JP6991857B2 - Tlr9アゴニストをチェックポイント阻害剤と共に用いるがんの治療 - Google Patents
Tlr9アゴニストをチェックポイント阻害剤と共に用いるがんの治療 Download PDFInfo
- Publication number
- JP6991857B2 JP6991857B2 JP2017519544A JP2017519544A JP6991857B2 JP 6991857 B2 JP6991857 B2 JP 6991857B2 JP 2017519544 A JP2017519544 A JP 2017519544A JP 2017519544 A JP2017519544 A JP 2017519544A JP 6991857 B2 JP6991857 B2 JP 6991857B2
- Authority
- JP
- Japan
- Prior art keywords
- cancer
- inhibitor
- immune checkpoint
- cell
- lymphoma
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
- A61K31/7115—Nucleic acids or oligonucleotides having modified bases, i.e. other than adenine, guanine, cytosine, uracil or thymine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/39—Medicinal preparations containing antigens or antibodies characterised by the immunostimulating additives, e.g. chemical adjuvants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/04—Antineoplastic agents specific for metastasis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/54—Medicinal preparations containing antigens or antibodies characterised by the route of administration
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/555—Medicinal preparations containing antigens or antibodies characterised by a specific combination antigen/adjuvant
- A61K2039/55511—Organic adjuvants
- A61K2039/55561—CpG containing adjuvants; Oligonucleotide containing adjuvants
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Medicinal Chemistry (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Dermatology (AREA)
- Biochemistry (AREA)
- Molecular Biology (AREA)
- Oncology (AREA)
- Microbiology (AREA)
- Mycology (AREA)
- Immunology (AREA)
- Hematology (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Medicinal Preparation (AREA)
Description
本出願は2014年10月10日出願の米国仮出願第62/062,274号及び2015年9月15日出願の米国仮出願第62/218,934号の優先権を主張する。上記出願(複数可)の全ての教示は参照により本明細書に援用される。
技術分野
本発明は概括的には腫瘍学の分野に関し、より詳細には、がんの治療における免疫療法の使用に関する。
用語「2’-置換ヌクレオシド」または「2’-置換アラビノシド」は一般に、ペントースまたはアラビノース部分の2’位のヒドロキシル基が置換されて2’-置換または2’-O-置換リボヌクレオシドが生じたヌクレオシドまたはアラビノヌクレオシドを含む。特定の実施形態において、かかる置換は、1~6の飽和または不飽和炭素原子を含む低級ヒドロカルビル基、ハロゲン原子または6~10の炭素原子を有するアリール基によるものであり、かかるヒドロカルビルまたはアリール基は、無置換であってもよく、または、例えば、ハロ、ヒドロキシ、トリフルオロメチル、シアノ、ニトロ、アシル、アシルオキシ、アルコキシ、カルボキシル、カルボアルコキシ、もしくはアミノ基で置換されていてもよい。2’-O-置換リボヌクレオシドまたは2’-O-置換アラビノシドの例としては、限定はされないが、2’-アミノ、2’-フルオロ、2’-アリル、2’-O-アルキル及び2’-プロパルギルリボヌクレオシドまたはアラビノシド、2’-O-メチルリボヌクレオシドまたは2’-O-メチルアラビノシドならびに2’-O-メトキシエトキシリボヌクレオシドまたは2’-O-メトキシエトキシアラビノシドが挙げられる。
本発明に係る化学物質は、自動DNA合成装置(OligoPilot II、AKTA、(アマシャム)及び/またはExpedite 8909(アプライドバイオシステム))を用いて、図1及び2に概略を示した直線型合成または並列型合成手順に従って、1μmol~0.1mMの規模で合成した。
BALB/cマウス(n=10)の右側腹部に、3×106のA20細胞を皮下注入により移植した。8日目に、2.5mg/kgのIMO-4の腫瘍内(i.t.)または皮下(s.c.)注入のいずれかによって治療を開始した。8日目、10日目、12日目及び14日目にIMO-4を与えた。プラセボ(PBS)対照及びIMO-4により治療した腫瘍を有するマウス由来の試料を、腫瘍移植後21日目に採取した。図3A及び3Bに示すように、腫瘍内投与したIMO-4は強力な抗腫瘍活性及びCD3+ TILの浸潤を誘導した。腫瘍内投与したIMO-4はまた、皮下投与と比較して、腫瘍チェックポイント発現を調節し、それによって1種または複数種のチェックポイント阻害剤との併用に対して腫瘍微小環境を感作した(データは示さず)。
BALB/cマウス(n=10)の左右の側腹部に、3×106のCT26細胞を皮下注入により移植した。8日目に、2.5mg/kgのIMO-4を用いた左側腹部における腫瘍内注入によって治療を開始した。8日目、10日目、12日目及び14日目にIMO-4を与えた。プラセボ(PBS)対照及びIMO-4により治療した腫瘍を有するマウス由来の試料を、腫瘍移植後21日目に採取した。図4に示すように、腫瘍内投与したIMO-4は治療した腫瘍小結節及び遠隔腫瘍小結節の両方において強力な抗腫瘍活性を誘導した。腫瘍内投与したIMO-4はまた、腫瘍チェックポイント発現を調節し、それによって1種または複数種のチェックポイント阻害剤との併用に対して腫瘍微小環境を感作した(データは示さず)。
BALB/cマウス(n=9)の左右の側腹部に、2×106のCT26細胞を皮下注入により移植した。7日目に、2.5mg/kgのIMO-4を用いた左側腹部における腫瘍内注入によって治療を開始した。7日目、9日目、11日目、13日目及び15日目にIMO-4を与えた。プラセボ(PBS)対照及びIMO-4により治療した腫瘍を有するマウス由来の試料を、腫瘍移植後27日目に採取した。図5に示すように、腫瘍内投与したIMO-4は治療した腫瘍小結節及び遠隔腫瘍小結節の両方において強力な抗腫瘍活性を誘導した。腫瘍内投与したIMO-4はまた、腫瘍チェックポイント発現を調節し、それによって1種または複数種のチェックポイント阻害剤との併用に対して腫瘍微小環境を感作した(データは示さず)。
BALB/cマウス(n=9)の左右の側腹部に、1×106のB16細胞を皮下注入により移植した。7日目に、2.5mg/kgのIMO-4を用いた左側腹部における腫瘍内注入によって治療を開始した。7日目、9日目、11日目、13日目及び15日目にIMO-4を与えた。プラセボ(PBS)対照及びIMO-4により治療した腫瘍を有するマウス由来の試料を、腫瘍移植後の22日目に採取した。図6に示すように、腫瘍内投与したIMO-4は治療した腫瘍小結節及び遠隔腫瘍小結節の両方において強力な抗腫瘍活性を誘導した。腫瘍内投与したIMO-4はまた、腫瘍チェックポイント発現を調節し、それによって1種または複数種のチェックポイント阻害剤との併用に対して腫瘍微小環境を感作した(データは示さず)。
BALB/cマウス(n=9)の右側腹部に、2×107のCT26細胞を皮下注入により移植した。次いで、肺転移を生じさせるために、マウスに3×106のCT26細胞を静脈内注入した。治療を5日目に開始した。2.5mg/kgのIMO-4を右側腹部のCT26固形腫瘍に腫瘍内投与し、10mg/kgの抗CTLA-4 mAbを腹腔内(i.p.)注入によって投与した。5日目、6日目、8日目及び9日目に、IMO-4または抗CTLA4 mAbのいずれかを単独で与えるか、またはこれらを同時投与した。PBS対照、IMO-4、抗CTLA-4 mAb、またはIMO-4及び抗CTLA-4 mAbで治療した、腫瘍を有するマウスの肺及び脾臓由来のT細胞を採取した。図7~9は、IMO-4及び抗CTLA-4 mAbの直接治療した腫瘍及び全身性肺転移に対する効果を示す。
BALB/cマウス(群当たりn=8)の右側腹部(腫瘍1)及び左側腹部(腫瘍2)に、1×107のマウス結腸癌CT26細胞を皮下注入により移植した。腫瘍容積が200~300mm3に達した7日目に治療を開始した。7日目、8日目、11日目及び12日目に計4回、2.5mg/kgのIMO-4(100μlのPBS中に50μg)を右側の腫瘍小結節に腫瘍内注入し、抗PD-1 mAb(10mg/kg、200μg/マウス)を腹腔内注入によって、単独で投与するかまたは同時投与するかのいずれかを行った。腫瘍結節を14日目に採取した。
BALB/cマウスの右側腹部に、1×107のB16.F10細胞を皮下注入により移植した。次いで、肺転移を生じさせるために、マウスに2×106のB16.F10細胞を静脈内注入した。5日目に治療を開始した。5mg/kgのIMO-4を右側腹部のB16固形腫瘍に腫瘍内投与し、15mg/kgの抗PD-1 mAbを腹腔内(i.p.)注入によって投与した。5日目、6日目、7日目、8日目、及び9日目に、IMO-4または抗PD-1 mAbのいずれかを単独で与えるか、またはこれらを同時投与した。対照、IMO-4、抗PD-1 mAb、またはIMO-4及び抗PD-1 mAbで治療した、腫瘍を有するマウス由来の試料を採取した。図12及び13は、IMO-4及び抗PD-1 mAbの直接治療した腫瘍及び全身性肺転移に対する効果を示す。
BALB/cマウスの右側腹部に、1×107のCT26細胞を皮下注入により移植した。次いで、肺転移を生じさせるために、マウスに3×106のCT26細胞を静脈内注入した。4日目に治療を開始した。2.5mg/kgのIMO-4を右側腹部の固形腫瘍に腫瘍内投与し、75mg/kgの抗IDO1阻害剤を経口投与(p.o.)した。4日目、5日目、7日目、及び8日目に、IMO-4またはIDO1阻害剤のいずれかを単独で与えるか、またはこれらを同時投与した。抗IDO1は2回投与した。対照、IMO-4、抗IDO1阻害剤、またはIMO-4及び抗IDO1阻害剤で治療した、腫瘍を有するマウス由来の試料を採取した。図14~17は、IMO-4及び抗IDO1阻害剤の直接治療した腫瘍及び全身性肺転移に対する効果を示す。
項1
1種または複数種のTLR9アゴニストと1種または複数種のチェックポイント阻害剤との同時投与を含み、前記1種または複数種のTLR9アゴニストが腫瘍内投与される、患者のがんの治療方法。
項2
前記1種または複数種のTLR9アゴニストと前記1種または複数種のチェックポイント阻害剤とがそれぞれ薬学上有効な量で投与される、項1に記載の方法。
項3
前記1種または複数種のTLR9アゴニストが、前記1種または複数種のチェックポイント阻害剤が前記患者に投与される前に投与される、項2に記載の方法。
項4
前記TLR9アゴニストがイムノマーである、項1に記載の方法。
項5
前記1種または複数種のチェックポイント阻害剤が、CTLA4、PD-1、PD-L1、LAG3、B7-H3、B7-H4、KIR、OX40、IgG、IDO-1、IDO-2、CEACAM1、TNFRSF4、BTLA、OX40L、及びTIM3からなる群より選択される免疫チェックポイントまたはそれらの組み合わせを標的とする、項1に記載の方法。
項6
前記1種または複数種のチェックポイント阻害剤が、CTLA-4、IDO-1、PD-L1、及びPD-1からなる群より選択される免疫チェックポイントまたはそれらの組み合わせを標的とする、項5に記載の方法。
項7
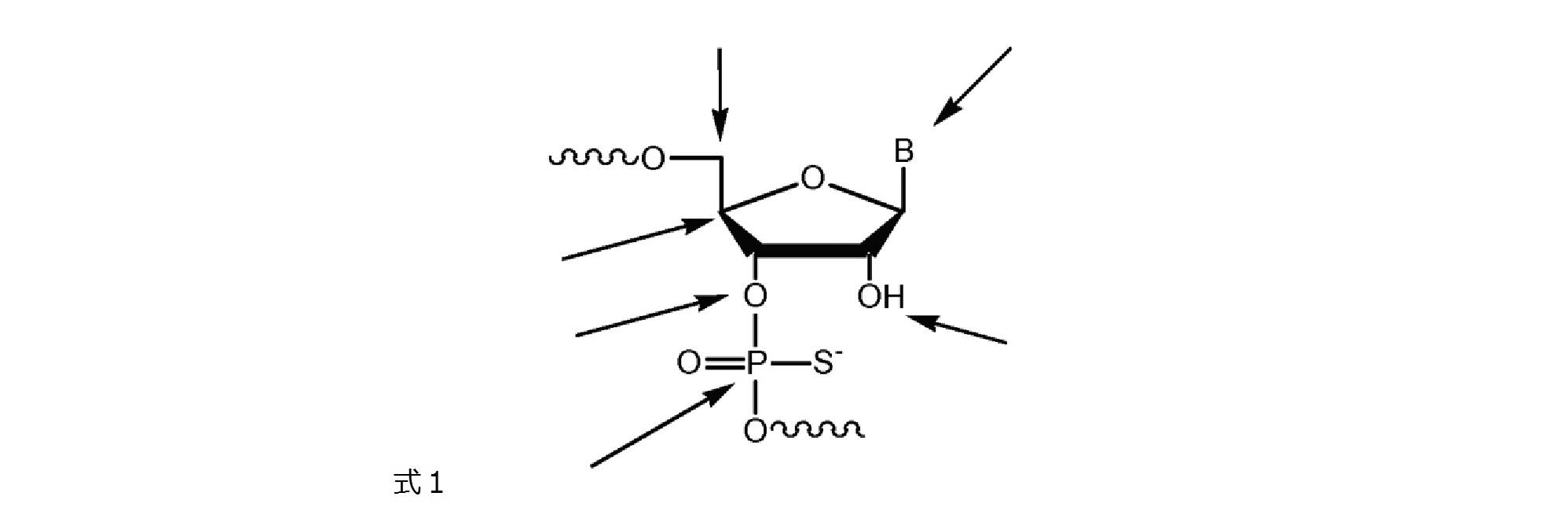
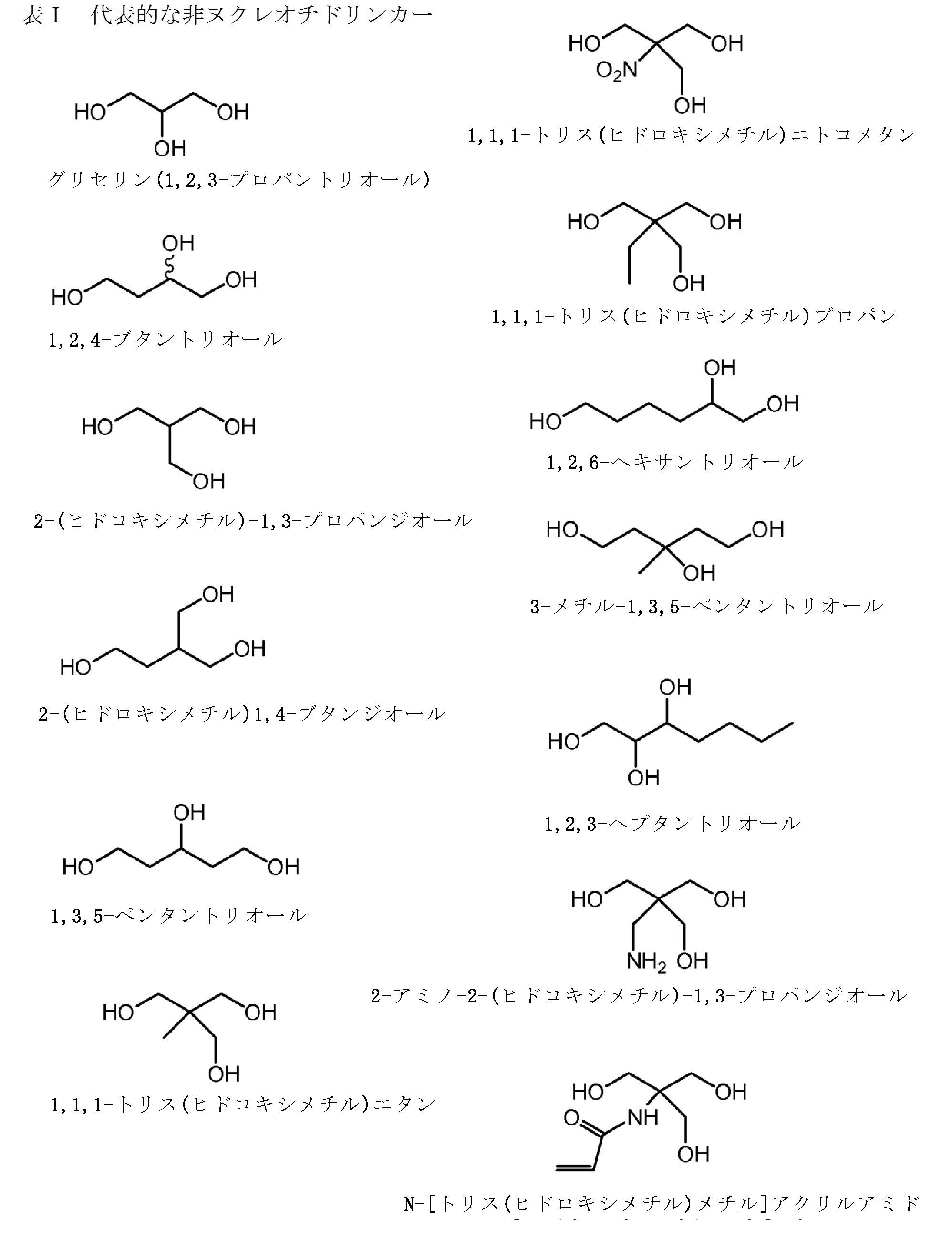
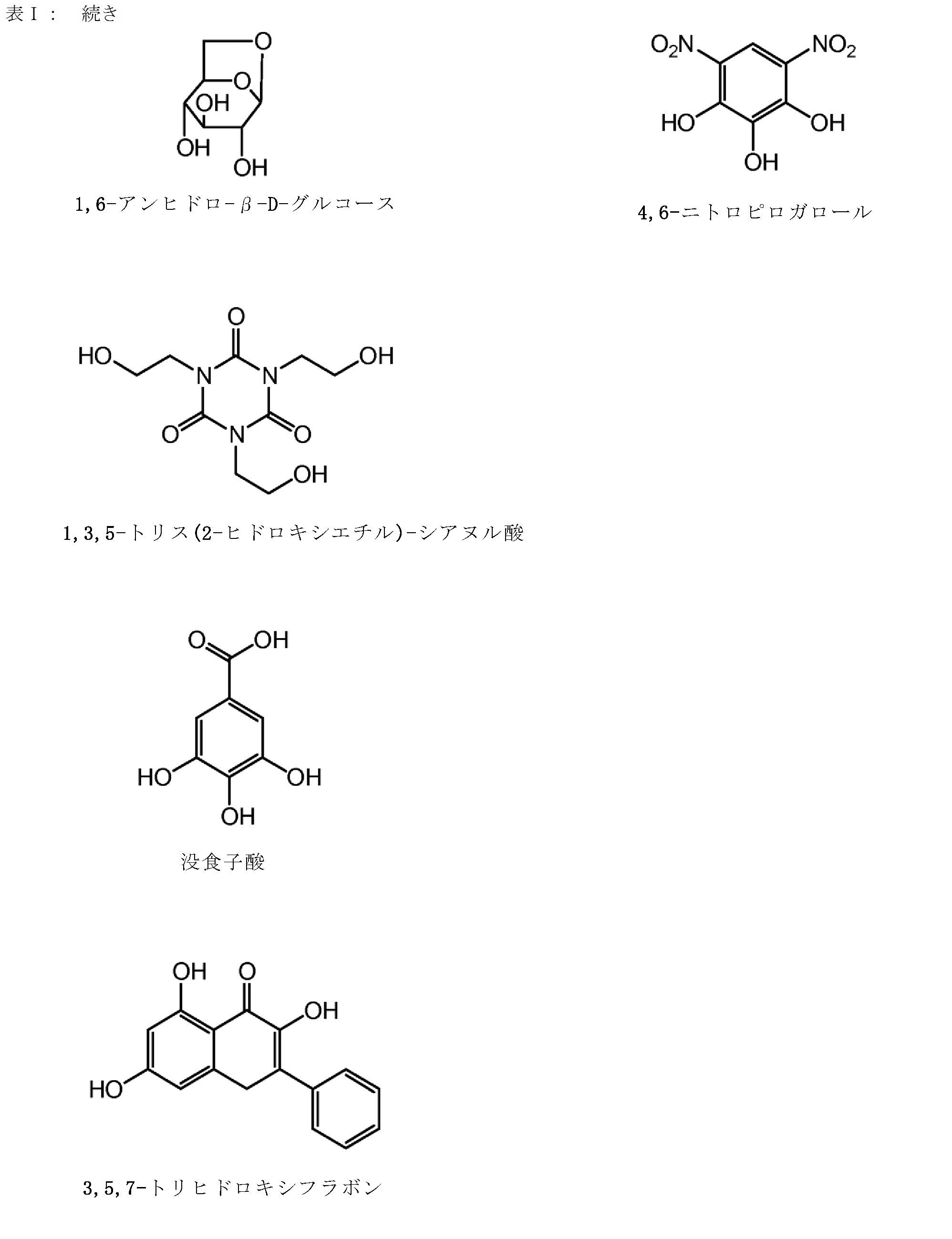
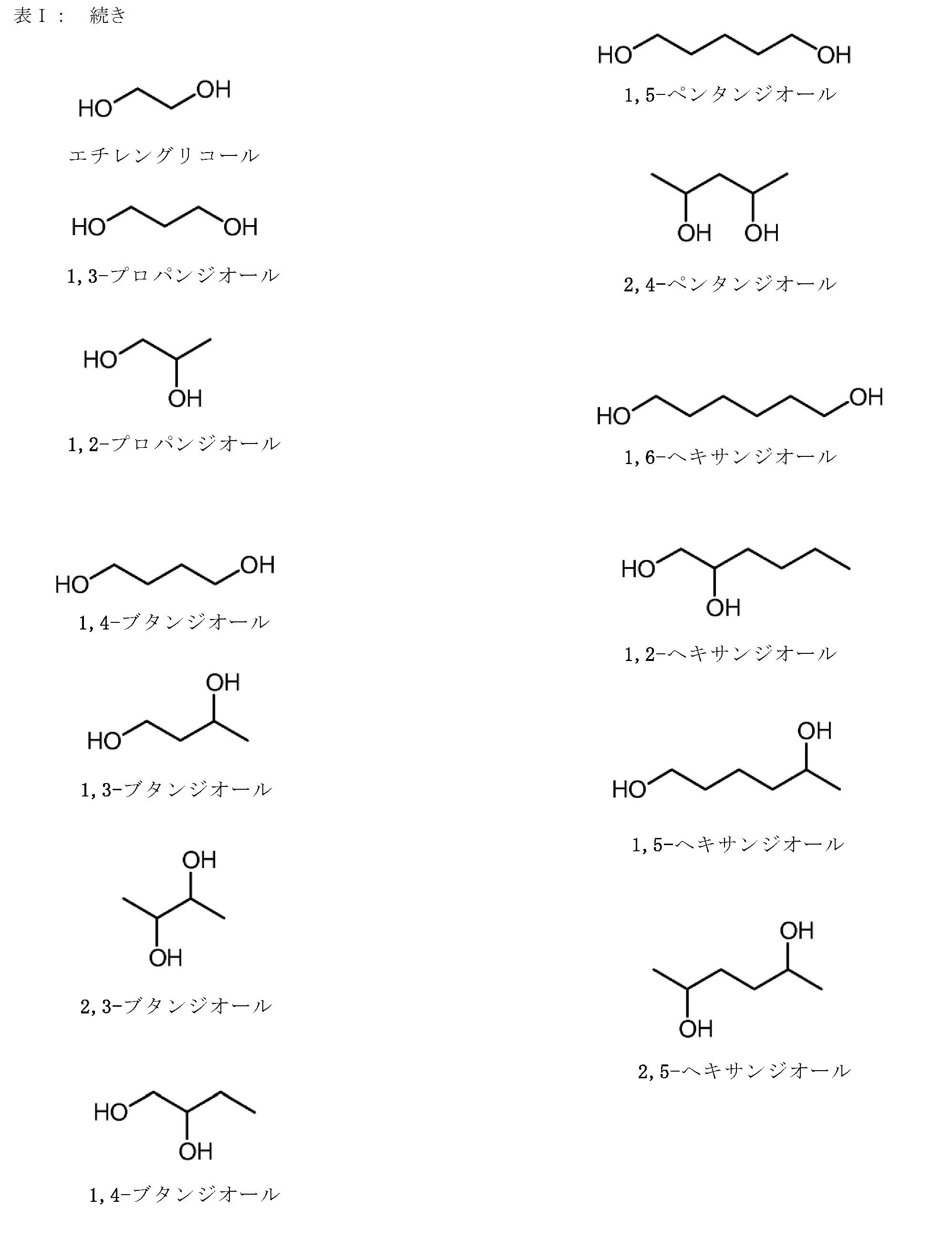
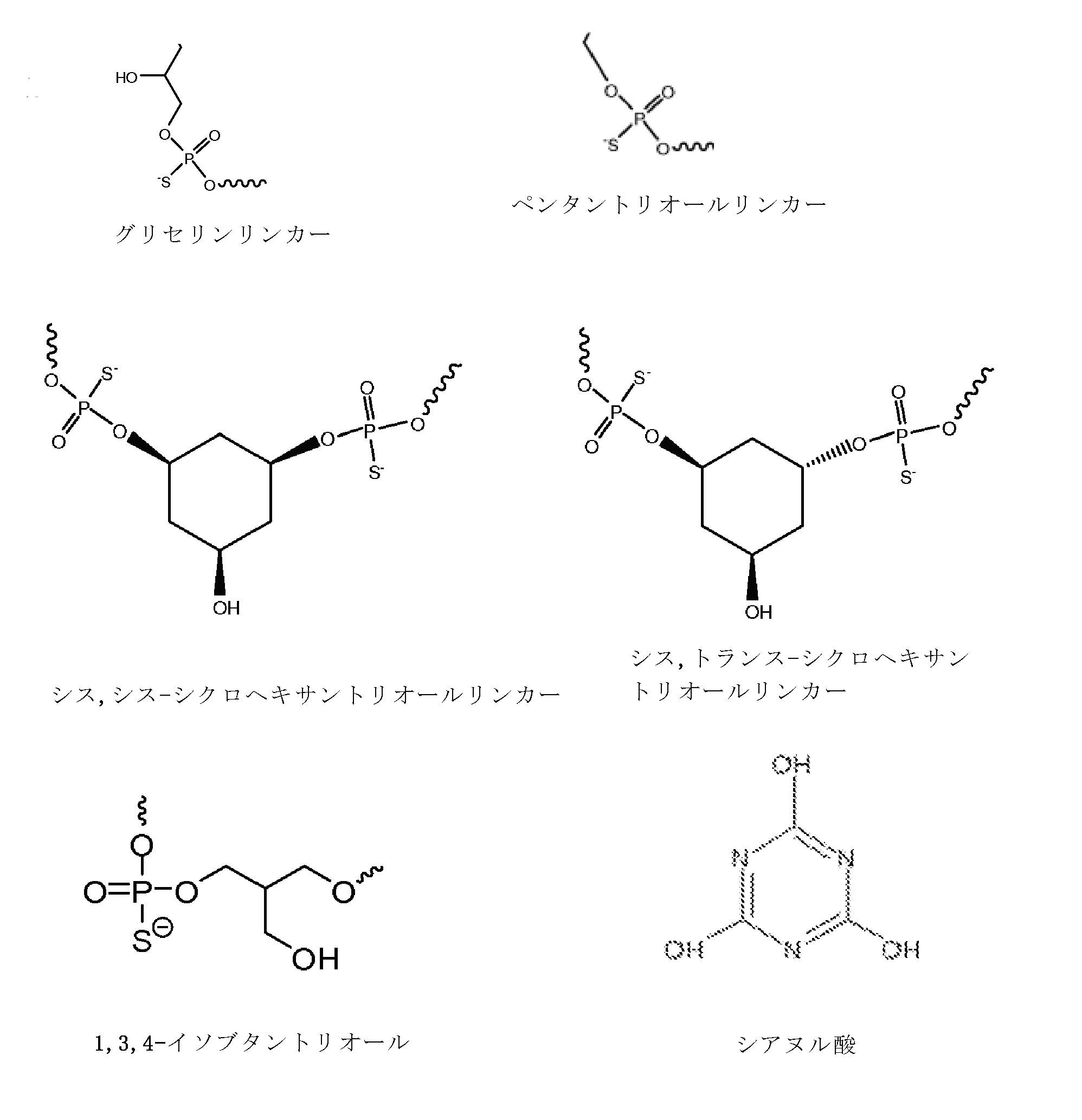
前記イムノマーが、5’-TCTGACG 1 TTCT-X-TCTTG 1 CAGTCT-5’、5’-TCTGTCG 1 TTCT-X-TCTTG 1 CTGTCT-5’、5-TCG 1 TCG 1 TTCTG-X-GTCTTG 1 CTG 1 CT-5’、5-TCG 1 AACG 1 TTCG 1 -X-G 1 CTTG 1 CAAG 1 CT-5’、5-CTGTCoG 2 TTCTC-X-CTCTTG 2 oCTGTC-5’、5-CTGTCG 2 TTCTCo-X-oCTCTTG 2 CTGTC-5’、5’-TCG 1 AACG 1 TTCG 1 -X-TCTTG 2 CTGTCT-5’、5-TCG 1 AACG 1 TTCG 1 -Y-GACAG 1 CTGTCT-5’、5-CAGTCG 2 TTCAG-X-GACTTG 2 CTGAC-5’、5-CAGTCG 1 TTCAG-X-GACTTG 1 CTGAC-5’、5’-TCG 1 AACG 1 TTCo[G]-Z-[G]oCTTG 1 CAAG 1 CT-5’、5’-TCG 1 AACG 1 TTCG 1 -Y 2 -TCTTG 1 CTGT[C]TTG 1 CT-5’、5’-TCG 1 AACG 1 TTCG 1 -Y 2 -TCTTG 1 CTG[U]CT-5’、5’-TCG 1 AACG 1 ToTCo[G]-m-[G]oCToTG 1 CAAG 1 CT-5’、5’-TCG 1 AACG 1 TTCo[G]-Y 3 -GACTTG 2 CTGAC-5’、5’-TCG 1 AACG 1 TTCG 1 -Y 4 -TGTTG 1 CTGT[C]TTG 1 CT-5’、5’-TCG 2 TCG 2 TTU 1 Y-M-YU 1 TTG 2 CTG 2 CT-5’、及び5’-CAGTCG 2 TTCAG-Y 3 -TCTTG 1 CTGTCT-5’からなる群より選択され、但し、G 1 は2’-デオキシ-7-デアザグアノシンであり、G 2 は2’-デオキシ-アラビノグアノシンであり、[G]/[C]/[U]は2’-O-メチルリボヌクレオチドであり、U 1 は2’-デオキシ-Uであり、oはホスホジエステル結合であり、Xはグリセリンリンカーであり、YはC3-リンカーであり、mはシス,トランス-1,3,5-シクロヘキサントリオールリンカーであり、Y 2 は1,3-プロパンジオールリンカーであり、Y 3 は1,4-ブタンジオールリンカーであり、Y 4 は1,5-ペンタンジオールリンカーであり、Zは1,3,5-ペンタントリオールリンカーであり、Mはシス,シス-1,3,5-シクロヘキサントリオールリンカーである、項4に記載の方法。
項8
前記イムノマーが配列5-TCG 1 AACG 1 TTCG 1 -X-G 1 CTTG 1 CAAG 1 CT-5を含み、但し、G 1 は2’-デオキシ-7-デアザグアノシンであり、Xはグリセリンリンカーである、項7に記載の方法。
項9
がんが、非ホジキンリンパ腫、B細胞リンパ腫、B細胞白血病、T細胞リンパ腫、T細胞白血病、急性リンパ球性白血病、慢性リンパ球性白血病、バーキットリンパ腫、ホジキンリンパ腫、毛状細胞白血病、急性骨髄球性白血病、慢性骨髄球性白血病、多発性骨髄腫、神経膠腫、ワルデンシュトレーム型マクログロブリン血症、癌腫、黒色腫、肉腫、神経膠腫、皮膚がん、口腔がん、消化管がん、結腸がん、胃がん、気道がん(pulmonary tract cancer)、肺がん、乳がん、卵巣がん、前立腺がん、子宮がん、子宮内膜がん、子宮頚がん、膀胱がん、膵臓がん、骨がん、肝臓がん、胆嚢がん、腎臓がん及び精巣がんからなる群より選択される、項1に記載の方法。
項10
前記がんが黒色腫である、項9に記載の方法。
項11
1種または複数種のTLR9アゴニスト、IDO1のチェックポイント阻害剤、及び1種または複数種の更なるチェックポイント阻害剤の同時投与を含み、前記1種または複数種のTLR9アゴニストが腫瘍内投与される、患者のがんの治療方法。
項12
前記1種または複数種のTLR9アゴニストと前記1種または複数種のチェックポイント阻害剤とがそれぞれ薬学上有効な量で投与される、項11に記載の方法。
項13
前記1種または複数種のTLR9アゴニストが、前記IDO1のチェックポイント阻害剤及び前記1種または複数種の更なるチェックポイント阻害剤が前記患者に投与される前に投与される、項12に記載の方法。
項14
前記TLR9アゴニストがイムノマーである、項11に記載の方法。
項15
前記1種または複数種の更なるチェックポイント阻害剤が、CTLA4、PD-1、PD-L1、LAG3、B7-H3、B7-H4、KIR、OX40、IgG、IDO-2、CEACAM1、TNFRSF4、BTLA、OX40L、及びTIM3からなる群より選択される免疫チェックポイントまたはそれらの組み合わせを標的とする、項11に記載の方法。
項16
前記1種または複数種のチェックポイント阻害剤が、CTLA-4、IDO-1、PD-L1、及びPD-1からなる群より選択される免疫チェックポイントまたはそれらの組み合わせを標的とする、項15に記載の方法。
項17
前記イムノマーが、5’-TCTGACG 1 TTCT-X-TCTTG 1 CAGTCT-5’、5’-TCTGTCG 1 TTCT-X-TCTTG 1 CTGTCT-5’、5-TCG 1 TCG 1 TTCTG-X-GTCTTG 1 CTG 1 CT-5’、5-TCG 1 AACG 1 TTCG 1 -X-G 1 CTTG 1 CAAG 1 CT-5’、5-CTGTCoG 2 TTCTC-X-CTCTTG 2 oCTGTC-5’、5-CTGTCG 2 TTCTCo-X-oCTCTTG 2 CTGTC-5’、5’-TCG 1 AACG 1 TTCG 1 -X-TCTTG 2 CTGTCT-5’、5-TCG 1 AACG 1 TTCG 1 -Y-GACAG 1 CTGTCT-5’、5-CAGTCG 2 TTCAG-X-GACTTG 2 CTGAC-5’、5-CAGTCG 1 TTCAG-X-GACTTG 1 CTGAC-5’、5’-TCG 1 AACG 1 TTCo[G]-Z-[G]oCTTG 1 CAAG 1 CT-5’、5’-TCG 1 AACG 1 TTCG 1 -Y 2 -TCTTG 1 CTGT[C]TTG 1 CT-5’、5’-TCG 1 AACG 1 TTCG 1 -Y 2 -TCTTG 1 CTG[U]CT-5’、5’-TCG 1 AACG 1 ToTCo[G]-m-[G]oCToTG 1 CAAG 1 CT-5’、5’-TCG 1 AACG 1 TTCo[G]-Y 3 -GACTTG 2 CTGAC-5’、5’-TCG 1 AACG 1 TTCG 1 -Y 4 -TGTTG 1 CTGT[C]TTG 1 CT-5’、5’-TCG 2 TCG 2 TTU 1 Y-M-YU 1 TTG 2 CTG 2 CT-5’、及び5’-CAGTCG 2 TTCAG-Y 3 -TCTTG 1 CTGTCT-5’からなる群より選択され、但し、G 1 は2’-デオキシ-7-デアザグアノシンであり、G 2 は2’-デオキシ-アラビノグアノシンであり、[G]/[C]/[U]は2’-O-メチルリボヌクレオチドであり、U 1 は2’-デオキシ-Uであり、oはホスホジエステル結合であり、Xはグリセリンリンカーであり、YはC3-リンカーであり、mはシス,トランス-1,3,5-シクロヘキサントリオールリンカーであり、Y 2 は1,3-プロパンジオールリンカーであり、Y 3 は1,4-ブタンジオールリンカーであり、Y 4 は1,5-ペンタンジオールリンカーであり、Zは1,3,5-ペンタントリオールリンカーであり、Mはシス,シス-1,3,5-シクロヘキサントリオールリンカーである、項14に記載の方法。
項18
前記イムノマーが配列5-TCG 1 AACG 1 TTCG 1 -X-G 1 CTTG 1 CAAG 1 CT-5を含み、但し、G 1 は2’-デオキシ-7-デアザグアノシンであり、Xはグリセリンリンカーである、項17に記載の方法。
項19
がんが、非ホジキンリンパ腫、B細胞リンパ腫、B細胞白血病、T細胞リンパ腫、T細胞白血病、急性リンパ球性白血病、慢性リンパ球性白血病、バーキットリンパ腫、ホジキンリンパ腫、毛状細胞白血病、急性骨髄球性白血病、慢性骨髄球性白血病、多発性骨髄腫、神経膠腫、ワルデンシュトレーム型マクログロブリン血症、癌腫、黒色腫、肉腫、神経膠腫、皮膚がん、口腔がん、消化管がん、結腸がん、胃がん、気道がん(pulmonary tract cancer)、肺がん、乳がん、卵巣がん、前立腺がん、子宮がん、子宮内膜がん、子宮頚がん、膀胱がん、膵臓がん、骨がん、肝臓がん、胆嚢がん、腎臓がん及び精巣がんからなる群より選択される、項11に記載の方法。
項20
前記がんの治療が更なる抗がん剤を投与することを含む、項1に記載の方法。
項21
前記更なる抗がん剤が、化学療法剤または放射線療法の中から選択される、項20に記載の方法。
項22
前記化学療法剤が、クロラムブシル、イホスファミド、ドキソルビシン、メサラジン、サリドマイド、レナリドマイド、テムシロリムス、エベロリムス、フルダラビン、フォスタマチニブ、パクリタキセル、ドセタキセル、オファツムマブ、リツキシマブ、デキサメタゾン、プレドニゾン、CAL-101、イブリツモマブ、トシツモマブ、ボルテゾミブ、ペントスタチン、エンドスタチン、またはそれらの組み合わせの中から選択される、項21に記載の方法。
項23
前記がんの治療が更なる抗がん剤を投与することを更に含む、項11に記載の方法。
項24
前記更なる抗がん剤が、化学療法剤または放射線療法の中から選択される、項23に記載の方法。
項25
前記化学療法剤が、クロラムブシル、イホスファミド、ドキソルビシン、メサラジン、サリドマイド、レナリドマイド、テムシロリムス、エベロリムス、フルダラビン、フォスタマチニブ、パクリタキセル、ドセタキセル、オファツムマブ、リツキシマブ、デキサメタゾン、プレドニゾン、CAL-101、イブリツモマブ、トシツモマブ、ボルテゾミブ、ペントスタチン、エンドスタチン、またはそれらの組み合わせの中から選択される、項24に記載の方法。
等価物
上述の発明は、明確さ及び理解を目的としてある程度詳細に記載してきたが、本開示を読めば、本発明及び添付の特許請求の範囲の真の範囲から逸脱することなく、形態及び詳細において、種々の変更がなされ得ることが当業者によって認識されよう。
Claims (11)
- TLR9アゴニストを含む、転移性がんの治療用組成物であって、
前記TLR9アゴニストが、下記構造:
5’-TCG1AACG1TTCG1-X-G1CTTG1CAAG1CT-5’
(式中、G1は2’-デオキシ-7-デアザグアノシンであり、Xはグリセリンリンカーである)
を有し、
抗CTLA-4モノクローナル抗体、抗PD-1モノクローナル抗体、及びそれらの組み合わせからなる群より選択される1種以上の免疫チェックポイント阻害剤と共投与され、
前記TLR9アゴニストが腫瘍内投与される、組成物。 - 前記TLR9アゴニストと、抗CTLA-4モノクローナル抗体、抗PD-1モノクローナル抗体、及びそれらの組み合わせからなる群より選択される1種以上の免疫チェックポイント阻害剤とがそれぞれ薬学上有効な量で投与される、請求項1に記載の組成物。
- 前記TLR9アゴニストが、抗CTLA-4モノクローナル抗体、抗PD-1モノクローナル抗体、及びそれらの組み合わせからなる群より選択される1種以上の免疫チェックポイント阻害剤の投与の前又は同時に投与される、請求項1又は2に記載の組成物。
- 前記転移性がんが、非ホジキンリンパ腫、B細胞リンパ腫、B細胞白血病、T細胞リンパ腫、T細胞白血病、急性リンパ球性白血病、慢性リンパ球性白血病、バーキットリンパ腫、ホジキンリンパ腫、毛状細胞白血病、急性骨髄球性白血病、慢性骨髄球性白血病、多発性骨髄腫、神経膠腫、ワルデンシュトレーム型マクログロブリン血症、癌腫、黒色腫、肉腫、神経膠腫、皮膚がん、口腔がん、消化管がん、結腸がん、胃がん、気道がん、肺がん、乳がん、卵巣がん、前立腺がん、子宮がん、子宮内膜がん、子宮頚がん、膀胱がん、膵臓がん、骨がん、肝臓がん、胆嚢がん、腎臓がん及び精巣がんからなる群より選択される、請求項1~3のいずれかに記載の組成物。
- 前記転移性がんが黒色腫である、請求項1~4のいずれかに記載の組成物。
- 前記転移性がんの治療が更なる抗がん剤の投与を更に含む、請求項1~5のいずれかに記載の組成物。
- 前記転移性がんの治療が、化学療法剤投与または放射線療法を更に含む、請求項6に記載の組成物。
- 前記化学療法剤が、クロラムブシル、イホスファミド、ドキソルビシン、メサラジン、サリドマイド、レナリドマイド、テムシロリムス、エベロリムス、フルダラビン、フォスタマチニブ、パクリタキセル、ドセタキセル、オファツムマブ、リツキシマブ、デキサメタゾン、プレドニゾン、CAL-101、イブリツモマブ、トシツモマブ、ボルテゾミブ、ペントスタチン、エンドスタチン、及びそれらの組み合わせから選択される、請求項7に記載の組成物。
- 前記TLR9アゴニストが、抗CTLA-4モノクローナル抗体及び抗PD-1モノクローナル抗体と共投与される、請求項1に記載の組成物。
- 前記TLR9アゴニストが、抗CTLA-4モノクローナル抗体と共投与される、請求項1に記載の組成物。
- 前記TLR9アゴニストが、抗PD-1モノクローナル抗体と共投与される、請求項1に記載の組成物。
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2021149233A JP2022000437A (ja) | 2014-10-10 | 2021-09-14 | Tlr9アゴニストをチェックポイント阻害剤と共に用いるがんの治療 |
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201462062274P | 2014-10-10 | 2014-10-10 | |
| US62/062,274 | 2014-10-10 | ||
| US201562218934P | 2015-09-15 | 2015-09-15 | |
| US62/218,934 | 2015-09-15 | ||
| PCT/US2015/054899 WO2016057898A1 (en) | 2014-10-10 | 2015-10-09 | Treatment of cancer using tlr9 agonist with checkpoint inhibitors |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2021149233A Division JP2022000437A (ja) | 2014-10-10 | 2021-09-14 | Tlr9アゴニストをチェックポイント阻害剤と共に用いるがんの治療 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2017531664A JP2017531664A (ja) | 2017-10-26 |
| JP2017531664A5 JP2017531664A5 (ja) | 2018-11-15 |
| JP6991857B2 true JP6991857B2 (ja) | 2022-01-13 |
Family
ID=55653841
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2017519544A Expired - Fee Related JP6991857B2 (ja) | 2014-10-10 | 2015-10-09 | Tlr9アゴニストをチェックポイント阻害剤と共に用いるがんの治療 |
| JP2021149233A Pending JP2022000437A (ja) | 2014-10-10 | 2021-09-14 | Tlr9アゴニストをチェックポイント阻害剤と共に用いるがんの治療 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2021149233A Pending JP2022000437A (ja) | 2014-10-10 | 2021-09-14 | Tlr9アゴニストをチェックポイント阻害剤と共に用いるがんの治療 |
Country Status (11)
| Country | Link |
|---|---|
| US (3) | US20160101128A1 (ja) |
| EP (2) | EP4029508A1 (ja) |
| JP (2) | JP6991857B2 (ja) |
| KR (1) | KR20170072244A (ja) |
| CN (2) | CN106999574B (ja) |
| AU (2) | AU2015330731B2 (ja) |
| CA (1) | CA2964155A1 (ja) |
| ES (1) | ES2908056T3 (ja) |
| IL (1) | IL251669B2 (ja) |
| MX (2) | MX391478B (ja) |
| WO (1) | WO2016057898A1 (ja) |
Families Citing this family (35)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| NZ753024A (en) | 2010-06-03 | 2020-08-28 | Pharmacyclics Llc | The use of inhibitors of bruton’s tyrosine kinase (btk) |
| CA2879570A1 (en) | 2012-07-24 | 2014-01-30 | Pharmacyclics, Inc. | Mutations associated with resistance to inhibitors of bruton's tyrosine kinase (btk) |
| CA2889182A1 (en) * | 2012-10-26 | 2014-05-01 | The University Of Chicago | Synergistic combination of immunologic inhibitors for the treatment of cancer |
| KR20160042917A (ko) | 2013-07-25 | 2016-04-20 | 엑시큐어, 인크. | 예방 및 치료 용도를 위한 면역자극제로서의 구형 핵산-기재 구축물 |
| WO2016095115A1 (en) * | 2014-12-17 | 2016-06-23 | Qualcomm Incorporated | Handover using dual active connections |
| WO2016138278A2 (en) | 2015-02-27 | 2016-09-01 | Idera Pharmaceuticals, Inc. | Compositions for inhibiting checkpoint gene expression and uses thereof |
| CN115109158A (zh) | 2015-05-07 | 2022-09-27 | 阿吉纳斯公司 | 抗ox40抗体及其使用方法 |
| EP3892284B1 (en) | 2015-05-29 | 2024-05-22 | Merck Sharp & Dohme LLC | Combination of a pd-1 antagonist and a cpg-c type oligonucleotide for treating cancer |
| USD805004S1 (en) | 2015-07-01 | 2017-12-12 | Amigo Mobility International, Inc. | Brake release handle |
| CN108368170B (zh) | 2015-07-13 | 2022-04-15 | 西托姆克斯治疗公司 | 抗pd-1抗体、可活化抗pd-1抗体及其使用方法 |
| CA3007233A1 (en) | 2015-12-02 | 2017-06-08 | Agenus Inc. | Antibodies and methods of use thereof |
| EP3452600A4 (en) * | 2016-05-06 | 2020-01-15 | Exicure, Inc. | SPHERIC NUCLEIC ACIDS TOWARDS TLR9 WITH STRONG ANTITUM ORACTIVITY |
| MY195089A (en) | 2016-05-27 | 2023-01-10 | Agenus Inc | Anti-Tim-3 Antibodies and Methods of use thereof |
| US11364304B2 (en) | 2016-08-25 | 2022-06-21 | Northwestern University | Crosslinked micellar spherical nucleic acids |
| JP7262774B2 (ja) | 2016-09-13 | 2023-04-24 | ノース カロライナ ステート ユニバーシティ | 治療剤送達のための血小板組成物及び方法 |
| US10463686B2 (en) * | 2016-09-15 | 2019-11-05 | Idera Pharmaceuticals, Inc. | Immune modulation with TLR9 agonists for cancer treatment |
| AU2017340913A1 (en) * | 2016-10-07 | 2019-04-18 | Abraxis Bioscience, Llc | Methods of treating biliary tract cancer |
| IL265800B2 (en) | 2016-10-11 | 2023-10-01 | Agenus Inc | Anti-LAG-3 antibodies and methods of using them |
| EP3538152A4 (en) | 2016-11-09 | 2020-09-30 | Agenus Inc. | ANTI-OX40 ANTIBODIES, ANTI-GITR ANTIBODIES, AND PROCESSES FOR USE |
| US20180271996A1 (en) | 2017-02-28 | 2018-09-27 | Mersana Therapeutics, Inc. | Combination therapies of her2-targeted antibody-drug conjugates |
| PT3600281T (pt) | 2017-03-23 | 2023-09-04 | QBiotics Pty Ltd | Terapia combinada para o tratamento ou prevenção de tumores |
| US11696954B2 (en) | 2017-04-28 | 2023-07-11 | Exicure Operating Company | Synthesis of spherical nucleic acids using lipophilic moieties |
| CN109893654B (zh) * | 2017-12-11 | 2021-07-27 | 江苏恒瑞医药股份有限公司 | Vegfr抑制剂治疗肿瘤的方法 |
| JP2021506968A (ja) * | 2017-12-18 | 2021-02-22 | デビオファーム・インターナショナル・エス・アーDebiopharm International S.A. | 癌を治療するためのアゴニスト抗PD−1抗体とGnRHアゴニスト又はアンタゴニストとの併用 |
| TW202011959A (zh) | 2018-04-14 | 2020-04-01 | 美商戴納瓦克斯技術公司 | 用於治療癌症之包括CpG-C 型寡核苷酸及組蛋白去乙醯酶抑制劑之組合 |
| EP3566718A1 (en) | 2018-05-07 | 2019-11-13 | Universitätsmedizin der Johannes Gutenberg-Universität Mainz | A pharmazeutical combination (treg depleting agent, checkpoint inhibitor, tlr9 agonist) for use in the treatment of cancer |
| DK3846824T3 (da) * | 2018-09-07 | 2025-09-22 | Massachusetts Gen Hospital | Sammensætninger og fremgangsmåder til immun-checkpoint-hæmning |
| TW202027792A (zh) * | 2018-09-29 | 2020-08-01 | 大陸商江蘇恆瑞醫藥股份有限公司 | Tlr激動劑與免疫檢查點抑制劑聯合在製備治療腫瘤的藥物中的用途 |
| EP3866849A4 (en) * | 2018-10-18 | 2022-10-05 | Idera Pharmaceuticals, Inc. | TLR9 MODULATORS FOR TREATMENT OF CANCER |
| WO2020128893A1 (en) * | 2018-12-21 | 2020-06-25 | Pfizer Inc. | Combination treatments of cancer comprising a tlr agonist |
| CN111057762B (zh) * | 2019-11-28 | 2022-11-08 | 中国人民解放军陆军军医大学第一附属医院 | 检测基因在制备检测食管鳞癌对顺铂敏感性的制剂中的用途 |
| KR102698700B1 (ko) * | 2020-07-03 | 2024-08-27 | 주식회사 레모넥스 | Rna 및 이를 포함하는 핵산 전달체 |
| DE112021004970T5 (de) * | 2020-09-22 | 2023-07-06 | Trisalus Life Sciences, Inc. | Krebstherapie unter verwendung von toll-like-rezeptor-agonisten |
| US11898147B2 (en) * | 2021-09-06 | 2024-02-13 | National Health Research Institutes | CpG-oligodeoxynucleotide compounds in combination with immune modulators for cancer immunotherapy |
| JP2025509266A (ja) | 2022-03-07 | 2025-04-11 | メルサナ セラピューティクス インコーポレイテッド | Stingアゴニストを含む抗体薬物コンジュゲート、組み合わせ、および使用方法 |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2010512421A (ja) | 2006-12-12 | 2010-04-22 | イデラ ファーマシューティカルズ インコーポレイテッド | Tlr9の合成アゴニスト |
| JP2010535242A (ja) | 2007-08-01 | 2010-11-18 | イデラ ファーマシューティカルズ インコーポレイテッド | Tlr9の新規な合成アゴニスト |
| US20130142815A1 (en) | 2010-02-09 | 2013-06-06 | Georiga Health Sciences Univ. Research Inst., Inc. | Alpha-methyl-tryptophan as an inhibitor of indoleamine dioxygenase |
Family Cites Families (100)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3512676A (en) * | 1967-08-07 | 1970-05-19 | Wsr Inc | Container end structure |
| US6303121B1 (en) | 1992-07-30 | 2001-10-16 | Advanced Research And Technology | Method of using human receptor protein 4-1BB |
| US6355476B1 (en) | 1988-11-07 | 2002-03-12 | Advanced Research And Technologyinc | Nucleic acid encoding MIP-1α Lymphokine |
| US6362325B1 (en) | 1988-11-07 | 2002-03-26 | Advanced Research And Technology Institute, Inc. | Murine 4-1BB gene |
| US5149797A (en) | 1990-02-15 | 1992-09-22 | The Worcester Foundation For Experimental Biology | Method of site-specific alteration of rna and production of encoded polypeptides |
| US5851795A (en) | 1991-06-27 | 1998-12-22 | Bristol-Myers Squibb Company | Soluble CTLA4 molecules and uses thereof |
| US6346614B1 (en) | 1992-07-23 | 2002-02-12 | Hybridon, Inc. | Hybrid oligonucleotide phosphorothioates |
| TW244371B (ja) | 1992-07-23 | 1995-04-01 | Tri Clover Inc | |
| US5652355A (en) | 1992-07-23 | 1997-07-29 | Worcester Foundation For Experimental Biology | Hybrid oligonucleotide phosphorothioates |
| US6084067A (en) | 1993-07-26 | 2000-07-04 | Dana-Farber Cancer Institute | CTLA4/CD28 ligands and uses therefor |
| US5811097A (en) | 1995-07-25 | 1998-09-22 | The Regents Of The University Of California | Blockade of T lymphocyte down-regulation associated with CTLA-4 signaling |
| US5855887A (en) | 1995-07-25 | 1999-01-05 | The Regents Of The University Of California | Blockade of lymphocyte down-regulation associated with CTLA-4 signaling |
| US6051227A (en) | 1995-07-25 | 2000-04-18 | The Regents Of The University Of California, Office Of Technology Transfer | Blockade of T lymphocyte down-regulation associated with CTLA-4 signaling |
| US5898031A (en) | 1996-06-06 | 1999-04-27 | Isis Pharmaceuticals, Inc. | Oligoribonucleotides for cleaving RNA |
| US5912332A (en) | 1996-07-26 | 1999-06-15 | Hybridon, Inc. | Affinity-based purification of oligonucleotides using soluble multimeric oligonucleotides |
| US6113881A (en) | 1996-09-20 | 2000-09-05 | Helene Curtis, Inc. | Hair styling mousse compositions comprising carboxylated polyurethane resins |
| PL188749B1 (pl) | 1996-10-11 | 2005-04-29 | Bristol Myers Squibb Co | Zastosowanie przeciwciała anty-4-1BB i kompozycjaleku |
| WO1998042752A1 (en) | 1997-03-21 | 1998-10-01 | Brigham And Women's Hospital Inc. | Immunotherapeutic ctla-4 binding peptides |
| US6506559B1 (en) | 1997-12-23 | 2003-01-14 | Carnegie Institute Of Washington | Genetic inhibition by double-stranded RNA |
| SI1141028T1 (sl) | 1998-12-23 | 2010-05-31 | Pfizer | Človeška monoklonska protitelesa proti CTLA |
| US7109003B2 (en) | 1998-12-23 | 2006-09-19 | Abgenix, Inc. | Methods for expressing and recovering human monoclonal antibodies to CTLA-4 |
| EE05627B1 (et) | 1998-12-23 | 2013-02-15 | Pfizer Inc. | CTLA-4 vastased inimese monoklonaalsed antikehad |
| FR2795937B1 (fr) | 1999-07-09 | 2001-09-14 | Air Liquide | Machine de croutage en ligne |
| DK1210428T3 (en) | 1999-08-23 | 2015-06-15 | Dana Farber Cancer Inst Inc | PD-1, a receptor for B7-4 AND USE THEREOF |
| JP4093757B2 (ja) | 1999-08-24 | 2008-06-04 | メダレックス, インコーポレイテッド | ヒトctla−4抗体およびその使用 |
| US7605238B2 (en) | 1999-08-24 | 2009-10-20 | Medarex, Inc. | Human CTLA-4 antibodies and their uses |
| US20040214783A1 (en) * | 2002-05-08 | 2004-10-28 | Terman David S. | Compositions and methods for treatment of neoplastic disease |
| DE19941311C1 (de) | 1999-08-31 | 2001-06-07 | Cryoelectra Ges Fuer Kryoelek | Bandfilter |
| AU1086501A (en) | 1999-10-15 | 2001-04-30 | Carnegie Institution Of Washington | Rna interference pathway genes as tools for targeted genetic interference |
| US7034121B2 (en) | 2000-01-27 | 2006-04-25 | Genetics Institue, Llc | Antibodies against CTLA4 |
| IL151928A0 (en) | 2000-03-30 | 2003-04-10 | Whitehead Biomedical Inst | Rna sequence-specific mediators of rna interference |
| BRPI0115814B8 (pt) | 2000-12-01 | 2021-05-25 | Europaeisches Laboratorium Fuer Molekularbiologie Embl | moléculas de rna de filamento duplo, seu método de preparação e composição farmacêutica compreendendo as mesmas |
| AR036993A1 (es) | 2001-04-02 | 2004-10-20 | Wyeth Corp | Uso de agentes que modulan la interaccion entre pd-1 y sus ligandos en la submodulacion de respuestas inmunologicas |
| EP1445264B1 (en) | 2001-07-31 | 2011-09-14 | Ono Pharmaceutical Co., Ltd. | Substance specific to pd-1 |
| WO2003086459A1 (en) | 2002-04-12 | 2003-10-23 | Medarex, Inc. | Methods of treatement using ctla-4 antibodies |
| EP2243493A1 (en) | 2002-07-03 | 2010-10-27 | Ono Pharmaceutical Co., Ltd. | Immunopotentiative composition |
| US6887673B2 (en) | 2002-07-30 | 2005-05-03 | Bristol-Myers Squibb Company | Humanized antibodies against human 4-1BB |
| MXPA05004022A (es) | 2002-10-17 | 2005-10-05 | Genmab As | Anticuerpos monoclonales humanos contra cd20. |
| US8039443B2 (en) | 2002-11-21 | 2011-10-18 | Archemix Corporation | Stabilized aptamers to platelet derived growth factor and their use as oncology therapeutics |
| BR0316880A (pt) | 2002-12-23 | 2005-10-25 | Wyeth Corp | Anticorpos contra pd-1 e usos dos mesmos |
| US7563869B2 (en) | 2003-01-23 | 2009-07-21 | Ono Pharmaceutical Co., Ltd. | Substance specific to human PD-1 |
| PL1897548T5 (pl) | 2003-02-28 | 2025-03-17 | The Johns Hopkins University | Regulacja komórek T |
| AU2004220036A1 (en) | 2003-03-12 | 2004-09-23 | Duke University | Oligonucleotide mimetics |
| US20050250106A1 (en) | 2003-04-24 | 2005-11-10 | David Epstein | Gene knock-down by intracellular expression of aptamers |
| CN100482673C (zh) * | 2003-07-15 | 2009-04-29 | 艾德拉药物股份有限公司 | 使用免疫刺激性寡核苷酸和/或免疫子化合物结合细胞因子和/或化疗剂或放射治疗对免疫系统的协同刺激作用 |
| EP2363141A1 (en) * | 2003-07-15 | 2011-09-07 | Idera Pharmaceuticals, Inc. | Compsition comprising two oligonucleotides linked directly at their 3'ends wherein at leat one oligonucleotide has an accessible 5'end and the compound further comprising IL-2 used for synergistically stimulating an immune response in a patient. |
| US7288638B2 (en) | 2003-10-10 | 2007-10-30 | Bristol-Myers Squibb Company | Fully human antibodies against human 4-1BB |
| CA2560919A1 (en) | 2004-03-26 | 2005-10-06 | Pfizer Products Inc. | Uses of anti-ctla-4 antibodies |
| HUE036894T2 (hu) * | 2004-06-15 | 2018-08-28 | Idera Pharmaceuticals Inc | Immunstimuláló oligonukleotid multimerek |
| CA2570812C (en) | 2004-06-18 | 2014-07-29 | The Regents Of The University Of California | Brassica indehiscent1 sequences |
| US8239749B2 (en) | 2004-06-25 | 2012-08-07 | Apple Inc. | Procedurally expressing graphic objects for web pages |
| US20090252741A1 (en) | 2004-09-08 | 2009-10-08 | Ohio State University Research Foundation | Human monoclonal anti-ctla4 antibodies in cancer treatment |
| CA2970873C (en) | 2005-05-09 | 2022-05-17 | E. R. Squibb & Sons, L.L.C. | Human monoclonal antibodies to programmed death 1 (pd-1) and methods for treating cancer using anti-pd-1 antibodies alone or in combination with other immunotherapeutics |
| MX351401B (es) | 2005-06-08 | 2017-10-13 | Dana Farber Cancer Inst Inc | Metodos y composiciones para el tratamiento de infecciones persistentes y cancer por inhibicion de la ruta de muerte celular programada (pd-1). |
| EA019344B1 (ru) | 2005-07-01 | 2014-03-31 | МЕДАРЕКС, Эл.Эл.Си. | Человеческие моноклональные антитела против лиганда-1 запрограммированной гибели клеток (pd-l1) и их применения |
| CN101268101A (zh) * | 2005-07-07 | 2008-09-17 | 科利制药集团公司 | 用于癌症治疗的抗-CTLA-4抗体和含有CpG基序的合成寡脱氧核苷酸联合治疗 |
| AU2006306521B2 (en) * | 2005-10-21 | 2011-12-22 | Medical College Of Georgia Research Institute, Inc. | The induction of indoleamine 2,3-dioxygenase in dendritic cells by TLR ligands and uses thereof |
| US8697087B2 (en) * | 2005-11-04 | 2014-04-15 | Novartis Ag | Influenza vaccines including combinations of particulate adjuvants and immunopotentiators |
| CN101379195B (zh) * | 2005-12-20 | 2012-05-09 | 艾德拉药物股份有限公司 | 含有不同长度回文片段的回文免疫调节性寡核苷酸(imotm)的免疫刺激活性 |
| CA2646671A1 (en) | 2006-03-30 | 2007-11-01 | University Of California | Methods and compositions for localized secretion of anti-ctla-4 antibodies |
| US20080031887A1 (en) * | 2006-06-30 | 2008-02-07 | Joseph Lustgarten | Conjugates for inducing targeted immune responses and methods of making and using same |
| CN101104640A (zh) | 2006-07-10 | 2008-01-16 | 苏州大学 | 抗人pd-l1单克隆抗体制备及应用 |
| JP2008066402A (ja) | 2006-09-05 | 2008-03-21 | Fujifilm Corp | 撮像素子および撮像装置 |
| US20100055111A1 (en) * | 2007-02-14 | 2010-03-04 | Med. College Of Georgia Research Institute, Inc. | Indoleamine 2,3-dioxygenase, pd-1/pd-l pathways, and ctla4 pathways in the activation of regulatory t cells |
| EP1987839A1 (en) | 2007-04-30 | 2008-11-05 | I.N.S.E.R.M. Institut National de la Sante et de la Recherche Medicale | Cytotoxic anti-LAG-3 monoclonal antibody and its use in the treatment or prevention of organ transplant rejection and autoimmune disease |
| SI2170959T1 (sl) | 2007-06-18 | 2014-04-30 | Merck Sharp & Dohme B.V. | Protitelesa proti receptorjem pd-1 za humano programirano smrt |
| KR20090010580A (ko) | 2007-07-24 | 2009-01-30 | 조용식 | 페트용 토이렛 |
| US9416165B2 (en) | 2007-10-26 | 2016-08-16 | The Regents Of The University Of California | Methods of inhibiting viral replication and improving T cell function employing soluble Tim-3 inhibitors |
| WO2009079597A1 (en) | 2007-12-17 | 2009-06-25 | Janssen Pharmaceutica N.V. | Piperazinyl derivatives useful as modulators of the neuropeptide y2 receptor |
| US8263073B2 (en) | 2008-02-04 | 2012-09-11 | Medarex, Inc. | Anti-CTLA-4 antibodies with reduced blocking of binding of CTLA-4 to B7 and uses thereof |
| GB2457346B (en) | 2008-02-12 | 2012-03-28 | Scott Cutters Uk Ltd | Cutting tools |
| WO2010001617A1 (en) | 2008-07-04 | 2010-01-07 | Ono Pharmaceutical Co., Ltd. | Use of an efficacy marker for optimizing therapeutic efficacy of an anti-human pd-1 antibody on cancers |
| AR072999A1 (es) | 2008-08-11 | 2010-10-06 | Medarex Inc | Anticuerpos humanos que se unen al gen 3 de activacion linfocitaria (lag-3) y los usos de estos |
| AU2009290543B2 (en) | 2008-09-12 | 2015-09-03 | Oxford University Innovation Limited | PD-1 specific antibodies and uses thereof |
| BRPI0919377A2 (pt) | 2008-09-26 | 2016-09-27 | Dana Farber Cancer Inst Inc | anticorpo isolado ou um fragmento ligante de antígeno do memso, ácido nucleico isolado, vetor, célula hospedeira, composição farmacêutica, método de produzir o referido anticorpo ou fragmento, uso dos mesmos, e composição compreendendo o referido anticorpo ou fragmento |
| FI4209510T3 (fi) | 2008-12-09 | 2024-03-22 | Hoffmann La Roche | Anti-PD-L1-vasta-aineita ja niiden käyttö T-solutoiminnan tehostamiseksi |
| ES2629337T3 (es) | 2009-02-09 | 2017-08-08 | Inserm - Institut National De La Santé Et De La Recherche Médicale | Anticuerpos contra PD-1 y anticuerpos contra PD-L1 y usos de los mismos |
| US8605955B2 (en) | 2009-06-29 | 2013-12-10 | DigitalOptics Corporation Europe Limited | Methods and apparatuses for half-face detection |
| JP5409275B2 (ja) | 2009-11-06 | 2014-02-05 | アズビル株式会社 | 監視制御システム |
| WO2011110604A1 (en) | 2010-03-11 | 2011-09-15 | Ucb Pharma, S.A. | Pd-1 antibody |
| TW201134488A (en) | 2010-03-11 | 2011-10-16 | Ucb Pharma Sa | PD-1 antibodies |
| CA2792561C (en) | 2010-04-06 | 2021-10-26 | Alnylam Pharmaceuticals, Inc. | Compositions and methods for inhibiting expression of cd274/pd-l1 gene |
| CN105648056A (zh) * | 2010-05-14 | 2016-06-08 | 综合医院公司 | 鉴定肿瘤特异性新抗原的组合物和方法 |
| JP2013532153A (ja) | 2010-06-18 | 2013-08-15 | ザ ブリガム アンド ウィメンズ ホスピタル インコーポレイテッド | 慢性免疫病に対する免疫治療のためのtim−3およびpd−1に対する二重特異性抗体 |
| FR2963448A1 (fr) | 2010-07-29 | 2012-02-03 | Sagem Defense Securite | Procede et systeme d'analyse de donnees de vol enregistrees au cours d'un vol d'un avion. |
| GB201103955D0 (en) | 2011-03-09 | 2011-04-20 | Antitope Ltd | Antibodies |
| RS57324B1 (sr) | 2011-04-20 | 2018-08-31 | Medimmune Llc | Antitela i drugi molekuli koji vezuju b7-h1 i pd-1 |
| US8841418B2 (en) | 2011-07-01 | 2014-09-23 | Cellerant Therapeutics, Inc. | Antibodies that specifically bind to TIM3 |
| US20130018499A1 (en) | 2011-07-12 | 2013-01-17 | The Boeing Company | Producibility analysis during engineering design of composite parts |
| TW202114735A (zh) | 2011-08-01 | 2021-04-16 | 美商建南德克公司 | 利用pd-1軸結合拮抗劑及mek抑制劑治療癌症之方法 |
| EP3939613A1 (en) | 2011-08-11 | 2022-01-19 | ONO Pharmaceutical Co., Ltd. | Therapeutic agent for autoimmune diseases comprising pd-1 agonist |
| HRP20220924T1 (hr) | 2011-10-17 | 2022-10-28 | Io Biotech Aps | Imunoterapija zasnovana na pd-l1 |
| EP3763741A1 (en) | 2011-11-28 | 2021-01-13 | Merck Patent GmbH | Anti-pd-l1 antibodies and uses thereof |
| EP2822957A1 (en) | 2012-03-07 | 2015-01-14 | Aurigene Discovery Technologies Limited | Peptidomimetic compounds as immunomodulators |
| CN104736168B (zh) | 2012-05-31 | 2018-09-21 | 索伦托治疗有限公司 | 与pd-l1结合的抗原结合蛋白 |
| EP3981791A1 (en) | 2012-08-30 | 2022-04-13 | Amgen Inc. | A method for treating melanoma using a herpes simplex virus and an immune checkpoint inhibitor |
| CN105163754B (zh) * | 2012-09-20 | 2018-01-05 | 王荣福 | 前列腺特异性肿瘤抗原及其用途 |
| US9308236B2 (en) | 2013-03-15 | 2016-04-12 | Bristol-Myers Squibb Company | Macrocyclic inhibitors of the PD-1/PD-L1 and CD80(B7-1)/PD-L1 protein/protein interactions |
| JP6792294B2 (ja) | 2015-05-29 | 2020-11-25 | ダイナバックス テクノロジーズ コーポレイション | 肺のがんを処置するためのポリヌクレオチドのToll様受容体9アゴニストの肺内投与 |
| EP3892284B1 (en) * | 2015-05-29 | 2024-05-22 | Merck Sharp & Dohme LLC | Combination of a pd-1 antagonist and a cpg-c type oligonucleotide for treating cancer |
-
2015
- 2015-10-09 JP JP2017519544A patent/JP6991857B2/ja not_active Expired - Fee Related
- 2015-10-09 US US14/879,573 patent/US20160101128A1/en not_active Abandoned
- 2015-10-09 EP EP21212650.2A patent/EP4029508A1/en not_active Withdrawn
- 2015-10-09 WO PCT/US2015/054899 patent/WO2016057898A1/en not_active Ceased
- 2015-10-09 IL IL251669A patent/IL251669B2/en unknown
- 2015-10-09 KR KR1020177012557A patent/KR20170072244A/ko not_active Ceased
- 2015-10-09 MX MX2017004708A patent/MX391478B/es unknown
- 2015-10-09 EP EP15848691.0A patent/EP3204040B1/en active Active
- 2015-10-09 CN CN201580067702.4A patent/CN106999574B/zh not_active Expired - Fee Related
- 2015-10-09 AU AU2015330731A patent/AU2015330731B2/en not_active Ceased
- 2015-10-09 CA CA2964155A patent/CA2964155A1/en not_active Abandoned
- 2015-10-09 CN CN202210493223.0A patent/CN115040532A/zh active Pending
- 2015-10-09 ES ES15848691T patent/ES2908056T3/es active Active
-
2017
- 2017-04-10 MX MX2022004374A patent/MX2022004374A/es unknown
- 2017-06-06 US US15/615,405 patent/US20170274004A1/en not_active Abandoned
-
2019
- 2019-12-13 US US16/714,127 patent/US20200101102A1/en not_active Abandoned
-
2020
- 2020-10-09 AU AU2020250293A patent/AU2020250293A1/en not_active Abandoned
-
2021
- 2021-09-14 JP JP2021149233A patent/JP2022000437A/ja active Pending
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2010512421A (ja) | 2006-12-12 | 2010-04-22 | イデラ ファーマシューティカルズ インコーポレイテッド | Tlr9の合成アゴニスト |
| JP2010535242A (ja) | 2007-08-01 | 2010-11-18 | イデラ ファーマシューティカルズ インコーポレイテッド | Tlr9の新規な合成アゴニスト |
| US20130142815A1 (en) | 2010-02-09 | 2013-06-06 | Georiga Health Sciences Univ. Research Inst., Inc. | Alpha-methyl-tryptophan as an inhibitor of indoleamine dioxygenase |
Non-Patent Citations (2)
| Title |
|---|
| Cancer Res., (2012), 72, [8 Suppl.], Abstract:LB-139, [online], 2012, [Retrieved on 2019-APR-02], Retrieved from the internet:<URL: http://cancerres.aacrjournals.org.content/72/8_Supplement/LB-139><DOI:10.1158/1538-7445.AM2012-LB-139> |
| History of Change for Study:NCT02254772, [online], 2014-OCT-01, ClinicalTrials.gov archive, NCT ID:NCT02254772 [Retrieved on 2019-JUL-11], Retrieved from the internet:<URL: https://clinicaltrials.gov/ct2/history/NCT02254772?A=1&B=1&C=merged> |
Also Published As
| Publication number | Publication date |
|---|---|
| CN115040532A (zh) | 2022-09-13 |
| CA2964155A1 (en) | 2016-04-14 |
| EP3204040B1 (en) | 2021-12-08 |
| IL251669B2 (en) | 2023-02-01 |
| WO2016057898A1 (en) | 2016-04-14 |
| JP2017531664A (ja) | 2017-10-26 |
| US20160101128A1 (en) | 2016-04-14 |
| EP3204040A4 (en) | 2018-04-11 |
| JP2022000437A (ja) | 2022-01-04 |
| KR20170072244A (ko) | 2017-06-26 |
| US20170274004A1 (en) | 2017-09-28 |
| EP3204040A1 (en) | 2017-08-16 |
| MX2017004708A (es) | 2017-10-12 |
| IL251669A0 (en) | 2017-06-29 |
| MX391478B (es) | 2025-03-21 |
| CN106999574A (zh) | 2017-08-01 |
| ES2908056T3 (es) | 2022-04-27 |
| US20200101102A1 (en) | 2020-04-02 |
| IL251669B (en) | 2022-10-01 |
| AU2020250293A1 (en) | 2021-01-14 |
| AU2015330731A1 (en) | 2017-05-04 |
| EP4029508A1 (en) | 2022-07-20 |
| AU2015330731B2 (en) | 2020-07-09 |
| MX2022004374A (es) | 2022-06-02 |
| CN106999574B (zh) | 2022-05-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6991857B2 (ja) | Tlr9アゴニストをチェックポイント阻害剤と共に用いるがんの治療 | |
| US20230374503A1 (en) | Combination | |
| JP6893608B2 (ja) | がんの処置のためのPD−1アンタゴニストとCpG−C型オリゴヌクレオチドの併用 | |
| JP6885867B2 (ja) | 組合せ腫瘍免疫療法 | |
| AU2001270134A1 (en) | Methods for enhancing antibody-induced cell lysis and treating cancer | |
| EP1296714A2 (en) | Methods for enhancing antibody-induced cell lysis and treating cancer | |
| WO2018192505A1 (en) | Immunomodulatory polynucleotides and uses thereof | |
| HK1242199B (en) | Treatment of cancer using tlr9 agonist with checkpoint inhibitors | |
| HK1238287B (en) | Combination | |
| HK1238287A1 (en) | Combination |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20181002 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20181002 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20190723 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20191021 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20191211 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20200526 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20200825 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20201023 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20210119 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20210419 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20210518 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20210914 |
|
| C60 | Trial request (containing other claim documents, opposition documents) |
Free format text: JAPANESE INTERMEDIATE CODE: C60 Effective date: 20210914 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20210914 |
|
| A911 | Transfer to examiner for re-examination before appeal (zenchi) |
Free format text: JAPANESE INTERMEDIATE CODE: A911 Effective date: 20211015 |
|
| C21 | Notice of transfer of a case for reconsideration by examiners before appeal proceedings |
Free format text: JAPANESE INTERMEDIATE CODE: C21 Effective date: 20211019 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20211109 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20211208 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6991857 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| LAPS | Cancellation because of no payment of annual fees |