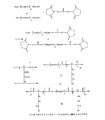
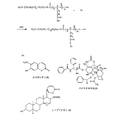
JP4365221B2 - 親水性ポリマー−マルチカルボキシルオリゴペプチドと薬剤分子との結合生成物、医薬組成物およびその薬学上の使用 - Google Patents
親水性ポリマー−マルチカルボキシルオリゴペプチドと薬剤分子との結合生成物、医薬組成物およびその薬学上の使用 Download PDFInfo
- Publication number
- JP4365221B2 JP4365221B2 JP2003573050A JP2003573050A JP4365221B2 JP 4365221 B2 JP4365221 B2 JP 4365221B2 JP 2003573050 A JP2003573050 A JP 2003573050A JP 2003573050 A JP2003573050 A JP 2003573050A JP 4365221 B2 JP4365221 B2 JP 4365221B2
- Authority
- JP
- Japan
- Prior art keywords
- group
- binding product
- product according
- hydrophilic polymer
- binding
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 239000003814 drug Substances 0.000 title claims abstract description 55
- 229940079593 drug Drugs 0.000 title claims abstract description 50
- 108010038807 Oligopeptides Proteins 0.000 title claims abstract description 40
- 102000015636 Oligopeptides Human genes 0.000 title claims abstract description 40
- 239000008194 pharmaceutical composition Substances 0.000 title description 4
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 claims abstract description 18
- 229930012538 Paclitaxel Natural products 0.000 claims abstract description 16
- 229960001592 paclitaxel Drugs 0.000 claims abstract description 16
- KLWPJMFMVPTNCC-UHFFFAOYSA-N Camptothecin Natural products CCC1(O)C(=O)OCC2=C1C=C3C4Nc5ccccc5C=C4CN3C2=O KLWPJMFMVPTNCC-UHFFFAOYSA-N 0.000 claims abstract description 10
- VSJKWCGYPAHWDS-FQEVSTJZSA-N camptothecin Chemical compound C1=CC=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 VSJKWCGYPAHWDS-FQEVSTJZSA-N 0.000 claims abstract description 10
- 229940127093 camptothecin Drugs 0.000 claims abstract description 10
- VSJKWCGYPAHWDS-UHFFFAOYSA-N dl-camptothecin Natural products C1=CC=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)C5(O)CC)C4=NC2=C1 VSJKWCGYPAHWDS-UHFFFAOYSA-N 0.000 claims abstract description 10
- 125000004400 (C1-C12) alkyl group Chemical group 0.000 claims abstract description 6
- 125000003710 aryl alkyl group Chemical group 0.000 claims abstract description 6
- 125000003107 substituted aryl group Chemical group 0.000 claims abstract description 6
- 125000004404 heteroalkyl group Chemical group 0.000 claims abstract description 5
- 125000000547 substituted alkyl group Chemical group 0.000 claims abstract description 5
- 239000002246 antineoplastic agent Substances 0.000 claims abstract 4
- 229920001223 polyethylene glycol Polymers 0.000 claims description 38
- 239000004220 glutamic acid Substances 0.000 claims description 26
- 229920001477 hydrophilic polymer Polymers 0.000 claims description 18
- 239000000203 mixture Substances 0.000 claims description 18
- -1 hydroxyl camptothecin Chemical compound 0.000 claims description 15
- MPDGHEJMBKOTSU-YKLVYJNSSA-N 18beta-glycyrrhetic acid Chemical compound C([C@H]1C2=CC(=O)[C@H]34)[C@@](C)(C(O)=O)CC[C@]1(C)CC[C@@]2(C)[C@]4(C)CC[C@@H]1[C@]3(C)CC[C@H](O)C1(C)C MPDGHEJMBKOTSU-YKLVYJNSSA-N 0.000 claims description 12
- 239000002202 Polyethylene glycol Substances 0.000 claims description 12
- 239000004480 active ingredient Substances 0.000 claims description 12
- RODXRVNMMDRFIK-UHFFFAOYSA-N scopoletin Chemical compound C1=CC(=O)OC2=C1C=C(OC)C(O)=C2 RODXRVNMMDRFIK-UHFFFAOYSA-N 0.000 claims description 12
- 239000000243 solution Substances 0.000 claims description 12
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims description 8
- XEHFSYYAGCUKEN-UHFFFAOYSA-N Dihydroscopoletin Natural products C1CC(=O)OC2=C1C=C(OC)C(O)=C2 XEHFSYYAGCUKEN-UHFFFAOYSA-N 0.000 claims description 6
- MPDGHEJMBKOTSU-UHFFFAOYSA-N Glycyrrhetinsaeure Natural products C12C(=O)C=C3C4CC(C)(C(O)=O)CCC4(C)CCC3(C)C1(C)CCC1C2(C)CCC(O)C1(C)C MPDGHEJMBKOTSU-UHFFFAOYSA-N 0.000 claims description 6
- 125000006367 bivalent amino carbonyl group Chemical group [H]N([*:1])C([*:2])=O 0.000 claims description 6
- 229960003720 enoxolone Drugs 0.000 claims description 6
- FWYIBGHGBOVPNL-UHFFFAOYSA-N scopoletin Natural products COC=1C=C2C=CC(OC2=C(C1)O)=O FWYIBGHGBOVPNL-UHFFFAOYSA-N 0.000 claims description 6
- 150000001413 amino acids Chemical class 0.000 claims description 5
- 229920001577 copolymer Polymers 0.000 claims description 5
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 5
- 108090000623 proteins and genes Proteins 0.000 claims description 5
- 102000004169 proteins and genes Human genes 0.000 claims description 5
- 239000004372 Polyvinyl alcohol Substances 0.000 claims description 4
- 239000002777 nucleoside Substances 0.000 claims description 4
- 229920001451 polypropylene glycol Polymers 0.000 claims description 4
- 229920002451 polyvinyl alcohol Polymers 0.000 claims description 4
- 239000000725 suspension Substances 0.000 claims description 4
- 230000008878 coupling Effects 0.000 claims description 3
- 238000010168 coupling process Methods 0.000 claims description 3
- 238000005859 coupling reaction Methods 0.000 claims description 3
- 229930003935 flavonoid Natural products 0.000 claims description 3
- 150000002215 flavonoids Chemical class 0.000 claims description 3
- 235000017173 flavonoids Nutrition 0.000 claims description 3
- 229930182478 glucoside Natural products 0.000 claims description 3
- 150000008131 glucosides Chemical class 0.000 claims description 3
- 229930015704 phenylpropanoid Natural products 0.000 claims description 3
- 239000000843 powder Substances 0.000 claims description 3
- 150000003431 steroids Chemical class 0.000 claims description 3
- 150000003505 terpenes Chemical class 0.000 claims description 3
- 125000004642 (C1-C12) alkoxy group Chemical group 0.000 claims description 2
- 108090000790 Enzymes Proteins 0.000 claims description 2
- 102000004190 Enzymes Human genes 0.000 claims description 2
- 239000000443 aerosol Substances 0.000 claims description 2
- 229930013930 alkaloid Natural products 0.000 claims description 2
- 150000001720 carbohydrates Chemical class 0.000 claims description 2
- VJJPUSNTGOMMGY-MRVIYFEKSA-N etoposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 VJJPUSNTGOMMGY-MRVIYFEKSA-N 0.000 claims description 2
- 229960005420 etoposide Drugs 0.000 claims description 2
- 239000007903 gelatin capsule Substances 0.000 claims description 2
- 150000003833 nucleoside derivatives Chemical class 0.000 claims description 2
- 150000007524 organic acids Chemical class 0.000 claims description 2
- 239000006187 pill Substances 0.000 claims description 2
- 239000000829 suppository Substances 0.000 claims description 2
- 239000003826 tablet Substances 0.000 claims description 2
- AZQWKYJCGOJGHM-UHFFFAOYSA-N 1,4-benzoquinone Chemical compound O=C1C=CC(=O)C=C1 AZQWKYJCGOJGHM-UHFFFAOYSA-N 0.000 claims 2
- YNAVUWVOSKDBBP-UHFFFAOYSA-N Morpholine Chemical compound C1COCCN1 YNAVUWVOSKDBBP-UHFFFAOYSA-N 0.000 claims 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 claims 1
- 239000003937 drug carrier Substances 0.000 claims 1
- 150000002995 phenylpropanoid derivatives Chemical class 0.000 claims 1
- 229920003169 water-soluble polymer Polymers 0.000 abstract description 3
- 231100000053 low toxicity Toxicity 0.000 abstract description 2
- 229940041181 antineoplastic drug Drugs 0.000 abstract 1
- 230000002708 enhancing effect Effects 0.000 abstract 1
- 125000005647 linker group Chemical group 0.000 abstract 1
- 239000000047 product Substances 0.000 description 52
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 33
- VHYFNPMBLIVWCW-UHFFFAOYSA-N 4-Dimethylaminopyridine Chemical compound CN(C)C1=CC=NC=C1 VHYFNPMBLIVWCW-UHFFFAOYSA-N 0.000 description 28
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 24
- 238000000034 method Methods 0.000 description 21
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 17
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 14
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 12
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 12
- 239000011541 reaction mixture Substances 0.000 description 12
- QOSSAOTZNIDXMA-UHFFFAOYSA-N Dicylcohexylcarbodiimide Chemical compound C1CCCCC1N=C=NC1CCCCC1 QOSSAOTZNIDXMA-UHFFFAOYSA-N 0.000 description 11
- 230000015572 biosynthetic process Effects 0.000 description 10
- 239000002244 precipitate Substances 0.000 description 10
- 238000002360 preparation method Methods 0.000 description 10
- 239000002904 solvent Substances 0.000 description 10
- 238000003786 synthesis reaction Methods 0.000 description 10
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 9
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 9
- 235000013922 glutamic acid Nutrition 0.000 description 9
- 108010016626 Dipeptides Proteins 0.000 description 8
- 230000021615 conjugation Effects 0.000 description 7
- 229910001873 dinitrogen Inorganic materials 0.000 description 7
- 125000000524 functional group Chemical group 0.000 description 7
- WGCNASOHLSPBMP-UHFFFAOYSA-N hydroxyacetaldehyde Natural products OCC=O WGCNASOHLSPBMP-UHFFFAOYSA-N 0.000 description 7
- 108090000765 processed proteins & peptides Proteins 0.000 description 7
- 238000002390 rotary evaporation Methods 0.000 description 7
- 239000007787 solid Substances 0.000 description 7
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 6
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 description 6
- 229920000642 polymer Polymers 0.000 description 6
- RYHBNJHYFVUHQT-UHFFFAOYSA-N 1,4-Dioxane Chemical compound C1COCCO1 RYHBNJHYFVUHQT-UHFFFAOYSA-N 0.000 description 5
- 125000003277 amino group Chemical group 0.000 description 5
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 5
- 150000001875 compounds Chemical class 0.000 description 5
- 238000001727 in vivo Methods 0.000 description 5
- 239000012452 mother liquor Substances 0.000 description 5
- 125000000217 alkyl group Chemical group 0.000 description 4
- 235000001014 amino acid Nutrition 0.000 description 4
- 230000008901 benefit Effects 0.000 description 4
- 238000006243 chemical reaction Methods 0.000 description 4
- 238000004255 ion exchange chromatography Methods 0.000 description 4
- 229920002643 polyglutamic acid Polymers 0.000 description 4
- 102000004196 processed proteins & peptides Human genes 0.000 description 4
- 235000018102 proteins Nutrition 0.000 description 4
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 3
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 3
- 239000004698 Polyethylene Substances 0.000 description 3
- 108010020346 Polyglutamic Acid Proteins 0.000 description 3
- PMZURENOXWZQFD-UHFFFAOYSA-L Sodium Sulfate Chemical compound [Na+].[Na+].[O-]S([O-])(=O)=O PMZURENOXWZQFD-UHFFFAOYSA-L 0.000 description 3
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 3
- 125000004432 carbon atom Chemical group C* 0.000 description 3
- 239000000969 carrier Substances 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 125000000753 cycloalkyl group Chemical group 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- 238000001914 filtration Methods 0.000 description 3
- 229930195712 glutamate Natural products 0.000 description 3
- 230000005847 immunogenicity Effects 0.000 description 3
- 229920000573 polyethylene Polymers 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- 239000000651 prodrug Substances 0.000 description 3
- 229940002612 prodrug Drugs 0.000 description 3
- UMJSCPRVCHMLSP-UHFFFAOYSA-N pyridine Natural products COC1=CC=CN=C1 UMJSCPRVCHMLSP-UHFFFAOYSA-N 0.000 description 3
- 230000002194 synthesizing effect Effects 0.000 description 3
- 231100000419 toxicity Toxicity 0.000 description 3
- 230000001988 toxicity Effects 0.000 description 3
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 3
- ZJIFDEVVTPEXDL-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) hydrogen carbonate Chemical compound OC(=O)ON1C(=O)CCC1=O ZJIFDEVVTPEXDL-UHFFFAOYSA-N 0.000 description 2
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 2
- KOSRFJWDECSPRO-WDSKDSINSA-N Glu-Glu Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(O)=O KOSRFJWDECSPRO-WDSKDSINSA-N 0.000 description 2
- OAKJQQAXSVQMHS-UHFFFAOYSA-N Hydrazine Chemical compound NN OAKJQQAXSVQMHS-UHFFFAOYSA-N 0.000 description 2
- 102000006992 Interferon-alpha Human genes 0.000 description 2
- 108010047761 Interferon-alpha Proteins 0.000 description 2
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- KOSRFJWDECSPRO-UHFFFAOYSA-N alpha-L-glutamyl-L-glutamic acid Natural products OC(=O)CCC(N)C(=O)NC(CCC(O)=O)C(O)=O KOSRFJWDECSPRO-UHFFFAOYSA-N 0.000 description 2
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 2
- 238000006065 biodegradation reaction Methods 0.000 description 2
- 230000004071 biological effect Effects 0.000 description 2
- PFYXSUNOLOJMDX-UHFFFAOYSA-N bis(2,5-dioxopyrrolidin-1-yl) carbonate Chemical compound O=C1CCC(=O)N1OC(=O)ON1C(=O)CCC1=O PFYXSUNOLOJMDX-UHFFFAOYSA-N 0.000 description 2
- 230000021523 carboxylation Effects 0.000 description 2
- 238000006473 carboxylation reaction Methods 0.000 description 2
- 125000000113 cyclohexyl group Chemical group [H]C1([H])C([H])([H])C([H])([H])C([H])(*)C([H])([H])C1([H])[H] 0.000 description 2
- 239000002552 dosage form Substances 0.000 description 2
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 2
- 108010055341 glutamyl-glutamic acid Proteins 0.000 description 2
- 229930182470 glycoside Natural products 0.000 description 2
- 150000002338 glycosides Chemical class 0.000 description 2
- 239000004615 ingredient Substances 0.000 description 2
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 2
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 2
- 238000011068 loading method Methods 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 2
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 2
- 125000001280 n-hexyl group Chemical group C(CCCCC)* 0.000 description 2
- 125000004123 n-propyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])* 0.000 description 2
- 125000003835 nucleoside group Chemical group 0.000 description 2
- 239000012044 organic layer Substances 0.000 description 2
- 238000006116 polymerization reaction Methods 0.000 description 2
- 229920001184 polypeptide Polymers 0.000 description 2
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 2
- 230000009257 reactivity Effects 0.000 description 2
- 150000003384 small molecules Chemical class 0.000 description 2
- 239000007974 sodium acetate buffer Substances 0.000 description 2
- 238000013268 sustained release Methods 0.000 description 2
- 239000012730 sustained-release form Substances 0.000 description 2
- 230000001225 therapeutic effect Effects 0.000 description 2
- FYSNRJHAOHDILO-UHFFFAOYSA-N thionyl chloride Chemical compound ClS(Cl)=O FYSNRJHAOHDILO-UHFFFAOYSA-N 0.000 description 2
- SPFMQWBKVUQXJV-BTVCFUMJSA-N (2r,3s,4r,5r)-2,3,4,5,6-pentahydroxyhexanal;hydrate Chemical compound O.OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C=O SPFMQWBKVUQXJV-BTVCFUMJSA-N 0.000 description 1
- ICLYJLBTOGPLMC-KVVVOXFISA-N (z)-octadec-9-enoate;tris(2-hydroxyethyl)azanium Chemical compound OCCN(CCO)CCO.CCCCCCCC\C=C/CCCCCCCC(O)=O ICLYJLBTOGPLMC-KVVVOXFISA-N 0.000 description 1
- LDXJRKWFNNFDSA-UHFFFAOYSA-N 2-(2,4,6,7-tetrahydrotriazolo[4,5-c]pyridin-5-yl)-1-[4-[2-[[3-(trifluoromethoxy)phenyl]methylamino]pyrimidin-5-yl]piperazin-1-yl]ethanone Chemical compound C1CN(CC2=NNN=C21)CC(=O)N3CCN(CC3)C4=CN=C(N=C4)NCC5=CC(=CC=C5)OC(F)(F)F LDXJRKWFNNFDSA-UHFFFAOYSA-N 0.000 description 1
- XZIIFPSPUDAGJM-UHFFFAOYSA-N 6-chloro-2-n,2-n-diethylpyrimidine-2,4-diamine Chemical compound CCN(CC)C1=NC(N)=CC(Cl)=N1 XZIIFPSPUDAGJM-UHFFFAOYSA-N 0.000 description 1
- 239000004322 Butylated hydroxytoluene Substances 0.000 description 1
- NLZUEZXRPGMBCV-UHFFFAOYSA-N Butylhydroxytoluene Chemical compound CC1=CC(C(C)(C)C)=C(O)C(C(C)(C)C)=C1 NLZUEZXRPGMBCV-UHFFFAOYSA-N 0.000 description 1
- SCULJPGYOQQXTK-OLRINKBESA-N Cinobufagin Chemical compound C=1([C@@H]2[C@@]3(C)CC[C@@H]4[C@@]5(C)CC[C@H](O)C[C@H]5CC[C@H]4[C@@]43O[C@@H]4[C@@H]2OC(=O)C)C=CC(=O)OC=1 SCULJPGYOQQXTK-OLRINKBESA-N 0.000 description 1
- SCULJPGYOQQXTK-UHFFFAOYSA-N Cinobufagin Natural products CC(=O)OC1C2OC22C3CCC4CC(O)CCC4(C)C3CCC2(C)C1C=1C=CC(=O)OC=1 SCULJPGYOQQXTK-UHFFFAOYSA-N 0.000 description 1
- BWGNESOTFCXPMA-UHFFFAOYSA-N Dihydrogen disulfide Chemical compound SS BWGNESOTFCXPMA-UHFFFAOYSA-N 0.000 description 1
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- PEEHTFAAVSWFBL-UHFFFAOYSA-N Maleimide Chemical compound O=C1NC(=O)C=C1 PEEHTFAAVSWFBL-UHFFFAOYSA-N 0.000 description 1
- 206010028980 Neoplasm Diseases 0.000 description 1
- 206010057249 Phagocytosis Diseases 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 150000008065 acid anhydrides Chemical class 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 150000001263 acyl chlorides Chemical class 0.000 description 1
- 239000002671 adjuvant Substances 0.000 description 1
- 238000005576 amination reaction Methods 0.000 description 1
- 150000004056 anthraquinones Chemical class 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 230000003078 antioxidant effect Effects 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 238000009835 boiling Methods 0.000 description 1
- 229940095259 butylated hydroxytoluene Drugs 0.000 description 1
- 235000010354 butylated hydroxytoluene Nutrition 0.000 description 1
- 125000002843 carboxylic acid group Chemical group 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 229960004106 citric acid Drugs 0.000 description 1
- 230000001268 conjugating effect Effects 0.000 description 1
- 239000012059 conventional drug carrier Substances 0.000 description 1
- 238000004132 cross linking Methods 0.000 description 1
- 125000001995 cyclobutyl group Chemical group [H]C1([H])C([H])([H])C([H])(*)C1([H])[H] 0.000 description 1
- 125000001559 cyclopropyl group Chemical group [H]C1([H])C([H])([H])C1([H])* 0.000 description 1
- 235000018417 cysteine Nutrition 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 201000010099 disease Diseases 0.000 description 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 1
- 238000004821 distillation Methods 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 125000004185 ester group Chemical group 0.000 description 1
- 238000005886 esterification reaction Methods 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 238000007429 general method Methods 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- 150000002334 glycols Chemical class 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 239000008297 liquid dosage form Substances 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 239000000693 micelle Substances 0.000 description 1
- 239000004005 microsphere Substances 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 239000003607 modifier Substances 0.000 description 1
- 150000002772 monosaccharides Chemical class 0.000 description 1
- 125000000740 n-pentyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 231100000956 nontoxicity Toxicity 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 239000012074 organic phase Substances 0.000 description 1
- 239000006174 pH buffer Substances 0.000 description 1
- 230000006320 pegylation Effects 0.000 description 1
- 230000008782 phagocytosis Effects 0.000 description 1
- 125000000286 phenylethyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])([H])* 0.000 description 1
- 239000008055 phosphate buffer solution Substances 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 150000004053 quinones Chemical class 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 239000012265 solid product Substances 0.000 description 1
- 229940035044 sorbitan monolaurate Drugs 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 229940124597 therapeutic agent Drugs 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- 229940117013 triethanolamine oleate Drugs 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/56—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule
- A61K47/59—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyureas or polyurethanes
- A61K47/60—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyureas or polyurethanes the organic macromolecular compound being a polyoxyalkylene oligomer, polymer or dendrimer, e.g. PEG, PPG, PEO or polyglycerol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/62—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being a protein, peptide or polyamino acid
- A61K47/65—Peptidic linkers, binders or spacers, e.g. peptidic enzyme-labile linkers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Epidemiology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Preparation (AREA)
- Peptides Or Proteins (AREA)
- Polyamides (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Other Resins Obtained By Reactions Not Involving Carbon-To-Carbon Unsaturated Bonds (AREA)
- Polyesters Or Polycarbonates (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CNB021066914A CN100475269C (zh) | 2002-03-05 | 2002-03-05 | 亲水性聚合物-谷氨酸寡肽与药物分子的结合物、包含该结合物的组合物及用途 |
| PCT/CN2003/000164 WO2003074586A1 (en) | 2002-03-05 | 2003-03-05 | Compound of hydrophilic polymer-polycarboxyl oligopeptide and medicines, medical composite comprising above compound and use of above compound in medicimes |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2005519122A JP2005519122A (ja) | 2005-06-30 |
| JP2005519122A5 JP2005519122A5 (enExample) | 2005-12-22 |
| JP4365221B2 true JP4365221B2 (ja) | 2009-11-18 |
Family
ID=27768504
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2003573050A Expired - Lifetime JP4365221B2 (ja) | 2002-03-05 | 2003-03-05 | 親水性ポリマー−マルチカルボキシルオリゴペプチドと薬剤分子との結合生成物、医薬組成物およびその薬学上の使用 |
Country Status (9)
| Country | Link |
|---|---|
| US (1) | US7498035B2 (enExample) |
| EP (1) | EP1489125B1 (enExample) |
| JP (1) | JP4365221B2 (enExample) |
| CN (2) | CN100475269C (enExample) |
| AT (1) | ATE456379T1 (enExample) |
| AU (1) | AU2003221208A1 (enExample) |
| DE (1) | DE60331135D1 (enExample) |
| ES (1) | ES2340374T3 (enExample) |
| WO (1) | WO2003074586A1 (enExample) |
Families Citing this family (28)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2003076490A1 (en) * | 2002-03-13 | 2003-09-18 | Beijing Jiankai Technology Co., Ltd. | Hydrophilic polymer derivate with y type branch and preparation method of it medical composite comprising above compound |
| CN1613868A (zh) * | 2003-11-07 | 2005-05-11 | 北京键凯科技有限公司 | 亲水性聚合物-黄杨木提取物的结合物及其药物组合物 |
| CN100374443C (zh) * | 2004-03-29 | 2008-03-12 | 北京键凯科技有限公司 | 亲水性聚合物-雷公藤提取物的结合物及其药物组合物 |
| CN100475837C (zh) * | 2004-06-11 | 2009-04-08 | 北京键凯科技有限公司 | 多叉分支的聚乙二醇-氨基酸寡肽及其活性衍生物和药物结合物 |
| JP4820758B2 (ja) * | 2004-09-22 | 2011-11-24 | 日本化薬株式会社 | 新規ブロック共重合体、ミセル調製物及びそれを有効成分とする抗癌剤 |
| KR20080106254A (ko) | 2006-03-28 | 2008-12-04 | 니폰 가야꾸 가부시끼가이샤 | 탁산류의 고분자 결합체 |
| RU2447095C2 (ru) | 2006-05-18 | 2012-04-10 | Ниппон Каяку Кабусики Кайся | Высокомолекулярный конъюгат подофиллотоксинов |
| WO2008034124A2 (en) * | 2006-09-15 | 2008-03-20 | Enzon Pharmaceuticals, Inc. | Targeted polymeric prodrugs containing multifunctional linkers |
| US20090239782A1 (en) * | 2006-10-03 | 2009-09-24 | Masaharu Nakamura | High-molecular weight conjugate of resorcinol derivatives |
| CN101168594B (zh) * | 2006-10-24 | 2011-07-27 | 北京键凯科技有限公司 | 寡肽为骨架的聚乙二醇活性衍生物、其制备方法及与药物分子的结合物 |
| US8334364B2 (en) | 2006-11-06 | 2012-12-18 | Nipon Kayaku Kabushiki Kaisha | High-molecular weight derivative of nucleic acid antimetabolite |
| US8188222B2 (en) | 2006-11-08 | 2012-05-29 | Nippon Kayaku Kabushiki Kaisha | High molecular weight derivative of nucleic acid antimetabolite |
| WO2009041570A1 (ja) * | 2007-09-28 | 2009-04-02 | Nippon Kayaku Kabushiki Kaisha | ステロイド類の高分子結合体 |
| CN101977631A (zh) | 2008-03-18 | 2011-02-16 | 日本化药株式会社 | 生理活性物质的高分子量偶联物 |
| JP5366940B2 (ja) | 2008-05-08 | 2013-12-11 | 日本化薬株式会社 | 葉酸若しくは葉酸誘導体の高分子結合体 |
| JP2011162569A (ja) * | 2008-05-23 | 2011-08-25 | Nano Career Kk | カンプトテシン高分子誘導体及びその用途 |
| ITRM20080551A1 (it) * | 2008-10-15 | 2010-04-16 | Univ Catania | Derivati anfifilici del poliossietilenglicole (peg), procedimento di preparazione e loro usi nella preparazione di sistemi farmaceutici. |
| WO2010131675A1 (ja) * | 2009-05-15 | 2010-11-18 | 日本化薬株式会社 | 水酸基を有する生理活性物質の高分子結合体 |
| JP2013234207A (ja) * | 2010-08-31 | 2013-11-21 | Jsr Corp | 新規重合体及び新規n−カルボキシアミノ酸無水物、ならびにそれらの製造方法 |
| US9018323B2 (en) | 2010-11-17 | 2015-04-28 | Nippon Kayaku Kabushiki Kaisha | Polymer derivative of cytidine metabolic antagonist |
| RU2623426C2 (ru) | 2011-09-11 | 2017-06-26 | Ниппон Каяку Кабусики Кайся | Способ получения блок-сополимера |
| CN103083680B (zh) * | 2011-11-07 | 2014-12-24 | 北京键凯科技有限公司 | 聚乙二醇-氨基酸寡肽-依诺替康药物结合物及其药物组合物 |
| US9919059B2 (en) * | 2012-12-10 | 2018-03-20 | Massachusetts Institute Of Technology | Multistage nanoparticle drug delivery system for the treatment of solid tumors |
| WO2014114262A1 (zh) * | 2013-01-28 | 2014-07-31 | 天津键凯科技有限公司 | 水溶性聚合物-氨基酸寡肽-药物结合物及其制备方法和用途 |
| US9700633B2 (en) | 2013-01-28 | 2017-07-11 | Jenkem Technology Co., Ltd., Tianjin Branch | Conjugates of water soluble polymer-amino acid oligopeptide-drug, preparation method and use thereof |
| WO2015081821A1 (zh) * | 2013-12-02 | 2015-06-11 | 北京键凯科技有限公司 | 聚乙二醇-多爪寡肽键合的雷帕霉素衍生物 |
| CN107033305B (zh) * | 2017-05-20 | 2019-03-01 | 西南大学 | 一种还原性响应的两亲性蠕虫状单分子前药的制备方法 |
| CN114948850B (zh) * | 2022-05-26 | 2024-02-27 | 中国药科大学 | 可载药寡肽及其可溶性分层微针的制备方法和应用 |
Family Cites Families (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS6096605A (ja) * | 1983-10-31 | 1985-05-30 | Mitsubishi Chem Ind Ltd | 低分子量の水溶性物質の分離方法 |
| DE3730797A1 (de) * | 1987-09-14 | 1989-03-23 | Siegel Rolf | Materialien mit nicht-thrombogener oberflaeche |
| EP0835931A4 (en) * | 1995-06-26 | 2000-11-15 | Daiichi Pure Chemicals Co Ltd | STABLE PLASMINE SOLUTION |
| US5672662A (en) * | 1995-07-07 | 1997-09-30 | Shearwater Polymers, Inc. | Poly(ethylene glycol) and related polymers monosubstituted with propionic or butanoic acids and functional derivatives thereof for biotechnical applications |
| CZ297979B6 (cs) * | 1996-03-12 | 2007-05-16 | Pg-Txl Company, L. P. | Kompozice obsahující protinádorové lécivo konjugované s ve vode rozpustným polymerem, její pouzití pro výrobu léciva a implantovatelná lékarská pomucka |
| CN1110681C (zh) * | 1996-04-10 | 2003-06-04 | 丰田自动车株式会社 | 有色金属熔化保持炉与有色金属熔液保持炉 |
| US6824766B2 (en) * | 1998-04-17 | 2004-11-30 | Enzon, Inc. | Biodegradable high molecular weight polymeric linkers and their conjugates |
| US7060708B2 (en) * | 1999-03-10 | 2006-06-13 | New River Pharmaceuticals Inc. | Active agent delivery systems and methods for protecting and administering active agents |
| JP2003528824A (ja) * | 2000-02-04 | 2003-09-30 | スプラテック ファーマ インコーポレイティド | 血管内皮成長因子受容体のためのリガンド |
| CN1376785A (zh) * | 2001-03-22 | 2002-10-30 | 上海博德基因开发有限公司 | 一种多肽——硫氨焦磷酸激酶-26.73和编码这种多肽的多核苷酸 |
| US6489153B1 (en) * | 2001-10-31 | 2002-12-03 | Pe Corporation (Ny) | Isolated human kinase proteins, nucleic acid molecules encoding human kinase proteins, and uses thereof |
| AU2003280592B2 (en) * | 2002-10-31 | 2008-12-18 | Nippon Kayaku Kabushiki Kaisha | High-molecular weight derivatives of camptothecins |
-
2002
- 2002-03-05 CN CNB021066914A patent/CN100475269C/zh not_active Expired - Lifetime
-
2003
- 2003-03-05 WO PCT/CN2003/000164 patent/WO2003074586A1/zh not_active Ceased
- 2003-03-05 AT AT03711787T patent/ATE456379T1/de not_active IP Right Cessation
- 2003-03-05 US US10/506,524 patent/US7498035B2/en not_active Expired - Lifetime
- 2003-03-05 EP EP03711787A patent/EP1489125B1/en not_active Expired - Lifetime
- 2003-03-05 AU AU2003221208A patent/AU2003221208A1/en not_active Abandoned
- 2003-03-05 ES ES03711787T patent/ES2340374T3/es not_active Expired - Lifetime
- 2003-03-05 JP JP2003573050A patent/JP4365221B2/ja not_active Expired - Lifetime
- 2003-03-05 DE DE60331135T patent/DE60331135D1/de not_active Expired - Lifetime
- 2003-12-31 CN CNB2003801109158A patent/CN100400657C/zh not_active Expired - Lifetime
Also Published As
| Publication number | Publication date |
|---|---|
| US7498035B2 (en) | 2009-03-03 |
| AU2003221208A1 (en) | 2003-09-16 |
| US20050147617A1 (en) | 2005-07-07 |
| EP1489125B1 (en) | 2010-01-27 |
| CN100475269C (zh) | 2009-04-08 |
| DE60331135D1 (de) | 2010-03-18 |
| CN1886500A (zh) | 2006-12-27 |
| CN100400657C (zh) | 2008-07-09 |
| EP1489125A4 (en) | 2006-09-13 |
| EP1489125A1 (en) | 2004-12-22 |
| WO2003074586A1 (en) | 2003-09-12 |
| ES2340374T3 (es) | 2010-06-02 |
| ATE456379T1 (de) | 2010-02-15 |
| CN1442440A (zh) | 2003-09-17 |
| JP2005519122A (ja) | 2005-06-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4365221B2 (ja) | 親水性ポリマー−マルチカルボキシルオリゴペプチドと薬剤分子との結合生成物、医薬組成物およびその薬学上の使用 | |
| JP4272537B2 (ja) | Y型分鎖親水性ポリマー誘導体、それらの調製方法、前記誘導体および薬剤分子の結合生成物、ならびに前記結合生成物を含む医薬組成物 | |
| WO2005121181A1 (en) | Multi-drop tree branching polyethylene glycol-oligopeptides and active derivates and pharmaceutical compounds there of | |
| CN103755949B (zh) | 多臂聚乙二醇衍生物及其与药物的结合物和凝胶 | |
| CA2208841C (en) | High molecular weight polymer-based prodrugs | |
| WO2010060260A1 (zh) | 新型的多臂聚乙二醇及其制备方法和应用 | |
| JP6790133B2 (ja) | マルチアームポリエチレングリコール及びその活性誘導体 | |
| US11180615B2 (en) | Cationic polyphosphazene compound, polyphosphazenes-drug conjugate compound and method for preparing same | |
| WO2010131675A1 (ja) | 水酸基を有する生理活性物質の高分子結合体 | |
| WO2013067767A1 (zh) | 聚乙二醇-氨基酸寡肽-依诺替康药物结合物及其药物组合物 | |
| WO2005092898A1 (en) | The conjugates of a hydrophilic polymer-tripterygium's extracts and the pharmaceutical compositions thereof | |
| US20050220753A1 (en) | Hydrophilic polymers-flavoids conjugates and pharmaceutical compositions comprising them | |
| CN101306203B (zh) | 亲水性聚合物-黄杨木提取物的结合物及其药物组合物 | |
| US7708978B2 (en) | Targeted hydrophilic polymer, binders with interferon and medical composite comprising above binders | |
| CN1259314C (zh) | 亲水性聚合物-黄酮结合物以及包含该结合物的药物组合物 | |
| CN110772643B (zh) | α-生育酚聚乙二醇琥珀酸酯修饰的强心苷类化合物前药 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20050401 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20050401 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20081127 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20090227 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20090409 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20090703 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20090728 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20090820 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20120828 Year of fee payment: 3 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 4365221 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20130828 Year of fee payment: 4 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| EXPY | Cancellation because of completion of term |