JP2015501641A - 関連するアクチノバクテリアの遺伝的形質転換のためのプラスミドとしての、アクチノプラネス属se50/110由来の新規な放線菌組込み接合エレメント - Google Patents
関連するアクチノバクテリアの遺伝的形質転換のためのプラスミドとしての、アクチノプラネス属se50/110由来の新規な放線菌組込み接合エレメント Download PDFInfo
- Publication number
- JP2015501641A JP2015501641A JP2014545206A JP2014545206A JP2015501641A JP 2015501641 A JP2015501641 A JP 2015501641A JP 2014545206 A JP2014545206 A JP 2014545206A JP 2014545206 A JP2014545206 A JP 2014545206A JP 2015501641 A JP2015501641 A JP 2015501641A
- Authority
- JP
- Japan
- Prior art keywords
- aice
- actinoplanes
- protein
- amino acids
- gene
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 241000187712 Actinoplanes sp. Species 0.000 title claims abstract description 15
- 230000002068 genetic effect Effects 0.000 title description 3
- 230000009466 transformation Effects 0.000 title description 3
- 239000013612 plasmid Substances 0.000 title description 2
- 241001156739 Actinobacteria <phylum> Species 0.000 title 1
- 230000013011 mating Effects 0.000 claims abstract description 5
- XUFXOAAUWZOOIT-SXARVLRPSA-N (2R,3R,4R,5S,6R)-5-[[(2R,3R,4R,5S,6R)-5-[[(2R,3R,4S,5S,6R)-3,4-dihydroxy-6-methyl-5-[[(1S,4R,5S,6S)-4,5,6-trihydroxy-3-(hydroxymethyl)-1-cyclohex-2-enyl]amino]-2-oxanyl]oxy]-3,4-dihydroxy-6-(hydroxymethyl)-2-oxanyl]oxy]-6-(hydroxymethyl)oxane-2,3,4-triol Chemical group O([C@H]1O[C@H](CO)[C@H]([C@@H]([C@H]1O)O)O[C@H]1O[C@@H]([C@H]([C@H](O)[C@H]1O)N[C@@H]1[C@@H]([C@@H](O)[C@H](O)C(CO)=C1)O)C)[C@@H]1[C@@H](CO)O[C@@H](O)[C@H](O)[C@H]1O XUFXOAAUWZOOIT-SXARVLRPSA-N 0.000 claims description 10
- 229960002632 acarbose Drugs 0.000 claims description 10
- XUFXOAAUWZOOIT-UHFFFAOYSA-N acarviostatin I01 Natural products OC1C(O)C(NC2C(C(O)C(O)C(CO)=C2)O)C(C)OC1OC(C(C1O)O)C(CO)OC1OC1C(CO)OC(O)C(O)C1O XUFXOAAUWZOOIT-UHFFFAOYSA-N 0.000 claims description 10
- 238000000034 method Methods 0.000 claims description 8
- 108091033319 polynucleotide Proteins 0.000 claims description 8
- 102000040430 polynucleotide Human genes 0.000 claims description 8
- 239000002157 polynucleotide Substances 0.000 claims description 8
- 238000002360 preparation method Methods 0.000 claims description 3
- 238000012258 culturing Methods 0.000 claims description 2
- 241001446247 uncultured actinomycete Species 0.000 claims 1
- 241000187844 Actinoplanes Species 0.000 abstract description 6
- 238000010353 genetic engineering Methods 0.000 abstract description 5
- 241000894007 species Species 0.000 abstract description 5
- 241000894006 Bacteria Species 0.000 abstract description 3
- 108091028043 Nucleic acid sequence Proteins 0.000 abstract description 2
- 108090000623 proteins and genes Proteins 0.000 description 69
- 102000004169 proteins and genes Human genes 0.000 description 48
- 150000001413 amino acids Chemical class 0.000 description 39
- 101100166100 Candida parapsilosis SAPP2 gene Proteins 0.000 description 22
- 210000004027 cell Anatomy 0.000 description 9
- 241000218953 Micromonospora aurantiaca Species 0.000 description 8
- 102100034343 Integrase Human genes 0.000 description 5
- 108010061833 Integrases Proteins 0.000 description 5
- 230000005540 biological transmission Effects 0.000 description 5
- 238000009792 diffusion process Methods 0.000 description 5
- 230000010354 integration Effects 0.000 description 5
- 230000010076 replication Effects 0.000 description 5
- 208000001072 type 2 diabetes mellitus Diseases 0.000 description 5
- 108020004566 Transfer RNA Proteins 0.000 description 4
- 102000004160 Phosphoric Monoester Hydrolases Human genes 0.000 description 3
- 108090000608 Phosphoric Monoester Hydrolases Proteins 0.000 description 3
- 241000187758 Streptomyces ambofaciens Species 0.000 description 3
- 230000001419 dependent effect Effects 0.000 description 3
- 239000002184 metal Substances 0.000 description 3
- 102000006040 nudix hydrolase Human genes 0.000 description 3
- 108020003260 nudix hydrolase Proteins 0.000 description 3
- 230000008520 organization Effects 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- 230000006798 recombination Effects 0.000 description 3
- 238000005215 recombination Methods 0.000 description 3
- 102000004190 Enzymes Human genes 0.000 description 2
- 108090000790 Enzymes Proteins 0.000 description 2
- 208000012766 Growth delay Diseases 0.000 description 2
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 2
- 241000218940 Micromonospora rosaria Species 0.000 description 2
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 2
- 101710195674 Replication initiator protein Proteins 0.000 description 2
- 101100301560 Streptomyces ambofaciens repSA gene Proteins 0.000 description 2
- 230000033228 biological regulation Effects 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 210000000349 chromosome Anatomy 0.000 description 2
- 238000002474 experimental method Methods 0.000 description 2
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 230000035897 transcription Effects 0.000 description 2
- 238000013518 transcription Methods 0.000 description 2
- 102000002735 Acyl-CoA Dehydrogenase Human genes 0.000 description 1
- 108010001058 Acyl-CoA Dehydrogenase Proteins 0.000 description 1
- 229940077274 Alpha glucosidase inhibitor Drugs 0.000 description 1
- 200000000007 Arterial disease Diseases 0.000 description 1
- 206010003211 Arteriosclerosis coronary artery Diseases 0.000 description 1
- 201000004569 Blindness Diseases 0.000 description 1
- 208000017667 Chronic Disease Diseases 0.000 description 1
- 108020004414 DNA Proteins 0.000 description 1
- 102000053602 DNA Human genes 0.000 description 1
- 102000004163 DNA-directed RNA polymerases Human genes 0.000 description 1
- 108090000626 DNA-directed RNA polymerases Proteins 0.000 description 1
- 102000004877 Insulin Human genes 0.000 description 1
- 108090001061 Insulin Proteins 0.000 description 1
- 102100024295 Maltase-glucoamylase Human genes 0.000 description 1
- 241000186359 Mycobacterium Species 0.000 description 1
- 102000001442 NUDIX hydrolase domains Human genes 0.000 description 1
- 108050009655 NUDIX hydrolase domains Proteins 0.000 description 1
- 108010076504 Protein Sorting Signals Proteins 0.000 description 1
- 208000001647 Renal Insufficiency Diseases 0.000 description 1
- 241000426681 Salinispora tropica Species 0.000 description 1
- 241000187747 Streptomyces Species 0.000 description 1
- 241001531149 Streptomyces cattleya NRRL 8057 = DSM 46488 Species 0.000 description 1
- 241000187432 Streptomyces coelicolor Species 0.000 description 1
- 241001180603 Streptomyces griseoflavus Tu4000 Species 0.000 description 1
- 101100096332 Streptomyces lividans spdA gene Proteins 0.000 description 1
- 101100096333 Streptomyces lividans spdB gene Proteins 0.000 description 1
- 241000187180 Streptomyces sp. Species 0.000 description 1
- 241001596104 Streptosporangium roseum DSM 43021 Species 0.000 description 1
- 108090000992 Transferases Proteins 0.000 description 1
- 241000065202 Verrucosispora maris AB-18-032 Species 0.000 description 1
- 239000012190 activator Substances 0.000 description 1
- 239000003888 alpha glucosidase inhibitor Substances 0.000 description 1
- 108010028144 alpha-Glucosidases Proteins 0.000 description 1
- 230000003321 amplification Effects 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 230000003197 catalytic effect Effects 0.000 description 1
- 230000032823 cell division Effects 0.000 description 1
- 230000002759 chromosomal effect Effects 0.000 description 1
- 238000003776 cleavage reaction Methods 0.000 description 1
- 230000001268 conjugating effect Effects 0.000 description 1
- 230000021615 conjugation Effects 0.000 description 1
- 208000029078 coronary artery disease Diseases 0.000 description 1
- 208000026758 coronary atherosclerosis Diseases 0.000 description 1
- 238000012217 deletion Methods 0.000 description 1
- 230000037430 deletion Effects 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- 239000010432 diamond Substances 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 238000004520 electroporation Methods 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 108091008053 gene clusters Proteins 0.000 description 1
- 102000034356 gene-regulatory proteins Human genes 0.000 description 1
- 108091006104 gene-regulatory proteins Proteins 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 238000009655 industrial fermentation Methods 0.000 description 1
- 229940125396 insulin Drugs 0.000 description 1
- 230000035990 intercellular signaling Effects 0.000 description 1
- 238000005304 joining Methods 0.000 description 1
- 201000006370 kidney failure Diseases 0.000 description 1
- 230000004807 localization Effects 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 230000001404 mediated effect Effects 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 238000002703 mutagenesis Methods 0.000 description 1
- 231100000350 mutagenesis Toxicity 0.000 description 1
- 238000003199 nucleic acid amplification method Methods 0.000 description 1
- 108020004707 nucleic acids Proteins 0.000 description 1
- 102000039446 nucleic acids Human genes 0.000 description 1
- 150000007523 nucleic acids Chemical class 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 238000012175 pyrosequencing Methods 0.000 description 1
- 230000008844 regulatory mechanism Effects 0.000 description 1
- 210000003705 ribosome Anatomy 0.000 description 1
- 230000000630 rising effect Effects 0.000 description 1
- 238000005096 rolling process Methods 0.000 description 1
- 230000007017 scission Effects 0.000 description 1
- 230000019491 signal transduction Effects 0.000 description 1
- 230000000638 stimulation Effects 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 210000001519 tissue Anatomy 0.000 description 1
- 230000002103 transcriptional effect Effects 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 238000011426 transformation method Methods 0.000 description 1
- 230000005945 translocation Effects 0.000 description 1
- 230000029663 wound healing Effects 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12P—FERMENTATION OR ENZYME-USING PROCESSES TO SYNTHESISE A DESIRED CHEMICAL COMPOUND OR COMPOSITION OR TO SEPARATE OPTICAL ISOMERS FROM A RACEMIC MIXTURE
- C12P19/00—Preparation of compounds containing saccharide radicals
- C12P19/26—Preparation of nitrogen-containing carbohydrates
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/195—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from bacteria
- C07K14/365—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from bacteria from Actinoplanes (G)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/52—Genes encoding for enzymes or proenzymes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/74—Vectors or expression systems specially adapted for prokaryotic hosts other than E. coli, e.g. Lactobacillus, Micromonospora
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/74—Vectors or expression systems specially adapted for prokaryotic hosts other than E. coli, e.g. Lactobacillus, Micromonospora
- C12N15/76—Vectors or expression systems specially adapted for prokaryotic hosts other than E. coli, e.g. Lactobacillus, Micromonospora for Actinomyces; for Streptomyces
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12P—FERMENTATION OR ENZYME-USING PROCESSES TO SYNTHESISE A DESIRED CHEMICAL COMPOUND OR COMPOSITION OR TO SEPARATE OPTICAL ISOMERS FROM A RACEMIC MIXTURE
- C12P19/00—Preparation of compounds containing saccharide radicals
- C12P19/26—Preparation of nitrogen-containing carbohydrates
- C12P19/28—N-glycosides
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Genetics & Genomics (AREA)
- Engineering & Computer Science (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biotechnology (AREA)
- General Engineering & Computer Science (AREA)
- Molecular Biology (AREA)
- Biochemistry (AREA)
- General Health & Medical Sciences (AREA)
- Biomedical Technology (AREA)
- Microbiology (AREA)
- Biophysics (AREA)
- Plant Pathology (AREA)
- Physics & Mathematics (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Gastroenterology & Hepatology (AREA)
- Medicinal Chemistry (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Enzymes And Modification Thereof (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
Description
原核生物アクチノプラネス属(Actinoplanes sp.) SE50/110は、2型糖尿病の治療において世界中で使用されている、α−グルコシダーゼ阻害剤アカルボースを産生する。2型糖尿病の発生率が世界的に急速に上昇しているという事実に基づいて、アカルボースの需要の増大が、将来的に予測されている。これらの期待に応えるために、株およびその誘導体の遺伝子操作は、アカルボース収量を増大させることを目指して行われなければならない。しかしながら、現在、この株のための遺伝子操作のためのツールは存在しておらず、菌株改良の過程を妨げている。
アクチノプラネス属(Actinoplanes sp.) SE50/110は、約9.25MBの大きさの高G+C含量のゲノムを有する、グラム陽性、好気性細菌である(Schwientek et al., 2012)。その医学的に重要な生物は、ヒトα−グルコシダーゼを阻害することが見出された様々な化学的に関連する物質の天然の生産者であり(Caspary and Graf, 1979)、医薬用途に特に適している(Frommer et al., 1975, 1977 a, 1977 b, 1979)。特に、十分に特徴付けられたアカルボース遺伝子クラスターにコードされる酵素によって合成される、シュードテトラサッカライドアカルボースは、2型糖尿病(インスリン非依存性)の治療に世界中で使用されている。
a)配列番号1の配列を有するポリヌクレオチド、
b)(a)で特定されるポリヌクレオチドにストリンジェントな条件下でハイブリダイズするポリヌクレオチド、および
c)配列番号1の配列と少なくとも90%同一性を有するポリヌクレオチド
からなる群から選択される。
a)有用な培地において上記宿主細胞を培養する段階、
b)培養物から産物を回収する段階、および
c)産物を単離および精製する段階
を含む、生物学的産物の調製のための方法において有用である。この方法における最も好ましい産物はアカルボースである。
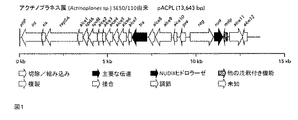
遺伝子int(ゲノム遺伝子座タグ:ACPL_6310)は、388アミノ酸の長さを有するAICEのインテグラーゼをコードする。その配列は、最初の383アミノ酸内で、ストレプトマイセス・グリセオフラブス(Streptomyces griseoflavus) Tu4000のインテグラーゼ(GenBank:EFL40120.1)に74%の類似性を示す。該タンパク質のインテグラーゼドメインは、アミノ酸182−365に位置し、Int/Topo IBシグネチャーモチーフ(保存ドメイン:cd01182)に高い類似性(e値 2.90e−21)を示す。該インテグラーゼは、2つの類似する、染色体上の付着部位attBおよびAICE上のattPの間で生じる、部位特異的組換えによる、tRNA遺伝子内への組込みの原因である(te Poele, Bolhuis, et al., 2008)。
Claims (6)
- d)配列番号1の配列を有するポリヌクレオチド、
e)(a)で特定されるポリヌクレオチドにストリンジェントな条件下でハイブリダイズするポリヌクレオチド、および
f)配列番号1の配列と少なくとも90%同一性を有するポリヌクレオチド
からなる群から選択される、放線菌組込み接合エレメント。 - 配列番号1の配列と少なくとも95%同一性を有する、請求項1の放線菌組込み接合エレメント。
- 配列番号1および2の放線菌組込み接合エレメントで形質転換された宿主細胞。
- 細胞がアクチノプラネス属(Actinoplanes sp.)である、請求項3の宿主細胞。
- d)有用な培地において請求項3または4の宿主細胞を培養する段階、
e)培養物から産物を回収する段階、および
f)産物を単離および精製する段階
を含む、生物学的産物の調製のための方法。 - 産物がアカルボースである、請求項5の方法。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP11192618.4 | 2011-12-08 | ||
| EP11192618 | 2011-12-08 | ||
| PCT/EP2012/074366 WO2013083566A1 (en) | 2011-12-08 | 2012-12-04 | New actinomycete integrative and conjugative element from actinoplanes sp. se50/110 as plasmid for genetic transformation of related actinobacteria |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| JP2015501641A true JP2015501641A (ja) | 2015-01-19 |
Family
ID=47278320
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2014545206A Pending JP2015501641A (ja) | 2011-12-08 | 2012-12-04 | 関連するアクチノバクテリアの遺伝的形質転換のためのプラスミドとしての、アクチノプラネス属se50/110由来の新規な放線菌組込み接合エレメント |
Country Status (12)
| Country | Link |
|---|---|
| US (2) | US9217154B2 (ja) |
| EP (1) | EP2788373B1 (ja) |
| JP (1) | JP2015501641A (ja) |
| KR (1) | KR20140109922A (ja) |
| CN (1) | CN104144942A (ja) |
| AR (1) | AR089117A1 (ja) |
| CA (1) | CA2858259A1 (ja) |
| ES (1) | ES2718331T3 (ja) |
| HK (1) | HK1203970A1 (ja) |
| IL (1) | IL232587A (ja) |
| IN (1) | IN2014CN04155A (ja) |
| WO (1) | WO2013083566A1 (ja) |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN104144942A (zh) | 2011-12-08 | 2014-11-12 | 拜耳知识产权有限责任公司 | 来自游动放线菌属菌种se50/110的新的放射菌整合和接合元件作为用于相关放线菌基因转化的质粒 |
| CN112522174B (zh) * | 2020-12-25 | 2022-08-09 | 上海交通大学 | 一种敲除负调控蛋白基因以提高阿卡波糖发酵水平的方法 |
| CN112592878B (zh) * | 2020-12-25 | 2022-08-26 | 上海交通大学 | 增强正调控蛋白基因表达以提高阿卡波糖发酵水平的方法 |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2010534489A (ja) * | 2007-07-27 | 2010-11-11 | ワイス・エルエルシー | 遺伝子クラスターまたはその一部分をクローニングするためのベクターおよび方法 |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE2064092C2 (de) | 1970-12-28 | 1983-06-01 | Bayer Ag, 5090 Leverkusen | Inhibitoren für Glykosidhydrolasen aus Actinomyceten |
| DE2209834C3 (de) | 1972-03-01 | 1978-04-20 | Bayer Ag, 5090 Leverkusen | Herstellung von Saccharase-Inhibitoren |
| US4062950A (en) | 1973-09-22 | 1977-12-13 | Bayer Aktiengesellschaft | Amino sugar derivatives |
| DE2347782C3 (de) | 1973-09-22 | 1979-10-11 | Bayer Ag, 5090 Leverkusen | Aminozuckerderivate, Verfahren zu ihrer Herstellung sowie diese Verbindungen enthaltende Arzneimittel |
| EP0796915A3 (de) * | 1996-03-22 | 1999-04-14 | Bayer Ag | Verfahren zur Herstellung sowie zur Verwendung von Acarviosyl-Transferase bei der Umwandlung von Acarbose-Homologen in Acarbose, zur Herstellung von Acarbose-Homologen |
| TW201217533A (en) * | 2010-08-04 | 2012-05-01 | Bayer Pharma AG | Genomics of actinoplanes utahensis |
| CN104144942A (zh) | 2011-12-08 | 2014-11-12 | 拜耳知识产权有限责任公司 | 来自游动放线菌属菌种se50/110的新的放射菌整合和接合元件作为用于相关放线菌基因转化的质粒 |
-
2012
- 2012-12-04 CN CN201280060526.8A patent/CN104144942A/zh active Pending
- 2012-12-04 ES ES12794734T patent/ES2718331T3/es active Active
- 2012-12-04 US US14/363,810 patent/US9217154B2/en not_active Expired - Fee Related
- 2012-12-04 EP EP12794734.9A patent/EP2788373B1/en not_active Not-in-force
- 2012-12-04 IN IN4155CHN2014 patent/IN2014CN04155A/en unknown
- 2012-12-04 WO PCT/EP2012/074366 patent/WO2013083566A1/en active Application Filing
- 2012-12-04 JP JP2014545206A patent/JP2015501641A/ja active Pending
- 2012-12-04 CA CA2858259A patent/CA2858259A1/en not_active Abandoned
- 2012-12-04 KR KR1020147018467A patent/KR20140109922A/ko not_active Application Discontinuation
- 2012-12-07 AR ARP120104603A patent/AR089117A1/es active Pending
-
2014
- 2014-05-13 IL IL232587A patent/IL232587A/en not_active IP Right Cessation
-
2015
- 2015-05-07 HK HK15104333.1A patent/HK1203970A1/xx unknown
- 2015-11-12 US US14/939,495 patent/US9562249B2/en not_active Expired - Fee Related
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2010534489A (ja) * | 2007-07-27 | 2010-11-11 | ワイス・エルエルシー | 遺伝子クラスターまたはその一部分をクローニングするためのベクターおよび方法 |
Non-Patent Citations (2)
| Title |
|---|
| JPN6016037946; Biotechnol Lett Vol. 30, No. 7, 2008, pp 1233-1238 * |
| JPN6016037947; Antimicrobial Agents and Chemotherapy Vol. 47, No. 2, 2003, pp.447-457 * |
Also Published As
| Publication number | Publication date |
|---|---|
| EP2788373A1 (en) | 2014-10-15 |
| AR089117A1 (es) | 2014-07-30 |
| CN104144942A (zh) | 2014-11-12 |
| IN2014CN04155A (ja) | 2015-07-17 |
| IL232587A0 (en) | 2014-06-30 |
| HK1203970A1 (en) | 2015-11-06 |
| US9562249B2 (en) | 2017-02-07 |
| EP2788373B1 (en) | 2019-01-23 |
| ES2718331T3 (es) | 2019-07-01 |
| IL232587A (en) | 2017-08-31 |
| US20160130622A1 (en) | 2016-05-12 |
| WO2013083566A1 (en) | 2013-06-13 |
| US9217154B2 (en) | 2015-12-22 |
| CA2858259A1 (en) | 2013-06-13 |
| US20140349346A1 (en) | 2014-11-27 |
| KR20140109922A (ko) | 2014-09-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN107083392B (zh) | 一种CRISPR/Cpf1基因编辑系统及其在分枝杆菌中的应用 | |
| KR102623312B1 (ko) | Ruvc 도메인이 존재하는 효소 | |
| Elias et al. | New antibiotic resistance cassettes suitable for genetic studies in Borrelia burgdorferi | |
| CN107142272A (zh) | 一种控制大肠杆菌中质粒复制的方法 | |
| JPS58192900A (ja) | 複合プラスミド | |
| DE60113448T2 (de) | Klonierungsvektoren und expressionsvektoren aus rhodococcus | |
| CN109706148A (zh) | 一种用于敲除BCL11A基因或者BCL11A基因增强子的gRNA、gRNA组合物以及电转方法 | |
| JP2015501641A (ja) | 関連するアクチノバクテリアの遺伝的形質転換のためのプラスミドとしての、アクチノプラネス属se50/110由来の新規な放線菌組込み接合エレメント | |
| CN105176899B (zh) | 构建生产或高产目的基因产物重组菌的方法及构建的重组菌与应用 | |
| Gopaul et al. | Characterization of the two Mycobacterium tuberculosis recA promoters | |
| CN108103025B (zh) | 一种造血干细胞及其制备方法和用途 | |
| CN118139979A (zh) | 具有hepn结构域的酶 | |
| CN1195062C (zh) | 非抗生素抗性穿梭质粒表达载体及其构建方法和应用 | |
| ES2543167T3 (es) | Microorganismos con mayor actividad de sacarosa mutasa | |
| TW201336992A (zh) | 源自游動放線菌se50/110並作為用於相關放線菌基因轉型之質體的新穎放線菌嵌合及接合單元 | |
| CN114480464B (zh) | 一种副溶血弧菌CRISPRi的双质粒构建方法 | |
| US9637748B2 (en) | Conjugative plasmids and methods of use thereof | |
| Srivastava et al. | Characterization of broad host range cryptic plasmid pCR1 from Corynebacterium renale | |
| WO2024199539A1 (zh) | 环庚三烯酚酮类化合物isatropolones生物合成基因簇及定向高产的菌株 | |
| KR100886358B1 (ko) | 파스튜렐라 멀토시다의 비병원성 약독화 균주 및 이를함유하는 백신 | |
| CN104817632A (zh) | 拟态弧菌ta系统毒素蛋白及其编码基因和应用 | |
| WO1998035029A1 (en) | Synthetic genes for recombinant mycobacterium proteins | |
| CN106636167A (zh) | 一种硫链丝菌素‑庆大霉素抗性基因体系及还有其的抗性表达盒和重组质粒 | |
| CN117210475A (zh) | 一种水产动物纤毛虫病原生物防控方法 | |
| Frazier | Efficiency of the TargeTron Gene Knockout System as a Transformative Protocol for the Mutagenesis of Listeria monocytogenes |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| RD03 | Notification of appointment of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7423 Effective date: 20150209 |
|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20150602 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20151113 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20160915 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20161004 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20161227 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20170516 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20171025 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20181101 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20190206 |