KR20110091594A - 라세믹 일라프라졸의 고체상 형태 - Google Patents
라세믹 일라프라졸의 고체상 형태 Download PDFInfo
- Publication number
- KR20110091594A KR20110091594A KR1020117016139A KR20117016139A KR20110091594A KR 20110091594 A KR20110091594 A KR 20110091594A KR 1020117016139 A KR1020117016139 A KR 1020117016139A KR 20117016139 A KR20117016139 A KR 20117016139A KR 20110091594 A KR20110091594 A KR 20110091594A
- Authority
- KR
- South Korea
- Prior art keywords
- burglar
- location
- racemic ilaprazole
- ilaprazole
- racemic
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 239000007790 solid phase Substances 0.000 title claims abstract description 16
- HRRXCXABAPSOCP-UHFFFAOYSA-N ilaprazole Chemical compound COC1=CC=NC(CS(=O)C=2NC3=CC(=CC=C3N=2)N2C=CC=C2)=C1C HRRXCXABAPSOCP-UHFFFAOYSA-N 0.000 title claims description 300
- 229950008491 ilaprazole Drugs 0.000 title claims description 187
- 239000008194 pharmaceutical composition Substances 0.000 claims abstract description 31
- 230000027119 gastric acid secretion Effects 0.000 claims abstract description 14
- 230000003111 delayed effect Effects 0.000 claims abstract description 8
- 239000003937 drug carrier Substances 0.000 claims abstract description 8
- 238000004482 13C cross polarization magic angle spinning Methods 0.000 claims abstract description 3
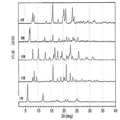
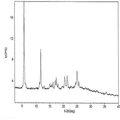
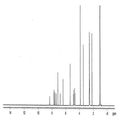
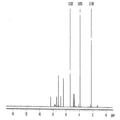
- 238000000634 powder X-ray diffraction Methods 0.000 claims description 61
- 239000013078 crystal Substances 0.000 claims description 57
- 238000000034 method Methods 0.000 claims description 34
- 230000002401 inhibitory effect Effects 0.000 claims description 4
- 239000000203 mixture Substances 0.000 abstract description 44
- 239000003814 drug Substances 0.000 abstract description 9
- 239000002253 acid Substances 0.000 abstract description 8
- 125000000475 sulfinyl group Chemical group [*:2]S([*:1])=O 0.000 abstract description 7
- 229940079593 drug Drugs 0.000 abstract description 6
- -1 4-methoxy-3-methyl-2-pyridinyl Chemical group 0.000 abstract description 5
- 208000018522 Gastrointestinal disease Diseases 0.000 abstract description 5
- HYZJCKYKOHLVJF-UHFFFAOYSA-N 1H-benzimidazole Chemical compound C1=CC=C2NC=NC2=C1 HYZJCKYKOHLVJF-UHFFFAOYSA-N 0.000 abstract description 3
- 238000002441 X-ray diffraction Methods 0.000 abstract description 2
- 150000007513 acids Chemical class 0.000 abstract 1
- 208000010643 digestive system disease Diseases 0.000 abstract 1
- 208000018685 gastrointestinal system disease Diseases 0.000 abstract 1
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 45
- 239000007787 solid Substances 0.000 description 41
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 34
- 239000007950 delayed release tablet Substances 0.000 description 20
- 238000010521 absorption reaction Methods 0.000 description 19
- 238000012937 correction Methods 0.000 description 19
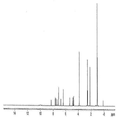
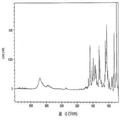
- 238000002329 infrared spectrum Methods 0.000 description 18
- 239000000243 solution Substances 0.000 description 18
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 16
- 238000001237 Raman spectrum Methods 0.000 description 16
- 238000004458 analytical method Methods 0.000 description 16
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 15
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 14
- 238000005259 measurement Methods 0.000 description 14
- 229940126409 proton pump inhibitor Drugs 0.000 description 14
- 239000000612 proton pump inhibitor Substances 0.000 description 14
- 238000001228 spectrum Methods 0.000 description 14
- 239000003826 tablet Substances 0.000 description 14
- 230000004580 weight loss Effects 0.000 description 14
- 125000004429 atom Chemical group 0.000 description 13
- 230000015572 biosynthetic process Effects 0.000 description 13
- 230000005855 radiation Effects 0.000 description 13
- 230000003595 spectral effect Effects 0.000 description 12
- 210000004027 cell Anatomy 0.000 description 11
- 238000009499 grossing Methods 0.000 description 11
- 239000000463 material Substances 0.000 description 11
- 238000000425 proton nuclear magnetic resonance spectrum Methods 0.000 description 11
- 238000005070 sampling Methods 0.000 description 11
- 238000003786 synthesis reaction Methods 0.000 description 11
- 238000011282 treatment Methods 0.000 description 11
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 10
- 238000001875 carbon-13 cross-polarisation magic angle spinning nuclear magnetic resonance spectrum Methods 0.000 description 10
- 238000001938 differential scanning calorimetry curve Methods 0.000 description 10
- 238000009472 formulation Methods 0.000 description 10
- 238000001757 thermogravimetry curve Methods 0.000 description 10
- HBAQYPYDRFILMT-UHFFFAOYSA-N 8-[3-(1-cyclopropylpyrazol-4-yl)-1H-pyrazolo[4,3-d]pyrimidin-5-yl]-3-methyl-3,8-diazabicyclo[3.2.1]octan-2-one Chemical class C1(CC1)N1N=CC(=C1)C1=NNC2=C1N=C(N=C2)N1C2C(N(CC1CC2)C)=O HBAQYPYDRFILMT-UHFFFAOYSA-N 0.000 description 9
- 208000021302 gastroesophageal reflux disease Diseases 0.000 description 9
- 230000008569 process Effects 0.000 description 9
- 238000012360 testing method Methods 0.000 description 9
- 238000002050 diffraction method Methods 0.000 description 8
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 8
- 230000003287 optical effect Effects 0.000 description 8
- 239000000843 powder Substances 0.000 description 8
- 239000002904 solvent Substances 0.000 description 8
- 238000005481 NMR spectroscopy Methods 0.000 description 7
- 239000008280 blood Substances 0.000 description 7
- 210000004369 blood Anatomy 0.000 description 7
- 201000010099 disease Diseases 0.000 description 7
- 238000004090 dissolution Methods 0.000 description 7
- 239000011521 glass Substances 0.000 description 7
- 238000004519 manufacturing process Methods 0.000 description 7
- 125000004430 oxygen atom Chemical group O* 0.000 description 7
- 230000000704 physical effect Effects 0.000 description 7
- 239000002002 slurry Substances 0.000 description 7
- 239000007909 solid dosage form Substances 0.000 description 7
- 229910052717 sulfur Inorganic materials 0.000 description 7
- 238000013268 sustained release Methods 0.000 description 7
- 239000012730 sustained-release form Substances 0.000 description 7
- 238000001069 Raman spectroscopy Methods 0.000 description 6
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 6
- 239000010949 copper Substances 0.000 description 6
- 238000005384 cross polarization magic-angle spinning Methods 0.000 description 6
- 238000013480 data collection Methods 0.000 description 6
- 210000004211 gastric acid Anatomy 0.000 description 6
- 230000002496 gastric effect Effects 0.000 description 6
- 229910052760 oxygen Inorganic materials 0.000 description 6
- 239000001301 oxygen Substances 0.000 description 6
- 239000004471 Glycine Substances 0.000 description 5
- 239000004677 Nylon Substances 0.000 description 5
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 5
- 239000004480 active ingredient Substances 0.000 description 5
- 238000001460 carbon-13 nuclear magnetic resonance spectrum Methods 0.000 description 5
- 238000002447 crystallographic data Methods 0.000 description 5
- 239000006185 dispersion Substances 0.000 description 5
- 229920001778 nylon Polymers 0.000 description 5
- 229940068196 placebo Drugs 0.000 description 5
- 239000000902 placebo Substances 0.000 description 5
- 238000002360 preparation method Methods 0.000 description 5
- 238000000926 separation method Methods 0.000 description 5
- 238000004467 single crystal X-ray diffraction Methods 0.000 description 5
- 230000001225 therapeutic effect Effects 0.000 description 5
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 4
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 4
- 208000017189 Gastrointestinal inflammatory disease Diseases 0.000 description 4
- 229920002472 Starch Polymers 0.000 description 4
- 239000000654 additive Substances 0.000 description 4
- 229910052782 aluminium Inorganic materials 0.000 description 4
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 4
- 239000007864 aqueous solution Substances 0.000 description 4
- DLGYNVMUCSTYDQ-UHFFFAOYSA-N azane;pyridine Chemical compound N.C1=CC=NC=C1 DLGYNVMUCSTYDQ-UHFFFAOYSA-N 0.000 description 4
- 230000008901 benefit Effects 0.000 description 4
- 125000003785 benzimidazolyl group Chemical group N1=C(NC2=C1C=CC=C2)* 0.000 description 4
- 230000027455 binding Effects 0.000 description 4
- 230000005540 biological transmission Effects 0.000 description 4
- 238000000576 coating method Methods 0.000 description 4
- 238000005388 cross polarization Methods 0.000 description 4
- 238000000113 differential scanning calorimetry Methods 0.000 description 4
- 239000003085 diluting agent Substances 0.000 description 4
- 229940000406 drug candidate Drugs 0.000 description 4
- 208000000718 duodenal ulcer Diseases 0.000 description 4
- 238000011049 filling Methods 0.000 description 4
- 238000002844 melting Methods 0.000 description 4
- 230000008018 melting Effects 0.000 description 4
- QSHDDOUJBYECFT-UHFFFAOYSA-N mercury Chemical compound [Hg] QSHDDOUJBYECFT-UHFFFAOYSA-N 0.000 description 4
- 229910052753 mercury Inorganic materials 0.000 description 4
- 229910052757 nitrogen Inorganic materials 0.000 description 4
- 239000002245 particle Substances 0.000 description 4
- 230000010287 polarization Effects 0.000 description 4
- 239000008107 starch Substances 0.000 description 4
- 229940032147 starch Drugs 0.000 description 4
- 235000019698 starch Nutrition 0.000 description 4
- 239000000126 substance Substances 0.000 description 4
- 238000002411 thermogravimetry Methods 0.000 description 4
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 4
- WSVLPVUVIUVCRA-KPKNDVKVSA-N Alpha-lactose monohydrate Chemical compound O.O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O WSVLPVUVIUVCRA-KPKNDVKVSA-N 0.000 description 3
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 3
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 3
- 241000282412 Homo Species 0.000 description 3
- 238000004566 IR spectroscopy Methods 0.000 description 3
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 3
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 3
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 3
- 208000007107 Stomach Ulcer Diseases 0.000 description 3
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 3
- 230000000996 additive effect Effects 0.000 description 3
- 239000011230 binding agent Substances 0.000 description 3
- 230000008033 biological extinction Effects 0.000 description 3
- 239000000872 buffer Substances 0.000 description 3
- 238000004364 calculation method Methods 0.000 description 3
- 239000002775 capsule Substances 0.000 description 3
- 238000012512 characterization method Methods 0.000 description 3
- 238000004296 chiral HPLC Methods 0.000 description 3
- 229940075614 colloidal silicon dioxide Drugs 0.000 description 3
- 239000000306 component Substances 0.000 description 3
- 150000001875 compounds Chemical class 0.000 description 3
- 238000009826 distribution Methods 0.000 description 3
- 239000002552 dosage form Substances 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- 238000010828 elution Methods 0.000 description 3
- 210000001035 gastrointestinal tract Anatomy 0.000 description 3
- 239000012535 impurity Substances 0.000 description 3
- 230000005764 inhibitory process Effects 0.000 description 3
- 230000000977 initiatory effect Effects 0.000 description 3
- 229960001375 lactose Drugs 0.000 description 3
- 239000008101 lactose Substances 0.000 description 3
- 229960001021 lactose monohydrate Drugs 0.000 description 3
- 230000007774 longterm Effects 0.000 description 3
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 3
- 235000012054 meals Nutrition 0.000 description 3
- 239000008108 microcrystalline cellulose Substances 0.000 description 3
- 229940016286 microcrystalline cellulose Drugs 0.000 description 3
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 3
- SYSQUGFVNFXIIT-UHFFFAOYSA-N n-[4-(1,3-benzoxazol-2-yl)phenyl]-4-nitrobenzenesulfonamide Chemical class C1=CC([N+](=O)[O-])=CC=C1S(=O)(=O)NC1=CC=C(C=2OC3=CC=CC=C3N=2)C=C1 SYSQUGFVNFXIIT-UHFFFAOYSA-N 0.000 description 3
- 229940021182 non-steroidal anti-inflammatory drug Drugs 0.000 description 3
- 239000012071 phase Substances 0.000 description 3
- 230000002265 prevention Effects 0.000 description 3
- 230000002829 reductive effect Effects 0.000 description 3
- 229910052710 silicon Inorganic materials 0.000 description 3
- 239000010703 silicon Substances 0.000 description 3
- FVAUCKIRQBBSSJ-UHFFFAOYSA-M sodium iodide Chemical compound [Na+].[I-] FVAUCKIRQBBSSJ-UHFFFAOYSA-M 0.000 description 3
- 238000007619 statistical method Methods 0.000 description 3
- 239000011593 sulfur Substances 0.000 description 3
- 125000004434 sulfur atom Chemical group 0.000 description 3
- 208000024891 symptom Diseases 0.000 description 3
- 239000000454 talc Substances 0.000 description 3
- 229910052623 talc Inorganic materials 0.000 description 3
- 229940033134 talc Drugs 0.000 description 3
- 235000012222 talc Nutrition 0.000 description 3
- VBICKXHEKHSIBG-UHFFFAOYSA-N 1-monostearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)CO VBICKXHEKHSIBG-UHFFFAOYSA-N 0.000 description 2
- 125000001462 1-pyrrolyl group Chemical group [*]N1C([H])=C([H])C([H])=C1[H] 0.000 description 2
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 2
- 238000012935 Averaging Methods 0.000 description 2
- 208000023665 Barrett oesophagus Diseases 0.000 description 2
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- 238000005079 FT-Raman Methods 0.000 description 2
- 238000005004 MAS NMR spectroscopy Methods 0.000 description 2
- 241000124008 Mammalia Species 0.000 description 2
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 2
- GLUUGHFHXGJENI-UHFFFAOYSA-N Piperazine Chemical compound C1CNCCN1 GLUUGHFHXGJENI-UHFFFAOYSA-N 0.000 description 2
- 102100021904 Potassium-transporting ATPase alpha chain 1 Human genes 0.000 description 2
- 108010083204 Proton Pumps Proteins 0.000 description 2
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 2
- 229930006000 Sucrose Natural products 0.000 description 2
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 2
- RAHZWNYVWXNFOC-UHFFFAOYSA-N Sulphur dioxide Chemical compound O=S=O RAHZWNYVWXNFOC-UHFFFAOYSA-N 0.000 description 2
- 239000008186 active pharmaceutical agent Substances 0.000 description 2
- 239000013543 active substance Substances 0.000 description 2
- 230000001154 acute effect Effects 0.000 description 2
- 239000000783 alginic acid Substances 0.000 description 2
- 235000010443 alginic acid Nutrition 0.000 description 2
- 229920000615 alginic acid Polymers 0.000 description 2
- 229960001126 alginic acid Drugs 0.000 description 2
- 150000004781 alginic acids Chemical class 0.000 description 2
- 238000000218 anomalous X-ray scattering Methods 0.000 description 2
- 230000002547 anomalous effect Effects 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- 150000001556 benzimidazoles Chemical class 0.000 description 2
- 239000013590 bulk material Substances 0.000 description 2
- 239000000969 carrier Substances 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 238000001514 detection method Methods 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- 239000007884 disintegrant Substances 0.000 description 2
- 238000009509 drug development Methods 0.000 description 2
- 239000002702 enteric coating Substances 0.000 description 2
- 238000009505 enteric coating Methods 0.000 description 2
- 230000029142 excretion Effects 0.000 description 2
- 235000013305 food Nutrition 0.000 description 2
- 201000000052 gastrinoma Diseases 0.000 description 2
- 239000007903 gelatin capsule Substances 0.000 description 2
- 229910052732 germanium Inorganic materials 0.000 description 2
- GNPVGFCGXDBREM-UHFFFAOYSA-N germanium atom Chemical compound [Ge] GNPVGFCGXDBREM-UHFFFAOYSA-N 0.000 description 2
- 239000003365 glass fiber Substances 0.000 description 2
- 238000000227 grinding Methods 0.000 description 2
- 230000035876 healing Effects 0.000 description 2
- 230000003993 interaction Effects 0.000 description 2
- 239000006194 liquid suspension Substances 0.000 description 2
- 230000005923 long-lasting effect Effects 0.000 description 2
- VTHJTEIRLNZDEV-UHFFFAOYSA-L magnesium dihydroxide Chemical compound [OH-].[OH-].[Mg+2] VTHJTEIRLNZDEV-UHFFFAOYSA-L 0.000 description 2
- 239000000347 magnesium hydroxide Substances 0.000 description 2
- 229910001862 magnesium hydroxide Inorganic materials 0.000 description 2
- 239000011159 matrix material Substances 0.000 description 2
- 239000011259 mixed solution Substances 0.000 description 2
- 150000007523 nucleic acids Chemical class 0.000 description 2
- 102000039446 nucleic acids Human genes 0.000 description 2
- 108020004707 nucleic acids Proteins 0.000 description 2
- 238000012856 packing Methods 0.000 description 2
- 239000000546 pharmaceutical excipient Substances 0.000 description 2
- 230000000144 pharmacologic effect Effects 0.000 description 2
- 229920001223 polyethylene glycol Polymers 0.000 description 2
- IOLCXVTUBQKXJR-UHFFFAOYSA-M potassium bromide Chemical compound [K+].[Br-] IOLCXVTUBQKXJR-UHFFFAOYSA-M 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 238000000746 purification Methods 0.000 description 2
- 239000008213 purified water Substances 0.000 description 2
- 238000011084 recovery Methods 0.000 description 2
- 230000004044 response Effects 0.000 description 2
- 229920006395 saturated elastomer Polymers 0.000 description 2
- 150000003335 secondary amines Chemical class 0.000 description 2
- 239000000779 smoke Substances 0.000 description 2
- 239000006104 solid solution Substances 0.000 description 2
- 239000012453 solvate Substances 0.000 description 2
- 238000001179 sorption measurement Methods 0.000 description 2
- 238000002336 sorption--desorption measurement Methods 0.000 description 2
- 239000003381 stabilizer Substances 0.000 description 2
- 239000005720 sucrose Substances 0.000 description 2
- 125000003375 sulfoxide group Chemical group 0.000 description 2
- 230000002459 sustained effect Effects 0.000 description 2
- 230000009885 systemic effect Effects 0.000 description 2
- 239000007916 tablet composition Substances 0.000 description 2
- 230000000699 topical effect Effects 0.000 description 2
- 239000000080 wetting agent Substances 0.000 description 2
- TZNQIYXOVOTIKQ-UHFFFAOYSA-N 2-[(4-methoxy-3-methylpyridin-2-yl)methylsulfanyl]-6-pyrrol-1-yl-1h-benzimidazole Chemical compound COC1=CC=NC(CSC=2NC3=CC=C(C=C3N=2)N2C=CC=C2)=C1C TZNQIYXOVOTIKQ-UHFFFAOYSA-N 0.000 description 1
- SUBDBMMJDZJVOS-UHFFFAOYSA-N 5-methoxy-2-{[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfinyl}-1H-benzimidazole Chemical compound N=1C2=CC(OC)=CC=C2NC=1S(=O)CC1=NC=C(C)C(OC)=C1C SUBDBMMJDZJVOS-UHFFFAOYSA-N 0.000 description 1
- 108091006112 ATPases Proteins 0.000 description 1
- 102000057290 Adenosine Triphosphatases Human genes 0.000 description 1
- 229920001817 Agar Polymers 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- 229910000809 Alumel Inorganic materials 0.000 description 1
- 239000005995 Aluminium silicate Substances 0.000 description 1
- 229920003084 Avicel® PH-102 Polymers 0.000 description 1
- 208000023514 Barrett esophagus Diseases 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- 241000282693 Cercopithecidae Species 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- 206010011224 Cough Diseases 0.000 description 1
- XDTMQSROBMDMFD-UHFFFAOYSA-N Cyclohexane Chemical compound C1CCCCC1 XDTMQSROBMDMFD-UHFFFAOYSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- 235000019739 Dicalciumphosphate Nutrition 0.000 description 1
- BWGNESOTFCXPMA-UHFFFAOYSA-N Dihydrogen disulfide Chemical compound SS BWGNESOTFCXPMA-UHFFFAOYSA-N 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 206010063655 Erosive oesophagitis Diseases 0.000 description 1
- 238000005033 Fourier transform infrared spectroscopy Methods 0.000 description 1
- 229910000530 Gallium indium arsenide Inorganic materials 0.000 description 1
- 208000007882 Gastritis Diseases 0.000 description 1
- 208000005577 Gastroenteritis Diseases 0.000 description 1
- 208000012671 Gastrointestinal haemorrhages Diseases 0.000 description 1
- 206010061459 Gastrointestinal ulcer Diseases 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 241000590002 Helicobacter pylori Species 0.000 description 1
- 239000004705 High-molecular-weight polyethylene Substances 0.000 description 1
- 102000003710 Histamine H2 Receptors Human genes 0.000 description 1
- 108090000050 Histamine H2 Receptors Proteins 0.000 description 1
- 229940122957 Histamine H2 receptor antagonist Drugs 0.000 description 1
- 240000007472 Leucaena leucocephala Species 0.000 description 1
- 235000010643 Leucaena leucocephala Nutrition 0.000 description 1
- 240000003183 Manihot esculenta Species 0.000 description 1
- 235000016735 Manihot esculenta subsp esculenta Nutrition 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- BZLVMXJERCGZMT-UHFFFAOYSA-N Methyl tert-butyl ether Chemical compound COC(C)(C)C BZLVMXJERCGZMT-UHFFFAOYSA-N 0.000 description 1
- 206010068052 Mosaicism Diseases 0.000 description 1
- 206010062501 Non-cardiac chest pain Diseases 0.000 description 1
- 206010030216 Oesophagitis Diseases 0.000 description 1
- 206010068319 Oropharyngeal pain Diseases 0.000 description 1
- 208000031481 Pathologic Constriction Diseases 0.000 description 1
- 208000008469 Peptic Ulcer Diseases 0.000 description 1
- 201000007100 Pharyngitis Diseases 0.000 description 1
- 239000004353 Polyethylene glycol 8000 Substances 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical compound [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 1
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 1
- 244000061456 Solanum tuberosum Species 0.000 description 1
- 235000002595 Solanum tuberosum Nutrition 0.000 description 1
- 229920003350 Spectratech® Polymers 0.000 description 1
- SSZBUIDZHHWXNJ-UHFFFAOYSA-N Stearinsaeure-hexadecylester Natural products CCCCCCCCCCCCCCCCCC(=O)OCCCCCCCCCCCCCCCC SSZBUIDZHHWXNJ-UHFFFAOYSA-N 0.000 description 1
- 208000025865 Ulcer Diseases 0.000 description 1
- 229910009372 YVO4 Inorganic materials 0.000 description 1
- 239000003655 absorption accelerator Substances 0.000 description 1
- 208000009956 adenocarcinoma Diseases 0.000 description 1
- 239000003463 adsorbent Substances 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 235000010419 agar Nutrition 0.000 description 1
- 235000012211 aluminium silicate Nutrition 0.000 description 1
- 150000003868 ammonium compounds Chemical class 0.000 description 1
- 238000000540 analysis of variance Methods 0.000 description 1
- 238000004164 analytical calibration Methods 0.000 description 1
- 230000001262 anti-secretory effect Effects 0.000 description 1
- 239000000010 aprotic solvent Substances 0.000 description 1
- 239000003125 aqueous solvent Substances 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 208000006673 asthma Diseases 0.000 description 1
- 239000000440 bentonite Substances 0.000 description 1
- 229910000278 bentonite Inorganic materials 0.000 description 1
- SVPXDRXYRYOSEX-UHFFFAOYSA-N bentoquatam Chemical compound O.O=[Si]=O.O=[Al]O[Al]=O SVPXDRXYRYOSEX-UHFFFAOYSA-N 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 230000002457 bidirectional effect Effects 0.000 description 1
- 238000011953 bioanalysis Methods 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 230000009141 biological interaction Effects 0.000 description 1
- 230000036765 blood level Effects 0.000 description 1
- 230000037396 body weight Effects 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- 235000010216 calcium carbonate Nutrition 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- CJZGTCYPCWQAJB-UHFFFAOYSA-L calcium stearate Chemical compound [Ca+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O CJZGTCYPCWQAJB-UHFFFAOYSA-L 0.000 description 1
- 239000008116 calcium stearate Substances 0.000 description 1
- 235000013539 calcium stearate Nutrition 0.000 description 1
- 238000004422 calculation algorithm Methods 0.000 description 1
- 239000007963 capsule composition Substances 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 229940105329 carboxymethylcellulose Drugs 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 229960000541 cetyl alcohol Drugs 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 208000013116 chronic cough Diseases 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 239000004927 clay Substances 0.000 description 1
- 238000011260 co-administration Methods 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 230000000295 complement effect Effects 0.000 description 1
- 238000013270 controlled release Methods 0.000 description 1
- 239000013256 coordination polymer Substances 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 238000005564 crystal structure determination Methods 0.000 description 1
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 description 1
- 230000001120 cytoprotective effect Effects 0.000 description 1
- 238000010908 decantation Methods 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 238000003795 desorption Methods 0.000 description 1
- 230000001627 detrimental effect Effects 0.000 description 1
- NEFBYIFKOOEVPA-UHFFFAOYSA-K dicalcium phosphate Chemical compound [Ca+2].[Ca+2].[O-]P([O-])([O-])=O NEFBYIFKOOEVPA-UHFFFAOYSA-K 0.000 description 1
- 229940038472 dicalcium phosphate Drugs 0.000 description 1
- 229910000390 dicalcium phosphate Inorganic materials 0.000 description 1
- 230000037213 diet Effects 0.000 description 1
- 235000005911 diet Nutrition 0.000 description 1
- 230000008034 disappearance Effects 0.000 description 1
- 208000035475 disorder Diseases 0.000 description 1
- 238000004821 distillation Methods 0.000 description 1
- MOTZDAYCYVMXPC-UHFFFAOYSA-N dodecyl hydrogen sulfate Chemical compound CCCCCCCCCCCCOS(O)(=O)=O MOTZDAYCYVMXPC-UHFFFAOYSA-N 0.000 description 1
- 229940043264 dodecyl sulfate Drugs 0.000 description 1
- 230000002183 duodenal effect Effects 0.000 description 1
- 230000008029 eradication Effects 0.000 description 1
- 208000006881 esophagitis Diseases 0.000 description 1
- 230000005284 excitation Effects 0.000 description 1
- 239000004744 fabric Substances 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 238000009093 first-line therapy Methods 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 239000011888 foil Substances 0.000 description 1
- 230000037406 food intake Effects 0.000 description 1
- 235000012631 food intake Nutrition 0.000 description 1
- 201000005917 gastric ulcer Diseases 0.000 description 1
- 208000015419 gastrin-producing neuroendocrine tumor Diseases 0.000 description 1
- 208000030304 gastrointestinal bleeding Diseases 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 229940014259 gelatin Drugs 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 229960001031 glucose Drugs 0.000 description 1
- 235000001727 glucose Nutrition 0.000 description 1
- YQEMORVAKMFKLG-UHFFFAOYSA-N glycerine monostearate Natural products CCCCCCCCCCCCCCCCCC(=O)OC(CO)CO YQEMORVAKMFKLG-UHFFFAOYSA-N 0.000 description 1
- SVUQHVRAGMNPLW-UHFFFAOYSA-N glycerol monostearate Natural products CCCCCCCCCCCCCCCCC(=O)OCC(O)CO SVUQHVRAGMNPLW-UHFFFAOYSA-N 0.000 description 1
- BCDGQXUMWHRQCB-UHFFFAOYSA-N glycine methyl ketone Natural products CC(=O)CN BCDGQXUMWHRQCB-UHFFFAOYSA-N 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 229940037467 helicobacter pylori Drugs 0.000 description 1
- BXWNKGSJHAJOGX-UHFFFAOYSA-N hexadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCO BXWNKGSJHAJOGX-UHFFFAOYSA-N 0.000 description 1
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 1
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 1
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 1
- 229960003943 hypromellose Drugs 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 229910052738 indium Inorganic materials 0.000 description 1
- APFVFJFRJDLVQX-UHFFFAOYSA-N indium atom Chemical compound [In] APFVFJFRJDLVQX-UHFFFAOYSA-N 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 1
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 235000012254 magnesium hydroxide Nutrition 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 238000007726 management method Methods 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 229960001855 mannitol Drugs 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 229940117841 methacrylic acid copolymer Drugs 0.000 description 1
- 229920003145 methacrylic acid copolymer Polymers 0.000 description 1
- 238000003801 milling Methods 0.000 description 1
- 230000000116 mitigating effect Effects 0.000 description 1
- 239000012046 mixed solvent Substances 0.000 description 1
- 210000004877 mucosa Anatomy 0.000 description 1
- 210000003097 mucus Anatomy 0.000 description 1
- 229940097496 nasal spray Drugs 0.000 description 1
- 239000007922 nasal spray Substances 0.000 description 1
- 229910052759 nickel Inorganic materials 0.000 description 1
- 239000002674 ointment Substances 0.000 description 1
- 229960000381 omeprazole Drugs 0.000 description 1
- 239000006186 oral dosage form Substances 0.000 description 1
- 229940041678 oral spray Drugs 0.000 description 1
- 239000000668 oral spray Substances 0.000 description 1
- RVTZCBVAJQQJTK-UHFFFAOYSA-N oxygen(2-);zirconium(4+) Chemical compound [O-2].[O-2].[Zr+4] RVTZCBVAJQQJTK-UHFFFAOYSA-N 0.000 description 1
- 210000000496 pancreas Anatomy 0.000 description 1
- 239000012188 paraffin wax Substances 0.000 description 1
- ATGAWOHQWWULNK-UHFFFAOYSA-I pentapotassium;[oxido(phosphonatooxy)phosphoryl] phosphate Chemical compound [K+].[K+].[K+].[K+].[K+].[O-]P([O-])(=O)OP([O-])(=O)OP([O-])([O-])=O ATGAWOHQWWULNK-UHFFFAOYSA-I 0.000 description 1
- 208000011906 peptic ulcer disease Diseases 0.000 description 1
- 239000005426 pharmaceutical component Substances 0.000 description 1
- 239000006187 pill Substances 0.000 description 1
- 229940068918 polyethylene glycol 400 Drugs 0.000 description 1
- 229940085678 polyethylene glycol 8000 Drugs 0.000 description 1
- 235000019446 polyethylene glycol 8000 Nutrition 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 229920006316 polyvinylpyrrolidine Polymers 0.000 description 1
- 230000002633 protecting effect Effects 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 238000005067 remediation Methods 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 239000012047 saturated solution Substances 0.000 description 1
- 230000003248 secreting effect Effects 0.000 description 1
- 238000010187 selection method Methods 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 150000004760 silicates Chemical class 0.000 description 1
- RMAQACBXLXPBSY-UHFFFAOYSA-N silicic acid Chemical compound O[Si](O)(O)O RMAQACBXLXPBSY-UHFFFAOYSA-N 0.000 description 1
- 229960004029 silicic acid Drugs 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 235000012239 silicon dioxide Nutrition 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- 239000001509 sodium citrate Substances 0.000 description 1
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 1
- 229960001790 sodium citrate Drugs 0.000 description 1
- 235000011083 sodium citrates Nutrition 0.000 description 1
- 229940023144 sodium glycolate Drugs 0.000 description 1
- 235000009518 sodium iodide Nutrition 0.000 description 1
- 235000019333 sodium laurylsulphate Nutrition 0.000 description 1
- 239000008109 sodium starch glycolate Substances 0.000 description 1
- 229940079832 sodium starch glycolate Drugs 0.000 description 1
- 229920003109 sodium starch glycolate Polymers 0.000 description 1
- 239000012265 solid product Substances 0.000 description 1
- 238000000371 solid-state nuclear magnetic resonance spectroscopy Methods 0.000 description 1
- 238000000527 sonication Methods 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 230000036262 stenosis Effects 0.000 description 1
- 208000037804 stenosis Diseases 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 229960004793 sucrose Drugs 0.000 description 1
- 208000011580 syndromic disease Diseases 0.000 description 1
- 229940124597 therapeutic agent Drugs 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- GZXOHHPYODFEGO-UHFFFAOYSA-N triglycine sulfate Chemical class NCC(O)=O.NCC(O)=O.NCC(O)=O.OS(O)(=O)=O GZXOHHPYODFEGO-UHFFFAOYSA-N 0.000 description 1
- JEJAMASKDTUEBZ-UHFFFAOYSA-N tris(1,1,3-tribromo-2,2-dimethylpropyl) phosphate Chemical compound BrCC(C)(C)C(Br)(Br)OP(=O)(OC(Br)(Br)C(C)(C)CBr)OC(Br)(Br)C(C)(C)CBr JEJAMASKDTUEBZ-UHFFFAOYSA-N 0.000 description 1
- 231100000397 ulcer Toxicity 0.000 description 1
- 238000000825 ultraviolet detection Methods 0.000 description 1
- 238000003828 vacuum filtration Methods 0.000 description 1
- 230000036642 wellbeing Effects 0.000 description 1
- 229910001928 zirconium oxide Inorganic materials 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D401/00—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom
- C07D401/14—Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms as the only ring hetero atoms, at least one ring being a six-membered ring with only one nitrogen atom containing three or more hetero rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/44—Non condensed pyridines; Hydrogenated derivatives thereof
- A61K31/4427—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems
- A61K31/4439—Non condensed pyridines; Hydrogenated derivatives thereof containing further heterocyclic ring systems containing a five-membered ring with nitrogen as a ring hetero atom, e.g. omeprazole
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Health & Medical Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Medicinal Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Epidemiology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Plural Heterocyclic Compounds (AREA)
- Medicinal Preparation (AREA)
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US87760806P | 2006-12-29 | 2006-12-29 | |
| US60/877,608 | 2006-12-29 | ||
| US88749907P | 2007-01-31 | 2007-01-31 | |
| US60/887,499 | 2007-01-31 |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020097013519A Division KR101052387B1 (ko) | 2006-12-29 | 2007-12-28 | 라세믹 일라프라졸의 고체상 형태 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20110091594A true KR20110091594A (ko) | 2011-08-11 |
Family
ID=39313094
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020117016139A Withdrawn KR20110091594A (ko) | 2006-12-29 | 2007-12-28 | 라세믹 일라프라졸의 고체상 형태 |
| KR1020097013519A Active KR101052387B1 (ko) | 2006-12-29 | 2007-12-28 | 라세믹 일라프라졸의 고체상 형태 |
| KR1020097013491A Ceased KR20090086121A (ko) | 2006-12-29 | 2007-12-28 | 용매화 일라프라졸의 결정형 |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020097013519A Active KR101052387B1 (ko) | 2006-12-29 | 2007-12-28 | 라세믹 일라프라졸의 고체상 형태 |
| KR1020097013491A Ceased KR20090086121A (ko) | 2006-12-29 | 2007-12-28 | 용매화 일라프라졸의 결정형 |
Country Status (18)
| Country | Link |
|---|---|
| US (4) | US7999110B2 (enExample) |
| EP (2) | EP2102192B1 (enExample) |
| JP (2) | JP5315253B2 (enExample) |
| KR (3) | KR20110091594A (enExample) |
| CN (2) | CN101687849B (enExample) |
| AU (2) | AU2007341984B2 (enExample) |
| CA (2) | CA2674358C (enExample) |
| DK (1) | DK2102192T3 (enExample) |
| ES (1) | ES2398846T3 (enExample) |
| IL (2) | IL199242A (enExample) |
| MX (2) | MX2009006529A (enExample) |
| MY (2) | MY145936A (enExample) |
| NO (2) | NO20092398L (enExample) |
| NZ (2) | NZ577509A (enExample) |
| PL (1) | PL2102192T3 (enExample) |
| PT (1) | PT2102192E (enExample) |
| RU (2) | RU2464270C2 (enExample) |
| WO (2) | WO2008083341A1 (enExample) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101961028B1 (ko) * | 2017-10-12 | 2019-03-21 | 중앙대학교 산학협력단 | 일라프라졸을 유효성분으로 함유하는 급성 위염의 예방 또는 치료용 약학 조성물 |
| KR102250509B1 (ko) * | 2020-12-09 | 2021-05-11 | 유니셀랩 주식회사 | 새로운 일라프라졸/자일리톨 공결정 |
| KR20210126951A (ko) * | 2020-04-13 | 2021-10-21 | 중앙대학교 산학협력단 | 일라프라졸을 유효성분으로 함유하는 장폐색증 예방 또는 치료용 약학조성물 |
Families Citing this family (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101144600B1 (ko) * | 2009-12-08 | 2012-05-16 | 일양약품주식회사 | 일라프라졸의 결정형 a, b의 제조방법 및 이들 결정형의 변환방법 |
| CN103204843B (zh) * | 2012-01-13 | 2014-12-31 | 丽珠医药集团股份有限公司 | 结晶型艾普拉唑钠乙醇化物及其制备方法 |
| CN103204842B (zh) * | 2012-01-13 | 2014-10-15 | 丽珠医药集团股份有限公司 | 结晶型艾普拉唑钠水合物及其制备方法 |
| WO2013114232A1 (en) * | 2012-02-02 | 2013-08-08 | Lupin Limited | Process for preparation of crystalline form l of ilaprazole |
| CN102746275B (zh) * | 2012-06-21 | 2014-07-16 | 丽珠医药集团股份有限公司 | 结晶型艾普拉唑钠及其制备方法 |
| CN102746277B (zh) * | 2012-06-21 | 2014-05-14 | 丽珠医药集团股份有限公司 | 一种结晶形式的艾普拉唑钠及其制备方法 |
| CN103172618B (zh) * | 2013-02-27 | 2014-09-03 | 丽珠医药集团股份有限公司 | 艾普拉唑晶型及其制备方法 |
| CN104370886B (zh) * | 2014-11-18 | 2016-05-18 | 宁夏康亚药业有限公司 | 艾普拉唑晶型及其制备方法和应用 |
| CN105055342A (zh) * | 2015-08-13 | 2015-11-18 | 青岛蓝盛洋医药生物科技有限责任公司 | 一种治疗消化性溃疡的药物艾普拉唑钠组合物冻干粉针剂 |
| CN105055343A (zh) * | 2015-08-31 | 2015-11-18 | 青岛蓝盛洋医药生物科技有限责任公司 | 一种治疗胃病的药物艾普拉唑钠组合物冻干粉针剂 |
| CN106749191A (zh) * | 2016-12-10 | 2017-05-31 | 珠海保税区丽珠合成制药有限公司 | 艾普拉唑晶型ⅱ及其制备方法 |
| KR20220065235A (ko) | 2020-11-13 | 2022-05-20 | 주식회사 파마코스텍 | 일라프라졸 혼형 결정의 신규한 제조방법 |
| KR20210019469A (ko) | 2021-02-02 | 2021-02-22 | 주식회사 파마코스텍 | 일라프라졸 나트륨 결정형 및 신규한 제조방법 |
| CN117050060A (zh) * | 2023-07-20 | 2023-11-14 | 浙江美诺华药物化学有限公司 | 一种艾普拉唑晶型f的制备方法 |
| KR20250156870A (ko) | 2024-04-25 | 2025-11-04 | 엠에프씨 주식회사 | 일라프라졸 마그네슘염 수화물의 제조방법 |
| CN119462610A (zh) * | 2024-11-06 | 2025-02-18 | 甘肃皓天科技股份有限公司 | 一种艾普拉唑新晶型及其制备方法 |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR0179401B1 (ko) * | 1994-02-28 | 1999-03-20 | 송택선 | 신규한 5-피롤릴-2-피리딜메틸설피닐벤즈이미다졸 유도체 |
| US6331539B1 (en) | 1999-06-10 | 2001-12-18 | 3M Innovative Properties Company | Sulfonamide and sulfamide substituted imidazoquinolines |
| RU2232159C2 (ru) * | 1999-07-29 | 2004-07-10 | Регентский Совет Университета Калифорнии | Производные бензимидазола и фармацевтические композиции, содержащие пролекарство ингибитора протонного насоса |
| US6627646B2 (en) * | 2001-07-17 | 2003-09-30 | Sepracor Inc. | Norastemizole polymorphs |
| AU2003224779A1 (en) | 2002-03-27 | 2003-10-13 | Teva Pharmaceutical Industries Ltd. | Lansoprazole polymorphs and processes for preparation thereof |
| ES2375298T3 (es) | 2005-03-25 | 2012-02-28 | Livzon Pharmaceutical Group Inc. | Procedimiento para preparar derivados de sulfóxido sustituidos. |
| WO2006118534A1 (en) * | 2005-05-04 | 2006-11-09 | Astrazeneca Ab | Proton pump inhibitors in the treatment of sleep disturbance due to silent gastro-esophageal reflux |
-
2007
- 2007-12-28 US US11/966,868 patent/US7999110B2/en active Active
- 2007-12-28 KR KR1020117016139A patent/KR20110091594A/ko not_active Withdrawn
- 2007-12-28 KR KR1020097013519A patent/KR101052387B1/ko active Active
- 2007-12-28 MX MX2009006529A patent/MX2009006529A/es active IP Right Grant
- 2007-12-28 MX MX2009006530A patent/MX2009006530A/es active IP Right Grant
- 2007-12-28 CN CN2007800488456A patent/CN101687849B/zh not_active Expired - Fee Related
- 2007-12-28 WO PCT/US2007/089137 patent/WO2008083341A1/en not_active Ceased
- 2007-12-28 CN CN2007800488403A patent/CN101687848B/zh active Active
- 2007-12-28 NZ NZ577509A patent/NZ577509A/en not_active IP Right Cessation
- 2007-12-28 JP JP2009544309A patent/JP5315253B2/ja active Active
- 2007-12-28 MY MYPI20092734A patent/MY145936A/en unknown
- 2007-12-28 KR KR1020097013491A patent/KR20090086121A/ko not_active Ceased
- 2007-12-28 EP EP07870091A patent/EP2102192B1/en active Active
- 2007-12-28 JP JP2009544310A patent/JP5315254B2/ja not_active Expired - Fee Related
- 2007-12-28 CA CA2674358A patent/CA2674358C/en not_active Expired - Fee Related
- 2007-12-28 ES ES07870091T patent/ES2398846T3/es active Active
- 2007-12-28 EP EP07866107A patent/EP2102191B1/en active Active
- 2007-12-28 DK DK07870091.1T patent/DK2102192T3/da active
- 2007-12-28 NZ NZ577129A patent/NZ577129A/en unknown
- 2007-12-28 WO PCT/US2007/089127 patent/WO2008083333A1/en not_active Ceased
- 2007-12-28 RU RU2009122242/04A patent/RU2464270C2/ru not_active IP Right Cessation
- 2007-12-28 PL PL07870091T patent/PL2102192T3/pl unknown
- 2007-12-28 MY MYPI20092733A patent/MY146462A/en unknown
- 2007-12-28 CA CA2674347A patent/CA2674347C/en active Active
- 2007-12-28 AU AU2007341984A patent/AU2007341984B2/en active Active
- 2007-12-28 PT PT78700911T patent/PT2102192E/pt unknown
- 2007-12-28 RU RU2009122241/04A patent/RU2466129C2/ru active
- 2007-12-28 US US11/966,896 patent/US7989632B2/en not_active Expired - Fee Related
- 2007-12-28 AU AU2007341992A patent/AU2007341992B2/en not_active Ceased
-
2009
- 2009-06-08 IL IL199242A patent/IL199242A/en not_active IP Right Cessation
- 2009-06-08 IL IL199234A patent/IL199234A/en active IP Right Grant
- 2009-06-24 NO NO20092398A patent/NO20092398L/no not_active Application Discontinuation
- 2009-06-29 NO NO20092464A patent/NO342530B1/no unknown
-
2010
- 2010-11-04 US US12/939,266 patent/US8592599B2/en not_active Expired - Fee Related
- 2010-11-04 US US12/939,276 patent/US8592600B2/en not_active Expired - Fee Related
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101961028B1 (ko) * | 2017-10-12 | 2019-03-21 | 중앙대학교 산학협력단 | 일라프라졸을 유효성분으로 함유하는 급성 위염의 예방 또는 치료용 약학 조성물 |
| KR20210126951A (ko) * | 2020-04-13 | 2021-10-21 | 중앙대학교 산학협력단 | 일라프라졸을 유효성분으로 함유하는 장폐색증 예방 또는 치료용 약학조성물 |
| KR102250509B1 (ko) * | 2020-12-09 | 2021-05-11 | 유니셀랩 주식회사 | 새로운 일라프라졸/자일리톨 공결정 |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR101052387B1 (ko) | 라세믹 일라프라졸의 고체상 형태 | |
| US20080200515A1 (en) | Solid state forms of enantiopure ilaprazole | |
| WO2017170623A1 (ja) | グリセオフルビン化合物 | |
| AU2017348598B2 (en) | Crystalline form of (1R,2R)-2-[4-(3-methyl-1H-pyrazol-5-yl)benzoyl]-N- (4-oxo-4,5,6,7-tetrahydropyrazolo[1,5-a]pyrazin-3- yl)cyclohexanecarboxamide | |
| BRPI0719624A2 (pt) | Formas de estado sólido do ilaprazol racêmico | |
| HK1140484B (en) | Solid state forms of racemic ilaprazole | |
| BRPI0719624B1 (pt) | Formas de estado sólido do ilaprazol racêmico | |
| HK1140483B (en) | Crystalline forms of solvated ilaprazole |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A107 | Divisional application of patent | ||
| PA0104 | Divisional application for international application |
Comment text: Divisional Application for International Patent Patent event code: PA01041R01D Patent event date: 20110712 |
|
| PG1501 | Laying open of application | ||
| PC1203 | Withdrawal of no request for examination | ||
| WITN | Application deemed withdrawn, e.g. because no request for examination was filed or no examination fee was paid |