WO2020054847A1 - アルミニウム合金の押出材、建具及びアルミニウム合金の押出材の製造方法 - Google Patents
アルミニウム合金の押出材、建具及びアルミニウム合金の押出材の製造方法 Download PDFInfo
- Publication number
- WO2020054847A1 WO2020054847A1 PCT/JP2019/036102 JP2019036102W WO2020054847A1 WO 2020054847 A1 WO2020054847 A1 WO 2020054847A1 JP 2019036102 W JP2019036102 W JP 2019036102W WO 2020054847 A1 WO2020054847 A1 WO 2020054847A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- aluminum alloy
- less
- extruded material
- aluminum
- weight
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C21/00—Alloys based on aluminium
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22F—CHANGING THE PHYSICAL STRUCTURE OF NON-FERROUS METALS AND NON-FERROUS ALLOYS
- C22F1/00—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working
- C22F1/04—Changing the physical structure of non-ferrous metals or alloys by heat treatment or by hot or cold working of aluminium or alloys based thereon
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25D—PROCESSES FOR THE ELECTROLYTIC OR ELECTROPHORETIC PRODUCTION OF COATINGS; ELECTROFORMING; APPARATUS THEREFOR
- C25D11/00—Electrolytic coating by surface reaction, i.e. forming conversion layers
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25D—PROCESSES FOR THE ELECTROLYTIC OR ELECTROPHORETIC PRODUCTION OF COATINGS; ELECTROFORMING; APPARATUS THEREFOR
- C25D11/00—Electrolytic coating by surface reaction, i.e. forming conversion layers
- C25D11/02—Anodisation
- C25D11/04—Anodisation of aluminium or alloys based thereon
-
- C—CHEMISTRY; METALLURGY
- C25—ELECTROLYTIC OR ELECTROPHORETIC PROCESSES; APPARATUS THEREFOR
- C25D—PROCESSES FOR THE ELECTROLYTIC OR ELECTROPHORETIC PRODUCTION OF COATINGS; ELECTROFORMING; APPARATUS THEREFOR
- C25D11/00—Electrolytic coating by surface reaction, i.e. forming conversion layers
- C25D11/02—Anodisation
- C25D11/04—Anodisation of aluminium or alloys based thereon
- C25D11/16—Pretreatment, e.g. desmutting
Definitions
- the present invention relates to an aluminum alloy extruded material, a fitting, and a method for producing an aluminum alloy extruded material.
- Priority is claimed on Japanese Patent Application No. 2018-172385 filed on Sep. 14, 2018, the content of which is incorporated herein by reference.
- Metal profiles are widely used as building materials such as sashes.
- sashes and the like have been required to have a high-class appearance, and for example, stainless steel members have been used as building materials.
- Stainless steel is excellent in glitter, but the cost of stainless steel, which is the material of the stainless steel, is high. For this reason, there has been a demand for a low-cost shape that can be produced instead of a stainless steel shape.
- a low-cost producible profile is an extruded aluminum alloy made of an aluminum alloy.
- an extruded material of an aluminum alloy is manufactured by extruding a billet of an aluminum alloy and subjecting the aluminum raw material obtained after the extrusion to anodization.
- the anodic oxidation treatment is a treatment for improving the corrosion resistance and wear resistance of the extruded material of the aluminum alloy.
- an oxide film is formed on the surface of the aluminum raw material.
- the oxide film is called an alumite film.
- the impurities contained in the aluminum alloy are precipitated in the alumite film.
- Typical impurities are iron (Fe) and silicon (Si).
- Precipitation of impurities in the alumite film causes the aluminum alloy extruded material to look cloudy, and reduces the glitter of the aluminum alloy extruded material.
- Patent Literature 1 discloses an aluminum alloy manufactured to improve glitter.
- the aluminum alloy of Patent Document 1 includes 0.35 to 0.45% by mass of magnesium (Mg), 0.35 to 0.45% by mass of Si, and 0.16 to 0.25% by mass of copper (Cu). ).
- the ratio between the Mg content and the Si content is 0.80 to 1.20.
- the aluminum alloy of Patent Document 1 contains 0.10% by mass or less of Fe as impurities and inevitable impurities of 0.05% by mass or less in total.
- a magnesium silicide (Mg 2 Si) compound having a particle size of 0.5 to 3 ⁇ m and 300 to 1500 particles / mm 2 exists in the aluminum alloy of Patent Document 1.
- Patent Literature 2 discloses a component including a metal substrate having a surface having a point and a sharp dent and an oxide layer (alumite film) disposed on the surface of the metal substrate.
- the thickness of the alumite film of Patent Document 2 is 10 ⁇ m to 20 ⁇ m.
- A6063 aluminum alloy (hereinafter referred to as A6063 alloy).
- the Cu content of the aluminum alloy described in Patent Document 1 is larger than that of the A6063 alloy.
- a high-purity aluminum raw material is used. Therefore, when an extruded material is manufactured using the aluminum alloy described in Patent Literature 1, the manufacturing cost increases.
- the thickness of the alumite film is limited to the range of 10 ⁇ m to 20 ⁇ m, but the component amount of the aluminum alloy is not controlled. Therefore, in the component described in Patent Document 2, sufficient glitter cannot be obtained.
- the present invention provides an aluminum alloy extruded material, a fitting, and a method for manufacturing an aluminum alloy extruded material which is made of the A6063 alloy and has high glitter.
- the extruded material of the aluminum alloy according to the present invention includes an aluminum base, and an alumite film formed on the surface of the base of the aluminum base.
- the extruded material of the aluminum alloy according to the present invention comprises 0.20 wt% to 0.53 wt% of Si, 0.18 wt% or less of Fe, 0.10 wt% or less of Cu, and 0.10 wt% or less of Cu. Wt% or less of Mn, 0.45 wt% or more and 0.9 wt% or less of Mg, 0.10 wt% or less of Cr, 0.10 wt% or less of Zn, and 0.04 wt% or less of And Ti, and further contains Al and inevitable impurities.
- a plurality of hairlines are formed on the surface of the substrate along the longitudinal direction of the aluminum substrate.
- the thickness of the alumite film is 4 ⁇ m or more and 10 ⁇ m or less.
- the fitting according to the present invention is configured to include the extruded material of the above-described aluminum alloy.
- the method for producing an aluminum alloy extruded material according to the present invention comprises the following steps: 0.20 wt% to 0.53 wt% of Si, 0.18 wt% or less of Fe, and 0.10 wt% or less of Cu; 0.10 wt% or less of Mn, 0.45 wt% or more and 0.9 wt% or less of Mg, 0.10 wt% or less of Cu, 0.10 wt% or less of Zn, and 0.04 wt% % Or less, and an aluminum alloy billet containing Al and unavoidable impurities is cast, the aluminum alloy billet is extruded to form an aluminum raw material, and the aluminum raw material surface is polished.
- Forming a plurality of hair lines on the polished surface of the raw material along the longitudinal direction of the aluminum raw material, anodizing the aluminum raw material on which the hair lines are formed, and forming a substrate surface of an aluminum base material Thickness includes forming a 10 ⁇ m following alumite film than 4 [mu] m.
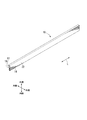
- FIG. 1 is a perspective view of an aluminum alloy extruded material of one embodiment of the present invention.
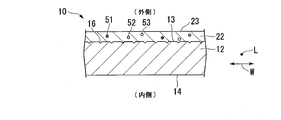
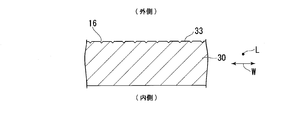
- FIG. 2 is a sectional view of a part of the extruded material of the aluminum alloy shown in FIG.
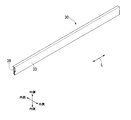
- FIG. 3 is a perspective view showing an example of a method for manufacturing an extruded aluminum alloy according to one embodiment of the present invention.
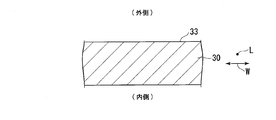
- FIG. 4 is a sectional view of a part of the aluminum raw material shown in FIG.
- FIG. 5 is a cross-sectional view illustrating an example of a method for manufacturing an extruded aluminum alloy according to one embodiment of the present invention.
- FIG. 6 is a cross-sectional view showing an example of a method for manufacturing an extruded aluminum alloy according to one embodiment of the present invention. It is a perspective view of the extruded material of the aluminum alloy experimentally manufactured in each example from Example 1 to Example 9.
- the extruded material 10 of an aluminum alloy extends along a predetermined direction (hereinafter, a longitudinal direction L) and has a predetermined cross-sectional shape.
- the use of the aluminum alloy extruded material 10 is, for example, a frame of a sash (fitting), a sash (fitting) for a store, a front sash (fitting) used for a facade, a door (fitting), an automatic door (fitting), and various other types. It is a building material and includes a wide range of applications to which A6063 alloy can be applied.
- the predetermined cross-sectional shape of the aluminum alloy extruded material 10 is appropriately designed according to the application, and is not particularly limited.
- FIG. 2 is a cross-sectional view of a portion 19 of the extruded material 10 of the aluminum alloy cut along a plane (not shown) orthogonal to the longitudinal direction L and viewed along the longitudinal direction L.
- the extruded material 10 of an aluminum alloy has an aluminum base 12 and an alumite coating 22 formed on a surface (base surface) 13 of the aluminum base 12.
- the extruded material 10 of an aluminum alloy satisfies at least the standard of A6063 of JIS.
- the extruded aluminum alloy material meeting the JIS A6063 standard (hereinafter, extruded A6063) has a Si content of 0.20% by weight or more and 0.6% by weight or less.
- the A6063 extruded material has a Fe content of 0.35% by weight or less.
- the A6063 extruded material has a Cu content of 0.10% by weight or less.
- the content of manganese (Mn) in the A6063 extruded material is 0.10% by weight or less.
- the content of Mg in the extruded A6063 is 0.45% by weight or more and 0.9% by weight or less.
- the content of chromium (Cr) in the extruded A6063 material is 0.10% by weight or less.
- the content of zinc (Zn) in the extruded material of A6063 is 0.10% by weight or less.
- the content of titanium (Ti) in the extruded material of A6063 is 0.10% by weight or less.
- the A6063 extruded material contains aluminum (Al) and unavoidable impurities, in addition to the plurality of metals described above.
- the lower limit of the content of each of Fe, Cu, Mn, Cr, Zn, and Ti for which the lower limit is not specified in the aforementioned A6063 extruded material is 0.00% by weight.
- the content of Fe in the extruded material 10 of the aluminum alloy according to the present embodiment is smaller than that of the extruded material of the aluminum alloy A6063, and is not less than 0.00% by weight and not more than 0.18% by weight.
- the glitter of the aluminum alloy extruded material 10 is enhanced. If the Fe content of the aluminum alloy extruded material 10 exceeds 0.18% by weight, the alumite film 22 becomes cloudy and the glitter of the aluminum alloy extruded material 10 is reduced.
- the L * a * b * color system is a standard of the color system by the International Commission on Illumination (hereinafter CIE).
- CIE International Commission on Illumination
- a color difference close to the color difference visually recognized by a user or an observer of the extruded material 10 of the aluminum alloy can be represented.
- the L * value is a value on the brightness axis in the L * a * b * color system.
- the L * value of the extruded aluminum alloy is such that the glitter of the extruded aluminum alloy is equivalent to the glitter of the stainless steel profile when viewed from an observer. Means that it is set in a range that can be determined.
- the L * value of the extruded material 10 of the aluminum alloy is 20.7 or less, the glittering property is enhanced, and an appearance excellent in aesthetics and design can be obtained.
- the L * value of the aluminum alloy extruded material 10 is measured using, for example, a spectrophotometer CM-M6 (manufactured by Konica Minolta Japan).
- CM-M6 spectrophotometer
- the light is applied to the extruded material 10 of the aluminum alloy at an incident angle of 45 ° with respect to an orthogonal plane (not shown) orthogonal to a reference plane (not shown) parallel to the surface 13.
- the predetermined light receiving angles include six types of light receiving angles of -15 °, + 15 °, + 25 °, + 45 °, + 75 °, and + 110 °.
- the six types of light receiving angles represent angles when an angle of 45 ° with respect to the orthogonal plane on the side opposite to the light incident side with respect to the orthogonal plane is 0 °.
- the signs of the six types of light receiving angles are positive (+) if the angle of the surface on the orthogonal surface side with respect to the surface with the light receiving angle of 0 ° is opposite to the surface opposite to the orthogonal surface with the light receiving angle of 0 °. Is negative (-) if the angle of the surface is.
- the L * value of the extruded material 10 of the aluminum alloy represents a value measured at a light receiving angle of + 110 °.
- the a * value and the b * value of the L * a * b * color system do not affect the glitter and appearance of the extruded material 10 of an aluminum alloy and the stainless steel material, and are not considered in this specification.
- the extruded material 10 of the aluminum alloy has a Si content of 0.20 to 0.53% by weight, and the extruded material 10 of an aluminum alloy has a Ti content of 0.004 to 0.04% by weight. It is.
- the cloudiness of the alumite coating 22 is suppressed, and the glitter of the aluminum alloy extruded material 10 is enhanced.
- the content of Cu in the aluminum alloy extruded material 10 is 0.00% by weight or more and 0.10% by weight or less.
- the content of Mn in the extruded material 10 of the aluminum alloy is 0.00% by weight or more and 0.10% by weight or less.
- the content of Mg in the extruded material 10 of the aluminum alloy is 0.45% by weight or more and 0.9% by weight or less.
- the content of Cr in the extruded material 10 of the aluminum alloy is 0.00% by weight or more and 0.10% by weight or less.
- the content of Zn in the aluminum alloy extruded material 10 is not less than 0.00% by weight and not more than 0.10% by weight.
- the Cu content of the extruded material 10 of the aluminum alloy is 0.03% by weight or less. Also, the glitter of the aluminum alloy extruded material 10 is enhanced, and the production cost of the aluminum alloy extruded material 10 can be suppressed by suppressing the amount of expensive Cu used.
- the aluminum base 12 extends along the longitudinal direction L and has the same cross-sectional shape as the aluminum alloy extruded material 10.
- the thickness of the aluminum base 12 is adjusted, for example, within a range of 1 mm or more and 3 mm or less.
- the thickness of the aluminum base 12 is appropriately set according to the use of the extruded material 10 of the aluminum alloy.
- the aluminum base 12 is configured based on an A6063 alloy.
- a plurality of hairlines 16 are formed on the surface 13 along the longitudinal direction L.
- the arithmetic average roughness Ra of the plurality of hairlines 16 is evaluated using, for example, an index defined in JIS B0601 (2013).
- the arithmetic average roughness Ra of the plurality of hairlines 16 is preferably 0.06 ⁇ m or more. It is preferable that the maximum height roughness Rz of the plurality of hairlines 16 is 0.63 ⁇ m or more.
- the average length RSm of the cross-sectional curve elements of the plurality of hairlines 16 is preferably 0.3 ⁇ m or less. In other words, the arithmetic average roughness Ra of the surface 13 on which the plurality of hairlines 16 are formed is preferably 0.06 ⁇ m or more. It is preferable that the maximum height roughness Rz of the surface 13 be 0.63 ⁇ m or more.
- the surface 13 means a surface of the aluminum substrate 12 facing at least outward.
- the outer side means a side of the extruded material 10 of the aluminum alloy in the use form, which is in the field of view of the user or the observer.
- the inner side means the side of the extruded material 10 of the aluminum alloy used in the use form, which is hidden from the view of the user or the observer.
- the alumite film 22 is formed on the surface 13 and the inner surface 14 of the aluminum base 12.
- a plurality of hairlines 16 and one alumite film 22 are formed on at least the surface 13. It is preferable that at least the alumite film 22 is formed on the surface 14 and a plurality of hairlines 16 are formed as necessary.
- the alumite film 22 is a film for improving the corrosion resistance and wear resistance of the extruded material 10 of the aluminum alloy.
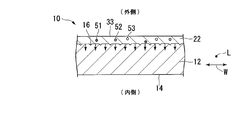
- the alumite film 22 is formed by performing anodizing treatment on the surface of the aluminum base material 12 including at least the surface 13. As shown in FIG. 2, a part of a plurality of impurities contained in the extruded material 10 of the aluminum alloy is precipitated on the alumite film 22.
- FIG. 2 shows only Fe51, Si52 and Ti53, and omits impurities other than Fe51, Si52 and Ti53. Fe51, Si52 and Ti53 precipitate on the alumite film 22, and have a great effect on the cloudiness of the alumite film 22 and the glitter of the extruded material 10 of the aluminum alloy.
- the alumite film 22 is a porous film.
- the alumite film 22 has a plurality of micropores that are opened on the outer surface 23 of the alumite film 22. In FIG. 2, a plurality of micro holes are omitted. Coloring metals, metal compounds, and the like are present in the plurality of micropores.
- the metal or metal compound for coloring is not limited to a specific substance.
- the thickness of the alumite film 22 is 4 ⁇ m or more and 10 ⁇ m or less. Thereby, the glitter of the extruded material 10 of the aluminum alloy having the Fe content of 0.18% by weight or less is enhanced.
- the thickness of the alumite film 22 is less than 4 ⁇ m, the corrosion resistance and wear resistance of the aluminum alloy extruded material 10 are insufficient, and the aluminum alloy extruded material 10 is not suitable for a desired use. If the thickness of the alumite film 22 is less than 4 ⁇ m, the corrosion resistance required for an extruded aluminum alloy based on the A6063 alloy may not be obtained.
- the thickness of the alumite film 22 is more than 10 ⁇ m, the cloudiness of the alumite film 22 due to Fe51 becomes remarkable, and the glitter of the extruded material 10 of the aluminum alloy may be reduced.
- a coating film (not shown) may be provided on the surface 23 in order to enhance the weather resistance and the design of the aluminum alloy extruded material 10.
- the method for manufacturing an extruded aluminum alloy material according to the present embodiment includes a casting step, an extrusion step, a polishing step, a hairline forming step, and a surface treatment step.
- an aluminum alloy billet (not shown) is cast.
- the content of Si in the aluminum alloy billet is from 0.20% by weight to 0.53% by weight.
- the Fe content of the aluminum alloy billet is 0.18% by weight or less.
- the Cu content of the aluminum alloy billet is 0.10% by weight or less.
- the Mn content of the aluminum alloy billet is 0.10% by weight or less.
- the Mg content of the aluminum alloy billet is 0.45% by weight or more and 0.9% by weight or less.
- the Cr content of the aluminum alloy billet is 0.10% by weight or less.
- the content of Zn in the aluminum alloy billet is 0.10% by weight or less.
- the Ti content of the aluminum alloy billet is 0.04% by weight or less.
- the remainder of the aluminum alloy billet other than the plurality of metals described above consists of Al and unavoidable impurities.
- the casting method of the aluminum alloy billet is the same as the conventional casting method of the A6063 alloy. However, when dissolving aluminum ingot, which is a raw material of the aluminum alloy billet, and blending and adjusting the additive metal (that is, the impurity metal added to the A6063 alloy), the amount of Fe added, the amount of Si added and The added amount of Ti is set within a predetermined range described below. The amount of Fe to be added to the entire aluminum alloy in the melting furnace is set to 0.00% by weight or more and 0.18% by weight or less.
- the amount of Si added to the entire aluminum alloy in the melting furnace is set to 0.20% by weight or more and 0.53% by weight or less.
- the amount of Ti added to the entire aluminum alloy in the melting furnace is set to 0.00% by weight or more and 0.04% by weight or less.
- the standard of A6063 of JIS is satisfied, and the content of Fe is 0.00% by weight to 0.18%.
- An aluminum alloy billet having a Si content of 0.20% to 0.53% by weight and a Ti content of 0.00% to 0.04% by weight is cast.
- the shape of the aluminum alloy billet is, for example, a columnar shape or a cylindrical shape having an annular cross section, and is appropriately adjusted according to the length and thickness required for extrusion in the subsequent extrusion step.
- FIG. 4 is a cross-sectional view of a part 39 of the aluminum raw material 30 taken along a plane (not shown) orthogonal to the longitudinal direction L and viewed along the longitudinal direction L.
- the aluminum raw material 30 is a precursor of the extruded material 10 of the aluminum alloy before the alumite film 22 is formed.
- the aluminum raw material 30 extends in the longitudinal direction L similarly to the aluminum alloy extruded material 10 and has the same cross-sectional shape as the aluminum alloy extruded material 10.
- the aluminum alloy billet is heated to a predetermined temperature, and is extruded at a high pressure into a mold formed in the same shape as the cross-sectional shape of the aluminum alloy extruded material 10.
- the predetermined temperature is, for example, 400 ° C. or more and 500 ° C. or less, but is appropriately adjusted.
- the aluminum raw material 30 extruded from the mold is cooled to room temperature. Thereafter, the aluminum raw material 30 may be subjected to aging at a temperature of, for example, 150 ° C. or more and 250 ° C. or less for 1 hour or more and 10 hours or less.
- the surface (raw material surface) 33 of the aluminum raw material 30 is polished.
- the method of polishing the surface 33 is not limited to a specific method as long as it can enhance the glitter of the extruded material 10 of the aluminum alloy.
- the glossiness of the surface 33 after polishing is 660 (%) or more and 900 (%) or less.
- the glossiness described above refers to a specular glossiness measured in accordance with the JIS standard with the incident angle to the surface 33 set at + 60 °.
- the glossiness of the surface 33 after polishing is lower than 660 (%), the design of the appearance of the extruded material 10 of the aluminum alloy is reduced. If the gloss of the surface 33 after polishing becomes higher than 900 (%), the gloss of the aluminum alloy extruded material 10 becomes excessively high as compared with the same gloss as the stainless steel material, and waste occurs in the polishing work, and the aluminum alloy is wasted.
- the production efficiency of the extruded material 10 decreases.
- the glossiness of the surface 33 after polishing is 660 (%) or more and 900 (%) or less, even if the glossiness of the surface 33 decreases when the operation of forming the next hairline is performed, the aluminum alloy is extruded. The reduction in the glitter of the material 10 is suppressed.
- the glossiness of the polished surface 33 is measured using, for example, a high gloss gloss checker IG-410 (manufactured by Horiba, Ltd.).
- a plurality of hairlines 16 are formed on the polished surface 33 along the longitudinal direction L, as shown in FIG.
- FIG. 5 is a cross-sectional view when a part of the aluminum raw material 30 after the plurality of hairlines 16 are formed is cut along a plane perpendicular to the longitudinal direction L and viewed along the longitudinal direction L.
- the distance between the centers of the hairlines 16 adjacent to each other in the width direction W is a predetermined distance, and the average value of the depths of the plurality of hairlines 16 is set to an appropriately designed depth. Any method may be used, and the method is not limited to a specific method.
- the aluminum raw material 30 on which the plurality of hairlines 16 are formed is subjected to anodizing treatment to form an alumite film 22 having a thickness of 4 ⁇ m or more and 10 ⁇ m or less on the surface 13 as shown in FIG.
- anodic oxidation treatment of the aluminum raw material 30 for example, electrolysis is performed in a sulfuric acid bath using the aluminum raw material 30 as an anode. Conditions for the electrolysis may be set as appropriate.
- oxygen is generated from the surface 33.
- the generated oxygen and Al contained in the aluminum raw material 30 are combined, and the alumite film 22 mainly composed of aluminum oxide is formed inside the surface 33 as shown in FIG. FIG.
- FIG. 6 is a cross-sectional view of a state in which the alumite film 22 is formed on a part of the aluminum raw material 30 after the hairline is formed, cut along a plane perpendicular to the longitudinal direction L and viewed along the longitudinal direction. .
- impurities such as Fe 51, Si 52, and Ti 53 precipitate from the aluminum raw material 30 on the alumite film 22 and are scattered in the alumite film 22.
- the electrolytic coloring and the electrodeposition coating may be performed after the alumite film 22 is formed by the surface treatment.
- electrolytic coloring and electrodeposition coating By electrolytic coloring and electrodeposition coating, the appearance of the aluminum alloy extruded material 10 is made closer to the appearance of a stainless steel profile, the weather resistance of the aluminum alloy extruded material 10 is enhanced, and the aluminum alloy extruded material is exposed to ultraviolet light when used outdoors. 10 discoloration can be prevented.
- Conditions for electrolytic coloring are appropriately set so that the L * value of at least the outside of the extruded material 10 of the aluminum alloy is 20.7 or less.
- an extruded material 10 of an aluminum alloy can be manufactured.
- the aluminum alloy extruded material 10 according to the present embodiment described above is made of an A6063 alloy that satisfies JIS A6063.
- the extruded material of aluminum alloy has a Fe content of 0.35% by weight or less. If the thickness of the alumite film exceeds 10 ⁇ m and the content of Fe exceeds 0.18% by weight, the alumite film may become cloudy and the glitter of the extruded aluminum alloy may be reduced.
- the content of Fe in the extruded material 10 of the aluminum alloy of the present embodiment is 0.18% by weight or less, the content of Si is 0.20% by weight or more and 0.53% by weight or less, and the content of Ti is 0.04% by weight or less.
- the thickness of the alumite film 22 is 4 ⁇ m or more and 10 ⁇ m or less. Under these conditions, the amounts of Fe51, Si52, and Ti53 in the alumite film 22 can be suppressed, and the cloudiness of the alumite film 22 can be suppressed. Therefore, the L * value of the extruded material 10 of the aluminum alloy is 20.7 or less, and the glitter of the extruded material 10 of the aluminum alloy can be enhanced, and a high glitter that is equal to or more than that of the conventional stainless steel material can be obtained. Can be.
- the L * value of the extruded material 10 of the aluminum alloy according to the present embodiment is 20.7 or less, it is possible to obtain at least one of high brilliancy and aesthetics equivalent to or higher than the conventional stainless steel material.
- the arithmetic average roughness Ra of the plurality of hairlines 16 is 0.06 ⁇ m or more, and the maximum height roughness Rz of the plurality of hairlines 16 is 0.63 ⁇ m or more.
- the average length RSm of the cross-sectional curve elements of the plurality of hairlines 16 is 0.3 ⁇ m or less.
- the content of Fe in the A6063 alloy is set to 0.18% by weight or less, and the content of Si is set to 0.20% to 0.53% by weight,
- An aluminum alloy billet in which the content of Ti is regulated to 0.04% by weight or less is cast.
- the aluminum raw material 30 is extruded using the aluminum alloy billet.
- the alumite film 22 is grown on the surface 33 and the alumite film 22 having a thickness of 4 ⁇ m or more and 10 ⁇ m or less is formed on the surface 13.
- the cloudiness of the alumite film 22 caused by Fe51, Si52, and Ti53 is suppressed.
- the glitter of the aluminum alloy extruded material 10 can be enhanced.
- a plurality of hairlines 16 and an alumite film 22 may be formed not only on the surface 13 but also on the surface 14 shown in FIGS. 2 and 6.
- the cross-sectional shape of the extruded material of the aluminum alloy according to the present invention is not limited to the cross-sectional shape illustrated in FIG.
- the extruded material of the aluminum alloy according to the present invention may be formed in a plate shape, or may be formed in a rod shape without forming a hollow portion.
- the shape of the extruded material of the aluminum alloy according to the present invention includes all shapes that can be realized by changing the shape of the mold and that can be extruded.
- electrolytic coloring and electrodeposition coating may be omitted. May be added.
- Example 1 to Example 6 According to the method for manufacturing an extruded aluminum alloy material of the above-described embodiment, the content of Fe is 0.18% by weight or less, and the content of Si is 0.20% by weight or more and 0.53% by weight or less, An aluminum raw material having a Ti content of 0.04% by weight or less was obtained. After forming a plurality of hair lines 16 on the aluminum raw material, the thickness of the alumite film 22 was changed to produce an aluminum alloy extruded material 50 shown in FIG.
- the aluminum alloy extruded material 50 has the same configuration as the aluminum alloy extruded material 10 of the above-described embodiment, except that it has a rectangular cross-sectional shape and is a hollow body. The thickness d of the aluminum alloy extruded material 50 was 1.7 mm.
- Table 1 shows the specifications of the extruded materials of the aluminum alloys of Examples 1 to 6 and Comparative Examples 1 to 4 and the L * values relating to the evaluation results of the glitter.
- L * values described in Table 1 were measured using a spectrophotometer CM-M6. In measuring these L * values, the light emitted from the spectrophotometer CM-M6 was applied to the alumite film of the extruded aluminum alloy in each example. “OK” of pass / fail described in Table 1 indicates that the L * value is 20.7 or less, and “NG” of pass / fail described in Table 1 indicates that the L * value is larger than 20.7. Represents
- the L * value of the extruded material 50 of the aluminum alloy of each of Examples 1 to 6 was 20.7 or less, and high glitter was obtained.
- the content of Fe in the extruded material 50 of the aluminum alloy is 0.00% by weight or more and 0.18% by weight or less, and the content of Si is 0.20% by weight. It is considered that the content of Ti was not less than 0.003 wt% and not more than 0.04 wt%.
- the thickness of the alumite film 22 was 4 ⁇ m or more and 10 ⁇ m or less.
- the content of Fe in the A6063 alloy is set to 0.000 to 0.18% by weight, and the content of Si is set to 0.20 to 0.53% by weight. Wt% or less, the content of Ti is set to 0.004 wt% or less and 0.04 wt% or less, and the thickness of the alumite film is set to 4 ⁇ m or more and 10 ⁇ m or less, thereby enhancing the glitter of the extruded aluminum alloy. did it.
- Example 7 to 9 The aluminum raw materials of the respective examples from Example 7 to Example 9 were obtained by the same method as the method for manufacturing the extruded material of the aluminum alloy of each of the examples 1 to 6. After forming a plurality of hairlines on the aluminum raw material, the thickness of the alumite film 22 was changed to produce an aluminum alloy extruded material 50 shown in FIG.
- the content of Si and the content of Mg of the extruded material 50 of the aluminum alloy were appropriately changed as shown in Table 2, The contents of Fe, Cu, Mn, Cr, and Ti were made constant as shown in Table 2.
- Table 2 shows the specification of the extruded material of the aluminum alloy of each of Examples 7 to 9 and Comparative Example 8, and the measurement results of the rating numbers with respect to the evaluation results of the corrosion resistance.
- the rating number described in Table 2 means the rating number specified in the JIS-H-8602-A2 CASS test. “OK” of pass / fail described in Table 2 indicates that the rating number of the extruded material of the aluminum alloy of each example is 9.5 or more, and “NG” of pass / fail is that of the extruded material of the aluminum alloy of each example. The rating number is smaller than 9.5.
- the extruded material 50 of the aluminum alloy of each of Examples 7 to 9 has a rating number of 9.5 or more, and the extruded material 50 of the aluminum alloy passes the CASS test. The required corrosion resistance was obtained. In each of Examples 7 to 9, it is considered that the thickness of the alumite film 22 was 4 ⁇ m or more and 10 ⁇ m or less.
- the extruded aluminum alloy of Comparative Example 8 did not have the corrosion resistance required to pass the CASS test. It is considered that in Comparative Example 8, the thickness of the alumite film was less than 4 ⁇ m.
- Corrosion resistance is an evaluation content from a different viewpoint from glitter, but as can be seen from the evaluation results of the above Examples and Comparative Examples, by setting the thickness of the alumite film to 4 ⁇ m or more and 10 ⁇ m or less, it is based on the A6063 alloy. The corrosion resistance required for extruded aluminum alloy was obtained.
- Aluminum alloy extruded material 12 Aluminum substrate 13 Surface (substrate surface) 16 Hairline 22 Alumite film 30 Aluminum raw material 33 Surface (raw material surface) 51 Iron (Fe) 52 Silicon (Si) 53 Titanium (Ti) L longitudinal direction
Landscapes
- Chemical & Material Sciences (AREA)
- Metallurgy (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Organic Chemistry (AREA)
- Electrochemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Mechanical Engineering (AREA)
- Physics & Mathematics (AREA)
- Crystallography & Structural Chemistry (AREA)
- Thermal Sciences (AREA)
- Extrusion Of Metal (AREA)
- Laminated Bodies (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2018-172385 | 2018-09-14 | ||
| JP2018172385A JP7248399B2 (ja) | 2018-09-14 | 2018-09-14 | アルミニウム形材、建具及びアルミニウム形材の製造方法 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2020054847A1 true WO2020054847A1 (ja) | 2020-03-19 |
Family
ID=69777121
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2019/036102 Ceased WO2020054847A1 (ja) | 2018-09-14 | 2019-09-13 | アルミニウム合金の押出材、建具及びアルミニウム合金の押出材の製造方法 |
Country Status (2)
| Country | Link |
|---|---|
| JP (2) | JP7248399B2 (enExample) |
| WO (1) | WO2020054847A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20210285120A1 (en) * | 2018-11-30 | 2021-09-16 | Uacj Corporation | Aluminum member and method of manufacturing aluminum member |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN112981194A (zh) * | 2021-02-09 | 2021-06-18 | 东莞市东联铝业有限公司 | 一种防起皱铝型材及制备方法 |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5366811A (en) * | 1976-11-27 | 1978-06-14 | Toyama Keikinzoku Kogyo Kk | Black spot preventive allmggsi based alloy and preventive treatment method of black spots |
| JPH09227978A (ja) * | 1996-02-16 | 1997-09-02 | Tateyama Alum Ind Co Ltd | アルミニウム合金 |
| JPH10306336A (ja) * | 1997-05-01 | 1998-11-17 | Sumitomo Light Metal Ind Ltd | 陽極酸化処理後の表面光沢性に優れたアルミニウム合金押出材およびその製造方法 |
| JP2012149335A (ja) * | 2010-12-27 | 2012-08-09 | Sankyo Tateyama Inc | アルミニウム合金 |
| JP2012255191A (ja) * | 2011-06-08 | 2012-12-27 | Sankyo Tateyama Inc | アルミニウム合金 |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS56161796A (en) * | 1980-05-19 | 1981-12-12 | Pioneer Electronic Corp | Speaker system of passive radiator system |
| JPS5915000A (ja) * | 1982-07-15 | 1984-01-25 | 株式会社勝光社 | アルミニウム基材の装飾法 |
| JP3609348B2 (ja) * | 2001-03-30 | 2005-01-12 | アイシン軽金属株式会社 | アルミニウム合金の電解着色方法 |
| JP4790564B2 (ja) * | 2006-10-16 | 2011-10-12 | Ykk Ap株式会社 | 表面処理アルミニウム材または表面処理アルミニウム合金材、及びそれを使用した建材製品 |
| JP5561181B2 (ja) * | 2011-01-18 | 2014-07-30 | トヨタ自動車株式会社 | 燃料残量測定装置 |
| JP5642597B2 (ja) * | 2011-03-22 | 2014-12-17 | 株式会社Lixil | アルミニウム押出材の陽極酸化塗装複合皮膜形成方法及びその陽極酸化塗装複合皮膜 |
| IN2014DN09152A (enExample) * | 2012-05-23 | 2015-05-22 | Nippon Steel & Sumitomo Metal Corp | |
| FR2996857B1 (fr) * | 2012-10-17 | 2015-02-27 | Constellium France | Elements de chambres a vide en alliage d'aluminium |
| JP6381435B2 (ja) * | 2014-12-17 | 2018-08-29 | 昭和電工株式会社 | ルーフレール材およびその製造方法 |
| JP2017029469A (ja) * | 2015-08-03 | 2017-02-09 | 株式会社オリンピア | 遊技機 |
| JP6705292B2 (ja) * | 2015-11-27 | 2020-06-03 | 日本軽金属株式会社 | 低反射アルミニウム材及びその製造方法 |
-
2018
- 2018-09-14 JP JP2018172385A patent/JP7248399B2/ja active Active
-
2019
- 2019-09-13 WO PCT/JP2019/036102 patent/WO2020054847A1/ja not_active Ceased
-
2023
- 2023-03-16 JP JP2023042348A patent/JP2023072081A/ja active Pending
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5366811A (en) * | 1976-11-27 | 1978-06-14 | Toyama Keikinzoku Kogyo Kk | Black spot preventive allmggsi based alloy and preventive treatment method of black spots |
| JPH09227978A (ja) * | 1996-02-16 | 1997-09-02 | Tateyama Alum Ind Co Ltd | アルミニウム合金 |
| JPH10306336A (ja) * | 1997-05-01 | 1998-11-17 | Sumitomo Light Metal Ind Ltd | 陽極酸化処理後の表面光沢性に優れたアルミニウム合金押出材およびその製造方法 |
| JP2012149335A (ja) * | 2010-12-27 | 2012-08-09 | Sankyo Tateyama Inc | アルミニウム合金 |
| JP2012255191A (ja) * | 2011-06-08 | 2012-12-27 | Sankyo Tateyama Inc | アルミニウム合金 |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20210285120A1 (en) * | 2018-11-30 | 2021-09-16 | Uacj Corporation | Aluminum member and method of manufacturing aluminum member |
| US12168835B2 (en) * | 2018-11-30 | 2024-12-17 | Uacj Corporation | Aluminum member and method of manufacturing aluminum member |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2020045506A (ja) | 2020-03-26 |
| JP2023072081A (ja) | 2023-05-23 |
| JP7248399B2 (ja) | 2023-03-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US9970080B2 (en) | Micro-alloying to mitigate the slight discoloration resulting from entrained metal in anodized aluminum surface finishes | |
| KR102213570B1 (ko) | 양극산화된-품질 알루미늄 합금 및 관련된 제품 및 방법 | |
| JP2019073802A (ja) | 高い強度及び美的訴求力を有するアルミニウム合金 | |
| JP2023072081A (ja) | アルミニウム形材、建具及びアルミニウム形材の製造方法 | |
| NO161517B (no) | Anordning for aa gi oppdrift til i det minste ett element av et undervannsproduksjonsstigeroer. | |
| NO121075B (enExample) | ||
| CN105063442A (zh) | 一种铝合金 | |
| JPH0347937A (ja) | 陽極酸化処理後の色調が白色のアルミニウム合金材料 | |
| AU2014307526A1 (en) | Al-coated steel sheet having excellent total reflection properties and corrosion resistance, and method for manufacturing same | |
| JP3314312B2 (ja) | アルミニウム合金材の結晶模様製造方法 | |
| JP2012149335A (ja) | アルミニウム合金 | |
| JP5629099B2 (ja) | 耐食性及び光輝性に優れた押出し用アルミニウム合金 | |
| JP3200523B2 (ja) | グレー発色用時効硬化型アルミニウム合金押出形材及びその製造方法 | |
| EP3500689B1 (en) | Anodized aluminum with dark gray color | |
| TWI565809B (zh) | 鋁合金之製造方法 | |
| US20170137956A1 (en) | Surface-treated aluminum material and zinc-supplemented aluminum alloy | |
| US3098724A (en) | Aluminous metal article | |
| JP3958182B2 (ja) | 後成形性良好なアルミニウム合金陽極酸化処理板 | |
| JP4942524B2 (ja) | 曲げ加工性及び陽極酸化処理後の光輝性に優れたアルミニウム合金ならびにその押出形材 | |
| WO2018221435A1 (ja) | アルミニウム積層体およびその製造方法 | |
| JP2005248213A (ja) | グレー発色アルミニウム合金 | |
| TWI758563B (zh) | 鋁積層體及其製造方法 | |
| JP2013173992A (ja) | 押出用高強度グレー発色合金 | |
| JP2021085039A (ja) | アルミニウム部材及びその製造方法 | |
| TWI467026B (zh) | 陽極用鋁合金片及其製造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 19858942 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 19858942 Country of ref document: EP Kind code of ref document: A1 |