JP4870354B2 - 硬化可能な熱可塑性エラストマーブレンド、その製造方法、およびその使用 - Google Patents
硬化可能な熱可塑性エラストマーブレンド、その製造方法、およびその使用 Download PDFInfo
- Publication number
- JP4870354B2 JP4870354B2 JP2004540346A JP2004540346A JP4870354B2 JP 4870354 B2 JP4870354 B2 JP 4870354B2 JP 2004540346 A JP2004540346 A JP 2004540346A JP 2004540346 A JP2004540346 A JP 2004540346A JP 4870354 B2 JP4870354 B2 JP 4870354B2
- Authority
- JP
- Japan
- Prior art keywords
- acrylate
- rubber
- meth
- copolymer
- polymer
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 239000000203 mixture Substances 0.000 title claims description 63
- 229920002725 thermoplastic elastomer Polymers 0.000 title claims description 27
- 238000000034 method Methods 0.000 title claims description 16
- 238000004519 manufacturing process Methods 0.000 title claims description 5
- 229920001971 elastomer Polymers 0.000 claims description 99
- -1 polyethylene Polymers 0.000 claims description 67
- 239000005060 rubber Substances 0.000 claims description 65
- 229920000642 polymer Polymers 0.000 claims description 46
- NIXOWILDQLNWCW-UHFFFAOYSA-M Acrylate Chemical compound [O-]C(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-M 0.000 claims description 41
- 239000000806 elastomer Substances 0.000 claims description 34
- 229920001577 copolymer Polymers 0.000 claims description 33
- 239000003999 initiator Substances 0.000 claims description 33
- 229920000728 polyester Polymers 0.000 claims description 31
- 239000004698 Polyethylene Substances 0.000 claims description 28
- 229920000573 polyethylene Polymers 0.000 claims description 28
- 150000001993 dienes Chemical class 0.000 claims description 25
- 229920001281 polyalkylene Polymers 0.000 claims description 23
- 238000004132 cross linking Methods 0.000 claims description 22
- XNGIFLGASWRNHJ-UHFFFAOYSA-L phthalate(2-) Chemical compound [O-]C(=O)C1=CC=CC=C1C([O-])=O XNGIFLGASWRNHJ-UHFFFAOYSA-L 0.000 claims description 22
- 229920000193 polymethacrylate Polymers 0.000 claims description 21
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 claims description 19
- 238000002156 mixing Methods 0.000 claims description 16
- 229920000058 polyacrylate Polymers 0.000 claims description 16
- 239000000155 melt Substances 0.000 claims description 15
- XFCMNSHQOZQILR-UHFFFAOYSA-N 2-[2-(2-methylprop-2-enoyloxy)ethoxy]ethyl 2-methylprop-2-enoate Chemical compound CC(=C)C(=O)OCCOCCOC(=O)C(C)=C XFCMNSHQOZQILR-UHFFFAOYSA-N 0.000 claims description 14
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 claims description 13
- 239000005977 Ethylene Substances 0.000 claims description 13
- 229920001707 polybutylene terephthalate Polymers 0.000 claims description 13
- 239000004636 vulcanized rubber Substances 0.000 claims description 13
- BAPJBEWLBFYGME-UHFFFAOYSA-N acrylic acid methyl ester Natural products COC(=O)C=C BAPJBEWLBFYGME-UHFFFAOYSA-N 0.000 claims description 12
- 229920001283 Polyalkylene terephthalate Polymers 0.000 claims description 11
- 239000012752 auxiliary agent Substances 0.000 claims description 10
- 229920001400 block copolymer Polymers 0.000 claims description 10
- 239000004721 Polyphenylene oxide Substances 0.000 claims description 9
- 229920000570 polyether Polymers 0.000 claims description 9
- LEJBBGNFPAFPKQ-UHFFFAOYSA-N 2-(2-prop-2-enoyloxyethoxy)ethyl prop-2-enoate Chemical compound C=CC(=O)OCCOCCOC(=O)C=C LEJBBGNFPAFPKQ-UHFFFAOYSA-N 0.000 claims description 8
- WGCNASOHLSPBMP-UHFFFAOYSA-N hydroxyacetaldehyde Natural products OCC=O WGCNASOHLSPBMP-UHFFFAOYSA-N 0.000 claims description 8
- 150000002148 esters Chemical class 0.000 claims description 7
- 238000001746 injection moulding Methods 0.000 claims description 6
- 238000005516 engineering process Methods 0.000 claims description 5
- QEQBMZQFDDDTPN-UHFFFAOYSA-N (2-methylpropan-2-yl)oxy benzenecarboperoxoate Chemical compound CC(C)(C)OOOC(=O)C1=CC=CC=C1 QEQBMZQFDDDTPN-UHFFFAOYSA-N 0.000 claims description 4
- KOMNUTZXSVSERR-UHFFFAOYSA-N 1,3,5-tris(prop-2-enyl)-1,3,5-triazinane-2,4,6-trione Chemical compound C=CCN1C(=O)N(CC=C)C(=O)N(CC=C)C1=O KOMNUTZXSVSERR-UHFFFAOYSA-N 0.000 claims description 4
- ODBCKCWTWALFKM-UHFFFAOYSA-N 2,5-bis(tert-butylperoxy)-2,5-dimethylhex-3-yne Chemical compound CC(C)(C)OOC(C)(C)C#CC(C)(C)OOC(C)(C)C ODBCKCWTWALFKM-UHFFFAOYSA-N 0.000 claims description 4
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 claims description 4
- 238000001125 extrusion Methods 0.000 claims description 4
- STVZJERGLQHEKB-UHFFFAOYSA-N ethylene glycol dimethacrylate Substances CC(=C)C(=O)OCCOC(=O)C(C)=C STVZJERGLQHEKB-UHFFFAOYSA-N 0.000 claims description 3
- 239000002202 Polyethylene glycol Substances 0.000 claims description 2
- 229920001223 polyethylene glycol Polymers 0.000 claims description 2
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 claims 3
- DBCAQXHNJOFNGC-UHFFFAOYSA-N 4-bromo-1,1,1-trifluorobutane Chemical compound FC(F)(F)CCCBr DBCAQXHNJOFNGC-UHFFFAOYSA-N 0.000 claims 1
- 229920001169 thermoplastic Polymers 0.000 description 31
- 239000004416 thermosoftening plastic Substances 0.000 description 29
- 238000007906 compression Methods 0.000 description 16
- 150000003254 radicals Chemical class 0.000 description 16
- 230000006835 compression Effects 0.000 description 15
- 150000002978 peroxides Chemical class 0.000 description 12
- 238000006243 chemical reaction Methods 0.000 description 9
- 239000000178 monomer Substances 0.000 description 8
- 239000003795 chemical substances by application Substances 0.000 description 7
- BJELTSYBAHKXRW-UHFFFAOYSA-N 2,4,6-triallyloxy-1,3,5-triazine Chemical compound C=CCOC1=NC(OCC=C)=NC(OCC=C)=N1 BJELTSYBAHKXRW-UHFFFAOYSA-N 0.000 description 5
- NIXOWILDQLNWCW-UHFFFAOYSA-N 2-Propenoic acid Natural products OC(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 5
- 238000002347 injection Methods 0.000 description 5
- 239000007924 injection Substances 0.000 description 5
- 150000001451 organic peroxides Chemical class 0.000 description 5
- 239000006185 dispersion Substances 0.000 description 4
- 239000002245 particle Substances 0.000 description 4
- 229920003023 plastic Polymers 0.000 description 4
- 239000004033 plastic Substances 0.000 description 4
- 229920000139 polyethylene terephthalate Polymers 0.000 description 4
- 239000005020 polyethylene terephthalate Substances 0.000 description 4
- 238000004073 vulcanization Methods 0.000 description 4
- IPJGAEWUPXWFPL-UHFFFAOYSA-N 1-[3-(2,5-dioxopyrrol-1-yl)phenyl]pyrrole-2,5-dione Chemical compound O=C1C=CC(=O)N1C1=CC=CC(N2C(C=CC2=O)=O)=C1 IPJGAEWUPXWFPL-UHFFFAOYSA-N 0.000 description 3
- SMZOUWXMTYCWNB-UHFFFAOYSA-N 2-(2-methoxy-5-methylphenyl)ethanamine Chemical compound COC1=CC=C(C)C=C1CCN SMZOUWXMTYCWNB-UHFFFAOYSA-N 0.000 description 3
- CERQOIWHTDAKMF-UHFFFAOYSA-N Methacrylic acid Chemical compound CC(=C)C(O)=O CERQOIWHTDAKMF-UHFFFAOYSA-N 0.000 description 3
- 230000000875 corresponding effect Effects 0.000 description 3
- 150000002009 diols Chemical class 0.000 description 3
- 230000014509 gene expression Effects 0.000 description 3
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 3
- 239000004615 ingredient Substances 0.000 description 3
- 238000002844 melting Methods 0.000 description 3
- 230000008018 melting Effects 0.000 description 3
- 238000006116 polymerization reaction Methods 0.000 description 3
- 229920000874 polytetramethylene terephthalate Polymers 0.000 description 3
- 238000012360 testing method Methods 0.000 description 3
- 239000004971 Cross linker Substances 0.000 description 2
- 239000004593 Epoxy Substances 0.000 description 2
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 125000005396 acrylic acid ester group Chemical group 0.000 description 2
- 125000005250 alkyl acrylate group Chemical group 0.000 description 2
- 150000001412 amines Chemical class 0.000 description 2
- 239000003963 antioxidant agent Substances 0.000 description 2
- 230000001588 bifunctional effect Effects 0.000 description 2
- WERYXYBDKMZEQL-UHFFFAOYSA-N butane-1,4-diol Chemical compound OCCCCO WERYXYBDKMZEQL-UHFFFAOYSA-N 0.000 description 2
- 239000003431 cross linking reagent Substances 0.000 description 2
- 230000007423 decrease Effects 0.000 description 2
- 238000004898 kneading Methods 0.000 description 2
- 229920005684 linear copolymer Polymers 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 238000000465 moulding Methods 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- 239000007858 starting material Substances 0.000 description 2
- 229920006342 thermoplastic vulcanizate Polymers 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- BQCIDUSAKPWEOX-UHFFFAOYSA-N 1,1-Difluoroethene Chemical compound FC(F)=C BQCIDUSAKPWEOX-UHFFFAOYSA-N 0.000 description 1
- PNJLSKTVCRNRBO-UHFFFAOYSA-N 1-tert-butylperoxy-2,5-dimethylhexane Chemical compound CC(C)CCC(C)COOC(C)(C)C PNJLSKTVCRNRBO-UHFFFAOYSA-N 0.000 description 1
- DMWVYCCGCQPJEA-UHFFFAOYSA-N 2,5-bis(tert-butylperoxy)-2,5-dimethylhexane Chemical compound CC(C)(C)OOC(C)(C)CCC(C)(C)OOC(C)(C)C DMWVYCCGCQPJEA-UHFFFAOYSA-N 0.000 description 1
- XMNIXWIUMCBBBL-UHFFFAOYSA-N 2-(2-phenylpropan-2-ylperoxy)propan-2-ylbenzene Chemical compound C=1C=CC=CC=1C(C)(C)OOC(C)(C)C1=CC=CC=C1 XMNIXWIUMCBBBL-UHFFFAOYSA-N 0.000 description 1
- IMSODMZESSGVBE-UHFFFAOYSA-N 2-Oxazoline Chemical compound C1CN=CO1 IMSODMZESSGVBE-UHFFFAOYSA-N 0.000 description 1
- HFCUBKYHMMPGBY-UHFFFAOYSA-N 2-methoxyethyl prop-2-enoate Chemical compound COCCOC(=O)C=C HFCUBKYHMMPGBY-UHFFFAOYSA-N 0.000 description 1
- BXAAQNFGSQKPDZ-UHFFFAOYSA-N 3-[1,2,2-tris(prop-2-enoxy)ethoxy]prop-1-ene Chemical compound C=CCOC(OCC=C)C(OCC=C)OCC=C BXAAQNFGSQKPDZ-UHFFFAOYSA-N 0.000 description 1
- LCFVJGUPQDGYKZ-UHFFFAOYSA-N Bisphenol A diglycidyl ether Chemical compound C=1C=C(OCC2OC2)C=CC=1C(C)(C)C(C=C1)=CC=C1OCC1CO1 LCFVJGUPQDGYKZ-UHFFFAOYSA-N 0.000 description 1
- 239000004970 Chain extender Substances 0.000 description 1
- 208000015943 Coeliac disease Diseases 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- JIGUQPWFLRLWPJ-UHFFFAOYSA-N Ethyl acrylate Chemical compound CCOC(=O)C=C JIGUQPWFLRLWPJ-UHFFFAOYSA-N 0.000 description 1
- 241001082241 Lythrum hyssopifolia Species 0.000 description 1
- CERQOIWHTDAKMF-UHFFFAOYSA-M Methacrylate Chemical compound CC(=C)C([O-])=O CERQOIWHTDAKMF-UHFFFAOYSA-M 0.000 description 1
- BMNSEGBVAJUJQM-UHFFFAOYSA-N OC(=O)C=C.C=CCCCCC=C Chemical compound OC(=O)C=C.C=CCCCCC=C BMNSEGBVAJUJQM-UHFFFAOYSA-N 0.000 description 1
- 239000004952 Polyamide Substances 0.000 description 1
- OKKRPWIIYQTPQF-UHFFFAOYSA-N Trimethylolpropane trimethacrylate Chemical compound CC(=C)C(=O)OCC(CC)(COC(=O)C(C)=C)COC(=O)C(C)=C OKKRPWIIYQTPQF-UHFFFAOYSA-N 0.000 description 1
- XTXRWKRVRITETP-UHFFFAOYSA-N Vinyl acetate Chemical compound CC(=O)OC=C XTXRWKRVRITETP-UHFFFAOYSA-N 0.000 description 1
- 150000001252 acrylic acid derivatives Chemical class 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 125000005907 alkyl ester group Chemical group 0.000 description 1
- RREGISFBPQOLTM-UHFFFAOYSA-N alumane;trihydrate Chemical compound O.O.O.[AlH3] RREGISFBPQOLTM-UHFFFAOYSA-N 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 230000003078 antioxidant effect Effects 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 150000001732 carboxylic acid derivatives Chemical class 0.000 description 1
- 125000002843 carboxylic acid group Chemical group 0.000 description 1
- 238000012668 chain scission Methods 0.000 description 1
- 238000010382 chemical cross-linking Methods 0.000 description 1
- FOCAUTSVDIKZOP-UHFFFAOYSA-M chloroacetate Chemical group [O-]C(=O)CCl FOCAUTSVDIKZOP-UHFFFAOYSA-M 0.000 description 1
- 229940089960 chloroacetate Drugs 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- 238000005859 coupling reaction Methods 0.000 description 1
- 238000002425 crystallisation Methods 0.000 description 1
- 230000008025 crystallization Effects 0.000 description 1
- 238000013036 cure process Methods 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 229910003460 diamond Inorganic materials 0.000 description 1
- 239000010432 diamond Substances 0.000 description 1
- 150000001991 dicarboxylic acids Chemical class 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 125000004185 ester group Chemical group 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- 239000005038 ethylene vinyl acetate Substances 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 239000012467 final product Substances 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- 229910052736 halogen Inorganic materials 0.000 description 1
- 150000002367 halogens Chemical class 0.000 description 1
- HCDGVLDPFQMKDK-UHFFFAOYSA-N hexafluoropropylene Chemical group FC(F)=C(F)C(F)(F)F HCDGVLDPFQMKDK-UHFFFAOYSA-N 0.000 description 1
- 229920006247 high-performance elastomer Polymers 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 230000005865 ionizing radiation Effects 0.000 description 1
- 239000012948 isocyanate Substances 0.000 description 1
- 150000002513 isocyanates Chemical class 0.000 description 1
- 150000002596 lactones Chemical class 0.000 description 1
- 239000010687 lubricating oil Substances 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- 150000002734 metacrylic acid derivatives Chemical class 0.000 description 1
- 125000005395 methacrylic acid group Chemical group 0.000 description 1
- RPQRDASANLAFCM-UHFFFAOYSA-N oxiran-2-ylmethyl prop-2-enoate Chemical compound C=CC(=O)OCC1CO1 RPQRDASANLAFCM-UHFFFAOYSA-N 0.000 description 1
- 239000008188 pellet Substances 0.000 description 1
- PNJWIWWMYCMZRO-UHFFFAOYSA-N pent‐4‐en‐2‐one Natural products CC(=O)CC=C PNJWIWWMYCMZRO-UHFFFAOYSA-N 0.000 description 1
- 125000005498 phthalate group Chemical class 0.000 description 1
- XNGIFLGASWRNHJ-UHFFFAOYSA-N phthalic acid Chemical class OC(=O)C1=CC=CC=C1C(O)=O XNGIFLGASWRNHJ-UHFFFAOYSA-N 0.000 description 1
- 150000003022 phthalic acids Chemical class 0.000 description 1
- LGRFSURHDFAFJT-UHFFFAOYSA-N phthalic anhydride Chemical class C1=CC=C2C(=O)OC(=O)C2=C1 LGRFSURHDFAFJT-UHFFFAOYSA-N 0.000 description 1
- 238000002464 physical blending Methods 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 239000000049 pigment Substances 0.000 description 1
- 229920002647 polyamide Polymers 0.000 description 1
- 150000004291 polyenes Chemical class 0.000 description 1
- 229920001225 polyester resin Polymers 0.000 description 1
- 239000004645 polyester resin Substances 0.000 description 1
- 239000004848 polyfunctional curative Substances 0.000 description 1
- 229920001228 polyisocyanate Polymers 0.000 description 1
- 239000005056 polyisocyanate Substances 0.000 description 1
- 230000000379 polymerizing effect Effects 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 239000007870 radical polymerization initiator Substances 0.000 description 1
- 238000010526 radical polymerization reaction Methods 0.000 description 1
- 229920005604 random copolymer Polymers 0.000 description 1
- 239000000376 reactant Substances 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- KKEYFWRCBNTPAC-UHFFFAOYSA-L terephthalate(2-) Chemical group [O-]C(=O)C1=CC=C(C([O-])=O)C=C1 KKEYFWRCBNTPAC-UHFFFAOYSA-L 0.000 description 1
- 229920001897 terpolymer Polymers 0.000 description 1
- 238000010998 test method Methods 0.000 description 1
- 229920006346 thermoplastic polyester elastomer Polymers 0.000 description 1
- 229920005992 thermoplastic resin Polymers 0.000 description 1
- 229920001187 thermosetting polymer Polymers 0.000 description 1
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 1
- 230000004580 weight loss Effects 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L67/00—Compositions of polyesters obtained by reactions forming a carboxylic ester link in the main chain; Compositions of derivatives of such polymers
- C08L67/02—Polyesters derived from dicarboxylic acids and dihydroxy compounds
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J5/00—Manufacture of articles or shaped materials containing macromolecular substances
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/04—Oxygen-containing compounds
- C08K5/14—Peroxides
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L33/00—Compositions of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical, or of salts, anhydrides, esters, amides, imides or nitriles thereof; Compositions of derivatives of such polymers
- C08L33/04—Homopolymers or copolymers of esters
- C08L33/06—Homopolymers or copolymers of esters of esters containing only carbon, hydrogen and oxygen, which oxygen atoms are present only as part of the carboxyl radical
- C08L33/08—Homopolymers or copolymers of acrylic acid esters
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L33/00—Compositions of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical, or of salts, anhydrides, esters, amides, imides or nitriles thereof; Compositions of derivatives of such polymers
- C08L33/04—Homopolymers or copolymers of esters
- C08L33/06—Homopolymers or copolymers of esters of esters containing only carbon, hydrogen and oxygen, which oxygen atoms are present only as part of the carboxyl radical
- C08L33/10—Homopolymers or copolymers of methacrylic acid esters
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L67/00—Compositions of polyesters obtained by reactions forming a carboxylic ester link in the main chain; Compositions of derivatives of such polymers
- C08L67/02—Polyesters derived from dicarboxylic acids and dihydroxy compounds
- C08L67/025—Polyesters derived from dicarboxylic acids and dihydroxy compounds containing polyether sequences
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08K—Use of inorganic or non-macromolecular organic substances as compounding ingredients
- C08K5/00—Use of organic ingredients
- C08K5/0008—Organic ingredients according to more than one of the "one dot" groups of C08K5/01 - C08K5/59
- C08K5/0025—Crosslinking or vulcanising agents; including accelerators
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L23/00—Compositions of homopolymers or copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond; Compositions of derivatives of such polymers
- C08L23/02—Compositions of homopolymers or copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond; Compositions of derivatives of such polymers not modified by chemical after-treatment
- C08L23/04—Homopolymers or copolymers of ethene
- C08L23/08—Copolymers of ethene
- C08L23/0846—Copolymers of ethene with unsaturated hydrocarbons containing atoms other than carbon or hydrogen
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L2666/00—Composition of polymers characterized by a further compound in the blend, being organic macromolecular compounds, natural resins, waxes or and bituminous materials, non-macromolecular organic substances, inorganic substances or characterized by their function in the composition
- C08L2666/02—Organic macromolecular compounds, natural resins, waxes or and bituminous materials
- C08L2666/14—Macromolecular compounds according to C08L59/00 - C08L87/00; Derivatives thereof
- C08L2666/18—Polyesters or polycarbonates according to C08L67/00 - C08L69/00; Derivatives thereof
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L33/00—Compositions of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical, or of salts, anhydrides, esters, amides, imides or nitriles thereof; Compositions of derivatives of such polymers
- C08L33/04—Homopolymers or copolymers of esters
- C08L33/06—Homopolymers or copolymers of esters of esters containing only carbon, hydrogen and oxygen, which oxygen atoms are present only as part of the carboxyl radical
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/31504—Composite [nonstructural laminate]
- Y10T428/31786—Of polyester [e.g., alkyd, etc.]
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Manufacturing & Machinery (AREA)
- Materials Engineering (AREA)
- Compositions Of Macromolecular Compounds (AREA)
- Processes Of Treating Macromolecular Substances (AREA)
- Extrusion Moulding Of Plastics Or The Like (AREA)
- Injection Moulding Of Plastics Or The Like (AREA)
Description
(a)15〜60重量パーセントの、ポリアルキレンフタレートポリエステルのポリマーまたはコポリマー、および
(b)40〜85重量パーセントの、架橋可能なポリ(メタ)アクリレートまたはポリエチレン/(メタ)アクリレート加硫ゴムを、前記硬化可能な熱可塑性エラストマーブレンドを押出成形または射出成形させる間に前記ゴムを架橋させるために、有効量のペルオキシドフリーラジカル開始剤と有機ジエン助剤とを組合せて含む。
(a)15〜60重量パーセントの、ポリアルキレンフタレートポリエステルのポリマーまたはコポリマーの連続相、および
(b)40〜85重量パーセントの、架橋可能なポリ(メタ)アクリレートまたはポリエチレン/(メタ)アクリレート加硫ゴム分散相を含み、前記ポリアクリレートゴムは、ペルオキシドフリーラジカル開始剤と有機ジエン助剤を用いて架橋される。
(a)架橋可能なポリ(メタ)アクリレートまたはポリエチレン/(メタ)アクリレート加硫ゴムとペルオキシドフリーラジカル開始剤と有機ジエン助剤とを、架橋を促進するには不十分な温度で、溶融押出機または溶融ブレンダーの中に添加して、混合する工程;
(b)前記溶融押出機または溶融ブレンダーにポリアルキレンフタレートポリエステルポリマーを添加し、前記ポリアルキレンフタレートポリエステルポリマーを、架橋前に前記架橋可能なポリ(メタ)アクリレートまたはポリエチレン/(メタ)アクリレート加硫ゴムと混合する工程;
(c)前記架橋可能なポリ(メタ)アクリレートまたはポリエチレン/(メタ)アクリレート加硫ゴムと、前記ポリアルキレンフタレートポリエステルポリマーと共にペルオキシドフリーラジカル開始剤と有機ジエン助剤を、前記架橋可能なポリ(メタ)アクリレートまたはポリエチレン/(メタ)アクリレート加硫ゴムを架橋させるのに充分な条件と温度で、さらに混合する工程;および
(d)連続相としての15〜60重量パーセントの前記ポリアルキレンフタレートポリエステルのポリマーまたはコポリマーと、分散相としての40〜85重量パーセントの、前記ペルオキシドフリーラジカル開始剤および前記有機ジエン助剤を用いて架橋させた前記ポリ(メタ)アクリレートまたはポリエチレン/(メタ)アクリレート加硫ゴムとを含む溶融加工可能な熱可塑性エラストマー組成物を回収する工程、を含む。
(a)15〜60重量パーセントの、ポリアルキレンフタレートポリエステルのポリマーまたはコポリマーの連続相、および
(b)40〜85重量パーセントの、ポリ(メタ)アクリレートまたはポリエチレン/(メタ)アクリレートゴム分散相を含み、前記ポリ(メタ)アクリレートまたはポリエチレン/(メタ)アクリレートゴムは、ペルオキシドフリーラジカル開始剤と有機ジエン助剤とを用いて架橋される。
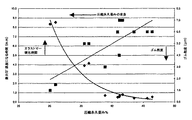
本発明による、一連の16種のブレンドを調製し、評価した。使用した熱可塑性プラスチックは、ポリエーテルエステルブロックコポリマーであって、そのメルトフロー速度(ISO133)は18g/10分、溶融温度(ISO3146C)は203℃、密度(ISO1183)は1.19g/cm3であった。そのポリエーテルエステルブロックコポリマーは、ポリブチレンテレフタレートの固い(結晶性)セグメントと、長鎖ポリエーテルグリコールの柔らかい(非晶質)セグメントとからなり、本願特許出願人から、商品名ハイトレル(Hytrel)(登録商標)として市販されているものである。使用したポリアクリレートゴムは、エチレンと63重量%のメチルアクリレートを共重合させて得られる、ポリエチレン/アクリレートエラストマー(ガムラバータイプ)であって、本願特許出願人から、商品名バマック(Vamac)(登録商標)として市販されているものである。各種のペルオキシドタイプのフリーラジカル開始剤を、選択したジエン助剤と各種の濃度で組合せてブレンドを調製したが、詳しくは下記の表1を参照されたい。さらにペルオキシドの特徴を示すために、1時間のフリーラジカル半減期に対応する、それぞれの温度を表1に示した。
実施例1〜16と同様な方法で、ポリエーテルエステルブロックコポリマーとポリエチレン/アクリレートエラストマーのさらに4種のブレンドのシリーズを調製し、評価した。4種の助剤は、1時間半減期となる温度が152℃と140℃と、比較的高く、その結果、最大G’速度になる時間が比較的長いことで示されるように、硬化速度が比較的遅いことが特徴である。この場合もまた、表2に示したように、最大G’速度になる時間が長い場合に、目的とする低い圧縮永久歪み値が得られるが、このことから、圧縮永久歪み値が高いサンプルに比較して、動的加硫の際の硬化が遅いことを示していることが判る。
実施例1〜16に記載の手順を使用して、下記の熱可塑性プラスチックをブレンドして得られる熱可塑性プラスチック相を用いて、下記の1組の動的に混合したエラストマー相を調製、架橋させた。
Claims (6)
- 硬化可能な熱可塑性エラストマー組成物であって:
(a)15〜60重量パーセントの、ポリアルキレンフタレートポリエステルのポリマー
またはコポリマー、および
(b)40〜85重量パーセントの、官能化されていない架橋可能なポリ(メタ)アクリレートまたはポリエチレン/(メタ)アクリレート加硫ゴムを、
前記組成物を押出成形または射出成形させる間に前記ゴムを架橋させるために、有効量のペルオキシドフリーラジカル開始剤と、ジエチレングリコールジアクリレート、エチレングリコールジメタクリレート、ジエチレングリコールジメタクリレート、ポリエチレングリコールジメタクリレート、N,N’−m−フェニレンジマレイミド、およびトリアリルイソシアヌレートからなる群より選択される有機ジエン助剤と、を組合せて含み、
前記官能化されていない架橋可能なポリ(メタ)アクリレートまたはポリエチレン/(メタ)アクリレート加硫ゴムと、前記ゴムを架橋させるための有効量のペルオキシドフリーラジカル開始剤および有機ジエン助剤とを組合せたものが、Alpha Technologies Advanced Polymer Analyzer APA2000を使用して、平行プレートダイを用い、ダイギャップが2.583mm、振動数が100.0cpm、歪みが0.500度で、180℃で測定したときに、最大G’速度になる時間が3.9分以上であり、
前記ポリアルキレンフタレートポリエステルのポリマーまたはコポリマーが、架橋後、連続相として存在し、前記ゴムが、架橋後、分散相として存在する、
ことを特徴とする硬化可能な熱可塑性エラストマー組成物。 - 前記ポリアルキレンフタレートポリエステルのポリマーまたはコポリマーが、ポリアルキレンテレフタレート、ポリアルキレンテレフタレートコポリマー、ポリアルキレンテレフタレートのポリエーテルエステル、またはポリアルキレンテレフタレートコポリマーのポリエーテルエステルであり;前記ゴムが、ポリアクリレートエラストマー、またはポリエチレン/アクリレートエラストマーであり;そして、前記開始剤が、2,5−ジメチル−2,5−ジ−(t−ブチルペルオキシ)ヘキシン−3、2,5−ジメチル−2,5−ジ−(t−ブチルペルオキシ)ヘキサン、またはt−ブチルペルオキシベンゾエートであることを特徴とする請求項1に記載の組成物。
- 前記ポリアルキレンフタレートポリエステルのポリマーまたはコポリマーが、ポリブチレンテレフタレートのセグメントとポリ(テトラメチレンエーテル)グリコールのセグメントとのブロックコポリマーであり、前記ゴムがエチレン/メチルアクリレートコポリマーエラストマーであり、前記開始剤が2,5−ジメチル−2,5−ジ−(t−ブチルペルオキシ)ヘキシン−3であり、そして前記助剤がジエチレングリコールジメタクリレートであることを特徴とする請求項2に記載の組成物。
- 溶融加工可能な熱可塑性エラストマー組成物を製造するための方法であって:
(a)官能化されていない架橋可能なポリ(メタ)アクリレートまたはポリエチレン/(メタ)アクリレート加硫ゴムとペルオキシドフリーラジカル開始剤と有機ジエン助剤とを、架橋を促進するには不十分な温度で、溶融押出機または溶融ブレンダーの中で混合する工程であって;
前記混合が、Alpha Technologies Advanced Polymer Analyzer APA2000を使用して、平行プレートダイを用い、ダイギャップが2.583mm、振動数が100.0cpm、歪みが0.500度で、180℃で測定したときに、最大G’速度になる時間が3.9分以上であることを特徴とする工程;
(b)前記溶融押出機または溶融ブレンダーにポリアルキレンフタレートポリエステルポリマーを添加し、前記ポリアルキレンフタレートポリエステルポリマーを、架橋前に前記官能化されていない架橋可能なポリ(メタ)アクリレートまたはポリエチレン/(メタ)アクリレート加硫ゴムと混合する工程;
(c)前記架橋可能なポリ(メタ)アクリレートまたはポリエチレン/(メタ)アクリレート加硫ゴムに、前記ポリアルキレンフタレートポリエステルポリマーと共にペルオキシドフリーラジカル開始剤と有機ジエン助剤を、前記官能化されていない架橋可能なポリ(メタ)アクリレートまたはポリエチレン/(メタ)アクリレート加硫ゴムを架橋させるのに充分な条件と温度で、さらに混合する工程;および
(d)連続相としての15〜60重量パーセントの前記ポリアルキレンフタレートポリエステルポリマーまたはコポリマーと、前記ペルオキシドフリーラジカル開始剤および前記有機ジエン助剤を用いて架橋させた、分散相としての40〜85重量パーセントの前記ポリ(メタ)アクリレートまたはポリエチレン/(メタ)アクリレート加硫ゴムと、からなる溶融加工可能な熱可塑性エラストマー組成物を回収する工程、を含み
前記ポリアルキレンフタレートポリエステルのポリマーまたはコポリマー、前記ゴム、前記有機ジエン助剤、および前記開始剤がそれぞれ、請求項2に記載のものであることを特徴とする方法。 - 前記ポリアルキレンフタレートポリエステルポリマーが、ポリブチレンテレフタレートのセグメントとポリ(テトラメチレンエーテル)グリコールのセグメントとのブロックコポリマーであり、前記ゴムが官能化されていないエチレン/メチルアクリレートコポリマーエラストマーであり、前記開始剤が2,5−ジメチル−2,5−ジ−(t−ブチルペルオキシ)ヘキシン−3であり、そして前記助剤がジエチレングリコールジメタクリレートであることを特徴とする請求項4に記載の方法。
- 請求項1、2、または3に記載の組成物から製造される造形品または成形品。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US41470802P | 2002-09-30 | 2002-09-30 | |
| US60/414,708 | 2002-09-30 | ||
| PCT/US2003/031230 WO2004029155A2 (en) | 2002-09-30 | 2003-09-30 | Curable thermoplastic elastomeric blend, method of manufacture, and use thereof |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2006501331A JP2006501331A (ja) | 2006-01-12 |
| JP2006501331A5 JP2006501331A5 (ja) | 2006-11-02 |
| JP4870354B2 true JP4870354B2 (ja) | 2012-02-08 |
Family
ID=32043404
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2004540346A Expired - Fee Related JP4870354B2 (ja) | 2002-09-30 | 2003-09-30 | 硬化可能な熱可塑性エラストマーブレンド、その製造方法、およびその使用 |
Country Status (12)
| Country | Link |
|---|---|
| US (1) | US7074857B2 (ja) |
| EP (1) | EP1551920B1 (ja) |
| JP (1) | JP4870354B2 (ja) |
| KR (1) | KR101008110B1 (ja) |
| CN (1) | CN100384929C (ja) |
| AU (1) | AU2003283988A1 (ja) |
| BR (1) | BR0314495A (ja) |
| CA (1) | CA2500141A1 (ja) |
| DE (1) | DE60333381D1 (ja) |
| MX (1) | MXPA05003336A (ja) |
| RU (1) | RU2339661C2 (ja) |
| WO (1) | WO2004029155A2 (ja) |
Families Citing this family (41)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8013067B2 (en) * | 2002-09-30 | 2011-09-06 | E.I. Du Pont De Nemours And Company | Curable thermoplastic elastomeric blend, method of manufacture, and use thereof |
| US20060223923A1 (en) * | 2005-02-07 | 2006-10-05 | Serge Cavalli | Thermoplastic vulcanisate blend |
| EP1710288A1 (en) * | 2005-04-06 | 2006-10-11 | E.I. du Pont de Nemours and Company | Sealing element |
| MX2007013604A (es) * | 2005-05-02 | 2007-12-10 | Du Pont | Combinacion de elastomero termoplastico, metodo de fabricacion y uso del mismo. |
| US7244790B2 (en) * | 2005-05-02 | 2007-07-17 | E.I. Du Pont De Nemours And Company | Thermoplastic elastomer blend, method of manufacture and use thereof |
| US20060247378A1 (en) * | 2005-05-02 | 2006-11-02 | Sunkara Hari B | Thermoplastic elastomer blend, method of manufacture and use thereof |
| WO2006132225A1 (ja) * | 2005-06-10 | 2006-12-14 | Nok Corporation | ゴム組成物 |
| JP2007086505A (ja) * | 2005-09-22 | 2007-04-05 | Du Pont Toray Co Ltd | 吸遮音材 |
| CA2598342C (en) * | 2006-09-08 | 2014-10-14 | Lanxess Inc. | Peroxide cured thermoplastic vulcanizates comprising butyl rubber |
| US7531593B2 (en) * | 2006-10-31 | 2009-05-12 | E.I. Du Pont De Nemours And Company | Thermoplastic elastomer blend composition |
| US7790790B2 (en) | 2006-11-14 | 2010-09-07 | E. I. Du Pont De Nemours And Company | Flame retardant thermoplastic elastomer compositions |
| US7399239B2 (en) | 2006-12-04 | 2008-07-15 | Acushnet Company | Use of engineering thermoplastic vulcanizates for golf ball layers |
| US7608216B2 (en) * | 2006-12-27 | 2009-10-27 | Freudenberg-Nok General Partnership | Methods for preparing articles from processable and dimensionally stable elastomer compositions |
| US20080161438A1 (en) * | 2006-12-28 | 2008-07-03 | Xingwang Wang | Composition comprising copolyetherester elastomer |
| EP2142820A2 (en) | 2007-05-01 | 2010-01-13 | E. I. Du Pont de Nemours and Company | Jounce bumpers made by corrugated extrusio |
| WO2010065805A2 (en) * | 2008-12-05 | 2010-06-10 | E. I. Du Pont De Nemours And Company | Method for reducing vibrations from a motor vehicle exhaust system |
| EP2364336A4 (en) * | 2008-12-05 | 2016-01-27 | Du Pont | THERMOPLASTIC ELASTOMER ABSORBING ENERGY |
| US8051947B2 (en) * | 2009-03-12 | 2011-11-08 | E.I. Du Pont De Nemours And Company | Energy absorbing thermoplastic elastomer |
| EP2462192A1 (en) | 2009-08-03 | 2012-06-13 | E. I. du Pont de Nemours and Company | Energy absorbing copolyetherester resin compositions |
| CN101812180A (zh) * | 2010-04-20 | 2010-08-25 | 北京化工大学 | 一种免硫化型丙烯酸酯橡胶的制备方法 |
| US20110315423A1 (en) * | 2010-06-29 | 2011-12-29 | E.I. Du Pont De Nemours And Company | Abrasion resistant and flame retardant thermoplastic vulcanizate compositions |
| US20120193851A1 (en) | 2010-08-12 | 2012-08-02 | E.I.Du Pont De Nemours And Company | Thermoplastic jounce bumpers |
| US8657271B2 (en) | 2010-08-12 | 2014-02-25 | E I Du Pont De Nemours And Company | Thermoplastic jounce bumpers |
| CN103068919A (zh) | 2010-08-17 | 2013-04-24 | 纳幕尔杜邦公司 | 热稳定无卤阻燃剂共聚酯热塑性弹性体组合物 |
| KR101379619B1 (ko) * | 2011-01-20 | 2014-04-01 | 코오롱플라스틱 주식회사 | 열가소성 폴리에테르에스테르 엘라스토머 수지 조성물 및 이로부터 제조된 탄성 모노 필라멘트 |
| US20120225291A1 (en) | 2011-03-02 | 2012-09-06 | E.I. Du Pont De Nemours And Company | Low smoke halogen free flame retardant thermoplastic vulcanizate compositions containing zeolites |
| US8781278B2 (en) | 2011-03-02 | 2014-07-15 | E I Du Pont De Nemours And Company | Low smoke halogen free flame retardant thermoplastic elastomer compositions containing zeolites |
| EP3034567B1 (de) * | 2014-12-19 | 2017-06-14 | Evonik Degussa GmbH | Covernetzersysteme für Verkapselungsfolien umfassend Ethylenglykoldi(meth)acrylatverbindungen |
| BR112017013889A2 (pt) | 2015-02-18 | 2018-03-06 | Du Pont | batente e sistema de suspensão automotiva |
| WO2017014154A1 (ja) * | 2015-07-22 | 2017-01-26 | 株式会社クラレ | (メタ)アクリル系ブロック共重合体 |
| TWI647083B (zh) | 2015-08-28 | 2019-01-11 | 耐基創新公司 | 用於由熱固性彈性體組成物模製物件之方法及模製之物件 |
| CN106589851B (zh) * | 2016-12-14 | 2020-11-13 | 四川晨光科新塑胶有限责任公司 | 一种消光改性热塑性聚酯弹性体组合物 |
| KR20180080454A (ko) * | 2017-01-04 | 2018-07-12 | (주) 데크카본 | 산소량이 감소된 고성능 탄화규소섬유의 제조방법 |
| CN109535452B (zh) * | 2018-10-11 | 2021-09-28 | 华南理工大学 | 一种可重复加工的硫磺硫化橡胶及其制备方法 |
| CN110698755A (zh) * | 2019-11-03 | 2020-01-17 | 南通林格橡塑制品有限公司 | 一种热塑性丙烯酸酯tpv材料 |
| JP2023508732A (ja) * | 2019-12-31 | 2023-03-03 | デュポン ポリマーズ インコーポレイテッド | ポリマーブレンド |
| CN112126150B (zh) * | 2020-09-27 | 2021-08-24 | 上海交通大学 | 可循环使用的POE vitrimer弹性体、发泡材料及其制备方法和应用 |
| EP4019586A3 (en) | 2020-12-21 | 2022-09-28 | Parker Hannifin Corp. | High temperature, oil-resistant thermoplastic vulcanizates |
| US12258471B2 (en) | 2020-12-21 | 2025-03-25 | Parker-Hannifin Corporation | Readily process-able, oil-resistant thermoplastic vulcanizates |
| TWI821988B (zh) * | 2021-04-16 | 2023-11-11 | 李長榮化學工業股份有限公司 | 熱塑性動態交聯材料、以其形成的物體及其形成方法 |
| TWI856357B (zh) * | 2022-02-25 | 2024-09-21 | 財團法人工業技術研究院 | 熱塑性硫化彈性體及其製備方法 |
Family Cites Families (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3396152A (en) * | 1964-03-23 | 1968-08-06 | Firestone Tire & Rubber Co | Process of curing an acrylic elastomer |
| GB1208585A (en) | 1967-12-11 | 1970-10-14 | Ici Ltd | Polyester compositions |
| BE792985A (fr) * | 1971-12-20 | 1973-04-16 | Asahi Chemical Ind | Compositions de matieres thermoplastiques |
| GB1603205A (en) * | 1977-04-12 | 1981-11-18 | Raychem Ltd | Polymer compositions |
| US4396746A (en) * | 1981-04-16 | 1983-08-02 | Daicel Chemical Industries, Ltd. | Thermoplastic polyester copolymer |
| IT1157308B (it) | 1982-06-01 | 1987-02-11 | Montedison Spa | Procedimento per preparare miscele di gomme acriliche con copolimeri stirene/acrilonitrile, dotate di migliorate caratteristiche di resistenza all'urto |
| US4739012A (en) * | 1986-02-21 | 1988-04-19 | E. I. Du Pont De Nemours And Company | Elastomer blends |
| IE873315L (en) | 1986-12-31 | 1988-06-30 | Flork Michel | Filled elastomer blends |
| US4981908A (en) * | 1988-02-02 | 1991-01-01 | E. I. Du Pont De Nemours And Company | Thermoplastic elastomer blends |
| AU610377B2 (en) | 1988-04-11 | 1991-05-16 | Advanced Elastomer Systems, L.P. | High temperature stable, low solvent swelling thermoplastic elastomer compositions |
| DE3911695A1 (de) * | 1989-04-10 | 1990-10-11 | Inventa Ag | Verbundwerkstoffe und ihre verwendung |
| DE4141319A1 (de) * | 1991-12-14 | 1993-10-14 | Roehm Gmbh | Hochschlagzähe Polymermischungen |
| RU2057772C1 (ru) * | 1992-04-09 | 1996-04-10 | Акционерное общество открытого типа "Научно-исследовательский институт пластических масс им.Г.С.Петрова с Опытным московским заводом пластмасс" | Полимерная композиция |
| DE69815032T2 (de) * | 1997-12-10 | 2004-04-01 | Advanced Elastomer Systems, L.P., Akron | Thermoplastische Vulkanisate aus Kondensationsharz und vernetztem Elastomer |
| US6528586B2 (en) | 2000-05-16 | 2003-03-04 | Gordon Mark Cohen | Compositions of elastomeric ethylene/(meth)acrylic (acid) ester copolymer and polylactone or polyether |
| JPWO2005042624A1 (ja) | 2003-10-31 | 2007-05-10 | 日本ゼオン株式会社 | 熱可塑性エラストマー組成物及び成形品 |
-
2003
- 2003-09-30 CA CA 2500141 patent/CA2500141A1/en not_active Abandoned
- 2003-09-30 BR BR0314495A patent/BR0314495A/pt not_active IP Right Cessation
- 2003-09-30 WO PCT/US2003/031230 patent/WO2004029155A2/en active Application Filing
- 2003-09-30 US US10/674,305 patent/US7074857B2/en not_active Expired - Lifetime
- 2003-09-30 CN CNB03823310XA patent/CN100384929C/zh not_active Expired - Fee Related
- 2003-09-30 MX MXPA05003336A patent/MXPA05003336A/es active IP Right Grant
- 2003-09-30 AU AU2003283988A patent/AU2003283988A1/en not_active Abandoned
- 2003-09-30 EP EP20030776215 patent/EP1551920B1/en not_active Expired - Lifetime
- 2003-09-30 JP JP2004540346A patent/JP4870354B2/ja not_active Expired - Fee Related
- 2003-09-30 RU RU2005113281A patent/RU2339661C2/ru active
- 2003-09-30 KR KR1020057005391A patent/KR101008110B1/ko not_active Expired - Fee Related
- 2003-09-30 DE DE60333381T patent/DE60333381D1/de not_active Expired - Lifetime
Also Published As
| Publication number | Publication date |
|---|---|
| WO2004029155A3 (en) | 2004-05-27 |
| KR20050057607A (ko) | 2005-06-16 |
| BR0314495A (pt) | 2005-08-02 |
| CA2500141A1 (en) | 2004-04-08 |
| RU2005113281A (ru) | 2006-01-27 |
| KR101008110B1 (ko) | 2011-01-13 |
| CN100384929C (zh) | 2008-04-30 |
| MXPA05003336A (es) | 2005-07-05 |
| EP1551920A2 (en) | 2005-07-13 |
| DE60333381D1 (de) | 2010-08-26 |
| US7074857B2 (en) | 2006-07-11 |
| JP2006501331A (ja) | 2006-01-12 |
| US20040115450A1 (en) | 2004-06-17 |
| RU2339661C2 (ru) | 2008-11-27 |
| AU2003283988A1 (en) | 2004-04-19 |
| EP1551920B1 (en) | 2010-07-14 |
| CN1777645A (zh) | 2006-05-24 |
| WO2004029155A2 (en) | 2004-04-08 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4870354B2 (ja) | 硬化可能な熱可塑性エラストマーブレンド、その製造方法、およびその使用 | |
| EP0924257B1 (en) | Fluorinated thermoplastic elastomers | |
| CN1057550C (zh) | 接枝改性的基本线型乙烯聚合物与其它热塑性聚合物的共混物 | |
| CN105111694B (zh) | 可交联组合物、由其可获得的热塑性弹性体以及它们的用途 | |
| EP3820941B1 (en) | A grafted polylactic acid | |
| JP3651900B2 (ja) | エチレンコポリマーで可塑化したpvcの改良製造方法 | |
| JPS63205337A (ja) | 溶融加工性熱可塑性組成物 | |
| JP4436753B2 (ja) | 押出し成形可能な熱可塑性組成物 | |
| CN103897260A (zh) | 乙烯-醋酸乙烯酯共聚物(eva)与聚乳酸(pla)共混组合物及其成型制品 | |
| US8013067B2 (en) | Curable thermoplastic elastomeric blend, method of manufacture, and use thereof | |
| EP0885928A1 (en) | Fluorinated thermoplastic elastomers and articles therefrom | |
| JP4068162B2 (ja) | ポリアクリレート/ポリオレフィンブレンドの製造方法 | |
| CN1239543C (zh) | 丙烯基抗冲增强剂的制备方法 | |
| EP1406937B1 (en) | Heat and oil resistant thermoplastic elastomer | |
| JPH01500201A (ja) | 熱可塑性ポリエステル樹脂用のオレフィン系衝撃改質剤および該樹脂とのブレンド | |
| JP5400309B2 (ja) | 熱可塑性エラストマー組成物およびその製造方法 | |
| EP2098570B1 (de) | Vernetzbare Zusammensetzungen, daraus erhältliche thermoplastische Elastomere und deren Verwendung | |
| EP4567070A1 (en) | Resin composition and biodegradable resin molded product comprising same | |
| JP2586830B2 (ja) | 熱可塑性エラストマー組成物 | |
| JPH01152133A (ja) | 加工方法 | |
| JPH01152134A (ja) | 加工方法 | |
| JPH0425546A (ja) | 水架橋性塩化ビニル樹脂組成物 | |
| JPH02107652A (ja) | ポリ塩化ビニル―アクリレートコポリマーと架橋エラストマーとの熱可塑性エラストマー |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20060913 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20060913 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20080807 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20081212 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20090312 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20090319 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20090413 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20090420 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20090512 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20090605 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20090907 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20090914 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20091005 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20091013 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20091105 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20091112 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20100216 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100616 |
|
| RD13 | Notification of appointment of power of sub attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7433 Effective date: 20100617 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20100617 |
|
| A911 | Transfer to examiner for re-examination before appeal (zenchi) |
Free format text: JAPANESE INTERMEDIATE CODE: A911 Effective date: 20100810 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20110204 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20110502 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20110512 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20110603 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20110610 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20110704 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20110711 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20110802 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20111111 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20111117 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 4870354 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20141125 Year of fee payment: 3 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |