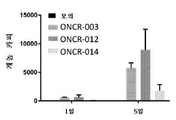
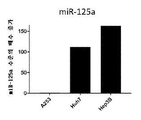
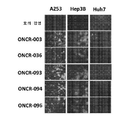
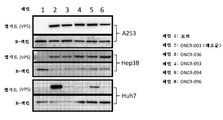
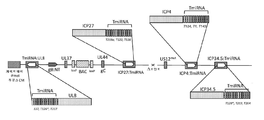
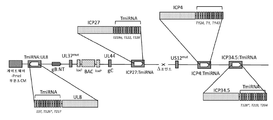
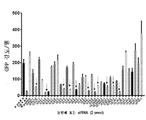
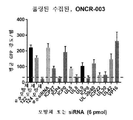
KR20200042904A - 종양용해성 바이러스 벡터 및 그의 용도 - Google Patents
종양용해성 바이러스 벡터 및 그의 용도 Download PDFInfo
- Publication number
- KR20200042904A KR20200042904A KR1020207005429A KR20207005429A KR20200042904A KR 20200042904 A KR20200042904 A KR 20200042904A KR 1020207005429 A KR1020207005429 A KR 1020207005429A KR 20207005429 A KR20207005429 A KR 20207005429A KR 20200042904 A KR20200042904 A KR 20200042904A
- Authority
- KR
- South Korea
- Prior art keywords
- mir
- virus
- tumor cell
- viral
- target sequence
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/85—Vectors or expression systems specially adapted for eukaryotic hosts for animal cells
- C12N15/86—Viral vectors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/66—Microorganisms or materials therefrom
- A61K35/76—Viruses; Subviral particles; Bacteriophages
- A61K35/763—Herpes virus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N7/00—Viruses; Bacteriophages; Compositions thereof; Preparation or purification thereof
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/14—Hydrolases (3)
- C12N9/16—Hydrolases (3) acting on ester bonds (3.1)
- C12N9/22—Ribonucleases [RNase]; Deoxyribonucleases [DNase]
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2710/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA dsDNA viruses
- C12N2710/00011—Details
- C12N2710/16011—Herpesviridae
- C12N2710/16611—Simplexvirus, e.g. human herpesvirus 1, 2
- C12N2710/16632—Use of virus as therapeutic agent, other than vaccine, e.g. as cytolytic agent
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2710/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA dsDNA viruses
- C12N2710/00011—Details
- C12N2710/16011—Herpesviridae
- C12N2710/16611—Simplexvirus, e.g. human herpesvirus 1, 2
- C12N2710/16641—Use of virus, viral particle or viral elements as a vector
- C12N2710/16643—Use of virus, viral particle or viral elements as a vector viral genome or elements thereof as genetic vector
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2710/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA dsDNA viruses
- C12N2710/00011—Details
- C12N2710/16011—Herpesviridae
- C12N2710/16611—Simplexvirus, e.g. human herpesvirus 1, 2
- C12N2710/16641—Use of virus, viral particle or viral elements as a vector
- C12N2710/16645—Special targeting system for viral vectors
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2710/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA dsDNA viruses
- C12N2710/00011—Details
- C12N2710/16011—Herpesviridae
- C12N2710/16611—Simplexvirus, e.g. human herpesvirus 1, 2
- C12N2710/16671—Demonstrated in vivo effect
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2710/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA dsDNA viruses
- C12N2710/00011—Details
- C12N2710/16011—Herpesviridae
- C12N2710/16711—Varicellovirus, e.g. human herpesvirus 3, Varicella Zoster, pseudorabies
- C12N2710/16761—Methods of inactivation or attenuation
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Genetics & Genomics (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Virology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- General Health & Medical Sciences (AREA)
- General Engineering & Computer Science (AREA)
- Microbiology (AREA)
- Biotechnology (AREA)
- Biomedical Technology (AREA)
- Medicinal Chemistry (AREA)
- Biochemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Molecular Biology (AREA)
- Epidemiology (AREA)
- Physics & Mathematics (AREA)
- Plant Pathology (AREA)
- Mycology (AREA)
- Biophysics (AREA)
- Immunology (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Peptides Or Proteins (AREA)
- Enzymes And Modification Thereof (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201762537359P | 2017-07-26 | 2017-07-26 | |
| US62/537,359 | 2017-07-26 | ||
| US201862686802P | 2018-06-19 | 2018-06-19 | |
| US62/686,802 | 2018-06-19 | ||
| PCT/US2018/043938 WO2019023483A1 (en) | 2017-07-26 | 2018-07-26 | ONCOLYTIC VIRAL VECTORS AND USES THEREOF |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20200042904A true KR20200042904A (ko) | 2020-04-24 |
Family
ID=65040409
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020207005429A Ceased KR20200042904A (ko) | 2017-07-26 | 2018-07-26 | 종양용해성 바이러스 벡터 및 그의 용도 |
Country Status (13)
| Country | Link |
|---|---|
| US (2) | US11612625B2 (enExample) |
| EP (1) | EP3658165A4 (enExample) |
| JP (2) | JP7249323B2 (enExample) |
| KR (1) | KR20200042904A (enExample) |
| CN (1) | CN111212656B (enExample) |
| AU (1) | AU2018306455A1 (enExample) |
| BR (1) | BR112020001559A2 (enExample) |
| CA (1) | CA3070299A1 (enExample) |
| IL (1) | IL271993A (enExample) |
| MX (1) | MX2020000993A (enExample) |
| RU (1) | RU2771110C2 (enExample) |
| SG (1) | SG11202000691XA (enExample) |
| WO (1) | WO2019023483A1 (enExample) |
Families Citing this family (28)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU2017212713B2 (en) * | 2016-01-27 | 2023-01-19 | Oncorus, Inc. | Oncolytic viral vectors and uses thereof |
| RU2771110C2 (ru) | 2017-07-26 | 2022-04-26 | Онкорус, Инк. | Онколитические вирусные векторы и их применение |
| EP3724334A1 (en) * | 2017-12-14 | 2020-10-21 | Unicyte EV AG | Pharmaceutical carriers containing mirnas for use in the treatment of renal cancer |
| CA3119801A1 (en) * | 2018-11-29 | 2020-06-04 | William Jia | Hsv vector with reduced neurotoxicity |
| CA3131529A1 (en) * | 2019-03-05 | 2020-09-10 | Amgen Inc. | Use of oncolytic viruses for the treatment of cancer |
| CN110283794B (zh) * | 2019-05-30 | 2021-04-23 | 伍泽堂 | 重组溶瘤病毒以及制备方法、应用和药物 |
| CA3150053A1 (en) * | 2019-08-05 | 2021-02-11 | Virogin Biotech Canada Ltd | Genetically modified enterovirus vectors |
| CN111235274A (zh) * | 2020-01-18 | 2020-06-05 | 山西医科大学第一医院 | 喉鳞癌血清外泌体标志物筛选方法和外泌体来源miR-941应用 |
| ES2856232B2 (es) * | 2020-03-25 | 2023-11-24 | Fundacion Para La Investig Biomedica Del Hospital 12 De Octubre | Biomarcadores para predecir la respuesta de un sujeto a una terapia con bcg, metodos y usos basados en los mismos |
| WO2021211902A2 (en) * | 2020-04-15 | 2021-10-21 | Humane Genomics | Artificial oncolytic viruses and related methods |
| TWI845834B (zh) * | 2020-04-23 | 2024-06-21 | 輔仁大學學校財團法人輔仁大學 | 癌症化療藥物促敏方法、其促敏劑組合物及其用途 |
| CN113583977B (zh) * | 2020-04-30 | 2025-06-06 | 杭州康万达医药科技有限公司 | 可受微小rna调控的分离的重组溶瘤痘病毒及其应用 |
| CN111494636A (zh) * | 2020-06-22 | 2020-08-07 | 中国人民解放军空军军医大学 | Mct1抑制剂的应用及含有其的药物组合物 |
| EP4179085A1 (en) * | 2020-07-07 | 2023-05-17 | Oriengene Biotechnology Ltd | Guide rna for hsv-1 gene editing and method thereof |
| US20230242994A1 (en) * | 2020-07-10 | 2023-08-03 | Shanghai Miran Biotech Co., Ltd. | Fluorescent cross-linked rnase h mutant conjugate, mirna combination and application thereof |
| US11505607B2 (en) * | 2020-11-13 | 2022-11-22 | Binhui Biopharmaceutical Co., Ltd. | Bispecific single-chain antibody, recombinant oncolytic virus for expressing same and virus composition |
| CN116744951A (zh) * | 2020-12-25 | 2023-09-12 | 苏州系统医学研究所 | 一种包含溶瘤病毒的组合物及其在肿瘤治疗中的应用 |
| WO2022151078A1 (zh) * | 2021-01-13 | 2022-07-21 | 嘉兴允英医学检验有限公司 | 溶瘤病毒及其应用 |
| JP2024509084A (ja) * | 2021-02-23 | 2024-02-29 | インターギャラクティック セラピューティクス インコーポレイテッド | 核酸ベクター及び使用方法 |
| JP2024512053A (ja) * | 2021-03-24 | 2024-03-18 | ヴァイロジン バイオテック カナダ リミテッド | 転写及び翻訳の二重調節を受ける腫瘍溶解性単純ヘルペスウイルスベクター |
| EP4395799A4 (en) * | 2021-08-31 | 2025-08-06 | Virogin Biotech Canada Ltd | GENETICALLY MODIFIED ONCOLYTIC HERPES VIRUS |
| EP4170022A1 (en) * | 2021-10-19 | 2023-04-26 | Consejo Superior De Investigaciones Científicas | Recombinant african swine fever virus and uses thereof |
| CN114010666B (zh) * | 2021-10-22 | 2024-05-07 | 上海交通大学 | 溶瘤病毒、parp抑制剂及pd-1抗体在制备抗肿瘤药物中的应用 |
| WO2023164442A2 (en) * | 2022-02-22 | 2023-08-31 | Vyro Bio Inc. | Oncolytic viruses and methods of use |
| CN117379389A (zh) * | 2022-07-12 | 2024-01-12 | 四川大学 | 血小板-溶瘤病毒复合物、其制备方法及其应用 |
| WO2024054293A1 (en) * | 2022-09-08 | 2024-03-14 | Research Institute At Nationwide Children's Hospital | Oncolytic virus combination to maximize oncolytic activity |
| AU2023409819A1 (en) * | 2022-12-20 | 2025-08-07 | Janssen Biotech, Inc. | Oncolytic virus and uses thereof |
| GB2634272A (en) * | 2023-10-04 | 2025-04-09 | Averywell Ltd | Methods |
Family Cites Families (112)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5244792A (en) | 1984-04-06 | 1993-09-14 | Chiron Corporation | Expression of recombinant glyoprotein B from herpes simplex virus |
| JPH0668B2 (ja) | 1985-08-30 | 1994-01-05 | 財団法人化学及血清療法研究所 | 単純ヘルペスウイルス遺伝子が組込まれた組換えプラスミド |
| GB8918616D0 (en) | 1989-08-15 | 1989-09-27 | Univ Glasgow | Herpes simplex virus type 1 mutant |
| US5725871A (en) | 1989-08-18 | 1998-03-10 | Danbiosyst Uk Limited | Drug delivery compositions comprising lysophosphoglycerolipid |
| US5707644A (en) | 1989-11-04 | 1998-01-13 | Danbiosyst Uk Limited | Small particle compositions for intranasal drug delivery |
| US5466468A (en) | 1990-04-03 | 1995-11-14 | Ciba-Geigy Corporation | Parenterally administrable liposome formulation comprising synthetic lipids |
| US5849571A (en) | 1990-10-10 | 1998-12-15 | University Of Pittsburgh Of The Commonwealth System Of Higher Education | Latency active herpes virus promoters and their use |
| US5849572A (en) | 1990-10-10 | 1998-12-15 | Regents Of The University Of Michigan | HSV-1 vector containing a lat promoter |
| US5399363A (en) | 1991-01-25 | 1995-03-21 | Eastman Kodak Company | Surface modified anticancer nanoparticles |
| US5756353A (en) | 1991-12-17 | 1998-05-26 | The Regents Of The University Of California | Expression of cloned genes in the lung by aerosol-and liposome-based delivery |
| ES2105262T3 (es) | 1992-05-18 | 1997-10-16 | Minnesota Mining & Mfg | Dispositivo de suministro de farmaco a traves de las mucosas. |
| US5658724A (en) | 1992-07-31 | 1997-08-19 | University Of Pittsburgh Of The Commonwealth System Of Higher Education | Herpes simplex virus strains deficient for the essential immediate early genes ICP4 and ICP27 and methods for their production, growth and use |
| US5879934A (en) | 1992-07-31 | 1999-03-09 | University Of Pittsburgh Of The Commonwealth System Of Higher Education | Herpes simplex virus strains for gene transfer |
| US5804413A (en) | 1992-07-31 | 1998-09-08 | University Of Pittsburgh Of The Commonwealth System Of Higher Education | Herpes simplex virus strains for gene transfer |
| US5543158A (en) | 1993-07-23 | 1996-08-06 | Massachusetts Institute Of Technology | Biodegradable injectable nanoparticles |
| GB9415319D0 (en) | 1994-07-29 | 1994-09-21 | Medical Res Council | HSV viral vector |
| GB9415369D0 (en) | 1994-07-29 | 1994-09-21 | Lynxvale Ltd | Mutant virus |
| IE80468B1 (en) | 1995-04-04 | 1998-07-29 | Elan Corp Plc | Controlled release biodegradable nanoparticles containing insulin |
| US6261552B1 (en) | 1997-05-22 | 2001-07-17 | University Of Pittsburgh Of The Commonwealth System Of Higher Education | Herpes simplex virus vectors |
| GB9700411D0 (en) | 1997-01-10 | 1997-02-26 | Univ London | Eukaryotic gene expression cassette and uses thereof |
| US6071742A (en) | 1997-03-05 | 2000-06-06 | Board Of Regents Of The University Of Nebraska | Coxsackie virus as a vector for delivery of anti-inflammatory cytokines |
| US5998174A (en) | 1997-05-12 | 1999-12-07 | University Of Pittsburgh Of The Commonwealth System Of Higher Education | Multigene vectors |
| AU8605598A (en) | 1997-07-31 | 1999-02-22 | University Of Pittsburgh | Targeted hsv vectors |
| NZ505500A (en) | 1997-12-17 | 2002-12-20 | Immunex Corp | Cell surface glycoproteins associated with human b cell lymphomas - ulbp, dna and polypeptides |
| GB9810423D0 (en) | 1998-05-15 | 1998-07-15 | Cancer Res Campaign Tech | Ionizing radiation or diathermy-switched gene therapy vectors and their use in antitumour therapy |
| EP1002864A1 (en) | 1998-11-10 | 2000-05-24 | Universita' degli studi di Bologna | HIgR and related V domain for the manufacture of a medicament for preventing or treating HSV-1, HSV-2 and BHV infections |
| US6897057B1 (en) | 1999-08-31 | 2005-05-24 | The General Hospital Corporation | Cell-specific and/or tumor-specific promoter retargeting of herpes γ 34.5 gene expression |
| GB9930418D0 (en) | 1999-12-22 | 2000-02-16 | Neurovex Ltd | Replication incompetent herpes virus vectors |
| ES2366608T3 (es) | 2001-03-27 | 2011-10-21 | The General Hospital Corporation | Vectores víricos y su uso en métodos terapéuticos. |
| WO2002088327A1 (en) | 2001-04-06 | 2002-11-07 | Mount Sinai School Of Medicine Of New York University | Methods for viral oncoapoptosis in cancer therapy |
| MXPA04003057A (es) | 2001-10-04 | 2005-06-20 | Immunex Corp | Proteina 4 de enlace ul16. |
| KR100900249B1 (ko) | 2001-12-07 | 2009-05-29 | 포항공과대학교 산학협력단 | SIVmac239의 면역원성 플라스미드 및 이들을 함유한AIDS DNA 백신 |
| EP1487983A4 (en) | 2002-03-01 | 2007-08-15 | Sloan Kettering Inst Cancer | PREVENTING THE REPEAT AND METASTASIS OF CANCER |
| US8927251B2 (en) | 2002-10-07 | 2015-01-06 | The University Of Chicago | Targeting of herpes simplex virus to specific receptors |
| US8741650B2 (en) | 2004-01-22 | 2014-06-03 | Dnavec Research Inc. | Methods for producing minus-strand RNA viral vectors using hybrid promoter comprising cytomegalovirus enhancer and chicken β-actin promoter |
| GB0406389D0 (en) | 2004-03-22 | 2004-04-21 | Istituto Superiore Di Sanito | Recombinant herpes simplex virus and uses therefor |
| US7473418B2 (en) | 2004-03-25 | 2009-01-06 | Cell Genesys, Inc. | Pan cancer oncolytic vectors and methods of use thereof |
| WO2006017914A1 (en) | 2004-08-20 | 2006-02-23 | Viralytics Limited | Methods and compositions for treatment of hematologic cancers |
| EP2377882A1 (en) | 2004-10-28 | 2011-10-19 | University of Pittsburgh of the Commonwealth System of Higher Education | Peripherally delivered glutamatic acid decarboxylase gene therapy for spinal cord injury pain |
| CN102166218A (zh) | 2005-01-17 | 2011-08-31 | 溶瘤病毒有限公司 | 治疗肿瘤的方法和组合物 |
| CN101287834B (zh) * | 2005-05-27 | 2016-11-09 | 圣拉法埃莱医院有限公司 | 基因载体 |
| DE602006015484D1 (de) | 2005-08-26 | 2010-08-26 | Danisco | Verwendung der crispr assoziierten gene (cas) |
| US8980246B2 (en) | 2005-09-07 | 2015-03-17 | Sillajen Biotherapeutics, Inc. | Oncolytic vaccinia virus cancer therapy |
| US20080008686A1 (en) | 2006-07-10 | 2008-01-10 | The Brigham And Women's Hospital, Inc. | Tetracycline repressor regulated oncolytic viruses |
| ZA200900787B (en) | 2006-08-11 | 2010-05-26 | Dow Agrosciences Llc | Zinc finger nuclease-mediated homologous recombination |
| US20100111873A1 (en) | 2007-02-20 | 2010-05-06 | Russell Stephen J | Treating cancer with viral nucleic acid |
| CA2687301C (en) | 2007-05-09 | 2016-08-23 | Board Of Supervisors Of Louisiana State University And Agricultural And Mechanical College | Synthetic herpes simplex viruses type-1 for treatment of cancers |
| US20080289058A1 (en) | 2007-05-14 | 2008-11-20 | University Of Pittsburgh - Of The Commonwealth System Of Higher Education | Targeted delivery of glycine receptors to excitable cells |
| WO2009111892A1 (en) | 2008-03-14 | 2009-09-17 | Ottawa Health Research Institute | Microrna mediated oncolytic targeting |
| GB0807424D0 (en) | 2008-04-23 | 2008-05-28 | Isis Innovation | Virus |
| ES2667622T3 (es) | 2008-05-29 | 2018-05-11 | Alma Mater Studiorum - Università di Bologna | Virus del herpes simple (VHS) con tropismo modificado, utilizaciones y procedimiento de preparación del mismo |
| WO2009148488A2 (en) | 2008-05-29 | 2009-12-10 | The General Hospital Corporation | Use of oncolytic herpes viruses for killing cancer stem cells |
| GB0810912D0 (en) | 2008-06-13 | 2008-07-23 | Inst Animal Health Ltd | Vector |
| US8546553B2 (en) | 2008-07-25 | 2013-10-01 | University Of Georgia Research Foundation, Inc. | Prokaryotic RNAi-like system and methods of use |
| US20100076057A1 (en) | 2008-09-23 | 2010-03-25 | Northwestern University | TARGET DNA INTERFERENCE WITH crRNA |
| WO2010054108A2 (en) | 2008-11-06 | 2010-05-14 | University Of Georgia Research Foundation, Inc. | Cas6 polypeptides and methods of use |
| RU2570562C2 (ru) | 2008-11-07 | 2015-12-10 | ДюПон НЬЮТРИШН БАЙОСАЙЕНСИЗ АпС | Последовательности crispr бифидобактерий |
| CN102625842A (zh) | 2009-03-13 | 2012-08-01 | 艾根股份有限公司 | 用于输送生物活性rna的组合物和方法 |
| US20100297072A1 (en) | 2009-05-19 | 2010-11-25 | Depinho Ronald A | Combinations of Immunostimulatory Agents, Oncolytic Virus, and Additional Anticancer Therapy |
| WO2011119925A2 (en) | 2010-03-25 | 2011-09-29 | Board Of Supervisors Of Louisiana State University And Agricultural And Mechanical College | Synthetic herpes simplex viruses for treatment of cancers |
| JP5652830B2 (ja) | 2010-04-09 | 2015-01-14 | 国立大学法人 東京大学 | マイクロrna制御組換えワクシニアウイルス及びその使用 |
| US9593347B2 (en) | 2010-04-16 | 2017-03-14 | University of Pittsburgh—of the Commonwealth System of Higher Education | Identification of mutations in herpes simplex virus envelope glycoproteins that enable or enhance vector retargeting to novel non-HSV receptors |
| WO2012006181A2 (en) | 2010-06-29 | 2012-01-12 | Mount Sinai School Of Medicine | Compositions and methods for inhibiting oncogenic micrornas and treatment of cancer |
| WO2012054726A1 (en) | 2010-10-20 | 2012-04-26 | Danisco A/S | Lactococcus crispr-cas sequences |
| SG194089A1 (en) | 2011-04-27 | 2013-11-29 | Amyris Inc | Methods for genomic modification |
| WO2012164565A1 (en) | 2011-06-01 | 2012-12-06 | Yeda Research And Development Co. Ltd. | Compositions and methods for downregulating prokaryotic genes |
| EP2543734A1 (en) | 2011-07-07 | 2013-01-09 | Deutsches Krebsforschungszentrum | Adeno-parvovirus chimera with enhanced oncolytical potential |
| PL3351261T3 (pl) | 2011-10-11 | 2021-12-06 | Universität Zürich | Lek złożony zawierający IL-12 i środek do blokowania cząsteczek hamujących komórki T w terapii nowotworów |
| US11951157B2 (en) | 2011-10-11 | 2024-04-09 | Universitat Zurich | Methods of treating malignant tumour with IL-12 and anti-PD-1 antibody |
| EP2591796A1 (en) | 2011-11-10 | 2013-05-15 | Universität Zürich | Combination medicament comprising IL-12 and an anti-CTLA-4 ligand for tumor therapy |
| US20130156808A1 (en) | 2011-11-22 | 2013-06-20 | Stipan Jonjic | Vaccine comprising beta-herpesvirus |
| GB201122458D0 (en) | 2011-12-30 | 2012-02-08 | Univ Wageningen | Modified cascade ribonucleoproteins and uses thereof |
| US20140363469A1 (en) * | 2012-01-19 | 2014-12-11 | Alnylam Pharmaceuticals, Inc. | Viral attenuation and vaccine production |
| KR20180009383A (ko) | 2012-02-24 | 2018-01-26 | 프레드 헛친슨 켄서 리서치 센터 | 이상혈색소증 치료를 위한 조성물 및 방법 |
| US9637739B2 (en) | 2012-03-20 | 2017-05-02 | Vilnius University | RNA-directed DNA cleavage by the Cas9-crRNA complex |
| WO2013141680A1 (en) | 2012-03-20 | 2013-09-26 | Vilnius University | RNA-DIRECTED DNA CLEAVAGE BY THE Cas9-crRNA COMPLEX |
| WO2014107599A2 (en) * | 2013-01-04 | 2014-07-10 | Cytomx Therapeutics, Inc. | Compositions and methods for detecting protease activity in biological systems |
| CA2915795C (en) * | 2013-06-17 | 2021-07-13 | The Broad Institute, Inc. | Delivery, use and therapeutic applications of the crispr-cas systems and compositions for targeting disorders and diseases using viral components |
| AU2014290568B2 (en) | 2013-07-17 | 2020-10-15 | University Of Pittsburgh - Of The Commonwealth System Of Higher Education | Non-toxic hsv vectors for efficient gene delivery applications and complementing cells for their production |
| PT3184641T (pt) | 2013-10-28 | 2020-08-21 | Univ Pittsburgh Commonwealth Sys Higher Education | Vetor de hsv oncolítico |
| SG11201607130RA (en) | 2014-02-27 | 2016-09-29 | Viralytics Ltd | Combination method for treatment of cancer |
| CN107073090A (zh) * | 2014-04-30 | 2017-08-18 | 哈佛学院董事会 | 结合的疫苗装置和杀死癌细胞的方法 |
| CA3190510A1 (en) | 2014-07-16 | 2016-01-21 | Transgene Sa | Oncolytic virus for expression of immune checkpoint modulators |
| EP3268034A4 (en) | 2015-03-05 | 2018-11-14 | Northwestern University | Non-neuroinvasive viruses and uses thereof |
| US10083137B2 (en) | 2015-04-02 | 2018-09-25 | Atmel Corporation | Peripheral interface circuit for serial memory |
| US9884704B2 (en) | 2015-08-04 | 2018-02-06 | Ernesto A. Aguero-Hernandez | Clip-clamp with top lock device and method |
| US10210575B1 (en) | 2015-08-12 | 2019-02-19 | State Farm Mutual Automobile Insurance Company | System and method of using an image object as a visual data container |
| WO2017059168A1 (en) * | 2015-10-01 | 2017-04-06 | Heat Biologics, Inc. | Compositions and methods for adjoining type i and type ii extracellular domains as heterologous chimeric proteins |
| JP6954648B2 (ja) | 2015-10-19 | 2021-10-27 | シージー オンコロジー, インコーポレイテッド | 併用療法による固形腫瘍又はリンパ系腫瘍の治療方法 |
| AU2016362495B2 (en) | 2015-12-02 | 2021-12-09 | Memorial Sloan-Kettering Cancer Center | Seneca valley virus (SVV) cellular receptor targeted oncotherapy |
| KR20180108590A (ko) * | 2015-12-17 | 2018-10-04 | 싸이오서스 테라퓨틱스 엘티디. | 항-tcr-복합체 항체 또는 단편을 암호화하는 바이러스 |
| EP3400293B2 (en) | 2016-01-08 | 2023-07-12 | Replimune Limited | Modified oncolytic virus |
| AU2017212713B2 (en) | 2016-01-27 | 2023-01-19 | Oncorus, Inc. | Oncolytic viral vectors and uses thereof |
| EP3426271B1 (en) | 2016-03-10 | 2025-08-27 | CG Oncology, Inc. | Methods of treating solid tumors by combination therapy |
| AU2016403583B2 (en) | 2016-04-22 | 2020-05-28 | Immvira Co., Limited | Construction of oncolytic herpes simplex viruses (oHSV) obligate vector and constructs for cancer therapy |
| EP3468588A4 (en) | 2016-06-14 | 2019-12-11 | University of Pittsburgh - of The Commonwealth System of Higher Education | EXPRESSION OF NKG2D-ACTIVATING LIGAND PROTEINS FOR THE SENSITIZATION OF CANCER CELLS FOR THE ATTACK THROUGH CYTOTOXIC IMMUNOCELLS |
| RU2021127872A (ru) * | 2016-06-30 | 2021-11-09 | Онкорус, Инк. | Доставка терапевтических полипептидов посредством псевдотипированных онколитических вирусов |
| ES2972406T3 (es) | 2016-08-01 | 2024-06-12 | Virogin Biotech Canada Ltd | Vectores del virus oncolítico del herpes simple que expresan moléculas estimuladoras del sistema inmunitario |
| JP2019527737A (ja) | 2016-08-09 | 2019-10-03 | アルケヤール, アルモハナッドALKAYYAL, Almohanad | Il12を発現する腫瘍溶解性ラブドウイルス |
| CN110214025B (zh) * | 2016-08-29 | 2024-08-02 | 阿卡米斯生物公司 | 携带双特异性t细胞衔接器的腺病毒 |
| TW201825674A (zh) | 2016-09-09 | 2018-07-16 | 美商艾斯合顧問有限公司 | 表現雙特異性接合分子的溶瘤病毒 |
| EP3534923A4 (en) | 2016-11-01 | 2020-05-27 | DNAtrix, Inc. | COMBINATION THERAPY FOR THE TREATMENT OF BRAIN CARCINOMAS |
| US11298420B2 (en) | 2016-12-21 | 2022-04-12 | Memgen, Llc | Armed oncolytic viruses |
| CN110650745A (zh) | 2016-12-21 | 2020-01-03 | 曼珍有限责任公司 | 武装复制型溶瘤腺病毒 |
| GB201700350D0 (en) | 2017-01-09 | 2017-02-22 | Replimune Ltd | Altered virus |
| US20200224220A1 (en) | 2017-07-14 | 2020-07-16 | Oncorus, Inc. | Encapsulated polynucleotides and methods of use |
| RU2771110C2 (ru) | 2017-07-26 | 2022-04-26 | Онкорус, Инк. | Онколитические вирусные векторы и их применение |
| US12263197B2 (en) | 2018-10-30 | 2025-04-01 | The University Of Tokyo | Oncolytic virus for cancer therapy |
| US20220160800A1 (en) | 2019-03-20 | 2022-05-26 | Turnstone Biologics Inc. | Methods for inducing an immune response against neoantigens |
| EP3941514A4 (en) | 2019-03-20 | 2023-08-30 | Turnstone Biologics Inc. | ONCOLYTIC VIROTHERAPY BY HETEROLOGY SEQUENTIAL BOOSTER |
| US20220380735A1 (en) | 2019-10-10 | 2022-12-01 | Oncorus, Inc. | Dual viruses and dual oncolytic viruses and methods of treatment |
| US20220409684A1 (en) | 2019-11-18 | 2022-12-29 | The Brigham And Women's Hospital, Inc. | Codon optimized new generation regulatable fusogenic oncolytic herpes simplex virus type 1 virus and methods of use |
-
2018
- 2018-07-26 RU RU2020108004A patent/RU2771110C2/ru active
- 2018-07-26 KR KR1020207005429A patent/KR20200042904A/ko not_active Ceased
- 2018-07-26 EP EP18837388.0A patent/EP3658165A4/en active Pending
- 2018-07-26 BR BR112020001559-8A patent/BR112020001559A2/pt not_active IP Right Cessation
- 2018-07-26 SG SG11202000691XA patent/SG11202000691XA/en unknown
- 2018-07-26 MX MX2020000993A patent/MX2020000993A/es unknown
- 2018-07-26 US US16/633,653 patent/US11612625B2/en active Active
- 2018-07-26 AU AU2018306455A patent/AU2018306455A1/en not_active Abandoned
- 2018-07-26 CN CN201880057565.XA patent/CN111212656B/zh active Active
- 2018-07-26 CA CA3070299A patent/CA3070299A1/en active Pending
- 2018-07-26 JP JP2020504236A patent/JP7249323B2/ja active Active
- 2018-07-26 WO PCT/US2018/043938 patent/WO2019023483A1/en not_active Ceased
-
2020
- 2020-01-12 IL IL271993A patent/IL271993A/en unknown
-
2023
- 2023-02-07 US US18/165,770 patent/US12208126B2/en active Active
- 2023-03-17 JP JP2023043321A patent/JP7652823B2/ja active Active
Also Published As
| Publication number | Publication date |
|---|---|
| JP2020532951A (ja) | 2020-11-19 |
| CN111212656B (zh) | 2024-10-18 |
| US20230241140A1 (en) | 2023-08-03 |
| MX2020000993A (es) | 2020-07-22 |
| SG11202000691XA (en) | 2020-02-27 |
| US11612625B2 (en) | 2023-03-28 |
| JP7652823B2 (ja) | 2025-03-27 |
| CA3070299A1 (en) | 2019-01-31 |
| RU2020108004A3 (enExample) | 2021-10-12 |
| US12208126B2 (en) | 2025-01-28 |
| RU2020108004A (ru) | 2021-08-26 |
| JP2023078309A (ja) | 2023-06-06 |
| CN111212656A (zh) | 2020-05-29 |
| IL271993A (en) | 2020-02-27 |
| US20200206285A1 (en) | 2020-07-02 |
| EP3658165A4 (en) | 2021-09-01 |
| WO2019023483A1 (en) | 2019-01-31 |
| JP7249323B2 (ja) | 2023-03-30 |
| AU2018306455A1 (en) | 2020-02-27 |
| BR112020001559A2 (pt) | 2020-08-11 |
| EP3658165A1 (en) | 2020-06-03 |
| RU2771110C2 (ru) | 2022-04-26 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7652823B2 (ja) | 腫瘍溶解性ウイルスベクター及びその使用 | |
| US20230115116A1 (en) | Oncolytic viral vectors and uses thereof | |
| JP5652830B2 (ja) | マイクロrna制御組換えワクシニアウイルス及びその使用 | |
| TW201842923A (zh) | 包含mir-302前驅體的組合物在製造用於肺癌治療之藥物上的用途 | |
| WO2017085175A1 (en) | Herpes simplex viruses | |
| US20250049867A1 (en) | Engineered oncolytic herpesviruses | |
| KR102679172B1 (ko) | 질환 세포-특이적인 miRNA에 의해 세포 생리 활성 조절 물질의 활성을 조절하는 복합체 및 이를 CRISPR/Cas 시스템에 적용한 질환 특이적 유전자 조작용 복합체 | |
| JP2024529400A (ja) | B型肝炎ウイルス(hbv)ノックアウト | |
| JP2023526905A (ja) | マイクロrnaで調節及び制御可能な単離された組換え腫瘍溶解性ポックスウイルス及びその使用 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20200225 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20210723 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20231128 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20240205 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20231128 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |