JP7009397B2 - 腐食防止コーティング - Google Patents
腐食防止コーティング Download PDFInfo
- Publication number
- JP7009397B2 JP7009397B2 JP2018564842A JP2018564842A JP7009397B2 JP 7009397 B2 JP7009397 B2 JP 7009397B2 JP 2018564842 A JP2018564842 A JP 2018564842A JP 2018564842 A JP2018564842 A JP 2018564842A JP 7009397 B2 JP7009397 B2 JP 7009397B2
- Authority
- JP
- Japan
- Prior art keywords
- tin
- layer
- binary
- ternary
- coating material
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 238000000576 coating method Methods 0.000 title claims description 174
- 239000011248 coating agent Substances 0.000 title claims description 161
- 238000005260 corrosion Methods 0.000 title claims description 79
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 claims description 90
- 239000000463 material Substances 0.000 claims description 89
- 229910052718 tin Inorganic materials 0.000 claims description 89
- 239000000758 substrate Substances 0.000 claims description 82
- 230000007797 corrosion Effects 0.000 claims description 78
- KAESVJOAVNADME-UHFFFAOYSA-N Pyrrole Chemical compound C=1C=CNC=1 KAESVJOAVNADME-UHFFFAOYSA-N 0.000 claims description 67
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims description 51
- 239000002131 composite material Substances 0.000 claims description 42
- 229910052799 carbon Inorganic materials 0.000 claims description 38
- 229910001128 Sn alloy Inorganic materials 0.000 claims description 35
- 239000003112 inhibitor Substances 0.000 claims description 33
- 239000000446 fuel Substances 0.000 claims description 32
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 claims description 23
- 239000011230 binding agent Substances 0.000 claims description 19
- 238000000151 deposition Methods 0.000 claims description 18
- 239000002184 metal Substances 0.000 claims description 15
- 238000000034 method Methods 0.000 claims description 15
- 229910052751 metal Inorganic materials 0.000 claims description 13
- 239000000203 mixture Substances 0.000 claims description 12
- 229910052759 nickel Inorganic materials 0.000 claims description 12
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 claims description 9
- 229910052787 antimony Inorganic materials 0.000 claims description 9
- 229910052802 copper Inorganic materials 0.000 claims description 9
- 239000010949 copper Substances 0.000 claims description 9
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 claims description 8
- 239000012964 benzotriazole Substances 0.000 claims description 8
- 229910052733 gallium Inorganic materials 0.000 claims description 8
- 229910052738 indium Inorganic materials 0.000 claims description 8
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Chemical compound C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 claims description 8
- GYHNNYVSQQEPJS-UHFFFAOYSA-N Gallium Chemical compound [Ga] GYHNNYVSQQEPJS-UHFFFAOYSA-N 0.000 claims description 7
- WATWJIUSRGPENY-UHFFFAOYSA-N antimony atom Chemical compound [Sb] WATWJIUSRGPENY-UHFFFAOYSA-N 0.000 claims description 7
- APFVFJFRJDLVQX-UHFFFAOYSA-N indium atom Chemical compound [In] APFVFJFRJDLVQX-UHFFFAOYSA-N 0.000 claims description 7
- 239000010935 stainless steel Substances 0.000 claims description 6
- 229910001220 stainless steel Inorganic materials 0.000 claims description 6
- YHMYGUUIMTVXNW-UHFFFAOYSA-N 1,3-dihydrobenzimidazole-2-thione Chemical compound C1=CC=C2NC(S)=NC2=C1 YHMYGUUIMTVXNW-UHFFFAOYSA-N 0.000 claims description 4
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 claims description 4
- 229910052782 aluminium Inorganic materials 0.000 claims description 4
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 claims description 4
- 239000006229 carbon black Substances 0.000 claims description 4
- 239000002041 carbon nanotube Substances 0.000 claims description 4
- 229910021393 carbon nanotube Inorganic materials 0.000 claims description 4
- 239000003610 charcoal Substances 0.000 claims description 4
- 239000003245 coal Substances 0.000 claims description 4
- 229910021389 graphene Inorganic materials 0.000 claims description 4
- 229910002804 graphite Inorganic materials 0.000 claims description 4
- 239000010439 graphite Substances 0.000 claims description 4
- 229910052742 iron Inorganic materials 0.000 claims description 4
- 239000010936 titanium Substances 0.000 claims description 4
- 229910052719 titanium Inorganic materials 0.000 claims description 4
- MARUHZGHZWCEQU-UHFFFAOYSA-N 5-phenyl-2h-tetrazole Chemical compound C1=CC=CC=C1C1=NNN=N1 MARUHZGHZWCEQU-UHFFFAOYSA-N 0.000 claims description 3
- 125000003354 benzotriazolyl group Chemical group N1N=NC2=C1C=CC=C2* 0.000 claims description 3
- JNMJQSIXSBWDRK-UHFFFAOYSA-N 4-amino-3-[(4-amino-5-oxo-1H-1,2,4-triazol-3-yl)methyl]-1H-1,2,4-triazol-5-one Chemical compound NN1C(=NN=C1O)CC1=NN=C(N1N)O JNMJQSIXSBWDRK-UHFFFAOYSA-N 0.000 claims description 2
- 229920000049 Carbon (fiber) Polymers 0.000 claims description 2
- 239000001273 butane Substances 0.000 claims description 2
- 239000004917 carbon fiber Substances 0.000 claims description 2
- IJDNQMDRQITEOD-UHFFFAOYSA-N n-butane Chemical compound CCCC IJDNQMDRQITEOD-UHFFFAOYSA-N 0.000 claims description 2
- OFBQJSOFQDEBGM-UHFFFAOYSA-N n-pentane Natural products CCCCC OFBQJSOFQDEBGM-UHFFFAOYSA-N 0.000 claims description 2
- 239000010410 layer Substances 0.000 description 92
- 210000004027 cell Anatomy 0.000 description 36
- 239000003575 carbonaceous material Substances 0.000 description 28
- 239000000976 ink Substances 0.000 description 21
- 238000012360 testing method Methods 0.000 description 15
- 239000000243 solution Substances 0.000 description 11
- 229910045601 alloy Inorganic materials 0.000 description 10
- 239000000956 alloy Substances 0.000 description 10
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 9
- 230000001133 acceleration Effects 0.000 description 8
- 150000001875 compounds Chemical class 0.000 description 6
- -1 formaldehyde, phenols Chemical class 0.000 description 6
- 238000007747 plating Methods 0.000 description 6
- QRUDEWIWKLJBPS-UHFFFAOYSA-N benzotriazole Chemical compound C1=CC=C2N[N][N]C2=C1 QRUDEWIWKLJBPS-UHFFFAOYSA-N 0.000 description 5
- 238000004140 cleaning Methods 0.000 description 5
- 238000004070 electrodeposition Methods 0.000 description 5
- 238000010438 heat treatment Methods 0.000 description 5
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical group CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 4
- 239000008367 deionised water Substances 0.000 description 4
- 229910021641 deionized water Inorganic materials 0.000 description 4
- 238000004090 dissolution Methods 0.000 description 4
- 230000007774 longterm Effects 0.000 description 4
- 239000012528 membrane Substances 0.000 description 4
- 239000003960 organic solvent Substances 0.000 description 4
- 229920000642 polymer Polymers 0.000 description 4
- 239000002243 precursor Substances 0.000 description 4
- 229920005989 resin Polymers 0.000 description 4
- 239000011347 resin Substances 0.000 description 4
- 238000007650 screen-printing Methods 0.000 description 4
- 229910000807 Ga alloy Inorganic materials 0.000 description 3
- 229910000846 In alloy Inorganic materials 0.000 description 3
- 229910000990 Ni alloy Inorganic materials 0.000 description 3
- DYHPIVADFQHIQZ-UHFFFAOYSA-N [Ni].[Sb].[Sn] Chemical compound [Ni].[Sb].[Sn] DYHPIVADFQHIQZ-UHFFFAOYSA-N 0.000 description 3
- BRMNETVQNQAIKC-UHFFFAOYSA-N [Sn].[In].[Sb] Chemical compound [Sn].[In].[Sb] BRMNETVQNQAIKC-UHFFFAOYSA-N 0.000 description 3
- GVFOJDIFWSDNOY-UHFFFAOYSA-N antimony tin Chemical compound [Sn].[Sb] GVFOJDIFWSDNOY-UHFFFAOYSA-N 0.000 description 3
- 239000007864 aqueous solution Substances 0.000 description 3
- 210000003850 cellular structure Anatomy 0.000 description 3
- 230000008021 deposition Effects 0.000 description 3
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Chemical group C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 3
- RHZWSUVWRRXEJF-UHFFFAOYSA-N indium tin Chemical compound [In].[Sn] RHZWSUVWRRXEJF-UHFFFAOYSA-N 0.000 description 3
- 238000004519 manufacturing process Methods 0.000 description 3
- 229910021645 metal ion Inorganic materials 0.000 description 3
- 239000000178 monomer Substances 0.000 description 3
- CLDVQCMGOSGNIW-UHFFFAOYSA-N nickel tin Chemical compound [Ni].[Sn] CLDVQCMGOSGNIW-UHFFFAOYSA-N 0.000 description 3
- 210000004872 soft tissue Anatomy 0.000 description 3
- HJGWFBQIRUSCQQ-UHFFFAOYSA-N 4-amino-3-[3-(4-amino-5-oxo-1H-1,2,4-triazol-3-yl)butan-2-yl]-1H-1,2,4-triazol-5-one Chemical compound NN1C(=NN=C1O)C(C(C)C1=NN=C(N1N)O)C HJGWFBQIRUSCQQ-UHFFFAOYSA-N 0.000 description 2
- KLSJWNVTNUYHDU-UHFFFAOYSA-N Amitrole Chemical group NC1=NC=NN1 KLSJWNVTNUYHDU-UHFFFAOYSA-N 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- 125000003118 aryl group Chemical group 0.000 description 2
- 239000012298 atmosphere Substances 0.000 description 2
- 230000004888 barrier function Effects 0.000 description 2
- 239000002134 carbon nanofiber Substances 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- ZYGHJZDHTFUPRJ-UHFFFAOYSA-N coumarin Chemical compound C1=CC=C2OC(=O)C=CC2=C1 ZYGHJZDHTFUPRJ-UHFFFAOYSA-N 0.000 description 2
- 230000007547 defect Effects 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 239000008240 homogeneous mixture Substances 0.000 description 2
- 229910001092 metal group alloy Inorganic materials 0.000 description 2
- 150000002739 metals Chemical class 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 239000011253 protective coating Substances 0.000 description 2
- 239000000376 reactant Substances 0.000 description 2
- 230000002829 reductive effect Effects 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 150000003536 tetrazoles Chemical group 0.000 description 2
- AGCPZMJBXSCWQY-UHFFFAOYSA-N 1,1,2,3,4-pentachlorobutane Chemical compound ClCC(Cl)C(Cl)C(Cl)Cl AGCPZMJBXSCWQY-UHFFFAOYSA-N 0.000 description 1
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonium chloride Substances [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 1
- VHUUQVKOLVNVRT-UHFFFAOYSA-N Ammonium hydroxide Chemical compound [NH4+].[OH-] VHUUQVKOLVNVRT-UHFFFAOYSA-N 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 1
- 239000004593 Epoxy Substances 0.000 description 1
- 101100084627 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) pcb-4 gene Proteins 0.000 description 1
- JCXJVPUVTGWSNB-UHFFFAOYSA-N Nitrogen dioxide Chemical class O=[N]=O JCXJVPUVTGWSNB-UHFFFAOYSA-N 0.000 description 1
- ZCQWOFVYLHDMMC-UHFFFAOYSA-N Oxazole Chemical group C1=COC=N1 ZCQWOFVYLHDMMC-UHFFFAOYSA-N 0.000 description 1
- 239000004952 Polyamide Substances 0.000 description 1
- 229920002560 Polyethylene Glycol 3000 Polymers 0.000 description 1
- WTKZEGDFNFYCGP-UHFFFAOYSA-N Pyrazole Chemical group C=1C=NNC=1 WTKZEGDFNFYCGP-UHFFFAOYSA-N 0.000 description 1
- 229910001245 Sb alloy Inorganic materials 0.000 description 1
- 229910020935 Sn-Sb Inorganic materials 0.000 description 1
- 229910008757 Sn—Sb Inorganic materials 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 1
- FZWLAAWBMGSTSO-UHFFFAOYSA-N Thiazole Chemical group C1=CSC=N1 FZWLAAWBMGSTSO-UHFFFAOYSA-N 0.000 description 1
- 125000000218 acetic acid group Chemical group C(C)(=O)* 0.000 description 1
- 150000001253 acrylic acids Chemical class 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 125000002723 alicyclic group Chemical group 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- 125000003342 alkenyl group Chemical group 0.000 description 1
- 125000003545 alkoxy group Chemical group 0.000 description 1
- 229920000180 alkyd Chemical class 0.000 description 1
- 125000000217 alkyl group Chemical group 0.000 description 1
- 125000000304 alkynyl group Chemical group 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-N ammonia Natural products N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 1
- 235000011114 ammonium hydroxide Nutrition 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 150000003851 azoles Chemical class 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 239000003054 catalyst Substances 0.000 description 1
- 229920002678 cellulose Chemical class 0.000 description 1
- 239000001913 cellulose Chemical class 0.000 description 1
- 150000001805 chlorine compounds Chemical class 0.000 description 1
- 239000008139 complexing agent Substances 0.000 description 1
- 230000001010 compromised effect Effects 0.000 description 1
- 239000004020 conductor Substances 0.000 description 1
- 238000007596 consolidation process Methods 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- 229960000956 coumarin Drugs 0.000 description 1
- 235000001671 coumarin Nutrition 0.000 description 1
- AFYCEAFSNDLKSX-UHFFFAOYSA-N coumarin 460 Chemical compound CC1=CC(=O)OC2=CC(N(CC)CC)=CC=C21 AFYCEAFSNDLKSX-UHFFFAOYSA-N 0.000 description 1
- 125000004122 cyclic group Chemical group 0.000 description 1
- 230000009089 cytolysis Effects 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000001627 detrimental effect Effects 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- 229920001971 elastomer Polymers 0.000 description 1
- 239000003792 electrolyte Substances 0.000 description 1
- 125000003700 epoxy group Chemical group 0.000 description 1
- 235000011087 fumaric acid Nutrition 0.000 description 1
- 150000002238 fumaric acids Chemical class 0.000 description 1
- 125000001475 halogen functional group Chemical group 0.000 description 1
- 125000001072 heteroaryl group Chemical group 0.000 description 1
- 229920001519 homopolymer Polymers 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 150000002430 hydrocarbons Chemical class 0.000 description 1
- 238000011835 investigation Methods 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- ZLTPDFXIESTBQG-UHFFFAOYSA-N isothiazole Chemical group C=1C=NSC=1 ZLTPDFXIESTBQG-UHFFFAOYSA-N 0.000 description 1
- CTAPFRYPJLPFDF-UHFFFAOYSA-N isoxazole Chemical group C=1C=NOC=1 CTAPFRYPJLPFDF-UHFFFAOYSA-N 0.000 description 1
- 150000002576 ketones Chemical class 0.000 description 1
- 150000002689 maleic acids Chemical class 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 230000003278 mimic effect Effects 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 150000002894 organic compounds Chemical class 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- WUHLVXDDBHWHLQ-UHFFFAOYSA-N pentazole Chemical group N=1N=NNN=1 WUHLVXDDBHWHLQ-UHFFFAOYSA-N 0.000 description 1
- 239000000546 pharmaceutical excipient Substances 0.000 description 1
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 1
- 239000000049 pigment Substances 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 229920002037 poly(vinyl butyral) polymer Polymers 0.000 description 1
- 229920002647 polyamide Polymers 0.000 description 1
- 239000004417 polycarbonate Substances 0.000 description 1
- 229920000515 polycarbonate Polymers 0.000 description 1
- 150000003071 polychlorinated biphenyls Chemical class 0.000 description 1
- 229920000647 polyepoxide Polymers 0.000 description 1
- 229920002635 polyurethane Polymers 0.000 description 1
- 239000004814 polyurethane Substances 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 125000000168 pyrrolyl group Chemical group 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 239000002356 single layer Substances 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 229910052717 sulfur Inorganic materials 0.000 description 1
- 239000011593 sulfur Substances 0.000 description 1
- 150000003467 sulfuric acid derivatives Chemical class 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- 239000012085 test solution Substances 0.000 description 1
- 150000003573 thiols Chemical class 0.000 description 1
- FAKFSJNVVCGEEI-UHFFFAOYSA-J tin(4+);disulfate Chemical compound [Sn+4].[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O FAKFSJNVVCGEEI-UHFFFAOYSA-J 0.000 description 1
- HPGGPRDJHPYFRM-UHFFFAOYSA-J tin(iv) chloride Chemical compound Cl[Sn](Cl)(Cl)Cl HPGGPRDJHPYFRM-UHFFFAOYSA-J 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 150000003852 triazoles Chemical group 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D11/00—Inks
- C09D11/52—Electrically conductive inks
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/02—Details
- H01M8/0202—Collectors; Separators, e.g. bipolar separators; Interconnectors
- H01M8/0204—Non-porous and characterised by the material
- H01M8/0223—Composites
- H01M8/0228—Composites in the form of layered or coated products
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D5/00—Coating compositions, e.g. paints, varnishes or lacquers, characterised by their physical nature or the effects produced; Filling pastes
- C09D5/08—Anti-corrosive paints
- C09D5/082—Anti-corrosive paints characterised by the anti-corrosive pigment
- C09D5/086—Organic or non-macromolecular compounds
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D5/00—Coating compositions, e.g. paints, varnishes or lacquers, characterised by their physical nature or the effects produced; Filling pastes
- C09D5/24—Electrically-conducting paints
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D7/00—Features of coating compositions, not provided for in group C09D5/00; Processes for incorporating ingredients in coating compositions
- C09D7/40—Additives
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09D—COATING COMPOSITIONS, e.g. PAINTS, VARNISHES OR LACQUERS; FILLING PASTES; CHEMICAL PAINT OR INK REMOVERS; INKS; CORRECTING FLUIDS; WOODSTAINS; PASTES OR SOLIDS FOR COLOURING OR PRINTING; USE OF MATERIALS THEREFOR
- C09D7/00—Features of coating compositions, not provided for in group C09D5/00; Processes for incorporating ingredients in coating compositions
- C09D7/40—Additives
- C09D7/60—Additives non-macromolecular
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23F—NON-MECHANICAL REMOVAL OF METALLIC MATERIAL FROM SURFACE; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL; MULTI-STEP PROCESSES FOR SURFACE TREATMENT OF METALLIC MATERIAL INVOLVING AT LEAST ONE PROCESS PROVIDED FOR IN CLASS C23 AND AT LEAST ONE PROCESS COVERED BY SUBCLASS C21D OR C22F OR CLASS C25
- C23F11/00—Inhibiting corrosion of metallic material by applying inhibitors to the surface in danger of corrosion or adding them to the corrosive agent
- C23F11/08—Inhibiting corrosion of metallic material by applying inhibitors to the surface in danger of corrosion or adding them to the corrosive agent in other liquids
- C23F11/10—Inhibiting corrosion of metallic material by applying inhibitors to the surface in danger of corrosion or adding them to the corrosive agent in other liquids using organic inhibitors
- C23F11/14—Nitrogen-containing compounds
- C23F11/149—Heterocyclic compounds containing nitrogen as hetero atom
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/64—Carriers or collectors
- H01M4/66—Selection of materials
- H01M4/665—Composites
- H01M4/667—Composites in the form of layers, e.g. coatings
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/02—Details
- H01M8/0202—Collectors; Separators, e.g. bipolar separators; Interconnectors
- H01M8/0204—Non-porous and characterised by the material
- H01M8/0206—Metals or alloys
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/02—Details
- H01M8/0202—Collectors; Separators, e.g. bipolar separators; Interconnectors
- H01M8/0204—Non-porous and characterised by the material
- H01M8/0213—Gas-impermeable carbon-containing materials
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D235/00—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, condensed with other rings
- C07D235/02—Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, condensed with other rings condensed with carbocyclic rings or ring systems
- C07D235/04—Benzimidazoles; Hydrogenated benzimidazoles
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D249/00—Heterocyclic compounds containing five-membered rings having three nitrogen atoms as the only ring hetero atoms
- C07D249/16—Heterocyclic compounds containing five-membered rings having three nitrogen atoms as the only ring hetero atoms condensed with carbocyclic rings or ring systems
- C07D249/18—Benzotriazoles
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/10—Fuel cells with solid electrolytes
- H01M2008/1095—Fuel cells with polymeric electrolytes
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/62—Selection of inactive substances as ingredients for active masses, e.g. binders, fillers
- H01M4/628—Inhibitors, e.g. gassing inhibitors, corrosion inhibitors
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/64—Carriers or collectors
- H01M4/66—Selection of materials
- H01M4/663—Selection of materials containing carbon or carbonaceous materials as conductive part, e.g. graphite, carbon fibres
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/64—Carriers or collectors
- H01M4/66—Selection of materials
- H01M4/668—Composites of electroconductive material and synthetic resins
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M8/00—Fuel cells; Manufacture thereof
- H01M8/18—Regenerative fuel cells, e.g. redox flow batteries or secondary fuel cells
- H01M8/184—Regeneration by electrochemical means
- H01M8/188—Regeneration by electrochemical means by recharging of redox couples containing fluids; Redox flow type batteries
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/30—Hydrogen technology
- Y02E60/50—Fuel cells
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- Electrochemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Materials Engineering (AREA)
- Manufacturing & Machinery (AREA)
- Sustainable Development (AREA)
- Sustainable Energy (AREA)
- Organic Chemistry (AREA)
- Wood Science & Technology (AREA)
- Composite Materials (AREA)
- Mechanical Engineering (AREA)
- Metallurgy (AREA)
- Fuel Cell (AREA)
- Laminated Bodies (AREA)
- Cell Electrode Carriers And Collectors (AREA)
- Non-Insulated Conductors (AREA)
- Other Surface Treatments For Metallic Materials (AREA)
- Electric Double-Layer Capacitors Or The Like (AREA)
- Conductive Materials (AREA)
- Inert Electrodes (AREA)
Description
炭素ベースの材料とアゾール含有腐食防止剤とを含む導電性コーティング材料を含む層と、
スズを含む層と、を含む。
本明細書に記載の基板と、
炭素ベースの材料とアゾール含有腐食防止剤とを含む導電性コーティング材料を含む層と、スズを含む層とを含む導電性複合コーティングと、を含む。
炭素ベースの材料とアゾール含有腐食防止剤とを含む導電性コーティング材料を含む2層以上の層と、
スズを含む2層以上の層と、を含む導電性複合コーティングを含み得る。
本明細書に記載のカーボンインクと、
アゾール含有腐食防止剤と、を含む。
本明細書に記載の炭素ベースの材料と、
本明細書に記載のアゾール含有腐食防止剤と、を含み、アゾール含有腐食防止剤は、ベンゾトリアゾール、2-メルカプトベンズイミダゾール、5-フェニルテトラゾール、ビス[4-アミノ-5-ヒドロキシ-1,2,4-トリアゾール-3-イル]メタンまたはビス[4-アミノ-5-ヒドロキシ-1,2,4-トリアゾール-3-イル]ブタン、またはそれらの混合物であり、好ましくは、アゾール含有腐食防止剤はベンゾトリアゾールである。
基板を用意するステップと、
基板の表面上にスズを含む層を堆積させるステップと、
炭素ベースの材料とアゾール含有腐食防止剤とを含む導電性コーティング材料を含む層を前記基板の表面上の層として堆積させるステップと、を含む。
被覆した基板の表面上にスズを含むさらなる層を堆積させるステップと、
炭素ベースの材料とアゾール含有腐食防止剤とを含む導電性コーティング材料を含むさらなる層を前記基板の表面上に堆積させるステップと、
をさらに含み得る。
電気化学的装置の部品の耐食コーティングとしての、
炭素ベースの材料とアゾール含有腐食防止剤とを含む導電性コーティング材料を含む層と、スズを含む層とを含む導電性複合コーティングの使用を提供する。
本明細書に記載の基板を用意するステップと、
基板の表面上にスズを含む層を堆積させるステップと、
本明細書に記載の導電性コーティング材料を含む層を前記基板の表面上に堆積させるステップと、を含む。
導電性複合コーティングを塗布する基板は銅クラッド複合板であり、可能なコーティング配置の断面を図1に示す。
基板をアセトンに5分間浸し、その後、脱イオン水(DI)で濯ぎ、軟らかいティッシュペーパーで乾燥した。
市販のSn浴、pH0.5~1を含むか、0.45M SnCl2.2H2Oおよび0.45M K4P2O7を含む電着用のスズめっき溶液を調製した。pHを水性アンモニア溶液で6に調整した。めっき中の浴温度を35℃に維持した。スズ合金用のめっき浴は、錯化剤(塩化物または硫酸塩など)、添加剤および合金化される他の金属イオン、例えばSb、Ga、InまたはNiと共にSn金属イオンを含む。Sn-6wt%Sbのためのめっき浴の例は、
5g/L SnSO4
5g/L Sb(SO4)3
100g/L濃縮H2SO4
1g/L PEG5800または2g/L PEG3000
2g/Lクマリン(7-ジエチルアミノ-4-メチルクマリン)
33g/L K4P2O7
からなる。上記めっき浴のpHは約1であり、浴温度は28℃に維持した。
Sunchemicalインクを、均質な混合物を達成するまで、1wt%ベンゾトリアゾールと混合した。導電性コーティング材料の層を堆積させるために、半自動スクリーンプリンターを使用し、被覆する表面上でブレードを4回通過させて約20~25μmの厚さを達成した。
導電性コーティング材料をスクリーン印刷した後、被覆した物品全体を予熱したオーブン内に約150℃で1時間置いた。1時間後、被覆した基板をオーブン内に保持し、室温まで冷却させた。
種々のコーティングについて検討を行い、腐食促進試験の一部として、コーティングを浸食環境で試験した。80℃で硫酸によってpH3に調整した1M Na2SO4溶液をこれらの試験のために選択した。溶液は脱気しなかった。電位をSHEに対して+0.6Vになるようにし、この電位に1時間維持した。コーティングを連続的に溶液に浸漬し、PEM燃料電池の動作を模倣する電気化学的試験を行った。そのような試験によって、最良のコーティングの選択が可能となる。
コーティングの3つの主要なグループを試験した。該グループは、導電性コーティング材料のみ(最大35 μm厚さ)、スズ層コーティングのみ(最大約5μm厚さ)、並びに導電性コーティング材料を含む層(最大約35μm厚さ)と、スズを含む層(最大約5 μm厚さ)とを含む導電性複合コーティングである。これらのコーティングを、実施例1に記載の方法に従って調製した。
本実施例では、導電性コーティング材料を含む2層とスズを含む2層とを含む複合コーティングをはるかに良好な性能のために調製した。複合コーティングを図1(b)に示す。該複合コーティングは、電着したSn層に続いて、導電性コーティング材料層のスクリーン印刷の後、Sn-6wt%Sb合金の電着および最終導電性コーティング材料層を含む。全体の厚さは最大約40μmであった。
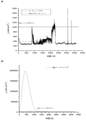
コーティングの性能を、1M Na2SO4,pH3を含む溶液での促進腐食試験で再び試験し、腐食電流がDoE目標を超えるまで、温度をR.T.と80℃との間で2hごとに循環させた。溶液は脱気しなかった。電位をSHEに対して+0.6Vとなるようにし、この電位で1時間保持した。コーティングの挙動を図5に示す。腐食電流密度は、18日を超えて非常に良好な挙動を示した。電流密度が時間と共に増加したが、DoE目標を超えなかった。
Claims (14)
- 基板と、
元素炭素とアゾール含有腐食防止剤とを含む導電性コーティング材料を含む層と、スズの層、あるいはニッケル、アンチモン、インジウムまたはガリウムの1つ以上とスズとの二元もしくは三元スズ合金の層、あるいはスズおよび前記二元もしくは三元スズ合金の層とを含む導電性複合コーティングと、
を含む被覆した物品を含み、
前記スズの層、あるいは前記二元もしくは三元スズ合金の層、あるいは前記スズおよび前記二元もしくは三元スズ合金の層は、前記基板と直接接触しており、
前記導電性コーティング材料を含む層は、前記スズの層、あるいは前記二元もしくは三元スズ合金の層、あるいは前記スズおよび前記二元もしくは三元スズ合金の層の上にあり、
a)前記導電性コーティング材料を含む層が1~50μm厚さであり、かつ/または
b)前記スズの層、あるいは前記二元もしくは三元スズ合金の層、あるいは前記スズおよび前記二元もしくは三元スズ合金の層が1~20μm厚さであることを特徴とする電気化学的装置。 - 前記導電性複合コーティングが、
元素炭素とアゾール含有腐食防止剤とを含む導電性コーティング材料を含む2層以上の層と、
スズの層、あるいはニッケル、アンチモン、インジウムまたはガリウムの1つ以上とスズとの二元もしくは三元スズ合金の層、あるいはスズおよび前記二元もしくは三元スズ合金の層の2層以上の層と、
を含む請求項1に記載の電気化学的装置。 - 前記導電性コーティング材料が、カーボンブラック、石炭、木炭、グラファイト、炭素繊維、カーボンナノチューブ、グラフェンまたはそれらの混合物を含む元素炭素を含む請求項1または2に記載の電気化学的装置。
- 前記導電性コーティング材料が有機バインダーをさらに含む請求項1~3のいずれか1項に記載の電気化学的装置。
- 前記導電性コーティング材料が、前記元素炭素と前記有機バインダーとを含むカーボンインクを含む請求項4に記載の電気化学的装置。
- 前記導電性コーティング材料が、前記元素炭素を50wt%~90wt%含む請求項1~5のいずれか1項に記載の電気化学的装置。
- 前記アゾール含有腐食防止剤が、ベンゾトリアゾール、2-メルカプトベンズイミダゾール、5-フェニルテトラゾール、ビス[4-アミノ-5-ヒドロキシ-1,2,4-トリアゾール-3-イル]メタン、ビス[4-アミノ-5-ヒドロキシ-1,2,4-トリアゾール-3-イル]ブタン、またはそれらの混合物である請求項1~6のいずれか1項に記載の電気化学的装置。
- 前記導電性コーティング材料中の前記アゾール含有腐食防止剤の存在量が、前記元素炭素および前記有機バインダーの量の10wt%以下である請求項4または5に記載の電気化学的装置。
- 前記導電性複合コーティングの面積固有電気抵抗(area specific electrical resistance)が10mΩcm2未満である請求項1~8のいずれか1項に記載の電気化学的装置
- 前記基板が、金属基板、好ましくは銅、鉄、チタン、アルミニウム、ニッケルまたはステンレス鋼板基板である請求項1~9のいずれか1項に記載の電気化学的装置。
- 前記基板が、燃料電池アセンブリ、バッテリー、レドックスフロー電池、電解槽またはスーパーキャパシタの部品である請求項1~10のいずれか1項に記載の電気化学的装置。
- 基板を用意するステップと;
前記基板の表面上に、スズの層、あるいはニッケル、アンチモン、インジウムまたはガリウムの1つ以上とスズとの二元もしくは三元スズ合金の層、あるいはスズおよび前記二元もしくは三元スズ合金の層を堆積させるステップと、
前記スズの層、前記二元もしくは三元スズ合金の層、あるいは前記スズおよび前記二元もしくは三元スズ合金の層上に元素炭素とアゾール含有腐食防止剤とを含む導電性コーティング材料を含む層を堆積させるステップと、
を含むことを特徴とする電気化学的装置用の被覆した物品を提供する方法。 - 前記元素炭素とアゾール含有腐食防止剤とを含む導電性コーティング材料を含む層上に、スズのさらなる層、あるいはニッケル、アンチモン、インジウムまたはガリウムの1つ以上とスズとの二元もしくは三元スズ合金のさらなる層、あるいはスズおよび前記二元もしくは三元スズ合金のさらなる層を堆積させるステップと、
前記スズのさらなる層、あるいは前記二元もしくは三元スズ合金のさらなる層、あるいは前記スズおよび前記二元もしくは三元スズ合金のさらなる層上に元素炭素とアゾール含有腐食防止剤とを含む導電性コーティング材料を含むさらなる層を前記基板の前記表面上に堆積させるステップと、
をさらに含む請求項12に記載の電気化学的装置用の被覆した物品を提供する方法。 - 電気化学的装置の基板または部品用の耐食コーティングとしての、元素炭素とアゾール含有腐食防止剤とを含む導電性コーティング材料を含む層と、スズの層、あるいはニッケル、アンチモン、インジウムまたはガリウムの1つ以上とスズとの二元もしくは三元スズ合金の層、あるいはスズおよび前記二元もしくは三元スズ合金の層とを含み、前記スズの層、あるいは前記二元もしくは三元スズ合金の層、あるいは前記スズおよび前記二元もしくは三元スズ合金の層が前記基板上に直接堆積されている導電性複合コーティングの使用。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB1610144.6 | 2016-06-10 | ||
| GB1610144.6A GB2551191B (en) | 2016-06-10 | 2016-06-10 | Electrically conductive composite coating with azole corrosion inhibitor |
| PCT/GB2017/051697 WO2017212295A1 (en) | 2016-06-10 | 2017-06-09 | Corrosion protection coating |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2019526147A JP2019526147A (ja) | 2019-09-12 |
| JP2019526147A5 JP2019526147A5 (ja) | 2020-02-06 |
| JP7009397B2 true JP7009397B2 (ja) | 2022-02-10 |
Family
ID=56894792
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2018564842A Active JP7009397B2 (ja) | 2016-06-10 | 2017-06-09 | 腐食防止コーティング |
Country Status (10)
| Country | Link |
|---|---|
| US (1) | US20190148741A1 (ja) |
| EP (1) | EP3469646B1 (ja) |
| JP (1) | JP7009397B2 (ja) |
| KR (1) | KR102379291B1 (ja) |
| CN (1) | CN109478656B (ja) |
| CA (1) | CA3026384C (ja) |
| ES (1) | ES2863237T3 (ja) |
| GB (1) | GB2551191B (ja) |
| WO (1) | WO2017212295A1 (ja) |
| ZA (1) | ZA201808560B (ja) |
Families Citing this family (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE102018204378A1 (de) * | 2018-03-22 | 2019-09-26 | Audi Ag | Ermittlung des Isolationswiderstands eines Brennstoffzellensystems |
| JP7006481B2 (ja) * | 2018-04-23 | 2022-02-10 | トヨタ自動車株式会社 | 燃料電池セパレータ |
| US11214693B2 (en) | 2019-08-09 | 2022-01-04 | The Boeing Company | Electrically conductive coating compositions with corrosion resistance |
| CN112341848A (zh) * | 2020-11-05 | 2021-02-09 | 上海理工大学 | 一种石墨烯涂层及石墨烯导电耐蚀涂层的制备方法 |
| DE102020130694A1 (de) * | 2020-11-20 | 2022-05-25 | Schaeffler Technologies AG & Co. KG | Bauteil für eine elektrochemische Zelle, sowie Redox-Flow-Zelle, Brennstoffzelle und Elektrolyseur |
| DE102020130693A1 (de) * | 2020-11-20 | 2022-05-25 | Schaeffler Technologies AG & Co. KG | Bauteil für eine elektrochemische Zelle, sowie Redox-Flow-Zelle, Brennstoffzelle und Elektrolyseur |
| DE102020130695A1 (de) * | 2020-11-20 | 2022-05-25 | Schaeffler Technologies AG & Co. KG | Bauteil einer elektrochemischen Zelle sowie elektrochemische Zellen |
| US20240322192A1 (en) | 2021-06-30 | 2024-09-26 | Schaeffler Technologies AG & Co. KG | Component for an electrochemical cell, redox flow cell, and electrolyser |
| DE102022112319A1 (de) | 2021-06-30 | 2023-01-05 | Schaeffler Technologies AG & Co. KG | Bauteil für eine elektrochemische Zelle, sowie Redox-Flow-Zelle, Brennstoffzelle und Elektrolyseur |
| DE102021128468B3 (de) * | 2021-11-02 | 2022-10-06 | Schaeffler Technologies AG & Co. KG | Schichtsystem, Elektrodenplatte mit einem solchen Schichtsystem, Verfahren zu deren Herstellung, sowie Brennstoffzelle, Elektrolyseur oder Redox-Flow-Zelle |
| KR102537766B1 (ko) * | 2022-09-21 | 2023-05-31 | 주식회사 아젠컴 | 스마트카드용 집적회로 칩과 그 제조방법 및 집적회로 칩을 포함하는 스마트카드 |
| US12431260B2 (en) * | 2022-09-23 | 2025-09-30 | Caterpillar Inc. | High-velocity air-fuel coatings for conductor corrosion resistance |
| CN120476487A (zh) | 2023-03-03 | 2025-08-12 | 舍弗勒技术股份两合公司 | 用于电化学电芯的构件以及氧化还原液流电芯、燃料电芯和电解槽 |
| DE102024105383A1 (de) | 2023-03-03 | 2024-09-05 | Schaeffler Technologies AG & Co. KG | Bauteil für eine elektrochemische Zelle, sowie Redox-Flow-Zelle, Brennstoffzelle und Elektrolyseur |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2005166442A (ja) | 2003-12-02 | 2005-06-23 | Matsushita Electric Ind Co Ltd | エネルギーデバイス及びその製造方法 |
| JP2006044233A (ja) | 2004-06-23 | 2006-02-16 | Ormecon Gmbh | 導電性重合体の被覆を持つ物品およびその製造方法 |
| JP2006127939A (ja) | 2004-10-29 | 2006-05-18 | Sumitomo Electric Ind Ltd | 電気導体部品及びその製造方法 |
| US20090061184A1 (en) | 2007-08-31 | 2009-03-05 | United Technologies Corporation | Processes for Applying a Conversion Coating with Conductive Additive(S) and the Resultant Coated Articles |
| JP2012082383A (ja) | 2010-09-15 | 2012-04-26 | Nitto Denko Corp | ペースト組成物および配線回路基板 |
| JP2012153800A (ja) | 2011-01-26 | 2012-08-16 | Nitto Denko Corp | ペースト組成物および配線回路基板 |
| WO2015104999A1 (ja) | 2014-01-07 | 2015-07-16 | 古河電気工業株式会社 | 電解銅箔、リチウムイオン二次電池用負極電極及びリチウムイオン二次電池、プリント配線板並びに電磁波シールド材 |
Family Cites Families (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS59227448A (ja) * | 1983-06-08 | 1984-12-20 | 株式会社村田製作所 | 金属の腐食防止構造 |
| JPH06333417A (ja) * | 1993-05-21 | 1994-12-02 | Hitachi Chem Co Ltd | 導電ペースト |
| JPH07262822A (ja) * | 1994-03-23 | 1995-10-13 | Hitachi Chem Co Ltd | 導電ペースト |
| WO1996024938A1 (en) * | 1995-02-08 | 1996-08-15 | Hitachi Chemical Co., Ltd. | Composite conductive powder, conductive paste, method of producing conductive paste, electric circuit and method of fabricating electric circuit |
| JP2000273317A (ja) * | 1999-02-12 | 2000-10-03 | Natl Starch & Chem Investment Holding Corp | エレクトロニクスデバイス用の電気的安定性をもつ導電性および抵抗性材料 |
| JP4633626B2 (ja) * | 2003-09-10 | 2011-02-16 | 三菱樹脂株式会社 | 燃料電池用セパレータ |
| US7211204B2 (en) * | 2003-12-12 | 2007-05-01 | Electrochemicals, Inc. | Additives to stop copper attack by alkaline etching agents such as ammonia and monoethanol amine (MEA) |
| KR20050081252A (ko) * | 2004-02-13 | 2005-08-18 | 고경현 | 다공성 금속 코팅 부재 및 저온 분사법을 이용한 그의제조 방법 |
| US8062386B2 (en) * | 2006-06-08 | 2011-11-22 | Eveready Battery Company, Inc. | Tin-plated anode casings for alkaline cells |
| CN102017254A (zh) * | 2008-02-27 | 2011-04-13 | 因派科特涂料公司 | 具有涂层的电极、其制造方法和材料的用途 |
| US9340697B2 (en) * | 2009-08-14 | 2016-05-17 | Nano-C, Inc. | Solvent-based and water-based carbon nanotube inks with removable additives |
| CN102468490B (zh) * | 2010-11-19 | 2014-04-16 | 中国科学院金属研究所 | 全钒液流电池不锈钢双极板表面碳化铬/石墨复合涂层 |
| CN102286271A (zh) * | 2011-09-19 | 2011-12-21 | 鞍钢集团矿业公司 | 矿用汽车发动机冷却液及其制备方法 |
| CN103319954B (zh) * | 2013-07-12 | 2014-10-01 | 珠海市乐通化工股份有限公司 | 一种石墨烯导电油墨及其制备方法 |
| CN103756534A (zh) * | 2013-12-27 | 2014-04-30 | 吴江市东泰电力特种开关有限公司 | 一种导电防腐涂料及其制备方法 |
| US9381369B2 (en) * | 2014-02-06 | 2016-07-05 | Cardiac Pacemakers, Inc. | Battery for use with medical devices |
-
2016
- 2016-06-10 GB GB1610144.6A patent/GB2551191B/en not_active Expired - Fee Related
-
2017
- 2017-06-09 JP JP2018564842A patent/JP7009397B2/ja active Active
- 2017-06-09 CN CN201780036113.9A patent/CN109478656B/zh active Active
- 2017-06-09 KR KR1020197000641A patent/KR102379291B1/ko active Active
- 2017-06-09 EP EP17730250.2A patent/EP3469646B1/en active Active
- 2017-06-09 ES ES17730250T patent/ES2863237T3/es active Active
- 2017-06-09 US US16/307,285 patent/US20190148741A1/en not_active Abandoned
- 2017-06-09 WO PCT/GB2017/051697 patent/WO2017212295A1/en not_active Ceased
- 2017-06-09 CA CA3026384A patent/CA3026384C/en active Active
-
2018
- 2018-12-19 ZA ZA2018/08560A patent/ZA201808560B/en unknown
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2005166442A (ja) | 2003-12-02 | 2005-06-23 | Matsushita Electric Ind Co Ltd | エネルギーデバイス及びその製造方法 |
| JP2006044233A (ja) | 2004-06-23 | 2006-02-16 | Ormecon Gmbh | 導電性重合体の被覆を持つ物品およびその製造方法 |
| JP2006127939A (ja) | 2004-10-29 | 2006-05-18 | Sumitomo Electric Ind Ltd | 電気導体部品及びその製造方法 |
| US20090061184A1 (en) | 2007-08-31 | 2009-03-05 | United Technologies Corporation | Processes for Applying a Conversion Coating with Conductive Additive(S) and the Resultant Coated Articles |
| JP2012082383A (ja) | 2010-09-15 | 2012-04-26 | Nitto Denko Corp | ペースト組成物および配線回路基板 |
| JP2012153800A (ja) | 2011-01-26 | 2012-08-16 | Nitto Denko Corp | ペースト組成物および配線回路基板 |
| WO2015104999A1 (ja) | 2014-01-07 | 2015-07-16 | 古河電気工業株式会社 | 電解銅箔、リチウムイオン二次電池用負極電極及びリチウムイオン二次電池、プリント配線板並びに電磁波シールド材 |
Also Published As
| Publication number | Publication date |
|---|---|
| GB2551191B (en) | 2020-01-15 |
| ES2863237T3 (es) | 2021-10-11 |
| CA3026384A1 (en) | 2017-12-14 |
| WO2017212295A1 (en) | 2017-12-14 |
| KR20190017914A (ko) | 2019-02-20 |
| ZA201808560B (en) | 2021-03-31 |
| CA3026384C (en) | 2023-08-29 |
| US20190148741A1 (en) | 2019-05-16 |
| CN109478656B (zh) | 2023-08-29 |
| EP3469646B1 (en) | 2021-01-20 |
| JP2019526147A (ja) | 2019-09-12 |
| GB201610144D0 (en) | 2016-07-27 |
| KR102379291B1 (ko) | 2022-03-28 |
| CN109478656A (zh) | 2019-03-15 |
| EP3469646A1 (en) | 2019-04-17 |
| GB2551191A (en) | 2017-12-13 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7009397B2 (ja) | 腐食防止コーティング | |
| Wei et al. | Design principle of insulating surface protective layers for metallic Zn anodes: a case study of ZrO2 | |
| Bi et al. | Multilayered Zr–C/aC film on stainless steel 316L as bipolar plates for proton exchange membrane fuel cells | |
| Fashu et al. | Influence of electrodeposition conditions on the microstructure and corrosion resistance of Zn–Ni alloy coatings from a deep eutectic solvent | |
| Zhang et al. | Corrosion behavior of TiN-coated stainless steel as bipolar plate for proton exchange membrane fuel cell | |
| Lu et al. | Characterization of Ti3SiC2-coating on stainless steel bipolar plates in simulated proton exchange membrane fuel cell environments | |
| Zhang et al. | Corrosion protection of 1Cr18Ni9Ti stainless steel by polypyrrole coatings in HCl aqueous solution | |
| Zhang et al. | Performance of Ti–Ag-deposited titanium bipolar plates in simulated unitized regenerative fuel cell (URFC) environment | |
| Tian et al. | Corrosion resistance and interfacial contact resistance of TiN coated 316L bipolar plates for proton exchange membrane fuel cell | |
| Chien et al. | Microstructure and properties of carbon–sulfur-containing chromium deposits electroplated in trivalent chromium baths with thiosalicylic acid | |
| Fashu et al. | Electrodeposition and characterization of Zn–Sn alloy coatings from a deep eutectic solvent based on choline chloride for corrosion protection | |
| Nikam et al. | Corrosion resistant low temperature carburized SS 316 as bipolar plate material for PEMFC application | |
| Li et al. | Performance of tantalum modified 316L stainless steel bipolar plate for proton exchange membrane fuel cell | |
| Gonzalez-Rodriguez et al. | Improvement on the corrosion protection of conductive polymers in pemfc environmets by adhesives | |
| Huang et al. | Improved electrochemical performance of Zn–air secondary batteries via novel organic additives | |
| Yang et al. | Electrodeposition of tin and antimony in 1-ethyl-3-methylimidazolium tetrafluoroborate ionic liquid | |
| Sievers et al. | Corrosion-protection of moulded graphite conductive plastic bipolar plates in PEM electrolysis by plasma processing | |
| Wu et al. | Unveiling the reaction mechanism of aluminum and its alloy anode in aqueous aluminum cells | |
| JP2004139951A (ja) | 燃料電池用セパレータおよびその製造方法 | |
| Rosalbino et al. | Electrochemical corrosion behaviour of Sn–Ag–Cu (SAC) eutectic alloy in a chloride containing environment | |
| Cui et al. | Electrochemical properties of niobium modified AISI316L stainless steel bipolar plates for direct formic acid fuel cell | |
| JP2018022598A (ja) | 導電性ペースト | |
| Lei et al. | Electrochemical mechanism of tin membrane electro‐deposition in chloride solutions | |
| Benzbiria et al. | Cathodic behavior of pure Al in sulfate media | |
| HK40006883B (en) | Corrosion protection coating |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20191220 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20191220 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20201117 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20201208 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20210304 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20210706 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20211005 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20220104 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20220112 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 7009397 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| S531 | Written request for registration of change of domicile |
Free format text: JAPANESE INTERMEDIATE CODE: R313531 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |