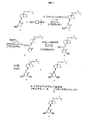
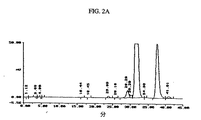
JP2004504295A - 安定化1α−ヒドロキシビタミンD - Google Patents
安定化1α−ヒドロキシビタミンD Download PDFInfo
- Publication number
- JP2004504295A JP2004504295A JP2002512124A JP2002512124A JP2004504295A JP 2004504295 A JP2004504295 A JP 2004504295A JP 2002512124 A JP2002512124 A JP 2002512124A JP 2002512124 A JP2002512124 A JP 2002512124A JP 2004504295 A JP2004504295 A JP 2004504295A
- Authority
- JP
- Japan
- Prior art keywords
- shvd
- hydroxyvitamin
- stabilized
- composition
- vitamin
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- OFHCOWSQAMBJIW-AVJTYSNKSA-N alfacalcidol Chemical compound C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@H](C)CCCC(C)C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C OFHCOWSQAMBJIW-AVJTYSNKSA-N 0.000 title claims abstract description 21
- 239000008194 pharmaceutical composition Substances 0.000 claims abstract description 9
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 claims description 42
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 claims description 36
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 claims description 33
- ZAFNJMIOTHYJRJ-UHFFFAOYSA-N Diisopropyl ether Chemical compound CC(C)OC(C)C ZAFNJMIOTHYJRJ-UHFFFAOYSA-N 0.000 claims description 25
- 238000006243 chemical reaction Methods 0.000 claims description 25
- 239000000203 mixture Substances 0.000 claims description 22
- 229930003316 Vitamin D Natural products 0.000 claims description 18
- QYSXJUFSXHHAJI-XFEUOLMDSA-N Vitamin D3 Natural products C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@H](C)CCCC(C)C)=C/C=C1\C[C@@H](O)CCC1=C QYSXJUFSXHHAJI-XFEUOLMDSA-N 0.000 claims description 18
- 235000019166 vitamin D Nutrition 0.000 claims description 18
- 239000011710 vitamin D Substances 0.000 claims description 18
- 229940046008 vitamin d Drugs 0.000 claims description 18
- TZIHFWKZFHZASV-UHFFFAOYSA-N methyl formate Chemical group COC=O TZIHFWKZFHZASV-UHFFFAOYSA-N 0.000 claims description 12
- 239000012535 impurity Substances 0.000 claims description 10
- 150000003710 vitamin D derivatives Chemical group 0.000 claims description 10
- 150000001875 compounds Chemical class 0.000 claims description 9
- -1 vitamin D compound Chemical class 0.000 claims description 9
- 238000004128 high performance liquid chromatography Methods 0.000 claims description 8
- 238000000034 method Methods 0.000 claims description 8
- 238000003860 storage Methods 0.000 claims description 8
- ZWEHNKRNPOVVGH-UHFFFAOYSA-N 2-Butanone Chemical compound CCC(C)=O ZWEHNKRNPOVVGH-UHFFFAOYSA-N 0.000 claims description 6
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 claims description 6
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 claims description 6
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims description 6
- 238000001953 recrystallisation Methods 0.000 claims description 6
- 239000007903 gelatin capsule Substances 0.000 claims description 5
- 239000007858 starting material Substances 0.000 claims description 5
- IMNFDUFMRHMDMM-UHFFFAOYSA-N N-Heptane Chemical compound CCCCCCC IMNFDUFMRHMDMM-UHFFFAOYSA-N 0.000 claims description 4
- OFBQJSOFQDEBGM-UHFFFAOYSA-N Pentane Chemical compound CCCCC OFBQJSOFQDEBGM-UHFFFAOYSA-N 0.000 claims description 4
- 239000004480 active ingredient Substances 0.000 claims description 4
- 239000002775 capsule Substances 0.000 claims description 4
- 239000003960 organic solvent Substances 0.000 claims description 4
- 239000007787 solid Substances 0.000 claims description 4
- 239000002904 solvent Substances 0.000 claims description 4
- 235000019864 coconut oil Nutrition 0.000 claims description 3
- 239000003240 coconut oil Substances 0.000 claims description 3
- 239000002552 dosage form Substances 0.000 claims description 3
- 230000000694 effects Effects 0.000 claims description 3
- 239000003921 oil Substances 0.000 claims description 3
- 235000019198 oils Nutrition 0.000 claims description 3
- 239000013557 residual solvent Substances 0.000 claims description 3
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 claims description 2
- FKCBLVCOSCZFHV-UHFFFAOYSA-N acetonitrile;ethanol Chemical compound CCO.CC#N FKCBLVCOSCZFHV-UHFFFAOYSA-N 0.000 claims description 2
- 239000003814 drug Substances 0.000 claims description 2
- WBJINCZRORDGAQ-UHFFFAOYSA-N formic acid ethyl ester Natural products CCOC=O WBJINCZRORDGAQ-UHFFFAOYSA-N 0.000 claims description 2
- QKGYJVXSKCDGOK-UHFFFAOYSA-N hexane;propan-2-ol Chemical compound CC(C)O.CCCCCC QKGYJVXSKCDGOK-UHFFFAOYSA-N 0.000 claims description 2
- 238000004519 manufacturing process Methods 0.000 claims description 2
- 239000000843 powder Substances 0.000 claims description 2
- 239000003826 tablet Substances 0.000 claims description 2
- 229940088594 vitamin Drugs 0.000 claims description 2
- 229930003231 vitamin Natural products 0.000 claims description 2
- 235000013343 vitamin Nutrition 0.000 claims description 2
- 239000011782 vitamin Substances 0.000 claims description 2
- 150000003722 vitamin derivatives Chemical group 0.000 claims description 2
- 201000010099 disease Diseases 0.000 claims 3
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims 3
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 claims 2
- 230000005856 abnormality Effects 0.000 claims 2
- 230000009102 absorption Effects 0.000 claims 2
- 238000010521 absorption reaction Methods 0.000 claims 2
- 229910052791 calcium Inorganic materials 0.000 claims 2
- 239000011575 calcium Substances 0.000 claims 2
- 230000003463 hyperproliferative effect Effects 0.000 claims 2
- 230000028993 immune response Effects 0.000 claims 2
- 230000028709 inflammatory response Effects 0.000 claims 2
- 230000004060 metabolic process Effects 0.000 claims 2
- 230000032258 transport Effects 0.000 claims 2
- NEETXMRNUNEBRH-GOTXBORWSA-N (1r,3s,5z)-5-[(2e)-2-[(1r,3as,7ar)-1-[(2r,5s)-5,6-dimethylheptan-2-yl]-7a-methyl-2,3,3a,5,6,7-hexahydro-1h-inden-4-ylidene]ethylidene]-4-methylidenecyclohexane-1,3-diol Chemical compound C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@H](C)CC[C@H](C)C(C)C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C NEETXMRNUNEBRH-GOTXBORWSA-N 0.000 claims 1
- 201000004681 Psoriasis Diseases 0.000 claims 1
- 238000001035 drying Methods 0.000 claims 1
- 239000008187 granular material Substances 0.000 claims 1
- 230000000640 hydroxylating effect Effects 0.000 claims 1
- 230000001678 irradiating effect Effects 0.000 claims 1
- 230000006641 stabilisation Effects 0.000 claims 1
- 238000011105 stabilization Methods 0.000 claims 1
- 239000000243 solution Substances 0.000 description 30
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 20
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 15
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 12
- CSNNHWWHGAXBCP-UHFFFAOYSA-L Magnesium sulfate Chemical compound [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 description 12
- 239000011653 vitamin D2 Substances 0.000 description 12
- MECHNRXZTMCUDQ-RKHKHRCZSA-N vitamin D2 Chemical compound C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@H](C)/C=C/[C@H](C)C(C)C)=C\C=C1\C[C@@H](O)CCC1=C MECHNRXZTMCUDQ-RKHKHRCZSA-N 0.000 description 11
- 238000012360 testing method Methods 0.000 description 10
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 9
- MECHNRXZTMCUDQ-UHFFFAOYSA-N Vitamin D2 Natural products C1CCC2(C)C(C(C)C=CC(C)C(C)C)CCC2C1=CC=C1CC(O)CCC1=C MECHNRXZTMCUDQ-UHFFFAOYSA-N 0.000 description 9
- 229960002061 ergocalciferol Drugs 0.000 description 9
- 238000009472 formulation Methods 0.000 description 9
- 239000000741 silica gel Substances 0.000 description 9
- 229910002027 silica gel Inorganic materials 0.000 description 9
- 235000001892 vitamin D2 Nutrition 0.000 description 9
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 8
- UIIMBOGNXHQVGW-UHFFFAOYSA-M Sodium bicarbonate Chemical class [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 8
- 238000002360 preparation method Methods 0.000 description 8
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 8
- 239000008186 active pharmaceutical agent Substances 0.000 description 7
- 229940088679 drug related substance Drugs 0.000 description 7
- 229960004756 ethanol Drugs 0.000 description 7
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 7
- YXFVVABEGXRONW-UHFFFAOYSA-N Toluene Chemical compound CC1=CC=CC=C1 YXFVVABEGXRONW-UHFFFAOYSA-N 0.000 description 6
- 239000013078 crystal Substances 0.000 description 6
- 239000012259 ether extract Substances 0.000 description 6
- 230000015572 biosynthetic process Effects 0.000 description 5
- 229960000935 dehydrated alcohol Drugs 0.000 description 5
- HKXBNHCUPKIYDM-CGMHZMFXSA-N doxercalciferol Chemical compound C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@H](C)/C=C/[C@H](C)C(C)C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C HKXBNHCUPKIYDM-CGMHZMFXSA-N 0.000 description 5
- 239000010410 layer Substances 0.000 description 5
- 229910052943 magnesium sulfate Inorganic materials 0.000 description 5
- 235000019341 magnesium sulphate Nutrition 0.000 description 5
- 239000011541 reaction mixture Substances 0.000 description 5
- 238000013112 stability test Methods 0.000 description 5
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 4
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical class [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 4
- 229910052786 argon Inorganic materials 0.000 description 4
- 238000010828 elution Methods 0.000 description 4
- 238000001914 filtration Methods 0.000 description 4
- 230000014759 maintenance of location Effects 0.000 description 4
- 239000012044 organic layer Substances 0.000 description 4
- 239000000047 product Substances 0.000 description 4
- 238000003756 stirring Methods 0.000 description 4
- 238000003786 synthesis reaction Methods 0.000 description 4
- WSLDOOZREJYCGB-UHFFFAOYSA-N 1,2-Dichloroethane Chemical compound ClCCCl WSLDOOZREJYCGB-UHFFFAOYSA-N 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- 239000012300 argon atmosphere Substances 0.000 description 3
- 239000012298 atmosphere Substances 0.000 description 3
- 239000000945 filler Substances 0.000 description 3
- 239000000706 filtrate Substances 0.000 description 3
- 150000002500 ions Chemical class 0.000 description 3
- 238000000746 purification Methods 0.000 description 3
- 235000017557 sodium bicarbonate Nutrition 0.000 description 3
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- GVJHHUAWPYXKBD-UHFFFAOYSA-N (±)-α-Tocopherol Chemical compound OC1=C(C)C(C)=C2OC(CCCC(C)CCCC(C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-UHFFFAOYSA-N 0.000 description 2
- VHYFNPMBLIVWCW-UHFFFAOYSA-N 4-Dimethylaminopyridine Chemical compound CN(C)C1=CC=NC=C1 VHYFNPMBLIVWCW-UHFFFAOYSA-N 0.000 description 2
- YYROPELSRYBVMQ-UHFFFAOYSA-N 4-toluenesulfonyl chloride Chemical compound CC1=CC=C(S(Cl)(=O)=O)C=C1 YYROPELSRYBVMQ-UHFFFAOYSA-N 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- 0 CC(C)[C@@](C)C=C[C@@](C(CC1)C(C)(CCC2)C1C2=CC=C(C[C@@](C1)O)C(C)[C@]1O)*=C Chemical compound CC(C)[C@@](C)C=C[C@@](C(CC1)C(C)(CCC2)C1C2=CC=C(C[C@@](C1)O)C(C)[C@]1O)*=C 0.000 description 2
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 2
- 108010010803 Gelatin Proteins 0.000 description 2
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 2
- DTXXSJZBSTYZKE-ZDQKKZTESA-N Maxacalcitol Chemical compound C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@@H](OCCC(C)(C)O)C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C DTXXSJZBSTYZKE-ZDQKKZTESA-N 0.000 description 2
- 238000005481 NMR spectroscopy Methods 0.000 description 2
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 2
- ZVQOOHYFBIDMTQ-UHFFFAOYSA-N [methyl(oxido){1-[6-(trifluoromethyl)pyridin-3-yl]ethyl}-lambda(6)-sulfanylidene]cyanamide Chemical compound N#CN=S(C)(=O)C(C)C1=CC=C(C(F)(F)F)N=C1 ZVQOOHYFBIDMTQ-UHFFFAOYSA-N 0.000 description 2
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 2
- 239000011612 calcitriol Substances 0.000 description 2
- DIOQZVSQGTUSAI-UHFFFAOYSA-N decane Chemical compound CCCCCCCCCC DIOQZVSQGTUSAI-UHFFFAOYSA-N 0.000 description 2
- 239000000284 extract Substances 0.000 description 2
- 239000008273 gelatin Substances 0.000 description 2
- 229920000159 gelatin Polymers 0.000 description 2
- 235000019322 gelatine Nutrition 0.000 description 2
- 235000011852 gelatine desserts Nutrition 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 238000002156 mixing Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 239000001301 oxygen Substances 0.000 description 2
- 229910052760 oxygen Inorganic materials 0.000 description 2
- DHRLEVQXOMLTIM-UHFFFAOYSA-N phosphoric acid;trioxomolybdenum Chemical compound O=[Mo](=O)=O.O=[Mo](=O)=O.O=[Mo](=O)=O.O=[Mo](=O)=O.O=[Mo](=O)=O.O=[Mo](=O)=O.O=[Mo](=O)=O.O=[Mo](=O)=O.O=[Mo](=O)=O.O=[Mo](=O)=O.O=[Mo](=O)=O.O=[Mo](=O)=O.OP(O)(O)=O DHRLEVQXOMLTIM-UHFFFAOYSA-N 0.000 description 2
- 238000006303 photolysis reaction Methods 0.000 description 2
- 230000015843 photosynthesis, light reaction Effects 0.000 description 2
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 2
- JPJALAQPGMAKDF-UHFFFAOYSA-N selenium dioxide Chemical compound O=[Se]=O JPJALAQPGMAKDF-UHFFFAOYSA-N 0.000 description 2
- 230000003595 spectral effect Effects 0.000 description 2
- 239000012258 stirred mixture Substances 0.000 description 2
- 230000001225 therapeutic effect Effects 0.000 description 2
- 238000002211 ultraviolet spectrum Methods 0.000 description 2
- PKFBWEUIKKCWEW-WEZTXPJVSA-N (1r,3r)-5-[(2e)-2-[(1r,3as,7ar)-1-[(2r)-6-hydroxy-6-methylheptan-2-yl]-7a-methyl-2,3,3a,5,6,7-hexahydro-1h-inden-4-ylidene]ethylidene]cyclohexane-1,3-diol Chemical compound C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@@H](CCCC(C)(C)O)C)=C\C=C1C[C@@H](O)C[C@H](O)C1 PKFBWEUIKKCWEW-WEZTXPJVSA-N 0.000 description 1
- DYLIWHYUXAJDOJ-OWOJBTEDSA-N (e)-4-(6-aminopurin-9-yl)but-2-en-1-ol Chemical compound NC1=NC=NC2=C1N=CN2C\C=C\CO DYLIWHYUXAJDOJ-OWOJBTEDSA-N 0.000 description 1
- SCYULBFZEHDVBN-UHFFFAOYSA-N 1,1-Dichloroethane Chemical compound CC(Cl)Cl SCYULBFZEHDVBN-UHFFFAOYSA-N 0.000 description 1
- NXXNVJDXUHMAHU-UHFFFAOYSA-N 1-anthracen-9-ylethanone Chemical compound C1=CC=C2C(C(=O)C)=C(C=CC=C3)C3=CC2=C1 NXXNVJDXUHMAHU-UHFFFAOYSA-N 0.000 description 1
- JRMXMUCILWKNNW-UHFFFAOYSA-N 1-butoxy-2-methylbenzene Chemical compound CCCCOC1=CC=CC=C1C JRMXMUCILWKNNW-UHFFFAOYSA-N 0.000 description 1
- 102100027324 2-hydroxyacyl-CoA lyase 1 Human genes 0.000 description 1
- 101001009252 Homo sapiens 2-hydroxyacyl-CoA lyase 1 Proteins 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 1
- UIIMBOGNXHQVGW-DEQYMQKBSA-M Sodium bicarbonate-14C Chemical class [Na+].O[14C]([O-])=O UIIMBOGNXHQVGW-DEQYMQKBSA-M 0.000 description 1
- 229930003427 Vitamin E Natural products 0.000 description 1
- 238000007792 addition Methods 0.000 description 1
- 238000007605 air drying Methods 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 230000003078 antioxidant effect Effects 0.000 description 1
- 235000006708 antioxidants Nutrition 0.000 description 1
- 230000008033 biological extinction Effects 0.000 description 1
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- LWQQLNNNIPYSNX-UROSTWAQSA-N calcipotriol Chemical compound C1([C@H](O)/C=C/[C@@H](C)[C@@H]2[C@]3(CCCC(/[C@@H]3CC2)=C\C=C\2C([C@@H](O)C[C@H](O)C/2)=C)C)CC1 LWQQLNNNIPYSNX-UROSTWAQSA-N 0.000 description 1
- 229960002882 calcipotriol Drugs 0.000 description 1
- GMRQFYUYWCNGIN-NKMMMXOESA-N calcitriol Chemical compound C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@@H](CCCC(C)(C)O)C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C GMRQFYUYWCNGIN-NKMMMXOESA-N 0.000 description 1
- 239000012159 carrier gas Substances 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 238000004440 column chromatography Methods 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 238000002425 crystallisation Methods 0.000 description 1
- 230000008025 crystallization Effects 0.000 description 1
- 229960000413 doxercalciferol Drugs 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 239000012467 final product Substances 0.000 description 1
- WIGCFUFOHFEKBI-UHFFFAOYSA-N gamma-tocopherol Natural products CC(C)CCCC(C)CCCC(C)CCCC1CCC2C(C)C(O)C(C)C(C)C2O1 WIGCFUFOHFEKBI-UHFFFAOYSA-N 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 238000004817 gas chromatography Methods 0.000 description 1
- 239000012362 glacial acetic acid Substances 0.000 description 1
- 235000011187 glycerol Nutrition 0.000 description 1
- LHGVFZTZFXWLCP-UHFFFAOYSA-N guaiacol Chemical compound COC1=CC=CC=C1O LHGVFZTZFXWLCP-UHFFFAOYSA-N 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 239000001307 helium Substances 0.000 description 1
- 229910052734 helium Inorganic materials 0.000 description 1
- SWQJXJOGLNCZEY-UHFFFAOYSA-N helium atom Chemical compound [He] SWQJXJOGLNCZEY-UHFFFAOYSA-N 0.000 description 1
- 230000033444 hydroxylation Effects 0.000 description 1
- 238000005805 hydroxylation reaction Methods 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 238000002329 infrared spectrum Methods 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 238000004806 packaging method and process Methods 0.000 description 1
- 229960000987 paricalcitol Drugs 0.000 description 1
- BPKAHTKRCLCHEA-UBFJEZKGSA-N paricalcitol Chemical compound C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@@H](\C=C\[C@H](C)C(C)(C)O)C)=C\C=C1C[C@@H](O)C[C@H](O)C1 BPKAHTKRCLCHEA-UBFJEZKGSA-N 0.000 description 1
- 239000000825 pharmaceutical preparation Substances 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- YUGCAAVRZWBXEQ-WHTXLNIXSA-N previtamin D3 Chemical compound C=1([C@@H]2CC[C@@H]([C@]2(CCC=1)C)[C@H](C)CCCC(C)C)\C=C/C1=C(C)CC[C@H](O)C1 YUGCAAVRZWBXEQ-WHTXLNIXSA-N 0.000 description 1
- 239000000651 prodrug Substances 0.000 description 1
- 229940002612 prodrug Drugs 0.000 description 1
- 239000000376 reactant Substances 0.000 description 1
- 230000035484 reaction time Effects 0.000 description 1
- 230000000630 rising effect Effects 0.000 description 1
- 238000003797 solvolysis reaction Methods 0.000 description 1
- 230000000087 stabilizing effect Effects 0.000 description 1
- 229910001220 stainless steel Inorganic materials 0.000 description 1
- 239000010935 stainless steel Substances 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 230000002459 sustained effect Effects 0.000 description 1
- 238000010189 synthetic method Methods 0.000 description 1
- CIHOLLKRGTVIJN-UHFFFAOYSA-N tert‐butyl hydroperoxide Chemical compound CC(C)(C)OO CIHOLLKRGTVIJN-UHFFFAOYSA-N 0.000 description 1
- 239000004408 titanium dioxide Substances 0.000 description 1
- 235000010215 titanium dioxide Nutrition 0.000 description 1
- 238000000870 ultraviolet spectroscopy Methods 0.000 description 1
- 239000011647 vitamin D3 Substances 0.000 description 1
- QYSXJUFSXHHAJI-YRZJJWOYSA-N vitamin D3 Chemical compound C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@H](C)CCCC(C)C)=C\C=C1\C[C@@H](O)CCC1=C QYSXJUFSXHHAJI-YRZJJWOYSA-N 0.000 description 1
- DIPPFEXMRDPFBK-JPWDPSJFSA-N vitamin D4 Chemical compound C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@H](C)CC[C@H](C)C(C)C)=C\C=C1\C[C@@H](O)CCC1=C DIPPFEXMRDPFBK-JPWDPSJFSA-N 0.000 description 1
- 235000019165 vitamin E Nutrition 0.000 description 1
- 229940046009 vitamin E Drugs 0.000 description 1
- 239000011709 vitamin E Substances 0.000 description 1
- 230000003442 weekly effect Effects 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C401/00—Irradiation products of cholesterol or its derivatives; Vitamin D derivatives, 9,10-seco cyclopenta[a]phenanthrene or analogues obtained by chemical preparation without irradiation
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/59—Compounds containing 9, 10- seco- cyclopenta[a]hydrophenanthrene ring systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/02—Nutrients, e.g. vitamins, minerals
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Epidemiology (AREA)
- Nutrition Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Diabetes (AREA)
- Hematology (AREA)
- Obesity (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Engineering & Computer Science (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US21906800P | 2000-07-18 | 2000-07-18 | |
| PCT/US2001/022729 WO2002006218A2 (en) | 2000-07-18 | 2001-07-18 | STABILIZED 1α-HYDROXY VITAMIN D |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2004504295A true JP2004504295A (ja) | 2004-02-12 |
| JP2004504295A5 JP2004504295A5 (enExample) | 2008-09-04 |
Family
ID=22817719
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2002512124A Pending JP2004504295A (ja) | 2000-07-18 | 2001-07-18 | 安定化1α−ヒドロキシビタミンD |
Country Status (7)
| Country | Link |
|---|---|
| US (2) | US6903083B2 (enExample) |
| EP (2) | EP1301479A2 (enExample) |
| JP (1) | JP2004504295A (enExample) |
| AU (2) | AU7895601A (enExample) |
| CA (1) | CA2414407A1 (enExample) |
| IL (2) | IL153378A0 (enExample) |
| WO (1) | WO2002006218A2 (enExample) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2005247803A (ja) * | 2004-03-08 | 2005-09-15 | Zeria Pharmaceut Co Ltd | 点眼剤及びその容器 |
| JP2014062131A (ja) * | 2008-03-12 | 2014-04-10 | Cytochroma Inc | 安定化1,25−ジヒドロキシビタミンd2及びその製造方法 |
| JP2018172299A (ja) * | 2017-03-31 | 2018-11-08 | ナガセ医薬品株式会社 | マキサカルシトール含有水溶液製剤 |
Families Citing this family (36)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6087350A (en) * | 1997-08-29 | 2000-07-11 | University Of Pittsburgh Of The Commonwealth System Of Higher Education | Use of pretreatment chemicals to enhance efficacy of cytotoxic agents |
| IL153378A0 (en) * | 2000-07-18 | 2003-07-06 | Bone Care Internat Inc | STABILIZED 1alpha-HYDROXY VITAMIN D |
| US20030191093A1 (en) * | 2001-12-03 | 2003-10-09 | Novacea, Inc. | Pharmaceutical compositions comprising active vitamin D compounds |
| US7148211B2 (en) * | 2002-09-18 | 2006-12-12 | Genzyme Corporation | Formulation for lipophilic agents |
| US20050101576A1 (en) * | 2003-11-06 | 2005-05-12 | Novacea, Inc. | Methods of using vitamin D compounds in the treatment of myelodysplastic syndromes |
| AU2003291810A1 (en) * | 2002-11-18 | 2004-06-15 | Teva Pharmaceutical Industries Ltd. | A crystallization method for purification of calcipotriene |
| US20050026877A1 (en) * | 2002-12-03 | 2005-02-03 | Novacea, Inc. | Pharmaceutical compositions comprising active vitamin D compounds |
| US20050020546A1 (en) * | 2003-06-11 | 2005-01-27 | Novacea, Inc. | Pharmaceutical compositions comprising active vitamin D compounds |
| AU2005309806B2 (en) * | 2004-11-22 | 2012-03-08 | Wisconsin Alumni Research Foundation | 2-methylene-19-nor-1alpha-hydroxy-17-ene-homopregnacalciferol and its uses |
| EP1945185B1 (en) * | 2005-10-12 | 2016-02-03 | Proventiv Therapeutics, LLC | Methods and articles for treating 25-hydroxyvitamin d insufficiency and deficiency |
| SI1993559T1 (sl) | 2006-02-03 | 2017-01-31 | Opko Renal, Llc | Zdravljenje nezadostnosti in pomanjkanja vitamina D s 25-hidroksivitaminon D2 in 25-hidroksivitaminom D3 |
| BRPI0621471A2 (pt) * | 2006-03-17 | 2012-09-11 | Leo Pharma As | métodos de isomerização de uma solução de um derivado de vitamina d, para produzir e preparar calcipotriol ou monoidrato de calcipotriol, e para a fabricação de uma formulação farmacêutica ou medicamento, e, uso de um reator foto-reator de fluxo direto ou foto-reator de fluxo contìnuo |
| DK3357496T3 (da) | 2006-06-21 | 2020-05-11 | Opko Ireland Global Holdings Ltd | Terapi ved brug af vitamin d-repletteringsmiddel og vitamin d-hormonsubstitutionsmiddel |
| CA2669914C (en) * | 2006-11-16 | 2017-11-07 | Kai Pharmaceuticals, Inc. | Polycationic calcium modulator peptides for the treatment of hyperparathyroidism and hypercalcemic disorders |
| PT2148661E (pt) | 2007-04-25 | 2013-03-06 | Proventiv Therapeutics Llc | Composições orais de libertação controlada compreendendo um composto de vitamina d e veículo ceroso |
| CA2684778C (en) * | 2007-04-25 | 2017-09-05 | Cytochroma Inc. | Methods and compounds for vitamin d therapy |
| ES3047207T3 (en) * | 2007-04-25 | 2025-12-03 | Opko Renal Llc | Method of safely and effectively treating and preventing secondary hyperparathyroidism in chronic kidney disease |
| PT2148684E (pt) * | 2007-04-25 | 2013-04-19 | Cytochroma Inc | Método de tratamento para a insuficiência e deficiência de vitamina d |
| EP2237837B1 (en) * | 2007-12-28 | 2012-08-22 | Wisconsin Alumni Research Foundation | (20r)-23,23-difluoro-2-methylene-19-nor-bishomopregnacalciferol-vitamin d analogs |
| CA2710954C (en) * | 2007-12-28 | 2015-07-21 | Wisconsin Alumni Research Foundation | (20s)-23,23-difluoro-2-methylene-19-nor-bishomopregnacalciferol-vitamin d analogs |
| CN106853250A (zh) | 2008-04-02 | 2017-06-16 | 赛特克罗公司 | 用于维生素d缺乏症和相关障碍的方法、组合物、用途和试剂盒 |
| US8013176B2 (en) * | 2008-09-11 | 2011-09-06 | Alphora Research Inc. | Paricalcitol purification |
| EP2376508A4 (en) * | 2008-11-26 | 2012-06-13 | Cytochroma Inc | PROCESS FOR SYNTHESIS OF VITAMIN D ANALOGUE |
| JP5270799B2 (ja) | 2009-07-29 | 2013-08-21 | カイ・ファーマシューティカルズ・インコーポレイテッド | 副甲状腺ホルモンを低減する治療剤 |
| US11672809B2 (en) | 2010-03-29 | 2023-06-13 | Eirgen Pharma Ltd. | Methods and compositions for reducing parathyroid levels |
| CA2698160C (en) * | 2010-03-30 | 2017-07-25 | Alphora Research Inc. | Stabilized doxercalciferol and process for manufacturing the same |
| US20120108554A1 (en) * | 2010-10-28 | 2012-05-03 | Formosa Laboratories, Inc. | PROCESS FOR PREPARING HIGH PURITY 1alpha-HYDROXY VITAMIN D2 |
| CA2837445C (en) | 2011-06-08 | 2022-10-04 | Kai Pharmaceuticals, Inc. | Therapeutic agents for regulating serum phosphorus |
| CN109985228A (zh) | 2011-11-10 | 2019-07-09 | 凯伊药品公司 | 拟钙剂及其使用方法 |
| KR101847947B1 (ko) | 2013-03-15 | 2018-05-28 | 옵코 아이피 홀딩스 Ⅱ 인코포레이티드 | 안정화되고 변형된 비타민 d 방출 제형 |
| US10220047B2 (en) | 2014-08-07 | 2019-03-05 | Opko Ireland Global Holdings, Ltd. | Adjunctive therapy with 25-hydroxyvitamin D and articles therefor |
| CN105348164B (zh) * | 2015-10-29 | 2017-03-29 | 无锡福祈制药有限公司 | 一种1α‑羟基维生素D2的制备方法 |
| WO2017182237A1 (en) | 2016-03-28 | 2017-10-26 | Opko Ireland Global Holdings, Limited | Methods of vitamin d treatment |
| CN106748642B (zh) * | 2016-11-28 | 2019-11-05 | 无锡福祈制药有限公司 | 一种度骨化醇类似物wxfq-65的合成方法 |
| CN106748942B (zh) * | 2016-11-28 | 2018-06-29 | 无锡福祈制药有限公司 | 度骨化醇类似物wxfq-65及其合成方法 |
| CN113666858A (zh) * | 2021-08-20 | 2021-11-19 | 江苏四环生物制药有限公司 | 一种度骨化醇及其制备方法 |
Citations (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS58126862A (ja) * | 1981-11-02 | 1983-07-28 | リサ−チ・インステイチユ−ト・フオア・メデイスン・アンド・ケミストリ−・インコ−ポレ−テツド | 1−ヒドロキシル化ビタミンd化合物の製法 |
| JPS62298572A (ja) * | 1986-06-19 | 1987-12-25 | Chugai Pharmaceut Co Ltd | 1α−ヒドロキシビタミンD3の精製法 |
| JPS63126891A (ja) * | 1986-11-14 | 1988-05-30 | Nisshin Flour Milling Co Ltd | ステロイド誘導体およびその製造方法 |
| JPS63126862A (ja) * | 1987-10-30 | 1988-05-30 | Nisshin Flour Milling Co Ltd | 1α−ヒドロキシビタミンD↓2の製造方法 |
| JPH01246276A (ja) * | 1988-03-25 | 1989-10-02 | Teikoku Chem Ind Corp Ltd | 1α−ヒドロキシ―6―(1,3−ベンゾジチオール−2−イル)オキシー3,5−シクロビタミンD↓3 |
| JPH02270860A (ja) * | 1988-03-25 | 1990-11-05 | Teikoku Chem Ind Corp Ltd | 1α―ヒドロキシ―ビタミンD↓3の製造方法 |
| JPH05279260A (ja) * | 1991-04-09 | 1993-10-26 | Takeda Chem Ind Ltd | 安定化されたビタミンd類製剤 |
| JPH05320127A (ja) * | 1992-03-27 | 1993-12-03 | Nisshin Flour Milling Co Ltd | 活性型ビタミンd誘導体 |
| JPH05339230A (ja) * | 1992-03-12 | 1993-12-21 | Nisshin Flour Milling Co Ltd | 活性型ビタミンd2及びその誘導体の製造法 |
| JPH06256301A (ja) * | 1992-11-04 | 1994-09-13 | Wisconsin Alumni Res Found | 1α,24−ジヒドロキシビタミンD類似体の製造方法 |
| JP2002535307A (ja) * | 1999-01-20 | 2002-10-22 | ウィスコンシン・アルムニ・リサーチ・ファウンデーション | 結晶1α−ヒドロキシビタミンD2とその精製方法 |
Family Cites Families (125)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2383446A (en) | 1941-06-04 | 1945-08-28 | Du Pont | Antirachitic materials and processes for their production |
| US3697559A (en) | 1971-02-25 | 1972-10-10 | Wisconsin Alumni Res Found | 1,25-dihydroxycholecalciferol |
| US3741996A (en) | 1971-12-02 | 1973-06-26 | Wisconsin Alumni Res Found | 1{60 -hydroxycholecalciferol |
| US5472957A (en) * | 1973-01-10 | 1995-12-05 | Research Institute For Medicine And Chemistry | Chemical compounds and process |
| US3907843A (en) | 1974-06-14 | 1975-09-23 | Wisconsin Alumni Res Found | 1{60 -Hydroxyergocalciferol and processes for preparing same |
| JPS5452717A (en) | 1977-09-29 | 1979-04-25 | Chugai Pharmaceut Co Ltd | Stable oily pharmaceuticals containing 1alpha-hydroxyvitamin d |
| US4202829A (en) | 1978-01-05 | 1980-05-13 | Wisconsin Alumni Research Foundation | Process for preparing 1α-hydroxylated compounds |
| US4195027A (en) | 1978-01-16 | 1980-03-25 | Wisconsin Alumni Research Foundation | Process for preparing 1α-hydroxylated compounds |
| US4260549A (en) | 1979-05-21 | 1981-04-07 | Wisconsin Alumni Research Foundation | Process for preparing 1α-hydroxylated compounds |
| US4160803A (en) | 1978-03-23 | 1979-07-10 | Corning Glass Works | Self packaged test kit |
| US4225596A (en) | 1978-10-13 | 1980-09-30 | Wisconsin Alumni Research Foundation | Method for treating calcium imbalance and improving calcium absorption in mammals |
| US4234495A (en) | 1979-09-10 | 1980-11-18 | Wisconsin Alumni Research Foundation | Process for preparing 1α-hydroxyvitamin D compounds from 1α-hydroxy-3,5-cyclovitamin D compounds |
| US4362710A (en) | 1980-07-04 | 1982-12-07 | Nissan Gosei Kogyo Co., Ltd. | Feeds for baby pigs, process for preparing the same and method of breeding baby pigs |
| JPS57149224A (en) | 1981-03-13 | 1982-09-14 | Chugai Pharmaceut Co Ltd | Tumor-suppressing agent |
| US4508651A (en) | 1983-03-21 | 1985-04-02 | Hoffmann-La Roche Inc. | Synthesis of 1α,25-dihydroxyergocalciferol |
| CH665834A5 (de) | 1983-05-09 | 1988-06-15 | Wisconsin Alumni Res Found | Verfahren zur herstellung von 1alpha,25-dihydroxyliertem vitamin d(2) und verwandten verbindungen. |
| US4689180A (en) | 1984-01-30 | 1987-08-25 | Wisconsin Alumni Research Foundation | 1α,25-dihydroxy-22Z-dehydroxyvitamin D compound |
| US4588716A (en) | 1984-05-04 | 1986-05-13 | Wisconsin Alumni Research Foundation | Method for treating metabolic bone disease in mammals |
| US4554106A (en) * | 1984-11-01 | 1985-11-19 | Wisconsin Alumni Research Foundation | Method for preparing 1α-hydroxyvitamin D compounds |
| US4555364A (en) | 1984-11-01 | 1985-11-26 | Wisconsin Alumni Research Foundation | Method for preparing 1-hydroxyvitamin D compounds |
| US4728643A (en) | 1984-11-02 | 1988-03-01 | The General Hospital Corporation | Method of treating psoriasis |
| US5037816A (en) | 1984-11-02 | 1991-08-06 | The General Hospital Corporation | Method of treating psoriasis |
| US4717721A (en) | 1985-05-30 | 1988-01-05 | Howard W. Bremer | Sidechain homo-vitamin D compounds with preferential anti-cancer activity |
| US4661294A (en) | 1985-03-18 | 1987-04-28 | The General Hospital Corporation | Biologically active 1-thio derivatives of vitamin D |
| IL78342A (en) | 1985-04-04 | 1991-06-10 | Gen Hospital Corp | Pharmaceutical composition for treatment of osteoporosis in humans comprising a parathyroid hormone or a fragment thereof |
| WO1987000834A1 (en) | 1985-08-02 | 1987-02-12 | Leo Pharmaceutical Products Ltd. A/S | Novel vitamin d analogues |
| US5554386A (en) | 1986-07-03 | 1996-09-10 | Advanced Magnetics, Inc. | Delivery of therapeutic agents to receptors using polysaccharides |
| US5338532A (en) | 1986-08-18 | 1994-08-16 | The Dow Chemical Company | Starburst conjugates |
| US5527524A (en) | 1986-08-18 | 1996-06-18 | The Dow Chemical Company | Dense star polymer conjugates |
| US4833125A (en) | 1986-12-05 | 1989-05-23 | The General Hospital Corporation | Method of increasing bone mass |
| US4902481A (en) | 1987-12-11 | 1990-02-20 | Millipore Corporation | Multi-well filtration test apparatus |
| US5087619A (en) | 1988-01-20 | 1992-02-11 | Hoffman-La Roche Inc. | Vitamin D3 analogs |
| US5145846A (en) | 1988-01-20 | 1992-09-08 | Hoffmann-La Roche Inc. | Vitamin D3 analogs |
| US5232836A (en) | 1988-05-04 | 1993-08-03 | Ire-Medgenix S.A. | Vitamin D derivatives: therapeutic applications and applications to assays of metabolites of vitamin D |
| US5104864A (en) | 1988-08-02 | 1992-04-14 | Bone Care International, Inc. | Method for treating and preventing loss of bone mass |
| US5602116A (en) | 1988-08-02 | 1997-02-11 | Bone Care International, Inc. | Method for treating and preventing secondary hyperparathyroidism |
| US5098899A (en) | 1989-03-06 | 1992-03-24 | Trustees Of Boston University | Method for therapeutically treating psoriatic arthritis using vitamin D analogues and metabolites |
| US5321018A (en) | 1989-03-09 | 1994-06-14 | Wisconsin Alumni Research Foundation | Use of 1α-hydroxylated-19-nor-vitamin D compounds to treat psoriasis |
| CA1333616C (en) | 1989-03-09 | 1994-12-20 | Hector F. Deluca | 19-nor-vitamin d compounds |
| US5372996A (en) | 1989-03-10 | 1994-12-13 | Endorecherche, Inc. | Method of treatment of androgen-related diseases |
| US4948789A (en) | 1989-03-28 | 1990-08-14 | Chugai Seiyaku Kabushiki Kaisha | Suppression of parathyroid hormone synthesis and secretion |
| JP2645130B2 (ja) | 1989-03-31 | 1997-08-25 | 日清製粉株式会社 | ステロイド誘導体 |
| US5063221A (en) | 1989-04-05 | 1991-11-05 | Chugai Seiyaku Kabushiki Kaisha | Treatment for hyperparathyroidism with use of vitamin d derivatives |
| US5219528A (en) | 1989-07-28 | 1993-06-15 | Pierce Chemical Company | Apparatus for rapid immunoassays |
| DE3933034A1 (de) | 1989-10-02 | 1991-04-11 | Schering Ag | 24-homo-vitamin-d-derivate, verfahren zu ihrer herstellung |
| US5260290A (en) | 1990-02-14 | 1993-11-09 | Wisconsin Alumni Research Foundation | Homologated vitamin D2 compounds and the corresponding 1α-hydroxylated derivatives |
| US5264618A (en) | 1990-04-19 | 1993-11-23 | Vical, Inc. | Cationic lipids for intracellular delivery of biologically active molecules |
| US5194248A (en) | 1990-06-21 | 1993-03-16 | Trustees Of Boston University | Compositions comprising vitamin D analog precursors and the use thereof |
| US5141719A (en) | 1990-07-18 | 1992-08-25 | Bio-Rad Laboratories, Inc. | Multi-sample filtration plate assembly |
| US5798345A (en) | 1990-09-21 | 1998-08-25 | Bone Care International, Inc. | Method of inhibiting the hyperproliferation of malignant cells |
| US5763428A (en) | 1990-09-21 | 1998-06-09 | Bone Care International, Inc. | Methods of treating skin disorders with novel 1a-hydroxy vitamin D4 compounds and derivatives thereof |
| BR9106062A (pt) | 1990-09-21 | 1993-03-09 | Lunar Corp | Nova 1 alfa-hidroxi vitamina d4 e novos intermediarios e analogos |
| US6251883B1 (en) | 1991-01-08 | 2001-06-26 | Bone Care International, Inc. | Methods for preparation and use of 1α,24(S)-dihydroxy vitamin D2 |
| DK0550702T3 (da) | 1991-01-08 | 1999-11-01 | Bone Care Int Inc | Fremgangsmåde til fremstilling og anvendelse af 1alfa,24-dihydroxy-vitamin D2 |
| DK0503630T3 (da) | 1991-03-13 | 1996-01-29 | Kuraray Co | Cyclohexantriolderivater |
| US5264184A (en) | 1991-03-19 | 1993-11-23 | Minnesota Mining And Manufacturing Company | Device and a method for separating liquid samples |
| US5417923A (en) | 1991-04-24 | 1995-05-23 | Pfizer Inc. | Assay tray assembly |
| EP0586521A1 (en) | 1991-05-28 | 1994-03-16 | The Procter & Gamble Company | Calcium, trace mineral, vitamin d and drug therapy combinations |
| ATE143007T1 (de) | 1991-07-05 | 1996-10-15 | Duphar Int Res | Vitamin-d derivat, verfahren zu dessen herstellung sowie zwischenprodukte dafür |
| US5205989A (en) | 1991-09-18 | 1993-04-27 | Minnesota Mining And Manufacturing Company | Multi-well filtration apparatus |
| ATE239484T1 (de) | 1991-10-24 | 2003-05-15 | Isis Pharmaceuticals Inc | Derivatisierte oligonukleotide mit verbessertem aufnahmevermögen |
| WO1993014763A1 (en) | 1992-01-29 | 1993-08-05 | Lunar Corporation | 1α-HYDROXY-24-EPI-VITAMIN D¿4? |
| EP0562497A1 (en) | 1992-03-27 | 1993-09-29 | Nisshin Flour Milling Co., Ltd. | 1 alpha-hydroxy vitamins D7 and D4' processes for the preparation thereof and pharmaceutical compositions |
| US6113946A (en) | 1992-04-03 | 2000-09-05 | The Regents Of The University Of California | Self-assembling polynucleotide delivery system comprising dendrimer polycations |
| US5962731A (en) | 1992-04-22 | 1999-10-05 | Ligand Pharmaceuticals Incorporated | Compounds having selective activity for retinoid X receptors, and means for modulation of processes mediated by retinoid X receptors |
| JP3722832B2 (ja) | 1992-06-22 | 2005-11-30 | ルーナー、コーポレーション | 経口用1α−ヒドロキシプレビタミンD |
| US5795882A (en) | 1992-06-22 | 1998-08-18 | Bone Care International, Inc. | Method of treating prostatic diseases using delayed and/or sustained release vitamin D formulations |
| DE4221961A1 (de) | 1992-06-30 | 1994-01-05 | Schering Ag | 22-En-25-oxa-Derivate in der Vitamin D-Reihe, Verfahren zu ihrer Herstellung, diese Derivate enthaltenen pharmazeutische Präparate sowie deren Verwendung als Arzneimittel |
| JP3179495B2 (ja) | 1992-08-28 | 2001-06-25 | ボーン ケア インターナショナル インコーポレイテッド | 1α,24(S)−ジヒドロキシビタミンD▲下2▼の調製及び使用方法 |
| IL103224A (en) * | 1992-09-18 | 1998-08-16 | Teva Pharma | Stabilized pharmaceutical compositions containing derivatives of vitamins d2 and d3 |
| CA2096105A1 (en) | 1992-10-07 | 1994-04-08 | Enrico Giuseppe Baggiolini (Deceased) | Vitamin d3 fluorinated analogs |
| US5753638A (en) | 1992-10-07 | 1998-05-19 | Hoffmann-La Roche Inc. | Method of treating hyperproliferative skin disease with Vitamin D3 fluorinated analogs |
| US5350745A (en) | 1993-01-29 | 1994-09-27 | Lunar Corporation | Treatment of myocardial failure |
| US5366965A (en) | 1993-01-29 | 1994-11-22 | Boehringer Mannheim Gmbh | Regimen for treatment or prophylaxis of osteoporosis |
| US5478816A (en) | 1993-07-02 | 1995-12-26 | Bristol-Myers Squibb Company | Liquid vitamin formulations containing vitamin D esters |
| PT667166E (pt) | 1993-09-01 | 2001-02-28 | Teijin Ltd | Composicao de emulsao de 1alfa,24-(oh)2 vitamina d3 |
| US5763429A (en) | 1993-09-10 | 1998-06-09 | Bone Care International, Inc. | Method of treating prostatic diseases using active vitamin D analogues |
| US6103709A (en) | 1993-12-23 | 2000-08-15 | The Regents Of The University Of California | Therapeutically effective 1α,25-dihydroxyvitamin D3 analogs and methods for treatment of vitamin D diseases |
| ES2120122T3 (es) | 1994-01-20 | 1998-10-16 | Duphar Int Res | Compuestos de vitamina d y metodo de preparar estos compuestos. |
| US5597575A (en) | 1994-06-06 | 1997-01-28 | Breitbarth; Richard | Composition for stimulating and inducing hair growth |
| WO1996002501A1 (en) * | 1994-07-18 | 1996-02-01 | Lunar Corporation | SYNTHESIS OF 1α-HYDROXY VITAMIN D |
| US6242434B1 (en) | 1997-08-08 | 2001-06-05 | Bone Care International, Inc. | 24-hydroxyvitamin D, analogs and uses thereof |
| US5739271A (en) | 1995-06-07 | 1998-04-14 | Gen-Probe Incorporated | Thiocationic lipids |
| US6221911B1 (en) | 1995-06-07 | 2001-04-24 | Karo Bio Ab | Uses for thyroid hormone compounds or thyroid hormone-like compounds |
| US5952317A (en) | 1995-09-21 | 1999-09-14 | Wisconsin Alumni Research Foundation | Calcitriol derivatives and their uses |
| JPH11514999A (ja) | 1995-10-10 | 1999-12-21 | ストルーブ,マリリン | ビタミンdおよびその誘導体による掻痒症治療 |
| ES2144183T3 (es) | 1995-10-30 | 2000-06-01 | Hoffmann La Roche | 1-alfa,26-dihidroxi-d-homo-vitamina d3. |
| US5691328A (en) | 1996-02-02 | 1997-11-25 | Clarion Pharmaceuticals Inc. | Phosphoethanolamine conjugates of vitamin D compounds |
| AU710931B2 (en) | 1996-02-28 | 1999-09-30 | Sumitomo Pharmaceuticals Company, Limited | Crystalline vitamin D derivative |
| ATE227564T1 (de) | 1996-04-04 | 2002-11-15 | Cilag Ag | Topische vitamin d zusammensetzung auf liposomenbasis |
| DE19619036A1 (de) | 1996-04-30 | 1997-11-13 | Schering Ag | Neue Vitamin D-Derivate mit carbo- oder heterocyclischen Substituenten an C-25, Verfahren zu ihrer Herstellung und die Verwendung zur Herstellung von Arzneimitteln |
| US5976784A (en) | 1996-09-20 | 1999-11-02 | Wisconsin Alumni Research Foundation | Calcitriol derivatives and their uses |
| US6599513B2 (en) | 1997-05-27 | 2003-07-29 | Sembiosys Genetics Inc. | Products for topical applications comprising oil bodies |
| US6372234B1 (en) | 1997-05-27 | 2002-04-16 | Sembiosys Genetics Inc. | Products for topical applications comprising oil bodies |
| US6359152B2 (en) | 1997-07-21 | 2002-03-19 | Wisconsin Alumni Research Foundation | 18-substituted-19-nor-vitamin D compounds |
| US6087350A (en) | 1997-08-29 | 2000-07-11 | University Of Pittsburgh Of The Commonwealth System Of Higher Education | Use of pretreatment chemicals to enhance efficacy of cytotoxic agents |
| EP2340840B1 (en) | 1998-03-27 | 2012-08-29 | Oregon Health Sciences University | Pulse dose Vitamin D drug for the treatment of hyperproliferative skin diseases |
| US6114317A (en) | 1998-05-21 | 2000-09-05 | Wisconsin Alumni Research Foundation | Method of locking 1α-OH of vitamin D compounds in axial orientation |
| US5972917A (en) | 1998-05-29 | 1999-10-26 | Bone Care Int Inc | 1 α-hydroxy-25-ene-vitamin D, analogs and uses thereof |
| US6552009B2 (en) | 1998-07-16 | 2003-04-22 | Gentrix Llc | Compositions and methods of treating abnormal cell proliferation |
| US20010002396A1 (en) | 1998-07-16 | 2001-05-31 | Charles Achkar | Compositions and methods of treating skin conditions |
| US6218430B1 (en) | 1998-08-24 | 2001-04-17 | Ligand Pharmaceuticals Incorporated | Vitamin D3 mimics |
| EP1123921A4 (en) | 1998-10-23 | 2003-08-20 | Teijin Ltd | VITAMIN D 3 DERIVATIVES? AND MEDICINES FOR INFLAMMATORY RESPIRATORY DISEASES CONTAINING THEM |
| US6548789B1 (en) * | 1999-04-22 | 2003-04-15 | Malden Mills Industries, Inc. | Electric resistance heating/warming fabric articles |
| US6524594B1 (en) | 1999-06-23 | 2003-02-25 | Johnson & Johnson Consumer Companies, Inc. | Foaming oil gel compositions |
| US6362350B1 (en) | 1999-07-01 | 2002-03-26 | Wisconsin Alumni Research Foundation | Crystalline 1α, 24(S)-dihydroxyvitamin D2 and method of purification thereof |
| PT1202957E (pt) | 1999-07-16 | 2005-01-31 | Leo Pharma As | Aminobenzofenonas como inibidoras de il-1beta e tnf-alpha |
| PL352944A1 (en) | 1999-07-16 | 2003-09-22 | Leo Pharma | Aminobenzophenones as inhibitors of il-1beta and tnf-alpha |
| DE19935771A1 (de) | 1999-07-23 | 2001-02-01 | Schering Ag | Neue Vitamin D-Derivate mit cyclischen Substrukturen in den Seitenketten, Verfahren und Zwischenprodukte zu ihrer Herstellung und die Verwendung zur Herstellung von Arzneimitteln |
| FR2798855B1 (fr) | 1999-09-28 | 2003-04-25 | Oreal | Utilisation de complexes inorganiques-organiques dans une composition a usage topique |
| AU7995300A (en) | 1999-10-05 | 2001-05-10 | Bethesda Pharmaceuticals, Inc. | Dithiolane derivatives |
| HUP0203813A3 (en) | 1999-12-06 | 2008-03-28 | Leo Pharma As | Aminobenzophenones as inhibitors of il-1betha and tnf-alpha, pharmaceutical compositions containing them and their use |
| US6989377B2 (en) | 1999-12-21 | 2006-01-24 | Wisconsin Alumni Research Foundation | Treating vitamin D responsive diseases |
| JP4803939B2 (ja) | 2000-04-19 | 2011-10-26 | 中外製薬株式会社 | ビタミンd誘導体 |
| US6251608B1 (en) * | 2000-04-20 | 2001-06-26 | Technion Research & Development Foundation, Ltd. | Method of determining a potential of a hyperglycemic patients of developing vascular complications |
| RU2270194C2 (ru) | 2000-05-22 | 2006-02-20 | Лео Фарма А/С | Бензофеноны как ингибиторы il-1бета и tnf-альфа, фармацевтическая композиция и способ лечения |
| US6395784B1 (en) | 2000-06-07 | 2002-05-28 | Bristol-Myers Squibb Company | Benzamide ligands for the thyroid receptor |
| CN100469764C (zh) | 2000-06-15 | 2009-03-18 | 中外制药株式会社 | 维生素d衍生物 |
| IL153378A0 (en) * | 2000-07-18 | 2003-07-06 | Bone Care Internat Inc | STABILIZED 1alpha-HYDROXY VITAMIN D |
| EP1366045A2 (en) * | 2000-09-22 | 2003-12-03 | Bristol-Myers Squibb Pharma Company | Efficient process for the preparation of a factor xa inhibitor |
| US20030149005A1 (en) | 2001-08-22 | 2003-08-07 | Posner Gary H. | 24-sulfur-substituted analogs of 1alpha, 25-dihydroxy vitamin D3 |
| ES2283593T3 (es) | 2001-10-12 | 2007-11-01 | Johns Hopkins University | Analogos de oxima poco calcemicos de 1alfa,25-dihidroxi vitamina d3. |
| DE10156596A1 (de) | 2001-11-13 | 2003-05-28 | Schering Ag | Vitamin D-Derivate mit Acyloxygruppen in der Seitenkette, Verfahren zu ihrer Herstellung und die Verwendung zur Herstellung von Arzneimitteln |
| US6924400B2 (en) | 2001-12-10 | 2005-08-02 | Galderma Research & Development, Snc | Triaromatic vitamin D analogues |
| WO2003086415A1 (en) | 2002-04-05 | 2003-10-23 | Merck & Co., Inc. | Method for inhibiting bone resorption with an alendronate and vitamin d formulation |
-
2001
- 2001-07-18 IL IL15337801A patent/IL153378A0/xx active IP Right Grant
- 2001-07-18 JP JP2002512124A patent/JP2004504295A/ja active Pending
- 2001-07-18 EP EP01957187A patent/EP1301479A2/en not_active Withdrawn
- 2001-07-18 WO PCT/US2001/022729 patent/WO2002006218A2/en not_active Ceased
- 2001-07-18 EP EP09002505A patent/EP2070911A2/en not_active Withdrawn
- 2001-07-18 AU AU7895601A patent/AU7895601A/xx active Pending
- 2001-07-18 AU AU2001278956A patent/AU2001278956B2/en not_active Ceased
- 2001-07-18 CA CA002414407A patent/CA2414407A1/en not_active Abandoned
-
2002
- 2002-08-20 US US10/223,986 patent/US6903083B2/en not_active Expired - Lifetime
- 2002-12-11 IL IL153378A patent/IL153378A/en not_active IP Right Cessation
-
2005
- 2005-02-24 US US11/065,429 patent/US20050148558A1/en not_active Abandoned
Patent Citations (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS58126862A (ja) * | 1981-11-02 | 1983-07-28 | リサ−チ・インステイチユ−ト・フオア・メデイスン・アンド・ケミストリ−・インコ−ポレ−テツド | 1−ヒドロキシル化ビタミンd化合物の製法 |
| JPS62298572A (ja) * | 1986-06-19 | 1987-12-25 | Chugai Pharmaceut Co Ltd | 1α−ヒドロキシビタミンD3の精製法 |
| JPS63126891A (ja) * | 1986-11-14 | 1988-05-30 | Nisshin Flour Milling Co Ltd | ステロイド誘導体およびその製造方法 |
| JPS63126862A (ja) * | 1987-10-30 | 1988-05-30 | Nisshin Flour Milling Co Ltd | 1α−ヒドロキシビタミンD↓2の製造方法 |
| JPH01246276A (ja) * | 1988-03-25 | 1989-10-02 | Teikoku Chem Ind Corp Ltd | 1α−ヒドロキシ―6―(1,3−ベンゾジチオール−2−イル)オキシー3,5−シクロビタミンD↓3 |
| JPH02270860A (ja) * | 1988-03-25 | 1990-11-05 | Teikoku Chem Ind Corp Ltd | 1α―ヒドロキシ―ビタミンD↓3の製造方法 |
| JPH05279260A (ja) * | 1991-04-09 | 1993-10-26 | Takeda Chem Ind Ltd | 安定化されたビタミンd類製剤 |
| JPH05339230A (ja) * | 1992-03-12 | 1993-12-21 | Nisshin Flour Milling Co Ltd | 活性型ビタミンd2及びその誘導体の製造法 |
| JPH05320127A (ja) * | 1992-03-27 | 1993-12-03 | Nisshin Flour Milling Co Ltd | 活性型ビタミンd誘導体 |
| JPH06256301A (ja) * | 1992-11-04 | 1994-09-13 | Wisconsin Alumni Res Found | 1α,24−ジヒドロキシビタミンD類似体の製造方法 |
| JP2002535307A (ja) * | 1999-01-20 | 2002-10-22 | ウィスコンシン・アルムニ・リサーチ・ファウンデーション | 結晶1α−ヒドロキシビタミンD2とその精製方法 |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2005247803A (ja) * | 2004-03-08 | 2005-09-15 | Zeria Pharmaceut Co Ltd | 点眼剤及びその容器 |
| JP2014062131A (ja) * | 2008-03-12 | 2014-04-10 | Cytochroma Inc | 安定化1,25−ジヒドロキシビタミンd2及びその製造方法 |
| JP2018172299A (ja) * | 2017-03-31 | 2018-11-08 | ナガセ医薬品株式会社 | マキサカルシトール含有水溶液製剤 |
Also Published As
| Publication number | Publication date |
|---|---|
| EP2070911A2 (en) | 2009-06-17 |
| US6903083B2 (en) | 2005-06-07 |
| AU2001278956B2 (en) | 2007-04-05 |
| US20030045509A1 (en) | 2003-03-06 |
| WO2002006218A3 (en) | 2002-07-18 |
| IL153378A (en) | 2007-02-11 |
| IL153378A0 (en) | 2003-07-06 |
| EP1301479A2 (en) | 2003-04-16 |
| US20050148558A1 (en) | 2005-07-07 |
| CA2414407A1 (en) | 2002-01-24 |
| AU7895601A (en) | 2002-01-30 |
| WO2002006218A2 (en) | 2002-01-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2004504295A (ja) | 安定化1α−ヒドロキシビタミンD | |
| AU2001278956A1 (en) | Stabilized 1alpha-hydroxy vitamin d | |
| US20070225513A1 (en) | Crystals of a vitamin d derivative and a method for the preparation thereof | |
| JP2004504295A5 (enExample) | ||
| CN114008023B (zh) | 索吡溴铵的晶型及其制备方法 | |
| Yamada et al. | Stereoselective synthesis of (5E)-and (5Z)-vitamin D3 19-alkanoic acids via vitamin D3-sulfur dioxide adducts | |
| IE49753B1 (en) | 24,24-difluoro-cyclovitamin d3 derivatives | |
| US11926583B2 (en) | Stabilized 1, 25-dihydroxyvitamin D2 and method of making same | |
| JPS6346728B2 (enExample) | ||
| Pietraszek et al. | Synthesis and Crystal Structure of Anhydrous Analog of 1, 25‐Dihydroxyvitamin D 3 | |
| KR100554560B1 (ko) | 비타민d유도체결정및그의제조방법 | |
| JPH0495082A (ja) | 1α,25―ジヒドロキシビタミンD↓3―26,23―ラクトール | |
| HK1225013B (en) | Stabilized 1,25-dihydroxyvitamin d2 and method of making same | |
| Cheng et al. | Preparation of 14C‐and 3H‐methoxyl‐labelled forms of ubiquinone by photochemical O‐demethylation and subsequent remethylation | |
| HK1018438B (en) | Crystals of vitamin d derivatives and process for the preparation thereof | |
| HK1225013A1 (en) | Stabilized 1,25-dihydroxyvitamin d2 and method of making same | |
| JP2000016987A (ja) | 活性型ビタミンd誘導体 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20080715 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20080715 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20110519 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20110518 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20111013 |