WO2015162838A1 - Électrode positive pour pile rechargeable non aqueuse, et pile rechargeable non aqueuse - Google Patents
Électrode positive pour pile rechargeable non aqueuse, et pile rechargeable non aqueuse Download PDFInfo
- Publication number
- WO2015162838A1 WO2015162838A1 PCT/JP2015/001140 JP2015001140W WO2015162838A1 WO 2015162838 A1 WO2015162838 A1 WO 2015162838A1 JP 2015001140 W JP2015001140 W JP 2015001140W WO 2015162838 A1 WO2015162838 A1 WO 2015162838A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- positive electrode
- active material
- electrode active
- secondary battery
- aqueous secondary
- Prior art date
Links
Images
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/48—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides
- H01M4/50—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of manganese
- H01M4/505—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of manganese of mixed oxides or hydroxides containing manganese for inserting or intercalating light metals, e.g. LiMn2O4 or LiMn2OxFy
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/48—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides
- H01M4/52—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of nickel, cobalt or iron
- H01M4/525—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of nickel, cobalt or iron of mixed oxides or hydroxides containing iron, cobalt or nickel for inserting or intercalating light metals, e.g. LiNiO2, LiCoO2 or LiCoOxFy
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/58—Selection of substances as active materials, active masses, active liquids of inorganic compounds other than oxides or hydroxides, e.g. sulfides, selenides, tellurides, halogenides or LiCoFy; of polyanionic structures, e.g. phosphates, silicates or borates
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/10—Energy storage using batteries
Definitions
- the present invention relates to a positive electrode for a non-aqueous secondary battery and a non-aqueous secondary battery.
- the present invention has been made in view of such circumstances, and it is an object to provide a positive electrode for a non-aqueous secondary battery and a non-aqueous secondary battery that can more effectively suppress a rapid voltage drop at the time of a short circuit. To do.
- the present inventor has developed a positive electrode for a non-aqueous secondary battery with a smaller voltage drop by devising the shape of the second positive electrode active material having a low charge / discharge potential such as LiFePO 4 .
- the positive electrode for a non-aqueous secondary battery according to the present invention is a positive electrode for a non-aqueous secondary battery having a first positive electrode active material and a second positive electrode active material having a charge / discharge potential lower than that of the first positive electrode active material.
- the average length of the long portion of the second positive electrode active material is L1
- the ratio of L1 / L2 is L2 when the average length of the short width portion of the second positive electrode active material is L2
- the aspect ratio is 1.5 or more.
- the nonaqueous secondary battery including the positive electrode for a nonaqueous secondary battery according to the present invention can more effectively suppress a rapid voltage drop at the time of a short circuit.
- the positive electrode for a non-aqueous secondary battery and the non-aqueous secondary battery according to an embodiment of the present invention will be described in detail.
- the positive electrode for a non-aqueous secondary battery of the present invention has a first positive electrode active material and a second positive electrode active material having a charge / discharge potential lower than that of the first positive electrode active material. Since the charge / discharge potential of the second positive electrode active material is lower than that of the first positive electrode active material, the resistance is high, and the movement speed of electrons and metal ions in the second positive electrode active material is slow.
- the shape of the second positive electrode active material has an aspect ratio of 1.5 or more. As shown in the examples described later, when the aspect ratio of the second positive electrode active material is 1.5 or more, the voltage drop at the time of short circuit is smaller than when the aspect ratio is less than 1.5. The reason is not clear, but it is thought as follows.
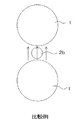
- FIG. 1A is an explanatory view showing the relationship between the first positive electrode active material 1 and the second positive electrode active material 2a of the present invention, and shows a case where the aspect ratio of the second positive electrode active material 2a is 1.5 or more.
- FIG. 1B is an explanatory diagram showing the relationship between the first positive electrode active material 1 and the second positive electrode active material 2b of the comparative example, and shows a case where the aspect ratio of the second positive electrode active material 2b is less than 1.5.
- the second positive electrode active materials 2 a and 2 b are both interposed between the first positive electrode active materials 1.
- the second positive electrode active material 2a forms particles (FIG. 1A) having an aspect ratio of 1.5 or more, spherical particles (FIG.
- the shape is flatter than the formed second positive electrode active material 2b.
- the flat second positive electrode active material 2a and the spherical second positive electrode active material 2b have the same mass per particle, the flat second positive electrode active material 2a is more than the spherical second positive electrode active material 2b.
- the shielding area which shields between the 1st positive electrode active materials 1 is large. Therefore, the contact between the first positive electrode active materials can be effectively prevented.
- the flat second positive electrode active material 2a suppresses the movement speed of more electrons and metal ions than the spherical second positive electrode active material 2b. Therefore, a rapid voltage drop in the positive electrode can be effectively suppressed.
- the average length L1 of the long part of the second positive electrode active material is obtained by measuring the average length of the longest part of the second positive electrode active material in cross-sectional observation, and measuring the length of the second positive electrode active material in the positive electrode. It is obtained by calculating the average.
- the average length L2 of the short width portion of the second positive electrode active material is measured by measuring the length of the longest portion in the direction orthogonal to the long direction of the long portion in the cross-sectional observation. It is obtained by calculating the average of the length of the positive electrode active material.
- Cross-sectional observation is based on an image obtained by measuring the cross section of the positive electrode of the present invention with a scanning electron microscope (SEM), transmission electron microscope (TEM), electron beam backscatter diffraction (EBSD), or the like. Good. You may analyze the said image using image analysis software.
- SEM scanning electron microscope
- TEM transmission electron microscope
- EBSD electron beam backscatter diffraction
- the “aspect ratio of the second positive electrode active material” is the ratio between the average length L1 of the long portion of the second positive electrode active material and the average length L2 of the short width portion of the second positive electrode active material ( L1 / L2).
- the aspect ratio of the second positive electrode active material is 1.5 or more.
- the aspect ratio of the second positive electrode active material is preferably 3 or more, more preferably 5 or more, and particularly preferably 8 or more.
- the shape of the second positive electrode active material is observed, for example, with a positive electrode scanning electron microscope (SEM), a transmission electron microscope (TEM), or the like.
- SEM positive electrode scanning electron microscope
- TEM transmission electron microscope
- the specific shape of the second positive electrode active material is often observed as a planar shape such as a rectangular shape, a needle shape, a fiber shape, an elliptical shape, or the like.
- the rectangular shape means a shape in which the average length L1 of the long portion of the second positive electrode active material is longer than the average length L2 of the short width portion, and the ratio of L1 / L2 is 1.5 or more.
- the needle shape is a shape in which the average length L1 of the long portion is longer than the rectangular shape with respect to the average length L2 of the short width portion, and the ratio of L1 / L2 exceeds 3 and is 10 or less.
- the fiber shape refers to a shape in which the average length L1 of the long portion is longer than the needle shape with respect to the average length L2 of the short width portion, and the L1 / L2 ratio exceeds 10 and is large.
- the elliptical shape refers to a circle having a major axis and a minor axis, the major axis corresponding to the average length of the long part, and the minor axis corresponding to the average length of the short part.
- the second positive electrode active material observed in various shapes as described above has various shapes in three dimensions, for example, a rectangular parallelepiped shape, a plate shape, a needle shape, a fiber shape, and the like. Often has the shape of
- the average length L1 of the long portion of the second positive electrode active material is preferably 0.1 ⁇ m or more, more preferably 1 ⁇ m or more, and most preferably 5 ⁇ m or more.
- the average length L1 of the long portion of the second positive electrode active material is preferably 10 ⁇ m or less. If the average length L1 of the long portion of the second positive electrode active material is too short, the shielding area of the second positive electrode active material blocking the first positive electrode active material is small, and the effect of suppressing the voltage drop may be reduced. If the average length L1 of the long portion of the second positive electrode active material is too long, the second positive electrode active material may be destroyed during electrode pressing.
- the second positive electrode active material is preferably dispersed between the first positive electrode active material.
- the shielding area which shields between the 1st positive electrode active materials of a 2nd positive electrode active material becomes large, and can reduce the voltage drop at the time of a short circuit.
- primary particles are preferably aggregated to form secondary particles.
- the average particle diameter of the secondary particles of the first positive electrode active material is preferably 1 ⁇ m or more and 10 ⁇ m or less, more preferably 3 ⁇ m or more and 8 ⁇ m or less, and most preferably 4 ⁇ m or more and 7 ⁇ m or less.
- the average particle size of the secondary particles of the first positive electrode active material is too small, the specific surface area of the first positive electrode active material is increased, side reaction with the electrolyte is increased, and the life may be deteriorated. .
- the average particle diameter of the secondary particles of the first positive electrode active material in this specification means the average value of the particle diameters of the secondary particles of the first positive electrode active material when measured by cross-sectional observation using an SEM or the like. To do.
- the ratio of L1 to L3 is 0. It is good that it is 0.01 or more and 10 or less, more preferably 0.1 or more and 5 or less, and most preferably 0.15 or more and 1 or less. If the ratio of L1 to L3 (L1 / L3) is too small, the shielding area of the second positive electrode active material blocking between the first positive electrode active materials is reduced, and the effect of suppressing the voltage drop at the time of short circuit may be reduced. There is. When the ratio of L1 to L3 (L1 / L3) is excessive, the second positive electrode active material may be easily broken.
- the second positive electrode active material is oriented between the adjacent first positive electrode active materials in a direction perpendicular to a straight line connecting the centers of the adjacent first positive electrode active materials.
- the second positive electrode active material can be blocked over a wide area while the second positive electrode active material is suppressed to the minimum amount. The voltage drop at the time of a short circuit can be suppressed effectively.
- the second positive electrode active material when the total mass of the first positive electrode active material and the second positive electrode active material is 100% by mass, the second positive electrode active material is more than 24.5% by mass and 35% by mass. It may be the following. Furthermore, it is preferable that it exceeds 24.5 mass% and is 28.7 mass% or less. If the second positive electrode active material is too small, the effect of reducing the voltage drop at the time of a short circuit due to the addition of the second positive electrode active material may be reduced. When the second positive electrode active material exceeds 35% by mass, the battery capacity and input / output characteristics of the entire positive electrode may be reduced.
- the first positive electrode active material is a material that functions as a positive electrode active material of a non-aqueous secondary battery.
- a 1st positive electrode active material what is necessary is just to employ
- a material that functions as a positive electrode active material of a lithium ion secondary battery may be used as the first positive electrode active material.
- a positive electrode active material that can occlude and release lithium ions can be used as the first positive electrode active material.
- Specific examples of the first positive electrode active material include a lithium metal composite oxide.
- the compound represented hereinafter sometimes referred to as “NCM”) is preferred.
- 0 ⁇ b ⁇ 1, 0 ⁇ c ⁇ 1, 0 ⁇ d ⁇ 1, and at least one of b, c, d is 0 ⁇ b ⁇ 80 /
- the ranges are 100, 0 ⁇ c ⁇ 70/100, 10/100 ⁇ d ⁇ 1, 10/100 ⁇ b ⁇ 68/100, 12/100 ⁇ c ⁇ 60/100, 20/100 ⁇ d.
- ⁇ 68/100 is more preferable, 25/100 ⁇ b ⁇ 60/100, 15/10 ⁇ C ⁇ 50/100, 25/100 ⁇ d ⁇ 60/100 are more preferable, and 1/3 ⁇ b ⁇ 50/100, 20/100 ⁇ c ⁇ 1/3, 30/100 ⁇ d.
- a is preferably in the range of 0.5 ⁇ a ⁇ 1.5, more preferably in the range of 0.7 ⁇ a ⁇ 1.3, still more preferably in the range of 0.9 ⁇ a ⁇ 1.2, A range of 1 ⁇ a ⁇ 1.1 is particularly preferable.
- the first positive electrode active material may be a lithium metal composite oxide having a spinel structure.
- the lithium metal composite oxide having a spinel structure has a general formula: Li x (A y Mn 2-y ) O 4 (A is Ca, Mg, S, Si, Na, K, Al, P, Ga, Ge) It is preferable that at least one element selected and at least one metal element selected from transition metal elements, 0 ⁇ x ⁇ 2.2, 0 ⁇ y ⁇ 1).
- the transition metal element that can constitute A in the general formula is, for example, at least one element selected from Fe, Cr, Cu, Zn, Zr, Ti, V, Mo, Nb, W, La, Ni, and Co. There should be.
- a specific example of the lithium metal composite oxide having a spinel structure is preferably at least one selected from LiMn 2 O 4 and LiNi 0.5 Mn 1.5 O 4 .
- the lithium metal composite oxide may contain a solid solution composed of a mixture of a layered rock salt structure and a spinel such as LiMn 2 O 4 .
- the second positive electrode active material is a material that can function as a positive electrode active material of a non-aqueous secondary battery and has a lower charge / discharge potential than the first positive electrode active material.
- the charge / discharge potential of the second positive electrode active material is lower than the charge / discharge potential of the first positive electrode active material.
- the positive electrode active material plays a role of charge / discharge of the positive electrode.
- the first positive electrode active material is a lithium metal composite oxide
- a lithium phosphate material or a lithium silicate material can be specifically used as the second positive electrode active material.
- lithium phosphate materials are preferred. This is because the lithium phosphate material has an olivine structure, and the positive electrode including this material recovers its voltage after a voltage drop due to a short circuit.
- Lithium phosphate-based materials are represented by the general formula: LiM h PO 4 (M is Mn, Fe, Co, Ni, Cu, Mg, Zn, V, Ca, Sr, Ba, Ti, Al, Si, B, Te and Mo.
- M is Mn, Fe, Co, Ni, Cu, Mg, Zn, V, Ca, Sr, Ba, Ti, Al, Si, B, Te and Mo.
- a material represented by at least one selected element, 0 ⁇ h ⁇ 2) may be mentioned.
- lithium phosphate materials that can be used as the second positive electrode active material include LiFePO 4 , LiMnPO 4 , LiVPO 4 , LiNiPO 4 , LiCoPO 4 , LiTePO 4 , LiV 2/3 PO 4 , LiFe 2/3. PO 4 and LiMn 7/8 Fe 1/8 PO 4 can be mentioned.
- LiFePO 4 is particularly preferable. The reason is as follows. LiFePO 4 exhibits a relatively flat discharge curve during discharge. Then, even if the positive electrode and the negative electrode of the lithium ion secondary battery are short-circuited and a sudden discharge occurs, a sudden potential difference associated with the discharge does not occur at the location where LiFePO 4 exists. Therefore, it is difficult to induce charge transfer from other parts in the electrode, and the occurrence of overcurrent can be suppressed. As a result, the heat generation of the secondary battery can be suitably suppressed.
- the lithium silicate-based material that can be used as the second positive electrode active material has a composition formula: Li 2 + ab Ab M 1- ⁇ M′ ⁇ Si 1 + ⁇ O 4 + c (where A is Na, K, Rb, and Is at least one element selected from the group consisting of Cs, M is at least one element selected from the group consisting of Fe, Co, Ni and Mn, and M ′ is Mg, Ca, Al, Nb. And at least one element selected from the group consisting of Ti, Cr, Cu, Zn, Zr, V, Mo and W. Each subscript is as follows: 0 ⁇ ⁇ ⁇ 0.2, 0 ⁇ ⁇ .
- the above composition formula indicates the basic composition of the lithium silicate material.
- a part of Li, A, M, M ′, Si, and O in the above composition formula may be substituted with another element. In the case of substitution with other elements, it is preferably performed within a range that does not adversely affect the capacity.
- Lithium silicate materials having a composition slightly deviating from the above composition formula due to unavoidable loss of Li, A, M, M ′, Si or O and oxidation of the compound are also included.
- the lithium silicate-based material include Li 2 FeSiO 4 , Li 2 MnSiO 4, Li 2 CoSiO 4 , and Li 2 NiSiO 4 .
- the positive electrode active material is preferably made of a lithium phosphate material.
- the second positive electrode active material it is preferable to employ a material whose surface is coated with a carbon material.
- the carbon material is preferably conductive, and for example, acetylene black (AB), ketjen black (KB), carbon black, carbon nanotube, graphene, carbon material fiber, graphite, or the like can be used.
- the positive electrode has the first positive electrode active material and the second positive electrode active material.
- the positive electrode preferably includes a positive electrode active material layer having the first positive electrode active material and the second positive electrode active material, and a current collector covered with the positive electrode active material layer.
- the total mass of the first positive electrode active material and the second positive electrode active material contained in the positive electrode active material layer is 85 mass% or more and 96 mass% or less. It is preferable.
- a positive electrode having this positive electrode active material layer is used for a battery, a sufficient battery capacity can be exhibited.
- the positive electrode active material layer includes the first positive electrode active material and the second positive electrode active material, and may further contain an additive as necessary.
- the additive include a conductive additive, a binder, and a dispersant.
- Conductive aid is added to increase the conductivity of the electrode. Therefore, the conductive auxiliary agent may be added arbitrarily when the electrode conductivity is insufficient, and may not be added when the electrode conductivity is sufficiently excellent.
- the conductive auxiliary agent may be any chemically inert electronic high conductor, such as carbon black, graphite, acetylene black, ketjen black (registered trademark), or vapor grown carbon fiber (Vapor Grown Carbon). Fiber: VGCF) and various metal particles are exemplified. These conductive assistants can be added to the positive electrode active material layer alone or in combination of two or more.
- the shape of the conductive auxiliary agent is not particularly limited, but it is preferable that the average particle diameter is small in view of its role.
- a preferable average particle diameter of the conductive auxiliary agent is preferably 10 ⁇ m or less, and more preferably in the range of 0.01 to 1 ⁇ m.
- the average particle diameter of a conductive support agent is the value of D50 at the time of measuring with a general particle size distribution measuring apparatus.
- the blending amount of the conductive additive in the positive electrode active material layer is preferably in the range of 0.5 to 10% by mass, more preferably in the range of 1 to 7% by mass, and particularly in the range of 2 to 5% by mass. preferable.
- the binder serves to bind the positive electrode active material and the conductive additive to the surface of the current collector.
- the binder include fluorine-containing resins such as polyvinylidene fluoride, polytetrafluoroethylene, and fluororubber, thermoplastic resins such as polypropylene and polyethylene, imide resins such as polyimide and polyamideimide, and alkoxysilyl group-containing resins. be able to.
- hydrophilic group of the polymer having a hydrophilic group examples include a carboxyl group, a sulfo group, a silanol group, an amino group, a hydroxyl group, and a phosphate group.
- Specific examples of the polymer having a hydrophilic group include polyacrylic acid, carboxymethylcellulose, polymethacrylic acid, and poly (p-styrenesulfonic acid).
- the amount of the binder in the positive electrode active material layer is preferably in the range of 0.5 to 10% by mass, more preferably in the range of 1 to 7% by mass, and particularly in the range of 2 to 5% by mass. preferable. If the blending amount of the binder is too small, the moldability of the layer may be lowered when the composition is used as a positive electrode active material layer. Moreover, when there are too many compounding quantities of a binder, since the quantity of the positive electrode active material in a positive electrode active material layer reduces, it is unpreferable.
- a current collector refers to a chemically inert electronic high conductor that keeps a current flowing through an electrode during discharge or charging of a lithium ion secondary battery.
- the current collector at least one selected from silver, copper, gold, aluminum, magnesium, tungsten, cobalt, zinc, nickel, iron, platinum, tin, indium, titanium, ruthenium, tantalum, chromium, molybdenum, and stainless steel Examples of such a metal material can be given.
- the current collector may be covered with a known protective layer.
- the current collector can take the form of a foil, a sheet, a film, a line, a bar, or the like. Therefore, metal foils, such as copper foil, nickel foil, aluminum foil, stainless steel foil, can be used suitably as a collector.
- the thickness is preferably in the range of 10 ⁇ m to 100 ⁇ m.
- Step a) is a step of producing a dispersion by mixing the first positive electrode active material, the second positive electrode active material having a lower charge / discharge potential than the first positive electrode active material, the additive, and the solvent.
- the solvent examples include N-methyl-2-pyrrolidone (hereinafter sometimes abbreviated as “NMP”), dimethylformamide, dimethylacetamide, methanol, acetone, methyl ethyl ketone, methyl isobutyl ketone, cyclohexanone, methyl acetate, acetic acid. Examples thereof include ethyl and tetrahydrofuran. These solvents may be used alone or in combination of two or more.
- the dispersion liquid of a) process consists of solid content other than a solvent and a solvent.
- Solid content other than a solvent means additives, such as a 1st positive electrode active material, a 2nd positive electrode active material, and a binder used as needed, a conductive support agent, and a dispersing agent.
- the blending amount of solids other than the solvent is preferably in the range of 30 to 90% by mass, more preferably in the range of 50 to 80% by mass. The range of 60 to 70% by mass is particularly preferable.
- each component may be added simultaneously or sequentially and mixed with a mixing device.
- the mixing apparatus include a mixing stirrer, a ball mill, a sand mill, a bead mill, a disperser, an ultrasonic disperser, a homogenizer, a homomixer, a planetary mixer, and a planetary stirring deaerator. What is necessary is just to set the mixing speed in a mixing apparatus suitably the speed
- Step b) is a step of forming a positive electrode active material layer by applying the dispersion liquid produced in step a) to a current collector and removing the solvent contained in the dispersion liquid.
- Specific methods for applying the dispersion liquid to the current collector include conventionally known methods such as a roll coating method, a dip coating method, a doctor blade method, a spray coating method, and a curtain coating method.
- a specific method for removing the solvent contained in the dispersion liquid there is a method of drying the dispersion liquid under a heating condition and / or a reduced pressure condition and removing the solvent contained in the dispersion liquid as a gas. it can.
- a conventionally known compressor may be used as the compression device.
- Specific examples of the compression device include a roll press, a vacuum press, a hydraulic press, and a hydraulic press.
- Examples of the compression pressure in the compression apparatus include a range of 1 to 5000 kN.
- the positive electrode of the present invention can be obtained simply.
- a positive electrode capable of effectively suppressing a voltage drop at the time of a short circuit can be manufactured.
- the second positive electrode active material can be interposed between the first positive electrode active materials by a simple method of mixing the first positive electrode active material and the second positive electrode active material.
- the non-aqueous secondary battery can be manufactured by using the positive electrode of the present invention.
- the non-aqueous secondary battery includes a positive electrode, a negative electrode, a separator, and an electrolytic solution as battery components.
- the negative electrode has a current collector and a negative electrode active material layer bound to the surface of the current collector.
- the negative electrode active material layer includes a negative electrode active material and, if necessary, a binder and / or a conductive aid.
- As the current collector, the binder, and the conductive additive those described for the positive electrode may be adopted. Further, styrene-butadiene rubber may be employed as a binder for the negative electrode active material layer.
- Examples of the negative electrode active material include a carbon-based material capable of inserting and extracting lithium, an element that can be alloyed with lithium, a compound having an element that can be alloyed with lithium, a polymer material, and the like.
- the carbon-based material examples include non-graphitizable carbon, natural graphite, artificial graphite, coke, graphite, glassy carbon, organic polymer compound fired body, carbon fiber, activated carbon, or carbon black.
- the organic polymer compound fired body refers to a material obtained by firing and carbonizing a polymer material such as phenols and furans at an appropriate temperature.
- elements that can be alloyed with lithium include Na, K, Rb, Cs, Fr, Be, Mg, Ca, Sr, Ba, Ra, Ti, Ag, Zn, Cd, Al, Ga, In, Si. , Ge, Sn, Pb, Sb, Bi can be exemplified, and Si or Sn is particularly preferable.
- Examples of compounds having elements that can be alloyed with lithium include ZnLiAl, AlSb, SiB 4 , SiB 6 , Mg 2 Si, Mg 2 Sn, Ni 2 Si, TiSi 2 , MoSi 2 , CoSi 2 , NiSi 2 , CaSi 2, CrSi 2, Cu 5 Si, FeSi 2, MnSi 2, NbSi 2, TaSi 2, VSi 2, WSi 2, ZnSi 2, SiC, Si 3 N 4, Si 2 N 2 O, SiO v (0 ⁇ v ⁇ 2), SnO w (0 ⁇ w ⁇ 2), SnSiO 3 , LiSiO 2 or LiSnO, and SiO x (0.3 ⁇ x ⁇ 1.6) is particularly preferable.
- examples of the compound having an element capable of alloying with lithium include tin compounds such as tin alloys (Cu—Sn alloy, Co—Sn alloy, etc.).
- the negative electrode active material may have Si element or Si compound.
- Si element or Si compound has a large irreversible capacity.
- Li ions are released from the second positive electrode active material having a low charge / discharge potential before the first positive electrode active material. Li ions released from the second positive electrode active material are occluded by the Si element or Si compound as the negative electrode active material, and make up for the irreversible capacity of the Si element or Si compound. For this reason, Li ions released from the first positive electrode active material after the second positive electrode active material function as a reversible proton transmission medium as the reversible capacity of the Si element or Si compound. Therefore, even if Si element or Si compound having a large irreversible capacity is the negative electrode active material, the original battery capacity of the first positive electrode active material itself can be exhibited.
- polymer material used for the negative electrode active material include polyacetylene and polypyrrole.
- the separator separates the positive electrode and the negative electrode and allows lithium ions to pass through while preventing a short circuit of current due to contact between the two electrodes.
- the separator include a porous film using one or more synthetic resins such as polytetrafluoroethylene, polypropylene, and polyethylene, or a ceramic porous film.
- the electrolytic solution has a non-aqueous solvent and an electrolyte dissolved in the non-aqueous solvent.
- cyclic esters examples include ethylene carbonate, propylene carbonate, butylene carbonate, gamma butyrolactone, vinylene carbonate, 2-methyl-gamma butyrolactone, acetyl-gamma butyrolactone, and gamma valerolactone.
- chain esters include dimethyl carbonate, diethyl carbonate, dibutyl carbonate, dipropyl carbonate, ethyl methyl carbonate, propionic acid alkyl ester, malonic acid dialkyl ester, and acetic acid alkyl ester.
- ethers examples include tetrahydrofuran, 2-methyltetrahydrofuran, 1,4-dioxane, 1,2-dimethoxyethane, 1,2-diethoxyethane, and 1,2-dibutoxyethane. These nonaqueous solvents may be used alone or in combination with the electrolyte.
- Examples of the electrolyte include lithium salts such as LiClO 4 , LiAsF 6 , LiPF 6 , LiBF 4 , LiCF 3 SO 3 , and LiN (CF 3 SO 2 ) 2 .
- a lithium salt such as LiClO 4 , LiPF 6 , LiBF 4 , LiCF 3 SO 3 in a nonaqueous solvent such as ethylene carbonate, dimethyl carbonate, propylene carbonate, and diethyl carbonate.
- a solution dissolved at a concentration of about 1 / l can be exemplified.
- a separator is sandwiched between a positive electrode and a negative electrode to form an electrode body.
- the electrode body may be either a stacked type in which the positive electrode, the separator and the negative electrode are stacked, or a wound type in which the positive electrode, the separator and the negative electrode are sandwiched.
- Non-aqueous secondary batteries can be mounted on vehicles.
- a lithium ion secondary battery maintains a large charge / discharge capacity and has excellent cycle performance, and thus a vehicle equipped with the lithium ion secondary battery is a high-performance vehicle.
- the vehicle may be a vehicle that uses electric energy from a battery as a whole or a part of a power source.
- a vehicle that uses electric energy from a battery as a whole or a part of a power source.
- an electric vehicle a hybrid vehicle, a plug-in hybrid vehicle, a hybrid railway vehicle, an electric forklift, an electric wheelchair, and an electric assist.
- Bicycles and electric motorcycles are examples.
- the charge / discharge potential of LiNi 5/10 Co 2/10 Mn 3/10 O 2 was 3.9 V on the basis of Li, and the charge / discharge potential of LiFePO 4 was 3.5 V on the basis of Li.
- AB acetylene black
- PVDF polyvinylidene fluoride
- Step b) An aluminum foil having a thickness of 20 ⁇ m was prepared as a current collector.
- the dispersion produced in step a) was placed on the surface of the aluminum foil, and applied using a doctor blade so that the dispersion became a film.
- the aluminum foil coated with the dispersion was dried at 80 ° C. for 20 minutes, whereby NMP was removed by volatilization, and a positive electrode active material layer was formed on the aluminum foil surface.
- FIG. 2 is an explanatory view showing a cross section of the positive electrode.
- FIG. 2 is a drawing drawn based on a SEM cross-sectional photograph.
- LiNi 5/10 Co 2/10 Mn 3/10 O 2 as the first positive electrode active material had primary particles aggregated to form secondary particles.
- the average particle diameter L3 of the secondary particles of LiNi 5/10 Co 2/10 Mn 3/10 O 2 during cross-sectional observation was 6 ⁇ m.
- LiFePO 4 whose surface is coated with a carbon material as the second positive electrode active material has an average length L1 of the long portion of 1 ⁇ m and an average length L2 of the short width portion of 0.3 ⁇ m during cross-sectional observation. It was needle-shaped particles.
- the average aspect ratio of the second positive electrode active material was 3.3. About half of the entire second positive electrode active material had an aspect ratio (L1 / L2) of 5 or more, and some of the second positive electrode active materials had an aspect ratio of less than 5. In the SEM cross-sectional photograph of the positive electrode, only the planar state of the cross section cut in one direction is observed, and the three-dimensional shape of the second positive electrode active material is not known. Even if the aspect ratio of the shape appearing in the cross section cut in one direction is small, the aspect ratio of the shape appearing in the cross section cut in the other direction may be large.
- some second positive electrode active materials are arranged in the gaps between the adjacent first positive electrode active materials, and are orthogonal to the straight line passing between the centers of the first positive electrode active materials.
- the second positive electrode active material was oriented in the direction to be.
- the ratio (L1 / L3) of the average length L1 of the long portion of the second positive electrode active material to the average particle size L3 of the secondary particles of the first positive electrode active material was 0.17.
- Example 1 Using the positive electrode of Example 1, a lithium ion secondary battery was produced as follows.
- the negative electrode was produced as follows. SiO x (0.3 ⁇ x ⁇ 1.6) and natural graphite were used as the negative electrode active material. Polyamideimide (PAI) was used as a binder. Acetylene black (AB) was used as a conductive aid. SiO x (0.3 ⁇ x ⁇ 1.6): natural graphite: AB: PAI is mixed at a mass ratio of 32: 50: 8: 10, and NMP is added to prepare a slurry-like negative electrode mixture. A liquid was obtained. The negative electrode mixture preparation liquid was applied to the surface of an aluminum foil having a thickness of 20 ⁇ m as a negative electrode current collector, and then, similarly to the positive electrode of Example 1, the negative electrode was obtained through a drying step and a compression step.
- PAI Polyamideimide
- AB Acetylene black
- Example 2 Using the positive electrode of Example 1 and the negative electrode, a laminate type lithium ion secondary battery was produced as follows.
- a rectangular sheet (50 ⁇ 90 mm, thickness 25 ⁇ m) made of a resin film having a three-layer structure of polypropylene / polyethylene / polypropylene as a separator was sandwiched between the positive electrode and the negative electrode to form an electrode plate group.
- the electrode plate group was covered with a set of two laminated films, and the three sides were sealed, and then an electrolyte solution was injected into the bag-like laminated film.
- As an electrolytic solution 1 mol of LiPF 6 was added to a solvent in which fluoroethylene carbonate (FEC), ethylene carbonate (EC), ethyl methyl carbonate (EMC) and dimethyl carbonate (DMC) were mixed at a volume ratio of 4: 26: 30: 40.
- the positive electrode and the negative electrode have a tab that can be electrically connected to the outside, and a part of the tab extends to the outside of the laminated lithium ion secondary battery.
- Comparative Example 1 The positive electrode of Comparative Example 1 is the same as Example 1 except that a spherical second positive electrode active material is used.
- the average length L1 of the long portion of the second positive electrode active material is 2 ⁇ m
- the average length L2 of the short width portion is 2 ⁇ m
- the aspect ratio (L1 / L2 ) was 1.
- the lithium ion secondary battery was charged at a constant voltage until stabilized at a potential of 4.5V.
- the charged lithium ion secondary battery (the discharge capacity is expected to be about 3.6 Ah) was placed on a constraining plate having a hole with a diameter of 20 mm.
- a restraint plate was placed on a press machine with a nail attached to the top. Until the nail penetrates the lithium ion secondary battery on the restraining plate and the tip of the nail is located inside the hole of the restraining plate, the nail is 20 mm / sec. Moved at a speed of. The surface temperature of the battery after penetrating the nail was measured.
- FIG. 3 shows a voltage profile in the nail penetration test.
- the horizontal axis represents the time (seconds) of the test elapsed
- the vertical axis represents the voltage (potential difference between the positive electrode and the negative electrode).
- the voltage of the battery of Example 1 dropped instantaneously from 4.5V to 3.8V by battery nail penetration.
- the voltage of the battery of Comparative Example 1 dropped instantaneously from 4.5V to 3.4V.
- the battery of Example 1 had less voltage drop during the nail penetration test than the battery of Comparative Example 1.
- the voltage returned to 4 V after the voltage drop.
- the battery using the needle-shaped second positive electrode active material like the battery of Example 1 had less voltage drop at the time of short circuit than when the spherical second positive electrode active material was used (Comparative Example 1). . It was found that the battery including the positive electrode using the second positive electrode active material having an aspect ratio of 1.5 or more has a small voltage drop and a small amount of heat generation.
- a lithium ion secondary battery was produced in the same manner as the lithium ion secondary battery of Example 1.
- the lithium ion secondary battery was subjected to a nail penetration test in the same manner as in Evaluation Example 1. Immediately after nail penetration, the voltage of the battery dropped to O (zero), and then the voltage did not return.
Landscapes
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Inorganic Chemistry (AREA)
- Battery Electrode And Active Subsutance (AREA)
Abstract
La présente invention concerne une électrode positive pour une pile rechargeable à électrolyte non aqueux qui a un premier matériau actif d'électrode positive et un deuxième matériau actif d'électrode positive qui a un potentiel de charge/décharge plus faible que celui du premier matériau actif d'électrode positive. Le facteur de forme L1/L2 est de 1,5 ou plus, où L1 représente la longueur moyenne d'une partie longue du deuxième matériau actif d'électrode positive et L2 représente la longueur moyenne d'une partie de largeur courte du deuxième matériau actif d'électrode positive. La présente invention permet de réduire au minimum une chute rapide de tension lors d'un court-circuit.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2014091341A JP6132164B2 (ja) | 2014-04-25 | 2014-04-25 | 非水系二次電池用正極及び非水系二次電池 |
| JP2014-091341 | 2014-04-25 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2015162838A1 true WO2015162838A1 (fr) | 2015-10-29 |
Family
ID=54332021
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2015/001140 WO2015162838A1 (fr) | 2014-04-25 | 2015-03-04 | Électrode positive pour pile rechargeable non aqueuse, et pile rechargeable non aqueuse |
Country Status (2)
| Country | Link |
|---|---|
| JP (1) | JP6132164B2 (fr) |
| WO (1) | WO2015162838A1 (fr) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN112534623A (zh) * | 2018-10-30 | 2021-03-19 | 松下知识产权经营株式会社 | 二次电池 |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6848199B2 (ja) * | 2016-04-06 | 2021-03-24 | 住友金属鉱山株式会社 | 非水系電解質二次電池用正極材料、該正極材料を用いた非水系電解質二次電池、および非水系電解質二次電池用正極材料の製造方法。 |
| JP7026433B2 (ja) * | 2016-09-02 | 2022-02-28 | 株式会社豊田自動織機 | 正極及びリチウムイオン二次電池 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2011076820A (ja) * | 2009-09-30 | 2011-04-14 | Hitachi Vehicle Energy Ltd | リチウム二次電池及びリチウム二次電池用正極 |
| JP2012256591A (ja) * | 2011-05-18 | 2012-12-27 | Fuji Heavy Ind Ltd | 蓄電デバイス及び蓄電デバイス用正極 |
| JP2013120724A (ja) * | 2011-12-08 | 2013-06-17 | Sony Corp | 電極、二次電池、電池パック、電動車両、電力貯蔵システム、電動工具および電子機器 |
| JP2015049997A (ja) * | 2013-08-30 | 2015-03-16 | 住友大阪セメント株式会社 | リチウムイオン電池用電極材料とその製造方法及びリチウムイオン電池用電極並びにリチウムイオン電池 |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5428125B2 (ja) * | 2005-11-24 | 2014-02-26 | 日産自動車株式会社 | 非水電解質二次電池用正極活物質、および、これを用いた非水電解質二次電池 |
| WO2008088180A1 (fr) * | 2007-01-18 | 2008-07-24 | Lg Chem, Ltd. | Matière active de cathode et batterie secondaire comprenant cette matière |
| JP5558109B2 (ja) * | 2007-01-24 | 2014-07-23 | エルジー・ケム・リミテッド | 安全性に優れた二次電池 |
| JP2012022995A (ja) * | 2010-07-16 | 2012-02-02 | Tdk Corp | 活物質、これを含む電極、当該電極を備えるリチウム二次電池、及び活物質の製造方法 |
| JP5557715B2 (ja) * | 2010-12-06 | 2014-07-23 | 株式会社日立製作所 | リチウムイオン二次電池用正極材料およびその製造方法,リチウムイオン二次電池用正極活物質,リチウムイオン二次電池用正極,リチウムイオン二次電池 |
| JP5807599B2 (ja) * | 2012-03-27 | 2015-11-10 | Tdk株式会社 | 活物質及びリチウムイオン二次電池 |
| JP6233828B2 (ja) * | 2012-09-21 | 2017-11-22 | 国立研究開発法人産業技術総合研究所 | リチウムイオン電池用負極、該負極を具備するリチウムイオン電池 |
-
2014
- 2014-04-25 JP JP2014091341A patent/JP6132164B2/ja not_active Expired - Fee Related
-
2015
- 2015-03-04 WO PCT/JP2015/001140 patent/WO2015162838A1/fr active Application Filing
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2011076820A (ja) * | 2009-09-30 | 2011-04-14 | Hitachi Vehicle Energy Ltd | リチウム二次電池及びリチウム二次電池用正極 |
| JP2012256591A (ja) * | 2011-05-18 | 2012-12-27 | Fuji Heavy Ind Ltd | 蓄電デバイス及び蓄電デバイス用正極 |
| JP2013120724A (ja) * | 2011-12-08 | 2013-06-17 | Sony Corp | 電極、二次電池、電池パック、電動車両、電力貯蔵システム、電動工具および電子機器 |
| JP2015049997A (ja) * | 2013-08-30 | 2015-03-16 | 住友大阪セメント株式会社 | リチウムイオン電池用電極材料とその製造方法及びリチウムイオン電池用電極並びにリチウムイオン電池 |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN112534623A (zh) * | 2018-10-30 | 2021-03-19 | 松下知识产权经营株式会社 | 二次电池 |
| US20220013789A1 (en) * | 2018-10-30 | 2022-01-13 | Panasonic Intellectual Property Management Co., Ltd. | Secondary battery |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2015210928A (ja) | 2015-11-24 |
| JP6132164B2 (ja) | 2017-05-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| DE112012004170B4 (de) | Hermetisch abgedichtete Lithiumsekundärbatterie | |
| JP6213315B2 (ja) | 表面処理した正極活物質、分散剤及び溶剤を含む組成物 | |
| JP6638286B2 (ja) | リチウムイオン二次電池用正極及びリチウムイオン二次電池 | |
| JP5448555B2 (ja) | リチウムイオン二次電池用負極、それを用いたリチウムイオン二次電池、リチウムイオン二次電池用の負極作製用のスラリー、リチウムイオン二次電池用負極の製造方法 | |
| JP6720488B2 (ja) | 複数の正極活物質、導電助剤、結着剤及び溶剤を含む組成物の製造方法 | |
| DE102016123898A1 (de) | Lithiumionenbatteriekomponenten | |
| JP6811404B2 (ja) | 非水電解質二次電池 | |
| JPWO2019168035A1 (ja) | リチウムイオン二次電池用正極材料、正極活物質層、及びリチウムイオン二次電池 | |
| JP5861896B2 (ja) | 第1正極活物質、第2正極活物質、分散剤及び溶剤を含む組成物 | |
| JP6120087B2 (ja) | 集電体本体への保護層形成方法、リチウムイオン二次電池用集電体、リチウムイオン二次電池用正極及びリチウムイオン二次電池 | |
| JP6061143B2 (ja) | リチウムイオン二次電池用正極及びリチウムイオン二次電池 | |
| WO2015025466A1 (fr) | Batterie secondaire lithium-ion possédant une électrode positive qui comprend une couche de suppression d'emballement thermique sur la couche de matériau actif d'électrode positive | |
| JP6132164B2 (ja) | 非水系二次電池用正極及び非水系二次電池 | |
| JP6229333B2 (ja) | 非水電解質二次電池 | |
| WO2015040891A1 (fr) | Cellule secondaire au lithium-ion | |
| JP6136849B2 (ja) | 表面改質活物質及び高抵抗金属化合物を含む正極 | |
| JP6600938B2 (ja) | リチウムイオン二次電池及びその製造方法 | |
| JP6056685B2 (ja) | リチウムイオン二次電池用正極活物質の処理方法、リチウムイオン二次電池用正極活物質及びリチウムイオン二次電池 | |
| WO2014112329A1 (fr) | Électrode positive pour batteries secondaires au lithium-ion et batteries secondaires au lithium-ion | |
| JP6202191B2 (ja) | 第1正極活物質及び第2正極活物質を有する正極活物質層、並びに該正極活物質層を具備する正極の製造方法 | |
| JP6187824B2 (ja) | 第1正極活物質、第2正極活物質、導電助剤、結着剤及び溶剤を含む組成物の製造方法 | |
| KR20230104899A (ko) | 이차전지용 음극, 음극용 슬러리 및 음극의 제조 방법 | |
| JP6338089B2 (ja) | 活物質 | |
| JP6703751B2 (ja) | 正極活物質及び溶剤を含む組成物 | |
| JP6607388B2 (ja) | リチウムイオン二次電池用正極及びその製造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 15783772 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 15783772 Country of ref document: EP Kind code of ref document: A1 |