WO2018084167A1 - 接着状態の細胞培養物の改変方法 - Google Patents
接着状態の細胞培養物の改変方法 Download PDFInfo
- Publication number
- WO2018084167A1 WO2018084167A1 PCT/JP2017/039476 JP2017039476W WO2018084167A1 WO 2018084167 A1 WO2018084167 A1 WO 2018084167A1 JP 2017039476 W JP2017039476 W JP 2017039476W WO 2018084167 A1 WO2018084167 A1 WO 2018084167A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- cell culture
- sheet
- cells
- hours
- culture
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/36—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix
- A61L27/38—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix containing added animal cells
- A61L27/3804—Materials for grafts or prostheses or for coating grafts or prostheses containing ingredients of undetermined constitution or reaction products thereof, e.g. transplant tissue, natural bone, extracellular matrix containing added animal cells characterised by specific cells or progenitors thereof, e.g. fibroblasts, connective tissue cells, kidney cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0652—Cells of skeletal and connective tissues; Mesenchyme
- C12N5/0658—Skeletal muscle cells, e.g. myocytes, myotubes, myoblasts
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N1/00—Microorganisms, e.g. protozoa; Compositions thereof; Processes of propagating, maintaining or preserving microorganisms or compositions thereof; Processes of preparing or isolating a composition containing a microorganism; Culture media therefor
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/0081—Purging biological preparations of unwanted cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/0081—Purging biological preparations of unwanted cells
- C12N5/0087—Purging against subsets of blood cells, e.g. purging alloreactive T cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0652—Cells of skeletal and connective tissues; Mesenchyme
- C12N5/0656—Adult fibroblasts
Definitions
- the present invention relates to a method for modifying an adherent cell culture, a method for producing a sheet-shaped cell culture including such modification, and the like.
- Non-Patent Documents 1 and 2 fetal cardiomyocytes, skeletal myoblasts, mesenchymal stem cells, cardiac stem cells, ES cells for the repair of myocardial tissue damaged by ischemic heart disease such as angina pectoris and myocardial infarction or dilated cardiomyopathy Etc. have been tried (Non-Patent Documents 1 and 2).
- Patent Document 1 Non-Patent Document 2
- sheet cell culture For the application of sheet cell culture to the treatment, use of cultured epidermis sheet for skin damage caused by burns, use of corneal epithelial sheet cell culture for corneal injury, oral mucosa sheet for endoscopic resection of esophageal cancer Studies on the use of cell cultures are underway, and some of them are in the clinical application stage.
- An object of the present invention is to provide a method for modifying an adhesive cell culture so that the content ratio of the cells constituting the adhesive cell culture is changed after the adhesive cell culture is formed.
- a sheet-like cell culture used for regenerative medicine and the like cells other than the target cell are considered to be contaminants.
- a cell culture for transplantation is preferably manufactured using autologous cells prepared by collecting a tissue piece from a recipient in order to reduce rejection, and so on. In many cases, it cannot be completely removed.
- there is no known method for changing the purity of a cell after manufacturing a sheet-shaped cell culture and thus, until now, a sheet-shaped cell using autologous cells adjusted to have the highest possible purity. By producing a cell culture, the purity of the target cells was increased.
- HBSS Hanks balanced salt solution
- the present invention relates to the following: ⁇ 1> A method for modifying an adherent cell culture, comprising immersing an adherent cell culture containing at least two types of cells in a hypotrophic isotonic solution, whereby The said method characterized by changing the content rate of the cell type which comprises a cell culture.
- the modification is to reduce the content of fibroblasts.
- ⁇ 5> The method according to ⁇ 4>, wherein the sheet-shaped cell culture is peeled from the culture substrate.
- ⁇ 6> The method according to ⁇ 5>, wherein the sheet-shaped cell culture is contracted upon peeling.
- ⁇ 7> The method according to ⁇ 5> or ⁇ 6>, wherein the sheet-shaped cell culture has an area of 6 cm 2 or more after peeling.
- ⁇ 8> The method according to ⁇ 4> to ⁇ 7>, wherein the sheet-shaped cell culture is a laminate of a plurality of single-layer sheet-shaped cell cultures.
- ⁇ 9> The method according to ⁇ 1> to ⁇ 8>, wherein the hypotrophic isotonic solution is Hanks balanced salt solution.
- ⁇ 10> The method according to ⁇ 1> to ⁇ 9>, wherein the immersion is performed for 24 to 150 hours.
- ⁇ 12> (a) seeding a cell population containing two or more types of cells on a culture substrate at a density capable of forming a sheet-like cell culture without substantially growing; (B) sheet-shaped culture of the seeded cell population to form a sheet-shaped cell culture, and (c) a sheet-shaped culture comprising immersing the formed sheet-shaped cell culture in a hypotrophic isotonic solution A method for producing a cell culture.
- ⁇ 13> The method according to ⁇ 12>, comprising peeling the formed sheet-shaped cell culture (c ′) before the step (c). ⁇ 14> After step (c) (C ′) The method according to ⁇ 12>, comprising peeling off the formed sheet-shaped cell culture. ⁇ 15> The method according to ⁇ 13> or ⁇ 14>, wherein in the step (c), the sheet-like cell culture shrinks upon peeling. ⁇ 16> The method according to ⁇ 13> to ⁇ 15>, wherein the peeled sheet-shaped cell culture has an area of 6 cm 2 or more. ⁇ 17> The method according to ⁇ 13> to ⁇ 16>, further comprising (c-2) laminating the peeled sheet-shaped cell culture after (c).
- ⁇ 18> (a) seeding a cell population containing a target cell on a culture substrate at a density capable of forming a sheet-like cell culture without substantially proliferating; (B) sheet-cultured the seeded cell population to form a sheet-like cell culture; (C) A method for producing a sheet-shaped cell culture, comprising peeling the formed sheet-shaped cell culture, and (d) immersing the peeled sheet-shaped cell culture in a hypotrophic isotonic solution.
- the present invention it is possible to improve the quality by further modifying the adherent cell culture after forming the adherent cell culture, for example, by forming into a sheet form. For this reason, it becomes possible to further improve the quality of the sheet-like cell culture produced by the conventional production method, and even if it is a sheet-like cell culture that should have been insufficient in the past, it can be prepared. Since it becomes possible to improve to a sufficient quality later, it is possible to eliminate waste of time and materials and reduce the burden on the recipient. Further, the modification means itself is very simple and can be used for a variety of cell cultures in various adhesive states.
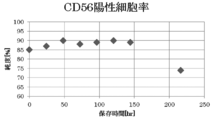
- FIG. 1 shows changes in the purity of skeletal myoblasts every 24 hours when a sheet-shaped cell culture containing human skeletal myoblasts and human fibroblasts is immersed in HBSS (+) under refrigerated conditions. It is a graph to show.
- CD56 positive cells mean skeletal myoblasts. The purity started to increase from the start of the immersion, and the purity reached about 90% in about 48 hours. Thereafter, the purity could be maintained until about 6 days after the start of the immersion.
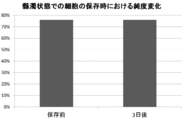
- FIG. 2 is a graph showing changes in the purity of skeletal myoblasts when HBSS (+) suspensions of human skeletal myoblasts and human fibroblasts are stored under refrigerated conditions. Three days after the start of storage, there was no change in the purity of skeletal myoblasts.
- modifying the adherent cell culture means changing the structure, function, characteristics, etc. of the adherent cell culture as compared to before treatment.
- Modification may include changing in a bad direction, but preferably in a good direction, ie more suitable for the intended use of the sheet cell culture.
- an “adherent cell culture” refers to a cell culture formed by cells adhering to other cells or a substrate.
- the cells may be linked to each other directly (including those via cell elements such as adhesion molecules) and / or via intervening substances.
- the intervening substance is not particularly limited as long as it is a substance that can connect cells at least physically (mechanically), and examples thereof include an extracellular matrix.
- the intervening substance is preferably derived from cells, in particular, derived from the cells constituting the cell culture.
- the cells are at least physically (mechanically) connected, but may be further functionally, for example, chemically or electrically connected.
- Adherent cell cultures include cell cultures that are adhered to the substrate, cell cultures that are adhered to each other while released from the substrate, and cells that are adhered to the substrate. Including cell cultures of the condition.
- Examples of the adherent cell culture include, but are not limited to, a sheet-like cell culture, a cell aggregate, an embryoid body, and a spheroid, and preferably a sheet-like cell culture.
- the adherent cell culture comprises a sheet-like cell culture adhered to a substrate.
- the adherent cell culture includes, but is not limited to, a cell culture that has been released from the substrate, for example, a sheet-like cell culture that has been detached from the substrate.
- the “sheet-shaped cell culture” refers to a cell-shaped cell connected to each other.
- the cells may be linked to each other directly (including those via cell elements such as adhesion molecules) and / or via intervening substances.
- the intervening substance is not particularly limited as long as it is a substance that can connect cells at least physically (mechanically), and examples thereof include an extracellular matrix.
- the intervening substance is preferably derived from cells, in particular, derived from the cells constituting the cell culture.
- the cells are at least physically (mechanically) connected, but may be further functionally, for example, chemically or electrically connected.
- the sheet-shaped cell culture is composed of one cell layer (single layer) or composed of two or more cell layers (laminated (multilayer) body, for example, two layers, three layers, 4 layers, 5 layers, 6 layers, etc.).
- the sheet-shaped cell culture preferably does not contain a scaffold (support). Scaffolds may be used in the art to attach cells on and / or within its surface and maintain the physical integrity of sheet-like cell cultures, for example, polyvinylidene difluoride ( PVDF) membranes and the like are known, but the sheet-like cell culture in the present invention may be capable of maintaining its physical integrity without such a scaffold.
- the sheet-like cell culture is preferably composed only of substances derived from the cells constituting the cell culture and does not contain any other substances.
- the cells constituting the adherent cell culture are not particularly limited as long as they can form an adherent cell culture, and include, for example, adherent cells (adherent cells).
- adherent cells include, for example, adherent somatic cells (eg, cardiomyocytes, fibroblasts, epithelial cells, endothelial cells, hepatocytes, pancreatic cells, kidney cells, adrenal cells, periodontal cells, gingival cells, periosteum cells, skin Cells, synovial cells, chondrocytes, etc.) and stem cells (eg, tissue stem cells such as myoblasts, cardiac stem cells, embryonic stem cells, pluripotent stem cells such as iPS (induced pluripotent stem) cells, mesenchymal stem cells, etc.) Etc.
- adherent somatic cells eg, cardiomyocytes, fibroblasts, epithelial cells, endothelial cells, hepatocytes, pancreatic cells, kidney cells, adrenal cells,
- Somatic cells may be stem cells, especially those differentiated from iPS cells (iPS cell-derived adherent cells).
- cells constituting an adherent cell culture include, for example, myoblasts (eg, skeletal myoblasts), mesenchymal stem cells (eg, bone marrow, adipose tissue, peripheral blood, skin, hair roots) , Muscle tissue, endometrium, placenta, cord blood, etc.), cardiomyocytes, fibroblasts, cardiac stem cells, embryonic stem cells, iPS cells, synovial cells, chondrocytes, epithelial cells (eg, oral mucosal epithelium) Cells, retinal pigment epithelial cells, nasal mucosal epithelial cells, etc.), endothelial cells (eg, vascular endothelial cells), hepatocytes (eg, liver parenchymal cells), pancreatic cells (eg, islet cells), kidney cells, adrenal glands Examples include cells, period
- Non-limiting examples of iPS cell-derived adherent cells include iPS cell-derived cardiomyocytes, fibroblasts, epithelial cells, endothelial cells, hepatocytes, pancreatic cells, kidney cells, adrenal cells, periodontal ligament cells, gingival cells, periosteal cells Skin cells, synovial cells, chondrocytes and the like.
- the cells constituting the adherent cell culture can be derived from any organism that can be treated with the adherent cell culture, particularly the sheet-like cell culture.
- organisms include, but are not limited to, humans, non-human primates, dogs, cats, pigs, horses, goats, sheep, rodents (eg, mice, rats, hamsters, guinea pigs, etc.), rabbits, and the like. Is included.
- the number of types of cells constituting the adherent cell culture, in particular, the sheet-like cell culture is not particularly limited, and may be usually only one, but the adherent cell culture of the present invention Uses two or more types of cells, preferably two types.
- the content ratio (purity) of the most cells is 50% or more, preferably 60% or more at the end of the formation of the adherent cell culture, More preferably, it is 70% or more, More preferably, it is 75% or more.
- the cells forming the adherent cell culture may be heterogeneous cells or allogeneic cells.
- heterologous cell as used herein means a cell derived from an organism of a species different from the recipient when an adherent cell culture, particularly a sheet-shaped cell culture, is used for transplantation.
- cells derived from monkeys or pigs correspond to xenogeneic cells.
- the “same species-derived cell” means a cell derived from an organism of the same species as the recipient.
- the human cell corresponds to the allogeneic cell.
- the allogeneic cells include autologous cells (also referred to as autologous cells or autologous cells), that is, cells derived from the recipient, and allogeneic non-autologous cells (also referred to as allogeneic cells). Autologous cells are preferable when used for transplantation because they do not cause rejection even after transplantation. However, it is also possible to use heterologous cells or allogeneic non-autologous cells. When using heterologous cells or allogeneic non-autologous cells, immunosuppressive treatment may be required to suppress rejection.
- cells other than autologous cells that is, heterologous cells and allogeneic nonautologous cells may be collectively referred to as nonautologous cells.
- the cells are autologous cells or allogeneic cells.
- the cell is an autologous cell.
- the cell is an allogeneic cell.
- the sheet-shaped cell culture can be produced by any known method (see, for example, Patent Document 1, Patent Document 2, Japanese Patent Application Laid-Open No. 2010-081829, Japanese Patent Application Laid-Open No. 2011-110368, etc.).
- the method for producing a sheet-shaped cell culture typically includes a step of seeding cells on a culture substrate, a step of forming the seeded cells into a sheet, and peeling the formed sheet-shaped cell culture from the culture substrate. Including but not limited to steps. Prior to the step of seeding the cells on the culture substrate, a step of freezing the cells and a step of thawing the cells may be performed. Further, a step of washing the cells may be performed after the step of thawing the cells.
- the production method of the present invention may include a step of producing a sheet-shaped cell culture.
- the step of producing the sheet-shaped cell culture is a step according to the method for producing the sheet-shaped cell culture as a substep. 1 or 2 or more may be included.
- the step of growing the cells before the step of seeding the cells on a culture substrate is not included.
- isotonic means a state in which the osmotic pressures of two kinds of liquids are equivalent, but in the present invention, unless otherwise specified, it means “physiological isotonic” and refers to intracellular fluid or blood. It has an osmotic pressure equivalent to a physiological fluid such as Therefore, in the present invention, “isotonic solution” is synonymous with “physiological isotonic solution” unless otherwise specified, and means a fluid having an osmotic pressure equivalent to a physiological fluid such as intracellular fluid or blood. .
- isotonic solutions include, but are not limited to, Hanks balanced salt solution, physiological saline, phosphate buffered saline, Ringer's solution, basal medium, and the like.
- undernutrition refers to a condition in which the number of cells in a cell population under that condition is maintained without being proliferated or killed for a predetermined period. This means that the number of cells in the period does not change substantially. “The number of cells does not change substantially” means that there is no substantial difference between the number A of cells at a certain time point and the number B of cells at another time point after a predetermined period of time.
- the cell number A may be about 70%, about 80%, about 90%, about 95%, about 100%, about 105%, about 110%, about 120%, about 130%, etc. Can be mentioned.
- the two time points may be arbitrarily selected, but when cultured under normal culture conditions, it is preferable that there is an interval enough to confirm cell growth. Such intervals are, for example, 3, 4, 5, 6, 7, etc.
- Undernutrition can be achieved by various conditions, and those skilled in the art can appropriately create undernutrition according to the target cells.
- Undernutrition requirements typically include, for example, the ability to supply the energy necessary to sustain a cell's life and / or lack of elements necessary for cell division.
- Specific examples include an environment in which energy such as a sugar necessary for metabolism can be supplied and / or an environment in which an essential amino acid required for cell division cannot be supplied.
- the undernutrition is low sugar.
- Low sugar means a state in which sugar is contained but its proportion is low, and thus low sugar does not include a sugar-free state.
- low sugar is low glucose, and low glucose is not glucose free.
- the low sugar include a composition containing less than 1000 mg / L of sugar.
- a low-sugar condition liquid for example, a condition in which the saccharide content is reduced to less than 1% as compared with the saccharide condition in a general culture solution not containing additional saccharide is included.
- the amount of sugar contained in the low-sugar solution is, for example, less than 1000 mg / L, preferably less than 500 mg / L, more preferably less than 200 mg / L, and even more preferably 100 mg / mL. Is less than.
- the undernutrition is in an amino acid free state, i.e. free of amino acids.
- an essential amino acid is not included, for example.
- undernutrition isotonic solution means an isotonic solution that is undernutrition.
- an isotonic solution containing a predetermined amount of carbohydrate and / or an isotonic solution incapable of supplying essential amino acids specifically, for example, containing a predetermined amount of sugar but containing amino acids (particularly essential amino acids)
- Non-isotonic solution, isotonic solution containing a predetermined amount of sugar and an essential amino acid synthesis inhibitor, and the like is typically a sugar, but is not limited thereto, and examples thereof include glucose, sucrose, maltose, fructose, galactose, etc.
- the predetermined sugar content may vary depending on the type of sugar contained and the required maintenance period (immersion time) of the cells.
- glucose from the viewpoint of the necessary amount of life support for cells, for example, about 100 mg / L or more, about 500 mg / L or more, about 1000 mg / L or more, about 1500 mg / L or more, about 2000 mg / L or more, etc. .
- isotonicity for example, about 50000 mg / L or less, about 40000 mg / L or less, about 30000 mg / L or less, about 25000 mg / L or less, about 20000 mg / L or less, about 15000 mg / L or less, about 10000 mg.
- the range of the content of glucose may be any combination of these upper limit value and lower limit value, and is not limited thereto, but is not limited thereto, for example, about 100 to 500,000 mg / L, about 500 to 25000 mg / L, about Examples include 500 to 4500 mg / L, about 1500 to 4500 mg / L, and about 500 to 1500 mg / L.
- a person skilled in the art can calculate the predetermined content according to the type of sugar contained.
- hypotonic isotonic solutions include isotonic solutions known as compositions containing a predetermined amount of sugar and not containing amino acids, such as Hank's balanced salt solution, Earl balanced salt solution, and glucose isotonic solution.
- a predetermined amount of carbohydrate may be added to an isotonic solution known as a composition containing no carbohydrate and amino acid, such as water, phosphate buffered saline, Ringer's solution and the like.
- the predetermined amount of carbohydrate generally contains, for example, 1000 to 4500 mg / L glucose in the case of Hank's balanced salt solution or Earl's balanced salt solution, and about 50,000 mg / L glucose in the case of glucose isotonic solution.
- the hypotrophic isotonic solution does not include a preservative solution conventionally known as a refrigerated storage solution for organs, such as UW solution, Euro-Collins solution, Hypothermosol (registered trademark).
- the undernutrition isotonic solution of the present invention may contain lactic acid. Thus, in one aspect of the invention, the undernutrition isotonic solution does not contain lactic acid.
- the hypotrophic isotonic solution of the present invention may also contain pyruvic acid. Thus, in one aspect of the invention, the hypotrophic isotonic solution does not contain pyruvic acid. Further, the hypotrophic isotonic solution of the present invention may contain ascorbic acid. Accordingly, in one aspect of the invention, the undernutrition isotonic solution comprises ascorbic acid. Moreover, the undernutrition isotonic solution of the present invention may contain a fatty acid. Thus, in one aspect of the invention, the undernutrition isotonic solution does not contain fatty acids.
- the undernutrition isotonic solution of the present invention may contain calcium.
- the hypotrophic isotonic solution comprises calcium, preferably at a concentration of about 1-2 mM, more preferably about 1.2-1.8 mM.
- the hypotrophic isotonic solution of the present invention may also contain cholesterol.
- the undernutrition isotonic solution does not contain cholesterol.
- the hypotrophic isotonic solution is free of lactic acid, pyruvate, fatty acids and cholesterol, contains ascorbic acid, and contains calcium at a concentration of about 1-2 mM, preferably about 1.2-1.8 mM. .
- “carbohydrate” is a general term for organic compounds having a monosaccharide as a structural unit, and includes sugar derivatives as well as monosaccharides, oligosaccharides and polysaccharides.
- sugar derivatives as well as monosaccharides, oligosaccharides and polysaccharides.
- sucrose or “sugar” refers to carbohydrates or components that are metabolized by cells and converted to energy, and typically represent monosaccharides, but are degraded in the system. And those that can be metabolized into cells.
- the present invention relates to a method for modifying an adherent cell culture comprising immersing an adherent cell culture containing at least two types of cells in a hypotrophic isotonic solution.
- an adherent cell culture containing two or more types of cells for example, a sheet-like cell culture containing skeletal myoblasts and fibroblasts, is used as a low nutrient isotonic solution such as Hank's balanced salt solution. It has been found that, when immersed, the content ratio of each cell type constituting the adherent cell culture changes, whereby the adherent cell culture can be modified. As shown in the Examples below, the change in the content ratio is a phenomenon that does not occur in a cell mixture in a suspension state, and is a phenomenon that is peculiar to an adherent cell culture.
- the adherent cell culture used in the method of the present invention contains at least two types of cells.
- the cells contained in the adherent cell culture include at least one kind of cells known in the art as cells constituting the adherent cell culture.
- Preferably, all cells contained in the sheet-shaped cell culture are selected from cells known in the art as cells constituting the sheet-shaped cell culture.
- the adherent cell culture of the present invention is useful for the treatment of various diseases, particularly diseases related to tissue abnormalities.
- the adherent cell culture is for use in the treatment of diseases associated with tissue abnormalities.
- the tissue to be treated is not limited, for example, myocardium, cornea, retina, esophagus, skin, joint, cartilage, liver, pancreas, gingiva, kidney, thyroid, skeletal muscle, middle ear, bone marrow, stomach, Examples include the digestive tract of the small intestine, duodenum, and large intestine.
- the disease to be treated is not limited, and for example, heart disease (eg, myocardial injury (myocardial infarction, cardiac injury), cardiomyopathy, etc.), corneal disease (eg, corneal epithelial stem cell exhaustion, cornea) Injury (heat / chemical corrosion), corneal ulcer, corneal opacity, corneal perforation, corneal scar, Stevens-Johnson syndrome, pemphigoid, etc., retinal diseases (eg retinitis pigmentosa, age-related macular degeneration) , Esophageal diseases (for example, prevention of esophageal inflammation / stenosis after esophageal surgery (esophageal cancer removal)), skin diseases (for example, skin damage (trauma, burn), etc.), joint diseases (for example, osteoarthritis, etc.) Cartilage disease (eg, cartilage damage), liver disease (eg, chronic liver disease), pancreatic disease (eg, diabetes), dental disease (eg, periodon,
- the adherent cell culture can be applied to the tissue to be treated and used to repair and regenerate it, but as a source of physiologically active substances such as hormones, the tissue to be treated It can also be transplanted to other sites (for example, subcutaneous tissue).
- the adherent cell culture can be used for the production of a pharmaceutical composition containing the cell culture.
- Such pharmaceutical compositions include various additional ingredients, such as pharmaceutically acceptable carriers, ingredients that enhance cell culture viability, engraftment and / or function, and other useful for the treatment of the target disease.
- An active ingredient etc. may be included. Any known additional components can be used, and those skilled in the art are familiar with these additional components.
- the pharmaceutical composition can be used in combination with a component that enhances the viability, engraftment and / or function of the cell culture in an adherent state, other active components useful for the treatment of the target disease, and the like.
- the adherent cell culture is an adherent cell culture used for the treatment of heart disease.
- the adherent cell culture used for the treatment of heart disease include, but are not limited to, a sheet cell culture containing skeletal myoblasts, a sheet cell culture containing cardiomyocytes, Examples thereof include a sheet-like cell culture containing vascular endothelial cells and a sheet-like cell culture containing mesenchymal stem cells.
- Preferred are a sheet-like cell culture containing skeletal myoblasts, a sheet-like cell culture containing cardiomyocytes, and a sheet-like cell culture containing vascular endothelial cells.
- Examples of other constituent cells that can be included in an adherent cell culture containing skeletal myoblasts include fibroblasts, vascular endothelial cells, and / or differentiated into them. Examples thereof include cells to be obtained (for example, stem cells and progenitor cells).
- Examples of other constituent cells that can be included in an adherent cell culture including cardiomyocytes (target cells), particularly a sheet-shaped cell culture include vascular endothelial cells, smooth muscle cells, and / or cells that can differentiate into them. (For example, stem cells and progenitor cells).
- Examples of other constituent cells that can be included in an adherent cell culture containing vascular endothelial cells (target cells), particularly a sheet-like cell culture, include skeletal myoblasts, fibroblasts, cardiomyocytes and / or the like. Examples thereof include cells that can differentiate (for example, stem cells and progenitor cells). Examples of other constituent cells that can be contained in an adherent cell culture containing mesenchymal stem cells (target cells), particularly a sheet-like cell culture, include precursor cells, adipocytes, macrophages, and vascular endothelial cells. .
- the “target cell” means a main constituent cell in the adherent cell culture of the present invention, and particularly a cell useful for treatment of a disease in a cell culture used for treatment of the disease. Means.
- the purity of a target cell can be increased by reducing the number of other constituent cells in an adherent cell culture, particularly a sheet-like cell culture. Therefore, the method of the present invention makes it possible to provide an adherent cell culture with high purity of target cells. For example, in the case of an adherent cell culture containing skeletal myoblasts and fibroblasts, particularly a sheet-like cell culture, the content of fibroblasts can be reduced by using the method of the present invention, and skeletal myoblasts can be reduced. The purity of can be increased.
- the adherent cell culture contains skeletal myoblasts and fibroblasts.
- the adherent cell culture, in particular the sheet cell culture comprises skeletal myoblasts, fibroblasts and mesenchymal stem cells.
- the adherent cell culture, in particular the sheet-shaped cell culture does not contain cells other than skeletal myoblasts and fibroblasts and cells differentiated from skeletal myoblasts or fibroblasts.
- the adherent cell culture, particularly the sheet-shaped cell culture comprises cardiomyocytes and vascular endothelial cells.
- the adherent cell culture does not contain cells other than cardiomyocytes and vascular endothelial cells and cells differentiated from vascular endothelial cells.
- the adherent cell culture, in particular the sheet-like cell culture comprises skeletal myoblasts, fibroblasts and vascular endothelial cells.
- the adherent cell culture, in particular the sheet cell culture is skeletal myoblasts, fibroblasts and vascular endothelial cells and cells differentiated from skeletal myoblasts, fibroblasts or vascular endothelial cells Contains no other cells.
- the adherent cell culture comprises mesenchymal stem cells and contaminating cells of the mesenchymal stem cells.
- contaminated cells of mesenchymal stem cells mean cells other than mesenchymal stem cells that are mixed when acquiring mesenchymal stem cells.
- the mesenchymal stem cell contaminated cells differ depending on the origin of the mesenchymal stem cells, and those skilled in the art can easily understand the types of contaminating cells to be mixed by the derived tissue.
- mesenchymal stem cells include bone marrow, adipose tissue, umbilical cord, umbilical cord blood, peripheral blood, pulp tissue, placenta, synovial tissue, periodontal ligament, dermal tissue, endometrium, wall-side decidua, etc.
- contaminating mesenchymal stem cells include precursor cells, adipocytes, macrophages, and vascular endothelial cells if derived from bone marrow, and precursor cells, adipocytes, fibroblasts, blood vessels and the like if derived from adipose tissue. Examples thereof include endothelial cells.
- the present invention relates to an adherent cell culture comprising skeletal myoblasts and fibroblasts, particularly a sheet-like cell culture, with reduced fibroblast content and improved skeletal myoblast purity.
- An adherent cell culture particularly a sheet-shaped cell culture, is provided.
- the present invention relates to an adherent cell culture containing cardiomyocytes and other cells, particularly a sheet-like cell culture, wherein the content of cells other than cardiomyocytes is reduced and the purity of cardiomyocytes is improved.
- a culture, particularly a sheet cell culture is provided.
- Non-limiting examples of cells other than cardiomyocytes include pluripotent stem cells, iPS cells, undifferentiated cells derived from iPS cells, and the like.
- the present invention relates to an adherent cell culture containing cardiomyocytes, vascular endothelial cells, and other cells, particularly a sheet-like cell culture, in which the purity of cells other than cardiomyocytes and vascular endothelial cells is reduced.
- an adherent cell culture particularly a sheet-shaped cell culture, in which the purity of endothelial cells is improved.
- Non-limiting examples of cells other than cardiomyocytes and vascular endothelial cells include pluripotent stem cells, iPS cells, and undifferentiated cells derived from iPS cells.
- the adherent cell culture includes cells induced to differentiate from pluripotent stem cells including iPS cells.
- undifferentiated cells remain when obtaining differentiation-inducing cells (for example, cardiomyocytes) from pluripotent stem cells, and this remaining undifferentiated cells may be unfavorable because of the risk of tumorigenesis. is there.
- the content of such remaining undifferentiated cells can be reduced.
- target cells for example, cardiomyocytes
- the adherent cell culture contains cardiomyocytes differentiated from iPS cells.
- examples of the other cells contained in the adherent cell culture include endothelial cells induced by differentiation from iPS cells, blood vessel constituent cells such as wall cells, and the like.
- the adherent cell culture comprises 30 to 70% iPS-derived cardiomyocytes (target cells), 0.1 to 20% iPS cell-derived vascular endothelial cells, 1 to 40% iPS cell-derived vascular wall.
- a cell culture containing cells preferably a sheet-shaped cell culture.
- the iPS cell-derived cardiomyocyte culture of this embodiment may contain about 5.0 ⁇ 10 4 cells / cm 2 or more, preferably about 1.0 ⁇ 10 5 cells / cm 2 or more.
- the number of cardiomyocytes is about 5.0 ⁇ 10 6 cells / cm 2 or less, preferably about 2.0 ⁇ 10 6 cells / cm 2 or less, more preferably about 1.0 ⁇ 10 6 cells / cm 2. It may contain 2 or less. Therefore, the number of cardiomyocytes that can be contained in the adherent cell culture of the present invention can be any combination of the above upper limit and lower limit, for example 5.0 ⁇ 10 4 cells / cm 2 to 5.
- It may be 0 ⁇ 10 6 pieces / cm 2 , preferably 1.0 ⁇ 10 5 pieces / cm 2 to 2.0 ⁇ 10 6 pieces / cm 2, and in another embodiment, 1.0 ⁇ 10 5 pieces / cm 2. Pieces / cm 2 to 1.0 ⁇ 10 6 pieces / cm 2 .
- the hypotrophic isotonic solution is Hank's balanced salt solution.
- Hank's balanced salt solution is an isotonic solution containing glucose at a concentration of 1000 mg / L, and its composition is known in the art.
- Hank's balanced salt solution includes HBSS (+) containing a divalent cation such as magnesium and calcium and HBSS (-) not containing them. In the present invention, HBSS (+) is more preferable.
- Typical composition of HBSS (+) is calcium chloride (anhydrous) 140 mg / L, magnesium chloride (hexahydrate) 100 mg / L, magnesium sulfate (7 hydrate) 100 mg / L, potassium chloride 400 mg / L, Potassium dihydrogen phosphate 60 mg / L, sodium hydrogen carbonate 350 mg / L, sodium chloride 8000 mg / L, sodium hydrogen phosphate 48 mg / L, glucose 1000 mg / L.
- the hypotrophic isotonic solution of the present invention may contain additional components to the extent that they do not adversely affect the adherent cell culture.
- additional component include, but are not limited to, a radical scavenger.
- radical scavengers that can be used in the hypotrophic isotonic solution of the present invention include vitamins such as vitamin C and vitamin E, and edaravone.
- the temperature at which the adherent cell culture is immersed can be appropriately selected within a range that does not damage the adherent cell culture.
- the immersion is performed at room temperature.
- the immersion is performed at refrigerated conditions, for example, about 2 ° C to 8 ° C.
- non-limiting examples of temperature conditions during immersion include about 2 to 40 ° C., about 2 to 35 ° C., about 2 to 30 ° C., about 2 to 25 ° C., about 2 to 20 ° C., about 2 to 15 ° C., about 2-10 ° C, about 2-8 ° C, about 2-4 ° C, about 8-40 ° C, about 10-40 ° C, about 15-40 ° C, about 20-40 ° C, about 25-40 ° C, about 30- Examples include 40 ° C., about 35 to 40 ° C., about 8 to 35 ° C., about 8 to 30 ° C., about 8 to 25 ° C., and about 15 to 40 ° C. In the case where the immersion time becomes long, refrigerated conditions are preferable from the viewpoint that damage to the cell culture in the adhered state can be reduced.
- the immersion time of the adherent cell culture is not particularly limited as long as it does not damage the adherent cell culture. However, since the liquid to be immersed is undernourished, soaking for a long time is not preferable. Accordingly, the upper limit of the immersion time is not limited to this, but for example, 2 days (48 hours) or less, 3 days (72 hours) or less, 100 hours or less, 5 days (120 hours) or less, 150 hours or less, 1 Weeks (168 hours) or less, 200 hours or less, etc. are mentioned.
- the lower limit of the immersing time is not limited to this, for example 48 Time or more, 24 hours or more, 12 hours or more, 10 hours or more, 8 hours or more, 6 hours or more, 4 hours or more.
- the range of the immersion time of the adherent cell culture may be any combination of these upper limit value and lower limit value, and is not limited to this, for example, 4 to 200 hours, 6 to 200 hours, 8 to 200 hours, 10 to 200 hours, 12 to 200 hours, 24 to 200 hours, 48 to 200 hours, 4 to 168 hours, 6 to 168 hours, 8 to 168 hours, 10 to 168 hours, 12 to 168 hours, 24-168 hours, 48-168 hours, 4-150 hours, 6-150 hours, 8-150 hours, 10-150 hours, 12-150 hours, 24-150 hours, 48-150 hours, 48-144 hours, 4 to 120 hours, 6 to 120 hours, 8 to 120 hours, 10 to 120 hours, 12 to 120 hours, 24 to 120 hours, 48 to 120 hours, 4 to 100 hours, 6 to 00 hours, 8-100 hours, 10-100 hours, 12-100 hours, 24-100 hours, 48-100 hours, 4-72 hours, 6-72 hours, 8-72 hours, 10-72 hours, 12- 72 hours, 24 to 72 hours, 4 to 48 hours, 6 to 48 hours, 8 to 48 to
- any combination of the above-described conditions for immersion may be used.
- the conditions for immersion can vary depending on the type of cell culture in the adhered state and the hypotonic isotonic solution to be immersed, and those skilled in the art can appropriately select optimal conditions.
- a non-limiting example in which a sheet-shaped cell culture containing skeletal myoblasts and fibroblasts is immersed in a hypotrophic isotonic solution is about 2 to 8 ° C. and about 4 to 200 hours in Hank's balanced salt solution.
- Hanks balanced salt at about 2-8 ° C Hanks balanced salt solution for about 8-150 hours, Hanks balanced salt solution for about 12-120 hours, Hanks balanced salt solution at about 2-8 ° C
- About 24 to 72 hours about 15 to 30 ° C., about 4 to 200 hours for Hanks balanced salt solution, about 15 to 30 ° C., about 8 to 150 hours about Hanks balanced salt solution, about 15 to about Undernutrition with glucose concentration of 4500 mg / L at 30 ° C, Hanks balanced salt solution for about 12-120 hours, about 15-30 ° C, Hanks balanced salt solution for about 24-72 hours, about 2-8 ° C Glucose concentration in an isotonic solution at about 2-8 ° C for about 4-200 hours 4500 mg / L hypotrophic isotonic solution for about 8 to 150 hours at about 2 to 8 ° C.
- Glucose concentration 4500 mg / L hypotrophic isotonic solution for about 12 to 120 hours at about 2 to 8 ° C. About 24 to 72 hours in a hypotrophic isotonic solution with a glucose concentration of 4500 mg / L, about 15 to 30 ° C. About 15 to 30 ° C.
- a hypotrophic isotonic solution with a glucose concentration of 4500 mg / L for about 4 to 200 hours
- a hypotrophic isotonic solution with a glucose concentration of 4500 mg / L, about 12 to 120 hours, about 15 to A hypotrophic isotonic solution with a glucose concentration of 4500 mg / L at 30 ° C. may include about 24 to 72 hours.
- the content (purity) of skeletal myoblasts in the sheet-shaped cell culture is improved, and the sheet-shaped cell culture becomes more preferable in the treatment of heart diseases by transplantation of the sheet-shaped cell culture.
- the adherent cell culture is a sheet-like cell culture.
- the sheet-shaped cell culture is formed on a culture substrate and then immersed in a hypotrophic isotonic solution without being peeled off.
- a low nutrient isotonic solution can be used as a medium used at the time of peeling.
- a hypotrophic isotonic solution is added to a sheet-shaped cell culture formed on a culture substrate and immersed and cooled as it is, or a cooled hypotrophic isotonic solution is added. Then, after being peeled off, it is immersed as it is, and then collected and used.
- the normal culture condition may be changed to a low nutrient state in the middle of formation, or the culture may be started in a low nutrient state at the start of formation.
- the state is changed from a low nutrient state to a normal culture condition. Switching between normal culture conditions and a low nutrient state may be achieved, for example, by adding a deficient component such as sugar or amino acid to a low nutrient isotonic solution, or may be achieved by liquid exchange.
- the adherent cell culture is a sheet-shaped cell culture, and the sheet-shaped cell culture is formed on the culture substrate and then detached from the culture substrate, Soak in nutrient isotonic solution. It is known that a sheet-shaped cell culture contracts to some extent when it is peeled from the culture substrate. The degree of such contraction varies depending on the cell type, content ratio, formation conditions, etc. constituting the sheet-shaped cell culture. For example, when a cell population including skeletal myoblasts and fibroblasts is formed using the sheet-forming culture of the present invention, the contraction ratio is, for example, a sheet diameter of about 30%, about 40%, or about 50%. , About 60%, about 70%, about 80%. In one embodiment of the present invention, the peeled (ie, contracted) sheet-like cell culture has an area of about 1 cm 2 or more, about 3 cm 2 or more, preferably about 6 cm 2 or more, more preferably about 10 cm 2 or more. possible.
- the sheet-shaped cell culture is a laminate of a plurality of single-layer sheet-shaped cell cultures.
- Such a laminated sheet-like cell culture can be formed by superposing the peeled monolayer sheet-like cell cultures.
- the sheet-like cell culture is immersed in a hypotrophic isotonic solution in the state of a monolayer sheet-like cell culture, and may be superposed to form a laminate, or the monolayer sheet-like cell culture is overlaid and laminated. After that, it may be immersed in a hypotrophic isotonic solution.
- a method for producing a sheet cell culture includes the following steps (a) to (d): (A) a step of seeding a cell population containing a target cell, preferably a cell population containing two or more types of cells, onto a culture substrate at a density capable of forming a sheet-shaped cell culture without substantially growing; (B) Incubating the seeded cell population to form a sheet-shaped cell culture, and (c) Immersing the formed sheet-shaped cell culture in a hypotrophic isotonic solution.
- the cell population to be seeded contains one or more cells selected from the cells constituting the sheet-shaped cell culture, preferably from the cells forming the sheet-shaped cell culture. It consists of two or more selected cells. Among the two or more types of cells forming the sheet-shaped cell culture, the content ratio (purity) of the most cells is 50% or more, preferably 60% or more, more preferably at the end of the formation of the sheet-shaped cell culture. Is 70% or more, more preferably 75% or more.
- the production method of the present invention includes a step of freezing a cell population and a step of thawing the frozen cell population before seeding the cells.
- the cell population obtained by thawing may be seeded after the cells are expanded, but in a preferred embodiment, between the step of thawing the frozen cell population and step (a). It does not include the step of growing the cells.
- the sheet-like cell culture produced in such an embodiment is more preferable than the sheet-like cell culture produced in an embodiment comprising a step of proliferating cells between the step of thawing a frozen cell population and the step (a). Activities such as cytokine production ability, engraftment ability, blood vessel induction ability and tissue regeneration ability are high.
- the high activity is not limited based on the activity of the sheet-like cell culture to be compared, for example, 5% or more, 10% or more, 20% or more, 30% or more, 40% or more, 50% or more, 60% or more, 70% or more, 80% or more, 90% or more, or 100% or more means high activity.
- the sheet-like cell culture containing skeletal myoblasts of the present invention contains 60% to 99% of skeletal myoblasts.
- the sheet-shaped cell culture containing cardiomyocytes of the present invention contains 50% to 70% cardiomyocytes.
- a cell population containing cells induced to differentiate from pluripotent stem cells such as iPS cells can be used as the cell population to be seeded.
- undifferentiated cells remain when obtaining differentiation-inducing cells (for example, cardiomyocytes) from pluripotent stem cells, and this remaining undifferentiated cells may be unfavorable because of the risk of tumorigenesis. is there.
- the content of such remaining undifferentiated cells can be reduced.
- target cells for example, cardiomyocytes
- the cell population to be seeded is selected from one or more cells selected from the cells constituting the adherent cell culture, preferably from the cells constituting the adherent cell culture.
- a step of forming intercellular adhesion for forming spheroids or embryoid bodies for seeding can be included separately from the sheet forming step (b) described later.
- an adherent cell culture such as a spheroid or embryoid body
- a method known in the art can be used. Non-limiting examples of such methods include the methods described in, for example, Miki et al., Cell Stem Cell 16, 699-711, June 4, 2015, WO2014 / 185358, WO2017 / 038562.
- the sheet-like cell culture produced by the production method of the present invention is preferably a sheet-like cell culture used for the treatment of heart diseases.
- the seeded cell population comprises skeletal myoblasts and fibroblasts.
- the seeded cell population comprises cardiomyocytes and vascular endothelial cells.
- the seeded cell population comprises skeletal myoblasts, fibroblasts and vascular endothelial cells.
- the density at which a sheet-like cell culture can be formed without substantially growing means that a sheet-like cell culture is formed when cultured in a non-proliferating culture medium substantially free of growth factors. It means the cell density that can be.
- This seeding density is higher than that in the method using a culture solution containing a growth factor, and may be equal to or higher than the density at which cells reach confluence.
- the density is not limited to this, but is, for example, 1.0 ⁇ 10 5 pieces / cm 2 or more.
- the upper limit of the seeding density is not particularly limited as long as the formation of the cell culture is not impaired and the cells do not shift to differentiation, but may be, for example, less than 3.4 ⁇ 10 6 cells / cm 2 .
- the “density at which cells can form a sheet-like cell culture without substantial growth” is at or above the density at which the cells reach confluence, or at or above the confluent density.
- the density at which cells can form a sheet-shaped cell culture without substantial growth is 1.0 ⁇ 10 5 to 3.4 ⁇ 10 6 cells / cm 2 in one embodiment, and 3. 0 ⁇ 10 5 to 3.4 ⁇ 10 6 pieces / cm 2 , in yet another embodiment, 3.5 ⁇ 10 5 to 3.4 ⁇ 10 6 pieces / cm 2 , and in yet another embodiment, 1.0 ⁇ 10 6 3.4 ⁇ 10 6 pieces / cm 2 , in another embodiment 3.0 ⁇ 10 5 to 1.7 ⁇ 10 6 pieces / cm 2 , and in another embodiment 3.5 ⁇ 10 5 to 1.7 ⁇ 10 6 pieces / cm 2 , and in yet another aspect, 1.0 ⁇ 10 6 to 1.7 ⁇ 10 6 pieces / cm 2 .
- the said range may include both an upper limit and a lower limit, or any one thereof. Therefore, the density is, for example, 3.0 ⁇ 10 5 pieces / cm 2 or more and less than 3.4 ⁇ 10 6 pieces / cm 2 (including the lower limit and not including the upper limit), 3.5 ⁇ 10 5 pieces / cm 2.
- the culture substrate is not particularly limited as long as the cells can form a sheet-like cell culture thereon, and those commonly used in the technical field can be used.
- a substrate include, but are not limited to, polyethylene, polypropylene, Teflon (registered trademark), polyethylene terephthalate, polymethyl methacrylate, nylon 6,6, polyvinyl alcohol, cellulose, silicon, polystyrene, glass, Examples thereof include polyacrylamide, polydimethylacrylamide, metal (for example, iron, stainless steel, aluminum, copper, brass).
- the culture substrate can include one surface of the culture container (for example, the bottom surface of the container), the surface of the cell culture scaffold, and the like.
- the surface of the culture substrate may be subjected to processing that is advantageous in the production of a sheet-shaped cell culture, including processing for enhancing cell adhesion, processing for facilitating peeling, and the like.
- processing include, but are not limited to, corona discharge treatment, ultraviolet irradiation treatment, coating with hydrophilic compounds such as collagen gel and hydrophilic polymer, collagen, fibronectin, laminin, vitronectin, proteoglycan, glyco
- hydrophilic compounds such as collagen gel and hydrophilic polymer, collagen, fibronectin, laminin, vitronectin, proteoglycan, glyco
- examples include coating with an extracellular matrix such as saminoglycan, cell adhesion factors such as cadherin family, selectin family, and integrin family, for example, coating with a material whose physical properties change in response to stimuli such as temperature and light.
- Materials whose physical properties change in response to stimuli such as temperature and light are not limited to these, but include, for example, (meth) acrylamide compounds, N-alkyl substituted (meth) acrylamide derivatives (eg, N-ethyl).
- the physical properties for example, hydrophilicity and hydrophobicity can be changed, and peeling of the cell culture adhered on the materials can be promoted.
- Culture dishes coated with a temperature-responsive material are commercially available (e.g. UpCell (R), Cellseed), they can be used in the production method of the present invention.
- the culture substrate may have various shapes, and can be performed in a container having an arbitrary size and shape, but is preferably flat.
- the area is not particularly limited, but is typically about 1 cm 2 to about 200 cm 2 , preferably about 2 cm 2 to about 100 cm 2 , more preferably about 3 cm 2 to about 50 cm 2 .
- step (b) the seeded cell population is cultured in a sheet to form a sheet-like cell culture.
- “Sheet culture” means culturing cells seeded on a culture substrate so as to form a sheet-like cell culture (ie, form a sheet).
- sheet culture cells that can form a sheet-shaped cell culture are typically seeded on a culture substrate, and the cells are cultured under the conditions for forming the sheet-shaped cell culture for a predetermined period of time to interact with each other. And by connecting the cells together.
- the period for culturing is not particularly limited as long as it is a period sufficient to form a sheet-like cell culture.
- the predetermined period is preferably within a period in which the cells do not shift to differentiation.
- the cells are maintained in an undifferentiated state during the culture period.
- the transition to cell differentiation can be evaluated by any method known to those skilled in the art. For example, in the case of skeletal myoblasts, MHC expression and cell multinucleation can be used as indicators of differentiation.
- the conditions for forming the sheet-shaped cell culture include any conditions that can form adhesion between cells and a substrate and cell-cell adhesion, and are not limited thereto.
- general cell culture Conditions are included. Examples of such conditions include culture at 37 ° C. and 5% CO 2 . Therefore, the sheeting process can be performed at room temperature (for example, 37 ° C.).
- the culture period varies depending on the type of cells (sheet-forming cells) forming the sheet-shaped cell culture from the viewpoint of a period sufficient to form the sheet-shaped cell culture.
- the sheet-forming cells are skeletal myoblasts, but not limited thereto, for example, 12 hours or more, 16 hours or more, 20 hours or more, 24 hours or more, 26 hours or more, 28 hours or more, 30 hours or more 32 hours or more and 36 hours or more, and from the viewpoint of preventing cell differentiation, but not limited thereto, for example, within 48 hours, within 44 hours, within 40 hours, and within 36 hours.
- the culture period can be exemplified by any combination of these upper and lower limits, and is not limited to this, but for example, 12 to 48 hours, 16 to 48 hours, 20 to 48 hours, 24 to 48 hours 26-48 hours, 28-48 hours, 30-48 hours, 32-48 hours, 36-48 hours, 12-44 hours, 16-44 hours, 20-44 hours, 24-44 hours, 26-44 hours 28-44 hours, 30-44 hours, 32-44 hours, 36-44 hours, 12-40 hours, 16-40 hours, 20-40 hours, 24-40 hours, 26-40 hours, 28-40 hours 30 to 40 hours, 32 to 40 hours, 36 to 40 hours, 12 to 36 hours, 16 to 36 hours, 20 to 36 hours, 24 to 36 hours, 26 to 36 hours, 28 to 36 hours, 30 to 36 hours 32-36 hours, and the like.
- the lower limit is, for example, 24 hours or more, 30 hours or more, 36 hours or more
- the upper limit is within 48 hours, within 72 hours, within 96 hours, and within 120 hours. Accordingly, the culture period can be exemplified by any combination of these upper and lower limits, and is not limited to this, but for example, 24-48 hours, 24-72 hours, 24-96 hours, 24-120 hours. 30 to 48 hours, 30 to 72 hours, 30 to 96 hours, 30 to 120 hours, 36 to 48 hours, 36 to 72 hours, 36 to 96 hours, 36 to 120 hours, and the like. A person skilled in the art can select optimal conditions according to the type of cells to be seeded. Non-limiting examples of sheet culture are described in, for example, Patent Document 1, JP 2010-081829, JP 2010-226991, JP 2011-110368, JP 2011-172925, WO 2014/185517, and the like.
- the sheeting medium used for sheeting is not particularly limited as long as it can induce sheeting of cells.
- physiological saline various physiological buffers (for example, PBS, HBSS, etc.), various cells Those based on a basal medium for culture can be used.
- a basal medium is not limited, for example, DMEM, MEM, F12, DME, RPMI 1640, MCDB (MCDB102, 104, 107, 120, 131, 153, 199 etc.), L15, SkBM, RITC80-7, DMEM / F12 and the like are included. Many of these basal media are commercially available, and their compositions are also known.
- the basal medium may be used in a standard composition (for example, as it is commercially available), or the composition may be appropriately changed according to the cell type and culture conditions. Therefore, the basal medium is not limited to those having a known composition, and includes one in which one or more components are added, removed, increased or decreased.
- the sheeting medium may contain additives such as serum (eg, bovine serum such as fetal bovine serum, horse serum, human serum, etc.), various growth factors (eg, FGF, EGF, VEGF, HGF, etc.).
- the cells are seeded at a density that does not substantially multiply, it is possible to obtain the desired size and shape without waiting for the cell culture to grow to the desired size as in the conventional method.
- a sheet-like cell culture can be obtained in a short period of time.
- the size and shape of the sheet-shaped cell culture can be adjusted by adjusting the size and shape of the cell adhesion surface of the culture substrate, or by installing a mold of the desired size and shape on the cell adhesion surface of the culture vessel. It can be arbitrarily adjusted by, for example, culturing cells therein.
- step (c) the detached sheet-shaped cell culture is immersed in a hypotonic isotonic solution. Accordingly, the sheet-shaped cell culture is immersed in a hypotrophic isotonic solution in a contracted state. Details of the soaking step are as described in the method for modifying a sheet-like cell culture.
- the method further includes the step (c ′) of peeling the formed sheet-shaped cell culture.
- Step (c ′) may be performed before or after step (c).
- a normal medium may be used as the peeling medium, or a low nutrient isotonic solution may be used.
- the step (c ′) may be carried out as it is after immersing in the hypotrophic isotonic solution in the step (c) or stripped in the step (c ′). Then, it is good also as a process (c) by immersing in a peeling medium as it is.
- the immersion of the sheet-shaped cell culture and the subsequent storage may be performed at normal temperature or under refrigerated conditions, but is preferably performed under refrigerated conditions.
- Peeling is not particularly limited as long as it can be peeled from the culture substrate while maintaining the structure of the sheet-like cell culture, and a method known in the art can be used. Specifically, for example, it can be performed by enzyme treatment with a proteolytic enzyme (for example, trypsin), mechanical treatment such as pipetting, physical peeling using a support, and the like.
- a proteolytic enzyme for example, trypsin
- mechanical treatment such as pipetting
- physical peeling using a support and the like.
- a culture substrate whose surface is coated with a material whose physical properties change in response to a stimulus such as temperature or light is used as the culture substrate, it is released non-enzymatically by applying a predetermined stimulus. You can also.
- a peeling medium (which may be the same as the above-mentioned sheeting medium) is added and cooled, or cooling By adding the peeled medium, the adhesiveness can be reduced and the film can be peeled off.
- the detachment process itself may be performed at room temperature (for example, a temperature around 37 ° C.) or under refrigerated conditions. You may go.
- the contraction ratio is, for example, a sheet diameter of about 30%, about 40%, or about 50%. , About 60%, about 70%, about 80%.
- the peeled (ie, contracted) sheet-like cell culture has an area of about 1 cm 2 or more, about 3 cm 2 or more, preferably about 6 cm 2 or more, more preferably about 10 cm 2 or more. possible.
- the peeled sheet-shaped cell culture When the peeled sheet-shaped cell culture is contracted, it may be contracted at normal temperature (for example, a temperature around 37 ° C.) or may be contracted under refrigerated conditions. Moreover, the washing
- the step (c ′′) further comprises laminating the peeled sheet-shaped cell culture.
- the step (c ′′) may be before or after the step (c) as long as it is after the step (c ′). That is, after dipping in a hypotrophic isotonic solution, peeling and laminating may be performed, after peeling, dipping in a low nutrient isotonic solution, and then laminating, or after peeling and laminating
- the sheet-shaped cell culture obtained as a laminate may be immersed in a hypotrophic isotonic solution.
- the sheet-shaped cell culture can be transported while being immersed in a hypotrophic isotonic solution.
- the sheet-like cell culture modified by the method of the present invention can be used for transplantation immediately after removal from the hypotrophic isotonic solution. Therefore, in one embodiment of the present invention, a sheet-shaped cell culture is produced, immersed in a hypotrophic isotonic solution, transported and stored in that state, and then taken out and used.
- the sheet-shaped cell culture of the present invention can be used for the production of a pharmaceutical composition as described above. Therefore, the present invention also includes a method for treating the above-mentioned various diseases using the sheet-shaped cell culture of the present invention.
- the treatment method of the present invention comprises the step of administering to a subject in need thereof an effective amount of a sheet cell culture or a pharmaceutical composition comprising it.
- the tissues and diseases to be treated are as described above for the sheet cell culture.
- a component that enhances the viability, engraftment and / or function of the sheet-shaped cell culture, other active ingredients useful for the treatment of the target disease, and the like are used in combination with the cell culture. be able to.
- Example 1 Modification test of sheet-like cell culture
- a sheet-like cell culture was prepared using skeletal myoblasts (including fibroblasts) prepared from human skeletal muscle by a conventional method. Temperature-responsive culture dish (UpCell (R) 12-well multi-well, Cellseed), the 20% human serum-containing DMEM / F12 medium (Thermo Fisher Scientific Inc.) human skeletal myoblasts suspended in cells and human fibroblasts
- the cell mixture was seeded at 3.7 ⁇ 10 6 cells / well and sheet-cultured at 37 ° C. under 5% CO 2 for 12 to 26 hours. After the sheet culture, the medium is removed, 700 ⁇ L of cooled HBSS (+) (Thermo Fisher Scientific Inc.) is added and left to stand for 10 minutes, and then gently pipetted to completely peel off the sheet-like cell culture. I let you.
- HBSS (+) was removed, and HBSS (+) at room temperature was newly added for rinsing. This washing process was performed 4 times. After removing HBSS (+), 1.55 mL of fresh HBSS (+) was added, and the mixture was allowed to stand at 2 to 8 ° C.
- the skeletal myoblast content (hereinafter referred to as “purity”) was measured every 24 hours for the sheet-like cell culture that was allowed to stand under refrigerated conditions in (1).
- the formed sheet-like cell culture was dissociated with trypsin-like proteolytic enzyme, centrifuged, and the supernatant was discarded.
- 0.5% BSA-containing PBS solution was added to rinse the cells, and then anti-human CD56 antibody (Becton Dickinson) diluted 10-fold with 0.5% BSA-containing PBS solution was added and mixed.
- a negative control antibody (Becton Dickinson) diluted 10-fold with 0.5% BSA-containing PBS solution was added and mixed.
- each antibody was mixed, it was immediately reacted in a cool dark place for about 1 hour, and a 0.5% BSA-containing PBS solution was added to rinse the cells. Then, a 0.5% BSA-containing PBS solution was added for analysis.
- a flow cytometer (Becton Dickinson) was used to measure the ratio of antibody-positive cells contained in cells mixed with each antibody. In the measurement, the positive rate of the negative control was corrected, and 5,000 to 10,000 cells were analyzed. After the analysis, the purity was determined from the difference in the ratio of the positive cell ratio of the cells mixed with each antibody.
- Example 2 1.55 mL of HBSS (+) was added to a mixture of human skeletal myoblasts and human fibroblasts in a state of not being made into a modified test sheet in the cell suspension, and the mixture was allowed to stand under refrigerated conditions at 2 to 8 ° C. The purity of skeletal myoblasts was measured at the start of the test and 72 hours later in the same manner as in Example 1 (2) above. The results are shown in FIG. When the sheet was not formed, no change was confirmed in the purity of skeletal myoblasts.
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- Biomedical Technology (AREA)
- Chemical & Material Sciences (AREA)
- Zoology (AREA)
- Organic Chemistry (AREA)
- Biotechnology (AREA)
- Genetics & Genomics (AREA)
- Wood Science & Technology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Health & Medical Sciences (AREA)
- Biochemistry (AREA)
- General Engineering & Computer Science (AREA)
- Microbiology (AREA)
- Cell Biology (AREA)
- Rheumatology (AREA)
- Molecular Biology (AREA)
- Medicinal Chemistry (AREA)
- Tropical Medicine & Parasitology (AREA)
- Virology (AREA)
- Urology & Nephrology (AREA)
- Botany (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Dermatology (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Epidemiology (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Hematology (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Materials For Medical Uses (AREA)
- Apparatus Associated With Microorganisms And Enzymes (AREA)
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| SG11201805456WA SG11201805456WA (en) | 2016-11-01 | 2017-11-01 | Modification method for sheet-shaped cell culture |
| JP2018521436A JP6426327B2 (ja) | 2016-11-01 | 2017-11-01 | 接着状態の細胞培養物の改変方法 |
| CN201780031342.1A CN109153963B (zh) | 2016-11-01 | 2017-11-01 | 粘附状态的细胞培养物的改变方法 |
| EP17867551.8A EP3406704B1 (en) | 2016-11-01 | 2017-11-01 | Modification method for adhesion-state cell culture |
| US16/188,630 US11395863B2 (en) | 2016-11-01 | 2018-11-13 | Modification method for sheet-shaped cell culture |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2016-214224 | 2016-11-01 | ||
| JP2016214224 | 2016-11-01 |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US16/188,630 Continuation US11395863B2 (en) | 2016-11-01 | 2018-11-13 | Modification method for sheet-shaped cell culture |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2018084167A1 true WO2018084167A1 (ja) | 2018-05-11 |
Family
ID=62076229
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2017/039476 Ceased WO2018084167A1 (ja) | 2016-11-01 | 2017-11-01 | 接着状態の細胞培養物の改変方法 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US11395863B2 (enExample) |
| EP (1) | EP3406704B1 (enExample) |
| JP (3) | JP6426327B2 (enExample) |
| CN (1) | CN109153963B (enExample) |
| SG (1) | SG11201805456WA (enExample) |
| WO (1) | WO2018084167A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2025183063A1 (ja) * | 2024-03-01 | 2025-09-04 | セントラル硝子株式会社 | 三次元細胞培養体の凍結物、三次元細胞培養体の凍結物を含む容器、細胞凍結キット、凍結保存液、三次元細胞培養体の凍結物の製造方法、三次元細胞培養体の凍結物の使用方法、および凍結保存液の使用方法 |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP3819373A4 (en) * | 2018-07-06 | 2022-05-18 | Myoridge Co. Ltd. | CELL LAYER PREPARATION METHOD, HEART MUSCLE LAYER AND KIT FOR MAKING A HEART MUSCLE LAYER |
| WO2021065989A1 (ja) * | 2019-09-30 | 2021-04-08 | テルモ株式会社 | Cd56陽性細胞の比率を高めるための方法 |
| CN115651906A (zh) * | 2022-09-14 | 2023-01-31 | 广东省科学院生物与医学工程研究所 | 一种将免疫引入骨修复的体外仿生组织评价模型及其应用 |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2007089442A (ja) * | 2005-09-28 | 2007-04-12 | Terumo Corp | 骨格筋芽細胞の分離方法 |
| JP2010099052A (ja) * | 2008-10-27 | 2010-05-06 | Olympus Corp | 細胞分離方法 |
| JP2012039906A (ja) * | 2010-08-17 | 2012-03-01 | Terumo Corp | 移植片移送用器具 |
| JP2016052270A (ja) * | 2014-09-03 | 2016-04-14 | テルモ株式会社 | シート状細胞培養物回収システムおよび方法 |
| JP2016052269A (ja) * | 2014-09-03 | 2016-04-14 | テルモ株式会社 | シート状細胞培養物回収システムおよび方法 |
Family Cites Families (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA2354978A1 (en) * | 2001-08-02 | 2003-02-02 | Universite Laval | Culture medium to improve the purity of myoblast culture |
| US20030211141A1 (en) * | 2001-12-11 | 2003-11-13 | Lebaron Richard G. | Genetic and protein manipulation of betaIG-H3 for the treatment and cure of muscular dystrophies |
| EP2266500B1 (en) | 2003-08-01 | 2015-04-01 | CellSeed Inc. | Three-dimensional tissue structure |
| KR101348325B1 (ko) * | 2006-01-31 | 2014-01-08 | 각고호우징 게이오기주크 | 줄기세포 및 태아에서 유래된 심근세포 및 예정 심근세포의제조방법 |
| CN101711277B (zh) * | 2007-07-31 | 2018-06-19 | 第一三共株式会社 | 心肌细胞的细胞块制备方法和该心肌细胞块的用途 |
| JP5378743B2 (ja) | 2008-09-30 | 2013-12-25 | テルモ株式会社 | 医療用細胞シートの製造方法 |
| JP5436905B2 (ja) | 2009-03-26 | 2014-03-05 | テルモ株式会社 | シート状細胞培養物の製造方法 |
| JP5667357B2 (ja) | 2009-11-30 | 2015-02-12 | テルモ株式会社 | シート状細胞培養物の製造方法 |
| JPWO2012118099A1 (ja) * | 2011-02-28 | 2014-07-07 | 学校法人東京女子医科大学 | サイトカイン産生細胞シートとその利用方法 |
| CN102191218B (zh) * | 2011-03-28 | 2013-07-24 | 遵义医学院附属医院 | 一种完全培养基及人羊膜间充质干细胞的培养方法 |
| US9822342B2 (en) | 2013-05-14 | 2017-11-21 | Kyoto University | Method of efficiently inducing cardiomyocytes |
| CN105229145B (zh) * | 2013-05-17 | 2020-08-28 | 泰尔茂株式会社 | 片状细胞培养物的制造方法 |
| EP3064578B1 (en) * | 2014-02-26 | 2021-01-13 | Terumo Kabushiki Kaisha | Method of producing cell population with high target cell purity |
| JP6495603B2 (ja) * | 2014-09-03 | 2019-04-03 | テルモ株式会社 | シート状細胞培養物とフィブリンゲルとの積層体の製造方法 |
| WO2016076368A1 (ja) * | 2014-11-12 | 2016-05-19 | テルモ株式会社 | 心筋細胞シート |
| WO2017038562A1 (ja) | 2015-08-31 | 2017-03-09 | 学校法人東京女子医科大学 | 多能性幹細胞を減少させる方法、多能性幹細胞を減少させた細胞集団の製造方法 |
-
2017
- 2017-11-01 SG SG11201805456WA patent/SG11201805456WA/en unknown
- 2017-11-01 JP JP2018521436A patent/JP6426327B2/ja active Active
- 2017-11-01 EP EP17867551.8A patent/EP3406704B1/en active Active
- 2017-11-01 CN CN201780031342.1A patent/CN109153963B/zh active Active
- 2017-11-01 WO PCT/JP2017/039476 patent/WO2018084167A1/ja not_active Ceased
-
2018
- 2018-10-24 JP JP2018199652A patent/JP2019030322A/ja active Pending
- 2018-11-13 US US16/188,630 patent/US11395863B2/en active Active
-
2022
- 2022-05-06 JP JP2022076414A patent/JP7282232B2/ja active Active
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2007089442A (ja) * | 2005-09-28 | 2007-04-12 | Terumo Corp | 骨格筋芽細胞の分離方法 |
| JP2010099052A (ja) * | 2008-10-27 | 2010-05-06 | Olympus Corp | 細胞分離方法 |
| JP2012039906A (ja) * | 2010-08-17 | 2012-03-01 | Terumo Corp | 移植片移送用器具 |
| JP2016052270A (ja) * | 2014-09-03 | 2016-04-14 | テルモ株式会社 | シート状細胞培養物回収システムおよび方法 |
| JP2016052269A (ja) * | 2014-09-03 | 2016-04-14 | テルモ株式会社 | シート状細胞培養物回収システムおよび方法 |
Non-Patent Citations (1)
| Title |
|---|
| REIS, M. V. P. ET AL.: "Effect of different storage media on root dentine composition and viability of fibroblasts evaluated by several assay methods", INTERNATIONAL ENDODONTIC JOURNAL, vol. 50, no. 12, 20 January 2017 (2017-01-20), pages 1185 - 1191, XP055481701 * |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2025183063A1 (ja) * | 2024-03-01 | 2025-09-04 | セントラル硝子株式会社 | 三次元細胞培養体の凍結物、三次元細胞培養体の凍結物を含む容器、細胞凍結キット、凍結保存液、三次元細胞培養体の凍結物の製造方法、三次元細胞培養体の凍結物の使用方法、および凍結保存液の使用方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| US11395863B2 (en) | 2022-07-26 |
| SG11201805456WA (en) | 2019-05-30 |
| CN109153963A (zh) | 2019-01-04 |
| CN109153963B (zh) | 2022-06-24 |
| JP7282232B2 (ja) | 2023-05-26 |
| JP2019030322A (ja) | 2019-02-28 |
| JPWO2018084167A1 (ja) | 2018-11-08 |
| JP6426327B2 (ja) | 2018-11-21 |
| EP3406704A1 (en) | 2018-11-28 |
| US20190076576A1 (en) | 2019-03-14 |
| JP2022105168A (ja) | 2022-07-12 |
| EP3406704A4 (en) | 2019-07-17 |
| EP3406704B1 (en) | 2021-10-20 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7282232B2 (ja) | 接着状態の細胞培養物の改変方法 | |
| CN105229145A (zh) | 片状细胞培养物的制造方法 | |
| JP2011172925A (ja) | 医療用積層体 | |
| WO2018097225A1 (ja) | 生細胞または生細胞を含む組成物の保存液 | |
| WO2018097228A1 (ja) | 生細胞または生細胞を含む組成物の保存液 | |
| JP5661048B2 (ja) | 脂肪細胞シート、その三次元構造体、及びそれらの製造方法 | |
| WO2018097226A1 (ja) | 生細胞または生細胞を含む組成物の保存液 | |
| EP3730604A1 (en) | Method for producing sheet-like cell culture | |
| WO2019189353A1 (ja) | 貫通構造を有するシート状細胞培養物 | |
| EP4086339A1 (en) | Bioadhesive sheet-like material for adhesion to organ surface | |
| JP6616559B2 (ja) | シート状細胞培養物の製造方法 | |
| JP7575392B2 (ja) | 心筋細胞のシート化方法 | |
| JP6843599B2 (ja) | 生細胞または生細胞を含む組成物の保存液 | |
| JP2021052661A (ja) | 多能性幹細胞を含む凍結保存細胞の希釈用緩衝液 | |
| JP7471069B2 (ja) | 移植片の活性を高める方法 | |
| WO2018097227A1 (ja) | 生細胞または生細胞を含む組成物の保存液 | |
| WO2020067434A1 (ja) | 細胞の凍結保存方法 | |
| WO2020067442A1 (ja) | 細胞の凍結保存方法 | |
| Soong | Development of a novel technology to engineer heart muscle for contractile and paracrine support in heart failure | |
| JP2019154361A (ja) | シート状細胞培養物の製造方法 | |
| JP2019154362A (ja) | シート状細胞培養物の製造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 2018521436 Country of ref document: JP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2017867551 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 2017867551 Country of ref document: EP Effective date: 20180823 |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 17867551 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |