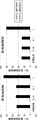WO2013062083A1 - 癌幹細胞特異的分子 - Google Patents
癌幹細胞特異的分子 Download PDFInfo
- Publication number
- WO2013062083A1 WO2013062083A1 PCT/JP2012/077714 JP2012077714W WO2013062083A1 WO 2013062083 A1 WO2013062083 A1 WO 2013062083A1 JP 2012077714 W JP2012077714 W JP 2012077714W WO 2013062083 A1 WO2013062083 A1 WO 2013062083A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- antibody
- cells
- cancer
- lgr5
- cell
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
-
- G01N33/57535—
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/47—Quinolines; Isoquinolines
- A61K31/4738—Quinolines; Isoquinolines ortho- or peri-condensed with heterocyclic ring systems
- A61K31/4745—Quinolines; Isoquinolines ortho- or peri-condensed with heterocyclic ring systems condensed with ring systems having nitrogen as a ring hetero atom, e.g. phenantrolines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/0011—Cancer antigens
- A61K39/001102—Receptors, cell surface antigens or cell surface determinants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/39533—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals
- A61K39/39558—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals against tumor tissues, cells, antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/04—Antineoplastic agents specific for metastasis
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/475—Growth factors; Growth regulators
- C07K14/51—Bone morphogenetic factor; Osteogenins; Osteogenic factor; Bone-inducing factor
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/30—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants from tumour cells
- C07K16/3046—Stomach, Intestines
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6844—Nucleic acid amplification reactions
- C12Q1/686—Polymerase chain reaction [PCR]
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6876—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes
- C12Q1/6883—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes for diseases caused by alterations of genetic material
- C12Q1/6886—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes for diseases caused by alterations of genetic material for cancer
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/574—Immunoassay; Biospecific binding assay; Materials therefor for cancer
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/574—Immunoassay; Biospecific binding assay; Materials therefor for cancer
- G01N33/57407—Specifically defined cancers
- G01N33/57419—Specifically defined cancers of colon
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/574—Immunoassay; Biospecific binding assay; Materials therefor for cancer
- G01N33/57484—Immunoassay; Biospecific binding assay; Materials therefor for cancer involving compounds serving as markers for tumor, cancer, neoplasia, e.g. cellular determinants, receptors, heat shock/stress proteins, A-protein, oligosaccharides, metabolites
- G01N33/57492—Immunoassay; Biospecific binding assay; Materials therefor for cancer involving compounds serving as markers for tumor, cancer, neoplasia, e.g. cellular determinants, receptors, heat shock/stress proteins, A-protein, oligosaccharides, metabolites involving compounds localized on the membrane of tumor or cancer cells
-
- G01N33/5759—
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/555—Medicinal preparations containing antigens or antibodies characterised by a specific combination antigen/adjuvant
- A61K2039/55511—Organic adjuvants
- A61K2039/55566—Emulsions, e.g. Freund's adjuvant, MF59
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/58—Medicinal preparations containing antigens or antibodies raising an immune response against a target which is not the antigen used for immunisation
- A61K2039/585—Medicinal preparations containing antigens or antibodies raising an immune response against a target which is not the antigen used for immunisation wherein the target is cancer
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/40—Immunoglobulins specific features characterized by post-translational modification
- C07K2317/41—Glycosylation, sialylation, or fucosylation
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/73—Inducing cell death, e.g. apoptosis, necrosis or inhibition of cell proliferation
- C07K2317/732—Antibody-dependent cellular cytotoxicity [ADCC]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/73—Inducing cell death, e.g. apoptosis, necrosis or inhibition of cell proliferation
- C07K2317/734—Complement-dependent cytotoxicity [CDC]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/76—Antagonist effect on antigen, e.g. neutralization or inhibition of binding
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/106—Pharmacogenomics, i.e. genetic variability in individual responses to drugs and drug metabolism
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/158—Expression markers
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/435—Assays involving biological materials from specific organisms or of a specific nature from animals; from humans
- G01N2333/705—Assays involving receptors, cell surface antigens or cell surface determinants
- G01N2333/72—Assays involving receptors, cell surface antigens or cell surface determinants for hormones
- G01N2333/726—G protein coupled receptor, e.g. TSHR-thyrotropin-receptor, LH/hCG receptor, FSH
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2800/00—Detection or diagnosis of diseases
- G01N2800/52—Predicting or monitoring the response to treatment, e.g. for selection of therapy based on assay results in personalised medicine; Prognosis
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Definitions
- the present invention relates to a cell surface molecule specific for Lgr5-positive cancer stem cells with high proliferation ability and Lgr5-negative cancer stem cells with low proliferation ability, and a pharmaceutical composition containing an antibody against these cell surface molecules as an active ingredient.
- the present invention further relates to a reagent for detecting cancer stem cells using the antibody and a method for screening cancer patients.
- CSC Cancer stem cells
- CSCs include acute myeloid leukemia (AML) (Non-Patent Documents 2 and 3), breast cancer (Non-Patent Document 4), glioma (Non-Patent Document 5), head and neck cancer (Non-Patent Document 6), pancreatic cancer ( Non-patent documents 7, 8), lung cancer (non-patent document 9), prostate cancer (non-patent documents 10, 11), mesenchymal neoplasms (non-patent document 12), and melanoma (non-patent documents 13, 14). It has been reported in some cancers. In colon cancer, CD133 is reported to be a CSC marker in early studies by O'Brien et al.
- Non-patent Document 15 Non-patent Document 15
- Non-patent Document 16 Ricci-Vitiani et al.
- CD44, EpCAM, CD166 (Non-Patent Document 17), and ALDH (Non-Patent Documents 18 and 19) were reported as additional markers by other research groups.
- Pang et al. Have shown that CD26 is a marker for a subpopulation of CSCs with metastatic activity (Non-patent Document 20).
- Non-patent Document 17 EpCAM high / CD44 + / CD166 +
- CD133 + / CD44 + Non-patent Document 21
- CD44 high / ALDH + Non-patent Document 18
- cell sorting approaches using combinations of CSC markers such as ALDH1 + / CD133 +
- Non-patent Document 22 in vitro spheroid (cell mass) cultures and direct xenotransplantation of cancer cells into immune-deficient mice have also been used for CSC enrichment.
- Non-patent Document 22 In order to further understand the nature of CSC, it was necessary to obtain cancer stem cells in high purity and in large quantities.
- Non-patent Document 29 Three-dimensional spheroid cultures are often used as a source of CSC. This spheroid culture can be directly adapted to clinically resected specimen tumor cells and the ability to maintain a heterogeneous population of CSCs may have certain advantages over xenotransplantation. However, due to its heterogeneity, the results in biochemical analysis often show the complex features of CSC. Although CSC selection using antibodies against cell surface marker proteins is widely used to isolate CSCs, the number and purity of cells obtained by this method is limited.
- xenografts as a source of CSC is also a common approach, since the xenograft phenotype remains stable after multiple passages.
- passage of xenografts in mice can only select cells that are viable in mice, thereby eliminating cells that are less susceptible to such an environment.
- CSCs present in xenograft tumors reflect the original characteristics of CSCs as long as they maintain the ability to self-renew and produce differentiated strains of the original tumor.
- Lgr5 leucine-rich-repeat-containing G protein-coupled receptor 5
- Non-patent Documents 23 and 24 Lgr5-positive columnar cells can regenerate any epithelial lineage (Non-patent document 25), and single Lgr5-positive cells can form crypto-viral organoids in vitro without a mesenchymal niche (Non-patent document 25). 26) clearly demonstrated that Lgr5-positive cells are stem cells in normal colon.
- Non-patent Document 27 Lgr5-positive cells had adenomas formed in the absence of Apc (Non-patent Document 27) and Lgr5 was expressed in colon cancer cell lines (Non-patent Document 25).
- Non-patent Document 25 Lgr5-positive cells are the origin of colorectal cancer (Non-patent Document 25).
- Wnt activity is essential for CSC proliferation in vitro and in vivo, and that exogenous HGF stimulates Wnt activity (28).
- Lgr5 has been identified as a normal colon stem cell marker and has been shown to be a marker for the origin of colon cancer (Patent Document 1, Non-Patent Document 30), and Lgr5 is a protein that is overexpressed in colon cancer stem cells. Although shown (Patent Document 2), the physiological role of Lgr5 in the development of colorectal cancer remains unclear.
- Non-patent Document 31 Non-patent Document 31
- the present invention has been made in view of such circumstances, and the present invention obtains two substantially uniform cancer stem cell populations characterized by using Lgr5, a cell surface marker, and these cancers. It is an object of the present invention to provide a cancer therapeutic drug using an antibody against these molecules by identifying cell membrane molecules specifically expressed in stem cells. Another object of the present invention is to provide a reagent for detecting cancer stem cells, a diagnosis of cancer patients, and a screening method using an antibody against a cell membrane molecule specifically expressed in cancer stem cells.
- CSC cancer stem cells
- the inventors apply in vitro monolayer culture (also referred to simply as adherent culture) using a serum-free stem cell medium.
- adherent culture also referred to simply as adherent culture
- the present inventors isolated and adherent cultured cells derived from moderately differentiated human colon cancer xenografts maintained in NOD / Shi-scid, IL-2R ⁇ null (NOG) mice. It was found that high-purity colon CSCs can be obtained in large quantities. Under these conditions, only colon CSCs were able to grow, survive, and expand, thereby obtaining highly pure and substantially uniform colon CSCs.
- Large intestine CSCs obtained by this method were stably maintained without changing the phenotype for more than one month by subcultured adherent culture using stem cell medium without serum.
- This cell expresses various colon cancer stem cell markers (CD133, CD44, EpCAM, CD166, CD24, CD26 and CD29) that have been reported so far, and shows tumor initiation activity at a frequency of almost 100%. Tumors with the same histopathological characteristics (hierarchical structure) as the primary tumors were reconstructed.
- the cells are also characterized by being highly proliferative under adherent culture conditions and positive for the cell surface marker Lgr5. Highly proliferative and Lgr5-positive cancer stem cells formed a tumor mass in organs such as the lung and liver when administered from the mouse tail vein, indicating that they play an important role in cancer metastasis. .
- cancer stem cells that are highly proliferative under adherent culture conditions and positive for cell surface marker Lgr5 with anticancer drugs such as irinotecan and 5-FU, they are low proliferative and negative for Lgr5 Cancer stem cells could be isolated. Furthermore, it was shown that cancer stem cells that are low proliferative and negative for Lgr5 were isolated and then cultured again under adherent culture conditions, thereby changing to cancer stem cells that were highly proliferative and positive for Lgr5. Therefore, it was shown that highly proliferative Lgr5-positive cancer stem cells and low-proliferative Lgr5-negative cancer stem cells can be converted into each other and have a self-alternating function.
- anticancer drugs such as irinotecan and 5-FU
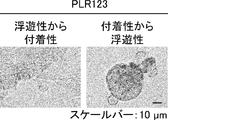
- Lgr5-positive colon CSCs that proliferated actively converted to a stationary Lgr5-negative state.
- Lgr5-negative CSC became Lgr5-positive CSC actively proliferating by separating and re-attaching the cells. These cells also showed tumor initiation activity with a frequency of almost 100%.
- Highly proliferative and Lgr5-positive cancer stem cells formed a tumor mass in organs such as the lung and liver when inoculated from the tail vein of mice, indicating that they play an important role in cancer metastasis. .
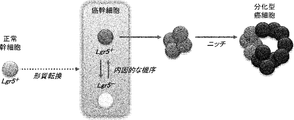
- both high-proliferation Lgr5-positive cancer stem cells and low-proliferation Lgr5-negative cancer stem cells are important for cancer development, formation, metastasis, recurrence, drug resistance, etc. It plays a role and can be a major target cell in the development of anticancer drugs.
- highly proliferative and Lgr5-positive cancer stem cells are involved in cancer formation and metastasis
- low-proliferative and Lgr5-negative cancer stem cells are considered to be involved in cancer recurrence.
- the present invention includes the following: [1] A pharmaceutical composition comprising as an active ingredient at least one antibody that binds to the protein described in SEQ ID NOs: 1 to 8, [2] The pharmaceutical composition according to [1], which is an anticancer agent, [3] The pharmaceutical composition according to [2], which is a cancer recurrence inhibitor, [4] The pharmaceutical composition according to [2], which is a cancer metastasis inhibitor or a postoperative adjuvant therapy agent, [5] The Lgr5-positive cancer metastasis inhibitor or postoperative adjuvant therapeutic agent, which contains at least one antibody that binds to the protein represented by SEQ ID NO: 1 to 6 as an active ingredient, [4] A pharmaceutical composition of [6] The pharmaceutical composition according to [2], which is a drug-resistant cancer therapeutic agent, [7] The pharmaceutical composition according to [6], which is a cancer therapeutic agent for Lgr5-negative cancer, comprising as an active ingredient at least one antibody that binds to the protein represented by SEQ ID NOs: 1 to 8.
- detecting a cancer comprising detecting the presence of at least one protein in a sample isolated from a cancer patient using at least one antibody that binds to the protein set forth in SEQ ID NOs: 1 to 8 Methods of diagnosing or screening for cancer patients (cancer testing, screening methods), [27] The method according to [26], wherein the antibody is at least one antibody that binds to the protein described in SEQ ID NOs: 1 to 6, diagnoses Lgr5-positive cancer, or screens cancer patients.
- the present invention also provides the following: [A1] A method for treating cancer, comprising administering to a subject at least one antibody that binds to the protein set forth in SEQ ID NOs: 1 to 8; [A2] at least one antibody that binds to the protein set forth in SEQ ID NOs: 1 to 8 for use in the treatment of cancer; [A3] Use of at least one antibody that binds to the protein set forth in SEQ ID NOs: 1 to 8 for producing an anticancer agent; [A4] a process for producing an anticancer agent, comprising using at least one antibody that binds to the protein set forth in SEQ ID NOs: 1 to 8; Is to provide.
- the cancer treatment is cancer recurrence suppression, cancer metastasis suppression, postoperative adjuvant therapy, drug resistant cancer treatment, cancer stem cell proliferation suppression, or cancer stem cell destruction
- It is a cancer recurrence inhibitor, a cancer metastasis inhibitor, a postoperative adjuvant therapy agent, a drug resistant cancer therapeutic agent, a cancer stem cell proliferation inhibitor, or a cancer stem cell destruction agent.
- the present invention provides the following: [B1] A reagent for detecting the presence of any one or more of the protein described in SEQ ID NOs: 1 to 8 and / or the polynucleotide encoding the protein, preferably the protein described in SEQ ID NOs: 1 to 8 A cancer stem cell detection reagent, a cancer diagnosis reagent, or a cancer patient, comprising at least one antibody that binds to a polynucleotide, or a polynucleotide encoding the protein described in SEQ ID NOs: 1 to 8 and / or a complementary chain thereof Or a reagent for confirming the effectiveness of the pharmaceutical composition of [1] to [22]; [B2] Preferably, at least one antibody that binds to the protein described in SEQ ID NO: 1 to 8, or a polynucleotide encoding the protein described in SEQ ID NO: 1 to 8 and / or a portion of its complementary strand And detecting the presence of any one or more of the
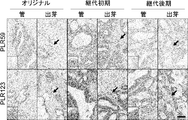
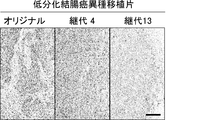
- Original refers to surgically excised tumor
- early passage refers to PLR59 and PLR123 xenografts passaged 4 times in NOG mice
- late passage refers to 15 times in MOG mice
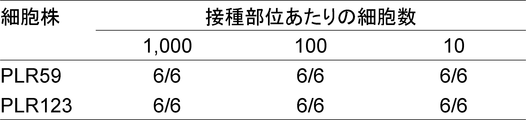
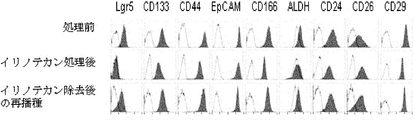
- the scale bar indicates 100 ⁇ m. It is a figure which shows the flow cytometry analysis result of the well-known CSC marker in the cell of the xenograft which subcultured PLR59 and PLR123 with the NOG mouse. Cells were stained with antibodies against the indicated markers and analyzed by flow cytometry.
- Gray indicates fluorescence intensity or ALDH activity after staining cells with the described antibodies, and white indicates fluorescence intensity or ALDH activity with ALDH inhibitors after staining cells with a control isotype antibody.
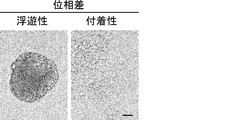
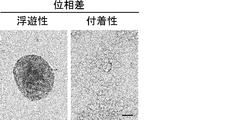
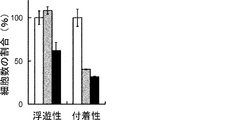
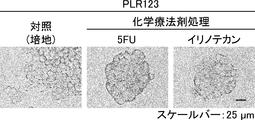
- Suspension cells interacted closely with each other to form spheroid-like structures, whereas adherent cells proliferated without forming cell clusters.
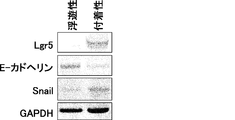
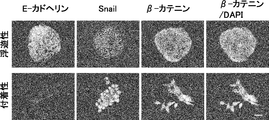
- the scale bar indicates 25 ⁇ m. It is a figure which shows the proliferation of floating CSC and adhering CSC (s) (PLR123 cell). The number of viable cells after culturing for 3 days (black column) is shown as a percentage of the number on day 0 (white column). Results are the average of 3 experiments. The bar at the top of each column indicates the standard deviation. It is a figure which shows the flow cytometry analysis result of the well-known CSC marker in a floating cell and an adherent cell (PLR123 cell).
- GAPDH as a reference protein for protein loading was also visualized. It is a figure which shows the growth inhibition of Lgr5-positive adhesion CSC (PLR123 cell) by FH535 (50 micromol) and cardamonin (50 micromol). The number of viable cells after 3 days culture with FH535 (grey column) and cardamonin (black column) is shown as a percentage of the number of DMSO only (white column). Results are the average of 3 experiments. The bar at the top of each column indicates the standard deviation. It is a figure which shows the proliferation of PLR123 cell in the presence or absence of EGF and FGF. Adherent CSCs were cultured for 3 days (black column) in the presence or absence.
- the number of viable cells is shown as a percentage of the number on day 0 (white column). Results are the average of 3 experiments. The bar at the top of each column indicates the standard deviation. It is a figure which shows the effect of a chemotherapeutic agent with respect to the proliferation of Lgr5-positive adhesion CSC and Lgr5-negative floating CSC (PLR123 cell).
- the number of viable cells after treatment with 5-FU (10 ⁇ g / mL, gray column) or irinotecan (10 ⁇ g / mL, black column) as a percentage of the number of viable cells cultured without chemotherapeutic agents (white column) Show. Results are the average of 3 experiments. The bar at the top of each column indicates the standard deviation.
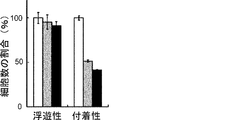
- the scale bar indicates 25 ⁇ m. It is a figure which shows the proliferation of floating CSC and adherent CSC (PLR59 cell). The number of viable cells after culturing for 3 days (black column) is shown as a percentage of the number on day 0 (white column). Results are the average of 3 experiments. The bar at the top of each column indicates the standard deviation. It is a figure which shows the result of the flow cytometry analysis of the CSC marker reported in the floating cell and the adherent cell (PLR59 cell). Adherent cells were positive for all reported markers, but suspension cells were negative for Lgr5 and ALDH.
- Gray indicates fluorescence intensity or ALDH activity after staining cells with the described antibodies, and white indicates fluorescence intensity or ALDH activity with ALDH inhibitors after staining cells with a control isotype antibody.
- It is a photograph showing the results of Western blot analysis of ⁇ -catenin, TCF1, TCF3, TCF4 and phosphorylated c-JUN protein in primary cells, floating CSCs, and adherent CSCs of PLR59 cells. Compared to primary cells, expression of all proteins was up-regulated in Lgr5-positive adherent CSCs. GAPDH as a reference for protein loading was also visualized.
- Gray indicates fluorescence intensity or ALDH activity after staining cells with the described antibodies, and white indicates fluorescence intensity or ALDH activity with ALDH inhibitors after staining cells with a control isotype antibody.
- Lgr5 mRNA levels in adherent and floating CSCs are shown as a ratio of PLR59 and PLR123 to the amount of Lgr5 mRNA in primary cells of xenograft tumors.
- Adherent CSC (right: white column) Lgr5 mRNA levels were significantly increased compared to levels in xenograft primary cells (left: black column), but not in floating CSC (middle: gray column) It was.
- the amount of Lgr5 mRNA was measured by normalization of GAPDH and ACTB expression and quantitative PCR.
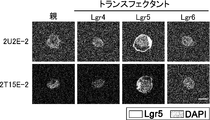
- 2T15E-2 which is an anti-human Lgr5 monoclonal antibody (mAb) in DG44 cell which transfected Lgr4, Lgr5, or Lgr6 * cDNA.
- Untransfected parental cells and transfectants (transfectants) were incubated with monoclonal 2T15E-2 antibody and analyzed by FACS.
- the 2T15E-2 antibody reacted with cells containing Lgr5 cDNA, but not with parental cells and cells containing Lgr4 or Lgr6gr cDNA. Expression of Lgr4, Lgr5, and Lgr6 in transfectants was confirmed by Western blot analysis.
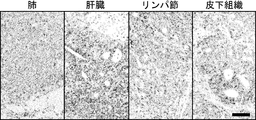
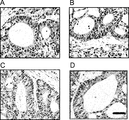
- the tumor cells showed undifferentiated tumor foci, but in the liver and other organs, the tumor cells showed tubular structures with multiple differentiation stages.
- the scale bar indicates 100 ⁇ m.
- CSC CSC
- the two distinct states of CSC alternate endogenously under environmental changes such as the presence of anticancer drugs.
- normal stem cells that express Lgr5 are considered to be transformed by multiple gene mutations to become CSCs.
- Highly proliferative CSCs express Lgr5 and undergo EMT. Under certain stress conditions, these can change to a quiescent state of Lgr5 negative. Involvement by the niche environment stimulates the transition of CSC to the differentiation stage.
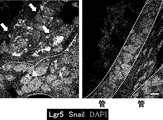
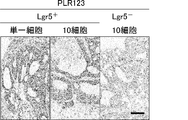
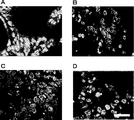
- FIG. 39D Is a photograph showing the immunostaining of Lgr5. Tissue sections were stained with anti-Lgr5 antibody. “Original” refers to a tumor surgically removed from a patient (FIG. 39A). The scale bar indicates 25 ⁇ m. It is a photograph which shows the result (HE stain) of the xenograft tumor derived from the Lgr5-positive cell of PLR59 (FIG. 40A and B) and PLR123 (FIG. 40C and D). 10 cells (FIGS. 40A and C) or 1 cell (FIGS. 40B and D) of Lgr5-positive cells from PLR59 and PLR123 adherent cultures were injected subcutaneously into NOG mice.
- the histopathological features of all tumors were quite similar to the original tumor.
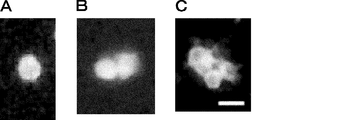
- the scale bar indicates 50 ⁇ m. It is a photograph showing symmetrical cell division of Lgr5-positive cells. Lgr5-positive cells stained with PKH67 dye and cultured for 72 hours were observed with a fluorescence microscope. 41A, B and C show stained images at 0 hour, 48 hours and 72 hours, respectively. The scale bar indicates 20 ⁇ m. 43 is a photograph showing symmetrical division of Lgr5-positive cells in the absence of Matrigel and serum (FIGS. 42A and B) and asymmetric division of Lgr5-positive cells in the presence of Matrigel and serum (FIGS. 42C and D). 42A and 42C show one split image, and FIGS.
- Lgr5 positive cells appear as late as 4 days after reseeding (FIG. 44C).
- Increased by 8 days after re-seeding shows an immunostained image.
- the scale bar indicates 50 ⁇ m. It is a graph which shows the measurement of the transcription amount of Lgr5 gene by quantitative real-time PCR.
- Lgr5 mRNA levels were high in Lgr5-positive cells in adherent culture, but decreased under spheroid culture conditions, and were hardly detected in Lgr-negative cells after irinotecan treatment.
- the mRNA level of the CK20 gene was less than the detection level in Lgr5-positive cells and negative cells in adherent culture, but increased in Lgr5-positive cells under the conditions of spheroid culture. It is a photograph which shows the expression of Lgr5 and CK20 protein confirmed by the tissue immunostaining. Slices of Lgr5-positive CSCs (PLR59 (FIG. 46A) and PLR123 (FIG.
- Lgr5-negative cells were completely resistant to both growth inhibitors. It is a figure which shows the expression of a CSC marker.
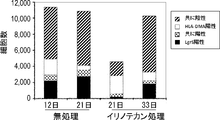
- “Before treatment” represents the expression of CSC markers in Lgr5-positive cells obtained from adherent cultures derived from PLR123 xenograft models
- “After irinotecan treatment” represents the expression of CSC markers in Lgr5-negative cells obtained by irinotecan treatment.
- Expressing expression, “re-seeding after removal of irinotecan” represents expression of CSC marker in Lgr5-negative cells re-seeded in a medium from which irinotecan has been removed.
- Gray indicates ALDH activity or fluorescence intensity after staining the cells with the described antibody, and white indicates ALDH activity in the presence of an ALDH inhibitor. It is a figure showing alternation of a Lgr5-positive cell and a negative cell. Lgr5-positive cells recovered by FACS and limitedly diluted were seeded and cultured in the presence of irinotecan for 3 days under adherent culture conditions. On the other hand, Lgr5-negative cells treated with irinotecan and subjected to limiting dilution were seeded and cultured under adherent culture conditions for 4 days in the absence of irinotecan.
- Lgr5 expression was visualized using PE-labeled anti-mouse IgG antibody (shown in red) or AlexaFluo 488-labeled anti-mouse IgG antibody (shown in green).
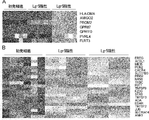
- 7 genes (A) whose expression is markedly up-regulated in Lgr5-negative cells compared to Lgr5-positive cells, and their expression in Lgr5-positive cells and Lgr5-negative cells compared to initial cells derived from xenografts It is a figure showing the heat map (Heat map) of 20 genes (B) which are rising.
- RNA prepared from Lgr5-positive and Lgr5-negative CSCs derived from PLR59 and PLR123 and primary cells isolated from xenografts were analyzed using Affymetrix U133. It is a photograph showing the binding of anti-HLA-DMA antibody and anti-EREG antibody to Lgr5-positive CSC and Lgr5-negative CSC by tissue immunostaining. Immobilized CSC (PLR123) was treated with anti-HLA-DMA antibody (Dako) and anti-EREG antibody (EP27).
- Tumors derived from Lgr5-negative CSCs in NOG mice injected with Lgr5-negative CSCs from PLR123 xenografts were stained with antibodies against Lgr5 (green), HLA-DMA (red) and EREG (green). On day 5, there were low Lgr5 expression, HLA-DMA positive, and EREG positive cells, as well as Lgr5 positive, HLA-DMA negative, and EREG positive cells.
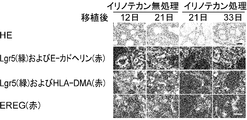
- the scale bar indicates 10 ⁇ m. It is a photograph which shows reconstruction of the tumor hierarchy from Lgr5-negative CSC.
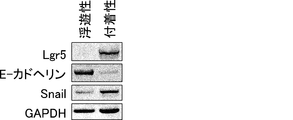
- the tissue structure (FIG. 53A) and immunofluorescence microscopic images using anti-Lgr5 antibody and anti-E-cadherin antibody are shown (FIG.
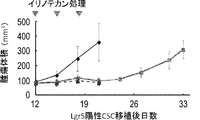
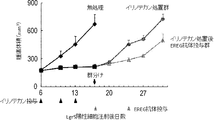
- mice were administered irinotecan or vehicle at 12, 15, and 18 days after tumor implantation.
- the scale bar indicates 25 ⁇ m.
- Tumors derived from Lgr5-positive CSC (PLR123) were administered to NOG mice at a dose of irinotecan (120 mg / kg / day) at day 12, 15 and 18 after transplantation.
- Each value represents an average value + standard deviation.
- It is a graph showing the number of Lgr5-positive and HLA-DMA-positive cells in a xenografted tumor tissue. After tissue slices were treated with Lgr5 and HLA-DMA antibodies, the number of Lgr5-positive and HLA-DMA-positive cells was counted. Numbers represent the total number of cells counted in each group (n 3). It is a graph which shows the antitumor effect of the EREG antibody after an irinotecan process. Irinotecan was administered at a dose of 100 mg / kg / day to SCID mice injected with PLR123-derived Lgr5-positive cells under the abdominal cavity at 6, 10 and 13 days after the injection of Lgr5-positive cells.
- FIG. 58A shows tumor tissue (HE) staining in a xenograft model in which Lgr5-positive cells derived from PLR123 were intravenously injected into NOG mice
- FIG. 58A (second row) uses EREG antibody. Shows tissue immunostaining.
- the arrows in the figure represent HE-stained images (first row) corresponding to EREG-expressing cells (second row) at the nodule portion.
- 58B and C represent the number of tumors formed in the lungs of SCID-beige mice injected intravenously with Lgr5-positive cells from the PLR123 xenograft model.
- the EREG antibody was administered 5 times once a week from 3 days after the injection of Lgr5-positive cells.
- the number of tumor nodules per animal in a slice of animal lung is shown in FIG. 58B.
- Each symbol (circle) indicated the number of tumor nodules in each animal in the group tested (FIG. 58B).
- the number of tumor nodules classified according to the size of tumor nodules in the antibody administration group and the control group is shown in FIG. 58C.
- FIG. 58C The tissue staining (HE staining) of the tumor in the EREG antibody administration and non-administration groups is shown in FIG. 58D.
- the scale bar represents 200 ⁇ m.
- Same sections of primary (primary) and liver metastatic colorectal cancer tissues isolated from patients are HE (2nd and 4th rows) or Lgr5 (green), HLA-DMA (red) and EREG (green) It is the photograph dye
- Lgr5-positive represents proliferative CSC
- EREG-positive and HLA-DMA-positive represent Lgr5-negative resting CSC.
- Lgr5-negative and positive CSCs were detected both in the tube structure and in the budding area of primary and liver metastatic tumors.
- Lgr5-positive CSCs were also found in the stromal region as single cells. Similar staining patterns were observed in multiple tumor tissues isolated from different patients.
- the arrow represents CSC.
- the scale bar represents 10 ⁇ m. It is a figure showing the anti-tumor effect with respect to CSC of an irinotecan non-treatment by an anti-CD70 antibody.
- the horizontal axis represents the antibody concentration, and the vertical axis represents the ratio of the cell growth inhibitory activity by the anti-CD70 antibody. It is a figure showing the anti-tumor effect with respect to CSC of an irinotecan non-treatment by an anti-EDAR antibody.
- the horizontal axis represents the antibody concentration, and the vertical axis represents the ratio of the cell growth inhibitory activity by the anti-EDAR antibody. It is a figure showing the antitumor effect with respect to CSC of an irinotecan non-treatment by an anti-FAS antibody.
- the horizontal axis represents the antibody concentration, and the vertical axis represents the ratio of the cell growth inhibitory activity by the anti-FAS antibody.
- the horizontal axis represents the antibody concentration, and the vertical axis represents the ratio of the cell growth inhibitory activity by the anti-PROCR antibody. It is a figure showing the anti-tumor effect with respect to CSC of an irinotecan non-treatment by an anti-EPCAM antibody.
- the horizontal axis represents the antibody concentration, and the vertical axis represents the ratio of the cell growth inhibitory activity by the anti-EPCAM antibody. It is a figure showing the anti-tumor effect with respect to CSC processed with the irinotecan by an anti- FAS antibody.
- the horizontal axis represents the antibody concentration, and the vertical axis represents the ratio of the cell growth inhibitory activity by the anti-FAS antibody.
- the horizontal axis represents the antibody concentration, and the vertical axis represents the ratio of the cell growth inhibitory activity by the anti-TNFSF9 antibody. It is a figure showing the anti-tumor effect with respect to CSC treated with irinotecan by an anti-PROCR antibody.
- the horizontal axis represents the antibody concentration, and the vertical axis represents the ratio of the cell growth inhibitory activity by the anti-PROCR antibody. It is a figure showing the anti-tumor effect with respect to CSC treated with irinotecan by an anti-EPCAM antibody.
- the horizontal axis represents the antibody concentration, and the vertical axis represents the ratio of the cell growth inhibitory activity by the anti-EPCAM antibody.
- FIG. 73A shows the expression level of CK20 in PLR59
- FIG. 73B shows the expression level of CK20 in PLR123.
- the present invention relates to a cell surface molecule specifically expressed in cancer stem cells, a pharmaceutical composition (anticancer agent or the like) using an antibody against the molecule, and a reagent for detecting cancer stem cells.
- cancer refers to or refers to a physiological condition in mammals that is typically characterized by unregulated cell growth.
- type of cancer is not particularly limited, but includes the following.
- Cancers epidermal cancers
- Sarcomas include liposarcoma, leiomyosarcoma, rhabdomyosarcoma, synovial sarcoma, angiosarcoma, fibrosarcoma, malignant peripheral nerve tumor, gastrointestinal stromal tumor, tendonoma, Ewing sarcoma, Osteosarcoma, chondrosarcoma, leukemia, lymphoma, myeloma, and other parenchymal tumors such as melanoma or brain tumor (Kumar V, Abbas AK, Fausio N. Robbins and Cotran Pathologic Basis of Disease. 7th Ed.
- a “tumor” refers to any tissue mass resulting from excessive cell growth or proliferation, benign (non-cancerous) or malignant (cancerous) including precancerous lesions.
- the cancer stem cell refers to a cell having the ability described in the following i) and / or ii).
- Self-replicating ability refers to the ability of one or both of two daughter cells that have divided to produce cells that retain the same ability and degree of differentiation as the parent cell in the cell lineage.
- Differentiate into multiple types of cancer cells that make up the cancer cell mass A plurality of types of cancer cells differentiated from cancer stem cells form a hierarchical structure having the cancer stem cells as apexes in the cell lineage, similar to normal stem cells. Cancer cell masses having various characteristics are formed by producing various types of cancer cells in stages from cancer stem cells.
- Cancer stem cells are cancer cells that have the ability to form cancer and have pluripotency and self-renewal ability as normal stem cells. Cancer stem cells form a hierarchical structure with cancer stem cells at the top. Cancer cell masses having various characteristics are formed by producing various types of cancer cells in stages from cancer stem cells. A cancer cell mass is a mass formed by cells adhering to each other in the same way as human tumor tissue. Cancer cells, cells other than cancer cells such as stromal cells and blood cells, collagen, laminin, etc. This refers to a mass that is constructed from the extracellular matrix of
- the origin of the cancer stem cells to be treated with the pharmaceutical composition of the present invention is not particularly limited, and is derived from mammals such as humans, monkeys, chimpanzees, dogs, cows, pigs, rabbits, rats, mice, and the like. Although a thing derived from a human tumor tissue is preferable, a thing derived from a human tumor tissue is more preferable.
- the cancer stem cells detected in the present invention are preferably a group of cells that reproduce the hierarchical structure of the cancer tissue.
- the cancer tissue from which the detected cancer stem cells are collected is preferably transplanted into a non-human animal and passaged. It is possible to confirm that the established cancer cell line produced by reappearing such a hierarchical structure of cancer tissue. More preferably, as a non-human animal, an NOG established by transplanting and substituting cancer tissue to an immunodeficient animal, most preferably a NOG mouse lacking functional T cells, B cells, natural killer cells, etc. It can be confirmed that the cancer cell line reproduces the hierarchical structure of such cancer tissue.
- the cancer stem cell detected in the present invention may be a spheroid (cell mass) formed by spheroid culture.
- Spheroid culture refers to cells that are three-dimensionally suspended after seeding cells in a culture vessel such as a non-adherent or low-adhesive culture flask, plate, or dish using a medium capable of culturing cancer stem cells.
- the cell mass formed by this method is called spheroid.
- NOG-established cancer cell lines can be prepared by methods known to those skilled in the art, such as the method described in Fujii E. et al., Pathol int. 2008; 58: 559-567, etc. It can be established by physically mining human colorectal cancer, stomach cancer, lung cancer, breast cancer, pancreatic cancer, etc. removed by surgery with scissors, and transplanting and subcultured in NOG mice. The NOG established cancer cell line maintains the characteristics of the original human cancer tissue even after passage.
- cancer stem cells can be selected using a cell marker.
- cell markers used in the present invention include Lgr5 (leucine-rich-repeat-containing G protein-coupled receptor 5), CD133, CD44, EpCAM, CD166, CD24, CD26, and CD29.
- the present invention relates to a molecule expressed in cancer stem cells, which is positive for the expression of Lgr5, a cell marker, is adherent and highly proliferative under serum-free culture conditions.
- Lgr5 a cell marker
- the cancer stem cell may be referred to as “Lgr5-positive highly proliferative cancer stem cell”.
- the present invention also relates to a molecule expressed in cancer stem cells, characterized in that Lgr5, which is a cell marker, is negatively expressed, is floating in serum-free culture conditions, and is low proliferative.
- Lgr5 which is a cell marker
- the cancer stem cells may be referred to as “Lgr5-negative hypoproliferative cancer stem cells”.
- the medium or culture medium used for culturing cancer stem cells of the present invention is not particularly limited as long as it is serum-free, as long as it can culture cancer stem cells.
- a conventionally known basic culture solution to which Glutamine and N-acetylcysteine are added or a mixture thereof can be used as the culture solution.
- the concentration of EGF is not particularly limited, but is 0.1 to 100 ng / mL, preferably 0.5 to 50 ng / mL, more preferably 1 to 20 ng / mL.
- the concentration of bFGF is not particularly limited, but is 0.1 to 100 ⁇ ng / mL, preferably 0.5 to 50 ⁇ ng / mL, more preferably 1 to 20 ⁇ ng / mL.
- the concentration of hLIF is not particularly limited, but is 0.1 to 100 ng / mL, preferably 0.5 to 50 ng / mL, more preferably 1 to 20 ng / mL.
- the concentration of HGF is not particularly limited, but is 0.1 to 100 ng / mL, preferably 1 to 50 ng / mL.
- the concentration of NGF is not particularly limited, but is 0.1 to 100 ng / mL, preferably 1 to 50 ng / mL.
- the concentration of NSF-1 is not particularly limited, but is 0.1 to 100 ng / mL, preferably 1 to 50 ng / mL.
- the concentration of TGF ⁇ is not particularly limited, but is 0.1 to 100 ng / mL, preferably 1 to 50 ng / mL.
- the concentration of TNF ⁇ is not particularly limited, but is 0.1 to 100 ng / mL, preferably 1 to 50 ng / mL.
- the concentration of Heparin is not particularly limited, but is 10 ⁇ g / mL to 10 ⁇ g / mL, preferably 2 to 5 ⁇ g / mL.
- the concentration of BSA is not particularly limited, but is 0.1 to 10 mg / mL, preferably 1 to 8 mg / mL.
- the concentration of insulin is not particularly limited, but is 1 to 100 ⁇ g / mL, preferably 10 to 50 ⁇ g / mL.
- the concentration of Transferrin is not particularly limited, but is 10 to 500 ⁇ g / mL, preferably 50 to 200 ⁇ g / mL.
- the concentration of Putrescine is not particularly limited, but is 1 to 50 ⁇ g / mL, preferably 10 to 20 ⁇ g / mL.
- the concentration of Selenite is not particularly limited, but is 1 to 50 nM, preferably 20 to 40 nM.
- the concentration of Progesterone is not particularly limited, but is 1 to 50 nM, preferably 10 to 30 nM.
- the concentration of Hydrocortisone is not particularly limited, but is 10 ng / mL to 10 ⁇ g / mL, preferably 100 ng / mL to 1 ⁇ g / mL.
- the concentration of D-(+)-Glucose is not particularly limited, but is 1 to 20 mg / mL, preferably 5 to 10 mg / mL.
- the concentration of Sodium Bicarbonate is not particularly limited, but is 0.1 to 5mLmg / mL, preferably 0.5 to 2 mg / mL.
- the concentration of HEPES is not particularly limited, but is 0.1-50 ⁇ mM, preferably 1-20 ⁇ mM.
- the concentration of L-Glutamine is not particularly limited, but is 0.1 to 10 mm, preferably 1 to 5 mm.
- the concentration of N-acetylcysteine is not particularly limited, but is 1 to 200 ⁇ g / mL, preferably 10 to 100 ⁇ g / mL.
- the known basal culture solution is not particularly limited as long as it is suitable for culturing cancer cells that are the basis of cancer stem cells.
- DMEM / F12 DMEM, F10, F12, IMDM, EMEM, RPMI-1640, MEM, BME, Mocoy's 5A, MCDB131 and the like.
- DMEM / F12 is preferable.
- the most preferred stem cell medium is DMEM / F12 medium with a final concentration of 20 ng / mL human EGF, 10 ng / mL ⁇ ⁇ human bFGF, 4 ⁇ g / mL heparin, 4 mg / mL BSA, 25 ⁇ g / mL human insulin. , And a medium supplemented with 2.9 mg / mL glucose.
- Lgr5-positive highly proliferative cancer stem cells exhibit the properties of mesenchymal cells (mesenchymal cells).
- Lgr5-negative hypoproliferative cancer stem cells exhibit the properties of epithelial cells.
- the epithelial cell in the present invention refers to a cell constituting an epithelial tissue in vivo.
- the origin of the cancer stem cells in the present invention is not particularly limited, but is preferably a solid cancer, more preferably a digestive organ cancer.
- digestive organ cancer include esophageal cancer, gastric cancer, duodenal cancer, pancreatic cancer, bile duct cancer, gallbladder cancer, biliary tract cancer, colon cancer, colon cancer, and rectal cancer, with colon cancer being preferred.
- the cancer stem cell is preferably positive for any one or more of cell markers CD133, CD44, EpCAM, CD166, CD24, CD26, CD29, more preferably CD133, CD44, EpCAM, CD166, CD24, CD26 and CD29 are positive.
- ALDH acetaldehyde dehydrogenase activity
- Lgr5-positive adherent cancer stem cells are positive for cell markers of ALDH activity
- Lgr5-negative cancer stem cells are negative for ALDH activity.
- any one or more of HLA-DMA, TMEM173, ZMAT3 and GPR110 can be used as a cell marker.
- Lgr5-positive adherent cancer stem cells are negative for HLA-DMA, TMEM173, ZMAT3 or GPR110 cell markers, and Lgr5-negative cancer stem cells are any of HLA-DMA, TMEM173, ZMAT3 or GPR110 cells The marker is positive.
- cancer stem cells having characteristics that reproduce the hierarchical structure of cancer tissue are preferred.
- the hierarchical structure means that a part of characteristic unique structure found in normal tissue is detected histopathologically in a tumor structure originating from the tissue.
- this hierarchical structure is reproduced more highly.
- tumors of glandular organs gastric cancer, colon cancer, pancreatic cancer, liver cancer, bile duct cancer, breast cancer, lung adenocarcinoma, prostate cancer etc.
- a tumor with squamous epithelial structure squamous cell carcinoma such as lung, skin, vaginal mucosa, etc.
- poorly differentiated cancers are said to be poorly reproduced in this hierarchical structure and rich in atypia (Kumar V, Abbas AK, Fausio N. Robbins and Cotran Pathologic Basis of Disease.
- cancer stem cells having epithelial-mesenchymal transition are preferable.
- the epithelial-mesenchymal transition ability includes both migration of epithelial cells by obtaining the properties of mesenchymal cells, or migration of mesenchymal cells by obtaining the properties of epithelial cells. EMT does not occur in normal cells except during the process of embryogenesis. Epithelial cells that are tightly bound to each other and exhibit polarity are associated more loosely with each other, giving rise to mesenchymal cells that exhibit a loss of polarity and have the ability to migrate.
- mesenchymal cells can not only diffuse into the tissues surrounding the primary tumor, but also separate from the tumor, infiltrate blood vessels and lymph vessels, migrate to new locations, and then divide there Additional tumors can be formed. Further tumor formation helps explain cancer drug resistance or metastasis or recurrence.
- the present invention also provides a pharmaceutical composition
- a pharmaceutical composition comprising as an active ingredient an antibody that binds to a molecule expressed in a substantially homogeneous cancer stem cell population, comprising the cancer stem cells of the present invention.
- substantially homogeneous means Hu Y & Smyth GK., J Immunol Methods. 2009 Aug 15; 347 (1-2): 70-8 and Ishizawa K & Rasheed ZA. Et al., Cell Stem Cell.
- the frequency of cancer stem cells is 1/20 or more, preferably 1/10 or more More preferably, it is 1/5 or more, more preferably 1/3 or more, still more preferably 1/2 or more, and most preferably 1/1.
- a cancer stem cell population can be prepared, for example, by culturing a cell containing cancer stem cells or a group of cells containing cancer stem cells described herein.
- adhesion culture refers to culturing and passaging cells in an attached state after seeding the cells in a culture vessel for adhesion culture, and is a culture excluding suspended cells.
- Cells grown to confluence are detached using Accutase, subcultured to a new adherent culture flask, adherent culture plate, and adherent culture dish, and continued to culture.
- the culture vessel for adherent culture is not particularly limited as long as it is a vessel used for adherent culture, a flask for adherent culture or highly adhesive, a plate for adherent culture or highly adherent, a flat bottom for adherent culture or highly adherent. A plate, an adherent culture or a highly adhesive dish can be appropriately selected and used.
- the medium used for the adhesion culture is not particularly limited, but it is preferable to use a serum-free stem cell medium.
- adheresiveness refers to the property of adhering to a culture vessel when cells are cultured in a culture vessel for adhesion culture.
- the floating culture refers to culturing and passage of cells in a floating state after seeding the cells in a culture vessel for floating culture, and is a culture from which adherent cells are removed. Confluent cells are subcultured to a new low adhesion cell culture flask, ultra low adhesion cell culture flask, low adhesion plate, ultra low adhesion plate, low adhesion dish, ultra low adhesion dish. to continue.
- the culture container for suspension culture is not particularly limited as long as it is a container used for suspension culture. Low adhesion cell culture flask, ultra-low adhesion cell culture flask, low adhesion plate, ultra-low adhesion plate, low adhesion Can be selected and used as appropriate.
- the medium used for the suspension culture is not particularly limited, but a serum-free stem cell medium is preferably used. In addition, it is preferable to proliferate the cell group containing a cancer stem cell before performing adhesion culture or suspension culture.
- the term “floating” refers to the property that when cells are cultured in a culture vessel for suspension culture, they can be cultured in a suspended state without adhering to the culture vessel.
- Propagating a cell group means, for example, growing by spheroid culture or transplanting to a non-human animal and subculture, but is not particularly limited thereto.
- an immunodeficient animal can be used for transplantation in that rejection is unlikely to occur as a non-human animal.
- Immunodeficient animals include non-human animals deficient in functional T cells, such as nude mice and rats, non-human animals deficient in functional T cells and B cells, such as SCID mice and NOD -SCID mice are preferred, and mice lacking T cells, B cells, and NK cells with excellent transplantability (including NOG mice, etc.) are more preferred. is there.
- non-human animals deficient in functional T cells such as nude mice and rats
- non-human animals deficient in functional T cells and B cells such as SCID mice and NOD -SCID mice are preferred, and mice lacking T cells, B cells, and NK cells with excellent transplantability (including NOG mice, etc.) are more preferred. is there.
- SCID mice, NOD / SCID mice, and NOG mice it is preferable to use nonhuman animals that are 4 to 100 weeks old.
- NOG mice can be prepared, for example, by the method described in WO 2002
- the cells to be transplanted can be cell masses, tissue pieces, individually dispersed cells, cells that have been cultured after isolation, cells that have been isolated from the animal after being transplanted to another animal, etc. A dispersed cell is preferable.
- the number of cells to be transplanted may be 10 6 or less, but a larger number of cells may be transplanted.
- Subcutaneous transplantation is a suitable transplantation site from the viewpoint that the transplantation technique is simple, but the transplantation site is not particularly limited, and it is preferable to appropriately select the transplantation site depending on the animal to be used.
- the transplantation operation of the NOG-established cancer cell line is not particularly limited, and can be performed according to a conventional transplantation operation.
- Cancer stem cells or cancer stem cell populations can be prepared, for example, by subjecting cancer tissues collected from patients to adherent culture or suspension culture using a serum-free stem cell medium. It can also be produced by spheroid culture of a cancer tissue collected from a patient, followed by adherent culture or suspension culture using a serum-free stem cell medium. It can also be produced by transplanting or subcultureing cancer tissue collected from a patient to a non-human animal, followed by adherent culture or suspension culture using a serum-free stem cell medium. Furthermore, it is also possible to use a method in which an NOG-established cancer cell line prepared by transplanting and subcultured cancer tissue collected from a patient into NOG mice is attached or cultured in a serum-free stem cell medium.
- the cancer stem cell or cancer stem cell population of the present invention can be used for screening methods for pharmaceuticals and anticancer agents.
- a method comprising the following steps (a) to (c) is provided: (A) providing a substantially homogeneous cancer stem cell population comprising Lgr5-positive adherent cancer stem cells; (B) contacting the cancer stem cell population or a cancer stem cell contained in the cancer stem cell population with a test substance; (C) A step of detecting a change in the biological characteristics of the cancer stem cell population or cancer stem cells brought into contact with the test substance.
- a substantially homogeneous cancer stem cell population containing Lgr5-positive adherent cancer stem cells or a substantially homogeneous cancer stem cell population containing Lgr5-negative cancer stem cells is prepared.
- the prepared cancer stem cell population or cancer stem cells contained in the cancer stem cell population is brought into contact with a test substance.
- the method for bringing a test substance into contact with a cancer stem cell population or cancer stem cells contained in the cancer stem cell population is not particularly limited.
- a cultured cell of a cancer stem cell population or a cancer stem cell contained in a cancer stem cell population can be contacted with a test substance.
- the treatment can be performed by adding a test substance to a cell culture solution or the cell extract.
- test substance is a protein
- a vector containing DNA encoding the protein is introduced into a cancer stem cell population or a cancer stem cell included in the cancer stem cell population, or the vector is introduced into the cancer stem cell population or cancer stem cell population. It can also be carried out by adding to the cell extract of cancer stem cells contained in. In addition, for example, a two-hybrid method using yeast or animal cells can be used.
- a change in biological characteristics of the cancer stem cell population or cancer stem cells treated with the test substance is then detected.
- changes in biological characteristics include, for example, changes in proliferative capacity, changes in the number of living cells, cancer stem cell populations or changes in tissue structure characteristically observed in the cancer progression process of cancer stem cells, the cancer stem cell population Or the change of the expression of DNA, RNA, protein, or a metabolite contained in a cancer stem cell can be mentioned.
- the detection of the change of biological characteristics can be performed by the following method, for example.
- the expression confirmation of DNA, RNA, protein, peptide and metabolite is not particularly limited, and can be performed according to a conventional expression confirmation method.
- RNA include microRNA, siRNA, tRNA, snRNA, mRNA, and non-coding RNA.
- the transcription level of each gene can be measured by extracting mRNA of each gene according to a standard method and performing Northern hybridization or RT-PCR using this mRNA as a template.
- the translation level of a gene can also be measured by collect
- Such a cancer stem cell population or DNA, RNA, or protein contained in the cancer stem cell population or cancer stem cell that is characteristically recognized in the cancer progression process of cancer stem cells is described in any one of SEQ ID NOs: 1 to 6
- a protein or polypeptide, or a polynucleotide encoding the protein or polypeptide is preferably mentioned.
- test substance when the cancer stem cell population after treatment with the test substance or the biological characteristics of the cancer stem cells shows no change or the rate of change is lower than that before the treatment, the test substance is cancerous. It is considered useful as a pharmaceutical (pharmaceutical composition) having a function of suppressing recurrence and metastasis (for example, cancer recurrence inhibitor, post-chemotherapy adjuvant, postoperative adjuvant therapy, anticancer agent, or cancer metastasis inhibitor). These test substances can be selected as effective substances having a therapeutic or prophylactic effect for cancer diseases.
- a pharmaceutical product (pharmaceutical composition) having a function of suppressing the progression of cancer is used as a cancer recurrence inhibitor, an adjuvant agent after chemotherapy, a postoperative adjuvant therapy agent, an anticancer agent, or a cancer metastasis inhibitor.
- the anticancer agent of the present invention may be used, for example, against cancer having resistance to drugs and chemotherapeutic agents. That is, the drug (pharmaceutical composition) of the present invention includes a drug resistant or chemotherapeutic drug resistant cancer therapeutic agent.
- the drug is not particularly limited to an anticancer agent or a metastasis or recurrence inhibitor, and can also be used as an angiogenesis inhibitor, a cell growth inhibitor, and the like.
- the pharmaceutical product (pharmaceutical composition) of the present invention may be used at the same time as the chemotherapeutic agent or after treatment with the chemotherapeutic agent.
- the drug is not particularly limited, and examples thereof include protein drugs, nucleic acid drugs, low molecular drugs, and cellular drugs.
- a method for screening a pharmaceutical product comprising the following steps (a) to (c) is provided: (A) producing a substantially homogeneous cancer stem cell population comprising Lgr5-negative suspension cancer stem cells; (B) contacting the test substance with the cancer stem cell population or a cancer stem cell contained in the cancer stem cell population (c) detecting a change in the biological characteristics of the cancer stem cell group or cancer stem cells contacted with the test substance Process.
- a substantially homogeneous cancer stem cell population containing Lgr5-negative suspension cancer stem cells is first prepared.
- the prepared cancer stem cell population or cancer stem cells contained in the cancer stem cell population is treated with a test substance.
- a change in the biological characteristics of the cancer stem cell population or cancer stem cells treated with the test substance is detected.
- Such a cancer stem cell population or DNA, RNA, or protein contained in the cancer stem cell population or cancer stem cell that is characteristically recognized in the cancer stem cell progression process is described in any one of SEQ ID NOs: 1 to 8
- a protein or polypeptide, or a polynucleotide encoding the protein or polypeptide is preferably mentioned.
- the protein or polypeptide described in SEQ ID NOs: 1 to 6 or a polynucleotide encoding the protein or polypeptide can be used.
- the protein or polypeptide set forth in SEQ ID NO: 7 or 8 or a polynucleotide encoding the protein or polypeptide can be used.
- the pharmaceutical product (pharmaceutical composition) obtained by the screening method is not particularly limited, but can be used as an anticancer agent. That is, when the cancer stem cell population after treatment with the test substance or the biological characteristics of the cancer stem cells shows no change or the rate of change is lower than that before the treatment, the test substance is cancerous. It is considered useful as a drug having a function of suppressing recurrence or metastasis (for example, a cancer recurrence inhibitor, an adjuvant agent after chemotherapy, a postoperative adjuvant therapy agent, an anticancer agent, or a cancer metastasis inhibitor).
- the test substance can be selected as an effective substance having a therapeutic or prophylactic effect for cancer diseases.
- a pharmaceutical product (pharmaceutical composition) having a function of suppressing the progression of cancer is used as a cancer recurrence inhibitor, an adjuvant agent after chemotherapy, a postoperative adjuvant therapy agent, an anticancer agent, or a cancer metastasis inhibitor.
- the pharmaceutical (pharmaceutical composition) of the present invention includes a cancer therapeutic agent for Lgr5-negative cancer, which contains as an active ingredient at least one antibody that binds to the protein described in SEQ ID NOs: 1 to 8.
- Lgr5-negative cancer includes cancer having drug resistance or resistance to chemotherapeutic agents.
- Still another embodiment of the screening method of the present invention includes a method using a cancer stem cell population of the present invention or a non-human animal administered with a cancer stem cell and a test substance contained in the cancer stem cell population.
- a method for screening a pharmaceutical product including the following steps (a) to (c) is provided; (A) producing a substantially homogeneous cancer stem cell population comprising Lgr5-positive adherent cancer stem cells; (B) A step of administering the cell population or a cancer stem cell and a test substance contained in the cancer stem cell population to a non-human animal (c) a step of detecting tumor formation in the non-human animal.
- a substantially homogeneous cancer stem cell population containing Lgr5-positive adherent cancer stem cells is prepared.
- the prepared cancer stem cell population or cancer stem cells contained in the cancer stem cell population and a test substance are administered to the non-human animal.
- the administration method of the test substance to the non-human animal is not particularly limited.
- oral administration or parenteral administration such as subcutaneous, intravenous, topical, transdermal or enteral (rectal) can be appropriately selected.
- the method of administering a cancer stem cell population or cancer stem cells to a non-human animal is not particularly limited.
- the administration method can be appropriately selected depending on the cell population to be administered, but subcutaneous administration or intravenous administration is preferred.
- the method detects tumor formation in the non-human animal.
- the test substance for the non-human animal, whether the cancer stem cell population or the tissue to which the cancer stem cell and the test substance are administered is removed, and the histological characteristics of the administered tissue are observed to confirm that the tumor is formed. This can be done by measuring whether or not.
- the test substance is considered to be useful as a pharmaceutical agent (for example, an anticancer agent or a cancer metastasis or cancer recurrence inhibitor) having a function of suppressing the progression or metastasis of cancer.
- the substance can be selected as an effective substance having a therapeutic or prophylactic effect for cancer diseases. That is, the pharmaceutical product (pharmaceutical composition) obtained by the screening method is not particularly limited, but can be used as an anticancer agent or a cancer metastasis or cancer recurrence inhibitor.
- test substance in the method of the present invention is not particularly limited.
- natural compounds, organic compounds, inorganic compounds, single compounds such as proteins, antibodies, peptides, amino acids, compound libraries, genes, etc.
- libraries include library expression products, cell extracts, cell culture supernatants, fermented microorganism products, marine organism extracts, plant extracts, prokaryotic cell extracts, eukaryotic single cell extracts, or animal cell extracts. .
- These may be purified products or crude purified products such as extracts from plants, animals, microorganisms, and the like.
- the method for producing the test substance is not particularly limited, and it may be isolated from a natural product, synthesized chemically or biochemically, or prepared by genetic engineering.
- test substance can be appropriately labeled and used as necessary.
- label include a radiolabel and a fluorescent label.
- a mixture obtained by mixing a plurality of these labels is also included in the test substance of the present invention.
- the present invention also provides a pharmaceutical product such as a vaccine comprising a partial peptide of any protein described in SEQ ID NOs: 1 to 8, and a method for screening a vaccine.
- a screening method a method for measuring cytotoxic activity targeting a cancer stem cell disclosed in the present invention using a cytotoxic T cell (CTL) or the like induced by the cancer vaccine of the present invention in vitro.
- CTL cytotoxic T cell
- adherent cells and non-adherent cells are separated from peripheral blood mononuclear cells (PBMC) collected by centrifuging human peripheral blood in a Ficoll-Conlay density gradient.
- PBMC peripheral blood mononuclear cells
- Adherent cells are incubated with 100 ng / ml GM-CSF (Novartis) and 10 IU / ml IL-4 (Gibco BRL) in AIM-V (Gibco). This cell is used as an antigen presenting cell (APC).
- the non-adherent cells are incubated with 30-100 IU / ml recombinant IL-4 (Ajinomoto) in AIM-V.
- a partial peptide final concentration 30 ⁇ g / ml
- any protein described in SEQ ID NOs: 1-8 provided by the present invention is added to APC.
- APC matures by adding recombinant TNF- ⁇ and IFN- ⁇ (Sumitomo Pharmaceutical).
- the irradiated APC and CD8 positive cells separated from autologous non-adherent cells are then mixed in AIM-V without IL-2.
- IL-2 Takeda Pharmaceutical
- CD8 positive cells are stimulated with autologous PHA blasts (PHA stimulated T cells) stimulated with PHA, a T cell mitogen, as APCs.
- PHA autologous PHA blasts
- PHA a T cell mitogen
- the Lgr5-positive highly proliferative cancer stem cell and the Lgr5-negative hypoproliferative cancer stem cell provided by the present invention can be used.
- Cytotoxic activity can be evaluated by measuring the uptake activity of 51 Cr-sodium chromate according to the assay method of ADCC activity.
- the drug selected by the screening method of the present invention can be obtained by conducting other drug efficacy tests and safety tests as necessary, and further by conducting clinical tests on patients with human cancer diseases. It can be selected as a prophylactically effective substance or therapeutically effective substance having a high effect and high practicality.
- the pharmaceutical product thus selected can also be industrially produced by chemical synthesis, biochemical synthesis (fermentation) or genetic manipulation based on the structural analysis result.
- High growth ability means that the doubling time is 6 days or less, preferably 4 days or less, more preferably 3 days or less when cultured in a serum-free medium supplemented with EGF and FGF using the method described herein. Say something.
- the low growth ability means that the doubling time is 7 days or more, preferably 14 days or more, and more preferably significant when cultured in a serum-free medium supplemented with EGF and FGF using the method described herein. It means not showing growth.
- Lgr5-positive highly proliferative cancer stem cells and Lgr5-negative low proliferative cancer stem cells they can be separated using Lgr5 which is a cell marker.
- a substantially homogenous cancer stem cell population was prepared by separating a cell population containing cancer stem cells using an Lgr5 antibody and by attaching or culturing the population containing cancer stem cells once.
- Lgr5-positive highly proliferative cancer stem cells can be prepared by separating cells from a cancer tissue that has been passaged for 3 generations or more in NOG mice and adherent culture in a serum-free stem cell medium.
- the obtained Lgr5-positive cancer stem cells are maintained under various stresses such as contact with a growth inhibitor such as irinotecan treatment (10 ⁇ g / ml irinotecan is added to serum-free stem cell medium and cultured for 3 days).
- irinotecan treatment 10 ⁇ g / ml irinotecan is added to serum-free stem cell medium and cultured for 3 days.
- the present invention provides a method for screening a pharmaceutical comprising contacting a test substance with cancer stem cells having different proliferation ability induced by the method provided by the present invention. That is, a cancer stem cell having a high proliferation ability, which is induced by a method of inducing a cancer stem cell having a low proliferation ability to a cancer stem cell having a high proliferation ability, or a cancer stem cell having a high proliferation ability to a cancer stem cell having a low proliferation ability. Or a method for screening a pharmaceutical comprising detecting a change in biological characteristics of a cancer stem cell by contacting a test substance with the cancer stem cell having low proliferation ability.
- cancer stem cells having low proliferative ability are obtained by maintaining cancer stem cells having high proliferative ability under various stresses such as suspension culture and contact with a growth inhibitor. be able to.
- cancer stem cells having high proliferation ability can be induced into cancer stem cells having high proliferation ability by suspension culture of cancer stem cells having high proliferation ability.
- cancer stem cells having high proliferative ability may be reduced in low adhesion or low adhesion plates, ultra low adhesion plates, low adhesion dishes, ultra low adhesion dishes, low adhesion flasks, ultra low adhesion cell culture flasks or the like.
- cancer stem cells having high proliferation ability can be induced into cancer stem cells having low proliferation ability. That is, cancer stem cells with high proliferation ability are treated with low adhesion such as low adhesion plates, ultra low adhesion plates, low adhesion dishes, ultra low adhesion dishes, low adhesion flasks, ultra low adhesion cell culture flasks, etc.
- cancer stem cells having a low proliferation ability can be produced by culturing in a cell culture vessel with an ultra-low adhesion.
- cancer stem cells having high proliferative ability can be induced into cancer stem cells having low proliferative ability. That is, cancer stem cells having low proliferation ability can be produced by exposing cancer stem cells having high proliferation ability to a growth inhibitor such as 5-FU or irinotecan. Exposure to a growth inhibitor can be carried out in any in vivo environment, such as in vitro cultured or transplanted non-human animals. In this case, those skilled in the art can appropriately select the exposure amount of the cancer stem cell to the growth inhibitor.
- a growth inhibitor such as 5-FU or irinotecan
- cancer stem cells having high proliferative ability can be produced by reseeding cancer stem cells having low proliferative ability in a medium that does not contain a growth inhibitor such as 5-FU or irinotecan.
- cancer stem cells having high proliferative ability can be produced by discontinuing administration of the growth inhibitor to non-human animals retaining cancer stem cells having low proliferative ability.
- cancer stem cells having low proliferation ability can be induced to cancer stem cells having high proliferation ability by adherent culture.
- low proliferation ability can be achieved by culturing cancer stem cells with low proliferation ability in a cell culture vessel with high adhesion, such as flat bottom plate, plate, adherent culture plate, adherent culture flask, dish, adherent culture dish, etc. It is also possible to induce cancer stem cells having a high proliferation ability into cancer stem cells.
- cancer stem cells with low proliferative ability can be proliferated by culturing them in cell culture vessels with high adhesion, such as flat-bottom plates, plates, adherent culture plates, adherent culture flasks, dishes, and adherent culture dishes. Cancer stem cells having the ability can also be produced.
- the present invention also relates to a reagent for detecting cancer cells.
- the cancer cell detection reagent of the present invention preferably binds to the protein described in SEQ ID NOs: 1 to 8 (protein consisting of the amino acid sequence described in any of SEQ ID NOs: 1 to 8) as an active ingredient. At least one antibody is included.
- Another embodiment of the reagent of the present invention includes a reagent for detecting Lgr5-positive cancer cells, and the reagent is preferably a protein described in SEQ ID NOs: 1 to 6 (SEQ ID NOs: 1 to 6). At least one antibody that binds to a protein consisting of the amino acid sequence described in any of the above.
- Still another embodiment of the reagent of the present invention includes a reagent for detecting Lgr5-negative cancer cells, and the reagent is preferably a protein (SEQ ID NO: 1 to SEQ ID NO: 1 to SEQ ID NO: 1 to 8). At least one antibody that binds to a protein comprising the amino acid sequence of any one of 8).
- the growth inhibitor may suitably include a DNA damaging agent, an anti-mitotic agent and / or an antimetabolite.
- the DNA damaging agent can be an alkylating agent, a topoisomerase inhibitor and / or a DNA intercalator.
- Carboplatin DNA alkylating agent
- etoposide inhibitor of topoisomerase II
- doxorubicin DNA intercalator
- docetaxel anti-mitotic agent
- Gemzar gemzar
- the alkylating agent can be selected from at least one of the following. Chlorambucil, cyclophosphamide, ifosfamide, mechlorethamine, melphalan, uracil mustard, thiotepa, busulfan, carmustine, lomustine, streptozocin, carboplatin, cisplatin, satraplatin, oxaliplatin, altretamine, ET-743, XL119 (becatecalin), Selected from dacarbazine, chlormethine, bendamustine, trofosfamide, uramustine, hotemstin, nimustine, predonimustine, ranimustine, semustine, nedaplatin, triplatin tetranitrate, mannosulfan, treosulphane, temozolomide, carbocon, triadicon, triethylene melamine and procarbazine At least one alkylating agent can be used
- the topoisomerase inhibitor can be selected from at least one of the following. Doxorubicin (Doxil), daunorubicin, epirubicin, idarubicin, anthracenedione (Novantrone), mitoxantrone, mitomycin C, bleomycin, dactinomycin, pricatomycin, irinotecan (camptosar), camptothecin, rubitecan, belotecan, etoposide and tenposide At least one topoisomerase inhibitor selected from (Hycamptin) and the like may be used.
- Doxorubicin Doxil
- daunorubicin epirubicin
- idarubicin anthracenedione
- Novantrone novantrone
- mitoxantrone mitoxantrone
- mitomycin C bleomycin
- dactinomycin pricatomycin
- irinotecan camptothecin
- At least one topoisomerase inhibitor selected from proflavine, doxorubicin (adriamycin), daunorubicin, dactinomycin, thalidomide and the like can be used.
- the anti-mitotic agent can be selected from at least one of the following. Paclitaxel (Abraxane) / Taxol, Docetaxel (Taxotere), BMS-275183, Xyotax, Tocosal, Vinorlebine, Vincristine, Vinblastine, Vindesine, Vinzolidine, Etoposide (VP-16), Teniposide (VM-26), Ixabepilone At least one topoisomerase inhibitor selected from ortataxel, tesetaxel, ispinesive and the like may be used.
- the antimetabolite can be selected from at least one of the following. Fluorouracil (5-FU), floxuridine (5-FUdR), methotrexate, Xeloda, Arranon, leucovorin, hydroxyurea, thioguanine (6-TG), mercaptopurine (6-MP), cytarabine, pentostatin, fludarabine phosphate
- Fluorouracil (5-FU) Fluorouracil
- floxuridine 5-FUdR
- methotrexate Xeloda
- Arranon leucovorin
- hydroxyurea hydroxyurea
- mercaptopurine (6-MP) cytarabine
- pentostatin fludarabine phosphate
- fludarabine phosphate At least one topoisomerase inhibitor selected from, for example, cladribine (2-CDA), asparaginase, gemcitabine, pemetrexe
- the present invention relates to a method for screening an anticancer agent using the cancer stem cells isolated and induced by the method of the present invention.
- the present invention relates to a compound evaluation method using cancer stem cells isolated and induced by the method of the present invention.
- the present invention also provides a method for detecting, identifying or quantifying the presence of the cancer stem cells of the present invention. Specifically, the present invention provides a method for detecting, identifying or quantifying the presence of a cancer stem cell of the present invention or a population of substantially homogeneous cancer stem cells comprising the following steps (a) and (b): (A) preparing a sample obtained from a cancer patient; (B) A step of bringing the sample into contact with the Lgr5 antibody.
- a sample obtained from a cancer patient is prepared.
- the “sample” is not particularly limited as long as it is an organ or tissue derived from a cancer patient, and a frozen or unfrozen organ or tissue can be used.
- a cancer (tumor) tissue obtained from a cancer patient can be mentioned.
- the sample and Lgr5 antibody are then contacted.
- the method for detecting / identifying or quantifying the presence of the cancer stem cells or the population of substantially homogeneous cancer stem cells of the present invention can be used for, for example, diagnosis of cancer, selection of cancer patients, efficacy of drugs (pharmaceutical compositions). It can be used for prediction, confirmation of efficacy, monitoring of treatment, and imaging of cancer.
- an organ or tissue can be removed from a cancer patient, a specimen can be prepared, and the presence of cancer stem cells can be detected, identified or quantified using the specimen.
- a known method can be appropriately used for preparing the specimen, for example, the PFA-AMeX-Paraffin method (WO 09/078386) can be used.
- the sample for example, a frozen or unfrozen organ or tissue can be used.
- a sample of a cancer patient is fixed with a PFA solution.
- a PFA solution is a cell fixing solution obtained by adding a buffer solution such as a phosphate buffer to a 1-6% aqueous solution of paraformaldehyde, preferably a 4% PFA fixing solution (4% paraformaldehyde / 0.01 M PBS (pH 7.4 )) Is used.
- Fixation with a PFA fixative solution is performed by adding a target organ or tissue to a PFA fixative solution containing 1 to 6%, preferably 4% paraformaldehyde, at a temperature of 0 to 8 ° C, preferably about 4 ° C. It can be carried out by immersing for a time, preferably 6 to 30 hours. Next, the fixed organ or tissue is washed with phosphate buffered saline or the like. At this time, the observed organ or tissue portion may be cut out and then washed.
- the organ or tissue thus prepared is then embedded in paraffin by the AMeX method.
- the AMeX method is a paraffin embedding method in which cold acetone fixation, dehydration with acetone, penetration with methyl benzoate and xylene, and paraffin embedding are performed as a series of operations. Specifically, it is immersed in acetone at ⁇ 25 to 8 ° C., preferably ⁇ 20 to 6 ° C. for 2 to 24 hours, preferably 4 to 16 hours, and then the acetone containing the tissue is returned to room temperature or at room temperature. After transferring the organ or tissue to acetone, it is dehydrated at room temperature for 0.5 to 5 hours, preferably 1 to 4 hours.
- the sample is immersed in methyl benzoate at room temperature for 0.5 to 3 hours, preferably 0.5 to 2 hours, and immersed in xylene at room temperature for 0.5 to 3 hours, preferably 0.5 to 2 hours.
- the organ or tissue paraffin block obtained by the PFA-AMeX method is stored at a low temperature until use.
- the paraffin block obtained above is prepared by using a microtome or the like to prepare a sliced slice, and the sliced slice is deparaffinized and hydrophilized.
- Deparaffinization and hydrophilization can be performed by a known method. For example, deparaffinization can be performed with xylene and toluene, and hydrophilization can be performed with alcohol and acetone.
- the sliced slices thus obtained are subjected to, for example, tissue staining, immunohistochemical staining, or enzyme histochemical staining, and subjected to detection / identification or quantification.
- any staining that can be performed with a normal paraffin-embedded section can be used (for example, PAS staining, Giemsa staining, toluidine blue staining, etc.).
- the enzyme histochemical staining staining possible on a section can be used (for example, various staining such as ALP, ACP, TRAP, esterase, etc.).
- Hematoxylin and eosin In order to stain the pathological tissue, hematoxylin and eosin (Hematoxylin-Eosin) staining; for collagen fibers, Van Gieson staining, Azan staining, Masson Trichrome ) Staining; For elastic fibers, Weigert staining, Elastica Van Gieson staining; For reticulofiber / basement membrane, Watanabe ⁇ silver staining, PAM staining (Periodic acid methenamine silver silver) Etc. can be used.
- Immunohistochemical staining or enzyme histochemical staining should be performed using a direct method using a primary antibody labeled with an enzyme or a labeling substance, or an indirect method in which a secondary antibody is labeled without labeling the primary antibody.
- the antibody can be labeled by a generally known method.
- the labeling substance include radioisotopes, enzymes, fluorescent substances, biotin / avidin, and the like. Commercially available labeling substances can be used for these labeling substances. Examples of the radioisotope include 32 P, 33 P, 131 I, 125 I, 3 H, 14 C, and 35 S.
- Examples of the enzyme include alkaline phosphatase, horseradish peroxidase, ⁇ -galactosidase, ⁇ -glucosidase and the like.
- Examples of the fluorescent substance include fluorescein isothiocyanate (FITC) and rhodamine. These can be obtained commercially and are labeled by known methods.
- the sliced slice is subjected to detection / identification or quantification by performing, for example, tissue staining, immunohistochemical staining or enzyme histochemical staining.
- detection / identification or quantification can also be performed for an organ or tissue specimen by quantifying DNA or RNA in cells in the organ or tissue specimen.
- expression confirmations are not particularly limited, and can be performed according to a conventional expression confirmation method.
- RNA include microRNA, siRNA, tRNA, snRNA, mRNA, and non-coding RNA.
- the transcription level of each gene can be measured by extracting Lgr5 mRNA according to a conventional method and performing Northern hybridization or RT-PCR using this mRNA as a template.
- a microdissection method particularly a laser microdissection (LMD) method can be used.
- the LMD method can collect target cells from living tissue, so it knows exactly how many of the various cells that make up a tissue express a specific gene. be able to.
- an AS-LMD system manufactured by Leica Microsystems
- Leica Microsystems can be used as an apparatus for performing microdissection.
- the present invention also includes detecting the presence of at least one protein in a sample isolated from a cancer patient using at least one antibody that binds to the protein described in SEQ ID NOs: 1 to 8.
- Methods are provided for diagnosing cancer, detecting cancer stem cells, or screening for cancer patients.
- At least one antibody that binds to the protein set forth in SEQ ID NOs: 1 to 6 is used to detect the presence of at least one protein of interest in a sample isolated from a cancer patient.
- a method for diagnosing cancer, detecting cancer stem cells, or screening for cancer patients is provided.
- By detecting the presence of the protein it is possible to detect the presence of Lgr5-positive cancer stem cells, but detection of the presence of Lgr5 together is not excluded by the present invention.
- detecting the presence of at least one protein of interest in a sample isolated from a cancer patient using at least one antibody that binds to the protein set forth in SEQ ID NOs: 1-8 is provided.
- a method for diagnosing cancer, detecting cancer stem cells, or screening cancer patients is provided.
- By detecting the presence of the protein it is possible to detect the presence of Lgr5-negative cancer stem cells, but detection of the presence of Lgr5 together is not excluded by the present invention.
- the present invention also provides a method for confirming the effectiveness of a pharmaceutical composition comprising at least one antibody that binds to the protein represented by SEQ ID NOs: 1 to 8, from a subject to whom the pharmaceutical composition has been administered.
- a method is provided that comprises detecting the presence of any one or more of the protein set forth in SEQ ID NOs: 1-8 and / or a polynucleotide encoding the protein in an isolated sample.
- a method for assessing the effectiveness of a treatment for cancer in a test subject in a first sample obtained from the subject prior to providing at least a portion of the treatment to the test subject.
- Expression of at least one of the protein set forth in SEQ ID NO: 1-8 and / or a polynucleotide encoding the protein as set forth in SEQ ID NO: 1-8 in a second sample obtained from the subject after provision of the therapeutic portion Comparing to the expression of at least one of said protein and / or a polynucleotide encoding said protein, said method comprising: significantly comparing said protein and / or said first sample of said polynucleotide in a second sample
- a method for monitoring the effectiveness in a subject of treatment with an antibody comprising: (I) obtaining a pre-dose sample from a subject prior to administration of the antibody; (Ii) detecting the level of expression in a pre-administration sample of at least one marker protein selected from the proteins set forth in SEQ ID NOs: 1 to 8, its mRNA, or its genomic DNA; (Iii) obtaining one or more post-administration samples from the subject; (Iv) detecting the level of expression or activity in a sample after administration of at least one marker protein selected from the proteins set forth in SEQ ID NOs: 1 to 8, its mRNA or its genomic DNA; (V) The level of expression or activity in the pre-administration sample of the marker protein, the mRNA, or the genomic DNA is compared with the marker protein, the mRNA, or the genomic DNA in the post-administration sample or sample (s).
- a method comprises a step consisting of altering the administration of the antibody to the subject accordingly.
- an increase in the administration of an antibody of the present invention results in expression of a marker (level of expression or activity in the pre-administration sample of the marker protein, mRNA, or genomic DNA) to a level higher than detected or It can be used to decrease activity, ie increase the effectiveness of the antibody.
- a method of monitoring the effectiveness in a subject of treatment with an antibody comprising: (I) detecting Lgr5-positive cancer stem cells in a pre-administration sample obtained from a subject before administration of the antibody; (Ii) detecting the level of expression in a pre-administration sample of at least one marker protein selected from the proteins set forth in SEQ ID NOs: 1 to 6, its mRNA, or its genomic DNA; (Iii) obtaining one or more post-administration samples from the subject; (Iv) detecting the level of expression or activity in the sample after administration of at least one marker protein selected from the proteins set forth in SEQ ID NOs: 1 to 6, its mRNA or its genomic DNA; (V) The level of expression or activity in the pre-administration sample of the marker protein, the mRNA, or the genomic DNA is compared with the marker protein, the mRNA, or the genomic DNA in the post-administration sample or sample (s).
- a method comprises a step consisting of altering the administration of the antibody to the subject accordingly.
- an increase in the administration of an antibody of the present invention results in expression of a marker (level of expression or activity in the pre-administration sample of the marker protein, mRNA, or genomic DNA) to a level higher than detected or It can be used to decrease activity, ie increase the effectiveness of the antibody.
- a method of monitoring the effectiveness in a subject of treatment with an antibody comprising: (I) detecting Lgr5-negative cancer stem cells in a pre-administration sample obtained from a subject before administration of the antibody; (Ii) detecting the level of expression in a pre-administration sample of at least one marker protein selected from the proteins set forth in SEQ ID NOs: 1 to 8, its mRNA, or its genomic DNA; (Iii) obtaining one or more post-administration samples from the subject; (Iv) detecting the level of expression or activity in a sample after administration of at least one marker protein selected from the proteins set forth in SEQ ID NOs: 1 to 8, its mRNA or its genomic DNA; (V) The level of expression or activity in the pre-administration sample of the marker protein, the mRNA, or the genomic DNA is compared with the marker protein, the mRNA, or the genomic DNA in the post-administration sample or sample (s).
- a method comprises a step consisting of altering the administration of the antibody to the subject accordingly.
- an increase in the administration of an antibody of the present invention results in expression of a marker (level of expression or activity in the pre-administration sample of the marker protein, mRNA, or genomic DNA) to a level higher than detected or It can be used to decrease activity, ie increase the effectiveness of the antibody.
- the cancer stem cell inhibitor means, for example, an agent having effects such as suppression of cancer stem cell proliferation, suppression of cancer stem cell metastasis or recurrence, cancer stem cell death, suppression of cancer cell proliferation, cancer cell metastasis Or you may have effects, such as suppression of recurrence and death of a cancer cell.
- suppress refers to any of biological activity when used in the context of biological activity such as, but not limited to, cancer stem cell proliferation or metastasis.
- control which can reduce or eliminate targeted functions such as protein production or molecular phosphorylation.
- inhibition can refer to a reduction of about 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90% or 95% of the targeted activity.
- the term refers to the prevention of the onset of symptoms, the reduction of symptoms, or the successful alleviation of a disease, condition or disorder.
- Metalsis refers to the process by which a cancerous lesion develops similar to a new location as the cancer spreads or moves from the primary site of the body to other areas.
- a “metastatic” or “metastatic” cell is a cell that loses adhesive contact with neighboring cells and migrates from the primary site of disease through the bloodstream or lymph to invade neighboring body structures.
- Relapse means that the same malignant tumor is reproduced in the remaining organ after partial excision of an organ for removing a malignant tumor from a cancer patient or after postoperative chemotherapy after the excision.
- an expression vector containing a gene containing DNA encoding the protein is prepared by any method known to those skilled in the art, and a transformant transformed with the expression vector is cultured.
- the protein can be easily prepared by generating and accumulating the protein and collecting the protein.
- the expression vector can be prepared according to a method known in the art. For example, (1) excising a DNA fragment containing a gene containing DNA encoding a protein; (2) ligating the DNA fragment downstream of a promoter in an appropriate expression vector; Can be manufactured.
- vectors examples include plasmids derived from E. coli (eg, pBR322, pBR325, pUC18, pUC118), plasmids derived from Bacillus subtilis (eg, pUB110, pTP5, pC194), plasmids derived from yeast (eg, pSH19, pSH15), ⁇ phage, etc.
- Animal viruses such as bacteriophages, retroviruses, vaccinia viruses and baculoviruses can be used.
- the promoter used in the present invention may be any promoter as long as it is suitable for the host used for gene expression.
- the host is Escherichia coli, trp promoter, lac promoter, recA promoter, ⁇ PL promoter, lpp promoter, etc.
- the host is Bacillus subtilis
- yeast such as SPO1, SPO2, promoter, penP promoter, etc.
- PHO5 promoter, PGK promoter, GAP promoter, ADH promoter and the like are preferable.
- SR ⁇ promoter, SV40 promoter, LTR promoter, CMV promoter, HSV-TK promoter and the like can be mentioned.
- an enhancer, a splicing signal, a poly A addition signal, a selection marker, an SV40 replication origin and the like known in the art can be added to the expression vector, if desired.
- the protein used in the present invention can be expressed as a fusion protein with other proteins (for example, glutathione S-transferase and protein A). Such a fusion protein can be cleaved using an appropriate protease and separated into each protein.
- host cells examples include Escherichia bacteria, Bacillus bacteria, yeast, insect cells, insects, and animal cells.
- Escherichia examples include Escherichia coli K12 / DH1 (Proc. Natl. Acad. Sci, USA, 60, 160 (1968)), JM103 (Nucleic Acids Research, 9, 309 ( 1981)), JA221 (Journal of Molecular Biology, Vol. 120, 517 (1978)), HB101 (Journal of Molecular Biology, Vol. 41, 459 (1969)) and the like are used.
- Bacillus genus bacteria examples include Bacillus subtilis MI114 (Gene, Vol. 24, Vol. 255 (1983)), 207-21 [Journal of Biochemistry, Vol. 95, Vol. 87 (1984)].
- yeasts examples include Saccharomyces cerevisiae AH22, AH22R-, NA87-11A, DKD-5D, 20B-12, Schizosaccaromyces pombe NCYC1913, NCYC2036, Pichia pastoris, etc. .
- animal cells examples include monkey cells COS-7, Vero, Chinese hamster cells CHO (hereinafter abbreviated as CHO cells), dhfr gene-deficient CHO cells, mouse L cells, mouse AtT-20 cells, mouse myeloma cells, rat GH3. Cells, human FL cells, etc. are used.
- Transformation of these host cells can be performed according to methods known in the art. For example, the following documents can be referred to. Proc. Natl. Acad. Sci. USA, 69, 2110 (1972); Gene, 17, 107 (1982); Molecular & General Genetics, 168, 111 (1979); Methods in Enzymology, 194, 182- 187 (1991); Proc. Natl. Acad. Sci. USA), 75, 1929 (1978); and Virology, 52, 456 (1973).
- the transformant thus obtained can be cultured according to a method known in the art.
- the culture is usually carried out at about 15 to 43 ° C. for about 3 to 24 hours, and if necessary, aeration or agitation can be added.
- the host is Bacillus
- the culture is usually carried out at about 30 to 40 ° C. for about 6 to 24 hours, and if necessary, aeration or agitation can be added.
- the culture When cultivating a transformant whose host is yeast, the culture is usually performed at a temperature of about 20 ° C. to 35 ° C. for about 24 to 72 hours using a medium whose pH is adjusted to about 5 to 8, and if necessary, aerated. Or agitation can be added.
- a crude protein extract is obtained by centrifugation or filtration.
- the buffer solution may contain a protein denaturant such as urea or guanidine hydrochloride, or a surfactant such as Triton X-100 TM .
- the protein thus obtained can be arbitrarily obtained by subjecting the protein produced by the recombinant to an appropriate protein modifying enzyme such as trypsin or chymotrypsin before or after purification by a known method or a method similar thereto. Modifications can be made and the polypeptide can be partially removed.
- the presence of the protein used in the present invention can be measured by various binding assays and enzyme immunoassays using specific antibodies.
- the antibody used in the present invention is not particularly limited as long as it binds to the protein used in the present invention, and can be obtained as a polyclonal or monoclonal antibody using a known means.
- a monoclonal antibody derived from a mammal is particularly preferable.
- Mammal-derived monoclonal antibodies include those produced by hybridomas and those produced by hosts transformed with expression vectors containing antibody genes by genetic engineering techniques.
- the antibody used in the present invention preferably specifically binds to the protein used in the present invention.
- Monoclonal antibody-producing hybridomas can be basically produced using known techniques as follows. That is, the protein used in the present invention is used as a sensitizing antigen, immunized according to a normal immunization method, and the obtained immune cells are fused with a known parent cell by a normal cell fusion method. It can be produced by screening monoclonal antibody-producing cells by a screening method. Specifically, the monoclonal antibody can be produced as follows.
- the gene sequence encoding the protein is inserted into a known expression vector system to transform an appropriate host cell, and then the protein is purified from the host cell or culture supernatant by a known method.
- the protein is used as a sensitizing antigen.
- a partial peptide of the protein can be used as a sensitizing antigen.
- the partial peptide can be obtained from the amino acid sequence of the protein by chemical synthesis by a general method known to those skilled in the art.
- the partial polypeptide of the protein for example, at least 10 or more, preferably 50 or more, more preferably 70 or more, more preferably 100 or more, most preferably, of the constituent amino acid sequences of the protein.
- a peptide having a sequence of 200 or more amino acids and having biological activity substantially equivalent to the function of the protein is used.
- the partial peptide is usually a carboxyl group (—COOH) or carboxylate (—COO—) at the C-terminus, but may be an amide (—CONH 2 ) or an ester (—COOR) at the C-terminus.
- the partial peptide includes those in which the amino group of the N-terminal methionine residue is protected with a protecting group, those in which the N-terminal side is cleaved in vivo and pyroglutamine oxidized, and amino acids in the molecule These include those in which a substituent on the side chain is protected with an appropriate protecting group, or a complex peptide such as a so-called glycopeptide to which a sugar chain is bound.
- the mammal to be immunized with the sensitizing antigen is not particularly limited, but is preferably selected in consideration of compatibility with the parent cell used for cell fusion. Animals such as mice, rats, hamsters and the like are used.
- Immunization of animals with a sensitizing antigen is performed according to a known method.
- a sensitizing antigen is injected into a mammal intraperitoneally or subcutaneously.
- the sensitizing antigen is diluted to an appropriate amount with PBS (Phosphate-Buffered Saline) or physiological saline, and mixed with an appropriate amount of an ordinary adjuvant such as Freund's complete adjuvant, if necessary, and emulsified.
- PBS Phosphate-Buffered Saline
- physiological saline physiological saline
- an ordinary adjuvant such as Freund's complete adjuvant
- an appropriate carrier can be used during immunization with the sensitizing antigen.
- immune cells are collected from the mammal and subjected to cell fusion.
- Mammalian myeloma cells are used as the other parent cell to be fused with the immune cells.
- This myeloma cell is known in various known cell lines such as P3 (P3x63Ag8.653) (J. Immunol. (1979) 123, 1548-1550), P3x63Ag8U.1 (Current Topics in Microbiology and Immunology (1978) 81, 1-7), NS-1 (Kohler. G. and Milstein, C. Eur. J. Immunol. (1976) 6, 511-519), MPC-11 (Margulies.
- the cell fusion between the immune cells and myeloma cells is basically performed by a known method such as the method of Kohler and Milstein et al. (Kohler. G. G and Milstein, C., Methods Enzymol. (1981) 73, 3- 46) etc.
- the cell fusion is performed, for example, in a normal nutrient culture medium in the presence of a cell fusion promoter.
- a cell fusion promoter for example, polyethylene glycol (PEG), Sendai virus (HVJ) or the like is used, and an auxiliary agent such as dimethyl sulfoxide can be added and used to increase the fusion efficiency as desired.
- the use ratio of immune cells and myeloma cells can be arbitrarily set.
- the number of immune cells is preferably 1 to 10 times that of myeloma cells.
- the culture medium used for the cell fusion for example, RPMI1640 culture medium suitable for growth of the myeloma cell line, MEM culture medium, and other normal culture liquids used for this type of cell culture can be used. Serum supplements such as fetal calf serum (FCS) can be used in combination.
- FCS fetal calf serum
- a predetermined amount of the immune cells and myeloma cells are mixed well in the culture medium, and a PEG solution (for example, an average molecular weight of about 1000-6000) preliminarily heated to about 37 ° C.
- the target fusion cell (hybridoma) is formed by adding at a concentration of w / v) and mixing. Subsequently, cell fusion agents and the like that are undesirable for the growth of the hybridoma are removed by sequentially adding an appropriate culture medium and centrifuging to remove the supernatant.
- the hybridoma thus obtained is selected by culturing in a normal selective culture solution, for example, a HAT culture solution (a culture solution containing hypoxanthine, aminopterin and thymidine). Culturing with the above HAT culture solution is continued for a sufficient time (usually several days to several weeks) for cells other than the target hybridoma (non-fused cells) to die. Subsequently, the usual limiting dilution method is performed, and the hybridoma producing the antibody used in the present invention is screened and single-cloned.
- a normal selective culture solution for example, a HAT culture solution (a culture solution containing hypoxanthine, aminopterin and thymidine). Culturing with the above HAT culture solution is continued for a sufficient time (usually several days to several weeks) for cells other than the target hybridoma (non-fused cells) to die. Subsequently, the usual limiting dilution method is performed, and the hybridom
- human lymphocytes are sensitized to the protein in vitro, and the sensitized lymphocytes are fused with human-derived myeloma cells having a permanent division ability.
- a desired human antibody having binding activity to the protein can be obtained (see Japanese Patent Publication No. 1-59878).
- an antibody-producing cell may be obtained by administering the protein as an antigen to a transgenic animal having all repertoires of human antibody genes, and a human antibody against the protein may be obtained from the immortalized cell. (See International Patent Application Publication Nos. WO 94/25585, WO 93/12227, WO 92/03918, WO 94/02602).
- a technique for obtaining a human antibody by panning using a human antibody library is also known.
- the V region of a human antibody is expressed as a single chain antibody (scFv) on the surface of the phage by the phage display method. Phages expressing scFv that bind to the antigen can be selected.
- the DNA sequence encoding the V region of the human antibody that binds to the antigen can be determined.
- the V region sequence is fused in-frame with the sequence of the desired human antibody C region, and then inserted into an appropriate expression vector, whereby an expression vector can be prepared.
- the human antibody is obtained by introducing the expression vector into a suitable expression cell as described above and expressing the gene encoding the human antibody.
- These methods are already known (see International Publication WO 1992/001047, WO 1992/020791, WO 1993/006213, WO 1993/011236, WO 1993/019172, WO 1995/001438, WO 1995/015388).
- the hybridoma producing the monoclonal antibody thus produced can be subcultured in a normal culture solution and can be stored for a long time in liquid nitrogen.
- the hybridoma is cultured according to a usual method and obtained as a culture supernatant thereof, or the hybridoma is administered to a mammal compatible therewith to proliferate, and its ascites
- the method obtained as follows is adopted.
- the former method is suitable for obtaining highly pure antibodies, while the latter method is suitable for mass production of antibodies.
- the monoclonal antibody used in the present invention is, for example, a recombinant antibody produced by cloning an antibody gene from a hybridoma, incorporating it into an appropriate vector, introducing it into a host, and producing it using a gene recombination technique.
- a recombinant antibody produced by cloning an antibody gene from a hybridoma, incorporating it into an appropriate vector, introducing it into a host, and producing it using a gene recombination technique.
- mRNA encoding the variable (V) region of the antibody is isolated from the hybridoma producing the antibody. Isolation of mRNA is performed by a known method such as guanidine ultracentrifugation (Chirgwin, JM et al., Biochemistry (1979) 18, 5294-5299), AGPC method (Chomczynski, P. et al., Anal). Biochem. (1987) 162, (156-159) etc. to prepare total RNA, and mRNA-Purification-Kit (manufactured by Pharmacia) etc. is used to prepare the target mRNA. Alternatively, mRNA can be directly prepared by using QuickPrep mRNA Purification Kit (Pharmacia).
- the antibody V region cDNA is synthesized from the obtained mRNA using reverse transcriptase. Synthesis of cDNA is performed using AMV Reverse Transcriptase First-strand DNA Synthesis Kit (manufactured by Seikagaku Corporation). For cDNA synthesis and amplification, 5'-Ampli FINDER RACE Kit (Clontech) and 5'-RACE method using PCR (Frohman, MAet al., Proc. Natl. Acad. Sci. USA) (1988) 85, 8998-9002, Belyavsky, A. et al., Le Nucleic Acids Res. 1989 (1989) 17, 2919-2932) and the like.
- the desired DNA fragment is purified from the obtained PCR product and ligated with vector DNA. Further, a recombinant vector is prepared from this, introduced into Escherichia coli, etc., and colonies are selected to prepare a desired recombinant vector. Then, the base sequence of the target DNA is confirmed by a known method such as the dideoxynucleotide chain termination method.
- an antibody gene is incorporated into an expression vector so that it is expressed under the control of an expression control region, for example, an enhancer or a promoter.
- an expression control region for example, an enhancer or a promoter.
- host cells are transformed with this expression vector to express the antibody.
- Expression of the antibody gene may be carried out by co-transforming host cells by separately incorporating DNAs encoding antibody heavy chains (H chains) or light chains (L chains) into expression vectors, or H chains and L chains.
- a host cell may be transformed by incorporating a DNA encoding s into a single expression vector (see WO94 / 11523).
- transgenic animals can be used for the production of recombinant antibodies.
- an antibody gene is inserted in the middle of a gene encoding a protein (such as goat casein) inherently produced in milk to prepare a fusion gene.
- a DNA fragment containing a fusion gene into which an antibody gene has been inserted is injected into a goat embryo, and the embryo is introduced into a female goat.
- the desired antibody is obtained from the milk produced by the transgenic goat born from the goat that received the embryo or its offspring.
- hormones may be used in the transgenic goat as appropriate (Ebert, KM et al., Bio / Technology (1994) 12, 699-702).
- genetically modified antibodies artificially modified for the purpose of reducing the heterologous antigenicity to humans for example, chimeric antibodies, humanized antibodies, human antibodies Can be used. These modified antibodies can be produced using known methods.
- the monoclonal antibodies of the present invention include not only the above-described animal-derived monoclonal antibodies but also genetically modified antibodies such as chimeric antibodies, humanized antibodies, and bispecific antibodies.
- the chimeric antibody can be obtained by ligating the DNA encoding the antibody V region obtained as described above with the DNA encoding the human antibody C region, incorporating it into an expression vector, introducing it into a host, and producing it. Using this known method, useful chimeric antibodies can be obtained.
- a humanized antibody is also referred to as a reshaped human antibody, which is a complementarity determining region (CDR) of a non-human mammal, for example, a mouse antibody, to a complement determining region of a human antibody. It is transplanted, and its general gene recombination technique is also known (see European Patent Application Publication No. EP 125023, WO 96/02576).
- CDR complementarity determining region
- a DNA sequence designed to link the CDR of a mouse antibody and the framework region (FR) of a human antibody has a portion that overlaps both terminal regions of CDR and FR.
- the prepared oligonucleotides are used as primers and synthesized by PCR (see the method described in WO98 / 13388).
- the framework region of the human antibody to be linked via CDR is selected such that the complementarity determining region forms a good antigen binding site. If necessary, the amino acid of the framework region in the variable region of the antibody may be substituted so that the complementarity determining region of the reshaped human antibody forms an appropriate antigen-binding site (Sato, K. et al., Cancer Res. (1993) 53, 851-856).
- human antibodies are used for the C region of the chimeric antibody and humanized antibody.
- CH1, CH2, CH3, and CH4 can be used for the H chain
- C ⁇ and C ⁇ can be used for the L chain.
- the human antibody C region may be modified in order to improve the stability of the antibody or its production.
- a chimeric antibody consists of a variable region of a non-human mammal-derived antibody and a constant region derived from a human antibody.
- a humanized antibody consists of a complementarity determining region of a non-human mammal-derived antibody, a framework region derived from a human antibody, and a C region. Since humanized antibodies have reduced antigenicity in the human body, they are useful as active ingredients of the therapeutic agent of the present invention.
- the antibody used in the present invention is not limited to the whole antibody molecule, and may be an antibody fragment or a modified product thereof as long as it binds to the protein used in the present invention, and includes both bivalent antibodies and monovalent antibodies. It is.
- antibody fragments include Fab, F (ab ′) 2, Fv, Fab / c having one Fab and a complete Fc, or a single chain in which Hv or L chain Fv is linked by an appropriate linker.
- Examples include Fv (scFv) and Diabody.
- the antibody is treated with an enzyme such as papain or pepsin to generate antibody fragments, or a gene encoding these antibody fragments is constructed and introduced into an expression vector, followed by appropriate host cells.
- ScFv can be obtained by linking antibody H chain V region and L chain V region.
- the H chain V region and the L chain V region are linked via a linker, preferably a peptide linker (Huston, J. S. et al., Proc. Natl. Acad. Sci. USA (1988) 85, 5879-5883).
- the H chain V region and L chain V region in scFv may be derived from any of those described as antibodies herein.
- the peptide linker for linking the V regions for example, any single chain peptide consisting of amino acid 12-19 residues such as (GGGGS) n is used.
- the scFv-encoding DNA is a DNA encoding the H chain or H chain V region of the antibody, and a DNA encoding the L chain or L chain V region.
- an expression vector containing them and a host transformed with the expression vector can be obtained according to a conventional method.
- ScFv can be obtained according to the method.
- Diabody is a dimerization by linking two fragments (for example, scFv etc.) obtained by linking a variable region and a variable region with a linker or the like, and usually contains two VLs and two VHs (P .Holliger et al., Proc.Natl.Acad.Sci.USA, 90, 6444-6448 (1993), EP 404097, WO 93/11161, Johnson et al., Method in Enzymology, 203, 88-98, (1991), Holliger et al., Protein Engineering, 9, 299-305, (1996), Perisic et al., Structure, 2, 1217-1226, (1994), John et al., Protein Engineering, 12 (7 ), 597-604, (1999), Holliger et al., Proc. Natl. Acad. Sci. USA., 90, 46444-6448, (1993), Atwell et al., Mol. Immunol., 33, 1301- 1312
- antibody fragments can be produced by the host by obtaining and expressing the gene in the same manner as described above.
- the “antibody” in the present invention includes fragments of these antibodies.
- the antibody of the present invention bound to various molecules can also be used as a modified antibody. It is also possible to bind a cytotoxic substance such as a radioisotope, a chemotherapeutic agent, or a bacterial toxin to the antibody.
- the “antibody” in the present invention includes these modified antibodies. Such a modified antibody can be obtained by chemically modifying the obtained antibody. Antibody modification methods have already been established in this field.
- the antibody used in the present invention may be a bispecific antibody.
- Bispecific antibodies are bispecific antibodies having antigen-binding sites that recognize different epitopes on the protein used in the present invention, and bispecific antibodies that recognize the protein used in the present invention and other proteins.
- one antigen binding site may recognize a protein used in the present invention, and the other antigen binding site may recognize a cytotoxic substance such as a chemotherapeutic agent or a cell-derived toxin.
- the cancer stem cells expressing the protein used in the present invention can be directly damaged by causing a cytotoxic substance to act specifically on the cancer stem cells, thereby suppressing the proliferation of the cancer stem cells.
- one antigen binding site recognizes a molecule constituting a T cell receptor complex such as CD3 expressed in cytotoxic T cells, and the other antigen binding site is represented by SEQ ID NO: 1 to 8 of the present invention.
- Bispecific antibodies that recognize epitopes present in any of the proteins described can also be used.
- Bispecific antibodies can be prepared by linking HL pairs of two types of antibodies, or by producing hybrid cells producing bispecific antibodies by fusing hybridomas that produce different monoclonal antibodies. it can.
- bispecific antibodies can be produced by genetic engineering techniques.
- the antibody gene constructed as described above can be expressed and obtained by a known method.
- it can be expressed by functionally binding a useful promoter commonly used, an antibody gene to be expressed, and a poly A signal downstream of the 3 ′ side thereof.
- the promoter / enhancer includes human cytomegalovirus early promoter / enhancer (human cytomegalovirus immediate-promoter / enhancer).
- viral promoters / enhancers such as retrovirus, polyoma virus, adenovirus, simian virus 40 (SV40), or human elongation factor 1 ⁇ (HEF1 ⁇ ).
- promoters / enhancers derived from mammalian cells.
- the origin of replication those derived from SV40, polyoma virus, adenovirus, bovine papilloma virus (BPV), etc. can be used. Furthermore, for the purpose of gene copy number amplification in the host cell system, the expression vector is used as a selection marker.
- An aminoglycoside transferase (APH) gene, thymidine kinase (TK) gene, E. coli xanthine guanine phosphoribosyltransferase (Ecogpt) gene, dihydrofolate reductase (dhfr) gene and the like can be included.
- the gene can be expressed by functionally combining a useful promoter commonly used, a signal sequence for antibody secretion, and an antibody gene to be expressed.
- the promoter include lacz promoter and araB promoter. When using the lacz promoter, the method of Ward et al. (Nature (1098) 341, 544-546; FASEB J. (1992) 6, 242-2427), or when using the araB promoter, the method of Better et al. (Science (1988) 240, 1041-1043).
- a pelB signal sequence (Lei, S.P. et al., J. Bacteriol. (1987) 169, 4379) may be used when the periplasm of E. coli is produced. Then, after separating the antibody produced in the periplasm, the structure of the antibody is appropriately refolded and used.
- a eukaryotic cell or a prokaryotic cell system can be used for the production of the antibody used in the present invention.
- eukaryotic cells include established mammalian cell systems, insect cell systems, filamentous fungal cells, and animal cells such as yeast cells.
- prokaryotic cells include bacterial cells such as E. coli cells.
- the antibodies used in the present invention are expressed in mammalian cells such as CHO, COS, myeloma, BHK, Vero, HeLa cells.
- the transformed host cell is cultured in vitro or in vivo to produce the desired antibody.
- Host cells are cultured according to a known method.
- DMEM, MEM, RPMI1640, and IMDM can be used as the culture medium
- serum supplements such as fetal calf serum (FCS) can be used in combination.
- the antibody expressed and produced as described above can be separated from cells and host animals and purified to homogeneity. Separation and purification of the antibody used in the present invention can be performed using an affinity column.
- an affinity column For example, as a column using a protein A column, Hyper® D, POROS, Sepharose® F.F. (Pharmacia) and the like can be mentioned.
- antibodies can be separated and purified by appropriately selecting and combining chromatography columns other than the affinity column, filters, ultrafiltration, salting out, dialysis, etc. (Antibodies A Laboratory Manual. Ed Harlow, David Lane) , Cold Spring Harbor Laboratory, 1988).
- Antigen binding activity of antibodies used in the present invention (Antibodies A Laboratory Manual. Ed Harlow, David Lane, Cold Spring Harbor Laboratory, 1988), ligand receptor binding inhibitory activity (Harada, A. et al., International Immunology (1993) 5, 681-690) can be measured using known means.
- ELISA enzyme-linked immunosorbent assay
- EIA enzyme immunoassay
- RIA radioimmunoassay
- fluorescent antibody method a sample containing the antibody, for example, a culture supernatant of the antibody-producing cells or a purified antibody is added to a plate coated with the protein used in the present invention. It is possible to evaluate the antigen binding activity by adding a secondary antibody labeled with an enzyme such as alkaline phosphatase, incubating the plate, washing, adding an enzyme substrate such as p-nitrophenyl phosphate and measuring the absorbance. it can.
- the antibody used in the present invention can be appropriately linked to the above-mentioned growth inhibitor, a toxic peptide, or a cytotoxic substance such as a radioactive chemical substance.
- a modified antibody (hereinafter referred to as antibody conjugate) can be obtained by chemically modifying the obtained antibody. That is, the linker molecule chemically bonds the growth inhibitor to the antibody (as described above) so that the growth inhibitor or cytotoxic agent and the antibody can be chemically coupled to each other (eg, can be covalently bonded).
- the binding agent (linker) is a cleavable linker. More preferably, the linker is cleaved under mild conditions (ie intracellular conditions such that drug activity is not affected).
- cleavable linkers examples include disulfide linkers, acid labile linkers, photolabile linkers, peptidase labile linkers, and esterase labile linkers.
- a linker containing a disulfide is a linker that is cleavable through disulfide exchange that can occur under physiological conditions.
- An acid labile linker is a linker that is cleavable at acidic pH. For example, certain intracellular compartments, such as endosomes and lysosomes, have acidic pH (pH 4-5) and provide suitable conditions for cleaving acid labile linkers.
- Photolabile linkers are useful on body surfaces and many body cavities that can be exposed to light.
- Peptidase labile linkers can be used to cleave specific peptides inside or outside cells (eg, Trouet et al. (Proc. Natl. Acad. Sci. USA (1982) 79, 626-629, and Umemoto Et al., Int. J. Cancer (1989) 43, 677-684).
- antibody modifications are bispecific antibodies designed using genetic recombination technology to recognize growth inhibitors, toxic peptides, or radioactive chemicals. Can be obtained as a molecular type. These antibodies are also included in the “antibody” in the present invention.
- modified antibodies provided by the present invention include ricin, abrin, ribonuclease, onconase, DNase I, Staphylococcal enterotoxin-A, pokeweed antiviral protein, gelonin, diphtheria toxin, Pseudomonas exotoxin, Pseudomonas endotoxin, L-asparaginase, PEG
- ricin abrin
- ribonuclease onconase
- DNase I Staphylococcal enterotoxin-A
- pokeweed antiviral protein pokeweed antiviral protein
- gelonin gelonin
- diphtheria toxin diphtheria toxin
- Pseudomonas exotoxin Pseudomonas endotoxin
- L-asparaginase L-asparaginase
- PEG Also exemplified are antibodies modified with toxic peptides such as As
- the binding between the antibody that binds to the protein described in at least one of SEQ ID NOs: 1 to 8 of the present invention and the above-described growth inhibitor, toxic peptide, or radioactive chemical substance is a covalent bond or a noncovalent bond.
- Methods for preparing modified antibodies bound with these chemotherapeutic agents are known.
- proteinaceous drugs and toxins can be bound to antibodies by genetic engineering techniques.
- DNA encoding the toxic peptide and DNA encoding an antibody that binds to at least one of the proteins described in SEQ ID NOs: 1 to 8 of the present invention are fused in frame.
- Recombinant vectors incorporated into expression vectors can be constructed.
- a transformed cell obtained by introducing the vector into an appropriate host cell is cultured, and the incorporated DNA is expressed, whereby a modified antibody to which a toxic peptide is bound can be obtained as a fusion protein.
- a proteinaceous drug or toxin is generally arranged on the C-terminal side of the antibody.
- a peptide linker can be interposed between the antibody and the proteinaceous drug or toxin.
- the antibody used in the present invention may have cytotoxic activity.
- the cytotoxic activity in the present invention include complement-dependent cytotoxicity (CDC) activity, antibody-dependent cell-mediated cytotoxicity (ADCC) activity and the like. Can do.
- CDC activity means cytotoxic activity by the complement system
- ADCC activity means that when a specific antibody is attached to a cell surface antigen of a target cell, an Fc ⁇ receptor-bearing cell (immune cell or the like) is attached to the Fc portion. ) Binds via the Fc ⁇ receptor and means an activity that damages the target cell.
- ADCC activity or CDC activity can be measured by a known method (for example, Current protocols in Immunology, Chapter7. Immunologic studies in humans, Editor , John E, Coligan et al., John Wiley & Sons, Inc., (1993)).
- the cytotoxic activity can be measured by the following method. -Preparation of effector cells
- the spleen is removed from a CBA / N mouse or the like, and the spleen cells are separated in RPMI1640 medium (GIBCO). After washing with the same medium containing 10% fetal bovine serum (FBS, HyClone), the cell concentration is adjusted to 5 ⁇ 10 6 / ml to prepare effector cells.
- FBS fetal bovine serum
- Protein expression cells (cancer stem cells, etc.) used in the present invention were mixed with 0.2 mCi of 51 Cr-sodium chromate (Amersham Pharmacia Biotech) in 10% FBS-containing DMEM medium at 37 ° C. Radiolabeling is performed by incubation for a period of time. After radiolabeling, the cells are washed three times with RPMI1640 medium containing 10% FBS, and the cell concentration is adjusted to 2 ⁇ 10 5 / ml to prepare target cells.
- ADCC activity To a 96-well U-bottom plate (manufactured by Becton Dickinson), 50 ⁇ l each of target cells and the antibody used in the present invention are added and allowed to react on ice for 15 minutes. Thereafter, 100 ⁇ l of effector cells are added and cultured for 4 hours in a carbon dioxide incubator. The final antibody concentration is 0 or 10 ⁇ g / ml. After the culture, 100 ⁇ l of the supernatant is collected, and the radioactivity is measured with a gamma counter (COBRAIIAUTO-GMMA, MODEL D5005, manufactured by Packard Instrument Company). Cytotoxic activity (%) is (AC) / (BC) ⁇ 100 It can ask for.
- COBRAIIAUTO-GMMA MODEL D5005
- A shows the radioactivity (cpm) in each sample
- B shows the radioactivity (cpm) in the sample added with 1% NP-40 (manufactured by Hanai)
- C shows the radioactivity (cpm) of the sample containing only the target cells.
- CDC activity To a 96-well flat bottom plate (Becton Dickinson), add 50 ⁇ l each of the target cells and the antibody used in the present invention, and allow to react on ice for 15 minutes. Thereafter, 100 ⁇ l of complement solution is added and cultured in a carbon dioxide incubator for 4 hours. The final antibody concentration is 0 or 3 ⁇ g / ml. After incubation, 100 ⁇ l of the supernatant is collected, and the radioactivity is measured with a gamma counter. Cytotoxic activity can be determined in the same manner as ADCC activity measurement.
- an antibody having a modified sugar chain can be appropriately used. It is known that the cytotoxic activity of an antibody can be enhanced by modifying the sugar chain of the antibody.
- antibodies with modified sugar chains for example, the following antibodies are known. -Antibodies with modified glycosylation (eg WO1999 / 054342) -Antibodies lacking fucose added to the sugar chain (WO2000 / 061739, WO2002 / 031140, etc.) -An antibody having a sugar chain having bisecting GlcNAc (bisecting N-acetylglucosamine) (WO2002 / 079255, etc.)
- the antibody of the present invention preferably includes an antibody whose sugar chain composition is modified so that the ratio of fucose-deficient antibody is high, or the ratio of bisecting N-acetylglucosamine added is high. It is.
- an antibody having neutralizing activity can also be used as appropriate.
- neutralizing activity refers to an exogenous molecule such as a virus or toxin, or an endogenous molecule such as a hormone or cytokine that inhibits the biological activity of a ligand that has biological activity on a cell.
- the substance having neutralizing activity refers to a substance that binds to the ligand or the receptor to which the ligand binds and inhibits the binding between the ligand and the receptor.
- a receptor that has been blocked from binding to a ligand by neutralizing activity cannot exhibit biological activity through the receptor.
- an antibody having such neutralizing activity is generally called a neutralizing antibody.
- the neutralizing activity of a test substance can be measured by comparing the biological activity in the presence of a ligand between conditions in the presence or absence of the test substance.
- EREG which is the target of the EP27 antibody described later in the examples.
- EGF receptor which is considered as the main receptor of EREG represented by SEQ ID NO: 3
- a dimer is formed by the binding of a ligand, and tyrosine kinase that is its own domain in the cell is activated.
- Activated tyrosine kinases form peptides containing phosphorylated tyrosines by autophosphorylation and associate them with various signaling accessory molecules. They are mainly PLC ⁇ (phospholipase C ⁇ ), Shc, Grb2, and the like.
- the former two are further phosphorylated by tyrosine kinases of the EGF receptor.
- the main pathway in signal transduction from the EGF receptor is a pathway through which phosphorylation is transmitted in the order of Shc, Grb2, Sos, Ras, Raf / MAPK kinase / MAP kinase.
- a route from PLC ⁇ to PKC which is a sub route. Since such intracellular signal cascades are different for each cell type, a target molecule can be appropriately set for each target cell of interest, and is not limited to the above factors.
- a commercially available kit for measuring in vivo signal activation can be used as appropriate (for example, a protein kinase C activity measurement system (GE Healthcare Bioscience Co., Ltd.)).
- activation of in vivo signals can also be detected using the transcription inducing action on a target gene present downstream of the in vivo signal cascade as an index.
- Changes in transcriptional activity can be detected by reporter assay principles. Specifically, a reporter gene such as GFP (Green Fluorescence Protein) or luciferase is placed downstream of the transcription factor or promoter region of the target gene, and the change in the transcription activity is measured as the reporter activity by measuring the reporter activity. can do.
- GFP Green Fluorescence Protein
- the EGF receptor usually works in the direction of promoting cell proliferation
- the activation of in vivo signaling can be evaluated by measuring the proliferation activity of the target cells.
- the latter cell proliferation activity is evaluated to evaluate the neutralizing activity of the neutralizing antibody of the present invention.
- the present invention is not limited to this method, and the method described above for each selected target cell. Can be suitably employed and evaluated.
- the neutralizing activity of the anti-EREG antibody can be evaluated or measured by measuring the following cell proliferation activity.
- a method is used in which the uptake of [ 3 H] -labeled thymidine added to the medium by living cells is measured as an indicator of DNA replication ability.
- a dye exclusion method in which the ability to exclude a dye such as trypan blue to the outside of cells is measured under a microscope, or an MTT method is used. The latter uses the ability of living cells to convert the tetrazolium salt MTT (3- (4,5-dimethylthiazol-2-yl) -2,5-diphenyl tetrazolium bromide) into a blue formazan product. Yes.
- the test antibody is added to the culture solution of the test cells, and after a predetermined time has elapsed, the MTT solution is added to the culture solution and allowed to stand for a fixed time, thereby allowing MTT to be taken into the cells.
- MTT which is a yellow compound
- MTT is converted to a blue compound by succinate dehydrogenase in the mitochondria in the cell.
- the blue product is dissolved and colored, and the absorbance is measured to obtain an index of the number of viable cells.
- reagents such as MTS, XTT, WST-1, and WST-8 are also commercially available (eg, nacalaitesque) and can be suitably used.
- a method for evaluating cell proliferation activity using cell ATP or cell culture impedance as an index is also known. In measuring the activity, a binding antibody that has the same isotype as the anti-EREG antibody and does not have the neutralizing activity is used as a control antibody in the same manner as the anti-EREG antibody, and the anti-EREG antibody is stronger than the control antibody. Activity can be determined by showing neutralization activity. *
- the cell in which the anti-EREG antibody suppresses the growth is not particularly limited as long as the cell expresses the EREG protein.
- Preferred EREG-expressing cells are, for example, cancer cells.
- cells derived from colorectal cancer, lung adenocarcinoma, pancreatic cancer, gastric cancer, and renal cancer are suitable as EREG-expressing cells in the present invention. According to the present invention, it is possible to obtain an effective cell growth inhibitory effect on both primary lesions and metastatic lesions of these cancers.
- Further preferred cancer cells are primary colorectal cancer, metastatic colorectal cancer, lung adenocarcinoma, pancreatic cancer, gastric cancer, and renal cancer.
- the anti-EREG antibody can be used for the purpose of treating or preventing diseases caused by cell proliferation, such as colon cancer, lung adenocarcinoma, pancreatic cancer, gastric cancer, renal cancer and the like. These cancers can be treated or prevented regardless of whether they are primary or metastatic. More preferably, an anti-EREG antibody can be used for the purpose of treating and / or preventing primary colorectal cancer, metastatic colorectal cancer, and pancreatic cancer. Furthermore, among these cancers, cancers that proliferate in an EREG-dependent manner are preferred as targets for treatment and / or prevention in the present invention.
- the effective dose of the cancer stem cell inhibitor of the present invention is selected in the range of 0.001 mg to 1000 mg per kg body weight at a time. Alternatively, a dose of 0.01 to 100,000 mg / body per patient can be selected. However, the inhibitor of the present invention is not limited to these doses. Moreover, as an administration time of the inhibitor of this invention, it can administer regardless of before and after the clinical symptom of a disease arises.
- the inhibitor of the present invention can be formulated according to a conventional method (Remington's Pharmaceutical Science, latest edition, Mark Publishing Company, Easton, USA), and contains both pharmaceutically acceptable carriers and additives. May be.
- Such carriers and pharmaceutical additives include water, pharmaceutically acceptable organic solvents, collagen, polyvinyl alcohol, polyvinyl pyrrolidone, carboxyvinyl polymer, sodium carboxymethylcellulose, sodium polyacrylate, sodium alginate, water-soluble dextran. , Sodium carboxymethyl starch, pectin, methylcellulose, ethylcellulose, xanthan gum, gum arabic, casein, agar, polyethylene glycol, diglycerin, glycerin, propylene glycol, petroleum jelly, paraffin, stearyl alcohol, stearic acid, human serum albumin (HSA), mannitol , Sorbitol, lactose, surfactants acceptable as pharmaceutical additives, and the like.
- water pharmaceutically acceptable organic solvents
- collagen collagen
- polyvinyl alcohol polyvinyl pyrrolidone
- carboxyvinyl polymer sodium carboxymethylcellulose
- sodium polyacrylate sodium alginate
- water-soluble dextran water-soluble de
- the actual additive is selected from the above alone or in appropriate combination depending on the dosage form of the inhibitor of the present invention, but of course is not limited thereto.
- a solvent such as physiological saline, buffer solution, glucose solution, etc.
- an adsorption inhibitor such as Tween 80, Tween 20, gelatin, human serum albumin, etc.
- Tween 80, Tween 20, gelatin, human serum albumin, etc. Can be used.
- it may be lyophilized to obtain a dosage form that is reconstituted before use, and as an excipient for lyophilization, for example, sugar alcohols or saccharides such as mannitol and glucose are used. be able to.
- the inhibitor of the present invention is usually administered by parenteral administration route, for example, injection (subcutaneous injection, intravenous injection, intramuscular injection, intraperitoneal injection, etc.), transdermal, transmucosal, nasal, transpulmonary, etc. Oral administration is also possible.
- parenteral administration route for example, injection (subcutaneous injection, intravenous injection, intramuscular injection, intraperitoneal injection, etc.), transdermal, transmucosal, nasal, transpulmonary, etc.
- parenteral administration route for example, injection (subcutaneous injection, intravenous injection, intramuscular injection, intraperitoneal injection, etc.), transdermal, transmucosal, nasal, transpulmonary, etc.
- parenteral administration route for example, injection (subcutaneous injection, intravenous injection, intramuscular injection, intraperitoneal injection, etc.), transdermal, transmucosal, nasal, transpulmonary, etc. Oral administration is also possible.
- “using in combination with a cancer stem cell inhibitor and an anticancer agent” means that these agents may be administered simultaneously, sequentially, or after one of them is administered first, and after a while. To do.
- the cancer stem cell inhibitor is used in various modes as indications such as cancer recurrence prevention, cancer recurrence suppression, cancer metastasis prevention, cancer metastasis inhibition, postoperative recurrence prevention adjuvant therapy, and the like.
- Any cancer stem cell inhibitor can be used as the cancer stem cell inhibitor of the present invention as long as it is used in the above embodiment, but preferred examples include, but are not limited to, cancer stem cell growth inhibitors and cancer stem cell disruptors.
- the cancer stem cell proliferation inhibitor provided by the present invention does not matter by what mechanism the proliferation of cancer stem cells is inhibited as long as the proliferation of targeted cancer stem cells is inhibited.
- cancer stem cell growth inhibitors include cancer stem cell growth inhibitors that contain as an active ingredient an antibody having neutralizing activity against proliferation or growth of cancer stem cells and cytotoxic activity against cancer stem cells. obtain.
- the cancer stem cell-disrupting agent provided by the present invention does not matter by what mechanism the cancer stem cell is destroyed as long as the target cancer stem cell is destroyed.
- a cancer stem cell proliferation inhibitor containing an antibody having cytotoxic activity and apoptotic activity against cancer stem cells as an active ingredient can be excised.
- Apoptotic activity is measured by using known methods including TUNEL (Terminal-deoxynucleotidyl-Transferase-Biotin-dUTP-Nick-End Labeling) assay, caspase activity (especially caspase-3) assay, and assay for fas ligand and annexin V.
- TUNEL Terminal-deoxynucleotidyl-Transferase-Biotin-dUTP-Nick-End Labeling
- caspase activity especially caspase-3
- assay for fas ligand and annexin V as ligand and annexin V.
- a person skilled in the art can determine whether or not the test cancer stem cell disrupting agent has apoptotic activity.
- Non-limiting preferred examples include cancer stem cell differentiation promoters and the like.
- Non-limiting examples of such differentiation promoting agents include BMP4, that is, the addition or deletion of one or more amino acids of the polypeptide represented by SEQ ID NO: 9 or the amino acid contained in the polypeptide, Substituted polypeptide equivalents may be exemplified.
- the polypeptide equivalent preferably has a differentiation-inducing activity equivalent to that of CSC possessed by the polypeptide represented by SEQ ID NO: 9. Whether the differentiation-inducing activity is equivalent is, for example, 10%, preferably 20%, and preferably 30%, more preferably 30%, more preferably CK20-inducing activity for CSC possessed by the polypeptide represented by SEQ ID NO: 9. It can be defined as equivalent if it is 40%, more preferably 50%.
- whether or not the differentiation-inducing activity is equivalent is, for example, 60%, preferably 70% of the CK20-inducing activity against CSC possessed by the polypeptide represented by SEQ ID NO: 9. And preferably 80%, more preferably 90%, and even more preferably 95%.
- Anticancer agents used in combination with the cancer stem cell inhibitor of the present invention include alkylating agents, antimetabolites, natural products, platinum complexes, and other drugs.
- Alkylating agents include nitrogen mustards (Nitrogen Mustards), ethyleneimines (Ethylenimines), methylmelamines (Methylmelamines), alkyl sulfonates (Alkyl Sulfonates), nitrosoureas (Nitrosoureas), triazenes (Triazens) Is mentioned.
- nitrogen mustards include mechlorethamine, cyclophosphamide, ifosfamide, melphalan, and chlorambucil.
- Examples of ethyleneimines and methylmelamines include hexamethylmelamine and thiotepa.
- alkyl sulfonates include busulfan.
- Examples of nitrosoureas include Carmustine (BCNU), Lomustine (CCNU), Semustine (methyl-CCNU), and Streptozocin.
- Examples of triazenes include dacarbazine (Dacarbazine: DTIC).
- Antimetabolites include folic acid analogs, pyrimidine analogs, and purine analogs. An example of a folic acid analog is methotrexate.
- Pyrimidine analogues include, for example, fluorouracil (5-FU), doxifluridine (5'-DFUR, trade name fluturon), capecitabine (trade name Xeloda), floxuridine (FudR), cytarabine (Cytarabine).
- purine analogs include mercaptopurine (Mercaptopurine: 6-MP), thioguanine (Thioguanine: TG), and pentostatin (Pentostatin).
- Natural products include vinca alkaloids, epipodophyllotoxins, and antibiotics. Examples of vinca alkaloids include vinblastine (VLB) and vincristine (VCR).
- Examples of epipodophyllotoxins include etoposide and teniposide.
- Examples of antibiotics include dactinomycin (Dactinomycin: actinomycin inD), daunorubicin (Daunorubicin), doxorubicin (Bleomycin), priomycin (Plicamycin), mitomycin (Mitomycin).
- the platinum complex refers to a platinum coordination complex, and examples thereof include cisplatin (CDDP) and carboplatin.
- topoisomerase inhibitors such as irinotecan and camptothecin
- taxols such as paclitaxel, docetaxel, anthracenedions, such as mitoxantrone, urea substitutes such as hydroxyurea And methyl hydrazines (Methyl Hydrazines), such as procarbazine hydrochloride (Procarbazine Hydrochloride), and vitamin A metabolites such as tretinoin (Tradenoin, product name Besanoid).
- rituximab alemtuzumab, trastuzumab, bevacizumab, cetuximab, panitumumab, tratuzumab, gemtuzumab, etc. are mentioned.
- Isolation of colonic CSCs and single cell suspensions of cancer cells from in vitro cultured xenografts were prepared by chopping the tissue with a razor, 37 in DPBS containing collagenase / dispase (Roche) and DNaseI (Roche) After incubation at 0 ° C. for 3 hours, the solution was filtered with a 40 ⁇ m cell strainer (BD Biosciences), suspended in lysis buffer (BD Biosciences), and erythrocytes were removed.
- the resulting xenograft-derived cells (referred to as primary, primary, or primary cells) are N-2 supplement (Invitrogen), 20 ng / mL at 37 ° C in a 5% CO 2 atmosphere.
- Human EGF (Invitrogen), 10 ng / mL human basic fibroblast growth factor (Sigma), 4 ⁇ g / mL heparin (Sigma), 4 mg / mL BSA (Invitrogen), 20 ⁇ g / mL human insulin, zinc solution ( Invitrogen), and cultured in DMEM / F12 medium (Invitrogen) containing 2.9 mg / mL glucose (Sigma) (Todaro M, et al. (2007) Cell Stem Cell 1; 389-402.).
- polystyrene-treated normal cell culture flasks (BD Biosciences) and ultra-low adhesion cell culture flasks (Corning) were used, respectively.
- Cell suspensions were prepared by serial dilution in vivo tumorigenesis analysis .
- 100 ⁇ L of cancer cell suspension in Hanks balanced salt solution (Invitrogen) was inoculated subcutaneously into the flank of mice using 50% Matrigel (BD Bioscience). Tumor development was monitored for 7 weeks.
- cells were labeled with FITC-labeled mouse anti-human CD326 (EpCAM) antibody (Miltenyi Biotec) and seeded on Terasaki plates (Termo Fisher Scientific). Single cells were confirmed under a fluorescence microscope. Single cells were inoculated into the flank of mice using 50 ⁇ l of 50% Matrigel. Tumor development was monitored for 10 weeks.
- Soluble Lgr5-Fc Protein Preparation Soluble Lgr5 (amino acids 1-555) protein was expressed as a fusion protein with the Fc portion of mouse IgG2a of CHO DG44. Transfectants were screened by sandwich ELISA using goat anti-mouse IgG2a (Bethyl labotratories) and HRP rat anti-mouse IgG2amAb (Serotec). The clone that produced the most abundant sLgr5-Fc was named 2D3. The 2D3 culture supernatant was collected, and the Lgr5-Fc protein was affinity purified by a protein A-Sepharose column (Pharmacia). Lgr5-Fc served as an antigen for protein immunization and ELISA screening.
- mice (Charles River Japan) were subcutaneously immunized with 50 ⁇ g of Lgr5-Fc emulsified in complete Freund's adjuvant. Two weeks later, weekly injections were repeated for 2 weeks with the same amount in Freund's incomplete adjuvant. Three days before cell fusion, mice were injected intravenously with 25 ⁇ g Lgr5Fc.
- Spleen lymphocytes derived from immunized mice were fused with P3-X63Ag8U1 mouse myeloma cells (ATCC) by conventional methods (Kremer L and Marquez G (2004) Methods Mol Biol., 239; 243-260).
- the hybridoma culture supernatant was screened for antibodies reactive with sLgr5-Fc using ELISA.
- Lgr5-specific mouse mAbs 2T15E-2 and 2U2E-2 were established.
- Flow cytometric analysis Colon CSCs were incubated with labeled antibodies and analyzed using EPICS ALTRA (Beckman Coulter) and FACSCalibur (Becton Dickinson).
- the antibodies used were PE-labeled mouse anti-human CD133 antibody (Miltenyi Biotec), PE-labeled mouse anti-human CD44 antibody (BD Pharmingen), FITC-labeled mouse anti-human CD326 (EpCAM) antibody (Miltenyi Biotec), PE-labeled mouse anti-human CD166.
- colon CSCs were incubated with mouse anti-human Lgr5 antibody (2T15E-2) and then with PR-labeled rat anti-mouse IgG antibody (Invitrogen). Aldehyde dehydrogenase activity was measured using AldeFluor® Kit (Stemcell® Technologies). Mouse cells and human colon CSCs were distinguished by staining with anti-mouse MHC class I antibody (Abcam) and PE or APC-labeled goat anti-human IgG2a antibody (BioLegend). Dead cells were also removed with 7-AAD Viability Dye (Beckman Coulter).
- Proteins were extracted using RIPA buffer (Sigma) supplemented with Complete Mini protease inhibitor cocktail (Roche). Proteins were fractionated on NuPAGE gel (Invitrogen) and transferred to PVDF membrane. After blocking with PBS containing 1% skim milk, the membrane was subjected to rabbit anti-human ⁇ -catenin antibody (Sigma), rabbit anti-human phospho c-JUN antibody (Sigma), rabbit anti-human TCF1 antibody (Cell Signaling), rabbit anti-human TCF3 antibody ( Cell Signaling), rabbit anti-human TCF4 antibody (Cell Signaling), rabbit anti-human Lgr5 antibody (Abcam), mouse anti-human E-cadherin antibody (Abcam), rabbit anti-human Snail antibody (Abcam), and mouse anti-human GAPDH antibody ( Probed with Santa Cruz). Reactive bands were detected using BCIP / NBT substrate (KPL).
- Lgr5 Forward primer 5′-AGTTTATCCTTCTGGTGGTAGTCC-3 ′ (SEQ ID NO: 10), Reverse primer 5′-CAAGATGTAGAGAAGGGGATTGA-3 ′ (SEQ ID NO: 11), GAPDH: Forward primer 5′-CTCTGCTCCTCCTGTTCGAC-3 ′ (SEQ ID NO: 12), Reverse primer 5′-ACGACCAAATCCGTTGACTC-3 ′ (SEQ ID NO: 13), ACTB: Forward primer 5′-AAGTCCCTTGCCATCCTAAAA-3 ′ (SEQ ID NO: 14), Reverse primer 5'-ATGCTATCACCTCCCCTGTG-3 '(SEQ ID NO: 15)
- Floating CSCs and adherent CSCs were seeded in 96-well plates at approximately 100 floating CSCs and 1 ⁇ 10 4 adherent CSCs per well, respectively. On days 0 and 3, viable cell counts were determined by Cell Counting Kit-8 assay (Doujindo) according to the manufacturer's protocol. The average absorbance on day 0 was expressed as 100%.
- floating CSCs and adherent CSCs were seeded in 96-well plates at approximately 100 floating CSCs and 4 adherent CSCs per well, respectively, and incubated for 24 hours, followed by 10 ⁇ g / mL 5-FU.
- Immunofluorescent staining for cultured cells and xenograft tissues For immunofluorescent cytochemistry, cells fixed with 4% paraformaldehyde and methanol were combined with mouse anti-human E-cadherin antibody (Abcam), rabbit anti-human Snail antibody (Abcam), Alternatively, incubation with a rabbit anti-human ⁇ -catenin antibody (Sigma) followed by visualization with AlexaFluor 488 labeled goat anti-mouse IgG antibody or goat anti-rabbit IgG antibody, respectively.
- Lgr5 protein was detected by goat anti-mouse antibody conjugated with polymer-HRP (DAKO) and visualized with AlexaFluor 488-labeled tyramide (Invitrogen). Snail protein was detected with biotinylated goat anti-rabbit antibody (VECTOR) and visualized with AlexaFluor 568 labeled streptavidin (Invitrogen). These cells and specimens were also stained with DAPI (Invitrogen).
- Example 1 Establishment of colorectal cancer xenografts Previous report (Fujii E. et al. (2008) Establishment and characterization of in vivo human tumor models in the NOD / SCID / gamma (c) (null) mouse. Pathol Int 58 : 559-567.) The present inventors established 11 human colorectal cancer xenografts using NOD / Shi-scid, IL-2R ⁇ null (NOG) mice (Table 1; immunodeficient NOG) Number of human colon cancer cell lines established in mice).
- 11 xenografts 10 were from grade 2 moderately differentiated adenocarcinoma and one was from grade 3 poorly differentiated adenocarcinoma.
- Ten of the 11 xenografts were from moderately differentiated colorectal cancer (MDCC) and the other was from poorly differentiated colorectal cancer (PDCC) (Table 2; 11 xenografts) Histopathological classification of the original human colorectal cancer used to establish the piece).
- MDCC moderately differentiated colorectal cancer
- PDCC poorly differentiated colorectal cancer
- MDCC xenografts and PDCC xenografts reconstructed almost the same histopathological morphology as the original tumor, but MDCC xenografts had distinct epithelial ducts with goblet cells and small budding clusters (It seems to undergo an epithelial to mesenchymal transition (EMT)). In contrast, PDCC xenografts did not show a clear epithelial tube structure (FIGS. 1 and 16).
- Example 2 Isolation of large intestine CSCs
- PLR59 and PLR123 Two types of MDCC xenografts, PLR59 and PLR123, to isolate large intestine CSCs. These xenografts were selected by the inventors because of their ability to reconstruct tumors that grow rapidly while having epithelial tracts and small budding clusters, even after 10 passages in NOG mice. (FIG. 1). Therefore, it was considered that stable CSCs can be obtained from these xenografts.
- asterisks indicate tumor xenografts established with NOG mice, and dagger marks indicate cell preparations.
- Primary refers to cells prepared by removing erythrocytes and mouse cells after collecting xenograft tumor tissue grown in NOG mice (primary cells).
- Floating refers to conditions in which primary cells are not attached.
- a cell cultured in vitro below is shown, and adhesion means a cell cultured in vitro under adhesion conditions.
- the plus sign (1) is the number of tumors formed and the plus sign (2) is the total number of inoculated sites. Parentheses indicate the percentage of tumor formation (carcinogenesis).
- LGR5 + indicates LGR5 positive, LGR5 - and shows the LGR5 negative.
- adherent and floating cells became highly uniform, as determined by colon CSC markers, and adherent cells were Lgr5 + , ALDH + , CD133 + , CD44 + , EpCAM + , CD166 + , CD24 + , CD26 + , and CD29 + .
- adherent cells were Lgr5 + , ALDH + , CD133 + , CD44 + , EpCAM + , CD166 + , CD24 + , CD26 + , and CD29 + .
- the floating cells were different from the adherent cells because they were Lgr5 ⁇ and ALDH ⁇ (FIGS. 6 and 20). Significant levels of Lgr5 mRNA were detected in adherent cells, but Lgr5 mRNA in floating cells was undetectable (FIG. 27).
- Lgr5 protein expression analysis In order to examine the expression of Lgr5 protein, the present inventors analyzed two types of Lgr5-specific monoclonal antibodies (2L36, 2T15E-) for immunohistochemical analysis and flow cytometry analysis, respectively. 2 and 2U2E-2) were prepared. Our antibodies were highly specific for Lgr5 but did not cross-react with Lgr4 and Lgr6, both of which have high homology to Lgr5 (FIGS. 28, 29). By using these antibodies, the inventors have demonstrated that Lgr5 is expressed on adherent CSCs.
- Lgr5-positive cells were detected through passage of the tumor tissue that was the source of PLR59 and PLR123 and their xenograft cancer tissue (FIG. 39).
- the frequency of Lgr5-positive cells in the original tumor tissue was low (0.01% for PLR59 and 0.04% for PLR123).
- the frequency of Lgr5-positive cells increased with passage, but there was no change after the 10th generation (FIG. 39).
- the ability of primary cells from the PLR123 xenograft model to reconstruct tumors also increased with passage.
- Estimated percentage of CSC in primary cells assessed from tumor remodeling ability was approximately 0.1% after passage 5, compared to approximately 0.4% at passage 14.
- Example 4 Tumor restructuring ability of Lgr5-positive and Lgr5-negative colon CSCs If the characteristic of the stem cell group of colon cancer is Wnt signaling, only Lgr5-positive adherent cells can form tumors in vivo. To confirm whether this is true, the inventors examined the tumorigenic potential of Lgr5-positive adherent cells and Lgr5-negative floating cells.
- Lgr5-positive adherent cells As a result, tumor-forming ability was stronger in Lgr5-positive adherent cells than Lgr5-negative floating cells, but both Lgr5-positive cells and Lgr5-negative cells retained tumor-forming ability in NOG mice.
- Subcutaneous injection of 10 Lgr5-positive cells resulted in tumors at all injection sites (6 out of 6), but Lgr5-negative cells had 2 out of 6 injection sites (PLR123-derived cells) or 1 (PLR59) Tumors were formed (derived cells) (Table 3).
- Lgr5-positive cells are reconstructed in 2 out of 12 injection sites (PLR123-derived cells) or 1 (PLR59-derived cells) even when only 1 cell is injected per inoculation site ( FIG.
- Lgr5-positive cells divided symmetrically under the conditions of adherent culture (FIG. 41).
- asymmetric cell division in the presence of Matrigel and serum was shown by Lgr5 protein partitioning into one of the two daughter cells in culture under the same conditions ( Figure 42C and D).
- One characteristic of CSC is symmetric cell division for self-renewal, and another prominent feature of CSC is non-target cell division.
- Lgr5-positive adherent cells divided symmetrically under adherent culture conditions (FIG. 41), while Lgr5 protein was distributed to one daughter cell in the presence of Matrigel and FBS (FIG. 42). It was shown that Lgr5-positive cells produced two different progeny by undergoing asymmetric division.
- Lgr5-positive and Lgr5-negative cells derived from PLR59 and PLR123 are high-purity colorectal CSCs, and Lgr5-positive and Lgr5-negative cells represent two distinct states of CSC in colorectal cancer Proved to be.
- Example 5 Effects of TCF and ⁇ -catenin Consistent with the expression of Lgr5, the levels of ⁇ -catenin, TCF1, TCF3, and TCF4 proteins were up-regulated in Lgr5-positive cells, but were observed in Lgr5-negative cells. None (FIGS. 7 and 21). On the other hand, phosphorylation of the N-terminal region of c-Jun was not detected in Lgr5-positive CSCs compared to Lgr5-negative CSCs (FIGS. 7 and 21).
- 50 ⁇ M FH535 significantly reduced the growth of Lgr5-positive colon CSCs, but did not affect the growth of Lgr5-negative colon CSCs (FIGS. 8 and 22).
- 50 ⁇ M cardamonin reduced the number of viable cells to 70% in Lgr5-positive colon CSC and to about 50% in Lgr5-negative colon CSC (FIGS. 8 and 22).
- TCF mediates the growth of Lgr5-positive cells and that ⁇ -catenin is involved in the survival of colon CSCs.
- Lgr5-positive cells proliferate without EGF and FGF supply (FIGS. 9 and 23), indicating that colon CSCs are endogenous / self responsible for activating Wnt signaling for their proliferation. It indicates that it includes a secretory mechanism.
- Example 6 The ability of colorectal CSCs to change from an Lgr5-positive state to an Lgr5-negative state Since one of the features of CSC is resistance to chemotherapeutic agents, the present inventors have introduced the large intestine against 5-FU and irinotecan. The sensitivity of CSC was examined. As described above, Lgr5-positive cells proliferated at a doubling time of about 2.5 days, while Lgr5-negative CSCs were quiescent from the viewpoint of proliferation.
- HLA-DMA, TMEM173, ZMAT3 and GPR110 were selected as markers for specifically detecting these Lgr5-negative normal CSCs, and immunostaining with specific antibodies against the same molecule was attempted. Staining images specific for colon CSCs that were exposed to the sun and negative for Lgr5 were obtained (FIG. 43). It was also confirmed that this immunostaining method can be applied to tissue sections obtained from generally used paraffin blocks (FIG. 43). From the above, it is shown that HLA-DMA, TMEM173, ZMAT3 and GPR110 can be specific markers of CSC in which Lgr5 is negative.
- tumors were formed in 2 and 1 mice in NOG mice by subcutaneous injection of 10 cells derived from PLR59 and PLR123, respectively (Table 4). ).
- Table 4 shows the tumorigenic activity of Lgr5-negative CSC 49 hours after inoculation.
- asterisks indicate tumor xenografts established with NOG mice. Plus (1) is the number of animals that showed a tumor, plus (2) is the total number of animals.
- the present inventors In order to confirm whether the Lgr5-negative colon CSC is changed to the Lgr5-positive state, the present inventors also performed adherent culture of the Lgr5-negative colon CSC prepared by irinotecan treatment again with a serum-free stem cell culture medium. It became Lgr5-positive, showed mesenchymal cell-like morphology (FIGS. 12 and 33), and started cell proliferation. On the other hand, when Lgr5-positive adherent colon CSCs were cultured on an ultra-low adhesion plate, the present inventors showed that some of the cells stopped growing, formed spheroid-like structures, and showed very low levels of Lgr5 mRNA. This was observed (FIGS. 12 and 33).
- the change from the Lgr5-positive state to the Lgr5-negative state (and vice versa) was also confirmed by observation with single cells in culture.
- a single Lgr5-positive cell was cultured in a multiwell plate, the cell shifted to an Lgr5-negative state within 3 days after irinotecan treatment.
- single Lgr5-negative cells obtained by irinotecan treatment were cultured in the absence of irinotecan in a multiwell plate, 19 to 43% of cells shifted to Lgr5-positive state within 4 days (FIG. 49 and Table). 5).
- Table 5 shows the ratio of the number of Lgr5 positive and negative cells stained as a result of immune cell staining using an anti-Lgr5 antibody (2L36 antibody). Numbers in parentheses indicate the percentage of Lgr5-positive or negative cells.
- colon CSCs alternate between Lgr5-positive and Lgr5-negative states, and that such changes do not require exogenous elements and niche environments.
- Example 7 In vitro and in vivo EMT of Lgr5-positive colon CSC Mesenchymal-like cells expressing nuclear ⁇ -catenin are thought to be mobile and metastatic CSCs that undergo EMT (Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T (2005) Opinion : migrating cancer stem cells-an integrated concept of malignant tumour progression. Nat Rev Cancer 5: 744-749.). Since the morphology of Lgr5-positive colon CSC resembles mesenchymal cells, the present inventors tested whether Lgr5-positive colon CSC corresponds to mobile CSC.
- Lgr5-positive large intestine CSCs form tumors in a plurality of tissues including lung, liver, lymph node, and subcutaneous.
- tumors with epithelial ductal structures were reconstructed in the liver, lymph nodes, and subcutaneous, but not in the lung (FIGS. 34, 35). ).
- Lgr5-negative CSCs directly generate a cancer hierarchy or first converted to Lgr5-positive cells in vivo.
- gene expression profiling was performed using Lgr5-positive cells, Lgr5-negative cells and xenograft tumor primary cells.
- HLA-DMA was selected from molecules that could be detected at a higher level in Lgr5-negative CSCs than in Lgr5-positive CSCs and initial cells (FIG. 50).
- Tissue immunostaining using Lgr5 antibody, HLA-DMA antibody and EREG antibody confirmed that HLA-DMA was specifically expressed in Lgr5-negative CSC (FIG. 51).
- HLA-DMA is also expressed in macrophages
- another marker expressed in CSC was also explored to eliminate the possibility that cells stained in tissue immunostaining with HLA-DMA antibodies are macrophages.
- EREG is expressed in both Lgr5-positive CSC and Lgr5-negative CSC by immunohistochemical staining of Lgr5-positive CSC and negative CSC using an antibody against EREG expressed in both Lgr5-positive CSC and negative CSC (FIG. 50). It was confirmed (FIG. 51). By detecting both markers in combination, it was confirmed that Lgr5-negative CSCs could be identified as cells positive for both HLA-DMA and EREG.
- Lgr-negative CSCs After a uniform population of Lgr-negative CSCs were injected into NOG mice, cells that slightly expressed Lgr5 but were still positive for HLA-DMA and EREG appeared for one day. By day 5 after the injection, HLA-DMA negative, Lgr5-positive and EREG-positive cells appeared (FIG. 52). Tumors derived from Lgr5-negative CSCs clearly had a tubular structure and contained Lgr5-positive cells (FIG. 53).
- Lgr5-positive cells In contrast, in control mice treated with vehicle, approximately 1/3 of the cancer cells in both the tubule and budding areas were Lgr5-positive (FIG. 54). Both Lgr5-positive cells and HLA-DMA positive and Lgr5-negative cells were EREG positive, and these were confirmed to be CSC (FIG. 54). After the irinotecan treatment, Lgr5-positive cells reappeared (FIG. 54). Combining these results, it was shown that Lgr5-negative CSCs originated from colorectal cancer after growth inhibitor treatment and reconstructed the cancer hierarchy via Lgr5-positive cells.
- Example 8 Identification of molecules specifically expressed in cancer stem cells Preparation of Lgr5-negative adherent cells by irinotecan treatment Lgr5-positive adherent cells were seeded in 6-well plates (BD, Cat. No. 353046) with 3 ⁇ 10 5 cells using stem cell medium. On the next day, irinotecan (Hospira, 61703-349-09) was added to the cells at a final concentration of 10 ⁇ g / mL, and the culture was continued. After 3 days of culture, cells resistant to irinotecan were observed.
- irinotecan Hospira, 61703-349-09
- mice mAb to human CD326 EpCAM
- AC133 PE labeled mouse mAb to human CD44
- PE labeled mouse mAb to human CD166 PE labeled mouse mAb to human CD24
- PE labeled mouse mAb to human CD26 PE labeled mouse mAb to human CD26
- PE labeled mouse mAb to human CD29 PE labeled mouse mAb to human CD29
- gene expression information was obtained using Affymetrix GeneChip (HG-U133 plus2). Data analysis was performed using Microsoft Excel and statistical software R (Statistics softwere R). Three types of cells (primary cells, Lgr5-positive cells, and Lgr5-negative cells) were compared with each other, and a list of genes whose expression was significantly increased individually was created. That is, GeneChip's raw data is standardized by GCRMA and converted to log with 2 as the bottom, and between different sample types (primary cells and Lgr5-positive cells, Lgr5-positive cells and Lgr5-negative cells, Lgr5-negative cells and primary cells) ) To calculate the expression difference. The following three types were used as criteria for extracting expression variable genes.
- a gene having GO: 0005886 [plasma membrane] was extracted in GeneOntology (GO). Furthermore, a gene that has GO: 0005576 [extracellular region], GO: 0009986 [cell surface], GO: 0016020 [membrane], or a transmembrane region predicted by the membrane protein prediction software TMHMM, predicts a signal peptide A gene that was predicted to have a signal peptide by soft SignalP and did not have GO: 0031090 [organelle membrane] was extracted. GeneChip data derived from normal colon tissue was also used to exclude genes with relatively high expression in normal tissues and primary cells, and genes with low rates of change in Lgr5-positive and Lgr5-negative cells.
- Table 6-2 is a continuation table of Table 6-1.
- Table 6-3 is a continuation table of Table 6-2.
- Table 6-4 is a continuation table of Table 6-3.
- Table 6-5 is a continuation table of Table 6-4.
- Table 6-6 is a continuation table of Table 6-5.
- Table 6-7 is a continuation of Table 6-6.
- Table 6-8 is a continuation table of Table 6-7.
- Table 6-9 is a continuation table of Table 6-8.
- Table 6-10 is a continuation of Table 6-9. (Numerical values in Tables 6-1 to 6-10 above show difference in expression (log2 ratio))
- Table 7-2 is a continuation table of Table 7-1.
- Table 7-3 is a continuation table of Table 7-2.
- Table 7-4 is a continuation table of Table 7-3.
- Table 7-5 is a continuation table of Table 7-4. (The numerical values in Tables 7-1 to 7-5 above show the difference in expression (log2 ratio))
- Table 8-2 is a continuation table of Table 8-1. (The numerical values in Tables 8-1 to 8-2 above show the difference in expression (log2 ratio))
- Lgr5-negative cells and Lgr5-positive cells have an average expression value greater than 64, and there are more than 4-fold changes in both Lgr5-negative cells and Lgr5-positive cells compared to primary cells, and t Genes for which a significant difference is observed by -test (Table 9).
- Lgr5-negative cells have an average expression value greater than 64
- primary cells and Lgr5-positive cells both have an average expression value of less than 64
- Lgr5-positive cells have an increase of more than 20 times in Lgr5-negative cells Genes that are present and have a significant difference in t-test (Table 10).
- FIG. 37 shows the results of flow cytometry analysis of EREG in PLR59 and PLR123 primary cells, Lgr5 + cancer stem cells, and Lgr5 ⁇ cancer stem cells.
- Cells were stained with an antibody against EREG and analyzed by flow cytometry. It was shown that there is a homogeneous cell population of EREG negative in primary cells, EREG positive in Lgr5 + cancer stem cells and Lgr5 ⁇ cancer stem cells. Gray indicates the fluorescence intensity after staining the cells with the described antibody, and white indicates the fluorescence intensity after staining the cells with the control isotype antibody.
- target cell suspension was prepared at the time of use at the time of the test. Centrifuge 1 x 10 6 cancer cell lines (1200 rpm, 5 min, room temperature) and add 200 ⁇ L of 0.2 mg / mL calcein-AM (Nacalai Tesque) / DMEM (10% FBS) medium to the cell pellet And suspended. Cell solution suspended with calcein -AM solution 5% CO 2 for 2 hours at a CO 2 incubator set at a temperature 37 ° C.. After washing once with 10% FBS / D-MEM, the cell density was adjusted to 2 ⁇ 10 5 / mL with 10% FBS / D-MEM to obtain a target cell suspension.
- Anti-EREG antibody was prepared at a concentration of 0.5 mg / mL, and further diluted with 10% FBS / D-MEM to prepare an antibody solution. Final concentrations were 0.4 ⁇ g / mL, 4 ⁇ g / mL, and 40 ⁇ g / mL. 50 ⁇ L of each concentration of antibody solution or 10% FBS / D-MEM was injected into a 96-well U-bottom plate. Next, 50 ⁇ L of the target cell suspension was added to all wells and allowed to stand at room temperature for 15 minutes.
- cytotoxicity (%) (AC) x 100 / (BC)
- A is the fluorescence intensity in each well
- B is the average fluorescence intensity in wells with 50 ⁇ L of 10% FBS / D-MEM and 50 ⁇ L of target cell suspension and 100 ⁇ L of NP-40 solution
- C is 50 ⁇ L 10% FBS Shown is the average fluorescence intensity in wells in which 50 ⁇ L of target cell suspension and 100 ⁇ L of 10% FBS / D-MEM are added to / D-MEM.
- Fig. 38 shows ADCC activity of PLR59 cells against Lgr5-positive cells and Lgr5-negative cells and PLR123 cells against Lgr5-positive cells and Lgr5-negative cells by anti-EREG antibody.
- EREG Lgr5-positive cells were administered into the peritoneal cavity of NOG mice.
- EREG was highly expressed in the early stage of tumor formation, but the tumor forms a clear tubular structure In the late stage, EREG expression was somewhat confined to budding clusters compared to the tubule structure.
- EREG positive cells were detected even after irinotecan was administered to mice bearing tumors (FIG. 54). Therefore, the antitumor activity of the EREG antibody after irinotecan treatment was examined. Since EREG antibody requires effector cells to exert ADCC activity, SCID mice were used as a model to be used for evaluation of drug efficacy of EREG antibody. Tumor growth was inhibited when the antibody was administered at 4 and 11 days after the last dose of irinotecan (FIG. 57).
- EREG expression in the metastasis model was first examined.
- Lgr5-positive cells were injected intravenously into NOG mice, tumors formed in multiple tissues including the lung. Most of the tumor cells formed in the lung were positive for EREG (FIG. 58A).
- the efficacy of EREG antibody was examined using SCID-Beige mice in which macrophages and monocytes can exert ADCC as effector cells.
- the number of tumor cells at the remote site was compared with that in the control mice in mice administered EREG antibody 5 times once a week starting 3 days later. (Fig. 58B).
- the size of each tumor was also shown to be extremely reduced in mice that received antibody (FIGS. 58C and D).
- Example 9 Presence of Lgr5-negative and positive CSCs in clinical tumor specimens Proliferating and resting CSCs were determined by tissue immunostaining using Lgr5 antibody (2U2E-2), HLA-DMA antibody and EREG antibody ( FIG. 59 and Table 11). Proliferative CSCs represent Lgr5-positive cells, and resting CSCs represent HLA-DMA positive and EREG positive cells (Table 11). Lgr5-positive cells that are both positive for HLA-DMA and EREG, and Lgr5-negative cells that are both positive for HLA-DMA and EREG are primary (primary) and metastatic from isolated colon cancer patients A small number of colon cancer specimens were present (FIG. 59).
- Lgr5-positive and negative CSCs were detected in the tube and budding area (FIG. 59).
- Lgr5-positive and negative CSCs in the tube were not observed in specific areas, but were randomly observed throughout the tube. *
- Example 10 Anti-tumor effect by various antibodies using Mab-ZAP and Rat-ZAP Targeted treatment targeting membrane proteins that are highly expressed in irinotecan-treated or untreated irinotecan PLR59 and PLR123 It was evaluated whether or not an antitumor effect could be exhibited.
- the binding activity of the commercially available antibodies listed in Table 12 to the antigens treated with irinotecan or expressed on the cell surface of irinotecan-untreated PLR59 or PLR123 was measured using flow cytometry (FCM). The results are summarized in Table 13.
- Mab-ZAP and Rat-ZAP are anti-mouse IgG antibody or anti-rat IgG antibody combined with saporin, a toxin that inhibits protein synthesis (manufactured by Advanced Targeting Systems).
- PLR59, PLR123 cells are seeded in each well of a 96-well plate at a cell density of 30000 cells / 80 ⁇ L / well, the final concentration of 0.01, Various antibody solutions were added at 0.1 and 1 ⁇ g / mL.
- a plate with Mab-ZAP or Rat-ZAP added to each well to a final concentration of 1 ⁇ g / mL was cultured in a CO 2 incubator at 37 ° C. for 72 hours.
- PLR59, PLR123 cells and 96-well plates with irinotecan added to each well at a final concentration of 15 ⁇ M are cultured for 72 hours in a 37 ° C CO 2 incubator It was done.
- the internalization activity of various antibodies against cells cultured in the presence or absence of irinotecan was evaluated.
- the internalization activity of various antibodies against cells present in each well replaced with a medium containing no irinotecan was evaluated as in the case of irinotecan untreated.
- 72 hours after addition of antibody, Mab-ZAP, and Rat-ZAP, 3% SDS (Nacalai Tesque) was added to each well at a dose of 10 ⁇ L / well.
- the plate was thoroughly dissolved by agitating. Thereafter, the luminescence signal of the mixed solution in each well to which CellTiter-Glo (registered trademark) Luminescent Cell Viability Assay (Promega) was added at a dose of 100 ⁇ L / well was measured.
- the resulting antitumor activity is shown in Table 14 and FIGS.
- the percentage of cell growth inhibition shown on the vertical axis in FIGS. 60 to 72 is the value of the luminescence signal of the mixed solution in the well to which only the test antibody was added (without adding Mab-ZAP and Rat-ZAP).
- the difference from the value of the luminescence signal of the mixture of wells not seeded with cells is taken as 100% (in the absence of Mab-ZAP and Rat-ZAP), in the wells to which only the test antibody was added It means the relative value of the difference between the value of the luminescence signal of the mixture and the value of the luminescence signal of the mixture in the well to which the test antibody, Mab-ZAP, and Rat-ZAP were added.
- the symbols-, +, ++ and +++ in Table 14 represent the relative values of internalization activity when the test antibody was tested at a concentration of 1 ⁇ g / ⁇ L.
- the relative value is the value of the luminescence signal of the mixture in the well to which only the test antibody is added (without adding Mab-ZAP and Rat-ZAP), and the luminescence of the mixture in the well where cells are not seeded.
- the value of the luminescence signal of the mixed solution in the well to which only the test antibody was added (without adding Mab-ZAP and Rat-ZAP), the test antibody, This means the relative value of the difference in the luminescence signal value of the mixed solution in the well to which Mab-ZAP and Rat-ZAP were added.
- -, +, ++ and +++ are each 5% Less than 5% or more and less than 15%, 15% or more and less than 25%, and 25% or more.
- anti-CD70 antibody and anti-FAS antibody were compared with irinotecan-untreated PLR59 and PLR123 under the condition that sufficient antitumor activity was confirmed with the anti-EPCAM antibody used as a positive control. More than 25% of internalization activity was shown (FIGS. 60 and 62). Also, against PLR59, the anti-EDAR antibody showed 15-25% internalization activity, and the anti-PVRL4 antibody and anti-PROCR antibody showed 5-15% internalization activity (FIGS. 61, 63 and 65).
- anti-FAS antibody and anti-TNFRSF9 antibody showed 25% or more internalization (internalization) activity of anti-PROM2 antibody (FIG. 67). , 70 and 68).
- Both the anti-PVRL4 antibody and the anti-PROCR antibody showed internalization activity against PLR59 and PLR123 treated with irinotecan, and the internalization activity against PLR59 was higher than that against PLR123 (FIGS. 69 and 71). From the above results, it was revealed that all the antibodies evaluated exhibited antitumor effects against PLR59 and PLR123.
- the differentiation promoting effect by BMP4 on PLR59 and PLR123 not treated with irinotecan and treated with irinotecan was evaluated.
- 5 ⁇ 10 5 cells / 1.5 mL / mL of PLR59 and PLR123 cells suspended in a medium supplemented with BMP4 (R & D Systems, final concentration 20 nM) or control buffer Each well of a 12-well plate was seeded at the cell density of the well. Subcultured by changing to the same medium 2, 4, and 7 days after seeding.
- a 12.5 mL culture flask seeded with PLR59 or PLR123 at a cell density of 17 x 10 5 cells / 5 mL / flask was given a final concentration of 15 ⁇ M the day after seeding.
- Irinotecan was added.
- the flask was incubated for 72 hours in a 37 ° C. CO 2 incubator. Subsequently, the medium in the flask was replaced with a medium to which BMP4 or control buffer was added, and the medium was further replaced with the same medium 2, 4, and 7 days after the replacement.
- RNA extracted from cells isolated 4 and 9 days after the first medium change using RNeasy Plus Mini Kit and RNase-Free DNase Set (QIAGEN) as a template RNA extracted from cells isolated 4 and 9 days after the first medium change using RNeasy Plus Mini Kit and RNase-Free DNase Set (QIAGEN) as a template.
- ThermoScript RT-PCR System CDNA was synthesized using Invitrogen.
- cell surface molecules that are specifically expressed in cancer stem cells have been identified, and new anticancer agents and cancer stem cell detection reagents have been provided by using antibodies against the molecules.
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Immunology (AREA)
- Organic Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Medicinal Chemistry (AREA)
- Molecular Biology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biochemistry (AREA)
- Genetics & Genomics (AREA)
- Oncology (AREA)
- Microbiology (AREA)
- Biophysics (AREA)
- Cell Biology (AREA)
- Analytical Chemistry (AREA)
- Zoology (AREA)
- Biomedical Technology (AREA)
- Pathology (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Urology & Nephrology (AREA)
- Hematology (AREA)
- Biotechnology (AREA)
- Physics & Mathematics (AREA)
- Wood Science & Technology (AREA)
- Hospice & Palliative Care (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Epidemiology (AREA)
- General Physics & Mathematics (AREA)
- Food Science & Technology (AREA)
- Mycology (AREA)
- General Engineering & Computer Science (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Gastroenterology & Hepatology (AREA)
Priority Applications (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US14/354,517 US20140302511A1 (en) | 2011-10-28 | 2012-10-26 | Cancer stem cell-specific molecule |
| EP19191052.0A EP3603671A3 (en) | 2011-10-28 | 2012-10-26 | Cancer stem cell-specific molecule |
| JP2013540843A JP6291254B2 (ja) | 2011-10-28 | 2012-10-26 | 癌幹細胞特異的分子 |
| EP12844389.2A EP2772268B8 (en) | 2011-10-28 | 2012-10-26 | Cancer stem cell-specific molecule |
| US15/042,548 US10934351B2 (en) | 2011-10-28 | 2016-02-12 | Cancer stem cell-specific molecule |
| US16/913,341 US11858987B2 (en) | 2011-10-28 | 2020-06-26 | Cancer stem cell-specific molecule |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2011-237438 | 2011-10-28 | ||
| JP2011237438 | 2011-10-28 | ||
| JP2012091142 | 2012-04-12 | ||
| JP2012-091142 | 2012-04-12 |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US14/354,517 A-371-Of-International US20140302511A1 (en) | 2011-10-28 | 2012-10-26 | Cancer stem cell-specific molecule |
| US15/042,548 Continuation US10934351B2 (en) | 2011-10-28 | 2016-02-12 | Cancer stem cell-specific molecule |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2013062083A1 true WO2013062083A1 (ja) | 2013-05-02 |
Family
ID=48167907
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2012/077714 Ceased WO2013062083A1 (ja) | 2011-10-28 | 2012-10-26 | 癌幹細胞特異的分子 |
Country Status (4)
| Country | Link |
|---|---|
| US (3) | US20140302511A1 (enExample) |
| EP (2) | EP3603671A3 (enExample) |
| JP (7) | JP6291254B2 (enExample) |
| WO (1) | WO2013062083A1 (enExample) |
Cited By (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016028569A (ja) * | 2014-07-16 | 2016-03-03 | 学校法人慶應義塾 | がん幹細胞集団の調製方法、異種移植片の調製方法、スクリーニング方法、miR−34aの発現量を低下させる方法及びがん幹細胞増殖抑制剤 |
| KR101779147B1 (ko) * | 2014-07-04 | 2017-09-20 | 연세대학교 산학협력단 | Kiaa1199를 이용한 췌장암의 진단 또는 치료용 조성물 |
| JP2017528114A (ja) * | 2014-06-25 | 2017-09-28 | テル ハショマー メディカル リサーチ インフラストラクチャー アンド サーヴィシーズ リミテッド | がん幹細胞の同定ならびに診断および処置のためのその使用 |
| US10018630B2 (en) | 2011-09-07 | 2018-07-10 | Chugai Seiyaku Kabushiki Kaisha | Cancer stem cell isolation |
| CN110546258A (zh) * | 2017-03-28 | 2019-12-06 | 学校法人庆应义塾 | 人体组织干细胞及其使用 |
| JPWO2020032162A1 (ja) * | 2018-08-08 | 2021-08-12 | 中外製薬株式会社 | がん組織またはがん組織に類似した組織の培養方法 |
| US11124773B2 (en) | 2010-10-06 | 2021-09-21 | Chugai Seiyaku Kabushiki Kaisha | Cancer stem cell population and method for production thereof |
| US11536713B2 (en) | 2009-12-25 | 2022-12-27 | Chugai Seiyaku Kabushiki Kaisha | Method for searching and screening for target of anti-cancer agent using non-human animal model having NOG established cancer cell line transplanted therein |
Families Citing this family (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20120141501A1 (en) | 2009-05-29 | 2012-06-07 | Forerunner Pharma Research Co. Ltd | Pharmaceutical Composition Containing Antagonist of EGF Family Ligand as Component |
| KR102049122B1 (ko) | 2011-06-30 | 2019-11-26 | 추가이 세이야쿠 가부시키가이샤 | 헤테로이량화 폴리펩티드 |
| WO2013062083A1 (ja) | 2011-10-28 | 2013-05-02 | ファーマロジカルズ・リサーチ プライベート リミテッド | 癌幹細胞特異的分子 |
| EP2817620B1 (en) * | 2012-02-22 | 2016-06-01 | Verik Bio, Inc. | A system for immunotherapy targeting tumor propagation and progression |
| WO2014104165A1 (ja) | 2012-12-27 | 2014-07-03 | 中外製薬株式会社 | ヘテロ二量化ポリペプチド |
| EP3015115A4 (en) * | 2013-06-24 | 2017-02-22 | Chugai Seiyaku Kabushiki Kaisha | Therapeutic agent comprising humanized anti-epiregulin antibody as active ingredient for non-small-cell lung carcinoma excluding adenocarcinoma |
| WO2017112811A1 (en) * | 2015-12-22 | 2017-06-29 | Abbvie Stemcentrx Llc | Novel anti-tnfsf9 antibodies and methods of use |
| JP7735291B2 (ja) | 2020-02-21 | 2025-09-08 | エーアールエス ファーマシューティカルズ、インコーポレイテッド | ネクチン-4抗体コンジュゲートおよびその使用 |
Citations (37)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0125023A1 (en) | 1983-04-08 | 1984-11-14 | Genentech, Inc. | Recombinant immunoglobulin preparations, methods for their preparation, DNA sequences, expression vectors and recombinant host cells therefor |
| JPH0159878B2 (enExample) | 1982-05-21 | 1989-12-20 | Yunibaashitei Obu Karifuorunia | |
| EP0404097A2 (de) | 1989-06-22 | 1990-12-27 | BEHRINGWERKE Aktiengesellschaft | Bispezifische und oligospezifische, mono- und oligovalente Rezeptoren, ihre Herstellung und Verwendung |
| WO1992001047A1 (en) | 1990-07-10 | 1992-01-23 | Cambridge Antibody Technology Limited | Methods for producing members of specific binding pairs |
| WO1992003918A1 (en) | 1990-08-29 | 1992-03-19 | Genpharm International, Inc. | Transgenic non-human animals capable of producing heterologous antibodies |
| WO1992020791A1 (en) | 1990-07-10 | 1992-11-26 | Cambridge Antibody Technology Limited | Methods for producing members of specific binding pairs |
| WO1993006213A1 (en) | 1991-09-23 | 1993-04-01 | Medical Research Council | Production of chimeric antibodies - a combinatorial approach |
| WO1993011236A1 (en) | 1991-12-02 | 1993-06-10 | Medical Research Council | Production of anti-self antibodies from antibody segment repertoires and displayed on phage |
| WO1993011161A1 (en) | 1991-11-25 | 1993-06-10 | Enzon, Inc. | Multivalent antigen-binding proteins |
| WO1993012227A1 (en) | 1991-12-17 | 1993-06-24 | Genpharm International, Inc. | Transgenic non-human animals capable of producing heterologous antibodies |
| WO1993019172A1 (en) | 1992-03-24 | 1993-09-30 | Cambridge Antibody Technology Limited | Methods for producing members of specific binding pairs |
| WO1994002602A1 (en) | 1992-07-24 | 1994-02-03 | Cell Genesys, Inc. | Generation of xenogeneic antibodies |
| WO1994011523A2 (en) | 1992-11-13 | 1994-05-26 | Idec Pharmaceuticals Corporation | Fully impaired consensus kozac sequences for mammalian expression |
| WO1994025585A1 (en) | 1993-04-26 | 1994-11-10 | Genpharm International, Inc. | Transgenic non-human animals capable of producing heterologous antibodies |
| WO1995001438A1 (en) | 1993-06-30 | 1995-01-12 | Medical Research Council | Sbp members with a chemical moiety covalently bound within the binding site; production and selection thereof |
| WO1995015388A1 (en) | 1993-12-03 | 1995-06-08 | Medical Research Council | Recombinant binding proteins and peptides |
| WO1996002576A1 (en) | 1994-07-13 | 1996-02-01 | Chugai Seiyaku Kabushiki Kaisha | Reconstituted human antibody against human interleukin-8 |
| WO1998013388A1 (en) | 1996-09-26 | 1998-04-02 | Chugai Seiyaku Kabushiki Kaisha | Antibody against human parathormone related peptides |
| WO1999054342A1 (en) | 1998-04-20 | 1999-10-28 | Pablo Umana | Glycosylation engineering of antibodies for improving antibody-dependent cellular cytotoxicity |
| WO2000061739A1 (fr) | 1999-04-09 | 2000-10-19 | Kyowa Hakko Kogyo Co., Ltd. | Methode de regulation de l'activite d'une molecule immunologiquement fonctionnelle |
| WO2002031140A1 (en) | 2000-10-06 | 2002-04-18 | Kyowa Hakko Kogyo Co., Ltd. | Cells producing antibody compositions |
| WO2002043477A1 (fr) | 2000-12-01 | 2002-06-06 | Central Institute For Experimental Animals | Methode d'obtention d'une souris adaptee a la prise, a la differenciation et a la proliferation de cellules heterogenes, souris obtenue selon cette methode et son utilisation |
| WO2002079255A1 (en) | 2001-04-02 | 2002-10-10 | Idec Pharmaceuticals Corporation | RECOMBINANT ANTIBODIES COEXPRESSED WITH GnTIII |
| JP2007530588A (ja) * | 2004-03-23 | 2007-11-01 | バイオジェン・アイデック・エムエイ・インコーポレイテッド | レセプターカップリング剤およびその治療用途 |
| WO2008047723A1 (fr) | 2006-10-12 | 2008-04-24 | Forerunner Pharma Research Co., Ltd. | Diagnostic et traitement du cancer à l'aide d'un anticorps anti-ereg |
| WO2008143954A2 (en) * | 2007-05-14 | 2008-11-27 | Biogen Idec Ma Inc. | Single-chain fc (scfc) regions, binding polypeptides comprising same, and methods related thereto |
| WO2009005809A2 (en) | 2007-07-02 | 2009-01-08 | Oncomed Pharmaceuticals, Inc. | Compositions and methods for treating and diagnosing cancer |
| JP2009502156A (ja) * | 2005-07-26 | 2009-01-29 | プロキュア・セラピューティクス・リミテッド | 幹細胞マーカー |
| JP2009509510A (ja) * | 2005-09-26 | 2009-03-12 | メダレックス インコーポレーティッド | Cd70に対するヒトモノクローナル抗体 |
| WO2009064301A1 (en) * | 2007-11-15 | 2009-05-22 | The Johns Hopkins University | Methods for detecting and monitoring circulating cancer stem cells |
| WO2009078386A1 (ja) | 2007-12-14 | 2009-06-25 | Chugai Seiyaku Kabushiki Kaisha | 組織形態保持および核酸品質保持に優れた新規標本作製法 |
| WO2009135181A2 (en) * | 2008-05-02 | 2009-11-05 | Seattle Genetics, Inc. | Methods and compositions for making antibodies and antibody derivatives with reduced core fucosylation |
| WO2010102244A1 (en) * | 2009-03-06 | 2010-09-10 | Kalobios Pharmaceuticals, Inc. | Treatment of leukemias and chronic myeloproliferative diseases with antibodies to epha3 |
| WO2010113117A2 (en) * | 2009-03-30 | 2010-10-07 | Edimer Biotech S.A. | Preparation of isolated agonist anti-edar monoclonal antibodies |
| WO2010123891A1 (en) * | 2009-04-20 | 2010-10-28 | Genentech, Inc. | Adjuvant cancer therapy |
| US20100275280A1 (en) | 2007-08-10 | 2010-10-28 | Hubrecht Institute | Method for identifying, expanding and removing adult stem cells and cancer stem cells |
| WO2010126074A1 (ja) * | 2009-04-28 | 2010-11-04 | 中外製薬株式会社 | Hlaクラスiを認識する抗体を有効成分として含有する維持療法用医薬組成物 |
Family Cites Families (55)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5573924A (en) * | 1992-09-08 | 1996-11-12 | Immunex Corporation | CD27 ligand |
| US6852318B1 (en) | 1998-05-08 | 2005-02-08 | The Regents Of The University Of California | Methods for detecting and inhibiting angiogenesis |
| US8044259B2 (en) | 2000-08-03 | 2011-10-25 | The Regents Of The University Of Michigan | Determining the capability of a test compound to affect solid tumor stem cells |
| US6984522B2 (en) | 2000-08-03 | 2006-01-10 | Regents Of The University Of Michigan | Isolation and use of solid tumor stem cells |
| US6946292B2 (en) | 2000-10-06 | 2005-09-20 | Kyowa Hakko Kogyo Co., Ltd. | Cells producing antibody compositions with increased antibody dependent cytotoxic activity |
| US7229960B2 (en) | 2000-11-03 | 2007-06-12 | University Of Vermont And State Agricultural College | Methods and compositions for inhibiting GRB7 |
| WO2003104401A2 (en) * | 2002-06-05 | 2003-12-18 | Avalon Pharmaceuticals, Inc. | Cancer-linked gene as target for chemotherapy |
| US7393531B2 (en) | 2003-01-21 | 2008-07-01 | Arius Research Inc. | Cytotoxicity mediation of cells evidencing surface expression of MCSP |
| JPWO2004101775A1 (ja) | 2003-05-16 | 2006-07-13 | 協和醗酵工業株式会社 | 新規な成体組織由来の幹細胞およびその用途 |
| JP2005035943A (ja) | 2003-07-16 | 2005-02-10 | Chemo Sero Therapeut Res Inst | 悪性腫瘍細胞増殖抑制剤 |
| CA2447400A1 (en) | 2003-09-12 | 2005-03-12 | The Hospital For Sick Children | Brain tumor stem cells |
| JPWO2005035740A1 (ja) | 2003-10-09 | 2006-12-21 | 協和醗酵工業株式会社 | 無血清馴化したゲノム改変細胞 |
| JP2005206508A (ja) * | 2004-01-22 | 2005-08-04 | Chemo Sero Therapeut Res Inst | 悪性腫瘍細胞増殖抑制抗体 |
| WO2005111076A1 (en) * | 2004-05-12 | 2005-11-24 | Institut National De La Sante Et De La Recherche Medicale (Inserm) | Nectin 4 (n4) as a marker for cancer prognosis |
| US20060135455A1 (en) | 2004-06-01 | 2006-06-22 | Reza Sheikhnejad | Methods and compositions for the inhibition of gene expression |
| US20090214517A1 (en) * | 2004-07-27 | 2009-08-27 | Justin Wong | Compositions and methods of use for modulators of nectin 4, semaphorin 4b, igsf9, and kiaa0152 in treating disease |
| JP2008514205A (ja) | 2004-09-29 | 2008-05-08 | 財団法人実験動物中央研究所 | Nogマウスを用いた高転移性細胞株の樹立 |
| WO2006051405A2 (en) | 2004-11-12 | 2006-05-18 | Cambridge University Technical Services Ltd. | Methods and means related to cancer stem cells |
| WO2006051984A1 (ja) | 2004-11-15 | 2006-05-18 | Kirin Beer Kabushiki Kaisha | 癌細胞の転移抑制方法およびそのために使用する医薬組成物 |
| AU2006236225C1 (en) * | 2005-04-19 | 2013-05-02 | Seagen Inc. | Humanized anti-CD70 binding agents and uses thereof |
| US20070105133A1 (en) | 2005-06-13 | 2007-05-10 | The Regents Of The University Of Michigan | Compositions and methods for treating and diagnosing cancer |
| EP1945754B1 (en) | 2005-10-31 | 2014-07-23 | The Regents Of The University Of Michigan | Compositions and methods for treating and diagnosing cancer |
| US20070220621A1 (en) | 2005-10-31 | 2007-09-20 | Clarke Michael F | Genetic characterization and prognostic significance of cancer stem cells in cancer |
| EP1792979A1 (en) | 2005-12-01 | 2007-06-06 | Stiftung Caesar Center of Advanced European Studies and Research | Cell culture system for the enrichment and expansion of stem cells |
| ES2548240T3 (es) | 2005-12-01 | 2015-10-15 | Pronai Therapeutics, Inc. | Terapias para el cáncer y composiciones farmacéuticas usadas en las mismas |
| WO2007124125A2 (en) * | 2006-04-21 | 2007-11-01 | Stowers Institute For Medical Research | Methods of identifying stem cells in normal and cancerous tissues and related progeny cells |
| WO2007132883A1 (ja) | 2006-05-17 | 2007-11-22 | Yokohama City University | 肝臓癌の鑑別、疾患ステージの判定又は予後の予測方法及びキット |
| CA2658003A1 (en) | 2006-06-06 | 2007-12-21 | University Of Tennessee Research Foundation | Compositions enriched in neoplastic stem cells and methods comprising same |
| US20110244501A1 (en) * | 2006-08-02 | 2011-10-06 | Biogen Idec Ma Inc. | Cancer stem cells |
| CA2659418C (en) | 2006-08-11 | 2018-05-22 | Ao-Forschungsinstitut Davos | Identification and selection of stem cells being committed to differentiate to a specific type for obtaining a homogeneous population of stem cells |
| AU2007292890A1 (en) * | 2006-09-05 | 2008-03-13 | Medarex, Inc. | Antibodies to bone morphogenic proteins and receptors therefor and methods for their use |
| WO2008033393A2 (en) | 2006-09-11 | 2008-03-20 | University Of Florida | Isolation, expansion and uses of tumor stem cells |
| GB2442059A (en) | 2006-09-19 | 2008-03-26 | Ist Superiore Sanita | Test for cancer of the gastrointestinal tract |
| JP2008102012A (ja) | 2006-10-19 | 2008-05-01 | Kanazawa Univ | 癌幹細胞の同定および単離方法 |
| US8309354B2 (en) | 2007-01-22 | 2012-11-13 | Macrogenics West, Inc. | Human cancer stem cells |
| JP2008182912A (ja) | 2007-01-29 | 2008-08-14 | Nippon Kayaku Co Ltd | 癌幹細胞の培養方法、および癌幹細胞 |
| WO2008149803A1 (ja) | 2007-06-06 | 2008-12-11 | The University Of Tokyo | 表面抗原マーカーを用いた急性リンパ性白血病における癌幹細胞の分離同定方法 |
| WO2009023194A2 (en) | 2007-08-10 | 2009-02-19 | Whitehead Institute For Biomedical Research | Hormone responsive tissue culture system and uses thereof |
| WO2009043159A1 (en) | 2007-10-01 | 2009-04-09 | The Hospital For Sick Children | Neural tumor stem cells and methods of use thereof |
| WO2009111067A2 (en) | 2008-03-07 | 2009-09-11 | Osi Pharmaceuticals, Inc. | Methods for the identification of agents that inhibit mesenchymal -like tumor cells or their formation |
| US20110111434A1 (en) | 2008-07-15 | 2011-05-12 | Huang Emina H | Colon stem cells associated with colitisand colorectal cancer and methods of use |
| WO2010016766A2 (en) | 2008-08-08 | 2010-02-11 | Koninklijke Nederlandse Akademie Van Wetenschappen | Antibodies recognizing endogenous human lgr5 and/or lgr6 |
| US20110301056A1 (en) * | 2008-12-12 | 2011-12-08 | Oncotherapy Science, Inc. | Nectin-4 for target genes of cancer therapy and diagnosis |
| US20120141501A1 (en) * | 2009-05-29 | 2012-06-07 | Forerunner Pharma Research Co. Ltd | Pharmaceutical Composition Containing Antagonist of EGF Family Ligand as Component |
| WO2011027308A1 (en) | 2009-09-03 | 2011-03-10 | Koninklijke Philips Electronics N.V. | Novel tumor markers |
| WO2011078301A1 (ja) | 2009-12-25 | 2011-06-30 | ファーマロジカルズ・リサーチ プライベート リミテッド | Nog樹立癌細胞株が移植された非ヒト動物モデルを用いた抗癌剤ターゲット探索及びスクリーニング法 |
| US9217032B2 (en) | 2010-01-08 | 2015-12-22 | Les Laboratoires Servier | Methods for treating colorectal cancer |
| SG189302A1 (en) | 2010-10-06 | 2013-05-31 | Pharmalogicals Res Pte Ltd | Cancer stem cell population and method for production thereof |
| WO2012096545A2 (ko) * | 2011-01-13 | 2012-07-19 | 연세대학교 산학협력단 | 췌장암 암줄기세포 특성을 이용한 췌장암 신규 바이오마커 및 그의 용도 |
| KR102049122B1 (ko) | 2011-06-30 | 2019-11-26 | 추가이 세이야쿠 가부시키가이샤 | 헤테로이량화 폴리펩티드 |
| EP2749641B1 (en) | 2011-09-07 | 2021-06-02 | Chugai Seiyaku Kabushiki Kaisha | Cancer stem cell isolation |
| WO2013062083A1 (ja) | 2011-10-28 | 2013-05-02 | ファーマロジカルズ・リサーチ プライベート リミテッド | 癌幹細胞特異的分子 |
| TWI593705B (zh) | 2011-12-28 | 2017-08-01 | Chugai Pharmaceutical Co Ltd | Humanized anti-epiregulin antibody and cancer therapeutic agent containing the antibody as an active ingredient |
| WO2014104165A1 (ja) | 2012-12-27 | 2014-07-03 | 中外製薬株式会社 | ヘテロ二量化ポリペプチド |
| US10504315B2 (en) | 2013-08-05 | 2019-12-10 | Ncr Corporation | Clamping of media items |
-
2012
- 2012-10-26 WO PCT/JP2012/077714 patent/WO2013062083A1/ja not_active Ceased
- 2012-10-26 US US14/354,517 patent/US20140302511A1/en not_active Abandoned
- 2012-10-26 EP EP19191052.0A patent/EP3603671A3/en active Pending
- 2012-10-26 JP JP2013540843A patent/JP6291254B2/ja active Active
- 2012-10-26 EP EP12844389.2A patent/EP2772268B8/en active Active
-
2016
- 2016-02-12 US US15/042,548 patent/US10934351B2/en active Active
-
2017
- 2017-11-29 JP JP2017228927A patent/JP6580112B2/ja active Active
-
2019
- 2019-05-31 JP JP2019102325A patent/JP6794496B2/ja active Active
-
2020
- 2020-06-26 US US16/913,341 patent/US11858987B2/en active Active
- 2020-11-11 JP JP2020187991A patent/JP6985486B2/ja active Active
-
2021
- 2021-11-25 JP JP2021191088A patent/JP7335310B2/ja active Active
-
2023
- 2023-08-17 JP JP2023133133A patent/JP7650923B2/ja active Active
-
2025
- 2025-03-12 JP JP2025038864A patent/JP2025102800A/ja active Pending
Patent Citations (37)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0159878B2 (enExample) | 1982-05-21 | 1989-12-20 | Yunibaashitei Obu Karifuorunia | |
| EP0125023A1 (en) | 1983-04-08 | 1984-11-14 | Genentech, Inc. | Recombinant immunoglobulin preparations, methods for their preparation, DNA sequences, expression vectors and recombinant host cells therefor |
| EP0404097A2 (de) | 1989-06-22 | 1990-12-27 | BEHRINGWERKE Aktiengesellschaft | Bispezifische und oligospezifische, mono- und oligovalente Rezeptoren, ihre Herstellung und Verwendung |
| WO1992001047A1 (en) | 1990-07-10 | 1992-01-23 | Cambridge Antibody Technology Limited | Methods for producing members of specific binding pairs |
| WO1992020791A1 (en) | 1990-07-10 | 1992-11-26 | Cambridge Antibody Technology Limited | Methods for producing members of specific binding pairs |
| WO1992003918A1 (en) | 1990-08-29 | 1992-03-19 | Genpharm International, Inc. | Transgenic non-human animals capable of producing heterologous antibodies |
| WO1993006213A1 (en) | 1991-09-23 | 1993-04-01 | Medical Research Council | Production of chimeric antibodies - a combinatorial approach |
| WO1993011161A1 (en) | 1991-11-25 | 1993-06-10 | Enzon, Inc. | Multivalent antigen-binding proteins |
| WO1993011236A1 (en) | 1991-12-02 | 1993-06-10 | Medical Research Council | Production of anti-self antibodies from antibody segment repertoires and displayed on phage |
| WO1993012227A1 (en) | 1991-12-17 | 1993-06-24 | Genpharm International, Inc. | Transgenic non-human animals capable of producing heterologous antibodies |
| WO1993019172A1 (en) | 1992-03-24 | 1993-09-30 | Cambridge Antibody Technology Limited | Methods for producing members of specific binding pairs |
| WO1994002602A1 (en) | 1992-07-24 | 1994-02-03 | Cell Genesys, Inc. | Generation of xenogeneic antibodies |
| WO1994011523A2 (en) | 1992-11-13 | 1994-05-26 | Idec Pharmaceuticals Corporation | Fully impaired consensus kozac sequences for mammalian expression |
| WO1994025585A1 (en) | 1993-04-26 | 1994-11-10 | Genpharm International, Inc. | Transgenic non-human animals capable of producing heterologous antibodies |
| WO1995001438A1 (en) | 1993-06-30 | 1995-01-12 | Medical Research Council | Sbp members with a chemical moiety covalently bound within the binding site; production and selection thereof |
| WO1995015388A1 (en) | 1993-12-03 | 1995-06-08 | Medical Research Council | Recombinant binding proteins and peptides |
| WO1996002576A1 (en) | 1994-07-13 | 1996-02-01 | Chugai Seiyaku Kabushiki Kaisha | Reconstituted human antibody against human interleukin-8 |
| WO1998013388A1 (en) | 1996-09-26 | 1998-04-02 | Chugai Seiyaku Kabushiki Kaisha | Antibody against human parathormone related peptides |
| WO1999054342A1 (en) | 1998-04-20 | 1999-10-28 | Pablo Umana | Glycosylation engineering of antibodies for improving antibody-dependent cellular cytotoxicity |
| WO2000061739A1 (fr) | 1999-04-09 | 2000-10-19 | Kyowa Hakko Kogyo Co., Ltd. | Methode de regulation de l'activite d'une molecule immunologiquement fonctionnelle |
| WO2002031140A1 (en) | 2000-10-06 | 2002-04-18 | Kyowa Hakko Kogyo Co., Ltd. | Cells producing antibody compositions |
| WO2002043477A1 (fr) | 2000-12-01 | 2002-06-06 | Central Institute For Experimental Animals | Methode d'obtention d'une souris adaptee a la prise, a la differenciation et a la proliferation de cellules heterogenes, souris obtenue selon cette methode et son utilisation |
| WO2002079255A1 (en) | 2001-04-02 | 2002-10-10 | Idec Pharmaceuticals Corporation | RECOMBINANT ANTIBODIES COEXPRESSED WITH GnTIII |
| JP2007530588A (ja) * | 2004-03-23 | 2007-11-01 | バイオジェン・アイデック・エムエイ・インコーポレイテッド | レセプターカップリング剤およびその治療用途 |
| JP2009502156A (ja) * | 2005-07-26 | 2009-01-29 | プロキュア・セラピューティクス・リミテッド | 幹細胞マーカー |
| JP2009509510A (ja) * | 2005-09-26 | 2009-03-12 | メダレックス インコーポレーティッド | Cd70に対するヒトモノクローナル抗体 |
| WO2008047723A1 (fr) | 2006-10-12 | 2008-04-24 | Forerunner Pharma Research Co., Ltd. | Diagnostic et traitement du cancer à l'aide d'un anticorps anti-ereg |
| WO2008143954A2 (en) * | 2007-05-14 | 2008-11-27 | Biogen Idec Ma Inc. | Single-chain fc (scfc) regions, binding polypeptides comprising same, and methods related thereto |
| WO2009005809A2 (en) | 2007-07-02 | 2009-01-08 | Oncomed Pharmaceuticals, Inc. | Compositions and methods for treating and diagnosing cancer |
| US20100275280A1 (en) | 2007-08-10 | 2010-10-28 | Hubrecht Institute | Method for identifying, expanding and removing adult stem cells and cancer stem cells |
| WO2009064301A1 (en) * | 2007-11-15 | 2009-05-22 | The Johns Hopkins University | Methods for detecting and monitoring circulating cancer stem cells |
| WO2009078386A1 (ja) | 2007-12-14 | 2009-06-25 | Chugai Seiyaku Kabushiki Kaisha | 組織形態保持および核酸品質保持に優れた新規標本作製法 |
| WO2009135181A2 (en) * | 2008-05-02 | 2009-11-05 | Seattle Genetics, Inc. | Methods and compositions for making antibodies and antibody derivatives with reduced core fucosylation |
| WO2010102244A1 (en) * | 2009-03-06 | 2010-09-10 | Kalobios Pharmaceuticals, Inc. | Treatment of leukemias and chronic myeloproliferative diseases with antibodies to epha3 |
| WO2010113117A2 (en) * | 2009-03-30 | 2010-10-07 | Edimer Biotech S.A. | Preparation of isolated agonist anti-edar monoclonal antibodies |
| WO2010123891A1 (en) * | 2009-04-20 | 2010-10-28 | Genentech, Inc. | Adjuvant cancer therapy |
| WO2010126074A1 (ja) * | 2009-04-28 | 2010-11-04 | 中外製薬株式会社 | Hlaクラスiを認識する抗体を有効成分として含有する維持療法用医薬組成物 |
Non-Patent Citations (97)
| Title |
|---|
| "Immunology", 1993, JOHN WILEY & SONS, INC., article "Immunologic studies in humans" |
| AL-HAJJ M; WICHA MS; BENITO-HERNANDEZ A; MORRISON SJ; CLARKE MF: "Prospective identification of tumorigenic breast cancer cells", PROC NATL ACAD SCI USA, vol. 100, 2003, pages 3983 - 3988 |
| ATWELL ET AL., MOL. IMMUNOL., vol. 33, 1996, pages 1301 - 1312 |
| BARKER N ET AL.: "Crypt stem cells as the cells-of-origin of intestinal cancer", NATURE, vol. 457, 2009, pages 608 - 611 |
| BARKER N ET AL.: "Identification of stem cells in small intestine and colon by marker gene Lgr5", NATURE, vol. 449, 2007, pages 1003 - 1007 |
| BARKER,N. ET AL.: "Identification of stem cells in small intestine and colon by marker gene Lgr5", NATURE, vol. 449, no. 25, 2007, pages 1003 - 1008, XP002457164 * |
| BELYAVSKY, A. ET AL., NUCLEIC ACIDS RES., vol. 17, 1989, pages 2919 - 2932 |
| BETTER ET AL., SCIENCE, vol. 240, 1988, pages 1041 - 1043 |
| BETTER, M.; HORWITZ, A. H.: "Methods in Enzymology", vol. 178, 1989, ACADEMIC PRESS, INC, pages: 476 - 496 |
| BIRD, R. E. ET AL., TIBTECH, vol. 9, 1991, pages 132 - 137 |
| BOIKO AD ET AL.: "Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271", NATURE, vol. 446, 2010, pages 133 - 137 |
| BONNET D; DICK JE: "Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell", NAT MED, vol. 3, 1997, pages 730 - 737 |
| BRABLETZ T; JUNG A; SPADERNA S; HLUBEK F; KIRCHNER T: "Opinion: migrating cancer stem cells - an integrated concept of malignant tumor progression", NAT REV CANCER, vol. 5, 2005, pages 744 - 749 |
| CHIRGWIN, J. M. ET AL., BIOCHEMISTRY, vol. 18, 1979, pages 5294 - 5299 |
| CHOMCZYNSKI, P. ET AL., ANAL. BIOCHEM., vol. 162, 1987, pages 156 - 159 |
| CHU P ET AL.: "Characterization of a subpopulation of colon cancer cells with stem cell-like properties", INT J CANCER, vol. 124, 2009, pages 1312 - 1321 |
| CLEVERS H: "The cancer stem cell: premises, promises and challenges", NAT MED, vol. 17, 2011, pages 313 - 319 |
| CO, M. S. ET AL., J. IMMUNOL., vol. 152, 1994, pages 2968 - 2976 |
| COLLINS AT; BERRY PA; HYDE C; STOWER MJ; MAITLAND NJ: "Prospective identification of tumorigenic prostate cancer stem cells", CANCER RES, vol. 65, 2005, pages 10946 - 10951 |
| CURRENT TOPICS IN MICROBIOLOGY AND IMMUNOLOGY, vol. 81, 1978, pages 1 - 7 |
| DALERBA P ET AL.: "Phenotypic characterization of human colorectal cancer stem cells", PROC NATL ACAD SCI USA, vol. 104, 2007, pages 10158 - 10163 |
| DE ST. GROTH, S. F. ET AL., J. IMMUNOL. METHODS, vol. 35, 1980, pages 1 - 21 |
| EBERT, K. M. ET AL., BIO/TECHNOLOGY, vol. 12, 1994, pages 699 - 702 |
| ED HARLOW; DAVID LANE: "Antibodies A Laboratory Manual", 1988, COLD SPRING HARBOR LABORATORY |
| ED HARLOW; DAVID LANE: "Antibodies A Laboratory Manual.", 1988, COLD SPRING HARBOR LABORATORY |
| ERAMO A ET AL.: "Identification and expansion of the tumorigenic lung cancer stem cell population", CELL DEATH DIFFER, vol. 15, 2008, pages 504 - 514 |
| FASEB J., vol. 6, 1992, pages 2422 - 2427 |
| FROHMAN, M. A. ET AL., PROC. NATL. ACAD. SCI. USA, vol. 85, 1988, pages 8998 - 9002 |
| FUJII E. ET AL., PATHOL INT., vol. 58, 2008, pages 559 - 567 |
| FUJII E. ET AL.: "Establishment and characterization of in vivo human tumor models in the NOD/SCID/gamma(c)(null) mouse", PATHOL INT, vol. 58, 2008, pages 559 - 567 |
| GALFRE, G. ET AL., NATURE, vol. 277, 1979, pages 131 - 133 |
| GENE, vol. 17, 1982, pages 107 |
| GENE, vol. 24, 1983, pages 255 |
| HARADA, A. ET AL., INTERNATIONAL IMMUNOLOGY, vol. 5, 1993, pages 681 - 690 |
| HARAGUCHI N ET AL.: "CD 133+CD44+ population efficiently enriches colon cancer initiating cells", ANN SURG ONCOL, vol. 15, 2008, pages 2927 - 2933 |
| HERMANN PC ET AL.: "Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer", CELL STEM CELL, vol. 1, 2007, pages 313 - 323 |
| HOLLIGER ET AL., PROC. NATL. ACAD. SCI. USA., vol. 90, 1993, pages 6444 - 6448 |
| HOLLIGER ET AL., PROTEIN ENGINEERING, vol. 9, 1996, pages 299 - 305 |
| HSU SY; LIANG SG; HSUEH AJ: "Characterization of two LGR genes homologous to gonadotropin and thyrotropin receptors with extracellular leucine-rich repeats and a G protein-coupled, seven-transmembrane region", MOL ENDCRINOL, vol. 12, 1998, pages 1830 - 1845 |
| HU Y; SMYTH GK., J IMMUNOL METHODS, vol. 347, no. 1-2, 15 August 2009 (2009-08-15), pages 70 - 8 |
| HUANG EH ET AL.: "Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis", CANCER RES, vol. 69, 2009, pages 3382 - 3389 |
| HUSTON, J. S. ET AL., PROC. NATL. ACAD. SCI. U.S.A., vol. 85, 1988, pages 5879 - 5883 |
| ISHIZAWA K ET AL.: "Tumor-initiating cells are rare in many human tumors", CELL STEM CELL, vol. 7, 2010, pages 279 - 282 |
| ISHIZAWA K; RASHEED ZA ET AL., CELL STEM CELL, vol. 7, no. 3, 3 September 2010 (2010-09-03), pages 279 - 82 |
| J. IMMNOL., vol. 123, 1979, pages 1548 - 1550 |
| JOHN ET AL., PROTEIN ENGINEERING, vol. 12, no. 7, 1999, pages 597 - 604 |
| JOHNSON ET AL., METHOD IN ENZYMOLOGY, vol. 203, 1991, pages 88 - 98 |
| JOURNAL OF BIOCHEMISTRY, vol. 95, 1984, pages 87 |
| JOURNAL OF MOLECULAR BIOLOGY, vol. 120, 1978, pages 517 |
| JOURNAL OF MOLECULAR BIOLOGY, vol. 41, 1969, pages 459 |
| KOHLER, G.; MILSTEIN, C., METHODS ENZYMOL., vol. 73, 1981, pages 3 - 46 |
| KOHLER, G; MILSTEIN, C., EUR. J. IMMUNOL., vol. 6, 1976, pages 511 - 519 |
| KOWALCZYK,C. ET AL.: "Molecular and therapeutic characterization of anti-ectodysplasin A receptor (EDAR) agonist monoclonal antibodies", THE JOURNAL OF BIOLOGICAL CHEMISTRY, vol. 286, no. 35, 2 September 2011 (2011-09-02), pages 30769 - 30779, XP055054469 * |
| KREMER L; MARQUEZ G, METHODS MOL BIOL., vol. 239, 2004, pages 243 - 260 |
| KUMAR V, ABBAS AK; FAUSIO N. ROBBINS; COTRAN: "General Pathology, 7: Neoplasia, Biology of tumor growth: Benign and malignant neoplasms", 2005, article "Pathologic Basis of Disease", pages: 269 - 342 |
| KUMAR V; ABBAS AK; FAUSIO N. ROBBINS; COTRAN: "General Pathology, 7: Neoplasia, Biology of tumor growth: Benign and malignant neoplasms", 2005, article "Pathologic Basis of Disease", pages: 272 - 281 |
| LAMOYI, E., METHODS IN ENZYMOLOGY, vol. 121, 1989, pages 652 - 663 |
| LAPIDOT T ET AL.: "A cell initiating human acute myeloid leukaemia after transplantation into SCID mice", NATURE, vol. 367, 1994, pages 645 - 648 |
| LI C ET AL.: "Identification of pancreatic cancer stem cells", CANCER RES, vol. 67, 2007, pages 1030 - 1037 |
| MARGULIES, D. H. ET AL., CELL, vol. 8, 1976, pages 405 - 415 |
| MCDONALD T ET AL.: "Identification and cloning of an orphan G protein-coupled receptor of the glycoprotein hormone receptor subfamily", BIOCHEM BIOPHYS RES COMMUN, vol. 247, 1998, pages 266 - 270 |
| METHODS IN ENZYMOLOGY, vol. 194, 1991, pages 182 - 187 |
| MIZUSHIMA ET AL., NUCLEIC ACIDS RES., vol. 18, 1990, pages 5322 |
| MOLECULAR & GENERAL GENETICS, vol. 168, 1979, pages 111 |
| MULLIGAN ET AL., NATURE, vol. 277, 1979, pages 108 |
| NUCLEIC ACIDS RESEARCH, vol. 9, 1981, pages 309 |
| O'BRIEN CA; POLLETT A; GALLINGER S; DICK JE: "A human colon cancer cell capable of initiating tumour growth in immunodeficient mice", NATURE, vol. 445, 2007, pages 106 - 110 |
| P. HOLLIGER ET AL., PROC. NATL. ACAD. SCI. USA, vol. 90, 1993, pages 6444 - 6448 |
| PANG R ET AL.: "A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer", CELL STEM CELL, vol. 6, 2010, pages 603 - 615 |
| PARK CY ET AL.: "Cancer stem cell-directed therapies:recent data from the laboratory and clinic", MOL THER, vol. 17, no. 2, 2009, pages 219 - 230 |
| PATRAWALA L ET AL.: "Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells", ONCOGENE, vol. 25, 2006, pages 1696 - 1708 |
| PERISIC ET AL., STRUCTURE, vol. 2, 1994, pages 1217 - 1226 |
| PLUECKTHUN, A.; SKERRA, A.: "Methods in Enzymology", vol. 178, 1989, ACADEMIC PRESS, INC., pages: 476 - 496 |
| PRINCE ME ET AL.: "Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma", PROC NATL ACAD SCI USA, vol. 104, 2007, pages 973 - 978 |
| PROC. NATL. ACAD. SCI, USA, vol. 60, 1968, pages 160 |
| PROC. NATL. ACAD. SCI. USA, vol. 69, 1972, pages 2110 |
| PROC. NATL. ACAD. SCI. USA, vol. 75, 1978, pages 1929 |
| REYA T; MORRISON SJ; CLARKE MF; WEISSMAN IL: "Stem cells, cancer, and cancer stem cells", NATURE, vol. 414, 2001, pages 105 - 111 |
| RICCI-VITIANI L ET AL.: "Identification and expansion of human colon-cancer-initiating cells", NATURE, vol. 445, 2007, pages 111 - 115 |
| ROUSSEAUX, J. ET AL., METHODS IN ENZYMOLOGY, vol. 121, 1989, pages 663 - 669 |
| SATO T ET AL.: "Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche", NATURE, vol. 459, 2009, pages 262 - 265 |
| SATO Y ET AL., AM J PATHOL, vol. 125, 1986, pages 431 - 435 |
| SATO Y ET AL., AM J PATHOL, vol. 140, 1992, pages 775 - 779 |
| SATO, K. ET AL., CANCER RES., vol. 53, 1993, pages 851 - 856 |
| SCHATTON T ET AL.: "Identification of cells initiating human melanomas", NATURE, vol. 451, 2008, pages 345 - 349 |
| SHULMAN, M. ET AL., NATURE, vol. 276, 1978, pages 269 - 270 |
| SINGH SK ET AL.: "Identification of human brain tumour initiating cells", NATURE, vol. 432, 2004, pages 396 - 401 |
| SUZUKI M ET AL., J TOXICOL SCI, vol. 27, 2002, pages 165 - 172 |
| TODARO M ET AL., CELL STEM CELL, vol. 1, 2007, pages 389 - 402 |
| TROUET ET AL., PROC. NATL. ACAD. SCI. USA, vol. 79, 1982, pages 626 - 629 |
| TROWBRIDGE, 1. S., J. EXP. MED., vol. 148, 1978, pages 313 - 323 |
| UMEMOTO ET AL., INT. J. CANCER, vol. 43, 1989, pages 677 - 684 |
| VANDAMME, A.M. ET AL., EUR. J. BIOCHEM., vol. 192, 1990, pages 767 - 775 |
| VERMEULEN L ET AL.: "Wnt activity defines colon cancer stem cells and is regulated by the microenvironment", NAT CELL BIOL, vol. 12, 2010, pages 468 - 476 |
| VIROLOGY, vol. 52, 1973, pages 456 |
| WARD ET AL., NATURE, vol. 341, October 1998 (1998-10-01), pages 544 - 546 |
| WU C ET AL.: "Side population cells isolated from mesenchymal neoplasms have tumor initiating potential", CANCER RES, vol. 1, 2007, pages 8216 - 8222 |
Cited By (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11536713B2 (en) | 2009-12-25 | 2022-12-27 | Chugai Seiyaku Kabushiki Kaisha | Method for searching and screening for target of anti-cancer agent using non-human animal model having NOG established cancer cell line transplanted therein |
| US11124773B2 (en) | 2010-10-06 | 2021-09-21 | Chugai Seiyaku Kabushiki Kaisha | Cancer stem cell population and method for production thereof |
| US11965180B2 (en) | 2010-10-06 | 2024-04-23 | Chugai Seiyaku Kabushiki Kaisha | Cancer stem cell population and method for production thereof |
| US10018630B2 (en) | 2011-09-07 | 2018-07-10 | Chugai Seiyaku Kabushiki Kaisha | Cancer stem cell isolation |
| JP2017528114A (ja) * | 2014-06-25 | 2017-09-28 | テル ハショマー メディカル リサーチ インフラストラクチャー アンド サーヴィシーズ リミテッド | がん幹細胞の同定ならびに診断および処置のためのその使用 |
| KR101779147B1 (ko) * | 2014-07-04 | 2017-09-20 | 연세대학교 산학협력단 | Kiaa1199를 이용한 췌장암의 진단 또는 치료용 조성물 |
| JP2016028569A (ja) * | 2014-07-16 | 2016-03-03 | 学校法人慶應義塾 | がん幹細胞集団の調製方法、異種移植片の調製方法、スクリーニング方法、miR−34aの発現量を低下させる方法及びがん幹細胞増殖抑制剤 |
| CN110546258A (zh) * | 2017-03-28 | 2019-12-06 | 学校法人庆应义塾 | 人体组织干细胞及其使用 |
| US11698366B2 (en) | 2017-03-28 | 2023-07-11 | Keio University | Human tissue stem cell and use thereof |
| JPWO2020032162A1 (ja) * | 2018-08-08 | 2021-08-12 | 中外製薬株式会社 | がん組織またはがん組織に類似した組織の培養方法 |
| JP7446224B2 (ja) | 2018-08-08 | 2024-03-08 | 中外製薬株式会社 | がん組織またはがん組織に類似した組織の培養方法 |
| JP2024037896A (ja) * | 2018-08-08 | 2024-03-19 | 中外製薬株式会社 | がん組織またはがん組織に類似した組織の培養方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| JP6291254B2 (ja) | 2018-03-14 |
| EP3603671A3 (en) | 2020-07-29 |
| JP7335310B2 (ja) | 2023-08-29 |
| JP7650923B2 (ja) | 2025-03-25 |
| US10934351B2 (en) | 2021-03-02 |
| EP2772268A4 (en) | 2015-12-02 |
| US11858987B2 (en) | 2024-01-02 |
| JPWO2013062083A1 (ja) | 2015-04-02 |
| US20160159904A1 (en) | 2016-06-09 |
| JP6985486B2 (ja) | 2021-12-22 |
| EP3603671A2 (en) | 2020-02-05 |
| EP2772268B8 (en) | 2020-01-08 |
| JP2025102800A (ja) | 2025-07-08 |
| JP2018083811A (ja) | 2018-05-31 |
| JP2022028866A (ja) | 2022-02-16 |
| US20140302511A1 (en) | 2014-10-09 |
| JP2019194190A (ja) | 2019-11-07 |
| EP2772268A1 (en) | 2014-09-03 |
| JP2023159269A (ja) | 2023-10-31 |
| EP2772268B1 (en) | 2019-09-04 |
| JP6794496B2 (ja) | 2020-12-02 |
| JP2021035971A (ja) | 2021-03-04 |
| US20200325222A1 (en) | 2020-10-15 |
| JP6580112B2 (ja) | 2019-09-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7650923B2 (ja) | 癌幹細胞特異的分子 | |
| JP6387383B2 (ja) | 癌幹細胞の分離 | |
| JP2011515497A (ja) | 骨髄性血液学的増殖性疾患と関連する免疫グロブリンおよび/またはToll様受容体タンパク質ならびにその使用 | |
| Qin et al. | Therapeutic strategies targeting uPAR potentiate anti–PD-1 efficacy in diffuse-type gastric cancer | |
| EP3287143A1 (en) | Ckap4-molecular-targeted antitumor agent | |
| JP2009544629A (ja) | Cd63の表面発現を証明する細胞の細胞傷害性への介在 | |
| CN112740043A (zh) | Vista受体 | |
| JP2022530339A (ja) | インテグリンα10および侵攻性癌型 | |
| EP4215211A1 (en) | Tumor immune microenvironment regulator, and preventive, diagnostic or therapeutic use thereof | |
| TW201827465A (zh) | 針對fzd10之單株抗體及其用途 | |
| US20200209247A1 (en) | Methods for identifying myeloma tumor-initiating cells and targeted therapy | |
| WO2024200505A1 (en) | Methods for predicting and improving therapeutic efficacy of cancer treatments and methods for cancer prognosis | |
| KOBAYASHI | Identification and Characterization of Cancer Stem Cells Responsible for Drug-Resistance and Metastasis | |
| WO2025175166A1 (en) | Combination therapies with btn1a1 binding proteins and chemotherapeutic agents | |
| JP6182149B2 (ja) | Ebag9に対するモノクローナル抗体、及びハイブリドーマ、並びにebag9に対するモノクローナル抗体の利用 | |
| Ruiu | Identification and Evaluation of Molecular Targets Associated to the Cancer Stem Cell Phenotype in Mammary Tumor |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 12844389 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2013540843 Country of ref document: JP Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 14354517 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| REEP | Request for entry into the european phase |
Ref document number: 2012844389 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2012844389 Country of ref document: EP |