RU2567811C2 - Способ очистки фактора свертывания крови viii - Google Patents
Способ очистки фактора свертывания крови viii Download PDFInfo
- Publication number
- RU2567811C2 RU2567811C2 RU2011102437/10A RU2011102437A RU2567811C2 RU 2567811 C2 RU2567811 C2 RU 2567811C2 RU 2011102437/10 A RU2011102437/10 A RU 2011102437/10A RU 2011102437 A RU2011102437 A RU 2011102437A RU 2567811 C2 RU2567811 C2 RU 2567811C2
- Authority
- RU
- Russia
- Prior art keywords
- fviii
- resin
- buffer
- multimodal
- elution
- Prior art date
Links
- 238000000034 method Methods 0.000 title claims abstract description 82
- 238000000746 purification Methods 0.000 title claims abstract description 44
- 108010054218 Factor VIII Proteins 0.000 title description 2
- 102000001690 Factor VIII Human genes 0.000 title description 2
- 101000911390 Homo sapiens Coagulation factor VIII Proteins 0.000 claims abstract description 248
- 102100026735 Coagulation factor VIII Human genes 0.000 claims abstract description 243
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 claims abstract description 115
- 239000011347 resin Substances 0.000 claims abstract description 115
- 229920005989 resin Polymers 0.000 claims abstract description 115
- 239000000872 buffer Substances 0.000 claims abstract description 70
- 239000013019 capto adhere Substances 0.000 claims abstract description 41
- 239000004475 Arginine Substances 0.000 claims abstract description 38
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 claims abstract description 38
- 150000001413 amino acids Chemical class 0.000 claims abstract description 30
- 150000003839 salts Chemical class 0.000 claims abstract description 28
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 claims abstract description 13
- 239000004472 Lysine Substances 0.000 claims abstract description 13
- 238000004587 chromatography analysis Methods 0.000 claims abstract description 11
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 claims abstract description 8
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 claims abstract description 7
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 claims abstract description 5
- 125000003277 amino group Chemical group 0.000 claims abstract description 4
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 claims description 158
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 claims description 144
- 239000011780 sodium chloride Substances 0.000 claims description 79
- 238000010828 elution Methods 0.000 claims description 57
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 claims description 55
- 229920000053 polysorbate 80 Polymers 0.000 claims description 55
- 108090000623 proteins and genes Proteins 0.000 claims description 51
- 102000004169 proteins and genes Human genes 0.000 claims description 51
- 229920002684 Sepharose Polymers 0.000 claims description 32
- 239000012149 elution buffer Substances 0.000 claims description 28
- 239000003446 ligand Substances 0.000 claims description 28
- 239000012528 membrane Substances 0.000 claims description 25
- 239000011534 wash buffer Substances 0.000 claims description 19
- 239000000243 solution Substances 0.000 claims description 18
- 238000005406 washing Methods 0.000 claims description 16
- 238000004140 cleaning Methods 0.000 claims description 14
- 230000002779 inactivation Effects 0.000 claims description 13
- 230000003993 interaction Effects 0.000 claims description 13
- 241000700605 Viruses Species 0.000 claims description 11
- 238000001042 affinity chromatography Methods 0.000 claims description 11
- 125000000129 anionic group Chemical group 0.000 claims description 10
- 239000003599 detergent Substances 0.000 claims description 10
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 claims description 9
- 238000012433 multimodal chromatography Methods 0.000 claims description 9
- 239000012564 Q sepharose fast flow resin Substances 0.000 claims description 8
- 239000003729 cation exchange resin Substances 0.000 claims description 8
- 239000012535 impurity Substances 0.000 claims description 8
- NWUYHJFMYQTDRP-UHFFFAOYSA-N 1,2-bis(ethenyl)benzene;1-ethenyl-2-ethylbenzene;styrene Chemical compound C=CC1=CC=CC=C1.CCC1=CC=CC=C1C=C.C=CC1=CC=CC=C1C=C NWUYHJFMYQTDRP-UHFFFAOYSA-N 0.000 claims description 7
- 240000004808 Saccharomyces cerevisiae Species 0.000 claims description 7
- 238000001914 filtration Methods 0.000 claims description 7
- 239000012634 fragment Substances 0.000 claims description 7
- 230000002209 hydrophobic effect Effects 0.000 claims description 7
- 239000013017 sartobind Substances 0.000 claims description 7
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 claims description 6
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 claims description 6
- 125000000524 functional group Chemical group 0.000 claims description 6
- 244000052769 pathogen Species 0.000 claims description 6
- 125000002091 cationic group Chemical group 0.000 claims description 5
- 150000002500 ions Chemical class 0.000 claims description 5
- 230000007935 neutral effect Effects 0.000 claims description 5
- 150000002894 organic compounds Chemical class 0.000 claims description 5
- 239000002904 solvent Substances 0.000 claims description 5
- 239000012505 Superdex™ Substances 0.000 claims description 4
- 239000013504 Triton X-100 Substances 0.000 claims description 4
- 229920004890 Triton X-100 Polymers 0.000 claims description 4
- 238000005341 cation exchange Methods 0.000 claims description 4
- 239000003153 chemical reaction reagent Substances 0.000 claims description 4
- 150000001875 compounds Chemical class 0.000 claims description 4
- 239000011159 matrix material Substances 0.000 claims description 4
- 238000000926 separation method Methods 0.000 claims description 4
- 239000003957 anion exchange resin Substances 0.000 claims description 3
- 239000007864 aqueous solution Substances 0.000 claims description 3
- 239000012928 buffer substance Substances 0.000 claims description 3
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims description 3
- 230000001717 pathogenic effect Effects 0.000 claims description 3
- 238000001542 size-exclusion chromatography Methods 0.000 claims description 3
- STCOOQWBFONSKY-UHFFFAOYSA-N tributyl phosphate Chemical compound CCCCOP(=O)(OCCCC)OCCCC STCOOQWBFONSKY-UHFFFAOYSA-N 0.000 claims description 3
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 claims description 2
- WOUANPHGFPAJCA-UHFFFAOYSA-N 2-[benzyl(methyl)amino]ethanol Chemical compound OCCN(C)CC1=CC=CC=C1 WOUANPHGFPAJCA-UHFFFAOYSA-N 0.000 claims description 2
- OACIMSFRSUBGQU-UHFFFAOYSA-N 2-benzamidobutanoic acid Chemical compound CCC(C(O)=O)NC(=O)C1=CC=CC=C1 OACIMSFRSUBGQU-UHFFFAOYSA-N 0.000 claims description 2
- VBAOEVKQBLGWTH-UHFFFAOYSA-N 2-pyridin-4-ylethanethiol Chemical compound SCCC1=CC=NC=C1 VBAOEVKQBLGWTH-UHFFFAOYSA-N 0.000 claims description 2
- PWLKDZUOOBKOAW-UHFFFAOYSA-N 3-[3-methyl-5-(oxolan-2-ylmethylamino)anilino]benzoic acid Chemical compound C=1C(NC=2C=C(C=CC=2)C(O)=O)=CC(C)=CC=1NCC1CCCO1 PWLKDZUOOBKOAW-UHFFFAOYSA-N 0.000 claims description 2
- 239000007995 HEPES buffer Substances 0.000 claims description 2
- 239000007987 MES buffer Substances 0.000 claims description 2
- 229920001213 Polysorbate 20 Polymers 0.000 claims description 2
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 claims description 2
- 238000010936 aqueous wash Methods 0.000 claims description 2
- 239000012539 chromatography resin Substances 0.000 claims description 2
- HQPMKSGTIOYHJT-UHFFFAOYSA-N ethane-1,2-diol;propane-1,2-diol Chemical compound OCCO.CC(O)CO HQPMKSGTIOYHJT-UHFFFAOYSA-N 0.000 claims description 2
- 229910052739 hydrogen Inorganic materials 0.000 claims description 2
- 239000001257 hydrogen Substances 0.000 claims description 2
- 230000000415 inactivating effect Effects 0.000 claims description 2
- 150000002632 lipids Chemical class 0.000 claims description 2
- 125000004344 phenylpropyl group Chemical group 0.000 claims description 2
- 230000001766 physiological effect Effects 0.000 claims description 2
- 229920001993 poloxamer 188 Polymers 0.000 claims description 2
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 claims description 2
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 claims description 2
- 239000011148 porous material Substances 0.000 claims description 2
- BDERNNFJNOPAEC-UHFFFAOYSA-N propan-1-ol Chemical compound CCCO BDERNNFJNOPAEC-UHFFFAOYSA-N 0.000 claims description 2
- 230000001105 regulatory effect Effects 0.000 claims description 2
- 239000001632 sodium acetate Substances 0.000 claims description 2
- 235000017281 sodium acetate Nutrition 0.000 claims description 2
- 239000001509 sodium citrate Substances 0.000 claims description 2
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 claims description 2
- 239000012736 aqueous medium Substances 0.000 claims 1
- 239000013043 chemical agent Substances 0.000 claims 1
- 230000000694 effects Effects 0.000 abstract description 29
- 239000000126 substance Substances 0.000 abstract description 9
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 abstract description 5
- 230000023555 blood coagulation Effects 0.000 abstract description 4
- 239000003814 drug Substances 0.000 abstract description 3
- 229960002885 histidine Drugs 0.000 description 57
- 239000007858 starting material Substances 0.000 description 54
- 239000000244 polyoxyethylene sorbitan monooleate Substances 0.000 description 53
- 229940068968 polysorbate 80 Drugs 0.000 description 53
- 235000018102 proteins Nutrition 0.000 description 43
- 239000000047 product Substances 0.000 description 41
- 108020004414 DNA Proteins 0.000 description 40
- 229960003121 arginine Drugs 0.000 description 35
- 235000009697 arginine Nutrition 0.000 description 35
- 210000004027 cell Anatomy 0.000 description 27
- 239000000706 filtrate Substances 0.000 description 23
- 229940024606 amino acid Drugs 0.000 description 20
- 235000001014 amino acid Nutrition 0.000 description 20
- 239000000523 sample Substances 0.000 description 19
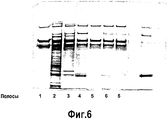
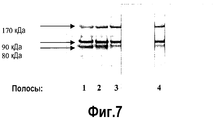
- 238000004458 analytical method Methods 0.000 description 18
- 239000000499 gel Substances 0.000 description 16
- 239000012460 protein solution Substances 0.000 description 16
- 238000011282 treatment Methods 0.000 description 14
- 238000009010 Bradford assay Methods 0.000 description 12
- 238000000605 extraction Methods 0.000 description 12
- 238000002474 experimental method Methods 0.000 description 10
- 239000000203 mixture Substances 0.000 description 10
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 9
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 9
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 9
- 229960002920 sorbitol Drugs 0.000 description 9
- 208000009292 Hemophilia A Diseases 0.000 description 8
- 239000013024 dilution buffer Substances 0.000 description 8
- 210000002381 plasma Anatomy 0.000 description 8
- 230000008569 process Effects 0.000 description 8
- 108090000765 processed proteins & peptides Proteins 0.000 description 7
- 108010025139 recombinant factor VIII SQ Proteins 0.000 description 7
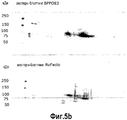
- 238000001262 western blot Methods 0.000 description 7
- KWTQSFXGGICVPE-UHFFFAOYSA-N 2-amino-5-(diaminomethylideneamino)pentanoic acid;hydron;chloride Chemical compound Cl.OC(=O)C(N)CCCN=C(N)N KWTQSFXGGICVPE-UHFFFAOYSA-N 0.000 description 6
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 6
- 239000011575 calcium Substances 0.000 description 6
- 238000011010 flushing procedure Methods 0.000 description 6
- 238000002523 gelfiltration Methods 0.000 description 6
- 238000004519 manufacturing process Methods 0.000 description 6
- 102000004196 processed proteins & peptides Human genes 0.000 description 6
- 229910052709 silver Inorganic materials 0.000 description 6
- 239000004332 silver Substances 0.000 description 6
- 208000031220 Hemophilia Diseases 0.000 description 5
- 241000282414 Homo sapiens Species 0.000 description 5
- 238000005571 anion exchange chromatography Methods 0.000 description 5
- 238000005277 cation exchange chromatography Methods 0.000 description 5
- 238000004113 cell culture Methods 0.000 description 5
- 230000015271 coagulation Effects 0.000 description 5
- 238000005345 coagulation Methods 0.000 description 5
- 230000000052 comparative effect Effects 0.000 description 5
- 230000009467 reduction Effects 0.000 description 5
- 239000000600 sorbitol Substances 0.000 description 5
- UXVMQQNJUSDDNG-UHFFFAOYSA-L Calcium chloride Chemical compound [Cl-].[Cl-].[Ca+2] UXVMQQNJUSDDNG-UHFFFAOYSA-L 0.000 description 4
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 4
- 230000008901 benefit Effects 0.000 description 4
- 239000001110 calcium chloride Substances 0.000 description 4
- 229910001628 calcium chloride Inorganic materials 0.000 description 4
- 239000006285 cell suspension Substances 0.000 description 4
- 239000012141 concentrate Substances 0.000 description 4
- 238000005516 engineering process Methods 0.000 description 4
- 230000036541 health Effects 0.000 description 4
- 238000004255 ion exchange chromatography Methods 0.000 description 4
- 238000011068 loading method Methods 0.000 description 4
- 238000002264 polyacrylamide gel electrophoresis Methods 0.000 description 4
- 229920001184 polypeptide Polymers 0.000 description 4
- 238000012545 processing Methods 0.000 description 4
- 238000011084 recovery Methods 0.000 description 4
- 108091023043 Alu Element Proteins 0.000 description 3
- KWTQSFXGGICVPE-WCCKRBBISA-N Arginine hydrochloride Chemical compound Cl.OC(=O)[C@@H](N)CCCN=C(N)N KWTQSFXGGICVPE-WCCKRBBISA-N 0.000 description 3
- 201000003542 Factor VIII deficiency Diseases 0.000 description 3
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 3
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 3
- 241001465754 Metazoa Species 0.000 description 3
- 239000000654 additive Substances 0.000 description 3
- 238000005349 anion exchange Methods 0.000 description 3
- 229960003589 arginine hydrochloride Drugs 0.000 description 3
- 235000003704 aspartic acid Nutrition 0.000 description 3
- 238000003556 assay Methods 0.000 description 3
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 3
- 229940023913 cation exchange resins Drugs 0.000 description 3
- 230000008859 change Effects 0.000 description 3
- 238000006243 chemical reaction Methods 0.000 description 3
- 238000011210 chromatographic step Methods 0.000 description 3
- 238000011161 development Methods 0.000 description 3
- 238000010790 dilution Methods 0.000 description 3
- 239000012895 dilution Substances 0.000 description 3
- 239000000975 dye Substances 0.000 description 3
- 238000001962 electrophoresis Methods 0.000 description 3
- 238000011156 evaluation Methods 0.000 description 3
- 239000012467 final product Substances 0.000 description 3
- 238000005227 gel permeation chromatography Methods 0.000 description 3
- 235000013922 glutamic acid Nutrition 0.000 description 3
- 239000004220 glutamic acid Substances 0.000 description 3
- 239000003456 ion exchange resin Substances 0.000 description 3
- 229920003303 ion-exchange polymer Polymers 0.000 description 3
- 230000010412 perfusion Effects 0.000 description 3
- 229920002401 polyacrylamide Polymers 0.000 description 3
- 229920000642 polymer Polymers 0.000 description 3
- 238000002360 preparation method Methods 0.000 description 3
- 238000001179 sorption measurement Methods 0.000 description 3
- 239000000725 suspension Substances 0.000 description 3
- TYMLOMAKGOJONV-UHFFFAOYSA-N 4-nitroaniline Chemical compound NC1=CC=C([N+]([O-])=O)C=C1 TYMLOMAKGOJONV-UHFFFAOYSA-N 0.000 description 2
- 108010088751 Albumins Proteins 0.000 description 2
- 102000009027 Albumins Human genes 0.000 description 2
- 208000002109 Argyria Diseases 0.000 description 2
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 2
- BHPQYMZQTOCNFJ-UHFFFAOYSA-N Calcium cation Chemical compound [Ca+2] BHPQYMZQTOCNFJ-UHFFFAOYSA-N 0.000 description 2
- 241000283707 Capra Species 0.000 description 2
- 108010074860 Factor Xa Proteins 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 2
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 2
- 241001494479 Pecora Species 0.000 description 2
- 238000010521 absorption reaction Methods 0.000 description 2
- 235000009582 asparagine Nutrition 0.000 description 2
- 229960001230 asparagine Drugs 0.000 description 2
- 229910001424 calcium ion Inorganic materials 0.000 description 2
- 239000013018 capto adhere resin Substances 0.000 description 2
- 239000002738 chelating agent Substances 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 238000011097 chromatography purification Methods 0.000 description 2
- 239000003593 chromogenic compound Substances 0.000 description 2
- 238000012364 cultivation method Methods 0.000 description 2
- 230000007812 deficiency Effects 0.000 description 2
- 230000001419 dependent effect Effects 0.000 description 2
- 238000007710 freezing Methods 0.000 description 2
- 230000008014 freezing Effects 0.000 description 2
- 239000011521 glass Substances 0.000 description 2
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 2
- 230000007062 hydrolysis Effects 0.000 description 2
- 238000006460 hydrolysis reaction Methods 0.000 description 2
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 2
- 238000005342 ion exchange Methods 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 239000002609 medium Substances 0.000 description 2
- 239000003147 molecular marker Substances 0.000 description 2
- 238000001728 nano-filtration Methods 0.000 description 2
- 238000001556 precipitation Methods 0.000 description 2
- 238000010188 recombinant method Methods 0.000 description 2
- 238000012552 review Methods 0.000 description 2
- 239000012679 serum free medium Substances 0.000 description 2
- 230000000087 stabilizing effect Effects 0.000 description 2
- 239000000758 substrate Substances 0.000 description 2
- ZOOGRGPOEVQQDX-UUOKFMHZSA-N 3',5'-cyclic GMP Chemical compound C([C@H]1O2)OP(O)(=O)O[C@H]1[C@@H](O)[C@@H]2N1C(N=C(NC2=O)N)=C2N=C1 ZOOGRGPOEVQQDX-UUOKFMHZSA-N 0.000 description 1
- LVSPDZAGCBEQAV-UHFFFAOYSA-N 4-chloronaphthalen-1-ol Chemical compound C1=CC=C2C(O)=CC=C(Cl)C2=C1 LVSPDZAGCBEQAV-UHFFFAOYSA-N 0.000 description 1
- 108010039627 Aprotinin Proteins 0.000 description 1
- 101100173896 Buchnera aphidicola subsp. Baizongia pistaciae (strain Bp) fliF gene Proteins 0.000 description 1
- 101100120255 Buchnera aphidicola subsp. Baizongia pistaciae (strain Bp) fliG gene Proteins 0.000 description 1
- 101100352594 Buchnera aphidicola subsp. Baizongia pistaciae (strain Bp) pmbA gene Proteins 0.000 description 1
- 102100035180 Coiled-coil domain-containing protein 62 Human genes 0.000 description 1
- 206010009944 Colon cancer Diseases 0.000 description 1
- 208000001333 Colorectal Neoplasms Diseases 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- 241000282575 Gorilla Species 0.000 description 1
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 description 1
- 241000282418 Hominidae Species 0.000 description 1
- 101000737082 Homo sapiens Coiled-coil domain-containing protein 62 Proteins 0.000 description 1
- 102000008100 Human Serum Albumin Human genes 0.000 description 1
- 108091006905 Human Serum Albumin Proteins 0.000 description 1
- PMMYEEVYMWASQN-DMTCNVIQSA-N Hydroxyproline Chemical compound O[C@H]1CN[C@H](C(O)=O)C1 PMMYEEVYMWASQN-DMTCNVIQSA-N 0.000 description 1
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 1
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 1
- 208000026350 Inborn Genetic disease Diseases 0.000 description 1
- 108090001061 Insulin Proteins 0.000 description 1
- 102000004877 Insulin Human genes 0.000 description 1
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 1
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 1
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 1
- 108090001090 Lectins Proteins 0.000 description 1
- 102000004856 Lectins Human genes 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 102000005741 Metalloproteases Human genes 0.000 description 1
- 108010006035 Metalloproteases Proteins 0.000 description 1
- 239000000020 Nitrocellulose Substances 0.000 description 1
- 241000282577 Pan troglodytes Species 0.000 description 1
- 108091005804 Peptidases Proteins 0.000 description 1
- 102000035195 Peptidases Human genes 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 241000282405 Pongo abelii Species 0.000 description 1
- 102000029797 Prion Human genes 0.000 description 1
- 108091000054 Prion Proteins 0.000 description 1
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 1
- 239000004365 Protease Substances 0.000 description 1
- 101000712605 Theromyzon tessulatum Theromin Proteins 0.000 description 1
- 108090000190 Thrombin Proteins 0.000 description 1
- 229940122388 Thrombin inhibitor Drugs 0.000 description 1
- 208000007536 Thrombosis Diseases 0.000 description 1
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 239000003929 acidic solution Substances 0.000 description 1
- 239000003463 adsorbent Substances 0.000 description 1
- 230000002776 aggregation Effects 0.000 description 1
- 238000004220 aggregation Methods 0.000 description 1
- 229940050528 albumin Drugs 0.000 description 1
- WNROFYMDJYEPJX-UHFFFAOYSA-K aluminium hydroxide Chemical compound [OH-].[OH-].[OH-].[Al+3] WNROFYMDJYEPJX-UHFFFAOYSA-K 0.000 description 1
- BFNBIHQBYMNNAN-UHFFFAOYSA-N ammonium sulfate Chemical compound N.N.OS(O)(=O)=O BFNBIHQBYMNNAN-UHFFFAOYSA-N 0.000 description 1
- 229910052921 ammonium sulfate Inorganic materials 0.000 description 1
- 235000011130 ammonium sulphate Nutrition 0.000 description 1
- 210000004102 animal cell Anatomy 0.000 description 1
- 239000003945 anionic surfactant Substances 0.000 description 1
- 150000001450 anions Chemical class 0.000 description 1
- 229960004405 aprotinin Drugs 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 238000012365 batch cultivation Methods 0.000 description 1
- 238000010923 batch production Methods 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 238000004166 bioassay Methods 0.000 description 1
- 230000008033 biological extinction Effects 0.000 description 1
- 239000002981 blocking agent Substances 0.000 description 1
- 239000006172 buffering agent Substances 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 125000003636 chemical group Chemical group 0.000 description 1
- 230000004186 co-expression Effects 0.000 description 1
- 229940105778 coagulation factor viii Drugs 0.000 description 1
- 238000004440 column chromatography Methods 0.000 description 1
- 239000013068 control sample Substances 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 235000018417 cysteine Nutrition 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000007850 degeneration Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000001687 destabilization Effects 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 238000000502 dialysis Methods 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- PMMYEEVYMWASQN-UHFFFAOYSA-N dl-hydroxyproline Natural products OC1C[NH2+]C(C([O-])=O)C1 PMMYEEVYMWASQN-UHFFFAOYSA-N 0.000 description 1
- 230000005684 electric field Effects 0.000 description 1
- 239000003480 eluent Substances 0.000 description 1
- -1 for example Chemical compound 0.000 description 1
- 238000005194 fractionation Methods 0.000 description 1
- 238000004108 freeze drying Methods 0.000 description 1
- 230000006870 function Effects 0.000 description 1
- 238000007306 functionalization reaction Methods 0.000 description 1
- 230000014509 gene expression Effects 0.000 description 1
- 238000007429 general method Methods 0.000 description 1
- 208000016361 genetic disease Diseases 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- 229960002897 heparin Drugs 0.000 description 1
- 229920000669 heparin Polymers 0.000 description 1
- 238000004191 hydrophobic interaction chromatography Methods 0.000 description 1
- 229910052588 hydroxylapatite Inorganic materials 0.000 description 1
- 229960002591 hydroxyproline Drugs 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 238000000338 in vitro Methods 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- ZPNFWUPYTFPOJU-LPYSRVMUSA-N iniprol Chemical compound C([C@H]1C(=O)NCC(=O)NCC(=O)N[C@H]2CSSC[C@H]3C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](C(N[C@H](C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=4C=CC(O)=CC=4)C(=O)N[C@@H](CC=4C=CC=CC=4)C(=O)N[C@@H](CC=4C=CC(O)=CC=4)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC=4C=CC=CC=4)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC2=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](CC=2C=CC=CC=2)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H]2N(CCC2)C(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N2[C@@H](CCC2)C(=O)N2[C@@H](CCC2)C(=O)N[C@@H](CC=2C=CC(O)=CC=2)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N2[C@@H](CCC2)C(=O)N3)C(=O)NCC(=O)NCC(=O)N[C@@H](C)C(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](C(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@H](C(=O)N1)C(C)C)[C@@H](C)O)[C@@H](C)CC)=O)[C@@H](C)CC)C1=CC=C(O)C=C1 ZPNFWUPYTFPOJU-LPYSRVMUSA-N 0.000 description 1
- 229940125396 insulin Drugs 0.000 description 1
- 238000001155 isoelectric focusing Methods 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 239000002523 lectin Substances 0.000 description 1
- 229960003646 lysine Drugs 0.000 description 1
- 239000006249 magnetic particle Substances 0.000 description 1
- 210000001161 mammalian embryo Anatomy 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 229940126601 medicinal product Drugs 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- 230000000813 microbial effect Effects 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 239000002808 molecular sieve Substances 0.000 description 1
- 239000010413 mother solution Substances 0.000 description 1
- FEMOMIGRRWSMCU-UHFFFAOYSA-N ninhydrin Chemical compound C1=CC=C2C(=O)C(O)(O)C(=O)C2=C1 FEMOMIGRRWSMCU-UHFFFAOYSA-N 0.000 description 1
- 229920001220 nitrocellulos Polymers 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- XYJRXVWERLGGKC-UHFFFAOYSA-D pentacalcium;hydroxide;triphosphate Chemical compound [OH-].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O XYJRXVWERLGGKC-UHFFFAOYSA-D 0.000 description 1
- 201000009981 periampullary adenocarcinoma Diseases 0.000 description 1
- 102000013415 peroxidase activity proteins Human genes 0.000 description 1
- 108040007629 peroxidase activity proteins Proteins 0.000 description 1
- 229920001467 poly(styrenesulfonates) Polymers 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 230000003234 polygenic effect Effects 0.000 description 1
- 230000001376 precipitating effect Effects 0.000 description 1
- 239000011546 protein dye Substances 0.000 description 1
- 230000006920 protein precipitation Effects 0.000 description 1
- 238000001742 protein purification Methods 0.000 description 1
- 238000003908 quality control method Methods 0.000 description 1
- 238000013139 quantization Methods 0.000 description 1
- 125000001453 quaternary ammonium group Chemical group 0.000 description 1
- 238000003753 real-time PCR Methods 0.000 description 1
- 239000013558 reference substance Substances 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- URGAHOPLAPQHLN-UHFFFAOYSA-N sodium aluminosilicate Chemical compound [Na+].[Al+3].[O-][Si]([O-])=O.[O-][Si]([O-])=O URGAHOPLAPQHLN-UHFFFAOYSA-N 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 239000013589 supplement Substances 0.000 description 1
- 238000010257 thawing Methods 0.000 description 1
- 229960004072 thrombin Drugs 0.000 description 1
- 239000003868 thrombin inhibitor Substances 0.000 description 1
- 210000001519 tissue Anatomy 0.000 description 1
- 238000004448 titration Methods 0.000 description 1
- FGMPLJWBKKVCDB-UHFFFAOYSA-N trans-L-hydroxy-proline Natural products ON1CCCC1C(O)=O FGMPLJWBKKVCDB-UHFFFAOYSA-N 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 238000000539 two dimensional gel electrophoresis Methods 0.000 description 1
- 238000001419 two-dimensional polyacrylamide gel electrophoresis Methods 0.000 description 1
- 238000010200 validation analysis Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K1/00—General methods for the preparation of peptides, i.e. processes for the organic chemical preparation of peptides or proteins of any length
- C07K1/14—Extraction; Separation; Purification
- C07K1/16—Extraction; Separation; Purification by chromatography
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/04—Peptides having up to 20 amino acids in a fully defined sequence; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/36—Blood coagulation or fibrinolysis factors
- A61K38/37—Factors VIII
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/55—Protease inhibitors
- A61K38/57—Protease inhibitors from animals; from humans
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D15/00—Separating processes involving the treatment of liquids with solid sorbents; Apparatus therefor
- B01D15/08—Selective adsorption, e.g. chromatography
- B01D15/26—Selective adsorption, e.g. chromatography characterised by the separation mechanism
- B01D15/38—Selective adsorption, e.g. chromatography characterised by the separation mechanism involving specific interaction not covered by one or more of groups B01D15/265 and B01D15/30 - B01D15/36, e.g. affinity, ligand exchange or chiral chromatography
- B01D15/3847—Multimodal interactions
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K1/00—General methods for the preparation of peptides, i.e. processes for the organic chemical preparation of peptides or proteins of any length
- C07K1/14—Extraction; Separation; Purification
- C07K1/36—Extraction; Separation; Purification by a combination of two or more processes of different types
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/745—Blood coagulation or fibrinolysis factors
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/745—Blood coagulation or fibrinolysis factors
- C07K14/755—Factors VIII, e.g. factor VIII C (AHF), factor VIII Ag (VWF)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/14—Hydrolases (3)
- C12N9/48—Hydrolases (3) acting on peptide bonds (3.4)
- C12N9/50—Proteinases, e.g. Endopeptidases (3.4.21-3.4.25)
- C12N9/64—Proteinases, e.g. Endopeptidases (3.4.21-3.4.25) derived from animal tissue
- C12N9/6421—Proteinases, e.g. Endopeptidases (3.4.21-3.4.25) derived from animal tissue from mammals
- C12N9/6424—Serine endopeptidases (3.4.21)
- C12N9/647—Blood coagulation factors not provided for in a preceding group or according to more than one of the proceeding groups
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Zoology (AREA)
- Genetics & Genomics (AREA)
- Engineering & Computer Science (AREA)
- Gastroenterology & Hepatology (AREA)
- Biochemistry (AREA)
- Molecular Biology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Hematology (AREA)
- Biophysics (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Immunology (AREA)
- Toxicology (AREA)
- Analytical Chemistry (AREA)
- Wood Science & Technology (AREA)
- Biomedical Technology (AREA)
- Microbiology (AREA)
- General Engineering & Computer Science (AREA)
- Biotechnology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Peptides Or Proteins (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US12940208P | 2008-06-24 | 2008-06-24 | |
| EP08158893.1 | 2008-06-24 | ||
| EP08158893 | 2008-06-24 | ||
| US61/129,402 | 2008-06-24 | ||
| PCT/EP2009/057883 WO2009156430A1 (en) | 2008-06-24 | 2009-06-24 | A process of purifying coagulation factor viii |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2015141849A Division RU2698392C2 (ru) | 2008-06-24 | 2009-06-24 | Способ очистки фактора свертывания крови viii |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2011102437A RU2011102437A (ru) | 2012-07-27 |
| RU2567811C2 true RU2567811C2 (ru) | 2015-11-10 |
Family
ID=40076764
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2015141849A RU2698392C2 (ru) | 2008-06-24 | 2009-06-24 | Способ очистки фактора свертывания крови viii |
| RU2011102437/10A RU2567811C2 (ru) | 2008-06-24 | 2009-06-24 | Способ очистки фактора свертывания крови viii |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2015141849A RU2698392C2 (ru) | 2008-06-24 | 2009-06-24 | Способ очистки фактора свертывания крови viii |
Country Status (19)
| Country | Link |
|---|---|
| US (1) | US8329871B2 (enExample) |
| EP (2) | EP2300497B1 (enExample) |
| JP (1) | JP5619738B2 (enExample) |
| KR (3) | KR101700722B1 (enExample) |
| CN (1) | CN102066417B (enExample) |
| AU (1) | AU2009264282B2 (enExample) |
| BR (1) | BRPI0914695B1 (enExample) |
| CA (1) | CA2728047C (enExample) |
| DK (2) | DK2537862T3 (enExample) |
| ES (2) | ES2538706T3 (enExample) |
| IL (3) | IL229583B (enExample) |
| MX (1) | MX2010013908A (enExample) |
| PL (1) | PL2300497T3 (enExample) |
| PT (1) | PT2300497E (enExample) |
| RU (2) | RU2698392C2 (enExample) |
| SI (1) | SI2300497T1 (enExample) |
| UA (1) | UA100901C2 (enExample) |
| WO (1) | WO2009156430A1 (enExample) |
| ZA (1) | ZA201009162B (enExample) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2734783C2 (ru) * | 2016-02-11 | 2020-10-23 | Октафарма Аг | Способ отделения фактора viii от продуктов крови |
| RU2800431C2 (ru) * | 2017-06-23 | 2023-07-21 | Такеда Фармасьютикал Компани Лимитед | Очистка подвидов фактора viii |
Families Citing this family (41)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5323072B2 (ja) | 2007-07-11 | 2013-10-23 | ノボ・ノルデイスク・エー/エス | 混合方式又は多様式樹脂を使用する第viii因子の精製 |
| EP2027875A1 (en) * | 2007-08-23 | 2009-02-25 | Octapharma AG | A Process for Isolation and Purification of a Target Protein free of Prion Protein (PrPSC) |
| BRPI0917656A2 (pt) | 2008-08-21 | 2017-07-11 | Octapharma Ag | Fatores viii e ix humanos produzidos de forma recombinante |
| SI2337580T1 (sl) * | 2008-09-03 | 2012-06-29 | Octapharma Ag | Stabilizirani sestavki za rekombinanto proizveden faktor VIII |
| WO2010030222A1 (en) * | 2008-09-12 | 2010-03-18 | Ge Healthcare Bio-Sciences Ab | Enhanced protein aggregate removal with multimodal anion exchangers in the presence of protein-excluded zwitterions |
| TR201911082T4 (tr) * | 2010-03-30 | 2019-08-21 | Octapharma Ag | K vitaminine bağımlı proteinlerin saflaştırılması için bir işlem. |
| KR101951446B1 (ko) | 2010-03-30 | 2019-02-22 | 옥타파마 아게 | 성장 인자 단백질을 정제하는 방법 |
| AU2011244348B2 (en) | 2010-04-20 | 2015-02-12 | Octapharma Ag | New stabilizing agent for pharmaceutical proteins |
| WO2012049285A1 (en) | 2010-10-14 | 2012-04-19 | Octapharma Ag | A method for the quantitative glycosylation analysis of proteins |
| MX342235B (es) | 2010-11-05 | 2016-09-21 | Hoffmann La Roche | Metodo optimizado para captura de anticuerpo por cromatografia de modo mixto. |
| EP3626737B1 (en) * | 2011-05-13 | 2023-11-29 | Octapharma AG | A method of increasing the productivity of eucaryotic cells in the production of recombinant fviii |
| CN103688177B (zh) | 2011-06-17 | 2017-07-04 | 学校法人东日本学园 | 狼疮抗凝物检测用血液凝固时间的测定方法 |
| US20130109807A1 (en) * | 2011-10-26 | 2013-05-02 | Bio-Rad Laboratories, Inc. | Removal of virucidal agents in mixed mode chromatography |
| WO2014066471A1 (en) * | 2012-10-24 | 2014-05-01 | Genzyme Corporation | Elution of biomolecules from multi-modal resins using mes and mops as mobile phase modifiers |
| US10188965B2 (en) | 2012-12-05 | 2019-01-29 | Csl Behring Gmbh | Hydrophobic charge induction chromatographic depletion of a protein from a solution |
| US20140154233A1 (en) | 2012-12-05 | 2014-06-05 | Csl Limited | Method of purifying therapeutic proteins |
| CN103880947B (zh) * | 2012-12-21 | 2016-01-13 | 武汉禾元生物科技股份有限公司 | 一种分离纯化高纯度重组人血清白蛋白的层析方法 |
| IL274646B (en) * | 2013-03-08 | 2022-09-01 | Genzyme Corp | Integrated continuous production of medicinal substances from medical protein |
| US20160130361A1 (en) * | 2013-06-13 | 2016-05-12 | Biogen Ma Inc. | Anti-factor viii antibodies or uses thereof |
| EP3019262A1 (en) | 2013-07-12 | 2016-05-18 | Merck Patent GmbH | Removal of fragments from a sample containing a target protein using activated carbon |
| US10947269B2 (en) | 2013-08-08 | 2021-03-16 | Bioverativ Therapeutics Inc. | Purification of chimeric FVIII molecules |
| CN105579468A (zh) | 2013-09-24 | 2016-05-11 | 辉瑞大药厂 | 包含重组人凝血因子Xa蛋白的异质性群体的组合物 |
| TWI709569B (zh) | 2014-01-17 | 2020-11-11 | 美商健臻公司 | 無菌層析樹脂及其用於製造方法的用途 |
| TWI709570B (zh) * | 2014-01-17 | 2020-11-11 | 美商健臻公司 | 無菌層析法及製法 |
| EP3097118B1 (en) | 2014-01-20 | 2018-07-18 | Octapharma AG | A process for manufacturing factor viii having an improved ratio of fviii:c/fviii:ag |
| HUE051470T2 (hu) * | 2014-01-24 | 2021-03-01 | Am Pharma Bv | Egy alkalikus foszfatáz feldolgozási folyamata |
| EP3102589A1 (en) * | 2014-02-04 | 2016-12-14 | Biogen MA Inc. | Use of cation-exchange chromatography in the flow-through mode to enrich post-translational modifications |
| CN104861060A (zh) * | 2014-02-21 | 2015-08-26 | 神州细胞工程有限公司 | 一种纯化凝血因子viii的方法 |
| WO2015183183A1 (en) | 2014-05-29 | 2015-12-03 | Agency For Science, Technology And Research | Protein Extraction Methods |
| US10626164B2 (en) * | 2014-07-25 | 2020-04-21 | Csl Limited | Purification of VWF |
| GB201506117D0 (en) * | 2015-04-10 | 2015-05-27 | Ge Healthcare Bio Sciences Ab | Method for chromatography |
| GB201506113D0 (en) | 2015-04-10 | 2015-05-27 | Ge Healthcare Bio Sciences Ab | Method for chromatography |
| WO2016207328A1 (en) * | 2015-06-24 | 2016-12-29 | Glycotope Gmbh | PROCESS FOR THE PURIFICATION OF γ-CARBOXYLATED POLYPEPTIDES |
| GB201617240D0 (en) * | 2016-10-11 | 2016-11-23 | Profactor Pharma Ltd | Purification process |
| JP7253505B2 (ja) * | 2017-06-23 | 2023-04-06 | 武田薬品工業株式会社 | 第viii因子亜種の精製 |
| CN107226859B (zh) * | 2017-08-10 | 2020-11-24 | 博雅生物制药集团股份有限公司 | 一种人凝血因子ⅷ的制备方法 |
| WO2019045149A1 (en) * | 2017-08-31 | 2019-03-07 | Green Cross Corporation | PROCESS FOR PURIFYING SULFATASE PROTEIN |
| CN111511800B (zh) | 2017-10-30 | 2023-11-28 | 武田药品工业株式会社 | 灭活脂包膜病毒的环境相容性去污剂 |
| BR112021003420A2 (pt) | 2018-08-31 | 2021-05-18 | Genzyme Corporation | resina cromatográfica estéril e uso da mesma em processos de manufatura |
| KR20220130692A (ko) * | 2020-01-20 | 2022-09-27 | 우시 바이올로직스 아일랜드 리미티드 | 친화성 크로마토그래피를 위한 신규한 세척 완충제 용액 |
| CN118217951B (zh) * | 2024-04-01 | 2025-05-23 | 山东建筑大学 | 一种水中的高纯度磷回收装置和方法 |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1994008686A1 (en) * | 1992-10-21 | 1994-04-28 | Cornell Research Foundation, Inc. | Multimodal chromatographic separation media and process for usingsame |
| WO2005082483A1 (en) * | 2004-02-27 | 2005-09-09 | Ge Healthcare Bio-Sciences Ab | A process for the purification of antibodies |
| WO2005121163A2 (en) * | 2004-06-07 | 2005-12-22 | Upfront Chromatography A/S | Isolation of plasma or serum proteins |
| RU2324495C1 (ru) * | 2006-08-31 | 2008-05-20 | Федеральное государственное унитарное предприятие "Научно-производственное объединение по медицинским иммунобиологическим препаратам "Микроген" Министерства здравоохранения Российской Федерации | Способ получения препарата фактора свертывания viii крови человека |
| WO2009007451A1 (en) * | 2007-07-11 | 2009-01-15 | Novo Nordisk A/S | Purification of factor viii using a mixed-mode or multimodal resin |
Family Cites Families (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4540573A (en) | 1983-07-14 | 1985-09-10 | New York Blood Center, Inc. | Undenatured virus-free biologically active protein derivatives |
| US5605884A (en) * | 1987-10-29 | 1997-02-25 | Rhone-Poulenc Rorer Pharmaceuticals Inc. | Factor VIII formulations in high ionic strength media |
| EP0711485B1 (en) | 1993-06-11 | 1997-04-16 | Nortel Networks Corporation | Method for providing user controlled call management services |
| DE4337573C1 (de) * | 1993-11-04 | 1995-05-18 | Octapharma Ag | Verfahren zur Herstellung einer virusinaktivierten Faktor VIII enthaltenden Fraktion mittels chromatographischer Methoden |
| SE9403915D0 (sv) | 1994-11-14 | 1994-11-14 | Annelie Almstedt | Process A |
| AUPR638801A0 (en) * | 2001-07-13 | 2001-08-09 | Life Therapeutics Limited | Factor viii separation |
| EP1707634A1 (en) | 2005-03-29 | 2006-10-04 | Octapharma AG | Method for isolation of recombinantly produced proteins |
| FR2887883B1 (fr) * | 2005-06-29 | 2007-08-31 | Lab Francais Du Fractionnement | Procede de separation des proteines fibrinogene, facteur xiii et colle biologique d'une fraction plasmatique solubilisee et de preparation de concentres lyophilises desdites proteines |
| EP1739179A1 (en) | 2005-06-30 | 2007-01-03 | Octapharma AG | Serum-free stable transfection and production of recombinant human proteins in human cell lines |
| NZ573639A (en) | 2006-07-14 | 2012-03-30 | Genentech Inc | Refolding of recombinant proteins |
| US8022187B2 (en) * | 2007-02-01 | 2011-09-20 | Baxter International Inc. | FVIII-independent FIX-mutant proteins for hemophilia A treatment |
| US9107902B2 (en) * | 2007-06-13 | 2015-08-18 | Csl Behring Gmbh | Use of VWF stabilized FVIII preparations and of VWF preparations without FVIII for extravascular administration in the therapy and prophylactic treatment of bleeding disorders |
| GB0723712D0 (en) * | 2007-12-04 | 2008-01-16 | Apitope Technology Bristol Ltd | Peptides |
| DE102008032361A1 (de) * | 2008-07-10 | 2010-01-21 | Csl Behring Gmbh | Der Einsatz von Faktor VIII und vWF bzw. vWF-enthaltenden Konzentraten zur Therapie der durch Thrombocyten-Inhibitoren induzierte Koagulopathie |
-
2009
- 2009-06-24 PL PL09769279T patent/PL2300497T3/pl unknown
- 2009-06-24 US US12/737,230 patent/US8329871B2/en active Active
- 2009-06-24 KR KR1020107028866A patent/KR101700722B1/ko active Active
- 2009-06-24 KR KR1020167023450A patent/KR20160104740A/ko not_active Abandoned
- 2009-06-24 RU RU2015141849A patent/RU2698392C2/ru active
- 2009-06-24 JP JP2011515372A patent/JP5619738B2/ja active Active
- 2009-06-24 ES ES12179389.7T patent/ES2538706T3/es active Active
- 2009-06-24 AU AU2009264282A patent/AU2009264282B2/en active Active
- 2009-06-24 BR BRPI0914695-4A patent/BRPI0914695B1/pt active IP Right Grant
- 2009-06-24 WO PCT/EP2009/057883 patent/WO2009156430A1/en not_active Ceased
- 2009-06-24 MX MX2010013908A patent/MX2010013908A/es active IP Right Grant
- 2009-06-24 PT PT09769279T patent/PT2300497E/pt unknown
- 2009-06-24 DK DK12179389.7T patent/DK2537862T3/en active
- 2009-06-24 EP EP09769279A patent/EP2300497B1/en active Active
- 2009-06-24 UA UAA201100699A patent/UA100901C2/ru unknown
- 2009-06-24 RU RU2011102437/10A patent/RU2567811C2/ru active
- 2009-06-24 ES ES09769279T patent/ES2391613T3/es active Active
- 2009-06-24 DK DK09769279.2T patent/DK2300497T3/da active
- 2009-06-24 CA CA2728047A patent/CA2728047C/en active Active
- 2009-06-24 SI SI200930362T patent/SI2300497T1/sl unknown
- 2009-06-24 EP EP12179389.7A patent/EP2537862B1/en not_active Revoked
- 2009-06-24 CN CN200980123974.6A patent/CN102066417B/zh active Active
- 2009-06-24 KR KR1020177024076A patent/KR101804136B1/ko active Active
-
2010
- 2010-12-05 IL IL229583A patent/IL229583B/en active IP Right Grant
- 2010-12-05 IL IL209758A patent/IL209758A/en active IP Right Grant
- 2010-12-21 ZA ZA2010/09162A patent/ZA201009162B/en unknown
-
2013
- 2013-11-24 IL IL229583A patent/IL229583A0/en unknown
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1994008686A1 (en) * | 1992-10-21 | 1994-04-28 | Cornell Research Foundation, Inc. | Multimodal chromatographic separation media and process for usingsame |
| WO2005082483A1 (en) * | 2004-02-27 | 2005-09-09 | Ge Healthcare Bio-Sciences Ab | A process for the purification of antibodies |
| WO2005121163A2 (en) * | 2004-06-07 | 2005-12-22 | Upfront Chromatography A/S | Isolation of plasma or serum proteins |
| RU2324495C1 (ru) * | 2006-08-31 | 2008-05-20 | Федеральное государственное унитарное предприятие "Научно-производственное объединение по медицинским иммунобиологическим препаратам "Микроген" Министерства здравоохранения Российской Федерации | Способ получения препарата фактора свертывания viii крови человека |
| WO2009007451A1 (en) * | 2007-07-11 | 2009-01-15 | Novo Nordisk A/S | Purification of factor viii using a mixed-mode or multimodal resin |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2734783C2 (ru) * | 2016-02-11 | 2020-10-23 | Октафарма Аг | Способ отделения фактора viii от продуктов крови |
| US10889630B2 (en) | 2016-02-11 | 2021-01-12 | Octapharma Ag | Method of separating Factor VIII from blood products |
| RU2800431C2 (ru) * | 2017-06-23 | 2023-07-21 | Такеда Фармасьютикал Компани Лимитед | Очистка подвидов фактора viii |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2567811C2 (ru) | Способ очистки фактора свертывания крови viii | |
| JP6216930B2 (ja) | 組換えadamts13および他のタンパク質を精製する方法ならびにそれらの組成物 | |
| EP2553095B1 (en) | A process for purifying vitamin K dependent proteins such as coagulation factor IX | |
| HK40103492B (en) | Methods of purifying recombinant adamts13 and other proteins and compositions thereof | |
| HK40103492A (en) | Methods of purifying recombinant adamts13 and other proteins and compositions thereof | |
| HK1241929A1 (en) | Methods of purifying recombinant adamts13 and other proteins and compositions thereof | |
| HK1171784B (en) | Method for purifying recombinant adamts13 and other proteins and compositions thereof | |
| HK1171784A (en) | Method for purifying recombinant adamts13 and other proteins and compositions thereof |