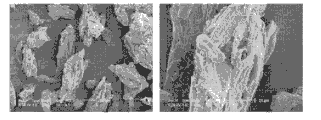
CN102215825A - 可直接压制的颗粒状的基于微晶纤维素的赋形剂、其制备方法及应用 - Google Patents
可直接压制的颗粒状的基于微晶纤维素的赋形剂、其制备方法及应用 Download PDFInfo
- Publication number
- CN102215825A CN102215825A CN2009801461591A CN200980146159A CN102215825A CN 102215825 A CN102215825 A CN 102215825A CN 2009801461591 A CN2009801461591 A CN 2009801461591A CN 200980146159 A CN200980146159 A CN 200980146159A CN 102215825 A CN102215825 A CN 102215825A
- Authority
- CN
- China
- Prior art keywords
- microcrystalline cellulose
- binder
- excipient
- slurry
- disintegrant
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2022—Organic macromolecular compounds
- A61K9/205—Polysaccharides, e.g. alginate, gums; Cyclodextrin
- A61K9/2054—Cellulose; Cellulose derivatives, e.g. hydroxypropyl methylcellulose
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/30—Macromolecular organic or inorganic compounds, e.g. inorganic polyphosphates
- A61K47/36—Polysaccharides; Derivatives thereof, e.g. gums, starch, alginate, dextrin, hyaluronic acid, chitosan, inulin, agar or pectin
- A61K47/38—Cellulose; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2022—Organic macromolecular compounds
- A61K9/2027—Organic macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyvinyl pyrrolidone, poly(meth)acrylates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2095—Tabletting processes; Dosage units made by direct compression of powders or specially processed granules, by eliminating solvents, by melt-extrusion, by injection molding, by 3D printing
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Epidemiology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Inorganic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Preparation (AREA)
- Polysaccharides And Polysaccharide Derivatives (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US11602508P | 2008-11-19 | 2008-11-19 | |
| US61/116,025 | 2008-11-19 | ||
| PCT/US2009/064498 WO2010059534A2 (en) | 2008-11-19 | 2009-11-16 | Directly compressible granular microcrystalline cellulose based excipient, manufacturing process and use thereof |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| CN102215825A true CN102215825A (zh) | 2011-10-12 |
Family
ID=42077071
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CN2009801461591A Pending CN102215825A (zh) | 2008-11-19 | 2009-11-16 | 可直接压制的颗粒状的基于微晶纤维素的赋形剂、其制备方法及应用 |
Country Status (13)
| Country | Link |
|---|---|
| US (1) | US20110288146A1 (OSRAM) |
| EP (1) | EP2376067A2 (OSRAM) |
| JP (1) | JP2012509329A (OSRAM) |
| KR (1) | KR20110089180A (OSRAM) |
| CN (1) | CN102215825A (OSRAM) |
| AU (1) | AU2009316812A1 (OSRAM) |
| BR (1) | BRPI0921507A2 (OSRAM) |
| CA (1) | CA2744142A1 (OSRAM) |
| IL (1) | IL212955A0 (OSRAM) |
| MX (1) | MX2011005168A (OSRAM) |
| SG (1) | SG171752A1 (OSRAM) |
| TW (1) | TW201023897A (OSRAM) |
| WO (1) | WO2010059534A2 (OSRAM) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN104189915A (zh) * | 2014-07-30 | 2014-12-10 | 上海新亚药业闵行有限公司 | 一种固体制剂预混剂及其制备方法 |
| CN104258411A (zh) * | 2014-09-19 | 2015-01-07 | 安徽山河药用辅料股份有限公司 | 一种硅化微晶纤维素复合辅料及其制备方法 |
| CN106265077A (zh) * | 2015-05-30 | 2017-01-04 | 天士力制药集团股份有限公司 | 一种中药浸膏雾化分散工艺及其制剂 |
| CN116036291A (zh) * | 2022-08-31 | 2023-05-02 | 瑞普(天津)生物药业有限公司 | 一种具有增溶作用的组合物及其制备方法与应用 |
Families Citing this family (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101298788B1 (ko) * | 2011-03-15 | 2013-08-22 | 보령제약 주식회사 | 안정성이 개선된 복합제제 |
| JP5879668B2 (ja) * | 2012-04-27 | 2016-03-08 | 日本コーンスターチ株式会社 | 澱粉顆粒の製造方法および口腔内崩壊錠 |
| US9492535B2 (en) | 2013-03-14 | 2016-11-15 | Aimmune Therapeutics, Inc. | Peanut formulations and uses thereof |
| ES2940686T3 (es) | 2013-03-14 | 2023-05-10 | Nestle Sa | Fabricación de formulaciones de cacahuete para la desensibilización oral |
| WO2015187736A1 (en) * | 2014-06-02 | 2015-12-10 | Allergen Research Corporation | Placebo formulations and uses thereof |
| JP6650750B2 (ja) * | 2015-12-22 | 2020-02-19 | アサヒグループ食品株式会社 | 錠剤の製造方法、錠剤原料用顆粒の製造方法、及び錠剤原料用顆粒 |
| WO2020037151A1 (en) | 2018-08-16 | 2020-02-20 | Aimmune Therapeutics, Inc. | Peanut oral immunotherapy with maintenance dose |
| JP2022514645A (ja) | 2018-12-20 | 2022-02-14 | アイミューン セラピューティクス,インコーポレイテッド | ピーナツ経口免疫療法において投与が欠落した場合の投与スケジュール |
| EP3965815A4 (en) | 2019-05-10 | 2023-05-31 | Société des Produits Nestlé S.A. | METHOD OF IMPROVING THE QUALITY OF LIFE IN A PEANUT ALLERGY PATIENT |
| PH12021553026A1 (en) | 2019-07-05 | 2023-08-23 | Swedish Match North Europe Ab | An oral pouched nicotine product including a filling material comprising nicotine-containing particles |
| PL4061155T3 (pl) | 2019-11-20 | 2024-01-29 | Swedish Match North Europe Ab | Doustny produkt nikotynowy w saszetce, zawierający materiał wypełniający zawierający cząstki nikotyny |
| PL4018848T3 (pl) | 2020-12-22 | 2024-11-25 | Swedish Match North Europe Ab | Wyrób w saszetkach do użytku doustnego zawierający materiał pokrywający przepuszczalny dla cieczy oraz materiał wypełniający |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1074228A (zh) * | 1991-12-30 | 1993-07-14 | Fmc有限公司 | 球团化微晶纤维素组合物 |
| WO2002062320A1 (en) * | 2001-02-05 | 2002-08-15 | R.P. Scherer Technologies, Inc. | Methods and compositions for reducing the taste of pharmaceutically active agents |
| CN1473054A (zh) * | 2000-11-06 | 2004-02-04 | ������������ʽ���� | 制剂用纤维素粒子 |
Family Cites Families (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE3505433A1 (de) * | 1985-02-16 | 1986-08-21 | Basf Ag, 6700 Ludwigshafen | Direkttablettierhilfsmittel |
| US5686107A (en) * | 1995-01-30 | 1997-11-11 | Fmc Corporation | Chewable pharmaceutical tablets |
| DE19628617A1 (de) * | 1996-07-16 | 1998-01-22 | Basf Ag | Direkttablettierhilfsmittel |
| EP1070740B1 (de) * | 1999-07-19 | 2008-01-02 | fit GmbH | Coprozessiertes Polysaccharidprodukt mit unlöslicher Carboxymethylcellulose |
| US8808735B2 (en) * | 2005-02-03 | 2014-08-19 | Takeda Nycomed As | Fast wet-massing method for the preparation of calcium-containing compositions |
| WO2008020990A1 (en) * | 2006-08-09 | 2008-02-21 | Mallinckrodt Baker, Inc. | New direct compressible excipient blend |
| US7998505B2 (en) * | 2006-10-27 | 2011-08-16 | Fmc Corporation | Dry granulation binders, products, and use thereof |
| SG185257A1 (en) * | 2007-10-10 | 2012-11-29 | Avantor Performance Mat Inc | Directly compressible high functionality granular microcrystalline cellulose based excipient, manufacturing process and use thereof |
-
2009
- 2009-11-16 AU AU2009316812A patent/AU2009316812A1/en not_active Abandoned
- 2009-11-16 EP EP09752702A patent/EP2376067A2/en not_active Ceased
- 2009-11-16 CA CA2744142A patent/CA2744142A1/en not_active Abandoned
- 2009-11-16 CN CN2009801461591A patent/CN102215825A/zh active Pending
- 2009-11-16 JP JP2011537526A patent/JP2012509329A/ja active Pending
- 2009-11-16 WO PCT/US2009/064498 patent/WO2010059534A2/en not_active Ceased
- 2009-11-16 US US12/998,659 patent/US20110288146A1/en not_active Abandoned
- 2009-11-16 SG SG2011035789A patent/SG171752A1/en unknown
- 2009-11-16 MX MX2011005168A patent/MX2011005168A/es not_active Application Discontinuation
- 2009-11-16 KR KR1020117013939A patent/KR20110089180A/ko not_active Ceased
- 2009-11-16 BR BRPI0921507A patent/BRPI0921507A2/pt not_active IP Right Cessation
- 2009-11-19 TW TW098139345A patent/TW201023897A/zh unknown
-
2011
- 2011-05-17 IL IL212955A patent/IL212955A0/en unknown
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1074228A (zh) * | 1991-12-30 | 1993-07-14 | Fmc有限公司 | 球团化微晶纤维素组合物 |
| CN1473054A (zh) * | 2000-11-06 | 2004-02-04 | ������������ʽ���� | 制剂用纤维素粒子 |
| WO2002062320A1 (en) * | 2001-02-05 | 2002-08-15 | R.P. Scherer Technologies, Inc. | Methods and compositions for reducing the taste of pharmaceutically active agents |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN104189915A (zh) * | 2014-07-30 | 2014-12-10 | 上海新亚药业闵行有限公司 | 一种固体制剂预混剂及其制备方法 |
| CN104189915B (zh) * | 2014-07-30 | 2016-08-10 | 上海新亚药业闵行有限公司 | 一种固体制剂预混剂及其制备方法 |
| CN104258411A (zh) * | 2014-09-19 | 2015-01-07 | 安徽山河药用辅料股份有限公司 | 一种硅化微晶纤维素复合辅料及其制备方法 |
| CN106265077A (zh) * | 2015-05-30 | 2017-01-04 | 天士力制药集团股份有限公司 | 一种中药浸膏雾化分散工艺及其制剂 |
| CN116036291A (zh) * | 2022-08-31 | 2023-05-02 | 瑞普(天津)生物药业有限公司 | 一种具有增溶作用的组合物及其制备方法与应用 |
Also Published As
| Publication number | Publication date |
|---|---|
| US20110288146A1 (en) | 2011-11-24 |
| CA2744142A1 (en) | 2010-05-27 |
| WO2010059534A2 (en) | 2010-05-27 |
| KR20110089180A (ko) | 2011-08-04 |
| AU2009316812A1 (en) | 2011-07-07 |
| BRPI0921507A2 (pt) | 2016-01-19 |
| MX2011005168A (es) | 2011-05-30 |
| SG171752A1 (en) | 2011-07-28 |
| EP2376067A2 (en) | 2011-10-19 |
| IL212955A0 (en) | 2011-07-31 |
| JP2012509329A (ja) | 2012-04-19 |
| TW201023897A (en) | 2010-07-01 |
| WO2010059534A3 (en) | 2011-01-20 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN102215825A (zh) | 可直接压制的颗粒状的基于微晶纤维素的赋形剂、其制备方法及应用 | |
| JP2014148556A (ja) | 直接圧縮可能で高機能性な顆粒状のリン酸水素カルシウムベースの共に処理された賦形剤 | |
| MX2011000277A (es) | Composiciones farmaceuticas que comprenden 5-cloro-n-({(5s)-2-oxo- 3-[4-(3-oxo-4-morfolinil)-fenil]-1,3-oxazolidin-5-il}-metil)-2-ti ofencarboxamida. | |
| US20170112768A1 (en) | Directly compressible granular microcrystalline cellulose based excipient, manufacturing process and use thereof | |
| JP2013514349A (ja) | 共加工された錠用賦形剤混合物、その調製及び使用 | |
| TWI498130B (zh) | 以可直接壓縮高功能性粒狀微晶為主之賦形劑,其製造方法及用途 | |
| JP5711969B6 (ja) | 直接圧縮可能な高機能性の顆粒状の微結晶性セルロースベースの賦形剤、製造工程およびその使用 | |
| US20120100211A1 (en) | Material and process for incorporation of low dosage active pharmaceutical ingredients and use thereof | |
| JP2011500565A5 (OSRAM) |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| RJ01 | Rejection of invention patent application after publication |
Application publication date: 20111012 |
|
| RJ01 | Rejection of invention patent application after publication |