WO2022014434A1 - 脂肪族-芳香族ポリエステル樹脂及びその成形品 - Google Patents
脂肪族-芳香族ポリエステル樹脂及びその成形品 Download PDFInfo
- Publication number
- WO2022014434A1 WO2022014434A1 PCT/JP2021/025593 JP2021025593W WO2022014434A1 WO 2022014434 A1 WO2022014434 A1 WO 2022014434A1 JP 2021025593 W JP2021025593 W JP 2021025593W WO 2022014434 A1 WO2022014434 A1 WO 2022014434A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- aliphatic
- dicarboxylic acid
- polyester resin
- aromatic polyester
- aromatic
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G63/00—Macromolecular compounds obtained by reactions forming a carboxylic ester link in the main chain of the macromolecule
- C08G63/02—Polyesters derived from hydroxycarboxylic acids or from polycarboxylic acids and polyhydroxy compounds
- C08G63/12—Polyesters derived from hydroxycarboxylic acids or from polycarboxylic acids and polyhydroxy compounds derived from polycarboxylic acids and polyhydroxy compounds
- C08G63/16—Dicarboxylic acids and dihydroxy compounds
- C08G63/20—Polyesters having been prepared in the presence of compounds having one reactive group or more than two reactive groups
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G63/00—Macromolecular compounds obtained by reactions forming a carboxylic ester link in the main chain of the macromolecule
- C08G63/02—Polyesters derived from hydroxycarboxylic acids or from polycarboxylic acids and polyhydroxy compounds
- C08G63/12—Polyesters derived from hydroxycarboxylic acids or from polycarboxylic acids and polyhydroxy compounds derived from polycarboxylic acids and polyhydroxy compounds
- C08G63/16—Dicarboxylic acids and dihydroxy compounds
- C08G63/18—Dicarboxylic acids and dihydroxy compounds the acids or hydroxy compounds containing carbocyclic rings
- C08G63/181—Acids containing aromatic rings
- C08G63/183—Terephthalic acids
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G63/00—Macromolecular compounds obtained by reactions forming a carboxylic ester link in the main chain of the macromolecule
- C08G63/02—Polyesters derived from hydroxycarboxylic acids or from polycarboxylic acids and polyhydroxy compounds
- C08G63/12—Polyesters derived from hydroxycarboxylic acids or from polycarboxylic acids and polyhydroxy compounds derived from polycarboxylic acids and polyhydroxy compounds
- C08G63/123—Polyesters derived from hydroxycarboxylic acids or from polycarboxylic acids and polyhydroxy compounds derived from polycarboxylic acids and polyhydroxy compounds the acids or hydroxy compounds containing carbocyclic rings
- C08G63/127—Acids containing aromatic rings
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G63/00—Macromolecular compounds obtained by reactions forming a carboxylic ester link in the main chain of the macromolecule
- C08G63/78—Preparation processes
- C08G63/82—Preparation processes characterised by the catalyst used
- C08G63/85—Germanium, tin, lead, arsenic, antimony, bismuth, titanium, zirconium, hafnium, vanadium, niobium, tantalum, or compounds thereof
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J5/00—Manufacture of articles or shaped materials containing macromolecular substances
- C08J5/18—Manufacture of films or sheets
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G2230/00—Compositions for preparing biodegradable polymers
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J2367/00—Characterised by the use of polyesters obtained by reactions forming a carboxylic ester link in the main chain; Derivatives of such polymers
- C08J2367/02—Polyesters derived from dicarboxylic acids and dihydroxy compounds
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J2367/00—Characterised by the use of polyesters obtained by reactions forming a carboxylic ester link in the main chain; Derivatives of such polymers
- C08J2367/02—Polyesters derived from dicarboxylic acids and dihydroxy compounds
- C08J2367/03—Polyesters derived from dicarboxylic acids and dihydroxy compounds the dicarboxylic acids and dihydroxy compounds having the hydroxy and the carboxyl groups directly linked to aromatic rings
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02W—CLIMATE CHANGE MITIGATION TECHNOLOGIES RELATED TO WASTEWATER TREATMENT OR WASTE MANAGEMENT
- Y02W90/00—Enabling technologies or technologies with a potential or indirect contribution to greenhouse gas [GHG] emissions mitigation
- Y02W90/10—Bio-packaging, e.g. packing containers made from renewable resources or bio-plastics
Definitions
- the present invention relates to an aliphatic-aromatic polyester resin having a high molecular weight, excellent moldability, and excellent mechanical properties such as tensile break point elongation of the obtained film, and a molded product and a film thereof.
- biodegradable polymer materials have been developed, and some of them have been commercialized as biodegradable packaging materials and biodegradable agricultural materials.
- biodegradable plastics are polylactic acid (hereinafter, may be abbreviated as "PLA”), polybutylene succinate (hereinafter, may be abbreviated as “PBS”), and polybutylene succinate.
- PBSA polybutylene adipate terephthalate
- PBAT polybutylene succinate terephthalate
- PBST polybutylene succinate terephthalate
- PBSeT Polybutylene succinate terephthalate
- PBSF polybutylene succinate furanoate
- PBAzT aliphatic-aromatic polyester resins
- PBAzT aliphatic-aromatic polyester resins
- the above biodegradable plastics do not have sufficient thermal stability during polymerization when trying to obtain a high molecular weight material as compared with aromatic polyester resins such as polyethylene terephthalate and polybutylene terephthalate, and the molecular weight is increased. In some cases it could not be raised.
- a high molecular weight material for example, an adipic acid or a derivative thereof or a mixture thereof and a mixture consisting of a terephthalic acid or an ester-forming derivative thereof or a mixture thereof and a sulfonate compound, and an alkanediol and carbon having 2 to 6 carbon atoms.
- polyesters obtained from dihydroxy compounds selected from the number 5 to 10 cycloalkanediols, compounds having at least three ester-forming groups, and polyesters further reacted with diisocyanate Patent Document 1). ..
- an aliphatic-aromatic polyester resin composed of an aliphatic dicarboxylic acid unit, an aromatic dicarboxylic acid unit, an aliphatic and / or alicyclic diol unit, and a constituent unit having a trifunctional or higher ester-forming group It is disclosed (Patent Document 2).
- the aliphatic-aromatic polyester disclosed in Patent Document 1 uses adipic acid or a derivative thereof as an essential component as the aliphatic dicarboxylic acid as a constituent unit, the thermal stability during polymerization is poor, and the obtained polymer is obtained. The thermal decomposition temperature of is low. Further, since it is difficult to obtain a polyester having a sufficiently high molecular weight in a one-step reaction, it is necessary to polymerize the polyester having a low molecular weight once and then increase the molecular weight by a chain length extension reaction using diisocyanate as a second step reaction.
- Patent Document 2 by allowing malic acid to be present in a predetermined ratio as a trifunctional or higher component serving as a branching agent in the polymerization reaction system, it is possible to increase the molecular weight in one step, and productivity, impact resistance, and flexibility are achieved. The property and tear resistance can be improved.
- the polymer containing the branched structure derived from malic acid undergoes an unintended increase due to the progress of the crosslinking reaction under high temperature conditions of the kneading and molding processes, as shown in Comparative Example 1 below. It has been found that stickiness occurs, which leads to a decrease in molding stability.
- the present invention has a high molecular weight, high thermal stability during heating, and as a result, excellent molding processing stability and excellent mechanical properties such as tensile break point elongation of the obtained film, an aliphatic-aromatic polyester.
- the purpose is to provide a resin.
- the present inventor has been introduced for the abundance ratio (molar ratio) of an aliphatic dicarboxylic acid unit and an aromatic dicarboxylic acid unit, a glass transition temperature, and a high molecular weight in a high molecular weight aliphatic-aromatic polyester resin.
- the abundance ratio (molar ratio) of the aliphatic dicarboxylic acid unit and the aromatic dicarboxylic acid unit is within a predetermined range, the glass transition temperature is equal to or higher than a predetermined value, and a specific branched structure exists.
- the gist of the present invention lies in the following [1] to [6].
- the abundance ratio (molar ratio) of the aromatic dicarboxylic acid unit is 15:85 to 85:15, the glass transition temperature is ⁇ 25 ° C. or higher, and it is represented by the following formulas (1) to (4).
- Ar 1 , Ar 2 , and Ar 3 each independently may have a substituent, and are divalent aromatic hydrocarbon ring groups having 4 to 12 carbon atoms. Alternatively, it represents an aromatic heterocyclic group.
- R represents a hydrogen atom or an alkyl group having 1 to 4 carbon atoms.
- N, m, and r are independently integers of 2 to 10).
- the total ratio of the branched structures represented by the above formulas (1) to (4) is 0.00001 mol% or more and less than 4.0 mol% with respect to the total 100 mol% of all the constituent units.
- the aliphatic dicarboxylic acid unit is a linear aliphatic dicarboxylic acid unit having 4 to 10 carbon atoms
- the aromatic dicarboxylic acid unit is an aromatic dicarboxylic acid unit having 6 to 8 carbon atoms.
- the aliphatic dicarboxylic acid unit is a succinic acid unit, the aromatic dicarboxylic acid unit is a terephthalic acid unit, and the aliphatic diol unit is a 1,4-butanediol unit, [1] to [ 3]
- the aliphatic-aromatic polyester resin according to any one of.
- [6] A film formed by molding the aliphatic-aromatic polyester resin according to any one of [1] to [4].
- the aliphatic-aromatic compound has a high molecular weight, has high thermal stability during heating, and therefore has excellent molding processing stability and excellent mechanical properties such as tensile break point elongation of the obtained film.
- a polyester resin and a molded product and a film made of the polyester resin are provided.
- FIG. 1 is a reaction system route diagram showing a process of forming a branched structure represented by the formulas (1) to (4) in the process of producing an aliphatic-aromatic polyester resin of the present invention.
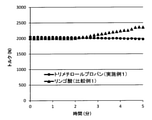
- FIG. 2 is a graph showing changes over time in torque at the time of melting of the aliphatic-aromatic polyester resin obtained in Example 1 and Comparative Example 1.
- the aliphatic-aromatic polyester resin of the present invention is an aliphatic-aromatic polyester resin whose main constituent units are an aliphatic dicarboxylic acid unit, an aromatic dicarboxylic acid unit, an aliphatic diol unit and / or an alicyclic diol unit.
- the abundance ratio (molar ratio) of the aliphatic dicarboxylic acid unit and the aromatic dicarboxylic acid unit is 15:85 to 85:15, the glass transition temperature is ⁇ 25 ° C. or higher, and the following formula ( It is characterized by having a branched structure shown in 1) to (4).
- Ar 1 , Ar 2 , and Ar 3 each independently may have a substituent, and are divalent aromatic hydrocarbon ring groups having 4 to 12 carbon atoms. Alternatively, it represents an aromatic heterocyclic group.
- R represents a hydrogen atom or an alkyl group having 1 to 4 carbon atoms.
- N, m, and r are independently integers of 2 to 10).
- the "aliphatic dicarboxylic acid unit” is derived from an aliphatic dicarboxylic acid and / or a derivative thereof (hereinafter, may be referred to as an "aliphatic dicarboxylic acid component”) and is an aliphatic-aromatic polyester. It is a repeating unit introduced into a resin.
- the "aromatic dicarboxylic acid unit” is derived from an aromatic dicarboxylic acid and / or a derivative thereof (hereinafter, may be referred to as an "aromatic dicarboxylic acid component") and is introduced into an aliphatic-aromatic polyester resin. It is a repeating unit.
- the aromatic dicarboxylic acid is an aromatic dicarboxylic acid in a broad sense including a heteroaromatic dicarboxylic acid.
- the "aliphatic diol unit” is a repeating unit derived from an aliphatic diol and introduced into an aliphatic-aromatic polyester resin.
- the "alicyclic diol unit” is a repeating unit derived from an alicyclic diol and introduced into an aliphatic-aromatic polyester resin.
- the main structural unit is a unit that occupies 55 mol% or more of the total structural unit 100 mol% constituting the aliphatic-aromatic polyester resin, preferably 75 mol% or more, and more preferably 85 to 100 mol%. It is a unit that occupies.
- branched structure (1)-(4) ratio In the aliphatic-aromatic polyester resin of the present invention, the branched structure represented by the above formulas (1) to (4) is obtained with respect to a total of 100 mol% of all the constituent units constituting the aliphatic-aromatic polyester resin.
- the ratio of the total of (hereinafter, this ratio may be simply referred to as “branched structure (1) to (4) ratio”) is not particularly limited, but is 0.00001 mol% or more and less than 4 mol%. Is preferable.
- the ratio of the branched structures (1) to (4) is less than 4.0 mol%, the crosslinking of the polymer does not proceed excessively during the production of the polymer, and the strands can be stably extracted, and the gel can be obtained during molding. It does not cause problems such as deterioration of moldability and deterioration of various physical properties.
- the ratio of the branched structures (1) to (4) is 0.00001 mol% or more, the reactivity of the polymerization reaction does not decrease, the bubbles during inflation molding are stable, and molding can be easily performed.
- the ratio of the branched structure (1) to (4) of the aliphatic-aromatic polyester resin of the present invention is more preferably 0.001 mol% or more and 2.0 mol% or less, still more preferably 0.005 mol% or more. It is 0 mol% or less, particularly preferably 0.01 mol% or more and 0.5 mol% or less, and most preferably 0.015 mol% or more and 0.4 mol% or less.
- branched structure (1) ratio The ratio of the branched structure represented by the above formula (1) to the total of 100 mol% of all the constituent units constituting the aliphatic-aromatic polyester resin of the present invention (hereinafter, may be referred to as “branched structure (1) ratio”). ) Is not particularly limited, but is preferably less than 1.0 mol%, more preferably 0.1 mol% or less, still more preferably 0.05 mol% or less, and particularly preferably 0.04 mol% or less. be.
- branched structure (2) ratio The ratio of the branched structure represented by the above formula (2) to the total of 100 mol% of all the constituent units constituting the aliphatic-aromatic polyester resin of the present invention (hereinafter, may be referred to as “branched structure (2) ratio”). ) Is not particularly limited, but is preferably less than 1.0 mol%, more preferably 0.5 mol% or less, still more preferably 0.1 mol% or less, and particularly preferably 0.08 mol% or less. be.
- branched structure (3) ratio The ratio of the branched structure represented by the above formula (3) to the total of 100 mol% of all the constituent units constituting the aliphatic-aromatic polyester resin of the present invention (hereinafter, may be referred to as “branched structure (3) ratio”). ) Is not particularly limited, but is preferably less than 1.0 mol%, more preferably 0.5 mol% or less, still more preferably 0.1 mol% or less, and particularly preferably 0.07 mol% or less. be.
- branched structure (4) ratio The ratio of the branched structure represented by the above formula (4) to the total of 100 mol% of all the constituent units constituting the aliphatic-aromatic polyester resin of the present invention (hereinafter, may be referred to as “branched structure (4) ratio”). ) Is not particularly limited, but is preferably less than 1.0 mol%, more preferably 0.1 mol% or less, still more preferably 0.05 mol% or less, and particularly preferably 0.04 mol% or less. be.
- branch structure (1) ratio, branch structure (2) ratio, branch structure (3) ratio, branch structure (4) ratio, and branch structure (1) to (4) ratio are described in the section of Examples described later. 1 It can be obtained by measuring the mole fraction by H-NMR.
- Ar 1 , Ar 2 , and Ar 3 each independently may have a substituent and is a divalent aromatic hydrocarbon ring group having 4 to 12 carbon atoms. Or it is an aromatic heterocyclic group.
- Ar 1 , Ar 2 , and Ar 3 are divalent aromatic hydrocarbon ring groups or aromatic heterocyclic groups having 4 to 6 carbon atoms, which may independently have a substituent.
- the divalent aromatic hydrocarbon ring group preferably includes a p-phenylene group or an m-phenylene group. Examples of the divalent aromatic heterocyclic group include a 2,5-furandyl group.
- terephthalic acid and / or a derivative thereof isophthalic acid and / or as raw material dicarboxylic acid and / or a derivative thereof (hereinafter, may be referred to as "dicarboxylic acid component") of an aliphatic-aromatic polyester resin, respectively.
- dicarboxylic acid component dicarboxylic acid component
- the derivative the flange carboxylic acid and / or the derivative thereof, it can be introduced into an aliphatic-aromatic polyester resin.
- Ar 1 , Ar 2 , and Ar 3 may have a substituent.
- substituents include a sulfonic acid group, a sulfonic acid base, an alkyl group, an alkoxy group, a halogen atom, a nitro group, an aromatic group and the like.
- R is a hydrogen atom or an alkyl group having 1 to 4 carbon atoms.
- alkyl group include a methyl group, an ethyl group, a propyl group, an isopropyl group and an n-butyl group.
- N, m, and r are independently integers of 2 to 10, preferably integers of 2 to 8.
- the branched structure represented by the formulas (1) to (4) is expressed by an ester forming reaction and / or a transesterification reaction as a raw material dicarboxylic acid component and a raw material diol component of the aliphatic-aromatic polyester resin of the present invention described later.
- the compound of the present invention is preferably used in a predetermined ratio so as to have the ratio of the above-mentioned branched structures (1) to (4), using the compounds into which the branched structures shown in (1) to (4) can be introduced. It can be introduced into aliphatic-aromatic polyester resins.
- the aliphatic dicarboxylic acid component constituting the aliphatic dicarboxylic acid unit which is the dicarboxylic acid unit constituting the aliphatic-aromatic polyester resin of the present invention is not particularly limited. From the viewpoint of cost, mechanical properties, thermophysical properties and biodegradability, the aliphatic dicarboxylic acid component preferably has 4 to 12 carbon atoms, particularly preferably an aliphatic dicarboxylic acid component having 4 to 10 carbon atoms, and has 4 to 10 carbon atoms. A linear aliphatic dicarboxylic acid component of 10 is particularly preferred.
- derivatives such as succinic acid, glutaric acid, adipic acid, suberic acid, sebacic acid, azelaic acid, dodecanedioic acid and the like and alkyl esters thereof.
- derivatives such as succinic acid, sebacic acid, adipic acid, azelaic acid and alkyl esters thereof are preferable, and succinic acid or its derivatives are particularly preferable.
- the derivative may be these acid anhydrides.
- One of these aliphatic dicarboxylic acid components may be used alone, or two or more thereof may be mixed and used.
- the aromatic dicarboxylic acid component constituting the aromatic dicarboxylic acid unit which is the dicarboxylic acid unit constituting the aliphatic-aromatic polyester resin of the present invention, is not particularly limited. From the balance of cost, mechanical properties, thermophysical properties and biodegradability, the aromatic dicarboxylic acid component has 4 to 14 carbon atoms, particularly 4 to 12 carbon atoms, particularly 4 to 8 carbon atoms, and particularly 4 to 6 carbon atoms. The aromatic dicarboxylic acid component of is preferred.
- terephthalic acid examples thereof include terephthalic acid, isophthalic acid, frangylcarboxylic acid, naphthalenedicarboxylic acid, diphenyldicarboxylic acid and lower alkyl esters thereof.
- These may be acid anhydrides.
- terephthalic acid, isophthalic acid, frangylcarboxylic acid, or their lower alkyl (for example, alkyl having 1 to 4 carbon atoms) ester is preferable, and terephthalic acid or its lower alkyl (for example, alkyl having 1 to 4 carbon atoms) thereof is particularly preferable.
- Esters are preferred.
- One of these aromatic dicarboxylic acid components may be used alone, or two or more thereof may be mixed and used.
- the ratio (absence ratio (molar ratio)) of the aliphatic dicarboxylic acid unit and the aromatic dicarboxylic acid unit constituting the dicarboxylic acid unit of the aliphatic-aromatic polyester resin of the present invention is 15:85 to 85:15. , Preferably 30:70 to 70:30, and more preferably 40:60 to 60:40.
- the proportion of the aliphatic dicarboxylic acid unit is less than the above lower limit and the proportion of the aromatic dicarboxylic acid unit is more than the above upper limit, the biodegradability of the aliphatic-aromatic polyester resin is impaired and the flexibility tends to be insufficient. There is.
- the proportion of the aliphatic dicarboxylic acid unit is larger than the above upper limit and the proportion of the aromatic dicarboxylic acid unit is smaller than the above lower limit, the thermal decomposition temperature is lowered and the elongation at the breaking point when formed into a film is small. It tends to be inflexible.
- the aliphatic-aromatic polyester resin of the present invention has the above-mentioned specific ratio in terms of biodegradability, heat resistance, flexibility, etc., when the abundance ratio (molar ratio) of the aliphatic dicarboxylic acid unit and the aromatic dicarboxylic acid unit is the above-mentioned specific ratio. , Shows excellent properties.
- This abundance ratio (molar ratio) can be controlled by the amount (ratio) of the aliphatic dicarboxylic acid component and the aromatic dicarboxylic acid component of the raw materials used in the method for producing an aliphatic-aromatic polyester resin described later.
- This abundance ratio (molar ratio) can be obtained by measuring the molar fraction by 1 H-NMR described in the section of Examples described later.
- Examples of the diol component constituting the aliphatic and / or alicyclic diol unit constituting the diol unit of the aliphatic-aromatic polyester resin of the present invention include ethylene glycol, diethylene glycol, triethylene glycol and 1,3-propane.
- Aliphatic diols having 2 to 10 carbon atoms such as diols, 1,2-propanediols, 1,4-butanediols, 1,5-pentanediols and 1,6-hexanediols, 1,4-cyclohexanediols, 1, Examples thereof include alicyclic diols having 3 to 12 carbon atoms such as 4-cyclohexanedimethanol.
- linear diols having 2 to 4 carbon atoms such as 1,4-butanediol, ethylene glycol, and 1,3-propanediol are preferable from the viewpoint of the physical properties of the obtained aliphatic-aromatic polyester resin.
- 1,4-butanediol and ethylene glycol are preferable, and 1,4-butanediol is particularly preferable.
- One of these may be used alone, or two or more thereof may be mixed and used.
- Trifunctional polyhydric alcohol In forming the branched structure represented by the above formulas (1) to (4) in the aliphatic-aromatic polyester resin of the present invention, in addition to the above components when producing the aliphatic-aromatic polyester resin, It is preferable to use a trifunctional polyhydric alcohol.
- the aliphatic-aromatic polyester resin of the present invention containing the branched structure represented by the formulas (1) to (4) has at least the above-mentioned aliphatic dicarboxylic acid component, aromatic dicarboxylic acid component, and aliphatic and / as raw materials. Alternatively, it can be produced by using an alicyclic diol and the above-mentioned trifunctional polyvalent alcohol through an esterification and / or transesterification reaction step and a subsequent polycondensation reaction step as described later.
- aliphatic dicarboxylic acid components aromatic dicarboxylic acid components, aliphatic and / or alicyclic diols, and trifunctional polyhydric alcohols used in the production of the aliphatic-aromatic polyester resin of the present invention are derived from petroleum raw materials or Either derived from a biomass resource may be used. It is preferable to use a component derived from a biomass resource as these components because it leads to suppression of the generation of carbon dioxide derived from a petroleum raw material generated during biodegradation or combustion.
- an esterification and / or transesterification reaction step and a subsequent polycondensation reaction are carried out in the presence of a catalyst using an aliphatic and / or alicyclic diol, an aliphatic dicarboxylic acid component and an aromatic dicarboxylic acid component.
- a catalyst using an aliphatic and / or alicyclic diol, an aliphatic dicarboxylic acid component and an aromatic dicarboxylic acid component.
- the "trifunctional polyhydric alcohol” is “trifunctional alcohol”
- the "aliphatic dicarboxylic acid component” is “aliphatic carboxylic acid”
- the "aromatic dicarboxylic acid component” is “aromatic dicarboxylic acid”.
- Each reaction pathway shown in FIG. 1 is an equilibrium reaction, and the reaction includes a difference in reactivity of an aromatic dicarboxylic acid component and an aliphatic dicarboxylic acid component with a trifunctional polyvalent alcohol (thermodynamic control) and an equilibrium reaction. Both the differences in product stability (thermodynamic control) in.
- thermodynamic control thermodynamic control
- the present inventor has found that the reaction product can be controlled by thermodynamic control by controlling the compounding ratio of each component, the reaction temperature and the reaction time.
- the supply amount of the trifunctional polyvalent alcohol is specified, and in the initial esterification and / or transesterification reaction step, the system is used.
- the abundance ratio (molar ratio) of the aliphatic dicarboxylic acid unit and the aromatic dicarboxylic acid unit By heating the temperature to 170 ° C. to 200 ° C. and reacting for 45 minutes to 1 hour while stirring the above formula (1) to the above formula (1), the abundance ratio (molar ratio) of the aliphatic dicarboxylic acid unit and the aromatic dicarboxylic acid unit.
- the content and ratio of the branched structure shown in (4) can be controlled.
- the intermediate dialcohols and monoalcohols completely react, and the reaction converges so as to have a branched structure represented by the above equations (1) to (4) without excess or deficiency.
- the ratio can be easily controlled to a desired value.
- the ratio of each of the branch structures of the above formulas (1) to (4) can be controlled by variously controlling the temperature, reaction time, etc. in the raw material preparation, the reaction process, and the like.
- the aliphatic-aromatic polyester resin of the present invention may have a repeating unit (aliphatic oxycarboxylic acid unit) derived from the aliphatic oxycarboxylic acid.
- aliphatic oxycarboxylic acid component that gives the aliphatic oxycarboxylic acid unit include lactic acid, glycolic acid, 2-hydroxy-n-butyric acid, 2-hydroxycaproic acid, 6-hydroxycaproic acid, and 2-hydroxy. Examples thereof include -3,3-dimethylbutyric acid, 2-hydroxy-3-methylbutyric acid, 2-hydroxyisocaproic acid and the like, or derivatives such as lower alkyl esters or intramolecular esters thereof.
- any of D-form, L-form or racemic form may be used, and the form may be any of solid, liquid or aqueous solution.
- particularly preferred are lactic acid or glycolic acid or derivatives thereof.
- One of these aliphatic oxycarboxylic acids may be used alone, or two or more thereof may be mixed and used.
- the aliphatic-aromatic polyester resin of the present invention contains these aliphatic oxycarboxylic acid units, the content thereof is 100 for all the constituent units constituting the aliphatic-aromatic polyester resin from the viewpoint of moldability.
- the mol% is preferably 20 mol% or less, more preferably 10 mol% or less, still more preferably 5 mol% or less, and most preferably 0 mol% (not included).
- a chain extender such as diisocyanate, diphenyl carbonate, dioxazoline or silicate ester may be used.
- diisocyanate include 2,4-tolylene diisocyanate, a mixture of 2,4-tolylene diisocyanate and 2,6-tolylene diisocyanate, diphenylmethane diisocyanate, 1,5-naphthylene diisocyanate, and xylylene diisocyanate.
- diisocyanates such as isocyanates, hydride xylylene diisocyanates, hexamethylene diisocyanates, and isophorone diisocyanates are exemplified.
- Specific examples of the silicic acid ester include tetramethoxysilane, dimethoxydiphenylsilane, dimethoxydimethylsilane, and diphenyldihydroxylane. These may be used alone or in admixture of two or more.
- the polyester terminal group may be sealed with a carbodiimide, an epoxy compound, a monofunctional alcohol or a carboxylic acid. Encapsulation of the polyester end group can be expected to improve the hydrolysis resistance of the aliphatic-aromatic polyester resin.
- the diol component and the dicarboxylic acid component react in substantially equal molar amounts, but since the diol component is distilled off during the esterification or transesterification reaction, it is usually 1 to 20 mol more than the dicarboxylic acid component. % Overused.
- the method for producing an aliphatic-aromatic polyester resin of the present invention will be described below by taking a continuous production method as an example.
- a method for producing an aliphatic-aromatic polyester resin by an esterification reaction step using an aliphatic diol and a trifunctional polyvalent alcohol, an aliphatic dicarboxylic acid and an aromatic dicarboxylic acid, and a subsequent polycondensation reaction step will be described.
- the esterification reaction step may be a transesterification reaction step or a step in which both the esterification reaction and the transesterification reaction are performed.
- the aliphatic diol may be an alicyclic diol.
- an esterification reaction step and a melt polycondensation reaction step of an aliphatic dicarboxylic acid and an aromatic dicarboxylic acid and an aliphatic diol and a trifunctional polyhydric alcohol are carried out using a plurality of continuous reaction tanks. After that, polyester pellets are continuously obtained.
- the method for producing an aliphatic-aromatic polyester resin of the present invention is not limited to the continuous method, and a conventionally known method for producing a polyester can be adopted.
- the esterification reaction step of reacting at least the dicarboxylic acid component with the diol component and the trifunctional polyhydric alcohol and the subsequent polycondensation reaction step may be carried out in a plurality of continuous reaction tanks or in a single reaction tank. can. In order to reduce the fluctuation of the physical properties of the obtained polyester, it is preferable to carry out in a plurality of continuous reaction tanks.
- the reaction temperature in the esterification reaction step is not particularly limited as long as it is a temperature at which the esterification reaction can be carried out.
- the reaction temperature is preferably 200 ° C. or higher, more preferably 210 ° C. or higher in that the reaction rate can be increased.
- the reaction temperature is preferably 270 ° C. or lower, more preferably 260 ° C. or lower, and particularly preferably 250 ° C. or lower. If the reaction temperature is too low, the esterification reaction rate is slow, the reaction time is required for a long time, and unfavorable reactions such as dehydration decomposition of aliphatic diols increase.
- the esterification reaction temperature is preferably a constant temperature.
- the esterification rate is stable when the temperature is constant.
- the constant temperature is a set temperature of ⁇ 5 ° C, preferably ⁇ 2 ° C.
- the temperature is raised to 170 ° C. to 200 ° C. and the reaction is carried out for 45 minutes to 1 hour in order to control the ratio of the branched structure.
- the reaction atmosphere is preferably an inert gas atmosphere such as nitrogen or argon.
- the reaction pressure is preferably 50 KPa to 200 KPa, more preferably 60 KPa or more, further preferably 70 KPa or more, still more preferably 130 KPa or less, still more preferably 110 KPa or less. If the reaction pressure is less than the above lower limit, scattered substances increase in the reaction vessel, the haze of the reactants increases, which tends to cause an increase in foreign substances, and the amount of the aliphatic diol distilled out of the reaction system increases, resulting in a polycondensation reaction rate. It is easy to cause a decline. When the reaction pressure exceeds the above upper limit, the dehydration decomposition of the aliphatic diol increases, which tends to cause a decrease in the polycondensation reaction rate.
- the reaction time is preferably 1 hour or more, and the upper limit is preferably 10 hours or less, more preferably 4 hours or less.
- the reaction molar ratio of the aliphatic diol to the total of the aliphatic dicarboxylic acid and the aromatic dicarboxylic acid undergoing the esterification reaction is the aliphatic dicarboxylic acid and the aromatic dicarboxylic acid present in the gas phase and the reaction liquid phase of the esterification reaction tank.
- the aliphatic dicarboxylic acid which represents the molar ratio of the aliphatic diol and the esterified aliphatic diol to the esterified aliphatic dicarboxylic acid and the aromatic dicarboxylic acid, and which is decomposed in the reaction system and does not contribute to the esterification reaction.
- the lower limit of the reaction molar ratio is usually 1.10 or more, preferably 1.12 or more, more preferably 1.15 or more, and particularly preferably 1.20 or more.
- the upper limit is usually 3.00 or less, preferably 2.50 or less, more preferably 2.30 or less, and particularly preferably 2.00 or less. If the reaction molar ratio is less than the above lower limit, the esterification reaction tends to be insufficient, the polycondensation reaction, which is a reaction in the subsequent step, does not proceed easily, and it is difficult to obtain a polyester having a high degree of polymerization.
- reaction molar ratio exceeds the above upper limit, the amount of decomposition of the aliphatic diol, the aliphatic dicarboxylic acid, and the aromatic dicarboxylic acid tends to increase.
- the amount of the trifunctional polyvalent alcohol used is the dicarboxylic acid component of the raw material in order to form the branched structure represented by the above formulas (1) to (4) at the above-mentioned preferable branched structure (1) to (4) ratio.
- the total of 100 mol% preferably 0.00002 mol% or more and less than 8.0 mol%, more preferably 0.002 mol% or more and 4.0 mol% or less, still more preferably 0.01 mol% or more 2 It is 0.0 mol% or less, particularly preferably 0.02 mol% or more and 1.0 mol% or less, and particularly preferably 0.03 mol% or more and 0.8 mol% or less.
- the terminal acid value of the ester oligomer obtained in the esterification reaction step and supplied to the next polycondensation reaction is 30 to 1000 eq. It is preferably / ton.
- the terminal acid value of the ester oligomer was changed to 30 eq. In order to make it lower than / ton, it is necessary to prolong the esterification reaction or increase the reaction molar ratio, which leads to an increase in the amount of by-product of decomposition products such as tetrahydrofuran. In addition, coloring due to deterioration of the terminal balance becomes remarkable. On the contrary, the terminal acid value of the ester oligomer was changed to 1000 eq.
- the terminal acid value supplied to the polycondensation reaction is 30 eq. / Ton ⁇ 1000eq. If it is / ton, the effect of the present invention can be sufficiently obtained.
- the terminal acid value is 30 to 1000 eq in the esterification reaction step.
- An ester oligomer of / ton was obtained, and the terminal acid value was 30 to 1000 eq.
- the / ton ester oligomer may be contacted with the phosphorus compound prior to feeding into the polycondensation reaction step. After that, by supplying the resin to the polycondensation reaction step, it is possible to obtain an aliphatic-aromatic polyester resin having a good color tone by suppressing the by-product of decomposition products such as tetrahydrofuran and suppressing the purification load of the plant.
- the terminal acid value of the ester oligomer is 50 to 800 eq. It is more preferably / ton, and 100 to 500 eq. It is more preferably / ton.
- reaction conditions such as the reaction molar ratio of the diol component to the dicarboxylic acid component, the reaction temperature, and the reaction pressure may be controlled. That is, when the reaction molar ratio of the diol component to the dicarboxylic acid component is increased, the terminal acid value of the obtained ester oligomer tends to be low, and when it is decreased, the terminal acid value of the obtained ester oligomer tends to be high.
- the terminal acid value of the obtained ester oligomer tends to be low, and conversely, when the reaction temperature is low and the reaction time is short, the terminal acid value of the obtained ester oligomer tends to be low. It tends to be higher. Therefore, by appropriately adjusting these conditions within the above-mentioned preferable range, the terminal acid value is 30 to 1000 eq.
- An ester oligomer of / ton can be obtained. Further, the terminal acid value of the ester oligomer can be controlled by appropriately setting the type and amount of the catalyst used in the esterification reaction step described later. The terminal acid value of the ester oligomer is measured by the method described in the Examples section below.
- the polycondensation reaction is carried out in the polycondensation reaction step.
- the ester oligomer obtained in the esterification reaction step may be brought into contact with the phosphorus compound before being supplied to the polycondensation reaction step.
- the phosphorus compound has a terminal acid value of 30 to 1000 eq obtained in the esterification reaction step. It is important that no phosphorus compound is present in the esterification reaction step, as it is in contact with the / ton ester oligomer.
- the phosphorus compound is preferably contacted with the ester oligomer together with the alkaline earth metal compound.
- the polycondensation reaction can be carried out under reduced pressure using a plurality of continuous reaction tanks. Therefore, contacting the phosphorus compound with the ester oligomer before the polycondensation reaction step corresponds to contacting the phosphorus compound with the ester oligomer before the reduced pressure condition.
- the reaction pressure of the final polycondensation reaction tank in the polycondensation reaction step is usually 0.01 KPa or more, preferably 0.03 KPa or more, and the upper limit is usually 1.4 KPa or less, preferably 0.4 kPa or less. If the pressure during the polycondensation reaction is too high, the polycondensation time becomes long, which causes a decrease in molecular weight and coloring due to thermal decomposition of the polyester, which tends to make it difficult to produce a polyester exhibiting practically sufficient characteristics.
- the method of manufacturing using an ultra-high vacuum polycondensation facility having a reaction pressure of less than 0.01 KPa is a preferable embodiment from the viewpoint of improving the polycondensation reaction rate, but it requires an extremely high capital investment. , It is economically disadvantageous.
- the lower limit of the reaction temperature is usually 215 ° C, preferably 220 ° C, and the upper limit is usually in the range of 270 ° C, preferably 260 ° C. If the reaction temperature is less than the above lower limit, the polycondensation reaction rate is slow, it takes a long time to produce a polyester having a high degree of polymerization, and a high-power stirrer is also required, which is economically disadvantageous. If the reaction temperature exceeds the above upper limit, thermal decomposition of the polyester during production tends to occur, and it tends to be difficult to produce a polyester having a high degree of polymerization.
- the lower limit of the reaction time is usually 1 hour, and the upper limit is usually 15 hours, preferably 10 hours, more preferably 8 hours. If the reaction time is too short, the reaction is insufficient and it is difficult to obtain polyester having a high degree of polymerization, and the mechanical properties of the molded product tend to be inferior. If the reaction time is too long, the molecular weight of the polyester will be significantly reduced due to thermal decomposition, and the mechanical properties of the molded product will tend to be inferior. In addition, the amount of carboxyl group ends that adversely affect the durability of the polyester will increase due to thermal decomposition. In some cases.
- Polyester with desired intrinsic viscosity can be obtained by controlling the polycondensation reaction temperature, time and reaction pressure.
- the aliphatic-aromatic polyester resin of the present invention is usually produced in the presence of a catalyst.
- a catalyst that can be used for producing a known polyester-based resin can be arbitrarily selected as long as the effect of the present invention is not significantly impaired.
- the polycondensation reaction catalyst may be added at any stage between the esterification reaction step and the polycondensation reaction step. Further, the polycondensation reaction catalyst may be added in a plurality of times between the esterification reaction step and the polycondensation reaction step.
- a compound containing at least one of the metal elements of Groups 1 to 14 of the periodic table is generally used.
- the metal elements include scandium, yttrium, samarium, titanium, zirconium, vanadium, chromium, molybdenum, tungsten, tin, antimony, cerium, germanium, zinc, cobalt, manganese, iron, aluminum, magnesium, and calcium. Examples include samarium, sodium and potassium.
- the metal elements of Groups 3 to 6 of the periodic table showing Lewis acidity are preferable. Specifically, it is scandium, titanium, zirconium, vanadium, molybdenum, and tungsten, and titanium and zirconium are particularly preferable from the viewpoint of easy availability, and titanium is preferable from the viewpoint of reaction activity.
- the periodic table refers to a long-periodic table (Nomenclature of Inorganic Chemistry IUPAC Recommendations 2005).
- a titanium compound is preferably used as a catalyst in the esterification reaction step.
- titanium compound tetraalkyl titanate and its hydrolyzate are preferable, and specifically, tetra-n-propyl titanate, tetraisopropyl titanate, tetra-n-butyl titanate, tetra-t-butyl titanate, tetraphenyl titanate, and the like.
- examples thereof include tetracyclohexyl titanate, tetrabenzyl titanate and mixed titanates thereof, and hydrolysates thereof.
- tetra-n-propyl titanate tetraisopropyl titanate
- tetra-n-butyl titanate titanium (oxy) acetylacetonate
- titaniumtetraacetylacetonate titanium bis (ammonium lactate) dihydroxydo
- polyhydroxytitanium steer titanium (oxy) acetylacetonate, titaniumtetraacetylacetonate, titanium bis (ammonium lactate) dihydroxydo, polyhydroxytitanium steer.
- titanium lactate, butyl titanate dimer are preferred, tetra-n-butyl titanate, titanium (oxy) acetylacetonate, titanium tetraacetylacetonate, polyhydroxytitanium stearate, titanium lactate, butyl titanate dimer are more preferred, especially Tetra-n-butyl titanate, polyhydroxytitanium stearate, titanium (oxy) acetylacetonate, and titanium tetraacetylacetonate are preferable.
- titanium compounds include alcohols such as methanol, ethanol, isopropanol and butanol, diols such as ethylene glycol, butanediol and pentanediol, ethers such as diethyl ether and tetrahydrofuran, nitriles such as acetonitrile, heptane, toluene and the like. It is supplied to the esterification reaction step as a catalytic solution prepared so that the titanium compound concentration is usually 0.05 to 5% by weight by using a solvent for dissolving the catalyst such as the hydrocarbon compound, water and a mixture thereof. Ru.
- alcohols such as methanol, ethanol, isopropanol and butanol
- diols such as ethylene glycol, butanediol and pentanediol
- ethers such as diethyl ether and tetrahydrofuran
- nitriles such as acetonitrile
- the terminal acid value obtained in the esterification reaction step is 30 to 1000 eq.
- Examples of the phosphorus compound to be contacted with the / ton ester oligomer include phosphorous acid, polyphosphorus, and trimethyl phosphate, triethyl phosphate, tri-n-butyl phosphate, trioctyl phosphate, triphenyl phosphate, and tricresyl phosphate.
- tris (triethylene glycol) phosphate ethyldiethylphosphonoacetate, methylacid phosphate, ethylacid phosphate, isopropylacid phosphate, butylacid phosphate, monobutyl phosphate, dibutyl phosphate, dioctyl phosphate, triethylene glycol acid phosphate, etc.
- trivalent phosphorus compounds such as phosphorus compounds, phosphorous acid, hypophosphite, diethylphosphite, trisdodecylphosphite, trisnonyldecylphosphite, and triphenylphosphite.
- an acidic phosphoric acid ester compound is preferable, and the acidic phosphoric acid ester compound has an ester structure of a phosphoric acid having at least one hydroxyl group represented by the following general formulas (I) and / or (II). Is preferably used.
- Ra , R b , and R c represent an alkyl group, a cyclohexyl group, an aryl group, or a 2-hydroxyethyl group having 1 to 6 carbon atoms, respectively.
- Ra and R b are the same. It may be different.
- an acidic phosphoric acid ester compound examples include methyl acid phosphate, ethyl acid phosphate, isopropyl acid phosphate, butyl acid phosphate, octyl acid phosphate and the like, with ethyl acid phosphate and butyl acid phosphate being preferred.
- One of these acidic phosphoric acid ester compounds may be used alone, or two or more thereof may be used in combination.
- Acidic phosphate ester compounds include monoesters (II) and diesters (I).
- a monoester or a mixture of monoesters and diesters can be used. It is preferable to use it.
- the mixed weight ratio of the monoester to the diester is preferably 80 or less: 20 or more, more preferably 70 or less: 30 or more, and particularly preferably 60 or less: 40 or more. 20 or more: 80 or less is preferable, more preferably 30 or more: 70 or less, and particularly preferably 40 or more: 60 or less.
- these phosphorus compounds may be used arbitrarily.
- the alkaline earth metal compound includes various compounds such as beryllium, magnesium, calcium, strontium, and barium. From the viewpoint of ease of handling and availability, and catalytic effect, magnesium and calcium compounds are preferable, and among them, magnesium compounds having excellent catalytic effect are preferable. Specific examples of the magnesium compound include magnesium acetate, magnesium hydroxide, magnesium carbonate, magnesium oxide, magnesium alcoholide, magnesium hydrogen phosphate and the like, and magnesium acetate is preferable among these.
- the alkaline earth metal compound may be added to the ester oligomer supplied to the polycondensation reaction step as a catalytic solution prepared to have a concentration of 0.02 to 9.7% by weight.
- the amount and ratio of the titanium compound used in the esterification reaction step and the phosphorus compound and alkaline earth metal compound used in the polycondensation reaction step are not particularly limited.
- the titanium compound is preferably used so that the amount added in terms of Ti to the produced polymer is 5 to 100 ppm by weight.
- the phosphorus compound is preferably used so that the P-converted molar ratio (P / Ti molar ratio) to the Ti-converted molar amount of the titanium compound is 0.5 to 2.5.
- the alkaline earth metal compound is preferably used so that the alkaline earth metal conversion addition molar ratio (alkaline earth metal / Ti molar ratio) to the Ti conversion addition molar amount of the titanium compound is 0.5 to 3.0. ..
- Too much of any catalytic compound is economically disadvantageous. Further, if the amount of the catalyst compound is too large, the reason is not yet clear, but the terminal acid value in the finally obtained polyester may be high. Therefore, the thermal stability of the polyester is increased by increasing the terminal acid value and the residual catalyst concentration. The property and hydrolysis resistance may decrease. If the amount of the catalyst compound is too small, the reaction activity is lowered, and as a result, thermal decomposition of the polyester is induced during the production of the polyester, and it becomes difficult to obtain a polyester having practically useful physical properties.
- the esterification reaction tank used in the present invention known ones can be used.
- the esterification reaction tank may be of any type such as a vertical stirring complete mixing tank, a vertical heat convection mixing tank, a tower type continuous reaction tank, and the same type or different types of tanks may be used as a single tank. It may be a plurality of tanks in series. Of these, a reaction vessel having a stirring device is preferable.
- a high-speed rotating type such as a turbine stator type high-speed rotary agitator, a disc mill type agitator, and a rotor mill type agitator shall also be used. Can be done.
- stirring there is no limitation on the form of stirring, and in addition to the usual stirring method in which the reaction solution in the reaction tank is directly stirred from the upper part, the lower part, the lateral part, etc. of the reaction tank, a part of the reaction liquid is piped to the outside of the reaction tank, etc. It is also possible to take it out and stir it with a line mixer or the like to circulate the reaction solution.
- stirring blades can be selected, and specific examples thereof include propeller blades, screw blades, turbine blades, fan turbine blades, disc turbine blades, Faudler blades, full zone blades, and max blend blades.
- the model of the polycondensation reaction tank used in the present invention is not particularly limited.
- a vertical stirring polymerization tank, a horizontal stirring polymerization tank, a thin film evaporation type polymerization tank and the like can be mentioned.
- the polycondensation reaction tank may be one unit, or may be a plurality of tanks in which a plurality of tanks of the same type or different types are connected in series. In the latter stage of polycondensation in which the viscosity of the reaction solution increases, it is preferable to select a horizontal stirring polymer having a thin film evaporation function having excellent interfacial renewal property, plug flow property and self-cleaning property as the polycondensation reaction tank.
- the molecular weight of the aliphatic-aromatic polyester resin of the present invention is usually 10,000 or more and the weight average molecular weight (Mw) of monodisperse polystyrene as a standard material measured by gel permeation chromatography (GPC) is 10,000 or more. It is less than 1,000,000.
- Mw of the aliphatic-aromatic polyester resin of the present invention is preferably 20,000 or more and 500,000 or less, more preferably 50,000 or more and 400,000 or less, still more preferably, because it is advantageous in terms of moldability and mechanical strength. Is 100,000 or more and 300,000 or less.
- the melt flow rate (MFR) of the aliphatic-aromatic polyester resin of the present invention is a value measured at 190 ° C. and a load of 2.16 kg based on JIS K7210 (2014), and is usually 0.1 g / 10 minutes or more and 100 g. / 10 minutes or less.
- the MFR of the aliphatic-aromatic polyester resin of the present invention is preferably 40 g / 10 minutes or less, more preferably 20 g / 10 minutes or less, and particularly preferably 10 g / 10 minutes or less. It is preferably 1.0 g / 10 minutes or more, and more preferably 2.0 g / 10 minutes or more.
- the MFR of the aliphatic-aromatic polyester resin can be adjusted by the molecular weight.
- the melting point of the aliphatic-aromatic polyester resin of the present invention is preferably 80 ° C. or higher, more preferably 100 ° C. or higher, preferably 180 ° C. or lower, more preferably 160 ° C. or lower, and particularly preferably lower than 140 ° C. Is.
- the glass transition temperature (Tg) of the aliphatic-aromatic polyester resin of the present invention is ⁇ 25 ° C. or higher, preferably ⁇ 20 ° C. or higher, and more preferably ⁇ 15 ° C. or higher.
- the Tg of the aliphatic-aromatic polyester resin of the present invention is preferably 5 ° C. or lower, more preferably 0 ° C. or lower. If the glass transition temperature is lower than ⁇ 25 ° C., the crystallization rate may decrease, which may lead to deterioration of moldability. The higher the glass transition temperature, the lower the impact strength tends to be.
- the method for adjusting the melting point and glass transition temperature of the aliphatic-aromatic polyester resin is not particularly limited. For example, it can be adjusted by selecting the type of the copolymerization component of the aliphatic dicarboxylic acid or the aromatic dicarboxylic acid, adjusting the copolymerization ratio of each, or combining them.
- the melting point and glass transition temperature of the aliphatic-aromatic polyester resin are measured by the method described in the section of Examples below.
- the aliphatic-aromatic polyester resin of the present invention has various additives such as a heat stabilizer, an antioxidant, an antioxidant, a crystal nucleating agent, a flame retardant, and an antistatic agent, as long as the characteristics are not impaired. Agents, mold release agents, UV absorbers and the like may be added.
- additives may be added to the reaction apparatus before the polymerization reaction, may be added to the transport apparatus from the start of the polymerization reaction to before the end of the polymerization reaction, or may be added to the transport apparatus after the completion of the polymerization reaction and before the extraction of the product. It may be added. Further, it may be added to the polyester after extraction.
- the aliphatic-aromatic polyester resin of the present invention can be molded by various molding methods applied to thermoplastic resins.
- Examples of the molding method include compression molding (compression molding, laminated molding, stampable molding), injection molding, extrusion molding and co-extrusion molding (film molding by inflation method and T-die method, laminating molding, pipe molding, electric wire / cable molding). , Deformed material molding), hollow molding (various blow molding), calendar molding, foam molding (melt foam molding, solid phase foam molding), solid molding (uniaxial stretching molding, biaxial stretching molding, roll rolling molding, stretching oriented non-woven fabric) Examples include molding, thermal molding (vacuum molding, pneumatic molding), plastic processing), powder molding (rotary molding), and various non-woven molding (dry method, bonding method, entanglement method, spunbond method, etc.).
- the molded product made of the aliphatic-aromatic polyester resin of the present invention has a wide range of packaging materials, agricultural materials, building materials, etc. for packaging liquids, powders and solids such as various foods, chemicals and miscellaneous goods. It is suitably used in applications. Specific applications include injection molded products (eg, fresh food trays, fast food containers, outdoor leisure products, etc.), extruded products (eg, films, fishing nets, fishing nets, vegetation nets, water retention sheets, etc.), hollows. Examples include molded products (bottles, etc.).
- injection molded products eg, fresh food trays, fast food containers, outdoor leisure products, etc.
- extruded products eg, films, fishing nets, fishing nets, vegetation nets, water retention sheets, etc.
- hollows examples include molded products (bottles, etc.).
- information electronic materials such as toner binders and ink binders for thermal transfer
- packaging materials such as packaging films, fruit & vegetable bags, shopping bags, compost bags, bags, trays, bottles, cushioning foams, fish boxes, etc.
- agricultural materials such as mulching film, tunnel film, house film, sun wrap, weed proof sheet, ridge sheet, germination sheet, vegetation mat, nursery bed, plant pot and the like.
- the molded product of the present invention is excellent in impact resistance, mechanical properties such as tear strength and tensile break point elongation, biodegradability, etc., and among the above applications, it is particularly preferable to be used for film applications. ..
- the thickness of the film of the present invention formed by molding the aliphatic-aromatic polyester resin of the present invention is appropriately designed according to its use, and is not particularly limited.
- the thickness of the film of the present invention is usually about 5 ⁇ m to 1 mm.
- the ratio of each branch structure and the ratios (1) to (4) of each branch structure (1) to (4) are determined from the integral ratio of the branch structure represented by (1): 0.95 ppm and the branch structure represented by the formula (4): 0.88 ppm. be able to.
- the aliphatic dicarboxylic acid unit and the aromatic dicarboxylic acid unit are the succinic acid unit and the flange carboxylic acid unit, respectively, from the integral ratio of 2.62 ppm and 7.20 ppm, the aliphatic dicarboxylic acid unit / aromatic dicarboxylic acid unit
- the molar ratio of a certain succinic acid unit / frangylcarboxylic acid unit can be determined.
- trimethylol propane is used as the trifunctional polyvalent alcohol, the branched structure represented by the formula (1): 1.04 ppm, the branched structure represented by the formula (2): 0.98 ppm, the formula (3).
- melt flow rate (MFR) was measured at 190 ° C. and 2.16 kg load based on JIS K7210 (2014).
- This biaxially stretched film was punched out to prepare a film test piece, and the test was carried out in accordance with ISO527-2 (2012).
- the long axis is expressed as MD and the short axis is expressed as TD, and the breaking point elongation MD (%) and the breaking point elongation TD (%) are measured, respectively, and the average value (the average value of the breaking point elongation MD and TD) is calculated. ..
- Example 1 In a reaction vessel equipped with a stirrer, nitrogen inlet, heating device, thermometer and decompression port, 100 parts by weight of succinic acid, 141 parts by weight of terephthalic acid, 168 parts by weight of 1,4-butanediol, and trimethylolpropane as raw materials. 0.73 parts by weight, 4 parts by weight of 1,4-butanediol solution in which tetra-n-butyl titanate was previously dissolved in 5% by weight, and 1,4 in which magnesium acetate tetrahydrate was previously dissolved in 2% by weight. -6.6 parts by weight of the butanediol solution was charged.
- the molar ratio of succinic acid to terephthalic acid is 50:50, the molar ratio of 1,4-butanediol to the total molar amount of succinic acid and terephthalic acid is 1.1, and trimethylolpropane is a constituent unit of the polymer produced. On the other hand, it was set to 0.16 mol%.
- Branched structure (1) to (4) ratio (mol%) and each branched structure ratio (mol%) in the obtained aliphatic-aromatic polyester crystallization peak temperature (° C.), crystallization peak area (J / g), melting point (° C), glass transition temperature (° C), weight average molecular weight, MFR (g / 10 minutes), breaking point elongation MD (%), breaking point elongation TD (%), breaking point elongation MD and TD
- the average value (%) and the rate of increase in torque during melting (after 5 minutes) (%) were measured.
- the measurement results are shown in Table 1. Further, FIG. 2 shows the change over time in the stirring torque during melting.
- Example 1 In Example 1, all the procedures were carried out in the same manner except that 0.73 part by weight of malic acid was used instead of 0.73 part by weight of trimethylolpropane. The results are shown in Table 1. Further, FIG. 2 shows the change over time in the stirring torque during melting.
- Example 2 an aliphatic-aromatic polyester resin was obtained under the same conditions except that 0.50 parts by weight of glycerol was used instead of 0.73 parts by weight of trimethylolpropane.
- Branched structure (1) to (4) ratio (mol%) and each branched structure ratio (mol%) in the obtained aliphatic-aromatic polyester crystallization peak temperature (° C.), crystallization peak area (J / g), melting point (° C), glass transition temperature (° C), weight average molecular weight, MFR (g / 10 minutes), breaking point elongation MD (%), breaking point elongation TD (%), breaking point elongation MD and TD The average value (%) was measured. The measurement results are shown in Table 1.
- Example 2 In Example 1, an aliphatic-aromatic polyester resin was obtained under the same conditions except that trimethylolpropane was 0.46 parts by weight and 0.101 mol% with respect to all the constituent units of the polymer to be produced. Branched structure (1) to (4) ratio (mol%) and each branched structure ratio (mol%), melting point (° C), glass transition temperature (° C), weight average molecular weight in the obtained aliphatic-aromatic polyester. , MFR (g / 10 minutes), breaking point elongation MD (%), breaking point elongation TD (%), and average value (%) of breaking point elongation MD and TD were measured. The results are shown in Table 2.
- Example 3 In Example 1, an aliphatic-aromatic polyester resin was obtained under the same conditions except that trimethylolpropane was 0.17 parts by weight and 0.037 mol% with respect to all the constituent units of the polymer to be produced. Branched structure (1) to (4) ratio (mol%) and each branched structure ratio (mol%), melting point (° C), glass transition temperature (° C), weight average molecular weight in the obtained aliphatic-aromatic polyester. , MFR (g / 10 minutes), breaking point elongation MD (%), breaking point elongation TD (%), and average value (%) of breaking point elongation MD and TD were measured. The results are shown in Table 2.
- Example 4 In Example 1, an aliphatic-aromatic polyester resin was obtained under the same conditions except that the amount was 0.068 parts by weight of trimethylolpropane and 0.015 mol% with respect to all the constituent units of the polymer to be produced. Branched structure (1) to (4) ratio (mol%) and each branched structure ratio (mol%), melting point (° C), glass transition temperature (° C), weight average molecular weight in the obtained aliphatic-aromatic polyester. , MFR (g / 10 minutes), breaking point elongation MD (%), breaking point elongation TD (%), and average value (%) of breaking point elongation MD and TD were measured. The results are shown in Table 2.
- Example 5 In Example 1, 80 parts by weight of succinic acid, 169 parts by weight of terephthalic acid, 0.75 parts by weight of trimethylolpropane, a molar ratio of succinic acid to terephthalic acid of 40:60, and all of the polymers producing trimethylolpropane.
- An aliphatic-aromatic polyester resin was obtained under the same conditions except that the ratio was 0.164 mol% with respect to the constituent unit.
- MFR g / 10 minutes
- breaking point elongation MD %
- breaking point elongation TD %
- average value (%) of breaking point elongation MD and TD were measured. The results are shown in Table 2.
- Example 6 In Example 1, 110 parts by weight of succinic acid, 127 parts by weight of terephthalic acid, 0.46 parts by weight of trimethylolpropane, a molar ratio of succinic acid to terephthalic acid of 55:45, and all of the polymers producing trimethylolpropane.
- An aliphatic-aromatic polyester resin was obtained under the same conditions except that the ratio was 0.100 mol% with respect to the constituent unit. Branched structure (1) to (4) ratio (mol%) and each branched structure ratio (mol%), melting point (° C), glass transition temperature (° C), weight average molecular weight in the obtained aliphatic-aromatic polyester. , MFR (g / 10 minutes), breaking point elongation MD (%), breaking point elongation TD (%), and average value (%) of breaking point elongation MD and TD were measured. The results are shown in Table 3.
- Example 7 In Example 1, 120 parts by weight of succinic acid, 113 parts by weight of terephthalic acid, 0.71 part by weight of trimethylolpropane, the molar ratio of succinic acid to terephthalic acid is 60:40, and all of the polymers producing trimethylolpropane.
- An aliphatic-aromatic polyester resin was obtained under the same conditions except that the ratio was 0.156 mol% with respect to the constituent unit. Branched structure (1) to (4) ratio (mol%) and each branched structure ratio (mol%), melting point (° C), glass transition temperature (° C), weight average molecular weight in the obtained aliphatic-aromatic polyester. , MFR (g / 10 minutes), breaking point elongation MD (%), breaking point elongation TD (%), and average value (%) of breaking point elongation MD and TD were measured. The results are shown in Table 3.
- Example 3 an aliphatic-aromatic polyester resin was obtained under the same conditions except that trimethylolpropane was added to 0 parts by weight and 0.00 mol% was added to the total constituent units of the polymer to be produced.
- MFR g / 10 minutes
- breaking point elongation MD %
- breaking point elongation TD %
- average value (%) of breaking point elongation MD and TD were measured. The results are shown in Table 2.
- Example 4 In Example 1, 180 parts by weight of succinic acid, 28 parts by weight of terephthalic acid, 0.75 parts by weight of trimethylolpropane, a molar ratio of succinic acid to terephthalic acid of 90:10, and all of the polymers producing trimethylolpropane.
- An aliphatic-aromatic polyester resin was obtained under the same conditions except that the ratio was 0.164 mol% with respect to the constituent unit. Branched structure (1) to (4) ratio (mol%) and each branched structure ratio (mol%), melting point (° C), glass transition temperature (° C), weight average molecular weight in the obtained aliphatic-aromatic polyester. , MFR (g / 10 minutes), breaking point elongation MD (%), breaking point elongation TD (%), and average value (%) of breaking point elongation MD and TD were measured. The results are shown in Table 3.
- Example 5 In Example 1, 20 parts by weight of succinic acid, 253 parts by weight of terephthalic acid, 0.75 parts by weight of trimethylolpropane, a molar ratio of succinic acid to terephthalic acid of 10:90, and all of the polymers producing trimethylolpropane.
- An aliphatic-aromatic polyester resin was obtained under the same conditions except that the ratio was 0.164 mol% with respect to the constituent unit. Branched structure (1) to (4) ratio (mol%) and each branched structure ratio (mol%), melting point (° C), glass transition temperature (° C), weight average molecular weight in the obtained aliphatic-aromatic polyester. was measured.
- This aliphatic-aromatic polyester resin had a high melting point and could not measure MFR, could not be molded, and could not measure MFR and tensile break point elongation. The results are shown in Table 3.
- Example 8 In Example 1, 80 parts by weight of succinic acid and 159 parts by weight of frangylcarboxylic acid were used instead of terephthalic acid to make 0.75 parts by weight of trimethylolpropane, and the molar ratio of succinic acid to frangylcarboxylic acid was 40:60.
- An aliphatic-aromatic polyester resin was obtained under the same conditions except that the amount of methylolpropane was 0.164 mol% with respect to all the constituent units of the produced polymer. Branched structure (1) to (4) ratio (mol%) and each branched structure ratio (mol%), melting point (° C), glass transition temperature (° C), weight average molecular weight in the obtained aliphatic-aromatic polyester. , MFR (g / 10 minutes), breaking point elongation MD (%), breaking point elongation TD (%), and average value (%) of breaking point elongation MD and TD were measured. The results are shown in Table 3.
- Example 8 an aliphatic-aromatic polyester resin was obtained under the same conditions except that trimethylolpropane was added to 0 parts by weight and 0.00 mol% was added to the total constituent units of the polymer to be produced.
- MFR g / 10 minutes
- breaking point elongation MD %
- breaking point elongation TD %
- average value (%) of breaking point elongation MD and TD were measured. The results are shown in Table 3.
- the crosslinking reaction proceeds during kneading and molding at a high temperature. It is presumed that this is due to the use of malic acid as a branching agent. As a result, the melt viscosity changes, resulting in molding defects. In addition, gelation causes poor appearance of the film such as fish eyes.
- the aliphatic-aromatic polyester resin of Example 1 containing the branched structures (1) to (4) has heat during heating as compared with Comparative Example 1 not containing the branched structures (1) to (4). It has high stability, can be molded without a cross-linking reaction, and is excellent in molding processing stability.
- the decrease in the crystallization peak temperature and the crystallization peak area are the same as those of the aliphatic-aromatic polyester resin of Example 1 including the branched structures (1) to (4). Since the size is smaller than that, it can be seen that the crystallinity is significantly reduced. It is presumed that this is due to the use of glycerol as a branching agent. As a result, the molding stability and the molding speed may be lowered, which may cause troubles such as fusion of the film after molding.
- the aliphatic-aromatic polyester resin of Example 1 containing the branched structures (1) to (4) has sufficient crystallinity as compared with Comparative Example 2 not containing the branched structures (1) to (4). It has excellent molding stability.
- the presence of the branched structures (1) to (4) in the aliphatic-aromatic polyester resin causes the melt viscosity of the aliphatic-aromatic polyester resin in the low shear region. Is increased, and MFR 10 g / 10 minutes or less, which is generally considered to be good for film and sheet molding, is realized. Further, as shown in Examples 1 to 8, a film having excellent elongation at break point, which is particularly important as a mechanical property of the film, can be obtained.
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Manufacturing & Machinery (AREA)
- Materials Engineering (AREA)
- Polyesters Or Polycarbonates (AREA)
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202180042728.9A CN115698122A (zh) | 2020-07-17 | 2021-07-07 | 脂肪族-芳香族聚酯树脂及其成形品 |
| JP2022536293A JPWO2022014434A1 (enExample) | 2020-07-17 | 2021-07-07 | |
| EP21841204.7A EP4183822A4 (en) | 2020-07-17 | 2021-07-07 | ALIPHATIC-AROMATIC POLYESTER RESIN AND MOLDED BODY THEREOF |
| US18/091,448 US20230140076A1 (en) | 2020-07-17 | 2022-12-30 | Aliphatic-aromatic polyester resin and molded article thereof |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2020-122966 | 2020-07-17 | ||
| JP2020122966 | 2020-07-17 |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US18/091,448 Continuation US20230140076A1 (en) | 2020-07-17 | 2022-12-30 | Aliphatic-aromatic polyester resin and molded article thereof |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2022014434A1 true WO2022014434A1 (ja) | 2022-01-20 |
Family
ID=79554816
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2021/025593 Ceased WO2022014434A1 (ja) | 2020-07-17 | 2021-07-07 | 脂肪族-芳香族ポリエステル樹脂及びその成形品 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US20230140076A1 (enExample) |
| EP (1) | EP4183822A4 (enExample) |
| JP (1) | JPWO2022014434A1 (enExample) |
| CN (1) | CN115698122A (enExample) |
| TW (1) | TWI891850B (enExample) |
| WO (1) | WO2022014434A1 (enExample) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2024128707A1 (ko) * | 2022-12-16 | 2024-06-20 | 코오롱인더스트리 주식회사 | 생분해성 폴리에스테르 수지 및 이의 제조 방법 |
| WO2024247988A1 (ja) | 2023-05-29 | 2024-12-05 | 三菱ケミカル株式会社 | 生分解性樹脂組成物、成形体、および積層体 |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH09152742A (ja) * | 1995-11-29 | 1997-06-10 | Unitika Ltd | トナーバインダー用ポリエステル樹脂およびこれを用いたトナー |
| JP2000066447A (ja) * | 1998-08-21 | 2000-03-03 | Mitsubishi Rayon Co Ltd | トナー用ポリエステル樹脂およびトナー |
| JP2000302888A (ja) * | 1999-04-16 | 2000-10-31 | Mitsubishi Rayon Co Ltd | ポリエステルシート |
| JP2001354758A (ja) * | 2000-06-09 | 2001-12-25 | Mitsubishi Rayon Co Ltd | ポリエステルシート |
| JP2002363271A (ja) * | 2001-06-13 | 2002-12-18 | Mitsubishi Rayon Co Ltd | ポリエステルシート |
| JP2012087291A (ja) * | 2010-09-21 | 2012-05-10 | Arakawa Chem Ind Co Ltd | 活性エネルギー線硬化皮膜付プラスチックフィルム用非水系アンダーコート剤、および活性エネルギー線硬化皮膜付プラスチックフィルム |
| JP2020122966A (ja) | 2004-09-16 | 2020-08-13 | 株式会社半導体エネルギー研究所 | 表示装置 |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN102558519A (zh) * | 2010-12-28 | 2012-07-11 | 上海轻工业研究所有限公司 | 芳香族-脂肪族共聚酯及其合成方法 |
| JP2014114418A (ja) * | 2012-12-12 | 2014-06-26 | Mitsubishi Chemicals Corp | 脂肪族ポリエステルの製造方法 |
| CN111234159B (zh) * | 2018-11-29 | 2022-01-04 | 中国石油化工股份有限公司 | 一种三重形状记忆聚合物及其制备方法和应用 |
-
2021
- 2021-07-07 JP JP2022536293A patent/JPWO2022014434A1/ja active Pending
- 2021-07-07 CN CN202180042728.9A patent/CN115698122A/zh active Pending
- 2021-07-07 EP EP21841204.7A patent/EP4183822A4/en active Pending
- 2021-07-07 WO PCT/JP2021/025593 patent/WO2022014434A1/ja not_active Ceased
- 2021-07-16 TW TW110126174A patent/TWI891850B/zh active
-
2022
- 2022-12-30 US US18/091,448 patent/US20230140076A1/en active Pending
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH09152742A (ja) * | 1995-11-29 | 1997-06-10 | Unitika Ltd | トナーバインダー用ポリエステル樹脂およびこれを用いたトナー |
| JP2000066447A (ja) * | 1998-08-21 | 2000-03-03 | Mitsubishi Rayon Co Ltd | トナー用ポリエステル樹脂およびトナー |
| JP2000302888A (ja) * | 1999-04-16 | 2000-10-31 | Mitsubishi Rayon Co Ltd | ポリエステルシート |
| JP2001354758A (ja) * | 2000-06-09 | 2001-12-25 | Mitsubishi Rayon Co Ltd | ポリエステルシート |
| JP2002363271A (ja) * | 2001-06-13 | 2002-12-18 | Mitsubishi Rayon Co Ltd | ポリエステルシート |
| JP2020122966A (ja) | 2004-09-16 | 2020-08-13 | 株式会社半導体エネルギー研究所 | 表示装置 |
| JP2012087291A (ja) * | 2010-09-21 | 2012-05-10 | Arakawa Chem Ind Co Ltd | 活性エネルギー線硬化皮膜付プラスチックフィルム用非水系アンダーコート剤、および活性エネルギー線硬化皮膜付プラスチックフィルム |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP4183822A4 |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2024128707A1 (ko) * | 2022-12-16 | 2024-06-20 | 코오롱인더스트리 주식회사 | 생분해성 폴리에스테르 수지 및 이의 제조 방법 |
| WO2024247988A1 (ja) | 2023-05-29 | 2024-12-05 | 三菱ケミカル株式会社 | 生分解性樹脂組成物、成形体、および積層体 |
Also Published As
| Publication number | Publication date |
|---|---|
| EP4183822A4 (en) | 2024-01-10 |
| US20230140076A1 (en) | 2023-05-04 |
| TWI891850B (zh) | 2025-08-01 |
| EP4183822A1 (en) | 2023-05-24 |
| TW202208489A (zh) | 2022-03-01 |
| JPWO2022014434A1 (enExample) | 2022-01-20 |
| CN115698122A (zh) | 2023-02-03 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11932725B2 (en) | Biodegradable polyester resin, preparation method thereof, and biodegradable polyester film comprising the same | |
| US5470941A (en) | Thermoplastic biodegradable resins and a process of preparation thereof | |
| JP5818185B2 (ja) | ポリ(ブチレンテレフタレート−co−アジペート)コポリマの製造方法 | |
| JPH10508640A (ja) | 生分解可能なポリマー、その製造及び生分解可能な成形体の製造のためのその使用 | |
| JP2008291244A (ja) | フラン構造を含むポリエステル樹脂の製造方法 | |
| JP2023024333A (ja) | 樹脂組成物及び樹脂組成物の製造方法 | |
| KR102671853B1 (ko) | 생분해성 폴리에스테르 수지, 및 이를 포함하는 생분해성 폴리에스테르 필름 및 적층체 | |
| JP2022136023A (ja) | 脂肪族芳香族ポリエステル組成物及びその製造方法 | |
| CN114929779B (zh) | 脂肪族芳香族聚酯的制造方法 | |
| US20230140076A1 (en) | Aliphatic-aromatic polyester resin and molded article thereof | |
| JP2022157778A (ja) | 生分解性樹脂組成物及び成形体 | |
| JP2001172488A (ja) | 生分解性ポリエステル樹脂組成物とその用途 | |
| KR101690082B1 (ko) | 생분해성 수지 조성물 및 그로부터 제조되는 생분해성 필름 | |
| JP2022149744A (ja) | 脂肪族芳香族ポリエステル樹脂 | |
| JP2008031456A (ja) | 脂肪族芳香族ポリエステル及びその樹脂組成物 | |
| JP2021191845A (ja) | 生分解性樹脂組成物及び生分解性樹脂成形体 | |
| JP2021161388A (ja) | ポリエステルの製造方法 | |
| JPH10237164A (ja) | ポリ乳酸系共重合体の製造方法及びポリ乳酸系共重合体 | |
| JP2676127B2 (ja) | 生分解性ポリエステルの製造方法 | |
| JP5135740B2 (ja) | ポリエステル及びその製造方法、並びにポリエステルブロック共重合体 | |
| JP2024010476A (ja) | ポリエステル樹脂、樹脂組成物、成形品、粒状成形品、フィルム、および、コーティング材 | |
| JP2752876B2 (ja) | ポリエステル製射出成形体 | |
| JP4171891B2 (ja) | ポリエステルカーボネート共重合体及びその製造法 | |
| JPH0873582A (ja) | 脂肪族ポリエステルの製造方法 | |
| JP2747196B2 (ja) | ポリエステル製射出中空成形体 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 21841204 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2022536293 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2021841204 Country of ref document: EP Effective date: 20230217 |