CN111363041B - 新型抗-pd-l1抗体 - Google Patents
新型抗-pd-l1抗体 Download PDFInfo
- Publication number
- CN111363041B CN111363041B CN202010174431.5A CN202010174431A CN111363041B CN 111363041 B CN111363041 B CN 111363041B CN 202010174431 A CN202010174431 A CN 202010174431A CN 111363041 B CN111363041 B CN 111363041B
- Authority
- CN
- China
- Prior art keywords
- antibody
- seq
- sequence
- binding
- human
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 102000008096 B7-H1 Antigen Human genes 0.000 claims abstract description 360
- 108010074708 B7-H1 Antigen Proteins 0.000 claims abstract description 357
- 238000000034 method Methods 0.000 claims abstract description 90
- 102000040430 polynucleotide Human genes 0.000 claims abstract description 46
- 108091033319 polynucleotide Proteins 0.000 claims abstract description 46
- 239000002157 polynucleotide Substances 0.000 claims abstract description 46
- 239000008194 pharmaceutical composition Substances 0.000 claims abstract description 28
- 230000027455 binding Effects 0.000 claims description 378
- 238000009739 binding Methods 0.000 claims description 368
- 210000004027 cell Anatomy 0.000 claims description 277
- 206010028980 Neoplasm Diseases 0.000 claims description 269
- 101001117317 Homo sapiens Programmed cell death 1 ligand 1 Proteins 0.000 claims description 194
- 239000000427 antigen Substances 0.000 claims description 167
- 108091007433 antigens Proteins 0.000 claims description 167
- 102000036639 antigens Human genes 0.000 claims description 167
- 239000012634 fragment Substances 0.000 claims description 124
- 102000048776 human CD274 Human genes 0.000 claims description 97
- 210000004881 tumor cell Anatomy 0.000 claims description 72
- 125000003275 alpha amino acid group Chemical group 0.000 claims description 42
- 238000001727 in vivo Methods 0.000 claims description 40
- 239000003814 drug Substances 0.000 claims description 37
- 238000011282 treatment Methods 0.000 claims description 37
- 210000001744 T-lymphocyte Anatomy 0.000 claims description 34
- 239000013598 vector Substances 0.000 claims description 34
- 201000011510 cancer Diseases 0.000 claims description 24
- 230000005764 inhibitory process Effects 0.000 claims description 24
- 229940124597 therapeutic agent Drugs 0.000 claims description 22
- 239000003795 chemical substances by application Substances 0.000 claims description 14
- 238000004519 manufacturing process Methods 0.000 claims description 14
- 108010003723 Single-Domain Antibodies Proteins 0.000 claims description 11
- 239000000539 dimer Substances 0.000 claims description 9
- 238000012216 screening Methods 0.000 claims description 9
- 206010009944 Colon cancer Diseases 0.000 claims description 8
- 206010035226 Plasma cell myeloma Diseases 0.000 claims description 8
- 210000001519 tissue Anatomy 0.000 claims description 8
- 101100450694 Arabidopsis thaliana HFR1 gene Proteins 0.000 claims description 7
- 102100033177 Vascular endothelial growth factor receptor 2 Human genes 0.000 claims description 7
- 238000012258 culturing Methods 0.000 claims description 7
- 208000015181 infectious disease Diseases 0.000 claims description 7
- 201000001441 melanoma Diseases 0.000 claims description 7
- 208000002250 Hematologic Neoplasms Diseases 0.000 claims description 6
- 101000851007 Homo sapiens Vascular endothelial growth factor receptor 2 Proteins 0.000 claims description 6
- 108010067060 Immunoglobulin Variable Region Proteins 0.000 claims description 6
- 102000017727 Immunoglobulin Variable Region Human genes 0.000 claims description 6
- 206010039491 Sarcoma Diseases 0.000 claims description 6
- 238000001959 radiotherapy Methods 0.000 claims description 6
- 208000031261 Acute myeloid leukaemia Diseases 0.000 claims description 5
- 206010006187 Breast cancer Diseases 0.000 claims description 5
- 208000033776 Myeloid Acute Leukemia Diseases 0.000 claims description 5
- 230000033115 angiogenesis Effects 0.000 claims description 5
- 238000002512 chemotherapy Methods 0.000 claims description 5
- 208000029742 colonic neoplasm Diseases 0.000 claims description 5
- 238000001415 gene therapy Methods 0.000 claims description 5
- 238000001794 hormone therapy Methods 0.000 claims description 5
- 238000009169 immunotherapy Methods 0.000 claims description 5
- 208000026310 Breast neoplasm Diseases 0.000 claims description 4
- 206010008342 Cervix carcinoma Diseases 0.000 claims description 4
- 208000035473 Communicable disease Diseases 0.000 claims description 4
- 208000031671 Large B-Cell Diffuse Lymphoma Diseases 0.000 claims description 4
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 claims description 4
- 201000010881 cervical cancer Diseases 0.000 claims description 4
- 208000013056 classic Hodgkin lymphoma Diseases 0.000 claims description 4
- 206010012818 diffuse large B-cell lymphoma Diseases 0.000 claims description 4
- 208000032839 leukemia Diseases 0.000 claims description 4
- 206010061289 metastatic neoplasm Diseases 0.000 claims description 4
- 238000009116 palliative therapy Methods 0.000 claims description 4
- 238000002626 targeted therapy Methods 0.000 claims description 4
- 208000003950 B-cell lymphoma Diseases 0.000 claims description 3
- 208000001333 Colorectal Neoplasms Diseases 0.000 claims description 3
- 208000000461 Esophageal Neoplasms Diseases 0.000 claims description 3
- 206010025323 Lymphomas Diseases 0.000 claims description 3
- 206010027406 Mesothelioma Diseases 0.000 claims description 3
- 208000034578 Multiple myelomas Diseases 0.000 claims description 3
- 206010030155 Oesophageal carcinoma Diseases 0.000 claims description 3
- 206010033128 Ovarian cancer Diseases 0.000 claims description 3
- 206010061535 Ovarian neoplasm Diseases 0.000 claims description 3
- 206010061902 Pancreatic neoplasm Diseases 0.000 claims description 3
- 208000006265 Renal cell carcinoma Diseases 0.000 claims description 3
- 208000005718 Stomach Neoplasms Diseases 0.000 claims description 3
- 230000001684 chronic effect Effects 0.000 claims description 3
- 201000004101 esophageal cancer Diseases 0.000 claims description 3
- 206010017758 gastric cancer Diseases 0.000 claims description 3
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 claims description 3
- 208000023356 medullary thyroid gland carcinoma Diseases 0.000 claims description 3
- 238000011499 palliative surgery Methods 0.000 claims description 3
- 201000002528 pancreatic cancer Diseases 0.000 claims description 3
- 208000008443 pancreatic carcinoma Diseases 0.000 claims description 3
- 201000011549 stomach cancer Diseases 0.000 claims description 3
- 208000024893 Acute lymphoblastic leukemia Diseases 0.000 claims description 2
- 208000014697 Acute lymphocytic leukaemia Diseases 0.000 claims description 2
- 208000010839 B-cell chronic lymphocytic leukemia Diseases 0.000 claims description 2
- 206010004146 Basal cell carcinoma Diseases 0.000 claims description 2
- 206010005003 Bladder cancer Diseases 0.000 claims description 2
- 208000005243 Chondrosarcoma Diseases 0.000 claims description 2
- 208000006332 Choriocarcinoma Diseases 0.000 claims description 2
- 208000031637 Erythroblastic Acute Leukemia Diseases 0.000 claims description 2
- 208000036566 Erythroleukaemia Diseases 0.000 claims description 2
- 208000006168 Ewing Sarcoma Diseases 0.000 claims description 2
- 208000016937 Extranodal nasal NK/T cell lymphoma Diseases 0.000 claims description 2
- 201000008808 Fibrosarcoma Diseases 0.000 claims description 2
- 208000018142 Leiomyosarcoma Diseases 0.000 claims description 2
- 208000031422 Lymphocytic Chronic B-Cell Leukemia Diseases 0.000 claims description 2
- 208000007054 Medullary Carcinoma Diseases 0.000 claims description 2
- 208000002030 Merkel cell carcinoma Diseases 0.000 claims description 2
- 206010029266 Neuroendocrine carcinoma of the skin Diseases 0.000 claims description 2
- 208000015914 Non-Hodgkin lymphomas Diseases 0.000 claims description 2
- 208000006664 Precursor Cell Lymphoblastic Leukemia-Lymphoma Diseases 0.000 claims description 2
- 206010065857 Primary Effusion Lymphoma Diseases 0.000 claims description 2
- 206010036711 Primary mediastinal large B-cell lymphomas Diseases 0.000 claims description 2
- 206010060862 Prostate cancer Diseases 0.000 claims description 2
- 208000000236 Prostatic Neoplasms Diseases 0.000 claims description 2
- 201000010208 Seminoma Diseases 0.000 claims description 2
- 208000024313 Testicular Neoplasms Diseases 0.000 claims description 2
- 208000000728 Thymus Neoplasms Diseases 0.000 claims description 2
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 claims description 2
- 208000008383 Wilms tumor Diseases 0.000 claims description 2
- 208000021841 acute erythroid leukemia Diseases 0.000 claims description 2
- 208000009956 adenocarcinoma Diseases 0.000 claims description 2
- 208000006990 cholangiocarcinoma Diseases 0.000 claims description 2
- 208000032852 chronic lymphocytic leukemia Diseases 0.000 claims description 2
- 208000017763 cutaneous neuroendocrine carcinoma Diseases 0.000 claims description 2
- 208000005017 glioblastoma Diseases 0.000 claims description 2
- 206010024627 liposarcoma Diseases 0.000 claims description 2
- 201000007270 liver cancer Diseases 0.000 claims description 2
- 208000014018 liver neoplasm Diseases 0.000 claims description 2
- 230000036210 malignancy Effects 0.000 claims description 2
- 230000001394 metastastic effect Effects 0.000 claims description 2
- 208000001611 myxosarcoma Diseases 0.000 claims description 2
- 208000025189 neoplasm of testis Diseases 0.000 claims description 2
- 208000002154 non-small cell lung carcinoma Diseases 0.000 claims description 2
- 201000008968 osteosarcoma Diseases 0.000 claims description 2
- 208000028591 pheochromocytoma Diseases 0.000 claims description 2
- 210000003720 plasmablast Anatomy 0.000 claims description 2
- 208000017805 post-transplant lymphoproliferative disease Diseases 0.000 claims description 2
- 201000009410 rhabdomyosarcoma Diseases 0.000 claims description 2
- 206010042863 synovial sarcoma Diseases 0.000 claims description 2
- 201000003120 testicular cancer Diseases 0.000 claims description 2
- 201000009377 thymus cancer Diseases 0.000 claims description 2
- 208000029729 tumor suppressor gene on chromosome 11 Diseases 0.000 claims description 2
- 201000005112 urinary bladder cancer Diseases 0.000 claims description 2
- 201000005787 hematologic cancer Diseases 0.000 claims 4
- 208000024200 hematopoietic and lymphoid system neoplasm Diseases 0.000 claims 4
- 206010000871 Acute monocytic leukaemia Diseases 0.000 claims 1
- 206010066476 Haematological malignancy Diseases 0.000 claims 1
- 208000035489 Monocytic Acute Leukemia Diseases 0.000 claims 1
- 208000024770 Thyroid neoplasm Diseases 0.000 claims 1
- 201000010536 head and neck cancer Diseases 0.000 claims 1
- 208000014829 head and neck neoplasm Diseases 0.000 claims 1
- 208000018964 sebaceous gland cancer Diseases 0.000 claims 1
- 201000008759 sweat gland cancer Diseases 0.000 claims 1
- 201000002510 thyroid cancer Diseases 0.000 claims 1
- 230000007935 neutral effect Effects 0.000 abstract description 41
- 230000002378 acidificating effect Effects 0.000 abstract description 32
- 239000013604 expression vector Substances 0.000 abstract description 7
- 108090000623 proteins and genes Proteins 0.000 description 103
- 241000699666 Mus <mouse, genus> Species 0.000 description 90
- 239000000872 buffer Substances 0.000 description 87
- 102100024216 Programmed cell death 1 ligand 1 Human genes 0.000 description 82
- 241000699670 Mus sp. Species 0.000 description 70
- 102000004169 proteins and genes Human genes 0.000 description 70
- 238000002965 ELISA Methods 0.000 description 68
- 235000018102 proteins Nutrition 0.000 description 68
- 230000000903 blocking effect Effects 0.000 description 60
- 230000001419 dependent effect Effects 0.000 description 56
- 125000000539 amino acid group Chemical group 0.000 description 47
- 239000011534 wash buffer Substances 0.000 description 47
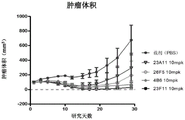
- 230000004614 tumor growth Effects 0.000 description 44
- 241000283707 Capra Species 0.000 description 42
- 229920001213 Polysorbate 20 Polymers 0.000 description 42
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 42
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 42
- 102100040678 Programmed cell death protein 1 Human genes 0.000 description 37
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 36
- 210000004408 hybridoma Anatomy 0.000 description 33
- 210000002966 serum Anatomy 0.000 description 33
- 108010047041 Complementarity Determining Regions Proteins 0.000 description 32
- 108090000765 processed proteins & peptides Proteins 0.000 description 32
- 239000006228 supernatant Substances 0.000 description 32
- 239000000243 solution Substances 0.000 description 31
- 101710089372 Programmed cell death protein 1 Proteins 0.000 description 29
- 210000004602 germ cell Anatomy 0.000 description 28
- 230000000694 effects Effects 0.000 description 27
- 239000013024 dilution buffer Substances 0.000 description 26
- 230000014509 gene expression Effects 0.000 description 26
- 235000001014 amino acid Nutrition 0.000 description 25
- 238000012360 testing method Methods 0.000 description 25
- 238000007792 addition Methods 0.000 description 24
- 150000001413 amino acids Chemical group 0.000 description 23
- 239000010432 diamond Substances 0.000 description 23
- 239000000203 mixture Substances 0.000 description 23
- 230000002401 inhibitory effect Effects 0.000 description 22
- 239000003981 vehicle Substances 0.000 description 22
- 230000006870 function Effects 0.000 description 21
- 102000004196 processed proteins & peptides Human genes 0.000 description 21
- 229910052717 sulfur Inorganic materials 0.000 description 21
- 102100035360 Cerebellar degeneration-related antigen 1 Human genes 0.000 description 20
- 101000611936 Homo sapiens Programmed cell death protein 1 Proteins 0.000 description 20
- 238000006243 chemical reaction Methods 0.000 description 20
- 238000002347 injection Methods 0.000 description 20
- 239000007924 injection Substances 0.000 description 20
- 238000002474 experimental method Methods 0.000 description 18
- 230000035772 mutation Effects 0.000 description 18
- 229920001184 polypeptide Polymers 0.000 description 18
- 229920002223 polystyrene Polymers 0.000 description 18
- 101001117316 Mus musculus Programmed cell death 1 ligand 1 Proteins 0.000 description 17
- 239000004793 Polystyrene Substances 0.000 description 17
- 239000000523 sample Substances 0.000 description 17
- 108020004414 DNA Proteins 0.000 description 16
- 102000005962 receptors Human genes 0.000 description 16
- 108020003175 receptors Proteins 0.000 description 16
- 230000003442 weekly effect Effects 0.000 description 16
- 230000000259 anti-tumor effect Effects 0.000 description 15
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 15
- 239000002609 medium Substances 0.000 description 15
- 108091026890 Coding region Proteins 0.000 description 14
- 239000012636 effector Substances 0.000 description 14
- 238000000746 purification Methods 0.000 description 14
- 210000004978 chinese hamster ovary cell Anatomy 0.000 description 13
- 239000011248 coating agent Substances 0.000 description 13
- 238000000576 coating method Methods 0.000 description 13
- 230000036961 partial effect Effects 0.000 description 13
- 238000006467 substitution reaction Methods 0.000 description 13
- 230000001225 therapeutic effect Effects 0.000 description 13
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 12
- 229940079593 drug Drugs 0.000 description 12
- 238000011156 evaluation Methods 0.000 description 12
- 102000048362 human PDCD1 Human genes 0.000 description 12
- 210000000056 organ Anatomy 0.000 description 12
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 12
- 230000000638 stimulation Effects 0.000 description 12
- 239000000126 substance Substances 0.000 description 12
- 238000005406 washing Methods 0.000 description 12
- 238000011740 C57BL/6 mouse Methods 0.000 description 11
- 108091028043 Nucleic acid sequence Proteins 0.000 description 11
- 201000010099 disease Diseases 0.000 description 11
- 238000011534 incubation Methods 0.000 description 11
- 238000010253 intravenous injection Methods 0.000 description 11
- 230000004044 response Effects 0.000 description 11
- 229910052720 vanadium Inorganic materials 0.000 description 11
- 238000012413 Fluorescence activated cell sorting analysis Methods 0.000 description 10
- 241000282567 Macaca fascicularis Species 0.000 description 10
- 239000000654 additive Substances 0.000 description 10
- 239000000499 gel Substances 0.000 description 10
- 229910052700 potassium Inorganic materials 0.000 description 10
- 239000002904 solvent Substances 0.000 description 10
- 238000001890 transfection Methods 0.000 description 10
- 241001465754 Metazoa Species 0.000 description 9
- 239000004698 Polyethylene Substances 0.000 description 9
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 9
- 230000003247 decreasing effect Effects 0.000 description 9
- 238000001514 detection method Methods 0.000 description 9
- 238000009472 formulation Methods 0.000 description 9
- 230000012010 growth Effects 0.000 description 9
- 230000001965 increasing effect Effects 0.000 description 9
- 238000011081 inoculation Methods 0.000 description 9
- 239000003446 ligand Substances 0.000 description 9
- 150000007523 nucleic acids Chemical group 0.000 description 9
- 230000002829 reductive effect Effects 0.000 description 9
- 238000002560 therapeutic procedure Methods 0.000 description 9
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 8
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 8
- 230000000996 additive effect Effects 0.000 description 8
- 230000037396 body weight Effects 0.000 description 8
- 229910052799 carbon Inorganic materials 0.000 description 8
- 230000008859 change Effects 0.000 description 8
- 238000012512 characterization method Methods 0.000 description 8
- 238000000113 differential scanning calorimetry Methods 0.000 description 8
- 239000000975 dye Substances 0.000 description 8
- 239000001963 growth medium Substances 0.000 description 8
- 239000007928 intraperitoneal injection Substances 0.000 description 8
- 239000013612 plasmid Substances 0.000 description 8
- 241000894007 species Species 0.000 description 8
- 229910052727 yttrium Inorganic materials 0.000 description 8
- 102000004127 Cytokines Human genes 0.000 description 7
- 108090000695 Cytokines Proteins 0.000 description 7
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 7
- 235000014680 Saccharomyces cerevisiae Nutrition 0.000 description 7
- 239000003963 antioxidant agent Substances 0.000 description 7
- 235000006708 antioxidants Nutrition 0.000 description 7
- 230000001588 bifunctional effect Effects 0.000 description 7
- 239000012472 biological sample Substances 0.000 description 7
- 238000002983 circular dichroism Methods 0.000 description 7
- 231100000673 dose–response relationship Toxicity 0.000 description 7
- 239000012091 fetal bovine serum Substances 0.000 description 7
- 206010073071 hepatocellular carcinoma Diseases 0.000 description 7
- 231100000844 hepatocellular carcinoma Toxicity 0.000 description 7
- 230000008595 infiltration Effects 0.000 description 7
- 238000001764 infiltration Methods 0.000 description 7
- 230000003993 interaction Effects 0.000 description 7
- 239000000047 product Substances 0.000 description 7
- 230000010512 thermal transition Effects 0.000 description 7
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 7
- 108091033409 CRISPR Proteins 0.000 description 6
- 102000004190 Enzymes Human genes 0.000 description 6
- 108090000790 Enzymes Proteins 0.000 description 6
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 6
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 6
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 6
- 108010002350 Interleukin-2 Proteins 0.000 description 6
- 102000000588 Interleukin-2 Human genes 0.000 description 6
- 241000283973 Oryctolagus cuniculus Species 0.000 description 6
- 239000012980 RPMI-1640 medium Substances 0.000 description 6
- SCJNCDSAIRBRIA-DOFZRALJSA-N arachidonyl-2'-chloroethylamide Chemical compound CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)NCCCl SCJNCDSAIRBRIA-DOFZRALJSA-N 0.000 description 6
- 238000003556 assay Methods 0.000 description 6
- 230000008901 benefit Effects 0.000 description 6
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 6
- 230000004071 biological effect Effects 0.000 description 6
- 238000005119 centrifugation Methods 0.000 description 6
- 210000004443 dendritic cell Anatomy 0.000 description 6
- 239000003995 emulsifying agent Substances 0.000 description 6
- 229940088598 enzyme Drugs 0.000 description 6
- 230000006801 homologous recombination Effects 0.000 description 6
- 238000002744 homologous recombination Methods 0.000 description 6
- 230000028993 immune response Effects 0.000 description 6
- 238000003018 immunoassay Methods 0.000 description 6
- 238000000338 in vitro Methods 0.000 description 6
- 239000007788 liquid Substances 0.000 description 6
- 229910052757 nitrogen Inorganic materials 0.000 description 6
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 6
- YBYRMVIVWMBXKQ-UHFFFAOYSA-N phenylmethanesulfonyl fluoride Chemical compound FS(=O)(=O)CC1=CC=CC=C1 YBYRMVIVWMBXKQ-UHFFFAOYSA-N 0.000 description 6
- 229910052698 phosphorus Inorganic materials 0.000 description 6
- 238000003752 polymerase chain reaction Methods 0.000 description 6
- 238000003127 radioimmunoassay Methods 0.000 description 6
- 230000009467 reduction Effects 0.000 description 6
- 150000003839 salts Chemical class 0.000 description 6
- 210000000952 spleen Anatomy 0.000 description 6
- 238000002198 surface plasmon resonance spectroscopy Methods 0.000 description 6
- 210000003171 tumor-infiltrating lymphocyte Anatomy 0.000 description 6
- 108090001008 Avidin Proteins 0.000 description 5
- 241000282693 Cercopithecidae Species 0.000 description 5
- 241000699800 Cricetinae Species 0.000 description 5
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 5
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 5
- 241000238631 Hexapoda Species 0.000 description 5
- 241000282412 Homo Species 0.000 description 5
- 101000914514 Homo sapiens T-cell-specific surface glycoprotein CD28 Proteins 0.000 description 5
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 5
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 5
- 206010027476 Metastases Diseases 0.000 description 5
- 101100407308 Mus musculus Pdcd1lg2 gene Proteins 0.000 description 5
- NWIBSHFKIJFRCO-WUDYKRTCSA-N Mytomycin Chemical compound C1N2C(C(C(C)=C(N)C3=O)=O)=C3[C@@H](COC(N)=O)[C@@]2(OC)[C@@H]2[C@H]1N2 NWIBSHFKIJFRCO-WUDYKRTCSA-N 0.000 description 5
- 108700030875 Programmed Cell Death 1 Ligand 2 Proteins 0.000 description 5
- 102100024213 Programmed cell death 1 ligand 2 Human genes 0.000 description 5
- 241000700159 Rattus Species 0.000 description 5
- 241000283984 Rodentia Species 0.000 description 5
- 230000006044 T cell activation Effects 0.000 description 5
- 102100027213 T-cell-specific surface glycoprotein CD28 Human genes 0.000 description 5
- 239000005557 antagonist Substances 0.000 description 5
- 239000011324 bead Substances 0.000 description 5
- 239000000969 carrier Substances 0.000 description 5
- 238000010367 cloning Methods 0.000 description 5
- 238000010790 dilution Methods 0.000 description 5
- 239000012895 dilution Substances 0.000 description 5
- 238000000684 flow cytometry Methods 0.000 description 5
- 230000004927 fusion Effects 0.000 description 5
- 239000008103 glucose Substances 0.000 description 5
- 230000003053 immunization Effects 0.000 description 5
- 238000002649 immunization Methods 0.000 description 5
- 230000002779 inactivation Effects 0.000 description 5
- 238000001990 intravenous administration Methods 0.000 description 5
- 210000003292 kidney cell Anatomy 0.000 description 5
- 239000008176 lyophilized powder Substances 0.000 description 5
- 239000000463 material Substances 0.000 description 5
- 230000001404 mediated effect Effects 0.000 description 5
- 230000009401 metastasis Effects 0.000 description 5
- 238000007799 mixed lymphocyte reaction assay Methods 0.000 description 5
- 230000004048 modification Effects 0.000 description 5
- 238000012986 modification Methods 0.000 description 5
- DNIAPMSPPWPWGF-UHFFFAOYSA-N monopropylene glycol Natural products CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 5
- 201000000050 myeloid neoplasm Diseases 0.000 description 5
- 102000039446 nucleic acids Human genes 0.000 description 5
- 108020004707 nucleic acids Proteins 0.000 description 5
- 238000012545 processing Methods 0.000 description 5
- 230000035755 proliferation Effects 0.000 description 5
- 230000005855 radiation Effects 0.000 description 5
- 238000010188 recombinant method Methods 0.000 description 5
- 239000011780 sodium chloride Substances 0.000 description 5
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 4
- LMDZBCPBFSXMTL-UHFFFAOYSA-N 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide Chemical compound CCN=C=NCCCN(C)C LMDZBCPBFSXMTL-UHFFFAOYSA-N 0.000 description 4
- NFGXHKASABOEEW-UHFFFAOYSA-N 1-methylethyl 11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate Chemical compound COC(C)(C)CCCC(C)CC=CC(C)=CC(=O)OC(C)C NFGXHKASABOEEW-UHFFFAOYSA-N 0.000 description 4
- STQGQHZAVUOBTE-UHFFFAOYSA-N 7-Cyan-hept-2t-en-4,6-diinsaeure Natural products C1=2C(O)=C3C(=O)C=4C(OC)=CC=CC=4C(=O)C3=C(O)C=2CC(O)(C(C)=O)CC1OC1CC(N)C(O)C(C)O1 STQGQHZAVUOBTE-UHFFFAOYSA-N 0.000 description 4
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 4
- 201000009030 Carcinoma Diseases 0.000 description 4
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 4
- 241000196324 Embryophyta Species 0.000 description 4
- 102000018071 Immunoglobulin Fc Fragments Human genes 0.000 description 4
- 108010091135 Immunoglobulin Fc Fragments Proteins 0.000 description 4
- 241000235649 Kluyveromyces Species 0.000 description 4
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 4
- 108700018351 Major Histocompatibility Complex Proteins 0.000 description 4
- 101100407306 Mus musculus Cd274 gene Proteins 0.000 description 4
- 102220552574 Phospholipase A2_N89T_mutation Human genes 0.000 description 4
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 4
- 101710120037 Toxin CcdB Proteins 0.000 description 4
- 241000700605 Viruses Species 0.000 description 4
- RJURFGZVJUQBHK-UHFFFAOYSA-N actinomycin D Natural products CC1OC(=O)C(C(C)C)N(C)C(=O)CN(C)C(=O)C2CCCN2C(=O)C(C(C)C)NC(=O)C1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)NC4C(=O)NC(C(N5CCCC5C(=O)N(C)CC(=O)N(C)C(C(C)C)C(=O)OC4C)=O)C(C)C)=C3N=C21 RJURFGZVJUQBHK-UHFFFAOYSA-N 0.000 description 4
- 238000012867 alanine scanning Methods 0.000 description 4
- 230000003698 anagen phase Effects 0.000 description 4
- 238000004458 analytical method Methods 0.000 description 4
- 239000004037 angiogenesis inhibitor Substances 0.000 description 4
- 239000003242 anti bacterial agent Substances 0.000 description 4
- 239000002246 antineoplastic agent Substances 0.000 description 4
- 210000004369 blood Anatomy 0.000 description 4
- 239000008280 blood Substances 0.000 description 4
- 238000004113 cell culture Methods 0.000 description 4
- 239000006285 cell suspension Substances 0.000 description 4
- 239000003153 chemical reaction reagent Substances 0.000 description 4
- 239000002299 complementary DNA Substances 0.000 description 4
- 229940127089 cytotoxic agent Drugs 0.000 description 4
- STQGQHZAVUOBTE-VGBVRHCVSA-N daunorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(C)=O)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 STQGQHZAVUOBTE-VGBVRHCVSA-N 0.000 description 4
- 229960000975 daunorubicin Drugs 0.000 description 4
- 238000013461 design Methods 0.000 description 4
- 230000029087 digestion Effects 0.000 description 4
- 208000035475 disorder Diseases 0.000 description 4
- 230000013595 glycosylation Effects 0.000 description 4
- 238000006206 glycosylation reaction Methods 0.000 description 4
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 4
- 230000016784 immunoglobulin production Effects 0.000 description 4
- 230000001976 improved effect Effects 0.000 description 4
- 239000003112 inhibitor Substances 0.000 description 4
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 4
- 230000002427 irreversible effect Effects 0.000 description 4
- 238000011031 large-scale manufacturing process Methods 0.000 description 4
- 210000004185 liver Anatomy 0.000 description 4
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 4
- 230000003211 malignant effect Effects 0.000 description 4
- 210000003071 memory t lymphocyte Anatomy 0.000 description 4
- 229930182817 methionine Natural products 0.000 description 4
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 4
- 210000001616 monocyte Anatomy 0.000 description 4
- 238000010172 mouse model Methods 0.000 description 4
- 230000003472 neutralizing effect Effects 0.000 description 4
- -1 platins (e.g. Chemical compound 0.000 description 4
- 229920001223 polyethylene glycol Polymers 0.000 description 4
- 238000000159 protein binding assay Methods 0.000 description 4
- 231100000628 reference dose Toxicity 0.000 description 4
- 230000010076 replication Effects 0.000 description 4
- 102220051014 rs141837529 Human genes 0.000 description 4
- 102200118280 rs33918343 Human genes 0.000 description 4
- 102200016993 rs35198096 Human genes 0.000 description 4
- 102220047535 rs587783040 Human genes 0.000 description 4
- 102200031793 rs800292 Human genes 0.000 description 4
- 238000012163 sequencing technique Methods 0.000 description 4
- 210000003491 skin Anatomy 0.000 description 4
- 239000007787 solid Substances 0.000 description 4
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 4
- 239000000758 substrate Substances 0.000 description 4
- 230000020382 suppression by virus of host antigen processing and presentation of peptide antigen via MHC class I Effects 0.000 description 4
- 239000000375 suspending agent Substances 0.000 description 4
- 239000000725 suspension Substances 0.000 description 4
- 102000017420 CD3 protein, epsilon/gamma/delta subunit Human genes 0.000 description 3
- 108050005493 CD3 protein, epsilon/gamma/delta subunit Proteins 0.000 description 3
- 238000010354 CRISPR gene editing Methods 0.000 description 3
- 241000282832 Camelidae Species 0.000 description 3
- 108010092160 Dactinomycin Proteins 0.000 description 3
- BWGNESOTFCXPMA-UHFFFAOYSA-N Dihydrogen disulfide Chemical compound SS BWGNESOTFCXPMA-UHFFFAOYSA-N 0.000 description 3
- 238000012286 ELISA Assay Methods 0.000 description 3
- 239000004471 Glycine Substances 0.000 description 3
- 108060003951 Immunoglobulin Proteins 0.000 description 3
- 102000008070 Interferon-gamma Human genes 0.000 description 3
- 108010074328 Interferon-gamma Proteins 0.000 description 3
- 108090000978 Interleukin-4 Proteins 0.000 description 3
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical compound SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 3
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- 241001529936 Murinae Species 0.000 description 3
- 229930182555 Penicillin Natural products 0.000 description 3
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 3
- 239000004365 Protease Substances 0.000 description 3
- 238000012228 RNA interference-mediated gene silencing Methods 0.000 description 3
- 229920002684 Sepharose Polymers 0.000 description 3
- 241000700584 Simplexvirus Species 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 3
- 229930006000 Sucrose Natural products 0.000 description 3
- 208000036142 Viral infection Diseases 0.000 description 3
- 210000001015 abdomen Anatomy 0.000 description 3
- 239000002671 adjuvant Substances 0.000 description 3
- 238000001042 affinity chromatography Methods 0.000 description 3
- 230000004520 agglutination Effects 0.000 description 3
- 235000004279 alanine Nutrition 0.000 description 3
- 230000003321 amplification Effects 0.000 description 3
- 229940088710 antibiotic agent Drugs 0.000 description 3
- 239000000611 antibody drug conjugate Substances 0.000 description 3
- 230000010056 antibody-dependent cellular cytotoxicity Effects 0.000 description 3
- 229940049595 antibody-drug conjugate Drugs 0.000 description 3
- 238000003782 apoptosis assay Methods 0.000 description 3
- 230000001580 bacterial effect Effects 0.000 description 3
- 229960002685 biotin Drugs 0.000 description 3
- 239000011616 biotin Substances 0.000 description 3
- 210000004556 brain Anatomy 0.000 description 3
- 210000000481 breast Anatomy 0.000 description 3
- 229910002091 carbon monoxide Inorganic materials 0.000 description 3
- 239000012930 cell culture fluid Substances 0.000 description 3
- 239000006143 cell culture medium Substances 0.000 description 3
- 230000010261 cell growth Effects 0.000 description 3
- 230000004663 cell proliferation Effects 0.000 description 3
- 238000004587 chromatography analysis Methods 0.000 description 3
- 210000001072 colon Anatomy 0.000 description 3
- 238000002648 combination therapy Methods 0.000 description 3
- 230000002860 competitive effect Effects 0.000 description 3
- 230000004540 complement-dependent cytotoxicity Effects 0.000 description 3
- 239000012228 culture supernatant Substances 0.000 description 3
- 235000018417 cysteine Nutrition 0.000 description 3
- 231100000433 cytotoxic Toxicity 0.000 description 3
- 230000001472 cytotoxic effect Effects 0.000 description 3
- 238000012217 deletion Methods 0.000 description 3
- 230000037430 deletion Effects 0.000 description 3
- 238000011161 development Methods 0.000 description 3
- 230000018109 developmental process Effects 0.000 description 3
- 238000000502 dialysis Methods 0.000 description 3
- 238000010494 dissociation reaction Methods 0.000 description 3
- 230000005593 dissociations Effects 0.000 description 3
- 239000003937 drug carrier Substances 0.000 description 3
- 210000001163 endosome Anatomy 0.000 description 3
- 239000002158 endotoxin Substances 0.000 description 3
- 210000003238 esophagus Anatomy 0.000 description 3
- 210000003527 eukaryotic cell Anatomy 0.000 description 3
- 238000002866 fluorescence resonance energy transfer Methods 0.000 description 3
- 210000000232 gallbladder Anatomy 0.000 description 3
- 230000009368 gene silencing by RNA Effects 0.000 description 3
- 238000011991 general safety test Methods 0.000 description 3
- 235000011187 glycerol Nutrition 0.000 description 3
- 210000000987 immune system Anatomy 0.000 description 3
- 230000005847 immunogenicity Effects 0.000 description 3
- 102000018358 immunoglobulin Human genes 0.000 description 3
- 238000003364 immunohistochemistry Methods 0.000 description 3
- 238000001114 immunoprecipitation Methods 0.000 description 3
- 238000001802 infusion Methods 0.000 description 3
- 238000002955 isolation Methods 0.000 description 3
- 210000003734 kidney Anatomy 0.000 description 3
- 210000000265 leukocyte Anatomy 0.000 description 3
- 230000000670 limiting effect Effects 0.000 description 3
- 238000011068 loading method Methods 0.000 description 3
- 210000004072 lung Anatomy 0.000 description 3
- 229960004857 mitomycin Drugs 0.000 description 3
- 238000004264 monolayer culture Methods 0.000 description 3
- 230000001613 neoplastic effect Effects 0.000 description 3
- 238000003199 nucleic acid amplification method Methods 0.000 description 3
- 210000001672 ovary Anatomy 0.000 description 3
- 230000003647 oxidation Effects 0.000 description 3
- 238000007254 oxidation reaction Methods 0.000 description 3
- 210000000496 pancreas Anatomy 0.000 description 3
- 239000006072 paste Substances 0.000 description 3
- 229940049954 penicillin Drugs 0.000 description 3
- 239000000546 pharmaceutical excipient Substances 0.000 description 3
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 3
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 3
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 3
- 238000002360 preparation method Methods 0.000 description 3
- 230000005522 programmed cell death Effects 0.000 description 3
- 210000001236 prokaryotic cell Anatomy 0.000 description 3
- 210000000664 rectum Anatomy 0.000 description 3
- 239000013074 reference sample Substances 0.000 description 3
- 238000013207 serial dilution Methods 0.000 description 3
- 239000011734 sodium Substances 0.000 description 3
- 210000002784 stomach Anatomy 0.000 description 3
- 238000010254 subcutaneous injection Methods 0.000 description 3
- 239000007929 subcutaneous injection Substances 0.000 description 3
- 239000005720 sucrose Substances 0.000 description 3
- 208000024891 symptom Diseases 0.000 description 3
- 230000008685 targeting Effects 0.000 description 3
- 238000000108 ultra-filtration Methods 0.000 description 3
- 241000701161 unidentified adenovirus Species 0.000 description 3
- 241001529453 unidentified herpesvirus Species 0.000 description 3
- 229960005486 vaccine Drugs 0.000 description 3
- 230000009385 viral infection Effects 0.000 description 3
- 239000013603 viral vector Substances 0.000 description 3
- 238000001262 western blot Methods 0.000 description 3
- IAKHMKGGTNLKSZ-INIZCTEOSA-N (S)-colchicine Chemical compound C1([C@@H](NC(C)=O)CC2)=CC(=O)C(OC)=CC=C1C1=C2C=C(OC)C(OC)=C1OC IAKHMKGGTNLKSZ-INIZCTEOSA-N 0.000 description 2
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 2
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 2
- FWBHETKCLVMNFS-UHFFFAOYSA-N 4',6-Diamino-2-phenylindol Chemical compound C1=CC(C(=N)N)=CC=C1C1=CC2=CC=C(C(N)=N)C=C2N1 FWBHETKCLVMNFS-UHFFFAOYSA-N 0.000 description 2
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 2
- 102220578235 ATP-dependent Clp protease proteolytic subunit, mitochondrial_L93Q_mutation Human genes 0.000 description 2
- 229920000936 Agarose Polymers 0.000 description 2
- 239000012103 Alexa Fluor 488 Substances 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- 208000023275 Autoimmune disease Diseases 0.000 description 2
- MLDQJTXFUGDVEO-UHFFFAOYSA-N BAY-43-9006 Chemical compound C1=NC(C(=O)NC)=CC(OC=2C=CC(NC(=O)NC=3C=C(C(Cl)=CC=3)C(F)(F)F)=CC=2)=C1 MLDQJTXFUGDVEO-UHFFFAOYSA-N 0.000 description 2
- 241000193830 Bacillus <bacterium> Species 0.000 description 2
- 241000894006 Bacteria Species 0.000 description 2
- 101100244725 Caenorhabditis elegans pef-1 gene Proteins 0.000 description 2
- 241000700199 Cavia porcellus Species 0.000 description 2
- 241000193403 Clostridium Species 0.000 description 2
- 108020004635 Complementary DNA Proteins 0.000 description 2
- 229920000858 Cyclodextrin Polymers 0.000 description 2
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 2
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 2
- 241000702421 Dependoparvovirus Species 0.000 description 2
- 241000255925 Diptera Species 0.000 description 2
- 241000588914 Enterobacter Species 0.000 description 2
- 241000588724 Escherichia coli Species 0.000 description 2
- 108010087819 Fc receptors Proteins 0.000 description 2
- 102000009109 Fc receptors Human genes 0.000 description 2
- 102000018233 Fibroblast Growth Factor Human genes 0.000 description 2
- 108050007372 Fibroblast Growth Factor Proteins 0.000 description 2
- 201000006353 Filariasis Diseases 0.000 description 2
- 206010017533 Fungal infection Diseases 0.000 description 2
- 108700028146 Genetic Enhancer Elements Proteins 0.000 description 2
- 108010017213 Granulocyte-Macrophage Colony-Stimulating Factor Proteins 0.000 description 2
- 102100039620 Granulocyte-macrophage colony-stimulating factor Human genes 0.000 description 2
- 239000007995 HEPES buffer Substances 0.000 description 2
- 241000711549 Hepacivirus C Species 0.000 description 2
- 208000017604 Hodgkin disease Diseases 0.000 description 2
- 208000021519 Hodgkin lymphoma Diseases 0.000 description 2
- 208000010747 Hodgkins lymphoma Diseases 0.000 description 2
- 101001117312 Homo sapiens Programmed cell death 1 ligand 2 Proteins 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 2
- 102000009786 Immunoglobulin Constant Regions Human genes 0.000 description 2
- 108010009817 Immunoglobulin Constant Regions Proteins 0.000 description 2
- 108010054477 Immunoglobulin Fab Fragments Proteins 0.000 description 2
- 102000001706 Immunoglobulin Fab Fragments Human genes 0.000 description 2
- 108090001061 Insulin Proteins 0.000 description 2
- 102000004877 Insulin Human genes 0.000 description 2
- 108090000174 Interleukin-10 Proteins 0.000 description 2
- 244000285963 Kluyveromyces fragilis Species 0.000 description 2
- 235000014663 Kluyveromyces fragilis Nutrition 0.000 description 2
- 241001138401 Kluyveromyces lactis Species 0.000 description 2
- 241000235058 Komagataella pastoris Species 0.000 description 2
- 239000005517 L01XE01 - Imatinib Substances 0.000 description 2
- 239000005411 L01XE02 - Gefitinib Substances 0.000 description 2
- 239000002147 L01XE04 - Sunitinib Substances 0.000 description 2
- 239000005511 L01XE05 - Sorafenib Substances 0.000 description 2
- 239000003798 L01XE11 - Pazopanib Substances 0.000 description 2
- 241000713666 Lentivirus Species 0.000 description 2
- GQYIWUVLTXOXAJ-UHFFFAOYSA-N Lomustine Chemical compound ClCCN(N=O)C(=O)NC1CCCCC1 GQYIWUVLTXOXAJ-UHFFFAOYSA-N 0.000 description 2
- TWRXJAOTZQYOKJ-UHFFFAOYSA-L Magnesium chloride Chemical compound [Mg+2].[Cl-].[Cl-] TWRXJAOTZQYOKJ-UHFFFAOYSA-L 0.000 description 2
- HPEUJPJOZXNMSJ-UHFFFAOYSA-N Methyl stearate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC HPEUJPJOZXNMSJ-UHFFFAOYSA-N 0.000 description 2
- 101100519207 Mus musculus Pdcd1 gene Proteins 0.000 description 2
- 201000003793 Myelodysplastic syndrome Diseases 0.000 description 2
- 241000221960 Neurospora Species 0.000 description 2
- 108091008606 PDGF receptors Proteins 0.000 description 2
- 108090000526 Papain Proteins 0.000 description 2
- 241001494479 Pecora Species 0.000 description 2
- 102100024616 Platelet endothelial cell adhesion molecule Human genes 0.000 description 2
- 102000011653 Platelet-Derived Growth Factor Receptors Human genes 0.000 description 2
- 101710094000 Programmed cell death 1 ligand 1 Proteins 0.000 description 2
- ZTHYODDOHIVTJV-UHFFFAOYSA-N Propyl gallate Chemical compound CCCOC(=O)C1=CC(O)=C(O)C(O)=C1 ZTHYODDOHIVTJV-UHFFFAOYSA-N 0.000 description 2
- 102220468699 Protein arginine N-methyltransferase 3_Y87F_mutation Human genes 0.000 description 2
- 241000589516 Pseudomonas Species 0.000 description 2
- 108700008625 Reporter Genes Proteins 0.000 description 2
- 241000607142 Salmonella Species 0.000 description 2
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 2
- 241000256251 Spodoptera frugiperda Species 0.000 description 2
- 241000187747 Streptomyces Species 0.000 description 2
- 230000006052 T cell proliferation Effects 0.000 description 2
- IQFYYKKMVGJFEH-XLPZGREQSA-N Thymidine Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 IQFYYKKMVGJFEH-XLPZGREQSA-N 0.000 description 2
- 241000960387 Torque teno virus Species 0.000 description 2
- 108010009583 Transforming Growth Factors Proteins 0.000 description 2
- 102000009618 Transforming Growth Factors Human genes 0.000 description 2
- 229920004890 Triton X-100 Polymers 0.000 description 2
- 244000000188 Vaccinium ovalifolium Species 0.000 description 2
- 108010073929 Vascular Endothelial Growth Factor A Proteins 0.000 description 2
- 102000005789 Vascular Endothelial Growth Factors Human genes 0.000 description 2
- 108010019530 Vascular Endothelial Growth Factors Proteins 0.000 description 2
- 241000251539 Vertebrata <Metazoa> Species 0.000 description 2
- JXLYSJRDGCGARV-WWYNWVTFSA-N Vinblastine Natural products O=C(O[C@H]1[C@](O)(C(=O)OC)[C@@H]2N(C)c3c(cc(c(OC)c3)[C@]3(C(=O)OC)c4[nH]c5c(c4CCN4C[C@](O)(CC)C[C@H](C3)C4)cccc5)[C@@]32[C@H]2[C@@]1(CC)C=CCN2CC3)C JXLYSJRDGCGARV-WWYNWVTFSA-N 0.000 description 2
- 208000033559 Waldenström macroglobulinemia Diseases 0.000 description 2
- 240000008042 Zea mays Species 0.000 description 2
- 235000005824 Zea mays ssp. parviglumis Nutrition 0.000 description 2
- 235000002017 Zea mays subsp mays Nutrition 0.000 description 2
- 238000002835 absorbance Methods 0.000 description 2
- RJURFGZVJUQBHK-IIXSONLDSA-N actinomycin D Chemical compound C[C@H]1OC(=O)[C@H](C(C)C)N(C)C(=O)CN(C)C(=O)[C@@H]2CCCN2C(=O)[C@@H](C(C)C)NC(=O)[C@H]1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)N[C@@H]4C(=O)N[C@@H](C(N5CCC[C@H]5C(=O)N(C)CC(=O)N(C)[C@@H](C(C)C)C(=O)O[C@@H]4C)=O)C(C)C)=C3N=C21 RJURFGZVJUQBHK-IIXSONLDSA-N 0.000 description 2
- OIRDTQYFTABQOQ-KQYNXXCUSA-N adenosine Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O OIRDTQYFTABQOQ-KQYNXXCUSA-N 0.000 description 2
- 230000001464 adherent effect Effects 0.000 description 2
- BSJGASKRWFKGMV-UHFFFAOYSA-L ammonia dichloroplatinum(2+) Chemical compound N.N.Cl[Pt+2]Cl BSJGASKRWFKGMV-UHFFFAOYSA-L 0.000 description 2
- 238000012870 ammonium sulfate precipitation Methods 0.000 description 2
- 229940121369 angiogenesis inhibitor Drugs 0.000 description 2
- RWZYAGGXGHYGMB-UHFFFAOYSA-N anthranilic acid Chemical compound NC1=CC=CC=C1C(O)=O RWZYAGGXGHYGMB-UHFFFAOYSA-N 0.000 description 2
- 230000030741 antigen processing and presentation Effects 0.000 description 2
- 239000012736 aqueous medium Substances 0.000 description 2
- 210000004436 artificial bacterial chromosome Anatomy 0.000 description 2
- 210000001106 artificial yeast chromosome Anatomy 0.000 description 2
- 210000003719 b-lymphocyte Anatomy 0.000 description 2
- 229960000686 benzalkonium chloride Drugs 0.000 description 2
- CADWTSSKOVRVJC-UHFFFAOYSA-N benzyl(dimethyl)azanium;chloride Chemical compound [Cl-].C[NH+](C)CC1=CC=CC=C1 CADWTSSKOVRVJC-UHFFFAOYSA-N 0.000 description 2
- 239000011230 binding agent Substances 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 235000020958 biotin Nutrition 0.000 description 2
- 210000004204 blood vessel Anatomy 0.000 description 2
- 239000001506 calcium phosphate Substances 0.000 description 2
- 229910000389 calcium phosphate Inorganic materials 0.000 description 2
- 235000011010 calcium phosphates Nutrition 0.000 description 2
- 239000001768 carboxy methyl cellulose Substances 0.000 description 2
- 230000003833 cell viability Effects 0.000 description 2
- 201000007455 central nervous system cancer Diseases 0.000 description 2
- 208000025997 central nervous system neoplasm Diseases 0.000 description 2
- 239000002738 chelating agent Substances 0.000 description 2
- OSASVXMJTNOKOY-UHFFFAOYSA-N chlorobutanol Chemical compound CC(C)(O)C(Cl)(Cl)Cl OSASVXMJTNOKOY-UHFFFAOYSA-N 0.000 description 2
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 2
- DQLATGHUWYMOKM-UHFFFAOYSA-L cisplatin Chemical compound N[Pt](N)(Cl)Cl DQLATGHUWYMOKM-UHFFFAOYSA-L 0.000 description 2
- 229960004316 cisplatin Drugs 0.000 description 2
- 230000000295 complement effect Effects 0.000 description 2
- 239000003636 conditioned culture medium Substances 0.000 description 2
- 238000013270 controlled release Methods 0.000 description 2
- 238000007796 conventional method Methods 0.000 description 2
- 235000005822 corn Nutrition 0.000 description 2
- 230000008878 coupling Effects 0.000 description 2
- 238000010168 coupling process Methods 0.000 description 2
- 238000005859 coupling reaction Methods 0.000 description 2
- 230000009260 cross reactivity Effects 0.000 description 2
- 229940097362 cyclodextrins Drugs 0.000 description 2
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 2
- 230000016396 cytokine production Effects 0.000 description 2
- 231100000599 cytotoxic agent Toxicity 0.000 description 2
- 229960000640 dactinomycin Drugs 0.000 description 2
- 238000004925 denaturation Methods 0.000 description 2
- 230000036425 denaturation Effects 0.000 description 2
- CFCUWKMKBJTWLW-UHFFFAOYSA-N deoliosyl-3C-alpha-L-digitoxosyl-MTM Natural products CC=1C(O)=C2C(O)=C3C(=O)C(OC4OC(C)C(O)C(OC5OC(C)C(O)C(OC6OC(C)C(O)C(C)(O)C6)C5)C4)C(C(OC)C(=O)C(O)C(C)O)CC3=CC2=CC=1OC(OC(C)C1O)CC1OC1CC(O)C(O)C(C)O1 CFCUWKMKBJTWLW-UHFFFAOYSA-N 0.000 description 2
- 229910003460 diamond Inorganic materials 0.000 description 2
- 238000007865 diluting Methods 0.000 description 2
- 239000003085 diluting agent Substances 0.000 description 2
- 238000011038 discontinuous diafiltration by volume reduction Methods 0.000 description 2
- 239000002270 dispersing agent Substances 0.000 description 2
- 229960004679 doxorubicin Drugs 0.000 description 2
- 238000012377 drug delivery Methods 0.000 description 2
- 238000004520 electroporation Methods 0.000 description 2
- 230000008030 elimination Effects 0.000 description 2
- 238000003379 elimination reaction Methods 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- DEFVIWRASFVYLL-UHFFFAOYSA-N ethylene glycol bis(2-aminoethyl)tetraacetic acid Chemical compound OC(=O)CN(CC(O)=O)CCOCCOCCN(CC(O)=O)CC(O)=O DEFVIWRASFVYLL-UHFFFAOYSA-N 0.000 description 2
- 208000006275 fascioliasis Diseases 0.000 description 2
- 229940126864 fibroblast growth factor Drugs 0.000 description 2
- 230000003176 fibrotic effect Effects 0.000 description 2
- 229960002584 gefitinib Drugs 0.000 description 2
- XGALLCVXEZPNRQ-UHFFFAOYSA-N gefitinib Chemical compound C=12C=C(OCCCN3CCOCC3)C(OC)=CC2=NC=NC=1NC1=CC=C(F)C(Cl)=C1 XGALLCVXEZPNRQ-UHFFFAOYSA-N 0.000 description 2
- 238000012224 gene deletion Methods 0.000 description 2
- 230000030279 gene silencing Effects 0.000 description 2
- 238000012226 gene silencing method Methods 0.000 description 2
- 239000003102 growth factor Substances 0.000 description 2
- 230000036541 health Effects 0.000 description 2
- 208000014951 hematologic disease Diseases 0.000 description 2
- BXWNKGSJHAJOGX-UHFFFAOYSA-N hexadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCO BXWNKGSJHAJOGX-UHFFFAOYSA-N 0.000 description 2
- 102000048119 human PDCD1LG2 Human genes 0.000 description 2
- 239000003906 humectant Substances 0.000 description 2
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 2
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 2
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 2
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 description 2
- 229960002411 imatinib Drugs 0.000 description 2
- KTUFNOKKBVMGRW-UHFFFAOYSA-N imatinib Chemical compound C1CN(C)CCN1CC1=CC=C(C(=O)NC=2C=C(NC=3N=C(C=CN=3)C=3C=NC=CC=3)C(C)=CC=2)C=C1 KTUFNOKKBVMGRW-UHFFFAOYSA-N 0.000 description 2
- 230000001900 immune effect Effects 0.000 description 2
- 230000002163 immunogen Effects 0.000 description 2
- 230000001506 immunosuppresive effect Effects 0.000 description 2
- 230000005917 in vivo anti-tumor Effects 0.000 description 2
- 230000000415 inactivating effect Effects 0.000 description 2
- 239000000411 inducer Substances 0.000 description 2
- 230000006698 induction Effects 0.000 description 2
- 239000004615 ingredient Substances 0.000 description 2
- 108091008042 inhibitory receptors Proteins 0.000 description 2
- 239000007972 injectable composition Substances 0.000 description 2
- 238000003780 insertion Methods 0.000 description 2
- 230000037431 insertion Effects 0.000 description 2
- 229940125396 insulin Drugs 0.000 description 2
- 229960003130 interferon gamma Drugs 0.000 description 2
- 230000004073 interleukin-2 production Effects 0.000 description 2
- 238000007912 intraperitoneal administration Methods 0.000 description 2
- 238000005342 ion exchange Methods 0.000 description 2
- 238000001155 isoelectric focusing Methods 0.000 description 2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 2
- 239000004816 latex Substances 0.000 description 2
- 229920000126 latex Polymers 0.000 description 2
- 238000001638 lipofection Methods 0.000 description 2
- 239000000314 lubricant Substances 0.000 description 2
- 210000001165 lymph node Anatomy 0.000 description 2
- 210000004698 lymphocyte Anatomy 0.000 description 2
- 210000003712 lysosome Anatomy 0.000 description 2
- 230000001868 lysosomic effect Effects 0.000 description 2
- 201000000564 macroglobulinemia Diseases 0.000 description 2
- 210000002540 macrophage Anatomy 0.000 description 2
- 235000019359 magnesium stearate Nutrition 0.000 description 2
- 239000003550 marker Substances 0.000 description 2
- 239000011159 matrix material Substances 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 239000012528 membrane Substances 0.000 description 2
- GLVAUDGFNGKCSF-UHFFFAOYSA-N mercaptopurine Chemical compound S=C1NC=NC2=C1NC=N2 GLVAUDGFNGKCSF-UHFFFAOYSA-N 0.000 description 2
- MYWUZJCMWCOHBA-VIFPVBQESA-N methamphetamine Chemical compound CN[C@@H](C)CC1=CC=CC=C1 MYWUZJCMWCOHBA-VIFPVBQESA-N 0.000 description 2
- CFCUWKMKBJTWLW-BKHRDMLASA-N mithramycin Chemical compound O([C@@H]1C[C@@H](O[C@H](C)[C@H]1O)OC=1C=C2C=C3C[C@H]([C@@H](C(=O)C3=C(O)C2=C(O)C=1C)O[C@@H]1O[C@H](C)[C@@H](O)[C@H](O[C@@H]2O[C@H](C)[C@H](O)[C@H](O[C@@H]3O[C@H](C)[C@@H](O)[C@@](C)(O)C3)C2)C1)[C@H](OC)C(=O)[C@@H](O)[C@@H](C)O)[C@H]1C[C@@H](O)[C@H](O)[C@@H](C)O1 CFCUWKMKBJTWLW-BKHRDMLASA-N 0.000 description 2
- 238000002156 mixing Methods 0.000 description 2
- BQJCRHHNABKAKU-KBQPJGBKSA-N morphine Chemical compound O([C@H]1[C@H](C=C[C@H]23)O)C4=C5[C@@]12CCN(C)[C@@H]3CC5=CC=C4O BQJCRHHNABKAKU-KBQPJGBKSA-N 0.000 description 2
- 229940126619 mouse monoclonal antibody Drugs 0.000 description 2
- 239000013642 negative control Substances 0.000 description 2
- 239000012457 nonaqueous media Substances 0.000 description 2
- 231100000252 nontoxic Toxicity 0.000 description 2
- 230000003000 nontoxic effect Effects 0.000 description 2
- 239000002773 nucleotide Substances 0.000 description 2
- 125000003729 nucleotide group Chemical group 0.000 description 2
- 239000006174 pH buffer Substances 0.000 description 2
- 229940055729 papain Drugs 0.000 description 2
- 235000019834 papain Nutrition 0.000 description 2
- 238000007911 parenteral administration Methods 0.000 description 2
- 229960000639 pazopanib Drugs 0.000 description 2
- CUIHSIWYWATEQL-UHFFFAOYSA-N pazopanib Chemical compound C1=CC2=C(C)N(C)N=C2C=C1N(C)C(N=1)=CC=NC=1NC1=CC=C(C)C(S(N)(=O)=O)=C1 CUIHSIWYWATEQL-UHFFFAOYSA-N 0.000 description 2
- 230000035515 penetration Effects 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 2
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 2
- 229960003171 plicamycin Drugs 0.000 description 2
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 2
- 229920000136 polysorbate Polymers 0.000 description 2
- 229950008882 polysorbate Drugs 0.000 description 2
- 229920000053 polysorbate 80 Polymers 0.000 description 2
- 229920002451 polyvinyl alcohol Polymers 0.000 description 2
- 235000019422 polyvinyl alcohol Nutrition 0.000 description 2
- 230000003389 potentiating effect Effects 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- AQHHHDLHHXJYJD-UHFFFAOYSA-N propranolol Chemical compound C1=CC=C2C(OCC(O)CNC(C)C)=CC=CC2=C1 AQHHHDLHHXJYJD-UHFFFAOYSA-N 0.000 description 2
- RXWNCPJZOCPEPQ-NVWDDTSBSA-N puromycin Chemical compound C1=CC(OC)=CC=C1C[C@H](N)C(=O)N[C@H]1[C@@H](O)[C@H](N2C3=NC=NC(=C3N=C2)N(C)C)O[C@@H]1CO RXWNCPJZOCPEPQ-NVWDDTSBSA-N 0.000 description 2
- 239000000985 reactive dye Substances 0.000 description 2
- 230000001105 regulatory effect Effects 0.000 description 2
- 125000006853 reporter group Chemical group 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 239000011347 resin Substances 0.000 description 2
- 229920005989 resin Polymers 0.000 description 2
- 230000000717 retained effect Effects 0.000 description 2
- 238000004007 reversed phase HPLC Methods 0.000 description 2
- 102200004706 rs1060505034 Human genes 0.000 description 2
- 102220316159 rs1553350080 Human genes 0.000 description 2
- 102200047334 rs193922289 Human genes 0.000 description 2
- 102220111532 rs572322476 Human genes 0.000 description 2
- 102220094509 rs876658977 Human genes 0.000 description 2
- 201000004409 schistosomiasis Diseases 0.000 description 2
- 230000035945 sensitivity Effects 0.000 description 2
- 239000012679 serum free medium Substances 0.000 description 2
- 238000003998 size exclusion chromatography high performance liquid chromatography Methods 0.000 description 2
- 150000003384 small molecules Chemical class 0.000 description 2
- 229910052708 sodium Inorganic materials 0.000 description 2
- 239000001632 sodium acetate Substances 0.000 description 2
- 235000017281 sodium acetate Nutrition 0.000 description 2
- UIIMBOGNXHQVGW-UHFFFAOYSA-M sodium bicarbonate Substances [Na+].OC([O-])=O UIIMBOGNXHQVGW-UHFFFAOYSA-M 0.000 description 2
- 229910000030 sodium bicarbonate Inorganic materials 0.000 description 2
- 238000000527 sonication Methods 0.000 description 2
- 229960003787 sorafenib Drugs 0.000 description 2
- 239000000600 sorbitol Substances 0.000 description 2
- 230000009870 specific binding Effects 0.000 description 2
- 238000004611 spectroscopical analysis Methods 0.000 description 2
- 239000003381 stabilizer Substances 0.000 description 2
- 210000000130 stem cell Anatomy 0.000 description 2
- 239000011550 stock solution Substances 0.000 description 2
- 229960005322 streptomycin Drugs 0.000 description 2
- 238000007920 subcutaneous administration Methods 0.000 description 2
- 235000000346 sugar Nutrition 0.000 description 2
- 229960001796 sunitinib Drugs 0.000 description 2
- WINHZLLDWRZWRT-ATVHPVEESA-N sunitinib Chemical compound CCN(CC)CCNC(=O)C1=C(C)NC(\C=C/2C3=CC(F)=CC=C3NC\2=O)=C1C WINHZLLDWRZWRT-ATVHPVEESA-N 0.000 description 2
- 238000001356 surgical procedure Methods 0.000 description 2
- 230000002459 sustained effect Effects 0.000 description 2
- 201000000596 systemic lupus erythematosus Diseases 0.000 description 2
- 239000003826 tablet Substances 0.000 description 2
- CWERGRDVMFNCDR-UHFFFAOYSA-N thioglycolic acid Chemical compound OC(=O)CS CWERGRDVMFNCDR-UHFFFAOYSA-N 0.000 description 2
- RWQNBRDOKXIBIV-UHFFFAOYSA-N thymine Chemical compound CC1=CNC(=O)NC1=O RWQNBRDOKXIBIV-UHFFFAOYSA-N 0.000 description 2
- WYWHKKSPHMUBEB-UHFFFAOYSA-N tioguanine Chemical compound N1C(N)=NC(=S)C2=C1N=CN2 WYWHKKSPHMUBEB-UHFFFAOYSA-N 0.000 description 2
- 238000013518 transcription Methods 0.000 description 2
- 230000035897 transcription Effects 0.000 description 2
- 230000010474 transient expression Effects 0.000 description 2
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 2
- 201000002311 trypanosomiasis Diseases 0.000 description 2
- 229910052721 tungsten Inorganic materials 0.000 description 2
- 241000701447 unidentified baculovirus Species 0.000 description 2
- 241001430294 unidentified retrovirus Species 0.000 description 2
- 229960003048 vinblastine Drugs 0.000 description 2
- JXLYSJRDGCGARV-XQKSVPLYSA-N vincaleukoblastine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 JXLYSJRDGCGARV-XQKSVPLYSA-N 0.000 description 2
- OGWKCGZFUXNPDA-XQKSVPLYSA-N vincristine Chemical compound C([N@]1C[C@@H](C[C@]2(C(=O)OC)C=3C(=CC4=C([C@]56[C@H]([C@@]([C@H](OC(C)=O)[C@]7(CC)C=CCN([C@H]67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)C[C@@](C1)(O)CC)CC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-XQKSVPLYSA-N 0.000 description 2
- 229960004528 vincristine Drugs 0.000 description 2
- OGWKCGZFUXNPDA-UHFFFAOYSA-N vincristine Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(OC(C)=O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-UHFFFAOYSA-N 0.000 description 2
- 230000003612 virological effect Effects 0.000 description 2
- 239000012224 working solution Substances 0.000 description 2
- QAPUWNJNUGPVPM-NTUXHYESSA-N (17z,23z)-3-butyl-4,6,8,10,12,14,16,27-octahydroxy-17,28-dimethyl-1-oxacyclooctacosa-17,19,21,23,25-pentaen-2-one Chemical compound CCCCC1C(O)CC(O)CC(O)CC(O)CC(O)CC(O)CC(O)\C(C)=C/C=CC=C\C=C/C=CC(O)C(C)OC1=O QAPUWNJNUGPVPM-NTUXHYESSA-N 0.000 description 1
- QUWIXWFOWIEFRQ-GASJEMHNSA-N (2R,3S,4R,5R)-1-sulfanylhexane-1,2,3,4,5,6-hexol Chemical compound SC(O)[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO QUWIXWFOWIEFRQ-GASJEMHNSA-N 0.000 description 1
- ALBODLTZUXKBGZ-JUUVMNCLSA-N (2s)-2-amino-3-phenylpropanoic acid;(2s)-2,6-diaminohexanoic acid Chemical compound NCCCC[C@H](N)C(O)=O.OC(=O)[C@@H](N)CC1=CC=CC=C1 ALBODLTZUXKBGZ-JUUVMNCLSA-N 0.000 description 1
- VEEGZPWAAPPXRB-BJMVGYQFSA-N (3e)-3-(1h-imidazol-5-ylmethylidene)-1h-indol-2-one Chemical compound O=C1NC2=CC=CC=C2\C1=C/C1=CN=CN1 VEEGZPWAAPPXRB-BJMVGYQFSA-N 0.000 description 1
- FELGMEQIXOGIFQ-CYBMUJFWSA-N (3r)-9-methyl-3-[(2-methylimidazol-1-yl)methyl]-2,3-dihydro-1h-carbazol-4-one Chemical compound CC1=NC=CN1C[C@@H]1C(=O)C(C=2C(=CC=CC=2)N2C)=C2CC1 FELGMEQIXOGIFQ-CYBMUJFWSA-N 0.000 description 1
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 1
- ICLYJLBTOGPLMC-KVVVOXFISA-N (z)-octadec-9-enoate;tris(2-hydroxyethyl)azanium Chemical compound OCCN(CCO)CCO.CCCCCCCC\C=C/CCCCCCCC(O)=O ICLYJLBTOGPLMC-KVVVOXFISA-N 0.000 description 1
- FPKVOQKZMBDBKP-UHFFFAOYSA-N 1-[4-[(2,5-dioxopyrrol-1-yl)methyl]cyclohexanecarbonyl]oxy-2,5-dioxopyrrolidine-3-sulfonic acid Chemical compound O=C1C(S(=O)(=O)O)CC(=O)N1OC(=O)C1CCC(CN2C(C=CC2=O)=O)CC1 FPKVOQKZMBDBKP-UHFFFAOYSA-N 0.000 description 1
- BJHCYTJNPVGSBZ-YXSASFKJSA-N 1-[4-[6-amino-5-[(Z)-methoxyiminomethyl]pyrimidin-4-yl]oxy-2-chlorophenyl]-3-ethylurea Chemical compound CCNC(=O)Nc1ccc(Oc2ncnc(N)c2\C=N/OC)cc1Cl BJHCYTJNPVGSBZ-YXSASFKJSA-N 0.000 description 1
- ULWKUJKHKUABSH-UHFFFAOYSA-N 1-butyl-3-methoxy-2-methylbenzene Chemical compound CCCCC1=CC=CC(OC)=C1C ULWKUJKHKUABSH-UHFFFAOYSA-N 0.000 description 1
- VBICKXHEKHSIBG-UHFFFAOYSA-N 1-monostearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)CO VBICKXHEKHSIBG-UHFFFAOYSA-N 0.000 description 1
- PWVUXRBUUYZMKM-UHFFFAOYSA-N 2-(2-hydroxyethoxy)ethyl octadecanoate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCCOCCO PWVUXRBUUYZMKM-UHFFFAOYSA-N 0.000 description 1
- FKOKUHFZNIUSLW-UHFFFAOYSA-N 2-Hydroxypropyl stearate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(C)O FKOKUHFZNIUSLW-UHFFFAOYSA-N 0.000 description 1
- QKNYBSVHEMOAJP-UHFFFAOYSA-N 2-amino-2-(hydroxymethyl)propane-1,3-diol;hydron;chloride Chemical compound Cl.OCC(N)(CO)CO QKNYBSVHEMOAJP-UHFFFAOYSA-N 0.000 description 1
- GOZMBJCYMQQACI-UHFFFAOYSA-N 6,7-dimethyl-3-[[methyl-[2-[methyl-[[1-[3-(trifluoromethyl)phenyl]indol-3-yl]methyl]amino]ethyl]amino]methyl]chromen-4-one;dihydrochloride Chemical compound Cl.Cl.C=1OC2=CC(C)=C(C)C=C2C(=O)C=1CN(C)CCN(C)CC(C1=CC=CC=C11)=CN1C1=CC=CC(C(F)(F)F)=C1 GOZMBJCYMQQACI-UHFFFAOYSA-N 0.000 description 1
- 206010069408 Acanthamoeba keratitis Diseases 0.000 description 1
- 206010063409 Acarodermatitis Diseases 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-N Acrylic acid Chemical compound OC(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 1
- 241000256118 Aedes aegypti Species 0.000 description 1
- 241000256173 Aedes albopictus Species 0.000 description 1
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 1
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- 208000004881 Amebiasis Diseases 0.000 description 1
- 206010001980 Amoebiasis Diseases 0.000 description 1
- 241000269627 Amphiuma means Species 0.000 description 1
- 206010002091 Anaesthesia Diseases 0.000 description 1
- 235000002198 Annona diversifolia Nutrition 0.000 description 1
- 208000002109 Argyria Diseases 0.000 description 1
- 201000002909 Aspergillosis Diseases 0.000 description 1
- 241000228212 Aspergillus Species 0.000 description 1
- 208000036641 Aspergillus infections Diseases 0.000 description 1
- 241000351920 Aspergillus nidulans Species 0.000 description 1
- 241000228245 Aspergillus niger Species 0.000 description 1
- 206010003571 Astrocytoma Diseases 0.000 description 1
- 241000201370 Autographa californica nucleopolyhedrovirus Species 0.000 description 1
- 208000032791 BCR-ABL1 positive chronic myelogenous leukemia Diseases 0.000 description 1
- 241000194108 Bacillus licheniformis Species 0.000 description 1
- 244000063299 Bacillus subtilis Species 0.000 description 1
- 235000014469 Bacillus subtilis Nutrition 0.000 description 1
- DWRXFEITVBNRMK-UHFFFAOYSA-N Beta-D-1-Arabinofuranosylthymine Natural products O=C1NC(=O)C(C)=CN1C1C(O)C(O)C(CO)O1 DWRXFEITVBNRMK-UHFFFAOYSA-N 0.000 description 1
- 206010004593 Bile duct cancer Diseases 0.000 description 1
- 206010005098 Blastomycosis Diseases 0.000 description 1
- 108010006654 Bleomycin Proteins 0.000 description 1
- 241000255789 Bombyx mori Species 0.000 description 1
- 241000409811 Bombyx mori nucleopolyhedrovirus Species 0.000 description 1
- 206010006143 Brain stem glioma Diseases 0.000 description 1
- COVZYZSDYWQREU-UHFFFAOYSA-N Busulfan Chemical compound CS(=O)(=O)OCCCCOS(C)(=O)=O COVZYZSDYWQREU-UHFFFAOYSA-N 0.000 description 1
- 239000004322 Butylated hydroxytoluene Substances 0.000 description 1
- NLZUEZXRPGMBCV-UHFFFAOYSA-N Butylhydroxytoluene Chemical compound CC1=CC(C(C)(C)C)=C(O)C(C(C)(C)C)=C1 NLZUEZXRPGMBCV-UHFFFAOYSA-N 0.000 description 1
- 239000002126 C01EB10 - Adenosine Substances 0.000 description 1
- 108010021064 CTLA-4 Antigen Proteins 0.000 description 1
- 102000008203 CTLA-4 Antigen Human genes 0.000 description 1
- 229940045513 CTLA4 antagonist Drugs 0.000 description 1
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical compound [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 description 1
- 241000282836 Camelus dromedarius Species 0.000 description 1
- 241000589876 Campylobacter Species 0.000 description 1
- 241000222120 Candida <Saccharomycetales> Species 0.000 description 1
- 241000222122 Candida albicans Species 0.000 description 1
- 206010007134 Candida infections Diseases 0.000 description 1
- 239000005461 Canertinib Substances 0.000 description 1
- 241000282465 Canis Species 0.000 description 1
- 101710128063 Carbohydrate oxidase Proteins 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- 108010022366 Carcinoembryonic Antigen Proteins 0.000 description 1
- 102100025475 Carcinoembryonic antigen-related cell adhesion molecule 5 Human genes 0.000 description 1
- DLGOEMSEDOSKAD-UHFFFAOYSA-N Carmustine Chemical compound ClCCNC(=O)N(N=O)CCCl DLGOEMSEDOSKAD-UHFFFAOYSA-N 0.000 description 1
- 102100035882 Catalase Human genes 0.000 description 1
- 108010053835 Catalase Proteins 0.000 description 1
- 206010007953 Central nervous system lymphoma Diseases 0.000 description 1
- 206010068051 Chimerism Diseases 0.000 description 1
- 208000010833 Chronic myeloid leukaemia Diseases 0.000 description 1
- 108020004705 Codon Proteins 0.000 description 1
- 108010071942 Colony-Stimulating Factors Proteins 0.000 description 1
- 102000007644 Colony-Stimulating Factors Human genes 0.000 description 1
- 108010060123 Conjugate Vaccines Proteins 0.000 description 1
- 108091035707 Consensus sequence Proteins 0.000 description 1
- 229920000742 Cotton Polymers 0.000 description 1
- 208000009798 Craniopharyngioma Diseases 0.000 description 1
- 208000011231 Crohn disease Diseases 0.000 description 1
- 208000008953 Cryptosporidiosis Diseases 0.000 description 1
- 206010011502 Cryptosporidiosis infection Diseases 0.000 description 1
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 description 1
- UHDGCWIWMRVCDJ-CCXZUQQUSA-N Cytarabine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1 UHDGCWIWMRVCDJ-CCXZUQQUSA-N 0.000 description 1
- 241000701022 Cytomegalovirus Species 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- 229920002271 DEAE-Sepharose Polymers 0.000 description 1
- GUBGYTABKSRVRQ-WFVLMXAXSA-N DEAE-cellulose Chemical compound OC1C(O)C(O)C(CO)O[C@H]1O[C@@H]1C(CO)OC(O)C(O)C1O GUBGYTABKSRVRQ-WFVLMXAXSA-N 0.000 description 1
- 102100033553 Delta-like protein 4 Human genes 0.000 description 1
- 229920002307 Dextran Polymers 0.000 description 1
- 206010012735 Diarrhoea Diseases 0.000 description 1
- LTMHDMANZUZIPE-AMTYYWEZSA-N Digoxin Natural products O([C@H]1[C@H](C)O[C@H](O[C@@H]2C[C@@H]3[C@@](C)([C@@H]4[C@H]([C@]5(O)[C@](C)([C@H](O)C4)[C@H](C4=CC(=O)OC4)CC5)CC3)CC2)C[C@@H]1O)[C@H]1O[C@H](C)[C@@H](O[C@H]2O[C@@H](C)[C@H](O)[C@@H](O)C2)[C@@H](O)C1 LTMHDMANZUZIPE-AMTYYWEZSA-N 0.000 description 1
- 241000255581 Drosophila <fruit fly, genus> Species 0.000 description 1
- 241000255601 Drosophila melanogaster Species 0.000 description 1
- 101100507451 Drosophila melanogaster sip3 gene Proteins 0.000 description 1
- 102000001301 EGF receptor Human genes 0.000 description 1
- 108060006698 EGF receptor Proteins 0.000 description 1
- 206010014096 Echinococciasis Diseases 0.000 description 1
- 208000009366 Echinococcosis Diseases 0.000 description 1
- MBYXEBXZARTUSS-QLWBXOBMSA-N Emetamine Natural products O(C)c1c(OC)cc2c(c(C[C@@H]3[C@H](CC)CN4[C@H](c5c(cc(OC)c(OC)c5)CC4)C3)ncc2)c1 MBYXEBXZARTUSS-QLWBXOBMSA-N 0.000 description 1
- 241000588921 Enterobacteriaceae Species 0.000 description 1
- 241000194033 Enterococcus Species 0.000 description 1
- 206010014967 Ependymoma Diseases 0.000 description 1
- 101800003838 Epidermal growth factor Proteins 0.000 description 1
- 241000283073 Equus caballus Species 0.000 description 1
- 241000588698 Erwinia Species 0.000 description 1
- 241000701959 Escherichia virus Lambda Species 0.000 description 1
- 241001524679 Escherichia virus M13 Species 0.000 description 1
- 102100029951 Estrogen receptor beta Human genes 0.000 description 1
- 108091008794 FGF receptors Proteins 0.000 description 1
- 229920001917 Ficoll Polymers 0.000 description 1
- 241000192125 Firmicutes Species 0.000 description 1
- 241000589565 Flavobacterium Species 0.000 description 1
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 description 1
- 239000012739 FreeStyle 293 Expression medium Substances 0.000 description 1
- 239000005715 Fructose Substances 0.000 description 1
- 229930091371 Fructose Natural products 0.000 description 1
- RFSUNEUAIZKAJO-ARQDHWQXSA-N Fructose Chemical compound OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O RFSUNEUAIZKAJO-ARQDHWQXSA-N 0.000 description 1
- 241000233866 Fungi Species 0.000 description 1
- 241000287828 Gallus gallus Species 0.000 description 1
- CEAZRRDELHUEMR-URQXQFDESA-N Gentamicin Chemical compound O1[C@H](C(C)NC)CC[C@@H](N)[C@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](NC)[C@@](C)(O)CO2)O)[C@H](N)C[C@@H]1N CEAZRRDELHUEMR-URQXQFDESA-N 0.000 description 1
- 229930182566 Gentamicin Natural products 0.000 description 1
- 241000626621 Geobacillus Species 0.000 description 1
- 206010018364 Glomerulonephritis Diseases 0.000 description 1
- 108010073178 Glucan 1,4-alpha-Glucosidase Proteins 0.000 description 1
- 102100022624 Glucoamylase Human genes 0.000 description 1
- UQJNXZSSGQIPIQ-FBCQKBJTSA-N Gly-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)CN UQJNXZSSGQIPIQ-FBCQKBJTSA-N 0.000 description 1
- 244000068988 Glycine max Species 0.000 description 1
- 235000010469 Glycine max Nutrition 0.000 description 1
- 241000282575 Gorilla Species 0.000 description 1
- 241000219146 Gossypium Species 0.000 description 1
- 108010026389 Gramicidin Proteins 0.000 description 1
- 208000031886 HIV Infections Diseases 0.000 description 1
- 241000589989 Helicobacter Species 0.000 description 1
- 208000006968 Helminthiasis Diseases 0.000 description 1
- 208000032843 Hemorrhage Diseases 0.000 description 1
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 description 1
- 241000700721 Hepatitis B virus Species 0.000 description 1
- 208000007514 Herpes zoster Diseases 0.000 description 1
- 201000002563 Histoplasmosis Diseases 0.000 description 1
- 241001272567 Hominoidea Species 0.000 description 1
- 101000872077 Homo sapiens Delta-like protein 4 Proteins 0.000 description 1
- 101001010910 Homo sapiens Estrogen receptor beta Proteins 0.000 description 1
- 101001034314 Homo sapiens Lactadherin Proteins 0.000 description 1
- 101000946889 Homo sapiens Monocyte differentiation antigen CD14 Proteins 0.000 description 1
- 101100519206 Homo sapiens PDCD1 gene Proteins 0.000 description 1
- 101000825949 Homo sapiens R-spondin-2 Proteins 0.000 description 1
- 101000825960 Homo sapiens R-spondin-3 Proteins 0.000 description 1
- 101000825962 Homo sapiens R-spondin-4 Proteins 0.000 description 1
- 108010001336 Horseradish Peroxidase Proteins 0.000 description 1
- 241000701044 Human gammaherpesvirus 4 Species 0.000 description 1
- 241001502974 Human gammaherpesvirus 8 Species 0.000 description 1
- 241000725303 Human immunodeficiency virus Species 0.000 description 1
- 241000713772 Human immunodeficiency virus 1 Species 0.000 description 1
- 241000701806 Human papillomavirus Species 0.000 description 1
- 241000829111 Human polyomavirus 1 Species 0.000 description 1
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 1
- 206010020751 Hypersensitivity Diseases 0.000 description 1
- 102100026120 IgG receptor FcRn large subunit p51 Human genes 0.000 description 1
- 101710177940 IgG receptor FcRn large subunit p51 Proteins 0.000 description 1
- OVPYIUNCVSOVNF-ZPFDUUQYSA-N Ile-Gln-Pro Natural products CC[C@H](C)[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(O)=O OVPYIUNCVSOVNF-ZPFDUUQYSA-N 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 229940076838 Immune checkpoint inhibitor Drugs 0.000 description 1
- 229940123309 Immune checkpoint modulator Drugs 0.000 description 1
- 102000006496 Immunoglobulin Heavy Chains Human genes 0.000 description 1
- 108010019476 Immunoglobulin Heavy Chains Proteins 0.000 description 1
- 206010062016 Immunosuppression Diseases 0.000 description 1
- 208000022559 Inflammatory bowel disease Diseases 0.000 description 1
- 102000006992 Interferon-alpha Human genes 0.000 description 1
- 108010047761 Interferon-alpha Proteins 0.000 description 1
- 102000003996 Interferon-beta Human genes 0.000 description 1
- 108090000467 Interferon-beta Proteins 0.000 description 1
- 102000014150 Interferons Human genes 0.000 description 1
- 108010050904 Interferons Proteins 0.000 description 1
- 102100020881 Interleukin-1 alpha Human genes 0.000 description 1
- 108090000177 Interleukin-11 Proteins 0.000 description 1
- 108010065805 Interleukin-12 Proteins 0.000 description 1
- 108010082786 Interleukin-1alpha Proteins 0.000 description 1
- 108010002386 Interleukin-3 Proteins 0.000 description 1
- 108010002616 Interleukin-5 Proteins 0.000 description 1
- 108090001005 Interleukin-6 Proteins 0.000 description 1
- 108010002586 Interleukin-7 Proteins 0.000 description 1
- 108090001007 Interleukin-8 Proteins 0.000 description 1
- 108010002335 Interleukin-9 Proteins 0.000 description 1
- 102000015696 Interleukins Human genes 0.000 description 1
- 108010063738 Interleukins Proteins 0.000 description 1
- 241000701460 JC polyomavirus Species 0.000 description 1
- 241000006384 Jeotgalibacillus marinus Species 0.000 description 1
- 239000007836 KH2PO4 Substances 0.000 description 1
- 208000007766 Kaposi sarcoma Diseases 0.000 description 1
- 241000588748 Klebsiella Species 0.000 description 1
- FADYJNXDPBKVCA-UHFFFAOYSA-N L-Phenylalanyl-L-lysin Natural products NCCCCC(C(O)=O)NC(=O)C(N)CC1=CC=CC=C1 FADYJNXDPBKVCA-UHFFFAOYSA-N 0.000 description 1
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 1
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 1
- 239000002136 L01XE07 - Lapatinib Substances 0.000 description 1
- 241000481961 Lachancea thermotolerans Species 0.000 description 1
- 102100039648 Lactadherin Human genes 0.000 description 1
- JVTAAEKCZFNVCJ-UHFFFAOYSA-M Lactate Chemical compound CC(O)C([O-])=O JVTAAEKCZFNVCJ-UHFFFAOYSA-M 0.000 description 1
- 241000186660 Lactobacillus Species 0.000 description 1
- 241000194036 Lactococcus Species 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 241000282838 Lama Species 0.000 description 1
- 108010000851 Laminin Receptors Proteins 0.000 description 1
- 102000002297 Laminin Receptors Human genes 0.000 description 1
- 239000004166 Lanolin Substances 0.000 description 1
- 208000004554 Leishmaniasis Diseases 0.000 description 1
- 102100020872 Leucyl-cystinyl aminopeptidase Human genes 0.000 description 1
- 206010024305 Leukaemia monocytic Diseases 0.000 description 1
- NNJVILVZKWQKPM-UHFFFAOYSA-N Lidocaine Chemical compound CCN(CC)CC(=O)NC1=C(C)C=CC=C1C NNJVILVZKWQKPM-UHFFFAOYSA-N 0.000 description 1
- 108060001084 Luciferase Proteins 0.000 description 1
- 239000005089 Luciferase Substances 0.000 description 1
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 1
- 239000006137 Luria-Bertani broth Substances 0.000 description 1
- 235000007688 Lycopersicon esculentum Nutrition 0.000 description 1
- 208000016604 Lyme disease Diseases 0.000 description 1
- PELXPRPDQRFBGQ-KKUMJFAQSA-N Lys-Tyr-Asn Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CCCCN)N)O PELXPRPDQRFBGQ-KKUMJFAQSA-N 0.000 description 1
- 241000282560 Macaca mulatta Species 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 208000037196 Medullary thyroid carcinoma Diseases 0.000 description 1
- 208000000172 Medulloblastoma Diseases 0.000 description 1
- RVYDCISQIGHAFC-ZPFDUUQYSA-N Met-Ile-Gln Chemical compound CSCC[C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(O)=O RVYDCISQIGHAFC-ZPFDUUQYSA-N 0.000 description 1
- 201000000090 Microsporidiosis Diseases 0.000 description 1
- VFKZTMPDYBFSTM-KVTDHHQDSA-N Mitobronitol Chemical compound BrC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CBr VFKZTMPDYBFSTM-KVTDHHQDSA-N 0.000 description 1
- 229930192392 Mitomycin Natural products 0.000 description 1
- 102100035877 Monocyte differentiation antigen CD14 Human genes 0.000 description 1
- 206010028116 Mucosal inflammation Diseases 0.000 description 1
- 201000010927 Mucositis Diseases 0.000 description 1
- 102000016943 Muramidase Human genes 0.000 description 1
- 108010014251 Muramidase Proteins 0.000 description 1
- 101100372766 Mus musculus Kdr gene Proteins 0.000 description 1
- 208000031888 Mycoses Diseases 0.000 description 1
- 208000033761 Myelogenous Chronic BCR-ABL Positive Leukemia Diseases 0.000 description 1
- 208000006123 Myiasis Diseases 0.000 description 1
- 108010062010 N-Acetylmuramoyl-L-alanine Amidase Proteins 0.000 description 1
- NQTADLQHYWFPDB-UHFFFAOYSA-N N-Hydroxysuccinimide Chemical compound ON1C(=O)CCC1=O NQTADLQHYWFPDB-UHFFFAOYSA-N 0.000 description 1
- 208000002454 Nasopharyngeal Carcinoma Diseases 0.000 description 1
- 206010061306 Nasopharyngeal cancer Diseases 0.000 description 1
- 206010028813 Nausea Diseases 0.000 description 1
- 241000588653 Neisseria Species 0.000 description 1
- 206010029260 Neuroblastoma Diseases 0.000 description 1
- 244000061176 Nicotiana tabacum Species 0.000 description 1
- 235000002637 Nicotiana tabacum Nutrition 0.000 description 1
- 239000004677 Nylon Substances 0.000 description 1
- 241001452677 Ogataea methanolica Species 0.000 description 1
- 201000010133 Oligodendroglioma Diseases 0.000 description 1
- 108020005187 Oligonucleotide Probes Proteins 0.000 description 1
- 241000243985 Onchocerca volvulus Species 0.000 description 1
- 208000010195 Onychomycosis Diseases 0.000 description 1
- 108700026244 Open Reading Frames Proteins 0.000 description 1
- BRUQQQPBMZOVGD-XFKAJCMBSA-N Oxycodone Chemical compound O=C([C@@H]1O2)CC[C@@]3(O)[C@H]4CC5=CC=C(OC)C2=C5[C@@]13CCN4C BRUQQQPBMZOVGD-XFKAJCMBSA-N 0.000 description 1
- 229940123751 PD-L1 antagonist Drugs 0.000 description 1
- 101150087384 PDCD1 gene Proteins 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 229930012538 Paclitaxel Natural products 0.000 description 1
- 241000282577 Pan troglodytes Species 0.000 description 1
- 206010033701 Papillary thyroid cancer Diseases 0.000 description 1
- 241001631646 Papillomaviridae Species 0.000 description 1
- 206010033767 Paracoccidioides infections Diseases 0.000 description 1
- 201000000301 Paracoccidioidomycosis Diseases 0.000 description 1
- 208000030852 Parasitic disease Diseases 0.000 description 1
- 235000019483 Peanut oil Nutrition 0.000 description 1
- 241000228143 Penicillium Species 0.000 description 1
- 102000057297 Pepsin A Human genes 0.000 description 1
- 108090000284 Pepsin A Proteins 0.000 description 1
- 108091005804 Peptidases Proteins 0.000 description 1
- 102000035195 Peptidases Human genes 0.000 description 1
- 239000001888 Peptone Substances 0.000 description 1
- 108010080698 Peptones Proteins 0.000 description 1
- 241000009328 Perro Species 0.000 description 1
- 240000007377 Petunia x hybrida Species 0.000 description 1
- 206010057249 Phagocytosis Diseases 0.000 description 1
- 101710114878 Phospholipase A-2-activating protein Proteins 0.000 description 1
- 108010004729 Phycoerythrin Proteins 0.000 description 1
- 208000007641 Pinealoma Diseases 0.000 description 1
- 102100022427 Plasmalemma vesicle-associated protein Human genes 0.000 description 1
- 101710193105 Plasmalemma vesicle-associated protein Proteins 0.000 description 1
- RVGRUAULSDPKGF-UHFFFAOYSA-N Poloxamer Chemical compound C1CO1.CC1CO1 RVGRUAULSDPKGF-UHFFFAOYSA-N 0.000 description 1
- 229920000805 Polyaspartic acid Polymers 0.000 description 1
- 208000008601 Polycythemia Diseases 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 239000004372 Polyvinyl alcohol Substances 0.000 description 1
- FUVBEZJCRMHWEM-FXQIFTODSA-N Pro-Asn-Ser Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(O)=O FUVBEZJCRMHWEM-FXQIFTODSA-N 0.000 description 1
- 102100033237 Pro-epidermal growth factor Human genes 0.000 description 1
- HCBIBCJNVBAKAB-UHFFFAOYSA-N Procaine hydrochloride Chemical compound Cl.CCN(CC)CCOC(=O)C1=CC=C(N)C=C1 HCBIBCJNVBAKAB-UHFFFAOYSA-N 0.000 description 1
- 102000007066 Prostate-Specific Antigen Human genes 0.000 description 1
- 108010072866 Prostate-Specific Antigen Proteins 0.000 description 1
- 108010029485 Protein Isoforms Proteins 0.000 description 1
- 102000001708 Protein Isoforms Human genes 0.000 description 1
- 108010076504 Protein Sorting Signals Proteins 0.000 description 1
- 241000588769 Proteus <enterobacteria> Species 0.000 description 1
- 102220543917 Protocadherin-10_L78M_mutation Human genes 0.000 description 1
- 208000010362 Protozoan Infections Diseases 0.000 description 1
- 241000589517 Pseudomonas aeruginosa Species 0.000 description 1
- 201000004681 Psoriasis Diseases 0.000 description 1
- 102100022763 R-spondin-2 Human genes 0.000 description 1
- 102100022766 R-spondin-3 Human genes 0.000 description 1
- 102100022759 R-spondin-4 Human genes 0.000 description 1
- 238000011529 RT qPCR Methods 0.000 description 1
- 208000032056 Radiation Fibrosis Syndrome Diseases 0.000 description 1
- 241000700157 Rattus norvegicus Species 0.000 description 1
- 101000959719 Rattus norvegicus AP-3 complex subunit mu-1 Proteins 0.000 description 1
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 1
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 1
- 201000000582 Retinoblastoma Diseases 0.000 description 1
- AUVVAXYIELKVAI-UHFFFAOYSA-N SJ000285215 Natural products N1CCC2=CC(OC)=C(OC)C=C2C1CC1CC2C3=CC(OC)=C(OC)C=C3CCN2CC1CC AUVVAXYIELKVAI-UHFFFAOYSA-N 0.000 description 1
- 235000018368 Saccharomyces fragilis Nutrition 0.000 description 1
- 241000293869 Salmonella enterica subsp. enterica serovar Typhimurium Species 0.000 description 1
- 241000447727 Scabies Species 0.000 description 1
- 206010039580 Scar Diseases 0.000 description 1
- 241000235347 Schizosaccharomyces pombe Species 0.000 description 1
- 241000607720 Serratia Species 0.000 description 1
- 241000607715 Serratia marcescens Species 0.000 description 1
- 241000607768 Shigella Species 0.000 description 1
- 206010041067 Small cell lung cancer Diseases 0.000 description 1
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 1
- 239000004141 Sodium laurylsulphate Substances 0.000 description 1
- 229920002125 Sokalan® Polymers 0.000 description 1
- 240000003768 Solanum lycopersicum Species 0.000 description 1
- 244000061456 Solanum tuberosum Species 0.000 description 1
- 235000002595 Solanum tuberosum Nutrition 0.000 description 1
- 241000191940 Staphylococcus Species 0.000 description 1
- 229920002472 Starch Polymers 0.000 description 1
- 235000021355 Stearic acid Nutrition 0.000 description 1
- 241000194017 Streptococcus Species 0.000 description 1
- ZSJLQEPLLKMAKR-UHFFFAOYSA-N Streptozotocin Natural products O=NN(C)C(=O)NC1C(O)OC(CO)C(O)C1O ZSJLQEPLLKMAKR-UHFFFAOYSA-N 0.000 description 1
- 241000244174 Strongyloides Species 0.000 description 1
- 108091027544 Subgenomic mRNA Proteins 0.000 description 1
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 1
- 239000012505 Superdex™ Substances 0.000 description 1
- 201000009594 Systemic Scleroderma Diseases 0.000 description 1
- 206010042953 Systemic sclerosis Diseases 0.000 description 1
- 230000024932 T cell mediated immunity Effects 0.000 description 1
- 229940123237 Taxane Drugs 0.000 description 1
- 241000255588 Tephritidae Species 0.000 description 1
- FOCVUCIESVLUNU-UHFFFAOYSA-N Thiotepa Chemical compound C1CN1P(N1CC1)(=S)N1CC1 FOCVUCIESVLUNU-UHFFFAOYSA-N 0.000 description 1
- SJPDTIQHLBQPFO-VLCNGCBASA-N Thr-Tyr-Trp Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O SJPDTIQHLBQPFO-VLCNGCBASA-N 0.000 description 1
- 241001149964 Tolypocladium Species 0.000 description 1
- 206010044269 Toxocariasis Diseases 0.000 description 1
- 201000005485 Toxoplasmosis Diseases 0.000 description 1
- 102000004338 Transferrin Human genes 0.000 description 1
- 108090000901 Transferrin Proteins 0.000 description 1
- 208000004938 Trematode Infections Diseases 0.000 description 1
- 206010044608 Trichiniasis Diseases 0.000 description 1
- 241000499912 Trichoderma reesei Species 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- 239000013504 Triton X-100 Substances 0.000 description 1
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 1
- 102100031988 Tumor necrosis factor ligand superfamily member 6 Human genes 0.000 description 1
- 108050002568 Tumor necrosis factor ligand superfamily member 6 Proteins 0.000 description 1
- 206010067584 Type 1 diabetes mellitus Diseases 0.000 description 1
- 102100039094 Tyrosinase Human genes 0.000 description 1
- 108060008724 Tyrosinase Proteins 0.000 description 1
- GBOGMAARMMDZGR-UHFFFAOYSA-N UNPD149280 Natural products N1C(=O)C23OC(=O)C=CC(O)CCCC(C)CC=CC3C(O)C(=C)C(C)C2C1CC1=CC=CC=C1 GBOGMAARMMDZGR-UHFFFAOYSA-N 0.000 description 1
- 241000202898 Ureaplasma Species 0.000 description 1
- 108010053099 Vascular Endothelial Growth Factor Receptor-2 Proteins 0.000 description 1
- 108010053100 Vascular Endothelial Growth Factor Receptor-3 Proteins 0.000 description 1
- 102100033179 Vascular endothelial growth factor receptor 3 Human genes 0.000 description 1
- 208000014070 Vestibular schwannoma Diseases 0.000 description 1
- 229940122803 Vinca alkaloid Drugs 0.000 description 1
- 101710145727 Viral Fc-gamma receptor-like protein UL119 Proteins 0.000 description 1
- 206010047700 Vomiting Diseases 0.000 description 1
- 208000010399 Wasting Syndrome Diseases 0.000 description 1
- 241000244005 Wuchereria bancrofti Species 0.000 description 1
- TVXBFESIOXBWNM-UHFFFAOYSA-N Xylitol Natural products OCCC(O)C(O)C(O)CCO TVXBFESIOXBWNM-UHFFFAOYSA-N 0.000 description 1
- 241000235015 Yarrowia lipolytica Species 0.000 description 1
- LWZFANDGMFTDAV-BURFUSLBSA-N [(2r)-2-[(2r,3r,4s)-3,4-dihydroxyoxolan-2-yl]-2-hydroxyethyl] dodecanoate Chemical compound CCCCCCCCCCCC(=O)OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O LWZFANDGMFTDAV-BURFUSLBSA-N 0.000 description 1
- 210000000683 abdominal cavity Anatomy 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- 208000004064 acoustic neuroma Diseases 0.000 description 1
- 208000017733 acquired polycythemia vera Diseases 0.000 description 1
- 229930183665 actinomycin Natural products 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 239000012190 activator Substances 0.000 description 1
- 230000004721 adaptive immunity Effects 0.000 description 1
- 229960005305 adenosine Drugs 0.000 description 1
- 210000000577 adipose tissue Anatomy 0.000 description 1
- 238000005377 adsorption chromatography Methods 0.000 description 1
- 239000000443 aerosol Substances 0.000 description 1
- 230000002776 aggregation Effects 0.000 description 1
- 238000004220 aggregation Methods 0.000 description 1
- 239000000556 agonist Substances 0.000 description 1
- 229930013930 alkaloid Natural products 0.000 description 1
- 229940100198 alkylating agent Drugs 0.000 description 1
- 239000002168 alkylating agent Substances 0.000 description 1
- 230000007815 allergy Effects 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 229940037003 alum Drugs 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 239000003708 ampul Substances 0.000 description 1
- 230000037005 anaesthesia Effects 0.000 description 1
- 229940035674 anesthetics Drugs 0.000 description 1
- 210000004102 animal cell Anatomy 0.000 description 1
- 239000003957 anion exchange resin Substances 0.000 description 1
- 150000001450 anions Chemical class 0.000 description 1
- 229940045799 anthracyclines and related substance Drugs 0.000 description 1
- 230000003527 anti-angiogenesis Effects 0.000 description 1
- 238000011122 anti-angiogenic therapy Methods 0.000 description 1
- 230000000844 anti-bacterial effect Effects 0.000 description 1
- 230000003474 anti-emetic effect Effects 0.000 description 1
- 230000000340 anti-metabolite Effects 0.000 description 1
- 230000000845 anti-microbial effect Effects 0.000 description 1
- 230000002927 anti-mitotic effect Effects 0.000 description 1
- 230000000692 anti-sense effect Effects 0.000 description 1
- 230000006023 anti-tumor response Effects 0.000 description 1
- 230000005875 antibody response Effects 0.000 description 1
- 229940125683 antiemetic agent Drugs 0.000 description 1
- 239000002111 antiemetic agent Substances 0.000 description 1
- 102000025171 antigen binding proteins Human genes 0.000 description 1
- 108091000831 antigen binding proteins Proteins 0.000 description 1
- 210000000612 antigen-presenting cell Anatomy 0.000 description 1
- 229940100197 antimetabolite Drugs 0.000 description 1
- 239000002256 antimetabolite Substances 0.000 description 1
- 239000003080 antimitotic agent Substances 0.000 description 1
- 229940034982 antineoplastic agent Drugs 0.000 description 1
- ATALOFNDEOCMKK-OITMNORJSA-N aprepitant Chemical compound O([C@@H]([C@@H]1C=2C=CC(F)=CC=2)O[C@H](C)C=2C=C(C=C(C=2)C(F)(F)F)C(F)(F)F)CCN1CC1=NNC(=O)N1 ATALOFNDEOCMKK-OITMNORJSA-N 0.000 description 1
- 229960001372 aprepitant Drugs 0.000 description 1
- 238000000149 argon plasma sintering Methods 0.000 description 1
- 210000004507 artificial chromosome Anatomy 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 201000009361 ascariasis Diseases 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 229960005070 ascorbic acid Drugs 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- 230000001363 autoimmune Effects 0.000 description 1
- 238000000376 autoradiography Methods 0.000 description 1
- 201000008680 babesiosis Diseases 0.000 description 1
- 230000003385 bacteriostatic effect Effects 0.000 description 1
- VVUBWCWVDFCEOP-UHFFFAOYSA-N benzene;styrene Chemical compound C1=CC=CC=C1.C=CC1=CC=CC=C1 VVUBWCWVDFCEOP-UHFFFAOYSA-N 0.000 description 1
- 102000005936 beta-Galactosidase Human genes 0.000 description 1
- 108010005774 beta-Galactosidase Proteins 0.000 description 1
- IQFYYKKMVGJFEH-UHFFFAOYSA-N beta-L-thymidine Natural products O=C1NC(=O)C(C)=CN1C1OC(CO)C(O)C1 IQFYYKKMVGJFEH-UHFFFAOYSA-N 0.000 description 1
- SQVRNKJHWKZAKO-UHFFFAOYSA-N beta-N-Acetyl-D-neuraminic acid Natural products CC(=O)NC1C(O)CC(O)(C(O)=O)OC1C(O)C(O)CO SQVRNKJHWKZAKO-UHFFFAOYSA-N 0.000 description 1
- 229960000397 bevacizumab Drugs 0.000 description 1
- 208000026900 bile duct neoplasm Diseases 0.000 description 1
- 238000001574 biopsy Methods 0.000 description 1
- 208000034158 bleeding Diseases 0.000 description 1
- 230000000740 bleeding effect Effects 0.000 description 1
- 229960001561 bleomycin Drugs 0.000 description 1
- OYVAGSVQBOHSSS-UAPAGMARSA-O bleomycin A2 Chemical compound N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC=C(N=1)C=1SC=C(N=1)C(=O)NCCC[S+](C)C)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1N=CNC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C OYVAGSVQBOHSSS-UAPAGMARSA-O 0.000 description 1
- 210000000601 blood cell Anatomy 0.000 description 1
- 210000001124 body fluid Anatomy 0.000 description 1
- RSIHSRDYCUFFLA-DYKIIFRCSA-N boldenone Chemical compound O=C1C=C[C@]2(C)[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 RSIHSRDYCUFFLA-DYKIIFRCSA-N 0.000 description 1
- 210000000988 bone and bone Anatomy 0.000 description 1
- 208000014581 breast ductal adenocarcinoma Diseases 0.000 description 1
- 201000010983 breast ductal carcinoma Diseases 0.000 description 1
- 208000003362 bronchogenic carcinoma Diseases 0.000 description 1
- 229960002092 busulfan Drugs 0.000 description 1
- 235000010354 butylated hydroxytoluene Nutrition 0.000 description 1
- 229940095259 butylated hydroxytoluene Drugs 0.000 description 1
- 210000004899 c-terminal region Anatomy 0.000 description 1
- 102220377136 c.10C>A Human genes 0.000 description 1
- 102220361728 c.224C>A Human genes 0.000 description 1
- 239000001110 calcium chloride Substances 0.000 description 1
- 229910001628 calcium chloride Inorganic materials 0.000 description 1
- 238000004422 calculation algorithm Methods 0.000 description 1
- 208000035269 cancer or benign tumor Diseases 0.000 description 1
- 201000003984 candidiasis Diseases 0.000 description 1
- 229950002826 canertinib Drugs 0.000 description 1
- OMZCMEYTWSXEPZ-UHFFFAOYSA-N canertinib Chemical compound C1=C(Cl)C(F)=CC=C1NC1=NC=NC2=CC(OCCCN3CCOCC3)=C(NC(=O)C=C)C=C12 OMZCMEYTWSXEPZ-UHFFFAOYSA-N 0.000 description 1
- 238000005251 capillar electrophoresis Methods 0.000 description 1
- 210000000234 capsid Anatomy 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 229960001631 carbomer Drugs 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 229960005243 carmustine Drugs 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 239000003729 cation exchange resin Substances 0.000 description 1
- 229940023913 cation exchange resins Drugs 0.000 description 1
- 230000022131 cell cycle Effects 0.000 description 1
- 230000003915 cell function Effects 0.000 description 1
- 238000012054 celltiter-glo Methods 0.000 description 1
- 230000005754 cellular signaling Effects 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 210000003169 central nervous system Anatomy 0.000 description 1
- 229940082500 cetostearyl alcohol Drugs 0.000 description 1
- 229960000541 cetyl alcohol Drugs 0.000 description 1
- QAPUWNJNUGPVPM-UHFFFAOYSA-N chainin Natural products CCCCC1C(O)CC(O)CC(O)CC(O)CC(O)CC(O)CC(O)C(C)=CC=CC=CC=CC=CC(O)C(C)OC1=O QAPUWNJNUGPVPM-UHFFFAOYSA-N 0.000 description 1
- NDAYQJDHGXTBJL-MWWSRJDJSA-N chembl557217 Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CC=3C4=CC=CC=C4NC=3)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CC=3C4=CC=CC=C4NC=3)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CC=3C4=CC=CC=C4NC=3)NC(=O)[C@@H](C(C)C)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](C(C)C)NC(=O)[C@H](C)NC(=O)[C@H](NC(=O)CNC(=O)[C@@H](NC=O)C(C)C)CC(C)C)C(=O)NCCO)=CNC2=C1 NDAYQJDHGXTBJL-MWWSRJDJSA-N 0.000 description 1
- 229960004630 chlorambucil Drugs 0.000 description 1
- JCKYGMPEJWAADB-UHFFFAOYSA-N chlorambucil Chemical compound OC(=O)CCCC1=CC=C(N(CCCl)CCCl)C=C1 JCKYGMPEJWAADB-UHFFFAOYSA-N 0.000 description 1
- 229960004926 chlorobutanol Drugs 0.000 description 1
- 235000012000 cholesterol Nutrition 0.000 description 1
- 229940107161 cholesterol Drugs 0.000 description 1
- 238000011098 chromatofocusing Methods 0.000 description 1
- 239000012501 chromatography medium Substances 0.000 description 1
- 208000019425 cirrhosis of liver Diseases 0.000 description 1
- 239000007979 citrate buffer Substances 0.000 description 1
- 239000013599 cloning vector Substances 0.000 description 1
- 238000000975 co-precipitation Methods 0.000 description 1
- 229960001338 colchicine Drugs 0.000 description 1
- 206010009887 colitis Diseases 0.000 description 1
- 229940047120 colony stimulating factors Drugs 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 201000010989 colorectal carcinoma Diseases 0.000 description 1
- 238000011284 combination treatment Methods 0.000 description 1
- 230000009137 competitive binding Effects 0.000 description 1
- 238000010668 complexation reaction Methods 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 238000005094 computer simulation Methods 0.000 description 1
- 238000012790 confirmation Methods 0.000 description 1
- 229940031670 conjugate vaccine Drugs 0.000 description 1
- 230000021615 conjugation Effects 0.000 description 1
- 239000000356 contaminant Substances 0.000 description 1
- 239000013068 control sample Substances 0.000 description 1
- 239000005289 controlled pore glass Substances 0.000 description 1
- 239000000599 controlled substance Substances 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- NKLPQNGYXWVELD-UHFFFAOYSA-M coomassie brilliant blue Chemical compound [Na+].C1=CC(OCC)=CC=C1NC1=CC=C(C(=C2C=CC(C=C2)=[N+](CC)CC=2C=C(C=CC=2)S([O-])(=O)=O)C=2C=CC(=CC=2)N(CC)CC=2C=C(C=CC=2)S([O-])(=O)=O)C=C1 NKLPQNGYXWVELD-UHFFFAOYSA-M 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- 235000005687 corn oil Nutrition 0.000 description 1
- 239000002285 corn oil Substances 0.000 description 1
- 235000012343 cottonseed oil Nutrition 0.000 description 1
- 239000002385 cottonseed oil Substances 0.000 description 1
- 239000006071 cream Substances 0.000 description 1
- 150000001896 cresols Chemical class 0.000 description 1
- 238000011461 current therapy Methods 0.000 description 1
- 238000005520 cutting process Methods 0.000 description 1
- 229960004397 cyclophosphamide Drugs 0.000 description 1
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 description 1
- 150000001945 cysteines Chemical class 0.000 description 1
- 229960000684 cytarabine Drugs 0.000 description 1
- GBOGMAARMMDZGR-TYHYBEHESA-N cytochalasin B Chemical compound C([C@H]1[C@@H]2[C@@H](C([C@@H](O)[C@@H]3/C=C/C[C@H](C)CCC[C@@H](O)/C=C/C(=O)O[C@@]23C(=O)N1)=C)C)C1=CC=CC=C1 GBOGMAARMMDZGR-TYHYBEHESA-N 0.000 description 1
- GBOGMAARMMDZGR-JREHFAHYSA-N cytochalasin B Natural products C[C@H]1CCC[C@@H](O)C=CC(=O)O[C@@]23[C@H](C=CC1)[C@H](O)C(=C)[C@@H](C)[C@@H]2[C@H](Cc4ccccc4)NC3=O GBOGMAARMMDZGR-JREHFAHYSA-N 0.000 description 1
- 210000001151 cytotoxic T lymphocyte Anatomy 0.000 description 1
- 239000002254 cytotoxic agent Substances 0.000 description 1
- 239000002619 cytotoxin Substances 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 125000001295 dansyl group Chemical group [H]C1=C([H])C(N(C([H])([H])[H])C([H])([H])[H])=C2C([H])=C([H])C([H])=C(C2=C1[H])S(*)(=O)=O 0.000 description 1
- 230000009615 deamination Effects 0.000 description 1
- 238000006481 deamination reaction Methods 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- RSIHSRDYCUFFLA-UHFFFAOYSA-N dehydrotestosterone Natural products O=C1C=CC2(C)C3CCC(C)(C(CC4)O)C4C3CCC2=C1 RSIHSRDYCUFFLA-UHFFFAOYSA-N 0.000 description 1
- 239000003398 denaturant Substances 0.000 description 1
- 238000000432 density-gradient centrifugation Methods 0.000 description 1
- 238000011033 desalting Methods 0.000 description 1
- 239000003599 detergent Substances 0.000 description 1
- 239000008121 dextrose Substances 0.000 description 1
- 239000008355 dextrose injection Substances 0.000 description 1
- LTMHDMANZUZIPE-PUGKRICDSA-N digoxin Chemical compound C1[C@H](O)[C@H](O)[C@@H](C)O[C@H]1O[C@@H]1[C@@H](C)O[C@@H](O[C@@H]2[C@H](O[C@@H](O[C@@H]3C[C@@H]4[C@]([C@@H]5[C@H]([C@]6(CC[C@@H]([C@@]6(C)[C@H](O)C5)C=5COC(=O)C=5)O)CC4)(C)CC3)C[C@@H]2O)C)C[C@@H]1O LTMHDMANZUZIPE-PUGKRICDSA-N 0.000 description 1
- 229960005156 digoxin Drugs 0.000 description 1
- LTMHDMANZUZIPE-UHFFFAOYSA-N digoxine Natural products C1C(O)C(O)C(C)OC1OC1C(C)OC(OC2C(OC(OC3CC4C(C5C(C6(CCC(C6(C)C(O)C5)C=5COC(=O)C=5)O)CC4)(C)CC3)CC2O)C)CC1O LTMHDMANZUZIPE-UHFFFAOYSA-N 0.000 description 1
- 229940042399 direct acting antivirals protease inhibitors Drugs 0.000 description 1
- 239000007884 disintegrant Substances 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 238000004821 distillation Methods 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 125000002228 disulfide group Chemical group 0.000 description 1
- 239000003534 dna topoisomerase inhibitor Substances 0.000 description 1
- 229960003722 doxycycline Drugs 0.000 description 1
- XQTWDDCIUJNLTR-CVHRZJFOSA-N doxycycline monohydrate Chemical compound O.O=C1C2=C(O)C=CC=C2[C@H](C)[C@@H]2C1=C(O)[C@]1(O)C(=O)C(C(N)=O)=C(O)[C@@H](N(C)C)[C@@H]1[C@H]2O XQTWDDCIUJNLTR-CVHRZJFOSA-N 0.000 description 1
- 238000001962 electrophoresis Methods 0.000 description 1
- 208000006036 elephantiasis Diseases 0.000 description 1
- 238000010828 elution Methods 0.000 description 1
- AUVVAXYIELKVAI-CKBKHPSWSA-N emetine Chemical compound N1CCC2=CC(OC)=C(OC)C=C2[C@H]1C[C@H]1C[C@H]2C3=CC(OC)=C(OC)C=C3CCN2C[C@@H]1CC AUVVAXYIELKVAI-CKBKHPSWSA-N 0.000 description 1
- 229960002694 emetine Drugs 0.000 description 1
- AUVVAXYIELKVAI-UWBTVBNJSA-N emetine Natural products N1CCC2=CC(OC)=C(OC)C=C2[C@H]1C[C@H]1C[C@H]2C3=CC(OC)=C(OC)C=C3CCN2C[C@H]1CC AUVVAXYIELKVAI-UWBTVBNJSA-N 0.000 description 1
- 230000012202 endocytosis Effects 0.000 description 1
- 238000011013 endotoxin removal Methods 0.000 description 1
- 206010014881 enterobiasis Diseases 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- 229940116977 epidermal growth factor Drugs 0.000 description 1
- 102000052116 epidermal growth factor receptor activity proteins Human genes 0.000 description 1
- 108700015053 epidermal growth factor receptor activity proteins Proteins 0.000 description 1
- 210000000981 epithelium Anatomy 0.000 description 1
- CAMHHLOGFDZBBG-UHFFFAOYSA-N epoxidized methyl oleate Natural products CCCCCCCCC1OC1CCCCCCCC(=O)OC CAMHHLOGFDZBBG-UHFFFAOYSA-N 0.000 description 1
- 239000006167 equilibration buffer Substances 0.000 description 1
- 230000008029 eradication Effects 0.000 description 1
- 210000003743 erythrocyte Anatomy 0.000 description 1
- 102000015694 estrogen receptors Human genes 0.000 description 1
- 108010038795 estrogen receptors Proteins 0.000 description 1
- 238000012869 ethanol precipitation Methods 0.000 description 1
- ZMMJGEGLRURXTF-UHFFFAOYSA-N ethidium bromide Chemical compound [Br-].C12=CC(N)=CC=C2C2=CC=C(N)C=C2[N+](CC)=C1C1=CC=CC=C1 ZMMJGEGLRURXTF-UHFFFAOYSA-N 0.000 description 1
- 229960005542 ethidium bromide Drugs 0.000 description 1
- VJJPUSNTGOMMGY-MRVIYFEKSA-N etoposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 VJJPUSNTGOMMGY-MRVIYFEKSA-N 0.000 description 1
- 229960005420 etoposide Drugs 0.000 description 1
- 206010016235 fasciolopsiasis Diseases 0.000 description 1
- 210000002458 fetal heart Anatomy 0.000 description 1
- 210000002950 fibroblast Anatomy 0.000 description 1
- 102000052178 fibroblast growth factor receptor activity proteins Human genes 0.000 description 1
- 239000012997 ficoll-paque Substances 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- GNBHRKFJIUUOQI-UHFFFAOYSA-N fluorescein Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 GNBHRKFJIUUOQI-UHFFFAOYSA-N 0.000 description 1
- 239000007850 fluorescent dye Substances 0.000 description 1
- 238000001215 fluorescent labelling Methods 0.000 description 1
- 229960002949 fluorouracil Drugs 0.000 description 1
- 239000006260 foam Substances 0.000 description 1
- 235000013355 food flavoring agent Nutrition 0.000 description 1
- 239000012537 formulation buffer Substances 0.000 description 1
- 238000005194 fractionation Methods 0.000 description 1
- 238000004108 freeze drying Methods 0.000 description 1
- 238000007710 freezing Methods 0.000 description 1
- 230000008014 freezing Effects 0.000 description 1
- 208000024386 fungal infectious disease Diseases 0.000 description 1
- 230000001408 fungistatic effect Effects 0.000 description 1
- 108020001507 fusion proteins Proteins 0.000 description 1
- 102000037865 fusion proteins Human genes 0.000 description 1
- 238000005227 gel permeation chromatography Methods 0.000 description 1
- 239000003193 general anesthetic agent Substances 0.000 description 1
- 230000002068 genetic effect Effects 0.000 description 1
- 229960002518 gentamicin Drugs 0.000 description 1
- 201000006592 giardiasis Diseases 0.000 description 1
- 210000004907 gland Anatomy 0.000 description 1
- 239000003862 glucocorticoid Substances 0.000 description 1
- 235000001727 glucose Nutrition 0.000 description 1
- 229940075529 glyceryl stearate Drugs 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 210000003714 granulocyte Anatomy 0.000 description 1
- 230000009036 growth inhibition Effects 0.000 description 1
- 239000003966 growth inhibitor Substances 0.000 description 1
- 201000009277 hairy cell leukemia Diseases 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 208000025750 heavy chain disease Diseases 0.000 description 1
- 210000002443 helper t lymphocyte Anatomy 0.000 description 1
- 201000002222 hemangioblastoma Diseases 0.000 description 1
- 229960002897 heparin Drugs 0.000 description 1
- 229920000669 heparin Polymers 0.000 description 1
- 210000003494 hepatocyte Anatomy 0.000 description 1
- 208000016356 hereditary diffuse gastric adenocarcinoma Diseases 0.000 description 1
- UBHWBODXJBSFLH-UHFFFAOYSA-N hexadecan-1-ol;octadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCO.CCCCCCCCCCCCCCCCCCO UBHWBODXJBSFLH-UHFFFAOYSA-N 0.000 description 1
- 235000019534 high fructose corn syrup Nutrition 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- 210000003630 histaminocyte Anatomy 0.000 description 1
- 210000003701 histiocyte Anatomy 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 210000005260 human cell Anatomy 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 239000008172 hydrogenated vegetable oil Substances 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 238000004191 hydrophobic interaction chromatography Methods 0.000 description 1
- 229910052588 hydroxylapatite Inorganic materials 0.000 description 1
- 239000001863 hydroxypropyl cellulose Substances 0.000 description 1
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 description 1
- 229940071676 hydroxypropylcellulose Drugs 0.000 description 1
- 238000003384 imaging method Methods 0.000 description 1
- 210000002865 immune cell Anatomy 0.000 description 1
- 239000012274 immune-checkpoint protein inhibitor Substances 0.000 description 1
- 238000003119 immunoblot Methods 0.000 description 1
- 230000002434 immunopotentiative effect Effects 0.000 description 1
- 230000003308 immunostimulating effect Effects 0.000 description 1
- 239000007943 implant Substances 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 238000000099 in vitro assay Methods 0.000 description 1
- 238000011503 in vivo imaging Methods 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 208000027866 inflammatory disease Diseases 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 150000002484 inorganic compounds Chemical class 0.000 description 1
- 229910010272 inorganic material Inorganic materials 0.000 description 1
- 230000010354 integration Effects 0.000 description 1
- 102000006495 integrins Human genes 0.000 description 1
- 108010044426 integrins Proteins 0.000 description 1
- 230000002687 intercalation Effects 0.000 description 1
- 238000009830 intercalation Methods 0.000 description 1
- 229940047124 interferons Drugs 0.000 description 1
- 229940047122 interleukins Drugs 0.000 description 1
- 230000000968 intestinal effect Effects 0.000 description 1
- 230000003834 intracellular effect Effects 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 206010073095 invasive ductal breast carcinoma Diseases 0.000 description 1
- 238000004255 ion exchange chromatography Methods 0.000 description 1
- 239000007951 isotonicity adjuster Substances 0.000 description 1
- 238000005304 joining Methods 0.000 description 1
- 229940031154 kluyveromyces marxianus Drugs 0.000 description 1
- 239000004310 lactic acid Substances 0.000 description 1
- 235000014655 lactic acid Nutrition 0.000 description 1
- 229940039696 lactobacillus Drugs 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 235000019388 lanolin Nutrition 0.000 description 1
- 229940039717 lanolin Drugs 0.000 description 1
- 229910052747 lanthanoid Inorganic materials 0.000 description 1
- 150000002602 lanthanoids Chemical class 0.000 description 1
- 229960004891 lapatinib Drugs 0.000 description 1
- BCFGMOOMADDAQU-UHFFFAOYSA-N lapatinib Chemical compound O1C(CNCCS(=O)(=O)C)=CC=C1C1=CC=C(N=CN=C2NC=3C=C(Cl)C(OCC=4C=C(F)C=CC=4)=CC=3)C2=C1 BCFGMOOMADDAQU-UHFFFAOYSA-N 0.000 description 1
- 208000028454 lice infestation Diseases 0.000 description 1
- 229960004194 lidocaine Drugs 0.000 description 1
- 210000003041 ligament Anatomy 0.000 description 1
- 239000002502 liposome Substances 0.000 description 1
- 239000006193 liquid solution Substances 0.000 description 1
- 239000003589 local anesthetic agent Substances 0.000 description 1
- 229960005015 local anesthetics Drugs 0.000 description 1
- 229960002247 lomustine Drugs 0.000 description 1
- 201000005202 lung cancer Diseases 0.000 description 1
- 210000005265 lung cell Anatomy 0.000 description 1
- 208000020816 lung neoplasm Diseases 0.000 description 1
- 210000001365 lymphatic vessel Anatomy 0.000 description 1
- 230000002132 lysosomal effect Effects 0.000 description 1
- 239000004325 lysozyme Substances 0.000 description 1
- 235000010335 lysozyme Nutrition 0.000 description 1
- 229960000274 lysozyme Drugs 0.000 description 1
- ZLNQQNXFFQJAID-UHFFFAOYSA-L magnesium carbonate Chemical compound [Mg+2].[O-]C([O-])=O ZLNQQNXFFQJAID-UHFFFAOYSA-L 0.000 description 1
- 239000001095 magnesium carbonate Substances 0.000 description 1
- 229910000021 magnesium carbonate Inorganic materials 0.000 description 1
- 229910001629 magnesium chloride Inorganic materials 0.000 description 1
- 239000004137 magnesium phosphate Substances 0.000 description 1
- 229910000157 magnesium phosphate Inorganic materials 0.000 description 1
- 229960002261 magnesium phosphate Drugs 0.000 description 1
- 235000010994 magnesium phosphates Nutrition 0.000 description 1
- 201000004792 malaria Diseases 0.000 description 1
- 210000004962 mammalian cell Anatomy 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 238000004949 mass spectrometry Methods 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 229960004961 mechlorethamine Drugs 0.000 description 1
- HAWPXGHAZFHHAD-UHFFFAOYSA-N mechlorethamine Chemical compound ClCCN(C)CCCl HAWPXGHAZFHHAD-UHFFFAOYSA-N 0.000 description 1
- 229960001924 melphalan Drugs 0.000 description 1
- SGDBTWWWUNNDEQ-LBPRGKRZSA-N melphalan Chemical compound OC(=O)[C@@H](N)CC1=CC=C(N(CCCl)CCCl)C=C1 SGDBTWWWUNNDEQ-LBPRGKRZSA-N 0.000 description 1
- 206010027191 meningioma Diseases 0.000 description 1
- 229960001428 mercaptopurine Drugs 0.000 description 1
- HEBKCHPVOIAQTA-UHFFFAOYSA-N meso ribitol Natural products OCC(O)C(O)C(O)CO HEBKCHPVOIAQTA-UHFFFAOYSA-N 0.000 description 1
- 229960000485 methotrexate Drugs 0.000 description 1
- 229920000609 methyl cellulose Polymers 0.000 description 1
- 235000010270 methyl p-hydroxybenzoate Nutrition 0.000 description 1
- 239000001923 methylcellulose Substances 0.000 description 1
- 238000002493 microarray Methods 0.000 description 1
- 239000003094 microcapsule Substances 0.000 description 1
- 238000000520 microinjection Methods 0.000 description 1
- 239000011859 microparticle Substances 0.000 description 1
- 238000000386 microscopy Methods 0.000 description 1
- 239000004005 microsphere Substances 0.000 description 1
- 230000003278 mimic effect Effects 0.000 description 1
- 239000002480 mineral oil Substances 0.000 description 1
- 235000010446 mineral oil Nutrition 0.000 description 1
- 239000007758 minimum essential medium Substances 0.000 description 1
- 229960005485 mitobronitol Drugs 0.000 description 1
- 229960001156 mitoxantrone Drugs 0.000 description 1
- KKZJGLLVHKMTCM-UHFFFAOYSA-N mitoxantrone Chemical compound O=C1C2=C(O)C=CC(O)=C2C(=O)C2=C1C(NCCNCCO)=CC=C2NCCNCCO KKZJGLLVHKMTCM-UHFFFAOYSA-N 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 201000006894 monocytic leukemia Diseases 0.000 description 1
- 229910000402 monopotassium phosphate Inorganic materials 0.000 description 1
- PJUIMOJAAPLTRJ-UHFFFAOYSA-N monothioglycerol Chemical compound OCC(O)CS PJUIMOJAAPLTRJ-UHFFFAOYSA-N 0.000 description 1
- 229960005181 morphine Drugs 0.000 description 1
- 201000006417 multiple sclerosis Diseases 0.000 description 1
- 210000003205 muscle Anatomy 0.000 description 1
- 210000001167 myeloblast Anatomy 0.000 description 1
- 206010028537 myelofibrosis Diseases 0.000 description 1
- YOHYSYJDKVYCJI-UHFFFAOYSA-N n-[3-[[6-[3-(trifluoromethyl)anilino]pyrimidin-4-yl]amino]phenyl]cyclopropanecarboxamide Chemical compound FC(F)(F)C1=CC=CC(NC=2N=CN=C(NC=3C=C(NC(=O)C4CC4)C=CC=3)C=2)=C1 YOHYSYJDKVYCJI-UHFFFAOYSA-N 0.000 description 1
- 239000002088 nanocapsule Substances 0.000 description 1
- 239000002105 nanoparticle Substances 0.000 description 1
- 239000002077 nanosphere Substances 0.000 description 1
- 201000003631 narcolepsy Diseases 0.000 description 1
- 201000011216 nasopharynx carcinoma Diseases 0.000 description 1
- 210000000581 natural killer T-cell Anatomy 0.000 description 1
- 210000000822 natural killer cell Anatomy 0.000 description 1
- 230000008693 nausea Effects 0.000 description 1
- 230000001537 neural effect Effects 0.000 description 1
- 108010087904 neutravidin Proteins 0.000 description 1
- 239000000346 nonvolatile oil Substances 0.000 description 1
- 238000012758 nuclear staining Methods 0.000 description 1
- 235000015097 nutrients Nutrition 0.000 description 1
- 229920001778 nylon Polymers 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 1
- 239000002674 ointment Substances 0.000 description 1
- 239000002751 oligonucleotide probe Substances 0.000 description 1
- 208000002042 onchocerciasis Diseases 0.000 description 1
- 230000000177 oncogenetic effect Effects 0.000 description 1
- 229960005343 ondansetron Drugs 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 201000008482 osteoarthritis Diseases 0.000 description 1
- DWAFYCQODLXJNR-BNTLRKBRSA-L oxaliplatin Chemical compound O1C(=O)C(=O)O[Pt]11N[C@@H]2CCCC[C@H]2N1 DWAFYCQODLXJNR-BNTLRKBRSA-L 0.000 description 1
- 229960001756 oxaliplatin Drugs 0.000 description 1
- 229960002085 oxycodone Drugs 0.000 description 1
- 239000006179 pH buffering agent Substances 0.000 description 1
- 229960001592 paclitaxel Drugs 0.000 description 1
- 230000036407 pain Effects 0.000 description 1
- 238000002638 palliative care Methods 0.000 description 1
- 208000004019 papillary adenocarcinoma Diseases 0.000 description 1
- 208000003154 papilloma Diseases 0.000 description 1
- 244000045947 parasite Species 0.000 description 1
- 208000014837 parasitic helminthiasis infectious disease Diseases 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 239000000312 peanut oil Substances 0.000 description 1
- 230000000149 penetrating effect Effects 0.000 description 1
- XYJRXVWERLGGKC-UHFFFAOYSA-D pentacalcium;hydroxide;triphosphate Chemical compound [OH-].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O XYJRXVWERLGGKC-UHFFFAOYSA-D 0.000 description 1
- 229960001412 pentobarbital Drugs 0.000 description 1
- WEXRUCMBJFQVBZ-UHFFFAOYSA-N pentobarbital Chemical compound CCCC(C)C1(CC)C(=O)NC(=O)NC1=O WEXRUCMBJFQVBZ-UHFFFAOYSA-N 0.000 description 1
- 229940111202 pepsin Drugs 0.000 description 1
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 1
- 235000019319 peptone Nutrition 0.000 description 1
- 210000005259 peripheral blood Anatomy 0.000 description 1
- 239000011886 peripheral blood Substances 0.000 description 1
- 210000001322 periplasm Anatomy 0.000 description 1
- 238000002823 phage display Methods 0.000 description 1
- 230000008782 phagocytosis Effects 0.000 description 1
- 230000003285 pharmacodynamic effect Effects 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- WVDDGKGOMKODPV-ZQBYOMGUSA-N phenyl(114C)methanol Chemical compound O[14CH2]C1=CC=CC=C1 WVDDGKGOMKODPV-ZQBYOMGUSA-N 0.000 description 1
- 239000008363 phosphate buffer Substances 0.000 description 1
- LFGREXWGYUGZLY-UHFFFAOYSA-N phosphoryl Chemical group [P]=O LFGREXWGYUGZLY-UHFFFAOYSA-N 0.000 description 1
- 230000026731 phosphorylation Effects 0.000 description 1
- 238000006366 phosphorylation reaction Methods 0.000 description 1
- 238000002428 photodynamic therapy Methods 0.000 description 1
- 239000006187 pill Substances 0.000 description 1
- 208000024724 pineal body neoplasm Diseases 0.000 description 1
- 201000004123 pineal gland cancer Diseases 0.000 description 1
- 210000002826 placenta Anatomy 0.000 description 1
- BLFWHYXWBKKRHI-JYBILGDPSA-N plap Chemical compound N([C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(O)=O)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(=O)[C@@H]1CCCN1C(=O)[C@H](CO)NC(=O)[C@@H](N)CCC(O)=O BLFWHYXWBKKRHI-JYBILGDPSA-N 0.000 description 1
- 229910052697 platinum Inorganic materials 0.000 description 1
- 229920001993 poloxamer 188 Polymers 0.000 description 1
- 108010054442 polyalanine Proteins 0.000 description 1
- 108010064470 polyaspartate Proteins 0.000 description 1
- 208000037244 polycythemia vera Diseases 0.000 description 1
- 230000000581 polycythemic effect Effects 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 229920000259 polyoxyethylene lauryl ether Polymers 0.000 description 1
- 239000000244 polyoxyethylene sorbitan monooleate Substances 0.000 description 1
- 229920002503 polyoxyethylene-polyoxypropylene Polymers 0.000 description 1
- 235000013824 polyphenols Nutrition 0.000 description 1
- 229940068968 polysorbate 80 Drugs 0.000 description 1
- 238000010837 poor prognosis Methods 0.000 description 1
- 239000013641 positive control Substances 0.000 description 1
- 238000002600 positron emission tomography Methods 0.000 description 1
- 238000012809 post-inoculation Methods 0.000 description 1
- 230000001323 posttranslational effect Effects 0.000 description 1
- 239000008057 potassium phosphate buffer Substances 0.000 description 1
- 230000003334 potential effect Effects 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 1
- 208000016800 primary central nervous system lymphoma Diseases 0.000 description 1
- 208000003476 primary myelofibrosis Diseases 0.000 description 1
- 229960004919 procaine Drugs 0.000 description 1
- MFDFERRIHVXMIY-UHFFFAOYSA-N procaine Chemical compound CCN(CC)CCOC(=O)C1=CC=C(N)C=C1 MFDFERRIHVXMIY-UHFFFAOYSA-N 0.000 description 1
- 229960001309 procaine hydrochloride Drugs 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 210000004765 promyelocyte Anatomy 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- 229960003712 propranolol Drugs 0.000 description 1
- 239000000473 propyl gallate Substances 0.000 description 1
- 235000010388 propyl gallate Nutrition 0.000 description 1
- 229940075579 propyl gallate Drugs 0.000 description 1
- 235000010232 propyl p-hydroxybenzoate Nutrition 0.000 description 1
- 229940093625 propylene glycol monostearate Drugs 0.000 description 1
- 230000009145 protein modification Effects 0.000 description 1
- 238000001742 protein purification Methods 0.000 description 1
- 230000017854 proteolysis Effects 0.000 description 1
- 208000005069 pulmonary fibrosis Diseases 0.000 description 1
- 229950010131 puromycin Drugs 0.000 description 1
- 230000002285 radioactive effect Effects 0.000 description 1
- 239000002287 radioligand Substances 0.000 description 1
- 239000011535 reaction buffer Substances 0.000 description 1
- 238000003259 recombinant expression Methods 0.000 description 1
- 238000001953 recrystallisation Methods 0.000 description 1
- 238000004064 recycling Methods 0.000 description 1
- 230000008929 regeneration Effects 0.000 description 1
- 238000011069 regeneration method Methods 0.000 description 1
- 238000002271 resection Methods 0.000 description 1
- 108091008146 restriction endonucleases Proteins 0.000 description 1
- 238000004366 reverse phase liquid chromatography Methods 0.000 description 1
- 238000003757 reverse transcription PCR Methods 0.000 description 1
- 206010039073 rheumatoid arthritis Diseases 0.000 description 1
- PYWVYCXTNDRMGF-UHFFFAOYSA-N rhodamine B Chemical compound [Cl-].C=12C=CC(=[N+](CC)CC)C=C2OC2=CC(N(CC)CC)=CC=C2C=1C1=CC=CC=C1C(O)=O PYWVYCXTNDRMGF-UHFFFAOYSA-N 0.000 description 1
- 102200091615 rs17887176 Human genes 0.000 description 1
- 102200097270 rs199472847 Human genes 0.000 description 1
- 102200044494 rs28903098 Human genes 0.000 description 1
- 102200110699 rs61491953 Human genes 0.000 description 1
- 239000012146 running buffer Substances 0.000 description 1
- CVHZOJJKTDOEJC-UHFFFAOYSA-N saccharin Chemical compound C1=CC=C2C(=O)NS(=O)(=O)C2=C1 CVHZOJJKTDOEJC-UHFFFAOYSA-N 0.000 description 1
- 235000002020 sage Nutrition 0.000 description 1
- 238000005185 salting out Methods 0.000 description 1
- 238000005070 sampling Methods 0.000 description 1
- 238000009738 saturating Methods 0.000 description 1
- 208000005687 scabies Diseases 0.000 description 1
- 230000037390 scarring Effects 0.000 description 1
- 201000008407 sebaceous adenocarcinoma Diseases 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 239000008159 sesame oil Substances 0.000 description 1
- 235000011803 sesame oil Nutrition 0.000 description 1
- SQVRNKJHWKZAKO-OQPLDHBCSA-N sialic acid Chemical compound CC(=O)N[C@@H]1[C@@H](O)C[C@@](O)(C(O)=O)OC1[C@H](O)[C@H](O)CO SQVRNKJHWKZAKO-OQPLDHBCSA-N 0.000 description 1
- 230000011664 signaling Effects 0.000 description 1
- 238000010898 silica gel chromatography Methods 0.000 description 1
- 238000009097 single-agent therapy Methods 0.000 description 1
- 238000005549 size reduction Methods 0.000 description 1
- 238000001542 size-exclusion chromatography Methods 0.000 description 1
- 208000000587 small cell lung carcinoma Diseases 0.000 description 1
- 229960004249 sodium acetate Drugs 0.000 description 1
- 239000007974 sodium acetate buffer Substances 0.000 description 1
- WBHQBSYUUJJSRZ-UHFFFAOYSA-M sodium bisulfate Chemical compound [Na+].OS([O-])(=O)=O WBHQBSYUUJJSRZ-UHFFFAOYSA-M 0.000 description 1
- 229910000342 sodium bisulfate Inorganic materials 0.000 description 1
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 1
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 1
- 239000008354 sodium chloride injection Substances 0.000 description 1
- 235000019333 sodium laurylsulphate Nutrition 0.000 description 1
- 239000012064 sodium phosphate buffer Substances 0.000 description 1
- AKHNMLFCWUSKQB-UHFFFAOYSA-L sodium thiosulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=S AKHNMLFCWUSKQB-UHFFFAOYSA-L 0.000 description 1
- 235000019345 sodium thiosulphate Nutrition 0.000 description 1
- VUFNRPJNRFOTGK-UHFFFAOYSA-M sodium;1-[4-[(2,5-dioxopyrrol-1-yl)methyl]cyclohexanecarbonyl]oxy-2,5-dioxopyrrolidine-3-sulfonate Chemical compound [Na+].O=C1C(S(=O)(=O)[O-])CC(=O)N1OC(=O)C1CCC(CN2C(C=CC2=O)=O)CC1 VUFNRPJNRFOTGK-UHFFFAOYSA-M 0.000 description 1
- 239000002195 soluble material Substances 0.000 description 1
- 238000000638 solvent extraction Methods 0.000 description 1
- 229940100515 sorbitan Drugs 0.000 description 1
- 229950006451 sorbitan laurate Drugs 0.000 description 1
- 235000011067 sorbitan monolaureate Nutrition 0.000 description 1
- 238000001228 spectrum Methods 0.000 description 1
- 210000004989 spleen cell Anatomy 0.000 description 1
- 210000004988 splenocyte Anatomy 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 230000010473 stable expression Effects 0.000 description 1
- 238000011255 standard chemotherapy Methods 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 239000008117 stearic acid Substances 0.000 description 1
- 238000011146 sterile filtration Methods 0.000 description 1
- 239000008223 sterile water Substances 0.000 description 1
- 230000004936 stimulating effect Effects 0.000 description 1
- 229960001052 streptozocin Drugs 0.000 description 1
- ZSJLQEPLLKMAKR-GKHCUFPYSA-N streptozocin Chemical compound O=NN(C)C(=O)N[C@H]1[C@@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O ZSJLQEPLLKMAKR-GKHCUFPYSA-N 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 125000000472 sulfonyl group Chemical group *S(*)(=O)=O 0.000 description 1
- 239000011593 sulfur Substances 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 238000004114 suspension culture Methods 0.000 description 1
- 238000013268 sustained release Methods 0.000 description 1
- 239000012730 sustained-release form Substances 0.000 description 1
- 201000010965 sweat gland carcinoma Diseases 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 239000006188 syrup Substances 0.000 description 1
- 235000020357 syrup Nutrition 0.000 description 1
- 229940037128 systemic glucocorticoids Drugs 0.000 description 1
- 208000004441 taeniasis Diseases 0.000 description 1
- 101150047061 tag-72 gene Proteins 0.000 description 1
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 1
- 210000002435 tendon Anatomy 0.000 description 1
- NRUKOCRGYNPUPR-QBPJDGROSA-N teniposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@@H](OC[C@H]4O3)C=3SC=CC=3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 NRUKOCRGYNPUPR-QBPJDGROSA-N 0.000 description 1
- 229960001278 teniposide Drugs 0.000 description 1
- 230000002381 testicular Effects 0.000 description 1
- 229960002372 tetracaine Drugs 0.000 description 1
- GKCBAIGFKIBETG-UHFFFAOYSA-N tetracaine Chemical compound CCCCNC1=CC=C(C(=O)OCCN(C)C)C=C1 GKCBAIGFKIBETG-UHFFFAOYSA-N 0.000 description 1
- MHXBHWLGRWOABW-UHFFFAOYSA-N tetradecyl octadecanoate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCCCCCCCCCCCCCC MHXBHWLGRWOABW-UHFFFAOYSA-N 0.000 description 1
- OULAJFUGPPVRBK-UHFFFAOYSA-N tetratriacontyl alcohol Natural products CCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCO OULAJFUGPPVRBK-UHFFFAOYSA-N 0.000 description 1
- MPLHNVLQVRSVEE-UHFFFAOYSA-N texas red Chemical compound [O-]S(=O)(=O)C1=CC(S(Cl)(=O)=O)=CC=C1C(C1=CC=2CCCN3CCCC(C=23)=C1O1)=C2C1=C(CCC1)C3=[N+]1CCCC3=C2 MPLHNVLQVRSVEE-UHFFFAOYSA-N 0.000 description 1
- 230000004797 therapeutic response Effects 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- RTKIYNMVFMVABJ-UHFFFAOYSA-L thimerosal Chemical compound [Na+].CC[Hg]SC1=CC=CC=C1C([O-])=O RTKIYNMVFMVABJ-UHFFFAOYSA-L 0.000 description 1
- 229940033663 thimerosal Drugs 0.000 description 1
- 229940035024 thioglycerol Drugs 0.000 description 1
- 229960001196 thiotepa Drugs 0.000 description 1
- 229940104230 thymidine Drugs 0.000 description 1
- 229940113082 thymine Drugs 0.000 description 1
- 210000001541 thymus gland Anatomy 0.000 description 1
- 208000013077 thyroid gland carcinoma Diseases 0.000 description 1
- 208000013818 thyroid gland medullary carcinoma Diseases 0.000 description 1
- 208000030045 thyroid gland papillary carcinoma Diseases 0.000 description 1
- 201000005882 tinea unguium Diseases 0.000 description 1
- 229960003087 tioguanine Drugs 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- 229940044693 topoisomerase inhibitor Drugs 0.000 description 1
- 239000003053 toxin Substances 0.000 description 1
- 231100000765 toxin Toxicity 0.000 description 1
- 108700012359 toxins Proteins 0.000 description 1
- 235000013619 trace mineral Nutrition 0.000 description 1
- 239000011573 trace mineral Substances 0.000 description 1
- 230000005026 transcription initiation Effects 0.000 description 1
- 230000005030 transcription termination Effects 0.000 description 1
- 239000012096 transfection reagent Substances 0.000 description 1
- 239000012581 transferrin Substances 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 230000009261 transgenic effect Effects 0.000 description 1
- 206010044412 transitional cell carcinoma Diseases 0.000 description 1
- 238000013519 translation Methods 0.000 description 1
- 238000012384 transportation and delivery Methods 0.000 description 1
- 208000003982 trichinellosis Diseases 0.000 description 1
- 201000007588 trichinosis Diseases 0.000 description 1
- 229940117013 triethanolamine oleate Drugs 0.000 description 1
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 1
- 230000004565 tumor cell growth Effects 0.000 description 1
- 230000005748 tumor development Effects 0.000 description 1
- 102000003390 tumor necrosis factor Human genes 0.000 description 1
- 230000005760 tumorsuppression Effects 0.000 description 1
- 230000002100 tumorsuppressive effect Effects 0.000 description 1
- 229940121358 tyrosine kinase inhibitor Drugs 0.000 description 1
- 239000005483 tyrosine kinase inhibitor Substances 0.000 description 1
- 241001515965 unidentified phage Species 0.000 description 1
- 230000003827 upregulation Effects 0.000 description 1
- 210000003932 urinary bladder Anatomy 0.000 description 1
- 230000002485 urinary effect Effects 0.000 description 1
- VBEQCZHXXJYVRD-GACYYNSASA-N uroanthelone Chemical compound C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(C)C)[C@@H](C)O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H](CCSC)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)CNC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CS)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CS)NC(=O)CNC(=O)[C@H]1N(CCC1)C(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O)C(C)C)[C@@H](C)CC)C1=CC=C(O)C=C1 VBEQCZHXXJYVRD-GACYYNSASA-N 0.000 description 1
- 238000010200 validation analysis Methods 0.000 description 1
- 210000005166 vasculature Anatomy 0.000 description 1
- 229950000578 vatalanib Drugs 0.000 description 1
- YCOYDOIWSSHVCK-UHFFFAOYSA-N vatalanib Chemical compound C1=CC(Cl)=CC=C1NC(C1=CC=CC=C11)=NN=C1CC1=CC=NC=C1 YCOYDOIWSSHVCK-UHFFFAOYSA-N 0.000 description 1
- 235000013311 vegetables Nutrition 0.000 description 1
- 210000003501 vero cell Anatomy 0.000 description 1
- 231100000747 viability assay Toxicity 0.000 description 1
- 238000003026 viability measurement method Methods 0.000 description 1
- 230000008673 vomiting Effects 0.000 description 1
- 238000003260 vortexing Methods 0.000 description 1
- 238000010792 warming Methods 0.000 description 1
- 239000008215 water for injection Substances 0.000 description 1
- 238000009736 wetting Methods 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
- 229940045860 white wax Drugs 0.000 description 1
- 239000000811 xylitol Substances 0.000 description 1
- 235000010447 xylitol Nutrition 0.000 description 1
- HEBKCHPVOIAQTA-SCDXWVJYSA-N xylitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)CO HEBKCHPVOIAQTA-SCDXWVJYSA-N 0.000 description 1
- 229960002675 xylitol Drugs 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2827—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against B7 molecules, e.g. CD80, CD86
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/39533—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals
- A61K39/39558—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals against tumor tissues, cells, antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2863—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against receptors for growth factors, growth regulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
- A61K2039/507—Comprising a combination of two or more separate antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/24—Immunoglobulins specific features characterized by taxonomic origin containing regions, domains or residues from different species, e.g. chimeric, humanized or veneered
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/33—Crossreactivity, e.g. for species or epitope, or lack of said crossreactivity
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/34—Identification of a linear epitope shorter than 20 amino acid residues or of a conformational epitope defined by amino acid residues
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
- C07K2317/565—Complementarity determining region [CDR]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
- C07K2317/567—Framework region [FR]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/73—Inducing cell death, e.g. apoptosis, necrosis or inhibition of cell proliferation
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/76—Antagonist effect on antigen, e.g. neutralization or inhibition of binding
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/77—Internalization into the cell
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/92—Affinity (KD), association rate (Ka), dissociation rate (Kd) or EC50 value
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/94—Stability, e.g. half-life, pH, temperature or enzyme-resistance
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Immunology (AREA)
- Organic Chemistry (AREA)
- Medicinal Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Biochemistry (AREA)
- Genetics & Genomics (AREA)
- Molecular Biology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biophysics (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Engineering & Computer Science (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biomedical Technology (AREA)
- Oncology (AREA)
- Microbiology (AREA)
- Mycology (AREA)
- Epidemiology (AREA)
- Peptides Or Proteins (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN2016077082 | 2016-03-23 | ||
| CNPCT/CN2016/077082 | 2016-03-23 | ||
| CN201780018886.4A CN108779180B (zh) | 2016-03-23 | 2017-02-10 | 新型抗-pd-l1抗体 |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201780018886.4A Division CN108779180B (zh) | 2016-03-23 | 2017-02-10 | 新型抗-pd-l1抗体 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN111363041A CN111363041A (zh) | 2020-07-03 |
| CN111363041B true CN111363041B (zh) | 2022-02-22 |
Family
ID=59900827
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202010174431.5A Active CN111363041B (zh) | 2016-03-23 | 2017-02-10 | 新型抗-pd-l1抗体 |
| CN201780018886.4A Active CN108779180B (zh) | 2016-03-23 | 2017-02-10 | 新型抗-pd-l1抗体 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201780018886.4A Active CN108779180B (zh) | 2016-03-23 | 2017-02-10 | 新型抗-pd-l1抗体 |
Country Status (8)
| Country | Link |
|---|---|
| US (2) | US10822416B2 (enExample) |
| EP (1) | EP3433277A4 (enExample) |
| JP (3) | JP7080213B2 (enExample) |
| KR (1) | KR20180127971A (enExample) |
| CN (2) | CN111363041B (enExample) |
| CA (1) | CA3007135A1 (enExample) |
| RU (1) | RU2744959C2 (enExample) |
| WO (1) | WO2017161976A1 (enExample) |
Families Citing this family (38)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN106243225B (zh) * | 2015-06-11 | 2021-01-19 | 智翔(上海)医药科技有限公司 | 新型抗-pd-l1抗体 |
| RU2744959C2 (ru) * | 2016-03-23 | 2021-03-17 | Мэбспейс Байосайнсиз (Сучжоу) Ко., Лтд | Новые анти-pd-l1 антитела |
| US10894823B2 (en) | 2016-03-24 | 2021-01-19 | Gensun Biopharma Inc. | Trispecific inhibitors for cancer treatment |
| CN109071656B (zh) | 2017-01-05 | 2021-05-18 | 璟尚生物制药公司 | 检查点调节物拮抗剂 |
| JP7021454B2 (ja) * | 2017-02-03 | 2022-02-17 | 株式会社三洋物産 | 遊技機 |
| EP3630838A1 (en) | 2017-06-01 | 2020-04-08 | CytomX Therapeutics, Inc. | Activatable anti-pdl1 antibodies, and methods of use thereof |
| JP7402158B2 (ja) * | 2017-11-30 | 2023-12-20 | ジェネンテック, インコーポレイテッド | 抗pd-l1抗体及びpd-l1を検出するためにそれを使用する方法 |
| CN109970857B (zh) * | 2017-12-27 | 2022-09-30 | 信达生物制药(苏州)有限公司 | 抗pd-l1抗体及其用途 |
| CN111542543B (zh) | 2017-12-28 | 2023-12-22 | 南京传奇生物科技有限公司 | 针对pd-l1的抗体及其变体 |
| WO2019228514A1 (en) | 2018-06-01 | 2019-12-05 | Tayu Huaxia Biotech Medical Group Co., Ltd. | Compositions and uses thereof for treating disease or condition |
| WO2019227490A1 (en) | 2018-06-01 | 2019-12-05 | Tayu Huaxia Biotech Medical Group Co., Ltd. | Compositions and methods for imaging |
| WO2020006516A1 (en) | 2018-06-29 | 2020-01-02 | Gensun Biopharma, Inc. | Antitumor immune checkpoint regulator antagonists |
| CN112996910A (zh) * | 2018-07-11 | 2021-06-18 | 动量制药公司 | 与靶向PD-L1的工程化Fc-抗原结合结构域构建体有关的组合物和方法 |
| WO2020151572A1 (en) * | 2019-01-23 | 2020-07-30 | Tayu Huaxia Biotech Medical Group Co., Ltd. | Anti-pd-l1 diabodies and the use thereof |
| US12421313B2 (en) | 2019-01-25 | 2025-09-23 | Chia Tia Tianqing Pharmaceutical Group Co., Ltd. | Combined pharmaceutical composition for treating tumor |
| CN109929037B (zh) * | 2019-04-01 | 2023-03-17 | 华博生物医药技术(上海)有限公司 | 针对程序性死亡配体的结合物及其应用 |
| CA3141531A1 (en) | 2019-05-24 | 2020-12-03 | Pfizer Inc. | Combination therapies using cdk inhibitors |
| JP2022534982A (ja) * | 2019-05-30 | 2022-08-04 | ブリストル-マイヤーズ スクイブ カンパニー | 細胞局在化シグネチャーおよびその使用 |
| CN110194798A (zh) * | 2019-05-31 | 2019-09-03 | 杭州科兴生物科技有限公司 | 抗pd-l1单克隆抗体、片段及其医药用途 |
| WO2020263312A1 (en) | 2019-06-28 | 2020-12-30 | Gensun Biopharma, Inc. | ANTITUMOR ANTAGONIST CONSISTING OF A MUTATED TGFβ1 - RII EXTRACELLULAR DOMAIN AND AN IMMUNOGLOBULIN SCAFFOLD |
| US20220356255A1 (en) * | 2019-07-15 | 2022-11-10 | Capella Bioscience Ltd | Anti-pd-l1 antibodies |
| US20230002492A1 (en) * | 2019-11-08 | 2023-01-05 | Simcere (Shanghai) Pharmaceutical Co., Ltd. | Anti-human programmed cell death ligand-1 (pd-l1) antibody and use thereof |
| WO2021197358A1 (zh) * | 2020-03-31 | 2021-10-07 | 普米斯生物技术(珠海)有限公司 | 一种抗pd-l1和pd-l2抗体及其衍生物和用途 |
| CN119462927B (zh) * | 2020-07-28 | 2025-07-29 | 乐普生物科技股份有限公司 | 靶向PD-L1和TGF-β的双功能分子 |
| CA3187850A1 (en) * | 2020-08-04 | 2022-02-10 | Bryan Glaser | Pd-l1 binding agents and uses thereof |
| CN111929447B (zh) * | 2020-09-24 | 2021-02-26 | 菁良基因科技(深圳)有限公司 | 一种pd-l1免疫组织化学参考品及其制备方法和应用 |
| CA3201518A1 (en) * | 2020-11-18 | 2022-05-27 | Suzhou Transcenta Therapeutics Co., Ltd. | Bi-functional molecules |
| CN112285366B (zh) * | 2020-12-25 | 2021-06-08 | 南京广祺医药科技有限公司 | 一种测定血清中双特异性抗体BsAb的ELISA方法及应用 |
| US20240352084A1 (en) | 2021-07-22 | 2024-10-24 | University Of Dundee | Therapeutic muteins |
| CN115521378B (zh) * | 2021-07-23 | 2023-12-22 | 南京吉盛澳玛生物医药有限公司 | Pd-l1抗体及其用途 |
| CN118215681A (zh) * | 2021-09-18 | 2024-06-18 | 江苏康宁杰瑞生物制药有限公司 | 包含pd-l1抗原结合片段的组合物及其用途 |
| WO2023057882A1 (en) | 2021-10-05 | 2023-04-13 | Pfizer Inc. | Combinations of azalactam compounds with a pd-1 axis binding antagonist for the treatment of cancer |
| WO2023079428A1 (en) | 2021-11-03 | 2023-05-11 | Pfizer Inc. | Combination therapies using tlr7/8 agonist |
| TW202333785A (zh) * | 2021-11-16 | 2023-09-01 | 中國大陸商蘇州創勝醫藥集團有限公司 | Claudin18.2拮抗劑和pd-1/pd-l1軸抑制劑的組合療法 |
| CN119213034A (zh) * | 2022-05-17 | 2024-12-27 | 苏州创胜医药集团有限公司 | 双功能蛋白质及其制剂和用途 |
| CN114939161B (zh) * | 2022-06-24 | 2023-02-28 | 南方医科大学南方医院 | Pd-1抗体在制备治疗肌腱损伤的药物中的应用 |
| CN120603849A (zh) * | 2023-01-18 | 2025-09-05 | 科望(苏州)生物医药科技有限公司 | 抗pdl1单域抗体、融合蛋白及其用途 |
| AR132623A1 (es) * | 2023-05-08 | 2025-07-16 | Hoffmann La Roche | PROTEÍNAS DE FUSIÓN DE INTERFERÓN a DIRIGIDAS Y MÉTODOS DE USO |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2013055404A1 (en) * | 2011-10-10 | 2013-04-18 | City Of Hope | Meditopes and meditope-binding antibodies and uses thereof |
| WO2013079174A1 (en) * | 2011-11-28 | 2013-06-06 | Merck Patent Gmbh | Anti-pd-l1 antibodies and uses thereof |
| WO2013173223A1 (en) * | 2012-05-15 | 2013-11-21 | Bristol-Myers Squibb Company | Cancer immunotherapy by disrupting pd-1/pd-l1 signaling |
| WO2014055897A2 (en) * | 2012-10-04 | 2014-04-10 | Dana-Farber Cancer Institute, Inc. | Human monoclonal anti-pd-l1 antibodies and methods of use |
| WO2014151006A2 (en) * | 2013-03-15 | 2014-09-25 | Genentech, Inc. | Biomarkers and methods of treating pd-1 and pd-l1 related conditions |
| WO2014165082A3 (en) * | 2013-03-13 | 2015-03-19 | Medimmune, Llc | Antibodies and methods of detection |
Family Cites Families (39)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| USRE30985E (en) | 1978-01-01 | 1982-06-29 | Serum-free cell culture media | |
| US4657760A (en) | 1979-03-20 | 1987-04-14 | Ortho Pharmaceutical Corporation | Methods and compositions using monoclonal antibody to human T cells |
| US4560655A (en) | 1982-12-16 | 1985-12-24 | Immunex Corporation | Serum-free cell culture medium and process for making same |
| US4657866A (en) | 1982-12-21 | 1987-04-14 | Sudhir Kumar | Serum-free, synthetic, completely chemically defined tissue culture media |
| GB8308235D0 (en) | 1983-03-25 | 1983-05-05 | Celltech Ltd | Polypeptides |
| US4816567A (en) | 1983-04-08 | 1989-03-28 | Genentech, Inc. | Recombinant immunoglobin preparations |
| US4767704A (en) | 1983-10-07 | 1988-08-30 | Columbia University In The City Of New York | Protein-free culture medium |
| US5807715A (en) | 1984-08-27 | 1998-09-15 | The Board Of Trustees Of The Leland Stanford Junior University | Methods and transformed mammalian lymphocyte cells for producing functional antigen-binding protein including chimeric immunoglobulin |
| US4879231A (en) | 1984-10-30 | 1989-11-07 | Phillips Petroleum Company | Transformation of yeasts of the genus pichia |
| US5206344A (en) | 1985-06-26 | 1993-04-27 | Cetus Oncology Corporation | Interleukin-2 muteins and polymer conjugation thereof |
| GB8516415D0 (en) | 1985-06-28 | 1985-07-31 | Celltech Ltd | Culture of animal cells |
| GB8607679D0 (en) | 1986-03-27 | 1986-04-30 | Winter G P | Recombinant dna product |
| US5225539A (en) | 1986-03-27 | 1993-07-06 | Medical Research Council | Recombinant altered antibodies and methods of making altered antibodies |
| US4927762A (en) | 1986-04-01 | 1990-05-22 | Cell Enterprises, Inc. | Cell culture medium with antioxidant |
| GB8610600D0 (en) | 1986-04-30 | 1986-06-04 | Novo Industri As | Transformation of trichoderma |
| EP0435911B1 (en) | 1988-09-23 | 1996-03-13 | Cetus Oncology Corporation | Cell culture medium for enhanced cell growth, culture longevity and product expression |
| US5530101A (en) | 1988-12-28 | 1996-06-25 | Protein Design Labs, Inc. | Humanized immunoglobulins |
| IL162181A (en) | 1988-12-28 | 2006-04-10 | Pdl Biopharma Inc | A method of producing humanized immunoglubulin, and polynucleotides encoding the same |
| EP0402226A1 (en) | 1989-06-06 | 1990-12-12 | Institut National De La Recherche Agronomique | Transformation vectors for yeast yarrowia |
| DE3920358A1 (de) | 1989-06-22 | 1991-01-17 | Behringwerke Ag | Bispezifische und oligospezifische, mono- und oligovalente antikoerperkonstrukte, ihre herstellung und verwendung |
| US5225212A (en) | 1989-10-20 | 1993-07-06 | Liposome Technology, Inc. | Microreservoir liposome composition and method |
| US5859205A (en) | 1989-12-21 | 1999-01-12 | Celltech Limited | Humanised antibodies |
| US5122469A (en) | 1990-10-03 | 1992-06-16 | Genentech, Inc. | Method for culturing Chinese hamster ovary cells to improve production of recombinant proteins |
| EP0590058B1 (en) | 1991-06-14 | 2003-11-26 | Genentech, Inc. | HUMANIZED Heregulin ANTIBODy |
| AU3178993A (en) | 1991-11-25 | 1993-06-28 | Enzon, Inc. | Multivalent antigen-binding proteins |
| EP0656946B2 (en) | 1992-08-21 | 2010-03-31 | Vrije Universiteit Brussel | Immunoglobulins devoid of light chains |
| US6005079A (en) | 1992-08-21 | 1999-12-21 | Vrije Universiteit Brussels | Immunoglobulins devoid of light chains |
| DE69427974T2 (de) | 1993-04-29 | 2001-12-06 | Unilever N.V., Rotterdam | Herstellung von antikörpern oder funktionstüchtig gemachten teilen davon, abgeleitet von schweren ketten von immunglobulinen von camelidae |
| EP0770628B9 (en) | 1994-07-13 | 2007-02-28 | Chugai Seiyaku Kabushiki Kaisha | Reconstituted human antibody against human interleukin-8 |
| WO2004006955A1 (en) | 2001-07-12 | 2004-01-22 | Jefferson Foote | Super humanized antibodies |
| KR101411165B1 (ko) * | 2005-07-01 | 2014-06-25 | 메다렉스, 엘.엘.시. | 예정 사멸 리간드 1 (피디-엘1)에 대한 인간 모노클로날항체 |
| US8217849B2 (en) | 2008-04-07 | 2012-07-10 | Intelleflex Corporation | Small profile antenna and RFID device having same |
| WO2010077634A1 (en) * | 2008-12-09 | 2010-07-08 | Genentech, Inc. | Anti-pd-l1 antibodies and their use to enhance t-cell function |
| US8741295B2 (en) * | 2009-02-09 | 2014-06-03 | Universite De La Mediterranee | PD-1 antibodies and PD-L1 antibodies and uses thereof |
| RU2416645C2 (ru) * | 2009-05-04 | 2011-04-20 | Общество с ограниченной ответственностью "Промоген-МАТ" | Одноцепочечное антитело, связывающее фактор некроза опухоли альфа, днк, плазмидная днк и способ получения одноцепочечного антитела |
| NZ599405A (en) | 2009-11-24 | 2014-09-26 | Medimmune Ltd | Targeted binding agents against b7-h1 |
| CN104736168B (zh) * | 2012-05-31 | 2018-09-21 | 索伦托治疗有限公司 | 与pd-l1结合的抗原结合蛋白 |
| AR093984A1 (es) * | 2012-12-21 | 2015-07-01 | Merck Sharp & Dohme | Anticuerpos que se unen a ligando 1 de muerte programada (pd-l1) humano |
| RU2744959C2 (ru) * | 2016-03-23 | 2021-03-17 | Мэбспейс Байосайнсиз (Сучжоу) Ко., Лтд | Новые анти-pd-l1 антитела |
-
2017
- 2017-02-10 RU RU2018124602A patent/RU2744959C2/ru active
- 2017-02-10 US US16/086,013 patent/US10822416B2/en active Active
- 2017-02-10 CA CA3007135A patent/CA3007135A1/en active Pending
- 2017-02-10 CN CN202010174431.5A patent/CN111363041B/zh active Active
- 2017-02-10 WO PCT/CN2017/073242 patent/WO2017161976A1/en not_active Ceased
- 2017-02-10 EP EP17769253.0A patent/EP3433277A4/en active Pending
- 2017-02-10 JP JP2019500712A patent/JP7080213B2/ja active Active
- 2017-02-10 CN CN201780018886.4A patent/CN108779180B/zh active Active
- 2017-02-10 KR KR1020187024767A patent/KR20180127971A/ko active Pending
-
2020
- 2020-09-28 US US17/033,970 patent/US11753473B2/en active Active
-
2022
- 2022-01-21 JP JP2022008113A patent/JP7345578B2/ja active Active
-
2023
- 2023-09-05 JP JP2023143484A patent/JP7624484B2/ja active Active
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2013055404A1 (en) * | 2011-10-10 | 2013-04-18 | City Of Hope | Meditopes and meditope-binding antibodies and uses thereof |
| WO2013079174A1 (en) * | 2011-11-28 | 2013-06-06 | Merck Patent Gmbh | Anti-pd-l1 antibodies and uses thereof |
| CN103987405A (zh) * | 2011-11-28 | 2014-08-13 | 默克专利股份公司 | 抗pd-l1抗体及其用途 |
| WO2013173223A1 (en) * | 2012-05-15 | 2013-11-21 | Bristol-Myers Squibb Company | Cancer immunotherapy by disrupting pd-1/pd-l1 signaling |
| WO2014055897A2 (en) * | 2012-10-04 | 2014-04-10 | Dana-Farber Cancer Institute, Inc. | Human monoclonal anti-pd-l1 antibodies and methods of use |
| WO2014165082A3 (en) * | 2013-03-13 | 2015-03-19 | Medimmune, Llc | Antibodies and methods of detection |
| WO2014151006A2 (en) * | 2013-03-15 | 2014-09-25 | Genentech, Inc. | Biomarkers and methods of treating pd-1 and pd-l1 related conditions |
Non-Patent Citations (2)
| Title |
|---|
| Antibody-Dependent Cellular Cytotoxicity Activity of a Novel Anti-PD-L1 Antibody Avelumab (MSB0010718C) on Human Tumor Cells;Benjamin Boyerinas;《Cancer Immunol Res.》;20151031;全文 * |
| Preclinical pharmacokinetics, pharmacodynamics, tissue distribution, and tumor penetration of anti-PD-L1 monoclonal antibody, an immune checkpoint inhibitor;Rong Deng等;《MAbs》;20160226;第8卷(第3期);全文 * |
Also Published As
| Publication number | Publication date |
|---|---|
| RU2744959C2 (ru) | 2021-03-17 |
| KR20180127971A (ko) | 2018-11-30 |
| WO2017161976A1 (en) | 2017-09-28 |
| JP2022068161A (ja) | 2022-05-09 |
| CN108779180B (zh) | 2020-10-16 |
| JP7080213B2 (ja) | 2022-06-03 |
| JP2023171733A (ja) | 2023-12-05 |
| CA3007135A1 (en) | 2017-09-28 |
| EP3433277A4 (en) | 2020-06-17 |
| JP7345578B2 (ja) | 2023-09-15 |
| US10822416B2 (en) | 2020-11-03 |
| US20190330348A1 (en) | 2019-10-31 |
| RU2018124602A (ru) | 2020-01-14 |
| RU2021106377A (ru) | 2021-03-31 |
| CN108779180A (zh) | 2018-11-09 |
| RU2018124602A3 (enExample) | 2020-03-06 |
| US20210009693A1 (en) | 2021-01-14 |
| RU2021106377A3 (enExample) | 2021-12-28 |
| JP7624484B2 (ja) | 2025-01-30 |
| EP3433277A1 (en) | 2019-01-30 |
| CN111363041A (zh) | 2020-07-03 |
| JP2019516394A (ja) | 2019-06-20 |
| US11753473B2 (en) | 2023-09-12 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN111363041B (zh) | 新型抗-pd-l1抗体 | |
| JP7520072B2 (ja) | Pd-l1特異的抗体およびそれを使用する方法 | |
| JP6976322B2 (ja) | 新規抗ctla4抗体 | |
| CN113563470B (zh) | 结合tigit抗原的抗体及其制备方法与应用 | |
| TW201837174A (zh) | 抗gprc5d抗體及包含該抗體之分子 | |
| CN112867735B (zh) | 双特异性抗原结合蛋白及其用途 | |
| AU2017379382A1 (en) | Anti-CD3 Antibody and Molecules Comprising the Antibody | |
| JP2021524251A (ja) | Cd3に特異的な抗体及びその使用 | |
| KR20230132544A (ko) | 신규한 항-그렘린1 항체 | |
| CN113045661B (zh) | 新型抗cd4抗体 | |
| KR20220042258A (ko) | 항 tigit 항체 및 그의 응용 | |
| JP7754506B2 (ja) | ポリオウイルス受容体(pvr)に対する抗体およびその使用 | |
| TW201916890A (zh) | 抗pd-1抗體和抗lag-3抗體聯合在製備治療腫瘤的藥物中的用途 | |
| CN114106182A (zh) | 抗tigit的抗体及其用途 | |
| HK40032298B (en) | Novel anti-pd-l1 antibodies | |
| HK40032298A (en) | Novel anti-pd-l1 antibodies | |
| RU2776121C2 (ru) | Новые анти-pd-l1 антитела | |
| CN113368232B (zh) | 多特异性抗原结合蛋白及其应用 | |
| HK1260737B (en) | Novel anti-pd-l1 antibodies | |
| HK1260737A1 (en) | Novel anti-pd-l1 antibodies | |
| WO2025172588A1 (en) | Bispecific constructs directed against fap and ltbr | |
| WO2025172587A1 (en) | Binding constructs | |
| HK40055449A (en) | Novel anti-cd4 antibodies | |
| CN118302200A (zh) | 用于治疗癌症的b7-h4抗体-药物缀合物 | |
| HK40055449B (en) | Novel anti-cd4 antibodies |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| CB02 | Change of applicant information | ||
| CB02 | Change of applicant information |
Address after: Room 501, building B6, biological nano Park, 218 Xinghu street, Wuzhong District, Suzhou City, Jiangsu Province Applicant after: MABSPACE BIOSCIENCES (SUZHOU) Co.,Ltd. Address before: Room 609, building B2, biological nano Park, 218 Xinghu street, Wuzhong District, Suzhou City, Jiangsu Province Applicant before: MABSPACE BIOSCIENCES (SUZHOU) Co.,Ltd. |
|
| REG | Reference to a national code |
Ref country code: HK Ref legal event code: DE Ref document number: 40032298 Country of ref document: HK |
|
| CB02 | Change of applicant information | ||
| CB02 | Change of applicant information |
Address after: Room 609, building B2, biological nano Park, 218 Xinghu street, Wuzhong District, Suzhou City, Jiangsu Province Applicant after: Suzhou ChuangSheng Pharmaceutical Group Co.,Ltd. Address before: Room 501, building B6, biological nano Park, 218 Xinghu street, Wuzhong District, Suzhou City, Jiangsu Province Applicant before: MABSPACE BIOSCIENCES (SUZHOU) Co.,Ltd. |
|
| GR01 | Patent grant | ||
| GR01 | Patent grant |