WO2016194659A1 - 塩化コバルト水溶液の精製方法 - Google Patents
塩化コバルト水溶液の精製方法 Download PDFInfo
- Publication number
- WO2016194659A1 WO2016194659A1 PCT/JP2016/065046 JP2016065046W WO2016194659A1 WO 2016194659 A1 WO2016194659 A1 WO 2016194659A1 JP 2016065046 W JP2016065046 W JP 2016065046W WO 2016194659 A1 WO2016194659 A1 WO 2016194659A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- cobalt chloride
- aqueous solution
- cobalt
- solution
- nickel
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01G—COMPOUNDS CONTAINING METALS NOT COVERED BY SUBCLASSES C01D OR C01F
- C01G51/00—Compounds of cobalt
- C01G51/08—Halides; Oxyhalides
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01G—COMPOUNDS CONTAINING METALS NOT COVERED BY SUBCLASSES C01D OR C01F
- C01G51/00—Compounds of cobalt
- C01G51/08—Halides; Oxyhalides
- C01G51/085—Chlorides; Oxychlorides
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B23/00—Obtaining nickel or cobalt
- C22B23/04—Obtaining nickel or cobalt by wet processes
- C22B23/0453—Treatment or purification of solutions, e.g. obtained by leaching
- C22B23/0461—Treatment or purification of solutions, e.g. obtained by leaching by chemical methods
- C22B23/0469—Treatment or purification of solutions, e.g. obtained by leaching by chemical methods by chemical substitution, e.g. by cementation
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B23/00—Obtaining nickel or cobalt
- C22B23/06—Refining
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22B—PRODUCTION AND REFINING OF METALS; PRETREATMENT OF RAW MATERIALS
- C22B3/00—Extraction of metal compounds from ores or concentrates by wet processes
- C22B3/20—Treatment or purification of solutions, e.g. obtained by leaching
- C22B3/44—Treatment or purification of solutions, e.g. obtained by leaching by chemical processes
- C22B3/46—Treatment or purification of solutions, e.g. obtained by leaching by chemical processes by substitution, e.g. by cementation
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/48—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides
- H01M4/52—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of nickel, cobalt or iron
- H01M4/525—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of nickel, cobalt or iron of mixed oxides or hydroxides containing iron, cobalt or nickel for inserting or intercalating light metals, e.g. LiNiO2, LiCoO2 or LiCoOxFy
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P10/00—Technologies related to metal processing
- Y02P10/20—Recycling
Definitions
- the present invention relates to a method for purifying an aqueous cobalt chloride solution.
- Cobalt is a rare metal and a valuable metal used as an alloy material. Further, as an application other than the alloy, cobalt is also used as a battery electrode material. For example, cobalt is also used for a positive electrode material of a lithium ion battery, which is a non-aqueous electrolyte secondary battery for vehicle use, which has been developed in recent years.
- salts for example, cobalt salts, such as cobalt sulfate and cobalt chloride
- the cobalt salt described above can be obtained as a by-product of the process of smelting nickel ore and the like. Specifically, wet processing is adopted for purification of impurities, and a cobalt salt is generated from a cobalt salt solution generated at that time.
- nickel ore and the like contain a wide variety of impurities such as manganese, iron, copper, and chromium in addition to nickel and cobalt. If impurities are also contained in the cobalt salt solution, impurities may be mixed into the cobalt salt. And if the cobalt salt containing an impurity is used for manufacture of a positive electrode material, an impurity may mix in a positive electrode material.
- the presence of impurities in the positive electrode material greatly affects the performance of the positive electrode material, that is, the battery characteristics.
- the lithium ion battery as described above has a high capacity and a high voltage, the presence of a trace amount of impurities greatly affects the battery characteristics, so the specifications of impurities of raw materials such as cobalt salts are very strictly controlled.
- the specifications of impurities of raw materials such as cobalt salts are very strictly controlled.
- copper is an important impurity that greatly affects the performance of the battery, it is required to strictly control the amount of copper contained in raw materials such as cobalt salts.
- Methods such as solvent extraction and electrolysis are known as methods for reducing impurities such as copper contained in cobalt salts. That is, if copper is removed from the cobalt salt solution by a solvent extraction method or an electrolytic method, the copper concentration in the cobalt salt solution, that is, the amount of copper contained in the cobalt salt can be reduced. However, these methods cannot reduce the lower limit concentration of copper that can be separated so much. In addition, these methods require a large-scale apparatus such as a solvent extraction apparatus such as a mixer-settler, an electrolytic cell or a power source, and thus have problems such as an increase in capital investment and a high processing cost.
- the precipitation method is a method for separating impurities by adding a neutralizing agent, a sulfurizing agent, or the like, and is widely used for wastewater treatment of heavy metals such as copper.
- the sulfidation method in which copper is precipitated and removed using a sulfiding agent, has very low copper sulfide solubility (water solubility: 18 ° C, 3.4 x 10 -4 g / L), and the concentration of copper in the solution. There is an advantage that can be greatly reduced.
- harmful hydrogen sulfide gas is used as a sulfiding agent, it is necessary to ensure the safety of workers and to take environmental measures.
- Various devices have been devised in order to control hydrogen sulfide (for example, Patent Document 1), but there is a problem that the cost of incidental equipment increases because the device configuration becomes complicated.
- the concentration of copper in the cobalt salt solution cannot be so low.
- the solubility of cobalt needs to be 100 g-Co / L or more. Since the solubility product of cobalt is 2.2 ⁇ 10 ⁇ 16 , the pH must be 6 or less. On the other hand, the solubility product of copper is 2.2 ⁇ 10 ⁇ 20 , and at pH 6, the solubility of copper is 14 mg-Cu / L, so that the copper separability is deteriorated.
- the cementation method is a method in which a metal ion to be removed is reduced and removed by an electrically base metal. For this reason, if a base metal is used rather than copper, copper can be removed from a solution. For example, since cobalt is a base metal than copper, if cobalt metal is used, copper in the cobalt chloride solution can be precipitated and removed.
- the base metal used is ionized and dissolved in the solution. Therefore, it is necessary to use a metal that can be dissolved, but the cobalt metal described above becomes a material for the positive electrode material. Even if it remains in the cobalt salt solution, the electrode performance is not affected.
- cobalt metal is usually distributed as a cobalt plate, and it is difficult to obtain it in a reactive powder or briquette form. That is, when copper is separated from a cobalt salt solution by cementation using cobalt metal, there is no choice but to use a cobalt plate having low reactivity, so that the efficiency of removing copper is deteriorated.
- a method of increasing the liquid temperature of the cobalt salt solution can be considered. That is, if cementation is performed with a cobalt plate while the cobalt salt solution is heated, the reactivity between the cobalt plate and copper may be increased.
- the energy for that and the installation for heating are required, and it leads to a cost increase.
- the cementation reaction is an exothermic reaction
- the temperature of the cobalt salt solution increases with the reaction.
- the liquid temperature may excessively increase due to heat generated by the reaction.
- the cementation reaction is further accelerated, and it is necessary to take measures against hydrogen gas generated during the reaction, and the precipitated copper tends to become fine powder.
- Even if copper which became fine powder precipitates it becomes easy to redissolve in a cobalt salt solution, and there is a possibility that the copper concentration in the cobalt salt solution cannot be sufficiently lowered.
- the tendency of the copper fine powder to re-dissolve is remarkable, and the copper deposited in the fine powder state is easily oxidized and easily generates heat after recovery.
- an object of the present invention is to provide a method for purifying an aqueous cobalt chloride solution that can efficiently remove impurities from a cobalt salt solution.
- the method for purifying an aqueous cobalt chloride solution of the first invention is a method of removing impurities by a cementation reaction by contacting metallic nickel with an aqueous solution containing cobalt chloride, wherein the metallic nickel is added to the aqueous solution containing cobalt chloride. Before contacting, the metallic nickel is washed with an acidic solution having a pH of 2.5 or less.
- the method for purifying a cobalt chloride aqueous solution according to the second invention is characterized in that, in the first invention, the metallic nickel is brought into contact with an aqueous solution containing the cobalt chloride at room temperature.
- the method for purifying a cobalt chloride aqueous solution of the third invention is characterized in that, in the first or second invention, the impurity is copper.
- the method for purifying the cobalt chloride aqueous solution of the fourth invention is the first, second or second 3.
- the invention according to claim 3, wherein the aqueous solution containing cobalt chloride from which impurities have been removed is a solution used as a raw material for a positive electrode material containing nickel and cobalt in the composition of a non-aqueous electrolyte secondary battery.
- the method for purifying a cobalt chloride aqueous solution according to a fifth invention is characterized in that, in the fourth invention, the aqueous solution containing cobalt chloride is a process liquid for a nickel smelting process.
- the passive film on the surface of the metallic nickel is removed. Since the passive film is removed from metallic nickel, impurities that are nobler than metallic nickel can be deposited by a cementation reaction when in contact with an aqueous solution containing cobalt chloride. In addition, since the metallic nickel is simply washed with an acid and brought into contact with an aqueous solution containing cobalt chloride, impurities can be easily removed from the aqueous solution containing cobalt chloride. According to the second invention, since the cobalt chloride aqueous solution is brought into contact at room temperature, there is no need to heat the cobalt chloride aqueous solution.
- the purified aqueous solution of cobalt chloride can be used for the production of a cobalt salt suitable for a raw material for producing a substance having an adverse effect on the presence of copper, such as a material for a non-aqueous electrolyte secondary battery.
- the impurity concentration in the cobalt chloride aqueous solution can be greatly reduced, while the cobalt chloride aqueous solution can be an aqueous solution containing nickel. Therefore, the refined aqueous solution of cobalt chloride can be used as a raw material for the positive electrode material containing nickel and cobalt in the composition of the non-aqueous electrolyte secondary battery.
- the purified cobalt chloride aqueous solution can be used as it is as a raw material for the positive electrode material containing nickel and cobalt in the composition of the non-aqueous electrolyte secondary battery. it can. Therefore, it is not necessary to produce a cobalt salt from the process liquid of the nickel smelting process, so that there is also an advantage that the production of the positive electrode material of the nonaqueous electrolyte secondary battery can be made more efficient.
- the method for purifying an aqueous cobalt chloride solution of the present invention is a method for removing impurities contained in an aqueous solution containing cobalt chloride, and the impurity concentration can be stably reduced without increasing capital investment. It has the characteristics.
- the aqueous solution (target aqueous solution) from which impurities are removed by the method for purifying an aqueous cobalt chloride solution of the present invention may be an aqueous solution containing cobalt chloride (hereinafter simply referred to as an aqueous cobalt chloride solution).
- aqueous solution intermediate process liquid for nickel smelting
- secondary raw materials such as used batteries
- An aqueous solution or the like generated when wet processing is performed to recover cobalt from sludge or the like generated from the treatment can be a target aqueous solution.
- the use of the aqueous solution (generated aqueous solution) produced by the method for purifying a cobalt chloride aqueous solution of the present invention is not particularly limited.
- it can be used as a raw material for producing electric cobalt or a cobalt salt, and can also be used as a raw material for a positive electrode material containing cobalt in its composition in a non-aqueous electrolyte secondary battery.
- the aqueous solution produced is an aqueous solution containing nickel, it can be used as a raw material for the positive electrode material containing nickel and cobalt in the composition of the non-aqueous electrolyte secondary battery.
- the resulting aqueous solution can be used as a raw material for the positive electrode material of a ternary (NCM) or nickel (NCA) lithium ion battery.
- the non-aqueous electrolyte 2 is used.
- the production of the positive electrode material for the secondary battery can be made more efficient.
- the positive electrode material of the non-aqueous electrolyte secondary battery is manufactured by firing a precursor of a metal hydroxide called a precursor prepared by neutralizing an aqueous solution of a metal salt mixed at a predetermined ratio.
- a precursor of a metal hydroxide called a precursor prepared by neutralizing an aqueous solution of a metal salt mixed at a predetermined ratio.
- the aqueous solution of the metal salt is prepared by dissolving solids (such as nickel salt and cobalt salt).
- the solid is produced from an intermediate process liquid of nickel smelting containing nickel salt, cobalt salt and the like.
- the solution is once converted into a solid, and then dissolved again to prepare an aqueous solution (raw material aqueous solution) of nickel salt or cobalt salt.
- the raw material aqueous solution is considered to have less impurities than the nickel smelting intermediate process liquid, but it can be considered that it takes extra labor and cost to generate solids and dissolve the solids.
- this product aqueous solution can be used as a raw material for the positive electrode material. Then, since the process of the production
- any metal that is nobler than nickel or cobalt can be removed as an impurity.
- copper or silver can be removed from the aqueous solution as an impurity.
- the purification method of the cobalt chloride aqueous solution of the present invention is used to remove copper from the cobalt chloride aqueous solution, it is possible to remove copper to a low concentration (for example, about 0.5 to 1.0 mg / L).
- cobalt suitable for a raw material for producing a substance that adversely affects the presence of copper such as a material for a non-aqueous electrolyte secondary battery. Salts can be produced.
- copper which is an important impurity that greatly affects the performance of the battery, is removed to a low concentration. The quality of the material is improved.
- the method for purifying a cobalt chloride aqueous solution of the present invention is a method of removing impurities contained in an aqueous solution containing cobalt chloride (cobalt chloride aqueous solution) by a cementation reaction.
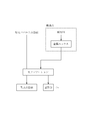
- FIG. 1 shows a schematic flow chart of the method for purifying an aqueous cobalt chloride solution of the present invention.
- metallic nickel is brought into contact with a cobalt chloride aqueous solution containing copper as a target aqueous solution, and copper is removed by a cementation reaction.
- the chemical formula of this cementation reaction is shown in Formula 1.
- Formula 1 As can be seen from Equation 1, by the cementation reaction, metallic nickel is dissolved to become nickel ions, and copper ions are precipitated as metallic copper. Ni + Cu 2+ ⁇ Ni 2+ + Cu (Formula 1)
- metallic nickel usually has a passive film that is an oxide on its surface, and the presence of this passive film inhibits dissolution of metallic nickel.
- a washing treatment with an acid is performed before the cobalt chloride aqueous solution is brought into contact with metallic nickel. Specifically, the washing treatment is performed with an acidic solution having a pH of 2.5 or less.
- the passive film on the surface of the metallic nickel is removed by the reaction shown in Formula 2, and nickel atoms are exposed on the surface of the metallic nickel.
- the above-mentioned cementation reaction can be easily caused by bringing the cobalt metal solution into contact with the metallic nickel. That is, since copper can be deposited instead of dissolving nickel in the cobalt chloride aqueous solution, the copper concentration (copper ion concentration) in the cobalt chloride aqueous solution can be reduced.
- the temperature of the aqueous solution must be kept at 60 ° C. or higher in order to cause a reaction between the aqueous solution and the passive film.
- the passive film is removed before contacting the nickel metal and the cobalt chloride aqueous solution (before causing the cementation reaction). For this reason, it became possible to maintain the cobalt chloride aqueous solution at a temperature at which a cementation reaction occurs. That is, copper can be deposited and removed by a cementation reaction while the aqueous cobalt chloride solution is kept at room temperature (about 10 to 30 ° C.). Then, since it is not necessary to heat the aqueous solution of cobalt chloride, there is an advantage that a facility for heating becomes unnecessary and it is not necessary to increase the capital investment.
- the aqueous solution of cobalt chloride is at room temperature, even if the liquid temperature of the aqueous solution of cobalt chloride is increased by the cementation reaction, the liquid temperature is not excessively high (60 ° C. or higher requiring heat-resistant equipment). Therefore, stable operation can be performed.
- an aqueous solution of cobalt chloride may be heated.
- the metallic nickel to be brought into contact with the cobalt chloride aqueous solution may have any shape.
- metallic nickel such as plate, powder, and briquette pulverized material can be used.
- a powder having a large specific surface area or a pulverized briquette is preferable.
- the method for bringing the cobalt chloride aqueous solution into contact with the metallic nickel is not particularly limited, as long as they come into contact with each other to the extent that a cementation reaction occurs at the interface where both are in contact.
- metallic nickel may be immersed in an aqueous cobalt chloride solution, or an aqueous cobalt chloride solution may be passed through the metallic nickel (in the case of powder or briquette pulverized product).
- you may flow cobalt chloride aqueous solution along the surface (in the case of plate shape) of metallic nickel.
- the acidic liquid is not particularly limited as long as it can remove the passive film of metallic nickel.
- an acidic solution such as hydrochloric acid, sulfuric acid, or nitric acid can be used.
- the acidic liquid is preferably hydrochloric acid.
- the pH of the acidic solution is not particularly limited as long as it can remove the passive film of metallic nickel.
- the passive film of metallic nickel can be removed if the pH is 2.5 or lower. If the pH is too low, even the metallic nickel under the passive film is dissolved, so that the metallic nickel contributing to cementation is reduced and the efficiency is deteriorated.
- the pH of the acidic liquid is preferably adjusted to be 1.5 or more and 2.5 or less, and more preferably 1.7 or more and 2.3 or less.
- the pH of the aqueous cobalt chloride aqueous solution is not particularly limited as long as it causes a cementation reaction. For example, if the pH is too low, metallic nickel is dissolved regardless of the cementation reaction. As a result, metallic nickel contributing to cementation is reduced and efficiency is deteriorated. In addition, since the amount of hydrogen generated per unit time increases, a separate safety device is required and the equipment cost increases. Therefore, the pH of the cobalt chloride aqueous solution is preferably adjusted to be 1.5 or more and 2.5 or less, and more preferably 1.7 or more and 2.3 or less.
- the aqueous solution of cobalt chloride has a pH of 0.3 by adding a 2 mol / L aqueous solution of sodium hydroxide to 400 mL of an aqueous solution of cobalt chloride having a copper concentration of 45 mg / L and a cobalt concentration of 67 g / L. What was adjusted to was used.
- As metallic nickel 40 g of a crushed nickel briquette was used.
- Example 1 A nickel briquette pulverized product (40 g) was immersed in 3 mol / L hydrochloric acid (40 ml) for 5 minutes to carry out an acid washing treatment (pickling treatment). The pulverized nickel briquette was added to an aqueous cobalt chloride solution at room temperature (20 ° C.) and mixed with stirring for 8 hours.
- Example 1 During stirring and mixing in each of the experiments of Example 1 to Comparative Example 2, the supernatant was sampled every hour, and the aqueous solution of cobalt chloride was measured using ICP emission analysis (measurement apparatus manufactured by Seiko Instruments Inc .: Model SPS3000). The copper concentration inside was confirmed.
- Example 1 despite the reaction at room temperature (20 ° C.), the copper concentration could be reduced to about 3 mg / L in the same time (1 hour) as in Comparative Example 2. That is, in Example 1, the same reaction rate as when the cobalt chloride aqueous solution was heated was obtained, and it was confirmed that a sufficient copper removing effect was obtained without heating.
- reaction time for removing copper from the cobalt chloride aqueous solution can be shortened by introducing a pickling step as a pre-step of the cementation step even if the reaction temperature is reduced.
- the method for purifying a cobalt chloride aqueous solution of the present invention is suitable for a method of removing impurities from a cobalt chloride aqueous solution used as a raw material for a non-aqueous electrolyte secondary battery.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Manufacturing & Machinery (AREA)
- Mechanical Engineering (AREA)
- Metallurgy (AREA)
- Materials Engineering (AREA)
- Inorganic Chemistry (AREA)
- General Life Sciences & Earth Sciences (AREA)
- Environmental & Geological Engineering (AREA)
- Life Sciences & Earth Sciences (AREA)
- Geochemistry & Mineralogy (AREA)
- Geology (AREA)
- Electrochemistry (AREA)
- Manufacture And Refinement Of Metals (AREA)
- Inorganic Compounds Of Heavy Metals (AREA)
- Battery Electrode And Active Subsutance (AREA)
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201680030091.0A CN107614711A (zh) | 2015-05-29 | 2016-05-20 | 氯化钴水溶液的纯化方法 |
| KR1020177037128A KR102460255B1 (ko) | 2015-05-29 | 2016-05-20 | 염화코발트 수용액의 정제 방법 |
| EP16803094.8A EP3305923B1 (en) | 2015-05-29 | 2016-05-20 | Aqueous cobalt chloride solution purification method |
| CA2986557A CA2986557C (en) | 2015-05-29 | 2016-05-20 | Aqueous cobalt chloride solution refinement method |
| US15/577,521 US10501334B2 (en) | 2015-05-29 | 2016-05-20 | Aqueous cobalt chloride solution refinement method |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2015110667A JP6218121B2 (ja) | 2015-05-29 | 2015-05-29 | 塩化コバルト水溶液の精製方法 |
| JP2015-110667 | 2015-05-29 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2016194659A1 true WO2016194659A1 (ja) | 2016-12-08 |
Family
ID=57440524
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2016/065046 Ceased WO2016194659A1 (ja) | 2015-05-29 | 2016-05-20 | 塩化コバルト水溶液の精製方法 |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US10501334B2 (enExample) |
| EP (1) | EP3305923B1 (enExample) |
| JP (1) | JP6218121B2 (enExample) |
| KR (1) | KR102460255B1 (enExample) |
| CN (1) | CN107614711A (enExample) |
| CA (1) | CA2986557C (enExample) |
| WO (1) | WO2016194659A1 (enExample) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10239764B2 (en) * | 2015-05-29 | 2019-03-26 | Sumitomo Metal Mining Co., Ltd. | Aqueous cobalt chloride solution purification method |
| WO2022209988A1 (ja) * | 2021-03-30 | 2022-10-06 | 日立金属株式会社 | リチウムイオン二次電池用正極活物質の製造方法 |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR102372482B1 (ko) | 2021-09-16 | 2022-03-10 | 주식회사 오스터 | 애완동물용 목욕장치 |
| KR102809278B1 (ko) | 2022-01-21 | 2025-05-22 | 강윤성 | 부착형 애완동물 목욕장치 겸용 캣타워 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5743628B2 (enExample) * | 1974-05-22 | 1982-09-16 | ||
| JPH1180986A (ja) * | 1997-09-03 | 1999-03-26 | Sumitomo Metal Mining Co Ltd | 含銅塩化ニッケル溶液からの銅の除去方法 |
| JP2000067862A (ja) * | 1998-08-24 | 2000-03-03 | Sumitomo Metal Mining Co Ltd | 非水系電解質二次電池用正極活物質とその製造方法 |
| JP2013194269A (ja) * | 2012-03-17 | 2013-09-30 | Mitsubishi Materials Corp | コバルト含有液の不純物除去方法 |
Family Cites Families (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR1536922A (fr) * | 1964-11-25 | 1968-09-02 | Republic Steel Corp | Procédé de récupération du nickel ou du cobalt métallique à partir de solutions de sels de ces deux métaux |
| US3950486A (en) * | 1970-05-26 | 1976-04-13 | Deepsea Ventures, Inc. | Method for separating metal constituents from ocean floor nodules |
| US3903236A (en) * | 1972-11-27 | 1975-09-02 | Deepsea Ventures Inc | Method for obtaining metal values by the halidation of a basic manganiferous ore with ferric chloride pre-treatment |
| FR2277894A1 (fr) * | 1974-07-10 | 1976-02-06 | Nickel Le | Procede hydrometallurgique pour le traitement des nodules polymetalliques des oceans |
| US4348224A (en) * | 1981-09-10 | 1982-09-07 | Gte Products Corporation | Method for producing cobalt metal powder |
| CN1115338A (zh) * | 1994-07-18 | 1996-01-24 | 西安建筑科技大学 | 一种从镍或钴的酸性溶液中除铜的方法 |
| JPH11131155A (ja) * | 1997-10-29 | 1999-05-18 | Sumitomo Metal Mining Co Ltd | コバルト溶液からの銅の除去方法 |
| JP2003020647A (ja) | 2001-07-05 | 2003-01-24 | Nippon Steel Corp | 回転杭貫入装置 |
| CN101629240A (zh) * | 2009-07-30 | 2010-01-20 | 浙江华友钴业股份有限公司 | 一种萃取净化钴溶液中镉制取高纯钴溶液的方法 |
| CN102534223B (zh) * | 2012-01-09 | 2014-09-17 | 湖南邦普循环科技有限公司 | 一种从废旧锂离子电池中回收有价金属的方法 |
-
2015
- 2015-05-29 JP JP2015110667A patent/JP6218121B2/ja active Active
-
2016
- 2016-05-20 CN CN201680030091.0A patent/CN107614711A/zh active Pending
- 2016-05-20 CA CA2986557A patent/CA2986557C/en active Active
- 2016-05-20 US US15/577,521 patent/US10501334B2/en active Active
- 2016-05-20 KR KR1020177037128A patent/KR102460255B1/ko active Active
- 2016-05-20 EP EP16803094.8A patent/EP3305923B1/en active Active
- 2016-05-20 WO PCT/JP2016/065046 patent/WO2016194659A1/ja not_active Ceased
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5743628B2 (enExample) * | 1974-05-22 | 1982-09-16 | ||
| JPH1180986A (ja) * | 1997-09-03 | 1999-03-26 | Sumitomo Metal Mining Co Ltd | 含銅塩化ニッケル溶液からの銅の除去方法 |
| JP2000067862A (ja) * | 1998-08-24 | 2000-03-03 | Sumitomo Metal Mining Co Ltd | 非水系電解質二次電池用正極活物質とその製造方法 |
| JP2013194269A (ja) * | 2012-03-17 | 2013-09-30 | Mitsubishi Materials Corp | コバルト含有液の不純物除去方法 |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP3305923A4 * |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10239764B2 (en) * | 2015-05-29 | 2019-03-26 | Sumitomo Metal Mining Co., Ltd. | Aqueous cobalt chloride solution purification method |
| WO2022209988A1 (ja) * | 2021-03-30 | 2022-10-06 | 日立金属株式会社 | リチウムイオン二次電池用正極活物質の製造方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| US10501334B2 (en) | 2019-12-10 |
| CA2986557C (en) | 2022-10-04 |
| EP3305923A4 (en) | 2018-11-14 |
| JP6218121B2 (ja) | 2017-10-25 |
| EP3305923B1 (en) | 2020-11-04 |
| JP2016222977A (ja) | 2016-12-28 |
| CA2986557A1 (en) | 2016-12-08 |
| KR20180014003A (ko) | 2018-02-07 |
| KR102460255B1 (ko) | 2022-10-27 |
| EP3305923A1 (en) | 2018-04-11 |
| US20180134577A1 (en) | 2018-05-17 |
| CN107614711A (zh) | 2018-01-19 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| TWI729543B (zh) | 鋰離子二次電池之正極活性物質廢棄物之處理方法 | |
| TWI406954B (zh) | Method for recovering valuable metals from IZO waste | |
| TWI432609B (zh) | Method for recovering valuable metal from indium - zinc oxide waste | |
| JP2022507019A (ja) | リチウムイオン電池から金属を抽出するためのプロセス | |
| CN111278998A (zh) | 从废锂基电池和其它进料中回收钴、锂和其它金属的方法 | |
| JP7198081B2 (ja) | 有価金属の回収方法 | |
| KR102188441B1 (ko) | 니켈, 코발트를 함유하는 용액의 제조 방법 | |
| JP2022094152A (ja) | リチウムイオン電池廃棄物の処理方法 | |
| JP6218121B2 (ja) | 塩化コバルト水溶液の精製方法 | |
| TW202134185A (zh) | 高純度硫酸鈷溶液之製造方法、及硫酸鈷之製造方法 | |
| WO2020137995A1 (ja) | 有価金属の回収方法 | |
| JP2018111858A (ja) | 酸化スカンジウムの製造方法 | |
| JP6929240B2 (ja) | 電池用硫酸コバルトの製造方法 | |
| JP2016222977A5 (enExample) | ||
| JP2021161448A (ja) | マンガンイオン除去方法 | |
| JP6201154B2 (ja) | 塩化コバルト水溶液の浄液方法 | |
| KR102576614B1 (ko) | 폐리튬이온전지로부터 유가 금속 회수 방법 | |
| JP2017226559A (ja) | 硫酸ニッケルと硫酸コバルトの低塩素濃度混合水溶液の製造方法 | |
| JP2006219719A (ja) | インジウムの回収方法 | |
| JP6565591B2 (ja) | カルシウムの分離方法 | |
| JP2016222976A5 (enExample) | ||
| JP7640810B2 (ja) | アルミニウム除去方法及び、金属回収方法 | |
| JP7627436B2 (ja) | 金属カドミウムの製造方法 | |
| JP2018197368A (ja) | 塩素浸出方法、電気ニッケルの製造方法 | |
| WO2025062730A1 (ja) | 金属回収方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 16803094 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2986557 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 15577521 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 20177037128 Country of ref document: KR Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2016803094 Country of ref document: EP |