WO2022158348A1 - 樹脂組成物、光学積層体、光学物品、レンズ及び眼鏡 - Google Patents
樹脂組成物、光学積層体、光学物品、レンズ及び眼鏡 Download PDFInfo
- Publication number
- WO2022158348A1 WO2022158348A1 PCT/JP2022/000699 JP2022000699W WO2022158348A1 WO 2022158348 A1 WO2022158348 A1 WO 2022158348A1 JP 2022000699 W JP2022000699 W JP 2022000699W WO 2022158348 A1 WO2022158348 A1 WO 2022158348A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- component
- resin composition
- photochromic
- active hydrogen
- group
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- G—PHYSICS
- G02—OPTICS
- G02C—SPECTACLES; SUNGLASSES OR GOGGLES INSOFAR AS THEY HAVE THE SAME FEATURES AS SPECTACLES; CONTACT LENSES
- G02C7/00—Optical parts
- G02C7/10—Filters, e.g. for facilitating adaptation of the eyes to the dark; Sunglasses
- G02C7/102—Photochromic filters
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/06—Layered products comprising a layer of synthetic resin as the main or only constituent of a layer, which is next to another layer of the same or of a different material
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/40—Layered products comprising a layer of synthetic resin comprising polyurethanes
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/08—Processes
- C08G18/16—Catalysts
- C08G18/22—Catalysts containing metal compounds
- C08G18/24—Catalysts containing metal compounds of tin
- C08G18/244—Catalysts containing metal compounds of tin tin salts of carboxylic acids
- C08G18/246—Catalysts containing metal compounds of tin tin salts of carboxylic acids containing also tin-carbon bonds
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/28—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the compounds used containing active hydrogen
- C08G18/2805—Compounds having only one group containing active hydrogen
- C08G18/2815—Monohydroxy compounds
- C08G18/283—Compounds containing ether groups, e.g. oxyalkylated monohydroxy compounds
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/28—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the compounds used containing active hydrogen
- C08G18/30—Low-molecular-weight compounds
- C08G18/38—Low-molecular-weight compounds having heteroatoms other than oxygen
- C08G18/3855—Low-molecular-weight compounds having heteroatoms other than oxygen having sulfur
- C08G18/3876—Low-molecular-weight compounds having heteroatoms other than oxygen having sulfur containing mercapto groups
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/28—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the compounds used containing active hydrogen
- C08G18/40—High-molecular-weight compounds
- C08G18/48—Polyethers
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/28—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the compounds used containing active hydrogen
- C08G18/40—High-molecular-weight compounds
- C08G18/48—Polyethers
- C08G18/4825—Polyethers containing two hydroxy groups
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/28—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the compounds used containing active hydrogen
- C08G18/40—High-molecular-weight compounds
- C08G18/48—Polyethers
- C08G18/4833—Polyethers containing oxyethylene units
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/28—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the compounds used containing active hydrogen
- C08G18/40—High-molecular-weight compounds
- C08G18/48—Polyethers
- C08G18/4854—Polyethers containing oxyalkylene groups having four carbon atoms in the alkylene group
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/28—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the compounds used containing active hydrogen
- C08G18/40—High-molecular-weight compounds
- C08G18/64—Macromolecular compounds not provided for by groups C08G18/42 - C08G18/63
- C08G18/6484—Polysaccharides and derivatives thereof
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/70—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the isocyanates or isothiocyanates used
- C08G18/72—Polyisocyanates or polyisothiocyanates
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/70—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the isocyanates or isothiocyanates used
- C08G18/72—Polyisocyanates or polyisothiocyanates
- C08G18/74—Polyisocyanates or polyisothiocyanates cyclic
- C08G18/75—Polyisocyanates or polyisothiocyanates cyclic cycloaliphatic
- C08G18/751—Polyisocyanates or polyisothiocyanates cyclic cycloaliphatic containing only one cycloaliphatic ring
- C08G18/752—Polyisocyanates or polyisothiocyanates cyclic cycloaliphatic containing only one cycloaliphatic ring containing at least one isocyanate or isothiocyanate group linked to the cycloaliphatic ring by means of an aliphatic group
- C08G18/753—Polyisocyanates or polyisothiocyanates cyclic cycloaliphatic containing only one cycloaliphatic ring containing at least one isocyanate or isothiocyanate group linked to the cycloaliphatic ring by means of an aliphatic group containing one isocyanate or isothiocyanate group linked to the cycloaliphatic ring by means of an aliphatic group having a primary carbon atom next to the isocyanate or isothiocyanate group
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/70—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the isocyanates or isothiocyanates used
- C08G18/72—Polyisocyanates or polyisothiocyanates
- C08G18/74—Polyisocyanates or polyisothiocyanates cyclic
- C08G18/75—Polyisocyanates or polyisothiocyanates cyclic cycloaliphatic
- C08G18/751—Polyisocyanates or polyisothiocyanates cyclic cycloaliphatic containing only one cycloaliphatic ring
- C08G18/752—Polyisocyanates or polyisothiocyanates cyclic cycloaliphatic containing only one cycloaliphatic ring containing at least one isocyanate or isothiocyanate group linked to the cycloaliphatic ring by means of an aliphatic group
- C08G18/753—Polyisocyanates or polyisothiocyanates cyclic cycloaliphatic containing only one cycloaliphatic ring containing at least one isocyanate or isothiocyanate group linked to the cycloaliphatic ring by means of an aliphatic group containing one isocyanate or isothiocyanate group linked to the cycloaliphatic ring by means of an aliphatic group having a primary carbon atom next to the isocyanate or isothiocyanate group
- C08G18/755—Polyisocyanates or polyisothiocyanates cyclic cycloaliphatic containing only one cycloaliphatic ring containing at least one isocyanate or isothiocyanate group linked to the cycloaliphatic ring by means of an aliphatic group containing one isocyanate or isothiocyanate group linked to the cycloaliphatic ring by means of an aliphatic group having a primary carbon atom next to the isocyanate or isothiocyanate group and at least one isocyanate or isothiocyanate group linked to a secondary carbon atom of the cycloaliphatic ring, e.g. isophorone diisocyanate
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/70—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the isocyanates or isothiocyanates used
- C08G18/72—Polyisocyanates or polyisothiocyanates
- C08G18/74—Polyisocyanates or polyisothiocyanates cyclic
- C08G18/75—Polyisocyanates or polyisothiocyanates cyclic cycloaliphatic
- C08G18/751—Polyisocyanates or polyisothiocyanates cyclic cycloaliphatic containing only one cycloaliphatic ring
- C08G18/752—Polyisocyanates or polyisothiocyanates cyclic cycloaliphatic containing only one cycloaliphatic ring containing at least one isocyanate or isothiocyanate group linked to the cycloaliphatic ring by means of an aliphatic group
- C08G18/757—Polyisocyanates or polyisothiocyanates cyclic cycloaliphatic containing only one cycloaliphatic ring containing at least one isocyanate or isothiocyanate group linked to the cycloaliphatic ring by means of an aliphatic group containing at least two isocyanate or isothiocyanate groups linked to the cycloaliphatic ring by means of an aliphatic group
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G18/00—Polymeric products of isocyanates or isothiocyanates
- C08G18/06—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen
- C08G18/70—Polymeric products of isocyanates or isothiocyanates with compounds having active hydrogen characterised by the isocyanates or isothiocyanates used
- C08G18/72—Polyisocyanates or polyisothiocyanates
- C08G18/74—Polyisocyanates or polyisothiocyanates cyclic
- C08G18/75—Polyisocyanates or polyisothiocyanates cyclic cycloaliphatic
- C08G18/758—Polyisocyanates or polyisothiocyanates cyclic cycloaliphatic containing two or more cycloaliphatic rings
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L75/00—Compositions of polyureas or polyurethanes; Compositions of derivatives of such polymers
- C08L75/04—Polyurethanes
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L75/00—Compositions of polyureas or polyurethanes; Compositions of derivatives of such polymers
- C08L75/04—Polyurethanes
- C08L75/08—Polyurethanes from polyethers
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K9/00—Tenebrescent materials, i.e. materials for which the range of wavelengths for energy absorption is changed as a result of excitation by some form of energy
- C09K9/02—Organic tenebrescent materials
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B1/00—Optical elements characterised by the material of which they are made; Optical coatings for optical elements
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B1/00—Optical elements characterised by the material of which they are made; Optical coatings for optical elements
- G02B1/04—Optical elements characterised by the material of which they are made; Optical coatings for optical elements made of organic materials, e.g. plastics
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B1/00—Optical elements characterised by the material of which they are made; Optical coatings for optical elements
- G02B1/04—Optical elements characterised by the material of which they are made; Optical coatings for optical elements made of organic materials, e.g. plastics
- G02B1/041—Lenses
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B5/00—Optical elements other than lenses
- G02B5/20—Filters
- G02B5/22—Absorbing filters
- G02B5/23—Photochromic filters
-
- G—PHYSICS
- G02—OPTICS
- G02C—SPECTACLES; SUNGLASSES OR GOGGLES INSOFAR AS THEY HAVE THE SAME FEATURES AS SPECTACLES; CONTACT LENSES
- G02C7/00—Optical parts
- G02C7/10—Filters, e.g. for facilitating adaptation of the eyes to the dark; Sunglasses
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2250/00—Layers arrangement
- B32B2250/02—2 layers
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/40—Properties of the layers or laminate having particular optical properties
- B32B2307/42—Polarizing, birefringent, filtering
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2375/00—Polyureas; Polyurethanes
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2551/00—Optical elements
Definitions
- the present invention relates to resin compositions, optical laminates, optical articles, lenses and spectacles.
- Photochromic spectacle lenses containing a photochromic compound are quickly colored and function as sunglasses outdoors where light including ultraviolet light such as sunlight is irradiated, and fade and become transparent indoors where such light is not irradiated. functions as a pair of glasses.
- the demand for photochromic optical articles having such photochromic properties has increased in recent years.
- the following methods are generally used to impart photochromic properties to spectacle lenses.
- the optical plate may be plastic or inorganic glass.
- Patent Literature 1 describes a dyed lens obtained by dyeing a urethane-based resin (molded body) as an article similar to a photochromic optical article.
- the urethane-based resin that forms this molded product uses thiol as a monomer and has excellent mechanical strength having (thio)urethane bonds in its molecular chain.
- the urethane resin used as the matrix has a (thio)urethane bond, so the monomer formulation needs to be specially adjusted. Otherwise, the molecular mobility of ring-opening/ring-closing of the photochromic compound may be inhibited.
- Patent Document 2 uses a bifunctional active hydrogen compound having a polypropylene glycol chain or the like, and Patent Document 3 uses a monofunctional active hydrogen compound. According to these methods, photochromic properties can be improved by forming spaces in the urethane matrix using a specific active hydrogen compound.
- the inventor of the present invention thought that it might be necessary to control the molecular mobility in the urethane resin by means of cross-linking molecules, rather than simply forming a free space. Then, it was found that a cured product (a urethane resin in which a photochromic compound is dispersed) that satisfies specific molecular mobility parameters can express good photochromic properties and mechanical properties, and the present invention has been completed. rice field.
- the present invention includes the following inventions. 1. Containing a urethane resin having a polyoxypropylene chain in the molecular chain and a photochromic compound, the maximum intensity of the signal within the range of 16 ppm or more and 20 ppm or less in the first spectrum by 13 C-PST/MAS nuclear magnetic resonance spectroscopy (PMI pst ); The ratio (PMI pst /PMI cp ) to the maximum intensity (PMI cp ) of the signal within the range of 16 ppm or more and 20 ppm or less in the second spectrum by 13 C-CP/MAS nuclear magnetic resonance spectroscopy is 8.0 or more and 40. 0 or less resin composition. 2.
- the ratio (EI pst /EI cp ) to the maximum intensity (EI cp ) of the signal within the range of 68 ppm or more and 72 ppm or less in the second spectrum is 5.0 or more and 20.0 or less. of the resin composition. 4.
- the urethane-based resin is (A) a polyiso(thio)cyanate component having two or more iso(thio)cyanate groups in the molecule selected from the group consisting of isocyanate groups and isothiocyanate groups; (B) an active hydrogen-containing component having an active hydrogen-containing group; A resin obtained by reacting Let nB be the total number of moles of active hydrogen-containing groups possessed by the active hydrogen-containing component (B), When the total number of moles of iso(thio)cyanate groups possessed by the (A) polyiso(thio)cyanate component is nA, The ratio (nA/nB) is 1.00 or more and 1.50 or less,
- the (B) active hydrogen-containing component is (B1) a polyfunctional active hydrogen-containing component having 3 or more active hydrogen-containing groups in one molecule; (B2) a first active hydrogen-containing component having one or two active hydrogen-containing groups in one molecule, 5.
- the resin composition according to item 7, wherein the (B2) first active hydrogen-containing component has the alkyl group and has 5 or more and 20 or less carbon atoms. 9. 8. The resin composition according to item 7, wherein the (B2) first active hydrogen-containing component has the polyoxyethylene chain, and the average value of repeating units thereof is 5 or more and 25 or less. 10.
- the (B1) polyfunctional active hydrogen-containing component is 10. The resin composition according to any one of the preceding items 5 to 9, which contains a compound having a quaternary carbon atom in the molecule and all groups bonded to the quaternary carbon atom having an active hydrogen-containing group. . 11.
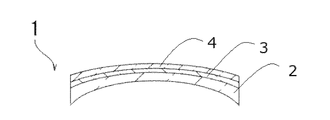
- an optical substrate The resin composition according to any one of the preceding items 1 to 10, which is laminated on at least one main surface of the optical substrate;
- An optical stack comprising: 12. 12.
- An optical article comprising the resin composition according to any one of 1 to 10 above.
- a lens comprising the resin composition according to any one of 1 to 10 above.
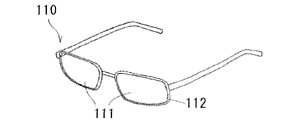
- 15. Spectacles comprising the lens according to 14 above.
- a resin composition having excellent photochromic properties and mechanical properties can be obtained.
- a resin composition having excellent photochromic properties and heat resistance can be obtained.
- BRIEF DESCRIPTION OF THE DRAWINGS Sectional drawing which shows schematically an example of the optical laminated body which concerns on embodiment.
- BRIEF DESCRIPTION OF THE DRAWINGS The perspective view which shows schematically an example of the spectacles which concern on embodiment.
- the resin composition is (i) a urethane resin having a polyoxypropylene chain in the molecular chain (component (i)), and (ii) a photochromic compound (component (ii)) including.
- the (i) urethane-based resin has a polyoxypropylene chain in its molecular chain, and when a urethane-based resin in which the molecular mobility of the polyoxypropylene chain satisfies a specific range is used as a matrix, it is excellent. It was found that the effect of Specifically, the motility of the portion containing the carbon atoms of the methyl group of the polyoxypropylene chain is evaluated by solid-state 13 CNMR measurement, and excellent photochromic is obtained by satisfying a specific range for the motility index. It results in a resin composition having properties and mechanical properties.
- Solid-state NMR measurement methods for carbon nuclei include Pulse Saturation Transfer/Magic Angle Spinning (PST/MAS) method and Cross Polarization/Magic Angle Spinning (CP/MAS) method.
- PST/MAS Pulse Saturation Transfer/Magic Angle Spinning
- CP/MAS Cross Polarization/Magic Angle Spinning
- the PST/MAS method is a technique for emphasizing and observing highly mobile parts (amorphous parts).
- the CP/MAS method is a technique for emphasizing and observing a portion with low mobility (crystalline portion). Therefore, by comparing the intensity of specific signals in these spectra, it is possible to evaluate the molecular mobility of the components constituting the urethane-based resin (matrix resin in which the photochromic compound is dispersed).
- the solid-state 13 C NMR spectrum of the resin composition by the PST/MAS method is also referred to as the first spectrum.
- a solid-state 13 C NMR spectrum of the resin composition by the CP/MAS method is also referred to as a second spectrum.
- the intensity of each peak measured by the PST/MAS method is I PST and the intensity of each peak measured by the CP/MAS method is I CP
- the value obtained by dividing I PST by I CP is I PST /I CP is an index of molecular mobility, and the higher the molecular mobility, the larger the I PST /I CP value.
- I PST /I CP 1
- a carbon atom of a C ⁇ O group appearing within the range of 163 to 168 ppm was taken as a reference peak.
- the (i) component essentially contains a polyoxypropylene chain. And it preferably contains an alkyl group and/or an oxyethylene chain.
- the chemical shift values of the carbon nucleus in each unit observed in the solid-state 13 C-NMR measurement are roughly as follows. 10-15 ppm: terminal methyl group in the alkyl group, 16-20 ppm: methyl group in the oxypropylene unit, 68 ppm to 72 ppm: ethylene group in oxyethylene unit 73 ppm to 80 ppm: ethylene group in oxypropylene unit (excluding methyl group).
- component (i) contains an alkyl group having 2 or more carbon atoms, a methyl group at the end of the alkyl group can be confirmed.
- a resin composition containing a photochromic compound or a photochromic optical article can be directly analyzed by NMR to determine the I PST /I CP value of each carbon nucleus.
- PMI pst is the maximum intensity, ie, maximum height, of a signal appearing within the range of 16 ppm to 20 ppm in the first spectrum.
- PMI cp is the maximum intensity, ie, maximum height, of a signal appearing within the range of 16 ppm to 20 ppm in the second spectrum.
- signals appearing within the range of 16 ppm to 20 ppm in the first and second spectra are signals that are believed to originate from the methyl group of the oxypropylene unit.
- the strength ratio (PMI pst /PMI cp ) of the resin composition is within a specific range.
- the urethane resin which constitutes the majority of the matrix of the resin composition, has polyoxypropylene chains in its molecular chains, free space is likely to be secured, and high photochromic properties can be exhibited. That is, since the matrix of the resin composition becomes flexible, the structural change of the photochromic compound is less likely to be hindered, and photochromic properties can be exhibited over a long period of time.
- the proportion of polyoxypropylene chains is excessively increased or the length of the introduced chains is too long, the mechanical properties may be degraded.
- the present inventors considered that it was necessary to evaluate the motility of component (i) itself in order to improve photochromic properties while maintaining high mechanical properties. Then, when the motility of the component (i) is measured by the NMR method described above, by setting the intensity ratio (PMI pst /PMI cp ) of the component (i) to 8.0 or more and 40.0 or less, It was found that excellent effects can be exhibited. In other words, not only does it form a free space, but also the (i) component itself has moderate mobility, so it is thought that the balance between the photochromic properties and the mechanical properties is excellent.
- the intensity ratio (PMI pst /PMI cp ) of component (i) is less than 8.0, the photochromic properties are inferior, which is not preferable.
- the mechanical properties, particularly the heat resistance, are degraded, which is not preferable. That is, if the strength ratio (PMI pst /PMI cp ) is less than 8.0, it may mean that the percentage of flexible polyoxypropylene chains with high molecular mobility in the resin composition is too low. In such a resin composition, the photochromic compound is less likely to undergo a structural change, and the photochromic properties of the photochromic compound, such as the fading rate and color density, are less likely to be exhibited.
- a strength ratio (PMI pst /PMI cp ) of more than 40.0 may mean that the ratio of highly crystalline polyoxypropylene chains in the resin composition is too low. Such resin compositions tend to have insufficient mechanical properties such as heat resistance.
- the intensity ratio (PMI pst /PMI cp ) is preferably 10.0 to 35.0, more preferably 10.0 to 15.0, or 20.0 in order to exhibit better characteristics. It is more preferable to set it to ⁇ 35.0.
- the intensity ratio (PMI pst /PMI cp ) can be 20.0 to 35.0. Especially preferred. When the monofunctional polymerizable monomer is not used, the degree of cross-linking increases, so it is considered that even if the strength ratio (PMI pst /PMI cp ) is high, excellent effects are exhibited. When a monofunctional polymerizable monomer is not used, the intensity ratio (PMI pst /PMI cp ) is preferably 23.5 to 35.0, more preferably 25.5, considering the balance of physical properties. ⁇ 35.0 is preferable.
- the intensity ratio (PMI pst /PMI cp ) is 10.0 to 15.0. And it is most preferable to use the monofunctional polymerizable monomer.
- a resin composition having excellent photochromic properties and mechanical properties is obtained by using a monofunctional polymerizable monomer and satisfying a strength ratio (PMI pst /PMI cp ) of 10.0 to 15.0. can do
- the intensity ratio (PMI pst /PMI cp ) is more preferably 11.0 to 15.0, more preferably 12.5 to 15.0.
- the intensity PMI pst is preferably 1.0 or more and 20.0 or less.
- a resin composition having a strength PMI pst within this range tends to have a sufficient amount of flexible polyoxypropylene chains with high molecular mobility.
- the intensity PMI pst is more preferably 1.1 or more and 17.5 or less, more preferably 1.1 or more and 3.5 or less, or 13.0 or more and 17.5 or less.
- the intensity PMI cp is preferably 0.1 or more and 1.0 or less.
- a resin composition having a strength PMI cp within this range tends to have sufficient polyoxypropylene chains with high crystallinity.
- the intensity PMI cp is more preferably 0.10 or more and 0.70 or less, more preferably 0.15 or more and 0.30 or less, or 0.50 or more and 0.70 or less.
- the component (i) preferably has an alkyl group in its molecule.
- This alkyl group is preferably a group having 5 or more and 20 or less carbon atoms, and is preferably a linear alkyl group.
- This alkyl group may constitute a side chain or a main chain of the component (i).
- Component (i) having an alkyl group in the molecule can be obtained, for example, by using a polymerizable monomer having an alkyl group as the monofunctional polymerizable monomer described above.
- the terminal group located opposite to the reactive group of the monofunctional polymerizable monomer is an alkyl group, preferably a linear alkyl group having 5 or more and 20 or less carbon atoms.
- AMI pst be the spectral intensity of the carbon atom of the methyl group at the end of the alkyl group measured by 13 C-PST/MAS NMR
- AMI cp the intensity ratio of AMI pst to AMI cp (AMI pst /AMI cp ) is preferably 7.0 or more and 23.0 or less.
- the resulting resin composition can have improved photochromic properties and mechanical properties.
- the intensity ratio (AMI pst /AMI cp ) is more preferably 11.5 or more and 20.0 or less, and more preferably 13.5 or more and 16.0 or less.
- the strength AMI pst is preferably 1.0 or more and 5.0 or less.
- a resin composition having a strength AMI pst within this range tends to have a sufficient number of flexible alkyl groups with high molecular mobility.
- the intensity AMI pst is more preferably 1.10 or more and 3.00 or less, and even more preferably 2.0 or more and 2.8 or less.
- the strength AMI cp is preferably 0.1 or more and 0.5 or less.
- a resin composition having a strength AMI cp within this range tends to have sufficient alkyl groups with high crystallinity.
- the strength AMI cp is more preferably 0.10 or more and 0.30 or less, and still more preferably 0.15 or more and 0.20 or less.
- component (i) has an oxyethylene chain in the molecule. Having a polyoxyethylene chain in the molecular chain, Let EI pst be the spectral intensity of the carbon atoms of the polyoxyethylene chain measured by 13 C-PST/MAS NMR, When the spectral intensity of the carbon atoms of the polyoxyethylene chain measured by 13 C-CP/MAS NMR is defined as EI cp , The intensity ratio of EI pst to EI cp (EI pst /EI cp ) is preferably 5.0 or more and 20.0 or less.
- the resulting resin composition can have improved photochromic properties and mechanical properties. More preferably, the intensity ratio (EI pst /EI cp ) is 6.5 or more and 12.0 or less. As noted above, signals believed to be due to ethylene groups in the oxyethylene units appear within the range of 68 ppm to 72 ppm in the first and second spectra.
- the intensity EI pst is preferably 4.0 or more and 20.0 or less. A resin composition having a strength EI pst within this range tends to have a sufficient amount of flexible oxyethylene chains with high molecular mobility.
- the intensity EI pst is more preferably 4.50 or more and 18.00 or less, and even more preferably 9.50 or more and 18.00 or less.
- the intensity EI cp is preferably 0.5 or more and 3.0 or less.
- a resin composition having an intensity EI cp within this range tends to have sufficient oxyethylene chains with high crystallinity.
- the intensity EI cp is more preferably 0.70 or more and 2.10 or less, and even more preferably 1.30 or more and 2.00 or less.
- a first spectrum of the resin composition is obtained by 13 C-PST/MAS NMR spectroscopy.
- a disc-shaped resin composition having a diameter of about 2 mm and a thickness of 1 mm is used as a sample.
- a 4 mm zirconia sample tube filled with this sample is used.
- Measurement conditions are, for example, as follows. Probe: 4 mm ⁇ CP/MAS probe (JEOL Ltd.). 13 C nuclear measurement frequency: 100.53 MHz.
- Measurement method CP/MAS method.
- Contact time 2 msec.
- Delay time 5 sec. Accumulated times: 5000 times.
- Sample amount about 80 mg.
- Sample rotation speed 6000 Hz.
- Temperature 25°C.
- External standard adamantane (29.5 ppm).
- Pre-saturation method Interval 10 msec.
- a second spectrum of the resin composition is obtained by 13 C-CP/MAS NMR spectroscopy.
- a second spectrum is obtained in the same manner as the first spectrum described above, except that the pre-saturation method is not used.
- the intensity and chemical shift of each signal are calculated using analysis software such as JEOL Delta v5.0.4.
- FIG. 1 is a graph showing an example of the first spectrum of the resin composition according to the embodiment.
- the graph shown in FIG. 1 is the first spectrum of the resin composition according to Example 15, which will be described later.
- the horizontal axis indicates the chemical shift
- the vertical axis indicates the intensity.
- the first spectrum shown in FIG. 1 includes a signal exhibiting a maximum PMI pst within a chemical shift range of 16 ppm to 20 ppm and a signal exhibiting a maximum EI pst within a chemical shift range of 68 ppm to 72 ppm.
- FIG. 2 is a graph showing an example of the second spectrum of the resin composition according to the embodiment.
- the graph shown in FIG. 2 is the second spectrum of the resin composition according to Example 15, which will be described later.
- the horizontal axis indicates the chemical shift
- the vertical axis indicates the intensity.
- the second spectrum shown in FIG. 2 includes a signal exhibiting a maximum PMI cp within a chemical shift range of 16 ppm to 20 ppm and a signal exhibiting a maximum EI cp within a chemical shift range of 68 ppm to 72 ppm.
- component (i) has a polyoxypropylene chain in the molecular chain, and if the strength ratio (PMI pst /PMI cp ) is 8.0 to 40.0, the production method is particularly limited. not to be Among them, it is preferable to use a polymerizable monomer having an oxypropylene chain for good polymerization moldability and easy production of a resin composition, and a polymerizable monomer having an oxypropylene chain and an oxyethylene chain. It is more preferable to use a polymerizable monomer (particularly preferably a monofunctional polymerizable monomer) having an oxypropylene chain, an oxyethylene chain, and an alkyl group having 5 to 20 carbon atoms. is preferred.
- photochromic compound (hereinafter also referred to as component (ii)) can be used without any particular limitation as long as it is a compound exhibiting photochromic properties. More than one species can be used in combination.
- photochromic compounds such as chromene compounds, fulgimide compounds, spirooxazine compounds, and spiropyran compounds can be used without any limitation.
- fulgimide compounds, spirooxazine compounds, spiropyran compounds and chromene compounds described above are described in, for example, JP-A-2-28154, JP-A-62-288830, WO94/22850 and WO96/14596. can be mentioned.
- chromene compounds in addition to those described in the above patent documents, chromene compounds having excellent photochromic properties are known, and such chromene compounds can be suitably used as component (ii).
- chromene compounds include JP-A-2001-031670, JP-A-2001-011067, JP-A-2001-011066, JP-A-2000-344761, JP-A-2000-327675, JP-A-2000-256347, JP-A-2000-229976, JP-A-2000-229975, JP-A-2000-229974, JP-A-2000-229973, JP-A-2000-229972, JP-A-2000-219678, JP-A-2000-219686, JP-A-11-322739, JP-A-11-286484, JP-A-11-279171, JP-A-09-218301, JP-A-09-124645, JP-A-08-295690, JP-A-
- chromene compounds having an indeno[2,1-f]naphtho[1,2-b]pyran skeleton are preferred from the viewpoint of photochromic properties such as color density, initial coloring property, durability, and fading speed. It is more preferable to use
- a photochromic compound having an oligomer chain group in the molecule can also be suitably used.
- photochromic compounds having such oligomer chain groups include WO2000/015630, WO2004/041961, WO2009/146509, WO2012/149599, WO2012/162725, WO2013/078086, and WO2019. /013249 pamphlet, WO2019/203205 pamphlet, and many other documents.
- photochromic compounds having an oligomer chain group in the molecule those having an oligomer chain group described in WO2019/013249 and WO2019/203205 pamphlets are preferred for exhibiting superior photochromic properties and durability. It is preferred to use photochromic compounds.
- the resin composition contains the component (i) and the component (ii).
- the mixing ratio of the component (i) and the component (ii) may be appropriately determined according to the intended use of the photochromic optical article. Among other things, considering the general use of It is preferable to contain 0.01 to 10 parts by mass of component (ii) with respect to 100 parts by mass of component (i).
- the component (i) forms a crosslinked structure
- the content of the component (i) is the total amount of polymerizable monomer components forming the component (i) in the photochromic curable composition.
- the resin composition may contain known additives in addition to the components (i) and (ii).
- ultraviolet absorbers antistatic agents, infrared absorbents, ultraviolet stabilizers, antioxidants, anti-coloring agents, antistatic agents, fluorescent dyes, dyes, pigments, additives such as fragrances, solvents, leveling agents , internal mold release agents, and polymerization modifiers such as thiols such as t-dodecylmercaptan.
- additives are preferably blended into the photochromic curable composition described in detail below.
- the total content of alkali metal ions and alkaline earth metal ions in the resin composition is preferably 500 ppm or less.
- a resin composition with a low content of alkali metal ions and alkaline earth metal ions is excellent in the durability of the photochromic compound.
- Alkali metal ions and alkaline earth metal ions are not particularly limited.
- Alkali metal ions include sodium ions, potassium ions, lithium ions, cesium ions, and the like.
- Alkaline earth metal ions include calcium ions, magnesium ions, barium ions, strontium ions, beryllium ions, and radium ions.
- Alkali metal ions and alkaline earth metal ions include, for example, at least one ion selected from the group consisting of sodium ions, potassium ions, cesium ions, and magnesium ions.
- the total content of alkali metal ions and alkaline earth metal ions in the resin composition can be measured by fluorescent X-ray analysis.
- a circular plate-shaped resin composition having a diameter of 40 mm and a thickness of 1 mm is used as a sample.
- a fluorescent X-ray spectrometer ZSX Primus IV
- a detection limit value of a fluorescent X-ray analyzer is, for example, 1 ppm.
- the total content of alkali metal ions and alkaline earth metal ions in the resin composition is preferably 200 ppm or less, more preferably 100 ppm or less.
- the lower limit of alkali metal ions and alkaline earth metal ions in the resin composition is 0 ppm or the detection limit of a fluorescent X-ray spectrometer.
- ⁇ Characteristics of resin composition Color density of 0.55 or more and color fading speed of 200 sec. , and a heat resistance (softening temperature) of 45°C or higher.
- the color density should be 0.60 or more and the color fading speed should be 95 sec. and heat resistance (softening temperature) of 50° C. or higher.
- the color development density is 0.75 or more, and the color fading speed is 80 sec. or lower, and heat resistance (softening temperature) can be 60° C. or higher.
- the heat resistance (softening temperature) can be 70°C or higher.
- the upper and lower limits of these physical properties are not particularly limited, the color density is 1.10 or less, and the color fading speed is 40 sec. Thus, the heat resistance is 90° C. or less.
- the method for producing the resin composition is not particularly limited as long as the urethane resin contained in the resin composition satisfies the requirements.
- component (i) may be impregnated with component (ii), or component (i) and component (ii) may be mixed.
- a photochromic curable composition containing the polymerizable monomer forming the urethane-based resin and the component (ii). is preferred.
- the photochromic curable composition can be easily produced, (A) a polyiso(thio)cyanate component having two or more iso(thio)cyanate groups in the molecule selected from the group consisting of isocyanate groups and isothiocyanate groups; (B) an active hydrogen-containing component having an active hydrogen-containing group; (ii) a component; It is preferred to provide a photochromic curable composition comprising: Then, it is preferable to produce a resin composition by curing (polymerizing) this photochromic curable composition.
- the blending ratio of the monomers forming component (i) is not particularly limited, but in order to stably obtain a resin composition, the blending ratio described later is recommended. is preferred.
- the photochromic curable composition will be explained.
- Photochromic curable composition As described above, (A) a polyiso(thio)cyanate component having two or more iso(thio)cyanate groups in the molecule selected from the group consisting of isocyanate groups and isothiocyanate groups; (B) an active hydrogen-containing component having an active hydrogen-containing group; (ii) a photochromic compound, is preferably included. Each component will be described below.
- polyiso(thio)cyanate component having two or more iso(thio)cyanate groups selected from the group consisting of isocyanate groups and isothiocyanate groups in the molecule>
- A In a polyiso(thio)cyanate component (component (A)) having two or more iso(thio)cyanate groups selected from the group consisting of isocyanate groups and isothiocyanate groups in the molecule, The number is not particularly limited as long as it is two or more. Among them, the number is preferably 2 to 6, more preferably 2 to 4, and even more preferably 2, from the viewpoint of facilitating control of polymerization.
- Polyiso(thio)cyanate compound refers to a group having two or more isocyanate groups and/or isothiocyanate groups.
- the component (A) includes aliphatic isocyanate compounds, alicyclic isocyanate compounds, aromatic isocyanate compounds, sulfur-containing heterocyclic isocyanate compounds, sulfur-containing aliphatic isocyanate compounds, aliphatic sulfide isocyanate compounds, and aromatic sulfide isocyanate compounds. , aliphatic sulfone-based isocyanate compounds, aromatic sulfone-based isocyanate compounds, sulfonic acid ester-based isocyanate compounds, and aromatic sulfonic acid amide-based isocyanate compounds.
- the isocyanate group of the isocyanate compound is at least selected from the group consisting of alcohols, lactams, phenols, oximes, pyrazoles, thiols, active methylene compounds, malonic acid diester compounds, and acetoacetic ester compounds. Also included are blocked isocyanate compounds blocked with one type of blocking agent.
- ((A) component; suitable polyisocyanate compound) compounds suitable for forming a resin composition having excellent transparency and mechanical strength, particularly suitable for producing a resin composition containing a photochromic compound, include the following formula Examples include compounds represented by (I) to (VIII).
- Preferred aliphatic isocyanate compounds include the following formula
- R 100 is an alkylene group having 1 to 10 carbon atoms, and may be a group in which part of the methylene groups in the chain of the alkylene group is substituted with a sulfur atom.
- R 100 It is preferred to use a compound represented by
- R 100 is an alkylene group having 1 to 10 carbon atoms and may be a linear or branched group.
- a pentamethylene group, a hexamethylene group, or a linear group of a heptamethylene group or an octamethylene group, or a part of hydrogen atoms of a pentamethylene group, a hexamethylene group, a heptamethylene group or an octamethylene group is methyl
- a branched group substituted with a group is preferred.
- the alkylene group in which a part of the methylene group is substituted with a sulfur atom is preferably a -CH 2 CH 2 SCH 2 CH 2 SCH 2 CH 2 - group.
- Specific examples of the compound represented by formula (I) include pentamethylene diisocyanate, hexamethylene diisocyanate, heptamethylene diisocyanate, octamethylene diisocyanate, 2,4,4-trimethylhexamethylene diisocyanate, 1,2-bis( 2-isocyanatoethylthio)ethane and the like. These compounds can be used alone, or two or more kinds of compounds can be used.
- aromatic isocyanate compound Preferred aromatic isocyanate compounds and alicyclic isocyanate compounds include the following formula (II) and the following formula (III)
- R 101 is each an alkyl group having 1 to 4 carbon atoms or a hydrogen atom, and may be the same group or different groups;
- R 102 is an alkyl group having 1 to 4 carbon atoms, and when a plurality of groups are present, they may be the same group or different groups, a 100 is an integer of 2 or 3, b 100 is an integer of 0 to 4, and c 100 is an integer of 0 to 4.
- a 100 is an integer of 2 or 3
- b 100 is an integer of 0 to 4
- c 100 is an integer of 0 to 4.
- the difference between the compound represented by the formula (II) and the compound represented by the formula (III) is that the compound having a phenyl group (the compound represented by the formula (II)) and the compound having a cyclohexane group (the compound represented by the formula (III) ) is a compound represented by ).
- the alkyl group having 1 to 4 carbon atoms may be a linear or branched group. Among them, R 101 is particularly preferably a hydrogen atom, a methyl group, or an ethyl group. In R 102 , the alkyl group having 1 to 4 carbon atoms may be a linear or branched group. Among them, R 102 is particularly preferably a methyl group or an ethyl group.
- the compound represented by formula (II) or formula (III) include isophorone diisocyanate, xylene diisocyanate (o-, m-, p-), 2,4-tolylene diisocyanate, 2, 6-tolylene diisocyanate, 1,3-bis(isocyanatomethyl)cyclohexane, 1,4-bis(isocyanatomethyl)cyclohexane and the like. These compounds can be used alone, or two or more kinds of compounds can be used.
- aromatic isocyanate compounds and alicyclic isocyanate compounds, the following formula (IV) and the following formula (V)
- Each R 103 is an alkyl group having 1 to 4 carbon atoms or a hydrogen atom, and may be the same group or different groups, and d 100 is an integer of 0 to 4.
- d 100 is an integer of 0 to 4.
- the difference between the compound represented by the formula (IV) and the compound represented by the formula (V) is that the compound having two phenyl groups (the compound represented by the formula (IV)) and the compound having two cyclohexane groups ( This is the difference from the compound represented by the formula (V)).
- the alkyl group having 1 to 4 carbon atoms may be a linear or branched group.
- R 103 is particularly preferably a hydrogen atom, a methyl group or an ethyl group.
- Specific examples of the compound represented by formula (IV) or formula (V) include 4,4'-diphenylmethane diisocyanate, dicyclohexylmethane-4,4'-diisocyanate, and the like. These compounds can be used alone, or two or more kinds of compounds can be used.
- Each R 104 is an alkyl group having 1 to 4 carbon atoms or a hydrogen atom, and may be the same group or different groups, and e 100 is an integer of 0 to 4. ) is preferably used.
- the alkyl group having 1 to 4 carbon atoms may be a linear or branched group.
- R 104 is particularly preferably a hydrogen atom, a methyl group or an ethyl group.
- the compound represented by the formula (VI) include norbornane diisocyanate, 2,5-bis(isocyanatomethyl)-bicyclo[2,2,1]-heptane, 2,6-bis(isocyanatomethyl) -bicyclo[2,2,1]-heptane. These compounds can be used alone, or two or more kinds of compounds can be used.
- sulfur-containing heterocyclic isocyanate compounds include the following formula (VII) and the following formula (VIII)
- R 105 is each an alkyl group having 1 to 4 carbon atoms or a hydrogen atom, and may be the same group or different groups;
- R 106 is a methylene group or a sulfur atom, and
- R 107 is an alkylene group having 1 to 6 carbon atoms, or a part of the methylene group in the chain of the alkylene group having 1 to 6 carbon atoms is substituted with a sulfur atom.
- f 100 is an integer of 0 to 2.
- compounds represented by formula (VII) or formula (VIII) include 2,5-bis(isocyanatomethyl)thiophene, 2,5-bis(isocyanatomethyl)-1,4 -dithiane, 3,4-bis(isocyanatomethyl)tetrahydrothiophene, 4,5-bis(isocyanatomethyl)-1,3-dithiolane and the like. These compounds can be used alone, or two or more kinds of compounds can be used.
- halogen-substituted, alkyl-substituted, alkoxy-substituted, and nitro-substituted polyisocyanates prepolymer-type modified products with polyhydric alcohols, carbodiimide-modified products, urea-modified products, biuret-modified products, dimerization or trimers Chemical reaction products and the like can also be used.
- Suitable (A) component; polyisothiocyanate compound examples include compounds in which the isocyanate group is replaced with an isothiocyanate group in the polyisocyanate compounds represented by the formulas (I) to (VIII). More specifically, aliphatic isothiocyanate compounds, alicyclic isothiocyanate compounds, aromatic isothiocyanate compounds, sulfur-containing heterocyclic isothiocyanate compounds, heterocyclic isothiocyanate compounds, sulfur-containing aliphatic isothiocyanate compounds, sulfur-containing Aromatic isothiocyanate compounds and the like can be mentioned.
- suitable compounds include aliphatic isothiocyanate compounds such as hexamethylene diisothiacynate, 1,2-diisothiocyanatoethane, 1,3-diisothiocyanatopropane, 1,4-diisothiocyanate, Thiocyanatobutane, 1,6-diisothiocyanatohexane, 2,4,4,-trimethylhexanemethylene diisothiacinate, thiobis(3-isothiocyanatopropane), thiobis(2-isothiocyanatoethane), dithiobis(2-iso thiocyanate ethane) and the like.
- aliphatic isothiocyanate compounds such as hexamethylene diisothiacynate, 1,2-diisothiocyanatoethane, 1,3-diisothiocyanatopropane, 1,4-diisothiocyanate, Thiocyanatobutane,
- Alicyclic isothiocyanate compounds and aromatic isothiocyanate compounds include p-phenylenediisopropylidene diisothiocyanate, 1,2-diisothiocyanatobenzene, 1,3-diisothiocyanatobenzene, 1,4-diisothiocyanate Benzene, 2,4-diisothiocyanatotoluene, isophorone diisothiocyanate, xylene diisothiocyanate (o-, m-, p-), 2,4-tolylene diisothiocyanate, 2,6-tolylene diisothiocyanate, cyclohexane diisothiocyanate and the like, and also 1,1′-methylenebis(4-isothiocyanatobenzene), 1,1′-methylenebis(4-isothiocyanate-2-methylbenzene), 1,1′-methylenebis(4- isothiocyanate 3-methylbenz
- Preferred alicyclic isothiocyanate compounds include 2,4-bis(isothiocyanatomethyl)norbornane, 2,5-bis(isothiocyanatomethyl)norbornane, and 2,6-bis(isothiocyanatomethyl)norbornane. , 3,5-bis(isothiocyanatomethyl)norbornane, norbornane diisothiocyanate, and the like.
- Preferred sulfur-containing heterocyclic isocyanate compounds include thiophene-2,5-diisothiocyanate, 1,4-dithiane-2,5-diisothiocyanate, 2,5-bis(isothiocyanatomethyl)-1,4- dithiane, 4,5-bis(isothiocyanatomethyl)-1,3-dithiolane and the like.
- Component (A) compound having an isocyanate group and an isothiocyanate group
- Compounds having both an isocyanate group and an isothiocyanate group as component (A) include the following compounds.
- at least one isocyanate group is an isothiocyanate group.
- at least one isothiocyanate group is a compound having an isocyanate group.
- a compound having an iso(thio)cyanate group blocked with a blocking agent is an alcohol, lactam, or , phenols, oximes, pyrazoles, thiols, active methylene compounds, malonic acid diester compounds, and acetoacetic ester compounds. can be done.
- the conditions for reacting the iso(thio)cyanate group with the blocking agent vary depending on the type of the blocking agent, and may be appropriately determined according to the selected blocking agent.
- the protection of the iso(thio)cyanate group by the blocking agent can be confirmed by Fourier transform infrared spectroscopy (FT-IR).
- the pot life of the photochromic composition can be extended.
- Preferred examples of component (A) above include pentamethylene diisocyanate, hexamethylene diisocyanate, heptamethylene diisocyanate, octamethylene diisocyanate, 1,3-bis(isocyanatomethyl)cyclohexane, 1,4-bis(isocyanatomethyl)cyclohexane, isophorone diisocyanate, norbornane diisocyanate, 2,5-bis(isocyanatomethyl)-bicyclo[2,2,1]-heptane, 2,6-bis(isocyanatomethyl)-bicyclo[2,2,1]-heptane, 1, 2-bis(2-isocyanato-ethylthio)ethane, xylene diisocyanate (o-, m-, p-), 2,4-tolylene diisocyanate, 2,6-tolylene diisocyanate, and 4,4'-diphenylmethane
- the active hydrogen-containing group in (B) the active hydrogen-containing component having an active hydrogen-containing group (component (B)) is a group containing active hydrogen. That is, the component (B) is an active hydrogen compound having a group containing active hydrogen.
- the active hydrogen-containing group mentioned above refers to a group capable of reacting with an isocyanate group, and includes, for example, a hydroxyl group, an amino group, a carboxyl group and/or a thiol group.
- the (B) component has a polyoxypropylene chain.
- component (B) preferably has an alkyl group having 5 to 20 carbon atoms and/or a polyoxyethylene chain in addition to the polyoxypropylene chain.
- the ratio (nA/nB) is preferably 1.00 or more and 1.50 or less, more preferably is 1.00 or more and 1.20 or less.
- nA/nB is 1.00 or more and 1.50 or less, a resin composition having a strength ratio (PMI pst /PMI cp ) of 8.0 or more and 40.0 or less can be easily produced.
- a resin composition having excellent photochromic properties and heat resistance can be obtained.
- nA/nB ratio When the nA/nB ratio is less than 1.00 or greater than 1.50, the degree of polymerization does not increase, resulting in low heat resistance.
- the mixing ratio of the component (A) and the component (B) is not particularly limited as long as the nA/nB ratio is 1.00 or more and 1.50 or less.
- the intensity ratio (PMI pst /PMI cp ) satisfies 8.0 or more and 40.0 or less, and considering the production of a resin composition excellent in photochromic properties and heat resistance, component (A),
- component (B) is preferably 50 to 70 parts by mass, more preferably 60 to 65 parts by mass.
- component (B) is (B1) polyfunctional active hydrogen-containing groups having 3 or more active hydrogen-containing groups in one molecule. It is desirable to include a component ((B1) component) and (B2) a first active hydrogen-containing component ((B2) component) having one or two active hydrogen-containing groups in one molecule.
- the (B1) component used in the photochromic curable composition is not particularly limited as long as it is a compound having 3 or more active hydrogen-containing groups in the molecule.
- a compound in which the total number of hydroxyl groups and thiol groups in the molecule is 3 or more is preferable.
- the number of hydroxyl groups and thiol groups is not particularly limited as long as it is 3 or more.
- (B1) component examples include aliphatic poly(thiol) compounds and aromatic poly(thiol) compounds. More specifically, the following compounds can be mentioned.
- Suitable poly(thio)ol compound examples include the following compounds. Specific examples include compounds represented by the following formulas (IX) to (XVII).
- Preferred aliphatic poly(thio)ol compounds include the following formula (IX)
- R 108 is a hydrogen atom, or the following formula (X)
- R 111 is an alkylene group having 1 to 6 carbon atoms.
- R 109 is each a hydrogen atom, a methyl group, or an ethyl group and may be the same or different
- R 110 is a hydrogen atom or an alkyl group having 1 to 6 carbon atoms, and when there are more than one, they may be the same or different
- o 100 is 0-2, p 100 is 1-6, q 100 is 0-10, r 100 is 2-4, and o 100 +r 100 is 4.
- ⁇ It is preferred to use a compound represented by
- R 111 is an alkylene group having 1 to 6 carbon atoms and may be a linear or branched group. Among them, R 111 is particularly preferably a methylene group, ethylene group, trimethylene group or propylene group.
- Specific examples of the compound represented by the formula (IX) include trimethylolpropane, pentaerythritol, trimethylolpropane tris(3-mercaptopropionate), pentaerythritol tetrakis(3-mercaptopropionate), and the like. mentioned.
- polyfunctional poly(thiol) compound having an ether bond is represented by the following formula (XI)
- F 100 is each an alkyl group of 1 to 6, or the following formula (XII)
- R 112 is a hydrogen atom or a group as defined in formula (X) above, and may be the same group or a different group;
- R 113 is each a hydrogen atom, a methyl group, or an ethyl group, and may be the same group or different groups, s 100 is 1-6 and t 100 is 0-10. ).
- ⁇ It is preferred to use a compound represented by
- At least two of the F 100 are groups represented by the formula (XII).
- Other groups include 1 to 6 alkyl groups, which may be chain or branched groups. Among them, F 100 is particularly preferably a methyl group, an ethyl group, a trimethyl group, or a propyl group.
- F 100 is particularly preferably a methyl group, an ethyl group, a trimethyl group, or a propyl group.
- two or more of F 100 are groups represented by the formula (XII), they may be the same group or different groups.
- Specific examples of the compound represented by the formula (XI) include ditrimethylolpropane, dipentaerythritol, ditrimethylolpropane tetrakis (3-mercaptopropionate), dipentaerythritol-ruhexakis (3-mercaptopropionate nate) and the like.
- polyfunctional polythiol compounds of the following formula (XIII) are preferred aliphatic poly(thiol) compounds.
- R 114 is a hydrogen atom, an alkyl group having 1 to 6 carbon atoms, or a group in which a part of the methylene group of the alkyl group having 1 to 6 carbon atoms is substituted with a sulfur atom, and when there are a plurality of R 114 may be the same group or different groups
- R 115 is an alkylene group having 1 to 10 carbon atoms, a group in which part of the methylene groups in the chain of the alkylene group having 1 to 10 carbon atoms is substituted with a sulfur atom, or the above 1 to 10 carbon atoms is a group in which a portion of the hydrogen atoms of the alkylene group of is substituted with a thiol group, and when there are a plurality of R 115 , they may be the same group or different groups, u 100 is an integer from 2 to 4, v 100 is an integer from 0 to 2, and u 100 +v 100 is 4. ) It is preferred to use a compound represented
- the alkyl group having 1 to 6 carbon atoms may be a linear or branched group, and R 114 is preferably a hydrogen atom, a methyl group, or an ethyl group.
- R 114 is preferably a hydrogen atom, a methyl group, or an ethyl group.
- Specific examples of groups in which a part of the methylene groups in the chain of an alkyl group having 1 to 6 carbon atoms are substituted with sulfur atoms include -CH 2 SCH 3 and the like.
- the alkylene group having 1 to 10 carbon atoms may be a linear or branched group.
- R 115 is particularly preferably a methylene group, an ethylene group, a trimethylene group or a propylene group.
- Specific groups in which part of the methylene groups in the chain of an alkylene group having 1 to 10 carbon atoms are substituted with sulfur atoms include -CH 2 S-, -CH 2 CH 2 S-, and -CH 2 CH 2 CH 2 S— and the like.
- groups such as —CH 2 SCH(SCH 2 SH)— are examples of the groups in which some of the hydrogen atoms of the alkyl group having 1 to 6 carbon atoms are substituted with thiol groups.
- Specific examples of the compound represented by the formula (XIII) include 4-mercaptomethyl-1,8-dimercapto-3,6-dithiaoctane, 1,1,1,1-tetrakis(mercaptomethyl)methane, 1 , 1,3,3-tetrakis(mercaptomethylthio)propane, 1,1,2,2-tetrakis(mercaptomethylthio)ethane, 4,7-bismercaptomethyl-3,6,9-trithio-1,11-undecane dithiol, 5,7-dimercaptomethyl-1,11-dimercapto-3,6,9-trithiundecane, 4,8-dimercaptomethyl-1,11-dimercapto-3,6,9-trithiundecane, etc. is mentioned.
- the phenyl group-containing polythiol compound is represented by the following formula (XIV)
- R 116 is an alkylene group having 1 to 6 carbon atoms, or a group in which a part of the methylene group in the chain of the alkylene group having 1 to 6 carbon atoms is substituted with a sulfur atom, and w 100 is 3; be.
- the alkylene group having 1 to 6 carbon atoms in R 116 may be a linear or branched group.
- R 116 is preferably a methylene group, ethylene group, trimethylene group or propylene group.
- the group in which a part of the methylene group in the chain of the alkylene group having 1 to 6 carbon atoms is substituted with a sulfur atom is specifically -CH 2 CH 2 CH 2 SCH 2 -, -CH 2 CH 2 SCH 2 -, -CH 2 SCH 2 - and the like.
- a specific example of the compound represented by the formula (XIV) is 1,3,5-tris(mercaptopropylthiomethyl)benzene.
- poly(thiol) compound having a triazine ring is represented by the following formula (XV)
- R 117 is each an alkyl group having 1 to 6 carbon atoms, or the following formula (XVI)
- R 118 and R 119 are an alkylene group having 1 to 6 carbon atoms, R 120 is an oxygen atom or a sulfur atom) wherein at least two of the R 117 are groups represented by the formula (XVI), and the R 117 may be the same group or different groups.
- R 118 and R 119 are an alkylene group having 1 to 6 carbon atoms, R 120 is an oxygen atom or a sulfur atom
- the alkylene group having 1 to 6 carbon atoms may be a linear or branched group.
- R 118 and R 119 are preferably methylene group, ethylene group, trimethylene group and propylene group.
- Specific examples of the compound represented by the formula (XV) include 2-mercaptomethanol and tris- ⁇ (3-mercaptopropionyloxy)-ethyl ⁇ -isocyanurate.
- a plurality of R 500 may be the same or different from each other, a hydrogen atom, an alkyl group, a cycloalkyl group, an alkoxy group, a phenyl group, and at least two hydroxyl groups in one molecule, and/or an organic group containing a thiol group, where n100 is an integer from 3 to 100 ).
- component (B1) can be used without any particular limitation, and can be used in combination in consideration of the photochromic properties and mechanical properties of the resulting photochromic cured product. Among them, in order to be able to produce a resin composition having excellent properties and to have a photochromic curable composition having excellent moldability and good handling properties, the component (B1) should contain 3 per molecule. It is preferred to use component (B1) having ⁇ 6 active hydrogen-containing groups.
- component (B1)a) the polyfunctional active hydrogen-containing component having 3 to 6 active hydrogen-containing groups in one molecule in component (B1) may be simply referred to as component (B1a).
- a component having 4 to 6 active hydrogen-containing groups per molecule is preferable, and a component having 6 active hydrogen-containing groups per molecule is most preferable.
- the active hydrogen-containing group is preferably a thiol group, considering the increase in viscosity when the photochromic curable composition is prepared.
- the component (B1) may consist only of the component (B1a), and may also contain a component having more than 6 active hydrogen-containing groups in one molecule, if necessary.
- this component is also simply referred to as the (B1b) component.
- the (B1a) component will be described.
- Examples of the (B1a) component which is a suitable component of the above (B1) component, include trimethylolpropane tris (3-mercaptopropionate), pentaerythritol tetrakis (3-mercaptopropionate), dipentaerythritol hexakis ( 3-mercaptopropionate), 4-mercaptomethyl-1,8-dimercapto-3,6-dithiaoctane, and tris- ⁇ (3-mercaptopropionyloxy)-ethyl ⁇ -isocyanurate, among which , trimethylolpropane tris (3-mercaptopropionate), pentaerythritol tetrakis (3-mercaptopropionate), and dipentaerythritol hexakis (3-mercaptopropionate).
- trimethylolpropane tris (3-mercaptopropionate
- pentaerythritol tetrakis (3
- dipentaerythritol hexakis (3-mercaptopropionate) is most preferable because it can improve the photochromic properties and mechanical properties of the resulting photochromic cured product (resin composition).
- other components (B1) may be mixed for viscosity adjustment.
- components (B1a) include trimethylolpropane tris(3-mercaptopropionate), pentaerythritol tetrakis(3-mercaptopropionate), 1,6-hexanediol bis(3-mercaptopropionate), 1, 2-bis[(2-mercaptoethyl)thio]-3-mercaptopropane, 2,2-bis(mercaptomethyl)-1,4-butanedithiol, 2,5-bis(mercaptomethyl)-1,4-dithiane , 4-mercaptomethyl-1,8-dimercapto-3,6-dithiaoctane, 1,1,1,1-tetrakis(mercaptomethyl)methane, 1,1,3,3-tetrakis(mercaptomethylthio)propane, 1, 1,2,2-tetrakis(mercaptomethylthio)ethane, 4,6-bis(mercaptomethylthio)-1,3-dithiane, tris
- component (B1) component In order that the strength ratio (PMI pst /PMI cp ) of component (i) satisfies 8.0 to 40.0 and the resin composition exhibits particularly excellent effects, ( B1) component. More specifically, it is preferable to use a component having more active hydrogen-containing groups in the molecule than the component (B1a). Among them, particularly preferred is a polyrotaxane component having a polyrotaxane structure and having 7 or more active hydrogen-containing groups in the molecule (hereinafter sometimes simply referred to as component (B1b)). .
- the photochromic curable composition preferably further contains a polyrotaxane component (component (B1b)) having more than 6 active hydrogen-containing groups in the molecule.
- component (B1b) the mobility of the polyrotaxane itself can enhance the photochromic properties of the resulting resin composition.
- incorporating an oxypropylene chain or the like into the polyrotaxane component facilitates adjustment of the strength ratio (PMI pst /PMI cp ) of component (i) to 8.0 to 40.0.
- the strength ratio (PMI pst /PMI cp ) can be easily increased by incorporating an oxypropylene chain into the polyrotaxane component.
- the resulting resin composition exhibits excellent photochromic properties.
- the (B1b) component is a known compound and has a composite molecular structure formed from a chain-like axial molecule and a cyclic molecule. That is, a chain-shaped axial molecule is enclosed by a plurality of cyclic molecules, and the axial molecule penetrates the inside of the ring possessed by the cyclic molecule. Therefore, the cyclic molecule can slide freely on the axial molecule, but bulky terminal groups are formed at both ends of the axial molecule to prevent the cyclic molecule from falling off from the axial molecule.
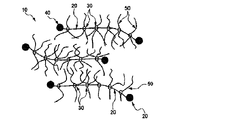
- the polyrotaxane component (B1b) component has a composite molecular structure formed from a chain-shaped axial molecule 20 and a cyclic molecule 30. As shown in FIG. More specifically, it has a structure in which a chain-shaped axial molecule 20 is enclosed by a plurality of cyclic molecules 30 , and the axial molecule 20 penetrates the inside of the ring of the cyclic molecule 30 .
- the cyclic molecule 30 can freely slide on the axial molecule 20, but bulky terminal groups 40 are formed at both ends of the axial molecule 20 to prevent the cyclic molecule 30 from falling off from the axial molecule 20. .
- the cyclic molecule 30 of the polyrotaxane 10 can slide on the axial molecule 20, so it is considered that the photochromic properties can be improved.
- the strength ratio (PMI pst /PMI cp ) in the resulting resin composition can be easily adjusted in the range of 8.0 to 40.0, and photochromic properties can be improved. can be improved.
- the polyrotaxane 10 shown in FIG. 3 also shows a side chain 50 introduced into the ring of the cyclic molecule 30 as necessary.
- (B1b) component is a known compound and can be synthesized by the method described in International Publication No. 2015/068798 and the like.
- the axial molecule is not particularly limited as long as it can penetrate the ring of the cyclic molecule, and may be linear or branched.
- This axial molecule is generally formed by a polymer. Suitable polymers forming the axial molecule include those exemplified in WO2015/068798, among which it is preferred to use polyethylene glycol.
- polyethylene glycol By using polyethylene glycol as the axial molecule, oxyethylene chains are introduced into the resulting resin composition, and the strength ratio (EI pst /EI cp ) can be easily adjusted to the range of 5.0 or more and 20.0 or less. can be adjusted to
- the bulky terminal groups formed at both ends of the axial molecule are not particularly limited as long as they are groups that prevent the cyclic molecule from detaching from the axial molecule.
- an adamantyl group is preferable.
- the weight average molecular weight (Mw) of the axial molecule is not particularly limited, but is preferably in the range of 1,000 to 100,000, more preferably in the range of 5,000 to 80,000, and even more preferably in the range of 10,000 to 50,000.
- the weight average molecular weight (Mw) of the axial molecule is 1000 or more, the mobility of the cyclic molecule tends to be improved.
- the mass average molecular weight (Mw) of the axial molecule is 100,000 or less, compatibility with other components tends to be improved.
- the strength ratio (EI pst /EI cp ) of the resulting resin composition can be easily adjusted to 5.0 or more and 20.0 or less. As a result, photochromic properties can be improved.
- the cyclic molecule has a ring that is large enough to enclose the axial molecule.
- Such rings include, for example, cyclodextrin rings.
- the cyclodextrin ring has ⁇ form (ring inner diameter: 0.45 to 0.6 nm), ⁇ form (ring inner diameter: 0.6 to 0.8 nm), and ⁇ form (ring inner diameter: 0.8 to 0.95 nm). ), with the ⁇ -cyclodextrin ring being preferred.
- one axial molecule encloses one or more cyclic molecules.
- the clathrate number of cyclic molecules is generally in the range of 0.001 to 0.6, and 0.002 to 0, when the maximum clathrate number of cyclic molecules that can be clathrated by one axial molecule is 1.0.
- a range of 0.5 is preferred, and a range of 0.003 to 0.4 is more preferred.
- the maximum number of inclusions of cyclic molecules with respect to one axial molecule can be calculated from the length of the axial molecule and the thickness of the ring of the cyclic molecule.
- the maximum inclusion number is calculated as follows. That is, two repeating units [--CH.sub.2-- CH.sub.2 O--] of polyethylene glycol are approximated to the thickness of one ⁇ -cyclodextrin ring.
- the number of repeating units is calculated from the molecular weight of this polyethylene glycol, and 1/2 of this number of repeating units is obtained as the maximum inclusion number of the cyclic molecule. Assuming that this maximum inclusion number is 1.0, the inclusion number of the cyclic molecule is adjusted within the range described above.
- a side chain may be introduced into the cyclic molecule.
- side chains When side chains are introduced in this way, a pseudo-crosslinked structure can be formed in the resulting resin composition (component (i)). Thereby, the mechanical properties of the resin composition can be improved, and the photochromic properties can be improved.
- the side chain is preferably formed of repeating units of organic groups having 3 to 20 carbon atoms.
- the weight average molecular weight (Mw) of the side chain is not particularly limited, but is preferably in the range of 200 to 10,000, more preferably in the range of 250 to 8,000, further preferably in the range of 300 to 5,000, and more preferably in the range of 300 to 1,500. Ranges are particularly preferred.
- the side chain can be introduced by utilizing the functional group (eg, hydroxyl group) of the ring of the cyclic molecule and modifying this functional group.
- the ⁇ -cyclodextrin ring has 18 hydroxyl groups as functional groups, through which side chains can be introduced. That is, up to 18 side chains can be introduced to one ⁇ -cyclodextrin ring.
- the degree of modification is 50%.
- the side chain may be linear or branched.
- the side chain reacts an appropriate compound to the ring possessed by the cyclic molecule using ring-opening polymerization; radical polymerization; cationic polymerization; anionic polymerization; living radical polymerization such as atom transfer radical polymerization, RAFT polymerization, NMP polymerization;
- a side chain of an appropriate size can be introduced by allowing the As the cyclic compound, cyclic lactones and cyclic carbonates are preferred, and ⁇ -caprolactone is more preferred.
- Propylene oxide incorporated into this side chain can be considered an oxypropylene chain. Therefore, particularly when propylene oxide is used to introduce highly reactive hydroxyl groups, the intensity ratio (PMI pst /PMI cp ) can be easily adjusted to 8.0 to 40.0.
- a polymerizable functional group selected from a hydroxyl group or a thiol group is introduced at the end of the side chain, and most preferably a polymerizable functional group selected from a hydroxyl group is introduced.
- the intensity ratio (AMI pst /AMI cp ) like the oxypropylene chain and the oxyethylene chain, has a peak of this alkyl group. included.
- the most suitably used (B1b) component satisfies the following requirements. Specifically, a cyclic molecule having an ⁇ -cyclodextrin ring having a polyethylene glycol attached to both ends by an adamantyl group as an axial molecule, and a hydroxyl group activated by propylene oxide is introduced into the cyclic molecule, A side chain having a terminal hydroxyl group is introduced into the cyclic molecule by the hydroxyl group and polycaprolactone.
- the axial molecule is an oxyethylene chain
- the molecular weight of the axial molecule is 8,000 to 30,000
- the introduction ratio of the ⁇ -cyclodextrin ring is in the range of 0.003 to 0.4
- the ⁇ -cyclodextrin ring is modified (degree of modification; side chain introduction rate) is preferably 30% or more and 70% or less.
- the root portion of the side chain is an oxypropylene unit.
- a side chain containing an oxypropylene unit and having an average molecular weight of 400 to 1,500 is introduced into the ⁇ -cyclodextrin ring.
- the component (B1b) has a weight average molecular weight of 100,000 to 200,000 and preferably contains 150 to 350 hydroxyl groups per molecule, although this is an average value.
- component (B1) it is preferable to use the above-exemplified polyfunctional active hydrogen-containing components.
- a polyfunctional active hydrogen-containing component having the following structure that is, component (B1) preferably contains a compound having a quaternary carbon atom in the molecule and all groups bonded to the quaternary carbon atom having active hydrogen-containing groups.
- component (B1) preferably contains a compound having a quaternary carbon atom in the molecule and all groups bonded to the quaternary carbon atom having active hydrogen-containing groups.
- FIG. 4 exemplifies a compound in which all groups bonded to a quaternary carbon atom have an active hydrogen-containing group (hereinafter sometimes simply referred to as "fully substituted compound”).
- FIG. 4A is an example of a "fully substituted compound” in which all four groups attached to a quaternary carbon atom have active hydrogen-containing groups.
- FIG. 5 illustrates a compound in which a part of the group that bonds to the quaternary carbon atom has an active hydrogen-containing group (hereinafter sometimes simply referred to as a "partially substituted compound").
- FIG. 4 exemplifies a compound in which all groups bonded to a quaternary carbon atom have an active hydrogen-containing group (hereinafter sometimes simply referred to as "fully substituted compound”).
- FIG. 4A is an example of a "fully substituted compound” in which all four groups attached to a quaternary carbon atom have active hydrogen-containing groups.
- FIG. 5 illustrates a compound in
- 5B is an example of a "partially substituted compound" in which three groups bonded to a quaternary carbon atom have active hydrogen-containing groups. 4 and 5, S is a simplified representation of an active hydrogen-containing group. Arrows indicate the direction of polymer growth.
- FIG. 4 shows an example in which all the groups bonded to one quaternary carbon atom have active hydrogen-containing groups, the same applies to the case of having four or more active hydrogen-containing groups.
- dipentaerythritol hexakis(3-mercaptopropionate) is a compound with two quaternary carbon atoms.
- the groups bound to the quaternary carbon atoms can then be viewed as three groups having one thiol group and one group having three thiol groups.
- dipentaerythritol hexakis(3-mercaptopropionate) can be considered a fully substituted compound.
- at least one quaternary carbon atom may be present in the molecule so that all four bonding groups are groups having active hydrogen-containing groups.
- the number of active hydrogen-containing groups in all substituted compounds is preferably 4 to 6.
- the photochromic curable composition preferably further contains a first active hydrogen-containing component having one or two active hydrogen-containing groups in one molecule as a component (B2). .
- a single type of compound or multiple types of compounds can be used as the first active hydrogen-containing component.
- Specific (B2) components include monoalkyl ether compounds having an oxypropylene chain (first active hydrogen-containing component having one active hydrogen-containing group per molecule), or glycol compounds having an oxypropylene chain (active A first active hydrogen-containing component having two hydrogen-containing groups in one molecule).
- monoalkyl ether compounds having an oxypropylene chain first active hydrogen-containing component having one active hydrogen-containing group per molecule
- glycol compounds having an oxypropylene chain active A first active hydrogen-containing component having two hydrogen-containing groups in one molecule.
- polyoxyethylene polyoxypropylene monoalkyl ether compounds or polyoxyethylene polyoxypropylene glycol compounds are mentioned.
- the component (B2) is not particularly limited, but the number average molecular weight is 500 or more, the NMR peak intensity ratio (PMI pst /PMI cp , AMI pst /AMI cp , EI pst /EI cp ), and can improve the photochromic properties of the resulting resin composition.
- the number average molecular weight of component (B2) is preferably 600 or more, more preferably 700 or more.
- the upper limit of the molecular weight is preferably 3000.
- l which is the average value of oxypropylene repeating units (--CH 2 CH(CH 3 )O--)
- m which is the average value of repeating units (-CH 2 CH 2 O-) of oxyethylene
- the component (B2) may also contain an alkyl group, and the alkyl group preferably has 5 or more and 20 or less carbon atoms.
- the obtained photochromic cured body (resin composition) not only exhibits excellent photochromic properties, but also exhibits the following characteristics.
- the photochromic curable composition containing the component (B2) is cured in a mold made of inorganic glass, the resulting photochromic cured product has improved releasability from the inorganic glass mold. This releasability improving effect is remarkably exhibited when a compound having one active hydrogen-containing group in one molecule is used.
- adhesion to other optical substrates can be improved.
- Other optical substrates include known plastic substrates and inorganic glass substrates. In particular, when the blending ratio of the component (A) is relatively large, the adhesion to the inorganic glass substrate can be improved.
- component (B2) a compound having one active hydrogen-containing group per molecule (hereinafter simply referred to as (B21) component) or a compound having two active hydrogen-containing groups per molecule (hereinafter simply (B22) is also referred to as component.) will be described in more detail.
- Component (B21) is not particularly limited, but it is preferable to use a compound represented by the following formula (XVIII).
- l', m', and n' are each an integer of 1 to 30.
- l' in the formula (XVIII) refers to a repeating unit of oxypropylene. This l' is an average value and is an integer of 1-30. Above all, the resin composition satisfying the strength ratio (PMI pst /PMI cp ) in the range of 8.0 to 40.0, preferably in the range of 10.0 to 15, can be easily produced and has excellent properties.
- l′ is preferably from 2 to 25, more preferably from 2 to 5, in order to have
- m' in the formula (XVIII) refers to a repeating unit of oxyethylene. And this m' is an average value and is an integer of 1-30. Among them, in order to easily produce a resin composition satisfying the strength ratio (EI pst /EI cp ) of 5.0 to 20.0 and to have excellent properties, m′ should be 5 to 25. and more preferably 7 to 12.
- n' in the formula (XVIII) indicates the number of carbon atoms in the terminal alkyl group. This n' is an integer of 1-30. Above all, in order to easily produce a resin composition satisfying the strength ratio (AMI pst /AMI cp ) of 7.0 to 23.0 and to have excellent properties, n′ should be 5 to 20. and more preferably 10 to 18.
- the polyoxyethylene-polyoxypropylene portion was shown as a block copolymer in the chemical formula, but the portion may be a random type copolymer.
- the site may be a block copolymer, but the intensity ratio (PMI pst /PMI cp ) satisfies 8.0 or more and 40.0 or less, preferably 10.0 or more and 15 or less.
- it is preferably a random copolymer.
- polyoxyethylene polyoxypropylene monolauryl ether polyoxyethylene polyoxypropylene monotridecyl ether, and polyoxyethylene polyoxypropylene monostearyl ether
- the polyoxyethylene polyoxypropylene moiety is a random copolymer. is preferred.
- the polymerization moldability of the photochromic curable composition can be further improved. That is, since the (B21) component is monofunctional, it can suppress a rapid increase in the viscosity of the photochromic curable composition.
- the photochromic curable composition is stored separately as component (A) and component (B). Then, when producing the resin composition, the components (A), (B), essential components, and optionally optional components are mixed to prepare a photochromic curable composition. . If the viscosity suddenly increases during mixing, it may become difficult to obtain a uniform resin composition. Therefore, it is preferable that the viscosity of the photochromic curable composition does not change until the conditions (for example, temperature) for initiating polymerization are reached. This temperature rise can be suppressed by using the (B21) component. For the above reasons, both the (B21) component and the (B22) component can be used simultaneously as the (B2) component, but it is preferable to use only the (B21) component.
- component (B22) is not particularly limited, it is preferable to use a compound represented by the following formula (XIX).
- l'', m'''+m'' are each an integer of 1 to 30.
- l′′ in the formula (XIX) represents a repeating unit of oxypropylene. This l'' is an average value and is an integer of 1-30. Among them, the strength ratio (PMI pst /PMI cp ) satisfies the range of 8.0 to 40.0, preferably 20.0 to 35.0. l′′ is preferably between 2 and 25, more preferably between 12 and 20, in order to have properties.
- m'' and m''' in the formula (XIX) refer to repeating units of oxyethylene. These m'' and m''' are average values, and m''+m''' is an integer of 1-30. Among them, in order to easily produce a resin composition satisfying the strength ratio (EI pst /EI cp ) of 5.0 to 20.0 and to have excellent properties, m''+m''' is preferably 5-25, more preferably 10-20.
- the component (B22) represented by the formula (XIX) is A block copolymer type polyoxyethylene polyoxypropylene glycol represented by the above formula (XIX) is exemplified.
- the mixing ratio of the component (A) and the component B is preferably within the following range.
- nA/nB is preferably from 1.00 to 1.50, more preferably from 1.00 to 1.20, still more preferably from 1.02 to 1.15, and from 1.04 to 1 0.10 or less is most preferred.
- the preferable ranges of PMI pst /PMI cp , AMI pst /AMI cp , and EI pst /EI cp are satisfied, and excellent photochromic properties are obtained. , durability and heat resistance can be obtained.
- nA/nB is 1.00 or more in order to enhance the releasability between the resulting photochromic cured body and the mold. 1.09 or less is particularly preferable. However, even when nA/nB exceeds 1.09, the releasability can be improved by blending the release agent described in detail below into the photochromic curable composition.
- nA/nB is preferably 1.10 or more and 1.50 or less, more preferably 1.10 or more, in order to improve adhesion (adhesion) with an optical substrate made of inorganic glass. It is preferable to be 1.40 or less.
- nA/nB is 1.00 or more and 1.50 or less, Adheres well to optical substrates.
- nA/nB is preferably 1.05 or more and 1.20 or less in order to obtain a laminate having excellent adhesiveness, photochromic properties, and mechanical properties.
- nA/nB is preferably in the following range. Specifically, when a polyvinyl alcohol-based polarizing film is present in the object to be laminated, nA/nB is preferably 1.10 or more and 1.50 or less, and 1.20 or more and 1.40 or less. It is preferable that
- nB1/nB2 is preferably 10.0 to 30.0, preferably 12.0 to 25.0. 0 is more preferred, and 12.0 to 22.0 is most preferred.
- the component (B1) contains the component (B1b)
- the number of moles of active hydrogen-containing groups in the component (B1b) is calculated from the hydroxyl value, for example.
- the number of moles of each of the photochromic curable compositions preferably satisfies the above range, and in terms of mass ratio, it preferably satisfies the following range.
- the mixing ratio of the components (A), (B1), and (B2) is not particularly limited, but in order to obtain excellent photochromic properties, durability, and heat resistance of the photochromic cured product, the following are required. It is preferable to satisfy the range. 20 to 74 parts by mass of component (A), 20 to 75 parts by mass of component (B1), and (B2) component with respect to a total of 100 parts by mass of component (A), component (B1), and (B2) It is preferably in the range of 5 to 40 parts by mass. Furthermore, it is preferable to satisfy the following compounding amounts.
- component 25 to 71 parts by mass of component (A), 23 to 67 parts by mass of component (B1), and (B2) component It is preferably in the range of 6 to 30 parts by mass, 25 to 69 parts by mass of component (A), 23 to 67 parts by mass of component (B1), and 6 to 30 parts by mass of component (B2). More preferably, 30 to 63 parts by mass of component (A), 30 to 60 parts by mass of component (B1), and 7 to 20 parts by mass of component (B2). 57 parts by mass, 35 to 60 parts by mass of component (B1), and 8 to 20 parts by mass of component (B2).
- the (B21) component is used as the (B2) component, and the mass ratio of the mass of the (B21) component to the mass of the (B1) component ((B21)/( B1)) is more preferably 0.35 or more and 0.65 or less, and more preferably 0.40 or more and 0.55 or less.
- the photochromic curable composition further contains various (C) polymerization curing accelerators (hereinafter also referred to as component (C)) in order to accelerate the polymerization and curing of the composition according to the type of the components described above. OK.
- component (C) polymerization curing accelerators
- a reaction catalyst or condensing agent for urethane or urea used for the reaction between hydroxyl groups and thiol groups and isocyanate groups and isothiacyanate groups is used as a polymerization curing accelerator.
- This reaction catalyst for urethane or urea is used in the formation of poly(thio)urethane bonds by the reaction of polyiso(thio)cyanate with polyol or polythiol.
- These urethane or urea reaction catalysts include tertiary amines and their corresponding inorganic or organic salts, phosphines, quaternary ammonium salts, quaternary phosphonium salts, Lewis acids, or organic sulfonic acids. Specific examples thereof include the following.
- the catalytic activity is too high depending on the type of the above-mentioned compound to be selected, it is possible to suppress the catalytic activity by using a mixture of a tertiary amine and a Lewis acid.
- Tertiary amines triethylamine, tri-n-propylamine, triisopropylamine, tri-n-butylamine, triisobutylamine, triethylamine, hexamethylenetetramine, N,N-dimethyloctylamine, N,N,N',N '-Tetramethyl-1,6-diaminohexane, 4,4'-trimethylenebis(1-methylpiperidine), 1,8-diazabicyclo-(5,4,0)-7-undecene.
- Phosphines trimethylphosphine, triethylphosphine, tri-n-propylphosphine, triisopropylphosphine, tri-n-butylphosphine, triphenylphosphine, tribenzylphosphine, 1,2-bis(diphenylphosphino)ethane, 1,2 - bis(dimethylphosphino)ethane.
- Quaternary ammonium salts tetramethylammonium bromide, tetrabutylammonium chloride, tetrabutylammonium bromide.
- Quaternary phosphonium salts tetramethylphosphonium bromide, tetrabutylphosphonium chloride, tetrabutylphosphonium bromide.
- Lewis acid triphenylaluminum, dimethyltin dichloride, dimethyltin bis (isooctylthioglycolate), dibutyltin dichloride, dibutyltin dilaurate, dibutyltin maleate, dibutyltin maleate polymer, dibutyltin diricinolate, dibutyltin bis (dodecylmerka dibutyltin bis(isooctylthioglycolate), dioctyltin dichloride, dioctyltin maleate, dioctyltin maleate polymer, dioctyltin bis(butyl maleate), dioctyltin dilaurate, dioctyltin
- Condensing agent Polymerization initiator that is a condensing agent
- the condensing agent include the following. Inorganic acids; hydrogen chloride, hydrogen bromide, sulfuric acid, phosphoric acid, and the like. Organic acids; p-toluenesulfonic acid, camphor-sulfonic acid and the like. Acidic ion exchange resins; Amberlite, Amberlyst, etc.; Carbodiimide; dicyclohexylcarbodiimide, 1-ethyl-3-(3-dimethylaminopyrrolyl)-carbodiimide.
- Each of the above-described various (C) components can be used alone or in combination of two or more.
- the amount used may be a so-called catalytic amount.
- a small amount in the range from 0.001 to 10 parts by weight, in particular from 0.01 to 5 parts by weight, can be used for a total of 100 parts by weight of the components.
- the photochromic curable composition may contain various additives known per se, such as UV absorbers, antistatic agents, infrared absorbers, UV stabilizers, antioxidants, anti-coloring agents, antistatic agents, as long as they do not impair the effect.
- Additives such as agents, fluorescent dyes, dyes, pigments, fragrances, solvents, leveling agents, internal release agents, and polymerization modifiers such as thiols such as t-dodecyl mercaptan, if necessary. can be done.
- an ultraviolet stabilizer in consideration of improving the durability of the photochromic compound.
- Hindered amine light stabilizers, hindered phenol antioxidants, sulfur-based antioxidants, and the like are known as such ultraviolet stabilizers.
- Particularly suitable UV stabilizers are bis(1,2,2,6,6-pentamethyl-4-piperidyl) sebacate, 2,6-di-t-butyl-4-methyl-phenol, ethylenebis(oxyethylene) bis[3-(5-t-butyl-4-hydroxy-m-tolyl)propionate] and the like, and commercially available products include Adekastab LA-52, LA-57, LA-62 manufactured by Asahi Denka Kogyo Co., Ltd.; LA-63, LA-67, LA-77, LA-82, LA-87, Ciba Specialty Chemicals IRGANOX 1010, 1035, 1075, 1098, 1135, 1141, 1222, 1330, 1425, 1520, 259 , 3
- an ultraviolet absorber considering the durability and photochromic properties of the photochromic compound, it is preferable to use an ultraviolet absorber.
- ultraviolet absorbers include benzotriazole-based ultraviolet absorbers, triazine-based ultraviolet absorbers, benzophenone-based ultraviolet absorbers, cyanoacrylate-based ultraviolet absorbers, diphenylacrylate-based ultraviolet absorbers, phenol-based ultraviolet absorbers, and oxanilide-based ultraviolet absorbers. UV absorbers, malonic ester UV absorbers, and cinnamate UV absorbers can be mentioned.
- cyanoacrylate UV absorbers diphenylacrylate UV absorbers, phenol UV absorbers, oxanilide UV absorbers, malonic acid ester UV absorbers, and cinnamate UV absorbers.
- a cinnamic acid ester-based ultraviolet absorber from the viewpoint that durability can be improved without impairing photochromic properties (especially color density) compared to when no ultraviolet absorber is used.
- an internal release agent can be used.
- any agent can be used as long as it has a mold release effect and does not impair physical properties such as the transparency of the resin, but surfactants are preferably used. Among these, phosphate surfactants are preferred.
- the internal release agent as used herein includes those having a release effect among the various catalysts described above, and may also include, for example, quaternary ammonium salts and quaternary phosphonium salts. These internal mold release agents are appropriately selected according to the combination with the monomer, polymerization conditions, economic efficiency, and ease of handling. Specific examples of the phosphate ester internal release agent are as follows.
- Alkyl acid phosphate mono-n-butyl phosphate, mono-2-ethylhexyl phosphate, mono-n-octyl phosphate, mono-n-butyl phosphate, bis(2-ethylhexyl) phosphate, di( 2-ethylhexyl), di-n-octyl phosphate, di-n-butyl phosphate, butyl acid phosphate (mono-, di-mixture), ethyl acid phosphate (mono-, di-mixture), butoxyethyl acid phosphate ( mono-, di-mixture), 2-ethylhexyl acid phosphate (mono-, di-mixture), isotridene acid phosphate (mono-, di-mixture), tetracosyl acid phosphate (mono-, di-mixture), stearyl Acid phosphate (mono-, di-mixture)
- Other phosphoric esters exe
- Each of the above-mentioned other compounding agents can be used alone or in combination of two or more. It can be used in an amount of 0.001 parts by weight to 10 parts by weight.
- the total amount of alkali metal ions and alkaline earth metal ions is preferably 500 ppm or less.
- the photochromic compound is excellent in color development durability, that is, a cured body in which the photochromic compound can be colored for a long period of time. can be realized. The reason for this is considered as follows.
- constituent components of photochromic curable compositions may use alkali metal salts or alkaline earth metal salts, or generate these metal salts, in the course of their synthesis. A trace amount of these metal salts may remain as impurities in the active hydrogen compound or the like after synthesis.
- Counter anions contained in alkali metal salts among others, carboxylate ions, act on the iso(thio)cyanate groups of polyiso(thio)cyanate compounds to form polyiso(thio)cyanurate in which the isocyanate groups are bonded to form a ring. compounds can be produced.
- the iso(thio)cyanate component when alkali metal salts and the like are contained in the photochromic curable composition, the iso(thio)cyanate component is excessively consumed, and polyiso(thio)cyanurate compounds and the like can be produced as by-products. Excessive consumption of the iso(thio)cyanate component results in excess active hydrogen compounds in the photochromic curable composition.
- active hydrogen groups such as thiol groups are excessive. These surplus active hydrogen groups can generate radicals when exposed to ultraviolet rays, which can cause deterioration of the cured product.
- deterioration of the cured product can be suppressed by reducing the amounts of the alkali metal salt and the alkaline earth metal salt in the photochromic curable composition.
- the amount of alkali metal salt and alkaline earth metal salt in the photochromic curable composition can be estimated from the amount of alkali metal ion and alkaline earth metal ion in the photochromic composition.
- the total amount of alkali metal ions and alkaline earth metal ions in the photochromic curable composition can be measured by Inductively Coupled Plasma (ICP) emission spectroscopy.
- ICP Inductively Coupled Plasma
- 10 g of the photochromic curable composition is dissolved in 20 g of chloroform to obtain 30 g of solution.
- 20 g of ultrapure water containing 1% HNO 3 is added to this solution to extract the supernatant.
- the extracted supernatant is used as a measurement sample.
- an ICP emission spectrometer iCAP6500DUO
- the detection limit of ICP emission analysis is, for example, 1 ppb.
- a calibration curve method is used to calculate the concentration of alkali metal ions and the like.
- the total content of alkali metal ions and alkaline earth metal ions in the photochromic curable composition is preferably 200 ppm or less, more preferably 100 ppm or less.
- the lower limit of alkali metal ions and alkaline earth metal ions in the photochromic composition is 0 ppm or the detection limit of an ICP emission spectrometer.
- the contents of alkali metal salts, alkaline earth metal salts, alkali metal ions, alkaline earth metal ions, and their counter anions in the photochromic curable composition are determined, for example, by washing with water, various adsorbents, ion exchange resins, and the like. It can be reduced by contacting treatment. By lengthening the water washing time of the photochromic curable composition, increasing the amounts of the adsorbent and the ion exchange resin, or lengthening the contact time with these, the amount of the alkali metal salt, etc. can be increased. can be reduced.
- the photochromic curable composition may be prepared using constituent components such as active hydrogen compounds subjected to the above-described reduction treatment as raw materials.
- the photochromic curable composition may be used by mixing the above-mentioned (ii) photochromic compound, (A) component, (B) component, and other ingredients by a known method without particular limitation.
- a photochromic curable composition can be obtained by dissolving component (ii) in component (A) and then adding component (B) and stirring.
- the stirring temperature is in the range of 0 to 100° C.
- the stirring time is in the range of 0.1 to 48 hours, which may be appropriately adjusted.
- the (B21) component it is possible to suppress an increase in viscosity during the production of this photochromic curable composition.
- the component (A) since the component (A) has an iso(thio)cyanate group in its molecule, it is preferably produced in an atmosphere of an inert gas such as argon or nitrogen in order to suppress the contamination of moisture.
- a photochromic optical article can be obtained by polymerizing a photochromic curable composition to form a photochromic cured body. It is usually polymerized by thermal polymerization. This cured product is a resin composition, and a photochromic optical article made of the resin composition can be obtained.
- the temperature particularly affects the properties of the resulting photochromic cured product. Since this temperature condition is affected by the type and amount of the thermal polymerization initiator and the type of compound, it cannot be unconditionally limited. preferred. As with temperature, the polymerization time also varies depending on various factors, so it is preferable to determine the optimum time according to these conditions in advance. preferred to choose. When obtaining a photochromic laminated sheet, it is preferable to carry out the polymerization at a temperature at which the reaction between the polymerizable functional groups proceeds, and to determine the optimum temperature and time so as to obtain the target molecular weight.
- the mode of obtaining a photochromic optical article by polymerizing the photochromic curable composition is not particularly limited.
- the mode of obtaining a photochromic optical article by polymerizing the photochromic curable composition is not particularly limited.
- the following known method can be adopted.
- the photochromic lens is produced by the kneading method
- the above photochromic composition is injected between inorganic glass molds held by elastomer gaskets or spacers, and after sufficient defoaming, it is placed in an air oven or
- a photochromic cured body (photochromic optical article) molded into the form of an optical material such as a lens can be obtained by cast polymerization by heating in water.
- an optical substrate such as a lens substrate is arranged so as to form a predetermined gap, a photochromic curable composition is injected into the gap, and in this state, casting polymerization is performed using an inner mold, in which polymerization is performed by heating.
- a photochromic lens laminate in which photochromic optical articles are laminated
- a photochromic layer is formed on the surface of an optical substrate can also be obtained by the method (manufacture of a laminate by a casting polymerization method).
- the optical substrate is not particularly limited, and optical substrates made of known plastics can be used. Specific examples include plastic materials such as (meth)acrylic resins, polycarbonate resins, allyl resins, thiourethane resins, urethane resins, and thioepoxy resins.
- the surface of the optical substrate is preliminarily treated with an alkaline solution, an acid solution, etc., subjected to chemical treatment, corona discharge, plasma discharge, polishing. Adhesion between the photochromic layer and the optical substrate can be enhanced by performing a physical treatment such as the above. Of course, it is also possible to provide a transparent adhesive resin layer on the surface of the optical substrate.
- a necessary amount of the photochromic curable composition is applied onto one optical substrate such as inorganic glass on which spacers are placed, and the other optical substrate such as glass is placed thereon, followed by photochromic curing applied. It is also possible to bond optical substrates such as a pair of inorganic glass optical materials by curing the optical composition.
- a photochromic lens laminate in which photochromic optical articles are laminated
- a photochromic sheet made of a photochromic curable composition is manufactured.
- the resulting photochromic sheet is sandwiched between two transparent sheets (optical sheets), and the above-described polymerization is performed to obtain a photochromic laminate having a photochromic layer as an adhesive layer.
- the photochromic sheet can also be produced by coating with a coating liquid obtained by dissolving a photochromic curable composition in an organic solvent.
- the photochromic laminate thus produced is mounted in a mold, and then a thermoplastic resin (for example, polycarbonate) for optical substrates such as lenses is injection-molded to obtain a photochromic laminate. is laminated to obtain a photochromic lens having a predetermined shape. Also, this photochromic laminate can be adhered to the surface of an optical substrate with an adhesive or the like, thereby obtaining a photochromic lens.
- a thermoplastic resin for example, polycarbonate
- optical substrates such as lenses
- this photochromic laminate can be adhered to the surface of an optical substrate with an adhesive or the like, thereby obtaining a photochromic lens.
- a urethane or urea-based polymerizable compound particularly a urethane-based polymerizable compound, is used as the polymerizable compound because it has particularly high adhesion to the optical substrate. , preferably adjusted to form a polyurethane.
- the resulting photochromic cured product/laminate can express photochromic properties excellent in color density, color fading speed, etc., and is effective in producing optical substrates with photochromic properties, such as photochromic lenses (photochromic optical articles). used for
- the photochromic cured product can be laminated with other functional layers or dyed with a dye such as a disperse dye, depending on the intended use, as long as the effect is not impaired.
- a hard coating film can be formed thereon using a silane coupling agent or a hard coating agent containing a sol such as silicon, zirconium, antimony, aluminum, tin or tungsten as a main component.
- thin films can be produced by vapor deposition of metal oxides such as SiO 2 , TiO 2 and ZrO 2 .
- An antireflection treatment can be performed with a thin film by applying an organic polymer.
- An antistatic treatment or the like can also be applied.
- lamination with other functional layers described above includes lamination of a polarizing film for the purpose of imparting polarizing properties to the obtained photochromic cured body.
- the position of the polarizing film is not particularly limited, and it may be laminated outside the photochromic cured body, between the photochromic cured body and another layer, or within the adhesive layer when an adhesive layer is used. A method of burying in the adhesive layer when using an adhesive layer made of thin film is preferred.
- the method of laminating the polarizing film is also not particularly limited, and a known method may be adopted.
- a polarizing film is placed between the front or rear mold and the photochromic curable composition, or within the photochromic composition. , followed by lamination by polymerizing the photochromic curable composition.
- thermosetting adhesive or ultraviolet (UV) curable adhesive it is preferable to laminate a polarizing film in advance on one surface of an optical substrate made of inorganic glass.
- thermosetting adhesive or ultraviolet (UV) curable adhesive it is possible to use a known thermosetting adhesive or ultraviolet (UV) curable adhesive to bond an optical substrate made of inorganic glass and a polarizing film.
- the polarizing film is not particularly limited, and commercially available polarizing films can be used.
- a polarizing film having a thickness of 20 to 100 ⁇ m can be suitably used.
- a polarizing film is formed by stretching polyvinyl alcohol dyed with a dichroic substance such as iodine or a dichroic dye.
- a commercially available dichroic dye can be used without limitation as the dichroic dye contained in the polarizing film.
- Examples thereof include azo-based and anthraquinone-based dyes. Specifically, Chloranthin Fast Red (CI 28160), Congo Red (CI 22120), Brilliant Blue B (CI 24410), Benzopurpurine (CI 23500), Chloranthin Sol Black BH (CI 22590), Direct Blue 2B (CI 22610), Diamine Green (CI 30295), Chrysophenine (CI 24895), Sirius Yellow (CI 29000) , Direct Fast Red (CI 23630), Acid Black (CI 20470), Direct Sky Blue (CI 24400), Solophenyl Blue 4GL (CI 34200), Direct Copper Blue 2B ( CI 24185), Nippon Brilliant Violet BKconc (CI 27885), and the like.
- Two or more color dyes can be selected from these dichroic dyes and used depending on the purpose.
- the photochromic curable composition described above is used, even a polarizing film having a luminous transmittance of 10 to 60% and a degree of polarization of 70.0 to 99.9, which is usually difficult to bond, can be strongly bonded. can be spliced.
- the polarizing film may have cellulose triacetate films laminated on both sides in order to enhance its function and adhesiveness.
- the thickness of the cellulose triacetate film is preferably 20-200 ⁇ m, more preferably 20-100 ⁇ m.
- An optical laminate according to an embodiment includes an optical substrate and a resin composition according to an embodiment laminated on at least one main surface of the optical substrate.
- FIG. 6 is a cross-sectional view schematically showing an example of the optical layered body according to the embodiment.
- the optical laminate 1 shown in FIG. 6 includes an optical substrate 2, a primer layer 3 provided on one main surface of the optical substrate 2, and a resin composition 4 provided on the primer layer 3. .
- the optical substrate 2 has an uneven shape.
- the primer layer 3 and the resin composition 4 cover the convex side of the optical substrate 2 .
- the primer layer 3 contains, for example, an adhesive containing polyurethane resin or the like.
- the primer layer 3 may be omitted.
- Eyeglasses according to embodiments include lenses according to embodiments.
- FIG. 7 is a perspective view schematically showing an example of spectacles according to the embodiment. Glasses 110 shown in FIG. 7 include two lenses 111 and a frame 112 fixing these lenses 111 . At least one of the two lenses 111 is a lens containing the resin composition according to the embodiment.
- NBDI norbornane diisocyanate.
- IPDI isophorone diisocyanate.
- 1,3-H6XDI 1,3-bis(isocyanatomethyl)cyclohexane.
- 1,4-H6XDI 1,4-bis(isocyanatomethyl)cyclohexane.
- TMMP trimethylolpropane tris(3-mercaptopropionate), having three thiol groups in one molecule.
- PEMP Pentaerythritol tetrakis(3-mercaptopropionate), having four thiol groups in the molecule.
- DPMP Dipentaerythritol-hexakis (3-mercaptopropionate), having 6 thiol groups in the molecule.
- RX-1 Polyrotaxane Polyrotaxane synthesized by the method described in International Publication No. 2015/068798.
- the axial molecule is formed of polyethylene glycol with a molecular weight of 11,000, the bulky groups at both ends are adamantyl groups, the cyclic molecule is ⁇ -cyclodextrin, and an average of 3.5 molecules of ⁇ -caprolactone are ring-opened via oxypropylene groups. It is what I did.
- the properties of RX-1 are shown below. Inclusion amount of ⁇ -cyclodextrin: 0.25. Modification degree of side chain: 0.5. Side chain molecular weight: about 450 on average. Weight average molecular weight: 180,000. Hydroxyl value: 85 mgKOH/g. From the above figures, the average number of hydroxyl groups in one molecule is 270.
- MPEG750 methoxy polyethylene glycol (average molecular weight 750)
- a photochromic curable composition was prepared by mixing each component according to the formulation shown in Tables 1 and 3.
- the content of alkali metal ions or alkaline earth metal ions in the photochromic curable composition was measured using an ICP emission spectrometer (iCAP6500DUO) manufactured by Thermo Fisher Scientific.
- ICP emission spectrometer iCAP6500DUO
- the prepared photochromic curable composition was sufficiently defoamed, it was injected into an inorganic glass mold provided with a gap of 2 mm, and the photochromic curable composition was polymerized by casting polymerization. Polymerization was carried out using an air furnace, and curing was carried out over 18 hours while gradually raising the temperature from 27°C to 120°C. After polymerization, the cured body was removed from the inorganic glass mold to obtain a photochromic cured body (resin composition) having a thickness of 2 mm. The obtained photochromic cured product was evaluated by the method shown below.
- Yellowness index ( ⁇ YI) The difference between the yellowness index (YI 96 ) after accelerated deterioration for 96 hours using the xenon weather meter X25 and the yellowness index (YI 0 ) before the test.
- a value measured by a touch panel type SM color computer SM-T manufactured by Suga Test Instruments Co., Ltd. was used.
- Heat-resistant As the softening temperature of the obtained photochromic cured product, a value measured by a thermomechanical analyzer TMA8311 manufactured by Rigaku Corporation (three-point bending method, heating rate: 10° C./min) was used.
- Alkali metal ion or alkaline earth metal ion The concentration of alkali metal ions or alkaline earth metal ions in the resulting photochromic cured product was measured using a circular flat plate with a diameter of 40 mm and a thickness of 1 mm, and an X-ray fluorescence spectrometer (ZSX Primus IV) manufactured by Rigaku Corporation. values were used. The lower limit of detection in this measurement is 1 ppm. Table 5 shows the evaluation results of the photochromic cured product.
- Examples 2 to 24 Comparative Examples 1 to 3> A photochromic cured product was produced in the same manner as in Example 1 according to the recipes shown in Tables 1 to 4 above, and evaluated. Similar to Example 1, evaluation results are shown in Tables 5 and 6.
- Photochromic curable compositions were prepared according to the formulations shown in Tables 1 to 4 above, except that 0.2 parts by mass of component PC1 was used. Corresponding examples and comparative examples are shown in Tables 7 and 8. Separately, a mold comprising an inorganic glass plate and a thiourethane plastic lens having a refractive index of 1.60 was prepared. The gap between the inorganic glass plate and the thiourethane plastic lens was set to 1 mm. After sufficiently defoaming the prepared photochromic curable composition, the photochromic curable composition was injected into the mold provided with a gap of 1 mm and polymerized.
- the photochromic curable compositions described in Tables 9-12 below were prepared.
- the prepared photochromic curable composition was used as an adhesive. That is, a laminate was produced by bonding a pair of optical substrates (plates) made of inorganic glass for optical articles with the adhesive. First, a photochromic curable composition was applied onto one of the inorganic glass plates for optical articles having spacers with a thickness of 0.1 mm arranged at the ends thereof. Then, the other plate made of the inorganic glass for optical articles was placed on the coated photochromic curable composition. The photochromic curable composition was then polymerized.
- the obtained photochromic laminate was evaluated for photochromic properties and durability in the same manner as in Example 1, and the evaluation results are shown in Tables 13 and 14.
- the motility of the component (i) was determined by curing the photochromic curable composition separately under the same conditions as in the above method, and measuring the obtained cured product.
- the heat resistance was not evaluated because of the influence of the plate made of inorganic glass.
- the photochromic curable compositions described in Tables 15-18 below were prepared.
- the prepared photochromic curable composition was used as an adhesive. That is, in a pair of optical substrates (plates) made of inorganic glass for optical articles, one having a polarizing film on the surface of one of the plates and the other of the plates are joined with the adhesive to laminate the manufactured the body.
- an acrylic adhesive was applied by spin coating to one surface of a plate made of inorganic glass for optical articles, and a polarizing film (thickness: 27 ⁇ m, luminous transmittance: 42.5%, degree of polarization: 99.2) was applied thereon.
- % gray color, polyvinyl alcohol base).
- UV irradiation was performed from the plate side to prepare an optical base material in which the polarizing film/the plate were laminated.
- a photochromic curable composition is applied onto a plate made of inorganic glass for optical articles and a spacer of 0.1 mm thickness is arranged at the edge, and the optical substrate having a polarizing film laminated thereon is obtained. , the polarizing film surface was placed in contact with the photochromic curable composition.
- the photochromic curable composition was then polymerized. Polymerization was carried out using an air furnace, and the temperature was gradually raised from 27°C to 120°C, and cured over 18 hours. A glass-bonded photochromic laminate was obtained. The obtained photochromic laminate was evaluated for photochromic properties and durability in the same manner as in Example 1, and the evaluation results are shown in Tables 19 and 20.
- the motility of the component (i) was determined by curing the photochromic curable composition separately under the same conditions as in the above method, and measuring the obtained cured product. The heat resistance was not evaluated because of the influence of the plate made of inorganic glass.
- a resin composition having a strength ratio (PMI pst /PMI cp ) of 8.0 or more and 40.0 or less could achieve excellent photochromic properties and mechanical properties by controlling molecular mobility with a crosslinked structure.
- a resin composition having excellent photochromic properties and heat resistance can be obtained.
- component (B1) By using a component having six thiol groups in the molecule as component (B1), a resin composition exhibiting excellent effects can be obtained. By using a component having six thiol groups in the molecule, the strength ratio (PMI pst /PMI cp ) of the obtained resin composition can be made a relatively high value.
- the resin composition according to the embodiment has excellent photochromic properties and particularly excellent heat resistance. From this, it is clear that photochromic properties can be evaluated from the intensity ratio (PMI pst /PMI cp ) for evaluating molecular mobility.
- the effect of improving heat resistance is that the number of hydrogen bonds per cross-linking point increases, resulting in Multivalent Interaction. ) is considered to have improved the heat resistance.
- the intensity ratio (PMI pst /PMI cp ) can be 10.0 or more and 15.0 or less. It can be preferably 11.0 to 15.0, more preferably 12.5 to 15.0. In this case, it is possible to keep the increase in viscosity of the photochromic curable composition low.
- PEMP component having 4 thiol groups in the molecule
- DPMP component having 6 thiol groups in the molecule
- TMMP component having three thiol groups in the molecule
- a comparison of these examples reveals that the use of PEMP and DPMP, which correspond to fully substituted compounds, exhibits particularly excellent photochromic properties as compared to the case of using TMMP, which corresponds to partially substituted compounds. rice field. From this, it is considered that when a fully substituted compound is used as the component (B1), a free space for molecular motion of the photochromic compound is effectively formed.
- durability can be improved by adjusting the content of alkali metal ions and alkaline earth metal ions contained in the photochromic composition of the present invention.
- the reason for this is considered as follows. That is, when the content of the alkali metal salt and alkaline earth metal salt contained in the photochromic composition is large, a side reaction such as isocyanuration occurs during the preparation or polymerization of the photochromic composition, resulting in Increases residual thiol groups. A thiol group generates radicals upon exposure to ultraviolet light, which is thought to promote deterioration of the photochromic compound and reduce durability.
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Physics & Mathematics (AREA)
- General Physics & Mathematics (AREA)
- Optics & Photonics (AREA)
- Ophthalmology & Optometry (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Compositions Of Macromolecular Compounds (AREA)
- Polyurethanes Or Polyureas (AREA)
- Eyeglasses (AREA)
Priority Applications (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP22742470.2A EP4282932A4 (en) | 2021-01-25 | 2022-01-12 | RESIN COMPOSITION, OPTICAL LAMINATE, OPTICAL ARTICLE, LENS AND GLASSES |
| CN202280010524.1A CN116868095A (zh) | 2021-01-25 | 2022-01-12 | 树脂组合物、光学层叠体、光学物品、透镜以及眼镜 |
| US18/271,925 US20240100810A1 (en) | 2021-01-25 | 2022-01-12 | Resin composition, optical laminate, optical article, lens and eyeglasses |
| MX2023008270A MX2023008270A (es) | 2021-01-25 | 2022-01-12 | Composicion de resina, laminado optico, articulo optico, lente, y anteojos. |
| KR1020237023465A KR20230137306A (ko) | 2021-01-25 | 2022-01-12 | 수지 조성물, 광학 적층체, 광학 물품, 렌즈 및 안경 |
| JP2022576620A JPWO2022158348A1 (enExample) | 2021-01-25 | 2022-01-12 |
Applications Claiming Priority (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2021009922 | 2021-01-25 | ||
| JP2021-009922 | 2021-01-25 | ||
| JP2021-047602 | 2021-03-22 | ||
| JP2021047602 | 2021-03-22 | ||
| JP2021-065523 | 2021-04-07 | ||
| JP2021065523 | 2021-04-07 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2022158348A1 true WO2022158348A1 (ja) | 2022-07-28 |
Family
ID=82548911
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2022/000699 Ceased WO2022158348A1 (ja) | 2021-01-25 | 2022-01-12 | 樹脂組成物、光学積層体、光学物品、レンズ及び眼鏡 |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US20240100810A1 (enExample) |
| EP (1) | EP4282932A4 (enExample) |
| JP (1) | JPWO2022158348A1 (enExample) |
| KR (1) | KR20230137306A (enExample) |
| MX (1) | MX2023008270A (enExample) |
| TW (1) | TW202237795A (enExample) |
| WO (1) | WO2022158348A1 (enExample) |
Citations (66)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS522189B1 (enExample) | 1970-06-30 | 1977-01-20 | ||
| JPS62288830A (ja) | 1986-05-01 | 1987-12-15 | ピルキントン・ブラザ−ズ・ピ−エルシ− | フォトクロミック化合物および該化合物を利用したプラスチック有機フォトクロミック物品 |
| JPH0228154A (ja) | 1987-11-10 | 1990-01-30 | Tokuyama Soda Co Ltd | 新規化合物及びそれを含む組成物 |
| WO1994022850A1 (en) | 1993-03-30 | 1994-10-13 | Pilkington Plc | Photochromic naphthopyran compounds |
| WO1996014596A1 (en) | 1994-11-03 | 1996-05-17 | Ppg Industries, Inc. | Novel photochromic indeno-fused naphthopyrans |
| JPH08157467A (ja) | 1994-12-02 | 1996-06-18 | Tokuyama Corp | クロメン化合物及びフォトクロミック材 |
| JPH08176139A (ja) | 1994-12-20 | 1996-07-09 | Tokuyama Corp | クロメン化合物 |
| JPH08295690A (ja) | 1995-04-26 | 1996-11-12 | Tokuyama Corp | クロメン化合物 |
| JPH09124645A (ja) | 1995-11-02 | 1997-05-13 | Tokuyama Corp | クロメン化合物 |
| US5645767A (en) | 1994-11-03 | 1997-07-08 | Transitions Optical, Inc. | Photochromic indeno-fused naphthopyrans |
| JPH09218301A (ja) | 1995-12-06 | 1997-08-19 | Tokuyama Corp | クロメン化合物 |
| US5658501A (en) | 1995-06-14 | 1997-08-19 | Transitions Optical, Inc. | Substituted naphthopyrans |
| US5961892A (en) | 1998-09-11 | 1999-10-05 | Ppg Industries Ohio, Inc. | Polyalkoxylated naphthopyrans |
| JPH11279171A (ja) | 1998-03-30 | 1999-10-12 | Tokuyama Corp | クロメン化合物 |
| JPH11286484A (ja) | 1998-03-31 | 1999-10-19 | Tokuyama Corp | クロメン化合物 |
| JPH11322739A (ja) | 1998-05-14 | 1999-11-24 | Tokuyama Corp | クロメン化合物 |
| JP2000219686A (ja) | 1999-01-29 | 2000-08-08 | Tokuyama Corp | クロメン化合物 |
| JP2000219678A (ja) | 1998-07-03 | 2000-08-08 | Sankyo Co Ltd | ベンゼン誘導体又はピリジン誘導体 |
| JP2000229972A (ja) | 1999-02-04 | 2000-08-22 | Tokuyama Corp | クロメン化合物 |
| JP2000229975A (ja) | 1999-02-04 | 2000-08-22 | Tokuyama Corp | クロメン化合物 |
| JP2000229974A (ja) | 1999-02-04 | 2000-08-22 | Tokuyama Corp | クロメン化合物 |
| JP2000229976A (ja) | 1999-02-05 | 2000-08-22 | Tokuyama Corp | クロメン化合物 |
| JP2000229973A (ja) | 1999-02-04 | 2000-08-22 | Tokuyama Corp | クロメン化合物 |
| JP2000256347A (ja) | 1999-03-12 | 2000-09-19 | Tokuyama Corp | クロメン化合物 |
| JP2000327675A (ja) | 1999-05-24 | 2000-11-28 | Tokuyama Corp | クロメン化合物 |
| WO2000071544A1 (en) | 1999-05-24 | 2000-11-30 | Tokuyama Corporation | Chromene compounds |
| JP2000344761A (ja) | 1999-05-28 | 2000-12-12 | Tokuyama Corp | クロメン化合物 |
| JP2001011067A (ja) | 1999-07-01 | 2001-01-16 | Tokuyama Corp | クロメン化合物 |
| JP2001011066A (ja) | 1999-07-02 | 2001-01-16 | Tokuyama Corp | クロメン化合物 |
| JP2001031670A (ja) | 1999-07-19 | 2001-02-06 | Tokuyama Corp | クロメン化合物 |
| WO2001060811A1 (en) | 2000-02-21 | 2001-08-23 | Tokuyama Corporation | Chromene compound |
| US6296785B1 (en) | 1999-09-17 | 2001-10-02 | Ppg Industries Ohio, Inc. | Indeno-fused photochromic naphthopyrans |
| WO2002090342A1 (en) | 2001-05-02 | 2002-11-14 | Tokuyama Corporation | Chromene compound |
| WO2003042203A1 (en) | 2001-11-16 | 2003-05-22 | Tokuyama Corporation | Chromene compound |
| JP2003277381A (ja) | 2002-03-25 | 2003-10-02 | Tokuyama Corp | クロメン化合物 |
| JP3471073B2 (ja) | 1994-04-27 | 2003-11-25 | 株式会社トクヤマ | フォトクロミック組成物 |
| WO2004041961A1 (en) | 2002-11-04 | 2004-05-21 | Polymers Australia Pty Limited | Photochromic compositions and light transmissible articles |
| WO2005028465A1 (ja) | 2003-09-18 | 2005-03-31 | Tokuyama Corporation | クロメン化合物 |
| JP2005112772A (ja) | 2003-10-07 | 2005-04-28 | Tokuyama Corp | クロメン化合物 |
| JP2005289812A (ja) | 2002-04-01 | 2005-10-20 | Tokuyama Corp | クロメン化合物 |
| JP3801386B2 (ja) | 1999-06-03 | 2006-07-26 | 株式会社トクヤマ | クロメン化合物 |
| WO2007086532A1 (ja) | 2006-01-25 | 2007-08-02 | Tokuyama Corporation | クロメン化合物 |
| JP3982770B2 (ja) | 1997-04-30 | 2007-09-26 | 株式会社トクヤマ | クロメン化合物 |
| WO2008023828A1 (en) | 2006-08-24 | 2008-02-28 | Tokuyama Corporation | Chromene compound |
| JP4157227B2 (ja) | 1999-06-01 | 2008-10-01 | 株式会社トクヤマ | クロメン化合物 |
| JP4157239B2 (ja) | 1999-10-19 | 2008-10-01 | 株式会社トクヤマ | クロメン化合物 |
| JP2009057300A (ja) | 2007-08-30 | 2009-03-19 | Tokuyama Corp | クロメン化合物 |
| JP2009067680A (ja) | 2007-09-10 | 2009-04-02 | Tokuyama Corp | クロメン化合物 |
| JP2009067754A (ja) | 2007-09-14 | 2009-04-02 | Tokuyama Corp | クロメン化合物 |
| JP2009120536A (ja) | 2007-11-15 | 2009-06-04 | Tokuyama Corp | クロメン化合物 |
| JP4301621B2 (ja) | 1999-01-29 | 2009-07-22 | 株式会社トクヤマ | クロメン化合物 |
| WO2009136668A1 (ja) | 2008-05-09 | 2009-11-12 | 株式会社トクヤマ | クロメン化合物 |
| WO2009146509A1 (en) | 2008-06-05 | 2009-12-10 | Advanced Polymerik Pty Ltd | Photochromic polymer and composition comprising photochromic polymer |
| JP4424981B2 (ja) | 2003-12-26 | 2010-03-03 | 株式会社トクヤマ | クロメン化合物 |
| WO2012149599A1 (en) | 2011-05-03 | 2012-11-08 | Advanced Polymerik Pty Ltd | Photochromic polymer |
| WO2012162725A1 (en) | 2011-06-03 | 2012-12-06 | Advanced Polymerik Pty Ltd | Photochromic polymers |
| WO2012176439A1 (ja) | 2011-06-23 | 2012-12-27 | 三井化学株式会社 | 重合性組成物 |
| WO2013078086A1 (en) | 2011-11-22 | 2013-05-30 | Transitions Optical, Inc. | Photochromic compounds having at least two photochromic moieties |
| WO2015068798A1 (ja) | 2013-11-11 | 2015-05-14 | 株式会社トクヤマ | フォトクロミック組成物 |
| WO2015115648A1 (ja) | 2014-02-03 | 2015-08-06 | 三井化学株式会社 | 光学材料用重合性組成物、当該組成物から得られる光学材料およびプラスチックレンズ |
| WO2016143910A1 (ja) | 2015-03-10 | 2016-09-15 | 株式会社トクヤマ | フォトクロミック硬化体の製造方法 |
| WO2017038865A1 (ja) * | 2015-09-03 | 2017-03-09 | 株式会社トクヤマ | ポリロタキサン及びその製法並びに該ポリロタキサンを含有する光学用組成物 |
| WO2019013249A1 (ja) | 2017-07-14 | 2019-01-17 | 株式会社トクヤマ | クロメン化合物、該化合物を含む硬化性組成物、および該硬化性組成物からなる硬化体を含む光学物品 |
| WO2019198664A1 (ja) * | 2018-04-12 | 2019-10-17 | 株式会社トクヤマ | フォトクロミック光学物品及びその製造方法 |
| WO2019203205A1 (ja) | 2018-04-17 | 2019-10-24 | 株式会社トクヤマ | フォトクロミック化合物、該フォトクロミック化合物を含む硬化性組成物及び光学物品 |
| WO2021241596A1 (ja) * | 2020-05-28 | 2021-12-02 | 株式会社トクヤマ | 光学材料用化合物、硬化性組成物、硬化体、及び光学物品 |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20210150386A (ko) * | 2019-04-03 | 2021-12-10 | 가부시끼가이샤 도꾸야마 | 포토크로믹 광학 물품 및 그의 제조 방법 |
-
2022
- 2022-01-12 JP JP2022576620A patent/JPWO2022158348A1/ja active Pending
- 2022-01-12 WO PCT/JP2022/000699 patent/WO2022158348A1/ja not_active Ceased
- 2022-01-12 US US18/271,925 patent/US20240100810A1/en active Pending
- 2022-01-12 KR KR1020237023465A patent/KR20230137306A/ko active Pending
- 2022-01-12 EP EP22742470.2A patent/EP4282932A4/en active Pending
- 2022-01-12 MX MX2023008270A patent/MX2023008270A/es unknown
- 2022-01-17 TW TW111101804A patent/TW202237795A/zh unknown
Patent Citations (76)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS522189B1 (enExample) | 1970-06-30 | 1977-01-20 | ||
| JPS62288830A (ja) | 1986-05-01 | 1987-12-15 | ピルキントン・ブラザ−ズ・ピ−エルシ− | フォトクロミック化合物および該化合物を利用したプラスチック有機フォトクロミック物品 |
| JPH0228154A (ja) | 1987-11-10 | 1990-01-30 | Tokuyama Soda Co Ltd | 新規化合物及びそれを含む組成物 |
| WO1994022850A1 (en) | 1993-03-30 | 1994-10-13 | Pilkington Plc | Photochromic naphthopyran compounds |
| JP3471073B2 (ja) | 1994-04-27 | 2003-11-25 | 株式会社トクヤマ | フォトクロミック組成物 |
| US5645767A (en) | 1994-11-03 | 1997-07-08 | Transitions Optical, Inc. | Photochromic indeno-fused naphthopyrans |
| WO1996014596A1 (en) | 1994-11-03 | 1996-05-17 | Ppg Industries, Inc. | Novel photochromic indeno-fused naphthopyrans |
| JPH08157467A (ja) | 1994-12-02 | 1996-06-18 | Tokuyama Corp | クロメン化合物及びフォトクロミック材 |
| JPH08176139A (ja) | 1994-12-20 | 1996-07-09 | Tokuyama Corp | クロメン化合物 |
| JPH08295690A (ja) | 1995-04-26 | 1996-11-12 | Tokuyama Corp | クロメン化合物 |
| US5658501A (en) | 1995-06-14 | 1997-08-19 | Transitions Optical, Inc. | Substituted naphthopyrans |
| JPH09124645A (ja) | 1995-11-02 | 1997-05-13 | Tokuyama Corp | クロメン化合物 |
| JPH09218301A (ja) | 1995-12-06 | 1997-08-19 | Tokuyama Corp | クロメン化合物 |
| JP3982770B2 (ja) | 1997-04-30 | 2007-09-26 | 株式会社トクヤマ | クロメン化合物 |
| JPH11279171A (ja) | 1998-03-30 | 1999-10-12 | Tokuyama Corp | クロメン化合物 |
| JPH11286484A (ja) | 1998-03-31 | 1999-10-19 | Tokuyama Corp | クロメン化合物 |
| JPH11322739A (ja) | 1998-05-14 | 1999-11-24 | Tokuyama Corp | クロメン化合物 |
| JP2000219678A (ja) | 1998-07-03 | 2000-08-08 | Sankyo Co Ltd | ベンゼン誘導体又はピリジン誘導体 |
| WO2000015630A1 (en) | 1998-09-11 | 2000-03-23 | Ppg Industries Ohio, Inc. | Polyalkoxylated naphthopyrans |
| US5961892A (en) | 1998-09-11 | 1999-10-05 | Ppg Industries Ohio, Inc. | Polyalkoxylated naphthopyrans |
| JP2000219686A (ja) | 1999-01-29 | 2000-08-08 | Tokuyama Corp | クロメン化合物 |
| JP4301621B2 (ja) | 1999-01-29 | 2009-07-22 | 株式会社トクヤマ | クロメン化合物 |
| JP2000229972A (ja) | 1999-02-04 | 2000-08-22 | Tokuyama Corp | クロメン化合物 |
| JP2000229975A (ja) | 1999-02-04 | 2000-08-22 | Tokuyama Corp | クロメン化合物 |
| JP2000229974A (ja) | 1999-02-04 | 2000-08-22 | Tokuyama Corp | クロメン化合物 |
| JP2000229973A (ja) | 1999-02-04 | 2000-08-22 | Tokuyama Corp | クロメン化合物 |
| JP2000229976A (ja) | 1999-02-05 | 2000-08-22 | Tokuyama Corp | クロメン化合物 |
| JP2000256347A (ja) | 1999-03-12 | 2000-09-19 | Tokuyama Corp | クロメン化合物 |
| JP4256985B2 (ja) | 1999-05-24 | 2009-04-22 | 株式会社トクヤマ | クロメン化合物 |
| JP2000327675A (ja) | 1999-05-24 | 2000-11-28 | Tokuyama Corp | クロメン化合物 |
| WO2000071544A1 (en) | 1999-05-24 | 2000-11-30 | Tokuyama Corporation | Chromene compounds |
| JP2000344761A (ja) | 1999-05-28 | 2000-12-12 | Tokuyama Corp | クロメン化合物 |
| JP4157227B2 (ja) | 1999-06-01 | 2008-10-01 | 株式会社トクヤマ | クロメン化合物 |
| JP3801386B2 (ja) | 1999-06-03 | 2006-07-26 | 株式会社トクヤマ | クロメン化合物 |
| JP2001011067A (ja) | 1999-07-01 | 2001-01-16 | Tokuyama Corp | クロメン化合物 |
| JP2001011066A (ja) | 1999-07-02 | 2001-01-16 | Tokuyama Corp | クロメン化合物 |
| JP4118458B2 (ja) | 1999-07-02 | 2008-07-16 | 株式会社トクヤマ | クロメン化合物 |
| JP2001031670A (ja) | 1999-07-19 | 2001-02-06 | Tokuyama Corp | クロメン化合物 |
| US6296785B1 (en) | 1999-09-17 | 2001-10-02 | Ppg Industries Ohio, Inc. | Indeno-fused photochromic naphthopyrans |
| JP4157239B2 (ja) | 1999-10-19 | 2008-10-01 | 株式会社トクヤマ | クロメン化合物 |
| JP2005289807A (ja) | 2000-02-21 | 2005-10-20 | Tokuyama Corp | クロメン化合物 |
| JP4157245B2 (ja) | 2000-02-21 | 2008-10-01 | 株式会社トクヤマ | クロメン化合物 |
| WO2001060811A1 (en) | 2000-02-21 | 2001-08-23 | Tokuyama Corporation | Chromene compound |
| JP4158881B2 (ja) | 2000-02-21 | 2008-10-01 | 株式会社トクヤマ | フォトクロミック材 |
| WO2002090342A1 (en) | 2001-05-02 | 2002-11-14 | Tokuyama Corporation | Chromene compound |
| JP4195615B2 (ja) | 2001-05-02 | 2008-12-10 | 株式会社トクヤマ | クロメン化合物 |
| WO2003042203A1 (en) | 2001-11-16 | 2003-05-22 | Tokuyama Corporation | Chromene compound |
| JP4369754B2 (ja) | 2001-11-16 | 2009-11-25 | 株式会社トクヤマ | クロメン化合物 |
| JP2003277381A (ja) | 2002-03-25 | 2003-10-02 | Tokuyama Corp | クロメン化合物 |
| JP2005289812A (ja) | 2002-04-01 | 2005-10-20 | Tokuyama Corp | クロメン化合物 |
| WO2004041961A1 (en) | 2002-11-04 | 2004-05-21 | Polymers Australia Pty Limited | Photochromic compositions and light transmissible articles |
| WO2005028465A1 (ja) | 2003-09-18 | 2005-03-31 | Tokuyama Corporation | クロメン化合物 |
| JP4424962B2 (ja) | 2003-10-07 | 2010-03-03 | 株式会社トクヤマ | クロメン化合物 |
| JP2005112772A (ja) | 2003-10-07 | 2005-04-28 | Tokuyama Corp | クロメン化合物 |
| JP4424981B2 (ja) | 2003-12-26 | 2010-03-03 | 株式会社トクヤマ | クロメン化合物 |
| WO2007086532A1 (ja) | 2006-01-25 | 2007-08-02 | Tokuyama Corporation | クロメン化合物 |
| WO2008023828A1 (en) | 2006-08-24 | 2008-02-28 | Tokuyama Corporation | Chromene compound |
| JP2008074832A (ja) | 2006-08-24 | 2008-04-03 | Tokuyama Corp | クロメン化合物 |
| JP2009057300A (ja) | 2007-08-30 | 2009-03-19 | Tokuyama Corp | クロメン化合物 |
| JP2009067680A (ja) | 2007-09-10 | 2009-04-02 | Tokuyama Corp | クロメン化合物 |
| JP2009067754A (ja) | 2007-09-14 | 2009-04-02 | Tokuyama Corp | クロメン化合物 |
| JP2009120536A (ja) | 2007-11-15 | 2009-06-04 | Tokuyama Corp | クロメン化合物 |
| WO2009136668A1 (ja) | 2008-05-09 | 2009-11-12 | 株式会社トクヤマ | クロメン化合物 |
| WO2009146509A1 (en) | 2008-06-05 | 2009-12-10 | Advanced Polymerik Pty Ltd | Photochromic polymer and composition comprising photochromic polymer |
| WO2012149599A1 (en) | 2011-05-03 | 2012-11-08 | Advanced Polymerik Pty Ltd | Photochromic polymer |
| WO2012162725A1 (en) | 2011-06-03 | 2012-12-06 | Advanced Polymerik Pty Ltd | Photochromic polymers |
| WO2012176439A1 (ja) | 2011-06-23 | 2012-12-27 | 三井化学株式会社 | 重合性組成物 |
| WO2013078086A1 (en) | 2011-11-22 | 2013-05-30 | Transitions Optical, Inc. | Photochromic compounds having at least two photochromic moieties |
| WO2015068798A1 (ja) | 2013-11-11 | 2015-05-14 | 株式会社トクヤマ | フォトクロミック組成物 |
| WO2015115648A1 (ja) | 2014-02-03 | 2015-08-06 | 三井化学株式会社 | 光学材料用重合性組成物、当該組成物から得られる光学材料およびプラスチックレンズ |
| WO2016143910A1 (ja) | 2015-03-10 | 2016-09-15 | 株式会社トクヤマ | フォトクロミック硬化体の製造方法 |
| WO2017038865A1 (ja) * | 2015-09-03 | 2017-03-09 | 株式会社トクヤマ | ポリロタキサン及びその製法並びに該ポリロタキサンを含有する光学用組成物 |
| WO2019013249A1 (ja) | 2017-07-14 | 2019-01-17 | 株式会社トクヤマ | クロメン化合物、該化合物を含む硬化性組成物、および該硬化性組成物からなる硬化体を含む光学物品 |
| WO2019198664A1 (ja) * | 2018-04-12 | 2019-10-17 | 株式会社トクヤマ | フォトクロミック光学物品及びその製造方法 |
| WO2019203205A1 (ja) | 2018-04-17 | 2019-10-24 | 株式会社トクヤマ | フォトクロミック化合物、該フォトクロミック化合物を含む硬化性組成物及び光学物品 |
| WO2021241596A1 (ja) * | 2020-05-28 | 2021-12-02 | 株式会社トクヤマ | 光学材料用化合物、硬化性組成物、硬化体、及び光学物品 |
Also Published As
| Publication number | Publication date |
|---|---|
| JPWO2022158348A1 (enExample) | 2022-07-28 |
| US20240100810A1 (en) | 2024-03-28 |
| EP4282932A1 (en) | 2023-11-29 |
| TW202237795A (zh) | 2022-10-01 |
| EP4282932A4 (en) | 2024-12-11 |
| MX2023008270A (es) | 2023-07-19 |
| KR20230137306A (ko) | 2023-10-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN108026240B (zh) | 光学材料用聚合性组合物、由该组合物得到的光学材料及塑料透镜 | |
| EP3103822B1 (en) | Polymerizable composition for optical material, and optical material and plastic lens obtained from said composition | |
| EP3109268B1 (en) | Method for manufacturing optical material | |
| JP7719772B2 (ja) | 光学材料用化合物、硬化性組成物、硬化体、及び光学物品 | |
| EP3521907B1 (en) | Photochromic lens and polymerizable composition | |
| CN108026241B (zh) | 成型体和光学材料用聚合性组合物 | |
| EP3950624A1 (en) | Photochromic optical article and method for manufacturing same | |
| CN111954832A (zh) | 光致变色光学物品及其制造方法 | |
| JP2020170095A (ja) | フォトクロミック光学物品 | |
| JP2019215450A (ja) | 光学材料用重合性組成物およびその用途 | |
| WO2022158348A1 (ja) | 樹脂組成物、光学積層体、光学物品、レンズ及び眼鏡 | |
| WO2021132559A1 (ja) | 光学材料用重合性組成物、当該組成物から得られる成形体およびその用途 | |
| US20250257162A1 (en) | Curable composition, cured body, laminate, optical article, lens, and eyeglasses | |
| KR101831889B1 (ko) | 플라스틱 렌즈용 폴리티올 조성물 | |
| CN116868095A (zh) | 树脂组合物、光学层叠体、光学物品、透镜以及眼镜 | |
| JP7344112B2 (ja) | フォトクロミック性接着組成物およびフォトクロミック光学物品 | |
| TWI908805B (zh) | 光學材料用化合物、硬化性組成物、硬化體、以及光學物品 | |
| EP4603522A1 (en) | Optical material composition, cured body, optical article, lens, and spectacles | |
| WO2024203896A1 (ja) | 光学部材および光学部材の製造方法 | |
| KR20180090721A (ko) | 플라스틱 렌즈용 폴리티올 조성물 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 22742470 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2022576620 Country of ref document: JP Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 18271925 Country of ref document: US Ref document number: MX/A/2023/008270 Country of ref document: MX |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 202280010524.1 Country of ref document: CN |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 202317048983 Country of ref document: IN |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2022742470 Country of ref document: EP |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2022742470 Country of ref document: EP Effective date: 20230825 |