EP2260124B1 - Elektrolysezelle zur chlorwasserstoffelektrolyse - Google Patents
Elektrolysezelle zur chlorwasserstoffelektrolyse Download PDFInfo
- Publication number
- EP2260124B1 EP2260124B1 EP09724561.7A EP09724561A EP2260124B1 EP 2260124 B1 EP2260124 B1 EP 2260124B1 EP 09724561 A EP09724561 A EP 09724561A EP 2260124 B1 EP2260124 B1 EP 2260124B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- nitrogen
- layer
- carbon nanotubes
- cathode
- weight
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Not-in-force
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B1/00—Electrolytic production of inorganic compounds or non-metals
- C25B1/01—Products
- C25B1/24—Halogens or compounds thereof
- C25B1/26—Chlorine; Compounds thereof
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B11/00—Electrodes; Manufacture thereof not otherwise provided for
- C25B11/04—Electrodes; Manufacture thereof not otherwise provided for characterised by the material
- C25B11/051—Electrodes formed of electrocatalysts on a substrate or carrier
- C25B11/055—Electrodes formed of electrocatalysts on a substrate or carrier characterised by the substrate or carrier material
- C25B11/057—Electrodes formed of electrocatalysts on a substrate or carrier characterised by the substrate or carrier material consisting of a single element or compound
- C25B11/065—Carbon
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B11/00—Electrodes; Manufacture thereof not otherwise provided for
- C25B11/04—Electrodes; Manufacture thereof not otherwise provided for characterised by the material
- C25B11/051—Electrodes formed of electrocatalysts on a substrate or carrier
- C25B11/073—Electrodes formed of electrocatalysts on a substrate or carrier characterised by the electrocatalyst material
- C25B11/091—Electrodes formed of electrocatalysts on a substrate or carrier characterised by the electrocatalyst material consisting of at least one catalytic element and at least one catalytic compound; consisting of two or more catalytic elements or catalytic compounds
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B15/00—Operating or servicing cells
- C25B15/02—Process control or regulation
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B15/00—Operating or servicing cells
- C25B15/08—Supplying or removing reactants or electrolytes; Regeneration of electrolytes
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25B—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES FOR THE PRODUCTION OF COMPOUNDS OR NON-METALS; APPARATUS THEREFOR
- C25B9/00—Cells or assemblies of cells; Constructional parts of cells; Assemblies of constructional parts, e.g. electrode-diaphragm assemblies; Process-related cell features
- C25B9/17—Cells comprising dimensionally-stable non-movable electrodes; Assemblies of constructional parts thereof
- C25B9/19—Cells comprising dimensionally-stable non-movable electrodes; Assemblies of constructional parts thereof with diaphragms
Definitions
- the invention relates to a device for hydrogen chloride electrolysis comprising an oxygen-consuming gas diffusion electrode based on nitrogen-doped carbon nanotubes ( NCNT ).
- NCNT nitrogen-doped carbon nanotubes
- Such oxygen-consuming gas diffusion electrodes often use catalysts to lower the necessary cell voltage.
- These catalysts in many cases comprise noble metals, noble metal salts or noble metal compounds such as platinum or rhodium, so that the catalysts are generally very expensive.
- a catalyst comprising rhodium sulfide (RhS x ) with which oxygen can be reduced is disclosed.
- the catalyst is applied to a conductive mesh, optionally together with a binder, thus forming an electrode suitable for the reduction of oxygen under application of a voltage.
- Rhodium is a rare and therefore expensive material, so that the use of the disclosed electrodes to the same economic disadvantages, such as those based on other precious metals.
- Another disadvantage of the rhodium-based electrodes is their property on the cathode side, that at high current densities, the selectivity for the oxygen reduction decreases and hydrogen can be formed as a by-product. This limits the technically achievable current density at which the oxygen reduction at the electrode can still be operated safely.
- noble metal catalysts are also disadvantageous because during operation of electrodes in connection with the hydrogen chloride electrolysis contact of the catalyst with chlorine and / or hydrochloric acid can not be reliably prevented on the cathode side and said materials on contact with chlorine and / or hydrochloric acid salts form, which can be washed out of the electrode material.
- the performance of the electrodes may deteriorate with the duration of operation, and the life of the electrodes is limited due to the consumption of catalyst material.
- WO 2005/035841 discloses a method for producing nitrogen-doped carbon nanotubes on a conductive surface, wherein the nitrogen-doped carbon nanotubes are deposited directly from a gas phase. This results in electrodes that can be used for oxygen reduction in batteries or fuel cells.
- the disclosed nitrogen-doped carbon nanotubes circumvent the need to use expensive noble or transition metals as catalysts.
- It is therefore an object to provide a device for hydrogen chloride electrolysis comprising an oxygen-consuming gas diffusion electrode which largely or completely dispenses with the use of expensive noble and / or transition metals and which comprises catalytic materials which are not consumed or inactivated in the course of operation, as well as over known materials an increased selectivity for the oxygen reduction at the Have electrode.
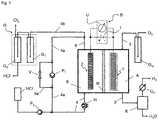
- a device for hydrogen chloride electrolysis comprises an electrode space ( A ) with an electrode ( 1 ) having a core ( 1a ) on which a layer ( 1b ) at least comprising a proportion of nitrogen-doped carbon nanotubes ( NCNT ). is applied and a further electrode space ( B ) with an electrode ( 2 ), wherein electrode space ( A ) and electrode space ( B ) by a membrane ( M ) are separated and the electrodes ( 1 and 2 ) via a power supply ( S ) electrically are conductively connected with each other, can solve this task.
- the invention particularly relates to a device for the electrolysis of hydrogen chloride gas or hydrogen chloride-containing solutions, in particular for the hydrochloric acid electrolysis with an HCl concentration in the range of 10 to 25 wt .-%, with an electrode space A with a cathode ( 1 ) and another electrode space ( B ) with an anode ( 2 ), wherein electrode space ( A ) and electrode space ( B ) are separated by an ion-conducting membrane ( M ) and the electrodes are electrically conductively connected to a power supply ( S ), characterized in that the cathode ( 1 ) has an electrically conductive core ( 1a ), on which a layer ( 1b ) at least comprising a proportion of nitrogen-doped carbon nanotubes and at least one perfluorinated polymer, and optionally a further layer ( 1c ) between core ( 1a ) and layer ( 1b ), is applied, wherein the nitrogen-doped carbon nanotubes have functional groups, the nitrogen
- the carbon nanotubes used according to the invention which have functional groups which contain nitrogen, are also referred to for short as nitrogen-doped carbon nanotubes.
- a preferred embodiment of the device is characterized in that electrode space ( A ) (cathode space) is provided with a feed line ( 3 ) for an aqueous and oxygen gas-containing electrolyte solution or for air or oxygen-containing gases.
- the electrode space ( B ) is preferably provided with a feed line ( 4 ) for hydrochloric acid or hydrogen chloride-containing gas.
- the catalyst for the preparation of the nitrogen-doped carbon nanotubes is based on manganese, cobalt, Al 2 O 3 and MgO, wherein Mn is present in a mass fraction of 2 to 65% and Co is present in a mass fraction of 2 to 80%, Al 2 O. 3 is present in a mass fraction of 5 to 75% and MgO is present in a mass fraction of 5 to 70%.
- a preferred form of the device is characterized in that the cathode ( 1 ) is electrically connected to a power distributor which is made of one or more materials selected from the list consisting of copper, graphite, titanium, noble metal-containing titanium alloy, in particular TiPd, and the Ni alloys Hastelloy and Incolloy is constructed.
- the layer ( 1b ) comprises a binder, in particular a binder based on fluorine-containing polymers, preferably PTFE.
- the layer ( 1b ) comprises at least 10% by weight of nitrogen-doped carbon nanotubes, more preferably at least 20% by weight, more preferably at least 40% by weight, very particularly preferably at least 60% by weight.
- the nitrogen-doped Kohlenstoffuanorschreibchen preferably contain a proportion of nitrogen of at least 1 wt .-%, preferably at least 3 wt .-%, particularly preferably at least 5 wt .-%.
- the layer thickness of the layer ( 1b ) is preferably at most 200 ⁇ m, preferably 1 ⁇ m to 150 ⁇ m, particularly preferably 10 ⁇ m to 100 ⁇ m.
- the ion-conducting membrane ( M ) is preferably a polymer membrane, more preferably the polymer membrane is based on polymeric perfluorosulfonic acids.
- the ion-conducting membrane ( M ) and the layer ( 1b ) of the cathode ( 1 ) have direct contact.
- a gas diffusion layer (1c) as a further layer particularly preferably, the further layer comprises (1c) at least one electrically conductive material, in particular graphite, and a hydrophobic material, especially PTFE.
- the invention also provides a process for hydrogen chloride electrolysis carried out in a device according to the invention.
- Electrode space ( A ) can be filled with an electrolyte solution comprising dissolved oxygen or with gas.
- electrode space ( A ) is filled with oxygen-containing gas.
- oxygen-containing gas Particularly preferably, pure oxygen or oxygen-air mixtures are supplied to the electrode space ( A ).
- the electrode space ( B ) is usually an electrolyte solution comprising hydrogen chloride or a gas comprising hydrogen chloride.
- Electrolytic solutions in the context of the present invention designate all solutions whose solvent is water and which comprise at least other ions as H + , H 3 O + and OH-. This is characterized by a higher specific conductivity than that of pure water.
- Non-conclusive examples are aqueous solutions of NaCl, MgCl 2 , but also acids which are soluble in water or miscible with it, such as H 2 SO 4 , HCl, etc.
- the core ( 1a ) of the cathode ( 1 ) according to the invention is usually used in the form of a rod, a plate, a mesh, mesh, non-woven or a fabric.
- the core ( 1a ) of the cathode ( 1 ) When the core ( 1a ) of the cathode ( 1 ) is used in the form of a rod or a plate, the core ( 1a ) may be porous or non-porous.
- the core ( 1a ) of the cathode ( 1 ) preferably has the form of a net, grid, fleece or fabric.
- the core ( 1a ) of the cathode ( 1 ) according to the invention is usually made of an electrically conductive material, which is preferably chemically stable to the electrolyte solutions comprising hydrogen chloride.
- a material is referred to that undergoes no chemical reaction with the surrounding electrolyte solutions comprising hydrogen chloride under the operating conditions of the device.
- Preferred electrically conductive, chemically stable materials are carbon black, graphite or coated metals.
- metals for example, titanium or titanium alloys, or the special metal alloys, which are known to the skilled worker under the name Hastelloy and Incolloy, are used.
- core (1a) of the cathode (1) are materials selected from the list graphite, titanium, titanium alloy, or the special metal alloys and Hastelloy Incolloy.
- the core ( 1a ) of the cathode ( 1 ) can also be a coated core ( 1a ').
- Possible coated cores ( 1a ') comprise the above-described core ( 1a ) and a coating of a transition metal transition metal oxide or transition metal mixed oxide having atomic numbers of 21 to 30 and / or transition metals having atomic numbers of 39 to 48 and / or transition metals atomic numbers 57 to 80. Preferred from the transition metals iridium and / or ruthenium and / or titanium.
- the layer ( 1b ) according to the invention is usually between 10 ⁇ m and 3 mm thick.
- the layer ( 1b ) is preferably between 30 ⁇ m and 1 mm thick.
- the layer ( 1b ) according to the invention comprises not only the proportion of nitrogen-doped carbon nanotubes ( NCNT ) but also a proportion of binder, and optionally a proportion of at least one metal.
- the binder is a perfluorinated polymer such as polytetrafluoroethylene. Preference is given to using proton-conducting polymers, such as polymeric perfluorosulfonic acids, for example the Nafion polymer marketed by DuPont.
- the nitrogen-doped carbon nanotubes ( NCNT ) may be present as such, or on a support in the layer. Should the nitrogen-doped carbon nanotubes ( NCNT ) are used on carriers, carriers with a high specific surface area, such as, for example, small-particle graphite, activated carbon, carbon black, etc., are preferred.
- the content of the nitrogen-doped carbon nanotubes ( NCNT ) in the layer ( 1b ) of the cathode ( 1 ) is usually at least 20% by weight. A proportion of at least 40% by weight, more preferably of at least 50% by weight, is preferred.
- Nitrogen-doped carbon nanotubes according to the invention are usually carbon nanotubes which comprise at least a proportion of 1% by weight of nitrogen.
- the nitrogen-doped carbon nanotubes comprise at least 3% by weight of nitrogen; more preferably at least 5% by weight of nitrogen.
- a low level of nitrogen causes the electrode potential to increase, requiring more electrical power to operate the device. More power is again economically disadvantageous.
- the metal is usually one of the metals selected from the list rhodium, platinum, iridum, rhenium, ruthenium and palladium, their sulfides and oxides, and mixed phases, in particular with molybdenum and / or selenium , Preference is given to a compound of ruthenium and selenium, particularly preferably rhodium sulfide (Rhl7S 15).
- the anode ( 2 ) according to the invention can consist of titanium or titanium alloys, for example titanium-palladium, and can be coated. If the anode ( 2 ) is coated, it is preferably coated with a mixed oxide comprising one or more of the metals ruthenium, iridium and titanium. Particularly preferred is a coating comprising a mixed oxide of ruthenium oxide and titanium oxide or a mixture of ruthenium oxide, iridium oxide and titanium oxide.
- the anode ( 2 ) according to the invention can also consist of graphite and other carbon materials such as diamond.
- Preferred are graphite electrodes, nitrogen-free and nitrogen-doped carbon nanotubes, boron-doped diamond and particularly preferably the aforementioned materials after oxidation, for example in nitric acid, or after activation in alkaline solution at temperatures above 30 ° C.
- the anode ( 2 ) according to the invention is usually used in the form of a rod, a plate or a mesh or grid.
- the anode ( 2 ) may be porous or non-porous.
- Anodes (2) are preferably in the form of a mesh or grid. Particularly preferred are porous graphite electrodes.
- the ion-conducting membrane ( M ) usually comprises a polymer membrane.
- Preferred polymer membranes are all polymer membranes, which the person skilled in the art generally knows under the generic term of the cation exchange membrane.
- Preferred membranes include polymeric perfluorosulfonic acids.
- the membranes ( M ) may also comprise reinforcing fabrics of other chemically stable materials, preferably fluorinated polymers, and more preferably polytetrafluoroethylene.
- the thickness of the ion-conducting membrane ( M ) is usually less than 1 mm.
- the thickness of the membrane ( M ) is less than 500 microns, more preferably less than 400 microns, most preferably less than 250 microns.
- the small thicknesses of the ion-conducting membrane are particularly advantageous, because in this way the necessary cell voltage in the device can be chosen to be lower, since the electrical resistance is reduced.
- a decrease in the membrane thickness is accompanied by an increase in the slip of chlorine through the ion-conducting membrane, whereby the cathode ( 1 ) located behind the ion-conducting membrane is loaded with chlorine. This could lead to corrosion of the cathode.
- the device of the invention comprises a layer ( 1b ) comprising NCNT which are chemically stable to chlorine, slippage of chlorine can be tolerated with lower cell voltage.
- the power supply ( S ), is usually operated so that cathode ( 1 ) forms the cathode and anode ( 2 ) forms the anode.
- the ion-conducting membrane ( M ) is applied directly to the layer comprising the nitrogen-doped carbon nanotubes ( 1b ) of the cathode ( 1 ).
- a further layer ( 1c ) is introduced between the layer comprising the nitrogen-doped carbon nanotubes ( 1b ) and the core ( 1a ) of the cathode ( 1 ) and comprises the ion-conducting membrane ( M ) on the layer the nitrogen-doped carbon nanotubes ( 1b ) applied directly.
- the further layer ( 1c ) usually comprises a mesh or fabric and / or a filling material.
- the mesh or fabric is usually made of a material that is chemically stable as defined above.
- Preferred is a fabric of carbon. Particularly preferably from graphitic carbon.
- the filler usually comprises a binder, as it is also used in the layer ( 1b ) according to the invention, and optionally carbon nanotubes.
- the filler material preferably comprises a binder, as is also used in the layer ( 1b ) according to the invention, and carbon nanotubes.
- Particularly preferred carbon nanotubes in the further layer ( 1c ) are nitrogen-doped carbon nanotubes ( NCNT ).
- the gas diffusion electrodes according to the invention are characterized by low material costs and high selectivity (no formation of hydrogen at high current densities).
- possible problems due to dissolution of noble metals or precious metal compounds by the corrosive medium hydrogen chloride and / or chlorine are eliminated.
- the inventive electrochemical cell comprising nitrogen-doped carbon nanotubes ( NCNT ) can be used for hydrogen chloride electrolysis .
- the device When used in the hydrogen chloride electrolysis, the device is usually operated with aqueous hydrochloric acid solution of a concentration of 0.5 mol / L to 10 mol / L, preferably from 3 mol / L to 6 mol / L.
- the operation is carried out at a temperature of 0-200 ° C, preferably 20-120 ° C and most preferably 40-90 ° C.
- hydrogen chloride electrolysis may also be carried out in the gas phase, i. the supply of hydrogen chloride takes place in the gaseous state with or without water.
- Oxygen ( O 2 ) is supplied to the electrode space ( A ) containing the cathode ( 1 ) via a feed line ( 3 ), which can be purified by means of a gas absorption device ( G o ) or saturated with water.
- a capacitor ( K ) is supplied.
- a hydrogen measuring device ( C H ) is installed above the condenser ( K ) in a safety discharge for such hydrogen ( H 2 ), which is controlled during the experiments and wherein, depending on the measured value displayed, the current and / or the voltage of the power supply ( S ) can be adjusted. From the condenser, a liquid comprising water ( H 2 O ) is withdrawn.
- the circulation stream ( 4a ) can additionally be adapted via a bypass flow ( 4a ') by suitable adjustment of a control valve ( V ).
- Example 1 Inventive electrochemical cell
- an electrochemical cell according to the invention is imaged. It consists of a cathode ( 1 ) and an anode ( 2 ), which are electrically connected via a current and voltage supply ( S ) with each other.
- the electrode spaces ( A and B ) are separated by a membrane ( M ) (Nafion®).
- M membrane
- In the cathode compartment A is an aqueous hydrochloric acid solution with 2 wt .-% HCl, which is permanently saturated with O 2
- the anode compartment ( B ) is an aqueous hydrochloric acid solution with 20 wt .-% HCl.
- the layer is prepared by spraying and drying a 5% solution of Nafion® in isopropanol in which NCNT is dispersed. Finally, a NCNT free solution of Nafion® in isopropanol is sprayed on and dried. Hiebei results in a loading of 8.0 g / m 2 Nafion®.
- the nitrogen-doped carbon nanotubes have a nitrogen content of 4.28 wt .-%.
- the nitrogen-doped carbon nanotubes are according to the text example 5 of the post-published German patent application DE 10 2007 062 421 produced.
- the anode ( 2 ) consists of porous graphite.
- the membrane ( M ) (Nafion®) is applied directly to the layer ( 1b ) of the cathode.
- the layer ( 1b ) comprises as binder Nafion® and a proportion of nitrogen-doped carbon nanotubes.
- the nitrogen-doped carbon nanotubes have a nitrogen content of 4.28 wt .-%.
- the cathode compartment ( A ) is open to the environment and consequently filled with room air. All other properties of the device according to Fig. 2 in this example correspond to those of Example 1, as already with reference to Fig. 1 was presented.
- a cathode constructed according to Example 2 is shown, which has been extended by a further layer ( 1c ) (gas diffusion layer).
- the further layer consists of a graphitic carbon fabric (Ballard company), on both sides as part of a gravure roll coating process
- the anode ( 2 ) consists of a ruthenium-titanium mixed metal oxide coated titanium-palladium alloy (TiPd0.2) in the form of an expanded metal.
- the cathode chamber ( A ) is further configured so that the gas can be introduced into the cathode rear space and at the bottom of the cell, the gas can be removed together with any liquid occurring reaction products.
- Example 4 HCl electrolysis in device according to the invention
- Fig. 4 is the cell voltage as a function of the current density in the production of chlorine from hydrogen chloride in the cell of the invention (see Fig. 3 Example 3).
- the liquid-filled gap between the surface of the anode ( 2 ) and membrane ( M ) was 2.5 mm.
- the active electrode area of anode and cathode was 100 cm 2 each and the membrane used was of the type Flemion® 133.
- Oxygen (> 99%) was in 3-fold stoichiometric excess (based on a current density of 5 kA / m 2 ) in the Cathode space at a pressure of 0-10 mbar above the ambient pressure passed and derived at the bottom together with the resulting water in the cathode as so-called condensate.
- the purity of the derived gaseous oxygen stream was controlled by means of a hydrogen sensor (sensitive from concentrations above 5 ppm hydrogen).
- the layers ( 1b ) and ( 1c ) of the cathode do not contain a noble metal. While chlorine is formed at the anode ( 2 ), oxygen reduction takes place at the noble metal-free cathode. In the entire measuring range up to current densities of 9 kA / m 2 electrode surface, no hydrogen was detected in the outflowed from the cell oxygen flow. The chlorine was produced over a period of 4 days of operation at a current density of 5 kA / m 2 at a cell voltage of 1.57 V, without an increase in the necessary cell voltage was recognizable.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Electrochemistry (AREA)
- Materials Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Automation & Control Theory (AREA)
- Electrolytic Production Of Non-Metals, Compounds, Apparatuses Therefor (AREA)
- Electrodes For Compound Or Non-Metal Manufacture (AREA)
- Carbon And Carbon Compounds (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE102008015901A DE102008015901A1 (de) | 2008-03-27 | 2008-03-27 | Elektrolysezelle zur Chlorwasserstoffelektrolyse |
| PCT/EP2009/002163 WO2009118162A1 (de) | 2008-03-27 | 2009-03-25 | Elektrolysezelle zur chlorwasserstoffelektrolyse |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP2260124A1 EP2260124A1 (de) | 2010-12-15 |
| EP2260124B1 true EP2260124B1 (de) | 2017-07-12 |
Family
ID=40810733
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP09724561.7A Not-in-force EP2260124B1 (de) | 2008-03-27 | 2009-03-25 | Elektrolysezelle zur chlorwasserstoffelektrolyse |
Country Status (9)
| Country | Link |
|---|---|
| US (1) | US8778148B2 (ja) |
| EP (1) | EP2260124B1 (ja) |
| JP (1) | JP5438092B2 (ja) |
| KR (1) | KR20110009091A (ja) |
| CN (1) | CN101981232B (ja) |
| DE (1) | DE102008015901A1 (ja) |
| IL (1) | IL207813A0 (ja) |
| TW (1) | TW201000678A (ja) |
| WO (1) | WO2009118162A1 (ja) |
Families Citing this family (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE102007062421A1 (de) * | 2007-12-20 | 2009-06-25 | Bayer Technology Services Gmbh | Verfahren zur Herstellung von Stickstoff-dotierten Kohlenstoffnanoröhrchen |
| KR101239966B1 (ko) * | 2010-11-04 | 2013-03-06 | 삼성전자주식회사 | 리튬 공기 전지용 양극, 그 제조방법 및 이를 채용한 리튬 공기 전지 |
| CN102010035B (zh) * | 2010-11-12 | 2012-07-04 | 山东农业大学 | 浸没式电解混合装置 |
| JP5557394B2 (ja) * | 2011-04-08 | 2014-07-23 | 株式会社オメガ | 排水処理方法 |
| US9136542B2 (en) | 2011-05-18 | 2015-09-15 | The Ohio State University | Catalysts for use in electrochemical applications and electrodes and devices using same |
| SA112330516B1 (ar) * | 2011-05-19 | 2016-02-22 | كاليرا كوربوريشن | انظمة وطرق هيدروكسيد كهروكيميائية مستخدمة لأكسدة المعدن |
| US9200375B2 (en) | 2011-05-19 | 2015-12-01 | Calera Corporation | Systems and methods for preparation and separation of products |
| KR101817271B1 (ko) | 2011-08-24 | 2018-01-10 | 모리나가 뉴교 가부시키가이샤 | 전해수 제조 장치 |
| CN103055966B (zh) * | 2012-12-26 | 2015-01-14 | 北京航空航天大学 | 基于分支型氧化铝纳米通道薄膜的纳流体二极管器件 |
| TWI633206B (zh) | 2013-07-31 | 2018-08-21 | 卡利拉股份有限公司 | 使用金屬氧化物之電化學氫氧化物系統及方法 |
| US10266954B2 (en) | 2015-10-28 | 2019-04-23 | Calera Corporation | Electrochemical, halogenation, and oxyhalogenation systems and methods |
| CN105461023B (zh) * | 2015-11-06 | 2018-08-10 | 北京航空航天大学 | 一种采用氧还原阴极的电解槽装置 |
| US10619254B2 (en) | 2016-10-28 | 2020-04-14 | Calera Corporation | Electrochemical, chlorination, and oxychlorination systems and methods to form propylene oxide or ethylene oxide |
| US10746686B2 (en) * | 2016-11-03 | 2020-08-18 | King Abdulaziz University | Electrochemical cell and a method of using the same for detecting bisphenol-A |
| EP3351505A1 (de) * | 2017-01-20 | 2018-07-25 | Covestro Deutschland AG | Verfahren zur flexiblen steuerung der verwendung von salzsäure aus chemischer produktion |
| WO2019060345A1 (en) | 2017-09-19 | 2019-03-28 | Calera Corporation | SYSTEMS AND METHODS USING LANTHANIDE HALIDE |
| US10590054B2 (en) | 2018-05-30 | 2020-03-17 | Calera Corporation | Methods and systems to form propylene chlorohydrin from dichloropropane using Lewis acid |
| WO2020216648A1 (de) | 2019-04-25 | 2020-10-29 | Basf Se | Verfahren zur herstellung von phosgen |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP3538271B2 (ja) * | 1995-09-12 | 2004-06-14 | ペルメレック電極株式会社 | 塩酸電解装置 |
| IT1282367B1 (it) * | 1996-01-19 | 1998-03-20 | De Nora Spa | Migliorato metodo per l'elettrolisi di soluzioni acquose di acido cloridrico |
| US6149782A (en) | 1999-05-27 | 2000-11-21 | De Nora S.P.A | Rhodium electrocatalyst and method of preparation |
| ITMI20010402A1 (it) | 2001-02-28 | 2002-08-28 | De Nora Elettrodi Spa | Nuova composizione elettrocatalitica per catodo depolarizzato ad ossigeno |
| DE10138215A1 (de) * | 2001-08-03 | 2003-02-20 | Bayer Ag | Verfahren zur elektrochemischen Herstellung von Chlor aus wässrigen Lösungen von Chlorwasserstoff |
| DE10335184A1 (de) | 2003-07-30 | 2005-03-03 | Bayer Materialscience Ag | Elektrochemische Zelle |
| US20070275160A1 (en) * | 2003-10-10 | 2007-11-29 | Stephen Maldonado | Carbon Nanostructure-Based Electrocatalytic Electrodes |
| JP3995051B2 (ja) * | 2003-12-26 | 2007-10-24 | 関西ティー・エル・オー株式会社 | 有機光触媒を用いた水の電気分解方法 |
| EP1926682A4 (en) * | 2005-06-21 | 2012-06-20 | Crosslink Polymer Res | DECONTAMINANT COATING ACTIVATED BY SIGNAL |
| US20100314261A1 (en) * | 2005-12-14 | 2010-12-16 | Perry Michael L | Oxygen-Consuming Zero-Gap Electrolysis Cells With Porous/Solid Plates |
| US8221937B2 (en) * | 2008-12-19 | 2012-07-17 | University Of Dayton | Metal-free vertically-aligned nitrogen-doped carbon nanotube catalyst for fuel cell cathodes |
-
2008
- 2008-03-27 DE DE102008015901A patent/DE102008015901A1/de not_active Withdrawn
-
2009
- 2009-03-25 WO PCT/EP2009/002163 patent/WO2009118162A1/de active Application Filing
- 2009-03-25 US US12/920,202 patent/US8778148B2/en not_active Expired - Fee Related
- 2009-03-25 EP EP09724561.7A patent/EP2260124B1/de not_active Not-in-force
- 2009-03-25 KR KR1020107021251A patent/KR20110009091A/ko active IP Right Grant
- 2009-03-25 CN CN2009801110576A patent/CN101981232B/zh not_active Expired - Fee Related
- 2009-03-25 JP JP2011501138A patent/JP5438092B2/ja not_active Expired - Fee Related
- 2009-03-26 TW TW098109820A patent/TW201000678A/zh unknown
-
2010
- 2010-08-26 IL IL207813A patent/IL207813A0/en unknown
Non-Patent Citations (1)
| Title |
|---|
| None * |
Also Published As
| Publication number | Publication date |
|---|---|
| DE102008015901A1 (de) | 2009-10-01 |
| TW201000678A (en) | 2010-01-01 |
| US20110005938A1 (en) | 2011-01-13 |
| CN101981232B (zh) | 2013-03-13 |
| WO2009118162A1 (de) | 2009-10-01 |
| CN101981232A (zh) | 2011-02-23 |
| IL207813A0 (en) | 2010-12-30 |
| US8778148B2 (en) | 2014-07-15 |
| JP2011515585A (ja) | 2011-05-19 |
| WO2009118162A8 (de) | 2010-10-07 |
| KR20110009091A (ko) | 2011-01-27 |
| EP2260124A1 (de) | 2010-12-15 |
| JP5438092B2 (ja) | 2014-03-12 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2260124B1 (de) | Elektrolysezelle zur chlorwasserstoffelektrolyse | |
| DE2844499C2 (de) | Verfahren zum Herstellen eines Halogens | |
| DE69901319T2 (de) | Katalysator für Gasdiffusionselektrode | |
| DE2847955C2 (de) | Verfahren zum Herstellen von Halogenen durch Elektrolyse wäßriger Alkalimetallhalogenide | |
| EP2398101B1 (de) | Gasdiffusionselektrode und Verfahren zu ihrer Herstellung | |
| DE2926560A1 (de) | Elektrolysezelle, membran-elektroden- einheit und verfahren zur herstellung von halogen und alkalimetallhydroxid | |
| DE102015203245A1 (de) | Abscheidung eines kupferhaltigen, Kohlenwasserstoffe entwickelnden Elektrokatalysators auf Nicht-Kupfer-Substraten | |
| EP2765222A1 (de) | Katalysatorbeschichtung und Verfahren zu ihrer Herstellung | |
| WO2010078952A2 (de) | Strukturierte gasdiffusionselektrode für elektrolysezellen | |
| DE3782464T2 (de) | An eine ionenaustauschermembran gebundene kathode fuer elektrolysezellen und entsprechendes elektrolyseverfahren. | |
| CH650032A5 (de) | Katalytisch aktive elektrodenstruktur fuer die elektrolyse von halogeniden. | |
| EP1463847B1 (de) | Elektroden für die elektrolyse in sauren medien | |
| DE4438275B4 (de) | Elektrolysezelle und Verfahren zur Elektrolyse einer wässrigen Kochsalzlösung | |
| EP2609649B1 (de) | Sauerstoffverzehrelektrode und verfahren zu ihrer herstellung | |
| EP3191620B1 (de) | Sauerstoffverzehrelektrode und verfahren zu ihrer herstellung | |
| DE10203689A1 (de) | Kathodischer Stromverteiler für Elektrolysezellen | |
| WO2009118124A1 (de) | Verfahren zur elektrolytischen sauerstoffreduktion | |
| WO2010020365A1 (de) | Elektrodenmaterial, elektrode und ein verfahren zur chlorwasserstoffelektrolyse | |
| DE3125173C2 (de) | Verwendung einer Kathode, die aus einer Einlagerungsverbindung besteht, zum Elektrolysieren von Alkalichloridsole | |
| EP2439314A2 (de) | Verfahren zur Herstellung von transport- und lagerstabilen Sauerstoffverzehrelektroden | |
| EP1106714B1 (de) | Verfahren zur Herstellung von Halogenen durch Gasphasenelektrolyse | |
| EP3597791B1 (de) | Verfahren zur leistungsverbesserung von nickelelektroden | |
| WO2017174563A1 (de) | Bifunktionelle elektrode und elektrolysevorrichtung für die chlor-alkali-elektrolyse | |
| DE10048004A1 (de) | Verfahren zur Elektrolyse wässriger Salzsäurelösungen |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20101027 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO SE SI SK TR |
|
| AX | Request for extension of the european patent |
Extension state: AL BA RS |
|
| DAX | Request for extension of the european patent (deleted) | ||
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: BAYER INTELLECTUAL PROPERTY GMBH Owner name: BAYER MATERIALSCIENCE AG |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: BAYER INTELLECTUAL PROPERTY GMBH |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: COVESTRO DEUTSCHLAND AG |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| INTG | Intention to grant announced |
Effective date: 20170321 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO SE SI SK TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D Free format text: NOT ENGLISH |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: REF Ref document number: 908400 Country of ref document: AT Kind code of ref document: T Effective date: 20170715 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D Free format text: LANGUAGE OF EP DOCUMENT: GERMAN |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 502009014147 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: MP Effective date: 20170712 |
|
| REG | Reference to a national code |
Ref country code: LT Ref legal event code: MG4D |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170712 Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20171012 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170712 Ref country code: SE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170712 Ref country code: NL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170712 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170712 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20171112 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20171013 Ref country code: BG Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20171012 Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170712 Ref country code: LV Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170712 Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170712 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R097 Ref document number: 502009014147 Country of ref document: DE |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: RO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170712 Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170712 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170712 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170712 Ref country code: EE Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170712 |
|
| 26N | No opposition filed |
Effective date: 20180413 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170712 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170712 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R119 Ref document number: 502009014147 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20180325 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170712 |
|
| REG | Reference to a national code |
Ref country code: BE Ref legal event code: MM Effective date: 20180331 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: MM4A |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20180325 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20181002 Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20180325 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20180331 Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20180331 Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20180331 Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20180325 Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20180325 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20180331 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MM01 Ref document number: 908400 Country of ref document: AT Kind code of ref document: T Effective date: 20180325 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20180325 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: TR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170712 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170712 Ref country code: HU Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT; INVALID AB INITIO Effective date: 20090325 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MK Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20170712 Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20170712 |