WO2022209158A1 - 電解液およびリチウムイオン二次電池 - Google Patents
電解液およびリチウムイオン二次電池 Download PDFInfo
- Publication number
- WO2022209158A1 WO2022209158A1 PCT/JP2022/001511 JP2022001511W WO2022209158A1 WO 2022209158 A1 WO2022209158 A1 WO 2022209158A1 JP 2022001511 W JP2022001511 W JP 2022001511W WO 2022209158 A1 WO2022209158 A1 WO 2022209158A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- ion secondary
- active material
- secondary battery
- lithium ion
- electrode active
- Prior art date
Links
- 239000008151 electrolyte solution Substances 0.000 title claims abstract description 172
- HBBGRARXTFLTSG-UHFFFAOYSA-N Lithium ion Chemical compound [Li+] HBBGRARXTFLTSG-UHFFFAOYSA-N 0.000 title claims description 250
- 229910001416 lithium ion Inorganic materials 0.000 title claims description 250
- 159000000002 lithium salts Chemical class 0.000 claims abstract description 75
- 229910003002 lithium salt Inorganic materials 0.000 claims abstract description 73
- 229940017219 methyl propionate Drugs 0.000 claims abstract description 69
- RJUFJBKOKNCXHH-UHFFFAOYSA-N Methyl propionate Chemical compound CCC(=O)OC RJUFJBKOKNCXHH-UHFFFAOYSA-N 0.000 claims abstract description 68
- -1 alkylene cyclic carbonate Chemical class 0.000 claims abstract description 52
- 239000003792 electrolyte Substances 0.000 claims abstract description 38
- 229910001290 LiPF6 Inorganic materials 0.000 claims abstract description 7
- 239000007774 positive electrode material Substances 0.000 claims description 90
- 239000007773 negative electrode material Substances 0.000 claims description 77
- 229910013870 LiPF 6 Inorganic materials 0.000 claims description 58
- 238000007789 sealing Methods 0.000 claims description 55
- 239000003125 aqueous solvent Substances 0.000 claims description 33
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims description 29
- 239000010439 graphite Substances 0.000 claims description 15
- 229910002804 graphite Inorganic materials 0.000 claims description 15
- 239000002904 solvent Substances 0.000 abstract description 22
- 239000010410 layer Substances 0.000 description 134
- 230000000052 comparative effect Effects 0.000 description 101
- 238000012360 testing method Methods 0.000 description 50
- 239000012452 mother liquor Substances 0.000 description 41
- KMTRUDSVKNLOMY-UHFFFAOYSA-N Ethylene carbonate Chemical compound O=C1OCCO1 KMTRUDSVKNLOMY-UHFFFAOYSA-N 0.000 description 39
- 239000012046 mixed solvent Substances 0.000 description 33
- 239000011888 foil Substances 0.000 description 31
- 229910052782 aluminium Inorganic materials 0.000 description 30
- 238000003860 storage Methods 0.000 description 30
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 28
- 210000000352 storage cell Anatomy 0.000 description 27
- 239000011230 binding agent Substances 0.000 description 24
- 238000011156 evaluation Methods 0.000 description 22
- 229940021013 electrolyte solution Drugs 0.000 description 21
- 239000000203 mixture Substances 0.000 description 21
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 19
- 239000011889 copper foil Substances 0.000 description 17
- VAYTZRYEBVHVLE-UHFFFAOYSA-N 1,3-dioxol-2-one Chemical compound O=C1OC=CO1 VAYTZRYEBVHVLE-UHFFFAOYSA-N 0.000 description 16
- 230000014759 maintenance of location Effects 0.000 description 14
- 238000000034 method Methods 0.000 description 14
- 239000011149 active material Substances 0.000 description 12
- 229920005989 resin Polymers 0.000 description 12
- 239000011347 resin Substances 0.000 description 12
- 239000000654 additive Substances 0.000 description 11
- 239000000126 substance Substances 0.000 description 11
- SECXISVLQFMRJM-UHFFFAOYSA-N N-Methylpyrrolidone Chemical compound CN1CCCC1=O SECXISVLQFMRJM-UHFFFAOYSA-N 0.000 description 10
- 239000010450 olivine Substances 0.000 description 10
- 229910052609 olivine Inorganic materials 0.000 description 10
- 125000001424 substituent group Chemical group 0.000 description 10
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 9
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 9
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 9
- 239000001768 carboxy methyl cellulose Substances 0.000 description 9
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 9
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 9
- 239000002002 slurry Substances 0.000 description 9
- 229920003048 styrene butadiene rubber Polymers 0.000 description 9
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 9
- 229910052799 carbon Inorganic materials 0.000 description 8
- 150000002148 esters Chemical class 0.000 description 8
- 239000007789 gas Substances 0.000 description 8
- 230000006872 improvement Effects 0.000 description 8
- 239000000463 material Substances 0.000 description 8
- 238000002844 melting Methods 0.000 description 8
- 230000008018 melting Effects 0.000 description 8
- 229910052751 metal Inorganic materials 0.000 description 8
- 239000002243 precursor Substances 0.000 description 8
- 238000003825 pressing Methods 0.000 description 8
- 125000005309 thioalkoxy group Chemical group 0.000 description 8
- KRHYYFGTRYWZRS-UHFFFAOYSA-N Fluorane Chemical compound F KRHYYFGTRYWZRS-UHFFFAOYSA-N 0.000 description 7
- 229910010707 LiFePO 4 Inorganic materials 0.000 description 7
- 239000004743 Polypropylene Substances 0.000 description 7
- 125000000217 alkyl group Chemical group 0.000 description 7
- 238000000576 coating method Methods 0.000 description 7
- 238000007599 discharging Methods 0.000 description 7
- 229910052736 halogen Inorganic materials 0.000 description 7
- 150000002367 halogens Chemical class 0.000 description 7
- 229910000040 hydrogen fluoride Inorganic materials 0.000 description 7
- 229910052742 iron Inorganic materials 0.000 description 7
- 230000007774 longterm Effects 0.000 description 7
- 125000003545 alkoxy group Chemical group 0.000 description 6
- 238000009835 boiling Methods 0.000 description 6
- 239000011248 coating agent Substances 0.000 description 6
- 125000001651 cyanato group Chemical group [*]OC#N 0.000 description 6
- 150000005676 cyclic carbonates Chemical class 0.000 description 6
- 125000000753 cycloalkyl group Chemical group 0.000 description 6
- 238000009792 diffusion process Methods 0.000 description 6
- IEJIGPNLZYLLBP-UHFFFAOYSA-N dimethyl carbonate Chemical compound COC(=O)OC IEJIGPNLZYLLBP-UHFFFAOYSA-N 0.000 description 6
- 230000000694 effects Effects 0.000 description 6
- JBTWLSYIZRCDFO-UHFFFAOYSA-N ethyl methyl carbonate Chemical compound CCOC(=O)OC JBTWLSYIZRCDFO-UHFFFAOYSA-N 0.000 description 6
- 229910052731 fluorine Inorganic materials 0.000 description 6
- 229910052748 manganese Inorganic materials 0.000 description 6
- 238000004519 manufacturing process Methods 0.000 description 6
- 239000002184 metal Substances 0.000 description 6
- 229910052759 nickel Inorganic materials 0.000 description 6
- 229920001155 polypropylene Polymers 0.000 description 6
- 229910052710 silicon Inorganic materials 0.000 description 6
- JGFBQFKZKSSODQ-UHFFFAOYSA-N Isothiocyanatocyclopropane Chemical compound S=C=NC1CC1 JGFBQFKZKSSODQ-UHFFFAOYSA-N 0.000 description 5
- 239000002033 PVDF binder Substances 0.000 description 5
- 239000006230 acetylene black Substances 0.000 description 5
- 125000003118 aryl group Chemical group 0.000 description 5
- WVQUCYVTZWVNLV-UHFFFAOYSA-N boric acid;oxalic acid Chemical class OB(O)O.OC(=O)C(O)=O WVQUCYVTZWVNLV-UHFFFAOYSA-N 0.000 description 5
- PWLNAUNEAKQYLH-UHFFFAOYSA-N butyric acid octyl ester Natural products CCCCCCCCOC(=O)CCC PWLNAUNEAKQYLH-UHFFFAOYSA-N 0.000 description 5
- 230000008859 change Effects 0.000 description 5
- 150000001875 compounds Chemical class 0.000 description 5
- 229910052802 copper Inorganic materials 0.000 description 5
- 239000010949 copper Substances 0.000 description 5
- 238000000354 decomposition reaction Methods 0.000 description 5
- 125000000623 heterocyclic group Chemical group 0.000 description 5
- 125000004435 hydrogen atom Chemical class [H]* 0.000 description 5
- 150000002500 ions Chemical class 0.000 description 5
- UUIQMZJEGPQKFD-UHFFFAOYSA-N n-butyric acid methyl ester Natural products CCCC(=O)OC UUIQMZJEGPQKFD-UHFFFAOYSA-N 0.000 description 5
- 229920002981 polyvinylidene fluoride Polymers 0.000 description 5
- 125000000547 substituted alkyl group Chemical group 0.000 description 5
- YCKRFDGAMUMZLT-UHFFFAOYSA-N Fluorine atom Chemical compound [F] YCKRFDGAMUMZLT-UHFFFAOYSA-N 0.000 description 4
- 239000004698 Polyethylene Substances 0.000 description 4
- 230000000996 additive effect Effects 0.000 description 4
- 150000001336 alkenes Chemical class 0.000 description 4
- 210000004027 cell Anatomy 0.000 description 4
- 230000006866 deterioration Effects 0.000 description 4
- 239000002270 dispersing agent Substances 0.000 description 4
- 239000011737 fluorine Substances 0.000 description 4
- 229910052739 hydrogen Inorganic materials 0.000 description 4
- 239000001257 hydrogen Substances 0.000 description 4
- 239000011254 layer-forming composition Substances 0.000 description 4
- 229910052749 magnesium Inorganic materials 0.000 description 4
- 239000011777 magnesium Substances 0.000 description 4
- 238000005259 measurement Methods 0.000 description 4
- 229910044991 metal oxide Inorganic materials 0.000 description 4
- 150000004706 metal oxides Chemical class 0.000 description 4
- 238000002156 mixing Methods 0.000 description 4
- 229920000573 polyethylene Polymers 0.000 description 4
- 230000002829 reductive effect Effects 0.000 description 4
- 125000005415 substituted alkoxy group Chemical group 0.000 description 4
- 125000005346 substituted cycloalkyl group Chemical group 0.000 description 4
- 229910052720 vanadium Inorganic materials 0.000 description 4
- 229910052725 zinc Inorganic materials 0.000 description 4
- 239000011701 zinc Substances 0.000 description 4
- HNAGHMKIPMKKBB-UHFFFAOYSA-N 1-benzylpyrrolidine-3-carboxamide Chemical compound C1C(C(=O)N)CCN1CC1=CC=CC=C1 HNAGHMKIPMKKBB-UHFFFAOYSA-N 0.000 description 3
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 3
- 229910010375 LiaMbPO4 Inorganic materials 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 3
- 229910001128 Sn alloy Inorganic materials 0.000 description 3
- KXKVLQRXCPHEJC-UHFFFAOYSA-N acetic acid trimethyl ester Natural products COC(C)=O KXKVLQRXCPHEJC-UHFFFAOYSA-N 0.000 description 3
- 230000004913 activation Effects 0.000 description 3
- OBNCKNCVKJNDBV-UHFFFAOYSA-N butanoic acid ethyl ester Natural products CCCC(=O)OCC OBNCKNCVKJNDBV-UHFFFAOYSA-N 0.000 description 3
- 229910052791 calcium Inorganic materials 0.000 description 3
- 239000011575 calcium Substances 0.000 description 3
- 230000007423 decrease Effects 0.000 description 3
- 238000010586 diagram Methods 0.000 description 3
- 239000007772 electrode material Substances 0.000 description 3
- 229910052738 indium Inorganic materials 0.000 description 3
- 239000005001 laminate film Substances 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 229910052744 lithium Inorganic materials 0.000 description 3
- 229910052750 molybdenum Inorganic materials 0.000 description 3
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 3
- 238000010248 power generation Methods 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- 239000003566 sealing material Substances 0.000 description 3
- 229910052718 tin Inorganic materials 0.000 description 3
- 239000011135 tin Substances 0.000 description 3
- UHOPWFKONJYLCF-UHFFFAOYSA-N 2-(2-sulfanylethyl)isoindole-1,3-dione Chemical compound C1=CC=C2C(=O)N(CCS)C(=O)C2=C1 UHOPWFKONJYLCF-UHFFFAOYSA-N 0.000 description 2
- BTBUEUYNUDRHOZ-UHFFFAOYSA-N Borate Chemical compound [O-]B([O-])[O-] BTBUEUYNUDRHOZ-UHFFFAOYSA-N 0.000 description 2
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 2
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 2
- 229910013275 LiMPO Inorganic materials 0.000 description 2
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 2
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 2
- 239000004642 Polyimide Substances 0.000 description 2
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 2
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 description 2
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 2
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 2
- 239000000853 adhesive Substances 0.000 description 2
- 230000001070 adhesive effect Effects 0.000 description 2
- 229910045601 alloy Inorganic materials 0.000 description 2
- 239000000956 alloy Substances 0.000 description 2
- 229910052787 antimony Inorganic materials 0.000 description 2
- 229920003235 aromatic polyamide Polymers 0.000 description 2
- 229910052788 barium Inorganic materials 0.000 description 2
- 229910052796 boron Inorganic materials 0.000 description 2
- 229910052794 bromium Inorganic materials 0.000 description 2
- 125000004432 carbon atom Chemical group C* 0.000 description 2
- 239000006229 carbon black Substances 0.000 description 2
- 239000003575 carbonaceous material Substances 0.000 description 2
- SYLNJGIBLUVXCG-UHFFFAOYSA-N carbonic acid;prop-1-yne Chemical compound CC#C.OC(O)=O SYLNJGIBLUVXCG-UHFFFAOYSA-N 0.000 description 2
- 150000005678 chain carbonates Chemical class 0.000 description 2
- 239000002800 charge carrier Substances 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- 229910052801 chlorine Inorganic materials 0.000 description 2
- 229910052804 chromium Inorganic materials 0.000 description 2
- 239000011651 chromium Substances 0.000 description 2
- 239000002131 composite material Substances 0.000 description 2
- 238000012790 confirmation Methods 0.000 description 2
- QXYJCZRRLLQGCR-UHFFFAOYSA-N dioxomolybdenum Chemical compound O=[Mo]=O QXYJCZRRLLQGCR-UHFFFAOYSA-N 0.000 description 2
- FKRCODPIKNYEAC-UHFFFAOYSA-N ethyl propionate Chemical compound CCOC(=O)CC FKRCODPIKNYEAC-UHFFFAOYSA-N 0.000 description 2
- YVIVRJLWYJGJTJ-UHFFFAOYSA-N gamma-Valerolactam Chemical compound CC1CCC(=O)N1 YVIVRJLWYJGJTJ-UHFFFAOYSA-N 0.000 description 2
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 2
- 229910052737 gold Inorganic materials 0.000 description 2
- 239000010931 gold Substances 0.000 description 2
- 239000008187 granular material Substances 0.000 description 2
- APFVFJFRJDLVQX-UHFFFAOYSA-N indium atom Chemical compound [In] APFVFJFRJDLVQX-UHFFFAOYSA-N 0.000 description 2
- 229910052740 iodine Inorganic materials 0.000 description 2
- 239000012528 membrane Substances 0.000 description 2
- ZKATWMILCYLAPD-UHFFFAOYSA-N niobium pentoxide Chemical compound O=[Nb](=O)O[Nb](=O)=O ZKATWMILCYLAPD-UHFFFAOYSA-N 0.000 description 2
- 239000011255 nonaqueous electrolyte Substances 0.000 description 2
- JRZJOMJEPLMPRA-UHFFFAOYSA-N olefin Natural products CCCCCCCC=C JRZJOMJEPLMPRA-UHFFFAOYSA-N 0.000 description 2
- 229910052760 oxygen Inorganic materials 0.000 description 2
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 2
- 229920001721 polyimide Polymers 0.000 description 2
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 2
- 239000004810 polytetrafluoroethylene Substances 0.000 description 2
- RUOJZAUFBMNUDX-UHFFFAOYSA-N propylene carbonate Chemical compound CC1COC(=O)O1 RUOJZAUFBMNUDX-UHFFFAOYSA-N 0.000 description 2
- 230000000452 restraining effect Effects 0.000 description 2
- 239000010703 silicon Substances 0.000 description 2
- 229910052709 silver Inorganic materials 0.000 description 2
- 239000004332 silver Substances 0.000 description 2
- 125000006850 spacer group Chemical group 0.000 description 2
- 239000010935 stainless steel Substances 0.000 description 2
- 229910001220 stainless steel Inorganic materials 0.000 description 2
- 229910052712 strontium Inorganic materials 0.000 description 2
- 125000000472 sulfonyl group Chemical group *S(*)(=O)=O 0.000 description 2
- 229910052717 sulfur Inorganic materials 0.000 description 2
- 229910052714 tellurium Inorganic materials 0.000 description 2
- 229910052719 titanium Inorganic materials 0.000 description 2
- 239000010936 titanium Substances 0.000 description 2
- 229910052723 transition metal Inorganic materials 0.000 description 2
- 150000003624 transition metals Chemical class 0.000 description 2
- 229910052721 tungsten Inorganic materials 0.000 description 2
- 125000004417 unsaturated alkyl group Chemical group 0.000 description 2
- 238000003466 welding Methods 0.000 description 2
- ZPFAVCIQZKRBGF-UHFFFAOYSA-N 1,3,2-dioxathiolane 2,2-dioxide Chemical compound O=S1(=O)OCCO1 ZPFAVCIQZKRBGF-UHFFFAOYSA-N 0.000 description 1
- NEAQRZUHTPSBBM-UHFFFAOYSA-N 2-hydroxy-3,3-dimethyl-7-nitro-4h-isoquinolin-1-one Chemical compound C1=C([N+]([O-])=O)C=C2C(=O)N(O)C(C)(C)CC2=C1 NEAQRZUHTPSBBM-UHFFFAOYSA-N 0.000 description 1
- AOCWQPKHSMJWPL-UHFFFAOYSA-N 3-methylpyrrolidin-2-one Chemical compound CC1CCNC1=O AOCWQPKHSMJWPL-UHFFFAOYSA-N 0.000 description 1
- ZTTYKFSKZIRTDP-UHFFFAOYSA-N 4,4-difluoro-1,3-dioxolan-2-one Chemical compound FC1(F)COC(=O)O1 ZTTYKFSKZIRTDP-UHFFFAOYSA-N 0.000 description 1
- DSMUTQTWFHVVGQ-UHFFFAOYSA-N 4,5-difluoro-1,3-dioxolan-2-one Chemical compound FC1OC(=O)OC1F DSMUTQTWFHVVGQ-UHFFFAOYSA-N 0.000 description 1
- RMYFSKOGEWSTQR-UHFFFAOYSA-N 4,5-difluoro-4,5-dimethyl-1,3-dioxolan-2-one Chemical compound CC1(F)OC(=O)OC1(C)F RMYFSKOGEWSTQR-UHFFFAOYSA-N 0.000 description 1
- QYIOFABFKUOIBV-UHFFFAOYSA-N 4,5-dimethyl-1,3-dioxol-2-one Chemical compound CC=1OC(=O)OC=1C QYIOFABFKUOIBV-UHFFFAOYSA-N 0.000 description 1
- RNNVXAXTORCUFA-UHFFFAOYSA-N 4-(fluoromethyl)-1,3-dioxolan-2-one Chemical compound FCC1COC(=O)O1 RNNVXAXTORCUFA-UHFFFAOYSA-N 0.000 description 1
- GKZFQPGIDVGTLZ-UHFFFAOYSA-N 4-(trifluoromethyl)-1,3-dioxolan-2-one Chemical compound FC(F)(F)C1COC(=O)O1 GKZFQPGIDVGTLZ-UHFFFAOYSA-N 0.000 description 1
- BJWMSGRKJIOCNR-UHFFFAOYSA-N 4-ethenyl-1,3-dioxolan-2-one Chemical compound C=CC1COC(=O)O1 BJWMSGRKJIOCNR-UHFFFAOYSA-N 0.000 description 1
- SBLRHMKNNHXPHG-UHFFFAOYSA-N 4-fluoro-1,3-dioxolan-2-one Chemical compound FC1COC(=O)O1 SBLRHMKNNHXPHG-UHFFFAOYSA-N 0.000 description 1
- PYKQXOJJRYRIHH-UHFFFAOYSA-N 4-fluoro-4-methyl-1,3-dioxolan-2-one Chemical compound CC1(F)COC(=O)O1 PYKQXOJJRYRIHH-UHFFFAOYSA-N 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-M Acrylate Chemical compound [O-]C(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-M 0.000 description 1
- 229920000856 Amylose Polymers 0.000 description 1
- 238000007088 Archimedes method Methods 0.000 description 1
- 229910001558 CF3SO3Li Inorganic materials 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 description 1
- 229910005187 FSO3Li Inorganic materials 0.000 description 1
- 108010022355 Fibroins Proteins 0.000 description 1
- 108010076876 Keratins Proteins 0.000 description 1
- 102000011782 Keratins Human genes 0.000 description 1
- 229910002986 Li4Ti5O12 Inorganic materials 0.000 description 1
- 229910013098 LiBF2 Inorganic materials 0.000 description 1
- 229910011157 LiMBO Inorganic materials 0.000 description 1
- 229910015645 LiMn Inorganic materials 0.000 description 1
- 229910015643 LiMn 2 O 4 Inorganic materials 0.000 description 1
- 229910015004 LiMnxFeyPO4 Inorganic materials 0.000 description 1
- 229910012258 LiPO Inorganic materials 0.000 description 1
- 229910012265 LiPO2F2 Inorganic materials 0.000 description 1
- 229910015868 MSiO Inorganic materials 0.000 description 1
- NTIZESTWPVYFNL-UHFFFAOYSA-N Methyl isobutyl ketone Chemical compound CC(C)CC(C)=O NTIZESTWPVYFNL-UHFFFAOYSA-N 0.000 description 1
- UIHCLUNTQKBZGK-UHFFFAOYSA-N Methyl isobutyl ketone Natural products CCC(C)C(C)=O UIHCLUNTQKBZGK-UHFFFAOYSA-N 0.000 description 1
- ZOKXTWBITQBERF-UHFFFAOYSA-N Molybdenum Chemical compound [Mo] ZOKXTWBITQBERF-UHFFFAOYSA-N 0.000 description 1
- 229910004008 NLi Inorganic materials 0.000 description 1
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 1
- 239000004952 Polyamide Substances 0.000 description 1
- 239000004962 Polyamide-imide Substances 0.000 description 1
- 239000004372 Polyvinyl alcohol Substances 0.000 description 1
- KJTLSVCANCCWHF-UHFFFAOYSA-N Ruthenium Chemical compound [Ru] KJTLSVCANCCWHF-UHFFFAOYSA-N 0.000 description 1
- 229910006145 SO3Li Inorganic materials 0.000 description 1
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 1
- 229920002125 Sokalan® Polymers 0.000 description 1
- 229930183415 Suberin Natural products 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 125000002252 acyl group Chemical group 0.000 description 1
- 125000004442 acylamino group Chemical group 0.000 description 1
- 125000004423 acyloxy group Chemical group 0.000 description 1
- 239000012790 adhesive layer Substances 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 1
- 150000001342 alkaline earth metals Chemical class 0.000 description 1
- 125000003342 alkenyl group Chemical group 0.000 description 1
- 125000004453 alkoxycarbonyl group Chemical group 0.000 description 1
- 125000004466 alkoxycarbonylamino group Chemical group 0.000 description 1
- 125000005370 alkoxysilyl group Chemical group 0.000 description 1
- 125000003282 alkyl amino group Chemical group 0.000 description 1
- 125000004414 alkyl thio group Chemical group 0.000 description 1
- 125000000304 alkynyl group Chemical group 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 125000004397 aminosulfonyl group Chemical group NS(=O)(=O)* 0.000 description 1
- WATWJIUSRGPENY-UHFFFAOYSA-N antimony atom Chemical compound [Sb] WATWJIUSRGPENY-UHFFFAOYSA-N 0.000 description 1
- 239000004760 aramid Substances 0.000 description 1
- 125000005162 aryl oxy carbonyl amino group Chemical group 0.000 description 1
- 125000005161 aryl oxy carbonyl group Chemical group 0.000 description 1
- 125000005110 aryl thio group Chemical group 0.000 description 1
- 125000004104 aryloxy group Chemical group 0.000 description 1
- 229910052797 bismuth Inorganic materials 0.000 description 1
- JCXGWMGPZLAOME-UHFFFAOYSA-N bismuth atom Chemical compound [Bi] JCXGWMGPZLAOME-UHFFFAOYSA-N 0.000 description 1
- 229910052795 boron group element Inorganic materials 0.000 description 1
- 150000001642 boronic acid derivatives Chemical class 0.000 description 1
- ZTCLFSRIWSZUHZ-UHFFFAOYSA-N but-1-yne;carbonic acid Chemical compound CCC#C.OC(O)=O ZTCLFSRIWSZUHZ-UHFFFAOYSA-N 0.000 description 1
- 229910052793 cadmium Inorganic materials 0.000 description 1
- BDOSMKKIYDKNTQ-UHFFFAOYSA-N cadmium atom Chemical compound [Cd] BDOSMKKIYDKNTQ-UHFFFAOYSA-N 0.000 description 1
- 125000003917 carbamoyl group Chemical group [H]N([H])C(*)=O 0.000 description 1
- 125000001951 carbamoylamino group Chemical group C(N)(=O)N* 0.000 description 1
- 229910052800 carbon group element Inorganic materials 0.000 description 1
- 239000002134 carbon nanofiber Substances 0.000 description 1
- 239000002041 carbon nanotube Substances 0.000 description 1
- 229910021393 carbon nanotube Inorganic materials 0.000 description 1
- WTDFOGSFVBZUNY-UHFFFAOYSA-N carbonic acid 3-fluoroprop-1-yne Chemical compound OC(O)=O.FCC#C WTDFOGSFVBZUNY-UHFFFAOYSA-N 0.000 description 1
- RDXQKAROFYREEQ-UHFFFAOYSA-N carbonic acid;hex-1-yne Chemical compound OC(O)=O.CCCCC#C RDXQKAROFYREEQ-UHFFFAOYSA-N 0.000 description 1
- DOAYJNMCHKRFLA-UHFFFAOYSA-N carbonic acid;hex-3-yne Chemical compound OC(O)=O.CCC#CCC DOAYJNMCHKRFLA-UHFFFAOYSA-N 0.000 description 1
- DYCIODWFEIGZLV-UHFFFAOYSA-N carbonic acid;oct-4-yne Chemical compound OC(O)=O.CCCC#CCCC DYCIODWFEIGZLV-UHFFFAOYSA-N 0.000 description 1
- VJRTZEWWUALMFH-UHFFFAOYSA-N carbonic acid;pent-1-yne Chemical compound OC(O)=O.CCCC#C VJRTZEWWUALMFH-UHFFFAOYSA-N 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 239000006182 cathode active material Substances 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 239000000919 ceramic Substances 0.000 description 1
- 239000006231 channel black Substances 0.000 description 1
- 229910017052 cobalt Inorganic materials 0.000 description 1
- 239000010941 cobalt Substances 0.000 description 1
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 description 1
- 239000004020 conductor Substances 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 230000008602 contraction Effects 0.000 description 1
- 230000007797 corrosion Effects 0.000 description 1
- 238000005260 corrosion Methods 0.000 description 1
- DMSZORWOGDLWGN-UHFFFAOYSA-N ctk1a3526 Chemical group NP(N)(N)=O DMSZORWOGDLWGN-UHFFFAOYSA-N 0.000 description 1
- 238000007766 curtain coating Methods 0.000 description 1
- 125000004122 cyclic group Chemical group 0.000 description 1
- 238000005262 decarbonization Methods 0.000 description 1
- 238000009831 deintercalation Methods 0.000 description 1
- 230000032798 delamination Effects 0.000 description 1
- 230000002542 deteriorative effect Effects 0.000 description 1
- 125000004663 dialkyl amino group Chemical group 0.000 description 1
- 238000007607 die coating method Methods 0.000 description 1
- 238000003618 dip coating Methods 0.000 description 1
- 238000010494 dissociation reaction Methods 0.000 description 1
- 230000005593 dissociations Effects 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 238000007606 doctor blade method Methods 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 239000012777 electrically insulating material Substances 0.000 description 1
- 230000005611 electricity Effects 0.000 description 1
- 239000011532 electronic conductor Substances 0.000 description 1
- 125000004494 ethyl ester group Chemical group 0.000 description 1
- 230000001747 exhibiting effect Effects 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 239000010419 fine particle Substances 0.000 description 1
- 229920001973 fluoroelastomer Polymers 0.000 description 1
- 239000006232 furnace black Substances 0.000 description 1
- 230000004927 fusion Effects 0.000 description 1
- 229910052733 gallium Inorganic materials 0.000 description 1
- 238000007429 general method Methods 0.000 description 1
- 229910052732 germanium Inorganic materials 0.000 description 1
- GNPVGFCGXDBREM-UHFFFAOYSA-N germanium atom Chemical compound [Ge] GNPVGFCGXDBREM-UHFFFAOYSA-N 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- 238000009499 grossing Methods 0.000 description 1
- 229910001849 group 12 element Inorganic materials 0.000 description 1
- 229910052735 hafnium Inorganic materials 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 125000000717 hydrazino group Chemical group [H]N([*])N([H])[H] 0.000 description 1
- 150000003949 imides Chemical class 0.000 description 1
- 125000001841 imino group Chemical group [H]N=* 0.000 description 1
- 239000011256 inorganic filler Substances 0.000 description 1
- 229910003475 inorganic filler Inorganic materials 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 238000009830 intercalation Methods 0.000 description 1
- 230000016507 interphase Effects 0.000 description 1
- 229910052741 iridium Inorganic materials 0.000 description 1
- JEIPFZHSYJVQDO-UHFFFAOYSA-N iron(III) oxide Inorganic materials O=[Fe]O[Fe]=O JEIPFZHSYJVQDO-UHFFFAOYSA-N 0.000 description 1
- 239000003273 ketjen black Substances 0.000 description 1
- 229910052745 lead Inorganic materials 0.000 description 1
- 229920005610 lignin Polymers 0.000 description 1
- 235000013490 limbo Nutrition 0.000 description 1
- 229910001540 lithium hexafluoroarsenate(V) Inorganic materials 0.000 description 1
- MHCFAGZWMAWTNR-UHFFFAOYSA-M lithium perchlorate Chemical compound [Li+].[O-]Cl(=O)(=O)=O MHCFAGZWMAWTNR-UHFFFAOYSA-M 0.000 description 1
- 229910001486 lithium perchlorate Inorganic materials 0.000 description 1
- 229910001496 lithium tetrafluoroborate Inorganic materials 0.000 description 1
- 239000011572 manganese Substances 0.000 description 1
- 239000007769 metal material Substances 0.000 description 1
- 239000002923 metal particle Substances 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- 150000004702 methyl esters Chemical class 0.000 description 1
- 238000010295 mobile communication Methods 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 239000011733 molybdenum Substances 0.000 description 1
- 229920005615 natural polymer Polymers 0.000 description 1
- 229910052758 niobium Inorganic materials 0.000 description 1
- 150000004767 nitrides Chemical group 0.000 description 1
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 description 1
- 239000004745 nonwoven fabric Substances 0.000 description 1
- 229910052763 palladium Inorganic materials 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 229910052698 phosphorus Inorganic materials 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 238000007747 plating Methods 0.000 description 1
- 229910052697 platinum Inorganic materials 0.000 description 1
- 229910052696 pnictogen Inorganic materials 0.000 description 1
- 239000004584 polyacrylic acid Substances 0.000 description 1
- 229920002239 polyacrylonitrile Polymers 0.000 description 1
- 229920002647 polyamide Polymers 0.000 description 1
- 229920002312 polyamide-imide Polymers 0.000 description 1
- 229920000728 polyester Polymers 0.000 description 1
- 229920013716 polyethylene resin Polymers 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 229920002451 polyvinyl alcohol Polymers 0.000 description 1
- 235000019422 polyvinyl alcohol Nutrition 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 239000011241 protective layer Substances 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 229910052702 rhenium Inorganic materials 0.000 description 1
- 229910052703 rhodium Inorganic materials 0.000 description 1
- 229910052707 ruthenium Inorganic materials 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 239000002210 silicon-based material Substances 0.000 description 1
- 125000003808 silyl group Chemical group [H][Si]([H])([H])[*] 0.000 description 1
- 239000002356 single layer Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000007784 solid electrolyte Substances 0.000 description 1
- 239000006104 solid solution Substances 0.000 description 1
- 238000007711 solidification Methods 0.000 description 1
- 230000008023 solidification Effects 0.000 description 1
- 238000005507 spraying Methods 0.000 description 1
- 125000000213 sulfino group Chemical group [H]OS(*)=O 0.000 description 1
- 125000000475 sulfinyl group Chemical group [*:2]S([*:1])=O 0.000 description 1
- 125000000020 sulfo group Chemical group O=S(=O)([*])O[H] 0.000 description 1
- 125000006296 sulfonyl amino group Chemical group [H]N(*)S(*)(=O)=O 0.000 description 1
- 229920003002 synthetic resin Polymers 0.000 description 1
- 239000000057 synthetic resin Substances 0.000 description 1
- 229910052715 tantalum Inorganic materials 0.000 description 1
- GUVRBAGPIYLISA-UHFFFAOYSA-N tantalum atom Chemical compound [Ta] GUVRBAGPIYLISA-UHFFFAOYSA-N 0.000 description 1
- RBYFNZOIUUXJQD-UHFFFAOYSA-J tetralithium oxalate Chemical compound [Li+].[Li+].[Li+].[Li+].[O-]C(=O)C([O-])=O.[O-]C(=O)C([O-])=O RBYFNZOIUUXJQD-UHFFFAOYSA-J 0.000 description 1
- 229920005992 thermoplastic resin Polymers 0.000 description 1
- ZMZDMBWJUHKJPS-UHFFFAOYSA-N thiocyanic acid Chemical compound SC#N ZMZDMBWJUHKJPS-UHFFFAOYSA-N 0.000 description 1
- 239000011366 tin-based material Substances 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- NDZWKTKXYOWZML-UHFFFAOYSA-N trilithium;difluoro oxalate;borate Chemical compound [Li+].[Li+].[Li+].[O-]B([O-])[O-].FOC(=O)C(=O)OF NDZWKTKXYOWZML-UHFFFAOYSA-N 0.000 description 1
- WFKWXMTUELFFGS-UHFFFAOYSA-N tungsten Chemical compound [W] WFKWXMTUELFFGS-UHFFFAOYSA-N 0.000 description 1
- 239000010937 tungsten Substances 0.000 description 1
- DZKDPOPGYFUOGI-UHFFFAOYSA-N tungsten dioxide Inorganic materials O=[W]=O DZKDPOPGYFUOGI-UHFFFAOYSA-N 0.000 description 1
- 238000004804 winding Methods 0.000 description 1
- 239000002759 woven fabric Substances 0.000 description 1
- 229910009207 xMxN Inorganic materials 0.000 description 1
- 229910052727 yttrium Inorganic materials 0.000 description 1
- 229910052726 zirconium Inorganic materials 0.000 description 1
Images
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/056—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes
- H01M10/0564—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes the electrolyte being constituted of organic materials only
- H01M10/0566—Liquid materials
- H01M10/0569—Liquid materials characterised by the solvents
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/052—Li-accumulators
- H01M10/0525—Rocking-chair batteries, i.e. batteries with lithium insertion or intercalation in both electrodes; Lithium-ion batteries
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/056—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes
- H01M10/0564—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes the electrolyte being constituted of organic materials only
- H01M10/0566—Liquid materials
- H01M10/0568—Liquid materials characterised by the solutes
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/58—Selection of substances as active materials, active masses, active liquids of inorganic compounds other than oxides or hydroxides, e.g. sulfides, selenides, tellurides, halogenides or LiCoFy; of polyanionic structures, e.g. phosphates, silicates or borates
- H01M4/583—Carbonaceous material, e.g. graphite-intercalation compounds or CFx
- H01M4/587—Carbonaceous material, e.g. graphite-intercalation compounds or CFx for inserting or intercalating light metals
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M2300/00—Electrolytes
- H01M2300/0017—Non-aqueous electrolytes
- H01M2300/002—Inorganic electrolyte
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M2300/00—Electrolytes
- H01M2300/0017—Non-aqueous electrolytes
- H01M2300/0025—Organic electrolyte
- H01M2300/0028—Organic electrolyte characterised by the solvent
- H01M2300/0037—Mixture of solvents
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/10—Energy storage using batteries
Definitions
- the present invention relates to an electrolyte that can be used in lithium ion secondary batteries, and a lithium ion secondary battery using the electrolyte.
- Lithium-ion secondary batteries which have excellent capacity, are used as power sources for mobile terminals, personal computers, and electric vehicles.
- decarbonization In recent years, there has been an increasing demand for decarbonization, and many efforts are being made to electrify the drive sources of various devices. Along with this, there is a demand for further improvement in the characteristics of lithium ion secondary batteries.
- the inventors of the present invention aimed to improve the characteristics of lithium-ion secondary batteries by optimizing the electrolyte of the lithium-ion secondary batteries.
- Patent Document 1 introduces an electrolytic solution in which LiPF 6 is dissolved at a concentration of 1 mol/L in a mixed non-aqueous solvent in which ethylene carbonate and ethyl methyl carbonate are mixed at a volume ratio of 3:7.
- Patent Document 2 introduces an electrolytic solution in which LiPF 6 is dissolved at a concentration of 1 mol/L in a mixed non-aqueous solvent in which ethylene carbonate, dimethyl carbonate, and ethyl methyl carbonate are mixed at a volume ratio of 3:2:5. .
- chain carbonate is used as the main solvent of the electrolytic solution in the above patent documents.
- the inventor of the present invention found that an alkylene cyclic carbonate and methyl propionate were used in combination as a non-aqueous solvent for the electrolytic solution.
- An application for an ion secondary battery has already been filed (Japanese Patent Application No. 2020-026926).
- the electrolytic solution described above can contribute to improving the characteristics of the lithium ion secondary battery.
- the inventor of the present invention was not satisfied with this, and conducted extensive research to further improve the characteristics.
- the present invention has been made in view of such circumstances, and an object thereof is to provide an electrolytic solution capable of imparting excellent characteristics to a lithium ion secondary battery, and a lithium ion secondary battery exhibiting excellent characteristics. do.
- the inventors of the present invention found that lithium It was found that the characteristics of the ion secondary battery are further improved.
- the inventors of the present invention completed the electrolytic solution of the present invention based on such findings.
- the applicant of the present invention has developed a structure of an electric storage cell and an electric storage device capable of suppressing a decrease in sealing performance as part of efforts to improve the durability of an electric storage device including a lithium ion secondary battery, and has already filed an application.
- Japanese Patent Application No. 2021-003409 Japanese Patent Application No. 2021-003409
- the inventors of the present invention also conducted various studies on the electrolytic solution in order to further improve the sealing performance of the storage cell in the lithium ion secondary battery having the structure. In the process, it was found that by combining the structure with the electrolytic solution of the present invention described above, the sealing property of the storage cell in the lithium ion secondary battery can be further improved, and the lithium ion secondary battery of the present invention completed.
- the electrolytic solution of the present invention that solves the above problems is having an electrolyte comprising a lithium salt and a non-aqueous solvent comprising an alkylene cyclic carbonate and methyl propionate;
- the electrolyte contains 30 mol% or more of a lithium salt other than LiPF 6 with respect to the total lithium salt,
- the nonaqueous solvent is an electrolytic solution containing 75% by volume or more of the methyl propionate.
- the lithium ion secondary battery of the present invention for solving the above problems is a positive electrode having a first current collector and a positive electrode active material layer provided on one surface of the first current collector; It has a second current collector and a negative electrode active material layer provided on one side of the second current collector, and is stacked on the positive electrode while facing the positive electrode active material layer. a negative electrode; a separator disposed between the positive electrode active material layer and the negative electrode active material layer; disposed between the first current collector and the second current collector to surround the positive electrode active material layer and the negative electrode active material layer; and a sealing portion that seals the electrolytic solution in the space between It is a lithium ion secondary battery using the electrolyte solution of the present invention described above as the electrolyte solution.
- the electrolyte solution of the present invention excellent characteristics can be imparted to lithium ion secondary batteries. Moreover, the lithium ion secondary battery of the present invention exhibits excellent characteristics, particularly excellent structural durability.
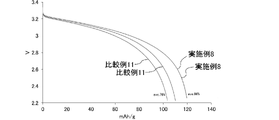
- FIG. 10 is a graph showing changes over time in CC discharge capacity in each lithium ion secondary battery of Example 8 and Comparative Example 11.
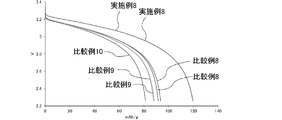
- FIG. 10 is a graph showing changes over time in CC discharge capacity in each lithium ion secondary battery of Example 8 and Comparative Examples 8 to 10.
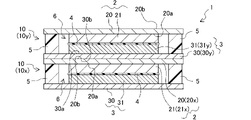
- FIG. 11 is an explanatory diagram schematically showing a lithium ion secondary battery of Example 9;
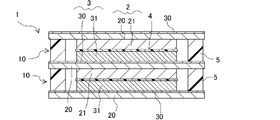
- FIG. 11 is an explanatory diagram schematically showing a lithium-ion secondary battery of Example 10;
- FIG. 11 is an explanatory diagram for explaining measurement positions of the natural peel length in Evaluation Example 9;
- 10 is a graph showing the natural peel length of each test piece in Evaluation Example 9.
- the numerical range "x to y" described in this specification includes the lower limit x and the upper limit y.
- a new numerical range can be formed by arbitrarily combining these upper and lower limits and the numerical values listed in the examples.
- numerical values arbitrarily selected from any of the above numerical ranges can be used as upper and lower numerical values of the new numerical range.
- the electrolytic solution of the present invention can improve the characteristics of lithium ion secondary batteries by providing all of the following (1) to (3).
- the non-aqueous solvent contains 75% by volume or more of methyl propionate.
- the above (1) can contribute particularly to improving the structural durability.
- the above (2) can contribute to smooth charging and discharging among the characteristics of the lithium ion secondary battery. Specifically, it is derived from alkylene cyclic carbonate, and lithium ions are smoothly generated and inserted into and detached from the negative electrode, and the disadvantages of alkylene cyclic carbonate such as high viscosity and high melting point are compensated by methyl propionate.
- the above (3) can contribute to the improvement of the capacity retention rate, output, and the like among the characteristics of the lithium ion secondary battery. It can be said that the electrolytic solution of the present invention can improve the characteristics of the lithium-ion secondary battery by these cooperation.
- the structural durability of the lithium ion secondary battery of the present invention can be improved by providing both (4) and (5) below.
- a positive electrode having a first current collector and a positive electrode active material layer provided on one surface of the first current collector; It has a second current collector and a negative electrode active material layer provided on one side of the second current collector, and is stacked on the positive electrode while facing the positive electrode active material layer.
- a negative electrode a separator disposed between the positive electrode active material layer and the negative electrode active material layer; disposed between the first current collector and the second current collector to surround the positive electrode active material layer and the negative electrode active material layer; and a sealing portion that seals the electrolytic solution in the space between (5)
- the electrolytic solution of the present invention described above is used.
- the above (4) can contribute to suppressing deterioration of the sealing performance among the characteristics of the lithium ion secondary battery.
- the above (5) can further contribute to the improvement of structural durability among the characteristics of the lithium ion secondary battery provided with the above (4), specifically, the improvement of sealing performance.
- the lithium-ion secondary battery of the present invention can realize improvement in its characteristics through these cooperation.
- the electrolytic solution and the lithium ion secondary battery of the present invention will be described below for each component.
- the lithium ion concentration in the electrolytic solution of the present invention is preferably in the range of 0.8 to 1.8 mol / L, more preferably in the range of 0.9 to 1.5 mol / L, from the viewpoint of ionic conductivity.
- the range of 0 to 1.4 mol/L is more preferable, and the range of 1.1 to 1.3 mol/L is particularly preferable.
- the electrolyte used in the electrolytic solution of the present invention contains a lithium salt, and contains 30 mol % or more of the lithium salt other than LiPF 6 with respect to the total lithium salt.
- the electrolytic solution of the present invention may contain LiPF 6 as a lithium salt or may not contain LiPF 6 .
- LiPF 6 is widely used as an electrolyte for electrolyte solutions for lithium ion secondary batteries, and is relatively inexpensive.
- the reason why the electrolytic solution of the present invention uses a lithium salt other than LiPF 6 is as follows.
- LiPF 6 It is known that the reaction of LiPF 6 with water produces hydrogen fluoride. Since a small amount of water is present in the electrolyte of a general lithium ion secondary battery, hydrogen fluoride may be contained in the electrolyte in the lithium ion secondary battery containing LiPF 6 in the electrolyte. It is also known that LiPF 6 is thermally unstable, forming PF 5 at temperatures above 60° C., for example. The PF5 can react with water to produce hydrogen fluoride.
- the inventors of the present invention have studied the composition of the electrolytic solution in order to improve the characteristics of the lithium ion secondary battery.
- hydrogen fluoride and PF 5 present in the electrolyte may corrode the electrodes and containers of the lithium-ion secondary battery, and corrosion of the electrodes and containers may reduce the durability of the lithium-ion secondary battery.
- the lithium ion secondary battery has a structure having a sealing portion between two current collectors as disclosed in Japanese Patent Application No.
- the surface of the metal portion contained in the current collectors corrodes ( Specifically, when fluorinated, the bonding strength between the sealing portion and the current collector is weakened, structurally deteriorating the battery, and the sealing performance of the sealing portion is likely to be reduced. This problem is particularly pronounced when the current collector contains aluminum.
- the inventors of the present invention aimed to suppress the above-mentioned problems caused by LiPF 6 in order to improve the structural durability of lithium ion secondary batteries. They have also found that the above problem is suppressed when the electrolyte contains a lithium salt other than LiPF 6 in an amount of 30 mol % or more with respect to the total lithium salt. Although details will be described in the section of Examples described later, as a result of actual tests by the inventors of the present invention, in a lithium ion secondary battery having a sealing portion between two current collectors, electrolytes other than LiPF 6 When the lithium salt of 30 mol % or more of the total lithium salt is contained, the durability is improved as compared with the case where only LiPF 6 is contained as the electrolyte.
- lithium salts other than LiPF 6 those represented by the following general formula (1) are particularly preferred. Lithium salts of this type are less likely to produce hydrogen fluoride and PF5 .
- R1X1 )( R2SO2 )NLi General formula ( 1 )
- R 1 is hydrogen, halogen, an optionally substituted alkyl group, an optionally substituted cycloalkyl group, an optionally substituted unsaturated alkyl group, a substituent
- R 2 is hydrogen, halogen, an optionally substituted alkyl group, an optionally substituted cycloalkyl group, an optionally substituted unsaturated alkyl group, or a substituent; optionally substituted unsaturated cycloalkyl group, optionally substituted aromatic group, optionally substituted heterocyclic group, optionally substituted alkoxy group, optionally substituted unsaturated alkoxy group, optionally substituted thioalkoxy group, optionally substituted unsaturated thioalkoxy group, CN, SCN, OCN be. Also, R 1 and R 2 may combine with each other to form a ring.
- R a and R b each independently represent hydrogen, halogen, an optionally substituted alkyl group, an optionally substituted cycloalkyl group, or an optionally substituted unsubstituted group.
- R a and R b may combine with R 1 or R 2 to form a ring.
- an optionally substituted alkyl group means an alkyl group in which one or more of the hydrogen atoms in the alkyl group is substituted with a substituent, or an unsubstituted alkyl group. do.
- substituents in the phrase "optionally substituted” include alkyl groups, alkenyl groups, alkynyl groups, cycloalkyl groups, unsaturated cycloalkyl groups, aromatic groups, heterocyclic groups, halogens, OH , SH, CN, SCN, OCN, nitro group, alkoxy group, unsaturated alkoxy group, amino group, alkylamino group, dialkylamino group, aryloxy group, acyl group, alkoxycarbonyl group, acyloxy group, aryloxycarbonyl group, acylamino group, alkoxycarbonylamino group, aryloxycarbonylamino group, sulfonylamino group, sulfamoyl group, carbamoyl group, alkylthio group, arylthio group, sulfonyl group, sulfinyl group, ureido group, phosphoramide group, sulfo group,
- the lithium salt is preferably represented by the following general formula (1-1).
- R 13 and R 14 are each independently C n Ha F b Cl c Br d I e (CN) f (SCN ) g (OCN) h .
- R c and R d each independently represent hydrogen, halogen, an optionally substituted alkyl group, an optionally substituted cycloalkyl group, or an optionally substituted unsubstituted group.
- R c and R d may combine with R 23 or R 24 to form a ring.
- n is preferably an integer of 0 to 6, more preferably an integer of 0 to 4, and particularly preferably an integer of 0 to 2.
- n is preferably an integer of 1 to 8, and 1 to 7 is more preferred, and integers from 1 to 3 are particularly preferred.
- the lithium salt is represented by the following general formula (1-2).
- R 15 SO 2 (R 16 SO 2 )NLi
- R 15 and R 16 are each independently C n Ha F b Cl c Br d Ie .
- n is preferably an integer of 0 to 6, more preferably an integer of 0 to 4, and particularly preferably an integer of 0 to 2.
- n is preferably an integer of 1 to 8, and 1 to 7 is more preferred, and integers from 1 to 3 are particularly preferred.
- the lithium salts represented by the general formula (1), (1-1) or (1-2) are (CF 3 SO 2 ) 2 NLi, (FSO 2 ) 2 NLi, (C 2 F 5 SO 2 ) at least one imide salt selected from 2NLi , FSO2 ( CF3SO2 ) NLi , ( SO2CF2CF2SO2 ) NLi , or ( SO2CF2CF2CF2SO2 ) NLi ; It is preferable to have Of these, (FSO 2 ) 2 NLi improves the output and durability of the lithium ion secondary battery. This is probably because the use of (FSO 2 ) 2 NLi reduces the viscosity of the electrolytic solution and forms a good film on the surfaces of the negative electrode and the positive electrode.
- the amount of the lithium salt other than LiPF 6 contained in the electrolytic solution of the present invention may be 30 mol% or more with respect to the total lithium salt, but the preferred range is 50 mol% with respect to the total lithium salt. As mentioned above, each range of 75 mol% or more and 90 mol% or more can be exemplified.
- the electrolytic solution of the present invention contains an alkylene cyclic carbonate and methyl propionate as a non-aqueous solvent.
- Alkylene cyclic carbonate is a non-aqueous solvent with a high dielectric constant, and is thought to contribute to dissolution and ion dissociation of the lithium salt. Further, it is generally known that an SEI (Solid Electrolyte Interphase) film is formed on the surface of the negative electrode by reductive decomposition of the alkylene cyclic carbonate during charging of the lithium ion secondary battery. It is believed that the presence of such an SEI coating enables reversible insertion and extraction of lithium ions into and from a negative electrode comprising graphite.
- the electrolytic solution of the present invention may use only one type of alkylene cyclic carbonate, or may use a plurality of types of alkylene cyclic carbonates in combination. Examples of alkylene cyclic carbonates include ethylene carbonate and propylene carbonate, with ethylene carbonate being particularly preferred.
- alkylene cyclic carbonates are useful as non-aqueous solvents for electrolytic solutions, they have high viscosity. Therefore, if the ratio of the alkylene cyclic carbonate is too high, the ionic conductivity of the electrolyte and the diffusion of lithium ions in the electrolyte may be adversely affected. In addition, since the alkylene cyclic carbonate has a relatively high melting point, if the proportion of the alkylene cyclic carbonate is too high, the electrolytic solution may solidify under low temperature conditions.
- methyl propionate is a non-aqueous solvent with low dielectric constant, low viscosity and low melting point.
- the coexistence of alkylene cyclic carbonate and methyl propionate offsets the disadvantage of alkylene cyclic carbonate with methyl propionate. That is, methyl propionate is considered to contribute to lowering the viscosity of the electrolytic solution, optimizing the ionic conductivity, optimizing the diffusion coefficient of lithium ions, and preventing solidification under low-temperature conditions.
- methyl acetate, ethyl acetate, ethyl propionate, methyl butyrate, and ethyl butyrate exist as esters having a chemical structure similar to that of methyl propionate.
- methyl ester is superior to ethyl ester in terms of physical properties of the electrolyte and battery characteristics.
- the melting points and boiling points of the methyl esters methyl propionate, methyl acetate, and methyl butyrate are as follows.
- Methyl propionate Melting point -88°C, boiling point 80°C Methyl acetate Melting point -98°C, boiling point 57°C Methyl butyrate Melting point -95°C, boiling point 102°C
- the non-aqueous solvent contained in the electrolytic solution preferably has a boiling point of 60°C or higher. From the point of view of the production environment, it is preferable that the boiling point of the non-aqueous solvent to be used is high.
- the number of carbon atoms in the ester increases, the lipophilicity of the ester increases, which is disadvantageous for dissolving and dissociating the lithium salt. Therefore, the number of carbon atoms in the ester is preferably as small as possible.
- the non-aqueous solvent in the electrolytic solution of the present invention contains 75% by volume or more of methyl propionate.
- the electrolytic solution of the present invention can improve both the charge/discharge capacity of the positive electrode and the charge/discharge capacity of the negative electrode in the lithium ion secondary battery. is possible.
- methyl propionate is preferably contained in the non-aqueous solvent in an amount of 85% by volume or less, more preferably 80% by volume or less.
- the non-aqueous solvent in the electrolytic solution of the present invention may contain other non-aqueous solvents in addition to the alkylene cyclic carbonate and methyl propionate, or may consist of alkylene cyclic carbonate and methyl propionate.
- the electrolytic solution of the present invention should contain 75% by volume or more of methyl propionate when the total non-aqueous solvent is taken as 100% by volume.
- 100% by volume of the entire non-aqueous solvent means the sum of the volumes of the non-aqueous solvents at normal temperature of 25° C. and normal pressure before mixing.
- the ratio of methyl propionate to the total volume of alkylene cyclic carbonate and methyl propionate is preferably in the range of 72 to 95% by volume, more preferably in the range of 75 to 90% by volume. is more preferred, most preferably in the range of 75 to 85% by volume.
- the ratio of the alkylene cyclic carbonate to the total volume of the alkylene cyclic carbonate and methyl propionate is preferably within the range of 5 to 28% by volume, more preferably within the range of 10 to 25% by volume, Most preferably it is in the range of 15-25% by volume.
- non-aqueous solvents mentioned above include fluorine-containing cyclic carbonates and unsaturated cyclic carbonates. These may be used alone or in combination. By using these non-aqueous solvents together with the alkylene cyclic carbonate and methyl propionate, the performance of the lithium ion secondary battery can be improved.
- Fluorine-containing cyclic carbonates include fluoroethylene carbonate, 4-(trifluoromethyl)-1,3-dioxolan-2-one, 4,4-difluoro-1,3-dioxolan-2-one, 4-fluoro-4 -methyl-1,3-dioxolan-2-one, 4-(fluoromethyl)-1,3-dioxolan-2-one, 4,5-difluoro-1,3-dioxolan-2-one, 4-fluoro- Examples include 5-methyl-1,3-dioxolan-2-one and 4,5-difluoro-4,5-dimethyl-1,3-dioxolan-2-one.
- unsaturated cyclic carbonates include vinylene carbonate, fluorovinylene carbonate, methyl vinylene carbonate, fluoromethyl vinylene carbonate, ethyl vinylene carbonate, propyl vinylene carbonate, butyl vinylene carbonate, dimethyl vinylene carbonate, diethyl vinylene carbonate, dipropyl vinylene carbonate, trifluoro Examples include methyl vinylene carbonate and vinyl ethylene carbonate. Particularly preferably, the electrolytic solution of the present invention contains vinylene carbonate.
- the amount of fluorine-containing cyclic carbonate and/or unsaturated cyclic carbonate added to the electrolytic solution of the present invention is in the range of 0.1 to 5% by mass, 0.3 to 4% by mass with respect to the total mass other than these. Within the range, within the range of 0.5 to 3% by mass, and within the range of 1 to 2% by mass can be exemplified.
- the electrolytic solution of the present invention may contain additives.
- the additive it is preferable to select an additive that initiates reductive decomposition at a potential higher than the potential at which other components of the electrolytic solution, specifically, alkylene cyclic carbonate and methyl propionate, initiate reductive decomposition.
- specific examples of additives include cyclic sulfates, oxalate borates, and dihalogenated phosphates. These additives may be used alone or in combination of multiple types.
- a cyclic sulfate is a compound represented by the following chemical formula.
- R--O--SO 2 --OR Two R's are alkyl groups and are bonded together to form a ring together with --O--S--O--.
- Examples of cyclic sulfate esters include 5- to 9-membered rings, 5- to 8-membered rings, and 5- to 7-membered rings. 4 can be exemplified.
- Lithium salts are preferred as oxalate borates.
- LiB(C 2 O 4 ) 2 and LiB(C 2 O 4 )X 2 (X is a halogen selected from F, Cl, Br and I) can be exemplified as specific oxalate borate salts.
- the borate oxalate is LiB(C 2 O 4 ) 2 , lithium bis(oxalate)borate and/or LiB(C 2 O 4 )F 2 , lithium difluoro(oxalate)borate.
- LiPO 2 X 2 (X is a halogen selected from F, Cl, Br and I) can be exemplified as a specific dihalogenated phosphate.
- the amount of the additive added to the electrolytic solution of the present invention is within the range of 0.1 to 5% by mass, within the range of 0.3 to 4% by mass, and 0.3% by mass to the total mass other than the additive. Within the range of 5 to 3% by mass and within the range of 1 to 2% by mass can be exemplified.
- the lithium ion secondary battery of the present invention using the electrolytic solution of the present invention will be described below.
- the lithium ion secondary battery of the present invention has a positive electrode, a negative electrode, a separator, a sealing portion and an electrolytic solution.
- the electrolytic solution is as described above.
- the positive electrode has a first current collector and a positive electrode active material layer provided on one surface of the first current collector.
- the negative electrode has a second current collector and a negative electrode active material layer provided on one surface of the second current collector.
- the negative electrode is stacked on the positive electrode with the negative electrode active material layer facing the positive electrode active material layer of the positive electrode.
- the first current collector and the second current collector are collectively referred to.
- the positive electrode active material and the negative electrode active material shall be collectively referred to, and when referring to the electrode active material layer, the positive electrode active material and the negative electrode active material shall be collectively referred to.
- a current collector is a chemically inactive electronic conductor that keeps current flowing through an electrode during discharging or charging of a lithium-ion secondary battery.
- metal materials such as The effect of improving durability, which is the effect of the lithium ion secondary battery of the present invention, is particularly remarkable when the first current collector, which is the current collector for the positive electrode, is made of aluminum. That is, it is particularly preferable that the first current collector is made of aluminum.
- the current collector may be covered with a known protective layer.
- a current collector whose surface has been treated by a known method may be used as the current collector.
- the current collector can be in the form of foil, sheet, film, wire, rod, mesh, etc. Therefore, metal foils such as copper foil, nickel foil, aluminum foil, and stainless steel foil can be preferably used as the current collector.
- the thickness is preferably in the range of 1 ⁇ m to 100 ⁇ m.
- the positive electrode active material should be capable of intercalating and deintercalating lithium ions .
- D is W, Mo, Re, Pd, Ba, Cr, B, Sb, Sr, Pb, Ga, Al, Nb, Mg, Ta, Ti, La, Zr, Cu, Ca, Ir , Hf, Rh, Fe, Ge, Zn, Ru, Sc, Sn, In, Y, Bi, S, Si, Na, K, P, V, 1.7 ⁇ f ⁇ 3)
- Lithium composite metal oxide represented by and Li 2 MnO 3 can be mentioned.
- a spinel-structured metal oxide such as LiMn 2 O 4
- a solid solution composed of a mixture of a spinel-structured metal oxide and a layered compound, LiMPO 4 , LiMVO 4 or Li 2 MSiO 4 in the formula is selected from at least one of Co, Ni, Mn and Fe.
- positive electrode active materials include taborite compounds represented by LiMPO 4 F (M is a transition metal) such as LiFePO 4 F, and borate compounds represented by LiMBO 3 (M is a transition metal) such as LiFeBO 3 . be able to.
- Any metal oxide used as a positive electrode active material may have the above compositional formula as a basic composition, and those in which the metal elements contained in the basic composition are replaced with other metal elements can also be used. Only one type of these positive electrode active materials may be used, or a plurality of types may be used in combination.
- positive electrode active materials having an olivine structure are suitable as positive electrode active materials for lithium ion secondary batteries because of their excellent thermal stability.
- a commercially available product may be purchased, or the method described in the following literature may be used as a reference.
- the positive electrode active material having an olivine structure one coated with carbon is preferable.
- LiaMbPO4 ( M is Mn , Fe, Co, Ni, Cu, Mg, Zn, V, Ca, Sr, Ba, Ti, Al; is at least one element selected from Si, B, Te, and Mo. a satisfies 0.9 ⁇ a ⁇ 1.2, and b satisfies 0.6 ⁇ b ⁇ 1.1).
- Examples of the range of a include 0.95 ⁇ a ⁇ 1.1 and 0.97 ⁇ a ⁇ 1.05.
- M in LiaMbPO4 is preferably at least one element selected from Mn, Fe, Co, Ni, Mg, V, and Te, and M is composed of two or more elements. is more preferred. More preferably M is selected from Mn, Fe and V. b preferably satisfies 0.95 ⁇ b ⁇ 1.05.
- the ranges of x and y are 0.5 ⁇ x ⁇ 0.9, 0.1 ⁇ y ⁇ 0.5, 0.6 ⁇ x ⁇ 0.8, 0.2 ⁇ y ⁇ 0.4, and Examples include 0.7 ⁇ x ⁇ 0.8 and 0.2 ⁇ y ⁇ 0.3.
- LiFePO 4 is widely used as a positive electrode active material having an olivine structure, but LiMn x Fe y PO 4 in which Mn and Fe coexist is known to have a higher reaction potential than LiFePO 4 .
- the positive electrode active material layer may contain additives such as a conductive aid, a binder, and a dispersant in addition to the positive electrode active material.
- additives such as a conductive aid, a binder, and a dispersant in addition to the positive electrode active material. Examples of the proportion of the positive electrode active material in the positive electrode active material layer are within the range of 70 to 99% by mass, within the range of 80 to 98% by mass, and within the range of 90 to 97% by mass.
- a conductive aid is added to increase the conductivity of the electrode. Therefore, the conductive aid may be added arbitrarily when the conductivity of the electrode is insufficient, and may not be added when the conductivity of the electrode is sufficiently excellent.
- the conductive aid may be any chemically inactive electron conductor, and examples include carbon black, graphite, vapor grown carbon fiber, carbon nanotube, and various metal particles, which are carbonaceous fine particles. be done. Examples of carbon black include acetylene black, Ketjenblack (registered trademark), furnace black, and channel black. These conductive aids can be added to the positive electrode active material layer singly or in combination of two or more.
- the blending amount of the conductive aid is not particularly limited.
- the proportion of the conductive aid in the positive electrode active material layer is preferably in the range of 1 to 7% by mass, more preferably in the range of 2 to 6% by mass, and even more preferably in the range of 3 to 5% by mass.
- Binders serve to bind the positive electrode active material and conductive aid to the surface of the current collector.
- Binders include fluorine-containing resins such as polyvinylidene fluoride, polytetrafluoroethylene, and fluororubber; thermoplastic resins such as polypropylene and polyethylene; imide resins such as polyimide and polyamideimide; alkoxysilyl group-containing resins; Examples include meth)acrylate resins, polyacrylic acid, polyvinyl alcohol, polyvinylpyrrolidone, carboxymethylcellulose, and styrene-butadiene rubber.
- the blending amount of the binder is not particularly limited.
- the proportion of the binder in the positive electrode active material layer is preferably in the range of 0.5 to 7% by mass, more preferably in the range of 1 to 5% by mass, and even more preferably in the range of 2 to 4% by mass.
- additives such as dispersants other than conductive aids and binders can be used.
- a material that can store and release charge carriers can be used as the negative electrode active material. Therefore, there is no particular limitation as long as it is a simple substance, alloy or compound that can occlude and release charge carriers such as lithium ions.
- the negative electrode active material Li, carbon, silicon, germanium, group 14 elements such as tin, aluminum, group 13 elements such as indium, zinc, group 12 elements such as cadmium, antimony, group 15 elements such as bismuth, magnesium , alkaline earth metals such as calcium, and Group 11 elements such as silver and gold may be used singly.
- alloys or compounds include tin-based materials such as Ag--Sn alloys, Cu--Sn alloys and Co--Sn alloys, carbon-based materials such as various types of graphite, and SiO x ( 0.3 ⁇ x ⁇ 1.6), silicon alone, or a composite of a silicon-based material and a carbon-based material.
- the ratio of the negative electrode active material in the negative electrode active material layer is in the range of 70 to 99% by mass, in the range of 80 to 98.5% by mass, in the range of 90 to 98% by mass, and in the range of 95 to 97.5% by mass.
- the inside can be exemplified.
- the negative electrode active material layer may contain additives such as binders and dispersants in addition to the negative electrode active material.
- additives such as binders and dispersants in addition to the negative electrode active material.
- the binder the one described for the positive electrode may be appropriately adopted.
- additives such as dispersants can be employed.
- the blending amount of the binder is not particularly limited.
- the proportion of the binder in the negative electrode active material layer is preferably in the range of 0.5 to 7% by mass, more preferably in the range of 1 to 5% by mass, and even more preferably in the range of 2 to 4% by mass.
- a conventionally known method such as a roll coating method, a die coating method, a dip coating method, a doctor blade method, a spray coating method, a curtain coating method, etc. is used to form a current collector.
- the active material may be applied to the surface of the body. Specifically, an active material, a solvent, and, if necessary, a binder and a conductive aid are mixed to produce a slurry composition for forming an active material layer, and the composition for forming an active material layer is collected. After coating on the surface of the electric body, it is dried.
- solvents include N-methyl-2-pyrrolidone, methanol, methyl isobutyl ketone, and water. In order to increase the electrode density, it may be compressed after drying.
- the active material layer may be formed using a manufacturing method disclosed in Japanese Patent Application Laid-Open No. 2015-201318. Specifically, a wet granule is obtained by granulating a mixture containing an active material, a binder, and a solvent. An aggregate of the granules is placed in a predetermined mold to obtain a flat molded body. After that, a transfer roll is used to adhere a flat plate-like molded body to the surface of the current collector, thereby forming an active material layer.
- an olivine structure can be selected as the positive electrode active material, and graphite can be selected as the negative electrode active material.
- a lithium ion secondary battery comprising a positive electrode comprising a positive electrode active material having an olivine structure and a negative electrode comprising graphite as a negative electrode active material can be said to have excellent thermal stability, but the capacity per unit volume of the electrode is low.
- the mass of the positive electrode active material layer existing on the area of 1 square centimeter on one side of the current collector foil of the positive electrode (hereinafter referred to as "positive weight basis weight )
- the mass of the negative electrode active material layer present on the area of 1 square centimeter on one side of the current collector foil of the negative electrode (hereinafter sometimes referred to as “the basis weight of the negative electrode”) increases. .
- the basis weight of the positive electrode is preferably 20 mg/cm 2 or more. Suitable positive electrode weight per unit area is 30 to 200 mg/cm 2 , 35 to 150 mg/cm 2 , 40 to 120 mg/cm 2 , and 50 to 100 mg/cm 2 . .
- the basis weight of the negative electrode is preferably 10 mg/cm 2 or more.
- suitable coating weight of the negative electrode are 15 to 100 mg/cm 2 , 17 to 75 mg/cm 2 , 20 to 60 mg/cm 2 , and 25 to 50 mg/cm 2 . .
- the charge / discharge capacity at a high rate is higher than the charge / discharge capacity at a low rate. If it becomes insufficient, a rate characteristic deterioration phenomenon occurs.
- the phenomenon of rate deterioration is believed to be related to the diffusion resistance of lithium ions in the lithium ion secondary battery, and the diffusion resistance of lithium ions is believed to be related to the viscosity of the electrolyte and the diffusion coefficient of lithium ions in the electrolyte. .
- the electrolytic solution of the present invention has a low viscosity due to the presence of methyl propionate, and is designed in consideration of the diffusion coefficient of lithium ions. Therefore, in the lithium ion secondary battery of the present invention, for example, the positive electrode is in the range of 30 to 200 mg/cm 2 and the negative electrode is in the range of 15 to 100 mg/cm 2 . The property deterioration phenomenon is suppressed to some extent.
- the lithium ion secondary battery of the present invention is not a so-called wound type lithium ion secondary battery in which the electrodes are wound and stored in a container, but a so-called cell stack type lithium ion secondary battery in which the electrodes are maintained in a stacked state without being wound.
- the lithium ion secondary battery is suitable as a lithium ion secondary battery having thick electrodes.
- a positive electrode active material layer is provided on one side of the first current collector, and a negative electrode active material is provided on one side of the second current collector.
- a positive electrode active material layer or a negative electrode active material layer may be provided on the other surface of the first current collector.
- a positive electrode active material layer or a negative electrode active material layer may be provided on the other surface of the second current collector.
- the other surface of the first current collector having one surface provided with the positive electrode active material layer and the other surface of the second current collector having one surface provided with the negative electrode active material layer were superimposed to integrate them.
- the first current collector and the second current collector in this case can be regarded as a current collector having a multilayer structure, which will be described later.
- the positive electrode active material layer is provided on one side (or the other side) of the two-layer current collector in which the first current collector and the second current collector are integrated, and the other side (or one side) is provided with a negative electrode active material layer.
- the electrode in the lithium ion secondary battery of the present invention has the same type of active material layer on each of both sides of the current collector, that is, the positive electrode active material layer and the positive electrode active material layer, or the negative electrode active material layer and the negative electrode active material layer. It may be an electrode provided with a layer.
- the electrode in the lithium ion secondary battery of the present invention is a bipolar electrode in which different active material layers, that is, a positive electrode active material layer and a negative electrode active material layer are provided on both sides of a current collector. It can be.
- the positive electrode and the negative electrode are stacked with the negative electrode active material layer facing the positive electrode active material layer, and in the direction in which the negative electrode active material layer and the positive electrode active material layer face each other (hereinafter referred to as the facing direction), A separator is arranged between the positive electrode active material layer and the negative electrode active material layer.
- Each of the first current collector and the second current collector may have a single-layer structure composed of a single metal, or may have a multi-layer structure composed of a plurality of dissimilar metals. Also, a multilayer structure in which the first current collector and the second current collector are laminated and integrated may be formed.
- the first current collector and the second current collector have the multilayer structure, for example, the first current collector (or the second current collector) is plated with the second current collector (or the first current collector).
- the second current collector (or the first current collector) may be roll-bonded to the first current collector (or the second current collector).
- the separately molded first current collector and second current collector may be joined together with a conductive adhesive or the like to be integrated.
- a metal foil obtained by plating an aluminum foil with copper or nickel is exemplified.
- the separator has a function of separating the positive electrode and the negative electrode and allowing lithium ions to pass therethrough while preventing a short circuit due to contact between the two electrodes.
- a known one may be adopted, and synthetic resins such as polytetrafluoroethylene, polypropylene, polyethylene, polyimide, polyamide, polyaramid (aromatic polyamide), polyester, polyacrylonitrile, polysaccharides such as cellulose and amylose, and fibroin. , natural polymers such as keratin, lignin and suberin, and porous bodies, non-woven fabrics, and woven fabrics using one or a plurality of electrically insulating materials such as ceramics.
- the separator may have a multilayer structure.
- the positive electrode active material layer and the negative electrode active material layer face each other.
- the positive electrode active material layer is formed on one surface of the first current collector
- the negative electrode active material layer is formed on one surface of the second current collector. disposed between the body and the second current collector.
- the lithium-ion secondary battery of the present invention includes a sealing portion between the first current collector and the second current collector in the facing direction. The sealing portion surrounds the positive electrode active material layer and the negative electrode active material layer, and has a function of sealing the electrolytic solution in the space between the first current collector and the second current collector.
- the lithium ion secondary battery of the present invention a storage cell having a positive electrode active material layer, a separator, a negative electrode active material layer and an electrolytic solution between a pair of first and second current collectors. is formed, and the storage cell is separated from the outside world by the seal.
- the lithium ion secondary battery of the present invention may include only one storage cell, or may include a plurality of storage cells.
- the lithium-ion secondary battery of the present invention having the structure described above can reduce the size and weight of the container, and reduce wiring such as lead wires. As a result, the lithium-ion secondary battery of the present invention having the structure described above has an improved energy density per unit volume and weight.
- the lithium ion secondary battery of the present invention having this structure is suitable for being embodied as a cell stack type lithium ion secondary battery, and can have thick electrodes as described above.
- the sealing portion functions as a sealing material for enclosing the electrolytic solution inside the storage cell, and is a spacer for electrically isolating the first current collector and the second current collector that constitute the same storage cell. also functions as The sealing portion that exhibits such a function may have a shape capable of accommodating the positive electrode active material layer and the negative electrode active material layer on the inner peripheral side, specifically, a ring shape or a cylindrical shape.
- the material for the sealing portion a material that can exhibit the above functions may be used, and a resin material is suitable.
- Olefins and acid-modified olefins are suitable as the material for the sealing portion, and specific examples thereof include polyethylene, polypropylene, acid-modified polyethylene, and acid-modified polypropylene.
- the sealing portion is preferably fixed to the first current collector and the second current collector by a general method such as adhesion or welding.
- the electrolyte solution is highly reliably sealed between the first current collector and the second current collector that constitute the same cell, and the relative position of the first current collector and the second current collector and the positive electrode This is for appropriately maintaining the relative positions of the active material layer, the separator, and the negative electrode active material layer.
- the method of fixing the sealing portion to the first current collector and the second current collector is not particularly limited, but methods such as adhesion, welding, and fusion bonding can be exemplified.
- the sealing portion may surround the positive electrode active material layer and the negative electrode active material layer, and may be in contact with or apart from the outer edge portion of the positive electrode active material layer and the outer edge portion of the negative electrode active material layer.
- the sealing portion should be the outer edge of the positive electrode active material layer and the negative electrode active material layer. It is preferably spaced from the outer edge of the material layer.
- a specific method for manufacturing a lithium ion secondary battery will be described.
- a positive electrode in which a positive electrode active material layer is formed on one side of a first current collector and a negative electrode in which a negative electrode active material layer is formed on one side of a second current collector are combined into a positive electrode active material layer and a negative electrode active material layer. face each other through a separator.
- a sealing portion is arranged between the first current collector and the second current collector so as to surround the positive electrode active material layer and the negative electrode active material layer, and the sealing portion serves as the first current collector. adheres to the body and the second current collector.
- the electrolytic solution is sealed inside the sealing portion.
- the first sealing portion is arranged between the first current collector and the separator, and the second sealing portion is arranged between the separator and the second current collector. Then, the first sealing portion and the second sealing portion may be integrated by a method such as adhesion.
- the lithium-ion secondary battery of the present invention may be mounted on a vehicle.
- the vehicle may be any vehicle that uses electrical energy from a lithium-ion secondary battery as a power source in whole or in part, and may be, for example, an electric vehicle or a hybrid vehicle.
- a lithium ion secondary battery is mounted on a vehicle, it is preferable to connect a plurality of lithium ion secondary batteries in series to form an assembled battery.
- Devices equipped with lithium ion secondary batteries include, in addition to vehicles, personal computers, mobile communication devices, various home electric appliances driven by batteries, office equipment, industrial equipment, and the like.
- the lithium ion secondary battery of the present invention is used for wind power generation, solar power generation, hydraulic power generation, and other power storage devices and power smoothing devices for power systems, power sources for ships and/or auxiliary equipment, aircraft, power source for spacecraft and/or auxiliary equipment, auxiliary power source for vehicles that do not use electricity as a power source, power source for mobile home robots, power source for system backup, power source for uninterruptible power supply, It may be used as a power storage device that temporarily stores electric power required for charging in a charging station for an electric vehicle.
- Electrolyte solutions 1 to 3 were prepared by dissolving (FSO 2 ) 2 NLi and LiPF 6 in a mixed solvent of ethylene carbonate and methyl propionate at a volume ratio of 30:70 at the ratios shown in Table 1 below.
- the viscosities of electrolyte solutions 1 to 3 were measured under the following conditions. Table 1 shows the results.
- ⁇ Viscosity> The viscosity of each electrolytic solution was measured at 25° C. with a Brookfield viscometer (Brookfield, DV2T) using a cone-shaped spindle. Note that the rotational speed of the cone-shaped spindle was 60 rpm.
- Electrolytes were produced using propyl propionate, methyl butyrate, and ethyl butyrate as esters with similar chemical structures to methyl propionate, and the effects of these esters on battery characteristics were investigated.
- a mother liquor was prepared by dissolving LiPF 6 at a concentration of 1.2 mol/L in a mixed solvent of ethylene carbonate and methyl propionate at a volume ratio of 15:85.
- LiDFOB Lithium difluoro (oxalate) borate
- LiDFOB is one aspect of oxalate borate) and an amount equivalent to 1% by mass of the mother liquor
- Electrolytic solution 4 was produced by adding and dissolving vinylene carbonate.
- PP ethylene carbonate and propyl propionate
- LiPF6 was dissolved at a concentration of 1.2 mol/L to obtain a mother liquor.
- Electrolytic solution 5 was prepared by adding LiDFOB in an amount corresponding to 1% by mass and vinylene carbonate in an amount corresponding to 1% by mass to the mother liquor and dissolving them.
- LiPF 6 was dissolved at a concentration of 1.2 mol/L in a mixed solvent of ethylene carbonate and methyl butyrate (hereinafter sometimes abbreviated as MB) at a volume ratio of 15:85 to obtain a mother liquor.
- Electrolytic solution 6 was prepared by adding and dissolving LiDFOB in an amount corresponding to 1% by mass and vinylene carbonate in an amount corresponding to 1% by mass with respect to the mother liquor.
- EB a mixed solvent of ethylene carbonate and ethyl butyrate
- Electrolytic solution 7 was prepared by adding LiDFOB in an amount corresponding to 1% by mass and vinylene carbonate in an amount corresponding to 1% by mass to the mother liquor and dissolving them.
- - Ethylene carbonate, ethyl methyl carbonate and dimethyl carbonate were mixed at a volume ratio of 30:30:40 to prepare a mixed solvent.
- LiPF 6 was dissolved in the mixed solvent to prepare a mother liquor having a LiPF 6 concentration of 1 mol/L.
- Electrolytic solution 8 was prepared by adding LiDFOB in an amount corresponding to 0.2 mol/L and vinylene carbonate in an amount corresponding to 1% by mass to the mother liquor and dissolving them.
- LiFePO 4 having an olivine structure coated with carbon as the positive electrode active material, acetylene black as the conductive aid, and polyvinylidene fluoride as the binder, the mass ratio of the positive electrode active material, conductive aid, and binder being 90:5: 5, and N-methyl-2-pyrrolidone was added as a solvent to prepare a composition for forming a positive electrode active material layer in slurry form.
- An aluminum foil was prepared as a positive electrode current collector.
- a positive electrode active material layer is formed on the surface of the aluminum foil by pressing the positive electrode precursor produced by applying the composition for forming the positive electrode active material layer in the form of a film on the surface of the aluminum foil and then removing the solvent, in the thickness direction. was formed on the positive electrode.
- the target weight of the positive electrode was 13.9 mg/cm 2 .
- Graphite as a negative electrode active material, carboxymethyl cellulose and styrene-butadiene rubber as binders were mixed so that the mass ratio of graphite, carboxymethyl cellulose and styrene-butadiene rubber was 97:0.8:2.2, and water was used as a solvent. It was added to prepare a slurry composition for forming a negative electrode active material layer.
- a copper foil was prepared as a current collector for the negative electrode.
- a negative electrode active material layer is formed on the surface of the copper foil by pressing the negative electrode precursor produced by applying the negative electrode active material layer forming composition to the surface of the copper foil in the form of a film and then removing the solvent, in the thickness direction. was formed on the negative electrode.
- the target weight of the negative electrode was 6.3 mg/cm 2 .
- a polypropylene porous membrane was prepared as a separator.
- An electrode body was formed by sandwiching a separator between the positive electrode and the negative electrode.
- the lithium ion secondary battery 4 was manufactured by putting this electrode body together with the electrolytic solution 4 in a bag-like laminate film and sealing it. Lithium ion secondary batteries 5 to 8 were similarly produced using electrolyte solutions 5 to 8.
- Lithium ion secondary batteries 4 to 8 were CC-CV charged to 4.0 V at a rate of 0.4 C, and the charge capacity at this time was used as a reference (SOC 100%).
- a storage test was performed by storing each lithium ion secondary battery at 40° C. for 11 days in the state of SOC 100. Capacity confirmation was performed before and after the storage test. Specifically, CC-CV charging was performed at a rate of 0.4C to 4.0V. Then, CC-CV discharge was performed at a rate of 1C to 2.5V. Thereby, the discharge capacity of each lithium ion secondary battery was confirmed.
- the percentage of the discharge capacity after the storage test to the discharge capacity before the storage test was defined as the capacity retention rate of each lithium ion secondary battery. Further, after the storage test, each lithium ion secondary battery adjusted to SOC 60% was discharged at a constant current rate for 5 seconds at 25° C., and the amount of voltage change was measured. The measurements were performed under multiple conditions with varying current rates. From the obtained results, the constant current (mA) at which the discharge time to a voltage of 2.5 V is 10 seconds was calculated for each lithium ion secondary battery with an SOC of 60%. A value obtained by multiplying the amount of voltage change from SOC 60% to 2.5 V by the calculated constant current was taken as the output. Table 2 shows the results of the above storage test.
- the lithium ion secondary battery 4 using methyl propionate as the non-aqueous solvent of the electrolyte is excellent in both capacity retention rate and output, and in particular in output, carbonate-based non-aqueous solvent It greatly exceeds the lithium ion secondary battery 8 using . This result supports the usefulness of selecting methyl propionate as the non-aqueous solvent.
- Example 1 The electrolytic solution and lithium ion secondary battery of Example 1 are described below.
- the lithium ion secondary battery of Example 1 has a general structure, unlike the lithium ion secondary battery of the present invention described above, except that the electrodes are thick.
- Graphite as a negative electrode active material, and carboxymethyl cellulose and styrene-butadiene rubber as binders were mixed so that the mass ratio of graphite, carboxymethyl cellulose, and styrene-butadiene rubber was 94.8:0.8:4.4.
- Water was added to prepare a slurry composition for forming a negative electrode active material layer.
- a copper foil was prepared as a current collector for the negative electrode.
- a negative electrode active material layer is formed on the surface of the copper foil by pressing the negative electrode precursor produced by applying the negative electrode active material layer forming composition to the surface of the copper foil in the form of a film and then removing the solvent, in the thickness direction. was formed on the negative electrode.
- the weight of the negative electrode was 26.5 mg/cm 2 .
- LiFePO 4 having an olivine structure coated with carbon as the positive electrode active material layer, acetylene black as the conductive aid, and polyvinylidene fluoride as the binder, the mass ratio of the positive electrode active material, conductive aid, and binder being 88.8. :5.1:6.1, and N-methyl-2-pyrrolidone was added as a solvent to prepare a composition for forming a positive electrode active material layer in slurry form.
- An aluminum foil was prepared as a positive electrode current collector.
- a positive electrode active material layer is formed on the surface of the aluminum foil by pressing the positive electrode precursor produced by applying the composition for forming the positive electrode active material layer in the form of a film on the surface of the aluminum foil and then removing the solvent, in the thickness direction. was formed on the positive electrode.
- the basis weight of the positive electrode was 55.5 mg/cm 2 .
- a polypropylene porous membrane was prepared as a separator.
- An electrode body was formed by sandwiching a separator between the positive electrode and the negative electrode.
- the lithium ion secondary battery of Example 1 was manufactured by putting this electrode body together with the electrolytic solution of Example 1 into a bag-like laminate film and sealing it.
- Example 2 The electrolytic solution of Example 2 is the same as the electrolytic solution of Example 1 except for the volume ratio of ethylene carbonate and methyl propionate. Specifically, in Example 2, (FSO 2 ) 2 NLi was dissolved at a concentration of 1.2 mol/L in a mixed solvent in which ethylene carbonate and methyl propionate were mixed at a volume ratio of 25:75, and the mixture was used as the mother liquor. did. An electrolytic solution of Example 2 was produced by adding and dissolving vinylene carbonate in an amount corresponding to 1% by mass with respect to the mother liquor.
- the lithium ion secondary battery of Example 2 is the same as the lithium ion secondary battery of Example 1 except that the electrolyte solution of Example 2 is used.
- Comparative Example 1 The electrolytic solution of Comparative Example 1 is the same as the electrolytic solution of Example 1 except for the volume ratio of ethylene carbonate and methyl propionate. Specifically, in Comparative Example 1, (FSO 2 ) 2 NLi was dissolved at a concentration of 1.2 mol/L in a mixed solvent in which ethylene carbonate and methyl propionate were mixed at a volume ratio of 30:70. did. An electrolytic solution of Comparative Example 1 was produced by adding and dissolving vinylene carbonate in an amount corresponding to 1% by mass with respect to the mother liquor. The lithium ion secondary battery of Comparative Example 1 is the same as the lithium ion secondary battery of Example 1 except that the electrolyte solution of Comparative Example 1 is used.
- Comparative example 2 The electrolytic solution of Comparative Example 2 is the same as the electrolytic solution of Example 1 except for the volume ratio of ethylene carbonate and methyl propionate. Specifically, in Comparative Example 2, (FSO 2 ) 2 NLi was dissolved at a concentration of 1.2 mol/L in a mixed solvent in which ethylene carbonate and methyl propionate were mixed at a volume ratio of 50:50. did. An electrolytic solution of Comparative Example 2 was produced by adding and dissolving vinylene carbonate in an amount corresponding to 1% by mass with respect to the mother liquor. The lithium ion secondary battery of Comparative Example 2 is the same as the lithium ion secondary battery of Example 1 except that the electrolyte solution of Comparative Example 2 is used.
- the lithium-ion secondary batteries of Examples 1 and 2 have all C rates of 0.4C to 4.0C compared to the lithium-ion secondary batteries of Comparative Examples 1 and 2. and exhibits excellent discharge characteristics. This is probably because the electrolyte in the lithium ion secondary batteries of Examples 1 and 2 contained 75% by volume or more of methyl propionate in the non-aqueous solvent.
- the lithium ion secondary battery of Example 1 has a structure different from that of the lithium ion secondary battery of the present invention described above, but needless to say, the effect of the electrolytic solution of Example 1 is the same as that of the lithium ion secondary battery of the present invention. The same effect is exhibited in the next battery. The same applies to each of the following examples.
- Example 3 The electrolytic solution and lithium ion secondary battery of Example 3 are described below.
- [Electrolyte] (FSO 2 ) 2 NLi was dissolved at a concentration of 1.2 mol/L in a mixed solvent of ethylene carbonate and methyl propionate at a volume ratio of 15:85 to obtain a mother liquor.
- 1,3,2-dioxathiolane-2,2-dioxide hereinafter sometimes abbreviated as DTD.
- DTD is one aspect of a cyclic sulfate ester) in an amount equivalent to 0.5% by mass relative to the mother liquor. was added and dissolved to prepare an electrolytic solution of Example 3.
- Graphite as a negative electrode active material, carboxymethyl cellulose and styrene-butadiene rubber as binders were mixed so that the mass ratio of graphite, carboxymethyl cellulose and styrene-butadiene rubber was 97:0.8:2.2, and water was used as a solvent. It was added to prepare a slurry composition for forming a negative electrode active material layer.
- a copper foil was prepared as a current collector for the negative electrode.
- a negative electrode active material layer is formed on the surface of the copper foil by pressing the negative electrode precursor produced by applying the negative electrode active material layer forming composition to the surface of the copper foil in the form of a film and then removing the solvent, in the thickness direction. was formed on the negative electrode.
- the weight of the negative electrode was 6.7 mg/cm 2 .
- LiFePO 4 having an olivine structure coated with carbon as the positive electrode active material layer, acetylene black as the conductive aid, and polyvinylidene fluoride as the binder, the mass ratio of the positive electrode active material, conductive aid and binder being 94:3. : 3, and N-methyl-2-pyrrolidone was added as a solvent to prepare a composition for forming a positive electrode active material layer in slurry form.
- An aluminum foil was prepared as a positive electrode current collector.
- a positive electrode active material layer is formed on the surface of the aluminum foil by pressing the positive electrode precursor produced by applying the composition for forming the positive electrode active material layer in the form of a film on the surface of the aluminum foil and then removing the solvent, in the thickness direction. was formed on the positive electrode.
- the basis weight of the positive electrode was 13.9 mg/cm 2 .
- a lithium-ion secondary battery of Example 3 was manufactured in the same manner as in Example 1 using the above positive electrode and negative electrode.
- Example 4 The electrolytic solution of Example 4 is the same as the electrolytic solution of Example 3 except that (FSO 2 ) 2 NLi and LiPF 6 are used as lithium salts. Specifically, in Example 4, (FSO 2 ) 2 NLi at a concentration of 0.6 mol/L and LiPF 6 at a concentration of 0.6 mol/L were added to a mixed solvent of ethylene carbonate and methyl propionate at a volume ratio of 15:85 It was dissolved at 0.6 mol/L to obtain a mother liquor. An electrolytic solution of Example 4 was produced by adding and dissolving DTD in an amount corresponding to 0.5% by mass with respect to the mother liquor.
- the lithium ion secondary battery of Example 4 is the same as the lithium ion secondary battery of Example 3 except that the electrolyte solution of Example 4 is used.
- Comparative Example 3 The electrolytic solution of Comparative Example 3 is the same as the electrolytic solution of Example 3 except that (FSO 2 ) 2 NLi and LiPF 6 are used as lithium salts. Specifically, in Comparative Example 3, LiPF 6 was dissolved at a concentration of 1.2 mol/L in a mixed solvent of ethylene carbonate and methyl propionate at a volume ratio of 15:85 to obtain a mother liquor. By adding (FSO 2 ) 2 NLi in an amount corresponding to 0.5% by mass and DTD in an amount corresponding to 0.5% by mass to the mother liquor and dissolving, the electrolytic solution of Comparative Example 3 was obtained. manufactured.
- the amount of (FSO 2 ) 2 NLi in the electrolytic solution of Comparative Example 3 was 0.03 mol/L, and the amount of (FSO 2 ) 2 NLi was the sum of (FSO 2 ) 2 NLi and LiPF 6 was 2.4 mol % with respect to
- the lithium ion secondary battery of Comparative Example 3 is the same as the lithium ion secondary battery of Example 3 except that the electrolytic solution of Comparative Example 3 was used.
- Comparative Example 4 The electrolytic solution of Comparative Example 3 is the same as the electrolytic solution of Example 3 except that only LiPF 6 was used as the lithium salt. Specifically, in Comparative Example 3, LiPF 6 was dissolved at a concentration of 1.2 mol/L in a mixed solvent of ethylene carbonate and methyl propionate at a volume ratio of 15:85 to obtain a mother liquor. An electrolytic solution of Comparative Example 4 was produced by adding and dissolving DTD in an amount corresponding to 0.5% by mass with respect to the mother liquor. The lithium ion secondary battery of Comparative Example 4 is the same as the lithium ion secondary battery of Example 3 except that the electrolyte solution of Comparative Example 4 is used.
- each lithium ion secondary battery adjusted to SOC 60% was discharged at a constant current rate for 10 seconds at 25° C., and the amount of change in voltage was measured. The measurements were performed under multiple conditions with varying current rates. From the obtained results, the constant current (mA) at which the discharge time to a voltage of 2.5 V is 10 seconds was calculated for each lithium ion secondary battery with an SOC of 60%. The initial output was obtained by multiplying the amount of voltage change from SOC 60% to 2.5 V by the calculated constant current (mA). Multiple tests of initial output were also performed. The percentage of the output value of each lithium ion secondary battery relative to the output value of the lithium ion secondary battery of Comparative Example 4 was calculated, and the value obtained by subtracting 100 (%) from the percentage was defined as the initial output increase rate (%). .
- Table 4 shows the initial output of each lithium ion secondary battery
- Example 4 compared to the lithium ion secondary battery of Comparative Example 4 in which LiPF 6 was used alone as the lithium salt, Comparative Example 3, Example 3 and Example 3 containing (FSO 2 ) 2 NLi as the lithium salt
- the lithium ion secondary battery of Example 4 is excellent in initial output.
- the (FSO 2 ) 2 NLi content is 30 mol % or more
- the (FSO 2 ) 2 NLi content is less than 30 mol %.
- the initial output is significantly increased.
- the lithium-ion secondary battery of Comparative Example 3 was comparable to Comparative Example 4 in charge-discharge cycle durability at high temperatures.
- the lithium ion secondary batteries of Comparative Examples 3 and 4 were also superior in charge/discharge cycle durability at high temperatures. From these results, (FSO 2 ) 2 NLi, that is, using an electrolytic solution containing 30 mol % or more of a lithium salt other than LiPF 6 with respect to the total lithium salt, the endurance of the lithium ion secondary battery, especially at high temperatures, was improved. It is confirmed that the quality is improved.
- the lithium ion secondary batteries of Examples 3 and 4 are superior to the lithium ion secondary batteries of Comparative Examples 3 and 4 in durability during storage at 40°C. was This result also confirms that the durability of lithium-ion secondary batteries, especially at high temperatures, is improved by using an electrolytic solution containing 30 mol% or more of a lithium salt other than LiPF 6 with respect to the total lithium salt. .
- Example 5 Using the same electrolytic solution of Example 5 as the electrolytic solution of Example 3, a lithium ion secondary battery of Example 5 was manufactured as follows.
- the electrolytic solution of Example 5 was prepared by dissolving (FSO 2 ) 2 NLi at a concentration of 1.2 mol/L in a mixed solvent of ethylene carbonate and methyl propionate at a volume ratio of 15:85. , and DTD in an amount corresponding to 0.5% by mass of the mother liquor is added and dissolved.
- Graphite as a negative electrode active material, carboxymethyl cellulose and styrene-butadiene rubber as binders were mixed so that the mass ratio of graphite, carboxymethyl cellulose and styrene-butadiene rubber was 97:0.8:2.2, and water was used as a solvent. It was added to prepare a slurry composition for forming a negative electrode active material layer.
- a copper foil was prepared as a current collector for the negative electrode.
- a negative electrode active material layer is formed on the surface of the copper foil by pressing the negative electrode precursor produced by applying the negative electrode active material layer forming composition to the surface of the copper foil in the form of a film and then removing the solvent, in the thickness direction. was formed on the negative electrode.
- the weight of the negative electrode was 6.24 mg/cm 2 .
- An aluminum foil was prepared as a positive electrode current collector.
- a positive electrode active material layer is formed on the surface of the aluminum foil by pressing the positive electrode precursor produced by applying the composition for forming the positive electrode active material layer in the form of a film on the surface of the aluminum foil and then removing the solvent, in the thickness direction. was formed on the positive electrode.
- the basis weight of the positive electrode was 13.87 mg/cm 2 .
- a lithium-ion secondary battery of Example 5 was manufactured in the same manner as in Example 1 using the above positive electrode and negative electrode.
- Comparative Example 5 A lithium ion secondary battery of Comparative Example 5 was manufactured in the same manner as in Example 5 using the same electrolytic solution of Comparative Example 5 as the electrolytic solution of Comparative Example 4.
- the electrolytic solution of Comparative Example 5 was prepared by dissolving LiPF 6 at a concentration of 1.2 mol/L in a mixed solvent of ethylene carbonate and methyl propionate at a volume ratio of 15:85 to obtain a mother liquor. DTD was added and dissolved in an amount corresponding to 0.5% by mass with respect to the
- Example 4 Evaluation of gas generation>
- the lithium ion secondary batteries of Example 5 and Comparative Example 5 were activated by charging at 0.05 C to 4.0 V and holding at 60° C. for 20 hours.
- the volume of each lithium ion secondary battery before and after activation was measured by the Archimedes method, and the amount of gas ( ⁇ L) generated by activation was calculated from the volume change of each lithium ion secondary battery before and after activation.
- the amount of gas generated in the lithium ion secondary battery of Example 5 is smaller than that in the lithium ion secondary battery of Comparative Example 5. From this result, it can be seen that gas generated during charging and discharging of the lithium ion secondary battery can be suppressed by using an electrolytic solution containing 30 mol % or more of lithium salt other than LiPF 6 with respect to the total lithium salt. In a lithium ion secondary battery using graphite for the negative electrode, it is considered that gas is generated by decomposition of the electrolytic solution at the negative electrode. In the lithium ion secondary battery of Example 5, (FSO 2 ) 2 NLi contained in the electrolytic solution is decomposed, so that a good film is formed on the surface of the negative electrode.
- Example 6 A lithium ion secondary battery of Example 6 was manufactured in the same manner as in Example 3 using the same electrolyte solution of Example 6 as that of Example 3.
- the electrolytic solution of Example 6 was prepared by dissolving (FSO 2 ) 2 NLi at a concentration of 1.2 mol/L in a mixed solvent of ethylene carbonate and methyl propionate at a volume ratio of 15:85. , and DTD in an amount corresponding to 0.5% by mass of the mother liquor is added and dissolved.
- Example 7 A lithium ion secondary battery of Example 7 was manufactured in the same manner as in Example 3 using the same electrolyte solution of Example 7 as that of Example 4.
- the electrolytic solution of Example 7 was prepared by adding (FSO 2 ) 2 NLi at a concentration of 0.6 mol/L and LiPF 6 to a mixed solvent of ethylene carbonate and methyl propionate at a volume ratio of 15:85. It was dissolved at a concentration of 0.6 mol/L to obtain a mother liquor, and DTD was added and dissolved in an amount corresponding to 0.5% by mass with respect to the mother liquor.
- Comparative Example 6 A lithium ion secondary battery of Comparative Example 6 was manufactured in the same manner as in Example 3 using the same electrolytic solution of Comparative Example 6 as the electrolytic solution of Comparative Example 3.
- the electrolytic solution of Comparative Example 6 was prepared by dissolving LiPF 6 at a concentration of 1.2 mol/L in a mixed solvent of ethylene carbonate and methyl propionate at a volume ratio of 15:85 to obtain a mother liquor.
- FSO 2 ) 2 NLi in an amount corresponding to 0.5% by mass and DTD in an amount corresponding to 0.5% by mass are added and dissolved.
- the amount of (FSO 2 ) 2 NLi in the electrolytic solution of Comparative Example 6 is 0.03 mol/L, and the amount of (FSO 2 ) 2 NLi is the total of (FSO 2 ) 2 NLi and LiPF 6 It was 2.4 mol %.
- Comparative Example 7 A lithium ion secondary battery of Comparative Example 7 was manufactured in the same manner as in Example 3 using the same electrolytic solution of Comparative Example 7 as that of Comparative Example 4.
- the electrolytic solution of Comparative Example 7 was prepared by dissolving LiPF 6 at a concentration of 1.2 mol/L in a mixed solvent of ethylene carbonate and methyl propionate at a volume ratio of 15:85 to obtain a mother liquor. DTD was added and dissolved in an amount corresponding to 0.5% by mass with respect to the
- the lithium ion secondary batteries of Examples 6 and 7 were superior to the lithium ion secondary batteries of Comparative Examples 6 and 7 in capacity retention rate. This result also confirms that the durability of lithium-ion secondary batteries, especially at high temperatures, is improved by using an electrolytic solution containing 30 mol% or more of a lithium salt other than LiPF 6 with respect to the total lithium salt. .
- the lithium ion secondary batteries of Examples 6 and 7 were superior to the lithium ion secondary batteries of Comparative Examples 6 and 7 in capacity retention rate, and after the start of the storage test, The difference was more pronounced as the number of days elapsed was longer. This result also confirms that the durability of lithium-ion secondary batteries, especially at high temperatures, is improved by using an electrolytic solution containing 30 mol% or more of a lithium salt other than LiPF 6 with respect to the total lithium salt. .
- Example 8 (FSO 2 ) 2 NLi was dissolved at a concentration of 1.2 mol/L in a mixed solvent of ethylene carbonate and methyl propionate at a volume ratio of 15:85 to obtain a mother liquor.
- An electrolytic solution of Example 8 was produced by adding and dissolving vinylene carbonate in an amount corresponding to 1.94% by mass with respect to the mother liquor.
- a lithium ion secondary battery of Example 8 was manufactured in the same manner as in Example 1 using the electrolyte solution of Example 8.
- Comparative Example 8 (FSO 2 ) 2 NLi was dissolved at a concentration of 1.2 mol/L in a mixed solvent of ethylene carbonate and dimethyl carbonate at a volume ratio of 15:85 to obtain a mother liquor.
- An electrolytic solution of Comparative Example 8 was produced by adding and dissolving vinylene carbonate in an amount corresponding to 1.94% by mass with respect to the mother liquor.
- a lithium ion secondary battery of Comparative Example 8 was manufactured in the same manner as in Example 1 using the electrolytic solution of Comparative Example 8.
- Comparative Example 9 (FSO 2 ) 2 NLi was dissolved at a concentration of 1.2 mol/L in a mixed solvent of ethylene carbonate, dimethyl carbonate, and ethyl methyl carbonate at a volume ratio of 15:65:20 to obtain a mother liquor.
- An electrolytic solution of Comparative Example 9 was produced by adding and dissolving vinylene carbonate in an amount corresponding to 1.94% by mass with respect to the mother liquor.
- a lithium ion secondary battery of Comparative Example 9 was manufactured in the same manner as in Example 1 using the electrolytic solution of Comparative Example 9.
- Comparative Example 10 (FSO 2 ) 2 NLi was dissolved at a concentration of 1.2 mol/L in a mixed solvent of ethylene carbonate, dimethyl carbonate and ethyl methyl carbonate at a volume ratio of 15:45:40 to obtain a mother liquor.
- An electrolytic solution of Comparative Example 10 was produced by adding and dissolving vinylene carbonate in an amount corresponding to 1.94% by mass with respect to the mother liquor.
- a lithium ion secondary battery of Comparative Example 10 was manufactured in the same manner as in Example 1 using the electrolytic solution of Comparative Example 10.
- Comparative Example 11 A mother liquor was prepared by dissolving LiPF 6 at a concentration of 1.2 mol/L in a mixed solvent in which ethylene carbonate and methyl propionate were mixed at a volume ratio of 15:85.
- An electrolytic solution of Comparative Example 11 was produced by adding and dissolving vinylene carbonate in an amount corresponding to 1% by mass and LiDFOB in an amount corresponding to 1% by mass with respect to the mother liquor.
- a lithium ion secondary battery of Comparative Example 8 was produced in the same manner as in Example 1.
- Example 7 Long-Term Discharge Test 1>
- FIG. 1 is a graph showing changes in CC discharge capacity over time in each of the lithium ion secondary batteries of Example 8 and Comparative Example 11.
- Table 10 shows the rate % of each lithium ion secondary battery of Example 8 and Comparative Example 11.
- the lithium ion secondary battery of Example 8 has a significantly higher capacity at the end of discharge than the ion secondary battery of Comparative Example 11, and can be said to be excellent in long-term discharge characteristics. Further, as shown in Table 10, the lithium ion secondary battery of Example 8 is significantly superior to the lithium ion secondary battery of Comparative Example 11 in rate % at the end of discharge.
- FIG. 2 shows a graph showing changes in CC discharge capacity over time in each of the lithium-ion secondary batteries of Example 8 and Comparative Examples 8-10.
- Table 11 shows the rate % of each lithium ion secondary battery of Example 8 and Comparative Examples 8-10.
- the lithium ion secondary battery of Example 8 has a significantly higher capacity at the end of discharge than the lithium ion secondary batteries of Comparative Examples 8 to 10, and has excellent long-term discharge characteristics. It can be said that there is
- the lithium ion secondary battery of Example 8 is also significantly superior to the lithium ion secondary batteries of Comparative Examples 8 to 10 in terms of rate % at the end of discharge. This result also reveals that the lithium ion secondary battery of Example 8 is excellent in long-term discharge characteristics.
- the reason why the lithium ion secondary battery of Example 8 is superior to the lithium ion secondary batteries of Comparative Examples 8 to 10 in long-term discharge characteristics is considered to be the composition of the non-aqueous electrolyte. This result further clarifies the usefulness of using methyl propionate in the non-aqueous electrolyte. Furthermore, from the results of Evaluation Examples 7 and 8, it was found that by using (FSO 2 ) 2 NLi as a lithium salt in combination with methyl propionate as a non-aqueous solvent, the battery characteristics for a lithium ion secondary battery were improved. It can be said that an electrolytic solution that can contribute to improvement is obtained.
- Example 9 The lithium ion secondary battery of Example 9 is the lithium ion secondary battery of the present invention.
- FIG. 3 shows an explanatory view schematically showing the lithium ion secondary battery of Example 9. As shown in FIG. The lithium ion secondary battery of Example 9 will be described below with reference to FIG.
- the lithium ion secondary battery 1 of Example 9 is obtained by stacking two storage cells 10 .
- Each storage cell 10 has a positive electrode 2 , a negative electrode 3 , a separator 4 , a sealing portion 5 and an electrolytic solution 6 .
- the positive electrode 2 has a first current collector 20 and a positive electrode active material layer 21 .
- the first current collector 20 is an aluminum foil and has one side 20a and the other side 20b.
- the one surface 20a and the other surface 20b are in a back-to-back relationship.
- the positive electrode active material layer 21 is the same as the positive electrode active material layer in the lithium ion secondary battery of Example 1, and is laminated on the central portion of the one surface 20 a of the first current collector 20 .
- the negative electrode 3 has a second current collector 30 and a negative electrode active material layer 31 .
- the second current collector 30 is a copper foil and has one side 30a and the other side 30b. The one surface 30a and the other surface 30b are in a back-to-back relationship.
- the negative electrode active material layer 31 is the same as the negative electrode active material layer in the lithium ion secondary battery of Example 1, and is laminated on the central portion of the one surface 30 a of the second current collector 30 .
- the negative electrode 3 is stacked on the positive electrode 2 in a state in which the negative electrode active material layer 31 faces the positive electrode active material layer 21 and the separator 4 is sandwiched between the negative electrode active material layer 31 and the positive electrode active material layer 21 .
- the negative electrode active material layer 31, the separator 4, and the positive electrode active material layer 21 are arranged between the first current collector 20 and the second current collector 30 that constitute the same storage cell 10 in the facing direction described above. is sandwiched.
- the separator 4 is the same as the separator in the lithium ion secondary battery of Example 1.
- the sealing part 5 is made of acid-modified olefin and has a substantially short cylindrical shape. Between the first current collector 20 and the second current collector 30, the sealing portion 5 is arranged to extend around the laminate of the negative electrode active material layer 31, the separator 4, and the positive electrode active material layer 21 in the circumferential direction of the laminate. It surrounds all around. The surface of the sealing portion 5 on the side of the first current collector 20 is heat-sealed to the first current collector 20, and the surface on the side of the second current collector 30 is heat-sealed to the second current collector 30. ing. Thereby, the sealing portion 5 liquid-tightly seals between the first current collector 20 and the second current collector 30 . The sealing portion 5 also functions as a spacer for maintaining the distance between the first current collector 20 and the second current collector 30 and breaking the direct electrical connection between the two.
- the electrolytic solution 6 is sealed in the space defined by the sealing portion 5 , the first current collector 20 and the second current collector 30 .
- the electrolytic solution 6 is the same as the electrolytic solution of the first embodiment.
- the lithium-ion secondary battery 1 of Example 9 has two storage cells 10 connected in series. Also, the first current collector 20x of one storage cell 10x and the second current collector 30y of the adjacent storage cell 10y are overlapped and directly electrically connected. Therefore, the first current collector 20x and the second current collector 30y can be regarded as one current collector having a two-layer structure, and the current collector has a cathode active material layer 21x on each of both surfaces thereof. and the negative electrode active material layer 31y can be regarded as a bipolar electrode.
- the positive electrode 2 and the negative electrode 3 are manufactured by the method described in Example 1.
- the sealing portion 5 is integrated with one of the positive electrode 2 and the negative electrode 3 .
- the sealing portion 5 is heat-sealed to one of the first current collector 20 and the second current collector 30 using an impulse sealing machine.
- the sealing portion 5 is integrated with the first current collector 20 or the second current collector 30 in a box shape, and the electrolytic solution 6 can be accommodated therein.
- the electrolytic solution 6 is injected into the inside of the sealing portion 5, and the other of the positive electrode 2 and the negative electrode 3 is integrated with this.
- the positive electrode active material layer 21 and the negative electrode active material layer 31 face each other and are stacked while sandwiching the separator 4 and the sealing portion 5 therebetween. Then, the sealing portion 5 is heat-sealed to the other of the first current collector 20 and the second current collector 30 using an impulse sealing machine.
- the storage cell 10 in the lithium ion secondary battery 1 of Example 9 was obtained. Two storage cells 10 are prepared, and a first current collector 20x of the first storage cell 10x, which is one of the storage cells 10, and a second current collector 30y of the second storage cell 10y, which is the other of the storage cells 10. are superimposed in the facing direction to stack the first current collector 20x and the second current collector 30y.
- the two stacked storage cells 10 are restrained by a restraining member (not shown), and a predetermined restraining load is applied to each storage cell 10 in the facing direction. Thereby, the first storage cell 10x and the second storage cell 10y can be maintained in a stacked state. Further, terminals (not shown) are fixed to the storage cells 10x and 10y. Thus, a lithium ion secondary battery of Example 9 was obtained.
- the sealing portion 5 is fixed to the first current collector 20 and the second current collector 30 . Therefore, each storage cell 10 can stably hold the electrolytic solution 6 therein.
- the lithium ion secondary battery of Example 10 is the lithium ion secondary battery of the present invention, and is substantially the same as the lithium ion secondary battery of Example 9 except for the structures of the first current collector and the second current collector. be.
- FIG. 4 is an explanatory view schematically showing the lithium ion secondary battery of Example 10. As shown in FIG. The lithium ion secondary battery of Example 10 will be described below with reference to FIG.
- the first current collector 20 of the positive electrode 2 and the second current collector 30 of the negative electrode 3 in the lithium ion secondary battery of Example 10 are integrally formed.
- the first current collector 20 is an aluminum foil
- the second current collector 30 is a copper-plated layer formed on the first current collector 20 .
- the lithium ion secondary battery of Example 10 also has excellent durability.
- Example 11 An electrolytic solution of Example 11 was prepared by dissolving (FSO 2 ) 2 NLi at a concentration of 1.2 mol/L in a mixed solvent of ethylene carbonate and methyl propionate at a volume ratio of 30:70.
- Example 12 (FSO 2 ) 2 NLi at a concentration of 1 mol/L and LiPF 6 at a concentration of 0.2 mol/L were dissolved in a mixed solvent in which ethylene carbonate and methyl propionate were mixed at a volume ratio of 30:70. A liquid was produced.
- the amount of (FSO 2 ) 2 NLi with respect to the total lithium salt is about 83.3 mol %.
- Example 13 (FSO 2 ) 2 NLi at a concentration of 0.8 mol/L and LiPF 6 at a concentration of 0.4 mol/L were dissolved in a mixed solvent of ethylene carbonate and methyl propionate at a volume ratio of 30:70. was manufactured.
- the amount of (FSO 2 ) 2 NLi with respect to the total lithium salt is about 66.7 mol %.
- Example 14 (FSO 2 ) 2 NLi at a concentration of 0.6 mol/L and LiPF 6 at a concentration of 0.6 mol/L were dissolved in a mixed solvent of ethylene carbonate and methyl propionate at a volume ratio of 30:70. was manufactured.
- the amount of (FSO 2 ) 2 NLi with respect to the total lithium salt is about 50 mol %.
- Example 15 (FSO 2 ) 2 NLi at a concentration of 0.4 mol/L and LiPF 6 at a concentration of 0.8 mol/L were dissolved in a mixed solvent of ethylene carbonate and methyl propionate at a volume ratio of 30:70. was manufactured.
- the amount of (FSO 2 ) 2 NLi with respect to the total lithium salt is about 33.3 mol %.
- Comparative Example 13 An electrolytic solution of Comparative Example 13 was prepared by dissolving LiPF 6 at a concentration of 1.2 mol/L in a mixed solvent of ethylene carbonate and methyl propionate at a volume ratio of 30:70.
- a test piece was prepared by heat-sealing an aluminum foil and a copper foil with a sealing material.
- the test piece was immersed in each of the electrolytic solutions of Examples 11 to 15 and Comparative Examples 12 and 13 to evaluate peeling.
- the dimensions of each test piece were 15 mm x 65 mm and the seal width was 18 mm.
- the sealing material is an acid-modified olefin-based material, and more specifically contains polyethylene resin with a low melting point.
- An impulse sealer was used for heat sealing, and the heating temperature was 110°C.
- An aluminum laminate film was made into a bag shape of 60 mm ⁇ 100 mm, 1 mL of electrolytic solution was injected into the bag, and a test piece was immersed therein.
- test piece and the electrolytic solution were sealed inside the bag. This was stored at 60°C for 72 hours. After that, the test piece taken out of the bag was washed with ethanol, and the natural peel length generated on the test piece was measured. A groove micrometer was used for the measurement.
- the length from the starting point of peeling between the resin 72 and the aluminum foil 70 to the terminal end of the resin 72 is defined as the natural peeling length L1 at the interface between the aluminum foil 70 and the resin 72 .
- the length from the starting point of peeling between the resin 72 and the copper foil 71 to the terminal end of the resin 72 was defined as the natural peeling length L2 at the interface between the copper foil 71 and the resin 72 .
- L1 and L2 were measured at a plurality of points, the average was calculated, and this was used as the natural peel length of the test piece for each electrolytic solution. The results are shown in FIG.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- Manufacturing & Machinery (AREA)
- Inorganic Chemistry (AREA)
- General Physics & Mathematics (AREA)
- Condensed Matter Physics & Semiconductors (AREA)
- Physics & Mathematics (AREA)
- Materials Engineering (AREA)
- Secondary Cells (AREA)
- Sealing Battery Cases Or Jackets (AREA)
- Battery Electrode And Active Subsutance (AREA)
Abstract
Description
例えば、特許文献1には、エチレンカーボネートとエチルメチルカーボネートを体積比3:7で混合した混合非水溶媒に、LiPF6を1mol/Lの濃度で溶解した電解液が紹介されている。
特許文献2には、エチレンカーボネートとジメチルカーボネートとエチルメチルカーボネートを体積比3:2:5で混合した混合非水溶媒に、LiPF6を1mol/Lの濃度で溶解した電解液が紹介されている。
本発明の発明者は、リチウムイオン二次電池の特性の向上を図る過程において、電解液の非水溶媒にアルキレン環状カーボネートとプロピオン酸メチルとを併用することを見出し、当該電解液を用いたリチウムイオン二次電池を既に出願した(特願2020-026926)。
本発明はかかる事情に鑑みて為されたものであり、リチウムイオン二次電池に優れた特性を付与し得る電解液、および、優れた特性を示すリチウムイオン二次電池を提供することを課題とする。
リチウム塩を含む電解質と、アルキレン環状カーボネートおよびプロピオン酸メチルを含む非水溶媒と、を有し、
前記電解質は、LiPF6以外のリチウム塩をリチウム塩の合計に対して30モル%以上含み、
前記非水溶媒は、前記プロピオン酸メチルを75体積%以上含む、電解液である。
第1集電体と、前記第1集電体の一方面に設けられた正極活物質層と、を有する正極と、
第2集電体と、前記第2集電体の一方面に設けられた負極活物質層と、を有し、前記負極活物質層を前記正極活物質層に対面させつつ前記正極に重ねられた負極と、
前記正極活物質層と前記負極活物質層との間に配置されたセパレータと、
前記第1集電体と前記第2集電体との間に配置され、前記正極活物質層及び前記負極活物質層の周囲を取り囲み、前記第1集電体と前記第2集電体との間の空間に電解液を封止する封止部と、を有し、
前記電解液として、上記した本発明の電解液を用いる、リチウムイオン二次電池である。
(1)
電解質としてLiPF6以外のリチウム塩をリチウム塩の合計に対して30モル%以上含む、
(2)
非水溶媒にアルキレン環状カーボネートおよびプロピオン酸メチルを含む、
(3)
非水溶媒はプロピオン酸メチルを75体積%以上含む。
また、上記(2)はリチウムイオン二次電池の特性のうち円滑な充放電に寄与し得る。具体的には、アルキレン環状カーボネートに由来してリチウムイオンの生成や負極への挿入及び脱離が円滑に行なわれ、かつ、高粘度や高融点等のアルキレン環状カーボネートの短所がプロピオン酸メチルによって補われる。
さらに、上記(3)はリチウムイオン二次電池の特性のうち容量維持率や出力等の向上に寄与し得る。
本発明の電解液は、これらの協働により、リチウムイオン二次電池の特性を向上させることが可能といえる。
(4)
第1集電体と、前記第1集電体の一方面に設けられた正極活物質層と、を有する正極と、
第2集電体と、前記第2集電体の一方面に設けられた負極活物質層と、を有し、前記負極活物質層を前記正極活物質層に対面させつつ前記正極に重ねられた負極と、
前記正極活物質層と前記負極活物質層との間に配置されたセパレータと、
前記第1集電体と前記第2集電体との間に配置され、前記正極活物質層及び前記負極活物質層の周囲を取り囲み、前記第1集電体と前記第2集電体との間の空間に電解液を封止する封止部と、を有する、
(5)
前記電解液として、上記した本発明の電解液を用いる。
上記(5)は、さらに、上記(4)を備えるリチウムイオン二次電池の特性のうち構造的な耐久性の向上、具体的には封止性の向上に寄与し得る。
本発明のリチウムイオン二次電池は、これらの協働により、その特性の向上を実現し得る。
以下、本発明の電解液及びリチウムイオン二次電池をその構成要素ごとに説明する。
また、LiPF6は熱的に不安定であり、例えば60℃を超える温度下で、PF5を生じることも知られている。当該PF5は水と反応してフッ化水素を生じ得る。
後述する実施例の欄で詳細を説明するが、本発明の発明者が実際に試験した結果、二つの集電体の間に封止部を有するリチウムイオン二次電池において、電解質としてLiPF6以外のリチウム塩をリチウム塩の合計に対して30モル%以上含む場合には、電解質としてLiPF6のみを含む場合に比較して、その耐久性が向上した。
(R1は、水素、ハロゲン、置換基で置換されていても良いアルキル基、置換基で置換されていても良いシクロアルキル基、置換基で置換されていても良い不飽和アルキル基、置換基で置換されていても良い不飽和シクロアルキル基、置換基で置換されていても良い芳香族基、置換基で置換されていても良い複素環基、置換基で置換されていても良いアルコキシ基、置換基で置換されていても良い不飽和アルコキシ基、置換基で置換されていても良いチオアルコキシ基、置換基で置換されていても良い不飽和チオアルコキシ基、CN、SCN、OCNから選択される。
R2は、水素、ハロゲン、置換基で置換されていても良いアルキル基、置換基で置換されていても良いシクロアルキル基、置換基で置換されていても良い不飽和アルキル基、置換基で置換されていても良い不飽和シクロアルキル基、置換基で置換されていても良い芳香族基、置換基で置換されていても良い複素環基、置換基で置換されていても良いアルコキシ基、置換基で置換されていても良い不飽和アルコキシ基、置換基で置換されていても良いチオアルコキシ基、置換基で置換されていても良い不飽和チオアルコキシ基、CN、SCN、OCNから選択される。
また、R1とR2は、互いに結合して環を形成しても良い。
X1は、SO2、C=O、C=S、RaP=O、RbP=S、S=O、Si=Oから選択される。
Ra、Rbは、それぞれ独立に、水素、ハロゲン、置換基で置換されていても良いアルキル基、置換基で置換されていても良いシクロアルキル基、置換基で置換されていても良い不飽和アルキル基、置換基で置換されていても良い不飽和シクロアルキル基、置換基で置換されていても良い芳香族基、置換基で置換されていても良い複素環基、置換基で置換されていても良いアルコキシ基、置換基で置換されていても良い不飽和アルコキシ基、置換基で置換されていても良いチオアルコキシ基、置換基で置換されていても良い不飽和チオアルコキシ基、OH、SH、CN、SCN、OCNから選択される。また、Ra、Rbは、R1又はR2と結合して環を形成しても良い。)
n、a、b、c、d、e、f、g、hはそれぞれ独立に0以上の整数であり、2n+1=a+b+c+d+e+f+g+hを満たす。
また、R23とR24は、互いに結合して環を形成しても良く、その場合は、2n=a+b+c+d+e+f+g+hを満たす。
X2は、SO2、C=O、C=S、RcP=O、RdP=S、S=O、Si=Oから選択される。
Rc、Rdは、それぞれ独立に、水素、ハロゲン、置換基で置換されていても良いアルキル基、置換基で置換されていても良いシクロアルキル基、置換基で置換されていても良い不飽和アルキル基、置換基で置換されていても良い不飽和シクロアルキル基、置換基で置換されていても良い芳香族基、置換基で置換されていても良い複素環基、置換基で置換されていても良いアルコキシ基、置換基で置換されていても良い不飽和アルコキシ基、置換基で置換されていても良いチオアルコキシ基、置換基で置換されていても良い不飽和チオアルコキシ基、OH、SH、CN、SCN、OCNから選択される。
また、Rc、Rdは、R23又はR24と結合して環を形成しても良い。)
n、a、b、c、d、eはそれぞれ独立に0以上の整数であり、2n+1=a+b+c+d+eを満たす。
また、R15とR16は、互いに結合して環を形成しても良く、その場合は、2n=a+b+c+d+eを満たす。)
このうち(FSO2)2NLiを用いる場合には、リチウムイオン二次電池の出力や耐久性が向上する。これは、(FSO2)2NLiを用いることで電解液の粘度が低下したり、負極や正極の表面に良好な被膜が形成されたりすることに由来すると考えられる。
また、一般に、アルキレン環状カーボネートがリチウムイオン二次電池の充電時に還元分解されることにより、負極表面にSEI(Solid Electrolyte Interphase)被膜が形成されることが知られている。かかるSEI被膜の存在に因り、黒鉛を備える負極に対して、リチウムイオンの可逆的な挿入及び離脱が可能になると考えられている。
本発明の電解液は、一種のみのアルキレン環状カーボネートを用いても良いし複数種のアルキレン環状カーボネートを併用しても良い。アルキレン環状カーボネートとしては、エチレンカーボネートやプロピレンカーボネートを例示でき、このうちエチレンカーボネートは特に好適である。
本発明の電解液においては、アルキレン環状カーボネートとプロピオン酸メチルが共存することで、アルキレン環状カーボネートの不利な点をプロピオン酸メチルが相殺する。すなわち、プロピオン酸メチルは、電解液の低粘度化、イオン伝導度の好適化、リチウムイオンの拡散係数の好適化及び低温条件下での固化防止に寄与していると考えられる。
ここで、特願2020-026926に開示するように、本発明の発明者は、メチルエステルはエチルエステルよりも電解液の物性及び電池特性の点で優れていることを知見した。
また、メチルエステルであるプロピオン酸メチル、酢酸メチル、酪酸メチルについて、これらの融点及び沸点は、以下のとおりである。
プロピオン酸メチル 融点-88℃、沸点80℃
酢酸メチル 融点-98℃、沸点57℃
酪酸メチル 融点-95℃、沸点102℃
特に好ましくは、本発明の電解液はビニレンカーボネートを含有するのが良い。
具体的な添加剤としては、環状硫酸エステル、オキサレート硼酸塩、ジハロゲン化リン酸塩を例示できる。これらの添加剤は、1種のみを用いても良いし複数種を併用しても良い。
R-O-SO2-O-R(2つのRはアルキル基であり、互いに結合して、-O-S-O-と共に環を形成している。)
環状硫酸エステルとしては、5~9員環、5~8員環、5~7員環のものを例示でき、また、環状硫酸エステルの炭素数としては、2~6、2~5、2~4を例示できる。
好ましくは、オキサレート硼酸塩はLiB(C2O4)2すなわちリチウムビス(オキサラート)ボラート及び/又はLiB(C2O4)F2すなわちリチウムジフルオロ(オキサラート)ボラートであるのが良い。
以下、本発明の電解液を用いた本発明のリチウムイオン二次電池について説明する。
負極は、第2集電体と、当該第2集電体の一方面に設けられた負極活物質層とを有する。負極は、負極活物質層を正極の正極活物質層に対面させつつ、当該正極に重ねられる。
本明細書において、単に集電体という場合には第1集電体と第2集電体とを総称するものとする。また、電極活物質という場合には正極活物質と負極活物質とを総称するものとし、電極活物質層という場合には正極活物質と負極活物質とを総称するものとする。
オリビン構造の正極活物質を準備するには、市販のものを購入してもよいし、以下の文献などに記載された方法を参考に製造してもよい。オリビン構造の正極活物質としては、炭素で被覆されているものが好ましい。
特開2002-198050号公報
特表2005-522009号公報
特開2012-79554号公報
正極活物質層における正極活物質の割合として、70~99質量%の範囲内、80~98質量%の範囲内、90~97質量%の範囲内を例示できる。
具体的には、活物質と結着剤と溶媒とを含む合剤を造粒することで湿潤状態の造粒体を得る。当該造粒体の集合物を予め定められた型枠に入れ、平板状の成形体を得る。その後、転写ロールを用いて平板状の成形体を集電体の表面に付着させることで活物質層を形成する方法である。
何れの場合にも、正極と負極とは負極活物質層を正極活物質層に対面させつつ重ねられ、負極活物質層と正極活物質層とが対面する方向(以下、対面方向という)において、正極活物質層と負極活物質層との間にはセパレータが配置される。
セパレータとしては、公知のものを採用すればよく、ポリテトラフルオロエチレン、ポリプロピレン、ポリエチレン、ポリイミド、ポリアミド、ポリアラミド(Aromatic polyamide)、ポリエステル、ポリアクリロニトリル等の合成樹脂、セルロース、アミロース等の多糖類、フィブロイン、ケラチン、リグニン、スベリン等の天然高分子、セラミックスなどの電気絶縁性材料を1種若しくは複数用いた多孔体、不織布、織布などを挙げることができる。また、セパレータは多層構造としてもよい。具体的には、電極とセパレータ間の高い接着性を実現するためにセパレータに接着層を設けた接着型のセパレータや、セパレータに無機フィラー等を含むコーティング膜を形成することで高温耐熱性を高めた塗布型セパレータなどを挙げることができる。
本発明のリチウムイオン二次電池は、対面方向において、第1集電体と第2集電体の間に封止部を備える。当該封止部は、正極活物質層および負極活物質層の周囲を取り囲み、第1集電体と第2集電体との間の空間に電解液を封止する機能を有する。換言すると、本発明のリチウムイオン二次電池においては、一対の第1集電体と第2集電体との間に、正極活物質層、セパレータ、負極活物質層および電解液を有する蓄電セルが形成され、当該蓄電セルは封止部によって外界から区画される。なお、本発明のリチウムイオン二次電池は、当該蓄電セルを1つのみ備えても良いし、複数備えても良い。
本発明のリチウムイオン二次電池は、上記の構造を有することにより、容器を小型化、軽量化でき、かつリード線等の配線を低減できる。これにより、上記構造を有する本発明のリチウムイオン二次電池では体積、重量あたりのエネルギー密度が向上する。さらに、当該構造を有する本発明のリチウムイオン二次電池は、セルスタック型のリチウムイオン二次電池として具現化するのに好適であり、既述したように、厚目付の電極を有し得る。
例えば、第1集電体の一方面に正極活物質層を形成した正極と、第2集電体の一方面に負極活物質層を形成した負極とを、正極活物質層および負極活物質層がセパレータを介して対面させる。このときさらに、第1集電体と第2集電体との間には、正極活物質層および負極活物質層を取り囲むように封止部を配置し、当該封止部を第1集電体および第2集電体に固着する。またこのとき封止部の内部に電解液を封入する。これによりリチウムイオン二次電池を製造することができる。
なお、セパレータの構造によっては、第1集電体とセパレータとの間に第1の封止部を配置し、かつ、セパレータと第2集電体との間に第2の封止部を配置して、第1の封止部と第2の封止部とを接着等の方法で一体化しても良い。
エチレンカーボネートとプロピオン酸メチルとを体積比30:70で混合した混合溶媒に、下記の表1の割合で(FSO2)2NLiおよびLiPF6を溶解し、電解液1~3を製造した。
電解液1~3につき、粘度を以下の条件で測定した。結果を表1に示す。
<粘度>
B型粘度計(Brookfield社、DV2T)にて、コーン型スピンドルを用いて25℃における各電解液の粘度を測定した。なお、コーン型スピンドルの回転速度は60rpmとした。
プロピオン酸メチルと化学構造が類似するエステルとして、プロピオン酸プロピル、酪酸メチルおよび酪酸エチルを用いた電解液を製造し、これらのエステルによる電池特性への影響を検討した。
〔電解液〕
・エチレンカーボネートとプロピオン酸メチルを体積比15:85で混合した混合溶媒に、LiPF6を濃度1.2mol/Lで溶解して母液とした。母液に対して、1質量%に相当する量のリチウムジフルオロ(オキサラート)ボラート(以下、LiDFOBと略すことがある。LiDFOBはオキサレート硼酸塩の一態様である。)及び1質量%に相当する量のビニレンカーボネートを加えて溶解することで、電解液4を製造した。
・エチレンカーボネートとプロピオン酸プロピル(以下、PPと略すことがある。)を体積比15:85で混合した混合溶媒に、LiPF6を濃度1.2mol/Lで溶解して母液とした。母液に対して1質量%に相当する量のLiDFOBと1質量%に相当する量のビニレンカーボネートとを加えて溶解することで、電解液5を製造した。
・エチレンカーボネートと酪酸メチル(以下、MBと略すことがある。)を体積比15:85で混合した混合溶媒に、LiPF6を濃度1.2mol/Lで溶解して母液とした。母液に対して1質量%に相当する量のLiDFOBと1質量%に相当する量のビニレンカーボネートとを加えて溶解することで、電解液6を製造した。
・エチレンカーボネートと酪酸エチル(以下、EBと略すことがある。)を体積比15:85で混合した混合溶媒に、LiPF6を濃度1.2mol/Lで溶解して母液とした。母液に対して1質量%に相当する量のLiDFOBと1質量%に相当する量のビニレンカーボネートとを加えて溶解することで、電解液7を製造した。
・エチレンカーボネート、エチルメチルカーボネート及びジメチルカーボネートを体積比30:30:40で混合して、混合溶媒とした。混合溶媒にLiPF6を溶解して、LiPF6の濃度が1mol/Lである母液を製造した。母液に対して0.2mol/Lに相当する量のLiDFOBと1質量%に相当する量のビニレンカーボネートとを加えて溶解することで、電解液8を製造した。
電解液4~8を用い、以下のようにリチウムイオン二次電池を製造した。
正極活物質として炭素で被覆されたオリビン構造のLiFePO4、導電助剤としてアセチレンブラック及び結着剤としてポリフッ化ビニリデンを、正極活物質と導電助剤と結着剤の質量比が90:5:5となるように混合し、溶剤としてN-メチル-2-ピロリドンを添加してスラリー状の正極活物質層形成用組成物とした。正極用集電体としてアルミニウム箔を準備した。アルミニウム箔の表面に正極活物質層形成用組成物を膜状に塗布した後に溶剤を除去して製造された正極前駆体を、厚み方向にプレスすることで、アルミニウム箔の表面に正極活物質層が形成された正極を製造した。
なお、正極の製造において、正極の目付け量13.9mg/cm2を目標とした。
なお、負極の製造において、負極の目付け量6.3mg/cm2を目標とした。
リチウムイオン二次電池4~8につき、0.4Cレートで4.0VまでCC-CV充電を行い、このときの充電容量を基準(SOC100%)とした。当該SOC100の状態で、各リチウムイオン二次電池を40℃で11日間保存することで、保存試験を行った。
保存試験の前後に容量確認を行った。具体的には、0.4Cレートで4.0VまでCC-CV充電を行った。次いで、1Cレートで2.5VまでCC-CV放電を行った。これにより、各リチウムイオン二次電池の放電容量を確認した。保存試験前の放電容量に対する、保存試験後の放電容量の百分率を、各リチウムイオン二次電池の容量維持率とした。
また、保存試験後、SOC60%に調整した各リチウムイオン二次電池に対して、25℃の条件下、一定電流レートで5秒間放電させた場合の電圧変化量を測定した。当該測定を、電流レートを変えた複数の条件下で行った。得られた結果から、SOC60%の各リチウムイオン二次電池につき、電圧2.5Vまでの放電時間が10秒となる一定電流(mA)を算出した。SOC60%から2.5Vまでの電圧変化量に算出された一定電流を乗じた値を出力とした。
以上の保存試験の結果を表2に示す。
実施例1の電解液およびリチウムイオン二次電池を以下に説明する。なお、実施例1のリチウムイオン二次電池は、既述した本発明のリチウムイオン二次電池とは異なり、電極が厚目付であること以外は一般的な構造を有するものである。
エチレンカーボネートとプロピオン酸メチルとを体積比15:85で混合した混合溶媒に、(FSO2)2NLiを濃度1.2mol/Lで溶解して母液とした。当該母液に対して1質量%に相当する量のビニレンカーボネートを加えて溶解することで、実施例1の電解液を製造した。
負極活物質として黒鉛、結着剤としてカルボキシメチルセルロース及びスチレンブタジエンゴムを、黒鉛とカルボキシメチルセルロースとスチレンブタジエンゴムの質量比が94.8:0.8:4.4となるように混合し、溶剤として水を添加してスラリー状の負極活物質層形成用組成物とした。負極用集電体として銅箔を準備した。銅箔の表面に負極活物質層形成用組成物を膜状に塗布した後に溶剤を除去して製造された負極前駆体を、厚み方向にプレスすることで、銅箔の表面に負極活物質層が形成された負極を製造した。
なお、負極の目付け量は26.5mg/cm2であった。
なお、正極の目付け量は55.5mg/cm2であった。
実施例2の電解液は、エチレンカーボネートとプロピオン酸メチルとの体積比以外は実施例1の電解液と同じものである。
具体的には、実施例2においては、エチレンカーボネートとプロピオン酸メチルとを体積比25:75で混合した混合溶媒に、(FSO2)2NLiを濃度1.2mol/Lで溶解して母液とした。当該母液に対して1質量%に相当する量のビニレンカーボネートを加えて溶解することで、実施例2の電解液を製造した。
実施例2のリチウムイオン二次電池は、実施例2の電解液を用いたこと以外は実施例1のリチウムイオン二次電池と同じものである。
比較例1の電解液は、エチレンカーボネートとプロピオン酸メチルとの体積比以外は実施例1の電解液と同じものである。
具体的には、比較例1においては、エチレンカーボネートとプロピオン酸メチルとを体積比30:70で混合した混合溶媒に、(FSO2)2NLiを濃度1.2mol/Lで溶解して母液とした。当該母液に対して1質量%に相当する量のビニレンカーボネートを加えて溶解することで、比較例1の電解液を製造した。
比較例1のリチウムイオン二次電池は、比較例1の電解液を用いたこと以外は実施例1のリチウムイオン二次電池と同じものである。
比較例2の電解液は、エチレンカーボネートとプロピオン酸メチルとの体積比以外は実施例1の電解液と同じものである。
具体的には、比較例2においては、エチレンカーボネートとプロピオン酸メチルとを体積比50:50で混合した混合溶媒に、(FSO2)2NLiを濃度1.2mol/Lで溶解して母液とした。当該母液に対して1質量%に相当する量のビニレンカーボネートを加えて溶解することで、比較例2の電解液を製造した。
比較例2のリチウムイオン二次電池は、比較例2の電解液を用いたこと以外は実施例1のリチウムイオン二次電池と同じものである。
実施例1、2および比較例1、2のリチウムイオン二次電池につき、0.4C、1.0C、2.0Cおよび4.0の4通りの放電レートで、SOC95%から電圧2.3Vとなるまで放電を行った。そして、放電レート毎に、各リチウムイオン二次電池の放電終止時の容量すなわちレート容量を比較することで、実施例1、2および比較例1、2のリチウムイオン二次電池のレート特性を評価した。なお、レート特性評価試験は、各Cレートにつきn=2で行い、その平均値を比較した。
各リチウムイオン二次電池につき、0.4Cレートで4.0VまでCC-CV充電を行ったときの充電容量をSOC100%とした。レート容量は、上記のSOC100%に対する百分率で表した。
結果を表3に示す。
なお、実施例1のリチウムイオン二次電池は、既述した本発明のリチウムイオン二次電池とは異なる構造であるが、言うまでもなく、実施例1の電解液による効果は本発明のリチウムイオン二次電池においても同様に発揮される。以下の各実施例についても同様である。
実施例3の電解液およびリチウムイオン二次電池を以下に説明する。
〔電解液〕
エチレンカーボネートとプロピオン酸メチルとを体積比15:85で混合した混合溶媒に、(FSO2)2NLiを濃度1.2mol/Lで溶解して母液とした。当該母液に対して0.5質量%に相当する量の1,3,2-ジオキサチオラン-2,2-ジオキシド(以下、DTDと略すことがある。DTDは環状硫酸エステルの一態様である。)を加えて溶解することで、実施例3の電解液を製造した。
負極活物質として黒鉛、結着剤としてカルボキシメチルセルロース及びスチレンブタジエンゴムを、黒鉛とカルボキシメチルセルロースとスチレンブタジエンゴムの質量比が97:0.8:2.2となるように混合し、溶剤として水を添加してスラリー状の負極活物質層形成用組成物とした。負極用集電体として銅箔を準備した。銅箔の表面に負極活物質層形成用組成物を膜状に塗布した後に溶剤を除去して製造された負極前駆体を、厚み方向にプレスすることで、銅箔の表面に負極活物質層が形成された負極を製造した。
なお、負極の目付け量は6.7mg/cm2であった。
なお、正極の目付け量は13.9mg/cm2であった。
実施例4の電解液は、リチウム塩として(FSO2)2NLiおよびLiPF6を用いたこと以外は実施例3の電解液と同じである。
具体的には、実施例4においては、エチレンカーボネートとプロピオン酸メチルとを体積比15:85で混合した混合溶媒に、(FSO2)2NLiを濃度0.6mol/LでおよびLiPF6を濃度0.6mol/Lで溶解して母液とした。当該母液に対して0.5質量%に相当する量のDTDを加えて溶解することで、実施例4の電解液を製造した。
実施例4のリチウムイオン二次電池は、実施例4の電解液を用いたこと以外は実施例3のリチウムイオン二次電池と同じものである。
比較例3の電解液は、リチウム塩として(FSO2)2NLiおよびLiPF6を用いたこと以外は実施例3の電解液と同じである。
具体的には、比較例3においては、エチレンカーボネートとプロピオン酸メチルとを体積比15:85で混合した混合溶媒に、LiPF6を濃度1.2mol/Lで溶解して母液とした。当該母液に対して0.5質量%に相当する量の(FSO2)2NLi、および、0.5質量%に相当する量のDTDを加えて溶解することで、比較例3の電解液を製造した。なお、比較例3の電解液における(FSO2)2NLiの量は、0.03mol/Lであり、当該(FSO2)2NLiの量は、(FSO2)2NLiとLiPF6との合計に対して2.4モル%であった。
比較例3のリチウムイオン二次電池は、比較例3の電解液を用いたこと以外は実施例3のリチウムイオン二次電池と同じものである。
比較例3の電解液は、リチウム塩としてLiPF6のみを用いたこと以外は実施例3の電解液と同じである。
具体的には、比較例3においては、エチレンカーボネートとプロピオン酸メチルとを体積比15:85で混合した混合溶媒に、LiPF6を濃度1.2mol/Lで溶解して母液とした。当該母液に対して0.5質量%に相当する量のDTDを加えて溶解することで、比較例4の電解液を製造した。
比較例4のリチウムイオン二次電池は、比較例4の電解液を用いたこと以外は実施例3のリチウムイオン二次電池と同じものである。
実施例3、4および比較例3、4のリチウムイオン二次電池に対して、高温充放電サイクル試験を行った。
まず、SOC60%に調整した各リチウムイオン二次電池に対して、25℃の条件下、一定電流レートで10秒間放電させた場合の電圧の変化量を測定した。当該測定を、電流レートを変えた複数の条件下で行った。得られた結果から、SOC60%の各リチウムイオン二次電池につき、電圧2.5Vまでの放電時間が10秒となる一定電流(mA)を算出した。SOC60%から2.5Vまでの電圧変化量に、算出された上記一定電流(mA)を乗じた値を、初期出力とした。初期出力の試験も複数回行った。
比較例4のリチウムイオン二次電池の出力値に対する各リチウムイオン二次電池の出力値の百分率を算出し、当該百分率から100(%)を引いた値を、初期出力増加率(%)とした。
その後、60℃で、1Cレートで4VまでCC-CV充電し、1CレートでSOD90%となるまでCC放電する高温充放電サイクルを100回繰り返した。なお、ここでいう充電とは、負極から正極にリチウムイオンが移動し正極と負極との電位差が大きくなることを意味する。
100回目の充放電終了後、上記の出力確認と同様に各リチウムイオン二次電池の出力確認を行った。比較例4のリチウムイオン二次電池の出力値に対する各リチウムイオン二次電池の出力値の百分率を算出し、当該百分率から100(%)を引いた値を、100サイクル後出力増加率(%)とした。
各リチウムイオン二次電池の初期出力を表4に示し、各リチウムイオン二次電池の100サイクル後の出力を表5に示す。なお、出力確認についてはn=4で、高温充放電サイクルについてはn=2で行い、表4および表5にはこれらの平均値を示した。
これらの結果から、(FSO2)2NLi、すなわちLiPF6以外のリチウム塩をリチウム塩全体に対して30モル%以上含む電解液を用いることで、リチウムイオン二次電池における特に高温下での耐久性が向上することが裏付けられる。
実施例3、4および比較例3、4のリチウムイオン二次電池につき、0.4Cレートで4.0VまでCC-CV充電を行い、このときの充電容量を基準(SOC100%)とした。当該SOC100の状態で、各リチウムイオン二次電池を40℃で12日間保存することで、保存試験を行った。
保存試験の前後に評価例2と同様に出力確認を行い、保存試験後の出力値につき、比較例4のリチウムイオン二次電池の出力値に対する各リチウムイオン二次電池の出力値の百分率を算出し、当該百分率から100(%)を引いた値を、保存後出力増加率(%)とした。
各リチウムイオン二次電池の100サイクル後の保存後の出力を表6に示す。なお、試験はn=2で行い、表6にはその平均値を示した。
実施例3の電解液と同じ実施例5の電解液を用い、以下のように実施例5のリチウムイオン二次電池を製造した。参考までに、実施例5の電解液は、エチレンカーボネートとプロピオン酸メチルとを体積比15:85で混合した混合溶媒に、(FSO2)2NLiを濃度1.2mol/Lで溶解して母液とし、当該母液に対して0.5質量%に相当する量のDTDを加えて溶解したものである。
負極活物質として黒鉛、結着剤としてカルボキシメチルセルロース及びスチレンブタジエンゴムを、黒鉛とカルボキシメチルセルロースとスチレンブタジエンゴムの質量比が97:0.8:2.2となるように混合し、溶剤として水を添加してスラリー状の負極活物質層形成用組成物とした。負極用集電体として銅箔を準備した。銅箔の表面に負極活物質層形成用組成物を膜状に塗布した後に溶剤を除去して製造された負極前駆体を、厚み方向にプレスすることで、銅箔の表面に負極活物質層が形成された負極を製造した。
なお、負極の目付け量は6.24mg/cm2であった。
なお、正極の目付け量は13.87mg/cm2であった。
比較例4の電解液と同じ比較例5の電解液を用い、実施例5と同様にして比較例5のリチウムイオン二次電池を製造した。参考までに、比較例5の電解液は、エチレンカーボネートとプロピオン酸メチルとを体積比15:85で混合した混合溶媒に、LiPF6を濃度1.2mol/Lで溶解して母液とし、当該母液に対して0.5質量%に相当する量のDTDを加えて溶解したものである。
実施例5および比較例5のリチウムイオン二次電池につき、4.0Vまで0.05Cで充電し、60℃で20時間保持することにより活性化を行った。活性化前後の各リチウムイオン二次電池の体積をアルキメデス法により測定し、活性化前後での各リチウムイオン二次電池の体積変化から、活性化により生じたガス量(μL)を算出した。
各リチウムイオン二次電池のガス発生量を表7に示す。なお、試験はn=3で行い、表7にはその平均値を示した。
なお、負極に黒鉛を用いたリチウムイオン二次電池においては、負極で電解液が分解されることによりガスが生じると考えられる。実施例5のリチウムイオン二次電池においては、電解液に含まれる(FSO2)2NLiが分解されることで負極の表面に良好な被膜が形成されると考えられ、当該被膜に因りに電解液の分解が抑制されたものと推測される。ガス発生を抑制することにより、充放電に伴うリチウムイオン二次電池の膨張量を低減でき、リチウムイオン二次電池に作用する応力を低減でき、ひいてはリチウムイオン二次電池の耐久性を向上させることが可能である。このガス発生の抑制効果は、容器として金属製の缶を用いない態様のリチウムイオン二次電池に特に有用であり、本発明リチウムイオン二次電池においても非常に有用である。
実施例3の電解液と同じ実施例6の電解液を用い、実施例3と同様にして実施例6のリチウムイオン二次電池を製造した。参考までに、実施例6の電解液は、エチレンカーボネートとプロピオン酸メチルとを体積比15:85で混合した混合溶媒に、(FSO2)2NLiを濃度1.2mol/Lで溶解して母液とし、当該母液に対して0.5質量%に相当する量のDTDを加えて溶解したものである。
実施例4の電解液と同じ実施例7の電解液を用い、実施例3と同様にして実施例7のリチウムイオン二次電池を製造した。参考までに、実施例7の電解液は、エチレンカーボネートとプロピオン酸メチルとを体積比15:85で混合した混合溶媒に、(FSO2)2NLiを濃度0.6mol/LでおよびLiPF6を濃度0.6mol/Lで溶解して母液とし、当該母液に対して0.5質量%に相当する量のDTDを加えて溶解したものである。
比較例3の電解液と同じ比較例6の電解液を用い、実施例3と同様にして比較例6のリチウムイオン二次電池を製造した。参考までに、比較例6の電解液は、エチレンカーボネートとプロピオン酸メチルとを体積比15:85で混合した混合溶媒に、LiPF6を濃度1.2mol/Lで溶解して母液とし、当該母液に対して0.5質量%に相当する量の(FSO2)2NLiおよび0.5質量%に相当する量のDTDを加えて溶解したものである。比較例6の電解液における(FSO2)2NLiの量は0.03mol/Lであり、当該(FSO2)2NLiの量は、(FSO2)2NLiとLiPF6との合計に対して2.4モル%であった。
比較例4の電解液と同じ比較例7の電解液を用い、実施例3と同様にして比較例7のリチウムイオン二次電池を製造した。参考までに、比較例7の電解液は、エチレンカーボネートとプロピオン酸メチルとを体積比15:85で混合した混合溶媒に、LiPF6を濃度1.2mol/Lで溶解して母液とし、当該母液に対して0.5質量%に相当する量のDTDを加えて溶解したものである。
実施例6、実施例7および比較例6、比較例5のリチウムイオン二次電池につき、0.4Cレートで4.0VまでCC-CV充電を行った。その後、1Cレートで2.5Vまで2時間かけてCC-CV放電を行った。このときの放電容量を初期容量とした。また、各リチウムイオン二次電池につき評価例2と同様の高温充放電サイクル試験を行った。さらに、50回目の充放電終了後、および、100回目の充放電終了後に、各リチウムイオン二次電池の放電容量を確認した。放電容量は、上記の初期容量と同様の方法で確認した。初期容量に対する50回目の充放電終了後の放電容量の百分率、および、100回目の充放電終了後の放電容量の百分率を計算し、各々、50サイクル後の容量維持率および100サイクル後の容量維持率とした。
各リチウムイオン二次電池の50サイクル後の容量維持率および100サイクル後の容量維持率を表8に示す。なお、各試験はn=2で行い、表8にはその平均値を示した。
実施例6、7および比較例6、7のリチウムイオン二次電池につき、評価例3と同様の方法で保存試験を行った。
保存試験前、保存試験開始7日経過後および保存試験開始12日経過後に、評価例2と同様に各リチウムイオン二次電池の放電容量を確認した。保存試験前の放電容量を初期容量とし、当該初期容量に対する保存試験開始7日経過後の放電容量の百分率、および、保存試験開始12日経過後の放電容量の百分率を計算し、各々、保存試験開始7日後の容量維持率、および、保存試験開始12日後の容量維持率とした。
各リチウムイオン二次電池の保存試験開始7日後の容量維持率、および、保存試験開始12日後の容量維持率を表9に示す。なお、各試験はn=2で行い、表9にはその平均値を示した。
この結果からも、LiPF6以外のリチウム塩をリチウム塩全体に対して30モル%以上含む電解液を用いることで、リチウムイオン二次電池における特に高温下での耐久性が向上することが裏付けられる。
エチレンカーボネートとプロピオン酸メチルとを体積比15:85で混合した混合溶媒に、(FSO2)2NLiを濃度1.2mol/Lで溶解して母液とした。当該母液に対して1.94質量%に相当する量のビニレンカーボネートを加えて溶解することで、実施例8の電解液を製造した。
実施例8の電解液を用い、実施例1と同様にして実施例8のリチウムイオン二次電池を製造した。
エチレンカーボネートとジメチルカーボネートとを体積比15:85で混合した混合溶媒に、(FSO2)2NLiを濃度1.2mol/Lで溶解して母液とした。当該母液に対して1.94質量%に相当する量のビニレンカーボネートを加えて溶解することで、比較例8の電解液を製造した。
比較例8の電解液を用い、実施例1と同様にして比較例8のリチウムイオン二次電池を製造した。
エチレンカーボネートとジメチルカーボネートとエチルメチルカーボネートとを体積比15:65:20で混合した混合溶媒に、(FSO2)2NLiを濃度1.2mol/Lで溶解して母液とした。当該母液に対して1.94質量%に相当する量のビニレンカーボネートを加えて溶解することで、比較例9の電解液を製造した。
比較例9の電解液を用い、実施例1と同様にして比較例9のリチウムイオン二次電池を製造した。
エチレンカーボネートとジメチルカーボネートとエチルメチルカーボネートとを体積比15:45:40で混合した混合溶媒に、(FSO2)2NLiを濃度1.2mol/Lで溶解して母液とした。当該母液に対して1.94質量%に相当する量のビニレンカーボネートを加えて溶解することで、比較例10の電解液を製造した。
比較例10の電解液を用い、実施例1と同様にして比較例10のリチウムイオン二次電池を製造した。
エチレンカーボネートとプロピオン酸メチルとを体積比15:85で混合した混合溶媒に、LiPF6を濃度1.2mol/Lで溶解して母液とした。当該母液に対して1質量%に相当する量のビニレンカーボネートおよび1質量%に相当する量のLiDFOBを加えて溶解することで、比較例11の電解液を製造した。
比較例11の電解液を用い、実施例1と同様にして比較例8のリチウムイオン二次電池を製造した。
実施例8および比較例11のリチウムイオン二次電池につき、1.1Cの放電レートでSOC95%から電圧2.2VとなるまでCC放電を行い、放電容量の変化を経時的に測定した。
別に、各リチウムイオン二次電池につき0.33Cの放電レートでSOC100%から3.0VとなるまでCC-CV放電を行い、このときの容量をCC-CV容量とした。各リチウムイオン二次電池につき上記したCC-CV容量に対するCC放電終止時の容量の百分率を算出してレート%とした。なお、試験はn=2で行い、CC放電終止時の容量としてはその平均値を用いた。
各リチウムイオン二次電池につき、0.2Cレートで3.75VまでCC-CV充電を行ったときの充電容量をSOC100%とした。
実施例8および比較例11の各リチウムイオン二次電池におけるCC放電容量の経時変化を表すグラフを図1に示す。また、実施例8および比較例11の各リチウムイオン二次電池のレート%を表10に示す。
また、表10に示すように、実施例8のリチウムイオン二次電池は、放電終止時のレート%についても、比較例11のリチウムイオン二次電池に比べて大幅に優れている。これらの結果は、リチウム塩として(FSO2)2NLiを用いることが長時間放電特性の向上に寄与することを示す。この結果は、基礎検討1にも示したように、リチウム塩の合計に対する(FSO2)2NLiの量が30モル%以上である場合に、電解液の粘度は低下し、イオン伝導率は向上することによるものと考えられる。
実施例8および比較例8~10のリチウムイオン二次電池につき、評価例7と同様にして放電容量の変化を経時的に測定した。
実施例8および比較例8~10の各リチウムイオン二次電池におけるCC放電容量の経時変化を表すグラフを図2に示す。また、実施例8および比較例8~10の各リチウムイオン二次電池のレート%を表11に示す。
実施例9のリチウムイオン二次電池は本発明のリチウムイオン二次電池である。実施例9のリチウムイオン二次電池を模式的に表す説明図を図3に示す。以下、図3を基に実施例9のリチウムイオン二次電池を説明する。
正極活物質層21は、実施例1のリチウムイオン二次電池における正極活物質層と同じものであり、第1集電体20の一方面20aにおける中央部分に積層形成されている。
負極活物質層31は、実施例1のリチウムイオン二次電池における負極活物質層と同じものであり、第2集電体30の一方面30aにおける中央部分に積層形成されている。
負極3は、負極活物質層31を正極活物質層21に対面させ、かつ、負極活物質層31と正極活物質層21との間にセパレータ4を挟んだ状態で、正極2に重ねられている。換言すると、既述した対面方向において、同じ蓄電セル10を構成する第1集電体20および第2集電体30の間には、負極活物質層31とセパレータ4と正極活物質層21との積層体が挟まれている。なお、セパレータ4は実施例1のリチウムイオン二次電池におけるセパレータと同じものである。
先ず、実施例1に説明した方法で正極2および負極3を製造する。
次いで封止部5を正極2と負極3との一方に一体化する。具体的には、インパルスシール機を用いて、第1集電体20と第2集電体30との一方に封止部5を熱融着する。これにより封止部5は、第1集電体20または第2集電体30上に箱状に一体化され、その内部に電解液6を収容可能になる。
次いで、当該封止部5の内部に電解液6を注入し、これに正極2と負極3との他方を一体化する。具体的には、正極活物質層21と負極活物質層31とを対面させ、間にセパレータ4および封止部5を挟みつつこれらを重ねる。そして、インパルスシール機を用いて、第1集電体20と第2集電体30との他方に封止部5を熱融着する。これにより、実施例9のリチウムイオン二次電池1における蓄電セル10が得られた。
二つの蓄電セル10を準備し、蓄電セル10の一方である第1蓄電セル10xの第1集電体20xと、蓄電セル10の他方である第2蓄電セル10yの第2集電体30yとを、対面方向に重ね合わせ、当該第1集電体20xと第2集電体30yとを積層する。積層された二つの蓄電セル10は図略の拘束部材によって拘束され、各蓄電セル10には、対面方向において、所定の拘束荷重が付加される。これにより、第1の蓄電セル10xと第2の蓄電セル10yとを、互いに積層された状態に維持できる。さらに、図略の端子を蓄電セル10x、10yに固着する。これにより、実施例9のリチウムイオン二次電池が得られた。
実施例10のリチウムイオン二次電池は本発明のリチウムイオン二次電池であり、第1集電体および第2集電体の構造以外は実施例9のリチウムイオン二次電池と概略同じものである。実施例10のリチウムイオン二次電池を模式的に表す説明図を図4に示す。以下、図4を基に実施例10のリチウムイオン二次電池を説明する。
実施例10のリチウムイオン二次電池もまた、実施例9のリチウムイオン二次電池と同様に耐久性に優れる。
エチレンカーボネートとプロピオン酸メチルとを体積比30:70で混合した混合溶媒に、(FSO2)2NLiを濃度1.2mol/Lで溶解して実施例11の電解液を製造した。
エチレンカーボネートとプロピオン酸メチルとを体積比30:70で混合した混合溶媒に、(FSO2)2NLiを濃度1mol/LおよびLiPF6を濃度0.2mol/Lで溶解して実施例12の電解液を製造した。なお、実施例12の電解液において、リチウム塩の合計に対する(FSO2)2NLiの量は約83.3モル%である。
エチレンカーボネートとプロピオン酸メチルとを体積比30:70で混合した混合溶媒に、(FSO2)2NLiを濃度0.8mol/LおよびLiPF6を濃度0.4mol/Lで溶解して実施例12の電解液を製造した。なお、実施例13の電解液において、リチウム塩の合計に対する(FSO2)2NLiの量は約66.7モル%である。
エチレンカーボネートとプロピオン酸メチルとを体積比30:70で混合した混合溶媒に、(FSO2)2NLiを濃度0.6mol/LおよびLiPF6を濃度0.6mol/Lで溶解して実施例14の電解液を製造した。なお、実施例14の電解液において、リチウム塩の合計に対する(FSO2)2NLiの量は約50モル%である。
エチレンカーボネートとプロピオン酸メチルとを体積比30:70で混合した混合溶媒に、(FSO2)2NLiを濃度0.4mol/LおよびLiPF6を濃度0.8mol/Lで溶解して実施例15の電解液を製造した。なお、実施例15の電解液において、リチウム塩の合計に対する(FSO2)2NLiの量は約33.3モル%である。
エチレンカーボネートとプロピオン酸メチルとを体積比30:70で混合した混合溶媒に、(FSO2)2NLiを濃度0.2mol/LおよびLiPF6を濃度1mol/Lで溶解して比較例12の電解液を製造した。なお、比較例12の電解液において、リチウム塩の合計に対する(FSO2)2NLiの量は約16.7モル%である。
エチレンカーボネートとプロピオン酸メチルとを体積比30:70で混合した混合溶媒に、LiPF6を濃度1.2mol/Lで溶解して比較例13の電解液を製造した。
アルミニウム箔と銅箔とをシール材で熱融着したテストピースを準備した。当該テストピースを実施例11~15および比較例12、13の各電解液に浸漬して剥離評価を行った。
詳しくは、各テストピースの寸法は15mm×65mmであり、シール幅は18mmであった。シール材は酸変性オレフィン系のものであり、より具体的には、低融点のポリエチレン樹脂を含む。熱融着にはインパルスシーラーを用い、加熱温度は110℃であった。
アルミニウムラミネートフィルムを60mm×100mmの袋状にし、その内部に電解液を1mL注入し、そこにテストピースを浸漬した。袋の開口をインパルスシーラーで熱融着することで、当該袋の内部にテストピースと電解液とを密封した。これを60℃で72時間保存した。その後、袋から取り出したテストピースをエタノールで洗浄し、当該テストピースに生じた自然剥離長を測定した。測定にはグルーブマイクロメーターを用いた。
既述したように、LiPF6は水と反応してフッ化水素を生じるため、比較例13の電解液を用いたテストピースにおいては、当該フッ化水素によってアルミニウム箔が劣化し、その結果、樹脂とアルミニウム箔との界面における剥離が進行したものと考えられる。そして、当該剥離は、実施例11~15のように、LiPF6以外のリチウム塩をリチウム塩の合計に対して30モル%以上含む電解液を用いる場合には、十分に抑制されるといえる。実施例12~15の電解液を用いたテストピースの自然剥離長がほぼ同じ値であったことから、剥離抑制の観点からは、電解液に含まれるLiPF6以外のリチウム塩の量は、リチウム塩の合計に対して30モル%以上であれば足るといい得る。
これに対して、本発明のリチウムイオン二次電池の電解液として、実施例11~15のように、LiPF6以外のリチウム塩をリチウム塩の合計に対して30モル%以上含む電解液を用いる場合には、第1集電体と封止部との界面における剥離が十分に抑制されるといい得る。
Claims (6)
- 第1集電体と、前記第1集電体の一方面に設けられた正極活物質層と、を有する正極と、
第2集電体と、前記第2集電体の一方面に設けられた負極活物質層と、を有し、前記負極活物質層を前記正極活物質層に対面させつつ前記正極に重ねられた負極と、
前記正極活物質層と前記負極活物質層との間に配置されたセパレータと、
前記第1集電体と前記第2集電体との間に配置され、前記正極活物質層及び前記負極活物質層の周囲を取り囲み、前記第1集電体と前記第2集電体との間の空間に電解液を封止する封止部と、を有し、
前記電解液として、
リチウム塩を含む電解質と、アルキレン環状カーボネートおよびプロピオン酸メチルを含む非水溶媒と、を有し、前記電解質は、LiPF6以外のリチウム塩を前記リチウム塩の合計に対して30モル%以上含み、
前記非水溶媒は、前記プロピオン酸メチルを75体積%以上含む、電解液を用いる、リチウムイオン二次電池。 - リチウム塩を含む電解質と、アルキレン環状カーボネートおよびプロピオン酸メチルを含む非水溶媒と、を有し、前記電解質は、LiPF6以外のリチウム塩を前記リチウム塩の合計に対して30モル%以上含み、
前記非水溶媒は、前記プロピオン酸メチルを75体積%以上含む、電解液。 - 前記LiPF6以外のリチウム塩は、(CF3SO2)2NLi、(FSO2)2NLi、(C2F5SO2)2NLi、FSO2(CF3SO2)NLi、(SO2CF2CF2SO2)NLi、又は(SO2CF2CF2CF2SO2)NLiである、請求項2に記載の電解液。
- 前記非水溶媒は、前記プロピオン酸メチルを80体積%以上含む、請求項2又は請求項3に記載の電解液。
- 前記電解質は、前記LiPF6以外のリチウム塩を前記リチウム塩の合計に対して50モル%以上含む、請求項2~請求項4の何れか一項に記載の電解液。
- 前記負極は、前記負極活物質層に黒鉛を含有する、請求項1に記載のリチウムイオン二次電池。
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP22779390.8A EP4318718A1 (en) | 2021-03-29 | 2022-01-18 | Electrolytic solution and lithium ion secondary battery |
| US18/284,376 US20240170725A1 (en) | 2021-03-29 | 2022-01-18 | Electrolytic solution and lithium ion secondary battery |
| CN202280026650.6A CN117099238A (zh) | 2021-03-29 | 2022-01-18 | 电解液和锂离子二次电池 |
| KR1020237020273A KR20230109691A (ko) | 2021-03-29 | 2022-01-18 | 전해액 및 리튬 이온 이차 전지 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2021-056292 | 2021-03-29 | ||
| JP2021056292A JP7563273B2 (ja) | 2021-03-29 | 2021-03-29 | 電解液およびリチウムイオン二次電池 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2022209158A1 true WO2022209158A1 (ja) | 2022-10-06 |
Family
ID=83458585
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2022/001511 WO2022209158A1 (ja) | 2021-03-29 | 2022-01-18 | 電解液およびリチウムイオン二次電池 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US20240170725A1 (ja) |
| EP (1) | EP4318718A1 (ja) |
| JP (1) | JP7563273B2 (ja) |
| KR (1) | KR20230109691A (ja) |
| CN (1) | CN117099238A (ja) |
| WO (1) | WO2022209158A1 (ja) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20230282799A1 (en) * | 2022-03-07 | 2023-09-07 | The University Of Hong Kong | Flexible and printable paper-based al ion batteries |
| WO2024184682A1 (en) * | 2023-03-08 | 2024-09-12 | Ses Holdings Pte. Ltd. | Electrolytes containing sulfonamide-type cyclic salts, and energy-storage cells and batteries made therewith |
Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2008258022A (ja) * | 2007-04-05 | 2008-10-23 | Bridgestone Corp | 電池用非水電解液及びそれを備えた非水電解液電池 |
| JP2010123300A (ja) | 2008-11-17 | 2010-06-03 | Toyota Central R&D Labs Inc | リチウム二次電池及びその使用方法 |
| JP2013140734A (ja) | 2012-01-05 | 2013-07-18 | Gs Yuasa Corp | 非水電解質二次電池 |
| JP2015201318A (ja) | 2014-04-07 | 2015-11-12 | トヨタ自動車株式会社 | 電極シートの製造方法 |
| JP2018092785A (ja) * | 2016-12-02 | 2018-06-14 | 日本電気株式会社 | リチウムイオン二次電池用電解液およびリチウムイオン二次電池 |
| WO2018179884A1 (ja) * | 2017-03-30 | 2018-10-04 | パナソニックIpマネジメント株式会社 | 非水電解液及び非水電解液二次電池 |
| JP2020026926A (ja) | 2018-08-13 | 2020-02-20 | 株式会社フジクラ | 熱交換器及び磁気ヒートポンプ装置 |
| JP2021003409A (ja) | 2019-06-27 | 2021-01-14 | 株式会社三洋物産 | 遊技機 |
-
2021
- 2021-03-29 JP JP2021056292A patent/JP7563273B2/ja active Active
-
2022
- 2022-01-18 EP EP22779390.8A patent/EP4318718A1/en active Pending
- 2022-01-18 CN CN202280026650.6A patent/CN117099238A/zh active Pending
- 2022-01-18 US US18/284,376 patent/US20240170725A1/en active Pending
- 2022-01-18 WO PCT/JP2022/001511 patent/WO2022209158A1/ja active Application Filing
- 2022-01-18 KR KR1020237020273A patent/KR20230109691A/ko unknown
Patent Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2008258022A (ja) * | 2007-04-05 | 2008-10-23 | Bridgestone Corp | 電池用非水電解液及びそれを備えた非水電解液電池 |
| JP2010123300A (ja) | 2008-11-17 | 2010-06-03 | Toyota Central R&D Labs Inc | リチウム二次電池及びその使用方法 |
| JP2013140734A (ja) | 2012-01-05 | 2013-07-18 | Gs Yuasa Corp | 非水電解質二次電池 |
| JP2015201318A (ja) | 2014-04-07 | 2015-11-12 | トヨタ自動車株式会社 | 電極シートの製造方法 |
| JP2018092785A (ja) * | 2016-12-02 | 2018-06-14 | 日本電気株式会社 | リチウムイオン二次電池用電解液およびリチウムイオン二次電池 |
| WO2018179884A1 (ja) * | 2017-03-30 | 2018-10-04 | パナソニックIpマネジメント株式会社 | 非水電解液及び非水電解液二次電池 |
| JP2020026926A (ja) | 2018-08-13 | 2020-02-20 | 株式会社フジクラ | 熱交換器及び磁気ヒートポンプ装置 |
| JP2021003409A (ja) | 2019-06-27 | 2021-01-14 | 株式会社三洋物産 | 遊技機 |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2022153187A (ja) | 2022-10-12 |
| CN117099238A (zh) | 2023-11-21 |
| JP7563273B2 (ja) | 2024-10-08 |
| EP4318718A1 (en) | 2024-02-07 |
| US20240170725A1 (en) | 2024-05-23 |
| KR20230109691A (ko) | 2023-07-20 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7232359B2 (ja) | 再充電可能なバッテリーセル用のso2ベースの電解質および再充電可能なバッテリーセル | |
| JP6070540B2 (ja) | 二次電池および電解液 | |
| US9118079B2 (en) | Nonaqueous electrolytic solution secondary battery, current collector and vehicle | |
| JP7268796B2 (ja) | リチウムイオン二次電池 | |
| WO2017094416A1 (ja) | リチウム二次電池用正極活物質、リチウム二次電池用正極及びリチウム二次電池、並びにそれらの製造方法 | |
| JP6279233B2 (ja) | リチウム二次電池 | |
| JP6623464B2 (ja) | リチウムイオン二次電池 | |
| JP7003394B2 (ja) | 二次電池用電解液、二次電池、電池パック、電動車両、電力貯蔵システム、電動工具および電子機器 | |
| JP6680293B2 (ja) | ハイドロフルオロエーテル化合物、非水電解液およびリチウムイオン二次電池 | |
| JP2006066341A (ja) | 非水電解質二次電池 | |
| JP6801722B2 (ja) | 二次電池、電池パック、電動車両、電力貯蔵システム、電動工具および電子機器 | |
| WO2017126276A1 (ja) | リチウム二次電池用正極活物質、リチウム二次電池用正極、リチウム二次電池及びこれらの製造方法 | |
| CN110036521B (zh) | 锂离子二次电池 | |
| WO2022209158A1 (ja) | 電解液およびリチウムイオン二次電池 | |
| JP6177042B2 (ja) | リチウム二次電池 | |
| KR20160055137A (ko) | 이차 전지 | |
| WO2023042262A1 (ja) | リチウム2次電池 | |
| WO2022254717A1 (ja) | リチウム2次電池 | |
| JP7565624B2 (ja) | リチウム2次電池 | |
| JP2016207447A (ja) | 非水電解液二次電池 | |
| JP5582573B2 (ja) | 二次電池およびそれに用いる二次電池用電解液 | |
| KR20230137979A (ko) | 충전식 배터리 셀 | |
| WO2012029645A1 (ja) | 二次電池およびそれに用いる二次電池用電解液 | |
| CN114616709A (zh) | 二次电池用电解液添加剂、锂二次电池用非水电解液及包含其的锂二次电池 | |
| JP2024115230A (ja) | 非水電解質二次電池 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 22779390 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 20237020273 Country of ref document: KR Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 18284376 Country of ref document: US |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 202280026650.6 Country of ref document: CN |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 202317068347 Country of ref document: IN |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2022779390 Country of ref document: EP |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2022779390 Country of ref document: EP Effective date: 20231030 |