WO2016035795A1 - ダイヤモンド複合材料、及び放熱部材 - Google Patents
ダイヤモンド複合材料、及び放熱部材 Download PDFInfo
- Publication number
- WO2016035795A1 WO2016035795A1 PCT/JP2015/074880 JP2015074880W WO2016035795A1 WO 2016035795 A1 WO2016035795 A1 WO 2016035795A1 JP 2015074880 W JP2015074880 W JP 2015074880W WO 2016035795 A1 WO2016035795 A1 WO 2016035795A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- diamond
- composite material
- powder
- metal
- group
- Prior art date
Links
- 229910003460 diamond Inorganic materials 0.000 title claims abstract description 407
- 239000010432 diamond Substances 0.000 title claims abstract description 407
- 239000002131 composite material Substances 0.000 title claims abstract description 280
- 229910052751 metal Inorganic materials 0.000 claims abstract description 212
- 239000002184 metal Substances 0.000 claims abstract description 212
- 239000002245 particle Substances 0.000 claims abstract description 201
- 239000001301 oxygen Substances 0.000 claims abstract description 122
- 229910052760 oxygen Inorganic materials 0.000 claims abstract description 122
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims abstract description 121
- 230000000737 periodic effect Effects 0.000 claims abstract description 88
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 claims abstract description 51
- 229910052709 silver Inorganic materials 0.000 claims abstract description 42
- 239000004332 silver Substances 0.000 claims abstract description 42
- 229910001316 Ag alloy Inorganic materials 0.000 claims abstract description 34
- 238000010438 heat treatment Methods 0.000 claims description 37
- 229910021480 group 4 element Inorganic materials 0.000 claims description 33
- 230000006866 deterioration Effects 0.000 claims description 12
- 238000004519 manufacturing process Methods 0.000 abstract description 55
- 239000000463 material Substances 0.000 abstract description 41
- 239000000843 powder Substances 0.000 description 193
- 239000010410 layer Substances 0.000 description 151
- 150000001875 compounds Chemical class 0.000 description 65
- 239000011159 matrix material Substances 0.000 description 51
- 238000000034 method Methods 0.000 description 48
- 238000012360 testing method Methods 0.000 description 42
- 239000010936 titanium Substances 0.000 description 40
- 239000007769 metal material Substances 0.000 description 38
- 239000002994 raw material Substances 0.000 description 38
- 239000011812 mixed powder Substances 0.000 description 35
- 238000001764 infiltration Methods 0.000 description 29
- 239000004065 semiconductor Substances 0.000 description 29
- 230000008595 infiltration Effects 0.000 description 28
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical group [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 26
- 229910052799 carbon Inorganic materials 0.000 description 26
- 238000013507 mapping Methods 0.000 description 26
- 238000011049 filling Methods 0.000 description 21
- 238000002156 mixing Methods 0.000 description 18
- 229910052719 titanium Inorganic materials 0.000 description 17
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 14
- 239000011148 porous material Substances 0.000 description 14
- 238000005755 formation reaction Methods 0.000 description 13
- 150000001247 metal acetylides Chemical class 0.000 description 13
- 230000015572 biosynthetic process Effects 0.000 description 12
- 239000010949 copper Substances 0.000 description 12
- 238000004453 electron probe microanalysis Methods 0.000 description 12
- 230000017525 heat dissipation Effects 0.000 description 12
- 239000000203 mixture Substances 0.000 description 12
- 239000012298 atmosphere Substances 0.000 description 11
- 238000005259 measurement Methods 0.000 description 11
- 238000002144 chemical decomposition reaction Methods 0.000 description 10
- 229910052802 copper Inorganic materials 0.000 description 10
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 10
- 238000006243 chemical reaction Methods 0.000 description 9
- 238000001816 cooling Methods 0.000 description 9
- 230000003647 oxidation Effects 0.000 description 9
- 238000007254 oxidation reaction Methods 0.000 description 9
- 229910001868 water Inorganic materials 0.000 description 9
- 238000005219 brazing Methods 0.000 description 8
- 239000000470 constituent Substances 0.000 description 8
- 238000002360 preparation method Methods 0.000 description 8
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 7
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 7
- 239000004372 Polyvinyl alcohol Substances 0.000 description 7
- 230000002093 peripheral effect Effects 0.000 description 7
- 229920002451 polyvinyl alcohol Polymers 0.000 description 7
- 238000003825 pressing Methods 0.000 description 7
- 230000001603 reducing effect Effects 0.000 description 7
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 6
- 239000000945 filler Substances 0.000 description 6
- 150000004678 hydrides Chemical class 0.000 description 6
- 238000002844 melting Methods 0.000 description 6
- 230000008018 melting Effects 0.000 description 6
- 239000000654 additive Substances 0.000 description 5
- 230000000996 additive effect Effects 0.000 description 5
- 229910045601 alloy Inorganic materials 0.000 description 5
- 239000000956 alloy Substances 0.000 description 5
- 239000011888 foil Substances 0.000 description 5
- 230000005484 gravity Effects 0.000 description 5
- 238000007731 hot pressing Methods 0.000 description 5
- 229910052739 hydrogen Inorganic materials 0.000 description 5
- 229910000679 solder Inorganic materials 0.000 description 5
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 4
- 239000002253 acid Substances 0.000 description 4
- 239000011230 binding agent Substances 0.000 description 4
- 238000000280 densification Methods 0.000 description 4
- 238000001035 drying Methods 0.000 description 4
- 239000007789 gas Substances 0.000 description 4
- 238000000227 grinding Methods 0.000 description 4
- 239000001257 hydrogen Substances 0.000 description 4
- 150000004767 nitrides Chemical class 0.000 description 4
- 238000007747 plating Methods 0.000 description 4
- 230000000630 rising effect Effects 0.000 description 4
- 229910017944 Ag—Cu Inorganic materials 0.000 description 3
- 238000000576 coating method Methods 0.000 description 3
- 238000005429 filling process Methods 0.000 description 3
- 229910052735 hafnium Inorganic materials 0.000 description 3
- 239000012535 impurity Substances 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 238000000465 moulding Methods 0.000 description 3
- 229910052757 nitrogen Inorganic materials 0.000 description 3
- 238000005498 polishing Methods 0.000 description 3
- 238000012545 processing Methods 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- 150000003568 thioethers Chemical class 0.000 description 3
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 2
- ZOXJGFHDIHLPTG-UHFFFAOYSA-N Boron Chemical compound [B] ZOXJGFHDIHLPTG-UHFFFAOYSA-N 0.000 description 2
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 2
- MWUXSHHQAYIFBG-UHFFFAOYSA-N Nitric oxide Chemical compound O=[N] MWUXSHHQAYIFBG-UHFFFAOYSA-N 0.000 description 2
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 2
- RAHZWNYVWXNFOC-UHFFFAOYSA-N Sulphur dioxide Chemical compound O=S=O RAHZWNYVWXNFOC-UHFFFAOYSA-N 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 229910052796 boron Inorganic materials 0.000 description 2
- 239000002775 capsule Substances 0.000 description 2
- 239000000919 ceramic Substances 0.000 description 2
- 239000011362 coarse particle Substances 0.000 description 2
- 239000011248 coating agent Substances 0.000 description 2
- 238000013329 compounding Methods 0.000 description 2
- 238000005520 cutting process Methods 0.000 description 2
- 238000007580 dry-mixing Methods 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 238000000921 elemental analysis Methods 0.000 description 2
- 239000010419 fine particle Substances 0.000 description 2
- VBJZVLUMGGDVMO-UHFFFAOYSA-N hafnium atom Chemical compound [Hf] VBJZVLUMGGDVMO-UHFFFAOYSA-N 0.000 description 2
- 238000007654 immersion Methods 0.000 description 2
- 239000011810 insulating material Substances 0.000 description 2
- 238000005304 joining Methods 0.000 description 2
- 230000001590 oxidative effect Effects 0.000 description 2
- 125000004430 oxygen atom Chemical group O* 0.000 description 2
- VSZWPYCFIRKVQL-UHFFFAOYSA-N selanylidenegallium;selenium Chemical compound [Se].[Se]=[Ga].[Se]=[Ga] VSZWPYCFIRKVQL-UHFFFAOYSA-N 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- 229910052717 sulfur Inorganic materials 0.000 description 2
- 239000011593 sulfur Substances 0.000 description 2
- 239000012085 test solution Substances 0.000 description 2
- 229910052726 zirconium Inorganic materials 0.000 description 2
- 238000007088 Archimedes method Methods 0.000 description 1
- 229910000881 Cu alloy Inorganic materials 0.000 description 1
- YCKRFDGAMUMZLT-UHFFFAOYSA-N Fluorine atom Chemical compound [F] YCKRFDGAMUMZLT-UHFFFAOYSA-N 0.000 description 1
- UCKMPCXJQFINFW-UHFFFAOYSA-N Sulphide Chemical compound [S-2] UCKMPCXJQFINFW-UHFFFAOYSA-N 0.000 description 1
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 229910052786 argon Inorganic materials 0.000 description 1
- 239000012300 argon atmosphere Substances 0.000 description 1
- 238000007664 blowing Methods 0.000 description 1
- -1 carbide Substances 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 239000007795 chemical reaction product Substances 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 239000003638 chemical reducing agent Substances 0.000 description 1
- 229910052804 chromium Inorganic materials 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 125000004122 cyclic group Chemical group 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 230000007812 deficiency Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000002542 deteriorative effect Effects 0.000 description 1
- 238000010894 electron beam technology Methods 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 239000012467 final product Substances 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 230000001771 impaired effect Effects 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 229910010272 inorganic material Inorganic materials 0.000 description 1
- 239000011147 inorganic material Substances 0.000 description 1
- 230000010354 integration Effects 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 238000003754 machining Methods 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 239000000155 melt Substances 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- 229910052759 nickel Inorganic materials 0.000 description 1
- 150000002926 oxygen Chemical class 0.000 description 1
- RVZRBWKZFJCCIB-UHFFFAOYSA-N perfluorotributylamine Chemical compound FC(F)(F)C(F)(F)C(F)(F)C(F)(F)N(C(F)(F)C(F)(F)C(F)(F)C(F)(F)F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F RVZRBWKZFJCCIB-UHFFFAOYSA-N 0.000 description 1
- 230000002250 progressing effect Effects 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 238000010079 rubber tapping Methods 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 238000007493 shaping process Methods 0.000 description 1
- 239000002356 single layer Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 238000004544 sputter deposition Methods 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 239000002344 surface layer Substances 0.000 description 1
- 230000003746 surface roughness Effects 0.000 description 1
- 229910052718 tin Inorganic materials 0.000 description 1
- 239000011135 tin Substances 0.000 description 1
- 238000001291 vacuum drying Methods 0.000 description 1
- 238000007740 vapor deposition Methods 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K5/00—Heat-transfer, heat-exchange or heat-storage materials, e.g. refrigerants; Materials for the production of heat or cold by chemical reactions other than by combustion
- C09K5/08—Materials not undergoing a change of physical state when used
- C09K5/14—Solid materials, e.g. powdery or granular
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F1/00—Metallic powder; Treatment of metallic powder, e.g. to facilitate working or to improve properties
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C1/00—Making non-ferrous alloys
- C22C1/04—Making non-ferrous alloys by powder metallurgy
- C22C1/0466—Alloys based on noble metals
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C1/00—Making non-ferrous alloys
- C22C1/10—Alloys containing non-metals
- C22C1/1036—Alloys containing non-metals starting from a melt
- C22C1/1073—Infiltration or casting under mechanical pressure, e.g. squeeze casting
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C26/00—Alloys containing diamond or cubic or wurtzitic boron nitride, fullerenes or carbon nanotubes
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C5/00—Alloys based on noble metals
- C22C5/06—Alloys based on silver
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C5/00—Alloys based on noble metals
- C22C5/06—Alloys based on silver
- C22C5/08—Alloys based on silver with copper as the next major constituent
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L21/00—Processes or apparatus adapted for the manufacture or treatment of semiconductor or solid state devices or of parts thereof
- H01L21/02—Manufacture or treatment of semiconductor devices or of parts thereof
- H01L21/04—Manufacture or treatment of semiconductor devices or of parts thereof the devices having potential barriers, e.g. a PN junction, depletion layer or carrier concentration layer
- H01L21/48—Manufacture or treatment of parts, e.g. containers, prior to assembly of the devices, using processes not provided for in a single one of the subgroups H01L21/06 - H01L21/326
- H01L21/4814—Conductive parts
- H01L21/4871—Bases, plates or heatsinks
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L23/00—Details of semiconductor or other solid state devices
- H01L23/34—Arrangements for cooling, heating, ventilating or temperature compensation ; Temperature sensing arrangements
- H01L23/36—Selection of materials, or shaping, to facilitate cooling or heating, e.g. heatsinks
- H01L23/373—Cooling facilitated by selection of materials for the device or materials for thermal expansion adaptation, e.g. carbon
- H01L23/3732—Diamonds
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L23/00—Details of semiconductor or other solid state devices
- H01L23/34—Arrangements for cooling, heating, ventilating or temperature compensation ; Temperature sensing arrangements
- H01L23/36—Selection of materials, or shaping, to facilitate cooling or heating, e.g. heatsinks
- H01L23/373—Cooling facilitated by selection of materials for the device or materials for thermal expansion adaptation, e.g. carbon
- H01L23/3735—Laminates or multilayers, e.g. direct bond copper ceramic substrates
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F7/00—Manufacture of composite layers, workpieces, or articles, comprising metallic powder, by sintering the powder, with or without compacting wherein at least one part is obtained by sintering or compression
- B22F7/06—Manufacture of composite layers, workpieces, or articles, comprising metallic powder, by sintering the powder, with or without compacting wherein at least one part is obtained by sintering or compression of composite workpieces or articles from parts, e.g. to form tipped tools
- B22F7/062—Manufacture of composite layers, workpieces, or articles, comprising metallic powder, by sintering the powder, with or without compacting wherein at least one part is obtained by sintering or compression of composite workpieces or articles from parts, e.g. to form tipped tools involving the connection or repairing of preformed parts
- B22F2007/066—Manufacture of composite layers, workpieces, or articles, comprising metallic powder, by sintering the powder, with or without compacting wherein at least one part is obtained by sintering or compression of composite workpieces or articles from parts, e.g. to form tipped tools involving the connection or repairing of preformed parts using impregnation
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F2301/00—Metallic composition of the powder or its coating
- B22F2301/25—Noble metals, i.e. Ag Au, Ir, Os, Pd, Pt, Rh, Ru
- B22F2301/255—Silver or gold
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F2302/00—Metal Compound, non-Metallic compound or non-metal composition of the powder or its coating
- B22F2302/05—Boride
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F2302/00—Metal Compound, non-Metallic compound or non-metal composition of the powder or its coating
- B22F2302/20—Nitride
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F2302/00—Metal Compound, non-Metallic compound or non-metal composition of the powder or its coating
- B22F2302/40—Carbon, graphite
- B22F2302/406—Diamond
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F2302/00—Metal Compound, non-Metallic compound or non-metal composition of the powder or its coating
- B22F2302/45—Others, including non-metals
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F2304/00—Physical aspects of the powder
- B22F2304/10—Micron size particles, i.e. above 1 micrometer up to 500 micrometer
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F2998/00—Supplementary information concerning processes or compositions relating to powder metallurgy
- B22F2998/10—Processes characterised by the sequence of their steps
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F2999/00—Aspects linked to processes or compositions used in powder metallurgy
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B22—CASTING; POWDER METALLURGY
- B22F—WORKING METALLIC POWDER; MANUFACTURE OF ARTICLES FROM METALLIC POWDER; MAKING METALLIC POWDER; APPARATUS OR DEVICES SPECIALLY ADAPTED FOR METALLIC POWDER
- B22F7/00—Manufacture of composite layers, workpieces, or articles, comprising metallic powder, by sintering the powder, with or without compacting wherein at least one part is obtained by sintering or compression
- B22F7/06—Manufacture of composite layers, workpieces, or articles, comprising metallic powder, by sintering the powder, with or without compacting wherein at least one part is obtained by sintering or compression of composite workpieces or articles from parts, e.g. to form tipped tools
- B22F7/08—Manufacture of composite layers, workpieces, or articles, comprising metallic powder, by sintering the powder, with or without compacting wherein at least one part is obtained by sintering or compression of composite workpieces or articles from parts, e.g. to form tipped tools with one or more parts not made from powder
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C26/00—Alloys containing diamond or cubic or wurtzitic boron nitride, fullerenes or carbon nanotubes
- C22C2026/005—Alloys containing diamond or cubic or wurtzitic boron nitride, fullerenes or carbon nanotubes with additional metal compounds being borides
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C26/00—Alloys containing diamond or cubic or wurtzitic boron nitride, fullerenes or carbon nanotubes
- C22C2026/006—Alloys containing diamond or cubic or wurtzitic boron nitride, fullerenes or carbon nanotubes with additional metal compounds being carbides
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C26/00—Alloys containing diamond or cubic or wurtzitic boron nitride, fullerenes or carbon nanotubes
- C22C2026/007—Alloys containing diamond or cubic or wurtzitic boron nitride, fullerenes or carbon nanotubes with additional metal compounds being nitrides
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C26/00—Alloys containing diamond or cubic or wurtzitic boron nitride, fullerenes or carbon nanotubes
- C22C2026/008—Alloys containing diamond or cubic or wurtzitic boron nitride, fullerenes or carbon nanotubes with additional metal compounds other than carbides, borides or nitrides
Definitions
- the present invention relates to a composite material in which diamond and metal are composited, a method for manufacturing the composite material, and a heat radiating member composed of the composite material.
- a heat radiating member (called a heat sink, a heat spreader, or the like) for expanding a heat radiating area is used for heat radiation of a semiconductor element, in addition to natural convection and forced air blowing.
- Patent Document 1 discloses a composite material of diamond and an Ag—Cu alloy.
- Patent Document 2 discloses a composite material of diamond and copper.
- Diamond is generally inferior in wettability with metal.
- pores are generated in the vicinity of the interface between the diamond and the metal, resulting in a decrease in the density and thermal conductivity of the composite material due to the pores. Therefore, it is desired to develop a composite material of diamond and metal that is used as a material for a heat radiating member such as a semiconductor element, which is fine with few pores and excellent in thermal conductivity.
- Patent Document 1 discloses that Ti powder is used as a raw material, diamond itself and Ti are reacted to form Ti carbide on the surface of diamond particles, and the Ti carbide and Ag—Cu alloy are wetted. Discloses a structure in which diamond particles and an Ag—Cu alloy are in close contact with each other through the carbide.
- elements of Group 4 of the periodic table such as Ti are generally easily bonded to oxygen, and an oxide film may be present on the surface of the Ti powder particles. This oxide film inhibits the reaction between diamond and Ti, so that the wettability cannot be sufficiently increased, and the density of the composite material and the thermal conductivity due to the pores can be reduced. Since the oxide can remain in the composite material, the thermal conductivity can be lowered.
- Patent Document 1 silver powder or a silver plate is used as a raw material. Since silver itself may contain oxygen, oxygen released from silver and elements of Group 4 of the periodic table such as Ti may be combined to form an oxide, which may inhibit the reaction between diamond and Ti. is there.
- oxides for example, oxides such as Cr and Fe
- oxides such as Cr and Fe may remain on the surface of the diamond powder particles. This oxide can also be a factor that hinders the reaction between diamond and Group 4 elements of the periodic table such as Ti.
- Citation 2 discloses a manufacturing method in which a green compact of diamond powder and copper powder is filled into a Mo capsule, sintered under ultra-high pressure, and then the capsule is ground and removed. According to this manufacturing method, a dense composite material is obtained, and no oxide is formed in copper. However, in this composite material, diamond and copper are only in contact with each other, and both are not bonded, and when used as a heat dissipation member, a gap occurs at the interface between diamond and copper due to repeated cooling and heating cycles, There is a risk of deteriorating thermal properties. In addition, this manufacturing method is inferior in manufacturability of composite materials because it requires equipment capable of generating and controlling ultra-high pressure. Accordingly, it is desired to develop a method for producing a diamond composite material that can reduce and remove oxides that may cause a decrease in thermal conductivity, while being a simpler production method.
- one of the objects of the present invention is to provide a dense diamond composite material and a heat radiating member that are excellent in thermal conductivity.
- Another object of the present invention is to provide a method for producing a diamond composite material that can produce a dense diamond composite material having excellent wettability between diamond and metal with high productivity.
- a diamond composite material bonds diamond particles, coated diamond particles that cover the surface of the diamond particles and includes a carbide layer containing an element of Group 4 of the periodic table, and the coated diamond particles.
- Silver or a silver alloy is provided, and the oxygen content is 0.1% by mass or less.
- Examples of the method for producing the diamond composite material include the following production methods.
- This method for manufacturing a diamond composite material includes the following preparation process, filling process, and infiltration process.
- Preparation step As raw materials, diamond powder, powder of one or more group 4 compounds selected from sulfides, nitrides, hydrides, borides containing elements of group 4 of the periodic table, and silver or silver alloy
- Preparing a metal material including (Filling step) A step of filling the mold with the diamond powder, the Group 4 compound powder and the metal material.
- (Infiltration step) A step of heating the filler filled in the mold and combining the diamond with the molten silver or silver alloy.
- the above diamond composite material is excellent in thermal conductivity and dense.
- the above-described method for producing a diamond composite material is excellent in wettability between diamond and metal and can easily produce a dense diamond composite material.
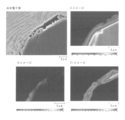
- Sample No. produced in Test Example 1 It is an image obtained by observing the cross section of the diamond composite material 1-3 with an electron beam microanalyzer (EPMA). The upper left is a reflected electron image, the lower left is an oxygen (O) mapping image, the upper right is a carbon (C) mapping image, and the lower right. Indicates a Ti mapping image.
- Sample No. produced in Test Example 1 The cross section of the diamond composite material of 1-102 is an image obtained by observing the vicinity of diamond particles with EPMA. The upper left shows a reflected electron image, the lower left shows an O mapping image, the upper right shows a C mapping image, and the lower right shows a Ti mapping image. .
- a diamond composite material includes a diamond particle, a coated diamond particle that covers the surface of the diamond particle and includes a carbide layer containing a group 4 element in the periodic table, and the coated diamond particles. And an oxygen content of 0.1% by mass or less.
- the periodic table refers to a long periodic table represented by the new IUPAC formula.
- the diamond composite material is dense and excellent in thermal conductivity from the following points.
- (Dense) The above-mentioned diamond composite material has an oxygen content of 0.1% by mass or less and a low oxygen content. Therefore, there is little oxygen over the entire composite material including the interface between the diamond particles and the carbide layer containing the elements of Group 4 of the periodic table and the vicinity thereof, preferably it does not exist, and the surface of the diamond particles and the carbide layer are not present. It can be said that there is almost no oxide. In such a diamond composite material, it is considered that the generation of pores that cause a decrease in density is sufficiently suppressed during the production process, and a carbide layer is easily formed on the surface of diamond.
- the diamond is in close contact with the carbide layer containing the Group 4 element.
- the elements of Group 4 of the periodic table existing around the diamond particles are mainly present as carbides.
- there is almost no oxide in silver or a silver alloy hereinafter sometimes referred to as a metal matrix.
- a metal matrix in such a diamond composite material, in the manufacturing process, the wettability between the carbide layer containing the elements of Group 4 of the periodic table and the molten metal forming the metal matrix is sufficiently enhanced, and pores that cause a decrease in density It is considered that the occurrence of the occurrence was sufficiently suppressed.
- the main components are diamond particles having a thermal conductivity of 1000 W / m ⁇ K or higher and silver or a silver alloy that tends to have a higher thermal conductivity than copper or a copper alloy.
- oxygen is low throughout the composite material including the vicinity of the diamond particles, preferably it is not present, that is, there is little oxide, preferably not present, that is inferior in thermal conductivity.
- Diamond particles are bonded together by the metal matrix and are dense, so the heat conduction path that connects the diamond particles, carbide, and metal matrix, and the heat that the carbides formed on the surface of the diamond particles are connected continuously. Conduction paths can be built well.
- the above-mentioned diamond composite material includes both diamond particles having a thermal expansion coefficient of about 2.3 ⁇ 10 ⁇ 6 / K or less and a metal matrix having a thermal expansion coefficient larger than that of diamond.
- the coefficient is close to the thermal expansion coefficient of a semiconductor element or a peripheral part of a semiconductor device (the difference is small and the matching is excellent). Therefore, the diamond composite material can be suitably used as a material for a heat dissipation member of a semiconductor element.
- the above form is dense, has few pores, can reduce a decrease in thermal conductivity due to the pores, and has high thermal conductivity.
- the average particle diameter of the diamond particles is 1 ⁇ m or more and 300 ⁇ m or less.
- the above form can suppress a decrease in thermal conductivity due to excessive diamond powder grain boundaries in the composite material because the diamond particles are too small, and has high thermal conductivity. And the said form can suppress the fall of workability, such as grinding by excessive diamond particle
- the above form is excellent in thermal conductivity because it contains sufficient diamond particles. And the said form can suppress degradation of the infiltration property (generation
- the thermal conductivity at room temperature is 500 W / m ⁇ K or more.
- Room temperature includes 20 ° C. or more and 27 ° C. or less under atmospheric pressure.
- the above embodiment can be suitably used for a material such as a heat radiating member of a semiconductor element which has a very high thermal conductivity and requires high heat dissipation.
- an average thermal expansion coefficient at 30 ° C. to 150 ° C. is 3 ⁇ 10 ⁇ 6 / K or more and 13 ⁇ 10 ⁇ 6 / K or less.
- the above form is excellent in consistency with the thermal expansion coefficient of the semiconductor element (for example, GaN: about 5.5 ⁇ 10 ⁇ 6 / K) and the thermal expansion coefficient of peripheral components such as a package, and the heat dissipation member of the semiconductor element It can use suitably for materials, such as.
- the thermal expansion coefficient of the semiconductor element for example, GaN: about 5.5 ⁇ 10 ⁇ 6 / K
- the thermal expansion coefficient of peripheral components such as a package
- the heat dissipation member of the semiconductor element It can use suitably for materials, such as.
- the thermal cycle resistance at ⁇ 60 ° C. to + 250 ° C. is 95% or more.
- the heat cycle resistance is (heat conductivity after the heat cycle / heat conductivity before the heat cycle) ⁇ 100.
- the above-mentioned diamond composite material with low oxygen content, denseness, and high thermal conductivity has little decrease in thermal conductivity even when subjected to a cooling cycle of ⁇ 60 ° C. to + 250 ° C. Conductivity can be maintained. Therefore, the said form can be utilized suitably for raw materials, such as a heat radiating member of the semiconductor element which receives a cooling cycle at the time of use.
- the deterioration rate of thermal conductivity after heating to 800 ° C. is less than 5%.
- the deterioration rate is ⁇ [(thermal conductivity before heating) ⁇ (thermal conductivity after heating)] / (thermal conductivity before heating) ⁇ ⁇ 100.
- the above-mentioned form maintains a high thermal conductivity even when heated to a high temperature such as 800 ° C. by being a diamond composite material having a low oxygen content, a denseness, and a high thermal conductivity as described above. It has excellent heat resistance.
- a form is a material such as a heat radiating member of a semiconductor element in which an insulating material made of ceramics or the like may be bonded using a high melting point bonding material such as a silver brazing material (melting point of about 780 ° C.), for example. Can be suitably used.
- the above form is easy to be smooth and has excellent surface properties by providing a metal layer. Further, when this form is used for a heat radiating member of a semiconductor element or the like, the semiconductor element and the heat radiating member can be firmly joined by using the metal layer as a base such as solder or brazing material.
- a heat dissipating member according to one aspect of the present invention is composed of the diamond composite material described in any one of (1) to (9) above.
- the heat dissipating member is composed of the above-mentioned diamond composite material that is dense and excellent in thermal conductivity, it is dense and excellent in thermal conductivity. Since said diamond composite material is excellent also in consistency with the thermal expansion coefficient of a semiconductor element, said heat radiating member can be utilized suitably for the heat radiating member of a semiconductor element.
- Examples of the method for producing the diamond composite material include the following production methods.
- M1 This method for producing a diamond composite material includes the following preparation process, filling process, and infiltration process.
- Preparation step As raw materials, diamond powder, powder of one or more group 4 compounds selected from sulfides, nitrides, hydrides, borides containing elements of group 4 of the periodic table, and silver or silver alloy
- Preparing a metal material including (Filling step) A step of filling the mold with the diamond powder, the Group 4 compound powder and the metal material.
- Infiltration step A step of heating the filler filled in the mold and combining the diamond with the molten silver or silver alloy.
- the manufacturing method of the above-mentioned diamond composite material does not use the elements of Group 4 of the periodic table as raw materials as in Patent Document 1, but rather the elements of Group 4 of the periodic table and specific elements, specifically sulfur, nitrogen.
- a Group 4 compound powder containing at least one element of hydrogen and boron is used as a raw material.
- the powder of the group 4 compound By using the powder of the group 4 compound, the oxidation of the elements of the group 4 of the periodic table in the raw material stage, the preparation process, the filling process, and the like can be suppressed. Due to this inhibition of oxidation, the surroundings of the elements in Group 4 of the periodic table are likely to be in a state where oxygen is low.
- the elements in Group 4 of the periodic table generated by the chemical decomposition of the Group 4 compound are oxidized by the surrounding oxygen. Can be suppressed. Further, some of the above specific elements have a reducing action.
- the reduction action means oxygen or oxides that can be contained in raw materials such as industrial diamond, silver, or silver alloy in the temperature rising process of the infiltration process, and the surroundings of elements of Group 4 of the periodic table generated by chemical decomposition. This is an action capable of reducing oxygen and oxides that may be present in the gas and removing them as gas (for example, water vapor).
- Oxidation suppressing action and reducing action of the specific element can effectively suppress oxidation of diamond, silver, and the like as well as the elements of Group 4 of the periodic table during the production process. From the above, it is possible to satisfactorily form a carbide that can react satisfactorily with the elements of Group 4 of the periodic table and diamond and enhance the wettability of diamond and molten metal, without excess or deficiency.
- a powder of the group 4 compound as a supply source of a carbide-forming element (group 4 element of the periodic table)
- the supply amount of the group 4 element of the periodic table is little or substantially not changed and stable.
- the thickness variation of the carbide layer hardly occurs.
- a composite material (typically, the diamond composite material according to the embodiment) having a small oxygen content, being dense, and having excellent thermal conductivity can be produced.
- the filler in the mold is a mixture (layer) of mixed powder and a metal material (layer)
- the above-mentioned group 4 compound tends to exist reliably around the diamond. Therefore, in the above embodiment, the group 4 element of the periodic table and diamond more easily react to easily form a carbide, the group 4 element of the periodic table that did not substantially react with diamond remains, It is easy to suppress the presence of an oxide.
- the weight of the molten metal can be increased.
- the molten metal Due to the weight of the molten metal, it can move automatically and easily to the layer side of the mixed powder and can be infiltrated well. Furthermore, it is easy to uniformly infiltrate the molten metal on the layer side of the mixed powder, and a diamond composite material in which a metal matrix is uniformly present is obtained as compared with the case where a small amount of molten metal is dispersed and produced in various places. It is considered easy.
- the metal material is made of metal powder and mixed with diamond powder, it can be said that the metal powder has a specific gravity larger than that of diamond and is difficult to mix uniformly with diamond powder.
- the said form should just mix the powder of a diamond and the powder of the said 4th group compound with a comparatively small specific gravity difference with a diamond, and is excellent in mixing workability
- a coated composite material including a metal layer composed of a metal having the same composition as that of the metal matrix on both sides of the diamond composite material can be manufactured.
- the above-mentioned form can form a metal layer simultaneously with infiltration, and can produce a coated composite material with high productivity, with fewer steps compared to the case where the metal layer is formed in a separate process such as joining of a metal foil or the like.
- the obtained coating composite material has a structure in which the metal matrix and the metal layer are continuous, the bonding strength is high, the metal layer is difficult to peel off, and the thermal conductivity is also excellent.
- this embodiment can obtain various effects described in the above (m2).
- the metal material is a metal powder
- the metal powder layer includes a Group 4 compound powder containing an element of Group 4 of the periodic table and a group of Periodic Table 4 Examples include a form containing at least one of the elemental powders.
- the metal material layer is a layer containing a powder of the Group 4 compound or a Group 4 element powder in addition to the metal powder
- the Group 4 compound contained in the metal powder layer is chemically decomposed.
- the elements of Group 4 of the periodic table and the elements of Group 4 of the periodic table contained in the metal powder layer are first taken into the molten metal formed by melting the metal powder in the temperature rising process of the infiltration process, and then Reacts with diamond to form carbides. If the reaction of diamond starts, such a reaction is likely to occur continuously thereafter.
- the above-described embodiment provides a state in which the elements of Group 4 of the periodic table are easily taken into the molten metal infiltrated into diamond, and as a result, the reaction between the diamond and the elements of Group 4 of the periodic table is facilitated. Even so, carbides can be formed better. Therefore, according to the said form, the diamond composite material which is denser and has higher thermal conductivity can be manufactured.
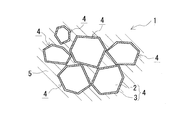
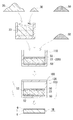
- Diamond composite material 1 includes a plurality of coatings including diamond particles 2 and a carbide layer 3 that covers the surface of the diamond particles 2 and includes a group 4 element of the periodic table as shown in FIG. Diamond particles 4 and a metal matrix 5 for bonding the coated diamond particles 4 to each other are provided.
- the metal matrix 5 is filled in the gap formed by the plurality of coated diamond particles 4, and the aggregated state of the diamond particles 2 is maintained by the metal matrix 5.
- the composite material 1 is a dense molded body having very few pores and filled with the metal matrix 5 without any gap (see the reflected electron image of EPMA in FIG. 2).
- One of the characteristics of the composite material 1 of the embodiment is that the oxygen content is low throughout.
- each component will be described in detail.
- the diamond composite material 1 includes a plurality of diamond particles 2 as one of main components.
- the composite material 1 having a thermal conductivity of 500 W / m ⁇ K or more can be obtained.
- the composite material 1 having a thermal expansion coefficient of 4 ⁇ 10 ⁇ 6 / K or more and 9.5 ⁇ 10 ⁇ 6 / K or less can be obtained, which is close to the thermal expansion coefficient of the semiconductor element and its peripheral components.
- the content of diamond particles 2 in the composite material 1 is preferably 30% by volume or more and 90% by volume, and more than 45% by volume. 85 volume% or less, 50 volume% or more and 80 volume% or less are more preferable. A method for measuring the content of the diamond particles 2 in the composite material 1 will be described later.
- the composite material 1 having a thermal conductivity of 500 W / m ⁇ K or more can be obtained.
- the particle size is not too large, it is excellent in workability such as grinding and can be easily adjusted to satisfy a predetermined dimensional tolerance. If the particle size is not too large, a thin composite material 1 can be obtained.
- the average particle size of the diamond particles 2 in the composite material 1 is preferably 1 ⁇ m to 300 ⁇ m, more preferably 1 ⁇ m to 100 ⁇ m, and more preferably 20 ⁇ m to 60 ⁇ m.
- Diamond powder can also be finely mixed.
- the composite material 1 containing finely mixed diamond powder is denser and has a higher relative density. A method for measuring the average particle diameter of the diamond particles 2 in the composite material 1 will be described later.
- each diamond particle 2 in the diamond composite material 1 is covered with a carbide containing an element of Group 4 of the periodic table, and each coated diamond particle 4 is formed of the carbide. Is provided.
- the carbide layer 3 is in close contact with both the diamond particles 2 and the metal matrix 5 (see the reflected electron image of EPMA in FIG. 2).
- the carbide layer 3 was easily formed on the diamond surface in the manufacturing process, and the carbide layer 3 was a molten metal. It is thought that it was able to adhere
- the composite material 1 including such a carbide layer 3 is dense with the diamond particles 2, the carbide layer 3, and the metal matrix 5 in close contact with each other without a gap.
- Various methods can be used for forming the carbide layer 3 as long as the gist of the present invention is not impaired.
- the carbide layer 3 is a carbide formed by combining constituent elements (carbon) in the surface side region of the diamond particles 2 and elements of Group 4 of the periodic table. It is preferable to be configured. In this case, since the carbide layer 3 includes the components of the diamond particles 2 themselves as constituent elements, the carbide layer 3 can be made into a denser composite material 1 that is more excellent in adhesion to the diamond particles 2.
- the main constituent components of the carbide layer 3 are carbon, preferably carbon derived from the diamond particles 2 and elements of Group 4 of the periodic table.
- Examples of the element of Group 4 of the periodic table included in the carbide layer 3 include at least one selected from titanium (Ti), zirconium (Zr), and hafnium (Hf).
- the carbide layer 3 can be in a form containing a plurality of kinds of elements in addition to a form containing only one kind of the listed elements.
- the carbide layer 3 is thin to some extent in consideration of thermal conductivity.
- the average thickness of the carbide layer 3 is preferably 5 ⁇ m or less, 3 ⁇ m or less, less than 3 ⁇ m, more preferably 1 ⁇ m or less, and can be nano-ordered.
- the thickness of the carbide layer 3 can be adjusted by adjusting the addition amount and size of the raw material.
- each coated diamond particle 4 is preferably a dense composite material 1 if it is 90 area% or more of the surface area of the diamond particle 2 and the entire surface of the diamond is covered with the above-mentioned carbide.
- the composite material 1 becomes more dense.
- it is allowed to include a portion on the surface of the diamond where a carbide containing an element of Group 4 of the periodic table does not exist the smaller this portion is, the more preferable.
- the diamond composite material 1 can have a portion in which at least a part of the carbide layer 3 provided in the adjacent coated diamond particles 4 is bonded and integrated (hereinafter, this portion may be referred to as a connecting portion). Both the form having a connecting part made of carbide and the form not having a connecting part (a form in which coated diamond particles are dispersed apart) are dense and excellent in thermal characteristics.
- the diamond composite material 1 has a metal matrix 5 as one of main components.
- the component of the metal matrix 5 is silver (so-called pure silver) or a silver alloy. If the metal matrix 5 is silver, the composite material 1 having a high thermal conductivity of 427 W / m ⁇ K and excellent thermal conductivity can be obtained.
- the silver alloy is an alloy containing Ag in excess of 50% by mass and an additive element, with the balance being inevitable impurities. In particular, a silver alloy containing 70% by mass or more of Ag and an additive element, and the balance being inevitable impurities, tends to have a low liquidus temperature while maintaining high thermal conductivity. Since it can be combined well even at low temperatures, it is excellent in manufacturability.
- the additive element of the silver alloy include Cu. The total content of additive elements is about 30% by mass or less.
- the oxygen content of the composite material 1 is 0.1% by mass or less. If the oxygen content of the entire composite material 1 is 0.1% by mass or less, oxides, pores and the like are sufficiently small in the vicinity of the surface side of the diamond particles 2, and are preferably substantially absent. Therefore, the composite material 1 can suppress a decrease in thermal conductivity between the diamond particles 2 and the metal matrix 5 due to the inclusion of an oxide or the like, and is excellent in thermal conductivity. Further, if the oxide is small, it can be said that the elements of Group 4 of the periodic table are present as carbides instead of oxides, and the dense composite material 1 can be obtained by interposing the carbide layer 3.
- the oxygen content is preferably as small as possible, more preferably 0.095% by mass or less, 0.090% by mass or less, and 0.080% by mass or less.
- the carbide layer 3 is present on the surface of the diamond particles 2 in the diamond composite material 1 except for the metal matrix 5. That is, when elemental analysis of the vicinity of the surface side of the diamond particle 2 is performed, it is preferable that carbon and the elements of Group 4 of the periodic table are mainly present, and other elements, particularly oxygen is small. When oxygen is present in the vicinity of the surface side of the diamond particle 2, it is considered that this oxygen is present, for example, as an oxide of a group 4 element in the periodic table. Since this oxide has low thermal conductivity and poor wettability with molten metal, when it is present in the vicinity of the surface of the diamond particle 2, it can be a composite material having poor thermal conductivity and denseness.
- the oxygen concentration in the vicinity of the surface side of the diamond particles 2 is also sufficiently low.
- a boundary between the diamond particles 2 and the carbide layer 3 is taken, and an annular region having a thickness of up to 5 ⁇ m is taken from this boundary toward the outer peripheral side (metal matrix 5 side).
- this annular region is defined as the outer peripheral region, those having an oxygen content of 0.1% by mass or less in the outer peripheral region can be mentioned.
- the boundary can be easily visualized by using element mapping of EPMA described later. Further, by using element mapping of EPMA, it is easily confirmed that the composite material 1 of the embodiment has very little oxygen, preferably substantially absent, in the vicinity of the boundary between the diamond particles 2 and the carbide layer 3. it can.
- Conceivable sources of oxygen that can be contained in the diamond composite material 1 are a raw material diamond powder 20 (FIG. 4), a silver or silver alloy metal material (metal powder 50 in FIG. 4), an atmosphere in the manufacturing process, and the like. . Therefore, oxygen can be contained in any location in the composite material 1.
- the oxygen concentration in the whole is in a specific range, and due to the fact that the whole oxygen is small, the portion near the boundary between the diamond and the substance adjacent to the diamond is likely to cause deterioration in thermal conductivity.
- there is very little oxygen By utilizing the method for producing a diamond composite material described later, oxygen can be reduced and removed well in the production process, and the composite material 1 having a low oxygen concentration throughout the vicinity including the vicinity of the diamond particles 2 can be produced.
- a metal layer 6 covering at least a part of the surface of the composite material 1 can be provided (the coated composite material 1 ⁇ / b> B in FIG. 6 is an example). ).
- the coated composite material 1B including the metal layer 6 is sufficiently wetted with the metal such as the metal layer 6 and the solder or brazing material. It is preferable that the covering composite material 1B and the like can be firmly bonded to the semiconductor element.
- the constituent metal of the metal layer 6 is not particularly limited as long as it is a metal that can withstand the use temperature of solder or brazing material.
- the metal layer 6 has, for example, a form that is the same component as the metal matrix 5, a form that has the same main component as the metal matrix 5 (for example, a form in which both the metal matrix 5 and the metal layer 6 are silver alloys and have different additive elements)
- the metal matrix 5 is silver and the metal layer 6 is a silver alloy), and the metal matrix 5 and the metal layer 6 are completely different components.
- specific metals include copper, gold, aluminum, nickel, zinc, tin, alloys of each element, and the like.
- the metal layer 6 can have a multilayer structure as well as a single layer structure.
- the method for forming the metal layer 6 is not particularly limited (see later).
- the metal layer 6 is preferably thin for the purpose of suppressing a decrease in the thermal conductivity of the entire coated composite material.
- the metal layer 6 has a thickness (total thickness in the case of a multilayer structure) of 300 ⁇ m or less, 200 ⁇ m or less, or 100 ⁇ m or less.
- the thickness of the metal layer 6 is 0.5 ⁇ m or more, 5 ⁇ m or more, or 20 ⁇ m or more for the purpose of the above-mentioned bonding base.
- the metal layer 6 may not be provided, and the thickness may be less than 0.5 ⁇ m.
- the diamond composite material 1 and the coated composite material 1B of the embodiment are excellent in thermal conductivity.
- the composite material 1 described above has a thermal conductivity at room temperature of 500 W / m ⁇ K or more (in the case of a coated composite material, the thermal conductivity in a state including the metal layer 6).
- the diamond composite material 1 and the coated composite material 1B according to the embodiment are mainly composed of diamond particles 2 having a small thermal expansion coefficient and a metal matrix 5 having a sufficiently larger thermal expansion coefficient than diamond, thereby having a thermal expansion coefficient.
- the intermediate value of both can be taken.
- the composite material 1 and the like satisfy an average coefficient of thermal expansion at 30 ° C. to 150 ° C. of 3 ⁇ 10 ⁇ 6 / K or more and 13 ⁇ 10 ⁇ 6 / K or less (in the case of a coated composite material, a metal layer) 6).
- the thermal expansion coefficient is 4 ⁇ 10 ⁇ 6 / K or more and 12 ⁇ 10 ⁇ 6 / K or less, 4.5 ⁇ 10 ⁇ 6 / K or more and 10 or more, although it depends on the content of the diamond particles 2 and the components of the metal matrix 5. X10 ⁇ 6 / K or less can be satisfied.
- Diamond composite material having a thermal conductivity at room temperature of 500 W / m ⁇ K or more and an average coefficient of thermal expansion at 30 ° C. to 150 ° C. of 3 ⁇ 10 ⁇ 6 / K to 13 ⁇ 10 ⁇ 6 / K 1 and the coated composite material 1B are excellent in thermal conductivity and excellent in consistency with the thermal expansion coefficient of the semiconductor element and its peripheral components, and therefore can be suitably used as a heat dissipation member for the semiconductor element.
- the diamond composite material 1 and the coated composite material 1B of the embodiment are excellent in thermal conductivity, and also have a low thermal conductivity and a high thermal conductivity even when subjected to a thermal cycle or heated to a high temperature.
- the rate can be maintained (in the case of a coated composite material, the thermal conductivity in a state including the metal layer 6).
- composite material 1 having a thermal cycle resistance at ⁇ 60 ° C. to + 250 ° C. of 95% or more can be given.
- Such a composite material 1 or the like can be suitably used as a heat radiating member of a semiconductor element that undergoes a cooling / heating cycle when in use since the decrease in thermal conductivity is as low as 5% or less even when subjected to a cooling / heating cycle.
- the composite material 1 etc. whose deterioration rate of thermal conductivity after heating at 800 degreeC is less than 5% are mentioned.
- the heat radiating member may be bonded to an insulating material made of ceramics or the like.
- a high melting point bonding material such as a silver brazing material may be used for this bonding.
- the heat radiating member is heated by the bonding material, and it is desired that the heat conductivity of the heat radiating member is less reduced by this heating.
- the composite material 1 having a thermal conductivity deterioration of less than 5% is excellent in heat resistance with little decrease in thermal conductivity even when exposed to high temperatures.
- the composite material 1 and the like can be suitably used for a heat radiating member of a semiconductor element in which a bonding material such as silver solder can be used.
- the region of the composite material 1 in the diamond composite material 1 or the coated composite material 1B of the embodiment has a small number of pores and is dense and has a high relative density. Since the metal layer 6 of the coated composite material 1B is dense with substantially no pores, the coated composite material 1B has a high relative density even when the metal layer 6 is included.
- the composite material 1 and the like satisfy a relative density of 96.5% or more. The higher the relative density is, the denser the material is, and the lower the thermal conductivity due to the pores is less likely to occur, and the higher the thermal conductivity, the higher the 96.7%, 97.0%, and 97.5%. More preferred.
- Typical shapes of the diamond composite material 1 and the coated composite material 1B of the embodiment include a flat plate shape.
- the composite material 1 having a desired planar shape or a three-dimensional shape can be formed by the shape of a mold used at the time of manufacturing, cutting, or the like.
- the size (thickness, width, length, etc.) of the composite material 1 can be selected as appropriate. When the thickness is thin (for example, 5 mm or less, 3 mm or less, and further 2.5 mm or less), a lightweight and thin composite material 1 can be obtained.
- the heat radiating member which concerns on embodiment is substantially comprised, such as a composite material 1 grade
- the diamond composite material 1 and the covering composite material 1B according to the embodiment can be manufactured by, for example, the following diamond composite material manufacturing method.
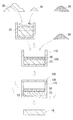
- the outline of this manufacturing method is as follows. As shown in FIGS. 4 and 6, the diamond powder 20 and a metal material that finally forms the metal matrix 5 (FIG. 1) (the metal powder 50 in FIGS. 4 and 6). Are prepared (preparation step), filled in the container 110 of the mold 100 (filling step), the filler is heated to melt the metal material, and the molten metal 52 is dissolved in the diamond powder 20. Immerse (infiltration process).
- This manufacturing method further uses, as a raw material, a Group 4 compound powder 30 containing a Group 4 element and a specific element as a raw material, and oxidation until the Group 4 element forms a carbide.
- the group 4 compound is chemically decomposed during the temperature rising process, and a specific element other than the group 4 of the periodic table generated by this chemical decomposition is caused to exert a reducing action, etc.
- diamond reacts with the elements of Group 4 of the periodic table generated by the chemical decomposition to form carbides.
- a diamond powder 20 As a raw material, a diamond powder 20, a Group 4 compound powder 30 containing a Group 4 element, and a metal material containing silver or a silver alloy are prepared.
- the size (average particle size) and content (volume ratio of the raw material) of the diamond powder 20 are the size (average particle size) of diamond particles in the diamond composite material 1A to be finally produced.
- the content (volume ratio in the composite material 1A) is selected so as to be a desired value (see the above-mentioned section of diamond).
- the surface side region of each powder particle constituting the diamond powder 20 is used for forming the carbide layer 3 (FIG. 1).
- the sheath content is different from the size and content of diamond in the composite material 1A.
- the carbide layer 3 is very thin as described above, it can be said that the size, content, shape, etc.
- the average particle diameter of the raw diamond powder is preferably 1 ⁇ m to 300 ⁇ m, more preferably 1 ⁇ m to 100 ⁇ m, and 20 ⁇ m to 60 ⁇ m.
- the average particle size of the coarse particles is preferably 2 times or more, more preferably 3 times or more and 4 times or more of the average particle size of the fine particles, and considering the thermal conductivity and workability, it is 300 ⁇ m.
- it is more preferably 100 ⁇ m or less and 60 ⁇ m or less.
- the average particle size of the fine particles may be smaller than the average particle size of the coarse particles, but considering densification and the like, it is preferably 1 ⁇ m or more, more preferably 5 ⁇ m or more, and 10 ⁇ m or more.
- the constituent components of the metal material are selected so that the metal matrix 5 in the diamond composite material 1A to be finally produced has a desired composition (see the above-mentioned section of the metal matrix).
- the metal material can be used in various forms, for example, metal powder 50.
- metal powder 50 When the metal powder 50 is heated in the infiltration process, individual powder particles are easily melted to form the molten metal 52. Further, the metal powder 50 can be easily mixed with the diamond powder 20, the Group 4 compound powder 30, the Group 4 element powder described later, and the like, and the mixed powder can be filled into the mold 100.
- size (average particle diameter) of the metal powder 50 can be selected suitably, for example, about 1 micrometer or more and 150 micrometers or less are mentioned. If it is in this range, it is considered that the metal powder 50 is easy to handle because it is not too small and is not too large.
- Plate materials and block bodies can be used as other metal materials.
- an appropriate size and shape may be used so that the mold 100 can be filled.
- the plate material and the block body can be easily stored in the mold 100 and have excellent workability.
- the content (volume ratio) of the metal material is selected so that the content (volume ratio) of the metal matrix 5 in the diamond composite material 1A to be finally produced has a desired value.
- the constituent components of the Group 4 compound powder containing the elements of Group 4 of the periodic table are the elements of Group 4 of the periodic table desired by the carbide layer 3 in the diamond composite material 1A to be finally produced (see above). (See the section on carbide layers).
- the Group 4 compound powder 30 includes one compound selected from sulfides, nitrides, hydrides, and borides containing one or more elements selected from Ti, Zr, and Hf. .
- the powder 30 can contain a plurality of types of compounds in addition to a form containing only one type of the listed compounds.
- a composite material including coated diamond particles including TiC and coated diamond particles including ZrC, a composite material including coated diamond particles covered with a composite carbide layer including Ti and Zr, and the like can be manufactured.
- TiH 2 is relatively easy to obtain, easy to store, and excellent in handleability, so it is easy to use.
- the component present in the diamond composite material 1A as the final product is substantially only the elements of Group 4 of the periodic table, and these elements mainly form carbides, and the carbide layer 3 (FIG. 1).
- the thickness of the carbide layer 3 varies depending on the amount of the Group 4 compound powder 30 added. As described above, if the carbide layer 3 is too thick, thermal conductivity is lowered due to excessive carbide. Therefore, it is preferable that the carbide layer 3 is not too thick in consideration of thermal conductivity.
- the content (volume ratio) of the Group 4 compound powder 30 may be adjusted so that the thickness of the carbide layer 3 has a desired value.
- the Group 4 compound powder 30 contains elements of Group 4 of the Periodic Table that are relatively easy to oxidize, but unlike the Group 4 element alone, the Periodic Table 4 is heated until it is heated in the infiltration step described below. Group elements are bonded to sulfur (S), nitrogen (N), hydrogen (H), or boron (B). For this reason, in this method for producing a diamond composite material, oxidation of the elements of Group 4 of the periodic table is difficult to occur during the process of producing the composite material, and the elements of Group 4 of the periodic table and carbon (here, particularly the diamond surface layer side region) The reaction can be performed well.
- each of the above-described elements bonded to the elements of Group 4 of the periodic table can be removed as a gas (for example, water, nitrogen monoxide, sulfur dioxide, etc.).
- a gas for example, water, nitrogen monoxide, sulfur dioxide, etc.
- the diamond powder 20, the Group 4 compound powder 30, and the metal material are filled into the container 110 of the mold 100.
- the filling form is, for example, a form in which the three parties are layered and the filling is a three-layer laminate, and when all three are powders, all powders are mixed and filled. And a form in which a powder obtained by mixing two of the three powders and the remaining one (not necessarily a powder) are layered to form a two-layer laminate. It is done.
- a mixed powder 23 including diamond powder 20 and Group 4 compound powder 30 and a metal material are arranged in layers in the container 110 of the mold 100 and laminated.
- a body 235 is formed.
- the group 4 compound can be present more reliably around the diamond
- molten metal can be generated from the metal material layer, and the molten metal having a relatively large weight is placed on the layer side of the mixed powder 23.
- the diamond powder 20 and the Group 4 compound powder 30 can be easily mixed, and the mixed powder 23 can be satisfactorily formed.
- a mixing apparatus that can be used for mixing powders of non-metallic inorganic materials (here, diamond powder 20 and group 4 compound powder 30) can be used as appropriate.
- known devices such as a Henschel mixer and a vacuum stirring device can be used.
- Any of wet mixing using a liquid binder typified by organic substances such as polyvinyl alcohol, water and alcohol, and dry mixing without using a binder can be used.
- a drying process for removing the binder may be provided after mixing or after the mixed powder 23 is filled in the mold 100, but the binder may be removed by heating in the infiltration process.
- water or alcohol when heating or vacuum drying is appropriately performed during mixing, and water or alcohol is gradually removed, separation of diamond from group 4 compounds due to specific gravity difference, etc. It is easy to mix uniformly.
- a laminate 235 having a two-layer structure By filling the prepared mixed powder 23 into the container 110 and filling a metal material such as the metal powder 50 thereon, a laminate 235 having a two-layer structure can be formed.
- a metal material having a large specific gravity is disposed on the layer of the mixed powder 23, when the metal material is melted in the next step, the molten metal 52 easily moves to the mixed powder 23 side of the lower layer due to the weight of the metal and infiltrates. it can.
- infiltration can proceed by a chemical reaction between the elements of Group 4 of the periodic table contained in the molten metal 52 and diamond.
- the laminated body 235 When the laminated body 235 is formed, for example, pressing is performed every time the powders 23 and 50 are filled (or a pressure as small as hand pressing), or tapping is performed by applying vibration to obtain a desired filling density.
- the lid 120 of the container 110 is closed.
- the molding die 100 can be provided with a box-shaped or bottomed cylindrical container 110 and a lid 120 that closes the opening of the container 110.
- the shape of the container 110 may be selected so that the diamond composite material 1A having a desired shape can be formed.
- As the mold 100 a mold having excellent heat resistance, strength and the like made of carbon can be suitably used.
- This process heats the filling (for example, laminated body 235) with which the shaping
- the heating temperature is a temperature at which the metal material melts, that is, a melting point of silver (961 ° C.) or higher, or a liquidus temperature of silver alloy or higher.
- the heating temperature may be 980 ° C. or higher and 1300 ° C. or lower.
- the holding time is about 10 minutes to 3 hours.
- the atmosphere is preferably a non-oxidizing atmosphere (for example, an argon atmosphere) or a low oxidizing atmosphere (for example, a vacuum atmosphere; the degree of vacuum is 10 kPa or less) in order to prevent the mixing and increase of oxygen. Since the lower the atmospheric pressure, the easier it is to infiltrate, a reduced-pressure atmosphere less than atmospheric pressure is preferable (for example, 10 kPa or less).
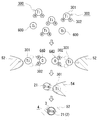
- TiH 2 is illustrated as a group 4 compound 300 among hydrides.
- oxygen 600 that can be contained in the metal powder is released and can be contained in the layer of the mixed powder (upper part of FIG. 5).
- the group 4 compound 300 is converted into an element 301 (Ti in FIG. 5) of the periodic table group 4 and an element 302 (FIG. 5) other than the group 4 element of the periodic table. Then, it is chemically decomposed into hydrogen (H)) (middle upper part of FIG. 5).
- the specific element 302 generated by the chemical decomposition is combined with the above-described oxygen 600 or, when an oxide is present on the raw material surface, the oxygen atom in the oxide and the like to form a gaseous compound 640 (in FIG. 5). Steam (water) is formed and released from the mixed powder. In this manner, oxygen that can be contained in the raw material or the like can be effectively reduced or removed by the specific element 302.
- the molten metal 52 in which the metal material has melted enters the layer side of the mixed powder and takes in the element 301 of the periodic table group 4 generated by the above-described chemical decomposition.
- the composite molten metal 54 incorporating the element 301 of the periodic table group 4 comes into contact with the diamond particles 21 in the mixed powder layer (the lower middle part of FIG. 5), the carbon in the surface side region of the diamond particles 21 and the group 4 of the periodic table
- the element 301 reacts (bonds) to form a carbide.
- the composite molten metal 54 is easily wetted with the diamond particles 21, and the formation reaction of the carbide with the group 301 element 301 of the periodic table proceeds continuously over the entire surface side region of the diamond particles 21.
- the element 301 of Group 4 of the periodic table in the composite molten metal 54 is consumed with the reaction with the diamond particles 21 and becomes a molten metal 52 of silver or a silver alloy.
- Infiltration of the molten metal 52 and the like proceeds with the formation of the carbide.
- the coated diamond particles 4 having the carbide layer 3 containing the elements of Group 4 of the periodic table can be formed on the surface of the diamond particles 2, and the molten metal 52 is filled in the gap formed between the coated diamond particles 4.
- a material can be formed. When the adjacent diamond particles 21 and 21 form carbides, a portion where the carbides are connected can be formed. In this case, a composite material having a connecting portion made of carbide can be manufactured.
- the specific element 302 generated by the chemical decomposition of the group 4 compound 300 may exist around the diamond particle 21 before the diamond particle 21 and the group 4 element 301 of the periodic table react.
- oxygen 600 and the like can be sufficiently reduced and removed, and the amount of oxygen in the finally obtained diamond composite material can be effectively reduced.
- the diamond composite material 1A (1) having a low oxygen concentration in the vicinity of the diamond particle 21 (2) can be obtained.
- the group 301 element of the periodic table generated by the chemical decomposition of the group 4 compound 300 is easy to react with the diamond particles 21 to form a carbide because oxygen is removed as described above.
- Most of the elements of Group 4 of the periodic table contained in the Group 4 compound powder 30 used as a raw material can be preferably made into a carbide. As a result, at least a part, preferably all, of the surface of the diamond particle 21 is covered with the carbide, and the wettability with the molten metal 52 (54) is enhanced. It is thought that such a phenomenon can similarly occur for any of the above-described filling forms.
- the rate of temperature increase is about 2 ° C./min or more and 20 ° C./min or less.
- a diamond composite material 1A having a low oxygen concentration, a dense, and excellent thermal conductivity can be obtained.
- This method for producing a diamond composite material uses the group 4 compound powder 30 containing the elements of the group 4 of the periodic table in this way to prevent oxidation of the elements of the group 4 of the periodic table, oxygen by reduction of oxygen and oxides, etc. Reduction, removal, good carbide formation, and improved wettability between diamond and molten metal.
- this manufacturing method can easily manufacture the composite material 1A without performing heat treatment a plurality of times for the combination of diamond and silver or a silver alloy, or without performing a high-pressure press described in Patent Document 2. Excellent productivity.
- the amount of the metal material is adjusted (increased) so that one surface of the composite material has the same components as the metal matrix 5 and is continuous.
- a coated composite material (one-side infiltrant) including the metal layer 6 having the texture thus formed can be formed.
- the container 110 is filled with a metal material such as the metal powder 50, then filled with the mixed powder 23, and filled with a metal material such as the last metal powder 50.
- a layered structure both-side metal laminate 2355) can be formed.
- various methods such as vapor deposition such as plating and sputtering, immersion in metal hot water, metal plate or metal foil, and metal powder heat bonding (hot press) can be used.
- vapor deposition such as plating and sputtering, immersion in metal hot water, metal plate or metal foil, and metal powder heat bonding (hot press)
- hot press metal powder heat bonding
- pressing pressure is 0.2ton / cm 2 or more 4.5ton / cm 2 or less (19.6 MPa above 441MPa or less) about the heating temperature is preferably degree 300 ° C. or higher 900 ° C. or less.
- a metal plate or the like is placed on one surface of the diamond composite material 1 and then pressed to produce a coated composite material on only one side. By disposing a pair of metal plates or the like so as to sandwich both surfaces of the composite material 1 and then pressing, a coated composite material 1B having metal layers 6 and 6 on both surfaces of the composite material 1 can be manufactured.
- the metal layer 6 having the same composition as the metal matrix 5 of the diamond composite material 1 but also the metal layer 6 having a different composition can be easily formed.
- the method for producing the diamond composite material may include a grinding step of polishing the surface of the composite material 1A not provided with the metal layer 6 or the surface of the coated composite material 1B provided with the metal layer 6. it can.
- a diamond composite material was prepared using a diamond powder, a metal material, and a powder containing an element of Group 4 of the periodic table as appropriate, and the thermal characteristics, relative density, and oxygen content were examined.
- a diamond powder having an average particle size of 50 ⁇ m, a silver (Ag) powder having an average particle size of 150 ⁇ m as a metal material, and an average particle size of 45 ⁇ m, and a powder ⁇ containing elements of Group 4 of the periodic table of materials shown in Table 1 are used.
- a powder ⁇ sample no. 1-1 to 1-12, Group 4 compound powder, Sample No. In 1-102 to 1-104, powders of elements of Group 4 of the periodic table were prepared.
- the average particle size of each powder is the median particle size.
- each powder was adjusted so that the diamond powder was 60% by volume, the silver powder was 38% by volume, and the powder ⁇ was 2% by volume with respect to the volume of 10 mm ⁇ in diameter and 2 mm in thickness.
- Sample No. In 1-101 powder ⁇ was not used, and diamond powder was 60% by volume and silver powder was 40% by volume.
- the above mixed powder was filled into a carbon mold container. After the filling, in order to level the surface of the mixed powder, after pressing at 40 kPa, the mixed powder layer is filled with silver powder, and a two-layered powder compact (including a laminate) is placed in the container. And the container was capped. In this test, a carbon punch was placed on the powder compact to facilitate infiltration, and a weight (300 g) was placed on the punch, but the weight was omitted. Natural infiltration is also possible. In a state where a 300 g load was applied to a molding die containing the powder molded body in which the punch and the weight were arranged in an argon (Ar) atmosphere (5 kPa), the temperature was increased to 1200 ° C.
- Ar argon
- the thermal conductivity and the thermal expansion coefficient were measured using a commercially available measuring instrument.
- the thermal conductivity was measured at room temperature (23 ° C.).
- the thermal expansion coefficient was an average value measured in the range of 30 ° C to 150 ° C.
- the relative density was determined by (actual density / theoretical density) ⁇ 100.
- the actual density was determined by the Archimedes method (underwater specific gravity method).
- the theoretical density is 100 / ⁇ (% by mass of diamond / density of diamond) + (% by mass of metal matrix / density of metal matrix) + (% by mass of group 4 element of periodic table / density of group 4 element of periodic table) ⁇ Sought by.
- the mass ratio of diamond, metal matrix (silver in this test), and periodic table group 4 was calculated using the volume ratio of the raw material composition. For example, in the sample using TiH 2 as the powder of the group 4 compound, the mass% of Ti was calculated from the amount of Ti formed by decomposition into Ti and H 2 . In addition, the said mass ratio is calculated
- the heat cycle resistance is an index representing the difficulty of lowering the thermal conductivity accompanying the temperature change in the substance, and was determined by (thermal conductivity after the thermal cycle / thermal conductivity before the thermal cycle) ⁇ 100.
- the thermal conductivity after the cooling / heating cycle is defined as one cycle: the infiltrant of each sample is immersed in a test solution maintained at ⁇ 60 ° C. for 10 minutes and then immersed in a test solution maintained at 250 ° C. for 10 minutes. The measurement was made after 1000 cycles of this cooling and heating cycle.
- the thermal conductivity after the cooling / heating cycle was measured at room temperature (23 ° C.) using the above-described commercially available measuring instrument.
- As the test liquid a fluorine-based inert liquid (“Galden (registered trademark)”, “Fluorinert (trade name)”, or the like can be used.
- the oxygen content was measured by separately preparing a test piece. Specifically, for each sample, a measurement material capable of collecting 5 or more 3 mm ⁇ 3 mm ⁇ 5 mm measurement test pieces was prepared in the same manner as each sample. Then, the measurement material was subjected to wire electric discharge machining, and a plurality of 3 mm ⁇ 3 mm ⁇ 5 mm measurement test pieces were cut out, and then acid washed to remove the wire component. After the acid washing, the oxygen concentration of each test specimen was measured using an oxygen / nitrogen analyzer (TC-600 type manufactured by LECO Japan GK). Table 1 shows the average value of five measurement specimens for each sample. The point regarding the measurement of the oxygen content is the same for the test examples described later. Note that the size of the measurement test piece is an example, and can be appropriately changed to a measurable size according to the specifications of the measurement apparatus. A measurement specimen may be taken from the composite material itself.
- a CP cross section was taken using a commercially available cross section polisher (CP) processing apparatus, and the structure observation and elemental analysis by EPMA were performed on this cross section.
- 2 and 3 show an observation image and an element mapping image (element image).
- the element mapping images by EPMA shown in FIGS. 2 and 3 show the levels of the extracted elements in different colors. White, red, orange, yellow, green, light blue, blue, black are shown in descending order of element concentration. A color scale is shown below the mapping image of each element.
- FIG. EPMA reflected electron images of the 1-3 infiltrated material show the oxygen mapping image, carbon mapping image, and titanium mapping image of EPMA in this order.
- a polygonal dark gray region indicates a diamond
- a light gray region indicates a metal matrix (here, silver).
- a film-like region exists between the polygonal dark gray region and the light gray region along the periphery of the polygonal region.
- the infiltrant 1-3 is filled with a metal matrix (here, silver) substantially without gaps between the diamond particles. Further, as apparent from the fact that the oxygen mapping image in the lower left of FIG. It can be seen that the 1-3 infiltrant is so small that oxygen is not substantially detected throughout.
- a metal matrix here, silver
- the polygonal particles are generally white to red to yellow, have a high carbon concentration, and can be identified as diamond. It can be seen that a region with a low carbon concentration (green region) is thin and circular along the contour of the polygonal particles. That is, it can be determined that the carbon concentration is low in the surface side region of the diamond particles. Looking at the titanium mapping image in the lower right of FIG. 2, it can be seen that a region having a high titanium concentration (generally a green to blue region) is thin and circular along the contour of the polygonal particles. When considered together with the above-described carbon mapping image, it can be seen that titanium exists in a ring shape along the contour of the diamond particles.
- an annular region having a relatively low carbon concentration and an annular region having a high titanium concentration substantially overlap, and oxygen overlapping this annular region is overlapped. It turns out that there is virtually no. From this, it can be determined that the thin annular region along the outline of the diamond particle is a region in which carbon and titanium are combined to form a carbide and oxygen is not substantially present. Since the carbon component of the carbide exists along the periphery of the diamond particle, it can be determined that it is caused by diamond. In addition, the average thickness of the cyclic
- Sample No. Sample Nos. 1-1, 1-2, 1-4 to 1-12 were also used for the infiltrant. Observation and analysis in the same manner as in 1-3 revealed that the gap formed between the diamond particles was filled with a metal matrix (here, silver) substantially without gaps, and that the surface side region of the diamond particles was of Group 4 of the periodic table. It has been confirmed that the carbide layer is thin and that the oxygen concentration is low throughout the infiltrant including the vicinity of the surface of the diamond particles.
- the infiltrant of 1-1 to 1-12 covers diamond particles and a carbide layer that covers the surface of the diamond particles and contains a group 4 element (in this case, the diamond particles and the group 4 element in particular are bonded to each other).
- the composite material is provided with coated diamond particles including a carbide layer and silver that bonds the coated diamond particles.
- sample no. As shown in Table 1, all of the composite materials 1-1 to 1-12 have a low oxygen content, are dense, and have excellent thermal characteristics. Specifically, Sample No. All of the composite materials 1-1 to 1-12 have an oxygen content of 0.1% by mass or less (here 0.06% by mass or less) and a high relative density (here 96.8% or more). The thermal conductivity is high (here, 580 W / m ⁇ K or more). Sample No. All of the composite materials 1-1 to 1-12 have excellent thermal cycle resistance (95% or more here), and 500 W / m ⁇ K or more (here 550 W / m ⁇ K) even after being subjected to the thermal cycle. The above thermal conductivity can be maintained.
- a diamond composite material having a low oxygen content, denseness, and excellent thermal properties, such as the composite material 1-1 to 1-12, can be easily obtained by an infiltration method using the above-mentioned Group 4 compound powder as a raw material. It can be seen that it can be manufactured.
- the coated diamond particles were extracted by removing silver with an acid or the like, and the average particle size (median particle size) was measured. The diameter was substantially maintained (about 45 ⁇ m). Further, the volume ratio of the extracted coated diamond particles to the composite material substantially maintained the blending ratio of the diamond powder used as the raw material (about 60% by volume). Considering that the carbide layer is extremely thin, it can be said that the particle size and volume ratio of the diamond particles in the composite material substantially maintain the state of the raw material stage. For diamond composite materials (low oxygen content, dense, and excellent thermal properties) prepared in the test examples described later, the metal matrix is removed with acid, etc., and the coated diamond particles are extracted to obtain an average particle size. The volume ratio is measured as described above, and the same result (maintenance of the raw material stage) is obtained.
- the reason why the above result is obtained is that the element of the group 4 of the periodic table is used as the raw material, so that the element of the group 4 of the periodic table may be oxidized in the raw material stage or exist in the manufacturing process of the infiltrant.
- Oxygen or the like causes oxidation of the Group 4 element of the periodic table, so that the carbide of the Group 4 element of the periodic table cannot be sufficiently formed (see also FIG. 3), resulting in insufficient wettability with the molten metal. Conceivable.
- sample No. 1-3 and the sample No. It can be considered that the difference from the oxygen amount of 1-102 is caused by the difference in the oxygen amount contained in the diamond particles and the oxide existing in the vicinity thereof, with reference to the oxygen mapping images shown in FIGS.
- a diamond composite material was produced in the same manner as 1-1 to 1-12.
- the outline is as follows. Tables 2 to 4 show diamond powders having an average particle diameter of 0.1 ⁇ m, 1 ⁇ m, 20 ⁇ m, 50 ⁇ m, 100 ⁇ m, 300 ⁇ m, and 400 ⁇ m, silver (Ag) powder having an average particle diameter of 150 ⁇ m, and an average particle diameter of 45 ⁇ m.
- a Group 4 compound powder was prepared. Adjustment was made so that the diamond powder was 60% by volume, the silver powder was 38% by volume, and the group 4 compound powder was 2% by volume with respect to the volume of 10 mm ⁇ in diameter and 2 mm in thickness.
- any of the infiltrant materials 2-1 to 2-88 covers diamond particles and a carbide layer that covers the surface of the diamond particles and contains an element of Group 4 of the periodic table (in particular, diamond particles and elements of Group 4 of the periodic table).
- the composite material includes a coated diamond particle including a TiC layer, a ZrC layer, or an HfC layer bonded to each other and silver that bonds the coated diamond particles to each other.
- sample no. As shown in Tables 2 to 4, all of the composite materials 2-1 to 2-88 have a low oxygen content, are dense, and have excellent thermal characteristics. Specifically, Sample No. No.
- the composite material of 2-1 to 2-88 has an oxygen content of 0.1% by mass or less (here, many samples are 0.06% by mass or less), and has a high relative density (here, many samples are 97.0% or more) and high thermal conductivity (here, many samples are 600 W / m ⁇ K or more). Furthermore, sample no.
- the composite material of 2-1 to 2-88 has excellent thermal cycle resistance (in this case, many samples are 96% or more), and many samples have a thermal cycle of 500 W / m ⁇ K or more. Fulfill. As shown in Tables 2 to 4, it can be seen that the larger the diamond particles, the better the thermal conductivity.
- a composite material having a thermal conductivity of 700 W / m ⁇ K or more for example, Sample Nos. 2-11, 34, 2-71, etc.
- a composite material having a thermal conductivity of 800 W / m ⁇ K or more for example, Sample Nos. 2-5, 2-42, 2-79, etc.
- a sample with too large diamond particles here, a sample using diamond powder having an average particle size of 400 ⁇ m
- the average particle size of diamond particles in the composite material is 400 ⁇ m. Less than 300 ⁇ m or less.
- the composite material of the sample using the fine coarse mixed powder as the diamond powder is likely to have a relatively high relative density and more dense than the sample not using the fine coarse mixed powder.
- a sample using a finely mixed powder is compared with a sample that is not used. For example, only a sample using a coarse powder of diamond powder having an average particle diameter of 50 ⁇ m and a diamond powder having an average particle diameter of 50 ⁇ m are used. When the coarsely mixed powder is used, it can be said that the thermal conductivity tends to be high and the thermal conductivity can be improved more easily.
- sample No Although the composite materials 2-101 to 2-124 use Group 4 compound powder as a raw material, the oxygen content is higher than 0.1% by mass and the thermal characteristics are lower than other samples. Yes. The reason for this is that the diamond particles are too small, the oxides that can be present in the diamond particles are relatively large and cannot be sufficiently reduced or removed, the oxides remain, and the diamond grain boundaries increase. In other words, the heat path becomes longer, the surface area of the diamond particles increases, and the heat loss at the interface between the diamond and Ag increases.
- sample No. 1 of Test Example 1 was mainly used except that the blending ratio of diamond powder and metal powder was changed from Test Example 1.
- a diamond composite material was produced in the same manner as 1-1 to 1-12.
- the particle diameter of the diamond powder and the material of the metal powder were also changed from Test Example 1.
- the outline is as follows. Diamond powder with an average particle diameter of 1 ⁇ m, 50 ⁇ m, 300 ⁇ m, silver (Ag) powder with an average particle diameter of 150 ⁇ m, or silver alloy (Ag-28 mass% Cu) powder containing 28 mass% Cu, with an average particle diameter of 45 ⁇ m Therefore, Group 4 compound powders having the materials shown in Tables 5 to 7 were prepared.
- Diamond powder is 25% by volume, 29% by volume, 30% by volume, 45% by volume, 60% by volume, 75% by volume, 90% by volume, 95% by volume, and silver powder with respect to a volume of 10 mm diameter and 2 mm thickness.
- the silver alloy powder was adjusted to the values shown in Tables 5 to 7 so that the Group 4 compound powder was 2% by volume.
- wet mixing of diamond powder and Group 4 compound powder ⁇ drying ⁇ filling mixed powder into carbon mold ⁇ pressing ⁇ filling silver powder or silver alloy powder ⁇ Ar atmosphere, 10 °C / min, 1200 °C ⁇ 2
- infiltrant (diameter 10 mm ⁇ , thickness 2 mm disk) was prepared (Sample Nos. 3-1 to 3-80, 3-101 to 3-104, 3-111 to 3- 114, 3-121 to 3-124).
- Sample No. Samples 3-125 to 3-127 are samples that do not use the powder of Group 4 compound. Specifically, a diamond powder having an average particle diameter of 1 ⁇ m, a silver alloy (Ag-28 mass% Cu) powder having an average particle diameter of 150 ⁇ m, an average particle diameter of 45 ⁇ m, and a powder of an element belonging to Group 4 of the periodic table (titanium ( Ti) powder, zirconium (Zr) powder, hafnium (Hf) powder). The volume was adjusted to 30% by volume of diamond powder, 68% by volume of silver alloy powder, and 2% by volume of group 4 element powder with respect to the volume of 10 mm diameter and 2 mm thickness. And sample no. Infiltrant (a disk having a diameter of 10 mm ⁇ and a thickness of 2 mm) was produced in the same manner as in 3-1.
- a diamond powder having an average particle diameter of 1 ⁇ m a silver alloy (Ag-28 mass% Cu) powder having an average particle diameter of 150 ⁇ m, an average particle diameter of
- Sample No. A sample 3-128 does not use the powder of the Group 4 compound. Specifically, diamond powder having an average particle diameter of 50 ⁇ m, silver alloy (Ag-28 mass% Cu) powder having an average particle diameter of 150 ⁇ m, and titanium (Ti) powder having an average particle diameter of 45 ⁇ m were prepared. Adjustments were made so that the diamond powder was 60% by volume, the silver alloy powder was 38% by volume, and the Ti powder was 2% by volume with respect to the volume of 10 mm ⁇ in diameter and 2 mm in thickness. And Ti powder and silver alloy powder were mixed. This mixing was dry mixing using a mixer mill.
- diamond powder is filled into a carbon mold ⁇ press ⁇ filled with a mixed powder of silver alloy powder and Ti powder ⁇ Ar atmosphere (5 kPa), 10 ° C./min, 1200 ° C. ⁇ 2 hours.
- An immersion material (a disk having a diameter of 10 mm ⁇ and a thickness of 2 mm) was produced.
- each of the infiltrators 3-1 to 3-80 covers diamond particles and a carbide layer that covers the surface of the diamond particles and contains elements of Group 4 of the periodic table (in particular, diamond particles and elements of Group 4 of the periodic table).
- Coated diamond particles comprising a TiC layer or a ZrC layer or an HfC layer bonded to each other, and silver or a silver alloy (sample Nos. 3-5, 3-33, 3-63, etc.) that bonds the coated diamond particles to each other. It was a composite material.
- sample no. As shown in Tables 5 to 7, all of the composite materials 3-1 to 3-80 are low in oxygen content, dense, and excellent in thermal characteristics. Specifically, Sample No. No.
- the composite material of 3-1 to 3-80 has an oxygen content of 0.1% by mass or less, a high relative density (here 96.5% or more), and a high thermal conductivity (here 500 W / m). ⁇ K or higher). Furthermore, sample no. No. All of the composite materials of 3-1 to 3-80 are excellent in thermal cycle resistance (95% or more here). Sample No. It can be seen that even when the metal matrix is a silver alloy such as composite materials such as 3-5, 3-33, and 3-63, the oxygen content is low, dense, and excellent in thermal characteristics. As shown in Tables 5 to 7, it can be seen that the greater the content of diamond particles, the better the thermal conductivity.
- the infiltrant materials 3-101 to 3-104, 3-111 to 3-114, and 3-121 to 3-124 have high oxygen concentrations of more than 0.1% by mass and low thermal characteristics. The reason for this is that the content of diamond having excellent thermal conductivity is small, and there is too much silver that can contain oxygen. As a result, there is too much oxygen, and the above-mentioned Group 4 compound powder is used as a raw material. However, it is considered that the oxide was present without sufficiently exerting the reducing action.
- sample No. 4 using a group obtained by adding a group 4 element of the periodic table to a silver alloy powder. In No. 3-128, an infiltrant was obtained, but sample no. It has a higher oxygen content, lower relative density, and poor thermal properties than 3-125.
- the raw material is not a single element of Group 4 of the periodic table, but a sulfide or nitride containing elements of Group 4 of the periodic table. It can be seen that it is preferable to use a Group 4 compound such as an oxide, a hydride, or a boride, and to use at least a part of the Group 4 compound powder mixed with the diamond powder.
- a diamond composite material having a low oxygen content, denseness, and excellent thermal properties such as a composite material of 3-1 to 3-80, has a diamond content in the composite material of more than 25% by volume and less than 95% by volume, Furthermore, it turns out that 30 volume% or more and 90 volume% or less are preferable.
- Test Example 4 A coated composite material having a metal layer was prepared by various methods, and thermal characteristics, relative density, oxygen content, and surface roughness were examined. The relative density was determined including the metal layer.
- the sample No. 1 prepared in Test Example 1 was used. 1-1 to 1-12 infiltrant, Sample No. Samples Nos. 2-1, 2-3, and 2-6, sample Nos.
- the infiltrant 3-2 was prepared, and a metal layer was formed on the surface of each infiltrant by metal plating, metal foil pressure bonding, or metal powder pressure bonding.
- the metal foil or metal powder was joined by hot pressing at a heating temperature of 400 ° C. and a pressure of 4 ton / cm 2 ⁇ 392 MPa.
- the metal plating utilized known conditions.
- Table 8 shows the sample number of the infiltrant used for the coating composite material of each sample, the material of the metal layer, and the method of forming the metal layer.
- the size of the coated composite material of each sample is a disk having a diameter of 10 mm ⁇ and a thickness of 2.2 mm in the state of being provided with a metal layer, and the infiltration is performed so that the thickness of the metal layer becomes a value shown in Table 8.
- the thickness of the material and the thickness of the metal layer were adjusted.
- the obtained sample No. Thermal conductivity (W / m ⁇ K), thermal expansion coefficient ( ⁇ 10 ⁇ 6 / K ppm / K), relative density (%), thermal cycle resistance (%) ),
- the oxygen content (oxygen content, mass%) was measured in the same manner as in Test Example 1. The results are shown in Table 8.
- the influence on the thermal characteristics due to the difference in the raw material of the metal layer such as metal foil and metal powder, the influence on the thermal characteristics due to the difference in the composition of the metal layer, the difference in the formation method of the metal layer such as hot press and plating It can be said that the effects on the thermal properties are small. Further, from this test, it can be said that the oxygen content may slightly increase when the metal layer has a composition that easily contains oxygen (for example, containing Cu) or the metal layer becomes thick.
- Test Example 5 The samples prepared in Test Examples 1 to 3 were examined for deterioration of thermal characteristics after being heated to a high temperature.
- 5-1 to 5-5 it can be seen that even when heated at 800 ° C., there is little decrease in thermal conductivity and excellent heat resistance. Specifically, even when any sample is heated to 800 ° C., the deterioration rate of the thermal conductivity is less than 5%. In this test, the deterioration rate is less than 5% even when subjected to heating twice.
- sample No. Sample No. 5 which is inferior in thermal characteristics to 5-1 No. 5-6 has a large decrease in thermal conductivity even when heated to 800 ° C., the deterioration rate is 5% or more, and 10% or more when subjected to heating twice.
- Sample No. A diamond composite material with a low oxygen content such as a composite material of 5-1 to 5-5, which is dense and has a high thermal conductivity, even when heated to a high temperature simulating the joining of a silver brazing material, Little decrease in thermal conductivity. It can be seen that such a composite material can be used for a heat radiating member and maintain high thermal conductivity even after a high melting point bonding material such as a silver brazing material is bonded.
- the heat radiating member of the present invention is a CPU (Central Processing Unit), GPU (Graphics Processing Unit), HEMT (High Electron Mobility Transistor), chip set, memory chip, etc. provided in supercomputers, other personal computers and mobile electronic devices, etc. It can be used as a heat dissipating member of a semiconductor element used in the above.
- the diamond composite material of the present invention can be used as a material for a heat dissipation member that requires high heat dissipation, such as the heat dissipation member of the semiconductor element.
- the method for producing a diamond composite material according to the present invention can be used for producing a diamond composite material mainly composed of diamond and silver or a silver alloy, which is dense and excellent in thermal conductivity.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Organic Chemistry (AREA)
- Mechanical Engineering (AREA)
- Metallurgy (AREA)
- Physics & Mathematics (AREA)
- Computer Hardware Design (AREA)
- General Physics & Mathematics (AREA)
- Condensed Matter Physics & Semiconductors (AREA)
- Microelectronics & Electronic Packaging (AREA)
- Power Engineering (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Combustion & Propulsion (AREA)
- Thermal Sciences (AREA)
- Manufacturing & Machinery (AREA)
- Ceramic Engineering (AREA)
- Cooling Or The Like Of Semiconductors Or Solid State Devices (AREA)
- Powder Metallurgy (AREA)
- Carbon And Carbon Compounds (AREA)
Abstract
Description
(準備工程)原料として、ダイヤモンドの粉末と、周期表4族の元素を含む硫化物、窒化物、水素化物、硼化物から選択される1種以上の4族化合物の粉末と、銀又は銀合金を含む金属材とを準備する工程。
(充填工程)前記ダイヤモンドの粉末と前記4族化合物の粉末と前記金属材とを成形型内に充填する工程。
(溶浸工程)前記成形型に充填した充填物を加熱して、前記ダイヤモンドと、溶融した前記銀又は銀合金とを複合する工程。
最初に本発明の実施態様を列記して説明する。
(緻密)
・上記のダイヤモンド複合材料は、酸素含有量が0.1質量%以下であり、酸素が少ない。そのため、ダイヤモンド粒子と周期表4族の元素を含む炭化物層との界面及びその近傍を含めた複合材料全体に亘って酸素が少ない、好ましくは存在せず、ダイヤモンド粒子の表面や上記炭化物層中にも酸化物がほとんど存在しないといえる。このような上記のダイヤモンド複合材料は、製造過程で、密度の低下の原因となる気孔の発生が十分に抑制され、ダイヤモンドの表面に炭化物層が健全に形成され易くなったと考えられる。その結果、ダイヤモンドと、周期表4族の元素を含む炭化物層とが密着している。
・上記炭化物層中に酸化物がほとんど存在しないことから、ダイヤモンド粒子の周囲に存在する周期表4族の元素が主として炭化物として存在するといえる。また、銀又は銀合金(以下、金属マトリクスと呼ぶことがある)中にも酸化物がほとんど存在しないといえる。このような上記のダイヤモンド複合材料は、製造過程で、周期表4族の元素を含む炭化物層と金属マトリクスを形成する溶融金属との濡れ性が十分に高められ、密度の低下の原因となる気孔の発生が十分に抑制されたと考えられる。その結果、周期表4族の元素を含む炭化物層と、金属マトリクスとが密着している。
・金属マトリクス中の気孔も十分に低減されている。
(熱伝導性)
・熱伝導率が1000W/m・K以上であるダイヤモンド粒子と、銅や銅合金よりも高い熱伝導率を有する傾向にある銀又は銀合金とを主成分とする。
・上述のようにダイヤモンド粒子の近傍を含めた複合材料全体に亘って酸素が少ない、好ましくは存在しない、即ち、熱伝導性に劣る酸化物が少ない、好ましくは存在しない。
・金属マトリクスによってダイヤモンド粒子同士が結合されると共に緻密であるため、ダイヤモンド粒子、炭化物、金属マトリクス間を繋ぐ熱伝導経路や、ダイヤモンド粒子の表面に形成される炭化物同士が連続的に繋がってなる熱伝導経路などを良好に構築できる。
(m1)このダイヤモンド複合材料の製造方法は、以下の準備工程と、充填工程と、溶浸工程とを備える。
(準備工程)原料として、ダイヤモンドの粉末と、周期表4族の元素を含む硫化物、窒化物、水素化物、硼化物から選択される1種以上の4族化合物の粉末と、銀又は銀合金を含む金属材とを準備する工程。
(充填工程)上記ダイヤモンドの粉末と上記4族化合物の粉末と上記金属材とを成形型内に充填する工程。
(溶浸工程)上記成形型に充填した充填物を加熱して、上記ダイヤモンドと、溶融した上記銀又は銀合金とを複合する工程。
更に上記の特定の元素は、還元作用を有するものがある。
ここでの還元作用とは、溶浸工程の昇温過程などにおいて、工業用ダイヤモンドや銀又は銀合金などの原料に含み得る酸素や酸化物、化学分解で生じた周期表4族の元素の周囲に存在し得る酸素や酸化物を還元して、気体(例えば水蒸気など)として除去可能な作用である。
上記の特定の元素が有する酸化抑制作用や還元作用によって、上記周期表4族の元素は勿論、ダイヤモンドや銀などが製造過程で酸化されることを効果的に抑制できる。
以上のことから、周期表4族の元素とダイヤモンドとが良好に反応でき、ダイヤモンドと溶融金属との濡れ性を高められる炭化物を健全に、かつ過不足なく十分に形成できる。特に、上記4族化合物の粉末を炭化物形成元素(周期表4族の元素)の供給源とすることで、周期表4族の元素の供給量の変動が少なく、又は実質的に生じず、安定して供給でき、炭化物層の厚さ変動が生じ難い。即ち、ダイヤモンド粒子の表面に、ダイヤモンド粒子の構成成分(炭素)と周期表4族の元素とが結合した炭化物層を均一的な厚さに一様に形成し易い。従って、上記のダイヤモンド複合材料の製造方法によれば、酸素含有量が少なく、緻密で、熱伝導性に優れる複合材料(代表的には、実施形態に係るダイヤモンド複合材料)を製造できる。
以下、図1を参照して、本発明の実施形態に係るダイヤモンド複合材料、放熱部材を詳細に説明し、図4~図6を参照して、実施形態のダイヤモンド複合材料を製造できるダイヤモンド複合材料の製造方法を詳細に説明する。
実施形態に係るダイヤモンド複合材料1は、図1に示すようにダイヤモンド粒子2と、ダイヤモンド粒子2の表面を覆い、周期表4族の元素を含む炭化物層3とを備える複数の被覆ダイヤモンド粒子4と、被覆ダイヤモンド粒子4同士を結合する金属マトリクス5とを備える。複数の被覆ダイヤモンド粒子4がつくる隙間に金属マトリクス5が充填されて、ダイヤモンド粒子2の集合状態が金属マトリクス5によって維持される。複合材料1は、気孔が非常に少なく、隙間なく金属マトリクス5が充填された緻密な成形体である(図2のEPMAの反射電子像参照)。実施形態の複合材料1はその全体に亘って酸素含有量が低いことを特徴の一つとする。以下、構成要素ごとを詳細に説明する。
・・・ダイヤモンド
ダイヤモンド複合材料1は、複数のダイヤモンド粒子2を主要構成要素の一つとする。複合材料1中のダイヤモンド粒子2の含有量が多いほど、熱伝導性に優れて好ましい。例えば、熱伝導率が500W/m・K以上を満たす複合材料1とすることができる。一方、上記含有量が多過ぎず、金属マトリクス5をある程度含むことで、複合材料1の熱膨張係数が小さくなり過ぎることを防止できる。例えば、熱膨張係数が4×10-6/K以上9.5×10-6/K以下程度の複合材料1とすることができ、半導体素子やその周辺部品の熱膨張係数に近い。また、上記含有量が多過ぎなければ、製造時、ダイヤモンド粒子間につくられる隙間に溶融金属が十分に溶浸できる。その結果、炭化物層3の介在による緻密化、複合化を良好に行えて、より緻密な複合材料1とすることができる。熱伝導性や半導体素子などとの熱膨張係数の整合性、緻密化などを考慮すると、複合材料1中のダイヤモンド粒子2の含有量は、30体積%以上90体積%が好ましく、45体積%以上85体積%以下、50体積%以上80体積%以下がより好ましい。複合材料1中のダイヤモンド粒子2の含有量の測定方法は、後述する。
ダイヤモンド複合材料1中の各ダイヤモンド粒子2の表面は、周期表4族の元素を含む炭化物で覆われており、各被覆ダイヤモンド粒子4は、上記炭化物から形成される炭化物層3を備える。この炭化物層3は、ダイヤモンド粒子2及び金属マトリクス5の双方に密着している(図2のEPMAの反射電子像参照)。上述のように複合材料1は、酸素含有量が極めて少なく、酸化物がほとんど存在しないため、製造過程で、炭化物層3がダイヤモンド表面で健全に形成され易かったこと、及び炭化物層3が溶融金属(複合材料1中では主として金属マトリクス5になる)との濡れ性に優れたことで密着できたと考えられる。このような炭化物層3を備える複合材料1は、ダイヤモンド粒子2と、炭化物層3と、金属マトリクス5との三者が隙間なく密着して緻密である。
炭化物層3の形成方法は、本発明の趣旨を損なわない限りにおいて、種々の方法を利用できる。ダイヤモンド粒子2との密着性をより高めるという観点からは、炭化物層3は、ダイヤモンド粒子2の表面側領域の構成元素(炭素)と周期表4族の元素とが結合して形成された炭化物で構成されていることが好ましい。この場合、炭化物層3は、ダイヤモンド粒子2自体の成分を構成要素とすることから、ダイヤモンド粒子2との密着性により優れて、より緻密な複合材料1とすることができる。
ダイヤモンド複合材料1は、金属マトリクス5を主要構成要素の一つとする。金属マトリクス5の構成成分は、銀(いわゆる純銀)又は銀合金とする。金属マトリクス5が銀であれば、熱伝導率が427W/m・Kと高く、熱伝導性に優れる複合材料1とすることができる。銀合金は、Agを50質量%超と、添加元素とを含み、残部が不可避不純物からなる合金である。特に、Agを70質量%以上と、添加元素とを含み、残部が不可避不純物である銀合金は、高い熱伝導性を維持しつつ、液相点温度が低い傾向にあり、製造時、溶浸温度を低くしても良好に複合化できるため、製造性に優れる。銀合金の添加元素は、Cuなどが挙げられる。添加元素の合計含有量は、30質量%以下程度が挙げられる。
ダイヤモンド複合材料1は、その全体において酸素が少ないことを特徴の一つとする。具体的には、複合材料1の酸素含有量は、0.1質量%以下である。複合材料1全体の酸素含有量が0.1質量%以下であれば、ダイヤモンド粒子2の表面側近傍に酸化物、気孔などが十分に少なく、好ましくは実質的に存在しない。そのため、複合材料1は、酸化物などの介在に起因するダイヤモンド粒子2と金属マトリクス5との間の熱伝導性の低下を抑制でき、熱伝導性に優れる。また、酸化物が少なければ、周期表4族の元素が酸化物ではなく炭化物として存在しているといえ、炭化物層3の介在によって緻密な複合材料1とすることができる。上記酸素含有量は、少ないほど好ましく、0.095質量%以下、0.090質量%以下、0.080質量%以下がより好ましい。
ダイヤモンド複合材料1の一例として、図6に示すように複合材料1の表面の少なくとも一部を覆う金属層6を備える形態とすることができる(図6の被覆複合材料1Bは一例)。複合材料1と半導体素子などとを半田やロウ材などで接合する場合に、金属層6を備える被覆複合材料1Bとすると、金属層6と半田やろう材などの金属とが十分に濡れて、被覆複合材料1Bなどと半導体素子などとを強固に接合できて好ましい。
・・・熱特性
実施形態のダイヤモンド複合材料1や被覆複合材料1Bなどは、熱伝導性に優れる。例えば、上記の複合材料1などは、室温における熱伝導率が500W/m・K以上を満たす(被覆複合材料の場合には金属層6を含めた状態での熱伝導率)。熱伝導率が高いほど、熱伝導性に優れる複合材料1などになり、放熱部材の素材に好ましいことから、520W/m・K以上、550W/m・K以上、600W/m・K以上がより好ましい。
一例として、-60℃~+250℃における冷熱サイクル耐性が95%以上である複合材料1などが挙げられる。このような複合材料1などは、冷熱サイクルを受けた場合にも熱伝導率の低下が5%以下と低いため、使用時に冷熱サイクルを受ける半導体素子の放熱部材に好適に利用できる。
又は、一例として、800℃に加熱した後における熱伝導率の劣化率が5%未満である複合材料1などが挙げられる。ここで、複合材料1などを半導体素子の放熱部材に利用する場合に、放熱部材とセラミックスなどからなる絶縁材などとを接合することがある。この接合に銀ロウ材といった高融点の接合材を用いることがある。この場合、放熱部材は、接合材によって加熱されることになるが、この加熱によって放熱部材の熱伝導率の低下が少ないことが望まれる。上記熱伝導率の劣化が5%未満である複合材料1などは、高温に曝された場合にも熱伝導率の低下が少なく、耐熱性に優れるといえる。この複合材料1などは、銀ロウなどの接合材が利用され得る半導体素子の放熱部材に好適に利用できる。
実施形態のダイヤモンド複合材料1や、被覆複合材料1Bなどにおける複合材料1の領域は、気孔が少なく緻密で相対密度が高い。被覆複合材料1Bの金属層6は気孔が実質的に存在せず緻密であることから、被覆複合材料1Bは、金属層6を含めた状態でも相対密度が高い。例えば、上記の複合材料1などは、相対密度が96.5%以上を満たす。相対密度が高いほど、緻密であり、気孔に起因する熱伝導性の低下が生じ難く、高い熱伝導性を有することから、96.7%以上、97.0%以上、97.5%以上がより好ましい。
実施形態のダイヤモンド複合材料1や被覆複合材料1Bなどの代表的な形状は、平板状が挙げられる。製造時に用いる成形型の形状や、切削加工などによって所望の平面形状、三次元形状の複合材料1などにすることができる。複合材料1などの大きさ(厚さ、幅、長さなど)は適宜選択できる。厚さが薄いと(例えば5mm以下、3mm以下、更に2.5mm以下)、軽量で薄型の複合材料1などとすることができる。
実施形態に係る放熱部材は、実施形態のダイヤモンド複合材料1や被覆複合材料1Bなどから構成されることで、複合材料1などの組成、組織、特性などを実質的に維持する。従って、実施形態の放熱部材は、酸素含有量が少なく(上述の酸素濃度の項参照)、緻密で(上述の相対密度の項参照)、熱伝導性に優れ(上述の熱特性の項参照)、半導体素子の放熱部材に好適に利用できる。
実施形態に係るダイヤモンド複合材料1や被覆複合材料1Bなどは、例えば、以下のダイヤモンド複合材料の製造方法によって製造することができる。この製造方法の概略を述べると、図4,図6に示すようにダイヤモンドの粉末20と、最終的に金属マトリクス5(図1)を形成する金属材(図4,図6では金属粉末50)とを含む原料を準備して(準備工程)、成形型100の容器110に充填し(充填工程)、充填物を加熱して金属材を溶融して、ダイヤモンドの粉末20に溶融金属52を溶浸する(溶浸工程)。この製造方法は、更に、原料に、周期表4族の元素と特定の元素とを含む4族化合物の粉末30を用いて、周期表4族の元素が炭化物を形成するまでの間の酸化を効果的に抑制し、溶浸工程では、昇温過程で4族化合物を化学分解させ、この化学分解で生じた周期表4族以外の特定の元素に還元作用などを発揮させて、ダイヤモンドの周囲に存在し得る酸素を低減、除去させながら、上記化学分解によって生じた周期表4族の元素とダイヤモンドとを反応させて炭化物を形成する。以下、工程ごとに説明する。
この工程では、原料として、ダイヤモンドの粉末20と、周期表4族の元素を含む4族化合物の粉末30と、銀又は銀合金を含む金属材とを準備する。
ダイヤモンドの粉末20の大きさ(平均粒径)、含有量(原料に占める体積割合)は、最終的に製造するダイヤモンド複合材料1A中のダイヤモンド粒子の大きさ(平均粒径)、含有量(複合材料1Aに占める体積割合)が所望の値(上述のダイヤモンドの項参照)となるように選択する。このダイヤモンド複合材料の製造方法では、ダイヤモンドの粉末20を構成する各粉末粒子の表面側領域が炭化物層3(図1)の形成に利用されるため、厳密に言うと、原料段階におけるダイヤモンドの大きさや含有量と、複合材料1A中のダイヤモンドの大きさや含有量とは異なる。しかし、炭化物層3は上述のように非常に薄いため、複合材料1A中のダイヤモンドの大きさ、含有量、形状などは、原料段階の大きさ、含有量、形状などを実質的に維持するといえる。原料のダイヤモンド粉末の平均粒径は、上述のように1μm以上300μm以下、更に1μm以上100μm以下、20μm以上60μm以下が好ましい。微粗混合とする場合には、粗粒の平均粒径は、微粒の平均粒径の2倍以上、更に3倍以上、4倍以上が好ましく、熱伝導性や加工性などを考慮すると、300μm以下、更に100μm以下、60μm以下が好ましい。微粒の平均粒径は、粗粒の平均粒径よりも小さければよいが、緻密化などを考慮すると、1μm以上、更に5μm以上、10μm以上が好ましい。
金属材の構成成分は、最終的に製造するダイヤモンド複合材料1A中の金属マトリクス5が所望の組成(上述の金属マトリクスの項参照)となるように選択する。
周期表4族の元素を含む4族化合物の粉末の構成成分は、最終的に製造するダイヤモンド複合材料1A中の炭化物層3が所望の周期表4族の元素(上述の炭化物層の項参照)を含むように選択する。具体的には、4族化合物の粉末30は、Ti,Zr及びHfから選択される1種以上の元素を含む硫化物、窒化物、水素化物、硼化物から選択される1種の化合物を含む。粉末30は、列挙した化合物を1種のみ含む形態の他、複数種の化合物を含むことができる。後者の場合、例えば、TiCを備える被覆ダイヤモンド粒子と、ZrCを備える被覆ダイヤモンド粒子とを含む複合材料、TiとZrとを含む複合炭化物層で覆われた被覆ダイヤモンド粒子を含む複合材料などを製造できる。水素化物のうちTiH2は、比較的容易に入手でき、保存などもし易く、取り扱い性に優れるため、利用し易い。
この工程では、ダイヤモンドの粉末20と4族化合物の粉末30と金属材とを成形型100の容器110内に充填する。充填形態は、例えば、三者を層状に充填して充填物を三層構造の積層体とする形態、三者が全て粉末の場合に全ての粉末を混合して充填した全混合粉末の充填物とする形態、三者のうち二者の粉末を混合した粉末と残り一者(粉末でなくてもよい)とを層状に充填して充填物を二層構造の積層体とする形態などが挙げられる。
この工程は、成形型100に充填した充填物(一例として積層体235)を加熱して、ダイヤモンドと、金属材を溶融した溶融金属52とを複合する。
・・金属層の形成
金属層6を備える被覆複合材料1Bなどを製造する場合、金属材を利用して、溶浸工程で複合化と同時に金属層6を形成する同時形成方法と、溶浸工程を経て作製した溶浸材の表面に金属層6を別途形成する別形成方法という二つの方法が利用できる。
その他、このダイヤモンド複合材料の製造方法は、金属層6を備えていない複合材料1Aの表面、又は金属層6を備える被覆複合材料1Bなどの表面に研磨を施す研削工程を備えることができる。
ダイヤモンドの粉末と、金属材と、適宜、周期表4族の元素を含む粉末とを用いて、ダイヤモンド複合材料を作製し、熱特性、相対密度、酸素量を調べた。
ダイヤモンドの粉末の粒径を異ならせて、種々のダイヤモンド複合材料を製造し、熱特性、相対密度、酸素量を調べた。
ダイヤモンドの粉末及び金属粉末の配合比を異ならせて、種々のダイヤモンド複合材料を作製し、熱特性、相対密度、酸素量を調べた。
種々の方法で金属層を有する被覆複合材料を作製し、熱特性、相対密度、酸素量、表面粗さを調べた。相対密度は、金属層を含めて求めた。
試験例1~試験例3で作製した試料について、高温に加熱した後の熱特性の劣化状態を調べた。
評価は、劣化率={[(加熱前の熱伝導率)-(加熱後の熱伝導率)]/(加熱前の熱伝導率)}×100を求めることで行った。ここでは、上述の条件で加熱を1回行った場合(熱処理1回目)と、上述の条件で加熱を2回行った場合(熱処理2回目)について、加熱後の熱伝導率(W/m・K)と劣化率(%)とを測定した。その結果を表9に示す。
2,21 ダイヤモンド粒子 3 炭化物層
4 被覆ダイヤモンド粒子 5 金属マトリクス 6 金属層
20 ダイヤモンドの粉末 30 4族化合物の粉末 23 混合粉末
50 金属粉末
235 積層体 2355 両側金属積層体
52 溶融金属 54 複合溶融金属
100 成形型 110 容器 120 蓋
300 4族化合物 301 周期表4族の元素
302 4族化合物の構成元素のうち、周期表4族の元素以外の元素
600 酸素 640 ガス状の化合物
Claims (10)
- ダイヤモンド粒子と、前記ダイヤモンド粒子の表面を覆い、周期表4族の元素を含む炭化物層とを備える被覆ダイヤモンド粒子と、
前記被覆ダイヤモンド粒子同士を結合する銀又は銀合金とを備え、
酸素含有量が0.1質量%以下であるダイヤモンド複合材料。 - 相対密度が96.5%以上である請求項1に記載のダイヤモンド複合材料。
- 前記ダイヤモンド粒子の平均粒径が1μm以上300μm以下である請求項1又は請求項2に記載のダイヤモンド複合材料。
- 前記ダイヤモンド粒子の含有量が30体積%以上90体積%以下である請求項1~請求項3のいずれか1項に記載のダイヤモンド複合材料。
- 室温における熱伝導率が500W/m・K以上である請求項1~請求項4のいずれか1項に記載のダイヤモンド複合材料。
- 30℃~150℃における平均の熱膨張係数が3×10-6/K以上13×10-6/K以下である請求項1~請求項5のいずれか1項に記載のダイヤモンド複合材料。
- -60℃~+250℃における冷熱サイクル耐性が95%以上である請求項1~請求項6のいずれか1項に記載のダイヤモンド複合材料。
- 800℃に加熱した後における熱伝導率の劣化率が5%未満である請求項1~請求項7のいずれか1項に記載のダイヤモンド複合材料。
- 前記ダイヤモンド複合材料の表面の少なくとも一部を覆う金属層を更に備え、前記金属層の厚さが1μm以上300μm以下である請求項1~請求項8のいずれか1項に記載のダイヤモンド複合材料。
- 請求項1~請求項9のいずれか1項に記載のダイヤモンド複合材料から構成される放熱部材。
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP15838762.1A EP3190198B1 (en) | 2014-09-02 | 2015-09-01 | Heat radiating member comprising diamond composite material |
| JP2016546659A JP6292688B2 (ja) | 2014-09-02 | 2015-09-01 | ダイヤモンド複合材料、及び放熱部材 |
| US15/327,269 US20170145280A1 (en) | 2014-09-02 | 2015-09-01 | Diamond composite material and heat radiating member |
| CN201580047186.9A CN106795596A (zh) | 2014-09-02 | 2015-09-01 | 金刚石复合材料和散热部件 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2014-178434 | 2014-09-02 | ||
| JP2014178434 | 2014-09-02 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2016035795A1 true WO2016035795A1 (ja) | 2016-03-10 |
Family
ID=55439855
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2015/074880 WO2016035795A1 (ja) | 2014-09-02 | 2015-09-01 | ダイヤモンド複合材料、及び放熱部材 |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US20170145280A1 (ja) |
| EP (1) | EP3190198B1 (ja) |
| JP (2) | JP6292688B2 (ja) |
| CN (3) | CN110656259A (ja) |
| WO (1) | WO2016035795A1 (ja) |
Cited By (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10115655B2 (en) | 2014-10-09 | 2018-10-30 | Superufo291 Tec | Heat dissipation substrate and method for producing heat dissipation substrate |
| JP2019071328A (ja) * | 2017-10-06 | 2019-05-09 | 株式会社豊田中央研究所 | 半導体実装基板、半導体モジュールおよび半導体実装基板の製造方法 |
| WO2019159694A1 (ja) | 2018-02-14 | 2019-08-22 | 住友電気工業株式会社 | 複合部材、及び複合部材の製造方法 |
| WO2019163721A1 (ja) * | 2018-02-21 | 2019-08-29 | 住友電気工業株式会社 | 複合材料、及び複合材料の製造方法 |
| WO2020012821A1 (ja) * | 2018-07-12 | 2020-01-16 | 住友電気工業株式会社 | 複合部材 |
| WO2020084903A1 (ja) | 2018-10-25 | 2020-04-30 | 住友電気工業株式会社 | 複合部材 |
| WO2020090213A1 (ja) | 2018-10-31 | 2020-05-07 | 住友電気工業株式会社 | 放熱部材 |
| WO2024157803A1 (ja) * | 2023-01-23 | 2024-08-02 | 旭ダイヤモンド工業株式会社 | 複合材料及びその製造方法 |
Families Citing this family (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP3950991A4 (en) * | 2019-03-29 | 2022-05-18 | Sumitomo Electric Industries, Ltd. | COMPOSITE MATERIAL |
| CN112625657B (zh) * | 2019-09-24 | 2022-01-14 | 华为技术有限公司 | 导热体、导热材料和半导体器件的封装结构 |
| WO2021205782A1 (ja) * | 2020-04-09 | 2021-10-14 | 住友電気工業株式会社 | 複合材料、ヒートシンク及び半導体装置 |
| CN111421141B (zh) * | 2020-04-20 | 2022-05-24 | 浙江工业大学 | 一种定向高导热金刚石/金属基复合材料的制备方法 |
| US20220025241A1 (en) * | 2020-07-27 | 2022-01-27 | Google Llc | Thermal interface material and method for making the same |
| KR102576792B1 (ko) * | 2021-06-08 | 2023-09-11 | 주식회사 더굿시스템 | 복합재료 및 방열부품 |
| CN114086047B (zh) * | 2021-11-22 | 2022-05-31 | 合肥哈瑞克机电科技有限公司 | 一种高导热复合材料及其制备方法 |
| KR102685109B1 (ko) * | 2021-12-13 | 2024-07-15 | 주식회사 더굿시스템 | 복합재료 및 이 복합재료를 포함하는 방열부품 |
| KR20230164796A (ko) | 2022-05-25 | 2023-12-05 | 주식회사 더굿시스템 | 복합재료 및 이 복합재료를 포함하는 방열부품 |
| WO2024210148A1 (ja) * | 2023-04-05 | 2024-10-10 | 積水化学工業株式会社 | ダイヤモンド複合粒子、樹脂組成物 |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2004197153A (ja) * | 2002-12-18 | 2004-07-15 | Allied Material Corp | ダイヤモンド−金属複合材料およびその製造方法 |
| JP2013115096A (ja) * | 2011-11-25 | 2013-06-10 | Tomei Diamond Co Ltd | ダイヤモンド含有ヒートシンク材及びその製法 |
Family Cites Families (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3650714A (en) * | 1969-03-04 | 1972-03-21 | Permattach Diamond Tool Corp | A method of coating diamond particles with metal |
| US5834115A (en) * | 1995-05-02 | 1998-11-10 | Technical Research Associates, Inc. | Metal and carbonaceous materials composites |
| US5976205A (en) * | 1996-12-02 | 1999-11-02 | Norton Company | Abrasive tool |
| JP2004200346A (ja) * | 2002-12-18 | 2004-07-15 | Sumitomo Electric Ind Ltd | 半導体素子収納用パッケージ、その製造方法及び半導体装置 |
| CN101035876A (zh) * | 2004-08-23 | 2007-09-12 | 莫门蒂夫性能材料股份有限公司 | 导热性组合物及其制备方法 |
| WO2007121052A2 (en) * | 2006-04-13 | 2007-10-25 | 3M Innovative Properties Company | Metal-coated superabrasive material and methods of making the same |
| CN1944698A (zh) * | 2006-10-24 | 2007-04-11 | 北京科技大学 | 一种超高导热、低热膨胀系数的复合材料及其制备方法 |
| WO2011049479A1 (en) * | 2009-10-21 | 2011-04-28 | Andrey Mikhailovich Abyzov | Composite material having high thermal conductivity and process of fabricating same |
| CN101985702B (zh) * | 2010-06-29 | 2013-02-06 | 北京科技大学 | 一种超高导热、低热膨胀系数金刚石复合材料及制备方法 |
| US9035448B2 (en) * | 2012-06-29 | 2015-05-19 | Materion Corporation | Semiconductor packages having metal composite base plates |
-
2015
- 2015-09-01 CN CN201910978537.8A patent/CN110656259A/zh active Pending
- 2015-09-01 JP JP2016546659A patent/JP6292688B2/ja active Active
- 2015-09-01 US US15/327,269 patent/US20170145280A1/en active Pending
- 2015-09-01 CN CN201580047186.9A patent/CN106795596A/zh active Pending
- 2015-09-01 WO PCT/JP2015/074880 patent/WO2016035795A1/ja active Application Filing
- 2015-09-01 EP EP15838762.1A patent/EP3190198B1/en active Active
- 2015-09-01 CN CN202111355188.8A patent/CN114032413A/zh active Pending
-
2018
- 2018-02-07 JP JP2018020004A patent/JP2018111883A/ja active Pending
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2004197153A (ja) * | 2002-12-18 | 2004-07-15 | Allied Material Corp | ダイヤモンド−金属複合材料およびその製造方法 |
| JP2013115096A (ja) * | 2011-11-25 | 2013-06-10 | Tomei Diamond Co Ltd | ダイヤモンド含有ヒートシンク材及びその製法 |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP3190198A4 * |
Cited By (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10115655B2 (en) | 2014-10-09 | 2018-10-30 | Superufo291 Tec | Heat dissipation substrate and method for producing heat dissipation substrate |
| JP2019071328A (ja) * | 2017-10-06 | 2019-05-09 | 株式会社豊田中央研究所 | 半導体実装基板、半導体モジュールおよび半導体実装基板の製造方法 |
| WO2019159694A1 (ja) | 2018-02-14 | 2019-08-22 | 住友電気工業株式会社 | 複合部材、及び複合部材の製造方法 |
| US11668009B2 (en) | 2018-02-14 | 2023-06-06 | Sumitomo Electric Industries, Ltd. | Composite member |
| KR20200119249A (ko) | 2018-02-14 | 2020-10-19 | 스미토모덴키고교가부시키가이샤 | 복합 부재 및, 복합 부재의 제조 방법 |
| KR102544898B1 (ko) | 2018-02-21 | 2023-06-16 | 스미토모덴키고교가부시키가이샤 | 복합 재료, 및 복합 재료의 제조 방법 |
| KR20200121311A (ko) * | 2018-02-21 | 2020-10-23 | 스미토모덴키고교가부시키가이샤 | 복합 재료, 및 복합 재료의 제조 방법 |
| JPWO2019163721A1 (ja) * | 2018-02-21 | 2021-04-08 | 住友電気工業株式会社 | 複合材料、及び複合材料の製造方法 |
| WO2019163721A1 (ja) * | 2018-02-21 | 2019-08-29 | 住友電気工業株式会社 | 複合材料、及び複合材料の製造方法 |
| JP7273374B2 (ja) | 2018-02-21 | 2023-05-15 | 住友電気工業株式会社 | 複合材料、及び複合材料の製造方法 |
| JP7189214B2 (ja) | 2018-07-12 | 2022-12-13 | 住友電気工業株式会社 | 複合部材 |
| WO2020012821A1 (ja) * | 2018-07-12 | 2020-01-16 | 住友電気工業株式会社 | 複合部材 |
| JPWO2020012821A1 (ja) * | 2018-07-12 | 2021-08-12 | 住友電気工業株式会社 | 複合部材 |
| WO2020084903A1 (ja) | 2018-10-25 | 2020-04-30 | 住友電気工業株式会社 | 複合部材 |
| KR20210079288A (ko) | 2018-10-25 | 2021-06-29 | 스미토모덴키고교가부시키가이샤 | 복합 부재 |
| JP7196193B2 (ja) | 2018-10-31 | 2022-12-26 | 住友電気工業株式会社 | 放熱部材 |
| JPWO2020090213A1 (ja) * | 2018-10-31 | 2021-10-07 | 住友電気工業株式会社 | 放熱部材 |
| WO2020090213A1 (ja) | 2018-10-31 | 2020-05-07 | 住友電気工業株式会社 | 放熱部材 |
| US12112993B2 (en) | 2018-10-31 | 2024-10-08 | A.L.M.T. Corp. | Heat radiation member |
| WO2024157803A1 (ja) * | 2023-01-23 | 2024-08-02 | 旭ダイヤモンド工業株式会社 | 複合材料及びその製造方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| EP3190198B1 (en) | 2019-11-06 |
| CN106795596A (zh) | 2017-05-31 |
| EP3190198A4 (en) | 2017-10-25 |
| CN114032413A (zh) | 2022-02-11 |
| JP2018111883A (ja) | 2018-07-19 |
| US20170145280A1 (en) | 2017-05-25 |
| CN110656259A (zh) | 2020-01-07 |
| EP3190198A1 (en) | 2017-07-12 |
| JP6292688B2 (ja) | 2018-03-14 |
| JPWO2016035795A1 (ja) | 2017-06-15 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6292688B2 (ja) | ダイヤモンド複合材料、及び放熱部材 | |
| JP6182082B2 (ja) | 緻密質複合材料、その製法及び半導体製造装置用部材 | |
| TWI600634B (zh) | 緻密質複合材料、其製法、接合體及半導體製造裝置用構件 | |
| JP6257575B2 (ja) | 半導体パッケージ、及び半導体装置 | |
| JP2011524466A (ja) | 金属浸潤炭化ケイ素チタンおよび炭化アルミニウムチタン体 | |
| CN111742073B (zh) | 复合材料和复合材料的制造方法 | |
| JP6714786B1 (ja) | 複合部材 | |
| JP2016028173A (ja) | Cu−Ga合金スパッタリングターゲット及びその製造方法 | |
| JP2015140456A (ja) | 複合材料、半導体装置、及び複合材料の製造方法 | |
| JP7196193B2 (ja) | 放熱部材 | |
| JP7350058B2 (ja) | 複合材料 | |
| JP2016065311A (ja) | スパッタリングターゲットおよびスパッタリングターゲットセット | |
| WO2021192916A1 (ja) | 複合材料、及び放熱部材 | |
| JP4594433B1 (ja) | 放熱部材 | |
| JP2011165811A (ja) | 半導体素子搭載部材とその製造方法ならびに半導体装置 | |
| JP5503474B2 (ja) | 放熱部材、半導体装置、放熱部材の製造方法 | |
| JP2013245374A (ja) | 複合材料、複合材料の製造方法、及び半導体装置 | |
| JP2009290136A (ja) | 複合部材及びその製造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 15838762 Country of ref document: EP Kind code of ref document: A1 |
|
| DPE1 | Request for preliminary examination filed after expiration of 19th month from priority date (pct application filed from 20040101) | ||
| ENP | Entry into the national phase |
Ref document number: 2016546659 Country of ref document: JP Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 15327269 Country of ref document: US |
|
| REEP | Request for entry into the european phase |
Ref document number: 2015838762 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2015838762 Country of ref document: EP |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |