JP4737931B2 - 医薬品の製造におけるヒドロキシオレイン酸及び類似化合物の使用 - Google Patents
医薬品の製造におけるヒドロキシオレイン酸及び類似化合物の使用 Download PDFInfo
- Publication number
- JP4737931B2 JP4737931B2 JP2003533923A JP2003533923A JP4737931B2 JP 4737931 B2 JP4737931 B2 JP 4737931B2 JP 2003533923 A JP2003533923 A JP 2003533923A JP 2003533923 A JP2003533923 A JP 2003533923A JP 4737931 B2 JP4737931 B2 JP 4737931B2
- Authority
- JP
- Japan
- Prior art keywords
- acid
- hydroxyoleic acid
- protein
- analogs
- hydroxyoleic
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- JBSOOFITVPOOSY-KTKRTIGZSA-N 2-hydroxyoleic acid Chemical compound CCCCCCCC\C=C/CCCCCCC(O)C(O)=O JBSOOFITVPOOSY-KTKRTIGZSA-N 0.000 title claims abstract description 96
- 239000003814 drug Substances 0.000 title abstract description 15
- 150000001875 compounds Chemical class 0.000 title abstract description 11
- 238000004519 manufacturing process Methods 0.000 title abstract description 4
- 238000011282 treatment Methods 0.000 claims abstract description 29
- 206010028980 Neoplasm Diseases 0.000 claims abstract description 17
- 201000011510 cancer Diseases 0.000 claims abstract description 10
- 206010020772 Hypertension Diseases 0.000 claims abstract description 8
- 208000008589 Obesity Diseases 0.000 claims abstract description 6
- 235000020824 obesity Nutrition 0.000 claims abstract description 6
- JUUBMADBGZQVFT-KHPPLWFESA-N (z)-2-methyloctadec-9-enoic acid Chemical compound CCCCCCCC\C=C/CCCCCCC(C)C(O)=O JUUBMADBGZQVFT-KHPPLWFESA-N 0.000 claims description 6
- ODURFHDFZAVGHC-KTKRTIGZSA-N (z)-2-aminooctadec-9-enoic acid Chemical compound CCCCCCCC\C=C/CCCCCCC(N)C(O)=O ODURFHDFZAVGHC-KTKRTIGZSA-N 0.000 claims description 4
- 239000008194 pharmaceutical composition Substances 0.000 claims 3
- 108091006027 G proteins Proteins 0.000 abstract description 42
- 239000012528 membrane Substances 0.000 abstract description 42
- 102000030782 GTP binding Human genes 0.000 abstract description 41
- 108091000058 GTP-Binding Proteins 0.000 abstract description 41
- 229940079593 drug Drugs 0.000 abstract description 14
- 230000033228 biological regulation Effects 0.000 abstract description 7
- 230000001404 mediated effect Effects 0.000 abstract description 4
- 201000010099 disease Diseases 0.000 abstract description 2
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 abstract description 2
- 230000004075 alteration Effects 0.000 abstract 1
- 230000000694 effects Effects 0.000 description 49
- 210000004027 cell Anatomy 0.000 description 27
- 230000036772 blood pressure Effects 0.000 description 18
- 235000014113 dietary fatty acids Nutrition 0.000 description 16
- 229930195729 fatty acid Natural products 0.000 description 16
- 239000000194 fatty acid Substances 0.000 description 16
- 150000004665 fatty acids Chemical class 0.000 description 14
- 241000700159 Rattus Species 0.000 description 12
- 102000005962 receptors Human genes 0.000 description 12
- 108020003175 receptors Proteins 0.000 description 12
- WRIDQFICGBMAFQ-UHFFFAOYSA-N (E)-8-Octadecenoic acid Natural products CCCCCCCCCC=CCCCCCCC(O)=O WRIDQFICGBMAFQ-UHFFFAOYSA-N 0.000 description 11
- LQJBNNIYVWPHFW-UHFFFAOYSA-N 20:1omega9c fatty acid Natural products CCCCCCCCCCC=CCCCCCCCC(O)=O LQJBNNIYVWPHFW-UHFFFAOYSA-N 0.000 description 11
- QSBYPNXLFMSGKH-UHFFFAOYSA-N 9-Heptadecensaeure Natural products CCCCCCCC=CCCCCCCCC(O)=O QSBYPNXLFMSGKH-UHFFFAOYSA-N 0.000 description 11
- ZQPPMHVWECSIRJ-UHFFFAOYSA-N Oleic acid Natural products CCCCCCCCC=CCCCCCCCC(O)=O ZQPPMHVWECSIRJ-UHFFFAOYSA-N 0.000 description 11
- 239000005642 Oleic acid Substances 0.000 description 11
- QXJSBBXBKPUZAA-UHFFFAOYSA-N isooleic acid Natural products CCCCCCCC=CCCCCCCCCC(O)=O QXJSBBXBKPUZAA-UHFFFAOYSA-N 0.000 description 11
- ZQPPMHVWECSIRJ-KTKRTIGZSA-N oleic acid Chemical compound CCCCCCCC\C=C/CCCCCCCC(O)=O ZQPPMHVWECSIRJ-KTKRTIGZSA-N 0.000 description 11
- 108090000623 proteins and genes Proteins 0.000 description 11
- 150000002632 lipids Chemical class 0.000 description 10
- SECPZKHBENQXJG-FPLPWBNLSA-N palmitoleic acid Chemical compound CCCCCC\C=C/CCCCCCCC(O)=O SECPZKHBENQXJG-FPLPWBNLSA-N 0.000 description 10
- 235000018102 proteins Nutrition 0.000 description 10
- 102000004169 proteins and genes Human genes 0.000 description 10
- 230000007704 transition Effects 0.000 description 10
- 239000002253 acid Substances 0.000 description 9
- 230000000259 anti-tumor effect Effects 0.000 description 9
- 239000002246 antineoplastic agent Substances 0.000 description 9
- STQGQHZAVUOBTE-UHFFFAOYSA-N 7-Cyan-hept-2t-en-4,6-diinsaeure Natural products C1=2C(O)=C3C(=O)C=4C(OC)=CC=CC=4C(=O)C3=C(O)C=2CC(O)(C(C)=O)CC1OC1CC(N)C(O)C(C)O1 STQGQHZAVUOBTE-UHFFFAOYSA-N 0.000 description 8
- WEAHRLBPCANXCN-UHFFFAOYSA-N Daunomycin Natural products CCC1(O)CC(OC2CC(N)C(O)C(C)O2)c3cc4C(=O)c5c(OC)cccc5C(=O)c4c(O)c3C1 WEAHRLBPCANXCN-UHFFFAOYSA-N 0.000 description 8
- 230000001684 chronic effect Effects 0.000 description 7
- STQGQHZAVUOBTE-VGBVRHCVSA-N daunorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(C)=O)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 STQGQHZAVUOBTE-VGBVRHCVSA-N 0.000 description 7
- 239000000203 mixture Substances 0.000 description 7
- 210000004881 tumor cell Anatomy 0.000 description 7
- 230000001154 acute effect Effects 0.000 description 6
- 230000001028 anti-proliverative effect Effects 0.000 description 6
- 230000037396 body weight Effects 0.000 description 6
- 230000004580 weight loss Effects 0.000 description 6
- 102100025064 Cellular tumor antigen p53 Human genes 0.000 description 5
- 241001465754 Metazoa Species 0.000 description 5
- 235000021319 Palmitoleic acid Nutrition 0.000 description 5
- 230000006907 apoptotic process Effects 0.000 description 5
- 230000027455 binding Effects 0.000 description 5
- 230000022131 cell cycle Effects 0.000 description 5
- SECPZKHBENQXJG-UHFFFAOYSA-N cis-palmitoleic acid Natural products CCCCCCC=CCCCCCCCC(O)=O SECPZKHBENQXJG-UHFFFAOYSA-N 0.000 description 5
- 235000013305 food Nutrition 0.000 description 5
- 229940088597 hormone Drugs 0.000 description 5
- 239000005556 hormone Substances 0.000 description 5
- 230000003993 interaction Effects 0.000 description 5
- 230000004952 protein activity Effects 0.000 description 5
- GWHCXVQVJPWHRF-KTKRTIGZSA-N (15Z)-tetracosenoic acid Chemical compound CCCCCCCC\C=C/CCCCCCCCCCCCCC(O)=O GWHCXVQVJPWHRF-KTKRTIGZSA-N 0.000 description 4
- 208000024172 Cardiovascular disease Diseases 0.000 description 4
- 102000016736 Cyclin Human genes 0.000 description 4
- 108050006400 Cyclin Proteins 0.000 description 4
- 241000282412 Homo Species 0.000 description 4
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 4
- XJXROGWVRIJYMO-SJDLZYGOSA-N Nervonic acid Natural products O=C(O)[C@@H](/C=C/CCCCCCCC)CCCCCCCCCCCC XJXROGWVRIJYMO-SJDLZYGOSA-N 0.000 description 4
- 230000032823 cell division Effects 0.000 description 4
- 239000003795 chemical substances by application Substances 0.000 description 4
- GWHCXVQVJPWHRF-UHFFFAOYSA-N cis-tetracosenoic acid Natural products CCCCCCCCC=CCCCCCCCCCCCCCC(O)=O GWHCXVQVJPWHRF-UHFFFAOYSA-N 0.000 description 4
- 238000002474 experimental method Methods 0.000 description 4
- 235000012631 food intake Nutrition 0.000 description 4
- 230000001631 hypertensive effect Effects 0.000 description 4
- 239000003112 inhibitor Substances 0.000 description 4
- 208000032839 leukemia Diseases 0.000 description 4
- 201000005202 lung cancer Diseases 0.000 description 4
- 208000020816 lung neoplasm Diseases 0.000 description 4
- 238000000034 method Methods 0.000 description 4
- -1 polyoxyethylene Polymers 0.000 description 4
- 230000026447 protein localization Effects 0.000 description 4
- 230000009467 reduction Effects 0.000 description 4
- 230000036186 satiety Effects 0.000 description 4
- 235000019627 satiety Nutrition 0.000 description 4
- 238000012360 testing method Methods 0.000 description 4
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 4
- 108060003345 Adrenergic Receptor Proteins 0.000 description 3
- 102000017910 Adrenergic receptor Human genes 0.000 description 3
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 3
- 102000012338 Poly(ADP-ribose) Polymerases Human genes 0.000 description 3
- 108010061844 Poly(ADP-ribose) Polymerases Proteins 0.000 description 3
- 229920000776 Poly(Adenosine diphosphate-ribose) polymerase Polymers 0.000 description 3
- 229940045799 anthracyclines and related substance Drugs 0.000 description 3
- 238000003782 apoptosis assay Methods 0.000 description 3
- 230000015572 biosynthetic process Effects 0.000 description 3
- 230000004531 blood pressure lowering effect Effects 0.000 description 3
- 230000015556 catabolic process Effects 0.000 description 3
- 101150073031 cdk2 gene Proteins 0.000 description 3
- 230000010261 cell growth Effects 0.000 description 3
- 210000000170 cell membrane Anatomy 0.000 description 3
- 230000004663 cell proliferation Effects 0.000 description 3
- 230000030570 cellular localization Effects 0.000 description 3
- 230000008859 change Effects 0.000 description 3
- 238000006243 chemical reaction Methods 0.000 description 3
- UWHZIFQPPBDJPM-FPLPWBNLSA-N cis-vaccenic acid Chemical compound CCCCCC\C=C/CCCCCCCCCC(O)=O UWHZIFQPPBDJPM-FPLPWBNLSA-N 0.000 description 3
- 238000006731 degradation reaction Methods 0.000 description 3
- 230000002526 effect on cardiovascular system Effects 0.000 description 3
- VJJPUSNTGOMMGY-MRVIYFEKSA-N etoposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 VJJPUSNTGOMMGY-MRVIYFEKSA-N 0.000 description 3
- 229960005420 etoposide Drugs 0.000 description 3
- 230000037406 food intake Effects 0.000 description 3
- 230000006870 function Effects 0.000 description 3
- 239000003102 growth factor Substances 0.000 description 3
- 230000005522 programmed cell death Effects 0.000 description 3
- 150000003839 salts Chemical class 0.000 description 3
- 230000004873 systolic arterial blood pressure Effects 0.000 description 3
- 230000001225 therapeutic effect Effects 0.000 description 3
- DJCQJZKZUCHHAL-UHFFFAOYSA-N (Z)-9-Pentadecensaeure Natural products CCCCCC=CCCCCCCCC(O)=O DJCQJZKZUCHHAL-UHFFFAOYSA-N 0.000 description 2
- 208000003174 Brain Neoplasms Diseases 0.000 description 2
- 102000005483 Cell Cycle Proteins Human genes 0.000 description 2
- 108010031896 Cell Cycle Proteins Proteins 0.000 description 2
- 102000002427 Cyclin B Human genes 0.000 description 2
- 108010068150 Cyclin B Proteins 0.000 description 2
- 102000006313 Cyclin D3 Human genes 0.000 description 2
- 108010058545 Cyclin D3 Proteins 0.000 description 2
- 102000004127 Cytokines Human genes 0.000 description 2
- 108090000695 Cytokines Proteins 0.000 description 2
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 2
- 108020004414 DNA Proteins 0.000 description 2
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 2
- 206010027476 Metastases Diseases 0.000 description 2
- 241000699670 Mus sp. Species 0.000 description 2
- MWUXSHHQAYIFBG-UHFFFAOYSA-N Nitric oxide Chemical group O=[N] MWUXSHHQAYIFBG-UHFFFAOYSA-N 0.000 description 2
- 102000003923 Protein Kinase C Human genes 0.000 description 2
- 108090000315 Protein Kinase C Proteins 0.000 description 2
- 102000014384 Type C Phospholipases Human genes 0.000 description 2
- 108010079194 Type C Phospholipases Proteins 0.000 description 2
- UWHZIFQPPBDJPM-FPLPWBNLSA-M Vaccenic acid Natural products CCCCCC\C=C/CCCCCCCCCC([O-])=O UWHZIFQPPBDJPM-FPLPWBNLSA-M 0.000 description 2
- MWRBNPKJOOWZPW-XPWSMXQVSA-N [3-[2-aminoethoxy(hydroxy)phosphoryl]oxy-2-[(e)-octadec-9-enoyl]oxypropyl] (e)-octadec-9-enoate Chemical compound CCCCCCCC\C=C\CCCCCCCC(=O)OCC(COP(O)(=O)OCCN)OC(=O)CCCCCCC\C=C\CCCCCCCC MWRBNPKJOOWZPW-XPWSMXQVSA-N 0.000 description 2
- 230000004913 activation Effects 0.000 description 2
- 230000003276 anti-hypertensive effect Effects 0.000 description 2
- 210000001130 astrocyte Anatomy 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 210000004556 brain Anatomy 0.000 description 2
- 230000025084 cell cycle arrest Effects 0.000 description 2
- 230000030833 cell death Effects 0.000 description 2
- 231100000762 chronic effect Toxicity 0.000 description 2
- 230000001276 controlling effect Effects 0.000 description 2
- 239000002537 cosmetic Substances 0.000 description 2
- 230000034994 death Effects 0.000 description 2
- 230000001419 dependent effect Effects 0.000 description 2
- 230000008034 disappearance Effects 0.000 description 2
- 239000012636 effector Substances 0.000 description 2
- 108010046094 fatty-acid amide hydrolase Proteins 0.000 description 2
- 230000012010 growth Effects 0.000 description 2
- 150000002373 hemiacetals Chemical class 0.000 description 2
- 238000000338 in vitro Methods 0.000 description 2
- 238000001727 in vivo Methods 0.000 description 2
- 239000000411 inducer Substances 0.000 description 2
- 230000001939 inductive effect Effects 0.000 description 2
- 230000002401 inhibitory effect Effects 0.000 description 2
- 230000005764 inhibitory process Effects 0.000 description 2
- 238000002372 labelling Methods 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 230000011278 mitosis Effects 0.000 description 2
- 230000000394 mitotic effect Effects 0.000 description 2
- 239000002858 neurotransmitter agent Substances 0.000 description 2
- 230000037361 pathway Effects 0.000 description 2
- 230000000144 pharmacologic effect Effects 0.000 description 2
- 230000035790 physiological processes and functions Effects 0.000 description 2
- 230000003389 potentiating effect Effects 0.000 description 2
- 230000004853 protein function Effects 0.000 description 2
- 230000001105 regulatory effect Effects 0.000 description 2
- 230000028327 secretion Effects 0.000 description 2
- 239000000600 sorbitol Substances 0.000 description 2
- 125000000446 sulfanediyl group Chemical group *S* 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- 230000004565 tumor cell growth Effects 0.000 description 2
- XOFLBQFBSOEHOG-UUOKFMHZSA-N γS-GTP Chemical compound C1=2NC(N)=NC(=O)C=2N=CN1[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OP(O)(O)=S)[C@@H](O)[C@H]1O XOFLBQFBSOEHOG-UUOKFMHZSA-N 0.000 description 2
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 1
- FSUQBXCPPDDGSI-QXMHVHEDSA-N (Z)-2-methyloctadec-9-ene Chemical compound CCCCCCCC\C=C/CCCCCCC(C)C FSUQBXCPPDDGSI-QXMHVHEDSA-N 0.000 description 1
- OQAGSRABDIVQJX-KHPPLWFESA-N (Z)-octadec-9-en-2-amine Chemical compound CCCCCCCC\C=C/CCCCCCC(C)N OQAGSRABDIVQJX-KHPPLWFESA-N 0.000 description 1
- SRXNGDFVUBMAOI-KHPPLWFESA-N (z)-octadec-9-en-2-ol Chemical compound CCCCCCCC\C=C/CCCCCCC(C)O SRXNGDFVUBMAOI-KHPPLWFESA-N 0.000 description 1
- KIHBGTRZFAVZRV-UHFFFAOYSA-N 2-hydroxyoctadecanoic acid Chemical compound CCCCCCCCCCCCCCCCC(O)C(O)=O KIHBGTRZFAVZRV-UHFFFAOYSA-N 0.000 description 1
- 238000010600 3H thymidine incorporation assay Methods 0.000 description 1
- ORILYTVJVMAKLC-UHFFFAOYSA-N Adamantane Natural products C1C(C2)CC3CC1CC2C3 ORILYTVJVMAKLC-UHFFFAOYSA-N 0.000 description 1
- 208000010507 Adenocarcinoma of Lung Diseases 0.000 description 1
- IVOMOUWHDPKRLL-KQYNXXCUSA-N Cyclic adenosine monophosphate Chemical group C([C@H]1O2)OP(O)(=O)O[C@H]1[C@@H](O)[C@@H]2N1C(N=CN=C2N)=C2N=C1 IVOMOUWHDPKRLL-KQYNXXCUSA-N 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 102000034286 G proteins Human genes 0.000 description 1
- 102000009465 Growth Factor Receptors Human genes 0.000 description 1
- 108010009202 Growth Factor Receptors Proteins 0.000 description 1
- 108010078321 Guanylate Cyclase Proteins 0.000 description 1
- 102000014469 Guanylate cyclase Human genes 0.000 description 1
- 208000001953 Hypotension Diseases 0.000 description 1
- 102000004310 Ion Channels Human genes 0.000 description 1
- 108090000862 Ion Channels Proteins 0.000 description 1
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical compound SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 1
- 108010092277 Leptin Proteins 0.000 description 1
- 102000016267 Leptin Human genes 0.000 description 1
- 239000000232 Lipid Bilayer Substances 0.000 description 1
- 102000004895 Lipoproteins Human genes 0.000 description 1
- 108090001030 Lipoproteins Proteins 0.000 description 1
- CNNRZPIZEKGCOI-UHFFFAOYSA-N OC(C(=O)O)CCCCCCC=C/CCCCCCCC.OC(C(=O)O)CCCCCCC=C/CCCCCCCC Chemical compound OC(C(=O)O)CCCCCCC=C/CCCCCCCC.OC(C(=O)O)CCCCCCC=C/CCCCCCCC CNNRZPIZEKGCOI-UHFFFAOYSA-N 0.000 description 1
- 102000035195 Peptidases Human genes 0.000 description 1
- 108091005804 Peptidases Proteins 0.000 description 1
- 108091000080 Phosphotransferase Proteins 0.000 description 1
- 206010060862 Prostate cancer Diseases 0.000 description 1
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 1
- 239000004365 Protease Substances 0.000 description 1
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 1
- 210000001744 T-lymphocyte Anatomy 0.000 description 1
- IVOMOUWHDPKRLL-UHFFFAOYSA-N UNPD107823 Natural products O1C2COP(O)(=O)OC2C(O)C1N1C(N=CN=C2N)=C2N=C1 IVOMOUWHDPKRLL-UHFFFAOYSA-N 0.000 description 1
- 102000004136 Vasopressin Receptors Human genes 0.000 description 1
- 108090000643 Vasopressin Receptors Proteins 0.000 description 1
- 238000002441 X-ray diffraction Methods 0.000 description 1
- 238000000333 X-ray scattering Methods 0.000 description 1
- WERKSKAQRVDLDW-ANOHMWSOSA-N [(2s,3r,4r,5r)-2,3,4,5,6-pentahydroxyhexyl] (z)-octadec-9-enoate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO WERKSKAQRVDLDW-ANOHMWSOSA-N 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 230000003213 activating effect Effects 0.000 description 1
- 102000030621 adenylate cyclase Human genes 0.000 description 1
- 108060000200 adenylate cyclase Proteins 0.000 description 1
- 239000000556 agonist Substances 0.000 description 1
- OFHCOWSQAMBJIW-AVJTYSNKSA-N alfacalcidol Chemical compound C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@H](C)CCCC(C)C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C OFHCOWSQAMBJIW-AVJTYSNKSA-N 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 239000002220 antihypertensive agent Substances 0.000 description 1
- 229940127088 antihypertensive drug Drugs 0.000 description 1
- 229940041181 antineoplastic drug Drugs 0.000 description 1
- 235000019789 appetite Nutrition 0.000 description 1
- 230000036528 appetite Effects 0.000 description 1
- 208000037849 arterial hypertension Diseases 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 230000033366 cell cycle process Effects 0.000 description 1
- 230000003915 cell function Effects 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 238000002512 chemotherapy Methods 0.000 description 1
- HSNQNPCNYIJJHT-ZCXUNETKSA-N cis-octadec-9-ene Chemical compound CCCCCCCC\C=C/CCCCCCCC HSNQNPCNYIJJHT-ZCXUNETKSA-N 0.000 description 1
- 238000004891 communication Methods 0.000 description 1
- 229940095074 cyclic amp Drugs 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- 235000018417 cysteine Nutrition 0.000 description 1
- 210000000172 cytosol Anatomy 0.000 description 1
- 230000007812 deficiency Effects 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 230000035487 diastolic blood pressure Effects 0.000 description 1
- 230000001614 effect on membrane Effects 0.000 description 1
- 239000012039 electrophile Substances 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 210000003743 erythrocyte Anatomy 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 125000004494 ethyl ester group Chemical group 0.000 description 1
- 210000002950 fibroblast Anatomy 0.000 description 1
- 238000000684 flow cytometry Methods 0.000 description 1
- GNBHRKFJIUUOQI-UHFFFAOYSA-N fluorescein Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 GNBHRKFJIUUOQI-UHFFFAOYSA-N 0.000 description 1
- 235000019525 fullness Nutrition 0.000 description 1
- 108091006104 gene-regulatory proteins Proteins 0.000 description 1
- 102000034356 gene-regulatory proteins Human genes 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- 239000004093 hydrolase inhibitor Substances 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- 208000021822 hypotensive Diseases 0.000 description 1
- 230000001077 hypotensive effect Effects 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 238000011835 investigation Methods 0.000 description 1
- 230000007794 irritation Effects 0.000 description 1
- 230000002045 lasting effect Effects 0.000 description 1
- 229940039781 leptin Drugs 0.000 description 1
- NRYBAZVQPHGZNS-ZSOCWYAHSA-N leptin Chemical compound O=C([C@H](CO)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)CNC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CC(C)C)CCSC)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](CS)C(O)=O NRYBAZVQPHGZNS-ZSOCWYAHSA-N 0.000 description 1
- 239000002502 liposome Substances 0.000 description 1
- 230000004807 localization Effects 0.000 description 1
- 201000005249 lung adenocarcinoma Diseases 0.000 description 1
- 201000002037 lung adenoma Diseases 0.000 description 1
- 102000006240 membrane receptors Human genes 0.000 description 1
- 108020004084 membrane receptors Proteins 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- 230000009401 metastasis Effects 0.000 description 1
- 230000003278 mimic effect Effects 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004879 molecular function Effects 0.000 description 1
- 239000003147 molecular marker Substances 0.000 description 1
- 230000009456 molecular mechanism Effects 0.000 description 1
- 108091006026 monomeric small GTPases Proteins 0.000 description 1
- 230000035407 negative regulation of cell proliferation Effects 0.000 description 1
- 231100000957 no side effect Toxicity 0.000 description 1
- 239000002736 nonionic surfactant Substances 0.000 description 1
- 108020004707 nucleic acids Proteins 0.000 description 1
- 102000039446 nucleic acids Human genes 0.000 description 1
- 150000007523 nucleic acids Chemical class 0.000 description 1
- 235000018343 nutrient deficiency Nutrition 0.000 description 1
- 235000015097 nutrients Nutrition 0.000 description 1
- 239000007764 o/w emulsion Substances 0.000 description 1
- FATBGEAMYMYZAF-KTKRTIGZSA-N oleamide Chemical compound CCCCCCCC\C=C/CCCCCCCC(N)=O FATBGEAMYMYZAF-KTKRTIGZSA-N 0.000 description 1
- FATBGEAMYMYZAF-UHFFFAOYSA-N oleicacidamide-heptaglycolether Natural products CCCCCCCCC=CCCCCCCCC(N)=O FATBGEAMYMYZAF-UHFFFAOYSA-N 0.000 description 1
- 238000011275 oncology therapy Methods 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 239000000825 pharmaceutical preparation Substances 0.000 description 1
- 229940127557 pharmaceutical product Drugs 0.000 description 1
- 238000011458 pharmacological treatment Methods 0.000 description 1
- 102000020233 phosphotransferase Human genes 0.000 description 1
- 239000013641 positive control Substances 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 230000027419 protein insertion into membrane Effects 0.000 description 1
- 230000007398 protein translocation Effects 0.000 description 1
- 238000001959 radiotherapy Methods 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 230000029865 regulation of blood pressure Effects 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 230000019491 signal transduction Effects 0.000 description 1
- 102000030938 small GTPase Human genes 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 230000004936 stimulating effect Effects 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 230000008093 supporting effect Effects 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 230000035488 systolic blood pressure Effects 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- LKOVPWSSZFDYPG-WUKNDPDISA-N trans-octadec-2-enoic acid Chemical compound CCCCCCCCCCCCCCC\C=C\C(O)=O LKOVPWSSZFDYPG-WUKNDPDISA-N 0.000 description 1
- PHYFQTYBJUILEZ-IUPFWZBJSA-N triolein Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC(OC(=O)CCCCCCC\C=C/CCCCCCCC)COC(=O)CCCCCCC\C=C/CCCCCCCC PHYFQTYBJUILEZ-IUPFWZBJSA-N 0.000 description 1
- 238000005303 weighing Methods 0.000 description 1
- 238000004260 weight control Methods 0.000 description 1
- 230000037221 weight management Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/185—Acids; Anhydrides, halides or salts thereof, e.g. sulfur acids, imidic, hydrazonic or hydroximic acids
- A61K31/19—Carboxylic acids, e.g. valproic acid
- A61K31/20—Carboxylic acids, e.g. valproic acid having a carboxyl group bound to a chain of seven or more carbon atoms, e.g. stearic, palmitic, arachidic acids
- A61K31/201—Carboxylic acids, e.g. valproic acid having a carboxyl group bound to a chain of seven or more carbon atoms, e.g. stearic, palmitic, arachidic acids having one or two double bonds, e.g. oleic, linoleic acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/185—Acids; Anhydrides, halides or salts thereof, e.g. sulfur acids, imidic, hydrazonic or hydroximic acids
- A61K31/19—Carboxylic acids, e.g. valproic acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/185—Acids; Anhydrides, halides or salts thereof, e.g. sulfur acids, imidic, hydrazonic or hydroximic acids
- A61K31/19—Carboxylic acids, e.g. valproic acid
- A61K31/195—Carboxylic acids, e.g. valproic acid having an amino group
- A61K31/197—Carboxylic acids, e.g. valproic acid having an amino group the amino and the carboxyl groups being attached to the same acyclic carbon chain, e.g. gamma-aminobutyric acid [GABA], beta-alanine, epsilon-aminocaproic acid or pantothenic acid
- A61K31/198—Alpha-amino acids, e.g. alanine or edetic acid [EDTA]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/08—Drugs for disorders of the urinary system of the prostate
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/04—Anorexiants; Antiobesity agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/12—Antihypertensives
Landscapes
- Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical & Material Sciences (AREA)
- Veterinary Medicine (AREA)
- Medicinal Chemistry (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Epidemiology (AREA)
- Cardiology (AREA)
- Heart & Thoracic Surgery (AREA)
- Urology & Nephrology (AREA)
- Obesity (AREA)
- Hematology (AREA)
- Diabetes (AREA)
- Vascular Medicine (AREA)
- Biomedical Technology (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Child & Adolescent Psychology (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Description
膜脂質は、タンパク質及び核酸に比べてより多くの二次構造をとることができる。生体膜の典型的な脂質二重層は、これらの二次的立体配置の一つに過ぎない。生細胞におけるその他の二次構造の存在度及び役割についてはほとんど知られていない。これらの構造の一機能、すなわち膜に対するGタンパク質の結合親和性を増加させる機能は、発明者他による以前の研究に記載されている(Escriba PV、Ozaita A、Ribas C、Miralles A、Fodor E、Farkas及びGarcia−Sevilla JA、Proceedings of the National Academy of Sciences of the USA、94巻、11375〜11380ページ、1997年)。
ヒドロキシオレイン酸及び関連化合物は、[35S]GTPγSの結合によって測定されるGタンパク質の活性を変調することができる(図5)。
膜構造を研究するのに最も効果的かつ強力な技法は、X線回折/散乱である。この技法を用い、我々は、2−ヒドロキシオレイン酸及びその類似体により膜の構造が変化することを立証した。層状構造から六方晶構造への遷移温度の低下は、膜における脂質分子の配置に対する重要な効果を示している。この配置の調節は、2−ヒドロキシオレイン酸及びその類似体によって発揮される効果の根幹を成している。一般式を満たす被験類似体はすべて、細胞増殖(癌における有効性)、血圧(心血管プロセスにおける有効性)及び体重(肥満における有効性)の膜変調及び制御の活性を有している。
ヒドロキシオレイン酸の抗増殖効果をヒト肺癌細胞A549及びヒト白血病細胞(ジャーカット)において証明した。図3は、腫瘍細胞が分裂するために必要なタンパク質cdk2、サイクリンB及びサイクリンD3の減少に伴う抗増殖性タンパク質p21の誘導を示している。同様の効果が、前述の一般構造式を満たす被験類似体すべてによって生じた。2−ヒドロキシオレイン酸及びその類似体のこの抗増殖効果は、培養液における腫瘍細胞の細胞密度の低下によって立証された(図4)。また、この抗増殖効果は、他の技法及び他の細胞タイプを用いても観察され、ラット一次星状細胞において、これらの脂肪酸は抗増殖効果を有し、トリチウム化チミジンの取り込みによって試験した。さらに、ヒドロキシオレイン酸及びその類似体は、ヒト癌細胞においてアポトーシスすなわちプログラム細胞死を引き起こすことができる。一方ではPARPの分解が(図3C)、他方では細胞形態の変化及び細胞残渣の存在が(図4)、細胞死の誘導物質としての2−ヒドロキシオレイン酸及びその類似体の効果を証明している。フローサイトメトリー実験を用いることにより、2−ヒドロキシオレイン酸の存在下で生きているヒト白血病細胞(ジャーカット)の数は、知られている抗腫瘍剤エトポシドにより生存し続ける細胞の10%に過ぎないことが立証された。
本発明の薬物とGタンパク質の活性と血圧の間には関連性がある。以前に記載したことを裏付ける結果は、ヒトで行われた研究であるが、高血圧患者に膜脂質のレベルに変化があることが認められた(表3)。膜脂質は層状−六方晶遷移に影響し、さらにGタンパク質の局在化及び機能を決定する。実際、高血圧患者において、我々は、膜に結合しているGタンパク質のレベルに、前述した膜脂質の変化及び六方晶相形成のしやすさに起因する変化を観察している。非層状膜構造の変調及び結果として生じるGタンパク質の再局在化が高血圧を引き起こすのであれば、膜脂質の層状−六方晶遷移を調節することにより、膜タンパク質の局在化及び、最終的には血圧の調節を行うことが可能である(図7)。
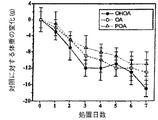
2−ヒドロキシオレイン酸は、ラットにおいて血圧の有意な低下を引き起こした(図9)。これらの低下は、急性型(治療2時間で19±6mmHg、P<0.01、n=6)及び慢性型(1週間、26±7mmHg、P<0.001、n=6)であった。さらに、1mg/kgから10mg/kgまでの急性及び慢性治療も、濃度依存性である血圧の有意な低下を引き起こした。
これらの分子で処置したラットは、慢性治療中(5〜17日)に体重が減った。これらの実験では、2−ヒドロキシオレイン酸又はその類似体、特にアミノオレイン酸で処置したラットには、処置ラットの対照群と同様、飼料と水を自由に与えた(図11)。これらの条件で、処置の初日から始まり、処置の7日目には17グラム(2〜3月齢Sprague−Dowleyラットの正常体重の5%)までラットの体重に漸進的減少が認められた。これらの動物に供給した飼料の重さを量ると、処置期間中は消費が低いことが判明し、本発明に関連する分子による処置は、動物に満腹の効果を生じることを裏付けた。2−ヒドロキシオレイン酸を用い、成体マウスに対して28日までの期間行った同様の実験は、対照マウス(溶媒で処置)に比べ、15%から25%の体重の減少を示す。
Claims (3)
- 2−ヒドロキシオレイン酸、2−メチル−オレイン酸又は2−アミノオレイン酸を含む、癌の治療のための、薬学的組成物。
- 2−ヒドロキシオレイン酸又は2−メチル−オレイン酸を含む、高血圧の治療のための、薬学的組成物。
- 2−ヒドロキシオレイン酸を含む、肥満の治療のための、薬学的組成物。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| ES200102269A ES2186576B1 (es) | 2001-10-11 | 2001-10-11 | Acido 2-hidroxioleico para utilizar como medicamento. |
| ESP200102269 | 2001-10-11 | ||
| PCT/ES2002/000475 WO2003030891A1 (es) | 2001-10-11 | 2002-10-09 | Utilizacion del acido hidroxioleico y compuestos analogos del mismo en la fabricacion de medicamentos |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2005527479A JP2005527479A (ja) | 2005-09-15 |
| JP2005527479A5 JP2005527479A5 (ja) | 2006-01-05 |
| JP4737931B2 true JP4737931B2 (ja) | 2011-08-03 |
Family
ID=8499164
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2003533923A Expired - Lifetime JP4737931B2 (ja) | 2001-10-11 | 2002-10-09 | 医薬品の製造におけるヒドロキシオレイン酸及び類似化合物の使用 |
Country Status (13)
| Country | Link |
|---|---|
| US (3) | US20050014831A1 (ja) |
| EP (1) | EP1435235B1 (ja) |
| JP (1) | JP4737931B2 (ja) |
| CN (3) | CN100471492C (ja) |
| AT (1) | ATE322261T1 (ja) |
| BR (1) | BRPI0213165B8 (ja) |
| CA (1) | CA2463348C (ja) |
| DE (1) | DE60210488T2 (ja) |
| ES (2) | ES2186576B1 (ja) |
| MX (1) | MXPA04003255A (ja) |
| PT (1) | PT1435235E (ja) |
| RU (1) | RU2310445C2 (ja) |
| WO (1) | WO2003030891A1 (ja) |
Families Citing this family (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ES2229935B1 (es) * | 2003-10-10 | 2006-10-01 | Universitat De Les Illes Balears | Utilizacion del acido hidroxioleico y compuestos analagos del mismo como aditivos alimentarios funcionales. |
| JP2009051732A (ja) * | 2005-12-13 | 2009-03-12 | Meiji Seika Kaisha Ltd | Pparリガンド活性を有する組成物 |
| CN101570481B (zh) * | 2008-04-30 | 2012-02-01 | 上海医药工业研究院 | 一种多羟基长链脂肪酸及其分离提取方法和在抑制芳香化酶活性中的应用 |
| ES2342997B1 (es) * | 2008-12-09 | 2011-06-06 | Universitat De Les Illes Balears | Alpha derivados de acidos grasos cis-monoinsaturados para ser usados como medicamento. |
| ES2345241B1 (es) * | 2009-03-16 | 2011-09-08 | Lipopharma Therapeutics | Uso de 2-hidroxiderivados de acidos grasos poliinsaturados como medicamentos. |
| WO2011089265A1 (en) | 2010-01-25 | 2011-07-28 | Bridge Bioresearch Plc | Use of fatty acid compounds for lowering blood glucose levels |
| CN101985420B (zh) * | 2010-01-29 | 2014-04-30 | 苏州润新生物科技有限公司 | 油酸酯及其制备方法和在制备用于治疗高血压及其并发症的药物中的应用 |
| ES2401629B1 (es) * | 2011-10-07 | 2014-03-04 | Universitat De Les Illes Balears | Enantiómeros de 2-hidroxiderivados de ácidos grasos y su uso como medicamentos. |
| EP2805717A4 (en) * | 2012-01-19 | 2015-06-10 | Nippon Suisan Kaisha Ltd | CUTTING HUNGER |
| WO2013132092A1 (en) | 2012-03-09 | 2013-09-12 | Pensieve International Plc | Use of monounsaturated fatty acid compounds for controlling the body weight of a dog or a cat |
| NZ706067A (en) * | 2012-09-26 | 2016-07-29 | Tangent Reprofiling Ltd | Modulators of androgen synthesis |
| GB201217296D0 (en) | 2012-09-27 | 2012-11-14 | Alta Innovations Ltd | Method of treatment and/or prevention |
| ES2731251T3 (es) | 2014-10-21 | 2019-11-14 | Univ Illes Balears | Compuestos de 2-hidroxi-triacilglicerol para uso en el tratamiento de una enfermedad |
| US10756968B2 (en) | 2015-01-26 | 2020-08-25 | Rapid7, Inc. | Network resource management devices methods and systems |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS6344843A (ja) * | 1986-08-13 | 1988-02-25 | Kao Corp | 油中水中油型乳化油脂組成物 |
| JPH02262514A (ja) * | 1988-11-16 | 1990-10-25 | Jyan Shue | 細胞親和性の不均質分子脂質(chml)およびその製造方法 |
| DE19727636C1 (de) * | 1997-06-28 | 1998-11-19 | Rwe Dea Ag | Verfahren zur Herstellung von 2-Alkylcarbonsäuren |
| JP2000513361A (ja) * | 1996-06-26 | 2000-10-10 | ザ スクリップス リサーチ インスティテュート | オレアミドヒドロラーゼ阻害剤 |
Family Cites Families (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS61297A (ja) * | 1984-06-12 | 1986-01-06 | 日本油脂株式会社 | オレイン酸の製造法 |
| GB8603621D0 (en) | 1986-02-14 | 1986-03-19 | Habib N | Modifying lipid structure of cell membranes |
| US5422371A (en) * | 1992-05-27 | 1995-06-06 | Arch Development Corp. | Methods and compositions for inhibiting 5α-reductase activity |
| CN1118228A (zh) * | 1995-03-16 | 1996-03-13 | 姜训书 | 松籽果酱 |
| CN1118218A (zh) * | 1995-03-16 | 1996-03-13 | 姜训书 | 松籽油的制作方法及松籽保健食用油 |
| GB9506837D0 (en) * | 1995-04-03 | 1995-05-24 | Scotia Holdings Plc | Triglycerides |
| DE69626927T2 (de) * | 1995-10-30 | 2003-11-20 | Oleoyl-Estrone Developments, S.I. | Oleat Monoester von Estrogenen zur Behandlung von Fettleibigkeit |
| CN1212867A (zh) * | 1997-09-29 | 1999-04-07 | 宋凤亭 | 防治心脑血管疾病的组合物 |
| EP0948963B1 (en) * | 1998-01-21 | 2003-05-07 | Fideline | Pig appeasing pheromones to decrease stress, anxiety and aggressiveness |
| US6214875B1 (en) * | 1998-04-14 | 2001-04-10 | Zhenhua Yang | Anticancer effects of specific branched-chain fatty acids and related production process |
| GB2389788B (en) * | 2000-12-23 | 2005-07-20 | Univ Creighton | Methods for inducing apoptosis and inhibiting proliferation in cancer cells |
| DE10130491A1 (de) * | 2001-06-25 | 2003-04-17 | Heirler Horst | Verwendung von mittelkettigen Triglyceriden zur Prävention und Therapie von Adipositas |
-
2001
- 2001-10-11 ES ES200102269A patent/ES2186576B1/es not_active Expired - Fee Related
-
2002
- 2002-10-09 CA CA2463348A patent/CA2463348C/en not_active Expired - Lifetime
- 2002-10-09 DE DE60210488T patent/DE60210488T2/de not_active Expired - Lifetime
- 2002-10-09 EP EP02783094A patent/EP1435235B1/en not_active Expired - Lifetime
- 2002-10-09 MX MXPA04003255A patent/MXPA04003255A/es active IP Right Grant
- 2002-10-09 ES ES02783094T patent/ES2261752T3/es not_active Expired - Lifetime
- 2002-10-09 US US10/488,726 patent/US20050014831A1/en not_active Abandoned
- 2002-10-09 BR BRPI0213165A patent/BRPI0213165B8/pt not_active IP Right Cessation
- 2002-10-09 JP JP2003533923A patent/JP4737931B2/ja not_active Expired - Lifetime
- 2002-10-09 CN CNB028201132A patent/CN100471492C/zh not_active Expired - Lifetime
- 2002-10-09 PT PT02783094T patent/PT1435235E/pt unknown
- 2002-10-09 WO PCT/ES2002/000475 patent/WO2003030891A1/es active IP Right Grant
- 2002-10-09 AT AT02783094T patent/ATE322261T1/de active
- 2002-10-09 CN CN2008100917937A patent/CN101259121B/zh not_active Expired - Fee Related
- 2002-10-09 RU RU2004114229/15A patent/RU2310445C2/ru active
- 2002-10-09 CN CN2008100917941A patent/CN101259122B/zh not_active Expired - Fee Related
-
2008
- 2008-04-15 US US12/082,938 patent/US7851507B2/en not_active Expired - Lifetime
-
2010
- 2010-11-04 US US12/939,641 patent/US8778995B2/en not_active Expired - Lifetime
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS6344843A (ja) * | 1986-08-13 | 1988-02-25 | Kao Corp | 油中水中油型乳化油脂組成物 |
| JPH02262514A (ja) * | 1988-11-16 | 1990-10-25 | Jyan Shue | 細胞親和性の不均質分子脂質(chml)およびその製造方法 |
| JP2000513361A (ja) * | 1996-06-26 | 2000-10-10 | ザ スクリップス リサーチ インスティテュート | オレアミドヒドロラーゼ阻害剤 |
| DE19727636C1 (de) * | 1997-06-28 | 1998-11-19 | Rwe Dea Ag | Verfahren zur Herstellung von 2-Alkylcarbonsäuren |
Also Published As
| Publication number | Publication date |
|---|---|
| EP1435235A1 (en) | 2004-07-07 |
| CN101259121A (zh) | 2008-09-10 |
| CN101259122A (zh) | 2008-09-10 |
| CN1688302A (zh) | 2005-10-26 |
| ES2186576A1 (es) | 2003-05-01 |
| DE60210488D1 (de) | 2006-05-18 |
| US8778995B2 (en) | 2014-07-15 |
| ATE322261T1 (de) | 2006-04-15 |
| CN101259121B (zh) | 2011-04-06 |
| ES2186576B1 (es) | 2004-09-16 |
| JP2005527479A (ja) | 2005-09-15 |
| EP1435235B1 (en) | 2006-04-05 |
| RU2004114229A (ru) | 2005-04-20 |
| US20050014831A1 (en) | 2005-01-20 |
| CA2463348A1 (en) | 2003-04-17 |
| BR0213165A (pt) | 2004-09-14 |
| US7851507B2 (en) | 2010-12-14 |
| CN101259122B (zh) | 2011-04-06 |
| MXPA04003255A (es) | 2005-01-25 |
| ES2261752T3 (es) | 2006-11-16 |
| RU2310445C2 (ru) | 2007-11-20 |
| US20090082446A1 (en) | 2009-03-26 |
| US20110136906A1 (en) | 2011-06-09 |
| PT1435235E (pt) | 2006-08-31 |
| CN100471492C (zh) | 2009-03-25 |
| WO2003030891A1 (es) | 2003-04-17 |
| DE60210488T2 (de) | 2006-12-14 |
| CA2463348C (en) | 2011-04-05 |
| BRPI0213165B8 (pt) | 2021-05-25 |
| BRPI0213165B1 (pt) | 2018-01-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US7851507B2 (en) | Use of hydroxyoleic acid and related compounds in the manufacture of drugs | |
| ES2363442T3 (es) | Combinación que comprende al menos una aminoácido y un inhibidor de pkr para utilizar en el tratamiento de la pérdida de masa muscular. | |
| CN101316584A (zh) | 用于预防及治疗由血管通透性亢进引起的眼病的医药 | |
| He et al. | Docosahexaenoic acid inhibits hepatic stellate cell activation to attenuate liver fibrosis in a PPARγ-dependent manner | |
| AU2018392985A1 (en) | Compositions and methods of treatment for neurological disorders comprising motor neuron diseases | |
| US20190274979A2 (en) | Use of (benzenesulfonamido)benzamide compounds for inhibiting liver fibrosis | |
| EP2931272A1 (en) | Methods of treating hepatic fibrosis and associated diseases by regulating rev-erb activity | |
| WO2020132378A2 (en) | Compositions and methods of treatment for neurological disorders comprising depression | |
| WO2004093910A1 (ja) | PPARδアゴニストによる脳神経変性疾患治療剤 | |
| CN109806249B (zh) | 类胡萝卜素在制备激活tmem16a离子通道的产品中的应用、激活剂、试剂盒和药物 | |
| WO2023242100A1 (en) | Novel ras inhibitors | |
| WO2016070798A1 (zh) | 一种抑制脂肪细胞分化和胰岛素耐受的药物 | |
| US20200261420A1 (en) | Enhancement of cancer treatment efficiency via the sphingosine-1-phosphate pathway | |
| EP2870135A1 (en) | Pharmaceutical composition comprising amino-phenyl- acetic acid octadec-(z)-9-enyl ester and use thereof for treating tumors | |
| EP4395775A1 (en) | Cyclopenta[4,5]furo[3,2-c]pyridine derivatives as ras inhibitors for use in the treatment of hyperproliferative diseases or genetic disorders | |
| WO2024156704A1 (en) | Novel mixtures as ras inhibitors for use in the treatment of proliferative and genetic diseases | |
| KR20240127619A (ko) | N-포르밀트립톨린을 유효성분으로 포함하는 방사선 내성 암의 예방, 개선 또는 치료용 조성물 | |
| WO2023242099A1 (en) | Novel ras inhibitors | |
| JP3981739B2 (ja) | 抗癌剤、癌細胞の増殖を抑制する方法 | |
| WO2006118299A1 (ja) | Gpcr阻害剤 | |
| JPWO2004056351A1 (ja) | Pka活性調節剤 | |
| MacCumber | Endothelin: Local synthesis and action in the brain, eye and peripheral tissues | |
| JP2014024807A (ja) | Trpv4活性抑制剤 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20050701 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20050701 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20090313 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20090611 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20090618 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20090710 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20090717 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20090812 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20090819 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20090911 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20090911 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20100323 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100607 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20101001 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20110124 |
|
| A911 | Transfer to examiner for re-examination before appeal (zenchi) |
Free format text: JAPANESE INTERMEDIATE CODE: A911 Effective date: 20110307 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20110408 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20110426 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 4737931 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20140513 Year of fee payment: 3 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| EXPY | Cancellation because of completion of term |