KR20150108948A - 양이온성-리포솜 매개된 핵산 전달을 이용하여 면역 반응을 생성시키는 방법 - Google Patents
양이온성-리포솜 매개된 핵산 전달을 이용하여 면역 반응을 생성시키는 방법 Download PDFInfo
- Publication number
- KR20150108948A KR20150108948A KR1020157025507A KR20157025507A KR20150108948A KR 20150108948 A KR20150108948 A KR 20150108948A KR 1020157025507 A KR1020157025507 A KR 1020157025507A KR 20157025507 A KR20157025507 A KR 20157025507A KR 20150108948 A KR20150108948 A KR 20150108948A
- Authority
- KR
- South Korea
- Prior art keywords
- complex
- hcv
- dna
- liposome
- nucleic acid
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 239000002502 liposome Substances 0.000 title claims abstract description 303
- 102000039446 nucleic acids Human genes 0.000 title claims description 164
- 108020004707 nucleic acids Proteins 0.000 title claims description 164
- 150000007523 nucleic acids Chemical class 0.000 title claims description 164
- 230000028993 immune response Effects 0.000 title abstract description 77
- 238000012384 transportation and delivery Methods 0.000 title abstract description 20
- 238000000034 method Methods 0.000 title description 105
- 230000001404 mediated effect Effects 0.000 title description 7
- 125000002091 cationic group Chemical group 0.000 claims abstract description 166
- 239000003446 ligand Substances 0.000 claims abstract description 102
- 108010067390 Viral Proteins Proteins 0.000 claims description 90
- 108010063738 Interleukins Proteins 0.000 claims description 77
- 102000015696 Interleukins Human genes 0.000 claims description 76
- 229940047122 interleukins Drugs 0.000 claims description 63
- 150000001875 compounds Chemical class 0.000 claims 1
- 239000000427 antigen Substances 0.000 abstract description 86
- 102000036639 antigens Human genes 0.000 abstract description 86
- 108091007433 antigens Proteins 0.000 abstract description 86
- 230000003612 virological effect Effects 0.000 abstract description 75
- 229960005486 vaccine Drugs 0.000 abstract description 74
- 201000010099 disease Diseases 0.000 abstract description 39
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 abstract description 39
- 230000008685 targeting Effects 0.000 abstract description 26
- 238000004519 manufacturing process Methods 0.000 abstract description 9
- 238000012377 drug delivery Methods 0.000 abstract description 5
- 108020004414 DNA Proteins 0.000 description 213
- 241000711549 Hepacivirus C Species 0.000 description 148
- 108090000623 proteins and genes Proteins 0.000 description 141
- 239000013612 plasmid Substances 0.000 description 115
- 108090000765 processed proteins & peptides Proteins 0.000 description 112
- 210000004185 liver Anatomy 0.000 description 109
- 235000018102 proteins Nutrition 0.000 description 98
- 102000004169 proteins and genes Human genes 0.000 description 98
- 241001465754 Metazoa Species 0.000 description 97
- 210000004027 cell Anatomy 0.000 description 88
- 150000002632 lipids Chemical class 0.000 description 77
- 239000000203 mixture Substances 0.000 description 77
- 210000001744 T-lymphocyte Anatomy 0.000 description 71
- 238000002347 injection Methods 0.000 description 64
- 239000007924 injection Substances 0.000 description 64
- 102000004196 processed proteins & peptides Human genes 0.000 description 64
- 241000282579 Pan Species 0.000 description 63
- 241000699670 Mus sp. Species 0.000 description 56
- 102000013462 Interleukin-12 Human genes 0.000 description 49
- 108010065805 Interleukin-12 Proteins 0.000 description 49
- 208000015181 infectious disease Diseases 0.000 description 49
- 229940117681 interleukin-12 Drugs 0.000 description 49
- 108090000695 Cytokines Proteins 0.000 description 46
- 241000700605 Viruses Species 0.000 description 46
- 102000004127 Cytokines Human genes 0.000 description 45
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 44
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 44
- 210000001519 tissue Anatomy 0.000 description 44
- 241000124008 Mammalia Species 0.000 description 43
- 102000000588 Interleukin-2 Human genes 0.000 description 41
- 108010002350 Interleukin-2 Proteins 0.000 description 41
- 238000002255 vaccination Methods 0.000 description 40
- 230000014509 gene expression Effects 0.000 description 38
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 37
- 230000004044 response Effects 0.000 description 35
- 238000011282 treatment Methods 0.000 description 35
- 239000013598 vector Substances 0.000 description 35
- 229920001184 polypeptide Polymers 0.000 description 34
- MWRBNPKJOOWZPW-CLFAGFIQSA-N dioleoyl phosphatidylethanolamine Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC(COP(O)(=O)OCCN)OC(=O)CCCCCCC\C=C/CCCCCCCC MWRBNPKJOOWZPW-CLFAGFIQSA-N 0.000 description 33
- 230000005867 T cell response Effects 0.000 description 32
- 238000001990 intravenous administration Methods 0.000 description 32
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 30
- 238000004458 analytical method Methods 0.000 description 30
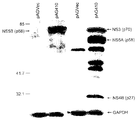
- 101710144111 Non-structural protein 3 Proteins 0.000 description 29
- 102000003812 Interleukin-15 Human genes 0.000 description 28
- 108090000172 Interleukin-15 Proteins 0.000 description 28
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 28
- 210000000612 antigen-presenting cell Anatomy 0.000 description 27
- 241000701161 unidentified adenovirus Species 0.000 description 27
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 26
- 238000002156 mixing Methods 0.000 description 26
- 239000000243 solution Substances 0.000 description 26
- 239000008121 dextrose Substances 0.000 description 24
- 238000009472 formulation Methods 0.000 description 24
- 210000003494 hepatocyte Anatomy 0.000 description 24
- 210000004369 blood Anatomy 0.000 description 23
- 239000008280 blood Substances 0.000 description 23
- 239000003814 drug Substances 0.000 description 23
- 238000011081 inoculation Methods 0.000 description 23
- 238000007918 intramuscular administration Methods 0.000 description 23
- 238000007912 intraperitoneal administration Methods 0.000 description 23
- 241000699666 Mus <mouse, genus> Species 0.000 description 22
- 101800001554 RNA-directed RNA polymerase Proteins 0.000 description 22
- 230000001965 increasing effect Effects 0.000 description 22
- 241000282577 Pan troglodytes Species 0.000 description 21
- 230000000694 effects Effects 0.000 description 21
- 238000001727 in vivo Methods 0.000 description 21
- 238000007917 intracranial administration Methods 0.000 description 21
- 230000002601 intratumoral effect Effects 0.000 description 21
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 20
- GZQKNULLWNGMCW-PWQABINMSA-N lipid A (E. coli) Chemical compound O1[C@H](CO)[C@@H](OP(O)(O)=O)[C@H](OC(=O)C[C@@H](CCCCCCCCCCC)OC(=O)CCCCCCCCCCCCC)[C@@H](NC(=O)C[C@@H](CCCCCCCCCCC)OC(=O)CCCCCCCCCCC)[C@@H]1OC[C@@H]1[C@@H](O)[C@H](OC(=O)C[C@H](O)CCCCCCCCCCC)[C@@H](NC(=O)C[C@H](O)CCCCCCCCCCC)[C@@H](OP(O)(O)=O)O1 GZQKNULLWNGMCW-PWQABINMSA-N 0.000 description 20
- -1 IL- 10 Proteins 0.000 description 19
- 101800001014 Non-structural protein 5A Proteins 0.000 description 19
- 230000001939 inductive effect Effects 0.000 description 19
- 206010022000 influenza Diseases 0.000 description 19
- 241000282412 Homo Species 0.000 description 18
- 238000003556 assay Methods 0.000 description 18
- PSLWZOIUBRXAQW-UHFFFAOYSA-M dimethyl(dioctadecyl)azanium;bromide Chemical compound [Br-].CCCCCCCCCCCCCCCCCC[N+](C)(C)CCCCCCCCCCCCCCCCCC PSLWZOIUBRXAQW-UHFFFAOYSA-M 0.000 description 18
- 229930182830 galactose Natural products 0.000 description 18
- 230000003053 immunization Effects 0.000 description 18
- 102000005962 receptors Human genes 0.000 description 18
- 108020003175 receptors Proteins 0.000 description 18
- 210000002966 serum Anatomy 0.000 description 18
- 206010028980 Neoplasm Diseases 0.000 description 17
- 238000002649 immunization Methods 0.000 description 17
- 108010074328 Interferon-gamma Proteins 0.000 description 16
- 108060001084 Luciferase Proteins 0.000 description 16
- 210000000952 spleen Anatomy 0.000 description 16
- 238000001262 western blot Methods 0.000 description 16
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 15
- 241000725303 Human immunodeficiency virus Species 0.000 description 15
- 102100037850 Interferon gamma Human genes 0.000 description 15
- 210000005228 liver tissue Anatomy 0.000 description 15
- 108020004999 messenger RNA Proteins 0.000 description 15
- 239000000523 sample Substances 0.000 description 15
- 239000000126 substance Substances 0.000 description 15
- 238000012360 testing method Methods 0.000 description 15
- 238000000684 flow cytometry Methods 0.000 description 14
- 210000005259 peripheral blood Anatomy 0.000 description 14
- 239000011886 peripheral blood Substances 0.000 description 14
- 230000000069 prophylactic effect Effects 0.000 description 14
- 230000001225 therapeutic effect Effects 0.000 description 14
- 201000003176 Severe Acute Respiratory Syndrome Diseases 0.000 description 13
- 208000002672 hepatitis B Diseases 0.000 description 13
- 239000008194 pharmaceutical composition Substances 0.000 description 13
- 239000000546 pharmaceutical excipient Substances 0.000 description 13
- 229940124597 therapeutic agent Drugs 0.000 description 13
- 229940021747 therapeutic vaccine Drugs 0.000 description 13
- 208000035473 Communicable disease Diseases 0.000 description 12
- 229930006000 Sucrose Natural products 0.000 description 12
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 12
- 206010064097 avian influenza Diseases 0.000 description 12
- 230000021615 conjugation Effects 0.000 description 12
- 230000036039 immunity Effects 0.000 description 12
- 210000000056 organ Anatomy 0.000 description 12
- 230000003204 osmotic effect Effects 0.000 description 12
- 238000007920 subcutaneous administration Methods 0.000 description 12
- 239000005720 sucrose Substances 0.000 description 12
- 230000000699 topical effect Effects 0.000 description 12
- 239000012581 transferrin Substances 0.000 description 12
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 11
- 239000007995 HEPES buffer Substances 0.000 description 11
- 239000007943 implant Substances 0.000 description 11
- 238000010253 intravenous injection Methods 0.000 description 11
- 238000012317 liver biopsy Methods 0.000 description 11
- 230000035772 mutation Effects 0.000 description 11
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 11
- 230000029812 viral genome replication Effects 0.000 description 11
- 238000011725 BALB/c mouse Methods 0.000 description 10
- 201000011001 Ebola Hemorrhagic Fever Diseases 0.000 description 10
- BJGNCJDXODQBOB-UHFFFAOYSA-N Fivefly Luciferin Natural products OC(=O)C1CSC(C=2SC3=CC(O)=CC=C3N=2)=N1 BJGNCJDXODQBOB-UHFFFAOYSA-N 0.000 description 10
- 108010014768 K(K(H)KKK)5-K(H)KKC Proteins 0.000 description 10
- 239000005089 Luciferase Substances 0.000 description 10
- 239000000443 aerosol Substances 0.000 description 10
- 238000013459 approach Methods 0.000 description 10
- 235000012000 cholesterol Nutrition 0.000 description 10
- 239000002131 composite material Substances 0.000 description 10
- 230000000875 corresponding effect Effects 0.000 description 10
- 238000000338 in vitro Methods 0.000 description 10
- 230000002458 infectious effect Effects 0.000 description 10
- 210000004072 lung Anatomy 0.000 description 10
- 239000002609 medium Substances 0.000 description 10
- 210000003071 memory t lymphocyte Anatomy 0.000 description 10
- 238000001890 transfection Methods 0.000 description 10
- 101710172711 Structural protein Proteins 0.000 description 9
- 102100036011 T-cell surface glycoprotein CD4 Human genes 0.000 description 9
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 9
- 206010073071 hepatocellular carcinoma Diseases 0.000 description 9
- 231100000844 hepatocellular carcinoma Toxicity 0.000 description 9
- 238000002360 preparation method Methods 0.000 description 9
- 230000010076 replication Effects 0.000 description 9
- 230000009385 viral infection Effects 0.000 description 9
- WQZGKKKJIJFFOK-SVZMEOIVSA-N (+)-Galactose Chemical compound OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@H]1O WQZGKKKJIJFFOK-SVZMEOIVSA-N 0.000 description 8
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 8
- 102000025850 HLA-A2 Antigen Human genes 0.000 description 8
- 108010074032 HLA-A2 Antigen Proteins 0.000 description 8
- 101710154606 Hemagglutinin Proteins 0.000 description 8
- 102000004388 Interleukin-4 Human genes 0.000 description 8
- 108090000978 Interleukin-4 Proteins 0.000 description 8
- 108091061960 Naked DNA Proteins 0.000 description 8
- 101710093908 Outer capsid protein VP4 Proteins 0.000 description 8
- 101710135467 Outer capsid protein sigma-1 Proteins 0.000 description 8
- 101710176177 Protein A56 Proteins 0.000 description 8
- 239000002671 adjuvant Substances 0.000 description 8
- 230000006907 apoptotic process Effects 0.000 description 8
- 210000001151 cytotoxic T lymphocyte Anatomy 0.000 description 8
- 238000001514 detection method Methods 0.000 description 8
- 238000011161 development Methods 0.000 description 8
- 230000018109 developmental process Effects 0.000 description 8
- 239000000185 hemagglutinin Substances 0.000 description 8
- 230000002440 hepatic effect Effects 0.000 description 8
- 230000008595 infiltration Effects 0.000 description 8
- 238000001764 infiltration Methods 0.000 description 8
- 210000005229 liver cell Anatomy 0.000 description 8
- 244000052769 pathogen Species 0.000 description 8
- 230000037452 priming Effects 0.000 description 8
- 229940023143 protein vaccine Drugs 0.000 description 8
- 238000000746 purification Methods 0.000 description 8
- 241000712461 unidentified influenza virus Species 0.000 description 8
- 208000030507 AIDS Diseases 0.000 description 7
- 102000004190 Enzymes Human genes 0.000 description 7
- 108090000790 Enzymes Proteins 0.000 description 7
- 102100026144 Transferrin receptor protein 1 Human genes 0.000 description 7
- 241000700618 Vaccinia virus Species 0.000 description 7
- 150000001413 amino acids Chemical class 0.000 description 7
- 230000037396 body weight Effects 0.000 description 7
- 230000001684 chronic effect Effects 0.000 description 7
- 210000002216 heart Anatomy 0.000 description 7
- 208000006454 hepatitis Diseases 0.000 description 7
- 210000003734 kidney Anatomy 0.000 description 7
- 210000001165 lymph node Anatomy 0.000 description 7
- 238000003753 real-time PCR Methods 0.000 description 7
- 238000013268 sustained release Methods 0.000 description 7
- 239000012730 sustained-release form Substances 0.000 description 7
- 239000013603 viral vector Substances 0.000 description 7
- WBSCNDJQPKSPII-UHFFFAOYSA-N 6-amino-2-[[6-amino-2-(2,6-diaminohexanoylamino)hexanoyl]amino]hexanoic acid Chemical compound NCCCCC(N)C(=O)NC(CCCCN)C(=O)NC(CCCCN)C(O)=O WBSCNDJQPKSPII-UHFFFAOYSA-N 0.000 description 6
- 102000003886 Glycoproteins Human genes 0.000 description 6
- 108090000288 Glycoproteins Proteins 0.000 description 6
- 108010067060 Immunoglobulin Variable Region Proteins 0.000 description 6
- 102000017727 Immunoglobulin Variable Region Human genes 0.000 description 6
- 208000002979 Influenza in Birds Diseases 0.000 description 6
- 102000003814 Interleukin-10 Human genes 0.000 description 6
- 108090000174 Interleukin-10 Proteins 0.000 description 6
- 108010002616 Interleukin-5 Proteins 0.000 description 6
- 102000000743 Interleukin-5 Human genes 0.000 description 6
- 108010002586 Interleukin-7 Proteins 0.000 description 6
- 102000000704 Interleukin-7 Human genes 0.000 description 6
- 102000004338 Transferrin Human genes 0.000 description 6
- 108090000901 Transferrin Proteins 0.000 description 6
- 108020000999 Viral RNA Proteins 0.000 description 6
- 239000013543 active substance Substances 0.000 description 6
- 230000001154 acute effect Effects 0.000 description 6
- 235000001014 amino acid Nutrition 0.000 description 6
- 239000003153 chemical reaction reagent Substances 0.000 description 6
- 239000003795 chemical substances by application Substances 0.000 description 6
- 230000009260 cross reactivity Effects 0.000 description 6
- 238000009826 distribution Methods 0.000 description 6
- 238000002296 dynamic light scattering Methods 0.000 description 6
- 238000002474 experimental method Methods 0.000 description 6
- 230000006870 function Effects 0.000 description 6
- 238000001476 gene delivery Methods 0.000 description 6
- 238000001415 gene therapy Methods 0.000 description 6
- 239000010410 layer Substances 0.000 description 6
- 239000007788 liquid Substances 0.000 description 6
- 210000004698 lymphocyte Anatomy 0.000 description 6
- 125000005439 maleimidyl group Chemical group C1(C=CC(N1*)=O)=O 0.000 description 6
- 230000007246 mechanism Effects 0.000 description 6
- 230000007935 neutral effect Effects 0.000 description 6
- 230000001717 pathogenic effect Effects 0.000 description 6
- 229940021993 prophylactic vaccine Drugs 0.000 description 6
- 238000011084 recovery Methods 0.000 description 6
- 230000002829 reductive effect Effects 0.000 description 6
- 238000011160 research Methods 0.000 description 6
- 210000003462 vein Anatomy 0.000 description 6
- 230000003442 weekly effect Effects 0.000 description 6
- 241001115402 Ebolavirus Species 0.000 description 5
- 208000005176 Hepatitis C Diseases 0.000 description 5
- 102000000589 Interleukin-1 Human genes 0.000 description 5
- 108010002352 Interleukin-1 Proteins 0.000 description 5
- 102000003816 Interleukin-13 Human genes 0.000 description 5
- 108090000176 Interleukin-13 Proteins 0.000 description 5
- 108050003558 Interleukin-17 Proteins 0.000 description 5
- 102000013691 Interleukin-17 Human genes 0.000 description 5
- 102000003810 Interleukin-18 Human genes 0.000 description 5
- 108090000171 Interleukin-18 Proteins 0.000 description 5
- 102000013264 Interleukin-23 Human genes 0.000 description 5
- 108010065637 Interleukin-23 Proteins 0.000 description 5
- 108010002386 Interleukin-3 Proteins 0.000 description 5
- 102000000646 Interleukin-3 Human genes 0.000 description 5
- 102000004889 Interleukin-6 Human genes 0.000 description 5
- 108090001005 Interleukin-6 Proteins 0.000 description 5
- 108090001007 Interleukin-8 Proteins 0.000 description 5
- 102000004890 Interleukin-8 Human genes 0.000 description 5
- 241000282560 Macaca mulatta Species 0.000 description 5
- 241000699660 Mus musculus Species 0.000 description 5
- 102000005348 Neuraminidase Human genes 0.000 description 5
- 108010006232 Neuraminidase Proteins 0.000 description 5
- 208000035415 Reinfection Diseases 0.000 description 5
- 108010033576 Transferrin Receptors Proteins 0.000 description 5
- 206010058874 Viraemia Diseases 0.000 description 5
- 238000001574 biopsy Methods 0.000 description 5
- 210000004556 brain Anatomy 0.000 description 5
- 229940030156 cell vaccine Drugs 0.000 description 5
- 230000001413 cellular effect Effects 0.000 description 5
- 235000018417 cysteine Nutrition 0.000 description 5
- 230000003247 decreasing effect Effects 0.000 description 5
- 238000005538 encapsulation Methods 0.000 description 5
- 238000005516 engineering process Methods 0.000 description 5
- 239000000284 extract Substances 0.000 description 5
- 238000000605 extraction Methods 0.000 description 5
- 210000001035 gastrointestinal tract Anatomy 0.000 description 5
- 231100000283 hepatitis Toxicity 0.000 description 5
- 230000001900 immune effect Effects 0.000 description 5
- 229960003971 influenza vaccine Drugs 0.000 description 5
- 210000001865 kupffer cell Anatomy 0.000 description 5
- 239000002479 lipoplex Substances 0.000 description 5
- 208000019423 liver disease Diseases 0.000 description 5
- 239000013642 negative control Substances 0.000 description 5
- 230000003472 neutralizing effect Effects 0.000 description 5
- 210000001672 ovary Anatomy 0.000 description 5
- 210000000496 pancreas Anatomy 0.000 description 5
- 230000008569 process Effects 0.000 description 5
- 239000000047 product Substances 0.000 description 5
- 238000011321 prophylaxis Methods 0.000 description 5
- 230000009885 systemic effect Effects 0.000 description 5
- 210000004881 tumor cell Anatomy 0.000 description 5
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 4
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 4
- 206010002091 Anaesthesia Diseases 0.000 description 4
- 102000017420 CD3 protein, epsilon/gamma/delta subunit Human genes 0.000 description 4
- 108050005493 CD3 protein, epsilon/gamma/delta subunit Proteins 0.000 description 4
- IGXWBGJHJZYPQS-SSDOTTSWSA-N D-Luciferin Chemical compound OC(=O)[C@H]1CSC(C=2SC3=CC=C(O)C=C3N=2)=N1 IGXWBGJHJZYPQS-SSDOTTSWSA-N 0.000 description 4
- CYCGRDQQIOGCKX-UHFFFAOYSA-N Dehydro-luciferin Natural products OC(=O)C1=CSC(C=2SC3=CC(O)=CC=C3N=2)=N1 CYCGRDQQIOGCKX-UHFFFAOYSA-N 0.000 description 4
- 238000011510 Elispot assay Methods 0.000 description 4
- 102100031181 Glyceraldehyde-3-phosphate dehydrogenase Human genes 0.000 description 4
- 241000713772 Human immunodeficiency virus 1 Species 0.000 description 4
- 108010002335 Interleukin-9 Proteins 0.000 description 4
- 102000000585 Interleukin-9 Human genes 0.000 description 4
- DDWFXDSYGUXRAY-UHFFFAOYSA-N Luciferin Natural products CCc1c(C)c(CC2NC(=O)C(=C2C=C)C)[nH]c1Cc3[nH]c4C(=C5/NC(CC(=O)O)C(C)C5CC(=O)O)CC(=O)c4c3C DDWFXDSYGUXRAY-UHFFFAOYSA-N 0.000 description 4
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 4
- 108090001074 Nucleocapsid Proteins Proteins 0.000 description 4
- 229910019142 PO4 Inorganic materials 0.000 description 4
- 241000315672 SARS coronavirus Species 0.000 description 4
- 229920002684 Sepharose Polymers 0.000 description 4
- 238000000692 Student's t-test Methods 0.000 description 4
- 238000010521 absorption reaction Methods 0.000 description 4
- 230000009471 action Effects 0.000 description 4
- 230000037005 anaesthesia Effects 0.000 description 4
- 230000000890 antigenic effect Effects 0.000 description 4
- 230000005540 biological transmission Effects 0.000 description 4
- 210000001185 bone marrow Anatomy 0.000 description 4
- 239000000872 buffer Substances 0.000 description 4
- 238000005119 centrifugation Methods 0.000 description 4
- 230000008859 change Effects 0.000 description 4
- 238000006243 chemical reaction Methods 0.000 description 4
- 238000000576 coating method Methods 0.000 description 4
- 238000004440 column chromatography Methods 0.000 description 4
- 230000009918 complex formation Effects 0.000 description 4
- 239000000470 constituent Substances 0.000 description 4
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 4
- 210000004443 dendritic cell Anatomy 0.000 description 4
- 239000000032 diagnostic agent Substances 0.000 description 4
- 229940039227 diagnostic agent Drugs 0.000 description 4
- 230000008030 elimination Effects 0.000 description 4
- 238000003379 elimination reaction Methods 0.000 description 4
- 238000001839 endoscopy Methods 0.000 description 4
- 238000003114 enzyme-linked immunosorbent spot assay Methods 0.000 description 4
- 102000054766 genetic haplotypes Human genes 0.000 description 4
- 108020004445 glyceraldehyde-3-phosphate dehydrogenase Proteins 0.000 description 4
- 208000005252 hepatitis A Diseases 0.000 description 4
- 238000003384 imaging method Methods 0.000 description 4
- 210000000987 immune system Anatomy 0.000 description 4
- 230000003993 interaction Effects 0.000 description 4
- 229910052757 nitrogen Inorganic materials 0.000 description 4
- 238000012634 optical imaging Methods 0.000 description 4
- 239000002245 particle Substances 0.000 description 4
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 4
- 239000010452 phosphate Substances 0.000 description 4
- 229920000642 polymer Polymers 0.000 description 4
- 210000003240 portal vein Anatomy 0.000 description 4
- 230000002265 prevention Effects 0.000 description 4
- 230000035755 proliferation Effects 0.000 description 4
- 238000012552 review Methods 0.000 description 4
- 239000002356 single layer Substances 0.000 description 4
- 238000010561 standard procedure Methods 0.000 description 4
- 239000000758 substrate Substances 0.000 description 4
- 125000003396 thiol group Chemical group [H]S* 0.000 description 4
- 231100000419 toxicity Toxicity 0.000 description 4
- 230000001988 toxicity Effects 0.000 description 4
- 238000011830 transgenic mouse model Methods 0.000 description 4
- TZCPCKNHXULUIY-RGULYWFUSA-N 1,2-distearoyl-sn-glycero-3-phosphoserine Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@H](COP(O)(=O)OC[C@H](N)C(O)=O)OC(=O)CCCCCCCCCCCCCCCCC TZCPCKNHXULUIY-RGULYWFUSA-N 0.000 description 3
- 102100036301 C-C chemokine receptor type 7 Human genes 0.000 description 3
- 210000001266 CD8-positive T-lymphocyte Anatomy 0.000 description 3
- 101100506090 Caenorhabditis elegans hil-2 gene Proteins 0.000 description 3
- 241000711573 Coronaviridae Species 0.000 description 3
- 108010041986 DNA Vaccines Proteins 0.000 description 3
- 238000007400 DNA extraction Methods 0.000 description 3
- 229940021995 DNA vaccine Drugs 0.000 description 3
- 108091029865 Exogenous DNA Proteins 0.000 description 3
- 206010016654 Fibrosis Diseases 0.000 description 3
- 241000711950 Filoviridae Species 0.000 description 3
- ZWZWYGMENQVNFU-UHFFFAOYSA-N Glycerophosphorylserin Natural products OC(=O)C(N)COP(O)(=O)OCC(O)CO ZWZWYGMENQVNFU-UHFFFAOYSA-N 0.000 description 3
- 101001018097 Homo sapiens L-selectin Proteins 0.000 description 3
- 101000914514 Homo sapiens T-cell-specific surface glycoprotein CD28 Proteins 0.000 description 3
- 102100034349 Integrase Human genes 0.000 description 3
- 102100033467 L-selectin Human genes 0.000 description 3
- JQSIGLHQNSZZRL-KKUMJFAQSA-N Lys-Lys-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)N JQSIGLHQNSZZRL-KKUMJFAQSA-N 0.000 description 3
- 101800001019 Non-structural protein 4B Proteins 0.000 description 3
- 102000011931 Nucleoproteins Human genes 0.000 description 3
- 108010061100 Nucleoproteins Proteins 0.000 description 3
- CTQNGGLPUBDAKN-UHFFFAOYSA-N O-Xylene Chemical compound CC1=CC=CC=C1C CTQNGGLPUBDAKN-UHFFFAOYSA-N 0.000 description 3
- 208000037581 Persistent Infection Diseases 0.000 description 3
- 108700008625 Reporter Genes Proteins 0.000 description 3
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 3
- 102100027213 T-cell-specific surface glycoprotein CD28 Human genes 0.000 description 3
- 210000004241 Th2 cell Anatomy 0.000 description 3
- 208000036142 Viral infection Diseases 0.000 description 3
- 201000011510 cancer Diseases 0.000 description 3
- 239000007979 citrate buffer Substances 0.000 description 3
- 238000010367 cloning Methods 0.000 description 3
- 239000011248 coating agent Substances 0.000 description 3
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 description 3
- 238000010790 dilution Methods 0.000 description 3
- 239000012895 dilution Substances 0.000 description 3
- 230000002708 enhancing effect Effects 0.000 description 3
- 210000002919 epithelial cell Anatomy 0.000 description 3
- 210000003743 erythrocyte Anatomy 0.000 description 3
- MHMNJMPURVTYEJ-UHFFFAOYSA-N fluorescein-5-isothiocyanate Chemical compound O1C(=O)C2=CC(N=C=S)=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 MHMNJMPURVTYEJ-UHFFFAOYSA-N 0.000 description 3
- 230000002068 genetic effect Effects 0.000 description 3
- 210000002149 gonad Anatomy 0.000 description 3
- 201000010536 head and neck cancer Diseases 0.000 description 3
- 208000014829 head and neck neoplasm Diseases 0.000 description 3
- 238000009396 hybridization Methods 0.000 description 3
- 208000037797 influenza A Diseases 0.000 description 3
- 238000001802 infusion Methods 0.000 description 3
- 230000005764 inhibitory process Effects 0.000 description 3
- 238000002372 labelling Methods 0.000 description 3
- 239000012528 membrane Substances 0.000 description 3
- 235000013336 milk Nutrition 0.000 description 3
- 239000008267 milk Substances 0.000 description 3
- 210000004080 milk Anatomy 0.000 description 3
- 238000000329 molecular dynamics simulation Methods 0.000 description 3
- 239000002114 nanocomposite Substances 0.000 description 3
- 108700025694 p53 Genes Proteins 0.000 description 3
- 230000008506 pathogenesis Effects 0.000 description 3
- 230000010412 perfusion Effects 0.000 description 3
- 239000013641 positive control Substances 0.000 description 3
- 238000002203 pretreatment Methods 0.000 description 3
- 238000012545 processing Methods 0.000 description 3
- 239000008213 purified water Substances 0.000 description 3
- 230000005855 radiation Effects 0.000 description 3
- 230000010837 receptor-mediated endocytosis Effects 0.000 description 3
- 230000009467 reduction Effects 0.000 description 3
- 150000003384 small molecules Chemical class 0.000 description 3
- 238000009987 spinning Methods 0.000 description 3
- 238000012353 t test Methods 0.000 description 3
- 238000002560 therapeutic procedure Methods 0.000 description 3
- 239000008096 xylene Substances 0.000 description 3
- RRBGTUQJDFBWNN-MUGJNUQGSA-N (2s)-6-amino-2-[[(2s)-6-amino-2-[[(2s)-6-amino-2-[[(2s)-2,6-diaminohexanoyl]amino]hexanoyl]amino]hexanoyl]amino]hexanoic acid Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(O)=O RRBGTUQJDFBWNN-MUGJNUQGSA-N 0.000 description 2
- 108700028369 Alleles Proteins 0.000 description 2
- 241000272525 Anas platyrhynchos Species 0.000 description 2
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 2
- 102100025064 Cellular tumor antigen p53 Human genes 0.000 description 2
- 208000000419 Chronic Hepatitis B Diseases 0.000 description 2
- 206010012239 Delusion Diseases 0.000 description 2
- 208000001490 Dengue Diseases 0.000 description 2
- 206010012310 Dengue fever Diseases 0.000 description 2
- 238000002965 ELISA Methods 0.000 description 2
- 101710091045 Envelope protein Proteins 0.000 description 2
- 241000588724 Escherichia coli Species 0.000 description 2
- 229920001917 Ficoll Polymers 0.000 description 2
- 241000710781 Flaviviridae Species 0.000 description 2
- 206010054261 Flavivirus infection Diseases 0.000 description 2
- 102000005720 Glutathione transferase Human genes 0.000 description 2
- 108010070675 Glutathione transferase Proteins 0.000 description 2
- 101710114810 Glycoprotein Proteins 0.000 description 2
- 108060003393 Granulin Proteins 0.000 description 2
- 229940033330 HIV vaccine Drugs 0.000 description 2
- 241000711557 Hepacivirus Species 0.000 description 2
- UMBKDWGQESDCTO-KKUMJFAQSA-N His-Lys-Lys Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(O)=O UMBKDWGQESDCTO-KKUMJFAQSA-N 0.000 description 2
- 101000716065 Homo sapiens C-C chemokine receptor type 7 Proteins 0.000 description 2
- 101001002657 Homo sapiens Interleukin-2 Proteins 0.000 description 2
- 101000611023 Homo sapiens Tumor necrosis factor receptor superfamily member 6 Proteins 0.000 description 2
- 206010061598 Immunodeficiency Diseases 0.000 description 2
- 108010054477 Immunoglobulin Fab Fragments Proteins 0.000 description 2
- 102000001706 Immunoglobulin Fab Fragments Human genes 0.000 description 2
- 108010064593 Intercellular Adhesion Molecule-1 Proteins 0.000 description 2
- 102100037877 Intercellular adhesion molecule 1 Human genes 0.000 description 2
- 206010023927 Lassa fever Diseases 0.000 description 2
- 101100006310 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) chol-1 gene Proteins 0.000 description 2
- 101100297828 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) chol-2 gene Proteins 0.000 description 2
- 101800001020 Non-structural protein 4A Proteins 0.000 description 2
- 108700026244 Open Reading Frames Proteins 0.000 description 2
- 241001494479 Pecora Species 0.000 description 2
- 239000004743 Polypropylene Substances 0.000 description 2
- 241000288906 Primates Species 0.000 description 2
- 229940124158 Protease/peptidase inhibitor Drugs 0.000 description 2
- 101710192141 Protein Nef Proteins 0.000 description 2
- 101710188315 Protein X Proteins 0.000 description 2
- 238000011530 RNeasy Mini Kit Methods 0.000 description 2
- 108020004459 Small interfering RNA Proteins 0.000 description 2
- PXIPVTKHYLBLMZ-UHFFFAOYSA-N Sodium azide Chemical compound [Na+].[N-]=[N+]=[N-] PXIPVTKHYLBLMZ-UHFFFAOYSA-N 0.000 description 2
- 101710167605 Spike glycoprotein Proteins 0.000 description 2
- 230000037453 T cell priming Effects 0.000 description 2
- 102100040403 Tumor necrosis factor receptor superfamily member 6 Human genes 0.000 description 2
- 101150010086 VP24 gene Proteins 0.000 description 2
- 101150026858 VP30 gene Proteins 0.000 description 2
- 101150077651 VP35 gene Proteins 0.000 description 2
- 101150036892 VP40 gene Proteins 0.000 description 2
- 241000710886 West Nile virus Species 0.000 description 2
- 241000021375 Xenogenes Species 0.000 description 2
- 208000003152 Yellow Fever Diseases 0.000 description 2
- 230000004913 activation Effects 0.000 description 2
- 230000004721 adaptive immunity Effects 0.000 description 2
- 239000011543 agarose gel Substances 0.000 description 2
- 125000000129 anionic group Chemical group 0.000 description 2
- 230000000692 anti-sense effect Effects 0.000 description 2
- 239000003146 anticoagulant agent Substances 0.000 description 2
- 229940127219 anticoagulant drug Drugs 0.000 description 2
- 239000004599 antimicrobial Substances 0.000 description 2
- 239000007864 aqueous solution Substances 0.000 description 2
- 230000002238 attenuated effect Effects 0.000 description 2
- 230000027455 binding Effects 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 238000010805 cDNA synthesis kit Methods 0.000 description 2
- 210000000234 capsid Anatomy 0.000 description 2
- 229920006317 cationic polymer Polymers 0.000 description 2
- 230000004709 cell invasion Effects 0.000 description 2
- 238000001516 cell proliferation assay Methods 0.000 description 2
- 239000013043 chemical agent Substances 0.000 description 2
- 208000016350 chronic hepatitis B virus infection Diseases 0.000 description 2
- 230000007882 cirrhosis Effects 0.000 description 2
- 208000019425 cirrhosis of liver Diseases 0.000 description 2
- 230000000052 comparative effect Effects 0.000 description 2
- 230000001268 conjugating effect Effects 0.000 description 2
- 238000005859 coupling reaction Methods 0.000 description 2
- 239000013078 crystal Substances 0.000 description 2
- 230000001461 cytolytic effect Effects 0.000 description 2
- 210000000805 cytoplasm Anatomy 0.000 description 2
- 231100000868 delusion Toxicity 0.000 description 2
- 208000025729 dengue disease Diseases 0.000 description 2
- 238000003745 diagnosis Methods 0.000 description 2
- 230000004069 differentiation Effects 0.000 description 2
- 241001493065 dsRNA viruses Species 0.000 description 2
- 108010048367 enhanced green fluorescent protein Proteins 0.000 description 2
- 238000011156 evaluation Methods 0.000 description 2
- 230000017188 evasion or tolerance of host immune response Effects 0.000 description 2
- 239000000499 gel Substances 0.000 description 2
- 239000001963 growth medium Substances 0.000 description 2
- 229920000669 heparin Polymers 0.000 description 2
- 208000010710 hepatitis C virus infection Diseases 0.000 description 2
- 231100000334 hepatotoxic Toxicity 0.000 description 2
- 230000003082 hepatotoxic effect Effects 0.000 description 2
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 2
- 150000002430 hydrocarbons Chemical group 0.000 description 2
- 238000010166 immunofluorescence Methods 0.000 description 2
- 239000002955 immunomodulating agent Substances 0.000 description 2
- 230000001976 improved effect Effects 0.000 description 2
- 238000011534 incubation Methods 0.000 description 2
- 230000009545 invasion Effects 0.000 description 2
- 230000007774 longterm Effects 0.000 description 2
- 238000003670 luciferase enzyme activity assay Methods 0.000 description 2
- 210000002540 macrophage Anatomy 0.000 description 2
- 239000003550 marker Substances 0.000 description 2
- WSFSSNUMVMOOMR-NJFSPNSNSA-N methanone Chemical compound O=[14CH2] WSFSSNUMVMOOMR-NJFSPNSNSA-N 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 210000001616 monocyte Anatomy 0.000 description 2
- 210000000822 natural killer cell Anatomy 0.000 description 2
- 229910052759 nickel Inorganic materials 0.000 description 2
- 238000005457 optimization Methods 0.000 description 2
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 2
- 210000001539 phagocyte Anatomy 0.000 description 2
- 229920003213 poly(N-isopropyl acrylamide) Polymers 0.000 description 2
- 229920001155 polypropylene Polymers 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- 239000003755 preservative agent Substances 0.000 description 2
- 238000011809 primate model Methods 0.000 description 2
- 230000002035 prolonged effect Effects 0.000 description 2
- XNSAINXGIQZQOO-SRVKXCTJSA-N protirelin Chemical compound NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)[C@H]1NC(=O)CC1)CC1=CN=CN1 XNSAINXGIQZQOO-SRVKXCTJSA-N 0.000 description 2
- 230000006798 recombination Effects 0.000 description 2
- 238000005215 recombination Methods 0.000 description 2
- 230000003362 replicative effect Effects 0.000 description 2
- 230000000717 retained effect Effects 0.000 description 2
- 230000002441 reversible effect Effects 0.000 description 2
- 238000005070 sampling Methods 0.000 description 2
- 230000028327 secretion Effects 0.000 description 2
- 230000035945 sensitivity Effects 0.000 description 2
- 210000003491 skin Anatomy 0.000 description 2
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 2
- 238000010186 staining Methods 0.000 description 2
- 235000000346 sugar Nutrition 0.000 description 2
- 150000008163 sugars Chemical class 0.000 description 2
- 239000004094 surface-active agent Substances 0.000 description 2
- 238000012385 systemic delivery Methods 0.000 description 2
- 210000001550 testis Anatomy 0.000 description 2
- 210000001541 thymus gland Anatomy 0.000 description 2
- 231100000041 toxicology testing Toxicity 0.000 description 2
- 238000013519 translation Methods 0.000 description 2
- 239000003981 vehicle Substances 0.000 description 2
- 244000052613 viral pathogen Species 0.000 description 2
- 238000005406 washing Methods 0.000 description 2
- 239000002023 wood Substances 0.000 description 2
- MZOFCQQQCNRIBI-VMXHOPILSA-N (3s)-4-[[(2s)-1-[[(2s)-1-[[(1s)-1-carboxy-2-hydroxyethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-3-[[2-[[(2s)-2,6-diaminohexanoyl]amino]acetyl]amino]-4-oxobutanoic acid Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN MZOFCQQQCNRIBI-VMXHOPILSA-N 0.000 description 1
- 102000040650 (ribonucleotides)n+m Human genes 0.000 description 1
- QCDWFXQBSFUVSP-UHFFFAOYSA-N 2-phenoxyethanol Chemical compound OCCOC1=CC=CC=C1 QCDWFXQBSFUVSP-UHFFFAOYSA-N 0.000 description 1
- FWBHETKCLVMNFS-UHFFFAOYSA-N 4',6-Diamino-2-phenylindol Chemical compound C1=CC(C(=N)N)=CC=C1C1=CC2=CC=C(C(N)=N)C=C2N1 FWBHETKCLVMNFS-UHFFFAOYSA-N 0.000 description 1
- 108020003589 5' Untranslated Regions Proteins 0.000 description 1
- 241000251468 Actinopterygii Species 0.000 description 1
- 101710186708 Agglutinin Proteins 0.000 description 1
- 102100036475 Alanine aminotransferase 1 Human genes 0.000 description 1
- 108010082126 Alanine transaminase Proteins 0.000 description 1
- 108010088751 Albumins Proteins 0.000 description 1
- 102000009027 Albumins Human genes 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- 241000710929 Alphavirus Species 0.000 description 1
- APKFDSVGJQXUKY-KKGHZKTASA-N Amphotericin-B Natural products O[C@H]1[C@@H](N)[C@H](O)[C@@H](C)O[C@H]1O[C@H]1C=CC=CC=CC=CC=CC=CC=C[C@H](C)[C@@H](O)[C@@H](C)[C@H](C)OC(=O)C[C@H](O)C[C@H](O)CC[C@@H](O)[C@H](O)C[C@H](O)C[C@](O)(C[C@H](O)[C@H]2C(O)=O)O[C@H]2C1 APKFDSVGJQXUKY-KKGHZKTASA-N 0.000 description 1
- 108010032595 Antibody Binding Sites Proteins 0.000 description 1
- 108020000948 Antisense Oligonucleotides Proteins 0.000 description 1
- 208000002109 Argyria Diseases 0.000 description 1
- 102000005427 Asialoglycoprotein Receptor Human genes 0.000 description 1
- 208000031504 Asymptomatic Infections Diseases 0.000 description 1
- 108010056594 Avian Proteins Proteins 0.000 description 1
- 238000000035 BCA protein assay Methods 0.000 description 1
- 108700031361 Brachyury Proteins 0.000 description 1
- 101710149858 C-C chemokine receptor type 7 Proteins 0.000 description 1
- 102100027207 CD27 antigen Human genes 0.000 description 1
- 102100036008 CD48 antigen Human genes 0.000 description 1
- 101100512078 Caenorhabditis elegans lys-1 gene Proteins 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 241000282472 Canis lupus familiaris Species 0.000 description 1
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 1
- 102000053642 Catalytic RNA Human genes 0.000 description 1
- 108090000994 Catalytic RNA Proteins 0.000 description 1
- 241000282693 Cercopithecidae Species 0.000 description 1
- 206010008909 Chronic Hepatitis Diseases 0.000 description 1
- 208000006154 Chronic hepatitis C Diseases 0.000 description 1
- 206010053567 Coagulopathies Diseases 0.000 description 1
- 108091035707 Consensus sequence Proteins 0.000 description 1
- 241000195493 Cryptophyta Species 0.000 description 1
- 101710118188 DNA-binding protein HU-alpha Proteins 0.000 description 1
- 108010016626 Dipeptides Proteins 0.000 description 1
- 108010053187 Diphtheria Toxin Proteins 0.000 description 1
- 102000016607 Diphtheria Toxin Human genes 0.000 description 1
- 208000000655 Distemper Diseases 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- 238000012286 ELISA Assay Methods 0.000 description 1
- 239000006145 Eagle's minimal essential medium Substances 0.000 description 1
- 102100038132 Endogenous retrovirus group K member 6 Pro protein Human genes 0.000 description 1
- 241000709661 Enterovirus Species 0.000 description 1
- 241000991587 Enterovirus C Species 0.000 description 1
- 241000283086 Equidae Species 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 241000710831 Flavivirus Species 0.000 description 1
- 229930182566 Gentamicin Natural products 0.000 description 1
- CEAZRRDELHUEMR-URQXQFDESA-N Gentamicin Chemical compound O1[C@H](C(C)NC)CC[C@@H](N)[C@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](NC)[C@@](C)(O)CO2)O)[C@H](N)C[C@@H]1N CEAZRRDELHUEMR-URQXQFDESA-N 0.000 description 1
- 208000032843 Hemorrhage Diseases 0.000 description 1
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 description 1
- 241000711553 Hepatitis C virus (isolate H) Species 0.000 description 1
- 108700039791 Hepatitis C virus nucleocapsid Proteins 0.000 description 1
- 102000008949 Histocompatibility Antigens Class I Human genes 0.000 description 1
- 108010088652 Histocompatibility Antigens Class I Proteins 0.000 description 1
- 102000018713 Histocompatibility Antigens Class II Human genes 0.000 description 1
- 241001272567 Hominoidea Species 0.000 description 1
- 101000914511 Homo sapiens CD27 antigen Proteins 0.000 description 1
- 101000716130 Homo sapiens CD48 antigen Proteins 0.000 description 1
- 101000674278 Homo sapiens Serine-tRNA ligase, cytoplasmic Proteins 0.000 description 1
- 101000674040 Homo sapiens Serine-tRNA ligase, mitochondrial Proteins 0.000 description 1
- 101000766306 Homo sapiens Serotransferrin Proteins 0.000 description 1
- 101000716102 Homo sapiens T-cell surface glycoprotein CD4 Proteins 0.000 description 1
- 101000946843 Homo sapiens T-cell surface glycoprotein CD8 alpha chain Proteins 0.000 description 1
- 101000914484 Homo sapiens T-lymphocyte activation antigen CD80 Proteins 0.000 description 1
- 101710146024 Horcolin Proteins 0.000 description 1
- 241000598171 Human adenovirus sp. Species 0.000 description 1
- 241000701085 Human alphaherpesvirus 3 Species 0.000 description 1
- 208000029462 Immunodeficiency disease Diseases 0.000 description 1
- 238000012404 In vitro experiment Methods 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- 102100026720 Interferon beta Human genes 0.000 description 1
- 108010047761 Interferon-alpha Proteins 0.000 description 1
- 102000006992 Interferon-alpha Human genes 0.000 description 1
- 108090000467 Interferon-beta Proteins 0.000 description 1
- 102000008070 Interferon-gamma Human genes 0.000 description 1
- 108020004684 Internal Ribosome Entry Sites Proteins 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 1
- 101150107380 LIPG gene Proteins 0.000 description 1
- 101150048797 LIPH gene Proteins 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 108091026898 Leader sequence (mRNA) Proteins 0.000 description 1
- 101710189395 Lectin Proteins 0.000 description 1
- 108090001090 Lectins Proteins 0.000 description 1
- 102000004856 Lectins Human genes 0.000 description 1
- 102000008072 Lymphokines Human genes 0.000 description 1
- 108010074338 Lymphokines Proteins 0.000 description 1
- FGMHXLULNHTPID-KKUMJFAQSA-N Lys-His-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CCCCN)C(O)=O)CC1=CN=CN1 FGMHXLULNHTPID-KKUMJFAQSA-N 0.000 description 1
- WBSCNDJQPKSPII-KKUMJFAQSA-N Lys-Lys-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(O)=O WBSCNDJQPKSPII-KKUMJFAQSA-N 0.000 description 1
- 108091054438 MHC class II family Proteins 0.000 description 1
- 241000282553 Macaca Species 0.000 description 1
- PEEHTFAAVSWFBL-UHFFFAOYSA-N Maleimide Chemical compound O=C1NC(=O)C=C1 PEEHTFAAVSWFBL-UHFFFAOYSA-N 0.000 description 1
- 101710179758 Mannose-specific lectin Proteins 0.000 description 1
- 101710150763 Mannose-specific lectin 1 Proteins 0.000 description 1
- 101710150745 Mannose-specific lectin 2 Proteins 0.000 description 1
- 241001115401 Marburgvirus Species 0.000 description 1
- 108091007491 NSP3 Papain-like protease domains Proteins 0.000 description 1
- 229930193140 Neomycin Natural products 0.000 description 1
- 101710144128 Non-structural protein 2 Proteins 0.000 description 1
- 101150038760 Ns3 gene Proteins 0.000 description 1
- 108010077850 Nuclear Localization Signals Proteins 0.000 description 1
- 101710199667 Nuclear export protein Proteins 0.000 description 1
- 108091028043 Nucleic acid sequence Proteins 0.000 description 1
- 108010038807 Oligopeptides Proteins 0.000 description 1
- 102000015636 Oligopeptides Human genes 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 101710160107 Outer membrane protein A Proteins 0.000 description 1
- 238000012408 PCR amplification Methods 0.000 description 1
- 238000010222 PCR analysis Methods 0.000 description 1
- 239000002033 PVDF binder Substances 0.000 description 1
- 206010061902 Pancreatic neoplasm Diseases 0.000 description 1
- 241001631646 Papillomaviridae Species 0.000 description 1
- 208000002606 Paramyxoviridae Infections Diseases 0.000 description 1
- 108091005804 Peptidases Proteins 0.000 description 1
- KHGNFPUMBJSZSM-UHFFFAOYSA-N Perforine Natural products COC1=C2CCC(O)C(CCC(C)(C)O)(OC)C2=NC2=C1C=CO2 KHGNFPUMBJSZSM-UHFFFAOYSA-N 0.000 description 1
- 241000710778 Pestivirus Species 0.000 description 1
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 1
- 241000709664 Picornaviridae Species 0.000 description 1
- 108010076039 Polyproteins Proteins 0.000 description 1
- 241000269324 Polypteridae Species 0.000 description 1
- 229920001213 Polysorbate 20 Polymers 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- 239000004365 Protease Substances 0.000 description 1
- 108010001267 Protein Subunits Proteins 0.000 description 1
- 102000002067 Protein Subunits Human genes 0.000 description 1
- 241000125945 Protoparvovirus Species 0.000 description 1
- 108700033844 Pseudomonas aeruginosa toxA Proteins 0.000 description 1
- 230000004570 RNA-binding Effects 0.000 description 1
- 108010092799 RNA-directed DNA polymerase Proteins 0.000 description 1
- 241000711798 Rabies lyssavirus Species 0.000 description 1
- 101900083372 Rabies virus Glycoprotein Proteins 0.000 description 1
- 241000700159 Rattus Species 0.000 description 1
- 108020004511 Recombinant DNA Proteins 0.000 description 1
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 1
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 1
- 241000702263 Reovirus sp. Species 0.000 description 1
- IWUCXVSUMQZMFG-AFCXAGJDSA-N Ribavirin Chemical compound N1=C(C(=O)N)N=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 IWUCXVSUMQZMFG-AFCXAGJDSA-N 0.000 description 1
- 241000702670 Rotavirus Species 0.000 description 1
- 108091005487 SCARB1 Proteins 0.000 description 1
- 238000011579 SCID mouse model Methods 0.000 description 1
- 238000012300 Sequence Analysis Methods 0.000 description 1
- 108010071390 Serum Albumin Proteins 0.000 description 1
- 102000007562 Serum Albumin Human genes 0.000 description 1
- 241000700584 Simplexvirus Species 0.000 description 1
- 101001039853 Sonchus yellow net virus Matrix protein Proteins 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- 230000024932 T cell mediated immunity Effects 0.000 description 1
- 230000006052 T cell proliferation Effects 0.000 description 1
- 102100034922 T-cell surface glycoprotein CD8 alpha chain Human genes 0.000 description 1
- 102100027222 T-lymphocyte activation antigen CD80 Human genes 0.000 description 1
- 238000012288 TUNEL assay Methods 0.000 description 1
- 210000000447 Th1 cell Anatomy 0.000 description 1
- 238000008050 Total Bilirubin Reagent Methods 0.000 description 1
- 108091023040 Transcription factor Proteins 0.000 description 1
- 102000040945 Transcription factor Human genes 0.000 description 1
- 102000004357 Transferases Human genes 0.000 description 1
- 108090000992 Transferases Proteins 0.000 description 1
- 102000004887 Transforming Growth Factor beta Human genes 0.000 description 1
- 108090001012 Transforming Growth Factor beta Proteins 0.000 description 1
- 101800004564 Transforming growth factor alpha Proteins 0.000 description 1
- 102400001320 Transforming growth factor alpha Human genes 0.000 description 1
- 108700019146 Transgenes Proteins 0.000 description 1
- 102000004142 Trypsin Human genes 0.000 description 1
- 108090000631 Trypsin Proteins 0.000 description 1
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 1
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 1
- 108010003533 Viral Envelope Proteins Proteins 0.000 description 1
- 108700005077 Viral Genes Proteins 0.000 description 1
- 108700022715 Viral Proteases Proteins 0.000 description 1
- HMNZFMSWFCAGGW-XPWSMXQVSA-N [3-[hydroxy(2-hydroxyethoxy)phosphoryl]oxy-2-[(e)-octadec-9-enoyl]oxypropyl] (e)-octadec-9-enoate Chemical compound CCCCCCCC\C=C\CCCCCCCC(=O)OCC(COP(O)(=O)OCCO)OC(=O)CCCCCCC\C=C\CCCCCCCC HMNZFMSWFCAGGW-XPWSMXQVSA-N 0.000 description 1
- 230000006978 adaptation Effects 0.000 description 1
- 230000033289 adaptive immune response Effects 0.000 description 1
- 229940021704 adenovirus vaccine Drugs 0.000 description 1
- 239000000910 agglutinin Substances 0.000 description 1
- WQZGKKKJIJFFOK-PHYPRBDBSA-N alpha-D-galactose Chemical compound OC[C@H]1O[C@H](O)[C@H](O)[C@@H](O)[C@H]1O WQZGKKKJIJFFOK-PHYPRBDBSA-N 0.000 description 1
- WNROFYMDJYEPJX-UHFFFAOYSA-K aluminium hydroxide Chemical compound [OH-].[OH-].[OH-].[Al+3] WNROFYMDJYEPJX-UHFFFAOYSA-K 0.000 description 1
- ILRRQNADMUWWFW-UHFFFAOYSA-K aluminium phosphate Chemical compound O1[Al]2OP1(=O)O2 ILRRQNADMUWWFW-UHFFFAOYSA-K 0.000 description 1
- APKFDSVGJQXUKY-INPOYWNPSA-N amphotericin B Chemical compound O[C@H]1[C@@H](N)[C@H](O)[C@@H](C)O[C@H]1O[C@H]1/C=C/C=C/C=C/C=C/C=C/C=C/C=C/[C@H](C)[C@@H](O)[C@@H](C)[C@H](C)OC(=O)C[C@H](O)C[C@H](O)CC[C@@H](O)[C@H](O)C[C@H](O)C[C@](O)(C[C@H](O)[C@H]2C(O)=O)O[C@H]2C1 APKFDSVGJQXUKY-INPOYWNPSA-N 0.000 description 1
- 229960003942 amphotericin b Drugs 0.000 description 1
- 230000003321 amplification Effects 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 230000000840 anti-viral effect Effects 0.000 description 1
- 230000030741 antigen processing and presentation Effects 0.000 description 1
- 239000000074 antisense oligonucleotide Substances 0.000 description 1
- 238000012230 antisense oligonucleotides Methods 0.000 description 1
- 230000036528 appetite Effects 0.000 description 1
- 235000019789 appetite Nutrition 0.000 description 1
- 229910052786 argon Inorganic materials 0.000 description 1
- 108010006523 asialoglycoprotein receptor Proteins 0.000 description 1
- 238000011717 athymic nude mouse Methods 0.000 description 1
- 230000003190 augmentative effect Effects 0.000 description 1
- 230000003305 autocrine Effects 0.000 description 1
- 239000003855 balanced salt solution Substances 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 229960001950 benzethonium chloride Drugs 0.000 description 1
- UREZNYTWGJKWBI-UHFFFAOYSA-M benzethonium chloride Chemical compound [Cl-].C1=CC(C(C)(C)CC(C)(C)C)=CC=C1OCCOCC[N+](C)(C)CC1=CC=CC=C1 UREZNYTWGJKWBI-UHFFFAOYSA-M 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 229960002685 biotin Drugs 0.000 description 1
- 239000011616 biotin Substances 0.000 description 1
- 230000000740 bleeding effect Effects 0.000 description 1
- 230000036772 blood pressure Effects 0.000 description 1
- 229910021538 borax Inorganic materials 0.000 description 1
- 239000006172 buffering agent Substances 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 235000011089 carbon dioxide Nutrition 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 238000012754 cardiac puncture Methods 0.000 description 1
- 150000001768 cations Chemical class 0.000 description 1
- 210000004970 cd4 cell Anatomy 0.000 description 1
- 230000020411 cell activation Effects 0.000 description 1
- 230000005779 cell damage Effects 0.000 description 1
- 208000037887 cell injury Diseases 0.000 description 1
- 230000030570 cellular localization Effects 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 230000035602 clotting Effects 0.000 description 1
- 238000011260 co-administration Methods 0.000 description 1
- 230000000536 complexating effect Effects 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 239000002872 contrast media Substances 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- 238000011461 current therapy Methods 0.000 description 1
- SUYVUBYJARFZHO-RRKCRQDMSA-N dATP Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@H]1C[C@H](O)[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O1 SUYVUBYJARFZHO-RRKCRQDMSA-N 0.000 description 1
- SUYVUBYJARFZHO-UHFFFAOYSA-N dATP Natural products C1=NC=2C(N)=NC=NC=2N1C1CC(O)C(COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O1 SUYVUBYJARFZHO-UHFFFAOYSA-N 0.000 description 1
- RGWHQCVHVJXOKC-SHYZEUOFSA-J dCTP(4-) Chemical compound O=C1N=C(N)C=CN1[C@@H]1O[C@H](COP([O-])(=O)OP([O-])(=O)OP([O-])([O-])=O)[C@@H](O)C1 RGWHQCVHVJXOKC-SHYZEUOFSA-J 0.000 description 1
- HAAZLUGHYHWQIW-KVQBGUIXSA-N dGTP Chemical compound C1=NC=2C(=O)NC(N)=NC=2N1[C@H]1C[C@H](O)[C@@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O1 HAAZLUGHYHWQIW-KVQBGUIXSA-N 0.000 description 1
- NHVNXKFIZYSCEB-XLPZGREQSA-N dTTP Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)C1 NHVNXKFIZYSCEB-XLPZGREQSA-N 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 238000012217 deletion Methods 0.000 description 1
- 230000037430 deletion Effects 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- 231100000673 dose–response relationship Toxicity 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 230000004064 dysfunction Effects 0.000 description 1
- 239000012636 effector Substances 0.000 description 1
- 210000003162 effector t lymphocyte Anatomy 0.000 description 1
- 235000013601 eggs Nutrition 0.000 description 1
- 210000001163 endosome Anatomy 0.000 description 1
- 210000002889 endothelial cell Anatomy 0.000 description 1
- 210000003191 femoral vein Anatomy 0.000 description 1
- 230000004761 fibrosis Effects 0.000 description 1
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 1
- 229940014144 folate Drugs 0.000 description 1
- OVBPIULPVIDEAO-LBPRGKRZSA-N folic acid Chemical compound C=1N=C2NC(N)=NC(=O)C2=NC=1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 OVBPIULPVIDEAO-LBPRGKRZSA-N 0.000 description 1
- 235000019152 folic acid Nutrition 0.000 description 1
- 239000011724 folic acid Substances 0.000 description 1
- 230000037406 food intake Effects 0.000 description 1
- 235000012631 food intake Nutrition 0.000 description 1
- 239000012634 fragment Substances 0.000 description 1
- 229940044627 gamma-interferon Drugs 0.000 description 1
- 229960002518 gentamicin Drugs 0.000 description 1
- 230000005182 global health Effects 0.000 description 1
- 229930195712 glutamate Natural products 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 239000011544 gradient gel Substances 0.000 description 1
- 230000012010 growth Effects 0.000 description 1
- 210000002443 helper t lymphocyte Anatomy 0.000 description 1
- ZFGMDIBRIDKWMY-PASTXAENSA-N heparin Chemical compound CC(O)=N[C@@H]1[C@@H](O)[C@H](O)[C@@H](COS(O)(=O)=O)O[C@@H]1O[C@@H]1[C@@H](C(O)=O)O[C@@H](O[C@H]2[C@@H]([C@@H](OS(O)(=O)=O)[C@@H](O[C@@H]3[C@@H](OC(O)[C@H](OS(O)(=O)=O)[C@H]3O)C(O)=O)O[C@@H]2O)CS(O)(=O)=O)[C@H](O)[C@H]1O ZFGMDIBRIDKWMY-PASTXAENSA-N 0.000 description 1
- 229960002897 heparin Drugs 0.000 description 1
- 231100000304 hepatotoxicity Toxicity 0.000 description 1
- 239000000833 heterodimer Substances 0.000 description 1
- 101150014702 hil-2 gene Proteins 0.000 description 1
- 230000036732 histological change Effects 0.000 description 1
- 238000000265 homogenisation Methods 0.000 description 1
- 210000005260 human cell Anatomy 0.000 description 1
- 229920001477 hydrophilic polymer Polymers 0.000 description 1
- 238000003018 immunoassay Methods 0.000 description 1
- 230000007813 immunodeficiency Effects 0.000 description 1
- 238000003125 immunofluorescent labeling Methods 0.000 description 1
- 229940121354 immunomodulator Drugs 0.000 description 1
- 238000009169 immunotherapy Methods 0.000 description 1
- 229940032219 immunotherapy vaccine Drugs 0.000 description 1
- 239000002596 immunotoxin Substances 0.000 description 1
- 230000002637 immunotoxin Effects 0.000 description 1
- 231100000608 immunotoxin Toxicity 0.000 description 1
- 229940051026 immunotoxin Drugs 0.000 description 1
- 238000000099 in vitro assay Methods 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 229940031551 inactivated vaccine Drugs 0.000 description 1
- 210000003000 inclusion body Anatomy 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 210000004969 inflammatory cell Anatomy 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 108700010900 influenza virus proteins Proteins 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 230000015788 innate immune response Effects 0.000 description 1
- 239000002054 inoculum Substances 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 230000004073 interleukin-2 production Effects 0.000 description 1
- 210000000936 intestine Anatomy 0.000 description 1
- 238000010255 intramuscular injection Methods 0.000 description 1
- 239000007927 intramuscular injection Substances 0.000 description 1
- 239000007928 intraperitoneal injection Substances 0.000 description 1
- 238000011835 investigation Methods 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 230000002045 lasting effect Effects 0.000 description 1
- 239000002523 lectin Substances 0.000 description 1
- 208000032839 leukemia Diseases 0.000 description 1
- 101150096059 lipC gene Proteins 0.000 description 1
- 230000003908 liver function Effects 0.000 description 1
- 208000018191 liver inflammation Diseases 0.000 description 1
- 230000007056 liver toxicity Effects 0.000 description 1
- 244000144972 livestock Species 0.000 description 1
- 230000004807 localization Effects 0.000 description 1
- 230000004904 long-term response Effects 0.000 description 1
- 231100001252 long-term toxicity Toxicity 0.000 description 1
- 238000000464 low-speed centrifugation Methods 0.000 description 1
- 239000012139 lysis buffer Substances 0.000 description 1
- 108010054155 lysyllysine Proteins 0.000 description 1
- 201000004792 malaria Diseases 0.000 description 1
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 description 1
- 238000007726 management method Methods 0.000 description 1
- 230000008774 maternal effect Effects 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 239000003607 modifier Substances 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 210000000865 mononuclear phagocyte system Anatomy 0.000 description 1
- 238000010172 mouse model Methods 0.000 description 1
- 231100000219 mutagenic Toxicity 0.000 description 1
- 230000003505 mutagenic effect Effects 0.000 description 1
- OHDXDNUPVVYWOV-UHFFFAOYSA-N n-methyl-1-(2-naphthalen-1-ylsulfanylphenyl)methanamine Chemical compound CNCC1=CC=CC=C1SC1=CC=CC2=CC=CC=C12 OHDXDNUPVVYWOV-UHFFFAOYSA-N 0.000 description 1
- 230000017074 necrotic cell death Effects 0.000 description 1
- 229960004927 neomycin Drugs 0.000 description 1
- 231100000957 no side effect Toxicity 0.000 description 1
- 238000003199 nucleic acid amplification method Methods 0.000 description 1
- 238000011580 nude mouse model Methods 0.000 description 1
- GSSMIHQEWAQUPM-AOLPDKKJSA-N ovalbumin peptide Chemical compound C([C@H](NC(=O)[C@H](C(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)[C@@H](C)CC)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C1=CN=CN1 GSSMIHQEWAQUPM-AOLPDKKJSA-N 0.000 description 1
- 230000002611 ovarian Effects 0.000 description 1
- 201000002528 pancreatic cancer Diseases 0.000 description 1
- 208000008443 pancreatic carcinoma Diseases 0.000 description 1
- 239000012188 paraffin wax Substances 0.000 description 1
- 230000007170 pathology Effects 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 229930192851 perforin Natural products 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 206010034674 peritonitis Diseases 0.000 description 1
- 102000013415 peroxidase activity proteins Human genes 0.000 description 1
- 108040007629 peroxidase activity proteins Proteins 0.000 description 1
- 230000002688 persistence Effects 0.000 description 1
- 230000002085 persistent effect Effects 0.000 description 1
- 238000002205 phenol-chloroform extraction Methods 0.000 description 1
- 229960005323 phenoxyethanol Drugs 0.000 description 1
- 239000008363 phosphate buffer Substances 0.000 description 1
- WTJKGGKOPKCXLL-RRHRGVEJSA-N phosphatidylcholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCC=CCCCCCCCC WTJKGGKOPKCXLL-RRHRGVEJSA-N 0.000 description 1
- 150000003904 phospholipids Chemical class 0.000 description 1
- 229910052698 phosphorus Inorganic materials 0.000 description 1
- 239000011574 phosphorus Substances 0.000 description 1
- 230000036470 plasma concentration Effects 0.000 description 1
- 239000013600 plasmid vector Substances 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 1
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 1
- 229940068977 polysorbate 20 Drugs 0.000 description 1
- 229920002981 polyvinylidene fluoride Polymers 0.000 description 1
- 230000023603 positive regulation of transcription initiation, DNA-dependent Effects 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 238000009021 pre-vaccination Methods 0.000 description 1
- 230000003449 preventive effect Effects 0.000 description 1
- 230000000750 progressive effect Effects 0.000 description 1
- 230000000770 proinflammatory effect Effects 0.000 description 1
- 230000009696 proliferative response Effects 0.000 description 1
- 210000002307 prostate Anatomy 0.000 description 1
- 208000023958 prostate neoplasm Diseases 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 229940124272 protein stabilizer Drugs 0.000 description 1
- 230000005180 public health Effects 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 238000010791 quenching Methods 0.000 description 1
- 230000000171 quenching effect Effects 0.000 description 1
- 230000002285 radioactive effect Effects 0.000 description 1
- 238000001959 radiotherapy Methods 0.000 description 1
- 239000011541 reaction mixture Substances 0.000 description 1
- 230000008929 regeneration Effects 0.000 description 1
- 238000011069 regeneration method Methods 0.000 description 1
- 208000023504 respiratory system disease Diseases 0.000 description 1
- 230000001177 retroviral effect Effects 0.000 description 1
- 238000003757 reverse transcription PCR Methods 0.000 description 1
- PYWVYCXTNDRMGF-UHFFFAOYSA-N rhodamine B Chemical compound [Cl-].C=12C=CC(=[N+](CC)CC)C=C2OC2=CC(N(CC)CC)=CC=C2C=1C1=CC=CC=C1C(O)=O PYWVYCXTNDRMGF-UHFFFAOYSA-N 0.000 description 1
- 229960000329 ribavirin Drugs 0.000 description 1
- HZCAHMRRMINHDJ-DBRKOABJSA-N ribavirin Natural products O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1N=CN=C1 HZCAHMRRMINHDJ-DBRKOABJSA-N 0.000 description 1
- 108091092562 ribozyme Proteins 0.000 description 1
- 238000005464 sample preparation method Methods 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 230000003248 secreting effect Effects 0.000 description 1
- 230000001568 sexual effect Effects 0.000 description 1
- 230000004905 short-term response Effects 0.000 description 1
- 231100001251 short-term toxicity Toxicity 0.000 description 1
- 231100000161 signs of toxicity Toxicity 0.000 description 1
- 229940083538 smallpox vaccine Drugs 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 235000010339 sodium tetraborate Nutrition 0.000 description 1
- 238000000527 sonication Methods 0.000 description 1
- 125000006850 spacer group Chemical group 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 210000004989 spleen cell Anatomy 0.000 description 1
- 210000004988 splenocyte Anatomy 0.000 description 1
- 230000002269 spontaneous effect Effects 0.000 description 1
- 238000007447 staining method Methods 0.000 description 1
- 238000007619 statistical method Methods 0.000 description 1
- 239000008227 sterile water for injection Substances 0.000 description 1
- 230000000638 stimulation Effects 0.000 description 1
- 210000002784 stomach Anatomy 0.000 description 1
- 238000010254 subcutaneous injection Methods 0.000 description 1
- 239000007929 subcutaneous injection Substances 0.000 description 1
- 125000004434 sulfur atom Chemical group 0.000 description 1
- 125000004354 sulfur functional group Chemical group 0.000 description 1
- 239000006228 supernatant Substances 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 238000007910 systemic administration Methods 0.000 description 1
- ZRKFYGHZFMAOKI-QMGMOQQFSA-N tgfbeta Chemical compound C([C@H](NC(=O)[C@H](C(C)C)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CCSC)C(C)C)[C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O)C1=CC=C(O)C=C1 ZRKFYGHZFMAOKI-QMGMOQQFSA-N 0.000 description 1
- 238000004448 titration Methods 0.000 description 1
- 238000013518 transcription Methods 0.000 description 1
- 230000035897 transcription Effects 0.000 description 1
- 238000010361 transduction Methods 0.000 description 1
- 230000026683 transduction Effects 0.000 description 1
- 230000001052 transient effect Effects 0.000 description 1
- 238000011269 treatment regimen Methods 0.000 description 1
- BSVBQGMMJUBVOD-UHFFFAOYSA-N trisodium borate Chemical compound [Na+].[Na+].[Na+].[O-]B([O-])[O-] BSVBQGMMJUBVOD-UHFFFAOYSA-N 0.000 description 1
- 229940031418 trivalent vaccine Drugs 0.000 description 1
- 239000012588 trypsin Substances 0.000 description 1
- 201000008827 tuberculosis Diseases 0.000 description 1
- 241001529453 unidentified herpesvirus Species 0.000 description 1
- 230000003827 upregulation Effects 0.000 description 1
- 230000002485 urinary effect Effects 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/87—Introduction of foreign genetic material using processes not otherwise provided for, e.g. co-transformation
- C12N15/88—Introduction of foreign genetic material using processes not otherwise provided for, e.g. co-transformation using microencapsulation, e.g. using amphiphile liposome vesicle
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/10—Dispersions; Emulsions
- A61K9/127—Synthetic bilayered vehicles, e.g. liposomes or liposomes with cholesterol as the only non-phosphatidyl surfactant
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/12—Viral antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/12—Viral antigens
- A61K39/29—Hepatitis virus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/16—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing nitrogen, e.g. nitro-, nitroso-, azo-compounds, nitriles, cyanates
- A61K47/18—Amines; Amides; Ureas; Quaternary ammonium compounds; Amino acids; Oligopeptides having up to five amino acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/28—Steroids, e.g. cholesterol, bile acids or glycyrrhetinic acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/69—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit
- A61K47/6905—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being a colloid or an emulsion
- A61K47/6911—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being a colloid or an emulsion the form being a liposome
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/69—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit
- A61K47/6905—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being a colloid or an emulsion
- A61K47/6911—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being a colloid or an emulsion the form being a liposome
- A61K47/6913—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being a colloid or an emulsion the form being a liposome the liposome being modified on its surface by an antibody
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/10—Dispersions; Emulsions
- A61K9/127—Synthetic bilayered vehicles, e.g. liposomes or liposomes with cholesterol as the only non-phosphatidyl surfactant
- A61K9/1271—Non-conventional liposomes, e.g. PEGylated liposomes or liposomes coated or grafted with polymers
- A61K9/1272—Non-conventional liposomes, e.g. PEGylated liposomes or liposomes coated or grafted with polymers comprising non-phosphatidyl surfactants as bilayer-forming substances, e.g. cationic lipids or non-phosphatidyl liposomes coated or grafted with polymers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
- A61P31/16—Antivirals for RNA viruses for influenza or rhinoviruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
- A61P31/18—Antivirals for RNA viruses for HIV
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/20—Antivirals for DNA viruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/51—Medicinal preparations containing antigens or antibodies comprising whole cells, viruses or DNA/RNA
- A61K2039/53—DNA (RNA) vaccination
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/54—Medicinal preparations containing antigens or antibodies characterised by the route of administration
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/54—Medicinal preparations containing antigens or antibodies characterised by the route of administration
- A61K2039/541—Mucosal route
- A61K2039/543—Mucosal route intranasal
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/555—Medicinal preparations containing antigens or antibodies characterised by a specific combination antigen/adjuvant
- A61K2039/55511—Organic adjuvants
- A61K2039/55522—Cytokines; Lymphokines; Interferons
- A61K2039/55527—Interleukins
- A61K2039/55538—IL-12
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/555—Medicinal preparations containing antigens or antibodies characterised by a specific combination antigen/adjuvant
- A61K2039/55511—Organic adjuvants
- A61K2039/55555—Liposomes; Vesicles, e.g. nanoparticles; Spheres, e.g. nanospheres; Polymers
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2730/00—Reverse transcribing DNA viruses
- C12N2730/00011—Details
- C12N2730/10011—Hepadnaviridae
- C12N2730/10111—Orthohepadnavirus, e.g. hepatitis B virus
- C12N2730/10134—Use of virus or viral component as vaccine, e.g. live-attenuated or inactivated virus, VLP, viral protein
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2740/00—Reverse transcribing RNA viruses
- C12N2740/00011—Details
- C12N2740/10011—Retroviridae
- C12N2740/16011—Human Immunodeficiency Virus, HIV
- C12N2740/16111—Human Immunodeficiency Virus, HIV concerning HIV env
- C12N2740/16134—Use of virus or viral component as vaccine, e.g. live-attenuated or inactivated virus, VLP, viral protein
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2760/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssRNA viruses negative-sense
- C12N2760/00011—Details
- C12N2760/16011—Orthomyxoviridae
- C12N2760/16111—Influenzavirus A, i.e. influenza A virus
- C12N2760/16134—Use of virus or viral component as vaccine, e.g. live-attenuated or inactivated virus, VLP, viral protein
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2770/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssRNA viruses positive-sense
- C12N2770/00011—Details
- C12N2770/20011—Coronaviridae
- C12N2770/20034—Use of virus or viral component as vaccine, e.g. live-attenuated or inactivated virus, VLP, viral protein
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2770/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssRNA viruses positive-sense
- C12N2770/00011—Details
- C12N2770/24011—Flaviviridae
- C12N2770/24111—Flavivirus, e.g. yellow fever virus, dengue, JEV
- C12N2770/24134—Use of virus or viral component as vaccine, e.g. live-attenuated or inactivated virus, VLP, viral protein
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2810/00—Vectors comprising a targeting moiety
- C12N2810/50—Vectors comprising as targeting moiety peptide derived from defined protein
- C12N2810/80—Vectors comprising as targeting moiety peptide derived from defined protein from vertebrates
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2810/00—Vectors comprising a targeting moiety
- C12N2810/50—Vectors comprising as targeting moiety peptide derived from defined protein
- C12N2810/80—Vectors comprising as targeting moiety peptide derived from defined protein from vertebrates
- C12N2810/85—Vectors comprising as targeting moiety peptide derived from defined protein from vertebrates mammalian
- C12N2810/859—Vectors comprising as targeting moiety peptide derived from defined protein from vertebrates mammalian from immunoglobulins
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Virology (AREA)
- Engineering & Computer Science (AREA)
- Epidemiology (AREA)
- Organic Chemistry (AREA)
- Immunology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Molecular Biology (AREA)
- Genetics & Genomics (AREA)
- Microbiology (AREA)
- Dispersion Chemistry (AREA)
- Communicable Diseases (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Mycology (AREA)
- Biotechnology (AREA)
- Oncology (AREA)
- Biophysics (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Biomedical Technology (AREA)
- General Engineering & Computer Science (AREA)
- Plant Pathology (AREA)
- Biochemistry (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Physics & Mathematics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- AIDS & HIV (AREA)
- Pulmonology (AREA)
- Tropical Medicine & Parasitology (AREA)
- Gastroenterology & Hepatology (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US92968507P | 2007-07-09 | 2007-07-09 | |
| US60/929,685 | 2007-07-09 | ||
| PCT/US2008/008394 WO2009009054A1 (en) | 2007-07-09 | 2008-07-09 | Methods for generating immune response using cationic-liposome-mediated nucleic acid delivery |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020107002754A Division KR101570886B1 (ko) | 2007-07-09 | 2008-07-09 | 양이온성-리포솜 매개된 핵산 전달을 이용하여 면역 반응을 생성시키는 방법 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20150108948A true KR20150108948A (ko) | 2015-09-30 |
Family
ID=40228915
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020157025507A Withdrawn KR20150108948A (ko) | 2007-07-09 | 2008-07-09 | 양이온성-리포솜 매개된 핵산 전달을 이용하여 면역 반응을 생성시키는 방법 |
| KR1020107002754A Expired - Fee Related KR101570886B1 (ko) | 2007-07-09 | 2008-07-09 | 양이온성-리포솜 매개된 핵산 전달을 이용하여 면역 반응을 생성시키는 방법 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020107002754A Expired - Fee Related KR101570886B1 (ko) | 2007-07-09 | 2008-07-09 | 양이온성-리포솜 매개된 핵산 전달을 이용하여 면역 반응을 생성시키는 방법 |
Country Status (15)
| Country | Link |
|---|---|
| US (1) | US20090053299A1 (enExample) |
| EP (1) | EP2175835B1 (enExample) |
| JP (2) | JP5890095B2 (enExample) |
| KR (2) | KR20150108948A (enExample) |
| CN (2) | CN106890142A (enExample) |
| AU (1) | AU2008275713B2 (enExample) |
| BR (1) | BRPI0814558A2 (enExample) |
| CA (1) | CA2692546C (enExample) |
| EA (1) | EA201070050A1 (enExample) |
| IL (1) | IL203204B (enExample) |
| MX (1) | MX2010000166A (enExample) |
| NZ (1) | NZ582414A (enExample) |
| SG (1) | SG182994A1 (enExample) |
| WO (1) | WO2009009054A1 (enExample) |
| ZA (1) | ZA201000038B (enExample) |
Families Citing this family (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5573111B2 (ja) * | 2009-11-06 | 2014-08-20 | ライオン株式会社 | イソプロピルメチルフェノール含有液体口腔用組成物 |
| US20110182976A1 (en) * | 2010-01-28 | 2011-07-28 | National Taiwan Ocean University | Lipoplex-patch based dna vaccine |
| WO2011105520A1 (ja) * | 2010-02-26 | 2011-09-01 | 国立大学法人 長崎大学 | 抗原または薬物送達複合体 |
| WO2012030901A1 (en) * | 2010-08-31 | 2012-03-08 | Novartis Ag | Small liposomes for delivery of immunogen-encoding rna |
| US8932575B2 (en) | 2010-09-21 | 2015-01-13 | University Of Miami | Compositions and methods for inducing migration by dendritic cells and an immune response |
| WO2012040266A2 (en) * | 2010-09-24 | 2012-03-29 | University Of Miami | Gene-based adjuvants and compositions thereof to increase antibody production in response to gene-based vaccines |
| TWI614034B (zh) * | 2012-09-19 | 2018-02-11 | 美國喬治城大學 | 經靶向之脂質體 |
| US20140120157A1 (en) | 2012-09-19 | 2014-05-01 | Georgetown University | Targeted liposomes |
| CN103405386B (zh) * | 2012-09-21 | 2016-05-18 | 上海泽润生物科技有限公司 | 一种脂质体的制备方法及制作脂质体佐剂的方法 |
| WO2014159917A2 (en) | 2013-03-14 | 2014-10-02 | Georgetown University | Treatment for exposure to nerve agent |
| CN105726485B (zh) * | 2016-03-24 | 2019-08-06 | 东华大学 | 一种半乳糖化壳聚糖修饰的免疫醇质体的制备方法 |
| KR20210100115A (ko) | 2018-12-03 | 2021-08-13 | 보드 오브 리전츠, 더 유니버시티 오브 텍사스 시스템 | 올리고-벤즈아미드 유사체 및 암 치료에서의 이의 용도 |
| CN111840538A (zh) * | 2020-05-29 | 2020-10-30 | 中山大学 | 一种水痘-带状疱疹病毒亚单位纳米疫苗的制备方法和应用 |
| CN117883382B (zh) * | 2023-11-27 | 2025-02-14 | 中山大学肿瘤防治中心(中山大学附属肿瘤医院、中山大学肿瘤研究所) | 高效递送疫苗的脂质体载体及脂质体疫苗的制备与用途 |
Family Cites Families (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4957735A (en) * | 1984-06-12 | 1990-09-18 | The University Of Tennessee Research Corporation | Target-sensitive immunoliposomes- preparation and characterization |
| US4946778A (en) * | 1987-09-21 | 1990-08-07 | Genex Corporation | Single polypeptide chain binding molecules |
| US5688488A (en) * | 1989-04-03 | 1997-11-18 | Purdue Research Foundation | Composition and method for tumor imaging |
| US5753258A (en) * | 1990-10-19 | 1998-05-19 | University Of Florida | Artificial viral envelopes |
| US6099842A (en) * | 1990-12-03 | 2000-08-08 | The United States Of America As Represented By The Department Of Health And Human Services | Recombinant immunotoxin composed of a single chain antibody reacting with the human transferrin receptor and diptheria toxin |
| CA2142007C (en) * | 1992-08-11 | 2007-10-30 | Robert Glen Urban | Immunomodulatory peptides |
| US5786214A (en) * | 1994-12-15 | 1998-07-28 | Spinal Cord Society | pH-sensitive immunoliposomes and method of gene delivery to the mammalian central nervous system |
| FR2730637B1 (fr) * | 1995-02-17 | 1997-03-28 | Rhone Poulenc Rorer Sa | Composition pharmaceutique contenant des acides nucleiques, et ses utilisations |
| US5977322A (en) * | 1995-06-14 | 1999-11-02 | The Regents Of The University Of California | High affinity human antibodies to tumor antigens |
| JP2001510457A (ja) * | 1996-11-12 | 2001-07-31 | ザ リージェンツ オブ ザ ユニバーシティ オブ カリフォルニア | インビボ送達に有効な、脂質―核酸複合体の安定な製剤の調製 |
| US6210707B1 (en) * | 1996-11-12 | 2001-04-03 | The Regents Of The University Of California | Methods of forming protein-linked lipidic microparticles, and compositions thereof |
| US6794128B2 (en) * | 1998-04-24 | 2004-09-21 | The Regents Of The University Of California | Methods of selecting internalizing antibodies |
| US20030022854A1 (en) * | 1998-06-25 | 2003-01-30 | Dow Steven W. | Vaccines using nucleic acid-lipid complexes |
| US7780882B2 (en) | 1999-02-22 | 2010-08-24 | Georgetown University | Simplified and improved method for preparing an antibody or an antibody fragment targeted immunoliposome for systemic administration of a therapeutic or diagnostic agent |
| US9034329B2 (en) * | 1999-02-22 | 2015-05-19 | Georgetown University | Preparation of antibody or an antibody fragment-targeted immunoliposomes for systemic administration of therapeutic or diagnostic agents and uses thereof |
| US7479276B1 (en) * | 1999-02-22 | 2009-01-20 | Synergene Therapeutics, Inc. | Antibody fragment-targeted immunoliposomes for systemic gene delivery |
| US20020103165A1 (en) * | 2000-02-29 | 2002-08-01 | Alliance Pharmaceutical Corp., | Engineered spray-dried lipid-based microparticles for cellular targeting |
| ES2350485T3 (es) | 2003-06-04 | 2011-01-24 | Georgetown University | Método para mejorar la estabilidad y la duración de conservación de complejos de liposomas. |
| WO2007047981A2 (en) * | 2005-10-20 | 2007-04-26 | Georgetown University | Tumor-targeted nanodelivery systems to improve early mri detection of cancer |
-
2008
- 2008-07-09 BR BRPI0814558-0A2A patent/BRPI0814558A2/pt not_active Application Discontinuation
- 2008-07-09 SG SG2012049664A patent/SG182994A1/en unknown
- 2008-07-09 CA CA2692546A patent/CA2692546C/en active Active
- 2008-07-09 AU AU2008275713A patent/AU2008275713B2/en not_active Ceased
- 2008-07-09 CN CN201710070091.XA patent/CN106890142A/zh active Pending
- 2008-07-09 NZ NZ582414A patent/NZ582414A/xx not_active IP Right Cessation
- 2008-07-09 KR KR1020157025507A patent/KR20150108948A/ko not_active Withdrawn
- 2008-07-09 KR KR1020107002754A patent/KR101570886B1/ko not_active Expired - Fee Related
- 2008-07-09 EP EP08780044.7A patent/EP2175835B1/en active Active
- 2008-07-09 WO PCT/US2008/008394 patent/WO2009009054A1/en not_active Ceased
- 2008-07-09 US US12/216,715 patent/US20090053299A1/en not_active Abandoned
- 2008-07-09 EA EA201070050A patent/EA201070050A1/ru unknown
- 2008-07-09 JP JP2010516041A patent/JP5890095B2/ja not_active Expired - Fee Related
- 2008-07-09 MX MX2010000166A patent/MX2010000166A/es active IP Right Grant
- 2008-07-09 CN CN200880105375A patent/CN101801344A/zh active Pending
-
2010
- 2010-01-04 ZA ZA2010/00038A patent/ZA201000038B/en unknown
- 2010-01-07 IL IL203204A patent/IL203204B/en active IP Right Grant
-
2014
- 2014-04-23 JP JP2014089131A patent/JP2014148533A/ja active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| CA2692546C (en) | 2018-03-13 |
| EA201070050A1 (ru) | 2010-10-29 |
| CN106890142A (zh) | 2017-06-27 |
| EP2175835A4 (en) | 2011-08-03 |
| US20090053299A1 (en) | 2009-02-26 |
| EP2175835B1 (en) | 2016-04-20 |
| KR20100044206A (ko) | 2010-04-29 |
| EP2175835A1 (en) | 2010-04-21 |
| MX2010000166A (es) | 2010-04-27 |
| ZA201000038B (en) | 2011-03-30 |
| CA2692546A1 (en) | 2009-01-15 |
| WO2009009054A1 (en) | 2009-01-15 |
| KR101570886B1 (ko) | 2015-11-23 |
| NZ582414A (en) | 2012-09-28 |
| JP2014148533A (ja) | 2014-08-21 |
| AU2008275713A1 (en) | 2009-01-15 |
| IL203204B (en) | 2018-05-31 |
| JP2010533178A (ja) | 2010-10-21 |
| CN101801344A (zh) | 2010-08-11 |
| HK1143532A1 (zh) | 2011-01-07 |
| JP5890095B2 (ja) | 2016-03-22 |
| AU2008275713B2 (en) | 2014-08-07 |
| BRPI0814558A2 (pt) | 2015-01-06 |
| SG182994A1 (en) | 2012-08-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5890095B2 (ja) | カチオン性リポソーム媒介核酸送達を用いて免疫応答を引き起こす方法 | |
| KR101790187B1 (ko) | 신드비스 바이러스 외피 당단백질로 가성형태화된 렌티바이러스 벡터 | |
| CN113521268A (zh) | 冠状病毒疫苗 | |
| CN115697298A (zh) | 脂质纳米颗粒 | |
| JP2023081859A (ja) | コロナウイルスワクチン | |
| ES2387141T3 (es) | Composición que comprende la proliproteína NS3/NS4 y el polipéptido NS5B del VHC, vectores de expresión que incluyen las secuencias nucleicas correspondientes y su utilización terapéutica | |
| US20140187752A1 (en) | Antigenic compositions and use of same in the targeted delivery of nucleic acids | |
| TW202328166A (zh) | 針對新型冠狀病毒感染之mRNA疫苗之組成物及方法 | |
| CN110520155A (zh) | 预防和治疗寨卡病毒感染的rna药物制剂 | |
| JP2024530047A (ja) | ワクチン抗原 | |
| Wang et al. | Innovative translational platforms for rapid developing clinical vaccines against COVID‐19 and other infectious disease | |
| CN121038810A (zh) | 针对冠状病毒感染、流行性感冒感染和/或rsv感染的组合疫苗 | |
| JP2023536269A (ja) | ワクチン組成物及びその使用方法 | |
| Yu et al. | Strategies for loading dendritic cells with hepatitis C NS5a antigen and inducing protective immunity | |
| US20230331815A1 (en) | Methods and compositions for treating viral infection | |
| HK1143532B (en) | Methods for generating immune response using cationic-liposome-mediated nucleic acid delivery | |
| CN116650633A (zh) | 冠状病毒疫苗 | |
| WO2022230971A1 (ja) | コロナウイルスに対する免疫応答を誘発する人工アジュバントベクター細胞、および当該細胞を含む医薬組成物、並びにそれらの使用 | |
| Koutsoumpli | Towards the rational design of therapeutic vaccines against hepatitis C virus infection | |
| WO2023215734A1 (en) | Multivalent particles compositions and methods of use | |
| CN115843270A (zh) | 包括缺失可变结构域的e2多肽的丙型肝炎核酸疫苗 | |
| HK40061489A (zh) | 冠状病毒疫苗 | |
| HK40061483A (en) | Coronavirus vaccine | |
| CN121197383A (zh) | 多价埃博拉病毒广谱mRNA疫苗及其制备方法与应用 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A107 | Divisional application of patent | ||
| PA0104 | Divisional application for international application |
Comment text: Divisional Application for International Patent Patent event code: PA01041R01D Patent event date: 20150916 Application number text: 1020107002754 Filing date: 20100208 |
|
| PG1501 | Laying open of application | ||
| PC1203 | Withdrawal of no request for examination | ||
| WITN | Application deemed withdrawn, e.g. because no request for examination was filed or no examination fee was paid |