KR20180098420A - 디파이브로타이드의 생물학적 활성 측정을 위한 유우글로불린에 기초한 방법 - Google Patents
디파이브로타이드의 생물학적 활성 측정을 위한 유우글로불린에 기초한 방법 Download PDFInfo
- Publication number
- KR20180098420A KR20180098420A KR1020187024264A KR20187024264A KR20180098420A KR 20180098420 A KR20180098420 A KR 20180098420A KR 1020187024264 A KR1020187024264 A KR 1020187024264A KR 20187024264 A KR20187024264 A KR 20187024264A KR 20180098420 A KR20180098420 A KR 20180098420A
- Authority
- KR
- South Korea
- Prior art keywords
- biological activity
- plasmin
- fibrotide
- substrate
- concentration
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
- A61K31/711—Natural deoxyribonucleic acids, i.e. containing only 2'-deoxyriboses attached to adenine, guanine, cytosine or thymine and having 3'-5' phosphodiester links
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/14—Hydrolases (3)
- C12N9/48—Hydrolases (3) acting on peptide bonds (3.4)
- C12N9/50—Proteinases, e.g. Endopeptidases (3.4.21-3.4.25)
- C12N9/64—Proteinases, e.g. Endopeptidases (3.4.21-3.4.25) derived from animal tissue
- C12N9/6421—Proteinases, e.g. Endopeptidases (3.4.21-3.4.25) derived from animal tissue from mammals
- C12N9/6424—Serine endopeptidases (3.4.21)
- C12N9/6435—Plasmin (3.4.21.7), i.e. fibrinolysin
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/34—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving hydrolase
- C12Q1/37—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving hydrolase involving peptidase or proteinase
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/56—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving blood clotting factors, e.g. involving thrombin, thromboplastin, fibrinogen
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y304/00—Hydrolases acting on peptide bonds, i.e. peptidases (3.4)
- C12Y304/21—Serine endopeptidases (3.4.21)
- C12Y304/21007—Plasmin (3.4.21.7), i.e. fibrinolysin
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/52—Use of compounds or compositions for colorimetric, spectrophotometric or fluorometric investigation, e.g. use of reagent paper and including single- and multilayer analytical elements
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/86—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving blood coagulating time or factors, or their receptors
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/10—Type of nucleic acid
- C12N2310/12—Type of nucleic acid catalytic nucleic acids, e.g. ribozymes
- C12N2310/127—DNAzymes
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/90—Enzymes; Proenzymes
- G01N2333/914—Hydrolases (3)
- G01N2333/948—Hydrolases (3) acting on peptide bonds (3.4)
- G01N2333/968—Plasmin, i.e. fibrinolysin
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Molecular Biology (AREA)
- General Health & Medical Sciences (AREA)
- Biochemistry (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Genetics & Genomics (AREA)
- Immunology (AREA)
- Biotechnology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Microbiology (AREA)
- Physics & Mathematics (AREA)
- General Engineering & Computer Science (AREA)
- Hematology (AREA)
- Analytical Chemistry (AREA)
- Biomedical Technology (AREA)
- Biophysics (AREA)
- Medicinal Chemistry (AREA)
- Urology & Nephrology (AREA)
- Neurosurgery (AREA)
- Pathology (AREA)
- General Physics & Mathematics (AREA)
- Food Science & Technology (AREA)
- Cell Biology (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Epidemiology (AREA)
- Pharmacology & Pharmacy (AREA)
- Plant Pathology (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
- Medicinal Preparation (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Peptides Or Proteins (AREA)
Abstract
Description
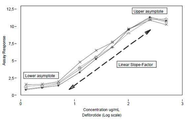
도 2는 디파이브로타이드의 표준물질 및 테스트 샘플에 상대적으로 일어나는 S자형 곡선(sigmoid)을 도시하는 플롯이다.
도 3은 적절한 선형 범위(예: 30 내지 35분)를 나타내는 표준 제제(standard preparation)(예를 들어, Sl_Ca, Sl_Cb, Sl_Cc)의 "흡광 대비 시간(absorbance versus time)"을 도시하는 플롯이다.
Claims (14)
- 디파이브로타이드(defibrotide)의 생물학적 활성의 측정방법에 있어서,
a) 디파이브로타이드와, 포유동물 유우글로불린(mammalian euglobulin)과, 플라스민의 반응을 통해 측정가능한 생성물을 제공하는 플라스민에 특이적인 기질을 접촉시키는 단계; 및
b) 연속해서 형성된 생성물의 양을 측정하여 상기 디파이브로타이드의 생물학적 활성을 측정하는 단계;를 포함하는, 디파이브로타이드의 생물학적 활성 측정 방법. - 제1항에 있어서,
상기 유우글로불린은 포유동물 유우글로불린인 것인, 디파이브로타이드의 생물학적 활성 측정 방법. - 제2항에 있어서,
상기 유우글로불린은 인간, 토끼 또는 소 유우글로불린인 것인, 디파이브로타이드의 생물학적 활성 측정 방법. - 제1항에 있어서,
상기 플라스민에 특이적 기질과 반응하는 플라스민은,
유우글로불린에 함유된 플라스미노겐(plasminogen)에 의하여 유리되는 것인, 디파이브로타이드의 생물학적 활성 측정 방법. - 제1항에 있어서,
상기 플라스민에 특이적 기질은,
색소생산 기질(chromogenic substrate)인 것인, 디파이브로타이드의 생물학적 활성 측정 방법. - 제1항에 있어서,
상기 플라스민에 특이적 기질은,
화학식 A1-A2-A3-X인 화합물로서,
여기서 A1 및 A2는 비-극성 아미노산, A3는 리신(lysine) 또는 아르기닌(arginine)이고, X는 측정가능한 생성물인 것인, 디파이브로타이드의 생물학적 활성 측정 방법. - 제6항에 있어서,
상기 측정가능한 생성물 X는 파라니트로아닐린(para-nitroaniline) 및 2-나프틸라민(2-naphthylamine)으로 구성된 군으로부터 선택되는 것인, 디파이브로타이드의 생물학적 활성 측정 방법. - 제4항에 있어서,
상기 플라스민에 특이적 기질은,
H-D-발릴-L-류실-L-리신-p-니트로아닐린(H-D-Valyl-L-Leucyl-L-Lysine-p-nitroaniline)인 것인, 디파이브로타이드의 생물학적 활성 측정 방법. - 제6항에 있어서,
상기 측정가능한 생성물 X는,
분광측정법(spectrophotometry) 또는 형광측정법(fluorimetry)에 의하여 측정되는 것인, 디파이브로타이드의 생물학적 활성 측정 방법. - 제2항에 있어서,
상기 포유동물 유우글로불린은, 원래 혈장과 동일 부피로 재구성되거나 적절한 버퍼로 1:10으로 희석되고, 색소생산/형광생성 기질(chromogenic/fluorogenic substrate)은 2.5 내지 3.5mM의 농도를 가지는 것인, 디파이브로타이드의 생물학적 활성 측정 방법. - 제1항에 있어서,
상기 디파이브로타이드의 생물학적 활성 측정 방법은,
pH 7 내지 8로 완충된 수용액인 반응 배지(reaction medium)에서 수행되는 것인, 디파이브로타이드의 생물학적 활성 측정 방법. - 제1항에 있어서,
35 내지 39℃로 온도가 유지되는 것인, 디파이브로타이드의 생물학적 활성 측정 방법. - 제1항에 있어서,
상기 플라스민 특이적 기질의 농도는,
0.3 내지 4 mM인 것인, 디파이브로타이드의 생물학적 활성 측정 방법. - 제1항에 있어서,
c) 표준 샘플과 테스트 샘플 모두의 효소 반응의 과정 동안, 상기 측정가능한 생성물의 유리 속도(rate of release)를 측정하는 단계; 및
d) 해당하는 디파이브로타이드 농도와 상기 유리 속도를 수학적으로 및/또는 그래프를 이용해서 상호 관련시켜, 디파이브로타이드의 테스트 샘플의 생물학적 활성을 구하는 단계;를 더 포함하는 것인, 디파이브로타이드의 생물학적 활성 측정 방법.
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PCT/IT2012/000193 WO2013190582A1 (en) | 2012-06-22 | 2012-06-22 | Euglobulin-based method for determining the biological activity of defibrotide |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR20157001763A Division KR20150044877A (ko) | 2012-06-22 | 2012-06-22 | 디파이브로타이드의 생물학적 활성 측정을 위한 유우글로불린에 기초한 방법 |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020197003854A Division KR102038357B1 (ko) | 2012-06-22 | 2012-06-22 | 디파이브로타이드의 생물학적 활성 측정을 위한 유우글로불린에 기초한 방법 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| KR20180098420A true KR20180098420A (ko) | 2018-09-03 |
| KR101948243B1 KR101948243B1 (ko) | 2019-05-21 |
Family
ID=46829846
Family Applications (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR20157001763A Ceased KR20150044877A (ko) | 2012-06-22 | 2012-06-22 | 디파이브로타이드의 생물학적 활성 측정을 위한 유우글로불린에 기초한 방법 |
| KR1020197028118A Ceased KR20190112197A (ko) | 2012-06-22 | 2012-06-22 | 디파이브로타이드의 생물학적 활성 측정을 위한 유우글로불린에 기초한 방법 |
| KR1020187024264A Active KR101948243B1 (ko) | 2012-06-22 | 2012-06-22 | 디파이브로타이드의 생물학적 활성 측정을 위한 유우글로불린에 기초한 방법 |
| KR1020197003854A Active KR102038357B1 (ko) | 2012-06-22 | 2012-06-22 | 디파이브로타이드의 생물학적 활성 측정을 위한 유우글로불린에 기초한 방법 |
Family Applications Before (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR20157001763A Ceased KR20150044877A (ko) | 2012-06-22 | 2012-06-22 | 디파이브로타이드의 생물학적 활성 측정을 위한 유우글로불린에 기초한 방법 |
| KR1020197028118A Ceased KR20190112197A (ko) | 2012-06-22 | 2012-06-22 | 디파이브로타이드의 생물학적 활성 측정을 위한 유우글로불린에 기초한 방법 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020197003854A Active KR102038357B1 (ko) | 2012-06-22 | 2012-06-22 | 디파이브로타이드의 생물학적 활성 측정을 위한 유우글로불린에 기초한 방법 |
Country Status (16)
| Country | Link |
|---|---|
| US (9) | US9902952B2 (ko) |
| EP (1) | EP2864496B2 (ko) |
| JP (1) | JP6198821B2 (ko) |
| KR (4) | KR20150044877A (ko) |
| CN (2) | CN110079580B (ko) |
| AU (1) | AU2012383169B2 (ko) |
| BR (1) | BR112014031934B1 (ko) |
| CA (1) | CA2874960C (ko) |
| DK (1) | DK2864496T4 (ko) |
| ES (1) | ES2660969T5 (ko) |
| IL (1) | IL236132B (ko) |
| IN (1) | IN2014DN10584A (ko) |
| MX (1) | MX352085B (ko) |
| RU (1) | RU2627177C2 (ko) |
| SG (1) | SG11201408481UA (ko) |
| WO (1) | WO2013190582A1 (ko) |
Families Citing this family (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2637672B1 (en) | 2010-11-12 | 2018-08-22 | Gentium S.r.l. | Defibrotide for use in prophylaxis and/or treatment of graft versus host disease (gvhd). |
| CA2874960C (en) | 2012-06-22 | 2021-05-18 | Gentium S.P.A. | Euglobulin-based method for determining the biological activity of defibrotide |
| EP3026122A1 (en) | 2014-11-27 | 2016-06-01 | Gentium S.p.A. | Cellular-based method for determining the potency of defibrotide |
| US10002713B1 (en) * | 2017-01-25 | 2018-06-19 | Kemet Electronics Corporation | Self-damping MLCC array |
| TW201909904A (zh) | 2017-08-03 | 2019-03-16 | 愛爾蘭商爵士製藥愛爾蘭有限責任公司 | 高濃度調配物 |
| CA3095335A1 (en) | 2018-04-12 | 2019-10-17 | Jazz Pharmaceuticals, Inc. | Defibrotide for the prevention and treatment of cytokine release syndrome and neurotoxicity associated with immunodepletion |
| WO2020118165A1 (en) | 2018-12-07 | 2020-06-11 | Jazz Pharmaceuticals Ireland Limited | Subcutaneous delivery of high concentration formulations |
| EP4110287A1 (en) | 2020-02-28 | 2023-01-04 | Jazz Pharmaceuticals Ireland Limited | Delivery of low viscosity formulations |
| WO2022234101A1 (en) | 2021-05-06 | 2022-11-10 | Jazz Pharmaceuticals Ireland Limited | Defibrotide for the treatment and prevention of acute respiratory distress syndrome |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20040090959A (ko) * | 2001-12-17 | 2004-10-27 | 젠티엄 에스피에이 | 디파이브로타이드의 생물학적 활성 측정 방법 |
Family Cites Families (72)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3899481A (en) | 1970-11-03 | 1975-08-12 | Crinos Industria Farmaco | Process for the controlled partial degradation of deoxyribonucleic acid extracted from animal organs |
| DE2154279A1 (de) | 1970-11-03 | 1972-05-25 | Crinos Industria Farmaco | Medikamente für das fibrinolytische System |
| IT1043823B (it) | 1970-11-03 | 1980-02-29 | Prephar | Procedimento per l estrazione di acidi nucleici da organi animali |
| DE2812943C3 (de) | 1978-03-23 | 1981-05-14 | Boehringer Mannheim Gmbh, 6800 Mannheim | Verfahren und Reagens zur Bestimmung der biologischen Aktivität von Heparin im Plasma |
| US4853221A (en) | 1980-11-13 | 1989-08-01 | Warner-Lambert Company | Method for treating non-small cell lung cancer, head and neck cancers and breast cancer |
| IT1170215B (it) | 1983-09-12 | 1987-06-03 | Crinos Industria Farmaco | Composizione farmaceutica per il trattamento di stati di insufficienza renale acuta |
| IT1170214B (it) * | 1983-09-12 | 1987-06-03 | Crinos Industria Farmaco | Composizione farmaceutica per la cura delle arteriopatie periferiche |
| IT1206341B (it) | 1984-02-16 | 1989-04-14 | Crinos Industria Farmaco | Composizione farmaceutica per il trattamento dell'ischemia acuta del miocardio. |
| US4694134A (en) | 1985-05-28 | 1987-09-15 | Ajax Magnethermic Corporation | Apparatus for overheating edges of skelp for the production of compression welded pipe |
| US5223609A (en) | 1986-04-17 | 1993-06-29 | Crinos Industria Farmacobiologica S.P.A. | Process for obtaining chemically defined and reproducible polydeoxyribonucleotides |
| IT1190313B (it) * | 1986-04-17 | 1988-02-16 | Crinos Industria Farmaco | Procedimento per l'ottenimento di polidesossiribonucleotidi chimicamente definiti e riproducibili e prodotto farmacologicamente attivo risultante |
| US5231006A (en) | 1986-10-16 | 1993-07-27 | Behringwerke Aktiengesellschaft | Method for the determination of plasminogen |
| US4753221A (en) | 1986-10-22 | 1988-06-28 | Intravascular Surgical Instruments, Inc. | Blood pumping catheter and method of use |
| JP2907447B2 (ja) | 1988-08-24 | 1999-06-21 | 中外製薬株式会社 | 抗血栓剤 |
| IT1231509B (it) | 1989-09-07 | 1991-12-07 | Crinos Industria Farmaco | Composizione farmceutica ad uso topico per la terapia della fragilita' capillare. |
| JPH0539280A (ja) * | 1990-07-20 | 1993-02-19 | Takeda Chem Ind Ltd | サツカロアスコルビン酸誘導体および血栓症予防治療剤 |
| US5199942A (en) | 1991-06-07 | 1993-04-06 | Immunex Corporation | Method for improving autologous transplantation |
| US6699985B2 (en) | 1991-08-21 | 2004-03-02 | Arsinur Burcoglu | Method of treating HIV infection and related secondary infections thereof |
| US5624912A (en) | 1991-08-21 | 1997-04-29 | Burcoglu; Arsinur | Method of treating HIV infection and related secondary infections with defibrotide |
| US5977083A (en) | 1991-08-21 | 1999-11-02 | Burcoglu; Arsinur | Method for using polynucleotides, oligonucleotides and derivatives thereof to treat various disease states |
| IT1252174B (it) | 1991-12-09 | 1995-06-05 | Crinos Industria Farmaco | Oligodesossimibonucleotidi ad attivita' antiischemica e procedimenti per il loro ottenimento |
| US5578716A (en) | 1993-12-01 | 1996-11-26 | Mcgill University | DNA methyltransferase antisense oligonucleotides |
| US6335356B1 (en) | 1994-01-07 | 2002-01-01 | Sugen, Inc. | Method of treating a patient by parenteral administration of a lipophilic compound |
| JPH08127539A (ja) | 1994-10-31 | 1996-05-21 | Ajinomoto Co Inc | ヒトil−11を含有する末梢血幹細胞増加剤 |
| EP0801076A4 (en) | 1994-11-30 | 1999-12-15 | Chugai Pharmaceutical Co Ltd | THROMBOCYTOTIC FACTOR |
| CA2260014A1 (en) | 1996-07-10 | 1998-01-15 | Meiji Milk Products Co., Ltd. | Novel use of mk family as hematopoietic factor |
| EP1202750A4 (en) | 1997-04-28 | 2002-10-16 | Arsinur Burcoglu | METHOD FOR THE TREATMENT OF HIV INFECTIONS AND THEIR OPPORTUNISTIC INFECTIONS |
| JP2002512508A (ja) | 1997-05-30 | 2002-04-23 | マクギル・ユニヴァーシティ | Dnaメチルトランスフェラーゼゲノミック配列およびアンチセンスオリゴヌクレオチド |
| US6177545B1 (en) | 1997-09-02 | 2001-01-23 | Insight Strategy & Marketing Ltd. | Heparanase specific molecular probes and their use in research and medical applications |
| DE19740384A1 (de) | 1997-09-08 | 1999-03-11 | Max Delbrueck Centrum | Antisense Oligodesoxynukleotide (ODN) gegen Proteinkinase C (PKC)-Isoformen, ihre Verwendung und pharmazeutische Zubereitungen dieser ODN |
| GB9719161D0 (en) * | 1997-09-09 | 1997-11-12 | Glaxo Group Ltd | New therapeutic method |
| US6573372B2 (en) | 1999-01-07 | 2003-06-03 | Heska Corporation | Feline immunoglobulin E molecules and compositions there of |
| JP2003503313A (ja) | 1999-06-03 | 2003-01-28 | ジェシー エル エス オウ | 細胞増殖及び細胞死を変調する方法及び組成物 |
| DK1059092T3 (da) | 1999-06-08 | 2006-03-27 | Gentium Spa | Anvendelse af komplekser af kationiske liposomer og polydeoxyribonukleotider som medikamenter |
| EP1147777A1 (en) | 2000-04-18 | 2001-10-24 | Crinos Industria Farmacobiologica S.p.A. | Combination of defibrotide and G-CSF and its use to activate haematopoietic progenitors |
| US8771663B2 (en) | 2000-04-18 | 2014-07-08 | Gentium Spa | Formulation having mobilising activity |
| CA2426540A1 (en) | 2000-10-20 | 2002-07-25 | Biocardia, Inc. | Leukocyte expression profiling |
| CA2430341A1 (en) | 2000-11-28 | 2002-06-06 | The University Of Chicago | Genetically engineered herpes virus for the treatment of cardiovascular disease |
| HUP0700079A2 (en) | 2000-12-29 | 2007-05-02 | Savient Pharmaceuticals | Isolated molecules comprising epitopes containing sulfated moieties, antibodies to such epitopes, and uses thereof |
| US7235358B2 (en) | 2001-06-08 | 2007-06-26 | Expression Diagnostics, Inc. | Methods and compositions for diagnosing and monitoring transplant rejection |
| US6770753B2 (en) | 2001-07-05 | 2004-08-03 | The Trustees Of Columbia University In The City Of New York | Phosphorothioate antisense heparanase oligonucleotides |
| US7514414B2 (en) | 2001-09-24 | 2009-04-07 | The United States Of America As Represented By The Department Of Health And Human Services | Suppressors of CpG oligonucleotides and methods of use |
| US6965025B2 (en) | 2001-12-10 | 2005-11-15 | Isis Pharmaceuticals, Inc. | Antisense modulation of connective tissue growth factor expression |
| US20050215498A1 (en) | 2002-05-31 | 2005-09-29 | Guenther Eissner | Method for the protection of endothelial and epithclial cells during chemotherapy |
| CN1304011C (zh) | 2002-05-31 | 2007-03-14 | 雷根斯堡大学医学院 | 保护性寡聚脱氧核糖核苷酸的制药用途 |
| JP2005536199A (ja) | 2002-07-01 | 2005-12-02 | サビエント ファーマシューティカルズ,インコーポレイティド | 抗体及びそれらの使用 |
| JP4483581B2 (ja) | 2002-08-06 | 2010-06-16 | 東レ株式会社 | 腎疾患治療又は予防剤及び腎疾患の診断方法 |
| US20050196382A1 (en) | 2002-09-13 | 2005-09-08 | Replicor, Inc. | Antiviral oligonucleotides targeting viral families |
| DE10244453A1 (de) | 2002-09-24 | 2004-04-01 | Phenomiques Gmbh | Hemmung der Proteinkinase C-alpha zur Behandlung von Krankheiten |
| US7803781B2 (en) | 2003-02-28 | 2010-09-28 | Isis Pharmaceuticals, Inc. | Modulation of growth hormone receptor expression and insulin-like growth factor expression |
| ITMI20031714A1 (it) | 2003-09-05 | 2005-03-06 | Gentium Spa | Formazioni ad azione antitumorale. |
| WO2005089503A2 (en) | 2004-03-19 | 2005-09-29 | Progenics Pharmaceuticals, Inc. | Cd4-igg2 formulations |
| AU2005289774B2 (en) * | 2004-09-22 | 2012-03-08 | The Regents Of The University Of Colorado | Methods for a global assay of coagulation and fibrinolysis |
| US7723127B2 (en) | 2005-03-03 | 2010-05-25 | Novx Systems Inc. | Immunoassay with extended dynamic range |
| CA2598613A1 (en) | 2005-03-03 | 2006-09-14 | Gentium S.P.A. | Defibrotide and/or oligodeoxyribonucleotides for treating angiogenesis-dependent tumors |
| WO2006119619A1 (en) | 2005-05-06 | 2006-11-16 | Replicor Inc. | Oligonucleotides inhibiting cell proliferation |
| EP1872787A1 (en) | 2006-06-27 | 2008-01-02 | Gentium S.p.A. | Use of defibrotide for the inhibition of heparanase |
| EP1982722A1 (en) | 2007-04-16 | 2008-10-22 | Gentium S.p.A. | Use of oligotide for the treatment of renal diseases |
| FR2917172B1 (fr) * | 2007-06-07 | 2014-01-03 | Inst Nat Sante Rech Med | Methode de mesure de l'activite plasmine des microparticules presentes dans un echantillon de fluide biologique et utilisation |
| EP2183598A1 (en) | 2007-07-13 | 2010-05-12 | Elan Pharmaceuticals Inc. | Compositions and methods for identifying substrate specificity of inhibitors of gamma secretase |
| EP2103689A1 (en) | 2008-03-19 | 2009-09-23 | Gentium S.p.A. | Synthetic phosphodiester oligonucleotides and therapeutical uses thereof |
| EP2385370A1 (en) | 2008-04-10 | 2011-11-09 | Massachusetts Institute of Technology (MIT) | Methods for identification and use of agents targeting cancer stem cells |
| CN101301306A (zh) | 2008-06-30 | 2008-11-12 | 广东天普生化医药股份有限公司 | Dft在制备治疗和预防休克药物中的应用 |
| PL2403865T3 (pl) | 2009-03-03 | 2016-01-29 | Grifols Therapeutics Inc | Sposoby wytwarzania plazminogenu |
| WO2010127099A2 (en) * | 2009-04-29 | 2010-11-04 | Amarin Corporation Plc | Pharmaceutical compositions comprising epa and a cardiovascular agent and methods of using the same |
| WO2011057143A1 (en) * | 2009-11-06 | 2011-05-12 | The Regents Of The University Of Colorado, A Body Corporate | Compositions, methods and uses for simultaneous assay of thrombin and plasmin generation |
| EP2637672B1 (en) * | 2010-11-12 | 2018-08-22 | Gentium S.r.l. | Defibrotide for use in prophylaxis and/or treatment of graft versus host disease (gvhd). |
| CA2874960C (en) | 2012-06-22 | 2021-05-18 | Gentium S.P.A. | Euglobulin-based method for determining the biological activity of defibrotide |
| EP3026122A1 (en) | 2014-11-27 | 2016-06-01 | Gentium S.p.A. | Cellular-based method for determining the potency of defibrotide |
| TW201909904A (zh) | 2017-08-03 | 2019-03-16 | 愛爾蘭商爵士製藥愛爾蘭有限責任公司 | 高濃度調配物 |
| WO2020118165A1 (en) | 2018-12-07 | 2020-06-11 | Jazz Pharmaceuticals Ireland Limited | Subcutaneous delivery of high concentration formulations |
| EP4110287A1 (en) | 2020-02-28 | 2023-01-04 | Jazz Pharmaceuticals Ireland Limited | Delivery of low viscosity formulations |
-
2012
- 2012-06-22 CA CA2874960A patent/CA2874960C/en active Active
- 2012-06-22 WO PCT/IT2012/000193 patent/WO2013190582A1/en not_active Ceased
- 2012-06-22 KR KR20157001763A patent/KR20150044877A/ko not_active Ceased
- 2012-06-22 US US14/408,272 patent/US9902952B2/en active Active
- 2012-06-22 AU AU2012383169A patent/AU2012383169B2/en active Active
- 2012-06-22 DK DK12756826.9T patent/DK2864496T4/da active
- 2012-06-22 JP JP2015517926A patent/JP6198821B2/ja active Active
- 2012-06-22 KR KR1020197028118A patent/KR20190112197A/ko not_active Ceased
- 2012-06-22 KR KR1020187024264A patent/KR101948243B1/ko active Active
- 2012-06-22 CN CN201910228570.9A patent/CN110079580B/zh active Active
- 2012-06-22 IN IN10584DEN2014 patent/IN2014DN10584A/en unknown
- 2012-06-22 SG SG11201408481UA patent/SG11201408481UA/en unknown
- 2012-06-22 RU RU2014149089A patent/RU2627177C2/ru active
- 2012-06-22 ES ES12756826T patent/ES2660969T5/es active Active
- 2012-06-22 EP EP12756826.9A patent/EP2864496B2/en active Active
- 2012-06-22 KR KR1020197003854A patent/KR102038357B1/ko active Active
- 2012-06-22 CN CN201280074120.5A patent/CN104619857A/zh active Pending
- 2012-06-22 BR BR112014031934-0A patent/BR112014031934B1/pt active IP Right Grant
- 2012-06-22 MX MX2014016114A patent/MX352085B/es active IP Right Grant
-
2014
- 2014-12-08 IL IL236132A patent/IL236132B/en active IP Right Grant
-
2017
- 2017-12-18 US US15/844,801 patent/US20180334672A1/en not_active Abandoned
-
2020
- 2020-03-12 US US16/816,741 patent/US11085043B2/en active Active
-
2021
- 2021-08-06 US US17/396,028 patent/US11746348B2/en active Active
- 2021-08-27 US US17/459,169 patent/US11236328B2/en active Active
-
2023
- 2023-07-12 US US18/351,241 patent/US20230357762A1/en not_active Abandoned
-
2024
- 2024-11-20 US US18/953,184 patent/US20250075206A1/en active Pending
-
2025
- 2025-04-08 US US19/173,652 patent/US12534722B2/en active Active
- 2025-04-08 US US19/173,657 patent/US12529052B2/en active Active
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20040090959A (ko) * | 2001-12-17 | 2004-10-27 | 젠티엄 에스피에이 | 디파이브로타이드의 생물학적 활성 측정 방법 |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR101948243B1 (ko) | 디파이브로타이드의 생물학적 활성 측정을 위한 유우글로불린에 기초한 방법 | |
| JP4348192B2 (ja) | デフィブロチドの生物学的活性を決定する方法 | |
| AU2002360945A2 (en) | A method for determining the biological activity of defibrotide | |
| RU2766143C2 (ru) | Жидкая композиция дефибротида для лечения и профилактики веноокклюзионной болезни | |
| TWI615473B (zh) | 用於測定去纖維蛋白多核苷酸的生物活性之基於優球蛋白的方法 | |
| HK1208503B (en) | Euglobulin-based method for determining the biological activity of defibrotide | |
| SA113350049B1 (ar) | طريقة تعتمد على جلوبولين حقيقي لتحديد النشاط الحيوي لديفيبروتيد |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A107 | Divisional application of patent | ||
| PA0104 | Divisional application for international application |
St.27 status event code: A-0-1-A10-A18-div-PA0104 St.27 status event code: A-0-1-A10-A16-div-PA0104 |
|
| A201 | Request for examination | ||
| PA0201 | Request for examination |
St.27 status event code: A-1-2-D10-D11-exm-PA0201 |
|
| PG1501 | Laying open of application |
St.27 status event code: A-1-1-Q10-Q12-nap-PG1501 |
|
| E701 | Decision to grant or registration of patent right | ||
| PE0701 | Decision of registration |
St.27 status event code: A-1-2-D10-D22-exm-PE0701 |
|
| PA0104 | Divisional application for international application |
St.27 status event code: A-0-1-A10-A18-div-PA0104 St.27 status event code: A-0-1-A10-A16-div-PA0104 |
|
| PR0701 | Registration of establishment |
St.27 status event code: A-2-4-F10-F11-exm-PR0701 |
|
| PR1002 | Payment of registration fee |
St.27 status event code: A-2-2-U10-U12-oth-PR1002 Fee payment year number: 1 |
|
| PG1601 | Publication of registration |
St.27 status event code: A-4-4-Q10-Q13-nap-PG1601 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 4 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 5 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 6 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 7 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 8 |
|
| U11 | Full renewal or maintenance fee paid |
Free format text: ST27 STATUS EVENT CODE: A-4-4-U10-U11-OTH-PR1001 (AS PROVIDED BY THE NATIONAL OFFICE) Year of fee payment: 8 |