JP2014201740A - イミドオリゴマー及びこれを加熱硬化させてなるポリイミド樹脂 - Google Patents
イミドオリゴマー及びこれを加熱硬化させてなるポリイミド樹脂 Download PDFInfo
- Publication number
- JP2014201740A JP2014201740A JP2013090734A JP2013090734A JP2014201740A JP 2014201740 A JP2014201740 A JP 2014201740A JP 2013090734 A JP2013090734 A JP 2013090734A JP 2013090734 A JP2013090734 A JP 2013090734A JP 2014201740 A JP2014201740 A JP 2014201740A
- Authority
- JP
- Japan
- Prior art keywords
- imide oligomer
- imide
- oligomer
- apb
- polyimide resin
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 150000003949 imides Chemical class 0.000 title claims abstract description 89
- 229920001721 polyimide Polymers 0.000 title claims abstract description 43
- 239000009719 polyimide resin Substances 0.000 title claims abstract description 32
- ZBMISJGHVWNWTE-UHFFFAOYSA-N 3-(4-aminophenoxy)aniline Chemical compound C1=CC(N)=CC=C1OC1=CC=CC(N)=C1 ZBMISJGHVWNWTE-UHFFFAOYSA-N 0.000 claims abstract description 4
- XROLBZOMVNMIFN-UHFFFAOYSA-N 1-(1-benzofuran-4-yl)propan-2-amine Chemical compound CC(N)CC1=CC=CC2=C1C=CO2 XROLBZOMVNMIFN-UHFFFAOYSA-N 0.000 claims description 31
- 239000002253 acid Substances 0.000 claims description 17
- FYYYKXFEKMGYLZ-UHFFFAOYSA-N 4-(1,3-dioxo-2-benzofuran-5-yl)-2-benzofuran-1,3-dione Chemical compound C=1C=C2C(=O)OC(=O)C2=CC=1C1=CC=CC2=C1C(=O)OC2=O FYYYKXFEKMGYLZ-UHFFFAOYSA-N 0.000 claims description 16
- 238000010438 heat treatment Methods 0.000 claims description 16
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 claims description 12
- 238000006116 polymerization reaction Methods 0.000 claims description 10
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 claims description 9
- 238000013007 heat curing Methods 0.000 claims description 9
- 239000000126 substance Substances 0.000 claims description 9
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 claims description 7
- -1 4-aminophenoxy Chemical group 0.000 claims description 6
- WFDIJRYMOXRFFG-UHFFFAOYSA-N Acetic anhydride Chemical compound CC(=O)OC(C)=O WFDIJRYMOXRFFG-UHFFFAOYSA-N 0.000 claims description 6
- 239000002966 varnish Substances 0.000 claims description 6
- SECXISVLQFMRJM-UHFFFAOYSA-N N-Methylpyrrolidone Chemical compound CN1CCCC1=O SECXISVLQFMRJM-UHFFFAOYSA-N 0.000 claims description 5
- 239000003795 chemical substances by application Substances 0.000 claims description 5
- 229920001823 Aquamid® Polymers 0.000 claims description 2
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 claims description 2
- 239000002798 polar solvent Substances 0.000 claims description 2
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 claims 3
- AXMANIZPMQZKTG-UHFFFAOYSA-N 4-(2-phenylethynyl)-2-benzofuran-1,3-dione Chemical compound O=C1OC(=O)C2=C1C=CC=C2C#CC1=CC=CC=C1 AXMANIZPMQZKTG-UHFFFAOYSA-N 0.000 claims 2
- FXHOOIRPVKKKFG-UHFFFAOYSA-N N,N-Dimethylacetamide Chemical compound CN(C)C(C)=O FXHOOIRPVKKKFG-UHFFFAOYSA-N 0.000 claims 2
- 239000006227 byproduct Substances 0.000 claims 1
- 238000006297 dehydration reaction Methods 0.000 claims 1
- 238000006358 imidation reaction Methods 0.000 claims 1
- 230000001376 precipitating effect Effects 0.000 claims 1
- UPGRRPUXXWPEMV-UHFFFAOYSA-N 5-(2-phenylethynyl)-2-benzofuran-1,3-dione Chemical compound C=1C=C2C(=O)OC(=O)C2=CC=1C#CC1=CC=CC=C1 UPGRRPUXXWPEMV-UHFFFAOYSA-N 0.000 abstract description 20
- 238000000465 moulding Methods 0.000 abstract description 8
- DKKYOQYISDAQER-UHFFFAOYSA-N 3-[3-(3-aminophenoxy)phenoxy]aniline Chemical compound NC1=CC=CC(OC=2C=C(OC=3C=C(N)C=CC=3)C=CC=2)=C1 DKKYOQYISDAQER-UHFFFAOYSA-N 0.000 abstract description 3
- JCRRFJIVUPSNTA-UHFFFAOYSA-N 4-[4-(4-aminophenoxy)phenoxy]aniline Chemical compound C1=CC(N)=CC=C1OC(C=C1)=CC=C1OC1=CC=C(N)C=C1 JCRRFJIVUPSNTA-UHFFFAOYSA-N 0.000 abstract description 3
- NBAUUNCGSMAPFM-UHFFFAOYSA-N 3-(3,4-dicarboxyphenyl)phthalic acid Chemical compound C1=C(C(O)=O)C(C(=O)O)=CC=C1C1=CC=CC(C(O)=O)=C1C(O)=O NBAUUNCGSMAPFM-UHFFFAOYSA-N 0.000 abstract 1
- 150000004985 diamines Chemical class 0.000 description 12
- 239000004642 Polyimide Substances 0.000 description 10
- GTDPSWPPOUPBNX-UHFFFAOYSA-N ac1mqpva Chemical compound CC12C(=O)OC(=O)C1(C)C1(C)C2(C)C(=O)OC1=O GTDPSWPPOUPBNX-UHFFFAOYSA-N 0.000 description 10
- 150000001875 compounds Chemical class 0.000 description 10
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 8
- 238000002844 melting Methods 0.000 description 7
- 230000008018 melting Effects 0.000 description 7
- 229920001187 thermosetting polymer Polymers 0.000 description 7
- 239000000463 material Substances 0.000 description 6
- 150000004984 aromatic diamines Chemical class 0.000 description 5
- 238000001723 curing Methods 0.000 description 5
- 125000006159 dianhydride group Chemical group 0.000 description 5
- 239000000203 mixture Substances 0.000 description 5
- 239000011347 resin Substances 0.000 description 5
- 229920005989 resin Polymers 0.000 description 5
- QQGYZOYWNCKGEK-UHFFFAOYSA-N 5-[(1,3-dioxo-2-benzofuran-5-yl)oxy]-2-benzofuran-1,3-dione Chemical group C1=C2C(=O)OC(=O)C2=CC(OC=2C=C3C(=O)OC(C3=CC=2)=O)=C1 QQGYZOYWNCKGEK-UHFFFAOYSA-N 0.000 description 4
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 4
- 239000011521 glass Substances 0.000 description 4
- 238000004519 manufacturing process Methods 0.000 description 4
- 229910052757 nitrogen Inorganic materials 0.000 description 4
- 229910052760 oxygen Inorganic materials 0.000 description 4
- 239000001301 oxygen Substances 0.000 description 4
- 230000000704 physical effect Effects 0.000 description 4
- 239000000843 powder Substances 0.000 description 4
- 239000000243 solution Substances 0.000 description 4
- 238000005979 thermal decomposition reaction Methods 0.000 description 4
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- 229920000049 Carbon (fiber) Polymers 0.000 description 3
- 239000004917 carbon fiber Substances 0.000 description 3
- 238000006243 chemical reaction Methods 0.000 description 3
- 238000010586 diagram Methods 0.000 description 3
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Chemical compound C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 description 3
- 238000000034 method Methods 0.000 description 3
- 239000003960 organic solvent Substances 0.000 description 3
- 239000012779 reinforcing material Substances 0.000 description 3
- 229910010271 silicon carbide Inorganic materials 0.000 description 3
- 238000003856 thermoforming Methods 0.000 description 3
- HLBLWEWZXPIGSM-UHFFFAOYSA-N 4-Aminophenyl ether Chemical compound C1=CC(N)=CC=C1OC1=CC=C(N)C=C1 HLBLWEWZXPIGSM-UHFFFAOYSA-N 0.000 description 2
- BEKFRNOZJSYWKZ-UHFFFAOYSA-N 4-[2-(4-aminophenyl)-1,1,1,3,3,3-hexafluoropropan-2-yl]aniline Chemical compound C1=CC(N)=CC=C1C(C(F)(F)F)(C(F)(F)F)C1=CC=C(N)C=C1 BEKFRNOZJSYWKZ-UHFFFAOYSA-N 0.000 description 2
- HESXPOICBNWMPI-UHFFFAOYSA-N 4-[2-[4-[2-(4-aminophenyl)propan-2-yl]phenyl]propan-2-yl]aniline Chemical compound C=1C=C(C(C)(C)C=2C=CC(N)=CC=2)C=CC=1C(C)(C)C1=CC=C(N)C=C1 HESXPOICBNWMPI-UHFFFAOYSA-N 0.000 description 2
- HHLMWQDRYZAENA-UHFFFAOYSA-N 4-[4-[2-[4-(4-aminophenoxy)phenyl]-1,1,1,3,3,3-hexafluoropropan-2-yl]phenoxy]aniline Chemical compound C1=CC(N)=CC=C1OC1=CC=C(C(C=2C=CC(OC=3C=CC(N)=CC=3)=CC=2)(C(F)(F)F)C(F)(F)F)C=C1 HHLMWQDRYZAENA-UHFFFAOYSA-N 0.000 description 2
- 0 C[C@@]12C=*CC1C2 Chemical compound C[C@@]12C=*CC1C2 0.000 description 2
- MQJKPEGWNLWLTK-UHFFFAOYSA-N Dapsone Chemical compound C1=CC(N)=CC=C1S(=O)(=O)C1=CC=C(N)C=C1 MQJKPEGWNLWLTK-UHFFFAOYSA-N 0.000 description 2
- 229910018540 Si C Inorganic materials 0.000 description 2
- 125000003277 amino group Chemical group 0.000 description 2
- 230000000052 comparative effect Effects 0.000 description 2
- 238000000748 compression moulding Methods 0.000 description 2
- 238000006482 condensation reaction Methods 0.000 description 2
- 238000004132 cross linking Methods 0.000 description 2
- 125000004427 diamine group Chemical group 0.000 description 2
- ZUOUZKKEUPVFJK-UHFFFAOYSA-N diphenyl Chemical compound C1=CC=CC=C1C1=CC=CC=C1 ZUOUZKKEUPVFJK-UHFFFAOYSA-N 0.000 description 2
- 238000001035 drying Methods 0.000 description 2
- 230000020169 heat generation Effects 0.000 description 2
- 230000009878 intermolecular interaction Effects 0.000 description 2
- 239000011159 matrix material Substances 0.000 description 2
- 238000012827 research and development Methods 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 238000003756 stirring Methods 0.000 description 2
- DDCJHFYXAPQYLA-UHFFFAOYSA-N (3-chlorophenyl)-phenylmethanol Chemical compound C=1C=CC(Cl)=CC=1C(O)C1=CC=CC=C1 DDCJHFYXAPQYLA-UHFFFAOYSA-N 0.000 description 1
- GTACSIONMHMRPD-UHFFFAOYSA-N 2-[4-[2-(benzenesulfonamido)ethylsulfanyl]-2,6-difluorophenoxy]acetamide Chemical compound C1=C(F)C(OCC(=O)N)=C(F)C=C1SCCNS(=O)(=O)C1=CC=CC=C1 GTACSIONMHMRPD-UHFFFAOYSA-N 0.000 description 1
- RYYUUQPLFHRZOY-UHFFFAOYSA-N 4-[2-(4-aminophenoxy)phenoxy]aniline Chemical compound C1=CC(N)=CC=C1OC1=CC=CC=C1OC1=CC=C(N)C=C1 RYYUUQPLFHRZOY-UHFFFAOYSA-N 0.000 description 1
- WUPRYUDHUFLKFL-UHFFFAOYSA-N 4-[3-(4-aminophenoxy)phenoxy]aniline Chemical compound C1=CC(N)=CC=C1OC1=CC=CC(OC=2C=CC(N)=CC=2)=C1 WUPRYUDHUFLKFL-UHFFFAOYSA-N 0.000 description 1
- DJQPGZPKGHRJOK-UHFFFAOYSA-N 4-[4-[1-[4-(4-aminophenoxy)phenyl]cyclohexyl]phenoxy]aniline Chemical compound C1=CC(N)=CC=C1OC1=CC=C(C2(CCCCC2)C=2C=CC(OC=3C=CC(N)=CC=3)=CC=2)C=C1 DJQPGZPKGHRJOK-UHFFFAOYSA-N 0.000 description 1
- 101710130081 Aspergillopepsin-1 Proteins 0.000 description 1
- 102100031007 Cytosolic non-specific dipeptidase Human genes 0.000 description 1
- 235000015842 Hesperis Nutrition 0.000 description 1
- 235000012633 Iberis amara Nutrition 0.000 description 1
- 125000004018 acid anhydride group Chemical group 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 150000008064 anhydrides Chemical class 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 239000012298 atmosphere Substances 0.000 description 1
- 239000004305 biphenyl Substances 0.000 description 1
- 235000010290 biphenyl Nutrition 0.000 description 1
- 238000009835 boiling Methods 0.000 description 1
- WKDNYTOXBCRNPV-UHFFFAOYSA-N bpda Chemical compound C1=C2C(=O)OC(=O)C2=CC(C=2C=C3C(=O)OC(C3=CC=2)=O)=C1 WKDNYTOXBCRNPV-UHFFFAOYSA-N 0.000 description 1
- 150000001244 carboxylic acid anhydrides Chemical group 0.000 description 1
- 239000007810 chemical reaction solvent Substances 0.000 description 1
- 238000004040 coloring Methods 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 238000007906 compression Methods 0.000 description 1
- 238000009833 condensation Methods 0.000 description 1
- 230000005494 condensation Effects 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- 238000005336 cracking Methods 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 239000012776 electronic material Substances 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 230000001747 exhibiting effect Effects 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- 238000005470 impregnation Methods 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 238000013035 low temperature curing Methods 0.000 description 1
- 229920002521 macromolecule Polymers 0.000 description 1
- 239000012299 nitrogen atmosphere Substances 0.000 description 1
- 238000011017 operating method Methods 0.000 description 1
- CGEXUOTXYSGBLV-UHFFFAOYSA-N phenyl benzenesulfonate Chemical compound C=1C=CC=CC=1S(=O)(=O)OC1=CC=CC=C1 CGEXUOTXYSGBLV-UHFFFAOYSA-N 0.000 description 1
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 1
- 229920003223 poly(pyromellitimide-1,4-diphenyl ether) Polymers 0.000 description 1
- 238000006068 polycondensation reaction Methods 0.000 description 1
- 229920006254 polymer film Polymers 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 238000010298 pulverizing process Methods 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 238000009864 tensile test Methods 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 238000001721 transfer moulding Methods 0.000 description 1
- 235000012431 wafers Nutrition 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
Images
Landscapes
- Macromolecular Compounds Obtained By Forming Nitrogen-Containing Linkages In General (AREA)
Abstract
【解決手段】2,3,3’、4’−ビフェニルテトラカルボン酸二無水化物、1,4−ビス(4−アミノフェノキシ)ベンゼン、3,4‘−ジアミノジフェニルエーテル、1,3−ビス(3−アミノフェノキシ)ベンゼンからなり、両末端が4−フェニルエチニルフタル酸無水物由来の残基であることを特徴とするイミドオリゴマー。
【選択図】なし
Description
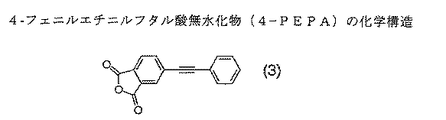
本発明において架橋性末端化合物として用いられる4−PEPAは、下記一般式(3)により表される化合物である。
その後、生成した酢酸とトリエチルアミン等を真空下留去して得たワニスを洗浄乾燥させたガラス板にカプトンテープを用い、成形型とし、ガラス棒を用い、均一にガラス板に塗布し、190℃で1時間乾燥させ、380℃で2時間加熱処理をした。冷却後、ナイフで切れ目を入れ、水の入ったビーカーにガラス板ごと付けた。剥離したフィルムを室温で一昼夜乾燥させ、幅3mm、長さ50mmに切り取り、膜厚測定後、力学試験を行った。
実施例1で重合したオリゴマー溶液をメタノール700mlに反応液を投入し、濾過後メタノールで洗浄濾過を数回繰り返し、80℃で一晩乾燥させ、黄色の粉末状イミドオリゴマーを得た。
表1に示したイミドナンバー1、3、4、5が比較例である。
Claims (7)
- 請求項1に記載のイミドオリゴマーにおいて、イミドオリゴマー全体の平均分子量が8,000以下であることを特徴とするイミドオリゴマー。
- 請求項3に記載のアミド酸オリゴマーにおいて、N−メチル−2−ピロリドン(NMP)、N,N−ジメチルアセトアミド(DMAc)、γ−ブチロタクトン、アクアミド(TM)等の極性溶媒存在下、加熱脱水反応によりイミド化し得られるイミドオリゴマー。
- 請求項3に記載のアミド酸オリゴマーで、60℃以下の低温でトリエチルアミン/無水酢酸等化学イミド化剤を用い、イミド化後、残存化学イミド化剤並びにその副生成物を減圧下留去して得られるイミドオリゴマーとそのワニス。
- 請求項1、2、4,5で得られたイミドオリゴマーを、メタノール等のアルコールを用いて沈殿させ得られる粉体イミドオリゴマー。
- 請求項1から6に記載オリゴマーを加熱硬化させてなることを特徴とするポリイミド樹脂。
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2013090734A JP2014201740A (ja) | 2013-04-05 | 2013-04-05 | イミドオリゴマー及びこれを加熱硬化させてなるポリイミド樹脂 |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2013090734A JP2014201740A (ja) | 2013-04-05 | 2013-04-05 | イミドオリゴマー及びこれを加熱硬化させてなるポリイミド樹脂 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2014201740A true JP2014201740A (ja) | 2014-10-27 |
| JP2014201740A5 JP2014201740A5 (ja) | 2016-08-18 |
Family
ID=52352476
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2013090734A Pending JP2014201740A (ja) | 2013-04-05 | 2013-04-05 | イミドオリゴマー及びこれを加熱硬化させてなるポリイミド樹脂 |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP2014201740A (ja) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN109162101A (zh) * | 2018-07-11 | 2019-01-08 | 中国航发北京航空材料研究院 | 一种低粘度高耐热聚酰亚胺纤维定型剂及其制备方法 |
| JP2021084929A (ja) * | 2019-11-26 | 2021-06-03 | 株式会社カネカ | ポリイミド樹脂、ポリイミド溶液、ポリイミドフィルム |
| CN114276544A (zh) * | 2022-02-18 | 2022-04-05 | 北京航空航天大学 | 一种耐高温的聚酰亚胺复合材料及其制备方法与应用 |
| JP7679005B2 (ja) | 2020-12-28 | 2025-05-19 | 株式会社カネカ | 特定の組成を有するアミド酸オリゴマー、ワニス、硬化物、複合材料 |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2012197403A (ja) * | 2011-03-19 | 2012-10-18 | Pi R & D Co Ltd | イミドオリゴマー及びこれを加熱硬化させてなるポリイミド樹脂 |
-
2013
- 2013-04-05 JP JP2013090734A patent/JP2014201740A/ja active Pending
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2012197403A (ja) * | 2011-03-19 | 2012-10-18 | Pi R & D Co Ltd | イミドオリゴマー及びこれを加熱硬化させてなるポリイミド樹脂 |
Non-Patent Citations (1)
| Title |
|---|
| SMITH,J.G.,JR.;CONNELL,J.W.;HERGENROTHER,P.M.;YOKOTA,R.;CRISS,J.M.: "High temperature transfer molding resins based on 2,3,3',4'-biphenyltetracarboxylic dianhydride", PLATFORM TO GLOVAL VALUE AND PERFORMANCE,BOOK 2, JPN6017001238, 2002, pages 316-327 * |
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN109162101A (zh) * | 2018-07-11 | 2019-01-08 | 中国航发北京航空材料研究院 | 一种低粘度高耐热聚酰亚胺纤维定型剂及其制备方法 |
| CN109162101B (zh) * | 2018-07-11 | 2021-03-26 | 中国航发北京航空材料研究院 | 一种低粘度高耐热聚酰亚胺纤维定型剂及其制备方法 |
| JP2021084929A (ja) * | 2019-11-26 | 2021-06-03 | 株式会社カネカ | ポリイミド樹脂、ポリイミド溶液、ポリイミドフィルム |
| JP7359662B2 (ja) | 2019-11-26 | 2023-10-11 | 株式会社カネカ | ポリイミド樹脂、ポリイミド溶液、ポリイミドフィルム |
| JP7679005B2 (ja) | 2020-12-28 | 2025-05-19 | 株式会社カネカ | 特定の組成を有するアミド酸オリゴマー、ワニス、硬化物、複合材料 |
| CN114276544A (zh) * | 2022-02-18 | 2022-04-05 | 北京航空航天大学 | 一种耐高温的聚酰亚胺复合材料及其制备方法与应用 |
| CN114276544B (zh) * | 2022-02-18 | 2022-12-06 | 北京航空航天大学 | 一种耐高温的聚酰亚胺复合材料及其制备方法与应用 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4133561B2 (ja) | ポリアミック酸オリゴマー、ポリイミドオリゴマー、溶液組成物、および繊維強化複合材料 | |
| US8846552B2 (en) | Soluble terminally modified imide oligomer using 2-phenyl-4, 4′-diaminodiphenyl ether, varnish, cured product thereof, imide prepreg thereof, and fiber-reinforced laminate having excellent heat resistance | |
| TWI475046B (zh) | 醯亞胺低聚合物及將該醯亞胺低聚合物加熱硬化而成之聚醯亞胺樹脂 | |
| US10017666B2 (en) | Polyimide resin composition and varnish produced from terminal-modified imide oligomer prepared using 2-phenyl-4,4′-diaminodiphenyl ether and thermoplastic aromatic polyimide prepared using oxydiphthalic acid, polyimide resin composition molded article and prepreg having excellent heat resistance and mechanical characteristic, and fiber-reinforced composite material thereof | |
| CN103547568B (zh) | 使用了2-苯基-4,4’-二氨基二苯基醚类的末端改性酰亚胺低聚物、其混合物、清漆以及固化树脂 | |
| CN101062980A (zh) | 一种含氟热塑性聚酰亚胺聚合物及其制备方法 | |
| JP5050269B2 (ja) | 末端変性イミドオリゴマーおよびワニス並びにその高弾性率硬化物 | |
| JP6786875B2 (ja) | ポリイミド樹脂組成物及びその製造方法 | |
| JP2013241553A (ja) | カルド型ジアミンを組成とする熱硬化性ポリイミド | |
| JP2014201740A (ja) | イミドオリゴマー及びこれを加熱硬化させてなるポリイミド樹脂 | |
| JPH02284923A (ja) | 末端変性イミドオリゴマー組成物 | |
| JP2009185204A (ja) | ポリイミドオリゴマー及びこれを加熱硬化させてなるポリイミド樹脂 | |
| WO2017195393A1 (ja) | 末端変性イミドオリゴマー、ワニス、それらの硬化物、フィルム、並びにそれらを用いたイミドプリプレグおよび繊維強化複合材料 | |
| Chen et al. | Effect of poly (etherimide) chemical structures on the properties of epoxy/poly (etherimide) blends and their carbon fiber‐reinforced composites | |
| JP2010121095A (ja) | イミドオリゴマー及びこれを加熱硬化させてなるポリイミド樹脂 | |
| JP2012197403A (ja) | イミドオリゴマー及びこれを加熱硬化させてなるポリイミド樹脂 | |
| CN112888726B (zh) | 用于模制聚酰亚胺复合材料的新型酰胺酸低聚物工艺 | |
| KR101288724B1 (ko) | 폴리아믹산 용액 및 폴리이미드 코팅층 | |
| KR101258432B1 (ko) | 고온에서의 열적 치수안정성이 우수한 폴리이미드 필름 및 그를 이용한 디스플레이 소자용 기판 | |
| JP2882114B2 (ja) | 末端変性イミドオリゴマ−組成物 | |
| JP5610335B2 (ja) | 機械的強度が向上した繊維強化ポリイミド材料の製造方法 | |
| JP2013256549A (ja) | イミドオリゴマー及びこれを加熱硬化させてなるポリイミド樹脂 | |
| JP2010143958A (ja) | イミドオリゴマー及びこれを加熱硬化させてなるポリイミド樹脂 | |
| Liu et al. | Development of Benzimidazole‐Containing Thermosetting Imide Oligomers With Enhanced Thermal Stability and Adhesive Properties | |
| JP2014201739A (ja) | イミドオリゴマ−及びこれを加熱硬化させてなるポリイミド樹脂 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A711 | Notification of change in applicant |
Free format text: JAPANESE INTERMEDIATE CODE: A711 Effective date: 20141119 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20141119 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20160331 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20160701 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20170113 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20170214 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20180130 |