KR20140084201A - 알칼리성 포스파타제 및/또는 나트륨이뇨 펩티드를 포함하는 조성물 및 그의 사용 방법 - Google Patents
알칼리성 포스파타제 및/또는 나트륨이뇨 펩티드를 포함하는 조성물 및 그의 사용 방법 Download PDFInfo
- Publication number
- KR20140084201A KR20140084201A KR1020147013214A KR20147013214A KR20140084201A KR 20140084201 A KR20140084201 A KR 20140084201A KR 1020147013214 A KR1020147013214 A KR 1020147013214A KR 20147013214 A KR20147013214 A KR 20147013214A KR 20140084201 A KR20140084201 A KR 20140084201A
- Authority
- KR
- South Korea
- Prior art keywords
- amino acid
- seq
- acid sequence
- polypeptide
- sequence
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 102000002260 Alkaline Phosphatase Human genes 0.000 title claims abstract description 48
- 108020004774 Alkaline Phosphatase Proteins 0.000 title claims abstract description 48
- 238000000034 method Methods 0.000 title claims description 149
- 239000000203 mixture Substances 0.000 title claims description 73
- 108020001621 Natriuretic Peptide Proteins 0.000 title description 3
- 102000004571 Natriuretic peptide Human genes 0.000 title description 3
- 239000000692 natriuretic peptide Substances 0.000 title description 3
- 108090000765 processed proteins & peptides Proteins 0.000 claims abstract description 415
- 102000004196 processed proteins & peptides Human genes 0.000 claims abstract description 394
- 229920001184 polypeptide Polymers 0.000 claims abstract description 387
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims abstract description 128
- 208000011580 syndromic disease Diseases 0.000 claims abstract description 91
- 208000035475 disorder Diseases 0.000 claims abstract description 79
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 claims abstract description 70
- 229910052708 sodium Inorganic materials 0.000 claims abstract description 70
- 239000011734 sodium Substances 0.000 claims abstract description 70
- 210000000988 bone and bone Anatomy 0.000 claims abstract description 66
- 101710158332 Diuretic hormone Proteins 0.000 claims abstract description 59
- 101710204261 Diuretic hormone class 2 Proteins 0.000 claims abstract description 59
- 102100027842 Fibroblast growth factor receptor 3 Human genes 0.000 claims abstract description 37
- 101710182396 Fibroblast growth factor receptor 3 Proteins 0.000 claims abstract description 35
- 208000003019 Neurofibromatosis 1 Diseases 0.000 claims abstract description 35
- 208000015100 cartilage disease Diseases 0.000 claims abstract description 34
- 208000024834 Neurofibromatosis type 1 Diseases 0.000 claims abstract description 30
- 208000020084 Bone disease Diseases 0.000 claims abstract description 29
- 210000002464 muscle smooth vascular Anatomy 0.000 claims abstract description 20
- 208000029578 Muscle disease Diseases 0.000 claims abstract description 18
- 208000021642 Muscular disease Diseases 0.000 claims abstract description 18
- 208000013403 hyperactivity Diseases 0.000 claims abstract description 16
- 108060003951 Immunoglobulin Proteins 0.000 claims abstract description 13
- 102000018358 immunoglobulin Human genes 0.000 claims abstract description 13
- 230000002232 neuromuscular Effects 0.000 claims abstract description 10
- 125000003275 alpha amino acid group Chemical group 0.000 claims description 413
- 125000000539 amino acid group Chemical group 0.000 claims description 384
- 229940024606 amino acid Drugs 0.000 claims description 233
- 235000001014 amino acid Nutrition 0.000 claims description 223
- 150000001413 amino acids Chemical class 0.000 claims description 180
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 claims description 103
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 claims description 67
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 claims description 60
- 201000010099 disease Diseases 0.000 claims description 49
- 241000282414 Homo sapiens Species 0.000 claims description 44
- 239000008194 pharmaceutical composition Substances 0.000 claims description 44
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 claims description 38
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 claims description 38
- 210000004899 c-terminal region Anatomy 0.000 claims description 38
- 239000012634 fragment Substances 0.000 claims description 38
- 238000003776 cleavage reaction Methods 0.000 claims description 34
- 230000007017 scission Effects 0.000 claims description 34
- 229910052720 vanadium Inorganic materials 0.000 claims description 32
- 102000014187 peptide receptors Human genes 0.000 claims description 26
- 239000000556 agonist Substances 0.000 claims description 25
- 235000003704 aspartic acid Nutrition 0.000 claims description 24
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 claims description 24
- 102000005600 Cathepsins Human genes 0.000 claims description 23
- 108010084457 Cathepsins Proteins 0.000 claims description 23
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 claims description 20
- 239000004471 Glycine Substances 0.000 claims description 19
- 235000013922 glutamic acid Nutrition 0.000 claims description 18
- 239000004220 glutamic acid Substances 0.000 claims description 18
- KJBQDYDTQCIAFO-ZLELNMGESA-N (2s)-2-amino-4-methylpentanoic acid;(2s)-2,6-diaminohexanoic acid Chemical compound CC(C)C[C@H](N)C(O)=O.NCCCC[C@H](N)C(O)=O KJBQDYDTQCIAFO-ZLELNMGESA-N 0.000 claims description 17
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 15
- 101710102159 Atrial natriuretic peptide receptor 2 Proteins 0.000 claims description 13
- 102100039341 Atrial natriuretic peptide receptor 2 Human genes 0.000 claims description 13
- 230000002378 acidificating effect Effects 0.000 claims description 13
- 150000001875 compounds Chemical class 0.000 claims description 10
- 239000000539 dimer Substances 0.000 claims description 10
- 108090000625 Cathepsin K Proteins 0.000 claims description 9
- 102000004171 Cathepsin K Human genes 0.000 claims description 9
- SEFLNGWGXSFZMH-DQZFIDPSSA-N (2s)-2-aminobutanedioic acid;(2s,3s)-2-amino-3-methylpentanoic acid Chemical compound CC[C@H](C)[C@H](N)C(O)=O.OC(=O)[C@@H](N)CC(O)=O SEFLNGWGXSFZMH-DQZFIDPSSA-N 0.000 claims description 8
- 230000000926 neurological effect Effects 0.000 claims description 8
- 230000008685 targeting Effects 0.000 claims description 8
- 230000001537 neural effect Effects 0.000 claims description 6
- 229920000805 Polyaspartic acid Polymers 0.000 claims description 5
- 125000000291 glutamic acid group Chemical group N[C@@H](CCC(O)=O)C(=O)* 0.000 claims description 5
- 108010064470 polyaspartate Proteins 0.000 claims description 5
- 108010020346 Polyglutamic Acid Proteins 0.000 claims description 4
- 229920002643 polyglutamic acid Polymers 0.000 claims description 4
- 230000029087 digestion Effects 0.000 claims description 3
- 150000007523 nucleic acids Chemical class 0.000 abstract description 50
- 102000039446 nucleic acids Human genes 0.000 abstract description 39
- 108020004707 nucleic acids Proteins 0.000 abstract description 38
- 238000011282 treatment Methods 0.000 abstract description 30
- 210000000845 cartilage Anatomy 0.000 abstract description 25
- 230000011164 ossification Effects 0.000 abstract description 4
- 108010076504 Protein Sorting Signals Proteins 0.000 description 288
- 125000005647 linker group Chemical group 0.000 description 99
- 101800002899 Soluble alkaline phosphatase Proteins 0.000 description 96
- 101000574445 Homo sapiens Alkaline phosphatase, tissue-nonspecific isozyme Proteins 0.000 description 75
- 102100025683 Alkaline phosphatase, tissue-nonspecific isozyme Human genes 0.000 description 73
- 102000012421 C-Type Natriuretic Peptide Human genes 0.000 description 60
- 101800000060 C-type natriuretic peptide Proteins 0.000 description 60
- 206010058314 Dysplasia Diseases 0.000 description 57
- 230000035772 mutation Effects 0.000 description 55
- 230000004927 fusion Effects 0.000 description 51
- 230000000694 effects Effects 0.000 description 39
- 241000699670 Mus sp. Species 0.000 description 37
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical compound C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 description 33
- -1 Phe Chemical compound 0.000 description 33
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 32
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 30
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 28
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 28
- 230000002354 daily effect Effects 0.000 description 28
- 125000000341 threoninyl group Chemical group [H]OC([H])(C([H])([H])[H])C([H])(N([H])[H])C(*)=O 0.000 description 28
- 125000002987 valine group Chemical group [H]N([H])C([H])(C(*)=O)C([H])(C([H])([H])[H])C([H])([H])[H] 0.000 description 28
- 108010085793 Neurofibromin 1 Proteins 0.000 description 27
- 210000004027 cell Anatomy 0.000 description 27
- 125000001909 leucine group Chemical group [H]N(*)C(C(*)=O)C([H])([H])C(C([H])([H])[H])C([H])([H])[H] 0.000 description 27
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical compound SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 26
- 108010041801 2',3'-Cyclic Nucleotide 3'-Phosphodiesterase Proteins 0.000 description 25
- 102100040458 2',3'-cyclic-nucleotide 3'-phosphodiesterase Human genes 0.000 description 25
- 208000009905 Neurofibromatoses Diseases 0.000 description 25
- 102100026379 Neurofibromin Human genes 0.000 description 25
- 201000004931 neurofibromatosis Diseases 0.000 description 25
- 125000003295 alanine group Chemical group N[C@@H](C)C(=O)* 0.000 description 24
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 23
- 108091035707 Consensus sequence Proteins 0.000 description 22
- 102000004190 Enzymes Human genes 0.000 description 22
- 108090000790 Enzymes Proteins 0.000 description 22
- 229940088598 enzyme Drugs 0.000 description 22
- 230000033558 biomineral tissue development Effects 0.000 description 21
- 230000001717 pathogenic effect Effects 0.000 description 21
- 206010072610 Skeletal dysplasia Diseases 0.000 description 20
- 101100328886 Caenorhabditis elegans col-2 gene Proteins 0.000 description 19
- 125000000174 L-prolyl group Chemical group [H]N1C([H])([H])C([H])([H])C([H])([H])[C@@]1([H])C(*)=O 0.000 description 19
- 108020001507 fusion proteins Proteins 0.000 description 19
- 102000037865 fusion proteins Human genes 0.000 description 19
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Chemical compound CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 18
- 125000000637 arginyl group Chemical group N[C@@H](CCCNC(N)=N)C(=O)* 0.000 description 18
- 125000001360 methionine group Chemical group N[C@@H](CCSC)C(=O)* 0.000 description 18
- 239000011780 sodium chloride Substances 0.000 description 18
- ODKSFYDXXFIFQN-BYPYZUCNSA-N L-arginine Chemical compound OC(=O)[C@@H](N)CCCN=C(N)N ODKSFYDXXFIFQN-BYPYZUCNSA-N 0.000 description 17
- 125000003607 serino group Chemical group [H]N([H])[C@]([H])(C(=O)[*])C(O[H])([H])[H] 0.000 description 17
- 108090000623 proteins and genes Proteins 0.000 description 16
- 230000001965 increasing effect Effects 0.000 description 15
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 14
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 14
- 238000001727 in vivo Methods 0.000 description 14
- 201000008482 osteoarthritis Diseases 0.000 description 14
- 206010061762 Chondropathy Diseases 0.000 description 13
- 208000011231 Crohn disease Diseases 0.000 description 13
- 102000018071 Immunoglobulin Fc Fragments Human genes 0.000 description 13
- 108010091135 Immunoglobulin Fc Fragments Proteins 0.000 description 13
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 13
- 108091028043 Nucleic acid sequence Proteins 0.000 description 13
- 235000018102 proteins Nutrition 0.000 description 13
- 102000004169 proteins and genes Human genes 0.000 description 13
- 238000006467 substitution reaction Methods 0.000 description 13
- 208000024891 symptom Diseases 0.000 description 13
- 102100039339 Atrial natriuretic peptide receptor 1 Human genes 0.000 description 12
- 230000006870 function Effects 0.000 description 12
- 125000003630 glycyl group Chemical group [H]N([H])C([H])([H])C(*)=O 0.000 description 12
- 125000001500 prolyl group Chemical group [H]N1C([H])(C(=O)[*])C([H])([H])C([H])([H])C1([H])[H] 0.000 description 12
- 210000003491 skin Anatomy 0.000 description 12
- 230000001225 therapeutic effect Effects 0.000 description 12
- 101710102163 Atrial natriuretic peptide receptor 1 Proteins 0.000 description 11
- 101001128963 Homo sapiens Protein Dr1 Proteins 0.000 description 11
- 241000699666 Mus <mouse, genus> Species 0.000 description 11
- 102100031227 Protein Dr1 Human genes 0.000 description 11
- 229910052799 carbon Inorganic materials 0.000 description 11
- 230000015556 catabolic process Effects 0.000 description 11
- 238000006731 degradation reaction Methods 0.000 description 11
- 239000001509 sodium citrate Substances 0.000 description 11
- HRXKRNGNAMMEHJ-UHFFFAOYSA-K trisodium citrate Chemical compound [Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O HRXKRNGNAMMEHJ-UHFFFAOYSA-K 0.000 description 11
- 229940038773 trisodium citrate Drugs 0.000 description 11
- ZHNUHDYFZUAESO-UHFFFAOYSA-N Formamide Chemical compound NC=O ZHNUHDYFZUAESO-UHFFFAOYSA-N 0.000 description 10
- 206010049933 Hypophosphatasia Diseases 0.000 description 10
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 10
- 208000020221 Short stature Diseases 0.000 description 10
- 238000007792 addition Methods 0.000 description 10
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 10
- 235000018417 cysteine Nutrition 0.000 description 10
- 125000000487 histidyl group Chemical group [H]N([H])C(C(=O)O*)C([H])([H])C1=C([H])N([H])C([H])=N1 0.000 description 10
- 125000003588 lysine group Chemical group [H]N([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 description 10
- 239000012528 membrane Substances 0.000 description 10
- 230000002829 reductive effect Effects 0.000 description 10
- 239000003981 vehicle Substances 0.000 description 10
- 125000000510 L-tryptophano group Chemical group [H]C1=C([H])C([H])=C2N([H])C([H])=C(C([H])([H])[C@@]([H])(C(O[H])=O)N([H])[*])C2=C1[H] 0.000 description 9
- 206010028980 Neoplasm Diseases 0.000 description 9
- 206010029748 Noonan syndrome Diseases 0.000 description 9
- 239000003795 chemical substances by application Substances 0.000 description 9
- 238000012217 deletion Methods 0.000 description 9
- 230000037430 deletion Effects 0.000 description 9
- 230000012010 growth Effects 0.000 description 9
- 238000009396 hybridization Methods 0.000 description 9
- 230000037417 hyperactivation Effects 0.000 description 9
- 230000009984 peri-natal effect Effects 0.000 description 9
- NGVDGCNFYWLIFO-UHFFFAOYSA-N pyridoxal 5'-phosphate Chemical compound CC1=NC=C(COP(O)(O)=O)C(C=O)=C1O NGVDGCNFYWLIFO-UHFFFAOYSA-N 0.000 description 9
- 235000007682 pyridoxal 5'-phosphate Nutrition 0.000 description 9
- 239000011589 pyridoxal 5'-phosphate Substances 0.000 description 9
- 208000007442 rickets Diseases 0.000 description 9
- 230000028327 secretion Effects 0.000 description 9
- 230000003442 weekly effect Effects 0.000 description 9
- 206010010356 Congenital anomaly Diseases 0.000 description 8
- 102000043136 MAP kinase family Human genes 0.000 description 8
- 108091054455 MAP kinase family Proteins 0.000 description 8
- 101100180983 Mus musculus Khdrbs3 gene Proteins 0.000 description 8
- 102000003729 Neprilysin Human genes 0.000 description 8
- 108090000028 Neprilysin Proteins 0.000 description 8
- 208000018737 Parkinson disease Diseases 0.000 description 8
- 241001415513 Salpida Species 0.000 description 8
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 8
- 235000004279 alanine Nutrition 0.000 description 8
- 230000018678 bone mineralization Effects 0.000 description 8
- 239000013078 crystal Substances 0.000 description 8
- 230000007812 deficiency Effects 0.000 description 8
- 231100000673 dose–response relationship Toxicity 0.000 description 8
- 230000014509 gene expression Effects 0.000 description 8
- WHUUTDBJXJRKMK-VKHMYHEASA-L glutamate group Chemical group N[C@@H](CCC(=O)[O-])C(=O)[O-] WHUUTDBJXJRKMK-VKHMYHEASA-L 0.000 description 8
- 238000002887 multiple sequence alignment Methods 0.000 description 8
- 230000037361 pathway Effects 0.000 description 8
- 239000000243 solution Substances 0.000 description 8
- 238000005406 washing Methods 0.000 description 8
- 229910052727 yttrium Inorganic materials 0.000 description 8
- 206010002961 Aplasia Diseases 0.000 description 7
- 108010044467 Isoenzymes Proteins 0.000 description 7
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 7
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 7
- SUHOOTKUPISOBE-UHFFFAOYSA-N O-phosphoethanolamine Chemical compound NCCOP(O)(O)=O SUHOOTKUPISOBE-UHFFFAOYSA-N 0.000 description 7
- 230000004913 activation Effects 0.000 description 7
- 238000003556 assay Methods 0.000 description 7
- 230000008901 benefit Effects 0.000 description 7
- 210000004271 bone marrow stromal cell Anatomy 0.000 description 7
- 210000003414 extremity Anatomy 0.000 description 7
- 229910052739 hydrogen Inorganic materials 0.000 description 7
- 125000000741 isoleucyl group Chemical group [H]N([H])C(C(C([H])([H])[H])C([H])([H])C([H])([H])[H])C(=O)O* 0.000 description 7
- 125000003729 nucleotide group Chemical group 0.000 description 7
- 230000001575 pathological effect Effects 0.000 description 7
- 150000003839 salts Chemical class 0.000 description 7
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 6
- 206010003591 Ataxia Diseases 0.000 description 6
- 241000283690 Bos taurus Species 0.000 description 6
- 241000282472 Canis lupus familiaris Species 0.000 description 6
- 206010010904 Convulsion Diseases 0.000 description 6
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 6
- 102100024193 Mitogen-activated protein kinase 1 Human genes 0.000 description 6
- 206010034010 Parkinsonism Diseases 0.000 description 6
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 6
- 241000700159 Rattus Species 0.000 description 6
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 6
- 239000004473 Threonine Substances 0.000 description 6
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 description 6
- 230000002411 adverse Effects 0.000 description 6
- 201000007201 aphasia Diseases 0.000 description 6
- 210000004369 blood Anatomy 0.000 description 6
- 239000008280 blood Substances 0.000 description 6
- 201000011510 cancer Diseases 0.000 description 6
- 210000001612 chondrocyte Anatomy 0.000 description 6
- 230000007547 defect Effects 0.000 description 6
- XPPKVPWEQAFLFU-UHFFFAOYSA-J diphosphate(4-) Chemical compound [O-]P([O-])(=O)OP([O-])([O-])=O XPPKVPWEQAFLFU-UHFFFAOYSA-J 0.000 description 6
- 235000011180 diphosphates Nutrition 0.000 description 6
- 238000009472 formulation Methods 0.000 description 6
- 231100000518 lethal Toxicity 0.000 description 6
- 230000001665 lethal effect Effects 0.000 description 6
- 210000000963 osteoblast Anatomy 0.000 description 6
- 229910052698 phosphorus Inorganic materials 0.000 description 6
- 239000003826 tablet Substances 0.000 description 6
- 238000002560 therapeutic procedure Methods 0.000 description 6
- 239000004474 valine Substances 0.000 description 6
- 239000013598 vector Substances 0.000 description 6
- ZOOGRGPOEVQQDX-UUOKFMHZSA-N 3',5'-cyclic GMP Chemical compound C([C@H]1O2)OP(O)(=O)O[C@H]1[C@@H](O)[C@@H]2N1C(N=C(NC2=O)N)=C2N=C1 ZOOGRGPOEVQQDX-UUOKFMHZSA-N 0.000 description 5
- 201000010028 Acrocephalosyndactylia Diseases 0.000 description 5
- 108020004414 DNA Proteins 0.000 description 5
- 108010016626 Dipeptides Proteins 0.000 description 5
- 206010013883 Dwarfism Diseases 0.000 description 5
- 208000029663 Hypophosphatemia Diseases 0.000 description 5
- 101100084030 Mus musculus Alpl gene Proteins 0.000 description 5
- 230000036772 blood pressure Effects 0.000 description 5
- 201000005043 chondromalacia Diseases 0.000 description 5
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 description 5
- 230000008021 deposition Effects 0.000 description 5
- 239000003112 inhibitor Substances 0.000 description 5
- 230000003902 lesion Effects 0.000 description 5
- 230000004048 modification Effects 0.000 description 5
- 238000012986 modification Methods 0.000 description 5
- 239000002773 nucleotide Substances 0.000 description 5
- 102000016914 ras Proteins Human genes 0.000 description 5
- 230000009467 reduction Effects 0.000 description 5
- 201000000980 schizophrenia Diseases 0.000 description 5
- 238000004904 shortening Methods 0.000 description 5
- 241000894007 species Species 0.000 description 5
- 230000002195 synergetic effect Effects 0.000 description 5
- 210000002303 tibia Anatomy 0.000 description 5
- 210000001519 tissue Anatomy 0.000 description 5
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 5
- 210000000689 upper leg Anatomy 0.000 description 5
- 230000002485 urinary effect Effects 0.000 description 5
- WMSPZRZYIGOEDS-QMMMGPOBSA-N 4-[[(1s)-5-amino-1-carboxypentyl]amino]-3,5-dinitrobenzoic acid Chemical compound NCCCC[C@@H](C(O)=O)NC1=C([N+]([O-])=O)C=C(C(O)=O)C=C1[N+]([O-])=O WMSPZRZYIGOEDS-QMMMGPOBSA-N 0.000 description 4
- 102100024321 Alkaline phosphatase, placental type Human genes 0.000 description 4
- 206010002198 Anaphylactic reaction Diseases 0.000 description 4
- 229920000623 Cellulose acetate phthalate Polymers 0.000 description 4
- 208000032170 Congenital Abnormalities Diseases 0.000 description 4
- 241000282326 Felis catus Species 0.000 description 4
- DHCLVCXQIBBOPH-UHFFFAOYSA-N Glycerol 2-phosphate Chemical compound OCC(CO)OP(O)(O)=O DHCLVCXQIBBOPH-UHFFFAOYSA-N 0.000 description 4
- 101000615334 Homo sapiens Antileukoproteinase Proteins 0.000 description 4
- 101000897494 Homo sapiens C-C motif chemokine 27 Proteins 0.000 description 4
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 4
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 4
- 239000004472 Lysine Substances 0.000 description 4
- 229910019142 PO4 Inorganic materials 0.000 description 4
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 4
- 101710118538 Protease Proteins 0.000 description 4
- 201000001718 Roberts syndrome Diseases 0.000 description 4
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 4
- 230000002159 abnormal effect Effects 0.000 description 4
- 230000005856 abnormality Effects 0.000 description 4
- 239000002253 acid Substances 0.000 description 4
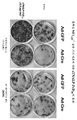
- HFVAFDPGUJEFBQ-UHFFFAOYSA-M alizarin red S Chemical compound [Na+].O=C1C2=CC=CC=C2C(=O)C2=C1C=C(S([O-])(=O)=O)C(O)=C2O HFVAFDPGUJEFBQ-UHFFFAOYSA-M 0.000 description 4
- 230000036783 anaphylactic response Effects 0.000 description 4
- 208000003455 anaphylaxis Diseases 0.000 description 4
- 125000000613 asparagine group Chemical group N[C@@H](CC(N)=O)C(=O)* 0.000 description 4
- 230000003197 catalytic effect Effects 0.000 description 4
- 229940081734 cellulose acetate phthalate Drugs 0.000 description 4
- 210000000968 fibrocartilage Anatomy 0.000 description 4
- 230000013595 glycosylation Effects 0.000 description 4
- 238000006206 glycosylation reaction Methods 0.000 description 4
- 230000000004 hemodynamic effect Effects 0.000 description 4
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 4
- 229910052500 inorganic mineral Inorganic materials 0.000 description 4
- 230000003834 intracellular effect Effects 0.000 description 4
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 4
- 229960000310 isoleucine Drugs 0.000 description 4
- 238000004519 manufacturing process Methods 0.000 description 4
- 229930182817 methionine Natural products 0.000 description 4
- 239000011707 mineral Substances 0.000 description 4
- 210000002569 neuron Anatomy 0.000 description 4
- 235000021317 phosphate Nutrition 0.000 description 4
- 108010031345 placental alkaline phosphatase Proteins 0.000 description 4
- 230000004481 post-translational protein modification Effects 0.000 description 4
- 210000002966 serum Anatomy 0.000 description 4
- 230000019491 signal transduction Effects 0.000 description 4
- 238000011287 therapeutic dose Methods 0.000 description 4
- URAYPUMNDPQOKB-UHFFFAOYSA-N triacetin Chemical compound CC(=O)OCC(OC(C)=O)COC(C)=O URAYPUMNDPQOKB-UHFFFAOYSA-N 0.000 description 4
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 3
- 206010067484 Adverse reaction Diseases 0.000 description 3
- RGCKGOZRHPZPFP-UHFFFAOYSA-N Alizarin Natural products C1=CC=C2C(=O)C3=C(O)C(O)=CC=C3C(=O)C2=C1 RGCKGOZRHPZPFP-UHFFFAOYSA-N 0.000 description 3
- 102100025677 Alkaline phosphatase, germ cell type Human genes 0.000 description 3
- 208000025490 Apert syndrome Diseases 0.000 description 3
- 239000004475 Arginine Substances 0.000 description 3
- 108091026890 Coding region Proteins 0.000 description 3
- 206010011878 Deafness Diseases 0.000 description 3
- 206010019280 Heart failures Diseases 0.000 description 3
- 241000282412 Homo Species 0.000 description 3
- 101000574440 Homo sapiens Alkaline phosphatase, germ cell type Proteins 0.000 description 3
- 208000009289 Jackson-Weiss syndrome Diseases 0.000 description 3
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 3
- 229940124647 MEK inhibitor Drugs 0.000 description 3
- 241000124008 Mammalia Species 0.000 description 3
- 208000001826 Marfan syndrome Diseases 0.000 description 3
- 208000034578 Multiple myelomas Diseases 0.000 description 3
- 208000012902 Nervous system disease Diseases 0.000 description 3
- 201000004404 Neurofibroma Diseases 0.000 description 3
- 102000008763 Neurofilament Proteins Human genes 0.000 description 3
- 108010088373 Neurofilament Proteins Proteins 0.000 description 3
- 208000008589 Obesity Diseases 0.000 description 3
- 201000004014 Pfeiffer syndrome Diseases 0.000 description 3
- 102000004160 Phosphoric Monoester Hydrolases Human genes 0.000 description 3
- 108090000608 Phosphoric Monoester Hydrolases Proteins 0.000 description 3
- 206010035226 Plasma cell myeloma Diseases 0.000 description 3
- 239000004365 Protease Substances 0.000 description 3
- DVEXZJFMOKTQEZ-JYFOCSDGSA-N U0126 Chemical compound C=1C=CC=C(N)C=1SC(\N)=C(/C#N)\C(\C#N)=C(/N)SC1=CC=CC=C1N DVEXZJFMOKTQEZ-JYFOCSDGSA-N 0.000 description 3
- 238000009825 accumulation Methods 0.000 description 3
- 230000006838 adverse reaction Effects 0.000 description 3
- 238000010171 animal model Methods 0.000 description 3
- 210000003423 ankle Anatomy 0.000 description 3
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 3
- 239000002775 capsule Substances 0.000 description 3
- 239000004202 carbamide Substances 0.000 description 3
- 210000000170 cell membrane Anatomy 0.000 description 3
- 230000002490 cerebral effect Effects 0.000 description 3
- 210000000038 chest Anatomy 0.000 description 3
- 238000000576 coating method Methods 0.000 description 3
- 238000012937 correction Methods 0.000 description 3
- 230000003247 decreasing effect Effects 0.000 description 3
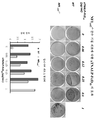
- 238000010586 diagram Methods 0.000 description 3
- OLSDWRNWUGHKSY-UHFFFAOYSA-J dicalcium;phosphonato phosphate;dihydrate Chemical compound O.O.[Ca+2].[Ca+2].[O-]P([O-])(=O)OP([O-])([O-])=O OLSDWRNWUGHKSY-UHFFFAOYSA-J 0.000 description 3
- 230000001882 diuretic effect Effects 0.000 description 3
- 239000003814 drug Substances 0.000 description 3
- 210000000959 ear middle Anatomy 0.000 description 3
- 201000010103 fibrous dysplasia Diseases 0.000 description 3
- 208000016354 hearing loss disease Diseases 0.000 description 3
- 208000003906 hydrocephalus Diseases 0.000 description 3
- 229910052588 hydroxylapatite Inorganic materials 0.000 description 3
- 208000011111 hypophosphatemic rickets Diseases 0.000 description 3
- 230000002401 inhibitory effect Effects 0.000 description 3
- 230000000968 intestinal effect Effects 0.000 description 3
- 238000001990 intravenous administration Methods 0.000 description 3
- 210000003734 kidney Anatomy 0.000 description 3
- 208000032839 leukemia Diseases 0.000 description 3
- 210000004185 liver Anatomy 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- 210000001872 metatarsal bone Anatomy 0.000 description 3
- 238000010369 molecular cloning Methods 0.000 description 3
- 239000000178 monomer Substances 0.000 description 3
- 210000005044 neurofilament Anatomy 0.000 description 3
- 235000020824 obesity Nutrition 0.000 description 3
- 230000002188 osteogenic effect Effects 0.000 description 3
- 208000005368 osteomalacia Diseases 0.000 description 3
- XYJRXVWERLGGKC-UHFFFAOYSA-D pentacalcium;hydroxide;triphosphate Chemical group [OH-].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O XYJRXVWERLGGKC-UHFFFAOYSA-D 0.000 description 3
- 108010011903 peptide receptors Proteins 0.000 description 3
- 238000002732 pharmacokinetic assay Methods 0.000 description 3
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 3
- 239000010452 phosphate Substances 0.000 description 3
- 239000013612 plasmid Substances 0.000 description 3
- 229910052700 potassium Inorganic materials 0.000 description 3
- 230000002028 premature Effects 0.000 description 3
- 238000000746 purification Methods 0.000 description 3
- 230000011664 signaling Effects 0.000 description 3
- 210000003625 skull Anatomy 0.000 description 3
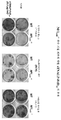
- 238000010186 staining Methods 0.000 description 3
- 238000007920 subcutaneous administration Methods 0.000 description 3
- 239000000725 suspension Substances 0.000 description 3
- 210000002700 urine Anatomy 0.000 description 3
- 230000004584 weight gain Effects 0.000 description 3
- 235000019786 weight gain Nutrition 0.000 description 3
- 238000001262 western blot Methods 0.000 description 3
- ZRMMVODKVLXCBB-UHFFFAOYSA-N 1-n-cyclohexyl-4-n-phenylbenzene-1,4-diamine Chemical compound C1CCCCC1NC(C=C1)=CC=C1NC1=CC=CC=C1 ZRMMVODKVLXCBB-UHFFFAOYSA-N 0.000 description 2
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 2
- 208000009304 Acute Kidney Injury Diseases 0.000 description 2
- 108010011170 Ala-Trp-Arg-His-Pro-Gln-Phe-Gly-Gly Proteins 0.000 description 2
- 208000008822 Ankylosis Diseases 0.000 description 2
- 201000001320 Atherosclerosis Diseases 0.000 description 2
- 208000000412 Avitaminosis Diseases 0.000 description 2
- 206010004146 Basal cell carcinoma Diseases 0.000 description 2
- 206010005003 Bladder cancer Diseases 0.000 description 2
- 208000010392 Bone Fractures Diseases 0.000 description 2
- 206010065687 Bone loss Diseases 0.000 description 2
- 206010006187 Breast cancer Diseases 0.000 description 2
- 208000026310 Breast neoplasm Diseases 0.000 description 2
- 206010007559 Cardiac failure congestive Diseases 0.000 description 2
- 208000024172 Cardiovascular disease Diseases 0.000 description 2
- 206010067380 Costello Syndrome Diseases 0.000 description 2
- ZZZCUOFIHGPKAK-UHFFFAOYSA-N D-erythro-ascorbic acid Natural products OCC1OC(=O)C(O)=C1O ZZZCUOFIHGPKAK-UHFFFAOYSA-N 0.000 description 2
- 208000018035 Dental disease Diseases 0.000 description 2
- 206010048768 Dermatosis Diseases 0.000 description 2
- 208000012239 Developmental disease Diseases 0.000 description 2
- NIQCNGHVCWTJSM-UHFFFAOYSA-N Dimethyl phthalate Chemical compound COC(=O)C1=CC=CC=C1C(=O)OC NIQCNGHVCWTJSM-UHFFFAOYSA-N 0.000 description 2
- 102100031480 Dual specificity mitogen-activated protein kinase kinase 1 Human genes 0.000 description 2
- 101710146526 Dual specificity mitogen-activated protein kinase kinase 1 Proteins 0.000 description 2
- 206010013935 Dysmenorrhoea Diseases 0.000 description 2
- 208000031213 Epidermal nevus syndrome Diseases 0.000 description 2
- 102000010834 Extracellular Matrix Proteins Human genes 0.000 description 2
- 108010037362 Extracellular Matrix Proteins Proteins 0.000 description 2
- 206010015995 Eyelid ptosis Diseases 0.000 description 2
- 206010016654 Fibrosis Diseases 0.000 description 2
- 206010017076 Fracture Diseases 0.000 description 2
- 102000018834 GTPase activity proteins Human genes 0.000 description 2
- 108040008737 GTPase activity proteins Proteins 0.000 description 2
- 206010051066 Gastrointestinal stromal tumour Diseases 0.000 description 2
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Chemical compound OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 2
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 2
- 229930186217 Glycolipid Natural products 0.000 description 2
- 206010053759 Growth retardation Diseases 0.000 description 2
- 208000028782 Hereditary disease Diseases 0.000 description 2
- 101000961040 Homo sapiens Atrial natriuretic peptide receptor 2 Proteins 0.000 description 2
- 206010020772 Hypertension Diseases 0.000 description 2
- 206010021135 Hypovitaminosis Diseases 0.000 description 2
- 208000026350 Inborn Genetic disease Diseases 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- 206010023198 Joint ankylosis Diseases 0.000 description 2
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 2
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 2
- LRQKBLKVPFOOQJ-YFKPBYRVSA-N L-norleucine Chemical compound CCCC[C@H]([NH3+])C([O-])=O LRQKBLKVPFOOQJ-YFKPBYRVSA-N 0.000 description 2
- 208000005101 LEOPARD Syndrome Diseases 0.000 description 2
- 208000020358 Learning disease Diseases 0.000 description 2
- 206010025323 Lymphomas Diseases 0.000 description 2
- XUMBMVFBXHLACL-UHFFFAOYSA-N Melanin Chemical compound O=C1C(=O)C(C2=CNC3=C(C(C(=O)C4=C32)=O)C)=C2C4=CNC2=C1C XUMBMVFBXHLACL-UHFFFAOYSA-N 0.000 description 2
- 208000024556 Mendelian disease Diseases 0.000 description 2
- VVQNEPGJFQJSBK-UHFFFAOYSA-N Methyl methacrylate Chemical compound COC(=O)C(C)=C VVQNEPGJFQJSBK-UHFFFAOYSA-N 0.000 description 2
- 208000010428 Muscle Weakness Diseases 0.000 description 2
- 206010028372 Muscular weakness Diseases 0.000 description 2
- 208000025966 Neurological disease Diseases 0.000 description 2
- 208000005890 Neuroma Diseases 0.000 description 2
- 101150083321 Nf1 gene Proteins 0.000 description 2
- 206010031243 Osteogenesis imperfecta Diseases 0.000 description 2
- 208000001132 Osteoporosis Diseases 0.000 description 2
- 108090000526 Papain Proteins 0.000 description 2
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 2
- 206010060862 Prostate cancer Diseases 0.000 description 2
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 2
- 208000001647 Renal Insufficiency Diseases 0.000 description 2
- 208000033626 Renal failure acute Diseases 0.000 description 2
- IQFYYKKMVGJFEH-XLPZGREQSA-N Thymidine Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 IQFYYKKMVGJFEH-XLPZGREQSA-N 0.000 description 2
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 description 2
- 229930003268 Vitamin C Natural products 0.000 description 2
- 230000001154 acute effect Effects 0.000 description 2
- 201000011040 acute kidney failure Diseases 0.000 description 2
- 208000012998 acute renal failure Diseases 0.000 description 2
- 235000008206 alpha-amino acids Nutrition 0.000 description 2
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 2
- 208000007502 anemia Diseases 0.000 description 2
- 210000000436 anus Anatomy 0.000 description 2
- 239000003125 aqueous solvent Substances 0.000 description 2
- 230000004872 arterial blood pressure Effects 0.000 description 2
- 230000002146 bilateral effect Effects 0.000 description 2
- 230000008468 bone growth Effects 0.000 description 2
- 210000004556 brain Anatomy 0.000 description 2
- 244000309464 bull Species 0.000 description 2
- 230000002308 calcification Effects 0.000 description 2
- 125000004432 carbon atom Chemical group C* 0.000 description 2
- 230000000747 cardiac effect Effects 0.000 description 2
- 230000001413 cellular effect Effects 0.000 description 2
- 208000020832 chronic kidney disease Diseases 0.000 description 2
- 208000024035 chronic otitis media Diseases 0.000 description 2
- 208000022831 chronic renal failure syndrome Diseases 0.000 description 2
- 238000012411 cloning technique Methods 0.000 description 2
- 239000011248 coating agent Substances 0.000 description 2
- 210000000860 cochlear nerve Anatomy 0.000 description 2
- 238000004440 column chromatography Methods 0.000 description 2
- 238000002648 combination therapy Methods 0.000 description 2
- 238000013270 controlled release Methods 0.000 description 2
- 230000002950 deficient Effects 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 230000018109 developmental process Effects 0.000 description 2
- 230000004882 diastolic arterial blood pressure Effects 0.000 description 2
- FLKPEMZONWLCSK-UHFFFAOYSA-N diethyl phthalate Chemical compound CCOC(=O)C1=CC=CC=C1C(=O)OCC FLKPEMZONWLCSK-UHFFFAOYSA-N 0.000 description 2
- 230000004069 differentiation Effects 0.000 description 2
- 239000002934 diuretic Substances 0.000 description 2
- 239000003937 drug carrier Substances 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- 239000002702 enteric coating Substances 0.000 description 2
- 238000009505 enteric coating Methods 0.000 description 2
- 230000002327 eosinophilic effect Effects 0.000 description 2
- 210000002744 extracellular matrix Anatomy 0.000 description 2
- 208000009618 familial spinal neurofibromatosis Diseases 0.000 description 2
- 230000004761 fibrosis Effects 0.000 description 2
- 201000011243 gastrointestinal stromal tumor Diseases 0.000 description 2
- 239000000499 gel Substances 0.000 description 2
- 239000007897 gelcap Substances 0.000 description 2
- 208000005017 glioblastoma Diseases 0.000 description 2
- 125000000404 glutamine group Chemical group N[C@@H](CCC(N)=O)C(=O)* 0.000 description 2
- 239000001087 glyceryl triacetate Substances 0.000 description 2
- 235000013773 glyceryl triacetate Nutrition 0.000 description 2
- 210000004349 growth plate Anatomy 0.000 description 2
- 231100000001 growth retardation Toxicity 0.000 description 2
- 231100000888 hearing loss Toxicity 0.000 description 2
- 230000010370 hearing loss Effects 0.000 description 2
- 208000019622 heart disease Diseases 0.000 description 2
- 238000004128 high performance liquid chromatography Methods 0.000 description 2
- 239000005556 hormone Substances 0.000 description 2
- 229940088597 hormone Drugs 0.000 description 2
- 210000002758 humerus Anatomy 0.000 description 2
- 230000007062 hydrolysis Effects 0.000 description 2
- 238000006460 hydrolysis reaction Methods 0.000 description 2
- 230000002209 hydrophobic effect Effects 0.000 description 2
- 229920003132 hydroxypropyl methylcellulose phthalate Polymers 0.000 description 2
- 229940031704 hydroxypropyl methylcellulose phthalate Drugs 0.000 description 2
- 230000001077 hypotensive effect Effects 0.000 description 2
- FDGQSTZJBFJUBT-UHFFFAOYSA-N hypoxanthine Chemical compound O=C1NC=NC2=C1NC=N2 FDGQSTZJBFJUBT-UHFFFAOYSA-N 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- 238000000338 in vitro Methods 0.000 description 2
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 2
- 201000006370 kidney failure Diseases 0.000 description 2
- 201000003723 learning disability Diseases 0.000 description 2
- 210000002414 leg Anatomy 0.000 description 2
- 230000000670 limiting effect Effects 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 239000011159 matrix material Substances 0.000 description 2
- 206010027191 meningioma Diseases 0.000 description 2
- 230000002503 metabolic effect Effects 0.000 description 2
- 230000005012 migration Effects 0.000 description 2
- 238000013508 migration Methods 0.000 description 2
- 235000013336 milk Nutrition 0.000 description 2
- 239000008267 milk Substances 0.000 description 2
- 210000004080 milk Anatomy 0.000 description 2
- 238000010172 mouse model Methods 0.000 description 2
- 230000002071 myeloproliferative effect Effects 0.000 description 2
- 208000006943 neurofibromatosis-pheochromocytoma-duodenal carcinoid syndrome Diseases 0.000 description 2
- 229910052757 nitrogen Inorganic materials 0.000 description 2
- 238000002638 palliative care Methods 0.000 description 2
- 229940055729 papain Drugs 0.000 description 2
- 235000019834 papain Nutrition 0.000 description 2
- 230000036961 partial effect Effects 0.000 description 2
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 2
- 230000026731 phosphorylation Effects 0.000 description 2
- 238000006366 phosphorylation reaction Methods 0.000 description 2
- 208000031223 plasma cell leukemia Diseases 0.000 description 2
- 239000004014 plasticizer Substances 0.000 description 2
- 235000017924 poor diet Nutrition 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- 230000035755 proliferation Effects 0.000 description 2
- 238000011321 prophylaxis Methods 0.000 description 2
- 230000007065 protein hydrolysis Effects 0.000 description 2
- 201000003004 ptosis Diseases 0.000 description 2
- 102000005962 receptors Human genes 0.000 description 2
- 108020003175 receptors Proteins 0.000 description 2
- 108010023714 recombinant human bone morphogenetic protein-2 Proteins 0.000 description 2
- 230000001105 regulatory effect Effects 0.000 description 2
- 230000004044 response Effects 0.000 description 2
- 208000037803 restenosis Diseases 0.000 description 2
- 206010039722 scoliosis Diseases 0.000 description 2
- 230000003248 secreting effect Effects 0.000 description 2
- 208000017520 skin disease Diseases 0.000 description 2
- 150000003384 small molecules Chemical class 0.000 description 2
- 235000012237 sodium aluminium phosphate Nutrition 0.000 description 2
- 208000005198 spinal stenosis Diseases 0.000 description 2
- 238000010561 standard procedure Methods 0.000 description 2
- 239000013589 supplement Substances 0.000 description 2
- 239000006188 syrup Substances 0.000 description 2
- 235000020357 syrup Nutrition 0.000 description 2
- 230000004873 systolic arterial blood pressure Effects 0.000 description 2
- 238000013519 translation Methods 0.000 description 2
- 229960002622 triacetin Drugs 0.000 description 2
- IQQWMJSNEUUJAY-UHFFFAOYSA-D trialuminum;sodium;dihydrogen phosphate;hydrogen phosphate;tetrahydrate Chemical compound O.O.O.O.[Na+].[Al+3].[Al+3].[Al+3].OP(O)([O-])=O.OP(O)([O-])=O.OP(O)([O-])=O.OP(O)([O-])=O.OP(O)([O-])=O.OP(O)([O-])=O.OP([O-])([O-])=O.OP([O-])([O-])=O IQQWMJSNEUUJAY-UHFFFAOYSA-D 0.000 description 2
- 125000000430 tryptophan group Chemical group [H]N([H])C(C(=O)O*)C([H])([H])C1=C([H])N([H])C2=C([H])C([H])=C([H])C([H])=C12 0.000 description 2
- 229910052721 tungsten Inorganic materials 0.000 description 2
- 125000001493 tyrosinyl group Chemical group [H]OC1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 description 2
- 241000701161 unidentified adenovirus Species 0.000 description 2
- 201000005112 urinary bladder cancer Diseases 0.000 description 2
- 210000004291 uterus Anatomy 0.000 description 2
- 230000000304 vasodilatating effect Effects 0.000 description 2
- 235000019154 vitamin C Nutrition 0.000 description 2
- 239000011718 vitamin C Substances 0.000 description 2
- 208000030401 vitamin deficiency disease Diseases 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- RBCOYOYDYNXAFA-UHFFFAOYSA-L (5-hydroxy-4,6-dimethylpyridin-3-yl)methyl phosphate Chemical compound CC1=NC=C(COP([O-])([O-])=O)C(C)=C1O RBCOYOYDYNXAFA-UHFFFAOYSA-L 0.000 description 1
- LDVVTQMJQSCDMK-UHFFFAOYSA-N 1,3-dihydroxypropan-2-yl formate Chemical compound OCC(CO)OC=O LDVVTQMJQSCDMK-UHFFFAOYSA-N 0.000 description 1
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 1
- PZBLUWVMZMXIKZ-UHFFFAOYSA-N 2-o-(2-ethoxy-2-oxoethyl) 1-o-ethyl benzene-1,2-dicarboxylate Chemical compound CCOC(=O)COC(=O)C1=CC=CC=C1C(=O)OCC PZBLUWVMZMXIKZ-UHFFFAOYSA-N 0.000 description 1
- HVCOBJNICQPDBP-UHFFFAOYSA-N 3-[3-[3,5-dihydroxy-6-methyl-4-(3,4,5-trihydroxy-6-methyloxan-2-yl)oxyoxan-2-yl]oxydecanoyloxy]decanoic acid;hydrate Chemical class O.OC1C(OC(CC(=O)OC(CCCCCCC)CC(O)=O)CCCCCCC)OC(C)C(O)C1OC1C(O)C(O)C(O)C(C)O1 HVCOBJNICQPDBP-UHFFFAOYSA-N 0.000 description 1
- UZOVYGYOLBIAJR-UHFFFAOYSA-N 4-isocyanato-4'-methyldiphenylmethane Chemical compound C1=CC(C)=CC=C1CC1=CC=C(N=C=O)C=C1 UZOVYGYOLBIAJR-UHFFFAOYSA-N 0.000 description 1
- ODHCTXKNWHHXJC-VKHMYHEASA-N 5-oxo-L-proline Chemical compound OC(=O)[C@@H]1CCC(=O)N1 ODHCTXKNWHHXJC-VKHMYHEASA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- 208000021969 Acromelia Diseases 0.000 description 1
- 208000028060 Albright disease Diseases 0.000 description 1
- 201000004384 Alopecia Diseases 0.000 description 1
- 241000272525 Anas platyrhynchos Species 0.000 description 1
- 206010002329 Aneurysm Diseases 0.000 description 1
- 206010003210 Arteriosclerosis Diseases 0.000 description 1
- 241000972773 Aulopiformes Species 0.000 description 1
- 208000008035 Back Pain Diseases 0.000 description 1
- DWRXFEITVBNRMK-UHFFFAOYSA-N Beta-D-1-Arabinofuranosylthymine Natural products O=C1NC(=O)C(C)=CN1C1C(O)C(O)C(CO)O1 DWRXFEITVBNRMK-UHFFFAOYSA-N 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-M Bicarbonate Chemical class OC([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-M 0.000 description 1
- 108010049931 Bone Morphogenetic Protein 2 Proteins 0.000 description 1
- 102000008143 Bone Morphogenetic Protein 2 Human genes 0.000 description 1
- 102100022545 Bone morphogenetic protein 8B Human genes 0.000 description 1
- 206010006002 Bone pain Diseases 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- 208000014644 Brain disease Diseases 0.000 description 1
- GOJCZVPJCKEBQV-UHFFFAOYSA-N Butyl phthalyl butylglycolate Chemical compound CCCCOC(=O)COC(=O)C1=CC=CC=C1C(=O)OCCCC GOJCZVPJCKEBQV-UHFFFAOYSA-N 0.000 description 1
- 208000000380 Cafe-au-Lait Spots Diseases 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 102000005701 Calcium-Binding Proteins Human genes 0.000 description 1
- 108010045403 Calcium-Binding Proteins Proteins 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- 206010007747 Cataract congenital Diseases 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 1
- 206010008690 Chondrocalcinosis pyrophosphate Diseases 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- 206010009269 Cleft palate Diseases 0.000 description 1
- 206010062344 Congenital musculoskeletal anomaly Diseases 0.000 description 1
- 208000025023 Cranial malformation Diseases 0.000 description 1
- 208000009283 Craniosynostoses Diseases 0.000 description 1
- 206010049889 Craniosynostosis Diseases 0.000 description 1
- 108010051219 Cre recombinase Proteins 0.000 description 1
- 201000001200 Crouzon syndrome-acanthosis nigricans syndrome Diseases 0.000 description 1
- 102000053602 DNA Human genes 0.000 description 1
- 208000013558 Developmental Bone disease Diseases 0.000 description 1
- 102100024746 Dihydrofolate reductase Human genes 0.000 description 1
- 208000007590 Disorders of Excessive Somnolence Diseases 0.000 description 1
- 102100023266 Dual specificity mitogen-activated protein kinase kinase 2 Human genes 0.000 description 1
- 101710146529 Dual specificity mitogen-activated protein kinase kinase 2 Proteins 0.000 description 1
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 1
- 208000013805 Dyggve-Melchior-Clausen disease Diseases 0.000 description 1
- 241001061260 Emmelichthys struhsakeri Species 0.000 description 1
- 208000032274 Encephalopathy Diseases 0.000 description 1
- 208000032541 Epidermal naevus Diseases 0.000 description 1
- 206010053177 Epidermolysis Diseases 0.000 description 1
- 241000283073 Equus caballus Species 0.000 description 1
- 239000001856 Ethyl cellulose Substances 0.000 description 1
- ZZSNKZQZMQGXPY-UHFFFAOYSA-N Ethyl cellulose Chemical compound CCOCC1OC(OC)C(OCC)C(OCC)C1OC1C(O)C(O)C(OC)C(CO)O1 ZZSNKZQZMQGXPY-UHFFFAOYSA-N 0.000 description 1
- 229920003134 Eudragit® polymer Polymers 0.000 description 1
- 108091008794 FGF receptors Proteins 0.000 description 1
- 102000044168 Fibroblast Growth Factor Receptor Human genes 0.000 description 1
- 102100023600 Fibroblast growth factor receptor 2 Human genes 0.000 description 1
- 101710182389 Fibroblast growth factor receptor 2 Proteins 0.000 description 1
- 206010061159 Foot deformity Diseases 0.000 description 1
- 208000034826 Genetic Predisposition to Disease Diseases 0.000 description 1
- 102100031181 Glyceraldehyde-3-phosphate dehydrogenase Human genes 0.000 description 1
- 239000007995 HEPES buffer Substances 0.000 description 1
- 208000006358 Hand Deformities Diseases 0.000 description 1
- 101000749809 Homo sapiens 2',3'-cyclic-nucleotide 3'-phosphodiesterase Proteins 0.000 description 1
- 101500027325 Homo sapiens Atrial natriuretic peptide Proteins 0.000 description 1
- 101000961044 Homo sapiens Atrial natriuretic peptide receptor 1 Proteins 0.000 description 1
- 101000899368 Homo sapiens Bone morphogenetic protein 8B Proteins 0.000 description 1
- 101500026735 Homo sapiens Brain natriuretic peptide 32 Proteins 0.000 description 1
- 101000796277 Homo sapiens C-type natriuretic peptide Proteins 0.000 description 1
- 101500027331 Homo sapiens Urodilatin Proteins 0.000 description 1
- 206010020751 Hypersensitivity Diseases 0.000 description 1
- 206010020880 Hypertrophy Diseases 0.000 description 1
- UGQMRVRMYYASKQ-UHFFFAOYSA-N Hypoxanthine nucleoside Natural products OC1C(O)C(CO)OC1N1C(NC=NC2=O)=C2N=C1 UGQMRVRMYYASKQ-UHFFFAOYSA-N 0.000 description 1
- 102000006496 Immunoglobulin Heavy Chains Human genes 0.000 description 1
- 108010019476 Immunoglobulin Heavy Chains Proteins 0.000 description 1
- 208000011327 Increased bone mineral density Diseases 0.000 description 1
- 108090001061 Insulin Proteins 0.000 description 1
- 102000004877 Insulin Human genes 0.000 description 1
- 208000035478 Interatrial communication Diseases 0.000 description 1
- 206010048469 Kidney enlargement Diseases 0.000 description 1
- 208000001182 Kniest dysplasia Diseases 0.000 description 1
- ZGUNAGUHMKGQNY-ZETCQYMHSA-N L-alpha-phenylglycine zwitterion Chemical class OC(=O)[C@@H](N)C1=CC=CC=C1 ZGUNAGUHMKGQNY-ZETCQYMHSA-N 0.000 description 1
- 229930182816 L-glutamine Natural products 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 208000006286 Legius syndrome Diseases 0.000 description 1
- 208000006824 Lichtenstein syndrome Diseases 0.000 description 1
- 241000219745 Lupinus Species 0.000 description 1
- 102000019149 MAP kinase activity proteins Human genes 0.000 description 1
- 108040008097 MAP kinase activity proteins Proteins 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- JLVVSXFLKOJNIY-UHFFFAOYSA-N Magnesium ion Chemical compound [Mg+2] JLVVSXFLKOJNIY-UHFFFAOYSA-N 0.000 description 1
- 208000035450 Malformed Nails Diseases 0.000 description 1
- 201000001853 McCune-Albright syndrome Diseases 0.000 description 1
- 101710113100 Membrane-bound alkaline phosphatase Proteins 0.000 description 1
- 208000036626 Mental retardation Diseases 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 206010062901 Multiple lentigines syndrome Diseases 0.000 description 1
- 101000899367 Mus musculus Bone morphogenetic protein 8A Proteins 0.000 description 1
- 208000007101 Muscle Cramp Diseases 0.000 description 1
- 102000008300 Mutant Proteins Human genes 0.000 description 1
- 108010021466 Mutant Proteins Proteins 0.000 description 1
- 241000475481 Nebula Species 0.000 description 1
- 206010071323 Neuropsychiatric syndrome Diseases 0.000 description 1
- 208000010708 Noonan syndrome with multiple lentigines Diseases 0.000 description 1
- 208000021384 Obsessive-Compulsive disease Diseases 0.000 description 1
- 206010030113 Oedema Diseases 0.000 description 1
- 208000000035 Osteochondroma Diseases 0.000 description 1
- 208000003076 Osteolysis Diseases 0.000 description 1
- 102000004316 Oxidoreductases Human genes 0.000 description 1
- 108090000854 Oxidoreductases Proteins 0.000 description 1
- 208000002193 Pain Diseases 0.000 description 1
- 206010033425 Pain in extremity Diseases 0.000 description 1
- 208000031481 Pathologic Constriction Diseases 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- 208000029082 Pelvic Inflammatory Disease Diseases 0.000 description 1
- 208000000450 Pelvic Pain Diseases 0.000 description 1
- 102000035195 Peptidases Human genes 0.000 description 1
- 108091005804 Peptidases Proteins 0.000 description 1
- 108091000080 Phosphotransferase Proteins 0.000 description 1
- 208000012641 Pigmentation disease Diseases 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 229920001710 Polyorthoester Polymers 0.000 description 1
- 208000004880 Polyuria Diseases 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- 101800001295 Putative ATP-dependent helicase Proteins 0.000 description 1
- 101800001006 Putative helicase Proteins 0.000 description 1
- 206010062237 Renal impairment Diseases 0.000 description 1
- 208000012635 Rhizomelia Diseases 0.000 description 1
- 208000027790 Rib fracture Diseases 0.000 description 1
- 208000005568 Robinow syndrome Diseases 0.000 description 1
- 206010039710 Scleroderma Diseases 0.000 description 1
- YIQKLZYTHXTDDT-UHFFFAOYSA-H Sirius red F3B Chemical compound C1=CC(=CC=C1N=NC2=CC(=C(C=C2)N=NC3=C(C=C4C=C(C=CC4=C3[O-])NC(=O)NC5=CC6=CC(=C(C(=C6C=C5)[O-])N=NC7=C(C=C(C=C7)N=NC8=CC=C(C=C8)S(=O)(=O)[O-])S(=O)(=O)[O-])S(=O)(=O)O)S(=O)(=O)O)S(=O)(=O)[O-])S(=O)(=O)[O-].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+] YIQKLZYTHXTDDT-UHFFFAOYSA-H 0.000 description 1
- 206010062348 Skull malformation Diseases 0.000 description 1
- 206010041549 Spinal cord compression Diseases 0.000 description 1
- 208000014151 Stomatognathic disease Diseases 0.000 description 1
- 206010042265 Sturge-Weber Syndrome Diseases 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- 241000282898 Sus scrofa Species 0.000 description 1
- 206010042778 Syndactyly Diseases 0.000 description 1
- 108010076818 TEV protease Proteins 0.000 description 1
- 108020005038 Terminator Codon Proteins 0.000 description 1
- 208000008312 Tooth Loss Diseases 0.000 description 1
- 208000002148 Transposition of Great Vessels Diseases 0.000 description 1
- 206010044443 Transposition of the great vessels Diseases 0.000 description 1
- 208000026911 Tuberous sclerosis complex Diseases 0.000 description 1
- ISAKRJDGNUQOIC-UHFFFAOYSA-N Uracil Chemical class O=C1C=CNC(=O)N1 ISAKRJDGNUQOIC-UHFFFAOYSA-N 0.000 description 1
- 241000469816 Varus Species 0.000 description 1
- 241000700605 Viruses Species 0.000 description 1
- 208000026724 Waardenburg syndrome Diseases 0.000 description 1
- 208000021017 Weight Gain Diseases 0.000 description 1
- PTFCDOFLOPIGGS-UHFFFAOYSA-N Zinc dication Chemical compound [Zn+2] PTFCDOFLOPIGGS-UHFFFAOYSA-N 0.000 description 1
- 230000021736 acetylation Effects 0.000 description 1
- 238000006640 acetylation reaction Methods 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 230000003213 activating effect Effects 0.000 description 1
- 239000004480 active ingredient Substances 0.000 description 1
- 239000000443 aerosol Substances 0.000 description 1
- 125000000217 alkyl group Chemical group 0.000 description 1
- 208000026935 allergic disease Diseases 0.000 description 1
- 150000001370 alpha-amino acid derivatives Chemical class 0.000 description 1
- 150000001371 alpha-amino acids Chemical class 0.000 description 1
- 230000009435 amidation Effects 0.000 description 1
- 238000007112 amidation reaction Methods 0.000 description 1
- 229920000469 amphiphilic block copolymer Polymers 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 230000019552 anatomical structure morphogenesis Effects 0.000 description 1
- 235000021120 animal protein Nutrition 0.000 description 1
- 230000002547 anomalous effect Effects 0.000 description 1
- 208000022531 anorexia Diseases 0.000 description 1
- 239000005557 antagonist Substances 0.000 description 1
- 208000008784 apnea Diseases 0.000 description 1
- 239000008365 aqueous carrier Substances 0.000 description 1
- 239000007900 aqueous suspension Substances 0.000 description 1
- 208000011775 arteriosclerosis disease Diseases 0.000 description 1
- 206010003246 arthritis Diseases 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 229940072107 ascorbate Drugs 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- 208000013914 atrial heart septal defect Diseases 0.000 description 1
- 210000002072 atrial myocyte Anatomy 0.000 description 1
- 206010003664 atrial septal defect Diseases 0.000 description 1
- 208000025261 autosomal dominant disease Diseases 0.000 description 1
- 208000021018 autosomal dominant inheritance Diseases 0.000 description 1
- 238000002869 basic local alignment search tool Methods 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- IQFYYKKMVGJFEH-UHFFFAOYSA-N beta-L-thymidine Natural products O=C1NC(=O)C(C)=CN1C1OC(CO)C(O)C1 IQFYYKKMVGJFEH-UHFFFAOYSA-N 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 230000031018 biological processes and functions Effects 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 210000004204 blood vessel Anatomy 0.000 description 1
- 230000037396 body weight Effects 0.000 description 1
- 238000007470 bone biopsy Methods 0.000 description 1
- 230000037182 bone density Effects 0.000 description 1
- 210000001185 bone marrow Anatomy 0.000 description 1
- 210000002805 bone matrix Anatomy 0.000 description 1
- 201000006715 brachydactyly Diseases 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 239000000337 buffer salt Substances 0.000 description 1
- 230000003139 buffering effect Effects 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- 230000000711 cancerogenic effect Effects 0.000 description 1
- 231100000315 carcinogenic Toxicity 0.000 description 1
- 210000005242 cardiac chamber Anatomy 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 210000003321 cartilage cell Anatomy 0.000 description 1
- 230000024245 cell differentiation Effects 0.000 description 1
- 230000010261 cell growth Effects 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 210000003109 clavicle Anatomy 0.000 description 1
- 210000000078 claw Anatomy 0.000 description 1
- 238000010367 cloning Methods 0.000 description 1
- 230000001149 cognitive effect Effects 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 230000000295 complement effect Effects 0.000 description 1
- 238000007906 compression Methods 0.000 description 1
- 230000006835 compression Effects 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 239000000599 controlled substance Substances 0.000 description 1
- 230000001054 cortical effect Effects 0.000 description 1
- 239000002537 cosmetic Substances 0.000 description 1
- 239000006071 cream Substances 0.000 description 1
- 210000002858 crystal cell Anatomy 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 231100000895 deafness Toxicity 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 206010061428 decreased appetite Diseases 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 208000002925 dental caries Diseases 0.000 description 1
- 210000001750 dental cementum Anatomy 0.000 description 1
- 210000004268 dentin Anatomy 0.000 description 1
- 239000003599 detergent Substances 0.000 description 1
- 239000008121 dextrose Substances 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- PCYQQSKDZQTOQG-NXEZZACHSA-N dibutyl (2r,3r)-2,3-dihydroxybutanedioate Chemical compound CCCCOC(=O)[C@H](O)[C@@H](O)C(=O)OCCCC PCYQQSKDZQTOQG-NXEZZACHSA-N 0.000 description 1
- 235000015872 dietary supplement Nutrition 0.000 description 1
- 238000009792 diffusion process Methods 0.000 description 1
- 108020001096 dihydrofolate reductase Proteins 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- FBSAITBEAPNWJG-UHFFFAOYSA-N dimethyl phthalate Natural products CC(=O)OC1=CC=CC=C1OC(C)=O FBSAITBEAPNWJG-UHFFFAOYSA-N 0.000 description 1
- 229960001826 dimethylphthalate Drugs 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 238000003182 dose-response assay Methods 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 238000012377 drug delivery Methods 0.000 description 1
- 230000009977 dual effect Effects 0.000 description 1
- 230000004064 dysfunction Effects 0.000 description 1
- 239000003792 electrolyte Substances 0.000 description 1
- 238000001493 electron microscopy Methods 0.000 description 1
- 230000008030 elimination Effects 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- 230000010102 embolization Effects 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 230000005221 enamel hypoplasia Effects 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- 238000002641 enzyme replacement therapy Methods 0.000 description 1
- 235000019325 ethyl cellulose Nutrition 0.000 description 1
- 229920001249 ethyl cellulose Polymers 0.000 description 1
- 230000007717 exclusion Effects 0.000 description 1
- 230000001747 exhibiting effect Effects 0.000 description 1
- 239000013604 expression vector Substances 0.000 description 1
- 239000012894 fetal calf serum Substances 0.000 description 1
- 210000002082 fibula Anatomy 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 210000003811 finger Anatomy 0.000 description 1
- 235000021323 fish oil Nutrition 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 235000013355 food flavoring agent Nutrition 0.000 description 1
- 235000003599 food sweetener Nutrition 0.000 description 1
- 210000002683 foot Anatomy 0.000 description 1
- 210000000245 forearm Anatomy 0.000 description 1
- 230000022244 formylation Effects 0.000 description 1
- 238000006170 formylation reaction Methods 0.000 description 1
- 230000037433 frameshift Effects 0.000 description 1
- UHBYWPGGCSDKFX-VKHMYHEASA-N gamma-carboxy-L-glutamic acid Chemical compound OC(=O)[C@@H](N)CC(C(O)=O)C(O)=O UHBYWPGGCSDKFX-VKHMYHEASA-N 0.000 description 1
- 210000004051 gastric juice Anatomy 0.000 description 1
- 210000001035 gastrointestinal tract Anatomy 0.000 description 1
- 238000001415 gene therapy Methods 0.000 description 1
- 230000002068 genetic effect Effects 0.000 description 1
- 108020004445 glyceraldehyde-3-phosphate dehydrogenase Proteins 0.000 description 1
- 235000011187 glycerol Nutrition 0.000 description 1
- 210000002288 golgi apparatus Anatomy 0.000 description 1
- 230000036449 good health Effects 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- 208000024963 hair loss Diseases 0.000 description 1
- 230000003676 hair loss Effects 0.000 description 1
- 210000003128 head Anatomy 0.000 description 1
- 210000002837 heart atrium Anatomy 0.000 description 1
- 210000001624 hip Anatomy 0.000 description 1
- 102000045328 human ALPL Human genes 0.000 description 1
- 102000047974 human CNP Human genes 0.000 description 1
- 239000000017 hydrogel Substances 0.000 description 1
- 230000009610 hypersensitivity Effects 0.000 description 1
- 206010020765 hypersomnia Diseases 0.000 description 1
- 230000002267 hypothalamic effect Effects 0.000 description 1
- 208000003532 hypothyroidism Diseases 0.000 description 1
- 230000002989 hypothyroidism Effects 0.000 description 1
- 210000001621 ilium bone Anatomy 0.000 description 1
- 229940072221 immunoglobulins Drugs 0.000 description 1
- 238000001114 immunoprecipitation Methods 0.000 description 1
- 238000009169 immunotherapy Methods 0.000 description 1
- 239000007943 implant Substances 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 229940125396 insulin Drugs 0.000 description 1
- 238000009830 intercalation Methods 0.000 description 1
- 230000002687 intercalation Effects 0.000 description 1
- 230000002452 interceptive effect Effects 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 201000005992 juvenile myelomonocytic leukemia Diseases 0.000 description 1
- 208000017169 kidney disease Diseases 0.000 description 1
- 230000005977 kidney dysfunction Effects 0.000 description 1
- 229940043355 kinase inhibitor Drugs 0.000 description 1
- 210000003127 knee Anatomy 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 210000000867 larynx Anatomy 0.000 description 1
- 231100000225 lethality Toxicity 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- 238000004811 liquid chromatography Methods 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 230000004777 loss-of-function mutation Effects 0.000 description 1
- 210000003141 lower extremity Anatomy 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 239000008176 lyophilized powder Substances 0.000 description 1
- 239000006166 lysate Substances 0.000 description 1
- 208000029791 lytic metastatic bone lesion Diseases 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 229910001425 magnesium ion Inorganic materials 0.000 description 1
- 206010025482 malaise Diseases 0.000 description 1
- 210000004962 mammalian cell Anatomy 0.000 description 1
- 238000005621 mannosylation reaction Methods 0.000 description 1
- 238000004949 mass spectrometry Methods 0.000 description 1
- 210000001595 mastoid Anatomy 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 239000002609 medium Substances 0.000 description 1
- 108020004999 messenger RNA Proteins 0.000 description 1
- 208000030159 metabolic disease Diseases 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 229910021645 metal ion Inorganic materials 0.000 description 1
- 210000003789 metatarsus Anatomy 0.000 description 1
- 230000011987 methylation Effects 0.000 description 1
- 238000007069 methylation reaction Methods 0.000 description 1
- 125000001570 methylene group Chemical group [H]C([H])([*:1])[*:2] 0.000 description 1
- 238000010603 microCT Methods 0.000 description 1
- 239000003595 mist Substances 0.000 description 1
- 201000006417 multiple sclerosis Diseases 0.000 description 1
- 210000000663 muscle cell Anatomy 0.000 description 1
- 208000001491 myopia Diseases 0.000 description 1
- 230000004379 myopia Effects 0.000 description 1
- JSOQIZDOEIKRLY-UHFFFAOYSA-N n-propylnitrous amide Chemical compound CCCNN=O JSOQIZDOEIKRLY-UHFFFAOYSA-N 0.000 description 1
- 239000005445 natural material Substances 0.000 description 1
- 239000006225 natural substrate Substances 0.000 description 1
- 210000005036 nerve Anatomy 0.000 description 1
- 210000000944 nerve tissue Anatomy 0.000 description 1
- 206010061311 nervous system neoplasm Diseases 0.000 description 1
- 208000007538 neurilemmoma Diseases 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 239000002687 nonaqueous vehicle Substances 0.000 description 1
- 230000006911 nucleation Effects 0.000 description 1
- 238000010899 nucleation Methods 0.000 description 1
- 235000016709 nutrition Nutrition 0.000 description 1
- 239000002674 ointment Substances 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 210000003463 organelle Anatomy 0.000 description 1
- 150000002895 organic esters Chemical class 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 201000008968 osteosarcoma Diseases 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 208000003278 patent ductus arteriosus Diseases 0.000 description 1
- 201000008785 pediatric osteosarcoma Diseases 0.000 description 1
- 230000006320 pegylation Effects 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 150000002994 phenylalanines Chemical class 0.000 description 1
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 1
- 102000020233 phosphotransferase Human genes 0.000 description 1
- 239000003757 phosphotransferase inhibitor Substances 0.000 description 1
- 210000002826 placenta Anatomy 0.000 description 1
- 229920000747 poly(lactic acid) Polymers 0.000 description 1
- 238000002264 polyacrylamide gel electrophoresis Methods 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 239000004626 polylactic acid Substances 0.000 description 1
- 108091033319 polynucleotide Proteins 0.000 description 1
- 102000040430 polynucleotide Human genes 0.000 description 1
- 239000002157 polynucleotide Substances 0.000 description 1
- 208000001061 polyostotic fibrous dysplasia Diseases 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 230000000750 progressive effect Effects 0.000 description 1
- 230000002062 proliferating effect Effects 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 210000004129 prosencephalon Anatomy 0.000 description 1
- 235000019419 proteases Nutrition 0.000 description 1
- 108020001580 protein domains Proteins 0.000 description 1
- 229940079889 pyrrolidonecarboxylic acid Drugs 0.000 description 1
- 238000001959 radiotherapy Methods 0.000 description 1
- 238000003259 recombinant expression Methods 0.000 description 1
- 230000000306 recurrent effect Effects 0.000 description 1
- 230000008085 renal dysfunction Effects 0.000 description 1
- 230000000241 respiratory effect Effects 0.000 description 1
- 238000003757 reverse transcription PCR Methods 0.000 description 1
- 235000019515 salmon Nutrition 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 208000005706 short rib-polydactyly syndrome Diseases 0.000 description 1
- 235000021309 simple sugar Nutrition 0.000 description 1
- 231100001055 skeletal defect Toxicity 0.000 description 1
- FGCSIJPPCNCQJB-FAOVPRGRSA-M sodium;(2r,3s,4r,5r)-2,3,4,5,6-pentahydroxyhexanal;chloride Chemical compound [Na+].[Cl-].OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C=O FGCSIJPPCNCQJB-FAOVPRGRSA-M 0.000 description 1
- VVRPOCPLIUDBSA-CNZCJKERSA-M sodium;(3r,5r)-7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylcarbamoyl)-5-propan-2-ylpyrrol-1-yl]-3,5-dihydroxyheptanoate Chemical compound [Na+].C=1C=CC=CC=1C1=C(C=2C=CC(F)=CC=2)N(CC[C@@H](O)C[C@@H](O)CC([O-])=O)C(C(C)C)=C1C(=O)NC1=CC=CC=C1 VVRPOCPLIUDBSA-CNZCJKERSA-M 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 230000037436 splice-site mutation Effects 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 230000036262 stenosis Effects 0.000 description 1
- 208000037804 stenosis Diseases 0.000 description 1
- 239000008174 sterile solution Substances 0.000 description 1
- 208000002254 stillbirth Diseases 0.000 description 1
- 231100000537 stillbirth Toxicity 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 210000002536 stromal cell Anatomy 0.000 description 1
- 238000010254 subcutaneous injection Methods 0.000 description 1
- 239000007929 subcutaneous injection Substances 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 230000019635 sulfation Effects 0.000 description 1
- 238000005670 sulfation reaction Methods 0.000 description 1
- 229910052717 sulfur Inorganic materials 0.000 description 1
- 230000009469 supplementation Effects 0.000 description 1
- 230000008093 supporting effect Effects 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 239000000375 suspending agent Substances 0.000 description 1
- 239000003765 sweetening agent Substances 0.000 description 1
- 208000019270 symptomatic heart failure Diseases 0.000 description 1
- 238000002636 symptomatic treatment Methods 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 230000002194 synthesizing effect Effects 0.000 description 1
- 238000011285 therapeutic regimen Methods 0.000 description 1
- 210000000115 thoracic cavity Anatomy 0.000 description 1
- 210000003813 thumb Anatomy 0.000 description 1
- 229940104230 thymidine Drugs 0.000 description 1
- RWQNBRDOKXIBIV-UHFFFAOYSA-N thymine Chemical class CC1=CNC(=O)NC1=O RWQNBRDOKXIBIV-UHFFFAOYSA-N 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 231100000027 toxicology Toxicity 0.000 description 1
- 238000001890 transfection Methods 0.000 description 1
- 238000006276 transfer reaction Methods 0.000 description 1
- 230000005945 translocation Effects 0.000 description 1
- 208000014903 transposition of the great arteries Diseases 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- YZWRNSARCRTXDS-UHFFFAOYSA-N tripropionin Chemical compound CCC(=O)OCC(OC(=O)CC)COC(=O)CC YZWRNSARCRTXDS-UHFFFAOYSA-N 0.000 description 1
- 201000008827 tuberculosis Diseases 0.000 description 1
- 208000009999 tuberous sclerosis Diseases 0.000 description 1
- 230000034512 ubiquitination Effects 0.000 description 1
- 238000010798 ubiquitination Methods 0.000 description 1
- IUCCYQIEZNQWRS-DWWHXVEHSA-N ularitide Chemical compound C([C@H]1C(=O)NCC(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](C(NCC(=O)N[C@@H](C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)NCC(=O)N[C@@H](CSSC[C@@H](C(=O)N1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](C)NC(=O)[C@@H](N)[C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)=O)[C@@H](C)CC)C1=CC=CC=C1 IUCCYQIEZNQWRS-DWWHXVEHSA-N 0.000 description 1
- 210000000623 ulna Anatomy 0.000 description 1
- 241001515965 unidentified phage Species 0.000 description 1
- 210000004509 vascular smooth muscle cell Anatomy 0.000 description 1
- 229940124549 vasodilator Drugs 0.000 description 1
- 239000003071 vasodilator agent Substances 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- 235000013343 vitamin Nutrition 0.000 description 1
- 239000011782 vitamin Substances 0.000 description 1
- 229930003231 vitamin Natural products 0.000 description 1
- 229940088594 vitamin Drugs 0.000 description 1
- 208000006542 von Hippel-Lindau disease Diseases 0.000 description 1
- 230000007279 water homeostasis Effects 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
- 210000000707 wrist Anatomy 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/575—Hormones
- C07K14/58—Atrial natriuretic factor complex; Atriopeptin; Atrial natriuretic peptide [ANP]; Cardionatrin; Cardiodilatin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/06—Anti-spasmodics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/14—Hydrolases (3)
- C12N9/16—Hydrolases (3) acting on ester bonds (3.1)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/22—Hormones
- A61K38/2278—Vasoactive intestinal peptide [VIP]; Related peptides (e.g. Exendin)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/43—Enzymes; Proenzymes; Derivatives thereof
- A61K38/46—Hydrolases (3)
- A61K38/465—Hydrolases (3) acting on ester bonds (3.1), e.g. lipases, ribonucleases
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/30—Non-immunoglobulin-derived peptide or protein having an immunoglobulin constant or Fc region, or a fragment thereof, attached thereto
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Genetics & Genomics (AREA)
- Zoology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Gastroenterology & Hepatology (AREA)
- Biomedical Technology (AREA)
- Biochemistry (AREA)
- Wood Science & Technology (AREA)
- Molecular Biology (AREA)
- Dermatology (AREA)
- Epidemiology (AREA)
- Cardiology (AREA)
- Endocrinology (AREA)
- Toxicology (AREA)
- Biotechnology (AREA)
- Biophysics (AREA)
- Urology & Nephrology (AREA)
- General Engineering & Computer Science (AREA)
- Microbiology (AREA)
- Immunology (AREA)
- Physical Education & Sports Medicine (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Peptides Or Proteins (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Enzymes And Modification Thereof (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201161549047P | 2011-10-19 | 2011-10-19 | |
| US61/549,047 | 2011-10-19 | ||
| US201261649717P | 2012-05-21 | 2012-05-21 | |
| US61/649,717 | 2012-05-21 | ||
| PCT/US2012/039004 WO2013058833A1 (en) | 2011-10-19 | 2012-05-22 | Compositions comprising alkaline phosphatase and/or natriuretic peptide and methods of use thereof |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20140084201A true KR20140084201A (ko) | 2014-07-04 |
Family
ID=48141235
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020147013214A Withdrawn KR20140084201A (ko) | 2011-10-19 | 2012-05-22 | 알칼리성 포스파타제 및/또는 나트륨이뇨 펩티드를 포함하는 조성물 및 그의 사용 방법 |
Country Status (8)
| Country | Link |
|---|---|
| EP (2) | EP3488861B1 (OSRAM) |
| JP (1) | JP6118807B2 (OSRAM) |
| KR (1) | KR20140084201A (OSRAM) |
| CA (1) | CA2852874A1 (OSRAM) |
| ES (2) | ES2714406T3 (OSRAM) |
| HK (1) | HK1201446A1 (OSRAM) |
| SG (1) | SG11201401605QA (OSRAM) |
| WO (2) | WO2013058833A1 (OSRAM) |
Families Citing this family (39)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ES2887039T3 (es) | 2004-04-21 | 2021-12-21 | Alexion Pharma Inc | Conjugados para administración ósea y procedimiento de uso de estos para dirigir proteínas al hueso |
| WO2012088608A1 (en) | 2010-12-27 | 2012-07-05 | Enobia Canada Limited Partnership | Compositions comprising natriuretic peptides and methods of use thereof |
| US10052366B2 (en) | 2012-05-21 | 2018-08-21 | Alexion Pharmaceuticsl, Inc. | Compositions comprising alkaline phosphatase and/or natriuretic peptide and methods of use thereof |
| WO2016007873A1 (en) | 2014-07-11 | 2016-01-14 | The Regents Of The University Of Michigan | Compositions and methods for treating craniosynostosis |
| BR112017011900A2 (pt) | 2014-12-05 | 2018-02-27 | Alexion Pharma Inc | tratamento de ataques com fosfatase alcalina recombinante |
| CA2973883A1 (en) | 2015-01-28 | 2016-08-04 | Alexion Pharmaceuticals, Inc. | Methods of treating a subject with an alkaline phosphatase deficiency |
| JP6693134B2 (ja) * | 2015-02-02 | 2020-05-13 | 東ソー株式会社 | リンカー配列を有する抗体及びそれを用いた測定法 |
| CA2981103A1 (en) | 2015-03-31 | 2016-10-06 | Vhsquared Limited | Polypeptides |
| KR102789821B1 (ko) * | 2015-03-31 | 2025-04-02 | 소리소 파마슈티컬스 인크. | 프로테아제-절단가능 링커를 갖는 펩티드 구조물 |
| DK4089113T3 (da) | 2015-03-31 | 2024-02-05 | Sorriso Pharmaceuticals Inc | Polypeptider |
| DK3328416T5 (da) | 2015-07-30 | 2024-10-07 | Biomarin Pharm Inc | Anvendelse af natriuretske peptidvarianter af c-typen til behandling af skeletdysplasi |
| AU2016308624B2 (en) * | 2015-08-17 | 2022-06-23 | Alexion Pharmaceuticals, Inc. | Manufacturing of alkaline phosphatases |
| JP6868617B2 (ja) * | 2015-09-28 | 2021-05-12 | アレクシオン ファーマシューティカルズ, インコーポレイテッド | 低ホスファターゼ血症の組織非特異的アルカリホスファターゼ(tnsalp)酵素補充療法に有効な投薬計画の特定 |
| WO2017074466A1 (en) | 2015-10-30 | 2017-05-04 | Alexion Pharmaceuticals, Inc. | Methods for treating craniosynostosis in a patient |
| US11065306B2 (en) | 2016-03-08 | 2021-07-20 | Alexion Pharmaceuticals, Inc. | Methods for treating hypophosphatasia in children |
| WO2017173395A1 (en) | 2016-04-01 | 2017-10-05 | Alexion Pharmaceuticals, Inc. | Methods for treating hypophosphatasia in adolescents and adults |
| US11186832B2 (en) * | 2016-04-01 | 2021-11-30 | Alexion Pharmaceuticals, Inc. | Treating muscle weakness with alkaline phosphatases |
| US20190218274A1 (en) * | 2016-05-06 | 2019-07-18 | Phasebio Pharmaceuticals, Inc. | Elp fusion proteins for controlled and sustained release |
| US10988744B2 (en) | 2016-06-06 | 2021-04-27 | Alexion Pharmaceuticals, Inc. | Method of producing alkaline phosphatase |
| JP7018933B2 (ja) * | 2016-08-18 | 2022-02-14 | アレクシオン ファーマシューティカルズ, インコーポレイテッド | 気管気管支軟化症の治療方法 |
| EP3519438A1 (en) | 2016-09-30 | 2019-08-07 | VHsquared Limited | Compositions |
| TWI787230B (zh) | 2017-01-20 | 2022-12-21 | 法商賽諾菲公司 | 抗TGF-β抗體及其用途 |
| TW202421659A (zh) * | 2017-01-20 | 2024-06-01 | 美商健臻公司 | 骨靶向抗體 |
| CN110719786A (zh) | 2017-03-31 | 2020-01-21 | 阿雷克森制药公司 | 用于治疗成人和青少年的低磷酸酯酶症(hpp)的方法 |
| US11338020B2 (en) | 2018-01-09 | 2022-05-24 | Synthetic Biologics, Inc. | Alkaline phosphatase agents for treatment of neurodevelopmental disorders |
| ES3032290T3 (en) | 2018-03-20 | 2025-07-16 | Theriva Biologics Inc | Intestinal alkaline phosphatase formulations |
| EP3773686B1 (en) | 2018-03-20 | 2023-06-07 | Theriva Biologics, Inc. | Alkaline phosphatase agents for treatment of radiation disorders |
| US11913039B2 (en) | 2018-03-30 | 2024-02-27 | Alexion Pharmaceuticals, Inc. | Method for producing recombinant alkaline phosphatase |
| EP3833377B1 (en) | 2018-08-10 | 2023-11-22 | Alexion Pharmaceuticals, Inc. | Bone healing at implants using alkaline phosphatase |
| CN113993541A (zh) | 2019-05-06 | 2022-01-28 | 合成生物制品有限公司 | 基于碱性磷酸酶的肿瘤学治疗 |
| AU2020296979A1 (en) | 2019-06-21 | 2022-02-24 | Sorriso Pharmaceuticals, Inc. | Polypeptides |
| EP3986571A1 (en) | 2019-06-21 | 2022-04-27 | Sorriso Pharmaceuticals, Inc. | Polypeptides |
| MX2022007114A (es) * | 2019-12-09 | 2022-07-11 | Alexion Pharma Inc | Polipeptidos de fosfatasa alcalina y metodos de uso de los mismos. |
| CN116848146A (zh) * | 2020-09-03 | 2023-10-03 | I·S·Y·陈 | 可溶性碱性磷酸酶构建体和包含编码可溶性碱性磷酸酶构建体的多核苷酸的表达载体 |
| BR112023016048A2 (pt) | 2021-02-12 | 2023-11-14 | Alexion Pharma Inc | Polipeptídeos de fosfatase alcalina e métodos de uso dos mesmos |
| AU2023232162A1 (en) * | 2022-03-11 | 2024-10-24 | Avirmax Biopharma Inc. | Compositions and methods for expressing therapeutics |
| WO2024094747A1 (en) | 2022-11-02 | 2024-05-10 | Novo Nordisk A/S | Cnp compounds |
| CN116286763B (zh) * | 2023-02-28 | 2024-06-25 | 西北工业大学 | 一种热稳定性提高的胱硫醚β-合成酶突变体及应用 |
| TW202513806A (zh) * | 2023-06-07 | 2025-04-01 | 美商拜奧馬林製藥公司 | 高通量篩選與身材矮小相關之基因變異體 |
Family Cites Families (32)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE69108830T2 (de) | 1990-04-20 | 1995-09-14 | Hisayuki Matsuo | Neue im schwein vorkommende physiologisch aktive peptide. |
| JP3026351B2 (ja) | 1990-07-13 | 2000-03-27 | 壽之 松尾 | ブタcnp遺伝子及び前駆体蛋白 |
| JP2930380B2 (ja) | 1990-07-13 | 1999-08-03 | 壽之 松尾 | ブタ由来新規生理活性ペプチド(cnp―53) |
| JP3026354B2 (ja) | 1990-09-27 | 2000-03-27 | 壽之 松尾 | ヒトcnp遺伝子及び前駆体蛋白 |
| US5626863A (en) | 1992-02-28 | 1997-05-06 | Board Of Regents, The University Of Texas System | Photopolymerizable biodegradable hydrogels as tissue contacting materials and controlled-release carriers |
| JP2809533B2 (ja) | 1991-01-31 | 1998-10-08 | 壽之 松尾 | Cnp類似体ペプチド |
| WO1994020534A1 (en) | 1993-03-03 | 1994-09-15 | Mayo Foundation For Medical Education And Research | Vasonatrin peptide and analogs thereof |
| AU4255397A (en) | 1996-09-06 | 1998-03-26 | Trustees Of The University Of Pennsylvania, The | Chimpanzee adenovirus vectors |
| ATE360082T1 (de) | 1999-11-16 | 2007-05-15 | Genzyme Corp | Vektoren und transgene mit regulatorischen elementen zur genverabreichung in leber |
| US6407211B1 (en) | 1999-12-17 | 2002-06-18 | Mayo Foundation For Medical Education And Research | Chimeric natriuretic peptides |
| JP2002178279A (ja) | 2000-12-12 | 2002-06-25 | Ulvac Japan Ltd | 基板搬送方法 |
| IL142118A0 (en) * | 2001-03-20 | 2002-03-10 | Prochon Biotech Ltd | Method and composition for treatment of skeletal dysplasias |
| EP1395293B1 (en) | 2001-05-14 | 2009-07-22 | Gbp Ip, Llc | Lentiviral vectors encoding clotting factors for gene therapy |
| BRPI0203172B8 (pt) | 2001-09-28 | 2021-05-25 | Nakao Kazuwa | composição farmacêutica para acondroplasia |
| SG2013034475A (en) | 2001-11-21 | 2016-10-28 | Univ Pennsylvania | Simian adenovirus nucleic acid and amino acid sequences, vectors containing same, and methods of use |
| CA2433479A1 (en) | 2002-07-22 | 2004-01-22 | F. Hoffmann-La Roche Ag | Conjugate of a tissue non-specific alkaline phosphatase and dextran, process for its production and use thereof |
| ATE459370T1 (de) | 2002-11-26 | 2010-03-15 | Biocon Ltd | Modifizierte natriuretic verbindungen, konjugate und ihre verwendungen |
| WO2005007809A2 (en) | 2003-05-30 | 2005-01-27 | Alexion Pharmaceuticals, Inc. | Antibodies and fusion proteins that include engineered constant regions |
| JP4825667B2 (ja) | 2004-03-31 | 2011-11-30 | 一和 中尾 | 関節炎症治療剤又は予防剤 |
| WO2005094890A1 (ja) | 2004-03-31 | 2005-10-13 | Kazuwa Nakao | 身長増加用組成物 |
| JP2005292718A (ja) | 2004-04-05 | 2005-10-20 | Furukawa Electric Co Ltd:The | 光導波路、光導波路モジュールおよび光導波路の作成方法 |
| ES2887039T3 (es) | 2004-04-21 | 2021-12-21 | Alexion Pharma Inc | Conjugados para administración ósea y procedimiento de uso de estos para dirigir proteínas al hueso |
| US20070081984A1 (en) | 2005-10-11 | 2007-04-12 | Shunji Tomatsu | Compositions and methods for treating hypophosphatasia |
| CN101068575A (zh) | 2004-12-01 | 2007-11-07 | 建新公司 | 向肝脏定向递送遗传物质的方法 |
| TW200833840A (en) * | 2006-10-25 | 2008-08-16 | Amgen Inc | Toxin peptide therapeutic agents |
| US20080181903A1 (en) | 2006-12-21 | 2008-07-31 | Pdl Biopharma, Inc. | Conjugate of natriuretic peptide and antibody constant region |
| AU2008250945B2 (en) | 2007-05-11 | 2013-12-12 | Alexion Pharmaceuticals, Inc. | Bone targeted alkaline phosphatase, kits and methods of use thereof |
| US20100310561A1 (en) | 2007-06-06 | 2010-12-09 | Boehringer Ingelheim International Gmbh | Natriuretic fusion proteins |
| CA2693303C (en) | 2007-07-20 | 2017-11-07 | Mayo Foundation For Medical Education And Research | Natriuretic polypeptides |
| US20100184680A1 (en) * | 2007-09-11 | 2010-07-22 | Dorian Bevec | Therapeutic uses of b-type natriuretic peptide and human growth hormone 1-43 |
| EP2217620A2 (en) * | 2007-11-21 | 2010-08-18 | BioMarin Pharmaceutical Inc. | Variants of c-type natriuretic peptide |
| TWI471137B (zh) | 2009-05-20 | 2015-02-01 | Biomarin Pharm Inc | C型利鈉胜肽變異體 |
-
2012
- 2012-05-22 WO PCT/US2012/039004 patent/WO2013058833A1/en not_active Ceased
- 2012-05-22 KR KR1020147013214A patent/KR20140084201A/ko not_active Withdrawn
- 2012-05-22 SG SG11201401605QA patent/SG11201401605QA/en unknown
- 2012-05-22 CA CA2852874A patent/CA2852874A1/en not_active Abandoned
- 2012-10-18 ES ES12842640T patent/ES2714406T3/es active Active
- 2012-10-18 HK HK15101938.6A patent/HK1201446A1/xx unknown
- 2012-10-18 EP EP18201378.9A patent/EP3488861B1/en active Active
- 2012-10-18 WO PCT/US2012/060869 patent/WO2013059491A1/en not_active Ceased
- 2012-10-18 EP EP12842640.0A patent/EP2768526B1/en active Active
- 2012-10-18 ES ES18201378T patent/ES2944087T3/es active Active
- 2012-10-18 JP JP2014537254A patent/JP6118807B2/ja active Active
Also Published As
| Publication number | Publication date |
|---|---|
| ES2714406T3 (es) | 2019-05-28 |
| HK1201446A1 (en) | 2015-09-04 |
| ES2944087T3 (es) | 2023-06-19 |
| WO2013058833A8 (en) | 2013-06-13 |
| EP2768526A1 (en) | 2014-08-27 |
| CA2852874A1 (en) | 2013-04-25 |
| WO2013059491A1 (en) | 2013-04-25 |
| JP6118807B2 (ja) | 2017-04-19 |
| WO2013058833A1 (en) | 2013-04-25 |
| EP2768526A4 (en) | 2015-04-15 |
| EP2768526B1 (en) | 2018-12-05 |
| EP3488861B1 (en) | 2023-02-15 |
| SG11201401605QA (en) | 2014-09-26 |
| EP3488861A1 (en) | 2019-05-29 |
| JP2015502336A (ja) | 2015-01-22 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR20140084201A (ko) | 알칼리성 포스파타제 및/또는 나트륨이뇨 펩티드를 포함하는 조성물 및 그의 사용 방법 | |
| US10052366B2 (en) | Compositions comprising alkaline phosphatase and/or natriuretic peptide and methods of use thereof | |
| JP6055779B2 (ja) | ナトリウム利尿ペプチドを含む組成物およびその使用方法 | |
| RU2592670C2 (ru) | Комбинированное применение ловушек gdf и активаторов рецепторов эритропоэтина для повышения содержания эритроцитов | |
| TWI748373B (zh) | 使用gdf阱以增加紅血球水平 | |
| KR20120062874A (ko) | ActRIIb 길항제들와 이의 투약 및 용도 | |
| EA033623B1 (ru) | ВАРИАНТЫ, ПРОИСХОДЯЩИЕ ИЗ ActRIIB, И ИХ ПРИМЕНЕНИЕ | |
| EA034563B1 (ru) | Способ лечения или предотвращения перегрузки железом у пациента с талассемией | |
| KR20130118203A (ko) | 매트릭스 무기물화 장애의 처리방법, 조성물 및 키트 | |
| AU2019406214A1 (en) | Use of annexins in preventing and treating muscle membrane injury | |
| CA3166820A1 (en) | Variant actriib proteins and uses thereof | |
| US20240366722A1 (en) | Compositions and methods for treating renal diseases or conditions | |
| JP2019531070A (ja) | ツイスティッド・ガストルレーション・ポリペプチドおよびその用途 | |
| CA3198957A1 (en) | Treatment of enpp1 deficiency and abcc6 deficiency | |
| RU2814047C2 (ru) | Комбинированное применение ловушек gdf и активаторов рецепторов эритропоэтина для повышения содержания эритроцитов | |
| KR20240167914A (ko) | Enpp1 결핍증 및 abcc6 결핍증의 치료 | |
| WO2025042995A1 (en) | Treatment of enpp1 deficiency and abcc6 deficiency in children | |
| BR122024002253A2 (pt) | Polipeptídeo compreendendo sequência de aminoácidos de actriib, ácido nucleico compreendendo uma sequência que codifica o dito polipeptídeo, vetor compreendendo o dito ácido nucléico, célula compreendendo polipeptídeo, ácido nucleico ou vetor, usos terapêuticos do dito polipeptídeo e do dito ácido nucléico e métodos para produzir um polupeptídeo, uma proteína ou uma heteromultímera, piroteína e heteromultímera compreendendo um polipeptídeo actriib variante |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20140516 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| N231 | Notification of change of applicant | ||
| PN2301 | Change of applicant |
Patent event date: 20160212 Comment text: Notification of Change of Applicant Patent event code: PN23011R01D |
|
| PC1203 | Withdrawal of no request for examination | ||
| WITN | Application deemed withdrawn, e.g. because no request for examination was filed or no examination fee was paid |