EP0752012B1 - Verfahren zur herstellung von elektroblechen mit einem glasüberzug - Google Patents
Verfahren zur herstellung von elektroblechen mit einem glasüberzug Download PDFInfo
- Publication number
- EP0752012B1 EP0752012B1 EP95912252A EP95912252A EP0752012B1 EP 0752012 B1 EP0752012 B1 EP 0752012B1 EP 95912252 A EP95912252 A EP 95912252A EP 95912252 A EP95912252 A EP 95912252A EP 0752012 B1 EP0752012 B1 EP 0752012B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- annealing separator
- additive
- mgo
- strip
- annealing
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 239000011521 glass Substances 0.000 title claims abstract description 24
- 238000000034 method Methods 0.000 title claims description 21
- 230000008569 process Effects 0.000 title description 10
- 238000000576 coating method Methods 0.000 title description 5
- 239000011248 coating agent Substances 0.000 title description 4
- 229910000831 Steel Inorganic materials 0.000 title description 3
- 239000010959 steel Substances 0.000 title description 3
- 239000000395 magnesium oxide Substances 0.000 claims abstract description 46
- CPLXHLVBOLITMK-UHFFFAOYSA-N magnesium oxide Inorganic materials [Mg]=O CPLXHLVBOLITMK-UHFFFAOYSA-N 0.000 claims abstract description 46
- AXZKOIWUVFPNLO-UHFFFAOYSA-N magnesium;oxygen(2-) Chemical compound [O-2].[Mg+2] AXZKOIWUVFPNLO-UHFFFAOYSA-N 0.000 claims abstract description 46
- 239000000654 additive Substances 0.000 claims abstract description 33
- 238000000137 annealing Methods 0.000 claims abstract description 26
- -1 sodium phosphate compound Chemical class 0.000 claims abstract description 15
- 239000001488 sodium phosphate Substances 0.000 claims abstract description 14
- 230000000996 additive effect Effects 0.000 claims abstract description 13
- 229910000162 sodium phosphate Inorganic materials 0.000 claims abstract description 10
- 239000006185 dispersion Substances 0.000 claims abstract description 6
- 238000004519 manufacturing process Methods 0.000 claims abstract description 5
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 claims description 21
- 239000004408 titanium dioxide Substances 0.000 claims description 10
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical compound [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 claims description 7
- MVMLTMBYNXHXFI-UHFFFAOYSA-H antimony(3+);trisulfate Chemical compound [Sb+3].[Sb+3].[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O MVMLTMBYNXHXFI-UHFFFAOYSA-H 0.000 claims description 6
- 239000002245 particle Substances 0.000 claims description 5
- VZWGHDYJGOMEKT-UHFFFAOYSA-J sodium pyrophosphate decahydrate Chemical compound O.O.O.O.O.O.O.O.O.O.[Na+].[Na+].[Na+].[Na+].[O-]P([O-])(=O)OP([O-])([O-])=O VZWGHDYJGOMEKT-UHFFFAOYSA-J 0.000 claims description 4
- 235000010339 sodium tetraborate Nutrition 0.000 claims description 4
- 229910021538 borax Inorganic materials 0.000 claims description 3
- UQGFMSUEHSUPRD-UHFFFAOYSA-N disodium;3,7-dioxido-2,4,6,8,9-pentaoxa-1,3,5,7-tetraborabicyclo[3.3.1]nonane Chemical compound [Na+].[Na+].O1B([O-])OB2OB([O-])OB1O2 UQGFMSUEHSUPRD-UHFFFAOYSA-N 0.000 claims description 3
- 239000004328 sodium tetraborate Substances 0.000 claims description 3
- FAPDDOBMIUGHIN-UHFFFAOYSA-K antimony trichloride Chemical compound Cl[Sb](Cl)Cl FAPDDOBMIUGHIN-UHFFFAOYSA-K 0.000 claims description 2
- 238000005097 cold rolling Methods 0.000 claims description 2
- JKWMSGQKBLHBQQ-UHFFFAOYSA-N diboron trioxide Chemical compound O=BOB=O JKWMSGQKBLHBQQ-UHFFFAOYSA-N 0.000 claims description 2
- 150000001399 aluminium compounds Chemical class 0.000 claims 3
- 229910052810 boron oxide Inorganic materials 0.000 claims 1
- 229910001510 metal chloride Inorganic materials 0.000 claims 1
- 239000010410 layer Substances 0.000 description 10
- 235000011008 sodium phosphates Nutrition 0.000 description 9
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 9
- 239000000853 adhesive Substances 0.000 description 8
- 230000001070 adhesive effect Effects 0.000 description 8
- 229910052782 aluminium Inorganic materials 0.000 description 8
- 239000003795 chemical substances by application Substances 0.000 description 8
- 238000005261 decarburization Methods 0.000 description 7
- 230000010287 polarization Effects 0.000 description 7
- 239000011734 sodium Substances 0.000 description 7
- 230000007547 defect Effects 0.000 description 6
- 238000009413 insulation Methods 0.000 description 6
- 230000005415 magnetization Effects 0.000 description 6
- 239000000203 mixture Substances 0.000 description 6
- 229910010413 TiO 2 Inorganic materials 0.000 description 5
- AZDRQVAHHNSJOQ-UHFFFAOYSA-N alumane Chemical class [AlH3] AZDRQVAHHNSJOQ-UHFFFAOYSA-N 0.000 description 5
- 229910000379 antimony sulfate Inorganic materials 0.000 description 5
- 229910052799 carbon Inorganic materials 0.000 description 5
- 238000009826 distribution Methods 0.000 description 5
- 239000011777 magnesium Substances 0.000 description 5
- 239000011241 protective layer Substances 0.000 description 5
- 239000000126 substance Substances 0.000 description 5
- 229910001224 Grain-oriented electrical steel Inorganic materials 0.000 description 4
- 229910004283 SiO 4 Inorganic materials 0.000 description 4
- 230000015572 biosynthetic process Effects 0.000 description 4
- 230000000694 effects Effects 0.000 description 4
- 230000007774 longterm Effects 0.000 description 4
- VTHJTEIRLNZDEV-UHFFFAOYSA-L magnesium dihydroxide Chemical compound [OH-].[OH-].[Mg+2] VTHJTEIRLNZDEV-UHFFFAOYSA-L 0.000 description 4
- 239000000347 magnesium hydroxide Substances 0.000 description 4
- 229910001862 magnesium hydroxide Inorganic materials 0.000 description 4
- 229910052757 nitrogen Inorganic materials 0.000 description 4
- FQENQNTWSFEDLI-UHFFFAOYSA-J sodium diphosphate Chemical compound [Na+].[Na+].[Na+].[Na+].[O-]P([O-])(=O)OP([O-])([O-])=O FQENQNTWSFEDLI-UHFFFAOYSA-J 0.000 description 4
- 229940048086 sodium pyrophosphate Drugs 0.000 description 4
- 229910052717 sulfur Inorganic materials 0.000 description 4
- 235000019818 tetrasodium diphosphate Nutrition 0.000 description 4
- 239000001577 tetrasodium phosphonato phosphate Substances 0.000 description 4
- 238000004458 analytical method Methods 0.000 description 3
- 238000011156 evaluation Methods 0.000 description 3
- 239000000843 powder Substances 0.000 description 3
- 229910018072 Al 2 O 3 Inorganic materials 0.000 description 2
- PNEYBMLMFCGWSK-UHFFFAOYSA-N Alumina Chemical class [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 2
- 229910019142 PO4 Inorganic materials 0.000 description 2
- 229910004298 SiO 2 Inorganic materials 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- 239000012790 adhesive layer Substances 0.000 description 2
- 230000000181 anti-adherent effect Effects 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 150000001639 boron compounds Chemical class 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- 150000001875 compounds Chemical class 0.000 description 2
- 230000018044 dehydration Effects 0.000 description 2
- 238000006297 dehydration reaction Methods 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- 239000003112 inhibitor Substances 0.000 description 2
- 238000002955 isolation Methods 0.000 description 2
- 230000035699 permeability Effects 0.000 description 2
- 235000021317 phosphate Nutrition 0.000 description 2
- 238000012549 training Methods 0.000 description 2
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- 206010037867 Rash macular Diseases 0.000 description 1
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 229940058905 antimony compound for treatment of leishmaniasis and trypanosomiasis Drugs 0.000 description 1
- 150000001463 antimony compounds Chemical class 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 229910001593 boehmite Inorganic materials 0.000 description 1
- 239000000919 ceramic Substances 0.000 description 1
- 230000015271 coagulation Effects 0.000 description 1
- 238000005345 coagulation Methods 0.000 description 1
- 239000011362 coarse particle Substances 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- BNIILDVGGAEEIG-UHFFFAOYSA-L disodium hydrogen phosphate Chemical compound [Na+].[Na+].OP([O-])([O-])=O BNIILDVGGAEEIG-UHFFFAOYSA-L 0.000 description 1
- CDMADVZSLOHIFP-UHFFFAOYSA-N disodium;3,7-dioxido-2,4,6,8,9-pentaoxa-1,3,5,7-tetraborabicyclo[3.3.1]nonane;decahydrate Chemical compound O.O.O.O.O.O.O.O.O.O.[Na+].[Na+].O1B([O-])OB2OB([O-])OB1O2 CDMADVZSLOHIFP-UHFFFAOYSA-N 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 229910052840 fayalite Inorganic materials 0.000 description 1
- 239000010419 fine particle Substances 0.000 description 1
- 230000000887 hydrating effect Effects 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 150000004679 hydroxides Chemical class 0.000 description 1
- FAHBNUUHRFUEAI-UHFFFAOYSA-M hydroxidooxidoaluminium Chemical compound O[Al]=O FAHBNUUHRFUEAI-UHFFFAOYSA-M 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 231100001231 less toxic Toxicity 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- CUXQLKLUPGTTKL-UHFFFAOYSA-M microcosmic salt Chemical compound [NH4+].[Na+].OP([O-])([O-])=O CUXQLKLUPGTTKL-UHFFFAOYSA-M 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 1
- 230000008092 positive effect Effects 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 238000005096 rolling process Methods 0.000 description 1
- 229910052710 silicon Inorganic materials 0.000 description 1
- 239000010703 silicon Substances 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 235000012239 silicon dioxide Nutrition 0.000 description 1
- 239000002002 slurry Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 150000003464 sulfur compounds Chemical class 0.000 description 1
- 238000010998 test method Methods 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 150000003609 titanium compounds Chemical class 0.000 description 1
- 229910000406 trisodium phosphate Inorganic materials 0.000 description 1
- 235000019801 trisodium phosphate Nutrition 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C21—METALLURGY OF IRON
- C21D—MODIFYING THE PHYSICAL STRUCTURE OF FERROUS METALS; GENERAL DEVICES FOR HEAT TREATMENT OF FERROUS OR NON-FERROUS METALS OR ALLOYS; MAKING METAL MALLEABLE, e.g. BY DECARBURISATION OR TEMPERING
- C21D8/00—Modifying the physical properties by deformation combined with, or followed by, heat treatment
- C21D8/12—Modifying the physical properties by deformation combined with, or followed by, heat treatment during manufacturing of articles with special electromagnetic properties
-
- C—CHEMISTRY; METALLURGY
- C21—METALLURGY OF IRON
- C21D—MODIFYING THE PHYSICAL STRUCTURE OF FERROUS METALS; GENERAL DEVICES FOR HEAT TREATMENT OF FERROUS OR NON-FERROUS METALS OR ALLOYS; MAKING METAL MALLEABLE, e.g. BY DECARBURISATION OR TEMPERING
- C21D8/00—Modifying the physical properties by deformation combined with, or followed by, heat treatment
- C21D8/12—Modifying the physical properties by deformation combined with, or followed by, heat treatment during manufacturing of articles with special electromagnetic properties
- C21D8/1277—Modifying the physical properties by deformation combined with, or followed by, heat treatment during manufacturing of articles with special electromagnetic properties involving a particular surface treatment
- C21D8/1283—Application of a separating or insulating coating
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22F—CHANGING THE PHYSICAL STRUCTURE OF NON-FERROUS METALS AND NON-FERROUS ALLOYS
- C22F1/00—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22F—CHANGING THE PHYSICAL STRUCTURE OF NON-FERROUS METALS AND NON-FERROUS ALLOYS
- C22F1/00—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working
- C22F1/10—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working of nickel or cobalt or alloys based thereon
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23D—ENAMELLING OF, OR APPLYING A VITREOUS LAYER TO, METALS
- C23D5/00—Coating with enamels or vitreous layers
- C23D5/02—Coating with enamels or vitreous layers by wet methods
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01F—MAGNETS; INDUCTANCES; TRANSFORMERS; SELECTION OF MATERIALS FOR THEIR MAGNETIC PROPERTIES
- H01F1/00—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties
- H01F1/01—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials
- H01F1/03—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials characterised by their coercivity
- H01F1/12—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials characterised by their coercivity of soft-magnetic materials
- H01F1/14—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials characterised by their coercivity of soft-magnetic materials metals or alloys
- H01F1/16—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials characterised by their coercivity of soft-magnetic materials metals or alloys in the form of sheets
- H01F1/18—Magnets or magnetic bodies characterised by the magnetic materials therefor; Selection of materials for their magnetic properties of inorganic materials characterised by their coercivity of soft-magnetic materials metals or alloys in the form of sheets with insulating coating
Definitions
- the invention relates to a method for producing Electrical sheets, especially grain-oriented ones Electrical sheets, with an evenly adhering Glass film and with improved magnetic properties, in which the hot strip initially produced and possibly annealed except for the final cold strip thickness with at least one Cold rolling stage is cold rolled, then to the an annealing separator rolled to the final thickness is applied and dried and then the cold-rolled strip coated in a high-temperature annealing is subjected, with an integral part of the Glow separator an aqueous magnesium oxide (MgO) dispersion and the glow separator is additionally at least one Has additive.
- MgO aqueous magnesium oxide
- decarburization annealing is carried out after rolling to the final thickness.
- the carbon is extracted from the material.
- An oxide layer forms as a base layer on the strip surface, the essential components of which are silicon dioxide (SiO 2 ) and fayalite (Fe 2 SiO 4 ).
- the strip is coated with a protective layer and subjected to a long-term annealing in the coil.
- the adhesive protection layer is intended to prevent the individual coil turns from sticking together during long-term annealing and, on the other hand, to form an insulation layer (glass film) with the base layer on the strip surface.
- the adhesive protection layer consists essentially of magnesium oxide (MgO).
- the MgO is slurried in water in the form of a powder, applied to the belt and dried. During this process, part of the magnesium oxide reacts with the water to form magnesium hydroxide (Mg (OH) 2 ). The amount of water bound to magnesium hydroxide, based on the total amount of oxide powder, is called the loss on ignition.
- Equation (I) shows the dehydration of the magnesium hydroxide, which starts at about 350 ° C. It is important for an optimally running process, both in terms of the insulation and the development of the magnetic properties, that the amount of water released is within certain limits.
- the water humidifies the predominantly hydrogen-containing annealing atmosphere and thus sets a corresponding oxidation potential.
- the annealing atmosphere must not be too dry because the glass film would be made too thin under such conditions. However, it must also not become too moist, because then it will be reoxidized too much and the glass film will have defects such as local flaking and poor adhesion.
- additives to MgO powder were introduced to improve the formation of the insulation layer and the magnetic properties of the finished product.
- These include titanium dioxide (TiO 2 ), boron compounds such as boron oxide (B 2 O 3 ) or sodium tetraborate (Na 2 B 4 O 7 ), as well as antimony compounds such as antimony sulfate (Sb 2 (SO 4 ) 3 ) in combination with a chloride, preferably antimony chloride SbCl 3 .
- TiO 2 titanium dioxide
- boron compounds such as boron oxide (B 2 O 3 ) or sodium tetraborate (Na 2 B 4 O 7 )
- antimony compounds such as antimony sulfate (Sb 2 (SO 4 ) 3 ) in combination with a chloride, preferably antimony chloride SbCl 3 .
- the additives used often also have disadvantages that reduce the product quality. Overall, the processing of such additives is cumbersome, since some of them have to be dissolved in previously
- the invention is based on the object of measures to meet, especially by modifying the Glow separators to the insulation properties and at the same time the magnetic properties of the Finished product to improve further.
- the Anti-adhesive layer can be applied more homogeneously quality-reducing phenomena, such as glow contours and to avoid local defects.
- one should easy handling can be guaranteed and the cost, am Standard measured, kept low.
- a readily water-soluble one Sodium phosphate compound is used.
- a readily water soluble sodium phosphate compound and a finely dispersed oxidic aluminum compound Glow separator can be added.
- the good water solubility of the sodium phosphate compound if necessary in combination with the finely dispersed distribution of the oxidic aluminum compound in the specified amounts ensure a homogeneous application of the adhesive protection, prevent coagulation within the aqueous Magnesium oxide dispersion and associated local Defects in the glass film and promote those in the Long-term annealing chemical reactions between the one on the belt surface Base layer and the adhesive protective layer to the glass film.
- a stronger than the standard Glass film formation that the interaction between the Annealing atmosphere and the strips are positively influenced the magnetic properties of the electrical sheets improved.
- a method with the generic measures has been known from EP 0 232 537 B1.
- a titanium compound such as TiO 2
- a boron compound such as B 2 O 3
- a sulfur compound such as SrS
- the MgO-based annealing separator is added to the MgO-based annealing separator as an additive, with the aim of improving the insulation properties, such as Adhesion and the appearance of the glass film to influence positively. This is achieved by hydrating the coating.
- the magnetic properties were also improved by the addition of such additives.
- JP-5-513 8021 describes a release agent based on MgO, which contains up to 50% by weight of Mg (OH) 2 and up to 5% by weight of Al (OH) 3 or Al (NO 3 ) 3 contains. It has been shown that the use of such a release agent for coating electrical sheets does not have an adverse effect on the magnetic properties of the product.
- JP-5-247 661 describes a process for the production of grain-oriented silicon steels which are coated with a glass layer.
- improvements in the magnetic properties and the surface properties of the coated steel are achieved by using release agent additives such as Sb 2 (SO 4 ) 3 , V 2 O s , SrS, Na 2 B 4 O 7 and Ca (H 2 PO 4 ) 2 .
- the positive influence on which the invention is based the magnetic properties is characteristic of the sodium phosphates.
- FIG. 1 shows the superiority of the samples produced by the method according to the invention with an MgO-based adhesive protection doped with sodium phosphate over other phosphate additives.
- HGO high permeability grain oriented tape samples were coated with MgO + 6% TiO 2 + the listed additives, dried and annealed.
- the sodium phosphates are readily water-soluble, allow thus an optimally homogeneous distribution within the Anti-adhesive layer.
- Sodium phosphates in the present case in particular using the example of Sodium pyrophosphate decahydrate are reported both the magnetic properties polarization and Magnetic loss, as well Isolation training improved.
- the Inhibitor test method is demonstrated that the Sodium pyrophosphate to a prematurely stronger one Glass film formation leads.
- the inhibitor test stops Process in which, in principle, high annealing certain annealing temperatures are canceled and the Samples can be assessed magnetically. In the present case the insulation training was also assessed.
- the magnetic properties of loss of magnetization P 1.7 and polarization J 800 were determined on the annealed strips.
- the aluminum compounds used as a further additive in addition to the sodium phosphate compound are aluminum oxides or hydroxides of the form Al 2 O 3 , AlO (OH) 3 and AL (OH), the effect of which is fully exploited when the corresponding particle sizes are small. The effect is particularly evident when the compounds are added in the form of sols (very fine particles / water mixtures).
- the average particle size should be less than 100 nm with the narrowest possible particle size distribution.
- the addition of these aluminum compounds leads to a considerable improvement in loss, similar to the case with the addition of titanium dioxide.
- the advantage of the aluminum compound as an additive over titanium dioxide is the lower dosage and the more homogeneous distribution of the particles. Another advantage lies in the fact that the aluminum compounds added also have the property of a ceramic binder, and the adhesive protective layer therefore adheres better to the tape.
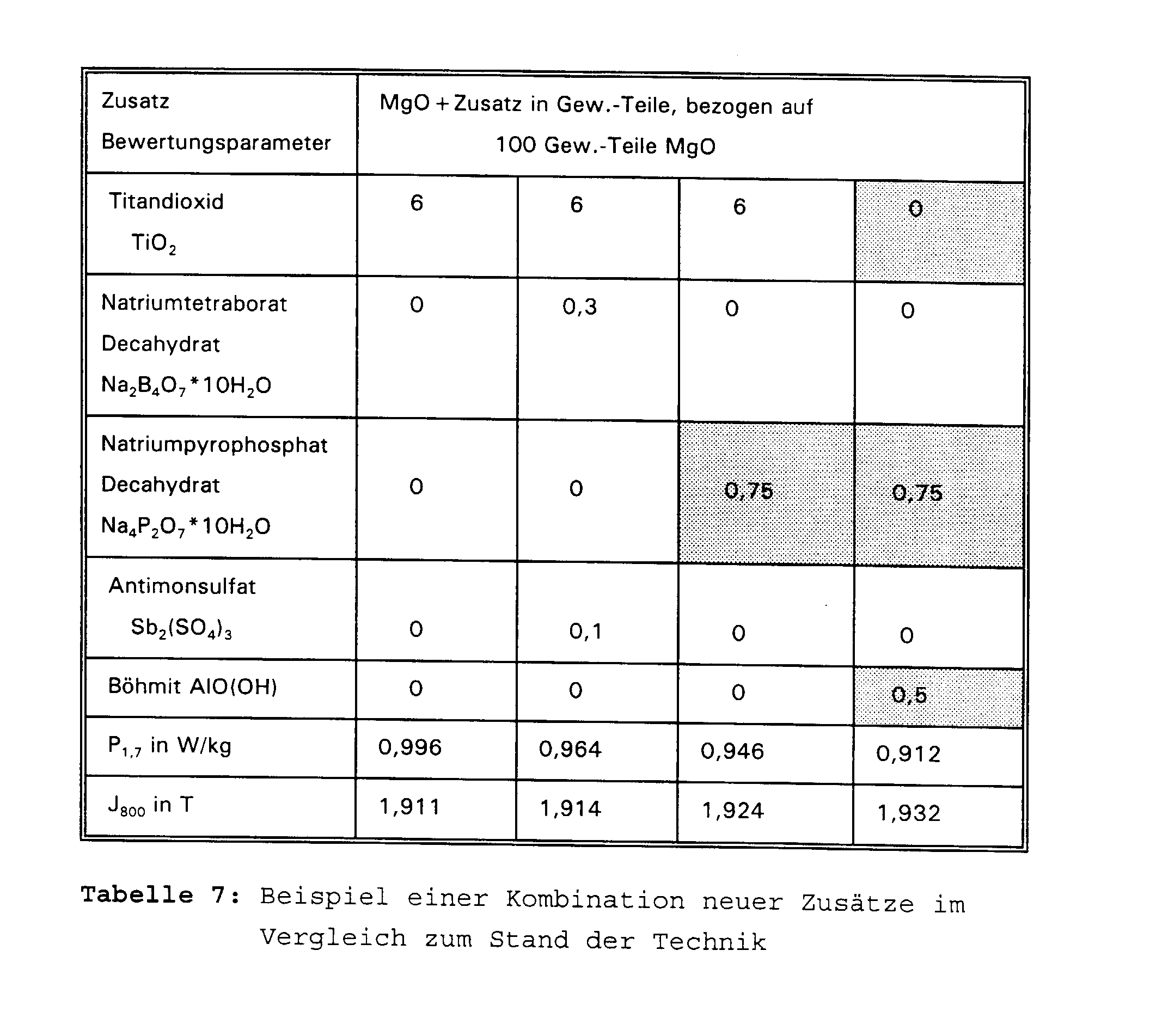
- Table 6 and Figure 3 show the influence of the selected aluminum compounds on the magnetic loss. Influence of different oxidic aluminum compounds on the magnetic properties and the glass film appearance
- Boehmite AIO (OH) 0 0.5 2nd Glass film appearance
- the effect of the above additives is optimized, if suitable combinations of additives are used. This also has positive effects in combination with additives already used, such as titanium dioxide, Antimony sulfate and sodium tetraborate achieved. Related to the slurry properties and thus the homogeneity of the MgO layer turns out to be a combination of one finely dispersed oxidic aluminum compound and one well water-soluble sodium phosphate as optimal because with these additives significantly fewer local defects to be observed.
- the magnetic properties of loss of magnetization P 1.7 and polarization J 800 were determined on the annealed strips.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Physics & Mathematics (AREA)
- Organic Chemistry (AREA)
- Metallurgy (AREA)
- Mechanical Engineering (AREA)
- Materials Engineering (AREA)
- Thermal Sciences (AREA)
- Crystallography & Structural Chemistry (AREA)
- Power Engineering (AREA)
- Electromagnetism (AREA)
- Dispersion Chemistry (AREA)
- Manufacturing & Machinery (AREA)
- Soft Magnetic Materials (AREA)
- Chemical Treatment Of Metals (AREA)
- Manufacturing Of Steel Electrode Plates (AREA)
- Insulating Bodies (AREA)
- Inorganic Insulating Materials (AREA)
- Glass Compositions (AREA)
- Laminated Bodies (AREA)
- Cell Separators (AREA)
Description
| Einfluß von Natriumpyrophosphat als Zusatz zum MgO auf die magnetischen Eigenschaften | ||
| Klebschutzzusammensetzung | 100 % MgO | 99,25 % MgO 0,75 % Na4P2O7*10H2O |
| J800 in T | 1,909 | 1,933 |
| P1,7 in W/kg | 1,118 | 0,995 |
| Si % | C % | Al % | Mn % | Sn % | N % | S % |
| 3,17- | 0,065- | 0,025- | 0,074- | 0,118- | 0,0077- | 0,025- |
| 3,29 | 0,070 | 0,026 | 0,080 | 0,120 | 0,0087 | 0,028 |
| Einfluß unterschiedlicher Natriumpyrophosphatkonzentrationen auf die magnetischen Eigenschaften und das Gladfilmaussehen | ||||
| Zusatz Bewertungsparameter | MgO + 6%TiO2 + Zusatz in Gew.-Teile, bezogen auf 100 Gew.-Teile MgO | |||
| Natriumpyrophosphat ecahydrat Na4P2O7*10H2O | 0 | 0,5 | 1 | 2 |
| Glasfilmassehen | Glühkonturen | frei von Glühkonturen | frei von Glühkonturen | fleckig |
| P1,7 in W/kg | 0,979 | 0,930 | 0,904 | 0,943 |
| J800 in T | 1,916 | 1,925 | 1,931 | 1,940 |
| Si % | C % | Al % | Mn % | Sn % | N % | S % |
| 3,13- | 0,063- | 0,024- | 0,072- | 0,075- | 0,0077- | 0,020- |
| 3,30 | 0,067 | 0,028 | 0,082 | 0,121 | 0,0090 | 0,027 |
| Si % | C % | Al % | Mn % | Sn % | N % | S % | |
| Probe 1 | 3,13 | 0,061 | 0,020 | 0,070 | 0,075 | 0,0078 | 0,024 |
| Probe 2 | 3,08 | 0,061 | 0,020 | 0,080 | 0,026 | 0,0076 | 0,023 |
| Einfluß unterschiedlicher Na-Phosphate auf die magnetischen Eigenschaften | ||||||
| Zusatz Bewertungsparameter | MgO + 6%TiO2 +Zusatz in Gew.-Teile bezogen auf 100 Gew.-Teile MgO | |||||
| Natriumtetraborat Decahydrat NA2B4O7 *10H2O | 0 | 0,3 | 0 | 0 | 0 | 0 |
| Natriumpyrophosphat Decahydrat Na4P2O7*10H2O | 0 | 0 | 1,5 | 0 | 0 | 0 |
| Di-Natriumhydrogen phosphat Na2HPO4*2H2O | 0 | 0 | 0 | 1,2 | 0 | 0 |
| Tri-Natriumphosphat Na3PO4*12H2O | 0 | 0 | 0 | 0 | 2,55 | 0 |
| Natriumammonium hydrogenphosphat NaNH4HPO4 | 0 | 0 | 0 | 0 | 0 | 1,4 |
| Antimonsulfat Sb2(SO4)3 | 0 | 0,1 | 0 | 0 | 0 | 0 |
| P1,7 in W/kg | 0,983 | 0,942 | 0,937 | 0 937 | 0,992 | 0,949 |
| J800 in T | 1,918 | 1,926 | 1,932 | 1,925 | 1,927 | 1,916 |
| Si % | C % | Al % | Mn % | Sn % | N % | S % |
| 3,23- | 0,065- | 0,025- | 0,073- | 0,117- | 0,0084- | 0,021- |
| 3,29 | 0,073 | 0,028 | 0,077 | 0,119 | 0,0090 | 0,027 |
| Einfluß unterschiedlicher oxidischer Aluminiumverbindungen auf die magnetischen Eigenschaften und das Glasfilmaussehen | ||||
| Zusatz Bewertungsparameter | MgO+Zusatz in Gew.-Teile, bezogen auf 100 Gew.-Teile MgO | |||
| Aluminiumoxid Al2O3 | 0 | 0,5 | 2 | 4 |
| Glasfilmaussehen | Glühkonturen | gleichmäßig | zu dünn | zu dünn |
| P1,7 in W/kg | 0,968 | 0,944 | 0,914 | 0,931 |
| J800 in T | 1,928 | 1,924 | 1,925 | 1,928 |
| Böhmit AIO(OH) | 0 | 0,5 | 2 | |
| Glasfilmaussehen | Glühkonturen | gleichmäßig | zu dünn | - |
| P1,7 in W/kg | 0,968 | 0,906 | 0,917 | |
| J800 in T | 1,928 | 1,931 | 1,928 | |
| Vergleich MgO+ Zusatz Titandioxid in Gew.-Teile, bezogen auf MgO | ||||
| Titandioxid | 0 | 6 | ||
| Glasfilmaussehen | Glühkonturen | Glühkonturen | ||
| P1,7 in W/kg | 0,968 | 0,913 | ||
| J800 in T | 1,928 | 1,919 |
Claims (7)
- Verfahren zur Herstellung von Elektroblechen, insbesondere von kornorientierten Elektroblechen, mit einem gleichmäßigen gut haftenden Glasfilm und mit verbesserten magnetischen Eigenschaften, bei dem das zunächst erzeugte und ggf. geglühte Warmband bis auf die Kaltband-Enddicke mit mindestens einer Kaltwalzstufe kaltgewalzt wird, anschließend auf das bis auf die Enddicke gewalzte Band ein Glühseparator aufgebracht und getrocknet wird und im Anschluß daran das so beschichtete Kaltband einer Hochtemperaturglühung unterworfen wird, wobei wesentlicher Bestandteil des Glühseparators eine wäßrige Magnesiumoxid(MgO)-Dispersion ist, die aus reaktivem MgO hergestellt wurde, und der Glühseparator zusätzlich mindestens ein Additiv aufweist,
dadurch gekennzeichnet, daß als mindestens ein Additiv eine gut wasserlösliche Natriumphosphatverbindung verwendet wird. - Verfahren nach Anspruch 1,
dadurch gekennzeichnet, daß mindestens zwei Additive verwendet werden, nämlich eine gut wasserlösliche Natriumphosphatverbindung und eine feindisperse oxidische Aluminiumverbindung . - Verfahren nach Anspruch 1 oder 2,
dadurch gekennzeichnet, daß dem Glühseparator als Additiv, bezogen auf die Menge MgO, 0,05 bis 4,0 % Natriumphosphat zugesetzt wird. - Verfahren nach Anspruch 1 oder 2,
dadurch gekennzeichnet, daß dem Glühseparator als Additiv, bezogen auf die Menge MgO, 0,3 bis 1,5 % Natriumpyrophosphat-Decahydrat zugesetzt wird. - Verfahren nach Anspruch 2, 3 oder 4,
dadurch gekennzeichnet, daß dem Glühseparator als Additiv, bezogen auf die Menge MgO, 0,05 bis 4,0 % der feindispersen oxidischen Aluminiumverbindung zugesetzt wird. - Verfahren nach Anspruch 2, 3, 4 oder 5,
dadurch gekennzeichnet, daß die oxidische Aluminiumverbindung mit einer Teilchengröße von kleiner als 100 nm verwendet wird. - Verfahren nach einem der Ansprüche 1 bis 6,
dadurch gekennzeichnet, daß dem Glühseparator weitere Additive wie Titanoxid, Boroxid, Natriumtetraborat, Antimonsulfat, Metallchlorid, vorzugsweise Antimonchlorid, zugesetzt werden.
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE4409691A DE4409691A1 (de) | 1994-03-22 | 1994-03-22 | Verfahren zur Herstellung von Elektroblechen mit einem Glasüberzug |
| DE4409691 | 1994-03-22 | ||
| PCT/EP1995/001020 WO1995025820A1 (de) | 1994-03-22 | 1995-03-18 | Verfahren zur herstellung von elektroblechen mit einem glasüberzug |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP0752012A1 EP0752012A1 (de) | 1997-01-08 |
| EP0752012B1 true EP0752012B1 (de) | 1998-08-26 |
Family
ID=6513410
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP95912252A Expired - Lifetime EP0752012B1 (de) | 1994-03-22 | 1995-03-18 | Verfahren zur herstellung von elektroblechen mit einem glasüberzug |
Country Status (10)
| Country | Link |
|---|---|
| US (1) | US5863356A (de) |
| EP (1) | EP0752012B1 (de) |
| JP (1) | JP3730254B2 (de) |
| KR (1) | KR100367985B1 (de) |
| AT (1) | ATE170226T1 (de) |
| CZ (1) | CZ292216B6 (de) |
| DE (2) | DE4409691A1 (de) |
| PL (1) | PL178890B1 (de) |
| RU (1) | RU2139945C1 (de) |
| WO (1) | WO1995025820A1 (de) |
Families Citing this family (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP3475258B2 (ja) * | 1994-05-23 | 2003-12-08 | 株式会社海水化学研究所 | セラミック被膜形成剤およびその製造方法 |
| DE19750066C1 (de) * | 1997-11-12 | 1999-08-05 | Ebg Elektromagnet Werkstoffe | Verfahren zum Beschichten von Elektrostahlbändern mit einem Glühseparator |
| US6835250B2 (en) * | 2000-05-01 | 2004-12-28 | Tateho Chemical Industries Co., Ltd. | Magnesium oxide particle aggregate |
| RU2271406C2 (ru) * | 2000-10-25 | 2006-03-10 | Татехо Кемикал Индастриз Ко., Лтд. | Агрегат частиц оксида магния |
| RU2298592C2 (ru) * | 2002-03-28 | 2007-05-10 | Ниппон Стил Корпорейшн | Листовая электротехническая сталь с ориентированными зернами, обладающая исключительно высокой адгезией пленки, и способ ее производства |
| DE102004014596A1 (de) * | 2004-03-23 | 2005-10-27 | Trithor Gmbh | Antihaftbeschichtung für die Herstellung von Kompositwerkstoff-Drähten |
| US7942982B2 (en) * | 2006-11-22 | 2011-05-17 | Nippon Steel Corporation | Grain-oriented electrical steel sheet excellent in coating adhesion and method of producing the same |
| JP5633178B2 (ja) * | 2010-04-27 | 2014-12-03 | Jfeスチール株式会社 | 方向性電磁鋼板用焼鈍分離剤 |
| DE102010038038A1 (de) * | 2010-10-07 | 2012-04-12 | Thyssenkrupp Electrical Steel Gmbh | Verfahren zum Erzeugen einer Isolationsbeschichtung auf einem kornorientierten Elektro-Stahlflachprodukt und mit einer solchen Isolationsbeschichtung beschichtetes Elektro-Stahlflachprodukt |
| CN102453793B (zh) * | 2010-10-25 | 2013-09-25 | 宝山钢铁股份有限公司 | 用于具有优良磁性能的镜面取向硅钢制备的退火隔离剂 |
| RU2562182C2 (ru) * | 2011-01-12 | 2015-09-10 | Ниппон Стил Энд Сумитомо Метал Корпорейшн | Лист из электротехнической стали с ориентированной зеренной структурой и способ его получения |
| JP5360272B2 (ja) * | 2011-08-18 | 2013-12-04 | Jfeスチール株式会社 | 方向性電磁鋼板の製造方法 |
| US9194016B2 (en) * | 2011-10-04 | 2015-11-24 | Jfe Steel Corporation | Annealing separator for grain-oriented electromagnetic steel sheet |
| DE102015114358B4 (de) | 2015-08-28 | 2017-04-13 | Thyssenkrupp Electrical Steel Gmbh | Verfahren zum Herstellen eines kornorientierten Elektrobands und kornorientiertes Elektroband |
| KR101909218B1 (ko) | 2016-12-21 | 2018-10-17 | 주식회사 포스코 | 방향성 전기강판용 소둔 분리제 조성물, 방향성 전기강판 및 방향성 전기강판의 제조방법 |
| JP6939767B2 (ja) * | 2018-12-27 | 2021-09-22 | Jfeスチール株式会社 | 方向性電磁鋼板用焼鈍分離剤および方向性電磁鋼板の製造方法 |
| JP6939766B2 (ja) * | 2018-12-27 | 2021-09-22 | Jfeスチール株式会社 | 方向性電磁鋼板用焼鈍分離剤および方向性電磁鋼板の製造方法 |
| CN111906142B (zh) * | 2020-06-24 | 2022-08-16 | 浙江博星工贸有限公司 | 一种控制冷轧不锈钢带力学性能的工艺 |
| CN114014529B (zh) * | 2021-12-17 | 2023-02-21 | 中国建筑材料科学研究总院有限公司 | 一种硼硅酸盐玻璃珠火抛用的隔离剂 |
| CN114854960B (zh) * | 2022-03-30 | 2023-09-05 | 武汉钢铁有限公司 | 一种减少取向硅钢表面缺陷的退火隔离剂及其使用方法 |
Family Cites Families (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3151000A (en) * | 1959-08-28 | 1964-09-29 | Hooker Chemical Corp | Method of applying highly heat resistant protective coatings to metallic surfaces |
| US3151997A (en) * | 1961-09-29 | 1964-10-06 | United States Steel Corp | Separating-medium coating for preparation of electrical steel strip for annealing |
| US3615918A (en) * | 1969-03-28 | 1971-10-26 | Armco Steel Corp | Method of annealing with a magnesia separator containing a decomposable phosphate |
| SU569653A1 (ru) * | 1976-01-04 | 1977-08-25 | Уральский научно-исследовательский институт черных металлов | Состав дл термоизол ционного покрыти |
| US4160681A (en) * | 1977-12-27 | 1979-07-10 | Allegheny Ludlum Industries, Inc. | Silicon steel and processing therefore |
| JPS55138021A (en) * | 1979-04-11 | 1980-10-28 | Nippon Steel Corp | Manufacture of annealing separation agent for electromagnetic steel plate |
| IT1127263B (it) * | 1978-11-28 | 1986-05-21 | Nippon Steel Corp | Sostanza di separazione da utilizzare nella fase di ricottura di strisce di acciaio al silicio a grani orientati |
| GB2130241B (en) * | 1982-09-24 | 1986-01-15 | Nippon Steel Corp | Method for producing a grain-oriented electrical steel sheet having a high magnetic flux density |
| US4582547A (en) * | 1984-05-07 | 1986-04-15 | Allegheny Ludlum Steel Corporation | Method for improving the annealing separator coating on silicon steel and coating therefor |
| JPS62156226A (ja) * | 1985-12-27 | 1987-07-11 | Nippon Steel Corp | 均一なグラス皮膜を有し磁気特性が優れた方向性電磁鋼板の製造方法 |
| US4909864A (en) * | 1986-09-16 | 1990-03-20 | Kawasaki Steel Corp. | Method of producing extra-low iron loss grain oriented silicon steel sheets |
| US4740251A (en) * | 1986-12-22 | 1988-04-26 | Calgon Corporation | Method for improving magnesium oxide steel coatings |
| JPH0649949B2 (ja) * | 1988-10-18 | 1994-06-29 | 新日本製鐵株式会社 | 打抜き性と磁気特性の優れた金属光沢を有する方向性電磁鋼板の製造方法 |
| EP0416420B1 (de) * | 1989-09-08 | 1994-12-14 | Armco Inc. | Magnesiumoxyd-Beschichtung für Elektrobleche und Beschichtungsverfahren |
| JPH05247661A (ja) * | 1992-03-04 | 1993-09-24 | Nippon Steel Corp | 均一なグラス被膜を有し、磁気特性の優れた方向性電磁鋼板の製造方法 |
| EP0577124B1 (de) * | 1992-07-02 | 2002-10-16 | Nippon Steel Corporation | Kornorientiertes Elektroblech mit hoher Flussdichte und geringen Eisenverlusten und Herstellungsverfahren |
-
1994
- 1994-03-22 DE DE4409691A patent/DE4409691A1/de not_active Withdrawn
-
1995
- 1995-03-18 PL PL95316139A patent/PL178890B1/pl unknown
- 1995-03-18 AT AT95912252T patent/ATE170226T1/de not_active IP Right Cessation
- 1995-03-18 DE DE59503345T patent/DE59503345D1/de not_active Expired - Lifetime
- 1995-03-18 KR KR1019960705227A patent/KR100367985B1/ko not_active Expired - Lifetime
- 1995-03-18 EP EP95912252A patent/EP0752012B1/de not_active Expired - Lifetime
- 1995-03-18 RU RU96119243A patent/RU2139945C1/ru active
- 1995-03-18 WO PCT/EP1995/001020 patent/WO1995025820A1/de not_active Ceased
- 1995-03-18 CZ CZ19962738A patent/CZ292216B6/cs not_active IP Right Cessation
- 1995-03-18 JP JP52437895A patent/JP3730254B2/ja not_active Expired - Lifetime
- 1995-03-18 US US08/704,579 patent/US5863356A/en not_active Expired - Lifetime
Also Published As
| Publication number | Publication date |
|---|---|
| KR970701795A (ko) | 1997-04-12 |
| KR100367985B1 (ko) | 2003-08-02 |
| PL178890B1 (pl) | 2000-06-30 |
| EP0752012A1 (de) | 1997-01-08 |
| CZ292216B6 (cs) | 2003-08-13 |
| ATE170226T1 (de) | 1998-09-15 |
| US5863356A (en) | 1999-01-26 |
| DE4409691A1 (de) | 1995-09-28 |
| PL316139A1 (en) | 1996-12-23 |
| JP3730254B2 (ja) | 2005-12-21 |
| CZ273896A3 (en) | 1997-04-16 |
| JPH09510503A (ja) | 1997-10-21 |
| RU2139945C1 (ru) | 1999-10-20 |
| WO1995025820A1 (de) | 1995-09-28 |
| DE59503345D1 (de) | 1998-10-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0752012B1 (de) | Verfahren zur herstellung von elektroblechen mit einem glasüberzug | |
| DE69006946T2 (de) | Herstellung von kornorientierten siliziumlegierten Feinblechen mit einer darauf erzeugten Isolierschicht. | |
| EP0090241B1 (de) | Thermostabile Eisenoxidpigmente | |
| DE2637591B2 (de) | Verfahren zum Ausbilden einer wärmebeständigen, isolierenden Beschichtung au/ einem orientierten Silicium-Stahlblech | |
| DE60020316T2 (de) | Orientierter elektromagnetischer Stahl mit sehr guter Beschichtigungsfähigkeit und Verfahren zur Herstellung | |
| EP3921454B1 (de) | Wässrige zusammensetzung zur beschichtung von kornorientiertem stahl | |
| DE2450850A1 (de) | Verfahren und mittel zum ausbilden einer isolierenden beschichtung auf einem orientierten si-stahlblech | |
| EP2586755B1 (de) | Hartmagnetischer La und Co dotierte hexagonale Strontiumferritwerkstoff | |
| DE102008008781A1 (de) | Verfahren zur Herstellung eines kornorientierten Elektrobands | |
| DE60024395T2 (de) | Verwendung eines Ferritsinterkörpers | |
| DE69618878T2 (de) | Verfahren zum Herstellen kornorientierter Siliziumstahlbleche und entkohlte Siliziumstahlbleche | |
| DE3026696A1 (de) | Ferromagnetische, im wesentlichen aus eisen bestehende metallteilchen mit einem oberflaechenueberzug, verfahren zu deren herstellung sowie ihre verwendung zur herstellung von magnetischen aufzeichnungstraegern | |
| DE2917235A1 (de) | Verfahren zum ausbilden von festhaftenden und gleichfoermigen isolationsschichten auf kornorientiertem siliciumstahlblech | |
| EP0199975A2 (de) | Eisenoxidrotpigmente mit verbesserten koloristischen Eigenschaften, Verfahren zu deren Herstellung sowie ihre Verwendung | |
| DE102020134301A1 (de) | Weichmagnetische Legierung und Verfahren zum Herstellen einer weichmagnetischen Legierung | |
| DE3044771A1 (de) | Magnetisches aufzeichnungsmedium und herstellung desselben | |
| DE69800332T2 (de) | Hematitteilchen und magnetischer Aufzeichnungsträger welcher die Hematitteilchen als nicht-magnitische Teilchen in einer nicht-magnetischen Unterschicht verwendet | |
| DE2810155A1 (de) | Ueberzugsloesung fuer elektrostahlbleche | |
| DE3218821A1 (de) | Stabile aufschlaemmung von inaktivem magnesiumoxid und verfahren zu ihrer herstellung | |
| DE4293604C2 (de) | Weichmagnetisches Stahlmaterial und Verfahren zu seiner Herstellung | |
| DE2856324A1 (de) | Siliziumstahl und verfahren zu dessen verarbeitung | |
| DE3784473T2 (de) | Trennmittel und verfahren zum gluehen eines siliziumstahls. | |
| EP3720913A1 (de) | Chrom- und phosphatfreie beschichtung zur elektrischen isolierung von elektroband | |
| WO1999024639A1 (de) | Verfahren zum beschichten von elektrostahlbändern mit einem glühseparator | |
| DE69513811T2 (de) | Verfahren zum herstellen eines kornorientierten elektrostahlblechs mit einer spiegeloberflache und mit geringem kernverlust |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 19960821 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE CH DE DK ES FR GB GR IE IT LI LU MC NL PT SE |
|
| 17Q | First examination report despatched |
Effective date: 19970402 |
|
| GRAG | Despatch of communication of intention to grant |
Free format text: ORIGINAL CODE: EPIDOS AGRA |
|
| GRAG | Despatch of communication of intention to grant |
Free format text: ORIGINAL CODE: EPIDOS AGRA |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| GRAH | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOS IGRA |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE CH DE DK ES FR GB GR IE IT LI LU MC NL PT SE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 19980826 Ref country code: GR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19980826 Ref country code: ES Free format text: THE PATENT HAS BEEN ANNULLED BY A DECISION OF A NATIONAL AUTHORITY Effective date: 19980826 |
|
| REF | Corresponds to: |
Ref document number: 170226 Country of ref document: AT Date of ref document: 19980915 Kind code of ref document: T |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| GBT | Gb: translation of ep patent filed (gb section 77(6)(a)/1977) |
Effective date: 19980827 |
|
| REF | Corresponds to: |
Ref document number: 59503345 Country of ref document: DE Date of ref document: 19981001 |
|
| ET | Fr: translation filed | ||
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D Free format text: GERMAN |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 19981126 Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 19981126 Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 19981126 |
|
| NLV1 | Nl: lapsed or annulled due to failure to fulfill the requirements of art. 29p and 29m of the patents act | ||
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990318 Ref country code: AT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990318 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990331 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990331 Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990331 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990504 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FD4D |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed | ||
| BERE | Be: lapsed |
Owner name: EBG G.- FUR ELEKTROMAGNETISCHE WERKSTOFFE M.B.H. Effective date: 19990331 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 19990930 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: IF02 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: CD |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20140319 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: IT Payment date: 20140321 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20140318 Year of fee payment: 20 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: FR Payment date: 20140318 Year of fee payment: 20 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R071 Ref document number: 59503345 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: PE20 Expiry date: 20150317 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GB Free format text: LAPSE BECAUSE OF EXPIRATION OF PROTECTION Effective date: 20150317 |