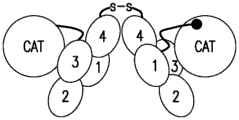
CN109476758B - 抗-凝血因子xi抗体 - Google Patents
抗-凝血因子xi抗体 Download PDFInfo
- Publication number
- CN109476758B CN109476758B CN201780037421.3A CN201780037421A CN109476758B CN 109476758 B CN109476758 B CN 109476758B CN 201780037421 A CN201780037421 A CN 201780037421A CN 109476758 B CN109476758 B CN 109476758B
- Authority
- CN
- China
- Prior art keywords
- amino acid
- seq
- antibody
- acid sequence
- fxi
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/36—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against blood coagulation factors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/39533—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals
- A61K39/3955—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals against proteinaceous materials, e.g. enzymes, hormones, lymphokines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/02—Antithrombotic agents; Anticoagulants; Platelet aggregation inhibitors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/58—Medicinal preparations containing antigens or antibodies raising an immune response against a target which is not the antigen used for immunisation
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/52—Constant or Fc region; Isotype
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
- C07K2317/565—Complementarity determining region [CDR]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/75—Agonist effect on antigen
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/76—Antagonist effect on antigen, e.g. neutralization or inhibition of binding
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/92—Affinity (KD), association rate (Ka), dissociation rate (Kd) or EC50 value
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Immunology (AREA)
- Hematology (AREA)
- Public Health (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Pharmacology & Pharmacy (AREA)
- Engineering & Computer Science (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Genetics & Genomics (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Molecular Biology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Microbiology (AREA)
- Epidemiology (AREA)
- Mycology (AREA)
- Endocrinology (AREA)
- Diabetes (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Peptides Or Proteins (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Management, Administration, Business Operations System, And Electronic Commerce (AREA)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202310501882.9A CN116425879A (zh) | 2016-06-14 | 2017-06-12 | 抗-凝血因子xi抗体 |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201662349888P | 2016-06-14 | 2016-06-14 | |
| US62/349888 | 2016-06-14 | ||
| PCT/US2017/036940 WO2017218371A1 (en) | 2016-06-14 | 2017-06-12 | Anti-coagulation factor xi antibodies |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202310501882.9A Division CN116425879A (zh) | 2016-06-14 | 2017-06-12 | 抗-凝血因子xi抗体 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN109476758A CN109476758A (zh) | 2019-03-15 |
| CN109476758B true CN109476758B (zh) | 2023-05-23 |
Family
ID=59078269
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN201780037421.3A Active CN109476758B (zh) | 2016-06-14 | 2017-06-12 | 抗-凝血因子xi抗体 |
| CN202310501882.9A Pending CN116425879A (zh) | 2016-06-14 | 2017-06-12 | 抗-凝血因子xi抗体 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN202310501882.9A Pending CN116425879A (zh) | 2016-06-14 | 2017-06-12 | 抗-凝血因子xi抗体 |
Country Status (29)
| Country | Link |
|---|---|
| US (6) | US10676536B2 (enExample) |
| EP (1) | EP3469002A1 (enExample) |
| JP (2) | JP7022081B2 (enExample) |
| KR (2) | KR102379580B1 (enExample) |
| CN (2) | CN109476758B (enExample) |
| AR (1) | AR108717A1 (enExample) |
| AU (3) | AU2017286432B2 (enExample) |
| BR (1) | BR112018075858A2 (enExample) |
| CA (2) | CA3172367A1 (enExample) |
| CL (2) | CL2018003565A1 (enExample) |
| CO (1) | CO2018013434A2 (enExample) |
| CR (1) | CR20180583A (enExample) |
| DO (1) | DOP2018000284A (enExample) |
| EA (1) | EA201892716A1 (enExample) |
| EC (1) | ECSP18091593A (enExample) |
| GE (1) | GEP20227382B (enExample) |
| IL (2) | IL315266A (enExample) |
| JO (1) | JOP20180121A1 (enExample) |
| MA (1) | MA45234A (enExample) |
| MX (2) | MX2018015757A (enExample) |
| MY (1) | MY201852A (enExample) |
| NI (1) | NI201800134A (enExample) |
| PE (1) | PE20190416A1 (enExample) |
| PH (1) | PH12018502586A1 (enExample) |
| SG (2) | SG10202103120UA (enExample) |
| TN (1) | TN2018000417A1 (enExample) |
| TW (2) | TWI802193B (enExample) |
| UA (1) | UA129793C2 (enExample) |
| WO (1) | WO2017218371A1 (enExample) |
Families Citing this family (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JOP20200312A1 (ar) | 2015-06-26 | 2017-06-16 | Novartis Ag | الأجسام المضادة للعامل xi وطرق الاستخدام |
| TW201802121A (zh) | 2016-05-25 | 2018-01-16 | 諾華公司 | 抗因子XI/XIa抗體之逆轉結合劑及其用途 |
| WO2017218371A1 (en) * | 2016-06-14 | 2017-12-21 | Merck Sharp & Dohme Corp. | Anti-coagulation factor xi antibodies |
| BR112019012667A2 (pt) | 2016-12-23 | 2020-02-11 | Novartis Ag | Anticorpos do fator xi e métodos de uso |
| CN110423278B (zh) * | 2019-08-08 | 2020-07-17 | 上海博槿生物科技有限公司 | 一种抗凝血因子XI活化形式因子XIa的抗体及其制备方法和应用 |
| AU2021302202A1 (en) * | 2020-07-02 | 2023-02-16 | Beijing Tuo Jie Biopharmaceutical Co. Ltd. | Anti-FXI/FXIa antibody, antigen-binding fragment thereof, and pharmaceutical use thereof |
| KR20230035079A (ko) * | 2020-07-03 | 2023-03-10 | 수조우 알파맵 씨오., 엘티디. | 응고인자 xi(fxi) 결합 단백질 |
| US20240043521A1 (en) | 2021-02-09 | 2024-02-08 | Arxx Therapeutics As | Anti-S100A4 Humanized Antibodies, Uses and Methods |
| EP4442273A4 (en) * | 2021-11-30 | 2025-12-24 | Suzhou Alphamab Co Ltd | METHOD FOR THE PREVENTION AND/OR TREATMENT OF THROMBOEMBOLIC DISEASES |
| EP4644426A1 (en) * | 2022-12-30 | 2025-11-05 | Gan & Lee Pharmaceuticals Co., Ltd. | Antibody against coagulation factor xi and/or activated form factor xia thereof, and use thereof |
| WO2025130748A1 (zh) * | 2023-12-22 | 2025-06-26 | 四川科伦博泰生物医药股份有限公司 | 一种抗凝血因子XI/XIa抗体制剂 |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN104684932A (zh) * | 2012-05-10 | 2015-06-03 | 拜耳药业股份公司 | 能够结合凝血因子XI和/或其活化形式因子XIa的抗体及其用途 |
Family Cites Families (91)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4447233A (en) | 1981-04-10 | 1984-05-08 | Parker-Hannifin Corporation | Medication infusion pump |
| US4439196A (en) | 1982-03-18 | 1984-03-27 | Merck & Co., Inc. | Osmotic drug delivery system |
| US4447224A (en) | 1982-09-20 | 1984-05-08 | Infusaid Corporation | Variable flow implantable infusion apparatus |
| US4487603A (en) | 1982-11-26 | 1984-12-11 | Cordis Corporation | Implantable microinfusion pump system |
| US4816567A (en) | 1983-04-08 | 1989-03-28 | Genentech, Inc. | Recombinant immunoglobin preparations |
| US4596556A (en) | 1985-03-25 | 1986-06-24 | Bioject, Inc. | Hypodermic injection apparatus |
| US4941880A (en) | 1987-06-19 | 1990-07-17 | Bioject, Inc. | Pre-filled ampule and non-invasive hypodermic injection device assembly |
| US4790824A (en) | 1987-06-19 | 1988-12-13 | Bioject, Inc. | Non-invasive hypodermic injection device |
| JP2749167B2 (ja) | 1988-06-21 | 1998-05-13 | ジェネンテク,インコーポレイテッド | 心筋梗塞の処置のための方法および治療用組成物 |
| US5530101A (en) | 1988-12-28 | 1996-06-25 | Protein Design Labs, Inc. | Humanized immunoglobulins |
| DE3920358A1 (de) | 1989-06-22 | 1991-01-17 | Behringwerke Ag | Bispezifische und oligospezifische, mono- und oligovalente antikoerperkonstrukte, ihre herstellung und verwendung |
| US5312335A (en) | 1989-11-09 | 1994-05-17 | Bioject Inc. | Needleless hypodermic injection device |
| US5064413A (en) | 1989-11-09 | 1991-11-12 | Bioject, Inc. | Needleless hypodermic injection device |
| CA2089661C (en) | 1990-08-29 | 2007-04-03 | Nils Lonberg | Transgenic non-human animals capable of producing heterologous antibodies |
| US5633425A (en) | 1990-08-29 | 1997-05-27 | Genpharm International, Inc. | Transgenic non-human animals capable of producing heterologous antibodies |
| US5874299A (en) | 1990-08-29 | 1999-02-23 | Genpharm International, Inc. | Transgenic non-human animals capable of producing heterologous antibodies |
| US5661016A (en) | 1990-08-29 | 1997-08-26 | Genpharm International Inc. | Transgenic non-human animals capable of producing heterologous antibodies of various isotypes |
| US5814318A (en) | 1990-08-29 | 1998-09-29 | Genpharm International Inc. | Transgenic non-human animals for producing heterologous antibodies |
| US5789650A (en) | 1990-08-29 | 1998-08-04 | Genpharm International, Inc. | Transgenic non-human animals for producing heterologous antibodies |
| US5545806A (en) | 1990-08-29 | 1996-08-13 | Genpharm International, Inc. | Ransgenic non-human animals for producing heterologous antibodies |
| US5770429A (en) | 1990-08-29 | 1998-06-23 | Genpharm International, Inc. | Transgenic non-human animals capable of producing heterologous antibodies |
| US5877397A (en) | 1990-08-29 | 1999-03-02 | Genpharm International Inc. | Transgenic non-human animals capable of producing heterologous antibodies of various isotypes |
| US5625126A (en) | 1990-08-29 | 1997-04-29 | Genpharm International, Inc. | Transgenic non-human animals for producing heterologous antibodies |
| EP1136556B1 (en) | 1991-11-25 | 2005-06-08 | Enzon, Inc. | Method of producing multivalent antigen-binding proteins |
| US5383851A (en) | 1992-07-24 | 1995-01-24 | Bioject Inc. | Needleless hypodermic injection device |
| JPH0656382A (ja) | 1992-08-04 | 1994-03-01 | Kawasaki Steel Corp | コークス乾式消火装置のバケット巻上機の吊ビーム装置 |
| MA24512A1 (fr) | 1996-01-17 | 1998-12-31 | Univ Vermont And State Agrienl | Procede pour la preparation d'agents anticoagulants utiles dans le traitement de la thrombose |
| GB9818110D0 (en) | 1998-08-19 | 1998-10-14 | Weston Medical Ltd | Needleless injectors and other devices |
| US6096002A (en) | 1998-11-18 | 2000-08-01 | Bioject, Inc. | NGAS powered self-resetting needle-less hypodermic jet injection apparatus and method |
| JP2002531466A (ja) | 1998-12-01 | 2002-09-24 | プロテイン デザイン ラブス, インコーポレイテッド | γ−インターフェロンに対するヒト化抗体 |
| JP5198704B2 (ja) | 2000-03-03 | 2013-05-15 | メディミューン リミティド | エオタキシンに対するヒト抗体及びそれらの使用 |
| EP1532175B1 (en) | 2002-03-22 | 2012-10-17 | Aprogen, Inc. | Humanized antibody and process for preparing the same |
| DK1648509T3 (da) | 2003-07-15 | 2013-01-07 | Amgen Inc | Humane anti-NGF-neutraliserende antistoffer som selektive NGF-pathway-inhibitorer |
| AR051805A1 (es) | 2004-12-21 | 2007-02-07 | Centocor Inc | Anticuerpos anti-il-12, epitopos, composiciones, metodos y usos |
| US8318906B2 (en) | 2005-04-15 | 2012-11-27 | The Regents Of The University Of California | EMP2 antibodies and their therapeutic uses |
| DK2161336T4 (en) | 2005-05-09 | 2017-04-24 | Ono Pharmaceutical Co | Human monoclonal antibodies for programmed death 1 (PD-1) and methods for treating cancer using anti-PD-1 antibodies alone or in combination with other immunotherapies |
| RS54271B1 (sr) | 2005-07-01 | 2016-02-29 | E. R. Squibb & Sons, L.L.C. | Humana monoklonska antitela za ligand programirane smrti 1 (pd-l1) |
| WO2007067730A2 (en) | 2005-12-08 | 2007-06-14 | Medarex, Inc. | Human monoclonal antibodies to protein tyrosine kinase 7 ( ptk7 ) and their use |
| WO2007067992A2 (en) | 2005-12-08 | 2007-06-14 | Medarex, Inc. | Human monoclonal antibodies to fucosyl-gm1 and methods for using anti-fucosyl-gm1 |
| JP5586952B2 (ja) | 2006-06-06 | 2014-09-10 | クルセル ホランド ベー ヴェー | 腸球菌に対する殺活性を有するヒトの結合分子及びその使用方法 |
| JP5362563B2 (ja) | 2006-08-03 | 2013-12-11 | アストラゼネカ・アクチエボラーグ | αVβ6に対する抗体およびその使用 |
| CL2007002567A1 (es) | 2006-09-08 | 2008-02-01 | Amgen Inc | Proteinas aisladas de enlace a activina a humana. |
| US7833527B2 (en) | 2006-10-02 | 2010-11-16 | Amgen Inc. | Methods of treating psoriasis using IL-17 Receptor A antibodies |
| EP2134749B1 (en) | 2007-03-22 | 2013-11-06 | The Regents of the University of California | Therapeutic monoclonal antibodies that neutralize botulinum neurotoxins |
| GB0708585D0 (en) | 2007-05-03 | 2007-06-13 | Queen Mary & Westfield College | Novel antibody and use in diagnosis and therapy of arthropathies |
| US7982016B2 (en) | 2007-09-10 | 2011-07-19 | Amgen Inc. | Antigen binding proteins capable of binding thymic stromal lymphopoietin |
| US8877688B2 (en) | 2007-09-14 | 2014-11-04 | Adimab, Llc | Rationally designed, synthetic antibody libraries and uses therefor |
| EP3753947A1 (en) | 2007-09-14 | 2020-12-23 | Adimab, LLC | Rationally designed, synthetic antibody libraries and uses therefor |
| KR101652125B1 (ko) | 2007-09-26 | 2016-08-29 | 우드라이 파마 게엠베하 | 헤파린-결합 표피 성장 인자-유사 성장 인자 항원 결합 단백질 |
| LT3002298T (lt) | 2007-11-21 | 2019-12-10 | Univ Oregon Health & Science | Monokloniniai antikūnai prieš faktorių xi ir jų panaudojimo būdai |
| AU2016203944A1 (en) | 2007-11-21 | 2016-07-07 | Oregon Health & Science University | Anti-factor xi monoclonal antibodies and methods of use thereof |
| US8188235B2 (en) | 2008-06-18 | 2012-05-29 | Pfizer Inc. | Antibodies to IL-6 and their uses |
| PL2297207T3 (pl) | 2008-06-19 | 2019-03-29 | Prothix Bv | Zastosowanie przeciwciał przeciw czynnikowi XI do zapobiegania lub leczenia tworzenia skrzepliny |
| US9000131B2 (en) | 2008-07-31 | 2015-04-07 | The Regents Of The University Of California | Antibodies that neutralize botulinum neurotoxins |
| SI2350304T1 (sl) | 2008-10-31 | 2017-04-26 | Janssen Biotech, Inc. | Antagonisti toll-u podobnega receptorja |
| DK2356270T3 (da) | 2008-11-07 | 2016-12-12 | Fabrus Llc | Kombinatoriske antistofbiblioteker og anvendelser deraf |
| US9212223B2 (en) | 2008-11-25 | 2015-12-15 | Alderbio Holdings Llc | Antagonists of IL-6 to prevent or treat thrombosis |
| US9452227B2 (en) | 2008-11-25 | 2016-09-27 | Alderbio Holdings Llc | Methods of treating or diagnosing conditions associated with elevated IL-6 using anti-IL-6 antibodies or fragments |
| DK2373691T3 (en) | 2008-12-18 | 2019-04-15 | Oregon Health&Science Univ | ANTI-FXI ANTIBODIES AND PROCEDURES FOR USE |
| WO2010072740A2 (en) | 2008-12-23 | 2010-07-01 | Astrazeneca Ab | TARGETED BINDING AGENTS DIRECTED TO α5β1 AND USES THEREOF |
| US8734796B2 (en) | 2009-03-20 | 2014-05-27 | Amgen Inc. | Carrier immunoglobulins |
| EP2419448A1 (en) | 2009-04-16 | 2012-02-22 | Abbott Biotherapeutics Corp. | Anti-tnf- antibodies and their uses |
| CN104558179A (zh) | 2009-04-27 | 2015-04-29 | 协和发酵麒麟株式会社 | 用于治疗血液肿瘤的抗IL-3Rα抗体 |
| US8435516B2 (en) | 2009-10-12 | 2013-05-07 | Pfizer Inc. | Cancer treatment |
| WO2011056997A1 (en) | 2009-11-04 | 2011-05-12 | Fabrus Llc | Methods for affinity maturation-based antibody optimization |
| WO2011079004A1 (en) | 2009-12-23 | 2011-06-30 | Schering Corporation | Cell line 3m |
| WO2011097329A2 (en) * | 2010-02-03 | 2011-08-11 | Trilogy Ip Holdings, Inc. | Mobile communication plan offerings |
| JP6266343B2 (ja) | 2010-07-16 | 2018-01-24 | アディマブ, エルエルシー | 抗体ライブラリー |
| US20120156130A1 (en) | 2010-08-06 | 2012-06-21 | Thore Hettmann | Use of her3 binding agents in prostate treatment |
| PH12013500990A1 (en) | 2010-11-19 | 2022-11-23 | Eisai R&D Man Co Ltd | Neutralizing anti-ccl20 antibodies |
| KR102502293B1 (ko) | 2011-01-06 | 2023-02-21 | 다케다 파머수티컬 컴패니 리미티드 | 혈장 칼리크레인 결합 단백질 |
| AU2012236068A1 (en) | 2011-04-01 | 2013-10-17 | Eureka Therapeutics, Inc. | T cell receptor-like antibodies specific for a WT1 peptide presented by HLA-A2 |
| RU2616881C2 (ru) | 2011-06-06 | 2017-04-18 | Ново Нордиск А/С | Терапевтические антитела |
| US8765385B2 (en) | 2011-10-27 | 2014-07-01 | Ravindra Kumar | Method of detection of neutralizing anti-actriib antibodies |
| ES2588484T3 (es) | 2012-04-13 | 2016-11-03 | Rottapharm Biotech S.R.L. | Anticuerpo anti-ADAMTS-5, derivados y usos del mismo |
| JP6441792B2 (ja) | 2012-05-17 | 2018-12-19 | ソレント・セラピューティクス・インコーポレイテッドSorrento Therapeutics, Inc. | Egfrに結合する抗原結合蛋白質 |
| US20140004121A1 (en) | 2012-06-27 | 2014-01-02 | Amgen Inc. | Anti-mesothelin binding proteins |
| US9498532B2 (en) | 2013-03-13 | 2016-11-22 | Novartis Ag | Antibody drug conjugates |
| EP3052523B1 (en) | 2013-10-01 | 2021-03-10 | Medimmune Limited | Methods of treating and diagnosing alpha-v-beta-6 overexpressing cancer |
| MX2016011885A (es) | 2014-03-20 | 2016-12-02 | Japan As Represented By Director-General Of Nat Inst Of Infectious Diseases | Anticuerpo con actividad inhibidora de inyeccion frente al virus de hepatitis c. |
| ES2894867T3 (es) | 2014-06-03 | 2022-02-16 | Xbiotech Inc | Composiciones y procedimientos de tratamiento y prevención de infecciones por estafilococo áureo |
| CA2955086A1 (en) | 2014-08-08 | 2016-02-11 | Alector Llc | Anti-trem2 antibodies and methods of use thereof |
| US9139653B1 (en) | 2015-04-30 | 2015-09-22 | Kymab Limited | Anti-human OX40L antibodies and methods of treatment |
| MX393951B (es) | 2015-04-07 | 2025-03-24 | Alector Llc | Anticuerpos antisortilina y métodos para su uso. |
| HK1252675A1 (zh) | 2015-06-12 | 2019-05-31 | Alector Llc | 抗cd33抗体及其使用方法 |
| JOP20200312A1 (ar) | 2015-06-26 | 2017-06-16 | Novartis Ag | الأجسام المضادة للعامل xi وطرق الاستخدام |
| EP3325010B1 (en) | 2015-07-23 | 2023-06-21 | The Regents of The University of California | Antibodies to coagulation factor xia and uses thereof |
| EP3356415B1 (en) | 2015-09-29 | 2024-05-01 | Amgen Inc. | Asgr inhibitors for reduzing cholesterol levels |
| AR107505A1 (es) | 2016-01-22 | 2018-05-09 | Merck Sharp & Dohme | Anticuerpos anti-factor de la coagulación xi |
| WO2017218371A1 (en) | 2016-06-14 | 2017-12-21 | Merck Sharp & Dohme Corp. | Anti-coagulation factor xi antibodies |
| WO2018054813A1 (en) | 2016-09-20 | 2018-03-29 | Bayer Pharma Aktiengesellschaft | Novel antibodies against factor xi and uses thereof |
-
2017
- 2017-06-12 WO PCT/US2017/036940 patent/WO2017218371A1/en not_active Ceased
- 2017-06-12 SG SG10202103120UA patent/SG10202103120UA/en unknown
- 2017-06-12 CN CN201780037421.3A patent/CN109476758B/zh active Active
- 2017-06-12 JP JP2018565042A patent/JP7022081B2/ja active Active
- 2017-06-12 TN TNP/2018/000417A patent/TN2018000417A1/en unknown
- 2017-06-12 CA CA3172367A patent/CA3172367A1/en active Pending
- 2017-06-12 US US15/619,620 patent/US10676536B2/en active Active
- 2017-06-12 PE PE2018003203A patent/PE20190416A1/es unknown
- 2017-06-12 IL IL315266A patent/IL315266A/en unknown
- 2017-06-12 MA MA045234A patent/MA45234A/fr unknown
- 2017-06-12 SG SG11201810763TA patent/SG11201810763TA/en unknown
- 2017-06-12 UA UAA201900311A patent/UA129793C2/uk unknown
- 2017-06-12 KR KR1020217004608A patent/KR102379580B1/ko active Active
- 2017-06-12 AR ARP170101603A patent/AR108717A1/es unknown
- 2017-06-12 EP EP17731444.0A patent/EP3469002A1/en active Pending
- 2017-06-12 MX MX2018015757A patent/MX2018015757A/es unknown
- 2017-06-12 MY MYPI2018002406A patent/MY201852A/en unknown
- 2017-06-12 EA EA201892716A patent/EA201892716A1/ru unknown
- 2017-06-12 IL IL263272A patent/IL263272B2/en unknown
- 2017-06-12 KR KR1020197001266A patent/KR102218714B1/ko active Active
- 2017-06-12 AU AU2017286432A patent/AU2017286432B2/en active Active
- 2017-06-12 CA CA3025869A patent/CA3025869C/en active Active
- 2017-06-12 CR CR20180583A patent/CR20180583A/es unknown
- 2017-06-12 TW TW110149482A patent/TWI802193B/zh active
- 2017-06-12 GE GEAP201714983A patent/GEP20227382B/en unknown
- 2017-06-12 CN CN202310501882.9A patent/CN116425879A/zh active Pending
- 2017-06-12 TW TW106119391A patent/TWI752964B/zh active
- 2017-06-12 BR BR112018075858-2A patent/BR112018075858A2/pt unknown
- 2017-06-16 JO JOP/2018/0121A patent/JOP20180121A1/ar unknown
-
2018
- 2018-12-06 PH PH12018502586A patent/PH12018502586A1/en unknown
- 2018-12-10 NI NI201800134A patent/NI201800134A/es unknown
- 2018-12-11 CL CL2018003565A patent/CL2018003565A1/es unknown
- 2018-12-11 EC ECSENADI201891593A patent/ECSP18091593A/es unknown
- 2018-12-12 CO CONC2018/0013434A patent/CO2018013434A2/es unknown
- 2018-12-13 DO DO2018000284A patent/DOP2018000284A/es unknown
- 2018-12-14 MX MX2023012740A patent/MX2023012740A/es unknown
-
2020
- 2020-05-01 US US16/864,559 patent/US11661460B2/en active Active
- 2020-05-01 US US16/864,577 patent/US11485794B2/en active Active
- 2020-05-01 US US16/864,570 patent/US11512142B2/en active Active
- 2020-05-01 US US16/864,583 patent/US11479615B2/en active Active
- 2020-07-30 AU AU2020210233A patent/AU2020210233B2/en active Active
-
2021
- 2021-03-26 JP JP2021053470A patent/JP7277500B2/ja active Active
-
2023
- 2023-04-18 US US18/302,033 patent/US12275800B2/en active Active
-
2024
- 2024-02-06 CL CL2024000367A patent/CL2024000367A1/es unknown
- 2024-02-20 AU AU2024201084A patent/AU2024201084A1/en active Pending
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN104684932A (zh) * | 2012-05-10 | 2015-06-03 | 拜耳药业股份公司 | 能够结合凝血因子XI和/或其活化形式因子XIa的抗体及其用途 |
Non-Patent Citations (1)
| Title |
|---|
| Two novel inhibitory anti-human factor XI antibodies prevent cessation of blood flow in a murine venous thrombosis model;Maurits L等;《Thromb Haemost》;20131130;第110卷(第5期);1065-1073 * |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7366988B2 (ja) | 抗凝固因子xi抗体 | |
| US12275800B2 (en) | Anti-coagulation factor XI antibodies |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PB01 | Publication | ||
| PB01 | Publication | ||
| SE01 | Entry into force of request for substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| TA01 | Transfer of patent application right |
Effective date of registration: 20220908 Address after: new jersey Applicant after: MERCK SHARP & DOHME B.V. Applicant after: ADIMAB, LLC Address before: new jersey Applicant before: MERCK SHARP & DOHME Corp. Applicant before: ADIMAB, LLC |
|
| TA01 | Transfer of patent application right | ||
| GR01 | Patent grant | ||
| GR01 | Patent grant | ||
| TG01 | Patent term adjustment | ||
| TG01 | Patent term adjustment |