EP2269738B1 - Vorrichtung zur PCR-Amplifizierung von Ziel-DNA-Sequenzen - Google Patents
Vorrichtung zur PCR-Amplifizierung von Ziel-DNA-Sequenzen Download PDFInfo
- Publication number
- EP2269738B1 EP2269738B1 EP10177401A EP10177401A EP2269738B1 EP 2269738 B1 EP2269738 B1 EP 2269738B1 EP 10177401 A EP10177401 A EP 10177401A EP 10177401 A EP10177401 A EP 10177401A EP 2269738 B1 EP2269738 B1 EP 2269738B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- cartridge
- reaction chambers
- reaction
- amplification
- reservoir
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 238000006243 chemical reaction Methods 0.000 title claims abstract description 148
- 230000003321 amplification Effects 0.000 title claims abstract description 91
- 238000003199 nucleic acid amplification method Methods 0.000 title claims abstract description 91
- 150000007523 nucleic acids Chemical group 0.000 title claims description 47
- 230000001419 dependent effect Effects 0.000 title description 5
- 239000012530 fluid Substances 0.000 claims abstract description 56
- 238000010438 heat treatment Methods 0.000 claims abstract description 39
- 238000011049 filling Methods 0.000 claims abstract description 14
- 239000000523 sample Substances 0.000 claims description 50
- 108020004707 nucleic acids Proteins 0.000 claims description 33
- 102000039446 nucleic acids Human genes 0.000 claims description 33
- 239000003153 chemical reaction reagent Substances 0.000 claims description 31
- 238000000034 method Methods 0.000 claims description 27
- 238000006073 displacement reaction Methods 0.000 claims description 23
- 238000005259 measurement Methods 0.000 claims description 14
- 230000005284 excitation Effects 0.000 claims description 11
- 230000003287 optical effect Effects 0.000 claims description 11
- 239000011248 coating agent Substances 0.000 claims description 3
- 238000000576 coating method Methods 0.000 claims description 3
- 125000004122 cyclic group Chemical group 0.000 claims description 2
- 238000006911 enzymatic reaction Methods 0.000 claims description 2
- 238000011534 incubation Methods 0.000 claims description 2
- 230000001737 promoting effect Effects 0.000 claims description 2
- 230000002255 enzymatic effect Effects 0.000 claims 1
- 108020004414 DNA Proteins 0.000 abstract description 11
- 108091028043 Nucleic acid sequence Proteins 0.000 abstract description 7
- 238000003752 polymerase chain reaction Methods 0.000 description 60
- 238000009396 hybridization Methods 0.000 description 24
- 238000004925 denaturation Methods 0.000 description 13
- 230000036425 denaturation Effects 0.000 description 13
- 230000033001 locomotion Effects 0.000 description 12
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 12
- 238000001514 detection method Methods 0.000 description 11
- 230000008901 benefit Effects 0.000 description 10
- 230000000295 complement effect Effects 0.000 description 8
- 238000007834 ligase chain reaction Methods 0.000 description 8
- 238000011109 contamination Methods 0.000 description 6
- 239000000203 mixture Substances 0.000 description 6
- 241000897276 Termes Species 0.000 description 5
- 230000000151 anti-reflux effect Effects 0.000 description 5
- 230000007423 decrease Effects 0.000 description 5
- 238000009826 distribution Methods 0.000 description 5
- 229920003023 plastic Polymers 0.000 description 5
- 239000011541 reaction mixture Substances 0.000 description 5
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 4
- TWRXJAOTZQYOKJ-UHFFFAOYSA-L Magnesium chloride Chemical compound [Mg+2].[Cl-].[Cl-] TWRXJAOTZQYOKJ-UHFFFAOYSA-L 0.000 description 4
- 230000000694 effects Effects 0.000 description 4
- 230000008020 evaporation Effects 0.000 description 4
- 238000001704 evaporation Methods 0.000 description 4
- 230000036541 health Effects 0.000 description 4
- 239000000463 material Substances 0.000 description 4
- 239000004033 plastic Substances 0.000 description 4
- 229910052697 platinum Inorganic materials 0.000 description 4
- 230000008569 process Effects 0.000 description 4
- 230000003612 virological effect Effects 0.000 description 4
- 108091034117 Oligonucleotide Proteins 0.000 description 3
- 108010006785 Taq Polymerase Proteins 0.000 description 3
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 3
- 238000001035 drying Methods 0.000 description 3
- GNBHRKFJIUUOQI-UHFFFAOYSA-N fluorescein Chemical class O1C(=O)C2=CC=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 GNBHRKFJIUUOQI-UHFFFAOYSA-N 0.000 description 3
- 230000002093 peripheral effect Effects 0.000 description 3
- 238000003757 reverse transcription PCR Methods 0.000 description 3
- 238000012360 testing method Methods 0.000 description 3
- 238000013518 transcription Methods 0.000 description 3
- 230000035897 transcription Effects 0.000 description 3
- 241000894006 Bacteria Species 0.000 description 2
- 102000053602 DNA Human genes 0.000 description 2
- 108010014303 DNA-directed DNA polymerase Proteins 0.000 description 2
- 102000016928 DNA-directed DNA polymerase Human genes 0.000 description 2
- AHCYMLUZIRLXAA-SHYZEUOFSA-N Deoxyuridine 5'-triphosphate Chemical compound O1[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)C[C@@H]1N1C(=O)NC(=O)C=C1 AHCYMLUZIRLXAA-SHYZEUOFSA-N 0.000 description 2
- 101710163270 Nuclease Proteins 0.000 description 2
- 241001354013 Salmonella enterica subsp. enterica serovar Enteritidis Species 0.000 description 2
- 238000012864 cross contamination Methods 0.000 description 2
- 238000003745 diagnosis Methods 0.000 description 2
- 201000010099 disease Diseases 0.000 description 2
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 2
- 229940082150 encore Drugs 0.000 description 2
- 235000013305 food Nutrition 0.000 description 2
- 230000002068 genetic effect Effects 0.000 description 2
- 235000003869 genetically modified organism Nutrition 0.000 description 2
- 230000005484 gravity Effects 0.000 description 2
- 239000000138 intercalating agent Substances 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 229910001629 magnesium chloride Inorganic materials 0.000 description 2
- 239000011159 matrix material Substances 0.000 description 2
- 230000001404 mediated effect Effects 0.000 description 2
- 230000008018 melting Effects 0.000 description 2
- 238000002844 melting Methods 0.000 description 2
- 239000013642 negative control Substances 0.000 description 2
- 239000004417 polycarbonate Substances 0.000 description 2
- 229920000515 polycarbonate Polymers 0.000 description 2
- 229920000136 polysorbate Polymers 0.000 description 2
- 238000011002 quantification Methods 0.000 description 2
- 238000003753 real-time PCR Methods 0.000 description 2
- 230000010076 replication Effects 0.000 description 2
- PYWVYCXTNDRMGF-UHFFFAOYSA-N rhodamine B Chemical class [Cl-].C=12C=CC(=[N+](CC)CC)C=C2OC2=CC(N(CC)CC)=CC=C2C=1C1=CC=CC=C1C(O)=O PYWVYCXTNDRMGF-UHFFFAOYSA-N 0.000 description 2
- 150000003839 salts Chemical class 0.000 description 2
- 108010037497 3'-nucleotidase Proteins 0.000 description 1
- 108700003860 Bacterial Genes Proteins 0.000 description 1
- 208000035143 Bacterial infection Diseases 0.000 description 1
- 238000000018 DNA microarray Methods 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 241001272720 Medialuna californiensis Species 0.000 description 1
- 241000607142 Salmonella Species 0.000 description 1
- 238000002105 Southern blotting Methods 0.000 description 1
- 239000004809 Teflon Substances 0.000 description 1
- 229920006362 Teflon® Polymers 0.000 description 1
- 241001080024 Telles Species 0.000 description 1
- 108700019146 Transgenes Proteins 0.000 description 1
- 108700005077 Viral Genes Proteins 0.000 description 1
- 108020000999 Viral RNA Proteins 0.000 description 1
- 208000036142 Viral infection Diseases 0.000 description 1
- 239000002390 adhesive tape Substances 0.000 description 1
- 239000000443 aerosol Substances 0.000 description 1
- 239000000427 antigen Substances 0.000 description 1
- 108091007433 antigens Proteins 0.000 description 1
- 102000036639 antigens Human genes 0.000 description 1
- 230000003416 augmentation Effects 0.000 description 1
- 208000022362 bacterial infectious disease Diseases 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 230000002860 competitive effect Effects 0.000 description 1
- 239000002299 complementary DNA Substances 0.000 description 1
- 238000009833 condensation Methods 0.000 description 1
- 230000005494 condensation Effects 0.000 description 1
- 239000000356 contaminant Substances 0.000 description 1
- 230000036461 convulsion Effects 0.000 description 1
- 230000001351 cycling effect Effects 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 238000002405 diagnostic procedure Methods 0.000 description 1
- 238000009792 diffusion process Methods 0.000 description 1
- 235000011180 diphosphates Nutrition 0.000 description 1
- 238000010494 dissociation reaction Methods 0.000 description 1
- 230000005593 dissociations Effects 0.000 description 1
- 239000000428 dust Substances 0.000 description 1
- 238000013399 early diagnosis Methods 0.000 description 1
- 230000001747 exhibiting effect Effects 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 238000005429 filling process Methods 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 230000002779 inactivation Effects 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 239000011229 interlayer Substances 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 239000006193 liquid solution Substances 0.000 description 1
- 108020004999 messenger RNA Proteins 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 230000001613 neoplastic effect Effects 0.000 description 1
- 239000013307 optical fiber Substances 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- -1 phosphoryl group Chemical group 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 235000020004 porter Nutrition 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 108090000623 proteins and genes Proteins 0.000 description 1
- 238000003908 quality control method Methods 0.000 description 1
- 238000010791 quenching Methods 0.000 description 1
- 230000000171 quenching effect Effects 0.000 description 1
- 239000012429 reaction media Substances 0.000 description 1
- 230000007420 reactivation Effects 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 230000003252 repetitive effect Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 238000007894 restriction fragment length polymorphism technique Methods 0.000 description 1
- 238000010839 reverse transcription Methods 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 108020004418 ribosomal RNA Proteins 0.000 description 1
- 210000003705 ribosome Anatomy 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 238000012163 sequencing technique Methods 0.000 description 1
- 230000002459 sustained effect Effects 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L3/00—Containers or dishes for laboratory use, e.g. laboratory glassware; Droppers
- B01L3/50—Containers for the purpose of retaining a material to be analysed, e.g. test tubes
- B01L3/502—Containers for the purpose of retaining a material to be analysed, e.g. test tubes with fluid transport, e.g. in multi-compartment structures
- B01L3/5027—Containers for the purpose of retaining a material to be analysed, e.g. test tubes with fluid transport, e.g. in multi-compartment structures by integrated microfluidic structures, i.e. dimensions of channels and chambers are such that surface tension forces are important, e.g. lab-on-a-chip
- B01L3/50273—Containers for the purpose of retaining a material to be analysed, e.g. test tubes with fluid transport, e.g. in multi-compartment structures by integrated microfluidic structures, i.e. dimensions of channels and chambers are such that surface tension forces are important, e.g. lab-on-a-chip characterised by the means or forces applied to move the fluids
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L3/00—Containers or dishes for laboratory use, e.g. laboratory glassware; Droppers
- B01L3/50—Containers for the purpose of retaining a material to be analysed, e.g. test tubes
- B01L3/502—Containers for the purpose of retaining a material to be analysed, e.g. test tubes with fluid transport, e.g. in multi-compartment structures
- B01L3/5025—Containers for the purpose of retaining a material to be analysed, e.g. test tubes with fluid transport, e.g. in multi-compartment structures for parallel transport of multiple samples
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L3/00—Containers or dishes for laboratory use, e.g. laboratory glassware; Droppers
- B01L3/50—Containers for the purpose of retaining a material to be analysed, e.g. test tubes
- B01L3/502—Containers for the purpose of retaining a material to be analysed, e.g. test tubes with fluid transport, e.g. in multi-compartment structures
- B01L3/5027—Containers for the purpose of retaining a material to be analysed, e.g. test tubes with fluid transport, e.g. in multi-compartment structures by integrated microfluidic structures, i.e. dimensions of channels and chambers are such that surface tension forces are important, e.g. lab-on-a-chip
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L3/00—Containers or dishes for laboratory use, e.g. laboratory glassware; Droppers
- B01L3/50—Containers for the purpose of retaining a material to be analysed, e.g. test tubes
- B01L3/502—Containers for the purpose of retaining a material to be analysed, e.g. test tubes with fluid transport, e.g. in multi-compartment structures
- B01L3/5027—Containers for the purpose of retaining a material to be analysed, e.g. test tubes with fluid transport, e.g. in multi-compartment structures by integrated microfluidic structures, i.e. dimensions of channels and chambers are such that surface tension forces are important, e.g. lab-on-a-chip
- B01L3/502715—Containers for the purpose of retaining a material to be analysed, e.g. test tubes with fluid transport, e.g. in multi-compartment structures by integrated microfluidic structures, i.e. dimensions of channels and chambers are such that surface tension forces are important, e.g. lab-on-a-chip characterised by interfacing components, e.g. fluidic, electrical, optical or mechanical interfaces
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L7/00—Heating or cooling apparatus; Heat insulating devices
- B01L7/52—Heating or cooling apparatus; Heat insulating devices with provision for submitting samples to a predetermined sequence of different temperatures, e.g. for treating nucleic acid samples
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L2300/00—Additional constructional details
- B01L2300/08—Geometry, shape and general structure
- B01L2300/0803—Disc shape
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L2300/00—Additional constructional details
- B01L2300/08—Geometry, shape and general structure
- B01L2300/0809—Geometry, shape and general structure rectangular shaped
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L2300/00—Additional constructional details
- B01L2300/08—Geometry, shape and general structure
- B01L2300/0861—Configuration of multiple channels and/or chambers in a single devices
- B01L2300/0864—Configuration of multiple channels and/or chambers in a single devices comprising only one inlet and multiple receiving wells, e.g. for separation, splitting
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L2300/00—Additional constructional details
- B01L2300/18—Means for temperature control
- B01L2300/1805—Conductive heating, heat from thermostatted solids is conducted to receptacles, e.g. heating plates, blocks
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L2400/00—Moving or stopping fluids
- B01L2400/04—Moving fluids with specific forces or mechanical means
- B01L2400/0403—Moving fluids with specific forces or mechanical means specific forces
- B01L2400/0406—Moving fluids with specific forces or mechanical means specific forces capillary forces
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L2400/00—Moving or stopping fluids
- B01L2400/04—Moving fluids with specific forces or mechanical means
- B01L2400/0475—Moving fluids with specific forces or mechanical means specific mechanical means and fluid pressure
- B01L2400/0487—Moving fluids with specific forces or mechanical means specific mechanical means and fluid pressure fluid pressure, pneumatics
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L2400/00—Moving or stopping fluids
- B01L2400/04—Moving fluids with specific forces or mechanical means
- B01L2400/0475—Moving fluids with specific forces or mechanical means specific mechanical means and fluid pressure
- B01L2400/0487—Moving fluids with specific forces or mechanical means specific mechanical means and fluid pressure fluid pressure, pneumatics
- B01L2400/049—Moving fluids with specific forces or mechanical means specific mechanical means and fluid pressure fluid pressure, pneumatics vacuum
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L7/00—Heating or cooling apparatus; Heat insulating devices
- B01L7/52—Heating or cooling apparatus; Heat insulating devices with provision for submitting samples to a predetermined sequence of different temperatures, e.g. for treating nucleic acid samples
- B01L7/525—Heating or cooling apparatus; Heat insulating devices with provision for submitting samples to a predetermined sequence of different temperatures, e.g. for treating nucleic acid samples with physical movement of samples between temperature zones
- B01L7/5255—Heating or cooling apparatus; Heat insulating devices with provision for submitting samples to a predetermined sequence of different temperatures, e.g. for treating nucleic acid samples with physical movement of samples between temperature zones by moving sample containers
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L7/00—Heating or cooling apparatus; Heat insulating devices
- B01L7/54—Heating or cooling apparatus; Heat insulating devices using spatial temperature gradients
Definitions
- the present invention relates to the field of genetics.
- the present invention relates to a device for the amplification of target nucleic sequences, and modes of use of this device.
- the object of the present invention is in particular to enable the detection and, where appropriate, the quantification in real time of target nucleic acid sequences in one or more samples.
- Detection of target nucleic acid sequences is an increasingly popular technique in many fields, and the range of applications of this technique is expected to expand as it becomes more reliable, cost-effective and more reliable. fast.
- the detection of certain nucleic acid sequences in certain cases allows a reliable and rapid diagnosis of viral or bacterial infections.
- the detection of certain genetic peculiarities can make it possible to identify susceptibilities to certain diseases, or to establish an early diagnosis of genetic or neoplastic diseases.
- the detection of target nucleic sequences is also used in the food industry, in particular to ensure the traceability of products, to detect the presence of genetically modified organisms and to identify them, or to carry out a health control of food.
- Nucleic acid-based detection methods almost invariably involve a molecular hybridization reaction between a target nucleic acid sequence and one or more sequences nucleic acid complementary to said target sequence.
- These methods have numerous variants such as the techniques known to those skilled in the art under the terms "transfer techniques” ( blot, dot block, Southern blot, Restriction Fragment Length Polymorphism, etc.), or else the miniaturized systems on which the complementary sequences of the target sequences ("biochips”) are prefixed.
- transfer techniques blot, dot block, Southern blot, Restriction Fragment Length Polymorphism, etc.
- biochips the complementary nucleic sequences are generally called probes.
- Another variant which may constitute in itself the basis of a diagnostic method or be only an additional step in one of the techniques mentioned above (in particular to increase the concentration of the target sequence and therefore the sensitivity of the diagnosis), consists in amplifying the targeted nucleic acid sequence.
- PCR Polymerase Chain Reaction
- PCR Polymerase Chain Rection
- primers complementary nucleic sequences of the target sequences
- the PCR reactions involve a repetition of cycles, the number of which generally ranges from 20 to 50, and which are each composed of three successive phases, namely: denaturation, hybridization, elongation.
- the first phase corresponds to the transformation of the double-stranded nucleic acids into single-stranded nucleic acids
- the second phase to the molecular hybridization between the target sequence and the complementary primers of said sequence
- the third phase to the elongation of the hybridized complementary primers. to the target sequence, by a DNA polymerase.
- phase are carried out at specific temperatures: generally 95 ° C for denaturation, 72 ° C for elongation, and between 30 ° C and 65 ° C for hybridization, depending on the hybridization temperature (Tm) of the primers used. It is also possible to perform the hybridization and elongation steps at the same temperature (generally 60 ° C).
- a PCR reaction therefore consists of a series of repetitive thermal cycles in which the number of target DNA molecules serving as template is theoretically doubled at each cycle.
- the yield r decreases during the PCR reaction, because of several factors, such as an amount mimicking at least one of the reagents necessary for the amplification, the inactivation of the polymerase by repeated passages. at 95 ° C, or its inhibition by the pyrophosphates produced by the reaction.
- the curve presenting the quantity of product, in logarithmic scale, as a function of the number of cycles, and a slope line (1 + r) and which intersects the y-axis with a value equal to the logarithm of the initial concentration.
- the real-time measurement of the quantity of product obtained can therefore make it possible to know the initial matrix concentration, which is particularly useful in a large number of applications, for example for measuring the viral load of a patient, or again to know the variability of a transcriptome.
- the PCRs involve reaction volumes ranging from 2 to 50 ⁇ l and are carried out in tubes, microtubes, capillaries or systems known to those skilled in the art under the term "microplates" (in fact sets of micro tubes secured).
- microplates in fact sets of micro tubes secured.
- Each batch of tubes or equivalent containers must be successively brought to the three temperatures corresponding to the different phases of the PCR, and as many times as desired cycles.
- oligonucleotides complementary to said sequence are based on oligonucleotides complementary to said sequence, and linked to couples of fluorophore or fluorophore / quencher moieties, such that hybridization of the probe to its target and subsequent amplification cycles result, depending on the case an increase or decrease in the total fluorescence of the mixture, in proportion to the amplification of the target sequence.
- Examples of useful probes for performing kinetic PCR include the TaqMan TM system (ABI ®), the AmpliSensor TM system (InGen) and the Sunrise TM system (Oncor ®, ® Appligene).
- the most used system today is the Taq Man TM system .
- This method combines the DNA polymerase and 5 ' ⁇ 3' nuclease activities of Taq polymerase during PCR. Its principle is as follows: in addition to the two primers of sequence complementary to that of the target to be quantified, a probe, called probe reporter, is added in the reaction medium. It has the ability to hybridize to the target in the body of the amplified sequence, but can not be amplified itself. Indeed, a phosphoryl group added to the 3 'end of the probe prevents its extension by Taq polymerase. A fluorescein derivative and a rhodamine derivative are incorporated into the probe at the 5 'and 3' ends, respectively. The probe is small, so the rhodamine derivative, located near the fluorescein, absorbs the energy emitted by the fluorescein subjected to a source of excitation ( quenching phenomenon).
- the Taq DNA polymerase attacks the probe by its 5 'activity. nuclease, releasing the quencher group and thereby restoring the fluorescence emission.
- the intensity of the fluorescence emitted is then proportional to the quantity of PCR products formed, which makes it possible to obtain a quantitative result.
- the fluorescence emitted is proportional to the number of starting target molecules. The kinetics of fluorescence development can be monitored in real time during the amplification reaction.
- This technique has the advantage of being easily automatable.
- An apparatus for carrying out this technique the ABI Prism 7700 TM , is marketed by Perkin-Elmer.
- This device combines a thermocycler and a fluorimeter. It is able to detect the increase in fluorescence generated during a quantification test according to the TaqMan TM process, thanks to optical fibers located below each tube and connected to a CCD camera that detects, in real time , the signal emitted by the fluorescent groups released during the PCR.
- the quantitative data are derived from the determination of the cycle at which the signal of the amplification product reaches a certain threshold determined by the user.
- this number of cycles is proportional to the amount of initial material (Gibson, Heid et al., 1996, Heid, Stevens et al., 1996, Williams, Giles et al., 1998).
- the objective of the present invention is to propose such a system which makes it possible to considerably reduce the number of manipulations required to implement an amplification method on a plurality of target sequences and, consequently, to reduce the time required. necessary for this operation.
- Another object of the present invention is to provide such a system which minimizes the risk of contamination from one container to another.
- Another objective of the present invention is to propose such a system which reduces the volumes of reagents involved and therefore the costs.
- Another objective of the present invention is to propose such a system which optimizes a homogeneous distribution in volume and in concentration of the reagents necessary for the PCR in the containers.
- Another objective is to provide all potential users, including hospitals, medical laboratories, agribusiness and health control laboratories, an easy-to-use and easy-to-use device to routine quantified nucleic acid amplifications in real time.
- the invention relates firstly to any device according to one of claims 1 to 16.
- the temperature of each zone of the platen can be homogeneous or, where appropriate, this temperature can vary according to a gradient.
- reaction mixture Several types of molecular biology reactions require placing the reaction mixture at different temperatures as a function of time. This is the case, for example, when it is desired to inactivate an enzyme after using it (for example, a restriction nuclease), or to test the stability of a complex. In the latter case, it is conceivable to place a complex (for example, an antigen / antibody complex, or receptor / ligand), one of which is coupled to a fluorophore and the other to a quencher of fluorescence, one of the reaction chambers of the device. The stage is then programmed to present several temperatures in increasing order, possibly in the form of a gradient.
- a complex for example, an antigen / antibody complex, or receptor / ligand
- the stability of the complex is then tested by moving the cartridge on the plate, so that the temperature of the reaction chamber rises gradually, and observing the increase in fluorescence, using excitation means measurement of the fluorescence placed opposite the reaction chamber.
- the increase in fluorescence then reflects the dissociation of the complex.
- the device of the invention is particularly suitable for reactions requiring a cyclic variation in the temperature of the reaction chambers, which is the case for certain nucleic acid amplification reactions, for example for polymerase chain reaction (PCR ), or for the ligase chain reaction (LCR).
- PCR polymerase chain reaction
- LCR ligase chain reaction
- the invention therefore relates in particular to a device for the thermo-dependent chain amplification of target nucleic acid sequences according to one of claims 1 to 16.
- Such a system according to the invention is less complex than the systems of the prior art, insofar as the temperatures required for the cycles of the chain amplification are provided by distinct zones of constant temperatures and not by a plate of which one must vary the temperature.
- thermo-dependent chain amplification reactions require the passage of samples at at least two temperatures.
- the CSF requires at each cycle a phase at about 95 ° C to denature the target DNA, then a phase between 55 and 65 ° C (depending on the Tm probes) to give rise to hybridization / ligation .
- each cycle generally breaks down into three phases, namely denaturation at about 95 ° C, hybridization whose temperature depends on the Tm of the probes, and elongation, usually performed at 72 ° C. .
- nucleic acids to be amplified it is possible to dispense, from a reservoir, a fluid containing a sample of nucleic acids to be analyzed and the reagents necessary for the PCR in a plurality of reaction chambers containing specific primers of target sequences. nucleic acids to be amplified, and to allow the amplification process by subjecting the contents of the chambers successively to different temperatures (namely those necessary for denaturation, hybridization and elongation) a multitude of times through a movement relative between the cartridge including said reaction chambers and said heating platen having two or three distinct zones that can be brought to different temperatures.
- the reaction chambers (13) may contain reagents necessary for a real-time PCR reaction other than the primers mentioned above.
- the reaction chambers also comprise, in addition to the primers, one or more specific probe (s) of the sequence to be amplified.
- the distribution of the probes in the reaction chambers may also be such that some chambers contain probes specific for the sequences to be amplified and other chambers comprise control probes, not recognizing a priori the sequence to be amplified. These probes may be labeled and, if several probes are present in the same reaction chamber (for example a probe specific for the sequence to be amplified and a control probe), these probes will preferably be labeled with different fluorophores.
- additional reagents such as dNTPs or salts, are initially deposited in the reaction chambers. These reagents will then be absent, or present in a smaller amount, in the fluid deposited in the reservoir (11). In the extreme case, all the reagents necessary for the PCR reaction, with the exception of the matrix, are deposited in the reaction chambers (13), and the fluid deposited in the reservoir (11) will then comprise only the sample. DNA (or RNA) to amplify.
- the variants described above assume that several reactions are carried out in parallel, with different primers and / or probes, on the same sample. It is therefore the characterization of a single sample (or a few samples if the reservoir is divided into a few sub-reservoirs) according to several criteria. In some applications, on the contrary, it is desired to characterize a multitude of samples according to a single criterion or a small number of criteria. This is the case for example in research, when it is desired to screen a library of phages or bacteria for the presence of a given gene. In this case, it is necessary to carry out a PCR on a large number of samples, from a given pair of primers.
- the device of the invention is also suitable for this type of manipulation.
- the samples are deposited in the reaction chambers (13).
- the primers can be introduced into the fluid deposited in the reservoir (11), along with the other reagents necessary for the PCR.
- this configuration does not exclude that certain reagents other than the sample to be analyzed are pre-deposited in the reaction chambers (13).
- each reagent deposited in the reaction chambers (13) can be advantageously deposited there by a simple liquid deposit, followed by drying.
- the arrival of the fluid from the reservoir (11) then allows the re-solution of these reagents.
- the amount of each reagent deposited is calculated as a function of the volume of fluid that will penetrate into each reaction chamber (13), so that the reactivation of the reagents will result in the desired final concentration for each of them.
- Cartridges as described above, in which at least a portion of the reaction chambers (13) comprise reagents which have been loaded therein by a liquid deposit, followed by drying, so that these reagents are returned to solution by the arrival of a fluid in these reaction chambers, are also an integral part of the invention.
- the device described above has the advantage of allowing a concomitant filling of all the reaction chambers, which reduces the preparation time and the risks of contamination from one chamber to another.
- This device also has the advantage of being able to be miniaturized and to involve the use of smaller volumes of reagents than in the state of the art.
- the invention makes it possible to accelerate PCR cycles, since it is not necessary to perform the various phases (denaturation, hybridization, elongation). to vary the temperature of the heating stage or of the atmosphere as in the state of the art, the relative movement between the cartridge and the plate making it possible to quickly and successively submit the contents of each of the chambers reaction at three distinct temperatures dedicated to each of these phases.
- the use of small reaction volumes, and a thin floor for the cartridge (1), also limit the thermal inertia at the reaction chambers, and therefore contribute to the speed of the reaction.
- the invention also relates to a device for thermo-dependent chain amplification of target nucleic acid sequences, measured in real time, characterized in that it comprises the same elements as any of the devices described above. above, and further comprising optical means (5) for excitation / measurement of fluorescence, arranged to excite and measure at each cycle the fluorescence of the contents of the reaction chambers.
- the diameter of the channels will preferably be chosen small enough not to allow a gravity distribution of the fluid present in the tank in the reaction chambers, so as to avoid non-reproducible filling of these chambers. This diameter will thus preferably be less than or equal to approximately 0.2 mm. With regard to this diameter, it will be noted that the section of the channels will preferably be circular but that it may also be of any other shape and in particular polygonal, the "diameter" of the channels then aiming at their greatest width in section.
- the reservoir intended to receive the sample of nucleic acids and the reagents necessary for the PCR may have a variable capacity, for example between about 0.1 ml and about 1 ml.
- the cartridge preferably comprises between about 20 and about 500 reaction chambers and, more preferably, between 60 and 100 reaction chambers.
- these rooms may also vary according to the embodiments.
- these chambers have a volume of between approximately 0.2 and 50 ⁇ l, preferably between 1 ⁇ l and 10 ⁇ l.
- the junction between the channels (12) and the reservoir (11) is at the periphery of the reservoir, and the bottom of said reservoir is inclined and / or convex, so as to ensure the distribution of a fluid contained in the tank at the entrance of the channels.
- a cartridge used according to the invention may have multiple forms. However, according to a preferred variant of the invention, this cartridge has a circular shape.
- the reservoir is provided substantially in the center of the cartridge, the reaction chambers being distributed in a circle around the reservoir, and the channels connecting the reservoir to the chambers being provided essentially radially.
- Such an architecture allows to optimize the filling of the reaction chambers from the central tank.
- the bottom of the tank (11) is conical.
- reaction chambers are provided relative to the periphery of said cartridge.
- said reaction chambers are provided relative to the periphery of said cartridge.
- such a cartridge comprises as many channels as there are reaction chambers.
- the cartridge preferably has a diameter of between about 1 and 10 cm.
- a variant of the cartridges of the invention described above whatever their geometry, consists in dividing the reservoir (11) into 2 to 20, preferably 2 to 8 sub-reservoirs, making it possible to simultaneously analyze several samples on a same cartridge.
- each of the reaction chambers (13) is connected to only one of these sub-tanks by a channel (12).
- the depth of the reaction chambers may also vary depending on the embodiments of the invention. According to a preferred variant, these chambers have a depth of between approximately 0.5 mm and 1.5 mm.
- this cartridge depends on several factors including the material constituting it.
- this cartridge is preferably made of plastic material, preferably polycarbonate, whose physical, optical and thermal properties are suitable for carrying out the present invention.
- the thickness of the cartridges of the invention is preferably between 0.5 and 5 mm.
- the thickness of the "floor” thereof will preferably be as low as possible. This thickness depends on the material used to make the cartridge. Preferably, it is between 0.05 and 0.5 mm, for example about 0.25 mm.
- reaction chambers of the cartridges of the invention are preferably closed by a transparent upper wall (17), for example of transparent plastic, in order to allow the excitation and the measurement of the fluorescence of the reaction fluid, in good conditions.
- the chambers are provided with vents (open system), allowing the air they contain to escape during their filling by the fluid from the tank.
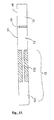
- the channels (12) preferably consist of at least two parts of different diameters (121 and 122), the diameter of the second part (122) being smaller than that of the first part (121), so as to create a pressure drop in the channel (12).
- the pressure drop phenomenon stops the progression of the fluid in the channel or channels whose first portion (121) is filled, until all the channels are filled in the same way. This allows to "pre-calibrate” the volumes for each channel, to ensure a homogeneous filling of the different reaction chambers.
- the second portion of the channel (122) may consist for example of a glass capillary, much smaller in diameter than the first portion (121), said capillary being included in a plastic cartridge.
- the cartridges described above provided for use in an open system or for use in a closed system, preferably comprise an opening that can be adapted to means (4) for modulating the pressure in the reservoir (11), making it possible to moving the fluid present in the reservoir to the reaction chambers.
- each channel (12) is equipped with an anti-reflux cavity (123) at its junction with the reservoir (11), said anti-reflux cavity consisting of a substantially vertical channel portion of a diameter greater than or equal to that of the channel (12).
- This variant has two main advantages. On the one hand, these anti-reflux cavities make it possible to prevent cross-contamination in the event of an inadvertent return of fluid to the reservoir (11), or in the case where all the fluid has not been engaged in the channels. On the other hand, these cavities make it possible to provide, in the devices of the invention, a plug whose serrations come to marry these vertical inlets, in order to plug the channels after the addressing of the editorial fluid but before the amplification reaction.
- each of the reaction chambers (13) comprises oligonucleotides. More preferably, each of the reaction chambers (13) comprises two primers specific for a nucleic acid sequence to be amplified and, optionally, one or more labeled probe (s) specific for said sequence.
- a probe may be labeled so that its signal is increased when it hybridizes to its target sequence (Sunrise TM system), or so that elongation from a strand on which it is hybridized causes a decrease or increase in the signal (AmpliSensor TM system or TaqMan TM system, respectively).
- probes in the reaction chambers makes it possible to carry out quantized amplifications in real time, with a device of the invention having means (5) for excitation / measurement of the fluorescence, as described above.
- Control probes nonspecific of the sequence to be amplified, and labeled in a different way from the specific probes, can also be used to detect possible contaminations.
- these different probes and primers will preferably be chosen such that their melting temperatures (Tm) respective ones are close.
- Tm melting temperatures
- the Tm of the different primers will preferably be in the same range of about 5 ° C.
- the different probes will preferably have a Tm within the same range of 5 ° C, which may be different from the range of Tm primers.
- the probes will be chosen so that their Tm is greater than that of the primers, the difference between Tm of the different categories of oligonucleotides then being preferably of the order of 5 ° C.
- the hybridization temperature chosen to carry out the amplification then corresponds to the lowest of the melting temperatures of the primers.
- the reaction chambers (13) of the cartridges of the invention may also comprise, in addition to the primers and any probes, one or more other reagents necessary for the PCR reaction or measurement of the amplification. It may be, for example, salts, dNTPs, or a fluorescent interlayer of double-stranded DNA, SybrGreen type (trademark). As mentioned above, all these reagents are advantageously deposited at the level of the reaction chambers (13) by the deposition of a liquid solution, followed by drying.
- the cartridges are intended for screening a large number of samples according to a small number of criteria. This implies that the user of these cartridges can easily deposit his samples in each of the reaction chambers (13).
- the cartridge can for example have a removable cover which, when removed, gives direct access to the reaction chambers.
- Such cartridges may also be pre-charged and comprise, in the reaction chambers (13), one or more reagents necessary for amplification and / or detection.
- the devices of the invention mentioned above may comprise one or more cartridges corresponding to any of the cartridges described above.
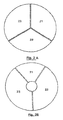
- the distinct heating zones of the heating plate (2) are preferentially distributed according to disk portions ( Figure 2A ) or crown ( Figure 2B ). Each portion may be heated to a different temperature to successively bring the contents of the reaction chambers to the desired different temperatures, by means of relative displacement means (3) between the cartridge (1) and the heating stage (2).
- the thermoblocks are preferably sufficiently wide to heat also part of the channels, as represented for example in FIG. figure 11 , as part of a rectangular cartridge.
- the number of separate heating zones can be two, three, or more.
- platinum may have a 95 ° C zone for denaturation of double-stranded nucleic acids, and a 60 ° C region for primer hybridization and elongation.
- the platinum will have an area at 95 ° C (denaturation), a region between 40 and 70 ° C (primer hybridization), and an area at 72 ° C (elongation).
- the plate may have a number of zones greater than three, for example to temporarily block the reaction at a given moment in each cycle.
- the platen can also have a number of zones that is a multiple of two or three, so that one revolution of the cartridge corresponds to several PCR cycles.
- the relative size of the different heating zones is advantageously chosen proportionally to the desired incubation time for the reaction fluid at the temperature of said zone.
- the thermoblock 21, dedicated to the denaturation step has a surface twice as small as that of the thermoblocks for the hybridization and elongation steps (blocks 22 and 23, respectively).
- the plate (2) is fixed and the cartridge (1) is moved by means of displacement (3).
- the displacement means (3) allow the rotation of said cartridge and / or said platen.
- a conductive element can be provided between the cartridge and the heating plate.
- said cartridge is in direct contact with said heating stage.
- said plate is advantageously provided with a coating promoting displacement between said cartridge and said plate.
- a coating may for example be made of Teflon (registered trademark).
- the system heating plate may have at least two or three zones that can be raised to different temperatures.
- this plate consists of two or three independent thermal blocks ("thermoblocks") connected to separate means for programming their temperature.
- thermoblocks independent thermal blocks
- the first of these thermoblocks (21) is heated to the denaturation temperature, the second (22) to the hybridization temperature, the third (23) to the elongation temperature.
- the use of such thermoblocks constant temperature simple realization of the heating stage.
- the relative displacement means of the cartridge relative to the platen can be realized in multiple forms.
- the cartridge (1) has on the underside a central projection (181) having a notch (182), so that the projecting portion (181) fits into the heating stage (2) and connects the cartridge (1).
- the displacement means (3) at a cleat or shaft (32) set in motion by a micromotor (31).
- the protruding part (181) thus makes it possible, on the one hand, to position the cartridge with respect to a plate (2) such as that represented in FIG. Figure 2B and, secondly, to ensure its connection with the moving means (3).
- the cartridge has at least one lug (183) and the displacement means (3) include at least one axis (32) cooperating with said lug to instill in said cartridge a rotary movement.
- the relative mode of movement between the plate and the cartridge may vary according to the embodiments. It may be a movement at continuous speed or in jerks. The speed of movement may be constant or vary over time.
- the system according to the invention also comprises optical means of excitation / fluorescence measurement, provided for example above or on the side of said cartridge.
- these means will constitute a single and fixed system.
- An advantage of a preferred variant of the invention according to which the cartridge is circular and moved in a rotary displacement is to be able to successively bring each reaction chamber under said optical system, thus reducing its complexity.
- a tracking system located for example on the cartridge (1), makes it possible to determine at each instant which reaction chamber is located opposite the optical system.
- the means for supplying the fluid present in said reservoir to said reaction chambers can be made in different forms. As has been described above, two categories of modes of addressing the fluid towards the reaction chambers can be distinguished: open system addressing, which assumes an increase in pressure at the reservoir and the presence of vents (14). ) at the level of the reaction chambers, and the addressing in closed system, which begins on the contrary by the establishment of a vacuum in the cartridge (1), followed by a recovery of this pressure.
- the means (4) for supplying the fluid into the reaction chambers differ according to the embodiment chosen.
- the fluid contained in the reservoir is distributed under pressure in the reaction chambers so as to allow uniform filling of these chambers.
- the feed means (4) preferably include a piston device (41) whose penetration rate in the tank will be calculated-to promote the proper filling of the reaction chambers, alternatively, these supply means include a pump connected to increase the pressure in the tank (11).
- another preferred variant of the invention involves working in a closed system.
- the fluid contained in the reservoir is then distributed in the reaction chambers as follows: in a first step, a vacuum is created inside the cartridge, where appropriate by a piston device or a pump (42), connected this time to reduce the pressure in the cartridge (1).
- the pressure is then restored, allowing the fluid to engage the channels and fill the peripheral reaction chambers.
- reagents necessary for the amplification reaction and / or the detection of the products of the amplification, and distinct from the primers and probes are pre-distributed in the reaction chambers (13). of the cartridge (1).
- the fluid introduced into the reservoir (11) does not then contain these reagents.
- the fluid distribution step in the reaction chambers (13) is carried out either by applying a vacuum inside the cartridge, then by restoring the pressure (closed system), or by increasing the pressure at the reservoir (11). ), provided that the reaction chambers are provided with vents (open system).
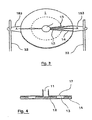
- the system for detecting and quantifying target nucleic sequences represented in figure 1 comprises a circular plastic cartridge 2 mm thick having a diameter of 5 cm.
- This cartridge (1) is provided with a central reservoir (11) and will be described in more detail with reference hereinafter to Figures 3 and 4 .
- the capacity of the reservoir is, in the context of the present embodiment, 400 .mu.l. Its floor is flat but it will be noted that in other embodiments it may be curved to facilitate the passage of fluid to the chambers without the formation of air bubbles, especially at the end of addressing when the reservoir is almost empty.
- the system further comprises a heating plate (2) in direct contact with the underside of the cartridge (1) and means (3) for moving the cartridge (1) relative to the heating plate (2).
- These displacement means include a micromotor (31) connected to two axes (32) which cooperate with two lugs (183) of the cartridge (1) to inculcate in it a rotary movement on the heating plate (2), the latter while remaining fixed.
- the described system also comprises a piston (41) intended to cooperate with said reservoir (11) as well as an optical device (5) for excitation / fluorescence measurement (emitting source allowing excitation at a given, programmable wavelength). and fluorescence receiver emitted) fixed and placed above the cartridge (1) and the heating plate (2).
- the heating plate (2) consists of three metal blocks (21, 22 and 23) (hereinafter referred to as thermoblocks) in the form of disk portions. Note that in this embodiment, these thermoblocks have substantially the same size but that, in other embodiments, they may have a different size, the size being understood as the occupied angular surface in top view.
- thermoblock (21, 22 and 23) is designed to be brought to a constant and programmable temperature, corresponding to one of the phases (denaturation, hybridization or elongation) amplification cycles (PCR), generally respectively 94 ° C for denaturation, 72 ° C for elongation, and between 30-40 and 65-70 ° C for hybridization according to the Tm (hybridization temperature) of the primers used, the temperatures of the thermoblocks can be controlled by all means known to those skilled in the art.
- phases denaturation, hybridization or elongation amplification cycles (PCR)
- Tm hybridization temperature
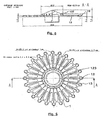
- the cartridge (1) is provided with a central tank (11) of capacity 400 ⁇ l connected to 36 reaction chambers (13) by as many channels (12), distributed uniformly over the entire periphery of the cartridge (on the figure 3 , we did not represent all the channels and rooms but only some of them).
- These reaction chambers (13) are further provided with vents (14) abutting on the edge of the cartridge (1).
- the channels have a diameter of 0.2 mm and the volume of the reaction chambers is 2.5 microliters. In other embodiments, this diameter and this volume may of course be different.
- this cartridge (1) is also provided with two lugs (183) each pierced with an orifice for passing an axis (32) connected to the micromotor (31).
- the reaction chambers have a depth of 1 mm. Their floor has a thickness of about 0.2 mm. This thickness is sufficiently small to facilitate good heat exchange between the chambers (13) and the thermoblocks (21, 22 and 23).
- the reaction chambers (13) are closed in their upper part by a wall (17) transparent, also forming the wall of the tank (11).
- Each chamber 10 except a few for negative control purposes, contains two primers specific for a target sequence to be amplified, and optionally one or more labeled probes, allowing a subsequent specific measurement of fluorescence.
- 10 ng of each primer were distributed in each chamber except for those serving as a negative control.
- the piston (41) After partially filling the reservoir (11) with the fluid whose volume is equal to the sum of the volumes of the chambers (the volume of a chamber is defined as being the product of its "floor” surface by its depth), the piston (41) is actuated to dispense this fluid into the plurality of reaction chambers (13).
- This piston increases the pressure within the reservoir (11) and allows the passage of fluid in the channels to the chambers.
- the speed of movement of the piston in the reservoir is about 1 mm per second and said displacement is stopped at a level that depends on the volume of fluid to be addressed in the chambers.
- the small diameter of the channels (12) makes it possible to prevent the diffusion of the fluid from the reservoir (11) to the channels (12) and the chambers (13) under the effect of gravity (at this scale, the processes are usually negligible as the capillary forces become pregnant, and in this case are enough to keep the fluid in the tank). Thanks to the vents (14), the air present in the chambers (13) is evacuated, which ensures the filling thereof.
- thermoblocks (21, 22, 23) are brought to the three temperatures corresponding to the three temperatures of the PCR phases (or at slightly higher temperatures given the possible heat losses between the heating stage (2) and the cartridge 1) and the displacement means (3) are implemented so as to animate the cartridge (1) by a gyratory movement in order to pass each reaction chamber successively and as many times as desired over the three thermoblocks.
- the block (21) is brought to the temperature corresponding to the denaturation phase (94 ° C.)
- the thermoblock (22) is brought to the temperature corresponding to the hybridization phase (36 ° C.)
- the thermoblock (23) is brought to the temperature corresponding to the phase of elongation (72 ° C).
- the micromotor (31) of the displacement means (3) is adapted to inculcate a rotation of 10 degrees every 2.5 seconds to the cartridge (1) (ie a 1.5 PCR cycle). min).
- this movement may have a different speed and be continuous instead of jerky.
- the optical device (5) is provided above the corresponding block 23 raised to a temperature corresponding to the elongation temperature, and more particularly to a location corresponding to the end of the elongation phase.
- the optical device (5) can be placed at a different location, chosen in particular according to the chemistry used. For example, using TaqMan TM chemistry or nonspecific fluorescence, it makes sense to perform the measurement at the end of the elongation phase, as described above.
- the use of a Molecular Beacons TM type chemistry implies that the measurement is done rather at the time of the hybridization.
- the system presented makes it possible to rapidly and reproducibly fill a large quantity of reaction chambers and to carry out PCR and fluorescence measurements on each PCR cycle.
- FIGS. 5 to 10 represent an example of a circular cartridge exhibiting certain modifications with respect to the cartridge of example 1.
- This cartridge is intended for use in a closed system, that is to say that the reaction chambers (13) have no other opening than the arrival of the channel (12).
- the cartridge consists of two elements that fit into each other: the lower part, or base, is represented in Figures 5 and 6 , and the upper part, or cover, is represented in Figures 7 and 8 . The assembly of the two is illustrated in figures 9 and 10 .
- This cartridge is loaded as follows:
- the fluid is no longer addressed by an increase in pressure but by depression, which presents the advantage of not requiring a vent and therefore working in a closed system.
- the bottom of the tank has a conical shape allowing the fluid to be distributed at its periphery, that is to say near the entrance of the channels.
- an anti-reflux system consisting of a vertical channel portion (123), which, on the one hand, prevents cross contamination in the event of inadvertent return of fluid to the central part or in the case where all the fluid would not be engaged in the channel and, on the other hand, allows once the addressing done but before the PCR, to come to plug the channels by means of a plug whose serrations come to marry these vertical entries, to work in closed system (no contamination, no evaporation).
- the cartridge is made of plastic, preferably polycarbonate because this polymer has interesting physical, optical and thermal behavior characteristics.
- the size of the channels is for example 0.4 x 0.2 mm (half-moon) in section.
- the size of the consumable is for example 100 mm (diameter), the number of chambers is 80, the number of sub-tanks is between 1 and 8.
- the cartridge (1) has on the underside a central projection (181) having a notch (182), so that the projecting portion (181) fits into the heating stage (2) and connects the cartridge (1). ) with the moving means (3) at a cleat or shaft (32) moved by a micromotor (31).
- the protruding part (181) thus makes it possible, on the one hand, to position the cartridge with respect to a plate (2) such as that represented in FIG. Figure 2B and, secondly, to ensure its connection with the moving means (3).
- the reaction chambers are loaded with primers specific for target sequences and, where appropriate, with TaqMan TM or other probes specific for said targets.
- the targets will be viral or bacterial genes, junctions between a transgene and the genome of a plant to detect and / or identify certain GMOs, etc.
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Clinical Laboratory Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Hematology (AREA)
- Analytical Chemistry (AREA)
- Dispersion Chemistry (AREA)
- Molecular Biology (AREA)
- Biochemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
- Apparatus Associated With Microorganisms And Enzymes (AREA)
- Physical Or Chemical Processes And Apparatus (AREA)
- Investigating Or Analysing Materials By The Use Of Chemical Reactions (AREA)
- Automatic Analysis And Handling Materials Therefor (AREA)
- Feeding, Discharge, Calcimining, Fusing, And Gas-Generation Devices (AREA)
- Saccharide Compounds (AREA)
- Compounds Of Unknown Constitution (AREA)
- Polymers With Sulfur, Phosphorus Or Metals In The Main Chain (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
Claims (18)
- Vorrichtung zur Durchführung enyzmatischer Reaktionen und/oder Molekularbiologie-Reaktionen, die zumindest zwei verschiedene Inkubationstemperaturen benötigen, umfassend zumindest eine kreisförmige Kartusche (1), die mehrere Reaktionskammern (13) aufweist, in denen vorab Initiatoren, ein Behälter (11) und Kanäle (12) angeordnet wurden, gekennzeichnet durch- diese Kartusche(1), die eine Rotationsgeometrie aufweist, wobei der Behälter (11) im Wesentlichen in der Mitte angeordnet ist, wobei die Reaktionskammern (13) kreisförmig um den Behälter verteilt sind und die Kanäle (12) den Behälter (11) mit den Kammern (13) verbinden, wobei die Kanäle insbesondere im Wesentlichen in radialer Richtung vorgesehen sind;- zumindest eine Heizplatte (2), die zumindest zwei unterschiedliche Zonen aufweist, die auf zumindest zwei verschiedene Temperaturen gebracht werden können, wobei die Heizplatte insbesondere drei unterschiedliche Bereiche aufweist, die auf drei verschiedene Temperaturen gebracht werden können;- Mittel (3) für die relative Bewegung zwischen der Kartusche und der Platte, um eine zyklische Veränderung der Temperatur der Reaktionskammern zu ermöglichen.
- Vorrichtung nach Anspruch 1, wobei die Reaktionskammern (13) am Umfang der Kartusche vorgesehen sind.
- Vorrichtung nach Anspruch 1 oder 2, wobei die enzymatische Reaktion eine temperaturabhängige Amplifikations-Kettenreaktion von Nukleinsäure-Sequenzen ist und wobei die Bereiche der Heizplatte (2) auf zumindest zwei verschiedene Temperaturen gebracht werden können, die den Phasen der Zyklen für die Amplifikation der Nukleinsäuren entsprechen.
- Vorrichtung nach Anspruch 3, dadurch gekennzeichnet, dass spezifische Initiatoren von zu amplifizierenden Zielsequenzen in den Reaktionskammern (13) vorab verteilt werden,
der Behälter (11) für die Aufnahme eines Fluids vorgesehen ist, das insbesondere von einer zu analysierenden Nukleinsäure-Probe und Reagenzien gebildet ist, die für eine Polymerase-Amplifikations-Kettenreaktion notwendig sind, mit Ausnahme der Initiatoren,
die Heizplatte (2) drei unterschiedliche Bereiche aufweist, die auf drei verschiedene Temperaturen gebracht werden können, die den drei Phasen der Zyklen für die Polymerase-Amplifikations-Kettenreaktion entsprechen. - Vorrichtung nach Anspruch 3 oder 4, für die temperaturabhängige Amplifikations-Kettenreaktion von Nukleinsäure-Sequenzen, die in Echtzeit gemessen wird, dadurch gekennzeichnet, dass sie optische Mittel (5) für die Anregung/Messung der Fluoreszenz umfasst, die derart angeordnet sind, dass sie bei jedem Zyklus die Fluoreszenz des Inhalts der Reaktionskammern anregen und messen.
- Vorrichtung nach einem der Ansprüche 1 bis 5, wobei die unterschiedlichen Heizbereiche der Platte (2) als zumindest zwei oder drei Scheibenteile aufgeteilt sind.
- Vorrichtung nach einem der Ansprüche 1 bis 5, wobei die unterschiedlichen Heizbereiche der Platte (2) als zumindest zwei oder drei Ringteile aufgeteilt sind.
- Vorrichtung nach einem der Ansprüche 1 bis 7, wobei die Bewegungsmittel (3) es ermöglichen, die Kartusche (1) und/oder die Heizplatte (2) in eine Drehung zu versetzen.
- Vorrichtung nach einem der Ansprüche 1 bis 8, wobei die Kartusche (1) in direktem Kontakt mit der Heizplatte (2) ist.
- Vorrichtung nach einem der Ansprüche 1 bis 9, wobei die Platte (2) mit einer Beschichtung versehen ist, welche die relative Bewegung zwischen der Kartusche (1) und der Platte (2) begünstigt.
- Vorrichtung nach einem der Ansprüche 1 bis 10, wobei die Heizplatte (2) zwei oder drei unterschiedliche Thermoblöcke (21, 22 und gegebenenfalls 23) aufweist, die mit Mitteln zur Programmierung ihrer Temperatur verbunden sind.
- Vorrichtung entsprechend einem der Ansprüche 1 bis 11, die optische Mittel (5) für die Anregung/Messung der Fluoreszenz aufweist, die oberhalb oder seitlich zur Kartusche angeordnet sind.
- Vorrichtung entsprechend einem der Ansprüche 1 bis 12, die ferner Mittel (4) für die Zuführung des in dem Behälter (11) vorhandenen Fluids in die Reaktionskammern (13) aufweist.
- Vorrichtung nach Anspruch 13, wobei die Zuführmittel (4) eine Kolben-Vorrichtung (41) einschließen und das Fluid in die Reaktionskammern durch eine Erhöhung des Drucks zugeführt wird.
- Vorrichtung nach Anspruch 13, wobei die Zuführmittel (4) eine Pumpe (41) einschließen und das Fluid in die Reaktionskammern durch eine Wiederherstellung des Drucks nach einer Erzeugung eines Unterdrucks zugeführt wird.
- Vorrichtung nach Anspruch 5, wobei die Reaktionskammern (13) der Kartusche (1) verschlossen sind.
- Verfahren zur Amplifikation von Nukleinsäure mit Hilfe einer Vorrichtung nach einem der Ansprüche 1 bis 16, das die folgenden Schritte aufweist:- zumindest teilweises Befüllen des Behälters (11) mit einem Fluid, das eine zu analysierende Nukleinsäure-Probe sowie all das für eine Amplifikations-Kettenreaktion Notwendige mit Ausnahme der Initiatoren und, gegebenenfalls, eine fluoreszierende Einlagerung der Nukleinsäuren enthält,- Verteilen des Fluids in den Reaktionskammern (13) der Kartusche (1), in denen vorab Initiatoren und, gegebenenfalls, eine oder mehrere markierte Sonden angeordnet wurden,- Einsetzen der Mittel (3) für die relative Bewegung zwischen der Kartusche und der Heizplatte, um nacheinander und so oft wie gewünscht den Inhalt jeder Reaktionskammer auf zwei, drei oder mehr Temperaturen zu bringen, die durch die zwei, drei oder mehr Bereiche der Heizplatte (2) festgelegt werden.
- Amplifikationsverfahren nach Anspruch 17, wobei der Schritt der Verteilung des Fluids in den Reaktionskammern (13) durchgeführt wird, indem ein Unterdruck im Innern der Kartusche angewendet wird und anschließend der Druck wiederhergestellt wird.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR0010029A FR2812306B1 (fr) | 2000-07-28 | 2000-07-28 | Systeme d'amplification en chaine par polymerse de sequences nucleiques cibles |
| EP01956628A EP1305115B1 (de) | 2000-07-28 | 2001-07-20 | Vorrichtung zur pcr-amplifizierung von ziel-dna-sequenzen |
Related Parent Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP01956628.0 Division | 2001-07-20 | ||
| EP01956628A Division EP1305115B1 (de) | 2000-07-28 | 2001-07-20 | Vorrichtung zur pcr-amplifizierung von ziel-dna-sequenzen |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP2269738A1 EP2269738A1 (de) | 2011-01-05 |
| EP2269738B1 true EP2269738B1 (de) | 2012-08-29 |
Family
ID=8853103
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP10177401A Expired - Lifetime EP2269738B1 (de) | 2000-07-28 | 2001-07-20 | Vorrichtung zur PCR-Amplifizierung von Ziel-DNA-Sequenzen |
| EP01956628A Expired - Lifetime EP1305115B1 (de) | 2000-07-28 | 2001-07-20 | Vorrichtung zur pcr-amplifizierung von ziel-dna-sequenzen |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP01956628A Expired - Lifetime EP1305115B1 (de) | 2000-07-28 | 2001-07-20 | Vorrichtung zur pcr-amplifizierung von ziel-dna-sequenzen |
Country Status (14)
| Country | Link |
|---|---|
| US (2) | US6821771B2 (de) |
| EP (2) | EP2269738B1 (de) |
| JP (2) | JP4979873B2 (de) |
| CN (1) | CN1248781C (de) |
| AT (1) | ATE532583T1 (de) |
| AU (2) | AU2001278554B2 (de) |
| BR (1) | BR0112789A (de) |
| CA (1) | CA2416756C (de) |
| DK (1) | DK2269738T3 (de) |
| EA (1) | EA004719B1 (de) |
| ES (2) | ES2372027T3 (de) |
| FR (1) | FR2812306B1 (de) |
| WO (1) | WO2002009877A1 (de) |
| ZA (1) | ZA200300700B (de) |
Families Citing this family (70)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2844522A1 (fr) * | 2002-09-13 | 2004-03-19 | Genesystems | Procede et sequences nucleotidiques pour la detection et l'identification de microorganismes dans un melange complexe |
| FR2844523B1 (fr) * | 2002-09-13 | 2011-01-14 | Genesystems | Procede et sequences nucleotidiques pour la detection et l'identification de microorganismes dans un melange complexe ou dans de l'eau |
| JP2005125311A (ja) * | 2003-09-30 | 2005-05-19 | Japan Science & Technology Agency | 化学反応装置 |
| DE10360220A1 (de) | 2003-12-20 | 2005-07-21 | Steag Microparts Gmbh | Mikrostrukturierte Anordnung zur blasenfreien Befüllung zumindest eines Systems zur Ableitung von Flüssigkeiten, Vorrichtung mit einer solchen Anordnung und Befüllungsverfahren |
| US7709249B2 (en) * | 2005-04-01 | 2010-05-04 | 3M Innovative Properties Company | Multiplex fluorescence detection device having fiber bundle coupling multiple optical modules to a common detector |
| US7507575B2 (en) * | 2005-04-01 | 2009-03-24 | 3M Innovative Properties Company | Multiplex fluorescence detection device having removable optical modules |
| US20060246493A1 (en) | 2005-04-04 | 2006-11-02 | Caliper Life Sciences, Inc. | Method and apparatus for use in temperature controlled processing of microfluidic samples |
| JP5086250B2 (ja) * | 2005-06-23 | 2012-11-28 | ビオカルティ ソシエテ アノニム | 自動医療診断用のカートリッジ、システム及び方法 |
| CN101213022B (zh) * | 2005-06-30 | 2010-06-09 | 拜欧卡蒂斯股份公司 | 自动医学诊断盒体 |
| US20070009382A1 (en) * | 2005-07-05 | 2007-01-11 | William Bedingham | Heating element for a rotating multiplex fluorescence detection device |
| US7527763B2 (en) | 2005-07-05 | 2009-05-05 | 3M Innovative Properties Company | Valve control system for a rotating multiplex fluorescence detection device |
| US11806718B2 (en) | 2006-03-24 | 2023-11-07 | Handylab, Inc. | Fluorescence detector for microfluidic diagnostic system |
| JP4626891B2 (ja) * | 2007-01-29 | 2011-02-09 | ヤマハ株式会社 | 温度制御装置 |
| JP5141039B2 (ja) * | 2007-02-22 | 2013-02-13 | 東洋紡株式会社 | 核酸増幅装置及び核酸増幅方法 |
| CN101802163A (zh) | 2007-05-23 | 2010-08-11 | 信诚医疗有限公司 | 反应液用容器,使用这种容器的反应促进装置,及其方法 |
| US8951732B2 (en) * | 2007-06-22 | 2015-02-10 | Advanced Liquid Logic, Inc. | Droplet-based nucleic acid amplification in a temperature gradient |
| US9186677B2 (en) | 2007-07-13 | 2015-11-17 | Handylab, Inc. | Integrated apparatus for performing nucleic acid extraction and diagnostic testing on multiple biological samples |
| JP5600293B2 (ja) | 2007-07-27 | 2014-10-01 | アール ツリー イノベーションズ エルエルシー | 椎間体移植システムおよびその方法 |
| EP2062982A1 (de) * | 2007-11-26 | 2009-05-27 | ImmunID | Verfahren zur Untersuchung der kombinatorischen Diversität von V(D)J. |
| US20160068905A1 (en) | 2007-11-26 | 2016-03-10 | Immunid | Method for Studying V(D)J Combinatory Diversity |
| JP2009240296A (ja) * | 2007-12-14 | 2009-10-22 | Ngk Insulators Ltd | 流体収容カートリッジ及びその利用 |
| US9121055B2 (en) * | 2008-04-24 | 2015-09-01 | 3M Innovative Properties Company | Analysis of nucleic acid amplification curves using wavelet transformation |
| JP5372418B2 (ja) * | 2008-06-23 | 2013-12-18 | 株式会社日立ハイテクノロジーズ | 核酸分析装置,自動分析装置、及び分析方法 |
| EP2143491A1 (de) * | 2008-07-10 | 2010-01-13 | Carpegen GmbH | Vorrichtung zum Analysieren einer chemischen oder biologischen Probe |
| AT507376B1 (de) * | 2008-08-29 | 2013-09-15 | Anagnostics Bioanalysis Gmbh | Vorrichtung zum temperieren eines rotationssymetrischen behältnisses |
| WO2012033396A1 (en) * | 2008-12-18 | 2012-03-15 | Universiti Sains Malaysia | A disposable multiplex polymerase chain reaction (pcr) chip and device |
| US9399219B2 (en) * | 2009-02-13 | 2016-07-26 | Frank Leo Spangler | Thermal Array |
| US20120028819A1 (en) * | 2009-04-07 | 2012-02-02 | Koninklijke Philips Electronics N.V. | Method for the detection and characterization of a toxinogenic clostridium difficile strain |
| KR101666947B1 (ko) * | 2009-05-20 | 2016-10-17 | 스미스 인터내셔널 인크. | 절삭 요소, 절삭 요소의 제조 방법 및, 절삭 요소를 포함하는 공구 |
| DE112010002222B4 (de) | 2009-06-04 | 2024-01-25 | Leidos Innovations Technology, Inc. (n.d.Ges.d. Staates Delaware) | Mehr-Proben-Mikrofluidchip fur DNA-Analyse |
| JP2011062119A (ja) * | 2009-09-16 | 2011-03-31 | Seiko Epson Corp | 生体試料定量用チップ |
| DE102009044431A1 (de) * | 2009-11-05 | 2011-06-22 | FRIZ Biochem Gesellschaft für Bioanalytik mbH, 82061 | Vorrichtung zur Durchführung einer PCR |
| US8906624B2 (en) * | 2010-03-30 | 2014-12-09 | Korea Advanced Institute Of Science And Technology (Kaist) | Rotational PCR equipment and PCR method using the same |
| JP5249988B2 (ja) * | 2010-05-07 | 2013-07-31 | 株式会社日立ハイテクノロジーズ | 核酸増幅装置及びそれを用いた核酸検査装置 |
| US20110312546A1 (en) * | 2010-06-17 | 2011-12-22 | Geneasys Pty Ltd | Loc device for pathogen detection and genetic analysis with chemical lysis, incubation and tandem nucleic acid amplification |
| WO2012051529A1 (en) | 2010-10-15 | 2012-04-19 | Lockheed Martin Corporation | Micro fluidic optic design |
| EP2450690A1 (de) * | 2010-11-04 | 2012-05-09 | Qiagen GmbH | Gefäss für exakte optische Messungen |
| CN104040238B (zh) * | 2011-11-04 | 2017-06-27 | 汉迪拉布公司 | 多核苷酸样品制备装置 |
| US9322054B2 (en) | 2012-02-22 | 2016-04-26 | Lockheed Martin Corporation | Microfluidic cartridge |
| US9303281B2 (en) | 2012-07-23 | 2016-04-05 | Pall Corporation | Compositions for detecting foodstuff spoilage microorganisms |
| US9382591B2 (en) | 2013-06-06 | 2016-07-05 | Pall Corporation | Compositions for detecting Alicyclobacillus microorganisms |
| EP2843057B1 (de) | 2013-09-03 | 2017-12-27 | Pall Genedisc Technologies | Verfahren zur Bestimmung des Vorhandenseins oder Nichtvorhandenseins von Shiga-Toxin produzierenden Escherichia Coli (STEC) in einer Lebensmittelprobe |
| KR101618113B1 (ko) * | 2014-02-10 | 2016-05-09 | 나노바이오시스 주식회사 | 일 방향 슬라이딩 구동 수단을 구비하는 pcr 장치 및 이를 이용하는 pcr 방법 |
| US10195610B2 (en) | 2014-03-10 | 2019-02-05 | Click Diagnostics, Inc. | Cartridge-based thermocycler |
| JP2016049064A (ja) * | 2014-09-01 | 2016-04-11 | 国立研究開発法人産業技術総合研究所 | マイクロチップを用いたpcr装置 |
| JP6778194B2 (ja) | 2014-12-31 | 2020-10-28 | ビスビュー メディカル,インコーポレイテッド | 分子診断試験のためのデバイス及び方法 |
| US9828626B2 (en) | 2015-07-10 | 2017-11-28 | Pall Corporation | Dendrimer conjugates for determining membrane retention level and/or pore structure |
| CN109642198A (zh) * | 2016-02-10 | 2019-04-16 | 卡尤迪生物科技(北京)有限公司 | 用于分析核酸的方法和系统 |
| US10987674B2 (en) | 2016-04-22 | 2021-04-27 | Visby Medical, Inc. | Printed circuit board heater for an amplification module |
| WO2017197040A1 (en) | 2016-05-11 | 2017-11-16 | Click Diagnostics, Inc. | Devices and methods for nucleic acid extraction |
| CN110325652A (zh) | 2016-06-29 | 2019-10-11 | 易捷仪器诊断股份有限公司 | 使用流动池检测分子的装置和方法 |
| USD800331S1 (en) | 2016-06-29 | 2017-10-17 | Click Diagnostics, Inc. | Molecular diagnostic device |
| USD800914S1 (en) | 2016-06-30 | 2017-10-24 | Click Diagnostics, Inc. | Status indicator for molecular diagnostic device |
| USD800913S1 (en) | 2016-06-30 | 2017-10-24 | Click Diagnostics, Inc. | Detection window for molecular diagnostic device |
| CN108738349A (zh) * | 2016-12-28 | 2018-11-02 | 达纳福股份有限公司 | 分析装置 |
| JP7239568B2 (ja) | 2017-11-09 | 2023-03-14 | ビスビュー メディカル,インコーポレイテッド | 携帯型分子診断デバイスおよび標的ウイルスの検出方法 |
| KR102206856B1 (ko) * | 2017-12-11 | 2021-01-25 | (주)바이오니아 | 중합효소 연쇄반응 시스템 |
| KR102065650B1 (ko) * | 2017-12-28 | 2020-02-11 | 에스디 바이오센서 주식회사 | 카트리지를 이용한 핵산 추출 방법 |
| KR102076220B1 (ko) | 2017-12-28 | 2020-02-11 | 에스디 바이오센서 주식회사 | 핵산 추출용 카트리지의 유로 구조 |
| MX2021002533A (es) | 2018-09-03 | 2021-05-27 | Visby Medical Inc | Dispositivos y metodos para pruebas de susceptibilidad a antibioticos. |
| KR102009505B1 (ko) * | 2019-01-17 | 2019-08-12 | 주식회사 엘지화학 | 유전자 증폭 모듈 |
| US11254979B2 (en) * | 2020-06-01 | 2022-02-22 | Shaheen Innovations Holding Limited | Systems and devices for infectious disease screening |
| US11730193B2 (en) | 2019-12-15 | 2023-08-22 | Shaheen Innovations Holding Limited | Hookah device |
| EP4085149A4 (de) | 2020-01-03 | 2024-03-06 | Visby Medical, Inc. | Vorrichtungen und verfahren zur prüfung von antibiotischer suszeptibilität |
| CN111607484A (zh) * | 2020-05-22 | 2020-09-01 | 东莞市东阳光诊断产品有限公司 | 一种核酸扩增装置及方法 |
| CA3180786A1 (en) | 2020-06-01 | 2021-05-28 | Imad Lahoud | An infectious disease screening system |
| IL298682B1 (en) | 2020-06-01 | 2025-10-01 | Shaheen Innovations Holding Ltd | Infectious disease scanning device |
| CN113583854B (zh) * | 2021-07-30 | 2024-02-27 | 广州和实生物技术有限公司 | 一种可家庭自测的核酸检测装置、检测方法及用途 |
| USD1064314S1 (en) | 2021-08-13 | 2025-02-25 | Visby Medical, Inc. | Molecular diagnostic device |
| GB202305080D0 (en) * | 2023-04-05 | 2023-05-17 | Anglia Ruskin Univ Higher Education Corporation | Methods and devices for nucleic acid amplification |
Family Cites Families (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1572596A (en) * | 1976-12-06 | 1980-07-30 | Opto Electronic Displays Ltd | Apparatus and method for innoculation |
| DE8813773U1 (de) * | 1988-11-03 | 1989-01-05 | Max-Planck-Gesellschaft zur Förderung der Wissenschaften eV, 37073 Göttingen | Gerät zum wahlweisen Einstellen der Temperatur einer Probe auf verschiedene Werte |
| FR2672231A1 (fr) * | 1991-02-01 | 1992-08-07 | Eibet | Appareil d'execution automatique repetee d'un cycle thermique, notamment pour l'amplification du nombre d'une sequence definie d'acide nucleique. |
| DE4234086A1 (de) * | 1992-02-05 | 1993-08-12 | Diagen Inst Molekularbio | Verfahren zur bestimmung von in vitro amplifizierten nukleinsaeuresequenzen |
| US5525300A (en) * | 1993-10-20 | 1996-06-11 | Stratagene | Thermal cycler including a temperature gradient block |
| US5639428A (en) * | 1994-07-19 | 1997-06-17 | Becton Dickinson And Company | Method and apparatus for fully automated nucleic acid amplification, nucleic acid assay and immunoassay |
| US5556771A (en) * | 1995-02-10 | 1996-09-17 | Gen-Probe Incorporated | Stabilized compositions of reverse transcriptase and RNA polymerase for nucleic acid amplification |
| US5609828A (en) * | 1995-05-31 | 1997-03-11 | bio M erieux Vitek, Inc. | Sample card |
| AU706862B2 (en) * | 1996-04-03 | 1999-06-24 | Applied Biosystems, Llc | Device and method for multiple analyte detection |
| WO1998005060A1 (en) * | 1996-07-31 | 1998-02-05 | The Board Of Trustees Of The Leland Stanford Junior University | Multizone bake/chill thermal cycling module |
| AUPO652997A0 (en) * | 1997-04-30 | 1997-05-29 | Kindconi Pty Limited | Temperature cycling device and method |
| US6063589A (en) * | 1997-05-23 | 2000-05-16 | Gamera Bioscience Corporation | Devices and methods for using centripetal acceleration to drive fluid movement on a microfluidics system |
| DE19810499A1 (de) * | 1998-03-11 | 1999-09-16 | Microparts Gmbh | Mikrotiterplatte |
| WO1999046045A1 (de) * | 1998-03-11 | 1999-09-16 | MICROPARTS GESELLSCHAFT FüR MIKROSTRUKTURTECHNIK MBH | Probenträger |
| DE19852835A1 (de) * | 1998-11-17 | 2000-05-18 | Stratec Biomedical Systems Ag | Probenträger |
| CA2255850C (en) * | 1998-12-07 | 2000-10-17 | Her Majesty The Queen In Right Of Canada As Represented By The Minister Of Agriculture And Agri-Food | Rotary thermocycling apparatus |
| US6303343B1 (en) * | 1999-04-06 | 2001-10-16 | Caliper Technologies Corp. | Inefficient fast PCR |
| US6706519B1 (en) * | 1999-06-22 | 2004-03-16 | Tecan Trading Ag | Devices and methods for the performance of miniaturized in vitro amplification assays |
| JP3623479B2 (ja) * | 1999-06-22 | 2005-02-23 | テカン トレーディング アーゲー | 小型化されたインビトロ増幅アッセイを行うための装置および方法 |
| US6272939B1 (en) * | 1999-10-15 | 2001-08-14 | Applera Corporation | System and method for filling a substrate with a liquid sample |
| US6720148B1 (en) * | 2001-02-22 | 2004-04-13 | Caliper Life Sciences, Inc. | Methods and systems for identifying nucleotides by primer extension |
-
2000
- 2000-07-28 FR FR0010029A patent/FR2812306B1/fr not_active Expired - Lifetime
-
2001
- 2001-07-20 BR BR0112789-6A patent/BR0112789A/pt not_active Application Discontinuation
- 2001-07-20 WO PCT/FR2001/002385 patent/WO2002009877A1/fr not_active Ceased
- 2001-07-20 AU AU2001278554A patent/AU2001278554B2/en not_active Expired
- 2001-07-20 ES ES01956628T patent/ES2372027T3/es not_active Expired - Lifetime
- 2001-07-20 AU AU7855401A patent/AU7855401A/xx active Pending
- 2001-07-20 CA CA002416756A patent/CA2416756C/fr not_active Expired - Lifetime
- 2001-07-20 ES ES10177401T patent/ES2389763T3/es not_active Expired - Lifetime
- 2001-07-20 CN CN01815876.5A patent/CN1248781C/zh not_active Expired - Lifetime
- 2001-07-20 AT AT01956628T patent/ATE532583T1/de active
- 2001-07-20 EP EP10177401A patent/EP2269738B1/de not_active Expired - Lifetime
- 2001-07-20 EA EA200300203A patent/EA004719B1/ru not_active IP Right Cessation
- 2001-07-20 JP JP2002515419A patent/JP4979873B2/ja not_active Expired - Lifetime
- 2001-07-20 DK DK10177401.6T patent/DK2269738T3/da active
- 2001-07-20 EP EP01956628A patent/EP1305115B1/de not_active Expired - Lifetime
- 2001-10-15 US US09/981,070 patent/US6821771B2/en not_active Expired - Lifetime
-
2003
- 2003-01-27 ZA ZA200300700A patent/ZA200300700B/en unknown
-
2004
- 2004-08-25 US US10/926,482 patent/US7732136B2/en not_active Expired - Fee Related
-
2011
- 2011-05-26 JP JP2011118127A patent/JP5202686B2/ja not_active Expired - Lifetime
Also Published As
| Publication number | Publication date |
|---|---|
| US6821771B2 (en) | 2004-11-23 |
| DK2269738T3 (da) | 2013-01-02 |
| WO2002009877A1 (fr) | 2002-02-07 |
| JP5202686B2 (ja) | 2013-06-05 |
| EA200300203A1 (ru) | 2003-06-26 |
| BR0112789A (pt) | 2003-09-09 |
| JP2011200245A (ja) | 2011-10-13 |
| EA004719B1 (ru) | 2004-08-26 |
| JP2004504828A (ja) | 2004-02-19 |
| FR2812306B1 (fr) | 2005-01-14 |
| EP2269738A1 (de) | 2011-01-05 |
| ES2389763T3 (es) | 2012-10-31 |
| AU2001278554B2 (en) | 2006-09-28 |
| US20050026277A1 (en) | 2005-02-03 |
| JP4979873B2 (ja) | 2012-07-18 |
| EP1305115B1 (de) | 2011-11-09 |
| US7732136B2 (en) | 2010-06-08 |
| ZA200300700B (en) | 2004-03-10 |
| FR2812306A1 (fr) | 2002-02-01 |
| US20020081669A1 (en) | 2002-06-27 |
| EP1305115A1 (de) | 2003-05-02 |
| ATE532583T1 (de) | 2011-11-15 |
| CN1458866A (zh) | 2003-11-26 |
| CA2416756C (fr) | 2010-01-19 |
| ES2372027T3 (es) | 2012-01-13 |
| AU7855401A (en) | 2002-02-13 |
| CA2416756A1 (fr) | 2002-02-07 |
| CN1248781C (zh) | 2006-04-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2269738B1 (de) | Vorrichtung zur PCR-Amplifizierung von Ziel-DNA-Sequenzen | |
| CN101275164B (zh) | 试样调制法及装置 | |
| US11142790B2 (en) | Microfluidic device | |
| US20220186325A1 (en) | Systems for sample analysis | |
| KR101851117B1 (ko) | 샘플-투-앤서 마이크로유체 카트리지 | |
| BE1020816A5 (fr) | Procede et dispositif pour la detection rapide de sequences nucleotidiques amplifiees. | |
| JP6190950B2 (ja) | 液滴保存方法 | |
| US20100173794A1 (en) | Device for amplification and detection of nucleic acids | |
| US20120053068A1 (en) | Real-time pcr of targets on a micro-array | |
| US20050196779A1 (en) | Self-contained microfluidic biochip and apparatus | |
| CN108479872A (zh) | 用于进行试验的集成微流体系统、方法和试剂盒 | |
| WO2012066239A1 (fr) | Cartouche microfluidique pour diagnostic moleculaire | |
| EP2107125A1 (de) | Echtzeit-PCR von Targets auf einem Mikroarray | |
| FR2950358A1 (fr) | Dispositif d'amplification d'acides nucleiques simplifie et son procede de mise en oeuvrei | |
| FR2902441A1 (fr) | Procede de determination de la concentration d'acides nucleiques | |
| EP1356303A1 (de) | Verfahren und vorrichtung zur kontinuerlichen durchführung einer biologischen, chemischen oder biochemischen reaktion | |
| US20230191407A1 (en) | Method for operating an analyzer, cartridge and analyzer | |
| FR3109585A1 (fr) | Plaquette de test et système de test biologique automatisé | |
| WO2002079508A1 (fr) | Dispositif integre et miniaturise de sequencage d'acides nucleiques | |
| HK1259903B (zh) | 用於进行试验的集成微流体系统、方法和试剂盒 | |
| FR2798604A1 (fr) | Dispositif pour la realisation de reactions chimiques ou biochimiques par cyclage thermique |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AC | Divisional application: reference to earlier application |
Ref document number: 1305115 Country of ref document: EP Kind code of ref document: P |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU MC NL PT SE TR |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: FESTOC, GABRIEL |
|
| 17P | Request for examination filed |
Effective date: 20110628 |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: PALL GENEDISC TECHNOLOGIES |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AC | Divisional application: reference to earlier application |
Ref document number: 1305115 Country of ref document: EP Kind code of ref document: P |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU MC NL PT SE TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D Free format text: NOT ENGLISH |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R082 Ref document number: 60147073 Country of ref document: DE Representative=s name: HOEGER, STELLRECHT & PARTNER PATENTANWAELTE MB, DE Ref country code: DE Ref legal event code: R082 Ref document number: 60147073 Country of ref document: DE Representative=s name: HOEGER, STELLRECHT & PARTNER PATENTANWAELTE, DE |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: NV Representative=s name: ISLER & PEDRAZZINI AG Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: REF Ref document number: 572768 Country of ref document: AT Kind code of ref document: T Effective date: 20120915 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D Free format text: LANGUAGE OF EP DOCUMENT: FRENCH |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 60147073 Country of ref document: DE Effective date: 20121018 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2389763 Country of ref document: ES Kind code of ref document: T3 Effective date: 20121031 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: T3 |
|
| REG | Reference to a national code |
Ref country code: SE Ref legal event code: TRGR |
|
| REG | Reference to a national code |
Ref country code: DK Ref legal event code: T3 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120829 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20121130 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20121231 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed |
Effective date: 20130530 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 60147073 Country of ref document: DE Effective date: 20130530 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120829 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120829 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R082 Ref document number: 60147073 Country of ref document: DE Representative=s name: HOEGER, STELLRECHT & PARTNER PATENTANWAELTE MB, DE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20130720 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 16 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 17 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 18 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R082 Ref document number: 60147073 Country of ref document: DE Representative=s name: HOEGER, STELLRECHT & PARTNER PATENTANWAELTE MB, DE |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20200612 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 20200617 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20200715 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IE Payment date: 20200709 Year of fee payment: 20 Ref country code: DE Payment date: 20200707 Year of fee payment: 20 Ref country code: TR Payment date: 20200720 Year of fee payment: 20 Ref country code: GB Payment date: 20200708 Year of fee payment: 20 Ref country code: ES Payment date: 20200803 Year of fee payment: 20 Ref country code: DK Payment date: 20200710 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20200610 Year of fee payment: 20 Ref country code: CH Payment date: 20200716 Year of fee payment: 20 Ref country code: SE Payment date: 20200710 Year of fee payment: 20 Ref country code: AT Payment date: 20200625 Year of fee payment: 20 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R071 Ref document number: 60147073 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: MK Effective date: 20210719 |
|
| REG | Reference to a national code |
Ref country code: DK Ref legal event code: EUP Expiry date: 20210720 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: PE20 Expiry date: 20210719 |
|
| REG | Reference to a national code |
Ref country code: BE Ref legal event code: MK Effective date: 20210720 |
|
| REG | Reference to a national code |
Ref country code: SE Ref legal event code: EUG |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: MK9A |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK07 Ref document number: 572768 Country of ref document: AT Kind code of ref document: T Effective date: 20210720 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20210720 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20210719 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20220128 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20210721 |