WO2019150640A1 - 過酸化水素濃度の測定システムおよび測定方法 - Google Patents
過酸化水素濃度の測定システムおよび測定方法 Download PDFInfo
- Publication number
- WO2019150640A1 WO2019150640A1 PCT/JP2018/033760 JP2018033760W WO2019150640A1 WO 2019150640 A1 WO2019150640 A1 WO 2019150640A1 JP 2018033760 W JP2018033760 W JP 2018033760W WO 2019150640 A1 WO2019150640 A1 WO 2019150640A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- dissolved oxygen
- hydrogen peroxide
- oxygen concentration
- water
- valve
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N31/00—Investigating or analysing non-biological materials by the use of the chemical methods specified in the subgroup; Apparatus specially adapted for such methods
- G01N31/22—Investigating or analysing non-biological materials by the use of the chemical methods specified in the subgroup; Apparatus specially adapted for such methods using chemical indicators
- G01N31/228—Investigating or analysing non-biological materials by the use of the chemical methods specified in the subgroup; Apparatus specially adapted for such methods using chemical indicators for peroxides
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/18—Water
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N31/00—Investigating or analysing non-biological materials by the use of the chemical methods specified in the subgroup; Apparatus specially adapted for such methods
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N31/00—Investigating or analysing non-biological materials by the use of the chemical methods specified in the subgroup; Apparatus specially adapted for such methods
- G01N31/10—Investigating or analysing non-biological materials by the use of the chemical methods specified in the subgroup; Apparatus specially adapted for such methods using catalysis
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T436/00—Chemistry: analytical and immunological testing
- Y10T436/20—Oxygen containing
- Y10T436/206664—Ozone or peroxide
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T436/00—Chemistry: analytical and immunological testing
- Y10T436/20—Oxygen containing
- Y10T436/207497—Molecular oxygen
- Y10T436/209163—Dissolved or trace oxygen or oxygen content of a sealed environment
Definitions
- the present invention relates to a measurement system and a measurement method for measuring a trace amount of hydrogen peroxide concentration in sample water.
- a method for analyzing the concentration of hydrogen peroxide a method using a test paper or a test reagent, a colorimetric method, a redox titration method, or the like is generally known.
- concentration analysis of hydrogen peroxide is used for various purposes.
- it can be used in a wastewater treatment facility for wastewater containing hydrogen peroxide.
- hydrogen peroxide is generated in an ultraviolet irradiation facility or the like in a production facility of pure water or ultrapure water, and the necessity of analyzing the concentration of hydrogen peroxide is being recognized.
- the colorimetric method and the oxidation-reduction titration method have complicated colorimetric analysis and titration operations, and are difficult to perform in-line automatic analysis. Not suitable for manufacturing equipment. Furthermore, since a reagent is required, the cost is increased due to chemical costs, maintenance, and waste liquid treatment after analysis.
- Patent Document 1 discloses a hydrogen peroxide analyzer and a hydrogen peroxide analysis method that can easily and highly sensitively analyze a small amount of hydrogen peroxide in water.
- the invention disclosed in Patent Document 1 is a method of calculating the hydrogen peroxide concentration in the sample water by means of hydrogen peroxide decomposition means and one or two dissolved oxygen concentration meters. Specifically, the hydrogen peroxide concentration in the sample water is calculated from the difference between the dissolved oxygen concentration in the sample water and the dissolved oxygen concentration in the treated water treated by the hydrogen peroxide decomposition means.
- FIG. 8 A configuration example of the embodiment of the apparatus is shown in FIG. 8 (corresponding to FIG. 6 of Patent Document 1) and FIG. 9 (corresponding to FIG. 3 of Patent Document 1).
- FIG. 8 the dissolved oxygen concentration contained in the sample water and the treated water is alternately measured with one dissolved oxygen concentration meter.
- FIG. 9 the dissolved oxygen concentration contained in the sample water and the treated water is measured simultaneously with two dissolved oxygen concentration meters.
- Patent Document 2 discloses a hydrogen peroxide concentration measuring device characterized by using a catalytic metal carrier in which a platinum group metal is supported on a monolithic organic porous anion exchanger as hydrogen peroxide decomposition means, and A measurement method is also disclosed.
- Patent Document 3 discloses a method for measuring the hydrogen peroxide concentration by a phenolphthalein colorimetric method using a solid color developing reagent.
- the dissolved oxygen concentration contained in the sample water and the treated water subjected to the decomposition treatment of hydrogen peroxide is alternately measured, and then the dissolved sample water and the treated water are dissolved. Since the hydrogen peroxide concentration is calculated from the difference in oxygen concentration, a time when the hydrogen peroxide concentration in the sample water cannot be measured frequently occurs. Further, the dissolved oxygen concentration in the sample water cannot be measured while the dissolved oxygen concentration contained in the treated water is measured. Further, by switching the sample water and the treated water, it takes some time until the dissolved oxygen concentration measurement value after the switching becomes stable.
- the dissolved oxygen concentration of the specimen water and the treated water is measured while simultaneously measuring the dissolved oxygen concentration of the specimen water and the treated water subjected to the decomposition treatment of hydrogen peroxide. Since the hydrogen peroxide concentration is calculated from the difference, the hydrogen peroxide concentration can be calculated continuously. There is also an advantage that the dissolved oxygen concentration in the sample water can be continuously measured. However, slight individual differences between the two dissolved oxygen concentration meters may lead to errors in the measured hydrogen peroxide concentration.
- an object of the present invention is to provide a method and a system for measuring a hydrogen peroxide concentration capable of continuously and accurately quantifying hydrogen peroxide in specimen water.
- a method for measuring the concentration of hydrogen peroxide includes: A method for measuring the concentration of hydrogen peroxide in sample water collected from a predetermined position in a water treatment process, A sampling process for collecting sample water; The dissolved oxygen concentration of the sample water or treated water obtained by treating the sample water with the hydrogen peroxide decomposing means is measured by the first dissolved oxygen concentration measuring means and the second dissolved oxygen concentration measuring means, and two dissolved oxygen concentration values are obtained.
- the hydrogen peroxide concentration measurement system is A hydrogen peroxide concentration measurement system that measures the concentration of hydrogen peroxide in sample water collected from a predetermined position in a water treatment process, A sample water collecting means for collecting the sample water; A path for measuring the dissolved oxygen concentration by introducing the collected sample water into the first dissolved oxygen concentration measuring means via the first pipe; A path for introducing the collected sample water into the hydrogen peroxide decomposition means and introducing the treated water decomposed with hydrogen peroxide into the second dissolved oxygen concentration measurement means via the second pipe; , A communication pipe communicating the first pipe and the second pipe; A first on-off valve disposed on the upstream side of a branch position between the communication pipe and at least one of the first pipe and the second pipe; A communication valve arranged in the communication pipe; Valve control means for opening and closing the first on-off valve and the communication valve; A measured value that is a difference between a dissolved oxygen concentration value of the sample water measured by the first dissolved oxygen concentration measuring means and a dissolved oxygen concentration value of the treated
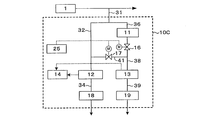
- FIG. 1 is a block diagram showing the configuration of a hydrogen peroxide concentration measurement system 10A according to an embodiment of the present invention.
- the hydrogen peroxide concentration measurement system 10 ⁇ / b> A is a part of water before or after being treated in a water treatment process 1 such as pure water or ultrapure water production equipment or wastewater treatment equipment.
- a sample water collection pipe sample water collection means
- the sample water is introduced into the first dissolved oxygen concentration meter (first dissolved oxygen concentration measuring means) 12 via the first pipe 32 and a path for measuring the dissolved oxygen concentration of the sample water, and the sample water is decomposed with hydrogen peroxide.
- the treated water introduced into the apparatus (hydrogen peroxide decomposition means) 11 and decomposed hydrogen peroxide is introduced into the second dissolved oxygen concentration meter (second dissolved oxygen concentration measuring means) 13 via the second pipe 38 and dissolved. Branching to a pipe 36 connected to a path for measuring the oxygen concentration.
- the first dissolved oxygen concentration meter 12 accepts the sample water as it is and measures the dissolved oxygen concentration in the sample water.
- the hydrogen peroxide decomposition apparatus 11 receives sample water, decomposes hydrogen peroxide in the sample water, and discharges it as treated water.
- the second dissolved oxygen concentration meter receives the discharged treated water and measures the dissolved oxygen concentration in the treated water.
- the specimen water collection pipe 31 is branched and divided into two measurement systems (paths).
- the sample water flowing into the first pipe 32 on one side of the branch is sent to the first dissolved oxygen concentration meter 12 via the first on-off valve 15.
- the sample water that has flowed into the other pipe 36 passes through the hydrogen peroxide decomposing apparatus 11 and undergoes a hydrogen peroxide decomposing process.
- the treated water passes through the second pipe 38 having the second on-off valve 16. It is sent to the second dissolved oxygen concentration meter 13 via.
- a communication pipe 41 that communicates the downstream side of the first on-off valve 15 of the pipe 32 and the downstream side of the second on-off valve 16 of the pipe 38 is provided.
- a communication valve 17 is provided in the communication pipe 41.
- the sample water collection pipe 31 may be provided with a gate valve used when sample water is not collected.
- a liquid feeding device such as a pump is unnecessary, but a liquid feeding device may be provided if necessary.
- the first on-off valve 15, the second on-off valve 16, and the communication valve 17 are all automatic open / close valves that are operated by electricity or compressed air, and are controlled to open and close by the valve control device (valve control means) 25 including the opening / closing timing.
- the dissolved oxygen concentration values measured by the first dissolved oxygen concentration meter 12 and the second dissolved oxygen concentration meter 13 are transmitted to the calculation unit (calculation means) 14 to calculate the hydrogen peroxide concentration in the sample water.
- a display device that displays the measured dissolved oxygen concentration value or the calculated hydrogen peroxide concentration value on a monitor screen or the like in real time, or an output device that appropriately prints on a printer or the like may be provided (not shown).
- Hydrogen peroxide contained in the sample water introduced into the hydrogen peroxide decomposition apparatus 11 is decomposed as follows.
- the generated oxygen dissolves in water and becomes dissolved oxygen. 2H 2 O 2 ⁇ 2H 2 O + O 2 (1)
- the hydrogen peroxide decomposition apparatus 11 may be constituted by a container or a column filled with a material having hydrogen peroxide decomposition ability.
- the material having the ability to decompose hydrogen peroxide is not particularly limited as long as it has the ability to decompose hydrogen peroxide in water into water and oxygen. Those having high ability and excellent durability are preferred. Further, in order to increase the contact efficiency with hydrogen peroxide, those having a large surface area such as granular, fibrous or porous are preferable. Examples of such materials include activated carbon, synthetic carbon-based adsorbent, ion exchange resin, metal catalyst (Pd, Pt, etc.), enzyme (catalase, etc.), enzyme carrier and the like.
- the hydrogen peroxide decomposition catalyst it is preferable to use a catalyst metal carrier (platinum group metal catalyst) on which a platinum group metal is supported.
- Hydrogen peroxide can be decomposed by catalytic decomposition by bringing hydrogen peroxide in the water to be treated into contact with a platinum group metal catalyst.
- the platinum group metal catalyst is supported on an anion exchanger.
- the anion exchanger may be a granular anion exchange resin.
- a catalytic metal carrier in which a platinum group metal is supported on a monolithic organic porous anion exchanger can decompose hydrogen peroxide even when water is passed at an SV (space velocity) exceeding 2000 h- 1 . For this reason, the hydrogen peroxide decomposition apparatus 11 can be easily downsized.
- the synergistic effect of the increase in SV and the downsizing of the hydrogen peroxide decomposition means enables high-speed water flow. For this reason, oxygen remaining in the catalyst itself and the packed column is easily removed, the system startup speed is improved, and rapid measurement is possible. Even when oxygen is mixed in through the joint, the increase in SV makes it easier to exclude oxygen, so that adverse effects on measurement accuracy can be suppressed.
- a Pd monolith in which Pd is supported on a monolithic organic porous anion exchanger as a platinum group metal water to be measured can be passed at high speed, the apparatus can be easily downsized.
- the influence can be suppressed, for example, also when air mixes from the upstream piping of the hydrogen peroxide decomposition apparatus. For example, when air is mixed intermittently, because of the high SV, the air is immediately pushed downstream and does not stay in the hydrogen peroxide decomposition apparatus for a long time. Even when air is continuously mixed, since the SV is large, the air is diluted and the influence on the measured value is mitigated. For this reason, analysis accuracy can be improved.
- air remains in the catalyst itself or packed column when the system is started up, it is necessary to wait until the air is removed and the measured value stabilizes. System startup time is reduced.
- the monolith anion exchanger are the A type and B type described below. Catalyst metal carriers in which a platinum group metal is supported on these monolith anion exchangers can be suitably applied to the hydrogen peroxide decomposition apparatus 11.
- the A type monolith anion exchanger is obtained by introducing an anion exchange group into the monolith.
- the macropores in the form of bubbles overlap each other, and the overlapping portion is in a wet state in water with an average diameter of 30 to 300 ⁇ m, preferably 30 A continuous macropore structure having an opening (mesopore) of ⁇ 200 ⁇ m, particularly preferably 40 to 100 ⁇ m.
- the average diameter of the A-type monolith anion exchanger opening is larger than the average diameter of the monolith opening because the entire monolith swells when an anion exchange group is introduced into the monolith.
- the average diameter of the openings in the water wet state is 30 ⁇ m or more, it is possible to easily prevent an increase in pressure loss during water flow.
- the average diameter of the openings in the water-wetting state is 300 ⁇ m or less, it is easy to ensure the contact between the water to be treated and the A-type monolith anion exchanger and the supported platinum group metal nanoparticles. Further, it is possible to easily prevent the degradation of hydrogen peroxide decomposition characteristics.
- the average diameter of the opening of the monolith intermediate in the dry state, the average diameter of the opening of the monolith in the dry state, and the average diameter of the opening of the monolith anion exchanger in the dry state mean values measured by the mercury intrusion method.
- the average diameter of the openings of the A-type monolith anion exchanger in the wet state is a value calculated by multiplying the average diameter of the openings of the A-type monolith anion exchanger in the dry state by the swelling rate. Further, the average diameter of the opening of the dried monolith before the introduction of the anion exchange group, and the swelling of the water-type A type monolith anion exchanger with respect to the dried monolith when the anion exchange group is introduced into the dried monolith When the ratio is known, the average diameter of the opening of the dry monolith can be multiplied by the swelling ratio to calculate the average diameter of the opening of the A-type monolith anion exchanger in the wet state.
- the skeleton part area appearing in the cross section is 25 to 50%, preferably 25 to 45% in the image region.
- the area of the skeleton part shown in the cross section is 25% or more in the image region, it can be easily prevented that the skeleton becomes a thin skeleton and the mechanical strength is lowered, and the monolith anion exchanger particularly when water is passed at a high flow rate. Can be easily prevented from being greatly deformed.
- the total pore volume of the A type monolith anion exchanger is 0.5 to 5 ml / g, preferably 0.8 to 4 ml / g.
- the total pore volume is 0.5 ml / g or more, it is possible to easily prevent an increase in pressure loss during water flow, and the amount of permeated fluid per unit cross-sectional area is reduced, resulting in a reduction in processing capacity. Can be easily prevented.
- the total pore volume is 5 ml / g or less, it is possible to easily prevent the mechanical strength from decreasing, and particularly when the water is passed at a high flow rate, the A type monolith anion exchanger is greatly deformed. Can be easily prevented.
- the total pore volume of the monolith means a value measured by mercury porosimetry.
- the total pore volume of the monolith is the same both in the dry state and in the water wet state.
- the pressure loss when water is permeated through the A-type monolith anion exchanger is the pressure loss when water is passed through a column packed with 1 m at a water flow velocity (LV) of 1 m / h (hereinafter, “In the range of 0.001 to 0.1 MPa / m ⁇ LV, particularly 0.005 to 0.05 MPa / m ⁇ LV is preferable.
- the A type monolith anion exchanger has an anion exchange capacity of 0.4 to 1.0 mg equivalent / ml per volume in a water-wet state.
- the anion exchange capacity per volume is 0.4 mg equivalent / ml or more, it is possible to easily prevent the supported amount of platinum group metal nanoparticles per volume.
- the anion exchange capacity per volume is 1.0 mg equivalent / ml or less, an increase in pressure loss during water passage can be easily prevented.
- the anion exchange capacity per weight of the A type monolith anion exchanger is not particularly limited. However, since the anion exchange group is uniformly introduced to the surface of the porous body and the inside of the skeleton, it is 3.5 to 4. 5 mg equivalent / g.
- the material constituting the skeleton of the continuous macropore structure is an organic polymer material having a crosslinked structure.
- the crosslink density of the polymer material is not particularly limited, but includes 0.3 to 10 mol%, preferably 0.3 to 5 mol% of cross-linked structural units with respect to all structural units constituting the polymer material. It is preferable.
- the cross-linking structural unit is 0.3 mol% or more, it is possible to easily prevent the mechanical strength from being insufficient.
- it is 10 mol% or less it is easy to introduce an anion exchange group. Can be prevented.
- aromatic vinyl polymers such as a polystyrene
- the polymer may be a polymer obtained by copolymerizing a single vinyl monomer and a crosslinking agent, a polymer obtained by polymerizing a plurality of vinyl monomers and a crosslinking agent, or a blend of two or more types of polymers. It may be what was done.
- the cross-linking weight of the aromatic vinyl polymer is easy due to the ease of forming a continuous macropore structure, the ease of introducing an anion exchange group and the high mechanical strength, and the high stability to acids or alkalis.
- a styrene-divinylbenzene copolymer and a vinylbenzyl chloride-divinylbenzene copolymer are particularly preferable materials.
- anion exchange groups of the A type monolith anion exchanger quaternary ammonium groups such as trimethylammonium group, triethylammonium group, tributylammonium group, dimethylhydroxyethylammonium group, dimethylhydroxypropylammonium group, methyldihydroxyethylammonium group, etc. Is mentioned.
- anion exchange groups are uniformly distributed” means that the distribution of anion exchange groups is uniformly distributed on the surface and inside the skeleton in the order of at least ⁇ m.
- the distribution state of the anion exchange group can be confirmed relatively easily by using EPMA after ion exchange of the counter anion with chloride ion, bromide ion or the like.
- the anion exchange groups are uniformly distributed not only on the surface of the monolith but also within the skeleton of the porous body, the physical and chemical properties of the surface and the interior can be made uniform, so that the swelling and shrinking The durability against is improved.
- the A type monolith anion exchanger swells greatly, for example, 1.4 to 1.9 times as thick as the bone monolith, since an anion exchange group is introduced into the bone monolith. For this reason, even if the opening diameter of the thick monolith is small, the opening diameter of the monolith ion exchanger generally increases at the above magnification. In addition, the total pore volume does not change even when the opening diameter increases due to swelling. Therefore, the A-type monolith ion exchanger has a high mechanical strength because it has a thick bone skeleton even though the opening diameter is remarkably large.
- the B type monolith anion exchanger has an average thickness composed of an aromatic vinyl polymer containing 0.3 to 5.0 mol% of a cross-linking structural unit in all constituent units into which an anion exchange group has been introduced.
- a co-continuous structure comprising a three-dimensionally continuous skeleton of 1 to 60 ⁇ m and three-dimensionally continuous pores having an average diameter of 10 to 100 ⁇ m in a wet state between the skeletons.
- the volume is 0.5 to 5 ml / g
- the ion exchange capacity per volume under water wet condition is 0.3 to 1.0 mg equivalent / ml
- the anion exchange group is uniform in the porous ion exchanger. Is distributed.
- the B type monolith anion exchanger has a three-dimensional continuous skeleton having an average thickness of 1 to 60 ⁇ m, preferably 3 to 58 ⁇ m in an wet state in which an anion exchange group is introduced, and an average diameter between the skeletons.
- a co-continuous structure comprising three-dimensionally continuous pores of 10 to 100 ⁇ m, preferably 15 to 90 ⁇ m, particularly preferably 20 to 80 ⁇ m in a wet state. That is, the co-continuous structure is a structure in which a continuous skeleton phase and a continuous vacancy phase are intertwined and both are three-dimensionally continuous.
- the skeleton thickness and pore diameter of the B type monolith anion exchanger are larger than the monolith skeleton thickness and pore diameter because the entire monolith swells when an anion exchange group is introduced into the monolith. It becomes.
- These continuous pores have higher continuity of the pores than the conventional open-cell type monolithic organic porous anion exchanger and particle aggregation type monolithic organic porous anion exchanger, and the size thereof is not biased. Therefore, an extremely uniform anion adsorption behavior can be achieved.
- the average diameter of the three-dimensionally continuous pores is 10 ⁇ m or more in a water-wet state, it is possible to easily prevent an increase in pressure loss during water flow.
- the average diameter is 100 ⁇ m or less
- the water to be treated and the organic porosity It is easy to ensure contact with the porous anion exchanger, and as a result, it is easy to remove dissolved oxygen from the water to be treated.
- the average thickness of the skeleton is 1 ⁇ m or more in a wet state of water, it is possible to easily prevent a decrease in anion exchange capacity per volume, a decrease in mechanical strength, especially when water is passed at a high flow rate.
- the B-type monolith anion exchanger can be easily prevented from being greatly deformed. Furthermore, it can prevent easily that the contact efficiency of to-be-processed water and a B type monolith anion exchanger falls, and a catalyst effect falls.
- the thickness of the skeleton is 60 ⁇ m or less, it is possible to easily prevent the skeleton from becoming thick and increasing pressure loss during water passage.
- the average diameter of the pores of the continuous structure in the water-wet state is a value calculated by multiplying the average diameter of the pores of the dry monolith anion exchanger measured by the mercury intrusion method and the swelling ratio.
- the average diameter of the pores of the dried monolith before the introduction of the anion exchange group, and the water-wet state B type monolith anion exchanger of the dried monolith when the anion exchange group is introduced into the dried monolith can be calculated by multiplying the average diameter of the pores of the dry monolith by the swelling ratio.
- the average thickness of the skeleton of the continuous structure in the water-wet state is determined by performing SEM observation of the dry B-type monolith anion exchanger at least three times and measuring the thickness of the skeleton in the obtained image. The average value is calculated by multiplying the swelling ratio. Further, the average thickness of the skeleton of the dried monolith before the introduction of the anion exchange group, and the water-wet state B type monolith anion exchanger of the dried monolith when the anion exchange group is introduced into the dried monolith. When the swelling ratio is known, the average thickness of the skeleton of the monolith anion exchanger in the water-wet state can be calculated by multiplying the average thickness of the skeleton of the monolith in the dry state by the swelling ratio.
- the skeleton has a rod-like shape and a circular cross-sectional shape, but may have a cross-section with a different diameter such as an elliptical cross-sectional shape.
- the thickness in this case is the average of the minor axis and the major axis.
- the total pore volume of the B type monolith anion exchanger is 0.5 to 5 ml / g.
- the total pore volume is 0.5 ml / g or more, it is possible to easily prevent an increase in pressure loss during water flow, and further, the amount of permeated water per unit cross-sectional area is reduced and the amount of treated water is reduced. Can be easily prevented.
- the total pore volume is 5 ml / g or less, it is possible to easily prevent a decrease in anion exchange capacity per volume and a decrease in the catalytic effect due to a decrease in the amount of platinum group metal nanoparticles supported. .
- the pressure loss when water is allowed to permeate through the B type monolith anion exchanger is the pressure loss when water is passed through a column filled with 1 m of a porous material at a water flow rate (LV) of 1 m / h (hereinafter referred to as “pressure loss”).
- pressure loss a water flow rate of 1 m / h
- pressure loss a water flow rate of 1 m / h
- the material constituting the skeleton of the co-continuous structure contains 0.3 to 5 mol%, preferably 0.5 to 3.0 mol% of cross-linked structural units in all the structural units. It is an aromatic vinyl polymer containing and is hydrophobic.
- the cross-linking structural unit is 0.3 mol% or more, it is possible to easily prevent the mechanical strength from being insufficient.
- the structure of the porous body deviates from the bicontinuous structure. Can be easily prevented.
- the polymer may be a polymer obtained by copolymerizing a single vinyl monomer and a crosslinking agent, a polymer obtained by polymerizing a plurality of vinyl monomers and a crosslinking agent, or a blend of two or more types of polymers. It may be what was done.
- organic polymer materials a styrene-divinylbenzene copolymer is obtained due to the ease of forming a co-continuous structure, the ease of introducing an anion exchange group and the high mechanical strength, and the high stability to acids or alkalis. And vinylbenzyl chloride-divinylbenzene copolymer is preferred.
- the B-type monolith anion exchanger has an ion exchange capacity of 0.3 to 1.0 mg equivalent / ml per anion volume in a wet state of water. Since the B type monolith anion exchanger has high continuity and uniformity of three-dimensionally continuous pores, the pressure loss does not increase so much even if the total pore volume is reduced. Therefore, the anion exchange capacity per volume can be dramatically increased while keeping the pressure loss low. When the anion exchange capacity per volume is 0.3 mg equivalent / ml or more, it is possible to easily prevent the supported amount of platinum group metal nanoparticles per volume. On the other hand, when the anion exchange capacity per volume is 1.0 mg equivalent / ml or less, an increase in pressure loss during water passage can be easily prevented.
- the anion exchange capacity per weight in the dry state of the B type monolith anion exchanger is not particularly limited, but the ion exchange groups are uniformly introduced to the skeleton surface and the skeleton inside the porous body. 5 to 4.5 mg equivalent / g.
- anion exchange group of the B type monolith anion exchanger examples include the same as those mentioned in the description of the A type monolith anion exchanger.
- distribution of anion exchange groups, the meaning of “anion exchange groups are uniformly distributed”, the method for confirming the distribution of anion exchange groups, and the anion exchange groups are porous as well as the surface of the monolith. The effect of even distribution within the body skeleton is the same as that of the A-type monolith anion exchanger.
- the kind of the polymer material of the monolith intermediate is the same as the kind of the polymer material of the monolith intermediate of the A type monolith anion exchanger, and the description thereof is omitted.
- the total pore volume of the monolith intermediate is more than 16 ml / g and not more than 30 ml / g, preferably more than 16 ml / g and not more than 25 ml / g.
- this monolith intermediate basically has a continuous macropore structure, but the opening (mesopore) that is the overlapping part of the macropore and the macropore is remarkably large, so that the skeleton constituting the monolith structure is primary from the two-dimensional wall surface. It has a structure as close as possible to the original rod-like skeleton.
- a porous body having a co-continuous structure is formed using the structure of the monolith intermediate as a mold.
- the ratio of monomer to water may be about 1:20 to 1:40.
- the average diameter of openings is 5 to 100 ⁇ m in a dry state.
- the average diameter of the openings is 5 ⁇ m or more in the dry state, it is possible to easily prevent the opening diameter of the monolith obtained after polymerizing the vinyl monomer from decreasing and the pressure loss during fluid permeation from increasing.
- it is 100 ⁇ m or less it is possible to easily prevent an increase in the opening diameter of the monolith obtained after polymerizing the vinyl monomer, and it is easy to ensure contact between the water to be treated and the monolith anion exchanger.
- Monolith intermediates preferably have a uniform structure with uniform macropore size and aperture diameter, but are not limited to this, and the uniform structure is dotted with nonuniform macropores larger than the size of the uniform macropore. You may do.
- B-type monolith anion exchangers swell 1.4 to 1.9 times larger than monoliths, for example, because anion exchange groups are introduced into monoliths having a co-continuous structure. Further, the total pore volume does not change even if the pore diameter becomes larger due to swelling. Therefore, the B type monolith anion exchanger has a high mechanical strength because it has a thick bone skeleton even though the size of three-dimensionally continuous pores is remarkably large. Further, since the skeleton is thick, the anion exchange capacity per volume in a water-wet state can be increased, and furthermore, the water to be treated can be passed for a long time at a low pressure and a large flow rate.
- sample water line the analysis line for measuring the dissolved oxygen concentration of the sample water
- treated water line the analysis line for measuring the dissolved oxygen concentration of the treated water
- the hydrogen peroxide decomposing apparatus 11 Since the hydrogen peroxide decomposing apparatus 11 is mounted, the pressure loss is larger and the number of joints is different. As a result, the measured values of the first dissolved oxygen concentration meter 12 and the second dissolved oxygen concentration meter 13 installed in the subsequent stage may be changed. Therefore, as in the hydrogen peroxide concentration measurement system 10B shown in FIG. 2, the condition adjustment unit 20 may be provided upstream of the first on-off valve 15 of the pipe 32 of the sample water line.
- the condition adjustment unit 20 is filled with a material (hereinafter referred to as a condition adjustment material) that matches the water flow conditions of the sample water line and the treated water line, such as causing a pressure loss similar to that of the hydrogen peroxide decomposition apparatus 11. It can be configured as a column (container).
- the condition adjustment unit 20 is not particularly limited as long as it does not have a function of generating oxygen, such as a hydrogen peroxide decomposition reaction, but is not dissolved in water and has excellent durability. Those are preferred.
- condition adjusting unit 20 may be a column packed only with the carrier as a condition adjusting material. it can.
- Such a condition adjustment unit 20 can also be applied to the configurations shown in FIGS.
- the column water flow rate is appropriately determined according to various conditions such as the type of packing material and the concentration of hydrogen peroxide in the sample water to be analyzed. It is determined.
- the flow rate is lowered, the contact time between the hydrogen peroxide and the packing material increases, the hydrogen peroxide decomposition rate increases, and the oxygen production rate tends to increase.
- the oxygen production rate through the column and piping system tends to increase. Permeation tends to cause an increase in dissolved oxygen concentration.
- the material of the column filled with the material having the ability to decompose hydrogen peroxide is not particularly limited, but a material having a low oxygen permeability is preferable. Moreover, it is transparent so that the presence or absence of air bubbles in the column can be confirmed at the time of starting the system, and preferably excellent in durability. Examples of such materials include acrylic, vinyl chloride, polycarbonate, and the like.
- the dissolved oxygen contained in the sample water and the treated water is measured by the first dissolved oxygen concentration meter 12 and the second dissolved oxygen concentration meter 13 (measurement value acquisition step).
- the two dissolved oxygen concentration meters 12 and 13 can be constituted by known dissolved oxygen meters.
- the two dissolved oxygen concentration meters 12 and 13 are not particularly limited, but are preferably of the same type and lot in order to minimize individual differences as much as possible.
- the measured values of the two dissolved oxygen concentration meters 12 and 13 are transmitted to the calculation unit 14, and the calculation unit 14 calculates the hydrogen peroxide concentration in the sample water based on a predetermined calculation formula.
- the hydrogen peroxide concentration from the dissolved oxygen concentration it can be obtained by the following calculation. That is, the difference between the dissolved oxygen concentrations in the treated water and the sample water (the numerical value obtained by subtracting the measured value of the first dissolved oxygen concentration meter 12 from the measured value of the second dissolved oxygen concentration meter 13) is as shown in the equation (1). It is derived from hydrogen peroxide. Accordingly, the concentration of hydrogen peroxide contained in the sample water can be calculated from the following equation based on the difference in the measured dissolved oxygen concentration.
- the difference ⁇ 1 between the dissolved oxygen concentration values in the treated water and the sample water (this difference ⁇ 1 obtained in the measurement value acquisition step is also referred to as “measured value”) is (treated water DO ⁇ sample water).
- 68 is the molecular weight of hydrogen peroxide on the left side of Formula (1) (doubled because it is 2 molecules)
- 32 is the molecular weight of oxygen on the right side of Formula (1).
- the unit on the left side is the same as the unit of DO on the right side.
- the dissolved oxygen concentration meter is adjusted so that there is the least error in the predetermined flow rate range, it is preferable to adjust the flow rate within that range. Therefore, it is preferable to provide a flow meter after the dissolved oxygen concentration meter. Thereby, the sample water and the treated water can be supplied to the hydrogen peroxide decomposition catalyst and the dissolved oxygen concentration meter at an appropriate flow rate.
- a mechanism for indicating the flow rate and stabilizing the flow rate at an appropriate value (hereinafter referred to as flow rate stabilizing means).
- a flow rate stabilization device (flow rate stabilization unit) 18 is provided in the downstream pipe 34 of the first dissolved oxygen concentration meter 12, and a flow rate stabilization device (flow rate stabilization unit) is provided in the downstream pipe 39 of the second dissolved oxygen concentration meter 13. ) 19 is provided.
- the flow rate stabilizing means 18 and 19 are not particularly limited, and examples thereof include a combination of a flow meter and a valve capable of adjusting the flow rate (flow rate indicating controller).
- the flow stabilizing devices 18 and 19 are preferably provided downstream of the dissolved oxygen concentration meter because oxygen may enter from the joint.
- a well-known configuration can be arbitrarily added to the process control system such as an alarm device.
- the sample water and the treated water whose dissolved oxygen concentration has been measured are drained after the flow rate measurement. Since no measurement reagent or the like is added, waste water treatment is easy.
- a dissolved oxygen concentration meter It is desirable to calibrate and use the dissolved oxygen concentration meter.
- calibration of a dissolved oxygen concentration meter is generally performed by atmospheric calibration when the sensor is exposed to the atmosphere and / or zero point calibration with an aqueous solution in which a reducing agent such as sodium sulfite is excessively dissolved to remove the dissolved oxygen concentration. .
- a reducing agent such as sodium sulfite
- the minute individual difference becomes more problematic.
- either one of the sample water or the treated water is simultaneously passed through both of the two dissolved oxygen concentration meters 12 and 13 to obtain a difference between the measured values (correction value acquisition step). ), A correction value for correcting individual differences between the dissolved oxygen concentration meters 12 and 13.
- the measured values of the two dissolved oxygen concentration meters 12 and 13 are transmitted to the calculation unit 14, and the calculation unit 14 calculates the individual difference between the two dissolved oxygen concentration meters 12 and 13 based on a predetermined calculation formula. Is calculated (calculation step).
- the measured value of the first dissolved oxygen concentration meter 12 is A
- the two dissolved oxygen concentration meters should be flowed at the same flow rate. Is preferred.
- a flow rate stabilizing means for stabilizing the flow rate at an appropriate value downstream of the dissolved oxygen concentration meter.
- valve opening / closing control will be described.
- the first on-off valve 15 is opened, the second on-off valve 16 is opened, and the communication valve 17 is closed.
- the first on-off valve 15 may be opened, the second on-off valve 16 may be closed, and the communication valve 17 may be opened (“ First correction value acquisition mode ”).
- the first on-off valve 15 may be closed, the second on-off valve 16 may be opened, and the communication valve 17 may be opened (“second correction value acquisition mode”).
- the first correction value acquisition mode and the second correction value acquisition mode are both “correction value acquisition modes”.
- the opening / closing control of each valve is configured to be performed using the valve control device 25, but each valve may be manually opened / closed without using the valve control device 25. The same applies to other embodiments.
- the communication valve 17 is not particularly limited, but a communication valve 17 having no restriction in the flow direction is preferable.
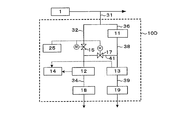
- FIG. 3 shows a hydrogen peroxide concentration measurement system 10C in the case where the sample water is measured using two dissolved oxygen concentration meters when calculating the correction value.
- This system 10C does not have the first on-off valve 15 as compared with FIG. 1, and the valve 16 serves as the first on-off valve.
- FIG. 4 shows a hydrogen peroxide concentration measurement system 10D in the case of measuring treated water using two dissolved oxygen concentration meters when calculating a correction value.
- This system 10D has only the first on-off valve 15 without the second on-off valve 16 as compared with FIG. Since other than that is the same as that of FIG. 1, detailed description is abbreviate
- the communication valve 17 in the measurement value acquisition mode, the communication valve 17 is closed and the first on-off valve 16 is opened. In the correction value acquisition mode, the communication valve 17 is open and the first on-off valve 16 is closed. In the configuration of FIG. 4, in the measurement value acquisition mode, the communication valve 17 is closed and the first on-off valve 15 is opened. In the correction value acquisition mode, the communication valve 17 is open and the first on-off valve 15 is closed.
- the number of valves can be reduced compared to the configuration of FIG. Thereby, the amount of oxygen entering the water from the valve can be reduced, and the maintenance of the valve can also be reduced.
- both the state in which the first on-off valve 15 is opened, the second on-off valve 16 is opened, and the communication valve 17 is closed at the time of normal hydrogen peroxide concentration measurement (measurement value acquisition mode) and the sample water are both used.
- the first open / close valve 15 is opened
- the second open / close valve 16 is closed
- the communication valve 17 is opened (correction value acquisition mode) when calculating the correction value by introducing it into the dissolved oxygen concentration meter
- the timing of valve switching will be described.
- Second correction value acquisition mode when switching from a normal hydrogen peroxide concentration measurement state (measurement value acquisition mode) to a state in which sample water is introduced into both dissolved oxygen concentration meters and a correction value is calculated (first correction value acquisition mode), 2
- the timing of switching the on-off valve 16 from opening to closing and the switching of the communication valve 17 from closing to opening can be arbitrarily set, and can be set at the same time. It is preferable to switch the second on-off valve 16 from open to closed after the switch to open is completed. This is because if the communication valve 17 is opened from the closed state after the second on-off valve 16 is switched from the open state to the closed state, the flow rate to the second dissolved oxygen concentration meter 13 is temporarily interrupted, and it takes time until the measured value becomes stable. This is because problems such as having
- the communication valve 17 is switched from open to closed after the switching of the second open / close valve 16 from closed to open is completed.
- the first on-off valve 15 and the communication valve 17 are switched at the same timing as described above.
- the timing for obtaining the correction value ⁇ 2 (the timing for performing the correction value acquisition step) can be set arbitrarily (regular or irregular), but is preferably between every day and every six months. With a frequency of every day or more, it can be easily prevented that the mode for frequently obtaining the correction value is switched and the time during which the hydrogen peroxide concentration cannot be output becomes long. In addition, when the frequency is less than half a year, the calibration frequency of the sensor is moderated, and it is possible to easily prevent the measurement value from becoming unreliable. It is preferable that the measurement value acquisition process is continuously performed during a period other than the period during which the correction value acquisition process is performed.
- sample water As the water to be measured when obtaining the correction value ⁇ 2.
- the dissolved oxygen concentration in the sample water is always output. That is, the dissolved oxygen concentration can be monitored even during the acquisition of the correction value ⁇ 2.
- a more reliable measurement value can be obtained by performing normal calibration (such as atmospheric calibration and / or zero point calibration) of the dissolved oxygen concentration meter as appropriate.
- the hydrogen peroxide concentration is calculated from equation (3), but it is possible to suppress variations by taking a moving average over a certain period of time.
- a degassing device (degassing means) 21 is provided in the pipe 31 for collecting the sample water to degas the oxygen dissolved in the sample water. Also good.
- a membrane type deaerator is used as the deaerator 21. The membrane type deaerator flows the water to be treated into one chamber partitioned by a gas separation membrane and depressurizes the other chamber so that the gas contained in the water to be treated passes through the gas separation membrane to the other chamber. A device that migrates and removes.
- a membrane in which a hydrophobic polymer membrane such as tetrafluoroethylene or silicone rubber is formed into an appropriate shape such as a hollow fiber membrane is usually used.
- a deaeration device is effective for measuring the hydrogen peroxide concentration with high accuracy when the dissolved oxygen concentration in the specimen water is high or fluctuates greatly.
- the deaeration device 21 can also be applied to the configurations of FIGS.
- the hydrogen peroxide concentration of pure water or ultrapure water is several tens of ⁇ g / L or less, so the dissolved oxygen concentration is 100 ⁇ g / L or less to reduce the blank value. It is particularly preferable to deaerate (deoxygenate) to 10 ⁇ g / L or less.
- the method of deaeration (deoxygenation) is not particularly limited, but the above-mentioned membrane type deaerator is preferable in that a high-grade deaeration process can be performed in a compact manner. If the hydrogen peroxide concentration in the sample water is high or analysis with high accuracy is not required, or if the dissolved oxygen concentration in the sample water is originally low, it is not always necessary to install a deaeration device.
- the pipes used in the hydrogen peroxide concentration measurement system described above especially the pipe from the sample collection point where the sample water is introduced to the dissolved oxygen concentration meter, use a material such as stainless steel or nylon with low gas permeability. It is preferable. These are preferable because of low oxygen permeability and less elution of impurities.
- the sample water collected by the pipe 31 is measured separately for the sample water line and the treated water line.
- the sample water for the sample water line and the sample water line may be collected independently (not shown).
- the conditions such as the dissolved oxygen concentration may be different if the collection position is far away, it is preferable to measure by branching the sample water collected at one place.
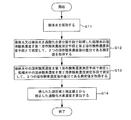
- sample water is collected (step S11 in FIG. 7).
- the dissolved oxygen concentration of the sample water or the treated water obtained by treating the sample water with the hydrogen peroxide decomposing means is measured by the first dissolved oxygen concentration measuring means and the second dissolved oxygen concentration measuring means, and the two dissolved oxygen concentrations are measured.
- a correction value that is a difference between the values is acquired (step S12).
- the dissolved oxygen concentration in the sample water is measured by the first dissolved oxygen concentration measuring means, the dissolved oxygen concentration in the treated water is measured by the second dissolved oxygen concentration measuring means, and the measurement is the difference between the two dissolved oxygen concentration values.
- a value is acquired (step S13).
- step S14 The hydrogen peroxide concentration corrected from the measured value and the correction value obtained at the end is calculated (step S14).
- Step 12 is appropriately performed, and step S13 and step S14 are performed simultaneously at other normal times. That is, the hydrogen peroxide concentration is calculated by correcting the measurement value obtained continuously continuously with the correction value immediately.
- hydrogen peroxide in the sample water can be quantified continuously and with high accuracy.
- the measurement of the hydrogen peroxide concentration in the sample water can be automated, it is possible to realize a hydrogen peroxide analysis system and method that are particularly suitable for pure water or ultrapure water production facilities that should avoid human intervention as much as possible.
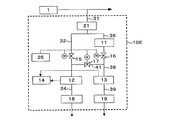
- Example 1 Four conditions of sample water with different hydrogen peroxide concentrations were introduced into the hydrogen peroxide concentration measurement system 10E shown in FIG. 5, and the hydrogen peroxide concentration was measured.
- the sample water was simultaneously passed through both the first dissolved oxygen concentration meter 12 and the second dissolved oxygen concentration meter 13 for 1 hour, both of which were in the latter half 30 minutes.
- the correction value ⁇ 2 for calculating the hydrogen peroxide concentration was calculated using the average value of the difference between the measured values. Thereafter, the hydrogen peroxide concentration was calculated by the formula (3).
- the dissolved oxygen concentration contained in the sample water and the treated water is measured simultaneously with two dissolved oxygen concentration meters, and the average value for 3 minutes after the measurement value is stabilized is calculated.
- the measured value of the hydrogen peroxide concentration was used.
- the hydrogen peroxide concentration in the sample water was analyzed by the phenolphthalein colorimetric method described in Patent Document 3.
- Example 1 the hydrogen peroxide concentration measurement result (Example 1) of the sample water according to the present invention and the hydrogen peroxide concentration measurement result (reference value) of the sample water by the phenolphthalein colorimetric method were obtained.
- the results are shown in Table 1. Comparing the measurement results of Example 1 and the reference value, the difference was 0.5 ⁇ g / L or less in all four conditions with different hydrogen peroxide concentrations in the sample water.
- the dissolved oxygen concentration contained in the sample water and the treated water is simultaneously measured by the calibrated first dissolved oxygen concentration meter 12 and the second dissolved oxygen concentration meter 13, respectively, and the equation (2) is used from the measured dissolved oxygen concentration.
- the hydrogen peroxide concentration was calculated.
- the dissolved oxygen concentration contained in the sample water and the treated water is measured simultaneously with the first dissolved oxygen concentration meter 12 and the second dissolved oxygen concentration meter 13 respectively, and the average value for 3 minutes after the measured value becomes stable is measured.
- the measured value of the hydrogen peroxide concentration in water was used. Further, the hydrogen peroxide concentration in the sample water was analyzed by the phenolphthalein colorimetric method described in Patent Document 3.
- Example 2 Ultrapure water containing hydrogen peroxide was introduced as sample water into the hydrogen peroxide concentration measurement system 10E shown in FIG. 5, and the hydrogen peroxide concentration was measured. Immediately before the measurement of the hydrogen peroxide concentration, the sample water was passed through both the first dissolved oxygen concentration meter 12 and the second dissolved oxygen concentration meter 13 simultaneously for 1 hour, and the difference between the measured values of the latter half 30 minutes was measured. A correction value ⁇ 2 for calculating the hydrogen peroxide concentration was calculated. Thereafter, the hydrogen peroxide concentration was calculated by the formula (3). The above results are shown in FIG.
Landscapes
- Life Sciences & Earth Sciences (AREA)
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Pathology (AREA)
- General Physics & Mathematics (AREA)
- Immunology (AREA)
- Physics & Mathematics (AREA)
- Analytical Chemistry (AREA)
- Biochemistry (AREA)
- General Health & Medical Sciences (AREA)
- Food Science & Technology (AREA)
- Medicinal Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Molecular Biology (AREA)
- Biophysics (AREA)
- Investigating Or Analyzing Non-Biological Materials By The Use Of Chemical Means (AREA)
- Investigating Or Analysing Materials By The Use Of Chemical Reactions (AREA)
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020207013127A KR102360586B1 (ko) | 2018-01-31 | 2018-09-12 | 과산화수소 농도의 측정 시스템 및 측정 방법 |
| CN201880088307.8A CN111670362B (zh) | 2018-01-31 | 2018-09-12 | 过氧化氢浓度的测定系统以及测定方法 |
| SG11202007246SA SG11202007246SA (en) | 2018-01-31 | 2018-09-12 | System and method for measuring hydrogen peroxide concentration |
| US16/966,631 US11408868B2 (en) | 2018-01-31 | 2018-09-12 | Measuring system and measuring method of hydrogen peroxide concentration |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2018-014416 | 2018-01-31 | ||
| JP2018014416A JP6590964B2 (ja) | 2018-01-31 | 2018-01-31 | 過酸化水素濃度の測定システムおよび測定方法 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2019150640A1 true WO2019150640A1 (ja) | 2019-08-08 |
Family
ID=67478957
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2018/033760 Ceased WO2019150640A1 (ja) | 2018-01-31 | 2018-09-12 | 過酸化水素濃度の測定システムおよび測定方法 |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US11408868B2 (enExample) |
| JP (1) | JP6590964B2 (enExample) |
| KR (1) | KR102360586B1 (enExample) |
| CN (1) | CN111670362B (enExample) |
| SG (1) | SG11202007246SA (enExample) |
| TW (1) | TWI772557B (enExample) |
| WO (1) | WO2019150640A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN115684532A (zh) * | 2022-12-30 | 2023-02-03 | 正大农业科学研究有限公司 | 水质监测方法、装置、系统、电子设备及存储介质 |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20230213537A1 (en) * | 2021-12-31 | 2023-07-06 | Baxter International, Inc. | Determination of oxidizing substances using peptide degradation |
| JP7615215B2 (ja) * | 2023-05-17 | 2025-01-16 | 野村マイクロ・サイエンス株式会社 | 測定装置、水処理装置、測定方法、及び水処理方法 |
| CN116380981B (zh) * | 2023-06-07 | 2023-09-05 | 中国电子工程设计院有限公司 | 一种气相定量测定亚ppb级污染物的方法 |
| JP7544210B1 (ja) * | 2023-07-28 | 2024-09-03 | 栗田工業株式会社 | 過酸化水素濃度測定システム及び過酸化水素濃度測定方法 |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2005274386A (ja) * | 2004-03-25 | 2005-10-06 | Japan Organo Co Ltd | 過酸化水素分析装置及び過酸化水素分析方法 |
| US20070238188A1 (en) * | 2006-03-29 | 2007-10-11 | Carr Gregory E | Peroxide monitor |
| JP2012063303A (ja) * | 2010-09-17 | 2012-03-29 | Japan Organo Co Ltd | 過酸化水素濃度の測定装置及び測定方法 |
| JP2013108916A (ja) * | 2011-11-24 | 2013-06-06 | Nomura Micro Sci Co Ltd | 超純水中の微量過酸化水素の濃度測定方法 |
| JP2017000970A (ja) * | 2015-06-11 | 2017-01-05 | 野村マイクロ・サイエンス株式会社 | 超純水製造システム及び超純水製造方法 |
Family Cites Families (35)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB894370A (en) * | 1959-11-24 | 1962-04-18 | Nat Res Dev | Method and apparatus for measuring the concentration of dissolved gas in a liquid |
| CA1082819A (en) * | 1974-12-09 | 1980-07-29 | Edward T. Armstrong | Waste treatment system |
| JPS55143437A (en) * | 1979-04-27 | 1980-11-08 | Toshiba Corp | Detection monitor for hydrogen peroxide and dissolved oxygen |
| JPS578779A (en) * | 1980-06-18 | 1982-01-18 | Kyowa Hakko Kogyo Co Ltd | Cultivation method |
| SU966673A1 (ru) * | 1981-03-12 | 1982-10-15 | Институт Кибернетики С Вычислительным Центром Ан Узсср | Способ автоматического управлени процессом непрерывного выращивани микроорганизмов в биореакторе и устройство дл его осуществлени |
| EP0227949B1 (en) * | 1985-11-26 | 1993-10-13 | Dai Nippon Insatsu Kabushiki Kaisha | Device for controlling the concentration of aqueous solution of alcohol |
| US5098547A (en) * | 1988-10-11 | 1992-03-24 | Bryan Avron I | Dissolved oxygen sensor calibration, monitoring and reporting system |
| JPH1096720A (ja) | 1996-09-25 | 1998-04-14 | Mitsubishi Gas Chem Co Inc | 微量過酸化水素定量用試薬 |
| JP3668575B2 (ja) * | 1996-12-18 | 2005-07-06 | キッコーマン株式会社 | ヒスタミンの定量方法及び定量装置 |
| JPH10290997A (ja) * | 1997-04-18 | 1998-11-04 | Hitachi Plant Eng & Constr Co Ltd | アンモニア性窒素濃度の検出装置 |
| US5889209A (en) * | 1997-12-18 | 1999-03-30 | The Regents Of The University Of California | Method and apparatus for preventing biofouling of aquatic sensors |
| DE19806854C2 (de) * | 1998-02-19 | 2000-02-03 | Maihak Ag | Verfahren und Vorrichtung zur Bestimmung des organischen Kohlenstoffs(TOC)-)Gehaltes in Flüssigkeiten, insbesondere Reinstwasser |
| KR20000072088A (ko) * | 2000-07-19 | 2000-12-05 | 조희섭 | 물 전기 분해에 의한 수소 및 산소 측정 실험장치 |
| JP3452897B2 (ja) * | 2000-12-13 | 2003-10-06 | 三星電子株式会社 | 不純物検出装置および不純物検出方法 |
| JP3655569B2 (ja) * | 2001-09-06 | 2005-06-02 | 大陽日酸株式会社 | ガス成分濃度測定方法及び装置 |
| MXPA05000941A (es) * | 2002-08-21 | 2005-05-16 | Univ Columbia | Medidor de arsenico. |
| JP2006071340A (ja) * | 2004-08-31 | 2006-03-16 | Kurita Water Ind Ltd | 液体中の溶存気体濃度の測定方法、測定装置及び窒素ガス溶解水の製造装置 |
| JP4406792B2 (ja) * | 2004-09-01 | 2010-02-03 | メタウォーター株式会社 | バイオセンサの校正方法 |
| WO2008105108A1 (ja) * | 2007-02-27 | 2008-09-04 | Shigenori Aono | 漂白繊維品の製法およびそれに用いる装置、並びにそれによって得られる漂白繊維品 |
| JP5430983B2 (ja) * | 2009-03-18 | 2014-03-05 | オルガノ株式会社 | 白金族金属担持触媒、過酸化水素の分解処理水の製造方法、溶存酸素の除去処理水の製造方法及び電子部品の洗浄方法 |
| CN103130303B (zh) * | 2009-06-01 | 2015-03-11 | 奥加诺株式会社 | 燃料电池的水处理装置 |
| JP5647842B2 (ja) * | 2010-09-17 | 2015-01-07 | オルガノ株式会社 | 純水または超純水の製造装置及び製造方法 |
| JP5753758B2 (ja) * | 2010-10-22 | 2015-07-22 | アークレイ株式会社 | オキシダーゼを用いた測定方法 |
| CN102809637A (zh) * | 2011-06-02 | 2012-12-05 | 伯英孚水质科技(苏州)有限公司 | 一种手持式水质测量仪 |
| WO2014006742A1 (ja) * | 2012-07-06 | 2014-01-09 | 株式会社日立製作所 | 塩排水の処理装置及びその処理方法 |
| CN103018415B (zh) * | 2012-10-30 | 2015-08-26 | 广州市市政工程设计研究院 | 污水厂有毒废水在线检测装置及检测方法 |
| MX390149B (es) * | 2013-12-17 | 2025-03-20 | Univ Autonoma De Baja California | Metodo para determinacion del peroxido de hidrogeno en soluciones alcalinas en la microelectronica. |
| CA2939094C (en) * | 2014-02-19 | 2022-06-14 | Mallinckrodt Hospital Products IP Limited | Methods for compensating long term sensitivity drift of electrochemical gas sensors exposed to nitric oxide |
| JP2016161664A (ja) * | 2015-02-27 | 2016-09-05 | 株式会社リコー | 画像形成装置 |
| CN104865979A (zh) * | 2015-03-02 | 2015-08-26 | 华南理工大学 | 一种污水处理过程自适应广义预测控制方法及系统 |
| US20160266084A1 (en) * | 2015-03-09 | 2016-09-15 | Scott Russell Burge | Unified Sampling and Analytical System for Monitoring Volatile Chemicals in Ground Water, Soil-Gas and Indoor Air Quality with Sample Collection For Laboratory Analysis |
| WO2018017672A1 (en) | 2016-07-19 | 2018-01-25 | Chemtreat, Inc. | Methods and systems for detecting and quantifying peroxyacid and peroxide in fluids |
| JP2018025454A (ja) * | 2016-08-09 | 2018-02-15 | 栗田工業株式会社 | 過酸化水素分析装置及び過酸化水素分析方法 |
| TW201815691A (zh) * | 2016-10-21 | 2018-05-01 | 野村微科學股份有限公司 | 超純水製造系統及超純水製造方法 |
| KR102844724B1 (ko) * | 2018-08-13 | 2025-08-08 | 에보쿠아 워터 테크놀로지스 엘엘씨 | 물의 조성을 측정하기 위한 시스템 및 방법 |
-
2018
- 2018-01-31 JP JP2018014416A patent/JP6590964B2/ja active Active
- 2018-09-12 WO PCT/JP2018/033760 patent/WO2019150640A1/ja not_active Ceased
- 2018-09-12 CN CN201880088307.8A patent/CN111670362B/zh active Active
- 2018-09-12 US US16/966,631 patent/US11408868B2/en active Active
- 2018-09-12 SG SG11202007246SA patent/SG11202007246SA/en unknown
- 2018-09-12 KR KR1020207013127A patent/KR102360586B1/ko active Active
- 2018-11-20 TW TW107141145A patent/TWI772557B/zh active
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2005274386A (ja) * | 2004-03-25 | 2005-10-06 | Japan Organo Co Ltd | 過酸化水素分析装置及び過酸化水素分析方法 |
| US20070238188A1 (en) * | 2006-03-29 | 2007-10-11 | Carr Gregory E | Peroxide monitor |
| JP2012063303A (ja) * | 2010-09-17 | 2012-03-29 | Japan Organo Co Ltd | 過酸化水素濃度の測定装置及び測定方法 |
| JP2013108916A (ja) * | 2011-11-24 | 2013-06-06 | Nomura Micro Sci Co Ltd | 超純水中の微量過酸化水素の濃度測定方法 |
| JP2017000970A (ja) * | 2015-06-11 | 2017-01-05 | 野村マイクロ・サイエンス株式会社 | 超純水製造システム及び超純水製造方法 |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN115684532A (zh) * | 2022-12-30 | 2023-02-03 | 正大农业科学研究有限公司 | 水质监测方法、装置、系统、电子设备及存储介质 |
Also Published As
| Publication number | Publication date |
|---|---|
| SG11202007246SA (en) | 2020-08-28 |
| CN111670362B (zh) | 2022-04-05 |
| KR102360586B1 (ko) | 2022-02-09 |
| TWI772557B (zh) | 2022-08-01 |
| CN111670362A (zh) | 2020-09-15 |
| JP6590964B2 (ja) | 2019-10-16 |
| JP2019132677A (ja) | 2019-08-08 |
| US11408868B2 (en) | 2022-08-09 |
| KR20200069327A (ko) | 2020-06-16 |
| TW201934999A (zh) | 2019-09-01 |
| US20210033581A1 (en) | 2021-02-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6590964B2 (ja) | 過酸化水素濃度の測定システムおよび測定方法 | |
| JP5484278B2 (ja) | 過酸化水素濃度の測定装置及び測定方法 | |
| JP5647842B2 (ja) | 純水または超純水の製造装置及び製造方法 | |
| WO1991006848A1 (fr) | Procede de mesure de la quantite totale de substances organiques dans de l'eau ultrapure et systeme de traitement d'eau ultrapure utilisant ce procede | |
| CN103025409A (zh) | 用于测试中空纤维膜过滤器的装置和方法 | |
| JP7020960B2 (ja) | 過酸化水素濃度の測定システムおよび測定方法 | |
| CN112051198A (zh) | 一种多孔膜孔径的量化评估方法 | |
| CN110997118B (zh) | 测试过滤器的方法 | |
| US12138593B2 (en) | Method for preparing water quality profile, method for inspecting separation membrane module, and water treatment apparatus | |
| US20250018343A1 (en) | State diagnosis method for separation membrane element | |
| JP5663410B2 (ja) | 超純水製造方法及び装置 | |
| CN213091444U (zh) | 一种多孔膜孔径的量化评估装置 | |
| JP7195097B2 (ja) | 過酸化水素濃度分析装置および過酸化水素濃度分析方法 | |
| JP2005274386A (ja) | 過酸化水素分析装置及び過酸化水素分析方法 | |
| JP2018025454A (ja) | 過酸化水素分析装置及び過酸化水素分析方法 | |
| JP7252044B2 (ja) | 濃度分析装置 | |
| JP7333706B2 (ja) | 濃度分析装置 | |
| JP2012063302A (ja) | 超純水中の全有機炭素含有量の測定システム及び方法 | |
| JP2021194587A (ja) | 超純水製造装置の運転管理システム、超純水製造装置、および超純水製造装置の運転方法 | |
| JP2006297244A (ja) | イオンクロマトグラフィー装置用前処理カラム、その回生方法及びイオンクロマトグラフィー装置 | |
| JP2013202580A (ja) | 中空糸膜の透水性能の評価方法 | |
| Taligrot et al. | Investigating the impact of low-pressure reverse osmosis membrane aging and heterogeneity on performance: A study of virus and microplastic retention | |
| JP2000065710A (ja) | 液体中の溶存気体濃度の測定方法及び測定装置 | |
| KR20240142068A (ko) | 초순수 검사 시스템 및 초순수 검사 방법 | |
| CN120142424A (zh) | 一种比较不同尺寸纳米通道内静电作用对离子传输速率影响的方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 18904290 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 20207013127 Country of ref document: KR Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 18904290 Country of ref document: EP Kind code of ref document: A1 |