EP1126888B1 - Blindungsvorrichtung für klinische prüfungen - Google Patents
Blindungsvorrichtung für klinische prüfungen Download PDFInfo
- Publication number
- EP1126888B1 EP1126888B1 EP99971721A EP99971721A EP1126888B1 EP 1126888 B1 EP1126888 B1 EP 1126888B1 EP 99971721 A EP99971721 A EP 99971721A EP 99971721 A EP99971721 A EP 99971721A EP 1126888 B1 EP1126888 B1 EP 1126888B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- container
- connection
- pharmaceutical
- intended
- administration
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 230000002745 absorbent Effects 0.000 claims description 8
- 239000002250 absorbent Substances 0.000 claims description 8
- 239000000463 material Substances 0.000 claims description 7
- 241000124008 Mammalia Species 0.000 claims description 6
- 238000013461 design Methods 0.000 claims description 3
- 239000004033 plastic Substances 0.000 claims description 2
- 230000002685 pulmonary effect Effects 0.000 claims 1
- 239000002552 dosage form Substances 0.000 description 33
- 239000003795 chemical substances by application Substances 0.000 description 11
- 239000000126 substance Substances 0.000 description 8
- 238000012360 testing method Methods 0.000 description 8
- 230000014759 maintenance of location Effects 0.000 description 7
- 238000000034 method Methods 0.000 description 5
- 239000007787 solid Substances 0.000 description 5
- 239000007788 liquid Substances 0.000 description 3
- 230000000717 retained effect Effects 0.000 description 3
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- 150000001875 compounds Chemical class 0.000 description 2
- 239000003580 lung surfactant Substances 0.000 description 2
- 239000007909 solid dosage form Substances 0.000 description 2
- 238000013022 venting Methods 0.000 description 2
- 229920001875 Ebonite Polymers 0.000 description 1
- 208000000203 Hyaline Membrane Disease Diseases 0.000 description 1
- 208000032571 Infant acute respiratory distress syndrome Diseases 0.000 description 1
- 206010028974 Neonatal respiratory distress syndrome Diseases 0.000 description 1
- 238000004026 adhesive bonding Methods 0.000 description 1
- 230000004069 differentiation Effects 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- -1 for example Substances 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 239000008297 liquid dosage form Substances 0.000 description 1
- 229940066294 lung surfactant Drugs 0.000 description 1
- 201000002652 newborn respiratory distress syndrome Diseases 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- 230000002028 premature Effects 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 238000007789 sealing Methods 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 239000011343 solid material Substances 0.000 description 1
- 238000009423 ventilation Methods 0.000 description 1
- 238000003466 welding Methods 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61D—VETERINARY INSTRUMENTS, IMPLEMENTS, TOOLS, OR METHODS
- A61D7/00—Devices or methods for introducing solid, liquid, or gaseous remedies or other materials into or onto the bodies of animals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61J—CONTAINERS SPECIALLY ADAPTED FOR MEDICAL OR PHARMACEUTICAL PURPOSES; DEVICES OR METHODS SPECIALLY ADAPTED FOR BRINGING PHARMACEUTICAL PRODUCTS INTO PARTICULAR PHYSICAL OR ADMINISTERING FORMS; DEVICES FOR ADMINISTERING FOOD OR MEDICINES ORALLY; BABY COMFORTERS; DEVICES FOR RECEIVING SPITTLE
- A61J1/00—Containers specially adapted for medical or pharmaceutical purposes
Definitions
- the invention relates to a device for blinding the administration of non-solid pharmaceutical dosage forms in clinical trials in mammals.
- the pharmaceutical dosage form is a colorless liquid
- the pharmaceutical dosage form may be replaced by a physiological sodium chloride solution, for example.
- an opaque application system can be used for the administration. It is understood that these methods are not suitable for intrapulmonary administration.
- the double-blind technique Double-Observer-Technique
- test substance is administered by persons who otherwise do not care for the patient during the clinical study. These persons are then subject to secrecy.
- the double-blind technique can only be carried out with great manpower and costs.
- WO97 / 05914 which discloses the closest prior art, describes an apparatus for blinding the administration of drugs by infusion based on syringes.
- Object of the present invention is the blinding of the administration of non-solid pharmaceutical dosage forms in clinical trials in a simple manner and without the use of the double-blind technique.
- the invention therefore provides a kit according to claim 1 and a container according to claim 2.
- a device consisting of an opaque container having at least two ports, one of which is suitable for connection to a dispensing means for the pharmaceutical dosage form and the other for connection to a Application means is suitable and wherein the container has in its interior means for retaining the pharmaceutical dosage form (hereinafter also referred to as "non flow type" configuration).
- the pharmaceutical administration form is retained in the container during administration - not recognizable by the person responsible for the administration.
- an opaque container having at least two ports, one of which is suitable for connection to a dispensing means for the pharmaceutical dosage form and the other is suitable for connection with an application agent and wherein the connections inside the container a continuous Compound, which ensures a transport of the pharmaceutical dosage form through the container (hereinafter also referred to as "flow type" configuration).
- the pharmaceutical dosage form - for the person responsible for the administration not recognizable - transported through the container and administered to the patient.
- Another object of the invention is therefore an opaque container having at least three ports, two of which are suitable for connection to a dispensing means for the pharmaceutical dosage form and the third is suitable for connection with an application agent, wherein the container in its interior means for Containment of the pharmaceutical dosage form and one of the terminals which are suitable for connection with a dispensing means in the interior of the container has a continuous connection with the terminal which is suitable for connection with the application means and the compound ensures a transport of the pharmaceutical dosage form through the container ,
- the pharmaceutical dosage form is either transported through the container and administered to the patient or retained in the container.
- the container may be rigid or flexible and made of any suitable solid material.
- it is a container which was made of plastic or rubber (eg film bag is made of PVC film).
- the means for retaining the pharmaceutical dosage form inside the container is preferably an absorbent material which can completely accommodate the non-solid pharmaceutical dosage form (e.g., absorbent tissue).
- the container may also be hollow or partially hollow in its interior. As a result, a retention can also be ensured.
- the container is preferably dimensioned so that it can completely absorb the test substance to be administered. In a preferred embodiment of the invention, the container is dimensioned so that it can catch 300 ml of liquid. Preference is given to containers which have an internal volume of from 500 ml to 3000 ml, particularly preferably those having an internal volume of from 1000 ml to 2000 ml.
- the container should, depending on the weight and amount of the test substance to be administered have a correspondingly higher weight.
- the container may have inside weight elements to ensure sufficient self-weight of the container.
- the weight elements may be hard rubber discs, for example.
- the connections which comprise the container are preferably commercially available Luerlock connections which are suitable for connection to the dispensing or application means.
- the terminals have different markings for differentiation.
- the dispensing means is preferably a commercially available syringe, which may be connected directly or via a hose connection with the designated connection to the container.
- the application agent is preferably an opaque application agent, for example an opaque catheter system, preferably a catheter system which is suitable for intrapulmonary application of a non-solid pharmaceutical dosage form. Those skilled in such catheter systems are known.
- the container which provides for retention of the pharmaceutical dosage form may further be connected to the port intended for connection to the dispensing means with a tube leading into the interior of the container and having an open end through which the pharmaceutical dosage form is discharged into the container interior.
- the hose has a plurality of openings in the hose line. This ensures a rapid delivery of the pharmaceutical dosage form into the container interior.
- the tube may also have a check valve which prevents a backflow of the dispensed into the container interior pharmaceutical dosage form into the tube.
- the provided for the connection with the application means connection can inside the container a Have closure, so that leakage of the retained pharmaceutical dosage form is excluded in the application means. If desired, this may be a closed tube leading into the interior of the container.
- the two tubes leading into the interior of the container can be connected to one another via a Y-connection piece, the tube leading to the connection provided for the application agent, tightly tied or tightly closed by a pinch or weld ,
- the third port of the Y-connector has an open cap through which the supplied pharmaceutical dosage form can flow into the blind bag.
- the container which effects a transport of the pharmaceutical dosage form through the container advantageously has a continuous hose connection between the connections inside the container.
- the container may be hollow or, if desired, also filled with suitable materials, such as absorbent materials and weight elements.
- the container is preferably designed according to the container, which causes a retention of the pharmaceutical dosage form in the container.
- the container may further include a vent (e.g., valve) to compensate for pressure differences resulting from the retention of the pharmaceutical dosage form in the container.
- a vent e.g., valve
- the containers, together with a dispensing agent and an application agent, are advantageously suitable for blinding the administration of non-solid, in particular liquid, administration forms by intrapulmonary administration.
- a liquid dosage form for example, pharmaceutical dosage forms may be mentioned which have the properties of natural lung surfactant.
- the administration forms described in WO95 / 32992 may be mentioned.
- containers are used in a clinical examination and containers without retention by the same person, so the various containers are preferably designed so that a distinction by the person is not possible.
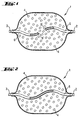
- Figure 1 shows a container 1 having a connector 2 suitable for connection to a dispensing means and a connector 3 suitable for connection to an application means.
- the container 1 is filled with an absorbent material 4 .
- the container 1 has at the terminal 2 a leading into the interior of the container 1 tube 5 , which is open at its end 5 ' .
- the connection 3 also has a hose 6 leading into the interior of the container, which is closed at its end 6 ' .
- Figure 2 shows a container 1 having a connector 2 suitable for connection to a dispensing means and a connector 3 suitable for connection to an application means.
- the ports 2 and 3 are connected inside the container 1 with a continuous tube 5 .
- the container 1 is filled with an absorbent material 4 .
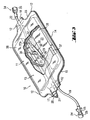
- An inventive blind bag 10 in a "non flow type" configuration shown for example in FIG. 3, has a film bag 11 , formed from two film blanks 13, 14 which are tightly connected to each other at the edge by means of welding, sealing or gluing seam 12 .
- the film bag 11 has at its opposite narrow sides 15, 16 neck-like projections 17, 18 , in which on the one hand a tube 19 for introducing a medium (eg test substance) and a venting device 20 and on the other hand, an outlet tube 22 are sealed.
- the venting device 20 is equipped with a conventional filter 21 for germ-free ventilation of the inner region 23 of the blind bag 10 .
- the inlet tube 19 has at its free outer end 24 a so-called Luerlock connection element 25 in "femal” design, which carries a cap-like connection adapter 26 .
- the bag inside end 27 of the inlet tube 19 is provided with a check valve 28 which prevents backflow of a medium in the inlet tube 19 .
- the check valve 28 is provided with a 1 .
- Terminal 29 of a Y-connector 32 is connected, which is connected with its second port 30 tightly connected to the outlet tube 22 .
- the outlet tube 22 has at its outer end 34 a so-called Luerlock connection element 35 in "male” design, which is connectable to a, for example, a patient in a suitably connected tube system (not shown).
- the outlet tube 22 is tied tight within the blind bag 10 or sealed by a pinch or weld 22a .
- an absorbent material 37 are provided in the interior 23 of the film bag 11 or blind bag 10 , which is capable of receiving a defined amount of eg a medium and to bind in itself.
- an open cap 36 is provided, through which the medium introduced via the inlet hose 19 can flow into the interior 23 of the blind bag 10 and be received by the absorbent structure 37 .
- two weight elements 38 are advantageously provided in the interior of the blind bag 10 , which obscure a concomitant increase in weight.
- An inventive blind bag 40 in "flow type" configuration shown in FIG. 4 has basically the same elements as the "non flow type" configuration according to FIG. 3, which is also characterized by the equivalence of the reference numbers used herein.
- the outlet tube 33 is open in this case so that a medium can flow through the blind bag 40 .
- a closed cap 41 is provided, that is, no medium can penetrate into the interior 23 of the blind bag.
Landscapes
- Veterinary Medicine (AREA)
- Life Sciences & Earth Sciences (AREA)
- Health & Medical Sciences (AREA)
- Public Health (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Medical Preparation Storing Or Oral Administration Devices (AREA)
- Infusion, Injection, And Reservoir Apparatuses (AREA)
- Bag Frames (AREA)
- Packages (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Automatic Analysis And Handling Materials Therefor (AREA)
- Measuring Pulse, Heart Rate, Blood Pressure Or Blood Flow (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DE19851119A DE19851119C2 (de) | 1998-11-06 | 1998-11-06 | Behälter und Vorrichtung zur Blindung der Verabreichung von nicht festen pharmazeutischen Darreichungsformen bei der klinischen Prüfung |
| DE19851119 | 1998-11-06 | ||
| PCT/EP1999/008476 WO2000027448A1 (de) | 1998-11-06 | 1999-11-05 | Blindungsvorrichtung für klinische prüfungen |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| EP1126888A1 EP1126888A1 (de) | 2001-08-29 |
| EP1126888B1 true EP1126888B1 (de) | 2006-02-08 |
Family
ID=7886853
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP99971721A Expired - Lifetime EP1126888B1 (de) | 1998-11-06 | 1999-11-05 | Blindungsvorrichtung für klinische prüfungen |
Country Status (9)
| Country | Link |
|---|---|
| US (1) | US6544250B1 (enExample) |
| EP (1) | EP1126888B1 (enExample) |
| JP (1) | JP2002529151A (enExample) |
| AT (1) | ATE317275T1 (enExample) |
| AU (1) | AU1380100A (enExample) |
| CA (1) | CA2350644C (enExample) |
| DE (2) | DE19851119C2 (enExample) |
| ES (1) | ES2258350T3 (enExample) |
| WO (1) | WO2000027448A1 (enExample) |
Families Citing this family (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CH696069A5 (de) * | 2002-12-19 | 2006-12-15 | Fisher Clinical Services Ag | Behälter und dessen Verwendung in einer Verpackung, beispielsweise Heilmittelverpackung. |
| DE202004007115U1 (de) * | 2004-05-03 | 2004-07-08 | Altana Pharma Ag | Blindungsvorrichtung |
| WO2011011035A2 (en) * | 2009-07-24 | 2011-01-27 | Millipore Corporation | Feed bag construction |
| JP2015506743A (ja) * | 2012-01-16 | 2015-03-05 | サノフィ−アベンティス・ドイチュラント・ゲゼルシャフト・ミット・ベシュレンクテル・ハフツング | 治験用の盲検キット |
| EP2804643B1 (en) * | 2012-01-16 | 2017-12-06 | Sanofi-Aventis Deutschland GmbH | Blinding kit for clinical trials |
Family Cites Families (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE255260C (enExample) | ||||
| DD255260A (enExample) * | ||||
| US3572340A (en) * | 1968-01-11 | 1971-03-23 | Kendall & Co | Suction drainage device |
| CH573026A5 (enExample) * | 1974-06-11 | 1976-02-27 | Kaiser Josef Ag Fahrzeugwerk | |
| CA1265963A (en) * | 1984-03-02 | 1990-02-20 | George Joseph Duffy | Injection device |
| IT221762Z2 (it) * | 1991-03-25 | 1994-10-20 | Salvatore Sapienza | Siringa opaca |
| KR100269731B1 (ko) * | 1993-07-15 | 2000-10-16 | 하루타 히로시 | 영상 프로세서용 광원장치 |
| IT1260685B (it) * | 1993-09-29 | 1996-04-22 | Sorin Biomedica Spa | Dispositivo per il contenimento di sangue |
| DE4418936A1 (de) | 1994-05-31 | 1996-02-08 | Byk Gulden Lomberg Chem Fab | Polypeptid |
| US5658248A (en) * | 1995-08-04 | 1997-08-19 | Localmed, Inc. | Double-blind infusion device and method |
| US5980834A (en) * | 1996-07-25 | 1999-11-09 | The United States Of America As Represented By The Secretary Of Commerce | Sample storage devices |
| US6287284B1 (en) * | 1999-09-27 | 2001-09-11 | Npt, Inc. | Silicone bag assembly |
| US6569122B2 (en) * | 2001-01-18 | 2003-05-27 | Ultradent Products, Inc | Syringe apparatus for delivering light activated materials |
-
1998
- 1998-11-06 DE DE19851119A patent/DE19851119C2/de not_active Expired - Fee Related
-
1999
- 1999-11-05 AU AU13801/00A patent/AU1380100A/en not_active Abandoned
- 1999-11-05 ES ES99971721T patent/ES2258350T3/es not_active Expired - Lifetime
- 1999-11-05 WO PCT/EP1999/008476 patent/WO2000027448A1/de not_active Ceased
- 1999-11-05 EP EP99971721A patent/EP1126888B1/de not_active Expired - Lifetime
- 1999-11-05 CA CA002350644A patent/CA2350644C/en not_active Expired - Fee Related
- 1999-11-05 DE DE59913117T patent/DE59913117D1/de not_active Expired - Lifetime
- 1999-11-05 US US09/830,427 patent/US6544250B1/en not_active Expired - Fee Related
- 1999-11-05 JP JP2000580675A patent/JP2002529151A/ja active Pending
- 1999-11-05 AT AT99971721T patent/ATE317275T1/de active
Also Published As
| Publication number | Publication date |
|---|---|
| DE59913117D1 (de) | 2006-04-20 |
| ATE317275T1 (de) | 2006-02-15 |
| JP2002529151A (ja) | 2002-09-10 |
| ES2258350T3 (es) | 2006-08-16 |
| CA2350644A1 (en) | 2000-05-18 |
| US6544250B1 (en) | 2003-04-08 |
| AU1380100A (en) | 2000-05-29 |
| DE19851119A1 (de) | 2000-05-18 |
| DE19851119C2 (de) | 2001-05-31 |
| EP1126888A1 (de) | 2001-08-29 |
| CA2350644C (en) | 2008-04-01 |
| WO2000027448A1 (de) | 2000-05-18 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP0757553B1 (de) | Einstückige appliziervorrichtung zur kontaminationsfreien verabreichung von arzneimitteln (zytostatika) | |
| AT505615B1 (de) | Medizinisches gerät in form eines katheters zum zu-, insbesondere jedoch abführen von fluid in, insbesondere aus körperhöhlen, insbesondere dem pleuraraum | |
| DE3513205C1 (de) | Konnektor fuer die Peritonealdialyse | |
| DE19982614B4 (de) | Aufnahmevorrichtung zum dichten Aufnehmen von Substanzen | |
| DE69226281T2 (de) | System zur entnahme von flüssigkeiten aus einem vorratsbehälter | |
| DE60315003T2 (de) | Flüssigkeitstransfer-anordnung | |
| DE69403572T2 (de) | Mehrkammerbehälter | |
| DE69421689T2 (de) | Vorrichtung zur in-line verabreichung von drogen | |
| DE3855267T2 (de) | Gerät zum Ansaugen von Blut | |
| DE69012152T2 (de) | Zweiseitige Nadelanordnung. | |
| DE69302188T2 (de) | Vorrichtung zum Vermischen | |
| DE1138186B (de) | Infusionsgeraet zum Sammeln und Verabreichen von medizinischen Fluessigkeiten | |
| EP0390898B1 (de) | Behälter zur getrennten, sterilen aufbewahrung mindestens zweier substanzen und zur vermischung dieser substanzen | |
| DE2819507A1 (de) | Beutel zum sammeln, speichern, verabreichen und filtern von blut, blutkomponenten, intravenoesen und aehnlichen fluessigkeiten | |
| DE60116409T2 (de) | Medizinische, vorgefertigte Einmalvorrichtung zum Verabreichen von mindestens zwei Medikamenten im vorgegebenen Verhältnis | |
| DE1087324B (de) | Infusionsgeraet | |
| DE2026086A1 (de) | Bentelvorrichtung für Transfusion von Blut oder Flüssigkeiten | |
| DD202390A5 (de) | Kartusche fuer die intravenoese verabreichung eines als trockenpraeparat vorliegenden medikamentes | |
| WO2016041948A1 (de) | Vorrichtung zum anschluss einer durchstechflasche an einen behälter oder an eine fluidleitung und überführung des inhaltes einer durchstechflasche in einen behälter oder in eine fluidleitung, sowie verfahren hierzu und verwendung einer solchen vorrichtung | |
| DE69316732T2 (de) | Behälter für Lösungen mit Verbindungsschlauch aus Kunststoff | |
| DE3780257T2 (de) | Passives arzneimittelverabreichungssystem. | |
| DE102008045692A1 (de) | Vorrichtung und Verfahren zum Anlegen eines Zugangs zu einem Hohlorgan, Werkzeug | |
| EP1126888B1 (de) | Blindungsvorrichtung für klinische prüfungen | |
| DE4314090C2 (de) | Medizinisches Besteck zur Herstellung einer Medikamentenlösung | |
| DE69001432T2 (de) | Parenterales zufuehrsystem. |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| 17P | Request for examination filed |
Effective date: 20010606 |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU MC NL PT SE |
|
| AX | Request for extension of the european patent |
Free format text: AL PAYMENT 20010606;LT PAYMENT 20010606;LV PAYMENT 20010606;MK PAYMENT 20010606;RO PAYMENT 20010606;SI PAYMENT 20010606 |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: ALTANA PHARMA AG |
|
| 17Q | First examination report despatched |
Effective date: 20030611 |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU MC NL PT SE |
|
| AX | Request for extension of the european patent |
Extension state: AL LT LV MK RO SI |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT;WARNING: LAPSES OF ITALIAN PATENTS WITH EFFECTIVE DATE BEFORE 2007 MAY HAVE OCCURRED AT ANY TIME BEFORE 2007. THE CORRECT EFFECTIVE DATE MAY BE DIFFERENT FROM THE ONE RECORDED. Effective date: 20060208 Ref country code: FI Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20060208 |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D Free format text: NOT ENGLISH |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D Free format text: LANGUAGE OF EP DOCUMENT: GERMAN |
|
| REF | Corresponds to: |
Ref document number: 59913117 Country of ref document: DE Date of ref document: 20060420 Kind code of ref document: P |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20060508 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: NV Representative=s name: E. BLUM & CO. PATENTANWAELTE |
|
| REG | Reference to a national code |
Ref country code: SE Ref legal event code: TRGR |
|
| GBT | Gb: translation of ep patent filed (gb section 77(6)(a)/1977) |
Effective date: 20060517 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PT Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20060710 |
|
| LTIE | Lt: invalidation of european patent or patent extension |
Effective date: 20060208 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FG2A Ref document number: 2258350 Country of ref document: ES Kind code of ref document: T3 |
|
| ET | Fr: translation filed | ||
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: MC Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20061130 |
|
| PLBE | No opposition filed within time limit |
Free format text: ORIGINAL CODE: 0009261 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: NO OPPOSITION FILED WITHIN TIME LIMIT |
|
| 26N | No opposition filed |
Effective date: 20061109 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PFA Owner name: NYCOMED GMBH Free format text: ALTANA PHARMA AG#BYK-GULDEN-STRASSE 2#78467 KONSTANZ (DE) -TRANSFER TO- NYCOMED GMBH#BYK-GULDEN-STRASSE 2#78467 KONSTANZ (DE) |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PFA Owner name: NYCOMED GMBH Free format text: NYCOMED GMBH#BYK-GULDEN-STRASSE 2#78467 KONSTANZ (DE) -TRANSFER TO- NYCOMED GMBH#BYK-GULDEN-STRASSE 2#78467 KONSTANZ (DE) |
|
| NLT1 | Nl: modifications of names registered in virtue of documents presented to the patent office pursuant to art. 16 a, paragraph 1 |
Owner name: NYCOMED GMBH |
|
| BECN | Be: change of holder's name |
Owner name: NYCOMED G.M.B.H. Effective date: 20060208 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20060509 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: CD |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: LU Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20061105 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CY Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20060208 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: NL Payment date: 20101110 Year of fee payment: 12 Ref country code: IE Payment date: 20101110 Year of fee payment: 12 Ref country code: FR Payment date: 20101123 Year of fee payment: 12 Ref country code: AT Payment date: 20101110 Year of fee payment: 12 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: DE Payment date: 20101104 Year of fee payment: 12 Ref country code: CH Payment date: 20101112 Year of fee payment: 12 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: GB Payment date: 20101103 Year of fee payment: 12 Ref country code: IT Payment date: 20101113 Year of fee payment: 12 Ref country code: SE Payment date: 20101111 Year of fee payment: 12 Ref country code: BE Payment date: 20101117 Year of fee payment: 12 |
|
| PGFP | Annual fee paid to national office [announced via postgrant information from national office to epo] |
Ref country code: ES Payment date: 20101217 Year of fee payment: 12 |
|
| BERE | Be: lapsed |
Owner name: NYCOMED G.M.B.H. Effective date: 20111130 |
|
| REG | Reference to a national code |
Ref country code: NL Ref legal event code: V1 Effective date: 20120601 |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: PL |
|
| REG | Reference to a national code |
Ref country code: SE Ref legal event code: EUG |
|
| GBPC | Gb: european patent ceased through non-payment of renewal fee |
Effective date: 20111105 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CH Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20111130 Ref country code: LI Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20111130 Ref country code: NL Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120601 |
|
| REG | Reference to a national code |
Ref country code: FR Ref legal event code: ST Effective date: 20120731 |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: MM4A |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20111105 Ref country code: BE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20111130 |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R119 Ref document number: 59913117 Country of ref document: DE Effective date: 20120601 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20111105 Ref country code: GB Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20111105 Ref country code: SE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20111106 |
|
| REG | Reference to a national code |
Ref country code: AT Ref legal event code: MM01 Ref document number: 317275 Country of ref document: AT Kind code of ref document: T Effective date: 20111105 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: FR Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20111130 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: AT Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20111105 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: DE Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20120601 |
|
| REG | Reference to a national code |
Ref country code: ES Ref legal event code: FD2A Effective date: 20131030 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF NON-PAYMENT OF DUE FEES Effective date: 20111106 |