WO2013162042A1 - 安定な眼内レンズ用重合性紫外線吸収色素 - Google Patents
安定な眼内レンズ用重合性紫外線吸収色素 Download PDFInfo
- Publication number
- WO2013162042A1 WO2013162042A1 PCT/JP2013/062537 JP2013062537W WO2013162042A1 WO 2013162042 A1 WO2013162042 A1 WO 2013162042A1 JP 2013062537 W JP2013062537 W JP 2013062537W WO 2013162042 A1 WO2013162042 A1 WO 2013162042A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- meth
- group
- compound
- polymerizable
- acrylate
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 0 *c(c(N=Nc1ccccc1)c1)cc(O)c1C(c1ccccc1)=O Chemical compound *c(c(N=Nc1ccccc1)c1)cc(O)c1C(c1ccccc1)=O 0.000 description 1
- OADXPBCLSVKKKO-BYYHNAKLSA-N CC(C(NCCc(cc1)ccc1/N=N/c(c(O)c1)cc(C(c2ccccc2)=O)c1O)=O)=C Chemical compound CC(C(NCCc(cc1)ccc1/N=N/c(c(O)c1)cc(C(c2ccccc2)=O)c1O)=O)=C OADXPBCLSVKKKO-BYYHNAKLSA-N 0.000 description 1
- IZWVXMVWAOUDNP-UHFFFAOYSA-N CC(C(NCc(cc1)ccc1[N+]([O-])=O)=O)=C Chemical compound CC(C(NCc(cc1)ccc1[N+]([O-])=O)=O)=C IZWVXMVWAOUDNP-UHFFFAOYSA-N 0.000 description 1
- XIPFMBOWZXULIA-UHFFFAOYSA-N CC(C)(C)C(N)=O Chemical compound CC(C)(C)C(N)=O XIPFMBOWZXULIA-UHFFFAOYSA-N 0.000 description 1
- OYOKEBJLTMGORC-UHFFFAOYSA-N CC(C)(C)OC(Nc1ccc(CCNC(C(C)=C)=O)cc1)=O Chemical compound CC(C)(C)OC(Nc1ccc(CCNC(C(C)=C)=O)cc1)=O OYOKEBJLTMGORC-UHFFFAOYSA-N 0.000 description 1
- ROGQELZLNBCXBI-UHFFFAOYSA-N CC(C)(C)OC(Nc1ccc(CCNC(C=C)=O)cc1)=O Chemical compound CC(C)(C)OC(Nc1ccc(CCNC(C=C)=O)cc1)=O ROGQELZLNBCXBI-UHFFFAOYSA-N 0.000 description 1
- JAFUHGPESJSRJX-UHFFFAOYSA-N CCOc(cc1)cc(O)c1C(c1ccccc1)=O Chemical compound CCOc(cc1)cc(O)c1C(c1ccccc1)=O JAFUHGPESJSRJX-UHFFFAOYSA-N 0.000 description 1
- YRUFISWPADUQAG-QVIHXGFCSA-N CC[O](C)c(c(/N=N/c1ccc(CCNC(C=C)=O)cc1)c1)cc(O)c1C(c1ccccc1)=O Chemical compound CC[O](C)c(c(/N=N/c1ccc(CCNC(C=C)=O)cc1)c1)cc(O)c1C(c1ccccc1)=O YRUFISWPADUQAG-QVIHXGFCSA-N 0.000 description 1
- ODVBBZFQPGORMJ-UHFFFAOYSA-N NCc(cc1)ccc1[N+]([O-])=O Chemical compound NCc(cc1)ccc1[N+]([O-])=O ODVBBZFQPGORMJ-UHFFFAOYSA-N 0.000 description 1
- ZXDDPOHVAMWLBH-UHFFFAOYSA-N Oc(cc1)cc(O)c1C(c1ccccc1)=O Chemical compound Oc(cc1)cc(O)c1C(c1ccccc1)=O ZXDDPOHVAMWLBH-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/50—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09B—ORGANIC DYES OR CLOSELY-RELATED COMPOUNDS FOR PRODUCING DYES, e.g. PIGMENTS; MORDANTS; LAKES
- C09B29/00—Monoazo dyes prepared by diazotising and coupling
- C09B29/0003—Monoazo dyes prepared by diazotising and coupling from diazotized anilines
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09B—ORGANIC DYES OR CLOSELY-RELATED COMPOUNDS FOR PRODUCING DYES, e.g. PIGMENTS; MORDANTS; LAKES
- C09B69/00—Dyes not provided for by a single group of this subclass
- C09B69/10—Polymeric dyes; Reaction products of dyes with monomers or with macromolecular compounds
- C09B69/106—Polymeric dyes; Reaction products of dyes with monomers or with macromolecular compounds containing an azo dye
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/14—Eye parts, e.g. lenses or corneal implants; Artificial eyes
- A61F2/16—Intraocular lenses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/14—Eye parts, e.g. lenses or corneal implants; Artificial eyes
- A61F2/16—Intraocular lenses
- A61F2002/16965—Lens includes ultraviolet absorber
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2430/00—Materials or treatment for tissue regeneration
- A61L2430/16—Materials or treatment for tissue regeneration for reconstruction of eye parts, e.g. intraocular lens, cornea
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/14—Macromolecular materials
- A61L27/16—Macromolecular materials obtained by reactions only involving carbon-to-carbon unsaturated bonds
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C245/00—Compounds containing chains of at least two nitrogen atoms with at least one nitrogen-to-nitrogen multiple bond
- C07C245/02—Azo compounds, i.e. compounds having the free valencies of —N=N— groups attached to different atoms, e.g. diazohydroxides
- C07C245/06—Azo compounds, i.e. compounds having the free valencies of —N=N— groups attached to different atoms, e.g. diazohydroxides with nitrogen atoms of azo groups bound to carbon atoms of six-membered aromatic rings
- C07C245/08—Azo compounds, i.e. compounds having the free valencies of —N=N— groups attached to different atoms, e.g. diazohydroxides with nitrogen atoms of azo groups bound to carbon atoms of six-membered aromatic rings with the two nitrogen atoms of azo groups bound to carbon atoms of six-membered aromatic rings, e.g. azobenzene
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F220/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical or a salt, anhydride ester, amide, imide or nitrile thereof
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F220/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical or a salt, anhydride ester, amide, imide or nitrile thereof
- C08F220/02—Monocarboxylic acids having less than ten carbon atoms; Derivatives thereof
- C08F220/52—Amides or imides
- C08F220/54—Amides, e.g. N,N-dimethylacrylamide or N-isopropylacrylamide
- C08F220/60—Amides, e.g. N,N-dimethylacrylamide or N-isopropylacrylamide containing nitrogen in addition to the carbonamido nitrogen
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F220/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical or a salt, anhydride ester, amide, imide or nitrile thereof
- C08F220/02—Monocarboxylic acids having less than ten carbon atoms; Derivatives thereof
- C08F220/52—Amides or imides
- C08F220/54—Amides, e.g. N,N-dimethylacrylamide or N-isopropylacrylamide
- C08F220/60—Amides, e.g. N,N-dimethylacrylamide or N-isopropylacrylamide containing nitrogen in addition to the carbonamido nitrogen
- C08F220/603—Amides, e.g. N,N-dimethylacrylamide or N-isopropylacrylamide containing nitrogen in addition to the carbonamido nitrogen and containing oxygen in addition to the carbonamido oxygen and nitrogen
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F220/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical or a salt, anhydride ester, amide, imide or nitrile thereof
- C08F220/62—Monocarboxylic acids having ten or more carbon atoms; Derivatives thereof
- C08F220/68—Esters
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L33/00—Compositions of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical, or of salts, anhydrides, esters, amides, imides or nitriles thereof; Compositions of derivatives of such polymers
- C08L33/24—Homopolymers or copolymers of amides or imides
- C08L33/26—Homopolymers or copolymers of acrylamide or methacrylamide
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09B—ORGANIC DYES OR CLOSELY-RELATED COMPOUNDS FOR PRODUCING DYES, e.g. PIGMENTS; MORDANTS; LAKES
- C09B29/00—Monoazo dyes prepared by diazotising and coupling
- C09B29/10—Monoazo dyes prepared by diazotising and coupling from coupling components containing hydroxy as the only directing group
- C09B29/12—Monoazo dyes prepared by diazotising and coupling from coupling components containing hydroxy as the only directing group of the benzene series
Definitions
- the present invention relates to a dye for coloring an intraocular lens, and more particularly to a polymerizable dye having an ability to absorb ultraviolet light and blue light.
- Cataract is a disease in which the entire visual field is fogged by turbidity and pigment formation and deposition in the lens.
- an operation is generally performed in which an opaque lens is removed and an intraocular lens (IOL) is inserted and placed in the lens capsule.
- IOL intraocular lens
- Many of the intraocular lens materials used for treatment are acrylic or silicon-based polymers, and in particular, polymethyl methacrylate (PMMA) has been used for some time.
- PMMA polymethyl methacrylate
- the original crystalline lens has the property of not transmitting ultraviolet rays, whereas the conventional polymer for intraocular lenses transmits ultraviolet rays, so there is a risk of damaging the retina.
- the original crystalline lens is slightly yellowish and has a property of suppressing transmission of part of light in the blue region.
- Patent Documents 1 to 4 Various types of such monomer compounds have been developed so far (Patent Documents 1 to 4), and other intraocular lens materials having a chromophore such as an azo group and an ultraviolet absorber such as a benzophenone skeleton in one molecule. Monomer compounds that can be copolymerized with other monomers have also been developed (Patent Documents 5 and 6).
- the polymerizable ultraviolet-absorbing dye monomers described in Patent Documents 5 and 6 have poor stability against pH change, particularly stability under alkaline conditions, and the dye sites (chromophores and UV-absorbing portions) are easily detached from the polymer. There is a problem. In particular, in the case of an intraocular lens material having a long intraocular implantation period, the risk of detachment of the pigment site is further increased. Therefore, an object of the present invention is to obtain a polymerizable ultraviolet-absorbing dye monomer that is stable even under alkaline conditions.
- the present inventor has found that the stability problem with respect to pH change is caused by the presence of an ester bond between the dye moiety in the monomer and the polymerizable group. . And it discovered that the polymerizable ultraviolet-ray-absorbing dye monomer compound represented by the following general formula (1) can solve the above problems. That is, the present invention is as follows.
- One embodiment of the present invention is a compound represented by the following general formula (1) (hereinafter also referred to as the dye compound of the present invention).
- R 1 is a hydrogen atom, a hydroxy group, a carboxy group, an alkyl group having 1 to 8 carbon atoms, an alkoxy group having 1 to 8 carbon atoms, a sulfonic acid group or a benzyloxy group, preferably Is a hydrogen atom, a methyl group or an ethyl group
- R 2 is a hydrogen atom, a hydroxy group or an alkoxy group having 1 to 4 carbon atoms, preferably a hydrogen atom, a hydroxy group, a methoxy group or an ethoxy group.
- R 3 is represented by the following formula (2).
- R 4 is a hydrogen atom or a methyl group.
- R 5 is a single bond or an alkylene group having 1 to 4 carbon atoms which may have a substituent, preferably a substituted group. (It is an alkylene group having 1 to 4 carbon atoms which does not have a group.)
- Another aspect of the present invention is a polymer obtained by copolymerizing the dye compound of the present invention and one or more other polymerizable monomers (hereinafter also referred to as the polymer of the present invention).
- another aspect of the present invention is an intraocular lens formed by molding the polymer of the present invention (hereinafter also referred to as the intraocular lens of the present invention).
- a polymerizable UV-absorbing dye monomer that is stable even under alkaline conditions.
- the compound represented by the general formula (1) has, in its molecule, a benzophenone skeleton having ultraviolet absorption performance, an azobenzene skeleton having blue light absorption performance, and a polymerizable group, so that it is copolymerized with other polymerizable monomers.
- a polymer can be obtained, and the polymer is useful as a material for an intraocular lens.
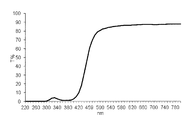
- 10 is a chart showing an ultraviolet-visible absorption spectrum of the polymer sheet obtained in Example 13.
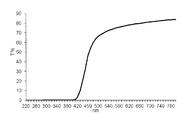
- 10 is a chart showing an ultraviolet-visible absorption spectrum of the polymer sheet obtained in Example 14.
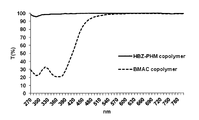
- FIG. It is a chart showing the ultraviolet visible absorption spectrum of the filtrate after alkali-treating a polymer.
- the dye compound of the present invention is represented by the following general formula (1).
- R 1 is a hydrogen atom, a hydroxy group, a carboxy group, an alkyl group having 1 to 8 carbon atoms, an alkoxy group having 1 to 8 carbon atoms, a sulfonic acid group, or a benzyloxy group.
- a hydrogen atom, a methyl group, or an ethyl group is preferable from the viewpoint of reaction efficiency in production.
- R 2 is a hydrogen atom, a hydroxy group, or an alkoxy group having 1 to 4 carbon atoms.
- the dye compound of the present invention has a benzophenone skeleton and an azobenzene skeleton, and these are dye parts.
- R 3 is represented by the following formula (2).
- R 4 is a hydrogen atom or a methyl group.
- R 5 is a C 1-4 alkylene group which may have a single bond or a substituent, and preferably a C 1-4 alkylene group which does not have a substituent. is there.
- the alkylene group having 1 to 4 carbon atoms means a methylene group, an ethylene group, a propylene group, or a butylene group.
- Such an alkylene group may or may not have a substituent, and in the case of having a substituent, the alkyl group having 1 to 2 carbon atoms, a halogen group, a carboxyl group, and a carboxy carbon atom having 1 to 2 carbon atoms.
- the substituent R 3 is a group in which a spacer is bonded to the polymerizable group (meth) acryloylamino group, and is a site involved in copolymerization in the dye compound of the present invention. Due to the structure represented by the general formula (2), the dye compound of the present invention has a property of high reaction efficiency with other polymerizable monomers.
- (meth) acryloyl means acryloyl or methacryloyl.
- the substituent R 3 is preferably bonded to the 3rd or 4th position of the azophenyl group.
- the dye compound of the present invention represented by the general formula (1) is not particularly limited, but preferred examples include those having the following structures.
- the dye compound of the present invention has, in one molecule, a benzophenone skeleton (ultraviolet absorption part) having ultraviolet absorption ability and an azobenzene skeleton (chromophore) having blue region light absorption ability. Due to the presence of these dye sites, the dye compound of the present invention has the performance (light absorption characteristics) of suppressing transmission in the ultraviolet region (wavelength 380 nm or less) and the blue region (wavelength 380 to 500 nm).
- the dye compound of the present invention is a conventional dye compound (for example, 2,4dihydroxy-5- (4- (2- (N-2-methacryloyloxyethyl) carbamoyloxy) ethylphenylazo) benzophenone described in Patent Document 6).
- a conventional dye compound for example, 2,4dihydroxy-5- (4- (2- (N-2-methacryloyloxyethyl) carbamoyloxy) ethylphenylazo) benzophenone described in Patent Document 6).
- BMAC blue-methacryloyloxyethyl
- the light absorption characteristics are more excellent. Specifically, when the ultraviolet-visible absorption spectrum is measured, the rising edge of the chart appears sharply around 420 to 500 nm, and the light transmission suppression ability in the ultraviolet / blue region is excellent.
- the dye compound of the present invention has an ultraviolet absorption part and a chromophore (collectively referred to as a dye part) in one molecule, the chromophore is damaged by ultraviolet rays, and the dye is faded over time. Hard to occur.
- the polymerizable group and the dye part are connected by an amide bond. Since such a bond is stable even under alkaline conditions, the dye moiety is not detached from the polymer of the present invention described later. As a result, the stability under alkaline conditions, which is poor with conventional dye compounds having ester bonds and copolymers using the same, is realized.
- the method for producing the coloring compound of the present invention is not particularly limited, and for example, it can be produced by the following synthesis methods 1 to 3.
- the amino group bonded to the aromatic group is protected with a protective group, or a compound having a protective group introduced in advance is used. It is preferable to remove the protecting group by acid treatment or the like.
- the dye compound of the present invention can be obtained by a protective step for introducing a protecting group into an aliphatic amine of an aminoaryl compound, a diazotization step for diazotizing an aminoaryl compound protected with an aliphatic amine to obtain a diazonium salt.
- the diazo coupling step of obtaining a diazo compound by diazo coupling of the diazonium salt and the benzophenone compound, the deprotection step of removing the protecting group of the aliphatic amine by acid treatment, and the resulting diazo compound with an acrylic acid compound or methacrylic acid It can also be produced by a synthesis method including an amidation step in which a compound or the like is reacted by an amidation reaction to introduce a polymerizable group.
- the diazotization process in each synthetic method mentioned above can be performed by a well-known method.
- the diazotizing agent sodium nitrite or sodium nitrite aqueous solution, potassium nitrite or potassium nitrite aqueous solution, isoamyl nitrite, and / or nitrosyl sulfuric acid (sulfuric acid solution) can be used.
- the amount of the diazotizing agent is not particularly limited, but is preferably 1.00 to 1.20 mol, more preferably 1.02 to 1.10 mol, per mol of the polymerizable group-containing aminoaryl compound. preferable.
- the reaction temperature in the diazotization step is in the range of ⁇ 78 ° C.
- the diazotization step is preferably carried out under neutral to acidic conditions, and an acid such as hydrochloric acid can be appropriately added to the reaction solvent.
- the diazo coupling step in the synthesis methods 2 and 3 is preferably carried out under weak base conditions using a weak base as a catalyst.
- a weak base such as sodium hydroxide or potassium hydroxide generally used in the diazo coupling reaction.
- a strong base such as sodium hydroxide or potassium hydroxide generally used in the diazo coupling reaction.
- the yield decreases, the use of a weak base can remarkably suppress the decomposition and ensure sufficient reactivity, and as a result, the reaction yield can be dramatically increased. is there.
- the vinyl group contained in the diazonium salt is also known to polymerize in the presence of the strong base as described above, which causes a significant decrease in yield, but a weak base can be used instead of the strong base.
- the weak base used here is a salt composed of a strong alkali and a weak acid, and the aqueous solution thereof does not hydrolyze an amide bond at 1 atm, 0 ° C. to 25 ° C., and does not cause a vinyl group polymerization reaction.
- a weak base such as sodium carbonate, sodium bicarbonate, potassium carbonate, sodium acetate or potassium acetate.
- the amount of the weak base used is preferably 4.0 to 10.0 mol, more preferably 6.0 to 8.0 mol per mol of diazonium salt in terms of sodium equivalent.
- the reaction temperature in the diazo coupling step is in the range of ⁇ 10 ° C. to 10 ° C., more preferably in the range of ⁇ 5 ° C. to 5 ° C.
- Reaction solvents in the diazo coupling step include organic solvents (alcohol solvents such as methanol, ethanol and isopropyl alcohol, amides such as N, N-dimethylacetamide, N, N-dimethylformamide and 1-methyl-2-pyrrolidone).
- organic solvents such as methanol, ethanol and isopropyl alcohol, amides such as N, N-dimethylacetamide, N, N-dimethylformamide and 1-methyl-2-pyrrolidone.
- Solvents such as sulfolane, sulfoxide solvents such as dimethyl sulfoxide, ureido solvents such as tetramethylurea, halogen solvents such as dichloromethane, chloroform, 1,2-dichloroethane, ester solvents such as ethyl acetate and butyl acetate, diethyl Ether solvents such as ether and tetrahydrofuran, pyridine solvents such as pyridine, ⁇ -picoline and 2,6-lutidine, etc.) alone or as a mixed system of a plurality of types, or in addition, a mixed system of an organic solvent and water and a single water system Can be used But is preferably an alcoholic solvent in these, It is also preferred to use a mixture of water thereto. Furthermore, depending on the reaction, in addition to the alcohol solvent and water, an amide solvent, ester solvent and ether solvent may be added and used.
- aminoaryl compound is represented by (i) or (v) in the above reaction formula
- nitroaryl compound is represented by (ix).
- polymerizable aminoaryl compound is represented by (vi) or (vii) in the above reaction formula
- polymerizable nitroaryl compound is represented by the same (x).
- the polymerizable aminoaryl compound or polymerizable nitroaryl compound is an amino-substituted aromatic amine or an amidation reaction between a nitro-substituted aromatic amine and (meth) acrylic acid, an amino-substituted aromatic alkylamine or a nitro-substituted aromatic alkylamine.
- An amidation reaction of (meth) acrylic acid with an amino-substituted aromatic amine or nitro-substituted aromatic amine and (meth) acrylic acid chloride, an amino-substituted aromatic alkylamine or a nitro-substituted aromatic alkylamine It can be obtained by an amidation reaction with (meth) acrylic acid chloride.
- the amino group is t-butoxycarbonyl group, benzyloxycarbonyl group, 9-fluorenylmethyloxycarbonyl group, 2,2,2-trichloroethoxycarbonyl group, allyloxycarbonyl group, p
- a protecting group such as a toluenesulfonyl group or 2-nitrobenzenesulfonyl group can be used.
- polymerizable aminoaryl compound examples include N- (4-aminophenyl) (meth) acrylamide, N-[(4-aminophenyl) methyl] (meth) acrylamide, and N- [2- (4-aminophenyl) ethyl.
- Examples of the polymerizable nitroaryl compound include N- (4-nitrophenyl) (meth) acrylamide, N-[(4-nitrophenyl) methyl] (meth) acrylamide, and N- [2- (4-nitrophenyl).
- a synthesis method in which an amidation step is performed before the diazo coupling step as in the synthesis method 2 or the synthesis method 3 is preferable.
- the diazotization step of synthesis method 1 there is a concern about the formation of a highly reactive by-product due to diazotization of an aliphatic amine. This is because the production of such a by-product can be avoided because the diazotization step is performed after the introduction by the azotization reaction.
- the reaction efficiency is good, and the dye compound of the present invention can be obtained with a high yield.
- the nitroaryl compound used as a starting material in the synthesis method 3 is less expensive than the aminoaryl compound. Furthermore, in the amidation reaction, when a polymerizable group is introduced, a by-product in which two molecules of the polymerizable group are introduced with respect to the amino group is formed, but this occurs when a nitroaryl compound is amidated. Removal of by-products is easier than when, for example, an aminoaryl compound is amidated. Therefore, in view of such economical points and simplicity of operation, the synthesis method 3 using a nitroaryl compound as a starting material is particularly preferable as a method for producing the dye compound of the present invention.
- the dye compound of the present invention can be industrially produced by the synthesis method 2 or the synthesis method 3, and can be used as a useful dye or a polymerizable ultraviolet absorbing dye monomer.
- the dye compound of the present invention can be copolymerized with one or more other copolymerization monomers to form a polymer.
- the dye compound of the present invention since the chromophore and the polymerizable group are sterically separated, the polymerization is not inhibited. Therefore, it can be used as a copolymerizable monomer having good reactivity with other copolymerizable monomers.
- limit especially as said other copolymerization monomer if it is normally used For example, the following are mentioned.
- (Meth) acrylate means “acrylate” or “methacrylate”, and the same applies to (meth) acrylic acid derivatives.
- one or more of the above copolymerization monomers may be selected and polymerized to form a macromonomer, which may be used as one of the copolymerization monomers for polymer production.
- the polymer of the present invention can be obtained by blending the dye compound of the present invention and one or more other copolymerization monomers in an arbitrary amount, mixing them uniformly, and then copolymerizing them.
- the proportion of the pigment compound of the present invention is influenced by the thickness of the use of the polymer, for example, an intraocular lens.
- the amount is preferably 0.001 to 5 parts by weight, more preferably 0.005 to 2 parts by weight, and still more preferably 0.01 to 0.06 parts by weight. If it is less than 0.001 part by weight, the color of the polymer may be deteriorated.
- the polymer of the present invention can be synthesized by a method commonly used in the art.
- the dye compound of the present invention and one or more other copolymerization monomers are uniformly mixed, and if necessary, a polymerization initiator is added and gradually heated in a temperature range from room temperature to about 130 ° C. Or by irradiation with electromagnetic waves such as microwaves, ultraviolet rays and radiation (gamma rays).
- a polymerization initiator is added and gradually heated in a temperature range from room temperature to about 130 ° C.
- electromagnetic waves such as microwaves, ultraviolet rays and radiation (gamma rays).
- various methods commonly used by those skilled in the art such as radical polymerization, bulk polymerization, or solvent polymerization, can be employed.
- heat polymerization the temperature is raised stepwise. You may let them.
- polymerization initiator examples include azobisisobutyronitrile, azobisdimethylvaleronitrile, 2,2′-azobis (2,4-dimethylvaleronitrile), benzoyl peroxide, tert-butyl hydroperoxide, cumene hydro Radical polymerization initiators such as peroxide and benzoyl peroxide can be mentioned, and one or more of these can be used.
- the amount used is preferably in the range of about 0.01 to 1 part by weight with respect to 100 parts by weight of all the copolymerization monomer mixtures.
- polymerizable ultraviolet absorbers mainly absorbing the ultraviolet part
- polymerizable dyes examples that absorb mainly light in the blue region without ultraviolet absorption ability
- a polymerizable ultraviolet absorbing dye can be used in combination.
- the combined use of these polymerizable UV absorbers, polymerizable dyes and polymerizable UV absorbing dyes makes it possible to finely adjust the balance between the UV absorption performance and the blue region light absorption performance of the finally obtained polymer.
- the polymer of the present invention is applied to an intraocular lens material as described later, such a combination is useful for adjusting the color tone of the intraocular lens and sufficiently imparting ultraviolet absorption ability. .
- the addition amount is preferably adjusted to be 0.01 parts by weight or more, more preferably 0.05 parts by weight or more with respect to 100 parts by weight of all the comonomer mixture, and a sufficient polymerization rate and degree of polymerization. Is preferably adjusted to 5 parts by weight or less, more preferably 3 parts by weight or less with respect to 100 parts by weight of all copolymerization monomers.
- polymerizable ultraviolet absorbers examples include benzophenone-based polymerizable ultraviolet absorbers disclosed in Japanese Patent Application Laid-Open No. 2003-253248 and benzotriazole-based polymerizable ultraviolet absorbers disclosed in Japanese Patent No. 2685980. Can be used. Specific examples include 2-hydroxy-4- (meth) acryloyloxybenzophenone, 2-hydroxy-4- (meth) acryloyloxy-5-t-butylbenzophenone, 2-hydroxy-4- (meth) acryloyloxy.
- Benzophenone-based polymerizable UV absorbers such as -2 ', 4'-dichlorobenzophenone, 2-hydroxy-4- (2'-hydroxy-3'-(meth) acryloyloxypropoxy) benzophenone; 2- (2'-hydroxy -5 ′-(meth) acryloyloxyethylphenyl) -2H-benzotriazole, 2- (2′-hydroxy-5 ′-(meth) acryloyloxyethylphenyl) -5-chloro-2H-benzotriazole, 2- ( 2'-hydroxy-5 '-(meth) acryloyloxypropylphenyl) -2H- Benzotriazole, 2- (2′-hydroxy-5 ′-(meth) acryloyloxypropyl-3′-t-butylphenyl) -5-chloro-2H-benzotriazole, 2- (2′-hydroxy-5′- Benzotriazole polymerizable ultraviolet absorbers such as (2 "
- Salicylic acid derivative-based polymerizable ultraviolet absorbers such as 2-cyano-3-phenyl-3- (3 ′-(meth) acryloyloxyphenyl) propenyl acid methyl ester, etc. These may be used alone or in combination of two or more. Can be used.
- polymerizable dyes examples include azo, anthraquinone, nitro, and phthalocyanine polymerizable dyes disclosed in JP-A-10-251537. These can be used alone or in admixture of two or more.
- Specific examples of the polymerizable azo dye include 1-phenylazo-4- (meth) acryloyloxynaphthalene, 1-phenylazo-2-hydroxy-3- (meth) acryloyloxynaphthalene, 1-naphthylazo-2-hydroxy.
- polymerizable anthraquinone dyes include, for example, 1,5-bis ((meth) acryloylamino) -9,10-anthraquinone, 1- (4′-vinylbenzoylamide) -9,10-anthraquinone, 4 -Amino-1- (4'-vinylbenzoylamide) -9,10-anthraquinone, 5-amino-1- (4'-vinylbenzoylamide) -9,10-anthraquinone, 8-amino-1- (4 ' -Vinylbenzoylamide) -9,10-anthraquinone, 4-nitro-1- (4'-vinylbenzoylamide) -9,10-anthraquinone, 4-hydroxy-1- (4'-vinylbenzoylamide) -9, 10-anthraquinone, 1- (3′-vinylbenzoylamide) -9,10-anthraquinone
- polymerizable nitro dye examples include o-nitroanilinomethyl (meth) acrylate and the like.
- polymerizable phthalocyanine dye examples include (meth) acryloylated tetraamino copper phthalocyanine, (meth) acryloylated (dodecanoylated tetraamino copper phthalocyanine), and the like.
- polymerizable UV-absorbing dyes that can be used in combination for the above purpose include, for example, 2,4-dihydroxy-3 (p-styrenoazo) benzophenone, 2,4-dihydroxy-5- (p-styrenoazo).
- a crosslinking agent in the copolymerization of the polymer of the present invention or by using a macromonomer having two or more polymerizable groups in the molecule as a copolymerization monomer, a tertiary is incorporated in the obtained polymer.
- An original cross-linked structure can be formed.
- the mechanical strength and hardness of the polymer can be improved, and the elution of the monomer (including the dye compound of the present invention) from the polymer can be suppressed.
- the polymer of the present invention is used as a material for an intraocular lens as described later, an intraocular lens that is uniform, transparent, and has excellent optical properties without distortion can be obtained. Chemical resistance, heat resistance, solvent resistance).
- the blending ratio is preferably used within a range of 0.01 to 10 parts by weight per 100 parts by weight of all copolymer monomer mixtures. If it is less than 0.01 part by weight, the effect is difficult to obtain, and if it exceeds 10 parts by weight, the resulting polymer tends to be brittle.
- the macromonomer as described above include butanediol di (meth) acrylate, ethylene glycol di (meth) acrylate, diethylene glycol di (meth) acrylate, triethylene glycol di (meth) acrylate, and propylene glycol di (meth).
- various functionalities can be imparted to the polymer of the present invention by selecting an appropriate copolymerization monomer.
- silicon-containing (meth) acrylates, silicon-containing monomers such as silicon-containing styrene derivatives, fluorine-containing alkyl (meth) acrylates, and the like are selected as copolymerization monomers.
- alkyl (meth) acrylates, styrene derivatives including styrene, (meth) acrylic acid, or the like may be selected as a copolymerization monomer.
- the anti-lipid contamination function is obtained when the polymer of the present invention is used as a material for an intraocular lens as described later. Can be provided.
- the copolymerization monomer such as hydroxy (meth) acrylates, (meth) acrylamides, aminoalkyl (meth) acrylates, (meth) acrylates, N-vinyl lactams, etc.
- a monomer having a hydrophilic group may be selected, and a water-containing flexible intraocular lens can be obtained when the polymer of the present invention is used as a material for an intraocular lens as described later.
- a monomer containing an aromatic ring such as a styrene monomer or an aromatic ring-containing (meth) acrylate
- the polymer of the present invention can be used as a lens material having a high refractive index.
- the copolymerization monomer for imparting various functions to the polymer of the present invention is selected and blended as described above, it is preferably 0.01 parts by weight or more with respect to 100 parts by weight of all copolymerization monomer mixtures. More preferably, it is 0.05 parts by weight or more, and is adjusted as appropriate so that it is preferably 5 parts by weight or less, more preferably 3 parts by weight or less with respect to 100 parts by weight of all the copolymerization monomer mixtures.
- the polymer of the present invention blocks ultraviolet light and absorbs blue region light.
- the strength can be reduced.
- the light transmittance decreases from around 500 nm, and it is preferable that the light transmittance is 0% below 400 nm. More preferably, when the ultraviolet-visible absorption spectrum is measured, the rising of the chart appears sharply at around 420 to 500 nm, and the light transmission suppression ability in the ultraviolet / blue region is superior to conventional dye compounds (such as BMAC).
- the dye compound of the present invention since the dye compound of the present invention is directly bonded to the polymer chain by copolymerization, the dye compound of the present invention does not elute from the polymer of the present invention. This can be confirmed by the fact that the light transmittance spectrum does not change before and after the polymer of the present invention is immersed in ethanol at 40 ° C. for 24 hours.
- the amide bond connecting the polymerizable group and the dye moiety is stable even under alkaline conditions (for example, pH 12 or higher), and therefore the dye moiety is detached from the polymer of the present invention. There is nothing.
- Intraocular lens of the present invention The polymer of the present invention can be used as an intraocular lens material.
- a dye compound increases its hardness when added to a polymer.
- the dye compound of the present invention is excellent in flexibility
- the inner lens can be expected to retain flexibility and can be easily handled during treatment.
- the polymer of the present invention exhibits excellent resistance to light and chemical agents, has high fastness, and does not elute the dye part from the polymer. Therefore, the polymer is excellent in safety and has no decolorization or discoloration. An inner lens can be obtained.
- the polymer of the present invention can be used as a material for glasses, sunglasses, contact lenses and the like, and can also be used for paints and building materials.
- the polymer of the present invention has a chemically stable dye moiety, and can be used without deterioration even outdoors or in severe environments where temperature changes and pH changes are expected to be severe.
- the polymer of the present invention When used as an intraocular lens material, it can be molded by a known method. For example, a polymerization reaction is performed in an appropriate mold or container to obtain a rod-like, block-like, or plate-like polymer, which is then processed into a desired shape by mechanical processing such as cutting or polishing, or desired shape A method of performing a polymerization reaction in a mold corresponding to the above to obtain a polymer molded product, and then mechanically finishing as required. Further, the support portion of the intraocular lens may be manufactured separately from the intraocular lens and attached later, or may be molded simultaneously (integrally) with the intraocular lens.
- surface modification treatment may be performed as necessary to make the lens surface hydrophilic, and plasma treatment or treatment with ultraviolet rays is preferable, and corona Discharge treatment, glow discharge treatment or ultraviolet / ozone treatment is more preferred.
- a processing apparatus and a processing method at that time a conventionally known normal apparatus and method can be used.
- N- [2- (4-Nitrophenyl) ethyl] methacrylamide (1.20 g) was then dissolved in ethanol (15 mL) and water (5 mL).
- Ammonium chloride (364 mg) and iron powder (933 mg) were added and refluxed at 80 ° C. for 4 hours.
- the iron powder was collected by filtration and concentrated with a rotary evaporator. Water and ethyl acetate were added, extraction operation was performed, and washing was performed with water and saturated brine.
- the extract was dried over sodium sulfate, and the desiccant was collected by filtration, followed by concentration, and the target product was obtained as a pale red oil. The yield was 1.01 g (97%).
- N- (4-nitrobenzyl) methacrylamide (811 mg) was then dissolved in ethanol (12 mL) and water (4 mL).
- Ammonium chloride (183 mg) and iron powder (573 mg) were added and refluxed at 80 ° C. for 4 hours.
- the iron powder was collected by filtration and concentrated with a rotary evaporator. Water and ethyl acetate were added, extraction operation was performed, and washing was performed with water and saturated brine.
- the extract was dried over sodium sulfate, and the desiccant was collected by filtration, followed by concentration, and the target product was obtained as a pale red oil. Yield was 580 mg (89%).
- the polymerizable ultraviolet absorbing dye of the present invention was synthesized using the polymerizable aminoaryl compound synthesized as described above. Examples 1 to 12 are shown below.
- N- [2- [4- (tert-butoxycarbonylamino) phenyl] ethyl] methacrylamide (609 mg) was dissolved in ethyl acetate (2 mL), and 4M hydrogen chloride in ethyl acetate (5 mL) was added. The mixture was stirred at room temperature for 40 minutes and then concentrated under reduced pressure. This concentrated residue was dissolved in 1M hydrochloric acid (4 mL), a solution of sodium nitrite (145 mg) in water (10 mL) was added dropwise under ice cooling, and the mixture was stirred at 4 ° C. for 40 minutes to prepare a diazonium salt.
- N- [2- [4- (tert-butoxycarbonylamino) phenyl] ethyl] acrylamide (581 mg) was acid-treated and then diazonium chlorided in the same manner as in Example 1 to obtain 2,4-dihydroxybenzophenone (428 mg) and diazo cup. Ringing was performed to obtain the target product. Yield was 313 mg (38%). With respect to the obtained compound, spectral data of 1 H-NMR (400 MHz, CDCl 3 ) are shown.
- N- [2- [4- (tert-butoxycarbonylamino) phenyl] ethyl] acrylamide (581 mg) was acid-treated and then diazonium-chlorinated to give 2-hydroxy-4-methoxybenzophenone (456 mg). Diazo coupling was performed to obtain the target product. Yield was 510 mg (59%). With respect to the obtained compound, spectral data of 1 H-NMR (400 MHz, CDCl 3 ) are shown.
- N- [2- [4- (tert-butoxycarbonylamino) phenyl] ethyl] acrylamide (581 mg) was acid-treated and then diazonium-chlorinated to give 4-ethoxy-2-hydroxybenzophenone (485 mg). Diazo coupling was performed to obtain the target product. The yield was 583 mg (66%). With respect to the obtained compound, spectral data of 1 H-NMR (400 MHz, CDCl 3 ) are shown.
- HBZ-PHM was synthesized by the following method different from Example 1.
- N- [2- [4-aminophenyl] ethyl] methacrylamide 930 mg
- 1M hydrochloric acid 15 mL
- a solution of sodium nitrite 355 mg
- water 5 mL
- the mixture was stirred at 0 ° C. to prepare a diazonium salt.
- 2,4-dihydroxybenzophenone (975 mg) was dissolved in ethanol (40 mL), and a solution of sodium carbonate (970 mg) in water (40 mL) was added.
- a solution containing the above diazonium salt was added dropwise to the mixture under ice cooling.
- N- [4- (tert-butoxycarbonylamino) benzyl] methacrylamide (581 mg) was subjected to acid treatment followed by diazonium chloride, followed by diazo coupling with 2-hydroxy-4-methoxybenzophenone (456 mg).
- the target product was obtained. Yield was 511 mg (59%).
- spectral data of 1 H-NMR 400 MHz, CDCl 3 ) are shown.
- MBZ-BZM was synthesized by the following method different from Example 11.
- N- [4-aminobenzyl] methacrylamide 574 mg
- 1M hydrochloric acid 9 mL
- a solution of sodium nitrite 210 mg
- 3 mL 3 mL
- a diazonium salt was prepared.
- 2,4-dihydroxybenzophenone (637 mg) was dissolved in ethanol (25 mL), and a solution of sodium carbonate (644 mg) in water (25 mL) was added.
- a solution containing the above diazonium salt was added dropwise to the mixture under ice cooling. The mixture was stirred at 4 ° C.
- Example 13 0.03 part by mass of the polymerizable ultraviolet absorbing dye (HBZ-PHM) obtained in Example 1, 60 parts by mass of 2-phenoxyethyl acrylate, 40 parts by mass of ethyl acrylate, 2,2′-azobis (2, 4-dimethylvaleronitrile) was uniformly blended and polymerized at 80 ° C. for 40 minutes to prepare a polymer sheet having a thickness of 1 mm.
- the light transmittance at a wavelength of 220 to 800 nm was measured.
- the results are shown in FIG. Further, this sample was immersed in ethanol at 40 ° C. for 24 hours for elution treatment, and the light transmittance was measured again. As a result, the spectrum did not change before and after the elution treatment. This indicates that the polymerizable UV-absorbing dye is chemically bonded in the material. Even if the dye compound of the present invention is used in combination with other UV absorbers for polymer synthesis, It was confirmed that no elution occurred.
- the light transmittance was measured using an ultraviolet-visible spectrophotometer (hereinafter the same).
- Example 14 Further 0.15 parts by mass of 2- [2′-hydroxy-5 ′-(2 ′′ -methacryloyloxyethoxy) -3′-t-butylphenyl] -5-methyl-2H-benzotriazole as a UV absorber A polymer sheet was produced in the same manner as in Example 13 except that.
- the light transmittance at a wavelength of 220 to 800 nm was measured in the same manner as in Example 13. The result is shown in FIG.
- the spectrum of light transmittance before and after the elution treatment does not change, and even if the polymerizable ultraviolet absorbing dye of the present invention is used in combination with other polymerizable ultraviolet absorbers, it is incorporated into the polymer as a copolymer component and polymerized. It was confirmed that it did not elute later.
- HBZ-PHM (1 part by weight) and methyl methacrylate (26 parts by weight) obtained in Example 1 were mixed with dioxane (52 parts by weight), N, N-dimethylformamide (22 parts by weight) and water (20 parts by weight).
- HBZ-PHM copolymer was prepared by adding 2.4 parts by weight of 2,2′-azobis (2,4-dimethylvaleronitrile) and polymerizing at 75 ° C. for 5 hours in an argon atmosphere.
- the filtrate after the alkali treatment of the HBZ-PHM copolymer transmits light of any wavelength, and even when the pH is set to 12 or more by the alkali treatment, the dye component is not liberated from the copolymer. It was confirmed that the polymer was more stable with respect to pH change than the polymer and hardly affected by pH change.
- a polymerizable ultraviolet-absorbing dye monomer that is stable even under alkaline conditions. Since the dye compound of the present invention has in its molecule a benzophenone skeleton having ultraviolet absorption capability, an azobenzene skeleton having blue region light absorption capability, and a polymerizable group, it is copolymerized with other polymerizable monomers to obtain a polymer.
- the polymer is useful as a material for intraocular lenses and the like.
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Medicinal Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Transplantation (AREA)
- Dermatology (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Epidemiology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Polymers & Plastics (AREA)
- Addition Polymer Or Copolymer, Post-Treatments, Or Chemical Modifications (AREA)
- Materials For Medical Uses (AREA)
- Prostheses (AREA)
Priority Applications (13)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| SG11201406978RA SG11201406978RA (en) | 2012-04-27 | 2013-04-30 | Stable polymerizable uv-absorbing colorant for intraocular lens |
| BR112014026853-3A BR112014026853B1 (pt) | 2012-04-27 | 2013-04-30 | corante que absorve uv polimerizável estável para lente intraocular |
| US14/397,352 US9365501B2 (en) | 2012-04-27 | 2013-04-30 | Stable polymerizable UV-absorbing colorant for intraocular lens |
| JP2014512734A JP5998208B2 (ja) | 2012-04-27 | 2013-04-30 | 安定な眼内レンズ用重合性紫外線吸収色素 |
| HK15104302.8A HK1203990B (en) | 2012-04-27 | 2013-04-30 | Stable polymerizable uv-absorbing colorant for intraocular lens |
| AU2013253368A AU2013253368B2 (en) | 2012-04-27 | 2013-04-30 | Stable polymerizable UV-absorbing colorant for intraocular lens |
| CN201380022411.4A CN104284942B (zh) | 2012-04-27 | 2013-04-30 | 稳定的人工晶体用聚合性紫外线吸收色素 |
| KR1020147032913A KR102089816B1 (ko) | 2012-04-27 | 2013-04-30 | 안정한 안내 렌즈용 중합성 자외선 흡수 색소 |
| IN9900DEN2014 IN2014DN09900A (enExample) | 2012-04-27 | 2013-04-30 | |
| RU2014147697A RU2635918C1 (ru) | 2012-04-27 | 2013-04-30 | Стабильный полимеризуемый уф-поглощающий краситель для интраокулярной линзы |
| ES13780703.8T ES2657477T3 (es) | 2012-04-27 | 2013-04-30 | Colorante absorbente de UV polimerizable estable para lente intraocular |
| EP13780703.8A EP2857459B1 (en) | 2012-04-27 | 2013-04-30 | Stable polymerizable uv-absorbing colorant for intraocular lens |
| IL235330A IL235330A (en) | 2012-04-27 | 2014-10-26 | Stable dye absorbs UV polymerizable lens for intraocular lens |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2012-102960 | 2012-04-27 | ||
| JP2012102960 | 2012-04-27 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2013162042A1 true WO2013162042A1 (ja) | 2013-10-31 |
Family
ID=49483336
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2013/062537 Ceased WO2013162042A1 (ja) | 2012-04-27 | 2013-04-30 | 安定な眼内レンズ用重合性紫外線吸収色素 |
Country Status (13)
| Country | Link |
|---|---|
| US (1) | US9365501B2 (enExample) |
| EP (1) | EP2857459B1 (enExample) |
| JP (1) | JP5998208B2 (enExample) |
| KR (1) | KR102089816B1 (enExample) |
| CN (1) | CN104284942B (enExample) |
| AU (1) | AU2013253368B2 (enExample) |
| BR (1) | BR112014026853B1 (enExample) |
| ES (1) | ES2657477T3 (enExample) |
| IL (1) | IL235330A (enExample) |
| IN (1) | IN2014DN09900A (enExample) |
| RU (1) | RU2635918C1 (enExample) |
| SG (1) | SG11201406978RA (enExample) |
| WO (1) | WO2013162042A1 (enExample) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2015064674A1 (ja) * | 2013-10-30 | 2015-05-07 | 興和株式会社 | 重合性紫外線吸収色素の製造方法 |
| WO2015064675A1 (ja) * | 2013-10-30 | 2015-05-07 | 興和株式会社 | 重合性紫外線吸収色素の製造方法 |
| JP2023519037A (ja) * | 2020-03-18 | 2023-05-10 | ジョンソン・アンド・ジョンソン・ビジョン・ケア・インコーポレイテッド | 高エネルギー可視光フィルタとして遷移金属錯体を含有する眼科用デバイス |
| JP2025532268A (ja) * | 2022-09-30 | 2025-09-29 | カール・ツアイス・メディテック・アーゲー | 複数のコモノマーグループを有する眼科用組成物及び眼科用レンズ |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2015170278A1 (en) | 2014-05-07 | 2015-11-12 | Tubitak | A formulation and lens manufacturing process for the production of intraocular lens (iol) |
| CN109641855B (zh) * | 2016-11-30 | 2022-04-29 | 东莞东阳光医疗智能器件研发有限公司 | 偶氮化合物、聚合物和它们的制备方法及用途 |
| WO2018177329A1 (zh) * | 2017-03-29 | 2018-10-04 | 东莞东阳光科研发有限公司 | 可聚合染料化合物、制备方法、含该染料的聚合物及用途 |
| IL275569B2 (en) * | 2018-01-31 | 2023-11-01 | Menicon Co Ltd | Material for intraocular lenses |
| US20220105011A1 (en) * | 2019-02-21 | 2022-04-07 | The Regents Of The University Of Colorado, A Body Corporate | Antimicrobial azo compounds and uses thereof |
| CN116970288B (zh) * | 2023-06-10 | 2024-10-18 | 水木聚力接枝纺织新技术(深圳)有限公司 | 一种水溶性丙烯酰胺型氨基c酸染料及其合成方法和用途 |
Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH02232056A (ja) | 1988-09-16 | 1990-09-14 | Menikon:Kk | 眼用レンズ用重合性紫外線吸収性色素並びにそれを用いた眼用レンズ材料 |
| JPH0588120A (ja) * | 1991-09-27 | 1993-04-09 | Tokyo Keikaku:Kk | ソフトコンタクトレンズ |
| JPH0728911B2 (ja) | 1988-05-06 | 1995-04-05 | 株式会社メニコン | 眼用レンズを着色するための重合性色素並びにそれを用いた着色眼用レンズ材料の製造法及び着色眼用レンズ材料 |
| JP2604799B2 (ja) | 1988-05-27 | 1997-04-30 | 株式会社メニコン | 眼内レンズ材料 |
| JP2685980B2 (ja) | 1990-11-26 | 1997-12-08 | 株式会社メニコン | 紫外線吸収性眼内レンズ |
| JPH10251537A (ja) | 1997-03-13 | 1998-09-22 | Menicon Co Ltd | 重合性色素及びそれを用いた着色眼用レンズ材料 |
| JP2003253248A (ja) | 2002-03-01 | 2003-09-10 | Dainichiseika Color & Chem Mfg Co Ltd | 紫外線吸収剤及び重合体結合ベンゾトリアゾール系紫外線吸収剤、製造方法、処理物品および処理方法 |
| JP2006291006A (ja) | 2005-04-08 | 2006-10-26 | Menicon Co Ltd | 新規重合性染料およびそれを含む眼用レンズ |
| JP2009034999A (ja) * | 2001-04-27 | 2009-02-19 | Millipore Corp | 架橋されたマルチポリマーコーティング |
Family Cites Families (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2604799Y2 (ja) | 1993-01-12 | 2000-06-05 | 株式会社クボタ | 金属管の端面防食ゴム |
| JPH0728911A (ja) | 1993-07-09 | 1995-01-31 | Fujitsu Social Sci Lab:Kk | 帳票送受信システムおよび帳票送受信システムにおける帳票送受信方法 |
| JP3449406B2 (ja) * | 1999-04-07 | 2003-09-22 | Hoyaヘルスケア株式会社 | 新規ピラゾロン化合物およびそれを用いた眼用プラスチックレンズ |
| JP4192091B2 (ja) | 2001-04-27 | 2008-12-03 | ミリポア・コーポレーシヨン | 架橋されたマルチポリマーコーティング |
| AU2007311172B2 (en) * | 2006-10-13 | 2013-01-31 | Alcon Inc. | Intraocular lenses with unique blue-violet cutoff and blue light transmission characteristics |
| WO2009044853A1 (ja) | 2007-10-04 | 2009-04-09 | Menicon Co., Ltd. | 眼用レンズ用着色剤、眼用レンズ用材料、眼用レンズの製造方法および眼用レンズ |
| TWI453199B (zh) | 2008-11-04 | 2014-09-21 | Alcon Inc | 用於眼用鏡片材料之紫外光/可見光吸收劑 |
| TWI473629B (zh) * | 2010-01-18 | 2015-02-21 | Alcon Inc | 用於眼用晶體材料之可見光吸收劑 |
| WO2015064675A1 (ja) * | 2013-10-30 | 2015-05-07 | 興和株式会社 | 重合性紫外線吸収色素の製造方法 |
-
2013
- 2013-04-30 IN IN9900DEN2014 patent/IN2014DN09900A/en unknown
- 2013-04-30 AU AU2013253368A patent/AU2013253368B2/en not_active Ceased
- 2013-04-30 RU RU2014147697A patent/RU2635918C1/ru active
- 2013-04-30 CN CN201380022411.4A patent/CN104284942B/zh not_active Expired - Fee Related
- 2013-04-30 EP EP13780703.8A patent/EP2857459B1/en not_active Not-in-force
- 2013-04-30 US US14/397,352 patent/US9365501B2/en not_active Expired - Fee Related
- 2013-04-30 BR BR112014026853-3A patent/BR112014026853B1/pt not_active IP Right Cessation
- 2013-04-30 SG SG11201406978RA patent/SG11201406978RA/en unknown
- 2013-04-30 ES ES13780703.8T patent/ES2657477T3/es active Active
- 2013-04-30 JP JP2014512734A patent/JP5998208B2/ja not_active Expired - Fee Related
- 2013-04-30 KR KR1020147032913A patent/KR102089816B1/ko not_active Expired - Fee Related
- 2013-04-30 WO PCT/JP2013/062537 patent/WO2013162042A1/ja not_active Ceased
-
2014
- 2014-10-26 IL IL235330A patent/IL235330A/en not_active IP Right Cessation
Patent Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0728911B2 (ja) | 1988-05-06 | 1995-04-05 | 株式会社メニコン | 眼用レンズを着色するための重合性色素並びにそれを用いた着色眼用レンズ材料の製造法及び着色眼用レンズ材料 |
| JP2604799B2 (ja) | 1988-05-27 | 1997-04-30 | 株式会社メニコン | 眼内レンズ材料 |
| JPH02232056A (ja) | 1988-09-16 | 1990-09-14 | Menikon:Kk | 眼用レンズ用重合性紫外線吸収性色素並びにそれを用いた眼用レンズ材料 |
| JP2685980B2 (ja) | 1990-11-26 | 1997-12-08 | 株式会社メニコン | 紫外線吸収性眼内レンズ |
| JPH0588120A (ja) * | 1991-09-27 | 1993-04-09 | Tokyo Keikaku:Kk | ソフトコンタクトレンズ |
| JPH10251537A (ja) | 1997-03-13 | 1998-09-22 | Menicon Co Ltd | 重合性色素及びそれを用いた着色眼用レンズ材料 |
| JP2009034999A (ja) * | 2001-04-27 | 2009-02-19 | Millipore Corp | 架橋されたマルチポリマーコーティング |
| JP2003253248A (ja) | 2002-03-01 | 2003-09-10 | Dainichiseika Color & Chem Mfg Co Ltd | 紫外線吸収剤及び重合体結合ベンゾトリアゾール系紫外線吸収剤、製造方法、処理物品および処理方法 |
| JP2006291006A (ja) | 2005-04-08 | 2006-10-26 | Menicon Co Ltd | 新規重合性染料およびそれを含む眼用レンズ |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2015064674A1 (ja) * | 2013-10-30 | 2015-05-07 | 興和株式会社 | 重合性紫外線吸収色素の製造方法 |
| WO2015064675A1 (ja) * | 2013-10-30 | 2015-05-07 | 興和株式会社 | 重合性紫外線吸収色素の製造方法 |
| JP2023519037A (ja) * | 2020-03-18 | 2023-05-10 | ジョンソン・アンド・ジョンソン・ビジョン・ケア・インコーポレイテッド | 高エネルギー可視光フィルタとして遷移金属錯体を含有する眼科用デバイス |
| JP2025532268A (ja) * | 2022-09-30 | 2025-09-29 | カール・ツアイス・メディテック・アーゲー | 複数のコモノマーグループを有する眼科用組成物及び眼科用レンズ |
Also Published As
| Publication number | Publication date |
|---|---|
| IN2014DN09900A (enExample) | 2015-08-07 |
| KR102089816B1 (ko) | 2020-03-16 |
| EP2857459A1 (en) | 2015-04-08 |
| JPWO2013162042A1 (ja) | 2015-12-24 |
| IL235330A (en) | 2017-10-31 |
| CN104284942A (zh) | 2015-01-14 |
| KR20150005650A (ko) | 2015-01-14 |
| AU2013253368B2 (en) | 2017-02-16 |
| EP2857459A4 (en) | 2016-02-24 |
| BR112014026853A2 (pt) | 2017-06-27 |
| JP5998208B2 (ja) | 2016-09-28 |
| US9365501B2 (en) | 2016-06-14 |
| HK1203990A1 (en) | 2015-11-06 |
| US20150094439A1 (en) | 2015-04-02 |
| EP2857459B1 (en) | 2017-12-27 |
| BR112014026853B1 (pt) | 2021-05-11 |
| SG11201406978RA (en) | 2015-01-29 |
| RU2635918C1 (ru) | 2017-11-17 |
| CN104284942B (zh) | 2016-09-07 |
| ES2657477T3 (es) | 2018-03-05 |
| AU2013253368A1 (en) | 2014-12-11 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5998208B2 (ja) | 安定な眼内レンズ用重合性紫外線吸収色素 | |
| JP4291296B2 (ja) | 新規重合性染料およびそれを含む眼用レンズ | |
| JP2685980B2 (ja) | 紫外線吸収性眼内レンズ | |
| JP5897906B2 (ja) | 高分子アントラキノン系色素、これを用いた眼用レンズ材料及び眼用レンズ | |
| JP6492368B2 (ja) | 重合性紫外線吸収色素の製造方法 | |
| JP4532243B2 (ja) | 眼用レンズ用着色剤、及び該着色剤を用いた着色眼用レンズ材料 | |
| EP0913713B1 (en) | Crosslinkable compound and an optical material employing it | |
| JP6258664B2 (ja) | 眼内レンズ用重合性紫外線吸収色素 | |
| JPH0728911B2 (ja) | 眼用レンズを着色するための重合性色素並びにそれを用いた着色眼用レンズ材料の製造法及び着色眼用レンズ材料 | |
| JP6492367B2 (ja) | 重合性紫外線吸収色素の製造方法 | |
| JP6265684B2 (ja) | 眼内レンズ用重合性紫外線吸収色素 | |
| JP2022071514A (ja) | コントラスト改善機能を有する眼科用材料 | |
| HK1203990B (en) | Stable polymerizable uv-absorbing colorant for intraocular lens | |
| JP3244851B2 (ja) | 眼用レンズ材料 | |
| JP2000109515A (ja) | 無水マレイン酸−スチレン系櫛形ポリマーおよびそれを用いてなる眼用レンズ材料 | |
| JPH02138151A (ja) | ビニルベンジルメタクリレート | |
| JPH09297285A (ja) | 眼用レンズおよびその製法 | |
| JPH1135524A (ja) | 架橋性化合物およびそれを用いてなる眼用レンズ材料 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 13780703 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2014512734 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 14397352 Country of ref document: US |
|
| ENP | Entry into the national phase |
Ref document number: 20147032913 Country of ref document: KR Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 2014147697 Country of ref document: RU Kind code of ref document: A |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: 112014026853 Country of ref document: BR |
|
| ENP | Entry into the national phase |
Ref document number: 2013253368 Country of ref document: AU Date of ref document: 20130430 Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 112014026853 Country of ref document: BR Kind code of ref document: A2 Effective date: 20141027 |