JP2005502369A - 分画細胞計数方法ならびにそれを実行するための関連する装置およびソフトウェア - Google Patents
分画細胞計数方法ならびにそれを実行するための関連する装置およびソフトウェア Download PDFInfo
- Publication number
- JP2005502369A JP2005502369A JP2003527562A JP2003527562A JP2005502369A JP 2005502369 A JP2005502369 A JP 2005502369A JP 2003527562 A JP2003527562 A JP 2003527562A JP 2003527562 A JP2003527562 A JP 2003527562A JP 2005502369 A JP2005502369 A JP 2005502369A
- Authority
- JP
- Japan
- Prior art keywords
- survey data
- cells
- cell
- data
- counting
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 238000000034 method Methods 0.000 title claims abstract description 256
- 210000004027 cell Anatomy 0.000 claims abstract description 354
- 230000003287 optical effect Effects 0.000 claims abstract description 137
- 238000010606 normalization Methods 0.000 claims abstract description 38
- 238000001914 filtration Methods 0.000 claims abstract description 26
- 210000000265 leukocyte Anatomy 0.000 claims description 59
- 238000011156 evaluation Methods 0.000 claims description 57
- 210000004369 blood Anatomy 0.000 claims description 30
- 239000008280 blood Substances 0.000 claims description 30
- 238000005286 illumination Methods 0.000 claims description 19
- 238000005070 sampling Methods 0.000 claims description 17
- 230000003628 erosive effect Effects 0.000 claims description 11
- 238000009826 distribution Methods 0.000 claims description 10
- 230000005670 electromagnetic radiation Effects 0.000 claims description 9
- 206010064127 Solar lentigo Diseases 0.000 claims description 7
- 230000036541 health Effects 0.000 claims description 7
- 238000003491 array Methods 0.000 claims description 6
- 230000002708 enhancing effect Effects 0.000 claims description 5
- 238000001228 spectrum Methods 0.000 claims description 5
- 230000010339 dilation Effects 0.000 claims description 4
- 238000011068 loading method Methods 0.000 claims description 4
- 101000738771 Homo sapiens Receptor-type tyrosine-protein phosphatase C Proteins 0.000 claims description 3
- 102100037422 Receptor-type tyrosine-protein phosphatase C Human genes 0.000 claims description 3
- 238000009499 grossing Methods 0.000 claims description 2
- 238000007619 statistical method Methods 0.000 claims description 2
- 230000003190 augmentative effect Effects 0.000 claims 2
- 238000012952 Resampling Methods 0.000 claims 1
- 239000006260 foam Substances 0.000 claims 1
- 239000000523 sample Substances 0.000 abstract description 63
- 210000000601 blood cell Anatomy 0.000 abstract description 13
- 238000002156 mixing Methods 0.000 abstract description 12
- 238000003672 processing method Methods 0.000 abstract description 12
- 238000003384 imaging method Methods 0.000 abstract description 9
- 239000000427 antigen Substances 0.000 abstract description 8
- 102000036639 antigens Human genes 0.000 abstract description 8
- 108091007433 antigens Proteins 0.000 abstract description 8
- 239000006101 laboratory sample Substances 0.000 abstract description 8
- 230000027455 binding Effects 0.000 abstract description 3
- 238000000265 homogenisation Methods 0.000 abstract description 2
- 239000010410 layer Substances 0.000 description 103
- 238000003556 assay Methods 0.000 description 59
- 239000011324 bead Substances 0.000 description 48
- 230000008569 process Effects 0.000 description 48
- 238000004458 analytical method Methods 0.000 description 31
- 239000000758 substrate Substances 0.000 description 28
- 230000006870 function Effects 0.000 description 26
- 238000004422 calculation algorithm Methods 0.000 description 24
- 238000006911 enzymatic reaction Methods 0.000 description 20
- 210000003743 erythrocyte Anatomy 0.000 description 19
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 18
- 239000010931 gold Substances 0.000 description 18
- 229910052737 gold Inorganic materials 0.000 description 18
- 239000002244 precipitate Substances 0.000 description 18
- 238000012545 processing Methods 0.000 description 18
- 238000004820 blood count Methods 0.000 description 17
- 210000004698 lymphocyte Anatomy 0.000 description 16
- 210000001772 blood platelet Anatomy 0.000 description 14
- 239000000463 material Substances 0.000 description 14
- 239000000853 adhesive Substances 0.000 description 13
- 230000001070 adhesive effect Effects 0.000 description 13
- 238000010586 diagram Methods 0.000 description 13
- 239000012530 fluid Substances 0.000 description 13
- 238000012360 testing method Methods 0.000 description 12
- 102100036011 T-cell surface glycoprotein CD4 Human genes 0.000 description 11
- 230000005540 biological transmission Effects 0.000 description 11
- 210000005087 mononuclear cell Anatomy 0.000 description 11
- 239000004033 plastic Substances 0.000 description 10
- 230000008859 change Effects 0.000 description 9
- 238000006243 chemical reaction Methods 0.000 description 9
- 230000009466 transformation Effects 0.000 description 9
- 230000005859 cell recognition Effects 0.000 description 8
- 238000001514 detection method Methods 0.000 description 8
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 8
- 208000015181 infectious disease Diseases 0.000 description 8
- 230000036961 partial effect Effects 0.000 description 8
- 239000002953 phosphate buffered saline Substances 0.000 description 8
- 230000008901 benefit Effects 0.000 description 7
- 239000012472 biological sample Substances 0.000 description 7
- 230000001413 cellular effect Effects 0.000 description 7
- 239000011248 coating agent Substances 0.000 description 7
- 238000000576 coating method Methods 0.000 description 7
- 238000000605 extraction Methods 0.000 description 7
- 210000000440 neutrophil Anatomy 0.000 description 7
- 239000003795 chemical substances by application Substances 0.000 description 6
- 238000007405 data analysis Methods 0.000 description 6
- 210000003979 eosinophil Anatomy 0.000 description 6
- 230000000873 masking effect Effects 0.000 description 6
- 230000007246 mechanism Effects 0.000 description 6
- 210000001616 monocyte Anatomy 0.000 description 6
- 239000004417 polycarbonate Substances 0.000 description 6
- 229920000515 polycarbonate Polymers 0.000 description 6
- 239000000126 substance Substances 0.000 description 6
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 5
- 208000032843 Hemorrhage Diseases 0.000 description 5
- 239000004793 Polystyrene Substances 0.000 description 5
- 229910052782 aluminium Inorganic materials 0.000 description 5
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 5
- 230000000712 assembly Effects 0.000 description 5
- 238000000429 assembly Methods 0.000 description 5
- 210000003651 basophil Anatomy 0.000 description 5
- 230000000740 bleeding effect Effects 0.000 description 5
- 238000004364 calculation method Methods 0.000 description 5
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 5
- 230000009977 dual effect Effects 0.000 description 5
- 238000011049 filling Methods 0.000 description 5
- 229910052751 metal Inorganic materials 0.000 description 5
- 239000002184 metal Substances 0.000 description 5
- 229920002223 polystyrene Polymers 0.000 description 5
- 102000001554 Hemoglobins Human genes 0.000 description 4
- 108010054147 Hemoglobins Proteins 0.000 description 4
- 108010090804 Streptavidin Proteins 0.000 description 4
- 239000003146 anticoagulant agent Substances 0.000 description 4
- 229940127219 anticoagulant drug Drugs 0.000 description 4
- 238000005119 centrifugation Methods 0.000 description 4
- 239000003086 colorant Substances 0.000 description 4
- 238000012937 correction Methods 0.000 description 4
- 238000013480 data collection Methods 0.000 description 4
- 230000007547 defect Effects 0.000 description 4
- 201000010099 disease Diseases 0.000 description 4
- 230000000694 effects Effects 0.000 description 4
- 239000011521 glass Substances 0.000 description 4
- 230000003993 interaction Effects 0.000 description 4
- 230000001788 irregular Effects 0.000 description 4
- 239000012528 membrane Substances 0.000 description 4
- 238000002310 reflectometry Methods 0.000 description 4
- 238000000926 separation method Methods 0.000 description 4
- 239000000725 suspension Substances 0.000 description 4
- 102100024222 B-lymphocyte antigen CD19 Human genes 0.000 description 3
- 229920002307 Dextran Polymers 0.000 description 3
- 101000980825 Homo sapiens B-lymphocyte antigen CD19 Proteins 0.000 description 3
- 206010027146 Melanoderma Diseases 0.000 description 3
- 206010028980 Neoplasm Diseases 0.000 description 3
- 210000001744 T-lymphocyte Anatomy 0.000 description 3
- 230000002159 abnormal effect Effects 0.000 description 3
- 238000013459 approach Methods 0.000 description 3
- 210000001185 bone marrow Anatomy 0.000 description 3
- 230000010354 integration Effects 0.000 description 3
- 230000002452 interceptive effect Effects 0.000 description 3
- 238000011835 investigation Methods 0.000 description 3
- 208000032839 leukemia Diseases 0.000 description 3
- 230000007774 longterm Effects 0.000 description 3
- 239000000203 mixture Substances 0.000 description 3
- 239000002245 particle Substances 0.000 description 3
- 238000011160 research Methods 0.000 description 3
- 230000004044 response Effects 0.000 description 3
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 2
- 206010025323 Lymphomas Diseases 0.000 description 2
- 206010025327 Lymphopenia Diseases 0.000 description 2
- 208000014767 Myeloproliferative disease Diseases 0.000 description 2
- 208000005485 Thrombocytosis Diseases 0.000 description 2
- 238000012984 biological imaging Methods 0.000 description 2
- 210000001124 body fluid Anatomy 0.000 description 2
- 239000010839 body fluid Substances 0.000 description 2
- 230000005983 bone marrow dysfunction Effects 0.000 description 2
- 201000011510 cancer Diseases 0.000 description 2
- 239000003153 chemical reaction reagent Substances 0.000 description 2
- 238000002512 chemotherapy Methods 0.000 description 2
- 229920001577 copolymer Polymers 0.000 description 2
- 238000005520 cutting process Methods 0.000 description 2
- 230000003247 decreasing effect Effects 0.000 description 2
- 238000000151 deposition Methods 0.000 description 2
- 230000008021 deposition Effects 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- 238000002059 diagnostic imaging Methods 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 238000005194 fractionation Methods 0.000 description 2
- 238000005534 hematocrit Methods 0.000 description 2
- 239000000017 hydrogel Substances 0.000 description 2
- 230000003100 immobilizing effect Effects 0.000 description 2
- 230000001965 increasing effect Effects 0.000 description 2
- 238000007689 inspection Methods 0.000 description 2
- 238000002955 isolation Methods 0.000 description 2
- 231100001023 lymphopenia Toxicity 0.000 description 2
- 238000001465 metallisation Methods 0.000 description 2
- 229920000609 methyl cellulose Polymers 0.000 description 2
- 239000001923 methylcellulose Substances 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 208000004235 neutropenia Diseases 0.000 description 2
- 230000009871 nonspecific binding Effects 0.000 description 2
- 238000010422 painting Methods 0.000 description 2
- 239000008188 pellet Substances 0.000 description 2
- 238000000053 physical method Methods 0.000 description 2
- 239000002985 plastic film Substances 0.000 description 2
- 229920006255 plastic film Polymers 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 238000004080 punching Methods 0.000 description 2
- 230000008707 rearrangement Effects 0.000 description 2
- 238000009877 rendering Methods 0.000 description 2
- 238000007789 sealing Methods 0.000 description 2
- 239000007790 solid phase Substances 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- -1 ACD Chemical compound 0.000 description 1
- 208000030507 AIDS Diseases 0.000 description 1
- 206010003445 Ascites Diseases 0.000 description 1
- 102000004274 CCR5 Receptors Human genes 0.000 description 1
- 108010017088 CCR5 Receptors Proteins 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- 206010053567 Coagulopathies Diseases 0.000 description 1
- 102000015612 Complement 3b Receptors Human genes 0.000 description 1
- 108010024114 Complement 3b Receptors Proteins 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 102000008946 Fibrinogen Human genes 0.000 description 1
- 108010049003 Fibrinogen Proteins 0.000 description 1
- 229920001917 Ficoll Polymers 0.000 description 1
- 229920000084 Gum arabic Polymers 0.000 description 1
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 description 1
- 101000917826 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor II-a Proteins 0.000 description 1
- 101000917824 Homo sapiens Low affinity immunoglobulin gamma Fc region receptor II-b Proteins 0.000 description 1
- 101000581981 Homo sapiens Neural cell adhesion molecule 1 Proteins 0.000 description 1
- 101000934346 Homo sapiens T-cell surface antigen CD2 Proteins 0.000 description 1
- 101000716102 Homo sapiens T-cell surface glycoprotein CD4 Proteins 0.000 description 1
- 101000946843 Homo sapiens T-cell surface glycoprotein CD8 alpha chain Proteins 0.000 description 1
- 206010020751 Hypersensitivity Diseases 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 208000028622 Immune thrombocytopenia Diseases 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- 102000007351 Interleukin-2 Receptor alpha Subunit Human genes 0.000 description 1
- 108010032774 Interleukin-2 Receptor alpha Subunit Proteins 0.000 description 1
- 108010013709 Leukocyte Common Antigens Proteins 0.000 description 1
- 102000017095 Leukocyte Common Antigens Human genes 0.000 description 1
- 102100029204 Low affinity immunoglobulin gamma Fc region receptor II-a Human genes 0.000 description 1
- 201000007224 Myeloproliferative neoplasm Diseases 0.000 description 1
- 102100027347 Neural cell adhesion molecule 1 Human genes 0.000 description 1
- 208000008589 Obesity Diseases 0.000 description 1
- 206010033661 Pancytopenia Diseases 0.000 description 1
- 208000005228 Pericardial Effusion Diseases 0.000 description 1
- 206010037660 Pyrexia Diseases 0.000 description 1
- 241000978776 Senegalia senegal Species 0.000 description 1
- 241000519995 Stachys sylvatica Species 0.000 description 1
- 102100025237 T-cell surface antigen CD2 Human genes 0.000 description 1
- 102100034922 T-cell surface glycoprotein CD8 alpha chain Human genes 0.000 description 1
- 208000031981 Thrombocytopenic Idiopathic Purpura Diseases 0.000 description 1
- 230000005856 abnormality Effects 0.000 description 1
- 239000000205 acacia gum Substances 0.000 description 1
- 235000010489 acacia gum Nutrition 0.000 description 1
- 239000012790 adhesive layer Substances 0.000 description 1
- 230000002776 aggregation Effects 0.000 description 1
- 238000004220 aggregation Methods 0.000 description 1
- 230000007815 allergy Effects 0.000 description 1
- 239000012491 analyte Substances 0.000 description 1
- 239000000538 analytical sample Substances 0.000 description 1
- 230000000511 anti-eosinophil effect Effects 0.000 description 1
- 230000000781 anti-lymphocytic effect Effects 0.000 description 1
- 230000000702 anti-platelet effect Effects 0.000 description 1
- 238000000149 argon plasma sintering Methods 0.000 description 1
- 201000003710 autoimmune thrombocytopenic purpura Diseases 0.000 description 1
- 210000003719 b-lymphocyte Anatomy 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 239000013060 biological fluid Substances 0.000 description 1
- 239000012620 biological material Substances 0.000 description 1
- 238000001574 biopsy Methods 0.000 description 1
- 208000034158 bleeding Diseases 0.000 description 1
- 239000002981 blocking agent Substances 0.000 description 1
- 238000009582 blood typing Methods 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 210000004970 cd4 cell Anatomy 0.000 description 1
- 210000003855 cell nucleus Anatomy 0.000 description 1
- 230000007969 cellular immunity Effects 0.000 description 1
- 238000005234 chemical deposition Methods 0.000 description 1
- 238000003759 clinical diagnosis Methods 0.000 description 1
- 230000035602 clotting Effects 0.000 description 1
- 230000006835 compression Effects 0.000 description 1
- 238000007906 compression Methods 0.000 description 1
- 230000021615 conjugation Effects 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 238000013479 data entry Methods 0.000 description 1
- 238000011157 data evaluation Methods 0.000 description 1
- 230000000779 depleting effect Effects 0.000 description 1
- 230000006866 deterioration Effects 0.000 description 1
- 208000035475 disorder Diseases 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 238000002651 drug therapy Methods 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 239000000428 dust Substances 0.000 description 1
- 229940012952 fibrinogen Drugs 0.000 description 1
- 230000002496 gastric effect Effects 0.000 description 1
- 230000002068 genetic effect Effects 0.000 description 1
- 230000005484 gravity Effects 0.000 description 1
- 230000003862 health status Effects 0.000 description 1
- 229960002897 heparin Drugs 0.000 description 1
- 229920000669 heparin Polymers 0.000 description 1
- 230000004727 humoral immunity Effects 0.000 description 1
- 210000000987 immune system Anatomy 0.000 description 1
- 238000010820 immunofluorescence microscopy Methods 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 230000003834 intracellular effect Effects 0.000 description 1
- 238000009533 lab test Methods 0.000 description 1
- 238000010030 laminating Methods 0.000 description 1
- 201000002364 leukopenia Diseases 0.000 description 1
- 231100001022 leukopenia Toxicity 0.000 description 1
- 238000001459 lithography Methods 0.000 description 1
- 210000003563 lymphoid tissue Anatomy 0.000 description 1
- 230000036210 malignancy Effects 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 238000002483 medication Methods 0.000 description 1
- 238000007431 microscopic evaluation Methods 0.000 description 1
- 230000000877 morphologic effect Effects 0.000 description 1
- 239000013642 negative control Substances 0.000 description 1
- 235000020824 obesity Nutrition 0.000 description 1
- 238000012634 optical imaging Methods 0.000 description 1
- 244000045947 parasite Species 0.000 description 1
- 238000005192 partition Methods 0.000 description 1
- 244000052769 pathogen Species 0.000 description 1
- 210000004912 pericardial fluid Anatomy 0.000 description 1
- 229920002120 photoresistant polymer Polymers 0.000 description 1
- 239000000049 pigment Substances 0.000 description 1
- 210000004180 plasmocyte Anatomy 0.000 description 1
- 210000004910 pleural fluid Anatomy 0.000 description 1
- 239000013641 positive control Substances 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 238000001959 radiotherapy Methods 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 230000002829 reductive effect Effects 0.000 description 1
- 238000012958 reprocessing Methods 0.000 description 1
- 108091008146 restriction endonucleases Proteins 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 238000004062 sedimentation Methods 0.000 description 1
- 238000010187 selection method Methods 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 230000000391 smoking effect Effects 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000001509 sodium citrate Substances 0.000 description 1
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 125000006850 spacer group Chemical group 0.000 description 1
- 210000000952 spleen Anatomy 0.000 description 1
- 238000010186 staining Methods 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 238000005728 strengthening Methods 0.000 description 1
- 239000006228 supernatant Substances 0.000 description 1
- 230000008961 swelling Effects 0.000 description 1
- 206010043554 thrombocytopenia Diseases 0.000 description 1
- 201000003067 thrombocytopenia due to platelet alloimmunization Diseases 0.000 description 1
- 230000002992 thymic effect Effects 0.000 description 1
- 210000001541 thymus gland Anatomy 0.000 description 1
- 230000000451 tissue damage Effects 0.000 description 1
- 231100000827 tissue damage Toxicity 0.000 description 1
- 238000000844 transformation Methods 0.000 description 1
- 238000002834 transmittance Methods 0.000 description 1
- 230000005740 tumor formation Effects 0.000 description 1
- 230000000007 visual effect Effects 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
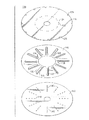
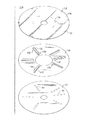
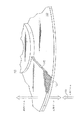
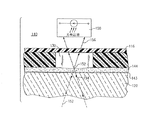
Images
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/5005—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells
- G01N33/5094—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving human or animal cells for blood cell populations
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N15/00—Investigating characteristics of particles; Investigating permeability, pore-volume or surface-area of porous materials
- G01N15/10—Investigating individual particles
- G01N15/14—Optical investigation techniques, e.g. flow cytometry
- G01N15/1429—Signal processing
- G01N15/1433—Signal processing using image recognition
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N35/00—Automatic analysis not limited to methods or materials provided for in any single one of groups G01N1/00 - G01N33/00; Handling materials therefor
- G01N35/00029—Automatic analysis not limited to methods or materials provided for in any single one of groups G01N1/00 - G01N33/00; Handling materials therefor provided with flat sample substrates, e.g. slides
- G01N35/00069—Automatic analysis not limited to methods or materials provided for in any single one of groups G01N1/00 - G01N33/00; Handling materials therefor provided with flat sample substrates, e.g. slides whereby the sample substrate is of the bio-disk type, i.e. having the format of an optical disk
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N15/00—Investigating characteristics of particles; Investigating permeability, pore-volume or surface-area of porous materials
- G01N15/10—Investigating individual particles
- G01N15/14—Optical investigation techniques, e.g. flow cytometry
- G01N2015/1486—Counting the particles
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Immunology (AREA)
- Engineering & Computer Science (AREA)
- Pathology (AREA)
- General Physics & Mathematics (AREA)
- General Health & Medical Sciences (AREA)
- Biochemistry (AREA)
- Analytical Chemistry (AREA)
- Physics & Mathematics (AREA)
- Biomedical Technology (AREA)
- Hematology (AREA)
- Molecular Biology (AREA)
- Cell Biology (AREA)
- Urology & Nephrology (AREA)
- Ecology (AREA)
- Dispersion Chemistry (AREA)
- Biotechnology (AREA)
- Tropical Medicine & Parasitology (AREA)
- Medicinal Chemistry (AREA)
- Food Science & Technology (AREA)
- Signal Processing (AREA)
- Microbiology (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Investigating Or Analysing Materials By Optical Means (AREA)
- Optical Measuring Cells (AREA)
- Automatic Analysis And Handling Materials Therefor (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
- Image Processing (AREA)
- Image Analysis (AREA)
Applications Claiming Priority (9)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US32286301P | 2001-09-12 | 2001-09-12 | |
| US35330002P | 2002-01-31 | 2002-01-31 | |
| US35392102P | 2002-01-31 | 2002-01-31 | |
| US35564402P | 2002-02-05 | 2002-02-05 | |
| US35530402P | 2002-02-08 | 2002-02-08 | |
| US35847902P | 2002-02-19 | 2002-02-19 | |
| US36394902P | 2002-03-12 | 2002-03-12 | |
| US40492102P | 2002-08-21 | 2002-08-21 | |
| PCT/US2002/028958 WO2003023571A2 (en) | 2001-09-12 | 2002-09-11 | Methods for differential cell counts including related apparatus and software for performing same |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2005502369A true JP2005502369A (ja) | 2005-01-27 |
| JP2005502369A5 JP2005502369A5 (enExample) | 2006-01-05 |
Family
ID=27575386
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2003527562A Pending JP2005502369A (ja) | 2001-09-12 | 2002-09-11 | 分画細胞計数方法ならびにそれを実行するための関連する装置およびソフトウェア |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US20030096324A1 (enExample) |
| EP (1) | EP1425696A2 (enExample) |
| JP (1) | JP2005502369A (enExample) |
| CN (1) | CN1575475A (enExample) |
| AU (1) | AU2002333589A1 (enExample) |
| WO (1) | WO2003023571A2 (enExample) |
Cited By (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2008249695A (ja) * | 2007-03-07 | 2008-10-16 | Canon Inc | 画像形成装置、及び画像形成方法 |
| JP2012531608A (ja) * | 2009-10-30 | 2012-12-10 | シーメンス アクチエンゲゼルシヤフト | 体液分析システムならびに体液分析のための画像処理装置および方法 |
| JP2013040772A (ja) * | 2011-08-11 | 2013-02-28 | Jvc Kenwood Corp | 光学的分析装置、光学的分析方法 |
| JP5630674B2 (ja) * | 2010-09-30 | 2014-11-26 | 日本電気株式会社 | 情報処理装置、情報処理システム、情報処理方法、プログラム及び記録媒体 |
| JPWO2016121065A1 (ja) * | 2015-01-29 | 2017-11-02 | オリンパス株式会社 | 細胞解析装置および方法 |
| JP2018512566A (ja) * | 2015-02-17 | 2018-05-17 | シーメンス・ヘルスケア・ダイアグノスティックス・インコーポレーテッドSiemens Healthcare Diagnostics Inc. | 検査室自動化のためのトップビューサンプルチューブ画像からのバーコードタグ状態の分類 |
| JP2018136297A (ja) * | 2017-02-22 | 2018-08-30 | 株式会社Jvcケンウッド | 分析装置及び分析方法 |
| US10438047B2 (en) | 2015-04-07 | 2019-10-08 | Olympus Corporation | Cell analysis device and cell analysis method |
| JP2023050994A (ja) * | 2021-09-30 | 2023-04-11 | 株式会社プロテリアル | 窒化珪素基板の評価方法、窒化珪素基板の評価装置、窒化珪素基板の評価システム、窒化珪素基板の製造方法、及びパワーモジュールの製造方法 |
| US12306086B2 (en) | 2019-11-22 | 2025-05-20 | Evident Corporation | Method for displaying cell count information, system, and computer-readable medium |
Families Citing this family (25)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2004106925A2 (en) * | 2003-03-03 | 2004-12-09 | Nagaoka & Co.Ltd. | Methods and apparatus for use in detection and quantitation of various cell types and use of optical bio-disc for performing same |
| US7471393B2 (en) * | 2004-03-06 | 2008-12-30 | Michael Trainer | Methods and apparatus for determining the size and shape of particles |
| US9297737B2 (en) | 2004-03-06 | 2016-03-29 | Michael Trainer | Methods and apparatus for determining characteristics of particles |
| WO2006017642A2 (en) * | 2004-08-04 | 2006-02-16 | Agilent Technologies, Inc. | Filtering of signals during array scanning |
| JP5058815B2 (ja) * | 2004-11-24 | 2012-10-24 | バッテル メモリアル インスティチュート | 細胞撮像用の光学システム |
| JP2007080327A (ja) * | 2005-09-13 | 2007-03-29 | Hitachi Ltd | 記録媒体欠陥検査装置及び欠陥検査方法 |
| US7518723B2 (en) * | 2005-09-19 | 2009-04-14 | Jmar Technologies, Inc. | Systems and methods for detecting radiation, biotoxin, chemical, and biological warfare agents using a multiple angle light scattering (MALS) instrument |
| US8609434B2 (en) * | 2008-02-14 | 2013-12-17 | Samsung Electronics Co., Ltd. | Bio-disc reading apparatus and assay method using same |
| EP2593771B1 (en) * | 2010-07-16 | 2019-09-04 | Luminex Corporation | Methods, storage mediums, and systems for analyzing particle quantity and distribution within an imaging region of an assay analysis system and for evaluating the performance of a focusing routing performed on an assay analysis system |
| US10114020B2 (en) | 2010-10-11 | 2018-10-30 | Mbio Diagnostics, Inc. | System and device for analyzing a fluidic sample |
| US9506935B2 (en) | 2012-06-01 | 2016-11-29 | Commissariat à l'énergie atomique et aux énergies alternatives | Method and system for estimating the quantity of an analyte contained in a liquid |
| FR2991457B1 (fr) * | 2012-06-01 | 2014-07-18 | Commissariat Energie Atomique | Procede et systeme de caracterisation de la vitesse de deplacement de particules contenues dans un liquide, telles que des particules sanguines |
| EP2911791A4 (en) | 2012-10-29 | 2016-11-02 | Mbio Diagnostics Inc | BIOPARTICLE IDENTIFICATION SYSTEM, CARTRIDGE AND RELATED METHODS |
| JP6427108B2 (ja) | 2012-12-31 | 2018-11-21 | ベックマン コールター, インコーポレイテッド | 凝集塊調節を伴う血小板計数のためのシステム及び方法 |
| JP6237417B2 (ja) * | 2014-03-31 | 2017-11-29 | 株式会社Jvcケンウッド | 分析装置及び分析方法 |
| CN104951782A (zh) * | 2015-07-01 | 2015-09-30 | 携程计算机技术(上海)有限公司 | 图像识别的背景过滤方法及系统 |
| CN106085829B (zh) * | 2016-07-06 | 2018-02-16 | 上海海洋大学 | 一种卵母细胞集聚器 |
| CN106644897A (zh) * | 2016-10-14 | 2017-05-10 | 北京海岸鸿蒙标准物质技术有限责任公司 | 一种用于颗粒计数标准物质的计数装置 |
| US10809178B2 (en) * | 2017-02-22 | 2020-10-20 | JVC Kenwood Corporation | Analysis device and analysis method |
| CN107123102A (zh) * | 2017-05-24 | 2017-09-01 | 天津工业大学 | 一种贴壁细胞生长融合度自动分析方法 |
| CN108169081B (zh) * | 2017-12-14 | 2021-06-04 | 四川大学华西医院 | 血细胞分析的差值核查方法及其应用方法 |
| CN109085332A (zh) * | 2018-09-29 | 2018-12-25 | 厦门大学 | 一种动态数据采集装置 |
| CN111539354B (zh) * | 2020-04-27 | 2020-12-15 | 易普森智慧健康科技(深圳)有限公司 | 一种液基细胞玻片扫描区域识别方法 |
| CN111832389B (zh) * | 2020-05-25 | 2022-12-09 | 中国人民解放军陆军军医大学第二附属医院 | 一种骨髓细胞形态学自动检测系统的计数及分析方法 |
| CN113554690B (zh) * | 2021-07-20 | 2024-04-05 | 北京邮电大学 | 一种基于归一化卷积的细胞膜等效半径识别方法 |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5952359A (ja) * | 1982-09-02 | 1984-03-26 | Hitachi Medical Corp | 画像間演算時の画像歪み自動補正装置 |
| JPH03255365A (ja) * | 1989-10-02 | 1991-11-14 | Louis A Kamentsky | 生物標本の複数の光学的特性を測定する方法および装置 |
| JPH05215750A (ja) * | 1992-02-06 | 1993-08-24 | Idemitsu Petrochem Co Ltd | 免疫学的分析方法 |
| JPH09503868A (ja) * | 1993-07-09 | 1997-04-15 | コンピュサイト コーポレイション | コンピュータ化された顕微鏡標本エンコーダ |
| JPH09173300A (ja) * | 1995-10-25 | 1997-07-08 | Toa Medical Electronics Co Ltd | 血液分析装置 |
| JPH10275150A (ja) * | 1997-03-28 | 1998-10-13 | Toa Medical Electronics Co Ltd | 画像ファイリングシステム |
| JPH11133021A (ja) * | 1997-10-28 | 1999-05-21 | Olympus Optical Co Ltd | 走査型サイトメータ |
Family Cites Families (41)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3736432A (en) * | 1971-03-22 | 1973-05-29 | Varian Associates | Bacterial colony counting method and apparatus |
| US3710323A (en) * | 1971-12-13 | 1973-01-09 | Ibm | Pattern-size normalizing for recognition apparatus |
| CA923621A (en) * | 1972-10-16 | 1973-03-27 | W. Moll Edward | Multi-stage queuer system |
| US3966322A (en) * | 1973-11-08 | 1976-06-29 | Vickers Limited | Device for use in producing a scanning beam of radiation and apparatus for use in investigating specimens |
| US4199748A (en) * | 1976-11-01 | 1980-04-22 | Rush-Presbyterian-St. Luke's Medical Center | Automated method and apparatus for classification of cells with application to the diagnosis of anemia |
| US4209548A (en) * | 1976-11-01 | 1980-06-24 | Rush-Presbyterian-St. Luke's Medical Center | Method for the preparation of blood samples for automated analysis |
| FR2535058B1 (fr) * | 1982-10-21 | 1987-08-21 | Materiel Biomedical | Dispositif pour la detection et la quantification d'agglutinats |
| FR2545610B1 (fr) * | 1983-05-02 | 1989-04-21 | Materiel Biomedical | Procede et dispositif pour la detection et la quantification d'agglutinats |
| US5112134A (en) * | 1984-03-01 | 1992-05-12 | Molecular Devices Corporation | Single source multi-site photometric measurement system |
| US4790640A (en) * | 1985-10-11 | 1988-12-13 | Nason Frederic L | Laboratory slide |
| US5087556A (en) * | 1989-05-17 | 1992-02-11 | Actimed Laboratories, Inc. | Method for quantitative analysis of body fluid constituents |
| US5143854A (en) * | 1989-06-07 | 1992-09-01 | Affymax Technologies N.V. | Large scale photolithographic solid phase synthesis of polypeptides and receptor binding screening thereof |
| JP3036049B2 (ja) * | 1990-10-31 | 2000-04-24 | スズキ株式会社 | 粒子凝集パターン判定方法 |
| US5548661A (en) * | 1991-07-12 | 1996-08-20 | Price; Jeffrey H. | Operator independent image cytometer |
| US6192320B1 (en) * | 1991-07-30 | 2001-02-20 | The University Of Virginia Patent Foundation | Interactive remote sample analysis system |
| US5366896A (en) * | 1991-07-30 | 1994-11-22 | University Of Virginia Alumni Patents Foundation | Robotically operated laboratory system |
| USH1183H (en) * | 1991-12-19 | 1993-05-04 | Laser scanner method for determining number and size of particles | |
| US5329461A (en) * | 1992-07-23 | 1994-07-12 | Acrogen, Inc. | Digital analyte detection system |
| WO1994007138A1 (en) * | 1992-09-14 | 1994-03-31 | Fodstad Oystein | Detection of specific target cells in specialized or mixed cell population and solutions containing mixed cell populations |
| US5747265A (en) * | 1992-10-30 | 1998-05-05 | T Cell Diagnostics, Inc. | Method for measuring the amount of a cell-associated molecule |
| US5478750A (en) * | 1993-03-31 | 1995-12-26 | Abaxis, Inc. | Methods for photometric analysis |
| US5793969A (en) * | 1993-07-09 | 1998-08-11 | Neopath, Inc. | Network review and analysis of computer encoded slides |
| US5537669A (en) * | 1993-09-30 | 1996-07-16 | Kla Instruments Corporation | Inspection method and apparatus for the inspection of either random or repeating patterns |
| EP0688113A2 (en) * | 1994-06-13 | 1995-12-20 | Sony Corporation | Method and apparatus for encoding and decoding digital audio signals and apparatus for recording digital audio |
| US6143510A (en) * | 1994-07-29 | 2000-11-07 | Iatron Laboratories Inc. | Measuring method using whole blood sample |
| US5978497A (en) * | 1994-09-20 | 1999-11-02 | Neopath, Inc. | Apparatus for the identification of free-lying cells |
| GB9418981D0 (en) * | 1994-09-21 | 1994-11-09 | Univ Glasgow | Apparatus and method for carrying out analysis of samples |
| US6327031B1 (en) * | 1998-09-18 | 2001-12-04 | Burstein Technologies, Inc. | Apparatus and semi-reflective optical system for carrying out analysis of samples |
| US5795716A (en) * | 1994-10-21 | 1998-08-18 | Chee; Mark S. | Computer-aided visualization and analysis system for sequence evaluation |
| US5585069A (en) * | 1994-11-10 | 1996-12-17 | David Sarnoff Research Center, Inc. | Partitioned microelectronic and fluidic device array for clinical diagnostics and chemical synthesis |
| US6143247A (en) * | 1996-12-20 | 2000-11-07 | Gamera Bioscience Inc. | Affinity binding-based system for detecting particulates in a fluid |
| US5871697A (en) * | 1995-10-24 | 1999-02-16 | Curagen Corporation | Method and apparatus for identifying, classifying, or quantifying DNA sequences in a sample without sequencing |
| US5741213A (en) * | 1995-10-25 | 1998-04-21 | Toa Medical Electronics Co., Ltd. | Apparatus for analyzing blood |
| US5798994A (en) * | 1996-01-16 | 1998-08-25 | Kamatani; Yasuo | Multi-layered optical disk reading method using liquid crystal diffraction device |
| DE69729101T2 (de) * | 1996-06-07 | 2005-05-12 | Immunivest Corp., Wilmington | Magnetische trennung mit hilfe von externen und internen gradienten |
| JP3679512B2 (ja) * | 1996-07-05 | 2005-08-03 | キヤノン株式会社 | 画像抽出装置および方法 |
| RO119751B1 (ro) * | 1997-02-28 | 2005-02-28 | Burstein Laboratories, Inc. | Disc optic, aparat pentru efectuarea unui control optic, metodă pentru detectarea prezenţei sau absenţei unui analit într-o probă şi metodă de control al unei probe bioligice, chimice sau biochimice |
| US6466695B1 (en) * | 1999-08-04 | 2002-10-15 | Eyematic Interfaces, Inc. | Procedure for automatic analysis of images and image sequences based on two-dimensional shape primitives |
| US6203992B1 (en) * | 1999-10-15 | 2001-03-20 | Abbott Laboratories | Nucleic acid primers and probes for detecting tumor cells |
| US6734401B2 (en) * | 2000-06-28 | 2004-05-11 | 3M Innovative Properties Company | Enhanced sample processing devices, systems and methods |
| US7087203B2 (en) * | 2000-11-17 | 2006-08-08 | Nagaoka & Co., Ltd. | Methods and apparatus for blood typing with optical bio-disc |
-
2002
- 2002-09-11 US US10/241,512 patent/US20030096324A1/en not_active Abandoned
- 2002-09-11 JP JP2003527562A patent/JP2005502369A/ja active Pending
- 2002-09-11 AU AU2002333589A patent/AU2002333589A1/en not_active Abandoned
- 2002-09-11 CN CN02820990.7A patent/CN1575475A/zh active Pending
- 2002-09-11 WO PCT/US2002/028958 patent/WO2003023571A2/en not_active Ceased
- 2002-09-11 EP EP02798217A patent/EP1425696A2/en not_active Withdrawn
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5952359A (ja) * | 1982-09-02 | 1984-03-26 | Hitachi Medical Corp | 画像間演算時の画像歪み自動補正装置 |
| JPH03255365A (ja) * | 1989-10-02 | 1991-11-14 | Louis A Kamentsky | 生物標本の複数の光学的特性を測定する方法および装置 |
| JPH05215750A (ja) * | 1992-02-06 | 1993-08-24 | Idemitsu Petrochem Co Ltd | 免疫学的分析方法 |
| JPH09503868A (ja) * | 1993-07-09 | 1997-04-15 | コンピュサイト コーポレイション | コンピュータ化された顕微鏡標本エンコーダ |
| JPH09173300A (ja) * | 1995-10-25 | 1997-07-08 | Toa Medical Electronics Co Ltd | 血液分析装置 |
| JPH10275150A (ja) * | 1997-03-28 | 1998-10-13 | Toa Medical Electronics Co Ltd | 画像ファイリングシステム |
| JPH11133021A (ja) * | 1997-10-28 | 1999-05-21 | Olympus Optical Co Ltd | 走査型サイトメータ |
Cited By (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2008249695A (ja) * | 2007-03-07 | 2008-10-16 | Canon Inc | 画像形成装置、及び画像形成方法 |
| JP2012531608A (ja) * | 2009-10-30 | 2012-12-10 | シーメンス アクチエンゲゼルシヤフト | 体液分析システムならびに体液分析のための画像処理装置および方法 |
| JP5630674B2 (ja) * | 2010-09-30 | 2014-11-26 | 日本電気株式会社 | 情報処理装置、情報処理システム、情報処理方法、プログラム及び記録媒体 |
| US9704018B2 (en) | 2010-09-30 | 2017-07-11 | Nec Corporation | Information processing apparatus, information processing system, information processing method, program, and recording medium |
| JP2013040772A (ja) * | 2011-08-11 | 2013-02-28 | Jvc Kenwood Corp | 光学的分析装置、光学的分析方法 |
| JPWO2016121065A1 (ja) * | 2015-01-29 | 2017-11-02 | オリンパス株式会社 | 細胞解析装置および方法 |
| JP2018512566A (ja) * | 2015-02-17 | 2018-05-17 | シーメンス・ヘルスケア・ダイアグノスティックス・インコーポレーテッドSiemens Healthcare Diagnostics Inc. | 検査室自動化のためのトップビューサンプルチューブ画像からのバーコードタグ状態の分類 |
| US10325182B2 (en) | 2015-02-17 | 2019-06-18 | Siemens Healthcare Diagnostics Inc. | Classification of barcode tag conditions from top view sample tube images for laboratory automation |
| US10438047B2 (en) | 2015-04-07 | 2019-10-08 | Olympus Corporation | Cell analysis device and cell analysis method |
| JP2018136297A (ja) * | 2017-02-22 | 2018-08-30 | 株式会社Jvcケンウッド | 分析装置及び分析方法 |
| US12306086B2 (en) | 2019-11-22 | 2025-05-20 | Evident Corporation | Method for displaying cell count information, system, and computer-readable medium |
| JP2023050994A (ja) * | 2021-09-30 | 2023-04-11 | 株式会社プロテリアル | 窒化珪素基板の評価方法、窒化珪素基板の評価装置、窒化珪素基板の評価システム、窒化珪素基板の製造方法、及びパワーモジュールの製造方法 |
| JP7760882B2 (ja) | 2021-09-30 | 2025-10-28 | 株式会社プロテリアル | 窒化珪素基板の評価方法、窒化珪素基板の評価装置、窒化珪素基板の評価システム、窒化珪素基板の製造方法、及びパワーモジュールの製造方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| AU2002333589A1 (en) | 2003-03-24 |
| EP1425696A2 (en) | 2004-06-09 |
| WO2003023571A2 (en) | 2003-03-20 |
| WO2003023571A3 (en) | 2004-03-04 |
| US20030096324A1 (en) | 2003-05-22 |
| CN1575475A (zh) | 2005-02-02 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2005502369A (ja) | 分画細胞計数方法ならびにそれを実行するための関連する装置およびソフトウェア | |
| JP5734838B2 (ja) | 分析チャンバ内に置かれた試料の面積を計測するための方法 | |
| EP2274612B1 (en) | Virtual separation of bound and free label in a ligand assay for performing immunoassays of biological fluids, including whole blood | |
| EP2519820B1 (en) | Method and apparatus for determining mean cell volume of red blood cells | |
| US7336812B2 (en) | System for microvolume laser scanning cytometry | |
| US7428200B2 (en) | Method for triggering through disc grooves and related optical analysis discs and system | |
| US20030104486A1 (en) | Methods and apparatus for detecting and quantifying lymphocytes with optical biodiscs | |
| JP2005502872A (ja) | 光バイオディスクシステムを使用した、核の形態に基づく白血球の型の識別および定量 | |
| JP2005509882A (ja) | 細胞分析のための光バイオディスクおよび流体回路ならびにそれに関連する方法 | |
| JP2005517154A (ja) | 細胞の定性および定量分析法ならびに関連する光バイオディスクシステム | |
| JP2007501407A (ja) | 多様な細胞タイプの検出および定量に使用される方法および装置並びにこれを行うための光バイオディスクの使用 | |
| US20050002827A1 (en) | Optical discs including equi-radial and/or spiral analysis zones and related disc drive systems and methods | |
| US20030077627A1 (en) | Transmissive optical disc assemblies for performing physical measurements and methods relating thereto | |
| WO2001008081A1 (en) | System for microvolume laser scanning cytometry | |
| JP2005509865A (ja) | 光バイオディスクを用いた血液タイピング(bloodtyping)の方法および装置 | |
| JP2005523684A (ja) | バイオドライブ用のセグメント領域検出器とその関連方法 | |
| US20050023765A1 (en) | Bio-safety features for optical analysis disc and disc system including same | |
| US20030143637A1 (en) | Capture layer assemblies for cellular assays including related optical analysis discs and methods |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A711 | Notification of change in applicant |
Free format text: JAPANESE INTERMEDIATE CODE: A711 Effective date: 20050526 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20050912 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20050912 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20080909 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20090224 |