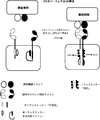
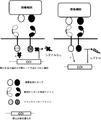
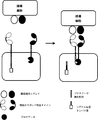
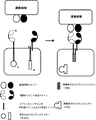
JP2017504601A - 免疫療法のためにマルチインプットシグナル感受性t細胞を操作する方法 - Google Patents
免疫療法のためにマルチインプットシグナル感受性t細胞を操作する方法 Download PDFInfo
- Publication number
- JP2017504601A JP2017504601A JP2016541194A JP2016541194A JP2017504601A JP 2017504601 A JP2017504601 A JP 2017504601A JP 2016541194 A JP2016541194 A JP 2016541194A JP 2016541194 A JP2016541194 A JP 2016541194A JP 2017504601 A JP2017504601 A JP 2017504601A
- Authority
- JP
- Japan
- Prior art keywords
- domain
- car
- cells
- cell
- seq
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/30—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants from tumour cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/46—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- C07K14/47—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2863—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against receptors for growth factors, growth regulators
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/46—Hybrid immunoglobulins
- C07K16/468—Immunoglobulins having two or more different antigen binding sites, e.g. multifunctional antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/60—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments
- C07K2317/62—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments comprising only variable region components
- C07K2317/622—Single chain antibody (scFv)
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/01—Fusion polypeptide containing a localisation/targetting motif
- C07K2319/03—Fusion polypeptide containing a localisation/targetting motif containing a transmembrane segment
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/30—Non-immunoglobulin-derived peptide or protein having an immunoglobulin constant or Fc region, or a fragment thereof, attached thereto
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2510/00—Genetically modified cells
- C12N2510/02—Cells for production
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Immunology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biochemistry (AREA)
- Molecular Biology (AREA)
- Genetics & Genomics (AREA)
- Biophysics (AREA)
- Cell Biology (AREA)
- Toxicology (AREA)
- Zoology (AREA)
- Gastroenterology & Hepatology (AREA)
- Pharmacology & Pharmacy (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Animal Behavior & Ethology (AREA)
- General Chemical & Material Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Peptides Or Proteins (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DKPA201370806 | 2013-12-20 | ||
| DKPA201370806 | 2013-12-20 | ||
| PCT/EP2014/078876 WO2015092024A2 (en) | 2013-12-20 | 2014-12-19 | Method of engineering multi-input signal sensitive t cell for immunotherapy |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2017504601A true JP2017504601A (ja) | 2017-02-09 |
| JP2017504601A5 JP2017504601A5 (enExample) | 2018-01-18 |
Family
ID=49884853
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2016541194A Pending JP2017504601A (ja) | 2013-12-20 | 2014-12-19 | 免疫療法のためにマルチインプットシグナル感受性t細胞を操作する方法 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US10239948B2 (enExample) |
| EP (2) | EP3083691A2 (enExample) |
| JP (1) | JP2017504601A (enExample) |
| AU (1) | AU2014368383B2 (enExample) |
| CA (1) | CA2934436A1 (enExample) |
| WO (1) | WO2015092024A2 (enExample) |
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2020512019A (ja) * | 2017-03-31 | 2020-04-23 | セレクティス ソシエテ アノニム | ユニバーサル抗cd22キメラ抗原受容体操作免疫細胞 |
| WO2020175366A1 (ja) * | 2019-02-25 | 2020-09-03 | 国立大学法人東京医科歯科大学 | キメラ抗原受容体 |
| JPWO2021131944A1 (enExample) * | 2019-12-27 | 2021-07-01 | ||
| JP2022520285A (ja) * | 2019-02-19 | 2022-03-29 | キングス カレッジ ロンドン | 低酸素応答性キメラ抗原受容体 |
| JP2022533806A (ja) * | 2018-11-06 | 2022-07-26 | イミュニティーバイオ、インコーポレイテッド | キメラ抗原受容体で修飾されたnk-92細胞 |
| JP2024001096A (ja) * | 2018-05-22 | 2024-01-09 | イミュニティーバイオ、インコーポレイテッド | FcイプシロンCAR |
| US12109238B2 (en) | 2018-11-06 | 2024-10-08 | Immunitybio, Inc. | Chimeric antigen receptor-modified NK-92 cells |
Families Citing this family (89)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| NZ723731A (en) | 2011-04-08 | 2020-05-29 | Baylor College Medicine | Reversing the effects of the tumor microenvironment using chimeric cytokine receptors |
| US9752113B2 (en) | 2012-03-15 | 2017-09-05 | Flodesign Sonics, Inc. | Acoustic perfusion devices |
| US9950282B2 (en) | 2012-03-15 | 2018-04-24 | Flodesign Sonics, Inc. | Electronic configuration and control for acoustic standing wave generation |
| US9745548B2 (en) | 2012-03-15 | 2017-08-29 | Flodesign Sonics, Inc. | Acoustic perfusion devices |
| US10704021B2 (en) | 2012-03-15 | 2020-07-07 | Flodesign Sonics, Inc. | Acoustic perfusion devices |
| US10967298B2 (en) | 2012-03-15 | 2021-04-06 | Flodesign Sonics, Inc. | Driver and control for variable impedence load |
| US9458450B2 (en) | 2012-03-15 | 2016-10-04 | Flodesign Sonics, Inc. | Acoustophoretic separation technology using multi-dimensional standing waves |
| US10689609B2 (en) | 2012-03-15 | 2020-06-23 | Flodesign Sonics, Inc. | Acoustic bioreactor processes |
| US10322949B2 (en) | 2012-03-15 | 2019-06-18 | Flodesign Sonics, Inc. | Transducer and reflector configurations for an acoustophoretic device |
| US10737953B2 (en) | 2012-04-20 | 2020-08-11 | Flodesign Sonics, Inc. | Acoustophoretic method for use in bioreactors |
| SG11201505858VA (en) | 2013-01-28 | 2015-09-29 | St Jude Childrens Res Hospital | A chimeric receptor with nkg2d specificity for use in cell therapy against cancer and infectious disease |
| US9745569B2 (en) | 2013-09-13 | 2017-08-29 | Flodesign Sonics, Inc. | System for generating high concentration factors for low cell density suspensions |
| CA2935960C (en) | 2014-01-08 | 2023-01-10 | Bart Lipkens | Acoustophoresis device with dual acoustophoretic chamber |
| ES2740903T3 (es) * | 2014-03-19 | 2020-02-07 | Cellectis | Receptores antigénicos quiméricos específicos de CD123 para inmunoterapia del cáncer |
| SG11201608393TA (en) | 2014-04-10 | 2016-11-29 | Seattle Children S Hospital Dba Seattle Children S Res Inst | Transgene genetic tags and methods of use |
| EP3131927B8 (en) | 2014-04-14 | 2020-12-23 | Cellectis | Bcma (cd269) specific chimeric antigen receptors for cancer immunotherapy |
| EP3143134B1 (en) | 2014-05-15 | 2020-10-28 | National University of Singapore | Modified natural killer cells and uses thereof |
| US9744483B2 (en) | 2014-07-02 | 2017-08-29 | Flodesign Sonics, Inc. | Large scale acoustic separation device |
| TWI805109B (zh) | 2014-08-28 | 2023-06-11 | 美商奇諾治療有限公司 | 對cd19具專一性之抗體及嵌合抗原受體 |
| HRP20240357T1 (hr) | 2014-12-24 | 2024-06-07 | Autolus Limited | Stanica |
| CA2973529A1 (en) | 2015-01-26 | 2016-08-04 | Cellectis | Cll1-specific multi-chain chimeric antigen receptor |
| WO2016126608A1 (en) * | 2015-02-02 | 2016-08-11 | Novartis Ag | Car-expressing cells against multiple tumor antigens and uses thereof |
| IL254817B2 (en) | 2015-04-08 | 2023-12-01 | Novartis Ag | Cd20 therapies, cd22 therapies, and combination therapies with a cd19 chimeric antigen receptor (car) - expressing cell |
| US11377651B2 (en) | 2016-10-19 | 2022-07-05 | Flodesign Sonics, Inc. | Cell therapy processes utilizing acoustophoresis |
| US11021699B2 (en) | 2015-04-29 | 2021-06-01 | FioDesign Sonics, Inc. | Separation using angled acoustic waves |
| US11708572B2 (en) | 2015-04-29 | 2023-07-25 | Flodesign Sonics, Inc. | Acoustic cell separation techniques and processes |
| KR20180029201A (ko) | 2015-05-18 | 2018-03-20 | 티씨알2 테라퓨틱스 인크. | 융합 단백질을 이용하는 tcr 리프로그래밍을 위한 조성물 및 방법 |
| MA42902A (fr) | 2015-07-08 | 2018-05-16 | Univ Johns Hopkins | Lymphocytes infiltrant la moelle (mil) en tant que source de lymphocytes t pour une thérapie par des récepteurs chimériques d'un antigène (car) |
| US11459540B2 (en) | 2015-07-28 | 2022-10-04 | Flodesign Sonics, Inc. | Expanded bed affinity selection |
| US11474085B2 (en) | 2015-07-28 | 2022-10-18 | Flodesign Sonics, Inc. | Expanded bed affinity selection |
| PT3331910T (pt) | 2015-08-03 | 2020-03-24 | Engmab Sarl | Anticorpos monoclonais contra o antigénio de maturação de células b (bcma) humano |
| US11458167B2 (en) * | 2015-08-07 | 2022-10-04 | Seattle Children's Hospital | Bispecific CAR T-cells for solid tumor targeting |
| SG10202112024PA (en) | 2016-01-11 | 2021-12-30 | Univ Leland Stanford Junior | Chimeric proteins and methods of immunotherapy |
| NZ743983A (en) * | 2016-01-11 | 2025-08-29 | Univ Leland Stanford Junior | Chimeric proteins and methods of regulating gene expression |
| DK3405490T3 (da) | 2016-01-21 | 2022-01-10 | Pfizer | Mono- og bispecifikke antistoffer mod epidermal vækstfaktorreceptor variant iii og cd3 og anvendelser deraf |
| US10259876B2 (en) * | 2016-01-21 | 2019-04-16 | Pfizer Inc. | Chimeric antigen receptors targeting epidermal growth factor receptor variant III |
| EP3202783A1 (en) * | 2016-02-02 | 2017-08-09 | Ecole Polytechnique Fédérale de Lausanne (EPFL) | Engineered antigen presenting cells and uses thereof |
| EP3420093B1 (en) * | 2016-02-26 | 2022-11-23 | Cellectis | Micelle based system nuclease encapsulation for in-vivo gene editing |
| CN107298715B (zh) * | 2016-04-15 | 2021-05-04 | 阿思科力(苏州)生物科技有限公司 | Slit2D2-嵌合抗原受体及其应用 |
| US11085035B2 (en) | 2016-05-03 | 2021-08-10 | Flodesign Sonics, Inc. | Therapeutic cell washing, concentration, and separation utilizing acoustophoresis |
| US11214789B2 (en) | 2016-05-03 | 2022-01-04 | Flodesign Sonics, Inc. | Concentration and washing of particles with acoustics |
| GB201609604D0 (en) * | 2016-06-01 | 2016-07-13 | Ucl Business Plc | Cell |
| KR20190019121A (ko) | 2016-06-17 | 2019-02-26 | 오사카 유니버시티 | 종양 내 정맥 형성 촉진제 |
| WO2018026953A1 (en) | 2016-08-02 | 2018-02-08 | TCR2 Therapeutics Inc. | Compositions and methods for tcr reprogramming using fusion proteins |
| CN108699163B (zh) * | 2016-09-28 | 2021-11-19 | 阿思科力(苏州)生物科技有限公司 | 一种多基因重组嵌合抗原受体分子及其应用 |
| IL302917A (en) | 2016-10-07 | 2023-07-01 | Tcr2 Therapeutics Inc | Compositions and methods for t-cell receptors reprogramming using fusion proteins |
| CN110494543A (zh) | 2016-10-19 | 2019-11-22 | 弗洛设计声能学公司 | 通过声学的亲和细胞提取 |
| WO2018073393A2 (en) | 2016-10-19 | 2018-04-26 | Cellectis | Tal-effector nuclease (talen) -modified allogenic cells suitable for therapy |
| US11124577B2 (en) | 2016-11-02 | 2021-09-21 | Engmab Sàrl | Bispecific antibody against BCMA and CD3 and an immunological drug for combined use in treating multiple myeloma |
| JP7291396B2 (ja) | 2016-11-22 | 2023-06-15 | ティーシーアール2 セラピューティクス インク. | 融合タンパク質を用いたtcrの再プログラミングのための組成物及び方法 |
| CN110291200B (zh) | 2016-12-12 | 2024-05-14 | 西雅图儿童医院(Dba西雅图儿童研究所) | 对哺乳动物细胞中转基因表达的药剂配体诱导具有增大的灵敏度的嵌合转录因子变异体 |
| SG11201908492PA (en) | 2017-03-27 | 2019-10-30 | Nat Univ Singapore | Truncated nkg2d chimeric receptors and uses thereof in natural killer cell immunotherapy |
| EP3601537A4 (en) | 2017-03-27 | 2021-01-13 | National University of Singapore | STIMULATING CELL LINES FOR EX VIVO EXPANSION AND ACTIVATION OF NATURAL KILLER CELLS |
| WO2018193231A1 (en) * | 2017-04-18 | 2018-10-25 | Autolus Limited | Cell |
| WO2018213332A1 (en) * | 2017-05-17 | 2018-11-22 | Seattle Children's Hospital (dba Seattle Children's Research Institute) | Generating mammalian t cell activation inducible synthetic promoters (syn+pro) to improve t cell therapy |
| JP2020530277A (ja) | 2017-06-30 | 2020-10-22 | セレクティスCellectis | 反復投与のための細胞免疫療法 |
| GB201711470D0 (en) * | 2017-07-17 | 2017-08-30 | Univ Oxford Innovation Ltd | Chimeric receptors |
| EP3662055A1 (en) * | 2017-08-02 | 2020-06-10 | Autolus Limited | Cells expressing a chimeric antigen receptor or engineered tcr and comprising a nucleotide sequence which is selectively expressed |
| WO2019072824A1 (en) | 2017-10-09 | 2019-04-18 | Cellectis | IMPROVED ANTI-CD123 CAR IN UNIVERSAL MODIFIED IMMUNE T LYMPHOCYTES |
| KR20220066413A (ko) | 2017-12-14 | 2022-05-24 | 프로디자인 소닉스, 인크. | 음향 트랜스듀서 구동기 및 제어기 |
| WO2019118902A2 (en) | 2017-12-15 | 2019-06-20 | The Board Of Trustees Of The Leland Stanford Junior University | Compositions and methods for inhibiting t cell exhaustion |
| CN111801348A (zh) | 2018-02-09 | 2020-10-20 | 新加坡国立大学 | 活化性嵌合受体及其在自然杀伤细胞免疫疗法中的用途 |
| KR20200138741A (ko) | 2018-04-02 | 2020-12-10 | 내셔널 유니버시티 오브 싱가포르 | 면역 세포에서 발현되는 막-결합 항-사이토카인 비-신호전달 결합제를 이용한 인간 사이토카인의 중화 |
| WO2019195586A1 (en) | 2018-04-06 | 2019-10-10 | The Regents Of The University Of California | Methods of treating egfrviii expressing glioblastomas |
| US12090170B2 (en) | 2018-04-06 | 2024-09-17 | The Regents Of The University Of California | Methods of treating glioblastomas |
| WO2020028744A1 (en) * | 2018-08-02 | 2020-02-06 | Asimov, Inc. | Universal chimeric receptors |
| CA3108710A1 (en) * | 2018-08-07 | 2020-02-13 | Purdue Research Foundation | Rejuvenation of car t cell |
| JP7560882B2 (ja) | 2018-08-29 | 2024-10-03 | ナショナル ユニヴァーシティー オブ シンガポール | 遺伝子修飾免疫細胞の生存及び増加を特異的に刺激するための方法 |
| JP7429222B2 (ja) * | 2018-08-31 | 2024-02-07 | アンヴェクティ エスアー | Carの機能を評価する方法 |
| US11542305B2 (en) * | 2018-08-31 | 2023-01-03 | California Institute Of Technology | Synthetic protein circuits detecting signal transducer activity |
| WO2020051562A2 (en) | 2018-09-07 | 2020-03-12 | Beam Therapeutics Inc. | Compositions and methods for improving base editing |
| AU2019374103A1 (en) | 2018-11-01 | 2021-05-20 | Juno Therapeutics, Inc. | Chimeric antigen receptors specific for G Protein-Coupled Receptor Class C Group 5 Member D (GPRC5D) |
| EP3773918A4 (en) | 2019-03-05 | 2022-01-05 | Nkarta, Inc. | ANTI-CD19 CHEMERIC ANTIGEN RECEPTORS AND THEIR USE IN IMMUNOTHERAPY |
| JP2022529380A (ja) | 2019-04-26 | 2022-06-21 | ザ ユニバーシティ オブ ノース カロライナ アット チャペル ヒル | キメラ抗原受容体構築物およびcar-t細胞におけるそれらの使用 |
| CN114945382A (zh) | 2019-11-26 | 2022-08-26 | 诺华股份有限公司 | Cd19和cd22嵌合抗原受体及其用途 |
| CA3161488A1 (en) * | 2019-12-11 | 2021-06-17 | Daniel Getts | Therapeutic cell compositions and methods for manufacture and uses thereof |
| EP4203980A1 (en) * | 2020-08-28 | 2023-07-05 | Innovative Cellular Therapeutics Holdings, Ltd. | Fusion protein enhancing cell therapy |
| EP4263600A1 (en) | 2020-12-18 | 2023-10-25 | Century Therapeutics, Inc. | Chimeric antigen receptor systems with adaptable receptor specificity |
| CA3232968A1 (en) | 2021-10-14 | 2023-04-20 | Jasper Williams | Immune cells having co-expressed shrnas and logic gate systems |
| WO2023070527A1 (zh) * | 2021-10-29 | 2023-05-04 | 上海鑫湾生物科技有限公司 | 一种条件控制的可剪接嵌合抗原受体分子及其应用 |
| US20250017960A1 (en) * | 2021-11-15 | 2025-01-16 | The Board Of Trustees Of The Leland Stanford Junior University | Composition and method of valency controlled receptor systems for cell engineering and therapy |
| WO2024015383A1 (en) * | 2022-07-12 | 2024-01-18 | Northwestern University | Engineered hypoxia biosensors and methods of using the same |
| EP4565262A2 (en) | 2022-08-05 | 2025-06-11 | Juno Therapeutics, Inc. | Chimeric antigen receptors specific for gprc5d and bcma |
| EP4573116A2 (en) * | 2022-08-15 | 2025-06-25 | The Regents Of The University Of California | Multi-chain synthetic receptors for simultaneous ligand-induced transcriptional regulation and membrane-proximal signal transduction |
| EP4593850A2 (en) | 2022-09-30 | 2025-08-06 | The Regents of the University of California | Compositions and methods for enhancing adoptive t cell therapeutics |
| CN119306846A (zh) * | 2023-07-14 | 2025-01-14 | 广州百吉生物制药有限公司 | 一种工程化免疫细胞及其制备和应用 |
| WO2025054489A1 (en) * | 2023-09-08 | 2025-03-13 | The Regents Of The University Of California | Chimeric antigen receptors with card domains |
| WO2025076422A1 (en) * | 2023-10-05 | 2025-04-10 | The Regents Of The University Of California | Regulated expression of card11-pik3r3 fusion protein |
| WO2025084881A1 (ko) * | 2023-10-20 | 2025-04-24 | 주식회사 이온셀 | Ct83 항원에 결합하는 car-t 세포 및 이의 용도 |
Citations (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2004524848A (ja) * | 2001-03-21 | 2004-08-19 | アイシス イノヴェイション リミテッド | アッセイ、方法および手段 |
| JP2008509209A (ja) * | 2004-08-09 | 2008-03-27 | キャンサー・リサーチ・テクノロジー・リミテッド | αケトグルタレート類および治療薬としてのその使用 |
| JP2009545310A (ja) * | 2006-07-31 | 2009-12-24 | ヴァスキュラー バイオジェニックス リミテッド | ポリペプチド、および、当該ポリペプチドをコードするポリヌクレオチド、および、虚血に関連する医学的状態の治療におけるそれらの使用 |
| WO2012099973A2 (en) * | 2011-01-18 | 2012-07-26 | The Trustees Of The University Of Pennsylvania | Compositions and methods for treating cancer |
| WO2013176915A1 (en) * | 2012-05-25 | 2013-11-28 | Roman Galetto | Methods for engineering allogeneic and immunosuppressive resistant t cell for immunotherapy |
| WO2014153270A1 (en) * | 2013-03-16 | 2014-09-25 | Novartis Ag | Treatment of cancer using humanized anti-cd19 chimeric antigen receptor |
| WO2014180306A1 (zh) * | 2013-05-08 | 2014-11-13 | 上海益杰生物技术有限公司 | 编码gpc3嵌合抗原受体蛋白的核酸及表达gpc3嵌合抗原受体蛋白的t淋巴细胞 |
| WO2014184143A1 (en) * | 2013-05-13 | 2014-11-20 | Cellectis | Cd19 specific chimeric antigen receptor and uses thereof |
| WO2014184744A1 (en) * | 2013-05-13 | 2014-11-20 | Cellectis | Methods for engineering highly active t cell for immunotherapy |
| WO2014184741A1 (en) * | 2013-05-13 | 2014-11-20 | Cellectis | Methods for engineering allogeneic and highly active t cell for immunotheraphy |
Family Cites Families (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB8611832D0 (en) | 1986-05-15 | 1986-06-25 | Holland I B | Polypeptide |
| US5037743A (en) | 1988-08-05 | 1991-08-06 | Zymogenetics, Inc. | BAR1 secretion signal |
| WO2006089133A2 (en) * | 2005-02-15 | 2006-08-24 | Duke University | Anti-cd19 antibodies and uses in oncology |
| JP2010532764A (ja) * | 2007-07-06 | 2010-10-14 | トゥルビオン・ファーマシューティカルズ・インコーポレーテッド | C末端に配置された特異的結合性ドメインを有する結合性ペプチド |
| PL3006459T3 (pl) * | 2008-08-26 | 2022-01-17 | City Of Hope | Sposób i kompozycje dla wzmocnionego działania efektorowego komórek t przeciw guzowi nowotworowemu |
| PH12013501201A1 (en) * | 2010-12-09 | 2013-07-29 | Univ Pennsylvania | Use of chimeric antigen receptor-modified t cells to treat cancer |
| CA2850411C (en) | 2011-09-28 | 2023-08-15 | Era Biotech, S.A. | Split inteins and uses thereof |
| JP6850528B2 (ja) | 2012-02-13 | 2021-03-31 | シアトル チルドレンズ ホスピタル ドゥーイング ビジネス アズ シアトル チルドレンズ リサーチ インスティテュート | 二重特異性キメラ抗原受容体およびその治療的使用 |
| US20130280220A1 (en) | 2012-04-20 | 2013-10-24 | Nabil Ahmed | Chimeric antigen receptor for bispecific activation and targeting of t lymphocytes |
| CN103483452B (zh) * | 2012-06-12 | 2021-08-13 | 上海细胞治疗集团有限公司 | 双信号独立的嵌合抗原受体及其用途 |
| AU2013312838B2 (en) * | 2012-09-04 | 2018-11-29 | Cellectis | Multi-chain chimeric antigen receptor and uses thereof |
| EP2970426B1 (en) * | 2013-03-15 | 2019-08-28 | Michael C. Milone | Targeting cytotoxic cells with chimeric receptors for adoptive immunotherapy |
| EP3925618A1 (en) * | 2013-07-29 | 2021-12-22 | 2seventy bio, Inc. | Multipartite signaling proteins and uses thereof |
-
2014
- 2014-12-19 AU AU2014368383A patent/AU2014368383B2/en not_active Ceased
- 2014-12-19 US US15/106,783 patent/US10239948B2/en active Active
- 2014-12-19 EP EP14830959.4A patent/EP3083691A2/en not_active Ceased
- 2014-12-19 WO PCT/EP2014/078876 patent/WO2015092024A2/en not_active Ceased
- 2014-12-19 CA CA2934436A patent/CA2934436A1/en not_active Abandoned
- 2014-12-19 JP JP2016541194A patent/JP2017504601A/ja active Pending
- 2014-12-19 EP EP20188659.5A patent/EP3808410A1/en active Pending
Patent Citations (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2004524848A (ja) * | 2001-03-21 | 2004-08-19 | アイシス イノヴェイション リミテッド | アッセイ、方法および手段 |
| JP2008509209A (ja) * | 2004-08-09 | 2008-03-27 | キャンサー・リサーチ・テクノロジー・リミテッド | αケトグルタレート類および治療薬としてのその使用 |
| JP2009545310A (ja) * | 2006-07-31 | 2009-12-24 | ヴァスキュラー バイオジェニックス リミテッド | ポリペプチド、および、当該ポリペプチドをコードするポリヌクレオチド、および、虚血に関連する医学的状態の治療におけるそれらの使用 |
| WO2012099973A2 (en) * | 2011-01-18 | 2012-07-26 | The Trustees Of The University Of Pennsylvania | Compositions and methods for treating cancer |
| WO2013176915A1 (en) * | 2012-05-25 | 2013-11-28 | Roman Galetto | Methods for engineering allogeneic and immunosuppressive resistant t cell for immunotherapy |
| WO2013176916A1 (en) * | 2012-05-25 | 2013-11-28 | Roman Galetto | Use of pre t alpha or functional variant thereof for expanding tcr alpha deficient t cells |
| US20140134142A1 (en) * | 2012-05-25 | 2014-05-15 | Cellectis | Multi-Chain Chimeric Antigen Receptor and Uses Thereof |
| WO2014153270A1 (en) * | 2013-03-16 | 2014-09-25 | Novartis Ag | Treatment of cancer using humanized anti-cd19 chimeric antigen receptor |
| WO2014180306A1 (zh) * | 2013-05-08 | 2014-11-13 | 上海益杰生物技术有限公司 | 编码gpc3嵌合抗原受体蛋白的核酸及表达gpc3嵌合抗原受体蛋白的t淋巴细胞 |
| WO2014184143A1 (en) * | 2013-05-13 | 2014-11-20 | Cellectis | Cd19 specific chimeric antigen receptor and uses thereof |
| WO2014184744A1 (en) * | 2013-05-13 | 2014-11-20 | Cellectis | Methods for engineering highly active t cell for immunotherapy |
| WO2014184741A1 (en) * | 2013-05-13 | 2014-11-20 | Cellectis | Methods for engineering allogeneic and highly active t cell for immunotheraphy |
Non-Patent Citations (3)
| Title |
|---|
| ANG SONNY O; OLIVARES SIMON; DENIGER DREW C; LEE DEAN A; CHAMPLIN RICHARD E; COOPER LAURENCE J: "CONDITIONAL ACTIVATION OF T CELLS TO SPECIFICALLY TARGET C-MET UNDER HYPOXIA", MOLECULAR THERAPY, vol. VOL:17, NR:SUPPL.1, JPN5017001555, May 2009 (2009-05-01), GB, pages 25 - 26, ISSN: 0004286322 * |
| ANG SONNY O; OLIVARES SIMON; SHPALL ELIZABETH J; LEE DEAN A; CHAMPLIN RICHARD, ET AL.: "CONDITIONAL T-CELL ACTIVATION FOR TUMOR UNDER HYPOXIA", BLOOD, vol. VOL:112, NR:11, JPN5017001556, November 2008 (2008-11-01), US, pages 1331 - 1332, ISSN: 0004286323 * |
| PLOS ONE, vol. 8(2), JPN6019029963, 2013, pages 57695 - 1, ISSN: 0004286324 * |
Cited By (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP7634045B2 (ja) | 2017-03-31 | 2025-02-20 | セレクティス ソシエテ アノニム | ユニバーサル抗cd22キメラ抗原受容体操作免疫細胞 |
| JP2020512019A (ja) * | 2017-03-31 | 2020-04-23 | セレクティス ソシエテ アノニム | ユニバーサル抗cd22キメラ抗原受容体操作免疫細胞 |
| JP7314115B2 (ja) | 2017-03-31 | 2023-07-25 | セレクティス ソシエテ アノニム | ユニバーサル抗cd22キメラ抗原受容体操作免疫細胞 |
| JP2023139070A (ja) * | 2017-03-31 | 2023-10-03 | セレクティス ソシエテ アノニム | ユニバーサル抗cd22キメラ抗原受容体操作免疫細胞 |
| US12435122B2 (en) | 2018-05-22 | 2025-10-07 | Immunitybio, Inc. | Fc-epsilon CAR |
| JP7748431B2 (ja) | 2018-05-22 | 2025-10-02 | イミュニティーバイオ、インコーポレイテッド | FcイプシロンCAR |
| JP2024001096A (ja) * | 2018-05-22 | 2024-01-09 | イミュニティーバイオ、インコーポレイテッド | FcイプシロンCAR |
| US12109238B2 (en) | 2018-11-06 | 2024-10-08 | Immunitybio, Inc. | Chimeric antigen receptor-modified NK-92 cells |
| JP7739389B2 (ja) | 2018-11-06 | 2025-09-16 | イミュニティーバイオ、インコーポレイテッド | キメラ抗原受容体で修飾されたnk-92細胞 |
| JP2022533806A (ja) * | 2018-11-06 | 2022-07-26 | イミュニティーバイオ、インコーポレイテッド | キメラ抗原受容体で修飾されたnk-92細胞 |
| JP2024037752A (ja) * | 2018-11-06 | 2024-03-19 | イミュニティーバイオ、インコーポレイテッド | キメラ抗原受容体で修飾されたnk-92細胞 |
| JP7536649B2 (ja) | 2018-11-06 | 2024-08-20 | イミュニティーバイオ、インコーポレイテッド | キメラ抗原受容体で修飾されたnk-92細胞 |
| JP2022520285A (ja) * | 2019-02-19 | 2022-03-29 | キングス カレッジ ロンドン | 低酸素応答性キメラ抗原受容体 |
| JP7684692B2 (ja) | 2019-02-19 | 2025-05-28 | キングス カレッジ ロンドン | 低酸素応答性キメラ抗原受容体 |
| JP7684691B2 (ja) | 2019-02-25 | 2025-05-28 | 国立大学法人東京科学大学 | キメラ抗原受容体 |
| JPWO2020175366A1 (enExample) * | 2019-02-25 | 2020-09-03 | ||
| WO2020175366A1 (ja) * | 2019-02-25 | 2020-09-03 | 国立大学法人東京医科歯科大学 | キメラ抗原受容体 |
| WO2021131944A1 (ja) * | 2019-12-27 | 2021-07-01 | 国立大学法人神戸大学 | 癌遺伝子治療薬 |
| JP7737708B2 (ja) | 2019-12-27 | 2025-09-11 | 国立大学法人神戸大学 | 癌遺伝子治療薬 |
| JPWO2021131944A1 (enExample) * | 2019-12-27 | 2021-07-01 | ||
| US12459984B2 (en) | 2019-12-27 | 2025-11-04 | National University Corporation Kobe University | Cancer gene therapy drug |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2015092024A2 (en) | 2015-06-25 |
| US20170073423A1 (en) | 2017-03-16 |
| AU2014368383A1 (en) | 2016-06-30 |
| WO2015092024A3 (en) | 2015-08-13 |
| US10239948B2 (en) | 2019-03-26 |
| EP3083691A2 (en) | 2016-10-26 |
| AU2014368383B2 (en) | 2020-01-16 |
| EP3808410A1 (en) | 2021-04-21 |
| CA2934436A1 (en) | 2015-06-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US10239948B2 (en) | Method of engineering multi-input signal sensitive T cell for immunotherapy | |
| AU2021201967B2 (en) | Methods for engineering allogeneic and immunosuppressive resistant T cell for immunotherapy | |
| JP6998917B2 (ja) | 免疫療法のために同種異系かつ高活性のt細胞を操作するための方法 | |
| CN111479921B (zh) | 用于以基因方式修饰且扩增淋巴细胞以及调节其活性的方法及组合物 | |
| CN110869046B (zh) | 通用型抗cd22嵌合抗原受体工程化的免疫细胞 | |
| AU2016313082B2 (en) | Chimeric antigen receptors with integrated controllable functions | |
| KR102170533B1 (ko) | 암 면역요법을 위한 cd33 특이적 키메라 항원 수용체들 | |
| EP2893004B1 (en) | Multi-chain chimeric antigen receptor and uses thereof | |
| AU2015367317A1 (en) | Inhibitory chimeric antigen receptor (iCAR or N-CAR) expressing non-T cell transduction domain | |
| CN115058395A (zh) | 嵌合抗原受体(car)、组合物及其使用方法 | |
| JP2020519267A (ja) | より安全な細胞免疫療法のためのプロテアーゼベースの切り替えキメラ抗原受容体 | |
| RU2775453C2 (ru) | Сконструированные универсальные иммунные клетки с анти-cd22 химерным антигенным рецептором | |
| HK40071469A (en) | Methods for engineering allogeneic and immunosuppressive resistant t cell for immunotherapy | |
| Zhu | Next Generation Receptors for the Enhanced Control of Cell-based Immunotherapies | |
| HK40001542A (en) | Methods for engineering allogeneic and immunosuppressive resistant t cell for immunotherapy | |
| HK40001542B (en) | Methods for engineering allogeneic and immunosuppressive resistant t cell for immunotherapy | |
| HK1208879B (en) | Methods for engineering allogeneic and immunosuppressive resistant t cell for immunotherapy | |
| HK1212728B (en) | Multi-chain chimeric antigen receptor and uses thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20160818 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20161012 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20171130 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20171130 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20181031 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20190115 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20190507 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20190805 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20191016 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20191219 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20200205 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20200617 |