WO2012066879A1 - 非水電解液二次電池 - Google Patents
非水電解液二次電池 Download PDFInfo
- Publication number
- WO2012066879A1 WO2012066879A1 PCT/JP2011/073546 JP2011073546W WO2012066879A1 WO 2012066879 A1 WO2012066879 A1 WO 2012066879A1 JP 2011073546 W JP2011073546 W JP 2011073546W WO 2012066879 A1 WO2012066879 A1 WO 2012066879A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- carbon atoms
- group
- aqueous electrolyte
- general formula
- positive electrode
- Prior art date
Links
Images
Classifications
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/056—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes
- H01M10/0564—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes the electrolyte being constituted of organic materials only
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/48—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides
- H01M4/50—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of manganese
- H01M4/505—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of manganese of mixed oxides or hydroxides containing manganese for inserting or intercalating light metals, e.g. LiMn2O4 or LiMn2OxFy
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/052—Li-accumulators
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/052—Li-accumulators
- H01M10/0525—Rocking-chair batteries, i.e. batteries with lithium insertion or intercalation in both electrodes; Lithium-ion batteries
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/056—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes
- H01M10/0564—Accumulators with non-aqueous electrolyte characterised by the materials used as electrolytes, e.g. mixed inorganic/organic electrolytes the electrolyte being constituted of organic materials only
- H01M10/0566—Liquid materials
- H01M10/0567—Liquid materials characterised by the additives
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/10—Energy storage using batteries
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02T—CLIMATE CHANGE MITIGATION TECHNOLOGIES RELATED TO TRANSPORTATION
- Y02T10/00—Road transport of goods or passengers
- Y02T10/60—Other road transportation technologies with climate change mitigation effect
- Y02T10/70—Energy storage systems for electromobility, e.g. batteries
Definitions
- the present invention relates to a non-aqueous electrolyte secondary battery, and in particular, a non-aqueous electrolysis having a positive electrode using a lithium-containing metal oxide containing manganese as a positive electrode active material and a non-aqueous electrolyte containing a specific fluorosilane compound.
- the present invention relates to a liquid secondary battery.
- non-aqueous electrolyte secondary batteries various additives for non-aqueous electrolyte solutions have been proposed in order to improve the stability and electrical characteristics of non-aqueous electrolyte secondary batteries.
- 1,3-propane sultone see, for example, Patent Document 1
- vinyl ethylene carbonate see, for example, Patent Document 2
- vinylene carbonate see, for example, Patent Document 3
- 1,3-propane sultone for example, refer to Patent Document 4
- vinylene carbonate for example, refer to Patent Document 5
- vinyl ethylene carbonate for example, refer to Patent Document 6
- lithium cobaltate has been widely used as a positive electrode active material.
- cobalt which is a raw material
- it is an inexpensive metal material other than cobalt.
- Development of a positive electrode active material using a positive electrode has been carried out, and the use of an inexpensive positive electrode using such a positive electrode active material has rapidly spread.
- lithium-containing metal oxides containing manganese are inexpensive, they have excellent performance in terms of the output of lithium secondary batteries, but manganese tends to elute at high temperatures, and the capacity of lithium secondary batteries decreases when used repeatedly. There is a problem of doing.
- conventionally known additives for non-aqueous electrolytes as described above cannot provide a sufficient effect on a positive electrode using a lithium-containing metal oxide containing manganese as a positive electrode active material. There was a need for further improvements.
- JP 63-102173 A Japanese Patent Laid-Open No. 04-87156 Japanese Patent Laid-Open No. 05-74486 Japanese Patent Laid-Open No. 10-50342 US Pat. No. 5,626,981 JP 2001-6729 A JP 2002-134169 A US Patent Application Publication No. 2004/0007688 US Patent Application Publication No. 2006/0269843 US Patent Application Publication No. 2007/0243470 US Patent Application Publication No. 2009/0197167
- an object of the present invention is to suppress elution of manganese from the positive electrode active material in a non-aqueous electrolyte secondary battery using a lithium-containing metal oxide containing manganese as the positive electrode active material, and to store at high temperature or at high temperature.
- An object of the present invention is to provide a non-aqueous electrolyte secondary battery that can maintain a small internal resistance and a high electric capacity even after charging and discharging.
- the present inventors have found that the above object can be achieved by using a nonaqueous electrolytic solution containing a fluorosilane compound having a specific structure, and completed the present invention.
- the present invention relates to a non-aqueous electrolyte secondary battery having a negative electrode capable of removing and inserting lithium ions, a positive electrode having a lithium-containing compound as a positive electrode active material, and a non-aqueous electrolyte in which a lithium salt is dissolved in an organic solvent.
- the lithium-containing compound is a lithium-containing metal oxide containing manganese
- the non-aqueous electrolyte contains a fluorosilane compound represented by the following general formula (1).
- a battery is provided.
- R 1 to R 3 each independently represents a fluorine atom or an alkyl group having 1 to 8 carbon atoms, and R 4 represents an alkylene group having 1 to 8 carbon atoms or an ether group having 4 to 8 carbon atoms
- R 1 to R 3 each independently represents a fluorine atom or an alkyl group having 1 to 8 carbon atoms
- R 4 represents an alkylene group having 1 to 8 carbon atoms or an ether group having 4 to 8 carbon atoms
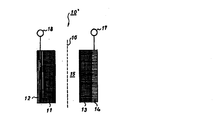
- FIG. 1 is a longitudinal sectional view schematically showing an example of the structure of a coin-type battery of the nonaqueous electrolyte secondary battery of the present invention.
- FIG. 2 is a schematic diagram showing a basic configuration of a cylindrical battery of the nonaqueous electrolyte secondary battery of the present invention.
- FIG. 3 is a perspective view showing the internal structure of the cylindrical battery of the nonaqueous electrolyte secondary battery of the present invention as a cross section.
- the present invention relates to a non-aqueous electrolyte secondary battery using a lithium-containing metal oxide containing manganese as a positive electrode active material, and a non-aqueous electrolyte containing the fluorosilane compound represented by the general formula (1). There is a feature in the place used. First, the positive electrode used in the present invention will be described.
- the positive electrode active material of the positive electrode used in the present invention is a lithium-containing metal oxide containing manganese which is a lithium-containing compound.
- the lithium-containing metal oxide containing manganese include a lithium manganese composite oxide and a compound obtained by substituting a part of the manganese atom of the lithium manganese composite oxide with another metal atom.
- the lithium-manganese composite oxide for example, LiMnO 2, LiMn 2 O 4 , Li 2 MnO 4, Li 2 MnO 3 and the like.
- a part of the manganese atom of the lithium manganese composite oxide is a transition metal atom other than the manganese atom, for example, aluminum, titanium, Compounds substituted with metal atoms such as vanadium, chromium, lithium, iron, cobalt, copper, zinc, magnesium, calcium, zirconium, niobium, such as LiNi 0.5 Mn 0.5 O 2 , LiNi 1/3 Co 1/3 Mn 1 / 5 O 2 , LiNi 0.5 Co 0.2 Mn 0.3 O 2 , LiMn 1.8 Ni 0.2 MnO 4 , LiMn 1.5 Ni 0.5 MnO 4 , LiMn 1.9 Mg 0.05 O 4 , Li 1.1 Mn 1.8 Mg 0.1 O 4 , Li 1.1 Mn 1.94 Mg 0.01 B 0.008 O 4 , Li 1.1 Mn 1.85 Al 0.05 O 4
- Li 1.1 Mn is excellent in performance as a positive electrode active material and has a large effect of preventing elution of manganese by the fluorosilane compound represented by the general formula (1).
- 1.8 Mg 0.1 O 4 Li 1.1 Mn 1.85 Al 0.05 O 4 , LiNi 1/3 Co 1/3 Mn 1/5 O 2 , LiNi 0.5 Co 0.2 Mn 0.3 O 2 are preferred.
- a negative electrode material such as the positive electrode active material, binder, conductive material and the like, which is slurried with a solvent, is applied to a current collector, dried, and rolled into a sheet shape as necessary. Used.
- the positive electrode active material binder examples include, but are not limited to, polyvinylidene fluoride, polytetrafluoroethylene, EPDM, SBR, NBR, fluororubber, and polyacrylic acid.
- the amount of the binder used is preferably 0.1 to 20 parts by mass, more preferably 0.5 to 10 parts by mass with respect to 100 parts by mass of the positive electrode active material.
- Examples of the conductive material for the positive electrode include graphite fine particles, carbon black such as acetylene black and ketjen black, amorphous carbon fine particles such as needle coke, and carbon nanofibers, but are not limited thereto.
- the amount of the conductive material used is preferably 0.01 to 20 parts by mass, more preferably 0.1 to 10 parts by mass with respect to 100 parts by mass of the positive electrode active material.
- As the solvent for forming a slurry an organic solvent or water that dissolves the binder is used.
- organic solvent examples include N-methylpyrrolidone, dimethylformamide, dimethylacetamide, methyl ethyl ketone, cyclohexanone, methyl acetate, methyl acrylate, diethyltriamine, NN-dimethylaminopropylamine, polyethylene oxide, tetrahydrofuran and the like.
- the amount of the solvent used is preferably 30 to 300 parts by mass, more preferably 50 to 200 parts by mass, with respect to 100 parts by mass of the positive electrode active material.
- aluminum, stainless steel, nickel-plated steel, or the like is used for the positive electrode current collector.
- the nonaqueous electrolytic solution used in the present invention contains a fluorosilane compound represented by the above general formula (1) in a nonaqueous electrolytic solution in which a lithium salt is dissolved in an organic solvent.
- R 1 to R 3 are each independently an alkyl group having 1 to 8 carbon atoms, an alkenyl group having 2 to 8 carbon atoms, a cycloalkyl group having 5 to 8 carbon atoms, or 6 to 6 carbon atoms.
- 8 represents an aryl group or a fluorine atom.
- alkyl group having 1 to 8 carbon atoms examples include methyl, ethyl, propyl, isopropyl, butyl, isobutyl, secondary butyl, t-butyl, pentyl, isopentyl, secondary pentyl, t-pentyl, hexyl, secondary hexyl, heptyl Secondary heptyl, octyl, secondary octyl, 2-methylpentyl, 2-ethylhexyl and the like can be mentioned.
- alkenyl group having 2 to 8 carbon atoms examples include vinyl, allyl, 3-butenyl, isobutenyl, 4-pentenyl, 5-hexenyl, 6-heptenyl, 7-octenyl and the like.
- Examples of the cycloalkyl group having 5 to 8 carbon atoms include cyclopentyl, cyclohexyl, cyclohexylmethyl and the like.
- aryl group having 6 to 8 carbon atoms examples include phenyl, toluyl, xylyl and the like.
- R 1 to R 3 are preferably a fluorine atom, methyl, or ethyl, and more preferably a fluorine atom or methyl, because they have little adverse effect on the movement of lithium ions and good charge characteristics.
- R 4 represents an alkylene group having 1 to 8 carbon atoms or an alkylene group having 4 to 8 carbon atoms having an ether group.
- alkylene group having 1 to 8 carbon atoms include methylene, ethylene, propylene, butylene, pentamethylene, hexamethylene, heptamethylene, octamethylene, 2-methylbutylene, etc., and an ether group having 4 to 8 carbon atoms.
- Examples of the alkylene group include 4-oxaheptylene, 5-oxanonylene and the like.
- R 4 is preferably ethylene, propylene, butylene, 2-methylbutylene, 4-oxaheptylene, more preferably ethylene, 4-oxaheptylene, because it has little adverse effect on the movement of lithium ions and good charging characteristics. Ethylene is most preferred.
- fluorosilane compound represented by the general formula (1) examples include 1,2-bis (difluorosilyl) methane, 1,1-bis (trifluorosilyl) ethane, and 1,2-bis (trifluorosilyl).
- Ethane 1,2-bis (difluoromethylsilyl) ethane, 1-trifluorosilyl-2-difluoromethylsilylethane, 1-fluorodimethylsilyl-2-difluoromethylsilylethane, 1,2-bis (difluoroethylsilyl) ) Ethane, 1-trifluorosilyl-2-difluoroethylsilylethane, 1-fluorodiethylsilyl-2-difluoroethylsilylethane, 1,2-bis (difluoropropylsilyl) ethane, 1-trifluorosilyl-2-difluoro Propylsilylethane, 1-fluorodipropyl
- the content of the fluorosilane compound represented by the general formula (1) is not limited to the non-aqueous electrolyte because the effect of increasing the amount of the non-aqueous electrolyte may be adversely affected.
- 0.001 to 5 mass% is preferable, 0.01 to 4 mass% is more preferable, and 0.03 to 3 mass% is most preferable.
- R 5 and R 6 each independently represents a hydrogen atom or an alkyl group having 1 to 8 carbon atoms
- R 7 represents an alkyl group having 1 to 8 carbon atoms
- carbon Represents an alkynyl group having 2 to 8 carbon atoms or a halogenated alkyl group having 1 to 8 carbon atoms.
- R 8 and R 9 each independently represents a hydrogen atom or an alkyl group having 1 to 8 carbon atoms
- R 10 represents an alkyl group having 1 to 8 carbon atoms
- carbon Represents an alkynyl group having 2 to 8 carbon atoms or a halogenated alkyl group having 1 to 8 carbon atoms
- n represents 1 or 2.
- R 5 and R 6 each independently represents a hydrogen atom or an alkyl group having 1 to 8 carbon atoms.
- the alkyl group having 1 to 8 carbon atoms include the alkyl groups having 1 to 8 carbon atoms exemplified in the description of R 1 to R 3 in the general formula (1).
- R 5 and R 6 are preferably a hydrogen atom, methyl, ethyl, or propyl, more preferably a hydrogen atom or methyl, and most preferably a hydrogen atom, since there is little adverse effect on the movement of lithium ions and good charging characteristics. .
- R 7 represents an alkyl group having 1 to 8 carbon atoms, an alkenyl group having 2 to 8 carbon atoms, an alkynyl group having 2 to 8 carbon atoms, or a halogenated alkyl group having 1 to 8 carbon atoms.
- Examples of the alkyl group having 1 to 8 carbon atoms and the alkenyl group having 2 to 8 carbon atoms include the alkyl groups having 1 to 8 carbon atoms exemplified as R 1 to R 3 in the general formula (1), and those having 2 to 8 carbon atoms. An alkenyl group etc. are mentioned.
- alkynyl group having 2 to 8 carbon atoms examples include ethynyl, 2-propynyl (also referred to as propargyl), 3-butynyl, 1-methyl-2-propynyl, 1,1-dimethyl-2-propynyl and the like.
- halogenated alkyl group having 1 to 8 carbon atoms examples include chloromethyl, trifluoromethyl, 2-fluoroethyl, 2-chloroethyl, 2,2,2-trifluoroethyl, 2,2,2-trichloroethyl, 1,1,2,2-tetrafluoroethyl, pentafluoroethyl, 3-fluoropropyl, 2-chloropropyl, 3-chloropropyl, 2-chloro-2-propyl, 3,3,3-trifluoropropyl, 2, , 2,3,3-tetrafluoropropyl, heptafluoropropyl, 2-chlorobutyl, 3-chlorobutyl, 4-chlorobutyl, 3-chloro-2-butyl, 1-chloro-2-butyl, 2-chloro-1,1 -Dimethylethyl, 3-chloro-2-methylpropyl, 5-chloropentyl, 3-chlor
- methyl, ethyl, propyl, isopropyl, butyl, pentyl, 2-propynyl, 3-chloropropyl, 3-chlorobutyl, and 4-chlorobutyl can be used.
- Methyl, ethyl, propyl and 2-propynyl are more preferable, and ethyl and 2-propynyl are most preferable.
- compounds in which R 5 and R 6 are hydrogen atoms include, for example, methylbis (2-propynyl) phosphate, ethylbis (2-propynyl) Phosphate, propyl bis (2-propynyl) phosphate, butyl bis (2-propynyl) phosphate, pentyl bis (2-propynyl) phosphate, allyl bis (2-propynyl) phosphate, tris (2-propynyl) phosphate, 2 -Chloroethylbis (2-propynyl) phosphate, 2,2,2-trifluoroethylbis (2-propynyl) phosphate, 2,2,2-trichloroethylbis (2-propynyl) phosphate, etc. .
- the compound in which R 5 is methyl and R 6 is a hydrogen atom includes, for example, methylbis (1-methyl-2-propynyl) phosphate.
- examples of the compounds in which R 5 and R 6 are methyl include methyl bis (1,1-dimethyl-2-propynyl) phosphate, ethyl bis (1,1-dimethyl-2-propynyl) phosphate, propylbis (1,1-dimethyl-2-propynyl) phosphate, butylbis (1,1-dimethyl-2-propynyl) phosphate, pentylbis (1,1 -Dimethyl-2-propynyl) phosphate, allylbis (1,1-dimethyl-2-propynyl) phosphate, 2-propynylbis (1,1-dimethyl-2-propynyl) phosphate, tris (1,1-dimethyl) -2-propynyl) phosphate, 2-chloroethylbis (1,1-dimethyl-2-propynyl) ) Phosphate
- Examples of the unsaturated phosphate compound represented by the general formula (2) include methyl bis (2-propynyl) phosphate, ethyl bis (2-propynyl) phosphate, propyl bis (2-propynyl) phosphate, butyl bis (2 -Propynyl) phosphate, pentylbis (2-propynyl) phosphate, tris (2-propynyl) phosphate, 2-chloroethylbis (2-propynyl) phosphate, ethyl bis (2-propynyl) phosphate, propylbis More preferred are (2-propynyl) phosphate, butyl bis (2-propynyl) phosphate, and tris (2-propynyl) phosphate, and most preferred are ethyl bis (2-propynyl) phosphate and tris (2-propynyl) phosphate. Preferred.
- R 8 and R 9 each independently represents a hydrogen atom or an alkyl group having 1 to 8 carbon atoms.
- the alkyl group having 1 to 8 carbon atoms include the alkyl groups having 1 to 8 carbon atoms exemplified in the description of R 1 to R 3 in the general formula (1).
- R 8 and R 9 are preferably a hydrogen atom, methyl, ethyl, or propyl, more preferably a hydrogen atom or methyl, and most preferably a hydrogen atom, since there is little adverse effect on the movement of lithium ions and good charge characteristics. .
- R 10 represents an alkyl group having 1 to 8 carbon atoms, an alkenyl group having 2 to 8 carbon atoms, an alkynyl group having 2 to 8 carbon atoms, or a halogenated alkyl group having 1 to 8 carbon atoms.
- Examples of the alkyl group having 1 to 8 carbon atoms and the alkenyl group having 2 to 8 carbon atoms include the alkyl groups having 1 to 8 carbon atoms exemplified in the description of R 1 to R 3 in the general formula (1), and 2 to 8 alkenyl groups and the like.
- alkynyl group having 2 to 8 carbon atoms and the halogenated alkyl group having 1 to 8 carbon atoms examples include the alkynyl groups having 2 to 8 carbon atoms and the 1 to 8 carbon atoms exemplified in the description of R 7 in the general formula (1). 8 halogenated alkyl groups and the like.
- methyl, ethyl, propyl, isopropyl, butyl, pentyl, 2-propynyl, 3-chloropropyl, 3-chlorobutyl, 4-chlorobutyl are used.
- methyl, ethyl, propyl and 2-propynyl are more preferable, and methyl and ethyl are most preferable.
- n 1 or 2.
- N is preferably 2 because the phosphoric acid ester reaction from the alkynediol as a raw material is easy and can be obtained in high yield.
- examples of the compound in which n is 1 include 2-butyne-1,4-diol tetramethyldiphosphate, 2-butyne-1 , 4-diol tetraethyl diphosphate, 2-butyne-1,4-diol tetrapropyl diphosphate, 2-butyne-1,4-diol tetraisopropyl diphosphate, 2-butyne-1,4-diol tetrabutyl Diphosphate, 2-butyne-1,4-dioltetrapentyldiphosphate, 2-butyne-1,4-dioltetrakis (2-propynyl) diphosphate, 2-butyne-1,4-dioltetrakis (3 -Chloropropyl) diphosphate, 2-butyne-1,4-dioltetrakis
- the compound in which n is 2 includes, for example, 2,4-hexadiyne-1,6-dioltetramethyldiphosphate, 2 , 4-hexadiyne-1,6-diol tetraethyl diphosphate, 2,4-hexadiyne-1,6-diol tetrapropyl diphosphate, 2,4-hexadiyne-1,6-diol tetraisopropyl diphosphate, 2 , 4-Hexadiyne-1,6-diol tetrabutyl diphosphate, 2,4-hexadiyne-1,6-diol tetrapentyl diphosphate, 2,4-hexadiyne-1,6-diol tetrakis (2-propynyl) Diphosphate, 2,4-hexadiyne-1,6-diol t
- the total content of the unsaturated phosphate compound represented by the general formula (2) and the unsaturated phosphate compound represented by the general formula (3) is If the amount is too small, a sufficient effect cannot be exhibited. If the amount is too large, not only the increase effect corresponding to the content cannot be obtained, but also the characteristics of the non-aqueous electrolyte may be adversely affected.
- the total content of the unsaturated phosphate ester compound represented by the general formula (2) and the unsaturated phosphate ester compound represented by the general formula (3) is 0. 001 to 5 mass% is preferable, 0.01 to 4 mass% is more preferable, and 0.03 to 3 mass% is most preferable.
- the unsaturated phosphate compound represented by the general formula (2) in terms of the availability of industrial raw materials, is preferable.
- the general formula (2) The mass ratio of the unsaturated phosphate ester compound represented by the general formula (3) to the unsaturated phosphate ester compound represented is preferably 0.05 to 10, and preferably 0.1 to 5. Is more preferable, and 0.2 to 3 is most preferable.
- the nonaqueous electrolytic solution according to the present invention preferably further contains a fluorosilane compound represented by the following general formula (4) in order to improve output characteristics at low temperatures.
- R 11 and R 12 are each independently an alkyl group having 1 to 8 carbon atoms, an alkenyl group having 2 to 8 carbon atoms, an alkynyl group having 2 to 8 carbon atoms, or a halogenated group having 1 to 8 carbon atoms.
- X 1 represents a fluorine atom, an alkyl group having 1 to 8 carbon atoms, An alkenyl group having 2 to 8 carbon atoms, an alkynyl group having 2 to 8 carbon atoms, a halogenated alkyl group having 1 to 8 carbon atoms, an aryl group having 6 to 18 carbon atoms, a halogenated aryl group having 6 to 18 carbon atoms, carbon (Indicates an aralkyl group of formula 7 to 18, a group represented by the following general formula (5), or a group represented by the following general formula (6).)
- R 11 and R 12 have the same meaning as in the general formula (4), and R 13 represents an alkylene group having 1 to 8 carbon atoms, an alkenylene group having 2 to 8 carbon atoms, and an alkynylene group having 2 to 8 carbon atoms. Represents a group or an arylene group having 6 to 18 carbon atoms.
- R 14 represents an alkylene group having 1 to 8 carbon atoms, an alkenylene group having 2 to 8 carbon atoms, an alkynylene group having 2 to 8 carbon atoms, or an arylene group having 6 to 18 carbon atoms
- R 15 represents the number of carbon atoms.
- X 2 represents an oxygen atom, a —C ( ⁇ O) —O— group or a —O—C ( ⁇ O) — group.
- R 11 and R 12 are each independently an alkyl group having 1 to 8 carbon atoms, an alkenyl group having 2 to 8 carbon atoms, an alkynyl group having 2 to 8 carbon atoms, or 1 to 8 represents a halogenated alkyl group, an aryl group having 6 to 18 carbon atoms, a halogenated aryl group having 6 to 18 carbon atoms, and an aralkyl group having 7 to 18 carbon atoms.
- Examples of the alkyl group having 1 to 8 carbon atoms and the alkenyl group having 2 to 8 carbon atoms include the alkyl groups having 1 to 8 carbon atoms exemplified in the description of R 1 to R 3 in the general formula (1), and 2 to 8 alkenyl groups and the like.
- Examples of the alkynyl group having 2 to 8 carbon atoms and the halogenated alkyl group having 1 to 8 carbon atoms include alkynyl groups having 2 to 8 carbon atoms and 1 to 8 carbon atoms exemplified in the description of R 7 in the general formula (2). And halogenated alkyl groups.
- aryl group having 6 to 18 carbon atoms examples include phenyl, methylphenyl, dimethylphenyl, ethylphenyl, trimethylphenyl, propylphenyl, isopropylphenyl, butylphenyl, t-butylphenyl, pentylphenyl, t-pentylphenyl, and hexyl.
- halogenated aryl group having 6 to 18 carbon atoms examples include 2-fluorophenyl, 3-fluorophenyl, 4-fluorophenyl, 2,4-difluorophenyl, 3,5-difluorophenyl, and 2,6-difluorophenyl. 2,3-difluorophenyl, 4,5-difluorophenyl, 2,4,6-trifluorophenyl, 2,3,4-trifluorophenyl, tetrafluorophenyl and the like.
- Examples of the aralkyl group having 7 to 18 carbon atoms include benzyl, 2-phenylethyl, 2-phenyl-2-propyl, 3-phenylpropyl, diphenylmethyl and the like.
- R 11 and R 12 are preferably methyl, ethyl, propyl, isopropyl, butyl, pentyl, 3-chloropropyl, 3-chlorobutyl, and 4-chlorobutyl because the internal resistance of the nonaqueous electrolyte secondary battery is reduced.
- Methyl, ethyl and propyl are more preferable, and methyl is most preferable.
- X 1 represents a fluorine atom, an alkyl group having 1 to 8 carbon atoms, an alkenyl group having 2 to 8 carbon atoms, an alkynyl group having 2 to 8 carbon atoms, or a halogenated group having 1 to 8 carbon atoms.
- An alkyl group, an aryl group having 6 to 18 carbon atoms, a halogenated aryl group having 6 to 18 carbon atoms, an aralkyl group having 7 to 18 carbon atoms, a group represented by the above general formula (5), or the above general formula (6) Represents a group represented by
- Examples of the alkyl group having 1 to 8 carbon atoms and the alkenyl group having 2 to 8 carbon atoms include the alkyl groups having 1 to 8 carbon atoms exemplified in the description of R 1 to R 3 in the general formula (1), and 2 to 8 alkenyl groups and the like.
- Examples of the alkynyl group having 2 to 8 carbon atoms and the halogenated alkyl group having 1 to 8 carbon atoms include alkynyl groups having 2 to 8 carbon atoms and 1 to 8 carbon atoms exemplified in the description of R 7 in the general formula (2). And halogenated alkyl groups.
- Examples of the aryl group having 6 to 18 carbon atoms, the halogenated aryl group having 6 to 18 carbon atoms, or the aralkyl group having 7 to 18 carbon atoms include carbons exemplified in the description of R 11 and R 12 in the general formula (4). Examples thereof include an aryl group having 6 to 18 carbon atoms, a halogenated aryl group having 6 to 18 carbon atoms, and an aralkyl group having 7 to 18 carbon atoms.
- R 11 and R 12 have the same meaning as in the general formula (4), and R 13 represents an alkylene group having 1 to 8 carbon atoms, an alkenylene group having 2 to 8 carbon atoms, and a carbon number of 2 Represents an alkynylene group having ⁇ 8 or an arylene group having 6 to 18 carbon atoms.
- Examples of the alkylene group having 1 to 8 carbon atoms include alkylene groups having 1 to 8 carbon atoms exemplified as R 4 in the general formula (1).
- Examples of the alkenylene group having 2 to 8 carbon atoms include 1,2-ethenediyl (also referred to as ethenylene or vinylene), 2-butene-1,4-diyl, 1,2-dimethyl-1,2-ethenediyl, and the like. It is done.
- Examples of the alkynylene group having 2 to 8 carbon atoms include 1,2-ethynediyl (also referred to as ethynylene), 2-butyne-1,4-diyl, and the like.
- Examples of the arylene group having 6 to 18 carbon atoms include 1,2-phenylene, 1,4-phenylene, (1,1′-biphenyl) -4,4′-diyl, and the like.
- ethylene, propylene, tetramethylene, pentamethylene, hexamethylene, heptamethylene, octamethylene, 2-methyltetramethylene, 1,2- Ethynediyl and 1,2-phenylene are preferred, ethylene, propylene and tetramethylene are more preferred, and ethylene is most preferred.
- R 14 represents an alkylene group having 1 to 8 carbon atoms, an alkenylene group having 2 to 8 carbon atoms, an alkynylene group having 2 to 8 carbon atoms, or an arylene group having 6 to 18 carbon atoms.
- Examples of the alkylene group having 1 to 8 carbon atoms include the alkylene groups having 1 to 8 carbon atoms exemplified in the description of R 4 in the general formula (1).
- Examples of the alkenylene group having 2 to 8 carbon atoms, the alkynylene group having 2 to 8 carbon atoms, and the arylene group having 6 to 18 carbon atoms include the groups exemplified in the description of R 13 in the general formula (5).
- ethylene, propylene, tetramethylene, pentamethylene, hexamethylene, heptamethylene, octamethylene, 2-methyltetramethylene, 1,2- Ethynediyl and 1,2-phenylene are preferred, ethylene, propylene and tetramethylene are more preferred, and ethylene is most preferred.
- R 15 represents an alkyl group having 1 to 8 carbon atoms, an alkenyl group having 2 to 8 carbon atoms, an alkynyl group having 2 to 8 carbon atoms, a halogenated alkyl group having 1 to 8 carbon atoms, carbon Represents an aryl group having 6 to 18 carbon atoms, a halogenated aryl group having 6 to 18 carbon atoms or an aralkyl group having 7 to 18 carbon atoms, and X 2 represents an oxygen atom, —C ( ⁇ O) —O— group or —O—. Represents a C ( ⁇ O) — group.
- Examples of the alkyl group having 1 to 8 carbon atoms and the alkenyl group having 2 to 8 carbon atoms include the alkyl groups having 1 to 8 carbon atoms exemplified in the description of R 1 to R 3 in the general formula (1), and 2 to 8 alkenyl groups and the like.
- Examples of the alkynyl group having 2 to 8 carbon atoms and the halogenated alkyl group having 1 to 8 carbon atoms include alkynyl groups having 2 to 8 carbon atoms and 1 to 8 carbon atoms exemplified in the description of R 7 in the general formula (2). And halogenated alkyl groups.
- Examples of the aryl group having 6 to 18 carbon atoms, the halogenated aryl group having 6 to 18 carbon atoms, or the aralkyl group having 7 to 18 carbon atoms include carbons exemplified in the description of R 11 and R 12 in the general formula (4). Examples thereof include an aryl group having 6 to 18 carbon atoms, a halogenated aryl group having 6 to 18 carbon atoms, and an aralkyl group having 7 to 18 carbon atoms.
- preferable compounds include, for example, butylmethyldifluorosilane, isobutylmethyldifluorosilane, pentylmethyldifluorosilane, and hexylmethyldifluorosilane.
- X 1 is an alkyl group having 1 to 8 carbon atoms, an alkenyl group having 2 to 8 carbon atoms, an alkynyl group having 2 to 8 carbon atoms, a halogenated alkyl group having 1 to 8 carbon atoms, an aryl group having 6 to 18 carbon atoms, Specific examples of preferable compounds among the compounds represented by the general formula (4), which are a halogenated aryl group having 6 to 18 carbon atoms or an aralkyl group having 7 to 18 carbon atoms, include, for example, trimethylfluorosilane, Ethyldimethylfluorosilane, propyldimethylfluorosilane, isopropyldimethylfluorosilane, butyldimethylfluorosilane, sec-butyldimethylfluorosilane, t-butyldimethylfluorosilane, pentyldimethylfluorosilane, hexyldimethylfluorosi
- X 1 is a group represented by the above general formula (5)
- specific examples of preferred compounds include, for example, 1,2-di (dimethyl). Fluorosilyl) ethane, 1,2-di (diethylfluorosilyl) ethane, 1,2-di (dipropylfluorosilyl) ethane, 1,2-di (dibutylfluorosilyl) ethane, 1,3-di (dimethylfluoro) Silyl) propane, 1,2-di (diethylfluorosilyl) propane, 1,3-di (dipropylfluorosilyl) propane, 1,3-di (dibutylfluorosilyl) propane, 1,4-di (dimethylfluorosilyl) ) Butane, 1,4-di (diethylfluorosilyl) butane, 1,4-di (dipropylfluorosilyl)
- X 1 is a group represented by the above general formula (6) and X 2 in the formula is an oxygen atom
- specific examples of preferred compounds are as follows. Is, for example, 3-methoxypropyldimethylfluorosilane, 3-ethoxypropyldimethylfluorosilane, 3-propoxypropyldimethylfluorosilane, 3-butoxypropyldimethylfluorosilane, 3-pentoxypropyldimethylfluorosilane, 3-hexoxypropyl Dimethylfluorosilane, 4-methoxybutyldimethylfluorosilane, 4-ethoxybutyldimethylfluorosilane, 4-propoxybutyldimethylfluorosilane, 4-butoxybutyldimethylfluorosilane, 4-pentoxybutyldimethylfluorosilane, 4-hexoxybutyl Dimethyl Fluorosilane etc. are mentioned.
- X 1 is a group represented by the general formula (6)
- X 2 in the formula is a —C ( ⁇ O) —O— group.
- specific examples of preferred compounds include, for example, 2- (dimethylfluorosilyl) ethyl acetate, 3- (dimethylfluorosilyl) propyl acetate, 3- (dimethylfluorosilyl) butyl acetate, and 3- (acetate).
- X 1 is a group represented by the general formula (6)
- X 2 in the formula is a —O—C ( ⁇ O) — group.
- preferred compounds include, for example, methyl dimethylfluorosilyl acetate, ethyl dimethylfluorosilyl acetate, butyl dimethylfluorosilyl acetate, pentyl dimethylfluorosilyl acetate, hexyl dimethylfluorosilyl acetate, 3- (dimethylfluorosilyl) propion Methyl acid, ethyl 3- (dimethylfluorosilyl) propionate, propyl 3- (dimethylfluorosilyl) propionate, butyl 3- (dimethylfluorosilyl) propionate, pentyl 3- (dimethylfluorosilyl) propionate, 3- ( Dimethylfluorosilyl) hexylpropionate, 4- (dimethylfluoro (
- the content of the fluorosilane compound represented by the general formula (4) when the content of the fluorosilane compound represented by the general formula (4) is too small, sufficient effects cannot be exhibited, and when the content is too large, the content is too small.
- the content of the fluorosilane compound represented by the general formula (4) is non-aqueous because not only an increase effect corresponding to the above can be obtained, but also the characteristics of the battery non-aqueous electrolyte may be adversely affected.
- 0.01 to 5% by mass is preferable, 0.03 to 4% by mass is more preferable, and 0.05 to 3% by mass is most preferable.
- the fluorosilane compound represented by the general formula (4) may be used alone or in combination of two or more.
- the nonaqueous electrolytic solution according to the present invention further includes an additive such as a cyclic carbonate compound having an unsaturated group, a chain carbonate compound, an unsaturated diester compound, a halogenated cyclic carbonate compound, a cyclic sulfite ester or a cyclic sulfate ester. It is preferable to do.
- an additive such as a cyclic carbonate compound having an unsaturated group, a chain carbonate compound, an unsaturated diester compound, a halogenated cyclic carbonate compound, a cyclic sulfite ester or a cyclic sulfate ester. It is preferable to do.
- Examples of the cyclic carbonate compound having an unsaturated group include vinylene carbonate, vinyl ethylene carbonate, propylidene carbonate, ethylene ethylidene carbonate, ethylene isopropylidene carbonate, and vinylene carbonate or vinyl ethylene carbonate is preferable.
- Examples of the chain carbonate compound include dipropargyl carbonate, propargyl methyl carbonate, ethyl propargyl carbonate, bis (1-methylpropargyl) carbonate, bis (1-dimethylpropargyl) carbonate, and the like.
- Examples of the unsaturated diester compounds include dimethyl maleate, diethyl maleate, dipropyl maleate, dibutyl maleate, dipentyl maleate, dihexyl maleate, diheptyl maleate, dioctyl maleate, dimethyl fumarate, diethyl fumarate, and fumaric acid.
- Examples include dihexyl, diheptyl acetylenedicarboxylate, and dioctyl acetylenedicarboxylate.
- halogenated cyclic carbonate compound examples include chloroethylene carbonate, dichloroethylene carbonate, fluoroethylene carbonate, difluoroethylene carbonate, and the like.
- cyclic sulfite examples include ethylene sulfite
- examples of the cyclic sulfate include propane sultone and butane sultone.
- vinylene carbonate, vinyl ethylene carbonate, dipropargyl carbonate, dimethyl acetylenedicarboxylate, diethyl acetylenedicarboxylate, chloroethylene carbonate, dichloroethylene carbonate, fluoroethylene carbonate, ethylene sulfite, propane sultone, butane sultone are preferable.
- More preferred are vinylene carbonate, dipropargyl carbonate, dimethyl acetylenedicarboxylate, chloroethylene carbonate, fluoroethylene carbonate, ethylene sulfite, propane sultone, and vinylene carbonate, dipropargyl carbonate, chloroethylene carbonate, fluoroethylene carbonate, ethylene sulfite, propane.
- Sulton is the most Masui.
- additives may be used alone or in combination of two or more.
- the content of these additives is preferably 0.005 to 10% by mass, and preferably 0.02 to 5% by mass in the non-aqueous electrolyte because it may adversely affect the characteristics of the non-aqueous electrolyte. Is more preferable, and 0.05 to 3% by mass is most preferable.
- organic solvent used in the non-aqueous electrolyte according to the present invention those usually used in non-aqueous electrolytes can be used alone or in combination of two or more. Specific examples include saturated cyclic carbonate compounds, saturated cyclic ester compounds, sulfoxide compounds, sulfone compounds, amide compounds, saturated chain carbonate compounds, chain ether compounds, cyclic ether compounds, and saturated chain ester compounds.
- saturated cyclic carbonate compounds saturated cyclic ester compounds, sulfoxide compounds, sulfone compounds and amide compounds have a high relative dielectric constant, and thus serve to increase the dielectric constant of non-aqueous electrolytes.
- Compounds are preferred.
- the saturated cyclic carbonate compound include ethylene carbonate, 1,2-propylene carbonate, 1,3-propylene carbonate, 1,2-butylene carbonate, 1,3-butylene carbonate, 1,1, -dimethylethylene carbonate, and the like. Is mentioned.
- saturated cyclic ester compound examples include ⁇ -butyrolactone, ⁇ -valerolactone, ⁇ -caprolactone, ⁇ -hexanolactone, and ⁇ -octanolactone.
- sulfoxide compound examples include dimethyl sulfoxide, diethyl sulfoxide, dipropyl sulfoxide, diphenyl sulfoxide, thiophene, and the like.
- sulfone compounds include dimethylsulfone, diethylsulfone, dipropylsulfone, diphenylsulfone, sulfolane (also referred to as tetramethylenesulfone), 3-methylsulfolane, 3,4-dimethylsulfolane, 3,4-diphenimethylsulfolane, sulfolene. , 3-methylsulfolene, 3-ethylsulfolene, 3-bromomethylsulfolene and the like, and sulfolane and tetramethylsulfolane are preferable.
- the amide compound include N-methylpyrrolidone, dimethylformamide, dimethylacetamide and the like.
- saturated chain carbonate compounds, chain ether compounds, cyclic ether compounds and saturated chain ester compounds can lower the viscosity of the non-aqueous electrolyte and increase the mobility of electrolyte ions. Battery characteristics such as output density can be made excellent. Moreover, since it is low-viscosity, the performance of the non-aqueous electrolyte at a low temperature can be enhanced, and among them, a saturated chain carbonate compound is preferable.
- saturated chain carbonate compound examples include dimethyl carbonate (DMC), ethyl methyl carbonate (EMC), diethyl carbonate (DEC), ethyl butyl carbonate, methyl-t-butyl carbonate, diisopropyl carbonate, t-butyl propyl carbonate, and the like. Is mentioned.
- Examples of the chain ether compound or cyclic ether compound include dimethoxyethane (DME), ethoxymethoxyethane, diethoxyethane, tetrahydrofuran, dioxolane, dioxane, 1,2-bis (methoxycarbonyloxy) ethane, 1,2- Bis (ethoxycarbonyloxy) ethane, 1,2-bis (ethoxycarbonyloxy) propane, ethylene glycol bis (trifluoroethyl) ether, propylene glycol bis (trifluoroethyl) ether, ethylene glycol bis (trifluoromethyl) ether, Examples include diethylene glycol bis (trifluoroethyl) ether, and among these, dioxolane is preferable.
- DME dimethoxyethane
- ethoxymethoxyethane diethoxyethane
- tetrahydrofuran dioxolane
- dioxane 1,2-bis (me
- saturated chain ester compound monoester compounds and diester compounds having a total number of carbon atoms in the molecule of 2 to 8 are preferable.
- Specific compounds include methyl formate, ethyl formate, methyl acetate, ethyl acetate, Propyl acetate, isobutyl acetate, butyl acetate, methyl propionate, ethyl propionate, methyl butyrate, methyl isobutyrate, methyl trimethyl acetate, ethyl trimethyl acetate, methyl malonate, ethyl malonate, methyl succinate, ethyl succinate, 3- Examples include methyl methoxypropionate, ethyl 3-methoxypropionate, ethylene glycol diacetyl, propylene glycol diacetyl, and the like.
- Methyl formate, ethyl formate, methyl acetate, ethyl acetate, propyl acetate, isobutyl acetate, butyl acetate, methyl propionate, propio Ethyl are preferred.
- acetonitrile acetonitrile, propionitrile, nitromethane and their derivatives can be used as the organic solvent.
- electrolyte salt used in the non-aqueous electrolyte according to the present invention a conventionally known electrolyte salt is used.
- a conventionally known electrolyte salt is used.
- the electrolyte salt is dissolved in the organic solvent so that the concentration in the non-aqueous electrolyte according to the present invention is 0.1 to 3.0 mol / L, particularly 0.5 to 2.0 mol / L. Is preferred. If the concentration of the electrolyte salt is less than 0.1 mol / L, a sufficient current density may not be obtained, and if it is more than 3.0 mol / L, the stability of the non-aqueous electrolyte may be impaired.
- halogen-based, phosphorus-based and other flame retardants can be appropriately added to the non-aqueous electrolyte according to the present invention in order to impart flame retardancy. If the amount of flame retardant added is too small, sufficient flame retarding effect cannot be exhibited.If the amount added is too large, an increase effect corresponding to the content cannot be obtained. Since the properties may be adversely affected, the content is preferably 5 to 100% by mass, more preferably 10 to 50% by mass with respect to the organic solvent constituting the nonaqueous electrolytic solution according to the present invention. .
- a negative electrode capable of removing and inserting lithium ions is used.
- the negative electrode from which lithium ions can be removed and inserted is not particularly limited as long as it can be used as a negative electrode for a lithium secondary battery.
- a negative electrode material such as a negative electrode active material and a binder that are slurried with a solvent is collected. It is applied to the body and dried to form a sheet.
- the negative electrode active material crystalline artificial graphite and natural graphite are used.
- Crystalline graphite with a crystal surface coated with another material, crystalline graphite formed into microcrystalline bulk particles, MCMB, soft graphite Carbon, hard carbon, silicon alloy, and tin alloy may be mixed and used.
- the binder for the negative electrode active material include the same as those for the positive electrode.
- the amount of the binder used is preferably 0.001 to 5 parts by mass, more preferably 0.05 to 3 parts by mass, and most preferably 0.01 to 2 parts by mass with respect to 100 parts by mass of the negative electrode active material.
- As the solvent for forming a slurry an organic solvent or water that dissolves the binder is used. Examples of the organic solvent are the same as those for the positive electrode.
- the amount of the solvent used is preferably 30 to 300 parts by mass, more preferably 50 to 200 parts by mass with respect to 100 parts by mass of the negative electrode active material.
- copper, nickel, stainless steel, nickel-plated steel, etc. are usually used for the negative electrode current collector.
- a separator between the positive electrode and the negative electrode it is preferable to use a separator between the positive electrode and the negative electrode, and a commonly used polymer microporous film can be used without particular limitation as the separator.
- the film include polyethylene, polypropylene, polyvinylidene fluoride, polyvinylidene chloride, polyacrylonitrile, polyacrylamide, polytetrafluoroethylene, polysulfone, polyethersulfone, polycarbonate, polyamide, polyimide, polyethylene oxide and polypropylene oxide.
- the microporosity method includes a phase separation method in which a polymer compound and a solvent solution are formed into a film while microphase separation is performed, and the solvent is extracted and removed to make it porous.
- the film is extruded and then heat treated, the crystals are arranged in one direction, and a “stretching method” or the like is performed by forming a gap between the crystals by stretching, and is appropriately selected depending on the film used.
- the electrode material, the non-aqueous electrolyte, and the separator include a phenol-based antioxidant, a phosphorus-based antioxidant, and a thioether-based antioxidant for the purpose of improving safety.
- a hindered amine compound or the like may be added.
- the shape of the nonaqueous electrolyte secondary battery of the present invention having the above configuration is not particularly limited, and can be various shapes such as a coin shape, a cylindrical shape, and a square shape.
- FIG. 1 shows an example of a coin-type battery of the nonaqueous electrolyte secondary battery of the present invention
- FIGS. 2 and 3 show examples of a cylindrical battery, respectively.
- 1 is a positive electrode capable of releasing lithium ions
- 1a is a positive electrode current collector
- 2 is a carbonaceous material capable of inserting and extracting lithium ions released from the positive electrode.
- a negative electrode current collector, 2a is a negative electrode current collector
- 3 is a non-aqueous electrolyte solution according to the present invention
- 4 is a stainless steel positive electrode case
- 5 is a stainless steel negative electrode case
- 6 is a polypropylene gasket
- 7 is polyethylene. It is a separator.
- 11 is a negative electrode
- 12 is a negative electrode current collector
- 13 is a positive electrode
- 14 is a positive electrode current collector
- 15 is the present invention.
- 16 is a separator
- 17 is a positive electrode terminal
- 18 is a negative electrode terminal
- 19 is a negative electrode plate
- 20 is a negative electrode lead
- 21 is a positive electrode plate
- 22 is a positive electrode lead
- 23 is a case
- 24 is an insulating plate
- 26 is a safety valve
- 27 is a PTC element.
- nonaqueous electrolyte secondary batteries lithium secondary batteries
- Preparation of positive electrode B 90 parts by mass of LiNi 0.5 Co 0.2 Mn 0.3 O 2 as a positive electrode active material, 5 parts by mass of acetylene black as a conductive material, and 5 parts by mass of polyvinylidene fluoride (PVDF) as a binder were mixed to obtain a positive electrode material.
- This positive electrode material was dispersed in 140 parts by mass of N-methyl-2-pyrrolidone (NMP) to form a slurry. This slurry was applied to a positive electrode current collector made of aluminum, dried and press-molded to obtain a positive electrode plate. Thereafter, the positive electrode plate was cut into a predetermined size to produce a disc-shaped positive electrode B.
- NMP N-methyl-2-pyrrolidone
- LiPF 6 was dissolved in a mixed solvent consisting of 30% by volume of ethylene carbonate, 40% by volume of ethyl methyl carbonate, 25% by volume of dimethyl carbonate and 5% by volume of propyl acetate to prepare an electrolyte solution A.
- LiPF 6 was dissolved in a mixed solvent composed of 30% by volume of ethylene carbonate, 40% by volume of ethyl methyl carbonate, and 30% by volume of dimethyl carbonate so as to have a concentration of 1 mol / L to prepare an electrolyte solution B.
- an initial characteristic test and a cycle characteristic test were performed by the following test methods.
- the initial characteristic test the discharge capacity ratio and the internal resistance ratio were obtained.
- the cycle characteristic test the discharge capacity maintenance rate and the internal resistance increase rate were obtained.
- the test results are shown in [Table 3] and [Table 4] below.
- it is a non-aqueous electrolyte secondary battery which is excellent in an initial characteristic, so that the numerical value of internal resistance ratio is so low that discharge capacity ratio is high.
- Method of measuring discharge capacity ratio A lithium secondary battery is placed in a constant temperature bath at 20 ° C., and a charging current of 0.3 mA / cm 2 (0.2 C) (current value equivalent to C) was charged at a constant current and constant voltage up to 4.2 V, and a constant current discharge up to 3.0 V at a discharge current of 0.3 mA / cm 2 (current value equivalent to 0.2 C) was performed five times. .
- Discharge capacity ratio (%) [(initial discharge capacity) / (initial discharge capacity in Example 1)] ⁇ 100
- ⁇ Initial characteristic test method for positive electrode B> The lithium secondary battery is placed in a constant temperature bath at 20 ° C., charged at a constant current and a constant voltage up to 4.3 V with a charging current of 0.3 mA / cm 2 (current value corresponding to 0.2 C), and a discharge current of 0.3 mA / cm 2. The operation of discharging a constant current to 3.0 V at cm 2 (current value corresponding to 0.2 C) was performed 5 times. Thereafter, 4.3 V until a constant current and constant voltage charging at a charging current 0.3 mA / cm 2, and a constant current discharge to 3.0V at a discharge current 0.3 mA / cm 2.
- the discharge capacity measured at the sixth time was taken as the initial discharge capacity of the battery, and the discharge capacity ratio (%) was determined in the same manner as in the case of the positive electrode A.
- the internal resistance ratio (%) was determined in the same manner as in the case of the positive electrode A.
- Method for measuring discharge capacity retention rate The lithium secondary battery after the initial characteristic test was placed in a constant temperature bath at 60 ° C., and a charging current of 1.5 mA / cm 2 (current value equivalent to 1 C, 1 C represents the battery capacity in 1 hour.
- the 250th discharge capacity is defined as the discharge capacity after the cycle test, and the discharge capacity retention rate (%) is obtained as a ratio of the discharge capacity after the cycle test when the initial discharge capacity is 100 as shown in the following formula. It was.
- Discharge capacity retention rate (%) [(discharge capacity after cycle test) / (initial discharge capacity)] ⁇ 100
- ⁇ Cycle characteristic test method for positive electrode B> The lithium secondary battery after the initial characteristic test is placed in a constant temperature bath at 60 ° C., and the charging current is 1.5 mA / cm 2 (current value equivalent to 1C, 1C is the current value at which the battery capacity is discharged in 1 hour).
- the discharge capacity at the 250th time was defined as the discharge capacity after the cycle test, and the discharge capacity retention rate (%) was determined in the same manner as in the case of the positive electrode A.
- the rate of increase in internal resistance (%) was determined in the same manner as in the positive electrode A.
- the non-aqueous electrolyte secondary battery of the present invention is a non-aqueous electrolyte secondary battery using a lithium-containing metal oxide containing manganese as a positive electrode active material and represented by the general formula (1).
- a non-aqueous electrolyte containing a fluorosilane compound By using a non-aqueous electrolyte containing a fluorosilane compound, the elution of manganese from the cathode active material can be suppressed, especially at high temperatures, and low internal resistance and high electricity can be achieved even after high-temperature storage and charge / discharge at high temperatures. Capacity can be maintained.
- the non-aqueous electrolyte secondary battery of the present invention includes a video camera, a digital camera, a portable music player, a sound recorder, a portable DVD player, a portable game machine, a notebook computer, an electronic dictionary, an electronic notebook, an electronic book, a mobile phone, and a portable TV. It can be used for various applications such as electric assist bicycles, battery cars, and hybrid cars. Among them, it can be suitably used for applications such as battery cars and hybrid cars that may be used at high temperatures.
Landscapes
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Manufacturing & Machinery (AREA)
- Inorganic Chemistry (AREA)
- Physics & Mathematics (AREA)
- Condensed Matter Physics & Semiconductors (AREA)
- General Physics & Mathematics (AREA)
- Materials Engineering (AREA)
- Secondary Cells (AREA)
Abstract
Description
上記リチウム含有化合物がマンガンを含有するリチウム含有金属酸化物であり、上記非水電解液が下記一般式(1)で表されるフルオロシラン化合物を含有することを特徴とする非水電解液二次電池を提供するものである。
本発明は、正極活物質としてマンガンを含有するリチウム含有金属酸化物を使用した非水電解液二次電池において、上記一般式(1)で表されるフルオロシラン化合物を含有する非水電解液を使用したところに特徴がある。初めに、本発明で使用される正極について説明する。
上記マンガンを含有するリチウム含有金属酸化物としては、リチウムマンガン複合酸化物、リチウムマンガン複合酸化物のマンガン原子の一部を他の金属原子で置換した化合物等が挙げられる。
上記リチウムマンガン複合酸化物としては、例えば、LiMnO2、LiMn2O4、Li2MnO4、Li2MnO3等が挙げられる。リチウムマンガン複合酸化物のマンガン原子の一部を他の金属原子で置換した化合物としては、リチウムマンガン複合酸化物のマンガン原子の一部を、マンガン原子以外の遷移金属原子、例えば、アルミニウム、チタン、バナジウム、クロム、リチウム、鉄、コバルト、銅、亜鉛、マグネシウム、カルシウム、ジルコニウム、ニオブ等の金属原子で置換した化合物、例えば、LiNi0.5Mn0.5O2、LiNi1/3Co1/3Mn1/5O2、LiNi0.5Co0.2Mn0.3O2、LiMn1.8Ni0.2MnO4、LiMn1.5Ni0.5MnO4、LiMn1.9Mg0.05O4、Li1.1Mn1.8Mg0.1O4、Li1.1Mn1.94Mg0.01B0.008O4、Li1.1Mn1.85Al0.05O4等が挙げられる。
スラリー化する溶媒としては、上記バインダーを溶解する有機溶剤又は水が使用される。該有機溶剤としては、例えば、N-メチルピロリドン、ジメチルホルムアミド、ジメチルアセトアミド、メチルエチルケトン、シクロヘキサノン、酢酸メチル、アクリル酸メチル、ジエチルトリアミン、N-N-ジメチルアミノプロピルアミン、ポリエチレンオキシド、テトラヒドロフラン等が挙げられるが、これに限定されない。上記溶媒の使用量は、上記正極活物質100質量部に対し、30~300質量部が好ましく、50~200質量部が更に好ましい。
正極の集電体には、通常、アルミニウム、ステンレス鋼、ニッケルメッキ鋼等が使用される。
ジフルオロエチルシリルブタン、1-フルオロジエチルシリル-4-ジフルオロエチルシリルブタン、1,4-ビス(ジフルオロプロピルシリル)ブタン、1-トリフルオロシリル-4-ジフルオロプロピルシリルブタン、1-フルオロジプロピルシリル-4-ジフルオロプロピルシリルブタン、1,4-ビス(ジフルオロブチルシリル)ブタン、1-トリフルオロシリル-4-ジフルオロブチルシリルブタン、1-フルオロジブチルシリル-4-ジフルオロブチルシリルブタン、1,4-ビス(ジフルオロペンチルシリル)ブタン、1-トリフルオロシリル-4-ジフルオロペンチルシリルブタン、1-フルオロジペンチルシリル-4-ジフルオロペンチルシリルブタン、1,4-ビス(ジフルオロヘキシルシリル)ブタン、1-トリフルオロシリル-4-ジフルオロヘキシルシリルブタン、1-フルオロジヘキシルシリル-4-ジフルオロヘキシルシリルブタン、1,4-ビス(ジフルオロヘプチルシリル)ブタン、1-トリフルオロシリル-4-ジフルオロヘプチルシリルブタン、1-フルオロジヘプチルシリル-4-ジフルオロヘプチルシリルブタン、1,4-ビス(ジフルオロオクチルシリル)ブタン、1-トリフルオロシリル-4-ジフルオロオクチルシリルブタン、1-フルオロジオクチルシリル-4-ジフルオロオクチルシリルブタン、1,4-ビス(トリフルオロシリル)-2-メチルブタン、1,4-ビス(ジフルオロメチルシリル)-2-メチルブタン、1-トリフルオロシリル-4-ジフルオロメチルシリルブタン、1-フルオロジメチルシリル-4-ジフルオロメチルシリル-2-メチルブタン、1,4-ビス(ジフルオロエチルシリル)-2-メチルブタン、1-トリフルオロシリル-4-ジフルオロエチルシリル-2-メチルブタン、1-フルオロジエチルシリル-4-ジフルオロエチルシリル-2-メチルブタン、1,4-ビス(ジフルオロプロピルシリル)-2-メチルブタン、1-トリフルオロシリル-4-ジフルオロプロピルシリル-2-メチルブタン、1-フルオロジプロピルシリル-4-ジフルオロプロピルシリル-2-メチルブタン、1,4-ビス(ジフルオロブチルシリル)-2-メチルブタン、1-トリフルオロシリル-4-ジフルオロブチルシリル-2-メチルブタン、1-フルオロジブチルシリル-4-ジフルオロブチルシリルブタン、1,4-ビス(ジフルオロペンチルシリル)-2-メチルブタン、1-トリフルオロシリル-4-ジフルオロペンチルシリル-2-メチルブタン、1-フルオロジペンチルシリル-4-ジフルオロペンチルシリル-2-メチルブタン、1,4-ビス(ジフルオロヘキシルシリル)-2-メチルブタン、1-トリフルオロシリル-4-ジフルオロヘキシルシリル-2-メチルブタン、1-フルオロジヘキシルシリル-4-ジフルオロヘキシルシリル-2-メチルブタン、1,4-ビス(ジフルオロヘプチルシリル)-2-メチルブタン、1-トリフルオロシリル-4-ジフルオロヘプチルシリル-2-メチルブタン、1-フルオロジヘプチルシリル-4-ジフルオロヘプチルシリル-2-メチルブタン、1,4-ビス(ジフルオロオクチルシリル)-2-メチルブタン、1-トリフルオロシリル-4-ジフルオロオクチルシリル-2-メチルブタン、1-フルオロジオクチルシリル-4-ジフルオロオクチルシリル-2-メチルブタン、1,6-ビス(トリフルオロシリル)ヘキサン、1,6-ビス(ジフルオロメチルシリル)ヘキサン、1-トリフルオロシリル-6-ジフルオロメチルシリルヘキサン、1-フルオロジメチルシリル-6-ジフルオロメチルシリルヘキサン、1,6-ビス(ジフルオロエチルシリル)ヘキサン、1-トリフルオロシリル-6-ジフルオロエチルシリルヘキサン、1-フルオロジエチルシリル-6-ジフルオロエチルシリルヘキサン、1,6-ビス(ジフルオロプロピルシリル)ヘキサン、1-トリフルオロシリル-6-ジフルオロプロピルシリルヘキサン、1-フルオロジプロピルシリル-6-ジフルオロプロピルシリルヘキサン、1,6-ビス(ジフルオロブチルシリル)ヘキサン、1-トリフルオロシリル-6-ジフルオロブチルシリルヘキサン、1-フルオロジブチルシリル-6-ジフルオロブチルシリルヘキサン、1,6-ビス(ジフルオロペンチルシリル)ヘキサン、1-トリフルオロシリル-6-ジフルオロペンチルシリルヘキサン、1-フルオロジペンチルシリル-6-ジフルオロペンチルシリルヘキサン、1,6-ビス(ジフルオロヘキシルシリル)ヘキサン、1-トリフルオロシリル-6-ジフルオロヘキシルシリルブヘキサン、1-フルオロジヘキシルシリル-6-ジフルオロヘキシルシリルヘキサン、1,6-ビス(ジフルオロヘプチルシリル)ヘキサン、1-トリフルオロシリル-6-ジフル
オロヘプチルシリルヘキサン、1-フルオロジヘプチルシリル-6-ジフルオロヘプチルシリルヘキサン、1,6-ビス(ジフルオロオクチルシリル)ヘキサン、1-トリフルオロシリル-6-ジフルオロオクチルシリルヘキサン、1-フルオロジオクチルシリル-6-ジフルオロオクチルシリルヘキサン、1-フルオロジメチルシリル-2-ジフルオロエチルシリルエタン等が挙げられる。
上記飽和鎖状エステル化合物としては、分子中の炭素数の合計が2~8であるモノエステル化合物及びジエステル化合物が好ましく、具体的な化合物としては、ギ酸メチル、ギ酸エチル、酢酸メチル、酢酸エチル、酢酸プロピル、酢酸イソブチル、酢酸ブチル、プロピオン酸メチル、プロピオン酸エチル、酪酸メチル、イソ酪酸メチル、トリメチル酢酸メチル、トリメチル酢酸エチル、マロン酸メチル、マロン酸エチル、コハク酸メチル、コハク酸エチル、3-メトキシプロピオン酸メチル、3-メトキシプロピオン酸エチル、エチレングリコールジアセチル、プロピレングリコールジアセチル等が挙げられ、ギ酸メチル、ギ酸エチル、酢酸メチル、酢酸エチル、酢酸プロピル、酢酸イソブチル、酢酸ブチル、プロピオン酸メチル、プロピオン酸エチルが好ましい。
以下の実施例及び比較例において、非水電解液二次電池(リチウム二次電池)は、以下の<作製手順>に従って作製された。
〔正極Aの作製〕
正極活物質としてLi1.1Mn1.8Mg0.1O490質量部、導電材としてアセチレンブラック5質量部、及びバインダーとしてポリフッ化ビニリデン(PVDF)5質量部を混合して、正極材料とした。この正極材料をN-メチル-2-ピロリドン(NMP)140質量部に分散させてスラリー状とした。このスラリーをアルミニウム製の正極集電体に塗布し、乾燥後、プレス成型して、正極板とした。その後、この正極板を所定の大きさにカットして円盤状正極Aを作製した。
正極活物質としてLiNi0.5Co0.2Mn0.3O290質量部、導電材としてアセチレンブラック5質量部、及びバインダーとしてポリフッ化ビニリデン(PVDF)5質量部を混合して、正極材料とした。この正極材料をN-メチル-2-ピロリドン(NMP)140質量部に分散させてスラリー状とした。このスラリーをアルミニウム製の正極集電体に塗布し、乾燥後、プレス成型して、正極板とした。その後、この正極板を所定の大きさにカットして円盤状正極Bを作製した。
負極活物質として人造黒鉛97質量部、及びバインダーとしてスチレンブタジエンゴム2質量部、増粘剤としてカルボキシメチルセルロース1質量部を混合して、負極材料とした。この負極材料を水120質量部に分散させてスラリー状とした。このスラリーを銅製の負極集電体に塗布し、乾燥後、プレス成型して、負極板とした。その後、この負極板を所定の大きさにカットし、円盤状負極を作製した。
エチレンカーボネート30体積%、エチルメチルカーボネート40体積%、ジメチルカーボネート25体積%及び酢酸プロピル5体積%からなる混合溶媒に、LiPF6を1mol/Lの濃度となるよう溶解し電解質溶液Aを調製した。
エチレンカーボネート30体積%、エチルメチルカーボネート40体積%、ジメチルカーボネート30体積%からなる混合溶媒に、LiPF6を1mol/Lの濃度となるよう溶解し電解質溶液Bを調製した。
電解液添加剤として、下記化合物A1~A4、化合物B1~B3、化合物C1~C5、化合物D1~D3、又は比較の化合物A‘1~A’2を、下記〔表1〕又は〔表2〕に示す割合で電解質溶液A又はBに溶解し、本発明に係る非水電解液及び比較の非水電解液を調製した。なお、〔表1〕及び〔表2〕中の( )内の数字は、非水電解液における濃度(質量%)を表す。
化合物A1:1,2-ビス(ジフルオロメチルシリル)エタン
化合物A2:1-フルオロジメチルシリル-2-ジフルオロメチルシリルエタン
化合物A3:1-トリフルオロシリル-2-ジフルオロメチルシリルエタン
化合物A4:1,7-ビス(ジフルオロメチルシリル)-4-オキサヘプタン
〔一般式(2)で表わされる不飽和リン酸エステル化合物〕
化合物B1:エチルビス(2-プロピニル)フォスフェート
化合物B2:トリス(2-プロピニル)フォスフェート
〔一般式(3)で表わされる不飽和リン酸エステル化合物〕
化合物B3:2,4-ヘキサジイン-1,6-ジオールテトラエチルジフォスフェート
〔一般式(4)で表わされるフルオロシラン化合物〕
化合物C1:n-ブチルフルオロジメチルシラン
化合物C2:1,2-ビス(フルオロジメチルシリル)エタン
化合物C3:3-メトキシプロピルジメチルフルオロシラン
化合物C4:(2-ジメチルフルオロシリル)プロピオン酸メチル
化合物C5:酢酸(3-ジメチルフルオロシリル)プロピル
〔不飽和基を有する環状カーボネート化合物〕
化合物D1:ビニレンカーボネート
〔環状硫酸エステル化合物〕
化合物D2:プロパンスルトン
〔ハロゲン化環状カーボネート化合物〕
化合物D3:フルオロエチレンカーボネート
〔比較のフルオロシラン化合物A’1〕
ジフルオロジフェニルシラン
〔比較のフルオロシラン化合物A’2〕
ジ-n-ブチルジフルオロシラン
得られた円盤状正極A又は正極Bと円盤状負極との間に、厚さ25μmのポリエチレン製の微多孔フィルムをはさんでケース内に保持した。その後、本発明に係る非水電解液又は比較の非水電解液と正極との組合せが〔表1〕又は〔表2〕となるように、それぞれの非水電解液をケース内に注入し、ケースを密閉、封止して、φ20mm、厚さ3.2mmのコイン型リチウム二次電池を製作し、実施例1~24又は比較例1~12の非水電解液二次電池とした。
a.放電容量比の測定方法
リチウム二次電池を、20℃の恒温槽内に入れ、充電電流0.3mA/cm2(0.2
C相当の電流値)で4.2Vまで定電流定電圧充電し、放電電流0.3mA/cm2(0.2C相当の電流値)で3.0Vまで定電流放電する操作を5回行った。その後、充電電流0.3mA/cm2で4.2Vまで定電流定電圧充電し、放電電流0.3mA/cm2で3.0Vまで定電流放電した。この6回目に測定した放電容量を、電池の初期放電容量とし、下記式に示すように、放電容量比(%)を、実施例1の初期放電容量を100とした場合の初期放電容量の割合として求めた。
放電容量比(%)=[(初期放電容量)/(実施例1における初期放電容量)]×100
上記6回目の放電容量を測定後のリチウム二次電池について、先ず、充電電流1.5mA/cm2(1C相当の電流値)でSOC60%になるように定電流充電し、交流インピーダンス測定装置(IVIUM TECHNOLOGIES製、商品名:モバイル型ポテンショスタットCompactStat)を用いて、周波数100kHz~0.02Hzまで走査し、縦軸に虚数部、横軸に実数部を示すコール-コールプロットを作成した。続いて、このコール-コールプロットにおいて、円弧部分を円でフィッティングして、この円の実数部分と交差する二点のうち、大きい方の値を、電池の初期内部抵抗とし、下記式に示すように、内部抵抗比(%)を、実施例1の初期内部抵抗を100とした場合の初期内部抵抗の割合として求めた。
内部抵抗比(%)=[(初期内部抵抗)/(実施例1における初期内部抵抗)]×100
リチウム二次電池を、20℃の恒温槽内に入れ、充電電流0.3mA/cm2(0.2C相当の電流値)で4.3Vまで定電流定電圧充電し、放電電流0.3mA/cm2(0.2C相当の電流値)で3.0Vまで定電流放電する操作を5回行った。その後、充電電流0.3mA/cm2で4.3Vまで定電流定電圧充電し、放電電流0.3mA/cm2で3.0Vまで定電流放電した。この6回目に測定した放電容量を、電池の初期放電容量とし、正極Aの場合と同様にして、放電容量比(%)を求めた。また、6回目の放電容量を測定後のリチウム二次電池について、正極Aの場合と同様にして、内部抵抗比(%)を求めた。
a.放電容量維持率の測定方法
初期特性試験後のリチウム二次電池を、60℃の恒温槽内に入れ、充電電流1.5mA/cm2(1C相当の電流値、1Cは電池容量を1時間で放電する電流値)で4.2Vまで定電流充電し、放電電流1.5mA/cm2で3.0Vまで定電流放電を行うサイクルを250回繰り返して行った。この250回目の放電容量をサイクル試験後の放電容量とし、下記式に示すように、放電容量維持率(%)を、初期放電容量を100とした場合のサイクル試験後の放電容量の割合として求めた。
放電容量維持率(%)=[(サイクル試験後の放電容量)/(初期放電容量)]×100
サイクル試験後、雰囲気温度を20℃に戻して、20℃における内部抵抗を、上記内部抵抗比の測定方法と同様にして測定し、この時の内部抵抗を、サイクル試験後の内部抵抗とし、下記式に示すように、内部抵抗増加率(%)を、各電池の初期内部抵抗を100とした場合のサイクル試験後の内部抵抗の増加の割合として求めた。
内部抵抗増加率(%)=[(サイクル試験後の内部抵抗-初期内部抵抗)/(初期内部抵抗)]×100
初期特性試験後のリチウム二次電池を、60℃の恒温槽内に入れ、充電電流1.5mA/cm2(1C相当の電流値、1Cは電池容量を1時間で放電する電流値)で4.3Vまで定電流充電し、放電電流1.5mA/cm2で3.0Vまで定電流放電を行うサイクルを250回繰り返して行った。この250回目の放電容量をサイクル試験後の放電容量とし、正極Aの場合と同様にして、放電容量維持率(%)を求めた。また、サイクル試験後のリチウム二次電池について、正極Aの場合と同様にして、内部抵抗増加率(%)を求めた。
正極活物質として、マンガンを含有するリチウム含有金属酸化物を使用した非水電解液二次電池において、上記一般式(1)で表されるフルオロシラン化合物を含有する非水電解液を使用した本発明の非水電解液二次電池は、比較のフルオロシラン化合物を含有する非水電解液を使用した比較例の非水電解液二次電池に比して、電池の初期における放電容量及び内部抵抗の両面で優れるだけでなく、60℃でのサイクル試験後においても、放電容量及び内部抵抗の両面で優れており、優れた電池特性を維持できることが確認できた。
サイクル試験後のリチウム二次電池を分解して、EDX-SEMを用いて負極へのマンガンの付着量を調べた。負極は、リチウム二次電池を分解して取り出た後、ジメチルカーボネートで洗浄し、乾燥してからEDX-SEM分析を行った。マンガン付着量は+~+++++まで5段階評価とし、+の数が多いほど正極からのマンガンの溶出が多かったことを示す。
1a 正極集電体
2 負極
2a 負極集電体
3 非水電解液
4 正極ケース
5 負極ケース
6 ガスケット
7 セパレータ
10 コイン型の非水電解液二次電池
10' 円筒型の非水電解液二次電池
11 負極
12 負極集電体
13 正極
14 正極集電体
15 非水電解液
16 セパレータ
17 正極端子
18 負極端子
19 負極板
20 負極リード
21 正極板
22 正極リード
23 ケース
24 絶縁板
25 ガスケット
26 安全弁
27 PTC素子
Claims (4)
- 上記非水電解液が、更に、下記一般式(2)で表される不飽和リン酸エステル化合物又は下記一般式(3)で表される不飽和リン酸エステル化合物を含有する請求項1に記載の非水電解液二次電池。
ル基又は炭素数1~8のハロゲン化アルキル基を表わす。)
- 上記非水電解液が、更に、下記一般式(4)で表されるフルオロシラン化合物を含有する請求項1又は2に記載の二次電解液二次電池。
- 請求項1~3の何れか1項に記載の非水電解液二次電池に使用される非水電解液。
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP11841545.4A EP2642580B1 (en) | 2010-11-16 | 2011-10-13 | Non-aqueous electrolyte secondary battery |
| CN201180043785.5A CN103098291B (zh) | 2010-11-16 | 2011-10-13 | 非水电解液二次电池 |
| US13/822,141 US9017866B2 (en) | 2010-11-16 | 2011-10-13 | Non-aqueous electrolyte secondary battery |
| KR1020137005879A KR101881450B1 (ko) | 2010-11-16 | 2011-10-13 | 비수 전해액 이차 전지 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2010-256225 | 2010-11-16 | ||
| JP2010256225A JP5781293B2 (ja) | 2010-11-16 | 2010-11-16 | 非水電解液二次電池 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2012066879A1 true WO2012066879A1 (ja) | 2012-05-24 |
Family
ID=46083819
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2011/073546 WO2012066879A1 (ja) | 2010-11-16 | 2011-10-13 | 非水電解液二次電池 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US9017866B2 (ja) |
| EP (1) | EP2642580B1 (ja) |
| JP (1) | JP5781293B2 (ja) |
| KR (1) | KR101881450B1 (ja) |
| CN (1) | CN103098291B (ja) |
| WO (1) | WO2012066879A1 (ja) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2013069529A1 (ja) * | 2011-11-10 | 2013-05-16 | 株式会社Adeka | 非水電解液及び該電解液を用いた非水電解液二次電池 |
| JPWO2016009808A1 (ja) * | 2014-07-16 | 2017-06-08 | 学校法人東京理科大学 | 非水電解液二次電池及び非水電解液 |
| WO2022070312A1 (ja) * | 2020-09-30 | 2022-04-07 | 昭和電工マテリアルズ株式会社 | 電解液及び電気化学デバイス |
Families Citing this family (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103545551B (zh) * | 2013-11-05 | 2015-12-02 | 张家港市国泰华荣化工新材料有限公司 | 一种适用于钛酸锂电池的电解液 |
| CN103730651A (zh) * | 2013-12-16 | 2014-04-16 | 广西科技大学 | 一种电池正极材料及其高温固相合成方法 |
| KR102476281B1 (ko) * | 2014-10-03 | 2022-12-09 | 실라트로닉스, 인크. | 관능화 실란 및 전해질 조성물 및 이들을 함유하는 전기화학 디바이스 |
| US10541444B2 (en) * | 2014-12-26 | 2020-01-21 | Samsung Sdi Co., Ltd. | Rechargeable lithium battery |
| JP6462125B2 (ja) * | 2015-06-12 | 2019-01-30 | 昭和電工株式会社 | 非水系電池電極用バインダー用組成物、非水系電池電極用バインダー、非水系電池電極用組成物、非水系電池電極、及び非水系電池 |
| CN105206874A (zh) * | 2015-10-19 | 2015-12-30 | 东莞市凯欣电池材料有限公司 | 一种含有炔基硅烷的锂离子电池电解液及使用该电解液的锂离子电池 |
| CN111029650B (zh) * | 2017-02-13 | 2023-05-02 | 宁德新能源科技有限公司 | 一种电解液及二次电池 |
| CN108878975B (zh) * | 2017-05-12 | 2020-02-21 | 宁德时代新能源科技股份有限公司 | 电解液以及包括该电解液的二次电池 |
| KR102576486B1 (ko) * | 2017-06-01 | 2023-09-07 | 가부시끼가이샤 레조낙 | 전해액 및 전기화학 디바이스 |
| EP3637529A4 (en) | 2017-06-01 | 2021-01-20 | Hitachi Chemical Company, Ltd. | ELECTROLYTE SOLUTION AND ELECTROCHEMICAL DEVICE |
| CN110679030B (zh) * | 2017-06-01 | 2022-08-16 | 昭和电工材料株式会社 | 电解液及电化学装置 |
| CN117790904A (zh) * | 2017-09-22 | 2024-03-29 | 三菱化学株式会社 | 非水系电解液、非水系电解液二次电池及能源装置 |
| KR102437872B1 (ko) * | 2018-04-25 | 2022-08-31 | 다이킨 고교 가부시키가이샤 | 전해액, 전기 화학 디바이스, 리튬 이온 이차 전지 및 모듈 |
| CN112310466B (zh) * | 2019-07-31 | 2023-03-10 | 深圳新宙邦科技股份有限公司 | 锂离子电池非水电解液及包含该电解液的锂离子电池 |
| EP4007018A4 (en) * | 2019-07-31 | 2022-11-23 | Mitsubishi Chemical Corporation | ANHYDROUS ELECTROLYTE SOLUTION AND ENERGY DEVICE |
| CN113130970B (zh) * | 2019-12-31 | 2023-07-11 | 深圳新宙邦科技股份有限公司 | 锂离子电池 |
| CN115513528B (zh) * | 2022-11-21 | 2023-05-05 | 广州天赐高新材料股份有限公司 | 非水电解液及二次电池 |
Citations (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS63102173A (ja) | 1986-10-16 | 1988-05-07 | Hitachi Maxell Ltd | リチウム二次電池 |
| JPH0487156A (ja) | 1990-07-26 | 1992-03-19 | Sanyo Electric Co Ltd | 非水系電解液電池 |
| JPH0574486A (ja) | 1991-09-10 | 1993-03-26 | Sanyo Electric Co Ltd | 非水系電解液電池 |
| US5626981A (en) | 1994-04-22 | 1997-05-06 | Saft | Rechargeable lithium electrochemical cell |
| JPH1050342A (ja) | 1996-08-01 | 1998-02-20 | Sony Corp | 非水電解質二次電池 |
| JP2001006729A (ja) | 1999-06-18 | 2001-01-12 | Mitsubishi Chemicals Corp | 非水系電解液二次電池 |
| JP2002134169A (ja) | 2000-10-30 | 2002-05-10 | Denso Corp | 非水電解液及び該電解液を用いた非水電解液二次電池 |
| JP2002198092A (ja) * | 2000-11-27 | 2002-07-12 | Wilson Greatbatch Ltd | 非水性電解液再充電可能な電池のホスフェート添加剤 |
| US20040007688A1 (en) | 2002-07-05 | 2004-01-15 | Denso Corporation | Nonaqueous electrolytic solution and nonaqueous secondary battery using the same |
| JP2004087459A (ja) * | 2002-06-25 | 2004-03-18 | Mitsubishi Chemicals Corp | 非水電解液二次電池 |
| JP2004171981A (ja) * | 2002-11-21 | 2004-06-17 | Mitsui Chemicals Inc | 非水電解液およびそれを用いた二次電池 |
| US20060269843A1 (en) | 2005-05-30 | 2006-11-30 | Denso Corporation | Nonaqueous electrolyte and nonaqueous electrolyte secondary battery using the same |
| US20070243470A1 (en) | 2006-04-17 | 2007-10-18 | Denso Corporation | Nonaqueous electrolyte solution and secondary battery using the electrolyte solution |
| JP2009512148A (ja) * | 2005-10-10 | 2009-03-19 | ゾルファイ フルーオル ゲゼルシャフト ミット ベシュレンクテル ハフツング | リチウムイオン電池用のフッ素化添加剤 |
| JP2010238506A (ja) * | 2009-03-31 | 2010-10-21 | Sanwa Yuka Kogyo Kk | 非水電解液及びそれを用いたリチウムイオン二次電池 |
| JP2011077029A (ja) * | 2009-09-07 | 2011-04-14 | Adeka Corp | 非水電解液及び該電解液を用いた非水電解液二次電池 |
| JP2011082001A (ja) * | 2009-10-06 | 2011-04-21 | Sony Corp | 非水電解質および非水電解質電池 |
| JP2011222450A (ja) * | 2010-04-14 | 2011-11-04 | Denso Corp | 二次電池用非水電解液及び該電解液を用いた非水電解液二次電池 |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6919141B2 (en) | 1998-10-22 | 2005-07-19 | Wilson Greatbatch Technologies, Inc. | Phosphate additives for nonaqueous electrolyte rechargeable electrochemical cells |
| KR102297569B1 (ko) * | 2005-10-20 | 2021-09-02 | 미쯔비시 케미컬 주식회사 | 리튬 2 차 전지 및 그것에 사용하는 비수계 전해액 |
-
2010
- 2010-11-16 JP JP2010256225A patent/JP5781293B2/ja active Active
-
2011
- 2011-10-13 US US13/822,141 patent/US9017866B2/en not_active Expired - Fee Related
- 2011-10-13 EP EP11841545.4A patent/EP2642580B1/en not_active Not-in-force
- 2011-10-13 CN CN201180043785.5A patent/CN103098291B/zh not_active Expired - Fee Related
- 2011-10-13 WO PCT/JP2011/073546 patent/WO2012066879A1/ja active Application Filing
- 2011-10-13 KR KR1020137005879A patent/KR101881450B1/ko active IP Right Grant
Patent Citations (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS63102173A (ja) | 1986-10-16 | 1988-05-07 | Hitachi Maxell Ltd | リチウム二次電池 |
| JPH0487156A (ja) | 1990-07-26 | 1992-03-19 | Sanyo Electric Co Ltd | 非水系電解液電池 |
| JPH0574486A (ja) | 1991-09-10 | 1993-03-26 | Sanyo Electric Co Ltd | 非水系電解液電池 |
| US5626981A (en) | 1994-04-22 | 1997-05-06 | Saft | Rechargeable lithium electrochemical cell |
| JPH1050342A (ja) | 1996-08-01 | 1998-02-20 | Sony Corp | 非水電解質二次電池 |
| JP2001006729A (ja) | 1999-06-18 | 2001-01-12 | Mitsubishi Chemicals Corp | 非水系電解液二次電池 |
| JP2002134169A (ja) | 2000-10-30 | 2002-05-10 | Denso Corp | 非水電解液及び該電解液を用いた非水電解液二次電池 |
| JP2002198092A (ja) * | 2000-11-27 | 2002-07-12 | Wilson Greatbatch Ltd | 非水性電解液再充電可能な電池のホスフェート添加剤 |
| JP2004087459A (ja) * | 2002-06-25 | 2004-03-18 | Mitsubishi Chemicals Corp | 非水電解液二次電池 |
| US20040007688A1 (en) | 2002-07-05 | 2004-01-15 | Denso Corporation | Nonaqueous electrolytic solution and nonaqueous secondary battery using the same |
| JP2004171981A (ja) * | 2002-11-21 | 2004-06-17 | Mitsui Chemicals Inc | 非水電解液およびそれを用いた二次電池 |
| US20060269843A1 (en) | 2005-05-30 | 2006-11-30 | Denso Corporation | Nonaqueous electrolyte and nonaqueous electrolyte secondary battery using the same |
| JP2009512148A (ja) * | 2005-10-10 | 2009-03-19 | ゾルファイ フルーオル ゲゼルシャフト ミット ベシュレンクテル ハフツング | リチウムイオン電池用のフッ素化添加剤 |
| US20090197167A1 (en) | 2005-10-10 | 2009-08-06 | Solvay Fluor Gmbh | Fluorinated Additives For Lithium Ion Batteries |
| US20070243470A1 (en) | 2006-04-17 | 2007-10-18 | Denso Corporation | Nonaqueous electrolyte solution and secondary battery using the electrolyte solution |
| JP2010238506A (ja) * | 2009-03-31 | 2010-10-21 | Sanwa Yuka Kogyo Kk | 非水電解液及びそれを用いたリチウムイオン二次電池 |
| JP2011077029A (ja) * | 2009-09-07 | 2011-04-14 | Adeka Corp | 非水電解液及び該電解液を用いた非水電解液二次電池 |
| JP2011082001A (ja) * | 2009-10-06 | 2011-04-21 | Sony Corp | 非水電解質および非水電解質電池 |
| JP2011222450A (ja) * | 2010-04-14 | 2011-11-04 | Denso Corp | 二次電池用非水電解液及び該電解液を用いた非水電解液二次電池 |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP2642580A4 * |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2013069529A1 (ja) * | 2011-11-10 | 2013-05-16 | 株式会社Adeka | 非水電解液及び該電解液を用いた非水電解液二次電池 |
| EP2779299A4 (en) * | 2011-11-10 | 2015-07-15 | Adeka Corp | WATER-FREE ELECTROLYTE SOLUTION AND SECONDARY BATTERY WITH A WATER-FREE ELECTROLYTE WITH THE ELECTROLYTE SOLUTION |
| US9419306B2 (en) | 2011-11-10 | 2016-08-16 | Adeka Corporation | Nonaqueous electrolyte and nonaqueous secondary battery using same |
| JPWO2016009808A1 (ja) * | 2014-07-16 | 2017-06-08 | 学校法人東京理科大学 | 非水電解液二次電池及び非水電解液 |
| WO2022070312A1 (ja) * | 2020-09-30 | 2022-04-07 | 昭和電工マテリアルズ株式会社 | 電解液及び電気化学デバイス |
Also Published As
| Publication number | Publication date |
|---|---|
| KR20130143557A (ko) | 2013-12-31 |
| US9017866B2 (en) | 2015-04-28 |
| KR101881450B1 (ko) | 2018-07-24 |
| CN103098291A (zh) | 2013-05-08 |
| JP5781293B2 (ja) | 2015-09-16 |
| JP2012109091A (ja) | 2012-06-07 |
| CN103098291B (zh) | 2016-04-27 |
| EP2642580B1 (en) | 2017-03-29 |
| EP2642580A4 (en) | 2014-07-30 |
| EP2642580A1 (en) | 2013-09-25 |
| US20130236777A1 (en) | 2013-09-12 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5781293B2 (ja) | 非水電解液二次電池 | |
| JP5781294B2 (ja) | 非水電解液二次電池 | |
| JP5955629B2 (ja) | 非水電解液二次電池 | |
| JP5506030B2 (ja) | 電池用非水電解液及び該電解液を用いた非水電解液二次電池 | |
| JP5604162B2 (ja) | 二次電池用非水電解液及び該電解液を用いた非水電解液二次電池 | |
| WO2012120597A1 (ja) | 電池用非水電解液及び該電解液を用いた非水電解液二次電池 | |
| JP6438299B2 (ja) | リチウムイオン二次電池 | |
| TWI674693B (zh) | 非水電解液及非水電解液二次電池 | |
| EP3352281B1 (en) | Non-aqueous electrolyte solution and non-aqueous electrolyte secondary battery | |
| JP2013145702A (ja) | 非水電解液二次電池及び二次電池用非水電解液 | |
| WO2016013480A1 (ja) | 非水電解液二次電池、非水電解液及び化合物 | |
| JP5897869B2 (ja) | 新規フルオロシラン化合物 | |
| JP5709574B2 (ja) | 二次電池用非水電解液及び該電解液を有する非水電解液二次電池 | |
| JP5823261B2 (ja) | 非水電解液及び該電解液を用いた非水電解液二次電池 | |
| KR20170069960A (ko) | 비수전해액 및 비수전해액 이차전지 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 201180043785.5 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 11841545 Country of ref document: EP Kind code of ref document: A1 |
|
| DPE1 | Request for preliminary examination filed after expiration of 19th month from priority date (pct application filed from 20040101) | ||
| ENP | Entry into the national phase |
Ref document number: 20137005879 Country of ref document: KR Kind code of ref document: A |
|
| REEP | Request for entry into the european phase |
Ref document number: 2011841545 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2011841545 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 13822141 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |