WO2012018074A1 - Ferritic stainless steel - Google Patents
Ferritic stainless steel Download PDFInfo
- Publication number
- WO2012018074A1 WO2012018074A1 PCT/JP2011/067850 JP2011067850W WO2012018074A1 WO 2012018074 A1 WO2012018074 A1 WO 2012018074A1 JP 2011067850 W JP2011067850 W JP 2011067850W WO 2012018074 A1 WO2012018074 A1 WO 2012018074A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- less
- stainless steel
- ferritic stainless
- mass
- content
- Prior art date
Links
- 229910001220 stainless steel Inorganic materials 0.000 title claims abstract description 47
- 229910052782 aluminium Inorganic materials 0.000 claims abstract description 28
- 229910052719 titanium Inorganic materials 0.000 claims abstract description 28
- 229910052791 calcium Inorganic materials 0.000 claims abstract description 23
- 229910052710 silicon Inorganic materials 0.000 claims abstract description 23
- 229910000831 Steel Inorganic materials 0.000 claims abstract description 18
- 239000010959 steel Substances 0.000 claims abstract description 18
- 239000012535 impurity Substances 0.000 claims abstract description 6
- 229910052757 nitrogen Inorganic materials 0.000 claims description 13
- 229910052799 carbon Inorganic materials 0.000 claims description 10
- 229910052796 boron Inorganic materials 0.000 claims description 7
- 229910052750 molybdenum Inorganic materials 0.000 claims description 7
- 229910052748 manganese Inorganic materials 0.000 claims description 6
- 229910052698 phosphorus Inorganic materials 0.000 claims description 6
- 229910052759 nickel Inorganic materials 0.000 claims description 5
- 229910052758 niobium Inorganic materials 0.000 claims description 5
- 229910052717 sulfur Inorganic materials 0.000 claims description 5
- 229910052726 zirconium Inorganic materials 0.000 claims description 3
- 206010027146 Melanoderma Diseases 0.000 abstract description 41
- 238000012360 testing method Methods 0.000 description 54
- 230000007797 corrosion Effects 0.000 description 51
- 238000005260 corrosion Methods 0.000 description 51
- 239000010936 titanium Substances 0.000 description 39
- 238000003466 welding Methods 0.000 description 33
- 239000011575 calcium Substances 0.000 description 30
- 241001016380 Reseda luteola Species 0.000 description 26
- 239000000203 mixture Substances 0.000 description 16
- PXHVJJICTQNCMI-UHFFFAOYSA-N nickel Substances [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 14
- 239000010955 niobium Substances 0.000 description 14
- 239000011651 chromium Substances 0.000 description 12
- JEIPFZHSYJVQDO-UHFFFAOYSA-N iron(III) oxide Inorganic materials O=[Fe]O[Fe]=O JEIPFZHSYJVQDO-UHFFFAOYSA-N 0.000 description 12
- 239000010949 copper Substances 0.000 description 9
- 230000000694 effects Effects 0.000 description 9
- 239000011324 bead Substances 0.000 description 8
- 239000011572 manganese Substances 0.000 description 8
- 238000000034 method Methods 0.000 description 8
- 239000000126 substance Substances 0.000 description 8
- XEEYBQQBJWHFJM-UHFFFAOYSA-N iron Substances [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 7
- 238000005259 measurement Methods 0.000 description 7
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 6
- 229910052804 chromium Inorganic materials 0.000 description 6
- 239000000463 material Substances 0.000 description 6
- 238000012545 processing Methods 0.000 description 6
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 6
- 239000007921 spray Substances 0.000 description 5
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 4
- 238000009826 distribution Methods 0.000 description 4
- 239000001301 oxygen Substances 0.000 description 4
- 229910052760 oxygen Inorganic materials 0.000 description 4
- 238000003860 storage Methods 0.000 description 4
- 229910000859 α-Fe Inorganic materials 0.000 description 4
- 229910000963 austenitic stainless steel Inorganic materials 0.000 description 3
- 239000011261 inert gas Substances 0.000 description 3
- 229910052742 iron Inorganic materials 0.000 description 3
- 229910052751 metal Inorganic materials 0.000 description 3
- 239000002184 metal Substances 0.000 description 3
- 230000001737 promoting effect Effects 0.000 description 3
- 239000011780 sodium chloride Substances 0.000 description 3
- 238000005728 strengthening Methods 0.000 description 3
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- 206010070834 Sensitisation Diseases 0.000 description 2
- 230000006399 behavior Effects 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 238000005097 cold rolling Methods 0.000 description 2
- 229910052802 copper Inorganic materials 0.000 description 2
- 238000004090 dissolution Methods 0.000 description 2
- 238000011156 evaluation Methods 0.000 description 2
- 238000010438 heat treatment Methods 0.000 description 2
- 238000003754 machining Methods 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 238000011084 recovery Methods 0.000 description 2
- 238000001953 recrystallisation Methods 0.000 description 2
- 238000007670 refining Methods 0.000 description 2
- 230000008313 sensitization Effects 0.000 description 2
- 239000010935 stainless steel Substances 0.000 description 2
- ZOXJGFHDIHLPTG-UHFFFAOYSA-N Boron Chemical compound [B] ZOXJGFHDIHLPTG-UHFFFAOYSA-N 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- 229910001208 Crucible steel Inorganic materials 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- PWHULOQIROXLJO-UHFFFAOYSA-N Manganese Chemical compound [Mn] PWHULOQIROXLJO-UHFFFAOYSA-N 0.000 description 1
- ZOKXTWBITQBERF-UHFFFAOYSA-N Molybdenum Chemical compound [Mo] ZOKXTWBITQBERF-UHFFFAOYSA-N 0.000 description 1
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 1
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 1
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- 230000001133 acceleration Effects 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 238000000137 annealing Methods 0.000 description 1
- 229910052786 argon Inorganic materials 0.000 description 1
- 239000004566 building material Substances 0.000 description 1
- 238000005336 cracking Methods 0.000 description 1
- 230000007547 defect Effects 0.000 description 1
- 230000006866 deterioration Effects 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 238000002845 discoloration Methods 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 239000011733 molybdenum Substances 0.000 description 1
- GUCVJGMIXFAOAE-UHFFFAOYSA-N niobium atom Chemical compound [Nb] GUCVJGMIXFAOAE-UHFFFAOYSA-N 0.000 description 1
- -1 outdoor equipment Substances 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 239000011574 phosphorus Substances 0.000 description 1
- 238000005554 pickling Methods 0.000 description 1
- 239000002994 raw material Substances 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- VSZWPYCFIRKVQL-UHFFFAOYSA-N selanylidenegallium;selenium Chemical compound [Se].[Se]=[Ga].[Se]=[Ga] VSZWPYCFIRKVQL-UHFFFAOYSA-N 0.000 description 1
- 239000010703 silicon Substances 0.000 description 1
- 239000002893 slag Substances 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000006104 solid solution Substances 0.000 description 1
- 238000007711 solidification Methods 0.000 description 1
- 230000008023 solidification Effects 0.000 description 1
- 238000004611 spectroscopical analysis Methods 0.000 description 1
- 238000004544 sputter deposition Methods 0.000 description 1
- 230000000087 stabilizing effect Effects 0.000 description 1
- 239000002436 steel type Substances 0.000 description 1
- 239000011593 sulfur Substances 0.000 description 1
- 239000002344 surface layer Substances 0.000 description 1
- WFKWXMTUELFFGS-UHFFFAOYSA-N tungsten Chemical compound [W] WFKWXMTUELFFGS-UHFFFAOYSA-N 0.000 description 1
- 229910052721 tungsten Inorganic materials 0.000 description 1
- 239000010937 tungsten Substances 0.000 description 1
- 229910052720 vanadium Inorganic materials 0.000 description 1
- LEONUFNNVUYDNQ-UHFFFAOYSA-N vanadium atom Chemical compound [V] LEONUFNNVUYDNQ-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/18—Ferrous alloys, e.g. steel alloys containing chromium
- C22C38/40—Ferrous alloys, e.g. steel alloys containing chromium with nickel
- C22C38/54—Ferrous alloys, e.g. steel alloys containing chromium with nickel with boron
-
- C—CHEMISTRY; METALLURGY
- C21—METALLURGY OF IRON
- C21D—MODIFYING THE PHYSICAL STRUCTURE OF FERROUS METALS; GENERAL DEVICES FOR HEAT TREATMENT OF FERROUS OR NON-FERROUS METALS OR ALLOYS; MAKING METAL MALLEABLE, e.g. BY DECARBURISATION OR TEMPERING
- C21D6/00—Heat treatment of ferrous alloys
- C21D6/002—Heat treatment of ferrous alloys containing Cr
-
- C—CHEMISTRY; METALLURGY
- C21—METALLURGY OF IRON
- C21D—MODIFYING THE PHYSICAL STRUCTURE OF FERROUS METALS; GENERAL DEVICES FOR HEAT TREATMENT OF FERROUS OR NON-FERROUS METALS OR ALLOYS; MAKING METAL MALLEABLE, e.g. BY DECARBURISATION OR TEMPERING
- C21D8/00—Modifying the physical properties by deformation combined with, or followed by, heat treatment
- C21D8/02—Modifying the physical properties by deformation combined with, or followed by, heat treatment during manufacturing of plates or strips
- C21D8/0221—Modifying the physical properties by deformation combined with, or followed by, heat treatment during manufacturing of plates or strips characterised by the working steps
- C21D8/0226—Hot rolling
-
- C—CHEMISTRY; METALLURGY
- C21—METALLURGY OF IRON
- C21D—MODIFYING THE PHYSICAL STRUCTURE OF FERROUS METALS; GENERAL DEVICES FOR HEAT TREATMENT OF FERROUS OR NON-FERROUS METALS OR ALLOYS; MAKING METAL MALLEABLE, e.g. BY DECARBURISATION OR TEMPERING
- C21D8/00—Modifying the physical properties by deformation combined with, or followed by, heat treatment
- C21D8/02—Modifying the physical properties by deformation combined with, or followed by, heat treatment during manufacturing of plates or strips
- C21D8/0221—Modifying the physical properties by deformation combined with, or followed by, heat treatment during manufacturing of plates or strips characterised by the working steps
- C21D8/0236—Cold rolling
-
- C—CHEMISTRY; METALLURGY
- C21—METALLURGY OF IRON
- C21D—MODIFYING THE PHYSICAL STRUCTURE OF FERROUS METALS; GENERAL DEVICES FOR HEAT TREATMENT OF FERROUS OR NON-FERROUS METALS OR ALLOYS; MAKING METAL MALLEABLE, e.g. BY DECARBURISATION OR TEMPERING
- C21D8/00—Modifying the physical properties by deformation combined with, or followed by, heat treatment
- C21D8/02—Modifying the physical properties by deformation combined with, or followed by, heat treatment during manufacturing of plates or strips
- C21D8/0247—Modifying the physical properties by deformation combined with, or followed by, heat treatment during manufacturing of plates or strips characterised by the heat treatment
- C21D8/0263—Modifying the physical properties by deformation combined with, or followed by, heat treatment during manufacturing of plates or strips characterised by the heat treatment following hot rolling
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/001—Ferrous alloys, e.g. steel alloys containing N
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/002—Ferrous alloys, e.g. steel alloys containing In, Mg, or other elements not provided for in one single group C22C38/001 - C22C38/60
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/004—Very low carbon steels, i.e. having a carbon content of less than 0,01%
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/02—Ferrous alloys, e.g. steel alloys containing silicon
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/04—Ferrous alloys, e.g. steel alloys containing manganese
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/06—Ferrous alloys, e.g. steel alloys containing aluminium
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/18—Ferrous alloys, e.g. steel alloys containing chromium
- C22C38/20—Ferrous alloys, e.g. steel alloys containing chromium with copper
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/18—Ferrous alloys, e.g. steel alloys containing chromium
- C22C38/22—Ferrous alloys, e.g. steel alloys containing chromium with molybdenum or tungsten
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/18—Ferrous alloys, e.g. steel alloys containing chromium
- C22C38/24—Ferrous alloys, e.g. steel alloys containing chromium with vanadium
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/18—Ferrous alloys, e.g. steel alloys containing chromium
- C22C38/26—Ferrous alloys, e.g. steel alloys containing chromium with niobium or tantalum
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/18—Ferrous alloys, e.g. steel alloys containing chromium
- C22C38/28—Ferrous alloys, e.g. steel alloys containing chromium with titanium or zirconium
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/18—Ferrous alloys, e.g. steel alloys containing chromium
- C22C38/32—Ferrous alloys, e.g. steel alloys containing chromium with boron
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/18—Ferrous alloys, e.g. steel alloys containing chromium
- C22C38/40—Ferrous alloys, e.g. steel alloys containing chromium with nickel
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/18—Ferrous alloys, e.g. steel alloys containing chromium
- C22C38/40—Ferrous alloys, e.g. steel alloys containing chromium with nickel
- C22C38/42—Ferrous alloys, e.g. steel alloys containing chromium with nickel with copper
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/18—Ferrous alloys, e.g. steel alloys containing chromium
- C22C38/40—Ferrous alloys, e.g. steel alloys containing chromium with nickel
- C22C38/44—Ferrous alloys, e.g. steel alloys containing chromium with nickel with molybdenum or tungsten
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/18—Ferrous alloys, e.g. steel alloys containing chromium
- C22C38/40—Ferrous alloys, e.g. steel alloys containing chromium with nickel
- C22C38/46—Ferrous alloys, e.g. steel alloys containing chromium with nickel with vanadium
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/18—Ferrous alloys, e.g. steel alloys containing chromium
- C22C38/40—Ferrous alloys, e.g. steel alloys containing chromium with nickel
- C22C38/48—Ferrous alloys, e.g. steel alloys containing chromium with nickel with niobium or tantalum
-
- C—CHEMISTRY; METALLURGY
- C22—METALLURGY; FERROUS OR NON-FERROUS ALLOYS; TREATMENT OF ALLOYS OR NON-FERROUS METALS
- C22C—ALLOYS
- C22C38/00—Ferrous alloys, e.g. steel alloys
- C22C38/18—Ferrous alloys, e.g. steel alloys containing chromium
- C22C38/40—Ferrous alloys, e.g. steel alloys containing chromium with nickel
- C22C38/50—Ferrous alloys, e.g. steel alloys containing chromium with nickel with titanium or zirconium
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F28—HEAT EXCHANGE IN GENERAL
- F28F—DETAILS OF HEAT-EXCHANGE AND HEAT-TRANSFER APPARATUS, OF GENERAL APPLICATION
- F28F21/00—Constructions of heat-exchange apparatus characterised by the selection of particular materials
- F28F21/08—Constructions of heat-exchange apparatus characterised by the selection of particular materials of metal
- F28F21/081—Heat exchange elements made from metals or metal alloys
- F28F21/082—Heat exchange elements made from metals or metal alloys from steel or ferrous alloys
- F28F21/083—Heat exchange elements made from metals or metal alloys from steel or ferrous alloys from stainless steel
Definitions
- the present invention relates to a ferritic stainless steel that generates less black spots in a TIG weld.
- Ferritic stainless steel generally has not only excellent corrosion resistance, but also has features such as a smaller thermal expansion coefficient than austenitic stainless steel and excellent resistance to stress corrosion cracking. For this reason, ferritic stainless steel is widely used for building exterior materials such as tableware, kitchen equipment and roofing materials, and materials for water storage and hot water storage. Furthermore, in recent years, due to soaring prices of Ni raw materials, there is also a great demand for switching from austenitic stainless steel, and its applications are becoming widespread.
- ferritic stainless steel has a problem in that its C and N solid solubility limit is small, so that sensitization occurs at the welded portion and corrosion resistance is lowered.
- a method of suppressing the sensitization of the weld metal part by reducing the amount of C and N or fixing C and N by adding a stabilizing element such as Ti or Nb has been proposed and widely used.
- a technique for forming an Al oxide film that improves the corrosion resistance of the weld heat affected zone on the surface layer of the steel during welding is disclosed.
- Patent Document 3 discloses a technique for improving the crevice corrosion resistance of a welded part by adding a certain amount or more of Si in addition to the combined addition of Al and Ti.
- Patent Document 4 describes that 4Al + Ti ⁇ 0.32 (Ti and Al in the formulas indicate the content of each component in the steel), thereby reducing the heat input during welding and reducing the welded portion.
- a technique for suppressing the generation of scale and improving the corrosion resistance of the welded portion is disclosed.
- the above-described prior art is intended to improve the corrosion resistance of the welded portion and the weld heat affected zone.
- Patent Document 5 As a means for improving the weather resistance and crevice corrosion resistance of the material itself, not the welded portion, there is a technique of positively adding P and adding appropriate amounts of Ca and Al (see, for example, Patent Document 5).
- Ca and Al are added to control the shape and distribution of nonmetallic inclusions in steel.
- P is added in an amount exceeding 0.04%, and Patent Document 5 does not describe any effect at the time of welding.
- black spots In conventional ferritic stainless steel, even if the shielding conditions in the welded part are optimized, black spots generally called black spots or slag spots may be scattered on the welded back bead after welding.
- a black spot is one in which Al, Ti, Si, and Ca, which have strong affinity for oxygen, form oxides and solidify on the weld metal during solidification of the weld metal in TIG (Tungsten Inert Gas) welding.
- TIG Transmissionungsten Inert Gas
- the generation of black spots is greatly influenced by welding conditions, particularly shielding conditions by inert gas, and more black spots are generated as the shielding is insufficient.
- the black spot itself is an oxide, there is no problem in the corrosion resistance and workability of the weld even if a small amount of black spot is scattered.
- black spots are generated in large quantities or continuously, not only the appearance of the welded part is used without being polished, but also the appearance of the black spot is removed when the welded part is processed. May occur.
- the black spot part is peeled off, there are cases where workability is deteriorated or a gap corrosion occurs between the black spot part and the peeled black spot part.
- Even if the processing is not performed after welding if the black spot is generated thickly, if the stress is applied to the welded portion due to the structure, the black spot may be peeled off and the corrosion resistance may be lowered.
- the corrosion resistance of the TIG welded part it is important not only to improve the corrosion resistance of the weld bead part and the weld scale part itself, but also to control the black spots generated in the welded part.
- scales with discoloration that occur during welding can be suppressed almost by the method of strengthening the shield condition of welding.
- the black spot generated in the TIG welded part is the conventional technology even if the shield condition is strengthened. Then, it was not able to suppress enough.
- This invention is made in view of such a situation, Comprising: It is hard to produce
- the present inventor conducted extensive research as shown below in order to suppress the amount of black spots generated. As a result, the inventors have found that by optimizing the amounts of Al, Ti, Si, and Ca, it is possible to suppress the generation of black spots in the TIG welded portion, and the present inventors have devised a ferritic stainless steel according to the present invention that generates less black spots.
- the gist of the present invention is as follows.
- the first aspect of the present invention is, in mass%, C: 0.020% or less, N: 0.025% or less, Si: 1.0% or less, Mn: 1.0% or less, P: 0.035. %: S: 0.01% or less, Cr: 16.0 to 25.0%, Al: 0.12% or less, Ti: 0.05 to 0.35%, Ca: 0.0015% or less
- the ferritic stainless steel is composed of Fe and inevitable impurities as the balance and satisfies the following formula (1).
- a second aspect of the present invention is the ferritic stainless steel according to the first aspect, and further includes ferritic stainless steel containing Nb: 0.6% or less by mass%.
- a third aspect of the present invention is a ferritic stainless steel according to the first or second aspect, and further includes ferritic stainless steel containing, by mass%, Mo: 3.0% or less.
- a fourth aspect of the present invention is the ferritic stainless steel according to any one of the first to third aspects, and further, by mass, Cu: 2.0% or less, Ni: 2.0% or less Ferritic stainless steel containing one or two selected from
- a fifth aspect of the present invention is the ferritic stainless steel according to any one of the first to fourth aspects, and further, by mass, V: 0.2% or less, Zr: 0.2% or less Ferritic stainless steel containing one or two selected from
- a sixth aspect of the present invention is a ferritic stainless steel according to any one of the first to fifth aspects, further comprising ferritic stainless steel containing, by mass%, B: 0.005% or less. is there.
- the present invention it is possible to provide a ferritic stainless steel in which black spots are unlikely to be generated in a TIG welded portion and excellent in corrosion resistance and workability of the TIG welded portion.
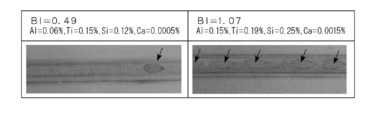
- FIG. 1A is a photograph showing the appearance of black spots generated on the back side during TIG welding.
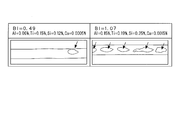
- FIG. 1B is a schematic diagram showing the appearance of a black spot generated on the back side during TIG welding, and corresponds to the photograph shown in FIG. 1A.
- FIG. 2A is a graph showing the results of AES measurement of the element depth profile (element concentration distribution in the depth direction) of the weld bead portion on the back side of the test piece.
- FIG. 2B is a graph showing the result of measuring the element depth profile (element concentration distribution in the depth direction) of the black spot on the back side of the test piece by AES.
- FIG. 3 is a graph showing the relationship between the BI value and the black spot generation length ratio.
- FIG. 4 is a graph showing the relationship between BI value and corrosion. Double circles ( ⁇ ) indicate excellent results, circles ( ⁇ ) indicate good results, and ⁇ indicate poor results.
- the ferritic stainless steel according to the present invention with little black spot formation satisfies the following formula (1).
- BI 3Al + Ti + 0.5Si + 200Ca ⁇ 0.8
- Al, Ti, Si, and Ca in the formula (1) are the content [% by mass] of each component in the steel.
- Al, Ti, Si, and Ca are elements that have particularly strong affinity with oxygen, and are elements that generate black spots during TIG welding. Further, as the content of Al, Ti, Si, and Ca contained in the steel is increased, black spots are easily generated.
- the coefficient of content of Al, Ti, Si, and Ca in the above formula (1) is determined based on the magnitude (strength) of the action that promotes the generation of black spots and the content in steel. More specifically, Al is an element that is contained in the black spot at the highest concentration and has a particularly large effect of promoting the generation of the black spot, as shown in an experimental example described later. For this reason, in the above equation (1), the coefficient of Al content is set to 3.
- Ca is an element that has a high effect of promoting the generation of black spots because it is contained in the black spots at a high concentration in spite of its low content in steel. For this reason, the coefficient of Ca content is set to 200.
- the generation of black spots becomes remarkable.
- the BI value is 0.8 or less, the generation of black spots in the TIG welded portion is sufficiently reduced and the corrosion resistance is excellent.
- the BI value is 0.6 or less, the generation of black spots can be more effectively suppressed, and when the BI value is 0.4 or less, the generation of black spots can be almost suppressed. Further, the corrosion resistance of the TIG welded portion can be further improved.
- the thickness of the black spots also increases, so it is estimated that they will be easily peeled off during processing, in which case peeling occurs in severe processing such as overhanging and becomes the starting point of corrosion. Conceivable.
- the occurrence rate of black spots is small, the thickness is reduced, and it is estimated that even if black spots are generated, it is difficult to peel off. Therefore, it is considered that the corrosion resistance of the welded portion can be improved by suppressing the occurrence of black spots.
- Aluminum (Al): 0.012% or less by mass% Al is important as a deoxidizing element, and also has the effect of controlling the composition of nonmetallic inclusions to refine the structure.
- Al is an element that contributes most to the generation of black spots.
- excessive addition of Al leads to coarsening of non-metallic inclusions, which may be a starting point for product wrinkling. Therefore, the upper limit value of the Al content is set to 0.12%.
- the Al content is more preferably 0.03% to 0.10%.
- Titanium (Ti): 0.05% to 0.35% by mass Ti is an extremely important element for fixing C and N and suppressing intergranular corrosion of the welded portion to improve workability.
- the range of Ti content is set to 0.05% to 0.35%. More desirably, it is 0.07% to 0.20%.
- Silicon (Si): 1.0% or less by mass% Si is an important element as a deoxidizing element, and is also effective in improving corrosion resistance and oxidation resistance.
- the upper limit of Si content is set to 1.0%.
- the Si content is more desirably 0.05% to 0.55%.
- Ca Calcium (Ca): 0.0015% or less in mass% Ca is very important as a deoxidizing element and is contained in a small amount in steel as a nonmetallic inclusion. However, since Ca is very easily oxidized, it becomes a major factor for generating black spots during welding. Moreover, Ca produces
- Mn Manganese
- MnS Manganese S
- the Mn content is set to 1.0% or less.
- Mn For deoxidation, it is preferable to contain 0.01% or more of Mn. More desirably, it is 0.05% to 0.5%. More desirably, it is 0.05% to 0.3%.
- Phosphorus (P): 0.035% or less in mass% P not only lowers weldability and workability, but also easily causes intergranular corrosion, so it needs to be kept low. Therefore, the content of P is set to 0.035% or less. More desirably, the content is 0.001% to 0.02%.
- S Sulfur: 0.01% or less by mass% S generates water-soluble inclusions that cause corrosion, such as CaS and MnS, and thus needs to be reduced. Therefore, the S content is 0.01% or less. However, excessive reduction causes cost deterioration. Therefore, the S content is more preferably 0.0001% to 0.005%.
- Chromium (Cr): 16.0-25.0% by mass Cr is the most important element for ensuring the corrosion resistance of stainless steel, and needs to be contained in an amount of 16.0% or more in order to stabilize the ferrite structure. However, Cr lowers the workability and manufacturability, so the upper limit was made 25.0%.
- the Cr content is desirably 16.5% to 23.0%, and more desirably 18.0% to 22.5%.
- Niobium (Nb): 0.6% or less by mass% Nb can be added alone or in combination with Ti due to its characteristics. When Nb is contained together with Ti, it is preferable to satisfy (Ti + Nb) / (C + N) ⁇ 6 (Ti, Nb, C, and N in the formula are contents [mass%] of each component in the steel).
- Nb is an element that fixes C and N like Ti and suppresses intergranular corrosion of the welded portion and improves workability. However, since excessive addition of Nb reduces workability, the upper limit of the Nb content is preferably 0.6%. Moreover, in order to improve said characteristic by containing Nb, it is preferable to contain Nb 0.05% or more. The Nb content is desirably 0.15% to 0.55%.
- Molybdenum (Mo): 3.0% or less by mass% Mo is an element that is effective in repairing a passive film and is very effective in improving corrosion resistance. Further, when Mo is contained together with Cr, there is an effect of effectively improving the pitting corrosion resistance. Moreover, Mo is effective together with Ni to improve flow rust resistance. However, when Mo is increased, the workability is lowered and the cost is increased. For this reason, it is preferable to make the upper limit of Mo content 3.0%. Moreover, in order to improve said characteristic by containing Mo, it is preferable to contain 0.30% or more of Mo. The Mo content is desirably 0.60% to 2.5%, and more desirably 0.9% to 2.0%.
- Ni Nickel (Ni): 2.0% or less by mass% Ni has an effect of suppressing the active dissolution rate and has excellent repassivation characteristics due to a small hydrogen overvoltage. However, excessive addition of Ni reduces workability and makes the ferrite structure unstable. For this reason, it is preferable to make the upper limit of Ni content 2.0%. Moreover, in order to improve said characteristic by containing Ni, it is preferable to contain Ni 0.05% or more.
- the Ni content is desirably 0.1% to 1.2%, and more desirably 0.2% to 1.1%.
- B Boron (B): 0.005% or less by mass% B is a grain boundary strengthening element effective for improving secondary work brittleness.
- B is a grain boundary strengthening element effective for improving secondary work brittleness.
- the lower limit is preferably 0.0001% and the upper limit is preferably 0.005%, and more preferably 0.0002% to 0.0020%.
- Test pieces made of ferritic stainless steel having the chemical components (compositions) shown in Table 1 were produced by the following method. First, a cast steel having a chemical composition (composition) shown in Table 1 was melted by vacuum melting to produce a 40 mm thick ingot, which was hot rolled to a thickness of 5 mm. Thereafter, heat treatment was performed at a temperature of 800 to 1000 ° C. for 1 minute based on each recrystallization behavior, the scale was ground and removed, and a steel sheet having a thickness of 0.8 mm was manufactured by cold rolling. Thereafter, as a final annealing, a heat treatment is performed at a temperature of 800 to 1000 ° C. for 1 minute based on each recrystallization behavior, and the surface oxide scale is removed by pickling. Test specimens 1 to 28 were produced. In addition, in the chemical component (composition) shown in Table 1, the content of each element is expressed by mass%, and the balance is iron and inevitable impurities. Underlined values indicate values outside the scope of the present invention.
- test specimens Nos. 1 to 28 thus obtained were subjected to TIG welding under the following welding conditions, and the black spot generation length ratio was calculated as follows. Further, the following corrosion tests were performed on the test pieces Nos. 1 to 28. "Welding conditions" TIG welding was performed by abutting the same steel type at a feed rate of 50 cm / min and a heat input of 550 to 650 J / cm 2 . Argon was used for the shield on the torch side and the back side.
- Black spot generation length ratio The black spot generation length ratio was determined as a standard representing the generation amount of black spots after TIG welding. The black spot generation length ratio was obtained by integrating the lengths in the welding direction of the black spots generated in the welded portion, and dividing the integrated value by the total weld length. Photographing the weld length of about 10cm with a digital camera, measuring the length of each black spot, and calculating the ratio of the total length of the black spots in the weld length to the weld length using image processing. Determined by
- Corrosion test As the corrosion test piece, a TIG welded portion was used. The overhanging condition was an Erichsen test condition based on JIS 2247, and a 20 mm ⁇ punch was used with the back side of the weld specimen as the surface. However, for the overhang height, the machining was stopped halfway to match the machining conditions. The stop height (overhang height) was unified at 6 mm and 7 mm. Corrosion evaluation was performed by performing a continuous spray test of 5% NaCl according to JIS Z 2371 and evaluating the presence or absence of flow rust after 48 hours.
- the black spot generation length ratio is small, and the black after TIG welding There was little generation of spots.
- the generation of black spots is further suppressed in Nos 1 to 15, 18, and 19 having a BI value of 0.6 or less, and the generation length is 10% or less in Nos 1 to 13 having a BI value of 0.4 or less. The occurrence was almost suppressed.
- the test pieces No. 1 to 21 having an overhang height of 6 mm no rust from the weld was observed in the continuous spray test of 5% NaCl on the corrosion resistance test piece after being processed by the Erichsen tester.
- test pieces No. 1 to 21 with a bulging height of 7 mm which are more severely processed, no rust is observed in the welded portion when the BI value is 0.4 or less, and rust is observed when the test piece exceeds 0.4. It was.
- test pieces Nos. 22, 24, and 26 to 28 having a BI value exceeding 0.8 the black spot generation length ratio after TIG welding was large, and rust from the weld was confirmed in all corrosion tests.
- the rust generating portions of the test pieces Nos. 22, 24, and 26 to 28 were magnified and observed with a magnifying glass, peeling was observed at the boundary between the black spot and the weld bead portion.
- Nos. 22, 26, 27, and 28 in which Al, Ti, Si, and Ca had a concentration higher than the specified level rust was generated in the corrosion test.
- test piece No25 where the composition ratio of Cr is less than 16% and test piece No23 where the composition ratio of Ti is less than 0.05% the occurrence of rust was observed in the corrosion test.
- Test material was manufactured in the same manner as the method for manufacturing the test piece of No. 1 except that a ferritic stainless steel having the chemical composition (composition) shown below was manufactured by cold rolling to produce a steel plate having a thickness of 1 mm. Using this, a test piece A and a test piece B were obtained.
- composition composition
- Test piece B C: 0.009%, N: 0.010%, Si: 0.25%, Mn: 0.15%, P: 0.21%, S: 0.001%, Cr: 20.2%, Al : 0.15%, Ti: 0.19%, Ca: 0.0015%, balance: iron and inevitable impurities
- the test piece A and test piece B thus obtained were the same as the test piece No1 TIG welding was performed under the welding conditions described above, and the appearance of black spots generated on the back side during TIG welding was observed. The results are shown in FIGS. 1A and 1B.
- FIG. 1A is a photograph showing the appearance of black spots generated on the back side during TIG welding.
- FIG. 1B is a schematic view showing the appearance of a black spot generated on the back side during TIG welding, and corresponds to the photograph shown in FIG. 1A. 1A and 1B, the left side is a photograph and drawing of a test piece A having a BI value of 0.49, and the right side is a photograph and drawing of a test piece B having a BI value of 1.07.
- spotted black spots are scattered on both the test piece A having a BI value of 0.49 and the test piece B having a BI value of 1.07.
- Auger electron spectroscopic analysis (AES) measurement was performed on the test piece B having a BI value of 1.07 at two locations, a weld bead portion and a black spot portion. The results are shown in FIGS. 2A and 2B.
- AES Auger electron spectroscopic analysis
- a scanning FE Auger electron spectrometer was used, and measurement was carried out under conditions of an acceleration voltage of 10 keV, a spot diameter of about 40 nm, and a sputtering rate of 15 nm / min until almost no oxygen intensity was observed.
- an error may occur depending on the measurement position, but this time it was adopted as an approximate thickness.
- FIG. 2A and 2B are graphs showing the results of AES measurement of the element depth profile (element concentration distribution in the depth direction) of the black spot and the weld bead on the back side of the test piece.
- FIG. 2A shows the result of the weld bead
- FIG. 2B shows the result of the black spot.
- the weld bead portion was mainly composed of Ti, and was an oxide having a thickness of several hundreds of microns including Al and Si.
- the black spots were mainly oxides of Al, and were thick oxides having a thickness of several thousand ⁇ ⁇ containing Ti, Si, and Ca.
- Al is contained at the highest concentration in the black spot
- Ca is contained in the black spot at a high concentration even though the content in the steel is small. It was confirmed that
- Example 2 C: 0.002 to 0.015%, N: 0.02 to 0.015%, Cr: 16.5 to 23%, Ni: 0 to 1.5%, Mo: 0 to 2.5%
- a ferritic stainless steel specimen having a composition and various chemical components (compositions) having different contents such as Al, Ti, Si, and Ca, which are the main components of the black spot, is manufactured in the same manner as the test piece A. Manufactured by. Using this, a plurality of test pieces were obtained. The plurality of test pieces thus obtained were subjected to TIG welding under the same welding conditions as for the No. 1 test piece, and the black spot generation length ratio was calculated in the same manner as for the No. 1 test piece.
- the black spot generation length ratio tended to increase as Al, Ti, Si, and Ca increased.
- These elements are elements having a particularly strong affinity with oxygen, and among these, the effect of Al is particularly great, and it has been found that Ca has a great influence on black spots despite its low content in steel. Further, it has been found that Ti and Si also contribute to the generation of black spots.
- a BI value represented by the following formula (1) was calculated for each of the plurality of test pieces, and the relationship with the black spot generation length ratio was examined.
- BI 3Al + Ti + 0.5Si + 200Ca ⁇ 0.8
- Al, Ti, Si, and Ca in the formula (1) are the content [% by mass] of each component in the steel.
- FIG. 3 is a graph showing the relationship between the BI value and the black spot generation length ratio. As shown in FIG. 3, it can be seen that the larger the BI value, the larger the black spot generation length ratio.
- FIG. 4 is a graph showing the relationship between the BI value and the corrosion resistance evaluation result after a spray test after processing.
- double circles ( ⁇ ) indicate excellent results
- circles ( ⁇ ) indicate good results
- ⁇ indicate poor results.
- FIG. 4 when the BI value is 0.8 or less, corrosion does not occur in the test piece with the overhang height of 6 mm, and particularly with a test piece with the overhang height of 7 mm when the BI value is 0.4 or less. Since corrosion was not observed, it was very good.
- Ferritic stainless steel of the present invention includes exterior materials, building materials, outdoor equipment, water storage and hot water storage tanks, home appliances, bathtubs, kitchen equipment, drain water recovery equipment for latent heat recovery type gas water heaters and their heat exchangers, various welding
- TIG welding such as pipes
- the ferritic stainless steel of the present invention is suitable for a member to be processed after TIG welding.
- the ferritic stainless steel of the present invention is excellent not only in corrosion resistance but also in workability of a TIG welded portion, it can be widely applied in severe processing applications.
Abstract
Description
本願は、2010年8月6日に、日本に出願された特願2010-177998号に基づき優先権を主張し、その内容をここに援用する。 The present invention relates to a ferritic stainless steel that generates less black spots in a TIG weld.
This application claims priority on August 6, 2010 based on Japanese Patent Application No. 2010-177998 filed in Japan, the contents of which are incorporated herein by reference.
また、特許文献2には、式P1=5Ti+20(Al-0.01)≧1.5(式中のTi,Alは鋼中のそれぞれの成分の含有量を示す)を満たすようにTiとAlを添加することで、溶接熱影響部の耐食性を改善するAl酸化皮膜を、溶接時の鋼の表層部に形成させる技術が開示されている。 In addition, regarding the corrosion resistance of welded parts of ferritic stainless steel, it is known that the scale part produced by heat input during welding deteriorates in corrosion resistance, and a shield with inert gas is sufficient compared to austenitic stainless steel. It is known to be important to implement.
Patent Document 2 describes that Ti and Al so as to satisfy the formula P1 = 5Ti + 20 (Al−0.01) ≧ 1.5 (Ti and Al in the formula indicate the content of each component in the steel). A technique for forming an Al oxide film that improves the corrosion resistance of the weld heat affected zone on the surface layer of the steel during welding is disclosed.
また、特許文献4には、4Al+Ti≦0.32(式中のTi、Alは鋼中のそれぞれの成分の含有量を示す)を満足することで、溶接時の入熱を低減させて溶接部のスケール生成を抑制し、溶接部の耐食性を向上させる技術が開示されている。
前述の従来技術は、溶接部や溶接熱影響部の耐食性を改善させることを目的としたものである。 Patent Document 3 discloses a technique for improving the crevice corrosion resistance of a welded part by adding a certain amount or more of Si in addition to the combined addition of Al and Ti.
Patent Document 4 describes that 4Al + Ti ≦ 0.32 (Ti and Al in the formulas indicate the content of each component in the steel), thereby reducing the heat input during welding and reducing the welded portion. A technique for suppressing the generation of scale and improving the corrosion resistance of the welded portion is disclosed.
The above-described prior art is intended to improve the corrosion resistance of the welded portion and the weld heat affected zone.
本発明の第1の態様は、質量%で、C:0.020%以下、N:0.025%以下、Si:1.0%以下、Mn:1.0%以下、P:0.035%以下、S:0.01%以下、Cr:16.0~25.0%、Al:0.12%以下、Ti:0.05~0.35%、Ca:0.0015%以下を含有し、残部としてFeおよび不可避的不純物からなり、下記(1)式を満足するフェライト系ステンレス鋼である。
BI=3Al+Ti+0.5Si+200Ca≦0.8 …(1)
(なお、(1)式中のAl、Ti、Si、Caは、鋼中の各成分の含有量[質量%]である。)
上記のフェライト系ステンレス鋼においては、溶接部のブラックスポット生成が少ない。 The gist of the present invention is as follows.
The first aspect of the present invention is, in mass%, C: 0.020% or less, N: 0.025% or less, Si: 1.0% or less, Mn: 1.0% or less, P: 0.035. %: S: 0.01% or less, Cr: 16.0 to 25.0%, Al: 0.12% or less, Ti: 0.05 to 0.35%, Ca: 0.0015% or less In addition, the ferritic stainless steel is composed of Fe and inevitable impurities as the balance and satisfies the following formula (1).
BI = 3Al + Ti + 0.5Si + 200Ca ≦ 0.8 (1)
(Al, Ti, Si, and Ca in the formula (1) are the content [% by mass] of each component in the steel.)
In the above ferritic stainless steel, there is little black spot generation at the weld.
本発明の第3の態様は、上記第1または第2の態様にかかるフェライト系ステンレス鋼であって、さらに、質量%で、Mo:3.0%以下を含むフェライト系ステンレス鋼である。
本発明の第4の態様は、上記第1から第3いずれかの態様にかかるフェライト系ステンレス鋼であって、さらに、質量%で、Cu:2.0%以下、Ni:2.0%以下から選ばれる一種又は二種を含むフェライト系ステンレス鋼である。
本発明の第5の態様は、上記第1から第4いずれかの態様にかかるフェライト系ステンレス鋼であって、さらに、質量%で、V:0.2%以下、Zr:0.2%以下から選ばれる一種又は二種を含むフェライト系ステンレス鋼である。
本発明の第6の態様は、上記第1から第5いずれかの態様にかかるフェライト系ステンレス鋼であって、さらに、質量%で、B:0.005%以下を含有するフェライト系ステンレス鋼である。 A second aspect of the present invention is the ferritic stainless steel according to the first aspect, and further includes ferritic stainless steel containing Nb: 0.6% or less by mass%.
A third aspect of the present invention is a ferritic stainless steel according to the first or second aspect, and further includes ferritic stainless steel containing, by mass%, Mo: 3.0% or less.
A fourth aspect of the present invention is the ferritic stainless steel according to any one of the first to third aspects, and further, by mass, Cu: 2.0% or less, Ni: 2.0% or less Ferritic stainless steel containing one or two selected from
A fifth aspect of the present invention is the ferritic stainless steel according to any one of the first to fourth aspects, and further, by mass, V: 0.2% or less, Zr: 0.2% or less Ferritic stainless steel containing one or two selected from
A sixth aspect of the present invention is a ferritic stainless steel according to any one of the first to fifth aspects, further comprising ferritic stainless steel containing, by mass%, B: 0.005% or less. is there.
本発明の溶接部のブラックスポットの生成の少ないフェライト系ステンレス鋼は、下記(1)式を満足するものである。
BI=3Al+Ti+0.5Si+200Ca≦0.8 …(1)
(なお、(1)式中のAl、Ti、Si、Caは、鋼中の各成分の含有量[質量%]である。) Hereinafter, the present invention will be described in detail.
The ferritic stainless steel according to the present invention with little black spot formation satisfies the following formula (1).
BI = 3Al + Ti + 0.5Si + 200Ca ≦ 0.8 (1)
(Al, Ti, Si, and Ca in the formula (1) are the content [% by mass] of each component in the steel.)
まず、上記(1)式を規定する各元素について説明する。
アルミニウム(Al):質量%で0.012%以下
Alは脱酸元素として重要であり,また非金属介在物の組成を制御して組織を微細化する効果もある。しかし、Alはブラックスポットの生成に最も寄与する元素である。また、Alの過剰な添加は、非金属介在物の粗大化を招き,製品の疵発生の起点になる恐れもある。そのため、Al含有量の上限値を0.12%とした。脱酸のためにはAlを0.01%以上含有させることが好ましい。Al含有量は、より望ましくは0.03%~0.10%である。 Next, the component composition of the ferritic stainless steel of the present invention will be described in detail.
First, each element that defines the above equation (1) will be described.
Aluminum (Al): 0.012% or less by mass% Al is important as a deoxidizing element, and also has the effect of controlling the composition of nonmetallic inclusions to refine the structure. However, Al is an element that contributes most to the generation of black spots. Moreover, excessive addition of Al leads to coarsening of non-metallic inclusions, which may be a starting point for product wrinkling. Therefore, the upper limit value of the Al content is set to 0.12%. For deoxidation, it is preferable to contain 0.01% or more of Al. The Al content is more preferably 0.03% to 0.10%.
Tiは、C、Nを固定し、溶接部の粒界腐食を抑制して加工性を向上させる上で非常に重要な元素である。しかしながら、Tiの過剰な添加は、ブラックスポットを生成させるだけでなく、製造時の表面疵の原因となる。このため、Ti含有量の範囲を0.05%~0.35%とした。より望ましくは0.07%~0.20%である。
ケイ素(Si):質量%で1.0%以下
Siは、脱酸元素として重要な元素であり、耐食性、耐酸化性の向上にも有効である。しかし、Siの過剰な添加はブラックスポットの生成を促進するだけでなく、加工性,製造性を低下させる。そのため、Siの含有量の上限値を1.0%とした。脱酸のためにはSiを0.01%以上含有させることが好ましい。Si含有量は、より望ましくは0.05%~0.55%である。 Titanium (Ti): 0.05% to 0.35% by mass
Ti is an extremely important element for fixing C and N and suppressing intergranular corrosion of the welded portion to improve workability. However, excessive addition of Ti not only generates black spots, but also causes surface defects during manufacturing. Therefore, the range of Ti content is set to 0.05% to 0.35%. More desirably, it is 0.07% to 0.20%.
Silicon (Si): 1.0% or less by mass% Si is an important element as a deoxidizing element, and is also effective in improving corrosion resistance and oxidation resistance. However, excessive addition of Si not only promotes the formation of black spots, but also reduces workability and manufacturability. Therefore, the upper limit of Si content is set to 1.0%. For deoxidation, it is preferable to contain 0.01% or more of Si. The Si content is more desirably 0.05% to 0.55%.
Caは脱酸元素として非常に重要であり、非金属介在物として鋼中に微量に含まれる。ただしCaは非常に酸化されやすいため、溶接時にブラックスポットを生成させる大きな要因となる。また、Caは、水溶性介在物を生成させて、耐食性を低下させる場合もある。このため、Caの含有量は極力低いことが望ましく、Caの含有量の上限値を0.0015%とした。より好ましくはCaの含有量は0.0012%以下である。 Calcium (Ca): 0.0015% or less in mass% Ca is very important as a deoxidizing element and is contained in a small amount in steel as a nonmetallic inclusion. However, since Ca is very easily oxidized, it becomes a major factor for generating black spots during welding. Moreover, Ca produces | generates a water-soluble inclusion and may reduce corrosion resistance. For this reason, it is desirable that the Ca content is as low as possible, and the upper limit of the Ca content is set to 0.0015%. More preferably, the Ca content is 0.0012% or less.
炭素(C):質量%で0.020%以下
Cは、耐粒界腐食性および加工性を低下させるため,その含有量を低減させる必要がある。このため,Cの含有量の上限値を0.020%とした。しかし、Cの含有量を過度に低減させると、精錬コストが悪化するため、Cの含有量は0.002%~0.015%であることがより望ましい。
窒素(N):質量%で0.025%以下
Nは、Cと同様に耐粒界腐食性,加工性を低下させるため,その含有量を低減させる必要がある。このため、Nの含有量の上限を0.025%とした。しかし、Nの含有量を過度に低減させると、精錬コストが悪化するため、0.002%~0.015%であることがより望ましい。 Next, other elements constituting the ferritic stainless steel of the present invention will be described.
Carbon (C): 0.020% or less in terms of mass% C reduces the intergranular corrosion resistance and workability, so its content needs to be reduced. For this reason, the upper limit of the C content is set to 0.020%. However, if the C content is excessively reduced, the refining cost deteriorates, so the C content is more preferably 0.002% to 0.015%.
Nitrogen (N): 0.025% or less in mass% N, like C, reduces intergranular corrosion resistance and workability, so its content needs to be reduced. For this reason, the upper limit of the N content is set to 0.025%. However, if the N content is excessively reduced, the refining cost deteriorates, so 0.002% to 0.015% is more desirable.
Mnは,脱酸元素として重要な元素であるが、過剰に添加すると腐食の起点となるMnSを生成しやすくなり、またフェライト組織を不安定化させる。このため、Mnの含有量を1.0%以下とした。脱酸のためにはMnを0.01%以上含有させることが好ましい。より望ましくは,0.05%~0.5%である。さらに望ましくは、0.05%~0.3%である。
燐(P):質量%で0.035%以下
Pは、溶接性,加工性を低下させるだけでなく,粒界腐食を生じやすくするため、低く抑える必要がある。そのためPの含有量を0.035%以下とした。より望ましくは0.001%~0.02%である。 Manganese (Mn): 1.0% or less by mass% Mn is an important element as a deoxidizing element, but if added excessively, it tends to generate MnS, which is the starting point of corrosion, and destabilizes the ferrite structure. Let Therefore, the Mn content is set to 1.0% or less. For deoxidation, it is preferable to contain 0.01% or more of Mn. More desirably, it is 0.05% to 0.5%. More desirably, it is 0.05% to 0.3%.
Phosphorus (P): 0.035% or less in mass% P not only lowers weldability and workability, but also easily causes intergranular corrosion, so it needs to be kept low. Therefore, the content of P is set to 0.035% or less. More desirably, the content is 0.001% to 0.02%.
Sは、CaSやMnS等の腐食の起点となる水溶性介在物を生成させるため,低減させる必要がある。そのため、Sの含有量は0.01%以下とする。ただし、過度の低減はコストの悪化を招く。このため、Sの含有量は、0.0001%~0.005%であることがより望ましい。 Sulfur (S): 0.01% or less by mass% S generates water-soluble inclusions that cause corrosion, such as CaS and MnS, and thus needs to be reduced. Therefore, the S content is 0.01% or less. However, excessive reduction causes cost deterioration. Therefore, the S content is more preferably 0.0001% to 0.005%.
Crは、ステンレス鋼の耐食性を確保する上で最も重要な元素であり、フェライト組織を安定化するために16.0%以上含有させる必要がある。しかし、Crは、加工性、製造性を低下させるため、上限を25.0%とした。Crの含有量は、望ましくは16.5%~23.0%であり、より望ましくは18.0%~22.5%である。 Chromium (Cr): 16.0-25.0% by mass
Cr is the most important element for ensuring the corrosion resistance of stainless steel, and needs to be contained in an amount of 16.0% or more in order to stabilize the ferrite structure. However, Cr lowers the workability and manufacturability, so the upper limit was made 25.0%. The Cr content is desirably 16.5% to 23.0%, and more desirably 18.0% to 22.5%.
Nbは、その特性上単独またはTiと複合して添加することが可能である。NbをTiとともに含有させる場合(Ti+Nb)/(C+N)≧6(式中のTi、Nb、C、Nは、鋼中の各成分の含有量[質量%]である。)を満たすことが好ましい。
Nbは、Tiと同様にC、Nを固定し、溶接部の粒界腐食を抑制して加工性を向上させる元素である。ただし、Nbの過剰な添加は、加工性を低下させるため、Nbの含有量の上限を0.6%とすることが好ましい。また、Nbを含有させることにより、上記の特性を向上させるためには、Nbを0.05%以上含有させることが好ましい。Nbの含有量は、望ましくは0.15%~0.55%である。 Niobium (Nb): 0.6% or less by mass% Nb can be added alone or in combination with Ti due to its characteristics. When Nb is contained together with Ti, it is preferable to satisfy (Ti + Nb) / (C + N) ≧ 6 (Ti, Nb, C, and N in the formula are contents [mass%] of each component in the steel). .
Nb is an element that fixes C and N like Ti and suppresses intergranular corrosion of the welded portion and improves workability. However, since excessive addition of Nb reduces workability, the upper limit of the Nb content is preferably 0.6%. Moreover, in order to improve said characteristic by containing Nb, it is preferable to contain Nb 0.05% or more. The Nb content is desirably 0.15% to 0.55%.
Moは、不働態皮膜の補修に効果があり、耐食性を向上させるのに非常に有効な元素である。また、MoはCrとともに含有されることにより耐孔食性を効果的に向上させる効果がある。またMoは、Niとともに含有されることにより耐流れさび性を改善する効果がある。しかし、Moを増加させると、加工性が低下し、コストが高くなる。このため、Moの含有量の上限を3.0%とすることが好ましい。また、Moを含有させることにより、上記の特性を向上させるためには、Moを0.30%以上含有させることが好ましい。Moの含有量は、望ましくは、0.60%~2.5%であり、より望ましくは0.9%~2.0%である。 Molybdenum (Mo): 3.0% or less by mass% Mo is an element that is effective in repairing a passive film and is very effective in improving corrosion resistance. Further, when Mo is contained together with Cr, there is an effect of effectively improving the pitting corrosion resistance. Moreover, Mo is effective together with Ni to improve flow rust resistance. However, when Mo is increased, the workability is lowered and the cost is increased. For this reason, it is preferable to make the upper limit of Mo content 3.0%. Moreover, in order to improve said characteristic by containing Mo, it is preferable to contain 0.30% or more of Mo. The Mo content is desirably 0.60% to 2.5%, and more desirably 0.9% to 2.0%.
Niは、活性溶解速度を抑制させる効果を有し、また水素過電圧が小さいために再不働態化特性に優れる。ただし、Niの過剰な添加は、加工性を低下させ、フェライト組織を不安定にする。このため、Niの含有量の上限を2.0%とすることが好ましい。また、Niを含有させることにより、上記の特性を向上させるためには、Niを0.05%以上含有させることが好ましい。Niの含有量は、望ましくは0.1%~1.2%であり、より望ましくは0.2%~1.1%である。 Nickel (Ni): 2.0% or less by mass% Ni has an effect of suppressing the active dissolution rate and has excellent repassivation characteristics due to a small hydrogen overvoltage. However, excessive addition of Ni reduces workability and makes the ferrite structure unstable. For this reason, it is preferable to make the upper limit of Ni content 2.0%. Moreover, in order to improve said characteristic by containing Ni, it is preferable to contain Ni 0.05% or more. The Ni content is desirably 0.1% to 1.2%, and more desirably 0.2% to 1.1%.
Cuは、Niと同様に活性溶解速度を低下させるだけでなく、再不働態化を促進する効果を有する。しかし、Cuの過剰な添加は、加工性を低下させる。このため、Cuを添加する場合は上限を2.0%とすることが好ましい。Cuを含有させることにより、上記の特性を向上させるためには、Cuは0.05%以上含有させることが好ましい。Cuの含有量は、望ましくは、0.2%~1.5%であり、更に望ましくは0.25%~1.1%である。 Copper (Cu): 2.0% or less by mass% Cu not only lowers the active dissolution rate in the same manner as Ni, but also has the effect of promoting repassivation. However, excessive addition of Cu reduces workability. For this reason, when adding Cu, it is preferable to make an upper limit into 2.0%. In order to improve said characteristic by containing Cu, it is preferable to contain Cu 0.05% or more. The Cu content is desirably 0.2% to 1.5%, and more desirably 0.25% to 1.1%.
VおよびZrは、耐候性や耐すき間腐食性を改善する。また、Cr、Moの使用を抑えてVを添加すれば優れた加工性も担保することができる。ただし、Vおよび/またはZrの過度の添加は加工性を低下させる上、耐食性向上効果も飽和するため、Vおよび/またはZrを含有する場合の含有量の上限を0.2%とすることが好ましい。また、Vおよび/またはZrを含有させることにより、上記の特性を向上させるためには、Vおよび/またはZrは0.03%以上含有させることが好ましい。また、Vおよび/またはZrの含有量は、より望ましくは0.05%~0.1%である。 Vanadium (V) and / or zirconium (Zr): 0.2% or less by mass% V and Zr improve weather resistance and crevice corrosion resistance. Moreover, if V is added while suppressing the use of Cr and Mo, excellent workability can be secured. However, excessive addition of V and / or Zr lowers workability and also saturates the effect of improving corrosion resistance, so the upper limit of the content when V and / or Zr is contained may be 0.2%. preferable. Moreover, in order to improve said characteristic by containing V and / or Zr, it is preferable to contain V and / or Zr 0.03% or more. Further, the content of V and / or Zr is more desirably 0.05% to 0.1%.
Bは二次加工脆性改善に有効な粒界強化元素であるが、過度の添加はフェライトを固溶強化して延性低下の原因になる。このため、Bを添加する場合は下限を0.0001%、上限を0.005%とすることが好ましく、0.0002%~0.0020%とすることがより望ましい。 Boron (B): 0.005% or less by mass% B is a grain boundary strengthening element effective for improving secondary work brittleness. However, excessive addition causes solid solution strengthening of ferrite and causes a decrease in ductility. Therefore, when B is added, the lower limit is preferably 0.0001% and the upper limit is preferably 0.005%, and more preferably 0.0002% to 0.0020%.
「溶接条件」
TIG溶接は、送り速度50cm/min、入熱550~650J/cm2で同鋼種を突合せて行った。シールドにはトーチ側、裏面側ともアルゴンを用いた。 The test specimens Nos. 1 to 28 thus obtained were subjected to TIG welding under the following welding conditions, and the black spot generation length ratio was calculated as follows. Further, the following corrosion tests were performed on the test pieces Nos. 1 to 28.
"Welding conditions"
TIG welding was performed by abutting the same steel type at a feed rate of 50 cm / min and a heat input of 550 to 650 J / cm 2 . Argon was used for the shield on the torch side and the back side.
ブラックスポット生成長さ比は、TIG溶接後のブラックスポットの生成量を表す基準として求めた。ブラックスポット生成長さ比は、溶接部に生じた各ブラックスポットの溶接方向の長さを積算し、この積算値を、全溶接長さで割って求めた。溶接長さ約10cm分をデジタルカメラで撮影して各ブラックスポットの長さを測定し、画像処理を用いて溶接長さ中におけるブラックスポットの長さの総和の溶接長さに対する比を計算させることにより求めた。 "Black spot generation length ratio"
The black spot generation length ratio was determined as a standard representing the generation amount of black spots after TIG welding. The black spot generation length ratio was obtained by integrating the lengths in the welding direction of the black spots generated in the welded portion, and dividing the integrated value by the total weld length. Photographing the weld length of about 10cm with a digital camera, measuring the length of each black spot, and calculating the ratio of the total length of the black spots in the weld length to the weld length using image processing. Determined by
腐食試験片は、TIG溶接部を張り出し加工したものを用いた。張り出し条件は、JIS2247に準拠したエリクセン試験条件で、溶接試験片の裏波側を表面として、20mmφのポンチを用いた。ただし張り出し高さは、加工条件を合わせるため、加工を途中で停止した。停止高さ(張り出し高さ)は、6mmおよび7mmで統一した。腐食性評価は、JIS Z 2371に準拠して、5%NaClの連続噴霧試験を実施し、48時間後の流れさびの有無で評価した。張り出し高さ6mmの加工材において5%NaClの連続噴霧試験で溶接部に流れさびが認められなかった場合を「良」、張り出し高さ7mmの加工材において同様にさびが認められなかったものを「優良」とした。連続噴霧試験で流れさびが発生した場合を「不良」とした。
表1の化学成分から求めたBI値、ブラックスポット成長長さ比、および腐食試験の結果を表2に示す。 "Corrosion test"
As the corrosion test piece, a TIG welded portion was used. The overhanging condition was an Erichsen test condition based on JIS 2247, and a 20 mmφ punch was used with the back side of the weld specimen as the surface. However, for the overhang height, the machining was stopped halfway to match the machining conditions. The stop height (overhang height) was unified at 6 mm and 7 mm. Corrosion evaluation was performed by performing a continuous spray test of 5% NaCl according to JIS Z 2371 and evaluating the presence or absence of flow rust after 48 hours. For workpieces with an overhang height of 6 mm, the case where no flow rust was observed in the weld in the continuous spray test of 5% NaCl was “good”, and for workpieces with an overhang height of 7 mm, no rust was observed. “Excellent”. The case where flow rust occurred in the continuous spray test was defined as “bad”.
Table 2 shows the BI value, the black spot growth length ratio, and the corrosion test results obtained from the chemical components in Table 1.
このうちBI値が0.6以下のNo1~15、18、19ではよりブラックスポットの生成が抑制されており、更にBI値が0.4以下のNo1~13ではその生成長さが10%以下とほぼその発生が抑制されていた。
さらに張り出し高さ6mmの試験片No1~21では、エリクセン試験機で加工した後の耐食性試験片における5%NaClの連続噴霧試験で溶接部からのさびは認めらなかった。更に、より加工の厳しい張り出し高さ7mmの試験片No1~21においては、BI値が0.4以下の試験片では溶接部のさびは認められず、0.4を超える試験片ではさびが認められた。 As shown in Table 2, in the test pieces No1 to 21 whose chemical composition (composition) is within the range of the present invention and the BI value is 0.8 or less, the black spot generation length ratio is small, and the black after TIG welding There was little generation of spots.
Among these, the generation of black spots is further suppressed in Nos 1 to 15, 18, and 19 having a BI value of 0.6 or less, and the generation length is 10% or less in Nos 1 to 13 having a BI value of 0.4 or less. The occurrence was almost suppressed.
Further, in the test pieces No. 1 to 21 having an overhang height of 6 mm, no rust from the weld was observed in the continuous spray test of 5% NaCl on the corrosion resistance test piece after being processed by the Erichsen tester. Furthermore, in test pieces No. 1 to 21 with a bulging height of 7 mm, which are more severely processed, no rust is observed in the welded portion when the BI value is 0.4 or less, and rust is observed when the test piece exceeds 0.4. It was.
また、Crの組成比が16%未満である試験片No25及びTiの組成比が0.05%未満である試験片No23では、腐食試験でさびの発生が認められた。 On the other hand, in test pieces Nos. 22, 24, and 26 to 28 having a BI value exceeding 0.8, the black spot generation length ratio after TIG welding was large, and rust from the weld was confirmed in all corrosion tests. When the rust generating portions of the test pieces Nos. 22, 24, and 26 to 28 were magnified and observed with a magnifying glass, peeling was observed at the boundary between the black spot and the weld bead portion. In Nos. 22, 26, 27, and 28 in which Al, Ti, Si, and Ca had a concentration higher than the specified level, rust was generated in the corrosion test.
Moreover, in test piece No25 where the composition ratio of Cr is less than 16% and test piece No23 where the composition ratio of Ti is less than 0.05%, the occurrence of rust was observed in the corrosion test.
以下に示す化学成分(組成)を有するフェライト系ステンレス鋼を、冷間圧延により厚み1mmの鋼板を製造したこと以外はNo1の試験片の製造方法と同様にして供試材を製造した。これを用いて試験片Aおよび試験片Bを得た。
「化学成分(組成)」
試験片A
C:0.007%、N:0.011%、Si:0.12%、Mn:0.18%,P:0.22%、S:0.001%、Cr:19.4%、Al:0.06%、Ti:0.15%、Ca:0.0005%、残部:鉄と不可避的不純物
試験片B
C:0.009%、N:0.010%、Si:0.25%、Mn:0.15%,P:0.21%、S:0.001%、Cr:20.2%、Al:0.15%、Ti:0.19%、Ca:0.0015%、残部:鉄と不可避的不純物
このようにして得られた試験片Aおよび試験片Bに対し、No1の試験片と同様の溶接条件でTIG溶接し、TIG溶接時に裏側に生じたブラックスポットの外観を観察した。
その結果を図1A、図1Bに示す。 "Experiment 1"
A test material was manufactured in the same manner as the method for manufacturing the test piece of No. 1 except that a ferritic stainless steel having the chemical composition (composition) shown below was manufactured by cold rolling to produce a steel plate having a thickness of 1 mm. Using this, a test piece A and a test piece B were obtained.
"Chemical composition (composition)"
Specimen A
C: 0.007%, N: 0.011%, Si: 0.12%, Mn: 0.18%, P: 0.22%, S: 0.001%, Cr: 19.4%, Al : 0.06%, Ti: 0.15%, Ca: 0.0005%, balance: iron and inevitable impurities Test piece B
C: 0.009%, N: 0.010%, Si: 0.25%, Mn: 0.15%, P: 0.21%, S: 0.001%, Cr: 20.2%, Al : 0.15%, Ti: 0.19%, Ca: 0.0015%, balance: iron and inevitable impurities The test piece A and test piece B thus obtained were the same as the test piece No1 TIG welding was performed under the welding conditions described above, and the appearance of black spots generated on the back side during TIG welding was observed.
The results are shown in FIGS. 1A and 1B.
図1Aおよび図1Bにおいて左側はBI値が0.49の試験片Aの写真、図面であり、右側はBI値が1.07の試験片Bの写真、図面である。
図1A、図1Bにおいて矢印で示すように、BI値が0.49の試験片A及びBI値が1.07の試験片Bの双方に、斑点状のブラックスポットが散見される。しかし、BI値が大きい試験片B(右側の写真)において、ブラックスポットはより多く発生しているのが分かる。 FIG. 1A is a photograph showing the appearance of black spots generated on the back side during TIG welding. FIG. 1B is a schematic view showing the appearance of a black spot generated on the back side during TIG welding, and corresponds to the photograph shown in FIG. 1A.
1A and 1B, the left side is a photograph and drawing of a test piece A having a BI value of 0.49, and the right side is a photograph and drawing of a test piece B having a BI value of 1.07.
As shown by arrows in FIGS. 1A and 1B, spotted black spots are scattered on both the test piece A having a BI value of 0.49 and the test piece B having a BI value of 1.07. However, it can be seen that more black spots are generated in the test piece B (right photo) having a large BI value.
なお、AES測定においては、走査型FEオージェ電子分光装置を用い、加速電圧10keV、スポット径約40nm、スパッタ速度15nm/minの条件で、酸素の強度が殆ど観測されなくなるまで測定を実施した。なお、AESの測定スポットは小さいため、測定位置により誤差が生じる場合があるが、概略の厚さを示すものとして今回採用した。 Further, Auger electron spectroscopic analysis (AES) measurement was performed on the test piece B having a BI value of 1.07 at two locations, a weld bead portion and a black spot portion. The results are shown in FIGS. 2A and 2B.
In the AES measurement, a scanning FE Auger electron spectrometer was used, and measurement was carried out under conditions of an acceleration voltage of 10 keV, a spot diameter of about 40 nm, and a sputtering rate of 15 nm / min until almost no oxygen intensity was observed. In addition, since the measurement spot of AES is small, an error may occur depending on the measurement position, but this time it was adopted as an approximate thickness.
図2Aに示すように、溶接ビード部は、Tiが主体であり、Al、Siを含む厚さ数百Åの酸化物であった。一方、図2Bに示すように、ブラックスポットは、Alが主体であり、Ti、Si、Caを含む厚さ数千Åの厚い酸化物であった。また、図2Bに示すブラックスポットのグラフより、Alは、ブラックスポットに最も高濃度で含まれており、Caは鋼中の含有量が少ないにもかかわらず、ブラックスポットに高濃度で含まれていることが確認できた。 2A and 2B are graphs showing the results of AES measurement of the element depth profile (element concentration distribution in the depth direction) of the black spot and the weld bead on the back side of the test piece. FIG. 2A shows the result of the weld bead, and FIG. 2B shows the result of the black spot.
As shown in FIG. 2A, the weld bead portion was mainly composed of Ti, and was an oxide having a thickness of several hundreds of microns including Al and Si. On the other hand, as shown in FIG. 2B, the black spots were mainly oxides of Al, and were thick oxides having a thickness of several thousand 含 む containing Ti, Si, and Ca. Further, from the black spot graph shown in FIG. 2B, Al is contained at the highest concentration in the black spot, and Ca is contained in the black spot at a high concentration even though the content in the steel is small. It was confirmed that
C:0.002~0.015%、N:0.02~0.015%、Cr:16.5~23%、Ni:0~1.5%、Mo:0~2.5%を基本組成とし、ブラックスポットの主成分であるAl、Ti、Si、Ca等の含有量の異なる種々の化学成分(組成)を有するフェライト系ステンレス鋼の供試材を、試験片Aと同様の製造方法により製造した。これを用いて、複数の試験片を得た。
このようにして得られた複数の試験片に対し、No1の試験片と同様の溶接条件でTIG溶接し、No1の試験片と同様にしてブラックスポット生成長さ比を算出した。 "Experimental example 2"
C: 0.002 to 0.015%, N: 0.02 to 0.015%, Cr: 16.5 to 23%, Ni: 0 to 1.5%, Mo: 0 to 2.5% A ferritic stainless steel specimen having a composition and various chemical components (compositions) having different contents such as Al, Ti, Si, and Ca, which are the main components of the black spot, is manufactured in the same manner as the test piece A. Manufactured by. Using this, a plurality of test pieces were obtained.
The plurality of test pieces thus obtained were subjected to TIG welding under the same welding conditions as for the No. 1 test piece, and the black spot generation length ratio was calculated in the same manner as for the No. 1 test piece.
BI=3Al+Ti+0.5Si+200Ca≦0.8 …(1)
(なお、(1)式中のAl、Ti、Si、Caは、鋼中の各成分の含有量[質量%]である。)
その結果を図3に示す。図3は、BI値とブラックスポット生成長さ比との関係を示したグラフである。図3に示すように、BI値が大きいほどブラックスポット生成長さ比が大きくなることが分かる。 In addition, a BI value represented by the following formula (1) was calculated for each of the plurality of test pieces, and the relationship with the black spot generation length ratio was examined.
BI = 3Al + Ti + 0.5Si + 200Ca ≦ 0.8 (1)
(Al, Ti, Si, and Ca in the formula (1) are the content [% by mass] of each component in the steel.)
The result is shown in FIG. FIG. 3 is a graph showing the relationship between the BI value and the black spot generation length ratio. As shown in FIG. 3, it can be seen that the larger the BI value, the larger the black spot generation length ratio.
Claims (11)
- 質量%で、C:0.020%以下、N:0.025%以下、Si:1.0%以下、Mn:1.0%以下、P:0.035%以下、S:0.01%以下、Cr:16.0~25.0%、Al:0.12%以下、Ti:0.05~0.35%、Ca:0.0015%以下を含有し、残部がFeおよび不可避的不純物からなり、下記(1)式を満足する、フェライト系ステンレス鋼。
BI=3Al+Ti+0.5Si+200Ca≦0.8 (1)
(なお、(1)式中のAl、Ti、Si、Caは、鋼中の各成分の含有量[質量%]である。) In mass%, C: 0.020% or less, N: 0.025% or less, Si: 1.0% or less, Mn: 1.0% or less, P: 0.035% or less, S: 0.01% Hereinafter, Cr: 16.0 to 25.0%, Al: 0.12% or less, Ti: 0.05 to 0.35%, Ca: 0.0015% or less, with the balance being Fe and inevitable impurities Ferritic stainless steel that satisfies the following formula (1).
BI = 3Al + Ti + 0.5Si + 200Ca ≦ 0.8 (1)
(Al, Ti, Si, and Ca in the formula (1) are the content [% by mass] of each component in the steel.) - 請求項1記載のフェライト系ステンレス鋼であって、さらに、質量%で、Nb:0.6%以下を含む、フェライト系ステンレス鋼。 The ferritic stainless steel according to claim 1, further comprising, by mass%, Nb: 0.6% or less.
- 請求項1記載のフェライト系ステンレス鋼であって、さらに、質量%で、Mo:3.0%以下を含む、フェライト系ステンレス鋼。 The ferritic stainless steel according to claim 1, further comprising, by mass%, Mo: 3.0% or less.
- 請求項2記載のフェライト系ステンレス鋼であって、さらに、質量%で、Mo:3.0%以下を含む、フェライト系ステンレス鋼。 The ferritic stainless steel according to claim 2, further comprising, by mass%, Mo: 3.0% or less.
- 請求項1ないし4いずれか一項記載のフェライト系ステンレス鋼であって、さらに、質量%で、Cu:2.0%以下、Ni:2.0%以下から選ばれる一種又は二種を含む、フェライト系ステンレス鋼。 The ferritic stainless steel according to any one of claims 1 to 4, further comprising, in mass%, one or two selected from Cu: 2.0% or less, Ni: 2.0% or less, Ferritic stainless steel.
- 請求項1ないし4いずれか一項記載のフェライト系ステンレス鋼であって、さらに、質量%で、V:0.2%以下、Zr:0.2%以下から選ばれる一種又は二種を含む、フェライト系ステンレス鋼。 The ferritic stainless steel according to any one of claims 1 to 4, further comprising, in mass%, one or two selected from V: 0.2% or less, Zr: 0.2% or less, Ferritic stainless steel.
- 請求項5記載のフェライト系ステンレス鋼であって、さらに、質量%で、V:0.2%以下、Zr:0.2%以下から選ばれる一種又は二種を含む、フェライト系ステンレス鋼。 The ferritic stainless steel according to claim 5, further comprising one or two kinds selected from V: 0.2% or less and Zr: 0.2% or less by mass%.
- 請求項1ないし4のいずれか一項に記載のフェライト系ステンレス鋼であって、さらに、質量%で、B:0.005%以下を含有する、フェライト系ステンレス鋼。 The ferritic stainless steel according to any one of claims 1 to 4, further comprising, by mass%, B: 0.005% or less.
- 請求項5記載のフェライト系ステンレス鋼であって、さらに、質量%で、B:0.005%以下を含有する、フェライト系ステンレス鋼。 The ferritic stainless steel according to claim 5, further comprising, in mass%, B: 0.005% or less.
- 請求項6記載のフェライト系ステンレス鋼であって、さらに、質量%で、B:0.005%以下を含有する、フェライト系ステンレス鋼。 The ferritic stainless steel according to claim 6, further comprising, in mass%, B: 0.005% or less.
- 請求項7記載のフェライト系ステンレス鋼であって、さらに、質量%で、B:0.005%以下を含有する、フェライト系ステンレス鋼。 The ferritic stainless steel according to claim 7, further comprising, in mass%, B: 0.005% or less.
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP11814699.2A EP2602351B1 (en) | 2010-08-06 | 2011-08-04 | Ferritic stainless steel |
| CN2011800382369A CN103052731A (en) | 2010-08-06 | 2011-08-04 | Ferritic stainless steel |
| KR1020137003262A KR20130034042A (en) | 2010-08-06 | 2011-08-04 | Ferritic stainless steel |
| AU2011286685A AU2011286685A1 (en) | 2010-08-06 | 2011-08-04 | Ferritic stainless steel |
| US13/813,511 US20130129560A1 (en) | 2010-08-06 | 2011-08-04 | Ferritic stainless steel |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2010-177998 | 2010-08-06 | ||
| JP2010177998A JP5793283B2 (en) | 2010-08-06 | 2010-08-06 | Ferritic stainless steel with few black spots |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2012018074A1 true WO2012018074A1 (en) | 2012-02-09 |
Family
ID=45559570
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2011/067850 WO2012018074A1 (en) | 2010-08-06 | 2011-08-04 | Ferritic stainless steel |
Country Status (8)
| Country | Link |
|---|---|
| US (1) | US20130129560A1 (en) |
| EP (1) | EP2602351B1 (en) |
| JP (1) | JP5793283B2 (en) |
| KR (1) | KR20130034042A (en) |
| CN (1) | CN103052731A (en) |
| AU (1) | AU2011286685A1 (en) |
| TW (1) | TWI526549B (en) |
| WO (1) | WO2012018074A1 (en) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20150020933A1 (en) * | 2012-03-30 | 2015-01-22 | Nippon Steel & Sumikin Stainless Steel Corporation | Heat-resistant cold rolled ferritic stainless steel sheet, hot rolled ferritic stainless steel sheet for cold rolling raw material, and methods for producing same |
| CN104903483A (en) * | 2012-11-20 | 2015-09-09 | 奥托库姆普联合股份公司 | Ferritic stainless steel |
| US9885099B2 (en) | 2012-03-09 | 2018-02-06 | Nippon Steel & Sumikin Stainless Steel Corporation | Ferritic stainless steel sheet |
| US10385429B2 (en) | 2013-03-27 | 2019-08-20 | Nippon Steel & Sumikin Stainless Steel Corporation | Hot-rolled ferritic stainless-steel plate, process for producing same, and steel strip |
Families Citing this family (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2014087648A1 (en) * | 2012-12-07 | 2014-06-12 | Jfeスチール株式会社 | Ferritic stainless steel sheet |
| JP5987821B2 (en) * | 2013-12-27 | 2016-09-07 | Jfeスチール株式会社 | Ferritic stainless steel |
| JP5935792B2 (en) * | 2013-12-27 | 2016-06-15 | Jfeスチール株式会社 | Ferritic stainless steel |
| JP5874864B1 (en) * | 2014-07-31 | 2016-03-02 | Jfeスチール株式会社 | Ferritic stainless steel sheet for plasma welding and welding method thereof |
| US20190106775A1 (en) * | 2016-03-29 | 2019-04-11 | Jfe Steel Corporation | Ferritic stainless steel sheet |
| WO2018003521A1 (en) * | 2016-06-27 | 2018-01-04 | Jfeスチール株式会社 | Ferritic stainless steel sheet |
| US11230756B2 (en) | 2016-09-02 | 2022-01-25 | Jfe Steel Corporation | Ferritic stainless steel |
| JP6699670B2 (en) | 2016-09-02 | 2020-05-27 | Jfeスチール株式会社 | Ferritic stainless steel |
| JP2019044255A (en) * | 2017-09-07 | 2019-03-22 | Jfeスチール株式会社 | Ferritic stainless steel sheet |
| JP7042057B2 (en) | 2017-10-25 | 2022-03-25 | 日鉄ステンレス株式会社 | Stainless steel materials and welded structural members with excellent slag spot generation suppression ability and their manufacturing methods |
| TWI801538B (en) * | 2018-03-27 | 2023-05-11 | 日商日鐵不銹鋼股份有限公司 | Ferritic stainless steel, method for producing the same, ferritic stainless steel sheet, method for producing the same, and members for fuel cell |
| ES2927078T3 (en) * | 2018-12-21 | 2022-11-02 | Outokumpu Oy | ferritic stainless steel |
| JP7118015B2 (en) * | 2019-01-16 | 2022-08-15 | 日鉄ステンレス株式会社 | Method for predicting and evaluating the amount of slag spots generated in stainless steel |
| KR102326044B1 (en) * | 2019-12-20 | 2021-11-15 | 주식회사 포스코 | Ferritic stainless steel with improved magnetization properties and manufacturing method thereof |
Citations (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5521102B2 (en) | 1975-02-01 | 1980-06-07 | ||
| JPH0570899A (en) | 1991-09-17 | 1993-03-23 | Nisshin Steel Co Ltd | Ferritic stainless steel excellent in corrosion resistance in weld zone |
| JPH0734205A (en) | 1993-05-19 | 1995-02-03 | Kawasaki Steel Corp | Ferritic stanless steel excellent in atmospheric corrosion resistance and crevice corrosion resistance |
| JPH08144021A (en) * | 1994-11-18 | 1996-06-04 | Sumitomo Metal Ind Ltd | Production of ferritic stainless steel and cold rolled sheet therefrom |
| JPH10102212A (en) * | 1996-09-30 | 1998-04-21 | Kawasaki Steel Corp | Ferritic stainless steel sheet excellent in penetration at welding |
| JP2004131796A (en) * | 2002-10-10 | 2004-04-30 | Nippon Steel Corp | Chromium-containing steel for vessel material, welding method therefor, and vessel material |
| JP2004149833A (en) * | 2002-10-29 | 2004-05-27 | Nippon Yakin Kogyo Co Ltd | Stainless steel excellent in corrosion resistance, weldability, and surface properties and its production method |
| JP2006241564A (en) | 2005-03-07 | 2006-09-14 | Nisshin Steel Co Ltd | Ferritic stainless steel for welded structure |
| JP2006263811A (en) * | 2005-02-28 | 2006-10-05 | Jfe Steel Kk | Ferritic stainless steel filler metal rod for tig welding |
| JP2007270290A (en) | 2006-03-31 | 2007-10-18 | Jfe Steel Kk | Ferritic stainless steel excellent in corrosion resistance of weld zone |
| JP2007302995A (en) * | 2006-04-10 | 2007-11-22 | Nisshin Steel Co Ltd | Ferritic stainless steel for warm water vessel with welded structure and warm water vessel |
| JP2009091654A (en) * | 2007-09-18 | 2009-04-30 | Jfe Steel Kk | Ferritic stainless steel having excellent weldability |
| JP2009174036A (en) * | 2008-01-28 | 2009-08-06 | Nippon Steel & Sumikin Stainless Steel Corp | High purity ferritic stainless steel having excellent corrosion resistance and workability and method for producing the same |
| JP2010177998A (en) | 2009-01-29 | 2010-08-12 | Nippon Telegr & Teleph Corp <Ntt> | User authentication method, user authentication system, user terminal, user authentication device, program for user terminal, and program for user authentication device |
| JP2010202973A (en) * | 2009-02-09 | 2010-09-16 | Nippon Steel & Sumikin Stainless Steel Corp | Ferritic stainless steel with low black spot generation |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH1012212A (en) * | 1996-06-18 | 1998-01-16 | Yuasa Corp | Sealed lead acid battery |
| JP3190290B2 (en) * | 1997-09-26 | 2001-07-23 | 日新製鋼株式会社 | Ferritic stainless steel with excellent corrosion resistance at welds |
| JP4465853B2 (en) * | 2000-10-30 | 2010-05-26 | Jfeスチール株式会社 | Ferritic stainless steel cold rolled steel for jar pot containers and ferritic stainless steel containers for jar pots with excellent corrosion resistance and scale adhesion |
| JP4397772B2 (en) * | 2004-09-24 | 2010-01-13 | 新日鐵住金ステンレス株式会社 | Manufacturing method of ferritic stainless steel sheet with excellent workability |
| ES2396221T3 (en) * | 2007-01-12 | 2013-02-20 | Jfe Steel Corporation | Ferritic stainless steel sheet for water heater with excellent corrosion resistance on a welded part and toughness of the steel sheet |
| JP5010301B2 (en) * | 2007-02-02 | 2012-08-29 | 日新製鋼株式会社 | Ferritic stainless steel for exhaust gas path member and exhaust gas path member |
| JP5111910B2 (en) * | 2007-03-23 | 2013-01-09 | 新日鐵住金ステンレス株式会社 | Ferritic stainless steel with low surface defects and excellent weldability and crevice corrosion resistance |
-
2010
- 2010-08-06 JP JP2010177998A patent/JP5793283B2/en active Active
-
2011
- 2011-08-04 TW TW100127716A patent/TWI526549B/en active
- 2011-08-04 EP EP11814699.2A patent/EP2602351B1/en active Active
- 2011-08-04 WO PCT/JP2011/067850 patent/WO2012018074A1/en active Application Filing
- 2011-08-04 CN CN2011800382369A patent/CN103052731A/en active Pending
- 2011-08-04 US US13/813,511 patent/US20130129560A1/en not_active Abandoned
- 2011-08-04 KR KR1020137003262A patent/KR20130034042A/en not_active Application Discontinuation
- 2011-08-04 AU AU2011286685A patent/AU2011286685A1/en not_active Abandoned
Patent Citations (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5521102B2 (en) | 1975-02-01 | 1980-06-07 | ||
| JPH0570899A (en) | 1991-09-17 | 1993-03-23 | Nisshin Steel Co Ltd | Ferritic stainless steel excellent in corrosion resistance in weld zone |
| JPH0734205A (en) | 1993-05-19 | 1995-02-03 | Kawasaki Steel Corp | Ferritic stanless steel excellent in atmospheric corrosion resistance and crevice corrosion resistance |
| JPH08144021A (en) * | 1994-11-18 | 1996-06-04 | Sumitomo Metal Ind Ltd | Production of ferritic stainless steel and cold rolled sheet therefrom |
| JPH10102212A (en) * | 1996-09-30 | 1998-04-21 | Kawasaki Steel Corp | Ferritic stainless steel sheet excellent in penetration at welding |
| JP2004131796A (en) * | 2002-10-10 | 2004-04-30 | Nippon Steel Corp | Chromium-containing steel for vessel material, welding method therefor, and vessel material |
| JP2004149833A (en) * | 2002-10-29 | 2004-05-27 | Nippon Yakin Kogyo Co Ltd | Stainless steel excellent in corrosion resistance, weldability, and surface properties and its production method |
| JP2006263811A (en) * | 2005-02-28 | 2006-10-05 | Jfe Steel Kk | Ferritic stainless steel filler metal rod for tig welding |
| JP2006241564A (en) | 2005-03-07 | 2006-09-14 | Nisshin Steel Co Ltd | Ferritic stainless steel for welded structure |
| JP2007270290A (en) | 2006-03-31 | 2007-10-18 | Jfe Steel Kk | Ferritic stainless steel excellent in corrosion resistance of weld zone |
| JP2007302995A (en) * | 2006-04-10 | 2007-11-22 | Nisshin Steel Co Ltd | Ferritic stainless steel for warm water vessel with welded structure and warm water vessel |
| JP2009091654A (en) * | 2007-09-18 | 2009-04-30 | Jfe Steel Kk | Ferritic stainless steel having excellent weldability |
| JP2009174036A (en) * | 2008-01-28 | 2009-08-06 | Nippon Steel & Sumikin Stainless Steel Corp | High purity ferritic stainless steel having excellent corrosion resistance and workability and method for producing the same |
| JP2010177998A (en) | 2009-01-29 | 2010-08-12 | Nippon Telegr & Teleph Corp <Ntt> | User authentication method, user authentication system, user terminal, user authentication device, program for user terminal, and program for user authentication device |
| JP2010202973A (en) * | 2009-02-09 | 2010-09-16 | Nippon Steel & Sumikin Stainless Steel Corp | Ferritic stainless steel with low black spot generation |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP2602351A4 |
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9885099B2 (en) | 2012-03-09 | 2018-02-06 | Nippon Steel & Sumikin Stainless Steel Corporation | Ferritic stainless steel sheet |
| US20150020933A1 (en) * | 2012-03-30 | 2015-01-22 | Nippon Steel & Sumikin Stainless Steel Corporation | Heat-resistant cold rolled ferritic stainless steel sheet, hot rolled ferritic stainless steel sheet for cold rolling raw material, and methods for producing same |
| US10260134B2 (en) | 2012-03-30 | 2019-04-16 | Nippon Steel & Sumikin Stainless Steel Corporation | Hot rolled ferritic stainless steel sheet for cold rolling raw material |
| CN104903483A (en) * | 2012-11-20 | 2015-09-09 | 奥托库姆普联合股份公司 | Ferritic stainless steel |
| US20160281184A1 (en) * | 2012-11-20 | 2016-09-29 | Outokumpu Oyj | Ferritic stainless steel |
| US10385429B2 (en) | 2013-03-27 | 2019-08-20 | Nippon Steel & Sumikin Stainless Steel Corporation | Hot-rolled ferritic stainless-steel plate, process for producing same, and steel strip |
Also Published As
| Publication number | Publication date |
|---|---|
| TW201213559A (en) | 2012-04-01 |
| EP2602351A4 (en) | 2017-04-05 |
| EP2602351A1 (en) | 2013-06-12 |
| US20130129560A1 (en) | 2013-05-23 |
| CN103052731A (en) | 2013-04-17 |
| AU2011286685A1 (en) | 2013-02-28 |
| JP2012036444A (en) | 2012-02-23 |
| EP2602351B1 (en) | 2019-10-02 |
| JP5793283B2 (en) | 2015-10-14 |
| TWI526549B (en) | 2016-03-21 |
| KR20130034042A (en) | 2013-04-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2012018074A1 (en) | Ferritic stainless steel | |
| JP5489759B2 (en) | Ferritic stainless steel with few black spots | |
| JP5050863B2 (en) | Ferritic stainless steel sheet for water heaters | |
| JP6206624B1 (en) | Ferritic stainless steel sheet | |
| JP5387802B1 (en) | Ferritic stainless steel | |
| JP2011190524A (en) | Ferritic stainless steel having excellent oxidation resistance, secondary processing brittleness resistance and weld zone toughness | |
| JPWO2019189871A1 (en) | Duplex stainless clad steel sheet and its manufacturing method | |
| JP5928726B2 (en) | Covered arc welding rod | |
| JP5703075B2 (en) | Ferritic stainless steel plate with excellent heat resistance | |
| JP5111910B2 (en) | Ferritic stainless steel with low surface defects and excellent weldability and crevice corrosion resistance | |
| TW201207128A (en) | Structural stainless steel sheet having excellent corrosion resistance at weld and method for manufacturing same | |
| JP4457492B2 (en) | Stainless steel with excellent workability and weldability | |
| JP5012194B2 (en) | Ferritic stainless steel sheet for water heater with high welded joint strength and manufacturing method thereof | |
| JP4465066B2 (en) | Welding materials for ferrite and austenitic duplex stainless steels | |
| JP2016199803A (en) | Ferritic stainless steel | |
| JP2005256121A (en) | Cr-CONTAINING ALLOY HAVING EXCELLENT STRAIN AGING RESISTANCE IN WELD ZONE |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 201180038236.9 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 11814699 Country of ref document: EP Kind code of ref document: A1 |
|
| DPE1 | Request for preliminary examination filed after expiration of 19th month from priority date (pct application filed from 20040101) | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 13813511 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 20137003262 Country of ref document: KR Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 2011286685 Country of ref document: AU Date of ref document: 20110804 Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2011814699 Country of ref document: EP |