WO2019035489A1 - 口唇用マイクロニードルアレイ - Google Patents
口唇用マイクロニードルアレイ Download PDFInfo
- Publication number
- WO2019035489A1 WO2019035489A1 PCT/JP2018/030587 JP2018030587W WO2019035489A1 WO 2019035489 A1 WO2019035489 A1 WO 2019035489A1 JP 2018030587 W JP2018030587 W JP 2018030587W WO 2019035489 A1 WO2019035489 A1 WO 2019035489A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- microneedle array
- microneedle
- lip
- lips
- water
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M37/00—Other apparatus for introducing media into the body; Percutany, i.e. introducing medicines into the body by diffusion through the skin
- A61M37/0015—Other apparatus for introducing media into the body; Percutany, i.e. introducing medicines into the body by diffusion through the skin by using microneedles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
- A61K9/0021—Intradermal administration, e.g. through microneedle arrays, needleless injectors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0053—Mouth and digestive tract, i.e. intraoral and peroral administration
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M37/00—Other apparatus for introducing media into the body; Percutany, i.e. introducing medicines into the body by diffusion through the skin
- A61M37/0015—Other apparatus for introducing media into the body; Percutany, i.e. introducing medicines into the body by diffusion through the skin by using microneedles
- A61M2037/0023—Drug applicators using microneedles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M37/00—Other apparatus for introducing media into the body; Percutany, i.e. introducing medicines into the body by diffusion through the skin
- A61M37/0015—Other apparatus for introducing media into the body; Percutany, i.e. introducing medicines into the body by diffusion through the skin by using microneedles
- A61M2037/0061—Methods for using microneedles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/02—General characteristics of the apparatus characterised by a particular materials
- A61M2205/0244—Micromachined materials, e.g. made from silicon wafers, microelectromechanical systems [MEMS] or comprising nanotechnology
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2210/00—Anatomical parts of the body
- A61M2210/06—Head
- A61M2210/0625—Mouth
Definitions
- the present invention relates to the technical field of topically applied microneedles to the lips, and more particularly to lip lightening and / or lip enrichment techniques.
- the microneedle preparation has high percutaneous absorbability, and development of cosmetics and medicines has been attempted.
- the application site of the microneedle preparation is the skin epidermis, but, for example, a microneedle patch as a vaccination by transoral buccal administration is known (Patent Document 1).
- the microneedle patch is designed to penetrate the outer layer of the inner buccal mucosa.
- a microneedle including a microneedle-like biocompatible matrix and porous particles on the surface or in the inside Patent Document 2.
- the microneedles can currently be used for the lower lip or the eight-fold crease, and are now shaped and sized to be able to adhere to the application site, such as the peroral area.
- the microneedle preparation can extend the epidermis to reduce the protective property of the epidermis and can administer cosmetics and medicines without penetrating the projection into the stratum corneum (Patent Document 3).
- the administration device further comprises a microprojection at the tip of the projection.
- An object of the present invention is to provide a composition that achieves lip lightening and / or moistening more effectively and simply.
- microneedles for intrabuccal administration or for the current or lower lip, but the lip is not keratinous and the outer mucosa covers the intradermal tissue. Insertion of microneedles into the lips to break the mucous membrane and deliver valuables to the skin should be avoided as it stimulates the skin and causes inflammation.
- the present inventors have made the microneedle itself and the substrate for standing it up of metal, a relatively soft material having a lower elastic modulus than silica, and By bringing into close contact and further finely adjusting the height so as to stay within the outer mucous membrane without breaking the mucous membrane of the lip, the inventors have arrived at the invention of a microneedle array suitable for the lip.
- the present invention is as follows. [1] A microneedle array applied locally to the lips for the purpose of lighting and / or enriching the lips, wherein the microneedle array contains a water-soluble polymer, and the height of the microneedles is 50 ⁇ m to 300 ⁇ m The microneedle array for the lips. [2] The microneedle array for lips according to [1], wherein the tip of the microneedle is a circle having a diameter of 5 to 150 ⁇ m or a flat surface having the same area. [3] The microneedle array for lips according to [1] or [2], wherein the density of the microneedles is 50 to 2000 needles / cm 2 .
- microneedle array for lips according to any one of [1] to [3], wherein the thickness of the substrate portion of the microneedle is 3 to 200 ⁇ m.
- the microneedle array is backed by a protective adhesive tape, the substrate of the adhesive tape is water-permeable, and the adhesive is partially coated on any of [1] to [5] Lip microneedle array as described.
- microneedle array for lips according to any one of [1] to [6], which contains one or more of a lightening component, a moisturizing component and an anti-inflammatory component in a microneedle portion.
- the topical lightening composition or the moisturizing composition exhibits high efficacy not previously possible.
- Specific strategies useful for the compositions and methods according to the present invention have resulted from the application of microneedles of water-soluble material to the lips.
- the microneedle is a known drug percutaneous absorption means.
- there have been no reports prior to the inventor of the present invention that predicted application of the microneedles to the lips to be extremely effective in lightening and enriching, and no reports have been tried. The reason is presumed to be as follows.
- the microneedle material there is mainly a very hard material such as stainless steel, silica, titanium, engineering plastic, etc., and the microneedles made of this material are soft and flexible. It may be because there is a sense of discomfort in applying to the lips.
- the microneedle array of the present invention is applied to the lips, since the lips do not have a stratum corneum, permeation of valuables is quicker than general skin, and therefore can be effective even in a short time application.
- microneedle array of the present invention is the nature of the material (being water soluble) and the needle shape.
- the needle-shaped lips of the microneedle array are free of keratin and the outer mucosa covers the intradermal tissue.
- the lip is a prominent organ, and inserting the microneedle to break the mucous membrane and deliver valuables into the skin should be avoided as it stimulates the skin and causes inflammation.
- the shape and height of the microneedles should be tightly controlled and designed so as not to be inserted into the skin. Specifically, it is desirable that the tip of the needle is a circle having a diameter of 5 ⁇ m or more or a flat surface having the same area. Tip areas less than 5 ⁇ m have the risk of piercing the lip and being inserted into the skin during application.
- the needle shape is preferably not a rod but a truncated cone or a cone.
- a truncated cone or a conedite shape when the needle portion is applied to the lips, the contact area with the outer mucous membrane is large and squeezes the lip, so that the extramucosal membrane is pulled and becomes thin, whereby the intradermal of valuables Delivery is advantageous.
- the contact area with the outer lip mucosa is small, which is disadvantageous for value delivery.
- the needle tip diameter is desirably 150 ⁇ m or less. When the tip diameter exceeds 150 ⁇ m, the effect of pulling the extramucosal mucosa is reduced and the function as a microneedle is lost.
- the needle height is preferably 50 ⁇ m or more and 300 ⁇ m or less, and more preferably 100 ⁇ m or more and 250 ⁇ m or less. If it is less than 50 ⁇ m, the degree of pressure on the lips is small, which is disadvantageous for the delivery of valuables. If it exceeds 300 ⁇ m, there is a risk that the needle may break the outer mucosa at the time of application. Needle density is preferably from 50 to 2,000 / cm 2, more preferably 100 to 1200 / cm 2. If the number is 50 / cm 2 or less, the content of valuables is low, and the delivery amount of valuables is insufficient. If it is 2000 bottles / cm 2 or more, it will be difficult to insert into the mucous membrane of soft lips.
- the thickness of the substrate portion is preferably 3 to 200 ⁇ m, and more preferably 5 to 50 ⁇ m. If it is 3 ⁇ m or less, the film formation toughness is weak and it is difficult to support the needle exactly. If it is 200 ⁇ m or more, the film becomes hard and it is difficult to follow the shape of the soft lip and it is difficult to apply. In addition, when water is externally applied to dissolve the whole including the substrate portion, a large amount of water or a long time is required.
- microneedle array It is important that the material of microneedle array is water soluble and low in hardness. When a microneedle array uniformly containing valuables using such a material is produced by a conventional method, valuables will be contained not only in the microneedle portion but also in the substrate portion. Valuables may be contained only in the microneedle portion according to the two-step filling method. When this microneedle array is applied to the lips, the microneedle portion squeezes the lips and pulls the outer lip mucosa to thin, thereby promoting intra-labial delivery of the contained value. When the microneedle array is made of a flexible material, the substrate part adheres to the lip following the bending of the lip and the substrate part is also compressed, and the value present there is also relatively smaller than the microneedle part However, it is delivered into the lips.
- Materials for microneedle arrays include hyaluronic acid and its derivatives (eg sodium salt, polyethylene oxide graft hyaluronic acid, hyaluronic acid propylene glycol ester, carboxymethylated sodium hyaluronate, acetyl hyaluronate sodium), collagen, proteoglycan, hydroxypropyl
- Water-soluble polymers such as cellulose, chondroitin sulfate, carboxymethyl cellulose and the like can be mentioned, and hyaluronic acid or a derivative thereof is preferable. The characteristics of these water soluble polymers are swellable when absorbing small amounts of water.
- the water-soluble polymer dissolved in the mucous membrane of the lip through the microneedle absorbs water and swells, resulting in lifting of the longitudinal wrinkles of the lip and moistening of the lip.
- Hyaluronic acid is a type of glycosaminoglycan (mucopolysaccharide), and has a structure in which disaccharide units of N-acetylglucosamine and glucuronic acid are linked.
- examples of hyaluronic acid include hyaluronic acid derived from organisms isolated from chicken crown, umbilical cord and the like, and hyaluronic acid derived from culture mass-produced by lactic acid bacteria, streptococcus and the like. Since hyaluronic acid of biological origin can not completely remove the collagen possessed by the organism from which it is derived, it may have a negative effect on the remaining collagen, and therefore, hyaluronic acid derived from a culture not containing collagen is preferred. Therefore, the hyaluronic acid preferably contains 50% by weight or more of hyaluronic acid derived from culture.
- the microneedle array molded from these polymer substances becomes harder as the weight average molecular weight decreases
- the weight average molecular weight is preferably 5,000 to 2,000,000.
- the microneedle array may be formed from a mixture of low molecular weight polymer substances having an average molecular weight of 50,000 or less.
- the weight average molecular weight of the high molecular weight polymer substance may be 50,000 or more, preferably 2,000,000 or less.
- the weight average molecular weight of the low molecular weight polymer substance may be 50,000 or less, preferably 1,000 or more.
- the weight average molecular weight is a value measured by gel permeation chromatography (GPC).
- the ratio in mixing the high molecular weight polymer substance and the low molecular weight polymer substance varies depending on the type and weight average molecular weight of each polymer substance, and accordingly, it may be appropriately determined so as to obtain preferable mechanical strength and hardness. Although it is preferable, it is generally preferable that the content is 1% by weight or more of the high molecular weight high molecular weight substance and 99% by weight or less of the low molecular weight high molecular weight substance.
- microneedle array of the present invention although the above-described materials and needle shapes are important and novel, known values can be used as the values to be impregnated into the microneedles.
- melanin synthetase such as hydroquinone, kojic acid, licorice and / or derivatives thereof, ascorbic acid / ethyl ascorbic acid, ascorbic acid derivatives such as ascorbic acid glucoside, arbutin (arbutin), etc.
- a substance effective for inhibition may be added.
- antioxidants such as astaxanthin, fullerene, coenzyme Q10, blood flow promoters such as vitamin E, retinol and retinal, components promoting turnover such as adenosine, components such as niacinamide to improve metabolism, lip roughening prevention Dipotassium glycyrrhizinate and the like, and cosmetically used fragrances may be added.
- moisturizing ingredients such as ceramide, vitamin A, E, and urea, and anti-inflammatory ingredients such as dipotassium glycyrrhizinate may be added. The amount added varies, depending on the type and nature of the substance and the degree of effect desired.
- the lightening agent, and the other additives are generally about 0.001 wt% to about 20 wt%, more preferably about 0.01 wt% to about 5 wt%, most preferably about 0.001 wt% to the total weight of the composition. It is present in an amount of from 0.1 wt% to about 2.5 wt%.
- a composition in which a lipstick component is added to the microneedles as valuables after or simultaneously with the lightening may be used.
- the amount added is in accordance with the lightening agent.
- pigments based on natural pigments such as safflower and cochineal, and pigments that are synthetic colorants (colorants) can be suitably used.
- colorants colorants
- hyaluronic acid and derivatives thereof For the purpose of moisturizing the lip, hyaluronic acid and derivatives thereof, collagen, chondroitin sulfate, proteoglycan, and high-molecular substances which swell in the skin such as placenta are used as valuables.
- hyaluronic acid and derivatives thereof are suitable as a material for microneedles, so that it is possible to achieve the purpose by microneedle molding with hyaluronic acid alone when the purpose is to enrich it. That is, in this case, it can be said that hyaluronic acid doubles as a material of the microneedle array and a valuable material.
- the method for producing the microneedle array of the present invention is not particularly limited, and may be produced by any conventionally known method.
- the above-mentioned high molecular weight hyaluronic acid and low in the mold in which the shape of the microneedle is drilled examples of the method include casting an aqueous solution of molecular weight hyaluronic acid and, if necessary, valuables, drying and peeling.
- the valuables are contained only in the microneedle portion, it is possible to use a manufacturing method (two-step filling manufacturing method) in which the valuables-containing raw material and the valuables-free raw material are divided into two steps and filled. After peeling, it is cut into the shape of a lip and used by backing with a protective adhesive tape.
- the microneedle array of the present invention is applied to the lips, but since the lips do not have a stratum corneum, permeation of valuables is faster than general skin and therefore can be effective even for a short time application.
- the topical composition (microneedle array) according to the present invention has various skin conditions such as age spots, dark spots, hyperpigmentation, post-inflammatory hyperpigmentation (eg, post-hypopigmentation), discoloration. It can be dealt with.
- the microneedle array of the present invention optionally comprises one or more of the following components: anesthetics, antiallergenic agents, antifungal agents, antiinflammatory agents, antiseptics, chelating agents, coloring agents, emollients, Exfollients, film formers, fragrances, moisturizers, insect repellents, lubricants, moisturizers, pharmaceuticals, preservatives, skin protectants, skin penetration enhancers, stabilizers, It may contain surfactants, thickeners, viscosity modifiers, or vitamins.
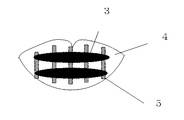
- FIG. 1 is a cross-sectional view showing an example of a method of manufacturing a microneedle array according to the present invention.
- a concave part 11 for forming a microneedle of a conyde type is formed by transferring a microneedle pattern of a conede type by electroforming after forming a microneedle pattern of a coniede type by a lithography method in which photosensitive resin is irradiated with light. Is the formed template.
- the microneedle-forming concave portions 11 are of a coned type having a root diameter of 0.6 mm, a tip diameter of 0.02 mm, and a depth of 0.2 mm, and are arranged in a grid at intervals of 0.6 mm.
- microneedle array of the present invention was obtained by setting in the central part of.
- the thickness of the base of the microneedle array was 60 ⁇ m.
- the microneedle array of the present invention was pressed together with the adhesive tape on the right side of the lips of two volunteers and fixed by the adhesive part, and peeled off after 30 minutes. Immediately after, the lip was lightly washed with water to wash away the red pigment adhering to the surface. When the lips were observed in that state, the right side was clearly more red on both sides and a plump feeling was observed as compared with the left side.
- Example 2 An oval microneedle array having a major axis of 5 cm and a minor axis of 0.8 cm was produced from the mold having the same composition as in Example 1 except that 2 parts by weight of arbutin was added. On the back of the microneedle array, an adhesive tape in which an adhesive was applied on one side of a non-woven fabric having a basis weight of 50 g / m 2 by pattern coating (longitudinal streak 2 mm width, interval 10 mm) was backed to obtain a microneedle array of the present invention. Refer to FIG. 2 for the specific aspect.
- microneedle array of the present invention Immediately after the microneedle array of the present invention is pressed together with the adhesive tape against the upper and lower lip of two volunteers and fixed with the adhesive tape, water is supplied from the non-woven fabric side with gauze, then the gauze is removed and the microneedle array attached part is massaged And peeled off after 15 minutes. After that, when observed in the same manner as in Example 1, red and plump feeling were clearly observed in the lip. When this sticking operation was repeated once a day for 7 days, the transparency of the sticking part was observed.
- Example 3 PEG-grafted hyaluronic acid is used instead of hyaluronic acid in Example 1, and 2% by weight of oxalic acid, 1% by weight of vitamin C ethyl, 0.1% by weight of dipotassium glycyrrhizinate, 0.1% by weight of red 218 % was added to 100 g of an aqueous solution to prepare a microneedle array as in Example 1.
- the effects of the product of the present invention were also obtained as in Example 1.
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Animal Behavior & Ethology (AREA)
- Dermatology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Anesthesiology (AREA)
- Medical Informatics (AREA)
- Hematology (AREA)
- Heart & Thoracic Surgery (AREA)
- Biomedical Technology (AREA)
- Chemical & Material Sciences (AREA)
- Epidemiology (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Physiology (AREA)
- Nutrition Science (AREA)
- Media Introduction/Drainage Providing Device (AREA)
- Cosmetics (AREA)
- Materials For Medical Uses (AREA)
- Medicinal Preparation (AREA)
Priority Applications (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AU2018317057A AU2018317057B2 (en) | 2017-08-17 | 2018-08-17 | Microneedle array for lips |
| EP18845645.3A EP3669929A4 (en) | 2017-08-17 | 2018-08-17 | MICRONADELFIELD FOR LIPS |
| KR1020197036709A KR102333037B1 (ko) | 2017-08-17 | 2018-08-17 | 입술용 마이크로니들 어레이 |
| CN201880040881.6A CN110769891A (zh) | 2017-08-17 | 2018-08-17 | 口唇用微针阵列 |
| US16/623,691 US20210106800A1 (en) | 2017-08-17 | 2018-08-17 | Microneedle array for lips |
| CA3067694A CA3067694C (en) | 2017-08-17 | 2018-08-17 | Microneedle array for lips |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2017157681 | 2017-08-17 | ||
| JP2017-157681 | 2017-08-17 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2019035489A1 true WO2019035489A1 (ja) | 2019-02-21 |
Family
ID=65362291
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2018/030587 Ceased WO2019035489A1 (ja) | 2017-08-17 | 2018-08-17 | 口唇用マイクロニードルアレイ |
Country Status (8)
| Country | Link |
|---|---|
| US (1) | US20210106800A1 (enExample) |
| EP (1) | EP3669929A4 (enExample) |
| JP (1) | JP7526976B2 (enExample) |
| KR (1) | KR102333037B1 (enExample) |
| CN (1) | CN110769891A (enExample) |
| AU (1) | AU2018317057B2 (enExample) |
| CA (1) | CA3067694C (enExample) |
| WO (1) | WO2019035489A1 (enExample) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN111803789A (zh) * | 2020-06-28 | 2020-10-23 | 王瑾 | 一种无创伤美唇方法 |
| WO2021125067A1 (en) * | 2019-12-16 | 2021-06-24 | L'oreal | Cosmetic process using microneedle sheet |
| US20220233833A1 (en) * | 2020-04-03 | 2022-07-28 | Cosmed Pharmaceutical Co., Ltd. | Fast-dissolving microneedle |
Families Citing this family (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB2586474A (en) * | 2019-08-20 | 2021-02-24 | Innoture Ip Ltd | Method of manufacturing microstructures |
| CN111408032A (zh) * | 2020-03-05 | 2020-07-14 | 优微(珠海)生物科技有限公司 | 一种透气透水微针贴片及其使用方法 |
| JP2021143146A (ja) * | 2020-03-11 | 2021-09-24 | コスメディ製薬株式会社 | 天然保湿因子含有貼付型化粧料 |
| CN115734798A (zh) | 2020-04-28 | 2023-03-03 | 提克纳有限责任公司 | 微针组件 |
| DE102021118997A1 (de) | 2021-07-22 | 2023-01-26 | Lts Lohmann Therapie-Systeme Ag. | Mikronadelarray mit Antiseptika |
| JP7235352B1 (ja) * | 2021-09-29 | 2023-03-08 | ラファス カンパニー リミテッド | 唇に適用するマイクロニードルパッチ製造用組成物およびこの組成物で製造されたマイクロニードルパッチ |
| US20230130124A1 (en) * | 2021-10-25 | 2023-04-27 | Cosmed Pharmaceutical Co., Ltd. | Cosmetic microneedle |
| KR102685131B1 (ko) * | 2023-10-25 | 2024-07-15 | 주식회사 스몰랩 | 안정성이 증진된 리라글루타이드-함유 마이크로니들용 조성물 및 이의 이용 |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2007130417A (ja) | 2005-11-08 | 2007-05-31 | Nano Device & System Research Inc | 経皮投与装置 |
| JP2008513132A (ja) * | 2004-09-16 | 2008-05-01 | ジュヴァ・メディカル・インコーポレーテッド | 組織増強装置 |
| JP2013075165A (ja) * | 2011-09-12 | 2013-04-25 | Kosumedei Seiyaku Kk | マイクロニードル迅速溶解法 |
| JP2015515474A (ja) | 2012-04-03 | 2015-05-28 | セラジェクト, インコーポレイテッド | ワクチンの経頬送達のための溶解性微小針アレイ |
| JP2015522342A (ja) * | 2012-06-29 | 2015-08-06 | イーエルシー マネージメント エルエルシー | 1つ以上の化粧品成分を含むマイクロニードル |
| JP2016087474A (ja) | 2014-11-10 | 2016-05-23 | スモール ラボ カンパニー リミテッドSMALL LAB Co., Ltd | マイクロニードル及びマイクロニードルパッチ |
Family Cites Families (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6256533B1 (en) * | 1999-06-09 | 2001-07-03 | The Procter & Gamble Company | Apparatus and method for using an intracutaneous microneedle array |
| US20070154494A1 (en) * | 2003-02-13 | 2007-07-05 | Becton Dickinson And Company | Anthrax vaccines and delivery methods |
| JP2006087474A (ja) * | 2004-09-21 | 2006-04-06 | Olympus Corp | 処置具システム及び内視鏡処置システム |
| US20060253078A1 (en) * | 2005-04-25 | 2006-11-09 | Wu Jeffrey M | Method of treating skin disorders with stratum corneum piercing device |
| WO2008020632A1 (en) | 2006-08-18 | 2008-02-21 | Toppan Printing Co., Ltd. | Microneedle and microneedle patch |
| WO2008053481A1 (en) * | 2006-11-01 | 2008-05-08 | Svip 6 Llc | Microneedle arrays |
| JP2009254756A (ja) * | 2008-04-14 | 2009-11-05 | Kosumedei Seiyaku Kk | マイクロニードルアレイ |
| JP5663792B2 (ja) * | 2009-08-07 | 2015-02-04 | 株式会社 メドレックス | 剣山型マイクロニードルのアプリケータデバイス |
| US20150223988A1 (en) * | 2010-03-04 | 2015-08-13 | Donald Spector | Bandage with a compressed layer that expands upon contact with liquid |
| CN103619384A (zh) * | 2011-01-18 | 2014-03-05 | 麻省理工学院 | 装置和其用途 |
| EP3574846A1 (en) * | 2012-06-15 | 2019-12-04 | University Of Washington Through Its Center For Commercialization | Microstructure-based wound closure devices |
| WO2015073919A1 (en) * | 2013-11-14 | 2015-05-21 | University Medical Pharmaceuticals Corporation | Microneedles for therapeutic agent delivery with improved mechanical properties |
| WO2015147040A1 (ja) * | 2014-03-26 | 2015-10-01 | コスメディ製薬株式会社 | 角質層に留まるマイクロニードル |
| JP2016189844A (ja) * | 2015-03-31 | 2016-11-10 | 日本写真印刷株式会社 | マイクロニードルパッチ |
| WO2017056893A1 (ja) | 2015-09-30 | 2017-04-06 | 富士フイルム株式会社 | 集合モールドの作製方法、パターンシートの製造方法、電鋳金型の作製方法、及び電鋳金型を用いた第2モールドの作製方法 |
-
2018
- 2018-08-17 US US16/623,691 patent/US20210106800A1/en not_active Abandoned
- 2018-08-17 JP JP2018153751A patent/JP7526976B2/ja active Active
- 2018-08-17 KR KR1020197036709A patent/KR102333037B1/ko active Active
- 2018-08-17 EP EP18845645.3A patent/EP3669929A4/en not_active Withdrawn
- 2018-08-17 CN CN201880040881.6A patent/CN110769891A/zh active Pending
- 2018-08-17 CA CA3067694A patent/CA3067694C/en active Active
- 2018-08-17 AU AU2018317057A patent/AU2018317057B2/en active Active
- 2018-08-17 WO PCT/JP2018/030587 patent/WO2019035489A1/ja not_active Ceased
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2008513132A (ja) * | 2004-09-16 | 2008-05-01 | ジュヴァ・メディカル・インコーポレーテッド | 組織増強装置 |
| JP2007130417A (ja) | 2005-11-08 | 2007-05-31 | Nano Device & System Research Inc | 経皮投与装置 |
| JP2013075165A (ja) * | 2011-09-12 | 2013-04-25 | Kosumedei Seiyaku Kk | マイクロニードル迅速溶解法 |
| JP2015515474A (ja) | 2012-04-03 | 2015-05-28 | セラジェクト, インコーポレイテッド | ワクチンの経頬送達のための溶解性微小針アレイ |
| JP2015522342A (ja) * | 2012-06-29 | 2015-08-06 | イーエルシー マネージメント エルエルシー | 1つ以上の化粧品成分を含むマイクロニードル |
| JP2016087474A (ja) | 2014-11-10 | 2016-05-23 | スモール ラボ カンパニー リミテッドSMALL LAB Co., Ltd | マイクロニードル及びマイクロニードルパッチ |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP3669929A4 |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2021125067A1 (en) * | 2019-12-16 | 2021-06-24 | L'oreal | Cosmetic process using microneedle sheet |
| US20220233833A1 (en) * | 2020-04-03 | 2022-07-28 | Cosmed Pharmaceutical Co., Ltd. | Fast-dissolving microneedle |
| CN111803789A (zh) * | 2020-06-28 | 2020-10-23 | 王瑾 | 一种无创伤美唇方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| CN110769891A (zh) | 2020-02-07 |
| EP3669929A4 (en) | 2021-05-12 |
| US20210106800A1 (en) | 2021-04-15 |
| AU2018317057A1 (en) | 2020-01-16 |
| CA3067694C (en) | 2023-01-03 |
| JP2019034151A (ja) | 2019-03-07 |
| EP3669929A1 (en) | 2020-06-24 |
| JP7526976B2 (ja) | 2024-08-02 |
| KR20200007016A (ko) | 2020-01-21 |
| AU2018317057B2 (en) | 2021-03-25 |
| CA3067694A1 (en) | 2019-02-21 |
| KR102333037B1 (ko) | 2021-12-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7526976B2 (ja) | 口唇用マイクロニードルアレイ | |
| JP4521492B2 (ja) | マイクロニードルアレイ及びその製造方法 | |
| US8167852B2 (en) | Microneedle device and method for producing the same | |
| JP5267910B2 (ja) | マイクロニードルアレイ | |
| KR101813735B1 (ko) | 프로테오글리칸을 함유하는 마이크로니들 어레이 | |
| BR112015005073B1 (pt) | Aplicador configurado para a administração de uma composição de melhoria de pele para uma pele e/ou subcútis de um sujeito, uso de um, microagulha e método para manufaturar uma microagulha | |
| TW201210646A (en) | Device having array provided with fine protrusions | |
| CN104826228B (zh) | 用于皮肤修复与保养的生物贴 | |
| US20230329999A1 (en) | Paste-type cosmetic containing natural moisturizing factor | |
| JP2009254756A (ja) | マイクロニードルアレイ | |
| CN113197814A (zh) | 一种玻尿酸微针贴片制备方法 | |
| JP2021509344A (ja) | 皮膚増強のための高装填マイクロニードル及び組成物 | |
| CN104921961B (zh) | 一种多效修复的可降解生物微针贴 | |
| JP7626382B2 (ja) | マイクロニードルによる顔全体の色調を明るくする美容方法 | |
| CN109044907A (zh) | 一种无痛透皮美容微针贴及其制备方法 | |
| JP7429458B2 (ja) | 抗シワ組成物、抗シワマイクロニードルパッチおよびその製造方法 | |
| US20230398040A1 (en) | A multi-layered sheet mask | |
| CN118717641B (zh) | 一种可溶性凝胶微针及其制备方法和应用 | |
| TWM643895U (zh) | 微針貼片 | |
| TW202442265A (zh) | 微針貼片及其製造方法 | |
| CN115715755A (zh) | 一种祛除黑眼圈的可溶性微针及其制备方法和其应用 | |
| KR20230067127A (ko) | 화장품용 마이크로니들 | |
| JP2022043919A (ja) | 化粧品用マイクロニードル | |
| BR102019011479A2 (pt) | Composição cosmética para emplastros cosméticos e emplastos cosméticos comprendendo a dita composição cosmética | |
| HK1175419A (en) | Proteoglycan-containing microneedle array |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 18845645 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 20197036709 Country of ref document: KR Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 3067694 Country of ref document: CA |
|
| ENP | Entry into the national phase |
Ref document number: 2018317057 Country of ref document: AU Date of ref document: 20180817 Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2018845645 Country of ref document: EP Effective date: 20200317 |