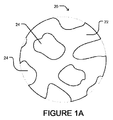
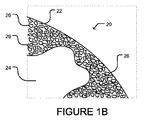
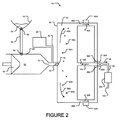
JP2012507562A - 微小球性多孔質生体適合性足場並びにその製造方法及び装置 - Google Patents
微小球性多孔質生体適合性足場並びにその製造方法及び装置 Download PDFInfo
- Publication number
- JP2012507562A JP2012507562A JP2011534806A JP2011534806A JP2012507562A JP 2012507562 A JP2012507562 A JP 2012507562A JP 2011534806 A JP2011534806 A JP 2011534806A JP 2011534806 A JP2011534806 A JP 2011534806A JP 2012507562 A JP2012507562 A JP 2012507562A
- Authority
- JP
- Japan
- Prior art keywords
- poly
- particle
- particles
- additive
- tissue
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J9/00—Working-up of macromolecular substances to porous or cellular articles or materials; After-treatment thereof
- C08J9/26—Working-up of macromolecular substances to porous or cellular articles or materials; After-treatment thereof by elimination of a solid phase from a macromolecular composition or article, e.g. leaching out
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K51/00—Preparations containing radioactive substances for use in therapy or testing in vivo
- A61K51/12—Preparations containing radioactive substances for use in therapy or testing in vivo characterised by a special physical form, e.g. emulsion, microcapsules, liposomes, characterized by a special physical form, e.g. emulsions, dispersions, microcapsules
- A61K51/1241—Preparations containing radioactive substances for use in therapy or testing in vivo characterised by a special physical form, e.g. emulsion, microcapsules, liposomes, characterized by a special physical form, e.g. emulsions, dispersions, microcapsules particles, powders, lyophilizates, adsorbates, e.g. polymers or resins for adsorption or ion-exchange resins
- A61K51/1255—Granulates, agglomerates, microspheres
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/5005—Wall or coating material
- A61K9/5021—Organic macromolecular compounds
- A61K9/5031—Organic macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyethylene glycol, poly(lactide-co-glycolide)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/5089—Processes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L24/00—Surgical adhesives or cements; Adhesives for colostomy devices
- A61L24/001—Use of materials characterised by their function or physical properties
- A61L24/0036—Porous materials, e.g. foams or sponges
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/36—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix
- A61L27/38—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix containing added animal cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/36—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix
- A61L27/38—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix containing added animal cells
- A61L27/3804—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix containing added animal cells characterised by specific cells or progenitors thereof, e.g. fibroblasts, connective tissue cells, kidney cells
- A61L27/3834—Cells able to produce different cell types, e.g. hematopoietic stem cells, mesenchymal stem cells, marrow stromal cells, embryonic stem cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/36—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix
- A61L27/38—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix containing added animal cells
- A61L27/3839—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix containing added animal cells characterised by the site of application in the body
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/50—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L27/56—Porous materials, e.g. foams or sponges
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/18—Drugs for disorders of the alimentary tract or the digestive system for pancreatic disorders, e.g. pancreatic enzymes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J9/00—Working-up of macromolecular substances to porous or cellular articles or materials; After-treatment thereof
- C08J9/28—Working-up of macromolecular substances to porous or cellular articles or materials; After-treatment thereof by elimination of a liquid phase from a macromolecular composition or article, e.g. drying of coagulum
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/87—Introduction of foreign genetic material using processes not otherwise provided for, e.g. co-transformation
- C12N15/88—Introduction of foreign genetic material using processes not otherwise provided for, e.g. co-transformation using microencapsulation, e.g. using amphiphile liposome vesicle
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2430/00—Materials or treatment for tissue regeneration
- A61L2430/36—Materials or treatment for tissue regeneration for embolization or occlusion, e.g. vaso-occlusive compositions or devices
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J2201/00—Foams characterised by the foaming process
- C08J2201/04—Foams characterised by the foaming process characterised by the elimination of a liquid or solid component, e.g. precipitation, leaching out, evaporation
- C08J2201/044—Elimination of an inorganic solid phase
- C08J2201/0444—Salts
- C08J2201/0446—Elimination of NaCl only
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J2201/00—Foams characterised by the foaming process
- C08J2201/04—Foams characterised by the foaming process characterised by the elimination of a liquid or solid component, e.g. precipitation, leaching out, evaporation
- C08J2201/054—Precipitating the polymer by adding a non-solvent or a different solvent
- C08J2201/0542—Precipitating the polymer by adding a non-solvent or a different solvent from an organic solvent-based polymer composition
- C08J2201/0544—Precipitating the polymer by adding a non-solvent or a different solvent from an organic solvent-based polymer composition the non-solvent being aqueous
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J2207/00—Foams characterised by their intended use
- C08J2207/10—Medical applications, e.g. biocompatible scaffolds
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Biomedical Technology (AREA)
- Epidemiology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Organic Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Dermatology (AREA)
- Zoology (AREA)
- Transplantation (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Genetics & Genomics (AREA)
- Cell Biology (AREA)
- Materials Engineering (AREA)
- Dispersion Chemistry (AREA)
- Botany (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Polymers & Plastics (AREA)
- Biotechnology (AREA)
- Wood Science & Technology (AREA)
- General Engineering & Computer Science (AREA)
- Physics & Mathematics (AREA)
- Vascular Medicine (AREA)
- Urology & Nephrology (AREA)
- Plant Pathology (AREA)
- Hematology (AREA)
- Heart & Thoracic Surgery (AREA)
- Molecular Biology (AREA)
- Surgery (AREA)
- Microbiology (AREA)
- Cardiology (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US19780308P | 2008-10-30 | 2008-10-30 | |
| US61/197,803 | 2008-10-30 | ||
| PCT/US2009/062744 WO2010062678A2 (en) | 2008-10-30 | 2009-10-30 | Micro-spherical porous biocompatible scaffolds and methods and apparatus for fabricating same |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2012507562A true JP2012507562A (ja) | 2012-03-29 |
| JP2012507562A5 JP2012507562A5 (enExample) | 2012-12-13 |
Family
ID=42026232
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2011534806A Pending JP2012507562A (ja) | 2008-10-30 | 2009-10-30 | 微小球性多孔質生体適合性足場並びにその製造方法及び装置 |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US20110212179A1 (enExample) |
| EP (1) | EP2344578A2 (enExample) |
| JP (1) | JP2012507562A (enExample) |
| CN (1) | CN102143996A (enExample) |
| WO (1) | WO2010062678A2 (enExample) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2015112262A (ja) * | 2013-12-11 | 2015-06-22 | 学校法人藤田学園 | 組織修復及び/又は再生促進材 |
| JP2018130322A (ja) * | 2017-02-15 | 2018-08-23 | ドリームメディカルパートナーズ株式会社 | 塞栓材及びその製造方法 |
| WO2021206440A1 (ko) * | 2020-04-09 | 2021-10-14 | 주식회사 피엘마이크로메드 | 색전 시술용 마이크로 비드 및 증식성 질환 치료용 조성물 |
Families Citing this family (73)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6660301B1 (en) | 1998-03-06 | 2003-12-09 | Biosphere Medical, Inc. | Injectable microspheres for dermal augmentation and tissue bulking |
| US7338657B2 (en) | 2001-03-15 | 2008-03-04 | Biosphere Medical, Inc. | Injectable microspheres for tissue construction |
| US6436424B1 (en) | 2000-03-20 | 2002-08-20 | Biosphere Medical, Inc. | Injectable and swellable microspheres for dermal augmentation |
| WO2001070289A2 (en) | 2000-03-20 | 2001-09-27 | Biosphere Medical, Inc. | Injectable and swellable microspheres for tissue bulking |
| KR20080046750A (ko) | 2000-03-24 | 2008-05-27 | 바이오스피어 메디칼 인코포레이티드 | 능동 색전화용 미소구 |
| MX2007013914A (es) | 2005-05-09 | 2008-02-22 | Biosphere Medical S A | Composiciones y metodos que usan microesferas y agentes de contraste no ionicos. |
| US20080033366A1 (en) * | 2006-01-30 | 2008-02-07 | Surgica Corporation | Compressible intravascular embolization particles and related methods and delivery systems |
| AU2014304191B2 (en) | 2013-08-09 | 2017-06-01 | Kimberly-Clark Worldwide, Inc. | Microparticles having a multimodal pore distribution |
| US10919229B2 (en) | 2013-08-09 | 2021-02-16 | Kimberly-Clark Worldwide, Inc. | Polymeric material for three-dimensional printing |
| CN105431479B (zh) | 2013-08-09 | 2018-11-16 | 金伯利-克拉克环球有限公司 | 用于选择性控制聚合物材料的孔隙率的方法 |
| MX364107B (es) | 2013-08-09 | 2019-04-11 | Kimberly Clark Co | Sistema de suministro para agentes activos. |
| EP3030605B1 (en) * | 2013-08-09 | 2019-08-07 | Kimberly-Clark Worldwide, Inc. | Flexible polymeric material with shape retention properties |
| JP2016528348A (ja) * | 2013-08-09 | 2016-09-15 | キンバリー クラーク ワールドワイド インコーポレイテッド | 異方性高分子材料 |
| WO2015038988A1 (en) | 2013-09-13 | 2015-03-19 | Modern Meadow, Inc. | Edible and animal-product-free microcarriers for engineered meat |
| CA2923753C (en) | 2013-09-19 | 2021-10-12 | Terumo Corporation | Polymer particles |
| BR112016005768B1 (pt) | 2013-09-19 | 2021-09-21 | Microvention, Inc | Películas de polímero |
| CN103550824B (zh) * | 2013-11-01 | 2016-03-02 | 南京医科大学附属口腔医院 | 一种引导骨组织再生的支架的制备方法 |
| EP3065719A4 (en) | 2013-11-08 | 2017-08-09 | Terumo Corporation | Polymer particles |
| AU2015214092B2 (en) | 2014-02-05 | 2018-11-15 | Fork & Goode, Inc. | Dried food products formed from cultured muscle cells |
| US20160220498A1 (en) * | 2015-01-29 | 2016-08-04 | Aneesh SONI | Fully or partially biodegradable device for controlled drug delivery |
| KR20240116531A (ko) | 2015-02-09 | 2024-07-29 | 슬링샷 바이오사이언시즈 인코포레이티드 | 튜닝가능한 광 특성을 갖는 하이드로겔 입자 및 이를 사용하기 위한 방법 |
| US9907880B2 (en) | 2015-03-26 | 2018-03-06 | Microvention, Inc. | Particles |
| US20180223067A1 (en) * | 2015-08-10 | 2018-08-09 | Kyoto University | Porous particle, method for producing porous particle, and block copolymer |
| WO2017026426A1 (ja) | 2015-08-10 | 2017-02-16 | 国立大学法人京都大学 | 有機ポリマー製多孔性粒子、有機ポリマー製多孔性粒子の製造方法およびブロックコポリマー |
| ES2842501T5 (es) | 2015-09-21 | 2023-04-13 | Modern Meadow Inc | Materiales compuestos de tejido reforzados con fibras |
| US10258590B2 (en) * | 2015-10-14 | 2019-04-16 | Alcresta Therapeutics, Inc. | Enteral feeding device and related methods of use |
| CN105175772A (zh) * | 2015-10-22 | 2015-12-23 | 山西科瑞迪生物科技有限公司 | 多肽/核苷酸合成用固相载体材料易官能化活性树脂的制备方法 |
| CN105327405B (zh) * | 2015-10-23 | 2019-01-29 | 华南理工大学 | 用于治疗宫颈炎的载药支架及其制备方法 |
| CN111303641A (zh) | 2016-02-15 | 2020-06-19 | 现代牧场股份有限公司 | 含有胶原原纤维的生物制造材料 |
| WO2017152127A1 (en) * | 2016-03-03 | 2017-09-08 | Amrita Vishwa Vidyapeetham | Mri and ct contrast-enabled composite implants for image-guided tissue regeneration and therapy |
| MX385370B (es) * | 2016-03-31 | 2025-03-18 | Immutrix Therapeutics Inc | Metodo para el tratamiento extracorporeo de preeclampsia y trastornos relacionados. |
| WO2017189308A1 (en) | 2016-04-19 | 2017-11-02 | The Broad Institute Inc. | Novel crispr enzymes and systems |
| KR102424476B1 (ko) | 2016-04-19 | 2022-07-25 | 더 브로드 인스티튜트, 인코퍼레이티드 | 신규한 crispr 효소 및 시스템 |
| US11286478B2 (en) | 2016-04-19 | 2022-03-29 | The Broad Institute, Inc. | Cpf1 complexes with reduced indel activity |
| CN107304251A (zh) * | 2016-04-22 | 2017-10-31 | 中国石油化工股份有限公司 | 用于选择性激光烧结的聚丙烯粉末及其制备 |
| US12421369B2 (en) * | 2016-06-14 | 2025-09-23 | The Regents Of The University Of California | Porous polymer scaffolds, and methods of making and using the same |
| WO2018035387A1 (en) | 2016-08-17 | 2018-02-22 | The Broad Institute, Inc. | Novel crispr enzymes and systems |
| US11352647B2 (en) | 2016-08-17 | 2022-06-07 | The Broad Institute, Inc. | Crispr enzymes and systems |
| CA3038642C (en) | 2016-09-28 | 2021-11-02 | Terumo Corporation | Polymer particles comprising polymerizable pharmaceutical agents |
| BR112019014280B1 (pt) * | 2017-01-31 | 2023-02-14 | Kimberly-Clark Worldwide, Inc | Material polimérico, fibra, manta não tecida, artigo absorvente, e, métodos para formação de um material polimérico e para formar uma fibra. |
| WO2018191274A1 (en) * | 2017-04-11 | 2018-10-18 | University Of Florida Research Foundation | Systems and methods for in-situ, bottom-up tissue generation |
| WO2018191750A2 (en) | 2017-04-14 | 2018-10-18 | The Broad Institute Inc. | Novel delivery of large payloads |
| WO2019135816A2 (en) | 2017-10-23 | 2019-07-11 | The Broad Institute, Inc. | Novel nucleic acid modifiers |
| WO2019094983A1 (en) | 2017-11-13 | 2019-05-16 | The Broad Institute, Inc. | Methods and compositions for treating cancer by targeting the clec2d-klrb1 pathway |
| AU2018253595A1 (en) | 2017-11-13 | 2019-05-30 | Modern Meadow, Inc. | Biofabricated leather articles having zonal properties |
| WO2019094984A1 (en) | 2017-11-13 | 2019-05-16 | The Broad Institute, Inc. | Methods for determining spatial and temporal gene expression dynamics during adult neurogenesis in single cells |
| WO2019126169A1 (en) | 2017-12-18 | 2019-06-27 | C. R. Bard, Inc. | Drug-loaded microbead compositions, embolization compositions and associated methods |
| US11590080B2 (en) | 2017-12-18 | 2023-02-28 | C.R. Bard, Inc. | Drug-loaded biodegradable microbead compositions including drug-containing vesicular agents |
| CN108126239B (zh) * | 2018-01-02 | 2020-11-10 | 四川大学 | 一种孔道结构可控的明胶细胞支架及其制备方法 |
| CN109106986B (zh) * | 2018-09-14 | 2020-12-25 | 广州润虹医药科技股份有限公司 | 一种药物控释磷酸钙骨水泥复合微球、其制备方法及应用 |
| AU2020209847B2 (en) | 2019-01-17 | 2024-10-17 | Modern Meadow, Inc. | Layered collagen materials and methods of making the same |
| CN109833509B (zh) * | 2019-01-18 | 2021-10-15 | 太阳雨林(厦门)生物医药有限公司 | 一种多重缓释血管栓塞载药组合物 |
| US12215382B2 (en) | 2019-03-01 | 2025-02-04 | The General Hospital Corporation | Liver protective MARC variants and uses thereof |
| WO2020191102A1 (en) | 2019-03-18 | 2020-09-24 | The Broad Institute, Inc. | Type vii crispr proteins and systems |
| WO2020236972A2 (en) | 2019-05-20 | 2020-11-26 | The Broad Institute, Inc. | Non-class i multi-component nucleic acid targeting systems |
| US12297426B2 (en) | 2019-10-01 | 2025-05-13 | The Broad Institute, Inc. | DNA damage response signature guided rational design of CRISPR-based systems and therapies |
| WO2021163131A1 (en) * | 2020-02-10 | 2021-08-19 | Youhealth Biotech, Limited | Device, kit, and method for islet transplantation |
| US20210299309A1 (en) * | 2020-03-31 | 2021-09-30 | Parasol Medical, Llc | Tent with antimicrobial treatment applied thereto and method of imparting antimicrobial properties to the tent |
| CN112890882A (zh) * | 2021-01-27 | 2021-06-04 | 广州跃金科技有限公司 | 一种注射装置及硅胶填充设备 |
| CA3235582A1 (en) | 2021-10-29 | 2023-05-04 | Anh Tuan Nguyen | Hydrogel particles as feeder cells and as synthetic antigen presenting cells |
| CN114191410A (zh) * | 2021-11-15 | 2022-03-18 | 深圳北航新兴产业技术研究院 | 一种诊疗一体化双亲微球缓释微球的制备方法 |
| CN114470308A (zh) * | 2022-03-01 | 2022-05-13 | 苏州森康微球医疗科技有限公司 | 一种大孔聚丙烯酸钠栓塞微球的制备工艺 |
| EP4503923A1 (en) | 2022-04-04 | 2025-02-12 | The Regents of the University of California | Genetic complementation compositions and methods |
| EP4519690A1 (en) | 2022-05-05 | 2025-03-12 | Slingshot Biosciences, Inc. | Engineered particles as red blood cell mimics and compositions containing same for hematology |
| CN114949348B (zh) * | 2022-06-30 | 2023-09-29 | 成都世联康健生物科技有限公司 | 藻酸盐-载hDPSCs的GelMA纤维微球及其制备方法 |
| CN120712108A (zh) * | 2022-10-26 | 2025-09-26 | 弹弓生物科学公司 | 具有可调整光学性质的大小可调整合成颗粒和用于使用其进行免疫细胞激活的方法 |
| WO2025072383A1 (en) | 2023-09-25 | 2025-04-03 | The Broad Institute, Inc. | Viral open reading frames, uses thereof, and methods of detecting the same |
| CN117018298B (zh) * | 2023-10-09 | 2023-12-26 | 北京华龛生物科技有限公司 | 一种降解时间可控的可注射多孔微球及其制备方法和应用 |
| WO2025097055A2 (en) | 2023-11-02 | 2025-05-08 | The Broad Institute, Inc. | Compositions and methods of use of t cells in immunotherapy |
| WO2025129158A1 (en) | 2023-12-15 | 2025-06-19 | The Broad Institute, Inc. | Engineered arc delivery vesicles and uses thereof |
| TW202525349A (zh) * | 2023-12-28 | 2025-07-01 | 白金科技股份有限公司 | 微球及其製備方法 |
| CN118406273B (zh) * | 2024-05-29 | 2024-11-19 | 广东安拓普聚合物科技股份有限公司 | 一种医用导管用tpu材料及其生产工艺 |
| WO2025250808A1 (en) | 2024-05-29 | 2025-12-04 | The Brigham And Women’S Hospital, Inc. | Anti-crispr delivery compositions and methods |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2001517615A (ja) * | 1997-09-24 | 2001-10-09 | アルカームズ コントロールド セラピューティックス,インコーポレイテッド | ポリマーを基剤とした制御された放出製剤の製造方法 |
| JP2002541925A (ja) * | 1999-04-16 | 2002-12-10 | ラトガーズ,ザ ステイト ユニバーシティ | 組織工学用多孔質ポリマー足場 |
| JP2004517699A (ja) * | 2001-01-30 | 2004-06-17 | ボード オブ リージェンツ ユニバーシティ オブ テキサス システム | 液体中への噴霧凍結によるナノ粒子およびミクロ粒子の製造方法 |
| JP2005533008A (ja) * | 2002-03-29 | 2005-11-04 | ボストン サイエンティフィック リミテッド | 薬物送達粒子 |
| JP2005535752A (ja) * | 2002-08-09 | 2005-11-24 | ボストン サイエンティフィック リミテッド | 塞栓術 |
| JP2005537070A (ja) * | 2002-08-30 | 2005-12-08 | ボストン サイエンティフィック リミテッド | 塞栓術 |
| JP2007506791A (ja) * | 2003-09-25 | 2007-03-22 | ラトガース,ザ ステート ユニバーシティ | 塞栓治療のための本質的に放射線不透過性であるポリマー生産物 |
Family Cites Families (78)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3673125A (en) * | 1969-05-10 | 1972-06-27 | Kanegafuchi Spinning Co Ltd | Method of producing polyvinyl acetal porous articles |
| GB1381258A (en) * | 1970-12-11 | 1975-01-22 | Porvair Ltd | Water vapour permeable materials |
| US3919411A (en) * | 1972-01-31 | 1975-11-11 | Bayvet Corp | Injectable adjuvant and compositions including such adjuvant |
| CA1134977A (en) * | 1978-09-07 | 1982-11-02 | Sumitomo Chemical Co., Ltd. | Method for preparing highly absorbent polymers |
| US4314032A (en) * | 1978-10-26 | 1982-02-02 | Kureha Kagaku Kogyo Kabushiki Kaisha | Crosslinked polyvinyl alcohol gel |
| US4268495A (en) * | 1979-01-08 | 1981-05-19 | Ethicon, Inc. | Injectable embolization and occlusion solution |
| US4306031A (en) * | 1979-08-14 | 1981-12-15 | Mitsubishi Chemical Industries Limited | Weakly acidic cation exchange resin and process for producing same |
| GB2088392B (en) * | 1980-12-03 | 1984-05-02 | Sumitomo Chemical Co | Production of hydrogels |
| CA1225585A (en) * | 1983-06-30 | 1987-08-18 | Maria T. Litvinova | Composition for embolization of blood vessels |
| GB8418772D0 (en) * | 1984-07-24 | 1984-08-30 | Geistlich Soehne Ag | Chemical substances |
| US5186922A (en) * | 1985-03-15 | 1993-02-16 | See/Shell Biotechnology, Inc. | Use of biodegradable microspheres labeled with imaging energy constrast materials |
| US4680171A (en) * | 1985-03-15 | 1987-07-14 | William Shell | Visualization of a bloodstream circulation with biodegradable microspheres |
| US4803075A (en) * | 1986-06-25 | 1989-02-07 | Collagen Corporation | Injectable implant composition having improved intrudability |
| JPS6317904A (ja) * | 1986-07-09 | 1988-01-25 | Mitsubishi Chem Ind Ltd | 多孔質架橋ポリビニルアルコ−ル粒子の製造法 |
| CA1329800C (en) * | 1987-12-29 | 1994-05-24 | Hiroaki Takayanagi | Composite separating agent |
| JPH064713B2 (ja) * | 1988-07-22 | 1994-01-19 | テルモ株式会社 | 生体適合性材料 |
| JPH0286838A (ja) * | 1988-09-22 | 1990-03-27 | Terumo Corp | 水不溶性ヒドロゲルおよびその製造方法 |
| US5162430A (en) * | 1988-11-21 | 1992-11-10 | Collagen Corporation | Collagen-polymer conjugates |
| US5510418A (en) * | 1988-11-21 | 1996-04-23 | Collagen Corporation | Glycosaminoglycan-synthetic polymer conjugates |
| US5258028A (en) * | 1988-12-12 | 1993-11-02 | Ersek Robert A | Textured micro implants |
| US5092883A (en) * | 1988-12-28 | 1992-03-03 | Eppley Barry L | Method for promoting soft connective tissue growth and repair in mammals |
| CA2017570C (en) * | 1989-05-31 | 2000-12-19 | James R. Gross | Porous structure of an absorbent polymer |
| US5007940A (en) * | 1989-06-09 | 1991-04-16 | American Medical Systems, Inc. | Injectable polymeric bodies |
| JPH0436328A (ja) * | 1990-06-01 | 1992-02-06 | Chuichi Hirayama | 大孔径ポリアミノ酸多孔質球状粒子とその製造方法 |
| US5202352A (en) * | 1990-08-08 | 1993-04-13 | Takeda Chemical Industries, Ltd. | Intravascular embolizing agent containing angiogenesis-inhibiting substance |
| US5410016A (en) * | 1990-10-15 | 1995-04-25 | Board Of Regents, The University Of Texas System | Photopolymerizable biodegradable hydrogels as tissue contacting materials and controlled-release carriers |
| FR2676927B1 (fr) * | 1991-05-29 | 1995-06-23 | Ibf | Microspheres utilisables pour les occlusions vasculaires therapeutiques et solutions injectables les contenant. |
| US5554658A (en) * | 1991-08-06 | 1996-09-10 | Rosenblatt; Solomon | Injection molded PVA Sponge |
| JP3356447B2 (ja) * | 1991-10-16 | 2002-12-16 | テルモ株式会社 | 乾燥高分子ゲルからなる血管病変塞栓材料 |
| BR9306038A (pt) * | 1992-02-28 | 1998-01-13 | Univ Texas | Hidrogéis biodegradáveis fotopolimerizáveis como materiais de contato de tecidos e condutores de liberação controlada |
| JPH05294839A (ja) * | 1992-04-20 | 1993-11-09 | Biomaterial Universe Kk | シスプラチン含有生体内分解吸収性高分子の微小球お よびその製造法 |
| US5629327A (en) * | 1993-03-01 | 1997-05-13 | Childrens Hospital Medical Center Corp. | Methods and compositions for inhibition of angiogenesis |
| CA2157793C (en) * | 1993-03-09 | 1999-07-13 | James E. Woiszwillo | Macromolecular microparticles and methods of production |
| US5583162A (en) * | 1994-06-06 | 1996-12-10 | Biopore Corporation | Polymeric microbeads and method of preparation |
| UA10911C2 (uk) * | 1994-08-10 | 1996-12-25 | Мале Впроваджувальне Підприємство "Іhтерфалл" | Біосумісhий гідрогель |
| US5955108A (en) * | 1994-12-16 | 1999-09-21 | Quadrant Healthcare (Uk) Limited | Cross-linked microparticles and their use as therapeutic vehicles |
| CA2207667A1 (en) * | 1995-01-27 | 1996-08-01 | Scimed Life Systems, Inc. | Embolizing system |
| US5752974A (en) * | 1995-12-18 | 1998-05-19 | Collagen Corporation | Injectable or implantable biomaterials for filling or blocking lumens and voids of the body |
| DE19601699A1 (de) * | 1996-01-18 | 1997-07-24 | Wacker Chemie Gmbh | Redispergierbare Polymerisatpulver und daraus erhältliche wäßrige Polymerisat-Dispersionen |
| US5785977A (en) * | 1996-02-07 | 1998-07-28 | Breithbarth; Richard | Non-metallic microparticle carrier materials |
| US5611344A (en) * | 1996-03-05 | 1997-03-18 | Acusphere, Inc. | Microencapsulated fluorinated gases for use as imaging agents |
| KR20000004975A (ko) * | 1996-03-27 | 2000-01-25 | 슈미트-하이트 모니카, 케르커 니콜레 | 포로겐을사용하여다공성중합체를제조하는방법 |
| US5823198A (en) * | 1996-07-31 | 1998-10-20 | Micro Therapeutics, Inc. | Method and apparatus for intravasculer embolization |
| US5925683A (en) * | 1996-10-17 | 1999-07-20 | Target Therapeutics, Inc. | Liquid embolic agents |
| US6048908A (en) * | 1997-06-27 | 2000-04-11 | Biopore Corporation | Hydrophilic polymeric material |
| US6565885B1 (en) * | 1997-09-29 | 2003-05-20 | Inhale Therapeutic Systems, Inc. | Methods of spray drying pharmaceutical compositions |
| CN1714783A (zh) * | 1998-03-06 | 2006-01-04 | 生物领域医疗公司 | 用于组织膨胀和治疗的可植入颗粒 |
| US6660301B1 (en) * | 1998-03-06 | 2003-12-09 | Biosphere Medical, Inc. | Injectable microspheres for dermal augmentation and tissue bulking |
| US5891470A (en) * | 1998-04-17 | 1999-04-06 | Advanced Polymer Systems, Inc. | Softgel formulation containing retinol |
| US6165193A (en) * | 1998-07-06 | 2000-12-26 | Microvention, Inc. | Vascular embolization with an expansible implant |
| FR2784580B1 (fr) * | 1998-10-16 | 2004-06-25 | Biosepra Inc | Microspheres de polyvinyl-alcool et procedes de fabrication de celles-ci |
| US6103255A (en) * | 1999-04-16 | 2000-08-15 | Rutgers, The State University | Porous polymer scaffolds for tissue engineering |
| US6710126B1 (en) * | 1999-11-15 | 2004-03-23 | Bio Cure, Inc. | Degradable poly(vinyl alcohol) hydrogels |
| KR100335866B1 (ko) * | 2000-01-06 | 2002-05-10 | 박호군 | 폴리아세트산비닐 코어/폴리비닐알코올 외피의 2중 구조를갖는 미세구형 색전재료 및 그의 제조방법 |
| AU2001245660B2 (en) * | 2000-03-13 | 2006-06-15 | Biocompatibles Uk Limited | Embolic compositions |
| US6652883B2 (en) * | 2000-03-13 | 2003-11-25 | Biocure, Inc. | Tissue bulking and coating compositions |
| US7338657B2 (en) * | 2001-03-15 | 2008-03-04 | Biosphere Medical, Inc. | Injectable microspheres for tissue construction |
| US6436424B1 (en) * | 2000-03-20 | 2002-08-20 | Biosphere Medical, Inc. | Injectable and swellable microspheres for dermal augmentation |
| WO2001070289A2 (en) * | 2000-03-20 | 2001-09-27 | Biosphere Medical, Inc. | Injectable and swellable microspheres for tissue bulking |
| KR20080046750A (ko) * | 2000-03-24 | 2008-05-27 | 바이오스피어 메디칼 인코포레이티드 | 능동 색전화용 미소구 |
| US20030212022A1 (en) * | 2001-03-23 | 2003-11-13 | Jean-Marie Vogel | Compositions and methods for gene therapy |
| US6537569B2 (en) * | 2001-02-14 | 2003-03-25 | Microvention, Inc. | Radiation cross-linked hydrogels |
| WO2002100444A1 (en) * | 2001-06-08 | 2002-12-19 | Biosphere Medical Inc. | Colloidal metal labelled microparticles, their production and use |
| US6911219B2 (en) * | 2001-09-27 | 2005-06-28 | Surgica Corporation | Partially acetalized polyvinyl alcohol embolization particles, compositions containing those particles and methods of making and using them |
| US7094369B2 (en) * | 2002-03-29 | 2006-08-22 | Scimed Life Systems, Inc. | Processes for manufacturing polymeric microspheres |
| US7053134B2 (en) * | 2002-04-04 | 2006-05-30 | Scimed Life Systems, Inc. | Forming a chemically cross-linked particle of a desired shape and diameter |
| US7838699B2 (en) * | 2002-05-08 | 2010-11-23 | Biosphere Medical | Embolization using degradable crosslinked hydrogels |
| US7303575B2 (en) * | 2002-08-01 | 2007-12-04 | Lumen Biomedical, Inc. | Embolism protection devices |
| US20040076582A1 (en) * | 2002-08-30 | 2004-04-22 | Dimatteo Kristian | Agent delivery particle |
| US7883490B2 (en) * | 2002-10-23 | 2011-02-08 | Boston Scientific Scimed, Inc. | Mixing and delivery of therapeutic compositions |
| US7588825B2 (en) * | 2002-10-23 | 2009-09-15 | Boston Scientific Scimed, Inc. | Embolic compositions |
| CA2504283A1 (en) * | 2002-10-31 | 2004-05-21 | Umd, Inc. | Therapeutic compositions for drug delivery to and through covering epithelia |
| JP2008504895A (ja) * | 2004-06-29 | 2008-02-21 | スパイン・ウェイブ・インコーポレーテッド | 椎間板の欠陥及び損傷を治療する方法 |
| MX2007013914A (es) * | 2005-05-09 | 2008-02-22 | Biosphere Medical S A | Composiciones y metodos que usan microesferas y agentes de contraste no ionicos. |
| EP1986706B1 (en) * | 2006-01-30 | 2011-08-17 | Biosphere Medical, Inc. | Porous intravascular embolization particles and methods of making them |
| US20080033366A1 (en) * | 2006-01-30 | 2008-02-07 | Surgica Corporation | Compressible intravascular embolization particles and related methods and delivery systems |
| US7888410B2 (en) * | 2007-04-24 | 2011-02-15 | Eastman Kodak Company | Method of making porous particles |
| EP2351779B1 (en) * | 2010-01-27 | 2019-04-24 | Biosphere Medical, Inc. | Microspheres and method of making the microspheres |
-
2009
- 2009-10-30 US US13/126,456 patent/US20110212179A1/en not_active Abandoned
- 2009-10-30 EP EP09745251A patent/EP2344578A2/en not_active Withdrawn
- 2009-10-30 JP JP2011534806A patent/JP2012507562A/ja active Pending
- 2009-10-30 WO PCT/US2009/062744 patent/WO2010062678A2/en not_active Ceased
- 2009-10-30 CN CN2009801344899A patent/CN102143996A/zh active Pending
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2001517615A (ja) * | 1997-09-24 | 2001-10-09 | アルカームズ コントロールド セラピューティックス,インコーポレイテッド | ポリマーを基剤とした制御された放出製剤の製造方法 |
| JP2002541925A (ja) * | 1999-04-16 | 2002-12-10 | ラトガーズ,ザ ステイト ユニバーシティ | 組織工学用多孔質ポリマー足場 |
| JP2004517699A (ja) * | 2001-01-30 | 2004-06-17 | ボード オブ リージェンツ ユニバーシティ オブ テキサス システム | 液体中への噴霧凍結によるナノ粒子およびミクロ粒子の製造方法 |
| JP2005533008A (ja) * | 2002-03-29 | 2005-11-04 | ボストン サイエンティフィック リミテッド | 薬物送達粒子 |
| JP2005535752A (ja) * | 2002-08-09 | 2005-11-24 | ボストン サイエンティフィック リミテッド | 塞栓術 |
| JP2005537070A (ja) * | 2002-08-30 | 2005-12-08 | ボストン サイエンティフィック リミテッド | 塞栓術 |
| JP2007506791A (ja) * | 2003-09-25 | 2007-03-22 | ラトガース,ザ ステート ユニバーシティ | 塞栓治療のための本質的に放射線不透過性であるポリマー生産物 |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2015112262A (ja) * | 2013-12-11 | 2015-06-22 | 学校法人藤田学園 | 組織修復及び/又は再生促進材 |
| JP2018130322A (ja) * | 2017-02-15 | 2018-08-23 | ドリームメディカルパートナーズ株式会社 | 塞栓材及びその製造方法 |
| US11253627B2 (en) | 2017-02-15 | 2022-02-22 | Dream Medical Partners Corporation | Embolic material and method for producing same |
| WO2021206440A1 (ko) * | 2020-04-09 | 2021-10-14 | 주식회사 피엘마이크로메드 | 색전 시술용 마이크로 비드 및 증식성 질환 치료용 조성물 |
Also Published As
| Publication number | Publication date |
|---|---|
| US20110212179A1 (en) | 2011-09-01 |
| WO2010062678A2 (en) | 2010-06-03 |
| EP2344578A2 (en) | 2011-07-20 |
| CN102143996A (zh) | 2011-08-03 |
| WO2010062678A3 (en) | 2011-04-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2012507562A (ja) | 微小球性多孔質生体適合性足場並びにその製造方法及び装置 | |
| US9308169B2 (en) | Microspheres for active embolization | |
| Yang et al. | Biodegradable magnesium-incorporated poly (L-lactic acid) microspheres for manipulation of drug release and alleviation of inflammatory response | |
| Go et al. | Multifunctional biodegradable microrobot with programmable morphology for biomedical applications | |
| Nigmatullin et al. | Polyhydroxyalkanoates, a family of natural polymers, and their applications in drug delivery | |
| JP6900416B2 (ja) | コア−シェル生分解性粒子の製造方法 | |
| Langer | Biomaterials in drug delivery and tissue engineering: one laboratory's experience | |
| US20060063732A1 (en) | Compositions and methods for gene therapy | |
| US20120063997A1 (en) | Delivery system with scaffolds | |
| JP2016530252A (ja) | 組成物および送達系 | |
| WO2001072280A2 (en) | Microspheres for gene therapy | |
| KR20190120655A (ko) | 최소 침습 골연골 재생용 자기 구동 마이크로지지체 | |
| CN109982689A (zh) | 递送装置及其制造方法 | |
| CN110035742A (zh) | 干细胞仿生微粒 | |
| Sable et al. | Applications of Electrospraying in Tissue Repair and Regeneration | |
| Zhu | Comparative analysis of delivery methods for stem cell therapy in liver diseases | |
| WO2024086572A1 (en) | Multi-phasic therapeutic delivery system | |
| Zorlutuna et al. | Biomaterials and tissue engineering research in Turkey: The METU Biomat Center experience | |
| Nair | Novel preparation of polymeric scaffolds for tissue engineering using phase separation with protein microbubble incorporation | |
| HK1107024A (en) | Microspheres for active embolization |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20121029 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20121029 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20131224 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20131226 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20140303 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20140310 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20140902 |