EP2269738B1 - Device for thermo-dependent chain reaction amplification of target nucleic acid sequences - Google Patents
Device for thermo-dependent chain reaction amplification of target nucleic acid sequences Download PDFInfo
- Publication number
- EP2269738B1 EP2269738B1 EP10177401A EP10177401A EP2269738B1 EP 2269738 B1 EP2269738 B1 EP 2269738B1 EP 10177401 A EP10177401 A EP 10177401A EP 10177401 A EP10177401 A EP 10177401A EP 2269738 B1 EP2269738 B1 EP 2269738B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- cartridge
- reaction chambers
- reaction
- amplification
- reservoir
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L3/00—Containers or dishes for laboratory use, e.g. laboratory glassware; Droppers
- B01L3/50—Containers for the purpose of retaining a material to be analysed, e.g. test tubes
- B01L3/502—Containers for the purpose of retaining a material to be analysed, e.g. test tubes with fluid transport, e.g. in multi-compartment structures
- B01L3/5027—Containers for the purpose of retaining a material to be analysed, e.g. test tubes with fluid transport, e.g. in multi-compartment structures by integrated microfluidic structures, i.e. dimensions of channels and chambers are such that surface tension forces are important, e.g. lab-on-a-chip
- B01L3/50273—Containers for the purpose of retaining a material to be analysed, e.g. test tubes with fluid transport, e.g. in multi-compartment structures by integrated microfluidic structures, i.e. dimensions of channels and chambers are such that surface tension forces are important, e.g. lab-on-a-chip characterised by the means or forces applied to move the fluids
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L3/00—Containers or dishes for laboratory use, e.g. laboratory glassware; Droppers
- B01L3/50—Containers for the purpose of retaining a material to be analysed, e.g. test tubes
- B01L3/502—Containers for the purpose of retaining a material to be analysed, e.g. test tubes with fluid transport, e.g. in multi-compartment structures
- B01L3/5025—Containers for the purpose of retaining a material to be analysed, e.g. test tubes with fluid transport, e.g. in multi-compartment structures for parallel transport of multiple samples
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L3/00—Containers or dishes for laboratory use, e.g. laboratory glassware; Droppers
- B01L3/50—Containers for the purpose of retaining a material to be analysed, e.g. test tubes
- B01L3/502—Containers for the purpose of retaining a material to be analysed, e.g. test tubes with fluid transport, e.g. in multi-compartment structures
- B01L3/5027—Containers for the purpose of retaining a material to be analysed, e.g. test tubes with fluid transport, e.g. in multi-compartment structures by integrated microfluidic structures, i.e. dimensions of channels and chambers are such that surface tension forces are important, e.g. lab-on-a-chip
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L3/00—Containers or dishes for laboratory use, e.g. laboratory glassware; Droppers
- B01L3/50—Containers for the purpose of retaining a material to be analysed, e.g. test tubes
- B01L3/502—Containers for the purpose of retaining a material to be analysed, e.g. test tubes with fluid transport, e.g. in multi-compartment structures
- B01L3/5027—Containers for the purpose of retaining a material to be analysed, e.g. test tubes with fluid transport, e.g. in multi-compartment structures by integrated microfluidic structures, i.e. dimensions of channels and chambers are such that surface tension forces are important, e.g. lab-on-a-chip
- B01L3/502715—Containers for the purpose of retaining a material to be analysed, e.g. test tubes with fluid transport, e.g. in multi-compartment structures by integrated microfluidic structures, i.e. dimensions of channels and chambers are such that surface tension forces are important, e.g. lab-on-a-chip characterised by interfacing components, e.g. fluidic, electrical, optical or mechanical interfaces
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L7/00—Heating or cooling apparatus; Heat insulating devices
- B01L7/52—Heating or cooling apparatus; Heat insulating devices with provision for submitting samples to a predetermined sequence of different temperatures, e.g. for treating nucleic acid samples
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L2300/00—Additional constructional details
- B01L2300/08—Geometry, shape and general structure
- B01L2300/0803—Disc shape
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L2300/00—Additional constructional details
- B01L2300/08—Geometry, shape and general structure
- B01L2300/0809—Geometry, shape and general structure rectangular shaped
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L2300/00—Additional constructional details
- B01L2300/08—Geometry, shape and general structure
- B01L2300/0861—Configuration of multiple channels and/or chambers in a single devices
- B01L2300/0864—Configuration of multiple channels and/or chambers in a single devices comprising only one inlet and multiple receiving wells, e.g. for separation, splitting
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L2300/00—Additional constructional details
- B01L2300/18—Means for temperature control
- B01L2300/1805—Conductive heating, heat from thermostatted solids is conducted to receptacles, e.g. heating plates, blocks
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L2400/00—Moving or stopping fluids
- B01L2400/04—Moving fluids with specific forces or mechanical means
- B01L2400/0403—Moving fluids with specific forces or mechanical means specific forces
- B01L2400/0406—Moving fluids with specific forces or mechanical means specific forces capillary forces
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L2400/00—Moving or stopping fluids
- B01L2400/04—Moving fluids with specific forces or mechanical means
- B01L2400/0475—Moving fluids with specific forces or mechanical means specific mechanical means and fluid pressure
- B01L2400/0487—Moving fluids with specific forces or mechanical means specific mechanical means and fluid pressure fluid pressure, pneumatics
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L2400/00—Moving or stopping fluids
- B01L2400/04—Moving fluids with specific forces or mechanical means
- B01L2400/0475—Moving fluids with specific forces or mechanical means specific mechanical means and fluid pressure
- B01L2400/0487—Moving fluids with specific forces or mechanical means specific mechanical means and fluid pressure fluid pressure, pneumatics
- B01L2400/049—Moving fluids with specific forces or mechanical means specific mechanical means and fluid pressure fluid pressure, pneumatics vacuum
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L7/00—Heating or cooling apparatus; Heat insulating devices
- B01L7/52—Heating or cooling apparatus; Heat insulating devices with provision for submitting samples to a predetermined sequence of different temperatures, e.g. for treating nucleic acid samples
- B01L7/525—Heating or cooling apparatus; Heat insulating devices with provision for submitting samples to a predetermined sequence of different temperatures, e.g. for treating nucleic acid samples with physical movement of samples between temperature zones
- B01L7/5255—Heating or cooling apparatus; Heat insulating devices with provision for submitting samples to a predetermined sequence of different temperatures, e.g. for treating nucleic acid samples with physical movement of samples between temperature zones by moving sample containers
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L7/00—Heating or cooling apparatus; Heat insulating devices
- B01L7/54—Heating or cooling apparatus; Heat insulating devices using spatial temperature gradients
Definitions
- the present invention relates to the field of genetics.
- the present invention relates to a device for the amplification of target nucleic sequences, and modes of use of this device.
- the object of the present invention is in particular to enable the detection and, where appropriate, the quantification in real time of target nucleic acid sequences in one or more samples.
- Detection of target nucleic acid sequences is an increasingly popular technique in many fields, and the range of applications of this technique is expected to expand as it becomes more reliable, cost-effective and more reliable. fast.
- the detection of certain nucleic acid sequences in certain cases allows a reliable and rapid diagnosis of viral or bacterial infections.
- the detection of certain genetic peculiarities can make it possible to identify susceptibilities to certain diseases, or to establish an early diagnosis of genetic or neoplastic diseases.
- the detection of target nucleic sequences is also used in the food industry, in particular to ensure the traceability of products, to detect the presence of genetically modified organisms and to identify them, or to carry out a health control of food.
- Nucleic acid-based detection methods almost invariably involve a molecular hybridization reaction between a target nucleic acid sequence and one or more sequences nucleic acid complementary to said target sequence.
- These methods have numerous variants such as the techniques known to those skilled in the art under the terms "transfer techniques” ( blot, dot block, Southern blot, Restriction Fragment Length Polymorphism, etc.), or else the miniaturized systems on which the complementary sequences of the target sequences ("biochips”) are prefixed.
- transfer techniques blot, dot block, Southern blot, Restriction Fragment Length Polymorphism, etc.
- biochips the complementary nucleic sequences are generally called probes.
- Another variant which may constitute in itself the basis of a diagnostic method or be only an additional step in one of the techniques mentioned above (in particular to increase the concentration of the target sequence and therefore the sensitivity of the diagnosis), consists in amplifying the targeted nucleic acid sequence.
- PCR Polymerase Chain Reaction
- PCR Polymerase Chain Rection
- primers complementary nucleic sequences of the target sequences
- the PCR reactions involve a repetition of cycles, the number of which generally ranges from 20 to 50, and which are each composed of three successive phases, namely: denaturation, hybridization, elongation.
- the first phase corresponds to the transformation of the double-stranded nucleic acids into single-stranded nucleic acids
- the second phase to the molecular hybridization between the target sequence and the complementary primers of said sequence
- the third phase to the elongation of the hybridized complementary primers. to the target sequence, by a DNA polymerase.
- phase are carried out at specific temperatures: generally 95 ° C for denaturation, 72 ° C for elongation, and between 30 ° C and 65 ° C for hybridization, depending on the hybridization temperature (Tm) of the primers used. It is also possible to perform the hybridization and elongation steps at the same temperature (generally 60 ° C).
- a PCR reaction therefore consists of a series of repetitive thermal cycles in which the number of target DNA molecules serving as template is theoretically doubled at each cycle.
- the yield r decreases during the PCR reaction, because of several factors, such as an amount mimicking at least one of the reagents necessary for the amplification, the inactivation of the polymerase by repeated passages. at 95 ° C, or its inhibition by the pyrophosphates produced by the reaction.
- the curve presenting the quantity of product, in logarithmic scale, as a function of the number of cycles, and a slope line (1 + r) and which intersects the y-axis with a value equal to the logarithm of the initial concentration.
- the real-time measurement of the quantity of product obtained can therefore make it possible to know the initial matrix concentration, which is particularly useful in a large number of applications, for example for measuring the viral load of a patient, or again to know the variability of a transcriptome.
- the PCRs involve reaction volumes ranging from 2 to 50 ⁇ l and are carried out in tubes, microtubes, capillaries or systems known to those skilled in the art under the term "microplates" (in fact sets of micro tubes secured).
- microplates in fact sets of micro tubes secured.
- Each batch of tubes or equivalent containers must be successively brought to the three temperatures corresponding to the different phases of the PCR, and as many times as desired cycles.
- oligonucleotides complementary to said sequence are based on oligonucleotides complementary to said sequence, and linked to couples of fluorophore or fluorophore / quencher moieties, such that hybridization of the probe to its target and subsequent amplification cycles result, depending on the case an increase or decrease in the total fluorescence of the mixture, in proportion to the amplification of the target sequence.
- Examples of useful probes for performing kinetic PCR include the TaqMan TM system (ABI ®), the AmpliSensor TM system (InGen) and the Sunrise TM system (Oncor ®, ® Appligene).
- the most used system today is the Taq Man TM system .
- This method combines the DNA polymerase and 5 ' ⁇ 3' nuclease activities of Taq polymerase during PCR. Its principle is as follows: in addition to the two primers of sequence complementary to that of the target to be quantified, a probe, called probe reporter, is added in the reaction medium. It has the ability to hybridize to the target in the body of the amplified sequence, but can not be amplified itself. Indeed, a phosphoryl group added to the 3 'end of the probe prevents its extension by Taq polymerase. A fluorescein derivative and a rhodamine derivative are incorporated into the probe at the 5 'and 3' ends, respectively. The probe is small, so the rhodamine derivative, located near the fluorescein, absorbs the energy emitted by the fluorescein subjected to a source of excitation ( quenching phenomenon).
- the Taq DNA polymerase attacks the probe by its 5 'activity. nuclease, releasing the quencher group and thereby restoring the fluorescence emission.
- the intensity of the fluorescence emitted is then proportional to the quantity of PCR products formed, which makes it possible to obtain a quantitative result.
- the fluorescence emitted is proportional to the number of starting target molecules. The kinetics of fluorescence development can be monitored in real time during the amplification reaction.
- This technique has the advantage of being easily automatable.
- An apparatus for carrying out this technique the ABI Prism 7700 TM , is marketed by Perkin-Elmer.
- This device combines a thermocycler and a fluorimeter. It is able to detect the increase in fluorescence generated during a quantification test according to the TaqMan TM process, thanks to optical fibers located below each tube and connected to a CCD camera that detects, in real time , the signal emitted by the fluorescent groups released during the PCR.
- the quantitative data are derived from the determination of the cycle at which the signal of the amplification product reaches a certain threshold determined by the user.
- this number of cycles is proportional to the amount of initial material (Gibson, Heid et al., 1996, Heid, Stevens et al., 1996, Williams, Giles et al., 1998).
- the objective of the present invention is to propose such a system which makes it possible to considerably reduce the number of manipulations required to implement an amplification method on a plurality of target sequences and, consequently, to reduce the time required. necessary for this operation.
- Another object of the present invention is to provide such a system which minimizes the risk of contamination from one container to another.
- Another objective of the present invention is to propose such a system which reduces the volumes of reagents involved and therefore the costs.
- Another objective of the present invention is to propose such a system which optimizes a homogeneous distribution in volume and in concentration of the reagents necessary for the PCR in the containers.
- Another objective is to provide all potential users, including hospitals, medical laboratories, agribusiness and health control laboratories, an easy-to-use and easy-to-use device to routine quantified nucleic acid amplifications in real time.
- the invention relates firstly to any device according to one of claims 1 to 16.
- the temperature of each zone of the platen can be homogeneous or, where appropriate, this temperature can vary according to a gradient.
- reaction mixture Several types of molecular biology reactions require placing the reaction mixture at different temperatures as a function of time. This is the case, for example, when it is desired to inactivate an enzyme after using it (for example, a restriction nuclease), or to test the stability of a complex. In the latter case, it is conceivable to place a complex (for example, an antigen / antibody complex, or receptor / ligand), one of which is coupled to a fluorophore and the other to a quencher of fluorescence, one of the reaction chambers of the device. The stage is then programmed to present several temperatures in increasing order, possibly in the form of a gradient.
- a complex for example, an antigen / antibody complex, or receptor / ligand
- the stability of the complex is then tested by moving the cartridge on the plate, so that the temperature of the reaction chamber rises gradually, and observing the increase in fluorescence, using excitation means measurement of the fluorescence placed opposite the reaction chamber.
- the increase in fluorescence then reflects the dissociation of the complex.
- the device of the invention is particularly suitable for reactions requiring a cyclic variation in the temperature of the reaction chambers, which is the case for certain nucleic acid amplification reactions, for example for polymerase chain reaction (PCR ), or for the ligase chain reaction (LCR).
- PCR polymerase chain reaction
- LCR ligase chain reaction
- the invention therefore relates in particular to a device for the thermo-dependent chain amplification of target nucleic acid sequences according to one of claims 1 to 16.
- Such a system according to the invention is less complex than the systems of the prior art, insofar as the temperatures required for the cycles of the chain amplification are provided by distinct zones of constant temperatures and not by a plate of which one must vary the temperature.
- thermo-dependent chain amplification reactions require the passage of samples at at least two temperatures.
- the CSF requires at each cycle a phase at about 95 ° C to denature the target DNA, then a phase between 55 and 65 ° C (depending on the Tm probes) to give rise to hybridization / ligation .
- each cycle generally breaks down into three phases, namely denaturation at about 95 ° C, hybridization whose temperature depends on the Tm of the probes, and elongation, usually performed at 72 ° C. .
- nucleic acids to be amplified it is possible to dispense, from a reservoir, a fluid containing a sample of nucleic acids to be analyzed and the reagents necessary for the PCR in a plurality of reaction chambers containing specific primers of target sequences. nucleic acids to be amplified, and to allow the amplification process by subjecting the contents of the chambers successively to different temperatures (namely those necessary for denaturation, hybridization and elongation) a multitude of times through a movement relative between the cartridge including said reaction chambers and said heating platen having two or three distinct zones that can be brought to different temperatures.
- the reaction chambers (13) may contain reagents necessary for a real-time PCR reaction other than the primers mentioned above.
- the reaction chambers also comprise, in addition to the primers, one or more specific probe (s) of the sequence to be amplified.
- the distribution of the probes in the reaction chambers may also be such that some chambers contain probes specific for the sequences to be amplified and other chambers comprise control probes, not recognizing a priori the sequence to be amplified. These probes may be labeled and, if several probes are present in the same reaction chamber (for example a probe specific for the sequence to be amplified and a control probe), these probes will preferably be labeled with different fluorophores.
- additional reagents such as dNTPs or salts, are initially deposited in the reaction chambers. These reagents will then be absent, or present in a smaller amount, in the fluid deposited in the reservoir (11). In the extreme case, all the reagents necessary for the PCR reaction, with the exception of the matrix, are deposited in the reaction chambers (13), and the fluid deposited in the reservoir (11) will then comprise only the sample. DNA (or RNA) to amplify.
- the variants described above assume that several reactions are carried out in parallel, with different primers and / or probes, on the same sample. It is therefore the characterization of a single sample (or a few samples if the reservoir is divided into a few sub-reservoirs) according to several criteria. In some applications, on the contrary, it is desired to characterize a multitude of samples according to a single criterion or a small number of criteria. This is the case for example in research, when it is desired to screen a library of phages or bacteria for the presence of a given gene. In this case, it is necessary to carry out a PCR on a large number of samples, from a given pair of primers.
- the device of the invention is also suitable for this type of manipulation.
- the samples are deposited in the reaction chambers (13).
- the primers can be introduced into the fluid deposited in the reservoir (11), along with the other reagents necessary for the PCR.
- this configuration does not exclude that certain reagents other than the sample to be analyzed are pre-deposited in the reaction chambers (13).
- each reagent deposited in the reaction chambers (13) can be advantageously deposited there by a simple liquid deposit, followed by drying.
- the arrival of the fluid from the reservoir (11) then allows the re-solution of these reagents.
- the amount of each reagent deposited is calculated as a function of the volume of fluid that will penetrate into each reaction chamber (13), so that the reactivation of the reagents will result in the desired final concentration for each of them.
- Cartridges as described above, in which at least a portion of the reaction chambers (13) comprise reagents which have been loaded therein by a liquid deposit, followed by drying, so that these reagents are returned to solution by the arrival of a fluid in these reaction chambers, are also an integral part of the invention.
- the device described above has the advantage of allowing a concomitant filling of all the reaction chambers, which reduces the preparation time and the risks of contamination from one chamber to another.
- This device also has the advantage of being able to be miniaturized and to involve the use of smaller volumes of reagents than in the state of the art.
- the invention makes it possible to accelerate PCR cycles, since it is not necessary to perform the various phases (denaturation, hybridization, elongation). to vary the temperature of the heating stage or of the atmosphere as in the state of the art, the relative movement between the cartridge and the plate making it possible to quickly and successively submit the contents of each of the chambers reaction at three distinct temperatures dedicated to each of these phases.
- the use of small reaction volumes, and a thin floor for the cartridge (1), also limit the thermal inertia at the reaction chambers, and therefore contribute to the speed of the reaction.
- the invention also relates to a device for thermo-dependent chain amplification of target nucleic acid sequences, measured in real time, characterized in that it comprises the same elements as any of the devices described above. above, and further comprising optical means (5) for excitation / measurement of fluorescence, arranged to excite and measure at each cycle the fluorescence of the contents of the reaction chambers.
- the diameter of the channels will preferably be chosen small enough not to allow a gravity distribution of the fluid present in the tank in the reaction chambers, so as to avoid non-reproducible filling of these chambers. This diameter will thus preferably be less than or equal to approximately 0.2 mm. With regard to this diameter, it will be noted that the section of the channels will preferably be circular but that it may also be of any other shape and in particular polygonal, the "diameter" of the channels then aiming at their greatest width in section.
- the reservoir intended to receive the sample of nucleic acids and the reagents necessary for the PCR may have a variable capacity, for example between about 0.1 ml and about 1 ml.
- the cartridge preferably comprises between about 20 and about 500 reaction chambers and, more preferably, between 60 and 100 reaction chambers.
- these rooms may also vary according to the embodiments.
- these chambers have a volume of between approximately 0.2 and 50 ⁇ l, preferably between 1 ⁇ l and 10 ⁇ l.
- the junction between the channels (12) and the reservoir (11) is at the periphery of the reservoir, and the bottom of said reservoir is inclined and / or convex, so as to ensure the distribution of a fluid contained in the tank at the entrance of the channels.
- a cartridge used according to the invention may have multiple forms. However, according to a preferred variant of the invention, this cartridge has a circular shape.
- the reservoir is provided substantially in the center of the cartridge, the reaction chambers being distributed in a circle around the reservoir, and the channels connecting the reservoir to the chambers being provided essentially radially.
- Such an architecture allows to optimize the filling of the reaction chambers from the central tank.
- the bottom of the tank (11) is conical.
- reaction chambers are provided relative to the periphery of said cartridge.
- said reaction chambers are provided relative to the periphery of said cartridge.
- such a cartridge comprises as many channels as there are reaction chambers.
- the cartridge preferably has a diameter of between about 1 and 10 cm.
- a variant of the cartridges of the invention described above whatever their geometry, consists in dividing the reservoir (11) into 2 to 20, preferably 2 to 8 sub-reservoirs, making it possible to simultaneously analyze several samples on a same cartridge.
- each of the reaction chambers (13) is connected to only one of these sub-tanks by a channel (12).
- the depth of the reaction chambers may also vary depending on the embodiments of the invention. According to a preferred variant, these chambers have a depth of between approximately 0.5 mm and 1.5 mm.
- this cartridge depends on several factors including the material constituting it.
- this cartridge is preferably made of plastic material, preferably polycarbonate, whose physical, optical and thermal properties are suitable for carrying out the present invention.
- the thickness of the cartridges of the invention is preferably between 0.5 and 5 mm.
- the thickness of the "floor” thereof will preferably be as low as possible. This thickness depends on the material used to make the cartridge. Preferably, it is between 0.05 and 0.5 mm, for example about 0.25 mm.
- reaction chambers of the cartridges of the invention are preferably closed by a transparent upper wall (17), for example of transparent plastic, in order to allow the excitation and the measurement of the fluorescence of the reaction fluid, in good conditions.
- the chambers are provided with vents (open system), allowing the air they contain to escape during their filling by the fluid from the tank.
- the channels (12) preferably consist of at least two parts of different diameters (121 and 122), the diameter of the second part (122) being smaller than that of the first part (121), so as to create a pressure drop in the channel (12).
- the pressure drop phenomenon stops the progression of the fluid in the channel or channels whose first portion (121) is filled, until all the channels are filled in the same way. This allows to "pre-calibrate” the volumes for each channel, to ensure a homogeneous filling of the different reaction chambers.
- the second portion of the channel (122) may consist for example of a glass capillary, much smaller in diameter than the first portion (121), said capillary being included in a plastic cartridge.
- the cartridges described above provided for use in an open system or for use in a closed system, preferably comprise an opening that can be adapted to means (4) for modulating the pressure in the reservoir (11), making it possible to moving the fluid present in the reservoir to the reaction chambers.
- each channel (12) is equipped with an anti-reflux cavity (123) at its junction with the reservoir (11), said anti-reflux cavity consisting of a substantially vertical channel portion of a diameter greater than or equal to that of the channel (12).
- This variant has two main advantages. On the one hand, these anti-reflux cavities make it possible to prevent cross-contamination in the event of an inadvertent return of fluid to the reservoir (11), or in the case where all the fluid has not been engaged in the channels. On the other hand, these cavities make it possible to provide, in the devices of the invention, a plug whose serrations come to marry these vertical inlets, in order to plug the channels after the addressing of the editorial fluid but before the amplification reaction.
- each of the reaction chambers (13) comprises oligonucleotides. More preferably, each of the reaction chambers (13) comprises two primers specific for a nucleic acid sequence to be amplified and, optionally, one or more labeled probe (s) specific for said sequence.
- a probe may be labeled so that its signal is increased when it hybridizes to its target sequence (Sunrise TM system), or so that elongation from a strand on which it is hybridized causes a decrease or increase in the signal (AmpliSensor TM system or TaqMan TM system, respectively).
- probes in the reaction chambers makes it possible to carry out quantized amplifications in real time, with a device of the invention having means (5) for excitation / measurement of the fluorescence, as described above.
- Control probes nonspecific of the sequence to be amplified, and labeled in a different way from the specific probes, can also be used to detect possible contaminations.
- these different probes and primers will preferably be chosen such that their melting temperatures (Tm) respective ones are close.
- Tm melting temperatures
- the Tm of the different primers will preferably be in the same range of about 5 ° C.
- the different probes will preferably have a Tm within the same range of 5 ° C, which may be different from the range of Tm primers.
- the probes will be chosen so that their Tm is greater than that of the primers, the difference between Tm of the different categories of oligonucleotides then being preferably of the order of 5 ° C.
- the hybridization temperature chosen to carry out the amplification then corresponds to the lowest of the melting temperatures of the primers.
- the reaction chambers (13) of the cartridges of the invention may also comprise, in addition to the primers and any probes, one or more other reagents necessary for the PCR reaction or measurement of the amplification. It may be, for example, salts, dNTPs, or a fluorescent interlayer of double-stranded DNA, SybrGreen type (trademark). As mentioned above, all these reagents are advantageously deposited at the level of the reaction chambers (13) by the deposition of a liquid solution, followed by drying.
- the cartridges are intended for screening a large number of samples according to a small number of criteria. This implies that the user of these cartridges can easily deposit his samples in each of the reaction chambers (13).
- the cartridge can for example have a removable cover which, when removed, gives direct access to the reaction chambers.
- Such cartridges may also be pre-charged and comprise, in the reaction chambers (13), one or more reagents necessary for amplification and / or detection.
- the devices of the invention mentioned above may comprise one or more cartridges corresponding to any of the cartridges described above.
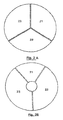
- the distinct heating zones of the heating plate (2) are preferentially distributed according to disk portions ( Figure 2A ) or crown ( Figure 2B ). Each portion may be heated to a different temperature to successively bring the contents of the reaction chambers to the desired different temperatures, by means of relative displacement means (3) between the cartridge (1) and the heating stage (2).
- the thermoblocks are preferably sufficiently wide to heat also part of the channels, as represented for example in FIG. figure 11 , as part of a rectangular cartridge.
- the number of separate heating zones can be two, three, or more.
- platinum may have a 95 ° C zone for denaturation of double-stranded nucleic acids, and a 60 ° C region for primer hybridization and elongation.
- the platinum will have an area at 95 ° C (denaturation), a region between 40 and 70 ° C (primer hybridization), and an area at 72 ° C (elongation).
- the plate may have a number of zones greater than three, for example to temporarily block the reaction at a given moment in each cycle.
- the platen can also have a number of zones that is a multiple of two or three, so that one revolution of the cartridge corresponds to several PCR cycles.
- the relative size of the different heating zones is advantageously chosen proportionally to the desired incubation time for the reaction fluid at the temperature of said zone.
- the thermoblock 21, dedicated to the denaturation step has a surface twice as small as that of the thermoblocks for the hybridization and elongation steps (blocks 22 and 23, respectively).
- the plate (2) is fixed and the cartridge (1) is moved by means of displacement (3).
- the displacement means (3) allow the rotation of said cartridge and / or said platen.
- a conductive element can be provided between the cartridge and the heating plate.
- said cartridge is in direct contact with said heating stage.
- said plate is advantageously provided with a coating promoting displacement between said cartridge and said plate.
- a coating may for example be made of Teflon (registered trademark).
- the system heating plate may have at least two or three zones that can be raised to different temperatures.
- this plate consists of two or three independent thermal blocks ("thermoblocks") connected to separate means for programming their temperature.
- thermoblocks independent thermal blocks
- the first of these thermoblocks (21) is heated to the denaturation temperature, the second (22) to the hybridization temperature, the third (23) to the elongation temperature.
- the use of such thermoblocks constant temperature simple realization of the heating stage.
- the relative displacement means of the cartridge relative to the platen can be realized in multiple forms.
- the cartridge (1) has on the underside a central projection (181) having a notch (182), so that the projecting portion (181) fits into the heating stage (2) and connects the cartridge (1).
- the displacement means (3) at a cleat or shaft (32) set in motion by a micromotor (31).
- the protruding part (181) thus makes it possible, on the one hand, to position the cartridge with respect to a plate (2) such as that represented in FIG. Figure 2B and, secondly, to ensure its connection with the moving means (3).
- the cartridge has at least one lug (183) and the displacement means (3) include at least one axis (32) cooperating with said lug to instill in said cartridge a rotary movement.
- the relative mode of movement between the plate and the cartridge may vary according to the embodiments. It may be a movement at continuous speed or in jerks. The speed of movement may be constant or vary over time.
- the system according to the invention also comprises optical means of excitation / fluorescence measurement, provided for example above or on the side of said cartridge.
- these means will constitute a single and fixed system.
- An advantage of a preferred variant of the invention according to which the cartridge is circular and moved in a rotary displacement is to be able to successively bring each reaction chamber under said optical system, thus reducing its complexity.
- a tracking system located for example on the cartridge (1), makes it possible to determine at each instant which reaction chamber is located opposite the optical system.
- the means for supplying the fluid present in said reservoir to said reaction chambers can be made in different forms. As has been described above, two categories of modes of addressing the fluid towards the reaction chambers can be distinguished: open system addressing, which assumes an increase in pressure at the reservoir and the presence of vents (14). ) at the level of the reaction chambers, and the addressing in closed system, which begins on the contrary by the establishment of a vacuum in the cartridge (1), followed by a recovery of this pressure.
- the means (4) for supplying the fluid into the reaction chambers differ according to the embodiment chosen.
- the fluid contained in the reservoir is distributed under pressure in the reaction chambers so as to allow uniform filling of these chambers.
- the feed means (4) preferably include a piston device (41) whose penetration rate in the tank will be calculated-to promote the proper filling of the reaction chambers, alternatively, these supply means include a pump connected to increase the pressure in the tank (11).
- another preferred variant of the invention involves working in a closed system.
- the fluid contained in the reservoir is then distributed in the reaction chambers as follows: in a first step, a vacuum is created inside the cartridge, where appropriate by a piston device or a pump (42), connected this time to reduce the pressure in the cartridge (1).
- the pressure is then restored, allowing the fluid to engage the channels and fill the peripheral reaction chambers.
- reagents necessary for the amplification reaction and / or the detection of the products of the amplification, and distinct from the primers and probes are pre-distributed in the reaction chambers (13). of the cartridge (1).
- the fluid introduced into the reservoir (11) does not then contain these reagents.
- the fluid distribution step in the reaction chambers (13) is carried out either by applying a vacuum inside the cartridge, then by restoring the pressure (closed system), or by increasing the pressure at the reservoir (11). ), provided that the reaction chambers are provided with vents (open system).
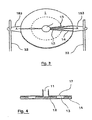
- the system for detecting and quantifying target nucleic sequences represented in figure 1 comprises a circular plastic cartridge 2 mm thick having a diameter of 5 cm.
- This cartridge (1) is provided with a central reservoir (11) and will be described in more detail with reference hereinafter to Figures 3 and 4 .
- the capacity of the reservoir is, in the context of the present embodiment, 400 .mu.l. Its floor is flat but it will be noted that in other embodiments it may be curved to facilitate the passage of fluid to the chambers without the formation of air bubbles, especially at the end of addressing when the reservoir is almost empty.
- the system further comprises a heating plate (2) in direct contact with the underside of the cartridge (1) and means (3) for moving the cartridge (1) relative to the heating plate (2).
- These displacement means include a micromotor (31) connected to two axes (32) which cooperate with two lugs (183) of the cartridge (1) to inculcate in it a rotary movement on the heating plate (2), the latter while remaining fixed.
- the described system also comprises a piston (41) intended to cooperate with said reservoir (11) as well as an optical device (5) for excitation / fluorescence measurement (emitting source allowing excitation at a given, programmable wavelength). and fluorescence receiver emitted) fixed and placed above the cartridge (1) and the heating plate (2).
- the heating plate (2) consists of three metal blocks (21, 22 and 23) (hereinafter referred to as thermoblocks) in the form of disk portions. Note that in this embodiment, these thermoblocks have substantially the same size but that, in other embodiments, they may have a different size, the size being understood as the occupied angular surface in top view.
- thermoblock (21, 22 and 23) is designed to be brought to a constant and programmable temperature, corresponding to one of the phases (denaturation, hybridization or elongation) amplification cycles (PCR), generally respectively 94 ° C for denaturation, 72 ° C for elongation, and between 30-40 and 65-70 ° C for hybridization according to the Tm (hybridization temperature) of the primers used, the temperatures of the thermoblocks can be controlled by all means known to those skilled in the art.
- phases denaturation, hybridization or elongation amplification cycles (PCR)
- Tm hybridization temperature
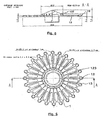
- the cartridge (1) is provided with a central tank (11) of capacity 400 ⁇ l connected to 36 reaction chambers (13) by as many channels (12), distributed uniformly over the entire periphery of the cartridge (on the figure 3 , we did not represent all the channels and rooms but only some of them).
- These reaction chambers (13) are further provided with vents (14) abutting on the edge of the cartridge (1).
- the channels have a diameter of 0.2 mm and the volume of the reaction chambers is 2.5 microliters. In other embodiments, this diameter and this volume may of course be different.
- this cartridge (1) is also provided with two lugs (183) each pierced with an orifice for passing an axis (32) connected to the micromotor (31).
- the reaction chambers have a depth of 1 mm. Their floor has a thickness of about 0.2 mm. This thickness is sufficiently small to facilitate good heat exchange between the chambers (13) and the thermoblocks (21, 22 and 23).
- the reaction chambers (13) are closed in their upper part by a wall (17) transparent, also forming the wall of the tank (11).
- Each chamber 10 except a few for negative control purposes, contains two primers specific for a target sequence to be amplified, and optionally one or more labeled probes, allowing a subsequent specific measurement of fluorescence.
- 10 ng of each primer were distributed in each chamber except for those serving as a negative control.
- the piston (41) After partially filling the reservoir (11) with the fluid whose volume is equal to the sum of the volumes of the chambers (the volume of a chamber is defined as being the product of its "floor” surface by its depth), the piston (41) is actuated to dispense this fluid into the plurality of reaction chambers (13).
- This piston increases the pressure within the reservoir (11) and allows the passage of fluid in the channels to the chambers.
- the speed of movement of the piston in the reservoir is about 1 mm per second and said displacement is stopped at a level that depends on the volume of fluid to be addressed in the chambers.
- the small diameter of the channels (12) makes it possible to prevent the diffusion of the fluid from the reservoir (11) to the channels (12) and the chambers (13) under the effect of gravity (at this scale, the processes are usually negligible as the capillary forces become pregnant, and in this case are enough to keep the fluid in the tank). Thanks to the vents (14), the air present in the chambers (13) is evacuated, which ensures the filling thereof.
- thermoblocks (21, 22, 23) are brought to the three temperatures corresponding to the three temperatures of the PCR phases (or at slightly higher temperatures given the possible heat losses between the heating stage (2) and the cartridge 1) and the displacement means (3) are implemented so as to animate the cartridge (1) by a gyratory movement in order to pass each reaction chamber successively and as many times as desired over the three thermoblocks.
- the block (21) is brought to the temperature corresponding to the denaturation phase (94 ° C.)
- the thermoblock (22) is brought to the temperature corresponding to the hybridization phase (36 ° C.)
- the thermoblock (23) is brought to the temperature corresponding to the phase of elongation (72 ° C).
- the micromotor (31) of the displacement means (3) is adapted to inculcate a rotation of 10 degrees every 2.5 seconds to the cartridge (1) (ie a 1.5 PCR cycle). min).
- this movement may have a different speed and be continuous instead of jerky.
- the optical device (5) is provided above the corresponding block 23 raised to a temperature corresponding to the elongation temperature, and more particularly to a location corresponding to the end of the elongation phase.
- the optical device (5) can be placed at a different location, chosen in particular according to the chemistry used. For example, using TaqMan TM chemistry or nonspecific fluorescence, it makes sense to perform the measurement at the end of the elongation phase, as described above.
- the use of a Molecular Beacons TM type chemistry implies that the measurement is done rather at the time of the hybridization.
- the system presented makes it possible to rapidly and reproducibly fill a large quantity of reaction chambers and to carry out PCR and fluorescence measurements on each PCR cycle.
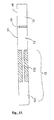
- FIGS. 5 to 10 represent an example of a circular cartridge exhibiting certain modifications with respect to the cartridge of example 1.
- This cartridge is intended for use in a closed system, that is to say that the reaction chambers (13) have no other opening than the arrival of the channel (12).
- the cartridge consists of two elements that fit into each other: the lower part, or base, is represented in Figures 5 and 6 , and the upper part, or cover, is represented in Figures 7 and 8 . The assembly of the two is illustrated in figures 9 and 10 .
- This cartridge is loaded as follows:
- the fluid is no longer addressed by an increase in pressure but by depression, which presents the advantage of not requiring a vent and therefore working in a closed system.
- the bottom of the tank has a conical shape allowing the fluid to be distributed at its periphery, that is to say near the entrance of the channels.
- an anti-reflux system consisting of a vertical channel portion (123), which, on the one hand, prevents cross contamination in the event of inadvertent return of fluid to the central part or in the case where all the fluid would not be engaged in the channel and, on the other hand, allows once the addressing done but before the PCR, to come to plug the channels by means of a plug whose serrations come to marry these vertical entries, to work in closed system (no contamination, no evaporation).
- the cartridge is made of plastic, preferably polycarbonate because this polymer has interesting physical, optical and thermal behavior characteristics.
- the size of the channels is for example 0.4 x 0.2 mm (half-moon) in section.
- the size of the consumable is for example 100 mm (diameter), the number of chambers is 80, the number of sub-tanks is between 1 and 8.
- the cartridge (1) has on the underside a central projection (181) having a notch (182), so that the projecting portion (181) fits into the heating stage (2) and connects the cartridge (1). ) with the moving means (3) at a cleat or shaft (32) moved by a micromotor (31).
- the protruding part (181) thus makes it possible, on the one hand, to position the cartridge with respect to a plate (2) such as that represented in FIG. Figure 2B and, secondly, to ensure its connection with the moving means (3).
- the reaction chambers are loaded with primers specific for target sequences and, where appropriate, with TaqMan TM or other probes specific for said targets.
- the targets will be viral or bacterial genes, junctions between a transgene and the genome of a plant to detect and / or identify certain GMOs, etc.
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Clinical Laboratory Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Analytical Chemistry (AREA)
- Hematology (AREA)
- Dispersion Chemistry (AREA)
- Molecular Biology (AREA)
- Biochemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
- Apparatus Associated With Microorganisms And Enzymes (AREA)
- Automatic Analysis And Handling Materials Therefor (AREA)
- Feeding, Discharge, Calcimining, Fusing, And Gas-Generation Devices (AREA)
- Physical Or Chemical Processes And Apparatus (AREA)
- Investigating Or Analysing Materials By The Use Of Chemical Reactions (AREA)
- Saccharide Compounds (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Compounds Of Unknown Constitution (AREA)
- Polymers With Sulfur, Phosphorus Or Metals In The Main Chain (AREA)
Abstract
Description
La présente invention concerne le domaine de la génétique.The present invention relates to the field of genetics.
Plus précisément, la présente invention concerne un dispositif pour l'amplification de séquences nucléiques cibles, et des modes d'utilisation de ce dispositif.More specifically, the present invention relates to a device for the amplification of target nucleic sequences, and modes of use of this device.
La présente invention a notamment pour objectif de permettre la détection et, le cas échéant, la quantification en temps réel, de séquences d'acide nucléique cibles dans un ou plusieurs échantillons.The object of the present invention is in particular to enable the detection and, where appropriate, the quantification in real time of target nucleic acid sequences in one or more samples.
La détection de séquences nucléiques cibles est une technique de plus en plus utilisée dans de nombreux domaines, et l'éventail des applications de cette technique est appelé à s'étendre au fur et à mesure qu'elle deviendra plus fiable, plus économique et plus rapide. Ainsi, en santé humaine, la détection de certaines séquences d'acide nucléique permet dans certains cas un diagnostic fiable et rapide d'infections virales ou bactériennes. De même, la détection de certaines particularités génétiques peut permettre d'identifier des susceptibilités à certaines maladies, ou d'établir un diagnostic précoce de maladies génétiques ou néoplasiques. La détection de séquences nucléiques cibles est aussi utilisée dans l'industrie agroalimentaire, notamment pour assurer la traçabilité des produits, pour détecter la présence d'organismes génétiquement modifiés et les identifier, ou pour effectuer un contrôle sanitaire des aliments.Detection of target nucleic acid sequences is an increasingly popular technique in many fields, and the range of applications of this technique is expected to expand as it becomes more reliable, cost-effective and more reliable. fast. Thus, in human health, the detection of certain nucleic acid sequences in certain cases allows a reliable and rapid diagnosis of viral or bacterial infections. Similarly, the detection of certain genetic peculiarities can make it possible to identify susceptibilities to certain diseases, or to establish an early diagnosis of genetic or neoplastic diseases. The detection of target nucleic sequences is also used in the food industry, in particular to ensure the traceability of products, to detect the presence of genetically modified organisms and to identify them, or to carry out a health control of food.
Les procédés de détection basés sur les acides nucléiques font quasi systématiquement intervenir une réaction d'hybridation moléculaire entre une séquence nucléique cible et une ou plusieurs séquences nucléiques complémentaires de ladite séquence cible. Ces procédés présentent de nombreuses variantes comme les techniques connues de l'homme de métier sous les expressions "techniques de transfert" (blot, dot bloc, Southern blot, Restriction Fragment Length Polymorphism, etc.), ou encore comme les systèmes miniaturisés sur lesquels sont préfixées les séquences complémentaires des séquences cibles ("biopuces"). Dans le cadre de ces techniques, les séquences nucléiques complémentaires sont généralement appelées sondes. Une autre variante, qui peut constituer en soi la base d'un procédé de diagnostic ou n'être qu'une étape supplémentaire dans une des techniques mentionnées ci-dessus (afin notamment d'augmenter la concentration de la séquence cible et donc la sensibilité du diagnostic), consiste à amplifier la séquence d'acide nucléique ciblée. Plusieurs techniques permettant l'amplification spécifique d'une séquence d'acide nucléique ont été décrites, dont la plus utilisée est l'Amplification en Chaîne par Polymérase (ACP), ou Polymerase Chain Rection (PCR). Dans le cadre de cette dernière technique, des séquences nucléiques complémentaires des séquences cibles, appelées amorces, sont utilisées pour amplifier lesdites séquences cibles.Nucleic acid-based detection methods almost invariably involve a molecular hybridization reaction between a target nucleic acid sequence and one or more sequences nucleic acid complementary to said target sequence. These methods have numerous variants such as the techniques known to those skilled in the art under the terms "transfer techniques" ( blot, dot block, Southern blot, Restriction Fragment Length Polymorphism, etc.), or else the miniaturized systems on which the complementary sequences of the target sequences ("biochips") are prefixed. In the context of these techniques, the complementary nucleic sequences are generally called probes. Another variant, which may constitute in itself the basis of a diagnostic method or be only an additional step in one of the techniques mentioned above (in particular to increase the concentration of the target sequence and therefore the sensitivity of the diagnosis), consists in amplifying the targeted nucleic acid sequence. Several techniques for the specific amplification of a nucleic acid sequence have been described, the most commonly used of which is Polymerase Chain Reaction (PCR) or Polymerase Chain Rection (PCR). In the context of this latter technique, complementary nucleic sequences of the target sequences, called primers, are used to amplify said target sequences.
Les réactions de PCR impliquent une répétition de cycles, dont le nombre varie généralement de 20 à 50, et qui sont composés chacun de trois phases successives, à savoir : dénaturation, hybridation, élongation. Respectivement, la première phase correspond à la transformation des acides nucléiques bicaténaires en acides nucléiques monocaténaires, la seconde phase à l'hybridation moléculaire entre la séquence cible et les amorces complémentaires de ladite séquence, et la troisième phase à l'élongation des amorces complémentaires hybridées à la séquence cible, par une ADN polymérase. Ces phases sont menées à des températures spécifiques : généralement 95°C pour la dénaturation, 72°C pour l'élongation, et entre 30°C et 65°C pour l'hybridation, selon la température d'hybridation (Tm) des amorces utilisées. Il est aussi possible d'effectuer les étapes d'hybridation et d'élongation à la même température (généralement 60 °C).The PCR reactions involve a repetition of cycles, the number of which generally ranges from 20 to 50, and which are each composed of three successive phases, namely: denaturation, hybridization, elongation. Respectively, the first phase corresponds to the transformation of the double-stranded nucleic acids into single-stranded nucleic acids, the second phase to the molecular hybridization between the target sequence and the complementary primers of said sequence, and the third phase to the elongation of the hybridized complementary primers. to the target sequence, by a DNA polymerase. These phases are carried out at specific temperatures: generally 95 ° C for denaturation, 72 ° C for elongation, and between 30 ° C and 65 ° C for hybridization, depending on the hybridization temperature (Tm) of the primers used. It is also possible to perform the hybridization and elongation steps at the same temperature (generally 60 ° C).
Une réaction de PCR consiste donc en un enchaînement de cycles thermiques répétitifs au cours duquel le nombre de molécules d'ADN cible servant de matrice est théoriquement doublé à chaque cycle. En réalité, le rendement de la PCR est inférieur à 100%, si bien que la quantité de produit Xn obtenu après n cycles est :
Xn-1 est la quantité de produit obtenu au cycle précédent, et rn le rendement de la PCR au cycle n (0 < rn ≤ 1).A PCR reaction therefore consists of a series of repetitive thermal cycles in which the number of target DNA molecules serving as template is theoretically doubled at each cycle. In fact, the yield of the PCR is less than 100%, so that the amount of product X n obtained after n cycles is:
X n-1 is the amount of product obtained in the previous cycle, and n the yield of the PCR at cycle n (0 <r n ≤ 1).
En considérant le rendement constant, c'est-à-dire identique pour chaque cycle, la quantité de produit Xn obtenu après n cycles à partir d'une quantité initiale X0 est alors :
En réalité, le rendement r diminue au cours de la réaction de PCR, du fait de plusieurs facteurs, tel qu'une quantité imitant d'au moins un des réactifs nécessaires à l'amplification, l'inactivation de la polymérase par ses passages répétés à 95°C, ou son inhibition par les pyrophosphates produits par la réaction.In reality, the yield r decreases during the PCR reaction, because of several factors, such as an amount mimicking at least one of the reagents necessary for the amplification, the inactivation of the polymerase by repeated passages. at 95 ° C, or its inhibition by the pyrophosphates produced by the reaction.
Du fait de cette diminution du rendement, la cinétique d'une réaction de PCR présente d'abord une phase exponentielle (tant que r est constant), qui évolue ensuite vers une phase de plateau lorsque r diminue.Because of this decrease in yield, the kinetics of a PCR reaction first exhibit an exponential phase (as long as r is constant), which then progresses to a plateau phase as r decreases.
Au cours de la phase exponentielle, l'équation (A) ci-dessus est valable, et peut aussi s'écrire :
Ainsi, dans la phase exponentielle de la PCR, la courbe présentant la quantité de produit, en échelle logarithmique, en fonction du nombre de cycles, et une droite de pente (1 + r) et qui coupe l'axe des ordonnées à une valeur égale au logarithme de la concentration initiale.Thus, in the exponential phase of the PCR, the curve presenting the quantity of product, in logarithmic scale, as a function of the number of cycles, and a slope line (1 + r) and which intersects the y-axis with a value equal to the logarithm of the initial concentration.
La mesure en temps réel, de la quantité de produit obtenu, peut donc permettre de connaître la concentration initiale de matrice, ce qui est particulièrement utile dans un grand nombre d'applications, par exemple pour mesurer la charge virale d'un malade, ou encore pour connaître la variabilité d'un transcriptome.The real-time measurement of the quantity of product obtained can therefore make it possible to know the initial matrix concentration, which is particularly useful in a large number of applications, for example for measuring the viral load of a patient, or again to know the variability of a transcriptome.
Généralement, les PCR impliquent des volumes réactionnels allant de 2 à 50 µl et sont effectuées dans des tubes, des microtubes, des capillaires ou des systèmes connus de l'homme de l'art sous le terme "microplaques" (en fait des ensembles de micro tubes solidarisés). Chaque lot de tubes ou de contenants équivalents doit donc être successivement porté aux trois températures correspondant aux différentes phases de la PCR, et ce autant de fois que de cycles désirés.Generally, the PCRs involve reaction volumes ranging from 2 to 50 μl and are carried out in tubes, microtubes, capillaries or systems known to those skilled in the art under the term "microplates" (in fact sets of micro tubes secured). Each batch of tubes or equivalent containers must be successively brought to the three temperatures corresponding to the different phases of the PCR, and as many times as desired cycles.
L'utilisation de tubes ou de systèmes s'y approchant oblige l'utilisateur à effectuer de multiples manipulations pour préparer autant de tubes et de solutions (connues de l'homme de l'art sous l'expression "mix PCR") que de séquences cibles qu'il souhaite amplifier même à partir d'un échantillon unique d'acides nucléiques, à l'exception des procédés d'amplification "multiplex", qui permettent l'amplification de plusieurs séquences cibles simultanément dans le même contenant, soit par l'utilisation d'amorces dites peu spécifiques qui peuvent s'hybrider avec plusieurs séquences cibles comme par exemple la technique RAPD - Random Amplified Polymorphism DNA, soit par l'utilisation d'amorces spécifiques mais en nombre plus important, chaque couple d'amorces utilisé permettant l'amplification d'une séquence cible. Ces amplifications multiplex correspondent à des cas particuliers et ne sont pas la norme. De surcroît, elles ne garantissent pas l'absence d'interactions d'une réaction d'amplification sur une autre, et pour des raisons notamment de possibles hybridations entre les amorces, ne peuvent qu'être très limitées dans le nombre de séquences cibles amplifiées par contenant.The use of tubes or systems approaching it requires the user to perform multiple manipulations to prepare as many tubes and solutions (known to those skilled in the art as "PCR mix") that target sequences that it wishes to amplify even from a single sample of nucleic acids, with the exception of "multiplex" amplification methods, which allow the amplification of several target sequences simultaneously in the same container, either by the use of so-called unspecific primers which can hybridize with several target sequences, for example the RAPD- Random Amplified Polymorphism DNA technique , or by the use of specific primers but in larger numbers, each pair of primers used to amplify a target sequence. These multiplex amplifications correspond to particular cases and are not the norm. Of moreover, they do not guarantee the absence of interactions of one amplification reaction on another, and for reasons including possible hybridizations between the primers, can only be very limited in the number of target sequences amplified by container.
Ces différentes manipulations impliquent de nombreux inconvénients.These different manipulations involve many disadvantages.
En premier lieu, elles sont consommatrices de temps. En second lieu, elles ne sont pas sans risques du point de vue des éventuelles contaminations d'un tube à l'autre ou depuis l'environnement extérieur (poussière, bactérie, aérosol ou tout autre contaminant susceptible de contenir des molécules d'acides nucléiques ou des molécules susceptibles d'influer sur l'efficacité de la réaction d'amplification). De plus, elles n'assurent pas une homogénéité de volume et de concentration en réactifs d'un tube à l'autre. Enfin, elles imposent l'utilisation de volumes manipulables manuellement, généralement supérieurs à 1µl, ce qui a une incidence sur les coûts liés à la réalisation des PCR, les réactifs utilisés étant chers.In the first place, they are time consuming. Second, they are not without risk from the point of view of possible contamination from one tube to another or from the external environment (dust, bacteria, aerosol or any other contaminant likely to contain nucleic acid molecules or molecules likely to influence the efficiency of the amplification reaction). In addition, they do not ensure homogeneity of volume and concentration of reagents from one tube to another. Finally, they require the use of manually manipulable volumes, generally greater than 1 .mu., which has an impact on the costs associated with carrying out the PCRs, the reagents used being expensive.
L'utilisation de dispositifs conçus pour automatiser au moins partiellement de telles manipulations permet de pallier certains de ces inconvénients. Toutefois, de tels automates sont relativement chers et leur utilisation ne se trouve donc généralement économiquement justifiée que dans le cas des PCR en grandes séries, par exemple pour le séquençage des génomes.The use of devices designed to automate at least partially such manipulations overcomes some of these disadvantages. However, such automata are relatively expensive and their use is therefore generally economically justified only in the case of large-scale PCRs, for example for the sequencing of genomes.
Il existe aussi certains automates permettant de réaliser des réactions de PCR cinétiques. Comme il a été vu plus haut, la réalisation d'une PCR cinétique nécessite de quantifier en temps réel, et de façon spécifique, la séquence cible amplifiée. L'utilisation d'un intercalant fluorescent dans le mélange réactionnel permet de mesurer l'augmentation de la quantité totale d'ADN double brin dans ledit mélange. Toutefois, cette méthode ne permet pas de discriminer l'amplification de la séquence cible par rapport au bruit de fond ou à une éventuelle amplification non spécifique. Plusieurs systèmes de sondes ont été décrits récemment pour permettre de mesurer spécifiquement l'amplification d'une séquence cible déterminée. Ils sont basés sur des oligonucléotides complémentaires de ladite séquence, et liés à des couples de groupements de fluorophores ou fluorophores/quenchers, de telle sorte que l'hybridation de la sonde à sa cible et les cycles d'amplification successifs entraînent, suivant les cas, une augmentation ou une diminution de la fluorescence totale du mélange, proportionnellement à l'amplification de la séquence cible.There are also some automata for performing kinetic PCR reactions. As has been seen above, carrying out a kinetic PCR requires quantifying in real time, and specifically, the amplified target sequence. The use of a fluorescent intercalant in the reaction mixture makes it possible to measure the increase in the total amount of double-stranded DNA in said mixture. However, this method does not discriminate amplification of the target sequence with respect to background noise or a possible non-specific amplification. Several probe systems have recently been described to specifically measure the amplification of a specific target sequence. They are based on oligonucleotides complementary to said sequence, and linked to couples of fluorophore or fluorophore / quencher moieties, such that hybridization of the probe to its target and subsequent amplification cycles result, depending on the case an increase or decrease in the total fluorescence of the mixture, in proportion to the amplification of the target sequence.
A titre d'exemples de sondes utilisables pour réaliser des PCR cinétiques, on peut citer le système TaqMan™ (ABI®), le système AmpliSensor™ (InGen), et le système Sunrise™ (Oncor®, Appligène®).Examples of useful probes for performing kinetic PCR include the TaqMan ™ system (ABI ®), the AmpliSensor ™ system (InGen) and the Sunrise ™ system (Oncor ®, ® Appligene).
Le système le plus utilisé actuellement est le système Taq Man™.The most used system today is the Taq Man ™ system .
Ce procédé associe les activités ADN polymérase et 5'→3' nucléase de la Taq polymérase au cours de la PCR. Son principe en est le suivant : en sus des deux amorces de séquence complémentaire de celle de la cible à quantifier, une sonde, appelée sonde reporter, est ajoutée dans le milieu réactionnel. Elle a la capacité de s'hybrider à la cible dans le corps de la séquence amplifiée, mais ne peut être amplifiée elle-même. En effet, un groupe phosphoryle ajouté à l'extrémité 3' de la sonde empêche son extension par la Taq polymérase. Un dérivé de la fluorescéine et un dérivé de la rhodamine sont incorporés dans la sonde, respectivement aux extrémités 5' et 3'. La sonde est de petite taille, aussi le dérivé de la rhodamine, situé à proximité de la fluorescéine, absorbe-t-il l'énergie émise par la fluorescéine soumise à une source d'excitation (phénomène de quenching).This method combines the DNA polymerase and 5 '→ 3' nuclease activities of Taq polymerase during PCR. Its principle is as follows: in addition to the two primers of sequence complementary to that of the target to be quantified, a probe, called probe reporter, is added in the reaction medium. It has the ability to hybridize to the target in the body of the amplified sequence, but can not be amplified itself. Indeed, a phosphoryl group added to the 3 'end of the probe prevents its extension by Taq polymerase. A fluorescein derivative and a rhodamine derivative are incorporated into the probe at the 5 'and 3' ends, respectively. The probe is small, so the rhodamine derivative, located near the fluorescein, absorbs the energy emitted by the fluorescein subjected to a source of excitation ( quenching phenomenon).
Une fois les amorces hybridées à la cible, au cours de la réaction d'extension, la Taq ADN polymérase attaque la sonde par son activité 5' nucléase, libérant le groupement quencher et rétablissant ainsi l'émission de fluorescence. L'intensité de la fluorescence émise est alors proportionnelle à la quantité de produits de PCR formés, ce qui permet d'obtenir un résultat quantitatif. La fluorescence émise est proportionnelle au nombre de molécules cibles de départ. La cinétique de développement de la fluorescence peut être suivie en temps réel au cours de la réaction d'amplification.Once the primers hybridized to the target, during the extension reaction, the Taq DNA polymerase attacks the probe by its 5 'activity. nuclease, releasing the quencher group and thereby restoring the fluorescence emission. The intensity of the fluorescence emitted is then proportional to the quantity of PCR products formed, which makes it possible to obtain a quantitative result. The fluorescence emitted is proportional to the number of starting target molecules. The kinetics of fluorescence development can be monitored in real time during the amplification reaction.
Cette technique présente l'avantage d'être facilement automatisable. Un appareil permettant de réaliser cette technique, l'ABI Prism 7700™, est commercialisé par la société Perkin-Elmer. Cet appareil combine un thermocycleur et un fluorimètre. Il est capable de détecter l'augmentation de fluorescence générée au cours d'un test de quantification selon le procédé TaqMan™, ceci grâce à des fibres optiques situées au-dessous de chaque tube et reliées à une caméra CCD qui détecte, en temps réel, le signal émis par les groupes fluorescents libérés au cours de la PCR. Les données quantitatives sont déduites à partir de la détermination du cycle auquel le signal du produit d'amplification atteint un certain seuil déterminé par l'utilisateur. Plusieurs études ont en effet montré que ce nombre de cycles était proportionnel à la quantité de matériel initial (Gibson, Heid et al. 1996; Heid, Stevens et al. 1996; Williams, Giles et al. 1998).This technique has the advantage of being easily automatable. An apparatus for carrying out this technique, the ABI Prism 7700 ™ , is marketed by Perkin-Elmer. This device combines a thermocycler and a fluorimeter. It is able to detect the increase in fluorescence generated during a quantification test according to the TaqMan ™ process, thanks to optical fibers located below each tube and connected to a CCD camera that detects, in real time , the signal emitted by the fluorescent groups released during the PCR. The quantitative data are derived from the determination of the cycle at which the signal of the amplification product reaches a certain threshold determined by the user. Several studies have shown that this number of cycles is proportional to the amount of initial material (Gibson, Heid et al., 1996, Heid, Stevens et al., 1996, Williams, Giles et al., 1998).
Le nombre d'applications potentielles d'un tel appareil est considérable, tant en santé humaine qu'en agroalimentaire et en contrôle qualité. Malheureusement, l'ABI Prism 7700™ et les quelques autres appareils concurrents commercialisés actuellement sont extrêmement chers, De plus, ils ne peuvent être utilisés que par un manipulateur qualifié. En pratique, de tels appareils ne sont donc utilisés que dans certaines structures très spécialisées.The number of potential applications of such a device is considerable, both in human health, agri-food and quality control. Unfortunately, the ABI Prism 7700 ™ and the few other competing devices currently on the market are extremely expensive, and they can only be used by a skilled manipulator. In practice, such devices are therefore used only in some very specialized structures.
Il existe donc aujourd'hui un réel besoin pour un système d'amplification d'acides nucléiques, le cas échéant mesurée en temps réel, et qui ne présente pas les inconvénients cités ci-dessus de l'état de la technique.There is therefore today a real need for a nucleic acid amplification system, if necessary measured in real time, and which does not have the drawbacks mentioned above of the state of the art.
L'objectif de la présente invention est de proposer un tel système qui permette de diminuer considérablement le nombre de manipulations nécessaires à la mise en oeuvre d'une méthode d'amplification sur une pluralité de séquences cibles et, en conséquence, de diminuer le temps nécessaire à cette opération.The objective of the present invention is to propose such a system which makes it possible to considerably reduce the number of manipulations required to implement an amplification method on a plurality of target sequences and, consequently, to reduce the time required. necessary for this operation.
Un autre objectif de la présente invention est de proposer un tel système qui minimise les risques de contamination d'un contenant à l'autre.Another object of the present invention is to provide such a system which minimizes the risk of contamination from one container to another.
Un autre objectif de la présente invention est de proposer un tel système qui réduise les volumes de réactifs mis en jeu et donc les coûts.Another objective of the present invention is to propose such a system which reduces the volumes of reagents involved and therefore the costs.
Un autre objectif de la présente invention est de proposer un tel système qui optimise une répartition homogène en volume et en concentration des réactifs nécessaires à la PCR dans les contenants.Another objective of the present invention is to propose such a system which optimizes a homogeneous distribution in volume and in concentration of the reagents necessary for the PCR in the containers.
Un autre objectif est de fournir à tous les utilisateurs potentiels, notamment aux centres hospitaliers, aux laboratoires d'analyses médicales, aux industriels de l'agroalimentaire et aux laboratoires de contrôle sanitaire, un appareil d'utilisation et de maintenance aisées, pour effectuer en routine des amplifications d'acides nucléiques quantifiées en temps réel.Another objective is to provide all potential users, including hospitals, medical laboratories, agribusiness and health control laboratories, an easy-to-use and easy-to-use device to routine quantified nucleic acid amplifications in real time.
Dans cette demande, plusieurs termes sont employés, dont la signification est la suivante :
- Une "réaction d'amplification d'acides nucléiques" fait référence à n'importe quelle méthode d'amplification d'acides nucléiques connue de l'homme du métier. On peut citer, à titre d'exemples et de façon non restrictive, l'Amplification en Chaîne par Polymérase (ACP), plus souvent désignée par l'homme du métier sous son acronyme anglophone, PCR (Polymerase Chain Reaction), la TMA (transcription mediated amplification), la NASBA (nucleic acid sequence base amplification), la 3SR (self sustained sequence replication), l'amplification par déplacement de brin, ou SDA (strand displacement amplifîcation) et la LCR (ligase chain reaction).
La matrice initiale de l'amplification peut être n'importe quel type d'acide nucléique, ADN ou ARN, génomique, plasmidique, recombinant, ADNc, ARNm, ARN ribosomal, ARN viral ou autre. Lorsque la matrice initiale est un ARN, une première étape de transcription réverse est en général réalisée pour obtenir une matrice ADN. Cette étape ne sera en général pas mentionnée dans ce texte, car l'homme du métier sait exactement quand et comment la réaliser. Il est bien entendu que les dispositifs de l'invention sont utilisables pour amplifier et éventuellement quantifier spécifiquement des séquences d'ARN aussi bien que d'ADN. Dans la suite du texte, le terme "PCR" sera donc le terme générique utilisé pour désigner aussi bien la PCR proprement dite que la RT-PCR (Reverse Ttranscription - Polymerase Chain Réaction). - Parmi les réactions d'amplification citées ci-dessus, certaines sont isothermes. D'autres, notamment la PCR et la LCR, impliquent de porter le mélange réactionnel à différentes températures au cours du temps, de façon cyclique. De telles réactions sont appelées ici "réactions d'amplification d'acides nucléiques thermo-dépendantes". Dans la suite de ce texte, le dispositif de l'invention sera principalement décrit dans son application à la PCR. Toutefois, il est bien évident que ce dispositif n'est pas limité à cette technique, et qu'il peut être utilisé aussi pour n'importe quelle réaction d'amplification d'acides nucléiques, voire pour d'autres réactions enzymatiques et/ou de biologie moléculaire. Ce dispositif est particulièrement adapté aux réactions qui nécessitent des volumes faibles et le passage cyclique du mélange réactionnel à plusieurs températures, comme cela apparaîtra clairement dans la suite.
- Un des objectifs de la présente invention est de fournir un nouveau dispositif pour effectuer des réactions d'amplification dites "quantitatives", c'est-à-dire permettant de déterminer la concentration de séquence cible initialement présente dans le mélange réactionnel. Plusieurs types de réactions d'amplification quantitatives ont été décrits. On peut distinguer les amplifications quantitatives basées sur l'emploi d'un standard externe, les amplifications compétitives, utilisant un standard interne, et enfin les amplifications cinétiques, dont le principe a été évoqué plus haut, et qui consistent à mesurer en temps réel, l'augmentation de la quantité de la séquence cible. Ce type d'amplification sera désigné ici indifféremment sous les termes "amplification cinétique (d'acides nucléiques)" "PCR cinétique", "amplification (d'acides nucléiques) quantifiée en temps réel", ou encore "PCR en temps réel". Les termes entre parenthèses sont parfois omis.
- Dans cette demande, le terme "réactif' doit être compris au sens large, comme désignant n'importe quel élément nécessaire soit à la réaction d'amplification proprement dite, soit à sa détection. Suivant cette définition, les sels, les dNTP, les amorces ou encore la polymérase, sont des réactifs nécessaires à la PCR. De même, un intercalant fluorescent, ou une sonde, sont ici considérés comme des réactifs participant à la détection des produits amplifiés, bien qu'ils ne réagissent pas au sens littéral.
- A "nucleic acid amplification reaction" refers to any method of nucleic acid amplification known to those skilled in the art. Nonlimiting examples include Polymerase Chain Amplification (PCR), plus frequently designated by those skilled in the art under its acronym, PCR ( Polymerase Chain Reaction ), TMA ( transcription mediated amplification), NASBA ( nucleic acid sequence base amplification ), 3SR ( self-sustained sequence replication ), amplification by strand displacement, or SDA ( strand displacement amplification ) and LCR ( ligase chain reaction ).
The initial template of the amplification can be any type of nucleic acid, DNA or RNA, genomic, plasmidic, recombinant, cDNA, mRNA, ribosomal RNA, viral RNA or other. When the initial template is an RNA, a first reverse transcription step is generally performed to obtain a DNA template. This step will generally not be mentioned in this text, because the skilled person knows exactly when and how to achieve it. It is understood that the devices of the invention are useful for amplifying and optionally quantifying specifically RNA sequences as well as DNA. In the remainder of the text, the term "PCR" will therefore be the generic term used to designate both the actual PCR and the RT-PCR ( Reverse Transcription - Polymerase Chain Reaction ). - Among the amplification reactions mentioned above, some are isothermal. Others, including PCR and LCR, involve carrying the reaction mixture at different temperatures over time, cyclically. Such reactions are referred to herein as "thermo-dependent nucleic acid amplification reactions". In the rest of this text, the device of the invention will be mainly described in its application to PCR. However, it is obvious that this device is not limited to this technique, and that it can also be used for any nucleic acid amplification reaction, or even for other enzymatic reactions and / or molecular biology. This device is particularly suitable for reactions that require low volumes and cycling the reaction mixture at several temperatures, as will become clear later.
- One of the objectives of the present invention is to provide a new device for performing so-called "quantitative" amplification reactions, that is to say, for determining the target sequence concentration initially present in the reaction mixture. Several types of quantitative amplification reactions have been described. We can distinguish the quantitative amplifications based on the use of an external standard, the competitive amplifications, using an internal standard, and finally the kinetic amplifications, the principle of which has been mentioned above, and which consist in measuring in real time, increasing the amount of the target sequence. This type of amplification will be referred to herein as "kinetic amplification (of nucleic acids)""kineticPCR","amplification (of nucleic acids) quantized in real time", or "real-time PCR". The terms in parentheses are sometimes omitted.
- In this application, the term "reagent" should be understood in a broad sense to mean any element necessary for either the amplification reaction itself or for its detection. The primers or the polymerase are necessary reagents for PCR, and a fluorescent intercalator, or a probe, are considered as reagents involved in the detection of the amplified products, although they do not react in the literal sense.
D'autres termes désignant certains éléments du dispositif de l'invention seront définis plus loin dans la description détaillée de invention.Other terms designating certain elements of the device of the invention will be defined later in the detailed description of invention.
Certains éléments du dispositif sont numérotés en référence aux dessins, qui illustrent quelques modes et variantes non limitatifs de réalisation l'invention, et dans lesquels :
- la
figure 1 représente une vue latérale d'une réalisation simplifiée du dispositif selon la présente invention ; - la
figure 2 représente une vue supérieure de la platine chauffante, dans le cas où les blocs (21 à 23) sont des secteurs de disque (figure 2A ), et dans le cas où ils sont constitués de secteurs de couronne (figure 2B ); - la
figure 3 représente une vue en perspective d'un premier exemple de réalisation de la cartouche (1), pourvue des chambres réactionnelles et d'une partie des moyens de déplacement ; - la
figure 4 représente une vue en coupe de cette cartouche en accord partiel avec l'invention selon la ligne AA ; - la
figure 5 représente une vue supérieure de la partie inférieure (socle) d'un deuxième mode de réalisation particulier de la cartouche selon l'invention. Les cotes sont données à titre purement indicatif, et ne sont en aucun cas limitatives; - la
figure 6 représente une vue en coupe de cette cartouche inférieure, selon la ligne AA de lafigure 5 ; - la
figure 7 représente une vue supérieure de la partie supérieure (couverte) de la cartouche représentée auxfigures 5 et 6 ; - la
figure 8 représente une vue en coupe de cette cartouche supérieure, suivant la ligne BB de lafigure 7 ; - la
figure 9 représente une cartouche complète, constituée du socle représenté auxfigures 5 et 6 (traits pleins), et du couvercle représenté auxfigures 7 et 8 (traits discontinus) ; - la
figure 10 montre trois modélisations de la cartouche de lafigure 9 , au-dessus de laquelle se trouvent les moyens d'excitation/mesure de la fluorescence (5) ; - la
figure 11 montre une coupe schématique d'un canal (12) possédant un dispositif de "perte de charge".
- the
figure 1 is a side view of a simplified embodiment of the device according to the present invention; - the
figure 2 represents an upper view of the heating plate, in the case where the blocks (21 to 23) are disk sectors (Figure 2A ), and in the case where they consist of crown sectors (Figure 2B ); - the
figure 3 is a perspective view of a first embodiment of the cartridge (1), provided with the reaction chambers and a portion of the displacement means; - the
figure 4 represents a sectional view of this cartridge in partial agreement with the invention along line AA; - the
figure 5 represents an upper view of the lower part (base) of a second particular embodiment of the cartridge according to the invention. The ratings are given for information only, and are in no way limiting; - the
figure 6 represents a sectional view of this lower cartridge, according to the line AA of thefigure 5 ; - the
figure 7 represents an upper view of the upper (covered) part of the cartridge shown inFigures 5 and 6 ; - the
figure 8 represents a sectional view of this upper cartridge, along the line BB of thefigure 7 ; - the
figure 9 represents a complete cartridge, consisting of the base shown inFigures 5 and 6 (solid lines), and the lid shown inFigures 7 and 8 (discontinuous lines); - the
figure 10 shows three modelizations of the cartridge of thefigure 9 above which are the means for exciting / measuring the fluorescence (5); - the
figure 11 shows a schematic section of a channel (12) having a "pressure drop" device.
L'invention concerne en premier lieu tout dispositif selon l'une queconque des revendications 1 à 16.The invention relates firstly to any device according to one of
La température de chaque zone de la platine peut être homogène ou, le cas échéant, cette température peut varier suivant un gradient.The temperature of each zone of the platen can be homogeneous or, where appropriate, this temperature can vary according to a gradient.
Plusieurs types de réactions de biologie moléculaire nécessitent de placer le mélange réactionnel à différentes températures en fonction du temps. Ceci est le cas par exemple lorsqu'on souhaite inactiver une enzyme après l'avoir utilisée (par exemple, une nucléase de restriction), ou pour tester la stabilité d'un complexe. Dans ce dernier cas, on peut envisager de placer un complexe (par exemple, un complexe antigène/anticorps, ou récepteur/ligand), dont l'un des éléments est couplé à un fluorophore et l'autre à un quencher de fluorescence, dans une des chambres réactionnelles du dispositif. La platine est alors programmée pour présenter plusieurs températures dans un ordre croissant, le cas échéant sous forme d'un gradient. La stabilité du complexe est alors testée en déplaçant la cartouche sur la platine, de façon à ce que la température de la chambre réactionnelle s'élève progressivement, et en observant l'augmentation de la fluorescence, à l'aide de moyens d'excitation / mesure de la fluorescence placés en regard de la chambre réactionnelle. L'augmentation de la fluorescence traduit alors la dissociation du complexe.Several types of molecular biology reactions require placing the reaction mixture at different temperatures as a function of time. This is the case, for example, when it is desired to inactivate an enzyme after using it (for example, a restriction nuclease), or to test the stability of a complex. In the latter case, it is conceivable to place a complex (for example, an antigen / antibody complex, or receptor / ligand), one of which is coupled to a fluorophore and the other to a quencher of fluorescence, one of the reaction chambers of the device. The stage is then programmed to present several temperatures in increasing order, possibly in the form of a gradient. The stability of the complex is then tested by moving the cartridge on the plate, so that the temperature of the reaction chamber rises gradually, and observing the increase in fluorescence, using excitation means measurement of the fluorescence placed opposite the reaction chamber. The increase in fluorescence then reflects the dissociation of the complex.
Le dispositif de l'invention est particulièrement adapté à des réactions nécessitant une variation cyclique de la température des chambres réactionnelles, ce qui est le cas pour certaines réactions d'amplifications d'acides nucléiques, par exemple pour l'amplification en chaîne par polymérase (PCR), ou pour la réaction en chaîne par ligase (LCR). The device of the invention is particularly suitable for reactions requiring a cyclic variation in the temperature of the reaction chambers, which is the case for certain nucleic acid amplification reactions, for example for polymerase chain reaction ( PCR ), or for the ligase chain reaction (LCR).
L'invention porte donc en particulier sur un dispositif pour l'amplification en chaîne thermo-dépendante de séquences d'acides nucléiques cibles selon l'une des revendications 1 à 16.The invention therefore relates in particular to a device for the thermo-dependent chain amplification of target nucleic acid sequences according to one of
Un tel système selon l'invention est moins complexe que les systèmes de l'art antérieur, dans la mesure où les températures nécessaires aux cycles de l'amplification en chaîne sont assurées par des zones distinctes de températures constantes et non par une platine dont on doit faire varier la température.Such a system according to the invention is less complex than the systems of the prior art, insofar as the temperatures required for the cycles of the chain amplification are provided by distinct zones of constant temperatures and not by a plate of which one must vary the temperature.
Il est important de noter que les réactions d'amplification en chaîne thermo-dépendantes nécessitent le passage des échantillons à au moins deux températures. Par exemple, la LCR nécessite à chaque cycle une phase à environ 95°C pour dénaturer l'ADN cible, puis une phase entre 55 et 65°C (en fonction du Tm des sondes), pour donner lieu à l'hybridation / ligation. En ce qui concerne la PCR, chaque cycle se décompose en général en trois phases, à savoir la dénaturation à environ 95°C, l'hybridation dont la température dépend du Tm des sondes, et l'élongation, habituellement réalisée à 72°C. Il est cependant possible de réaliser des PCR ayant des cycles simplifiés, dans lesquels l'hybridation et l'élongation se font à la même température, si bien que chaque cycle nécessite seulement deux températures différentes.It is important to note that thermo-dependent chain amplification reactions require the passage of samples at at least two temperatures. For example, the CSF requires at each cycle a phase at about 95 ° C to denature the target DNA, then a phase between 55 and 65 ° C (depending on the Tm probes) to give rise to hybridization / ligation . With regard to PCR, each cycle generally breaks down into three phases, namely denaturation at about 95 ° C, hybridization whose temperature depends on the Tm of the probes, and elongation, usually performed at 72 ° C. . However, it is possible to perform PCRs with simplified cycles, in which the hybridization and elongation are at the same temperature, so that each cycle requires only two different temperatures.
Il pourra être envisagé différentes variantes du dispositif décrit ci-dessus. Selon une variante préférentielle de l'invention, le système comprend les caractéristiques suivantes :
- des amorces spécifiques de séquences cibles à amplifier sont pré-réparties dans les chambres réactionnelles (13),
- le réservoir (11) est destiné à recevoir un fluide composé notamment d'un échantillon d'acides nucléiques à analyser et des réactifs nécessaires à une réaction d'amplification en chaîne par polymérase, à l'exception des amorces,
- la platine de chauffage (2) présente trois zones distinctes pouvant être portées à trois températures différentes, correspondant aux trois phases des cycles d'amplification en chaîne par polymérase.
- primers specific for target sequences to be amplified are pre-distributed in the reaction chambers (13),
- the reservoir (11) is intended to receive a fluid composed in particular of a sample of nucleic acids to be analyzed and reagents necessary for a polymerase chain reaction reaction, with the exception of the primers,
- the heating stage (2) has three distinct zones which can be raised to three different temperatures, corresponding to the three phases of the polymerase chain amplification cycles.
Selon cette variante préférentielle, il est possible de distribuer, à partir d'un réservoir, un fluide contenant un échantillon d'acides nucléiques à analyser et les réactifs nécessaires à la PCR dans une pluralité de chambres réactionnelles contenant des amorces spécifiques de séquences cibles d'acides nucléiques à amplifier, et d'autoriser le processus d'amplification en soumettant le contenu des chambres successivement à différentes températures (à savoir celles nécessaires à la dénaturation, l'hybridation et l'élongation) une multitude de fois grâce à un mouvement relatif entre la cartouche incluant lesdites chambres réactionnelles et ladite platine de chauffage présentant deux ou trois zones distinctes pouvant être portées à des températures différentes.According to this preferred embodiment, it is possible to dispense, from a reservoir, a fluid containing a sample of nucleic acids to be analyzed and the reagents necessary for the PCR in a plurality of reaction chambers containing specific primers of target sequences. nucleic acids to be amplified, and to allow the amplification process by subjecting the contents of the chambers successively to different temperatures (namely those necessary for denaturation, hybridization and elongation) a multitude of times through a movement relative between the cartridge including said reaction chambers and said heating platen having two or three distinct zones that can be brought to different temperatures.
Le cas échéant, les chambres réactionnelles (13) peuvent contenir des réactifs nécessaires à une réaction de PCR en temps réel autres que les amorces mentionnées ci-dessus. Dans une réalisation préférée du dispositif de l'invention, les chambres réactionnelles comportent également, en plus des amorces, une ou plusieurs sonde(s) spécifique(s) de la séquence à amplifier. La distribution des sondes dans les chambres réactionnelles peut aussi être telle que certaines chambres comportent des sondes spécifiques des séquences à amplifier et d'autres chambres comportent des sondes contrôles, ne reconnaissant pas a priori la séquence à amplifier. Ces sondes peuvent être marquées et, si plusieurs sondes sont présentes dans une même chambre réactionnelle (par exemple une sonde spécifique de la séquence à amplifier et une sonde contrôle), ces sondes seront de préférence marquées par des fluorophores différents.If desired, the reaction chambers (13) may contain reagents necessary for a real-time PCR reaction other than the primers mentioned above. In a preferred embodiment of the device of the invention, the reaction chambers also comprise, in addition to the primers, one or more specific probe (s) of the sequence to be amplified. The distribution of the probes in the reaction chambers may also be such that some chambers contain probes specific for the sequences to be amplified and other chambers comprise control probes, not recognizing a priori the sequence to be amplified. These probes may be labeled and, if several probes are present in the same reaction chamber (for example a probe specific for the sequence to be amplified and a control probe), these probes will preferably be labeled with different fluorophores.
Dans une autre variante du dispositif, des réactifs supplémentaires, tels que les dNTP ou des sels, sont initialement déposés dans les chambres réactionnelles. Ces réactifs seront alors absents, ou présents en moindre quantité, dans le fluide déposé dans le réservoir (11). Dans le cas extrême, tous les réactifs nécessaires à la réaction de PCR, à l'exception de la matrice, sont déposés dans les chambres réactionnelles (13), et le fluide déposé dans le réservoir (11) comprendra alors uniquement l'échantillon d'ADN (ou d'ARN) à amplifier.In another variant of the device, additional reagents, such as dNTPs or salts, are initially deposited in the reaction chambers. These reagents will then be absent, or present in a smaller amount, in the fluid deposited in the reservoir (11). In the extreme case, all the reagents necessary for the PCR reaction, with the exception of the matrix, are deposited in the reaction chambers (13), and the fluid deposited in the reservoir (11) will then comprise only the sample. DNA (or RNA) to amplify.
Les variantes décrites ci-dessus supposent que plusieurs réactions soient réalisées en parallèle, avec des amorces et/ou des sondes différentes, sur un même échantillon. Il s'agit donc de la caractérisation d'un échantillon unique (ou de quelques échantillons si le réservoir est divisé en quelques sous-réservoirs) suivant plusieurs critères. Dans certaines applications, on souhaite au contraire caractériser une multitude d'échantillons suivant un critère unique ou un faible nombre de critères. Ceci est le cas par exemple en recherche, lorsqu'on souhaite cribler une banque de phages ou de bactéries pour la présence d'un gène donné. Il est dans ce cas nécessaire d'effectuer une PCR sur un grand nombre d'échantillons, à partir d'un couple d'amorces donné. Le dispositif de l'invention est adapté aussi à ce type de manipulations. Pour cela, les échantillons sont déposés dans les chambres réactionnelles (13). Les amorces peuvent être introduites dans le fluide déposé dans le réservoir (11), avec les autres réactifs nécessaires à la PCR. Bien entendu, cette configuration n'exclut pas non plus que certains réactifs autres que l'échantillon à analyser soient pré-déposés dans les chambres réactionnelles (13).The variants described above assume that several reactions are carried out in parallel, with different primers and / or probes, on the same sample. It is therefore the characterization of a single sample (or a few samples if the reservoir is divided into a few sub-reservoirs) according to several criteria. In some applications, on the contrary, it is desired to characterize a multitude of samples according to a single criterion or a small number of criteria. This is the case for example in research, when it is desired to screen a library of phages or bacteria for the presence of a given gene. In this case, it is necessary to carry out a PCR on a large number of samples, from a given pair of primers. The device of the invention is also suitable for this type of manipulation. For this, the samples are deposited in the reaction chambers (13). The primers can be introduced into the fluid deposited in the reservoir (11), along with the other reagents necessary for the PCR. Of course, this configuration does not exclude that certain reagents other than the sample to be analyzed are pre-deposited in the reaction chambers (13).
Quelle que soit la variante du dispositif choisie, et quels que soient les réactifs déposés dans les chambres réactionnelles (13), ils peuvent y être déposés avantageusement par un simple dépôt liquide, suivi d'un séchage. L'arrivée du fluide en provenance du réservoir (11) permet ensuite la remise en solution de ces réactifs. La quantité de chaque réactif déposée est calculée en fonction du volume de fluide qui pénétrera dans chaque chambre réactionnelle (13), de telle sorte que la remise en solution des réactifs aboutisse à la concentration finale souhaitée pour chacun d'entre eux. Des cartouches telles que décrites ci-dessus, dans lesquelles au moins une partie des chambres réactionnelles (13) comportent des réactifs qui y ont été chargés par un dépôt liquide, suivi d'un séchage, de telle sorte que ces réactifs soient remis en solution par l'arrivée d'un fluide dans ces chambres réactionnelles, sont également partie intégrante de l'invention.Whatever the variant of the device chosen, and whatever the reagents deposited in the reaction chambers (13), they can be advantageously deposited there by a simple liquid deposit, followed by drying. The arrival of the fluid from the reservoir (11) then allows the re-solution of these reagents. The amount of each reagent deposited is calculated as a function of the volume of fluid that will penetrate into each reaction chamber (13), so that the reactivation of the reagents will result in the desired final concentration for each of them. Cartridges as described above, in which at least a portion of the reaction chambers (13) comprise reagents which have been loaded therein by a liquid deposit, followed by drying, so that these reagents are returned to solution by the arrival of a fluid in these reaction chambers, are also an integral part of the invention.
Le dispositif décrit ci-dessus présente l'avantage d'autoriser un remplissage concomitant de toutes les chambres réactionnelles, ce qui diminue le temps de préparation et les risques de contamination d'une chambre à l'autre. Ce dispositif présente également l'avantage de pouvoir être miniaturisé et d'impliquer l'utilisation de volumes de réactifs plus faibles que dans l'état de la technique.The device described above has the advantage of allowing a concomitant filling of all the reaction chambers, which reduces the preparation time and the risks of contamination from one chamber to another. This device also has the advantage of being able to be miniaturized and to involve the use of smaller volumes of reagents than in the state of the art.
Enfin on notera aussi que, grâce à la platine de chauffage spécifique qu'elle préconise, l'invention permet d'accélérer les cycles de PCR, puisqu'il n'est pas nécessaire pour effectuer les différentes phases (dénaturation, hybridation, élongation) de faire varier la température de la platine chauffante ou de l'atmosphère comme dans l'état de la technique, le mouvement relatif entre la cartouche et la platine permettant de soumettre rapidement et successivement le contenu de chacune des chambres réactionnelles à trois températures distinctes dédiées à chacune de ces phases. L'utilisation de faibles volumes réactionnels, et d'un plancher de faible épaisseur pour la cartouche (1), permettent aussi de limiter l'inertie thermique au niveau des chambres réactionnelles, et contribuent donc à la rapidité de la réaction.Finally, it will also be noted that, thanks to the specific heating platinum that it recommends, the invention makes it possible to accelerate PCR cycles, since it is not necessary to perform the various phases (denaturation, hybridization, elongation). to vary the temperature of the heating stage or of the atmosphere as in the state of the art, the relative movement between the cartridge and the plate making it possible to quickly and successively submit the contents of each of the chambers reaction at three distinct temperatures dedicated to each of these phases. The use of small reaction volumes, and a thin floor for the cartridge (1), also limit the thermal inertia at the reaction chambers, and therefore contribute to the speed of the reaction.
L'invention porte également sur un dispositif pour l'amplification en chaîne thermo-dépendante de séquences d'acides nucléiques cibles, mesurée en temps réel, caractérisé en ce qu'il comprend les mêmes éléments que l'un quelconque des dispositifs décrits ci-dessus, et comportant en outre des moyens optiques (5) d'excitation / mesure de la fluorescence, disposés de manière à exciter et mesurer à chaque cycle la fluorescence du contenu des chambres réactionnelles.The invention also relates to a device for thermo-dependent chain amplification of target nucleic acid sequences, measured in real time, characterized in that it comprises the same elements as any of the devices described above. above, and further comprising optical means (5) for excitation / measurement of fluorescence, arranged to excite and measure at each cycle the fluorescence of the contents of the reaction chambers.
Un des éléments particulièrement originaux des dispositifs décrits ci-dessus est l'élément appelé indifféremment plaque ou cartouche réactionnelle (1). Cet élément peut être recyclable ou, de façon préférée, consommable, et constitue en soi un aspect de la présente invention. Ainsi, l'invention de préférence utilise une cartouche réactionnelle comportant plusieurs chambres réactionnelles (13) et au moins un réservoir (11) et présentant les caractéristiques suivantes :
- chaque chambre réactionnelle est reliée au réservoir par un canal (12) ayant une section droite comprise dans un cercle de diamètre inférieur à 3 mm,
- la capacité du réservoir est inférieure à 10 ml,
- la disposition des chambres réactionnelles et des canaux par rapport au réservoir permet de répartir un fluide de façon homogène dans les chambres réactionnelles, à partir du réservoir.
- each reaction chamber is connected to the reservoir by a channel (12) having a cross-section included in a circle of diameter less than 3 mm,
- the capacity of the tank is less than 10 ml,
- the arrangement of the reaction chambers and the channels with respect to the reservoir makes it possible to distribute a fluid homogeneously in the reaction chambers from the reservoir.
Le diamètre des canaux sera préférentiellement choisi suffisamment faible pour ne pas autoriser une distribution par gravité du fluide présent dans le réservoir dans les chambres réactionnelles, ceci de façon à éviter un remplissage non reproductible de ces chambres. Ce diamètre sera ainsi préférentiellement inférieur ou égal à environ 0,2 mm. Au sujet de ce diamètre, on notera que la section des canaux sera préférentiellement circulaire mais qu'elle pourra être également de toute autre forme et notamment polygonale, le "diamètre" des canaux visant alors leur plus grande largeur en section.The diameter of the channels will preferably be chosen small enough not to allow a gravity distribution of the fluid present in the tank in the reaction chambers, so as to avoid non-reproducible filling of these chambers. This diameter will thus preferably be less than or equal to approximately 0.2 mm. With regard to this diameter, it will be noted that the section of the channels will preferably be circular but that it may also be of any other shape and in particular polygonal, the "diameter" of the channels then aiming at their greatest width in section.
Le réservoir destiné à recevoir l'échantillon d'acides nucléiques et les réactifs nécessaires à la PCR pourra présenter une capacité variable, comprise par exemple entre environ 0,1 ml et environ 1 ml.The reservoir intended to receive the sample of nucleic acids and the reagents necessary for the PCR may have a variable capacity, for example between about 0.1 ml and about 1 ml.
La cartouche comprend de préférence entre environ 20 et environ 500 chambres réactionnelles et, de manière encore préférée, entre 60 et 100 chambres réactionnelles.The cartridge preferably comprises between about 20 and about 500 reaction chambers and, more preferably, between 60 and 100 reaction chambers.
Le volume de ces chambres pourra également varier selon les modes de réalisation. Avantageusement, ces chambres présentent un volume compris entre environ 0,2 et 50 µl, de préférence entre 1 µl et 10 µl.The volume of these rooms may also vary according to the embodiments. Advantageously, these chambers have a volume of between approximately 0.2 and 50 μl, preferably between 1 μl and 10 μl.
Dans les cartouches, la jonction entre les canaux (12) et le réservoir (11) se fait à la périphérie du réservoir, et le fond dudit réservoir est incliné et/ou convexe, de façon à assurer la répartition d'un fluide contenu dans le réservoir au niveau de l'entrée des canaux.In the cartridges, the junction between the channels (12) and the reservoir (11) is at the periphery of the reservoir, and the bottom of said reservoir is inclined and / or convex, so as to ensure the distribution of a fluid contained in the tank at the entrance of the channels.
On notera qu'une cartouche utilisée selon l'invention peut présenter de multiples formes. Toutefois, selon une variante préférentielle de l'invention, cette cartouche présente une forme circulaire. Le réservoir est prévu sensiblement au centre de la cartouche, les chambres réactionnelles étant réparties en cercle autour du réservoir, et les canaux reliant le réservoir aux chambres étant prévus essentiellement radialement. Une telle architecture permet d'optimiser le remplissage des chambres réactionnelles à partir du réservoir central.It will be noted that a cartridge used according to the invention may have multiple forms. However, according to a preferred variant of the invention, this cartridge has a circular shape. The reservoir is provided substantially in the center of the cartridge, the reaction chambers being distributed in a circle around the reservoir, and the channels connecting the reservoir to the chambers being provided essentially radially. Such an architecture allows to optimize the filling of the reaction chambers from the central tank.
Dans une réalisation particulière des cartouches circulaires de l'invention, le fond du réservoir (11) est conique.In a particular embodiment of the circular cartridges of the invention, the bottom of the tank (11) is conical.
Egalement préférentiellement, lesdites chambres réactionnelles sont prévues relativement à la périphérie de ladite cartouche. Ainsi, il est possible d'optimiser le nombre de chambres réactionnelles pouvant être prévues sur la cartouche et remplies à partir du réservoir central.Also preferably, said reaction chambers are provided relative to the periphery of said cartridge. Thus, it is possible to optimize the number of reaction chambers that can be provided on the cartridge and filled from the central tank.
Selon une variante de l'invention, une telle cartouche comprend autant de canaux que de chambres réactionnelles. Toutefois, dans certains modes de réalisation, on pourra prévoir des tronçons de canaux communs à plusieurs chambres réactionnelles.According to one variant of the invention, such a cartridge comprises as many channels as there are reaction chambers. However, in some embodiments, it will be possible to provide channel sections common to several reaction chambers.
L'un des avantages de la présente invention est d'autoriser une miniaturisation du dispositif qu'elle propose. Ainsi, avantageusement, la cartouche, présente de préférence un diamètre compris entre environ 1 et 10 cm.One of the advantages of the present invention is to allow a miniaturization of the device that it proposes. Thus, advantageously, the cartridge preferably has a diameter of between about 1 and 10 cm.
Une variante des cartouches de l'invention décrites ci-dessus, quelle que soit leur géométrie, consiste à diviser le réservoir (11) en 2 à 20, de préférence 2 à 8 sous-réservoirs, permettant d'analyser simultanément plusieurs échantillons sur une même cartouche. Dans ce cas, chacune des chambres réactionnelles (13) est reliée à un seul de ces sous-réservoirs par un canal (12).A variant of the cartridges of the invention described above, whatever their geometry, consists in dividing the reservoir (11) into 2 to 20, preferably 2 to 8 sub-reservoirs, making it possible to simultaneously analyze several samples on a same cartridge. In this case, each of the reaction chambers (13) is connected to only one of these sub-tanks by a channel (12).
La profondeur des chambres réactionnelles (par rapport aux canaux) peut aussi varier en fonction des modes de réalisation de invention. Selon une variante préférentielle, ces chambres présentent une profondeur comprise entre environ 0,5 mm et 1,5 mm.The depth of the reaction chambers (with respect to the channels) may also vary depending on the embodiments of the invention. According to a preferred variant, these chambers have a depth of between approximately 0.5 mm and 1.5 mm.
On notera par ailleurs que l'épaisseur de la cartouche dépend de plusieurs facteurs et notamment du matériau la constituant. En pratique, cette cartouche est préférentiellement constituée en matière plastique, de préférence en polycarbonate, dont les propriétés physiques, optiques et thermiques, sont appropriées pour la réalisation de la présente invention. L'épaisseur des cartouches de l'invention est de préférence comprise entre 0,5 et 5 mm.Note also that the thickness of the cartridge depends on several factors including the material constituting it. In practice, this cartridge is preferably made of plastic material, preferably polycarbonate, whose physical, optical and thermal properties are suitable for carrying out the present invention. The thickness of the cartridges of the invention is preferably between 0.5 and 5 mm.
Afin de faciliter les échanges thermiques entre le contenu des chambres réactionnelles et la platine, l'épaisseur du "plancher" de celles-ci devra être préférentiellement aussi faible que possible. Cette épaisseur dépend du matériau utilisé pour réaliser la cartouche. Préférentiellement, elle est comprise entre 0,05 et 0,5 mm, par exemple environ 0,25 mm.In order to facilitate the heat exchange between the contents of the reaction chambers and the platinum, the thickness of the "floor" thereof will preferably be as low as possible. This thickness depends on the material used to make the cartridge. Preferably, it is between 0.05 and 0.5 mm, for example about 0.25 mm.
Les chambres réactionnelles des cartouches de l'invention sont de préférence fermées par une paroi supérieure transparente (17), par exemple en plastique transparent, afin de permettre l'excitation et la mesure de la fluorescence du fluide réactionnel, dans des bonnes conditions.The reaction chambers of the cartridges of the invention are preferably closed by a transparent upper wall (17), for example of transparent plastic, in order to allow the excitation and the measurement of the fluorescence of the reaction fluid, in good conditions.
Dans une réalisation particulière de l'invention, les chambres sont pourvues d'évents (système ouvert), permettant à l'air qu'elles contiennent de s'échapper lors de leur remplissage par le fluide provenant du réservoir.In a particular embodiment of the invention, the chambers are provided with vents (open system), allowing the air they contain to escape during their filling by the fluid from the tank.
Dans le cas ci-dessus, où les chambres (13) sont pourvues d'évents (14), les canaux (12) sont préférentiellement constitués d'au moins deux parties de diamètres différents (121 et 122), le diamètre de la deuxième partie (122) étant inférieur à celui de la première partie (121), de façon à créer une perte de charge dans le canal (12). Ainsi, si un canal se remplit plus vite qu'un autre sous l'effet de la pression, le phénomène de perte de charge permet d'arrêter la progression du fluide dans le ou les canaux dont la première partie (121) est remplie, jusqu'à ce que tous les canaux soient remplis de la même façon. Ceci permet de « pré-calibrer » les volumes pour chaque canal, afin d'assurer un remplissage homogène des différentes chambres réactionnelles. La deuxième partie du canal (122) peut être constituée par exemple d'un capillaire de verre, de diamètre beaucoup plus faible que la première partie (121), ledit capillaire étant inclus dans une cartouche en plastique.In the above case, where the chambers (13) are provided with vents (14), the channels (12) preferably consist of at least two parts of different diameters (121 and 122), the diameter of the second part (122) being smaller than that of the first part (121), so as to create a pressure drop in the channel (12). Thus, if one channel fills faster than another under the effect of pressure, the pressure drop phenomenon stops the progression of the fluid in the channel or channels whose first portion (121) is filled, until all the channels are filled in the same way. This allows to "pre-calibrate" the volumes for each channel, to ensure a homogeneous filling of the different reaction chambers. The second portion of the channel (122) may consist for example of a glass capillary, much smaller in diameter than the first portion (121), said capillary being included in a plastic cartridge.
Il est aussi possible de prévoir des enceintes (15) dans lesquelles débouchent les évents (14) des chambres réactionnelles. Ces enceintes possèdent une ouverture (16) vers l'extérieur de la cartouche (système ouvert), et présentent l'intérêt, d'une part, de récupérer sans pollution les éventuels surplus de fluide qui sortiraient des chambres réactionnelles par les évents (14) et, d'autre part, de pouvoir se fermer après le remplissage des chambres réactionnelles. Cette fermeture peut se faire par exemple en utilisant une bande adhésive, et permet de passer en système clos pour effectuer l'amplification proprement dite. Ceci permet d'éviter ou au moins limiter l'évaporation du fluide contenu dans la cartouche (1). Cette réalisation de l'invention est illustrée à la
Alternativement, il est possible de travailler en système clos dès le remplissage des chambres réactionnelles, en provoquant dans la cartouche une dépression suivie d'un rétablissement de la pression, comme cela sera détaillé plus loin. Des cartouches dans lesquelles les chambres réactionnelles ne possèdent pas d'autre ouverture que l'arrivée du canal (12) (chambres réactionnelles dites "closes") font donc également partie de l'invention.Alternatively, it is possible to work in a closed system as soon as the reaction chambers are filled, causing the cartridge to depressurise followed by a reestablishment of the pressure, as will be detailed below. Cartridges in which the reaction chambers have no other opening than the arrival of the channel (12) (so-called "closed" reaction chambers) are also part of the invention.
Les cartouches décrites ci-dessus, prévues soit pour un usage en système ouvert, soit pour un usage en système fermé, comportent de préférence une ouverture adaptable à des moyens (4) de modulation de la pression dans le réservoir (11), permettant de déplacer le fluide présent dans le réservoir vers les chambres réactionnelles.The cartridges described above, provided for use in an open system or for use in a closed system, preferably comprise an opening that can be adapted to means (4) for modulating the pressure in the reservoir (11), making it possible to moving the fluid present in the reservoir to the reaction chambers.
L'invention porte également sur un procédé de remplissage en système clos des chambres réactionnelles (13) d'une cartouche (1) telle que décrite au paragraphe précédent, dans la variante où les chambres réactionnelles sont closes, lequel procédé comporte les étapes suivantes :
- emplir au moins partiellement le réservoir (11) avec un fluide,
- connecter la cartouche (1) aux moyens (4) de modulation de la pression,
- appliquer une dépression à l'intérieur de la cartouche, puis rétablir la pression.
- at least partially filling the reservoir (11) with a fluid,
- connect the cartridge (1) to the means (4) for modulating the pressure,
- apply a vacuum inside the cartridge, then restore the pressure.
Dans une variante des cartouches de invention, chaque canal (12) est équipé d'une cavité anti-reflux (123) au niveau de sa jonction avec le réservoir (11), ladite cavité anti-reflux étant constituée d'une portion de canal sensiblement verticale, d'un diamètre supérieur ou égal à celui du canal (12). Cette variante présente deux principaux avantages. D'une part, ces cavités anti-reflux permettent de prévenir les contaminations croisées en cas de retour intempestif de fluide vers le réservoir (11), ou au cas où tout le fluide ne se serait pas engagé dans les canaux. D'autre part, ces cavités permettent de prévoir, dans les dispositifs de l'invention, un bouchon dont les dentelures viennent épouser ces entrées verticales, afin de boucher les canaux après l'adressage du fluide rédactionnel mais avant la réaction d'amplification. Ceci permet de travailler en système parfaitement clos, et donc d'éviter tout risque de contamination et d'évaporation. Toutefois, il est important de noter que les cavités anti-reflux, et l'utilisation d'un bouchon au niveau du réservoir pour boucher l'entrée des canaux du côté du réservoir, peuvent être employées aussi dans le cas de systèmes ouverts tels que décrits ci-dessus, où les chambres réactionnelles sont pourvues d'évents.In a variant of the cartridges of the invention, each channel (12) is equipped with an anti-reflux cavity (123) at its junction with the reservoir (11), said anti-reflux cavity consisting of a substantially vertical channel portion of a diameter greater than or equal to that of the channel (12). This variant has two main advantages. On the one hand, these anti-reflux cavities make it possible to prevent cross-contamination in the event of an inadvertent return of fluid to the reservoir (11), or in the case where all the fluid has not been engaged in the channels. On the other hand, these cavities make it possible to provide, in the devices of the invention, a plug whose serrations come to marry these vertical inlets, in order to plug the channels after the addressing of the editorial fluid but before the amplification reaction. This allows to work in perfectly closed system, and thus to avoid any risk of contamination and evaporation. However, it is important to note that the anti-reflux cavities, and the use of a plug at the reservoir to plug the entrance of the channels on the tank side, can be used also in the case of open systems such as described above, where the reaction chambers are provided with vents.
Dans une réalisation préférée des cartouches de l'invention, une partie au moins des chambres réactionnelles (13) comporte des oligonucléotides. De façon encore préférée, chacune des chambres réactionnelles (13) comporte deux amorces spécifiques d'une séquence d'acide nucléique à amplifier et, facultativement, une ou plusieurs sonde(s) marquée(s) spécifique(s) de ladite séquence. Une telle sonde peut être marquée de façon à ce que sont signal augmente lorsqu'elle s'hybride à sa séquence cible (système Sunrise™), ou de façon à ce que l'élongation à partir d'un brin sur laquelle elle est hybridée entraîne une diminution ou une augmentation du signal (système AmpliSensor™ ou système TaqMan™, respectivement). La présence de telles sondes dans les chambres réactionnelles permet de réaliser des amplifications quantifiées en temps réel, avec un dispositif de l'invention disposant de moyens (5) d'excitation / mesure de la fluorescence, tel que décrit plus haut. Des sondes contrôles, non spécifiques de la séquence à amplifier, et marquées d'une façon différente des sondes spécifiques, peuvent aussi être utilisées, pour détecter d'éventuelles contaminations.In a preferred embodiment of the cartridges of the invention, at least a portion of the reaction chambers (13) comprise oligonucleotides. More preferably, each of the reaction chambers (13) comprises two primers specific for a nucleic acid sequence to be amplified and, optionally, one or more labeled probe (s) specific for said sequence. Such a probe may be labeled so that its signal is increased when it hybridizes to its target sequence (Sunrise ™ system), or so that elongation from a strand on which it is hybridized causes a decrease or increase in the signal (AmpliSensor ™ system or TaqMan ™ system, respectively). The presence of such probes in the reaction chambers makes it possible to carry out quantized amplifications in real time, with a device of the invention having means (5) for excitation / measurement of the fluorescence, as described above. Control probes, nonspecific of the sequence to be amplified, and labeled in a different way from the specific probes, can also be used to detect possible contaminations.
Dans la réalisation de l'invention décrite ci-dessus, où les chambres réactionnelles comportent des amorces et, facultativement une ou plusieurs sonde(s), ces différentes sondes et amorces seront choisies de préférence de telle sorte que leurs températures de fusion (Tm) respectives soient proches. En particulier, le Tm des différentes amorces sera de préférence compris dans un même intervalle d'environ 5°C. De même, les différentes sondes auront de préférence un Tm compris dans un même intervalle de 5°C, qui peut être différent de l'intervalle des Tm des amorces. Dans ce cas, les sondes seront choisies de telle sorte que leur Tm soit supérieur à celui des amorces, la différence entre de Tm des différentes catégories d'oligonucléotides étant alors de préférence de l'ordre de 5°C. La température d'hybridation retenue pour effectuer l'amplification correspond alors à la plus basse des températures de fusion des amorces.In the embodiment of the invention described above, where the reaction chambers comprise primers and, optionally one or more probe (s), these different probes and primers will preferably be chosen such that their melting temperatures (Tm) respective ones are close. In particular, the Tm of the different primers will preferably be in the same range of about 5 ° C. Similarly, the different probes will preferably have a Tm within the same range of 5 ° C, which may be different from the range of Tm primers. In this case, the probes will be chosen so that their Tm is greater than that of the primers, the difference between Tm of the different categories of oligonucleotides then being preferably of the order of 5 ° C. The hybridization temperature chosen to carry out the amplification then corresponds to the lowest of the melting temperatures of the primers.
Les chambres réactionnelles (13) des cartouches de l'invention peuvent aussi comporter, outre les amorces et les sondes éventuelles, un ou plusieurs autres réactifs nécessaires à la réaction de PCR ou à la mesure de l'amplification. Il peut s'agir, par exemple, de sels, de dNTP, ou d'un intercalant fluorescent de l'ADN double brin, de type SybrGreen (marque déposée). Comme mentionné plus haut, tous ces réactifs sont avantageusement déposés au niveau des chambres réactionnelles (13) par le dépôt d'une solution liquide, suivi d'un séchage.The reaction chambers (13) of the cartridges of the invention may also comprise, in addition to the primers and any probes, one or more other reagents necessary for the PCR reaction or measurement of the amplification. It may be, for example, salts, dNTPs, or a fluorescent interlayer of double-stranded DNA, SybrGreen type (trademark). As mentioned above, all these reagents are advantageously deposited at the level of the reaction chambers (13) by the deposition of a liquid solution, followed by drying.
Selon un mode de réalisation alternatif des cartouches de l'invention, les cartouches sont prévues pour le criblage d'un grand nombre d'échantillons suivant un faible nombre de critères. Ceci implique que l'utilisateur de ces cartouches puisse facilement déposer ses échantillons dans chacune des chambres réactionnelles (13). Pour cela, la cartouche peut par exemple posséder un couvercle amovible qui, lorsqu'il est enlevé, donne accès directement aux chambres réactionnelles. De telles cartouches peuvent aussi être pré-chargées et comporter, au niveau des chambres réactionnelles (13), un ou plusieurs réactifs nécessaire(s) à l'amplification et / ou à sa détection.According to an alternative embodiment of the cartridges of the invention, the cartridges are intended for screening a large number of samples according to a small number of criteria. This implies that the user of these cartridges can easily deposit his samples in each of the reaction chambers (13). For this, the cartridge can for example have a removable cover which, when removed, gives direct access to the reaction chambers. Such cartridges may also be pre-charged and comprise, in the reaction chambers (13), one or more reagents necessary for amplification and / or detection.
Bien entendu, les dispositifs de l'invention mentionnés plus haut peuvent comprendre une ou plusieurs cartouches correspondant à n'importe laquelle des cartouches décrites ci-dessus.Of course, the devices of the invention mentioned above may comprise one or more cartridges corresponding to any of the cartridges described above.
Dans la réalisation particulière du dispositif de l'invention, où la cartouche est circulaire, les zones distinctes de chauffage de la platine de chauffage (2) sont préférentiellement réparties selon des portions de disque (
Il est important de noter que le nombre de zones de chauffage distinctes peut être égal à deux, trois, ou davantage. Par exemple, dans le cas d'une PCR à deux températures, la platine pourra présenter une zone à 95°C pour la dénaturation des acides nucléiques double brin, et une zone à 60°C pour l'hybridation des amorces et l'élongation. Dans le cas d'une PCR à trois températures, la platine présentera une zone à 95°C (dénaturation), une zone entre 40 et 70°C (hybridation des amorces), et une zone à 72°C (élongation). Enfin, la platine peut présenter un nombre de zones supérieur à trois, par exemple pour bloquer provisoirement la réaction à un moment donné de chaque cycle. La platine peut aussi présenter un nombre de zones qui soit un multiple de deux ou trois, de telle façon qu'un tour de la cartouche corresponde à plusieurs cycles PCR. Enfin, il est important de noter que la taille relative des différentes zones de chauffage est avantageusement choisie proportionnellement à la durée d'incubation désirée pour le fluide réactionnel à la température de ladite zone. Ainsi, dans la platine représentée à a
Au sujet des moyens de déplacement on notera que, selon un mode préférentiel de réalisation de l'invention, la platine (2) est fixe et la cartouche (1) est mue grâce aux moyens de déplacement (3).With regard to the displacement means, it will be noted that, according to a preferred embodiment of the invention, the plate (2) is fixed and the cartridge (1) is moved by means of displacement (3).
Toutefois, dans d'autres modes de réalisation, on pourra également prévoir une cartouche fixe et une platine de chauffage mise en mouvement grâce audits moyens de déplacement.However, in other embodiments, it will also be possible to provide a fixed cartridge and a heating plate set in motion by means of displacement means.
Dans le mode de réalisation particulièrement préféré de l'invention, selon lequel la cartouche est circulaire, les moyens de déplacement (3) autorisent la mise en rotation de ladite cartouche et/ou de ladite platine.In the particularly preferred embodiment of the invention, wherein the cartridge is circular, the displacement means (3) allow the rotation of said cartridge and / or said platen.
On peut prévoir un élément conducteur entre la cartouche et la platine chauffante. Toutefois, selon une variante préférentielle de l'invention, ladite cartouche est en contact direct avec ladite platine chauffante. Dans ce cas, ladite platine est avantageusement pourvue d'un revêtement favorisant le déplacement entre ladite cartouche et ladite platine. Un tel revêtement peut par exemple être constitue en Téflon (marque déposée).A conductive element can be provided between the cartridge and the heating plate. However, according to a preferred embodiment of the invention, said cartridge is in direct contact with said heating stage. In this case, said plate is advantageously provided with a coating promoting displacement between said cartridge and said plate. Such a coating may for example be made of Teflon (registered trademark).
Comme indiqué ci-dessus, la platine de chauffage du système peut présenter au moins deux ou trois zones pouvant être portées à des températures distinctes. Préférentiellement, cette platine est constituée de deux ou trois blocs thermiques indépendants ("thermoblocs") distincts reliés à des moyens de programmation de leur température. Dans le cas où la platine comporte trois thermoblocs (21 à 23), le premier de ces thermoblocs (21) est chauffé à la température de dénaturation, le deuxième (22) à la température d'hybridation, le troisième (23) à la température d'élongation. L'utilisation de tels thermoblocs de température constante simple la réalisation de la platine chauffante.As indicated above, the system heating plate may have at least two or three zones that can be raised to different temperatures. Preferably, this plate consists of two or three independent thermal blocks ("thermoblocks") connected to separate means for programming their temperature. In the case where the plate comprises three thermoblocks (21 to 23), the first of these thermoblocks (21) is heated to the denaturation temperature, the second (22) to the hybridization temperature, the third (23) to the elongation temperature. The use of such thermoblocks constant temperature simple realization of the heating stage.
Les moyens de déplacement relatif de la cartouche par rapport à la platine pourront être réalisés sous de multiples formes. Selon un mode de réalisation préféré, illustré à la
Selon un mode de réalisation alternatif, représenté aux
Le mode de déplacement relatif entre la platine et la cartouche pourra varier selon les modes de réalisation. Il pourra s'agir d'un déplacement à vitesse continu ou par à-coups. La vitesse de déplacement pourra être constante ou varier dans le temps.The relative mode of movement between the plate and the cartridge may vary according to the embodiments. It may be a movement at continuous speed or in jerks. The speed of movement may be constant or vary over time.
Avantageusement, le système selon l'invention comprend également des moyens optiques d'excitation/mesure de la fluorescence, prévus par exemple au-dessus ou sur le côté de ladite cartouche. Selon une variante préférentielle de l'invention, ces moyens constitueront un système unique et fixe. Un avantage d'une variante préférentielle de l'invention selon laquelle la cartouche est circulaire et mue selon un déplacement rotatif est de pouvoir amener successivement chaque chambre réactionnelle sous ledit système optique, réduisant ainsi sa complexité. Un système de repérage, situé par exemple sur la cartouche (1), permet de déterminer à chaque instant quelle chambre réactionnelle est située en regard du système optique.Advantageously, the system according to the invention also comprises optical means of excitation / fluorescence measurement, provided for example above or on the side of said cartridge. According to a preferred variant of the invention, these means will constitute a single and fixed system. An advantage of a preferred variant of the invention according to which the cartridge is circular and moved in a rotary displacement is to be able to successively bring each reaction chamber under said optical system, thus reducing its complexity. A tracking system, located for example on the cartridge (1), makes it possible to determine at each instant which reaction chamber is located opposite the optical system.
Les moyens d'amenée du fluide présent dans ledit réservoir vers lesdites chambres réactionnelles peuvent être réalisés sous différentes formes. Comme cela a été décrit plus haut, on peut distinguer deux catégories de modes d'adressage du fluide vers les chambres réactionnelles: l'adressage en système ouvert, qui suppose une augmentation de pression au niveau du réservoir et la présence d'évents (14) au niveau des chambres réactionnelles, et l'adressage en système clos, qui débute au contraire par l'établissement d'une dépression dans la cartouche (1), suivi d'un rétablissement de cette pression.The means for supplying the fluid present in said reservoir to said reaction chambers can be made in different forms. As has been described above, two categories of modes of addressing the fluid towards the reaction chambers can be distinguished: open system addressing, which assumes an increase in pressure at the reservoir and the presence of vents (14). ) at the level of the reaction chambers, and the addressing in closed system, which begins on the contrary by the establishment of a vacuum in the cartridge (1), followed by a recovery of this pressure.
Les moyens (4) d'amenée du fluide dans les chambre réactionnelles diffèrent suivant le mode de réalisation choisi. Ainsi, en système ouvert, le fluide contenu dans le réservoir est distribué sous pression dans les chambres réactionnelles de façon à permettre un remplissage uniforme de ces chambres. Dans ce cas, les moyens d'amenée (4) incluent préférentiellement un dispositif à piston (41) dont la vitesse de pénétration dans le réservoir sera calculée-pour favoriser le bon remplissage des chambres réactionnelles, Alternativement, ces moyens d'amenée incluent une pompe branchée de façon à augmenter la pression dans le réservoir (11).The means (4) for supplying the fluid into the reaction chambers differ according to the embodiment chosen. Thus, in an open system, the fluid contained in the reservoir is distributed under pressure in the reaction chambers so as to allow uniform filling of these chambers. In this case, the feed means (4) preferably include a piston device (41) whose penetration rate in the tank will be calculated-to promote the proper filling of the reaction chambers, alternatively, these supply means include a pump connected to increase the pressure in the tank (11).
Comme il a été vu plus haut, une autre variante préférée de l'invention implique de travailler en système clos. Le fluide contenu dans le réservoir est alors distribué dans les chambres réactionnelles de la façon suivante : dans un premier temps, une dépression est créée à l'intérieur de la cartouche, le cas échéant par un dispositif à piston ou une pompe (42), branchée cette fois de manière à diminuer la pression dans la cartouche (1). La pression est ensuite rétablie, ce qui permet au fluide de s'engager dans les canaux et de remplir les chambres réactionnelles périphériques.As has been seen above, another preferred variant of the invention involves working in a closed system. The fluid contained in the reservoir is then distributed in the reaction chambers as follows: in a first step, a vacuum is created inside the cartridge, where appropriate by a piston device or a pump (42), connected this time to reduce the pressure in the cartridge (1). The pressure is then restored, allowing the fluid to engage the channels and fill the peripheral reaction chambers.
L'invention concerne également tout procédé d'amplification d'acide nucléique grâce à un système tel que décrit ci-dessus, caractérisé en ce qu'il comprend les étapes consistant à :
- remplir au moins partiellement le réservoir (11) avec un fluide contenant un échantillon d'acides nucléiques à analyser ainsi que tout le nécessaire à une réaction d'amplification, à l'exception des amorces, et facultativement, un intercalant fluorescent des acides nucléiques ;
- répartir ledit fluide dans les chambres réactionnelles (13) prévues dans la cartouche (1), dans lesquelles sont pré-réparties des amorces et, facultativement, une ou plusieurs sondes marquées spécifiques de la séquence nucléique cible;
- mettre en oeuvre les moyens de déplacement relatif entre la cartouche et la platine chauffante pour amener successivement, et autant de fois que désiré, le contenu de chaque chambre aux températures définies par les deux, trois ou davantage zones de ladite platine de chauffage.
- at least partially filling the reservoir (11) with a fluid containing a nucleic acid sample to be analyzed as well as all the necessary for an amplification reaction, with the exception of the primers, and optionally, a fluorescent intercalator of the nucleic acids;
- distributing said fluid in the reaction chambers (13) provided in the cartridge (1), in which primers and, optionally, one or more labeled probes specific for the target nucleic sequence are pre-distributed;
- implementing the relative displacement means between the cartridge and the heating plate to successively bring, as many times as desired, the contents of each chamber to the temperatures defined by the two, three or more zones of said heating plate.
Dans une variante du procédé ci-dessus, des réactifs nécessaires à la réaction d'amplification et/ou à la détection des produits de l'amplification, et distincts des amorces et des sondes, sont pré-répartis dans les chambres réactionnelles (13) de la cartouche (1). Le fluide introduit dans le réservoir (11) ne contient alors pas ces réactifs.In a variant of the above process, reagents necessary for the amplification reaction and / or the detection of the products of the amplification, and distinct from the primers and probes, are pre-distributed in the reaction chambers (13). of the cartridge (1). The fluid introduced into the reservoir (11) does not then contain these reagents.
L'étape de répartition du fluide dans les chambres réactionnelles (13) est effectuée soit en appliquant une dépression à l'intérieur de la cartouche, puis en rétablissant la pression (système clos), soit en augmentant la pression au niveau du réservoir (11), à condition que les chambres réactionnelles soient pourvues d'évents (système ouvert).The fluid distribution step in the reaction chambers (13) is carried out either by applying a vacuum inside the cartridge, then by restoring the pressure (closed system), or by increasing the pressure at the reservoir (11). ), provided that the reaction chambers are provided with vents (open system).
L'invention, ainsi que les différents avantages qu'elle présente, seront mieux compris grâce à la description qui va suivre de quelques modes non limitatifs de réalisation de celle-ci, illustrés dans les figures.The invention, as well as the various advantages that it presents, will be better understood thanks to the following description of some non-limiting embodiments thereof, illustrated in the figures.
Le système de détection et de quantification de séquences nucléiques cibles représentés à la
Le système comprend par ailleurs une platine chauffante (2) en contact direct avec la face inférieure de la cartouche (1) et des moyens de déplacement (3) de la cartouche (1) par rapport à la platine chauffante (2). Ces moyens de déplacement incluent un micromoteur (31) relié à deux axes (32) qui coopèrent avec deux oreilles (183) de la cartouche (1) pour inculquer à celle-ci un mouvement rotatif sur la platine chauffante (2), celle-ci restant quant à elle fixe.The system further comprises a heating plate (2) in direct contact with the underside of the cartridge (1) and means (3) for moving the cartridge (1) relative to the heating plate (2). These displacement means include a micromotor (31) connected to two axes (32) which cooperate with two lugs (183) of the cartridge (1) to inculcate in it a rotary movement on the heating plate (2), the latter while remaining fixed.
Le système décrit comprend également un piston (41) destiné à coopérer avec ledit réservoir (11) ainsi qu'un dispositif optique (5) d'excitation/mesure de fluorescence (source émettrice permettant une excitation à une longueur d'onde donnée et programmable et récepteur de la fluorescence émise) fixe et placé au dessus de la cartouche (1) et de la platine chauffante (2).The described system also comprises a piston (41) intended to cooperate with said reservoir (11) as well as an optical device (5) for excitation / fluorescence measurement (emitting source allowing excitation at a given, programmable wavelength). and fluorescence receiver emitted) fixed and placed above the cartridge (1) and the heating plate (2).
Comme on peut le voir sur la
En référence à la
Comme déjà précisé, cette cartouche (1) est également pourvue de deux oreilles (183) percées chacune d'un orifice pour laisser passer un axe (32) relié au micromoteur (31).As already stated, this cartridge (1) is also provided with two lugs (183) each pierced with an orifice for passing an axis (32) connected to the micromotor (31).
Selon la
L'utilisation du dispositif représenté est la suivante :
- Le réservoir central (11) est destiné à recevoir l'échantillon d'acides nucléiques à analyser ainsi que tout le nécessaire à une réaction d'amplification, et facultativement un intercalant fluorescent des acides nucléiques (l'ensemble est ci-après dénommé fluide), à l'exception des amorces préréparties dans chaque chambre réactionnelle périphérique 10.
- The central reservoir (11) is intended to receive the sample of nucleic acids to be analyzed as well as all the necessary for an amplification reaction, and optionally a fluorescent intercalant of the nucleic acids (the assembly is hereinafter referred to as a fluid). with the exception of pre-distributed primers in each peripheral reaction chamber 10.
Dans le cadre du présent mode de réalisation, l'utilisateur place dans le réservoir central 90 µl (c'est-à-dire 36 fois 2,5 µl) de fluide, dont 75 ng d'acides nucléiques. Les concentrations en réactifs dudit fluide sont les suivantes :
- dNTP : 200 µM
- Tampon Taq:1x
- MgCl2 : 1,5 mM
- Taq : 4U
- SybrGreen (marque déposée) : 1 x
- H2O : qsp
- dNTP: 200 μM
- Taq buffer: 1x
- MgCl 2 : 1.5 mM
- Taq: 4U
- SybrGreen (registered trademark): 1 x
- H 2 O: qs
Chaque chambre 10, sauf quelques-unes à des fins de témoins négatifs, contient deux amorces spécifiques d'une séquence cible à amplifier, et facultativement une ou plusieurs sondes marquées, permettant une mesure spécifique ultérieure de fluorescence. Dans le présent mode de réalisation, ont été répartis 10 ng de chaque amorce dans chaque chambre sauf dans celles servant de témoin négatif.Each chamber 10, except a few for negative control purposes, contains two primers specific for a target sequence to be amplified, and optionally one or more labeled probes, allowing a subsequent specific measurement of fluorescence. In the present embodiment, 10 ng of each primer were distributed in each chamber except for those serving as a negative control.
Après avoir rempli partiellement le réservoir (11) avec le fluide dont le volume est égal à la somme des volumes des chambres (le volume d'une chambre est défini comme étant le produit de sa surface de "plancher" par sa profondeur), le piston (41) est actionné pour distribuer ce fluide dans la pluralité de chambres réactionnelles (13). Ce piston permet d'augmenter la pression au sein du réservoir (11) et permet le passage du fluide dans les canaux vers les chambres. La vitesse de déplacement du piston dans le réservoir est d'environ 1 mm par seconde et ledit déplacement est stoppé à un niveau qui dépend du volume de fluide à adresser dans les chambres.After partially filling the reservoir (11) with the fluid whose volume is equal to the sum of the volumes of the chambers (the volume of a chamber is defined as being the product of its "floor" surface by its depth), the piston (41) is actuated to dispense this fluid into the plurality of reaction chambers (13). This piston increases the pressure within the reservoir (11) and allows the passage of fluid in the channels to the chambers. The speed of movement of the piston in the reservoir is about 1 mm per second and said displacement is stopped at a level that depends on the volume of fluid to be addressed in the chambers.
Le faible diamètre des canaux (12) permet d'empêcher la diffusion du fluide depuis le réservoir (11) vers les canaux (12) et les chambres (13) sous l'effet de la gravité (à cette échelle, les processus habituellement négligeables comme les forces de capillarité deviennent prégnants, et dans le cas présent suffisent à maintenir le fluide dans le réservoir). Grâce aux évents (14), l'air présent dans les chambres (13) est évacué, ce qui assure le remplissage de celles-ci.The small diameter of the channels (12) makes it possible to prevent the diffusion of the fluid from the reservoir (11) to the channels (12) and the chambers (13) under the effect of gravity (at this scale, the processes are usually negligible as the capillary forces become pregnant, and in this case are enough to keep the fluid in the tank). Thanks to the vents (14), the air present in the chambers (13) is evacuated, which ensures the filling thereof.
Les thermoblocs (21, 22, 23) sont portés aux trois températures correspondant aux trois températures des phases de la PCR (ou à des températures légèrement supérieures compte tenu des éventuelles déperditions thermiques entre la platine chauffante (2) et la cartouche 1) et les moyens de déplacement (3) sont mis en oeuvre de façon à animer d'un mouvement giratoire la cartouche (1) pour faire passer successivement et autant de fois que désiré chaque chambre réactionnelle au-dessus des trois thermoblocs.The thermoblocks (21, 22, 23) are brought to the three temperatures corresponding to the three temperatures of the PCR phases (or at slightly higher temperatures given the possible heat losses between the heating stage (2) and the cartridge 1) and the displacement means (3) are implemented so as to animate the cartridge (1) by a gyratory movement in order to pass each reaction chamber successively and as many times as desired over the three thermoblocks.
Plus précisément, le bloc (21) est porté à la température correspondant à la phase de dénaturation (94°C), le thermobloc (22) est porté à la température correspondant à la phase d'hybridation (36°C) et le thermobloc (23) est porté à la température correspondant à la phase d'élongation (72°C).More precisely, the block (21) is brought to the temperature corresponding to the denaturation phase (94 ° C.), the thermoblock (22) is brought to the temperature corresponding to the hybridization phase (36 ° C.) and the thermoblock (23) is brought to the temperature corresponding to the phase of elongation (72 ° C).
Dans le présent mode de réalisation, le micromoteur (31) des moyens de déplacement (3) est conçu pour inculquer une rotation de 10 degrés toutes les 2,5 secondes à la cartouche (1) (soit un cycle de PCR en 1,5 mn). Toutefois, dans d'autres modes de réalisation, ce mouvement pourra présenter une vitesse différente et être continu au lieu d'être saccadé.In the present embodiment, the micromotor (31) of the displacement means (3) is adapted to inculcate a rotation of 10 degrees every 2.5 seconds to the cartridge (1) (ie a 1.5 PCR cycle). min). However, in other embodiments, this movement may have a different speed and be continuous instead of jerky.
On notera que le dispositif optique (5) est prévu au dessus du bloc correspondant 23 porté à une température correspondant à la température d'élongation, et plus particulièrement à un emplacement qui correspond à la fin de la phase d'élongation. Bien entendu, le dispositif optique (5) peut être placé à un emplacement différent, choisi notamment en fonction de la chimie utilisée. Par exemple, en utilisant la chimie TaqMan™ ou de la fluorescence non spécifique, il est logique d'effectuer la mesure à la fin de la phase d'élongation, comme décrit ci-dessus. En revanche, l'utilisation d'une chimie du type Molecular Beacons™ implique que la mesure se fasse plutôt au moment de l'hybridation.It will be noted that the optical device (5) is provided above the corresponding
Le système présenté permet de remplir rapidement et de façon reproductible une grande quantité de chambres réactionnelles et d'effectuer sur le contenu de celles-ci une PCR et des mesures de fluorescence à chaque cycle de la PCR.The system presented makes it possible to rapidly and reproducibly fill a large quantity of reaction chambers and to carry out PCR and fluorescence measurements on each PCR cycle.
Le mode de réalisation ici décrit n'a pas pour objet de réduire la portée de l'invention. Il pourra donc y être apporté de nombreuses modifications sans sortir du cadre de celle-ci.The embodiment described here is not intended to reduce the scope of the invention. It can therefore be made many changes without departing from the scope thereof.
Les
Cette cartouche est prévue pour une utilisation en système clos, c'est-à-dire que les chambres réactionnelles (13) n'ont pas d'autre ouverture que l'arrivée du canal (12). La cartouche est constituée de deux éléments qui s'emboîtent l'un dans l'autre : la partie inférieure, ou socle, est représentée aux
Le chargement de cette cartouche s'effectue de la façon suivante :This cartridge is loaded as follows:
L'utilisateur place dans le réservoir central l'extrait d'acides nucléiques à analyser. Il place le consommable dans l'automate. Ce dernier crée une dépression à l'intérieur de la cartouche (P = 0,05 bar approximativement), par exemple par l'utilisation d'une pompe (42). La pression est ensuite rétablie , ce qui permet aux fluides de s'engager dans les canaux et de remplir les chambres réactionnelles périphériques. Ainsi, par rapport au dispositif de l'exemple 1, le fluide n'est plus adressé par une augmentation de la pression mais par dépression, ce qui présente l'avantage de ne pas nécessiter d'évent et donc de travailler en système clos.The user places in the central reservoir the nucleic acid extract to be analyzed. He places the consumable in the automaton. The latter creates a vacuum inside the cartridge (P = 0.05 bar approximately), for example by the use of a pump (42). The pressure is then reestablished, allowing the fluids to engage the channels and fill the peripheral reaction chambers. Thus, compared to the device of Example 1, the fluid is no longer addressed by an increase in pressure but by depression, which presents the advantage of not requiring a vent and therefore working in a closed system.
Le cas échéant, on peut prévoir plusieurs sous-réservoirs et non plus un réservoir unique, ce qui présente l'avantage de traiter simultanément plusieurs échantillons.If necessary, several sub-tanks can be provided and no longer a single tank, which has the advantage of simultaneously processing several samples.
Le fond du réservoir a une forme conique permettant au fluide de se répartir à sa périphérie, c'est-à-dire près de l'entrée des canaux.The bottom of the tank has a conical shape allowing the fluid to be distributed at its periphery, that is to say near the entrance of the channels.
A la jonction entre les canaux et le réservoir, se trouve un système anti-reflux, constitué par une portion de canal vertical (123), ce qui, d'une part, prévient les contaminations croisées en cas de retour intempestif de fluide vers la partie centrale ou au cas où tout le fluide ne se serait pas engagé dans le canal et, d'autre part, permet une fois l'adressage effectué mais avant la PCR, de venir boucher les canaux au moyen d'un bouchon dont les dentelures viennent épouser ces entrées verticales, afin de travailler en système clos (pas de contamination, pas d'évaporation).At the junction between the channels and the reservoir, there is an anti-reflux system consisting of a vertical channel portion (123), which, on the one hand, prevents cross contamination in the event of inadvertent return of fluid to the central part or in the case where all the fluid would not be engaged in the channel and, on the other hand, allows once the addressing done but before the PCR, to come to plug the channels by means of a plug whose serrations come to marry these vertical entries, to work in closed system (no contamination, no evaporation).
La cartouche est en plastique, préférentiellement en polycarbonate car ce polymère présente des caractéristiques physiques, optiques et de comportement thermique intéressantes.The cartridge is made of plastic, preferably polycarbonate because this polymer has interesting physical, optical and thermal behavior characteristics.
La taille des canaux est par exemple de 0,4 x 0,2 mm (demi-lune) en section.The size of the channels is for example 0.4 x 0.2 mm (half-moon) in section.
La taille du consommable est par exemple de 100 mm (diamètre), le nombre de chambres est de 80, le nombre de sous-réservoirs est compris entre 1 et 8.The size of the consumable is for example 100 mm (diameter), the number of chambers is 80, the number of sub-tanks is between 1 and 8.
Comme illustré à la
Les chambres réactionnelles sont chargées avec des amorces spécifiques de séquences cibles et, le cas échéant, avec des sondes de type TaqMan™ ou autre spécifiques desdites cibles. Suivant les applications, les cibles seront des gènes viraux ou bactériens, des jonctions entre un transgène et le génome d'une plante pour détecter et/ou identifier certains OGM, etc.The reaction chambers are loaded with primers specific for target sequences and, where appropriate, with TaqMan ™ or other probes specific for said targets. Depending on the applications, the targets will be viral or bacterial genes, junctions between a transgene and the genome of a plant to detect and / or identify certain GMOs, etc.
Une variante de la cartouche décrite ci-dessus, comportant 36 chambres réactionnelles d'un volume de 8 µl et des canaux d'un diamètre de 0,3 mm, a été utilisée pour effectuer un test de détection de bactéries Salmonelles, 288 µl (soit 36 fois 8 µl) de la solution suivante ont été placés dans le réservoir central:
- DUTP 400 µM
- dNTP : 200 µM
- Tampon Taq : 1 x
- MgCl2 : 3 mM
- Taq: 15 U
- TWEEN (marque déposée) : 0,007 %
- SybrGreen (marque déposée) : 0,1 x
- ADN génomique de Salmonella enteritidis : 1 ng
- H2O: qsp
- DUTP 400 μM
- dNTP: 200 μM
- Taq buffer: 1 x
- MgCl 2 : 3 mM
- Taq: 15 U
- TWEEN (registered trademark): 0.007%
- SybrGreen (registered trademark): 0.1 x
- Genomic DNA of Salmonella enteritidis: 1 ng
- H 2 O: qs
1,6 picomole des amorces FinA1 et FinA2 décrites dans Cohen, Mechanda et al. 1996 ont été déposées dans les chambres réactionnelles.1.6 picomole FinA1 and FinA2 primers described in Cohen, Mechanda et al. 1996 were deposited in the reaction chambers.
Cette expérience a donné des résultats positifs, comme attendu.This experiment gave positive results, as expected.
-
Cohen, H. J., S. M. Mechanda, et al. (1996). "PCR amplification of the fimA gene sequence of Salmonella typhimurium, a specific method for detection of Salmonella spp." Appl Environ Microbiol 62(12): 4303-8 Cohen, HJ, SM Mechanda, et al. (1996). "PCR amplification of the fimA gene sequence of Salmonella typhimurium, a specific method for detection of Salmonella spp." Appl Environ Microbiol 62 (12): 4303-8 -
Gibson, U. E., C. A. Heid, et al. (1996). "A novel method for real time quantitative RT-PCR." Genome Res 6(10): 995-1001 Gibson, UE, CA Heid, et al. (1996). "A novel method for real time quantitative RT-PCR." Genome Res 6 (10): 995-1001 -
Heid, C. A., J. Stevens, et al. (1996). "Real time quantitative PCR." Genome Res 6(10): 986-94 Heid, CA, J. Stevens, et al. (1996). "Real time quantitative PCR." Genome Res 6 (10): 986-94 -
Williams, P. M., T. Giles, et al. (1998). "Development and application of real-time quantitative PCR." In F; Ferré (Ed.), Gene Quantification. Birkhäuser, BostWilliams, P. M., T. Giles, et al. (1998). "Development and application of real-time quantitative PCR." In F; Ferré (Ed.), Gene Quantification. Birkhäuser, Bost
Claims (18)
- Device for carrying out enzymatic and/or molecular biological reactions requiring at least two different incubation temperatures, comprising at least one cartridge (1) of circular shape comprising a plurality of reaction chambers (13), in which primers are pre-disposed, a reservoir (11) and channels (12), characterized by:- this cartridge (1) having a geometry of revolution, in which the reservoir (11) is positioned substantially at the centre, the reaction chambers (13) being distributed in a circle around said reservoir, and the channels (12) connecting said reservoir (11) to said chambers (13), the channels being provided, in particular, essentially radially;- at least one heating plate (2) having at least two distinct zones that can be heated to at least two different temperatures,
in particular, the heating plate having three distinct zones that can be heated to three different temperatures;- means (3) for relative displacement between said cartridge and said plate, allowing a cyclic variation of the temperature of the reaction chambers. - Device according to claim 1, in which said reaction chambers (13) are placed at the periphery of said cartridge.
- Device according to claim 1 or 2, in which the enzymatic reaction is a thermodependent chain amplification of nucleic acid sequences, and in which the zones of the heating plate (2) can be heated to at least two different temperatures, corresponding to the phases of the nucleic acid amplification cycles.
- Device according to claim 3, characterized in that:- primers specific for target sequences to be amplified are pre-distributed in the reaction chambers (13),- the reservoir (11) is intended to receive a fluid composed, in particular, of a sample of nucleic acids to be analysed and reagents required for a polymerase chain amplification reaction, with the exception of the primers,- the heating plate (2) has three distinct zones that can be heated to three different temperatures corresponding to the three phases of the polymerase chain amplification cycles.
- Device according to claim 3 or 4, for thermodependent chain amplification of nucleic acid sequences, measured in real-time, characterized in that it comprises optical means (5) for fluorescence excitation/measurement, disposed so as to excite and measure the fluorescence of the contents of the reaction chambers for each cycle.
- Device according to any one of claims 1 to 5, in which the distinct heating zones of the plate (2) are distributed corresponding to at least two or three sections of a disk.
- Device according to any one of claims 1 to 5, in which the distinct heating zones of the plate (2) are distributed corresponding to sections of a ring.
- Device according to any one of claims 1 to 7, in which said displacement means (3) allow rotation of said cartridge (1) and/or said heating plate (2).
- Device according to any one of claims 1 to 8, in which the cartridge (1) is in direct contact with the heating plate (2).
- Device according to any one of claims 1 to 9, in which the plate (2) is provided with a coating promoting relative displacement between said cartridge (1) and said plate (2).
- Device according to any one of claims 1 to 10, in which the heating plate (2) comprises two or three distinct thermoblocks (21, 22 and, optionally, 23) connected to means for programming their temperature.
- Device according to any one of claims 1 to 11, comprising optical means (5) for fluorescence excitation/measurement disposed above or to the side of the cartridge.
- Device according to any one of claims 1 to 12, further comprising means (4) for supplying the fluid present in the reservoir (11) to the reaction chambers (13).
- Device according to claim 13, in which said supply means (4) include a piston device (41), and the fluid is supplied to the reaction chambers by increasing the pressure.
- Device according to claim 13, in which said supply means (4) include a pump (41), and the fluid is supplied to the reaction chambers by reestablishing the pressure after establishing an underpressure.
- Device according to claim 5, in which the reaction chambers (13) of the cartridge (1) are closed.
- Method for nucleic acid amplification using a device according to any one of claims 1 to 16, comprising the following steps:- at least partially filling the reservoir (11) with a fluid containing a sample of nucleic acids to be analysed and all that is required for an amplification reaction, with the exception of the primers, and, optionally, a fluorescent nucleic acid reporter;- distributing said fluid to the reaction chambers (13) of the cartridge (1), in which are pre-disposed primers and, optionally, one or more labelled probes;- employing the means (3) for relative displacement between the cartridge and the heating plate to successively bring the contents of each reaction chamber to the two, three or more temperatures defined by the two, three or more zones of said heating plate (2) as many times as is desired.
- Amplification method according to claim 17, in which the step for distributing the fluid to the reaction chambers (13) is carried out by applying an underpressure to the interior of the cartridge, then reestablishing the pressure.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| FR0010029A FR2812306B1 (en) | 2000-07-28 | 2000-07-28 | POLYMERSIS CHAIN AMPLIFICATION SYSTEM OF TARGET NUCLEIC SEQUENCES |
| EP01956628A EP1305115B1 (en) | 2000-07-28 | 2001-07-20 | Device for heat-dependent chain amplification of target nucleic acid sequences |
Related Parent Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP01956628.0 Division | 2001-07-20 | ||
| EP01956628A Division EP1305115B1 (en) | 2000-07-28 | 2001-07-20 | Device for heat-dependent chain amplification of target nucleic acid sequences |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP2269738A1 EP2269738A1 (en) | 2011-01-05 |
| EP2269738B1 true EP2269738B1 (en) | 2012-08-29 |
Family
ID=8853103
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP01956628A Expired - Lifetime EP1305115B1 (en) | 2000-07-28 | 2001-07-20 | Device for heat-dependent chain amplification of target nucleic acid sequences |
| EP10177401A Expired - Lifetime EP2269738B1 (en) | 2000-07-28 | 2001-07-20 | Device for thermo-dependent chain reaction amplification of target nucleic acid sequences |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP01956628A Expired - Lifetime EP1305115B1 (en) | 2000-07-28 | 2001-07-20 | Device for heat-dependent chain amplification of target nucleic acid sequences |
Country Status (14)
| Country | Link |
|---|---|
| US (2) | US6821771B2 (en) |
| EP (2) | EP1305115B1 (en) |
| JP (2) | JP4979873B2 (en) |
| CN (1) | CN1248781C (en) |
| AT (1) | ATE532583T1 (en) |
| AU (2) | AU2001278554B2 (en) |
| BR (1) | BR0112789A (en) |
| CA (1) | CA2416756C (en) |
| DK (1) | DK2269738T3 (en) |
| EA (1) | EA004719B1 (en) |
| ES (2) | ES2372027T3 (en) |
| FR (1) | FR2812306B1 (en) |
| WO (1) | WO2002009877A1 (en) |
| ZA (1) | ZA200300700B (en) |
Families Citing this family (66)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2844523B1 (en) * | 2002-09-13 | 2011-01-14 | Genesystems | METHOD AND NUCLEOTIDE SEQUENCES FOR THE DETECTION AND IDENTIFICATION OF MICROORGANISMS IN A COMPLEX MIXTURE OR IN WATER |
| FR2844522A1 (en) * | 2002-09-13 | 2004-03-19 | Genesystems | Multiplex detection and identification of bacteria in complex mixtures, useful for testing food and environmental samples, by simultaneous, but spatially resolved amplification |
| JP2005125311A (en) * | 2003-09-30 | 2005-05-19 | Japan Science & Technology Agency | Chemical reactor |
| DE10360220A1 (en) | 2003-12-20 | 2005-07-21 | Steag Microparts Gmbh | Fine structure arrangement in fluid ejection system, has predetermined region in transitional zone between inlet and discharge ports, at which capillary force is maximum |
| US7709249B2 (en) * | 2005-04-01 | 2010-05-04 | 3M Innovative Properties Company | Multiplex fluorescence detection device having fiber bundle coupling multiple optical modules to a common detector |
| US7507575B2 (en) * | 2005-04-01 | 2009-03-24 | 3M Innovative Properties Company | Multiplex fluorescence detection device having removable optical modules |
| US20060246493A1 (en) | 2005-04-04 | 2006-11-02 | Caliper Life Sciences, Inc. | Method and apparatus for use in temperature controlled processing of microfluidic samples |
| US9568424B2 (en) | 2005-06-23 | 2017-02-14 | Biocartis Nv | Cartridge, system and method for automated medical diagnostics |
| JP5049274B2 (en) * | 2005-06-30 | 2012-10-17 | ビオカルティ ソシエテ アノニム | Cartridge for automated medical diagnosis |
| US7527763B2 (en) | 2005-07-05 | 2009-05-05 | 3M Innovative Properties Company | Valve control system for a rotating multiplex fluorescence detection device |
| US20070009382A1 (en) * | 2005-07-05 | 2007-01-11 | William Bedingham | Heating element for a rotating multiplex fluorescence detection device |
| JP4626891B2 (en) * | 2007-01-29 | 2011-02-09 | ヤマハ株式会社 | Temperature control device |
| JP5141039B2 (en) * | 2007-02-22 | 2013-02-13 | 東洋紡株式会社 | Nucleic acid amplification apparatus and nucleic acid amplification method |
| WO2008146754A1 (en) | 2007-05-23 | 2008-12-04 | Trust Co., Ltd. | Container for liquid reaction mixture, reaction-promoting device using the same and method therefor |
| US8951732B2 (en) * | 2007-06-22 | 2015-02-10 | Advanced Liquid Logic, Inc. | Droplet-based nucleic acid amplification in a temperature gradient |
| JP5600293B2 (en) | 2007-07-27 | 2014-10-01 | アール ツリー イノベーションズ エルエルシー | Intervertebral body transplantation system and method |
| US20160068905A1 (en) | 2007-11-26 | 2016-03-10 | Immunid | Method for Studying V(D)J Combinatory Diversity |
| EP2062982A1 (en) * | 2007-11-26 | 2009-05-27 | ImmunID | Method for studying the V(D)J combinatorial diversity. |
| JP2009240296A (en) * | 2007-12-14 | 2009-10-22 | Ngk Insulators Ltd | Fluid receiving cartridge and utilization of the same |
| CA2722484A1 (en) * | 2008-04-24 | 2009-10-29 | Peter D. Ludowise | Analysis of nucleic acid amplification curves using wavelet transformation |
| JP5372418B2 (en) * | 2008-06-23 | 2013-12-18 | 株式会社日立ハイテクノロジーズ | Nucleic acid analyzer, automatic analyzer, and analysis method |
| EP2143491A1 (en) * | 2008-07-10 | 2010-01-13 | Carpegen GmbH | Device for analysing a chemical or biological sample |
| AT507376B1 (en) * | 2008-08-29 | 2013-09-15 | Anagnostics Bioanalysis Gmbh | DEVICE FOR TEMPERING A ROTATION SYMETRIC CONTAINER |
| WO2012033396A1 (en) * | 2008-12-18 | 2012-03-15 | Universiti Sains Malaysia | A disposable multiplex polymerase chain reaction (pcr) chip and device |
| US9399219B2 (en) * | 2009-02-13 | 2016-07-26 | Frank Leo Spangler | Thermal Array |
| JP5859424B2 (en) | 2009-04-07 | 2016-02-10 | コーニンクレッカ フィリップス エヌ ヴェKoninklijke Philips N.V. | Method for detection and characterization of toxin-producing Clostridium difficile |
| WO2010135605A2 (en) * | 2009-05-20 | 2010-11-25 | Smith International, Inc. | Cutting elements, methods for manufacturing such cutting elements, and tools incorporating such cutting elements |
| GB2483402B (en) | 2009-06-04 | 2014-04-09 | Lockheed Corp | Multiple-sample microfluidic chip for DNA analysis |
| JP2011062119A (en) | 2009-09-16 | 2011-03-31 | Seiko Epson Corp | Chip for quantitatively determining biological sample |
| DE102009044431A1 (en) * | 2009-11-05 | 2011-06-22 | FRIZ Biochem Gesellschaft für Bioanalytik mbH, 82061 | Device for carrying out a PCR |
| US8906624B2 (en) * | 2010-03-30 | 2014-12-09 | Korea Advanced Institute Of Science And Technology (Kaist) | Rotational PCR equipment and PCR method using the same |
| JP5249988B2 (en) * | 2010-05-07 | 2013-07-31 | 株式会社日立ハイテクノロジーズ | Nucleic acid amplification apparatus and nucleic acid test apparatus using the same |
| US20110312541A1 (en) * | 2010-06-17 | 2011-12-22 | Geneasys Pty Ltd | Loc for detection of hybridization of nucleic acid sequences with primer-linked linear probes |
| CA2814720C (en) | 2010-10-15 | 2016-12-13 | Lockheed Martin Corporation | Micro fluidic optic design |
| EP2450690A1 (en) * | 2010-11-04 | 2012-05-09 | Qiagen GmbH | Vessel for Accurate Optical Measurements |
| CN104040238B (en) * | 2011-11-04 | 2017-06-27 | 汉迪拉布公司 | Polynucleotides sample preparation apparatus |
| US9322054B2 (en) | 2012-02-22 | 2016-04-26 | Lockheed Martin Corporation | Microfluidic cartridge |
| US9303281B2 (en) | 2012-07-23 | 2016-04-05 | Pall Corporation | Compositions for detecting foodstuff spoilage microorganisms |
| US9382591B2 (en) | 2013-06-06 | 2016-07-05 | Pall Corporation | Compositions for detecting Alicyclobacillus microorganisms |
| EP2843057B1 (en) | 2013-09-03 | 2017-12-27 | Pall Genedisc Technologies | Method for determining the presence or absence of shiga toxin-producing Escherichia coli (STEC) in a food sample |
| KR101618113B1 (en) * | 2014-02-10 | 2016-05-09 | 나노바이오시스 주식회사 | Device for polymerase chain reaction comprising driving element for one-direction sliding, and method for polymerase chain reaction using the same |
| US10195610B2 (en) | 2014-03-10 | 2019-02-05 | Click Diagnostics, Inc. | Cartridge-based thermocycler |
| JP2016049064A (en) * | 2014-09-01 | 2016-04-11 | 国立研究開発法人産業技術総合研究所 | Pcr device using microchip |
| EP4029606A1 (en) | 2014-12-31 | 2022-07-20 | Visby Medical, Inc. | Molecular diagnostic testing |
| US9828626B2 (en) | 2015-07-10 | 2017-11-28 | Pall Corporation | Dendrimer conjugates for determining membrane retention level and/or pore structure |
| WO2017139447A1 (en) * | 2016-02-10 | 2017-08-17 | Coyote Bioscience Usa Inc. | Methods and systems for analyzing nucleic acids |
| WO2017185067A1 (en) | 2016-04-22 | 2017-10-26 | Click Diagnostics, Inc. | Printed circuit board heater for an amplification module |
| WO2017197040A1 (en) | 2016-05-11 | 2017-11-16 | Click Diagnostics, Inc. | Devices and methods for nucleic acid extraction |
| USD800331S1 (en) | 2016-06-29 | 2017-10-17 | Click Diagnostics, Inc. | Molecular diagnostic device |
| MX2018015889A (en) | 2016-06-29 | 2019-05-27 | Click Diagnostics Inc | Devices and methods for the detection of molecules using a flow cell. |
| USD800913S1 (en) | 2016-06-30 | 2017-10-24 | Click Diagnostics, Inc. | Detection window for molecular diagnostic device |
| USD800914S1 (en) | 2016-06-30 | 2017-10-24 | Click Diagnostics, Inc. | Status indicator for molecular diagnostic device |
| JP7044715B2 (en) * | 2016-12-28 | 2022-03-30 | 株式会社Mirai Genomics | Analysis equipment |
| CA3078976A1 (en) | 2017-11-09 | 2019-05-16 | Visby Medical, Inc. | Portable molecular diagnostic device and methods for the detection of target viruses |
| KR102206856B1 (en) * | 2017-12-11 | 2021-01-25 | (주)바이오니아 | Polymerase Chain Reaction System |
| KR102065650B1 (en) * | 2017-12-28 | 2020-02-11 | 에스디 바이오센서 주식회사 | Method for extracting nucleic acid using cartridge |
| KR102076220B1 (en) | 2017-12-28 | 2020-02-11 | 에스디 바이오센서 주식회사 | Flow structure of cartridge for extracting nucleic acid |
| KR102009505B1 (en) * | 2019-01-17 | 2019-08-12 | 주식회사 엘지화학 | Module for polymerase chain reaction of sample |
| US11254979B2 (en) * | 2020-06-01 | 2022-02-22 | Shaheen Innovations Holding Limited | Systems and devices for infectious disease screening |
| US11730193B2 (en) | 2019-12-15 | 2023-08-22 | Shaheen Innovations Holding Limited | Hookah device |
| WO2021138544A1 (en) | 2020-01-03 | 2021-07-08 | Visby Medical, Inc. | Devices and methods for antibiotic susceptibility testing |
| CN111607484A (en) * | 2020-05-22 | 2020-09-01 | 东莞市东阳光诊断产品有限公司 | Nucleic acid amplification device and method |
| AU2021285406A1 (en) | 2020-06-01 | 2023-01-19 | Shaheen Innovations Holding Limited | An infectious disease screening device |
| AU2021285405A1 (en) | 2020-06-01 | 2023-01-19 | Shaheen Innovations Holding Limited | An infectious disease screening system |
| CN113583854B (en) * | 2021-07-30 | 2024-02-27 | 广州和实生物技术有限公司 | Nucleic acid detection device capable of performing home self-test, detection method and application |
| GB202305080D0 (en) * | 2023-04-05 | 2023-05-17 | Anglia Ruskin Univ Higher Education Corporation | Methods and devices for nucleic acid amplification |
Family Cites Families (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1572596A (en) * | 1976-12-06 | 1980-07-30 | Opto Electronic Displays Ltd | Apparatus and method for innoculation |
| DE8813773U1 (en) * | 1988-11-03 | 1989-01-05 | Max-Planck-Gesellschaft zur Förderung der Wissenschaften eV, 37073 Göttingen | Device for optionally setting the temperature of a sample to different values |
| FR2672231A1 (en) * | 1991-02-01 | 1992-08-07 | Eibet | Apparatus for repeated automatic execution of a heat cycle, especially for the amplification of the number of a defined nucleic acid sequence |
| DE4234086A1 (en) * | 1992-02-05 | 1993-08-12 | Diagen Inst Molekularbio | METHOD FOR DETERMINING NUCLEIC ACID SEQUENCES AMPLIFIED IN VITRO |
| US5525300A (en) * | 1993-10-20 | 1996-06-11 | Stratagene | Thermal cycler including a temperature gradient block |
| US5639428A (en) * | 1994-07-19 | 1997-06-17 | Becton Dickinson And Company | Method and apparatus for fully automated nucleic acid amplification, nucleic acid assay and immunoassay |
| US5556771A (en) * | 1995-02-10 | 1996-09-17 | Gen-Probe Incorporated | Stabilized compositions of reverse transcriptase and RNA polymerase for nucleic acid amplification |
| US5609828A (en) * | 1995-05-31 | 1997-03-11 | bio M erieux Vitek, Inc. | Sample card |
| EP0889751B1 (en) * | 1996-04-03 | 1999-09-08 | The Perkin-Elmer Corporation | Device and method for multiple analyte detection |
| WO1998005060A1 (en) * | 1996-07-31 | 1998-02-05 | The Board Of Trustees Of The Leland Stanford Junior University | Multizone bake/chill thermal cycling module |
| AUPO652997A0 (en) * | 1997-04-30 | 1997-05-29 | Kindconi Pty Limited | Temperature cycling device and method |
| WO1998053311A2 (en) * | 1997-05-23 | 1998-11-26 | Gamera Bioscience Corporation | Devices and methods for using centripetal acceleration to drive fluid movement in a microfluidics system |
| DE19810499A1 (en) * | 1998-03-11 | 1999-09-16 | Microparts Gmbh | Micro-titration plate suitable for a range of automated optical test procedures |
| DE59905743D1 (en) * | 1998-03-11 | 2003-07-03 | Steag Microparts Gmbh | SAMPLE CARRIER |
| DE19852835A1 (en) * | 1998-11-17 | 2000-05-18 | Stratec Biomedical Systems Ag | Sample holder |
| CA2255850C (en) * | 1998-12-07 | 2000-10-17 | Her Majesty The Queen In Right Of Canada As Represented By The Minister Of Agriculture And Agri-Food | Rotary thermocycling apparatus |
| US6303343B1 (en) * | 1999-04-06 | 2001-10-16 | Caliper Technologies Corp. | Inefficient fast PCR |
| EP1192006B1 (en) * | 1999-06-22 | 2008-05-14 | Tecan Trading AG | Devices for the performance of miniaturised in vitro amplification assays |
| US6706519B1 (en) * | 1999-06-22 | 2004-03-16 | Tecan Trading Ag | Devices and methods for the performance of miniaturized in vitro amplification assays |
| US6272939B1 (en) * | 1999-10-15 | 2001-08-14 | Applera Corporation | System and method for filling a substrate with a liquid sample |
| US6720148B1 (en) * | 2001-02-22 | 2004-04-13 | Caliper Life Sciences, Inc. | Methods and systems for identifying nucleotides by primer extension |
-
2000
- 2000-07-28 FR FR0010029A patent/FR2812306B1/en not_active Expired - Lifetime
-
2001
- 2001-07-20 EA EA200300203A patent/EA004719B1/en not_active IP Right Cessation
- 2001-07-20 ES ES01956628T patent/ES2372027T3/en not_active Expired - Lifetime
- 2001-07-20 CN CN01815876.5A patent/CN1248781C/en not_active Expired - Lifetime
- 2001-07-20 DK DK10177401.6T patent/DK2269738T3/en active
- 2001-07-20 ES ES10177401T patent/ES2389763T3/en not_active Expired - Lifetime
- 2001-07-20 AU AU2001278554A patent/AU2001278554B2/en not_active Expired
- 2001-07-20 EP EP01956628A patent/EP1305115B1/en not_active Expired - Lifetime
- 2001-07-20 BR BR0112789-6A patent/BR0112789A/en not_active Application Discontinuation
- 2001-07-20 WO PCT/FR2001/002385 patent/WO2002009877A1/en active Application Filing
- 2001-07-20 EP EP10177401A patent/EP2269738B1/en not_active Expired - Lifetime
- 2001-07-20 AU AU7855401A patent/AU7855401A/en active Pending
- 2001-07-20 AT AT01956628T patent/ATE532583T1/en active
- 2001-07-20 CA CA002416756A patent/CA2416756C/en not_active Expired - Lifetime
- 2001-07-20 JP JP2002515419A patent/JP4979873B2/en not_active Expired - Lifetime
- 2001-10-15 US US09/981,070 patent/US6821771B2/en not_active Expired - Lifetime
-
2003
- 2003-01-27 ZA ZA200300700A patent/ZA200300700B/en unknown
-
2004
- 2004-08-25 US US10/926,482 patent/US7732136B2/en not_active Expired - Fee Related
-
2011
- 2011-05-26 JP JP2011118127A patent/JP5202686B2/en not_active Expired - Lifetime
Also Published As
| Publication number | Publication date |
|---|---|
| AU7855401A (en) | 2002-02-13 |
| JP2004504828A (en) | 2004-02-19 |
| US7732136B2 (en) | 2010-06-08 |
| EP1305115B1 (en) | 2011-11-09 |
| EP1305115A1 (en) | 2003-05-02 |
| US20020081669A1 (en) | 2002-06-27 |
| CA2416756A1 (en) | 2002-02-07 |
| ATE532583T1 (en) | 2011-11-15 |
| BR0112789A (en) | 2003-09-09 |
| CN1458866A (en) | 2003-11-26 |
| JP2011200245A (en) | 2011-10-13 |
| DK2269738T3 (en) | 2013-01-02 |
| US6821771B2 (en) | 2004-11-23 |
| CA2416756C (en) | 2010-01-19 |
| ZA200300700B (en) | 2004-03-10 |
| AU2001278554B2 (en) | 2006-09-28 |
| EA200300203A1 (en) | 2003-06-26 |
| CN1248781C (en) | 2006-04-05 |
| JP4979873B2 (en) | 2012-07-18 |
| EP2269738A1 (en) | 2011-01-05 |
| US20050026277A1 (en) | 2005-02-03 |
| ES2372027T3 (en) | 2012-01-13 |
| ES2389763T3 (en) | 2012-10-31 |
| WO2002009877A1 (en) | 2002-02-07 |
| FR2812306B1 (en) | 2005-01-14 |
| FR2812306A1 (en) | 2002-02-01 |
| JP5202686B2 (en) | 2013-06-05 |
| EA004719B1 (en) | 2004-08-26 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2269738B1 (en) | Device for thermo-dependent chain reaction amplification of target nucleic acid sequences | |
| US11142790B2 (en) | Microfluidic device | |
| BE1020816A5 (en) | METHOD AND DEVICE FOR RAPID DETECTION OF AMPLIFIED NUCLEOTIDE SEQUENCES. | |
| US20220186325A1 (en) | Systems for sample analysis | |
| US20100173794A1 (en) | Device for amplification and detection of nucleic acids | |
| JP6190950B2 (en) | Droplet storage method | |
| US20050196779A1 (en) | Self-contained microfluidic biochip and apparatus | |
| EP2640517A1 (en) | Microfluid cartridge for molecular diagnostics | |
| JP5298718B2 (en) | Centrifugal device for filling biological sample reaction chip with reaction solution | |
| JP2004504828A5 (en) | ||
| US8597882B2 (en) | Method and apparatus for conducting an assay | |
| EP2107125A1 (en) | Real-time PCR of targets on a micro-array | |
| FR2950358A1 (en) | SIMPLIFIED NUCLEIC ACID AMPLIFICATION DEVICE AND ITS IMPLEMENTATION METHOD | |
| FR2902441A1 (en) | METHOD FOR DETERMINING THE CONCENTRATION OF NUCLEIC ACIDS | |
| EP1356303A1 (en) | Method and system for performing in continuous flow a biological, chemical or biochemical protocol | |
| US20230409629A1 (en) | Time-based cluster imaging | |
| FR2798604A1 (en) | Microfluidics device with continuous flow system, useful e.g. for nucleic acid amplification or sequencing, providing temperature cycling of sample |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| AC | Divisional application: reference to earlier application |
Ref document number: 1305115 Country of ref document: EP Kind code of ref document: P |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU MC NL PT SE TR |
|
| RIN1 | Information on inventor provided before grant (corrected) |
Inventor name: FESTOC, GABRIEL |
|
| 17P | Request for examination filed |
Effective date: 20110628 |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: PALL GENEDISC TECHNOLOGIES |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AC | Divisional application: reference to earlier application |
Ref document number: 1305115 Country of ref document: EP Kind code of ref document: P |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU MC NL PT SE TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D Free format text: NOT ENGLISH |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R082 Ref document number: 60147073 Country of ref document: DE Representative=s name: HOEGER, STELLRECHT & PARTNER PATENTANWAELTE MB, DE Ref country code: DE Ref legal event code: R082 Ref document number: 60147073 Country of ref document: DE Representative=s name: HOEGER, STELLRECHT & PARTNER PATENTANWAELTE, DE |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: NV Representative=s name: ISLER & PEDRAZZINI AG Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: REF Ref document number: 572768 Country of ref document: AT Kind code of ref document: T Effective date: 20120915 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D Free format text: LANGUAGE OF EP DOCUMENT: FRENCH |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 60147073 Country of ref document: DE Effective date: 20121018 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2389763 Country of ref document: ES Kind code of ref document: T3 Effective date: 20121031 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: T3 |
|
| REG | Reference to a national code |
Ref country code: SE Ref legal event code: TRGR |
|
| REG | Reference to a national code |
Ref country code: DK Ref legal event code: T3 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120829 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20121130 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20121231 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed |
Effective date: 20130530 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 60147073 Country of ref document: DE Effective date: 20130530 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120829 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20120829 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R082 Ref document number: 60147073 Country of ref document: DE Representative=s name: HOEGER, STELLRECHT & PARTNER PATENTANWAELTE MB, DE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20130720 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 16 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 17 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: PLFP Year of fee payment: 18 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R082 Ref document number: 60147073 Country of ref document: DE Representative=s name: HOEGER, STELLRECHT & PARTNER PATENTANWAELTE MB, DE |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20200612 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: BE Payment date: 20200617 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20200715 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IE Payment date: 20200709 Year of fee payment: 20 Ref country code: DE Payment date: 20200707 Year of fee payment: 20 Ref country code: TR Payment date: 20200720 Year of fee payment: 20 Ref country code: GB Payment date: 20200708 Year of fee payment: 20 Ref country code: ES Payment date: 20200803 Year of fee payment: 20 Ref country code: DK Payment date: 20200710 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20200610 Year of fee payment: 20 Ref country code: CH Payment date: 20200716 Year of fee payment: 20 Ref country code: SE Payment date: 20200710 Year of fee payment: 20 Ref country code: AT Payment date: 20200625 Year of fee payment: 20 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R071 Ref document number: 60147073 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: MK Effective date: 20210719 |
|
| REG | Reference to a national code |
Ref country code: DK Ref legal event code: EUP Expiry date: 20210720 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: PE20 Expiry date: 20210719 |
|
| REG | Reference to a national code |
Ref country code: BE Ref legal event code: MK Effective date: 20210720 |
|
| REG | Reference to a national code |
Ref country code: SE Ref legal event code: EUG |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: MK9A |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MK07 Ref document number: 572768 Country of ref document: AT Kind code of ref document: T Effective date: 20210720 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20210720 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20210719 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20220128 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20210721 |