |
| WO2008023362A2 (en) * | 2006-08-21 | 2008-02-28 | Can-Fite Biopharma Ltd. | Use of a combination of methotrexate and an a3ar agonist for the treatment of cancer |
| WO2008045330A2 (en) * | 2006-10-06 | 2008-04-17 | The Trustees Of The University Of Pennsylvania | Effective delivery of cross-species a3 adenosine-receptor antagonists to reduce intraocular pressure |
| GB0625100D0 (en) * | 2006-12-15 | 2007-01-24 | Univ Murcia | Epigallocatechin-3-gallate compositions for cancer therapy and chemoprotection |
| US20090088403A1 (en) * | 2007-05-07 | 2009-04-02 | Randy Blakely | A3 adenosine receptors as targets for the modulation of central serotonergic signaling |
| CA2694983A1 (en) * | 2007-07-17 | 2009-01-22 | Combinatorx, Incorporated | Treatments of b-cell proliferative disorders |
| TW200914048A (en) * | 2007-07-17 | 2009-04-01 | Combinatorx Inc | Combinations for the treatment of B-cell proliferative disorders |
| WO2009151569A2 (en) * | 2008-06-09 | 2009-12-17 | Combinatorx, Incorporated | Beta adrenergic receptor agonists for the treatment of b-cell proliferative disorders |
| WO2010027935A1 (en) * | 2008-09-08 | 2010-03-11 | Merck Sharp & Dohme Corp. | Ahcy hydrolase inhibitors for treatment of hyper homocysteinemia |
| AU2010250759B2 (en) | 2009-05-17 | 2013-03-14 | Can-Fite Biopharma Ltd. | A3 adenosine receptor agonists for the reduction of intraocular pressure |
| EP2456419B1 (en) | 2009-07-21 | 2016-11-23 | Oradin Pharmaceutical Ltd. | A3 adenosine receptor ligands for modulation of pigmentation |
| WO2011074903A2 (ko) * | 2009-12-17 | 2011-06-23 | 이화여자대학교 산학협력단 | A3 아데노신 수용체 효능제를 포함하는 약제학적 조성물 |
| EA025415B1 (ru) | 2010-01-11 | 2016-12-30 | Инотек Фармасьютикалз Корпорейшн | Комбинация, набор и способ снижения внутриглазного давления |
| KR20130072189A (ko) | 2010-03-03 | 2013-07-01 | 더 가번먼트 오브 더 유나이티드 스테이츠 오브 아메리카, 레프리젠티드 바이 더 세크러터리, 디파트먼트 오브 헬쓰 앤드 휴먼 서비스즈 | 포도막염의 치료를 위한 a3ar 작동약 |
| EP2569325A4 (en) | 2010-03-26 | 2013-10-09 | Inotek Pharmaceuticals Corp | METHOD FOR REDUCING INNER EYE PRESSURE IN HUMANS USING N6-CYCLOPENTYLADENOSINE (CPA), CPA DERIVATIVES OR PRODRUGS THEREOF |
| US20130109645A1 (en) | 2010-03-31 | 2013-05-02 | The united States of America,as represented by Secretary,Dept.,of Health and Human Services | Adenosine receptor agonists for the treatment and prevention of vascular or joint capsule calcification disorders |
| AU2012358803C1 (en) | 2011-12-22 | 2019-12-19 | Janssen Biopharma, Inc. | Substituted nucleosides, nucleotides and analogs thereof |
| HK1202053A1 (en) * | 2012-01-23 | 2015-09-18 | Can-Fite Biopharma Ltd. | Treatment of liver conditions |
| US9278991B2 (en) | 2012-01-26 | 2016-03-08 | Inotek Pharmaceuticals Corporation | Anhydrous polymorphs of [(2R,3S,4R,5R)-5-(6-(cyclopentylamino)-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)} methyl nitrate and processes of preparation thereof |
| US9441007B2 (en) | 2012-03-21 | 2016-09-13 | Alios Biopharma, Inc. | Substituted nucleosides, nucleotides and analogs thereof |
| USRE48171E1 (en) | 2012-03-21 | 2020-08-25 | Janssen Biopharma, Inc. | Substituted nucleosides, nucleotides and analogs thereof |
| US8822460B2 (en) | 2012-04-06 | 2014-09-02 | Janssen Pharmaceutica Nv | Fused cyclopentyl antagonists of CCR2 |
| ES2627010T3 (es) | 2012-07-19 | 2017-07-26 | Janssen Pharmaceutica, N.V. | Antagonistas de octahidro-ciclopentapirrolilo de CCR2 |
| BR112015002697A8 (pt) | 2012-08-09 | 2018-01-16 | Can Fite Biopharma Ltd | uso de ligante de receptor de adenosina a3 (a3ar), ligante de a3ar, e, composição farmacêutica para tratar disfunção sexual em um indivíduo |
| CA2903114A1 (en) | 2013-03-15 | 2014-09-25 | Inotek Pharmaceuticals Corporation | Ophthalmic formulations |
| IL242723B (en) | 2015-11-23 | 2019-12-31 | Can Fite Biopharma Ltd | A3 adenosine receptor ligand for the treatment of ectopic fat accumulation |
| KR102445887B1 (ko) | 2016-04-21 | 2022-09-21 | 아스트로사이트 파마슈티컬스, 인코포레이티드 | 신경계 및 심혈관계 병태 치료를 위한 화합물 및 방법 |
| IL254535A0 (en) * | 2017-09-17 | 2017-11-30 | Can Fite Biopharma Ltd | Adenosine a3 receptor ligand for use in the management of cytokine release syndrome |
| EP3749322A4 (en) | 2018-02-09 | 2021-11-17 | Astrocyte Pharmaceuticals, Inc. | COMPOUNDS AND METHODS FOR THE TREATMENT OF ADDICTION AND RELATED DISORDERS |
| EP3856741A4 (en) | 2018-09-26 | 2022-06-22 | Astrocyte Pharmaceuticals, Inc. | POLYMORPHOUS COMPOUNDS AND USES THEREOF |
| IL264112A (en) | 2019-01-06 | 2020-07-30 | Fishman Pnina | Adenosine a3 receptor ligand for use in lowering adipocyte levels |
| IT202300014340A1 (it) | 2023-07-10 | 2025-01-10 | Consiglio Nazionale Ricerche | Trattamento di malattie associate alla disfunzione del gene ocrl. |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1995002604A1 (en) * | 1993-07-13 | 1995-01-26 | The United States Of America, Represented By The Secretary, Department Of Health And Human Services | A3 adenosine receptor agonists |
| RU2071771C1 (ru) * | 1995-01-11 | 1997-01-20 | Владислав Алексеевич Шматко | Способ лечения рака молочной железы |
| RU2071770C1 (ru) * | 1994-12-14 | 1997-01-20 | Владислав Алексеевич Шматко | Способ лечения рака шейки матки и яичников |
| WO1998050047A1 (en) * | 1997-05-09 | 1998-11-12 | Trustees Of The University Of Pennsylvania | Methods and compositions for reducing ischemic injury of the heart by administering adenosine receptor agonists and antagonists |
| WO1999002143A2 (en) * | 1997-07-10 | 1999-01-21 | Can-Fite Technologies Ltd. | Medicament comprising adenosine |
| WO1999006053A1 (en) * | 1997-07-29 | 1999-02-11 | Medco Research, Inc. | N6-substituted-adenosine-5'-uronamides as adenosine receptor modulators |
| WO1999020284A1 (en) * | 1997-10-23 | 1999-04-29 | Trustees Of The University Of Pennsylvania | Methods for reducing ischemic injury of the heart via the sequential administration of monophosphoryl lipid a and adenosine receptor agents |
Family Cites Families (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5443836A (en) * | 1993-03-15 | 1995-08-22 | Gensia, Inc. | Methods for protecting tissues and organs from ischemic damage |
| US5688774A (en) * | 1993-07-13 | 1997-11-18 | The United States Of America As Represented By The Department Of Health And Human Services | A3 adenosine receptor agonists |
| GB2289218A (en) * | 1994-05-06 | 1995-11-15 | Merck & Co Inc | Inhibition of TNFalpha production with agonists of the A2b subtype of the adenosine receptor |
| US6048865A (en) * | 1997-07-29 | 2000-04-11 | Medco Research, Inc. | N6 -substituted-adenosine-5'-uronamides as adenosine receptor modulator |
| EP1011608A4 (en) * | 1998-06-08 | 2002-05-15 | Epigenesis Pharmaceuticals Inc | COMPOSITION AND METHOD FOR THE PREVENTION AND TREATMENT OF HEART AND NEUTRAL FAILURE OR DAMAGE RELATED TO ISCHEMIA, ENDOTOXIN RELEASE, ARDS OR CALLED BY THE ADDITION OF SPECIFIC MEDICINES |
| US6448253B1 (en) | 1998-09-16 | 2002-09-10 | King Pharmaceuticals Research And Development, Inc. | Adenosine A3 receptor modulators |
| IL127947A0 (en) | 1999-01-07 | 1999-11-30 | Can Fite Technologies Ltd | Pharmaceutical use of adenosine agonists |
| BR0007864A (pt) * | 1999-02-01 | 2001-11-06 | Univ Virginia | Composto, e, uso de um composto |
| IL131096A (en) | 1999-07-26 | 2004-09-27 | Can Fite Technologies Ltd | Method for preparing a composition derived from muscle tissue |
-
1999
- 1999-12-23 IL IL13368099A patent/IL133680A0/xx not_active IP Right Cessation
-
2000
- 2000-09-08 BR BR0013905-0A patent/BR0013905A/pt not_active Application Discontinuation
- 2000-09-08 HU HU0203870A patent/HU226913B1/hu unknown
- 2000-09-08 WO PCT/IL2000/000550 patent/WO2001019360A2/en not_active Ceased
- 2000-09-08 EP EP00956773A patent/EP1261322B1/en not_active Expired - Lifetime
- 2000-09-08 JP JP2001522994A patent/JP4980530B2/ja not_active Expired - Lifetime
- 2000-09-08 KR KR1020027003164A patent/KR100584797B1/ko not_active Expired - Lifetime
- 2000-09-08 KR KR1020067006473A patent/KR100674529B1/ko not_active Expired - Lifetime
- 2000-09-08 MX MXPA02002546A patent/MXPA02002546A/es active IP Right Grant
- 2000-09-08 RU RU2002109228/15A patent/RU2239455C2/ru active
- 2000-09-08 US US09/700,751 patent/US7064112B1/en not_active Expired - Lifetime
- 2000-09-08 CA CA002384111A patent/CA2384111C/en not_active Expired - Lifetime
- 2000-09-08 HK HK03105018.4A patent/HK1052653B/zh not_active IP Right Cessation
- 2000-09-08 DK DK00956773T patent/DK1261322T3/da active
- 2000-09-08 AU AU68634/00A patent/AU782826B2/en not_active Expired
- 2000-09-08 ES ES00956773T patent/ES2296637T3/es not_active Expired - Lifetime
- 2000-09-08 PL PL356469A patent/PL199852B1/pl unknown
- 2000-09-08 DE DE60037277T patent/DE60037277T2/de not_active Expired - Lifetime
- 2000-09-08 PT PT00956773T patent/PT1261322E/pt unknown
- 2000-09-08 CN CNB008148007A patent/CN100358512C/zh not_active Expired - Lifetime
-
2005
- 2005-11-25 US US11/286,376 patent/US20060084626A1/en not_active Abandoned
-
2007
- 2007-05-14 JP JP2007128137A patent/JP2007204496A/ja active Pending
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1995002604A1 (en) * | 1993-07-13 | 1995-01-26 | The United States Of America, Represented By The Secretary, Department Of Health And Human Services | A3 adenosine receptor agonists |
| RU2071770C1 (ru) * | 1994-12-14 | 1997-01-20 | Владислав Алексеевич Шматко | Способ лечения рака шейки матки и яичников |
| RU2071771C1 (ru) * | 1995-01-11 | 1997-01-20 | Владислав Алексеевич Шматко | Способ лечения рака молочной железы |
| WO1998050047A1 (en) * | 1997-05-09 | 1998-11-12 | Trustees Of The University Of Pennsylvania | Methods and compositions for reducing ischemic injury of the heart by administering adenosine receptor agonists and antagonists |
| WO1999002143A2 (en) * | 1997-07-10 | 1999-01-21 | Can-Fite Technologies Ltd. | Medicament comprising adenosine |
| WO1999006053A1 (en) * | 1997-07-29 | 1999-02-11 | Medco Research, Inc. | N6-substituted-adenosine-5'-uronamides as adenosine receptor modulators |
| WO1999020284A1 (en) * | 1997-10-23 | 1999-04-29 | Trustees Of The University Of Pennsylvania | Methods for reducing ischemic injury of the heart via the sequential administration of monophosphoryl lipid a and adenosine receptor agents |
Non-Patent Citations (3)
| Title |
|---|
| Противоопухолевая терапия. Справочник/Под ред. Н.И.Переводчиковой - М., 1993 с.192 и 202. * |
| Реферат из АБД Medline: Ezeamuzie CI et al. Adenosine A3 receptors on human eosinophils mediate inhibition of degranulation and superoxide anion release. Br J Pharmacol. - 1999, May; 127(1): 188-94. Справочник по онкологии. - М.: Медицина 1964 с.63. * |
| Реферат из АБД Medline: Yao Y et al. Adenosine A3 receptor agonists protect HL-60 and U-937 cells from apoptosis induced by A3 antagonists Biochem Biophys Res Commun. 1997 Mar 17; 232(2):317-22. * |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2391103C2 (ru) * | 2004-10-15 | 2010-06-10 | Си Ви Терапьютикс, Инк. | Способ предупреждения и лечения ремоделирования дыхательных путей и воспаления легких с применением антагонистов аденозиновых рецепторов a2b |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2239455C2 (ru) | Фармацевтические композиции, содержащие агонист или антагонист аденозинового рецептора | |
| US6579857B1 (en) | Combination cancer therapy comprising adenosine and deaminase enzyme inhibitors | |
| DE60202278T2 (de) | Aktivierung natürlicher killerzellen durch adenosin-a3-rezeptoragonisten | |
| PT1385498E (pt) | Ácidos gordos como factores de sobrevivência e activação de neutrófilos | |
| Roberts et al. | Phase I study of bryostatin 1 and fludarabine in patients with chronic lymphocytic leukemia and indolent (non-Hodgkin's) lymphoma | |
| EP1272897A2 (en) | Adenosine a2a receptor antagonists for treating and preventing hepatic fibrosis, cirrhosis and fatty liver | |
| AU2001238124A1 (en) | Adenosine a2a receptor antagonists for treating and preventing hepatic fibrosis,cirrhosis and fatty liver | |
| US6638914B1 (en) | Pharmaceutical administration of adenosine agonists | |
| US6790839B2 (en) | Pharmaceutical administration of adenosine agonists | |
| US20080090848A1 (en) | Substituted Purinyl Derivatives With Immunomodulator And Chemoprotective Activity And Use Alone Or With Medium-Chain Length Fatty Acids Or Glycerides | |
| JP2020176071A (ja) | 血液がんの新規治療法及び新規治療剤 | |
| HK1118707A (en) | Pharmaceutical compositions comprising an adenosine receptor agonist or antagonist | |
| US20020037871A1 (en) | Pharmaceutical use of adenosine agonists | |
| US11957701B2 (en) | Therapy and new therapeutic agent for blood cancer | |
| Alberts et al. | Phase I trial of gemcitabine and CPT-11 given weekly for four weeks every six weeks | |
| CN101164548A (zh) | 包含腺苷受体激动剂或拮抗剂的药物组合物 | |
| HK40061085A (en) | Novel therapeutic method and novel therapeutic agent for hematological cancer |