WO2012018074A1 - Acier inoxydable ferritique - Google Patents
Acier inoxydable ferritique Download PDFInfo
- Publication number
- WO2012018074A1 WO2012018074A1 PCT/JP2011/067850 JP2011067850W WO2012018074A1 WO 2012018074 A1 WO2012018074 A1 WO 2012018074A1 JP 2011067850 W JP2011067850 W JP 2011067850W WO 2012018074 A1 WO2012018074 A1 WO 2012018074A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- less
- stainless steel
- ferritic stainless
- mass
- content
- Prior art date
Links
- 229910001220 stainless steel Inorganic materials 0.000 title claims abstract description 47
- 229910052782 aluminium Inorganic materials 0.000 claims abstract description 28
- 229910052719 titanium Inorganic materials 0.000 claims abstract description 28
- 229910052791 calcium Inorganic materials 0.000 claims abstract description 23
- 229910052710 silicon Inorganic materials 0.000 claims abstract description 23
- 229910000831 Steel Inorganic materials 0.000 claims abstract description 18
- 239000010959 steel Substances 0.000 claims abstract description 18
- 239000012535 impurity Substances 0.000 claims abstract description 6
- 229910052757 nitrogen Inorganic materials 0.000 claims description 13
- 229910052799 carbon Inorganic materials 0.000 claims description 10
- 229910052796 boron Inorganic materials 0.000 claims description 7
- 229910052750 molybdenum Inorganic materials 0.000 claims description 7
- 229910052748 manganese Inorganic materials 0.000 claims description 6
- 229910052698 phosphorus Inorganic materials 0.000 claims description 6
- 229910052759 nickel Inorganic materials 0.000 claims description 5
- 229910052758 niobium Inorganic materials 0.000 claims description 5
- 229910052717 sulfur Inorganic materials 0.000 claims description 5
- 229910052726 zirconium Inorganic materials 0.000 claims description 3
- 206010027146 Melanoderma Diseases 0.000 abstract description 41
- 238000012360 testing method Methods 0.000 description 54
- 230000007797 corrosion Effects 0.000 description 51
- 238000005260 corrosion Methods 0.000 description 51
- 239000010936 titanium Substances 0.000 description 39
- 238000003466 welding Methods 0.000 description 33
- 239000011575 calcium Substances 0.000 description 30
- 241001016380 Reseda luteola Species 0.000 description 26
- 239000000203 mixture Substances 0.000 description 16
- PXHVJJICTQNCMI-UHFFFAOYSA-N nickel Substances [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 14
- 239000010955 niobium Substances 0.000 description 14
- 239000011651 chromium Substances 0.000 description 12
- JEIPFZHSYJVQDO-UHFFFAOYSA-N iron(III) oxide Inorganic materials O=[Fe]O[Fe]=O JEIPFZHSYJVQDO-UHFFFAOYSA-N 0.000 description 12
- 239000010949 copper Substances 0.000 description 9
- 230000000694 effects Effects 0.000 description 9
- 239000011324 bead Substances 0.000 description 8
- 239000011572 manganese Substances 0.000 description 8
- 238000000034 method Methods 0.000 description 8
- 239000000126 substance Substances 0.000 description 8
- XEEYBQQBJWHFJM-UHFFFAOYSA-N iron Substances [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 7
- 238000005259 measurement Methods 0.000 description 7
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 6
- 229910052804 chromium Inorganic materials 0.000 description 6
- 239000000463 material Substances 0.000 description 6
- 238000012545 processing Methods 0.000 description 6
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 6
- 239000007921 spray Substances 0.000 description 5
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 4
- 238000009826 distribution Methods 0.000 description 4
- 239000001301 oxygen Substances 0.000 description 4
- 229910052760 oxygen Inorganic materials 0.000 description 4
- 238000003860 storage Methods 0.000 description 4
- 229910000859 α-Fe Inorganic materials 0.000 description 4
- 229910000963 austenitic stainless steel Inorganic materials 0.000 description 3
- 239000011261 inert gas Substances 0.000 description 3
- 229910052742 iron Inorganic materials 0.000 description 3
- 229910052751 metal Inorganic materials 0.000 description 3
- 239000002184 metal Substances 0.000 description 3
- 230000001737 promoting effect Effects 0.000 description 3
- 239000011780 sodium chloride Substances 0.000 description 3
- 238000005728 strengthening Methods 0.000 description 3
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- 206010070834 Sensitisation Diseases 0.000 description 2
- 230000006399 behavior Effects 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 238000005097 cold rolling Methods 0.000 description 2
- 229910052802 copper Inorganic materials 0.000 description 2
- 238000004090 dissolution Methods 0.000 description 2
- 238000011156 evaluation Methods 0.000 description 2
- 238000010438 heat treatment Methods 0.000 description 2
- 238000003754 machining Methods 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 238000011084 recovery Methods 0.000 description 2
- 238000001953 recrystallisation Methods 0.000 description 2
- 238000007670 refining Methods 0.000 description 2
- 230000008313 sensitization Effects 0.000 description 2
- 239000010935 stainless steel Substances 0.000 description 2
- ZOXJGFHDIHLPTG-UHFFFAOYSA-N Boron Chemical compound [B] ZOXJGFHDIHLPTG-UHFFFAOYSA-N 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- 229910001208 Crucible steel Inorganic materials 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- PWHULOQIROXLJO-UHFFFAOYSA-N Manganese Chemical compound [Mn] PWHULOQIROXLJO-UHFFFAOYSA-N 0.000 description 1
- ZOKXTWBITQBERF-UHFFFAOYSA-N Molybdenum Chemical compound [Mo] ZOKXTWBITQBERF-UHFFFAOYSA-N 0.000 description 1
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 1
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 1
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- 230000001133 acceleration Effects 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 238000000137 annealing Methods 0.000 description 1
- 229910052786 argon Inorganic materials 0.000 description 1
- 239000004566 building material Substances 0.000 description 1
- 238000005336 cracking Methods 0.000 description 1
- 230000007547 defect Effects 0.000 description 1
- 230000006866 deterioration Effects 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 238000002845 discoloration Methods 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 239000011733 molybdenum Substances 0.000 description 1
- GUCVJGMIXFAOAE-UHFFFAOYSA-N niobium atom Chemical compound [Nb] GUCVJGMIXFAOAE-UHFFFAOYSA-N 0.000 description 1
- -1 outdoor equipment Substances 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 239000011574 phosphorus Substances 0.000 description 1
- 238000005554 pickling Methods 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- VSZWPYCFIRKVQL-UHFFFAOYSA-N selanylidenegallium;selenium Chemical compound [Se].[Se]=[Ga].[Se]=[Ga] VSZWPYCFIRKVQL-UHFFFAOYSA-N 0.000 description 1
- 239000010703 silicon Substances 0.000 description 1
- 239000002893 slag Substances 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000006104 solid solution Substances 0.000 description 1
- 238000007711 solidification Methods 0.000 description 1
- 230000008023 solidification Effects 0.000 description 1
- 238000004611 spectroscopical analysis Methods 0.000 description 1
- 238000004544 sputter deposition Methods 0.000 description 1
- 230000000087 stabilizing effect Effects 0.000 description 1
- 239000002436 steel type Substances 0.000 description 1
- 239000011593 sulfur Substances 0.000 description 1
- 239000002344 surface layer Substances 0.000 description 1
- WFKWXMTUELFFGS-UHFFFAOYSA-N tungsten Chemical compound [W] WFKWXMTUELFFGS-UHFFFAOYSA-N 0.000 description 1
- 229910052721 tungsten Inorganic materials 0.000 description 1
- 239000010937 tungsten Substances 0.000 description 1
- 229910052720 vanadium Inorganic materials 0.000 description 1
- LEONUFNNVUYDNQ-UHFFFAOYSA-N vanadium atom Chemical compound [V] LEONUFNNVUYDNQ-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/18—Ferrous alloys, e.g. steel alloys containing chromium
- C22C38/40—Ferrous alloys, e.g. steel alloys containing chromium with nickel
- C22C38/54—Ferrous alloys, e.g. steel alloys containing chromium with nickel with boron
-
- C—CHEMISTRY; METALLURGY
- C21—METALLURGY OF IRON
- C21D—MODIFYING THE PHYSICAL STRUCTURE OF FERROUS METALS; GENERAL DEVICES FOR HEAT TREATMENT OF FERROUS OR NON-FERROUS METALS OR ALLOYS; MAKING METAL MALLEABLE, e.g. BY DECARBURISATION OR TEMPERING
- C21D6/00—Heat treatment of ferrous alloys
- C21D6/002—Heat treatment of ferrous alloys containing Cr
-
- C—CHEMISTRY; METALLURGY
- C21—METALLURGY OF IRON
- C21D—MODIFYING THE PHYSICAL STRUCTURE OF FERROUS METALS; GENERAL DEVICES FOR HEAT TREATMENT OF FERROUS OR NON-FERROUS METALS OR ALLOYS; MAKING METAL MALLEABLE, e.g. BY DECARBURISATION OR TEMPERING
- C21D8/00—Modifying the physical properties by deformation combined with, or followed by, heat treatment
- C21D8/02—Modifying the physical properties by deformation combined with, or followed by, heat treatment during manufacturing of plates or strips
- C21D8/0221—Modifying the physical properties by deformation combined with, or followed by, heat treatment during manufacturing of plates or strips characterised by the working steps
- C21D8/0226—Hot rolling
-
- C—CHEMISTRY; METALLURGY
- C21—METALLURGY OF IRON
- C21D—MODIFYING THE PHYSICAL STRUCTURE OF FERROUS METALS; GENERAL DEVICES FOR HEAT TREATMENT OF FERROUS OR NON-FERROUS METALS OR ALLOYS; MAKING METAL MALLEABLE, e.g. BY DECARBURISATION OR TEMPERING
- C21D8/00—Modifying the physical properties by deformation combined with, or followed by, heat treatment
- C21D8/02—Modifying the physical properties by deformation combined with, or followed by, heat treatment during manufacturing of plates or strips
- C21D8/0221—Modifying the physical properties by deformation combined with, or followed by, heat treatment during manufacturing of plates or strips characterised by the working steps
- C21D8/0236—Cold rolling
-
- C—CHEMISTRY; METALLURGY
- C21—METALLURGY OF IRON
- C21D—MODIFYING THE PHYSICAL STRUCTURE OF FERROUS METALS; GENERAL DEVICES FOR HEAT TREATMENT OF FERROUS OR NON-FERROUS METALS OR ALLOYS; MAKING METAL MALLEABLE, e.g. BY DECARBURISATION OR TEMPERING
- C21D8/00—Modifying the physical properties by deformation combined with, or followed by, heat treatment
- C21D8/02—Modifying the physical properties by deformation combined with, or followed by, heat treatment during manufacturing of plates or strips
- C21D8/0247—Modifying the physical properties by deformation combined with, or followed by, heat treatment during manufacturing of plates or strips characterised by the heat treatment
- C21D8/0263—Modifying the physical properties by deformation combined with, or followed by, heat treatment during manufacturing of plates or strips characterised by the heat treatment following hot rolling
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/001—Ferrous alloys, e.g. steel alloys containing N
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/002—Ferrous alloys, e.g. steel alloys containing In, Mg, or other elements not provided for in one single group C22C38/001 - C22C38/60
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/004—Very low carbon steels, i.e. having a carbon content of less than 0,01%
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/02—Ferrous alloys, e.g. steel alloys containing silicon
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/04—Ferrous alloys, e.g. steel alloys containing manganese
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/06—Ferrous alloys, e.g. steel alloys containing aluminium
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/18—Ferrous alloys, e.g. steel alloys containing chromium
- C22C38/20—Ferrous alloys, e.g. steel alloys containing chromium with copper
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/18—Ferrous alloys, e.g. steel alloys containing chromium
- C22C38/22—Ferrous alloys, e.g. steel alloys containing chromium with molybdenum or tungsten
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/18—Ferrous alloys, e.g. steel alloys containing chromium
- C22C38/24—Ferrous alloys, e.g. steel alloys containing chromium with vanadium
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/18—Ferrous alloys, e.g. steel alloys containing chromium
- C22C38/26—Ferrous alloys, e.g. steel alloys containing chromium with niobium or tantalum
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/18—Ferrous alloys, e.g. steel alloys containing chromium
- C22C38/28—Ferrous alloys, e.g. steel alloys containing chromium with titanium or zirconium
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/18—Ferrous alloys, e.g. steel alloys containing chromium
- C22C38/32—Ferrous alloys, e.g. steel alloys containing chromium with boron
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/18—Ferrous alloys, e.g. steel alloys containing chromium
- C22C38/40—Ferrous alloys, e.g. steel alloys containing chromium with nickel
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/18—Ferrous alloys, e.g. steel alloys containing chromium
- C22C38/40—Ferrous alloys, e.g. steel alloys containing chromium with nickel
- C22C38/42—Ferrous alloys, e.g. steel alloys containing chromium with nickel with copper
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/18—Ferrous alloys, e.g. steel alloys containing chromium
- C22C38/40—Ferrous alloys, e.g. steel alloys containing chromium with nickel
- C22C38/44—Ferrous alloys, e.g. steel alloys containing chromium with nickel with molybdenum or tungsten
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/18—Ferrous alloys, e.g. steel alloys containing chromium
- C22C38/40—Ferrous alloys, e.g. steel alloys containing chromium with nickel
- C22C38/46—Ferrous alloys, e.g. steel alloys containing chromium with nickel with vanadium
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/18—Ferrous alloys, e.g. steel alloys containing chromium
- C22C38/40—Ferrous alloys, e.g. steel alloys containing chromium with nickel
- C22C38/48—Ferrous alloys, e.g. steel alloys containing chromium with nickel with niobium or tantalum
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/18—Ferrous alloys, e.g. steel alloys containing chromium
- C22C38/40—Ferrous alloys, e.g. steel alloys containing chromium with nickel
- C22C38/50—Ferrous alloys, e.g. steel alloys containing chromium with nickel with titanium or zirconium
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F28—HEAT EXCHANGE IN GENERAL
- F28F—DETAILS OF HEAT-EXCHANGE AND HEAT-TRANSFER APPARATUS, OF GENERAL APPLICATION
- F28F21/00—Constructions of heat-exchange apparatus characterised by the selection of particular materials
- F28F21/08—Constructions of heat-exchange apparatus characterised by the selection of particular materials of metal
- F28F21/081—Heat exchange elements made from metals or metal alloys
- F28F21/082—Heat exchange elements made from metals or metal alloys from steel or ferrous alloys
- F28F21/083—Heat exchange elements made from metals or metal alloys from steel or ferrous alloys from stainless steel
Definitions
- the present invention relates to a ferritic stainless steel that generates less black spots in a TIG weld.
- Ferritic stainless steel generally has not only excellent corrosion resistance, but also has features such as a smaller thermal expansion coefficient than austenitic stainless steel and excellent resistance to stress corrosion cracking. For this reason, ferritic stainless steel is widely used for building exterior materials such as tableware, kitchen equipment and roofing materials, and materials for water storage and hot water storage. Furthermore, in recent years, due to soaring prices of Ni raw materials, there is also a great demand for switching from austenitic stainless steel, and its applications are becoming widespread.
- ferritic stainless steel has a problem in that its C and N solid solubility limit is small, so that sensitization occurs at the welded portion and corrosion resistance is lowered.
- a method of suppressing the sensitization of the weld metal part by reducing the amount of C and N or fixing C and N by adding a stabilizing element such as Ti or Nb has been proposed and widely used.
- a technique for forming an Al oxide film that improves the corrosion resistance of the weld heat affected zone on the surface layer of the steel during welding is disclosed.
- Patent Document 3 discloses a technique for improving the crevice corrosion resistance of a welded part by adding a certain amount or more of Si in addition to the combined addition of Al and Ti.
- Patent Document 4 describes that 4Al + Ti ⁇ 0.32 (Ti and Al in the formulas indicate the content of each component in the steel), thereby reducing the heat input during welding and reducing the welded portion.
- a technique for suppressing the generation of scale and improving the corrosion resistance of the welded portion is disclosed.
- the above-described prior art is intended to improve the corrosion resistance of the welded portion and the weld heat affected zone.
- Patent Document 5 As a means for improving the weather resistance and crevice corrosion resistance of the material itself, not the welded portion, there is a technique of positively adding P and adding appropriate amounts of Ca and Al (see, for example, Patent Document 5).
- Ca and Al are added to control the shape and distribution of nonmetallic inclusions in steel.
- P is added in an amount exceeding 0.04%, and Patent Document 5 does not describe any effect at the time of welding.
- black spots In conventional ferritic stainless steel, even if the shielding conditions in the welded part are optimized, black spots generally called black spots or slag spots may be scattered on the welded back bead after welding.
- a black spot is one in which Al, Ti, Si, and Ca, which have strong affinity for oxygen, form oxides and solidify on the weld metal during solidification of the weld metal in TIG (Tungsten Inert Gas) welding.
- TIG Transmissionungsten Inert Gas
- the generation of black spots is greatly influenced by welding conditions, particularly shielding conditions by inert gas, and more black spots are generated as the shielding is insufficient.
- the black spot itself is an oxide, there is no problem in the corrosion resistance and workability of the weld even if a small amount of black spot is scattered.
- black spots are generated in large quantities or continuously, not only the appearance of the welded part is used without being polished, but also the appearance of the black spot is removed when the welded part is processed. May occur.
- the black spot part is peeled off, there are cases where workability is deteriorated or a gap corrosion occurs between the black spot part and the peeled black spot part.
- Even if the processing is not performed after welding if the black spot is generated thickly, if the stress is applied to the welded portion due to the structure, the black spot may be peeled off and the corrosion resistance may be lowered.
- the corrosion resistance of the TIG welded part it is important not only to improve the corrosion resistance of the weld bead part and the weld scale part itself, but also to control the black spots generated in the welded part.
- scales with discoloration that occur during welding can be suppressed almost by the method of strengthening the shield condition of welding.
- the black spot generated in the TIG welded part is the conventional technology even if the shield condition is strengthened. Then, it was not able to suppress enough.
- This invention is made in view of such a situation, Comprising: It is hard to produce
- the present inventor conducted extensive research as shown below in order to suppress the amount of black spots generated. As a result, the inventors have found that by optimizing the amounts of Al, Ti, Si, and Ca, it is possible to suppress the generation of black spots in the TIG welded portion, and the present inventors have devised a ferritic stainless steel according to the present invention that generates less black spots.
- the gist of the present invention is as follows.
- the first aspect of the present invention is, in mass%, C: 0.020% or less, N: 0.025% or less, Si: 1.0% or less, Mn: 1.0% or less, P: 0.035. %: S: 0.01% or less, Cr: 16.0 to 25.0%, Al: 0.12% or less, Ti: 0.05 to 0.35%, Ca: 0.0015% or less
- the ferritic stainless steel is composed of Fe and inevitable impurities as the balance and satisfies the following formula (1).
- a second aspect of the present invention is the ferritic stainless steel according to the first aspect, and further includes ferritic stainless steel containing Nb: 0.6% or less by mass%.
- a third aspect of the present invention is a ferritic stainless steel according to the first or second aspect, and further includes ferritic stainless steel containing, by mass%, Mo: 3.0% or less.
- a fourth aspect of the present invention is the ferritic stainless steel according to any one of the first to third aspects, and further, by mass, Cu: 2.0% or less, Ni: 2.0% or less Ferritic stainless steel containing one or two selected from
- a fifth aspect of the present invention is the ferritic stainless steel according to any one of the first to fourth aspects, and further, by mass, V: 0.2% or less, Zr: 0.2% or less Ferritic stainless steel containing one or two selected from
- a sixth aspect of the present invention is a ferritic stainless steel according to any one of the first to fifth aspects, further comprising ferritic stainless steel containing, by mass%, B: 0.005% or less. is there.
- the present invention it is possible to provide a ferritic stainless steel in which black spots are unlikely to be generated in a TIG welded portion and excellent in corrosion resistance and workability of the TIG welded portion.
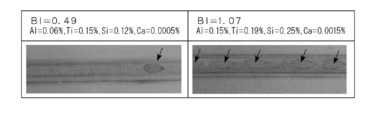
- FIG. 1A is a photograph showing the appearance of black spots generated on the back side during TIG welding.
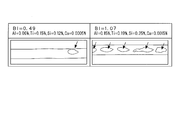
- FIG. 1B is a schematic diagram showing the appearance of a black spot generated on the back side during TIG welding, and corresponds to the photograph shown in FIG. 1A.
- FIG. 2A is a graph showing the results of AES measurement of the element depth profile (element concentration distribution in the depth direction) of the weld bead portion on the back side of the test piece.
- FIG. 2B is a graph showing the result of measuring the element depth profile (element concentration distribution in the depth direction) of the black spot on the back side of the test piece by AES.
- FIG. 3 is a graph showing the relationship between the BI value and the black spot generation length ratio.
- FIG. 4 is a graph showing the relationship between BI value and corrosion. Double circles ( ⁇ ) indicate excellent results, circles ( ⁇ ) indicate good results, and ⁇ indicate poor results.
- the ferritic stainless steel according to the present invention with little black spot formation satisfies the following formula (1).
- BI 3Al + Ti + 0.5Si + 200Ca ⁇ 0.8
- Al, Ti, Si, and Ca in the formula (1) are the content [% by mass] of each component in the steel.
- Al, Ti, Si, and Ca are elements that have particularly strong affinity with oxygen, and are elements that generate black spots during TIG welding. Further, as the content of Al, Ti, Si, and Ca contained in the steel is increased, black spots are easily generated.
- the coefficient of content of Al, Ti, Si, and Ca in the above formula (1) is determined based on the magnitude (strength) of the action that promotes the generation of black spots and the content in steel. More specifically, Al is an element that is contained in the black spot at the highest concentration and has a particularly large effect of promoting the generation of the black spot, as shown in an experimental example described later. For this reason, in the above equation (1), the coefficient of Al content is set to 3.
- Ca is an element that has a high effect of promoting the generation of black spots because it is contained in the black spots at a high concentration in spite of its low content in steel. For this reason, the coefficient of Ca content is set to 200.
- the generation of black spots becomes remarkable.
- the BI value is 0.8 or less, the generation of black spots in the TIG welded portion is sufficiently reduced and the corrosion resistance is excellent.
- the BI value is 0.6 or less, the generation of black spots can be more effectively suppressed, and when the BI value is 0.4 or less, the generation of black spots can be almost suppressed. Further, the corrosion resistance of the TIG welded portion can be further improved.
- the thickness of the black spots also increases, so it is estimated that they will be easily peeled off during processing, in which case peeling occurs in severe processing such as overhanging and becomes the starting point of corrosion. Conceivable.
- the occurrence rate of black spots is small, the thickness is reduced, and it is estimated that even if black spots are generated, it is difficult to peel off. Therefore, it is considered that the corrosion resistance of the welded portion can be improved by suppressing the occurrence of black spots.
- Aluminum (Al): 0.012% or less by mass% Al is important as a deoxidizing element, and also has the effect of controlling the composition of nonmetallic inclusions to refine the structure.
- Al is an element that contributes most to the generation of black spots.
- excessive addition of Al leads to coarsening of non-metallic inclusions, which may be a starting point for product wrinkling. Therefore, the upper limit value of the Al content is set to 0.12%.
- the Al content is more preferably 0.03% to 0.10%.
- Titanium (Ti): 0.05% to 0.35% by mass Ti is an extremely important element for fixing C and N and suppressing intergranular corrosion of the welded portion to improve workability.
- the range of Ti content is set to 0.05% to 0.35%. More desirably, it is 0.07% to 0.20%.
- Silicon (Si): 1.0% or less by mass% Si is an important element as a deoxidizing element, and is also effective in improving corrosion resistance and oxidation resistance.
- the upper limit of Si content is set to 1.0%.
- the Si content is more desirably 0.05% to 0.55%.
- Ca Calcium (Ca): 0.0015% or less in mass% Ca is very important as a deoxidizing element and is contained in a small amount in steel as a nonmetallic inclusion. However, since Ca is very easily oxidized, it becomes a major factor for generating black spots during welding. Moreover, Ca produces
- Mn Manganese
- MnS Manganese S
- the Mn content is set to 1.0% or less.
- Mn For deoxidation, it is preferable to contain 0.01% or more of Mn. More desirably, it is 0.05% to 0.5%. More desirably, it is 0.05% to 0.3%.
- Phosphorus (P): 0.035% or less in mass% P not only lowers weldability and workability, but also easily causes intergranular corrosion, so it needs to be kept low. Therefore, the content of P is set to 0.035% or less. More desirably, the content is 0.001% to 0.02%.
- S Sulfur: 0.01% or less by mass% S generates water-soluble inclusions that cause corrosion, such as CaS and MnS, and thus needs to be reduced. Therefore, the S content is 0.01% or less. However, excessive reduction causes cost deterioration. Therefore, the S content is more preferably 0.0001% to 0.005%.
- Chromium (Cr): 16.0-25.0% by mass Cr is the most important element for ensuring the corrosion resistance of stainless steel, and needs to be contained in an amount of 16.0% or more in order to stabilize the ferrite structure. However, Cr lowers the workability and manufacturability, so the upper limit was made 25.0%.
- the Cr content is desirably 16.5% to 23.0%, and more desirably 18.0% to 22.5%.
- Niobium (Nb): 0.6% or less by mass% Nb can be added alone or in combination with Ti due to its characteristics. When Nb is contained together with Ti, it is preferable to satisfy (Ti + Nb) / (C + N) ⁇ 6 (Ti, Nb, C, and N in the formula are contents [mass%] of each component in the steel).
- Nb is an element that fixes C and N like Ti and suppresses intergranular corrosion of the welded portion and improves workability. However, since excessive addition of Nb reduces workability, the upper limit of the Nb content is preferably 0.6%. Moreover, in order to improve said characteristic by containing Nb, it is preferable to contain Nb 0.05% or more. The Nb content is desirably 0.15% to 0.55%.
- Molybdenum (Mo): 3.0% or less by mass% Mo is an element that is effective in repairing a passive film and is very effective in improving corrosion resistance. Further, when Mo is contained together with Cr, there is an effect of effectively improving the pitting corrosion resistance. Moreover, Mo is effective together with Ni to improve flow rust resistance. However, when Mo is increased, the workability is lowered and the cost is increased. For this reason, it is preferable to make the upper limit of Mo content 3.0%. Moreover, in order to improve said characteristic by containing Mo, it is preferable to contain 0.30% or more of Mo. The Mo content is desirably 0.60% to 2.5%, and more desirably 0.9% to 2.0%.
- Ni Nickel (Ni): 2.0% or less by mass% Ni has an effect of suppressing the active dissolution rate and has excellent repassivation characteristics due to a small hydrogen overvoltage. However, excessive addition of Ni reduces workability and makes the ferrite structure unstable. For this reason, it is preferable to make the upper limit of Ni content 2.0%. Moreover, in order to improve said characteristic by containing Ni, it is preferable to contain Ni 0.05% or more.
- the Ni content is desirably 0.1% to 1.2%, and more desirably 0.2% to 1.1%.
- B Boron (B): 0.005% or less by mass% B is a grain boundary strengthening element effective for improving secondary work brittleness.
- B is a grain boundary strengthening element effective for improving secondary work brittleness.
- the lower limit is preferably 0.0001% and the upper limit is preferably 0.005%, and more preferably 0.0002% to 0.0020%.
- Test pieces made of ferritic stainless steel having the chemical components (compositions) shown in Table 1 were produced by the following method. First, a cast steel having a chemical composition (composition) shown in Table 1 was melted by vacuum melting to produce a 40 mm thick ingot, which was hot rolled to a thickness of 5 mm. Thereafter, heat treatment was performed at a temperature of 800 to 1000 ° C. for 1 minute based on each recrystallization behavior, the scale was ground and removed, and a steel sheet having a thickness of 0.8 mm was manufactured by cold rolling. Thereafter, as a final annealing, a heat treatment is performed at a temperature of 800 to 1000 ° C. for 1 minute based on each recrystallization behavior, and the surface oxide scale is removed by pickling. Test specimens 1 to 28 were produced. In addition, in the chemical component (composition) shown in Table 1, the content of each element is expressed by mass%, and the balance is iron and inevitable impurities. Underlined values indicate values outside the scope of the present invention.
- test specimens Nos. 1 to 28 thus obtained were subjected to TIG welding under the following welding conditions, and the black spot generation length ratio was calculated as follows. Further, the following corrosion tests were performed on the test pieces Nos. 1 to 28. "Welding conditions" TIG welding was performed by abutting the same steel type at a feed rate of 50 cm / min and a heat input of 550 to 650 J / cm 2 . Argon was used for the shield on the torch side and the back side.
- Black spot generation length ratio The black spot generation length ratio was determined as a standard representing the generation amount of black spots after TIG welding. The black spot generation length ratio was obtained by integrating the lengths in the welding direction of the black spots generated in the welded portion, and dividing the integrated value by the total weld length. Photographing the weld length of about 10cm with a digital camera, measuring the length of each black spot, and calculating the ratio of the total length of the black spots in the weld length to the weld length using image processing. Determined by
- Corrosion test As the corrosion test piece, a TIG welded portion was used. The overhanging condition was an Erichsen test condition based on JIS 2247, and a 20 mm ⁇ punch was used with the back side of the weld specimen as the surface. However, for the overhang height, the machining was stopped halfway to match the machining conditions. The stop height (overhang height) was unified at 6 mm and 7 mm. Corrosion evaluation was performed by performing a continuous spray test of 5% NaCl according to JIS Z 2371 and evaluating the presence or absence of flow rust after 48 hours.
- the black spot generation length ratio is small, and the black after TIG welding There was little generation of spots.
- the generation of black spots is further suppressed in Nos 1 to 15, 18, and 19 having a BI value of 0.6 or less, and the generation length is 10% or less in Nos 1 to 13 having a BI value of 0.4 or less. The occurrence was almost suppressed.
- the test pieces No. 1 to 21 having an overhang height of 6 mm no rust from the weld was observed in the continuous spray test of 5% NaCl on the corrosion resistance test piece after being processed by the Erichsen tester.
- test pieces No. 1 to 21 with a bulging height of 7 mm which are more severely processed, no rust is observed in the welded portion when the BI value is 0.4 or less, and rust is observed when the test piece exceeds 0.4. It was.
- test pieces Nos. 22, 24, and 26 to 28 having a BI value exceeding 0.8 the black spot generation length ratio after TIG welding was large, and rust from the weld was confirmed in all corrosion tests.
- the rust generating portions of the test pieces Nos. 22, 24, and 26 to 28 were magnified and observed with a magnifying glass, peeling was observed at the boundary between the black spot and the weld bead portion.
- Nos. 22, 26, 27, and 28 in which Al, Ti, Si, and Ca had a concentration higher than the specified level rust was generated in the corrosion test.
- test piece No25 where the composition ratio of Cr is less than 16% and test piece No23 where the composition ratio of Ti is less than 0.05% the occurrence of rust was observed in the corrosion test.
- Test material was manufactured in the same manner as the method for manufacturing the test piece of No. 1 except that a ferritic stainless steel having the chemical composition (composition) shown below was manufactured by cold rolling to produce a steel plate having a thickness of 1 mm. Using this, a test piece A and a test piece B were obtained.
- composition composition
- Test piece B C: 0.009%, N: 0.010%, Si: 0.25%, Mn: 0.15%, P: 0.21%, S: 0.001%, Cr: 20.2%, Al : 0.15%, Ti: 0.19%, Ca: 0.0015%, balance: iron and inevitable impurities
- the test piece A and test piece B thus obtained were the same as the test piece No1 TIG welding was performed under the welding conditions described above, and the appearance of black spots generated on the back side during TIG welding was observed. The results are shown in FIGS. 1A and 1B.
- FIG. 1A is a photograph showing the appearance of black spots generated on the back side during TIG welding.
- FIG. 1B is a schematic view showing the appearance of a black spot generated on the back side during TIG welding, and corresponds to the photograph shown in FIG. 1A. 1A and 1B, the left side is a photograph and drawing of a test piece A having a BI value of 0.49, and the right side is a photograph and drawing of a test piece B having a BI value of 1.07.
- spotted black spots are scattered on both the test piece A having a BI value of 0.49 and the test piece B having a BI value of 1.07.
- Auger electron spectroscopic analysis (AES) measurement was performed on the test piece B having a BI value of 1.07 at two locations, a weld bead portion and a black spot portion. The results are shown in FIGS. 2A and 2B.
- AES Auger electron spectroscopic analysis
- a scanning FE Auger electron spectrometer was used, and measurement was carried out under conditions of an acceleration voltage of 10 keV, a spot diameter of about 40 nm, and a sputtering rate of 15 nm / min until almost no oxygen intensity was observed.
- an error may occur depending on the measurement position, but this time it was adopted as an approximate thickness.
- FIG. 2A and 2B are graphs showing the results of AES measurement of the element depth profile (element concentration distribution in the depth direction) of the black spot and the weld bead on the back side of the test piece.
- FIG. 2A shows the result of the weld bead
- FIG. 2B shows the result of the black spot.
- the weld bead portion was mainly composed of Ti, and was an oxide having a thickness of several hundreds of microns including Al and Si.
- the black spots were mainly oxides of Al, and were thick oxides having a thickness of several thousand ⁇ ⁇ containing Ti, Si, and Ca.
- Al is contained at the highest concentration in the black spot
- Ca is contained in the black spot at a high concentration even though the content in the steel is small. It was confirmed that
- Example 2 C: 0.002 to 0.015%, N: 0.02 to 0.015%, Cr: 16.5 to 23%, Ni: 0 to 1.5%, Mo: 0 to 2.5%
- a ferritic stainless steel specimen having a composition and various chemical components (compositions) having different contents such as Al, Ti, Si, and Ca, which are the main components of the black spot, is manufactured in the same manner as the test piece A. Manufactured by. Using this, a plurality of test pieces were obtained. The plurality of test pieces thus obtained were subjected to TIG welding under the same welding conditions as for the No. 1 test piece, and the black spot generation length ratio was calculated in the same manner as for the No. 1 test piece.
- the black spot generation length ratio tended to increase as Al, Ti, Si, and Ca increased.
- These elements are elements having a particularly strong affinity with oxygen, and among these, the effect of Al is particularly great, and it has been found that Ca has a great influence on black spots despite its low content in steel. Further, it has been found that Ti and Si also contribute to the generation of black spots.
- a BI value represented by the following formula (1) was calculated for each of the plurality of test pieces, and the relationship with the black spot generation length ratio was examined.
- BI 3Al + Ti + 0.5Si + 200Ca ⁇ 0.8
- Al, Ti, Si, and Ca in the formula (1) are the content [% by mass] of each component in the steel.
- FIG. 3 is a graph showing the relationship between the BI value and the black spot generation length ratio. As shown in FIG. 3, it can be seen that the larger the BI value, the larger the black spot generation length ratio.
- FIG. 4 is a graph showing the relationship between the BI value and the corrosion resistance evaluation result after a spray test after processing.
- double circles ( ⁇ ) indicate excellent results
- circles ( ⁇ ) indicate good results
- ⁇ indicate poor results.
- FIG. 4 when the BI value is 0.8 or less, corrosion does not occur in the test piece with the overhang height of 6 mm, and particularly with a test piece with the overhang height of 7 mm when the BI value is 0.4 or less. Since corrosion was not observed, it was very good.
- Ferritic stainless steel of the present invention includes exterior materials, building materials, outdoor equipment, water storage and hot water storage tanks, home appliances, bathtubs, kitchen equipment, drain water recovery equipment for latent heat recovery type gas water heaters and their heat exchangers, various welding
- TIG welding such as pipes
- the ferritic stainless steel of the present invention is suitable for a member to be processed after TIG welding.
- the ferritic stainless steel of the present invention is excellent not only in corrosion resistance but also in workability of a TIG welded portion, it can be widely applied in severe processing applications.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Mechanical Engineering (AREA)
- Materials Engineering (AREA)
- Metallurgy (AREA)
- Organic Chemistry (AREA)
- Physics & Mathematics (AREA)
- Thermal Sciences (AREA)
- Crystallography & Structural Chemistry (AREA)
- General Engineering & Computer Science (AREA)
- Heat Treatment Of Sheet Steel (AREA)
- Arc Welding In General (AREA)
Abstract
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP11814699.2A EP2602351B1 (fr) | 2010-08-06 | 2011-08-04 | Acier inoxydable ferritique |
| CN2011800382369A CN103052731A (zh) | 2010-08-06 | 2011-08-04 | 铁素体系不锈钢 |
| US13/813,511 US20130129560A1 (en) | 2010-08-06 | 2011-08-04 | Ferritic stainless steel |
| KR1020137003262A KR20130034042A (ko) | 2010-08-06 | 2011-08-04 | 페라이트계 스테인리스 강 |
| AU2011286685A AU2011286685A1 (en) | 2010-08-06 | 2011-08-04 | Ferritic stainless steel |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2010177998A JP5793283B2 (ja) | 2010-08-06 | 2010-08-06 | ブラックスポットの生成の少ないフェライト系ステンレス鋼 |
| JP2010-177998 | 2010-08-06 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2012018074A1 true WO2012018074A1 (fr) | 2012-02-09 |
Family
ID=45559570
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2011/067850 WO2012018074A1 (fr) | 2010-08-06 | 2011-08-04 | Acier inoxydable ferritique |
Country Status (8)
| Country | Link |
|---|---|
| US (1) | US20130129560A1 (fr) |
| EP (1) | EP2602351B1 (fr) |
| JP (1) | JP5793283B2 (fr) |
| KR (1) | KR20130034042A (fr) |
| CN (1) | CN103052731A (fr) |
| AU (1) | AU2011286685A1 (fr) |
| TW (1) | TWI526549B (fr) |
| WO (1) | WO2012018074A1 (fr) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20150020933A1 (en) * | 2012-03-30 | 2015-01-22 | Nippon Steel & Sumikin Stainless Steel Corporation | Heat-resistant cold rolled ferritic stainless steel sheet, hot rolled ferritic stainless steel sheet for cold rolling raw material, and methods for producing same |
| CN104903483A (zh) * | 2012-11-20 | 2015-09-09 | 奥托库姆普联合股份公司 | 铁素体不锈钢 |
| US9885099B2 (en) | 2012-03-09 | 2018-02-06 | Nippon Steel & Sumikin Stainless Steel Corporation | Ferritic stainless steel sheet |
| US10385429B2 (en) | 2013-03-27 | 2019-08-20 | Nippon Steel & Sumikin Stainless Steel Corporation | Hot-rolled ferritic stainless-steel plate, process for producing same, and steel strip |
Families Citing this family (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN104685089B (zh) * | 2012-12-07 | 2016-08-17 | 杰富意钢铁株式会社 | 铁素体系不锈钢板 |
| JP5935792B2 (ja) * | 2013-12-27 | 2016-06-15 | Jfeスチール株式会社 | フェライト系ステンレス鋼 |
| JP5987821B2 (ja) * | 2013-12-27 | 2016-09-07 | Jfeスチール株式会社 | フェライト系ステンレス鋼 |
| KR20170018457A (ko) * | 2014-07-31 | 2017-02-17 | 제이에프이 스틸 가부시키가이샤 | 플라즈마 용접용 페라이트계 스테인리스 강판 및 그 용접 방법 |
| US20190106775A1 (en) * | 2016-03-29 | 2019-04-11 | Jfe Steel Corporation | Ferritic stainless steel sheet |
| ES2835273T3 (es) | 2016-06-27 | 2021-06-22 | Jfe Steel Corp | Lámina de acero inoxidable ferrítico |
| JP6418338B2 (ja) | 2016-09-02 | 2018-11-07 | Jfeスチール株式会社 | フェライト系ステンレス鋼 |
| JP6699670B2 (ja) | 2016-09-02 | 2020-05-27 | Jfeスチール株式会社 | フェライト系ステンレス鋼 |
| JP2019044255A (ja) * | 2017-09-07 | 2019-03-22 | Jfeスチール株式会社 | フェライト系ステンレス鋼板 |
| JP7042057B2 (ja) | 2017-10-25 | 2022-03-25 | 日鉄ステンレス株式会社 | スラグスポット発生抑止能に優れるステンレス鋼材並びに溶接構造部材およびその製造法 |
| TWI801538B (zh) | 2018-03-27 | 2023-05-11 | 日商日鐵不銹鋼股份有限公司 | 肥粒鐵系不鏽鋼及其製造方法、肥粒鐵系不鏽鋼板及其製造方法、以及燃料電池用構件 |
| EP3670692B1 (fr) * | 2018-12-21 | 2022-08-10 | Outokumpu Oyj | Acier inoxydable ferritique |
| JP7118015B2 (ja) * | 2019-01-16 | 2022-08-15 | 日鉄ステンレス株式会社 | ステンレス鋼材のスラグスポット発生量の予測評価方法 |
| JP2022539597A (ja) | 2019-07-05 | 2022-09-12 | スタミカーボン・ベー・フェー | 尿素プラントのフェライト鋼部品 |
| KR102326044B1 (ko) * | 2019-12-20 | 2021-11-15 | 주식회사 포스코 | 자화특성이 향상된 페라이트계 스테인리스강 및 그 제조 방법 |
Citations (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5521102B2 (fr) | 1975-02-01 | 1980-06-07 | ||
| JPH0570899A (ja) | 1991-09-17 | 1993-03-23 | Nisshin Steel Co Ltd | 溶接部耐食性に優れるフエライト系ステンレス鋼 |
| JPH0734205A (ja) | 1993-05-19 | 1995-02-03 | Kawasaki Steel Corp | 耐候性、耐隙間腐食性に優れたフェライト系ステンレス鋼 |
| JPH08144021A (ja) * | 1994-11-18 | 1996-06-04 | Sumitomo Metal Ind Ltd | フェライトステンレス鋼およびその冷延鋼板の製造方法 |
| JPH10102212A (ja) * | 1996-09-30 | 1998-04-21 | Kawasaki Steel Corp | 溶接溶け込み性に優れるフェライト系ステンレス鋼板 |
| JP2004131796A (ja) * | 2002-10-10 | 2004-04-30 | Nippon Steel Corp | 容器材料用クロム含有鋼およびその溶接方法、ならびに容器材料 |
| JP2004149833A (ja) * | 2002-10-29 | 2004-05-27 | Nippon Yakin Kogyo Co Ltd | 耐食性、溶接性および表面性状に優れるステンレス鋼およびその製造方法 |
| JP2006241564A (ja) | 2005-03-07 | 2006-09-14 | Nisshin Steel Co Ltd | 溶接構造物用フェライト系ステンレス鋼 |
| JP2006263811A (ja) * | 2005-02-28 | 2006-10-05 | Jfe Steel Kk | ティグ溶接用フェライト系ステンレス鋼溶加棒 |
| JP2007270290A (ja) | 2006-03-31 | 2007-10-18 | Jfe Steel Kk | 溶接部の耐食性に優れたフェライト系ステンレス鋼。 |
| JP2007302995A (ja) * | 2006-04-10 | 2007-11-22 | Nisshin Steel Co Ltd | 溶接構造温水容器用フェライト系ステンレス鋼および温水容器 |
| JP2009091654A (ja) * | 2007-09-18 | 2009-04-30 | Jfe Steel Kk | 溶接性に優れたフェライト系ステンレス鋼 |
| JP2009174036A (ja) * | 2008-01-28 | 2009-08-06 | Nippon Steel & Sumikin Stainless Steel Corp | 耐食性と加工性に優れた高純度フェライト系ステンレス鋼およびその製造方法 |
| JP2010177998A (ja) | 2009-01-29 | 2010-08-12 | Nippon Telegr & Teleph Corp <Ntt> | ユーザ認証方法、ユーザ認証システム、ユーザ端末、ユーザ認証装置、ユーザ端末用プログラム及びユーザ認証装置用プログラム |
| JP2010202973A (ja) * | 2009-02-09 | 2010-09-16 | Nippon Steel & Sumikin Stainless Steel Corp | ブラックスポットの生成の少ないフェライト系ステンレス鋼 |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH1012212A (ja) * | 1996-06-18 | 1998-01-16 | Yuasa Corp | 密閉形鉛蓄電池 |
| JP3190290B2 (ja) * | 1997-09-26 | 2001-07-23 | 日新製鋼株式会社 | 溶接部の耐食性に優れるフェライト系ステンレス鋼 |
| JP4465853B2 (ja) * | 2000-10-30 | 2010-05-26 | Jfeスチール株式会社 | 耐食性および耐水垢付着性に優れたジャーポット容器用フェライト系ステンレス冷延鋼板およびジャーポット用フェライト系ステンレス鋼製容器 |
| JP4397772B2 (ja) * | 2004-09-24 | 2010-01-13 | 新日鐵住金ステンレス株式会社 | 加工性に優れるフェライト系ステンレス鋼板の製造方法 |
| EP2100983B1 (fr) * | 2007-01-12 | 2012-10-31 | JFE Steel Corporation | Tôle d'acier inoxydable ferritique pour chauffe-eau, présentant une excellente résistance à la corrosion au niveau d'une partie soudée et une excellente ténacité de tôle |
| JP5010301B2 (ja) * | 2007-02-02 | 2012-08-29 | 日新製鋼株式会社 | 排ガス経路部材用フェライト系ステンレス鋼および排ガス経路部材 |
| JP5111910B2 (ja) * | 2007-03-23 | 2013-01-09 | 新日鐵住金ステンレス株式会社 | 溶接部加工性および耐すき間腐食性に優れた表面疵の少ないフェライト系ステンレス鋼 |
-
2010
- 2010-08-06 JP JP2010177998A patent/JP5793283B2/ja active Active
-
2011
- 2011-08-04 WO PCT/JP2011/067850 patent/WO2012018074A1/fr active Application Filing
- 2011-08-04 CN CN2011800382369A patent/CN103052731A/zh active Pending
- 2011-08-04 TW TW100127716A patent/TWI526549B/zh active
- 2011-08-04 EP EP11814699.2A patent/EP2602351B1/fr active Active
- 2011-08-04 US US13/813,511 patent/US20130129560A1/en not_active Abandoned
- 2011-08-04 AU AU2011286685A patent/AU2011286685A1/en not_active Abandoned
- 2011-08-04 KR KR1020137003262A patent/KR20130034042A/ko not_active Application Discontinuation
Patent Citations (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5521102B2 (fr) | 1975-02-01 | 1980-06-07 | ||
| JPH0570899A (ja) | 1991-09-17 | 1993-03-23 | Nisshin Steel Co Ltd | 溶接部耐食性に優れるフエライト系ステンレス鋼 |
| JPH0734205A (ja) | 1993-05-19 | 1995-02-03 | Kawasaki Steel Corp | 耐候性、耐隙間腐食性に優れたフェライト系ステンレス鋼 |
| JPH08144021A (ja) * | 1994-11-18 | 1996-06-04 | Sumitomo Metal Ind Ltd | フェライトステンレス鋼およびその冷延鋼板の製造方法 |
| JPH10102212A (ja) * | 1996-09-30 | 1998-04-21 | Kawasaki Steel Corp | 溶接溶け込み性に優れるフェライト系ステンレス鋼板 |
| JP2004131796A (ja) * | 2002-10-10 | 2004-04-30 | Nippon Steel Corp | 容器材料用クロム含有鋼およびその溶接方法、ならびに容器材料 |
| JP2004149833A (ja) * | 2002-10-29 | 2004-05-27 | Nippon Yakin Kogyo Co Ltd | 耐食性、溶接性および表面性状に優れるステンレス鋼およびその製造方法 |
| JP2006263811A (ja) * | 2005-02-28 | 2006-10-05 | Jfe Steel Kk | ティグ溶接用フェライト系ステンレス鋼溶加棒 |
| JP2006241564A (ja) | 2005-03-07 | 2006-09-14 | Nisshin Steel Co Ltd | 溶接構造物用フェライト系ステンレス鋼 |
| JP2007270290A (ja) | 2006-03-31 | 2007-10-18 | Jfe Steel Kk | 溶接部の耐食性に優れたフェライト系ステンレス鋼。 |
| JP2007302995A (ja) * | 2006-04-10 | 2007-11-22 | Nisshin Steel Co Ltd | 溶接構造温水容器用フェライト系ステンレス鋼および温水容器 |
| JP2009091654A (ja) * | 2007-09-18 | 2009-04-30 | Jfe Steel Kk | 溶接性に優れたフェライト系ステンレス鋼 |
| JP2009174036A (ja) * | 2008-01-28 | 2009-08-06 | Nippon Steel & Sumikin Stainless Steel Corp | 耐食性と加工性に優れた高純度フェライト系ステンレス鋼およびその製造方法 |
| JP2010177998A (ja) | 2009-01-29 | 2010-08-12 | Nippon Telegr & Teleph Corp <Ntt> | ユーザ認証方法、ユーザ認証システム、ユーザ端末、ユーザ認証装置、ユーザ端末用プログラム及びユーザ認証装置用プログラム |
| JP2010202973A (ja) * | 2009-02-09 | 2010-09-16 | Nippon Steel & Sumikin Stainless Steel Corp | ブラックスポットの生成の少ないフェライト系ステンレス鋼 |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP2602351A4 |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9885099B2 (en) | 2012-03-09 | 2018-02-06 | Nippon Steel & Sumikin Stainless Steel Corporation | Ferritic stainless steel sheet |
| US20150020933A1 (en) * | 2012-03-30 | 2015-01-22 | Nippon Steel & Sumikin Stainless Steel Corporation | Heat-resistant cold rolled ferritic stainless steel sheet, hot rolled ferritic stainless steel sheet for cold rolling raw material, and methods for producing same |
| US10260134B2 (en) | 2012-03-30 | 2019-04-16 | Nippon Steel & Sumikin Stainless Steel Corporation | Hot rolled ferritic stainless steel sheet for cold rolling raw material |
| CN104903483A (zh) * | 2012-11-20 | 2015-09-09 | 奥托库姆普联合股份公司 | 铁素体不锈钢 |
| US20160281184A1 (en) * | 2012-11-20 | 2016-09-29 | Outokumpu Oyj | Ferritic stainless steel |
| US10385429B2 (en) | 2013-03-27 | 2019-08-20 | Nippon Steel & Sumikin Stainless Steel Corporation | Hot-rolled ferritic stainless-steel plate, process for producing same, and steel strip |
Also Published As
| Publication number | Publication date |
|---|---|
| CN103052731A (zh) | 2013-04-17 |
| TWI526549B (zh) | 2016-03-21 |
| KR20130034042A (ko) | 2013-04-04 |
| JP2012036444A (ja) | 2012-02-23 |
| JP5793283B2 (ja) | 2015-10-14 |
| TW201213559A (en) | 2012-04-01 |
| EP2602351A4 (fr) | 2017-04-05 |
| AU2011286685A1 (en) | 2013-02-28 |
| EP2602351B1 (fr) | 2019-10-02 |
| US20130129560A1 (en) | 2013-05-23 |
| EP2602351A1 (fr) | 2013-06-12 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2012018074A1 (fr) | Acier inoxydable ferritique | |
| JP5489759B2 (ja) | ブラックスポットの生成の少ないフェライト系ステンレス鋼 | |
| JP5050863B2 (ja) | 温水器用フェライト系ステンレス鋼板 | |
| JP6206624B1 (ja) | フェライト系ステンレス鋼板 | |
| JP5387802B1 (ja) | フェライト系ステンレス鋼 | |
| JP2011190524A (ja) | 耐酸化性、二次加工脆性および溶接部の靭性に優れたフェライト系ステンレス鋼 | |
| JPWO2019189871A1 (ja) | 二相ステンレスクラッド鋼板およびその製造方法 | |
| JP5928726B2 (ja) | 被覆アーク溶接棒 | |
| JP4784239B2 (ja) | ティグ溶接用フェライト系ステンレス鋼溶加棒 | |
| JP5111910B2 (ja) | 溶接部加工性および耐すき間腐食性に優れた表面疵の少ないフェライト系ステンレス鋼 | |
| TW201207128A (en) | Structural stainless steel sheet having excellent corrosion resistance at weld and method for manufacturing same | |
| JP4457492B2 (ja) | 加工性と溶接性に優れたステンレス鋼 | |
| JP5012194B2 (ja) | 溶接継手強度が高い温水器用フェライト系ステンレス鋼板およびその製造方法 | |
| JPWO2018066573A1 (ja) | オーステナイト系耐熱合金およびそれを用いた溶接継手 | |
| JP4465066B2 (ja) | フェライト・オーステナイト二相系ステンレス鋼用溶接材料 | |
| JP2016199803A (ja) | フェライト系ステンレス鋼 | |
| JP2005256121A (ja) | 溶接部の耐歪時効特性に優れたCr含有合金 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 201180038236.9 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 11814699 Country of ref document: EP Kind code of ref document: A1 |
|
| DPE1 | Request for preliminary examination filed after expiration of 19th month from priority date (pct application filed from 20040101) | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 13813511 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 20137003262 Country of ref document: KR Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 2011286685 Country of ref document: AU Date of ref document: 20110804 Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2011814699 Country of ref document: EP |