WO2015122264A1 - 蒸気発生設備のスケール除去方法及びスケール除去剤 - Google Patents
蒸気発生設備のスケール除去方法及びスケール除去剤 Download PDFInfo
- Publication number
- WO2015122264A1 WO2015122264A1 PCT/JP2015/052012 JP2015052012W WO2015122264A1 WO 2015122264 A1 WO2015122264 A1 WO 2015122264A1 JP 2015052012 W JP2015052012 W JP 2015052012W WO 2015122264 A1 WO2015122264 A1 WO 2015122264A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- salt
- scale
- water
- steam generating
- polyacrylic acid
- Prior art date
Links
- 238000000034 method Methods 0.000 title claims abstract description 40
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims abstract description 112
- 150000003839 salts Chemical class 0.000 claims abstract description 84
- 229920002125 Sokalan® Polymers 0.000 claims abstract description 57
- 239000004584 polyacrylic acid Substances 0.000 claims abstract description 57
- 229920002845 Poly(methacrylic acid) Polymers 0.000 claims abstract description 47
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 claims abstract description 43
- 229910052742 iron Inorganic materials 0.000 claims abstract description 20
- 239000003795 chemical substances by application Substances 0.000 abstract description 21
- 238000005260 corrosion Methods 0.000 abstract description 10
- 230000007797 corrosion Effects 0.000 abstract description 10
- 238000012360 testing method Methods 0.000 description 32
- 230000000694 effects Effects 0.000 description 23
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 16
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 16
- 238000012546 transfer Methods 0.000 description 13
- KWYUFKZDYYNOTN-UHFFFAOYSA-M Potassium hydroxide Chemical compound [OH-].[K+] KWYUFKZDYYNOTN-UHFFFAOYSA-M 0.000 description 12
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 12
- 239000002738 chelating agent Substances 0.000 description 10
- 230000000052 comparative effect Effects 0.000 description 10
- 239000000377 silicon dioxide Substances 0.000 description 8
- 229910000029 sodium carbonate Inorganic materials 0.000 description 8
- 229910001220 stainless steel Inorganic materials 0.000 description 8
- 239000010935 stainless steel Substances 0.000 description 8
- 239000000126 substance Substances 0.000 description 7
- 239000002253 acid Substances 0.000 description 6
- 239000011575 calcium Substances 0.000 description 6
- 230000007423 decrease Effects 0.000 description 6
- 239000011777 magnesium Substances 0.000 description 6
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 6
- 229910000831 Steel Inorganic materials 0.000 description 5
- 239000003513 alkali Substances 0.000 description 5
- 229910000288 alkali metal carbonate Inorganic materials 0.000 description 5
- 150000008041 alkali metal carbonates Chemical class 0.000 description 5
- 150000008044 alkali metal hydroxides Chemical class 0.000 description 5
- 239000002270 dispersing agent Substances 0.000 description 5
- 239000003814 drug Substances 0.000 description 5
- 229940079593 drug Drugs 0.000 description 5
- 229920001444 polymaleic acid Polymers 0.000 description 5
- 239000010959 steel Substances 0.000 description 5
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 4
- 150000007513 acids Chemical class 0.000 description 4
- 239000007864 aqueous solution Substances 0.000 description 4
- 239000008367 deionised water Substances 0.000 description 4
- 229910021641 deionized water Inorganic materials 0.000 description 4
- 229920000193 polymethacrylate Polymers 0.000 description 4
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 3
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 3
- WMFOQBRAJBCJND-UHFFFAOYSA-M Lithium hydroxide Chemical compound [Li+].[OH-] WMFOQBRAJBCJND-UHFFFAOYSA-M 0.000 description 3
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 3
- 229940123973 Oxygen scavenger Drugs 0.000 description 3
- 239000000654 additive Substances 0.000 description 3
- 230000000996 additive effect Effects 0.000 description 3
- 229910000318 alkali metal phosphate Inorganic materials 0.000 description 3
- 150000001412 amines Chemical class 0.000 description 3
- 239000002585 base Substances 0.000 description 3
- 229910052791 calcium Inorganic materials 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 238000004140 cleaning Methods 0.000 description 3
- 229910052749 magnesium Inorganic materials 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- 239000000203 mixture Substances 0.000 description 3
- 230000003472 neutralizing effect Effects 0.000 description 3
- 229920000058 polyacrylate Polymers 0.000 description 3
- 229920000642 polymer Polymers 0.000 description 3
- 229910000027 potassium carbonate Inorganic materials 0.000 description 3
- 230000002265 prevention Effects 0.000 description 3
- 239000008400 supply water Substances 0.000 description 3
- RGHNJXZEOKUKBD-SQOUGZDYSA-N D-gluconic acid Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C(O)=O RGHNJXZEOKUKBD-SQOUGZDYSA-N 0.000 description 2
- OAKJQQAXSVQMHS-UHFFFAOYSA-N Hydrazine Chemical compound NN OAKJQQAXSVQMHS-UHFFFAOYSA-N 0.000 description 2
- QIGBRXMKCJKVMJ-UHFFFAOYSA-N Hydroquinone Chemical compound OC1=CC=C(O)C=C1 QIGBRXMKCJKVMJ-UHFFFAOYSA-N 0.000 description 2
- UQSXHKLRYXJYBZ-UHFFFAOYSA-N Iron oxide Chemical compound [Fe]=O UQSXHKLRYXJYBZ-UHFFFAOYSA-N 0.000 description 2
- YNAVUWVOSKDBBP-UHFFFAOYSA-N Morpholine Chemical compound C1COCCN1 YNAVUWVOSKDBBP-UHFFFAOYSA-N 0.000 description 2
- LSNNMFCWUKXFEE-UHFFFAOYSA-N Sulfurous acid Chemical compound OS(O)=O LSNNMFCWUKXFEE-UHFFFAOYSA-N 0.000 description 2
- PAFZNILMFXTMIY-UHFFFAOYSA-N cyclohexylamine Chemical compound NC1CCCCC1 PAFZNILMFXTMIY-UHFFFAOYSA-N 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- 238000001704 evaporation Methods 0.000 description 2
- 230000008020 evaporation Effects 0.000 description 2
- 230000001771 impaired effect Effects 0.000 description 2
- -1 iron ions Chemical class 0.000 description 2
- MGFYIUFZLHCRTH-UHFFFAOYSA-N nitrilotriacetic acid Chemical compound OC(=O)CN(CC(O)=O)CC(O)=O MGFYIUFZLHCRTH-UHFFFAOYSA-N 0.000 description 2
- 239000003002 pH adjusting agent Substances 0.000 description 2
- XAEFZNCEHLXOMS-UHFFFAOYSA-M potassium benzoate Chemical compound [K+].[O-]C(=O)C1=CC=CC=C1 XAEFZNCEHLXOMS-UHFFFAOYSA-M 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 230000000717 retained effect Effects 0.000 description 2
- 239000002455 scale inhibitor Substances 0.000 description 2
- 239000003352 sequestering agent Substances 0.000 description 2
- 159000000000 sodium salts Chemical class 0.000 description 2
- 229920002554 vinyl polymer Polymers 0.000 description 2
- 238000004065 wastewater treatment Methods 0.000 description 2
- BJEPYKJPYRNKOW-REOHCLBHSA-N (S)-malic acid Chemical compound OC(=O)[C@@H](O)CC(O)=O BJEPYKJPYRNKOW-REOHCLBHSA-N 0.000 description 1
- HXKKHQJGJAFBHI-UHFFFAOYSA-N 1-aminopropan-2-ol Chemical compound CC(O)CN HXKKHQJGJAFBHI-UHFFFAOYSA-N 0.000 description 1
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 1
- 229940058020 2-amino-2-methyl-1-propanol Drugs 0.000 description 1
- BFSVOASYOCHEOV-UHFFFAOYSA-N 2-diethylaminoethanol Chemical compound CCN(CC)CCO BFSVOASYOCHEOV-UHFFFAOYSA-N 0.000 description 1
- FAXDZWQIWUSWJH-UHFFFAOYSA-N 3-methoxypropan-1-amine Chemical compound COCCCN FAXDZWQIWUSWJH-UHFFFAOYSA-N 0.000 description 1
- RJWLLQWLBMJCFD-UHFFFAOYSA-N 4-methylpiperazin-1-amine Chemical compound CN1CCN(N)CC1 RJWLLQWLBMJCFD-UHFFFAOYSA-N 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-M Bisulfite Chemical compound OS([O-])=O LSNNMFCWUKXFEE-UHFFFAOYSA-M 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- CIWBSHSKHKDKBQ-DUZGATOHSA-N D-araboascorbic acid Natural products OC[C@@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-DUZGATOHSA-N 0.000 description 1
- RGHNJXZEOKUKBD-UHFFFAOYSA-N D-gluconic acid Natural products OCC(O)C(O)C(O)C(O)C(O)=O RGHNJXZEOKUKBD-UHFFFAOYSA-N 0.000 description 1
- RPNUMPOLZDHAAY-UHFFFAOYSA-N Diethylenetriamine Chemical compound NCCNCCN RPNUMPOLZDHAAY-UHFFFAOYSA-N 0.000 description 1
- 229920001174 Diethylhydroxylamine Polymers 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 1
- BJEPYKJPYRNKOW-UHFFFAOYSA-N alpha-hydroxysuccinic acid Natural products OC(=O)C(O)CC(O)=O BJEPYKJPYRNKOW-UHFFFAOYSA-N 0.000 description 1
- CBTVGIZVANVGBH-UHFFFAOYSA-N aminomethyl propanol Chemical compound CC(C)(N)CO CBTVGIZVANVGBH-UHFFFAOYSA-N 0.000 description 1
- 125000000129 anionic group Chemical group 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- 229960005070 ascorbic acid Drugs 0.000 description 1
- 238000005452 bending Methods 0.000 description 1
- 238000007664 blowing Methods 0.000 description 1
- 230000009172 bursting Effects 0.000 description 1
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 1
- XEVRDFDBXJMZFG-UHFFFAOYSA-N carbonyl dihydrazine Chemical compound NNC(=O)NN XEVRDFDBXJMZFG-UHFFFAOYSA-N 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 239000013522 chelant Substances 0.000 description 1
- 230000009920 chelation Effects 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 238000012790 confirmation Methods 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- FVCOIAYSJZGECG-UHFFFAOYSA-N diethylhydroxylamine Chemical compound CCN(O)CC FVCOIAYSJZGECG-UHFFFAOYSA-N 0.000 description 1
- BNIILDVGGAEEIG-UHFFFAOYSA-L disodium hydrogen phosphate Chemical compound [Na+].[Na+].OP([O-])([O-])=O BNIILDVGGAEEIG-UHFFFAOYSA-L 0.000 description 1
- 229910000397 disodium phosphate Inorganic materials 0.000 description 1
- 235000019800 disodium phosphate Nutrition 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 239000004318 erythorbic acid Substances 0.000 description 1
- 235000010350 erythorbic acid Nutrition 0.000 description 1
- 239000000446 fuel Substances 0.000 description 1
- KWMLJOLKUYYJFJ-VFUOTHLCSA-N glucoheptonic acid Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)[C@@H](O)C(O)=O KWMLJOLKUYYJFJ-VFUOTHLCSA-N 0.000 description 1
- 239000000174 gluconic acid Substances 0.000 description 1
- 235000012208 gluconic acid Nutrition 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- 150000002443 hydroxylamines Chemical class 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 238000005342 ion exchange Methods 0.000 description 1
- 235000014413 iron hydroxide Nutrition 0.000 description 1
- NCNCGGDMXMBVIA-UHFFFAOYSA-L iron(ii) hydroxide Chemical compound [OH-].[OH-].[Fe+2] NCNCGGDMXMBVIA-UHFFFAOYSA-L 0.000 description 1
- 229940026239 isoascorbic acid Drugs 0.000 description 1
- 239000001630 malic acid Substances 0.000 description 1
- 235000011090 malic acid Nutrition 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 150000002772 monosaccharides Chemical class 0.000 description 1
- LNOPIUAQISRISI-UHFFFAOYSA-N n'-hydroxy-2-propan-2-ylsulfonylethanimidamide Chemical compound CC(C)S(=O)(=O)CC(N)=NO LNOPIUAQISRISI-UHFFFAOYSA-N 0.000 description 1
- 235000019645 odor Nutrition 0.000 description 1
- 150000007524 organic acids Chemical class 0.000 description 1
- 238000013021 overheating Methods 0.000 description 1
- RECVMTHOQWMYFX-UHFFFAOYSA-N oxygen(1+) dihydride Chemical group [OH2+] RECVMTHOQWMYFX-UHFFFAOYSA-N 0.000 description 1
- 238000010979 pH adjustment Methods 0.000 description 1
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 1
- 239000011574 phosphorus Substances 0.000 description 1
- 229910052698 phosphorus Inorganic materials 0.000 description 1
- 238000005498 polishing Methods 0.000 description 1
- 229920001495 poly(sodium acrylate) polymer Polymers 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- SBMSLRMNBSMKQC-UHFFFAOYSA-N pyrrolidin-1-amine Chemical compound NN1CCCC1 SBMSLRMNBSMKQC-UHFFFAOYSA-N 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 230000002829 reductive effect Effects 0.000 description 1
- 238000001223 reverse osmosis Methods 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 235000002639 sodium chloride Nutrition 0.000 description 1
- 239000001488 sodium phosphate Substances 0.000 description 1
- NNMHYFLPFNGQFZ-UHFFFAOYSA-M sodium polyacrylate Chemical compound [Na+].[O-]C(=O)C=C NNMHYFLPFNGQFZ-UHFFFAOYSA-M 0.000 description 1
- 235000019832 sodium triphosphate Nutrition 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- 230000009897 systematic effect Effects 0.000 description 1
- 229920001864 tannin Polymers 0.000 description 1
- 239000001648 tannin Substances 0.000 description 1
- 235000018553 tannin Nutrition 0.000 description 1
- 238000005979 thermal decomposition reaction Methods 0.000 description 1
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical compound [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 description 1
- 229910000406 trisodium phosphate Inorganic materials 0.000 description 1
- 235000019801 trisodium phosphate Nutrition 0.000 description 1
- 238000011144 upstream manufacturing Methods 0.000 description 1
- 238000005303 weighing Methods 0.000 description 1
- 230000004580 weight loss Effects 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F5/00—Softening water; Preventing scale; Adding scale preventatives or scale removers to water, e.g. adding sequestering agents
- C02F5/08—Treatment of water with complexing chemicals or other solubilising agents for softening, scale prevention or scale removal, e.g. adding sequestering agents
- C02F5/10—Treatment of water with complexing chemicals or other solubilising agents for softening, scale prevention or scale removal, e.g. adding sequestering agents using organic substances
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B08—CLEANING
- B08B—CLEANING IN GENERAL; PREVENTION OF FOULING IN GENERAL
- B08B3/00—Cleaning by methods involving the use or presence of liquid or steam
- B08B3/02—Cleaning by the force of jets or sprays
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B08—CLEANING
- B08B—CLEANING IN GENERAL; PREVENTION OF FOULING IN GENERAL
- B08B3/00—Cleaning by methods involving the use or presence of liquid or steam
- B08B3/02—Cleaning by the force of jets or sprays
- B08B3/022—Cleaning travelling work
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B08—CLEANING
- B08B—CLEANING IN GENERAL; PREVENTION OF FOULING IN GENERAL
- B08B3/00—Cleaning by methods involving the use or presence of liquid or steam
- B08B3/04—Cleaning involving contact with liquid
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B08—CLEANING
- B08B—CLEANING IN GENERAL; PREVENTION OF FOULING IN GENERAL
- B08B3/00—Cleaning by methods involving the use or presence of liquid or steam
- B08B3/04—Cleaning involving contact with liquid
- B08B3/041—Cleaning travelling work
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F120/00—Homopolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical or a salt, anhydride, ester, amide, imide or nitrile thereof
- C08F120/02—Monocarboxylic acids having less than ten carbon atoms; Derivatives thereof
- C08F120/04—Acids; Metal salts or ammonium salts thereof
- C08F120/06—Acrylic acid; Methacrylic acid; Metal salts or ammonium salts thereof
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L33/00—Compositions of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and only one being terminated by only one carboxyl radical, or of salts, anhydrides, esters, amides, imides or nitriles thereof; Compositions of derivatives of such polymers
- C08L33/02—Homopolymers or copolymers of acids; Metal or ammonium salts thereof
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/16—Organic compounds
- C11D3/20—Organic compounds containing oxygen
- C11D3/2075—Carboxylic acids-salts thereof
- C11D3/2082—Polycarboxylic acids-salts thereof
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/16—Organic compounds
- C11D3/37—Polymers
- C11D3/3746—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds
- C11D3/3757—(Co)polymerised carboxylic acids, -anhydrides, -esters in solid and liquid compositions
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/16—Organic compounds
- C11D3/37—Polymers
- C11D3/3746—Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds
- C11D3/3757—(Co)polymerised carboxylic acids, -anhydrides, -esters in solid and liquid compositions
- C11D3/3765—(Co)polymerised carboxylic acids, -anhydrides, -esters in solid and liquid compositions in liquid compositions
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D7/00—Compositions of detergents based essentially on non-surface-active compounds
- C11D7/22—Organic compounds
- C11D7/26—Organic compounds containing oxygen
- C11D7/265—Carboxylic acids or salts thereof
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23F—NON-MECHANICAL REMOVAL OF METALLIC MATERIAL FROM SURFACE; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL; MULTI-STEP PROCESSES FOR SURFACE TREATMENT OF METALLIC MATERIAL INVOLVING AT LEAST ONE PROCESS PROVIDED FOR IN CLASS C23 AND AT LEAST ONE PROCESS COVERED BY SUBCLASS C21D OR C22F OR CLASS C25
- C23F14/00—Inhibiting incrustation in apparatus for heating liquids for physical or chemical purposes
- C23F14/02—Inhibiting incrustation in apparatus for heating liquids for physical or chemical purposes by chemical means
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F22—STEAM GENERATION
- F22B—METHODS OF STEAM GENERATION; STEAM BOILERS
- F22B37/00—Component parts or details of steam boilers
- F22B37/02—Component parts or details of steam boilers applicable to more than one kind or type of steam boiler
- F22B37/48—Devices for removing water, salt, or sludge from boilers; Arrangements of cleaning apparatus in boilers; Combinations thereof with boilers
- F22B37/52—Washing-out devices
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F2303/00—Specific treatment goals
- C02F2303/22—Eliminating or preventing deposits, scale removal, scale prevention
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D2111/00—Cleaning compositions characterised by the objects to be cleaned; Cleaning compositions characterised by non-standard cleaning or washing processes
- C11D2111/10—Objects to be cleaned
- C11D2111/14—Hard surfaces
- C11D2111/20—Industrial or commercial equipment, e.g. reactors, tubes or engines
Definitions
- the present invention relates to a scale removal method and a scale remover for efficiently removing scale adhered to a boiler can of a steam generation facility.
- scale components such as calcium, magnesium, silica, and iron brought into the boiler can scale and adhere to the heat transfer surface with a high heat load, causing expansion, bending, and bursting due to overheating of the steel material.
- scale components such as calcium, magnesium, silica, and iron brought into the boiler can scale and adhere to the heat transfer surface with a high heat load, causing expansion, bending, and bursting due to overheating of the steel material.
- An anti-scaling agent prevents the hardness component brought into the aqueous system from being scaled.
- Phosphate salts such as trisodium phosphate and sodium tripolyphosphate, and polymers such as sodium polyacrylate are used as scale inhibitors.
- Patent Document 1 describes a scale removal method by chemical cleaning using a chelating agent such as high-concentration ethylenediaminetetraacetic acid (EDTA) or an organic acid such as sulfamic acid.
- EDTA high-concentration ethylenediaminetetraacetic acid
- organic acid such as sulfamic acid.
- Patent Document 2 proposes a method for removing the scale without stopping the operation of the boiler.
- a specific chelating agent such as EDTA, nitrilotriacetic acid (NTA) or diethylenetriamine and a specific dispersing agent such as polymaleic acid are added to the boiler can, and the scale is removed while the boiler is operating. To do.
- the chelating agent used in the scale removal method of Patent Document 2 acts on iron, which is a base material of a boiler, and corrosion occurs.
- Patent Document 3 proposes a method in which a chelating agent and an anticorrosive agent are used in combination.
- the scale is removed by the chelating agent while the corrosion is suppressed by the chelating agent and aldonic acid or a salt thereof.
- Patent Document 4 proposes a method for removing the hardness scale without using a chelating agent.
- a composition comprising a mixture of a polymeric sequestering agent and another water-soluble anionic vinyl polymer dispersant is used.
- Patent Document 4 describes that adhesion of a hardness scale can be prevented or an effect of removing the adhered scale can be obtained by adding a high concentration of the sequestering agent and dispersant. Has been.
- This invention is made
- the scale adhering to the inside of a boiler can etc. can be efficiently removed by the chemical addition amount which can be economically accepted, without corroding a boiler. It is an object to provide a descaling method and a descaling agent.
- An object of the present invention is to provide a scale removal method and a scale remover that can efficiently remove scale adhered to a boiler can even in equipment operated with water supply containing high concentration of iron. To do.
- the present inventors have efficiently used a scale attached to the system with a small addition concentration by using polyacrylic acid and / or a salt thereof in a specific molecular weight range. It has been found that it can be removed. As a result of further research, even when drain is collected and reused as feed water, or when steel is used in highly corrosive economizers, even when iron is contained in a high concentration in the feed water The present inventors have found that the above-described problems can be solved without reducing the scale removal efficiency by using polymethacrylic acid and / or a salt thereof in a specific molecular weight range together.
- the gist of the present invention is as follows.
- a scale removal method for removing scale adhered to a system of a steam generation facility polyacrylic acid having a weight average molecular weight of more than 20,000 and not more than 170,000 and / or a salt thereof is contained in the steam generation facility.
- a method for removing the scale of a steam generating facility comprising adding water or feed water of the steam generating facility.
- the concentration of the polyacrylic acid and / or salt thereof in the water of the steam generation unit of the steam generation facility is 1 to 1,000 mg / L in [1].
- the method for removing the scale of the steam generating facility is characterized in that it is added as described above.
- the water supply of the steam generation facility contains iron, and further, polymethacrylic acid having a weight average molecular weight of more than 1,000 and not more than 100,000 and / or a salt thereof is added to the steam generation facility.
- a method for removing the scale of the steam generating facility which comprises adding the water to the water in the water or the feed water of the steam generating facility.
- the concentration of the polymethacrylic acid and / or salt thereof in the water of the steam generation unit of the steam generation facility is 1 to 1,000 mg / L in [3].
- the method for removing the scale of the steam generating facility is characterized in that it is added as described above.
- the weight concentration ratio of polyacrylic acid and / or a salt thereof to polymethacrylic acid and / or a salt thereof in the water in the steam generating part of the steam generating facility is from 1: 100 to A method for removing scale from a steam generating facility, comprising adding polyacrylic acid and / or a salt thereof and polymethacrylic acid and / or a salt thereof so as to be 100: 1.
- a scale remover that removes the scale attached to the system of the steam generating facility, polyacrylic acid having a weight average molecular weight of more than 20,000 and not more than 170,000 and / or a salt thereof, and a weight average molecular weight of 1,000.
- a scale remover for steam generating equipment comprising more than 100,000 and not more than 100,000 polymethacrylic acid and / or a salt thereof.
- scale attached to the system during operation of the steam generation facility can be efficiently removed with a relatively small amount of chemicals used without using a chelating agent and without corroding the system. Moreover, even if high concentration iron exists in the water supply, a high scale removal effect can be obtained.
- the polyacrylic acid having a weight average molecular weight of more than 20,000 and not more than 170,000 is attached to the scale attached to the boiler can, that is, the steam generation unit.
- / or a salt thereof hereinafter referred to as “polyacrylic acid (salt)”
- polyacrylic acid (salt) is added to the water in the steam generation facility or the feed water of the steam generation facility.
- polymethacrylic acid having a weight average molecular weight of more than 1,000 and not more than 100,000 and / or a salt thereof (hereinafter referred to as “polymethacrylic acid (salt)”. ) In combination.
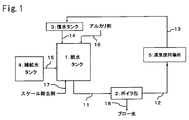
- FIG. 1 is a system diagram showing an embodiment of a steam generation facility for carrying out the present invention.
- 1 is a water supply tank
- 2 is a boiler can (steam generation unit)
- 3 is a condensate tank
- 4 is makeup water.
- Tank 5 is a place where steam is used.
- the water supply in the water supply tank 1 is fed from the water supply line 11 to the boiler can 2.
- the steam generated in the boiler can 2 is fed from the steam line 12 to the steam use place 5, and the condensate is circulated to the feed water tank 1 through the circulation line 13, the condensate tank 3 and the condensate line 14.
- the water supply tank 1 is supplied with supply water in the supply water tank 4 from the supply water line 15, added with an alkali agent from the alkali agent addition line 16, and added with a scale remover from the scale remover line 17. Is done. Blow water is discharged from the boiler can 2 through the blow line 18.
- a polyacrylic acid (salt) or a scale remover containing polyacrylic acid (salt) and polymethacrylic acid (salt) is added to the water supply in the water supply tank 1.
- the scale removing agent may be added to the makeup water tank 4, may be added to the condensate tank 3, the transfer line of each water system, or may be added to these two or more locations.
- polyacrylic acid (salt) and polymethacrylic acid (salt) these may be added to a separate location and may be added to the same location.
- polyacrylic acid (salt) and polymethacrylic acid (salt) may be mixed and added in advance, or may be added separately. The same applies to other optional components described later.
- FIG. 1 shows a circulation type steam generation facility, but the present invention is not limited to the circulation type and can be applied to a once-through type and other steam generation facilities.
- raw water treated with a reverse osmosis membrane raw water softened, raw water ion exchange treated, or the like can be used.
- the operating conditions of the steam generating facility are not particularly limited, but the operating pressure is preferably 0.2 to 4 MPa, more preferably 0.2 to 3 MPa. If it is lower than 0.2 MPa, a sufficient scale removal effect cannot be obtained. When the pressure is higher than 4 PMa, polymers such as polyacrylic acid (salt) and polymethacrylic acid (salt) are affected by thermal decomposition, and the scale removal effect is reduced.
- polyacrylic acid (salt) used as a scale removing component polyacrylic acid (salt) used as a scale removing component
- polymethacrylic acid (salt) used in combination with polyacrylic acid (salt) polymethacrylic acid (salt)
- Other optional additive components that can be used in combination will be described.
- the polyacrylic acid is not particularly limited, and a polyacrylic acid that satisfies the condition of the weight average molecular weight described later can be used.
- examples of the polyacrylate include sodium salt and potassium salt of the polyacrylic acid.
- Polyacrylic acid salt can be produced by adding together with polyacrylic acid, alkali metal hydroxides such as sodium hydroxide and potassium hydroxide, alkali metal carbonates such as sodium carbonate and potassium carbonate, and the like.
- the weight average molecular weight of the polyacrylic acid used in the present invention is more than 20,000 and not more than 170,000, preferably more than 50,000 and not more than 120,000.
- weight average molecular weight of polyacrylic acid is less than 20,000, a sufficient scale removal effect cannot be obtained. Even if the weight average molecular weight of polyacrylic acid exceeds 170,000, the descaling effect decreases.
- the weight average molecular weight of the polyacrylic acid serving as the base of the polyacrylate satisfies the above conditions.
- the amount of polyacrylic acid (salt) added should be such that the concentration in the water of the steam generating part of the steam generating equipment, that is, the concentration in the boiler water is 1 to 1,000 mg / L, particularly 10 to 500 mg / L. Is preferred.
- polyacrylic acid salt
- concentration in the boiler water is in the above range according to the concentration rate of the steam generating facility.
- the polyacrylic acid (salt) is preferably added as an aqueous solution having a concentration of 0.1 to 30% by weight, particularly 0.5 to 10% by weight, using deionized water.
- Iron in water is usually insoluble in water such as iron hydroxide or iron oxide, while water-soluble is dissolved and dissociated in water and exists as iron ions. is doing.
- iron when iron is included in the water supply in excess of 0.3 mg / L, including suspended and dissolved, for example, when it is contained at a high concentration of 0.4 to 5.0 mg / L It is preferable to use polymethacrylic acid (salt) together with polyacrylic acid (salt).
- Polymethacrylic acid is not particularly limited, and those satisfying the following weight average molecular weight conditions are preferably used.
- the polymethacrylate include sodium salt and potassium salt of the polymethacrylic acid.
- Polymethacrylate can be produced by adding together with polymethacrylic acid, alkali metal hydroxides such as sodium hydroxide and potassium hydroxide, alkali metal carbonates such as sodium carbonate and potassium carbonate, and the like.
- the weight average molecular weight of the polymethacrylic acid used in the present invention is preferably 1,000 or more and 100,000 or less, more preferably 5,000 or more and 60,000 or less.
- weight average molecular weight of polymethacrylic acid is less than 1,000, sufficient iron scale prevention effect may not be obtained, and if the weight average molecular weight of polymethacrylic acid exceeds 100,000, the effect decreases.
- polymethacrylate it is only necessary that the weight average molecular weight of polymethacrylic acid serving as a base of polymethacrylate satisfies the above conditions.
- the amount of polymethacrylic acid (salt) added is such that the concentration in the water of the steam generating part of the steam generating facility, that is, the concentration in the boiler water is 1 to 1,000 mg / L, particularly 10 to 500 mg / L. It is preferable.
- polymethacrylic acid salt
- concentration in the boiler water is in the above range according to the concentration ratio of the steam generating facility.
- the polymethacrylic acid (salt) is preferably added as an aqueous solution having a concentration of 0.1 to 30% by weight, particularly 0.5 to 20% by weight, prepared using deionized water.
- additive components such as a pH adjuster, an oxygen scavenger, and an anticorrosion agent are provided at any point in the system of the steam generation facility as necessary.
- An effective amount of an agent, a scale dispersant and the like can be added.
- the pH of the boiler water is preferably 11.0 or more, and preferably 12.0 or less from the viewpoint of preventing corrosion in the boiler can or the steam generating equipment system.
- Examples of a method for adjusting the pH of boiler water to 11.0 or higher include a method of adding an alkaline agent and a method of adjusting the concentration rate by adjusting the blow amount and / or the amount of water supply. Among these, a method of adding an alkaline agent is preferable from the viewpoint of easy pH adjustment.
- alkali agents for adjusting pH examples include alkali metal hydroxides, alkali metal carbonates, alkali metal phosphates, neutralizing amines, and the like.
- Examples of the alkali metal hydroxide include sodium hydroxide, potassium hydroxide, and lithium hydroxide.
- Examples of the alkali metal carbonate include sodium carbonate and potassium carbonate.
- Examples of the alkali metal phosphate include phosphorus. Examples thereof include trisodium acid and sodium hydrogen phosphate.
- neutralizing amines examples include monoethanolamine, cyclohexylamine, morpholine, diethylethanolamine, monoisopropanolamine, 3-methoxypropylamine, 2-amino-2-methyl-1-propanol and the like.
- alkali agents neutralizing amines migrate to the steam condensate system, so if added at a high concentration, odors are generated in the steam and condensate, or the pH of the steam condensate system rises too much, and there is a copper-based material in the system. May cause corrosion.
- an alkali agent an alkali metal hydroxide, an alkali metal carbonate, and an alkali metal phosphate are preferable, and sodium hydroxide, potassium hydroxide, sodium carbonate, etc. are more preferable from an economical viewpoint.
- the above alkaline agents can be used singly or in combination of two or more.
- Alkaline agents are also preferably added to make-up water or water supply, similar to the scale remover.
- the steam generating facility is a circulation type, it may be added to the condensate.
- the steam generation facility preferably has pH measuring means on the upstream side and / or the downstream side of the boiler can.
- oxygen scavengers include various hydroxylamines such as hydrazine, carbohydrazide and diethylhydroxylamine, N-amino heterocyclic compounds such as 1-aminopyrrolidine and 1-amino-4-methylpiperazine, hydroquinone, hydrolyzed and condensed types Tannins (acids) and salts thereof, erythorbic acid and ascorbic acid and salts thereof, aldonic acids and salts thereof such as gluconic acid and alpha glucoheptonic acid, sugars such as glucose (monosaccharides and polysaccharides), sulfurous acid and meta Examples thereof include sulfite-based substances such as bisulfite and salts thereof. You may use these individually by 1 type or in combination of 2 or more types.
- anticorrosive examples include polycarboxylic acids such as succinic acid, citric acid and malic acid, oxycarboxylic acids and salts thereof. You may use these individually by 1 type or in combination of 2 or more types.
- the scale remover of the present invention contains various water treatment agent components such as the aforementioned pH adjuster, oxygen scavenger, anticorrosive, and scale dispersant as required, as long as the object of the present invention is not impaired. Also good.
- the scale remover of the present invention may be one obtained by integrating polyacrylic acid (salt) and polymethacrylic acid (salt), and these may be supplied separately.
- polyacrylic acid salt
- polymethacrylic acid salt
- polymethacrylic acid is usually dissolved in deionized water and used as an aqueous solution having a concentration of 0.1 to 30% by weight, particularly 0.5 to 20% by weight.
- CaCl 2 is used as Ca hardness
- MgCl 2 is used as Mg hardness
- Na 2 SiO 3 is used as silica
- FeCl 2 is used as Fe.
- Synthetic water A Synthetic water with Ca hardness 20 mg CaCO 3 / L, Mg hardness 10 mg CaCO 3 / L, silica concentration 15 mg / L, sodium carbonate concentration 30 mg / L
- Synthetic water B silica concentration 15 mg / L, weight average shown in Table 1 Synthetic water having a molecular weight of polyacrylic acid or polymaleic acid concentration of 10 mg / L and sodium carbonate concentration of 32 mg / L
- a strainer with a diameter of 20 mm and 60 mesh was installed in the blow line.
- This stainless steel test boiler was adjusted to have a pressure of 2.0 MPa, an evaporation amount of 9.0 L / h, a blow rate of 1.0 L / h, and a concentration factor of 10 times while supplying synthetic water A, and was operated for 24 hours. .
- the heat transfer tube with the scale attached after operation was taken out and weighed to calculate the scale attached amount. Thereafter, the heat transfer tube was again inserted into the stainless steel test boiler, and the scale removal step was performed by operating with synthetic water B under the same operating conditions for 3 days.
- the heat transfer tube was taken out and weighed to calculate the scale adhesion amount, and the scale removal rate was calculated from the scale adhesion amount before and after the scale removal step.
- the scale removal rate is low when the weight average molecular weight of the polyacrylic acid is 20,000 or less, and the scale removal rate is high when the weight average molecular weight exceeds 20,000 and 170,000 or less. It can be seen that the scale removal effect is high.
- polymaleic acid was found to have a certain degree of scale removal effect, but a large amount of deposits were observed on the blow strainer, and most of the strainer was covered, and it was just before the blockage. This was presumed to be due to the gelled product produced by the reaction between the hardness component and polymaleic acid.
- Synthetic water C Synthetic water with Ca hardness 40 mg CaCO 3 / L, Mg hardness 20 mg CaCO 3 / L, silica concentration 30 mg / L, sodium carbonate concentration 30 mg / L, Fe concentration 1 mg / L
- Synthetic water D silica concentration 30 mg / L, chemical Synthetic water having a polyacrylic acid concentration of 5 mg / L of the weight average molecular weight described in Table 2 as 1 and a concentration of 5 mg / L, 2 mg of Fe concentration of 1 mg / L of Fe, and 32 mg / L of sodium carbonate as drug 2 (however, In Comparative Example II-1, Drug 1 and Drug 2 are not added, and in Comparative Examples II-2 to 8-8, Drug 2 is not added.)
- This stainless steel test boiler was adjusted to a pressure of 0.7 MPa, an evaporation amount of 11.7 L / h, a blow rate of 1.3 L / h, and a concentration factor of 10 times while supplying synthetic water C, and was operated for 21 hours. .
- the heat transfer tube with the scale attached after operation was taken out and weighed to calculate the scale attached amount. Thereafter, the heat transfer tube was inserted again into the stainless steel test boiler, and tested with synthetic water D under the same operating conditions, and the scale removal step was performed. Similarly, after the operation, the heat transfer tube was taken out and weighed to calculate the scale adhesion amount, and the scale removal amount was calculated from the scale adhesion amount before and after the scale removal step.
- the amount of scale removal is evaluated by taking out the heat transfer tube every 3 days, weighing it, and inserting it again, performing the scale removal process for a total of 9 days, and removing the scale after the scale removal process for 9 days (scale removal)
- scale removal The ratio of the total scale that could be removed in 9 days relative to the scale attached at the start of the process was calculated.
- Table 2 shows the following.
- Comparative Example II-11 uses two types of polymers together, but its removal effect is low. Similarly, Comparative Examples II-9 and II-10 have a low removal effect.
- the scale is removed at a constant rate even after the number of days has passed. This is because by using polymethacrylic acid in combination, the scale removal effect by polyacrylic acid having a high weight average molecular weight is kept constant while preventing iron scale adhesion.
- Test Example III Examples III-1 to 6, Comparative Examples III-1 to 4
- a test piece made of steel SPCC, 15 ⁇ 50 ⁇ 10 mm, # 400 polishing
- the synthetic water contained in was supplied under the same conditions as in Test Example II, and a corrosive confirmation test was conducted.
- the pH in the boiler can was adjusted to 11.3.
- Corrosion rate (mdd) test piece corrosion weight loss (mg) / (Surface area of test piece (dm 2 ) ⁇ test period (day)) (1)
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Health & Medical Sciences (AREA)
- General Chemical & Material Sciences (AREA)
- Mechanical Engineering (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Wood Science & Technology (AREA)
- Polymers & Plastics (AREA)
- Medicinal Chemistry (AREA)
- General Engineering & Computer Science (AREA)
- Thermal Sciences (AREA)
- Hydrology & Water Resources (AREA)
- Environmental & Geological Engineering (AREA)
- Water Supply & Treatment (AREA)
- Physics & Mathematics (AREA)
- Emergency Medicine (AREA)
- Materials Engineering (AREA)
- Metallurgy (AREA)
- Preventing Corrosion Or Incrustation Of Metals (AREA)
- Detergent Compositions (AREA)
- Cleaning And De-Greasing Of Metallic Materials By Chemical Methods (AREA)
- Air Humidification (AREA)
Abstract
Description
本発明の蒸気発生設備のスケール除去方法では、蒸気発生設備において、ボイラ缶即ち蒸気発生部等の系内に付着したスケールを、重量平均分子量が20,000を超え170,000以下のポリアクリル酸及び/又はその塩(以下、「ポリアクリル酸(塩)」と称す。)を該蒸気発生設備内の水又は該蒸気発生設備の給水に添加する。蒸気発生設備の給水が鉄を含む場合には、好ましくは重量平均分子量1,000を超え100,000以下のポリメタクリル酸及び/又はその塩(以下、「ポリメタクリル酸(塩)」と称す。)を併用添加する。
ポリアクリル酸は特に限定されず、後述する重量平均分子量の条件を満たすものを用いることが可能である。ポリアクリル酸塩としては、前記ポリアクリル酸のナトリウム塩、カリウム塩等が挙げられる。ポリアクリル酸塩は、ポリアクリル酸とともに、水酸化ナトリウム、水酸化カリウム等のアルカリ金属水酸化物、炭酸ナトリウム、炭酸カリウム等のアルカリ金属炭酸塩等を添加することにより生成させることができる。
蒸気発生設備の給水中に鉄が含まれる場合、上記のポリアクリル酸(塩)と共にポリメタクリル酸(塩)を併用することが好ましい。
本発明においては、本発明の目的が損なわれない範囲で、必要に応じて、蒸気発生設備の系内の何れかの箇所で、各種の添加成分、例えば、pH調整剤、脱酸素剤、防食剤、スケール分散剤等を有効量添加することできる。これらの添加成分は1種単独で又は2種以上を組み合わせて用いることができる。
本発明の蒸気発生設備のスケール除去剤は、重量平均分子量20,000を超え170,000以下、好ましくは50,000を超え120,000以下のポリアクリル酸(塩)と、重量平均分子量1,000を超え100,000以下、好ましくは5,000を超え60,000以下のポリメタクリル酸(塩)とを含むことを特徴とするものであり、好ましくはポリアクリル酸(塩)とポリメタクリル酸(塩)とをポリアクリル酸(塩):ポリメタクリル酸(塩)=1:100~100:1、より好ましくは1:50~10:1の重量比で含むものである。
以下の試験装置及び給水を用い、以下の条件で以下のスケール除去試験を行い、ポリアクリル酸とポリマレイン酸のスケール除去効果の評価を行った。
ステンレス製テストボイラ(保有水量5L)
合成水A:Ca硬度20mgCaCO3/L、Mg硬度10mgCaCO3/L、シリカ濃度15mg/L、炭酸ナトリウム濃度30mg/Lの合成水
合成水B:シリカ濃度15mg/L、表1に記載の重量平均分子量のポリアクリル酸又はポリマレイン酸濃度10mg/L、炭酸ナトリウム濃度32mg/Lの合成水
給水温度:40℃
運転圧力:2.0MPa
給水量:10L/h
濃縮倍率:10倍
ボイラ水pH:11.4
伝熱チューブ(鋼材製、表面積200cm2×3本)を秤量して記録した後、ステンレス製テストボイラに挿入した。
表1より、ポリアクリル酸の重量平均分子量が20,000以下ではスケール除去率は低く、20,000を超え170,000以下でスケール除去率が高くなり、特に50,000を超え120,000以下でスケール除去効果が高いことが分かる。
以下の試験装置及び給水を用い、以下の条件で以下のスケール除去試験を行い、ポリアクリル酸の重量平均分子量とポリメタクリル酸の併用がスケール除去効果に与える影響を評価した。
ステンレス製テストボイラ(保有水量5L)
合成水C:Ca硬度40mgCaCO3/L、Mg硬度20mgCaCO3/L、シリカ濃度30mg/L、炭酸ナトリウム濃度30mg/L、Fe濃度1mg/Lの合成水
合成水D:シリカ濃度30mg/L、薬剤1として表2に記載の重量平均分子量のポリアクリル酸濃度5mg/L、薬剤2として表2に示すものを濃度5mg/L、Fe濃度1mg/L、炭酸ナトリウム濃度32mg/Lの合成水(ただし、比較例II-1では、薬剤1と薬剤2を添加せず、比較例II-2~8では薬剤2を添加せず。)
給水温度:40℃
運転圧力:0.7MPa
給水量:13L/h
濃縮倍率:10倍
ボイラ水pH:11.5
伝熱チューブ(鋼材製、表面積200cm2×3本)を秤量して記録した後、ステンレス製テストボイラに挿入した。
表2より、以下のことが分かる。
試験例IIにおいて、テストボイラの缶内に鋼材製のテストピース(SPCC、15×50×10mm、♯400研磨)を設置し、表3に示す薬剤を表3に示すボイラ缶内濃度となるように含む合成水を試験例IIと同様の条件で給水して腐食性の確認試験を行った。ボイラ缶内のpHは11.3に調整した。
腐食速度(mdd)=テストピースの腐食減量(mg)/
(テストピースの表面積(dm2)×試験期間(day))…(1)
本出願は、2014年2月13日付で出願された日本特許出願2014-025459に基づいており、その全体が引用により援用される。
Claims (7)
- 蒸気発生設備の系内に付着したスケールを除去するスケール除去方法において、
重量平均分子量が20,000を超え170,000以下のポリアクリル酸及び/又はその塩を該蒸気発生設備内の水又は該蒸気発生設備の給水に添加することを特徴とする蒸気発生設備のスケール除去方法。 - 請求項1において、前記ポリアクリル酸及び/又はその塩を前記蒸気発生設備の蒸気発生部の水中の該ポリアクリル酸及び/又はその塩の濃度が1~1,000mg/Lとなるように添加することを特徴とする蒸気発生設備のスケール除去方法。
- 請求項1又は2において、前記蒸気発生設備の給水が鉄を含み、更に重量平均分子量1,000を超え100,000以下のポリメタクリル酸及び/又はその塩を該蒸気発生設備内の水又は該蒸気発生設備の給水に添加することを特徴とする蒸気発生設備のスケール除去方法。
- 請求項3において、前記ポリメタクリル酸及び/又はその塩を前記蒸気発生設備の蒸気発生部の水中の該ポリメタクリル酸及び/又はその塩の濃度が1~1,000mg/Lとなるように添加することを特徴とする蒸気発生設備のスケール除去方法。
- 請求項3又は4において、前記蒸気発生設備の蒸気発生部の水中のポリアクリル酸及び/又はその塩とポリメタクリル酸及び/又はその塩との重量濃度比が1:100~100:1となるように、ポリアクリル酸及び/又はその塩とポリメタクリル酸及び/又はその塩を添加することを特徴とする蒸気発生設備のスケール除去方法。
- 蒸気発生設備の系内に付着したスケールを除去するスケール除去剤において、重量平均分子量20,000を超え170,000以下のポリアクリル酸及び/又はその塩と、重量平均分子量1,000を超え100,000以下のポリメタクリル酸及び/又はその塩とを含むことを特徴とする蒸気発生設備のスケール除去剤。
- 請求項6において、前記ポリアクリル酸及び/又はその塩と、前記ポリメタクリル酸及び/又はその塩とを、1:100~100:1の重量比で含むことを特徴とする蒸気発生設備のスケール除去剤。
Priority Applications (11)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020167021274A KR20160120723A (ko) | 2014-02-13 | 2015-01-26 | 증기 발생 설비의 스케일 제거 방법 및 스케일 제거제 |
| SG11201606348VA SG11201606348VA (en) | 2014-02-13 | 2015-01-26 | Scale removal method and scale removal agent for steam generating facilities |
| US15/115,493 US10384966B2 (en) | 2014-02-13 | 2015-01-26 | Method for removing scale and scale remover in steam generating facility |
| PL15748848.7T PL3106439T3 (pl) | 2014-02-13 | 2015-01-26 | Sposób usuwania kamienia kotłowego dla instalacji do wytwarzania pary |
| MYPI2016702811A MY183247A (en) | 2014-02-13 | 2015-01-26 | Method for removing scale and scale remover in steam generating facility |
| BR112016017693-6A BR112016017693B1 (pt) | 2014-02-13 | 2015-01-26 | Removedor de incrustação para remover incrustações depositadas em um sistema de uma instalação de geração de vapor |
| CN201580008475.8A CN105980317A (zh) | 2014-02-13 | 2015-01-26 | 蒸气产生设备的垢去除方法和垢去除剂 |
| ES15748848T ES2943022T3 (es) | 2014-02-13 | 2015-01-26 | Método de eliminación de incrustación para instalaciones de generación de vapor |
| EP15748848.7A EP3106439B1 (en) | 2014-02-13 | 2015-01-26 | Scale removal method for steam generating facilities |
| PH12016501568A PH12016501568B1 (en) | 2014-02-13 | 2016-08-08 | Method for removing scale and scale remover in steam generating facility |
| US15/935,850 US10703659B2 (en) | 2014-02-13 | 2018-03-26 | Scale remover in steam generating facility |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2014025459A JP5800044B2 (ja) | 2014-02-13 | 2014-02-13 | 蒸気発生設備のスケール除去方法及びスケール除去剤 |
| JP2014-025459 | 2014-02-13 |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US15/115,493 A-371-Of-International US10384966B2 (en) | 2014-02-13 | 2015-01-26 | Method for removing scale and scale remover in steam generating facility |
| US15/935,850 Division US10703659B2 (en) | 2014-02-13 | 2018-03-26 | Scale remover in steam generating facility |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2015122264A1 true WO2015122264A1 (ja) | 2015-08-20 |
Family
ID=53800012
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2015/052012 WO2015122264A1 (ja) | 2014-02-13 | 2015-01-26 | 蒸気発生設備のスケール除去方法及びスケール除去剤 |
Country Status (13)
| Country | Link |
|---|---|
| US (2) | US10384966B2 (ja) |
| EP (1) | EP3106439B1 (ja) |
| JP (1) | JP5800044B2 (ja) |
| KR (1) | KR20160120723A (ja) |
| CN (1) | CN105980317A (ja) |
| BR (1) | BR112016017693B1 (ja) |
| ES (1) | ES2943022T3 (ja) |
| MY (1) | MY183247A (ja) |
| PH (1) | PH12016501568B1 (ja) |
| PL (1) | PL3106439T3 (ja) |
| SG (1) | SG11201606348VA (ja) |
| TW (1) | TWI642636B (ja) |
| WO (1) | WO2015122264A1 (ja) |
Families Citing this family (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5800044B2 (ja) | 2014-02-13 | 2015-10-28 | 栗田工業株式会社 | 蒸気発生設備のスケール除去方法及びスケール除去剤 |
| CN105753182A (zh) * | 2016-03-08 | 2016-07-13 | 佛山市聚成生化技术研发有限公司 | 一种苯乙烯酮-马来酸酐-丁二烯-丁烯醇阻垢剂的制备方法及所制备的阻垢剂 |
| CN106838874B (zh) * | 2016-12-25 | 2019-01-18 | 大庆让胡路区轩鸿科技有限公司 | 一种无损型锅炉除垢的方法 |
| CN109323237B (zh) * | 2018-09-14 | 2020-01-07 | 福建宁德核电有限公司 | 核级聚丙烯酸分散剂用于核电厂蒸汽发生器湿保养的方法 |
| CN111748147B (zh) * | 2019-03-28 | 2022-04-05 | 合肥杰事杰新材料股份有限公司 | 一种阻垢聚丙烯材料及其制备方法 |
| KR102144295B1 (ko) * | 2019-04-19 | 2020-08-13 | 최영환 | 보일러 자동 세관 시스템 |
| JP7050840B2 (ja) * | 2020-03-12 | 2022-04-08 | 栗田工業株式会社 | ボイラにおける蒸発管の腐食疲労の抑制方法 |
| JP7083365B2 (ja) * | 2020-03-12 | 2022-06-10 | 栗田工業株式会社 | ボイラにおける蒸発管の腐食疲労の抑制方法 |
| CN115448472A (zh) * | 2022-08-17 | 2022-12-09 | 中核武汉核电运行技术股份有限公司 | 一种核级聚丙烯酸高温高压最优在线添加工艺 |
| CN116947224A (zh) * | 2023-09-21 | 2023-10-27 | 山东上远环保科技有限公司 | 一种循环水处理剂及其制备方法 |
Citations (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS59189998A (ja) * | 1983-04-12 | 1984-10-27 | ケメツド・コ−ポレ−シヨン | スケ−ルの除去方法 |
| JPS6365999A (ja) * | 1981-11-05 | 1988-03-24 | ナルコ ケミカル カンパニー | ボイラー系のスケール防止用ポリマー組成物 |
| JP2000154996A (ja) * | 1998-08-19 | 2000-06-06 | Miura Co Ltd | ボイラのスケ―ル除去方法 |
| WO2005116296A1 (ja) * | 2004-05-25 | 2005-12-08 | Kurita Water Industries Ltd. | 冷却水の処理方法及び処理薬剤 |
| JP2008006369A (ja) * | 2006-06-29 | 2008-01-17 | Kurita Water Ind Ltd | スケール防止方法 |
| JP2010172816A (ja) * | 2009-01-29 | 2010-08-12 | Kurita Water Ind Ltd | スケール防止剤、およびスケール防止方法 |
| JP2013022535A (ja) * | 2011-07-22 | 2013-02-04 | Kurita Water Ind Ltd | ボイラ水系のスケール除去方法 |
| WO2013058115A1 (ja) * | 2011-10-18 | 2013-04-25 | 栗田工業株式会社 | 蒸気発生器の水側缶内における鉄スケール防止方法 |
| WO2013140913A1 (ja) * | 2012-03-19 | 2013-09-26 | 栗田工業株式会社 | エコノマイザを有するボイラの水処理方法 |
| WO2014162992A1 (ja) * | 2013-04-02 | 2014-10-09 | 栗田工業株式会社 | 蒸気発生設備のスケール除去方法 |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA863696A (en) | 1971-02-16 | L. Salutsky Murrell | Control of scaling in evaporators | |
| GB1433221A (en) | 1973-03-27 | 1976-04-22 | Ciba Geigy Ag | Water treatment |
| CA2042341C (en) * | 1990-05-23 | 2001-06-12 | Judy H. Bardsley | Silica scale inhibition |
| JP2923039B2 (ja) | 1990-11-28 | 1999-07-26 | 三菱重工業株式会社 | スケールの除去組成物 |
| CN1068313A (zh) * | 1991-07-08 | 1993-01-27 | 化学工业部天津化工研究院 | 锅炉水处理剂 |
| JP2002273478A (ja) | 2001-03-21 | 2002-09-24 | Jsr Corp | スケール防止方法 |
| DE102007019428A1 (de) * | 2006-07-07 | 2008-10-30 | Henkel Ag & Co. Kgaa | Wasch-, Reinigungs- und Pflegemittel 2 |
| DE102006047229A1 (de) * | 2006-10-04 | 2008-04-10 | Henkel Kgaa | Wasch- oder Reinigungsmittelabgabesystem |
| JP5786277B2 (ja) | 2010-03-31 | 2015-09-30 | 栗田工業株式会社 | スケール除去方法及びスケール除去剤 |
| DE102013204824A1 (de) * | 2013-03-15 | 2014-09-18 | Henkel Ag & Co. Kgaa | Reinigungsmittel für harte Oberflächen enthaltend Phosphorsäureester eines Polyether-modifizierten Alkylalkohols |
| JP5800044B2 (ja) | 2014-02-13 | 2015-10-28 | 栗田工業株式会社 | 蒸気発生設備のスケール除去方法及びスケール除去剤 |
-
2014
- 2014-02-13 JP JP2014025459A patent/JP5800044B2/ja active Active
-
2015
- 2015-01-26 ES ES15748848T patent/ES2943022T3/es active Active
- 2015-01-26 BR BR112016017693-6A patent/BR112016017693B1/pt active IP Right Grant
- 2015-01-26 US US15/115,493 patent/US10384966B2/en active Active
- 2015-01-26 WO PCT/JP2015/052012 patent/WO2015122264A1/ja active Application Filing
- 2015-01-26 SG SG11201606348VA patent/SG11201606348VA/en unknown
- 2015-01-26 PL PL15748848.7T patent/PL3106439T3/pl unknown
- 2015-01-26 CN CN201580008475.8A patent/CN105980317A/zh active Pending
- 2015-01-26 MY MYPI2016702811A patent/MY183247A/en unknown
- 2015-01-26 KR KR1020167021274A patent/KR20160120723A/ko not_active Application Discontinuation
- 2015-01-26 EP EP15748848.7A patent/EP3106439B1/en active Active
- 2015-02-04 TW TW104103753A patent/TWI642636B/zh active
-
2016
- 2016-08-08 PH PH12016501568A patent/PH12016501568B1/en unknown
-
2018
- 2018-03-26 US US15/935,850 patent/US10703659B2/en active Active
Patent Citations (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS6365999A (ja) * | 1981-11-05 | 1988-03-24 | ナルコ ケミカル カンパニー | ボイラー系のスケール防止用ポリマー組成物 |
| JPS59189998A (ja) * | 1983-04-12 | 1984-10-27 | ケメツド・コ−ポレ−シヨン | スケ−ルの除去方法 |
| JP2000154996A (ja) * | 1998-08-19 | 2000-06-06 | Miura Co Ltd | ボイラのスケ―ル除去方法 |
| WO2005116296A1 (ja) * | 2004-05-25 | 2005-12-08 | Kurita Water Industries Ltd. | 冷却水の処理方法及び処理薬剤 |
| JP2008006369A (ja) * | 2006-06-29 | 2008-01-17 | Kurita Water Ind Ltd | スケール防止方法 |
| JP2010172816A (ja) * | 2009-01-29 | 2010-08-12 | Kurita Water Ind Ltd | スケール防止剤、およびスケール防止方法 |
| JP2013022535A (ja) * | 2011-07-22 | 2013-02-04 | Kurita Water Ind Ltd | ボイラ水系のスケール除去方法 |
| WO2013058115A1 (ja) * | 2011-10-18 | 2013-04-25 | 栗田工業株式会社 | 蒸気発生器の水側缶内における鉄スケール防止方法 |
| WO2013140913A1 (ja) * | 2012-03-19 | 2013-09-26 | 栗田工業株式会社 | エコノマイザを有するボイラの水処理方法 |
| WO2014162992A1 (ja) * | 2013-04-02 | 2014-10-09 | 栗田工業株式会社 | 蒸気発生設備のスケール除去方法 |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP3106439A4 * |
Also Published As
| Publication number | Publication date |
|---|---|
| US10384966B2 (en) | 2019-08-20 |
| JP5800044B2 (ja) | 2015-10-28 |
| EP3106439A4 (en) | 2017-08-02 |
| PH12016501568A1 (en) | 2016-09-14 |
| TWI642636B (zh) | 2018-12-01 |
| KR20160120723A (ko) | 2016-10-18 |
| SG11201606348VA (en) | 2016-09-29 |
| US10703659B2 (en) | 2020-07-07 |
| EP3106439B1 (en) | 2023-03-22 |
| TW201538438A (zh) | 2015-10-16 |
| BR112016017693B1 (pt) | 2022-08-09 |
| PL3106439T3 (pl) | 2023-06-19 |
| BR112016017693A2 (ja) | 2017-08-08 |
| MY183247A (en) | 2021-02-18 |
| CN105980317A (zh) | 2016-09-28 |
| PH12016501568B1 (en) | 2016-09-14 |
| ES2943022T3 (es) | 2023-06-08 |
| US20170050873A1 (en) | 2017-02-23 |
| EP3106439A1 (en) | 2016-12-21 |
| US20180215637A1 (en) | 2018-08-02 |
| JP2015150484A (ja) | 2015-08-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5800044B2 (ja) | 蒸気発生設備のスケール除去方法及びスケール除去剤 | |
| EP3063311B1 (en) | Corrosion inhibiting compositions and methods | |
| JP5773091B2 (ja) | 蒸気発生設備のスケール除去方法 | |
| JP5786277B2 (ja) | スケール除去方法及びスケール除去剤 | |
| JP5970884B2 (ja) | 防食方法 | |
| JP5891630B2 (ja) | ボイラ水系のスケール除去方法 | |
| JP5900064B2 (ja) | エコノマイザを有するボイラの水処理方法 | |
| JP6314560B2 (ja) | 蒸気発生設備の水処理方法 | |
| JP2003159597A (ja) | 水処理剤 | |
| JP5909956B2 (ja) | ボイラにおけるエコノマイザの腐食抑制方法 | |
| JP2013068341A (ja) | ボイラにおけるエコノマイザの防食方法 | |
| JP5691697B2 (ja) | 蒸気発生設備の水処理方法 | |
| JP5862193B2 (ja) | 蒸気発生器の水側缶内における鉄スケール防止方法 | |
| JP5879699B2 (ja) | ボイラ給水系の防食方法 | |
| WO2014199523A1 (ja) | 蒸気発生設備の水処理方法 | |
| JP2014059076A (ja) | ボイラ水系のスケール除去方法 | |
| JP5640608B2 (ja) | 酸素除去方法及び酸素除去剤 | |
| JP2009299161A (ja) | 水系の金属腐食抑制方法 | |
| JP2012184465A (ja) | ボイラ給水系及びボイラ缶内の防食方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 15748848 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 15115493 Country of ref document: US |
|
| ENP | Entry into the national phase |
Ref document number: 20167021274 Country of ref document: KR Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 12016501568 Country of ref document: PH |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: 112016017693 Country of ref document: BR |
|
| REEP | Request for entry into the european phase |
Ref document number: 2015748848 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2015748848 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: IDP00201605346 Country of ref document: ID |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 112016017693 Country of ref document: BR Kind code of ref document: A2 Effective date: 20160729 |